Introduction
Obesity is a disease in which fat accumulates in the
body due to an imbalance between food intake and energy
expenditure. It is known to increase the risk of metabolic
disorders such as type 2 diabetes, arteriosclerosis, hypertension
and various cancers (1-3). Global research on obesity prevention
and treatment is underway and various methods such as diet,
exercise, surgery and drug therapy are being proposed (4-6).
However, it is not easy to lose weight with behavioral therapy
alone and drugs and surgery cause side effects such as mental
disorders and depression (7).
Obesity is induced by the accumulation of triglyceride in cells
through adipogenesis, in which preadipocytes differentiate into
mature adipocytes. Methods for preventing and treating obesity by
regulating adipogenesis, one of the causes of obesity, are being
studied (8,9).
The standard adipogenesis process of 3T3-L1
preadipocytes consists of three steps: Contact inhibition, mitotic
clonal expansion (MCE) and terminal adipogenic differentiation
(10). After contact inhibition
and growth arrest, 3T3-L1 preadipocytes reenter the cell cycle
using a differentiation medium, MDI [dexamethasone,
3-isobutyl-1-methylxanthine (IBMX), insulin]. Then, the cells
undergo a few mitotic cycles during MCE, which is an essential step
in the early stage of adipocyte differentiation (11). Therefore, anti-obesity therapies
that inhibit adipocyte differentiation through MCE regulation are
being studied (12-15). Transcription factors that regulate
adipogenesis include peroxisome proliferator-activated receptors
(PPARs) and CCAAT/enhancer-binding proteins (C/EBPs). During MCE,
early adipogenic factors, C/EBPβ and C/EBPδ are temporally
expressed and this event stimulates late adipogenic factors such as
C/EBPα and peroxisome proliferator-activated receptor γ (PPARγ).
C/EBPα and PPARγ coordinately induce terminal differentiation into
a mature adipocyte phenotype by activating adipocyte-specific genes
including adipocyte protein 2 (FABP4), a terminal marker of
adipogenesis (16-18). In addition, the expression of the
regulators has been reported to be associated with the expression
of cyclin-dependent kinase inhibitors, including p27 (19,20).
CypB is a protein present in the endoplasmic
reticulum. It has prolyl isomerase activity (PPIase) and the
ability to inhibit endoplasmic reticulum (ER) stress and oxidative
stress. It is involved in protein folding and acts as a chaperone
to prevent or inhibit protein folding (21). CypB expression has been reported
to increase in the serum of patients with metabolic disorders more
than in healthy subjects and in various organs of ob/ob mice
(obesity-induced mice) (22).
Sanglifehrin-based cyclophilin inhibitor suppresses the carcinoma
formation and progression of non-alcoholic fatty liver (23). Similar to CypB, CypA, which is
known as a member of the PPIase family, has been reported as a
novel adipogenic factor (24).
Therefore, it is fascinating to study the role of cyclophilin
family proteins in the progression of adipogenesis.
CHOP has been reported as a negative regulator that
inhibits adipocyte differentiation (25-27). It has been reported that eIF2α
phosphorylation increases CHOP production to repress adipocyte
differentiation in response to ER stress in vitro and in
vivo (28). It has been
proposed that an elevated rate of protein synthesis would in turn
increase phosphorylation of eIF2α in differentiation and later
induce downstream target molecules such as CHOP. Therefore,
inhibiting ER stress activation may be the key to reducing
metabolic syndrome events such as adipogenesis (28). Further studies are needed to
dissect the cellular and molecular mechanisms involved in the
regulation of adipogenesis and ER stress.
The mammalian target of rapamycin (mTOR) is a
phosphoinositide 3-kinase (PI3K)-like serine/threonine-protein
kinase that controls protein and lipid synthesis, cell size,
proliferation, differentiation, autophagy and metabolism according
to intracellular and extracellular cues (29). mTOR is the catalytic core of two
distinct multiprotein complexes, mTOR complex 1 (mTORC1) and 2
(mTORC2), which differ in their components, regulation, function
and sensitivity to rapamycin (30). Insulin and insulin-like growth
factors (IGFs) bind to the insulin receptor (IR) or IGF receptor
(IGF-IR), thereby activating the receptor tyrosine kinase (31). Then, activated IR substrate
proteins trigger PI3K/AKT signaling and promote cell proliferation
and lipid synthesis (32).
PI3K/AKT/mTOR signaling pathway and its downstream, ribosomal
protein S6 kinase (P70S6K) are reported to be critical regulators
of adipogenesis (33). In
particular, mTOR complex 1 (mTORC1) phosphorylates P70S6K and
upregulates the adipogenic transcription factors PPARγ and C/EBPα
(34-36).
The present study, for the first time to the best of
the authors' knowledge, investigated the molecular mechanism of
CypB in 3T3-L1 adipocyte differentiation, with a focus on the
early-stage MCE process of adipogenesis and the AKT/mTOR signaling
pathway.
Materials and methods
Reagents
The 3T3-L1 cell was purchased from the Korean Cell
Line Bank. Dulbecco's modified Eagle's medium (DMEM) and calf serum
(CS) used for cell culture were purchased from HyClone (Cytiva) and
penicillin/streptomycin was purchased from Corning, Inc. MDI used
for differentiation was purchased from MilliporeSigma, fetal bovine
serum (FBS) HyClone (Cytiva). Cyclosporine A (CsA) used as an
inhibitor of CypB was purchased from Cell Signaling Technology,
Inc. and Rapamycin used as an mTOR inhibitor was purchased from
MilliporeSigma. RNA extraction was performed using
TRIzol® (Invitrogen; Thermo Fisher Scientific, Inc.) and
cDNA synthesis was performed using a kit (Thermo Fisher Scientific,
Inc.). Reverse transcription PCR was performed using a 7500 Reverse
transcription PCR system (Applied Biosystems; Thermo Fisher
Scientific, Inc.) and with Power SYBR® Green PCR Master
Mix (Applied Biosystems; Thermo Fisher Scientific, Inc.). All
reagents used for Oil Red O Staining were purchased from
MilliporeSigma. For cell cycle analysis, propidium iodide solution
and RNase A solution were purchased from MilliporeSigma. Cell
proliferation assay was performed using Chromo-CK cell viability
assay reagent (Monobio). Antibodies used in the present study
included mouse anti-β-actin; cat. no. sc-47778, mouse
anti-vinculin; cat. no. sc-25336, mouse anti-C/EBPβ; cat. no.
sc-7962, mouse anti-C/EBPα; cat. no. sc-166258, goat anti-FABP4;
cat. no. sc-18661, rabbit anti-Adiponectin; cat. no. PA1-054
(Thermo Fisher Scientific, Inc.), mouse anti-hemagglutinin (HA);
cat. no. sc-7392, mouse anti-CyclinD; cat. no. sc-8396, mouse
anti-CyclineE; cat. no. sc247, mouse anti-CyclinA; cat. no.
sc-271645, rabbit anti-AKT; cat. no. sc-8312 (Santa Cruz
Biotechnology, Inc.), rabbit anti-PPAR; cat. no. 2443s, rabbit
anti-CDK4; cat. no. 12790s, rabbit anti-p27; cat. no. 2552s, rabbit
anti-pRB; cat. no. 8516s, rabbit anti-pAKT; cat. no. 9271s, rabbit
anti-pmTOR; cat. no. 5536s, rabbit anti-mTOR; cat. no. 2983s,
rabbit anti-phosphorylated (p-) p70S6K; cat. no. 9204L, rabbit
anti-p70S6K; cat. no. 9202L (Cell Signaling Technology, Inc.),
rabbit anti-CypB; cat. no. ab16045 (Abcam). Secondary antibodies
used were goat anti-rabbit IgG; cat. no. 31460, goat anti-mouse
IgG; cat. no. M32607 (Invitrogen; Thermo Fisher Scientific, Inc.)
and rabbit anti goat IgG; cat. no. SA007-500 (GenDEPOT, LLC).
Cell culture and differentiation
3T3-Ll cells were cultured in DMEM supplemented with
10% CS and 1% P/S at 37°C in a 5% CO2 atmosphere. For
3T3-L1 cell differentiation, the cells when confluent were
incubated for 2 more days (day 0) and then treated with MDI (500
µM IBMX, 0.25 µM dexamethasone, 10 µg/ml
insulin) for 48 h at 37°C to induce differentiation. At 48 h after
initiating 3T3-L1 cell differentiation, the DMEM medium (10% FBS,
1% P/S) was supplemented with 10 µg/ml insulin for 2 days
(day 4). Day 4 after 3T3-L1 cell differentiation, DMEM medium (10%
FBS, 1% P/S) was supplemented for 3 days (day 7). On day 7 of
differentiation, differentiating cells were observed by capturing
five different random fields of views for each well using a light
microscopy at ×40 magnification (model IX73; Olympus Corporation).
CsA (a CypB inhibitor) and mTOR-specific inhibitor rapamycin were
dissolved in DMSO and added into the medium at 5 µg/ml (CsA)
and 0.5 µM (Rapamycin), respectively at 37°C.
