Introduction
Esophageal cancer is the 7th most common type of
cancer with ~572,000 estimated new cases and ~509,000 deaths in
2018 (1) and a five-year
survival rate of <20% (2).
The predominant histological subtype is esophageal squamous cell
carcinoma (ESCC), which accounts for >85% of esophageal cancers
(3). There are various known
risk factors for ESCC, including alcohol consumption, cigarette
smoking, chewing tobacco, hot drinks, indoor air pollution,
pickles, preserved meat, a low intake of fruits and vegetables
(4). The mainstay treatment for
early-stage ESCC is surgery (endoscopic resection or
esophagectomy). For advanced ESCC, surgery is frequently combined
with neoadjuvant treatments, such as chemotherapy or
chemoradiotherapy (5). However,
the poor prognosis of ESCC highlights the urgent requirement for
the development of novel therapeutics.
To aid the discovery of novel drug candidates or the
repurposing of approved drugs, the National Institutes of Health
(NIH) have launched the Library of Integrated Network-based
Cellular Signatures (LINCS) (6).
Using L1000 technology, LINCS has detected transcriptional
signatures for 1.4 million genetic (CRISPR knock-out, shRNA
knockdown and open reading frame overexpression) and small-molecule
(drug and tool compound) perturbations spanning 50 cell types of
varied lineages (6). It has been
proven by several studies that LINCS is useful for drug discovery.
For head and neck squamous cell carcinoma with mutant p53, the
phosphatidylinositol-3-kinase α-selective inhibitor alpelisib was
found, using the LINCS approach, to disrupt the interaction of MYC,
mutant p53 and Yes-associated protein with MYC target promoters
(7). A recent study indicated
that crizotinib, celastrol and GSK1059615 may be combined with
erlotinib to inhibit erlotinib-resistant PC9 lung cancer cells
(8). In addition, it was
discovered that aurora inhibitors and JQ1 synergistically inhibit
glioblastoma and that mitoxantrone or imatinib may synergize with
gemcitabine (9). ZhangScore is
an algorithm that is able to evaluate the similarity of
transcriptomes based on drug-perturbated gene expression data. Lin
et al (10) indicated
that the ZhangScore method (11), which may provide the significance
level of a small molecule by bootstrapping, is superior to other
methods and has high accuracy in identifying potential drugs.
NF-κB signaling is an important pathway that
regulates various biological processes, including inflammation,
survival, proliferation and immune response (12). NF-κB signaling is frequently
constitutively activated in numerous cancer types, which relies on
the phosphorylation and degradation of its specific inhibitor IκB
protein by the IκB kinase (IKK) complex (13-15). Degradation of IκB protein leads
to the release of the p65/RELA-p50 (canonical) or p52-RELB
complexes into the nucleus, inducing the transcription of multiple
target genes involved in pro-inflammation, angiogenesis, cell
proliferation, anti-apoptosis and cell-matrix remodeling (16). Thus, targeting NF-κB signaling is
considered a promising strategy for cancer treatment.
2-[(Aminocarbonyl)amino]-5-(4-fluorop henyl)-3-thiophenecarboxamide
(TPCA-1) is a potent selective inhibitor of IKKβ (17). It was reported that flubendazole,
an inhibitor of NF-κB signaling, may induce apoptosis in ESCC cells
(15). However, whether TPCA-1
is able to inhibit ESCC has remained elusive.
In the present study, the extensive data on the
small moleculeperturbed transcription signatures in the LINCS
project were used to identify potential small inhibitory molecules
for ESCC. A list of 50 small molecules was compiled and
prioritized, and the four top-scoring candidate molecules
(nutlin-3a, vemurafenib, TPCA-1 and CID2858522) were experimentally
verified to inhibit ESCC cell viability. In particular, it was
determined that TPCA-1, which targets the IKKβ protein, was able to
inhibit the viability of ESCC cells more effectively than that of
non-tumorigenic esophageal epithelial cells.
Materials and methods
Dataset collection
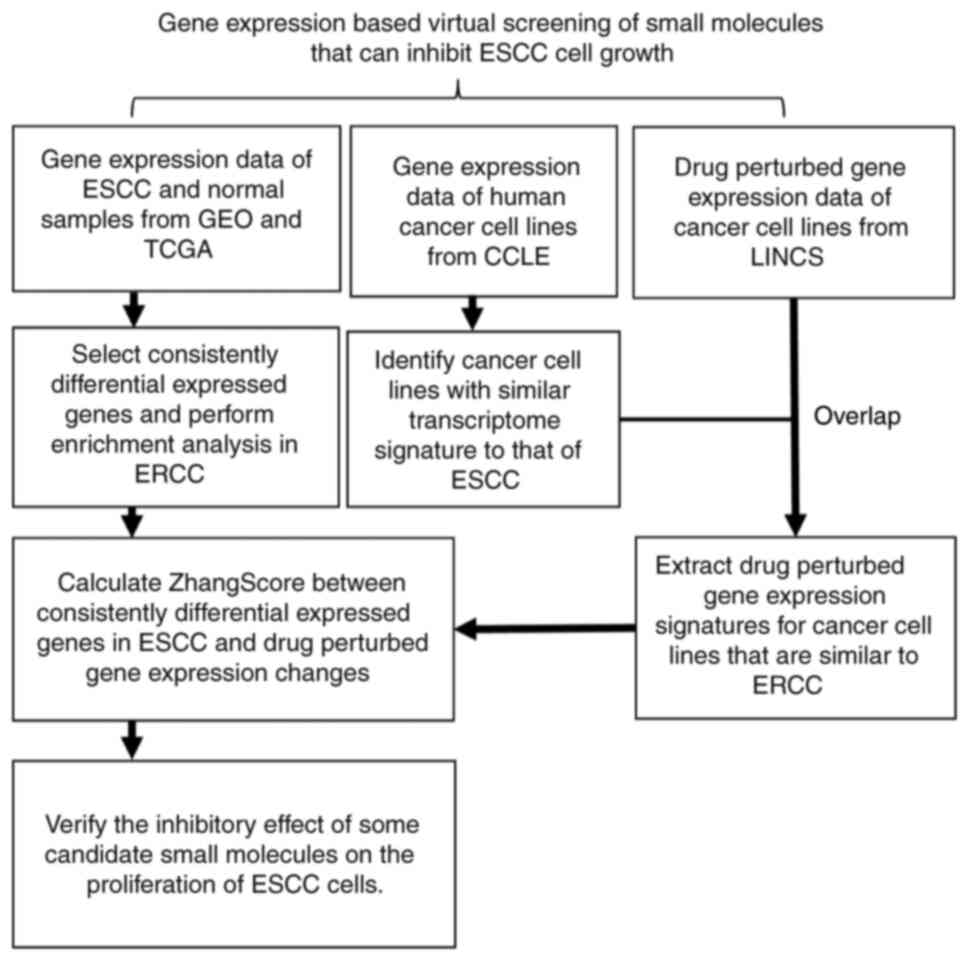
The analytical workflow of the present study is
illustrated in Fig. 1. A total
of 20 gene expression datasets of ESCC were obtained from the Xena
(https://xena.ucsc.edu/), Sequence Read Archive
(SRA, https://www.ncbi.nlm.nih.gov/sra) and Gene Expression
Omnibus (GEO, https://www.ncbi.nlm.nih.gov/geo) databases. A total
of 911 samples were included, of which 515 were tumor samples or
tumor cell lines and 396 were normal esophagus or nontumorigenic
cell lines. Gene expression data and corresponding annotation files
for >1,000 human cancer cell lines were downloaded from the
Cancer Cell Line Encyclopedia (CCLE, https://portals.broadinstitute.org/ccle). The
preprocessed level 5 GCTX format data of small-molecule-perturbed
gene expression files, as well as the corresponding cell annotation
and experimental annotation files, were downloaded from the GEO
database (accession no. GSE92742).
Identification of the consensus
differentially expressed genes (DEGs) in ESCC
For ESCC expression chips, the Limma package
(18) was used to perform
background correction, standardization and differential expression
analysis. For RNAseq data from the SRA database, align-free aligner
Salmon (19) was used to perform
quantification. Gene-level expression data were then imported into
the R environment by the tximport package (20) and DEG analysis was performed
using the DESeq2 (21) package.
For RNAseq data from The Cancer Genome Atlas (TCGA), the expression
data were obtained from Xena and gene expression data were
extracted for ESCC and normal tissues. DESeq2 was then used to
perform DEG analysis. DEGs were defined as adjusted P<0.05 and
|log2 FoldChange|>1 in each dataset. To obtain the consistent
transcriptional changes in ESCC, DEGs that were shared by >50%
of the 20 datasets were further identified as consensus DEGs. The
ClusterProfiler (22) package
was used to perform Gene Ontology (GO) and Kyoto Encyclopedia of
Genes and Genome (KEGG) enrichment analysis and visualization for
DEGs.
