Introduction
Chronic obstructive pulmonary disease (COPD) is one
of the major causes of death worldwide today, and its related
morbidity has been predicted to show an increase in the following
years (1). It is a preventable
and treatable disease which shows progressive and not fully
reversible airflow limitation, and it is associated with chronic
inflammatory responses and oxidative stress to poisonous particles
or gases in the airway and lung (2,3).
Tobacco smoking, including second-hand smoke exposure, is the main
risk factor for COPD, contributing to 90% of COPD-related deaths
(4). The other risk factor for
COPD include environmental conditions, such as dust and fumes.
Downregulating airway smooth-muscle tone using bronchodilators
and/or reducing pulmonary inflammation using inhaled
corticosteroids or phosphodiesterase type 4 inhibitors (e.g.,
roflumilast) is the major strategy for the treatment of COPD at
present (5). These drugs are
expensive and display adverse side effects, which limit their
clinical use (6,7). Previous studies indicate that
microRNAs (miRNAs) are involved in the pathogenetic and therapeutic
progression of COPD (8), which
provide a promising new therapeutic approach targeting at the
inflammatory response and/or oxidative stress to prevent and treat
patients with COPD.
Recent studies have shown that Danshen, a Chinese
herbal medicine and the dried root of Savia miltiorrhiza, is
a potential drug for the treatment of inflammation-related lung
diseases (9,10). Danshen has been widely used in
many countries including China, Japan and the US to treat various
diseases, including cardiovascular diseases, chronic liver
diseases, bronchitis and stroke (11). Tanshinone IIA (TIIA), the main
pharmacological component from Danshen, has been shown to exert
profound anti-inflammatory and anti-oxidative effects in lung and
cardiac diseases in animal studies (12-14). In addition, a recent study showed
that TIIA inhalation exerted protective effects against cigarette
smoke (CS) and lipopolysaccharide (LPS) exposure-induced COPD in
mice, attenuating lung function decline, airspace enlargement,
mucus production, bronchial collagen deposition, inflammatory
responses and oxidative stress through downregulation of cystic
fibrosis transmembrane conductance regulator (10). These advances suggest that TIIA
is a potential drug for the treatment of COPD with high efficiency
and low side effects. Sodium tanshinone IIA sulfonate (STS) is a
water-soluble derivative of TIIA. Similar to TIIA, STS has been
reported to have anti-oxidative and anti-inflammatory activities
(15), which makes it a
promising therapeutic agent for COPD. However, the underlying
mechanism of STS involved in the protective effects against COPD is
largely unknown.
Different from Western medicine, Chinese medicine
consistently shows multiple target and systemic effects through
which it exerts protective effects against diseases, suggesting
that the systemic effects of TIIA may contribute to its protective
effects against COPD. Exosomes, small (30-100 nm) endogenous
membrane vesicles secreted by most cell types, play important roles
in mediating cell-to-cell communication and crosstalk between
organs via shuttling proteins, mRNAs, and non-coding RNAs (such as
miRNAs) (16-18). Exosomes have emerged as novel
elements that mediate the therapeutic effects of various strategies
(19-21). The role of exosomes in the
mediation of the effects of Chinese medicine has also been examined
(22). A study by Ruan et
al demonstrated that Suxiao Jiuxin pill treatment significantly
increased exosome secretion (23). Maremanda et al
demonstrated that exosomes derived from mesenchymal stem cells
showed a protective effect to cigarette smoke-induced mitochondrial
dysfunction in mice (24). These
advances suggest that exosomes may play a role in the mediation of
the protective effects of TIIA against COPD. Here, we examined the
role of exosomes in the protective effects of TIIA in COPD mice,
and found that TIIA protects against COPD via exosome-shuttled
miR-486-5p.
Materials and methods
Induction of COPD in C57 mice and
treatment
A total of 132 wild-type C57 male mice (age 6-8
weeks and weight 20±3 g) were purchased from the Beijing Vital
River Laboratory Animal Technology Co., Ltd. and used in the
induction of COPD. All animals were housed in a specified
pathogen-free and temperature controlled (24±2°C temperature and
55% humidity) condition with 12 h-12 h light/dark cycles and free
access to food and water. Animal experiments were approved by the
Animal Care and Use Committee of Yan'an University (Yan'an,
Shaanxi, China) following ICUAC guidelines. COPD was induced as
reported previously (10).
Briefly, the COPD model was established using CS exposure plus
intranasal inhalation of lipopolysaccharide (LPS). LPS (7.5
µg/mouse in 50 µl saline; L8643, Sigma-Aldrich) or
saline (vehicle control) was administered to the mice by intranasal
inhalation on the 1st and 14th day. CS (9 cigarettes/h, 2
h/session, twice/day and 6 days/week in a whole-body exposure
chamber) was administered to the mice from day 0 to 60 except for
the days giving LPS. The cigarettes used were Plum brand filtered
cigarettes (Guangdong Tobacco Industry) and each cigarette yields
11 mg tar, 1.0 mg nicotine and 13 mg carbon monoxide.
For TIIA treatment, sodium tanshinone IIA sulfonate
(STS) (Jiangsu Carefree Pharmaceutical), a water-soluble substance
derived from TIIA, was used. Mice were administered saline or STS
(5 mg/kg, 30 min per session, twice per day) by airway inhalation
with a PARI Nebuliser (PARI GmbH) in a whole-body exposure chamber
for 30 min daily before being exposed to CS. Finally, all mice were
subjected to lung function analysis, and sacrificed at day 61.
For exosome treatment, exosomes purified from equal
volumes (1 ml) of plasma was intravenous injected into mice at an
interval of every 7 days from the 1st day of COPD induction. For
miR-486-5p intervention, agomir or antagomir of miR-486-5p (1 nmol)
was intravenously injected into mice at an interval of every 7 days
from the 1st day of COPD induction. For sacrifice, the mice were
euthanized using CO2 exposure with 3 liters/min (~45%
volume/min) flow rate, and CO2 exposure lasted for more
than 1 min after breathing was determined to be arrested.
Measurement of lung function
Lung function was evaluated using the Forced
Pulmonary Maneuver System (Buxco Research Systems) as described
previously (10). Mice were
anesthetized with pentobarbital (50 mg/kg body weight). The
breathing frequency was set at 150 breaths/min. Three semiautomatic
maneuvers were used. These included: Boyle's law functional
residual capacity maneuver to detect functional residual capacity,
quasi-static pressure volume maneuver to detect total lung capacity
and chord compliance, and fast flow volume maneuver to detect
forced expiration volume in 50 msec and forced vital capacity.
Hematocrit measurement
Capillary tubes (0.5-mm outside diameter, VWR
Scientific) was used to collect blood via right ventricle puncture
with K2EDTA as an anticoagulant. The collected blood was
centrifuged at 300 × g for 5 min, and read using a hematocrit chart
(VWR Scientific).
Histopathology
The left lung specimens of the mice were isolated
and fixed in 10% neutral buffered formalin for 24 h, embedded in
paraffin wax, and cut into 4-µm thick slices. The slices
were stained with hematoxylin and eosin (H&E; Nanjing Jiancheng
Bioengineering) to evaluate morphological changes and Masson
Trichrome (Nanjing Jiancheng Bioengineering) to detect collagen
deposition of small airways according to manufacturer's
instructions.
Plasma exosome isolation and
characterization
Blood samples were taken from mice with COPD. Plasma
exosomes were isolated using the ExoQuick Plasma prep and the
Exosome Precipitation kit according to the manufacturer's
instructions (System Biosciences). The isolated exosomes were
resuspended in PBS for further experiments. Transmission electron
microscopy was conducted as previously described (22), and Nanoparticle tracking analysis
(NTA) was carried out using a NanoSight NS300 (Marvel).
miRNA sequencing
miRNA sequencing was performed by Guangzhou RiboBio
Co., Ltd. Briefly, adaptors were added to the 30 and 50 end of
total RNAs extracted from plasma. Then, products of reverse
transcription polymerase chain reaction (RT-PCR) derived from 18-
to 30- nt RNAs were purified. The Illumina Hiseq 2500 platform
(Illumina, Inc.) was used for the sequencing.
Cell culture
Human bronchial epithelial 16HBE cells were
purchased from the Cell Bank of the Chinese Academy of Sciences
(Shanghai, China) and cultured with DMEM (Biological Industries
Israel Beit Haemek, Ltd.) supplemented with 10% FBS (Thermo Fisher
Scientific, Inc.), 100 µg/ml penicillin and 100 µg/ml
streptomycin (Beyotime Institute of Biotechnology) in a humidified
incubator at 37°C with 95% (v/v) air and 5% (v/v)
CO2.
Cigarette smoke extract (CSE) preparation
and exposure
CSE was freshly prepared from Plum brand filtered
cigarettes within 30 min prior to treatments as reported previously
(10). The acquired CSE
suspension in yellowish color with an optical density (OD) at 405
nm (0.506±0.008) was adjusted to pH 7.4, passed through a
0.22-µm filter to remove bacteria and particles and
considered as concentration 100% in cell treatments. The cells were
exposed to CSE (2%) for 12 h.
