Introduction
Breast cancer is the leading cause of cancer-related
mortality among women globally (1,2).
Bone is one of the most common target organs for breast cancer
metastasis (2-5). Bone metastases are incurable and can
cause skeletal-related events, including bone pain, pathological
fractures, spinal cord compression and hypercalcemia, which affect
the survival and quality of life in patients with advanced-stage
breast cancer (2,5). Bone metastasis is the result of
complex communications between tumor and stromal cells, including
osteoclasts and osteoblasts, in the bone microenvironment (5-7).
When breast cancer cells colonize the bone, they produce various
factors that stimulate osteoclast formation and activity to induce
massive bone degradation (5-7). A
better understanding of the biological mechanisms through which
cancer cells communicate with bone stromal cells in the bone
microenvironment can lead to the development of potential targets
for the treatment or prevention of the osteolytic bone metastasis
of breast cancer.
Accumulating evidence indicates that exosomes are
critical mediators of intercellular communication during cancer
progression and metastasis (8,9).
Exosomes, also known as small extracellular vesicles, are nanosized
membrane-bound vesicles surrounded by a lipid bilayer and released
upon fusion of multi-vesicular bodies with the plasma membrane
(10-12). Exosomes are rich in signaling
molecules, including microRNAs (miRNAs/miRs), proteins, metabolites
and lipids, and can deliver these signaling molecules to target
cells, thereby contributing to the physiological and pathological
state of target cells (11,12). Tumor-derived exosomes regulate
cell proliferation and migration, angiogenesis,
epithelial-mesenchymal transition and immune responses by
participating in cell-cell communication in the tumor
microenvironment (13,14).
RAS signaling plays a central role in the biogenesis
and release of exosomes and sorting of miRNAs into exosomes in a
number of types of cancer (15-17). The activation of KRAS regulates
the loading of specific miRNAs into exosomes, resulting in the
exosomal release of miRNAs (17,18). Oncogenic mutations in RAS
genes (HRAS, KRAS and NRAS) are rare in human breast
cancer (19). However, the
hyperactivation of RAS signaling via the alternative mechanisms,
including the overexpression of RAS proteins and hyperactivation of
receptor tyrosine kinases, such as human epidermal growth factor
receptor 2 (HER2), contributes to human breast cancer initiation
and metastasis (20,21). For example, basal-like breast
cancer overexpresses NRAS, which promotes tumor formation and
progression (22,23), and RAS activation is a key
determinant of metastasis in luminal breast cancer (21). However, the role of the
interaction between RAS signaling and exosomal miRNAs in osteolytic
bone metastasis of breast cancer remains unclear. Therefore, it was
hypothesized that the exosomal miRNAs secreted upon the activation
of RAS signaling in breast cancer cells may regulate
osteoclastogenesis to promote osteolytic bone metastasis in the
bone microenvironment.
The present study investigated whether the
activation of RAS signaling enhances the secretion of exosomal
osteoclastogenic miRNAs in human breast cancer cells. Exosomes from
wild-type RAS and RAS-activated breast cancer cells were prepared,
and their effects on RANKL-induced osteoclastogenesis were
compared. Using a miRNA microarray, it was found that the exosomal
expression levels of osteoclastogenic miRNAs, such as
miR-494-3p, increased upon the activation of RAS signaling.
The functional role of miR-494-3p in osteoclastogenesis was also
investigated in vitro and in an animal model in
vivo.
Materials and methods
Cells and cell culture
All the cell lines used in the present study were
obtained from the American Type Culture Collection (ATCC). The
MCF-7 (ATCC HTB-22) and T47D (ATCC HTB-133) cell lines are estrogen
receptor (ER)-positive, progesterone receptor (PR)-positive and
HER2-negative. The MDA-MB-231 (ATCC HTB-26) cell line is
ER-negative, PR-negative and HER2-negative. The 4T1-Luc2 (ATCC
CRL-2539-LUC2) cells are a luciferase-expressing mouse mammary
carcinoma cell line. The MCF-7, T47D and 4T1-Luc2 cells were
maintained in RPMI medium (cat. no. L0498-500; Biowest)
supplemented with 10% fetal bovine serum (FBS; cat. no. S1480;
BioWest) and 1% penicillin/streptomycin (cat. no. 15140-122; Gibco;
Thermo Fisher Scientific, Inc.). The MDA-MB-231, RAW264.7 (ATCC
TIB-71) murine macrophages, and C2C12 (ATCC CRL-1772) murine
myoblast cell lines were cultured in DMEM (BioWest, #L0103-500)
supplemented with 10% FBS and 1% penicillin/streptomycin. Bone
marrow-derived macrophages (BMMs) were isolated from the tibias and
femurs of 6-week-old male ICR mice (DBL Co. Ltd.), as previously
described (24). The protocol was
approved by the Institutional Animal Care and Use Committee (IACUC)
of Kangwon National University (IACUC approval no. KW-210914-1,
September 23, 2021). In brief, bone marrow cells were isolated from
the femurs and tibias of ICR mice (total, six mice) and cultured in
MEM (cat. no. SH30601.01; HyClone; Cytiva) containing 10% FBS, and
1% penicillin/streptomycin. Floating cells were collected and
cultured for 3 days with macrophage colony-stimulating factor
(M-CSF, 30 ng/ml; cat. no. cyt-439; Prospec-Tany TechnoGene, Ltd.).
Cells adhering to the bottom of the culture dish were classified as
BMMs. All cells were maintained at 37°C in a humidified incubator
with 5% CO2.
Ras inhibitor, plasmids, miRNAs, siRNA
and transfection
The pan-RAS inhibitor, salirasib, was purchased from
MilliporeSigma (cat. no. SML1166). The miR-494-3p mimic
(5′-UGA AAC AUA CAC GGG AAA CCU C-3′, miRBase Accession no.
MIMAT0002816), miR-1915-3p mimic (5′-CCC CAG GGC GAC GCG GCG
GG-3′, miRBase Accession no. MIMAT0007892), miR-4508 mimic
(5′-GCG GGG CUG GGC GCG CG-3′, miRBase Accession no. MIMAT0019045),
miR-4516 mimic (5′-GGG AGA AGG GUC GGG GC-3′, miRBase
Accession no. MIMAT0019053), miR-6088 mimic (5′-AGA GAU GAA
GCG GGG GGG CG-3′, miRBase Accession no. MIMAT0023713),
miR-6869-5p mimic (5′-GUG AGU AGU GGC GCG CGG CGG C-3′,
miRBase Accession no. MIMAT0027638), miR-494-3p inhibitor
(5′-ACU UUG UAU GUG CCC UUU GGAG-3′), miRNA mimic negative control
(cat. no. SMC-2003), and miRNA inhibitor negative control (cat. no.
SMC-2103) were all purchased from Bioneer Corporation. The
pMT3-HRASV12, pMT3-NRASV12 and pMT3-KRASV12 constructs were kindly
provided by Professor Lee Kwang-Yeol (Cheonnam National University,
Kwangju, Korea). The HER2 expression vector (cat. no. HG10004-UT)
was purchased from Sino Biological. The control siRNA (cat. no.
sc-37007) and siRNA for leucine-rich repeat-containing G-protein
coupled receptor 4 (LGR4; cat. no. sc-62558) were purchased
from Santa Cruz Biotechnology, Inc. The cells were seeded in a cell
culture dish (2.5×105 cells/ml), cultured for 24 h, and
then transfected with nucleic acids (plasmids, 1 µg per
transfection; siRNAs or miRNAs, 10 or 100 µM) using
Lipofectamine 3000 (for plasmids, cat. no. L3000-015) or RNAiMAX
(for siRNAs and miRNAs, cat. no. 13778-150) according to the
manufacturer's instructions (Thermo Fisher Scientific, Inc.) After
48 h, the transfected cells were used for further experiments.
miRNA microarray
Exosomes isolated from the MCF-7 cells transfected
with control or KRASV12 were used for miRNA microarray
analysis by Macrogen Inc. The miRNA microarray system with
Affymetrix GeneChip® 4.0 array (Affymetrix; Thermo
Fisher Scientific, Inc.) containing 2,578 human mature miRNA
oligonucleotide probes was used according to the manufacturer's
recommended protocol (Affymetrix; Thermo Fisher Scientific, Inc.).
Raw data were extracted in the Affymetrix data extraction protocol
using the software provided by Affymetrix GeneChip®
Command Console® Software. The CEL files import, miRNA
level RMA+DABG-All analysis and result export were conducted using
Affymetrix® Power Tools Software. Array data were
filtered using probes for annotated species. Comparative analysis
between the control and test samples was carried out using fold
changes. For a significant DEmiRNA set, hierarchical cluster
analysis was performed using complete linkage and Euclidean
distance as measures of similarity. All statistical tests and
visualization of differentially expressed genes were performed
using the R statistical language v. 3.3.2 (https://www.r-project.org).
In vitro osteoclastogenesis and bone
resorption assays
BMMs transfected with the indicated miRNAs were
plated in 96-wells plate at a density of 5×105 cells/ml
and then stimulated with M-CSF (30 ng/ml; cat. no. cyt-439;
Prospec-Tany TechnoGene, Ltd.) and receptor activator of nuclear
factor-κB ligand (RANKL, 100 ng/ml; cat. no. 462-TEC-010; R&D
Systems, Inc.) for 6 days with a change in medium every 2 days. The
RAW264.7 cells (5×104 cells/ml) were seeded in a 96-well
plate and stimulated with RANKL (100 ng/ml) for 4 days with a
change in medium every 2 days. At the end of the incubation (6 days
for BMMs, 4 days for RAW264.7 cells) at 37°C, the
tartrate-resistant acid phosphatase (TRAP) staining of osteoclasts
was performed using a leukocyte acid phosphatase staining kit (cat.
no. 387A; MilliporeSigma). TRAP-positive multinucleated cells with
more than five nuclei were quantified as mature osteoclasts. For
the bone resorption assay, BMMs transfected with the indicated
miRNAs were cultured in OsteoAssay Surface 96-well plates (cat. no.
3989; Corning, Inc.) and primed with M-CSF and RANKL as described
above. After 6-7 days, the cells were removed from the wells with
sodium hypochlorite solution and washed with distilled water.
Resorption pits were captured using a model H550L microscope (Nikon
Corporation) and quantified using ImageJ software [Java 1.6.0_20
(64 bit); National Institutes of Health].
Alkaline phosphatase (ALP) activity
To induce osteoblast differentiation, the C2C12
cells (105 cells/ml) were seeded in a 48-well plate.
Upon reaching confluency, the cells were transfected with the
indicated miRNAs and stimulated with bone morphogenetic protein 2
(BMP2; 30 ng/ml, cat. no. 355-BM; R&D Systems, Inc.) for 3
days. ALP activity assay was performed following the manufacturer's
instructions (cat. no. MAK447; MilliporeSigma).
