1. Introduction
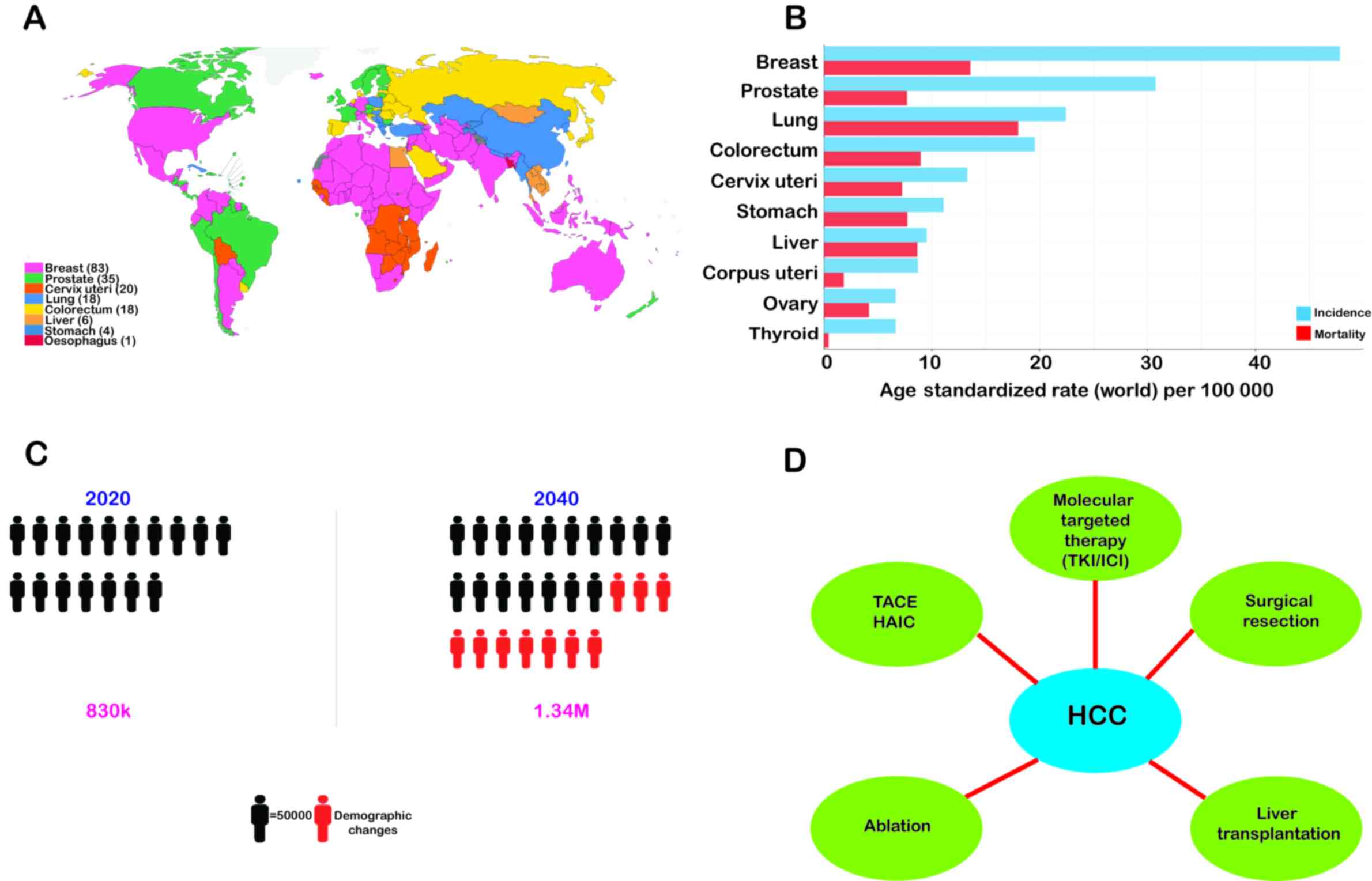
Liver cancer is the sixth most prevalent form of
cancer worldwide and accounts for the third most frequent cause of
cancer-associated mortality (1,2)
(Fig. 1). Hepatocellular
carcinoma (HCC) is the most predominant form of malignant liver
cancer, which is responsible for ~90% of all non-metastatic liver
cancers and has been reported to be associated with cirrhosis and
hepatitis B or C virus (HBV/HCV) infection (3,4).
Other risk factors include obesity, iron overload, alcohol
consumption, diabetes, fatty liver disease and smoking (5).
However, according to statistics, only 25% of
patients with HCC are diagnosed in the initial stages at the onset
of the disease (6). A probable
reason for this may be the absence of initial symptoms and frequent
overlap with other diseases, thus making it difficult to
distinguish HCC from other clinical conditions. The survival rates
of cancer patients may be markedly enhanced by timely and precise
diagnosis at the initial stages (7). As with the late detection of
advanced-stage HCC, the diagnosis of cancer at a late stage
suggests that the patient has reached a stage that is non-curative.
Henceforth, the chances of survival are greatly minimized, and such
patients are placed under palliative care due to the high rate of
metastasis and relapse, as occurs in patients with late-stage HCC
(8,9).
At present, the most common strategies for the
treatment of HCC include ablation, surgical resection, liver
transplantation, chemotherapy, transarterial chemoembolization
(TACE), radiotherapy and combination therapy depending on the
disease staging and patient's profile (Fig. 1) (10). However, these conventional
therapies have multiple limitations that compromise the quality of
life of patients receiving these therapies. For example, undesired
effects of radiotherapy and the development of resistance to
chemotherapy due to long-term treatment and transplantation lead to
long-term immunosuppressive therapy (11). Although substantial advancements
have been made over the past decade in the management and treatment
of HCC, including liver resection or transplantation and ablation,
only ~15% of patients with early-stage HCC without cirrhosis are
eligible for surgical removal. TACE is another available treatment
option for patients with intermediate-stage HCC that results in a
23% increase in the 2-year survival rate when compared to
traditional treatment therapies (12).
Most HCC cases are diagnosed predominantly in the
late stages of the disease, which renders both surgical (resection
and transplantation) and locoregional treatment (chemoembolization)
inadequate for the overall survival of patients. As a result, there
is an urgent need for the development of an effective therapy for
patients with advanced-stage HCC.
In the SHARP randomized controlled trial, sorafenib
as a monotherapy was shown to be efficacious for advanced HCC. With
a high safety profile, the sorafenib-treated group exhibited an
overall survival rate of 10.7 months compared to 7.9 months for the
placebo group (13) (Fig. 2). Sorafenib is currently the only
approved prescribed option for the treatment of patients with
advanced-stage HCC. Patients with HCC who have not responded to
earlier treatments are recommended to use sorafenib, which received
authorization by the US Food and Drug Administration (FDA) in 2007
(14). The anticancer effect of
sorafenib is based on its ability to obstruct cell proliferation
and angiogenesis, which inhibits tumor growth (15). However, sorafenib treatment is
beneficial to only a limited number of patients and is often
accompanied by drug resistance within 6 months of commencing the
treatment. Moreover, the use of sorafenib is also associated with
side-effects, such as nausea, alopecia and hypertension (14).
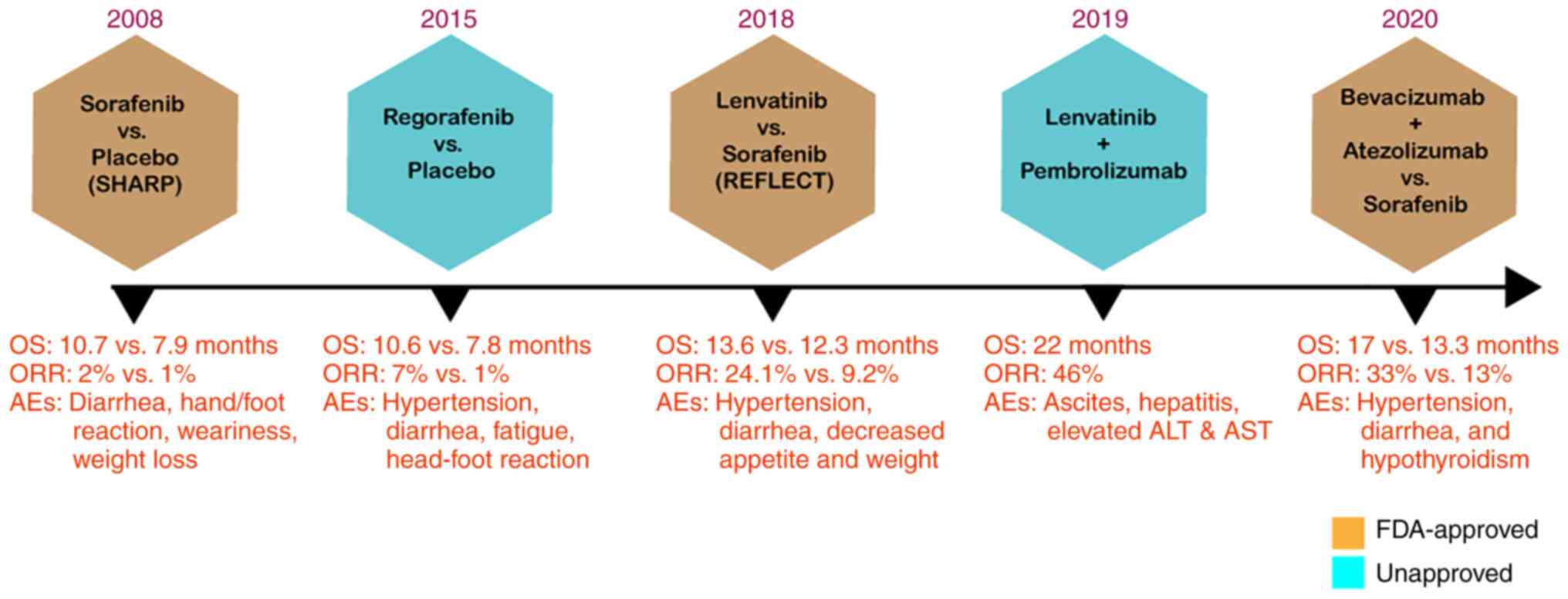 | Figure 2Timeline of FDA-approved drugs for
HCC. The SHARP trial (13)
demonstrated the effectiveness of sorafenib against HCC. Compared
to the placebo group, the sorafenib-treated group exhibited a
markedly longer OS (mOS 10.7 vs. 7.9 months; HR, 0.69; 95% CI,
0.55-0.87; P<0.001). In the REFLECT trial (16), lenvatinib displayed
non-inferiority in OS corresponding to sorafenib monotherapy (mOS,
13.6 vs. 12.3 months; HR, 0.92; 95% CI, 0.79-1.06). In the
IMbrave150 trial (17), the
efficacy of bevacizumab combined with atezolizumab was compared
with that of sorafenib. The combination treatment resulted in a
markedly improved outcome than sorafenib monotherapy, exhibiting
prolonged OS and PFS (mOS 19.2 vs. 13.4 months; HR, 0.66; 95% CI,
0.52-0.85; P=0.0009). FDA, Food and Drug Administration; HCC,
hepatocellular carcinoma; OS, overall survival; HR, hazard ratio;
Mos, median OS; CI, confidence interval; ORR, objective response
rate; PFS, progress free survival; AEs, adverse effects. |
It took >10 years following the approval of
sorafenib before a second first-line targeted drug for HCC was
developed. As per the outcome from REFLECT trial (16), a randomized phase III
non-inferiority trial reported by Kudo et al (16), led to the approval of lenvatinib
to be used as the first-line treatment for advanced HCC.
In terms of overall survival, this randomized phase
III trial in 2018 demonstrated that lenvatinib was not inferior to
sorafenib showing overall survival of 13.6 months compared to 12.6
months for the sorafenib-treated group (16). A recent milestone in the
development of HCC first-line drug development was achieved in 2020
when the FDA approved bevacizumab plus atezolizumab, an antibody
combination strategy, as a first-line treatment for patients with
unresectable HCC on the basis of safety and efficacy determined in
the IMbrave150 trial (17). In
this phase III study, 501 patients with HCC who had not previously
received systemic treatment were compared to the effectiveness of
bevacizumab coupled with atezolizumab against sorafenib. By
significantly improving the overall survival by 12.6% at 12 months,
the combination treatment significantly outperformed sorafenib
monotherapy (17). Although all
three drugs approved by the FDA for first-line therapy had led to
an improved response and survival rate, they are all associated
with multiple adverse effects (Fig.
2).
Despite advancements being made in several first-
and second-line drugs for the treatment of HCC, the use of these
drugs still presents the issue of a compromised lifestyle with the
provision of less benefit overall. The quality of life of patients
receiving therapy with these drugs does not appear to be improving,
and there is also the issue of the high costs of these drugs.
Moreover, these targeted therapies are associated with the drawback
of an inadequate objective response rate (ORR) and
adaptive/acquired resistance (18). Furthermore, the long-term usage of
such chemotherapeutic drugs may pose the issue of toxicity, as well
as drug inefficacy. Therefore, it is imperative that a novel
treatment strategy be developed with the aim of developing targeted
therapy that can be applied to patients with HCC at any stage of
treatment without posing any side-effects with longer
durability.
The present review article illustrates the
innovative exosome-based therapy as a delivery agent of two
potential anticancer candidates, i.e., tumor necrosis
factor-related apoptosis-inducing ligand (TRAIL) and microRNA
(miRNA/miR)-335. The summary and discussion of scientific
investigations highlights the immense potential of harnessing the
ability of exosomes for developing an effective anticancer drug
therapy for patients with liver cancer and their role in addressing
the issue of drug resistance development conferred by existing
chemotherapeutics.
2. MicroRNA-335: A novel candidate for
anticancer treatment
miRNAs are non-coding RNAs whose function is to
perform post-transcriptional gene regulation. They play a key role
in tumor development by modulating the expression of various
oncogenes and tumor suppressor genes (19). miRNAs are poorly regulated in
multiple types of cancers and, as per their function, they function
either as oncogenes or tumor suppressors. Oncomirs, such as miR-21
stimulate tumor growth by impeding tumor suppressor genes (20). On the contrary, tumor suppressor
miRNAs, such as miR-145 restrict the progression of tumors by
blocking oncogenes (21).
