Introduction
Acetaminophen (APAP) is a widely used analgesic and
antipyretic drug (1). Although
APAP is generally safe and effective, excessive APAP use can cause
severe liver injury that eventually develops into liver failure
(2). The APAP-induced liver
injury (AILI) animal model displays characteristics similar to
those observed in patients and has been widely used to study the
mechanisms underlying this condition in order to identify potential
therapeutic intervention strategies (3,4).
The main pathogenic mechanism of AILI is that excessive APAP
produces toxic metabolites of N-acetyl-p-benzoquinone
imine (NAPQI) through cytochrome P450 (CYP) metabolism (mainly
CYP2E1). The accumulated NAPQI depletes the intracellular
glutathione levels. Any glutathione that is not bound to NAPQI can
then bind with mitochondrial membrane proteins, leading to
mitochondrial permeability transition. This can directly impair
mitochondrial function in hepatocytes, causing lipid peroxidation,
oxidative stress and DNA fragmentation, thus resulting in
hepatocyte necrosis (5-7). In addition, it has also been shown
that, following APAP treatment, a large number of neutrophils
infiltrate the site of injury, further aggravating liver damage
(8).
Fingolimod (FTY720) is a novel type of
immunosuppressive drug derived from the ascomycete Cordyceps
sinensis (9). Different from
conventional immunosuppressive compounds, such as cyclosporine,
tacrolimus and sirolimus, FTY720 acts by inducing lymphocyte
apoptosis and homing (10).
FTY720 is a sphingosine-1-phosphate (S1P) analogue, which can be
phosphorylated by sphingosine kinase. Phosphorylated (p)-FTY720 is
transported by S1P transporter proteins across the membrane to the
outside of the cell and competitively binds to the S1P receptor,
thus antagonizing downstream S1P signaling (11).
The JAK2/STAT3 signaling pathway can be activated by
various cytokines and participates in numerous important biological
processes, such as cell proliferation, differentiation and immune
regulation (12-14). Previous studies have found that
hepatocyte necrosis was closely related to the JAK2/STAT3 signaling
pathway (15-17). However, it is unclear whether
FTY720 exerts its immunosuppressive effect through the JAK2/STAT3
signaling pathway. Therefore, in order to further explore the
preventive and therapeutic effect of FTY720 in vivo, an AILI
animal model was used. The specific mechanism underlying the effect
of FTY720 on the liver was evaluated by examining serum liver
function markers and pathological changes in hepatocytes (18-20).
Materials and methods
Experimental animals and experimental
design
Male C57BL/6 mice (8-9 weeks of age) were purchased
from the Animal Experimental Center of China Medical University
(Shenyang, China) and housed in a pathogen-free environment
(temperature, 23±2°C; humidity, 55±5%) with a 12-h light/dark cycle
and free access to food and water. Mice weighing 18-22 g (a total
of 192 mice; 64 mice used for 3 cycles of experiments) were
randomly separated into four groups after overnight fasting as
follows: i) Control group, mice were administered 200 μl
PBS; ii) FTY720 group, mice received 5 mg/kg FTY720 [Cayman
Chemical Company; molecular formula,
C19H34NO5P (Fig. 1A); molecular weight, 387.5;
purity ≥98%; solubility in chloroform, 0.5 mg/ml; slightly soluble
in DMSO)] intraperitoneally (i.p.); iii) APAP group, mice received
300 mg/kg APAP (MilliporeSigma) i.p.; and iv) FTY720 + APAP group,
mice were administered FTY720 again after APAP challenge for 30
min. Mice were anesthetized with 200 mg/kg ketamine and 10 mg/kg
xylazine i.p., then euthanized 6 h (n=8/group) or 12 h (n=8/group)
after APAP injection. Serum was collected and livers were
harvested. In addition, AG490 was used to inhibit the JAK2/STAT3
signaling pathway in mice. Mice weighing 18-22 g (a total of 120
mice; 30 mice used for 3 cycles of experiments) were randomly
separated into four groups after overnight fasting as follows: Each
group received 300 mg/kg APAP i.p; i) Control group, mice were
administered 200 μl PBS; ii) FTY720 group, mice received 5
mg/kg FTY720; iii) AG490 group, mice received 50 μM AG490;
and iv) FTY720 + AG490 group, mice were administered FTY720 and
AG490 again after APAP challenge for 30 min. Mice were anesthetized
with 200 mg/kg ketamine and 10 mg/kg xylazine i.p., then euthanized
6 h (n=10/group) or 12 h (n=8/group) after APAP injection. Serum
was collected and livers were harvested. The experimental protocols
were approved (approval no. CMU2021076) by the Institutional Animal
Care and Use Committee and conformed to the Guidelines for the Care
and Use of Laboratory Animals of China Medical University
(Shenyang, China).
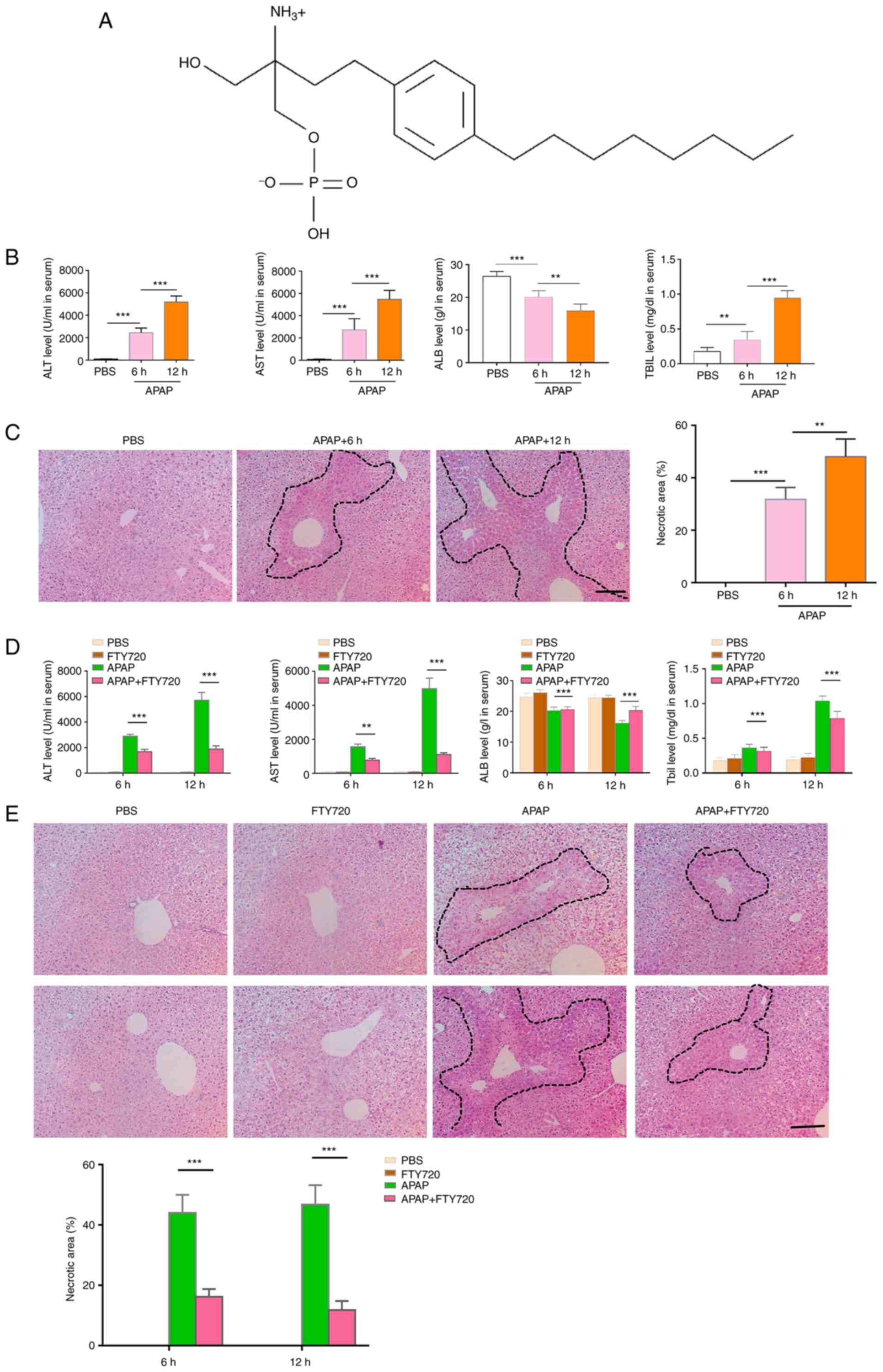 | Figure 1FTY720 alleviates APAP-induced liver
injury. (A) Molecular structure of FTY720. (B) ALT, AST, ALB and
TBIL levels. Mice were treated with APAP and serum was harvested 6
and 12 h post-APAP treatment. (C) H&E staining and necrotic
area quantification of liver sections from APAP-treated mice. Scale
bar, 200 μm. (D) Serum ALT, AST, ALB and TBIL levels in
control, APAP, FTY720 and APAP + FTY720 mice. (E) H&E staining
and necrotic area quantification of liver sections from control,
APAP, FTY720 and APAP + FTY720 mice. Scale bar, 200 μm. The
data are presented as the mean ± SEM. **P<0.01 and
***P<0.001; two-tailed, unpaired Student's t-test.
APAP, acetaminophen; H&E, hematoxylin and eosin; ALT, alanine
transaminase; AST, aspartate transaminase; ALB, albumin; TBIL,
total bilirubin. |
Liver histopathology
The mice were anesthetized with 200 mg/kg ketamine
and 10 mg/kg xylazine i.p., then sacrificed by cervical
dislocation. For histopathological examination, the liver tissues
were fixed in 10% formalin at 25°C for 24 h, then embedded in
paraffin, sectioned at 5-μm thickness and stained with
H&E at 25°C for 10 min. To estimate the extent of necrosis, the
specimen was observed under a light microscope, using ImageJ
software (version 1.8.0; National Institutes of Health) to
calculate the necrotic area.