Immunofluorescence staining and confocal
microscopy
The cells were washed with phosphate-buffered saline
(PBS), fixed in 4% paraformaldehyde for 10 min at 25°C and
permeabilized in 0.1% Triton X-100 for 30 min. After being washed
with PBS, the cells on the glass coverslips were incubated with
anti-nuclear factor of activated T-cells (NFATc1; dilution, 1:500;
cat. no. 8032; Cell Signaling Technology, Inc.) or RelA/p65
(dilution, 1:500; cat. no ; sc8008; Santa Cruz Biotechnology, Inc.)
antibodies at 4°C overnight, and washed with PBS three times. The
cells were then incubated with anti-rabbit secondary Alexa
(dilution, 1:500; cat. no. A11008, Thermo Fisher Scientific, Inc.)
or anti-mouse secondary Alexa 488 antibody (dilution, 1:500; cat.
no. A11001, Thermo Fisher Scientific, Inc.) for 4 h at room
temperature. Cell nuclei were counterstained with
4',6-diamidino-2-phenylindole (DAPI; cat. no. D9542;
MilliporeSigma) for 5 min at room temperature and mounted with a
mounting solution (cat. no. M01; Biomeda Corp.). Confocal images
were acquired using a confocal laser microscope (LSM 880; Airyscan,
Carl Zeiss AG).
Western blot analysis and antibodies
Cells and exosomes were lysed in lysis buffer [50 mM
Tris-HCl (pH 7.4), 1 mM ethylenediaminetetraacetic acid (EDTA), 1%
NP 40, 150 mM sodium chloride, 5 mM sodium orthovanadate and
protease inhibitor cocktail; cat. no. 11836145010, Roche;
MilliporeSigma], and centrifuged at 20,000 × g for 15 min at 4°C.
The lysates (30 µg/sample) were loaded equally and separated
using 8-12% sodium dodecyl sulfate-polyacrylamide gel
electrophoresis. The proteins on the gel were then transferred to a
Hybond-P membrane (cat. no. 10600023; Cytiva), followed by
incubation with blocking solution (PBS containing 5% skim milk or
BSA) for 4 h at room temperature. The membrane was then incubated
with primary antibody with shaking at 4°C overnight. After being
washed with PBS containing tween-20 (cat. no. P7949;
Sigma-Aldrich), the membrane was probed with the appropriate
secondary antibody conjugated to horseradish peroxidase. The
primary and secondary antibodies used in the present study are
listed in Table SI. The protein
signal was observed using an enhanced chemiluminescence system
(cat. no. LR 01-01; BioNote).
Exosome isolation, nanoparticle tracking
analysis (NTA) and transmission electron microscopy (TEM)
Exosomes were collected from the cell culture
supernatant using differential ultracentrifugation, as previously
described (25). The culture
supernatant was centrifuged at 300 × g for 15 min at 4°C to remove
cellular debris and subsequently centrifuged at 10,000 × g for 30
min at 4°C. The supernatant was centrifuged at 100,000 × g for 70
min at 4°C using an Optimal LE-80K ultracentrifuge (SW55Ti rotor,
Beckman Coulter, Inc.). The pellet was resuspended in PBS and
centrifuged at 100,000 × g for 70 min at 4°C. The supernatant was
discarded, and the precipitated exosomes were used for further
experiments. The size distribution and concentration of exosomes
were analyzed using NTA with NanoSight NS300 (Malvern Panalytical,
Ltd.) as previously described (25). TEM was performed to observe the
morphology of purified exosomes, as previously described (25). The suspension (20 µl) was
applied to a carbon-coated grid that was previously glow-discharged
(Harrick Plasma, Inc.) for 3 min in air, followed by negative
staining with 2% uranyl acetate. The prepared grids were identified
using TEM on a JEM 2100F microscope (JEOL) operating at 200 kV.
Images were acquired using a one view camera (Gatan, Inc.).
In vitro exosome uptake assay
The purified exosomes were labeled with PKH26
according to the manufacturer's protocol (cat. no. PKH26GL-1KT;
MilliporeSigma). PKH26 was added to exosomes in a total volume of
400 µl of diluent C and the mixture was incubated for 5 min
at 37°C. The labeling reaction was terminated by the addition of an
equal volume of 1% BSA. The labeled exosomes were collected using
ultracentrifugation at 100,000 × g for 70 min at 4°C. The
supernatant was removed, and the pellet was resuspended in 50
µl PBS. The BMMs and RAW264.7 cells cultured on glass
coverslips for 24 h were incubated with PKH26-labeled exosomes for
12 h at 37°C with 5% CO2. At the end of the incubation
period, the cells were washed with PBS, fixed with 4%
paraformaldehyde for 10 min at room temperature and then mounted
with a mounting solution. Images were captured using a confocal
laser 21 microscope (LSM 880 with Airyscan, Carl Zeiss AG).
Reverse transcription-quantitative PCR
(RT-qPCR)
Total RNA was isolated using TRIzol reagent
according to the manufacturer's instructions (cat. no. FATRR001;
Favorgen Biotech Corp.). For the analysis of mRNA expression, total
RNA was reverse transcribed into cDNA using the Maxime RT PreMix
kit (cat. no. 25081; Intron Biotechnology, Inc.). For the analysis
of miRNA expression, cDNA was synthesized using the miScript II RT
kit (cat. no. 218161; Qiagen, Inc.). Quantitative PCR (qPCR) was
performed on StepOne Real-time PCR System (Applied Biosystems;
Thermo Fisher Scientific, Inc.) using 2X Fast Q-PCR Mastermix (cat.
no. TG1210; SMOBIO Technology, Inc.). The optimized qPCR conditions
were as follows: 95°C for 20 sec, followed by 40 cycles at 95°C for
10 sec, 55°C for 10 sec, and 72°C for 20 sec. GAPDH was used
as a normalization control for mRNA expression in cells. U6 small
nuclear RNA (RNU6-1) was used as a normalization control for
miRNA expression in the cells and exosomes. All the primer
sequences used in the present study are listed in Tables SII and SIII. The qPCR reverse
primer for all miRNAs including U6 used was the universal reverse
primer provided with the kit (cat. no. 218161; Qiagen, Inc.).
Blood samples and analysis of miRNA
expression in serum
Blood samples from Korean patients with a confirmed
diagnoses of ductal carcinoma (n=19) and triple-negative breast
cancer (n=15) were collected at Kangwon National University
Hospital (Chuncheon, Korea). The present study was performed
following the guiding principles of the Declaration of Helsinki. In
addition, it was approved by the Institutional Review Board of
Kangwon National University Hospital (approval no.
KNUH-2022-04-011, approval date, May 4, 2022). The requirement for
informed consent was waived by the Institutional Review Board of
Kangwon National University Hospital owing to the retrospective
nature of the study. The clinicopathological characteristics of the
breast tumor tissues from the patients are presented in Table SIV. Blood in containers was kept
at 4°C for 4 h to ensure serum separation. Serum samples were
centrifuged at 1,000 × g for 10 min and stored at -80°C until use.
RNA was extracted from serum using TRIzol reagent according to the
manufacturer's protocol (cat. no. FATRR001; Favorgen Biotech
Corp.). The expression levels of miRNAs were determined using qPCR,
as described above. U6 small nuclear RNA (RNU6-1) was used
as a normalization control for miRNA expression in serum.
Animal experiment
Female BALB/c mice (6 weeks old; n=24; weight,
18.5-20.3 g) were purchased from DBL Co., Ltd. All experimental
protocols were approved by the Institutional Animal Care and Use
Committee (IACUC) of Kangwon National University (IACUC approval
no. KW-210914-1, September 23, 2021). The mice were housed under
standard laboratory conditions (light cycle, 12 h dark/12 h light;
temperature, 22±2°C; humidity, 55±2.5%), and were provided with
ad libitum access to food and water. For intratibial
injection, the mice were anesthetized by an intraperitoneal
injection of 100 mg/kg ketamine and 10 mg/kg xylazine. To mimic
established breast cancer bone metastases, the mice (n=24) were
treated with exosomes derived from 4T1-Luc2 cells (109
particles/mouse) by tail vein injection once a day. After 14 days,
1×105 4T1-Luc2 cells in 10 µl sterile PBS were
injected once into the intratibial bone marrow cavity of the mice.
The mice were then randomly assigned to three groups (n=8 in each
group) as follows: The control group treated with PBS plus negative
control (NC) miRNA (0.06 nmol/mouse); the exosome group treated
with exosomes derived from 4T1-Luc2 cells (109
particles/mouse) plus NC (0.06 nmol/mouse); and the exosome plus
inhibitor (IN) group treated with exosomes derived from 4T1-Luc2
cells (109 particles/mouse) plus miR-494-3p IN
(0.06 nmol/mouse). Subsequently, the mice were administered PBS
plus NC, exosomes plus NC, or exosomes plus IN intravenously via
the tail vein three times a week (at 2- to 3-day intervals) for 2
weeks. The NC and IN were incubated with exosomes for 30 min at
room temperature prior to the tail vein injection. Tumor growth in
the tibia was measured using bioluminescent imaging with Spectral
Instruments Imaging following the intraperitoneal injection of 150
mg/kg sterile d-luciferin (cat. no. P1042; Promega Corporation).
All experimental animals were monitored once every 2 days, and if
they lost food or water intake, activity, or weight, they were
euthanized. The euthanasia of animals progressed to cervical
dislocation following an anesthetic injection (100 mg/kg ketamine
and 10 mg/kg xylazine). Tibiae were collected at the end of the
experiment. For histological analysis, the tibiae were fixed in 4%
paraformaldehyde for 1 day at room temperature, decalcified in 12%
EDTA for 3 weeks, and embedded in paraffin. Sections of 7 µm
thickness were prepared and stained with hematoxylin (cat. no.
HHS16; Sigma-Aldrich) and eosin (cat. no. E4009) for 1 min at room
temperature, and TRAP for 1 h at 37°C. The stained sections were
scanned using the Grundium Ocus scanner (Grandium).
Statistical analysis
Data are expressed as the mean ± standard deviation
(SD). Statistical analyses to determine the significance between
groups were performed using one-way analysis of variance (for
comparisons of more than two groups) followed by Tukey's multiple
comparisons test or an unpaired Student's t-test (for comparisons
between two groups). All statistical analyses were performed using
GraphPad Prism 9 software (Dotmatics). A P-value ≤0.05 was
considered to indicate a statistically significant difference.
Results
Exosomes derived from RAS-activated
breast cancer cells stimulate RANKL-induced osteoclast formation in
vitro
First, to investigate the role of RAS activation in
the exosome-mediated osteolytic bone metastases of breast cancer
cells, the KRAS mutant MDA-MD-231 cell line was used, which
forms osteolytic bone metastases, as a model system. Exosomes from
MDA-MB-231 cells were isolated and analyzed for their physical
features and the expression of marker proteins. TEM analysis
revealed that exosomes purified from MDA-MB-231 cells had a typical
exosome morphology, exhibiting round and cup shapes with sizes
ranging from 50 to 150 nm (Fig.
1A). In addition, NTA revealed that the average size of the
exosomes was 109.9±3.6 nm (Fig.
1B), indicating that most of the exosomes isolated from
MDA-MB-231 cells were distributed within the range of the exosome
diameter. Exosomal markers, including apoptosis-linked gene
2-interacting protein X (ALIX), tumor susceptibility gene 101
(TSG101) and syntenin-1, were detected; however, Golgi matrix
protein 130 (GM130), a Golgi marker, was not detected in the
exosomes isolated from the MDA-MB-231 cells (Fig. 1C), suggesting that the exosomes
were successfully isolated from the MDA-MB-231 cells.