Furthermore, miRNAs are involved in several biological processes,
including cell proliferation, apoptosis, metastasis and
angiogenesis (22,23), which are the key features in
cancer progression.
miRNAs as a novel class of regulatory factors are
crucial to the biological and pathological processes in several
cases of human solid tumors (24). According to numerous recent
studies, as a tumor suppressor gene, miR-335-5p has been linked to
the emergence and growth of several tumors, including colorectal
cancer (25), non-small cell lung
cancer (26) and HCC (27). Furthermore, hepatic stellate
cell-derived miR-335-5p exosomes may be used as prospective miRNA
biomarkers in HBV-related HCC (28) and thus have potential therapeutic
significance in HCC (29).
Previous research has demonstrated that miR-335 is a
suppressor of tumor formation, invasion and metastasis, and is
responsible for the regulation of apoptosis and thus has prognostic
value in HCC (30). miR-335-3p,
sometimes referred to as miR-335*, is generated concurrently with
miR-335. Notably, these miRNAs have also been shown to be
responsible for inducing the activation of the p53 tumor suppressor
pathway to prevent cellular proliferation and neoplastic cell
transformation (31). Emerging
evidence has depicted the role of miR-335 in the majority of
oncogenic signaling pathways responsible for cell growth and
survival. It has been discovered that the downregulation of miR-335
in lung cancer enhances cell proliferation by activating the
AKT/mTOR signaling pathway, which is one of the most commonly
dysregulated signaling pathways in human malignancies (32). This characteristic of miR-335
could be exploited in therapeutic applications for restricting HCC
progression and controlling metastasis.
miR-335-5p reportedly targets downstream genes to
modulate the biological activity of cancer cells. For example,
miR-335-5p overexpression has been shown to suppress the
proliferation, migration and invasion of non-small cell lung cancer
by targeting CPNE1 (33). In the
case of HCC, miR-335-5p has been reported to significantly
attenuate the development of this type of cancer. For example, a
previous study demonstrated how the
circ_0009910/miR-335-5p/Rho-associated coiled-coil-containing
protein kinase 1 (ROCK1) axis is crucial to the onset and
development of HCC (34). In that
study, miR-335-5p inhibited HCC cell proliferation, migration and
invasion by targeting ROCK1. By enhancing the inhibitory effects of
miR-335-5p on the expression of ROCK1 in HCC, circ_0009910
knockdown exhibited anticancer properties. In addition to that, it
was found that the invasiveness of cancer cells was positively
associated with ROCK1 (34). In a
similar context, Liu et al (35) demonstrated that miR-335 was
involved in suppressing HCC cell proliferation, migration and
invasion by downregulating ROCK1 expression. Their research
investigating molecular mechanisms revealed that ROCK1, which is
associated with cell movement and invasion in various cancer types,
was a target gene for miR-335 for modulating the proliferation and
metastasis of HCC cells (35).
Reportedly, ROCK1 functions as an oncogene in HCC and promotes the
development of HCC (36).
The significance of miR-335 was further validated by
another study which demonstrated that miR-335 restoration inhibited
hepatic stellate cell (HSC) migration (37). The findings of that study
concluded that miR-335 considerably decreased during HSC
activation. Restoring miR-335 expression markedly decreased
collagen type I and α-smooth muscle actin levels and prevented cell
migration, at least in part through the downregulation of
tenascin-C, an extracellular matrix glycoprotein involved in cell
migration. The overexpression of miR-335 in HSC may thus provide a
novel strategy for the treatment of hepatic fibrosis (37). Another recent study by Yang et
al (38) revealed that a
newly characterized circular RNA, circ_0005075, promoted HCC
progression by suppressing the function of miR-335. Circ_0005075
was discovered to be upregulated in HCC tissues where it was found
that the downregulation of circ_0005075 inhibited HCC progression
(38). As per their study
mitogen-activated protein kinase 1 (MAPK1) was shown to be
regulated by miR-335, divulging it as one of the downstream
regulatory targets of circ_0005075. It was also shown that the
upregulation of circ_0005075 may be responsible for the elevated
level of MAPK1 (38). As a
crucial member of the MAPK family, the main function of MAPK1
involves cell proliferation, gene expression, differentiation,
mitosis, cell survival and apoptosis (39). Likewise, their role is also
imperative in tumorigenesis. With the overexpression of MAPK1 in
multiple types of cancer, including breast cancer (40), they could be the likely candidates
to be used for the prognosis of patients with HCC (38). To sum up, Yang et al
(38) demonstrated that miR-335
may target and inhibit MAPK1 (38). Moreover, Ji et al (41) identified octamer-binding
transcription factor 4 (OCT4) as another target gene of miR-335-5p.
miR-335-5p was demonstrated to prevent OCT4 gene expression to
restrict the downstream activation of the Akt signaling pathway,
which was demonstrated to be a canonical regulator in the
development of liver cancer (41).
Notably, a number of scientific investigations have
also discovered a pertinent link between miR-335 expression and the
survival of cancer patients (26,42). Furthermore, several findings have
indicated that miR-335 influences the chemotherapeutic response in
patients receiving standard treatment (43,44). For example, the study by Cui et
al (45) demonstrated that
serum miR-335 levels can be utilized as a marker to identify the
status of disease progression, apart from clinical outcome in
patients receiving TACE therapy. TACE therapy is the standard of
care treatment for patients with large or multinodular HCC whose
treatment response varies and still lacks any prognostic marker.
However, the study by Cui et al (45) indicated that low levels of miR-335
in patient serum were associated with a low survival rate with a
poor treatment response. Chen and Xia (46) demonstrated the role of miR-335 as
a biomarker for HCC treatment and demonstrated its function
responsible for regulating sensitivity to sorafenib in HCC. They
depicted the fact that miR-335 regulates sorafenib sensitivity in
HCC cells by inhibiting the AKT pathway ia targeting C-MET, which
is a tyrosine kinase protein involved in the development of cancer
(46).
Dohi et al (47) first reported that miR-335, which
is located within the intron of its protein-coding host gene, MEST,
was downregulated due to aberrant promoter hypermethylation.
Primary HCC tissues exhibited considerably higher levels of
miR-335/MEST methylation and miR-335 expression was much lower in
tumors compared to the non-tumor tissue counterparts (47). Their finding suggested that
aberrant DNA methylation in primary HCC was the cause of the
decreased miR-335 expression. Furthermore, their findings suggested
that a decreased expression of miR-335 may be linked to distant
metastases in HCC (47). Similar
findings were also provided on the expression of miR-335 in tumor
tissues, which was reported to be much lower than in non-tumor
tissues compare to HCC patient samples (48). The recent study by Nie et
al (49) demonstrated ROCK1
as a target gene of miR-335-5p, where circ_0064288 enhanced ROCK1
expression by competitively binding with miR-335-5p. They suggested
that circ_0064288, which is highly expressed in HCC, functions as
an oncogene by inhibiting miR-335-5p expression and promoting ROCK1
expression, which is responsible for regulating cell motility. A
list of various studies investigating the clinical significance of
miR-335 in HCC and their molecular mechanisms is presented in
Table I. Thus, potential
diagnostic and treatment options for HCC may be provided via the
modulation of miR-335.
 | Table IClinical function and mechanisms of
action of miRNA-335 in HCC. |
Table I
Clinical function and mechanisms of
action of miRNA-335 in HCC.
| Clinical
significance | Mechanisms | Recipient
cells | (Refs.) |
|---|
| Suppresses HCC cell
proliferation, migration and invasion | By enhancing the
inhibitory effects of miR-335-5p on the expression of ROCK1 in
HCC | HepG2, Hep3B,
HCCLM3, MHCC97 (human HCC cell lines) | (34) |
| Restricts the
proliferation, migration and invasion of HCC cells | Via downregulating
the Rho-associated coiled-coil-containing protein kinase 1 | HuH7 cells and
HepG2 | (35) |
| circ_0005075
promotes HCC proliferation, migration, invasion, anti-apoptosis,
and chemotherapeutic resistance | Via repressing the
function of miR-335 | HepG2 and SMMC-7721
cells | (38) |
| Restricts the
proliferation of Huh-7 liver cancer cells | Via targeting the
OCT4/Akt pathway | Huh7 human liver
cancer cells | (41) |
| miR-335 contributes
to the sensitizing effects of anticancer drugs | SIAH2 is the target
of miR-335 by enhancing the expression of HDAC3 | SNU387R, Malme3MR,
SNU387Rtaxol, Malme3MR-Taxol, SNU387-R Vinblastine | (43) |
| Prognostic
marker | Via aberrant
promoter hypermethylation | Patients with
HCC | (45) |
| Modulates sorafenib
resistance | Via suppressing the
c-Met-Akt pathway through lncRNA NEAT1 | HepG2/Bel7404 | (46) |
| Diminishes the
expression of miR-335, which may be associated with distant
metastasis in HCC | DNA
hypermethylation of CpG islands within promoter regions of
protein-coding host gene, MEST | Huh1, Huh7, HLE,
HLF and HepG2 | (48) |
| miR-335-5p
overexpression partly counteracts the effect of circ_0064288
responsible for HCC cell growth and migration | Circ_0064288
facilitates HCC cell growth and migration by regulating the
miR-335-5p/ROCK1 axis | Huh7, Hep3B,
HCCLM3, and MHCC97-L | (49) |
| Obstructs the
proliferation and invasion, and increases the apoptosis of HCC
cells | Shuttle between
hepatoma cells and HSCs, downregulate mRNA targets for miR-335 | MHCC97L, MHCC97H,
Huh7 and HepG2 cells | (137) |
3. Significance of TRAIL in HCC
treatment
TRAIL is a pro-apoptotic ligand that has received
increasing attention owing to its property of inducing apoptosis in
multiple types of cancer cells without affecting normal cells
(50). This unique feature of
TRAIL has allowed several researchers to investigate the
development of TRAIL-receptor agonists as a form of anticancer
therapy (51,52). Additionally, TRAIL-based
therapeutics are independent of p53 in tumor cells, unlike other
chemotherapeutic drugs, which renders them unique in terms of the
cell death pathway (53,54).
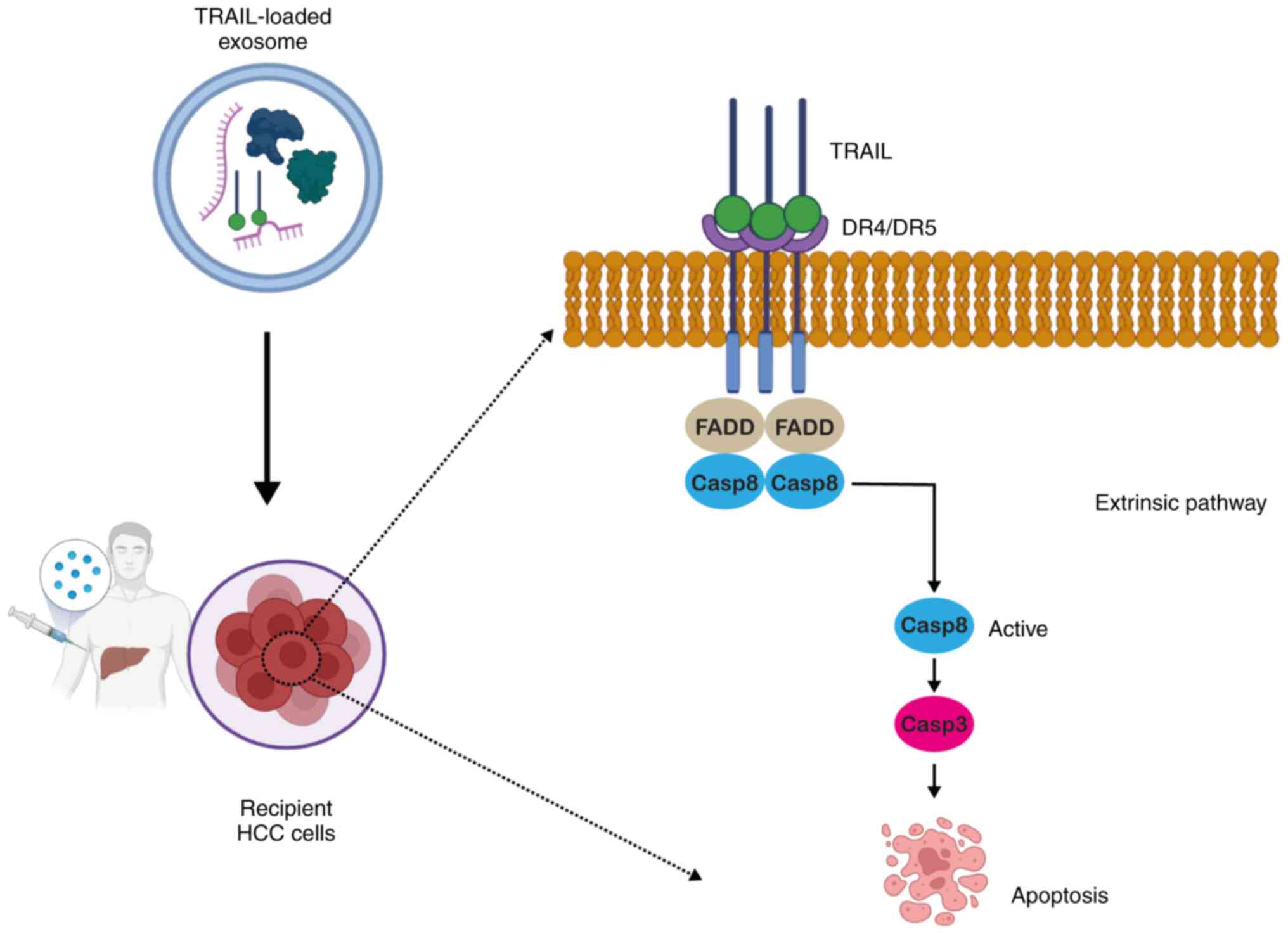
TRAIL is a transmembrane protein reported to be
found on natural killer (NK) cells and cytotoxic T-cell surfaces
with a predominant expression in tissues, including the prostate,
lungs and spleen (55). TRAIL can
be secreted in the soluble form, which is non-toxic to normal
cells, while healthy adult plasma contains a trace quantity of
endogenous TRAIL (100 pg/ml) (56). Multiple types of cancer cells
overexpress the death receptors (DRs), DR4 and DR5 (57). Upon secretion from NK cells, TRAIL
binds to DR4 and DR5 (58) and
upon binding, it leads to the recruitment of caspase-8 to the
Fas-associated death domain adaptor protein. Following activation,
it culminates in apoptotic signaling via caspase-3 activation,
ultimately leading to cell death (Fig. 3) (59).