RT-qPCR
Total RNA was extracted from plasma by an RNA
isolation kit (AccuRef Scientific) according to the manufacturer's
instructions. Equal amounts of RNA were added to a reverse
transcriptase reaction mix (Takara), with oligo-dT as a primer. The
resulting templates were subjected to PCR using SYBR Green Master
Mix kit (AccuRef Scientific) in ABI 7500 Real-Time PCR system
(Applied Biosystems; Thermo Fisher Scientific, Inc.) with the
following conditions: 95°C for 10 min and 40 cycles of 95°C for 5
sec, 58°C for 20 sec, and 72°C for 10 sec. Using actin (mRNA
specific) or U6 (miRNA) as the internal control, the relative
expression of genes was calculated using the 2−ΔΔCq
method (25). Specific primers
used are shown in Table SI.
Cell transfection
miR-22-3p mimic (5′-AAG CUG CCA GUU GAA GAA CUG
U-3′), miR-486-5p mimic (5′-UCC UGU ACU GAG CUG CCC CGA G-3′),
miR-16-5p mimic (5′-UAG CAG CAC GUA AAU AUU GGC G-3′), miR-10b-5p
mimic (5′-UAC CCU GUA GAA CCG AAU UUG UG-3′), miR-27b-3p mimic
(5′-UUC ACA GUG GCU AAG UUC UGC-3′), miRNA negative control
(miR-NC; cat. #B04001), agomir-NC (cat. #B04008), agomiR-486-5p
(cat. #B06001), antagomiR-NC (cat. #B04007), and antagomiR-486-5p
(cat. #B05001) were synthesized by GenePharma Company (Shanghai,
China). miRNA mimic (100 nmol/l) or miR-NC (100 nmol/l) was
transfected using Lipofectamine™ 3000 (Invitrogen; Thermo Fisher
Scientific, Inc.) according to the manufacturer's protocol. After
48 h of transfection, cells were harvested and used for further
experiments.
Dual-luciferase reporter assay
Wild-type (Wt) and mutant (Mt) PIK3R1 3′ UTR
sequence was obtained by PCR amplification using template and
primers, and then cloned into SpeI and HindIII sites
of the pMir-Report Luciferase vector (Applied Biosystems; Thermo
Fisher Scientific, Inc.). The resulting construct was transfected
(5 ng) into macrophages with 20 nM control mimics or 20 nM mimics
for miR-486-5p using Lipofectamine 2000 (Invitrogen; Thermo Fisher
Scientific, Inc.) according to the manufacturer's instructions.
After 24 h of transfection, luciferase activity in the cells was
determined using a Luciferase Assay System (Promega Corp.).
Apoptosis analysis
After transfected with miRNA mimic/NC, PIK3R1,
and/or treatment with CSE, the apoptotic cells were measured using
the Annexin V-FITC/PI apoptosis Detection kit (Beyotime Institute
of Biotechnology) according to the manufacturer's protocol.
Briefly, adherent and suspension cultured 16HBE cells were
harvested and centrifuged at 1,000 × g at 4°C for 5 min followed by
cold PBS rinse for three times. Then, cells were re-suspended in
200 µl of suspension buffer and incubated with 5 µl
of Annexin V-FITC and 5 µl of PI at room temperature for 20
min in the dark. Following this, suspension buffer was added to 1
ml and analyzed using flow cytometry (Beckman Coulter).
CCK-8 assay
After transfected with miRNA mimic/NC or PIK3R1 for
48 h, the cells were seeded into 96-well plates at a density of
1.0×104 per well and cultured for 24 h. Then, the cells
were treated with CSE or/and STS for 48 h. After incubation, 10
µl of CCK-8 solution (Beyotime Institute of Biotechnology)
was added to each well and incubated at 37°C for 2 h. Then, the
optical density (OD) value of each well was detected at 450 nm
using a microplate reader (BioTek).
Western blot analysis
Protein expression was measured using western blot
analysis. Briefly, cells and tissues were lysed on ice for 30 min
using RIPA lysis buffer and centrifuged at 4°C and 12,000 × g for
10 min, and proteins (the supernatants) were quantified using the
BCA method (AccuRef Scientific). Then, 25 µg protein for
each sample was diluted in loading buffer (EXINNO) and subjected to
12% SDS-PAGE followed by electronic transferring to PVDF membrane.
Subsequently, the membranes were blocked with 5% skimmed milk
solution at room temperature for 30 min and probed with anti-CD63
(dilution: 1:200; cat. no. SAB4301607; Sigma-Adrich; Merck KGaA),
anti-CD81 (dilution: 1:1,000; cat. no.ab109201; Abcam),
anti-phosphoinositide-3-kinase regulatory subunit 1 (PIK3R1,
dilution: 1:1,000; cat. no. ab191606; Abcam) or anti-β-actin
(dilution: 1:1,000; cat. no. ab8226; Abcam) overnight at 4°C
followed by incubation with the corresponding anti-mouse secondary
antibody (dilution: 1:5,000; cat. no. ab6728; Abcam) or anti-rabbit
secondary antibody (dilution: 1:5,000; cat. no. ab6721; Abcam) at
room temperature for 1 h. The blots were visualized with ECL-Plus
Reagent (AccuRef Scientific). Finally, the protein bands on
membranes were quantified using ImageJ software (version 1.49, NIH)
for further statistical analysis.
Statistical analysis
All values are presented as mean ± standard
deviation. Data between two groups were compared using an unpaired
t-test. Data among multiple groups were compared using one-way
analysis of variance (ANOVA) followed by Turkey's post hoc with
Bonferroni's correction to reduce the dominance of type-I error.
Differences were considered significant at P<0.05.
Results
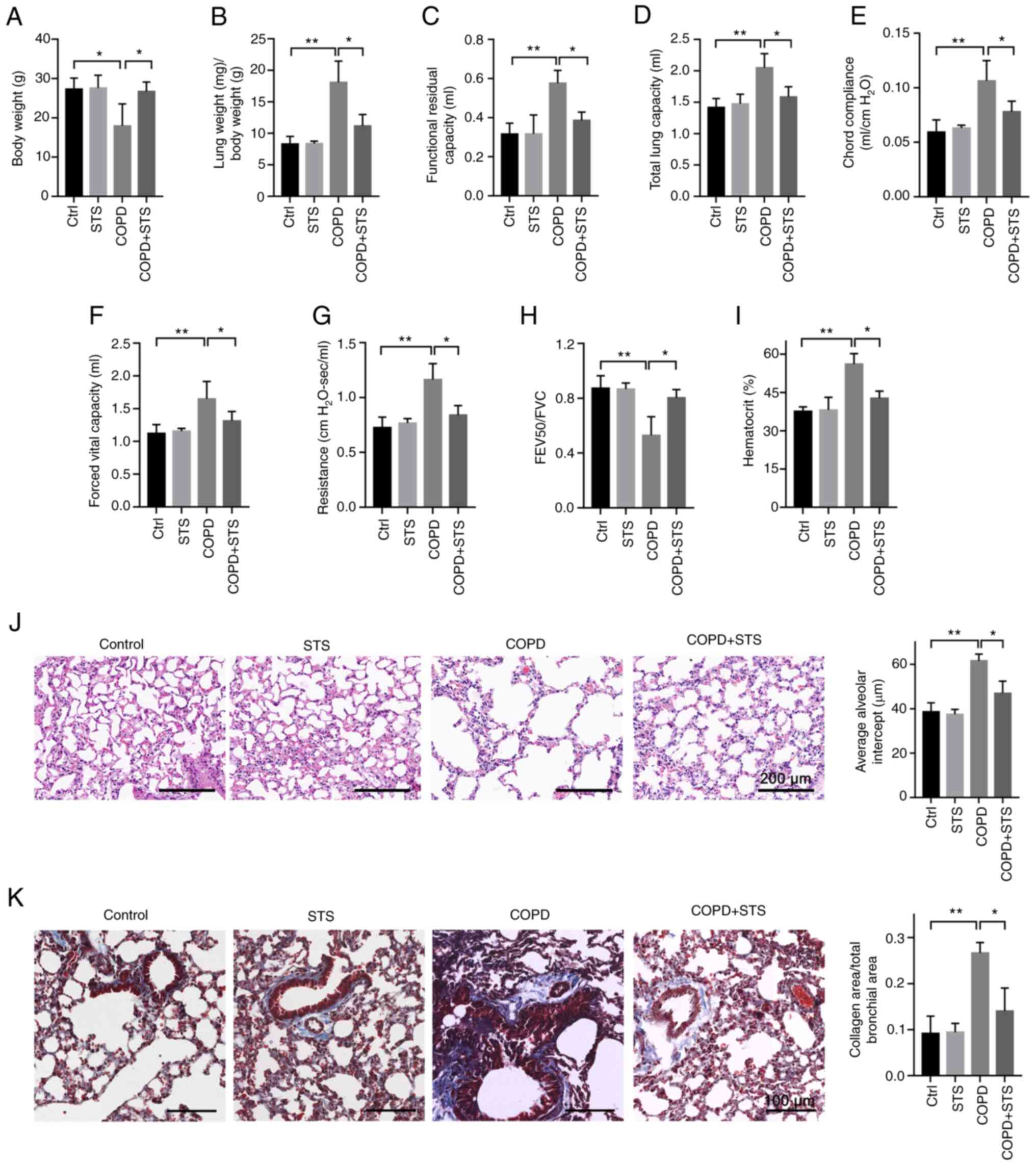
STS inhalation protects against COPD
After 60 days of induction, COPD mice displayed
typical clinical manifestations, including decreased body weight
(Fig. 1A), increased lung weight
(Fig. 1B), impaired lung
function (Fig. 1C-H) and
increased hematocrit value in blood (Fig. 1I). An impairment of lung function
was evidenced by increases in functional residual capacity
(Fig. 1C), total lung capacity
(Fig. 1D), chord compliance
(Fig. 1E), forced vital capacity
(Fig. 1F) and lung resistance
(Fig. 1G), a decrease in forced
expiratory volume at 50 msec (Fig.