Screening for the cancer cells most
similar to ESCC cells at the gene expression level
As the current version of LINCS has no
small-molecule-perturbed experiment performed on ESCC cells, the
cell lines most similar to ESCC cells at the transcriptome level
were first identified using the CCLE dataset. The R-package GGally
(23) was used to first identify
the most representative ESCC cell lines with a high pair-wise
Pearson correlation coefficient (PCC). The mean PCC between one
ESCC cell line and all other representative ESCC cell lines was
>0.7. The mean value for each gene was then calculated among the
representative cell lines as the representative profile of ESCC.
The PCCs between the representative profile of ESCC and no-ESCC
cell lines were computed in LINCS. The cancer cell lines with a PCC
>0.83 (determined by the inflection point presented in Fig. S1B) were defined as similar to
ESCC cells at the transcriptome level. Finally, six cell lines
(A375, CORL23, HT115, HT29, SNUC5 and SW620) were indicated to be
similar to ESCC cells and to have small-molecule perturbation data
in LINCS.
Candidate small molecule screening using
LINCS data
The SignatureSearch (24) package was used to parse and
extract gene expression data prior to and after small molecule
treatment for the six cell lines similar to ESCC cells in the
LINCS. Next, the RCSM (10)
package was used to calculate the ZhangScore (11) between DEGs of ESCC (query gene
signatures) and the small-molecule-perturbed gene expression
(reference gene signatures). The molecules were ordered by
ZhangScore in ascending order for each of the six cell lines.
Finally, RobustRankAggreg (RRA) (25) was used to integrate the ranking
results of molecules from the six cell lines. The significant level
of each of the top-ranking small molecules among all six rank lists
was obtained. The final list was ranked based on the sum of the
ZhangScore across the six cell lines in ascending order.
Cell culture
KYSE-150, KYSE-180 and KYSE-450 cell lines were from
German collection of microorganisms and cell cultures. TE-1 cell
line was from the RIKEN BRC cell bank. Eca-109 cell line was from
China center for type culture collection. The immortalized human
esophageal epithelial cell line, Het-1A was from American type
culture collection. All cell lines used in the present study were
cultured at 37°C in a thermostatic cell incubator (Thermo Fisher
Scientific, Inc.) with saturated humidity and 5% CO2.
Cells were cultured in RPMI-1640 medium (Gibco; Thermo Fisher
Scientific, Inc.) supplemented with 10% FBS (HyClone; Cytiva) and
1% double resistance (100 U/ml penicillin and 100 µg/ml
streptomycin; HyClone; Cytiva).
Cell viability assay
ESCC cells were seeded in 96-well plates at a
density of 2,000 cells/100 µl and cultured overnight at 37°C
with 5% CO2. Following treatment with the indicated
concentrations of small molecules for different durations, their
survival was measured with an MTS kit (Thermo Fisher Scientific,
Inc.) using an enzyme labeler (Varioskan Flash; Thermo Fisher
Scientific, Inc.). The absorbance was measured at a wavelength of
490 nm and cell viability was calculated as follows:
Viability=[Optical density
(OD)Drug−ODBlank]/(ODControl−ODBlank).
Colony-forming assay
Cells (700/well) were exposed to various
concentrations (0, 0.5, 1, 2 and 4 µM) of TPCA-1
(MedChemExpress, Inc.) in 6-well plates and then cultured for 2
weeks at 37°C in an incubator with 5% CO2. Following
gentle washing with PBS once at room temperature, 1 ml methanol was
used to fix each well for 10 min at room temperature. Each well was
then dyed with 1 ml crystalline purple for 10 min at room
temperature. Finally, each well was washed several times with PBS.
A gel imager (ChemiDoc XRS Plus; Bio-Rad Laboratories, Inc.) with
bright light optics was used to acquire images.
Cell apoptosis analysis
KYSE-450 cells were treated with different
concentrations of TPCA-1 (0, 0.5, 1, 2 and 4 µM) for 48 h.
Cells were washed twice with pre-cooled PBS, digested with trypsin
(Gibco; Thermo Fisher Scientific, Inc.) and centrifuged at 1,000 ×
g for 5 min at room temperature. The supernatant was then discarded
and 195 ml Annexin V-FITC binding buffer was added to gently
resuspend the cells. Finally, 5 ml Annexin V-FITC and 10 ml
propidium iodide staining solution (Beyotime Institute of
Biotechnology) were added to the cell suspension with gentle
mixing. Following incubation at room temperature (20-25°C) in the
dark for 10 min, stained cells were ana lyzed using a flow
cytometer (FACSCalibur, BD Biosciences).
Western blot analysis
KYSE-450 and Het-1A cells were treated with
different concentrations of TPCA-1 (0, 0.5, 1, 2 and 4 µM)
for 48 h. Cells were scraped down and centrifuged in pre-warmed
PBS. After extracting the cell lysate with lysis buffer (50 mM
Tris·HCl pH 8.0, 250 mM NaCl, 40 mM NaF, 0.5 mM NaVO4,
0.5% Nonidet P-40, 1 mM PMSF, 2 µg/ml leupeptin and 2
µg/ml aprotinin), the total protein concentration was
measured with Bradford reagent (B6916; MilliporeSigma) using a
spectrophotometer (Thermo Fisher Scientific, Inc.). Equal amounts
of total protein (50 µg for each lane) were subjected to
SDS-PAGE separation with 10% polyacrylamide gel, followed by
electro-transfer to a polyvinylidene difluoride membrane (Bio-Rad
Laboratories, Inc.) in a Mini-PROTEAN Tetra vertical
electrophoresis cell (Bio-Rad Laboratories, Inc.). The membrane was
then blocked with 5% skimmed milk (cat. no. P0126; Beyotime
Institute of Biotechnology) for 1 h at room temperature and then
incubated with specific antibodies at 4°C for 16 h. The primary
antibodies for IκBα (cat. no. 4814; 1:1,000 dilution),
phosphorylated (p)-IKKβ (cat. no. 2697; 1:1,000 dilution), IKKβ
(cat. no. 2678; 1:1,000 dilution), p-P65 (cat. no. 3033; 1:1,000
dilution), P65 (cat. no. 8242; 1:1,000 dilution), caspase 3 (cat.
no. 9662; 1:1,000 dilution) and cleaved caspase 3 (cat. no. 9662;
1:1,000 dilution) were purchased from Cell Signaling Technology,
Inc. Antibodies for GAPDH (cat. no. 0037; 1:3,000 dilution) and Bim
(cat. no. AY1380; 1:1,000 dilution) were purchased from Abways
Technology. The HRP-conjugated goat anti-mouse (cat. no. sc-2005;
1:3,000 dilution) and goat anti-rabbit IgG antibodies (cat. no.
sc-2004; 1:1,000 dilution) were purchased from Santa Cruz
Biotechnology, Inc. The bands were visualized by enhanced
chemiluminescence (cat. no. P10100; New Cell & Molecular
Biotech Co., Ltd.) in a gel imager (ChemiDoc XRS Plus; Bio-Rad
Laboratories, Inc.).
Statistical analysis
All bioinformatics analyses and calculations were
performed in R. Values are expressed as the mean ± standard error
of the mean. All statistical analysis was performed using GraphPad
Prism 8 software (GraphPad Software, Inc.). An unpaired Student's
t-test was used to determine the significance of differences
between groups with and without TCPA-1 treatment in each cell line.
Two-way ANOVA followed by Tukey's post-hoc test was used to
determine the significance of differential responses of KYSE-450
and Het-1A cells to TPCA-1 treatment. P<0.05 was considered to
indicate a statistically significant difference.
