Introduction
Autophagy is a self-digesting and auto-metabolic
process that serves to stabilize intracellular metabolism under
stressful conditions, such as hypoxia, infection and nutrient
deficiency (1). A number of
reports have suggested that autophagy is involved in a variety of
physiological and pathophysiological processes in the dental pulp
tissue, including regeneration (2), odontogenic differentiation
(3,4), cellular aging (5) and stress adaptation (6-8).
In particular, a previous study has demonstrated that
lipopolysaccharide can induce autophagy in human dental pulp cells
(HDPCs) (9).
Autophagy has been reported to be a selective
process under certain circumstances, such that mitochondrial
autophagy (mitophagy) is considered to be a specialized form of
autophagy, which maintains mitochondrial quality and homeostasis
(10). Mitophagy is activated by
two pathways, namely the ubiquitin-dependent and receptor-dependent
pathways (11). FUN14
domain-containing 1 (FUNDC1) is a receptor that has been previously
found to activate hypoxia-induced mitophagy (12). FUNDC1 is an integral molecule
localized to the outer mitochondrial membrane that can interact
with the LC3 protein to activate mitophagy, by altering its
phosphorylation states (12).
Accumulating evidence has revealed that FUNDC1-related mitophagy is
closely associated with the initiation, development and prognosis
of various diseases, such as cardiovascular diseases (13), cancer (14) and sepsis (15). These findings suggest that
mitophagy and FUNDC1 can serve as a potential prognostic marker or
as a promising therapeutic target. Previously, FUNDC1 has also been
demonstrated to promote the proliferation, migration and
differentiation of breast cancer cells (16). However, to the best of our
knowledge, the role of FUNDC1 in dental pulp diseases has not been
reported previously.
Dental pulp tissue forms the soft component of the
tooth that mainly consists of dental pulp cells and odontoblasts
(17). HDPCs include unique
mesenchymal stem cells with pluripotent potential (18). In response to injury or
infection, HDPCs can differentiate into odontoblasts and migrate to
the site of damage, where they mediate injury repair (19). Surrounded by the dentin and
nutritionally supported by the blood vessels through the apical
foramen, the dental pulp tissue is particularly susceptible to
hypoxia after the occurrence of inflammation (20). Therefore, it was hypothesized
that autophagy, mitophagy, proliferation, migration and odontogenic
differentiation in HDPCs may all be influenced by hypoxia, a
process in which FUNDC1 may also serve a potential role.
Given that injury repair and protection are of
significance when the dental pulp is damaged, the present study
aimed to explore the relationship between FUNDC1-related mitophagy
and the regeneration of HDPCs under hypoxic conditions.
Materials and methods
Specimen collection
All samples were obtained from The Hospital of
Stomatology, Guanghua School of Stomatology, Sun Yat-sen University
(Guangzhou, China) between September 2019 and March 2020. Written
informed consent was received from all patients, parent or guardian
of the patients involved. The present study was approved by the
Ethics Committee of Hospital of Stomatology and Guanghua School of
Stomatology, affiliated to Sun Yat-sen University [protocol no.
ERC-(2017)-27; Guangzhou, China].
Healthy human premolars or third molars were
obtained from 15 orthodontic patients aged 13-29 years of either
sex (sex, 8 males, 7 females; mean age, 23.9±4.3 years) from
orthodontic extraction. By contrast, inflamed teeth were collected
from 15 patients (sex, 8 males, 7 females; age range, 13-29 years;
mean age, 23.1±4.4 years) who were diagnosed with irreversible
pulpitis. The inclusion criteria were mature permanent teeth with
history of spontaneous pain or severe, prolonged pain to thermal
stimulation. The exclusion criteria were any teeth with periodontal
diseases or radiographic evidence of internal or external
resorption, inter-radicular bone loss or periapical pathology. The
pulps of the healthy and inflamed groups were obtained by splitting
the teeth longitudinally. In total, five tissues from each group
were randomly selected for immunohistochemical staining and fixed
at 25°C with 4% (w/v) paraformaldehyde (Sigma-Aldrich; Merck KGaA)
for 24 h. Tissues were then embedded in paraffin and
5-µm-thick serial sections were prepared. Samples destined
for reverse transcription-quantitative PCR (RT-qPCR) were preserved
at −80°C.
RT-qPCR
The extraction and purification of total RNA from
pulp tissues were performed according to the manufacturer's
protocols of the RNA-Quick Purification kit (Shanghai Yishan
Biotechnology Co., Ltd.). A NanoDrop® 2000 instrument
(Thermo Fisher Scientific, Inc.) was used to measure the
concentration and quality of the RNA samples. RNA (2 µg) was
reverse transcribed into cDNA using an Evo M-MLV RT Premix kit
(cat. no. AG11706; Hunan Aikerui Biological Engineering Co., Ltd.)
by applying the following three stages: 37°C for 15 min, 85°C for 5
sec and then cooling at 4°C. Subsequently, qPCR was performed using
the SYBR® Green Premix Pro Taq HS qPCR kit (cat. no.
AG11701; Hunan Aikerui Biological Engineering Co., Ltd.) in a
LightCycler 480 system (Roche Diagnostics), using a 20-µl
reaction system. The PCR primers were synthesized by Sangon Biotech
Co., Ltd. (Table I). The
thermocycling conditions were as follows: 95°C for 5 min, followed
by 40 cycles of 95°C for 10 sec, 60°C for 20 sec and then 72°C for
20 sec; melting at 95°C for 5 sec, 65°C for 60 sec and 97°C for 1
sec and cooling at 40°C for 10 sec. The expression levels of each
gene were normalized to β-actin using the 2−ΔΔCq method
(21).
 | Table IPrimer sequences. |
Table I
Primer sequences.
| Gene | Primer sequence,
5′-3′ |
|---|
| IL-1β | Forward:
GCCAGTGAAATGATGGCTTATT |
| Reverse:
AGGAGCACTTCATCTGTTTAGG |
| IL-6 | Forward:
CACTGGTCTTTTGGAGTTTGAG |
| Reverse:
GGACTTTTGTACTCATCTGCAC |
| IL-8 | Forward:
AACTGAGAGTGATTGAGAGTGG |
| Reverse:
ATGAATTCTCAGCCCTCTTCAA |
| TNF-α | Forward:
TGGCGTGGAGCTGAGAGATAACC |
| Reverse:
CGATGCGGCTGATGGTGTGG |
| HIF-1α | Forward:
ACGTTCCTTCGATCAGTTGTCACC |
| Reverse:
GGCAGTGGTAGTGGTGGCATTAG |
| FUNDC1 | Forward:
GCAGTAGGTGGTGGCTTTC |
| Reverse:
TGCTTTGTTCGCTCGTTT |
| β-actin | Forward:
GCATGGGTCAGAAGGATTCCT |
| Reverse:
TCGTCCCAGTTGGTGACGAT |
Immunohistochemical staining
Immunohistochemical staining was performed to
evaluate the expression levels of hypoxia-inducible factor-1α
(HIF-1α) and FUNDC1 in the healthy and inflamed groups as described
previously (22). The sections
were dewaxed and rehydrated with xylene and ethanol, then had their
antigens retrieved in citrate buffer for 15 min in a microwave oven
at 98°C. According to the manufacturer's protocol for the SP Rabbit
& Mouse HRP kit (DAB; cat. no. CW2069M; CoWin Biosciences),
endogenous peroxidase was blocked by using hydrogen peroxide
(Solution A) for 10 min at room temperature. The sections were then
blocked at room temperature for 30 min in goat serum (Solution B;
contained in the kit) and incubated overnight at 4°C with the
primary antibodies. The primary antibodies used were: Anti-HIF-1α
(1:150 dilution; cat. no. AF1009; Affinity Biosciences) and rabbit
polyclonal anti-FUNDC1 (1:150 dilution; cat. no. ab224722; Abcam).
After washing with PBS (Gibco; Thermo Fisher Scientific, Inc.), the
sections were incubated with biotinylated goat anti-rabbit
immunoglobulin G secondary antibody (Solution C; contained in the
kit) for 10 min at room temperature and stained using the
streptavidin-biotin-peroxidase complex (Solution D). The
chromophore used was 3,3′-diaminobenzidine (DAB). The stained
sections were counterstained with hematoxylin (cat. no. C0105S;
Beyotime Institute of Biotechnology) for 1 min at room temperature.
For immunohistochemical staining, the slides were scanned using a
light microscope (×20 and ×80 magnification; Leica Aperio AT2
instrument; Leica Microsystems, Inc.).
Isolation and culture of HDPCs
After splitting the healthy teeth, the pulp tissues
were carefully separated and transferred onto a tissue culture
dish, rinsed three times with sterile PBS (Gibco; Thermo Fisher
Scientific, Inc.) containing 2% penicillin-streptomycin (Gibco;
Thermo Fisher Scientific, Inc.), gently cut into small pieces (1
mm2) and digested with 3 mg/ml collagenase I and 4 mg/ml
dispase II (Sigma-Aldrich; Merck KGaA) at 37°C for 30 min. By the
enzymatic digestion method, the resultant cell suspensions were
filtered through a 70-µm cell strainer (Corning, Inc.) at
room temperature after centrifugation at 300 × g for 5 min at 37°C
and then cultivated in α-modified minimum essential medium (α-MEM;
Gibco; Thermo Fisher Scientific, Inc.) containing 20%
heat-inactivated FBS (Gibco; Thermo Fisher Scientific, Inc.) and 1%
penicillin-streptomycin in a 25-cm2 cell culture flask
(Corning, Inc.) at 37°C in an incubator (Thermo Fisher Scientific,
Inc.). The cultured cells were incubated in media mentioned above
that was refreshed every 3 days and passaged with TrypLE Express
(Gibco; Thermo Fisher Scientific, Inc.) into new flasks when 80-90%
confluence was reached and sub-cultured with α-MEM and 10% FBS.
Cells at passages 3-5 were used for experiments. HDPCs cultured
with 21% O2 and 5% CO2 at 37°C were used as
the normoxic group, whilst cells cultured in 1% O2, 5%
CO2 and 94% N2 at 37°C were used as the
hypoxic group.
Immunofluorescence staining
HPDCs were seeded into 15-mm laser confocal dishes
(Biosharp Life Sciences) at a density of 1×103 cells per
dish and cultured at 37°C for 24 h. Subsequently, the media were
removed and replaced with 4% paraformaldehyde for fixation at room
temperature. After 15 min, the cells were washed three times with
PBS and permeabilized with 0.3% Triton X-100 (Sigma-Aldrich; Merck
KGaA) for another 15 min, followed by blocking with 5% BSA
(Biofroxx; neoFroxx GmbH) for 30 min at room temperature.
Subsequently, the HPDCs were separately incubated with the rabbit
anti-vimentin antibody (1:150 dilution; cat. no. AF7013; Affinity
Biosciences) or rabbit anti-cytokeratin-14 antibody (1:150
dilution; cat. no. AF5370; Affinity Biosciences) overnight at 4°C.
The secondary antibodies used (both 1:100 dilution; EarthOx Life
Sciences) were goat anti-rabbit IgG (H + L) Dylight 594 (1:100
dilution; cat. no. E032420-02) and goat anti-rabbit IgG (H + L)
Dylight 488 (1:100 dilution; cat. no. E032220-02) for 1 h at room
temperature. DAPI (cat. no. C1006; Beyotime Institute of
Biotechnology) was then applied for 5 min at room temperature to
identify the nucleus. Finally, the cells were imaged under an
LSM780 confocal microscope (×200 magnification; Carl Zeiss AG).