1H), and an increased hematocrit value in blood (Fig. 1I). All of these manifestations
were attenuated in mice with STS inhalation when compared to the
vehicle-treated mice (Ctrl) (Fig,
1A-I). In addition, the protective effects of STS were further
manifested by a decrease in pulmonary structural damage as detected
by H&E staining (Fig. 1J),
as well as a decrease in collagen deposition in the small airway as
detected by Masson staining (Fig.
1K). The lung of COPD mice displayed damaged alveolar walls,
pulmonary bullae and increased collagen deposition which were
attenuated in STS-treated mice (Fig.
1J and K). These results demonstrated that STS inhalation
exerted protective effects against CS-LPS exposure-induced
COPD.
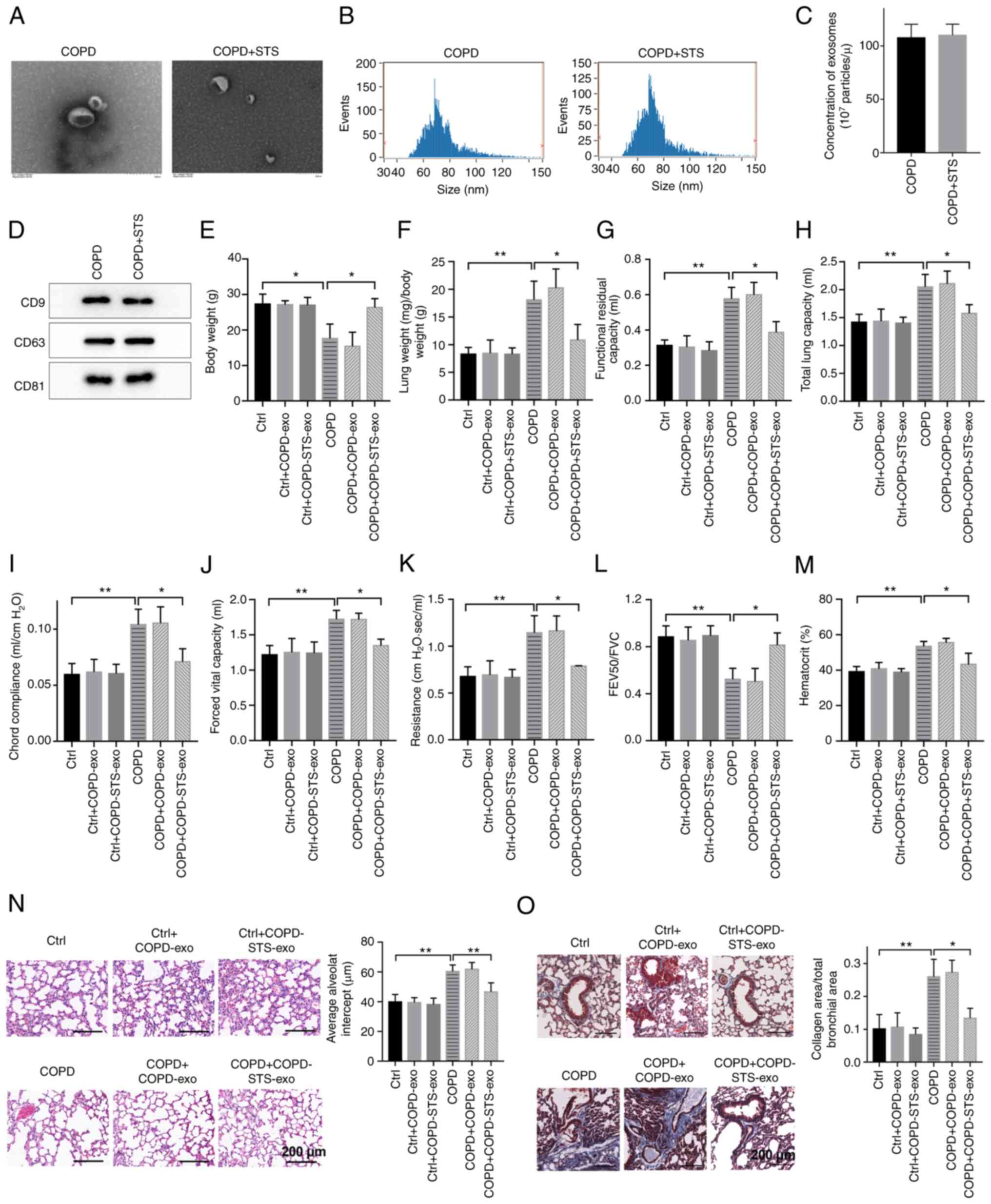
Exosomes derived from STS-treated COPD
mice exert protective effects against COPD
To test whether exosomes contribute to the
protective effects of STS, exosomes were purified from the plasma
of COPD mice treated with or without STS. Electron microscopy
revealed typical rounded particles (50-100 nm in diameter) in
isolated fractions (Fig. 2A).
Nanoparticle tracking showed no differences in size distribution
and plasma concentration between exosomes derived from untreated
COPD and STS-treated COPD mice (Fig.
2B and C), and the results were further confirmed by western
blot analysis (Fig. 2D). Then,
purified exosomes were administrated to COPD mice to test whether
these exosomes exert protective effects against COPD.
Interestingly, exosomes purified from STS-treated COPD mice exerted
protective effects against COPD. Exosome treatment increased body
weight (Fig. 2E), improved lung
function (Fig. 2F-L) and
decreased hematocrit value in blood (Fig. 2M) in mice with COPD, while
exosomes treatment showed little effects in the control (Ctrl) mice
(Fig. 2F-M). In addition, the
protective effects of exosomes were further manifested by a
decrease in pulmonary structural damage as detected by H&E
staining (Fig. 2N), as well as a
decrease in collagen deposition in the small airway as detected by
Masson staining (Fig. 2O).
Particularly, exosomes purified from STS-treated COPD mice showed
higher protective effects than that from the untreated COPD mice
(Fig. 2E-O), suggesting that
there were endogenous protective factors in the exosomes of COPD
mice and STS treatment enhanced their protective effects.
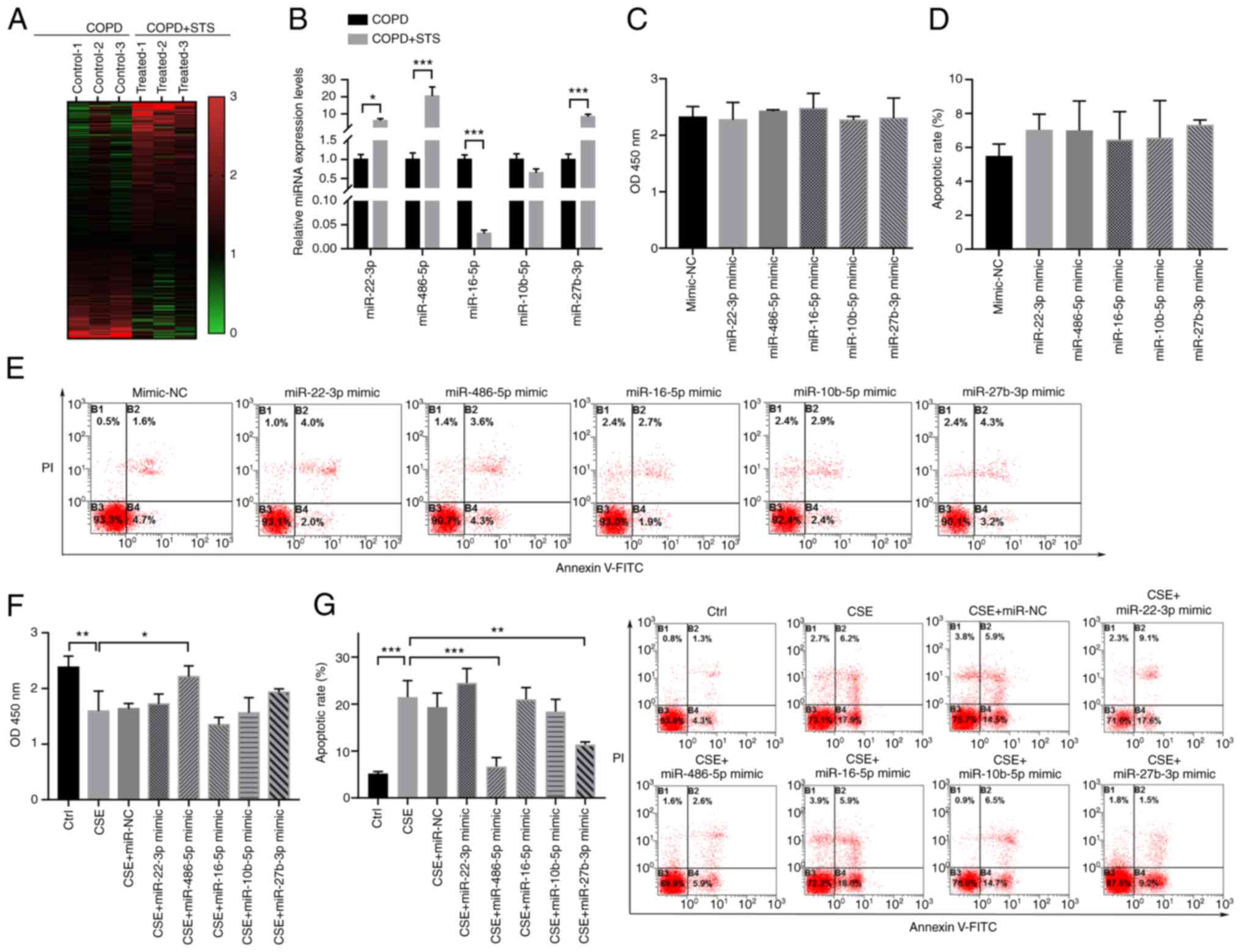
Exosome-shuttled miR-486-5p protects lung
cells in vitro
To explore the possibility of miRNA(s) contributing
to exosome-mediated protective effects against COPD, a miRNA
profiling assay comparing the differences between exosomes purified
from untreated COPD and STS-treated COPD mice was conducted using
Illumina HiSeq 2500 high-throughput sequencing. A total of 417
differentially expressed miRNAs (fold change >1.5; P<0.05;
Fig. 3A) were detected, and the
top 5 were further confirmed by RT-qPCR (Fig. 3B). Among these miRNAs, miR-22-3p,
miR-486-5p, and miR-27b-3p were upregulated in the exosomes
purified from the STS-treated COPD mice compared with those in the
untreated COPD mice, while miR-16-5p was significantly
downregulated in the exosome derived from the STS treated COPD mice
compared with the COPD mice (Fig.