Immunohistochemistry
Paraffin tissue sections of mouse livers were baked
at 60°C for 1 h. Next, the samples were dewaxed in gradient xylene,
and placed in water after dewaxing. Subsequently, 10 parts of
distilled water to 1 part of 30% H2O2 was
added, and then the sections were washed 3 times with distilled
water at room temperature for 10 min each time. The sections were
then immersed in 0.01 M citric acid buffer, heated to boiling at
maximum power in a microwave (98-100°C), cooled (5-10 min), and
repeated twice. The slices were cooled naturally at room
temperature and washed 3 times with PBS for 5 min each time. Next,
the sections were sealed with 5% BSA at room temperature for 20
min, and excess liquid was removed. The primary antibody (F4/80;
1:500; product no. 70076; Cell Signaling Technology, Inc.) was then
added dropwise at 37°C for 1 h or 4°C overnight. The sections were
then washed 3 times with PBS for 3 min each time. Subsequently, the
secondary antibody (HRP-conjugated goat anti-mouse IgG; product no.
91196; 1:5,000; Cell Signaling Technology, Inc.) was added dropwise
at 37°C for 15-30 min. The sections were then washed 3 times with
PBS for 3 min each time. The streptavidin-biotin complex (SABC) was
then added dropwise at 37°C for 30 min. Next, the sections were
washed 3 times again with PBS for 5 min each time. The color
developing agent was then added dropwise in 1 ml distilled water
and mixed well. After the DAB chromogenic agent was prepared, it
was added onto the sections at room temperature, and the reaction
time was observed under a microscope (~5 min). The sections were
then rinsed with tap water and then with distilled water. The
sections were counterstained with hematoxylin for 2 min and then
rinsed with tap water. Finally, the film was sealed by gradient
dehydration and neutral resin. The film was observed and images
were captured under a light microscope (OLYMPUS IX73; Olympus
Corporation).
Plasma assay
Plasma alanine transaminase (ALT; cat. no.
C009-2-1), aspartate transaminase (AST; cat. no. C010-2-1) and
albumin (ALB; cat. no. A028-2-1; all from Nanjing Jiancheng
Bioengineering Institute), and total bilirubin (TBIL) activities
(cat. no. Hhzt-4576; Shanghai Huzheng Biology Pharmaceutical Co.,
Ltd.) were measured according to the manufacturer's protocol.
Myeloperoxidase (MPO) ELISA
The levels of MPO in the liver tissue were
determined using an MPO ELISA kit [cat. no. ZC-38698; Shanghai Zhuo
Cai Technology Co., Ltd. (ZCIBIO)] according to the manufacturer's
protocol. Briefly, liver tissue samples were washed with PBS and
homogenized in RIPA (cat. no. 89900; Thermo Scientific, Inc.)
buffer using a tissue homogenizer (Ultra-Turrax). The homogenate
was then centrifuged at 5,000 × g for 5 min at 4°C and immediately
used for ELISA. The sample readings were obtained using an ELx800
universal microplate reader (Biotek Instruments, Inc.).
Isolation and culture of primary liver
cells
Mouse hepatocytes were isolated using a two-step
collagenase perfusion method, as previously described (21). The cells were re-suspended in
high-glucose DMEM (HyClone; Cytiva), then filtered using a
100-μm pore size cell strainer to remove undigested tissue
fragments and connective tissue. The samples were then centrifuged
at 50 × g for 3 min at 4°C. The supernatant was discarded and the
cells were then re-suspended in 20 ml high-glucose DMEM. An equal
volume of 90% Percoll (Cytiva) was added and mixed. The samples
were then centrifuged at 200 × g for 10 min at 4°C. The dead cells
at the top of the gradient were discarded, and the cell pellet was
re-suspended in 30 ml high-glucose DMEM. The centrifugation (200 ×
g for 3 min at 4°C) was then repeated. The isolated hepatocytes
were cultured in DMEM containing 10% FBS (Gibco; Thermo Fisher
Scientific, Inc.) and 1% penicillin/streptomycin (Gibco; Thermo
Fisher Scientific, Inc.) at 37°C. Cell viability was determined
using 0.4% Trypan blue staining at room temperature for 5 min.
PI staining
Primary liver cells were cultured in a 24-well
culture plate for 24 h, and then stimulated with APAP
(concentration, 10 mM) or FTY720 (concentration, 5 μM), or
APAP + FTY720 combined for 6 h, and dead cells were stained with 1
μg/ml PI (cat. no. P4170; Sigma-Aldrich; Merck KGaA) for 5
min. The nuclei were then stained with DAPI at room temperature for
5 min. Images were obtained using Nikon eclipse TE 2000-S
fluorescence microscope (Nikon Corporation). The binary processing
function in ImageJ software (version 1.8.0) was used to process
immunofluorescence images and measure the fluorescence parameters
of cells.
Preparation of murine livers for laser
scanning intravital microscopy
The mice were anesthetized with 200 mg/kg ketamine
and 10 mg/kg xylazine (22). A
midline and lateral incision along the costal margin to the
midaxillary line was used to expose the liver. The mouse was placed
in the right lateral position on the heating plate, and the
ligaments connecting the liver to the diaphragm were transected to
allow the liver to become exteriorized onto a glass coverslip. The
blood flow was maintained. To prevent dehydration, exposed
abdominal tissues were covered with a saline-soaked gauze.
Neutrophils of mice at normal body temperature were stained using a
rhodamine-conjugated anti-Ly-6G antibody (1:200; cat. no. PE-65140;
ProteinTech Group, Inc.) for 30 min. Intravital microscopy was
performed using an LSM-880 inverted microscope (Zeiss AG).
Cell viability measurement
Hepatocytes were seeded at a density of
2×103 cells/well and treated with APAP (10 mM) and
FTY720 (5 μM) for 6 h in a 96-well plate incubated at 37°C
with 5% CO2. A 10-μl volume of Cell Counting
Kit-8 (Dojindo Laboratories, Inc.) solution was added to the cells,
which were then incubated at 37°C with 5% CO2 for 2 h.
The optical density was measured at a wavelength of 450 nm.
Reverse transcription-quantitative PCR
(RT-qPCR)
Total RNA was extracted from liver tissue in
TRIzol® (Takara Bio, Inc.) according to the
manufacturer's protocol. First-strand cDNA synthesis was carried
out using a reverse transcription kit (Takara Bio, Inc.) according
to the manufacturer's protocol. SYBR® Green was used to
set up the PCR using an CFX 96 Touch System (Bio-Rad Laboratories,
Inc.) according to the manufacturer's instructions. The
amplification conditions included 32 cycles at 95°C for 20 sec,
57.2°C for 30 sec, 72°C for 30 sec and 95°C for 2 min. qPCR was
carried out using the following primers: TNF-α forward, 5′-ACG GCA
TGG ATC TCA AAG AC-3′ and reverse, 5′-AGA TAG CAA ATC GGC TGA
CG-3′; IL-6 forward, 5′-ACA ACC ACG GCC TTC CCT AC-3′ and reverse,
5′-TCC ACG ATT TCC CAG AGA ACA-3′; IL-1β forward, 5′-TGT CTT GGC
CGA GGA CTA AGG-3′ and reverse, 5′-TGG GCT GGA CTG TTT CTA ATG
C-3′; C-C motif chemokine receptor (CCR)2 forward, 5′-TGG TAA ATT
CTT CAG CTT TTC C-3′ and reverse, 5′-TCC ACA ACC TGA TAA AGC CTC
C-3′; CCR5 forward, 5′-CTG GCC ATC TCT GAC CTG TTT TTC CTC C-3′ and
reverse, 5′-CAG CCC TGT GCC TCT TCT TCT CAT TTC-3′; C-X-C motif
chemokine ligand (CXCL)9 forward, 5′-TCC TTT TGG GCA TCA TCT TC-3′
and reverse, 5′-TTC CCC CTC TTT TGC TTT TT-3′; C-X-C motif
chemokine receptor (CXCR)2 forward, 5′-ATG CCC TCT ATT CTG CCA
GAT-3′ and reverse, 5′-GTG CTC CGG TTG TAT AAG ATG AC-3′; catalase
(CAT) forward, 5′-AGG CTC AGC TGA CAC AGT TC-3′ and reverse, 5′-ATG
GAG AGA CTC GGG ACG AA-3′; glutathione peroxidase (GSH-PX) forward,
5′-TAC AAC ATG TCG GAC CCA CG-3′ and reverse, 5′-CCA GGT GGA ATG
AGG GCA AT-3′; total superoxide dismutase (T-SOD) forward, 5′-GCC
CAA ACC TAT CGT GTC CA-3′ and reverse, 5′-AGG GAA CCC TAA ATG CTG
CC-3′; and GAPDH forward, 5′-TGC AGT GGC AAA GTG GAG ATT-3′ and
reverse, 5′-TCG CTC CTG GAA GAT GGT GAT-3′. qPCR assays were
conducted in triplicate for each sample, and the expression levels
were calculated using the 2−ΔΔCq method (23). GAPDH was used as the housekeeping
gene.
