Introduction
Obesity is characterized by a chronic energy
imbalance caused by factors such as excessive dietary intake, lack
of physical activity and genetic predisposition (1). Obesity is associated with a high
risk of mortality globally and is associated with development of
various diseases, including diabetes, dyslipidemia, fatty liver
disease, hypertension and cancer (2). Despite efforts to combat obesity,
its prevalence continues to increase globally, posing a threat to
public health (2). While it is
well-established that diet and exercise are essential for
preventing and managing obesity, pharmacotherapy may also be
considered when diet and exercise are ineffective (3,4).
Drugs have been developed and used for the treatment of obesity,
but their use has been limited due to serious side effects such as
insomnia, headache, tremor, and abdominal pain (3). Consequently, there has been growing
interest in the development of natural products with fewer side
effects for the treatment of obesity (5,6).
Paeonia lactiflora (PL), a medicinal plant
belonging to the Paeoniaceae family, has been used as a
traditional herbal medicine in countries such as China, South
Korea, Japan, Taiwan and Thailand for the treatment of inflammatory
diseases such as rheumatoid arthritis, hepatitis and systemic lupus
erythematosus (7). In addition to
its therapeutic effects on inflammatory disease, PL has been shown
to have anti-allergic, analgesic, antimicrobial and
anti-melanogenic effects (8,9).
Furthermore, PL has been reported to inhibit adipogenesis in
adipocytes via activation of PPAR-α and Wnt/β-catenin signaling,
indicating its anti-obesity effects (10). Although the anti-obesity activity
of PL has been demonstrated (10), the precise mechanism by which it
exerts this effect remains unclear. Therefore, the present study
aimed to elucidate the mechanisms underlying the anti-obesity
activity of PL.
Materials and methods
Chemicals
Dexamethasone, 3-isobutyl-1-methylxanthine (IBMX),
insulin, oil red O (ORO) and compound C (AMPK inhibitor) were
purchased from Sigma-Aldrich (Merck KGaA). The primary antibodies
against adipose triacylglycerol lipase (ATGL; cat. no. 2138),
hormone-sensitive lipase (HSL; cat. no. 4107), phosphorylated
(p-)HSL (cat. no. 4137), perilipin-1 (cat. no. 9349), AMP-activated
protein kinase (AMPK; cat. no. 5831), p-AMPK (cat. no. 2535),
uncoupling protein 1 (UCP-1; cat. no. 14670) and β-actin (cat. no.
5125) and secondary antibodies horseradish peroxidase-linked
anti-rabbit (cat. no. 7074) and anti-mouse IgG (cat. no. 7076) were
purchased from Cell Signaling Technology, Inc. The primary
antibodies against PPAR-γ coactivator-1α (PGC-1α; cat. no.
sc-518025) and PR domain-containing 16 (PRDM16; cat. no. ab106410)
were purchased from Santa Cruz Biotechnology, Inc. and Abcam,
respectively.
Sample preparation
As the root is the most commonly utilized part of PL
in both herbal medicine and food applications (7), it was selected for the present
study. PL root (PLR) was obtained from the Bonghwa Medicinal Herb
Research Institute, Gyeongsangbuk-do Agricultural Research &
Extension Service (Bonghwa, South Korea). A total of 10 g powdered
PLR was dried at 40°C for 3 days, then soaked and extracted in 200
ml distilled water at 40°C for 24 h. Following extraction, the
clear supernatant was recovered by centrifugation at 15,000 × g at
4°C for 10 min. Then, the recovered extract supernatant was
lyophilized to obtain water extract from PLR. The freeze-dried PLR
was stored at −80°C and dissolved in sterilized water (50 mg PLR/ml
DH2O) before 3T3-L1 cells were treated.
Cell culture
The 3T3-L1 pre-adipocytes were purchased from the
American Type Culture Collection. Before the experiments, 3T3-L1
cells were maintained in DMEM/F-12 (Hyclone; Cytiva) supplemented
with 10% bovine calf serum (Gibco; Thermo Fisher Scientific, Inc.)
and penicillin/streptomycin (100 U/100 µg/ml) at 37°C with
5% CO2. For the differentiation of 3T3-L1 cells,
DMEM/F-12 supplemented with 10% fetal bovine serum (FBS, Gibco) and
penicillin/streptomycin (100 U/100 µg/ml) was used.
Differentiation of 3T3-L1 cells
3T3-L1 cells were cultured for 2 days in a 6-well
plate at 100% confluence. After 2 days (D0), the 3T3-L1 cells were
treated with 50 µM IBMX, 1 µM dexamethasone and 10
µg/ml insulin (DMI) for 2 days (D2). Subsequently, 3T3-L1
cells were treated with 10 µg/ml insulin for 2 days (D4).
3T3-L1 cells were cultured for 4 days, with media refreshed once
every 2 days (D6 and D8). All procedures were performed at 37°C
with 5% CO2.
Measurement of cell number and
viability
The 3T3-L1 cells were cultured for 2 days in a
6-well plate at 100% confluence at 37°C with 5% CO2.
After 2 days, the 3T3-L1 cells were treated with PLR (200
µg/ml) in the presence (differentiation) or absence (no
differentiation) of 50 µM IBMX, 1 µM dexamethasone
and 10 µg/ml insulin (DMI) and 10 µg/ml insulin for
2-6 days (D0-D6) at 37°C with 5% CO2. At 2, 4 and 6 days
after PLR treatment, the total cell number and viability were
measured using a NucleoCounter NC-250 instrument (Chemometec)
following the manufacturer's protocols. This experiment was
repeated thrice.
ORO staining
3T3-L1 cells were fixed with 10% formalin at room
temperature for 1 h. The 3T3-L1 cells were washed three times with
distilled water and dehydrated with 60% isopropanol at room
temperature for 5 min. The dried 3T3-L1 cells were then stained
with 60% ORO staining solution at room temperature for 20 min to
visualize LDs. After staining, the 3T3-L1 cells were washed five
times with distilled water and observed under a light microscope
(400× magnification; Olympus Corporation). After visualizing the
LDs, accumulated LDs were quantified by extracting ORO from the
stained LDs in completely dried 3T3-L1 cells using 100% isopropanol
and measuring absorbance at 500 nm using a microplate reader
(SpectraMax M2, Molecular Devices). The experiment was repeated
thrice.
Measurement of glycerol content
The differentiated 3T3-L1 cells were treated with
PLR (200 µg/ml) and cultured for 2 days. All procedures were
performed at 37°C with 5% CO2. At 2 days after PLR
treatment, free glycerol content was measured using a Glycerol
Cell-Based Assay kit (cat. no. 10011725, Cayman Chemical Company)
according to the manufacturer's protocol. The cell culture medium
was mixed with reconstituted free glycerol assay reagent in a 1:4
ratio and incubated at room temperature for 15 min. Absorbance was
measured at 540 nm using a microplate reader (Human Cop.,
Xma-3000PC). This experiment was repeated three times.