Results
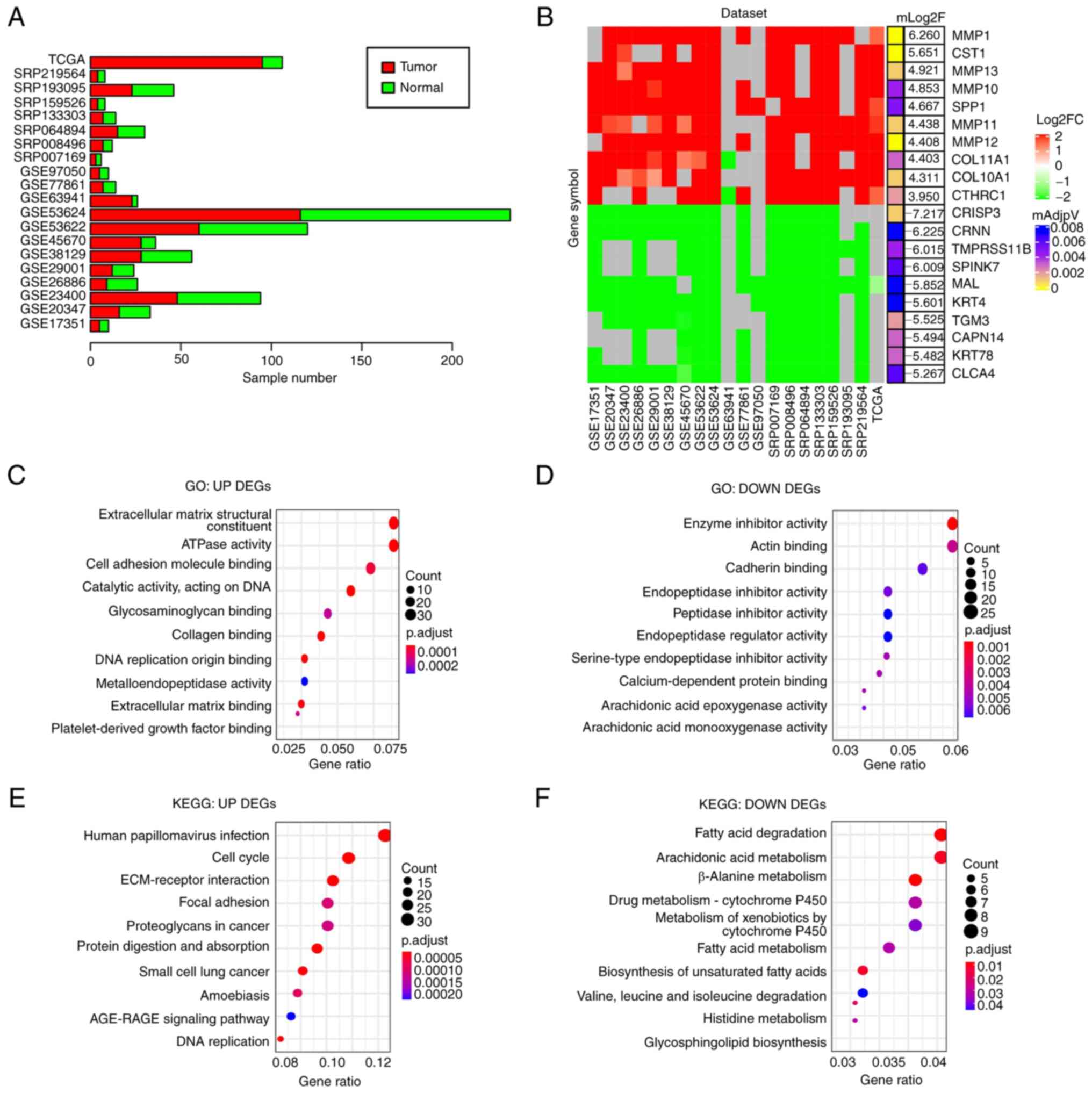
Consensus DEGs in ESCC
To obtain DEGs in ESCC compared with normal
controls, 20 ESCC gene expression datasets from the GEO, SRA or
Xena databases consisting of 911 samples (515 tumor and 396 normal
samples) were analyzed (Fig. 2A)
and DEGs were identified in each dataset (adjusted P<0.05 and
|log2FoldChange|>1). DEGs identified in at least 10 datasets
(≥50%) were considered as DEGs of ESCC in the present study. In
total, 521 downregulated and 460 upregulated genes were identified
(Table SI). Of note, several
cell matrix-related proteins, including MMP-1, -13 and -10, were
among the top 10 upregulated DEGs (Fig. 2B), suggesting that the alteration
of cell matrix-related gene expression may have an important role
in ESCC development.
GO enrichment analyses indicated that the
upregulated DEGs were mainly related to extracellular matrix
(Fig. 2C) and the downregulated
DEGs were mainly associated with peptidase inhibitor and
arachidonic acid epoxygenase (Fig.
2D). KEGG pathway analysis suggested that the upregulated DEGs
were primarily enriched in extracellular matrix-receptor
interaction and cell cycle regulators (Fig. 2E) and the downregulated DEGs in
fatty acid degradation and cytochrome P450 (Fig. 2F). Both the arachidonic acid
epoxygenase and fatty acid degradation pathways are associated with
inflammation (26,27), suggesting that disrupted immune
response may be involved in ESCC development.
Identification of cancer cell lines with
similar gene expression patterns to ESCC cells
Although LINCS (28) has transcriptome profiles derived
from a variety of human cancer cells treated with various small
molecules, there is a lack of perturbation data for ESCC cells.
Thus, it was hypothesized that non-ESCC cell lines that display
transcriptome profiles similar to that of ESCC in LINCS may have
similar cellular responses to treatment of ESCC cells with small
molecules and that the small molecules that inhibit the viability
of these non-ESCC cells may also inhibit the viability of ESCC
cells. To do this, the gene expression data of >1,000 human
cancer cell lines from CCLE were analyzed (29), including 22 ESCC cell lines. Gene
expression datasets derived from both RNAseq and microarrays were
used to calculate the PCC in each pair from a total of 22 ESCC cell
lines. As presented in Fig.
S1A, the PCCs of seven ESCC cell lines, including OE21,
KYSE-510, TE-11, KYSE-70, KYSE-520, KYSE-180 and TE-14, exhibited
high pairwise similarities (PCC>0.7). The mean expression values
of each gene in the seven cell lines were calculated and then used
as a reference profile for ESCC cells to calculate the PCCs between
this reference profile and the transcriptome profiles of non-ESCC
cancer cells in CCLE. A total of 70 non-ESCC cancer cell lines with
a PCC>0.83 (determined by inflection point) exhibited a similar
transcriptome profile to that of ESCC cells (Fig. S1B). Among them, transcriptome
profiles of small-molecule perturbations were available for six
cell lines, including A375 human melanoma, CORL23 human lung large
cell carcinoma, HT115 human colon carcinoma, HT29 human colorectal
cancer, SNUC5 human cecum adenocarcinoma and SW620 human colon
adenocarcinoma cell lines (Fig.
S1C) in LINCS. Of note, data for 31,398 perturbations were
available for the six cell lines (Fig. S1D).