Western blot analysis
Hepatocytes were cultured in six-well plates and
exposed to APAP treatment or FTY720/PBS. The cells were lysed using
RIPA lysis buffer with 1 mM PMSF and 1 mM phosphatase inhibitors
for 30 min on ice, then centrifuged at 500 × g for 15 min at 4°C.
The supernatant was diluted in SDS-PAGE loading buffer (the
proteins were determined using BCA kits), boiled at 100°C for 10
min, and then 20 μg of protein was loaded on 8-12% gels and
transferred to a PVDF membrane (MilliporeSigma). The membranes were
blocked with 5% BSA in PBS with Tween-20 (1:2,000 in PBS) for 1 h
at room temperature, then incubated with primary antibodies against
phosphorylated (p)-JAK2, JAK2, p-STAT3, STAT3, BAX, BCL-2 and GAPDH
overnight at 4°C. The membranes were washed 3-4 times with PBST,
incubated with HRP-conjugated goat anti-mouse IgG secondary
antibody (cat. no. 91196; 1:5,000; Cell Signaling Technology, Inc.)
for 1-2 h at room temperature and washed again 3-4 times with PBST.
The protein bands were visualized using enhanced chemiluminescence
reagent (PerkinElmer, Inc.). A western blotting imaging system
(Model, JP-K300; Shanghai Jiapeng Technology Co., Ltd.) was used to
obtain all images. Densitometric analysis was performed using
ImageJ software version 1.8.0. The list of antibodies is presented
in Table I.
 | Table IList of antibodies. |
Table I
List of antibodies.
| Primary antibodies
(western blotting and immunofluorescence) | Dilution | Product no. | Company |
|---|
| F4/80 | 1:500 | 70076 | Cell Signaling
Technology, Inc. |
| p-JAK2 | 1:1,000 | 4406 | Cell Signaling
Technology, Inc. |
| JAK2 | 1:1,000 | 3230 | Cell Signaling
Technology, Inc. |
| p-STAT3 | 1:1,000 | 52075 | Cell Signaling
Technology, Inc. |
| STAT3 | 1:1,000 | 12640 | Cell Signaling
Technology, Inc. |
| BAX | 1:1,000 | 14796 | Cell Signaling
Technology, Inc. |
| BCL-2 | 1:1,000 | 3498 | Cell Signaling
Technology, Inc. |
| GAPDH | 1:1,000 | 5174 | Cell Signaling
Technology, Inc. |
| p-p65 | 1:1,000 | 3033 | Cell Signaling
Technology, Inc. |
| p65 | 1:1,000 | 8242 | Cell Signaling
Technology, Inc. |
ROS staining
Primary hepatocytes were cultured in a 24-well
culture plate for 24 h, and then stimulated with APAP
(concentration, 10 mM) or FTY720 (concentration, 5 μM), or
APAP + FTY720 + AG490 (concentration, 5 μM; cat. no.
CSN13724; CSNpharm, Inc.) combined at 37°C for 6 h. The primary
hepatocytes were stained with 10 μmol/ml ROS-DHE (cat. no.
D6883; Sigma-Aldrich; Merck KGaA) in a dark incubator at 37°C for
30 min. ROS levels were deter- mined by measuring the fluorescence
under a fluorescence microscope (Olympus Corporation) and analyzed
using ImageJ software (version 1.8.0).
Statistical analysis
The data are presented as the mean ± SD. Data were
analyzed using unpaired two-tailed Student's t-tests. Statistical
analysis was carried out using SPSS software (version 23.0 for
Windows; IBM Corp.). P<0.05 was considered to indicate a
statistically significant difference.
Results
FTY720 alleviates APAP-induced liver
injury
The serum levels of ALT and AST were upregulated 6
and 12 h following treatment with APAP (Fig. 1B), which indicated that excessive
APAP could induce liver injury. Additionally, APAP treatment
resulted in the destruction of liver tissue structure. In fact, as
evidenced by H&E staining, the area of liver injury increased
significantly (Fig. 1C).
However, FTY720 significantly reduced the serum levels of AST and
ALT at 6 and 12 h following treatment with APAP (Fig. 1D). H&E staining also
indicated that FTY720 significantly reduced the area of liver
injury at 6 and 12 h following treatment with APAP (Fig. 1E). These data indicated that
FTY720 could alleviate the liver damage induced by APAP.
FTY720 reduces APAP-induced
inflammation
Previous studies (24-26) have demonstrated that APAP-induced
hepatocyte necrosis causes local inflammation and activates cells
to express adhesion molecules, which promotes the recruitment of
immune cells and the release of pro-inflammatory factors and
chemokines, thereby aggravating liver injury.
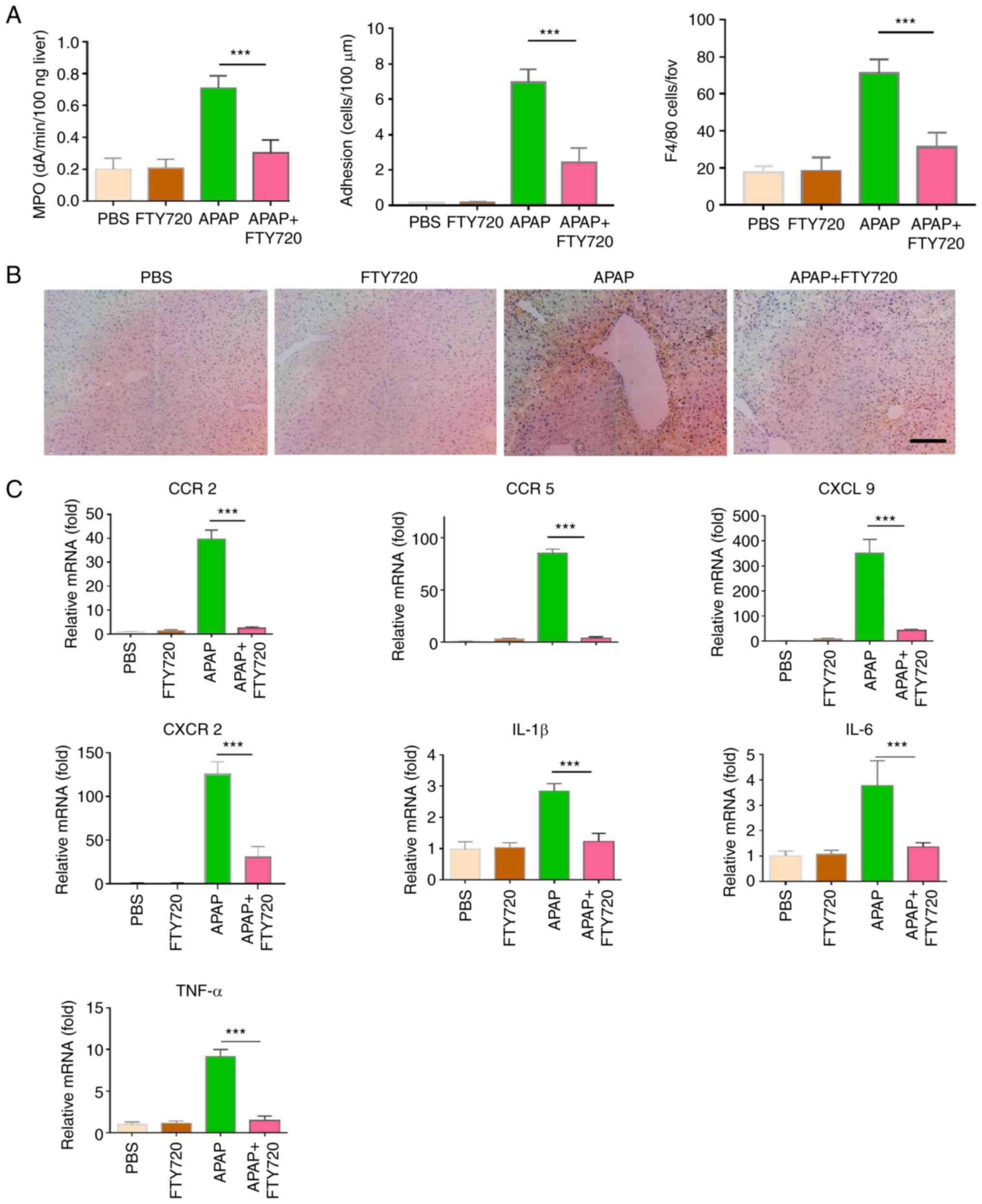
The expression of MPO in the FTY720 + APAP group was
significantly reduced compared with the APAP group (Fig. 2A). Furthermore, the effect of
FTY720 on neutrophil and macrophage recruitment in APAP-induced
liver injury was examined. Compared with the APAP group, macrophage
recruitment was significantly reduced in the FTY720 + APAP group
(Fig. 2B). In vivo
imaging was used to directly observe the dynamic recruitment of
neutrophils under different treatment conditions. A large number of
neutrophils was recruited and adhered to the liver in APAP-treated
mice. However, in the FTY720 + APAP group, recruitment and adhesion
of neutrophils to the liver was reduced. Neutrophils were not
recruited in mice treated with PBS or FTY720 alone (Videos SI-IV).
