1. Introduction
Severe acute respiratory syndrome-coronavirus 2
(SARS-CoV-2) is the pathogen responsible for causing SARS-CoV-2
pneumonia [coronavirus disease 2019 (COVID-19)]. The initial
clinical symptoms of viral infection are frequently atypical and
include mild coughing and headaches (1,2).
The increase in SARS-CoV-2 infection cases has also led to the
emergence of a number of characteristic stomach symptoms. According
to a multicenter retrospective study by Rizvi et al
(3), 3,229 (18.5%) of the 17,462
hospitalized patients exhibited various gastrointestinal
manifestations. During follow-up, these manifestations included
gastrointestinal bleeding, pancreatitis and conditions arising from
the manifestations, such as malnutrition. Although the exact
mechanism of SARS-CoV-2 infection in the gastrointestinal tract
remains unknown, it is generally accepted that the expression of
angiotensin-converting enzyme II (ACE2) and transmembrane serine
protease II (TMPRSS2) in the stomach, liver, pancreas, duodenum and
colon is essential for infection to occur (4). Through the tethering of the virus to
both these proteins via its spike (S) protein, the virus is able to
enter the digestive tract.
ACE2 is a vital part of the
renin-angiotensin-aldosterone system, being produced from the
conversion of ACE (5). ACE2 acts
as a key effector peptide that causes vasodilation (5). TMPRSS2 is a type II transmembrane
protein that acts as a serine protease (6,7).
The principal mediators of viral S protein binding are these two
proteins. Several factors, such as age, sex, obesity, smoking,
Helicobacter pylori (H. pylori) infection and tumors,
have been indicated to be capable of promoting the upregulation of
the genes encoding ACE2 and TMPRSS2 in the digestive system, making
it easier for SARS-CoV-2 to enter the body (5,7).
For instance, two recent studies revealed that stomach and
colorectal adenocarcinomas have high levels of ACE2 and TMPRSS2
expression (8,9). In addition, Viveiros et al
(10) reported that older male
mice expressed higher levels of ACE2 compared with younger mice.
Furthermore, Da Eira et al (11) observed that obese mice have
comparatively higher levels of ACE2 and TMPRSS2 expression.
Taken together, the findings of previous studies
have established that the entry of SARS-CoV-2 into the digestive
tract, which causes diarrhea, nausea, vomiting and lack of
appetite, depends on ACE2 and TMPRSS2. In addition, SARS-CoV-2 is
able to influence the severity, prognosis and outcome of conditions
such as liver damage and severe pancreatitis. It also has a direct
or indirect association with a number of common digestive ailments.
Through introducing the microstructure of SARS-CoV-2 and detailing
the process via which SARS-CoV-2 enters the human body through ACE2
and TMPRSS2, the present review summarizes the effects of
SARS-CoV-2 infection on the digestive system and the expression
characteristics of ACE2 and TMPRSS2 in major target organs. Several
potential strategies for the diagnosis, identification and
treatment of digestive system diseases during the COVID-19 pandemic
are also described.
2. Structural features and molecular
mechanism of SARS-CoV-2 entry into cells
Classification and microstructure of
SARS-CoV-2
At present, coronavirus has been divided into four
major subclades, namely the α-, β-, γ- and δ-coronaviruses.
SARS-CoV-2 belongs to the β-coronavirus group and mainly arises in
humans and mammals. It belongs to the same branch of coronaviruses
as SARS-CoV, SARS, human coronavirus (HCoV)-OC43, HCoV-HKU1 and
Middle East Respiratory Syndrome coronavirus (MERS-CoV) (6,12,13). SARS-CoV-2 and SARS-like
coronavirus share 88% homology, whereas SARS-CoV-2 and SARS-CoV
share 79% homology, and SARS-CoV-2 and MERS-CoV share a relatively
low homology of 50%. However, a computational analysis of the
crystal structure of SARS-CoV-2 revealed that its manner of
attachment to host ACE2 was comparable with that of the SARS-CoV
and HCoV-NL63 viruses (13).
The viral particles of SARS-CoV-2 are
single-stranded positive RNA envelope particles, having a spherical
shape and a size between 100 and 160 nm. Its genome, 29,903 bp in
length, has been demonstrated to have a highly conserved structure
(12,14,15). The genome includes three regions.
The open reading frames (ORFs) ORF1a and ORF1b comprise the first
two-thirds of the genome, whereas the final third of the genome
encodes structural proteins, including the S protein, envelope (E)
protein, membrane (M) protein and nucleocapsid (N) protein
(Fig. 1) (12).
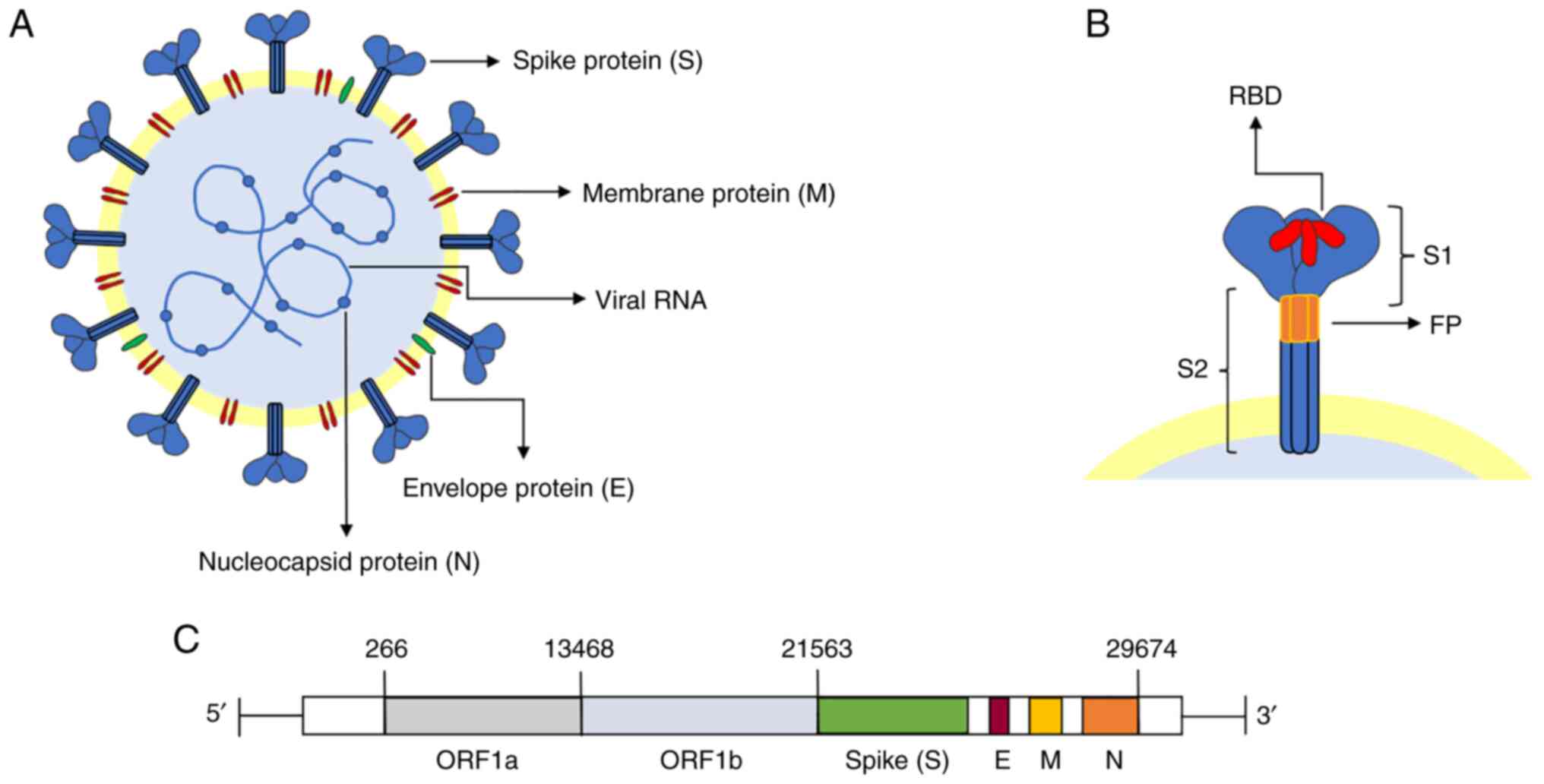 | Figure 1Molecular structure of SARS-CoV-2.
(A) SARS-CoV-2 viral particles are single-stranded positive RNA
spherical envelope viruses. The viral membrane is composed of the
spike protein, membrane protein, envelope protein, nucleocapsid
protein and genetic material (RNA). (B) The two subunits that make
up each monomer of the spike protein, S1 and S2, are arranged in a
trimer shape. The RBD subunit in the S1 subunit is in charge of
receptor binding. The S2 subunit's FP structure is responsible for
attachment to the target cell membrane. (C) The genome's length is
29,903 base pairs and it comprises open reading frames ORF1a and
ORF1b, as well as the structural S, E, M and N proteins.
SARS-CoV-2, severe acute respiratory syndrome-coronavirus 2; FP,
fusion peptide; ORF, open reading frame; S, spike protein; M,
membrane protein; E, envelope protein; N, nucleocapsid protein;
RBD, receptor-binding domain. |
The S protein, one of the four abovementioned
structural proteins, is essential for viral entrance into the host
cells. The S protein is a homotrimeric class I membrane protein
(6,16). Located on the surface of the virus
particles, the protein forms a crown (17) and, indeed, SARS-CoV-2 acquired its
name for this reason (Fig. 1B).
There are 22 N-linked glycosylation sites on each monomer of the
highly glycosylated protein (66 in total) (18). The S protein contains an
N-terminal signal peptide, the S1 subunit (responsible for receptor
binding) and the S2 subunit (responsible for membrane fusion), with
a total length of 1,273 amino acid residues (18). Receptor binding is performed by
the S1 (N-terminal) subunit, whereas membrane fusion is
accomplished by the S2 (C-terminal) subunit (16). The N-terminal domain,
receptor-binding domain (RBD) and C-terminal domain 1 (CTD1) and
CTD2 are other divisions of the S1 subunit. Regarding the S2
subunit, this comprises a central helix, junction region,
heptapeptide repeat sequence 1 (HR1) and HR2, fusion peptide (FP),
FP proximal region, transmembrane fragment and cytoplasmic tail
(18). According to recent
studies, RBD and FP are the structures specifically linked to viral
invasion.
