Diabetes mellitus (DM) is a metabolic disease
characterized by three primary metabolic disorders, namely
carbohydrate, lipid and protein metabolism disorders, all of which
are caused by inadequate insulin production (1). Patients are usually diagnosed only
when serious complications have occurred due to the ignorance of
symptoms of diabetes in the early stages. Therefore, early
detection, diagnosis and treatment can benefit a patient's health
and quality of life, while also reducing the financial burden
(2).
The complications of diabetes, including diabetic
nephropathy, diabetic retinopathy, diabetic neuropathy (DN),
cardiovascular complications, liver fibrosis and other
complications, seriously affect the prognosis of diabetic patients
(3). Diabetic foot ulcers (DFUs),
one of the most prevalent and serious complications of diabetes
(4), are common in diabetic
patients and can lead to amputation or even death when the
condition worsens (5).
Ceramides are a type of sphingolipid, the major
lipid component of cell membranes (6). Ceramides not only act as the second
messenger molecule in the sphingolipid signaling pathway, but also
take part in the formation process of the stratum corneum. The
stratum corneum, which is the primary portion of the intercellular
matrix containing 40-50% of intercellular lipids, plays a crucial
function in maintaining the water balance of the skin (7). Some studies have found that
ceramides can inhibit glucose uptake and increase adipose ectopic
deposition, while inhibiting the enzymes required for ceramide
synthesis can improve the progression of diabetes (8-10).
A single study has shown significant differences in the
concentration of ceramides in the skin of the feet of diabetic and
non-diabetic patients (11).
Ceramides can be divided into several types according to their
chemical composition. Different chemical structures correspond to
different biological functions (12). Ceramides and their metabolites can
decrease insulin sensitivity, blood vessel reactivity and
pancreatic cell function, as well as maintaining the skin's barrier
function, regulating hydration, exerting anti-aging effects and
acting as future therapeutic targets for some diseases, such as
insulin resistance, obesity, type 2 diabetes, Parkinson's disease,
autoimmune rheumatic disease, ventilator-induced lung injury and
cancer (especially breast cancer) (9,13-18).
All the aforementioned findings suggest that
ceramides may have a close connection with the development of DFUs.
The aim of the present review is to assess the role of ceramides in
diabetes, the skin and atherosclerosis, and to discuss their
possible mechanisms in DFUs. The hope is that these findings will
translate into new screening methods and treatments to alleviate
and possibly prevent or cure diabetes and DFUs.
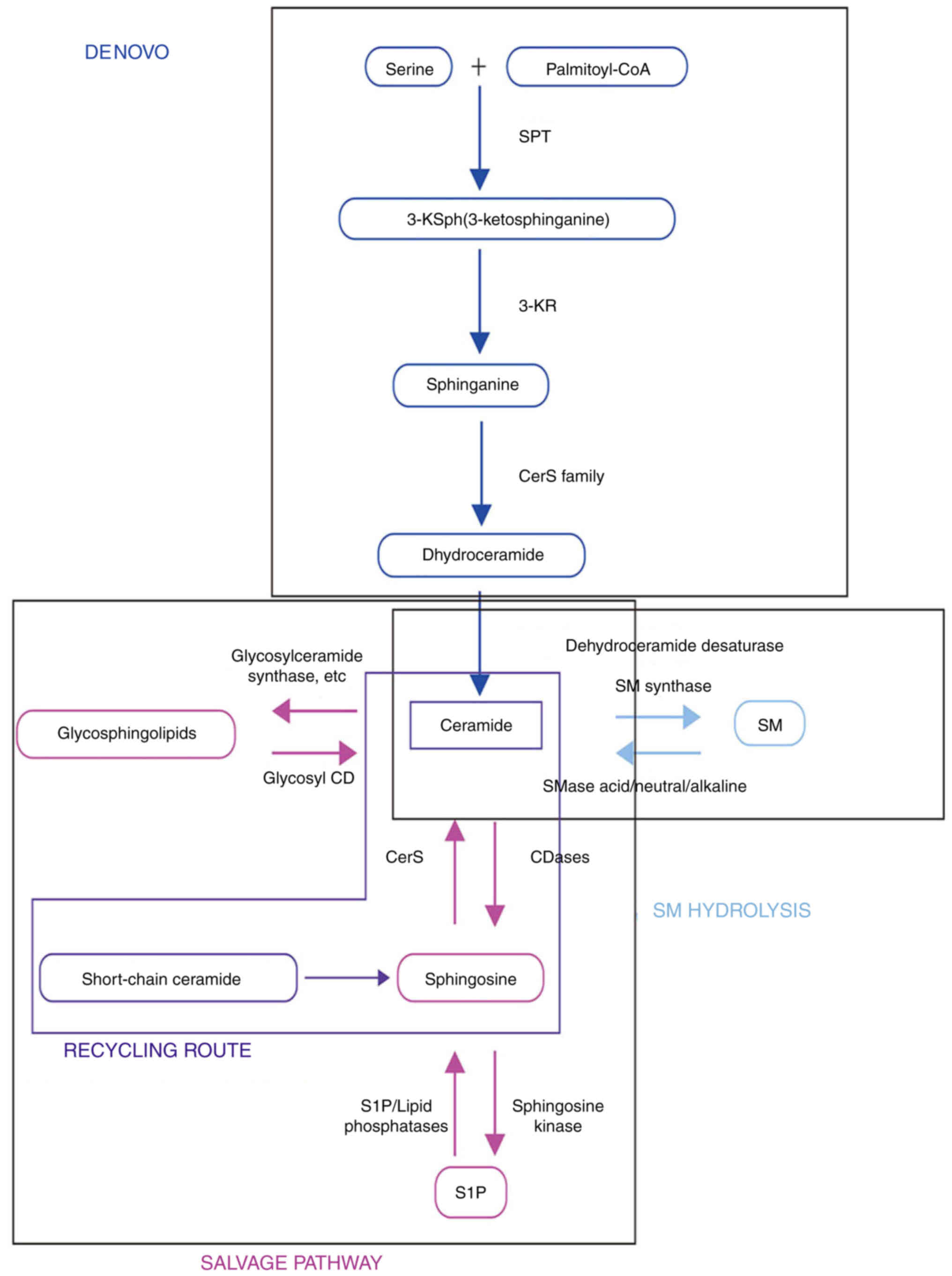
Second, in the sphingomyelinase (SMase) pathway,
ceramides are produced by hydrolysis of sphingomyelin (SM)
catalyzed by SMases, which are divided into neutral SMases (nSMases
1, 2 and 3), acid SMases (aSMase) and alkaline SMases (20). When cellular stress occurs in a
particular compartment, the level of ceramide is quickly increased
multiple times through this pathway (21).
The third pathway is the salvage pathway, in which
sphingolipids degraded to sphingosine (Sph) are reutilized by
reacylation to produce ceramides. Lipid phosphate phosphatases or
Sph-1-phosphate (S1P) phosphatase are used to obtain Sph (22). CerSs may acylate Sph to form
ceramides. Ceramides can be digested by ceramidases in the opposite
direction, resulting in Sph. Sph kinase (SphK) phosphorylates Sph,
resulting in S1P, which then reenters sphingolipid metabolism by
the SphK and/or one of the six CerSs (23) (Fig.
1).
Each CerS controls the production of endogenous
ceramides from scratch. Although certain CerSs are found throughout
the body, other isoforms create tissue-specific synthesis of
ceramides. CerS1 specifically synthesizes C18 ceramide, and while
it is highly expressed in the skeletal muscles, it is nearly
undetectable in other tissues (24). CerS2 is mostly expressed in the
kidneys and liver, and synthesizes C22-24 ceramides (25). Ceramides with varying acyl chain
lengths may trigger diverse cell responses; hence, different CerS
isoforms may create different ceramide species (17).
Ceramides are broken down into free fatty acids
(FFAs) and Sph by ceramidases. Five distinct ceramidases have been
previously reported in humans, each with a different catalytic pH
optimum, namely, acid ceramidase, neutral ceramidase, and alkaline
ceramidases 1, 2 and 3, which are encoded by five separate genes
(ASAH1, ASAH2, ACER1, ACER2 and ACER3, respectively) (30).
Ceramide species produced in the endoplasmic
reticulum (ER) of the stratum spinosum inside the epidermis are
subsequently acylated by linoleoyl-CoA to generate acyl-ceramides
in the skin. In healthy skin, the acyl chain length spans from C16
to C36, with C24 being the most common. Ceramides with distinct
molecular architectures have different activities, implying that
they play a variety of roles in skin homeostasis (31). Acyl-ceramides are glycosylated in
the Golgi apparatus and subsequently transported with other lipids
into lamellar bodies. The lamellar bodies, containing glycosylated
ceramides, acyl ceramides, cholesterol and very-long-chain FAs, are
secreted into the extracellular space of the stratum corneum, where
the ceramides are covalently linked to structural skin proteins,
such as involucrin, filaggrin and small proline-rich proteins, to
form the epidermal permeability barrier (32).
Ceramide levels are significantly higher in
insulin-resistant patients than in healthy individuals (33); therefore, the content level of the
ceramides may be somehow related to skin disorders in diabetics.
Ceramides containing omega carbons from linoleic acid esterified to
omega hydroxy fatty acids are found only in the epidermis and help
build a multilayered membrane that plays a key role in the skin
barrier (34). The primary
ceramides generated by the de novo pathway play a role in
the development of the epidermal permeability barrier. The skin
barrier is composed of extracellular ceramides. Intracellular
ceramides, on the other hand, signal without O-acylation and have
an acyl chain length of 16 to 18 carbon atoms (35).
The skin of mammals serves as their body's first
line of protection against external threats, making it one of the
most important organs of the human body (36).
Both internal and external factors influence the
epidermal ceramide expression profile. For example, downregulating
CerS3 can reduce the proportion of unsaturated long-chain ceramides
(37). Furthermore, AZGP1, an
adipokine, has been shown to improve epidermal barrier function in
Alzheimer's disease mice by increasing ceramide 3 (NP), ceramide 5
(AS) and ceramide 1 (EOS) (38).
The composition of ceramides in the stratum corneum
is changing with age. Children have more ceramide 8 (NH), and less
α-hydroxy and esterified ω-hydroxy ceramides than adults. There is
less ceramide 1 in older people compared with that in younger
people. Moreover, the degree of fatty acid chain saturation of
ceramide 1 is associated with the season, being reduced in autumn
and winter, which participates in lamellar and lateral lipid
organization. Deficiency of this type of ceramide may be associated
with seasonal skin dryness (39).
Exposure to ultraviolet irradiation can increase plasma ceramide
levels and decrease SM levels in mice (40). Therefore, the level of epidermal
ceramides is maintained in dynamic equilibrium by control from a
number of factors and plays an important role in skin homeostasis
(41).
Although ceramides account for the highest
proportion of skin lipids, they are no more important than other
lipids. Previous studies found that perturbation of the skin with
ceramides alone or mixtures of ceramides and FFAs delayed skin
barrier recovery, and normal recovery was only possible with
equimolar mixtures of all key lipids, as determined by
transepidermal water loss (42,43). These findings demonstrate that
FFAs, cholesterol and ceramides are hydrophilic extracellular lipid
matrices that are indispensable for skin permeability (44).
Despite the lack of direct evidence, ceramides have
been hypothesized to regulate keratinocyte proliferation and
differentiation in the skin. Both in vitro and in
vivo, keratinocyte differentiation is reported to be
accompanied by an increase in ceramide production (45).