The ability of TRAIL to induce tumor-specific cell
death renders it a promising candidate for antitumor therapy. In
numerous in vitro studies, TRAIL protein in its soluble
recombinant form has been widely explored as an anticancer agent
(57,60-62). A number of previous publications
with the aim of developing antitumor therapy have centered their
findings on the in vitro and in vivo tumoricidal
activity of TRAIL protein; they demonstrated a peculiar feature of
apoptosis induction by recombinant, soluble TRAIL protein in a
broad range of cancer cell lines, although they demonstrated no
activity against normal cells (63-65). The safe use of TRAIL as a ligand
for therapeutic purposes, displaying no noticeable cytotoxicity to
normal tissues, has also been validated in mouse models (57) and in humans (66).
The study by Grisendi et al (67) on adipose-derived (AD)-mesenchymal
stem cells (MSCs) producing TRAIL revealed that when AD-MSCs loaded
with TRAIL were injected into mice, they localized to the tumor
site and induced apoptosis without causing any significant toxicity
to normal tissues. Those authors also proposed that using stably
genetically modified AD-MSCs to deliver TRAIL alone or in
conjunction with sensitizing drugs may provide new treatment
options for malignancies that remain incurable (67). Furthermore, El-Shemi et al
(61) revealed TRAIL and
inhibitor of growth 4 (ING4) as potent apoptosis-inducing genes in
an orthotopic mouse model of human HCC bearing utilizing oncolytic
adenoviruses as a gene delivery agent. Their study found that the
combination of these drugs significantly reduced tumor-driven
angiogenesis and neovascularization, and also triggered apoptosis
and immune responses, without exhibiting any overlapping toxicities
(61). Another study by Liu et
al (65) revealed that TRAIL
plasmid DNA delivered via HCC-targeted
lipid/calcium/phosphate/protamine nanoparticles in conjunction with
traditional sorafenib therapy decreased HCC development, as well as
liver fibrosis in a mouse model of HCC. Overall, these findings
provide a promising treatment strategy for cancer based on TRAIL
that may be applied in clinical settings.
Another study recently demonstrated the underlying
mechanisms of action of TRAIL, which involved causing substantial
cytotoxicity to tumor cells only, but seldom affecting
non-transformed cells (68). That
research revealed an interaction between TRAIL and immediate early
response gene (IER3), which is expressed in a number of human
tissues, and appears to be downregulated in cancer cells, and its
overexpression can stimulate the apoptosis of cancer cells and
enhance their sensitivity to chemotherapeutic drugs (68). It was demonstrated that these two
proteins may be responsible for directing HCC cells to undergo
apoptosis and interrupt with their capacity to proliferate and
migrate. These findings demonstrate that TRAIL can partly influence
the pathogenesis of HCC by interacting with IER3 to reduce
Wnt/-catenin signaling (68). A
number of TRAIL-based therapeutics for the treatment of HCC are
currently undergoing or have undergone clinical trials, as
demonstrated in Table II.
 | Table IIList of clinical trials conducted on
TRAIL-based therapy against various types of cancer. |
Table II
List of clinical trials conducted on
TRAIL-based therapy against various types of cancer.
| Cancer type | Mechanism | Settings | Clinical
trial/status |
|---|
| Advanced-stage
HCC | Monoclonal antibody
targeting TRAIL-R1 (mapatumumab) | Combination therapy
(sorafenib) | Phase II completed
(143) (NCT01258608) |
| Advanced non-small
cell lung cancer | Apoptosis-inducing
recombinant TRAIL via DR4 and DR5 activation (mapatumumab,) | Combination therapy
(Paclitaxel and Carboplatin) | Phase III completed
in 2018 (144)
(NCT00583830) |
| Relapsed and
refractory multiple myeloma | Recombinant TRAIL
triggering apoptosis via the activation of DR4 and DR5 (CPT) | Combination therapy
(thalidomide) | Phase III completed
in 2014 (ChiCTRONC-1200206) |
| Advanced-stage
HCC | TRAIL receptor
agonists against TRAIL-R1(DR4) | Combination of
mapatumumab with sorafenib | Phase II (completed
in 2013) (NCT01258608) |
| Metastatic
triple-negative breast cancer | Monoclonal antibody
targeting TRAIL-R2 (tigatuzumab) | Combination therapy
(abraxane) | Phase II (completed
in 2017) (NCT01307891) |
| Non-small cell lung
cancer | Targeted stem cells
expressing TRAIL (MSC TRAIL) | Combination therapy
(pemetrexed/cisplatin chemotherapy) | Phase III clinical
trial estimated to be completed in September, 2025
(NCT03298763) |
| B-cell
non-Hodgkin's lymphoma | Recombinant TRAIL
triggering apoptosis via activation of DR4 and DR5
(dulanermin) | Combination therapy
(rituximab) | Phase II (completed
in 2010) (145)
(NCT00118209) |
| Advanced solid
tumors | TRAIL receptor
agonists against DR5 (DS-8273a) | Monotherapy | Phase I (completed
in 2017) (146)
(NCT02076451) |
| Colorectal cancer
non-small cell lung cancer, triple-negative breast cancer, renal
cell carcinoma, gastric cancer, pancreatic cancer | Equal mixture of
two humanized non-competing DR5-specific monoclonal antibodies
(GEN1029) | Monotherapy | Phase III clinical
trial estimated to be completed in March, 2022 (NCT03576131)
(terminated) |
Challenges with TRAIL therapy
Unfortunately, despite being such a prominent
feature of being specific to tumor cells, TRAIL-based therapy still
has a long way to go for successful clinical translation.
TRAIL-based therapy, including recombinant or agonistic monoclonal
antibodies against DR4/DR5 (69,70), has exhibited limited efficacy in a
number of clinical trials of different stages due to the short
plasma half-life of recombinant TRAIL, limited bioavailability and
undesirable systemic toxicity (69). Moreover, TRAIL-based therapy is
associated with several challenges, including the development of
TRAIL-mediated cell death resistance that leads to TRAIL-induced
apoptosis being ineffective in HCC. The root cause of the
development of therapeutic resistance may be intrinsic resistance
in some highly malignant tumors and acquired resistance
post-frequent exposure to TRAIL (71). Other factors may include the
activation of anti-apoptotic molecules and multiple receptors of
various signaling pathways (71-73).
Developing agonist monoclonal antibodies (mAbs)
targeting DR4 or DR5 receptors has been the most prevailing
approach due to their long serum half-lives in vivo. A
number of clinical trials, have been conducted on several agonists,
such as mapatumumab (NCT01258608), lexatumumab (NCT00428272) and
tigatuzumab (NCT01307891); however, none of them displayed any
antitumor response rates in cancer patients (69). Similarly, in preclinical tumor
xenograft mouse models, mapatumumab and lexatumumab have failed to
eradicate tumors (74,75). The dimeric structure of the
antibody is most likely to blame for the failure of all agonist mAb
clinical trials. Since the binding of ligand on DR4 and DR5 induces
receptor trimerization following the activation of the extrinsic
pathway, maintaining its trimeric structure will be required in the
future to enable its functional mechanism of apoptosis
induction.
The strategy employing recombinant human native
TRAIL appears to be more feasible as it allows the preservation of
the original trimeric structure with full functional efficacy.
Dulanermin, which is Amgen's version of TRAIL, was not found to be
effective against cancer in human clinical trials, even though it
was effective in preclinical tumor xenograft models (76). The probable reason for the
inefficacy of dulanermin may be related to its poor pharmacokinetic
profile due to its very short half-life in mammals. Given its low
molecular weight and its non-covalently linked trimeric structure
instability, which may cause rapid renal elimination. All these
facts illustrate the requirement of a novel approach to tackle this
challenge.
To obtain such biologically active TRAIL, many
expression systems, such as His, Flag tag or the incorporation of
trimerization domains, such as leucine zipper or isoleucine zipper
and the stabilization of trimers with cations, as zinc were
identified. It has been validated that to assemble and maintain a
functionally folded ligand trimer of TRAIL, zinc chelation is
critical (77,78). However, it appears to have its own
set of drawbacks. For example, both leucine zipper-fused TRAIL and
an N-terminus His-tagged TRAIL stabilized by insertion mutation are
likely to be immunogenic in humans (57). In addition, particularly the
His-tagged version, has been linked to hepatotoxicity not observed
with native TRAIL (79,80). Other strategies for prolonging the
half-life of TRAIL, which include albumin-conjugated TRAIL
nanoparticles or liposome conjugated TRAIL, present the issue of
production limitation (81,82).
The recent study by Naval et al (83) demonstrated the significance of the
oligomerization of TRAIL receptors in TRAIL-induced apoptosis. They
stated that TRAIL, as a transmembrane protein, exhibited more
potent pro-apoptotic properties than its soluble form (83). In immune system cells, TRAIL is
expressed as a type II membrane protein in the plasma membrane
(84,85) or is enclosed within microvesicles
(86,87). DR5 is solely triggered by the
membrane-bound form of TRAIL, whereas DR4 can be activated by both
the soluble and membrane-bound forms of the ligand (88). Given the property of TRAIL of
being naturally secreted as a membrane protein in exosomes, a
number of nanocarriers, such as liposomes, whose lipid composition
replicates natural exosomes with surface-bound TRAIL, have been
investigated in several pre-clinical studies on its anticancer
properties. Various in vitro and in vivo studies have
demonstrated that this membrane-bound form of TRAIL has greater
antitumor activity than the soluble form against hematological and
solid tumors (89-91). In comparison to soluble TRAIL,
this liposomal formulation with TRAIL attached to the liposome
surface produces improved DR5 clustering and increased DISC
recruitment, resulting in a greater apoptotic signal (90). As TRAIL produces high-order TRAIL
oligomers on the lipid nanoparticle surface, improved DR5
clustering and higher DISC recruitment is accomplished (92). Furthermore, TRAIL-encapsulated
liposomes and nanoparticles face hurdles with agent release from
carriers (93,94). In summary, the therapeutic
benefits of TRAIL therapy have been limited, possibly due to the
resistance displayed by HCC cells and poor pharmacokinetics. Thus,
an effective delivery agent is required for the delivery of TRAIL,
which can increase its circulation time in the human body with
optimal encapsulation.
4. Exosomes: A novel approach for drug
delivery
Conventional anticancer drugs display limited
efficacy owing to their short half-life, poor solubility and
inefficacious delivery, resulting in the development of drug
resistance and substantial systemic toxicity (95). Apart from this, poor drug delivery
is also a major contributor to treatment failure. After entering
the blood circulation by injection, the drug faces a variety of
hurdles before reaching and acting on the target site (96). To improve the efficacy of HCC
chemotherapeutics, a drug delivery system with active targeting and
local, controlled, and continuous drug release is urgently
required.
Extracellular vesicles (EV)-based therapeutics are a
promising drug delivery system, since they can penetrate tissues
and even cross the blood-brain barrier as natural nanoscale agents
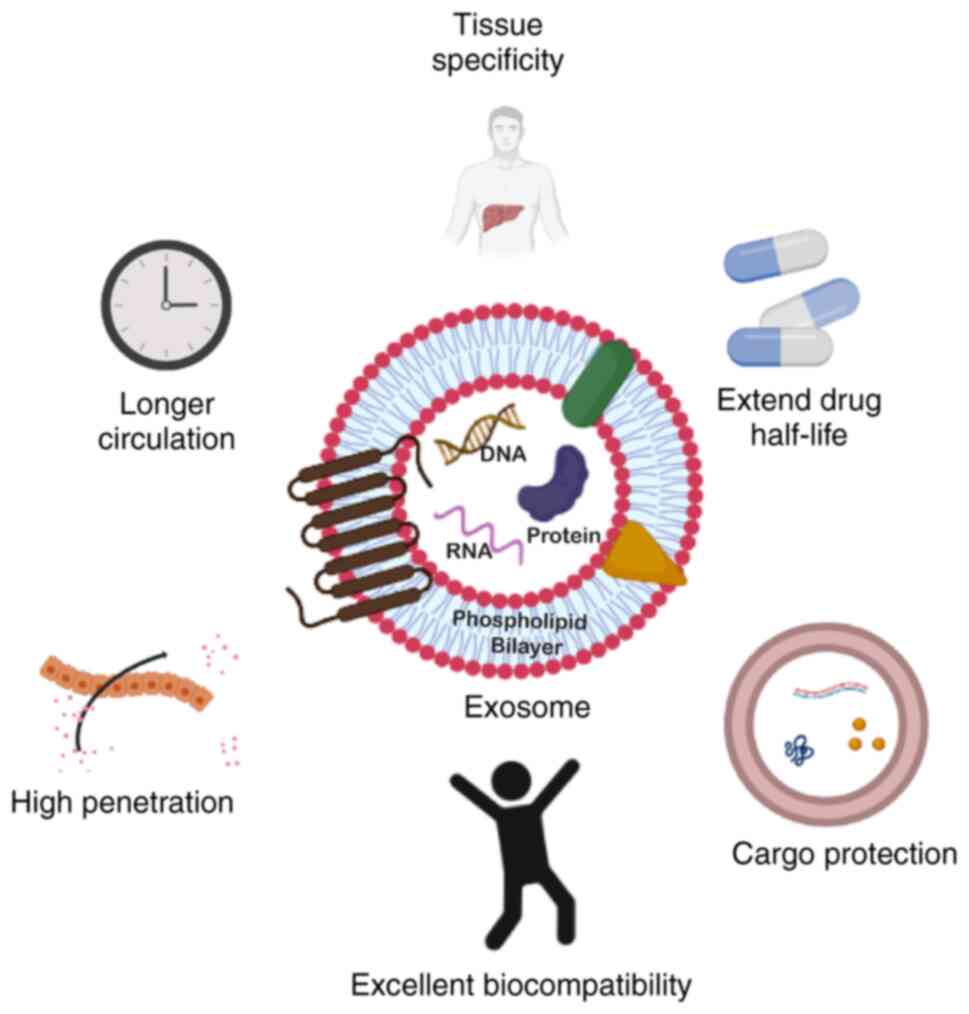
(97,98). Exosomes in particular, offer
numerous benefits as drug delivery vehicles, which include a small
size, low cytotoxicity, long half-life in the circulation, and the
ability to load various cargoes with high a biocompatibility
(Fig. 4) (99,100). Conventional drug delivery
strategies frequently fall short of the intended results for
several reasons, including the rapid in vivo degradation of
miRNAs, the loss of native structure in proteins and the potential
for severe toxicity in normal cells. However, these issues may be
resolved by using exosomes as carriers and thus, by deploying
exosomes to deliver drugs to tumor sites, an effective and
promising approach may be made available for targeted cancer
therapy.