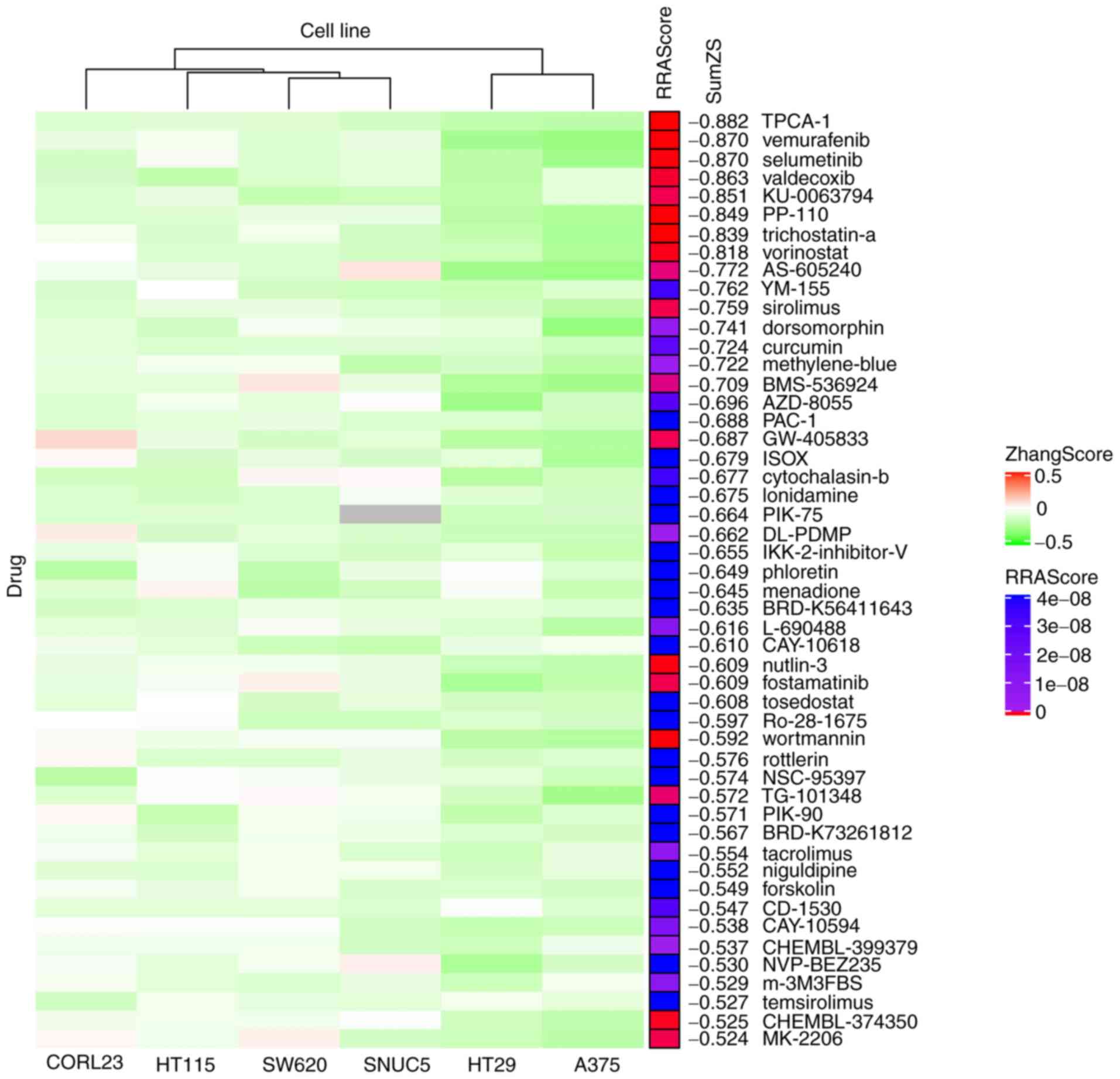
ZhangScore and RRA methods for ranking
candidate small molecules that may reverse the aberrant gene
expression of ESCC
The ZhangScore algorithm is able to calculate the
reversing extent of a small-molecule-perturbed signature, termed
reference signature, compared with a disease signature, termed
query signature. The more negative the ZhangScore, the higher the
potential of the small molecule to reverse the altered expression
of malignant cells back to their non-malignant state (11). Therefore, DEGs shared in ESCC
were used as the query signature and small-molecule-perturbed
signatures were used as the reference signatures (each reference
signature was derived from the transcriptome of cells treated with
a combination of small molecule, concentration and duration) from
six cell lines identified above. The ZhangScore was first
calculated for each small-molecule-perturbed signature from each
cell line. The smallest negative ZhangScore of each small molecule,
among various combinations of concentrations and durations, was
used for candidates ranking in each cell line (Table SII). The RRA method (25) was then used to integrate the
ranking results from the six cell lines. The final ranking was
based on the sum of ZhangScores (SumZS) derived from the six cell
lines and presented in ascending order (Table I presents the top 50 molecules).
In Fig. 3, the heatmap
illustrates that the majority of the top 50 small molecules had a
negative ZhangScore (in green color) in all the six cell lines,
suggesting that these small molecules were likely able to reverse
the expression of DEGs upon small molecule perturbation. The RRA
scores labeled in red indicate the top-ranking small molecules that
had the most consistent effects among all six cell lines.
 | Table ITargets and current status in
clinical trials of the top 50 small molecules. |
Table I
Targets and current status in
clinical trials of the top 50 small molecules.
| Rank | Molecule | Target protein
(classes) | PMID |
Indications/clinical
trials/classification |
|---|
| 1 | TPCA-1 | IKKβ
(inhibitor) | 15316093 | Unknown |
| 2 | Vemurafenib |
BRAFV600E (inhibitor) | 35045748 | Melanoma |
| 3 | Selumetinib | MAP2K1
(inhibitor) | 32504375 |
Melanoma/neurofibromatosis type 1/thyroid
cancer |
| 4 | Valdecoxib | PTGS2
(inhibitor) | 23517091 | Rheumatoid
arthritis [withdrawn] |
| 5 | KU-0063794 | mTOR
(inhibitor) | 19402821 | Mild atopic asthma
(Phase 2) |
| 6 | PP-110 | Unknown | Not available | Unknown |
| 7 | Trichostatin-a | HDAC
(inhibitor) | 25923331 | Relapsed or
refractory hematologic malignancies (Phase 1) |
| 8 | Vorinostat | HDAC
(inhibitor) | 35158962 | Cutaneous T-cell
lymphoma/ESCC (NCT00537121, Phase 1) |
| 9 | AS-605240 | PIK3CG
(inhibitor) | 24935930 | Unknown |
| 10 | YM-155 | Survivin
(inhibitor) | 21737502 | Melanoma (Phase
2)/metastatic breast cancer (Phase 2) |
| 11 | Sirolimus | mTOR
(inhibitor) | 34415233 | Organ
rejection/prostate cancer (Phase 2)/pancreatic cancer (Phase
2) |
| 12 | Dorsomorphin | AMPK
(inhibitor) | 18026094 | Unknown |
| 13 | Curcumin | PPARG
(inhibitor) | 23534763 | Periodontitis
(Phase 4)/advanced pancreatic cancer (Phase 2)/prostate cancer
(Phase 3) |
| 14 | Methylene-blue | GUCY1A2
(inhibitor) | 30666126 |
Methaemoglobinaemia/colorectal cancer
(Phase 3) |
| 15 | BMS-536924 | IGF1R
(inhibitor) | 16134929 | Unknown |
| 16 | AZD-8055 | mTOR
(inhibitor) | 21333749 | Recurrent Gliomas
(Phase 1)/advanced tumors (Phase 1) |
| 17 | PAC-1 | Caspase-3
(activator) | 19281821 | Uveal melanoma
(Phase 2)/advanced malignancies (Phase 1) |
| 18 | GW-405833 | Unknown | 20863899 | Unknown |
| 19 | ISOX | HDAC
(inhibitor) | 29867749 | Unknown |
| 20 | Cytochalasin-b | Unknown | Not available | Unknown |
| 21 | lonidamine | HK
(inactivator) | 16986056 | Prostatic
hyperplasia (Phase 2/3, terminated) |
| 22 | PIK-75 | PI3K
(inhibitor) | 17362206 | Unknown |
| 23 | DL-PDMP | Unknown | 21571032 | Unknown |
| 24 |
IKK-2-inhibitor-V | IKKβ
(inhibitor) | 23211970 | Unknown |
| 25 | Phloretin | Unknown | 14019989 | Flavors |
| 26 | Menadione | Cytochrome P450
(suppressor) | 27167070 | Vitamins |
| 27 | BRD-K56411643 | Unknown | Not available | Unknown |
| 28 | L-690488 | Unknown | Not available | Unknown |
| 29 | CAY-10618 | Unknown | Not available | Unknown |
| 30 | Nutlin-3a | MDM2
(antagonist) | 14704432 | Unknown |
| 31 | Fostamatinib | SYK
(inhibitor) | 16946104 | Advanced
colorectal, non-small cell lung, head and neck hepatocellular and
renal cell carcinomas among other cancers (Phase
2)/thrombocytopenia (Phase 2)/COVID-19 (Phase 2/3) |
| 32 | Tosedostat | aminopeptidase
(inhibitor) | 18701491 | Acute myeloid
leukaemia (phase 1/2) |
| 33 | Ro-28-1675 | Unknown | 20161845 | Unknown |
| 34 | Wortmannin | PIK3CG
(inhibitor) | 24654606 |
Radiation-sensitizing/insulin
antagonists/immunosuppressive |
| 35 | Rottlerin | Protein kinase
(inhibitor) | 8123051 | Unknown |
| 36 | NSC-95397 | Cdc25
(inhibitor) | 11901209 | Unknown |
| 37 | TG-101348 | JAK2
(inhibitor) | 32346607 | Primary or
secondary myelofibrosis |
| 38 | PIK-90 | PI3K
(inhibitor) | 16864657 | Unknown |
| 39 | BRD-K73261812 | β-catenin
(inhibitor) | 19382889 | Unknown |
| 40 | Tacrolimus | FKBP1A
(inhibitor) | 23228564 | Transplant
rejection/leukemia or lymphoma (Phase 3) |
| 41 | Niguldipine | Calcium Channel
(Blockers) | 2548881 |
Antineoplastic/antihypertensive/calcium
channel blockers |
| 42 | Forskolin | AC (activator) | 24559688 |
Cardiotonic/vasodilator/bronchodilator/immunologic |
| 43 | CD-1530 | RARG (agonist) | 27336223 | Unknown |
| 44 | CAY-10594 | Phospholipase D2
inhibitor | 31076618 | Unknown |
| 45 | CHEMBL-399379 | Unknown | Not available | Unknown |
| 46 | NVP-BEZ235 | mTOR
(inhibitor) | 20804212 | Orally bioavailable
antineoplastic agents |
| 47 | m-3M3FBS | PLCβ
(activator) | 34625112 | Unknown |
| 48 | Temsirolimus | mTOR
(inhibitor) | 18543327 | Advanced renal-cell
carcinoma/mantle cell lymphoma |
| 49 | CID2858522 | PKC
(inhibitor) | 23469385 | Unknown |
| 50 | MK-2206 | Akt
(inhibitor) | 23917345 | Orally bioavailable
antineoplastic activity |
The top-ranking small molecules included several
known and clinically used anti-cancer drugs, such as vemurafenib
(ranked 2nd) for the treatment of melanoma (30) and vorinostat (ranked 8th) for the
treatment of cutaneous T-cell lymphoma (31). Several drugs identified are
involved in immune regulation, such as sirolimus (ranked 11th) and
tacrolimus (ranked 40th), which are used to counteract organ
rejection (32). Of note,
certain natural products from edible plants or their analogs, such
as curcumin (ranked 13th), phloretin (ranked 25th) and menadione
(ranked 26th), were also identified.
