Introduction
Cerebral ischemia is caused by blood vessel
blockage, resulting in reduced cerebral blood flow and brain
damage. The longer that the decrease in cerebral blood flow lasts,
the more severe is the brain damage that occurs (1). Reperfusion of blood flow in ischemic
areas can also cause brain tissue damage and associated dysfunction
and this process is termed cerebral ischemia-reperfusion injury
(CIRI) (2). CIRI is characterized
by high morbidity, high disability and high mortality rates
(3) and the timely restoration of
blood-flow reperfusion in the ischemic area is the primary
treatment method for this type of disease. However, the mechanism
of CIRI is complex and the aim of the present study was to gain
further insights into the pathogenesis of CIRI.
Phosphodiesterase (PDEs) are metal-dependent
phosphohydrolases that metabolize the second messenger cyclic
adenosine phosphate (cAMP) and cyclic guanosine phosphate (cGMP)
into their respective inactive 5′-phosphates (4). PDEs are subdivided into 11
structurally related, but functionally distinct families (PDE1-11)
based on different post-translational modifications and cyclic
nucleotide substrates (5). In
general, PDEs are involved in numerous physiological processes,
including cell proliferation and differentiation, gene expression,
inflammation, apoptosis and metabolism (6). The isozyme PDE2, as a member of the
PDE family, is mainly distributed in the central nervous system of
the human body and is highly expressed in the cerebral cortex and
hippocampus (7). Previous studies
have shown that PDE2 inhibitors are able to inhibit PDE2 protein
activity, thereby increasing the levels of cAMP and cGMP and
enhancing long-term synaptic transmission and ultimately improving
cognitive and memory functions in patients with Alzheimer's disease
and aging brain populations (8,9).
In addition, the PDE2 inhibitor Bay 60-7550 has been demonstrated
to ameliorate Aβ-induced cognitive and memory impairment in mice
through regulating the hypothalamic-pituitary-adrenal axis, or HPA
axis (10). Moreover, the novel
PDE2 inhibitor Hcyb1 is shown to produce neuroprotective and
antidepressant-like effects, probably mediated through the
cAMP/cGMP-CREB-BDNF signaling axis (11). However, it remains to be
elucidated whether PDE2 is able to inhibit neuroinflammation and
neuronal apoptosis during CIRI.
Therefore, the aim of the present study was to
explore the role of PDE2 in CIRI and the underlying mechanism(s)
through in vivo and in vitro experiments. The results
obtained provided a theoretical basis for the treatment of
CIRI.
Materials and methods
Establishment of the middle cerebral
artery occlusion (MCAO) model
Male C57BL/6J mice (n=15; 8 weeks old; 18-22 g)
provided by Department of Laboratory Animal Science, Peking
University Health Science Center were housed in an environment with
50±10% humidity, constant (25°C) temperature with regular 12-h
light/dark cycle and given ad libitum access to water and
food for 1 week prior to modeling. Mice were then randomly divided
into the control group, the MCAO group and the MCAO + Bay-607550
group; for the latter group, the mice were treated with Bay-607550
(cat. no. 439083-90-6; Merck KGaA), an inhibitor of PDE2. Each
group contained five mice. Mice in the MCAO + Bay-607550 group were
administered Bay-607550 (1 mg/kg/day) by gavage for 14 days.
Subsequently, all mice were anesthetized with sodium pentobarbital
(40 mg/kg, intraperitoneal injection) and in the MCAO and MCAO +
Bay-607550 groups, a midline incision in the neck was made to
expose the right common carotid artery along with the vagus nerve,
followed by blunt dissection of the internal carotid artery,
together with the external carotid artery. Focal cerebral ischemia
was then induced by the occlusion of the MCA with a nylon fishing
line (0.38 mm diameter) for 2 h. The filament was removed 1 h after
MCA occlusion and reperfusion was allowed to proceed for 24 h. Mice
in the control group underwent the same procedure, but without the
filament insertion procedure. After successful modeling, the mice
were sacrificed by cervical dislocation (mortality was confirmed
when the mice stopped breathing, their hearts stopped beating,
their muscles relaxed, their body temperature dropped and they did
not respond to external stimuli). All these animal experiments were
approved by the ethics committee of Wuxi Higher Health Vocational
Technology School and the NIH Guide for the Care and Use of
Laboratory Animals, 8th (grants.nih.gov/grants/olaw/Guide-for-the-Care-and-use-of-laboratory-animals.pdf)
was followed rigorously.
Reverse transcription-quantitative
(RT-q)PCR
Total RNA was extracted from samples which are
inoculated into 6-well plates at a density of 1×103
cells/well using TRIzol® (Thermo Fisher Scientific,
Inc.) from cells or brain tissues according to the manufacturer's
protocols. cDNA synthesis from 1 µg total RNA in a reaction
volume of 20 µl was performed using the miScript
SYBR® Green PCR kit (DBI Bioscience) according to the
manufacturer's protocols. The amplification was performed using
Real-time PCR Detection System (Mx3000P qPCR system; Agilent
Technologies, Inc.) according to the manufacture's protocol. Each
experiment was replicated three times. The relative RNA expression
levels were quantified using the 2−ΔΔCq method (12). GAPDH was used as the control RNA
species. The primer sequences used were as follows: mice: PDE2
forward, 5′-CAG ACT GGT GTG TGA GGA CC-3′ and reverse, 5′-ACG TGT
TCA TCC TCA TCT GTG A-3′; TNF-α forward, 5′-TCC TCA CCC ACA CCG TCA
G-3′ and reverse, 5′-GCT GAG TTG GTC CCC CTT C-3′; IL-1β forward,
5′-TTC TCG CAG CAG CAC ATC-3′ and reverse, 5′-CAG CAG GTT ATC ATC
ATC ATC C-3′; IL-6 forward, 5′-TCC ATC CAG TTG CCT TCT T-3′ and
reverse, 5′-TTT CTC ATT TCC ACG ATT TCC-3′; MCP-1(CCL2) forward,
5′-CTG TTC ACA GTT GCC GGC TG-3′ and reverse, 5′-AGC TTC TTT GGG
ACA CCT GCT-3′; Iba-1(AIF1) forward, 5′-TGA GGA GAT TTC AAC AGA AGC
TGA-3′ and reverse, 5′-CCT CAG ACG CTG GTT GTC TT-3′; and GAPDH
forward, 5′-CCC TTA AGA GGG ATG CTG CC-3′ and reverse, 5′-ACT GTG
CCG TTG AAT TTG CC-3′. Human: PDE2 forward, 5′-CAC CAC ATC CTC ATC
GCT GT-3′ and reverse, 5′-AGG CAT CCA GGT CGT AGA GT-3′; TNF-α
forward, 5′-CTG GGC AGG TCT ACT TTG GG-3′ and reverse, 5′-CTG GAG
GCC CCA GTT TGA AT-3′; IL-1β forward, 5′-AGC CAT GGC AGA AGT ACC
TG-3′ and reverse, 5′-TGA AGC CCT TGC TGT AGT GG-3′; IL-6 forward,
5′-GTC CAG TTG CCT TCT CCC TGG-3′ and reverse, 5′-CCC ATG CTA CAT
TTG CCG AAG-3′; MCP-1 forward, 5′-GAT CTC AGT GCA GAG GCT CG-3′ and
reverse, 5′-TTT GCT TGT CCA GGT GGT CC-3′; Iba-1 forward, 5′-CTC
CAG CTT GGA GGA AAA GC-3′ and reverse, 5′-TGG AGG GCA GAT CCT CAT
CA-3′; and GAPDH forward, 5′-AAT GGG CAG CCG TTA GGA AA-3′ and
reverse, 5′-GCG CCC AAT ACG ACC AAA TC-3′.
