Introduction
Sarcopenia has been well-established to have a
harmful clinical impact on malnutrition in patients with
advanced-stage chronic liver disease, and has been implicated in
the poor quality of life and negative prognostic outcomes of
patients with cirrhosis (1-3).
Although sarcopenia varies in prevalence due to different
definitions and diagnostic methods, it has been estimated to affect
up to 70% of patients with cirrhosis (4,5).
Liver cirrhosis-based skeletal muscle wasting invokes a
multifactorial pathogenesis, including malnutrition;
hyperammonemia; changes in the levels of hormones, including
insulin growth factor-1 (IGF-1); chronic inflammation; increased
resting energy expenditure; and decreased physical activity
(1,2,6-10). Notably, the tripartite
inter-organ crosstalk between the gut, liver and skeletal muscle
(i.e., the gut-liver-muscle axis) has recently been suggested to
contribute to the development of sarcopenia in patients with
cirrhosis (11). Therefore, the
identification of therapeutic targets for sarcopenia in patients
with cirrhosis has often been laborious.
L-carnitine or β-hydroxy-g-N-trimethyl aminobutyric
acid is an endogenous molecule involved in the β-oxidation of fatty
acids and is biosynthesized from the amino acids, L-lysine and
L-methionine, within the brain, kidneys and liver (12). L-carnitine is known to play a key
role in cellular energy metabolism through the mitochondrial
transport of long-chain fatty acids (13). Clinically, the supplementation of
L-carnitine has been suggested to forestall skeletal muscle loss
through its anti-inflammatory and antioxidant effects (14,15). Moreover, recent studies have
demonstrated that L-carnitine has the potential to prevent the
progression of sarcopenia, by attenuating hyperammonemia in
patients with cirrhosis (16,17). Although monotherapy with
L-carnitine supplementation appears to ameliorate the impairment of
muscle mass and function, this is partial and insufficient.
Therefore, combining L-carnitine with another agent may prove to be
more practical for treating sarcopenia accompanied by liver
cirrhosis.
Rifaximin, which is a minimally-absorbed antibiotic
with a broad-spectrum activity against aerobic and anaerobic
Gram-positive and -negative bacteria, has been clinically used to
attenuate hyperammonemia in patients with cirrhosis and hepatic
encephalopathy (HE) (18-20).
Notably, a recent study that used a model of preclinical sarcopenia
in cirrhosis with portosystemic shunts revealed that the
rifaximin-mediated decrease in ammonia levels had the potential to
reverse skeletal muscle loss (21). Moreover, previous clinical and
basic studies by the authors have demonstrated that rifaximin can
improve gut hyperpermeability and prevent hepatic exposure to
endogenous lipopolysaccharide (LPS), which plays a detrimental role
in skeletal muscle homeostasis (22,23). However, the effects of rifaximin
and L-carnitine on skeletal muscle atrophy, as well as the
underlying mechanisms that are particularly associated with the
modulation of the gut-liver-muscle axis, remain obscure.
The present study thus aimed to investigate the
combined effects of rifaximin and L-carnitine supplementation on
skeletal muscle atrophy and explored the therapeutic mechanisms
associated with the gut-liver-muscle axis in a rodent model of
cirrhosis.
Materials and methods
Animals and treatment
Fischer 344 rats (6 weeks old, male; body weight,
150±20 g; CLEA Japan) were randomly divided into five groups and
treated for 12 weeks as follows (Fig. 1A; n=10 in each group): i) With a
choline-sufficient amino acid-defined (CSAA) diet (Research Diets,
Inc.) with lactose hydrate (FUJIFILM, Wako Pure Chemical
Corporation) as the vehicle; ii) with a choline-deficient l-amino
acid-defined (CDAA) diet (Research Diets Inc.) with the vehicle;
iii) with the CDAA diet with rifaximin (ASKA Pharmaceutical Co.
Ltd.; 100 mg/kg); iv) with the CDAA diet and L-carnitine (Otsuka
Pharmaceutical Co. Ltd.; 200 mg/kg); and v) with the CDAA diet with
rifaximin and L-carnitine, as previously described (23,24). All drugs were administered by
intragastric gavage once a day. The rats were housed in plastic
cages (2 rats/cage) in a pathogen-free room and were provided with
free access to their diet and drinking water, and were kept under
controlled, stable ambient conditions (23±3°C/12-h light/dark cycle
with 50±20% humidity). At the end of the experiment, all rats
underwent the following procedures: Euthanasia by an
intraperitoneal injection of pentobarbital sodium (200 mg/kg), the
opening of the abdominal cavity, blood collection via puncture of
the aorta and harvesting of the liver, ileum and gastrocnemius
muscle for histological and molecular evaluation. The anesthetized
rats were then decapitated for assuring death. The present study
was approved by the Animal Ethics Committee of Nara Medical
University (approval no. 12764), and all protocols were performed
in accordance with the National Institutes of Health Guidelines for
the Care and Use of Laboratory Animals.
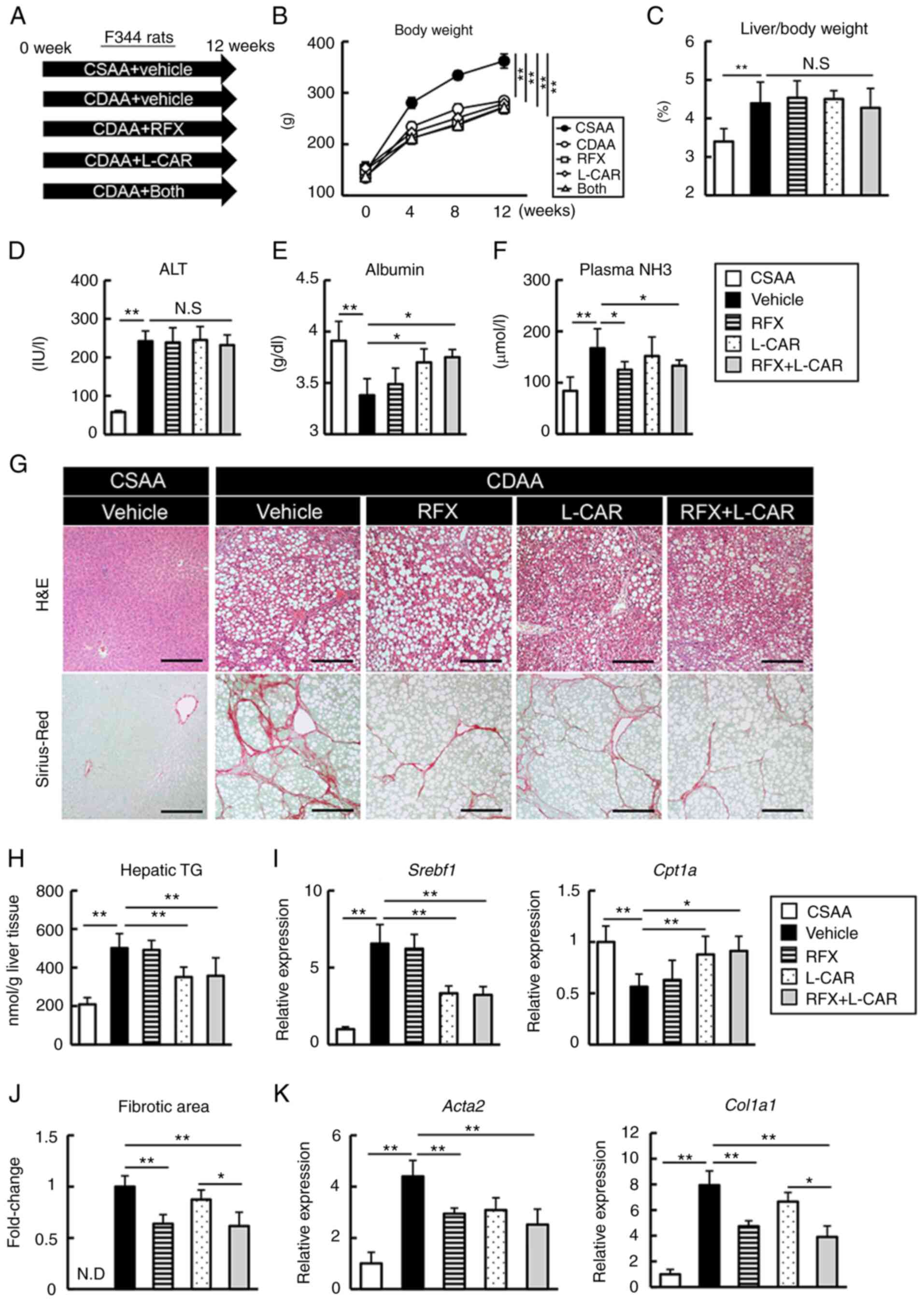 | Figure 1Effects of rifaximin and L-carnitine
on hepatic phenotypes in CDAA-fed rats. (A) Experimental protocols.
(B) Changes in the body weight of the rats during the experimental
period. The comparison was performed between the groups at the last
time point. (C) Ratio of liver weight to body weight at the end of
the experiment. (D-F) Blood levels of ALT, albumin and NH3. (G)
Representative microphotographs of liver sections stained with
H&E and Sirius Red in the experimental groups. Scale bar, 50
μm. (H) Hepatic concentrations of TG. (I) Relative mRNA
expression levels of Srebf1 and Cpt1a in the liver of
experimental rats. (J) Semi-quantification of Sirius Red-stained
fibrotic area in high-power field using ImageJ software. (K)
Relative mRNA expression levels of Acta2 and Col1a1
in the livers of experimental rats. The mRNA expression levels were
measured using reverse transcription-quantitative PCR, and
Gapdh was used as an internal control. Histochemical
quantitative analyses included five fields per section. (I-K)
Quantitative values are indicated as fold changes relative to the
values of CSAA group. Data are the mean ± SD (n=10),
*P<0.05 and **P<0.01, significant
difference between groups. N.S, not significant; N.D, not detected;
CDAA, choline-deficient L-amino acid-defined diet; CSAA,
choline-sufficient amino acid-defined diet; ALT, alanine
aminotransferase; NH3, ammonia; H&E, hematoxylin and eosin; TG,
triglycerides; RFX, rifaximin; L-CAR, L-carnitine; Srebf1,
sterol regulatory element binding transcription factor 1;
Cpt1a, carnitine palmitoyltransferase 1A; Acta2,
actin alpha 2, smooth muscle; Col1a1, collagen type i alpha
1 chain. |
Rat myoblast culture
L6 rat skeletal muscle myoblasts (cat. no. JCRB9081,
Japanese Collection of Research Bioresources Cell Bank) were
cultured and differentiated into myotubes as previously described
(25,26). Briefly, the cells were cultured
in Dulbecco's modified Eagle's medium (DMEM, Nacalai Tesque, Inc.)
supplemented with 10% fetal bovine serum (Gibco; Thermo Fisher
Scientific, Inc.) and antibiotics (1% penicillin and streptomycin)
at 37°C in a 5% CO2 air environment. To induce
differentiation into myocytes, the cells were further cultured in
DMEM containing 2% horse serum for 8 days. The cells were
supplemented with fresh medium every 48 h and were used at the
stage of myotubes (60-70%) (25,27,28). Myogenic differentiation into
myotubes was confirmed by using a AE2000-1080M microscope (Shimadzu
Corporation) based on the morphological alignment, elongation and
fusion (data not shown). Mycoplasma testing was performed using a
MycoProbe® Mycoplasma Detection kit (R&D Systems,
Inc.) according to the manufacturer's protocol. The differentiated
myotubes were stimulated with LPS (O55:B5; MilliporeSigma) or tumor
necrosis factor-α (TNF-α) at various concentrations and treated
with LPS (1.0 μg/ml) or TNF-α (20 ng/ml) and L-carnitine (5
mM) and/or rifaximin (10 μM) for 48 h.