Exosomes are endosomal-derived EVs with a size of
30-200 nm that have been reported to be released by a multitude of
cell types. They have the characteristic cargo-loading capacity of
carrying heterogenous biomolecules, such as DNA, RNA, proteins and
lipids, and transferring them to recipient cells, thus acting as
intercellular messengers. Exosomes are formed by the intraluminal
budding of multivesicular bodies (MVBs) of an intact cell and are
released into the extracellular environment when these MVBs fuse
with the plasma membrane of the recipient cell (101).
Exosomes have the potential to mirror the intricacy
of the parental cell with the innate capacity to regulate multitude
of roles in crucial biological activities (102). Consequently, this characteristic
led to exosome-based applications in cancer treatment and diagnoses
being more feasible. Exosomes contain molecules with a wide range
of functions, but lack the complexity of cells and organs; as a
result, exosomes are regarded as excellent tools for use in the
treatment of a variety of disorders, including cancer. Exosomes
also have a number of advantages in terms of biocompatibility,
stability, cellular uptake mechanism, biodistribution,
pharmacokinetics and immunogenicity, rendering them promising
anticancer candidates. These characteristics can raise the
therapeutic index of exosome-based cancer treatments by
preferentially targeting tumor cells, while reducing undesirable
side-effects. Below is a brief illustration of key facts of the
therapeutic potential of exosomes over present drug delivery
platforms.
Source and safety
MSCs have been reported to be the most favorable
selection for the commercial production of exosomes due to the ease
of availability and are reported to produce an excessive number of
exosomes (EVs) with consistent sustainability and reproducibility
compared to other cell lines (103). Most importantly, applying
MSC-exosomes as an agent to deliver drugs has been reported to have
no safety concerns, including the chances of inducing
tumorigenicity (104,105). MSC-derived EVs exhibit
significant flexibility for in vitro and in vivo
modifications (106), as well as
a high stability in human plasma and at storage at 20°C (107,108).
Furthermore, MSC-derived EVs have been demonstrated
to be well-tolerated in a variety of animal models, aside from
possessing therapeutic benefits as proven in the treatment of
myocardial infarction, chronic kidney disease, wound healing and
liver injury in mouse models (109,110). Previous studies have employed
EVs as an efficient systemic natural gene carrier for transporting
anticancer miRNAs and proteins (111,112). A number of phase I clinical
trials have validated the safety of EV administration; no reports
of grade II toxicity were reported with the determination of the
maximal tolerated dose (113-115).
Drug delivery and cellular uptake
As aforementioned, exosomes, being a natural
cellular messenger, provide the benefit of a heterogeneous
cargo-loading capacity and specificity. Exosomes are known to
possess homing properties that can deliver cargo even to distant
targets and in between cells with suitable biocompatibility, and to
regulate the functions of targeted cells transiently (116). Reportedly, the interaction of
exosomes with target cells involves multiple mechanisms, as
exosomes can directly bind to membrane receptors of the recipient
cell for content internalization, or they can transport bioactive
cargo by fusing with the plasma membrane of the target cell.
Presently, a number of drugs face the issue of not
being able to cross the blood-brain barrier, limiting the efficacy
of several therapies, including cancer therapies. However, exosomes
can cross the blood-brain barrier to increase intracranial drug
concentration (117). For
example, exosomes have been shown to carry medicines or siRNA to
the brains of mice with Alzheimer's disease (111,118). Unlike the traditional approach
of drug administration, delivery using exosomes does not have the
drawback of drug toxicity, intracranial infection and imprecise
absorption (118).
Cargo protection and improved
durability
The ideal delivery agent does not only perform the
site-specific transportation of enclosed therapeutics, but should
also be capable of protecting the enclosed material and avoiding
premature degradation by the body's immune system. Exosomes have a
lipid bilayer structure that not only aids in transport efficiency
and supports the load of hydrophobic or hydrophilic drugs, but also
protects the encapsulated material (119). Furthermore, being able to have a
reduced clearance rate can sustain the drugs in the body's
circulation. Exosomes, being natural products of the body, do not
invoke any immune response and possess a longer circulation
half-life, which can prevent therapeutic cargo from degrading too
rapidly (118).
Stability
Exosomes are well-known for their stability, as they
preserve the identity of their parental cells, while maintaining
their long-term innate integrity (107). Multiple freeze-thaw cycles have
been shown to have no effect on their size, indicating that
freezing has no effect on the quality of exosomes stored (107). Kalra et al (108) demonstrated the stability of
colon cancer-derived exosomes and noted that the majority of
samples retained their integrity even without protease inhibitors
for 3 months, and that the highest stability was found at 80°C.
This property of being stable for a long period of time in storage
at 80°C suggests another advantage of exosomes over existing
anticancer drugs (120).
Furthermore, a previous study found that therapeutic
exosomes maintained antitumor activity even after being frozen for
at least 5 months (121).
Exosomes can shield therapeutic nucleic acids and proteins from
RNases and proteinase degradation as they contain fragile bioactive
molecules within a lipid bilayer membrane (122). In addition, under both
physiological and pathological conditions, exosomes appear to
display an enhanced stability in the blood, that facilitates
long-distance travel within the body. Thus, exosome stability
covers not just the human body, but also storage in the field. In
addition to this, they have also been shown to have improved
stability in the blood, allowing them to cover long distances
throughout the body under both normal and pathological conditions
(123).
Therapeutic significance and drug
resistance
It has been demonstrated that drugs encapsulated
with exosomes lead to an enhanced chemotherapeutic efficacy
(124). Exosomes appear to have
a much higher potential (>10-fold) in targeting cancer cells
compared to liposomes of similar size (125). Furthermore, currently, the major
barrier to an effective therapy or complete cure is multi-drug
resistance (MDR). This resistance is commonly shown by all cancer
patients undergoing long-term chemotherapy. The exosome is one such
natural nanocarrier which has proven to be efficient in overcoming
the issue of MDR in tumors. Kim et al (126) confirmed this by integrating
paclitaxel (PTX) into exosomes released from macrophages for the
treatment of MDR cancer. They determined that exosomes augmented
cytotoxicity in drug-resistant cells by >50-fold when compared
to exosome-free drugs (126).
Furthermore, when doxorubicin (DOX)-loaded exosomes were
administered intranasally to animals with pulmonary metastasis,
confocal fluorescence microscopy revealed an almost perfect
co-localization with cancer cells. These findings suggest that
PTX-loaded exosomes inhibit MDR tumors and pulmonary metastasis
growth more effectively (126).
5. Challenges, synergism, and strategies
against liver cancer
Several drug delivery strategies have been developed
over the years for the treatment of cancer; however, only a few of
these have obtained clinical approval. The likelihood of an
efficacious drug delivery to cancer tissues following in
vivo administration is <0.7% (127). The expected accomplishments in
the case of HCC drug development may not be as satisfactory as in
the case of other types of cancer. Furthermore, based upon the
outcome of recent clinical trials, a single drug therapy appears to
be inadequate in the case of treatment for advanced-stage HCC
(128). Thus, combination
therapy is a major area of research for the treatment of
advanced-stage HCC.
Surprisingly, formulations containing recombinant
TRAIL have encountered considerable difficulties in being evaluated
for human application due to its undesirable systemic toxicity, the
short plasma half-life of recombinant TRAIL, or the activation of
anti-apoptotic proteins (62,69,94,129). However, the exosome-based
encapsulation of TRAIL protein can convey better pharmacokinetic
characteristics, greater bioavailability and the ability to cross
target tissues to the enclosed protein/drug (130). For example, in a previous study,
TRAIL was transduced into leukemia K562 cells with human membrane
TRAIL and produced TRAIL secreted exosomes, which were shown to
trigger the apoptosis of melanoma and lymphoma cell lines in
vitro (131). These findings
reveal that cells that have been genetically engineered to express
TRAIL can secrete exosomes that contain the pro-apoptotic ligand in
an active form in their membranes. Although therapeutic success
varied in the different tumor models studied, TRAIL exosomes
exhibited potent killing activity in vitro and in
vivo, in both local and systemic therapy modalities (131).
Another study by Yuan et al (132) demonstrated that TRAIL-loaded
exosomes were more efficient in inducing cell death than
recombinant soluble TRAIL. It was shown that the fluidic nature of
the lipid bilayer membrane in exosomes harboring TRAIL may allow
higher order TRAIL oligomerization and, as a result, the stronger
clustering of its receptors, which is a crucial signal for
effective extrinsic death pathway activation (132). It was demonstrated that the
limited bioavailability of TRAIL, the low activity and cell
resistance to TRAIL ligand can be overcome by TRAIL-expressing EVs
derived from MSCs, thereby improving the clinical efficacy of
TRAIL. This EV-loaded TRAIL effectively induced apoptosis in a
variety of cancer cell lines, including lung (A549, NCI-H460 and
NCI-H727), neuroblastoma (SHEP-TET), breast (M231), kidney (RCC10)
and malignant pleural mesothelioma lines (H2795). While there was
no toxicity to control healthy cells, TRAIL+ exosomes
were capable of triggering apoptosis in TRAIL-resistant cancer
cells (132).
The TRAIL receptor binding to target cells, which
activates the caspase cascade and results in death, was suggested
as the therapeutic mechanism of TRAIL-MSC-EVs. Furthermore,
TRAIL-MSC-EVs have exhibited therapeutic efficacy in
TRAIL-resistant cancer cell lines, which is noteworthy (132). The study by Shamili et al
(133) also demonstrated the
anti-tumor activity of TRAIL-transfected MSC-derived exosomes in a
mouse model of melanoma. Their findings suggested that when
TRAIL-expressing exosomes were injected into mice, they delayed the
appearance of tumors and attenuated tumor growth (133). Of note, they proposed that a
combination of TRAIL-exosomes with another chemotherapeutic may be
explored as a promising therapeutic tool (133). The diagrammatic representation
of how TRAIL-expressing exosomes would lead to cell death in
recipient HCC cells is presented in Fig. 3. A list of studies demonstrating
the exosomal delivery by TRAIL and its clinical significance is
presented Table III.
 | Table IIIList of recent studies on exosomal
delivery of TRAIL for cancer treatment. |
Table III
List of recent studies on exosomal
delivery of TRAIL for cancer treatment.
| Cancer types | Donor cells | Results | (Refs.) |
|---|
| Melanoma and
lymphoma | K562 cells
(lymphoblasts) | Induction of
apoptosis in cancer cells and control tumor progression in
vivo | (131) |
| Lung cancer (in
vitro), pleural mesothelioma (in vitro), renal cancer
(in vitro), breast adenocarcinoma (in vitro),
neuroblastoma (in vitro) | MSCs | Highly efficient at
selectively inducing apoptosis in cancer cells and TRAIL delivery
by MSC-EVs at least partially overcomes TRAIL resistance in cancer
cells | (132) |
| Melanoma | MSCs | Delay in the
appearance of tumors and attenuation of tumor growth | (133) |
| Lymphoma | Myeloid leukemia
cells | Increased apoptosis
of leukemia cells | (147) |
| Human lung
adenocarcinoma | 293T cells | Dinaciclib and
TRAIL exert synergistic effects on TRAIL-mediated apoptosis | (148) |
| Lung cancer | MSCs | EV-encapsulated
TRAIL and dinaciclib can overcome the drug-resistance of lung
cancer cells and are highly efficient for inducing the apoptosis of
the TRAIL-resistant A549 cell line | (149) |
| Malignant
melanoma | RAW 264.7
(macrophage cell line) |
TRAIL-Exo/triptolide improved tumor
targetability, enhanced cellular uptake, inhibited
theproliferation, invasion, and migration, and induced the
apoptosis of A375 cells | (150) |
Several studies have found a synergistic effect
between TRAIL and sorafenib, suggesting that combined treatment
with these agents may lead to the development of effective therapy
for overcoming TRAIL resistance in cancer cells. For example, a
previous study demonstrated the synergistic effects of sorafenib
and TRAIL, where Sorafenib considerably increased the cytotoxicity
TRAIL to HCC cells (134). The
enhancement in cytotoxicity may be obtained from the downregulation
of anti-apoptotic proteins by sorafenib. Similarly, another study
by Chen et al (135)
revealed that sorafenib sensitized TRAIL-resistant HCC cells to
TRAIL-induced apoptosis by inhibiting STAT3 (135).
Moreover, the application of Sorafenib enclosed
within exosomes into the target site offers a number of benefits as
opposed to oral administration. As depicted by a previous study,
exosome-encapsulated DOX delivery increased the therapeutic index
in breast and ovarian cancer mouse models compared to exosome-free
DOX (124). It was also
demonstrated by in vitro and in vivo experiments that
exosomes loaded with DOX limited heart toxicity by partially
reducing the passage of DOX through cardiac endothelial cells
(124).
The major issue with the safe, specific and
efficient delivery of miRNAs is their property of being easily
degradable before reaching the target organ. The lipid bilayer
membrane of EVs protects the enclosed miRNA, preventing it from
degradation and facilitating its effective delivery to the target
site (136). Previous research
has demonstrated that exosomes can safely enclose and carry miRNA
to target cells of multiple types of cancer. For example, Almanza
et al (137) demonstrated
that EVs containing miR-335 effectively and long-lastingly restored
the endogenous miR-335 pool in human triple-negative breast cancer
cells, suppressing the expression of the miR-335 target gene SOX4
transcription factor, and significantly reducing tumor development
in vivo.
Previously, another group (138) reported utilizing EVs as a
delivery agent for miR-335 both in vivo and in vitro.