3B). We further tested these 5 miRNAs in 16HBE cells. The
results demonstrated that overexpression of these five miRNAs had
no obvious effect on the cell viability and apoptosis of the 16HBE
cells (Fig. 3C-E). However,
overexpression of miR-486-5p significantly increase the cell
viability and decreased apoptosis of the 16HBE cells after exposure
to CSE (Fig. 3F and G). To
validate the interaction between exosome and lung epithelial cells,
16HBE cells were treated with exosomes purified from COPD mice
(COPD-exo) or STS-treated COPD mice (COPD+STS-exo). We found
significantly increased viability in the COPD+STS-exo group when
compared with the viability in the exosomes derived from COPD mice
(COPD-exo) group (Fig. S1).
These results suggest that exosome-shuttled miR-486-5p may mediate
the protective effects of STS against COPD.
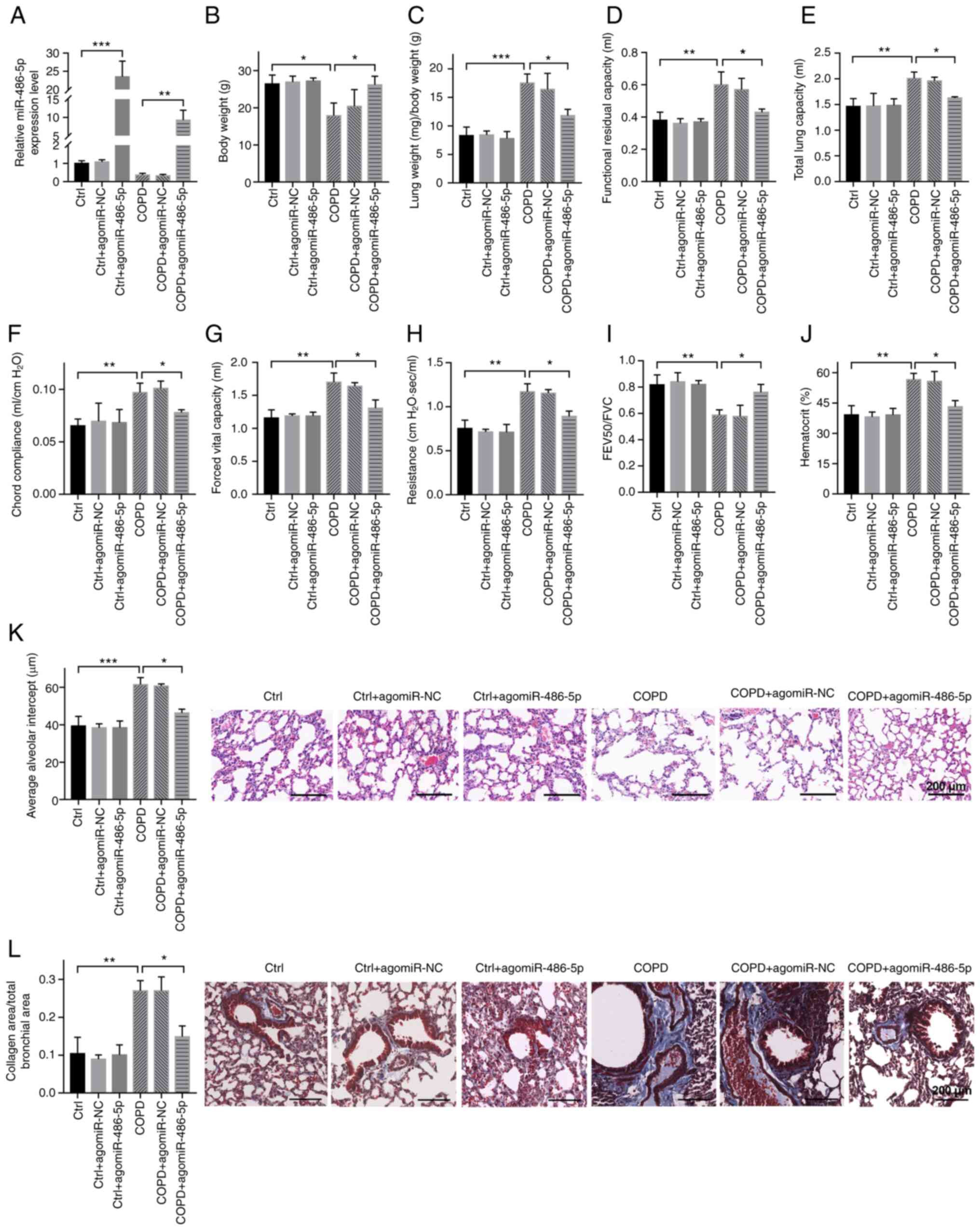
miR-486-5p protects against COPD
Agomir of miR-486-5p was used by intravenous
injection to upregulate the level of miR-486-5p in COPD mice to
test whether miR-486-5p protects against COPD. As shown in Fig. 4A, miR-486-5p was upregulated in
both the control (Ctrl) and COPD mice, suggesting that the
injection of miR-486-5p agomir was successfully performed (Fig. 4B-I). Further analyses showed that
agomiR-486-5p treatment increased body weight (Fig. 4B), improved lung function
(Fig. 4C-I) and decreased the
hematocrit value in blood (Fig.
4J) in COPD mice compared with that in untreated COPD mice,
while agomiR-486-5p treatment showed little effects in the control
mice (Fig. 4B-J). In addition,
the protective effects of agomiR-486-5p were further confirmed by a
decrease in pulmonary structural damage as detected by H&E
staining (Fig. 4K), as well as a
decrease in collagen deposition in the small airway as detected by
Masson staining (Fig. 4L). These
results demonstrated that miR-486-5p protects against COPD in
mice.
STS protects against COPD through
upregulation of miR-486-5p
Antagomir of miR-486-5p was used by intra-venous
injection to downregulate the level of miR-486-5p in STS-treated
COPD mice to test whether miR-486-5p contributes to the protective
effects of STS against COPD. As shown in Fig. 5A, miR-486-5p was downregulated in
STS-treated COPD mice by antagomiR-486-5p treatment, and
downregulation of miR-486-5p in STS-treated COPD mice attenuated
the protective effects of STS against COPD (Fig. 5B-I). AntagomiR-486-5p treatment
decreased body weight in the STS-treated COPD mice, while it showed
little effects on body weight in COPD mice without STS treatment
(Fig. 4B). AntagomiR-486-5p
treatment decreased lung function (Fig. 5C-I) and increased the hematocrit
value in blood (Fig. 5J) in the
STS-treated COPD mice. In addition, the effects of antagomiR-486-5p
were further confirmed by an increase in pulmonary structural
damage as detected by H&E staining (Fig. 5K), as well as an increase in
collagen deposition in the small airway as detected by Masson
staining (Fig. 5L). These
results demonstrated that STS protects against COPD through
upregulation of miR-486-5p.