Molecular mechanism through which
SARS-CoV-2 enters the human body
The RBD, a protein mainly composed of four cysteine
residues that form disulfide bonds and seven β-fragments, is able
to bind to ACE2 (17,19). Viruses may enter host cells in two
different ways (Fig. 2A). In the
first scenario, if the host cell only expresses ACE2 but does not
express TMPRSS2 (or if the expression of TMPRSS2 is insufficient),
the viral S protein may bind to ACE2 to change the conformation of
the S1 subunit, thereby exposing the S2 subunit; subsequently, via
the process of reticulin-mediated endocytosis, the entire viral
molecule enters the cell and after the S2 subunit is dissociated by
cathepsins, channels are opened for the release of viral RNA
(6). Alternatively, if the host
cell co-expresses ACE2 and TMPRSS2, viral RNA enters the cell
through membrane fusion. First, ACE2 combines with RBD, resulting
in a change in the conformation of the S protein (Fig. 2B). At this stage, the furin
protease, a proprotein conversion enzyme that is able to activate
the S protein, recognizes and cleaves the polybase insertion (also
termed 'PRRAR') site in S1/S2, and S1/S2 subunit dissociation
occurs (20,21). After S1 is separated from S2, the
conformation of the S2 subunit undergoes a change, thereby exposing
the S2' cleavage site, which is recognized and cleaved by TMPRSS2
(or cathepsin B/L), and then the exposed fusion peptide is inserted
into the target cell membrane (12,22). Subsequently, HR1 and HR2 of the S2
subunit form a stable six-helix bundle fusion core that binds the
viral and cellular membranes together to form a fusion pore to
release the RNA (Fig. 2C and D)
(15).
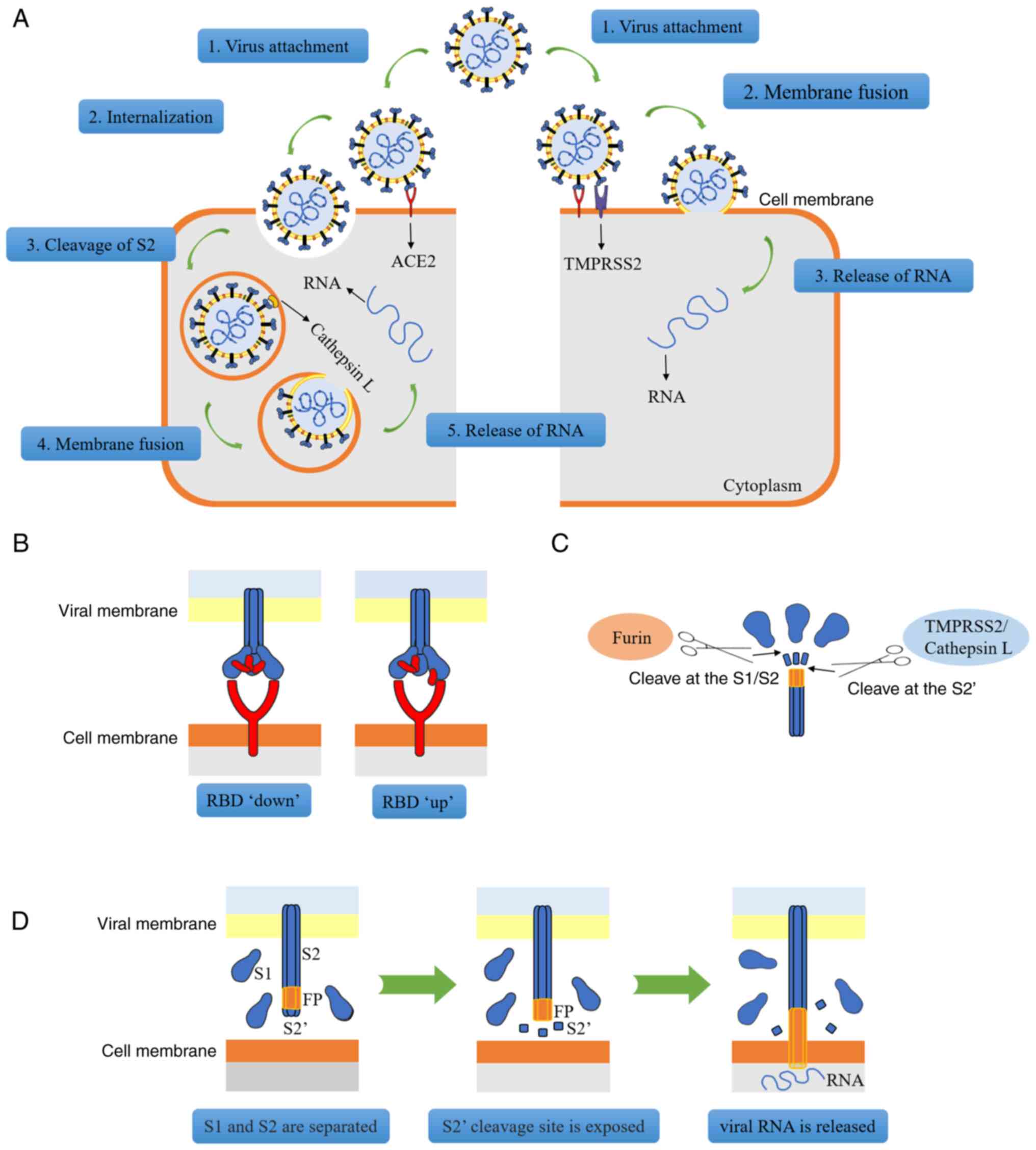 | Figure 2Mode of SARS-CoV-2 infection of
target cells. (A) On the left, when TMPRSS2 is not fully expressed
in the cells, the viral spike protein interacts with ACE2, changing
the structure of the S1 subunit. At this point, the S1 and S2
subunits are broken down by furin protease, which allows reticular
protein to facilitate endocytosis. Cathepsin disintegrates the S2′
subunit, exposing its FP sequence, which then causes FP to release
viral RNA in the cells. On the right, the stimulatory protein
associated with ACE2, the furin protease, lyses the S1-S2 site and
exposes the S2′ site. TMPRSS2 lyses the S2′ site, the exposed FP is
inserted into the cell membrane, membrane fusion occurs on the cell
surface and viral RNA is released. (B) The spike protein binds to
ACE2 via the RBD. When the RBD is not associated with ACE2, it is
in a 'downward' position; however, when the RBD is bound to ACE2,
its conformation changes and it is then in an 'upward' position.
(C) RNA must be released from spinous proteins by a two-step
cleavage process. Furin protease cleaves at the S1/S2 subunit site;
TMPRSS2 or cathepsin cleave at the S2′ site. (D) The process via
which FP attaches to the cell membrane. After S1 and S2 are
separated, the S2′ cleavage site is exposed. In order to create a
fusion hole (membrane fusion), FP is exposed and is then inserted
into the cell membrane. As the S2′ cleavage site is segmented,
viral RNA is released from the fusion hole. SARS-CoV-2, severe
acute respiratory syndrome-coronavirus 2; TMPRSS2, transmembrane
serine protease II; ACE2, angiotensin-converting enzyme II; RBD,
receptor-binding domain; FP, fusion peptide; S, spike protein. |
Previous studies have also indicated that mutant
strains may enhance their ability to bind to ACE2 due to their own
mutations, and for other reasons. For instance, the B.1.1.7,
B.1.351, P.1 and B.1.617.2 mutant variants of SARS-CoV-2 have a
high affinity for ACE2 and these mutant strains express multiple
mutations of the S protein. These mutations may change the
structure of the protein, resulting in greater infectivity
(23-26). Storti et al (27) discovered that the B.1.1.7 strain
is able to internalize faster and this accelerated internalization
may be directly associated with the N501Y mutation of the S
protein, which enhances the binding of RBD to ACE2. When the RBD
gene is mutated, it may lead to an increase in the infection rate
of SARS-CoV-2 (28). For
instance, the D164G mutation was indicated to change the structure
of the S protein, thereby increasing the affinity between RBD and
ACE2 (29). Although limited
studies have been published on the infection of the digestive
system by mutant strains of SARS-CoV-2, the potential threat cannot
be denied.
3. Clinical manifestations of SARS-CoV-2
infection and gastrointestinal expression of ACE2 and TMPRSS2
Clinical features of SARS-CoV-2 that
damage the digestive system
SARS-CoV-2 causes atypical
gastrointestinal symptoms, including bleeding
Since SARS-CoV-2 swept across the globe, a sizable
number of digestive symptoms or disorders have been linked to
viruses, primarily the most widespread and fundamental of digestive
symptoms. SARS-CoV-2 has been positively identified in stool tests
of numerous patients with COVID-19, which may provide evidence of
the replication and presence of the virus in the digestive tract
(30). In another SARS-CoV-2 RNA
sample test, 44 (29%) of 153 stool samples were found to be
positive for SARS-CoV-2 (31).
Certain patients with COVID-19 have tested positive for the virus
in their stool, even after throat swab tests were negative
(32,33). In addition, SARS-CoV-2 RNA was
found in the stool of 53% of hospitalized patients with COVID-19,
and biopsies of the stomach, duodenum and rectum tested positive
for the viral nucleocapsid, suggesting that the virus may infect
the digestive tract (34). Xiao
et al (35) performed a
gastrointestinal endoscopy on a patient with COVID-19 and found
that the esophageal mucosa was damaged; a subsequent histological
examination revealed the presence of a large number of plasma cells
and lymphocyte infiltration in the lamina propria of the stomach,
duodenum and rectum. In addition, viral nucleocapsid proteins were
detected in the cytoplasm of these sites. The prevalence of
gastrointestinal symptoms in individuals with COVID-19 ranges from
12 to 61%, and gastrointestinal symptoms were manifested in the
digestive tract more frequently in patients with a longer disease
course (36). The signs and
symptoms include diarrhea, nausea and vomiting. Diarrhea is the
most typical symptom (37).
