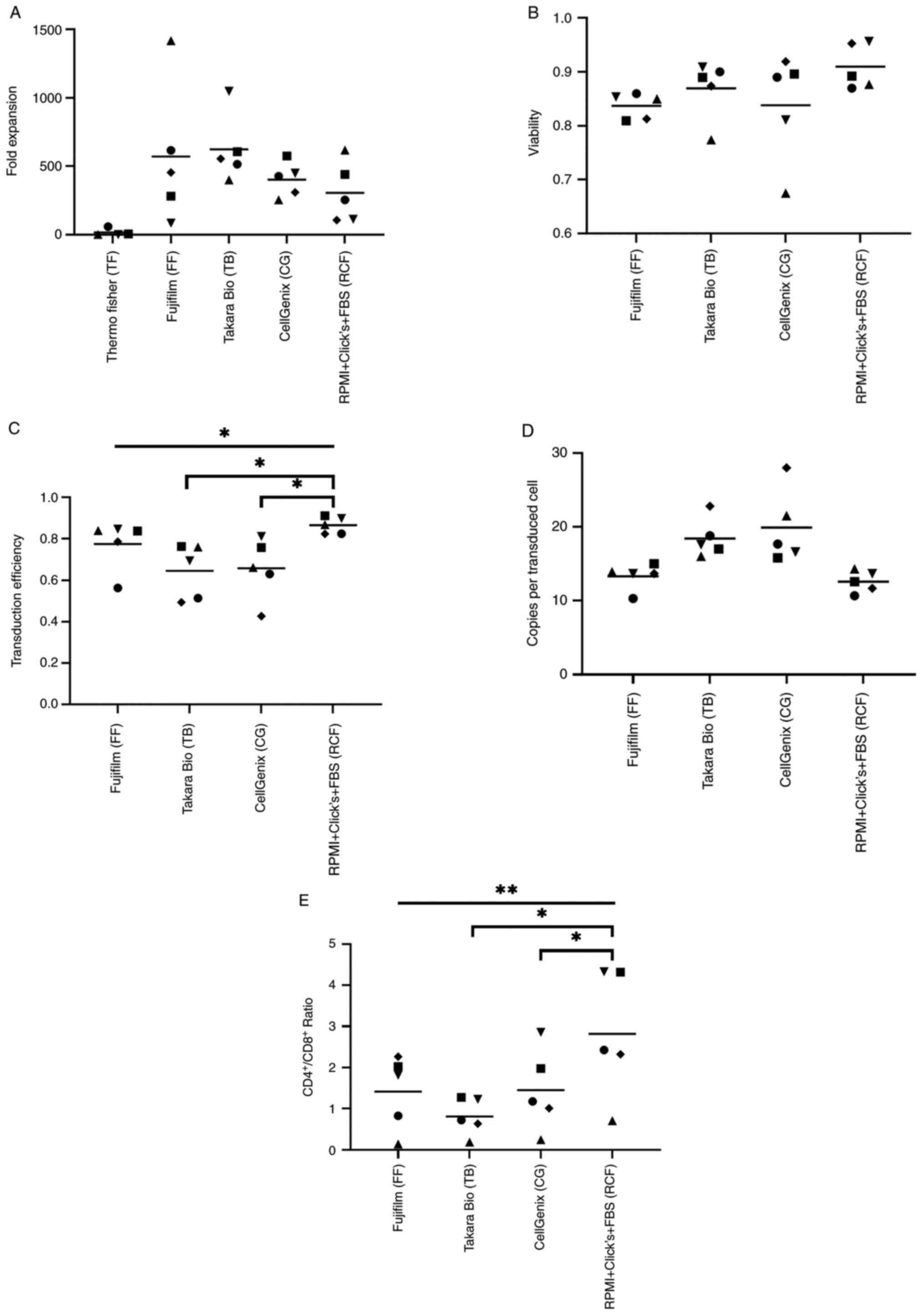
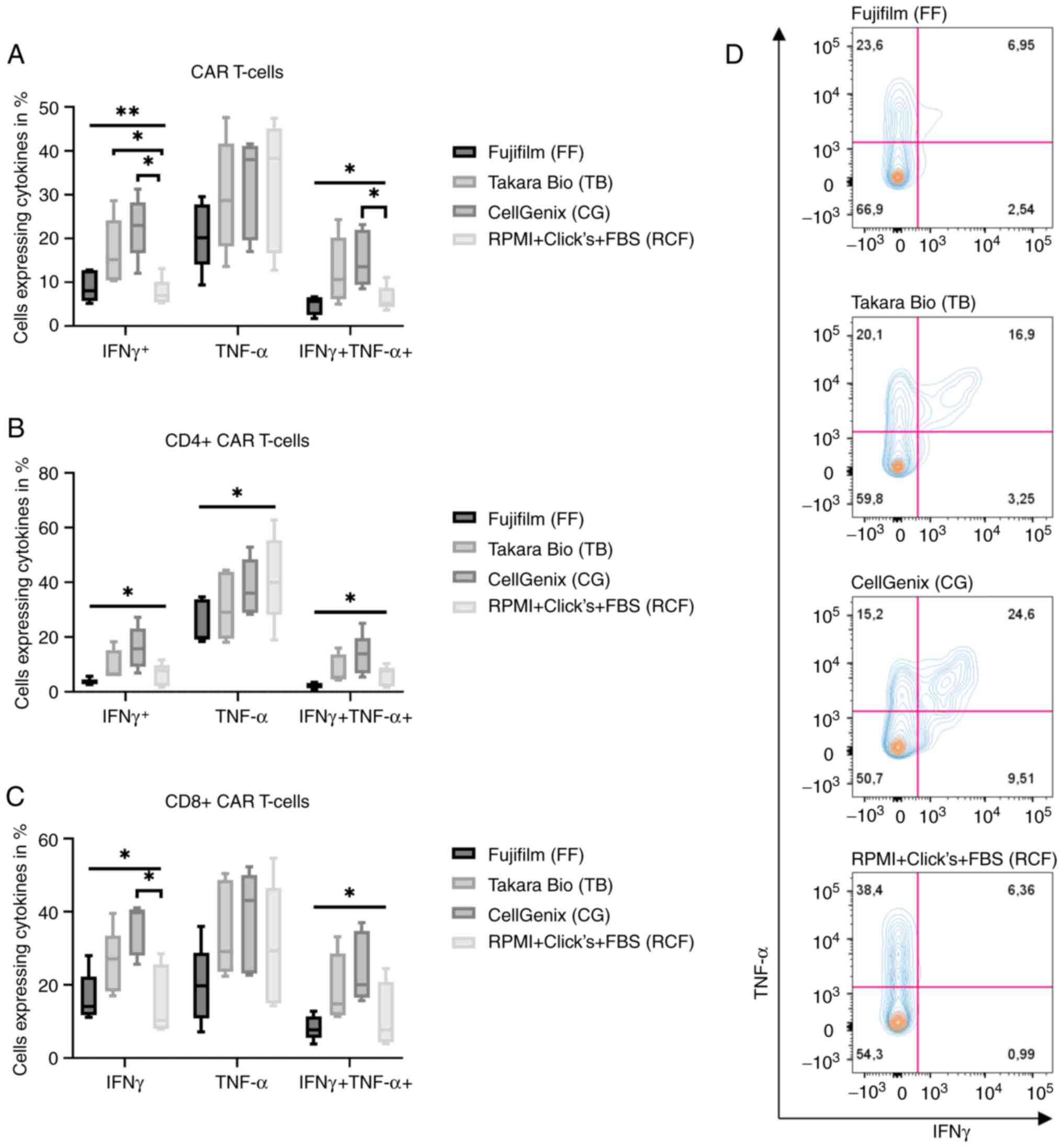
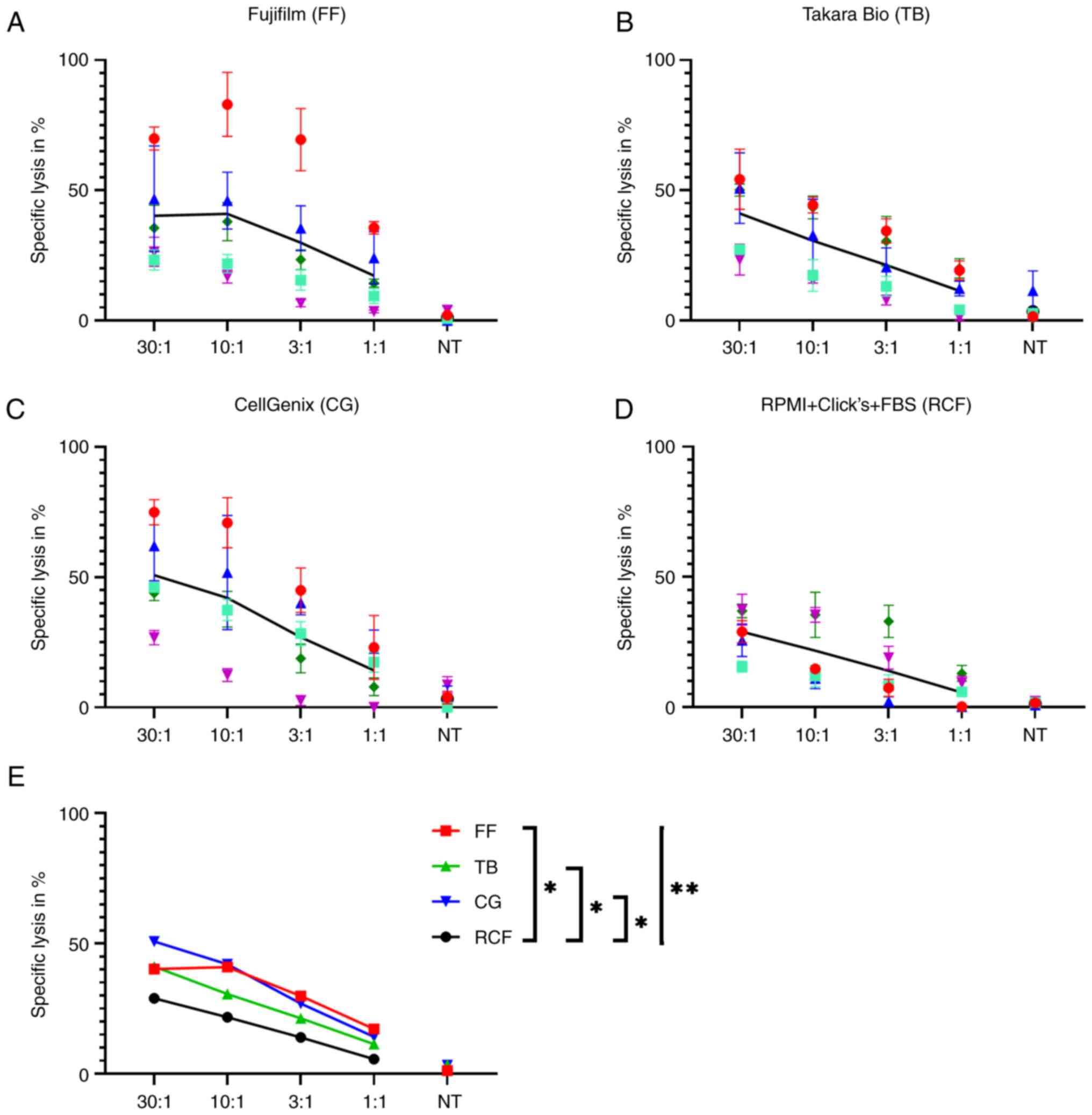
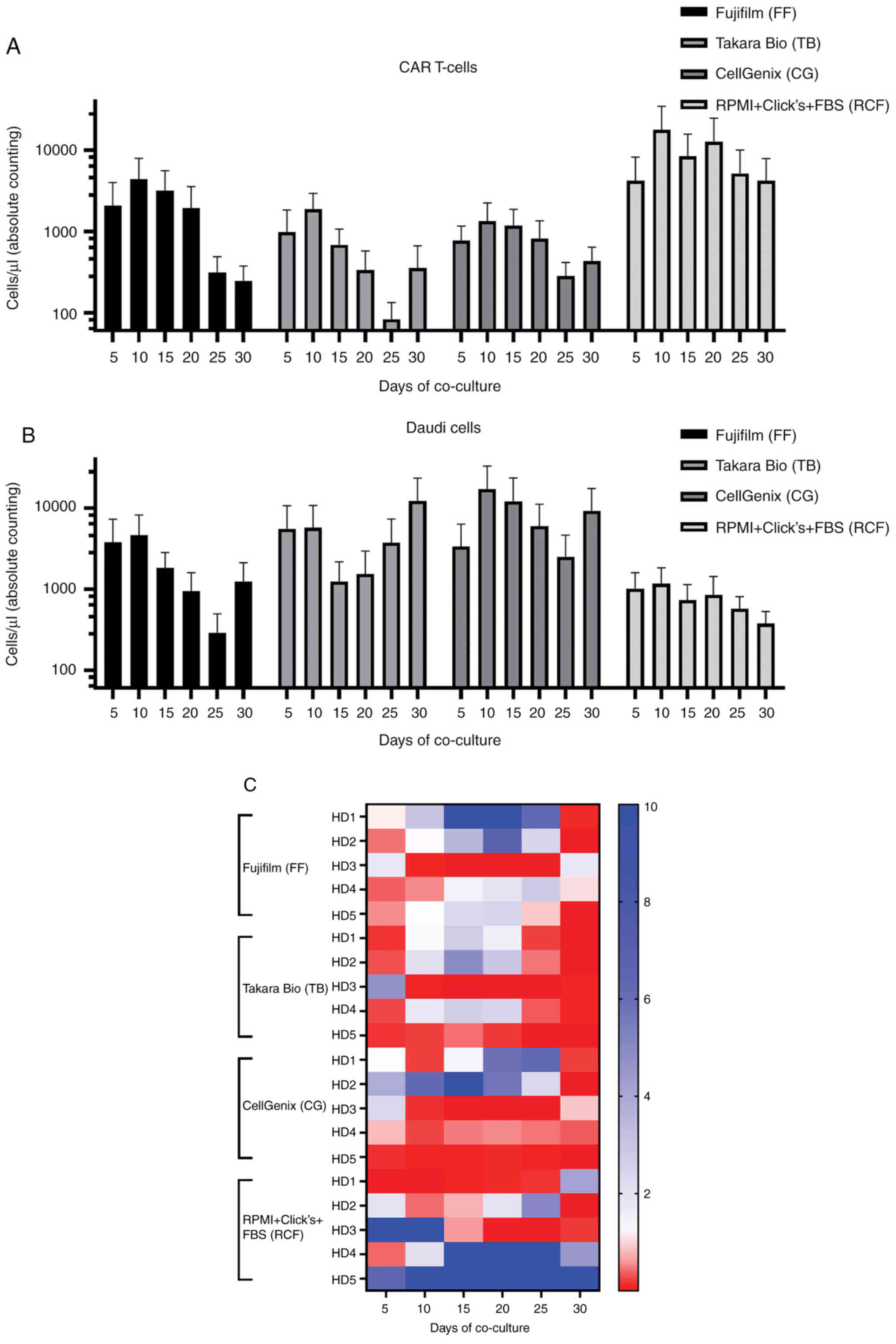
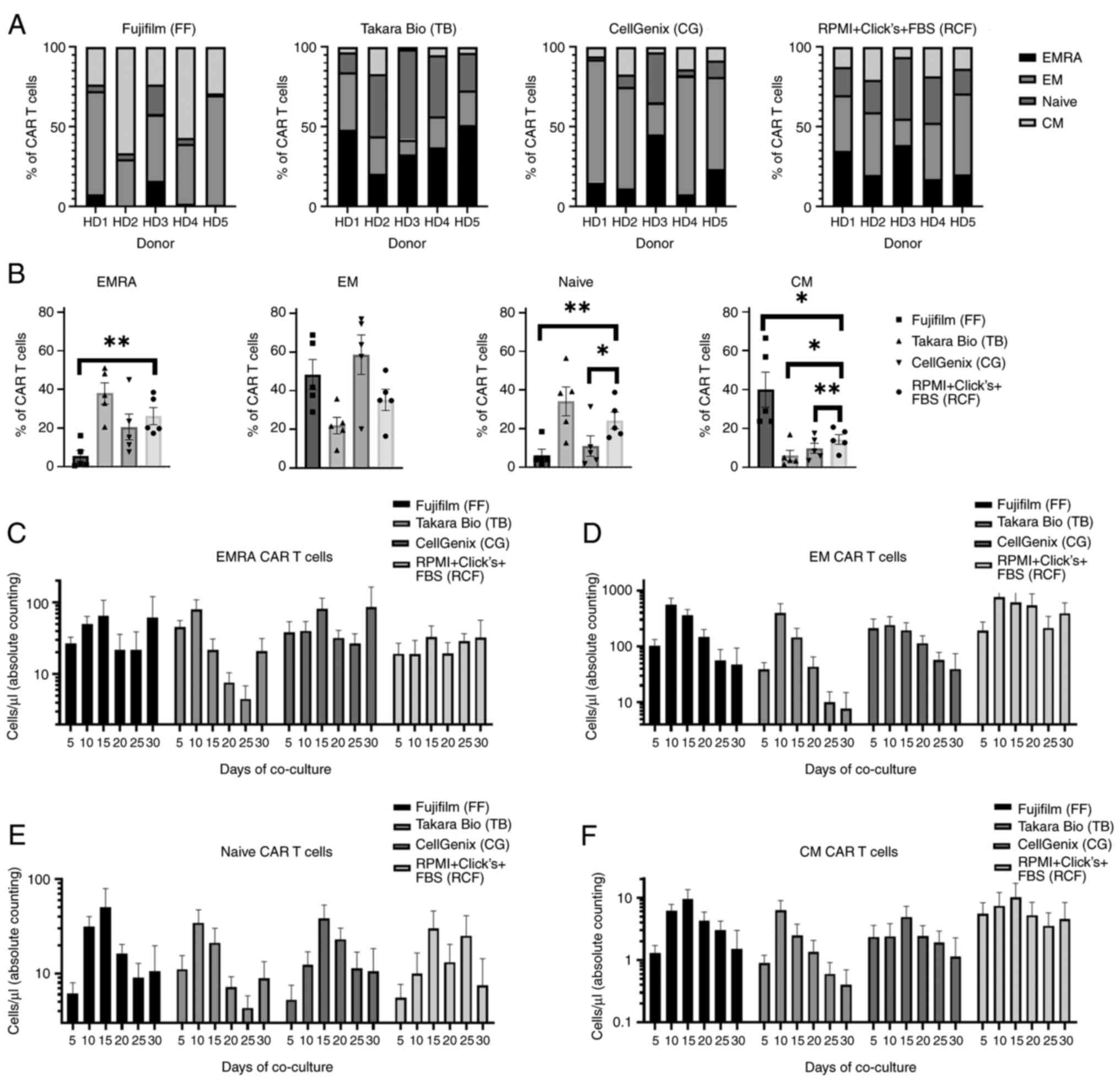
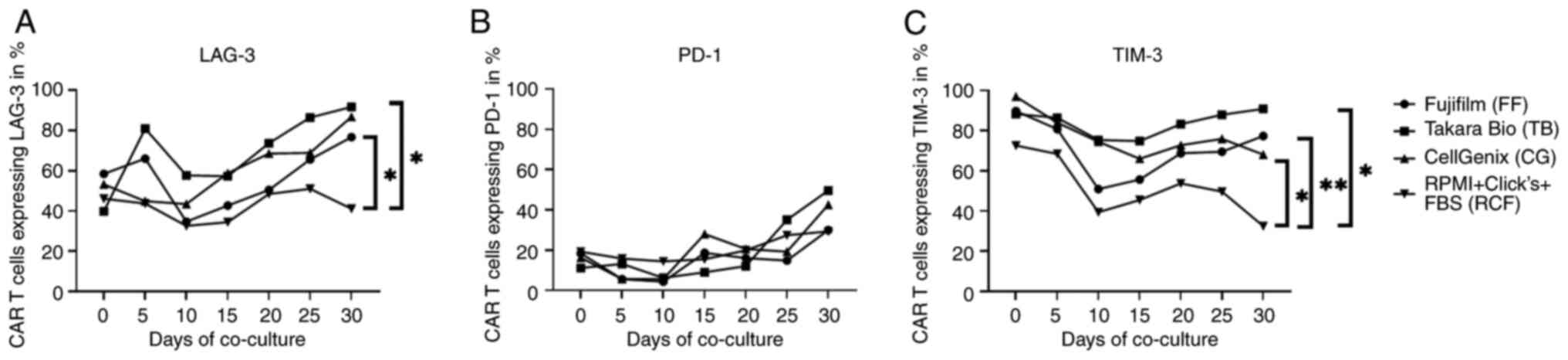
|
1
|
Maude SL, Laetsch TW, Buechner J, Rives S,
Boyer M, Bittencourt H, Bader P, Verneris MR, Stefanski HE, Myers
GD, et al: Tisagenlecleucel in Children and young adults with
B-cell lymphoblastic leukemia. N Engl J Med. 378:439–448. 2018.