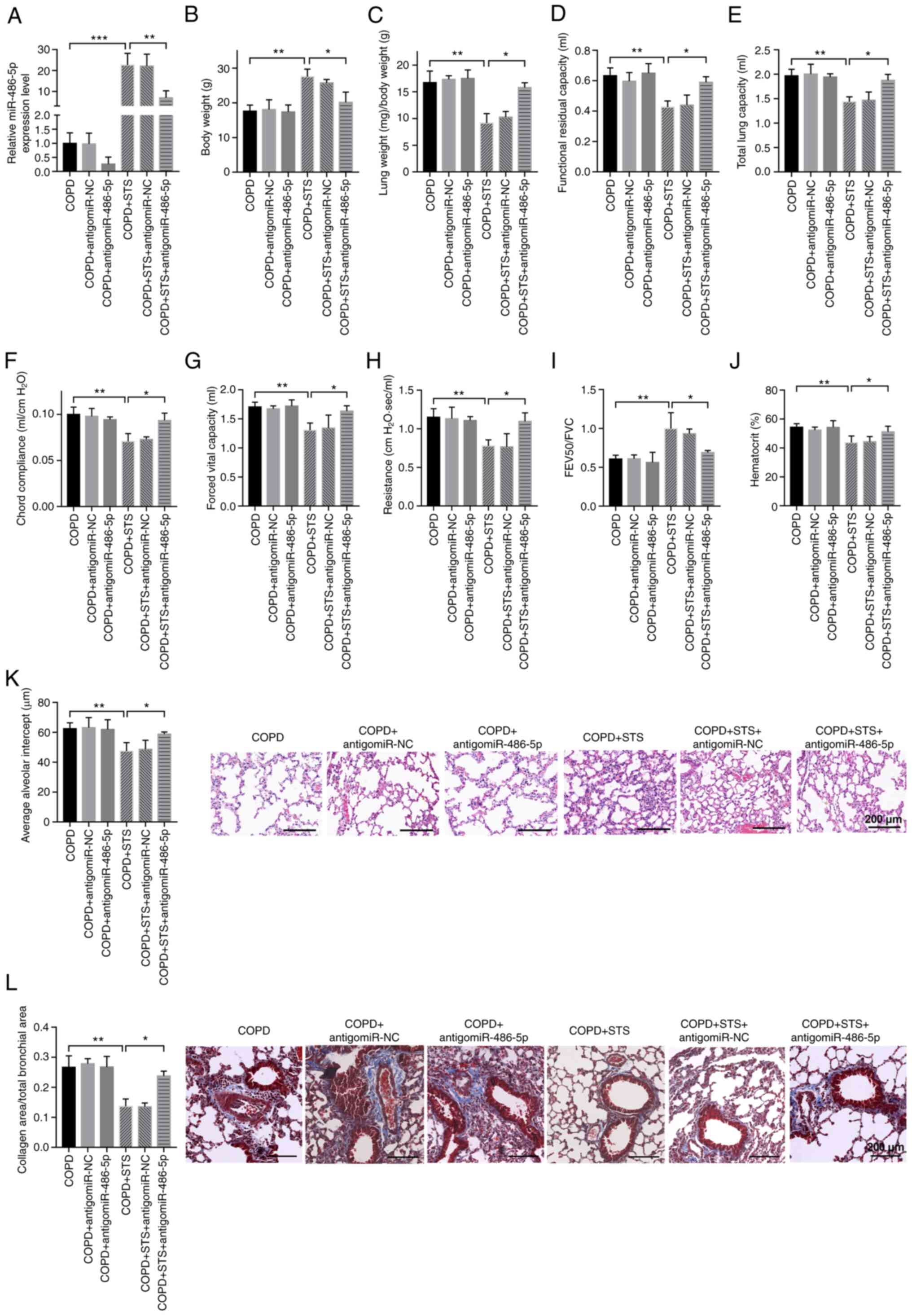 | Figure 5STS protects against COPD through
upregulation of miR-486-5p. (A) miR-486-5p was downregulated in
STS-treated COPD mice by antagomiR-486-5p treatment. (B-J)
Downregulation of miR-486-5p in STS-treated COPD mice attenuated
the protective effects of STS against COPD. Body weight (B), lung
weight (C), functional residual capacity (D), total lung capacity
(E), chord compliance (F), forced vital capacity (G), lung
resistance (H), forced expiratory volume at 50 msec (I), and
hematocrit value in blood (J) are shown. (K) AntagomiR-486-5p
increased pulmonary structural damage as detected by H&E
staining in STS-treated COPD mice. (L) AntagomiR-486-5p increased
collagen deposition in the small airway as detected by Masson
staining in STS-treated COPD mice. n=6. *P<0.05;
**P<0.01 ***P<0.001. COPD, chronic
obstructive pulmonary disease; STS, sodium tanshinone IIA
sulfonate. |
miR-486-5p exerts protective effects
against COPD via targeting PIK3R1
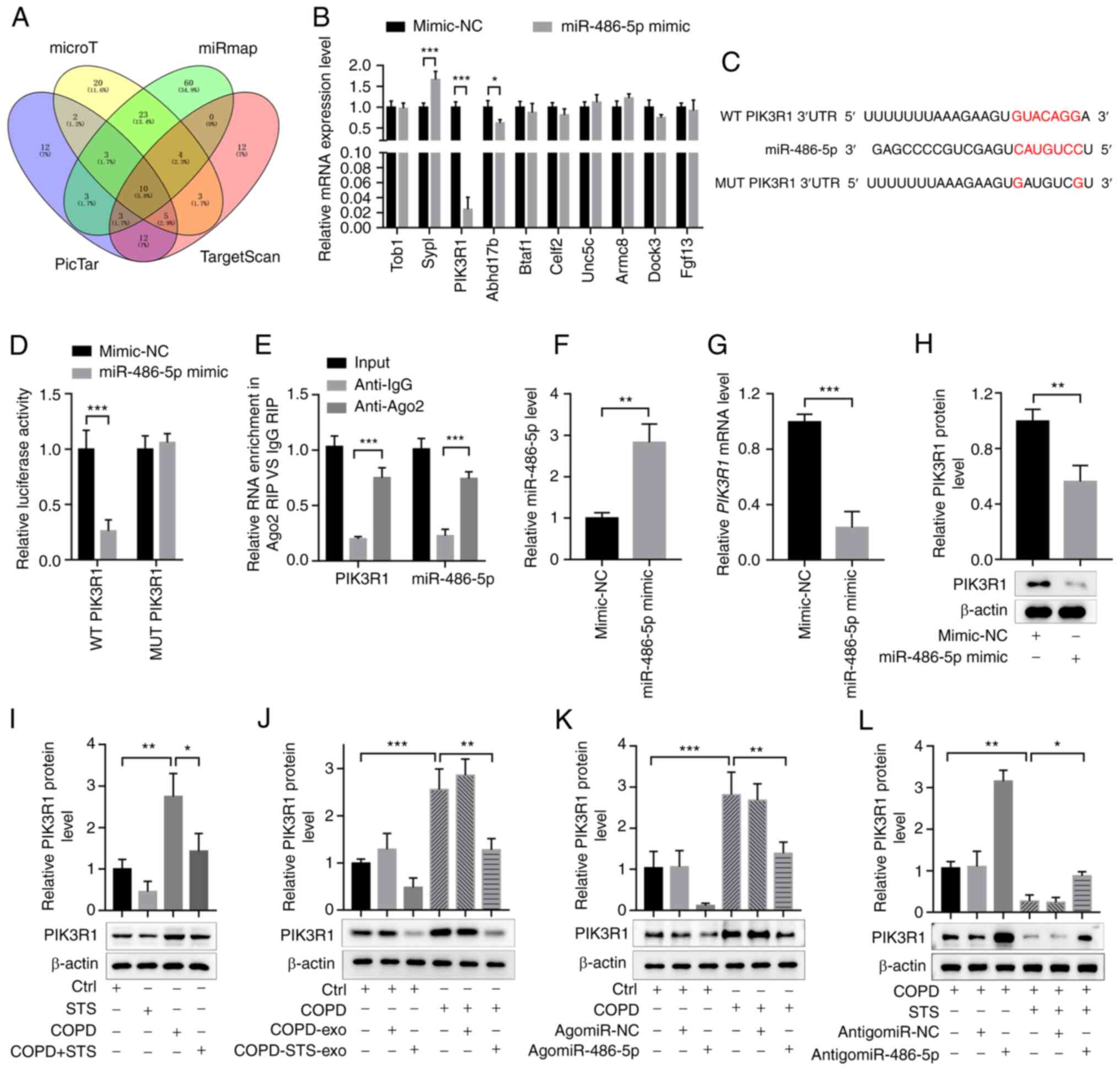
Target genes of miR-486-5p were predicted by miRmap
(http://mirnamap.mbc.nctu.edu.tw/),
microT (http://diana.imis.athena-innovation.gr/DianaTools/index.php?r=microT_CDS/index),
TargetScan (http://www.targetscan.org/mamm_31/) and PicTar
(https://pictar.mdc-berlin.de/). There
were 10 candidates (Fig. 6A).
The miR-486-5p mimic decreased PIK3R1 mRNA level more
significantly in 16HBE cells (Fig.
6B). PIK3R1 is a subunit of phosphatidylinositol 3-kinase
(PI3K) and regulates PI3K activity. The binding sites for
miR-486-5p in the 3′-untranslated regions (3′UTRs) of PIK3R1
were further examined using a luciferase reporter assay. Either
wild-type (WT) 3′UTRs or mutant (MUT) 3′UTRs in putative miR-486-5p
binding sites were cloned into a reporter plasmid and assessed
their responsiveness to miR-486-5p in 293T cells. The results
showed that miR-486-5p reduced luciferase activity for PIK3R1
wild-type 3′UTR constructs but had no effect when the miR-486-5p
binding sites were mutated (Fig. 6C
and D). RIP assay showed that PIK3R1 could directly interact
with miR-486-5p (Fig. 6E).
Following this, expression of PIK3R1 was determined in 16HBE cells
after transfection with miR-486-5p (Fig. 6F). The results showed that
overexpression of miR-486-5p could significantly inhibit
PIK3R1 mRNA expression (Fig.