Retrospective case studies have indicated that diarrhea, nausea,
vomiting and anorexia are the most typical digestive symptoms in
patients with COVID-19 (38-40). In addition, evidence suggests that
nausea frequently starts at an early stage in patients infected
with SARS-CoV-2, indicating that the gastrointestinal tract may be
infected by the virus. Nausea was associated with the first case of
COVID-19 in both China and the USA (41). In excess of 12,000 patients with
COVID-19 were included in the analysis of 41 studies by Andrews
et al (42), and the
findings revealed a median incidence of nausea and diarrhea of 10.5
and 11%, respectively. Therefore, patients with SARS-CoV-2
infection should be vigilant of symptoms of both nausea and
diarrhea with similar care. Epidemiological data have also
indicated that nausea should be taken into consideration as a
potential early symptom of SARS-CoV-2 (41). The most frequent abdomen computed
tomography findings for imaging viral invasion of the digestive
tract in patients with COVID-19 were found to be colonic thickening
and edema, gastritis and small intestine gas buildup (43).
Several SARS-CoV-2-positive individuals have also
experienced gastrointestinal hemorrhage. Carvalho et al
(44) reported on a 71-year-old
female patient with hemorrhagic colitis brought to the hospital on
account of SARS-CoV-2 infection; endoscopic investigation
identified a patchy localized erythema without any ulcer in the
gut. The lamina propria had modest enlargement and edema, as
observed by H&E staining of the colon and rectal biopsy. In
addition, the particular case of a 77-year-old male patient with
upper gastrointestinal hemorrhage was reported by Li et al
(45). Lymphocyte infiltration
and the presence of SARS-CoV-2 RNA in the samples taken from this
patient provided conclusive evidence that the upper
gastrointestinal bleeding in this case was caused by esophageal
SARS-CoV-2 infection. A total of 95 patients with COVID-19 were
studied by Lin et al (38), six of whom received endoscopies
due to gastrointestinal symptoms. A severely ill patient also
experienced esophageal hemorrhage, erosion and ulceration. In
addition, SARS-CoV-2 RNA was found in the rectum, duodenum, stomach
and esophagus of two seriously ill patients.
In the event of an epidemic breakout, symptoms that
may be encountered in clinical practice include nausea, diarrhea
and stomach pain. It is fitting to contemplate the connection
between these manifestations and SARS-CoV-2, if other possible
causes that may account for the gastrointestinal symptoms have been
ruled out. For hospitalized patients, it is also important to look
out for SARS-CoV-2 infection to prevent the virus's impact on the
onset and prognosis of digestive illnesses.
Effects of SARS-CoV-2 on the digestive
system may be prolonged
Hospitalization of patients with COVID-19 with
SARS-CoV-2 gastrointestinal infections led to a prolongation of the
symptoms in 104 patients in China, according to a retrospective
cohort research study (46). Hu
et al (47) tested for the
virus in 289 patients with COVID-19 and 21 (7.3%) of these patients
were readmitted after discharge due to re-detection of SARS-CoV-2.
In the positive retest, the percentage of the readmitted patients
for whom the virus was detected by anal swab was 71.4% (15/21). The
subsequent phylogenetic analysis of the patients' full-length
SARS-CoV-2 genome revealed that the virus detected in the retest
had evolved from the parental virus involved in the initial
infection. Therefore, the virus was indicated to participate in the
primary, rather than the secondary infection. Hu et al
(47) also determined that the
presence of SARS-CoV-2 may remain undetected and that the virus may
be replicated at low levels, mainly in the gastrointestinal tract,
for a long time; furthermore, periodic shedding of the virus may
lead to a resurgence of the virus, as mainly found in the anal swab
samples. In patients with acute diarrhea, Noviello et al
(48) discovered that moderate
gastrointestinal symptoms persisted for ~5 months after SARS-CoV-2
infection. In addition, these researchers considered that acute
SARS-CoV-2 infection may also have an impact on the brain-gut axis,
resulting in symptoms such as headaches, backaches, irregular sleep
patterns, low mood and anxiety (48).
Furthermore, patients with underlying
gastrointestinal problems may potentially be impacted by
SARS-CoV-2. In a retrospective analysis of surgical resection
specimens from patients with gastrointestinal cancer, the specimens
were tested for SARSCoV-2 to determine whether the patients had
COVID-19. The results indicated that among 52 patients with
gastrointestinal cancer, the mortality rate of patients with early
or asymptomatic COVID-19 following surgery was 16.7%, which was
much higher compared with that of patients without COVID-19
(49). In addition, the presence
of certain underlying conditions, such as inflammatory bowel
disease (IBD), was indicated to boost the expression of ACE2, with
the result that the patients may have been more susceptible to
SARS-CoV-2 (50). There is
evidence that patients with IBD express ACE2 and TMPRSS2 more
frequently in the colorectum compared with non-IBD patients
(51,52). The study by Tao et al
(53) revealed that patients with
COVID-19 with IBD were more likely to experience symptoms of
diarrhea and abdominal pain, and have elevated levels of biomarkers
compared with patients without IBD. Due to viral infection, colitis
is easily induced via direct damage caused to the intestinal
epithelial cells. As a result, patients with IBD may be more
vulnerable to intestinal damage caused by COVID-19 (54). Viganò et al (55) surveyed 709 patients with IBD, 53
of whom were also infected with COVID-19. They found that the
probability of diarrhea was 49%, significantly higher than the
probability for patients with IBD alone (42.2%). Furthermore,
active IBD has been indicated to be significantly associated with
COVID-19. Derikx et al (56) also found that ~38.6% of patients
with IBD who were infected with COVID-19 had diarrhea symptoms. IBD
patients with COVID-19 are also more likely to develop digestive
diseases than those infected with COVID-19 or IBD alone, suggesting
that COVID-19 may be associated either with the aggravation of IBD
symptoms or with the promotion of its transition to the active
phase.
The gastrointestinal tract may become infected with
SARS-CoV-2 on a recurring, periodic and persistent basis. Patients
with COVID-19 must continue to be monitored in order to assess
whether the virus is still active after their gastrointestinal
symptoms have improved. In addition, as far as possible, SARS-CoV-2
infection should always be avoided by those with fundamental
digestive problems, in order to prevent the condition from getting
worse or changing the prognosis.
SARS-CoV-2 with TMPRSS2 and ACE2
expression in the gastrointestinal tract
Connection between ACE2, SARS-CoV-2
and the stomach
Both healthy individuals and SARS-CoV-2-infected
patients have digestive organs that express ACE2 and TMPRSS2. ACE2
is expressed in stomach tissues, according to recent investigations
(57,58). According to Lee et al
(59), who performed single-cell
RNA sequencing (scRNA-seq) analyses of various parts of the
gastrointestinal tract, the expression ratios of ACE2 in the upper
digestive tract (esophagus, stomach and duodenum) and the lower
digestive tract (ileum and colorectum) were 1.04% (1,084/104,174
cells) and 14.06% (4,754/33,808 cells), respectively. In the
gastrointestinal tract, the co-expression of ACE2 and TMPRSS2 was
found to be the highest in the small intestine and colorectum. More
than 20% of intestinal epithelial cells and ~5% of colon cells were
found to co-express ACE2 and TMPRSS2 (59). According to An et al
(60), the main cells of the
stomach manufacture pepsinogen and express ACE2 at a higher level
in gastric tissue than in parietal cells, which may help to explain
why patients with SARS-CoV-2 who have anorexia display this
clinical characteristic. Due to the fact that ACE2 is still
expressed in the gastric mucosa, further investigations have
discovered that H. pylori infection and intestinal
metaplasia may render subjects vulnerable to SARS-CoV-2 (61). Finally, Sun et al (62) developed a human ACE2
(hACE2)-expressing mouse model and demonstrated that hACE2 mice are
sensitive to SARS-CoV-2 infection.
Characteristics of SARS-CoV-2 intestinal
infection and expression of ACE2 and TMPRSS2 in intestinal
cells
Numerous studies have confirmed that the intestinal
tract is one of the susceptible sites for SARS-CoV-2.
SARS-CoV-2-infected gastrointestinal tracts were indicated to shed
a large number of infectious viruses, according to an African green
monkey viral infection experiment. Furthermore, infectious viruses
were repeatedly isolated over time from mucosal swabs, including
from the rectum (63). Using
monkey experiments, Jiao et al (64) also indicated that SARS-COV-2
infection led to an inhibition of gastrointestinal goblet cell
proliferation, with the induction of apoptosis. Goblet cells
secrete mucins, which prevent the entry of pathogens into the
cells; a lack of mucins renders hosts more susceptible to
pathogens. Therefore, SARS-CoV-2 infection has been shown to result
in an inhibition of the proliferation of goblet cells and a
decrease in the levels of mucins, which induces apoptosis, leading
to the destruction of the intestinal barrier and further infection
of multiple tissues (64).
Regarding the human gut, Livanos et al
(65) observed strong expression
of ACE2 on the brush border of the small intestine in both
uninfected and SARS-CoV-2-infected patients. In addition, using
immunofluorescence and electron microscopic experiments, these
researchers detected viral nucleocapsid proteins in the small
intestinal epithelial cells of 11 out of 12 COVID-19 patients
(mainly goblet cells), suggesting that these cells were infected
with the virus. In addition, Lee et al (59) found that ACE2 and TMPRSS2 are
mainly co-expressed in the intestinal epithelial cells of the lower
digestive tract, with the highest co-expression rates occurring in
progenitor and stem-like epithelial cells, particularly in the
small intestine, suggesting a potential mechanism for the
gastrointestinal manifestations of acute COVID-19 infection. As far
as the large intestine is concerned, studies have confirmed that
ACE2 is mainly expressed on the membrane and in the cytoplasm of
goblet cells, and the expression of ACE2 on the basal side of the
colonic epithelium was found to be lower than that on the luminal
side. Since colonic basal cells are able to regenerate and
differentiate into mature functional glandular epithelial cells,
the damaged colonic epithelial cells will be repaired after the
SARS-CoV-2 virus is cleared, which may provide the explanation for
the self-limiting diarrhea found in patients with COVID-19
(60). Qi et al (66) analyzed scRNA-seq data from the
digestive system and found that ACE2 and TMPRSS2 are highly
expressed in goblet cells throughout the intestine (both small
intestine and large intestine), thereby identifying the intestine
as a high-risk organ.