Measurement of psoas muscle mass index
(PMI) and forelimb grip strength
According to the clinical criteria for sarcopenia
assessment, the PMI (cross-sectional area/height2) was
assessed on a single computed tomography (CT) slice (image) at the
level of the L3 pedicle using Slice-O-Matic (Tomovision) (29). All rats underwent an abdominal CT
scan before and at 4, 8, and 12 weeks after the start of CDAA
feeding and/or rifaximin/L-carnitine treatment using CosmoScan FX
(Rigaku Corporation) as previously described (26). The forelimb grip strength of the
experimental rats was simultaneously measured using a grip strength
meter (MK-380Si; Muromachi Kikai, Co., Ltd.) as previously
described (26). During the grip
strength test, the rats were allowed to use their front paws to
grab a horizontal bar mounted on the gauge, and the tail was slowly
pulled back. The peak tension was recorded at the time the mouse
released the grip on the bar. Measurements were repeated three
times, and the mean of three measurements was recorded.
Serum alanine aminotransferase (ALT) and
albumin measurement in rats
The serum ALT and albumin concentrations in the rats
were measured using a Rat Alanine Aminotransferase ELISA kit (cat.
no. ab285264, Abcam) and Rat Albumin ELISA kit (cat. no. ab108789,
Abcam), respectively. All samples were processed and assayed
according to the manufacturer's protocol.
Serum and hepatic IGF-1 measurement in
rats
The serum IGF-1 concentrations in the rats were
measured using a Mouse/Rat IGF-I/IGF-1 Quantikine ELISA kit (cat.
no. MG100, R&D Systems, Inc.). All samples were processed and
assayed according to the manufacturer's protocol.
Plasma and muscle ammonia
measurement
The concentrations of ammonia in plasma and muscle
(100 mg of rat gastrocnemius muscle tissue homogenate) were
measured using the Ammonia Assay kit (cat. no. ab83360, Abcam),
according to the manufacturer's protocol.
Hepatic triglyceride (TG)
concentration
The intrahepatic TG concentrations in 100 mg frozen
liver tissue per mouse were measured using the Triglyceride-Glo™
Assay (Promega Corporation), according to the manufacturer's
instructions.
Rat muscle myostatin measurements
The levels of myostatin in 100 mg rat gastrocnemius
muscle tissue homogenate were measured using a myostatin ELISA kit
(cat. no. DGDF80, R&D Systems, Inc.) following the
manufacturer's instructions.
Measurement of in vivo intestinal
permeability
A 4-kDa fluorescein isothiocyanate (FITC)-dextran
(MilliporeSigma) solution was used for the intestinal permeability
measurement, as previously described (30). Another set of rats (n=5) in each
group was applied to evaluate in vivo intestinal
permeability. Briefly, 6 h after initiating fasting conditions
(only fasted from food), FITC-dextran was gently administered via
oral gavage to the rats at 40 mg/kg, 200 μl body weight.
Blood was collected from the portal vein at 1 h after the
FITC-dextran administration. To evaluate the degree of gut
permeability, blood was analyzed by the fluorescence measurement of
the concentration of FITC-labeled dextran at an excitation
wavelength of 490 nm and an emission wavelength of 520 nm using a
NanoDrop 3300 fluorospectrometer (Thermo Fisher Scientific,
Inc.).
Histological, immunohistochemical and
immunofluorescent analyses
The liver, ileum and gastrocnemius specimens were
fixed in 10% formalin, incubated overnight at room temperature and
embedded in paraffin. Sections of 5-μm thickness were
stained with hematoxylin and eosin (H&E) and Sirius Red
(performed at Narabyouri Research Co., Nara, Japan). For
immunohistochemical staining, the liver tissue sections were
blocked with 10% goat serum (Abcam) for 30 min following
deparaffinization and antigen retrieval, and then incubated
overnight at 4°C with mouse-monoclonal CD68 antibody (1:100; cat.
no. GTX41868, GeneTex, Inc.). The sections were washed three times
with phosphate-buffered saline and subsequently incubated with a
goat anti-mouse IgG (H+L) HRP-conjugated secondary antibody
(1:2,000; cat. no. 62-6520, Thermo Fisher Scientific, Inc.) for 30
min at room temperature. The slides were developed with DAB until
the signal clearly appeared, and the nuclei were stained with
hematoxylin for 5 min at room temperature, and images were obtained
using a BX53 microscope (Olympus Corporation).
For immunofluorescence, the ileum sections were
deparaffinized and rehydrated and blocked in a similar manner to
immunohistochemical staining, and then rabbit-polyclonal Zonula
occludens-1 (ZO-1; 1:100; cat. no. 61-7300, Invitrogen; Thermo
Fisher Scientific, Inc.) and rabbit-polyclonal Occludin (1:100;
cat. no. 71-1500, Invitrogen; Thermo Fisher Scientific, Inc.) were
used as primary antibodies. Following overnight incubation at 4°C,
the immunofluorescence detection of the primary antibodies was
performed using goat anti-rabbit IgG (H+L) Alexa Fluor-conjugated
secondary antibodies (1:200; cat. nos. R37116 and A-21207, Thermo
Fisher Scientific, Inc.) for 1 h at room temperature. The sections
were mounted on Vectashield mounting medium with
4′,6-diamidino-2-phenylindole Fluoromount-G mounting medium for
fluorescent nucleic acid staining (Vector Laboratories, Inc.) and
images were captured using a BZ-X700 microscope (Keyence
Corporation). Semi-quantitative analysis was performed for five
fields per section in high-power fields at ×400 magnification using
ImageJ software version 64 (National Institutes of Health).
RNA extraction and reverse
transcription-quantitative polymerase chain reaction (RT-qPCR)
Total RNA was isolated from the liver and muscle
tissues and cultured rat L6 myocyte cells. The RNeasy Mini kit
(Qiagen GmbH) was used for the liver tissues and L6 cells, and the
RNeasy Fibrous Tissue Mini kit (Qiagen GmbH) was used for the
muscle tissues. The RNA samples were then treated with DNase in
order to remove DNA contamination with TURBO DNA-free™ DNase
(Invitrogen; Thermo Fisher Scientific, Inc.) and total RNA (2
μg) was reverse transcribed into complementary DNA (cDNA)
using the High-Capacity RNA-to-cDNA kit (Applied Biosystems; Thermo
Fisher Scientific, Inc.) by applying the following three stages:
37°C for 15 min, 85°C for 5 sec and then cooling at 4°C. qPCR was
performed using the primer pairs listed in Table SI, and SYBR™-Green PCR Master
Mix (Applied Biosystems; Thermo Fisher Scientific, Inc.), and an
Applied Biosystems StepOnePlus™ Real-Time PCR® system
(Applied Biosystems; Thermo Fisher Scientific, Inc.). The
thermocycling conditions were as follows: 95°C for 5 min, followed
by 40 cycles of 95°C for 10 sec, 60°C for 20 sec and then 72°C for
20 sec; melting at 95°C for 5 sec, 65°C for 60 sec and 97°C for 1
sec and cooling at 40°C for 10 sec. Relative expression was
normalized to Gapdh expression, and estimated using the
2-ΔΔCq method, and presented as the fold change relative
to the control (31).
Protein extraction and western blot
analysis
Whole cell lysate proteins were extracted from the
intestinal and muscle tissues, and cultured rat L6 myocyte cells
using tissue-protein extraction reagent (T-PER) supplemented with
proteinase and phosphatase inhibitors (all from Thermo Fisher
Scientific, Inc.). The protein concentration was measured using a
protein assay (Bio-Rad Laboratories, Inc.). In total, 50 μg
whole cell lysates were separated by sodium dodecyl
sulfate-polyacrylamide gel electrophoresis (NuPAGE™ 4-12%,
Bis-Tris; Thermo Fisher Scientific, Inc.) and transferred to
Invitrolon polyvinylidene difluoride membranes (Thermo Fisher
Scientific, Inc.), which were subsequently blocked for 1 h with 5%
bovine serum albumin (Thermo Fisher Scientific, Inc.) in
Tris-buffered saline supplemented with Tween-20 (Cell Signaling
Technology, Inc.). The membranes were then incubated overnight at
4°C with antibodies against rabbit-polyclonal ZO-1 (1:500; cat. no.
61-7300) and rabbit-polyclonal Occludin (1:125; cat. no. 71-1500)
(from Invitrogen; Thermo Fisher Scientific, Inc.), p70S6K (1:1,000,
cat. no. 9202), phosphorylated (p-) p70S6K (Thr389; 1:1,000, cat.
no. 9205), nuclear factor-κB (NF-κB) p65 (1:1,000, cat. no. 8242),
p-NF-κB p65 (Ser536; 1:1,000, cat. no. 3033), GAPDH (1:1,000, cat.
no. 2118) and actin (1:1,000, cat. no. 4967) (from Cell Signaling
Technology, Inc.). The membranes were washed and incubated at room
temperature for 1 h with Amersham ECL horseradish
peroxidase-conjugated immunoglobulin G F(ab)2 fragment antibody
(1:5,000 dilution; cat. no. NA931, GE Healthcare; Cytiva) and
developed using Clarity Western enhanced chemiluminescence
substrate (Bio-Rad Laboratories, Inc.). Immunoblotting bands were
densitometrically analyzed using ImageJ 64-bit Java 1.8.0 software
(National Institutes of Health).