Ceramides and their derivatives play a crucial role
in controlling inflammation and the immune system. The activation
of dendritic cells (DCs) with proinflammatory agents like
lipopolysaccharide, TNF or interleukin 1 (IL1A and IL1B) has been
shown to increase the intracellular concentrations of ceramides by
promoting the stability of Toll-like receptor 4 (TLR4) (46). Changes in the transcriptional
levels of ceramide synthetase and ceramidase in the lipid skeleton
of TLR9-stimulated wild-type DCs were found to regulate the
ceramide ratio (47). Ceramides
are intracellular modulators of the NLR family, pyrin domain
containing 3 (NLRP3) inflammasome assembly, and studies in
microglia have shown that they can promote the release of IL1B
(48). A single study has found
that in mouse models, the presence of Staphylococcus
epidermidis increases the content of ceramides in the epidermis
by secreting SMase, which interferes with the growth of other
pathogenic microorganisms and affects the immune pathway (49). Ceramides are therefore considered
to be bioactive signaling molecules active in a number of
inflammatory signaling pathways.
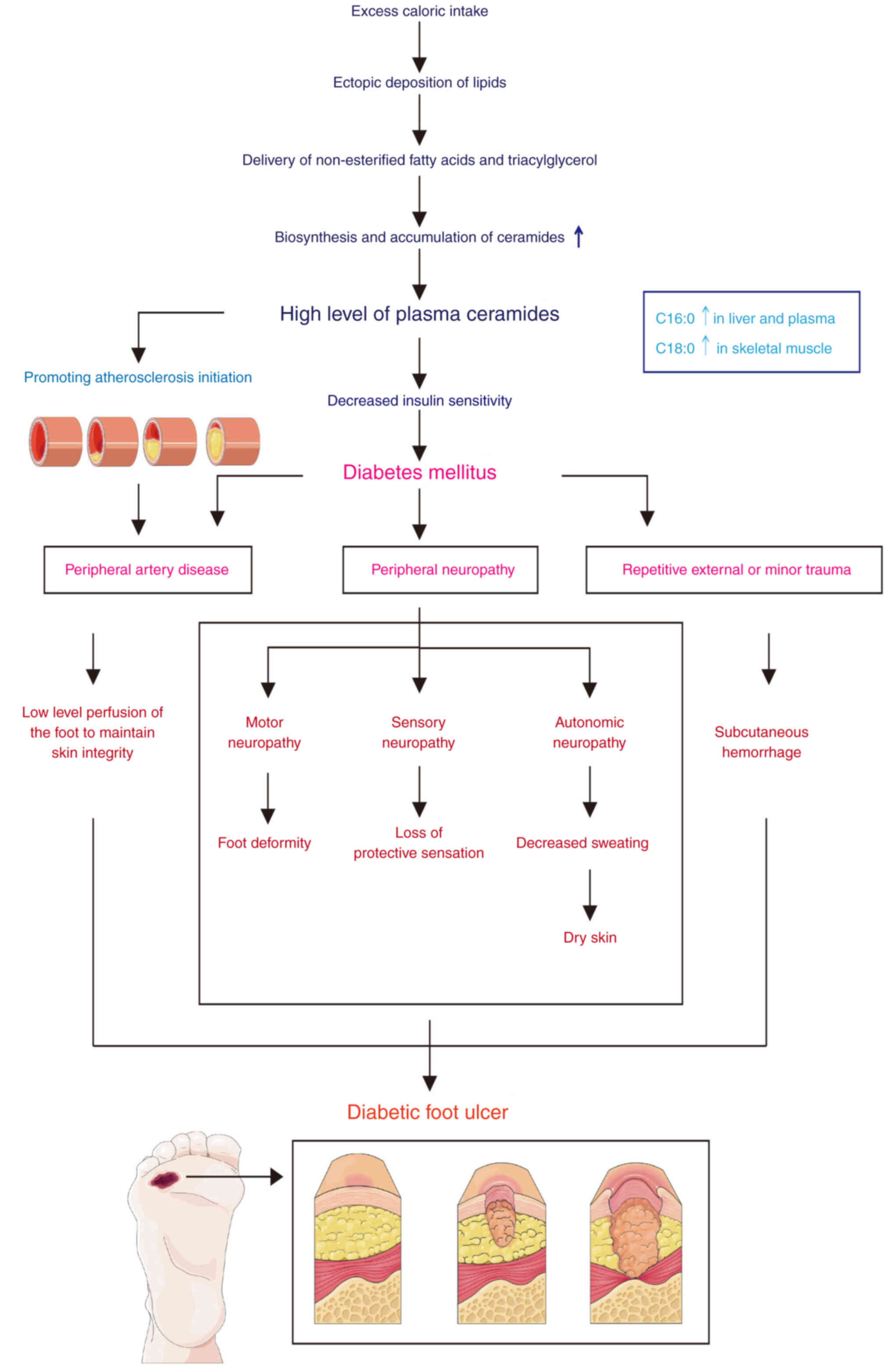
One of the major chronic complications of diabetes,
and the main cause of disability and mortality, is DFUs (50). Approximately 15-25% of diabetic
patients will develop DFUs. Severe DFUs progress to gangrene and
infection, leading to amputation (4). Despite the fact that DFUs can be
treated, they can return up to 40% in the first year and almost
100% in the next decade (51).
Numerous risk factors affect DFUs, but they are not independent
causes of foot ulcers. Age, blood pressure, blood sugar levels,
smoking, various types of nerve injury, impaired vascular
circulation and diabetes over an extended period of time are all
risk factors (52). DFUs are
caused by peripheral neuropathy, peripheral artery disease, and
repetitive external and minor trauma (Fig. 2) (53).
Ceramides, a crucial part of the keratinocyte
membrane and a factor in the incidence and progression of several
skin illnesses, are associated with the skin's barrier and
permeability functions (35).
Ceramides are also closely associated with insulin resistance,
diabetes, and the microvascular and macrovascular side effects of
diabetes, including atherosclerosis and DN (10). These findings raise the question
of whether ceramides are crucial lipid molecules in diabetic
patients who develop vascular disease, neuropathy and foot
ulcers.
The etiology of DFUs involves peripheral artery
occlusive disease, neuropathy and trauma with subsequent infection
(54).
In addition, foot deformities, trauma and secondary
infections accompanied by weakened resistance further aggravate
DFUs, forming a vicious cycle (65).
Regulation of vascular tone requires the production
of vasoactive substances, such as NO, by endothelial cells
(66). According to the current
evidence, ceramides have a detrimental effect on endothelial
function. Endothelial dysfunction is caused by decreased NO
synthesis or increased NO breakdown due to the generation of ROS
(67). Ceramides can activate
NADPH oxidase, increasing ROS, leading to increased oxidative
stress, which degrades ceramidase (68). Ceramides have been shown to
inhibit the de novo pathway of ceramides in diet-induced
obese C57Bl/6 mice, which produces normalization of endothelial
dysfunction and systemic hypertension (69). However, endothelium-dependent
vasodilation is impaired when the endothelium is briefly exposed to
exogenous ceramides. This finding indicates that ceramides are
crucial in the impairment of the endothelium caused by inflammation
or obesity. Exogenous C16 ceramide-mediated endothelial NO synthase
(NOS) uncoupling and dysregulated protein phosphatase 2 (PP2A) were
found to damage human aortic endothelial cells in a study of
atherosclerotic patients (70).
Another study in patients with coronary arteries also found that
ceramides were associated with coronary endothelial dysfunction
(71). However, more mechanistic
studies are needed to confirm the findings. Karakashian et
al (72) demonstrated that
nSMase activity persistently increases with aging, resulting in
greater ceramide and endothelial NOS (eNOS) inactivation, as well
as a reduction in NO production. In addition, nsMase-derived
ceramides participate in the conversion of NO to
H2O2, which leads to the reduction of NO and
the formation of coronary artery disease (73). Ceramides have been shown to
decrease eNOS3 (NOS3; also known as eNOS) activity, either under
baseline conditions or after stimulation (74). On the other hand, in high-fat diet
mice, inhibiting the synthesis of ceramides by the de novo
pathway may indirectly improve endothelial dysfunction (75). Moreover, a research study has
demonstrated that ceramides can activate NADPH oxidase (76), but other studies contend that
ceramides can directly influence the mitochondrial electron
transport chain to increase ROS in various cell types, including
endothelial cells (77,78). Following a harmful self-amplifying
cascade of events, the resultant O2 can ultimately cause
the decoupling of NOS3 and the generation of ROS in endothelial
cells. Last but not least, ceramides have the ability to activate
the NLPR3 inflammasomes and release inflammatory cytokines, thereby
contributing to atherosclerotic lesions and vascular dysfunction
(48).
Ceramides have been found to be connected with
vasoactivity. Ceramides can not only contract the blood vessels,
but also vasodilate them (74).
However, the mechanisms by which ceramides induce vasoactivity
remain unclear.
A single study has shown that ceramide-induced
vasodilation is partially endothelium-dependent via activation of
NOS3 and subsequent NO generation, as aforementioned, by impairing
endothelium function (79).
Angiotensin II (ANGII) may indirectly increase ceramide production,
thereby activating SMases in the peripheral vasculature to control
vascular function (80).
However, a considerable amount of research has found
that ceramides can promote vasoconstriction (74,81,82). Total ceramide levels in the aortic
tissue of hypertensive rats and humans were much higher than those
in normotensive mice and humans, with the main changes being
increases in the levels of ceramides C24:1 and C24:0 (83). However, a correlation between
increased blood pressure and increased ceramides in plasma does not
indicate a causal relationship (84). Notably, it has been suggested that
nSMase-derived ceramides promote the vasoconstriction caused by
changes in the levels of thromboxane A2, ANG II and oxygen tension
(85,86).
In short, the vascular effects of ceramides appear
to be complicated and remain unclear. This vasoactivity may be
influenced by a variety of variables. Numerous exogenous and
endogenous processes can produce ceramides, and can also transform
them into other active molecules. The different vascular effects
may result from the different types of vessels and their various
diameters, as well as the cell types they act on. Notably,
different lengths of ceramides have different pathological
functions (87).
Diabetes and cardiovascular disease are mostly
caused by lipotoxicity, an abnormal accumulation of lipids in
non-adipose tissue. The most dangerous sphingolipids are those that
promote cell death, insulin resistance and decreased insulin gene
expression (88). A key component
of the metabolism of sphingolipids is ceramides (29,30). Furthermore, previous studies have
provided clear evidence that sphingolipids, particularly ceramides,
have an important role in T1DM and the complications of T2DM
(89,90). Indeed, several metabolic diseases,
including DM, cardiomyopathy, insulin resistance and
atherosclerosis, can be treated by preventing ceramide production
or accelerating ceramide breakdown (87).
TNF, IL1B and interferon γ are examples of
inflammatory agents producing cytotoxicity to pancreatic β-cells by
increasing the levels of sphingolipids, particularly ceramides,
which are either exogenously delivered or produced endogenously
(91). By contrast, several
studies have shown that reducing ceramide production, such as
inhibition of SPT or inhibition of CerS inhibitors, reduces
cytotoxicity to rodent (92) and
human (93) β-cells. In fact,
there is evidence of a very small increase in ceramides following
FFA therapy in trials involving cell apoptosis (94). However, some studies have shown
that inhibiting ceramide synthesis reduces β-cell apoptosis
(95-98). There may be three reasons to
explain this result. First, the increased ceramide level induces
apoptosis only at the designated site, namely the mitochondria,
without changing the total ceramide mass (93). Second, upon triggering apoptosis,
ceramides are changed into another sphingolipid metabolite
(glucosylceramide) (99). Third,
distinct ceramide isoforms may have different apoptotic potentials.
In order to promote apoptosis, hyperglycemia increases the levels
of the harmful isoforms of ceramides, namely C22:0, C24:1 and C18:0
(100).
There is increasing evidence that ceramides play an
important role in insulin resistance. Insulin resistance is a
pathophysiological state characterized by hyperinsulinemia, high
blood glucose levels and decreased responsiveness of peripheral
tissues to insulin. Numerous studies have shown that excessive
consumption of FFAs, the use of glucocorticoids, corpulence and
decreased physical activity are some of the major contributors to
insulin resistance, in which ceramides serve as a key intermediary
(101). Increased ceramide
accumulation from palmitate exposure occurs in several cells,
including muscle cells (102),
adipocytes (103) and
cardiomyocytes (104).
