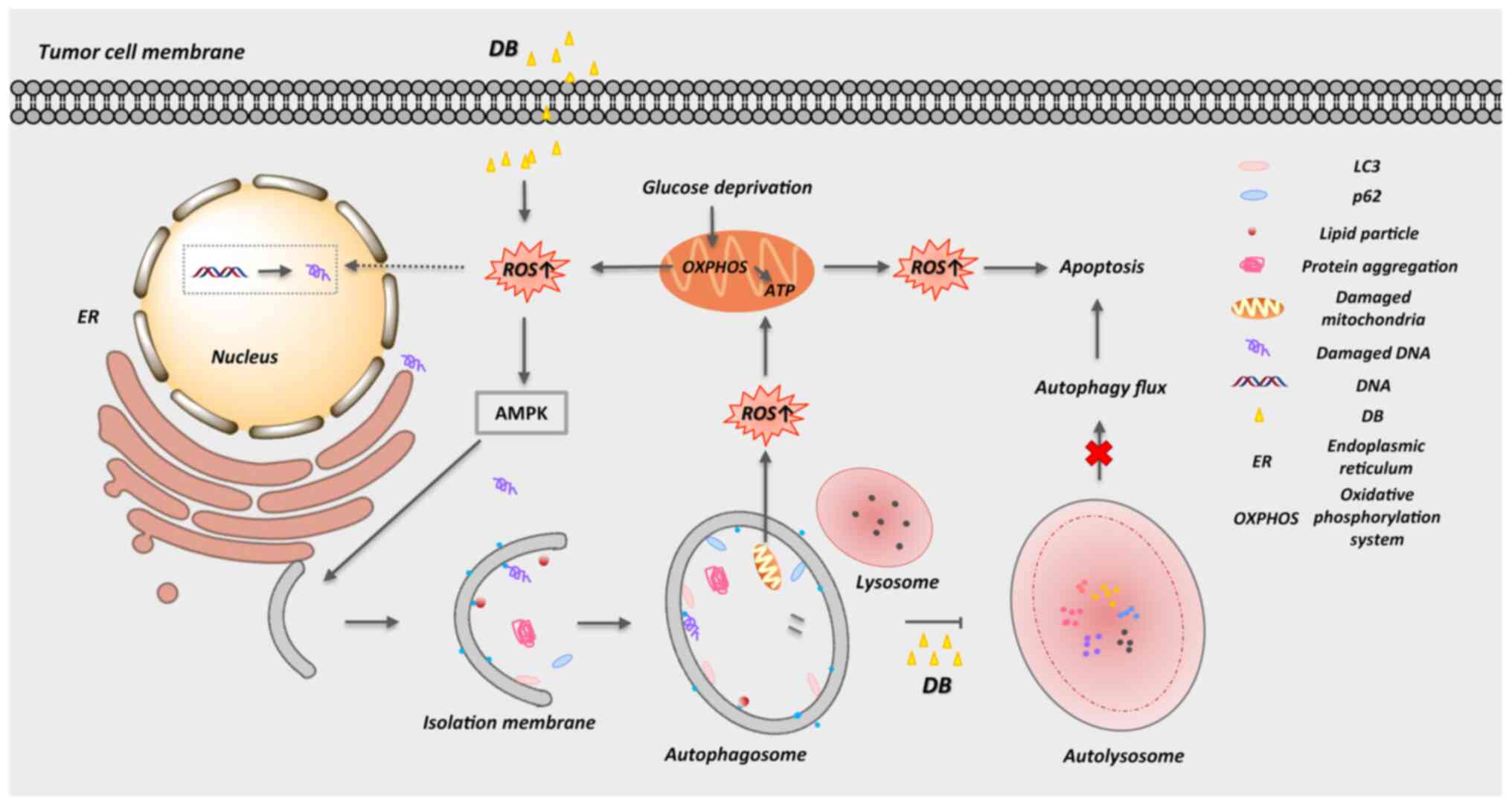
Introduction
Breast cancer is known as one of the leading causes
of cancer-related mortality and morbidity among women worldwide
(1-3). There is strong evidence for
considering early-stage breast cancer potentially curable; however,
concerning late-stage or metastatic breast cancer, currently
available therapeutic approaches are only able to prolong the
survival and maintain the quality of life of patients. Due to its
highly drug-resistant and invasive nature, and its proclivity for
recurrence and metastasis, triple-negative breast cancer [human
epidermal growth factor receptor-2 (HER2)-, estrogen receptor (ER)-
and prostaglandin receptor (PgR)-] is particularly lethal, with no
effective treatments available (4-7).
Autophagy is an ubiquitous catabolic process in
animal cells. It has been suggested that autophagy plays a crucial
role in the growth and development of a variety of cells (8,9).
As an evolutionary conserved adaptive process, autophagy can
sequester long-lived, aggregated and misfolded proteins along with
damaged organelles via the formation of autophagosomes, which then
fuse with lysosomes, in which cargos are degraded and recycled
(10,11). The biological and clinical
significance of autophagy in cancer stems from its complex role in
the tumor microenvironment (12).
Autophagy has been revealed to suppress early cancer development,
while facilitating advanced tumor progression (13-17). The pharmacological regulation of
autophagy as a valid strategy in certain types of cancer, including
breast cancer, and has been demonstrated to enhance the efficacy of
therapeutics and to overcome resistance (18-21).
Marine microbes are a significant source of lead
compounds in drugs that have been scientifically validated to exert
marked anticancer, anti-bacterial and pro-apoptotic effects, and to
regulate immunity in cell and animal models (22,23). There is accumulating evidence to
indicate that purified natural therapeutics may remove impurities
and toxic components, and enhance the curative effects in relation
to fully synthetic therapeutics. In addition, various natural
Chinese medicines such as Genkwadaphnin, dihydroartemisinin, etc.
when combined with radiotherapy and chemotherapy, have been
reported to not only reduce the side-effects of drugs, but to also
significantly enhance their antitumor effects (24). Therefore, there is considerable
interest in investigating marine natural products. Since 2010,
dicitrinone A-F (DA-DF) has been successively discovered from the
volcano ash-derived or marine-derived fungus, Penicillium
citrinum. Those novel carbon-bridged citrinin dimers, as
natural polyketones, have anti-tumor, anti-bacterial, anti-oxidant
and microtubule targeting properties (25-28). Previous studies have demonstrated
that DA-DD has a similar structure and can significantly inhibit
the proliferation of HL-60, MOLT-4, A-549, BEL-7402 and SPC-A1
cells, of which dicitrinone B (DB) has the best anti-tumor activity
(25-27). The structures of DE and DF are
relatively different with those of DA-DD, and they have no
antitumor activity (28).
Previous research by the authors has revealed that DB induces the
apoptosis of human malignant melanoma A375 cells by increasing
reactive oxygen species (ROS) generation, and this process is
related to the regulation of Bcl-2 family proteins (25). However, the anticancer effects of
DB and its detailed mechanisms of action in breast cancer remain
unclear. The novel findings of the present study (to the best of
our knowledge) indicate that DB may be a potential autophagy
inhibitor with anticancer activity; however, further preclinical
research is required in order to develop effective treatments for
breast cancer.
Materials and methods
Reagents and antibodies
The compound DB was separated from the fermentation
product of Penicillium citrinum and purified by various
separation and purification methods, such as extraction, column
chromatography, and high-performance liquid chromatography, the
purity of which was >95%, dissolved in dimethyl sulphoxide
(DMSO, cat. no. 196055, MP Biomedicals, LLC) to yield a stock
solution at 20 mM and stored at -20°C. Chloroquine (CQ; C129284)
was purchased from Shanghai Aladdin Biochemical Technology Co.,
Ltd. and dissolved in phosphate-buffered saline (PBS). Adriamycin
(ADM; cat. no. D807083) was purchased from Shanghai Macklin
Biochemical Co., Ltd. and dissolved in PBS. Rapamycin (RAPA; cat.
no. HY-10219) was purchased from MedChemExpress and dissolved in
PBS. Acridine orange (AO; cat. no. cM07364) was purchased from
Beijing Bai'aolaibo Technology Co., Ltd. LysoTracker Red (cat. no.
C1046) and N-acetyl-L-cysteine (NAC; cat. no. S0077) were purchased
from the Beyotime Institute of Biotechnology. Bicinchoninic acid
(BCA; cat. no. MPK002) was purchased from MACGENE Biotechnology.
Microtubule-associated protein 1 light chain 3 beta (LC3 B),
microtubule-associated protein 1 light chain 3 alpha/beta (LC3A/B),
p62, Bcl-2, Bax, poly(ADP-ribose) polymerase (PARP), mTOR, Akt and
cleaved PARP antibodies (cat. nos. 3868S, 12741S, 16177S, 3498S,
5023S, 9532S, 2983S, 4691S and 5625S, respectively; rabbit) and
HRP-linked goat anti-rabbit IgG, HRP-linked anti-mouse IgG (cat.
nos. 7074P2 and 7076P2, respectively) were purchased from Cell
Signaling Technology, Inc. Cathepsin D (CTSD; cat. no. BM1577;
mouse), β-actin (cat. no. BM3873; rabbit), cofilin (cat. no.
PB9033; rabbit) and cathepsin B (CTSB; cat. no. A01456-3, rabbit)
antibodies were purchased from Wuhan Boster Biological Technology,
Ltd. FITC-linked goat anti-rabbit IgG (cat. no. ZF-0311) was
purchased from Beijing Zhongshan Jinqiao Biotechnology Co., Ltd.
Puromycin was purchased from InvivoGen (cat. no. ant-pr-1).
Cells and cell culture
The human breast cancer cell line MCF7 (cat. no.
TCHu 74) was purchased from the Shanghai Cell Resource Center and
cultured in Dulbecco's modified Eagle's medium (HyClone; Cytiva)
supplemented with 10% fetal bovine serum (Gemini Bio Products; cat.
no. 900-108) and 1.0% penicillin/streptomycin (cat. no. MA0110;
Dalian Meilun Biology Technology Co., Ltd.). The MDA-MB-231 cells
were a gift from Dr. Liu (Fujian Cancer Hospital, Fujian, China)
and were maintained in RPMI-1640 medium (HyClone; Cytiva)
containing 10% fetal bovine serum (Gemini Bio Products; cat. no.
900-108) and 1% penicillin/streptomycin (cat. no. MA0110; Dalian
Meilun Biotech, Co., Ltd.). All cells were cultured at 37°C in a
humidified atmosphere with 5% CO2.