Mitochondrial DNA (mtDNA) copy
number
Total DNA was obtained from the gastrocnemius muscle
tissue using a DNA Extractor® TIS kit (FUJIFILM, Wako
Pure Chemical Corporation). The mtDNA copy number was assessed
using RT-qPCR as described in above according to mtDNA
(Rnr2)/nDNA (Gapdh). The primer sequences used were
as follows: RNR2 forward, 5′-AGC TAT TAA TGG TTC GTT TGT-3′ and
reverse, 5′-AGG AGG CTC CAT TTC TCT TGT-3′; and nuclear-encoded
GAPDH forward, 5′-GGA AAG ACA GGT GTT TTG CA-3′ and reverse, 5′-AGG
TCA GAG TGA GCA GGA CA-3′. The PCR amplification process was as
follows: One cycle at 95°C for 10 min, 40 cycles at 95°C for 15 sec
and 60°C for 1 min. Both mtDNA and nDNA threshold cycle (CT)
average values were obtained, and the mtDNA content was calculated
relative to nDNA as mtDNA/nDNA=2(CTnDNA-CTmtDNA).
Mitochondrial respiration and
extracellular acidification
The oxygen consumption rate (OCR) was measured using
the XFe96 extracellular flux analyzer (Agilent Technologies, Inc.)
as previously described (32).
Briefly, the L6 cells were seeded at 8,000 cells per well in a
96-well tissue culture plate 24 h before running the flux analyzer.
To assess the mitochondrial respiration rate, the cells were
incubated in XF base medium (Agilent Technologies, Inc.)
supplemented with 10 mM glucose, 1 mM pyruvate and 2 mM glutamine
for 1.5 h at 37°C. Subsequently, the OCR was measured following the
addition of 1 μM oligomycin to inhibit ATP synthesis from
oxidative phosphorylation, 1.5 μM carbonyl cyanide
4-(trifluoromethoxy) phenylhydrazone (FCCP) to uncouple the
mitochondrial membrane that stimulates respiration, and a mixture
containing 0.5 μM each of antimycin A and rotenone (A+R) to
inhibit complex I and III that terminates mitochondrial oxidative
phosphorylation. After the seahorse measurements were completed,
total cellular content was measured using a CyQUANT Cell
Proliferation Assay kit (Thermo Fisher Scientific, Inc.), and the
OCR values were normalized to the cellular mitochondrial content
(number of cells x mtDNA/nDNA). The basal OCR was calculated as
follows: [OCR(initial)-OCR(A + R)]. The
maximum OCR was computed as follows:
[OCR(FCCP)-OCR(A + R)].
Measurement of mitochondrial membrane
potential
Tetramethylrhodamine methyl ester (TMRM; FUJIFILM,
Wako Pure Chemical Corporation) were used to assess the
mitochondrial membrane potential. The L6 cells were stained by
addition of the dye to the culture medium at 100 nM for 30 min at
37°C. Subsequently, the cells were stained with the Hoechst 33342
nuclear dye (Thermo Fisher Scientific, Inc.) at a 1:500 dilution in
PBS at room temperature for 10 min, washed with PBS, and visualized
using a BZ-X700 (Keyence Corporation). The intensity of the
fluorescence signal was quantified using ImageJ software version 64
(National Institutes of Health).
Statistical analyses
Statistical analyses were performed using Prism,
version 9.1.2 (GraphPad Software, Inc.). Data were analyzed using
one-way ANOVA followed by Tukey's test as a post hoc test. Values
are presented as the mean ± standard deviation. A value of
P<0.05 was considered to indicate a statistically significant
difference.
Results
Effects of the combined use of rifaximin
and L-carnitine on CDAA-induced hepatic steatosis and fibrosis
The experimental design of the study is illustrated
in Fig. 1A. The CDAA-fed rats
exhibited a significant decrease in body weight and hepatomegaly as
compared with the CSAA-fed rats; however, neither rifaximin nor
L-carnitine inhibited these physical impairments (Fig. 1B and C). Serological analysis
revealed elevated alanine aminotransferase (ALT) levels, as well as
a decreased albumin level in the CDAA-fed rats; neither rifaximin
nor L-carnitine significantly affected the ALT levels; however,
L-carnitine suppressed the progression of hypoalbuminemia (Fig. 1D and E). Rifaximin also mildly
improved CDAA-induced hyperammonemia (Fig. 1F). The histological assessment
demonstrated that hepatic lipid accumulation in the CDAA-fed rats
was suppressed by L-carnitine, but not by rifaximin (Fig. 1G). Concomitantly, treatment with
L-carnitine decreased the hepatic TG levels, with the decreased
expression of the lipogenesis-related gene, sterol regulatory
element binding transcription factor 1 (Srebf1), and the
increased expression of carnitine palmitoyltransferase 1A
(Cpt1a) (Fig. 1H and I).
Moreover, Sirius Red staining revealed that hepatic fibrosis
progression was prevented by rifaximin, which was consistent with
the reduced expression mRNA levels of hepatic profibrotic markers
[i.e., actin alpha 2, smooth muscle (Acta2) and collagen
type i alpha 1 chain (Col1a1)]; however, these events were
not significantly mediated by L-carnitine (Fig. 1G-K).
Rifaximin inhibits the CDAA-induced
expansion of hepatic macrophages and the LPS/Toll-like receptor 4
(TLR4)-mediated inflammatory response
Based on the suppression of liver fibrosis following
rifaximin treatment, the present study then examined the
pro-inflammatory status and the hepatic LPS/TLR4 pathway. The
results revealed an extensive hepatic infiltration of CD68-positive
macrophages in the CDAA-fed rats, which was attenuated by treatment
with rifaximin (Fig. 2A and B).
Corresponding with the upregulated expression of hepatic
LPS-binding protein (Lbp), the mRNA levels of Tlr4
and its coreceptor, Cd14, were increased in the CDAA-fed
rats. Notably, treatment with rifaximin inhibited these effects
(Fig. 2C and D). In this
context, rifaximin treatment significantly reduced the hepatic mRNA
levels of pro-inflammatory cytokines, including Tnfa Il1b
and Il6 (Fig. 2E). On the
other hand, treatment with L-carnitine alone did not affect the
inflammatory response (Fig.
2A-E).
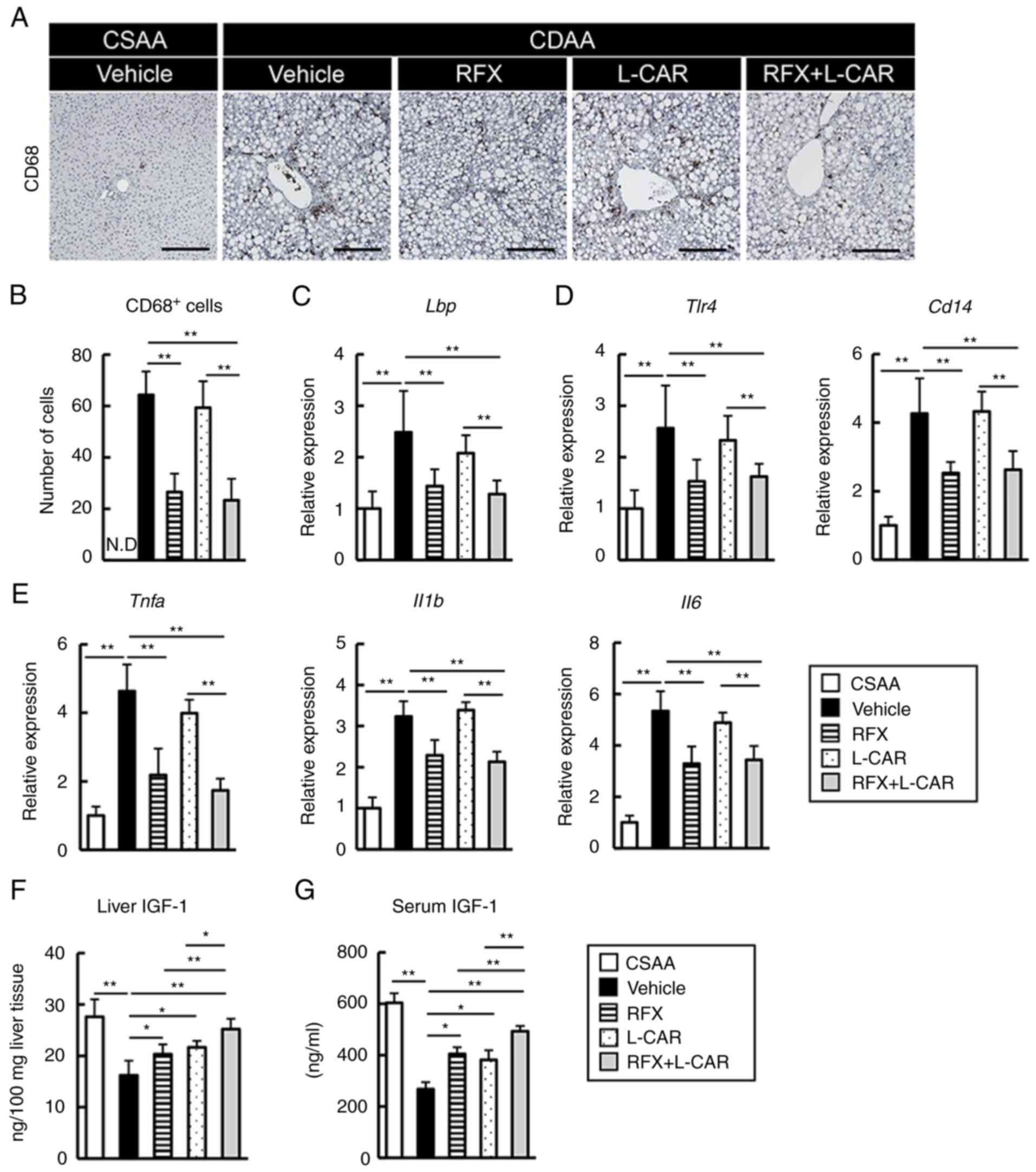 | Figure 2Effects of rifaximin and L-carnitine
on the LPS/TLR4-mediated inflammatory response and IGF-1 levels in
CDAA-fed rats. (A) Representative microphotographs of liver
sections stained with CD68 in the experimental groups. Scale bar,
50 μm. (B) Semi-quantification of CD68-positive cells in a
high-power field using ImageJ software. (C-E) Relative mRNA
expression level of (C) Lbp, (D) Tlr4 and
Cd14, Tnfa, and (E) Il1b and Il6 in the
livers of experimental rats. The mRNA expression levels were
measured using reverse transcription-quantitative PCR, and
Gapdh was used as an internal control. (F and G) Hepatic and
serum levels of IGF-1. Data are the mean ± SD (n=10).