Western blot analysis
The cells were collected using RIPA buffer (Boston
BioProducts) and left to stand at 4°C for 30 min. Following
centrifugation at 15,000 × g at 4°C for 30 min, protein extract was
obtained. Following protein quantification using BCA protein assay
kit (Thermo Fisher Scientific, Inc.), an equal amount of protein
(30 µg/well) was subjected to electrophoresis on a 12%
(p-HSL, HSL, ATGL, perlipin-1, UCP-1, and PGC-1α or 8% acrylamide
gel for PRDM16 at 150 V and 400 A for 1 h. Proteins separated on
the acrylamide gel were transferred onto a nitrocellulose membrane
(Thermo Fisher Scientific, Inc.) for 2 h at 100 V and 300 A. After
blocking with 5% non-fat milk at room temperature for 1 h, the
membranes were incubated with primary antibodies (1:1,000) at 4°C
overnight. Membranes were incubated with secondary antibodies
(1:1,000) at room temperature for 1 h. After treating the membrane
with ECL Prime Western Blotting Detection Reagents (Amersham
Biosciences Corp.), protein bands were visualized using a LI-COR
C-DiGit Blot Scanner (LI-COR Biosciences). Quantitative analysis of
the visualized protein bands was performed using UN-SCAN-IT gel
software version 5.1 (Silk Scientific, Inc.). This experiment was
repeated three times.
High-performance liquid chromatography
(HPLC) analysis of bioactive compounds
The bioactive compounds in PLR were analyzed using
HPLC. A Waters 2695 Separation Module and Waters 2996 Photodiode
Array Detector. The column was equipped with an XBridge R C18
column (250.0×4.6 mm). The binary mobile phase comprised
acetonitrile (solvent A) and water containing 0.5% acetic acid
(solvent B). The flow rate was maintained at 1.0 ml/min for a total
run time of 55 min. The mobile phase was programmed consecutively
in a linear gradient as follows: 0-15 min (10% A:90% B); 15-30 min
(20% A:80% B); 30-45 min (35% A:65% B) and 45-55 min (50% A:50% B).
The injection volume of the extract was 10 µl. Elution was
monitored at 230 nm. Paeoniflorin in the PLR was identified using a
chromatogram of the analytical Paeoniflorin standard
(Sigma-Aldrich; Merck KGaA). This experiment was repeated three
times.
Statistical analysis
All experiments were repeated at least three times.
Statistical analysis was performed using GraphPad Prism version 5.0
(GraphPad Software, Inc.) and data are presented as the mean ±
standard deviation. Data were analyzed using one-way analysis of
variance followed by Bonferroni's post hoc test. P<0.05 was
considered to indicate a statistically significant difference.
Results
PLR decreases accumulation of LDs and
triacylglycerol in 3T3-L1 cells
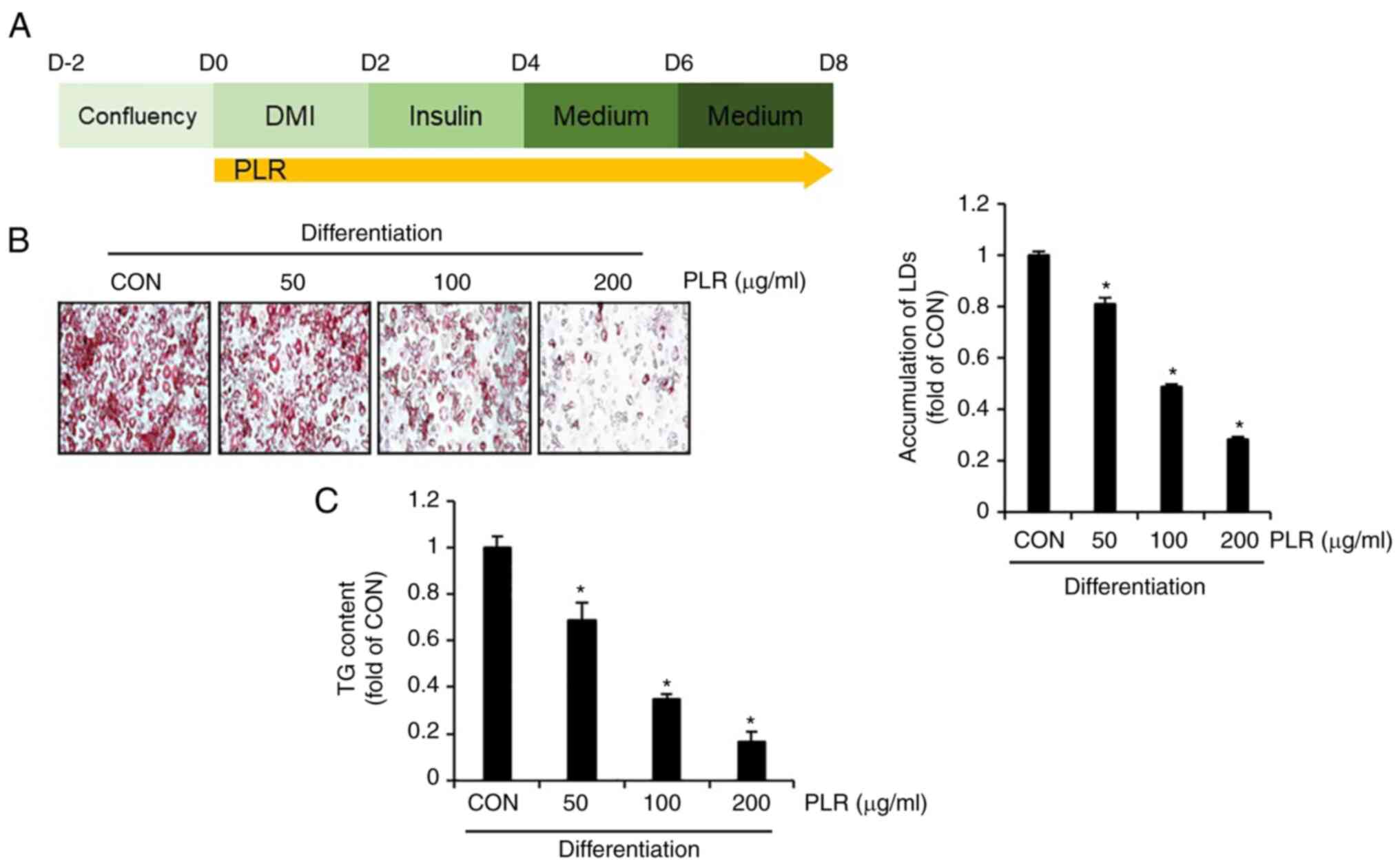
To examine whether PLR exerts anti-obesity effects,
3T3-L1 cells differentiated by DMI were treated with PLR from D0 to
D8 and the number of LDs and amount of triacylglycerol (TG) were
analyzed (Fig. 1A). Compared with
3T3-L1 cells that had been differentiated without PLR treatment
(CON group), there was a significant dose-dependent decrease in the
accumulation of LDs and TG in PLR-treated 3T3-L1 cells (Fig. 1B and C).
PLR inhibits the accumulation of LDs at
all phases of adipocyte differentiation in 3T3-L1 cells
To investigate the timing of LD accumulation during
adipocyte differentiation and the effect of PLR, 3T3-L1 cells
undergoing differentiation were treated with PLR from D0 to D8 and
LD accumulation was assessed using ORO staining (Fig. 2A). LD accumulation began on D4
following initiation of differentiation, and the extent of LD
accumulation increased until D8 (Fig.
2B). However, minimal LD accumulation was observed in 3T3-L1
cells treated with PLR. To investigate at which phases of adipocyte
differentiation, such as early (D0-D2), intermediate (D2-D6) and
late phases (D6-D8), PLR inhibits the accumulation of LDs, 3T3-L1
cells undergoing differentiation were treated with PLR from D0 to
D8, D0 to D2, D2 to D6, or D6 to D8 and the extent of LD
accumulation at D8 was evaluated using ORO staining (Fig. 2C). PLR at the early (D0-D2),
intermediate (D2-D6) and late phases (D6-D8) of differentiation
significantly reduced the accumulation of LDs compared with the
group without PLR treatment (Fig.