In addition, FTY720 significantly reduced the expression levels of
the pro-inflammatory cytokines TNF-α, IL-6 and IL-1β in damaged
liver tissue (Fig. 2C).
Similarly, FTY720 significantly reduced the mRNA expression of the
chemokines and chemokine receptors CCR2, CCR5 and CXCL9 (Fig. 2C). These findings indicated that
FTY720 reduces APAP-induced immune cell recruitment and release of
pro-inflammatory cytokines and chemokines, thereby attenuating
AILI.
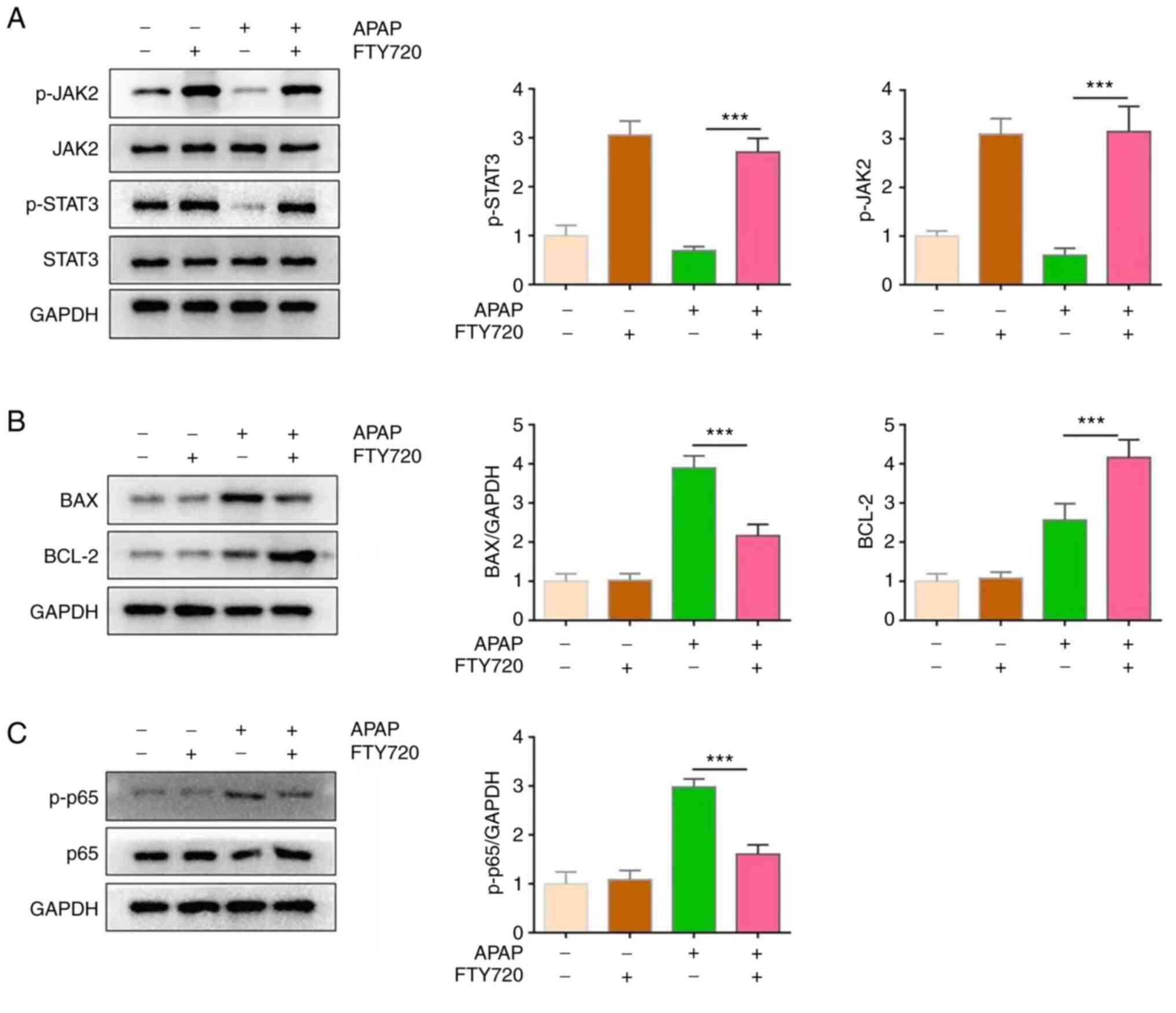
FTY720 can activate JAK2/STAT3 signaling
and inhibit apoptosis and inflammation
Previous studies have suggested that the JAK2/STAT3
signaling pathway is involved in important biological processes,
such as cell proliferation, differentiation, apoptosis and immune
regulation (12-14). The effect of FTY720 on the
JAK2/STAT3 signaling pathway was therefore examined. APAP + FTY720
co-treatment significantly activated the JAK2/STAT3 signaling
pathway compared with APAP treatment alone (Fig. 3A). Furthermore, the BAX protein
expression levels were downregulated, while those of BCL-2 were
upregulated in the APAP + FTY720 group compared with the APAP
treatment group (Fig. 3B). In
addition, FTY720 significantly reduced the activation of p65 in
liver tissue compared with APAP treatment (Fig. 3C). These results indicated that
FTY720 could inhibit apoptosis in hepatocytes.
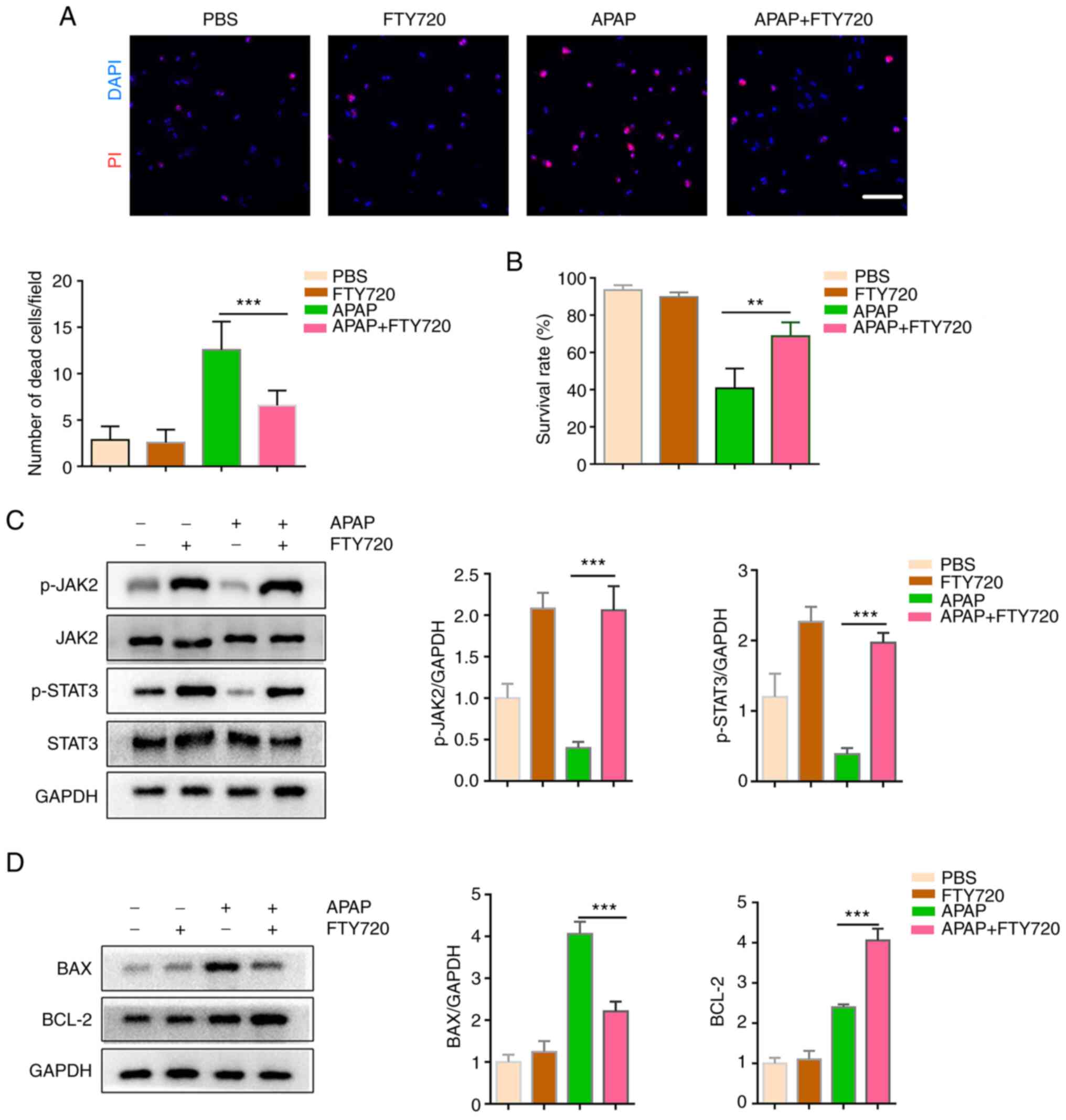
FTY720 can directly protect hepatocytes
against APAP-induced hepatotoxicity
To confirm whether FTY720 has a direct protective
effect on hepatocytes, primary hepatocytes were treated with APAP,
either alone or with FTY720. At the 6-h time-point, APAP induced
hepatocyte apoptosis; however, FTY720 reversed this effect
(Fig. 4A). Compared with APAP
treatment, the viability of primary hepatocytes co-treated with
APAP and FTY720 was increased (Fig.