A large number of previously published studies
(60,65) have otherwise demonstrated that
ACE2 and TMPRSS2 are fully expressed in numerous intestinal
regions, particularly in goblet cells. The severity of a viral
infection in the intestine may be directly correlated with the
expression of these two proteases. Consequently, close attention
should be paid to the areas where these proteases are highly
expressed while a clinical diagnosis is being made.
4. SARS-CoV-2 and the liver
General characteristics of liver damage
with SARS-CoV-2
According to the study by Wang et al
(67), 303 (46.1%) of 657
patients with COVID-19 experienced liver injury, with the frequency
of severe and critical cases being greater [148/257 (57.6%)]
compared with that of moderate cases [155/400 (38.8%)]. Males
[192/303 (63.4%)] were also found to be substantially more likely
to experience liver damage [111/303 (36.6%)] as compared to
females. Zhang et al (68)
discovered that male patients were more likely to experience liver
injury than female patients in a multivariate analysis of 218
patients performed at Wuhan Central Hospital. This increased
likelihood of liver injury in male patients may have been due to
the liver-protecting effects of increased estrogen levels in women.
High levels of D-dimer and neutrophils are additional risk factors
for liver damage in individuals with COVID-19, in addition to their
sex. The prognosis may be poor for individuals who have underlying
liver illness, such as alcoholic liver disease or cirrhosis, if
they contract SARS-CoV-2 (69).
Yang et al (70) noted in
in vivo studies that the livers of hamsters infected with
SARS-CoV-2 displayed structural abnormalities and had large
vacuoles. The expression of the viral protein was compatible with
the placement of large vacuoles in hepatocytes, as revealed by
immunohistochemical nucleoprotein staining, indicating viral
replication in the liver. A previous study also revealed that
patients with COVID-19 who had liver injury had a high viral load
during the early stages of infection, suggesting that the virus may
harm the liver directly (71).
When SARS-CoV-2 infects cells, a huge number of inflammatory
mediator and chemokine molecules are released, which leads to the
aggregation of neutrophils. In addition, their secretions,
containing cytokines and chemokines, encourage immune cell
aggregation and the immune response (72). According to another previously
published study, the inflammatory cytokine storm that SARS-CoV-2
generates raises patients' levels of interleukin (IL)-1β, IL-2,
IL-6, IL-8, IL-10, IL-17, interferon, interferon-γ-induced protein
10 and monocyte and results in damage to the liver (73).
The prognosis is typically poor once a patient is
infected with SARS-CoV-2, whether or not there is an underlying
liver condition. The liver is more susceptible to injury in males
due to their levels of estrogen being lower than those in females.
Therefore, when male patients are infected with SARS-CoV-2, more
focus should be placed on liver protection.
SARS-CoV-2 infection is able to cause
liver damage that may result in increased liver enzyme levels
In a large tertiary care health system in Detroit
(USA), out of 1,935 patients who were hospitalized with COVID-19,
1,031 (53.2%) of them had mildly elevated levels of liver enzymes
and 396 (20.5%) had liver damage (74). According to a meta-analysis study
performed by Wijarnpreecha et al (75), a quarter of all patients with
COVID-19 had increased levels of liver enzymes and the extents of
the increases were associated with the severity of the condition.
Previous studies have also demonstrated that liver damage, which
manifests as high levels of the enzymes alanine aminotransferase
(ALT), aspartate aminotransferase (AST), lactate dehydrogenase and
total bilirubin, was observed in numerous patients with COVID-19,
particularly in those who were critically ill. Typically, the
levels peak in 8-9 days, whereas in patients only moderately
affected by COVID-19, the increase in these indicators was barely
perceptible (41,76,77). According to a retrospective study
by Gomi et al (78) that
included 216 subjects, patients with mild and moderate COVID-19
infection were more likely to have impaired liver function. In
addition, it is possible to distinguish COVID-19 from other
illnesses using elevated ALT and AST without alkaline phosphatase
or γ-glutamyl transpeptidase. These studies, in addition to others,
have found that it is the activation of hepatic infiltrating
lymphocytes, which results in the rise of hepatic cytokine levels,
that causes the indirect liver harm resulting from SARS-CoV-2
infection.
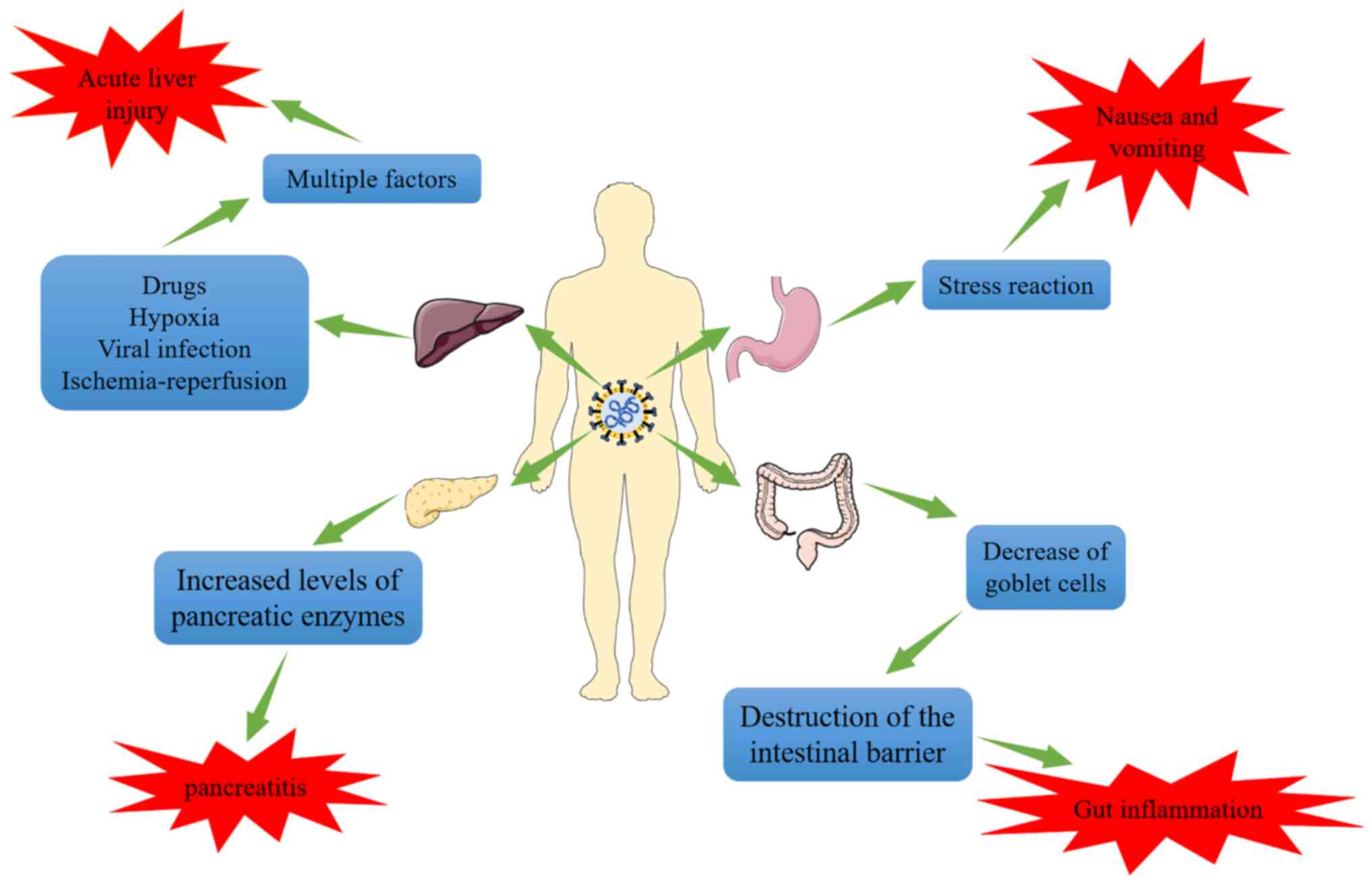
Types and multiple factors of liver
injury induced by SARS-CoV-2
There is evidence that liver injury in patients with
COVID-19 is not only caused by SARS-CoV-2, but also that SARS-CoV-2
infection is associated with drug-induced liver injury, secondary
liver injury caused by hypoxia, viral liver injury and liver
ischemia-reperfusion injury (Fig.
3) (73,79,80). Studies have indicated that
SARS-CoV-2 may infect endothelial cells and cause diffuse
dermatitis. Subsequently, microvascular dysfunction may lead to
hypercoagulable states, tissue edema and organ ischemia (81,82). In addition, a pathological
examination of one case of mortality resulting from COVID-19
revealed moderate microvascular steatosis and mild active
inflammation of the hepatic lobule-portal vein region in the liver,
which indicated that the liver injury of COVID-19 is frequently
secondary damage caused by hypoxia (83). In vitro experiments also
suggested that the expression and activity of ACE2 increased
markedly in hepatocytes and cholangiocytes under hypoxic conditions
(84). A retrospective analysis
of 551 patients with COVID-19 in New York (USA) examined liver
function during the time that they were hospitalized, and this
analysis revealed that SARS-CoV-2 and other factors may have
contributed to liver injury (85). According to the study by Chew
et al (86), anomalies in
liver tests linked with COVID-19 were predominantly found to be
secondary to ischemia or drug-induced liver injury. As localized
necrosis and liver neutrophil and Kupffer cell growth were observed
in the autopsies of individuals who had died from COVID-19,
Vishwajeet et al (87)
concluded that SARS-CoV-2 and other factors probably contribute to
liver damage in patients with COVID-19. Del Nonno et al
(77) discovered from liver
biopsies from three patients with coronavirus pneumonia and the
autopsies of three individuals infected with SARS-CoV-2 that in an
increase in the amount of iron in the liver. It is hypothesized
that SARS-CoV-2 may elevate serum ferritin levels and cause damage
to the liver. Multifactorial liver injury, however, has increased
the difficulty of treating liver injury during the SARS-CoV-2
pandemic.