Plasmid and short interfering (si)RNAs
transfection
The 3T3-L1 cells were seeded at a density of
5.5×105 cells/ml in a 60 mm dish or 12×105
cells/ml in a 100 mm dish and then incubated for 24 h at 37°C in a
5% CO2 atmosphere. Afterward, plasmid or siRNA
transfection was performed using X-tremeGene® HP DNA
Transfection Reagent (MilliporeSigma) or X-tremeGENE siRNA
Transfection Reagent (MilliporeSigma) according to the
manufacturer's instructions and treated for 48 h at 37°C in a 5%
CO2 atmosphere. Subsequent experimentation was then
performed 2 days later. CypB was HA-tagged at its 5' end and
subcloned into a pcDNA vector (HA tag; 5′-TAC CCA TAC GAC GTC CCA
GAC TAC GCT-3′). CypB-specific siRNA (5′GGA AAG ACU GUU CCA AAA
A3′; D-001136-01-20) and scrambled siRNA (5′UAA GGC UAU GAA GAG AUA
C3′) were purchased from Dharmacon.
Western blot analysis
Cells were lysed in RIPA buffer (50 mM Tris-HCl; pH
7.6; 150 mM NaCl; 1% Triton X-100; 1% Sodium deoxycholate). Protein
concentrations was determined by BCA assay. Equal amounts of
protein (20 µg) were separated with 6-15% SDS-PAGE gel and
transferred to BioTrace NT nitro-cellulose membrane (Pall Life
Sciences) and incubated with 5% blocking solution [(BSA; cat. no.
A0100-010; GenDEPOT, LLC) or skimmed milk (cat. no. MB-S1667; MB
cell)] for 1 h at room temperature. Afterward, the membrane was
incubated with primary antibody overnight at 4°C. Next, the
reaction was performed at room temperature for 1 h using a
secondary antibody matching the primary antibody. The primary
antibodies and secondary antibodies concentration used in the
experiment were 1:1,000 and 1:10,000, respectively. The bands were
detected with the enhanced chemiluminescence (Clarity™ Western ECL
Substrate; cat. no. 170-5061; Bio-Rad Laboratories, Inc.), and the
density and size of the bands were quantified using ImageJ 1.50i
software (National Institutes of Health) by normalizing to β-actin
and Vinculin.
Reverse transcription-quantitative (RT-q)
PCR
The 3T3-L1 cells were seeded at a density of
5.5×105 cells/ml in a 60 mm dish. Total RNAs were
extracted by TRIzol® (Invitrogen; Thermo Fisher
Scientific, Inc.). cDNA was synthesized from 0.2 µg total
RNA using RevertAid First Strand cDNA Synthesis kit (Invitrogen;
Thermo Fisher Scientific, Inc.). Reverse transcription PCR was
performed using 7500 Real Time PCR system with SYBR-Green PCR
Master Mix according to the manufacturer's instructions. The Real
Time PCR cycling conditions consisted of an initial activation step
of 95°C for 15 min, denaturation of 95°C for 15 sec, annealing of
60°C for 30 sec, extension for 72°C for 30 sec; for 40 cycles. The
primer sequences used are shown in Table I. The data were quantified using
the 2−ΔΔCq method and were normalized against the levels
of GAPDH (37). These experiments
were performed in triplicate and repeated five times with similar
results.
 | Table IA list of primer sequences used in
current study. |
Table I
A list of primer sequences used in
current study.
| Gene | Direction | Sequence |
|---|
| CypB | Forward |
TATGAAGGTGCTCTTCGCCG |
| Reverse |
AGTATACCTTGACTGTGACTTTAGG |
| GAPDH | Forward |
ATGGTGAAGGTCGGTGTGAA |
| Reverse |
TGGAAGATGGTGATGGGCTT |
| C/EBPβ | Forward |
AGCTGAGCGACGAGTACAAG |
| Reverse |
AGCTGCTCCACCTTCTTCTG |
| C/EBPα | Forward |
GCCATGTGGTAGGAGACAGA |
| Reverse |
CAAGTTCCTTCAGCAACAGC |
| PPARγ | Forward |
ATCTTAACTGCCGGATCCAC |
| Reverse |
TGGTGATTTGTCCGTTGTCT |
|
Adiponectin | Forward |
TGTTCCTCTTAATCCTGCCCA |
| Reverse |
CCAACCTGCACAAGTTCCTT |
| FABP4 | Forward |
AAAGAAGTGGGAGTGGGC |
| Reverse |
CTGTCGTCTGCGGTGATT |
Oil Red O staining
After day 7, differentiated 3T3-L1 cells were washed
three times with phosphate-buffered saline (PBS) and then fixed
with 3.7% paraformaldehyde for 1 h at room temperature and washed
thrice again with PBS. The cells were stained with 0.5% Oil Red O
in isopropanol for 1 h at room temperature. Stained cells were
washed three times with distilled water. Then, the stained cells
were observed by capturing five different random fields of views
for each well using a light microscopy at ×100 magnification (model
IX73; Olympus Corporation) and lipid droplets were measured with a
microplate reader (Molecular Devices, LLC.) at 510 nm.
Flow cytometric cell cycle analysis
The cells were harvested after MDI treatment for 48
h. Harvested cells were fixed 70% cold ethanol overnight at -20°C
and washed with cold PBS. The fixed cells were centrifuged at 500 ×
g for 10 min at room temperature. The centrifuged cell pellet was
resuspended in 10 µg/ml of RNase A at 37°C for 1 h. Then, 40
µg/ml propidium iodine solution was added at 37°C for 10 min
in the dark. Cells were analyzed using a FACSCalibur system (BD
Biosciences) according to the manufacturer's instructions. The
results were averaged, and the percentage of cell cycle progression
was calculated using the BD CellQuest™ Pro software (version 5.1;
BD Biosciences).
Statistical analysis
Results were presented as the mean ± standard
deviation. Error bars represent the mean ± standard deviation of
three or five independent experiments performed in triplicate.
Depending on the design of the experiment, data were analyzed
one-way ANOVA with post hoc multicomparison analysis (Tukey's test)
and comparisons between the two groups were performed using an
unpaired two-tailed Student's t-test. P<0.05 was considered to
indicate a statistically significant difference.
Results
Expression of CypB during adipogenesis in
3T3-L1 cells
Post confluent 3T3-L1 pre-adipocytes were treated
with MDI, an adipogenic inducer and were observed for 7 days to
determine the expression level of CypB during adipocyte
differentiation and adipogenesis. Fig. 1A shows the morphology image of
3T3-L1 cells during the differentiation period under a micro-scope
and Fig. 1B shows that the lipid
droplets were stained with Oil Red O staining assay for each day,
confirming that the differentiation was normal. For reference, the
expression levels of key adipogenic markers such as C/EBPβ, PPARγ,
C/EBPα, FABP4 and adiponectin were monitored. The C/EBPβ gene is
expressed in the early stage of adipogenesis and at the middle and
later stages, PPARγ, C/EBPα, FABP4 and Adiponectin are expressed
(38). As shown in Fig. 1C, CypB and C/EBPβ were transiently
induced on day 2 and the protein expression levels of PPARγ,
C/EBPα, FABP4 and Adiponectin were significantly increased after
day 4. Notably, CypB protein was upregulated by 5-6 fold after day
2. mRNA levels of CypB were measured by RT-qPCR during adipocyte
differentiation (Fig. 1D).
Consistent with previous reports (16,38), the mRNA levels of adipogenic genes
including C/EBPβ, PPARγ, C/EBPα and FABP4 were increased after
treatment with MDI. The mRNA level of CypB was also significantly
upregulated during MDI-induced adipocyte differentiation,
consistent with the CypB protein expression data.