Simultaneous Akt inhibition results in decreased insulin
sensitivity (105). Sphingolipid
recycling or the salvage pathway can cause a buildup of ceramides
in response to an excessive supply of saturated or unsaturated FAs.
Ceramides are an obligatory intermediate in saturated FA-induced
insulin resistance (101).
Zalewska et al (106)
showed that mice administered a high-fat diet expressed higher
levels of CerS than mice fed a regular diet. However, Holland et
al (107) found that
infusion of both highly saturated and highly unsaturated FAs
reduced glucose uptake and Akt activation in rats, but infusion of
highly saturated FAs alone increased ceramide levels. Both
saturated fats and unsaturated fats can promote insulin resistance
through different mechanisms, but ceramides only participate in
saturated fat-induced insulin resistance (107). However, unsaturated FAs were not
associated with increased levels of ceramides, which are not the
only lipids responsible for insulin resistance. In line with this,
overexpression of acid ceramidase can lower ceramide levels,
prevent the buildup of ceramides caused by palmitate and enhance
insulin signaling (108).
Glucocorticoids can increase the levels of
ceramides, which may be the mechanism for inducing insulin
resistance (107,109). By activating enzymes such as
SPT, SMase and CerS, the commonly used glucocorticoid dexamethasone
increases ceramide levels in a number of cell types and animal
species, such as 3T3-L1 cells, wild-type mice and Sprague-Dawley
rats (107,110,111). Pretreating C57Bl6/J mice with
myriocin, a potent inhibitor of SPT, was shown to avoid some of
these effects (112). However,
the study involved a low dose of myriocin, so the alterations
cannot be ruled out as a result of subsequent changes in the gut
microbiota. Peroxisome proliferator-activated receptor α (PPARA) is
activated by ceramide accumulation, and thus genetic disruption of
PPARA, or other damage causing decreased hepatic PPARA expression,
has been reported to inhibit insulin resistance in some conditions
(113).
Obesity is known to contribute to T2DM by
influencing glucose homeostasis and insulin sensitivity (114). However, the levels of ceramides
in the skeletal muscles of rats, mice and diabetic patients have
been reduced with long-term aerobic training (115,116). In other studies, for some
unknown reason, even after exercise training in rats and people,
the level of muscle ceramides does not significantly decrease
(115,117,118).
Sphingolipids are bioactive lipids found in
atherosclerotic plaques, which have been linked to both the
development and progression of atherosclerosis. Although their
specific effects in human atherosclerotic plaques are still
unclear, ceramides are an important component in sphingolipids that
are also associated with atherosclerosis (71). By promoting their aggregation
through ceramide-ceramide interactions, SM may be converted to
ceramides by aSMase on low-density lipoprotein (LDL) surfaces, thus
accelerating the onset of atherosclerosis. Additionally, the
pro-atherogenic pathways may be stimulated by the S1P produced from
ceramides (119).
Ceramides from human plaques can cause plaque
inflammation and cell death. Ceramide levels were higher in plaques
connected to the inflammatory response, as determined by examining
the histology and measuring cytokine levels of the plaques
(120). Similar findings were
obtained in mouse models (121).
Inhibiting SPT in the ceramide biosynthesis pathway
with myriocin has been shown to decrease the progress of
atherosclerosis in rodent models (122). Ceramides have been shown to have
more precise roles in the pathophysiology of cardiovascular disease
than other existing lipid biomarkers, making them a promising
biomarker for prediction purposes. Also, in mouse models, the
inhibition of enzymes involved in ceramide synthesis can reduce
cardiovascular complications. However, it is not easy to understand
the possible mechanism of atherosclerosis without more research. On
study found that ACER2 is a target gene for HIF-2a, which reduces
ceramide levels in fat cells and improves atherosclerosis (123). However, the study cannot rule
out other mechanisms involved in protecting atherosclerosis.
Due to its preservation of the integrity of the
internal environment, apoptosis is described as genetically
programmed cell death. The mechanism of apoptosis is complex. There
are three known apoptotic signaling mechanisms: The intrinsic
mitochondrial, intrinsic ER and extrinsic death receptor pathways.
A number of studies have shown that ceramides are associated with
the induction of β-cell apoptosis (124). Zhang et al (125) found that amyloid peptides induce
β-cell apoptosis, partly through the production of ceramides by
activating aSMase through activation of K+ channels on
the cell membrane, which is a marker of cell apoptosis.
Ceramides have also been shown to cause apoptosis
through their effect on the mitochondria, which controls regional
levels of certain lipids like sphingolipids to detect cellular
stress (126). BAX and ceramides
work together to synergistically induce mitochondrial outer
membrane permeabilization (MOMP). According to Ganesan et al
(127), when ceramides are
present, they are the key molecules in mitochondrial
permeabilization, suggesting that the inhibition of ceramides
without activating BAX-induced mitochondrial permeabilization,
blocks the induction of MOMP, resulting in the death of yeast
cells. According to the study by James et al (128), ceramides may be suggested in a
new pathway leading to cell apoptosis by inducing the generation of
Creola bodies, a marker of apoptotic lung epithelial cells.
However, this was found in mouse lung tissues, and the mechanisms
in other tissues require more investigation. Meanwhile, ROS also
increased as a result of the increased ceramide level, which can
contribute to apoptosis. Studies have found that ceramide-mediated
insulin resistance-induced apoptosis was associated with the
DNA-damage response and mitogen-activated protein kinase pathways,
and that ceramides could mediate mitochondrial dysfunction
(129,130). ROS are primarily produced by the
mitochondria, and impairment of the mitochondria is generally
considered to be associated with increased ROS. Apoptosis-inducing
factors are generated when ceramides are being synthesized.
Ceramides may inhibit the activation of mitochondrial NADPH
oxidase, thus blocking electron transport at complex I and complex
III of the respiratory chain, and inducing apoptosis by increasing
ROS production (131,132).
Ceramides are a mediator of palmitate-induced cell
toxicity, according to certain studies (133-135). One hypothesis that has been
postulated is that ceramides can induce β-cell death by activating
protein kinase C δ-type (PKCD), which is necessary for apoptosis in
numerous cell types (136).
Another possible way to induce β-cell apoptosis is
to inhibit the function of Akt, which is a serine/threonine kinase.
First, it was shown that Akt could increase cellular proliferation,
but that its inhibition could induce apoptosis (142). Second, Akt upregulates CDKN1B to
trigger apoptosis while adversely regulating the transcriptional
activity of FOXO1 (143). Third,
Akt promotes mTOR/p70S6K signaling and directly phosphorylates and
inactivates BCL2 members, including BAD, BAX and BID, to cause
apoptosis (144). In addition,
AKT induce apoptosis by activating cell signaling such as that of
c-Jun N-terminal kinase (JNK) and extracellular signal-regulated
kinase (98).
The phosphorylation of insulin receptor substrate-1
(IRS1), a mediator protein that links the binding of insulin and
insulin growth factor 1 to related intracellular receptors of the
insulin pathways, can activate ceramides to lead to impaired islet
signaling (145). The increased
IRS1 level induces serine 307 phosphorylation to inhibit insulin
signaling (146). Ceramides can
also suppress IRS1 expression by triggering the PKR/JNK/Prep1/p160
axis and/or the c-Jun amino-terminal kinase/PBX regulatory protein
1 axis (89). In addition,
glucosylceramides have been demonstrated to impair insulin
signaling by inhibiting insulin receptors (101,147).
As aforementioned, ceramides can inhibit AKT to
cause β-cell apoptosis, and other studies have shown that they can
also mediate the secretion of insulin (98,148,149). Ceramides first catalyze the
dephosphorylation of AKT by activating PP2A (150). Ceramide secondly prevent the
translocation of AKT to the PIP3-PDK1 complex in the plasma
membrane. Ceramides can activate PKCZ (151), and then ceramide-induced
phosphorylation inhibits this at the serine 473 or threonine 308
residue (24,102). This can form a stable AKT-PKCZ
complex to prevent its interactions with PIP3. In further studies,
PKC inhibitors were shown to improve insulin sensitivity and block
the ceramide-induced loss of AKT activity (152-154). Ceramides were also elevated in
other animal models such as Zucker diabetic fatty (ZDF) rats
(155) and ob/ob mice (156), as well as in obese humans
(157). If ceramides cause
insulin resistance by inhibiting AKT, the proximal insulin
signaling should be intact; however, there are also abnormalities
in this proximal insulin signaling. In addition, the presence of
the HIF-2α-neuraminidase 3-ceramide pathway was also observed in
mice administered a high-fat diet, and insulin sensitivity was
improved in mice ablated with enteric-specific HIF-2α. However,
this only occurred in the absence of oxygen (158).
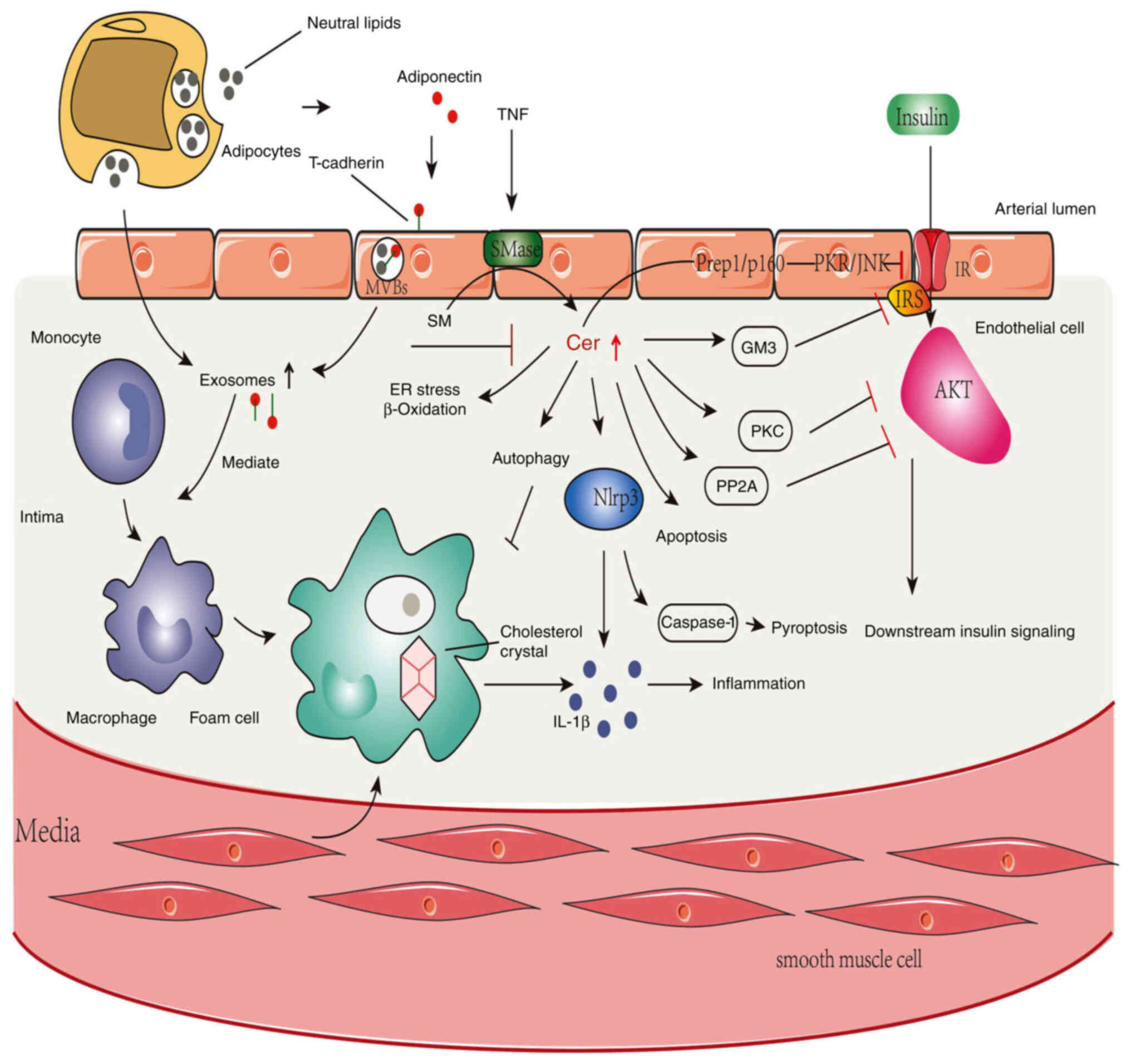
Exosome secretion and/or biogenesis may be impacted
by ceramides. The protein adiponectin, which is released by
adipocytes, stimulates the synthesis of exosomes in skeletal muscle
and endothelial cells, and induces the expression of cadherin 13
(159). Given that research
indicates that the microRNA profiles of exosomes were changed in
patients with T2DM, Santovito et al (160) reported that exosomes, a
metabolic mediator, produced by adipose tissue macrophages, impact
insulin sensitivity in mice. Lean mice developed glucose
intolerance and insulin resistance after exposure to exosomes from
obese mice. By contrast, exosomes from adipose tissue macrophages
in lean mice reduced insulin resistance and glucose intolerance in
obese animals (161). Exosomes
may also convert monocytes into macrophages, lead to inflammation
and disrupt insulin signaling to cause T2DM (162).