6G). Protein levels in 16HBE cells detected by western blot
analysis showed the same trends consistent with the results of the
mRNA levels (Fig. 6H),
indicating PIK3R1 as a potential target of miR-486-5p. We also
tested the expression levels of PIK3R1 in mice. PIK3R1 expression
was upregulated in the COPD mice, and STS treatment decreased
PIK3R1 expression in the COPD mice (Fig. 6I). Exosomes purified from
untreated and STS-treated COPD mice also decreased PIK3R1
expression in the COPD mice (Fig.
6J). AgomiR-486-5p decreased PIK3R1 expressions and
antagomir-486-5p increased PIK3R1 expressions in the COPD mice
(Fig. 6K and L). These results
demonstrated that miR-486-5p exerts protective effects against COPD
via targeting PIK3R1.
Overexpression of PIK3R1 attenuates the
protective effect of STS-induced miR-486-5p in CSE-exposed 16HBE
cells
To further explore the role of PIK3R1 in COPD,
PIK3R1 was overexpressed in 16HBE cells and exposed to CSE followed
by cell viability and apoptosis analyses. The result demonstrated
that PIK3R1 was significantly upregulated in the 16HBE cells and
CSE exposure could further enhance the expression of PIK3R1 in
16HBE cells (Fig. 7A). Cell
viability analysis showed that overexpression of PIK3R1 could
significantly inhibit the viability of 16HBE cells and CSE exposure
markedly enhanced this reduction in the cell viability of 16HBE
cells (Fig. 7B). Western blot
analysis demonstrated that overexpression of PIK3R1 significantly
enhanced the expression of cleaved-PARP, cleaved-caspase-3, and
cleaved-caspase-9, but had no obvious effect on the expression of
pro-PARP, pro-caspase-3, and pro-caspase-9 in 16HBE cells exposed
to CSE (Fig. 7C). In addition,
further flow cytometric analysis showed that PIK3R1 expression
significantly increased the apoptosis of 16HBE cells and CSE
exposure could significantly aggregate the apoptosis of the
PIK3R1-overexpressed 16HBE cells (Fig. 7D). These findings demonstrated
that overexpression of PIK3R1 enhanced the CSE-induced injury in
16HBE cells.
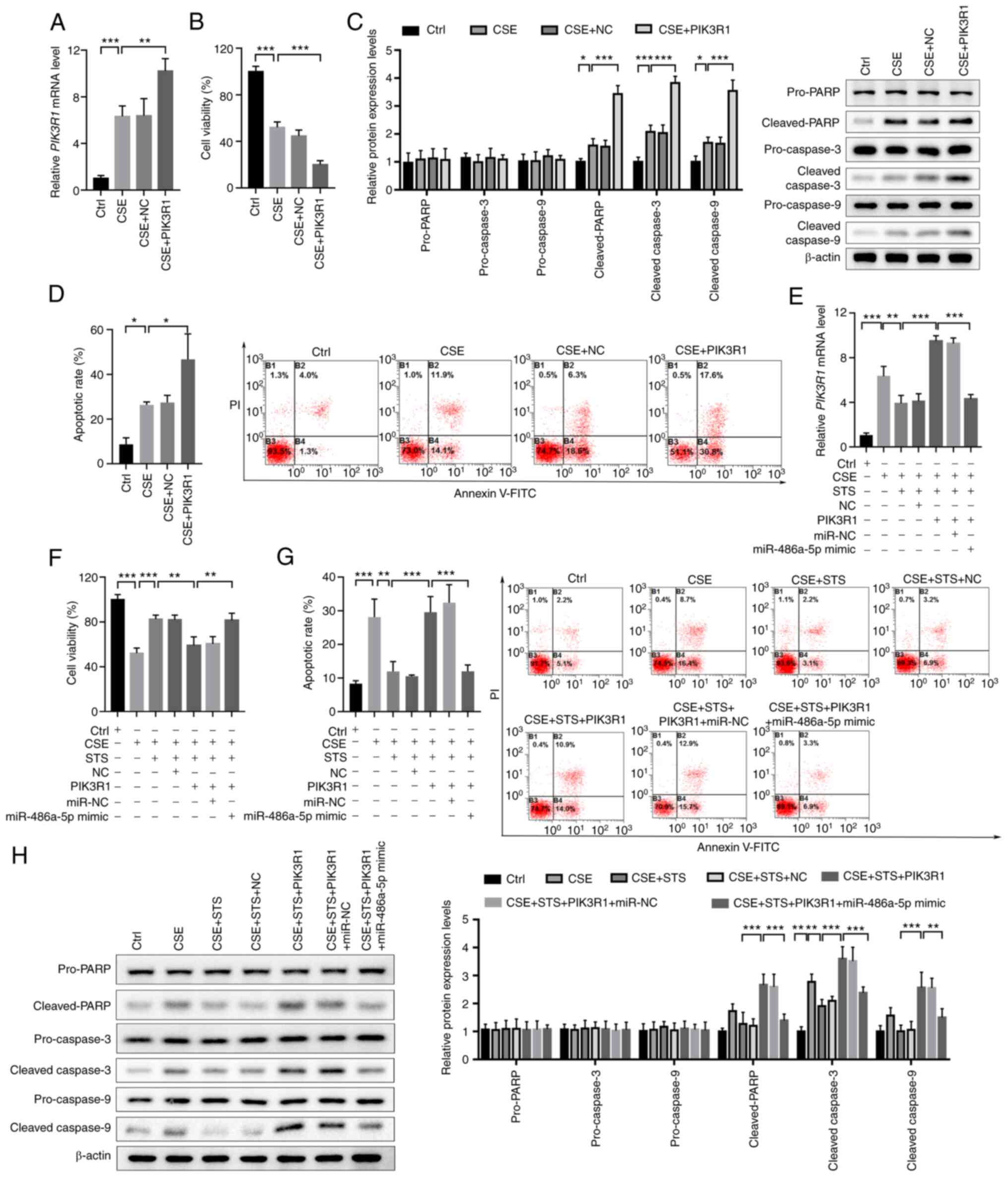 | Figure 7STS protects 16HBE cells against
CSE-induced injury via the miR-486-5p/PIK3R1 axis. (A) Confirmation
of PIK3R1 overexpression in CSE-exposed 16HBE cells. (B)
Cell viability of CSE-exposed 16HBE cells with or without
PIK3R1 overexpression. (C) Expression of apoptotic markers
in CSE-exposed 16HBE cells with or without PIK3R1
overexpression. (D) Apoptosis of CSE-exposed 16HBE cells with or
without PIK3R1 overexpression. (E) Expression of
PIK3R1 in 16HBE cells after exposure to CSE, treatment with
STS, PIK3R1 overexpression, or miR-486-5p overexpression. (F and G)
Cell viability (F) and apoptosis (G) of 16HBE cells after exposure
to CSE, treatment with STS, PIK3R1 overexpression, or
miR-486-5p overexpression. (H) Expression of apoptotic markers in
16HBE cells after exposure to CSE, treatment with STS,
PIK3R1 overexpression, or miR-486-5p overexpression.
*P<0.05, **P<0.01 and
***P<0.001. COPD, chronic obstructive pulmonary
disease; PIK3R1, phosphoinositide-3-kinase regulatory subunit 1;
STS, sodium tanshinone IIA sulfonate; CSE, cigarette smoke
extract. |
To reveal whether STS could alleviate COPD injury
via the miR-486-5p/PIK3R1 axis, miR-486-5p and PIK3R1 were
co-transfected into CSE-exposed 16HBE cells and treated with STS.
The result presented that STS treatment could obviously abort
PIK3R1 upregulation induced by CSE and overexpression of PIK3R1
could reverse the effect of STS, but overexpression of miR-486-5p
remarkably decreased PIK3R1 expression in the PIK3R1-overexpressing
16HBE cells after exposure to CSE and treatment with STS (Fig. 7E). Cell viability analysis showed
that STS treatment could significantly promote the viability of
CSE-exposed 16HBE cells and PIK3R1 could attenuate the effect of
STS on the viability of 16HBE cells, while overexpression of
miR-486-5p could abort PIK3R1 effect on the viability of CSE and
STS co-treated 16HBE cells (Fig.
7F). Flow cytometry also demonstrated that STS could decrease
the apoptosis of 16HBE cells exposed to CSE and overexpression of
PIK3R1 could attenuate the protective effect of STS; however,
miR-486-5p reversed the effect of PIK3R1 and rescued the protective
effect of STS (Fig. 7G). In
addition, western blot analysis presented that STS treatment
significantly decreased the upregulation of cleaved-PARP,
cleaved-caspase-3, and cleaved-caspase-9 induced by CSE, but had no
marked effect on the expression of pro-PARP, pro-caspase-3, and
pro-caspase-9 in the 16HBE cells. However, PIK3R1 overexpression
could obviously attenuate the protective effect of STS and
miR-485-5p could attenuate the effect of PIK3R1 to rescue the
protective effect of STS (Fig.
7H). All of these data demonstrated that STS alleviated the
CSE-induced injury in 16HBE cells via regulating the
miR-485-5p/PIK3R1 axis.