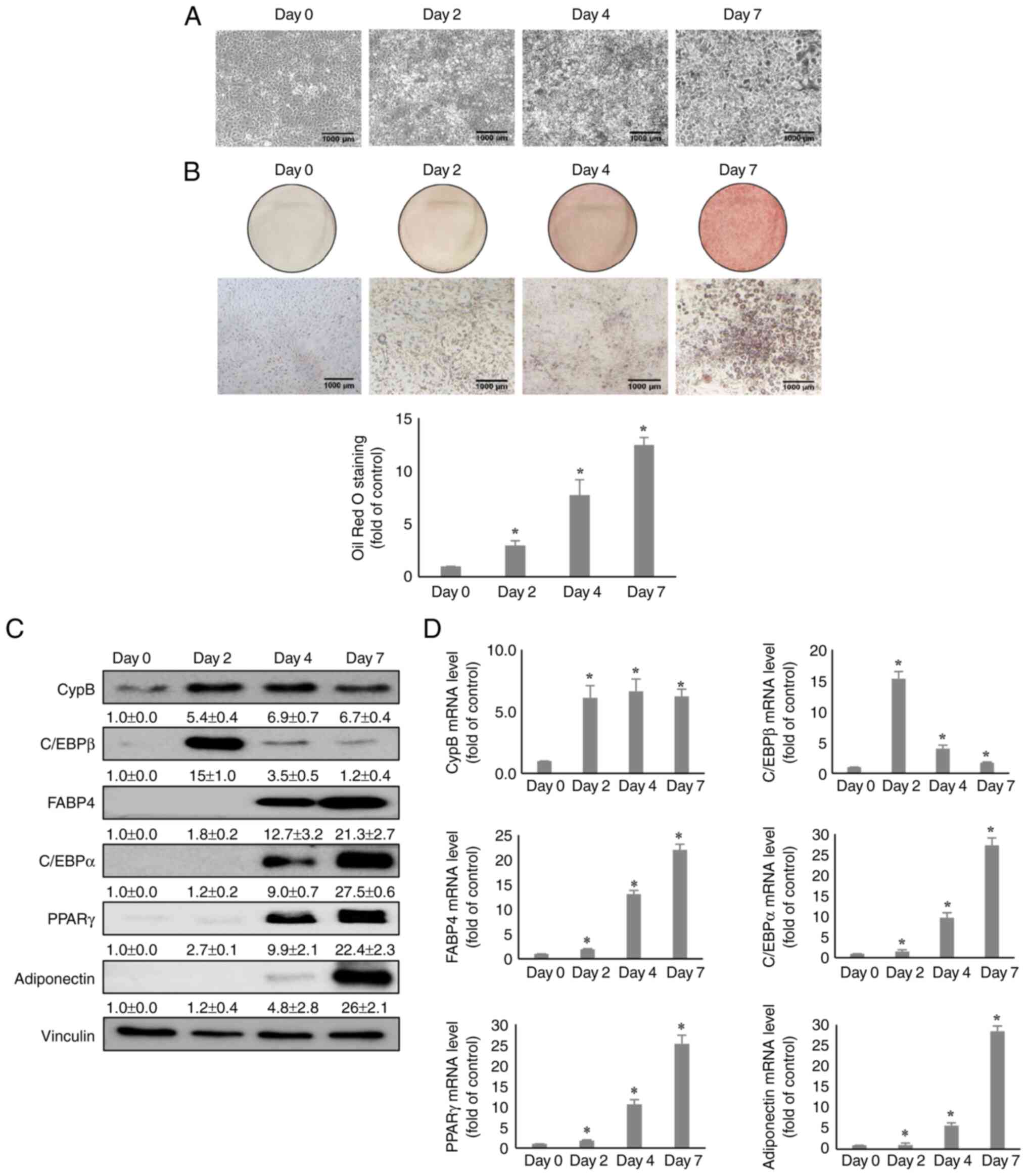 | Figure 1Expression of CypB during 3T3-L1 cell
adipogenesis. (A) The morphology images of 3T3-L1 cells during the
adipogenesis period. (B) During adipogenesis, cells (Day 0, 2, 4
and 7) were stained with lipid droplets through Oil Red O staining
assay. Quantification of the stained lipid droplets was performed
by measuring the absorbance at 510 nm. (C) At 0, 2, 4 and 7 days
after induction of 3T3-L1 cell adipogenesis, protein levels of CypB
and adipogenic markers (C/EBPβ, PPARγ, C/EBPα, FABP4 and
adiponectin) during 3T3-L1 cell adipogenesis was measured by
western blotting. Vinculin was used as a loading control. (D) The
mRNA levels of CypB and adipogenic marker genes (C/EBPβ, PPARγ,
C/EBPα, FABP4 and adiponectin) were measured by reverse
transcription-quantitative PCR. The values were shown as the mean ±
standard deviation of three independent experiments.
*P<0.001 vs. the data of Day 0. CypB, cyclophilin B;
C/EBP, CCAAT-enhancer binding protein; PPARγ, peroxisome
proliferator-activated receptor γ; FABP4, fatty acid binding
protein 4. |
The influence of CypB knockdown on
adipocyte differentiation
To investigate the functional roles of CypB in
3T3-L1 cell adipogenesis, the effects of CypB gene silencing were
monitored using siRNA on adipocyte differentiation. Following
CypB-knockdown cells were treated with MDI for 48 h to induce
differentiation, Oil Red O staining assay was performed to evaluate
the effects of CypB knockdown on lipid accumulation during
adipocyte differentiation. The results showed that lipid
accumulation was reduced by >50% in siCypB, compared to
siControl (Fig. 2A). Next,
western blot analysis was performed to monitor a key early-stage
adipogenic marker, C/EBPβ. As shown in Fig. 2B, both CypB and C/EBPβ were
significantly suppressed in CypB knockdown, compared with
siControl. Other key adipogenic factors such as FABP4, C/EBPα and
PPARγ were significantly downregulated in CypB knockdown, compared
with siControl (Fig. 2C).
Likewise, mRNA levels of key adipogenic markers such as FABP4,
C/EBPα and PPARγ were significantly decreased in siCypB, compared
to siControl (Fig. 2D). To
confirm these results, CsA was used. Consistent with the CypB
knockdown data, treatment with CsA inhibited lipid accumulation by
>50%, compared to the DMSO (Fig.
2E). In addition, the CsA treatment led to a reduction the
expression levels of key adipogenic markers such as FABP4, C/EBPα
and PPARγ (Fig. 2F). These data
suggested that CypB was required for adipogenesis in 3T3-L1
cells.
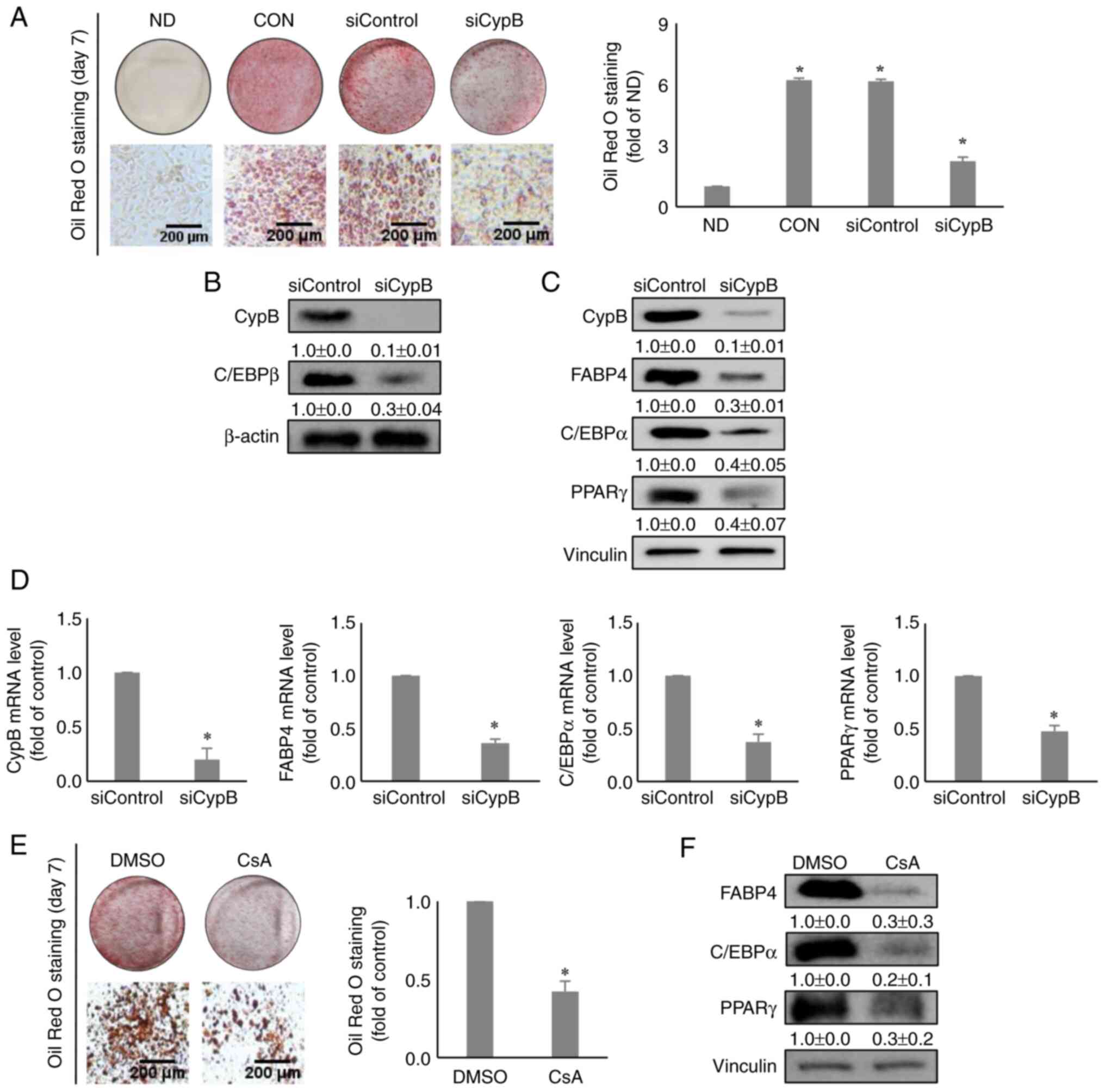 | Figure 2Effect of knockdown of CypB on
adipogenesis in 3T3-L1 cells. 3T3-L1 cells at 70-80% confluence
were transfected with scrambled siRNA (siControl) or CypB siRNA
(siCypB at 37°C for 48 h. Then the fully confluent cells were
differentiated for 2 to 7 days. (A) The differentiated cells (ND,
CON, siControl and siCypB) were stained with Oil Red O to detect
cytoplasmic lipid droplets. Quantification of the stained lipid
droplets was performed by measuring the absorbance at 510 nm. CypB
knockdown efficiency was monitored by western blotting and reverse
transcription-quantitative PCR. β-actin and vinculin were used as
loading controls. (B) The expression of C/EBPβ, an early adipogenic
factor was analyzed by western blotting 2 days after induction of
adipogenesis. β-actin was used as a loading control. (C) The
expressions of late adipogenic factors (C/EBPα, PPARγ and FABP4)
were analyzed by western blotting 7 days after induction of
adipogenesis. Vinculin was used as a loading control. (D) At 7 days
after induction of adipogenesis, the mRNA levels of CypB and
adipogenic marker genes (C/EBPβ, PPARγ, C/EBPα and FABP4) were
measured by reverse transcription-quantitative PCR. The values were
shown as the mean ± standard deviation of five independent
experiments. *P<0.001 vs. siControl. (E) The
CsA-treated differentiated cells were stained with Oil Red O to
detect cytoplasmic lipid droplets. Quantification of the stained
lipid droplets was performed as aforementioned. (F) After treatment
with 5 µg/ml CsA (dissolved in DMSO), 3T3-L1 cells were
induced to differentiate into adipocytes. The expressions of late
adipogenic factors (C/EBPα, PPARγ and FABP4) were analyzed by
western blotting 7 days after induction of adipogenesis. Vinculin
was used as a loading control. The control was treated with DMSO.