The most frequent cardiovascular consequence of T2DM
is atherosclerosis, which develops in big and medium-sized arteries
(163); it is characterized by
inflammation, lipid and macrophage buildup, cell death and fibrosis
(164). In addition,
sphingolipids, including ceramides, are elevated in human
atherosclerotic lesions, and it is widely recognized that chronic
inflammation is the constant sign of T2DM and atherosclerosis
(101). Additionally,
sphingolipid metabolites are important in inflammatory signaling.
Sphingolipids, particularly ceramides (the center of sphingolipid
metabolism), have been proved to have an association with cell
death, insulin resistance, inflammation and lipotoxicity, as
previously described (6). The
most prevalent and harmful metabolites in mammalian tissue are C16-
and C18-ceramides (165). It was
reported that ceramides cause inflammation in smooth muscle cells
of the human coronary arteries (10). However, myriocin could suppress
SPT by preventing ceramide de novo synthesis, which reduced
atherosclerosis in apolipoprotein E-knockout mice (122). Atherosclerosis is also
associated with lipoprotein aggregation. However, hydrolysis of SM
to ceramides, especially nSMaes2, has been found to cause
lipoprotein aggregation and participate in atherogenesis (166).
ER stress and the NLRP3 inflammasome have a close
association with the development of atherosclerosis (167). As aforementioned, patients with
T2DM may have defects in the downstream insulin signaling pathway
that affect glucose transport due to activation of the IRS1
tyrosine phosphorylation/phosphoinositide 3 (PI-3) kinase axis
(168). In addition, since the
same PI-3 kinase pathway also activates NOS3, less NO is produced,
which impairs endothelial function and accelerates atherosclerosis
(169).
NFKBIB and NFKB are connected in the cytoplasm.
Increased NFKBIB/NFKB signaling activity may be a significant
factor in T2DM inflammation and insulin resistance (170). Fatty acyl-CoAs are an example of
an inflammatory factor that activates NFKBIB kinase, phosphorylates
NFKBIB and then translocates to the nucleus to bind to target
genes, thus increasing the production of inflammatory cytokines
that are involved in atherosclerosis (TNF, IL1B, IL6 and PKC)
(171,172). Ceramides have the ability to
activate certain plasma membrane receptors, including TLR4, which
might lead to inflammation and insulin resistance (173). The TLR4 mRNA/protein levels are
also elevated in the muscle of obese patients and those with T2DM,
and they closely correspond with NFKBIB/NFKB activation, which is
another mechanism for promoting atherosclerosis. Therefore, it is
likely that ceramides are closely related to this development
(174).
Since it has been shown that sphingolipid production
is inhibited, atherosclerosis is reduced by the decreased
expression of sterol-regulatory element binding transcription
factors (SREBFs), which include SREBF1A, SREBF1C and SREBF2
(175). However, in animals
orally treated with myriocin (an inhibitor of SPT and a necessary
enzyme for the synthesis of ceramides), SREBF1C levels decreased,
which was potentially due to decreased very-LDL particle size.
Myriocin was found to be anti-atherosclerotic. These effects may be
partly correlated with SREBF (176,177).
In conclusion, ceramides are closely associated with
diabetes and atherosclerosis, and the mechanism of action may
involve the induction of DFUs (Fig.
3). Additional mechanisms of action of ceramides need to be
found to support ceramides as the underlying molecule involved in
the advent of DFUs.
A certain degree of common pathogenesis is shared
between diabetic complications and DFUs.
Diabetic nephropathy and diabetic retinopathy have
the same pathogenesis, which is caused by the dysfunction of
microvascular endothelial function (178). Consistent with DFUs,
hyperglycemia through various pathways such as the polyol pathway,
the AGE/receptor for AGE axis and the PKC pathway increases ROS
production, causes oxidative stress and a series of inflammatory
responses, and eventually leads to the accumulation of AGEs and
endothelial dysfunction. At the same time, recent studies have
found that renal cells can produce exosomes, which promote the
development of inflammation and lead to endothelial cell damage
(179-181). This is supported by the finding
that the transplantation of new endothelial cells can prevent the
development of a diabetic foot (182).
Diabetic peripheral neuropathy is involved in the
development of DFUs, as aforementioned. The same diabetes leads to
increased ROS levels and mitochondrial dysfunction, leading to
impairment of axonal transport function in peripheral nerves,
especially Schwann cells. However, the mechanism of diabetic
peripheral neuropathy is still unclear (65).
Cardiac autonomic neuropathy and DFUs have also been
recently linked, although the mechanism of cardiac autonomic
neuropathy is still unclear. At present, ROS and AGEs are suspected
to be associated with the occurrence of inflammation and
microvascular lesions, which is also consistent with the occurrence
of DFUs (183).
There is no evidence for a common mechanism of
liver fibrosis and DFUs. In only one case report, excluding those
on hepatitis and autoimmune liver diseases, was there a record of a
patient with liver fibrosis that was associated with
microangiopathy of the liver, as well as diabetic nephropathy and
DFUs (184). This still needs
more research and discussion.
Research has shown that ceramides are important in
diabetes and cardiovascular disease. Ceramides are precursors of
complex sphingolipids that form the epidermal barrier structure and
are involved in maintaining skin homeostasis. Direct experimental
evidence suggests that the plasma levels of ceramides are higher in
diabetics, that ceramides antagonize insulin signaling, and that
the inhibition or elimination of every ceramide
biosynthesis-related enzyme is consistent with insulin
sensitization, anti-atherosclerosis and heart protection. DFUs are
a chronic complication of diabetes and an important cause of
disability and death from diabetes, mainly caused by peripheral
artery disease, peripheral neuropathy and recurrent external or
mild trauma. Most of the existing reviews describe the association
between ceramides and DM. The present review attempts to identify
the function of ceramides in the occurrence and development of DFUs
by elaborating on the association between ceramides and DM. The
hope is that these findings will translate into new screening
methods and treatments to alleviate and possibly prevent or cure
diabetes and DFUs.
Not applicable.
YW conceived the topic and wrote the first draft.
ZS, GZ, LZ and ZW revised the manuscript and figures. All authors
read and approved the final manuscript. Data authentication is not
applicable.
Not applicable.
Not applicable.
The authors declare that they have no competing
interests.
Not applicable.
This study was supported by the National Natural Science
Foundation of China (grant no. 82070455), the related Foundation of
Jiangsu Province (grant nos. BK20201225), the Medical Innovation
Team Project of Jiangsu Province (grant no. CXTDA2017010).
|
1
|
Galicia-Garcia U, Benito-Vicente A, Jebari
S, Larrea-Sebal A, Siddiqi H, Uribe KB, Ostolaza H and Martín C:
Pathophysiology of type 2 diabetes mellitus. Int J Mol Sci.
21:62752020. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Zheng Y, Ley SH and Hu FB: Global
aetiology and epidemiology of type 2 diabetes mellitus and its
complications. Nat Rev Endocrinol. 14:88–98. 2018. View Article : Google Scholar
|
|
3
|
Demir S, Nawroth PP, Herzig S and Ekim
Üstünel B: Emerging targets in type 2 diabetes and diabetic
complications. Adv Sci (Weinh). 8:21002752021. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Everett E and Mathioudakis N: Update on
management of diabetic foot ulcers:. Ann N Y Acad Sci.
1411:153–165. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Wolf SJ, Melvin WJ and Gallagher K:
Macrophage-mediated inflammation in diabetic wound repair. Semin
Cell Dev Biol. 119:111–118. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Gomez-Larrauri A, Presa N,
Dominguez-Herrera A, Ouro A, Trueba M and Gomez-Muñoz A: Role of
bioactive sphingolipids in physiology and pathology. Essays
Biochem. 64:579–589. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Castro BM, Prieto M and Silva LC:
Ceramide: A simple sphingolipid with unique biophysical properties.
Prog Lipid Res. 54:53–67. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Summers SA: Editorial: The role of
ceramides in diabetes and cardiovascular disease. Front Endocrinol
(Lausanne). 12:6678852021. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Raichur S, Brunner B, Bielohuby M, Hansen
G, Pfenninger A, Wang B, Bruning JC, Larsen PJ and Tennagels N: The
role of C16:0 ceramide in the development of obesity and type 2
diabetes: CerS6 inhibition as a novel therapeutic approach. Mol
Metab. 21:36–50. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Field BC, Gordillo R and Scherer PE: The
role of ceramides in diabetes and cardiovascular disease regulation
of ceramides by adipokines. Front Endocrinol (Lausanne).
11:5692502020. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Lechner A, Akdeniz M, Tomova-Simitchieva
T, Bobbert T, Moga A, Lachmann N, Blume-Peytavi U and Kottner J:
Comparing skin characteristics and molecular markers of xerotic
foot skin between diabetic and non-diabetic subjects: An
exploratory study. J Tissue Viability. 28:200–209. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Summers SA, Chaurasia B and Holland WL:
Metabolic messengers: Ceramides. Nat Metab. 1:1051–1058. 2019.
View Article : Google Scholar
|
|
13
|
Custodia A, Aramburu-Núñez M, Correa-Paz
C, Posado-Fernández A, Gómez-Larrauri A, Castillo J, Gómez-Muñoz A,
Sobrino T and Ouro A: Ceramide metabolism and Parkinson's
disease-therapeutic targets. Biomolecules. 11:9452021. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Alexandropoulou I, Grammatikopoulou MG,
Gkouskou KK, Pritsa AA, Vassilakou T, Rigopoulou E, Lindqvist HM
and Bogdanos DP: Ceramides in autoimmune rheumatic diseases:
Existing evidence and therapeutic considerations for diet as an
anticeramide treatment. Nutrients. 15:2292023. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Mandell EW and Savani RC: Ceramides,
autophagy, and apoptosis mechanisms of ventilator-induced lung
injury and potential therapeutic targets. Am J Respir Crit Care
Med. 199:687–689. 2019. View Article : Google Scholar :
|
|
16
|
Pal P, Atilla-Gokcumen GE and Frasor J:
Emerging roles of ceramides in breast cancer biology and therapy.