Western blot analysis
RIPA lysate (Thermo Fisher Scientific, Inc.) was
added to SH-SY5Y cells and brain tissues to extract the proteins,
following the manufacturer's protocol. A BCA protein assay kit
(Beyotime Institute of Biotechnology) was used to determine the
protein concentration. The proteins (20 µg per lane) were
separated using SDS-PAGE (10% gels) and subsequently transferred to
PVDF membranes (Bio-Rad Laboratories, Inc.) which were blocked by
5% non-fat milk for 2 h at room temperature. The membrane was
incubated with the corresponding primary antibody PDE2 (1:1,000;
cat. no. ab271673); Iba-1 (1:1,000; cat. no. ab178846); p-p65
(1:1,000; cat. no. ab76302); p65 (1:1,000; cat. no. ab76311);
p-IkB-α (1:1,000; cat. no. ab133462); IkB-α (1:1,000; cat. no.
ab32518); Bax (1:1,000; cat. no. ab32503); cleaved caspase3
(1:1,000; cat. no. ab214430); caspase3 (1:1,000; cat. no.
ab184787); cleaved PARP (1:1,000; cat. no. ab32064); PARP (1:1,000;
cat. no. ab191217); PKA (1:1,000; cat. no. ab32390) and GAPDH
(1:1,000; cat. no. ab8245) all from Abcam, overnight at 4°C. The
following day, the Goat Anti-Mouse IgG H&L (Alexa Fluor 647;
1:5,000; cat. no. ab150115, Abcam) was incubated with the membranes
at room temperature for 2 h. The protein bands were visualized
using chemiluminescence (ECL; Merck KGaA) assay and the blots were
semi-quantified using ImageJ software (version 146; National
Institutes of Health).
Hematoxylin and eosin (H&E)
staining
Brain tissues of mice were isolated and fixed in 10%
formalin (Beyotime Institute of Biotechnology) at room temperature
for 24 h. The tissues were dehydrated with 90% ethanol for 2 min
and embedded with paraffin for 5 min and then cut into
5-µm-thick sections for H&E staining.
Neurological score
Behavioral tests were performed at 3 days after
reperfusion. The five-point scale was as follows: 0, no
neurological deficits; 1, failure to extend the contralateral
forepaw fully; 2, circling to the ipsilateral side when held by the
tail; 3, falling to the contralateral side; and 4, did not walk
spontaneously and had a reduced level of consciousness. Higher
neurological deficit scores indicated more severe impairment of
motor injury.
Brain water (BW) content
The two hemispheres were evaluated on an electronic
balance for wet weight and subsequently dried in an oven for 24 h
at 100°C for determination of the dry weight. BW was calculated as
follows: BW = (wet weight-dry weight)/wet weight ×100%.
2,3,5-Triphenyltertrazolium chloride
(TTC) staining
The brain sections were incubated in 2% TTC
phosphate buffer (pH 7.4) for 30 min at 37°C and subsequently fixed
with 10% formaldehyde solution for 24 h to enhance the color
contrast. Cerebral sections were assessed for the observed levels
of ischemia and unstained areas were measured with ImageJ software
(Version 146; National Institutes of Health) to calculate the
percentage of grayish-white areas. The white parts represented
ischemic areas.
Immunofluorescence (IF) analysis
Brain slices (20 µm) were fixed with 4%
paraformaldehyde for 15 min at room temperature and then separately
permeabilized with 0.5% Triton X-100 in PBS for 10 min at room
temperature. After the slices had been blocked with 2% BSA for 2 h
at room temperature, they were stained with anti-IBA1 antibody
(1:200; cat. no. GT10312, Thermo Fisher Scientific, Inc.) for 1 h
at 37°C. Subsequently, brain slices were incubated with goat
anti-mouse secondary antibody (1 µg/ml; cat. no. A-11001,
Invitrogen; Thermo Fisher Scientific, Inc.) for 1 h at 37°C.
Finally, the brain slices were stained with 100 nM DAPI
(MilliporeSigma) for 15 min at room temperature and samples were
observed under a fluorescence microscope (Zeiss GmbH).
TUNEL analysis
A One Step TUNEL Apoptosis Assay kit (Beyotime
Institute of Biotechnology) was used to assess the extent of
apoptosis, according to the manufacturer's instructions.
Transfected cells were fixed using 4% paraformaldehyde for 1 h at
4°C and then 0.1% Triton X-100 was added at room temperature for 5
min. Cells were washed with PBS and incubated with TUNEL reagent
for 1 h at 37°C. Then, 50 µl DAB color development was
performed for 10 min at 15°C according to the manufacturer's
instructions. The cells were stained with DAPI for 10 min at room
temperature in the dark. Stained cells were observed under a glass
coverslip with PBS using a fluorescent microscope (magnification,
×200; Carl Zeiss AG). Subsequently, a fluorescence microscope
(Nikon Corporation) was used to capture images of the cells and to
count the TUNEL-positive cells. ImageJ software (Version 1.8.0;
National Institutes of Health) was used to count the total cells
and the TUNEL+ cells. The number of apoptotic cells was
calculated to be the average number of positive cells out of the
total number of cells in six fields of view per slide.
Cell culture
SH-SY5Y cells obtained from Cell Bank of the Chinese
Academy of Sciences were cultured in DMEM supplemented with 10% FBS
and 100 U/ml penicillin (all from Gibco; Thermo Fisher Scientific,
Inc.) at 37°C in an atmosphere of 5% CO2. STR profiling
was carried out in order to verify the authenticity of the SH-SY5Y
cell line.
Oxygen-glucose deprivation (OGD) and
reoxygenation (OGD/R)
SH-SY5Y cells were cultured in Hank's Balanced Salt
Solution (HBSS; Merck KGaA) and then incubated with a gaseous
mixture of 5% CO2/95% N2 for 60 min at 37°C.
The OGD treatment was stopped by replacing HBSS with 15% FBS. The
cells were then returned to normoxic conditions for 24 h for
re-oxygenation. Control culture cells were incubated with the HBSS
buffer supplemented with 15 mM glucose in a humidified incubator
under normoxic conditions for the same period of time as the OGD
cultures. The control cells group was treated the same as the OGD/R
group, with the exception that the culture gas was different. Cells
were pretreated with 1 µM Bay-607550 for 30 min.
Cell transfection
Small interfering (si) RNAs for PDE2 (siRNA-PDE2-1
and siRNA-PDE2-2) or siRNA negative control (siRNA-NC) were
synthesized by Guangzhou RiboBio Co., Ltd. and transfections into
the SH-SY5Y cells at 20 nM were performed with Lipofectamine
2000® reagent (Invitrogen; Thermo Fisher Scientific,
Inc.) at room temperature for 20 min. Cells were harvested at 48 h
after the transfection for further study. si-PDE2-1: sense 5′-AUA
UUG AAG GAC UUU GAG CUG-3′, antisense 5′-GCU CAA AGU CCU UCA AUA
UCU-3′; si-PDE2-2: sense 5′-UAU GAU ACA UCA UCA UCU CAU-3′,
antisense 5′-GAG AUG AUG AUG UAU CAU AUG-3′; si-NC: sense 5′-GAC
GUA AAC GGC CAC AAG UTC-3′, antisense 5′-ACU UGU GGC CGU UUA CGU
CGC-3′.
Statistical analysis
Data were analyzed with GraphPad Prism version 8.0
(GraphPad Software, Inc.) and expressed as the mean ± SD.
Comparisons among multiple groups were performed with one-way ANOVA
followed by a Tukey's post hoc test. The neurological scores were
presented as median and IQR, and statistically analyzed using
Kruskal-Wallis and Dunn's post hoc test. P<0.05 was considered
to indicate a statistically significant difference.