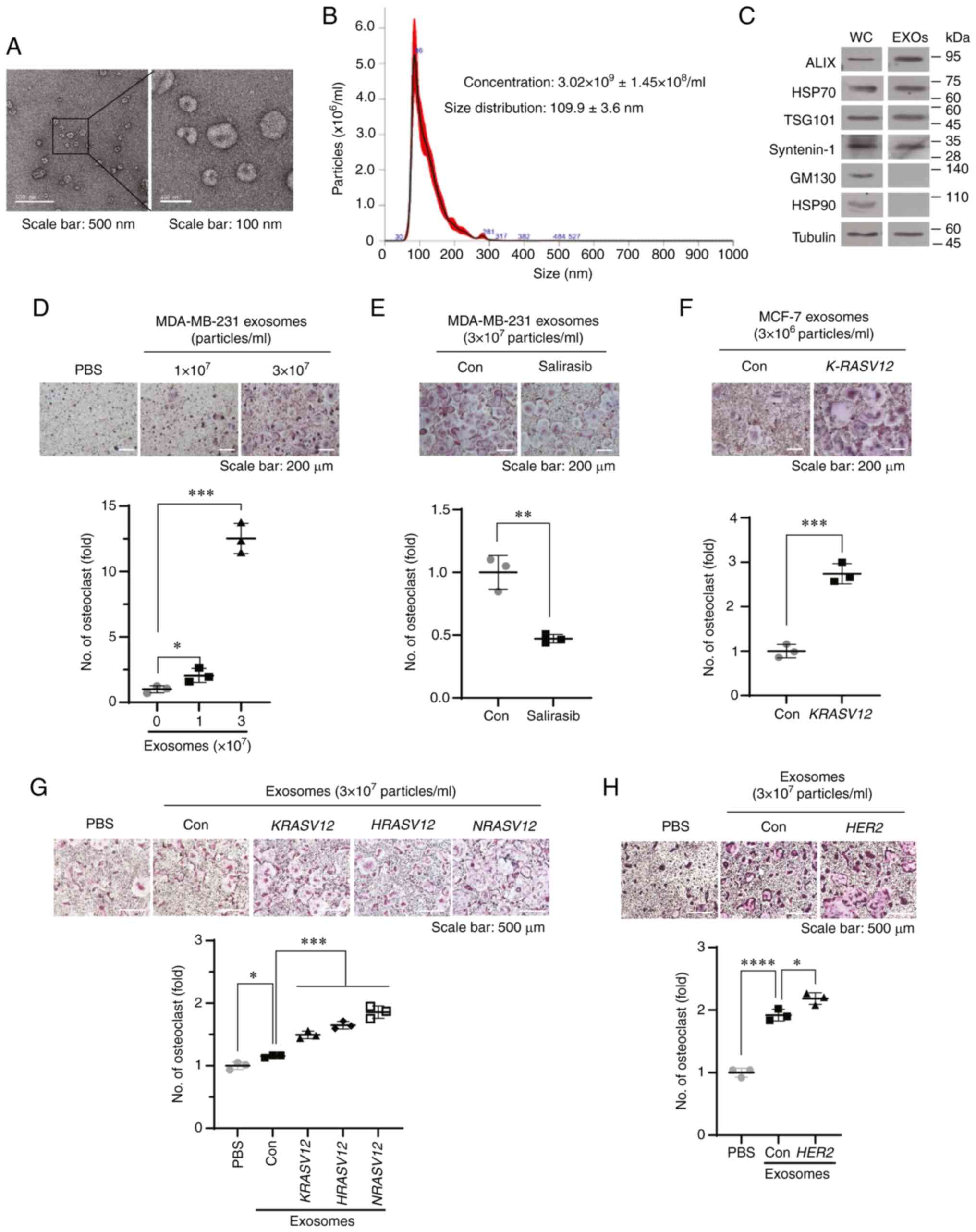 | Figure 1Exosomes derived from MDA-MB-231
cells stimulate RANKL-induced osteoclastogenesis in vitro.
(A) Representative transmission electron microscopy images of
exosomes derived from MDA-MB-231 cells. Scale bar, 500 nm (left
panel) and 100 nm (right panel). (B) Nanoparticle tracking analysis
of exosomes derived from MDA-MB-231 cells. (C) Western blot
analysis of whole cell lysates (WC) and exosomes (EXOs) prepared
from MDA-MB-231 cells. (D) BMMs were treated with exosomes derived
from MDA-MB-231 cells in the presence of RANKL and M-CSF for 4
days. Representative TRAP staining images and quantification of
TRAP-positive multinucleated cells. *P<0.05 and
***P<0.001. (E) BMMs were treated with exosomes
derived from MDA-MB-231 cells treated with vehicle (Con) or the pan
RAS inhibitor, salirasib (10 µM), in the presence of RANKL
and M-CSF for 6 days. Representative TRAP staining images and
quantification of TRAP-positive multinucleated cells.
**P<0.01. (F) BMMs were treated with exosomes derived
from MCF-7 cells transfected with control vector (Con) or K-RASV12
vector in the presence of RANKL and M-CSF for 6 days.
Representative TRAP staining images and number of TRAP-positive
multinucleated cells. ***P<0.001. (G) BMMs were
treated with exosomes derived from T47D cells transfected with
control vector (Con), K-RASV12, H-RASV12, or N-RASV12
vector in the presence of RANKL and M-CSF for 6 days.
Representative TRAP staining images and number of TRAP-positive
multinucleated cells. *P<0.05 and
***P<0.001. (H) BMMs were treated with exosomes
derived from control vector (Con) or HER2 vector-transfected T47D
cells in the presence of RANKL and M-CSF for 6 days.
*P<0.05 and ****P<0.0001. RANKL,
receptor activator of nuclear factor-κB ligand; BMMs, bone
marrow-derived macrophages; TRAP, tartrate-resistant acid
phosphatase; M-CSF, macrophage colony-stimulating factor; EXOs,
exosomes; ALIX, apoptosis-linked gene 2-interacting protein X; HSP,
heat shock protein; TSG101, tumor susceptibility 101; GM130, Golgi
matrix protein 130; HER2, human epidermal growth factor receptor
2. |
Subsequently, the present study determined whether
exosomes derived from the MDA-MB-231 cells stimulated RANKL-induced
osteoclast formation. Treatment of the BMMs with exosomes derived
from MDA-MB-231 cells significantly increased the number of
TRAP-positive multinucleated osteoclasts (Fig. 1D). By contrast, treatment with
exosomes derived from the MDA-MB-231 cells treated with the pan RAS
inhibitor, salirasib, significantly decreased the number of
TRAP-positive osteoclasts, compared with that in the control group
(Fig. 1E). To investigate whether
the activation of RAS signaling contributes to exosome-mediated
osteoclastogenesis, the MCF-7 and T47D cell lines, which carry
wild-type RAS and do not overexpress HER2, were transfected with
mutant RAS or HER2. Transfection of the MCF-7 cells with KRASV12
increased the expression levels of pan-RAS and p-ERK (Fig. S1A). Treatment of the BMMs with
exosomes derived from MCF-7 cells transfected with a KRASV12
expression vector (MCF-7/KRASV12) significantly promoted
RANKL-induced osteoclastogenesis compared with the exosomes derived
from the MCF-7 cells transfected with a control vector (MCF-7/Con
MCF-7/Con cells) (Fig. 1F). In
addition, the effect of RAS signaling on exosome-mediated
osteoclastogenesis in T47D cells following the overexpression of
KRASV12, HRASV12, NRASV12, or HER2 was
also determined (Fig. S1B and
C). Exosomes derived from T47D cells transfected with
KRASV12, HRASV12, NRASV12, or HER2
stimulated RANKL-induced osteoclastogenesis (Fig. 1G and H). NTA revealed that the
enforced expression of KRASV12, HRASV12, or
NRASV12 in the MCF-7 or T47D cells increased the
concentration of exosomes (Fig. S1D
and E). Moreover, the transfection of HER2 into the T47D
cells also increased the concentration of exosomes (Fig. S1F). By contrast, treatment of the
MDA-MB-231 cells with salirasib decreased the concentration of
exosomes (Fig. S1G). These
results indicated that RAS activation stimulated exosome-mediated
osteoclastogenesis by regulating the release of exosomes and/or
loading cargo into exosomes in breast cancer cells.
Identification of RAS-dependent
osteoclastogenic miRNAs in exosomes
To identify miRNAs that could be loaded into
exosomes via RAS activation, miRNA expression profiles were
compared between exosomes derived from MCF-7/KRASV12 and MCF-7/Con
cells using a miRNA microarray. A total of 23 miRNAs were
upregulated, and five miRNAs were downregulated in the exosomes
derived from the MCF-7/KRASV12 cells (Fig. S2A-C). To confirm the expression
of these 28 miRNAs in exosomes, their expression levels in exosomes
were analyzed using RT-qPCR. In total, 11 miRNAs whose expression
levels were high were selected, and their expression levels in
exosomes derived from the MCF-7/KRASV12 and MCF-7/Con cells were
compared (Fig. 2A and B). The
levels of eight miRNAs among these were significantly increased in
the exosomes derived from the MCF-7/KRASV12 cells compared to the
exosomes from the MCF-7/Con cells (Fig. 2B). However, the cellular
expression levels of these miRNAs were not significantly increased
by KRASV12 (Fig. 2C). Treatment
of the MDA-MB-231 cells with salirasib significantly decreased the
levels of seven miRNAs in exosomes (Fig. 2D). Moreover, the transfection of
NRASV12 into the T47D cells increased the expression levels of six
miRNAs (miR-494-3p, miR-1915-3p, miR-4508, miR-4516,
miR-6088 and miR-6869-5p) in exosomes, apart from
miR-4530 (Fig. 2E),
suggesting that RAS activation increased the loading of these
miRNAs into exosomes.