View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Schuster SJ, Svoboda J, Chong EA, Nasta
SD, Mato AR, Anak Ö, Brogdon JL, Pruteanu-Malinici I, Bhoj V,
Landsburg D, et al: Chimeric antigen receptor T cells in refractory
B-cell lymphomas. N Engl J Med. 377:2545–2554. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Neelapu SS, Locke FL, Bartlett NL, Lekakis
LJ, Miklos DB, Jacobson CA, Braunschweig I, Oluwole OO, Siddiqi T,
Lin Y, et al: Axicabtagene ciloleucel CAR T-cell therapy in
refractory large B-cell lymphoma. N Engl J Med. 377:2531–2544.
2017. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Locke FL, Ghobadi A, Jacobson CA, Miklos
DB, Lekakis LJ, Oluwole OO, Lin Y, Braunschweig I, Hill BT,
Timmerman JM, et al: Long-term safety and activity of axicabtagene
ciloleucel in refractory large B-cell lymphoma (ZUMA-1): A
single-arm, multicentre, phase 1-2 trial. Lancet Oncol. 20:31–42.
2019. View Article : Google Scholar :
|
|
5
|
Kochenderfer JN, Dudley ME, Kassim SH,
Somerville RP, Carpenter RO, Stetler-Stevenson M, Yang JC, Phan GQ,
Hughes MS, Sherry RM, et al: Chemotherapy-refractory diffuse large
B-cell lymphoma and indolent B-cell malignancies can be effectively
treated with autologous T cells expressing an anti-CD19 chimeric
antigen receptor. J Clin Oncol. 33:540–549. 2015. View Article : Google Scholar :
|
|
6
|
Beyar-Katz O and Gill S: Advances in
chimeric antigen receptor T cells. Curr Opin Hematol. 27:368–377.
2020. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Braendstrup P, Levine BL and Ruella M: The
long road to the first FDA-approved gene therapy: Chimeric antigen
receptor T cells targeting CD19. Cytotherapy. 22:57–69. 2020.
View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Crees ZD and Ghobadi A: Cellular therapy
updates in B-cell lymphoma: The state of the CAR-T. Cancers
(Basel). 13. pp. 51812021, View Article : Google Scholar
|
|
9
|
Schubert ML, Schmitt A, Sellner L, Neuber
B, Kunz J, Wuchter P, Kunz A, Gern U, Michels B, Hofmann S, et al:
Treatment of patients with relapsed or refractory CD19+ lymphoid
disease with T lymphocytes transduced by RV-SFG.CD19.CD28.4-1BBzeta
retroviral vector: A unicentre phase I/II clinical trial protocol.
BMJ Open. 9:e0266442019. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Abramson JS: Anti-CD19 CAR T-cell therapy
for B-cell non-Hodgkin lymphoma. Transfus Med Rev. 34:29–33. 2020.