Results
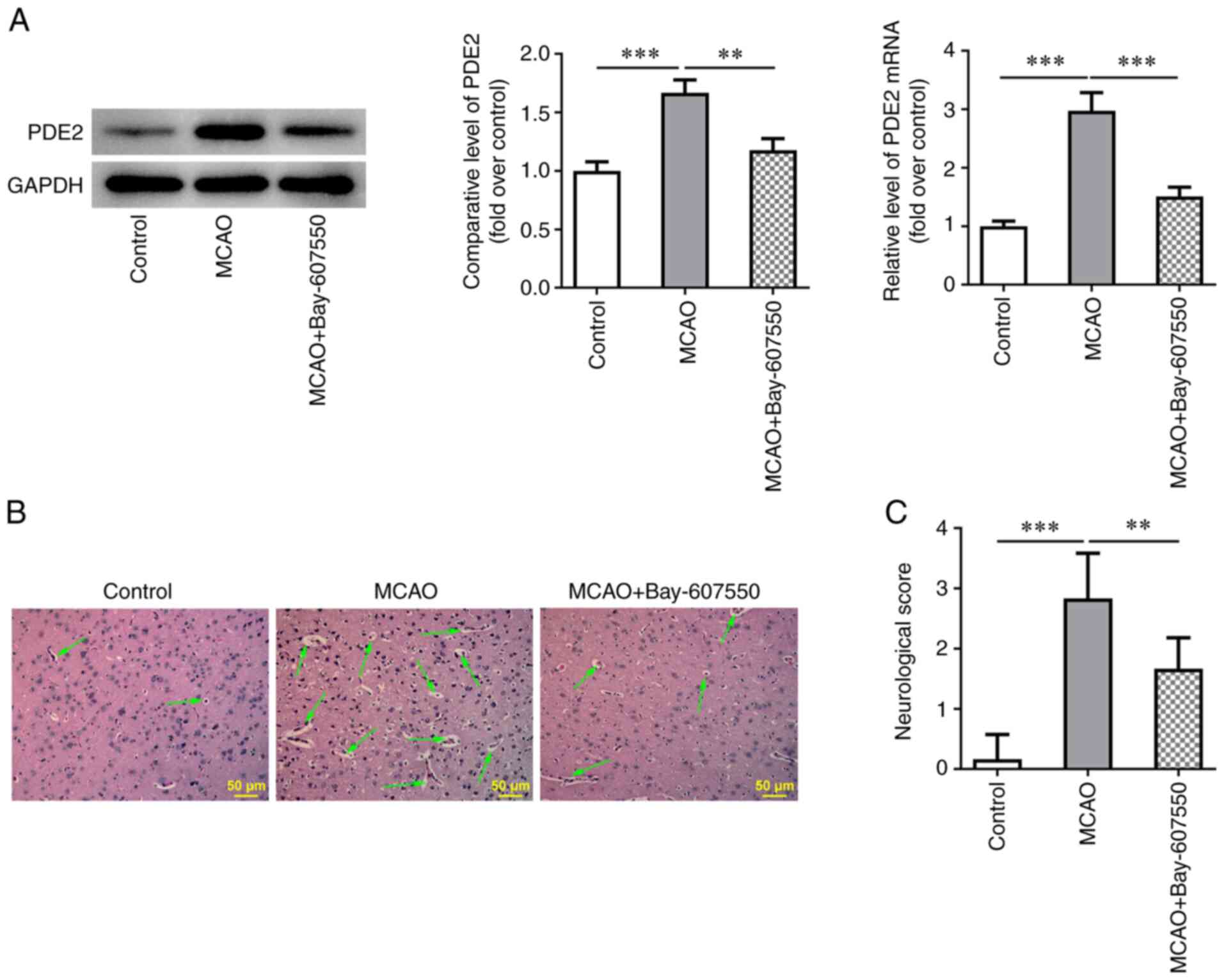
A PDE2 inhibitor, Bay-607550, reduces
histopathological damage in CIRI tissue
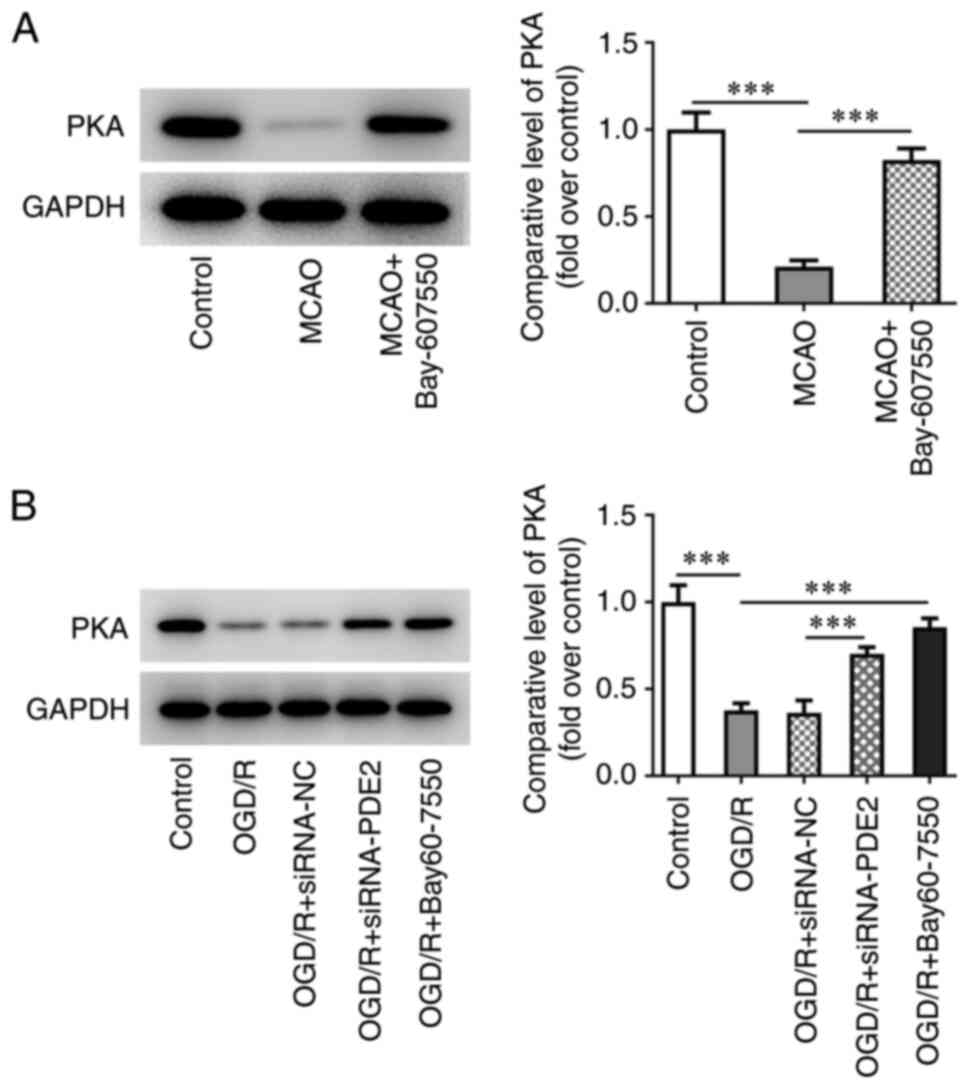
The expression of PDE2 in mouse brain tissues was
detected using RT-qPCR and western blot analysis. The results
obtained showed that the expression of PDE2 was significantly
increased in the MCAO group compared with the control group. By
contrast, the increases in PDE2 expression were reversed after
treatment with the PDE2 inhibitor, Bay-607550, compared with the
MACO group (Fig. 1A).
Histopathological damage was subsequently detected by H&E
staining. The results obtained showed that the histopathological
damage of mice in the MCAO group was severe. However, after the
MCAO-modeled mice were treated with Bay-607550, the
histopathological damage of brain tissue was found to be
significantly ameliorated (Fig.
