Introduction
Acquired immunodeficiency syndrome (AIDS)-associated
malignancies are a major cause of co-morbidity among patients with
AIDS worldwide. Kaposi's sarcoma-associated herpes virus
(KSHV)/human herpesvirus 8 (HHV-8) is an important herpes viral
agent associated with viral malignancy, related to HIV-AIDS
(1,2). In the case of KSHV, both the latency
and lytic phases play critical roles in the process of oncogenesis,
as with other viral malignancies. In a KSHV-associated malignant
environment, the virus maintains a delicate spon-taneous balance
between these two phases. Depending on the need for the maintenance
of the oncogenic process, the virus can switch between the two
phases and maintain a persistent infection inside the host in
response to varied stress exposures by controlling several cellular
events (3,4). Several factors influence this
lytic-latency balance in a KSHV-infected tumor microenvironment.
Reactive oxygen species (ROS) have been found as the key
determining factor for maintaining the balance between the two
phases of the KSHV life cycle at different stages of KSHV
infection. In response to varying levels of intracellular ROS, KSHV
can modulate several cellular ROS regulatory pathways required for
malignancy (5). The virus
strategically maintains a moderate ROS level during latency,
allowing unregulated cell proliferation. While there is a slight
increase in the ROS level, the virion senses it and converts it
towards lytic reactivation. This avoids the maintenance of a
stressed cell; the virus utilizes the stress influence to secure
its own persistence. The replicative cells again utilize the high
ROS level and infect new healthy cells (6,7).
KSHV has been found to overread the cellular mechanisms under
hypoxic conditions and perform hypoxia-induced viral reactivation.
During this process, viral and cellular replication machineries are
strategically protected from KSHV-encoded latency-associated
nuclear antigen (LANA) cellular degradation. This interactive
understanding of the hypoxic stress response in virus-associated
oncogenesis clearly reveals a continuous oncogenic stress
management system, which is maintained inside the KSHV-infected
cell for its pathogenesis (8,9).
Due to this viral stress responsive strategy, a
number of potent anticancer drugs are not applicable against
KSHV-associated malignancies. A number of cytotoxic drugs are
capable of inducing significant cell death in KSHV malignant cells;
however, in parallel, they can reactivate the virus by moderating
the increase in cellular ROS levels (10). Alternatively, cytotoxic drugs can
induce the cell death process, triggering oxidative stress, which
exerts a toxic effect on the normal cellular environment.
Therefore, the long-term chemotherapeutic application of such drugs
results in severe side-effects in the case of several malignancies,
including KSHV-associated ones (11,12). Thus, potential targeted
therapeutic strategies need to be developed against the 'cancer
cell oxidative stress balancing system', which can effectively
break the ROS homeostasis inside the cancer cell, along with a
minimal effect of chemotherapeutic stress on the body.
Antioxidants have been reported as oxidative stress
balancers, which can function both as pro- and antioxidant agents
and are widely applied in anticancer therapeutics (13). On this note, some naturally
derived bioactive compounds are well reported as ROS scavengers.
However, in the majority of cases, it has been observed that either
these compounds are used as adjuvants or are supplemented along
with chemotherapy (14,15). Due to a lack of knowledge about
their stress balance mechanisms in an oncogenic environment with
different ROS levels during cancer and chemotherapy, these natural
antioxidants are very limited as regards their clinical
applicability. The application of ROS scavengers is dependent on
the status of ROS in cancer cells and the malignant environment, as
they can be associated with cancer progression and cancer
inhibition (13). KSHV-infected
malignant cells have a flexible stress management system of the
virus, which maintains a lytic-latency balance for a continuous
oncogenic process, and may be an ideal model which may be used to
understand the role of ROS scavengers as anticancer compounds. The
present study aimed to determine the mechanisms through which two
very common flavonoid-based antioxidant compounds from natural
sources with an effective ROS scavenging capacity can respond
against the critical KSHV oncogenic stress balance strategy, and
lead to a potential anticancer therapeutic effect.
Materials and methods
Cell lines and cell culture
Two KSHV-infected malignant cell lines (JSC-1 and
BC-3) and a non-KSHV cell line (293T) were used in the present
study; all cell lines were kindly provided by Dr Erle Robertson
from University of Pennsylvania (Philadelphia, PA, USA) and Dr
David Blackbourn, from the University of Surrey (Guildford, UK).
The non-KSHV control cell line, BJAB, was collected from Dr Avhik
Saha (Presidency University, Kolkata, India) and Dr Avra Roy
(Calcutta University, Kolkata, India). The cells were grown and
maintained in complete RPMI-1640 medium (Gibco; Thermo Fisher
Scientific, Inc.) supplemented with 10% FBS and 1%
penicillin/streptomycin in 5% CO2 at 37°C in a
humidified atmosphere. Contamination-free cell lines at passage
number 3-10 after thawing were used in the experiments.
Peripheral blood mononuclear cell (PBMC)
isolation and processing
PBMCs were isolated using Histopaque (RNBB8622;
MilliporeSigma) from blood collected from healthy individuals. The
selected healthy individuals were had an average age of 33 years,
had a proper height-to-weight ratio (a standard/ideal weight
according to an individual's height) and the following
clinicopathological characteristics: Hematological parameters
(range): Red blood cells (mill/cmm), ~3.9; white blood cells (Cmm),
~6,500; hemoglobin (g/dl), ~12.9; platelets (Lakh/cmm), ~2.88;
neutrophils (%), ~60.29; lymphocytes (%), ~32.83; eosinophils (%),
~4; monocytes (%), ~3.36; basophils (%), 0. Liver parameters
(range): Cholesterol, ~128.6; aspartate aminotransferase (U/l),
~25; alanine aminotransferase (U/l), ~17; albumin(g/dl), ~4.53.
Renal parameters (range): Creatinine, ~0.66; total protein (g/dl)
~7.2; blood glucose (mg/dl), ~98. The PBMCs were maintained in
complete RPMI-1640 medium for 24 h. Both the treatment and
non-treatment conditions used for KSHV-positive cell lines were
maintained for these PBMCs. During FACS analysis, the lymphocyte
population was gated from whole PBMCs by the bivariate FSC vs. SSC
dot plot. The gated population was used for flowcytometry-based
cell death analysis.
Chemical compounds
For the present study, two flavonoid-based naturally
derived compounds were selected. Theaflavin was purchased from
MilliporeSigma (tea extract from Camellia sinensis, T5550,
contains ≥80% theaflavins) and a stock solution of 5 mg/ml was
prepared by dissolving it into 50% EtOH in distilled water. The
other group of flavonoids, the novel curcumin derivatives (the Cl
and F derivatives) were used and were synthesized from mother
curcumin (C1386; MilliporeSigma). A stock solution (5 mg/ml each)
was prepared in a similar manner by 50% EtOH in a distilled water
solution for the following experimental purposes. The detailed
synthesis process and structure of a series of curcumin derivatives
has been previously described (16); the two compounds,
2-Amino-4-(4-chlorophenyl)-5-((E)-3-(4-hydroxy-3-methoxyphenyl)acryloyl)-6-((E)-4-hydroxy-3-methoxystyryl)-4H-pyran-3-carbonitrile
(Cl derivative) and
2-Amino-4-(4-fluorophenyl)-5-((E)-3-(4-hydroxy-3-methoxyphenyl)acryloyl)-6-((E)-4-hydroxy-3-methoxystyryl)-4H-pyran-3-carbonitrile
(F derivative) were selected from this series for the present
study.
Establishment of stress induced in vitro
cell line model
The KSHV-associated malignancy cycle is completely
dependent on a fine balance between the maintenance of the viral
latency and lytic phases inside the cell (17,18). This fine biphasic balance
maintenance is stress responsive. A transient serum stress in
vitro model was thus utilized in different experiments to
analyze this varied stress response in a natural manner resembling
the oncogenic stress management system during KSHV malignancy. For
the present study, the latently infected KSHV cells (B lymphocytes)
were grown in serum-free (without FBS) RPMI-1640 medium for 24 h.
The surviving cells were revived by growing in complete RPMI-1640
(+10% FBS) medium for the following 24 h. These stressed and
recovered KSHV cells (as an in vitro model) were used in
further experiments with all three different drug treatments at
their respective concentrations. A similar cell line-based stress
induced model for viral malignancy was previously used in
HIV-associated cancers (19). It
has been observed that alterations in serum levels in the growth
medium affects various virus-associated factors, such as
infectivity, replication etc., in virus-infected cell lines
(20,21). Thus, the stress-altered cell line
model may be a useful model which may be used to understand the
stress responsive behavior of KSHV-infected oncogenic cells and to
examine any alteration(s) with antioxidant treatments.
Cell viability assay
Different KSHV-infected cell lines in treated and
non-treated conditions [use of antioxidants at various
concentrations (10, 20, 30, 40 and 50 µg/ml) and time
durations (6, 12 and 24 h) were subjected to cell viability assay.
Cells (1×106) in each set treated and incubated (37°C in
5% CO2) for this analysis. These cells were then washed
with PBS and incubated with 50 µg/ml propidium iodide (PI)
at room temperature for 15 min. Following treatment, the cells were
analyzed using a Bio-Rad ZOE fluorescent cell imager (by air drying
the treated suspension cells over slides) red channel (excitation,
556/20 nm; emission, 615/61 nm; Bio-Rad Laboratories, Inc.) and a
flow cytometer (BD FACSVerse; BD Biosciences) in a specific PI
channel. The percentage of PI-positive cells was measured with the
loss of membrane integrity and viability compared to untreated
(EtOH-treated) control cells using BD FACSuite™ software (version
1.0.6; BD Biosciences).
Annexin V-FITC apoptosis detection using
flow cytometry
KSHV cells from different treatment sets and control
cells were incubated (37°C in 5% CO2) for 24 h with
their effective low concentration and a comparatively high
effective concentration (low concentrations: Theaflavin, 30
µg/ml; curcumin derivatives, 10 µg/ml; high
concentrations: Theaflavin, 50 µg/ml; curcumin derivatives,
30 µg/ml). Following incubation, they were washed with PBS
and subsequently prepared for Annexin V-PI staining following the
manufacturer's protocol (FITC Annexin V apoptosis detection kit,
51-6710AK, BD Biosciences). The cells were then analyzed using a BD
FACSVerse flow cytometer and data were analyzed using BD FACSuite™
software (version 1.0.6) by the FITC vs. the PI densitometry plot.
To examine specific the caspase-mediated cell death process, the
caspase inhibitor, Z-VAD-FMK (50377, BD Biosciences), was used
along with the different treatments.
ROS determination
KSHV-infected and non-KSHV cells (in both the latent
condition and exposed to serum stress) were seeded with or without
various concentrations doses of the drugs (the three compounds) and
the FDA-approved antioxidant, N-acetyl cysteine (NAC;
MilliporeSigma) for different periods of time. Subsequently,
following incubation (37°C for 6, 12 and 24 h), the cells were
washed in PBS and resuspended in serum-free RPMI-1640 medium with
50 µM 2′,7′-dichlorofluoresin diacetate (DCFH-DA; D6883;
MilliporeSigma) for 30 min at 37°C in the dark. Following
incubation, the fluorescence intensity of DCFH-DA was determined
using a flow cytometer (BD FACSVerse; BD Biosciences) at 488 nm
excitation and 530 nm emission (FITC channel).
Cell cycle detection
To analyze the different cell cycle stages, the
treated and non-treated KSHV (latent, serum starved and revived)
cells were incubated (24 h at 37°C in 5% CO2) with or
without the respective drugs. They were then washed with PBS and
fixed with chilled 70% EtOH on ice for 15-30 min. Following
fixation, the cells were washed with PBS again. The cells were then
stained with 10 µg/ml PI in Triton X-100 (RM845; HiMedia)
buffer and RNase (DS0003; HiMedia) for 30 min at room temperature
and examined using a BD FACSVerse flow cytometer. The DNA contents
at different phases of the cell cycle were assessed using BD
FACSuite™ software (version 1.0.6).
Mitochondrial membrane potential
analysis
Latent, serum stress-exposed cells were subjected to
the various treatment sets for different periods of time followed
by analyses using a flow cytometry-based mitochondrial membrane
potential detection kit (BD Mito Screen, JC-1 kit, 55132; BD
Biosciences). The cells were washed with PBS and then processed for
JC-1 staining following the manufacturer's protocol. The cells were
then examined using a BD FACSVerse flow cytometer and analyzed
using BD FACSuite™ software (version 1.0).