View Article : Google Scholar
|
|
11
|
Enblad G, Karlsson H, Gammelgård G, Wenthe
J, Lövgren T, Amini RM, Wikstrom KI, Essand M, Savoldo B, Hallböök
H, et al: A phase I/IIa trial using CD19-targeted third-generation
CAR T cells for lymphoma and leukemia. Clin Cancer Res.
24:6185–6194. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Wang L, Gong W, Wang S, Neuber B, Sellner
L, Schubert ML, Hückelhoven-Krauss A, Kunz A, Gern U, Michels B, et
al: Improvement of in vitro potency assays by a resting step for
clinical-grade chimeric antigen receptor engineered T cells.
Cytotherapy. 21:566–578. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Heger JI, Froehlich K, Pastuschek J and
Schmidt A, Baer C, Mrowka R, Backsch C, Schleußner E, Markert UR
and Schmidt A: Human serum alters cell culture behavior and
improves spheroid formation in comparison to fetal bovine serum.
Exp Cell Res. 365:57–65. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Brentjens RJ, Rivière I, Park JH, Davila
ML, Wang X, Stefanski J, Taylor C, Yeh R, Bartido S, Borquez-Ojeda
O, et al: Safety and persistence of adoptively transferred
autologous CD19-targeted T cells in patients with relapsed or
chemotherapy refractory B-cell leukemias. Blood. 118:4817–4828.
2011. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Hollyman D, Stefanski J, Przybylowski M,
Bartido S, Borquez-Ojeda O, Taylor C, Yeh R, Capacio V, Olszewska
M, Hosey J, et al: Manufacturing validation of biologically
functional T cells targeted to CD19 antigen for autologous adoptive
cell therapy. J Immunother. 32:169–180. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Kalos M, Levine BL, Porter DL, Katz S,
Grupp SA, Bagg A and June CH: T cells with chimeric antigen
receptors have potent antitumor effects and can establish memory in
patients with advanced leukemia. Sci Transl Med. 3:95ra732011.
View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Alnabhan R, Gaballa A, Mörk LM, Mattsson
J, Uhlin M and Magalhaes I: Media evaluation for production and
expansion of anti-CD19 chimeric antigen receptor T cells.
Cytotherapy. 20:941–951. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Ghassemi S, Martinez-Becerra FJ, Master
AM, Richman SA, Heo D, Leferovich J, Tu Y, García-Cañaveras JC,
Ayari A, Lu Y, et al: Enhancing chimeric antigen receptor T cell
anti-tumor function through advanced media design. Mol Ther Methods
Clin Dev. 18:595–606. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Smith C, Økern G, Rehan S, Beagley L, Lee
SK, Aarvak T, Schjetne KW and Khanna R: Ex vivo expansion of human
T cells for adoptive immunotherapy using the novel xeno-free CTS
immune cell serum replacement. Clin Transl Immunology. 4:e312015.
View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Coeshott C, Vang B, Jones M and Nankervis
B: Large-scale expansion and characterization of CD3+
T-cells in the quantum® cell expansion system. J Transl
Med. 17:2582019. View Article : Google Scholar
|
|
21
|
Lamoreaux L, Roederer M and Koup R:
Intracellular cytokine optimization and standard operating
procedure. Nat Protoc. 1:1507–1516. 2006. View Article : Google Scholar
|
|
22
|
Kunz A, Gern U, Schmitt A, Neuber B, Wang
L, Hückelhoven-Krauss A, Michels B, Hofmann S, Müller-Tidow C,
Dreger P, et al: Optimized assessment of qPCR-based vector copy
numbers as a safety parameter for GMP-grade CAR T cells and
monitoring of frequency in patients. Mol Ther Methods Clin Dev.
17:448–454. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Livark KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(-Delta Delta C(T)) method. Methods. 25:402–408. 2001.
View Article : Google Scholar
|
|
24
|
Díez JM, Bauman E, Gajardo R and Jorquera
JI: Culture of human mesenchymal stem cells using a candidate
pharmaceutical grade xeno-free cell culture supplement derived from
industrial human plasma pools. Stem Cell Res Ther. 6:282015.