4B). In addition, the JAK2/STAT3 signaling pathway was
activated in primary hepatocytes co-treated with APAP and FTY720
(Fig. 4C). It was also observed
that FTY720 downregulated the expression of the pro-apoptotic
protein BAX and increased that of the anti-apoptotic protein BCL-2
(Fig. 4D). Thus, consistent with
the in vivo results, FTY720 reduced hepatocyte
apoptosis.
Inhibition of JAK2/STAT3 signaling
attenuates the effect of FTY720 on hepatocyte apoptosis
AG490 (50 μM; 48 h), an inhibitor of the
JAK2/STAT3 signaling pathway, was used in mice following the
administration of APAP, either alone or together with FTY720
(Fig. 5A). In both groups, the
inhibition of JAK2/STAT3 signaling resulted in a significant
increase in ALT and AST (Fig.
5B). H&E staining also demonstrated that, following the
inhibition of the JAK2/STAT3 signaling pathway, the area of damaged
liver tissue significantly increased in both groups (Fig. 5C). In addition, neutrophil
recruitment and the survival rate were also significantly increased
(Fig. 5D and E, respectively).
These data suggested that the JAK2/STAT3 signaling pathway may
mediate the effects of FTY720 on hepatocytes.
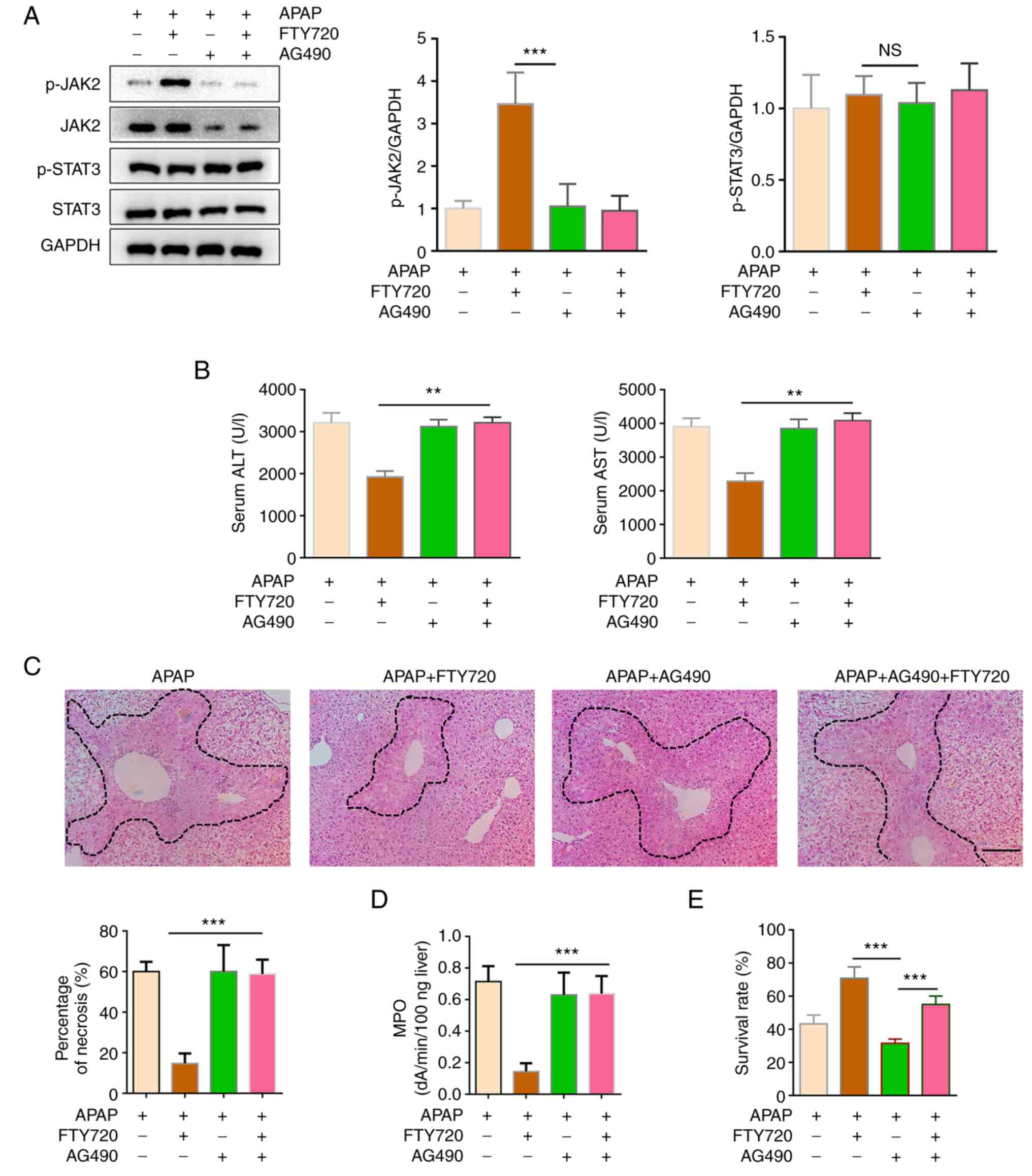 | Figure 5Inhibition of JAK2/STAT3 weakens the
protective effect of FTY720. Mice were treated with APAP, with or
without FTY720 and AG490. (A) p-JAK2, total JAK2, p-STAT3 and total
STAT3 protein abundance were analyzed by western blotting. (B)
Serum ALT and AST levels were assayed by commercial kits. (C)
Hematoxylin and eosin staining and necrotic area quantification of
liver sections. Scale bar, 200 μm. (D) MPO levels were
determined in liver tissue lysates using ELISA. (E) Cell viability
was analyzed using Cell Counting Kit-8 assays. The data are
presented as the mean ± SEM. **P<0.01 and
***P<0.001; two-tailed, unpaired Student's t-test.
APAP, acetaminophen; JAK2, Janus kinase 2; ALT, alanine
transaminase; AST, aspartate transaminase; MPO, myeloperoxidase;
NS, not significant. |
Inhibition of JAK2/STAT3 signaling
attenuates the effect of FTY720 on oxidative stress
Oxidative stress resulting from APAP-induced
hepatocyte apoptosis can aggravate liver injury. The oxidative
stress level of primary hepatocytes decreased significantly
following co-treatment with APAP and FTY720, although this effect
was reversed by AG490 (Fig. 6A).
Moreover, the mRNA expression levels of CAT, GSH-PX and T-SOD,
which are markers of oxidative stress, increased significantly
following FTY720 treatment and were significantly downregulated by
AG490 (Fig. 6B). These data
suggested that FTY720 inhibits APAP-induced oxidative stress by
regulating the JAK2/STAT3 signaling pathway. In summary, APAP led
to hepatocyte apoptosis and increased neutrophil and macrophage
recruitment, further aggravating liver injury. FTY720 can reverse
APAP-induced liver cell apoptosis, excessive oxidative stress
injury and neutrophil and macrophage recruitment by the activating
JAK2/STAT3 signaling pathway, thereby attenuating liver injury
(Fig. 6C).