In addition, the subcellular locations of LC3 and
translocase of outer mitochondrial membrane 20 (TOMM20) in HDPCs
after being cultured in normoxia or hypoxia for 18 h at 37°C were
examined by immunofluorescence staining as aforementioned. The
primary antibodies used for overnight at 4°C were as follows:
Rabbit anti-LC3 (1:100 dilution; cat. no. 3868S; Cell Signaling
Technology, Inc.) and mouse anti-TOMM20 (1:100 dilution; cat. no.
ab56783; Abcam). The corresponding secondary antibodies (both 1:100
dilution; EarthOx Life Sciences) were goat anti-rabbit IgG (H + L)
Dylight 594 (cat. no. E032420-02) and goat anti-mouse IgG (H + L)
Dylight 488, which were used for 1 h at room temperature. These
cells were imaged using an LSM780 confocal microscope (×100 and
×400 magnification; Carl Zeiss AG).
Flow cytometry analysis of the surface
markers of HDPCs
Surface markers of HDPCs were identified by flow
cytometry. After collecting ~1×106 cells from the third
passage, HDPCs were washed twice with PBS and then suspended in
PBS, before being blocked with 5% BSA for 30 min at room
temperature to remove nonspecific binding. The antibodies,
including CD13-PE (1:40 dilution; cat. no. 555394; BD Biosciences),
CD14-FITC (1:40 dilution; cat. no. 555397; BD Biosciences),
CD29-FITC (1:40 dilution; cat. no. 11-0291-82; Thermo Fisher
Scientific, Inc.), CD34-PE (1:40 dilution; cat. no. 560941; BD
Biosciences), CD45-PE-Cy5 (1:40 dilution; cat. no. 560974; BD
Biosciences), CD73-FITC (1:10 dilution; cat. no. 561254; BD
Biosciences), CD90-PE-Cy5 (1:40 dilution; cat. no. 555597; BD
Biosciences) and CD105-PE (1:40 dilution; cat. no. 560839; BD
Biosciences), were added and cells were incubated for 90 min at
37°C. IgG1-PE (1:40 dilution; cat. no. 556650; BD Biosciences),
IgG1-FITC (1:40 dilution; cat. no. 556649; BD Biosciences) and
IgG1-PE-Cy5 (1:40 dilution; cat. no. 550618; BD Biosciences) were
also used as isotype controls with incubation for 90 min at 37°C.
After washing with PBS, suspended HDPCs were transferred to FACS
tubes and analyzed using a MOFlo™ XDP high-performance cell sorter
(Beckman Coulter, Inc.) and SUMMIT version 5.0 software (Beckman
Coulter, Inc.) according to the manufacturer's instructions.
Evaluation of the osteogenic,
chondrogenic and adipogenic capabilities of HDPCs
HDPCs (2×105 cells/well) in passage 3
were seeded into 6-well plates (Corning, Inc.). When the cells
reached 80% confluence, the previous medium was replaced with
differentiation-inducing medium and replaced every 3 days according
to the type of differentiation.
Osteogenic medium [10% FBS, 10 nM dexamethasone
(Beijing Solarbio Science & Technology Co., Ltd.), 10 mM
β-glycerophosphate (Beijing Solarbio Science & Technology Co.,
Ltd.) and 50 µg/ml ascorbic acid (Beijing Solarbio Science
& Technology Co., Ltd.) in α-MEM (Gibco; Thermo Fisher
Scientific, Inc.)] was applied for 28 days on HDPCs at 37°C before
the mineralized nodules were revealed using Alizarin Red (Cyagen
Biosciences, Inc.) for 30 min staining at room temperature.
For chondrogenic differentiation, HPDCs were induced
with serum-free α-MEM containing 10 nM dexamethasone, 50
µg/ml ascorbic acid, 40 ng/ml proline (Sigma-Aldrich; Merck
KGaA), 0.1 ng/ml TGF-β1 (Sigma-Aldrich; Merck KGaA) and 3 µM
insulin-transferrin-selenium supplement (Sigma-Aldrich; Merck KGaA)
for 21 days at 37°C. Finally, the extent of chondrogenic
differentiation was assessed using Alcian Blue (Cyagen Biosciences,
Inc.) for 30 min staining at room temperature.
For adipogenic differentiation, HPDCs were cultured
with induction medium consisting of 10% FBS, 50 nM dexamethasone,
0.1 mM indomethacin (Sigma-Aldrich; Merck KGaA), 5 µg/ml
insulin (Sigma-Aldrich; Merck KGaA) and 0.25 mM
3-isobutyl-methylxanthine (Sigma-Aldrich; Merck KGaA) in α-MEM for
28 days at 37°C (23). The
observation of lipid droplets was achieved by Oil Red O (Cyagen
Biosciences, Inc.) for 30 min staining at room temperature.
Images of the aforementioned morphological features
were captured using a light microscope (×40, ×40 and ×80
magnification; Axio Observer Z1; Carl Zeiss AG).
Cell transfection
HDPCs were first cultured in α-MEM supplemented with
10% FBS. Small interfering RNA (siRNA) targeting FUNDC1 was
designed and synthesized by Guangzhou RiboBio Co., Ltd.
Subsequently, cell transfection was performed with Lipofectamine
3000 (Invitrogen; Thermo Fisher Scientific, Inc.) according to the
manufacturer's protocols. After 15 min incubation at room
temperature, siRNA at a final concentration of 50 nM was used to
transfect cells at 70% confluency. The sequences of si-RNA1,
si-RNA2 and si-RNA3 for FUNDC1 were as follows: si-RNA1 sense,
5-CAA GCA GAA CAU UGU GAU AdT dT-3 and antisense, 5-UAU CAC AAU GUU
CUG CUU GdT dT-3; si-RNA2 sense, 5-GUA GCU ACC CAG AUU GUA AdT
dT-3, and antisense, 5-UUA CAA UCU GGG UAG CUA CdT dT-3 and si-RNA
3 sense, 5-GCA GCA CCU GAA AUC AAC AdT dT-3 and antisense, 5-UGU
UGA UUU CAG GUG CUG CdT dT-3. The corresponding non-specific
control sequence was obtained from the commercial reagent (cat. no.
siN0000001-1-5; Guangzhou RiboBio Co., Ltd.). After 48 h at 37°C,
knockdown efficiency was evaluated by western blotting.
Cell viability assay
HPDCs were inoculated into 96-well plates (Corning,
Inc.) at a density of 3×103 cells/well and incubated
overnight at 37°C before treatment. Subsequently, the cells were
treated under normoxic or hypoxic conditions for different
durations (0, 6, 12, 18 and 24 h) at 37°C and harvested for further
analysis. Cell proliferation was measured according to the
manufacturer's protocols of the Cell Counting Kit-8 (CCK-8; Dojindo
Molecular Technologies, Inc.). After adding 10 µl CCK-8
solution into each well of the plate and incubation for 1 h at
37°C, the absorbance at a wavelength of 450 nm was measured by a
microplate reader with BioTek Gen5 system (BioTek Instruments,
Inc.).
Migration assays
The migration of HDPCs after treatment with normoxia
or hypoxia was assessed by wound healing and Transwell migration
assays.
For the wound healing assay, HPDCs or transfected
cells were seeded into 6-well plates in α-MEM supplemented with 10%
FBS and incubated to obtain a 90% cell confluence. A sterile 1-ml
pipette tip was used to generate equal-width scratch wounds. After
washing away the detached cells with PBS, serum-free α-MEM was
added before recording the initial wounds using an EVOS M5000
microscope (Thermo Fisher Scientific, Inc.). During treatment with
hypoxia at 37°C, the wound was imaged under a light microscope (×10
magnification) every 6 h for 24 h. Wound closure was defined as a
mean percentage of the migrated width compared with the initial
wound width. The calculation formula was: Healing rate (%)=(width
of the wound at 0 h-width of the wound at 24 h)/width of the wound
at 0 h ×100%.
Transwell migration assays were performed using
Transwell chambers with 8-µm pores (Corning, Inc.). HPDCs or
transfected HPDCs (2×105 cells/ml) suspended in 100
µl serum-free α-MEM were seeded into the upper compartment
whereas 500 µl culture medium supplemented with 10% FBS was
added into the lower chamber. After treatment with normoxia or
hypoxia for 18 h at 37°C, migrated cells on the underside of the
chamber were fixed with 4% paraformaldehyde for 15 min at room
temperature, stained with 1% crystal violet (Beyotime Institute of
Biotechnology) for 30 min at room temperature, imaged and counted
in three random fields under an inverted light microscope (×100
magnification; Carl Zeiss AG).
Alkaline phosphatase (ALP) staining and
activity assays
ALP staining was achieved using a BCIP/NBT ALP kit
(cat. no. C3206; Beyotime Institute of Biotechnology). According to
the manufacturer's protocols, HDPCs were seeded into 6-well plates
at a density of 1×105 cells/well in α-MEM supplemented
with 10% FBS. After incubation overnight at 37°C, the cells were
cultured with the osteogenic medium as aforementioned and treated
with normoxia or hypoxia for 3 days at 37°C. Subsequently, HDPCs
were fixed with 4% paraformaldehyde for 30 min at room temperature,
washed with PBS three times and stained with the aforementioned kit
for 30 min at room temperature. Images were captured using an
optical light microscope (×50 magnification; Carl Zeiss AG).
In addition, ALP activity was also examined using an
ALP detection kit (cat. no. P0321S; Beyotime Institute of
Biotechnology) according to the manufacturer's protocols.
Protein isolation and western
blotting
HPDCs or transfected HPDCs were seeded into 6-well
plates and treated with normoxia or hypoxia at 37°C before total
protein was collected using RIPA buffer (cat. no. P0013B; Beyotime
Institute of Biotechnology) supplemented with protease inhibitor
and phosphatase inhibitor (CoWin Biosciences) every 6 h for 24 h.
For mineralization assay, HPDCs cultured with osteogenic medium
were treated with normoxia or hypoxia at 37°C for 3 days.
Subsequently, the total protein was extracted as aforementioned.
The sample protein content was quantified using a BCA protein assay
kit (CoWin Biosciences) before immunoblotting. Equal amounts (25
µg) of protein were separated by 10% SDS-PAGE and
transferred onto nitrocellulose membranes (MilliporeSigma). The
membranes were blocked with 5% skimmed milk or 5% BSA for 1 h at
25°C. Subsequently, the membranes were incubated overnight at 4°C
with the following primary antibodies: Rabbit anti-HIF-1α (1:1,000
dilution; cat. no. AF1009; Affinity Biosciences), rabbit
anti-sequestosome 1 (SQSTM1)/p62 (1:1,000 dilution; cat. no.