Cell viability assay
Cell growth inhibition activity was examined using a
CellTiter 96® AQueous One Solution Cell Proliferation
Assay (cat. no. G3581, Promega Corporation) following the
manufacturer's instructions. Briefly, ~5,000 MCF7 or MDA-MB-231
cells were seeded per well in three replicates into 96-well plates
and treated with DMSO or serial dilutions of DB (5, 10, 20, 40 and
60 µM), ADM (0.5, 2.5 and 10 µM) or PTX (0.05, 0.1,
0.2 and 0.4 µM) for 24 or 48 h, cells were incubated at 37°C
in a humidified atmosphere with 5% CO2. In addition,
~5,000 MCF7 or MDA-MB-231 cells were seeded per well in three
replicates into 96-well plates and treated with DB (10 µM),
ADM (0.2 µM) and PTX (0.005 µM) alone or in
combination with DB (10 µM), ADM (0.2 µM) or PTX
(0.005 µM) for 24 and 48 h. Following treatment, MTS
solution (provided with the kit) was used to evaluate the viability
of cells. The absorbance was detected at 490 nm using a microplate
reader (SH-1000; Corona Electric Co., Ltd.).
Western blot analysis
Western blot analysis was performed as previously
described with minor modifications (25). In brief, the MCF7 or MDA-MB-231
cells were scraped in modified RIPA buffer (Wuhan Boster Biological
Technology, Ltd.) containing 1 mM PMSF (cat. no. P0100, Beijing
Solarbio Science & Technology Co., Ltd.) to prepare whole-cell
lysates. BCA Protein Assay kit (cat. no. P0012, Beyotime Institute
of Biotechnology) was used for protein quantification. Equal
aliquots of protein (30 µg) were separated on 12 or 15%
SDS-PAGE gels, transferred to a nitrocellulose membrane using the
wet transfer method, blocked using blocking buffer (1X TBST with 5%
w/v non-fat dry milk; purchased from Cell Signaling Technology,
Inc.; cat. no. 9999) for 1 h at room temperature and incubated with
antibodies specific for LC3B, p62, Bcl-2, Bax, PARP, cleaved PARP,
mTOR, Akt, CTSD, CTSB, β-actin and cofilin (all antibody cat. nos.
as described above; 1:1,000) overnight at 4°C and finally incubated
with HRP-linked goat anti-rabbit IgG (1:3,000) or HRP-linked goat
anti-mouse IgG (1:3,000) (all antibody cat. nos. as described
above) for 1 h at room temperature. Bands were automatically
visualized using a FluorChem E digital darkroom system
(ProteinSimple; Bio-Techne). Band intensities were quantified using
Image J 1.8.0.1 (National Institutes of Health), and densitometric
analysis was performed using GraphPad Prism 7.0 software (GraphPad
Software, Inc.).
Immunofluorescence assay
The MCF7 or MDA-MB-231 cells were grown on
coverslips in 12-well plates and treated with 20 µM DB or 5
µM RAPA or 60 µM CQ for 6 h. Following treatment, the
cells were fixed with 100% methanol for 5 min, blocked with BSA
(5%) (cat. no 9048-46-8; Beijing bai'aolaibo Technology Co., Ltd.)
and incubated with anti-LC3A/B (cat. no. D3U4C; 1:200) and
sequestosome 1 (SQSTM1)/p62 (Abcam, cat. no. D6M5X; 1:200) primary
antibodies at 4°C overnight. The coverslips were then incubated
with FITC-linked goat anti-rabbit IgG (1:100) at room temperature
for 1 h in the dark and examined under an Olympus inverted
fluorescence microscope (Olympus Corporation). Images were randomly
captured.
Analysis of autophagic flux
To analyze autophagic flux, ~10,000 MCF7 and
MDA-MB-231 cells were plated in 96-well plates, incubated at 37°C
in a humidified atmosphere with 5% CO2 for 24 h, then
transfected with the lentivirus pGMLV-CMV-RFP-GFP-hLC3-Puro (cat.
no. GM-3394LV, Genomeditech Co., Ltd.) at a MOI of 30 for 72 h at
37°C and then treated with 20 µM DB in the presence or
absence of 5 µM RAPA (MedChemExpress, cat. no. HY-10219) or
60 µM CQ (Aladdin, cat. no. C129284) for a further 6 h. The
MCF7 and MDA-MB-231 cells were pre-treated with 10 mM NAC (Beyotime
Instittue of Biotechnology, cat. no. S0077) for 1 h and incubated
with 20 µM DB for 6 h at 37°C. The stably transfected cells
were constructed by puromycin selection (cells were cultured in
culture medium with additional 10 µg/ml puromycin).
Puromycin was purchased from InvivoGen (cat. no. ant-pr-1).
Brightfield microscopy
Cell monolayers were cultured for 24 h in 12-well
glass-covered chamber slides and treated with 0, 5, 10 or 20
µM DB for 6 h. Micrographs were obtained using an Olympus
inverted fluorescence microscope (Olympus Corporation).
Apoptosis assay
An apoptosis assay was performed as we previously
described (29). In brief,
~1×106 MCF7 or MDA-MB-231 cells treated with 20
µM DB for 48 h (incubated at 37°C in a humidified atmosphere
with 5% CO2) were collected and washed twice with 1X
Annexin V binding buffer and resuspended in 100 µl of 1X
Annexin V binding buffer. Subsequently, the cells were stained
using an Annexin V-FITC/PI kit (BD Biosciences, cat. no. 556547).
The staining conditions were as follows: Incubation for 15 min at
room temperature in the dark. The samples were analyzed using a
FACScan flow cytometer (BD Biosciences). Statistical analysis was
performed using BD FlowJo™ V10 software.
AO staining
The MCF7 or MDA-MB-231 cells were cultured in
12-well glass-covered chamber slides and then treated with either
DB or CQ for 6 h. Following treatment, the cells were incubated
with AO for 15 min at 37°C in the dark and fixed with 100%
methanol. Fluorescent images were obtained using an Olympus
inverted fluorescence microscope (Olympus Corporation).
DCFH-DA and LysoTracker Red staining
The MCF7 or MDA-MB-231 cells (~1×106)
were collected and incubated with pre-warmed LysoTracker Red or
DCFH-DA (cat. no. C1046, Beyotime Institute of Biotechnology) for
30 min at 37°C in the dark. The cells were then washed twice in PBS
and resuspended in PBS. Fluorescence was measured with a flow
cytometer in the PI channel (emission=530 nm).
Transmission electron microscopy
(TEM)
Untreated and DB-treated cells were carefully
digested using trypsin (HyClone; Cytiva), washed with PBS, and
fixed in 2.5% glutaraldehyde (Phygene) for 24 h. Following washing
with PBS three times, the samples were dehydrated in graded acetone
solutions (30-100%) and then embedded in low-viscosity resin.
Subsequently, ultrathin sections were cut using the Leica Ultracut
UCT ultramicrotome (Leica Microsystems, Inc.) and stained with
uranyl acetate (Weill Corning Medicine) for 5 min at room
temperature. Samples were observed by using a FEI Tecnai 12 BioTwin
transmission electron microscope (FEI Company).
In vivo xenograft experiment
In this experiment, the mice used were female
BALB/cJ nude mice (5 to 6 weeks old, weighting 15-18 g, n=20 in
total) purchased from Gempharmatech Co., Ltd. All mice were kept in
an environment at 24°C with a 12-h light/dark cycle, with water and
food freely available. The human endpoints of the study were
primarily determined by tumors that should not exceed 20 mm in any
dimension, and also that mouse weight loss should be <20%. All
procedures complied with the standards of euthanasia according to
Laboratory Animal Guidelines (GB/T 39760-2021) issued by the
National Standardization Management Committee. The animal
experiments were reviewed and approved by The Animal Care and Use
Committee of Fujian Medical University (approval no. 2020-CAARM015)
and were carried out in accordance with the National Institutes of
Health Guide for Care and Use of Laboratory Animals. Mice used in
these studies were maintained in a clean, modified-barrier animal
facility, fed regular commercial mouse diet (Gempharmatech) under a
controlled light/dark cycle (12/12 h) and a controlled temperature
(20-23°C). In order to relieve mouse pain, inhalation anesthesia
was preferred; the mice inhaled 5% isoflurane (cat. no. 792632,
MilliporeSigma) until death. The mice were observed for a lack of a
heartbeat and respiration and for graying of mucous membranes for
at least 10 min to confirm death. Subsequently, 5×106
cells were injected subcutaneously into the left hindlimbs of the
nude mice. After 7 days of culture, the tumor volume was ~100
mm3. The mice were randomly divided into four groups
[PBS control, DB, adriamycin (ADM) and DB + ADM; 5 mice per group].
During this experiment, mouse health and behavior were monitored
daily. The injection dose of DB and ADM (Shanghai Macklin
Biochemical Co., Ltd.) was 10 mg/kg. Intratumoral injection was
used. The injection regimen was administered once every 2 days for
10 consecutive injections. The tumor length and width were measured
every 3 days, and volume was calculated according to the formula
(tumor volume=shortest diameter2 × longest diameter/2).
The body weight of the mice was measured every 3 days. All
experiments involving living mice followed Chinese experimental
animal welfare and ethical guidelines and made every effort to
minimize the pain of the animals. At the end of the experiment, the
mice were euthanized by intravenous injection of 2% sodium
pentobarbital (150 mg/kg). Death was verified by respiratory arrest
and dilated pupils.
Histological examination
After 21 days, the tumors were removed, and the
tumor tissue was fixed with 4% tumor tissue fixation solution.
Finally, the tissue was sent to Wuhan Sevicebio Technology Co.,
Ltd. for the immunohistochemical detection of Ki-67, TUNEL and
LC3.
Statistical analysis
Statistical analysis was performed using GraphPad
Prism 7.0 software (GraphPad Software, Inc.). All data are
expressed as the mean ± standard error of the mean (SEM) values,
and each experiment was performed at least three times. One-way
analysis of variance (ANOVA) followed by the Bonferroni's post hoc
test and the unpaired Student t-test were used to assess
statistical significance where appropriate. P<0.05 was
considered to indicate a statistically significant difference.
Results
DB is a novel autophagy modulator of MCF7
and MDA-MB-231 cells
To discover novel autophagy modulators for human
breast cancer, compounds isolated and purified from the marine
fungus, Penicillium citrinum, were all screened and it was
observed that treatment with one of the compounds, DB (Fig. 1A) for 6 h culminated in an
increase in cellular perinuclear vacuoles in a
concentration-dependent manner in MCF7 breast cancer cells and
MDA-MB-231 triple-negative breast cancer cells (Fig. 1B). Thus, it was hypothesized that
DB may be a potent autophagy modulator candidate and was thus used
for further analysis.