1B). The neurological function of the mice was then scored and
this analysis revealed that the MCAO group experienced the highest
and most severe neurological injury scores compared with the
control group. However, compared with the MCAO group, the MCAO +
Bay-607550 group did not have such pronounced neurological injury
scores (Fig. 1C).
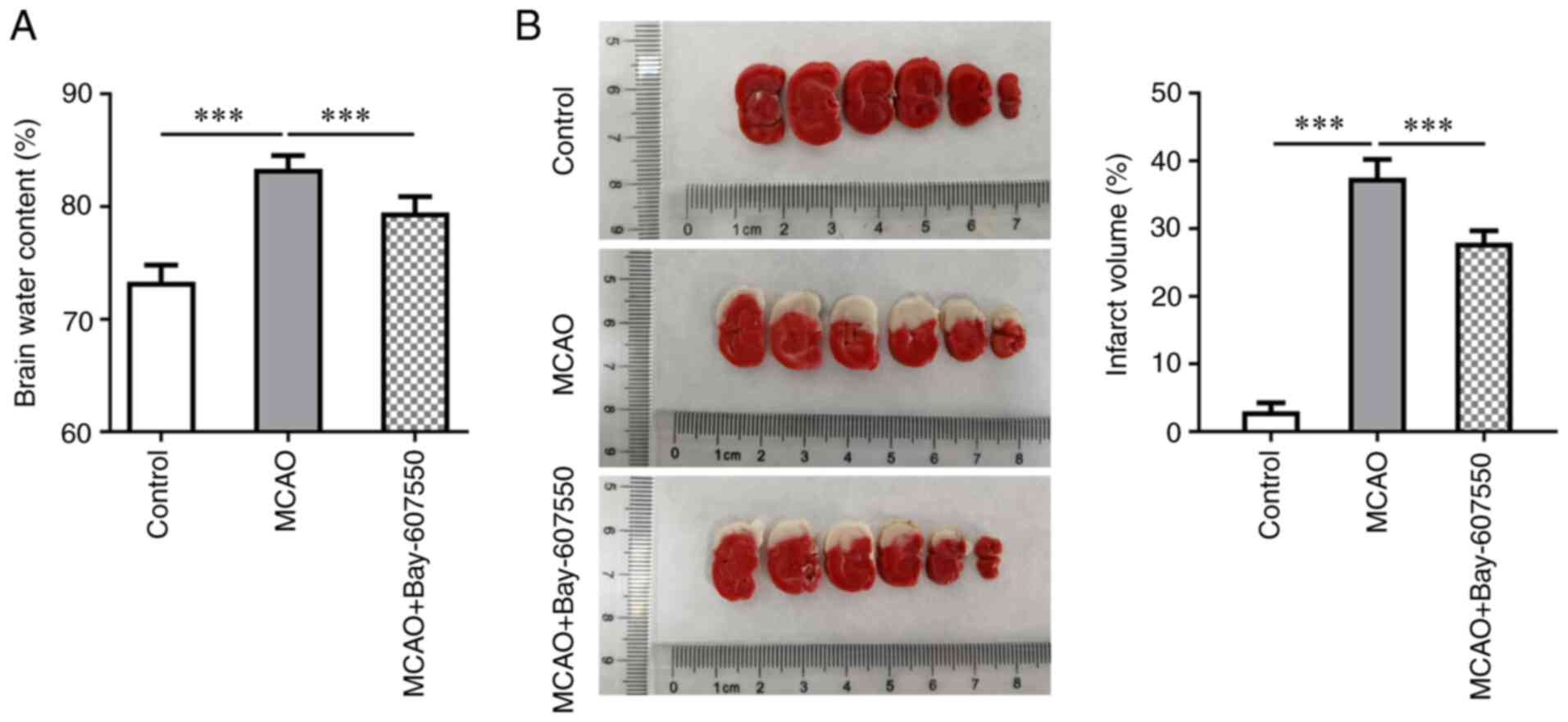
The PDE2 inhibitor Bay-607550 reduces
cerebral edema and ischemic area in CIRI mice
Subsequently, the dry weight/wet weight ratio of the
mice was measured in order to observe the cerebral edema of mice.
These results showed that cerebral edema in the MCAO group was more
severe compared with the control group. Furthermore, compared with
the MCAO group, cerebral edema was significantly improved in the
MCAO + Bay-607550 group (Fig.
2A). Subsequently, TTC staining was used to detect cerebral
ischemia. These results are shown in Fig. 2B; specifically, no clear cerebral
ischemia was identified in the control group mice. By contrast,
cerebral ischemia in the MCAO group was found to be more extensive
compared with that in the control group. However, following
administration of Bay-607550 to the model mice, the extent of
cerebral ischemic damage was found to be significantly ameliorated
(Fig. 2B).
The PDE2 inhibitor Bay-607550 reduces
inflammation and the activation of microglia in CIRI mice
Subsequently, RT-qPCR was used to detect the
expression of inflammatory factors in the ischemic site of mice.
These experiments showed that the expression levels of tumor
necrosis factor-α (TNF-α), interleukin (IL)-1β, IL-6 and monocyte
chemoattractant protein-1 (MCP-1) were significantly increased in
the MCAO model mice compared with the control group. Compared with
the MCAO group, the levels of TNF-α, IL-1β, IL-6 and MCP-1 were
markedly inhibited in the MCAO + Bay-607550 group (Fig. 3A). IF staining, western blotting
and RT-qPCR experiments were subsequently used to detect the
expression of ionized calcium binding adaptor molecule 1 (Iba-1), a
marker of microglia activation and these results showed that,
compared with the control group, the expression of Iba-1 in the
MCAO group was significantly increased. However, compared with the
MCAO group, the expression of Iba-1 was significantly inhibited
following Bay-607550 administration (Fig. 3B and C). Subsequently, western
blot analysis was used to detect the protein expression levels of
the inflammation-associated proteins P65, phosphorylated (p)-P65,
NF-κB inhibitor α (IkB-α) and p-IkB-α in cells. These experiments
showed that the trends in the alterations of the expression trends
of these proteins were consistent with those of the inflammatory
factors TNF-α, IL-1β, IL-6 and MCP-1 (Fig. 3D).