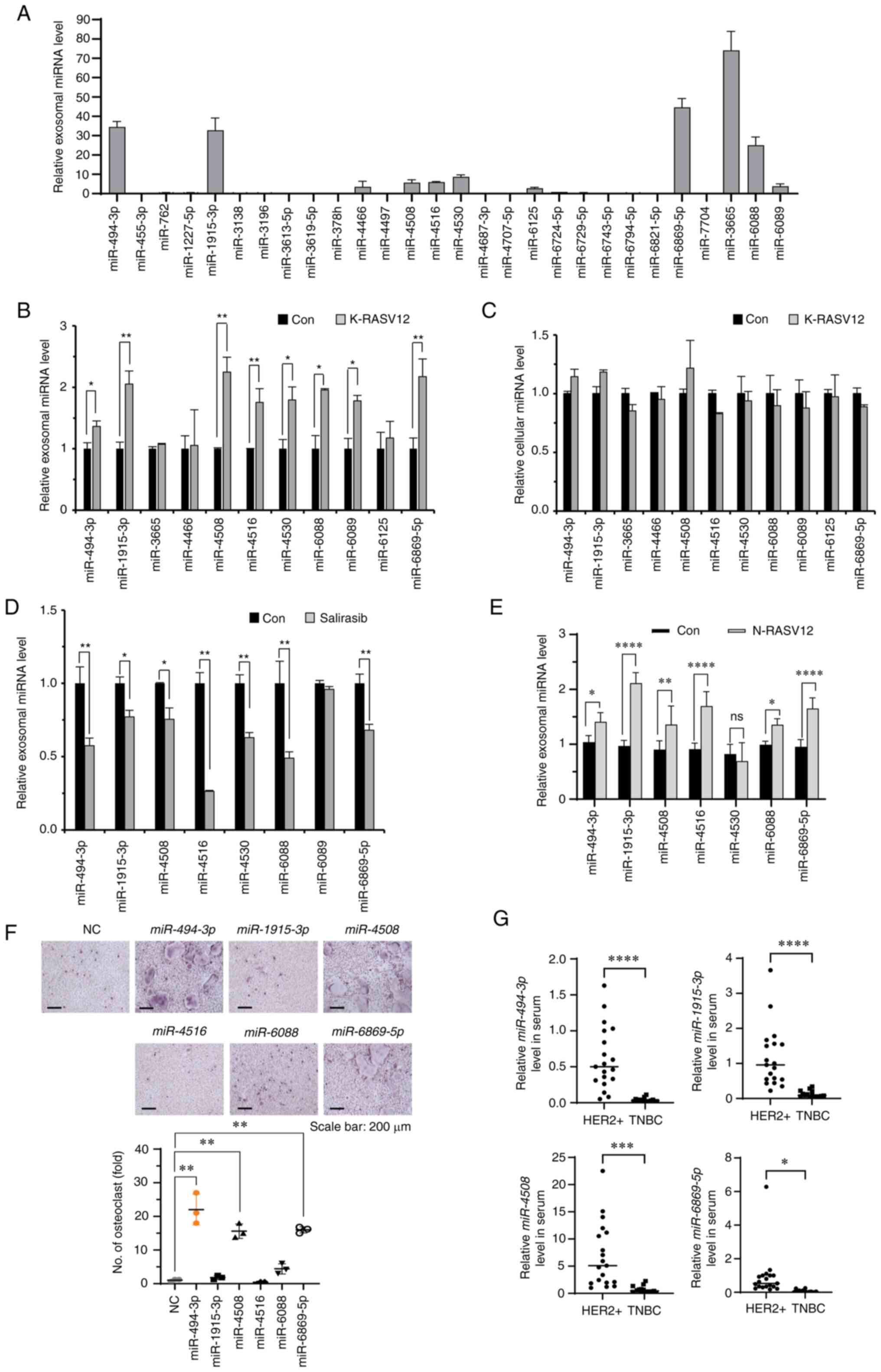 | Figure 2Identification of osteoclastogenic
miRNAs in exosomes induced by RAS activation. (A) Expression levels
of 28 miRNAs in exosomes derived from MCF-7 cells were determined
using RT-qPCR. The results were normalized to U6 snRNA. (B) The
expression levels of 11 selected miRNAs in exosomes derived from
control or KRASV12 vector-transfected MCF-7 cells were determined
using RT-qPCR. The results were normalized to U6 snRNA.
*P<0.05 and **P<0.01. (C) Cellular
expression levels of 11 selected miRNAs in MCF-7 cells transfected
with the control or K-RASV12 vector were determined using RT-qPCR.
The results were normalized to U6 snRNA. (D) The expression levels
of eight selected miRNAs in exosomes derived from MDA-MB-231 cells
treated with the control or salirasib (10 µM) were
determined using RT-qPCR. The results were normalized to U6 snRNA.
*P<0.05 and **P<0.01. (E) The
expression levels of seven selected miRNAs in exosomes derived from
T47D cells transfected with the control vector (Con) or N-RASV12
vector were determined using RT-qPCR. The results were normalized
to U6 snRNA. *P<0.05, **P<0.01 and
****P<0.0001. (F) Representative images of
TRAP-positive osteoclasts in BMMs transfected with the indicated
miRNAs. BMMs transfected with the indicated miRNAs (20 nM each)
were stimulated with RANKL and M-CSF for 4 days. NC, miRNA mimic
negative control. **P<0.01. (G) The expression levels
of miR-494-3p, miR-1915-3p, miR-4508 and miR-6869-5p
in sera derived from patients with HER2-positive breast cancer
(n=19) and triple-negative breast cancer (n=15). The results were
normalized to U6 snRNA. *P<0.05,
***P<0.001 and ****P<0.0001. RT-qPCR,
reverse transcription-quantitative PCR; M-CSF, macrophage
colony-stimulating factor; HER2, human epidermal growth factor
receptor 2; TNBC, triple-negative breast cancer; BMMs, bone
marrow-derived macrophages. |
Subsequently, the effects of these six miRNAs on
RANKL-induced osteoclastogenesis in BMMs and BMP2-induced
osteoblastogenesis in C2C12 cells were determined. Transfection of
these miRNA mimics into BMMs significantly increased their
expression levels (Fig. S2D).
Three miRNAs (miR-494-3p, miR-4508 and miR-6869-5p)
significantly promoted RANKL-induced osteoclast formation in BMMs
(Fig. 2F), and two miRNAs
(miR-494-3p and miR-1915-3p) significantly suppressed
BMP2-induced osteoblastogenesis (Fig. S2E). The present study also
determined the expression levels of these four miRNAs in the serum
of patients with HER2-positive and triple-negative breast cancer.
The levels of miR-494-3p, miR-1915-3p, miR-4508 and
miR-6869-5p in serum from patients with HER2-positive breast
cancer were significantly higher than those in the serum of
patients with triple-negative breast cancer (Fig. 2G). Therefore, miR-494-3p
was selected for further analyses based on its RAS-dependent
expression in exosomes and potent regulatory effects on
osteoclastogenesis and osteoblastogenesis.
miR-494-3p in exosomes derived from
breast cancer cells is taken up into osteoclast precursors, and
promotes RANKL-induced osteoclastogenesis
PKH-labeled exosomes derived from the MDA-MB-231
cells were internalized into BMMs and RAW264.7 cells (Fig. 3A) and treatment of the BMMs with
exosomes derived from the MDA-MB-231 cells significantly increased
the expression of miR-494-3p in BMMs (Fig. 3B), indicating that exosomes
derived from the MDA-MB-231 cells were effectively taken up by
osteoclast precursors. Treatment of the BMMs with exosomes
significantly increased the number of TRAP-positive osteoclasts,
whereas treatment with miR-494-3p inhibitor inhibited the
exosome-mediated increase in osteoclast formation (Fig. 3C). The functional role of
miR-494-3p in RANKL-induced osteoclastogenesis was then
examined. Stimulation of the BMMs with RANKL increased the
expression of miR-494-3p (Fig.
3D), and treatment with miR-494-3p mimic promoted
formation of TRAP-positive osteoclasts (Fig. 3E). In contrast, treatment with
miR-494-3p inhibitor decreased the expression of
miR-494-3p (Fig. 3F) and
inhibited RANKL-induced osteoclastogenesis (Fig. 3G). Treatment with
miR-494-3p mimic increased the expression of CtsK, an
osteoclast marker, and the formation of resorption pits by mature
osteoclasts in response to RANKL, whereas treatment with
miR-494-3p inhibitor suppressed these effects (Fig. 3H and I). Moreover, treatment with
miR-494-3p mimic increased the RANKL-induced expression of
NFATc1 target genes, including ACP5, acid phosphatase 5, tartrate
resistant (ACP5), cathepsins K (CtsK) and dendrocyte
expressed seven transmembrane protein (DC-STAMP) (Fig. 3J). These results suggested that
miR-494-3p may be a positive regulator of RANKL-induced
osteoclastogenesis in BMMs and exosomes derived from breast cancer
cells stimulated osteoclast differentiation, at least in part, by
transferring exosomal miR-494-3p to osteoclast
precursors.
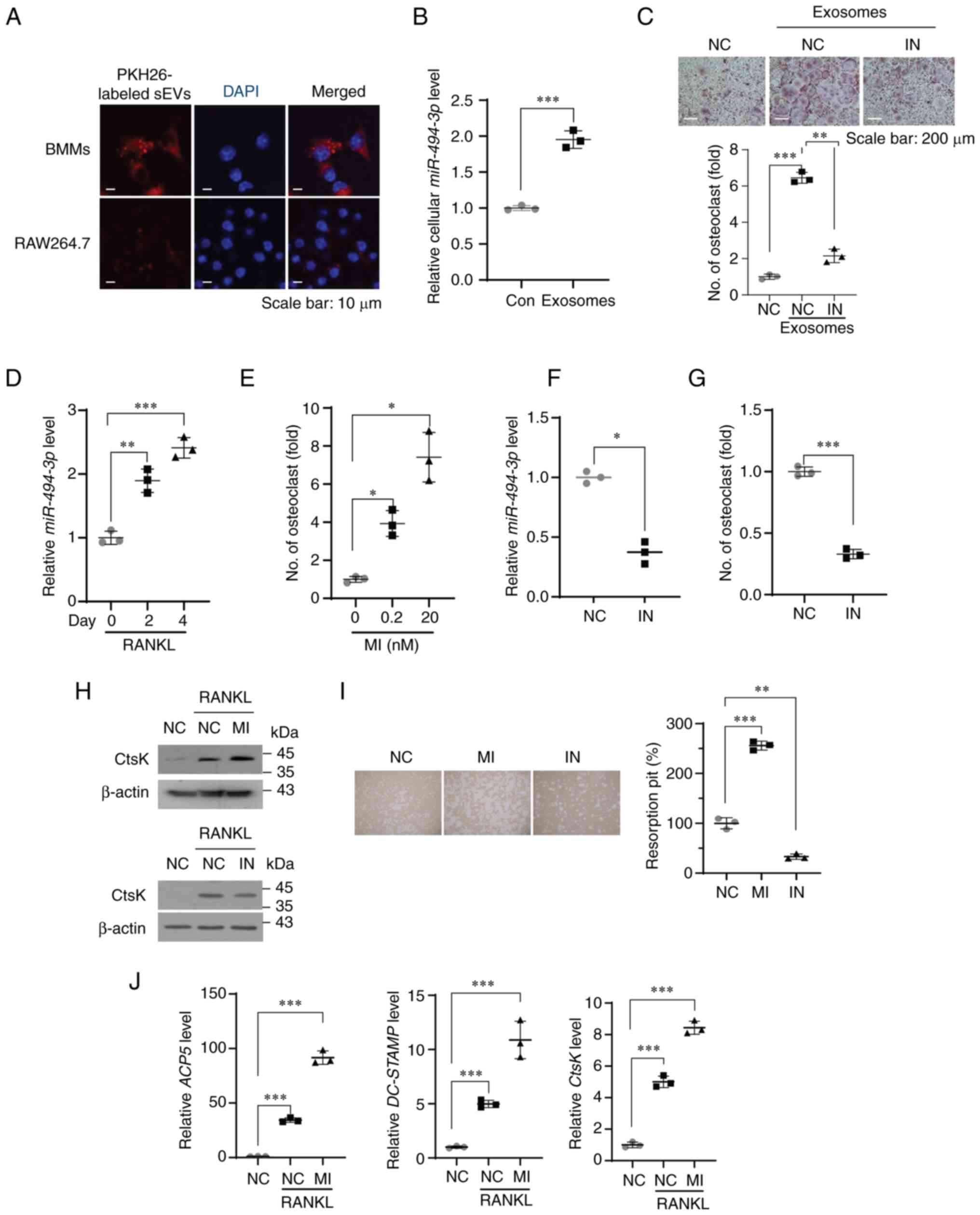 | Figure 3miR-494-3p in exosomes derived from
breast cancer cells stimulates RANKL-induced osteoclast
differentiation. (A) Representative confocal microscopy images of
BMMs (upper panels) and RAW264.7 cells (lower panels) treated with
PKH26-labeled exosomes derived from MDA-MB-231 cells for 24 h. Cell
nuclei were stained with DAPI (blue). (B) Expression level of
miR-494-3p in BMMs treated with PBS (Con) and exosomes
(3×107 particles/ml) derived from MDA-MB-231cells for 24 h was
determined using RT-qPCR (n=3). ***P<0.001. (C) BMMs
were transfected with miRNA inhibitor negative control miRNA (NC)
or miR-494-3p inhibitor (IN, 100 nM) and sunsequently stimulated
with exosomes (3×107 particles/ml) derived from MDA-MB-231 cells in
the presence of M-CSF and RANKL for 5 days. Representative image of
TRAP staining. Graph represents the number of TRAP-positive
multinucleated osteoclasts (n=3). Scale bar, 200 µm.