Autophagy detection
Treated and untreated cells were incubated for
different periods of time (12 and 24 h) with theaflavin, Cl and F
curcumin at their respective concentrations and stained with
monodansylcadaverine (MDC; BCBX0277; MilliporeSigma) at 50
µM for 30 min and the fluorescence intensity was measured
using a Bio-Rad ZOE fluorescent cell imager (by air drying the
treated suspension cells over slides) in the blue channel
(excitation, 355/40 nm; emission, 433/36 nm) and a flow cytometer
(BD FACSVerse) at a wavelength of 488 nm. The autophagy inhibitor,
bafilomycin-A1 (B1793; MilliporeSigma; 20 nM working concentration)
was used to mark the specific autophagic process inside the cell,
following different treatment conditions in stress-exposed and
revived cells.
Western blot analysis
Cells were lysed using RIPA buffer containing
protease inhibitor cocktail (P8340, Millipore Sigma) and following
centrifugation (14,000 × g for 15 min at 4°C), the supernatant
containing protein samples was collected. The protein concentration
was determined using a Lowry protein assay kit (23240, Thermo
Fisher Scientific, Inc.). An equal amount of protein lysate from
each sample (~20 µg) for each lane was loaded and subjected
for separation by SDS-PAGE electrophoresis. Following transfer to a
nitrocellulose membrane, the membrane was blocked (using 5% skim
milk at room temperature for 1 h) and probed with specific primary
antibodies (overnight incubation at 4°C) followed by HRP-tagged
secondary antibody (1 h of incubation), at room temperature.
Following proper incubation, the membrane was visualized using a
Chemidoc XRS+ image system (Bio-Rad Laboratories, Inc.) using
Bio-Rad ECL chemiluminescent substrate and captured using Image Lab
software (version 5.2). The intensity of the protein band was
analyzed and normalized to the loading control (mouse mAb GAPDH;
1:1,000, cat. no. sc-166545, Santa Cruz Biotechnology, Inc.) and
finally with the untreated control using ImageJ software (version
1.8.0, National Institutes of Health). Primary antibodies against
rabbit mAb BAX (cat. no. 2772T), rabbit mAb Bcl-2 (cat. no. 4223T),
mouse mAB p53 (cat. no. 2524S) (all from Cell Signaling Technology,
Inc.) were used at a dilution of 1:1,000. Goat anti-S6 K
(S6 kinase) antibody was obtained from Boster Biological
Technology (cat. no. A0147, 1:500 dilution). Rabbit anti-Beclin1
(6176, IMGENEX India Pvt Ltd.), mouse anti-p21 (80191; IMGENEX
India Pvt Ltd.) were used at a dilution of 1:1,000. Mouse mAb
anti-checkpoint kinase (Chk)-1 (sc-8408) and mouse mAb anti-Chk-2
(sc-5278) were obtained from Santa Cruz Biotechnology, Inc.
(1:1,000 dilution). 12% SDS PAGE was used for identifying these
proteins. For the detection of the autophagy representative marker
protein, LCIIIB, rabbit pAb anti LCIIIB was used (cat. no. NB100
2220; Novus Biologicals, Ltd.) at a dilution of 1:500 with 10% SDS
PAGE. Anti-mouse (cat. no. 11-301; Abgenex); anti-rabbit (cat. no.
AS014; ABclonal Biotech Co., Ltd.); anti-goat (cat. no. 20401;
Imgenex) HRP-tagged secondary antibodies were used at a 1:10,000
dilution.
Reverse transcription-quantitative PCR
(RT-qPCR)
Total RNA from different KSHV-associated cell lines
with various treatment and experimental conditions were isolated
using the TRIzol-based method (TRI reagent; T9424; MilliporeSigma).
cDNA was synthesized from isolated cellular RNA using the reverse
transcription method according to the manufacturer′s protocol
(Bio-Rad iScript cDNA Synthesis kit; cat. no. 170-8891; Bio-Rad
Laboratories, Inc.). The cDNA was then subjected to amplification
using specific primers (LANA forward, 5′-CAT ACG AAC TCC AGG TCT
GTG-3′ and reverse, 5′-GGT GGA AGA GCC CAT AAT CT-3′; vFLIP K13
forward, 5′-GGA TGC CCT AAT GTC AT GC-3′ and reverse, 5′-GGC GAT
AGT GTT GGA GTG T-3′; replication and transcription activator (RTA)
forward, 5′-CAG ACG GTG TCA GTC AAG GC-3′ and reverse, 5′-ACA TGA
CGT CAG GAA AGA GC-3′; and GAPDH forward, 5′-CCA CAT CGC TGA GAC
ACC AT-3′ and reverse, 5′-TTC CCG TTC TCA GCC TTG AC-3′) used in a
previous study, purchased from Integrated DNA Technologies
(22,23). qPCR was performed done using
SYBR-Green Supermix (Bio-Rad iTaq Universal SYBR-Green Supermix,
cat. no. 172-5124, Bio-Rad Laboratories, Inc.) on a Bio-Rad CFX
connect Real-Time PCR System. Thermal cycling has been performed
with an initial denaturation at 95°C for 3 min, following 40 cycles
of 95°C for 10 sec; 55°C for 30 sec and 72°C for 30 sec. mRNA
expression levels were analyzed using Bio-Rad CFX Manager with the
2−∆∆Cq method from the fold change triplicate values,
considering the fold change of untreated cells as the fold
induction (24). The expression
for each was normalized using GAPDH as the reference gene.
Statistical analysis
All statistical analyses were performed using Graph
Pad Prism software (version 6; GraphPad Software, Inc.). The data
presented are the average of obtained from triplicate values for
each independent experiment repeated three times. The differences