5. SARS-CoV-2-infected pancreatic symptoms
and expression of associated proteins
Acute pancreatitis may be caused by
SARS-CoV-2
Acute pancreatitis, and even abrupt onset diabetes
with ketoacidosis, are common in adult patients with COVID-19, and
numerous case reports over the course of the last two years have
demonstrated that SARS-CoV-2 appears to be able to affect
pancreatic (exocrine and endocrine) cells (88-90). Elevated lipase or amylase levels,
or other potential explanations, may be eliminated when patients
with COVID-19 experience abdominal pain and do not also have any
underlying digestive illnesses. In a case study involving three
family members, the mother and daughter both experienced severe
acute pancreatitis (thereby eliminating other potential causes of
pancreatitis) and had increased levels of pancreatic lipase
(91). In a different cohort
study, 83 patients hospitalized with COVID-19 also exhibited
increased lipase levels (>3 times the upper limit of normal),
which were regarded as a separate indicator of severe illness
(92). According to a
retrospective cohort analysis, out of 48,012 hospitalized patients,
11,883 (24.75%) tested positive for SARS-CoV-2 at admission and 189
(point prevalence, 0.39%) met the diagnostic criteria for
pancreatitis. Of these 189 individuals, 32 (17%) tested positive
for SARS-CoV-2. The point prevalence of pancreatitis among COVID-19
hospitalized patients was found to be 0.27% (93). Children have also been reported to
have acute pancreatitis. According to a case report by Samies et
al (94), three children who
were hospitalized for severe pancreatitis also tested positive for
SARS-CoV-2 in blood tests. Although pancreatitis is not a direct
result of SARS-CoV-2 infection, there appears to be an association
between pancreatitis and the COVID-19 diagnostic timeframe. In
addition, subjects who already have pancreatitis may be affected by
SARS-CoV-2. In a multicenter investigation, Pandanaboyana et
al (95) discovered that,
among 1,777 individuals with acute pancreatitis, the incidences of
severe pancreatitis, morbidity and death were significantly higher
when SARS-CoV-2 infection was present.
SARS-CoV-2 may induce acute pancreatitis, or is a
risk factor for acute pancreatitis, impacting its severity and
prognosis, even though the connection between acute pancreatitis
and SARS-CoV-2 infection is still being investigated.
Correlation between SARS-CoV-2, ACE2,
TMPRSS2 and pancreatic infection
Pancreatic cell expression of
SARS-CoV-2-associated proteins
It has been reported that ACE2 is expressed in
pancreatic exocrine and endocrine tissues (96). Co-expression of ACE2 and TMPRSS2
in pancreatic duct and acinar cells has been demonstrated in
numerous studies to be essential for effective viral entry into
cells. Pancreatic damage is induced by viral attachment to ACE2
(59,97,98). In a previous study, researchers
examined the expression of TMPRSS2 in 30 normal human tissues and
discovered that the target organs of SARS-CoV-2 infection, namely
the stomach, pancreas, lung, small intestine and salivary glands,
had the highest levels of TMPRSS2 expression (99). This protease is considered to be
the primary serine protease required for SARS-CoV-2 infection,
since studies have revealed that TMPRSS2 is expressed at a
relatively higher level in duct and acinar cells compared with
β-cells (100). Previous studies
also indicated that TMPRSS2 is primarily expressed in duct cells,
although ACE2 and TMPRSS2 are only infrequently co-expressed in
pancreatic duct and endocrine cells. ACE2 is primarily expressed in
islets and exocrine tissue capillaries, including pericytes and a
subset of duct cells (59,101).
An et al (60) used
immunohistochemistry staining to identify the expression levels of
the ACE2 protein in the colon, stomach, liver and pancreas. This
group discovered that islet cells were stained with ACE2 more
strongly than acinar cells. In addition, it has also been
previously demonstrated that SARS-CoV-2 is able to enter islet
cells and infect them, resulting in diabetes, which may be
attributed to the selective expression of the cell-surface
receptors neuropilin-1 (NRP1) and transferrin receptor (TFRC) in
cells (22). According to this
study, the enhancement in the levels of NRP1 and TFRC may be a
potential mechanism for the SARS-CoV-2 tropism of cells.
SARS-CoV-2-induced pancreatic
injury-associated protein phenotypes
In the islets of six of 11 patients with COVID-19,
Steenblock et al (102)
employed RNA in situ hybridization to identify the RNA of
the virus SARS-CoV-2. Another study (103) identified that SARS-CoV-2 may
penetrate and infect induced pluripotent stem cell-derived
pancreatic cells, including endocrine and exocrine cell types. This
resulted in morphological abnormalities and impaired expression of
critical markers, which corresponds to inflammatory features. In
addition, the postmortem pancreas examination of a patient who died
from SARS-CoV-2-19 revealed SARS-CoV-2-19 infection of the
pancreatic tissue. Therefore, taken together, these findings
suggest that pancreatic cells may be directly infected by
SARS-CoV-2. Drug-induced pancreatic injury cannot be ruled out;
however, certain patients with digestive issues who are treated in
hospitals also have a history of medicine use (96). According to data from one study,
ACE2 is not expressed in pancreatic acinar cells, but only in
islets and duct cells (60).
83.6% (56/67) of the biopsy islet tissues were found to be labeled
positively for ACE2, whereas 15.3% (20/131) of the biopsy
pancreatic acinar cells were mildly stained for ACE2 (60). In addition, capillary endothelial
cells on 98.5% (129/131) of the acinar cells stained positive for
ACE2. It is possible that SARS-CoV-2 may spread through the duct
system cells in pancreatic tissue, eventually causing islet
destruction and aberrant blood glucose levels. Therefore, this
phenotype would result from the unique expression of SARS-CoV-2 in
the pancreas following infection. Consequently, SARS-CoV-2
infection, and the ensuing damage, may increase due to the elevated
expression level of ACE2 in the pancreas (60). According to other studies,
SARS-CoV-2 infection and pancreatic cell inflammation may activate
pancreatic stellate cells and cause fibrosis, as seen in infected
non-human primate and human pancreas (97). As a result, ACE2 expression is
essential for pancreatic SARS-CoV-2 infection.
6. Conclusions
Investigations are ongoing to determine how
SARS-CoV-2 infects the gastrointestinal tract. It is known that the
SARS-CoV-2 S protein binds to ACE2 of the host cell, cleaves the S
protein with the help of proteases such as TMPRSS2 and then forms
fusion pores to release RNA into the cytoplasm. The virus is
multiplied in infected cells, which sets off an inflammatory
reaction. It is not possible to rule out the possibility of
SARS-CoV-2 infection when diagnosing digestive illnesses in the
setting of the outbreak. The risk of SARS-CoV-2 cannot be denied,
regardless of whether it directly affects the target organ, causing
severe pancreatitis, gastrointestinal hemorrhage or liver damage,
or whether it indirectly aggravates these conditions. Secondly, it
is important to take into account both drug and viral harm to
target organs while treating digestive system disorders, and to
minimize the combined effects of medications. It is possible to
investigate how to lessen the injury caused by SARS-CoV-2 to the
digestive system by moderately decreasing the expression of ACE2
and TMPRSS2, or by creating medications that block the viral S
protein. If there are further entry points into the digestive
system, this will require extensive testing. Finally, the damage
that SARS-CoV-2 causes to the digestive system should always be a
concern.
Availability of data and materials
Not applicable.
Authors' contributions
LZhe and LZha drafted the manuscript and contributed
equally. YZ participated in the literature search and analysis of
the data to be included in the review. JA and HJ were involved in
the design of the study and assisted in the preparation of the
figures and tables. GW and BT edited and revised the manuscript.
All authors have read and approved the final version of the
manuscript. Data authentication is not applicable.
Ethics approval and consent to
participate
Not applicable.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
Acknowledgments
Not applicable.
Funding
The present study was supported by grants from the National
Natural Science Foundation of China (grant nos. 82073087 and
81960507) and the Collaborative Innovation Center of Chinese
Ministry of Education (grant no. 2020-39).
References
|
1
|
Li J, Li C, Wang X, Wang Y and Zhou Y:
Considerations and perspectives on digestive diseases during the
COVID-19 pandemic: A narrative review. Ann Palliat Med.
10:4858–4867. 2021. View Article : Google Scholar
|
|
2
|
Delgado-Gonzalez P, Gonzalez-Villarreal
CA, Roacho-Perez JA, Quiroz-Reyes AG, Islas JF, Delgado-Gallegos
JL, Arellanos-Soto D, Galan-Huerta KA and Garza-Treviño EN:
Inflammatory effect on the gastrointestinal system associated with
COVID-19. World J Gastroenterol. 27:4160–4171. 2021. View Article : Google Scholar
|
|
3
|
Rizvi A, Patel Z, Liu Y, Satapathy SK,
Sultan K and Trindade AJ; Northwell Health COVID-19 Research
Consortium: Gastrointestinal sequelae 3 and 6 months after
hospitalization for coronavirus disease 2019. Clin Gastroenterol
Hepatol. 19:2438–2440.e1. 2021. View Article : Google Scholar :
|
|
4
|
Fang LG and Zhou Q: Remarkable
gastrointestinal and liver manifestations of COVID-19: A clinical
and radiologic overview. World J Clin Cases. 9:4969–4979. 2021.
View Article : Google Scholar :
|
|
5
|
Gkogkou E, Barnasas G, Vougas K and
Trougakos IP: Expression profiling meta-analysis of ACE2 and
TMPRSS2, the putative anti-inflammatory receptor and priming
protease of SARS-CoV-2 in human cells, and identification of
putative modulators. Redox Biol. 36:1016152020. View Article : Google Scholar
|
|
6
|
Jackson CB, Farzan M, Chen B and Choe H:
Mechanisms of SARS-CoV-2 entry into cells. Nat Rev Mol Cell Biol.
23:3–20. 2022. View Article : Google Scholar
|
|
7
|
Parmar MS: TMPRSS2: An equally important
protease as ACE2 in the pathogenicity of SARS-CoV-2 Infection. Mayo
Clin Proc. 96:2748–2752. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Huang X, He C, Hua X, Kan A, Sun S, Wang J
and Li S: Bioinformatic Analysis of correlation between immune
infiltration and COVID-19 in cancer patients. Int J Biol Sci.
16:2464–2476. 2020. View Article : Google Scholar :
|
|
9
|
Hoang T, Nguyen TQ and Tran TTA: Genetic
Susceptibility of ACE2 and TMPRSS2 in six common cancers and
possible impacts on COVID-19. Cancer Res Treat. 53:650–656. 2021.