*P<0.05 and **P<0.01, significant
difference between groups. N.D, not detected; LPS,
lipopolysaccharide; TLR4, Toll-like receptor 4; Lbp,
LPS-binding protein; IGF-1, insulin-like growth factor-1; CDAA,
choline-deficient L-amino acid-defined diet; CSAA,
choline-sufficient amino acid-defined diet; RFX, rifaximin; L-CAR,
L-carnitine. |
Subsequently, the present study assessed the changes
in the status of IGF-1, which is a potent myotrophic factor. Both
the hepatic and serum concentrations of IGF-1 were decreased in the
CDAA-fed rats, compared with the CSAA-fed rats (Fig. 2F and G). It was found that
L-carnitine suppressed the CDAA-induced decrease in IGF-1
production (Fig. 2F and G). Of
note, combination treatment with L-carnitine and rifaximin
effectively enhanced the suppressive effects of L-carnitine on the
reduction of the liver and serum IGF-1 levels in the CDAA-fed rats
by reducing the LPS overload, which was recognized to suppress the
hepatic production of IGF-1 (Fig. 2F
and G).
Rifaximin attenuates CDAA-induced
intestinal hyperpermeability
To elucidate the functional mechanisms of the
rifaximin-mediated prevention of the hepatic overload by endogenous
LPS, intestinal permeability was then evaluated. Immunofluorescence
staining revealed that the areas that were immuno-positive for ZO-1
and Occludin, the representative markers of tight junction proteins
(TJPs), were profoundly less in number in the CDAA-fed group than
in the CSAA-fed group; notably, this deprivation of TJPs was
effectively restored by treatment with rifaximin (Fig. 3A-C). The results of western blot
analysis supported these findings; rifaximin replenished the TJP
protein levels (Fig. 3D and E).
In addition, the leakage of plasma FITC-dextran was augmented by
>2.5-fold by the CDAA diet and was inversely proportional with
the deprivation of TJPs (Fig.
3F). Treatment with rifaximin led to a marked decreased in the
leakage of FITC-dextran in the CDAA-fed rats (Fig. 3F). Treatment with L-carnitine
alone did not affect the impairment of intestinal barrier function
(Fig. 3).
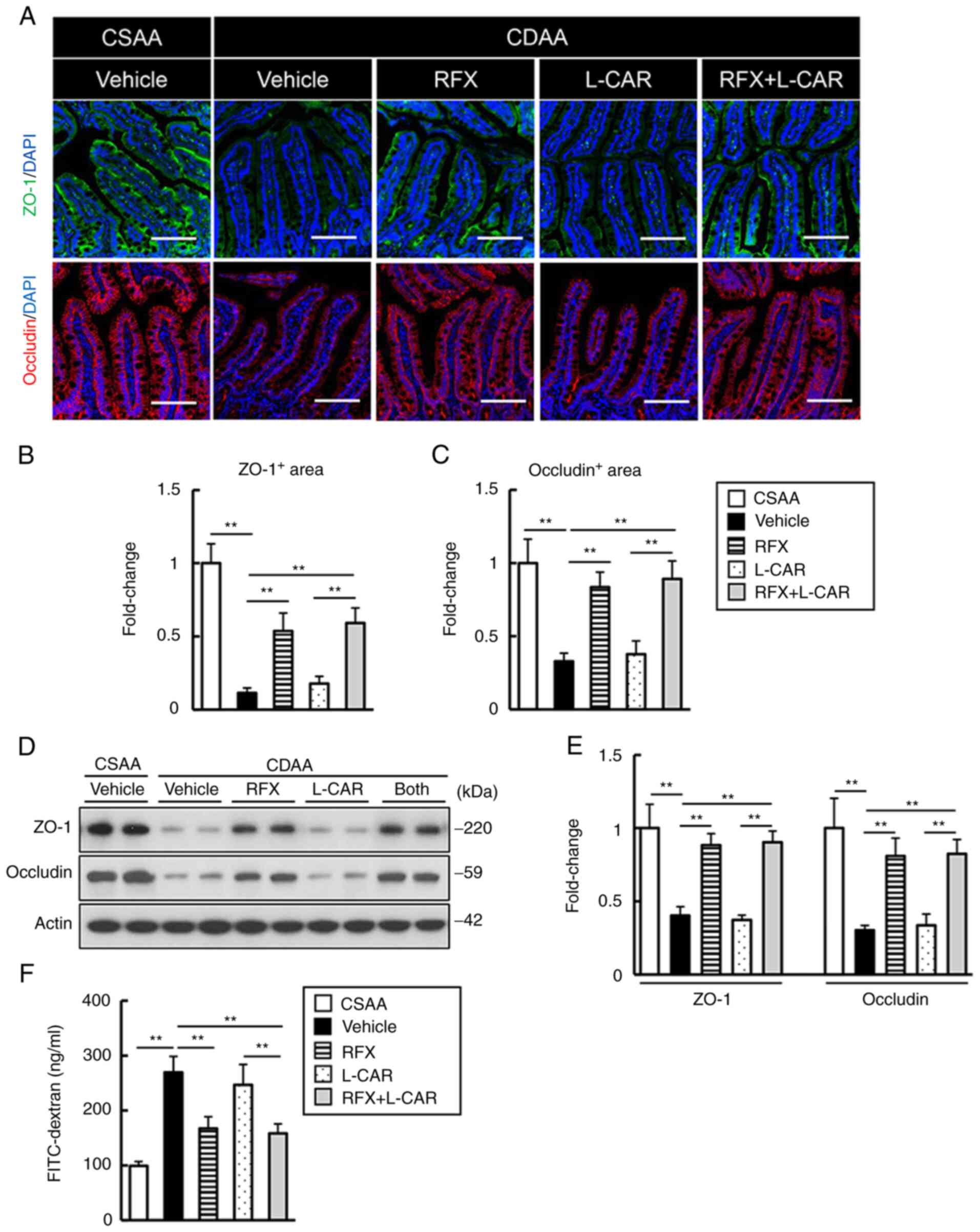 | Figure 3Effects of rifaximin and L-carnitine
on intestinal barrier function in CDAA-fed rats. (A) Representative
microphotographs of ileum sections stained with ZO-1 and occludin
in the experimental groups. Nuclei were counterstained with DAPI.
Scale bar, 50 μm. (B and C) Semi-quantification of ZO-1 and
occludin immunopositive areas in a high-power field using ImageJ
software. (D) Western blots for ZO-1 and occludin in the ilea of
experimental mice. Actin was used as an internal control. (E)
Densitometric quantification of the protein expression of ZO-1 and
occludin. (F) Blood levels of FITC-dextran (4 kDa) at 4 h after the
oral administration. (B, C and E) Quantitative values are indicated
as fold changes to the values of CSAA group Data are the mean ± SD.
(B and C) n=10, (E) n=4, (F) n=5). **P<0.01,
significant difference between groups. ZO-1, zonula occludens-1;
CDAA, choline-deficient L-amino acid-defined diet; CSAA,
choline-sufficient amino acid-defined diet; RFX, rifaximin; L-CAR,
L-carnitine. |
Inhibitory effects of rifaximin and
L-carnitine on CDAA-induced skeletal muscle atrophy and
strength
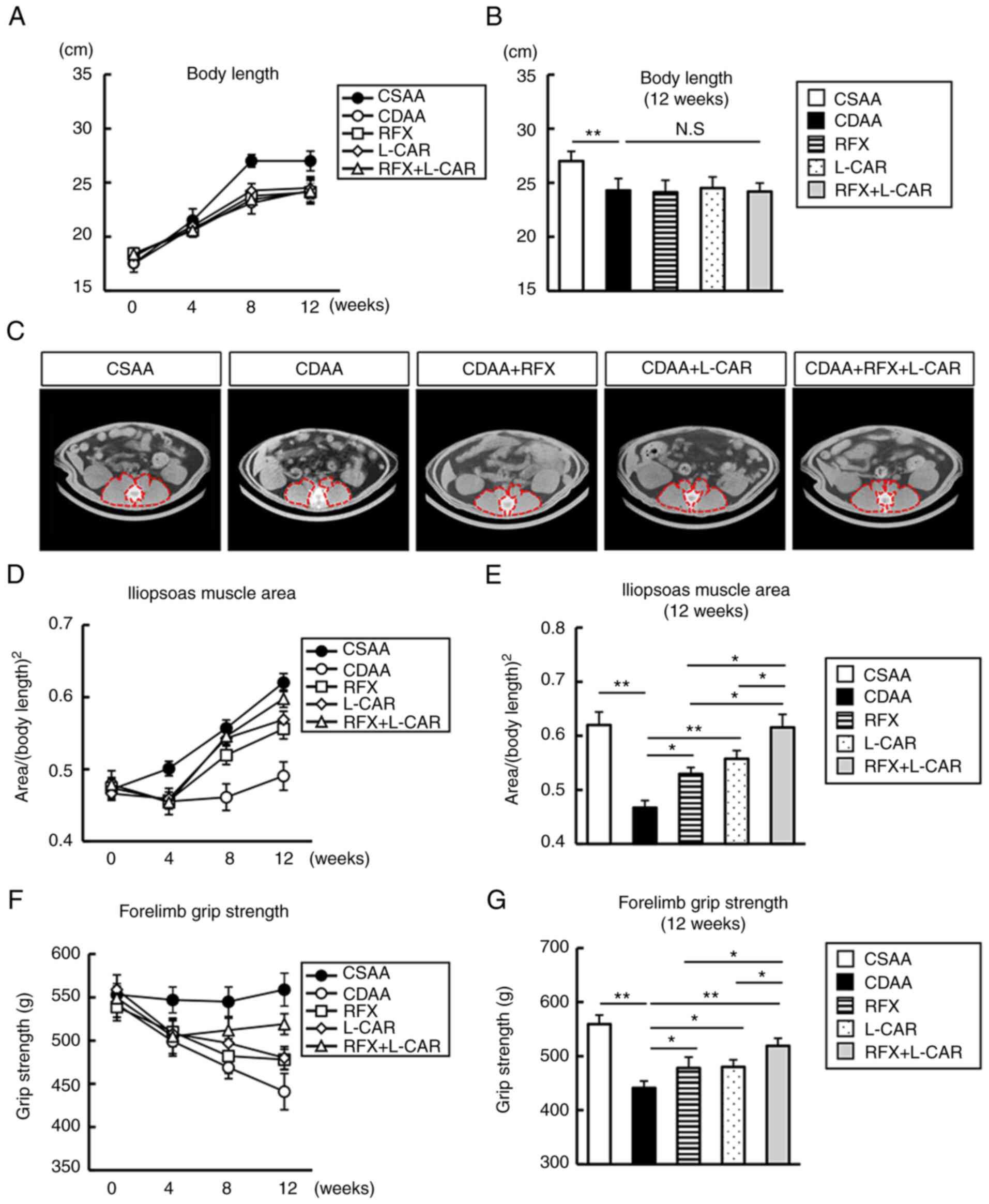
Considering the marked weight loss of the rats, it
was hypothesized that the CDAA-fed rats would undergo skeletal
muscle atrophy. After 12 weeks of feeding, the body length of the
rats was significantly shortened in the CDAA-fed group, and
rifaximin or L-carnitine treatment did not reverse this change
(Fig. 4A and B). After 4 weeks
of feeding, the value of PMI, determined as the cross-sectional
area/height2, significantly decreased in the CDAA-fed
group (Fig. 4C and D). Notably,
rifaximin and L-carnitine suppressed the CDAA-induced decrease in
PMI after 8 weeks of treatment, which was enhanced after 12 weeks
of combined treatment (Fig.