They were successful in demonstrating that the safe administration
of fibroblast-derived EVs that were loaded with miR-195 may
concentrate inside the tumor, reduce the size of tumors, and
increase the longevity of treated rats in a rat model of
cholangiocarcinoma (138). Wang
et al (139) demonstrated
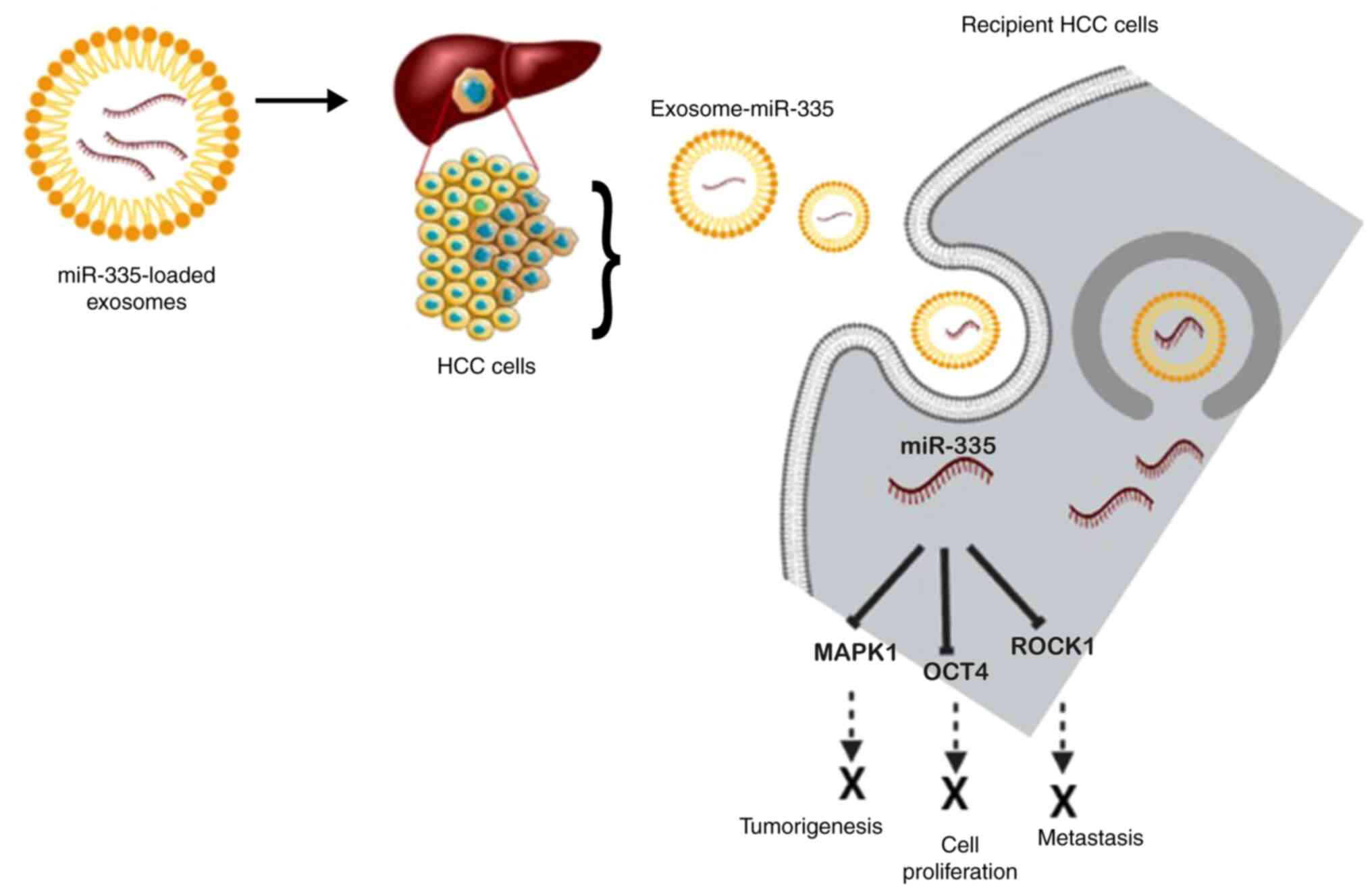
that miR335-5p could be successfully supplied to hepatoma cells by
utilizing exosomes as a delivery agent both in vivo and
in vitro. They observed the progression of HCC cell
development when stellate cells were co-cultured with HCC cells due
to exosomal transfer and noted the downregulated expression of
miR-3355p in both cells and exosomes (139). The target genes identified in
terms of HCC are CDC42, NRG1, EIF5, CDK2, EIF2C2, LIMK1, PLK2,
RGS19, THBS1, YBX1 and TCF3. However, upregulating the expression
of miR-335-5p in stellate cell-derived exosomes has been shown to
restrict HCC cell proliferation and invasion in vitro, and
cause tumor shrinkage in mouse models (139). A schematic diagram of the
exosomal delivery of miR-335 into recipient HCC cells and the mode
of action based on the afore-mentioned investigations is presented
in Fig. 5.
It is also noteworthy that miR-335 modulates the
sensitivity of sorafenib against HCC cells. As shown in the study
by Kim et al (43), Siah
E3 ubiquitin protein ligase 2 (SIAH2) is the target of miR-335,
where miR-335 contributes to the sensitizing effect of anticancer
drugs via the expression enhancement of histone deacetylase 3
(HDAC3). SIAH2 overexpression was shown to result in anticancer
drug resistance due to its effect on HDAC3 expression and
ubiquitination (43). These
findings suggest that miR-335 may be a promising anticancer agent.
When combined with sorafenib, encapsulating it in exosomes
increases its sensitivity to the drug, thus enhancing the
therapeutic efficacy and preventing degradation.
6. Development of exosome-based TRAIL +
miR-335 therapy
Although exosome-based cancer therapy displays
exceptional therapeutic potential, there are still a number of
substantial challenges that need to be resolved in order for its
use to be feasible in clinical applications. The technology of
exosome mass production is not yet standardized. Although
small-scale GMP exosome production has been shown to be viable,
there are still numerous obstacles in large-scale production
(121,140). Numerous companies are still
struggling to produce exosome on mass scale level. However, few
companies, such as CK Exogene, a Korean biotechnology firm, have
managed to overcome the issue of low exosome yield and have
acquired the patented technology (10-2020-0062365) for exosome mass
production (141) and this
company is currently developing exosome-based anticancer drug for
patients with liver cancer using the aforementioned candidates
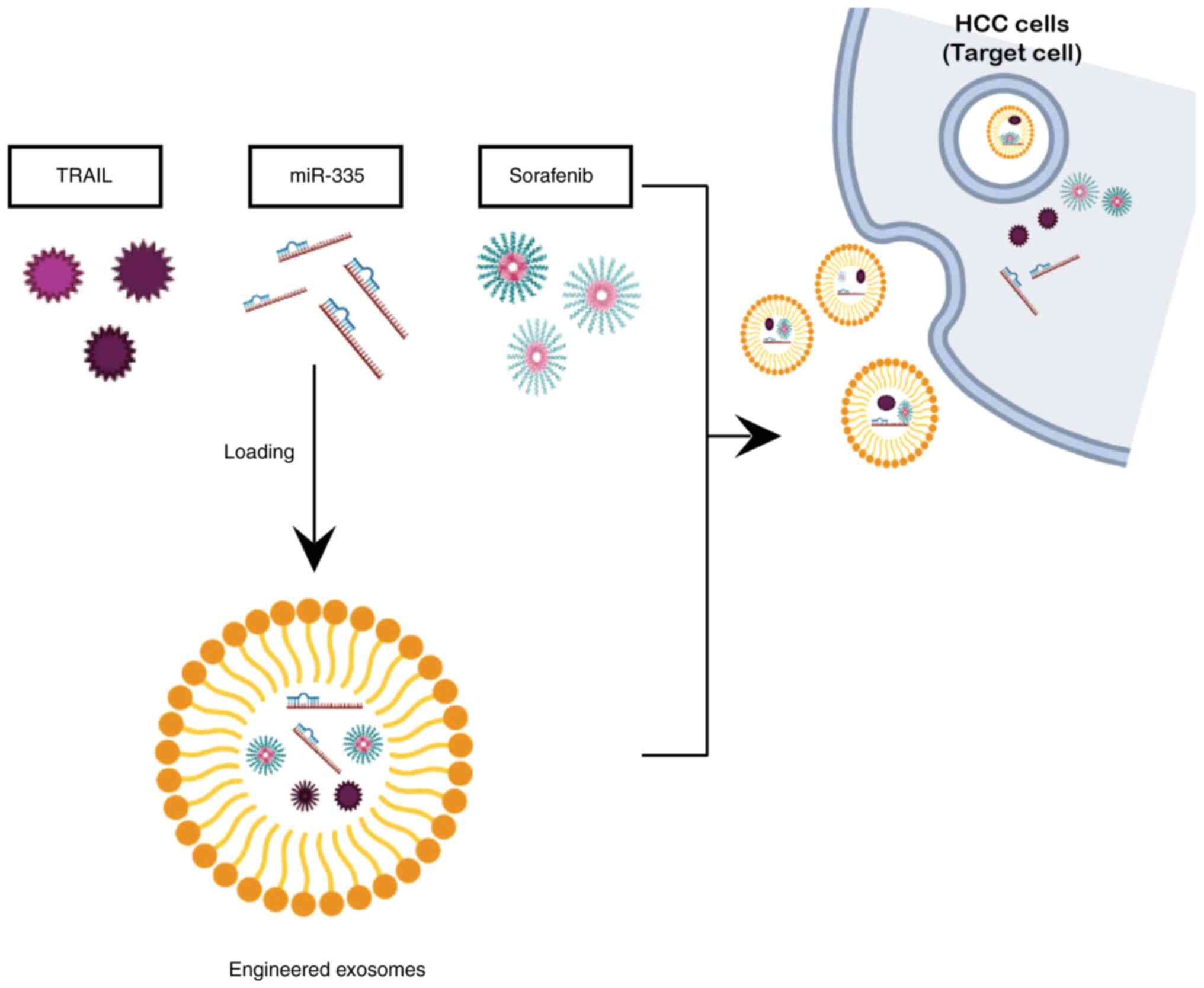
i.e., TRAIL and miR-335. A schematic overview of anticancer
candidates, including dorafenib encapsulated in exosomed as a
delivery agent is presented in Fig.
6).
To the best of our knowledge, the present review is
the first of its kind, exploring and combining the cutting-edge
feature of exosome with novel anticancer candidates (TRAIL and
miR-335). The aim of the present review article was to present the
compiled investigations of TRAIL and miR-335, both of which have
been extensively explored in the past, along with the added benefit
of utilizing exosomes as a carrier. Exosomes encapsulating TRAIL
have the potential to overcome the challenging issue of resistance
among HCC cells towards TRAIL-induced apoptosis, thus rendering
TRAIL more effective in killing cancer cells. Such a strategy not
only provides long-term and effective anticancer treatment for
patients with HCC, but it has also been reported to overcome the
issue of drug resistance, which is the major challenge to current
drug therapy for liver cancer.
Additionally, to receive the successful outcome of
any drug treatment, the accessibility of the drug to the target
organ is imperative and necessitates the requirement of an
effective delivery route. Even though drugs targeted against HCC
comprise various delivery routes, including the direct injection
into the liver, intra-arterial drug delivery is an effective
technique for targeting the tumor site with multiple agents.
Compared to intravenous delivery, intra-arterial drug
administration expedites the systemic clearance and enhances the
intra-tumor drug concentration (142). Owing to such high magnitude of
benefits conferred by exosome-based TRAIL-miR-335 delivery from
preventing metastasis to inducing cytotoxicity in cancer cells
specifically, this approach has the prospects to be provided to
patients with all stages of liver cancer from stages 0 to 4.
7. Conclusions
Exosomes offer the versatile characteristics of an
efficient delivery system for both TRAIL and miR-335 as anticancer
candidates. The incorporation of the benefits of exosomes, with
them being a natural cellular carrier and the combination of these
novel candidates with a standard drug, such as sorafenib would have
a sensitizing effect and has been proven to yield a synergistic
anti-cytotoxicity effect on TRAIL-resistant cancer cells. Moreover,
this strategy also has the potential to overcome resistance to
sorafenib, the most prevalent issue of the current drug treatment
program among patients with HCC. The most prominent significance of
exosome-based technology lies in the fact that this approach can be
applied to all types of cancer and encompasses the benefit of
overcoming drug resistance, which is the most prevalent issue in
current drug treatment regimen. The present review thus provides an
insight into the development of exosome-based therapy and the
possibility of its bench-to-bed translation for providing an
exceptional anticancer treatment.
Availability of data and materials
Data sharing is not applicable to this article, as
no data sets were generated or analyzed during the current
study.
Authors' contributions
All authors (NT, YJC, KHY, TBW, DK, DC and JK) were
involved in the drafting and revision of the manuscript, and in
critically revising the manuscript for important intellectual
content. All authors have read and approved the final manuscript.
Data authentication is not applicable.
Ethics approval and consent to
participate
Not applicable.
Patient consent for publication
Not applicable.
Competing interests
The purification strategy for the mass production
of highly purified and concentrated exosomes is subject to Korean
patent application no. 10-2020-0062365, associated with CK-Exogene,
Inc. JK and NT are employees of CK-Exogene, Inc. The other authors
(YJC, KHY, TBW, DK and DC) are not associated with CK-Exogene, Inc.
and declare that they have no competing interests.
Acknowledgments
Not applicable.
Funding
No funding was received.
References
|
1
|
Bray F, Ferlay J, Soerjomataram I, Siegel
RL, Torre LA and Jemal A: Global cancer statistics 2018: GLOBOCAN
estimates of incidence and mortality worldwide for 36 cancers in
185 countries. CA Cancer J Clin. 68:394–424. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Singal AG and El-Serag HB: Hepatocellular
carcinoma from epidemiology to prevention: Translating knowledge
into practice. Clin Gastroenterol Hepatol. 13:2140–2151. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Dasgupta P, Henshaw C, Youlden DR, Clark
PJ, Aitken JF and Baade PD: Global trends in incidence rates of
primary adult liver cancers: A systematic review and meta-analysis.
Front Oncol. 10:1712020. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Gomes MA, Priolli DG, Tralhão JG and
Botelho MF: Hepatocellular carcinoma: Epidemiology, biology,
diagnosis, and therapies. Rev Assoc Med Bras (1992). 59:514–524.