Experimental validation of the effects of
selective molecules on ESCC cell viability
To verify the effectiveness of the screening methods
used in the present study, the effects of TPCA-1 (IKKβ inhibitor;
ranked 1st), vemurafenib (BRAFV600E inhibitor, Food and
Drug Administration-approved for the treatment of melanoma; ranked
2nd), nutlin-3a (MDM2 antagonists; ranked 30th) and CID2858522 (PKC
inhibitor; ranked 49th) on the viability of ESCC cells and a
non-tumorigenic human epithelial Het-1A cells were examined. These
results indicated that, although different ESCC cells exhibited
variable sensitivity to TPCA-1, nutlin-3a, vemurafenib or
CID2858522, these four small molecules markedly inhibited ESCC cell
viability in a dose-dependent manner (Fig. 4A-D, Table II). Of note, while KYSE-450
cells appeared to be somewhat resistant, Het-1A cells exhibited
marked resistance to the treatment with these molecules (Table II). Furthermore, TPCA-1 was more
potent in inhibiting ESCC cell viability than nutlin-3a,
vemurafenib or CID2858522, as evidenced by the lowest
IC50 values (Table
II). Of note, TPCA-1 significantly inhibited the growth of ESCC
cells (Fig. 4E-H) and had higher
selectivity for inducing death of KYSE-450 cells compared with
non-tumorigenic Het-1A cells (Fig.
S2). To substantiate the differential inhibitory effects of
TPCA-1 on ESCC and non-tumorigenic epithelial cells, colony
formation assays were performed. As indicated in Fig. 4I, 0.5 µM TPCA-1
significantly inhibited the colony formation ability of KYSE-450
cells. By contrast, the same dose of TPCA-1 only had a minor effect
on the colony formation ability of Het-1A cells. These results
indicated that TPCA-1 inhibits KYSE-450 cells more significantly
than non-tumorigenic epithelial Het-1A cells.
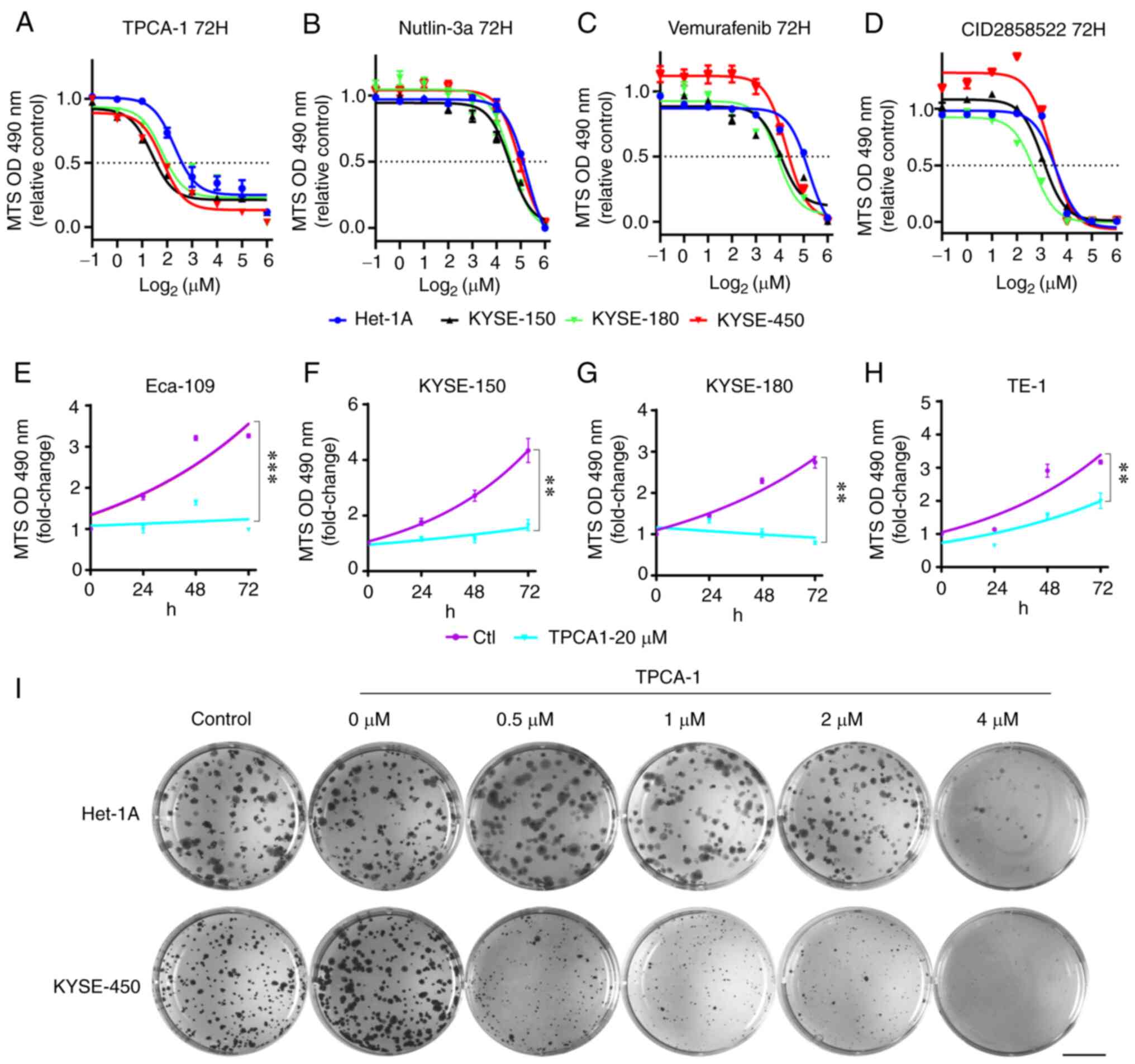 | Figure 4Effects of TPCA-1, nutlin-3a,
vemurafenib or CID2858522 on the viability and growth of ESCC cells
and Het-1A cells. (A-D) Reduction of the viability of ESCC or
Het-1A cells by indicated small molecules. (A) TPCA-1, (B)
nutlin-3a, (C) vemurafenib or (D) CID2858522. IC50
values are listed in Table II.