**P<0.01 and ***P<0.001. (D) BMMs were
stimulated with RANKL and M-CSF for the indicated period.
Expression level of miR-494-3p was determined using RT-qPCR (n=3).
The results were normalized to U6 snRNA. **P<0.01 and
***P<0.001. (E) BMMs were transfected with miRNA
mimic negative control (NC) and miR-494-3p mimic (MI, 0.2
and 20 nM) in the presence of M-CSF and RANKL for 5 days. The graph
represents the number of TRAP-positive multinucleated osteoclasts
(n=3). *P<0.05. (F) BMMs were transfected with miRNA
inhibitor negative control (NC) and miR-494-3p inhibitor (IN, 100
nM). The expression level of miR-494-3p in BMMs was determined
using RT-qPCR (n=3). The results were normalized to U6 snRNA.
*P<0.05. (G) BMMs were transfected with miRNA
inhibitor negative control (NC) and miR-494-3p inhibitor (IN, 100
nM) in the presence of M-CSF and RANKL for 5 days. The graph
represents the number of TRAP-positive multinucleated osteoclasts
(n=3). ***P<0.001. (H) BMMs transfected with negative
control miRNA, miR-494-3p mimic (MI, 20 nM), or miR-494-3p
inhibitor (IN, 100 nM) were stimulated with RANKL and M-CSF for 4
days. Western blot analysis was performed to determine the
expression levels of CtsK and β-actin. (I) BMMs transfected with
negative control miRNA, miR-494-3p mimic (MI, 20 nM), or
miR-494-3p inhibitor (IN, 100 nM) were stimulated with M-CSF
and RANKL for 6 days. Pit resorption area was measured using ImageJ
software (n=3). **P<0.01 and
***P<0.001. (J) BMMs were transfected with miRNA
mimic negative control (NC) or miR-494-3p mimic (MI, 20 nM) and
subsequently stimulated with M-CSF and RANKL for 6 days. RT-qPCR
was performed to determine the mRNA expression levels of CtsK,
ACP5 and DC-STAMP (n=3). ***P<0.001.
RT-qPCR, reverse transcription-quantitative PCR; RANKL, receptor
activator of nuclear factor-κB ligand; BMMs, bone marrow-derived
macrophages; TRAP, tartrate-resistant acid phosphatase; M-CSF,
macrophage colony-stimulating factor; CtsK, cathepsin K; ACP5, acid
phosphatase 5, tartrate resistant; DC-STAMP, dendrocyte expressed
seven transmembrane protein. |
LGR4 is a potential target of miR-494-3p
in osteoclast precursors
To elucidate the molecular mechanisms by which
miR-494-3p regulates osteoclast differentiation, potential
target genes of miR-494-3p were analyzed using two most
widely used miRNA target prediction databases: miRDB (mirdb.org) and TargetScan (targetscan.org). LGR4, a negative regulator of
osteoclast differentiation (26),
was identified as a potential target gene of miR-494-3p in
osteoclast precursors (Fig. 4A).
Treatment with miR-494-3p mimic significantly suppressed
both the mRNA and protein expression of LGR4, whereas treatment
with miR-494-3p inhibitor markedly increased the mRNA and
protein expression of LGR4 (Fig. 4B
and C). Consistent with the LGR4 expression levels, treatment
with miR-494-3p inhibitor decreased the expression of
miR-494-3p, whereas treatment with miR-494-3p mimic
increased the expression of miR-494-3p in RAW264.7 cells
(Fig. S3A). Treatment of the
RAW264.7 cells with exosomes derived from the MDA-MB-231 cells
resulted in a significant decrease in the protein expression of
LGR4; however, treatment with miR-494-3p inhibitor reversed
the exosome-mediated downregulation of LGR4 (Fig. 4D).
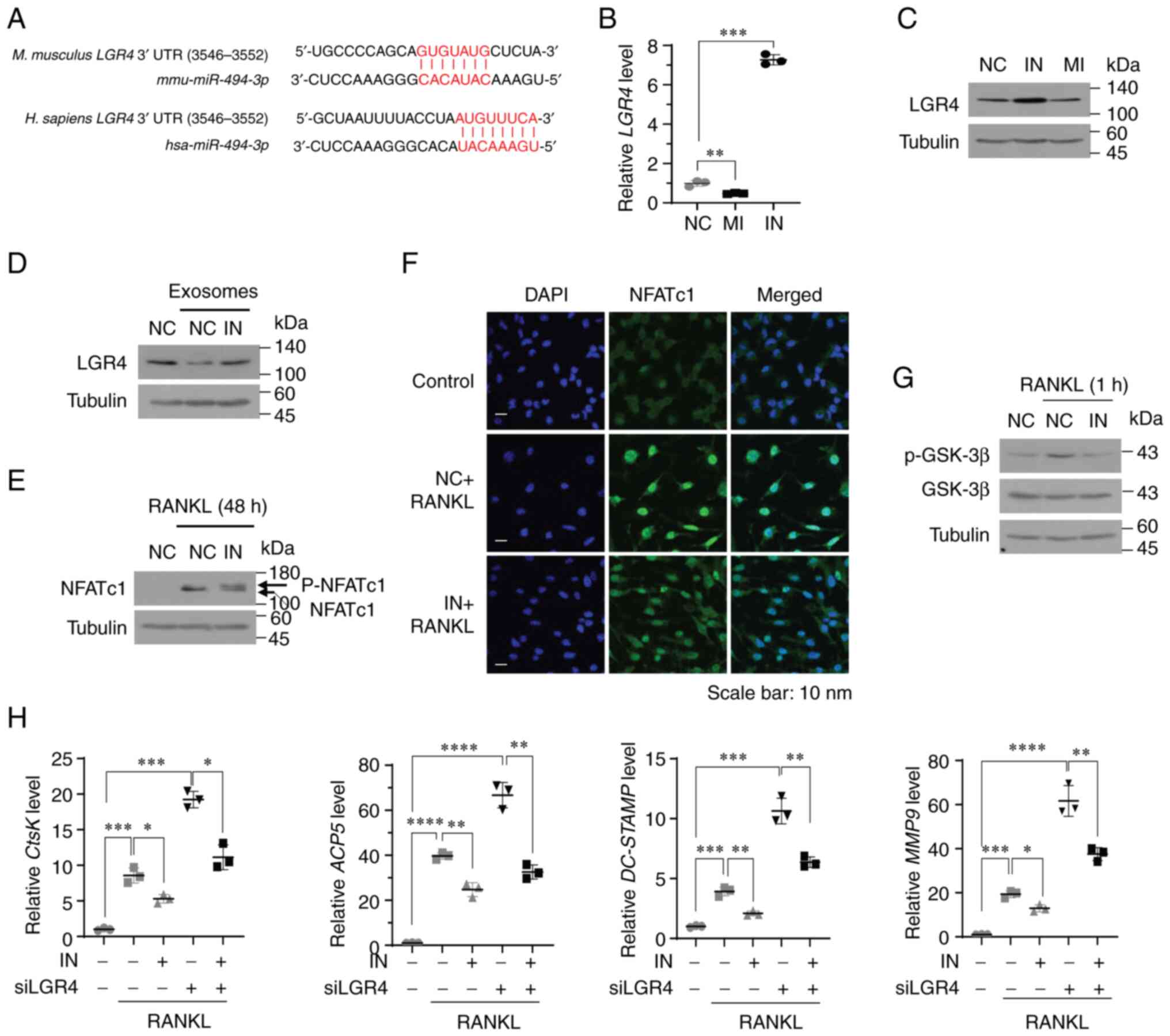 | Figure 4LGR4 is a potential target of
miR-494-3p in osteoclasts. (A) miR-494-3p binding sites in
human and murine LGR4 mRNA. (B and C) RAW264.7 cells were
transfected with miRNA negative control (NC), miR-494-3p mimic (MI,
20 nM), or miR-494-3p inhibitor (IN, 100 nM). The LGR4 mRNA (B) and
protein (C) expression levels were determined using (B) RT-qPCR and
(C) western blot analysis. (D) RAW264.7 cells were transfected with
miRNA negative control miRNA (NC) or miR-494-3p inhibitor
(IN, 100 nM) and subsequently incubated with exosomes
(3×107 particles/ml) derived from MDA-MB-231 cells for
24 h. The expression level of LGR4 was determined using western
blot analysis. (E) RAW264.7 cells were transfected with miRNA
negative control miRNA (NC) or miR-494-3p inhibitor (IN, 100
nM) and subsequently stimulated with RANKL (100 ng/ml) for 48 h.
The expression level of NFATc1 was determined using western blot
analysis. (F) BMMs transfected with miRNA negative control miRNA
(NC) or miR-494-3p inhibitor (IN, 100 nM) were stimulated
with RANKL and M-CSF for 4 days. The cells were stained with an
anti-NFATc1 antibody. DAPI was used to stain the nuclei. (G)
RAW264.7 cells transfected with NC miRNA (NC) or miR-494-3p
inhibitor (IN, 100 nM) were stimulated with RANKL for 1 h. The
expression level of phospho-GSK-3β was determined using western
blot analysis. (H) RAW264.7 cells transfected with
miR-494-3p inhibitor (IN, 100 nM) and/or LGR4 siRNA (siLGR4)
were stimulated with RANKL for 3 days. The expression levels of
CtsK, ACP5, DC-STAMP and MMP9 were determined using
RT qPCR. *P<0.05, **P<0.01,
***P<0.001 and ****P<0.0001. RT-qPCR,
reverse transcription-quantitative PCR; LGR4, leucine-rich
repeat-containing G-protein coupled receptor 4; RANKL, receptor
activator of nuclear factor-κB ligand; BMMs, bone marrow-derived
macrophages; NFATc1, nuclear factor of activated T-cells 1; GSK-3β,
glycogen synthase kinase 3β. |
LGR4 is a receptor for RANKL and negatively
regulates RANKL-induced osteoclast differentiation by inhibiting
the RANKL-induced activation of NF-κB and NFATc1 (26). RANKL binding to LGR4 activates Gq
protein α-subunit, resulting in the inhibition of the glycogen
synthase kinase (GSK)-3b-mediated activation of NFATc1 (26). Thus, the present study then
determined the effects of miR-494-3p on the RANKL-induced
activation of NF-κB and NFATc1. Treatment with miR-494-3p
inhibitor significantly blocked the RANKL-induced degradation of
IκBα and the nuclear translocation of the RelA/p65 subunit of NF-κB
(Fig. S3B and C). In addition,
miR-494-3p inhibitor blocked the RANKL-induced nuclear
translocation and dephosphorylation of NFATc1 (Fig. 4E and F) and GSK-3β phosphorylation
(Fig. 4G). LGR4 knockdown
markedly increased the RANKL-induced expression of NFATc1 target
genes, including CtsK, ACP5, DC-STAMP and
MMP-9 (Figs. 4H and
S3D), whereas treatment with
miR-494-3p inhibitor impaired these effects (Fig. 4H). Overall, these findings suggest
that miR-494-3p in exosomes stimulates RANKL-induced
osteoclastogenesis by downregulating the expression of LGR4 in
osteoclast precursors.
miR-494-3p inhibits osteoblastogenesis by
targeting semaphorin 3A (SEMA3A)
SEMA3A was also identified as a potential
target of miR-494-3p (Fig.