The values were shown as the mean ± standard deviation of five
independent experiments. *P<0.001 vs. DMSO. CypB,
cyclophilin B; si, short interfering; ND, non-differentiation; CON,
untreated differentiation control; C/EBP, CCAAT-enhancer binding
protein; PPARγ, peroxisome proliferator-activated receptor γ;
FABP4, fatty acid binding protein 4. |
The influence of CypB overexpression
during 3T3-L1 differentiation
To investigate the functional role of CypB in
adipogenesis, 3T3-L1 pre-adipocytes were transfected with a CypB
expression vector. After the pre-adipocytes were treated with MDI
for 2 days, Oil Red O staining assay was performed to evaluate the
effects of overexpression of CypB on lipid accumulation during
adipocyte differentiation. The results showed that overexpression
of CypB increased lipid accumulation by almost two-fold, compared
to pcDNA (Fig. 3A). Next, western
blot analysis was performed to monitor the levels of a key
early-stage adipogenic marker, C/EBPβ. As shown in Fig. 3B, C/EBPβ was significantly
increased during CypB-overexpression, compared with pcDNA. Other
key adipogenic factors such as FABP4, C/EBPα and PPARγ were
significantly upregulated under the conditions of
CypB-overexpression on day 7, compared with pcDNA (Fig. 3C). Likewise, CypB overexpression
significantly increased mRNA levels of key adipogenic markers such
as FABP4, C/EBPα and PPARγ, compared to pcDNA (Fig. 3D).
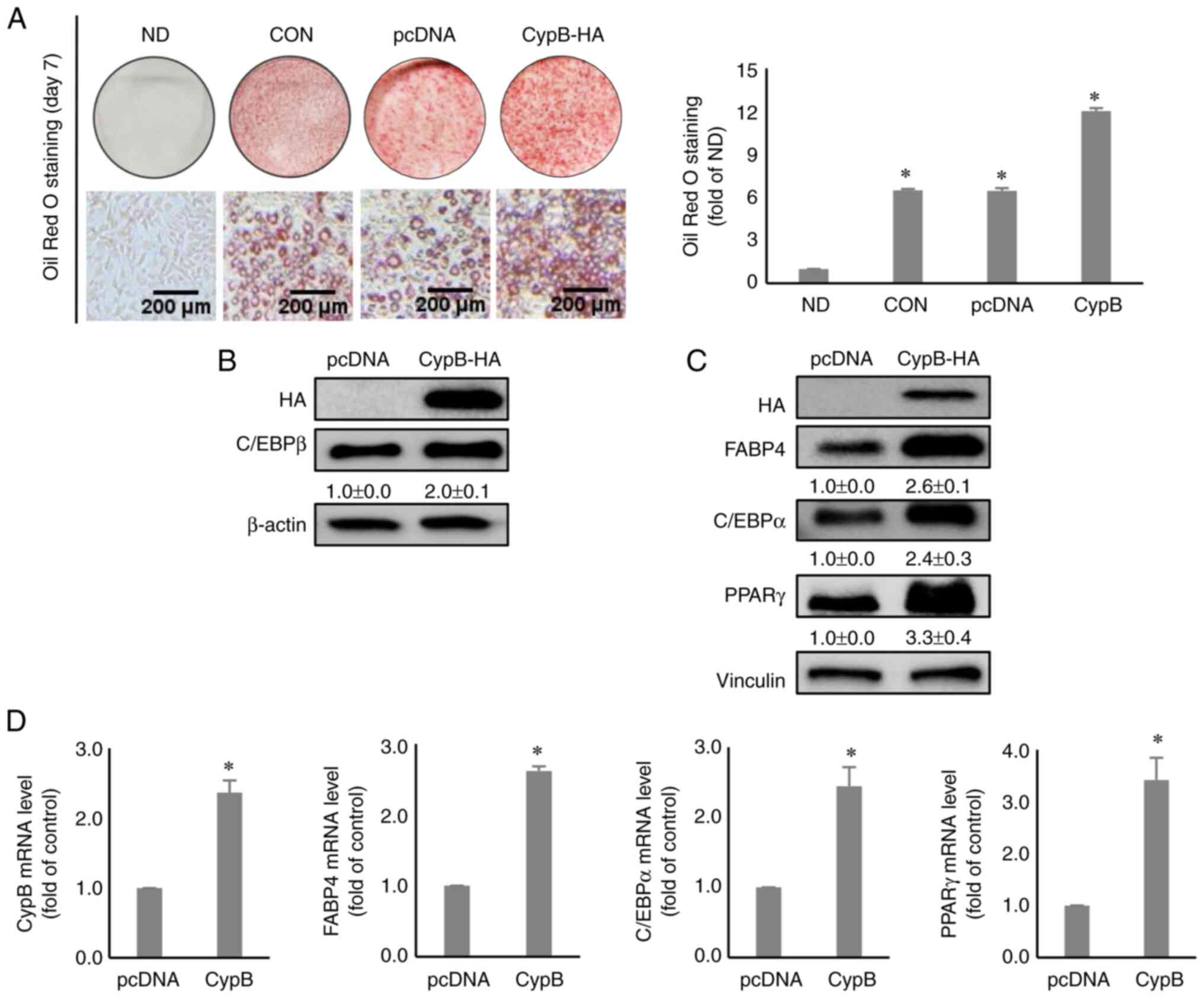 | Figure 3Effect of CypB overexpression on
adipogenesis in 3T3-L1 cells. 3T3-L1 cells at 70-80% confluence
were transfected with pcDNA or CypB-HA at 37°C for 48 h. Then the
fully confluent cells were differentiated for 2 to 7 days. (A) The
CypB-overexpressed and differentiated cells were stained with Oil
Red O to detect cytoplasmic lipid droplets. Quantification of the
stained lipid droplets was performed as before. CypB overexpression
efficiency was monitored by western blotting. (B) The expression of
C/EBPβ, an early adipogenic factor was analyzed by western blotting
2 days after induction of adipogenesis. β-actin was used as a
loading control. (C) The expressions of late adipogenic factors
(C/EBPα, PPARγ and FABP4) were analyzed by western blotting 7 days
after induction of adipogenesis. Vinculin was used as a loading
control. (D) At 7 days after induction of adipogenesis, the mRNA
levels of CypB and adipogenic marker genes (C/EBPβ, PPARγ, C/EBPα
and FABP4) were measured by reverse transcription-quantitative PCR.
The values were shown as the mean ± standard deviation of five
independent experiments. *P<0.001 vs. pcDNA. CypB, cyclophilin
B; si, short interfering; ND, non-differentiation; CON, untreated
differentiation control; C/EBP, CCAAT-enhancer binding protein;
PPARγ, peroxisome proliferator-activated receptor γ; FABP4, fatty
acid binding protein 4. |
Effect of CypB on the regulation of the
AKT/mTOR signaling pathway during 3T3-L1 differentiation
AKT/mTOR plays an important role in adipocyte
differentiation, which affects lipid metabolism through the insulin
pathway (39). To examine whether
CypB affects the AKT/mTOR pathway in adipocyte differentiation,
CypB-HA was overexpressed in 3T3-L1 preadipocytes before inducing
the differentiation of 3T3-L1 cells with MDI, followed by western
blotting. As shown in Fig. 4A,
CypB overexpression activated the AKT/mTOR pathway. P70S6 kinase
(P70S6K) is a mitogen-activated Ser/Thr protein kinase that is
required for cell growth and G1 cell cycle progression.
CypB overexpression increased the expression of p-P70S6K. Next, the
effects of CypB gene silencing were tested using siRNA on the
AKT/mTOR pathway by western blotting (Fig. 4B). The expression levels of p-AKT,
p-mTOR and p-P70S6K were significantly reduced in CypB knockdown.
To confirm these results, rapamycin was used in the assay.
Treatment with rapamycin negated the effects of overexpressed CypB
on the AKT/mTOR pathway (Fig.
4C). These results suggested that CypB plays an important role
in the AKT/mTOR pathway involved in adipocyte differentiation.