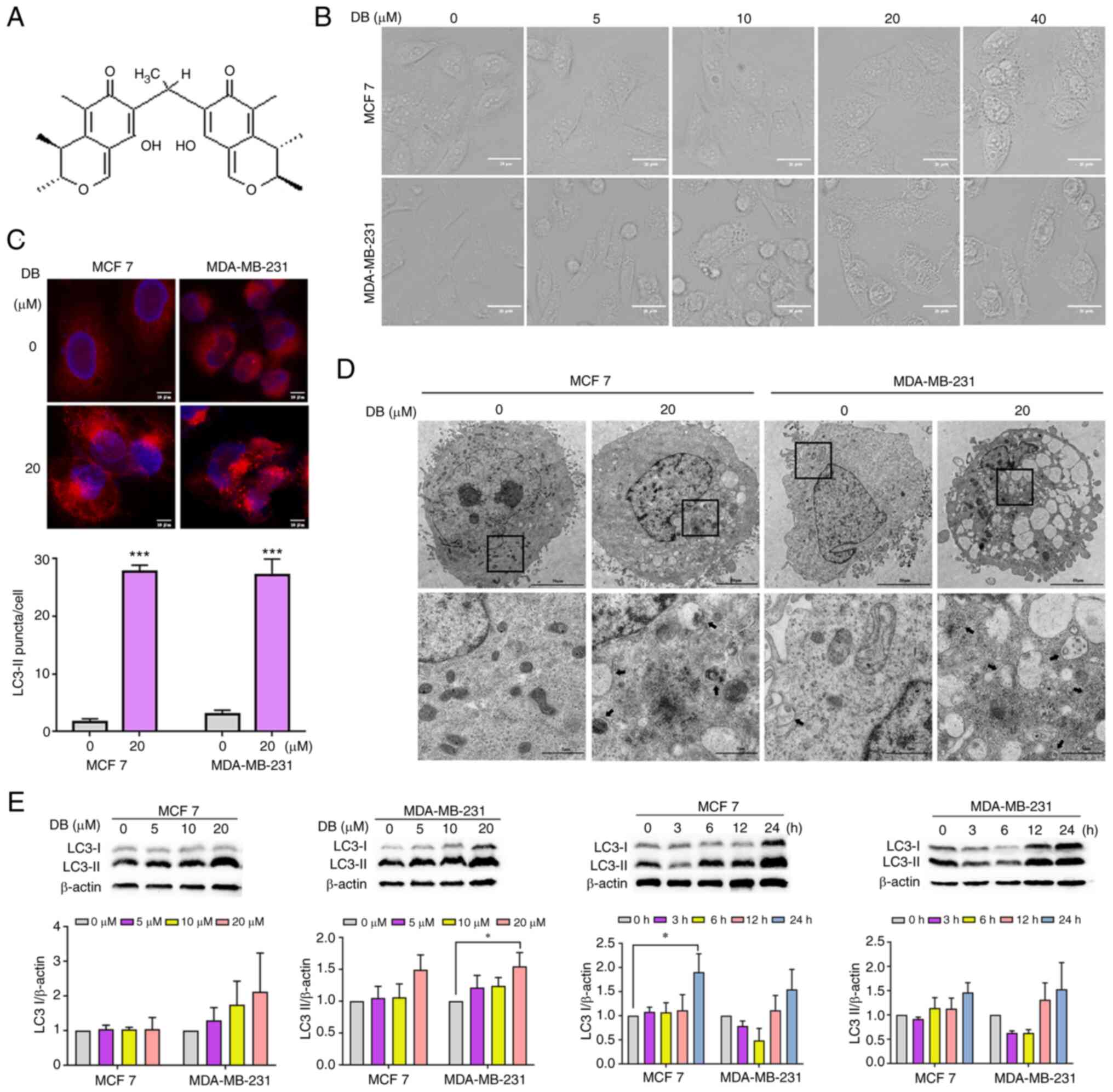 | Figure 1DB modulates autophagy in MCF7 and
MDA-MB-231 cells. (A) Chemical structure of DB. The molecular
weight of DB is 438 g/mol. (B) Representative microscopy images of
cells treated with increasing concentrations (0-40 µM) of DB
for 6 h. Scale bars, 20 µm. (C) Microscopy images of MCF7
and MDA-MB-231 cells following exposure to 20 µM DB for 6 h.
Scale bar, 10 µm. Representative fluorescence images of MCF7
and MDA-MB-231 cells expressing RFP-LC3 following treatment with DB
(10 µM) for 6 h. The numbers of RFP-LC3 puncta in each cell
were quantified using Image J 1.8.0.1 software. Scale bars, 10
µm. n=five microscopic fields per group;
***P<0.001 vs. the control. (D) Transmission electron
micrographs of MCF7 and MDA-MB-231 cells following incubation with
20 µM DB for 6 h; autophagic vacuoles are indicated by black
arrows. The bottom panels (scale bars, 1 µm) represent a
magnified image of the boxed region in the top panels (scale bars,
50 µm). (E) Representative western blots and corresponding
protein quantification plots of LC3 I/II protein expression in MCF7
and MDA-MB-231 cells incubated with increasing concentrations of DB
for 6 h or DB (20 µM) for various periods of time. β-actin
was used as the loading control. *P<0.05 vs. the
control. DB, dicitrinone B; RFP, red fluorescent protein; LC3,
microtubule associated protein 1 light chain 3; LC3 I, cytoplasmic
LC3; LC3 II, membrane-bound LC3. |
To explore this hypothesis, the analyses of LC3
immunofluorescence were performed in the MCF7 and MDA-MB-231 cells.
Presenting on the autophagosome during and after its formation, LC3
has been extensively studied as an autophagosome marker to monitor
autophagy (30,31). The results of the present study
demonstrated that treatment with DB for 6 h led to an increase in
the cell punctate number of fluorescence as compared with diffuse
fluorescence in the controls (Fig.
1C), confirming the influence of DB on the autophagic process.
Representative TEM photomicrographs depicted that DB increased the
number of double-membrane autophagosomes containing undigested
cytoplasmic cargos, further highlighting the capacity of DB to
modulate autophagy (Fig. 1D). For
a comparative and more detailed evaluation of DB to modulate
autophagy in MCF7 and MDA-MB-231 cells, western blot analysis of
cytoplasmic LC3 (LC3-I) and membrane-bound LC3 (LC3-II) was
performed. Notably, treatment with DB resulted in a marked increase
in LC3-II levels in both MCF7 and MDA-MB-231 cells in a
concentration- and time-dependent manner with respect to the
control cells, indicating the accumulation of autophagosomes
(Fig. 1E).
Inhibition of autophagic flux by DB in
MCF7 and MDA-MB-231 cells
The accumulation of autophagosomes can be associated
either with the induction of autophagy or the inhibition of
late-stage autophagy (32). To
distinguish the role of DB in autophagy in MCF7 and MDA-MB-231
cells, the levels of LC3-II in the presence and absence of the
autophagy inducer, RAPA, or the autophagy inhibitor, CQ, were
examined. As demonstrated in Fig.
2A, compared with cells treated with RAPA only, cells treated
with both RAPA and DB exhibited elevated LC3-II levels. By
contrast, co-incubation with DB and CQ did not result in a
significant increase in LC3-II levels, as compared with those in
cells treated with CQ only, indicating that DB is not an autophagy
inducer.
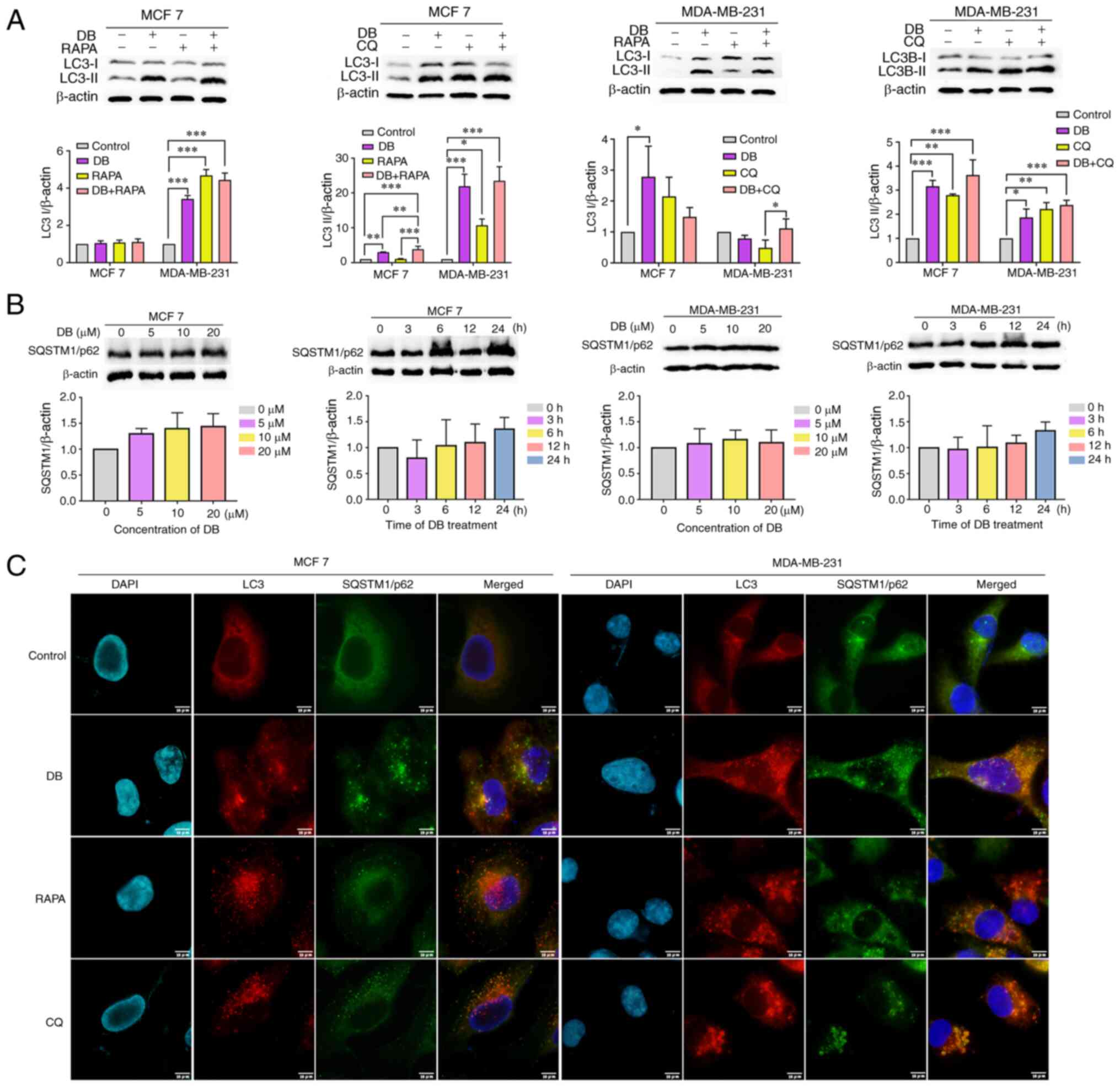 | Figure 2DB disrupts the autophagic flux. (A)
Representative western blots and corresponding protein
quantification plots of LC3-I/II protein expression in MCF7 and
MDA-MB-231 cells following treatment with DB in the presence or
absence of 5 µM RAPA or 60 µM CQ for 6 h. β-actin was
used as the loading control. *P<0.05,
**P<0.01 and ***P<0.001. (B)
Representative western blots and corresponding protein
quantification plots of SQSTM1/p62 protein expression in MCF7 and
MDA-MB-231 cells following treatment with increasing concentrations
of DB for 6 h or for different time periods (0-24 h) with 20
µM DB. β-actin was used as a loading control. (C)
Representative images of MCF7 and MDA-MB-231 cells treated with 20
µM DB in the presence or absence of 5 µM RAPA or 60
µM CQ for 6 h and subjected to anti-LC3-II (red),
anti-SQSTM1/p62 (green) and 4,6-DAPI (blue) staining. Scale bars,
10 µm. LC3, microtubule associated protein 1 light chain 3;
RAPA, rapamycin; CQ, chloroquine; DB, dicitrinone B; SQSTM1,
sequestosome 1; DAPI, diamidino-2-phenylindole. |
To further explore whether DB is an autophagy
inhibitor, the expression levels of SQSTM1/p62 were monitored using
western blot analysis. SQSTM1/p62 is known as an autophagy
inhibited marker, which is implicated in autophagic cargo
recognition and was lost in autolysosome degradation (33). Correspondingly, a concentration-
or time-dependent increase in the level of SQSTM1/p62 was observed
in the MCF7 and MDA-MB-231 cells following treatment with DB,
suggesting that DB probably blocked the autophagic flux (Fig. 2B). This phenomenon was further
verified using immunofluorescence-based assays of intracellular
accumulation of LC3-II and SQSTM1/p62 puncta. As depicted in
Fig. 2C, LC3-II colocalized well
with SQSTM1/p62 during DB treatment, similar to the finding in
cells treated with CQ, a late-stage inhibitor. By contrast, the
RAPA-treated cells exhibited a separated localization of LC3-II and
SQSTM1/p62, suggesting the autophagy-inducing role of RAPA. Based
on these findings, it was hypothesized that DB may modulate
autophagy by blocking the fusion of autophagosomes with lysosomes.