View Article : Google Scholar
|
|
10
|
Viveiros A, Gheblawi M, Aujla PK,
Sosnowski DK, Seubert JM, Kassiri Z and Oudit GY: Sex- and
age-specific regulation of ACE2: Insights into severe COVID-19
susceptibility. J Mol Cell Cardiol. 164:13–16. 2022. View Article : Google Scholar
|
|
11
|
Da Eira D, Jani S and Ceddia RB:
Obesogenic and ketogenic diets distinctly regulate the SARS-CoV-2
Entry Proteins ACE2 and TMPRSS2 and the Renin-angiotensin system in
rat lung and heart tissues. Nutrients. 13:33572021. View Article : Google Scholar
|
|
12
|
Rando HM, MacLean AL, Lee AJ, Lordan R,
Ray S, Bansal V, Skelly AN, Sell E, Dziak JJ, Shinholster L, et al:
Pathogenesis, symptomatology, and transmission of SARS-CoV-2
through analysis of viral genomics and structure. mSystems.
6:e00095212021. View Article : Google Scholar
|
|
13
|
Saied EM, El-Maradny YA, Osman AA, Darwish
AMG, Abo Nahas HH, Niedbala G, Piekutowska M, Abdel-Rahman MA,
Balbool BA and Abdel-Azeem AM: A comprehensive review about the
molecular structure of severe acute respiratory syndrome
coronavirus 2 (SARS-CoV-2): Insights into natural products against
COVID-19. Pharmaceutics. 13:17592021. View Article : Google Scholar :
|
|
14
|
Salem R, El-Kholy AA, Waly FR, Ayman D,
Sakr A and Hussein M: Generation and utility of a single-chain
fragment variable monoclonal antibody platform against a
baculovirus expressed recombinant receptor binding domain of
SARS-CoV-2 spike protein. Mol Immunol. 141:287–296. 2022.
View Article : Google Scholar
|
|
15
|
Tai L, Zhu G, Yang M, Cao L, Xing X, Yin
G, Chan C, Qin C, Rao Z, Wang X, et al: Nanometer-resolution in
situ structure of the SARS-CoV-2 postfusion spike protein. Proc
Natl Acad Sci USA. 118:e21127031182021. View Article : Google Scholar :
|
|
16
|
Grishin AM, Dolgova NV, Landreth S,
Fisette O, Pickering IJ, George GN, Falzarano D and Cygler M:
Disulfide bonds play a critical role in the structure and function
of the receptor-binding domain of the SARS-CoV-2 spike antigen. J
Mol Biol. 434:1673572022. View Article : Google Scholar
|
|
17
|
Chen Y, Guo Y, Pan Y and Zhao ZJ:
Structure analysis of the receptor binding of 2019-nCoV. Biochem
Biophys Res Commun. 525:135–140. 2020. View Article : Google Scholar :
|
|
18
|
Zhang J, Xiao T, Cai Y and Chen B:
Structure of SARS-CoV-2 spike protein. Curr Opin Virol. 50:173–182.
2021. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Edenfield RC and Easley CA IV:
Implications of testicular ACE2 and the renin-angiotensin system
for SARS-CoV-2 on testis function. Nat Rev Urol. 19:116–127. 2022.
View Article : Google Scholar
|
|
20
|
Li D, Liu X, Zhang L, He J, Chen X, Liu S,
Fu J, Fu S, Chen H, Fu J and Cheng J: COVID-19 disease and
malignant cancers: The impact for the furin gene expression in
susceptibility to SARS-CoV-2. Int J Biol Sci. 17:3954–3967. 2021.
View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Peacock TP, Goldhill DH, Zhou J, Baillon
L, Frise R, Swann OC, Kugathasan R, Penn R, Brown JC, Sanchez-David
RY, et al: The furin cleavage site in the SARS-CoV-2 spike protein
is required for transmission in ferrets. Nat Microbiol. 6:899–909.
2021. View Article : Google Scholar
|
|
22
|
Wu CT, Lidsky PV, Xiao Y, Lee IT, Cheng R,
Nakayama T, Jiang S, Demeter J, Bevacqua RJ, Chang CA, et al:
SARS-CoV-2 infects human pancreatic β cells and elicits β cell
impairment. Cell Metab. 33:1565–1576.e5. 2021. View Article : Google Scholar
|
|
23
|
Yele V, Sanapalli BKR and Mohammed AA:
Imidazoles and benzimidazoles as putative inhibitors of SARS-CoV-2
B.1.1.7 (Alpha) and 1 (Gamma) variant spike glycoproteins: A
computational approach. Chem Zvesti. 76:1107–1117. 2022.
|
|
24
|
Liu C, Zhou D, Nutalai R, Duyvesteyn HME,
Tuekprakhon A, Ginn HM, Dejnirattisai W, Supasa P, Mentzer AJ, Wang
B, et al: The antibody response to SARS-CoV-2 Beta underscores the
antigenic distance to other variants. Cell Host Microbe.
30:53–68.e12. 2022. View Article : Google Scholar
|
|
25
|
Moss DL and Rappaport J: SARS-CoV-2 beta
variant substitutions alter spike glycoprotein receptor binding
domain structure and stability. J Biol Chem. 297:1013712021.
View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Sanches PRS, Charlie-Silva I, Braz HLB,
Bittar C, Freitas Calmon M, Rahal P and Cilli EM: Recent advances
in SARS-CoV-2 Spike protein and RBD mutations comparison between
new variants Alpha (B.1.1.7, United Kingdom), Beta (B.1.351, South
Africa), Gamma (1Brazil) and Delta (B.1.617.2, India). J Virus
Erad. 7:1000542021. View Article : Google Scholar
|
|
27
|
Storti B, Quaranta P, Di Primio C,
Clementi N, Mancini N, Criscuolo E, Spezia PG, Carnicelli V,
Lottini G, Paolini E, et al: A spatial multi-scale fluorescence
microscopy toolbox discloses entry checkpoints of SARS-CoV-2
variants in Vero E6 cells. Comput Struct Biotechnol J.
19:6140–6156. 2021. View Article : Google Scholar :
|
|
28
|
Alaofi AL and Shahid M: Mutations of
SARS-CoV-2 RBD may alter its molecular structure to improve its
infection efficiency. Biomolecules. 11:12732021. View Article : Google Scholar :
|
|
29
|
Bhattacharya M, Chatterjee S, Sharma AR,
Agoramoorthy G and Chakraborty C: D614G mutation and SARS-CoV-2:
Impact on S-protein structure, function, infectivity, and immunity.
Appl Microbiol Biotechnol. 105:9035–9045. 2021. View Article : Google Scholar :
|
|
30
|
Holshue ML, DeBolt C, Lindquist S, Lofy
KH, Wiesman J, Bruce H, Spitters C, Ericson K, Wilkerson S, Tural
A, et al: First Case of 2019 novel coronavirus in the United
States. N Engl J Med. 382:929–936. 2020. View Article : Google Scholar :
|
|
31
|
Wang W, Xu Y, Gao R, Lu R, Han K, Wu G and
Tan W: Detection of SARS-CoV-2 in different types of clinical
specimens. JAMA. 323:1843–1844. 2020.
|
|
32
|
Xu Y, Li X, Zhu B, Liang H, Fang C, Gong
Y, Guo Q, Sun X, Zhao D, Shen J, et al: Characteristics of
pediatric SARS-CoV-2 infection and potential evidence for
persistent fecal viral shedding. Nat Med. 26:502–505. 2020.
View Article : Google Scholar
|
|
33
|
Chen L, Lou J, Bai Y and Wang M: COVID-19
disease with positive fecal and negative pharyngeal and sputum
viral tests. Am J Gastroenterol. 115:7902020. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Burgueño JF, Reich A, Hazime H, Quintero
MA, Fernandez I, Fritsch J, Santander AM, Brito N, Damas OM,
Deshpande A, et al: Expression of SARS-CoV-2 Entry Molecules ACE2
and TMPRSS2 in the Gut of Patients With IBD. Inflamm Bowel Dis.
26:797–808. 2020. View Article : Google Scholar
|
|
35
|
Xiao F, Tang M, Zheng X, Liu Y, Li X and
Shan H: Evidence for gastrointestinal infection of SARS-CoV-2.
Gastroenterology. 158:1831–1833.e3. 2020. View Article : Google Scholar
|
|
36
|
Gupta A, Madhavan MV, Sehgal K, Nair N,
Mahajan S, Sehrawat TS, Bikdeli B, Ahluwalia N, Ausiello JC, Wan
EY, et al: Extrapulmonary manifestations of COVID-19. Nat Med.
26:1017–1032. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Mohamed DZ, Ghoneim ME, Abu-Risha SE,
Abdelsalam RA and Farag MA: Gastrointestinal and hepatic diseases
during the COVID-19 pandemic: Manifestations, mechanism and
management. World J Gastroenterol. 27:4504–4535. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Lin L, Jiang X, Zhang Z, Huang S, Zhang Z,
Fang Z, Gu Z, Gao L, Shi H, Mai L, et al: Gastrointestinal symptoms
of 95 cases with SARS-CoV-2 infection. Gut. 69:997–1001. 2020.
View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Elmunzer BJ, Spitzer RL, Foster LD,
Merchant AA, Howard EF, Patel VA, West MK, Qayed E, Nustas R,
Zakaria A, et al: Digestive manifestations in patients hospitalized
with coronavirus disease 2019. Clin Gastroenterol Hepatol.
19:1355–1365.e4. 2021. View Article : Google Scholar
|
|
40
|
Ferm S, Fisher C, Pakala T, Tong M, Shah
D, Schwarzbaum D, Cooley V, Hussain S and Kim SH: Analysis of
gastrointestinal and hepatic manifestations of SARS-CoV-2 infection
in 892 patients in queens, NY. Clin Gastroenterol Hepatol.
18:2378–2379.e1. 2020. View Article : Google Scholar
|
|
41
|
Wang MK, Yue HY, Cai J, Zhai YJ, Peng JH,
Hui JF, Hou DY, Li WP and Yang JS: COVID-19 and the digestive
system: A comprehensive review. World J Clin Cases. 9:3796–3813.
2021. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Andrews PLR, Cai W, Rudd JA and Sanger GJ:
COVID-19, nausea, and vomiting. J Gastroenterol Hepatol.