AF5384; Affinity Biosciences), rabbit anti-LC3B (1:1,000 dilution;
cat. no. 3868S; Cell Signaling Technology, Inc.), mouse
anti-dynamin-related protein 1 (DRP1; 1:1,000 dilution; cat. no.
ab56788; Abcam), rabbit anti-translocase of inner mitochondrial
membrane 23 (TIMM23; 1:1,000 dilution; cat. no. ab116329; Abcam),
mouse anti-TOMM20 (1:1,000 dilution; cat. no. ab56783; Abcam),
rabbit anti-Beclin-1 (1:1,000 dilution; cat. no. 3495S; Cell
Signaling Technology, Inc.), rabbit anti-autophagy related 5 (ATG5;
1:1,000 dilution; cat. no. 12994S; Cell Signaling Technology,
Inc.), rabbit anti-FUNDC1 (1:500 dilution; cat. no. ab224722;
Abcam), rabbit anti-phosphorylated (p-) FUNDC1 (Ser 17; 1:500
dilution; cat. no. AF0001; Affinity Biosciences), mouse
anti-β-actin (1:5,000 dilution; cat. nos. E021021-01; EarthOx Life
Sciences), rabbit anti-collagen type I (Col I; 1:1,000 dilution;
cat. no. AF7001; Affinity Biosciences), rabbit anti-osterix (OSX;
1:1,000 dilution; cat. no. DF7731; Affinity Biosciences), rabbit
anti-osteopontin (OPN; 1:1,000 dilution; cat. no. AF0227; Affinity
Biosciences) and rabbit anti-runt-related transcription factor 2
(RUNX2; 1:1,000 dilution; cat. no. AF5186; Affinity Biosciences).
The membranes were washed with TBS with 0.1% Tween-20 buffer (CoWin
Biosciences) for 30 min and incubated with the appropriate
secondary antibodies conjugated with HRP (both 1:5,000 dilution;
cat. nos. E030120-01 and E030110-01; both EarthOx Life Sciences)
for 1 h at room temperature, followed by visualization using
MilliporeSigma™ Immobilon™ Western Chemiluminescent HRP Substrate
(ECL; MilliporeSigma). Densitometric analysis of the protein bands
was performed using the ImageJ version 1.5.0 software (National
Institutes of Health).
Transmission electron microscopy
(TEM)
The samples were washed and harvested after normoxia
or hypoxia treatment for 18 h at 37°C. Subsequently, the
preparation of cells for TEM was performed as previously described
(9). Briefly, cells were fixed
with 2.5% glutaraldehyde (cat. no. A17876; Thermo Fisher
Scientific, Inc.) for 2 h at temperature, washed six times in PBS
(30 min each) and fixed in 1% osmic acid for 2 h at room
temperature. The fixed samples were dehydrated through an
increasing graded series of ethanol (30-100%), infiltrated and
embedded in eponate 12 resin (cat. no. GP18010; Ted Pella, Inc.) at
38°C for 4 h. After the process of curing (38°C for 6 h, 60°C for 6
h and 80°C for 12 h), the embedded blocks were cut into ultrathin
sections (70 nm) using a Leica Ultramicrotome EM UC7 (Leica
Microsystems, Inc.). The sections were then stained with 2% uranyl
acetate for 30 min at room temperature and 3.5% lead citrate for 15
min at room temperature. After that, ultrathin sections (70 nm)
were viewed at 100 kV with a transmission electron microscope
(magnification, ×30,000; JEM-1400 PLUS; Jeol, Ltd.).
Statistical analysis
The experiments were repeated three times
independently and the data are presented as the mean ± SD. Data
analysis was performed using GraphPad Prism version 9.0 software
(GraphPad Software, Inc.). Two-group comparisons were assessed by
unpaired Student's t-test, whilst multiple comparisons were
analyzed by one-way ANOVA followed by Fisher's least significant
difference post hoc test or Bonferroni's correction. P<0.05 was
considered to indicate a statistically significant difference.
Results
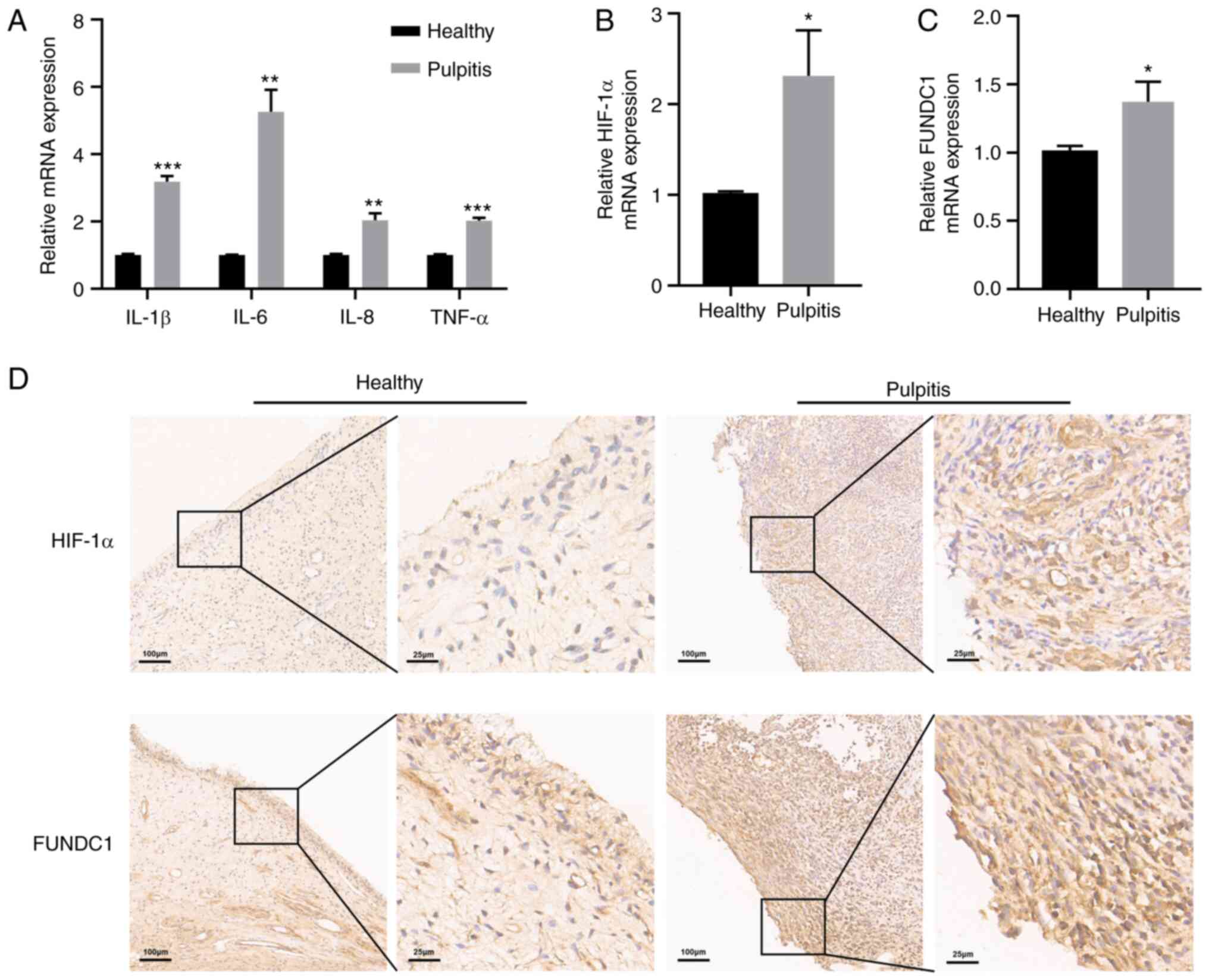
HIF-1α and FUNDC1 expression is
upregulated in inflammatory human dental pulp tissues
To confirm the inflammatory status of human dental
pulp tissues, RT-qPCR was performed to detect the mRNA expression
levels of inflammatory cytokines in healthy and pulpitis tissues
separately. Pulpitis tissues exhibited statistically significantly
higher levels of IL-1β, IL-6, IL-8 and TNF-α expression compared
with those in the healthy group (Fig. 1A). The present study also
revealed significantly higher levels of HIF-1α and FUNDC1 mRNA
expression in pulpitis tissues compared with those in the healthy
group (Fig. 1B and C). To
measure the protein expression levels of HIF-1α and FUNDC1 further,
immunohistochemistry was performed, which revealed that HIF-1α and
FUNDC1 protein expression was also marked higher in pulpitis
tissues compared with that in the healthy group (Fig. 1D).
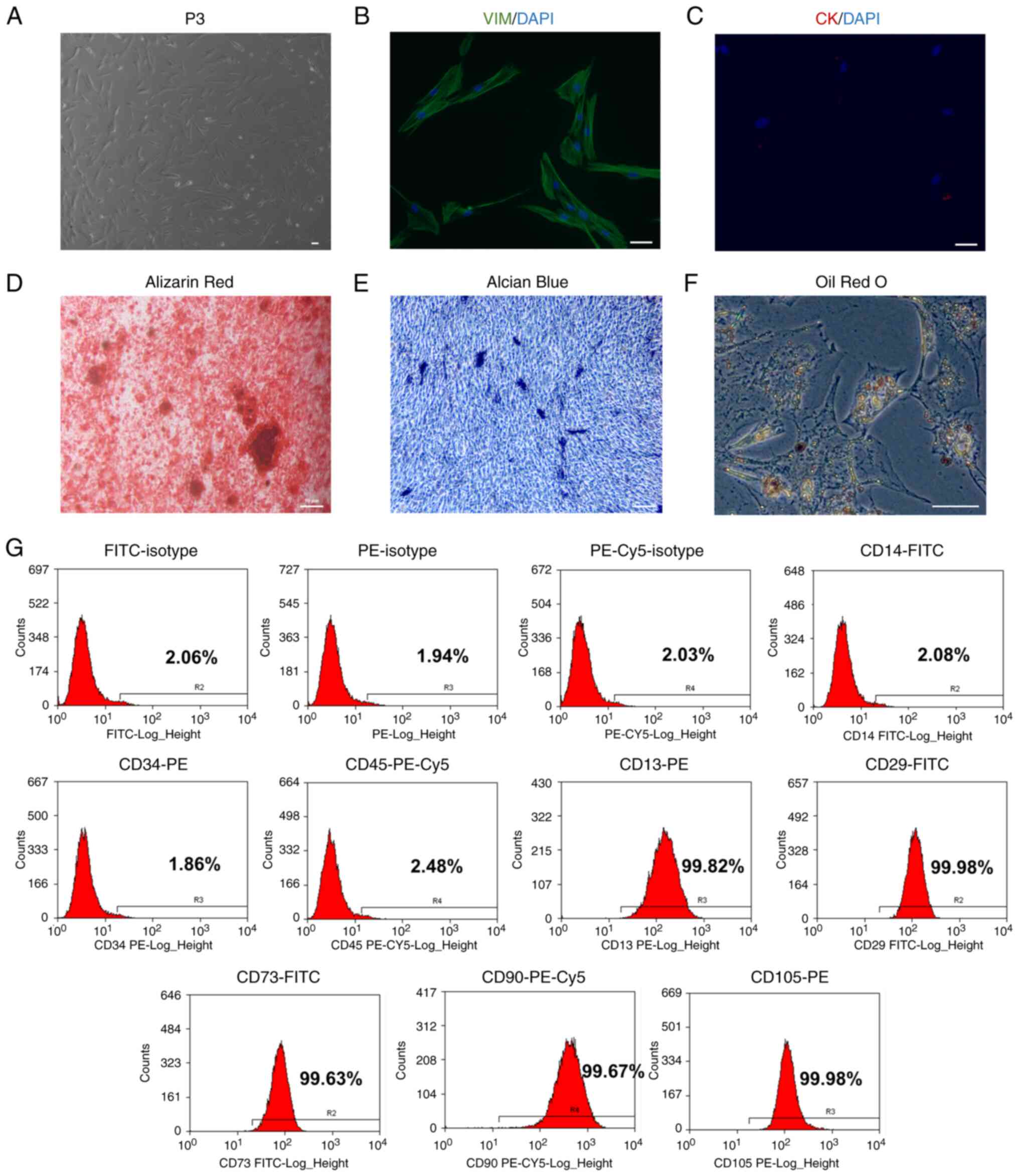
Identification of HDPCs
HDPCs were isolated from the human dental pulp
tissues before analysis of cell morphology was performed (Fig. 2A). The morphology of cells was
relatively uniform, showing a fibrous/fusiform shape.