Taken together, these results demonstrated that DB interrupted the
autophagic flux at the late stage in MCF7 and MDA-MB-231 cells,
leading to autophagosome accumulation.
DB has no effect on the pH of
lysosomes
Considering that the blockade of autophagic flux may
be caused by the inhibition of autophagosome-lysosome fusion or the
impairment of lysosomal degradation, the effects of DB on the
autophagic flux were further investigated using a tandem
RFP-GFP-LC3 reporter. GFP is sensitive to the acidic environment of
lysosomes or autophagolysosomes, whereas RFP fluorescence remains
stable (34,35). Thus, autolysosomes can be observed
using RFP-LC3 fluorescence. The results revealed that similar to
treatment with the positive control CQ, DB treatment alone evoked
the accumulation of both RFP and GFP puncta, visible as yellow
fluorescence, in the MCF7 and MDA-MB-231 cells. Moreover, the
autophagosome/autolysosome ratios were further increased when CQ
was combined with DB treatment compared to DB treatment only,
indicating that most autophagosomes and lysosomes cannot fuse
(Fig. 3A).
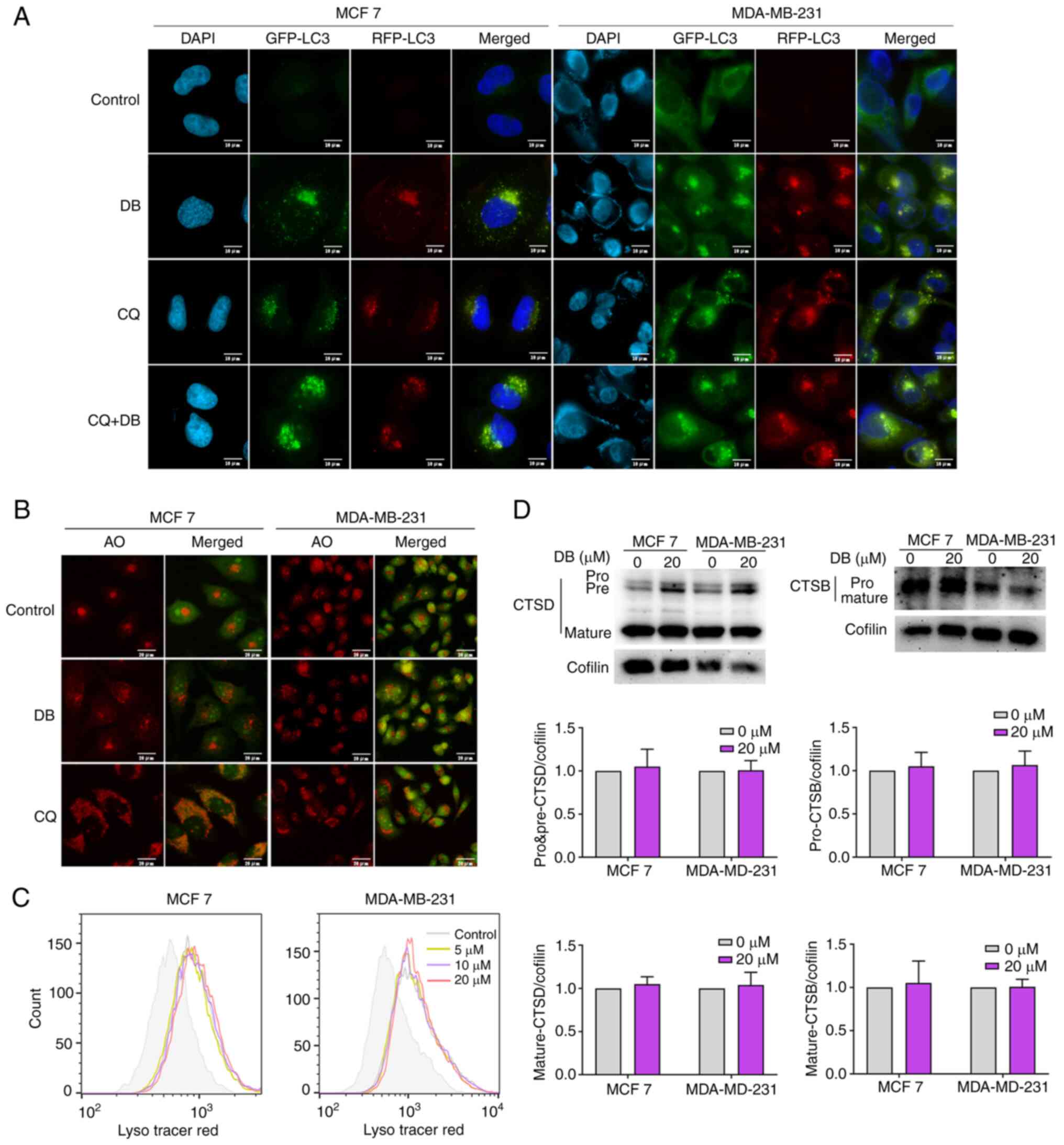 | Figure 3DB does not affect the pH or the
hydrolytic function of lysosomes. (A) Fluorescence images of MCF7
and MDA-MB-231 cells transfected with double fluorescent
mRFP-GFP-LC3 lentivirus and treated with DB, CQ alone or in
combination for 6 h. Scale bars, 10 µm. (B) Fluorescence
microscopy with AO staining. MCF7 and MDA-MB-231 cells were treated
with 20 µM DB or 60 µM CQ for 6 h and then stained
with AO. The red fluorescence represents the acidic vesicles. Scale
bars, 20 µm. (C) Flow cytometry for LysoTracker Red in MCF7
and MDA-MB-231 cells treated with DB (0, 5, 10 and 20 µM)
for 6 h. (D) Representative western blots and corresponding protein
quantification plots of CTSD and CTSB protein expression in MCF7
and MDA-MB-231 cells treated with 20 µM DB for 6 h. Cofilin
was used as a loading control. DB, dicitrinone B; RFP, red
fluorescent protein; GFP, green fluorescent protein; LC3,
microtubule associated protein 1 light chain 3; AO, acridine
orange; CQ, chloroquine; DB, dicitrinone B; CTSD, cathepsin D;
CTSB, cathepsin B. |
To explore the detailed mechanisms of DB in the
autophagic inhibition in MCF7 and MDA-MB-231 cells, the present
study then examined whether DB can alter the lysosomal pH. The MCF7
and MDA-MB-231 cells were stained with AO following exposure to DB
for 6 h. A large number of acidic vesicles with red fluorescence
were observed to be significantly augmented in response to DB
treatment, suggesting that DB could not inhibit lysosomal
acidification (Fig. 3B). To
further corroborate that the lysosomal pH was not altered by DB,
flow cytometry was used to evaluate the LysoTracker Red
fluorescence, which is used for labeling lysosomes and can be
quenched by increasing the pH. As demonstrated in Fig. 3C, compared with CQ treatment, DB
treatment resulted in a considerable increase in the acidic
compartment, suggesting that lysosomal pH was unaltered.
CTSD is known to hydrolyze proteins in the acidic
environment of lysosomes. Within the pH range of 2.8-5, CTSD can
degrade hormones, polypeptide precursors, polypeptides, structural
and functional proteins; however, it loses activity when the pH is
greater than 5.5. CTSB is a cysteine proteolytic enzyme in
lysosomes. It is active at pH 3.0~7.0 and is irreversibly
inactivated under alkaline conditions (36,37). Herein, to investigate the effects
of DB on lysosomal function, the levels of CTSD and CTSB in MCF7
and MDA-MB-231 cells were measured, and it was observed that the
expression levels of the pro-form and the mature forms of CTSD and
CTSB in cells treated with DB were not significantly different from
those in control cells (Fig. 3D).
Collectively, the findings of the present study suggested that the
DB-mediated blockade of autophagosome-lysosome fusion was not due
to impaired pH or lysosomal functionality.
DB inhibits autophagy via the
overproduction of ROS
It is worth mentioning that the natural compound,
DB, has previously been demonstrated to significantly augment
cellular ROS in the human malignant melanoma cell line, A375
(25). Therefore, it was
hypothesized that the role of DB in autophagy inhibition may be
associated with an increase in intracellular ROS production. To
verify this hypothesis, the ROS levels after DB treatment were
examined in MCF7 and MDA-MB-231 cells using the DCFH-DA probe. Data
from flow cytometric analysis revealed that cell exposure to DB led
to a consistent increase in the level of intracellular ROS
(Fig. 4A and B). In order to
verify further whether ROS is related to the PI3K/Akt pathway, the
expression levels of the PI3K/Akt pathway related proteins, Akt and
mTOR, were evaluated in MCF7 and MDA-MB-231 cells following DB
treatment. The results demonstrated that the expression of Akt and
mTOR in both cell lines increased along with DB concentration,
particularly in MDA-MB-231 cells (Fig. 4C). In addition, NAC, a scavenger
of ROS, was administered to determine whether excess ROS is
responsible for blocking autophagy by DB treatment. Flow cytometric
and western blot analyses of LC3-II revealed that pre-treatment
with NAC markedly reduced the conversion of LC3B-I to LC3B-II
(Fig. 4D).