2013.In English, Portuguese. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Center MM and Jemal A: International
trends in liver cancer incidence rates. Cancer Epidemiol Biomarkers
Prev. 20:2362–2368. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Farinati F, Sergio A, Baldan A, Giacomin
A, Di Nolfo MA, Del Poggio P, Benvegnu L, Rapaccini G, Zoli M,
Borzio F, et al: Early and very early hepatocellular carcinoma:
When and how much do staging and choice of treatment really matter?
A multi-center study. BMC Cancer. 9:332009. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Kakushadze Z, Raghubanshi R and Yu W:
Estimating cost savings from early cancer diagnosis. Data.
2:302017. View Article : Google Scholar
|
|
8
|
Finn RS: Emerging targeted strategies in
advanced hepatocellular carcinoma. Semin Liver Dis. 33(Suppl 1):
S11–S19. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
WHO's International Agency for Research on
Cancer (IARC): World Cancer Report 2014. Stewart BW and Kleihues P:
IARC Press; Lyon: 2014
|
|
10
|
Dimitroulis D, Damaskos C, Valsami S,
Davakis S, Garmpis N, Spartalis E, Athanasiou A, Moris D,
Sakellariou S, Kykalos S, et al: From diagnosis to treatment of
hepatocellular carcinoma: An epidemic problem for both developed
and developing world. World J Gastroenterol. 23:5282–5294. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Arruebo M, Vilaboa N, Saez-Gutierrez B,
Lambea J, Tres A, Valladares M and González-Fernández A: Assessment
of the evolution of cancer treatment therapies. Cancers (Basel).
3:3279–3330. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
El-Serag HB, Marrero JA, Rudolph L and
Reddy KR: Diagnosis and treatment of hepatocellular carcinoma.
Gastroenterology. 134:1752–1763. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Llovet JM, Ricci S, Mazzaferro V, Hilgard
P, Gane E, Blanc JF, de Oliveira AC, Santoro A, Raoul JL, Forner A,
et al: Sorafenib in advanced hepatocellular carcinoma. N Engl J
Med. 359:378–390. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Kane RC, Farrell AT, Madabushi R, Booth B,
Chattopadhyay S, Sridhara R, Justice R and Pazdur R: Sorafenib for
the treatment of unresectable hepatocellular carcinoma. Oncologist.
14:95–100. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Liu L, Cao Y, Chen C, Zhang X, McNabola A,
Wilkie D, Wilhelm S, Lynch M and Carter C: Sorafenib blocks the
RAF/MEK/ERK pathway, inhibits tumor angiogenesis, and induces tumor
cell apoptosis in hepatocellular carcinoma model PLC/PRF/5. Cancer
Res. 66:11851–11858. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Kudo M, Finn RS, Qin S, Han KH, Ikeda K,
Piscaglia F, Baron A, Park JW, Han G, Jassem J, et al: Lenvatinib
versus sorafenib in first-line treatment of patients with
unresectable hepatocellular carcinoma: A randomised phase 3
non-inferiority trial. Lancet. 391:1163–1173. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Finn RS, Qin S, Ikeda M, Galle PR, Ducreux
M, Kim TY, Kudo M, Breder V, Merle P, Kaseb AO, et al: Atezolizumab
plus bevacizumab in unresectable hepatocellular carcinoma. N Engl J
Med. 382:1894–1905. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Ikeda K, Kudo M, Kawazoe S, Osaki Y, Ikeda
M, Okusaka T, Tamai T, Suzuki T, Hisai T, Hayato S, et al: Phase 2
study of lenvatinib in patients with advanced hepatocellular
carcinoma. J Gastroenterol. 52:512–519. 2017. View Article : Google Scholar :
|
|
19
|
Calin GA and Croce CM: MicroRNA signatures
in human cancers. Nat Rev Cancer. 6:857–866. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Meng F, Henson R, Wehbe-Janek H, Ghoshal
K, Jacob ST and Patel T: MicroRNA-21 regulates expression of the
PTEN tumor suppressor gene in human hepatocellular cancer.
Gastroenterology. 133:647–658. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Jin C, Wang A, Liu L, Wang G, Li G and Han
Z: miR-145-5p inhibits tumor occurrence and metastasis through the
NF-κB signaling pathway by targeting TLR4 in malignant melanoma. J
Cell Biochem. Jan 30–2019.Epub ahead of print. View Article : Google Scholar
|
|
22
|
Su Z, Yang Z, Xu Y, Chen Y and Yu Q:
MicroRNAs in apoptosis, autophagy and necroptosis. Oncotarget.
6:8474–8490. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Hydbring P, Wang Y, Fassl A, Li X, Matia
V, Otto T, Choi YJ, Sweeney KE, Suski JM, Yin H, et al:
Cell-cycle-targeting MicroRNAs as therapeutic tools against
refractory cancers. Cancer Cell. 31:576–590.e8. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Nagy Á, Lánczky A, Menyhárt O and Győrffy
B: Validation of miRNA prognostic power in hepatocellular carcinoma
using expression data of independent datasets. Sci Rep. 8:92272018.
View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Wang L, Zhao Y, Xu M, Zhou F and Yan J:
Serum miR-1301-3p, miR-335-5p, miR-28-5p and their target B7-H3 may
serve as novel biomarkers for colorectal cancer. J BUON.
24:1120–1127. 2019.PubMed/NCBI
|
|
26
|
Du W, Tang H, Lei Z, Zhu J, Zeng Y, Liu Z
and Huang JA: miR-335-5p inhibits TGF-β1-induced
epithelial-mesenchymal transition in non-small cell lung cancer via
ROCK1. Respir Res. 20:2252019. View Article : Google Scholar
|
|
27
|
Xu X, Tao Y, Shan L, Chen R, Jiang H, Qian
Z, Cai F, Ma L and Yu Y: The role of MicroRNAs in hepatocellular
carcinoma. J Cancer. 9:3557–3569. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Wang G, Dong F, Xu Z, Sharma S, Hu X, Chen
D, Zhang L, Zhang J and Dong Q: MicroRNA profile in HBV-induced
infection and hepatocellular carcinoma. BMC Cancer. 17:8052017.
View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Gougelet A: Exosomal microRNAs as a
potential therapeutic strategy in hepatocellular carcinoma. World J
Hepatol. 10:785–789. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Ye L, Wang F, Wu H, Yang H, Yang Y, Ma Y,
Xue A, Zhu J, Chen M, Wang J and Zhang QA: Functions and targets of
miR-335 in cancer. Onco Targets Ther. 14:3335–3349. 2021.
View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Scarola M, Schoeftner S, Schneider C and
Benetti R: miR-335 directly targets Rb1 (pRb/p105) in a proximal
connection to p53-dependent stress response. Cancer Res.
70:6925–6933. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Liu J, Bian T, Feng J, Qian L, Zhang J,
Jiang D, Zhang Q, Li X, Liu Y and Shi J: miR-335 inhibited cell
proliferation of lung cancer cells by target Tra2β. Cancer Sci.
109:289–296. 2018. View Article : Google Scholar
|
|
33
|
Tang H, Zhu J, Du W, Liu S, Zeng Y, Ding
Z, Zhang Y, Wang X, Liu Z and Huang J: CPNE1 is a target of
miR-335-5p and plays an important role in the pathogenesis of
non-small cell lung cancer. J Exp Clin Cancer Res. 37:1312018.
View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Li HW and Liu J: Circ_0009910 promotes
proliferation and metastasis of hepatocellular carcinoma cells
through miR-335-5p/ROCK1 axis. Eur Rev Med Pharmacol Sci.
24:1725–1735. 2020.PubMed/NCBI
|
|
35
|
Liu H, Li W, Chen C, Pei Y and Long X:
MiR-335 acts as a potential tumor suppressor miRNA via
downregulating ROCK1 expression in hepatocellular carcinoma. Tumour
Biol. 36:6313–6319. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Chen K and Zhang L: LINC00339 regulates
ROCK1 by miR-152 to promote cell proliferation and migration in
hepatocellular carcinoma. J Cell Biochem. 120:14431–14443. 2019.
View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Chen C, Wu CQ, Zhang ZQ, Yao DK and Zhu L:
Loss of expression of miR-335 is implicated in hepatic stellate
cell migration and activation. Exp Cell Res. 317:1714–1725. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Yang X, Song H, Zi Z, Kou J, Chen S, Dai
Y, Wang J, Yuan L and Gao K: Circ_0005075 promotes hepatocellular
carcinoma progression by suppression of microRNA-335. J Cell
Physiol. 234:21937–21946. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Pearson G, Robinson F, Beers Gibson T, Xu
BE, Karandikar M, Berman K and Cobb MH: Mitogen-activated protein
(MAP) kinase pathways: Regulation and physiological functions.
Endocr Rev. 22:153–183. 2001.PubMed/NCBI
|
|
40
|
Zhang D, Li X, Yao Z, Wei C, Ning N and Li
J: GABAergic signaling facilitates breast cancer metastasis by
promoting ERK1/2-dependent phosphorylation. Cancer Lett.
348:100–108. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Ji YY, Song Y and Wang AN: MiR-335-5p
inhibits proliferation of Huh-7 liver cancer cells via targeting
the Oct4/Akt pathway. Eur Rev Med Pharmacol Sci. 25:1853–1860.
2021.PubMed/NCBI
|
|
42
|
Zhang BJ, Gong HY, Zheng F, Liu DJ and Liu
HX: Up-regulation of miR-335 predicts a favorable prognosis in
esophageal squamous cell carcinoma. Int J Clin Exp Pathol.
7:6213–6218. 2014.PubMed/NCBI
|
|
43
|
Kim Y, Kim H, Park D and Jeoung D: miR-335
targets SIAH2 and confers sensitivity to anti-cancer drugs by
increasing the expression of HDAC3. Mol Cells. 38:562–572. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Cheng Y and Shen P: miR-335 acts as a
tumor suppressor and enhances ionizing radiation-induced tumor
regression by targeting ROCK1. Front Oncol. 10:2782020. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Cui L, Hu Y, Bai B and Zhang S: Serum
miR-335 level is associated with the treatment response to
trans-arterial chemoembolization and prognosis in patients with
hepatocellular carcinoma. Cell Physiol Biochem. 37:276–283. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Chen S and Xia X: Long noncoding RNA NEAT1
suppresses sorafenib sensitivity of hepatocellular carcinoma cells
via regulating miR-335-c-Met. J Cell Physiol. Apr 1–2019.Epub ahead
of print.
|
|
47
|
Dohi O, Yasui K, Gen Y, Takada H, Endo M,
Tsuji K, Konishi C, Yamada N, Mitsuyoshi H, Yagi N, et al:
Epigenetic silencing of miR-335 and its host gene MEST in
hepatocellular carcinoma. Int J Oncol. 42:411–418. 2013. View Article : Google Scholar :
|
|
48
|
Shang X, Li G, Liu H, Li T, Liu J, Zhao Q
and Wang C: Comprehensive circular RNA profiling reveals that hsa_
circ_0005075, a new circular RNA biomarker, is involved in
hepatocellular crcinoma development. Medicine (Baltimore).
95:e38112016. View Article : Google Scholar
|
|
49
|
Nie Y, Zhu X, Bu N, Jiang Y, Su Y, Pan K
and Li S: Circ_0064288 acts as an oncogene of hepatocellular
carcinoma cells by inhibiting miR-335-5p expression and promoting
ROCK1 expression. BMC Cancer. 22:2652022. View Article : Google Scholar : PubMed/NCBI
|
|
50
|
Ashkenazi A and Dixit VM: Apoptosis
control by death and decoy receptors. Curr Opin Cell Biol.
11:255–260. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
51
|
Wajant H: Molecular mode of action of
TRAIL receptor agonists-common principles and their translational
exploitation. Cancers (Basel). 11:9542019. View Article : Google Scholar : PubMed/NCBI
|
|
52
|
Amarante-Mendes GP and Griffith TS:
Therapeutic applications of TRAIL receptor agonists in cancer and
beyond. Pharmacol Ther. 155:117–131. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
53
|
Willms A, Schittek H, Rahn S, Sosna J,
Mert U, Adam D and Trauzold A: Impact of p53 status on
TRAIL-mediated apoptotic and non-apoptotic signaling in cancer
cells. PLoS One. 14:e02148472019. View Article : Google Scholar : PubMed/NCBI
|
|
54
|
Micheau O, Shirley S and Dufour F: Death
receptors as targets in cancer. Br J Pharmacol. 169:1723–1744.
2013. View Article : Google Scholar : PubMed/NCBI
|
|
55
|
Lim B, Allen JE, Prabhu VV, Talekar MK,
Finnberg NK and El-Deiry WS: Targeting TRAIL in the treatment of
cancer: New developments. Expert Opin Ther Targets. 19:1171–1185.
2015. View Article : Google Scholar : PubMed/NCBI
|
|
56
|
Graves JD, Kordich JJ, Huang TH, Piasecki
J, Bush TL, Sullivan T, Foltz IN, Chang W, Douangpanya H, Dang T,
et al: Apo2L/TRAIL and the death receptor 5 agonist antibody AMG
655 cooperate to promote receptor clustering and antitumor
activity. Cancer Cell. 26:177–189. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
57
|
Walczak H, Miller RE, Ariail K, Gliniak B,
Griffith TS, Kubin M, Chin W, Jones J, Woodward A, Le T, et al:
Tumoricidal activity of tumor necrosis factor-related
apoptosis-inducing ligand in vivo. Nat Med. 5:157–163. 1999.
View Article : Google Scholar : PubMed/NCBI
|
|
58
|
Zamai L, Ahmad M, Bennett IM, Azzoni L,
Alnemri ES and Perussia B: Natural killer (NK) cell-mediated
cytotoxicity: Differential use of TRAIL and Fas ligand by immature
and mature primary human NK cells. J Exp Med. 188:2375–2380. 1998.
View Article : Google Scholar : PubMed/NCBI
|
|
59
|
Huang Y, Yang X, Xu T, Kong Q, Zhang Y,
Shen Y, Wei Y, Wang G and Chang KJ: Overcoming resistance to
TRAIL-induced apoptosis in solid tumor cells by simultaneously
targeting death receptors, c-FLIP and IAPs. Int J Oncol.