Discussion
Danshen and its derivative products including its
water soluble form, sodium tanshinone IIA sulfonate (STS), have
been applied to treat cardiovascular and cerebrovascular diseases
in the clinic. Recent research has shown that STS exerted
protective effects against COPD in rodents (10). The present study demonstrated
that STS inhalation attenuated lung dysfunction in mice with COPD,
and its protective effects were mediated by the elevation of
circulating miR-486-5p via targeting PIK3R1. The results suggest
that STS is a potential drug for the clinical treatment of
COPD.
STS has been described as an antioxidant to reduce
oxidative stress and inflammatory responses, which scavenges
oxygen-free radicals, prevents lipid peroxidation, inhibits low
density lipoprotein oxidation, increases Zn superoxide dismutase
(SOD) activity as well as mRNA and protein expression, activates
the Nrf2 pathway and inhibits NF-κB and MAPK signaling pathways
(26-28). In the present study, we found
that STS treatment significantly reduced lung injury responses to
CS exposure. These findings are consistent with a previous study
(10). A broad spectrum of
anti-inflammatory drugs, including inhibitors of the
pro-inflammatory enzymes PDE4, Janus kinases, NF-κB kinase, p38
mitogen-activated protein kinase, and PI3 kinase-γ and -δ, have
been developed for COPD treatment, but their side effects limit
their clinical application (29). Unlike significant advances in the
development of long-acting bronchodilators, it has proven difficult
to find safe and effective anti-inflammatory treatments for COPD.
As a medicine used in traditional Chinese medicine, the side
effects and safety of Danshen and Danshen products have been fully
evaluated in clinical practice (11). Indeed, we found that the dose of
STS used in the study to treat COPD did not cause a detectable
toxic effect. Based on our findings, STS is a potential drug for
preventing COPD, which could be conveniently delivered by aerosol
inhalation.
It has been shown that STS protects COPD through
downregulation of cystic fibrosis transmembrane conductance
regulator in local lungs (10).
Our study extended the findings that STS protects against COPD
through upregulation of miR-486-5p which is derived from
circulation, suggesting that various factors both from local and
circulation are involved in the protective effects of STS against
COPD. miRNAs are endogenous noncoding small RNAs (~22 nt) that
modulate the activity of mRNA by hybridizing to complementary
sequences in the 3′-untranslated region (UTR) of specific targets
(30). Numerous studies have
demonstrated that miRNAs participate in various cell biological
processes, including cell growth, differentiation, and cell
apoptosis and various interventions exert beneficial effects
through regulation of miRNAs (31,32). Thus, it is possible for STS to
exert beneficial effects through regulation of miRNAs. Indeed,
there are reports which show the contribution of miRNAs in the
beneficial effects of Chinese medicine (33-35). Here, our miRNA sequencing data
revealed that miR-22-3p, miR-486-5p, and miR-27b-3p were
upregulated, while miR-16-5p was downregulated in the exosomes
derived from STS-treated COPD mice. Among the significantly altered
miRNAs, miR-22-3p and miR-486-5p were reported to have
anti-inflammatory effects (36-38), while miR-27b-3p and miR-16-5p are
involved in the initiation of inflammation (39,40). In the present study, we found
that STS exerted beneficial effects through upregulation of
miR-486-5p. Our ongoing research will be to investigate other
exosome-derived miRNAs found in this study, under which the
potential mechanisms would provide further understanding of the
beneficial effect of STS for preventing COPD.
miR-486-5p has been extensively studied in tumors,
including lung cancer. Various studies have shown that miR-486-5p
is downregulated in lung cancers (41-44). Upregulation of miR-486-5p reduces
tumor proliferation and migration. PIK3R1 is a well-established
target of miR-486-5p in various pathological conditions. PIK3R1 is
a subunit of PI3K which plays an important role in the regulation
of a vast array of fundamental cellular processes, including
proliferation, adhesion, cell size, and protection from apoptosis
(45,46). Although PI3K signaling exerts
protective effects in various diseases, recent studies demonstrated
that PI3K signaling is prominently activated in COPD and correlates
with an increased susceptibility of patients to lung infections
(47,48). PI3K isoforms have emerged as
promising alternative drug targets for respiratory diseases,
including COPD, and a wide array of pan-isoform and
isoform-selective inhibitors have been tested in preclinical models
and are currently being evaluated in clinical studies (47). Consistently with previous
studies, our results showed that PI3K is overactivated in COPD as
evidenced by the increased expression of PIK3R1. Although the role
of PIK3R1 has not been explored in COPD, its role in lung cancer
has been extensively studied. PIK3R1 is the 11th most
commonly mutated gene across cancer lineages in the TCGA database
(49). Various studies have
shown that miR-486-5p is downregulated and PIK3R1 is upregulated in
lung cancers (41-44). Upregulation of miR-486-5p or
PIK3R1 abrogation reduces tumor proliferation and migration. These
advances reinforce the notion that the miR-486-5p/PIK3R1 axis plays
an important role in the regulation of lung function.
Taken together, these results demonstrated that STS
inhalation effectively attenuated CS-induced lung dysfunction in
COPD, and STS-elevated miR-486-5p contributes to its protective
effects against COPD via targeting PIK3R1. These findings extend
the current knowledge that STS exerts protective effects through
systemic alterations. Thus, STS is a potential drug for the
treatment of COPD.
Supplementary Data
Availability of data and materials
The datasets used and/or analyzed during the present
study are available from the corresponding author upon reasonable
request.
Authors' contributions
DT and CZ conceived and designed the study. DT, YM,
WH, NY, PW and QG performed the experiments and statistical
analyses. DT and CZ wrote the paper. YM, WH, NY, and PW reviewed
and edited the manuscript. All authors read and approved the
manuscript and agree to be accountable for all aspects of the
research in ensuring that the accuracy or integrity of any part of
the work, including the data, are appropriately investigated and
resolved.
Ethics approval and consent to
participate
Animal experiments were approved by the Animal Care
and Use Committee of Yan'an University (Yan'an, Shaanxi, China)
following ICUAC guidelines.
Patient consent for publication
Not applicable.
Competing interests
All the authors declare that they have no competing
interests.
Acknowledgments
Not applicable.
Funding
This study was supported by the National Natural Science
Foundation of China (no. 81860014).
References
|
1
|
Lozano R, Naghavi M, Foreman K, Lim S,
Shibuya K, Aboyans V, Abraham J, Adair T, Aggarwal R, Ahn SY, et
al: Global and regional mortality from 235 causes of death for 20
age groups in 1990 and 2010: A systematic analysis for the global
burden of disease study 2010. Lancet. 380:2095–2128. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Miravitlles M, Calle M and Soler-Cataluña
JJ: Clinical phenotypes of COPD: Identification, definition and
implications for guidelines. Arch Bronconeumol. 48:86–98. 2012.In
English, Spanish. View Article : Google Scholar
|
|
3
|
Vestbo J: COPD: Definition and phenotypes.
Clin Chest Med. 35:1–6. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Salvi S: Tobacco smoking and environmental
risk factors for chronic obstructive pulmonary disease. Clin Chest
Med. 35:17–27. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Calverley PM: New treatments for COPD:
Many miles still to go. Lancet Respir Med. 2:6–7. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Tamimi A, Serdarevic D and Hanania NA: The
effects of cigarette smoke on airway inflammation in asthma and
COPD: Therapeutic implications. Respir Med. 106:319–328. 2012.
View Article : Google Scholar
|
|
7
|
Page CP and Spina D: Selective PDE
inhibitors as novel treatments for respiratory diseases. Curr Opin
Pharmacol. 12:275–286. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
De Smet EG, Mestdagh P, Vandesompele J,
Brusselle GG and Bracke KR: Non-coding RNAs in the pathogenesis of
COPD. Thorax. 70:782–791. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Li J, Zheng Y, Li MX, Yang CW and Liu YF:
Tanshinone IIA alleviates lipopolysaccharide-induced acute lung
injury by downregulating TRPM7 and pro-inflammatory factors. J Cell
Mol Med. 22:646–654. 2018. View Article : Google Scholar
|
|
10
|
Li D, Wang J, Sun D, Gong X, Jiang H, Shu
J, Wang Z, Long Z, Chen Y, Zhang Z, et al: Tanshinone IIA sulfonate
protects against cigarette smoke-induced COPD and down-regulation
of CFTR in mice. Sci Rep. 8:3762018. View Article : Google Scholar
|
|
11
|
Zhou L, Zuo Z and Chow MS: Danshen: An
overview of its chemistry, pharmacology, pharmacokinetics, and
clinical use. J Clin Pharmacol. 45:1345–1359. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Long R, You Y, Li W, Jin N, Huang S, Li T,
Liu K and Wang Z: Sodium tanshinone IIA sulfonate ameliorates
experimental coronary no-reflow phenomenon through down-regulation
of FGL2. Life Sci. 142:8–18. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Han JY, Fan JY, Horie Y, Miura S, Cui DH,
Ishii H, Hibi T, Tsuneki H and Kimura I: Ameliorating effects of
compounds derived from Salvia miltiorrhiza root extract on
microcirculatory disturbance and target organ injury by ischemia
and reperfusion. Pharmacol Ther. 117:280–295. 2008. View Article : Google Scholar
|
|
14
|
Xu M, Cao F, Liu L, Zhang B, Wang Y, Dong
H, Cui Y, Dong M, Xu D, Liu Y, et al: Tanshinone IIA-induced
attenuation of lung injury in endotoxemic mice is associated with
reduction of hypoxia-inducible factor 1alpha expression. Am J
Respir Cell Mol Biol. 45:1028–1035. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Cheng J, Chen T, Li P, Wen J, Pang N,
Zhang L and Wang L: Sodium tanshinone IIA sulfonate prevents
lipopolysaccharide-induced inflammation via suppressing nuclear
factor-kappaB signaling pathway in human umbilical vein endothelial
cells. Can J Physiol Pharmacol. 96:26–31. 2018. View Article : Google Scholar
|
|
16
|
Pascual M, Ibanez F and Guerri C: Exosomes
as mediators of neuron-glia communication in neuroinflammation.