2D).
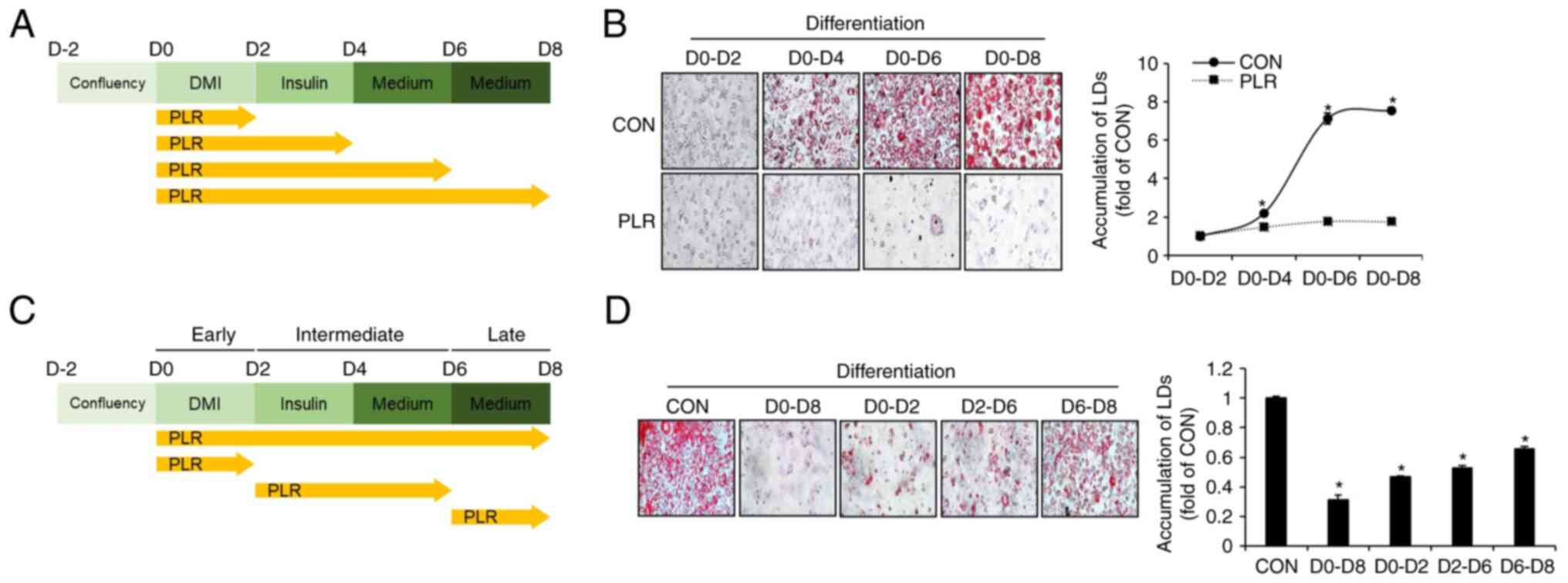 | Figure 2Effect of PLR on phases of adipocyte
differentiation in 3T3-L1 cells. (A) Experimental design. 3T3-L1
cells were treated with PLR (200 µg/ml) for D0-D2, D0-D4,
D0-D6, or D0-D8. (B) ORO staining at D2-8 following PLR (200
µg/ml) treatment. *P<0.05 vs. D0-D2 group. (C)
Experimental design. 3T3-L1 cells were treated with PLR (200
µg/ml) for D0-D8, D0-D2, D2-D6, or D6-D8. (D) ORO staining
(x 400) at D8 following PLR (200 µg/ml) treatment from D0 to
D8. *P<0.05 vs. CON. CON, control; DMI,
dexamethasone, 3-isobutyl-1-methylxanthine, insulin; LD, lipid
droplet; PLR, Paeonia lactiflora root. |
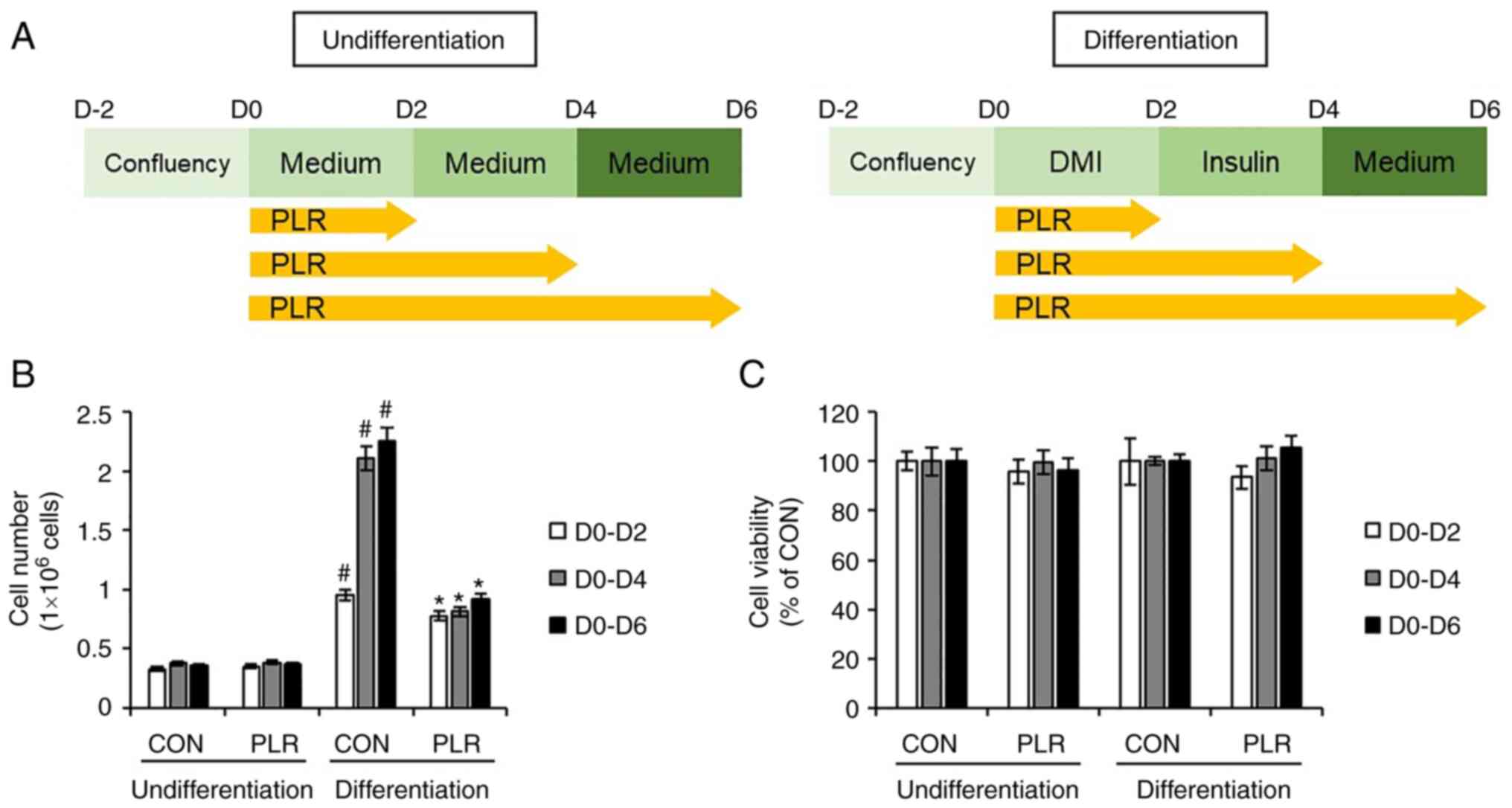
PLR inhibits proliferation but not
viability of 3T3-L1 cells
To investigate the effects of PLR on the
proliferation and viability of 3T3-L1 cells, 3T3-L1 cells were
treated with PLR from D0 to D6 during differentiation because the
proliferation of undifferentiated 3T3-L1 cells occurred during the
early (D0-D2) to intermediate phases (D2-D6) of the differentiation
process (Fig. 3A). Although PLR
did not affect the number of undifferentiated 3T3-L1 cells, PLR
treatment of differentiated 3T3-L1 cells led to a significant
decrease in the number of cells (Fig.