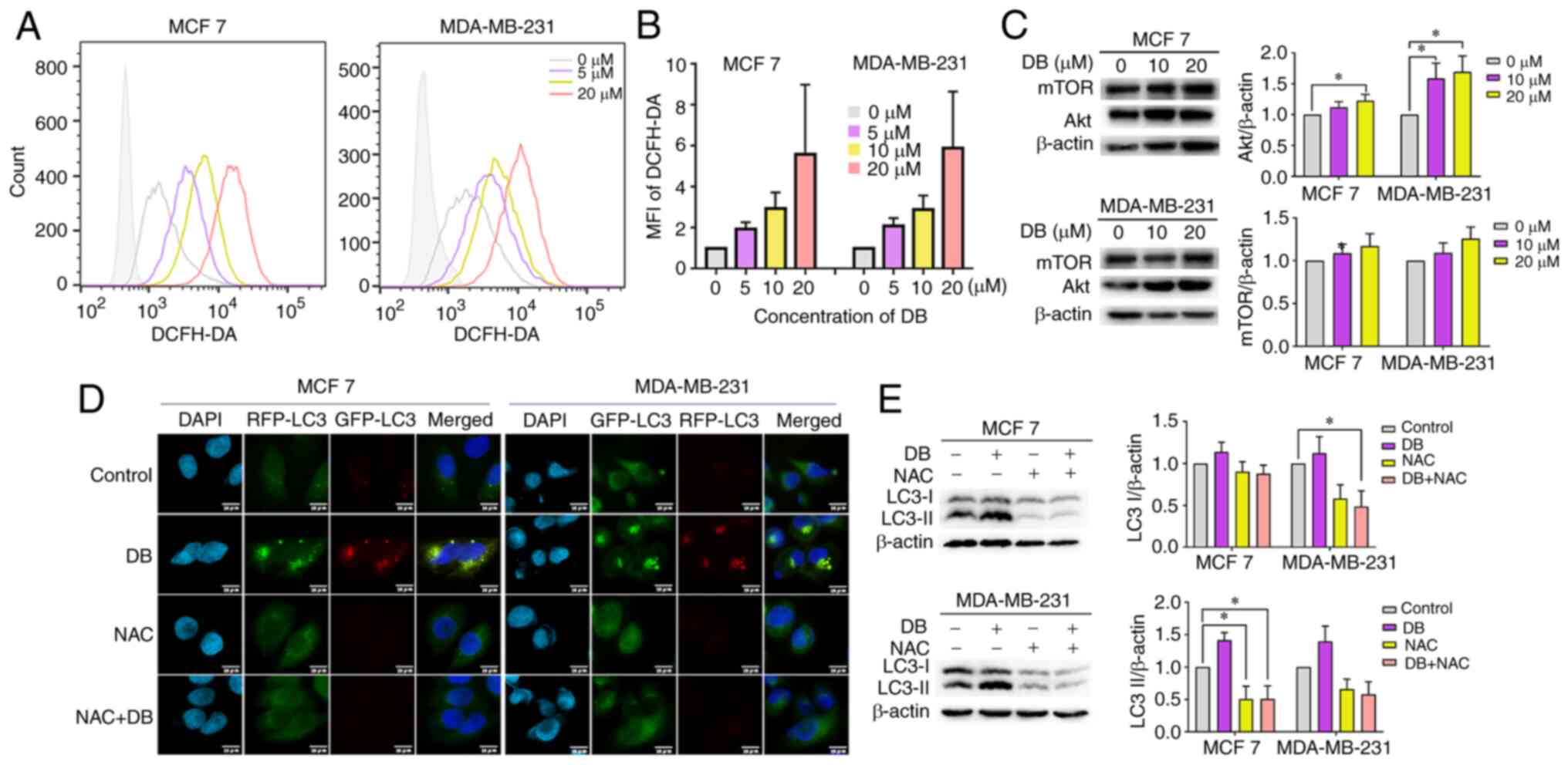 | Figure 4Promotion of intracellular ROS
generation is required for DB-induced autophagy inhibition. (A)
Flow cytometry of ROS levels in DB (0, 5, 10 and 20 µM 6
h)-treated MCF7 and MDA-MB-231 cells. (B) The fluorescence
intensity of each component was calculated according to the median
value of Fig. 4A. (C)
Representative western blots and corresponding protein
quantification plots of Akt and mTOR protein expression in MCF7 and
MDA-MB-231 cells, treated with 10, 20 µM DB for 6 h. β-actin
was used as the loading control. *P<0.05. (D)
Fluorescence images of mRFP-GFP-LC3 puncta in MCF7 and MDA-MB-231
cells treated with DB or NAC only or in combination. Scale bars, 10
µm. (E) The cells of MCF7 and MDA-MB-231 were pre-treated
with NAC (10 mM) for 1 h and incubated with 20 µM DB for 6
h. Representative western blots and corresponding protein
quantification plots of LC3-I and LC3-II expression levels were
examined using western blot analysis. β-actin was used as a loading
control. *P<0.05. ROS, reactive oxygen species; DB,
dicitrinone B; RFP, red fluorescent protein; GFP, green fluorescent
protein; NAC, N-acetyl-L-cysteine; LC3, microtubule associated
protein 1 light chain 3; LC3 I, cytoplasmic LC3; LC3 II,
membrane-bound LC3. |
To corroborate the findings from western blot
analysis, the present study then investigated the effects on
autophagic flux using tandem monomeric RFP-GFP-tagged LC3 in
response to NAC. Consistently, immunofluorescence micros-copy
revealed that pre-treatment with NAC significantly delayed the
formation of yellow fluorescence induced by DB in both MCF7 and
MDA-MB-231 cells (Fig. 4E),
revealing the significant involvement of ROS regulatory mechanisms.
Taken together, the results of the present study suggested that
DB-induced autophagy inhibition in breast cancer cells was in fact
modulated via the overproduction of ROS.
DB induces MCF7 and MDA-MB-231 cell
apoptosis via autophagy inhibition
To reveal the cell fate of MCF7 and MDA-MB-231 after
autophagy inhibition and ROS explosion evoked by DB treatment, MTS
assays were then performed to evaluate the cytotoxicity of DB in
MCF7 and MDA-MB-231 cells. The data demonstrated that the number of
MCF7 and MDA-MB-231 cells and their metabolic activity markedly
decreased with the increasing concentrations and treatment times
(Fig. 5A). The IC50 values of DB
for MCF7 and MDA-MB-231 cells were 14.29 and 15.70 µM,
respectively, for 48 h.
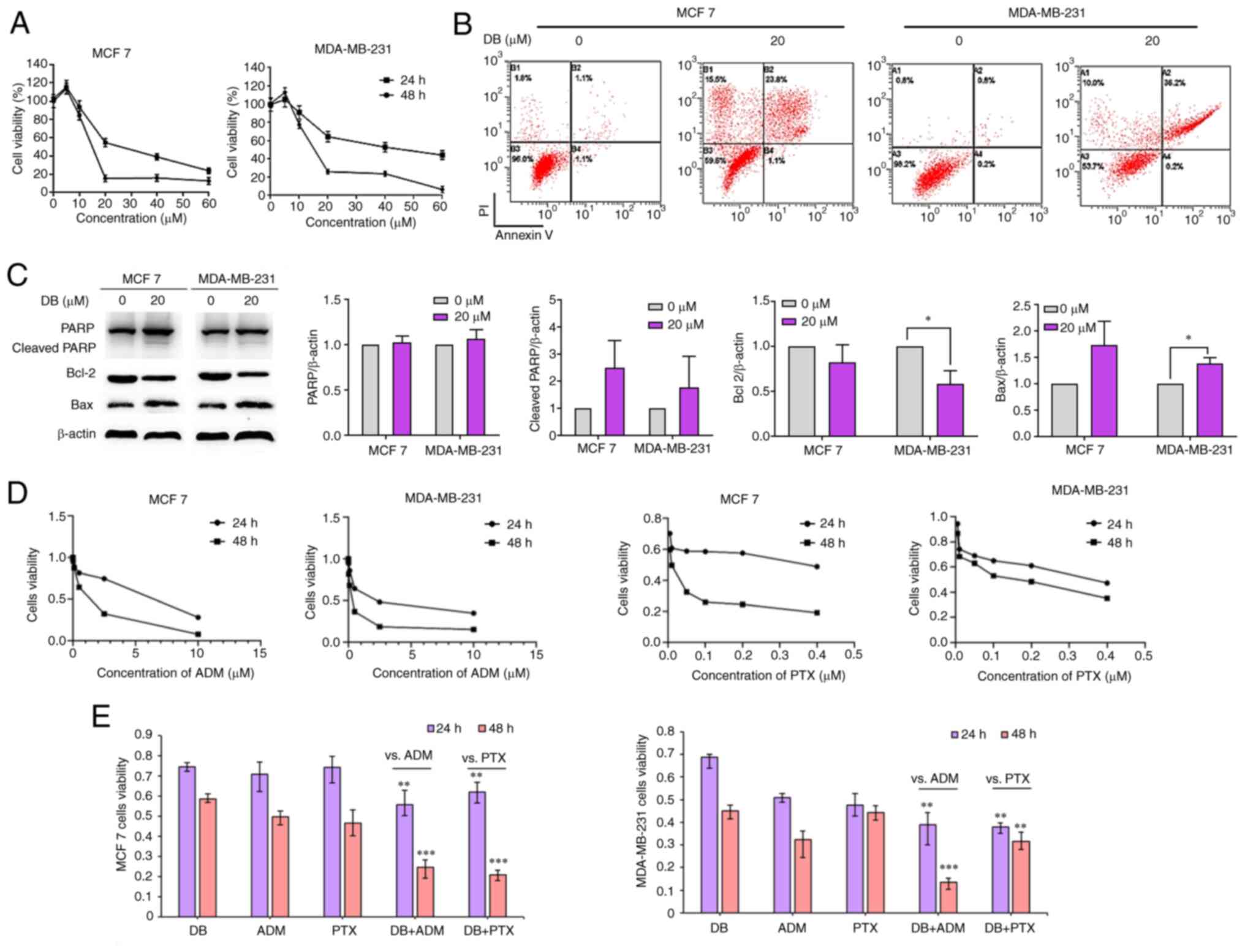 | Figure 5Effect of DB on apoptosis when singly
used or in combination with conventional chemotherapeutic drugs in
MDA-MB-231 and MCF7 cells. (A) CellTiter 96® AQueous One
Solution Cell Proliferation assay was used to detect the cell
survival rate of MDA-MB-231 and MCF7 cells treated with DB (20
µM) for 24 h. (B) Representative images of Annexin V/PI
staining assay using flow cytometry in control cells and cells
treated with DB (0 and 20 µM, 48 h). (C) Representative
western blots and corresponding protein quantification plots of
PARP, cleaved PARP, Bcl-2 and Bax protein expression in MCF-7 and
MDA-MB-231 cells treated with DB (20 µM) for 6 h.