5A). SEMA3A stimulates osteoblast differentiation and inhibits
osteoclast differentiation (27).
As osteoblasts mainly express SEMA3A (27,28), the present study then investigated
whether miR-494-3p regulates BMP2-induced osteoclastogenesis
by targeting SEMA3A in C2C12 cells. Treatment of the C2C12
cells with miR-494-3p mimic or miR-494-3p inhibitor
effectively modulated decreased the expression level of
miR-494-3p (Fig. S4A and
B). Treatment with miR-494-3p mimic decreased the
protein and mRNA expression of SEMA3A, whereas treatment with
miR-494-3p inhibitor increased the expression of SEMA3A in
C2C12 cells (Fig. 5B and C).
Treatment of the C2C12 cells with exosomes derived from MDA-MB-231
cells decreased the protein and mRNA expression levels of SEMA3A
(Fig. 5D and E). The expression
of miR-494-3p was downregulated by treatment with BMP2 in
C2C12 cells (Fig. 5F). Treatment
of the C2C12 cells with exosomes derived from the MDA-MB-231 cells
reduced BMB2-induced osteoblast formation (Fig. 5G). Moreover, treatment with
miR-494-3p mimic suppressed BMP2-induced osteoblastogenesis,
whereas treatment with miR-494-3p inhibitor reversed this
effect (Fig. 5H). On the whole,
these results indicated that miR-494-3p in exosomes impaired
BMP2-induced osteoblastogenesis by targeting SEMA3A.
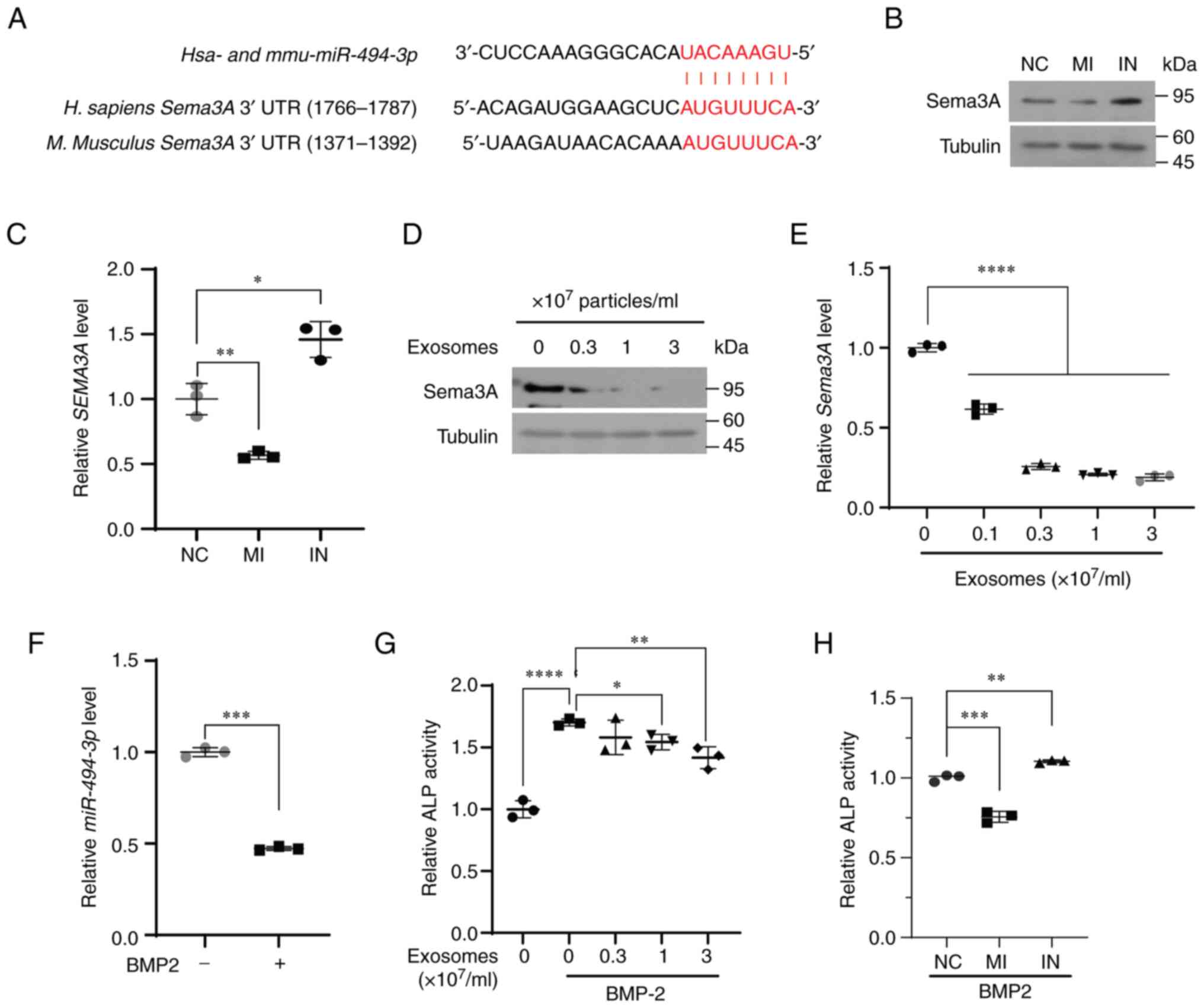 | Figure 5SEMA3A is a potential target
of miR-494-3p in osteoblasts. (A) miR-494-3p binding
sites in human and murine SEMA3A mRNA. (B and C) C2C12 cells
were transfected with negative control miRNA (NC),
miR-494-3p mimic (MI, 20 nM), or miR-494-3p inhibitor
(IN, 100 nM). The expression level of SEMA3A was determined using
(B) western blot analysis and (C) RT-qPCR. *P<0.05.
(D and E) C2C12 cells were incubated with exosomes derived from
MDA-MB-231 cells at the indicated concentrations for 24 h. The
expression level of SEMA3A was determined using (D) western blot
analysis and (E) RT-qPCR (n=3). ****P<0.0001. (F) The
C2C12 cells were stimulated with BMP2 (30 ng/ml) for 3 days. The
expression level of miR-494-3p was determined using RT-qPCR (n=3),
***P<0.001. (G) C2C12 cells incubated with exosomes
derived from MDA-MB-231 cells at the indicated concentrations for
24 h were stimulated with BMP2 for 3 days. ALP activity was
determined (n=3). *P<0.05, **P<0.01 and
****P<0.0001. (H) C2C12 cells transfected with NC
miRNA (NC), miR-494-3p mimic (MI, 20 nM), and
miR-494-3p inhibitor (IN, 100 nM) were incubated with BMP2
for 3 days. The activity of ALP was determined (n=3),
**P<0.01 and ***P<0.001. SEMA3A,
semaphorin 3A; RT-qPCR, reverse transcription-quantitative PCR;
BMP2, bone morphogenetic protein 2; ALP, alkaline phosphatase. |
Exosomes stimulate tumor growth in the
tibia, whereas treatment with miR-494-3p inhibitor suppresses
it
Mouse 4T1-Luc2 cell-derived exosomes abundantly
expressed miR-494-3p and stimulated RANKL-induced osteoclast
formation (Fig. 6A and B). Thus,
the present study then investigated whether 4T1-Luc2 cell-derived
exosomes and miR-494-3p regulate osteolytic tumor growth in
the bone microenvironment, by injecting 4T1-Luc2 cells into the
bone marrow cavity of the tibias of BALB/c mice. Quantification of
the bioluminescence signal revealed that the exosome-treated mice
exhibited an increased tumor growth in the tibia compared with the
control mice, whereas exosome-induced tumor growth in the tibia was
significantly suppressed by treatment with miR-494-3p
inhibitor (Fig. 6C).
Immunochemical staining of the tibiae revealed that the
exosome-treated mice had a significantly increased number of
TRAP-positive osteoclasts at the tumor-bone interface; however,
treatment with miR-494-3p inhibitor reversed these effects
(Fig. 6D). These results
suggested that the exosome-mediated transfer of miR-494-3p
from breast cancer cells to bone cells induced osteolytic bone
metastases by stimulating osteoclastogenesis in the bone
microenvironment.