3B). However, PLR did not affect the viability of 3T3-L1 cells
during differentiation (Fig.
3C).
PLR induces lipolysis in 3T3-L1
cells
To examine whether PLR induces lipolysis, 3T3-L1
cells undergoing differentiation were treated with PLR (50-200
µg/ml) from D0 to D8 (Fig.
4A) and levels of proteins associated with lipolysis, including
p-HSL, HSL, ATGL and perilipin-1, were analyzed by western
blotting. PLR treatment (50-200 µg/ml) notably upregulated
the levels of p-HSL, HSL, and ATGL protein (Fig. 4B). Perilipin-1 was significantly
decreased by PLR treatment (100 and 200 µg/ml). To verify
whether PLR induced breakdown of accumulated LDs, differentiated
3T3-L1 cells were treated with PLR from D8 to D10 (Fig. 4C). Decreased LD accumulation and
an increase in glycerol levels were observed in PLR-treated 3T3-L1
cells (Fig. 4D). To confirm
whether these changes in PLR-treated 3T3-L1 cells were due to
alterations in lipolysis-associated proteins, protein levels of
ATGL and HSL were examined by western blotting. PLR treatment
significantly increased ATGL and HSL and decreased perilipin-1
(Fig. 4E).
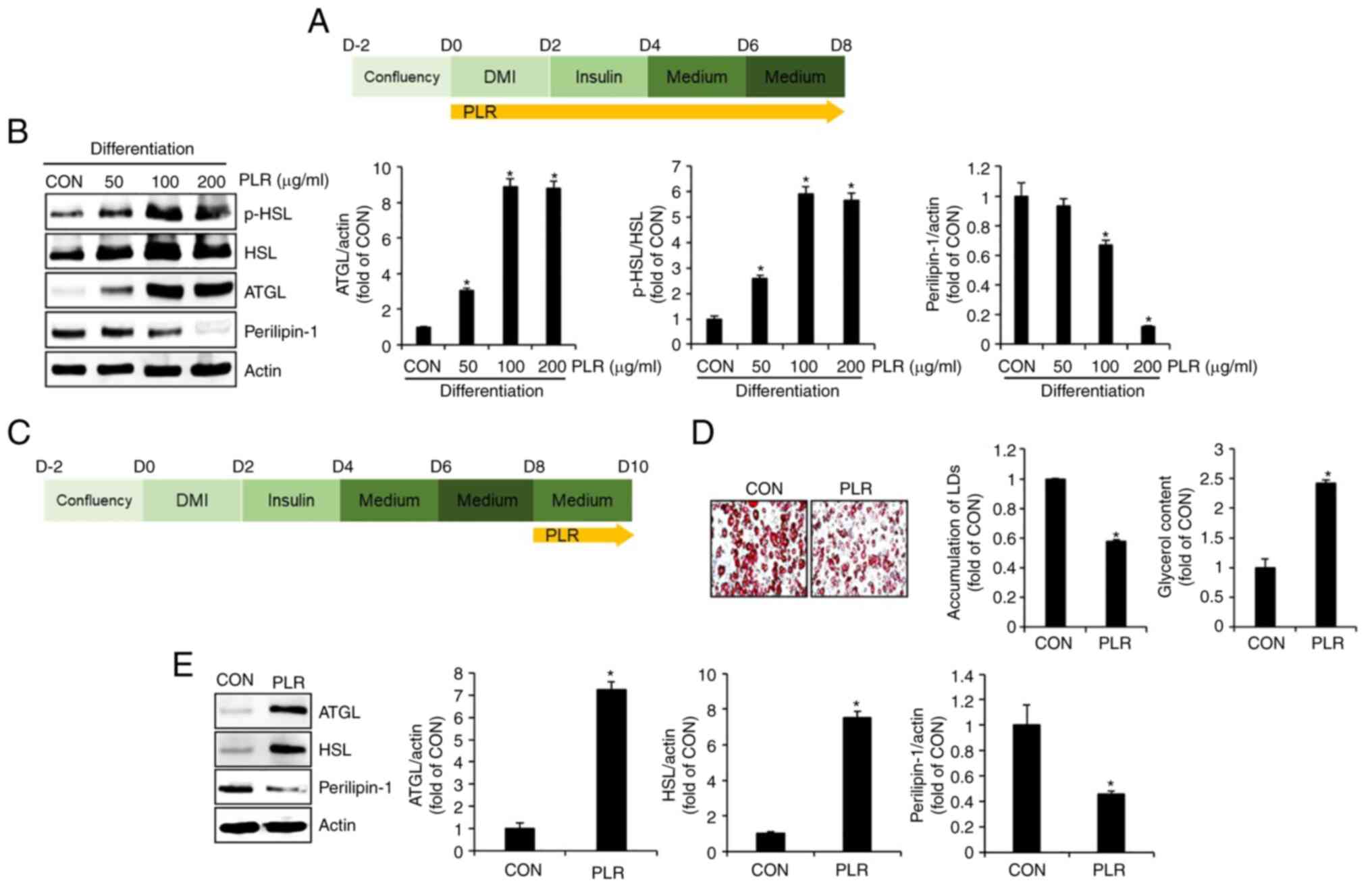 | Figure 4Effect of PLR on levels of proteins
associated with lipolysis in 3T3-L1 cells. (A) Experimental design.
3T3-L1 cells were treated with PLR (200 µg/ml) for D0-D8.