4C-E).
The present study further investigated the efficacy
of both agents on forelimb grip strength. The CDAA diet
significantly weakened forelimb grip strength after 4 weeks
(Fig. 4F and G). In accordance
with the effects observed on muscle atrophy, monotherapy with
rifaximin and L-carnitine prevented the CDAA-induced decrease in
forelimb grip strength, and the combination of these agents
augmented these preventive effects after 12 weeks (Fig. 4F and G).
Effects of rifaximin and L-carnitine on
skeletal muscle protein metabolism in CDAA-fed rats
To elucidate the effects of rifaximin and
L-carnitine on skeletal muscle protein metabolism, the changes in
protein synthesis and degradation in the gastrocnemius muscle
tissues were further analyzed. As illustrated in Fig. 5A-C, the histological assessment
of gastrocnemius muscle fiber revealed a marked decrease in fiber
length and density in the CDAA-fed group. Treatment with either
rifaximin or L-carnitine efficiently ameliorated muscle fiber
atrophy, and the combination of both agents potentiated these
effects (Fig. 5A-C). The
phosphoinositide 3-kinase/Akt/mammalian target of rapamycin (mTOR)
pathway is a pivotal process in muscle protein synthesis. In
CDAA-fed group, the phosphorylation of intramuscular p70S6K
(Thr389), a hallmark of activation by mTOR, was significantly
diminished as compared to CSAA-fed group (Fig. 5D and E). This CDAA-induced
decrease in p70S6K phosphorylation was suppressed by rifaximin and
L-carnitine treatment (Fig. 5D and
E).
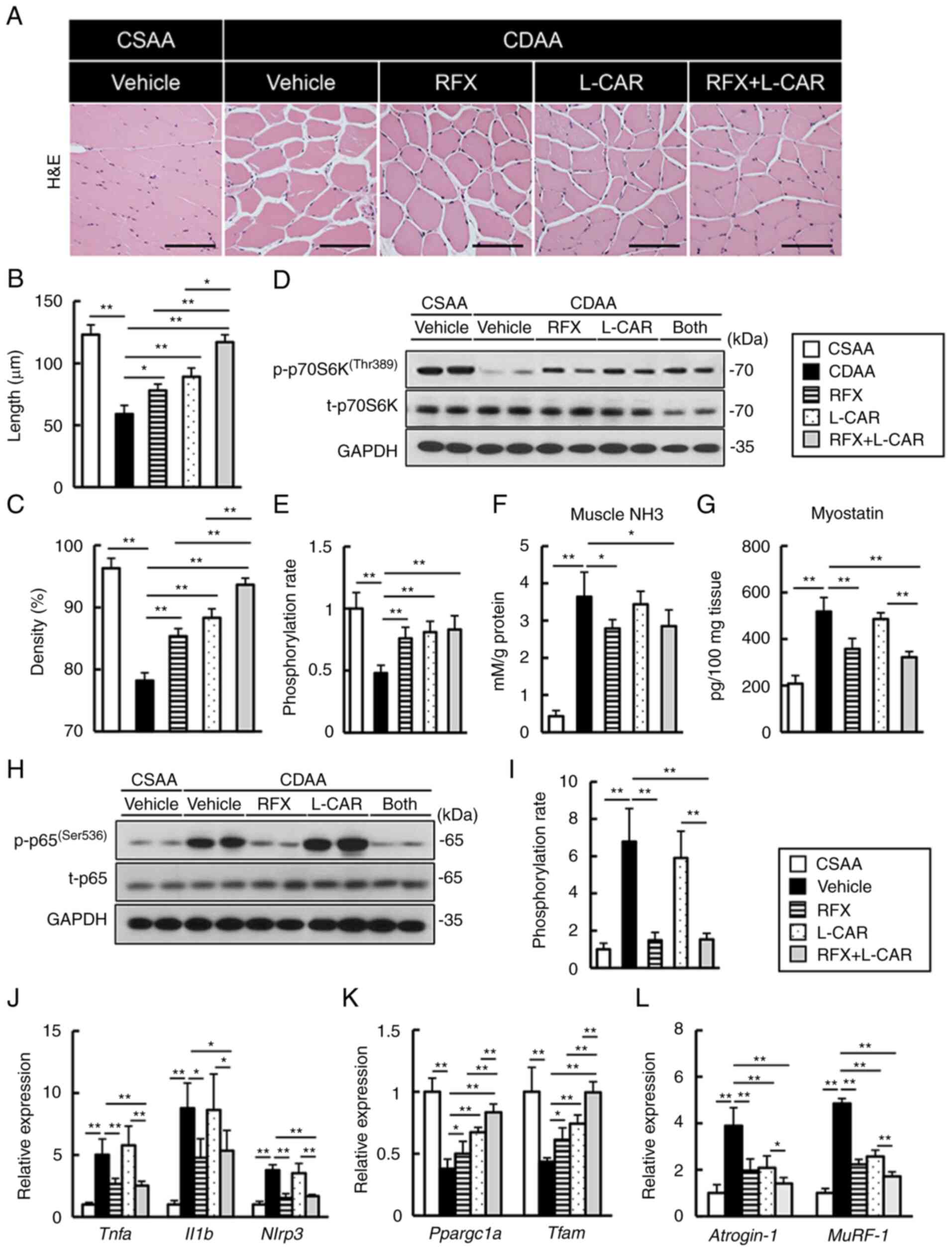 | Figure 5Effects of rifaximin and L-carnitine
on protein synthesis and the degradation of gastrocnemius muscle in
CDAA-fed rats. (A) Representative microphotographs of gastrocnemius
muscle sections stained with H&E in the experimental groups.
Scale bar, 50 μm. (B and C) Summary data of myocyte
cross-sectional length and density. (D) Western blots for p70S6K
phosphorylation in gastrocnemius muscle tissues. (E) Quantification
of phosphorylated p70S6K/total p70S6K. (F) Ammonia levels in
gastrocnemius muscle tissues of experimental rats. (G) Myostatin
concentrations in gastrocnemius muscle tissues of experimental
rats. (H) Western blots for NF-κB p65 phosphorylation in
gastrocnemius muscle tissues. (I) Quantification of phosphorylated
p65/total p65. (J-L) Relative mRNA expression levels of (J)
Tnfa, Il1b and Nlrp3, (K) Ppargc1a and
Tfam, and (L) Atrogin-1 and MuRF-1 in the
gastrocnemius muscle of experimental rats. (D and H) GAPDH was used
as the loading control for western blot analysis. The mRNA
expression levels were measured using reverse
transcription-quantitative PCR and Gapdh was used as an
internal control. Quantitative values are indicated as fold changes
to the values of CSAA group (E-G and I-L). Data are the mean ± SD.
(B, C, F, G and J-L) n=10, (E and I) n=4. *P<0.05 and
**P<0.01, significant difference between groups.
H&E, hematoxylin and eosin; CDAA, choline-deficient L-amino
acid-defined diet; CSAA, choline-sufficient amino acid-defined
diet; RFX, rifaximin; L-CAR, L-carnitine; Ppargc1a,
peroxisome proliferator-activated receptor γ coactivator-1α;
Tfam, mitochondrial transcription factor A; MuRF-1,
muscle RING-finger protein-1. |
The present study then evaluated the muscle levels
of ammonia and myostatin, which is a myokine that inhibits muscle
cell growth. In the CDAA-fed rats, the intramuscular ammonia levels
were elevated along with changes in serum ammonia levels; this
effect was attenuated by rifaximin treatment (Fig. 5F). Consistently, the marked
increase in the myostatin levels in the CDAA-fed rats was
suppressed by rifaximin treatment (Fig. 5G). Furthermore, in the CDAA-fed
rats, there was an upregulation in the levels of the inflammatory
mediators, Tnfa, Il1b and NLR family pyrin domain
containing 3 (Nlrp3), following NF-κB activation in the
skeletal muscle, and rifaximin potently suppressed the activation
of these pro-inflammatory pathways (Fig. 5H-J). These effects were not
observed in the muscles of the L-carnitine-treated rats (Fig. 5F-J).
Based on the fact that mitochondrial function
dictates muscle fiber homeostasis, the present study investigated
mitochondrial biogenesis in the gastrocnemius muscle tissue. The
CDAA-fed rats manifested a prominent decrease in the intramuscular
levels of mitochondrial biogenesis-related genes, peroxisome
proliferator-activated receptor γ coactivator-1α (PGC-1α;
Ppargc1a) and mitochondrial transcription factor A (TFAM;
Tfam) (Fig. 5K). Of note,
these decreases were efficiently restored by treatment with either
rifaximin or L-carnitine; this was augmented by the combination of
both agents (Fig. 5K).
Following the changes in these regulatory factors,
the intramuscular mRNA expression levels of Atrogin-1 and
muscle RING-finger protein-1 (MuRF-1), which are the pivotal
markers of the ubiquitin-proteasome system (UPS), were upregulated
in the CDAA-fed group; treatment with rifaximin and L-carnitine
attenuated these effects (Fig.
5L).
L-carnitine inhibits the LPS- or
TNF-α-stimulated impairment of mitochondrial biogenesis and the UPS
in skeletal muscle cells
In the model in the present study, it was found that
L-carnitine supplementation attenuated the impairment of
mitochondrial biogenesis and the progression of UPS in the course
of muscle degradation. Therefore, the present study then examined
whether L-carnitine could directly affect differentiated L6 rat
myotubes in vitro (Fig.
6A). As shown in Fig. S1A and
B, LPS stimulation decreased the mRNA levels of Ppargc1a
and Tfam in a concentration-dependent manner, and conversely
increased those of Atrogin-1 and MuRF-1 in rat
myotubes. Of note, L-carnitine suppressed the LPS-induced decrease
in the levels of mitochondrial biogenesis-related markers, and
consequently inhibited the UPS stimulation by LPS (Fig. 6B and C). To further evaluate
mitochondrial biogenesis, the cellular mitochondrial content was
measured by quantifying mtDNA over nuclear DNA (mtDNA/nDNA). The
results revealed a a marked reduction in mtDNA/nDNA in the
LPS-stimulated L6 myotubes, and treatment with L-carnitine restored
the LPS-stimulated reduction in mtDNA/nDNA (Fig. 6D). Moreover, mitochondrial
membrane potential, an indicator of mitochondrial function, was
assessed by the fluorescence intensity of TMRM that accumulates in
the mitochondria. As shown in Fig.