between the means of two groups were analyzed using an unpaired
t-test and ordinary one-way ANOVA used to analyze differences
between multiple groups. Two-way ANOVA was performed followed by
Bonferroni's post hoc test to assess statistical significance, in
the case of analyzing comparisons or inter-relationships between
more than two set of groups/multiple set of groups. A probability
(P)-value <0.05 was considered to indicate a statistically
significant difference. All the statistical values in the different
experiments represent the mean ± SD, where n=3.
Chemical structure of the compounds
The 3D structure of theaflavin was obtained from
PubChem (ID 135403798). The structure of the novel curcumin
derivatives (Cl and F) was drawn using ChemDraw (16).
Results
The compounds exert cytotoxic effects on
and the efficient caspase-mediated apoptosis against different KSHV
cell lines
In order to examine whether the selected flavonoid
compounds exert any cytotoxicity against KSHV-positive cells, a
cell viability assay was performed to determine cell cytotoxicity
in a concentration- and time-dependent manner, with all three
compounds in the preliminary screening. Treatment with all three
compounds efficiently led to a decrease in cell viability in a
concentration- and time-dependent manner. The in vitro PI
cytotoxic assay clearly revealed that all three compounds exerted
significant cytotoxic effects against two different KSHV-infected
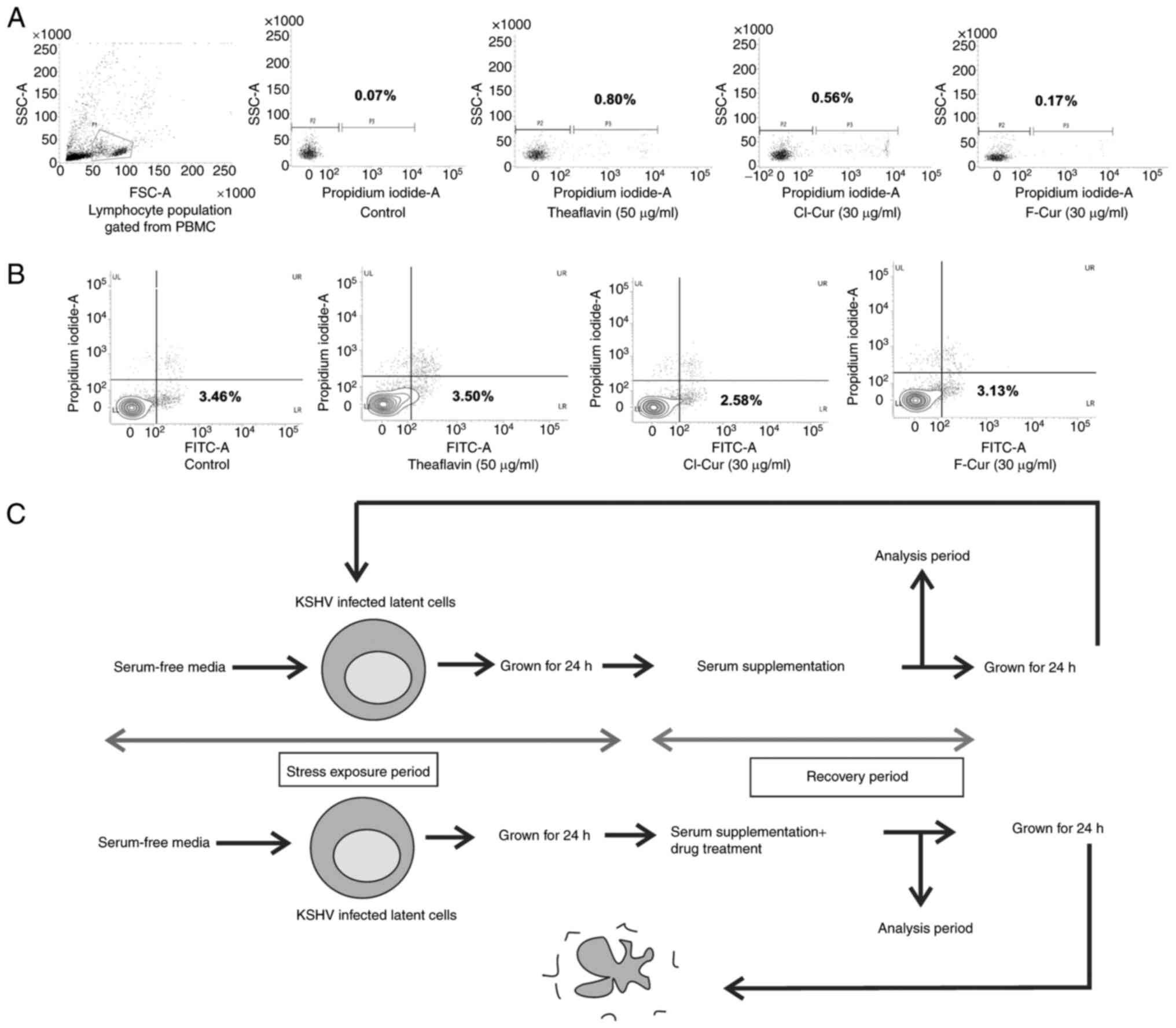
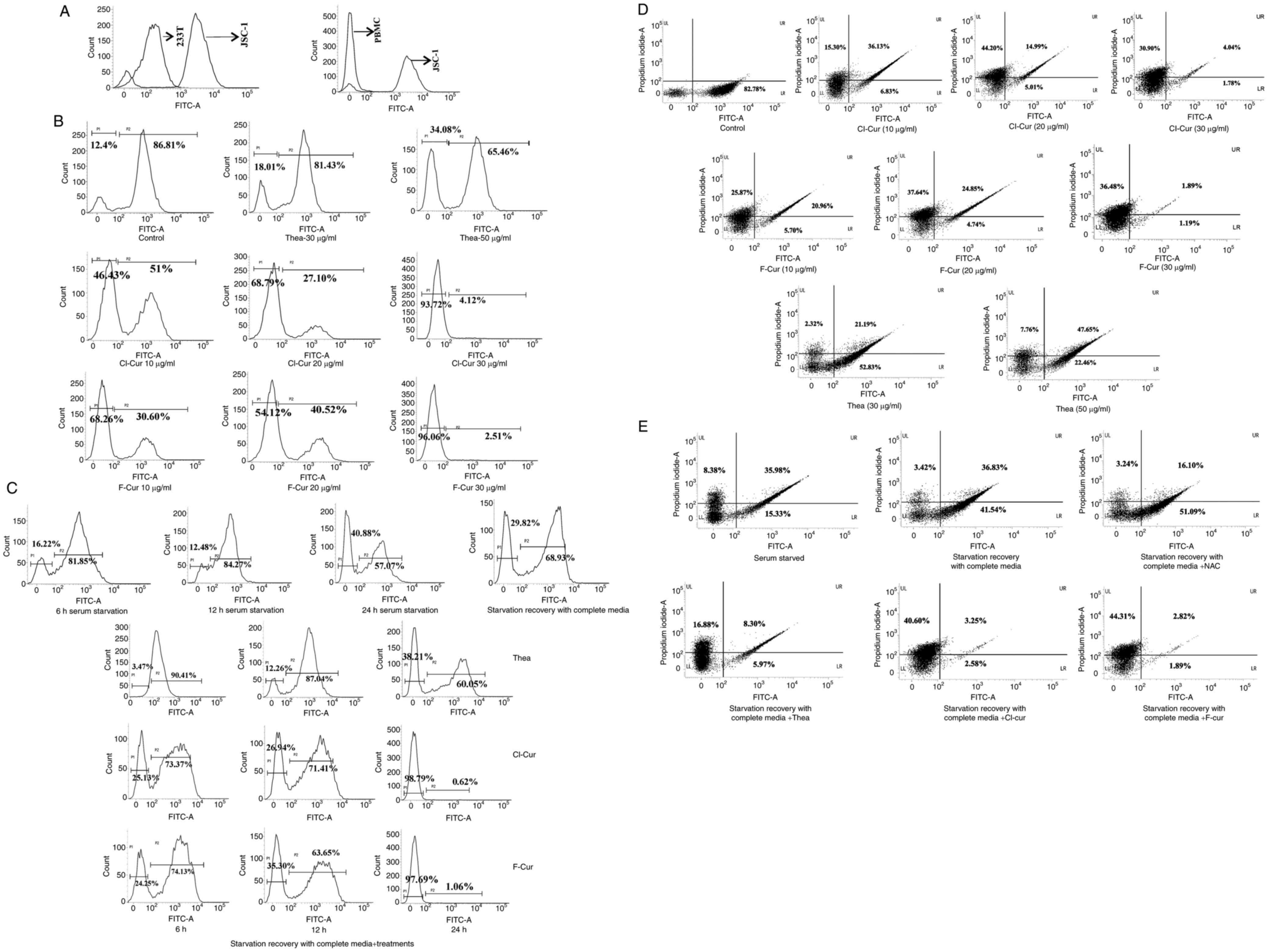
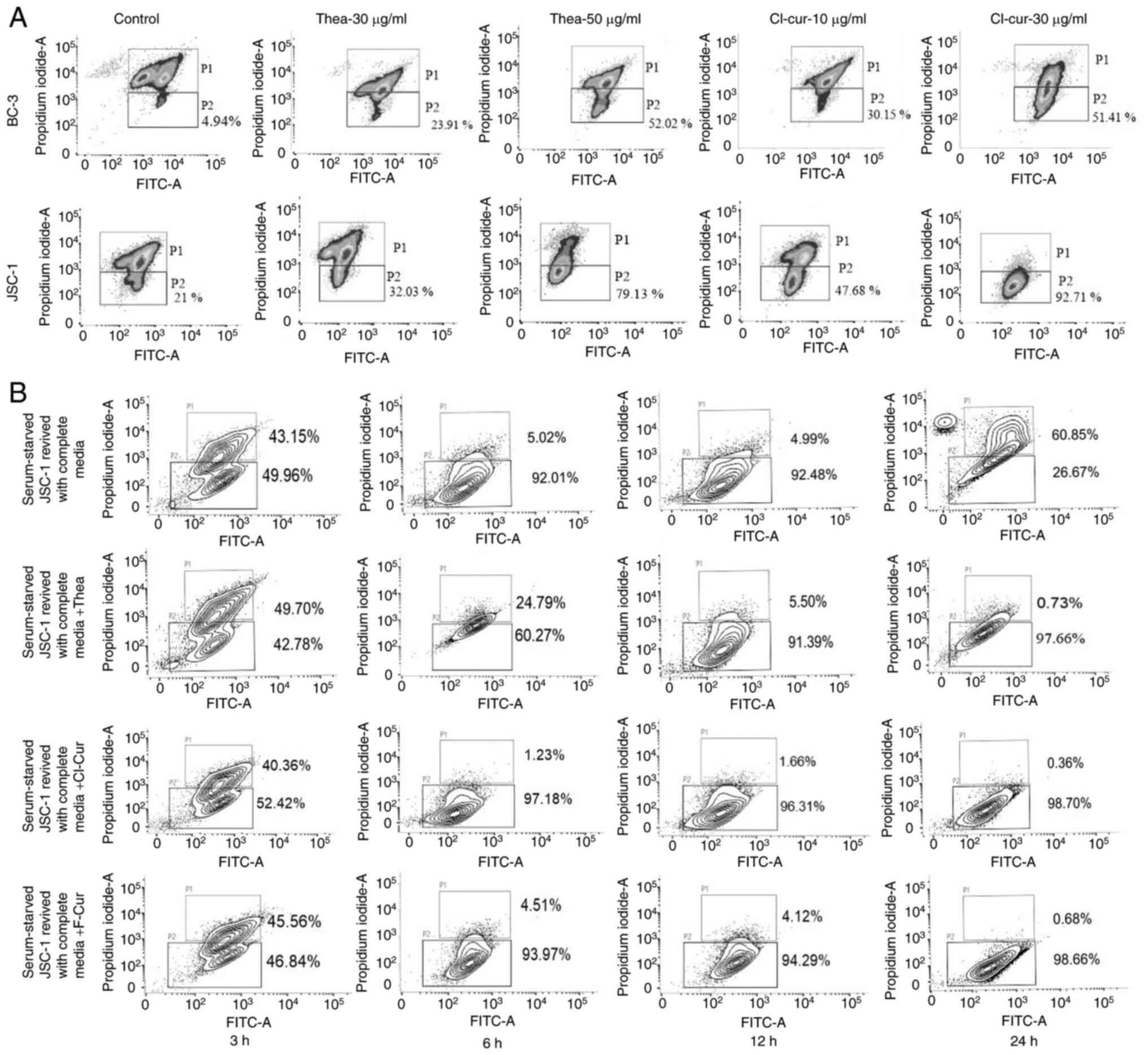
cell lines compared to the control (Fig. 1A and B).
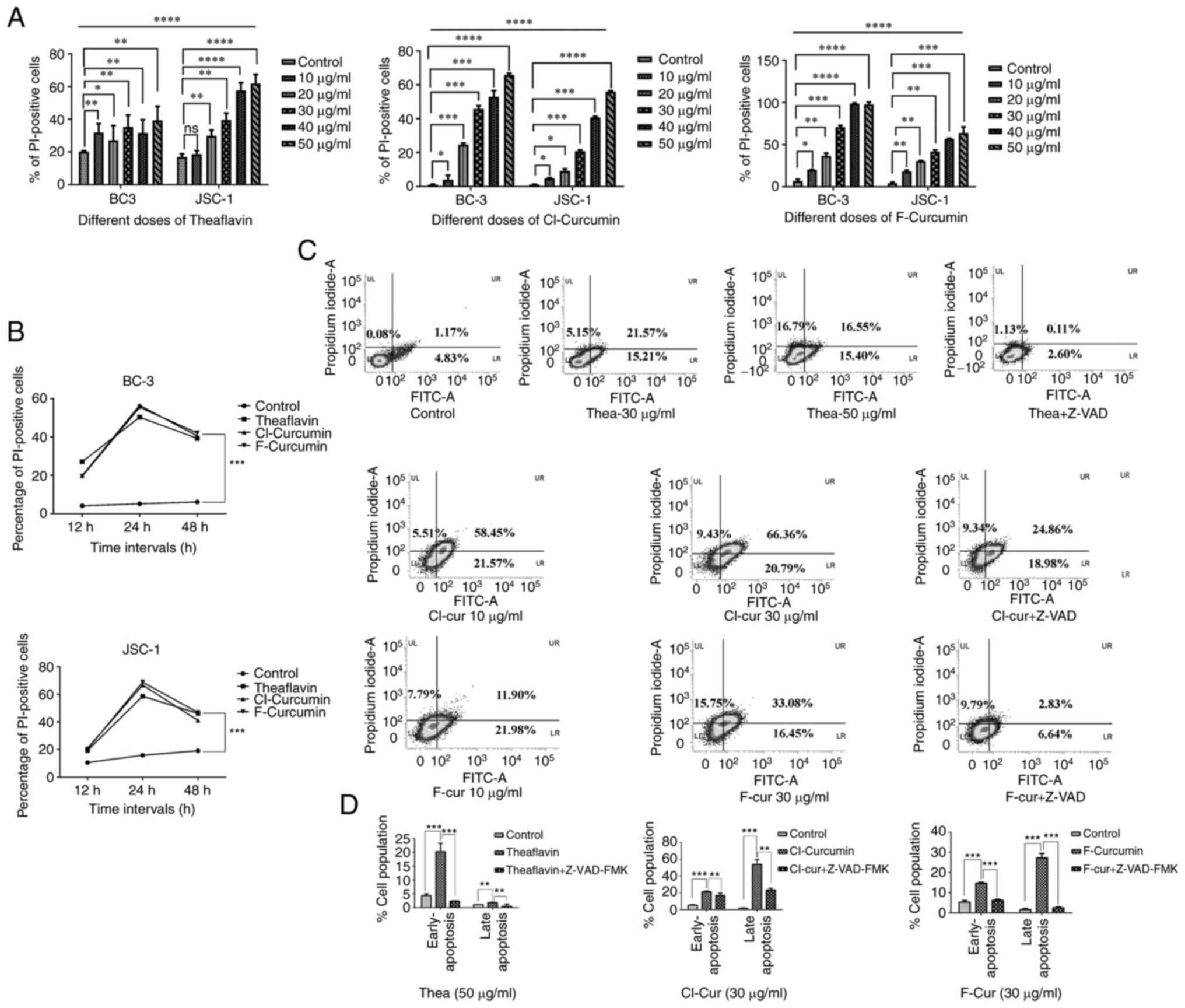 | Figure 1Cell viability and apoptosis
assessment in different KSHV-positive cell lines with treatment.
(A) Concentration-dependent increased loss of cell viability with
an increase in PI positivity with all the three compounds in both
BC-3 and JSC-1 cells treated for 24 h. nsP>0.05,
*P≤0.05 **P≤0.01 ***P≤0.001,
****P≤0.0001 significant differences for all data sets
vs. the control. (B) Time kinetic increase in cell viability with
an increasing PI florescence intensity with all the three compounds
at their respective concentrations (theaflavin, 50 µg/ml;
both curcumin derivatives, 30 µg/ml) against BC-3 and JSC-1
cells. Significant change in compound treated cells compared to
control is indicated by ***P≤0.001, significant
differences vs. the control. (C) FITC-Annexin V apoptosis analysis
in JSC-1 cells treated with the three compounds represented by
densitometry plots (one low dose and one high dose). Apoptosis
assay following treatment with the compounds along with Z-VAD-FMK
was performed to examine caspase dependency. All the FITC-Annexin V
plots with the percentages of the cell population in each section
are mentioned, representing the apoptotic event. (D) Bar charts
demonstrating the comparative pro- and late apoptotic cell
percentage following treatment with the three compounds alone (at
their particular effective concentration as indicated) and
treatment along with Z-VAD-FMK application compared to the control
**P≤0.01 and ***P≤0.001, significant
differences vs. control and differences with respect to treatment
in the case of Z-VAD). KSHV, Kaposi's sarcoma-associated herpes
virus; Thea, theaflavin; Cl-cur, Cl-curcumin; and F-cur,
F-curcumin. |
The FITC Annexin-V assay clearly demonstrated that
the cell death process was mediated through caspase-dependent
mechanisms in the presence of all three compounds. The densitometry
plots of the concentration-dependent treatment indicated the
gradual shifting of the pro-apoptotic population towards late
apoptosis. These plots also demonstrated that the caspase inhibitor
with three of the test compounds successfully restricted the
late-stage apoptotic event (Fig. 1C
and D). The bar charts representing the comparative assessment
of apoptosis with the inhibitor, indicate a significant increase in
the apoptotic cell population in the treated sets only, which was
decreased with the inhibitor (Fig. 1C
and D). These results strongly suggest that both theaflavin and
curcumin derivatives are capable of inducing the specific
caspase-mediated death of KSHV-infected cells. All the compounds
led to significant cell death from their starting concentrations to
the higher concentrations (apart from the 10 µg/ml
concentration of theaflavin against JSC-1 cells). Thus, among these
concentrations, considering the more significant results, the
minimum effective concentrations (low dose) and higher effective
concentration (almost 50% cell death; high concentration) for these
drugs can be predicted from the aforementioned results. For
theaflavin, the lower concentration of 30 µg/ml can be the
starting level for the cell death process, which reaches >50%,
and the effective concentration is 50 µg/ml. The
concentrations were comparatively much lower for the curcumin
derivatives, starting from 10 µg/ml with the significant
effectiveness being observed and reaches high at 30 µg/ml.