(B) Western blot analysis in 3T3-L1 cells treated with PLR from D0
to D8. (C) Experimental design. 3T3-L1 cells were treated with PLR
(200 µg/ml) for D8-D10. (D) ORO staining (x 400) and
glycerol content and (E) The 3T3-L1 cells differentiated from D0 to
D8 were treated with PLR (200 µg/ml) for 2 days. western
blot analysis in 3T3-L1 cells treated with PLR (200 µg/ml)
from D8 to D10. *P<0.05 vs. CON. CON, control; DMI,
dexamethasone, 3-isobutyl-1-methylxanthine, insulin; LD, lipid
droplet; PLR, Paeonia lactiflora root; ATGL, adipose
triglyceride lipase; p-, phosphorylated; HSL, hormone-sensitive
lipase; ORO, oil red O. |
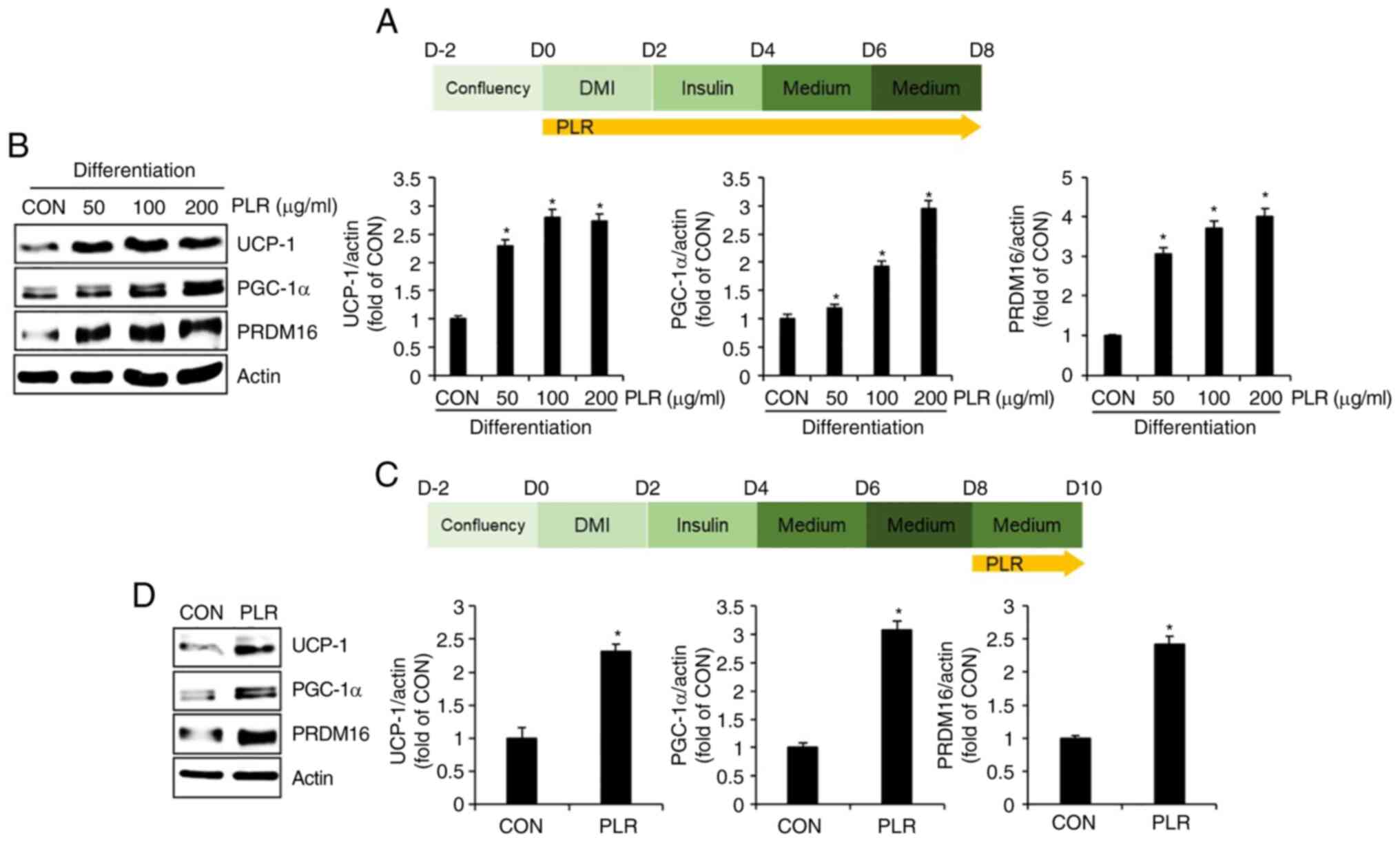
PLR induces thermogenesis in 3T3-L1
cells
To examine whether PLR induces thermogenesis, 3T3-L1
cells undergoing differentiation were treated with PLR from D0 to
D8 (Fig. 5A); subsequently,
levels of proteins associated with thermogenesis, including UCP-1,
PGC-1α, and PRDM16, were analyzed by western blotting. UCP-1,
PGC-1a and PRDM16 protein were upregulated in PLR-treated 3T3-L1
cells compared with cells treated with DMI and insulin alone (CON
group) (Fig. 5B). To investigate
whether thermogenesis was involved in decreasing accumulation of
LDs by PLR, levels of thermogenesis-associated proteins were
examined by western blotting at D8-D10 in 3T3-L1 cells with
accumulated LDs (Fig. 5C).
Protein levels of UCP-1, PGC-1 and PRDM16 increased in 3T3-L1 cells
treated with PLR (Fig. 5D).
PLR-mediated lipolysis and thermogenesis
are dependent on AMPK activation in 3T3-L1 cells
To investigate whether AMPK activation affects
PLR-induced lipolysis and thermogenesis, 3T3-L1 cells were treated
with PLR from D0 to D4 during the differentiation process and AMPK
phosphorylation was measured using western blotting (Fig. 6A). Phosphorylation of AMPK
occurred in 3T3-L1 cells treated with PLR from D2 (Fig. 6B). Therefore, the effect of
PLR-mediated AMPK activation on levels of lipolysis-related factors
such as ATGL and HSL, as well as thermogenesis-associated factors
such as PGC-1α and UCP-1 was investigated. The 3T3-L1 cells were
treated with PLR from D0 to D4 in the presence of the AMPK
inhibitor Compound C and the levels of ATGL, HSL, PGC-1α and UCP-1
were analyzed by western blotting (Fig. 6C). Treatment of 3T3-L1 cells with
PLR in the absence of Compound C resulted in significantly
increased levels of ATGL, HSL, PGC-1α and UCP-1 (Fig. 6D). However, AMPK inhibition by
Compound C attenuated PLR-mediated increases in levels of ATGL,
HSL, PGC-1α and UCP-1.