49:153–163. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
60
|
Ashkenazi A, Pai RC, Fong S, Leung S,
Lawrence DA, Marsters SA, Blackie C, Chang L, McMurtrey AE, Hebert
A, et al: Safety and antitumor activity of recombinant soluble Apo2
ligand. J Clin Invest. 104:155–162. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
61
|
Galal El-Shemi A, Mohammed Ashshi A, Oh E,
Jung BK, Basalamah M, Alsaegh A and Yun CO: Efficacy of combining
ING4 and TRAIL genes in cancer-targeting gene virotherapy strategy:
First evidence in preclinical hepatocellular carcinoma. Gene Ther.
25:54–65. 2018. View Article : Google Scholar :
|
|
62
|
Herbst RS, Eckhardt SG, Kurzrock R,
Ebbinghaus S, O'Dwyer PJ, Gordon MS, Novotny W, Goldwasser MA,
Tohnya TM, Lum BL, et al: Phase I dose-escalation study of
recombinant human Apo2L/TRAIL, a dual proapoptotic receptor
agonist, in patients with advanced cancer. J Clin Oncol.
28:2839–2846. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
63
|
Wiley SR, Schooley K, Smolak PJ, Din WS,
Huang CP, Nicholl JK, Sutherland GR, Smith TD, Rauch C, Smith CA,
et al: Identification and characterization of a new member of the
TNF family that induces apoptosis. Immunity. 3:673–682. 1995.
View Article : Google Scholar : PubMed/NCBI
|
|
64
|
Pitti RM, Marsters SA, Ruppert S, Donahue
CJ, Moore A and Ashkenazi A: Induction of apoptosis by Apo-2
ligand, a new member of the tumor necrosis factor cytokine family.
J Biol Chem. 271:12687–12690. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
65
|
Liu CH, Chern GJ, Hsu FF, Huang KW, Sung
YC, Huang HC, Qiu JT, Wang SK, Lin CC, Wu CH, et al: A
multifunctional nanocarrier for efficient TRAIL-based gene therapy
against hepatocellular carcinoma with desmoplasia in mice.
Hepatology. 67:899–913. 2018. View Article : Google Scholar
|
|
66
|
Kim CY, Jeong M, Mushiake H, Kim BM, Kim
WB, Ko JP, Kim MH, Kim M, Kim TH, Robbins PD, et al: Cancer gene
therapy using a novel secretable trimeric TRAIL. Gene Ther.
13:330–338. 2006. View Article : Google Scholar
|
|
67
|
Grisendi G, Bussolari R, Cafarelli L,
Petak I, Rasini V, Veronesi E, De Santis G, Spano C, Tagliazzucchi
M, Barti-Juhasz H, et al: Adipose-derived mesenchymal stem cells as
stable source of tumor necrosis factor-related apoptosis-inducing
ligand delivery for cancer therapy. Cancer Res. 70:3718–3729. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
68
|
Liu S, Qiu J, He G, He W, Liu C, Cai D and
Pan H: TRAIL promotes hepatocellular carcinoma apoptosis and
inhibits proliferation and migration via interacting with IER3.
Cancer Cell Int. 21:632021. View Article : Google Scholar : PubMed/NCBI
|
|
69
|
Lemke J, von Karstedt S, Zinngrebe J and
Walczak H: Getting TRAIL back on track for cancer therapy. Cell
Death Differ. 21:1350–1364. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
70
|
Holland PM: Death receptor agonist
therapies for cancer, which is the right TRAIL? Cytokine Growth
Factor Rev. 25:185–193. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
71
|
Zhang L, Gu J, Lin T, Huang X, Roth JA and
Fang B: Mechanisms involved in development of resistance to
adenovirus-mediated proapoptotic gene therapy in DLD1 human colon
cancer cell line. Gene Ther. 9:1262–1270. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
72
|
Hinz S, Trauzold A, Boenicke L, Sandberg
C, Beckmann S, Bayer E, Walczak H, Kalthoff H and Ungefroren H:
Bcl-XL protects pancreatic adenocarcinoma cells against CD95- and
TRAIL-receptor-mediated apoptosis. Oncogene. 19:5477–5486. 2000.
View Article : Google Scholar : PubMed/NCBI
|
|
73
|
Eggert A, Grotzer MA, Zuzak TJ, Wiewrodt
BR, Ho R, Ikegaki N and Brodeur GM: Resistance to tumor necrosis
factor-related apoptosis-inducing ligand (TRAIL)-induced apoptosis
in neuroblastoma cells correlates with a loss of caspase-8
expression. Cancer Res. 61:1314–1319. 2001.PubMed/NCBI
|
|
74
|
Marini P, Denzinger S, Schiller D, Kauder
S, Welz S, Humphreys R, Daniel PT, Jendrossek V, Budach W and Belka
C: Combined treatment of colorectal tumours with agonistic TRAIL
receptor antibodies HGS-ETR1 and HGS-ETR2 and radiotherapy:
Enhanced effects in vitro and dose-dependent growth delay in vivo.
Oncogene. 25:5145–5154. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
75
|
Pukac L, Kanakaraj P, Humphreys R,
Alderson R, Bloom M, Sung C, Riccobene T, Johnson R, Fiscella M,
Mahoney A, et al: HGS-ETR1, a fully human TRAIL-receptor 1
monoclonal antibody, induces cell death in multiple tumour types in
vitro and in vivo. Br J Cancer. 92:1430–1441. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
76
|
Kelley SK, Harris LA, Xie D, Deforge L,
Totpal K, Bussiere J and Fox JA: Preclinical studies to predict the
disposition of Apo2L/tumor necrosis factor-related
apoptosis-inducing ligand in humans: characterization of in vivo
efcacy, pharmacokinetics, and safety. J Pharmacol Exp Ther.
299:31–38. 2001.PubMed/NCBI
|
|
77
|
Hymowitz SG, O'Connell MP, Ultsch MH,
Hurst A, Totpal K, Ashkenazi A, de Vos AM and Kelley RF: A unique
zinc-binding site revealed by a high-resolution X-ray structure of
homotrimeric Apo2L/TRAIL. Biochemistry. 39:633–640. 2000.
View Article : Google Scholar : PubMed/NCBI
|
|
78
|
Mérino D, Lalaoui N, Morizot A, Solary E
and Micheau O: TRAIL in cancer therapy: Present and future
challenges. Expert Opin Ther Targets. 11:1299–1314. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
79
|
Lawrence D, Shahrokh Z, Marsters S,
Achilles K, Shih D, Mounho B, Hillan K, Totpal K, DeForge L, Schow
P, et al: Differential hepatocyte toxicity of recombinant
Apo2L/TRAIL versions. Nat Med. 7:383–385. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
80
|
Ashley DM, Riffkin CD, Lovric MM, Mikeska
T, Dobrovic A, Maxwell JA, Friedman HS, Drummond KJ, Kaye AH, Gan
HK, et al: In vitro sensitivity testing of minimally passaged and
uncultured gliomas with TRAIL and/or chemotherapy drugs. Br J
Cancer. 99:294–304. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
81
|
Bae S, Ma K, Kim TH, Lee ES, Oh KT, Park
ES, Lee KC and Youn YS: Doxorubicin-loaded human serum albumin
nanoparticles surface-modified with TNF-related apoptosis-inducing
ligand and transferrin for targeting multiple tumor types.
Biomaterials. 33:1536–1546. 2012. View Article : Google Scholar
|
|
82
|
Mitchell MJ, Wayne E, Rana K, Schaffer CB
and King MR: TRAIL-coated leukocytes that kill cancer cells in the
circulation. Proc Natl Acad Sci USA. 111:930–935. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
83
|
Naval J, de Miguel D, Gallego-Lleyda A,
Anel A and Martinez-Lostao L: Importance of TRAIL molecular anatomy
in receptor oligomerization and signaling Implications for cancer
therapy. Cancers (Basel). 11:4442019. View Article : Google Scholar
|
|
84
|
Griffith T, Wiley SR, Kubin MZ, Sedger LM,
Maliszewski CR and Fanger NA: Monocyte-mediated tumoricidal
activity via the tumor necrosis factor-related cytokine, TRAIL. J
Exp Med. 189:1343–1354. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
85
|
Kayagaki N, Yamaguchi N, Nakayama M,
Kawasaki A, Akiba H, Okumura K and Yagita H: Involvement of
TNF-related apoptosis-inducing ligand in human CD4+ T cell-mediated
cytotoxicity. J Immunol. 162:2639–2647. 1999.PubMed/NCBI
|
|
86
|
Monleón I, Martínez-Lorenzo MJ, Anel A,
Lasierra P, Larrad L, Pineiro A, Naval J and Alava MA: CD59
cross-linking induces secretion of APO2 ligand in overactivated
human T cells. Eur J Immunol. 30:1078–1087. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
87
|
Monleón I, Martinez-Lorenzo MJ, Monteagudo
L, Lasierra P, Taulés M, Iturralde M, Piñeiro A, Larrad L, Alava
MA, Naval J and Anel A: Differential secretion of Fas ligand- or
APO2 ligand/TNF-related apoptosis-inducing ligand-carrying
microvesicles during activation-induced death of human T cells. J
Immunol. 167:6736–6744. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
88
|
Wajant H, Moosmayer D, Wüest T, Bartke T,
Gerlach E, Schönherr U, Peters N, Scheurich P and Pfizenmaier K:
Differential activation of TRAIL-R1 and -2 by soluble and membrane
TRAIL allows selective surface antigen-directed activation of
TRAIL-R2 by a soluble TRAIL derivative. Oncogene. 20:4101–4106.
2001. View Article : Google Scholar : PubMed/NCBI
|
|
89
|
De Miguel D, Basáñez G, Sánchez D, Malo
PG, Marzo I, Larrad L, Naval J, Pardo J, Anel A and Martinez-Lostao
L: Liposomes decorated with Apo2L/TRAIL overcome chemoresistance of
human hematologic tumor cells. Mol Pharm. 10:893–904. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
90
|
De Miguel D, Gallego-Lleyda A, Anel A and
Martinez-Lostao L: Liposome-bound TRAIL induces superior DR5
clustering and enhanced DISC recruitment in histiocytic lymphoma
U937 cells. Leuk Res. 39:657–666. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
91
|
De Miguel D, Gallego-Lleyda A, Ayuso JM,
Erviti-Ardanaz S, Pazo-Cid R, del Agua C, Fernández LJ, Ochoa I,
Anel A and Martinez-Lostao L: TRAIL-coated lipid-nanoparticles
overcome resistance to soluble recombinant TRAIL in non-small cell
lung cancer cells. Nanotechnology. 27:1851012016. View Article : Google Scholar : PubMed/NCBI
|
|
92
|
De Miguel D, Gallego-Lleyda A, Ayuso JM,
Pejenaute-Ochoa D, Jarauta V, Marzo I, Fernández LJ, Ochoa I, Conde
B, Anel A and Martinez-Lostao L: High-order TRAIL oligomer
formation in TRAIL-coated lipid nanoparticles enhances DR5
cross-linking and increases antitumour effect against colon cancer.
Cancer Lett. 383:250–260. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
93
|
Kim TH, Jiang HH, Park CW, Youn YS, Lee S,
Chen X and Lee KC: PEGylated TNF-related apoptosis-inducing ligand
(TRAIL)-loaded sustained release PLGA microspheres for enhanced
stability and antitumor activity. J Control Release. 150:63–69.
2011. View Article : Google Scholar
|
|
94
|
Wang S and El-Deiry WS: TRAIL and
apoptosis induction by TNF-family death receptors. Oncogene.
22:8628–8633. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
95
|
Chinnappan M, Srivastava A, Amreddy N,
Razaq M, Pareek V, Ahmed R, Mehta M, Peterson JE, Munshi A and
Ramesh R: Exosomes as drug delivery vehicle and contributor of
resistance to anticancer drugs. Cancer Lett. 486:18–28. 2020.
View Article : Google Scholar : PubMed/NCBI
|
|
96
|
Wang J, Yeung BZ, Cui M, Peer CJ, Lu Z,
Figg WD, Guillaume Wientjes M, Woo S and Au JL: Exosome is a
mechanism of inter-cellular drug transfer: Application of
quantitative pharmacology. J Control Release. 268:147–158. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
97
|
Lee Y, El Andaloussi S and Wood MJ:
Exosomes and microvesicles: Extracellular vesicles for genetic
information transfer and gene therapy. Hum Mol Genet. 21:R125–R134.
2012. View Article : Google Scholar : PubMed/NCBI
|
|
98
|
Lee JK, Park SR, Jung BK, Jeon YK, Lee YS,
Kim MK, Kim YG, Jang JY and Kim CW: Exosomes derived from
mesenchymal stem cells suppress angiogenesis by down-regulating
VEGF expression in breast cancer cells. PLoS One. 8:e842562013.
View Article : Google Scholar
|
|
99
|
Syn NL, Wang L, Chow EK, Lim CT and Goh
BC: Exosomes in cancer nanomedicine and immunotherapy: Prospects
and challenges. Trends Biotechnol. 35:665–676. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
100
|
Ha D, Yang N and Nadithe V: Exosomes as
therapeutic drug carriers and delivery vehicles across biological
membranes: Current perspectives and future challenges. Acta Pharm
Sin B. 6:287–296. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
101
|
Farooqi AA, Desai NN, Qureshi MZ,
Librelotto DRN, Gasparri ML, Bishayee A, Nabavi SM, Curti V and
Daglia M: Exosome biogenesis, bioactivities and functions as new
delivery systems of natural compounds. Biotechnol Adv. 36:328–334.
2018. View Article : Google Scholar
|
|
102
|
Maia J, Caja S, Strano Moraes MC, Couto N
and Costa-Silva B: Exosome-based cell-cell communication in the
tumor microenvironment. Front Cell Dev Biol. 6:182018. View Article : Google Scholar : PubMed/NCBI
|
|
103
|
Yeo RW, Lai RC, Zhang B, Tan SS, Yin Y,
The BJ and Lim SK: Mesenchymal stem cell: An efficient mass
producer of exosomes for drug delivery. Adv Drug Deliv Rev.
65:336–341. 2013. View Article : Google Scholar
|
|
104
|
Lou G, Chen Z, Zheng M and Liu Y:
Mesenchymal stem cell-derived exosomes as a new therapeutic
strategy for liver diseases. Exp Mol Med. 49:e3462017. View Article : Google Scholar : PubMed/NCBI
|
|
105
|
Cho BS, Kim JO, Ha DH and Yi YW: Exosomes
derived from human adipose tissue-derived mesenchymal stem cells
alleviate atopic dermatitis. Stem Cell Res Ther. 9:1872018.