Thus, for theaflavin, the effective low concentration was selected
at 30 µg/ml and the effective high concentration at 50
µg/ml, whereas in the case of curcumin derivatives, the
effective low concentration was selected at 10 µg/ml. and
the effective high concentration at 30 µg/ml. The threshold
timeline for the drugs was found to be 12 h, reaching optimum
levels at 24 h, and finally decreasing to a constitutive level
after 48 h. Thus, in further experiments, incubation for 24 h was
selected for the assessment of the drug effectiveness.
The compounds are not cytotoxic against
healthy lymphocytes and non-KSHV lymphoma cells
KSHV malignancy has often been found to be
associated with HIV. Highly active antiretroviral therapy (HART)
therapy is the only effective therapeutic option for both cases.
However, prolonged HART treatment leads to a stressful effect and
creates an extra burden of drug-induced cytotoxic after-effects
which hamper the normal cellular environment with a dysfunctional
anti-oxidative system along with a distorted immune response.
Immune reconstitution inflammatory syndrome (IRIS) is the most
common example in such cases (25). This rare incident is associated
with the deterioration of the body's response against previous
subclinical or present clinical conditions and as a result, the
risks of developing further pathogenic co-infections, such as KSHV
are augmented (11,26). Thus, the present study then
assessed whether the compounds used for treatments exert any
cytotoxic effects against normal healthy lymphocytic cells. The
isolated PBMCs treated with each of the three test compounds at
their effective concentrations for 24 h were subjected to cell
death analysis. The lymphocyte population from the control set was
isolated (Fig. 2A) and this
population was selected for determining the effects of the
compounds. The results clearly indicated that the compounds
eliminated the chance of any toxic stress burst and helped towards
maintaining a normal healthy cellular environment along with
treatment (Fig. 2A).
Similarly, the non-KSHV B cell lymphoma cell line,
BJAB, was also investigated with the same test compounds, which did
not exert any notable apoptosis-inducing effects compared with the
aforementioned KSHV-positive cell lines (Fig. 2B). This indicates that, apoptotic
induction is specific against virus induced altered cell death
mechanisms.
Status of oncogenic stress response in
KSHV-infected cells following treatment with antioxidants
KSHV-infected cells maintain a redox homeostasis via
a stress responsive antioxidant mechanism during different phases
of pathogenesis; this begins from infection, is then maintained and
is reactivated until neoplasty, resulting in the regulation of the
pathogenesis process. The resulting oncogenic stress management
system eventually helps throughout the malignancy process. As a
result, the total cellular oxidative stress is in an unbalanced
condition throughout the process of oncogenesis. The role of the
anti-oxidative therapeutic approach against this malignant
environment with a distorted stress balance needs to be fully
understood as a stress responsive anti-oncogenic strategy.
The present study thus aimed to understand the
status of oxidative stress with the application of different
antioxidants in KSHV-infected cells. The estimation of
intercellular ROS generation in KSHV-infected cells clearly
revealed that the KSHV-infected latent cells expressed a
significant high level of ROS, which was higher than normal
non-cancerous human PBMCs (Fig.
3A). PBMCs are a primary cell model with a heterogeneous cell
population, used for various research purposes (27). Thus, in order to supplement the
finding with a control cell line, 293T cells were used as a
non-viral non-cancer control cell line in this experiment,
exhibiting low intracellular ROS levels compared to KSHV-infected
cells (Fig. 3A). 293T cells are a
highly transfectable daughter cell line variant of 293, which also
supports high viral gene expression This high level of cellular ROS
was completely decreased following treatment with the curcumin
derivatives at their respective concentrations, whereas in the case
of theaflavin, it was observed that a moderate level of ROS was
maintained in the treated cells. Thus, in order to examine the ROS
alteration pattern, a concentration-dependent assay was performed
against the KSHV-infected cells with the three antioxidants. The
gradual increment of the ROS-negative population from the histogram
plots clearly revealed the concentration-dependent efficacy of the
compounds to suppress the intracellular ROS generation (Fig. 3B).
Following the confirmation of this alteration in ROS
levels in latent KSHV cells, the present study aimed to confirm
whether the therapeutic treatment indeed targets the stress
balancing system of the virus-infected cells. The latently infected
cells were subjected to serum stress for the generation of a time
kinetic stress-induced in vitro model and to mimic the
situation of varied oxidative stress levels during reactivation,
cytotoxicity induction, or infection to a new cell (Fig. 2C). It was observed that following
the starvation period, an extra stressful cellular environment was
created, which tended to markedly decline after 24 h. After 24 h of
stress exposure when these cells were supplemented with complete
growth medium, the intracellular ROS levels increased, gradually
balanced and maintained at a moderately high level, as found in
latent cells (Fig. 3C). When
these stress-exposed cells were revived with normal growth medium
along with treatment, the stress balance mechanism was initiated
without any further elevation in the intracellular ROS levels.
Collectively, these results indicate that the compounds can
interact with the oncogenic stress response of the virus-infected
cells along with the cell death process, by utilizing the existing
oxidative stress response.
Of note, up to this point, these results
demonstrated that the antioxidants induced apoptosis with a
reduction in intracellular ROS levels. Thus, there may be a
contradictory fact between these two events of ROS alteration and
apoptosis induction. In order to further clarify this fact, a ROS
vs. cell death percentage assay was performed on the same cells
using a flow cytometer. This analysis clearly revealed that the
JSC-1 cells without treatment exhibited only a positive ROS signal.
When they were treated with both theaflavin and curcumin
derivatives, the total cell population gradually tended to switch
towards the both PI- and ROS-positive zone and ultimately, towards
only the PI-positive zone (Fig.
3D). This finding was then verified and supplemented, utilizing
the stress-induced in vitro model. It was clearly revealed
that the stress-induced cells were distributed in the ROS-PI
dual-positive zone, which indicated stress-induced cell death. This
event was recovered by complete medium supplementation, after which
the cells were observed to be redistributed more in only the
ROS-positive area. Following the treatment of these cells at the
significant concentration the whole population shifted towards only
the PI-positive area. The activity of these antioxidants was also
analyzed in comparison with the FDA-approved antioxidant, NAC. NAC
has been reported to exert effective chemopreventive and inhibitory
effects on angiogenesis in an in vivo oxidative
stress-induced KSHV model (28,29). It was observed that, in the in
vitro stress-induced cell line model, NAC supplementation
recovered stress-induced cell death and led to the distribution of
the cells in only the ROS-positive zone (Fig. 3E).
These experiments supplement the fact that
antioxidants drive all the ROS-positive latent KSHV-infected cells
towards death and at their higher concentration, a total cellular
destruction occurs, represented by only the PI-positive cell
population. From these aforementioned results, it was confirmed
that the theaflavin and curcumin derivatives can effectively
control KSHV-infected cell growth by specifically targeting the
oncogenic stress response of these cells. It was clearly observed
that the initial pattern of stress management by activating the
antioxidative strategy in stress-exposed KSHV-infected cells was
similar for both serum-recovered, and serum-recovered cells with
treatment. However, recovery from stress exposure with normal
growth supplement gradually balanced this by switching it to an
oncogenic stress balance, which maintained the latency. However,
when these cells were treated with antioxidants, a successful
stress response towards apoptosis was observed. This result
strongly revealed that the virus was evolved in such a manner,
which utilized the cellular antioxidant mechanism for its survival
and the antioxidants utilized the same mechanism, but sensitized
these virus-infected cells against stress, leading to cell death.
The difference was in the case of theaflavin, of which the
significant effective concentration was higher compared to that of
curcumin.
The compounds exert a significant
alteration in mitochondrial membrane potential
The mitochondria are the major cellular energy hub
for different cellular functioning and naturally release a certain
amount of ROS as by-product. However, hypoxic pressure, or stress,
influence the mitochondria to generate high levels of ROS, which
are stabilized with time. The cells continue to grow with these
elevated ROS levels by escaping senescence and death. These
persistent dysfunctional cells with high mitochondrial ROS levels
induce mutations in DNA, eventually resulting in malignant
transformation. Thus, dysfunctional mitochondrial functions elicit
malignant transformation (30,31). Pathogenic cancer cells, such as
Epstein Barr virus-associated cancers, have been reported to
persist with such dysfunctional mitochondria and to exhibit an
unregulated cell proliferation (32). Thus, the status of the
mitochondria following cancer treatment against needs to be
understood in the case of the antioxidative therapeutic approach.
Therefore, the present study further proceeded to analyze the
status of mitochondrial membrane potential following the treatment
of the normal and stress-exposed KSHV-positive cell lines with the
antioxidants. It was observed that all the three test compounds
initiated the deterioration process of the damaged mitochondria at
their respective effective concentrations. The compounds
effectively increased the mitochondrial membrane potential in a
concentration-dependent manner in the latent KSHV-infected cells
(Fig. 4A). When the
stress-exposed cells were revived with complete growth medium,
initially they exhibited a destroyed mitochondrial load, which was
maintained in the same manner for few more hours of
supplementation, which later stabilized. Ultimately, the latently
infected cells exhibited a normal balanced mitochondrial potential
and continued to grow. While these stress-exposed cells were grown
with the stress balancers (both theaflavin and curcumin
derivatives), they were successfully able to switch the rescue
process of the damaged mitochondria inside the oncogenic cell, with
a notable loss of mitochondrial membrane potential in a gradual
time-dependent manner, which drove the cell towards apoptosis
(Fig. 4B).
The antioxidants can break the evolved
tactics of the virus to utilize stress-induced cell cycle check
points
The oncogenic herpes virus has been evolved to
utilize the stress-induced cell cycle regulatory check points for
efficient viral replication, leading to cell cycle arrest during
malignancy in a p53-mediated manner (33). Whenever there is certain type of
stress induction, such as drug or metabolic stress and nutrient
deprivation, the affected cell has an indigenous management system
which halts the cells and does not allow the cells to undergo
further growth with the stress-affected defects. However, the virus
is evolved in such manner that it utilizes this stress for its
replication. The results of the present study clearly indicated
that with the stress induction to the latent cells, there was a
halt in the cell cycle process, which was recovered after 24 h of
growth supplementation; this is how the virus maintains the
latency. The serum-stressed cells underwent normal cell cycle
phases after they were replaced with complete growth media. The
graphical representation of different phases of the cell cycle
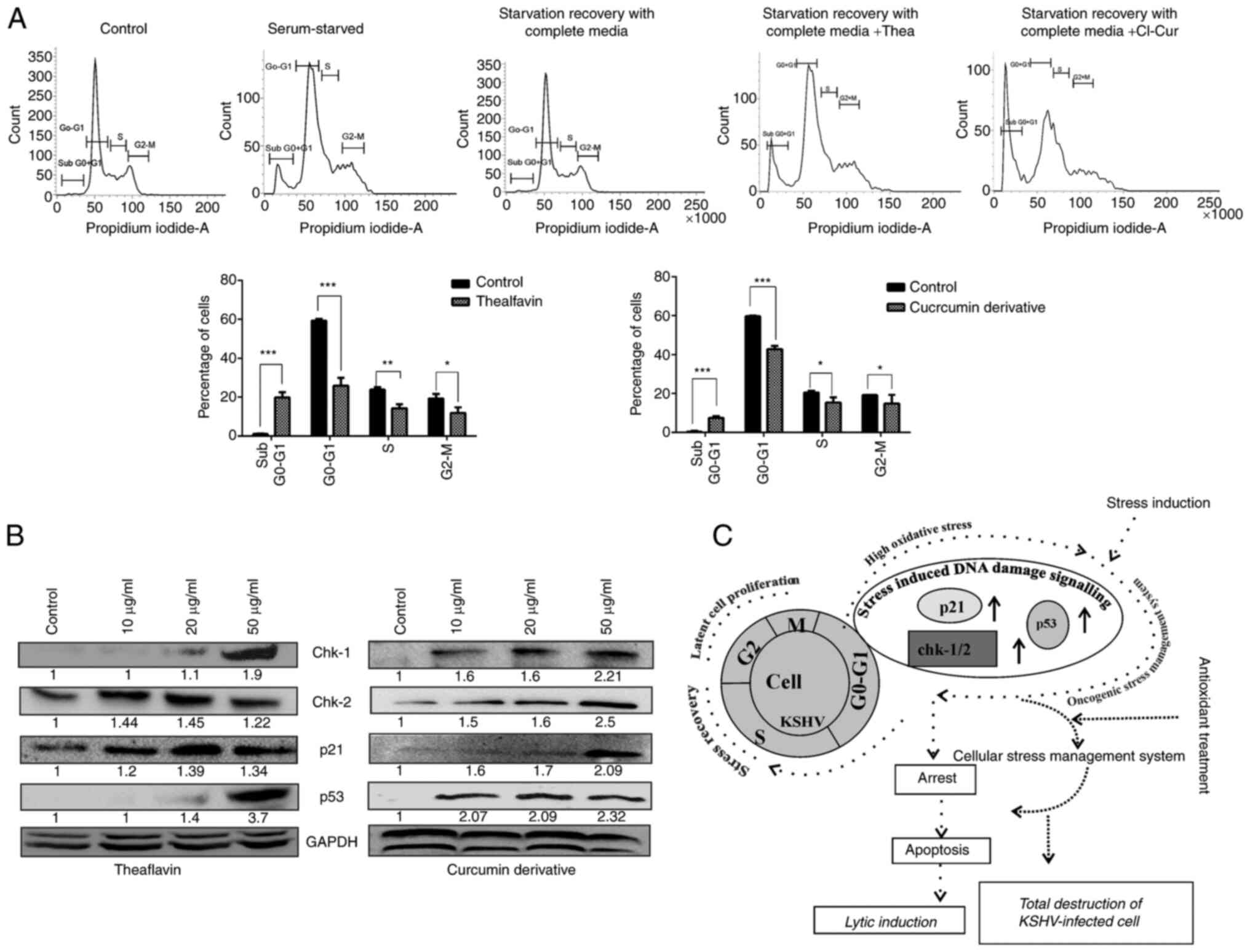
clearly revealed with significant difference that the cells treated
with theaflavin and curcumin that were stress-exposed exhibited a
restricted cell cycle arrest at the sub G0-G1
phase compared to stress recovered nontreated control cells
(Fig. 5A). No such proper
synthesis phase was also observed in these cases. On the other
hand, more specifically, the expression of the DNA damage-induced
cell cycle regulatory proteins, Chk-1 and Chk2-, was found to be
enhanced in a concentration-dependent manner (Fig. 5B). We have also found a
significant increase of p53 and p21 protein which indicates the
upregulation of stress induced DNA damage signaling in a p53
dependent manner (Fig. 5B). These
results suggest that the virus can manipulate the stress-induced
cell cycle regulation, so following a stress exposure, with
supplementation, they are able to manage the cell growth with KSHV
infection. However, when the cells were treated with the
antioxidants, cell growth was arrested at a particular stage; the
compounds rendered the cells sensitive to the existing high ROS
levels and the stress-induced cell cycle restricting kinases were
then activated, leading to apoptosis (Fig. 5C).