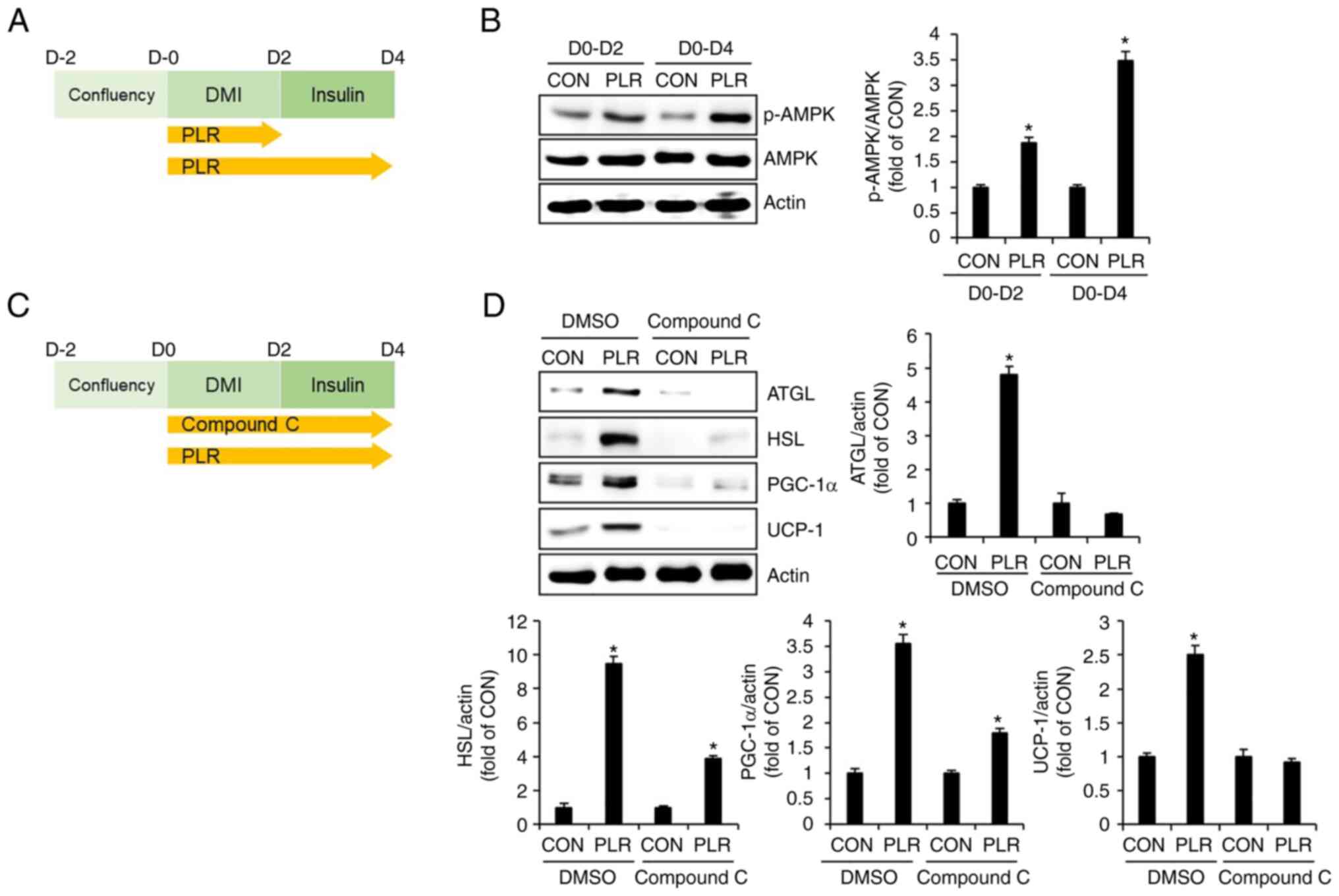 | Figure 6Effect of AMPK activation on
PLR-mediated lipolysis and thermogenesis in 3T3-L1 cells. (A)
Experimental design. 3T3-L1 cells were treated with PLR (200
µg/ml) for D0-D2 or D0-D4. (B) Western blot analysis of
3T3-L1 cells treated with PLR (200 µg/ml) from D0 to D4. (C)
Experimental design. 3T3-L1 cells were treated with PLR (200
µg/ml) for D0-D4 in presence or absence of Compound C. (D)
Western blot analysis in 3T3-L1 cells treated with PLR (200
µg/ml) from D0 to D4 in the presence or absence of Compound
C (10 µM). *P<0.05 vs. CON. CON, control; DMI,
dexamethasone, 3-isobutyl-1-methylxanthine, insulin; PLR,
Paeonia lactiflora root; UCP-1, uncoupling protein 1;
PGC-1α, peroxisome proliferator-activated receptor-γ coactivator
1α; PRDM16, PR domain-containing 16. p-, phosphorylated; AMPK,
AMP-activated protein kinase; ATGL, adipose triglyceride lipase;
HSL, hormone-sensitive lipase. |
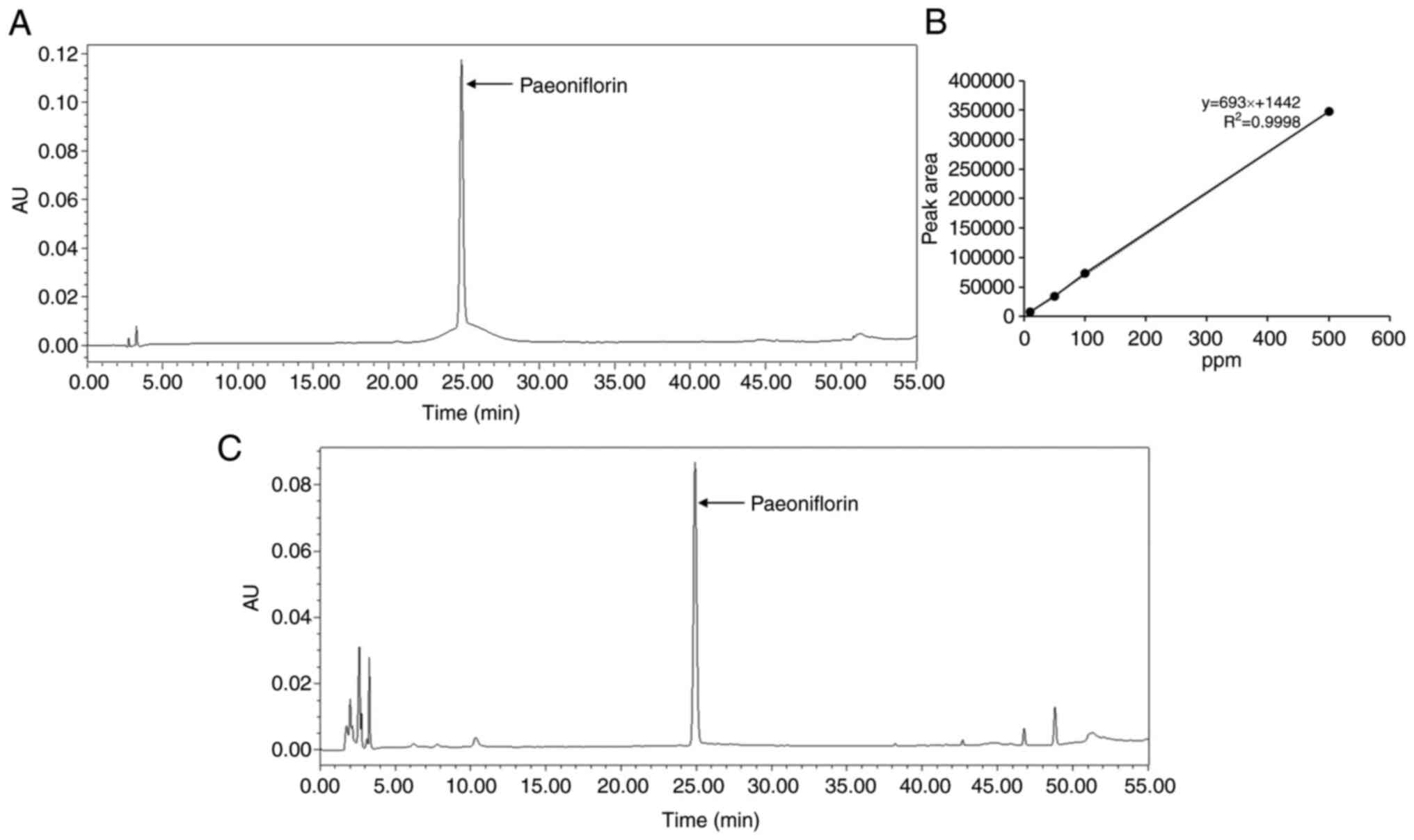
Analysis of bioactive compounds from
PLR
HPLC analysis of PLR was performed to identify the
components that exerted anti-obesity activity. Paeoniflorin was
detected in the PLR (Fig. 7).