(E-H) Inhibition of ESCC cell growth by TPCA-1, a potent inhibitor
of IKKβ protein. (E) Eca-109, (F) KYSE-150, (G) KYSE-180 and (H)
TE-1. P-values were calculated based on the fold changes of cell
viability between 20 µM TPCA-1 treated cells and untreated
cells at 72 h. (I) Effects of TPCA-1 on the growth of Het-1A or
KYSE-450 cells by colony-formation assay. Images of cells in each
well (35 mm in diameter) are provided (scale bar, 10 mm). Data are
derived from three independent experiments and presented as the
mean ± standard error of the mean. **P<0.01,
***P<0.001. TPCA-1, 2-[(aminocarbonyl)
amino]-5-(4-fluorophenyl)-3-thiophenecarboxamide; ESCC, esophageal
squamous cell carcinoma; OD490, optical density at 490 nm. |
 | Table IIIC50 values (µM) of
esophageal squamous cell carcinoma cell lines treated with the
indicated small molecules for 72 h. |
Table II
IC50 values (µM) of
esophageal squamous cell carcinoma cell lines treated with the
indicated small molecules for 72 h.
| Molecule | Het-1A | KYSE-150 | KYSE-180 | KYSE-450 |
|---|
| TPCA-1 | 4.26
(3.43-5.41) | 2.63
(2.30-3.03) | 3.45
(2.62-4.61) | 3.56
(3.00-4.15) |
| Nutlin-3a | 38.30
(35.68-41.28) | 25.43
(23.73-27.19) | 26.84
(24.67-29.11) | 35.38
(31.59-39.64) |
| Vemurafenib | 37.17
(34.08-40.66) | 18.51
(13.30-27.42) | 15.37
(12.34-18.70) | 19.36
(17.50-21.51) |
| CID2858522 | 11.77
(10.85-13.05) | 8.26
(7.59-9.01) | 6.45
(5.80-7.12) | 10.28
(8.93-11.85) |
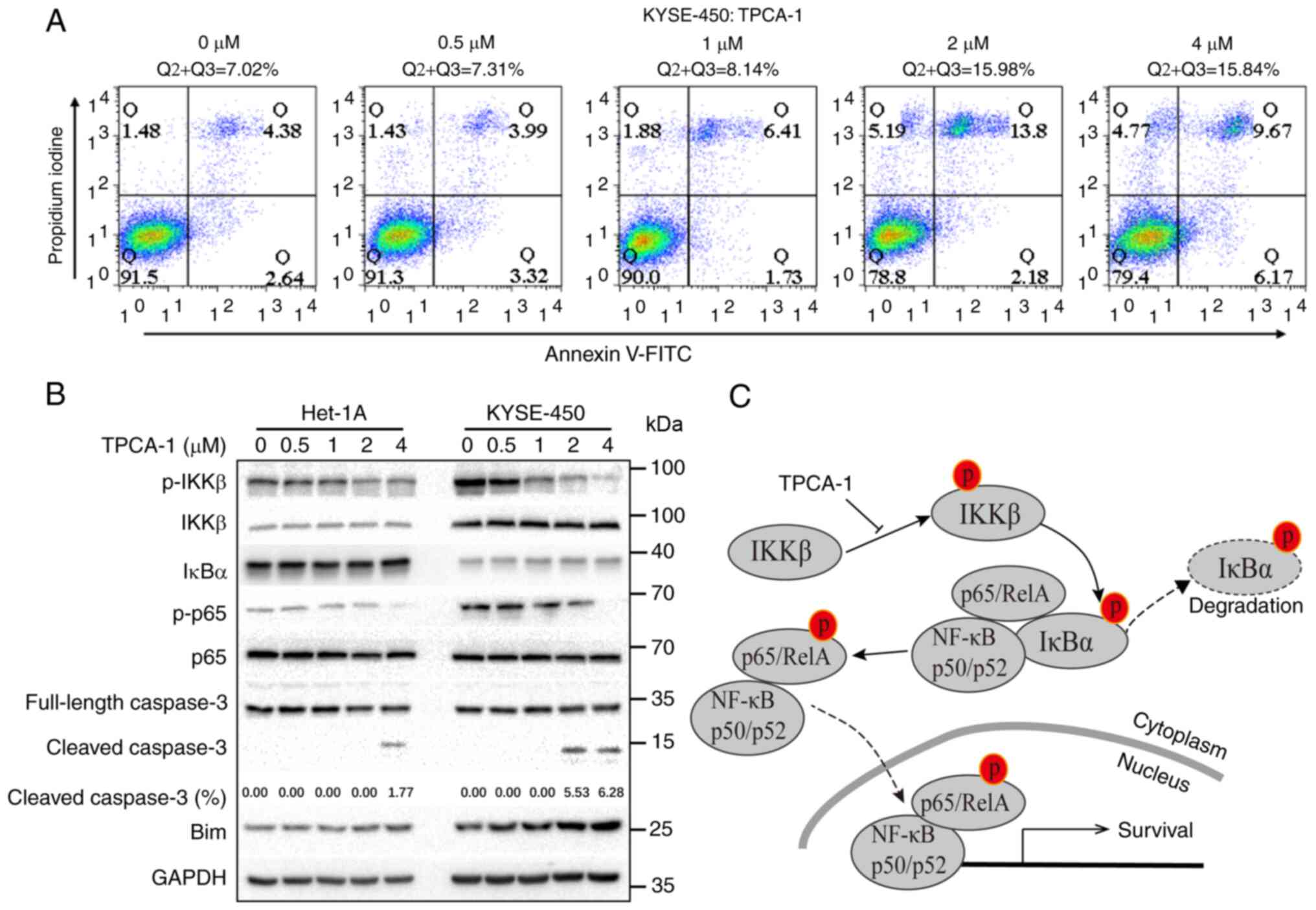
TPCA-1 induced apoptosis in ESCC cells
through inhibition of NF-κB signaling
To explore the mechanisms through which TPCA-1
inhibits the growth of ESCC cells, the effects of TPCA-1 on
KYSE-450 cell apoptosis were examined using flow cytometry. As
presented in Fig. 5A, TPCA-1
significantly induced KYSE-450 cell apoptosis in a dose-dependent
manner. Of note, as presented in Fig. 5B, the NF-κB pathway was
significantly activated in KYSE-450 but not in Het-1A cells, as
evidenced by the lower IκBα expression and higher levels of p-KKβ
and p-p65 compared with Het-1A cells. Treatment of KYSE-450 cells
with TPCA-1 led to the inhibition of both p-IKKβ and p-p65,
ultimately resulting in increased Bim protein expression, cleaved
caspase 3 and apoptosis. Despite the observation that TPCA-1 also
inhibited IKKβ phosphorylation in Het-1A cells, the expression
level of IκBα remained high and suppression of p-p65 was barely
observed. Of note, treatment with 4 µM TPCA-1 was still able
to trigger apoptosis in Het-1A cells, suggesting that TPCA-1
promotes apoptosis in Het-1A cells only when used at higher doses.
Collectively, these results indicated that Het-1A cells are less
dependent on the activation of NF-κB signaling for survival and are
thus less sensitive to the TPCA-1-mediated inhibition of IKKβ.
Discussion
The LINCS project has generated millions of
small-molecule-perturbed transcription signatures in a variety of
different cell lines (28,33). Using a LINCS-based approach, it
was previously determined that crizotinib, celastrol or GSK1059615
may be combined with erlotinib to inhibit the growth of
erlotinib-tolerant PC9 lung cancer cells (8), while mitoxantrone or imatinib may
synergize with gemcitabine to inhibit glioblastoma (9). However, LINCS does not currently
contain the transcription signatures of small-molecule
perturbations in the ESCC cell lines. Thus, the
small-molecule-perturbed transcription signatures cannot be used to
screen for putative drugs against ESCC directly using the LINCS
datasets. It may therefore be hypothesized that non-ESCC cell lines
that display transcriptome profiles similar to that of ESCC in the
LINCS may have similar cellular responses to small-molecule
treatment in ESCC cells and that identifying small molecules that
inhibit the viability of these non-ESCC cells may also inhibit the
viability of ESCC cells. In the present study, the LINCS datasets,
Zhang Score and RRA methods were used to screen for the small
molecules that may reverse the transcriptome aberration of ESCC
cells. Among the 50 top-ranking molecules, four molecules were
selected for experimental verification: TPCA-1 (IKKβ inhibitor;
ranked 1st), vemurafenib (BRAFV600E inhibitor; ranked
2nd), nutlin-3a (MDM2 antagonists; ranked 30th) and CID2858522 (PKC
inhibitor; ranked 49th). It was indicated that all four molecules
were able to significantly inhibit the growth and viability of
multiple ESCC cells, suggesting that the approach used in the
present study may be applicable in small molecule screening for
different cancer types without small-molecule-perturbated profiles
in the LINCS.
The top-ranking small molecules may be divided into
several categories, including inhibitors of growth signaling
pathways, molecules targeting epigenetic modification and immune
modulation drugs. These pathways are known to be important for
cancer progression and treatment. Of note, several small molecules
are clinically approved drugs or currently part of a clinical trial
for cancer treatment. For instance, vemurafenib (ranked 2nd) is a
BRAFV600E inhibitor used for the treatment of melanoma
(34). Selumetinib (ranked 3rd)
is a MAP2K1 inhibitor used for the treatment of melanoma,
neurofibromatosis type 1 and thyroid cancer (35). Vorinostat (ranked 8th) is an HDAC
inhibitor that has completed a phase I clinical trial on cutaneous
T-cell lymphoma (31). Of note,
several further drugs have been approved for the treatment of other
diseases, such as valdecoxib (ranked 4th) which targets PTGS2 and
is used for the treatment of rheumatoid arthritis (36). Furthermore, it has been reported
that certain molecules may inhibit ESCC cells in vitro or in
animal models, such as curcumin (37,38) and menadione (39).