Immunofluorescence revealed that the HDPCs were positive for the
expression of the mesenchymal marker vimentin (Fig. 2B) but negative for the epithelial
marker cytokeratin (Fig. 2C).
HDPCs were subsequently induced in osteogenic, chondrogenic and
adipogenic media. The multipotent differentiation capabilities of
these HDPCs were confirmed by the Alizarin Red staining of
osteoblasts, Alcian Blue staining of chondroblasts and Oil Red O
staining of fat droplets (Fig.
2D-F). Flow cytometry analysis of surface markers revealed that
these HDPCs were positive for CD13 (99.82%), CD29 (99.98%), CD73
(99.63%), CD90 (99.67%) and CD105 (99.98%). However, they were
tested negative for CD14 (2.08%), CD34 (1.86%) and CD45 (2.48%).
These flow cytometry profiles are consistent with the
characteristics of mesenchymal stem cells (Fig. 2G) (24).
Hypoxia promotes proliferation, migration
and odontoblastic differentiation in HDPCs
To explore the effects of hypoxia on the
proliferation and differentiation of HDPCs, cells in the hypoxia
group were cultured in 1% O2 in an incubator for 6, 12,
18 and 24 h, whilst cells in the normoxic group were cultured in
normoxic conditions. CCK-8 assay results indicated that hypoxia
significantly promoted HDPC proliferation after 18 h of stimulation
compared with that in the normoxic group (Fig. 3E). Although the wound healing
assay demonstrated that 24 h stimulation increased the migration,
HDPCs in the 18 h hypoxia group exhibited higher horizontal and
vertical migration ability compared with that in the normoxic group
(Fig. 3A-D). Additionally, 3
days after odontogenic induction, ALP staining and activity were
markedly enhanced in the hypoxic group (Fig. 3F-G). Western blotting also
revealed significantly upregulated protein expression levels of
RUNX2, Col I, OSX, OPN in the hypoxic treatment group compared with
those in the normoxic group (Fig.
3H-I).
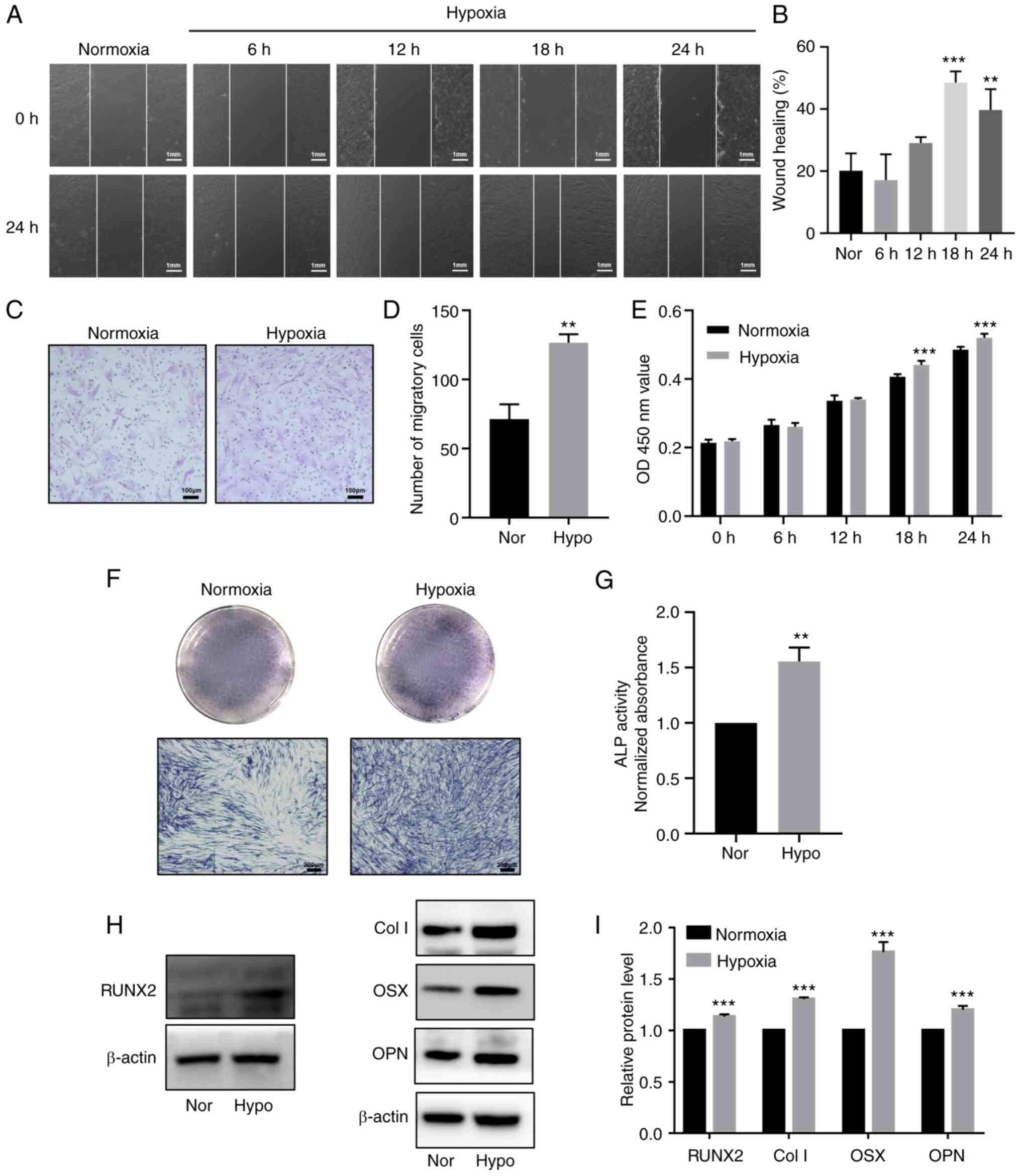 | Figure 3Hypoxia promotes proliferation,
migration and odontoblastic differentiation in HDPCs. (A)
Representative images of wound healing assays in the normoxic or
hypoxic group. Scale bar, 1 mm. (B) Percentages of wound closure
from three independent experiments are quantified. (C)
Representative images of migratory cells stained with crystal
violet. (D) Statistical quantification of the number of migratory
cells from three independent experiments are shown. Scale bar, 100
µm. (E) Cell viability of HDPCs was detected using Cell
Counting Kit-8 analysis. (F) ALP staining and (G) activity in the
normoxic or hypoxic group on day 3. Scale bar, 200 µm. (H)
Representative western blotting images showing the protein
expression levels of RUNX2, Col I, OSX and OPN in the normoxic or
hypoxic group on day 3, (I) which were quantified. Results are
presented as the means ± SD from ≥ three independent experiments.
**P<0.01 and ***P<0.001 vs. normoxia.
Nor, normoxia; Hypo, hypoxia; OD, optical density; ALP, alkaline
phosphatase; RUNX2, runt-related transcription factor 2; Col I,
collagen type I; OSX, osterix; OPN, osteopontin. |
Hypoxia-induces mitophagy in HDPCs
To investigate the effects of hypoxia on autophagy,
TEM, western blotting and immunofluorescence staining were
performed. TEM was utilized to examine the ultrastructure of HDPCs
following hypoxia or normoxia treatment for 18 h. Hypoxia induced
the formation of higher numbers of autophagic vacuoles in HDPCs
(Fig. 4B) compared with those in
the normoxic group (Fig. 4A).
Furthermore, a series of processes during autophagic vesicle
formation in the hypoxic group, including the early, mid and late
stages, could be clearly observed (Fig. 4C-F). During the early stages
(Fig. 4C), the formation of
double membrane-bound vacuoles was a characteristic feature of
autophagosomes (25). After
fusing with lysosomes to form autolysosomes, the contents of the
vacuoles were indistinct, typical of mid-stage of autophagy
(Fig. 4D and E) (25). The complete degradation of
organelles could be observed during late-stage autophagosome
formation (Fig. 4F).
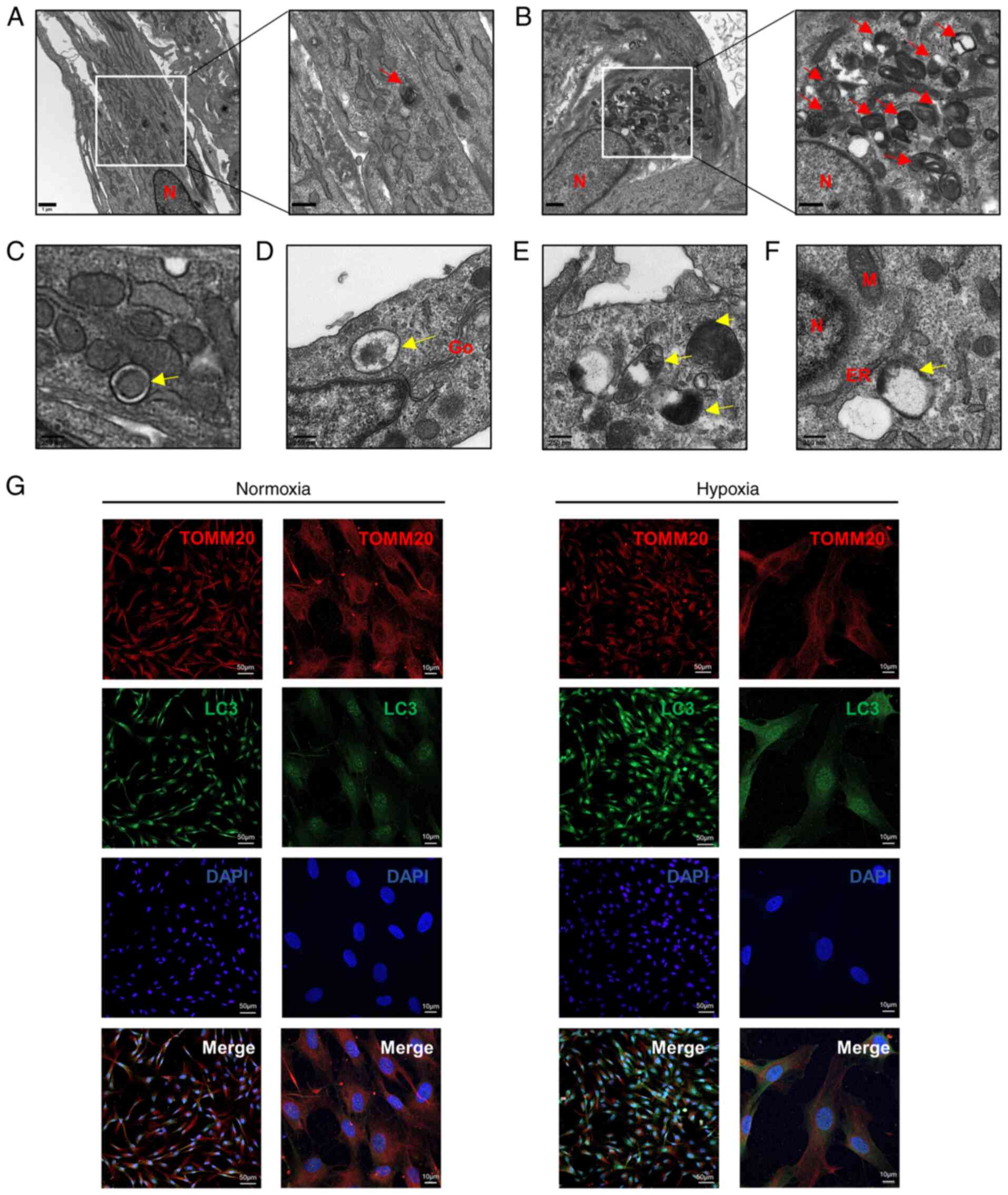 | Figure 4Hypoxia induces mitophagy in HDPCs.