Int J Mol Sci. 23:111782022. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Wattenberg BW: The long and the short of
ceramides. J Biol Chem. 293:9922–9923. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Cha HJ, He C, Zhao H, Dong Y, An IS and An
S: Intercellular and intracellular functions of ceramides and their
metabolites in skin (Review). Int J Mol Med. 38:16–22. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Magnan C and Le Stunff H: Role of
hypothalamic de novo ceramides synthesis in obesity and associated
metabolic disorders. Mol Metab. 53:1012982021. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Insausti-Urkia N, Solsona-Vilarrasa E,
Garcia-Ruiz C and Fernandez-Checa JC: Sphingomyelinases and liver
diseases. Biomolecules. 10:14972020. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Taniguchi M and Okazaki T: Role of
ceramide/sphingomyelin (SM) balance regulated through 'SM cycle' in
cancer. Cell Signal. 87:1101192021. View Article : Google Scholar
|
|
22
|
Hammerschmidt P and Brüning JC:
Contribution of specific ceramides to obesity-associated metabolic
diseases. Cell Mol Life Sci. 79:3952022. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Bhattacharya N, Sato WJ, Kelly A,
Ganguli-Indra G and Indra AK: Epidermal lipids: Key mediators of
atopic dermatitis pathogenesis. Trends Mol Med. 25:551–562. 2019.
View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Roszczyc-Owsiejczuk K and Zabielski P:
Sphingolipids as a culprit of mitochondrial dysfunction in insulin
resistance and type 2 diabetes. Front Endocrinol (Lausanne).
12:6351752021. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Aldoghachi AF, Baharudin A, Ahmad U, Chan
SC, Ong TA, Yunus R, Razack AH, Yusoff K and Veerakumarasivam A:
Evaluation of CERS2 gene as a potential biomarker for bladder
cancer. Dis Markers. 2019:38751472019. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Polubothu S, Glover M, Holder SE and
Kinsler VA: Uniparental disomy as a mechanism for CERS3-mutated
autosomal recessive congenital ichthyosis. Br J Dermatol.
179:1214–1215. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Sheridan M and Ogretmen B: The role of
ceramide metabolism and signaling in the regulation of mitophagy
and cancer therapy. Cancers (Basel). 13:24752021. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Kurz J, Parnham MJ, Geisslinger G and
Schiffmann S: Ceramides as novel disease biomarkers. Trends Mol
Med. 25:20–32. 2019. View Article : Google Scholar
|
|
29
|
Mullen TD, Hannun YA and Obeid LM:
Ceramide synthases at the centre of sphingolipid metabolism and
biology. Biochem J. 441:789–802. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Parveen F, Bender D, Law SH, Mishra VK,
Chen CC and Ke LY: Role of ceramidases in sphingolipid metabolism
and human diseases. Cells. 8:15732019. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Li Q, Fang H, Dang E and Wang G: The role
of ceramides in skin homeostasis and inflammatory skin diseases. J
Dermatol Sci. 97:2–8. 2020. View Article : Google Scholar
|
|
32
|
Jung K, Kim SH, Joo KM, Lim SH, Shin JH,
Roh J, Kim E, Park W and Kim W: Oral intake of enzymatically
decomposed AP collagen peptides improves skin moisture and ceramide
and natural moisturizing factor contents in the stratum corneum.
Nutrients. 13:43722021. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Ramírez-Vélez R, Martínez-Velilla N,
Correa-Rodríguez M, Sáez de Asteasu ML, Zambom-Ferraresi F,
Palomino-Echeverria S, García-Hermoso A and Izquierdo M: Lipidomic
signatures from physically frail and robust older adults at
hospital admission. Geroscience. 44:1677–1688. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Coderch L, López O, de la Maza A and Parra
JL: Ceramides and skin function. Am J Clin Dermatol. 4:107–129.
2003. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Badhe Y, Gupta R and Rai B: Structural and
barrier properties of the skin ceramide lipid bilayer: A molecular
dynamics simulation study. J Mol Model. 25:1402019. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Vollmer DL, West VA and Lephart ED:
Enhancing skin health: By oral administration of natural compounds
and minerals with implications to the dermal microbiome. Int J Mol
Sci. 19:30592018. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Kim B, Shon JC, Seo HS, Liu KH, Lee JW,
Ahn SK and Hong SP: Decrease of ceramides with long-chain fatty
acids in psoriasis: Possible inhibitory effect of interferon gamma
on chain elongation. Exp Dermatol. 31:122–132. 2022. View Article : Google Scholar
|
|
38
|
Wang L, Liu M, Ning D, Zhu H, Shan G, Wang
D, Ping B, Yu Y, Yang H, Yan K, et al: Low serum ZAG levels
correlate with determinants of the metabolic syndrome in Chinese
subjects. Front Endocrinol (Lausanne). 11:1542020. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Fujiwara A, Morifuji M, Kitade M, Kawahata
K, Fukasawa T, Yamaji T, Itoh H and Kawashima M: Age-related and
seasonal changes in covalently bound ceramide content in forearm
stratum corneum of Japanese subjects: Determination of molecular
species of ceramides. Arch Dermatol Res. 310:729–735. 2018.
View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Łuczaj W, Jastrząb A, do Rosário Domingues
M, Domingues P and Skrzydlewska E: Changes in phospholipid/ceramide
profiles and eicosanoid levels in the plasma of rats irradiated
with UV rays and treated topically with cannabidiol. Int J Mol Sci.
22:87002021. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Fujii M: The pathogenic and therapeutic
implications of ceramide abnormalities in atopic dermatitis. Cells.
10:23862021. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Meckfessel MH and Brandt S: The structure,
function, and importance of ceramides in skin and their use as
therapeutic agents in skin-care products. J Am Acad Dermatol.
71:177–184. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Draelos ZD: The science behind skin care:
Moisturizers. J Cosmet Dermatol. 17:138–144. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Wertz PW: Roles of lipids in the
permeability barriers of skin and oral mucosa. Int J Mol Sci.
22:52292021. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Bocheńska K and Gabig-Cimińska M:
Unbalanced sphingolipid metabolism and its implications for the
pathogenesis of psoriasis. Molecules. 25:11302020. View Article : Google Scholar
|
|
46
|
Santinha DR, Marques DR, Maciel EA, Simões
CS, Rosa S, Neves BM, Macedo B, Domingues P, Cruz MT and Domingues
MR: Profiling changes triggered during maturation of dendritic
cells: A lipidomic approach. Anal Bioanal Chem. 403:457–471. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Paget C, Deng S, Soulard D, Priestman DA,
Speca S, von Gerichten J, Speak AO, Saroha A, Pewzner-Jung Y,
Futerman AH, et al: TLR9-mediated dendritic cell activation
uncovers mammalian ganglioside species with specific ceramide
backbones that activate invariant natural killer T cells. PLoS
Biol. 17:e30001692019. View Article : Google Scholar : PubMed/NCBI
|
|
48
|
Scheiblich H, Schlütter A, Golenbock DT,
Latz E, Martinez-Martinez P and Heneka MT: Activation of the NLRP3
inflammasome in microglia: The role of ceramide. J Neurochem.
143:534–550. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
49
|
Zheng Y, Hunt RL, Villaruz AE, Fisher EL,
Liu R, Liu Q, Cheung GYC, Li M and Otto M: Commensal staphylococcus
epidermidis contributes to skin barrier homeostasis by generating
protective ceramides. Cell Host Microbe. 30:301–313.e9. 2022.
View Article : Google Scholar : PubMed/NCBI
|
|
50
|
Wang Y, Shao T, Wang J, Huang X, Deng X,
Cao Y, Zhou M and Zhao C: An update on potential biomarkers for
diagnosing diabetic foot ulcer at early stage. Biomed Pharmacother.
133:1109912021. View Article : Google Scholar
|
|
51
|
Abbott CA, Chatwin KE, Foden P, Hasan AN,
Sange C, Rajbhandari SM, Reddy PN, Vileikyte L, Bowling FL, Boulton
AJM and Reeves ND: Innovative intelligent insole system reduces
diabetic foot ulcer recurrence at plantar sites: A prospective,
randomised, proof-of-concept study. Lancet Digit Health.
1:e308–e318. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
52
|
Kim EJ and Han K: Factors related to
self-care behaviours among patients with diabetic foot ulcers. J
Clin Nurs. 29:1712–1722. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
53
|
Bandyk DF: The diabetic foot:
Pathophysiology, evaluation, and treatment. Semin Vasc Surg.
31:43–48. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
54
|
Aldana PC, Cartron AM and Khachemoune A:
Reappraising diabetic foot ulcers: A focus on mechanisms of
ulceration and clinical evaluation. Int J Low Extrem Wounds.
21:294–302. 2022. View Article : Google Scholar
|
|
55
|
Rubitschung K, Sherwood A, Crisologo AP,
Bhavan K, Haley RW, Wukich DK, Castellino L, Hwang H, La Fontaine
J, Chhabra A, et al: Pathophysiology and molecular imaging of
diabetic foot infections. Int J Mol Sci. 22:115522021. View Article : Google Scholar : PubMed/NCBI
|
|
56
|
Armstrong DG, Boulton AJM and Bus SA:
Diabetic foot ulcers and their recurrence. N Engl J Med.
376:2367–2375. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
57
|
Feldman EL, Callaghan BC, Pop-Busui R,
Zochodne DW, Wright DE, Bennett DL, Bril V, Russell JW and
Viswanathan V: Diabetic neuropathy. Nat Rev Dis Primers. 5:422019.
View Article : Google Scholar : PubMed/NCBI
|
|
58
|
Volpe CMO, Villar-Delfino PH, dos Anjos
PMF and Nogueira-Machado JA: Cellular death, reactive oxygen
species (ROS) and diabetic complications. Cell Death Dis.
9:1192018. View Article : Google Scholar : PubMed/NCBI
|
|
59
|
Bönhof GJ, Herder C, Strom A, Papanas N,
Roden M and Ziegler D: Emerging biomarkers, tools, and treatments
for diabetic polyneuropathy. Endocr Rev. 40:153–192. 2019.
View Article : Google Scholar
|
|
60
|
Hammad SM, Baker NL, El Abiad JM,
Spassieva SD, Pierce JS, Rembiesa B, Bielawski J, Lopes-Virella MF
and Klein RL; DCCT/EDIC Group of Investigators: Increased plasma
levels of select deoxy-ceramide and ceramide species are associated
with increased odds of diabetic neuropathy in type 1 diabetes: A
pilot study. Neuromolecular Med. 19:46–56. 2017. View Article : Google Scholar :
|
|
61
|
Strain WD and Paldánius PM: Diabetes,
cardiovascular disease and the microcirculation. Cardiovasc
Diabetol. 17:572018. View Article : Google Scholar : PubMed/NCBI
|
|
62
|
Criqui MH, Matsushita K, Aboyans V, Hess
CN, Hicks CW, Kwan TW, McDermott MM, Misra S, Ujueta F; American
Heart Association Council on Epidemiology and Prevention; et al:
Lower extremity peripheral artery disease: Contemporary
epidemiology, management gaps, and future directions: A scientific
statement from the american heart association. Circulation. 144.
pp. e171–e191. 2021, View Article : Google Scholar
|
|
63
|
He X and Schuchman EH: Ceramide and
ischemia/reperfusion injury. J Lipids. 2018:36467252018. View Article : Google Scholar : PubMed/NCBI
|
|
64
|
Davis FM, Kimball A, Boniakowski A and
Gallagher K: Dysfunctional wound healing in diabetic foot ulcers:
New crossroads. Curr Diab Rep. 18:22018. View Article : Google Scholar : PubMed/NCBI
|
|
65
|
Sloan G, Selvarajah D and Tesfaye S:
Pathogenesis, diagnosis and clinical management of diabetic
sensorimotor peripheral neuropathy. Nat Rev Endocrinol. 17:400–420.