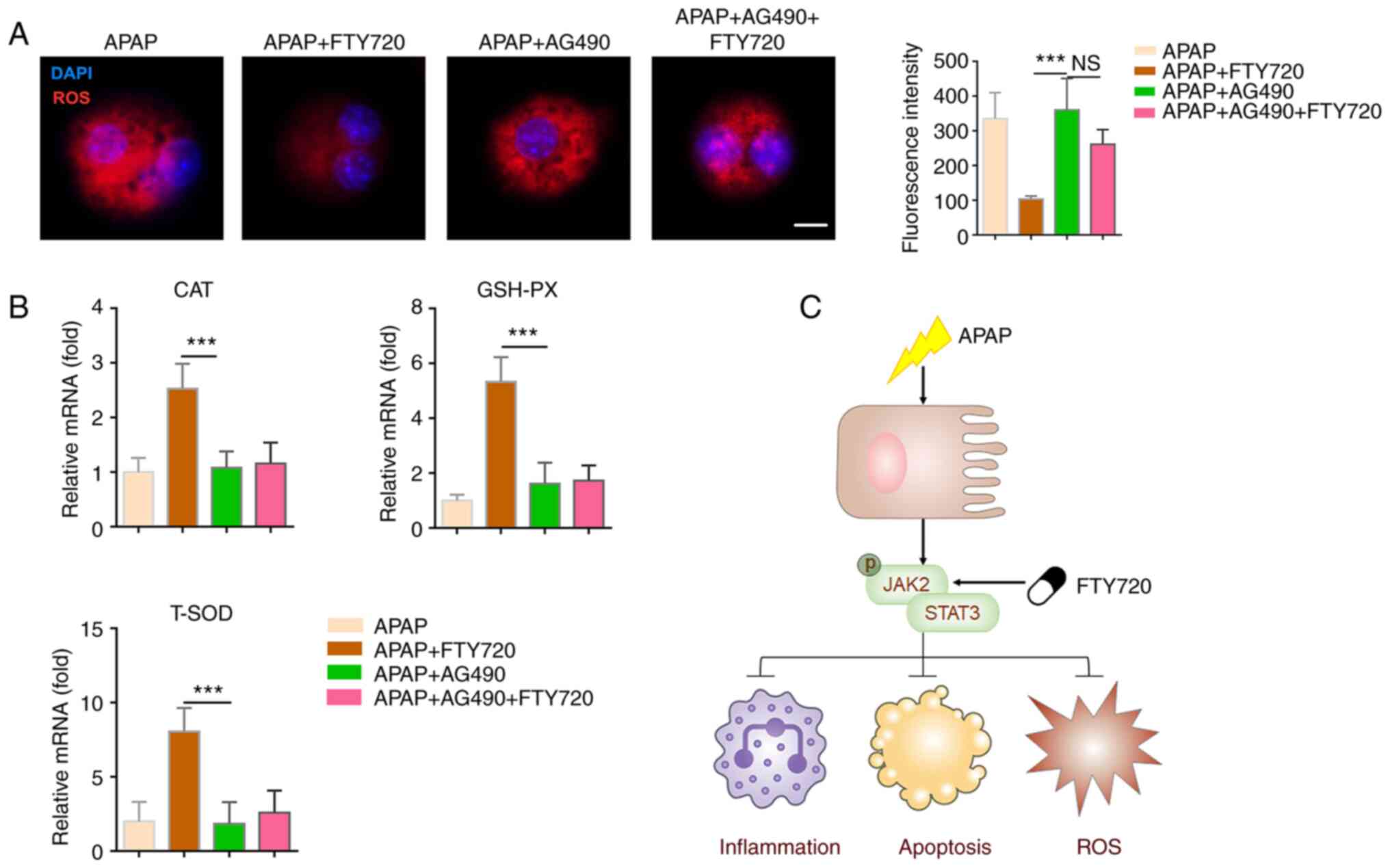 | Figure 6Inhibition of JAK2/STAT3 signaling
attenuates the effects of FTY720. (A) Primary murine hepatocytes
were treated with APAP, with or without FTY720 and AG490. The
levels of ROS were analyzed using a commercial kit. Scale bar, 20
μm. (B) mRNA levels of CAT, GSH-PX and T-SOD in liver
tissue. (C) Schematic illustration of the putative signaling
mechanism. The data are presented as the mean ± SEM.
***P<0.001; two-tailed, unpaired Student's t-test.
APAP, acetaminophen; JAK2, Janus kinase 2; GSH-PX, glutathione
peroxidase; T-SOD, total superoxide dismutase; ROS, reactive oxygen
species; CAT, catalase; NS, not significant. |
Discussion
The improper use of health products, such as
prescription medicines, over-the-counter drugs and traditional
Chinese medicines, can cause varying degrees of liver damage
(27). The incidence of
drug-induced liver injury (DILI) has been increasing in recent
years (28). In the process of
DILI, drugs and their metabolites combine with endogenous
hepatocyte components to form new complexes, which activate
hepatocytes and lead to hepatocyte injury or even death (29,30). In China, DILI accounts for ~10%
of hospitalized patients with acute hepatitis. Clinical screening
also suggests that 20-50% of the adults with elevated transaminase
levels suffer from DILI (31).
Therefore, DILI has become a common clinical liver disease.
It has been reported that excessive APAP intake
leads to the accumulation of the toxic metabolite NAPQI in
hepatocytes (32). The
accumulation of NAPQI downregulates glutathione in hepatocytes;
NAQPI also binds to outer mitochondrial membrane proteins (4,33). The covalent binding of NAPQI to
mitochondrial proteins leads to oxidative stress and the opening of
mitochondrial membrane permeability transition pore, which leads to
reduced mitochondrial membrane potential, mitochondrial function
and ATP production in hepatocytes. This results in lipid
peroxidation, oxidative stress and DNA fragmentation, leading to
the necrosis of hepatocytes (24). Following hepatocyte necrosis, a
large number of damage-associated molecular patterns and chemokines
are released, thereby activating Kupffer cells located in the liver
and recruiting macrophages and neutrophils from the peripheral
circulation to the site of liver injury (25,26). This results in aseptic
inflammation, further aggravating liver injury (7,34). Taken together all aforementioned
studies have revealed that APAP-induced liver injury is a very
complex pathophysiological process, involving inflammation,
apoptosis and neutrophil infiltration.
In the present study, neutrophil infiltration into
the liver was observed following intraperitoneal injection of APAP
in mice. However, when FTY720 and APAP were co-administered, the
number of neutrophils infiltrating the liver was significantly
reduced compared with that of the APAP group. Another study has
suggested that FTY720 induces neutrophil extracellular traps
through an NADPH oxidase-independent pathway and that this compound
could represent a potential antibacterial drug (35). FTY720 is an S1P analog and a
potent S1P receptor modulator. It induces the internalization of
S1P1 and consequently inhibits S1P activity. Previous studies have
also confirmed that S1P prevents egress of hematopoietic stem cells
from the liver to reduce fibrosis and suppresses TLR-induced CXCL8
secretion from human T cells (36,37). Liang et al (38) reported that CXCL9/10 possesses
antifibrotic roles on liver non-parenchymal cells. In previous
studies the depletion of peripheral T cells by FTY720 resulted in
increased infiltration of innate immune cells concomitant to
reduced T-cell infiltration and exacerbation of
hypoxic-ischemic-induced brain injury, which indicates that
neonatal T cells may promote endogenous neuroprotection in the
term-born equivalent hypoxic-ischemic brain potentially providing
new opportunities for therapeutic intervention (39,40). It may be hypothesized that FTY720
could directly affect the recruitment of neutrophils and
macrophages that express S1P receptors. FTY720 is a novel type of
immunosuppressant derived from the ascomycete Cordyceps
sinensis, which plays an important role in immunosuppression by
inducing lymphocyte apoptosis and homing (41,42). Furthermore, it has been reported
that rat spleen cells cultured with FTY720 displayed the typical
characteristics of apoptosis, including disappearance of microvilli
on the cell surface, concentration of chromatin and the formation
of apoptotic bodies (43). Human
lymphocytes co-treated with FTY720 exhibit the same features.
Nagahara et al (44)
suggested that FTY720 induced lymphocyte apoptosis through changes
in membrane permeability and the release of cytochrome c
from cells. However, other studies have shown that the molecular
mechanism of lymphocyte apoptosis was complex and resulted from a
variety of factors (45,46). Due to the downregulation of the
BCL-2 protein and upregulation of the BAX protein, it may be
suggested that the BCL-2 family is involved in FTY720-induced
lymphocyte apoptosis. In the present study, FTY720 reversed the
BAX/BCL-2 ratio and APAP-induced oxidative stress. It has been
demonstrated that FTY720 could inhibit the proliferation of
glomerular mesangial cells by inducing cell cycle arrest and
apoptosis, possibly via the regulation of the expression of cell
cycle-related genes and BAX/BCL-2 (47). In addition, exosomes could
ameliorate the morphology of neurons, reduce inflammatory
infiltration and edema, decrease the expression of BAX and AQP-4,
upregulate the expression of claudin-5 and BCL-2 and inhibit cell
apoptosis (48).