View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Olsen H, Andersen A, Nordbø A, Kongsgaard
UE and Børmer OP: Pharmaceutical-grade albumin: Impaired
drug-binding capacity in vitro. BMC Clin Pharmacol. 4:42004.
View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Park J, Kim MS, Park T, Kim YH and Shin
DH: Crystal structure of pharmaceutical-grade human serum albumin.
Int J Biol Macromol. 166:221–228. 2021. View Article : Google Scholar
|
|
27
|
Sumi A, Okuyama K, Kobayashi K, Ohtani W,
Ohmura T and Yokoyama K: Purification of recombinant human serum
albumin efficient purification using STREAMLINE. Bioseparation.
8:195–200. 1999. View Article : Google Scholar
|
|
28
|
Ohtani W, Masaki A, Ikeda Y, Hirose M,
Chuganji M, Takeshima K, Kondo M, Sumi A and Ohmura T: Structure of
recombinant human serum albumin from Pichia pastoris. Yakugaku
Zasshi. 117:220–232. 1997.In Japanese. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Watanabe H, Yamasaki K, Kragh-Hansen U,
Tanase S, Harada K, Suenaga A and Otagiri M: In vitro and in vivo
properties of recombinant human serum albumin from Pichia pastoris
purified by a method of short processing time. Pharm Res.
18:1775–1781. 2001. View Article : Google Scholar
|
|
30
|
European Medicines Agency: Guideline on
potency testing of cell based immunotherapy medicinal products for
the treatment of cancer. 2016.
|
|
31
|
Food and Drug Administration: Guidance for
industry: Potency tests for cellular and gene therapy products.
2011.
|
|
32
|
Brudno JN, Lam N, Vanasse D, Shen YW, Rose
JJ, Rossi J, Xue A, Bot A, Scholler N, Mikkilineni L, et al: Safety
and feasibility of anti-CD19 CAR T cells with fully human binding
domains in patients with B-cell lymphoma. Nat Med. 26:270–280.
2020. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Oliveira G, Ruggiero E, Stanghellini MT,
Cieri N, D'Agostino M, Fronza R, Lulay C, Dionisio F, Mastaglio S,
Greco R, et al: Tracking genetically engineered lymphocytes
long-term reveals the dynamics of T cell immunological memory. Sci
Transl Med. 7:317ra1982015. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Hoffmann JM, Schubert ML, Wang L,
Hückelhoven A, Sellner L, Stock S, Schmitt A, Kleist C, Gern U,
Loskog A, et al: Differences in expansion potential of naive
chimeric antigen receptor T cells from healthy donors and untreated
chronic lymphocytic leukemia patients. Front Immunol. 8:19562018.
View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Ghassemi S, Nunez-Cruz S, O'Connor RS,
Fraietta JA, Patel PR, Scholler J, Barrett DM, Lundh SM, Davis MM,
Bedoya F, et al: Reducing ex vivo culture improves the antileukemic
activity of chimeric antigen receptor (CAR) T cells. Cancer Immunol
Res. 6:1100–1109. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Blaeschke F, Stenger D, Kaeuferle T,
Willier S, Lotfi R, Kaiser AD, Assenmacher M, Döring M, Feucht J
and Feuchtinger T: Induction of a central memory and stem cell
memory phenotype in functionally active CD4+ and
CD8+ CAR T cells produced in an automated good
manufacturing practice system for the treatment of CD19+
acute lymphoblastic leukemia. Cancer Immunol Immunother.
67:1053–1066. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Wherry EJ and Kurachi M: Molecular and
cellular insights into T cell exhaustion. Nat Rev Immunol.
15:486–499. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Jochems CE, van der Valk JB, Stafleu FR
and Baumans V: The use of fetal bovine serum: Ethical or scientific
problem? Altern Lab Anim. 30:219–227. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Xu H, Wang N, Cao W, Huang L, Zhou J and
Sheng L: Influence of various medium environment to in vitro human
T cell culture. In Vitro Cell Dev Biol Anim. 54:559–566. 2018.
View Article : Google Scholar : PubMed/NCBI
|