Quantitative analysis of the content of paeoniflorin in PLR showed
that it contained 11.52% paeoniflorin/g extract (data not
shown).
Discussion
Obesity is a key issue that must be controlled
because it can lead to metabolic disorders such as diabetes,
dyslipidemia, fatty liver disease, hypertension and cancer that are
directly associated with mortality (2). Despite the development of numerous
anti-obesity drugs such as Phentermine, Diethylpropion, Zonisamide,
and Topiramate, their clinical use is limited because of side
effects (3). Therefore, the
development of natural products with minimal side effects is
necessary (5). PL, which has been
used as an herbal medicine for the treatment of inflammatory
diseases, has shown potential as an anti-obesity agent (10).
The present study confirmed that PLR effectively
inhibited accumulation of LDs and triacylglycerol in 3T3-L1 cells.
These findings provide evidence that PLR can be utilized for the
development of natural anti-obesity agents. The present study also
confirmed that PLR inhibited accumulation of LDs in adipocytes
during all phases of differentiation. The ability of PLR to inhibit
LD accumulation in the early phase of differentiation implies its
potential for obesity prevention, while suppression of LD
accumulation by PLR in the intermediate and late phases shows its
potential for obesity treatment.
Obesity is characterized by abnormal expansion of
white adipose tissue due to an increase in the number and size of
adipocytes (11). Moreover, the
increase in the number of adipocytes is due to proliferation of
adipocytes that differentiate from pre-adipocytes into mature
adipocytes in a process known as adipogenesis (12). Thus, controlling the number of
adipocytes is a potential therapeutic approach to obesity (13). Here, PLR had no effect on
proliferation and survival of undifferentiated 3T3-L1 cells, but
significantly reduced proliferation of differentiating 3T3-L1
cells. The finding that PLR reduced the number of differentiating
adipocytes is consistent with a previous report (10). When 3T3-L1 preadipocytes reach
100% confluence, they undergo growth arrest due to contact
inhibition. Growth-arrested 3T3-L1 cells reenter the cell cycle
upon treatment with DMI, initiate proliferation and eventually
differentiate into mature adipocytes (14). Therefore, the lack of
proliferation in undifferentiated cells may have occurred because
undifferentiated 3T3-L1 cells reached 100% confluence, causing
growth arrest through contact inhibition.
In addition to inhibiting adipogenesis, there are
other therapeutic approaches for obesity. Lipolysis is one target
and numerous natural plants such as fish oil, Salacia
reticulate, and Rubus idaeus that induce lipolysis have
potential as anti-obesity agents (15,16). Lipolysis is a process in which
triacylglycerol is broken down into one glycerol molecule and three
fatty acids and is mediated by the ATGL and HSL enzymes (17). ATGL removes one fatty acid from
triacylglycerol to form diacylglycerol, which is subsequently
hydrolyzed by HSL (17).
Perilipin-1, which surrounds LDs in adipocytes, is a key regulator
of lipolysis and its knockdown promotes lipolysis (18). The aforementioned studies suggest
that an increase in ATGL and HSL and a decrease in perilipin-1
serve as important markers associated with lipolysis induction. The
present study demonstrated that PLR increased ATGL and HSL levels
and decreased perilipin-1 levels in differentiating or fully
differentiated 3T3-L1 adipocytes. PLR treatment of fully
differentiated 3T3-L1 adipocytes increased glycerol content. The
results of the present study provide evidence that PLR promoted
lipolysis, thereby inhibiting excessive lipid accumulation in
adipocytes.
As obesity is caused by excessive energy intake
compared with expenditure (1),
increasing energy expenditure can be a treatment for obesity
(19). The strategy to increase
expenditure is to induce the browning of white adipose tissue, as
white adipose tissue stores energy, whereas brown adipose tissue
consumes stored energy as heat through thermogenesis (20,21). Thus, the browning of white
adipocytes is a potential therapeutic strategy against obesity
(18). White adipocytes are
converted into brown thermogenic adipocytes by the action of
various molecules, such as UCP-1, PGC-1α and PRDM16 (22). Therefore, increased levels of
these molecules serve as molecular markers for white adipocyte
browning (22). In the present
study, PLR treatment of differentiating or fully differentiated
3T3-L1 cells resulted in increased UCP-1, PGC-1α and PRDM16 levels.
These results suggested that PLR can convert white adipocytes into
brown thermogenic adipocytes, providing evidence for the underlying
mechanism of PLR-induced inhibition of LD accumulation.
AMPK is a key regulator of energy homeostasis that
serves as a molecular target for drugs in the treatment of various
metabolic disorders, including obesity (23,24). AMPK activation induces lipolysis
by increasing levels of lipolytic factors, such as ATGL and HSL
(25). In addition, AMPK has been
reported to induce browning of white adipocytes by increasing
levels of thermogenic factors, such as PGC-1α and PRDM16 (26). The aforementioned reports provide
evidence that AMPK activation serves a central role in the
induction of lipolysis and thermogenesis. The present study
demonstrated that the increase in lipolytic (ATGL and HSL) and
thermogenic factors (PGC-1α and UCP-1) induced by PLR was reversed
by Compound C-mediated inhibition of AMPK. These results suggested
that PLR induced lipolysis and thermogenesis through AMPK
activation in adipocytes. However, signaling pathways other than
the AMPK pathway were not analyzed. Therefore, further mechanistic
studies on the anti-obesity activity of PLR are necessary.
The present study confirmed that PLR contained
paeoniflorin. Although PL contains various components, albiflorin
and paeoniflorin are primary active ingredients with
pharmacological activities (27).
Albiflorin and paeoniflorin have been reported to exert
anti-obesity effects (28,29).
According to previous studies, paeoniflorin, a water-soluble
monoterpene glycoside, is the most abundant component and accounts
for >90% of components in PL (30,31). Although the present study did not
use paeoniflorin as a positive control to evaluate its role in PLR,
considering previous studies on the anti-obesity activity of
paeoniflorin and its solubility (29-31), it can be hypothesized that
paeoniflorin contributes to the antiplatelet activity of PLR. In
addition, lack of data on other components in PLR, including
albiflorin, is a limitation of the present study. Therefore, it is
necessary to conduct a thorough analysis of other components,
including albiflorin, that exhibit anti-obesity activity.
The present study demonstrated that the PLR induced
lipolysis and thermogenesis by regulating lipolytic and thermogenic
factors via AMPK activation in adipocytes, which contributed to its
anti-obesity activity. The present study provides further evidence
of the anti-obesity activity of PLR and the mechanism underlying
this activity. PLR may have potential value as a natural agent for
obesity control. A limitation of the present study was the lack of
in vivo studies using animal models as only in vitro
results using 3T3-L1 cells were obtained. Therefore, in vivo
studies are required to assess the clinical applicability of PLR as
an anti-obesity agent. Furthermore, the present study did not
investigate the effects of different doses of PLR. Therefore,
research is needed to address this.
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
JWC, HJC and GHR wrote the manuscript, performed
cell experiments and analyzed the data. JWL, JKB and EJK performed
HPLC analysis. JBJ designed the experiments and wrote and edited
the manuscript. JWC, HJC, GHR, JWL, JKB, EJK and JBJ confirm the
authenticity of all the raw data. All the authors have read and
approved the final manuscript.