In the present study, it was determined that TPCA-1,
a molecule targeting IKKβ, exhibits a greater inhibitory effect on
ESCC KYSE-450 cells than non-tumorigenic epithelial Het-1A cells.
The results indicated that NF-κB signaling was activated in
KYSE-450 cells, in keeping with the observation that NF-κB
signaling is frequently constitutively activated in ESCC (40). The activation of NF-κB signaling
relies on the phosphorylation of IKKβ, which in turn promotes the
phosphorylation and degradation of IκBα, resulting in the
activation and nuclear localization of the NF-κB complex, thus
facilitating cell survival (14,15). The present study demonstrated
that TPCA-1 potently inhibits NF-κB signaling in KYSE-450 cells,
resulting in apoptosis. By contrast, treatment with low-dose TPCA-1
(0.5-2 µM) failed to influence the levels of IκBα protein
and phosphorylated p65 in Het-1A cells, which may explain why
TPCA-1 is more potent in killing KYSE-450 cells than Het-1A cells.
Of note, a higher dose of TPCA-1 may still trigger apoptosis of
Het-1A.
Among the tested ESCC cell lines, KYSE-450 cells
exhibited a certain amount of resistance to the four tested
molecules. By searching the cancer dependency map (41), a unique missense mutation of
SMAD4, SMAD4D255N, was identified in KYSE-450, but not
in KYSE-150 or KYSE-180 cells. SMAD4 is a critical tumor
suppressor. The inactivation of SMAD4 by point mutations or
deletions has been indicated to cause drug resistance in colon
cancer (42) and pancreatic
ductal adenocarcinoma (43). Low
SMAD4 expression is also associated with cetuximab resistance in
head and neck squamous cell carcinoma, a cancer type similar to
ESCC (44). Taken together, the
SMAD4D255N mutation may contribute to the drug
resistance observed in KYSE-450 cells, which should be further
investigated.
Several limitations of the approach used in the
present study require to be noted. First, the ranking of small
molecules in the present study was primarily based on the drugs'
potential to reverse the transcriptome change of ESCC. This
strategy does not consider the regulation of gene expression levels
other than transcription, such as mRNA translation,
post-translational modification and protein stability. Furthermore,
the screening process is unable to take the targets of drugs into
consideration, which may lead to the truly valuable drugs for
clinical application being missed.
In conclusion, based on small-molecule-perturbed
transcriptome profiling of cell lines that are similar to ESCC
cells, candidate molecules that may perturb the transcriptome
signatures in ESCC were identified. A list of the top-ranking 50
small molecules that may potentially reverse the aberrant
transcriptome alterations of ESCC was compiled. Numerous candidates
are known to target crucial pathways in ESCC, including the
RAS/RAF/MEK/MAPK, PI3K/AKT/mTOR and NF-κB pathways. It was
experimentally verified that four candidates, TPCA-1, nutlin-3a,
vemurafenib and CID2858522, were able to inhibit the viability of
multiple ESCC cell lines. TPCA-1 (ranked 1st), a potent inhibitor
of IKKβ, exerted a higher inhibitory effect on ESCC cells compared
with non-tumorigenic epithelial Het-1A cells. Mechanistically,
TPCA-1 exerts its anti-tumor activity by suppressing the NF-κB
signaling pathway. Of note, NF-κB signaling is activated in ESCC
cells, which are sensitive to the inhibition of TPCA-1. The high
expression of IκBα in Het-1A cells may confer resistance to drug
treatment. Thus, the potent and selective killing of ESCC cells may
make TPCA-1 a potential small molecule in ESCC treatment. The
screening procedure used in the present study may serve as a novel
virtual screening method for drug discovery.
Supplementary Data
Availability of data and materials
The raw data used in the present study were derived
from the following public repositories: Xena (https://xena.ucsc.edu), the Sequence Read Archive
(https://www.ncbi.nlm.nih.gov/sra), the
Library of Integrated Network-based Cellular Signatures (https://lincsproject.org/LINCS/data/overview), Gene
Expression Omnibus (https://www.ncbi.nlm.nih.gov/geo) and the Cancer Cell
Line Encyclopedia (https://portals.broadinstitute.org/ccle) databases.
The datasets generated during the present study are available from
the corresponding author on reasonable request.
Authors' contributions
LJZ and JY prepared the draft of the original
manuscript. ZYL, JY and ZXX participated in writing, reviewing and
editing the manuscript. LJZ and ZYL performed data curation,
experiments and formal analyses. ZXX supervised the study. ZXX and
JY were responsible for project administration. ZXX and JY acquired
funding. ZXX, JY and ZYL confirm the authenticity of all the raw
data. All authors have read and agreed to the final version of the
manuscript to be published.
Ethics approval and consent to
participate
Not applicable.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
Acknowledgments
The authors would like to thank Professor Haoyang
Cai of Sichuan University (Chengdu, China) for providing the
computational resources.
Funding
This research was funded in part by the National Natural Science
Foundation of China (grant nos. 32100441 and 81830108) and the
Sichuan University Postdoctoral Interdisciplinary Innovation Fund
(grant no. 0020404153020).
References
|
1
|
Bray F, Ferlay J, Soerjomataram I, Siegel
RL, Torre LA and Jemal A: Global cancer statistics 2018: GLOBOCAN
estimates of incidence and mortality worldwide for 36 cancers in
185 countries. CA Cancer J Clin. 68:394–424. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Montgomery EA: Oesophageal cancer. World
Cancer Report 2014. Stewart BW and Wild CP: World Health
Organization, International Agency for Research on Cancer; Lyon:
pp. 528–543. 2014
|
|
3
|
Thrift AP: Global burden and epidemiology
of Barrett oesophagus and oesophageal cancer. Nat Rev Gastroenterol
Hepatol. 18:432–443. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Sheikh M, Poustchi H, Pourshams A, Etemadi
A, Islami F, Khoshnia M, Gharavi A, Hashemian M, Roshandel G,
Khademi H, et al: Individual and combined effects of environmental
risk factors for esophageal cancer based on results from the
golestan cohort study. Gastroenterology. 156:1416–1427. 2019.
View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Watanabe M, Otake R, Kozuki R, Toihata T,
Takahashi K, Okamura A and Imamura Y: Recent progress in
multidisciplinary treatment for patients with esophageal cancer.
Surg Today. 50:12–20. 2020. View Article : Google Scholar :
|
|
6
|
Subramanian A, Narayan R, Corsello SM,
Peck DD, Natoli TE, Lu X, Gould J, Davis JF, Tubelli AA, Asiedu JK,
et al: A next generation connectivity map: L1000 platform and the
first 1000000 profiles. Cell. 171:1437–1452. 2017. View Article : Google Scholar
|
|
7
|
Ganci F, Pulito C, Valsoni S, Sacconi A,
Turco C, Vahabi M, Manciocco V, Mazza EMC, Meens J, Karamboulas C,
et al: PI3K inhibitors curtail myc-dependent mutant p53
gain-of-function in head and neck squamous cell carcinoma. Clin
Cancer Res. 26:2956–2971. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Aissa AF, Islam A, Ariss MM, Go CC, Rader
AE, Conrardy RD, Gajda AM, Rubio-Perez C, Valyi-Nagy K, Pasquinelli
M, et al: Single-cell transcriptional changes associated with drug
tolerance and response to combination therapies in cancer. Nature
Commun. 12:16282021. View Article : Google Scholar
|
|
9
|
Stathias V, Jermakowicz AM, Maloof ME,
Forlin M, Walters W, Suter RK, Durante MA, Williams SL, Harbour JW,
Volmar CH, et al: Drug and disease signature integration identifies
synergistic combinations in glioblastoma. Nat Commun. 9:53152018.
View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Lin K, Li L, Dai Y, Wang H, Teng S, Bao X,
Lu ZJ and Wang D: A comprehensive evaluation of connectivity
methods for L1000 data. Brief Bioinform. 21:2194–2205. 2020.
View Article : Google Scholar
|
|
11
|
Zhang SD and Gant TW: A simple and robust
method for connecting small-molecule drugs using gene-expression
signatures. BMC Bioinformatics. 9:2582008. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Oeckinghaus A and Ghosh S: The NF-kappaB
family of transcription factors and its regulation. Cold Spring
Harb Perspect Biol. 1:a0000342009. View Article : Google Scholar
|
|
13
|
Yu H, Lin L, Zhang Z, Zhang H and Hu H:
Targeting NF-kappaB pathway for the therapy of diseases: Mechanism
and clinical study. Signal Transduct Target Ther. 5:2092020.