2021. View Article : Google Scholar : PubMed/NCBI
|
|
66
|
Zweier JL and Ilangovan G: Regulation of
nitric oxide metabolism and vascular tone by cytoglobin. Antioxid
Redox Signal. 32:1172–1187. 2020. View Article : Google Scholar :
|
|
67
|
Sun HJ, Wu ZY, Nie XW and Bian JS: Role of
endothelial dysfunction in cardiovascular diseases: The link
between inflammation and hydrogen sulfide. Front Pharmacol.
10:15682020. View Article : Google Scholar : PubMed/NCBI
|
|
68
|
Chabowski DS, Cohen KE, Abu-Hatoum O,
Gutterman DD and Freed JK: Crossing signals: Bioactive lipids in
the microvasculature. Am J Physiol Heart Circ Physiol.
318:H1185–H1197. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
69
|
Zhang QJ, Holland WL, Wilson L, Tanner JM,
Kearns D, Cahoon JM, Pettey D, Losee J, Duncan B, Gale D, et al:
Ceramide mediates vascular dysfunction in diet-induced obesity by
PP2A-mediated dephosphorylation of the eNOS-Akt complex. Diabetes.
61:1848–1859. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
70
|
Akawi N, Checa A, Antonopoulos AS,
Akoumianakis I, Daskalaki E, Kotanidis CP, Kondo H, Lee K,
Yesilyurt D, Badi I, et al: Fat-secreted ceramides regulate
vascular redox state and influence outcomes in patients with
cardiovascular disease. J Am Coll Cardiol. 77:2494–2513. 2021.
View Article : Google Scholar : PubMed/NCBI
|
|
71
|
Akhiyat N, Vasile V, Ahmad A, Sara JD,
Nardi V, Lerman LO, Jaffe A and Lerman A: Plasma ceramide levels
are elevated in patients with early coronary atherosclerosis and
endothelial dysfunction. J Am Heart Assoc. 11:e0228522022.
View Article : Google Scholar : PubMed/NCBI
|
|
72
|
Karakashian AA, Giltiay NV, Smith GM and
Nikolova-Karakashian MN: Expression of neutral sphingomyelinase-2
(NSMase-2) in primary rat hepatocytes modulates IL-beta-induced JNK
activation. FASEB J. 18:968–970. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
73
|
Parker BA, Walton CM, Carr ST, Andrus JL,
Cheung ECK, Duplisea MJ, Wilson EK, Draney C, Lathen DR, Kenner KB,
et al: β-Hydroxybutyrate elicits favorable mitochondrial changes in
skeletal muscle. Int J Mol Sci. 19:22472018. View Article : Google Scholar
|
|
74
|
Cogolludo A, Villamor E, Perez-Vizcaino F
and Moreno L: Ceramide and regulation of vascular tone. Int J Mol
Sci. 20:4112019. View Article : Google Scholar : PubMed/NCBI
|
|
75
|
Sletten AC, Peterson LR and Schaffer JE:
Manifestations and mechanisms of myocardial lipotoxicity in
obesity. J Intern Med. 284:478–491. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
76
|
Arsenault EJ, McGill CM and Barth BM:
Sphingolipids as regulators of neuro-inflammation and NADPH oxidase
2. Neuromolecular Med. 23:25–46. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
77
|
Patwardhan GA, Beverly LJ and Siskind LJ:
Sphingolipids and mitochondrial apoptosis. J Bioenerg Biomembr.
48:153–168. 2016. View Article : Google Scholar
|
|
78
|
Colombini M: Ceramide channels and
mitochondrial outer membrane permeability. J Bioenerg Biomembr.
49:57–64. 2017. View Article : Google Scholar
|
|
79
|
Cantalupo A, Sasset L, Gargiulo A,
Rubinelli L, Del Gaudio I, Benvenuto D, Wadsack C, Jiang XC, Bucci
MR and Di Lorenzo A: Endothelial sphingolipid de novo synthesis
controls blood pressure by regulating signal transduction and NO
via ceramide. Hypertension. 75:1279–1288. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
80
|
Pérez-Villavicencio R, Flores-Estrada J,
Franco M, Escalante B, Pérez-Méndez O, Mercado A and Bautista-Pérez
R: Effect of empagliflozin on sphingolipid catabolism in diabetic
and hypertensive rats. Int J Mol Sci. 23:28832022. View Article : Google Scholar : PubMed/NCBI
|
|
81
|
Lin YH, Jewell BE, Gingold J, Lu L, Zhao
R, Wang LL and Lee DF: Osteosarcoma: Molecular pathogenesis and
iPSC modeling. Trends Mol Med. 23:737–755. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
82
|
Altura BM, Gebrewold A, Carella A, Shah
NC, Shah GJ, Resnick LM and Altura BT: Why vasculitis probably can
be ameliorated with magnesium and antagonists of ceramides and
platelet-activating factor. MOJ Anat Physiol. 6:120–123. 2019.
|
|
83
|
Borodzicz-Jażdżyk S, Jażdżyk P, Łysik W,
Cudnoch-Jedrzejewska A and Czarzasta K: Sphingolipid metabolism and
signaling in cardiovascular diseases. Front Cardiovasc Med.
9:9159612022. View Article : Google Scholar
|
|
84
|
Zhang Y, Zhao H, Liu B, Shu H, Zhang L,
Bao M, Yi W, Tan Y, Ji X, Zhang C, et al: Human serum metabolomic
analysis reveals progression for high blood pressure in type 2
diabetes mellitus. BMJ Open Diabetes Res Care. 9:e0023372021.
View Article : Google Scholar : PubMed/NCBI
|
|
85
|
Li X, Wang HF, Li XX and Xu M:
Contribution of acid sphingomyelinase to angiotensin II-induced
vascular adventitial remodeling via membrane rafts/Nox2 signal
pathway. Life Sci. 219:303–310. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
86
|
Liu A, Chu YJ, Wang X, Yu R, Jiang H, Li
Y, Zhou H, Gong LL, Yang WQ and Ju J: Serum metabolomics study
based on LC-MS and antihypertensive effect of uncaria on
spontaneously hypertensive rats. Evid Based Complement Alternat
Med. 2018:92819462018. View Article : Google Scholar : PubMed/NCBI
|
|
87
|
Shu H, Peng Y, Hang W, Li N, Zhou N and
Wang DW: Emerging roles of ceramide in cardiovascular diseases.
Aging Dis. 13:232–245. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
88
|
Choi SR, Lim JH, Kim MY, Kim EN, Kim Y,
Choi BS, Kim YS, Kim HW, Lim KM, Kim MJ and Park CW: Adiponectin
receptor agonist AdipoRon decreased ceramide, and lipotoxicity, and
ameliorated diabetic nephropathy. Metabolism. 85:348–360. 2018.
View Article : Google Scholar : PubMed/NCBI
|
|
89
|
Yaribeygi H, Bo S, Ruscica M and Sahebkar
A: Ceramides and diabetes mellitus: An update on the potential
molecular relationships. Diabet Med. 37:11–19. 2020. View Article : Google Scholar
|
|
90
|
Kane JP, Pullinger CR, Goldfine ID and
Malloy MJ: Dyslipidemia and diabetes mellitus: Role of lipoprotein
species and inter-related pathways of lipid metabolism in diabetes
mellitus. Curr Opin Pharmacol. 61:21–27. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
91
|
Guitton J, Bandet CL, Mariko ML, Tan-Chen
S, Bourron O, Benomar Y, Hajduch E and Le Stunff H:
Sphingosine-1-phosphate metabolism in the regulation of
obesity/type 2 diabetes. Cells. 9:16822020. View Article : Google Scholar : PubMed/NCBI
|
|
92
|
Miller LG Jr, Young JA, Ray SK, Wang G,
Purohit S, Banik NL and Dasgupta S: Sphingosine toxicity in EAE and
MS: Evidence for ceramide generation via
serine-palmitoyltransferase activation. Neurochem Res.
42:2755–2768. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
93
|
Siskind LJ: Mitochondrial ceramide and the
induction of apoptosis. J Bioenerg Biomembr. 37:143–153. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
94
|
Mancini A, Imperlini E, Nigro E,
Montagnese C, Daniele A, Orrù S and Buono P: Biological and
nutritional properties of palm oil and palmitic acid: Effects on
health. Molecules. 20:17339–17361. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
95
|
Park IB, Kim MH, Han JS and Park WJ:
Gryllus bimaculatus extract protects against palmitate-induced
β-cell death by inhibiting ceramide synthesis. Appl Biol Chem.
65:722022. View Article : Google Scholar
|
|
96
|
Tong X, Chaudhry Z, Lee CC, Bone RN,
Kanojia S, Maddatu J, Sohn P, Weaver SA, Robertson MA, Petrache I,
et al: Cigarette smoke exposure impairs β-cell function through
activation of oxidative stress and ceramide accumulation. Mol
Metab. 37:1009752020. View Article : Google Scholar
|
|
97
|
Xu YN, Wang Z, Zhang SK, Xu JR, Pan ZX,
Wei X, Wen HH, Luo YS, Guo MJ and Zhu Q: Low-grade elevation of
palmitate and lipopolysaccharide synergistically induced β-cell
damage via inhibition of neutral ceramidase. Mol Cell Endocrinol.
539:1114732022. View Article : Google Scholar
|
|
98
|
Šrámek J, Němcová-Fürstová V and Kovář J:
Molecular mechanisms of apoptosis induction and its regulation by
fatty acids in pancreatic β-cells. Int J Mol Sci. 22:42852021.
View Article : Google Scholar
|
|
99
|
Canals D, Salamone S and Hannun YA:
Visualizing bioactive ceramides. Chem Phys Lipids. 216:142–151.
2018. View Article : Google Scholar : PubMed/NCBI
|
|
100
|
Marra F and Svegliati-Baroni G:
Lipotoxicity and the gut-liver axis in NASH pathogenesis. J
Hepatol. 68:280–295. 2018. View Article : Google Scholar
|
|
101
|
Meikle PJ and Summers SA: Sphingolipids
and phospholipids in insulin resistance and related metabolic
disorders. Nat Rev Endocrinol. 13:79–91. 2017. View Article : Google Scholar
|
|
102
|
Bandet CL, Tan-Chen S, Bourron O, Stunff
HL and Hajduch E: Sphingolipid metabolism: New insight into
ceramide-induced lipotoxicity in muscle cells. Int J Mol Sci.
20:4792019. View Article : Google Scholar : PubMed/NCBI
|
|
103
|
Fang Z, Pyne S and Pyne NJ: Ceramide and
sphingosine 1-phosphate in adipose dysfunction. Prog Lipid Res.
74:145–159. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
104
|
Bekhite M, González-Delgado A, Hübner S,
Haxhikadrija P, Kretzschmar T, Müller T, Wu JMF, Bekfani T, Franz
M, Wartenberg M, et al: The role of ceramide accumulation in human
induced pluripotent stem cell-derived cardiomyocytes on
mitochondrial oxidative stress and mitophagy. Free Radic Biol Med.
167:66–80. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
105
|
Chavez JA, Knotts TA, Wang LP, Li G,
Dobrowsky RT, Florant GL and Summers SA: A role for ceramide, but
not diacylglycerol, in the antagonism of insulin signal
transduction by saturated fatty acids. J Biol Chem.
278:10297–10303. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
106
|
Zalewska A, Maciejczyk M, Szulimowska J,
Imierska M and Błachnio-Zabielska A: High-fat diet affects ceramide
content, disturbs mitochondrial redox balance, and induces
apoptosis in the submandibular glands of mice. Biomolecules.
9:8772019. View Article : Google Scholar : PubMed/NCBI
|
|
107
|
Holland WL, Brozinick JT, Wang LP, Hawkins
ED, Sargent KM, Liu Y, Narra K, Hoehn KL, Knotts TA, Siesky A, et
al: Inhibition of ceramide synthesis ameliorates glucocorticoid-,
saturated-fat-, and obesity-induced insulin resistance. Cell Metab.