36:646–656. 2021. View Article : Google Scholar
|
|
43
|
Boraschi P, Giugliano L, Mercogliano G,
Donati F, Romano S and Neri E: Abdominal and gastrointestinal
manifestations in COVID-19 patients: Is imaging useful? World J
Gastroenterol. 27:4143–4159. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Carvalho A, Alqusairi R, Adams A, Paul M,
Kothari N, Peters S and DeBenedet AT: SARS-CoV-2 gastrointestinal
infection causing hemorrhagic colitis: Implications for detection
and transmission of COVID-19 disease. Am J Gastroenterol.
115:942–946. 2020. View Article : Google Scholar
|
|
45
|
Li X, Huang S, Lu J, Lai R, Zhang Z, Lin
X, Zheng X and Shan H: Upper Gastrointestinal Bleeding Caused by
SARS-CoV-2 Infection. Am J Gastroenterol. 115:1541–1542. 2020.
View Article : Google Scholar
|
|
46
|
Xu Z, Tang M, Chen P, Cai H and Xiao F:
SARS-CoV-2 gastro-intestinal infection prolongs the time to recover
from COVID-19. Front Med (Lausanne). 8:6835512021. View Article : Google Scholar
|
|
47
|
Hu F, Chen F, Ou Z, Fan Q, Tan X, Wang Y,
Pan Y, Ke B, Li L, Guan Y, et al: A compromised specific humoral
immune response against the SARS-CoV-2 receptor-binding domain is
related to viral persistence and periodic shedding in the
gastrointestinal tract. Cell Mol Immunol. 17:1119–1125. 2020.
View Article : Google Scholar
|
|
48
|
Noviello D, Costantino A, Muscatello A,
Bandera A, Consonni D, Vecchi M and Basilisco G: Functional
gastrointestinal and somatoform symptoms five months after
SARS-CoV-2 infection: A controlled cohort study. Neurogastroenterol
Motil. 34:e141872022. View Article : Google Scholar
|
|
49
|
Liu YL, Ren J, Yuan JP, Zhang ZJ, Guo WY,
Guan Y, Moeckel G, Ahuja N and Fu T: Postoperative onset and
detection of SARS-CoV-2 in surgically resected specimens from
gastrointestinal cancer patients with pre/asymptomatic COVID-19.
Ann Surg. 272:e321–e328. 2020. View Article : Google Scholar
|
|
50
|
Nabil A, Elshemy MM, Uto K, Soliman R,
Hassan AA, Shiha G and Ebara M: Coronavirus (SARS-CoV-2) in
gastroenterology and its current epidemiological situation: An
updated review until January 2021. EXCLI J. 20:366–385.
2021.PubMed/NCBI
|
|
51
|
McAllister MJ, Kirkwood K, Chuah SC,
Thompson EJ, Cartwright JA, Russell CD, Dorward DA, Lucas CD and Ho
GT: Intestinal protein characterisation of SARS-CoV-2 entry
molecules ACE2 and TMPRSS2 in inflammatory bowel disease (IBD) and
Fatal COVID-19 Infection. Inflammation. 45:567–572. 2022.
View Article : Google Scholar
|
|
52
|
Suárez-Fariñas M, Tokuyama M, Wei G, Huang
R, Livanos A, Jha D, Levescot A, Irizar H, Kosoy R, Cording S, et
al: Intestinal inflammation modulates the expression of ACE2 and
TMPRSS2 and potentially overlaps with the pathogenesis of
SARS-CoV-2-related disease. Gastroenterology. 160:287–301.e20.
2021. View Article : Google Scholar
|
|
53
|
Tao SS, Wang XY, Yang XK, Liu YC, Fu ZY,
Zhang LZ, Wang ZX, Ni J, Shuai ZW and Pan HF: COVID-19 and
inflammatory bowel disease crosstalk: From emerging association to
clinical proposal. J Med Virol. 94:5640–5652. 2022. View Article : Google Scholar
|
|
54
|
Shen S, Gong M, Wang G, Dua K, Xu J, Xu X
and Liu G: COVID-19 and gut injury. Nutrients. 14:44092022.
View Article : Google Scholar
|
|
55
|
Viganò C, Massironi S, Pirola L,
Cristoferi L, Fichera M, Bravo M, Mauri M, Redaelli AE, Dinelli ME
and Invernizzi P: COVID-19 in patients with inflammatory bowel
disease: A single-center observational study in Northern Italy.
Inflamm Bowel Dis. 26:e138–e139. 2020. View Article : Google Scholar
|
|
56
|
Derikx LAAP, Lantinga MA, de Jong DJ, van
Dop WA, Creemers RH, Römkens TEH, Jansen JM, Mahmmod N, West RL,
Tan ACITL, et al: Clinical Outcomes of Covid-19 in patients with
inflammatory bowel disease: A nationwide cohort study. J Crohns
Colitis. 15:529–539. 2021. View Article : Google Scholar
|
|
57
|
Zhou L, Niu Z, Jiang X, Zhang Z, Zheng Y,
Wang Z, Zhu Y, Gao L, Huang H, Wang X and Sun Q: SARS-CoV-2 Targets
by the pscRNA Profiling of ACE2, TMPRSS2 and Furin Proteases.
iScience. 23:1017442020. View Article : Google Scholar : PubMed/NCBI
|
|
58
|
Qi F, Qian S, Zhang S and Zhang Z: Single
cell RNA sequencing of 13 human tissues identify cell types and
receptors of human coronaviruses. Biochem Biophys Res Commun.
526:135–140. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
59
|
Lee JJ, Kopetz S, Vilar E, Shen JP, Chen K
and Maitra A: Relative Abundance of SARS-CoV-2 entry genes in the
enterocytes of the lower gastrointestinal tract. Genes (Basel).
11:6452020. View Article : Google Scholar : PubMed/NCBI
|
|
60
|
An X, Lin W, Liu H, Zhong W, Zhang X, Zhu
Y, Wang X, Li J and Sheng Q: SARS-CoV-2 Host Receptor ACE2 protein
expression atlas in human gastrointestinal tract. Front Cell Dev
Biol. 9:6598092021. View Article : Google Scholar
|
|
61
|
Zhang M, Feng C, Zhang X, Hu S, Zhang Y,
Min M, Liu B, Ying X and Liu Y: Susceptibility factors of stomach
for SARS-CoV-2 and treatment implication of mucosal protective
agent in COVID-19. Front Med (Lausanne). 7:5979672021. View Article : Google Scholar : PubMed/NCBI
|
|
62
|
Sun SH, Chen Q, Gu HJ, Yang G, Wang YX,
Huang XY, Liu SS, Zhang NN, Li XF, Xiong R, et al: A Mouse Model of
SARS-CoV-2 Infection and Pathogenesis. Cell Host Microbe.
28:124–133.e4. 2020. View Article : Google Scholar
|
|
63
|
Hartman AL, Nambulli S, McMillen CM, White
AG, Tilston-Lunel NL, Albe JR, Cottle E, Dunn MD, Frye LJ,
Gilliland TH, et al: SARS-CoV-2 infection of African green monkeys
results in mild respiratory disease discernible by PET/CT imaging
and shedding of infectious virus from both respiratory and
gastrointestinal tracts. PLoS Pathog. 16:e10089032020. View Article : Google Scholar : PubMed/NCBI
|
|
64
|
Jiao L, Li H, Xu J, Yang M, Ma C, Li J,
Zhao S, Wang H, Yang Y, Yu W, et al: The gastrointestinal tract is
an alternative route for SARS-CoV-2 Infection in a nonhuman primate
model. Gastroenterology. 160:1647–1661. 2021. View Article : Google Scholar
|
|
65
|
Livanos AE, Jha D, Cossarini F,
Gonzalez-Reiche AS, Tokuyama M, Aydillo T, Parigi TL, Ladinsky MS,
Ramos I, Dunleavy K, et al: Intestinal host response to SARS-CoV-2
Infection and COVID-19 outcomes in patients with gastrointestinal
symptoms. Gastroenterology. 160:2435–2450.e34. 2021. View Article : Google Scholar
|
|
66
|
Qi J, Zhou Y, Hua J, Zhang L, Bian J, Liu
B, Zhao Z and Jin S: The scRNA-seq Expression Profiling of the
Receptor ACE2 and the cellular protease TMPRSS2 reveals human
organs susceptible to SARS-CoV-2 Infection. Int J Environ Res
Public Health. 18:2842021. View Article : Google Scholar : PubMed/NCBI
|
|
67
|
Wang M, Yan W, Qi W, Wu D, Zhu L, Li W,
Wang X, Ma K, Ni M, Xu D, et al: Clinical characteristics and risk
factors of liver injury in COVID-19: A retrospective cohort study
from Wuhan, China. Hepatol Int. 14:723–732. 2020. View Article : Google Scholar
|
|
68
|
Zhang H, Liao YS, Gong J, Liu J and Zhang
H: Clinical characteristics and risk factors for liver injury in
COVID-19 patients in Wuhan. World J Gastroenterol. 26:4694–4702.
2020. View Article : Google Scholar : PubMed/NCBI
|
|
69
|
Wisniewska H, Skowron M, Bander D, Hornung
M, Jurczyk K, Karpinska E, Laurans Ł, Socha Ł, Czajkowski Z and
Wawrzynowicz-Syczewska M: Nosocomial COVID-19 Infection and Severe
COVID-19 pneumonia in patients hospitalized for alcoholic liver
disease: A case report. Am J Case Rep. 21:e9274522020. View Article : Google Scholar : PubMed/NCBI
|
|
70
|
Yang SJ, Wei TC, Hsu CH, Ho SN, Lai CY,
Huang SF, Chen YY, Liu SJ, Yu GY and Dou HY: Characterization of
virus replication, pathogenesis, and cytokine responses in syrian
hamsters inoculated with SARS-CoV-2. J Inflamm Res. 14:3781–3795.