Ethics approval and consent to
participate
Not applicable.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
Acknowledgments
Not applicable.
Funding
The present study was supported by Cooperative Research Program
for Agriculture Science and Technology Development (grant no.
PJ017090052022), Rural Development Administration, Republic of
Korea.
References
|
1
|
Haslam DW and James WP: Obesity. Lancet.
366:1197–1209. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Withrow D and Alter DA: The economic
burden of obesity worldwide: A systematic review of the direct
costs of obesity. Obes Rev. 12:131–141. 2011. View Article : Google Scholar
|
|
3
|
Kang JG and Park CY: Anti-obesity drug: A
review about their effects and safety. Diabetes Metab J. 36:13–25.
2012. View Article : Google Scholar
|
|
4
|
Price S, Le QN and White ND: Lifestyle and
pharmacotherapy for weight loss in preventing or delaying diabetes.
Am J Lifestyle Med. 12:34–37. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Fu C, Jiang Y, Guo J and Su Z: Natural
products with anti-obesity effects and different mechanisms of
action. J Agric Food Chem. 64:9571–9585. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Cho YR, Lee JA, Kim YY, Kang JS, Lee JH
and Ahn EK: Anti-obesity effects of Clausena excavata in high-fat
diet-induced obese mice. Biomed Pharmacother. 99:253–260. 2018.
View Article : Google Scholar : PubMed/NCBI
|
|
7
|
He DY and Dai SM: Anti-inflammatory and
immunomodulatory effects of Paeonia lactiflora pall., a traditional
Chinese herbal medicine. Front Pharmacol. 2:102011. View Article : Google Scholar :
|
|
8
|
Choi EM and Lee YS: Paeoniflorin isolated
from Paeonia lactiflora attenuates osteoblast cytotoxicity induced
by antimycin A. Food Funct. 4:1332–1338. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Li XL, Thakur K, Zhang YY, Tu XF, Zhang
YS, Zhu DY, Zhang JG and Wei ZJ: Effects of different chemical
modifications on the antibacterial activities of polysaccharides
sequentially extracted from peony seed dreg. Int J Biol Macromol.
116:664–675. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Kim B: The activation of PPAR-α and
Wnt/β-catenin by Paeonia lactiflora root supercritical carbon
dioxide extract. J Korean Applied Sci Technol. 36:1136–1142.
2019.
|
|
11
|
Haczeyni F, Bell-Anderson KS and Farrell
GC: Causes and mechanisms of adipocyte enlargement and adipose
expansion. Obes Rev. 19:406–420. 2018. View Article : Google Scholar
|
|
12
|
Haider N and Larose L: Harnessing
adipogenesis to prevent obesity. Adipocyte. 8:98–104. 2019.
View Article : Google Scholar
|
|
13
|
Jakab J, Miškić B, Mikšić Š, Juranić B,
Ćosić V, Schwarz D and Včev A: Adipogenesis as a potential
anti-obesity target: A review of pharmacological treatment and
natural products. Diabetes Metab Syndr Obes. 14:67–83. 2021.
View Article : Google Scholar :
|
|
14
|
Sarruf DA, Iankova I, Abella A, Assou S,
Miard S and Fajas L: Cyclin D3 promotes adipogenesis through
activation of peroxisome proliferator-activated receptor γ. Mol
Cell Biol. 25:9985–9995. 2005. View Article : Google Scholar
|
|
15
|
Duncan RE, Ahmadian M, Jaworski K,
Sarkadi-Nagy E and Sul HS: Regulation of lipolysis in adipocytes.
Annu Rev Nutr. 27:79–101. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Yun JW: Possible anti-obesity therapeutics
from nature-a review. Phytochemistry. 71:1625–1641. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Gaidhu MP, Anthony NM, Patel P, Hawke TJ
and Ceddia RB: Dysregulation of lipolysis and lipid metabolism in
visceral and subcutaneous adipocytes by high-fat diet: role of
ATGL, HSL, and AMPK. Am J Physiol Cell Physiol. 298:C961–C971.
2010. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Hansen JS, de Maré S, Jones HA, Göransson
O and Lindkvist-Petersson K: Visualization of lipid directed
dynamics of perilipin 1 in human primary adipocytes. Sci Rep.
7:150112017. View Article : Google Scholar :
|
|
19
|
Arch JRS and Trayhurn P: Detection of
thermogenesis in rodents in response to anti-obesity drugs and
genetic modification. Front Pysiol. 4:642013.
|
|
20
|
Fenzl A and Kiefer FW: Brown adipose
tissue and thermogenesis. Horm Mol Biol Clin Investig. 19:25–37.
2014.
|
|
21
|
Lee YH, Mottillo EP and Granneman JG:
Adipose tissue plasticity from WAT to BAT and in between. Biochim
Biophys Acta. 1842:358–369. 2014. View Article : Google Scholar
|
|
22
|
Song NJ, Chang SH, Li DY, Villanueva CJ
and Park KW: Induction of thermogenic adipocytes: molecular targets
and thermogenic small molecules. Exp Mol Med. 49:e3532017.
View Article : Google Scholar :
|
|
23
|
Kola B, Grossman AB and Korbonits M: The
role of AMP-activated protein kinase in obesity. Front Horm Res.
36:198–211. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Xio B, Sanders MJ, Carmena D, Bright NJ,
Haire LF, Underwood E, Patel BR, Heath RB, Walker PA, Hallen S, et
al: Structural basis of AMPK regulation by small molecule
activators. Nat Commun. 4:30172013. View Article : Google Scholar
|
|
25
|
Bu S, Yuan CY, Xue Q, Chen Y and Cao F:
Bilobalide suppresses adipogenesis in 3T3-L1 adipocytes via the
AMPK signaling pathway. Molecules. 24:35032019. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Kim K, Nam KH, Yi SA, Park JW, Han JW and
Lee J: Ginsenoside Rg3 induces browning of 3T3-L1 adipocytes by
activating AMPK signaling. Nutrients. 12:4272020. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Gao LN, Zhang Y, Cui YL and Akinyi OM:
Comparison of paeoniflorin and albiflorin on human CYP3A4 and
CYP2D6. Evid Based Complement Alternat Med. 2015:4702192015.
View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Jeong MY, Park J, Youn DH, Jung Y, Kang
JW, Lim S, Kang MW, Kim HL, So HS, Park R, et al: Albiflorin
ameliorates obesity by inducing thermogenic genes via AMPK and
PI3K/AKT in vivo and in vitro. Metabolism. 73:85–99. 2017.
View Article : Google Scholar
|
|
29
|
Rabie BM and Ho JK: The mechanism of
action of Lipiburn on fat metabolism. Front Biosci. 24:427–434.
2019. View Article : Google Scholar
|
|
30
|
Ikeda N, Fukuda T, Jyo H, Shimada Y,
Murakami N, Saka M and Yoshikawa M: Quality evaluation on Paeoniae
Radix. I. Quantitative analysis of monoterpene glycosides
constituents of Paeoniae Radix by means of high performance liquid
chromatography. Comparative characterization of the external
figures, processing method and the cultivated areas. Yakugaku
Zasshi. 116:138–147. 1996. View Article : Google Scholar
|
|
31
|
Yoo JS, Song MC, Ahn EM, Lee YH, Rho YD
and Baek NI: Quantitative analysis of paeoniflorin from Paeonia
lactiflora using 1H-NMR. Nat Prod Sci. 12:237–240.
2006.
|