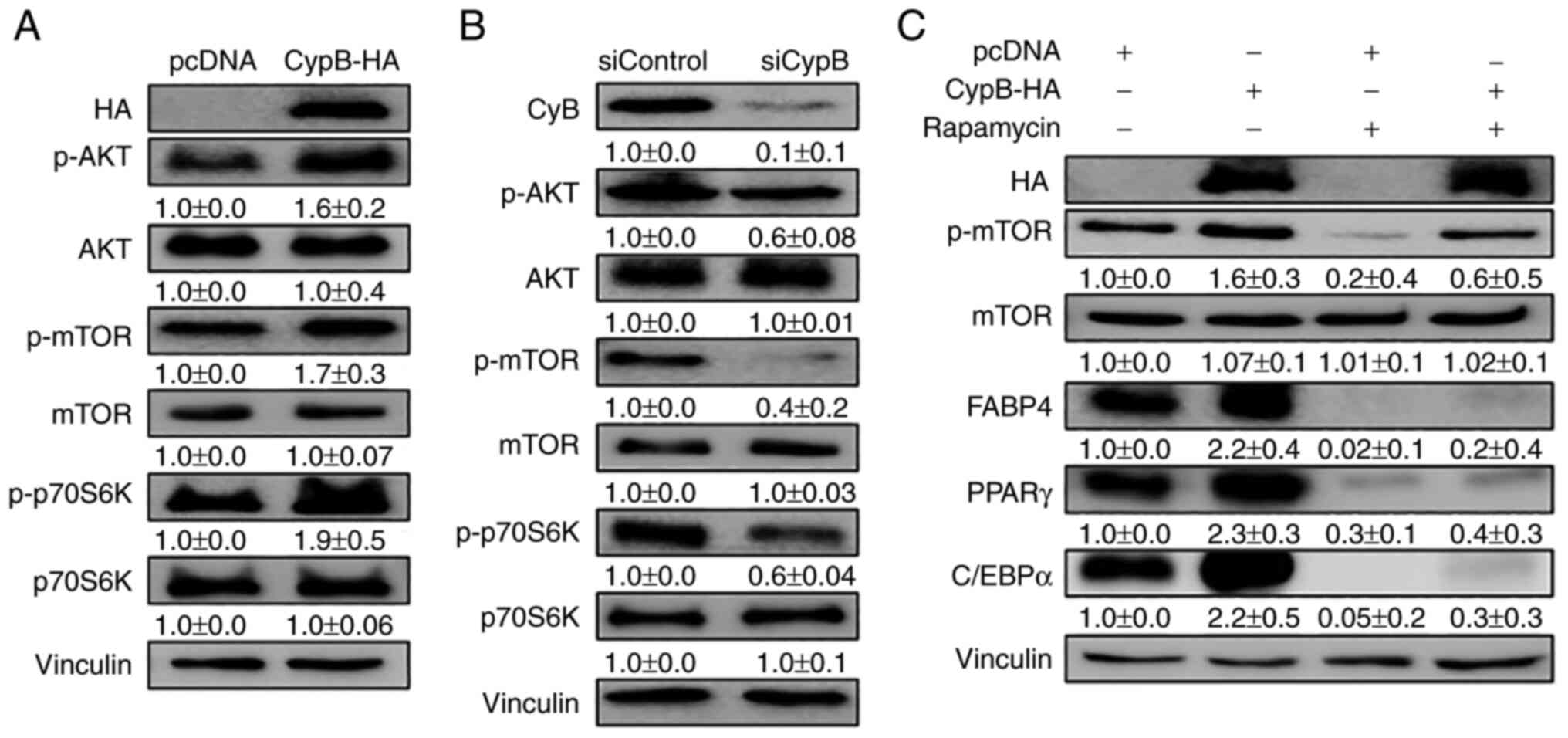 | Figure 4CypB regulates the AKT/mTOR signaling
pathway in 3T3-L1 adipocytes. 3T3-L1 cells at 70-80% confluence
were transfected with plasmids or siRNA at 37°C for 48 h. Then the
fully confluent cells were differentiated with MDI treatment for 24
h. CypB overexpression and CypB knockdown efficiency were monitored
by western blotting. The protein band intensities were quantified
by ImageJ and were normalized to the expression of vinculin. (A)
Following transfection with CypB-HA or pcDNA, 3T3-L1 preadipocytes
were differentiated into adipocytes for 24 h. The expression of
AKT/mTOR signaling pathway molecules (p-AKT, p-mTOR and p-p70S6K)
was monitored by Western blotting. (B) After gene knockdown by
siCypB or siControl, 3T3-L1 preadipocytes were differentiated into
adipocytes for 24 h. AKT/mTOR signaling pathway molecules (p-AKT,
p-mTOR and p-p70S6K) were monitored by western blotting. (C)
Following transfection with CypB-HA or pcDNA, 3T3-L1 preadipocytes
were differentiated into adipocytes in the presence or absence of
rapamycin and mTOR inhibitor for 7 days and subjected to western
blotting with antibodies against late adipogenic factors such as
C/EBPα, PPARγ and FABP4. Vinculin was used as a loading control.
The control was pcDNA (-rapamycin). The values were shown as the
mean ± standard deviation of three independent experiments. CypB,
cyclophilin B; AKT, protein kinase B; mTOR, mammalian target of
rapamycin; p-phosphorylated; si, short interfering; C/EBP,
CCAAT-enhancer binding protein; PPARγ, peroxisome
proliferator-activated receptor γ; FABP4, fatty acid binding
protein 4; ND, non-differentiation; CON, untreated differentiation
control. |
CypB promotes mitotic clonal expansion in
adipogenesis
Next, how cell cycle events during mitotic clonal
expansion are affected by CypB were examined.
G1-arrested 3T3-L1 cells synchronously re-enter the cell
cycle and undergo mitotic clonal expansion during adipogenesis
(11). As shown in Fig. 5A, CypB knockdown (siCypB) reduced
the G2/M population during adipocyte differentiation,
compared to siControl. The DNA content of the cells in the
G1 phase in the siControl and siCypB groups was 37.6 and
54.0%, respectively, while that of the cells in the G2/M
phase in the two groups was 32.7 and 16.8%, respectively. Next, the
expression of cell cycle-associated genes such as CyclinD,
CylclinE, CyclinA, CDK4, p27 and pRB in CypB knockdown were
monitored (Fig. 5B). Compared to
siControl, expression levels of CyclinD, CylclinE, CyclinA, CDK4
and pRB were significantly reduced in siCypB, while p27 expression
level was increased in siCypB by two-fold. These results suggested
that the cell cycle transition from G1 into the
G2/M phase was inhibited in CypB knockdown. As shown in
Fig. 5C, overexpression of CypB
increased the G2/M population during adipocyte
differentiation, compared to pcDNA control. The DNA content of the
cells in the G1 phase in pcDNA and CypB-HA groups was
36.6 and 23.8%, respectively, while that of the cells in the
G2/M phase in the two groups was 35.7 and 53.5%,
respectively. Compared to pcDNA, expression levels of CyclinD,
CylclinE, CyclinA, CDK4 and pRB were significantly increased by
CypB overexpression, while p27 expression level was significantly
decreased in CypB overexpression. These results suggested that the
cell cycle transition from G1 into the G2/M
phase was facilitated by CypB overexpression. Altogether, the data
suggested that CypB regulates adipocyte differentiation by
modulation of cell cycle progression.
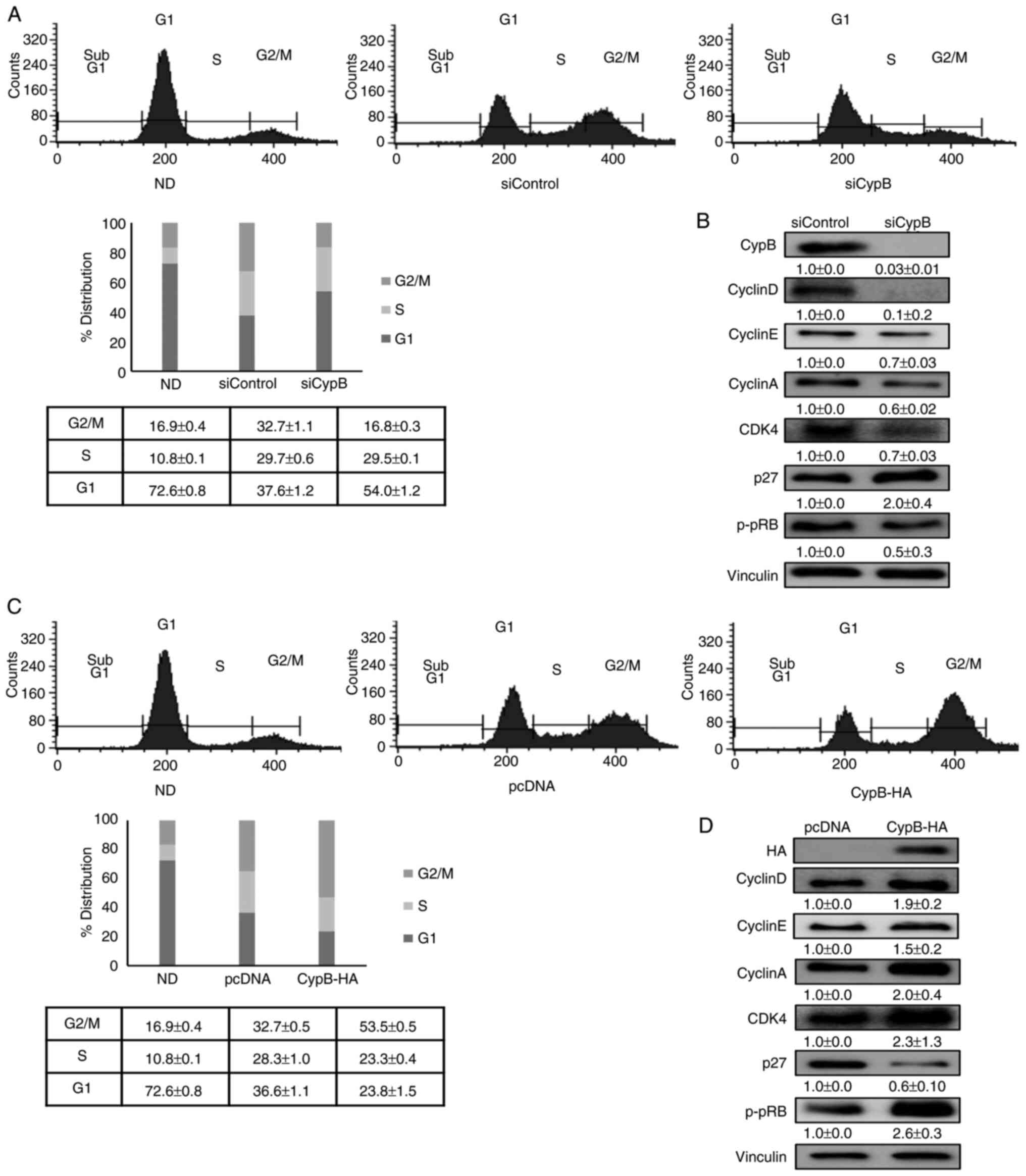 | Figure 5Effect of CypB on cell cycle
progression during 3T3-L1 cell differentiation. (A) 3T3-L1 cells at
70-80% confluence were transfected with scrambled siRNA (siControl)
or CypB siRNA (siCypB). Then the fully confluent cells were
differentiated into adipocyte with MDI treatment for 24 h. Cell
cycle progression was monitored with flow cytometric analysis.