5:167–179. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
108
|
Petersen MC and Shulman GI: Mechanisms of
insulin action and insulin resistance. Physiol Rev. 98:2133–2223.
2018. View Article : Google Scholar : PubMed/NCBI
|
|
109
|
Gasparini SJ, Swarbrick MM, Kim S, Thai
LJ, Henneicke H, Cavanagh LL, Tu J, Weber MC, Zhou H and Seibel MJ:
Androgens sensitise mice to glucocorticoid-induced insulin
resistance and fat accumulation. Diabetologia. 62:1463–1477. 2019.
View Article : Google Scholar : PubMed/NCBI
|
|
110
|
Linn SC, Kim HS, Keane EM, Andras LM, Wang
E and Merrill AH Jr: Regulation of de novo sphingolipid
biosynthesis and the toxic consequences of its disruption. Biochem
Soc Trans. 29:831–835. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
111
|
Choi KM, Lee YS, Choi MH, Sin DM, Lee S,
Ji SY, Lee MK, Lee YM, Yun YP, Hong JT and Yoo HS: Inverse
relationship between adipocyte differentiation and ceramide level
in 3T3-L1 cells. Biol Pharm Bull. 34:912–916. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
112
|
Li Y, Talbot CL, Chandravanshi B, Ksiazek
A, Sood A, Chowdhury KH, Maschek JA, Cox J, Babu AKS, Paz HA, et
al: Cordyceps inhibits ceramide biosynthesis and improves insulin
resistance and hepatic steatosis. Sci Rep. 12:72732022. View Article : Google Scholar : PubMed/NCBI
|
|
113
|
Kumar DP, Caffrey R, Marioneaux J,
Santhekadur PK, Bhat M, Alonso C, Koduru SV, Philip B, Jain MR,
Giri SR, et al: The PPAR α/γ agonist saroglitazar improves insulin
resistance and steatohepatitis in a diet induced animal model of
nonalcoholic fatty liver disease. Sci Rep. 10:93302020. View Article : Google Scholar
|
|
114
|
Kucuk S, Niven J, Caamano J, Jones SW,
Camacho-Muñoz D, Nicolaou A and Mauro C: Unwrapping the mechanisms
of ceramide and fatty acid-initiated signals leading to
immune-inflammatory responses in obesity. Int J Biochem Cell Biol.
135:1059722021. View Article : Google Scholar : PubMed/NCBI
|
|
115
|
Gilbert M: Role of skeletal muscle lipids
in the pathogenesis of insulin resistance of obesity and type 2
diabetes. J Diabetes Investig. 12:1934–1941. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
116
|
Reidy PT, Mahmassani ZS, McKenzie AI,
Petrocelli JJ, Summers SA and Drummond MJ: Influence of exercise
training on skeletal muscle insulin resistance in aging: Spotlight
on muscle ceramides. Int J Mol Sci. 21:15142020. View Article : Google Scholar : PubMed/NCBI
|
|
117
|
Coen PM and Goodpaster BH: Role of
intramyocelluar lipids in human health. Trends Endocrinol Metab.
23:391–398. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
118
|
Galadari S, Rahman A, Pallichankandy S,
Galadari A and Thayyullathil F: Role of ceramide in diabetes
mellitus: Evidence and mechanisms. Lipids Health Dis. 12:982013.
View Article : Google Scholar : PubMed/NCBI
|
|
119
|
Choi RH, Tatum SM, Symons JD, Summers SA
and Holland WL: Ceramides and other sphingolipids as drivers of
cardiovascular disease. Nat Rev Cardiol. 18:701–711. 2021.
View Article : Google Scholar : PubMed/NCBI
|
|
120
|
Edsfeldt A, Dunér P, Ståhlman M, Mollet
IG, Asciutto G, Grufman AHM, Nitulescu M, Persson AF, Fisher RM,
Melander O, et al: Proinflammatory role of sphingolipids and
glycosphingolipids in the human atherosclerotic plaque.
Arterioscler Thromb Vasc Biol. 36:1132–1140. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
121
|
Wang P, Zeng G, Yan Y, Zhang SY, Dong Y,
Zhang Y, Zhang X, Liu H, Zhang Z, Jiang C and Pang Y: Disruption of
adipocyte HIF-1 α improves atherosclerosis through the inhibition
of ceramide generation. Acta Pharm Sin B. 12:1899–1912. 2022.
View Article : Google Scholar : PubMed/NCBI
|
|
122
|
Yang RX, Pan Q, Liu XL, Zhou D, Xin FZ,
Zhao ZH, Zhang RN, Zeng J, Qiao L, Hu CX, et al: Therapeutic effect
and autophagy regulation of myriocin in nonalcoholic
steatohepatitis. Lipids Health Dis. 18:1792019. View Article : Google Scholar : PubMed/NCBI
|
|
123
|
Zhang X, Zhang Y, Wang P, Zhang SY, Dong
Y, Zeng G, Yan Y, Sun L, Wu Q, Liu H, et al: Adipocyte
hypoxia-inducible factor 2α suppresses atherosclerosis by promoting
adipose ceramide catabolism. Cell Metab. 30:937–951.e5. 2019.
View Article : Google Scholar
|
|
124
|
Dany M, Gencer S, Nganga R, Thomas RJ,
Oleinik N, Baron KD, Szulc ZM, Ruvolo P, Kornblau S, Andreeff M and
Ogretmen B: Targeting FLT3-ITD signaling mediates
ceramide-dependent mitophagy and attenuates drug resistance in AML.
Blood. 128:1944–1958. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
125
|
Zhang Y, Huang NQ, Yan F, Jin H, Zhou SY,
Shi JS and Jin F: Diabetes mellitus and Alzheimer's disease: GSK-3β
as a potential link. Behav Brain Res. 339:57–65. 2018. View Article : Google Scholar
|
|
126
|
Yang Y, Xu G, Xu Y, Cheng X, Xu S, Chen S
and Wu L: Ceramide mediates radiation-induced germ cell apoptosis
via regulating mitochondria function and MAPK factors in
caenorhabditis elegans. Ecotoxicol Environ Saf. 208:1115792021.
View Article : Google Scholar : PubMed/NCBI
|
|
127
|
Ganesan V, Perera MN, Colombini D,
Datskovskiy D, Chadha K and Colombini M: Ceramide and activated Bax
act synergistically to permeabilize the mitochondrial outer
membrane. Apoptosis. 15:553–562. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
128
|
James BN, Oyeniran C, Sturgill JL, Newton
J, Martin RK, Bieberich E, Weigel C, Maczis MA, Palladino END,
Lownik JC, et al: Ceramide in apoptosis and oxidative stress in
allergic inflammation and asthma. J Allergy Clin Immunol.
147:1936–1948.e9. 2021. View Article : Google Scholar :
|
|
129
|
Römer A, Linn T and Petry SF: Lipotoxic
impairment of mitochondrial function in β-cells: A review.
Antioxidants (Basel). 10:2932021. View Article : Google Scholar
|
|
130
|
Onyango AN: Cellular stresses and stress
responses in the pathogenesis of insulin resistance. Oxid Med Cell
Longev. 2018:43217142018. View Article : Google Scholar : PubMed/NCBI
|
|
131
|
Ueda N: A rheostat of ceramide and
sphingosine-1-phosphate as a determinant of oxidative
stress-mediated kidney injury. Int J Mol Sci. 23:40102022.
View Article : Google Scholar : PubMed/NCBI
|
|
132
|
Poole LP and Macleod KF: Mitophagy in
tumorigenesis and metastasis. Cell Mol Life Sci. 78:3817–3851.
2021. View Article : Google Scholar : PubMed/NCBI
|
|
133
|
Srivastava S and Chan C: Hydrogen peroxide
and hydroxyl radicals mediate palmitate-induced cytotoxicity to
hepatoma cells: Relation to mitochondrial permeability transition.
Free Radic Res. 41:38–49. 2007. View Article : Google Scholar
|
|
134
|
Law BA, Liao X, Moore KS, Southard A,
Roddy P, Ji R, Szulc Z, Bielawska A, Schulze PC and Cowart LA:
Lipotoxic very-long-chain ceramides cause mitochondrial
dysfunction, oxidative stress, and cell death in cardiomyocytes.
FASEB J. 32:1403–1416. 2018. View Article : Google Scholar :
|
|
135
|
Botta A, Elizbaryan K, Tashakorinia P, Lam
NH and Sweeney G: An adiponectin-S1P autocrine axis protects
skeletal muscle cells from palmitate-induced cell death. Lipids
Health Dis. 19:1562020. View Article : Google Scholar : PubMed/NCBI
|
|
136
|
Simon JN, Chowdhury SAK, Warren CM,
Sadayappan S, Wieczorek DF, Solaro RJ and Wolska BM:
Ceramide-mediated depression in cardiomyocyte contractility through
PKC activation and modulation of myofilament protein
phosphorylation. Basic Res Cardiol. 109:4452014. View Article : Google Scholar : PubMed/NCBI
|
|
137
|
Kim C and Kim B: Anti-cancer natural
products and their bioactive compounds inducing ER stress-mediated
apoptosis: A review. Nutrients. 10:10212018. View Article : Google Scholar : PubMed/NCBI
|
|
138
|
Hu H, Tian M, Ding C and Yu S: The C/EBP
homologous protein (CHOP) transcription factor functions in
endoplasmic reticulum stress-induced apoptosis and microbial
infection. Front Immunol. 9:30832019. View Article : Google Scholar : PubMed/NCBI
|
|
139
|
Xiang C, Wang Y, Zhang H and Han F: The
role of endoplasmic reticulum stress in neurodegenerative disease.
Apoptosis. 22:1–26. 2017. View Article : Google Scholar
|
|
140
|
Szpigel A, Hainault I, Carlier A,
Venteclef N, Batto AF, Hajduch E, Bernard C, Ktorza A, Gautier JF,
Ferré P, et al: Lipid environment induces ER stress, TXNIP
expression and inflammation in immune cells of individuals with
type 2 diabetes. Diabetologia. 61:399–412. 2018. View Article : Google Scholar
|
|
141
|
Xu G, Chen J, Jing G, Grayson TB and
Shalev A: miR-204 targets PERK and regulates UPR signaling and
β-cell apoptosis. Mol Endocrinol. 30:917–924. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
142
|
Ojo OA, Grant S, Amanze JC, Oni AI, Ojo
AB, Elebiyo TC, Obafemi TO, Ayokunle DI and Ogunlakin AD: Annona
muricata L. peel extract inhibits carbohydrate metabolizing enzymes
and reduces pancreatic β-cells, inflammation, and apoptosis via
upregulation of PI3K/AKT genes. PLoS One. 17:e02769842022.
View Article : Google Scholar
|
|
143
|
Wang Y, Liu J, Akatsu C, Zhang R, Zhang H,
Zhu H, Liu K, Zhu HY, Min Q, Meng X, et al: LAPTM5 mediates
immature B cell apoptosis and B cell tolerance by regulating the
WWP2-PTEN-AKT pathway. Proc Natl Acad Sci USA. 119:e22056291192022.
View Article : Google Scholar : PubMed/NCBI
|
|
144
|
Hsu CM, Lin JJ, Su JH and Liu CI:
13-Acetoxysarcocrassolide induces apoptosis in human hepatocellular
carcinoma cells through mitochondrial dysfunction and suppression
of the PI3K/AKT/mTOR/p70S6K signalling pathway. Pharm Biol.
60:2276–2285. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
145
|
Cui F and He X: IGF-1 ameliorates
streptozotocin-induced pancreatic β cell dysfunction and apoptosis
via activating IRS1/PI3K/Akt/FOXO1 pathway. Inflamm Res.
71:669–680. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
146
|
Denhez B, Rousseau M, Spino C, Dancosst
DA, Dumas MÈ, Guay A, Lizotte F and Geraldes P: Saturated fatty
acids induce insulin resistance in podocytes through inhibition of
IRS1 via activation of both IKKβ and mTORC1. Sci Rep. 10:216282020.