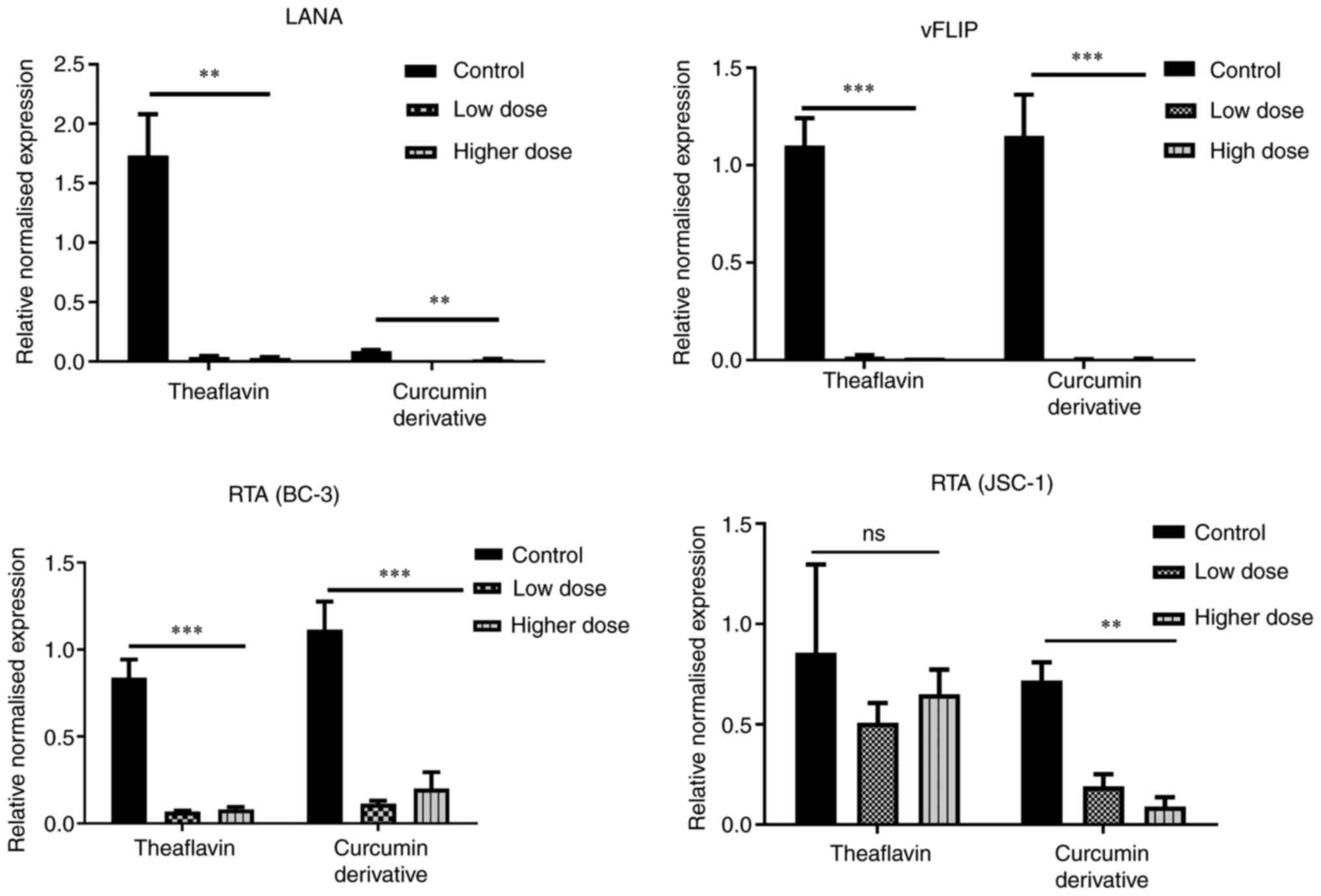
Status of lytic-latency gene in
KSHV-positive cells
A marked imbalance in the oxidative stress response
is a major consequence of viral pathogenesis. The aforementioned
findings demonstrated that KSHV latency was evolved in such a
manner that it maintained a redox homeostasis against the
infection-induced oxidative stress response by interacting with
inbuilt defense and death-associated cellular mechanisms, such as
apoptosis. It managed to maintain a moderate intercellular ROS
concentration and allowed the cells to proliferate. On the other
hand, when the latency-associated cells are exposed to various
stress inducers, they are unable to proliferate by managing the
stress; they better decide to degrade the cell instead of
maintaining it and are directed to be reactivated (34). This high oxidative stress favors
the process until the establishment of infection. Thus, there is a
continuous fluctuation of ROS in a KSHV-infected cancer cell, which
is associated with a cycle of latency and lytic stages and thus,
both latency and lytic genes correlate with each other to help in
sustaining the virus. Likewise, the KSHV latency-associated gene,
LANA, regulates the expression of the lytic-associated gene, RTA,
and protects the infected cell from hypoxia-induced damage. RTA in
turn, following infection, regulates the expression of LANA for the
maintenance of latency (9). Thus,
when an antioxidative approach is designed against an infected
cancer cell to disrupt the oxidative balance, both lytic and
latency gene profiles need to be analyzed well. In the case of KSHV
malignancy, the role of two major latency-associated genes of KSHV,
LANA and vFLIP is critical (5).
The present study found that the expression of both LANA and vFLIP
was decreased following treatment with theaflavin and curcumin
derivatives, and exhibited significant differences in the latent
KSHV-infected cells with respect to the untreated control cells
(Fig. 6, upper panel). On the
other hand, to understand whether lytic upregulation is associated
with cell death, the present study also examined whether the
antioxidants can specifically lead to an imbalance in the oncogenic
stress response of the infected cells and induce any early lytic
gene expression. Increased RTA and KSHV reactivation is known to be
associated with oxidative stress induced by several anticancer
drugs (3,6). The results clearly demonstrated that
there was no such expression of the early lytic associated protein,
RTA, with any of the drug treatments with respect to
stress-recovered untreated control cells; the downregulation of RTA
was instead observed, which may be due the probable antiviral
mechanism of the compounds effective against stress recovered cells
showing slight high RTA level (Fig.
6, lower panel). Collectively, these results prove the fact
that both theaflavin and curcumin derivatives successfully destroy
the latency-associated viral proteins and eliminate the chance of
latency-mediated stress-induced viral reactivation and
maintenance.
Autophagy-apoptosis switching functions
as the intermediate regulatory mechanism behind the cell death
process
Viruses need a functional host cell for survival and
multiplication; this is the reason they evolve in such a manner,
where they are not intended to exert any harmful effect to their
host. Viruses utilize several host cell mechanisms (3). Autophagy is one such mechanism of
the host cell, which is a ROS responsive process and known as the
stress balancer of the cell. It has been reported that in
KSHV-infected cells, a basal level of autophagy is always present
that helps in the maintenance of latency by balancing the harmful
consequences of infection and maintaining the host cell to a
certain level of healthy state so that the virus can reside inside
the cell and receive an adequate nutrient supply (35). On the other hand, increased
autophagy is observed during viral reactivation, eventually helping
in KSHV replication (36-39). Thus, the present study then aimed
to examine the autophagic status during the therapeutic
applications of the test antioxidants to determine the role of
autophagy in this stress balance mechanism.
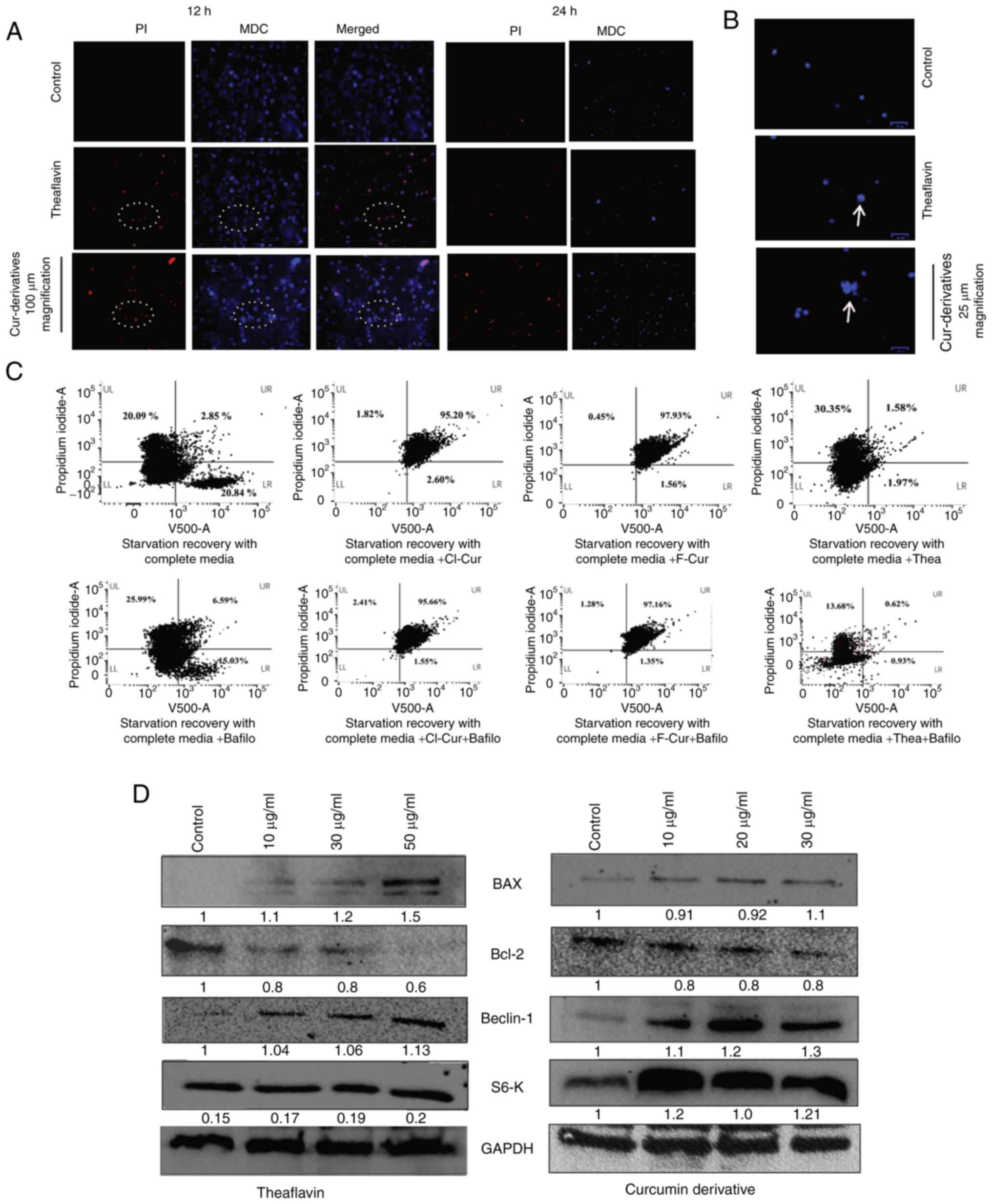
For this purpose, an autophagy vs. cytotoxicity
assay was performed using a microscope, It was found that with
treatment, the cell viability increased, and all thePI-positive
cells were also positive for the autophagy indicative MDC stain,
indicating an autophagy-associated increase in cytotoxicity in the
KSHV-infected cells. It was also observed that the control cells
also stained positive for MDC, representing the basal level of
autophagy in latent cells (Fig.