*P<0.05. (D) The viability of MCF7 and MDA-MB-231
cells treated with ADM and PTX. (E) CellTiter 96®
AQueous One Solution Cell Proliferation Assay was used to determine
the survival rate of MDA-MB-231 cells and MCF7 cells treated with
DB, ADM and PTX alone or in combination with DB, ADM or PTX (10
µM DB, 0.2 µM ADM, 0.005 µM PTX, 24 and 48 h).
**P<0.01 vs. ADM or PTX, ***P<0.001 vs.
ADM or PTX. DB, dicitrinone B; PARP, poly (ADP-ribose) polymerase;
ADM, adriamycin; PTX, paclitaxel. |
Considering that apoptosis is the major pathway of
cell death mediated by chemotherapeutics, it was further attempted
to identify the association between apoptosis and DB-induced
autophagy inhibition in MCF7 and MDA-MB-231 cells. The results of
the flow cytometric Annexin V-FITC/propidium iodide staining assay
results demonstrated that apoptotic cell numbers increased with DB
concentration in MCF7 and MDA-MB-231 cells (Fig. 5B). Apoptotic rates were markedly
increased up to ~24.9 and ~36.4% in the MCF7 and MDA-MB-231 cells,
respectively, after the cells were exposed to DB for 48 h,
indicating that DB-induced autophagy inhibition may eventually lead
to apoptosis.
The expression of the apoptosis-related proteins,
PARP, Bax and Bcl-2, were then evaluated in DB-treated cells using
western blot analysis. As demonstrated in Fig. 5C, DB treatment resulted in an
upregulation of cleaved PARP and Bax and a downregulation of Bcl-2
expression, confirming that DB could promote apoptosis by blocking
autophagy in MCF7 and MDA-MB-231 cells.
It is known that autophagy inhibition can enhance
the sensitivity of tumor cells to chemotherapeutic drugs or
targeted drugs (38). The change
in the sensitivity of MCF7 and MDA-MB-231 cells to the conventional
chemotherapeutics, ADM and paclitaxel (PTX) was also investigated
(Fig. 5D). The results revealed
that the inhibitory effects observed following combined treatment
with DB and ADM or PTX were significantly greater than those
observed with DB, ADM or PTX treatment alone in MDA-MB-231 and MCF7
cells (Fig. 5E), indicating that
DB-induced autophagy inhibition may enhance the sensitivity of MCF7
and MDA-MB-231 cells to ADM and PTX.
Antitumor efficacy of combined treatment
with DB and the chemotherapeutic drug, ADM in vivo
To explore the antitumor efficacy of DB in
vivo, a nude mouse subcutaneous planting tumor model was
constructed using MDA-MB-231 cells. In the experiment,
5×106 cells were injected subcutaneously into the left
hindlimb of nude mice. After 7 days, the tumor volumes reached ~100
mm3, and the mice were randomly divided into four groups
with 5 mice in each group (PBS, DB, ADM and DB + ADM). The mice
were treated with DB and/or ADM via an intratumoral injection every
2 days at a dose of 10 mg/kg (Fig.
6A). Following 12 days of treatment, the body weight of mice in
the control group began to decrease significantly, while that of
the mice in the DB group instead slightly increased, indicating
that the use of DB in vivo could be considered safe
(Fig. 6B). After 21 days of
administration (10 consecutive injections), the mice were
sacrificed, and the tumors were dissected. It was found that the
tumors in the blank control group were the largest, while the
tumors in the combined treatment group were the smallest in size.
Moreover, the efficacy of DB treatment and ADM treatment
demonstrated almost no difference, indicating that when used as a
single therapeutic, DB can be comparable to ADM, and there is a
good synergistic effect when used in combination (Fig. 6C-E). The results of
immunohistochemical analysis revealed that compared with the
control group, the number of TUNEL-positive cells increased
significantly in the combined group and increased slightly in the
DB or ADM treatment group (Fig. 6F
and G). Simultaneously, the number of Ki-67-positive cells
decreased significantly in the combined group and decreased
slightly in the DB or ADM treatment group (Fig. 6F and G). These data suggested that
DB or ADM treatment can promote tumor apoptosis and inhibit tumor
proliferation, and the combination of DB and ADM may enhance the
antitumor efficacy. Of note, the results of the immunohistochemical
analysis of both the DB and combined administration groups revealed
more LC3-positive cells, indicating the blockade of autophagy after
DB treatment in tumor cells (Fig. 6F
and G), which was consistent with the experimental results
obtained in vitro. Taken together, the aforementioned
results revealed that DB was safe and effective as an antitumor
drug by regulating autophagy in vivo.
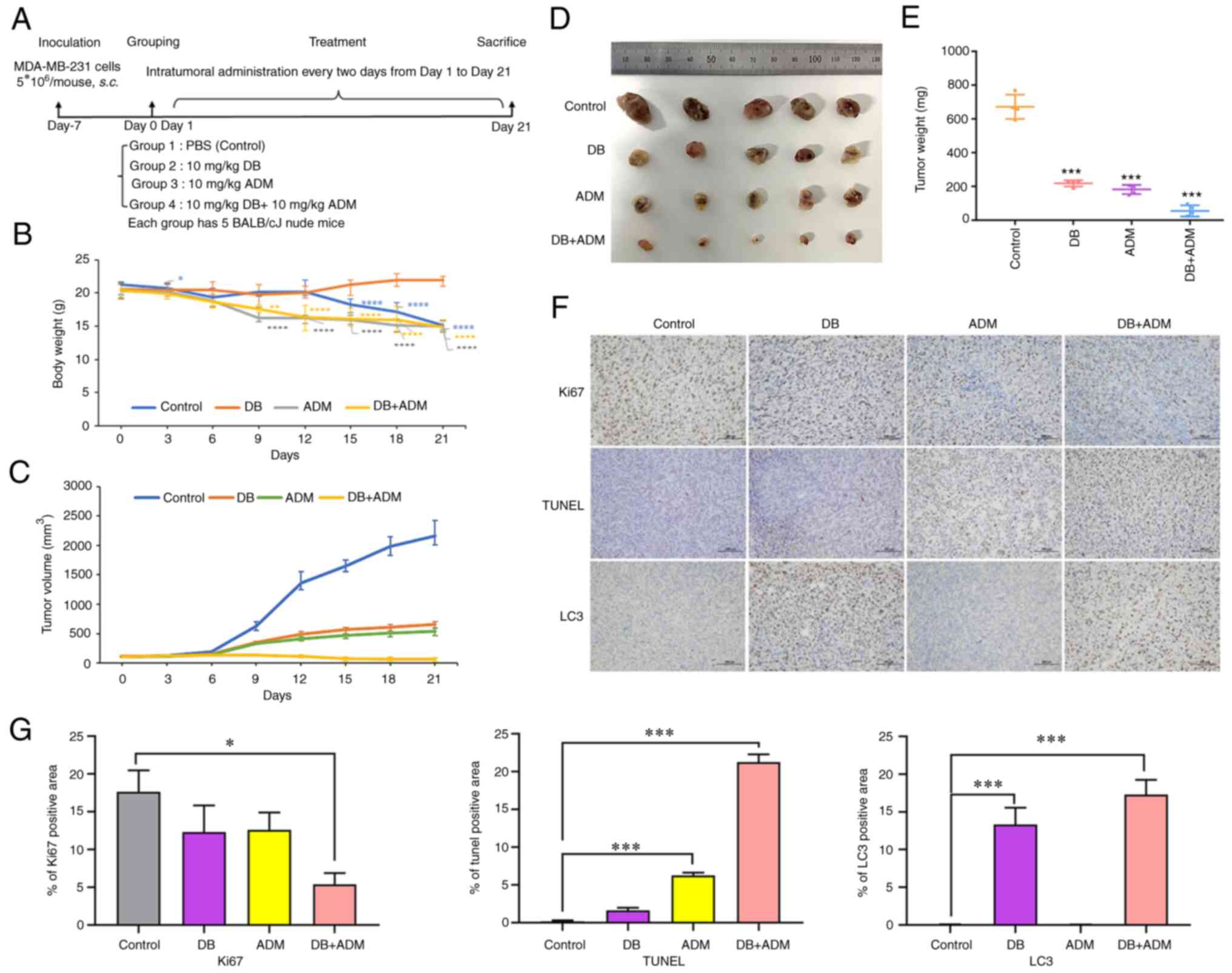 | Figure 6Efficacy of DB combined with the
conventional chemotherapeutic drug, ADM, in a MDA-MB-231 cell
line-derived tumor mouse model. (A) Mouse experimental scheme map.
(B) Changes in tumor volume in different groups of mice during
treatment. Calculation formula: tumor volume=shortest
diameter2 × longest diameter/2. *P<0.05,
**P<0.01 and ****P<0.0001 vs. the
control. (C) Changes in body weight in different groups of mice
during treatment. (D) Images of tumors removed from nude mice after
21 days of treatment. (E) Tumor tissue weight of mice in different
groups (control, DB, ADM and DB + ADM) after 21 days of treatment.
(F) On day 21, all mice were sacrificed, and tumors were isolated
for histopathological examination with TUNEL, Ki-67 and LC3 B
staining assays (scale bar, 100 µm). The dose of DB and ADM
was 10 mg/kg. DB, dicitrinone B; ADM, Adriamycin; LC3 B,
microtubule associated protein 1 light chain 3 beta.
***P<0.001 vs. the control. (G) Relative expression
quantification of histopathological examination with TUNEL, Ki-67
and LC3 B staining assays. *P<0.05 and
***P<0.001. |
Discussion
As a highly heritable heterogeneous disease, breast
cancer has diverse histological and molecular subtypes. The
prognosis of triple-negative breast cancer patients is particularly
poor due to the lack of effective targeted therapy, and in a large
number of patients, advanced breast cancer eventually becomes
refractory or relapses due to invasion and metastasis (39). In this context, targeting
autophagy inhibition may represent a new therapeutic strategy for
human breast cancer cells with apoptosis resistance or highly
frequent metastasis. Previous studies have suggested that autophagy
is a survival-promoting mechanism in cancer, particularly in
advanced tumors (38,40,41). It can help cancer cells evade
various environmental stressors by removing damaged organelles and
recycling nutrients. The autophagy inhibitors, CQ and
hydroxychloroquine (HCQ), have shown promising efficacy in breast
cancer and triple-negative breast cancer pre-clinical models or in
clinical trials when combined with other conventional
chemotherapies (42,43). However, the retinal toxicity of
CQ/HCQ has also attracted increased attention (44-47). Therefore, the research and
development of novel autophagy inhibitors is of utmost clinical
significance in cancer treatment. In the present study, the novel
natural product, DB, was examined, which may impair autophagic flux
and lead to a therapeutic benefit for breast cancer.