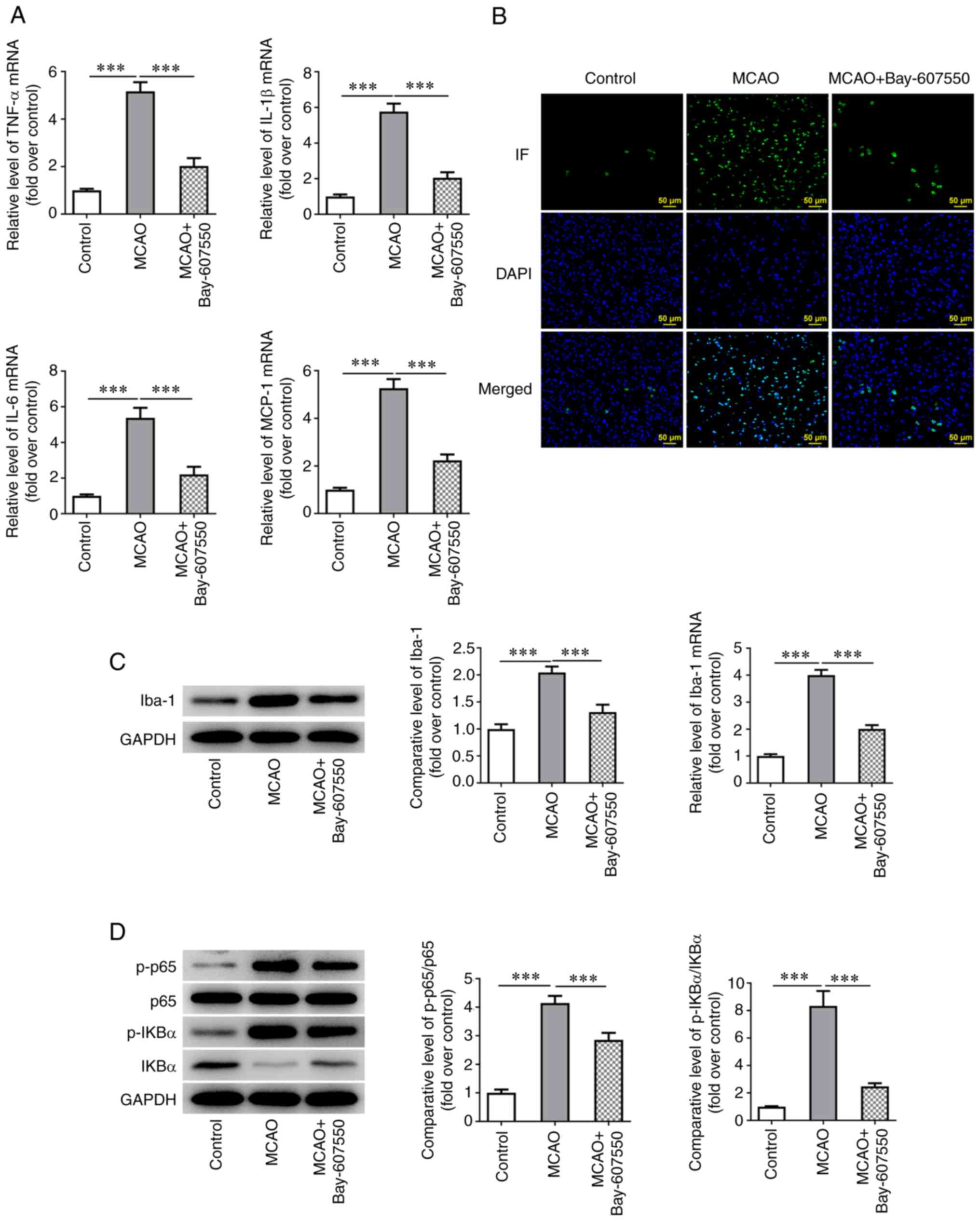 | Figure 3PDE2 inhibitor reduces inflammatory
and the activation of microglia in CIRI mice. (A) RT-qPCR detected
the expression of TNF-α, IL-1β, IL-6 and MCP-1. (B) The expression
of Iba was detected by IF staining. (C) The expression of Iba was
detected by western blotting and RT-qPCR. (D) Western blotting was
used to detect the expression of p-P65, p-IkB-α, P65 and IkB-α.
***P<0.001. PDE, phosphodiesterase; CIRI, cerebral
ischemia-reperfusion injury; RT-qPCR, reverse
transcription-quantitative PCR; TNF, tumor necrosis factor; IL,
interleukin; MCP, monocyte chemoattractant protein; Iba, ionized
calcium binding adaptor; IF, Immunofluorescence; p-,
phosphorylated; IkB-α, NF-κB inhibitor α. |
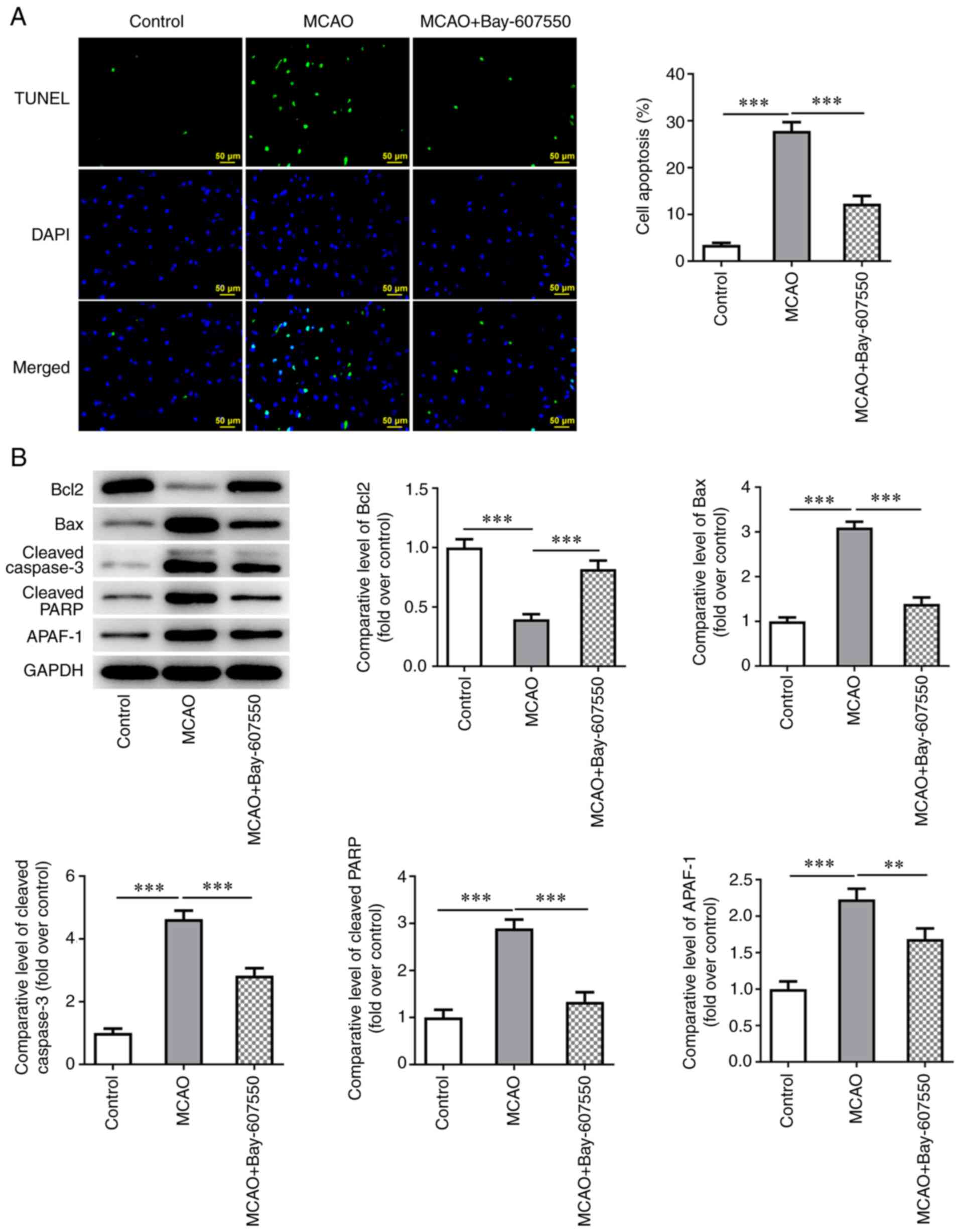
The PDE2 inhibitor Bay-607550 reduces the
apoptosis of nerve cells in CIRI mice
Finally, the effects of the PDE2 inhibitor
Bay-607550 were investigated in a series of in vivo animal
experiments, The extent of apoptosis was determined in the nerve
cells of the mouse brain tissue. The TUNEL assay and western
blotting results showed that, compared with the control group, the
level of apoptosis was significantly increased in the MCAO group,
as shown by the increased expression levels of Bax, cleaved
caspase-3 and cleaved poly (ADP-ribose) polymerase (PARP) and a
decrease in Bcl-2 expression. Compared with the MCAO group,
administration of Bay-607550 led to a significant reduction in the
levels of apoptosis observed in the nerve cells, accompanied by
decreased expression levels of Bax, cleaved caspase-3 and cleaved
PARP and an increase in Bcl-2 expression (Fig. 4A and B).
The PDE2 inhibitor Bay-607550 reduces
OGD/R-induced neuronal inflammation and apoptosis
In the cell experiments, SH-SY5Y neurons were
selected and the OGD/R model was established. As shown in Fig. 5A, the expression levels of PDE2
were increased in a time-dependent manner as the induction time of
OGD/R was increased (Fig. 5A).