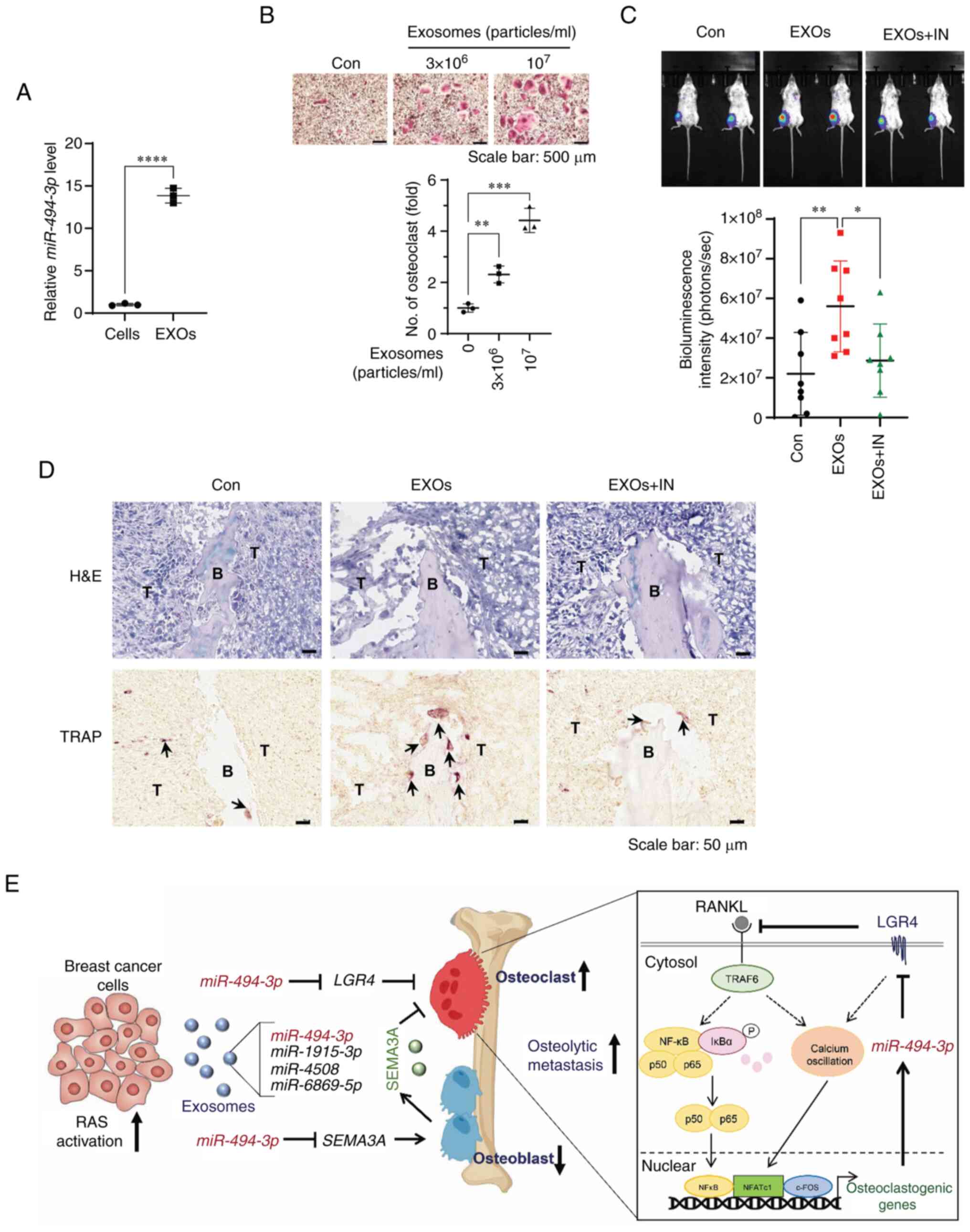 | Figure 6miR-494-3p inhibitor treatment
suppresses the osteolytic bone metastasis of 4T1 breast cancer
cells in a mouse model. (A) Expression levels of miR-494-3p
in 4T1-Luc2 cells and exosomes (EXOs) derived from 4T1-Luc2 cells
as determined using RT-qPCR. ****P<0.0001. (B) BMMs
were treated with exosomes derived from 4T1-Luc2 cells in the
presence of M-CSF and RANKL for 5 days. Representative images of
TRAP staining. The graph represents the number of TRAP-positive
multinucleated osteoclasts (n=3). Scale bar, 500 µm.
**P<0.01 and ***P<0.001. (C)
Representative bioluminescence images (upper panel) and
quantification of bioluminescence intensity (lower panel) showing
4T1-Luc2 cell localization after 2 weeks in recipient mice with
different treatments (n=8). Con, PBS treatment; EXOs, exosome
treatment; EXOs + IN, exosomes puls miR-494-3p inhibitor
treatment. *P<0.05 and **P<0.01. (D)
Representative images of H&E and TRAP staining of bones
isolated from the indicated mice on day 14 after exosomes (EXOs)
or/and miR-494-3p inhibitor (IN) treatment. Arrows indicate
TRAP-positive osteoclasts. B, bone; T, tumor. Scale bar, 50
µm. (E) Working model of exosomal miR-494-3p secreted
from RAS-ativated breast cancer cells in regulating osteolytic bone
metastasis of breast cancer cells. RANKL, receptor activator of
nuclear factor-κB ligand; BMMs, bone marrow-derived macrophages;
TRAP, tartrate-resistant acid phosphatase; H&E, hematoxylin and
eosin; SEMA3A, semaphorin 3A; LGR4, leucine-rich repeat-containing
G-protein coupled receptor 4. |
Discussion
Understanding the molecular mechanisms underlying
bone metastasis in breast cancer is critical for the development of
novel strategies with which to treat and/or prevent bone
metastasis. The present demonstrated that RAS activation in breast
cancer cells stimulated the secretion of osteoclastogenic
miR-494-3p, miR-4508 and miR-6869-5p on
exosomes to promote osteolytic bone metastasis. In addition, the
functional role of exosomal miR-494-3p in remodeling the
bone microenvironment to induce osteolytic bone metastasis in
vitro and in vivo was described.
Breast cancer cells secrete various
pro-osteoclastogenic factors, including interleukin (IL)-6, IL-1β,
and parathyroid hormone-related protein, to activate osteoclast
differentiation and function, triggering a vicious cycle in
osteolytic bone metastasis (5,7,29).
Accumulating evidence indicates that exosomes secreted by breast
cancer cells participate in bone metastasis by enhancing osteoclast
formation and function (14,30,31). miRNAs in exosomes may be critical
mediators for generating a bone microenvironment suitable for tumor
growth through crosstalk between cancer cells and the bone
microenvironment (14,30-32). For example, it has been
demonstrated that exosomal miR-21 derived from SCP28 breast
cancer cells contributes to pre-metastatic niche formation by
transferring miR-21 to osteoclasts, resulting in the
promotion of osteolytic bone metastasis (31). miR-20a-5p derived from
MDA-MB-231 cells also promotes the proliferation and
differentiation of osteoclasts by targeting SRC kinase signaling
inhibitor 1 (32). In the present
study, RAS activation in breast cancer cells stimulated
exosome-mediated osteoclastogenesis. In total, three
miRNAs-miR-494-3p, miR-4508 and miR-6869-5p were
identified in exosomes as osteoclastogenic miRNAs whose expression
increased upon RAS activation. miR-494-3p and
miR-1915-3p suppressed BMP2-induced osteoblast
differentiation. Notably, a miR-494-3p inhibitor suppressed
the stimulatory effect of exosomes on RNAKL-induced
osteoclastogenesis in vitro and the exosome-induced tumor
growth in the tibia in vivo. These findings suggested that
the activation of RAS signaling in breast cancer cells played an
essential role in increasing the load of osteoclastogenic and
anti-osteoblastogenic miRNAs, including miR-494-3p on
exosomes.
Breast cancer is generally classified into four
molecular subtypes based on the ER, PR and HER2 status: Luminal A
(ER+ and/or PR+, HER2−), luminal B
(ER+ and/or PR+, HER2+), HER2
(ER− and/or PR−, HER2+) and
basal-type (triple-negative) tumors (3). The bone is a common site of
metastasis for all breast cancer subtypes, apart from basal-like
tumors (4,33). The incidence of bone metastasis is
highest in the luminal B subtype (4,33).
In the present study, it was found that the levels of
miR-494-3p, miR-1915-3p, miR-4508 and
miR-6869-5p in the serum of patients with HER2-positive
human breast cancer were significantly higher than those in the
serum of patients with triple-negative breast cancer. These
findings indicated that the activation of RAS signaling via HER2
overexpression may induce osteolytic bone metastasis by increasing
the release of osteoclastogenic and anti-osteoblastogenic miRNAs,
including miR-494-3p from breast cancer cells in certain
breast cancer subtypes, such as luminal B.
miR-494-3p is an onco-miRNA regulating cell
proliferation, migration and invasion by targeting PTEN,
PTPN12, or SOX7 in different types of cancer cells
(25,34-36). However, the functions of
miR-494-3p in osteoclastogenesis and osteolytic bone
metastasis remain unclear. In the present study, it was found that
miR-494-3p promoted RANKL-induced osteoclastogenesis by
targeting LGR4 in osteoclast precursors, whereas it
suppressed bone-forming osteoblast differentiation by targeting
SEMA3A. In addition, treatment with miR-494-3p
inhibitor suppressed exosome-mediated tumor growth and osteoclast
formation in the tibia in vivo. These findings indicated
that exosomal miR-494-3p secreted from breast cancer cells
was a critical mediator of osteoclytic bone metastasis. LGR4
functions as a RANKL receptor that suppresses osteoclast
differentiation and bone formation by activating Gαq-GSK-3β
signaling pathway and inhibiting NF-κB activation, which results in
the expression of and activity of NFATc1 during osteoclastogenesis
(26). In the present study,
treatment with miR-494-3p mimic downregulated LGR4 mRNA and
protein expression, whereas that with miR-494-3p inhibitor
reversed this effect in osteoclast precursors. Moreover, treatment
with miR-494-3p inhibitor suppressed RANKL-induced
activation of NF-κB and NFATc1, suggesting that miR-494-3p
promoted osteoclast differentiation, at least in part, by targeting
LGR4. SEMA3A regulates neuronal guidance, bone remodeling,
cancer progression, and immune disorders (37). It is mainly expressed by
osteoblasts, whereas its receptor, neuropilin 1, is expressed in
osteoclast precursors (37).
SEMA3A positively regulates osteoblast differentiation through the
Wnt/β-catenin pathway, but suppresses osteoclast differentiation by
suppressing phospholipase Cγ activation and calcium oscillation
(27,28). Therefore, the findings presented
herein suggested that miR-494-3p suppressed SEMA3A
expression in osteoblast precursors, which may contribute to the
stimulation of osteolytic bone lesion formation in the bone
microenvironment.
In spite of these novel findings, the present study
has several limitations. First, osteolytic bone metastasis in
vivo is a complex process that is regulated by complex
communications between cancer cells and stromal cells in the bone
microenvironment. Moreover, a single miRNA can target multiple
genes. The present study focused on the effects of
miR-494-3p on osteoclastogenesis and osteoblastogenesis.
Further studies are required to investigate other target genes of
miR-494-3p in the bone microenvironment. Second, the present
study only investigated the effects of miR-494-3p on
osteolytic bone metastasis in female mice. Although male breast
cancer is rare, it remains to be elucidated whether
miR-494-3p may regulate osteolytic bone metastasis in male
mice.
In conclusion, the present study found that RAS
activation promoted exosome-mediated osteoclastogenesis by
increasing the loading of osteoclastogenic miRNAs, including
miR-494-3p, into exosomes. miR-494-3p is a major
osteoclastogenic miRNA secreted by RAS-activated breast cancer
cells. miR-494-3p promoted RANKL-induced osteoclastogenesis
by targeting LGR4 and SEMA3A in the bone
microenvironment, and treatment with miR-494-3p inhibitor
suppressed osteolytic lesions in an animal model. Overall, the
results presented herein suggested that miRNAs in exosomes,
including miR-494-3p, derived from RAS-activated breast
cancer cells were critical mediators in the induction of osteolytic
bone metastasis in human breast cancer.
Supplementary Data
Availability of data and materials
The datasets used in the current study are
available from the corresponding author on reasonable request. The
original miRNA array data generated in the present study were
submitted to the GEO repository (accession no. GSE235802). This
accession no. is currently private and is scheduled to be released
on June 16, 2024.
Authors' contributions
OK, PTT and MG performed the research. SJL and SHN
contributed to the analyses of miRNAs in the serum of patients. CH
analyzed the data. JHL designed the experiments and wrote the
manuscript. OK, PTT and JHL confirm the authenticity of all the raw
data. All authors have read and approved the final manuscript.