Similar to CQ/HCQ, in the present study, DB induced
the accumulation of autophagosomes by inhibiting
autophagosome-lysosome fusion. It was noted that the results of the
immunofluorescence assay of LC3 appeared to be more notable in the
MDA-MB-231 cells; however, the western blot results looked not so
obvious. It was hypothesized that this may be attributed to the
different morphological characteristics of the autophagosomes in
MDA-MB-231 cells and MCF7 cells. The autophagosomes formed in
MDA-MB-231 cells were usually of larger size than that those in
MCF7 cells, leading to the potential confusion that the level of
LC3 and p62 was higher in MDA-MB-231 cells. Actually, the effect of
DB on autophagosome accumulation in MDA-MB-231 and MCF7 cells was
relatively similar, based on the results of western blot analysis.
Various studies have revealed that alkalization of lysosomes,
defects in lysosomal proteolytic activity, and delayed trafficking
of autophagosomes to lysosomes easily lead to the limitation of
autophagosomes-lysosome fusion (48,49). However, the data from the AO and
LysoTracker Red staining assays in the present study demonstrated
that the pH was not altered in response to DB treatment. It is
believed that lysosomal hydrolases, known as cathepsins, are the
main tool for autolysosomes to decompose their contents (50-52). Western blot analysis of the
hydrolases CTSD and CTSB indicated that although DB treatment
blocked the autophagic flux and increased autophagosomes, it did
not alter the activity and function of lysosomal cathepsins. To
date, most compounds that affect autophagy in cancer cells perform
this by locking the fusion of autophagosomes and lysosomes
(53). Among these compounds,
bafilomycin A1 can raise the pH of lysosomes, which leads to the
inhibition of the activity of resident hydrolases and further
blocks the fusion of autophagosomes and lysosomes (54). Liensinine, as an autophagy
inhibitor, is able to affect the recruitment of the small GTP
binding protein RAB7A to lysosomes but does not affect lysosomal
pH, which in turn blocks the fusion of autophagosomes and lysosomes
in breast cancer cells (55).
CQ/HCQ decreases autophagosome lysosome fusion by interfering with
autophagosomal SNARE protein SNAP29 recruitment and the Golgi
complex without substantially changing lysosomal acidity (56,57). It cannot be excluded that DB may
preferentially destabilize auxilin, which is involved in the fusion
of autophagosomes and lysosomes, and consequently lead to
autophagosome aggregation and disruption of autophagy (58).
As a type of highly reactive oxygen-free radical or
non-radical molecules, ROS play an essential role in deciding cell
fate. A previous study by the authors have revealed that the levels
of ROS in A375 human malignant melanoma cells was obviously
increased after DB treatment, due to the significant decrease in
mitochondrial membrane potential induced by DB (25). In the present study, DB also
caused an increase in ROS generation in MCF7 breast cancer and
MDA-MB-231 TNBC cells. ROS perform important functions and are
associated with numerous signaling pathways in cells (59). To determine the association
between ROS and autophagy, the ROS scavenger, NAC, was used to
treat the cells prior to DB-inhibited autophagy. Of note, the
DB-induced autophagic flux inhibition was abolished by NAC.
Therefore, the effect of DB on ROS generation may be related to the
blocking of the autophagic flow in MCF7 and MDA-MB-231 cells. To
date, the association linking autolysosomal accumulation and ROS
accumulation remains largely unclear. It has been reported that
high levels of intracellular ROS may stem from damaged mitochondria
in the cytoplasm or undegraded mitochondria in autophagosomes
(60,61). The accumulation of autophagosomes
containing defective mitochondria and dysfunctional mitochondria
after DB treatment may promote ROS release. Simultaneously, high
levels of ROS trigger the formation of new autophagosomes and
cellular damage, potentially initiating a vicious cycle that
eventually leads to apoptosis. In addition, it has been reported
that Akt, a downstream protein of the PI3K pathway, inhibits
autophagy by activating rapamycin complex 1 (mTORC1) in response to
the increase in ROS levels, and mTORC1 and rapamycin complex 2
(mTORC2) inhibit autophagy at medium ROS levels; however, mTORC2
can promote cell aging through autophagy at high ROS levels
(62). The present study revealed
that DB upregulated Akt and mTOR protein levels in breast cancer
cells, particularly in MDA-MB-231 cells. This indicated that the
blocking effect of DB on the autophagy of MCF7 and MDA-MB-231 cells
may be related to ROS and PI3K pathways.
The present study only detected the anti-tumor and
autophagy inhibitory activity of DB in two human breast cancer cell
lines (MCF7 and MDA-MB-231) without making comparisons with a
normal control cell line. Therefore, it was not possible to
evaluate the toxicity of DB to normal cells at the cellular level.
However, the mouse experiments revealed that the body weight of the
mice in the doxorubicin group decreased significantly, while that
of the mice in the DB group was not markedly altered, indicating
that DB is safe to use in vivo.
In conclusion, the present study provides
biochemical evidence of a novel (to the best of our knowledge)
autophagy modulator, DB, that can inhibit autophagy and induce
apoptosis via the accumulation of autophagosomes and the promotion
of ROS production in MCF7 breast cancer and MDA-MB-231 TNBC cells
(Fig. 7). In view of its safety
and efficacy in vivo and the necessity to enhance the
sensitivity of tumors to chemotherapeutic drugs, DB is expected to
become a new-generation antitumor drug.
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
QL, YY and QZ contributed to the experimental
design. QL, YY, FC, MC and SC contributed to the experiments. QL,
YY, YS and LC contributed to data analysis. QL, YY and LC
contributed to the original manuscript preparation. YS and LC
contributed to the supervision of the present study. YS and LC
contributed to the manuscript review and editing. YS and LC confirm
the authenticity of all the raw data. All authors contributed to
drafting the manuscript. All authors have read and approved the
final manuscript.
Ethics approval and consent to
participate
The animal experiments were reviewed and approved by
The Animal Care and Use Committee of Fujian Medical University
(approval no. 2020-CAARM015) and were carried out in accordance
with the National Institutes of Health Guide for Care and Use of
Laboratory Animals.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
Acknowledgments
The authors would like to thank Dr Liu Shijia from
Fujian Cancer Hospital (Fuzhou, China) for the generous gift of the
MDA-MB-231 cells.
Funding
The present study was funded by grants from the National Natural
Science Foundation of China (no. 81873045), The Natural Science
Foundation of Fujian Province (nos. 2020J011118, 2020J011115 and
2021J01608), the Fujian Province Health Care Young and Middle-aged
Backbone Talents Training Project (no. 2020GGA015), the Startup
Fund for Scientific Research, Fujian Medical University (no.
2019QH1197), the Fujian Provincial Clinical Research Center for
Cancer Radiotherapy and Immunotherapy (no. 2020Y2012) and the
Fuzhou University Testing Fund of Precious Apparatus (no.
2022T041).
References
|
1
|
Ghoncheh M, Pournamdar Z and Salehiniya H:
Incidence and mortality and epidemiology of breast cancer in the
world. Asian Pac J Cancer Prev. 17:43–56. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Kocarnik JM, Compton K, Dean FE, Fu W, Gaw
BL, Harvey JD, Henrikson HJ, Lu D, Pennini A, Xu R, et al: Cancer
incidence, mortality, years of life lost, years lived with
disability, and disability-adjusted life years for 29 cancer groups
from 2010 to 2019: A systematic analysis for the global burden of
disease study 2019. JAMA Oncol. 8:420–444. 2021.PubMed/NCBI
|
|
3
|
Bray F, Ferlay J, Soerjomataram I, Siegel
RL, Torre LA and Jemal A: Global cancer statistics 2018: GLOBOCAN
estimates of incidence and mortality worldwide for 36 cancers in
185 countries. CA Cancer J Clin. 68:394–424. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Gadi VK and Davidson NE: Practical
approach to triple-negative breast cancer. J Oncol Pract.
13:293–300. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Harbeck N and Gnant M: Breast cancer.
Lancet. 389:1134–1150. 2017. View Article : Google Scholar
|
|
6
|
Harbeck N, Penault-Llorca F, Cortes J,
Gnant M, Houssami N, Poortmans P, Ruddy K, Tsang J and Cardoso F:
Breast cancer. Nat Rev Dis Primers. 5:662019. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Kwapisz D: Pembrolizumab and atezolizumab
in triple-negative breast cancer. Cancer Immunol Immunother.
70:607–617. 2021. View Article : Google Scholar
|
|
8
|
Glick D, Barth S and Macleod KF:
Autophagy: Cellular and molecular mechanisms. J Pathol. 221:3–12.
2010. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Onorati AV, Dyczynski M, Ojha R and
Amaravadi RK: Targeting autophagy in cancer. Cancer. 124:3307–3318.
2018. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Mizushima N and Klionsky DJ: Protein
turnover via autophagy: Implications for metabolism. Annu Rev Nutr.
27:19–40. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Morishita H and Mizushima N: Diverse
cellular roles of autophagy. Annu Rev Cell Dev Biol. 35:453–475.
2019. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Camuzard O, Santucci-Darmanin S, Carle GF
and Pierrefite-Carle V: Autophagy in the crosstalk between tumor
and microenvironment. Cancer Lett. 490:143–153. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Li YJ, Lei YH, Yao N, Wang CR, Hu N, Ye
WC, Zhang DM and Chen ZS: Autophagy and multidrug resistance in
cancer. Chin J Cancer. 36:522017. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Zhang B and Liu L: Autophagy is a
double-edged sword in the therapy of colorectal cancer. Oncol Lett.
21:3782021. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Parzych KR and Klionsky DJ: An overview of
autophagy: Morphology, mechanism, and regulation. Antioxid Redox
Signal. 20:460–473. 2014. View Article : Google Scholar :
|
|
16
|
Saha S, Panigrahi DP, Patil S and Bhutia
SK: Autophagy in health and disease: A comprehensive review. Biomed
Pharmacother. 104:485–495. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Singh SS, Vats S, Chia AY, Tan TZ, Deng S,
Ong MS, Arfuso F, Yap CT, Goh BC, Sethi G, et al: Dual role of
autophagy in hallmarks of cancer. Oncogene. 37:1142–1158. 2018.
View Article : Google Scholar
|
|
18
|
Mowers EE, Sharifi MN and Macleod KF:
Autophagy in cancer metastasis. Oncogene. 36:1619–1630. 2017.