7A). The merged images at 12 h of these cells indicated a few
autophagy apoptotic co-expressed cells (indicated within the white
circles in Fig. 7A). At 24 h, a
decreased number of cells was observed due to highly active
apoptosis. The magnified view of these cells (MDC-stained) with
treatment represents an increased size compared to the control with
clear punctures inside, marked by white arrows (Fig. 7B). A notable change in
fluorescence intensity and specifically, for the autophagy event
with identifying vesicles was not clearly detected in the
microscopy images, as all KSHV-positive cells express a moderate
level of autophagy for the maintenance of malignancy. Thus, to
determine the role of endogenous or overexpressed autophagy in the
cell death process, the present study then performed an MDC vs. PI
assay using a flow cytometer in the stress-induced in vitro
cell line model. The result clearly demonstrated that there was a
proper separate MDC-positive population (upregulated autophagy) in
the stress-recovered cells with complete medium supplementation.
However, a decreased MDC positivity was observed in the cells
treated with the autophagy inhibitor (bafilomycin A1) with an
increased cell death. This indicated that stress recovery in
KSHV-infected cells was achieved by autophagy and the inhibition of
autophagy accelerated the cell death process. However, this
autophagy cannot protect the cells from drug-induced stress
followed by cell death by apoptosis. All the latent KSHV-infected
cells with a moderate level of autophagy underwent cell death
following treatment, exhibiting a dual MDC-PI positivity. In the
case of curcumin derivatives, the apoptosis was not markedly
altered with bafilomycin A1 treatment, indicating an
autophagy-associated apoptosis, which became independent of
autophagy at the respective concentration. However, in the case of
theaflavin, the cell death process was completely switched to cell
death and was autophagy-dependent (Fig. 7C). Thus, collectively, it was
confirmed that ROS responsive autophagy was active in KSHV-infected
cells and the cell death process was mediated by autophagic
involvement in latent cells. However, there was no such significant
autophagic induction in favor of the virus for protection, as this
switched towards apoptosis post treatment.
Subsequently, in order to intersect and characterize
this autophagy apoptotic switching, a molecular investigation of
autophagy-dependent cell death in the theaflavin- and
curcumin-treated KSHV-positive cells was performed. Therefore, the
expression of specific mitochondria-mediated intrinsic apoptotic
protein markers (BAX and Bcl-2) was investigated, in latently
infected KSHV cells treated with theaflavin and one of the curcumin
derivatives. It was observed that with the loss of mitochondrial
membrane potential, BAX expression was increased with a concomitant
decrease in Bcl-2 protein expression in the KSHV infected cells
compared to the control cells. The anti-apoptotic Bcl-2 oncogene
has been reported to negatively regulate the autophagic gene,
Beclin-1, and thus contribute to the inhibition of apoptosis
(40,41). Moreover, Bcl-2 protein expression
in the endoplasmic reticulum and mitochondria can be a stress
sensor and can regulate Beclin-1 and caspase-mediated
autophagy-apoptosis (42). Thus,
after observing Bcl-2 downregulation in the KSHV-positive cells,
the present study wished to examine autophagy-mediated apoptosis by
investigating the expression level of the Beclin-1 gene. From the
results, it was confirmed that there was a concentration-dependent
increase in the expression of Beclin-1 autophagy protein,
indicating an autophagy to apoptotic switch in the KSHV-positive
cell lines treated with both theaflavin and curcumin derivatives
(Fig. 7D). The autophagic marker
protein, LCIII-B, was also investigated. It was found only found to
be expressed in stressed and stress-recovered cells treated with
complete medium, but not in cells treated with theaflavin and
curcumin derivatives; this supports and clarifies the fact that
autophagy is upregulated and plays a vital role in support of the
KSHV-infected cancer cells recovering after serum stress, and
completely switching towards apoptosis with treatment, thus failing
to recover from drug-induced stress and apoptosis (Fig. S1). This type of event can be
compared with recent findings on incomplete autophagy supporting
severe acute respiratory syndrome coronavirus-2 replication
machinery (43). Likewise, in the
present study, there was an induction of incomplete cellular
autophagy-mediated apoptosis, required during stress recovery where
autophagy plays a cytoprotective role, and the complete switch of
that autophagy to apoptosis leads to cell demise in treatment sets
(44,45). Kinase cascades are ROS-responsive,
and this has been reported to modulate and regulate autophagy
accordingly during cancers. mTOR is a major connecting pathway
between ROS and autophagy in cancer cells, the downregulation of
which has been found to restrict cancer progression (46). Activated mTOR is responsible to
activate S6K by phosphorylation. Rapamycin, which has been reported
to restrict KSHV malignancy by mTOR inhibition, has been found to
minimize the phosphorylation of S6K, hence restricting the
activated S6K (47,48). Both theaflavin and curcumin have
been previously reported as potent mTOR inhibitors (49-51). In the present study, the results
of western blot results analysis revealed the visible
overexpression of mTOR-regulated intact S6
post-treatment with the test compounds. The expression of this key
kinase is relevant to the present study, by suggesting mTOR
inhibition by the test antioxidant compounds (Fig. 7D).
Discussion
Flavonoid compounds, with their structural
specificities, are biologically active and exhibit various
anticancer, as well as cancer manifesting activities. These
compounds are potential ROS scavengers, which can act both as anti-
and pro-oxidant compounds by decreasing ROS generation and
sensitizing cancer cell unbalanced ROS levels, leading to apoptotic
cell death (52). On this note,
during viral pathogenesis, the oncogenic stress management system
plays a crucial role, where the oncogenic virus takes control over
the cellular stress management mechanisms and a continuous
oncogenic stress management system persists (Fig. 8A) (53). Thus, a clear understanding of
these stress balance tactics at different stages of the cancer
progression with a varied ROS response is required to target this
mechanism and combat cancer progression. Keeping this in mind, in
the present study, two flavonoid based compounds, theaflavin and
synthetic curcumin derivatives, were selected, which have been
reported as natural antioxidants against various diseases,
including cancer. Both theaflavin and curcumin have been reported
as natural antioxidants and are therapeutically applied in cancers
(49,54). Theaflavin has not been previously
reported against any KSHV-associated malignancy, at least to the
best of our knowledge; however, curcumin has been found have
potential anti-KSHV malignant activity by inducing STAT3 activation
and caspase-mediated apoptosis. It is also known that curcumin is a
potent inhibitor of APE1-mediated redox signaling in the process of
replication, invasiveness and angiogenesis during KSHV malignancy
(55-57). Thus, in the present study, two
different flavonoid compounds were selected, namely theaflavin and
modified curcumin derivatives (Cl-curcumin and F-curcumin) for
analyzing their bioactivity over the varied level of oncogenic
stress balance mechanism of KSHV-infected cells. The novel modified
bioactive derivatives were used in the present study, with an
intention to magnify the biological efficacy of mother compound
curcumin, with increased bioavailability, which is a major drawback
of the clinical applicability of this compound.
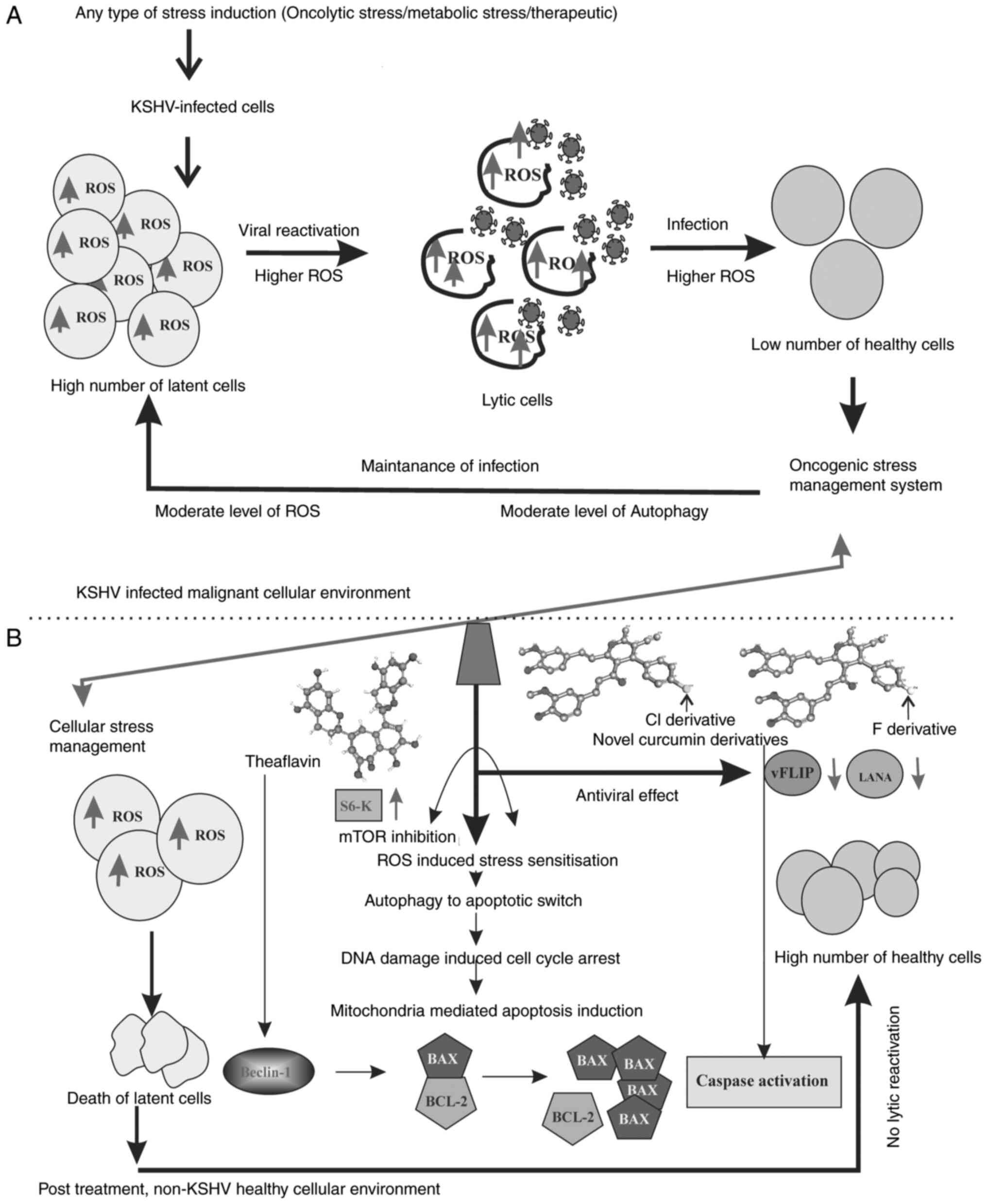 | Figure 8Hypothetical therapeutic regulatory
model of the antioxidant activity against KSHV-positive cells. (A)
Literature-based model (3-8,35,36)
of the oncogenic stress management system inside the KSHV infected
malignant cell environment. The latent cell maintain a moderate
level of ROS, whereas these levels re higher during lytic
reactivation. Further during the infection of new cells, the ROS
level is high and during the establishment of infection it is
balanced to a moderately high level. This is how the virus makes
the cells insensitive to cell death in infected cells by
controlling over various stress-related cellular functions and thus
respond with different stress responses. (B) Summarized
experimental outcomes, representing the antioxidant induced
sensitization of cellular stress balance mechanism. The results
suggest that theaflavin and curcumin derivatives are potent ROS
responders. The therapeutic approach renders the infected cells
sensitive against high ROS. As a result, intermediate mTOR
signaling and autophagy occur in infected cells; cells undergo
senescence and apoptosis in a Beclin-Bcl-2-dependent manner. The
ROS responsive mitochondrial damage further initiates
caspase-mediated cell death. The downregulation of two major
latency associated proteins, LANA and vFLIP, with treatment
strengthens the process of cancer cell death without any
reactivation or further infection. KSHV, Kaposi's
sarcoma-associated herpes virus; ROS, reactive oxygen species;
LANA, latency-associated nuclear antigen. |
From the results presented herein, it was observed
that both the compounds exhibited potential anticancer activity
against the KSHV-associated cell lines via caspase-dependent
apoptosis induction. They potentially handle the stress balancing
capability of the oncogenic cells as their cancer management
mechanism. The present study clearly demonstrated that the
constitutive expression of moderate levels of ROS during KSHV
latency, which helps in the maintenance of the virus, was
sensitized by these bioactive antioxidant compounds. Moreover, it
was revealed that when there was a serum stress induction in the
virus-infected cells, this induction was successfully utilized to
destroy the energy-deprived cell. When, these stress-exposed
KSHV-associated latent cells were supplemented with favorable
growth conditions, cellular growth was reverted by managing the
high level of ROS, utilizing the same stress balance strategy as a
normal cell. When they were grown with theaflavin or the curcumin
derivatives, the existing stress balance process inside the cells
was destroyed, and cell death occurred. The regulatory mechanism,
which controls several downstream signaling pathways for the
continuous maintenance of the balance of the latency and lytic
phases for the successful survival of the virus, can reside inside
the host cell, and is destroyed by these compounds.