6E and F, mitochondrial membrane potential was decreased in the
LPS-stimulated L6 myotubes, and this decrease was suppressed by
treatment with L-carnitine.
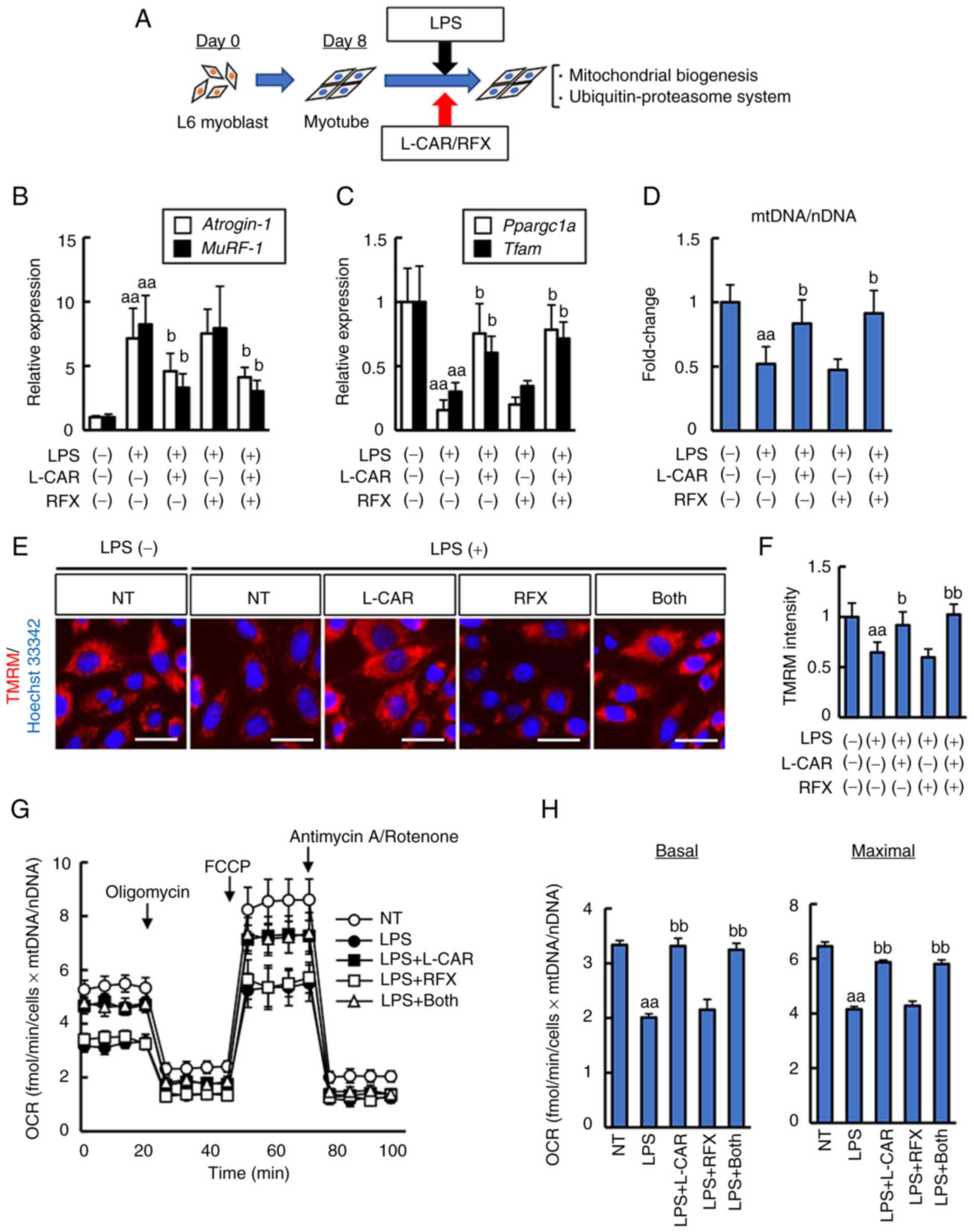 | Figure 6Cellular mitochondrial biogenesis in
LPS-stimulated rat L6 myocytes. (A) In vitro experimental
protocol. (B and C) Effects of L-carnitine and/or rifaximin on the
mRNA expression levels of (B) Atrogin-1 and MuRF-1,
and (C) Ppargc1a and Tfam in LPS-stimulated rat L6
myocytes. The mRNA expression levels were measured using reverse
transcription-quantitative PCR, and Gapdh was used as an
internal control. (D) The mitochondrial content was assessed using
quantitative PCR as described in the Materias and methods. (E)
Representative images of TMRM live stains corresponding to
mitochondrial membrane potential. Scale bar, 50 μm. (F)
Quantification of TMRM intensity per cell; data shown as the mean ±
SD for 100 cells per condition in three representative experiments.
Nuclei were stained with Hoechst 33342. (G) Measurements of OCR
using a seahorse extracellular flux analyzer. (H) Calculations of
the basal and maximal respiration rates. Cells were treated with
LPS (1.0 μg/ml) and L-carnitine (5 mM) and/or rifaximin (10
μM) for 48 h. Quantitative values are indicated as fold
changes to the values of non-treated (NT) groups (B, C and D). Data
are the mean ± SD; (B, C and D) n=8, (F) n=3, or the mean ± SEM (G
and H) n=5. aaP<0.01 vs. LPS (-)/L-CAR (-)/RFX (-),
bP<0.05 and bbP<0.01 vs. LPS (+)/L-CAR
(-)/RFX (-). LPS, lipopolysaccharide; TMRM, tetramethylrhodamine
methyl ester; OCR, oxygen consumption rate; CSAA,
choline-sufficient amino acid-defined diet; RFX, rifaximin; L-CAR,
L-carnitine; Ppargc1a, peroxisome proliferator-activated
receptor γ coactivator-1α; Tfam, mitochondrial transcription
factor A; MuRF-1, muscle RING-finger protein-1. |
Subsequently, the cellular effects of both agents on
mitochondrial respiration were examined using seahorse analyses to
measure the OCR of L6 myotubes stimulated with LPS. The OCR values
were normalized to the cellular mitochondrial content and the basal
and maximal respiration rates were calculated. The results revealed
that stimulation with LPS downregulated both the basal OCR and
maximal respiratory capacity in L6 myotubes, which were restored by
treatment with L-carnitine (Fig. 6G
and H).
Inflammatory cytokines have been reported to impair
intramuscular mitochondrial biogenesis. Thus, the present study
also examined the effects of both agents on the TNF-α-induced
impairment of mitochondrial biogenesis. TNF-α administration
exacerbated the impairment of mitochondrial biogenesis,
mitochondrial membrane potential and respiratory capacity and
promoted the UPS in the rat myotubes in L6 myotubes (Figs. S1C and D, and S2C-F). Notably,
L-carnitine significantly prevented this sequence of muscle
degradation induced by TNF-α stimulation (Fig. S2A-F). By contrast, rifaximin did
not directly affect myotubes. These findings suggest that
L-carnitine reversed the LPS- or TNF-α-induced impairment of
mitochondrial biogenesis in myotubes.
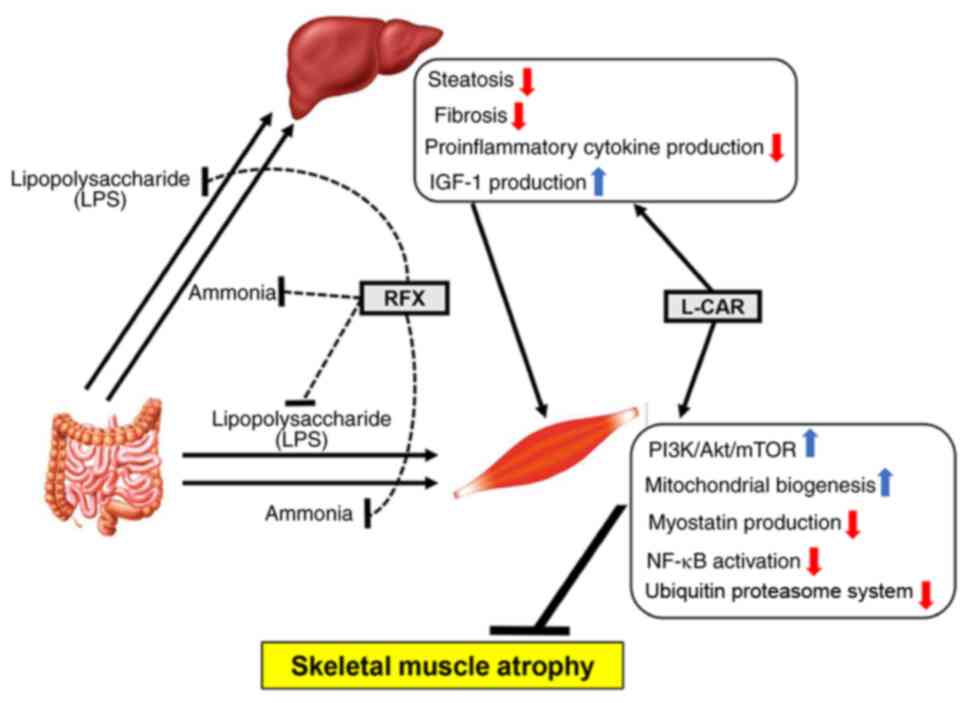
Taken together, the results demonstrated that
rifaximin enhanced the inhibitory effects of L-carnitine
supplementation on skeletal muscle atrophy in CDAA-fed cirrhotic
rats via multifunctional mechanisms based on the modulation of the
gut-liver-muscle axis (Fig.
7).
Discussion
The present study demonstrated that the additional
administration of rifaximin effectively potentiated the preventive
effects of L-carnitine supplementation on skeletal muscle wasting
in a CDAA-fed rat model. To date, studies have proposed several
experimental models of cirrhosis-based sarcopenia in rodents.
Giusto et al (33)
observed skeletal muscle myopenia in both bile duct ligation and
carbon tetrachloride-induced cirrhosis. Moreover, choline has been
reported to be required for skeletal muscle homeostasis based on
its proper modulation of fat and protein metabolism and
counteraction of inflammation, apoptosis and autophagy (34). Indeed, a recent study by the
authors suggested that CDAA feeding induced marked steatohepatitis
and fibrosis, accompanied by significant skeletal muscle atrophy in
rats (26). Moreover, it has
also been found that CDAA-fed rats exhibit gut hyperpermeability
(35). Therefore, this model was
assumed to be suitable to address the therapeutic interventions
through the gut-liver-muscle axis.