View Article : Google Scholar :
|
|
19
|
Mowers EE, Sharifi MN and Macleod KF:
Functions of autophagy in the tumor microenvironment and cancer
metastasis. FEBS J. 285:1751–1766. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Smith AG and Macleod KF: Autophagy, cancer
stem cells and drug resistance. J Pathol. 247:708–718. 2019.
View Article : Google Scholar :
|
|
21
|
Vempati RK and Malla RR: Autophagy-induced
drug resistance in liver cancer. Crit Rev Oncog. 25:21–30. 2020.
View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Demain AL and Vaishnav P: Natural products
for cancer chemotherapy. Microb Biotechnol. 4:687–699. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Talmadge JE: Natural product derived
immune-regulatory agents. Int Immunopharmacol. 37:5–15. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Yang Y, Liu Q, Shi X, Zheng Q, Chen L and
Sun Y: Advances in plant-derived natural products for antitumor
immunotherapy. Arch Pharm Res. 44:987–1011. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Chen L, Gong MW, Peng ZF, Zhou T, Ying MG,
Zheng QH, Liu QY and Zhang QQ: The marine fungal metabolite,
dicitrinone B, induces A375 cell apoptosis through the ROS-related
caspase pathway. Mar Drugs. 12:1939–1958. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Chen L, Zhao YY, Lan RF, Du L, Wang BS,
Zhou T, Li YP, Zhang QQ, Ying MG, Zheng QH, et al: Dicitrinone D,
an antimitotic polyketide isolated from the marine-derived fungus
Penicillium citrinum. Tetrahedron. 73:5900–5911. 2017. View Article : Google Scholar
|
|
27
|
Du L, Li D, Zhang G, Zhu T, Ai J and Gu Q:
Novel carbon-bridged citrinin dimers from a volcano ash-derived
fungus Penicillium citrinum and their cytotoxic and cell cycle
arrest activities. Tetrahedron. 66:9286–9290. 2010. View Article : Google Scholar
|
|
28
|
Wang L, Li C, Yu G, Sun Z, Zhang G, Gu Q,
Zhu T, Che Q, Guan H and Li D: Dicitrinones E and F, citrinin
dimers from the marine derived fungus Penicillium citrinum
HDN-152-088. Tetrahedron Letters. 60:151182–151189. 2019.
View Article : Google Scholar
|
|
29
|
Zhao H, Zhang X, Wang M, Lin Y and Zhou S:
Stigmasterol simultaneously induces apoptosis and protective
autophagy by inhibiting Akt/mTOR pathway in gastric cancer cells.
Front Oncol. 11:6290082021. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Lee YK and Lee JA: Role of the mammalian
ATG8/LC3 family in autophagy: Differential and compensatory roles
in the spatiotemporal regulation of autophagy. BMB Rep. 49:424–430.
2016. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Tanida I, Ueno T and Kominami E: LC3
conjugation system in mammalian autophagy. Int J Biochem Cell Biol.
36:2503–2518. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Harris H and Rubinsztein DC: Control of
autophagy as a therapy for neurodegenerative disease. Nat Rev
Neurol. 8:108–117. 2012. View Article : Google Scholar
|
|
33
|
Rogov V, Dotsch V, Johansen T and Kirkin
V: Interactions between autophagy receptors and ubiquitin-like
proteins form the molecular basis for selective autophagy. Mol
Cell. 53:167–178. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Yang K, Tang M, Chang HH, Kanamala M,
Davidson AJ and Wu Z: Mannosylation of pH-sensitive liposomes
promoted cytoplasmic delivery of protein to macrophages: Green
fluorescent protein (GFP) performed as an endosomal escape tracer.
Pharm Dev Technol. 26:1000–1009. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Mizuno H, Sawano A, Eli P, Hama H and
Miyawaki A: Red fluorescent protein from Discosoma as a fusion tag
and a partner for fluorescence resonance energy transfer.
Biochemistry. 40:2502–2510. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Qiao S, Tao S, de la Vega M, Park SL,
Vonderfecht AA, Jacobs SL, Zhang DD and Wondrak GT: The
antimalarial amodiaquine causes autophagic-lysosomal and
proliferative blockade sensitizing human melanoma cells to
starvation- and chemotherapy-induced cell death. Autophagy.
9:2087–2102. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Jakoš T, Pišlar A, Jewett A and Kos J:
Cysteine cathepsins in tumor-associated immune cells. Front
Immunol. 10:20372019. View Article : Google Scholar
|
|
38
|
Dikic I, Johansen T and Kirkin V:
Selective autophagy in cancer development and therapy. Cancer Res.
70:3431–3434. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Abramson VG, Lehmann BD, Ballinger TJ and
Pietenpol JA: Subtyping of triple-negative breast cancer:
Implications for therapy. Cancer. 121:8–16. 2015. View Article : Google Scholar
|
|
40
|
Liu B, Wen X and Cheng Y: Survival or
death: Disequilibrating the oncogenic and tumor suppressive
autophagy in cancer. Cell Death Dis. 4:e8922013. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Dower CM, Wills CA, Frisch SM and Wang HG:
Mechanisms and context underlying the role of autophagy in cancer
metastasis. Autophagy. 14:1110–1128. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Rojas-Sanchez G, Garcia-Miranda A,
Montes-Alvarado JB, Cotzomi-Ortega I, Sarmiento-Salinas FL,
Jimenez-Ignacio EE, Ramirez-Ramirez D, Romo-Rodriguez RE,
Reyes-Leyva J, Vallejo-Ruiz V, et al: Chloroquine induces
ROS-mediated macrophage migration inhibitory factor secretion and
epithelial to mesenchymal transition in ER-positive breast cancer
cell lines. J Mammary Gland Biol Neoplasia. 26:341–355. 2021.
View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Dong J, Zhu C, Zhang F, Zhou Z and Sun M:
'Attractive/adhesion force' dual-regulatory nanogels capable of
CXCR4 antagonism and autophagy inhibition for the treatment of
metastatic breast cancer. J Control Release. 341:892–903. 2022.
View Article : Google Scholar
|
|
44
|
Ruamviboonsuk P, Lai TYY, Chang A, Lai CC
and Mieler WF; Lam DSC and for Asia-Pacific Vitreo-Retina Society:
Chloroquine and hydroxychloroquine retinal toxicity consideration
in the treatment of COVID-19. Asia Pac J Ophthalmol (Phila).
9:85–87. 2020. View Article : Google Scholar
|
|
45
|
Muller R: Systemic toxicity of chloroquine
and hydroxychloroquine: Prevalence, mechanisms, risk factors,
prognostic and screening possibilities. Rheumatol Int.
41:1189–1202. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Doyno C, Sobieraj DM and Baker WL:
Toxicity of chloroquine and hydroxychloroquine following
therapeutic use or overdose. Clin Toxicol (Phila). 59:12–23. 2021.
View Article : Google Scholar
|
|
47
|
Askarian F, Firoozi Z, Ebadollahi-Natanzi
A, Bahrami S and Rahimi HR: A review on the pharmacokinetic
properties and toxicity considerations for chloroquine and
hydroxychloroquine to potentially treat coronavirus patients.
Toxicol Res. 38:137–148. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
48
|
Abokyi S, Shan SW, Lam CHI, Catral KP, Pan
F, Chan HHL, To CH and Tse DYY: Targeting lysosomes to reverse
hydroquinone-induced autophagy defects and oxidative damage in
human retinal pigment epithelial cells. Int J Mol Sci. 22:90422021.
View Article : Google Scholar : PubMed/NCBI
|
|
49
|
Chen R, Jaattela M and Liu B: Lysosome as
a central hub for rewiring PH homeostasis in tumors. Cancers.
12:24372020. View Article : Google Scholar :
|
|
50
|
Hossain MI, Marcus JM, Lee JH, Garcia PL,
Singh V, Shacka JJ, Zhang J, Gropen TI, Falany CN and Andrabi SA:
Restoration of CTSD (cathepsin D) and lysosomal function in stroke
is neuroprotective. Autophagy. 17:1330–1348. 2021. View Article : Google Scholar :
|
|
51
|
Di YQ, Han XL, Kang XL, Wang D, Chen CH,
Wang JX and Zhao XF: Autophagy triggers CTSD (cathepsin D)
maturation and localization inside cells to promote apoptosis.
Autophagy. 17:1170–1192. 2021. View Article : Google Scholar :
|
|
52
|
Cao M, Luo X, Wu K and He X: Targeting
lysosomes in human disease: From basic research to clinical
applications. Signal Transduct Target Ther. 6:3792021. View Article : Google Scholar : PubMed/NCBI
|
|
53
|
Li Z, Si W, Jin W, Yuan Z, Chen Y and Fu
L: Targeting autophagy in colorectal cancer: An update on
pharmacological small-molecule compounds. Drug Discov Today.
27:2373–2385. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
54
|
Feng X, Zhang H, Meng L, Song H, Zhou Q,
Qu C, Zhao P, Li Q, Zou C, Liu X and Zhang Z: Hypoxia-induced
acetylation of PAK1 enhances autophagy and promotes brain
tumorigenesis via phosphorylating ATG5. Autophagy. 17:723–742.
2021. View Article : Google Scholar :
|
|
55
|
Zhou J, Li G, Zheng Y, Shen HM, Hu X, Ming
QL, Huang C, Li P and Gao N: A novel autophagy/mitophagy inhibitor
liensinine sensitizes breast cancer cells to chemotherapy through
DNM1L-mediated mitochondrial fission. Autophagy. 11:1259–1279.
2015. View Article : Google Scholar : PubMed/NCBI
|
|
56
|
Tian X, Teng J and Chen J: New insights
regarding SNARE proteins in autophagosome-lysosome fusion.
Autophagy. 17:2680–2688. 2021. View Article : Google Scholar :
|
|
57
|
Ganley IG, Wong PM, Gammoh N and Jiang X:
Distinct autophagosomal-lysosomal fusion mechanism revealed by
thapsigargin-induced autophagy arrest. Mol Cell. 42:731–743. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
58
|
Vidyadhara DJ, Lee JE and Chandra SS: Role
of the endolysosomal system in Parkinson's disease. J Neurochem.
150:487–506. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
59
|
Gibson SB: A matter of balance between
life and death: Targeting reactive oxygen species (ROS)-induced
autophagy for cancer therapy. Autophagy. 6:835–837. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
60
|
Kubota C, Torii S, Hou N, Saito N,
Yoshimoto Y, Imai H and Takeuchi T: Constitutive reactive oxygen
species generation from autophagosome/lysosome in neuronal
oxidative toxicity. J Biol Chem. 285:667–674. 2010. View Article : Google Scholar :
|
|
61
|
Scherz-Shouval R and Elazar Z: ROS,
mitochondria and the regulation of autophagy. Trends Cell Biol.
17:422–427. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
62
|
Kma L and Baruah TJ: The interplay of ROS
and the PI3K/Akt pathway in autophagy regulation. Biotechnol Appl
Biochem. 69:248–264. 2022. View Article : Google Scholar
|