In the present study, AILI led to hepatocyte
apoptosis and upregulation of pro-inflammatory factors, indicative
of activation of the innate immune system. IL family members (IL-6,
-13 and -22) can effectively activate STAT3 during the process of
liver repair. STAT3 is an essential signaling molecule that can
directly or indirectly regulate the expression of important genes
in the process of liver repair (49). STAT3 is mainly coupled with JAK
tyrosine protein kinases and participates in the signal
transduction process downstream of extracellular signals, such as
ILs; with significant anti-apoptotic and pro-mitotic effects
(50). It has also been found
that pro-inflammatory factors can activate and phosphorylate JAK2,
further induce STAT3 phosphorylation, thus participating in the
regulation of gene transcription and mediating the expression of a
variety of pro-inflammatory factors (51). Therefore, detecting the serum
levels of IL-6, -13 and -22, as well as the expression of STAT3 and
JAK2 in liver tissue can provide insight into the effect of FTY720
on AILI.
However, there is a limitation in the present study.
FTY720 hydrochloride is an analog of sphingosine and a potent
modulator of S1P receptors. FTY720 hydrochloride is phosphorylated
by sphingosine kinases, particularly by SK2, and then binds S1PR1,
3, 4, and 5. FTY720 hydrochloride induces the internalization of
S1P1, and consequently, inhibits S1P activity (52-54). S1P was not analyzed in the
present study. Therefore, S1P could be analyzed in the future.
In conclusion, FTY720 could significantly ameliorate
AILI. FTY720 was able to inhibit the metabolism of APAP oxidase and
reduce the production of NAPQI; it could also inhibit the oxidative
stress produced by mitochondrial permeability transition to reduce
hepatocyte death. FTY720 also reduced the infiltration of
neutrophils into the liver, thereby reducing AILI in mice. FTY720
may serve this protective role by regulating the JAK2/STAT3
signaling pathway. The present findings revealed a novel mechanism
through which FTY720 attenuates AILI and provide a theoretical
basis for the clinical treatment of AILI using this compound.
Supplementary Data
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
XH and BC designed the study and carried out the
experiments. KK, DP and YS analyzed the data, as well as wrote and
edited the manuscript. All authors have read and approved the final
manuscript and confirm the authenticity of all the raw data.
Ethics approval and consent to
participate
Experiments were conducted under protocols approved
(approval no. CMU2021076) by the Institutional Animal Care and Use
Committee and conformed to the Guidelines for the Care and Use of
Laboratory Animals of China Medical University (Shenyang,
China).
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
Acknowledgments
Not applicable.
Funding
No funding was received.
References
|
1
|
Shan S, Shen Z and Song F: Autophagy and
acetaminophen-induced hepatotoxicity. Arch Toxicol. 92:2153–2161.
2018. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
[2] Bernal W and
Wendon J: Acute liver failure. N Engl J Med. 369:2525–2534. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
3
|
[3]
Bunchorntavakul C and Reddy KR: Acetaminophen (APAP or
N-Acetyl-p-Aminophenol) and acute liver failure. Clin Liver Dis.
22:325–346. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
[4] Fisher ES and
Curry SC: Evaluation and treatment of acetaminophen toxicity. Adv
Pharmacol. 85:263–272. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
[5]
Jóźwiak-Bebenista M and Nowak JZ: Paracetamol: Mechanism of action,
applications and safety concern. Acta Pol Pharm. 71:11–23.
2014.
|
|
6
|
[6] Ramachandran
A and Jaeschke H: Acetaminophen toxicity: Novel insights into
mechanisms and future perspectives. Gene Expr. 18:19–30. 2018.
View Article : Google Scholar :
|
|
7
|
[7] Ramachandran
A and Jaeschke H: Acetaminophen hepatotoxicity. Semin Liver Dis.
39:221–234. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
[8] He Y, Feng D,
Li M, Gao Y, Ramirez T, Cao H, Kim SJ, Yang Y, Cai Y, Ju C, et al:
Hepatic mitochondrial DNA/Toll-like receptor 9/MicroRNA-223 forms a
negative feedback loop to limit neutrophil overactivation and
acetaminophen hepatotoxicity in mice. Hepatology. 66:220–234. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
9
|
[9] Guo XD, Ji J,
Xue TF, Sun YQ, Guo RB, Cheng H and Sun XL: FTY720 exerts
anti-glioma effects by regulating the glioma microenvironment
through increased CXCR4 internalization by glioma-associated
microglia. Front Immunol. 11:1782020. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
[10] Dragun D,
Fritsche L, Boehler T, Peters H, Budde K and Neumayer HH: FTY720:
Early clinical experience. Transplant Proc. 36(2 Suppl): 544S–548S.
2004. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
[11] Marciniak A,
Camp SM, Garcia JGN and Polt R: An update on
sphingosine-1-phosphate receptor 1 modulators. Bioorg Med Chem
Lett. 28:3585–3591. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
[12] Cheng X,
Yeung PKK, Zhong K, Zilundu PLM, Zhou L and Chung SK: Astrocytic
endothelin-1 overexpression promotes neural progenitor cells
proliferation and differentiation into astrocytes via the
Jak2/Stat3 pathway after stroke. J Neuroinflammation. 16:2272019.
View Article : Google Scholar : PubMed/NCBI
|
|
13
|
[13] Jo S, Wang
SE, Lee YL, Kang S, Lee B, Han J, Sung IH, Park YS, Bae SC and Kim
TH: IL-17A induces osteoblast differentiation by activating
JAK2/STAT3 in ankylosing spondylitis. Arthritis Res Ther.
20:1152018. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
[14] Zhang L and
Wei W: Anti-inflammatory and immunoregulatory effects of
paeoniflorin and total glucosides of paeony. Pharmacol Ther.
207:1074522020. View Article : Google Scholar
|
|
15
|
[15] Hata T,
Rehman F, Hori T and Nguyen JH: GABA, γ-aminobutyric acid, protects
against severe liver injury. J Surg Res. 236:172–183. 2019.
View Article : Google Scholar : PubMed/NCBI
|
|
16
|
[16] Yu HC, Qin
HY, He F, Wang L, Fu W, Liu D, Guo FC, Liang L, Dou KF and Han H:
Canonical notch pathway protects hepatocytes from
ischemia/reperfusion injury in mice by repressing reactive oxygen
species production through JAK2/STAT3 signaling. Hepatology.
54:979–988. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
[17] Zai W, Chen
W, Luan J, Fan J, Zhang X, Wu Z, Ding T, Ju D and Liu H:
Dihydroquercetin ameliorated acetaminophen-induced hepatic
cytotoxicity via activating JAK2/STAT3 pathway and autophagy. Appl
Microbiol Biotechnol. 102:1443–1453. 2018. View Article : Google Scholar
|
|
18
|
[18] Freitas MC,
Uchida Y, Zhao D, Ke B, Busuttil RW and Kupiec-Weglinski JW:
Blockade of Janus kinase-2 signaling ameliorates mouse liver damage
due to ischemia and reperfusion. Liver Transpl. 16:600–610. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
19
|
[19] Zhou HC,
Wang H, Shi K, Li JM, Zong Y and Du R: Hepatoprotective effect of
baicalein against acetaminophen-induced acute liver injury in mice.
Molecules. 24:1312018. View Article : Google Scholar
|
|
20
|
[20] Hong SS,
Choi JH, Lee SY, Park YH, Park KY, Lee JY, Kim J, Gajulapati V, Goo
JI, Singh S, et al: A novel small-molecule inhibitor targeting the
IL-6 receptor β subunit, glycoprotein 130. J Immunol. 195:237–245.
2015. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
[21] Wright MC,
Issa R, Smart DE, Trim N, Murray GI, Primrose JN, Arthur MJ,
Iredale JP and Mann DA: Gliotoxin stimulates the apoptosis of human
and rat hepatic stellate cells and enhances the resolution of liver
fibrosis in rats. Gastroenterology. 121:685–698. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
[22] Liew PX, Lee
WY and Kubes P: iNKT cells orchestrate a switch from inflammation
to resolution of sterile liver injury. Immunity. 47:752–765.e5.
2017. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
[23] Livak KJ and
Schmittgen TD: Analysis of relative gene expression data using
real-time quantitative PCR and the 2(-Delta Delta C(T)) method.