The present study demonstrated that monotherapy with
rifaximin exerted an inhibitory effect on the atrophic changes in
the skeletal muscle of CDAA-fed rats. In this context, the
involvement of multifunctional mechanisms was suggested (Fig. 7). First, rifaximin attenuated
hyperammonemia in the CDAA-fed rats according to its
pharmacological property. Hyperammonemia has been recognized to
trigger skeletal muscle wasting related with liver cirrhosis
(1,2). In patients with cirrhosis, muscle
ammonia levels are often elevated and can lead to the induction of
NF-κB, and thus to a further increase in myostatin expression,
followed by the inhibition of myogenesis and an increase in
autophagy (36). Likewise, Kumar
et al (21) reported that
combination therapy with rifaximin and L-ornithine L-aspartate
reversed sarcopenia in a rat model of portocaval anastomosis by
suppressing the GCN2-dependent hyperammonemic cellular stress
response. Consistently, the present study demonstrated a reduction
in intramuscular ammonia levels, as well as improved hyperammonemia
following rifaximin treatment of the CDAA-fed rats. This suggested
that lowering the ammonia level partially contributed to the
rifaximin-mediated inhibition of the development of sarcopenia. On
the other hand, sarcopenia has been noted to be irreversible
following liver transplantation despite normal ammonia metabolism
in the graft, suggesting that the withdrawal of ammonia alone is
insufficient for the recovery of sarcopenia (37). In this respect, rifaximin was
likely to more potently attenuate skeletal muscle atrophy than
hyperammonemia in the model used herein. Thus, this suggests the
possible involvement of another functional mechanism in this
anti-myopenic effect.
The present study focused on the impact of rifaximin
on the status of endogenous LPS. Endotoxemia is exacerbated due to
an impaired intestinal barrier integrity and dysbiosis in liver
cirrhosis, particularly when related to alcoholic liver injury and
non-alcoholic steatohepatitis (38,39). In line with a previous study by
the authors, the rifaximin-mediated blunting of intestinal
hyperpermeability suppressed the CDAA-induced hepatic
proinflammatory response and fibrogenesis by inhibiting hepatic the
LPS/TLR4 signaling pathway (23). Subsequently, the upregulation in
the levels of inflammatory mediators was suppressed by rifaximin
treatment in skeletal muscle. Given that TNF-α is a key mediator of
liver fibrosis-induced muscle atrophy, the rifaximin-mediated
anti-sarcopenic effect was also relevant to the improved hepatic
pathology (40). Previous
studies have documented that an LPS stimulus decreases the
circulating levels and hepatic expression of IGF-1, and leads to
the inhibition of skeletal muscle protein synthesis (41,42). In the present study, the CDAA-fed
rats exhibited a reduction in both serum and hepatic levels of
IGF-1 along with the hepatic overload of LPS. Of note, rifaximin
significantly suppressed the decrease in the IGF-1 level,
corroborating the likelihood that rifaximin could also prevent
sarcopenia by shielding from exposure to gut-derived LPS.
The present study underscored the results on the
enhanced effect of L-carnitine supplementation on skeletal muscle
atrophy with the addition of rifaximin. Previous studies have
documented that L-carnitine increases the plasma IGF-1 level and
results in the suppression of the UPS-induced myofibrillar protein
degradation in humans and rodents (43,44). Consistently, the findings of the
present study demonstrated that L-carnitine exerted a limited
effect on hepatic and gut phenotypes in the CDAA-fed rats, while it
significantly improved lipid metabolism in the liver, increased
hepatic and serum levels of IGF-1, and promoted mTOR signaling
activation and mitochondrial biogenesis in the skeletal muscle,
resulting in the suppression of muscle atrophy. Moreover, the
results suggested that L-carnitine increased the levels of key
regulators involved in skeletal muscle mitochondrial biogenesis in
the CDAA-fed rats. To reflect this, the in vitro experiments
demonstrated that L-carnitine directly improved mitochondrial
biogenesis and oxygen respiratory function, and consequently
attenuated the UPS in rat myotubes stimulated with both LPS and
TNF-α. Moreover, rifaximin did not directly affect myotubes,
suggesting that it predominantly suppressed skeletal muscle atrophy
by reducing the exposure of LPS and inflammatory cytokines, as well
as ammonia to muscle tissue. These findings strongly suggest that
L-carnitine is available as a conventional therapy for
cirrhosis-related sarcopenia and that its combined use with
rifaximin has the potential to be a novel effective therapy.
The present study had several limitations which
should be noted. First, the effect of rifaximin on microbial
profiles was obscure in the present model. In this context,
previous research has indicated the impact of rifaximin on the gut
microbiota. Patel et al (45) demonstrated that rifaximin reduced
the mucin-degrading sialidase-rich species, leading to gut barrier
repair in patients with cirrhosis with HE. Kitagawa et al
(46) demonstrated that
rifaximin attenuated ethanol-induced liver injury in mice followed
by a decrease in Erysipelotrichales and an increase in
Bacteroidales. Given these findings, further analyses are
required to identify the interaction between microbial alterations
by rifaximin and the therapeutic effects in the current model.
Second, although the doses of rifaximin (100 mg/kg/day) and
L-carnitine (200 mg/kg/day) for use in the in vivo
experiments were selected based on previous studies (23,24), the results did not disclose that
these selected doses were relevant to the clinical dose. The
package insert from ASKA Pharmaceutical Co. Ltd. has documented
that when rifaximin is used at the dose used in the present study
and orally administered to male rats, the maximum plasma
concentration is almost equal to that observed with the clinical
dose (1,100 mg/day) orally administered to patients with liver
cirrhosis for 14 days. Moreover, since rifaximin is a poorly
absorbable drug and specifically affects the gastrointestinal
tract, it is relatively difficult to determine the dose equivalent
to clinical doses by measuring the blood concentration. A previous
study demonstrated that the dose of rifaximin (100 mg/kg/day)
sufficiently affected the intestine and colon in rats (47). Additionally, it was observed that
these doses of both drugs did not exhibit hepatic and renal
toxicity in rats (data not shown). Thus, these doses are considered
to be within tolerance for use in in vivo experiments.
However, further pharmacokinetics analyses are required to evaluate
whether the doses used in the present study were relevant to the
clinical dose. Third, the present study did not observe the
suppression of body weight loss in the CDAA-fed rats by treatment
with both agents, in spite of the improvement of skeletal muscle
atrophy. In this regard, it was hypothesized that both agents would
possibly reduce visceral fat, as well as increase skeletal muscle
in the CDAA-fed rats. However, this is merely a speculation and
further investigations are required to confirm this hypothesis by
evaluating the changes in visceral fat following treatment with
both agents.
In conclusion, the present study demonstrated that
rifaximin enhanced the anti-myopenic properties of L-carnitine
supplementation in the skeletal muscle of CDAA-fed rats. Notably,
this effect of rifaximin was based on the modulation of the
gut-liver-muscle axis through the suppression of the endogenous LPS
overload by maintaining intestinal barrier function, in addition to
its ammonia-lowering properties. Of note, both drugs are clinically
available for patients with chronic liver diseases and that the
aforementioned effects on skeletal muscle wasting were achieved
without observing any drug toxicity. Therefore, these findings
suggested that this combination regimen may provide a clinical
benefit for liver cirrhosis-related sarcopenia.
Supplementary Data
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
KM, KK, TN, TA and HY contributed to the conception
and design of the present study. KM, KK, NN, ME, YFujimoto, ST, YT,
YFujinaga, HT and HK performed the experiments and analyzed the
data. KM, KK and HY wrote and revised the manuscript. KM, KK and NN
confirm the authenticity of all the raw data. All authors have read
and approved the final manuscript.
Ethics approval and consent to
participate
The present study was approved by the Animal Ethics
Committee of Nara Medical University (approval no. 12764), and all
protocols were performed in accordance with the National Institutes
of Health Guidelines for the Care and Use of Laboratory
Animals.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
Acknowledgements
Not applicable.
Funding
No funding was received.
References
|
1
|
Dasarathy S and Merli M: Sarcopenia from
mechanism to diagnosis and treatment in liver disease. J Hepatol.
65:1232–1244. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Bhanji RA, Montano-Loza AJ and Watt KD:
Sarcopenia in cirrhosis: Looking beyond the skeletal muscle loss to
see the systemic disease. Hepatology. 70:2193–2203. 2019.
View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Tandon P, Montano-Loza AJ, Lai JC,
Dasarathy S and Merli M: Sarcopenia and frailty in decompensated
cirrhosis. J Hepatol. 75(Suppl 1): S147–S162. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Vasques J, Guerreiro CS, Sousa J, Pinto M
and Cortez-Pinto H: Nutritional support in cirrhotic patients with
sarcopenia. Clin Nutr ESPEN. 33:12–17. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Ebadi M, Bhanji RA, Mazurak VC and
Montano-Loza AJ: Sarcopenia in cirrhosis: From pathogenesis to
interventions. J Gastroenterol. 54:845–859. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Ascenzi F, Barberi L, Dobrowolny G, Villa
Nova Bacurau A, Nicoletti C, Rizzuto E, Rosenthal N, Scicchitano BM
and Musarò A: Effects of IGF-1 isoforms on muscle growth and
sarcopenia. Aging Cell. 18:e129542019. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Nishikawa H, Enomoto H, Nishiguchi S and
Iijima H: Liver cirrhosis and sarcopenia from the viewpoint of
dysbiosis. Int J Mol Sci. 21:52542020. View Article : Google Scholar :
|
|
8
|
West J, Gow PJ, Testro A, Chapman B and
Sinclair M: Exercise physiology in cirrhosis and the potential
benefits of exercise interventions: A review. J Gastroenterol
Hepatol. 36:2687–2705. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Dos Santos ALS and Anastácio LR: The
impact of L-branched-chain amino acids and L-leucine on
malnutrition, sarcopenia, and other outcomes in patients with
chronic liver disease. Expert Rev Gastroenterol Hepatol.
15:181–194. 2021. View Article : Google Scholar
|
|
10
|
Sinclair M, Grossmann M, Angus PW,
Hoermann R, Hey P, Scodellaro T and Gow PJ: Low testosterone as a
better predictor of mortality than sarcopenia in men with advanced
liver disease. J Gastroenterol Hepatol. 31:661–667. 2016.
View Article : Google Scholar
|
|
11
|
Ponziani FR, Picca A, Marzetti E, Calvani
R, Conta G, Del Chierico F, Capuani G, Faccia M, Fianchi F, Funaro
B, et al: Characterization of the gut-liver-muscle axis in
cirrhotic patients with sarcopenia. Liver Int. 41:1320–1334. 2021.
View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Kendler BS: Carnitine: An overview of its
role in preventive medicine. Prev Med. 15:373–390. 1986. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Houten SM, Wanders RJA and Ranea-Robles P:
Metabolic interactions between peroxisomes and mitochondria with a
special focus on acylcarnitine metabolism. Biochim Biophys Acta Mol
Basis Dis. 1866:1657202020. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Hanai T, Shiraki M, Imai K, Suetugu A,
Takai K and Shimizu M: Usefulness of carnitine supplementation for
the complications of liver cirrhosis. Nutrients. 12:19152020.