Ethics approval and consent to
participate
All animal experimental protocols were approved by
the Institutional Animal Care and Use Committee (IACUC) of Kangwon
National University (KW-210914-1, September 23, 2021), and all
experiments were performed in accordance with relevant regulation
and guideline. Blood collection from breast cancer patients was
performed following the guiding principles of the Declaration of
Helsinki and was approved by the Institutional Review Board of
Kangwon National University Hospital (KNUH-2022-04-011; approved
date: May 4, 2022). The requirement for informed consent was waived
by the Institutional Review Board of Kangwon National University
Hospital owing to the retrospective nature of the study.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
Abbreviations:
|
ALIX
|
apoptosis-linked gene 2-interacting
protein X
|
|
ALP
|
alkaline phosphatase
|
|
BMMs
|
bone marrow-derived macrophages
|
|
DAPI
|
4′,6-diamidino-2-phenylindole
|
|
ER
|
estrogen receptor
|
|
FEB
|
fetal bovine serum
|
|
GM130
|
Golgi matrix protein 130
|
|
HER2
|
human epidermal growth factor
receptor 2
|
|
M-CSF
|
macrophage colony-stimulating
factor
|
|
NTA
|
nanoparticle tracking analysis
|
|
PBS
|
phosphate-buffered saline
|
|
PR
|
progesterone receptor
|
|
qPCR
|
quantitative polymerase chain
reaction
|
|
RANKL
|
receptor activator of nuclear
factor-κB ligand
|
|
TEM
|
transmission electron microscopy
|
|
TRAP
|
tartrate-resistant acid
phosphatase
|
Acknowledgments
The biospecimens and data used in the present study
were provided by the Biobank of Kangwon University Hospital, a
member of the Korea Biobank Network. The authors would like to
acknowledge Professor Kwang-Yeol Lee, Chonnam National University,
Republic of Korea, for the RAS constructs.
Funding
The present study was supported by grants from the National
Research Foundation (NRF) of Korea (2020R1A5A8019180).
References
|
1
|
Torre LA, Islami F, Siegel RL, Ward EM and
Jemal A: Global cancer in women: Burden and trends. Cancer
Epidemiol Biomarkers Prev. 26:444–457. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Hernandez RK, Wade SW, Reich A, Pirolli M,
Liede A and Lyman GH: Incidence of bone metastases in patients with
solid tumors: Analysis of oncology electronic medical records in
the United States. BMC Cancer. 18:442018. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Perou CM, Sørlie T, Eisen MB, van de Rijn
M, Jeffrey SS, Rees CA, Pollack JR, Ross DT, Johnsen H, Akslen LA,
et al: Molecular portraits of human breast tumours. Nature.
406:747–752. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Kennecke H, Yerushalmi R, Woods R, Cheang
MC, Voduc D, Speers CH, Nielsen TO and Gelmon K: Metastatic
behavior of breast cancer subtypes. J Clin Oncol. 28:3271–3277.
2010. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Coleman RE, Croucher PI, Padhani AR,
Clézardin P, Chow E, Fallon M, Guise T, Colangeli S, Capanna R and
Costa L: Bone metastases. Nat Rev Dis Primers. 6:832020. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Waning DL and Guise TA: Molecular
mechanisms of bone metastasis and associated muscle weakness. Clin
Cancer Res. 20:3071–3077. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Weilbaecher KN, Guise TA and McCauley LK:
Cancer to bone: A fatal attraction. Nat Rev Cancer. 11:411–425.
2011. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Wortzel I, Dror S, Kenific CM and Lyden D:
Exosome-mediated metastasis: Communication from a distance. Dev
Cell. 49:347–360. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Möller A and Lobb RJ: The evolving
translational potential of small extracellular vesicles in cancer.
Nat Rev Cancer. 20:697–709. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Théry C, Witwer KW, Aikawa E, Alcaraz MJ,
Anderson JD, Andriantsitohaina R, Antoniou A, Arab T, Archer F,
Atkin-Smith GK, et al: Minimal information for studies of
extracellular vesicles 2018 (MISEV2018): A position statement of
the international society for extracellular vesicles and update of
the MISEV2014 guidelines. J Extracell Vesicles. 7:15357502018.
View Article : Google Scholar
|
|
11
|
Colombo M, Raposo G and Théry C:
Biogenesis, secretion, and intercellular interactions of exosomes
and other extracellular vesicles. Annu Rev Cell Dev Biol.
30:255–289. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Kalluri R and LeBleu VS: The biology,
function, and biomedical applications of exosomes. Science.
367:eaau69772020. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Dai J, Su Y, Zhong S, Cong L, Liu B, Yang
J, Tao Y, He Z, Chen C and Jiang Y: Exosomes: Key players in cancer
and potential therapeutic strategy. Signal Transduct Target Ther.
5:1452020. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Wu M, Wang G, Hu W, Yao Y and Yu XF:
Emerging roles and therapeutic value of exosomes in cancer
metastasis. Mol Cancer. 18:532019. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Lee TH, Chennakrishnaiah S, Audemard E,
Montermini L, Meehan B and Rak J: Oncogenic ras-driven cancer cell
vesiculation leads to emission of double-stranded DNA capable of
interacting with target cells. Biochem Biophys Res Commun.
451:295–301. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Lobb RJ, Hastie ML, Norris EL, van
Amerongen R, Gorman JJ and Möller A: Oncogenic transformation of
lung cells results in distinct exosome protein profile similar to
the cell of origin. Proteomics. 17:16004322017. View Article : Google Scholar
|
|
17
|
Cha DJ, Franklin JL, Dou Y, Liu Q,
Higginbotham JN, Demory Beckler M, Weaver AM, Vickers K, Prasad N,
Levy S, et al: KRAS-dependent sorting of miRNA to exosomes. Elife.
4:e071972015. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
McKenzie AJ, Hoshino D, Hong NH, Cha DJ,
Franklin JL, Coffey RJ, Patton JG and Weaver AM: KRAS-MEK signaling
controls Ago2 sorting into exosomes. Cell Rep. 15:978–987. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Rochlitz CF, Scott GK, Dodson JM, Liu E,
Dollbaum C, Smith HS and Benz CC: Incidence of activating ras
oncogene mutations associated with primary and metastatic human
breast cancer. Cancer Res. 49:357–360. 1989.PubMed/NCBI
|
|
20
|
Galiè M: RAS as supporting actor in breast
cancer. Front Oncol. 9:11992019. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Wright KL, Adams JR, Liu JC, Loch AJ, Wong
RG, Jo CE, Beck LA, Santhanam DR, Weiss L, Mei X, et al: Ras
signaling is a key determinant for metastatic dissemination and
poor survival of luminal breast cancer patients. Cancer Res.
75:4960–4972. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Zheng ZY, Tian L, Bu W, Fan C, Gao X, Wang
H, Liao YH, Li Y, Lewis MT, Edwards D, et al: Wild-type N-Ras,
overexpressed in basal-like breast cancer, promotes tumor formation
by inducing IL-8 secretion via JAK2 activation. Cell Rep.
12:511–524. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Banys-Paluchowski M, Milde-Langosch K,
Fehm T, Witzel I, Oliveira-Ferrer L, Schmalfeldt B and Müller V:
Clinical relevance of H-RAS, K-RAS, and N-RAS mRNA expression in
primary breast cancer patients. Breast Cancer Res Treat.
179:403–414. 2020. View Article : Google Scholar
|
|
24
|
Gal M, Kim O, Tran PT, Huong LT, Nhiem NX,
Van Kiem P, Dang NH and Lee JH: Mussaendoside O, a N-triterpene
cycloartane saponin, attenuates RANKL-induced osteoclastogenesis
and inhibits lipopolysaccharide-induced bone loss. Phytomedicine.
105:1543782022. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Kim O, Hwangbo C, Tran PT and Lee JH:
Syntenin-1-mediated small extracellular vesicles promotes cell
growth, migration, and angiogenesis by increasing onco-miRNAs
secretion in lung cancer cells. Cell Death Dis. 13:1222022.
View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Luo J, Yang Z, Ma Y, Yue Z, Lin H, Qu G,
Huang J, Dai W, Li C, Zheng C, et al: LGR4 is a receptor for RANKL
and negatively regulates osteoclast differentiation and bone
resorption. Nat Med. 22:539–546. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Hayashi M, Nakashima T, Taniguchi M,
Kodama T, Kumanogoh A and Takayanagi H: Osteoprotection by
semaphorin 3A. Nature. 485:69–74. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Li Z, Hao J, Duan X, Wu N, Zhou Z, Yang F,
Li J, Zhao Z and Huang S: The role of semaphorin 3A in bone
remodeling. Front Cell Neurosci. 11:402017.PubMed/NCBI
|
|
29
|
Mundy GR: Metastasis to bone: Causes,
consequences and therapeutic opportunities. Nat Rev Cancer.
2:584–593. 2002. View
Article : Google Scholar : PubMed/NCBI
|
|
30
|
Jabbari N, Akbariazar E, Feqhhi M,
Rahbarghazi R and Rezaie J: Breast cancer-derived exosomes: Tumor
progression and therapeutic agents. J Cell Physiol. 235:6345–6356.
2020. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Yuan X, Qian N, Ling S, Li Y, Sun W, Li J,
Du R, Zhong G, Liu C, Yu G, et al: Breast cancer exosomes
contribute to pre-metastatic niche formation and promote bone
metastasis of tumor cells. Theranostics. 11:1429–1445. 2021.
View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Guo L, Zhu Y, Li L, Zhou S, Yin G, Yu G
and Cui H: Breast cancer cell-derived exosomal miR-20a-5p promotes
the proliferation and differentiation of osteoclasts by targeting
SRCIN1. Cancer Med. 8:5687–5701. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Arciero CA, Guo Y, Jiang R, Behera M,
O'Regan R, Peng L and Li X: ER+/HER2+ breast cancer has different
metastatic patterns and better survival than ER-/HER2+ breast
cancer. Clin Breast Cancer. 19:236–245. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Faversani A, Amatori S, Augello C, Colombo
F, Porretti L, Fanelli M, Ferrero S, Palleschi A, Pelicci PG,
Belloni E, et al: miR-494-3p is a novel tumor driver of lung
carcinogenesis. Oncotarget. 8:7231–7247. 2017. View Article : Google Scholar :
|
|
35
|
Wu C, Yang J, Li R, Lin X and Wu J and Wu
J: LncRNA WT1-AS/miR-494-3p regulates cell proliferation,
apoptosis, migration and invasion via PTEN/PI3K/AKT signaling
pathway in non-small cell lung cancer. Onco Targets Ther.
14:891–904. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
He H, Liao X, Yang Q, Liu Y, Peng Y, Zhong
H, Yang J, Zhang H, Yu Z, Zuo Y, et al: MicroRNA-494-3p promotes
cell growth, migration, and invasion of nasopharyngeal carcinoma by
targeting Sox7. Technol Cancer Res Treat. 17:15330338188099932018.
View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Behar O, Golden JA, Mashimo H, Schoen FJ
and Fishman MC: Semaphorin III is needed for normal patterning and
growth of nerves, bones and heart. Nature. 383:525–528. 1996.
View Article : Google Scholar : PubMed/NCBI
|




