Since the increases in PDE2 levels were the most marked when the
OGD/R model was induced for 24 h, this time point was selected for
subsequent experiments. After cell transfection was performed to
inhibit the expression levels of PDE2 in the cells, the inhibitory
effect of siRNA-PDE2-1, as detected by RT-qPCR and western blot
experiments, was found to be the most significant; therefore,
siRNA-PDE2-1 was selected as the interfering plasmid of choice for
the subsequent experiments (Fig.
5B). The cells were divided into control, OGD/R, OGD/R +
siRNA-NC, OGD/R + siRNA-PDE2-1 and OGD/R + Bay607550 groups. The
RT-qPCR and western blotting results showed that, compared with the
control group, the expression of TNF-α, IL-1β, IL-6, MCP-1 and
p-P65 and p-IKBα were significantly increased after OGD/R
induction. Compared with the OGD/R group, the expression levels of
TNF-α, IL-1β, IL-6, MCP-1 and p-P65 and p-IKBα were significantly
inhibited in the OGD/R + Bay-607550 group. Compared with the OGD/R
group at 24 h with siRNA-NC treatment, the expression levels of
TNF-α, IL-1β, IL-6, MCP-1 and p-P65 and p-IKBα were inhibited in
the OGD/R + siRNA-PDE2-1 group (Fig.
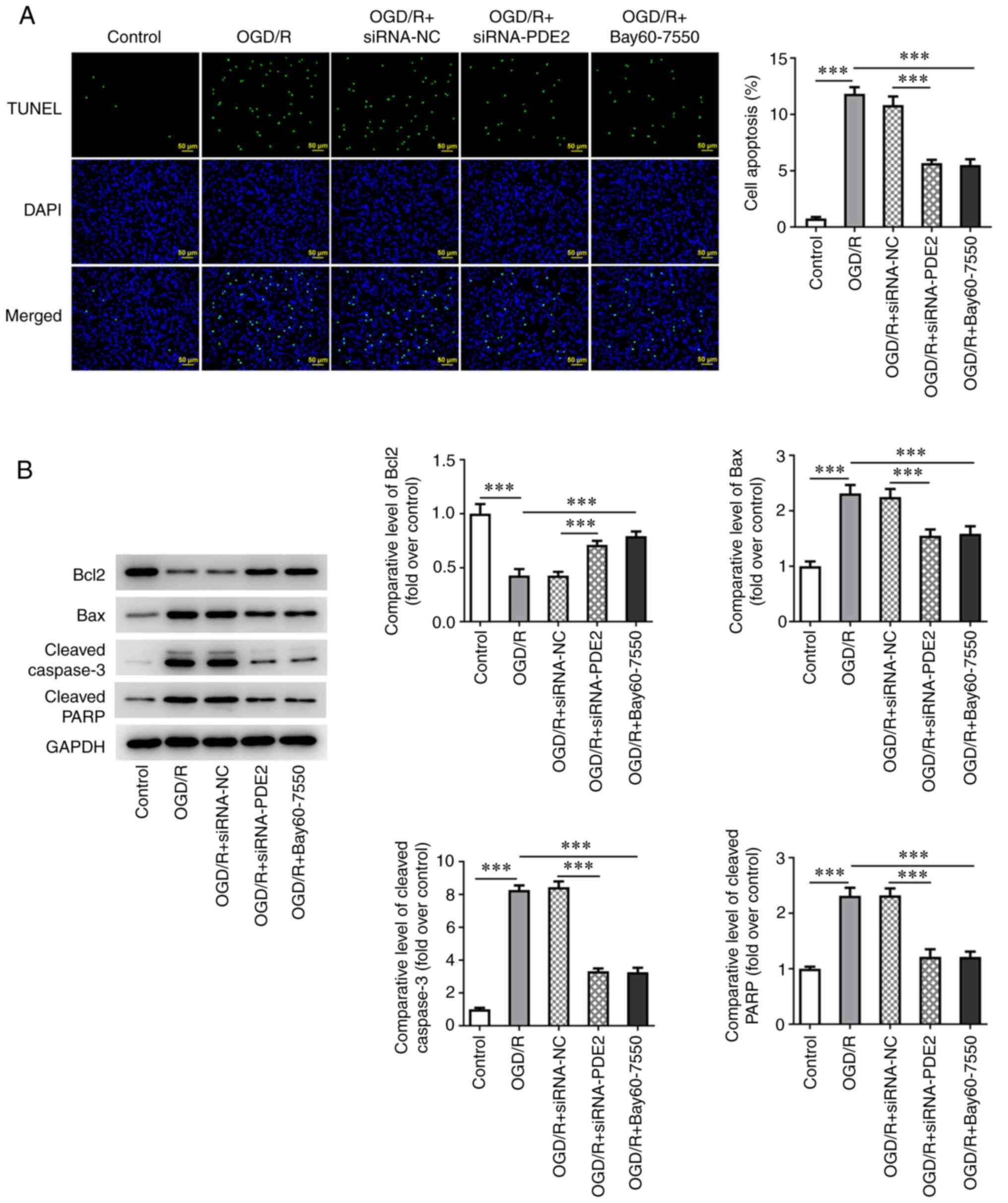
5C and D). Subsequently, the apoptotic levels of the cells were
detected and the results of the TUNEL assay and western blotting
experiments showed that, compared with the control group, the level
of apoptosis was significantly increased, as assessed from the
significant increases in expression of Bax, cleaved caspase-3 and
cleaved PARP in the OGD/R group and marked decrease in Bcl-2
expression. By contrast, compared with the OGD/R group, apoptosis
was inhibited, the expression levels of Bax, cleaved caspase-3 and
cleaved PARP were decreased and that of Bcl-2 was increased in
OGD/R-induced cells that were treated with Bay60-7550. Finally,
compared with the OGD/R + siRNA-NC group, the extent of apoptosis
was also inhibited in the OGD/R + siRNA-PDE2-1 group, which
exhibited decreased expression levels of cleaved caspase-3 and
cleaved PARP and an increased expression of Bcl-2 (Fig. 6A and B).