(A) Cells in the normoxic group show fewer autolysosomes, which are
marked by red arrows. Scale bars are 1 µm and 500 nm,
respectively. (B) Cells in the hypoxic group show increased numbers
of autolysosomes, which are marked by red arrows. Scale bars are 1
µm and 500 nm respectively. (C-F) The developmental process
of autolysosomes. (C) Early stage of autophagosomes marked by
yellow arrows in HDPCs from the hypoxic group, showing the envelope
structure in the vesicles remaining intact. (D) Mid-stage
autophagy, showing that the autophagosomes were fused with
lysosomes to form autolysosomes and (E) the encapsulated contents
were degraded to form indistinct structure. (F) Late-stage
autophagy, showing the complete degradation of organelles in the
vacuoles. Scale bar, 250 nm. (G) Immunofluorescence staining with
the anti-TOMM20 antibody (red) and anti-LC3 antibody (green) was
performed in HDPCs. DAPI (blue) staining indicated cell nuclei.
Scale bars are 50 and 10 µm, respectively. TOMM20,
translocase of outer mitochondrial membrane 20; LC3,
microtubule-associated protein 1 light chain 3; DAPI,
4′,6-diamidino-2-phenylindole; N, nucleus; M, mitochondrion; Go,
Golgi; ER, endoplasmic reticulum. |
Immunofluorescence analysis demonstrated markedly
higher LC3 expression and lower TOMM20 expression levels following
18-h hypoxia stimulation compared with those in the normoxic group
(Fig. 4G). Additionally, the
expression levels of mitochondrial membrane proteins (TOMM20 and
TIMM23) and autophagy-related proteins (LC3II, SQSTM1/p62, Beclin-1
and ATG5) were detected in HDPCs after hypoxia treatment at 6-h
intervals for 24 h by western blotting. Compared with those in the
normoxic group, significantly increased protein expression levels
of HIF-1α, LC3II and DRP1, coupled with the significantly decreased
protein expression levels of SQSTM1/p62, TIMM23 and TOMM20, were
observed in the hypoxia groups from 12 h onwards (Fig. 5A-I). Specifically, a plateau in
LC3II expression and a trough in SQSTM1/p62 expression were reached
at 18 h. Additionally, a slight by significant increase in Beclin-1
and ATG5 expression could be observed at 24 h (Fig. 5A-I). Overall, these results
suggest that hypoxia markedly induced mitochondrial autophagy in
HDPCs.
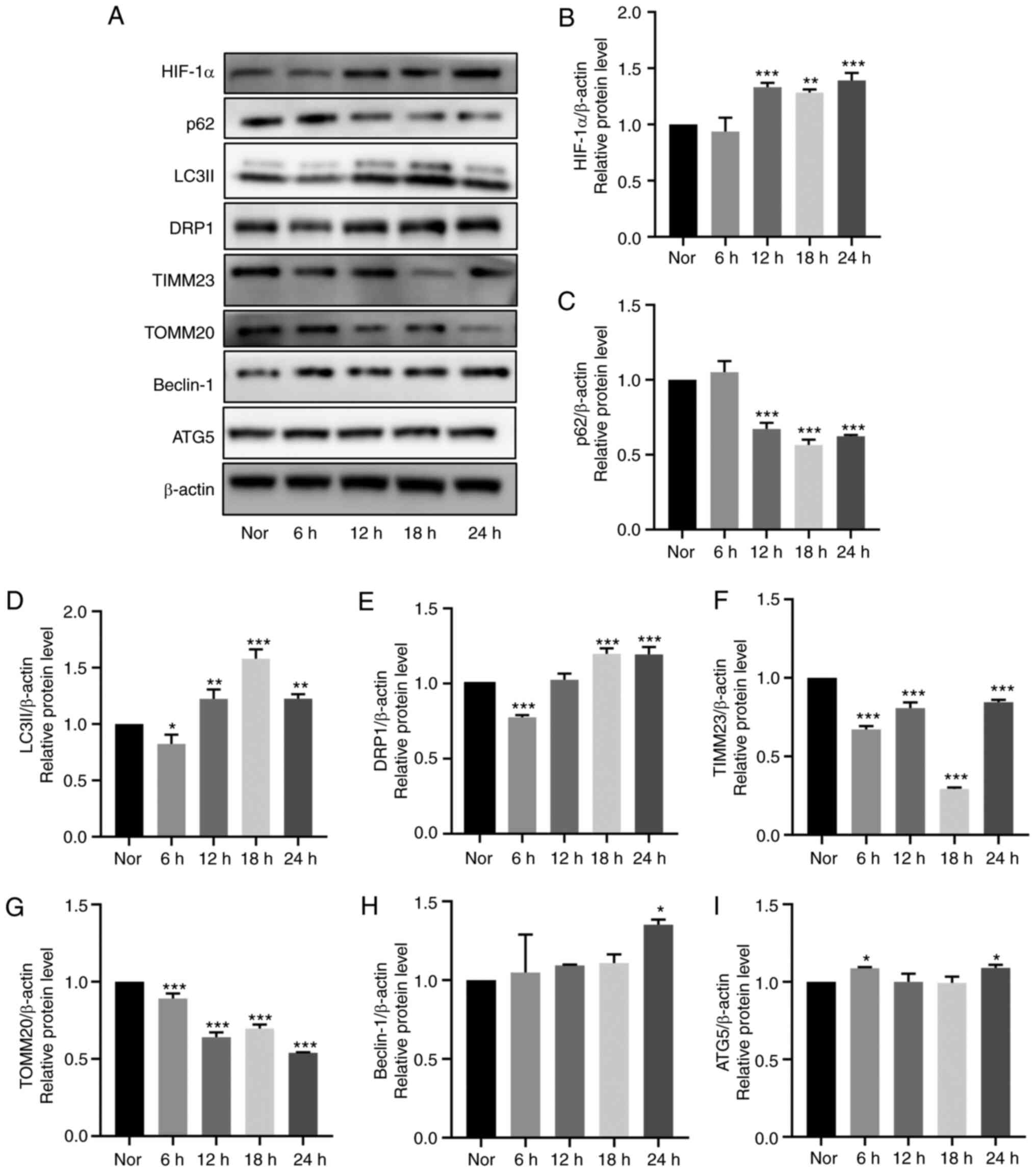 | Figure 5Effects of hypoxia on mitophagy in
cultured HDPCs. (A) Representative western blotting images. Protein
expression of (B) HIF-1α, (C) p62, (D) LC3II, (E) DRP1, (F) TIMM23,
(G) TOMM20, (H) Beclin-1 and (I) ATG5 in HDPCs cultured in hypoxia
were quantified. The expression of the target proteins was measured
by quantifying the intensity of the bands and normalized to that of
β-actin. The results are presented as the means ± SD from ≥ three
independent experiments. *P<0.05,
**P<0.01 and ***P<0.001 vs. normoxia.
Nor, normoxia; HIF-1α, hypoxia-inducible factor-1α; DRP1,
dynamin-related protein 1; TIMM23, translocase of inner
mitochondrial membrane 23; TOMM20, translocase of outer
mitochondrial membrane 20; ATG5, autophagy related 5. |
Hypoxia promotes the phosphorylation of
FUNDC1 in HDPCs
After hypoxia treatment for 24 h at 6-h intervals,
the protein expression levels of FUNDC1 were slightly decreased
(Fig. 6A and B). However, the
ratio of p-/total FUNDC1 was significantly increased from 12 h
onwards after hypoxia stimulation compared with that in the
normoxic group (Fig. 6C).
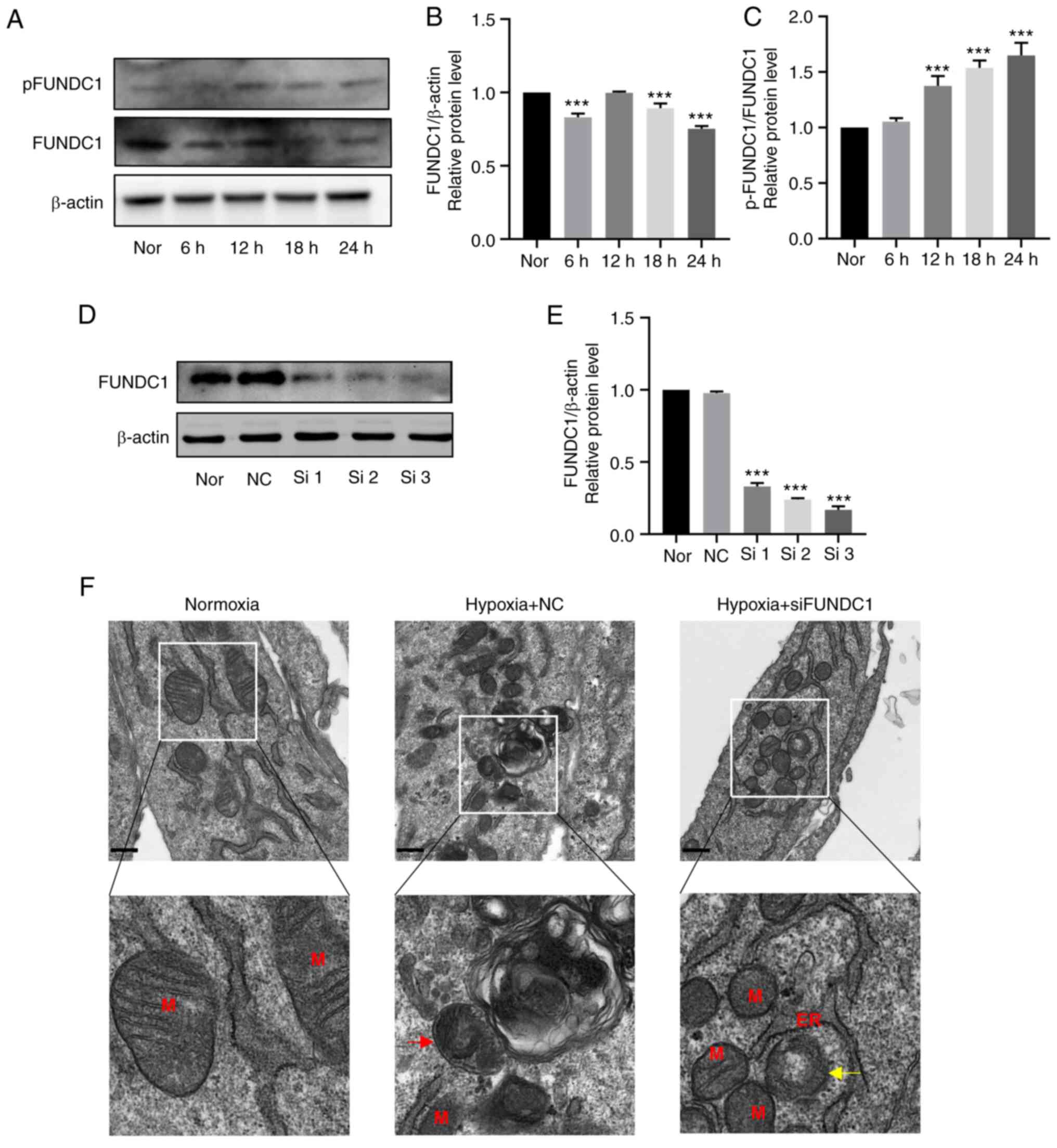 | Figure 6Hypoxia promotes the phosphorylation
of FUNDC1 but the knockdown of FUNDC1 influences mitochondria
morphology. (A) Representative western blotting images. (B) Protein
expression of FUNDC1 and (C) phosphorylation of FUNDC1 (Ser 17) in
HDPCs cultured in hypoxia were quantified. (D) Protein expression
of FUNDC1 in HDPCs cultured in normoxia after transfection with
siNC or three different siRNAs, (E) which was quantified. The
results are presented as the means ± SD from ≥ three independent
experiments. (F) Transmission electron microscopy images of normal
mitochondria in the normoxic group, single-membrane autolysosomes
containing partially degraded mitochondria in the hypoxia + NC
group (red arrow) and blurred mitochondrial cristae (yellow arrow)
in the hypoxia + siFUNDC1 group. Scale bars, 500 nm.