View Article : Google Scholar
|
|
147
|
Jennemann R, Kaden S, Volz M, Nordström V,
Herzer S, Sandhoff R and Gröne HJ: Gangliosides modulate insulin
secretion by pancreatic beta cells under glucose stress.
Glycobiology. 30:722–734. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
148
|
Benito-Vicente A, Jebari-Benslaiman S,
Galicia-Garcia U, Larrea-Sebal A, Uribe KB and Martin C: Molecular
mechanisms of lipotoxicity-induced pancreatic β-cell dysfunction.
Int Rev Cell Mol Biol. 359:357–402. 2021. View Article : Google Scholar
|
|
149
|
Huang X, Liu G, Guo J and Su Z: The
PI3K/AKT pathway in obesity and type 2 diabetes. Int J Biol Sci.
14:1483–1496. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
150
|
Obanda DN, Ribnicky D, Yu Y, Stephens J
and Cefalu WT: An extract of Urtica dioica L. mitigates obesity
induced insulin resistance in mice skeletal muscle via protein
phosphatase 2A (PP2A). Sci Rep. 6:222222016. View Article : Google Scholar : PubMed/NCBI
|
|
151
|
Li J, Huang J, Lu J, Guo Z, Li Z, Gao H,
Wang P, Luo W, Cai S, Hu Y, et al: Sirtuin 1 represses PKC-ζ
activity through regulating interplay of acetylation and
phosphorylation in cardiac hypertrophy. Br J Pharmacol.
176:416–435. 2019.
|
|
152
|
Ivey RA, Sajan MP and Farese RV:
Requirements for pseudosubstrate arginine residues during
autoinhibition and phosphatidylinositol 3,4,5-(PO4)3-dependent
activation of atypical PKC. J Biol Chem. 289:25021–25030. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
153
|
Campana M, Bellini L, Rouch C, Rachdi L,
Coant N, Butin N, Bandet CL, Philippe E, Meneyrol K, Kassis N, et
al: Inhibition of central de novo ceramide synthesis restores
insulin signaling in hypothalamus and enhances β-cell function of
obese Zucker rats. Mol Metab. 8:23–36. 2018. View Article : Google Scholar
|
|
154
|
Wali JA, Jarzebska N, Raubenheimer D,
Simpson SJ, Rodionov RN and O'Sullivan JF: Cardio-metabolic effects
of high-fat diets and their underlying mechanisms-a narrative
review. Nutrients. 12:15052020. View Article : Google Scholar : PubMed/NCBI
|
|
155
|
Huang H, Aminian A, Hassan M, Dan O,
Axelrod CL, Schauer PR, Brethauer SA and Kirwan JP: Gastric bypass
surgery improves the skeletal muscle ceramide/S1P ratio and
upregulates the AMPK/SIRT1/PGC-1α pathway in Zucker diabetic fatty
rats. Obes Surg. 29:2158–2165. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
156
|
Matsuzaka T, Kuba M, Koyasu S, Yamamoto Y,
Motomura K, Arulmozhiraja S, Ohno H, Sharma R, Shimura T, Okajima
Y, et al: Hepatocyte ELOVL fatty acid elongase 6 determines
ceramide Acyl-chain length and hepatic insulin sensitivity in mice.
Hepatology. 71:1609–1625. 2020. View Article : Google Scholar
|
|
157
|
Yazıcı D and Sezer H: Insulin resistance,
obesity and lipotoxicity. Engin AB and Engin A: Obesity and
Lipotoxicity. Advances in Experimental Medicine and Biology. 960.
Springer International Publishing; pp. 277–304. 2017, View Article : Google Scholar
|
|
158
|
Xia QS, Lu FE, Wu F, Huang ZY, Dong H, Xu
LJ and Gong J: New role for ceramide in hypoxia and insulin
resistance. World J Gastroenterol. 26:2177–2186. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
159
|
Obata Y, Kita S, Koyama Y, Fukuda S,
Takeda H, Takahashi M, Fujishima Y, Nagao H, Masuda S, Tanaka Y, et
al: Adiponectin/T-cadherin system enhances exosome biogenesis and
decreases cellular ceramides by exosomal release. JCI Insight.
3:e996802018. View Article : Google Scholar : PubMed/NCBI
|
|
160
|
Santovito D, De Nardis V, Marcantonio P,
Mandolini C, Paganelli C, Vitale E, Buttitta F, Bucci M, Mezzetti
A, Consoli A and Cipollone F: Plasma exosome microRNA profiling
unravels a new potential modulator of adiponectin pathway in
diabetes: Effect of glycemic control. J Clin Endocrinol Metab.
99:E1681–E1685. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
161
|
Ying W, Riopel M, Bandyopadhyay G, Dong Y,
Birmingham A, Seo JB, Ofrecio JM, Wollam J, Hernandez-Carretero A,
Fu W, et al: Adipose tissue macrophage-derived exosomal miRNAs can
modulate in vivo and in vitro insulin sensitivity. Cell.
171:372–384.e12. 2017. View Article : Google Scholar
|
|
162
|
Tian F, Tang P, Sun Z, Zhang R, Zhu D, He
J, Liao J, Wan Q and Shen J: miR-210 in exosomes derived from
macrophages under high glucose promotes mouse diabetic obesity
pathogenesis by suppressing NDUFA4 expression. J Diabetes Res.
2020:68946842020. View Article : Google Scholar : PubMed/NCBI
|
|
163
|
Ruiz-León AM, Lapuente M, Estruch R and
Casas R: Clinical advances in immunonutrition and atherosclerosis:
A review. Front Immunol. 10:8372019. View Article : Google Scholar : PubMed/NCBI
|
|
164
|
Geovanini GR and Libby P: Atherosclerosis
and inflammation: Overview and updates. Clin Sci (Lond).
132:1243–1252. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
165
|
Ho QWC, Zheng X and Ali Y: Ceramide Acyl
chain length and its relevance to intracellular lipid regulation.
Int J Mol Sci. 23:96972022. View Article : Google Scholar : PubMed/NCBI
|
|
166
|
Sindhu S, Leung YH, Arefanian H, Madiraju
SRM, Al-Mulla F, Ahmad R and Prentki M: Neutral sphingomyelinase-2
and cardiometabolic diseases. Obes Rev. 22:e132482021. View Article : Google Scholar : PubMed/NCBI
|
|
167
|
Chen X, Guo X, Ge Q, Zhao Y, Mu H and
Zhang J: ER stress activates the NLRP3 inflammasome: A novel
mechanism of atherosclerosis. Oxid Med Cell Longev.
2019:34625302019. View Article : Google Scholar : PubMed/NCBI
|
|
168
|
Alaaeldin R, Abdel-Rahman IAM, Hassan HA,
Youssef N, Allam AE, Abdelwahab SF, Zhao QL and Fathy M:
Carpachromene ameliorates insulin resistance in HepG2 cells via
modulating IR/IRS1/PI3k/Akt/GSK3/FoxO1 pathway. Molecules.
26:76292021. View Article : Google Scholar : PubMed/NCBI
|
|
169
|
Gündüz D, Troidl C, Tanislav C, Rohrbach
S, Hamm C and Aslam M: Role of PI3K/Akt and MEK/ERK signalling in
cAMP/Epac-mediated endothelial barrier stabilisation. Front
Physiol. 10:13872019. View Article : Google Scholar : PubMed/NCBI
|
|
170
|
Prasad M, Gatasheh MK, Alshuniaber MA,
Krishnamoorthy R, Rajagopal P, K rishnamoor thy K, Periyasamy V,
Veeraraghavan VP and Jayaraman S: Impact of glyphosate on the
development of insulin resistance in experimental diabetic rats:
Role of NFκB signalling pathways. Antioxidants (Basel).
11:24362022. View Article : Google Scholar
|
|
171
|
Wright CJ, McKenna S, De Dios R, Boehmer
BH, Nguyen L, Ghosh S, Sandoval J and Rozance PJ: Lower threshold
to NFκB activity sensitizes murine β-cells to streptozotocin. J
Endocrinol. 249:163–175. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
172
|
Lin Z, Ge J, Wang Z, Ren J, Wang X, Xiong
H, Gao J, Zhang Y and Zhang Q: Let-7e modulates the inflammatory
response in vascular endothelial cells through ceRNA crosstalk. Sci
Rep. 7:424982017. View Article : Google Scholar : PubMed/NCBI
|
|
173
|
Olona A, Hateley C, Muralidharan S, Wenk
MR, Torta F and Behmoaras J: Sphingolipid metabolism during
Toll-like receptor 4 (TLR4)-mediated macrophage activation. Br J
Pharmacol. 178:4575–4587. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
174
|
Engin A: The pathogenesis of
obesity-associated adipose tissue inflammation. Obesity and
Lipotoxicity. Advances in Experimental Medicine and Biology. Engin
AB and Engin A: 960. Springer International Publishing; pp.
221–245. 2017, View Article : Google Scholar
|
|
175
|
Berg M, Polyzos KA, Agardh H, Baumgartner
R, Forteza MJ, Kareinen I, Gisterå A, Bottcher G, Hurt-Camejo E,
Hansson GK and Ketelhuth DFJ: 3-Hydroxyanthralinic acid metabolism
controls the hepatic SREBP/lipoprotein axis, inhibits inflammasome
activation in macrophages, and decreases atherosclerosis in Ldlr-/-
mice. Cardiovasc Res. 116:1948–1957. 2020. View Article : Google Scholar
|
|
176
|
Hornemann T and Worgall TS: Sphingolipids
and atherosclerosis. Atherosclerosis. 226:16–28. 2013. View Article : Google Scholar
|
|
177
|
Dekker MJ, Baker C, Naples M, Samsoondar
J, Zhang R, Qiu W, Sacco J and Adeli K: Inhibition of sphingolipid
synthesis improves dyslipidemia in the diet-induced hamster model
of insulin resistance: Evidence for the role of sphingosine and
sphinganine in hepatic VLDL-apoB100 overproduction.
Atherosclerosis. 228:98–109. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
178
|
Yang J and Liu Z: Mechanistic pathogenesis
of endothelial dysfunction in diabetic nephropathy and retinopathy.
Front Endocrinol (Lausanne). 13:8164002022. View Article : Google Scholar : PubMed/NCBI
|
|
179
|
Sharma S, Schaper N and Rayman G:
Microangiopathy: Is it relevant to wound healing in diabetic foot
disease? Diabetes Metab Res Rev. 36(Suppl 1): e32442020. View Article : Google Scholar
|
|
180
|
Wang S, Lei B, Zhang E, Gong P, Gu J, He
L, Han L and Yuan Z: Targeted therapy for inflammatory diseases
with mesenchymal stem cells and their derived exosomes: From basic
to clinics. Int J Nanomedicine. 17:1757–1781. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
181
|
Gil CL, Hooker E and Larrivée B: Diabetic
kidney disease, endothelial damage, and podocyte-endothelial
crosstalk. Kidney Med. 3:105–115. 2020. View Article : Google Scholar
|
|
182
|
Zhao WN, Xu SQ, Liang JF, Peng L, Liu HL,
Wang Z, Fang Q, Wang M, Yin WQ, Zhang WJ and Lou JN: Endothelial
progenitor cells from human fetal aorta cure diabetic foot in a rat
model. Metabolism. 65:1755–1767. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
183
|
Basra R, Papanas N, Farrow F, Karalliedde
J and Vas P: Diabetic foot ulcers and cardiac autonomic neuropathy.
Clin Ther. 44:323–330. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
184
|
King RJ, Harrison L, Gilbey SG,
Santhakumar A, Wyatt J, Jones R and Bodansky HJ: Diabetic
hepatosclerosis: Another diabetes microvascular complication?
Diabet Med. 33:e5–e7. 2016. View Article : Google Scholar
|