Compounds with antioxidative properties are
generally considered to minimize the oxidative stress of the cell;
however, in some cases, specifically in cancer cells, they can play
prooxidative role, particularly when there is a hyper ROS response
which drives the cells towards cell death (58,59). How a viral cancer cell responds
with ROS fluctuation has been reported during different phases of
cancer; however, how this fluctuation is managed by the
pathogen-altered cancer cells has not been fully elucidated. Of
note, the present study critically analyzed this altered version of
cellular stress management in a tumor microenvironment. The present
study reveals the mechanism through which the oncogenic stress
balance system combats this varied ROS response and how the same
standpoint can be an effective therapeutic strategy. These
experiments were performed using a stress-induced in vitro
model with the comparative analysis of different antioxidants.
Curcumin and theaflavin have been reported to enhance the ROS level
in cancer cells for the induction of apoptosis (60). In the present study, these
compounds followed a similar pattern of cell death mechanisms
against the KSHV-infected cells. More critically, it was found that
against the KSHV-infected cells, theaflavin and curcumin
derivatives enhanced the stress-induced cell death process,
sensitizing the moderate level of ROS in the latent cells.
The present study clearly demonstrates the
regulatory mechanisms behind the stress management and cell death
process, where autophagy was found to be the vital player.
Autophagy is the major cellular mechanism which is modulated KSHV
during malignancy. A moderate level of autophagy helps in latency
maintenance and is increased during the lytic phase, assisting in
virus reactivation and infection. This autophagy is ROS-responsive
and it can also regulate the cellular ROS level. The present study
demonstrated that the KSHV stress balance system utilized the
existing autophagy, increasing the levels to manage the extra
stress induced by serum starvation during latency (Fig. 7C). However, following treatment
with theaflavin and curcumin derivatives, the moderate level of
autophagy was switched towards apoptosis. Of note, these two
antioxidants successfully managed to overcome this
autophagy-mediated stress recovery. Moreover, the curcumin
derivatives induced autophagy-independent apoptosis at the
respective concentrations. The molecular analysis of autophagy in
the treated cells revealed that autophagy was actually sensitizing
the cells towards death. In both cases of drug treatment, different
stress regulatory signaling pathways were altered and drove the
cells towards death. The stress-induced DNA damage signaling was
found to be activated, where Chk-1 and Chk-2 levels were found to
be notably altered. The damaged mitochondria in cancer cells
continue to persist and produce excess amounts of reactive oxygen
during cancer progression (30).
Both compounds used herein were found to destroy these mitochondria
and induce mitochondria-mediated caspase-dependent apoptosis.
Lastly, mTOR signaling, which is the sensory signaling between the
varied ROS response and autophagy, has been found to be (Fig. 7D) altered during treatment. The
present study demonstrated that an enhanced cellular autophagy
represented with its identifying markers (Figs. 7C and S1) was required for the cancer cell
stress management in a cellular environment lacking apoptotic
modulators. This autophagy is converted to apoptosis by the altered
interplay of several decisive switching molecules, such as p53,
Beclin-1, BAX, Bcl-2 and caspase following treatment with the
compounds (Figs. 1, and 7). Overall, it was clearly demonstrated
that different cellular stress responsive targets, which are
utilized by the virus and act in favor of cancer progression, are
specifically targeted by the antioxidants for the destruction of
the cancer cells. Moreover, in relation to this, different KSHV
viral genes have been recently reported to protect the viral
replication process during hypoxia-induced stress (9,61).
In association to these findings, the present study clearly
emphasized the fact that these stress-tolerant oncogenic cells can
be targeted by the antioxidants, which specifically affects the
stress responsive adaptive regulatory pathways, utilizes the same
mechanisms and makes the cells sensitive to the cellular stress
response leading to apoptosis (Fig.
8B).
KSHV-associated malignancies are the major secondary
type of cancer in patients with HIV. On the other hand,
tuberculosis is another common co-infection among patients with
HIV. Such an association between these three entities is very
common in India (62). It has
been found that patients with HIV are in a state of high oxidative
stress expression, due to the dual role of infection and the extra
burden of prolonged antiretroviral therapy for both tuberculosis
and HIV treatment. Thus, this high ROS response in the system
enhances the chance of further infections, such as KSHV, which lead
to a very poor prognosis without any further treatment (11,25,63). Strategically, treatment modalities
need to be identified which can nullify this stressful
multi-infectious malignant environment. The findings presented
herein demonstrate that the antioxidative approach can withstand
such stress recovery mechanisms by the virus. These compounds can
efficiently overcome the oncogenic stress management system. In the
field of antioxidative anticancer therapy, NAC is an effective
FDA-approved agent. NAC has been reported to minimize the KSHV
invasiveness in vivo, but it is limited in its clinical
application with chemotherapy against this malignancy (29). The present study with the
comparison of NAC also supports the pharmacological efficacy of
these compounds as antioxidants. Collectively, the present study
proves the affectivity of the antioxidants against KSHV malignancy
which eliminate any chance of deleterious side-effects by
chemotherapeutics and their further use as a natural anti KSHV
malignant strategy. A limitation of the present study is that only
an in vitro cell line model was used. Understanding the
therapeutic efficacy of these antioxidants against KSHV-infected
in vivo models with varied stress responses is mandatory.
Thus, further studies are required to fully determine the clinical
applicability of these compounds.
Supplementary Data
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
PD contributed to the conception and design of the
study, and was involved in conducting the experiment and in data
acquisition. PD also analyzed the data and drafted the manuscript.
TC performed data interpretation, correction, and the editing and
reviewing of the manuscript. GB assisted with the design, synthesis
and characterization, as well as with the supply of the novel
curcumin derivatives. KC assisted in evaluating the results and
reviewing the article. All the authors in the study made
substantial contributions to the study and have read and approved
the final version of the manuscript. PD and TC confirm the
authenticity of all the raw data.
Ethics approval and consent to
participate
The present study was performed following the
guidelines of the and following the approval of the Visva-Bharati
University Human Ethical Committee (Ref. no. IECHR/VB/8017/18).
Healthy individuals participated in the study. Written informed
consent was obtained from each individual participant for sample
collection and for utilization of the data for research
purposes.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
Acknowledgments
The authors are thankful to the organic chemistry
laboratory at the Department of Chemistry, Visva-Bharati
University, Santiniketan, India, where the synthesis of the novel
curcumin derivatives was performed. The authors would like to
acknowledge Dr Jason S. Knight (Division of Rheumatology,
University of Michigan, Ann Arbor, MI, USA), for kindly assisting
in supervising the scientific writing during the preparation of the
manuscript. The authors also sincerely acknowledge the laboratory
attendant, Mr. Laltu Hazra, who assisted with the general technical
procedures during the study.
Funding
The authors are thankful to the Council of Scientific and
Industrial Research (CSIR. Government of India) for assisting with
the CSIR-SRF fellowship [Fellowship ID: 09/202(0075) 2K18 EMRI] and
the contingency grant (DBT Project Contingency Grant No:
BT/PR7044/MED/29/B55/2014).
References
|
1
|
Yarchoan R and Uldrick TS: HIV-associated
cancers and related diseases. N Eng J Med. 378:1029–1041. 2018.
View Article : Google Scholar
|
|
2
|
Stewart A, Chan Carusone S, To K,
Schaefer-McDaniel N, Halman M and Grimes R: Causes of death in HIV
patients and the evolution of an AIDS hospice: 1988-2008. AIDS Res
Treat. 2012:3904062012.PubMed/NCBI
|
|
3
|
Aneja KK and Yuan Y: Reactivation and
lytic replication of Kaposi's sarcoma-associated herpesvirus: An
update. Front Microbiol. 8:6132017. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Miller G, Heston L, Grogan E, Gradoville
L, Rigsby M, Sun R, Shedd D, Kushnaryov VM, Grossberg S and Chang
Y: Selective switch between latency and lytic replication of
Kaposi's sarcoma herpesvirus and Epstein-Barr virus in dually
infected body cavity lymphoma cells. J Virol. 71:314–324. 1997.
View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Ye F, Zhou F, Bedolla RG, Jones T, Lei X,
Kang T, Guadalupe M and Gao SJ: Reactive oxygen species hydrogen
peroxide mediates Kaposi's sarcoma-associated herpesvirus
reactivation from latency. PLoS Pathog. 7:e10020542011. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Li X, Feng J and Sun R: Oxidative stress
induces reactivation of Kaposi's sarcoma-associated herpesvirus and
death of primary effusion lymphoma cells. J Virol. 85:715–724.
2011. View Article : Google Scholar :
|
|
7
|
Bottero V, Chakraborty S and Chandran B:
Reactive oxygen species are induced by Kaposi's sarcoma-associated
herpesvirus early during primary infection of endothelial cells to
promote virus entry. J Virol. 87:1733–1749. 2013. View Article : Google Scholar :
|
|
8
|
Davis DA, Rinderknecht AS, Zoeteweij JP,
Aoki Y, Read-Connole EL, Tosato G, Blauvelt A and Yarchoan R:
Hypoxia induces lytic replication of Kaposi sarcoma-associated
herpesvirus. Blood. 97:3244–3250. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Singh RK, Lamplugh ZL, Lang F, Yuan Y,
Lieberman P, You J and Robertson ES: KSHV-encoded LANA protects the
cellular replication machinery from hypoxia induced degradation.
PLOS Pathogens. 15. pp. e10080252019, View Article : Google Scholar
|
|
10
|
Granato M, Gilardini Montani MS,
Angiolillo C, D'Orazi G, Faggioni A and Cirone M: Cytotoxic drugs
activate KSHV lytic cycle in latently infected PEL cells by
inducing a moderate ROS increase controlled by HSF1, NRF2 and
p62/SQSTM1. Viruses. 11:82018. View Article : Google Scholar
|
|
11
|
Sharma B: Oxidative stress in HIV patients
receiving antiretroviral therapy. Curr HIV Res. 12:13–21. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Saavedra-García P, Roman-Trufero M,
Al-Sadah HA, Blighe K, López-Jiménez E, Christoforou M, Penfold L,
Capece D, Xiong X, Miao Y, et al: Systems level profiling of
chemotherapy-induced stress resolution in cancer cells reveals
druggable trade-offs. Proc Natl Acad Sci. 118:e20182291182021.
View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Singh K, Bhori M, Kasu YA, Bhat G and
Marar T: Antioxidants as precision weapons in war against cancer
chemotherapy induced toxicity - Exploring the armoury of obscurity.