3T3-L1 cells were stained with propidium iodide nuclear dye under
the indicated conditions. Cellular DNA content was analyzed by flow
cytometric analysis and cells were distributed in the three phases
of the cycle (G1. S and G2/M). Results were
presented in percentage for each plot and are representative of
three independent experiments. (B) The expressions of cell
cycle-associated genes (CyclinD, CylclinE, CyclinA, CDK4, p27 and
pRB) were analyzed in CypB knockdown by western blotting. Vinculin
was used as a loading control. (C) 3T3-L1 cells transfected with
pcDNA or CypB-HA were induced to differentiate into adipocytes.
Following CypB overexpression, cell cycle progression was monitored
as before. (D) Following overexpression of CypB, the expression of
cell cycle-associated proteins (CyclinD, CylclinE, CyclinA, CDK4,
p27 and pRB) was analyzed by western blotting. The values were
shown as the mean ± standard deviation of three independent
experiments. CypB, cyclophilin B; si, short interfering;
p-phosphorylated. |
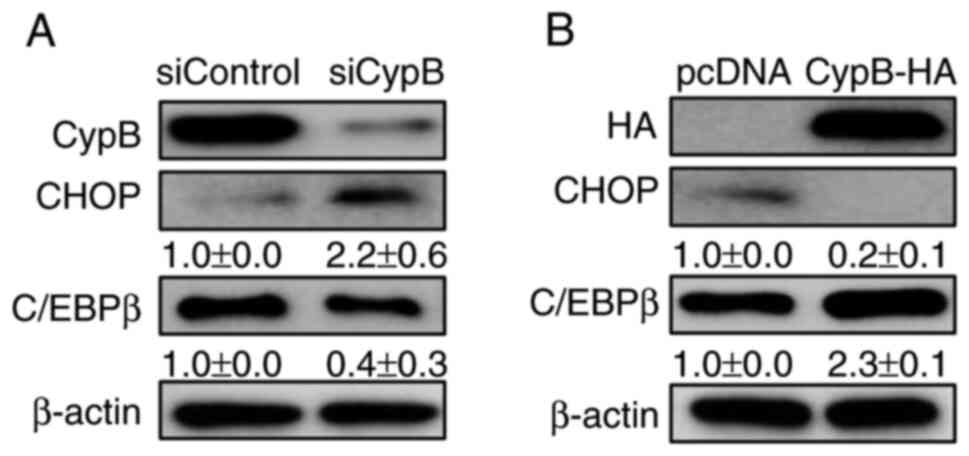
CypB modulates the expression of CHOP, a
negative regulator in adipogenesis
Our previous study reported that CypB interacts with
p300 to degrade CHOP in tumor cells (40). It has also been reported that CHOP
inhibits adipogenesis and p300 is the transcriptional co-activator
of C/EBPα (25,41). Therefore, whether CypB regulates
the expression of CHOP in 3T3-L1 cells was tested by western
blotting. CypB knockdown decreased the expression level of C/EBPβ
and increased the expression level of CHOP (Fig. 6A). Compared with pc-DNA,
overexpression of CypB increased the expression level of C/EBPβ and
significantly decreased the expression level of CHOP (Fig. 6B). By contrast, CypB knockdown
decreased the expression level of C/EBPβ and increased the
expression level of CHOP (Fig.
6B). As expected, these data suggested that CypB regulates
adipogenesis by regulating CHOP.
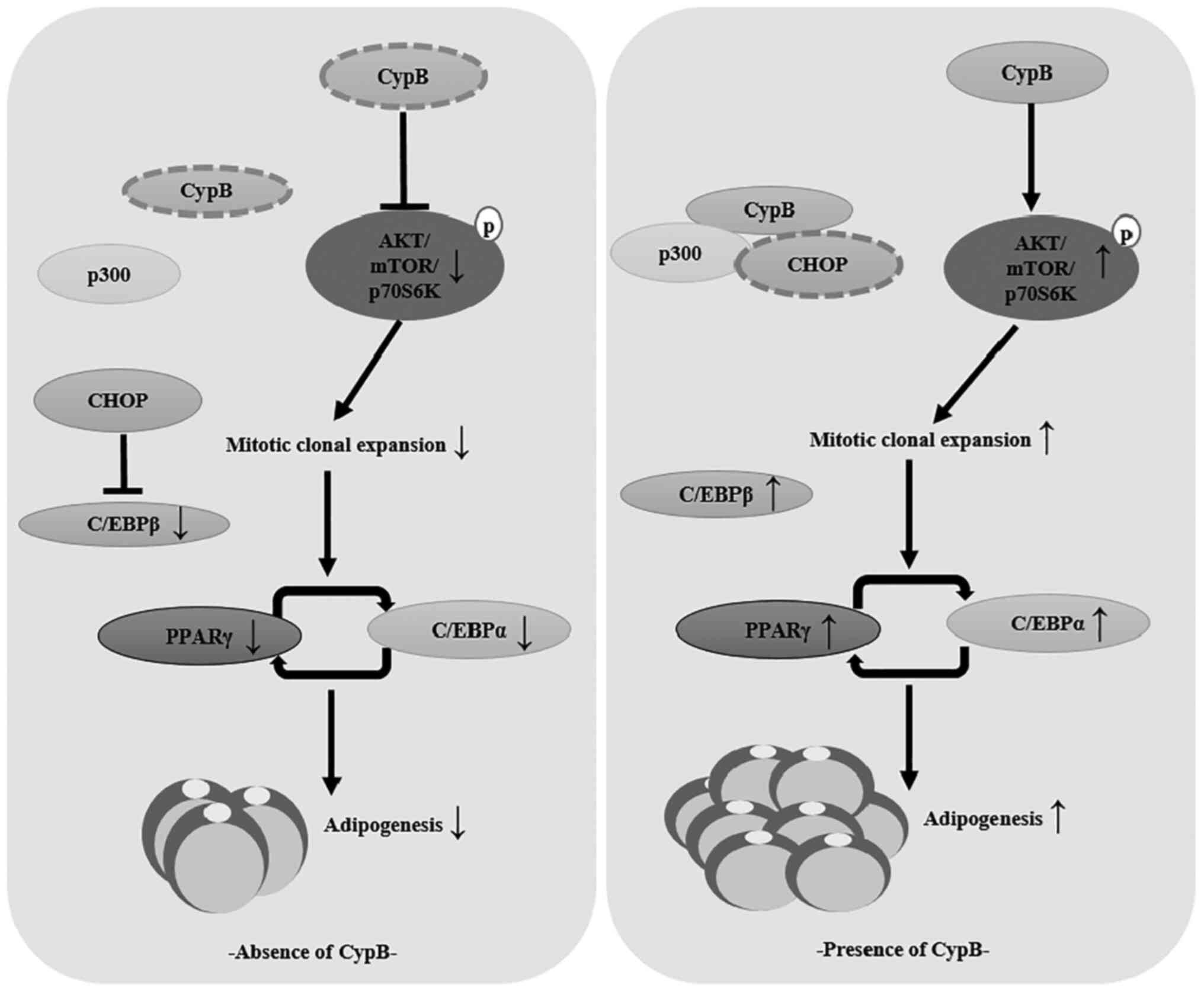
Schematic model of the role played by
CypB in 3T3-L1 adipocyte differentiation
The present study showed that CypB regulated
adipocyte differentiation through modulation of mitotic clonal
expansion and AKT/mTOR signaling pathway (Fig. 7).
Discussion
The present study described that CypB regulated
adipogenesis in 3T3-L1 cells. At present, CypB is known to be
related to cell collagen formation and the growth of various cancer
cells (42,43). However, it has been reported that
CypA, which has similar properties to CypB, serves an important
role in adipocyte differentiation (24). Also, it has been reported that the
expression level of CypB was only enhanced in the liver, visceral
and subcutaneous adipose tissue rather than in the lung and kidney
of ob/ob mice. Moreover, serum CypB expression levels were more
associated with hypertriglyceridemia and decreased HDL cholesterol
levels than with hypertension and DM or hyperglycemia, among all
components of metabolic syndrome, further suggesting the
relationship between CypB and lipid metabolism (22). Although cyclophilin family
proteins have been speculated to be involved in adipogenesis and
lipogenesis, the present study is the first one to show that CypB
plays important role in adipocyte differentiation, to the best of
the authors' knowledge.