View Article : Google Scholar
|
|
14
|
Tian F, Fan T, Zhang Y, Jiang Y and Zhang
X: Curcumin potentiates the antitumor effects of 5-FU in treatment
of esophageal squamous carcinoma cells through downregulating the
activation of NF-κB signaling pathway in vitro and in vivo. Acta
Biochim Biophys Sin (Shanghai). 44:847–855. 2012. View Article : Google Scholar
|
|
15
|
Tao J, Zhao H, Xie X, Luo M, Gao Z, Sun H
and Huang Z: The anthelmintic drug flubendazole induces cell
apoptosis and inhibits NF-kappaB signaling in esophageal squamous
cell carcinoma. Onco Targets Ther. 12:471–478. 2019. View Article : Google Scholar :
|
|
16
|
Taniguchi K and Karin M: NF-κB,
inflammation, immunity and cancer: Coming of age. Nat Rev Immunol.
18:309–324. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Podolin PL, Callahan JF, Bolognese BJ, Li
YH, Carlson K, Davis TG, Mellor GW, Evans C and Roshak AK:
Attenuation of murine collagen-induced arthritis by a novel,
potent, selective small molecule inhibitor of IkappaB kinase 2,
TPCA-1
(2-[(aminocarbonyl)amino]-5-(4-fluorophenyl)-3-thiophenecarboxamide),
occurs via reduction of proinflammatory cytokines and
antigen-induced T cell proliferation. J Pharmacol Exp Ther.
312:373–381. 2005. View Article : Google Scholar
|
|
18
|
Ritchie ME, Phipson B, Wu D, Hu Y, Law CW,
Shi W and Smyth GK: Limma powers differential expression analyses
for RNA-sequencing and microarray studies. Nucleic Acids Res.
43:e472015. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Patro R, Duggal G, Love MI, Irizarry RA
and Kingsford C: Salmon provides fast and bias-aware quantification
of transcript expression. Nat Methods. 14:417–419. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Soneson C, Love MI and Robinson MD:
Differential analyses for RNA-seq: Transcript-level estimates
improve gene-level inferences. F1000Res. 4:15212015. View Article : Google Scholar
|
|
21
|
Love MI, Huber W and Anders S: Moderated
estimation of fold change and dispersion for RNA-seq data with
DESeq2. Genome Biol. 15:5502014. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Yu G, Wang LG, Han Y and He QY:
clusterProfiler: An R package for comparing biological themes among
gene clusters. OMICS. 16:284–287. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Barret Schloerke DC, Joseph Larmarange,
Briatte Francois, Marbach Moritz, Thoen Edwin, Amos Elberg and
Crowley Jason: GGally: Extension to 'ggplot2'. R package version
2.0.0. https://CRAN.R-project.org/package=GGally.
|
|
24
|
Duan Y, Evans DS, Miller RA, Schork NJ,
Cummings SR and Girke T: signatureSearch: Environment for gene
expression signature searching and functional interpretation.
Nucleic Acids Res. 48:e1242020. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Kolde R, Laur S, Adler P and Vilo J:
Robust rank aggregation for gene list integration and
meta-analysis. Bioinformatics. 28:573–580. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Spector AA: Arachidonic acid cytochrome
P450 epoxygenase pathway. J Lipid Res. 50(Suppl): S52–S56. 2009.
View Article : Google Scholar :
|
|
27
|
Khasawneh J, Schulz MD, Walch A, Rozman J,
de Angelis M, Klingenspor M, Buck A, Schwaiger M, Saur D, Schmid
RM, et al: Inflammation and mitochondrial fatty acid beta-oxidation
link obesity to early tumor promotion. Proc Natl Acad Sci USA.
106:3354–3359. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Keenan AB, Jenkins SL, Jagodnik KM, Koplev
S, He E, Torre D, Wang Z, Dohlman AB, Silverstein MC, Lachmann A,
et al: The library of integrated network-based cellular signatures
NIH program: System-level cataloging of human cells response to
perturbations. Cell Syst. 6:13–24. 2018. View Article : Google Scholar
|
|
29
|
Ghandi M, Huang FW, Jane-Valbuena J,
Kryukov GV, Lo CC, McDonald ER III, Barretina J, Gelfand ET,
Bielski CM, Li H, et al: Next-generation characterization of the
cancer cell line encyclopedia. Nature. 569:503–508. 2019.
View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Seth R, Messersmith H, Kaur V, Kirkwood
JM, Kudchadkar R, McQuade JL, Provenzano A, Swami U, Weber J,
Alluri KC, et al: Systemic therapy for melanoma: ASCO guideline. J
Clin Oncol. 38:3947–3970. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Argnani L, Broccoli A and Zinzani PL:
Cutaneous T-cell lymphomas: Focusing on novel agents in relapsed
and refractory disease. Cancer Treat Rev. 61:61–69. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Ciancio G, Burke GW, Gaynor JJ, Mattiazzi
A, Roth D, Kupin W, Nicolas M, Ruiz P, Rosen A and Miller J: A
randomized long-term trial of tacrolimus and sirolimus versus
tacrolimus and mycophenolate mofetil versus cyclosporine (NEORAL)
and sirolimus in renal transplantation. I. Drug interactions and
rejection at one year. Transplantation. 77:244–251. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Cheng L and Li L: Systematic quality
control analysis of LINCS data. CPT Pharmacometrics Syst Pharmacol.
5:588–598. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Bollag G, Hirth P, Tsai J, Zhang J,
Ibrahim PN, Cho H, Spevak W, Zhang C, Zhang Y, Habets G, et al:
Clinical efficacy of a RAF inhibitor needs broad target blockade in
BRAF-mutant melanoma. Nature. 467:596–599. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Markham A and Keam SJ: Selumetinib: First
approval. Drugs. 80:931–937. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Bensen W, Weaver A, Espinoza L, Zhao WW,
Riley W, Paperiello B and Recker DP: Efficacy and safety of
valdecoxib in treating the signs and symptoms of rheumatoid
arthritis: A randomized, controlled comparison with placebo and
naproxen. Rheumatology (Oxford). 41:1008–1016. 2002. View Article : Google Scholar
|
|
37
|
Liu Y, Wang X, Zeng S, Zhang X, Zhao J,
Zhang X, Chen X, Yang W, Yang Y, Dong Z, et al: The natural
polyphenol curcumin induces apoptosis by suppressing STAT3
signaling in esophageal squamous cell carcinoma. J Exp Clin Cancer
Res. 37:3032018. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Tung LN, Song S, Chan KT, Choi MY, Lam HY,
Chan CM, Chen Z, Wang HK, Leung HT, Law S, et al: Preclinical study
of novel curcumin analogue SSC-5 using orthotopic tumor xenograft
model for esophageal squamous cell carcinoma. Cancer Res Treat.
50:1362–1377. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Liu W, Xie L, He YH, Wu ZY, Liu LX, Bai
XF, Deng DX, Xu XE, Liao LD, Lin W, et al: Large-scale and
high-resolution mass spectrometry-based proteomics profiling
defines molecular subtypes of esophageal cancer for therapeutic
targeting. Nat Commun. 12:49612021. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Li B, Li YY, Tsao SW and Cheung AL:
Targeting NF-kappaB signaling pathway suppresses tumor growth,
angiogenesis, and metastasis of human esophageal cancer. Mol Cancer
Ther. 8:2635–2644. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Boehm JS and Golub TR: An ecosystem of
cancer cell line factories to support a cancer dependency map. Nat
Rev Genet. 16:373–374. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Papageorgis P, Cheng K, Ozturk S, Gong Y,
Lambert AW, Abdolmaleky HM, Zhou JR and Thiagalingam S: Smad4
inactivation promotes malignancy and drug resistance of colon
cancer. Cancer Res. 71:998–1008. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Chen YW, Hsiao PJ, Weng CC, Kuo KK, Kuo
TL, Wu DC, Hung WC and Cheng KH: SMAD4 loss triggers the phenotypic
changes of pancreatic ductal adenocarcinoma cells. BMC Cancer.
14:1812014. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Ozawa H, Ranaweera RS, Izumchenko E,
Makarev E, Zhavoronkov A, Fertig EJ, Howard JD, Markovic A, Bedi A,
Ravi R, et al: SMAD4 loss is associated with cetuximab resistance
and induction of MAPK/JNK activation in head and neck cancer cells.
Clin Cancer Res. 23:5162–5175. 2017. View Article : Google Scholar : PubMed/NCBI
|