***P<0.001 vs. normoxia. Nor, normoxia; N, nucleus;
M, mitochondrion; Go, Golgi; ER, endoplasmic reticulum; NC,
negative control; si, small-interfering; p, phosphorylated; FUNDC1,
FUN14 domain-containing. |
FUNDC1 knockdown suppresses
hypoxia-induced mitophagy, weakening the promotion of
hypoxia-induced proliferation, migration and odontoblastic
differentiation
To identify the effects of FUNDC1 on hypoxia-induced
mitophagy, cell proliferation, migration and differentiation,
FUNDC1 expression was silenced by siRNA transfection. FUNDC1
expression in HDPCs was significantly knocked down by siRNA number
three compared with that in the NC group (Fig. 6D and E). This siRNA was therefore
used in the subsequent 18-h hypoxia stimulation experiments. TEM
was first used to assess mitochondria morphology. The
ultrastructure of mitochondria in the normoxic group was clear,
including intact membranes, dense mitochondrial cristae and clear
mitochondrial matrices (Fig.
6F). Under hypoxia treatment, there appeared to be
autophagosomes containing partially degraded mitochondria (Fig. 6F), whilst the FUNDC1-knockdown
group exhibited damaged mitochondria with blurred, disordered or
even vacuolized mitochondrial cristae (Fig. 6F).
After FUNDC1 expression was knocked down,
hypoxia-induced mitophagy was inactivated, which was demonstrated
by western blotting with decreased protein expression levels of
LC3II and significantly increased protein expression levels of
SQSTM1/p62, TIMM23 and TOMM20 (Fig.
7A-I). Based on these findings, FUNDC1 knockdown appeared to
have reversed hypoxia-induced mitophagy. CCK-8 assay (Fig. 8E), migration assays (Fig. 8A-D), ALP staining (Fig. 8F) and activity assays (Fig. 8G) and western blotting (Fig. 8H and I) were utilized to detect
the proliferation, migration and odontoblastic differentiation in
the FUNDC1-knockdown group. The western blotting results showed
that the protein levels of RUNX2, Col I, OSX, OPN in
hypoxia-siFUNDC1 group were significantly downregulated. Compared
with those in the hypoxia-NC group, FUNDC1 knockdown markedly
reversed hypoxia-induced proliferation, migration and odontoblastic
differentiation.
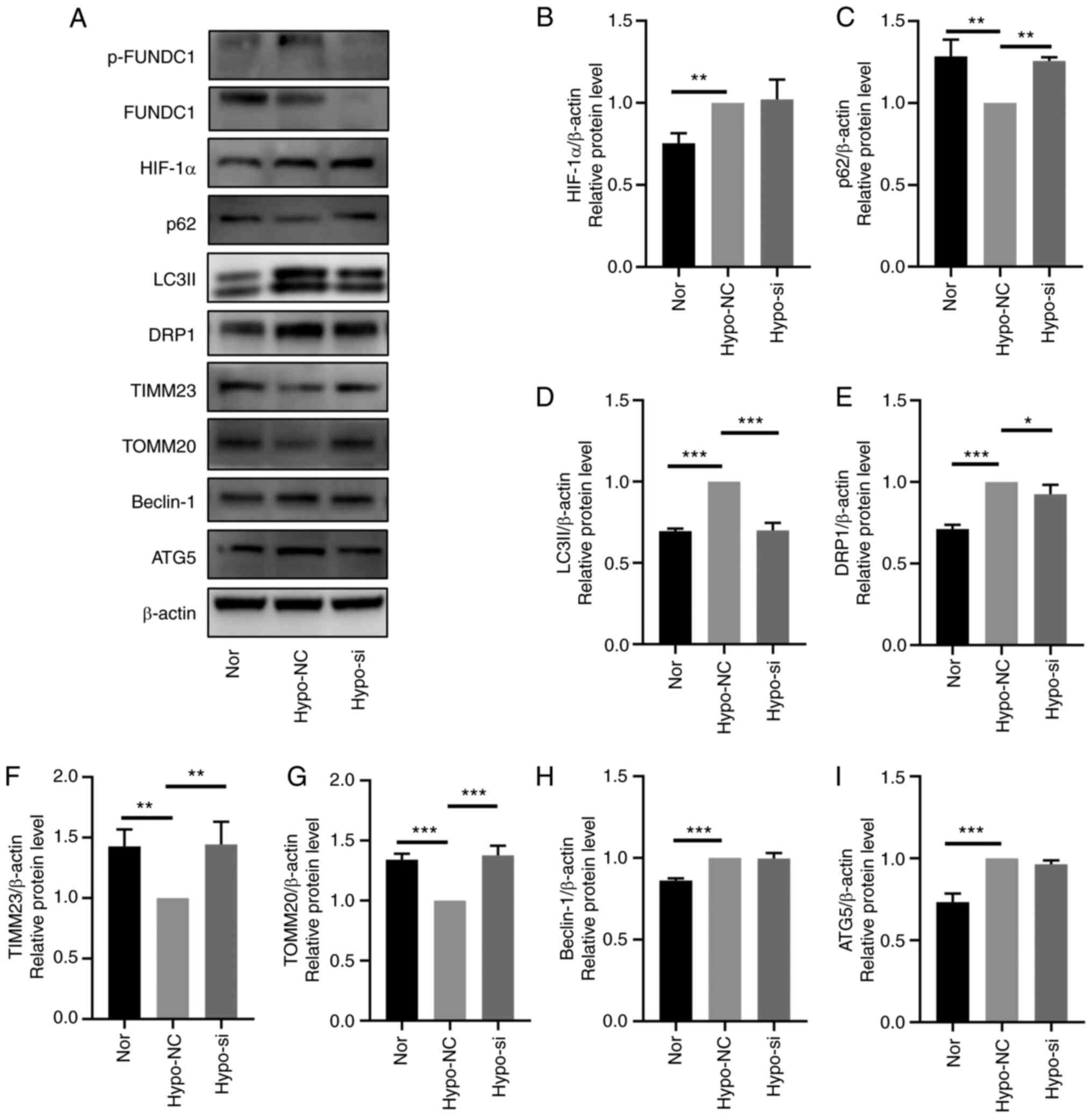 | Figure 7FUNDC1 knockdown prevents
hypoxia-induced mitophagy. (A) Representative western blotting
images. The protein expression of (B) HIF-1α, (C) p62, (D) LC3II,
(E) DRP1, (F) TIMM23, (G) TOMM20, (H) Beclin-1 and (I) ATG5 in
HDPCs after hypoxic culture and transfection with NC or siRNA was
all quantified. The results are presented as the means ± SD from ≥
three independent experiments. *P<0.05,
**P<0.01 and ***P<0.001. FUNDC1, FUN14
domain-containing; p-, phosphorylated; Nor, normoxia; Hypo,
hypoxia; NC, negative siRNA; si, small interfering; HIF-1α,
hypoxia-inducible factor-1α; DRP1, dynamin-related protein 1;
TIMM23, translocase of inner mitochondrial membrane 23; TOMM20,
translocase of outer mitochondrial membrane 20; ATG5, autophagy
related 5. |
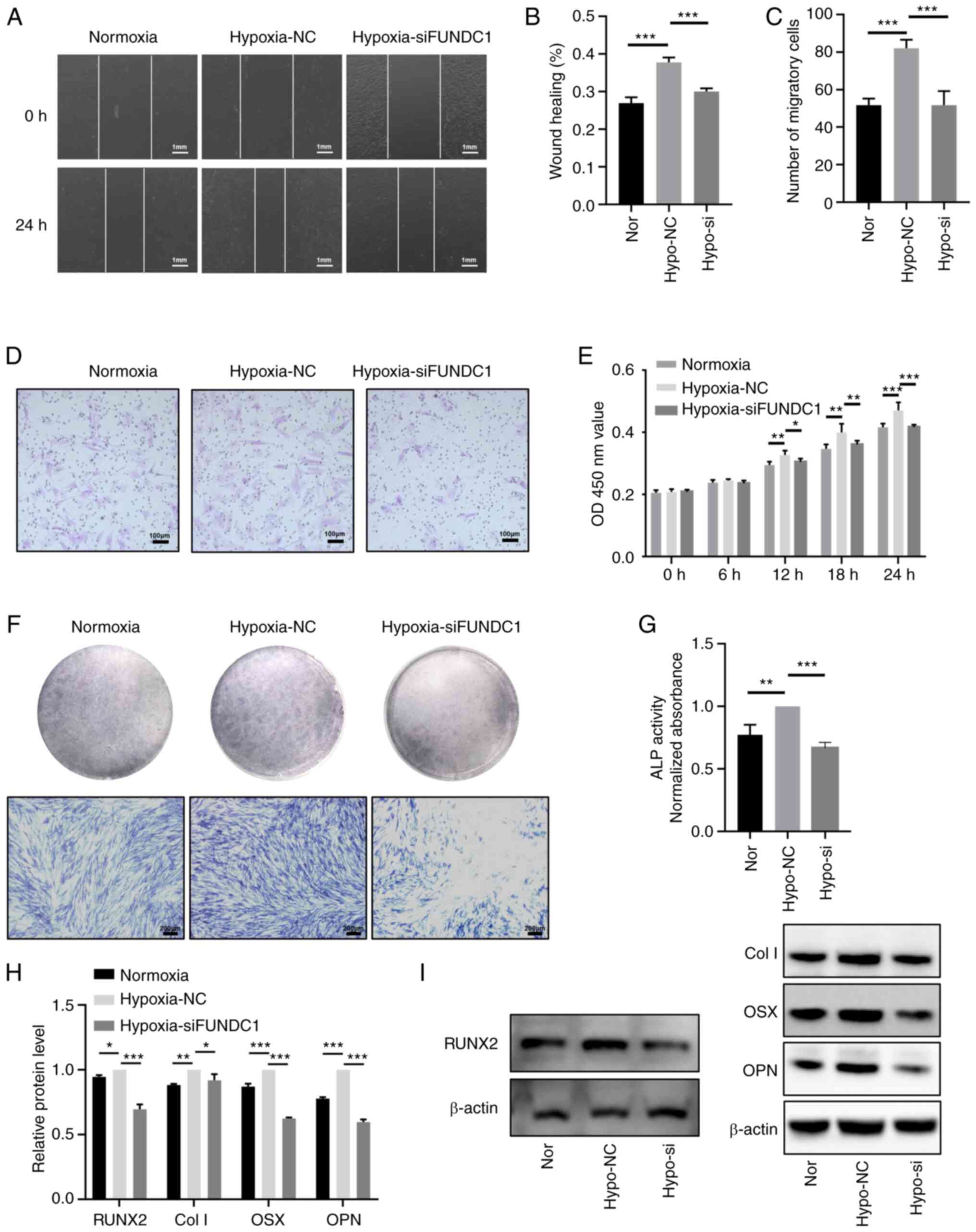 | Figure 8Knocking down FUNDC1 expression
weakens the promotion of hypoxia-induced proliferation, migration
and odontoblastic differentiation. (A) Representative images of the
wound healing assay (scale bar, 1 mm) and (B) percentages of wound
closure from three independent experiments are quantified. (C) The
number of migratory cells stained with crystal violet solution from
three independent experiments was quantified. (D) Representative
images of migratory cells are shown. Scale bar, 100 µm. (E)
Viability of HDPCs was detected by Cell Counting Kit-8 analysis.