View Article : Google Scholar : PubMed/NCBI
|
|
106
|
El-Andaloussi S, Lee Y, Lakhal-Littleton
S, Li J, Seow Y, Gardiner C, Alvarez-Erviti L, Sargent IL and Wood
MJ: Exosome-mediated delivery of siRNA in vitro and in vivo. Nat
Protoc. 7:2112–2126. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
107
|
Sokolova V, Ludwig AK, Hornung S, Rotan O,
Horn PA, Epple M and Giebel B: Characterisation of exosomes derived
from human cells by nanoparticle tracking analysis and scanning
electron microscopy. Colloids Surf B Biointerfaces. 87:146–150.
2011. View Article : Google Scholar : PubMed/NCBI
|
|
108
|
Kalra H, Adda CG, Liem M, Ang CS, Mechler
A, Simpson RJ, Hulett MD and Mathivanan S: Comparative proteomics
evaluation of plasma exosome isolation techniques and assessment of
the stability of exosomes in normal human blood plasma. Proteomics.
13:3354–3364. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
109
|
Rani S, Ryan AE, Griffin MD and Ritter T:
Mesenchymal stem cell-derived extracellular vesicles: Toward
cell-free therapeutic applications. Mol Ther. 23:812–823. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
110
|
Sun L, Xu R, Sun X, Duan Y, Han Y, Zhao Y,
Qian H, Zhu W and Xu W: Safety evaluation of exosomes derived from
human umbilical cord mesenchymal stromal cell. Cytotherapy.
18:413–422. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
111
|
Alvarez-Erviti L, Seow Y, Yin H, Betts C,
Lakhal S and Wood MJ: Delivery of siRNA to the mouse brain by
systemic injection of targeted exosomes. Nat Biotechnol.
29:341–345. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
112
|
El Andaloussi S, Lakhal S, Mäger I and
Wood MJ: Exosomes for targeted siRNA delivery across biological
barriers. Adv Drug Deliv Rev. 65:391–397. 2013. View Article : Google Scholar
|
|
113
|
Escudier B, Dorval T, Chaput N, André F,
Caby MP, Novault S, Flament C, Leboulaire C, Borg C, Amigorena S,
et al: Vaccination of metastatic melanoma patients with autologous
dendritic cell (DC) derived-exosomes: Results of thefirst phase I
clinical trial. J Transl Med. 3:102005. View Article : Google Scholar : PubMed/NCBI
|
|
114
|
Morse MA, Garst J, Osada T, Khan S,
Hobeika A, Clay TM, Valente N, Shreeniwas R, Sutton MA, Delcayre A,
et al: A phase I study of dexosome immunotherapy in patients with
advanced non-small cell lung cancer. J Transl Med. 3:92005.
View Article : Google Scholar : PubMed/NCBI
|
|
115
|
Dai S, Wei D, Wu Z, Zhou X, Wei X, Huang H
and Li G: Phase I clinical trial of autologous ascites-derived
exosomes combined with GM-CSF for colorectal cancer. Mol Ther.
16:782–790. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
116
|
Iranifar E, Seresht BM, Momeni F, Fadaei
E, Mehr MH, Ebrahimi Z, Rahmati M, Kharazinejad E and Mirzaei H:
Exosomes and microRNAs: New potential therapeutic candidates in
Alzheimer disease therapy. J Cell Physiol. 234:2296–2305. 2019.
View Article : Google Scholar
|
|
117
|
Qu M, Lin Q, Huang L, Fu Y, Wang L, He S,
Fu Y, Yang S, Zhang Z, Zhang L and Sun X: Dopamine-loaded blood
exosomes targeted to brain for better treatment of Parkinson's
disease. J Control Release. 287:156–166. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
118
|
Wang H, Sui H, Zheng Y, Jiang Y, Shi Y,
Liang J and Zhao L: Curcumin-primed exosomes potently ameliorate
cognitive function in AD mice by inhibiting hyperphosphorylation of
the Tau protein through the AKT/GSK-3β pathway. Nanoscale.
11:7481–7496. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
119
|
Kim MS, Haney MJ, Zhao Y, Yuan D, Deygen
I, Klyachko NL, Kabanov AV and Batrakova EV: Engineering
macro-phage-derived exosomes for targeted paclitaxel delivery to
pulmonary metastases: In vitro and in vivo evaluations.
Nanomedicine. 14:195–204. 2018. View Article : Google Scholar
|
|
120
|
Azmi AS, Bao B and Sarkar FH: Exosomes in
cancer development, metastasis, and drug resistance: A
comprehensive review. Cancer Metastasis Rev. 32:623–642. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
121
|
Mendt M, Kamerkar S, Sugimoto H, McAndrews
KM, Wu CC, Gagea M, Yang S, Blanko EVR, Peng Q, Ma X, et al:
Generation and testing of clinical-grade exosomes for pancreatic
cancer. JCI Insight. 3:e992632018. View Article : Google Scholar : PubMed/NCBI
|
|
122
|
Wang Y, Balaji V, Kaniyappan S, Krüger L,
Irsen S, Tepper K, Chandupatla R, Maetzler W, Schneider A,
Mandelkow E and Mandelkow EM: The release and trans-synaptic
transmission of Tau via exosomes. Mol Neurodegener. 12:52017.
View Article : Google Scholar : PubMed/NCBI
|
|
123
|
Jiang XC and Gao JQ: Exosomes as novel
bio-carriers for gene and drug delivery. Int J Pharm. 521:167–175.
2017. View Article : Google Scholar : PubMed/NCBI
|
|
124
|
Hadla M, Palazzolo S, Corona G, Caligiuri
I, Canzonieri V, Toffoli G and Rizzolio F: Exosomes increase the
therapeutic index of doxorubicin in breast and ovarian cancer mouse
models. Nanomedicine (Lond). 11:2431–2441. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
125
|
Smyth TJ, Redzic JS, Graner MW and
Anchordoquy TJ: Examination of the specificity of tumor cell
derived exosomes with tumor cells in vitro. Biochim Biophys Acta.
1838:2954–2965. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
126
|
Kim MS, Haney MJ, Zhao Y, Mahajan V,
Deygen I, Klyachko NL, Inskoe E, Piroyan A, Sokolsky M, Okolie O,
et al: Development of exosome-encapsulated paclitaxel to overcome
MDR in cancer cells. Nanomedicine. 12:655–664. 2016. View Article : Google Scholar
|
|
127
|
Wilhelm S, Tavares AJ, Dai Q, Ohta S,
Audet J, Dvorak HF and Chan WCW: Analysis of nanoparticle delivery
to tumours. Nat Rev Mater. 1:160142016. View Article : Google Scholar
|
|
128
|
Faivre S, Rimassa L and Finn RS: Molecular
therapies for HCC: Looking outside the box. J Hepatol. 72:342–352.
2020. View Article : Google Scholar : PubMed/NCBI
|
|
129
|
Trivedi R and Mishra DP: Trailing TRAIL
resistance: Novel targets for TRAIL sensitization in cancer cells.
Front Oncol. 5:692015. View Article : Google Scholar : PubMed/NCBI
|
|
130
|
Sterzenbach U, Putz U, Low LH, Silke J,
Tan SS and Howitt J: Engineered exosomes as vehicles for
biologically active proteins. Mol Ther. 25:1269–1278. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
131
|
Rivoltini L, Chiodoni C, Squarcina P,
Tortoreto M, Villa A, Vergani B, Bürdek M, Botti L, Arioli I, Cova
A, et al: TNF-related apoptosis-inducing ligand (TRAIL)-armed
exosomes deliver proapoptotic signals to tumor site. Clin Cancer
Res. 22:3499–3512. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
132
|
Yuan Z, Kolluri KK, Gowers KH and Janes
SM: TRAIL delivery by MSC-derived extracellular vesicles is an
effective anticancer therapy. J Extracell Vesicles. 6:12652912017.
View Article : Google Scholar : PubMed/NCBI
|
|
133
|
Shamili FH, Bayegi HR, Salmasi Z, Sadri K,
Mahmoudi M, Kalantari M, Ramezani M and Abnous K: Exosomes derived
from TRAIL-engineered mesenchymal stem cells with effective
anti-tumor activity in a mouse melanoma model. Int J Pharm.
549:218–229. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
134
|
Nojiri K, Sugimoto K, Shiraki K, Tameda M,
Inagaki Y, Ogura S, Kasai C, Kusagawa S, Yoneda M, Yamamoto N, et
al: Sorafenib and TRAIL have synergistic effect on hepatocellular
carcinoma. Int J Oncol. 42:101–108. 2013. View Article : Google Scholar
|
|
135
|
Chen KF, Tai WT, Liu TH, Huang HP, Lin YC,
Shiau CW, Li PK, Chen PJ and Cheng AL: Sorafenib overcomes TRAIL
resistance of hepatocellular carcinoma cells through the inhibition
of STAT3. Clin Cancer Res. 16:5189–5199. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
136
|
Munir J, Yoon JK and Ryu S: Therapeutic
miRNA-enriched extracellular vesicles: Current approaches and
future prospects. Cells. 9:22712020. View Article : Google Scholar : PubMed/NCBI
|
|
137
|
Almanza G, Rodvold JJ, Tsui B, Jepsen K,
Carter H and Zanetti M: Extracellular vesicles produced in B cells
deliver tumor suppressor miR-335 to breast cancer cells disrupting
oncogenic programming in vitro and in vivo. Sci Rep. 8:175812018.
View Article : Google Scholar : PubMed/NCBI
|
|
138
|
Li L, Piontek K, Ishida M, Fausther M,
Dranoff JA, Fu R, Mezey E, Gould SJ, Fordjour FK, Meltzer SJ, et
al: Extracellular vesicles carry microRNA-195 to intrahepatic
cholangiocarcinoma and improve survival in a rat model. Hepatology.
65:501–514. 2017. View Article : Google Scholar
|
|
139
|
Wang F, Li L, Piontek K, Sakaguchi M and
Selaru FM: Exosome miR-335 as a novel therapeutic strategy in
hepatocellular carcinoma. Hepatology. 67:940–954. 2018. View Article : Google Scholar
|
|
140
|
Andriolo G, Provasi E, Lo Cicero V,
Brambilla A, Soncin S, Torre T, Milano G, Biemmi V, Vassalli G,
Turchetto L, et al: Exosomes from human cardiac progenitor cells
for therapeutic applications: Development of a GMP-grade
manufacturing method. Front Physiol. 9:11692018. View Article : Google Scholar : PubMed/NCBI
|
|
141
|
Yoo KH, Thapa N, Kim BJ, Lee JO, Jang YN,
Chwae YJ and Kim J: Possibility of exosome-based coronavirus
disease 2019 vaccine (review). Mol Med Rep. 25:262022. View Article : Google Scholar
|
|
142
|
Cooke JN, Ellis JA, Hossain S, Nguyen J,
Bruce JN and Joshi S: Computational pharmacokinetic rationale for
intra-arterial delivery to the brain. Drug Deliv Transl Res.
6:622–629. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
143
|
Ciuleanu T, Bazin I, Lungulescu D, Miron
L, Bondarenko I, Deptala A, Rodriguez-Torres M, Giantonio B, Fox
NL, Wissel P, et al: A randomized, double-blind, placebo-controlled
phase II study to assess the efficacy and safety of mapatumumab
with sorafenib in patients with advanced hepatocellular carcinoma.
Ann Oncol. 27:680–687. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
144
|
von Pawel J, Harvey JH, Spigel DR, Dediu
M, Reck M, Cebotaru CL, Humphreys RC, Gribbin MJ, Fox NL and
Camidge DR: Phase II trial of mapatumumab, a fully human agonist
monoclonal anti-body to tumor necrosis factor-related
apoptosis-inducing ligand receptor 1 (TRAIL-R1), in combination
with paclitaxel and carboplatin in patients with advanced
non-small-cell lung cancer. Clin Lung Cancer. 15:188–196.e2. 2014.
View Article : Google Scholar
|
|
145
|
Davies A, Sage B, Kolluri K, Alrifai D,
Graham R, Weil B, Rego R, Bain O, Patrick PS, Champion K, et al:
TACTICAL: A phase I/II trial to assess the safety and efficacy of
MSCTRAIL in the treatment of metastatic lung adenocarcinoma. J Clin
Oncol. 37:TPS91162019. View Article : Google Scholar
|
|
146
|
Forero A, Bendell JC, Kumar P, Janisch L,
Rosen M, Wang Q, Copigneaux C, Desai M, Senaldi G and Maitland ML:
First-in-human study of the antibody DR5 agonist DS-8273a in
patients with advanced solid tumors. Invest New Drugs. 35:298–306.
2017. View Article : Google Scholar : PubMed/NCBI
|
|
147
|
Xie Y, Bai O, Zhang H, Yuan J, Zong S,
Chibbar R, Slattery K, Qureshi M, Wei Y, Deng Y and Xiang J:
Membrane-bound HSP70-engineered myeloma cell-derived exosomes
stimulate more efficient CD8(+) CTL- and NK-mediated antitumour
immunity than exosomes released from heat-shocked tumour cells
expressing cytoplasmic HSP70. J Cell Mol Med. 14:2655–2666. 2010.
View Article : Google Scholar
|
|
148
|
Ke C, Hou H, Li J, Su K, Huang C, Lin Y,
Lu Z, Du Z, Tan W and Yuan Z: Extracellular vesicle delivery of
TRAIL eradicates resistant tumor growth in combination with CDK
inhibition by dinaciclib. Cancers (Basel). 12:11572020. View Article : Google Scholar : PubMed/NCBI
|
|
149
|
Yuan Q, Su K, Li S, Long X, Liu L, Yang M,
Yuan X, Sun J, Hu J, Li Q, et al: Pulmonary delivery of
extracellular vesicle-encapsulated dinaciclib as an effective lung
cancer therapy. Cancers (Basel). 14:35502022. View Article : Google Scholar : PubMed/NCBI
|
|
150
|
Jiang L, Gu Y, Du Y, Tang X, Wu X and Liu
J: Engineering exosomes endowed with targeted delivery of
triptolide for malignant melanoma therapy. ACS Appl Mater
Interfaces. 13:42411–42428. 2021. View Article : Google Scholar : PubMed/NCBI
|