Neural Regen Res. 15:796–801. 2020. View Article : Google Scholar :
|
|
17
|
Rong S, Wang L, Peng Z, Liao Y, Li D, Yang
X, Nuessler AK, Liu L, Bao W and Yang W: The mechanisms and
treatments for sarcopenia: Could exosomes be a perspective research
strategy in the future? J Cachexia Sarcopenia Muscle. 11:348–365.
2020. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Li X, Li C, Zhang L, Wu M, Cao K, Jiang F,
Chen D, Li N and Li W: The significance of exosomes in the
development and treatment of hepatocellular carcinoma. Mol Cancer.
19:12020. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Ibrahim A and Marbán E: Exosomes:
Fundamental biology and roles in cardiovascular physiology. Ann Rev
Physiol. 78:67–83. 2016. View Article : Google Scholar
|
|
20
|
Yao X, Wei W, Wang X, Chenglin L,
Björklund M and Ouyang H: Stem cell derived exosomes: microRNA
therapy for age-related musculoskeletal disorders. Biomaterials.
224:1194922019. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Cheng N, Du D, Wang X, Liu D, Xu W, Luo Y
and Lin Y: Recent advances in biosensors for detecting
cancer-derived exosomes. Trends Biotechnol. 37:1236–1254. 2019.
View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Ruan XF, Li YJ, Ju CW, Shen Y, Lei W, Chen
C, Li Y, Yu H, Liu YT, Kim IM, et al: Exosomes from Suxiao Jiuxin
pill-treated cardiac mesenchymal stem cells decrease H3K27
demethylase UTX expression in mouse cardiomyocytes in vitro. Acta
Pharmacol Sin. 39:579–586. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Ruan XF, Ju CW, Shen Y, Liu YT, Kim IM, Yu
H, Weintraub N, Wang XL and Tang Y: Suxiao Jiuxin pill promotes
exosome secretion from mouse cardiac mesenchymal stem cells in
vitro. Acta Pharmacol Sin. 39:569–578. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Maremanda KP, Sundar IK and Rahman I:
Protective role of mesenchymal stem cells and mesenchymal stem
cell-derived exosomes in cigarette smoke-induced mitochondrial
dysfunction in mice. Toxicol Appl Pharmacol. 385:1147882019.
View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expressiion data using real-time quantitative PCR and
the 2(-Delta Delta C(T)) method. Methods. 25:402–408. 2001.
View Article : Google Scholar
|
|
26
|
Niu XL, Ichimori K, Yang X, Hirota Y,
Hoshiai K, Li M and Nakazawa H: Tanshinone II-A inhibits low
density lipoprotein oxidation in vitro. Free Radic Res. 33:305–312.
2000. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Tang F, Wu X, Wang T, Wang P, Li R, Zhang
H, Gao J, Chen S, Bao L, Huang H and Liu P: Tanshinone II A
attenuates atherosclerotic calcification in rat model by inhibition
of oxidative stress. Vascul Pharmacol. 46:427–438. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Yin X, Yin Y, Cao FL, Chen YF, Peng Y, Hou
WG, Sun SK and Luo ZJ: Tanshinone IIA attenuates the inflammatory
response and apoptosis after traumatic injury of the spinal cord in
adult rats. PLoS One. 7:e383812012. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Barnes PJ: Development of new drugs for
COPD. Curr Med Chem. 20:1531–1540. 2013. View Article : Google Scholar
|
|
30
|
Bartel DP: MicroRNAs: Genomics,
biogenesis, mechanism, and function. Cell. 116:281–297. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Ambros V: MicroRNA pathways in flies and
worms: Growth, death, fat, stress, and timing. Cell. 113:673–676.
2003. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Kloosterman WP and Plasterk RH: The
diverse functions of microRNAs in animal development and disease.
Dev Cell. 11:441–450. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Hu H, Zhu X and Lin X: GuaLou GuiZhi
decoction represses LPS-induced BV2 activation via miR-155 induced
inflammatory signals. Pak J Pharm Sci. 33:403–408. 2020.PubMed/NCBI
|
|
34
|
Lin F, Chen HW, Zhao GA, Li Y, He XH,
Liang WQ, Shi ZL, Sun SY, Tian PP, Huang MY and Liu C: Advances in
research on the circRNA-miRNA-mRNA network in coronary heart
disease treated with traditional Chinese medicine. Evid Based
Complement Alternat Med. 17:80486912020.
|
|
35
|
Tang C, Zhao R, Ni H, Zhao K, He Y, Fang S
and Chen Q: Molecule mechanisms of Ganoderma lucidum treated
hepatocellular carcinoma based on the transcriptional profiles and
miRNA-target network. Biomed Pharmacother. 125:1100282020.
View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Wang X, Chi J, Dong B, Xu L, Zhou Y, Huang
Y, Sun S, Wei F, Liu Y, Liu C, et al: miR-223-3p and miR-22-3p
inhibit monosodium urate-induced gouty inflammation by targeting
NLRP3. Int J Rheum Dis. 24:599–607. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Hu Z, Lv X, Chen L, Gu X, Qian H,
Fransisca S, Zhang Z, Liu Q and Xie P: Protective effects of
microRNA-22-3p against retinal pigment epithelial inflammatory
damage by targeting NLRP3 inflammasome. J Cell Physiol.
234:18849–18857. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Chai X, Si H, Song J, Chong Y, Wang J and
Zhao G: miR-486-5p inhibits inflammatory response, matrix
degradation and apoptosis of nucleus pulposus cells through
directly targeting FOXO1 in inter-vertebral disc degeneration. Cell
Physiol Biochem. 52:109–118. 2019. View Article : Google Scholar
|
|
39
|
Yamada K, Takizawa S, Ohgaku Y, Asami T,
Furuya K, Yamamoto K, Takahashi F, Hamajima C, Inaba C, Endo K, et
al: MicroRNA 16-5p is upregulated in calorie-restricted mice and
modulates inflammatory cytokines of macrophages. Gene.
725:1441912020. View Article : Google Scholar
|
|
40
|
Ruan C, Cong RJ, Wang M, Wang L, Yu Y, Li
X and Lv H: miR-27b-3p targeting BDNF inhibits TrkB/CREB signaling
pathway and improves IL-1 β induced chondrocytic inflammation.
Preprints: 2021020602. 2021.10.20944/preprints202102.0602.v1.
|
|
41
|
Tian F, Wang J, Ouyang T, Lu N, Lu J, Shen
Y, Bai Y, Xie X and Ge Q: miR-486-5p serves as a good biomarker in
nonsmall cell lung cancer and suppresses cell growth with the
involvement of a target PIK3R1. Front Genet. 10:6882019. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Yang S, Sui J, Liu T, Wu W, Xu S, Yin L,
Pu Y, Zhang X, Zhang Y, Shen B and Liang G: Expression of
miR-486-5p and its significance in lung squamous cell carcinoma. J
Cell Biochem. 120:13912–13923. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Zhang Y, Fu J, Zhang Z and Qin H:
miR-486-5p regulates the migration and invasion of colorectal
cancer cells through targeting PIK3R1. Oncol Lett. 15:7243–7248.
2018.PubMed/NCBI
|
|
44
|
Huang XP, Hou J, Shen XY, Huang CY, Zhang
XH, Xie YA and Luo XL: MicroRNA-486-5p which is downregulated in
hepatocellular carcinoma, suppresses tumor growth by targeting
PIK3R1. FEBS J. 282:579–594. 2015. View Article : Google Scholar
|
|
45
|
Mirza-Aghazadeh-Attari M, Ekrami EM,
Aghdas SAM, Mihanfar A, Hallaj S, Yousefi B, Safa A and Majidinia
M: Targeting PI3K/Akt/mTOR signaling pathway by polyphenols:
Implication for cancer therapy. Life Sci. 255:1174812020.
View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Sun K, Luo J, Guo J, Yao X, Jing X and Guo
F: The PI3K/AKT/mTOR signaling pathway in osteoarthritis: A
narrative review. Osteoarthritis Cartilage. 28:400–409. 2020.
View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Pirozzi F, Ren K, Murabito A and Ghigo A:
PI3K signaling in chronic obstructive pulmonary disease:
Mechanisms, targets, and therapy. Curr Med Chem. 26:2791–2800.
2019. View Article : Google Scholar
|
|
48
|
Marwick JA, Chung KF and Adcock IM:
Phosphatidylinositol 3-kinase isoforms as targets in respiratory
disease. Ther Adv Respir Dis. 4:19–34. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
49
|
Cerami E, Gao J, Dogrusoz U, Gross BE,
Sumer SO, Aksoy BA, Jacobsen A, Byrne CJ, Heuer ML, Larsson E, et
al: The cBio cancer genomics portal: an open platform for exploring
multidimensional cancer genomics data. Cancer Discov. 2:401–404.
2012. View Article : Google Scholar : PubMed/NCBI
|