CHOP is generally expressed when ER stress occurs in
cells and is known to induce cell death through apoptosis (44,45). It has also been reported as a
negative regulator of adipogenic factors such as C/EBPβ and C/EBPα
(25,27,45). Previously, we have shown that CypB
cooperates with p300 to degrade CHOP (40). Consistently, the data showed that
the expression of CHOP was also suppressed by CypB overexpression
or increased by CypB knockdown in adipocytes (Fig. 6). It is suggestive that the
molecular mechanism of CypB function in CHOP degradation is good
evidence for the role of CypB in adipogenesis.
Several signaling pathways are involved in
adipogenesis (46-49). AKT/mTOR serves an important role
in cell proliferation and adipogenesis. The present study showed
that CypB regulated adipocyte differentiation via the
AKT/mTOR/p70S6K pathway (Fig. 4).
In particular, mTOR is known to regulate cell cycle progression by
P70S6K. STAT3 knockdown and kaempferol treatment suppress
adipogenesis by inhibiting or delaying cell cycle progression
during adipocyte differentiation (50,51). In the present study, CypB
knockdown inhibited the cell cycle transition from G1 to
G2/M phase, thereby suppressing adipogenesis. A previous
report demonstrated that p27 protein levels decrease during phase
transitions of 3T3-L1 clonal expansion by the 26S proteasome
(52). Consistently, the present
study showed that the expression level of p27 was reduced by CypB
overexpression (Fig. 5D).
Increased p27 in CypB knockdown appeared to inhibit the CDK4/Cyclin
D, CDK2/Cyclin E and CDK2/Cyclin A complexes, thereby leading to
reduced levels of phospho-pRB (Fig.
5B).
The present study discovered a novel function of
CypB in adipocytes and clearly showed that the reduction of CypB
inhibited adipogenesis. CsA, a well-known inhibitor of CypB, has
been shown to inhibit adipogenic differentiation through the
reduced expression of phosphorylation levels of PPARγ and C/EBPα,
which is consistent with the present study (53). Our previous study reported that
honokiol, a natural compound, inhibited the migration of human
hepatocellular carcinoma by decreasing CypB expression in
hepatocarcinoma cells (54).
Consistently, in a recent study, it was reported that HNK inhibits
adipogenesis and promotes the browning of white adipose tissues
(55). However, further studies
are needed to determine whether adipogenesis is indeed suppressed
by HNK-induced CypB reduction.
Accumulated reports have demonstrated that ER stress
can induce dysregulation of metabolism in adipocytes. A total of
two FDA-approved chaperones, 4-phenylbutyric acid (4-PBA) and
taurine-conjugated ursodeoxycholic acid have been investigated in
adipocytes and β-cells for their ability to reduce ER-stress
induced dysfunction (56). The
two chaperones have been reported to reduce activation of ER stress
in obese mice (57). However,
further studies are needed to elucidate the mechanism of
intertwining ER stress and adipogenesis.
Taken together, it was proved that CypB plays an
important role as a novel adipogenesis regulator in 3T3-L1 cells
and this is expected to be helpful in obesity treatment
studies.
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
JY and WC participated in study design, performed
all experiments, collected data, and drafted the manuscript. IK,
JH, SK, and WC analyzed and interpreted the data and revised the
manuscript. JY and WC confirm the authenticity of all the raw data.
All authors have read and approved the final manuscript.
Ethics approval and consent to
participate
Not applicable.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
Abbreviations:
|
CypB
|
cyclophilin B
|
|
C/EBP
|
CCAAT-enhancer binding protein
|
|
CHOP
|
C/EBP homologous protein
|
|
PPARγ
|
peroxisome proliferator-activated
receptor γ
|
|
p300
|
histone acetyltransferase p300
|
|
FABP4
|
fatty acid binding protein 4
|
|
mTOR
|
mammalian target of rapamycin
|
|
p70S6K
|
p70S6 kinase
|
|
pRB
|
retinoblastoma protein
|
|
MCE
|
mitotic clonal expansion
|
|
IBMX
|
3-Isobutyl-1-methylxanthine
|
|
PPIase
|
prolyl isomerase
|
|
ob/ob mice
|
obesity-induced mice
|
|
ER
|
endoplasmic reticulum
|
|
PI3K
|
phosphoinositide 3-kinases
|
|
mTORc1
|
mammalian target of rapamycin complex
1
|
|
CS
|
calf serum
|
|
FBS
|
fetal bovine serum
|
|
CypA
|
cyclophilin A
|
|
CsA
|
cyclosporine
|
|
AKT
|
protein kinase B, HA,
hemagglutinin
|
Acknowledgments
Not applicable.
Funding
The present study was supported by Basic Science Research
Program through the National Research Foundation of Korea funded by
the Ministry of Education of the Korean government (grant no.
NRF-2018R1A6A1A030525124).
References
|
1
|
Mokdad AH, Ford ES, Bowman BA, Dietz WH,
Vinicor F, Bales VS and Marks JS: Prevalence of obesity, diabetes,
and obesity-related health risk factors, 2001. JAMA. 289:76–79.
2003. View Article : Google Scholar
|
|
2
|
Smith PD, O'Halloran P, Hahn DL, Grasmick
M and Radant L: Screening for obesity: Clinical tools in evolution,
a WREN study. WMJ. 109:274–278. 2010.PubMed/NCBI
|
|
3
|
Spiegelman BM and Flier JS: Obesity and
the regulation of energy balance. Cell. 104:531–543. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Tak YJ and Lee SY: Anti-Obesity Drugs:
Long-Term efficacy and safety: An updated review. World J Mens
Health. 39:208–221. 2021. View Article : Google Scholar :
|
|
5
|
Colman E, Golden J, Roberts M, Egan A,
Weaver J and Rosebraugh C: The FDA's assessment of two drugs for
chronic weight management. N Engl J Med. 367:1577–1579. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Schauer PR, Bhatt DL, Kirwan JP, Wolski K,
Aminian A, Brethauer SA, Navaneethan SD, Singh RP, Pothier CE,
Nissen SE, et al: Bariatric surgery versus intensive medical
therapy for diabetes-5-year outcomes. N Engl J Med. 376:641–651.
2017. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Every-Palmer S, Romans SE, Stubbs R,
Tomlinson A, Gandhi S and Huthwaite M: Experiences of weight-loss
surgery in people with serious mental Illness: A qualitative study.
Front Psychiatry. 11:4192020. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Gesta S, Tseng YH and Kahn CR:
Developmental origin of fat: Tracking obesity to its source. Cell.
131:242–256. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Chang E and Kim CY: Natural products and
obesity: A focus on the regulation of mitotic clonal expansion
during adipogenesis. Molecules. 24:11572019. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Mitterberger MC and Zwerschke W:
Mechanisms of resveratrol-induced inhibition of clonal expansion
and terminal adipogenic differentiation in 3T3-L1 preadipocytes. J
Gerontol A Biol Sci Med Sci. 68:1356–1376. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Tang QQ, Otto TC and Lane MD: Mitotic
clonal expansion: A synchronous process required for adipogenesis.
Proc Natl Acad Sci USA. 100:44–49. 2003. View Article : Google Scholar :
|
|
12
|
Jang MK, Yun YR, Kim JH, Park MH and Jung
MH: Gomisin N inhibits adipogenesis and prevents high-fat
diet-induced obesity. Sci Rep. 7:403452017. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Ferguson BS, Nam H and Morrison RF:
Curcumin Inhibits 3T3-L1 preadipocyte proliferation by mechanisms
involving post-transcriptional p27 regulation. Biochem Biophys Rep.
5:16–21. 2016.
|
|
14
|
Maki C, Funakoshi-Tago M, Aoyagi R, Ueda
F, Kimura M, Kobata K, Tago K and Tamura H: Coffee extract inhibits
adipogenesis in 3T3-L1 preadipocyes by interrupting insulin
signaling through the downregulation of IRS1. PLoS One.
12:e01732642017. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Kang HJ, Seo HA, Go Y, Oh CJ, Jeoung NH,
Park KG and Lee IK: Dimethylfumarate suppresses adipogenic
differentiation in 3T3-L1 preadipocytes through inhibition of STAT3
activity. PLoS One. 8:e614112013. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Wu Z, Rosen ED, Brun R, Hauser S, Adelmant
G, Troy AE, McKeon C, Darlington GJ and Spiegelman BM:
Cross-regulation of C/EBP alpha and PPAR gamma controls the
transcriptional pathway of adipogenesis and insulin sensitivity.
Mol Cell. 3:151–158. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Lee JE, Schmidt H, Lai B and Ge K:
Transcriptional and epigenomic regulation of adipogenesis. Mol Cell
Biol. 39:e00601–18. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Ghaben AL and Scherer PE: Adipogenesis and
metabolic health. Nat Rev Mol Cell Biol. 20:242–258. 2019.
View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Muller C, Calkhoven CF, Sha X and Leutz A:
The CCAAT enhancer-binding protein alpha (C/EBPalpha) requires a
SWI/SNF complex for proliferation arrest. J Biol Chem.
279:7353–7358. 2004. View Article : Google Scholar
|
|
20
|
Morrison RF and Farmer SR: Role of
PPARgamma in regulating a cascade expression of cyclin-dependent
kinase inhibitors, p18(INK4c) and p21(Waf1/Cip1), during
adipogenesis. J Biol Chem. 274:17088–17097. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Price ER, Zydowsky LD, Jin MJ, Baker CH,
McKeon FD and Walsh CT: Human cyclophilin B: A second cyclophilin
gene encodes a peptidyl-prolyl isomerase with a signal sequence.