2021. View Article : Google Scholar
|
|
71
|
Wong GL, Yip TC, Wong VW, Tse YK, Hui DS,
Lee SS, Yeoh EK, Chan HL and Lui GC: SARS-CoV-2 viral persistence
based on cycle threshold value and liver injury in patients with
COVID-19. Open Forum Infect Dis. 8:ofab2052021. View Article : Google Scholar
|
|
72
|
Lei HY, Ding YH, Nie K, Dong YM, Xu JH,
Yang ML, Liu MQ, Wei L, Nasser MI, Xu LY, et al: Potential effects
of SARS-CoV-2 on the gastrointestinal tract and liver. Biomed
Pharmacother. 133:1110642021. View Article : Google Scholar
|
|
73
|
Zhong P, Xu J, Yang D, Shen Y, Wang L,
Feng Y, Du C, Song Y, Wu C, Hu X and Sun Y: COVID-19-associated
gastrointestinal and liver injury: Clinical features and potential
mechanisms. Signal Transduct Target Ther. 5:2562020. View Article : Google Scholar :
|
|
74
|
Siddiqui MA, Suresh S, Simmer S,
Abu-Ghanimeh M, Karrick M, Nimri F, Musleh M, Mediratta V,
Al-Shammari M, Russell S, et al: Increased morbidity and mortality
in COVID-19 patients with liver injury. Dig Dis Sci. 67:2577–2583.
2021. View Article : Google Scholar
|
|
75
|
Wijarnpreecha K, Ungprasert P,
Panjawatanan P, Harnois DM, Zaver HB, Ahmed A and Kim D: COVID-19
and liver injury: A meta-analysis. Eur J Gastroenterol Hepatol.
33:990–995. 2021. View Article : Google Scholar
|
|
76
|
Wang Q, Zhao H, Liu LG, Wang YB, Zhang T,
Li MH, Xu YL, Gao GJ, Xiong HF, Fan Y, et al: Pattern of liver
injury in adult patients with COVID-19: A retrospective analysis of
105 patients. Mil Med Res. 7:282020.PubMed/NCBI
|
|
77
|
Del Nonno F, Nardacci R, Colombo D,
Visco-Comandini U, Cicalini S, Antinori A, Marchioni L, D'Offizi G,
Piacentini M and Falasca L: Hepatic failure in COVID-19: Is iron
overload the dangerous trigger? Cells. 10:11032021. View Article : Google Scholar : PubMed/NCBI
|
|
78
|
Gomi K, Ito T, Yamaguchi F, Kamio Y, Sato
Y, Mori H, Endo K, Abe T, Sakakura S, Kobayashi K, et al: Clinical
features and mechanism of liver injury in patients with mild or
moderate coronavirus disease 2019. JGH Open. 5:888–895. 2021.
View Article : Google Scholar
|
|
79
|
Ma C, Cong Y and Zhang H: COVID-19 and the
digestive system. Am J Gastroenterol. 115:1003–1006. 2020.
View Article : Google Scholar : PubMed/NCBI
|
|
80
|
Zhang Q, Li J, Zhang Y, Gao J, Wang P, Ai
M, Ding W and Tan X: Differences in clinical characteristics and
liver injury between suspected and confirmed COVID-19 patients in
Jingzhou, Hubei Province of China. Medicine (Baltimore).
100:e259132021. View Article : Google Scholar : PubMed/NCBI
|
|
81
|
Ackermann M, Verleden SE, Kuehnel M,
Haverich A, Welte T, Laenger F, Vanstapel A, Werlein C, Stark H,
Tzankov A, et al: Pulmonary vascular endothelialitis, thrombosis,
and angiogenesis in Covid-19. N Engl J Med. 383:120–128. 2020.
View Article : Google Scholar :
|
|
82
|
Varga Z, Flammer AJ, Steiger P, Haberecker
M, Andermatt R, Zinkernagel AS, Mehra MR, Schuepbach RA, Ruschitzka
F and Moch H: Endothelial cell infection and endotheliitis in
COVID-19. Lancet. 395:1417–1418. 2020. View Article : Google Scholar :
|
|
83
|
Xu Z, Shi L, Wang Y, Zhang J, Huang L,
Zhang C, Liu S, Zhao P, Liu H, Zhu L, et al: Pathological findings
of COVID-19 associated with acute respiratory distress syndrome.
Lancet Respir Med. 8:420–422. 2020. View Article : Google Scholar :
|
|
84
|
Paizis G, Tikellis C, Cooper ME, Schembri
JM, Lew RA, Smith AI, Shaw T, Warner FJ, Zuilli A, Burrell LM and
Angus PW: Chronic liver injury in rats and humans upregulates the
novel enzyme angiotensin converting enzyme 2. Gut. 54:1790–1796.
2005. View Article : Google Scholar
|
|
85
|
Bender JM and Worman HJ: Coronavirus
Disease 2019 and liver injury: A retrospective analysis of
hospitalized patients in New York City. J Clin Transl Hepatol.
9:551–558. 2021.PubMed/NCBI
|
|
86
|
Chew M, Tang Z, Radcliffe C, Caruana D,
Doilicho N, Ciarleglio MM, Deng Y and Garcia-Tsao G: Significant
liver injury during hospitalization for COVID-19 is not associated
with liver insufficiency or death. Clin Gastroenterol Hepatol.
19:2182–2191.e7. 2021. View Article : Google Scholar
|
|
87
|
Vishwajeet V, Purohit A, Kumar D, Parag V,
Tripathi S, Kanchan T, Kothari N, Dutt N, Elhence PA, Bhatia PK, et
al: Evaluation of pathological findings of COVID-19 by minimally
invasive autopsies: A single tertiary care center experience from
India. J Lab Physicians. 13:97–106. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
88
|
Tollard C, Champenois V, Delemer B,
Carsin-Vu A and Barraud S: An inaugural diabetic ketoacidosis with
acute pancreatitis during COVID-19. Acta Diabetol. 58:389–391.
2021. View Article : Google Scholar
|
|
89
|
Kumaran NK, Karmakar BK and Taylor OM:
Coronavirus disease-19 (COVID-19) associated with acute necrotising
pancreatitis (ANP). BMJ Case Rep. 13:e2379032020. View Article : Google Scholar : PubMed/NCBI
|
|
90
|
Alves AM, Yvamoto EY, Marzinotto MAN,
Teixeira ACS and Carrilho FJ: SARS-CoV-2 leading to acute
pancreatitis: An unusual presentation. Braz J Infect Dis.
24:561–564. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
91
|
Hadi A, Werge M, Kristiansen KT, Pedersen
UG, Karstensen JG, Novovic S and Gluud LL: Coronavirus Disease-19
(COVID-19) associated with severe acute pancreatitis: Case report
on three family members. Pancreatology. 20:665–667. 2020.
View Article : Google Scholar
|
|
92
|
Barlass U, Wiliams B, Dhana K, Adnan D,
Khan SR, Mahdavinia M and Bishehsari F: Marked elevation of lipase
in COVID-19 Disease: A cohort study. Clin Transl Gastroenterol.
11:e002152020. View Article : Google Scholar
|
|
93
|
Inamdar S, Benias PC, Liu Y, Sejpal DV,
Satapathy SK and Trindade AJ; Northwell COVID-19 Research
Consortium: Prevalence, risk factors, and outcomes of hospitalized
patients with coronavirus disease 2019 presenting as acute
pancreatitis. Gastroenterology. 159:2226–2228.e2. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
94
|
Samies NL, Yarbrough A and Boppana S:
Pancreatitis in pediatric patients with COVID-19. J Pediatric
Infect Dis Soc. 10:57–59. 2021. View Article : Google Scholar
|
|
95
|
Pandanaboyana S, Moir J, Leeds JS, Oppong
K, Kanwar A, Marzouk A, Belgaumkar A, Gupta A, Siriwardena AK,
Haque AR, et al: SARS-CoV-2 infection in acute pancreatitis
increases disease severity and 30-day mortality: COVID PAN
collaborative study. Gut. 70:1061–1069. 2021. View Article : Google Scholar
|
|
96
|
Liu F, Long X, Zhang B, Zhang W, Chen X
and Zhang Z: ACE2 expression in pancreas may cause pancreatic
damage after SARS-CoV-2 Infection. Clin Gastroenterol Hepatol.
18:2128–2130.e2. 2020. View Article : Google Scholar :
|
|
97
|
Qadir MMF, Bhondeley M, Beatty W, Gaupp
DD, Doyle-Meyers LA, Fischer T, Bandyopadhyay I, Blair RV, Bohm R,
Rappaport J, et al: SARS-CoV-2 infection of the pancreas promotes
thrombofibrosis and is associated with new-onset diabetes. JCI
Insight. 6:e1515512021. View Article : Google Scholar :
|
|
98
|
Jablonska B, Olakowski M and Mrowiec S:
Association between acute pancreatitis and COVID-19 infection: What
do we know? World J Gastrointest Surg. 13:548–562. 2021. View Article : Google Scholar :
|
|
99
|
Cao W, Feng Q and Wang X: Computational
analysis of TMPRSS2 expression in normal and SARS-CoV-2-infected
human tissues. Chem Biol Interact. 346:1095832021. View Article : Google Scholar :
|
|
100
|
Kusmartseva I, Wu W, Syed F, Van Der Heide
V, Jorgensen M, Joseph P, Tang X, Candelario-Jalil E, Yang C, Nick
H, et al: Expression of SARS-CoV-2 entry factors in the pancreas of
normal organ donors and individuals with COVID-19. Cell Metab.
32:1041–1051.e6. 2020. View Article : Google Scholar :
|
|
101
|
Coate KC, Cha J, Shrestha S, Wang W,
Goncalves LM, Almaca J, Kapp ME, Fasolino M, Morgan A, Dai C, et
al: SARS-CoV-2 cell entry factors ACE2 and TMPRSS2 are expressed in
the microvasculature and ducts of human pancreas but are not
enriched in β cells. Cell Metab. 32:1028–1040.e4. 2020. View Article : Google Scholar
|
|
102
|
Steenblock C, Richter S, Berger I, Barovic
M, Schmid J, Schubert U, Jarzebska N, von Mässenhausen A,
Linkermann A, Schürmann A, et al: Viral infiltration of pancreatic
islets in patients with COVID-19. Nat Commun. 12:35342021.
View Article : Google Scholar
|
|
103
|
Shaharuddin SH, Wang V, Santos RS, Gross
A, Wang Y, Jawanda H, Zhang Y, Hasan W, Garcia G Jr, Arumugaswami V
and Sareen D: Deleterious Effects of SARS-CoV-2 infection on human
pancreatic cells. Front Cell Infect Microbiol. 11:6784822021.
View Article : Google Scholar
|