Saudi Pharm J. 26:177–190. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Mut-Salud N, Álvarez PJ, Garrido JM,
Carrasco E, Aránega A and Rodríguez-Serrano F: Antioxidant intake
and antitumor therapy: Toward nutritional recommendations for
optimal results. Oxid Med Cell Longev. 2016:67195342016. View Article : Google Scholar
|
|
15
|
Bouayed J and Bohn T: Exogenous
antioxidants--Double-edged swords in cellular redox state: Health
beneficial effects at physiologic doses versus deleterious effects
at high doses. Oxid Med Cell Longev. 3:228–237. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Brahmachari G and Mandal M: One-pot
multicomponent synthesis of a new series of curcumin-derived
4H-pyrans under ambient conditions. J Heterocycl Chem. 57:744–750.
2020. View Article : Google Scholar
|
|
17
|
Broussard G and Damania B: Regulation of
KSHV latency and lytic reactivation. Viruses. 12:10342020.
View Article : Google Scholar :
|
|
18
|
Gam Ze Letova C, Kalt I, Shamay M and
Sarid R: Latently KSHV-infected cells promote further establishment
of latency upon superinfection with KSHV. Int J Mol Sci.
22:119942021. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Raja R, Lata S, Trivedi S and Banerjea AC:
Serum deprivation/starvation leads to reactivation of HIV-1 in
latently infected monocytes via activating ERK/JNK pathway. Sci
Rep. 8:144962018. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Iki S, Yokota S, Okabayashi T, Yokosawa N,
Nagata K and Fujii N: Serum-dependent expression of promyelocytic
leukemia protein suppresses propagation of influenza virus.
Virology. 343:106–115. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Palmisano I, Della Chiara G, D'Ambrosio
RL, Huichalaf C, Brambilla P, Corbetta S, Riba M, Piccirillo R,
Valente S, Casari G, et al: Amino acid starvation induces
reactivation of silenced transgenes and latent HIV-1 provirus via
down-regulation of histone deacetylase 4 (HDAC4). Proc Natl Acad
Sci USA. 109:E2284–E2293. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Kumar A, Mohanty S, Das P, Sahu SK,
Rajasubramaniam S and Choudhuri T: 1, 25(OH)2 D3 induces
reactivation and death of Kaposi's sarcoma-associated herpesvirus
of primary effusion lymphoma cells. Sci Rep. 7:124382017.
View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Mohanty S, Kumar A, Das P, Sahu SK and
Choudhuri T: Multi-targeted therapy of everolimus in Kaposi's
sarcoma associated herpes virus infected primary effusion lymphoma.
Apoptosis. 22:1098–1115. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(-Delta Delta C(T)) method. Methods. 25:402–408. 2001.
View Article : Google Scholar
|
|
25
|
Popoola TD and Awodele O: Interplay
between antiretroviral therapy and oxidative stress in HIV
seropositive patients. Afr J Med Med Sci. 45:5–21. 2016.PubMed/NCBI
|
|
26
|
Mandas A, Iorio EL, Congiu MG, Balestrieri
C, Mereu A, Cau D, Dessì S and Curreli N: Oxidative imbalance in
HIV-1 infected patients treated with antiretroviral therapy. J
Biomed Biotechnol. 2009:7495752009. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Acosta Davila JA and Hernandez De Los Rios
A: An overview of peripheral blood mononuclear cells as a model for
immunological research of toxoplasma gondii and other apicomplexan
parasites. Front Cell Infect Microbiol. 9:24. 2019. View Article : Google Scholar :
|
|
28
|
Albini A, Morini M, D'Agostini F, Ferrari
N, Campelli F, Arena G, Noonan DM, Pesce C and De Flora S:
Inhibition of angiogenesis-driven Kaposi's sarcoma tumor growth in
nude mice by oral N-Acetylcysteine. Cancer Res. 61:8171–8178.
2001.PubMed/NCBI
|
|
29
|
Ma Q, Cavallin LE, Yan B, Zhu S, Duran EM,
Wang H, Hale LP, Dong C, Cesarman E, Mesri EA and
Goldschmidt-Clermont PJ: Antitumorigenesis of antioxidants in a
transgenic Rac1 model of Kaposi's sarcoma. Proc Natl Acad Sci.
106:8683–8688. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Ralph SJ, Rodríguez-Enríquez S, Neuzil J,
Saavedra E and Moreno-Sánchez R: The causes of cancer revisited:
'mitochondrial malignancy' and ROS-induced oncogenic
transformation-why mitochondria are targets for cancer therapy. Mol
Aspects Med. 31:145–170. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Wang CH, Wu SB, Wu YT and Wei YH:
Oxidative stress response elicited by mitochondrial dysfunction:
Implication in the pathophysiology of aging. Exp Biol Med
(Maywood). 238:450–460. 2013. View Article : Google Scholar
|
|
32
|
Gilardini Montani MS, Santarelli R,
Granato M, Gonnella R, Torrisi MR, Faggioni A and Cirone M: EBV
reduces autophagy, intracellular ROS and mitochondria to impair
monocyte survival and differentiation. Autophagy. 15:652–667. 2019.
View Article : Google Scholar :
|
|
33
|
Balistreri G, Viiliäinen J, Turunen M,
Diaz R, Lyly L, Pekkonen P, Rantala J, Ojala K, Sarek G, Teesalu M,
et al: Oncogenic herpesvirus utilizes stress-induced cell cycle
checkpoints for efficient lytic replication. PLoS Pathog.
12:e10054242016. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
McGeoch DJ and Davison AJ: The descent of
human herpesvirus 8. Semin Cancer Biol. 9:201–209. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Leidal AM, Cyr DP, Hill RJ, Lee PW and
McCormick C: Subversion of autophagy by Kaposi's sarcoma-associated
herpesvirus impairs oncogene-induced senescence. Cell Host Microbe.
11:167–180. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Vescovo T, Pagni B, Piacentini M, Fimia GM
and Antonioli M: Regulation of autophagy in cells infected with
oncogenic human viruses and its impact on cancer development. Front
Cell Dev Biol. 8:472020. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Liang C: Viral FLIPping autophagy for
longevity. Cell Host Microbe. 11:101–103. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Granato M, Santarelli R, Filardi M,
Gonnella R, Farina A, Torrisi MR, Faggioni A and Cirone M: The
activation of KSHV lytic cycle blocks autophagy in PEL cells.
Autophagy. 11:1978–1986. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Wen HJ, Yang Z, Zhou Y and Wood C:
Enhancement of autophagy during lytic replication by the Kaposi's
sarcoma-associated herpesvirus replication and transcription
activator. J Virol. 84:7448–7458. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Pattingre S and Levine B: Bcl-2 inhibition
of autophagy: A new route to cancer? Cancer Res. 66:2885–2888.
2006. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Ciechomska IA, Goemans GC, Skepper JN and
Tolkovsky AM: Bcl-2 complexed with Beclin-1 maintains full
anti-apoptotic function. Oncogene. 28:2128–2141. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Yang B, Liu Q and Bi Y: Autophagy and
apoptosis are regulated by stress on Bcl2 by AMBRA1 in the
endoplasmic reticulum and mitochondria. Theor Biol Med Model.
16:182019. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Qu Y, Wang X, Zhu Y, Wang W, Wang Y, Hu G,
Liu C, Li J, Ren S, Xiao MZ, et al: ORF3a-mediated incomplete
autophagy facilitates severe acute respiratory syndrome
coronavirus-2 replication. Front Cell Dev Biol. 9:7162082021.
View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Yousefi S, Perozzo R, Schmid I, Ziemiecki
A, Schaffner T, Scapozza L, Brunner T and Simon HU:
Calpain-mediated cleavage of Atg5 switches autophagy to apoptosis.
Nat Cell Biol. 8:1124–1132. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Galluzzi L, Vitale I, Abrams JM, Alnemri
ES, Baehrecke EH, Blagosklonny MV, Dawson TM, Dawson VL, El-Deiry
WS, Fulda S, et al: Molecular definitions of cell death
subroutines: Recommendations of the nomenclature committee on cell
death. 2012.Cell Death Differ. 19:107–120. 2012. View Article : Google Scholar
|
|
46
|
Paquette M, El-Houjeiri L and Pause A:
mTOR pathways in cancer and autophagy. Cancers (Basel). 10:182018.
View Article : Google Scholar
|
|
47
|
Zhang J, Gao Z and Ye J: Phosphorylation
and degradation of S6K1 (p70S6K1) in response to persistent JNK1
activation. Biochim Biophys Acta. 1832:1980–1988. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
48
|
Nichols LA, Adang LA and Kedes DH:
Rapamycin blocks production of KSHV/HHV8: Insights into the
anti-tumor activity of an immunosuppressant drug. PLoS One.
6:e145352011. View Article : Google Scholar : PubMed/NCBI
|
|
49
|
O'Neill EJ, Termini D, Albano A and Tsiani
E: Anti-cancer properties of theaflavins. Molecules. 26:9872021.
View Article : Google Scholar : PubMed/NCBI
|
|
50
|
Kuo CJ, Huang CC, Chou SY, Lo YC, Kao TJ,
Huang NK, Lin C, Lin HC, Lin HC and Lee YC: Potential therapeutic
effect of curcumin, a natural mTOR inhibitor, in tuberous sclerosis
complex. Phytomedicine. 54:132–139. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
51
|
Beevers CS, Zhou H and Huang S: Hitting
the golden TORget: Curcumin's effects on mTOR signaling. Anticancer
Agents Med Chem. 13:988–994. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
52
|
Kopustinskiene DM, Jakstas V, Savickas A
and Bernatoniene J: Flavonoids as anticancer agents. Nutrients.
12:4572020. View Article : Google Scholar :
|
|
53
|
Liou GY and Storz P: Reactive oxygen
species in cancer. Free Radic Res. 44:479–496. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
54
|
Tomeh MA, Hadianamrei R and Zhao X: A
review of curcumin and its derivatives as anticancer agents. Int J
Mol Sci. 20:10332019. View Article : Google Scholar :
|
|
55
|
Uddin S, Hussain AR, Manogaran PS,
Al-Hussein K, Platanias LC, Gutierrez MI and Bhatia KG: Curcumin
suppresses growth and induces apoptosis in primary effusion
lymphoma. Oncogene. 24:7022–7030. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
56
|
Zhong C, Xu M, Wang Y, Xu J and Yuan Y: An
APE1 inhibitor reveals critical roles of the redox function of APE1
in KSHV replication and pathogenic phenotypes. PLoS Pathog.
13:e10062892017. View Article : Google Scholar : PubMed/NCBI
|
|
57
|
Li H, Zhong C, Wang Q, Chen W and Yuan Y:
Curcumin is an APE1 redox inhibitor and exhibits an antiviral
activity against KSHV replication and pathogenesis. Antiviral Res.
167:98–103. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
58
|
Stone WL, Krishnan K, Campbell SE and
Palau VE: The role of antioxidants and pro-oxidants in colon
cancer. World J Gastrointest Oncol. 6:55–66. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
59
|
Gerhardt T, Jones R, Park J, Lu R, Chan
HW, Fang Q, Singh N and Lai H: Effects of antioxidants and
pro-oxidants on cytotoxicity of dihydroartemisinin to molt-4 human
leukemia cells. Anticancer Res. 35:18672015.PubMed/NCBI
|
|
60
|
Adhikary A, Mohanty S, Lahiry L, Hossain
DMS, Chakraborty S and Das T: Theaflavins retard human breast
cancer cell migration by inhibiting NF-κB via p53-ROS cross-talk.
FEBS Lett. 584:7–14. 2010. View Article : Google Scholar
|
|
61
|
Kumar Singh R, Pei Y, Bose D, Lamplugh ZL,
Sun K, Yuan Y, Lieberman P, You J and Robertson ES: KSHV-encoded
vCyclin can modulate HIF1α levels to promote DNA replication in
hypoxia. Elife. 10:e574362021. View Article : Google Scholar
|
|
62
|
Das P, Roy Chattopadhyay N, Chatterjee K
and Choudhuri T: Kaposi's sarcoma-associated herpesvirus related
malignancy in India, a rare but emerging member to be considered.
VirusDisease. 31:209–219. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
63
|
Tasca KI, Caleffi JT, Correa CR, Gatto M,
Tavares FC, Camargo CC, Sartori A, Biasin M and de Souza LD:
Antiretroviral therapy initiation alters the redox system of
asymptomatic HIV-infected individuals: A longitudinal study. Oxid
Med Cell Longev. 2017:98348032017. View Article : Google Scholar : PubMed/NCBI
|