Methods. 25:402–408. 2001. View Article : Google Scholar
|
|
24
|
[24] Athersuch
TJ, Antoine DJ, Boobis AR, Coen M, Daly AK, Possamai L, Nicholson
JK and Wilson ID: Paracetamol metabolism, hepatotoxicity,
biomarkers and therapeutic interventions: A perspective. Toxicol
Res (Camb). 7:347–357. 2018. View Article : Google Scholar
|
|
25
|
[25] Mitchell JR,
Jollow DJ, Potter WZ, Gillette JR and Brodie BB:
Acetaminophen-induced hepatic necrosis. IV. Protective role of
glutathione. J Pharmacol Exp Ther. 187:211–217. 1973.PubMed/NCBI
|
|
26
|
[26] Giustarini
G, Kruijssen L, van Roest M, Bleumink R, Weaver RJ,
Bol-Schoenmakers M, Smit J and Pieters R: Tissue influx of
neutrophils and monocytes is delayed during development of
trovafloxacin-induced tumor necrosis factor-dependent liver injury
in mice. J Appl Toxicol. 38:753–765. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
[27] Lesiński W,
Mnich K, Golińska AK and Rudnicki WR: Integration of human cell
lines gene expression and chemical properties of drugs for drug
induced liver injury prediction. Biol Direct. 16:22021. View Article : Google Scholar
|
|
28
|
[28] Kong X, Guo
D, Liu S, Zhu Y and Yu C: Incidence, characteristics and risk
factors for drug-induced liver injury in hospitalized patients: A
matched case-control study. Br J Clin Pharmacol. 87:4304–4312.
2021. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
[29] Katarey D
and Verma S: Drug-induced liver injury. Clin Med (Lond). 16(Suppl
6): S104–S109. 2016. View Article : Google Scholar
|
|
30
|
[30] Fisher K,
Vuppalanchi R and Saxena R: Drug-induced liver injury. Arch Pathol
Lab Med. 139:876–887. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
[31] Shen T, Liu
Y, Shang J, Xie Q, Li J, Yan M, Xu J, Niu J, Liu J, Watkins PB, et
al: Incidence and etiology of drug-induced liver injury in mainland
China. Gastroenterology. 156:2230–2241.e11. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
[32] Jaeschke H,
Murray FJ, Monnot AD, Jacobson-Kram D, Cohen SM, Hardisty JF,
Atillasoy E, Hermanowski-Vosatka A, Kuffner E, Wikoff D, et al:
Assessment of the biochemical pathways for acetaminophen toxicity:
Implications for its carcinogenic hazard potential. Regul Toxicol
Pharmacol. 120:1048592021. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
[33] Papp S,
Moderzynski K, Rauch J, Heine L, Kuehl S, Richardt U, Mueller H,
Fleischer B and Osterloh A: Liver necrosis and lethal systemic
inflammation in a murine model of rickettsia typhi Infection: Role
of neutrophils, macrophages and NK cells. PLoS Negl Trop Dis.
10:e00049352016. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
[34] Ishitsuka Y,
Kondo Y and Kadowaki D: Toxicological property of acetaminophen:
The dark side of a safe antipyretic/analgesic drug. Biol Pharm
Bull. 43:195–206. 2020. View Article : Google Scholar
|
|
35
|
[35] Zhang L, Gao
H, Yang L, Liu T, Zhang Q, Xun J, Li C, Cui L and Wang X: FTY720
induces neutrophil extracellular traps via a NADPH
oxidase-independent pathway. Arch Biochem Biophys. 711:1090152021.
View Article : Google Scholar : PubMed/NCBI
|
|
36
|
[36] King A,
Houlihan DD, Kavanagh D, Haldar D, Luu N, Owen A, Suresh S, Than
NN, Reynolds G, Penny J, et al: Sphingosine-1-phosphate prevents
egress of hematopoietic stem cells from liver to reduce fibrosis.
Gastroenterology. 153:233–248.e16. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
[37] Sharma N,
Akhade AS and Qadri A: Sphingosine-1-phosphate suppresses
TLR-induced CXCL8 secretion from human T cells. J Leukoc Biol.
93:521–528. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
[38] Liang YJ,
Luo J, Lu Q, Zhou Y, Wu HW, Zheng D, Ren YY, Sun KY, Wang Y and
Zhang ZS: Gene profile of chemokines on hepatic stellate cells of
schistosome-infected mice and antifibrotic roles of CXCL9/10 on
liver non-parenchymal cells. PLoS One. 7:e424902012. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
[39] Herz J,
Köster C, Crasmöller M, Abberger H, Hansen W, Felderhoff-Müser U
and Bendix I: Peripheral T cell depletion by FTY720 exacerbates
hypoxic-ischemic brain injury in neonatal mice. Front Immunol.
9:16962018. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
[40] Sola A,
Weigert A, Jung M, Vinuesa E, Brecht K, Weis N, Brüne B, Borregaard
N and Hotter G: Sphingosine-1-phosphate signalling induces the
production of Lcn-2 by macrophages to promote kidney regeneration.
J Pathol. 225:597–608. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
[41] Tsai HC and
Han MH: Sphingosine-1-Phosphate (S1P) and S1P signaling pathway:
Therapeutic targets in autoimmunity and inflammation. Drugs.
76:1067–1079. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
[42] Zhao M, Yang
C, Chai S, Yuan Y, Zhang J, Cao P, Wang Y, Xiao X, Wu K, Yan H, et
al: Curcumol and FTY720 synergistically induce apoptosis and
differentiation in chronic myelomonocytic leukemia via multiple
signaling pathways. Phytother Res. 35:2157–2170. 2021. View Article : Google Scholar
|
|
43
|
[43] Takai K,
Takahara S, Isoyama N, Tsuchida M, Matsumura M, Kishi Y, Uchiyama K
and Naito K: Effects of FTY720 on rat lymphoid organs. Transplant
Proc. 36:2453–2456. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
[44] Nagahara Y,
Enosawa S, Ikekita M, Suzuki S and Shinomiya T: Evidence that
FTY720 induces T cell apoptosis in vivo. Immunopharmacology.
48:75–85. 2000. View Article : Google Scholar
|
|
45
|
[45] Tuckermann
JP, Kleiman A, McPherson KG and Reichardt HM: Molecular mechanisms
of glucocorticoids in the control of inflammation and lymphocyte
apoptosis. Crit Rev Clin Lab Sci. 42:71–104. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
46
|
[46]
Sordo-Bahamonde C, Lorenzo-Herrero S, Payer ÁR, Gonzalez S and
López-Soto A: Mechanisms of apoptosis resistance to NK
cell-mediated cytotoxicity in cancer. Int J Mol Sci. 21:37262020.
View Article : Google Scholar :
|
|
47
|
[47] Jiang J,
Huang X, Wang Y, Deng A and Zhou J: FTY720 induces cell cycle
arrest and apoptosis of rat glomerular mesangial cells. Mol Biol
Rep. 39:8243–8250. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
48
|
[48] Chen J,
Zhang C, Li S, Li Z, Lai X and Xia Q: Exosomes derived from nerve
stem cells loaded with FTY720 promote the recovery after spinal
cord injury in rats by PTEN/AKT signal pathway. J Immunol Res.
2021:81002982021. View Article : Google Scholar : PubMed/NCBI
|
|
49
|
[49] Hu Z, Han Y,
Liu Y, Zhao Z, Ma F, Cui A, Zhang F, Liu Z, Xue Y, Bai J, et al:
CREBZF as a key regulator of STAT3 pathway in the control of liver
regeneration in mice. Hepatology. 71:1421–1436. 2020. View Article : Google Scholar
|
|
50
|
[50] Ozaki M:
Cellular and molecular mechanisms of liver regeneration:
Proliferation, growth, death and protection of hepatocytes. Semin
Cell Dev Biol. 100:62–73. 2020. View Article : Google Scholar
|
|
51
|
[51] Zegeye MM,
Lindkvist M, Fälker K, Kumawat AK, Paramel G, Grenegård M, Sirsjö A
and Ljungberg LU: Activation of the JAK/STAT3 and PI3K/AKT pathways
are crucial for IL-6 trans-signaling-mediated pro-inflammatory
response in human vascular endothelial cells. Cell Commun Signal.
16:552018. View Article : Google Scholar : PubMed/NCBI
|
|
52
|
[52] Foster AD,
Vicente D, Clark N, Leonhardt C, Elster EA, Davis TA and Bradley
MJ: FTY720 effects on inflammation and liver damage in a rat model
of renal ischemia-reperfusion injury. Mediators Inflamm.
2019:34968362019. View Article : Google Scholar : PubMed/NCBI
|
|
53
|
[53] Zhao Y, Man
K, Lo CM, Ng KT, Li XL, Sun CK, Lee TK, Dai XW and Fan ST:
Attenuation of small-for-size liver graft injury by FTY720:
Significance of cell-survival Akt signaling pathway. Am J
Transplant. 4:1399–1407. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
54
|
[54] Kaneko T,
Murakami T, Kawana H, Takahashi M, Yasue T and Kobayashi E:
Sphingosine-1-phosphate receptor agonists suppress concanavalin
A-induced hepatic injury in mice. Biochem Biophys Res Commun.
345:85–92. 2006. View Article : Google Scholar : PubMed/NCBI
|