Proc Natl Acad Sci USA. 88:1903–1907. 1991. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Zhang H, Fan Q, Xie H, Lu L, Tao R, Wang
F, Xi R, Hu J, Chen Q, Shen W, et al: Elevated serum cyclophilin B
levels are associated with the prevalence and severity of metabolic
syndrome. Front Endocrinol (Lausanne). 8:3602017. View Article : Google Scholar
|
|
23
|
Kuo J, Serrano SS, Gronberg A, Massoumi R,
Hansson MJ and Gallay P: Cyclophilin inhibitor NV556 reduces
fibrosis and hepatocellular carcinoma development in mice with
Non-alcoholic steatohepatitis. Front Pharmacol. 10:11292019.
View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Zhang L, Li Z, Zhang B, He H and Bai Y:
PPIA is a novel adipogenic factor implicated in obesity. Obesity
(Silver Spring). 23:2093–2100. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Tang QQ and Lane MD: Role of C/EBP
homologous protein (CHOP-10) in the programmed activation of
CCAAT/enhancer-binding protein-beta during adipogenesis. Proc Natl
Acad Sci USA. 97:12446–12450. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Batchvarova N, Wang XZ and Ron D:
Inhibition of adipogenesis by the stress-induced protein CHOP
(Gadd153). EMBO J. 14:4654–4661. 1995. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Huang X, Ordemann J, Muller JM and Dubiel
W: The COP9 signalosome, cullin 3 and Keap1 supercomplex regulates
CHOP stability and adipogenesis. Biol Open. 1:705–710. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Han J, Murthy R, Wood B, Song B, Wang S,
Sun B, Malhi H and Kaufman RJ: ER stress signalling through eIF2α
and CHOP, but not IRE1α, attenuates adipogenesis in mice.
Diabetologia. 56:911–924. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Albert V and Hall MN: mTOR signaling in
cellular and organismal energetics. Curr Opin Cell Biol. 33:55–66.
2015. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Bracho-Valdes I, Moreno-Alvarez P,
Valencia-Martinez I, Robles-Molina E, Chavez-Vargas L and
Vazquez-Prado J: mTORC1- and mTORC2-interacting proteins keep their
multifunctional partners focused. IUBMB Life. 63:896–914. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Nissley SP, Haskell JF, Sasaki N, De
Vroede MA and Rechler MM: Insulin-like growth factor receptors. J
Cell Sci Suppl. 3:39–51. 1985. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Wong RH and Sul HS: Insulin signaling in
fatty acid and fat synthesis: A transcriptional perspective. Curr
Opin Pharmacol. 10:684–691. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Chen J, Crawford R, Chen C and Xiao Y: The
key regulatory roles of the PI3K/Akt signaling pathway in the
functionalities of mesenchymal stem cells and applications in
tissue regeneration. Tissue Eng Part B Rev. 19:516–528. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Shockley KR, Rosen CJ, Churchill GA and
Lecka-Czernik B: PPARgamma2 regulates a molecular signature of
marrow mesenchymal stem cells. PPAR Res. 2007:812192007. View Article : Google Scholar
|
|
35
|
Lieberthal W and Levine JS: The role of
the mammalian target of rapamycin (mTOR) in renal disease. J Am Soc
Nephrol. 20:2493–2502. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Showkat M, Beigh MA and Andrabi KI: mTOR
signaling in protein translation regulation: Implications in cancer
genesis and therapeutic interventions. Mol Biol Int.
2014:6869842014. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(-Delta Delta C(T)) Method. Methods. 25:402–408. 2001.
View Article : Google Scholar
|
|
38
|
Farmer SR: Transcriptional control of
adipocyte formation. Cell Metab. 4:263–273. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Hemmings BA and Restuccia DF: PI3K-PKB/Akt
pathway. Cold Spring Harb Perspect Biol. 4:a0111892012. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Jeong K, Kim H, Kim K, Kim SJ, Hahn BS,
Jahng GH, Yoon KS, Kim SS, Ha J, Kang I and Choe W: Cyclophilin B
is involved in p300-mediated degradation of CHOP in tumor cell
adaptation to hypoxia. Cell Death Differ. 21:438–450. 2014.
View Article : Google Scholar :
|
|
41
|
Erickson RL, Hemati N, Ross SE and
MacDougald OA: p300 coactivates the adipogenic transcription factor
CCAAT/enhancer-binding protein alpha. J Biol Chem. 276:16348–16355.
2001. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Fang F, Zheng J, Galbaugh TL, Fiorillo AA,
Hjort EE, Zeng X and Clevenger CV: Cyclophilin B as a co-regulator
of prolactin-induced gene expression and function in breast cancer
cells. J Mol Endocrinol. 44:319–329. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Choi JW, Sutor SL, Lindquist L, Evans GL,
Madden BJ, Bergen HR III, Hefferan TE, Yaszemski MJ and Bram RJ:
Severe osteogenesis imperfecta in cyclophilin B-deficient mice.
PLoS Genet. 5:e10007502009. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Hu H, Tian M, Ding C and Yu S: The C/EBP
homologous protein (CHOP) transcription factor functions in
endoplasmic reticulum stress-induced apoptosis and microbial
infection. Front Immunol. 9:30832019. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Lei Y, Wang S, Ren B, Wang J, Chen J, Lu
J, Zhan S, Fu Y, Huang L and Tan J: CHOP favors endoplasmic
reticulum stress-induced apoptosis in hepatocellular carcinoma
cells via inhibition of autophagy. PLoS One. 12:e01836802017.
View Article : Google Scholar : PubMed/NCBI
|
|
46
|
El-Chaar D, Gagnon A and Sorisky A:
Inhibition of insulin signaling and adipogenesis by rapamycin:
Effect on phosphorylation of p70 S6 kinase vs eIF4E-BP1. Int J Obes
Relat Metab Disord. 28:191–198. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Ahmad B, Serpell CJ, Fong IL and Wong EH:
Molecular mechanisms of adipogenesis: The Anti-adipogenic role of
AMP-Activated protein kinase. Front Mol Biosci. 7:762020.
View Article : Google Scholar : PubMed/NCBI
|
|
48
|
Kang MJ, Kim KK, Son BY, Nam SW, Shin PG
and Kim GD: The anti-adipogenic activity of a new cultivar,
pleurotus eryngii var. ferulae 'Beesan No. 2', through
Down-Regulation of PPAR ү and C/EBP α in 3T3-L1 Cells. J Microbiol
Biotechnol. 26:1836–1844. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
49
|
Christodoulides C, Lagathu C, Sethi JK and
Vidal-Puig A: Adipogenesis and WNT signalling. Trends Endocrinol
Metab. 20:16–24. 2009. View Article : Google Scholar
|
|
50
|
Lee YJ, Choi HS, Seo MJ, Jeon HJ, Kim KJ
and Lee BY: Kaempferol suppresses lipid accumulation by inhibiting
early adipogenesis in 3T3-L1 cells and zebrafish. Food Funct.
6:2824–2833. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
51
|
Yuan Y, Xi Y, Chen J, Zhu P, Kang J, Zou
Z, Wang F and Bu S: STAT3 stimulates adipogenic stem cell
proliferation and cooperates with HMGA2 during the early stage of
differentiation to promote adipogenesis. Biochem Biophys Res
Commun. 482:1360–1366. 2017. View Article : Google Scholar
|
|
52
|
Auld CA, Fernandes KM and Morrison RF:
Skp2-mediated p27(Kip1) degradation during S/G2 phase progression
of adipocyte hyperplasia. J Cell Physiol. 211:101–111. 2007.
View Article : Google Scholar
|
|
53
|
Qu Y, Lin Q, Yuan Y, Sun Z, Li P, Wang F,
Jiang H and Chen T: Cyclosporin A inhibits adipogenic
differentiation and regulates immunomodulatory functions of murine
mesenchymal stem cells. Biochem Biophys Res Commun. 498:516–522.
2018. View Article : Google Scholar : PubMed/NCBI
|
|
54
|
Lee YS, Jeong S, Kim KY, Yoon JS, Kim S,
Yoon KS, Ha J, Kang I and Choe W: Honokiol inhibits hepatoma
carcinoma cell migration through downregulated Cyclophilin B
expression. Biochem Biophys Res Commun. 552:44–51. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
55
|
Ding Y, Zhang L, Yao X, Zhang H, He X, Fan
Z and Song Z: Honokiol alleviates high-fat diet-induced obesity of
mice by inhibiting adipogenesis and promoting white adipose tissue
browning. Animals (Basel). 11:14932021. View Article : Google Scholar : PubMed/NCBI
|
|
56
|
Nakatani Y, Kaneto H, Kawamori D,
Yoshiuchi K, Hatazaki M, Matsuoka TA, Ozawa K, Ogawa S, Hori M,
Yamasaki Y and Matsuhisa M: Involvement of endoplasmic reticulum
stress in insulin resistance and diabetes. J Biol Chem.
280:847–851. 2005. View Article : Google Scholar
|
|
57
|
Ozcan U, Yilmaz E, Ozcan L, Furuhashi M,
Vaillancourt E, Smith RO, Görgün CZ and Hotamisligil GS: Chemical
chaperones reduce ER stress and restore glucose homeostasis in a
mouse model of type 2 diabetes. Science. 313:1137–1140. 2006.
View Article : Google Scholar : PubMed/NCBI
|