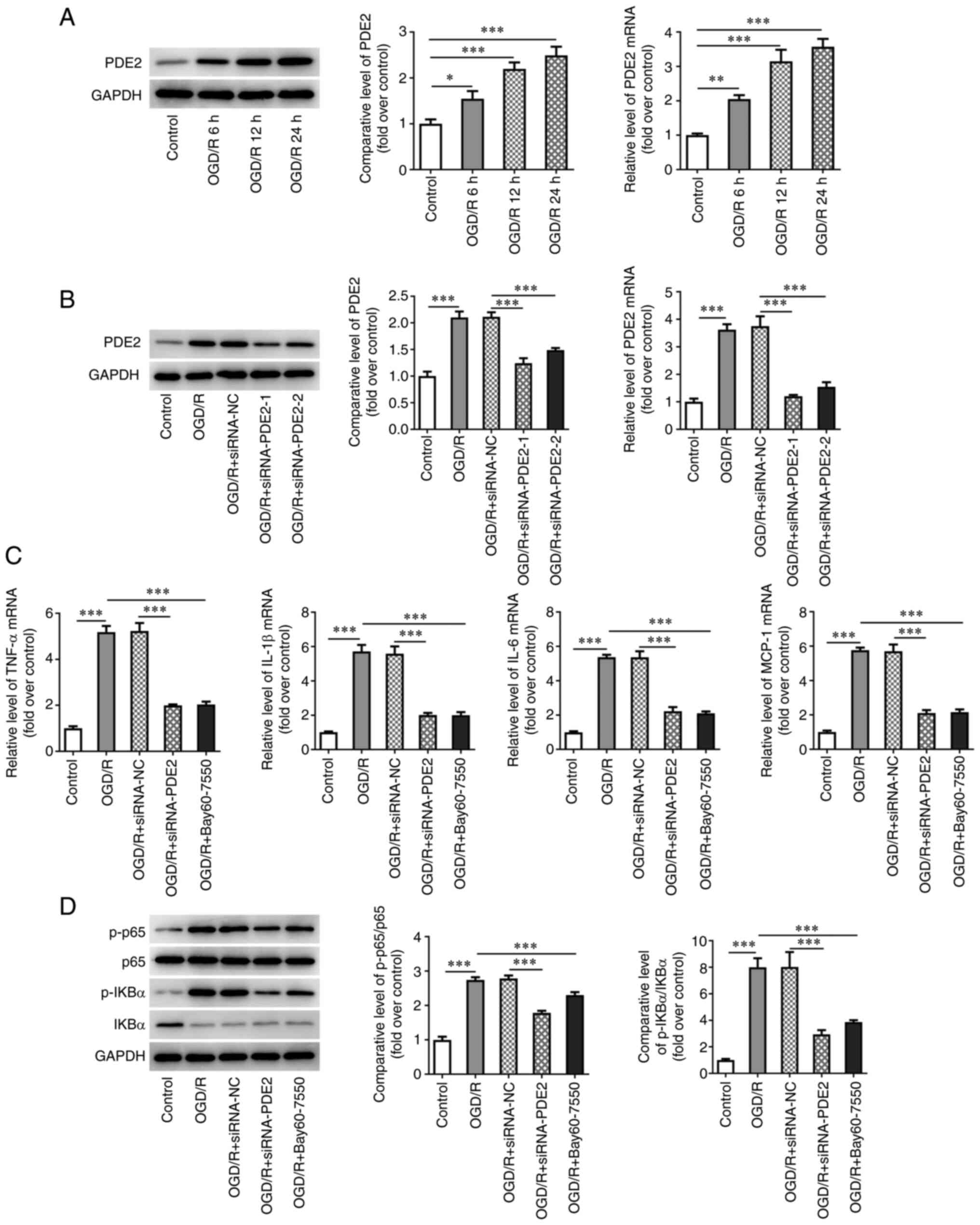 | Figure 5PDE2 inhibitor reduced OGD/R induced
neuronal inflammation. (A) Western blotting and RT-qPCR detected
the expression of PDE2 in OGD/R induced SH-SY5Y cells. (B) Western
blotting and RT-qPCR detected the expression of PDE2 after cell
transfection in OGD/R induced SH-SY5Y cells. (C) RT-qPCR detected
the expression of TNF-α, IL-1β, IL-6 and MCP-1. (D) Western
blotting was used to detect the expression of p-P65, p-IkB-α, P65
and IkB-α. *P<0.05, **P<0.01,
***P<0.001. PDE, phosphodiesterase; OGD/R,
oxygen-glucose deprivation and reoxygenation; RT-qPCR, reverse
transcription-quantitative PCR; TNF, tumor necrosis factor; IL,
interleukin; MCP, monocyte chemoattractant protein; Iba, ionized
calcium binding adaptor; IF, Immunofluorescence; p-,
phosphorylated; IkB-α, NF-κB inhibitor α. |
The PDE2 inhibitor Bay-607550 activates
the expression of protein kinase A (PKA) both in vivo and in vitro
in CIRI mice
In the animal experiments, it was found that,
compared with the control group, the expression of PKA in
ischemia-reperfusion tissues in the MCAO group was significantly
decreased. However, after further administration of Bay-607550, the
change in expression level of PKA was found to be reversed
(Fig. 7A). Subsequently, western
blot analysis was used to detect the expression of PKA in in
vitro experiments. The results of these experiments showed
that, compared with the control group, the expression of PKA in the
OGD/R group was significantly decreased, whereas the observed
change in PKA expression was reversed in the OGD/R-induced
Bay-607550 cells. Furthermore, the expression level of PKA in the
OGD/R + siRNA-PDE2 group was also significantly increased compared
with the OGD/R + siRNA-NC group (Fig.
7B).
Discussion
The pathophysiological changes associated with nerve
injury in CIRI have yet to be fully understood and are currently
irreversible; therefore, it is of great value to identify
therapeutic targets that could effectively protect patients from
the nerve injury that results from reperfusion. The present study
has exploited the fact that abnormal expression of PDE2 is known to
be closely associated with the occurrence and development of CIRI
(11).
Members of the PDE family are involved in numerous
physiological processes in organisms (13,14). A previously published study showed
that the PDE4 inhibitor Roflilast prevents ischemic stroke-induced
neuronal injury by inhibiting glycogen synthase kinase 3β-mediated
oxidative stress and the IRE1α/TRAF2/JNK pathway (15). PDE5 inhibitors inhibit microglia
activation and release of inflammatory factors during CIRI
(16). K-134 is a PDE3 inhibitor
that prevents brain injury via inhibiting thrombosis in a rat
cerebral infarction model (17).
In addition, PDE7 inhibitors ameliorate brain injury and
neuroinflammation during CIRI (18). However, to the to the best of the
authors' knowledge, the role of PDE2 in CIRI has yet to be studied
in any great depth. In addition, a previous study showed that,
following the change in phenotype of the microglia in neural
tissues, the expression of PDE2 in the cells change significantly
and this phenomenon serves an important role in brain function
(19). Sildenafil, a PDE2
inhibitor, inhibits nerve cell apoptosis and inflammation in
Alzheimer's disease (20).
Therefore, an investigation of the role of PDE2 in CIRI came to be
the focal point of the present study. The expression of PDE2 was
found to be significantly decreased in MCAO mice and OGD/R-induced
neurons. The PDE2 inhibitor Bay-607550 was shown to significantly
inhibit brain tissue damage in CIRI-modeled mice, inhibit microglia
activation and ameliorate the damaged caused by brain tissue
ischemia in mice. The present study only focused on the cerebral
ischemia area and did not make as much observation on the penumbra.
Special attention will be paid to penumbra in future experiments
related to cerebral ischemia.
Possible mechanisms of CIRI include, among other
possibilities, the inflammatory response, oxidative stress,
intra-cellular calcium overload, apoptosis and a decreased
secretion of neurotrophic factors (21). The early manifestations of CIRI
include free radical production, mitochondrial dysfunction,
enhanced inflammatory response and neuronal apoptosis, among which
neuroinflammation and neuronal apoptosis are the non-negligible
causes of CIRI (22). Therefore,
the present study focused on the specific role and influence of
PDE2 regulation on the inflammatory response and neuronal apoptosis
in CIRI disease. The PDE2 inhibitor Bay-607550 was found to inhibit
the inflammatory response and apoptosis of neurons in CIRI.
A previous study showed that PDE2 exerts a role
through PKA signaling, activating the expression of PKA after PDE2
inhibition (9). Activation of PKA
in the peri-ischemic area has an important neuroprotective role in
ischemic stroke models (23).
Activation of PKA is also shown to reduce neuronal inflammation and
apoptosis during CIRI (24).
Moreover, PKA can simultaneously affect NF-κB-mediated regulation
of inflammation and apoptosis (25). Therefore, a further goal of the
present study was to explore whether PDE2 inhibition could activate
the expression of PKA signaling protein. It was shown that the PDE2
inhibitor Bay60-7550 could significantly promote the expression of
PKA in a CIRI model. In addition, inhibition of PDE2 expression in
OGD/R-induced cells significantly promoted the expression of PKA.
Therefore, it was possible to tentatively conclude that PDE2 may
regulate the inflammatory response and apoptosis in CIRI through
regulating the expression of PKA2. It is aimed to further explore
and verify the underlying mechanism in future studies.