View Article : Google Scholar :
|
|
15
|
Ohashi K, Ishikawa T, Hoshii A, Hokari T,
Suzuki M, Noguchi H, Hirosawa H, Koyama F, Kobayashi M, Hirosawa S,
et al: Effect of levocarnitine administration in patients with
chronic liver disease. Exp Ther Med. 20:942020. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Ohara M, Ogawa K, Suda G, Kimura M,
Maehara O, Shimazaki T, Suzuki K, Nakamura A, Umemura M, Izumi T,
et al: L-carnitine suppresses loss of skeletal muscle mass in
patients with liver cirrhosis. Hepatol Commun. 2:906–918. 2018.
View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Hiramatsu A, Aikata H, Uchikawa S, Ohya K,
Kodama K, Nishida Y, Daijo K, Osawa M, Teraoka Y, Honda F, et al:
Levocarnitine use is associated with improvement in sarcopenia in
patients with liver cirrhosis. Hepatol Commun. 3:348–355. 2019.
View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Bass NM, Mullen KD, Sanyal A, Poordad F,
Neff G, Leevy CB, Sigal S, Sheikh MY, Beavers K, Frederick T, et
al: Rifaximin treatment in hepatic encephalopathy. N Engl J Med.
362:1071–1081. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Lyon KC, Likar E, Martello JL and Regier
M: Retrospective cross-sectional pilot study of rifaximin dosing
for the prevention of recurrent hepatic encephalopathy. J
Gastroenterol Hepatol. 32:1548–1552. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Rahimi RS, Brown KA, Flamm SL and Brown RS
Jr: Overt hepatic encephalopathy: Current pharmacologic treatments
and improving clinical outcomes. Am J Med. 134:1330–1338. 2021.
View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Kumar A, Davuluri G, Silva RNE, Engelen
MPKJ, Ten Have GAM, Prayson R, Deutz NEP and Dasarathy S: Ammonia
lowering reverses sarcopenia of cirrhosis by restoring skeletal
muscle proteostasis. Hepatology. 65:2045–2058. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Kaji K, Saikawa S, Takaya H, Fujinaga Y,
Furukawa M, Kitagawa K, Ozutsumi T, Kaya D, Tsuji Y, Sawada Y, et
al: Rifaximin alleviates endotoxemia with decreased serum levels of
soluble CD163 and mannose receptor and partial modification of gut
microbiota in cirrhotic patients. Antibiotics (Basel). 9:1452020.
View Article : Google Scholar
|
|
23
|
Fujinaga Y, Kawaratani H, Kaya D, Tsuji Y,
Ozutsumi T, Furukawa M, Kitagawa K, Sato S, Nishimura N, Sawada Y,
et al: Effective combination therapy of angiotensin-II receptor
blocker and rifaximin for hepatic fibrosis in rat model of
nonalcoholic steatohepatitis. Int J Mol Sci. 21:55892020.
View Article : Google Scholar :
|
|
24
|
Demiroren K, Dogan Y, Kocamaz H, Ozercan
IH, Ilhan S, Ustundag B and Bahcecioglu IH: Protective effects of
L-carnitine, N-acetylcysteine and genistein in an experimental
model of liver fibrosis. Clin Res Hepatol Gastroenterol. 38:63–72.
2014. View Article : Google Scholar
|
|
25
|
Horinouchi T, Hoshi A, Harada T, Higa T,
Karki S, Terada K, Higashi T, Mai Y, Nepal P, Mazaki Y and Miwa S:
Endothelin-1 suppresses insulin-stimulated Akt phosphorylation and
glucose uptake via GPCR kinase 2 in skeletal muscle cells. Br J
Pharmacol. 173:1018–1032. 2016. View Article : Google Scholar
|
|
26
|
Takeda S, Kaji K, Nishimura N, Enomoto M,
Fujimoto Y, Murata K, Takaya H, Kawaratani H, Moriya K, Namisaki T,
et al: Angiotensin receptor blockers potentiate the protective
effect of branched-chain amino acids on skeletal muscle atrophy in
cirrhotic rats. Mol Nutr Food Res. 65:e21005262021. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Doyle A, Zhang G, Abdel Fattah EA, Eissa
NT and Li YP: Toll-like receptor 4 mediates
lipopolysaccharide-induced muscle catabolism via coordinate
activation of ubiquitin-proteasome and autophagy-lysosome pathways.
FASEB J. 25:99–110. 2011. View Article : Google Scholar :
|
|
28
|
Girven M, Dugdale HF, Owens DJ, Hughes DC,
Stewart CE and Sharples AP: l-Glutamine improves skeletal muscle
cell differentiation and prevents myotube atrophy after cytokine
(TNF-α) stress via reduced p38 MAPK signal transduction. J Cell
Physiol. 231:2720–2732. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Nishikawa H, Shiraki M, Hiramatsu A,
Moriya K, Hino K and Nishiguchi S: Japan society of hepatology
guidelines for sarcopenia in liver disease (1st edition):
Recommendation from the working group for creation of sarcopenia
assessment criteria. Hepatol Res. 46:951–963. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Gao JH, Wen SL, Tong H, Wang CH, Yang WJ,
Tang SH, Yan ZP, Tai Y, Ye C, Liu R, et al: Inhibition of
cyclooxygenase-2 alleviates liver cirrhosis via improvement of the
dysfunctional gut-liver axis in rats. Am J Physiol Gastrointest
Liver Physiol. 310:G962–G372. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(−Delta Delta C(T)) method. Methods. 25:402–408. 2001.
View Article : Google Scholar
|
|
32
|
Shahini A, Rajabian N, Choudhury D,
Shahini S, Vydiam K, Nguyen T, Kulczyk J, Santarelli T, Ikhapoh I,
Zhang Y, et al: Ameliorating the hallmarks of cellular senescence
in skeletal muscle myogenic progenitors in vitro and in vivo. Sci
Adv. 7:eabe56712021. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Giusto M, Barberi L, Di Sario F, Rizzuto
E, Nicoletti C, Ascenzi F, Renzi A, Caporaso N, D'Argenio G, Gaudio
E, et al: Skeletal muscle myopenia in mice model of bile duct
ligation and carbon tetrachloride-induced liver cirrhosis. Physiol
Rep. 5:e131532017. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Moretti A, Paoletta M, Liguori S, Bertone
M, Toro G and Iolascon G: Choline: An essential nutrient for
skeletal muscle. Nutrients. 12:21442020. View Article : Google Scholar :
|
|
35
|
Sawada Y, Kawaratani H, Kubo T, Fujinaga
Y, Furukawa M, Saikawa S, Sato S, Seki K, Takaya H, Okura Y, et al:
Combining probiotics and an angiotensin-II type 1 receptor blocker
has beneficial effects on hepatic fibrogenesis in a rat model of
non-alcoholic steatohepatitis. Hepatol Res. 49:284–295. 2019.
View Article : Google Scholar
|
|
36
|
Qiu J, Thapaliya S, Runkana A, Yang Y,
Tsien C, Mohan ML, Narayanan A, Eghtesad B, Mozdziak PE, McDonald
C, et al: Hyperammonemia in cirrhosis induces transcriptional
regulation of myostatin by an NF-κB-mediated mechanism. Proc Natl
Acad Sci USA. 110:18162–18167. 2013. View Article : Google Scholar
|
|
37
|
Saiman Y and Serper M: Frailty and
sarcopenia in patients pre- and post-liver transplant. Clin Liver
Dis. 25:35–51. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Gao B and Bataller R: Alcoholic liver
disease: Pathogenesis and new therapeutic targets.
Gastroenterology. 141:1572–1585. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Leung C, Rivera L, Furness JB and Angus
PW: The role of the gut microbiota in NAFLD. Nat Rev Gastroenterol
Hepatol. 13:412–425. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Rosa CGS, Colares JR, da Fonseca SRB,
Martins GDS, Miguel FM, Dias AS, Marroni CA, Picada JN, Lehmann M
and Marroni NAP: Sarcopenia, oxidative stress and inflammatory
process in muscle of cirrhotic rats-action of melatonin and
physical exercise. Exp Mol Pathol. 121:1046622021. View Article : Google Scholar
|
|
41
|
Thomsen KL, Nielsen SS, Grønbiek H,
Flyvbjerg A and Vilstrup H: Effects of lipopolysaccharide endotoxin
on the insulin-like growth factor I system in rats with cirrhosis.
In Vivo. 22:655–661. 2008.
|
|
42
|
Fang WY, Tseng YT, Lee TY, Fu YC, Chang
WH, Lo WW, Lin CL and Lo YC: Triptolide prevents LPS-induced
skeletal muscle atrophy via inhibiting NF-κB/TNF-α and regulating
protein synthesis/degradation pathway. Br J Pharmacol.
178:2998–3016. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Sawicka AK, Hartmane D, Lipinska P,
Wojtowicz E, Lysiak-Szydlowska W and Olek RA: l-Carnitine
supplementation in older women. A pilot study on aging skeletal
muscle mass and function. Nutrients. 10:2552018. View Article : Google Scholar :
|
|
44
|
Keller J, Couturier A, Haferkamp M, Most E
and Eder K: Supplementation of carnitine leads to an activation of
the IGF-1/PI3K/Akt signalling pathway and down regulates the E3
ligase MuRF1 in skeletal muscle of rats. Nutr Metab (Lond).
10:282013. View Article : Google Scholar
|
|
45
|
Patel VC, Lee S, McPhail MJW, Da Silva K,
Guilly S, Zamalloa A, Witherden E, Støy S, Manakkat Vijay GK, Pons
N, et al: Rifaximin-α reduces gut-derived inflammation and mucin
degradation in cirrhosis and encephalopathy: RIFSYS randomised
controlled trial. J Hepatol. 76:332–342. 2022. View Article : Google Scholar
|
|
46
|
Kitagawa R, Kon K, Uchiyama A, Arai K,
Yamashina S, Kuwahara-Arai K, Kirikae T, Ueno T and Ikejima K:
Rifaximin prevents ethanol-induced liver injury in obese
KK-Ay mice through modulation of small intestinal
microbiota signature. Am J Physiol Gastrointest Liver Physiol.
317:G707–G715. 2019. View Article : Google Scholar
|
|
47
|
Cellai L, Colosimo M, Marchi E, Venturini
AP and Zanolo G: Rifaximin (L/105), a new topical intestinal
antibiotic: Pharmacokinetic study after single oral administration
of 3H-rifaximin to rats. Chemioterapia. 3:373–377. 1984.PubMed/NCBI
|