(F) ALP staining and (G) activity in the normoxic, hypoxia + NC and
hypoxia + siFUNDC1 groups on days 3. Scale bar, 200 µm. (H)
Protein expression levels of RUNX2, Col I, OSX and OPN in the
normoxic or hypoxic group on day 3 were quantified, (I) the
corresponding representative western blotting images of which were
shown. Results are presented as the means ± SD from ≥ three
independent experiments. *P<0.05,
**P<0.01 and ***P<0.001. FUNDC1, FUN14
domain-containing 1; Nor, normoxia; Hypo, hypoxia; NC, negative
control; si, small interfering; ALP, alkaline phosphatase; RUNX2,
runt-related transcription factor 2; Col I, collagen type I; OSX,
osterix; OPN, osteopontin. |
Discussion
Hypoxia may be caused by inflammation resulting from
bacterial infection or trauma, which frequently occurs in injured
dental pulp tissues (20). Using
RT-qPCR and immunohistochemistry, the present study verified the
increased expression levels of inflammatory cytokines IL-1β, IL-6,
IL-8 and TNF-α in addition to HIF-1α in pulpitis tissues,
suggesting the presence of a hypoxic environment after the
inflammation occurred. In some tissues, hypoxia induces cell
proliferation, migration or angiogenesis to promote cell survival
and tissue repair (22). HDPCs
can differentiate into odontoblasts and migrate to the site of
damage, where they then activate their stimulatory effects after
injury or infection (19). A
marked increase in the proliferation of HDPCs under hypoxic
conditions was previously observed by Sakdee et al (26). In addition, Du et al
(27) revealed that the
migration and odontoblastic differentiation of HDPCs were enhanced
by treatment with 10 µM deferoxamine, a hypoxia-mimetic
agent. HIF-1α knockdown in stem cells isolated from human
exfoliated deciduous teeth (SHEDs) resulted in reduced
proliferative and migratory capabilities, which in turn impaired
the paracrine angiogenic effects (28). Therefore, the present study aimed
to investigate the influence of 1% O2 hypoxia on the
proliferation, migration and odontoblastic differentiation of
HDPCs. The results suggest that 1% O2 hypoxia could
promote all of these aforementioned processes. However, other
studies have also revealed opposite effects. Chen et al
(29) found that cobalt
chloride, a hypoxia-mimetic agent, inhibited the osteogenic
differentiation of SHEDs. Hu et al (30) revealed that hypoxia (5%
O2) diminished cell survival whilst suppressing the
osteogenic differentiation of dental pulp stem cells. These
discrepancies are likely to be due to the use of different cell
lines, oxygen deprivation protocols and/or the hypoxia treatment
time. Zhou et al (31)
previously demonstrated that hypoxia serves a key role in
maintaining the stemness and differentiation capacity of
periodontal ligament and dental pulp cells. In addition, another
study reported that hypoxia can induce the differentiation of stem
cells from the apical papilla into cells of osteogenic and
neurogenic lineages (32). HDPCs
are capable for multilineage differentiation, but the effects of
hypoxia on chondrogenesis or differentiation into other lineages
warrant further study, which is a limitation of the present
study.
The intracellular mechanism underlying the
stimulation by hypoxia in HDPCs remain poorly understood. Autophagy
is a degradative pathway that can regulate various physiological
processes, including cell proliferation, migration, angiogenesis
and odontoblastic differentiation (33-35). Gong et al (36) reported that HIF-1α overexpression
can trigger mitochondrial autophagy, which promotes neuron
survival, whereas Song et al (37) demonstrated that overexpression of
HIF-1α promoted papillary thyroid carcinoma progression by inducing
autophagy during hypoxia stress. Zhang et al (38) revealed that hypoxia increased the
migration of epidermal keratinocytes during wound healing through
BCL2 interacting protein 3-induced autophagy, highlighting the
reactive oxygen species-mediated activation of p38 and JNK MAPK
signaling in the process. Additionally, a previous study reported
that typical ultrastructure of autophagosomes could be observed in
radicular cysts and periapical granulomas, suggesting that
autophagy is activated in inflamed and hypoxic periapical lesions
(39). As observed using
electron microscopy in the present study, higher numbers of
autophagic vacuoles were present in hypoxic HDPCs. Several markers
of autophagy are involved in this process, including LC3, Beclin-1,
p62 and ATG5 (40). LC3-II,
Beclin-1 and ATG5 are directly associated with autophagic activity
at various stages, whereas p62 expression levels are inversely
associated with this (41).
Since TOMM20 and TIMM23 are markers of the mitochondrial membrane,
their protein expression levels can be used to directly reflect the
number of mitochondria. As a pro-fission protein, DRP1 is involved
in the modulation of the dynamic mitochondrial cycle, which can
coordinate with the autophagic machinery to mediate mitophagy
(42). The present study
demonstrated that the protein expression levels of LC3-II, Beclin-1
and DRP1 were increased, whereas those of p62, TOMM20 and TIMM23
were decreased, in HDPCs cultured under hypoxic conditions.
Subsequently, double immunofluorescence staining of LC3 and TOMM20
supported these western blotting findings. These results suggest
that hypoxia can induce mitophagy in HPDCs.
The involvement of mitochondrial dysfunction and
mitophagy in diseases has been previously reported (43) As a receptor for activating
hypoxia-induced mitophagy, FUNDC1 expression has been demonstrated
to be upregulated in patients with chronic obstructive pulmonary
disease (44) and breast cancer
(16). The present study first
reported the elevated mRNA and protein levels of FUNDC1 in pulpitis
tissues whereas the in vitro experiments revealed that
hypoxia upregulated phosphorylation of Ser 17 of FUNDC1 in HDPCs.
Increasing the phosphorylation of Ser 17 on FUNDC1 enhances the
binding of FUNDC1 to LC3, leading to increased mitophagy (41). In addition, silencing FUNDC1
expression reversed hypoxia-induced mitophagy in HDPCs. These
results suggest that FUNDC1-mediated mitophagy is therefore
activated by hypoxia, where FUNDC1 served a key role in the
process. Furthermore, it was revealed that not only was mitophagy
inhibited, silencing FUNDC1 expression also reversed
hypoxia-induced proliferation, migration and odontoblastic
differentiation. FUNDC1-mediated mitophagy is associated with
various cell activities (45,46). Wu et al (16) previously showed that upregulating
the expression of FUNDC1 can stimulate human breast cancer cell
proliferation and migration by increasing Ca2+ influx.
By contrast, Wu et al (47) previously demonstrated that
knocking down FUNDC1 or DRP1 expression suppressed mitophagy under
hypoxic conditions. In cervical cancer cell lines, FUNDC1 knockdown
inhibited cell proliferation (48). Lampert et al (49) previously revealed that
FUNDC1-mediated mitophagy was required for mitochondrial network
remodeling during cardiac progenitor cell differentiation. To
initiate protective effects following injury or infection, HDPCs
can differentiate into odontoblasts and migrate to the site of
damage (19). Based on the
aforementioned results, FUNDC1-mediated mitophagy may be associated
with the proliferation, migration and odontoblastic differentiation
in HDPCs, which may be a mechanism underlying the activities of
HDPCs.
In conclusion, the present results suggested that
FUNDC1-mediated mitophagy can serve as a key factor for the
physiology of HDPCs. However, further studies are necessary to
explore the pathways underlying this association and to investigate
how these activities can be modulated during the responses of HDPCs
to hypoxia.
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
YL and LC performed the experiments, collected and
visualized the data. YL, QG and YH analyzed the data. HJ and YH
conceptualized the study. YL and YH confirm the authenticity of all
the raw data. All authors have read and approved the final version
of the manuscript.
Ethics approval and consent to
participate
Written informed consent was received from all
patients, parent or guardian of the patients involved. The present
study was approved by the Ethics Committee of Hospital of
Stomatology and Guanghua School of Stomatology, affiliated to Sun
Yat-sen University [protocol no. ERC-(2017)-27; Guangzhou,
China].
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
Acknowledgments
Not applicable.
Funding
The present study was supported by funds from the National
Natural Science Foundation of China (grant nos. 81700957 and
81870750) and Natural Science Foundation of Guangdong Province
(grant no. 2016A030310197).
References
|
1
|
Yin Z, Pascual C and Klionsky DJ:
Autophagy: Machinery and regulation. Microb Cell. 3:588–596. 2016.
View Article : Google Scholar
|
|
2
|
Yang JW, Zhang YF, Wan CY, Sun ZY, Nie S,
Jian SJ, Zhang L, Song GT and Chen Z: Autophagy in SDF-1a-mediated
DPSC migration and pulp regeneration. Biomaterials. 44:11–23. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Pantovic A, Krstic A, Janjetovic K, Kocic
J, Harhaji-Trajkovic L, Bugarski D and Trajkovic V: Coordinated
time-dependent modulation of AMPK/Akt/mTOR signaling and autophagy
controls osteogenic differentiation of human mesenchymal stem
cells. Bone. 52:524–531. 2013. View Article : Google Scholar
|
|
4
|
Pan Y, Li Z, Wang Y, Yan M, Wu J, Beharee
RG and Yu J: Sodium fluoride regulates the osteo/odontogenic
differentiation of stem cells from apical papilla by modulating
autophagy. J Cell Physiol. Feb 14–2019.Epub ahead of print.