Collectively, results of the present study
demonstrated that inhibition of PDE2 could reduce inflammation and
apoptosis during CIRI through regulating PKA. These findings have
provided a basis both for understanding the underlying mechanism of
CIRI and for the development of targeted therapies for CIRI in the
clinic.
Availability of data and materials
The analyzed data sets generated during the present
study are available from the corresponding author on reasonable
request.
Authors' contributions
DW, JF and PW designed the study. DW, XW, JF, PW and
HX performed the experiments. JF and PW revised the manuscript for
important intellectual content. DW, XW and HX collected the
clinical data and analyzed the data. DW, XW and HX confirm the
authenticity of all the raw data. All authors have read and
approved the final manuscript.
Ethics approval and consent to
participate
All animal procedures were operated according with
the NIH Guide for the Care and Use of Laboratory Animals approved
by the ethical guidelines of Wuxi Higher Health Vocational
Technology School.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
Acknowledgments
Not applicable.
Funding
The present study was supported by the Qinglan Project of
Jiangsu Province in 2019.
References
|
1
|
Shin TH, Lee DY, Basith S, Manavalan B,
Paik MJ, Rybinnik I, Mouradian MM, Ahn JH and Lee G: Metabolome
changes in cerebral ischemia. Cells. 9:16302020. View Article : Google Scholar :
|
|
2
|
Wang H, Chen S, Zhang Y, Xu H and Sun H:
Electroacupuncture ameliorates neuronal injury by
Pink1/Parkin-mediated mitophagy clearance in cerebral
ischemia-reperfusion. Nitric Oxide. 91:23–34. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Wu MY, Yiang GT, Liao WT, Tsai AP, Cheng
YL, Cheng PW, Li CY and Li CJ: Current mechanistic concepts in
ischemia and reperfusion injury. Cell Physiol Biochem.
46:1650–1667. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Weber S, Zeller M, Guan K, Wunder F,
Wagner M and El-Armouche A: PDE2 at the crossway between cAMP and
cGMP signalling in the heart. Cell Signal. 38:76–84. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
5
|
He Y, Huang Y, Mai C, Pan H, Luo HB, Liu L
and Xie Y: The immunomodulatory role of PDEs inhibitors in immune
cells: Therapeutic implication in rheumatoid arthritis. Pharmacol
Res. 161:1051342020.2020. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Maurice DH, Ke H, Ahmad F, Wang Y, Chung J
and Manganiello VC: Advances in targeting cyclic nucleotide
phosphodiesterases. Nat Rev Drug Discov. 13:290–314. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Sadek MS, Cachorro E, El-Armouche A and
Kämmerer S: Therapeutic implications for PDE2 and cGMP/cAMP
mediated crosstalk in cardiovascular diseases. Int J Mol Sci.
21:74622020. View Article : Google Scholar :
|
|
8
|
Zhou Y, Li J, Yuan H, Su R, Huang Y, Huang
Y, Li Z, Wu Y, Luo H, Zhang C and Huang L: Design, synthesis, and
evaluation of dihydropyranopyrazole derivatives as novel PDE2
inhibitors for the treatment of Alzheimer's disease. Molecules.
26:30342021. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Chen L, Liu K, Wang Y, Liu N, Yao M, Hu J,
Wang G, Sun Y and Pan J: Phosphodiesterase-2 inhibitor reverses
post-traumatic stress induced fear memory deficits and behavioral
changes via cAMP/cGMP pathway. Eur J Pharmacol. 891:1737682021.
View Article : Google Scholar
|
|
10
|
Ruan L, Du K, Tao M, Shan C, Ye R, Tang Y,
Pan H, Lv J, Zhang M and Pan J: Phosphodiesterase-2 inhibitor bay
60-7550 ameliorates abeta-induced cognitive and memory impairment
via regulation of the HPA axis. Front Cell Neurosci. 13:4322019.
View Article : Google Scholar
|
|
11
|
Soares LM, Meyer E, Milani H, Steinbusch
HW, Prickaerts J and de Oliveira RM: The phosphodiesterase type 2
inhibitor BAY 60-7550 reverses functional impairments induced by
brain ischemia by decreasing hippocampal neurodegeneration and
enhancing hippocampal neuronal plasticity. Eur J Neurosci.
45:510–520. 2017. View Article : Google Scholar
|
|
12
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(-Delta Delta C(T)) method. Methods. 25:402–408. 2001.
View Article : Google Scholar
|
|
13
|
Kokkonen K and Kass DA: Nanodomain
regulation of cardiac cyclic nucleotide signaling by
phosphodiesterases. Annu Rev Pharmacol Toxicol. 57:455–479. 2017.
View Article : Google Scholar
|
|
14
|
Francis SH, Blount MA and Corbin JD:
Mammalian cyclic nucleotide phosphodiesterases: Molecular
mechanisms and physiological functions. Physiol Rev. 91:651–690.
2011. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Xu B, Xu J, Cai N, Li M, Liu L, Qin Y, Li
X and Wang H: Roflumilast prevents ischemic stroke-induced neuronal
damage by restricting GSK3beta-mediated oxidative stress and
IRE1alpha/TRAF2/JNK pathway. Free Radic Biol Med. 163:281–296.
2021. View Article : Google Scholar
|
|
16
|
Moretti R, Leger PL, Besson VC, Csaba Z,
Pansiot J, Di Criscio L, Gentili A, Titomanlio L, Bonnin P, Baud O
and Charriaut-Marlangue C: Sildenafil, a cyclic GMP
phosphodiesterase inhibitor, induces microglial modulation after
focal ischemia in the neonatal mouse brain. J Neuroinflammation.
13:952016. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Yoshida H, Ashikawa Y, Itoh S, Nakagawa T,
Asanuma A, Tanabe S, Inoue Y and Hidaka H: K-134, a
phosphodiesterase 3 inhibitor, prevents brain damage by inhibiting
thrombus formation in a rat cerebral infarction model. PLoS One.
7:e464322012. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Redondo M, Zarruk JG, Ceballos P, Pérez
DI, Pérez C, Perez-Castillo A, Moro MA, Brea J, Val C, Cadavid MI,
et al: Neuroprotective efficacy of quinazoline type
phosphodiesterase 7 inhibitors in cellular cultures and
experimental stroke model. Eur J Med Chem. 47:175–185. 2012.
View Article : Google Scholar
|
|
19
|
Bender AT and Beavo JA: Specific localized
expression of cGMP PDEs in Purkinje neurons and macrophages.
Neurochem Int. 45:853–857. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Sanders O: Sildenafil for the treatment of
Alzheimer's disease: A systematic review. J Alzheimers Dis Rep.
4:91–106. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Orellana-Urzua S, Rojas I, Libano L and
Rodrigo R: Pathophysiology of ischemic stroke: Role of oxidative
stress. Curr Pharm Des. 26:4246–4260. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Polderman KH: Mechanisms of action,
physiological effects, and complications of hypothermia. Crit Care
Med. 37:S186–S202. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Li H, Wang J, Wang P, Rao Y and Chen L:
Resveratrol reverses the synaptic plasticity deficits in a chronic
cerebral hypoperfusion rat model. J Stroke Cerebrovasc Dis.
25:122–128. 2016. View Article : Google Scholar
|
|
24
|
Liu Y, Zhang J, Zan J, Zhang F, Liu G and
Wu A: Lidocaine improves cerebral ischemia-reperfusion injury in
rats through cAMP/PKA signaling pathway. Exp Ther Med. 20:495–499.
2020. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Hu X, Li S, Doycheva DM, Huang L, Lenahan
C, Liu R, Huang J, Gao L, Tang J, Zuo G and Zhang JH: Rh-CSF1
attenuates oxidative stress and neuronal apoptosis via the
CSF1R/PLCG2/PKA/UCP2 signaling pathway in a rat model of neonatal
HIE. Oxid Med Cell Longev. 2020:68015872020. View Article : Google Scholar : PubMed/NCBI
|