View Article : Google Scholar
|
|
5
|
Couve E, Osorio R and Schmachtenberg O:
Mitochondrial autophagy and lipofuscin accumulation in aging
odontoblasts. J Dent Res. 91:696–701. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Park SY, Sun EG, Lee Y, Kim MS, Kim JH,
Kim WJ and Jung JY: Autophagy induction plays a protective role
against hypoxic stress in human dental pulp cells. J Cell Biochem.
119:1992–2002. 2018. View Article : Google Scholar
|
|
7
|
Roa-Mansergas X, Fado R, Atari M, Mir JF,
Muley H, Serra D and Casals N: CPT1C promotes human mesenchymal
stem cells survival under glucose deprivation through the
modulation of autophagy. Sci Rep. 8:69972018. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Zhuang H, Hu D, Singer D, Walker JV, Nisr
RB, Tieu K, Ali K, Tredwin C, Luo S, Ardu S and Hu B: Local
anesthetics induce autophagy in young permanent tooth pulp cells.
Cell Death Discov. 1:150242015. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Huang Y, Li X, Liu Y, Gong Q, Tian J and
Jiang H: LPS-induced autophagy in human dental pulp cells is
associated with p38. J Mol Histol. 52:919–928. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Yang F, Li Y, Duan H, Wang H, Pei F, Chen
Z and Zhang L: Activation of mitophagy in inflamed odontoblasts.
Oral Dis. 25:1581–1588. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Wang Y, Liu N and Lu B: Mechanisms and
roles of mitophagy in neurodegenerative diseases. CNS Neurosci
Ther. 25:859–875. 2019.PubMed/NCBI
|
|
12
|
Liu L, Feng D, Chen G, Chen M, Zheng Q,
Song P, Ma Q, Zhu C, Wang R, Qi W, et al: Mitochondrial
outer-membrane protein FUNDC1 mediates hypoxia-induced mitophagy in
mammalian cells. Nat Cell Biol. 14:177–185. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Zhang W, Siraj S, Zhang R and Chen Q:
Mitophagy receptor FUNDC1 regulates mitochondrial homeostasis and
protects the heart from I/R injury. Autophagy. 13:1080–1081. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Yuan Q, Sun N, Zheng J, Wang Y, Yan X, Mai
W, Liao Y and Chen X: Prognostic and Immunological role of FUN14
domain containing 1 in pan-cancer: Friend or foe? Front Oncol.
9:15022019. View Article : Google Scholar
|
|
15
|
Yan M and Yu Y, Mao X, Feng J, Wang Y,
Chen H, Xie K and Yu Y: Hydrogen gas inhalation attenuates
sepsis-induced liver injury in a FUNDC1-dependent manner. Int
Immunopharmacol. 71:61–67. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Wu L, Zhang D, Zhou L, Pei Y, Zhuang Y,
Cui W and Chen J: FUN14 domain-containing 1 promotes breast cancer
proliferation and migration by activating calcium-NFATC1-BMI1 axis.
EBioMedicine. 41:384–394. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Veis A: The role of dental pulp-thoughts
on the session on pulp repair processes. J Dent Res. 64:Spec No.
552–554. 1985. View Article : Google Scholar
|
|
18
|
Kim SA, Choi HS and Ahn SG: Pin1 induces
the ADP-induced migration of human dental pulp cells through P2Y1
stabilization. Oncotarget. 7:85381–85392. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Li C and Jiang H: Altered expression of
circular RNA in human dental pulp cells during odontogenic
differentiation. Mol Med Rep. 20:871–878. 2019.PubMed/NCBI
|
|
20
|
Heyeraas KJ and Berggreen E: Interstitial
fluid pressure in normal and inflamed pulp. Crit Rev Oral Biol Med.
10:328–336. 1999. View Article : Google Scholar
|
|
21
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(-Delta Delta C(T)) method. Methods. 25:402–408. 2001.
View Article : Google Scholar
|
|
22
|
Gong Q, Jiang H, Wei X, Ling J and Wang J:
Expression of erythropoietin and erythropoietin receptor in human
dental pulp. J Endod. 36:1972–1977. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Luke AM, Patnaik R, Kuriadom S, Abu-Fanas
S, Mathew S and Shetty KP: Human dental pulp stem cells
differentiation to neural cells, osteocytes and adipocytes-An in
vitro study. Heliyon. 6:e030542020. View Article : Google Scholar :
|
|
24
|
Al Madhoun A, Sindhu S, Haddad D, Atari M,
Ahmad R and Al-Mulla F: Dental pulp stem cells derived from adult
human third molar tooth: A brief review. Front Cell Dev Biol.
9:7176242021. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Chakraborty J, Caicci F, Roy M and Ziviani
E: Investigating mitochondrial autophagy by routine transmission
electron microscopy: Seeing is believing? Pharmacol Res.
160:1050972020. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Sakdee JB, White RR, Pagonis TC and
Hauschka PV: Hypoxia-amplified proliferation of human dental pulp
cells. J Endod. 35:818–823. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Du R, Zhao J, Wen Y, Zhu Y and Jiang L:
Deferoxamine enhances the migration of dental pulp cells via
hypoxia-inducible factor 1alpha. Am J Transl Res. 13:4780–4787.
2021.
|
|
28
|
Han Y, Chen Q, Zhang L and Dissanayaka WL:
Indispensable role of HIF-1a signaling in post-implantation
survival and angio-/vasculogenic properties of SHED. Front Cell Dev
Biol. 9:6550732021. View Article : Google Scholar
|
|
29
|
Chen Y, Zhao Q, Yang X, Yu X, Yu D and
Zhao W: Effects of cobalt chloride on the stem cell marker
expression and osteogenic differentiation of stem cells from human
exfoliated deciduous teeth. Cell Stress Chaperones. 24:527–538.
2019. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Hu HM, Mao MH, Hu YH, Zhou XC, Li S, Chen
CF, Li CN, Yuan QL and Li W: Artemisinin protects DPSC from hypoxia
and TNF-alpha mediated osteogenesis impairments through CA9 and Wnt
signaling pathway. Life Sci. 277:1194712021. View Article : Google Scholar
|
|
31
|
Zhou Y, Fan W and Xiao Y: The effect of
hypoxia on the stemness and differentiation capacity of PDLC and
DPC. Biomed Res Int. 2014:8906752014. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Vanacker J, Viswanath A, De Berdt P,
Everard A, Cani PD, Bouzin C, Feron O, Diogenes A, Leprince JG and
des Rieux A: Hypoxia modulates the differentiation potential of
stem cells of the apical papilla. J Endod. 40:1410–1418. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Xu H, Xu F, Zhao J, Zhou C and Liu J:
Platelet-rich plasma induces autophagy and promotes regeneration in
human dental pulp cells. Front Bioeng Biotechnol. 9:6597422021.
View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Li Y, Zhao X, He B, Wu W, Zhang H, Yang X
and Cheng W: Autophagy activation by hypoxia regulates angiogenesis
and apoptosis in oxidized low-density lipoprotein-induced
preeclampsia. Front Mol Biosci. 8:7097512021. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Pei F, Wang HS, Chen Z and Zhang L:
Autophagy regulates odontoblast differentiation by suppressing
NF-kappaB activation in an inflammatory environment. Cell Death
Dis. 7:e21222016. View Article : Google Scholar
|
|
36
|
Gong G, Hu L, Liu Y, Bai S, Dai X, Yin L,
Sun Y, Wang X and Hou L: Upregulation of HIF-1alpha protein induces
mitochondrial autophagy in primary cortical cell cultures through
the inhibition of the mTOR pathway. Int J Mol Med. 34:1133–1140.
2014. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Song H, Chen X, Jiao Q, Qiu Z, Shen C,
Zhang G, Sun Z, Zhang H and Luo QY: HIF-1alpha-mediated telomerase
reverse transcriptase activation inducing autophagy through
mammalian target of rapamycin promotes papillary thyroid carcinoma
progression during hypoxia stress. Thyroid. 31:233–246. 2021.
View Article : Google Scholar
|
|
38
|
Zhang J, Zhang C, Jiang X, Li L, Zhang D,
Tang D, Yan T, Zhang Q, Yuan H, Jia J, et al: Involvement of
autophagy in hypoxia-BNIP3 signaling to promote epidermal
keratinocyte migration. Cell Death Dis. 10:2342019. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Huang HY, Wang WC, Lin PY, Huang CP, Chen
CY and Chen YK: The roles of autophagy and hypoxia in human
inflammatory periapical lesions. Int Endod J. 51(Suppl 2):
e125–e145. 2018. View Article : Google Scholar
|
|
40
|
Ren C, Xu Y, Liu H, Wang Z, Ma T, Li Z,
Sun L, Huang Q, Zhang K, Zhang C, et al: Effects of runt-related
transcription factor 2 (RUNX2) on the autophagy of
rapamycin-treated osteoblasts. Bioengineered. 13:5262–5276. 2022.
View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Wang L, Wang P, Dong H, Wang S, Chu H, Yan
W and Zhang X: Ulk1/FUNDC1 prevents nerve cells from
hypoxia-induced apoptosis by promoting cell autophagy. Neurochem
Res. 43:1539–1548. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Wu W, Li W, Chen H, Jiang L, Zhu R and
Feng D: FUNDC1 is a novel mitochondrial-associated-membrane (MAM)
protein required for hypoxia-induced mitochondrial fission and
mitophagy. Autophagy. 12:1675–1676. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
De R, Mazumder S and Bandyopadhyay U:
Mediators of mitophagy that regulate mitochondrial quality control
play crucial role in diverse pathophysiology. Cell Biol Toxicol.
37:333–366. 2021. View Article : Google Scholar
|
|
44
|
Wen W, Yu G, Liu W, Gu L, Chu J, Zhou X,
Liu Y and Lai G: Silencing FUNDC1 alleviates chronic obstructive
pulmonary disease by inhibiting mitochondrial autophagy and
bronchial epithelium cell apoptosis under hypoxic environment. J
Cell Biochem. 120:17602–17615. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Liu H, Zang C, Yuan F, Ju C, Shang M, Ning
J, Yang Y, Ma J, Li G, Bao X and Zhang D: The role of FUNDC1 in
mitophagy, mitochondrial dynamics and human diseases. Biochem
Pharmacol. 197:1148912022. View Article : Google Scholar
|
|
46
|
Zhang W: The mitophagy receptor FUN14
domain-containing 1 (FUNDC1): A promising biomarker and potential
therapeutic target of human diseases. Genes Dis. 8:640–654. 2021.
View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Wu W, Lin C, Wu K, Jiang L, Wang X, Li W,
Zhuang H, Zhang X, Chen H, Li S, et al: FUNDC1 regulates
mitochondrial dynamics at the ER-mitochondrial contact site under
hypoxic conditions. EMBO J. 35:1368–1384. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
48
|
Hou H, Er P, Cheng J, Chen X, Ding X, Wang
Y, Chen X, Yuan Z, Pang Q, Wang P and Qian D: High expression of
FUNDC1 predicts poor prognostic outcomes and is a promising target
to improve chemoradiotherapy effects in patients with cervical
cancer. Cancer Med. 6:1871–1881. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
49
|
Lampert MA, Orogo AM, Najor RH, Hammerling
BC, Leon LJ, Wang BJ, Kim T, Sussman MA and Gustafsson ÅB:
BNIP3L/NIX and FUNDC1-mediated mitophagy is required for
mitochondrial network remodeling during cardiac progenitor cell
differentiation. Autophagy. 15:1182–1198. 2019. View Article : Google Scholar : PubMed/NCBI
|