Introduction
The incidence of segmental bone defects, slow bone
healing and bone nonunion due to trauma, inflammation or tumors is
increasing (1). The current
treatment for such patients is bone grafting using autografts or
allografts; however, both types have significant disadvantages and
limitations (2). With the
development of modern molecular biology and regenerative medicine,
stem cell therapy has advanced the treatments for these diseases.
Mesenchymal stem cells (MSCs) are multipotent cells of mesodermal
and neural crest origin with stromal properties (3). MSCs are currently the most
frequently utilized stem cells in preclinical and clinical studies
of skeletal diseases (4). These
cells can be extracted from a range of sources, including the bone
marrow, adipose tissue, urine, placenta and umbilical cord. In
addition, they exhibit three different biological properties,
namely differentiation potential, secretion of growth-promoting
factors and immunomodulation, which render them highly suitable as
seed cells for cell therapy (5).
Therefore, promoting osteogenic differentiation of MSCs may help
accelerate fracture healing and treat bone defect and nonunion.
Bone formation requires the generation of numerous
osteoblasts, which are mainly derived from bone marrow MSCs
(BMSCs). Numerous regulatory factors can affect the osteogenic
differentiation of BMSCs and several studies have indicated that
the Wnt/β-catenin signaling pathway has an instrumental role in
bone production (6-8). The binding of Wnt ligands to
Frizzled and lipoprotein receptor-related protein 5/6 receptors
activates Dishevelled (Dvl), which promotes β-catenin
stabilization. Subsequently, β-catenin binds to T-cell
factor/lymphoid enhancing factor (LEF) DNA-binding proteins to
regulate the transcription of osteogenic-related target genes
(9). Furthermore, several studies
have demonstrated that the activation of the Wnt/β-catenin pathway
through the use of small molecule drugs or growth factors can
promote systemic and local bone formation (10,11). This suggests that targeting the
Wnt/β-catenin signaling pathway is an effective strategy for bone
regeneration.
Genetic and functional studies have shown that the
metabolism of glucose, fatty acids, and amino acids is an essential
regulator of BMSCs differentiation. This is because BMSCs require
large amounts of energy to maintain bone homeostasis. The balance
between bone formation and resorption is disrupted when the energy
metabolism of BMSCs is dysregulated (12). Glucose metabolism has a
significant role in the differentiation of BMSCs and is primarily
divided into glycolysis and mitochondrial oxidative phosphorylation
(OXPHOS). Recent studies have revealed that differentiated
osteoblasts have higher levels of glycolysis and OXPHOS than
undifferentiated cells. As cells differentiate, their rate of
oxygen consumption decreases, while their rate of glycolysis
increases, indicated by a decrease in the ratio of oxygen
consumption rate (OCR) to extracellular acidification rate (ECAR)
(13). This phenomenon is known
as aerobic glycolysis and is similar to the Warburg effect observed
in tumor cells. Aerobic glycolysis is a significant source of
energy that promotes the differentiation of MSCs, and inhibition of
glycolysis inhibits osteogenic differentiation of BMSCs (14,15). It was indicated that Wnt can
enhance the levels of glucose transporter isoform 1 and
hexokinase-2, leading to an increased consumption of glucose
(16). In addition, Wnt promotes
aerobic glycolysis by upregulating L-lactate dehydrogenase A (LDHA)
and 3-phosphoinositide-dependent protein kinase 1 to increase
lactate production rather than pyruvate production (17). Furthermore, knockdown of
mitofusin-2 enhances the Wnt/β-catenin signaling pathway in induced
pluripotent stem cell-derived MSCs, thereby enhancing aerobic
glycolysis and osteogenic differentiation (18). All of the above-mentioned studies
suggest that glycolysis may have a key role in the future treatment
of different diseases using BMSCs and that the Wnt/β-catenin
signaling pathway stimulates aerobic glycolysis in MSCs.
Toxoplasma gondii excretory/secretory
proteins (TgESPs) are a class of proteins secreted by Toxoplasma
gondii (T. gondii) that can circulate and remain on the
surface of cells (19). In
previous studies, a proteomics study detected ~512 proteins in
TgESPs with various functions, such as extracellular proteases,
hormones, neurotransmitters and antimicrobial peptides (20). They also participate in cell
adhesion, migration and intercellular communication. TgESPs are
therefore not only critical for the immune response of T.
gondii but also enter the cells more readily (20,21). Recent studies have found that
TgESPs can alter the metabolism of host cells, most of which
involve the tricarboxylic acid (TCA) cycle, purine metabolism and
tryptophan metabolism (22).
Various metabolism-related proteins have also been identified in
TgESPs (23). This indicates that
TgESPs may affect the energy metabolism of cells after entering
them. However, it is unclear whether TgESPs can affect the energy
metabolism and osteogenic differentiation of BMSCs.
The present study aimed to reveal the molecular
mechanisms through which TgESPs affect the metabolism and
osteogenic differentiation of BMSCs. The study may help in
developing a new therapeutic approach for bone repair.
Materials and methods
Isolation and culture of human BMSCs
(hBMSCs)
The experimental protocol conforms to the ethical
standards of the Declaration of Helsinki and was approved by the
ethics committee of the Affiliated Hospital of Guangdong Medical
University (Zhanjiang, Guangdong, China). Bone marrow samples were
obtained from the femoral cavity during the operation of five
female patients aged 35 to 45 years with traumatic femoral head
fractures. Each sample was treated individually and used for
subsequent experiments. All participants provided written informed
consent. The bone marrow samples were collected from each
participant and hBMSCs were isolated using Ficoll density gradient
centrifugation. Subsequently, the bone marrow samples were
transferred to a 15-ml centrifuge tube and diluted with PBS at a
ratio of 1:1. An equal volume of Histopaque®-1077
(Sigma-Aldrich; Merck KGaA) was then added to the diluted bone
marrow sample and centrifuged at 400 × g and room temperature for
20 min without accelerating or braking. Cells in the middle layer
were extracted and further resuspended in Modified Eagle's Medium α
(α-MEM) supplemented with 10% (v/v) fetal bovine serum (FBS), 10
U/ml penicillin G and 10 mg/ml streptomycin (all from Gibco; Thermo
Fisher Scientific, Inc.). The obtained cells were incubated at 37°C
in an incubator containing air with 5% CO2 and saturated
humidity. The medium was changed every three days; when the cells
reached ~90% confluence, they were digested with 0.25% trypsin-EDTA
(Gibco; Thermo Fisher Scientific, Inc.) and passaged at a 1:3
ratio. The cells from passages five to six were used in all
subsequent experiments.
Identification of cell phenotype
The cells from passage three were used to identify
phenotypes. When the cells had reached 80% confluence, they were
digested with 0.25% trypsin-EDTA (Gibco; Thermo Fisher Scientific,
Inc.), centrifuged at 800 × g for 3 min at room temperature and
resuspended in PBS. The cell surface antigens CD90, CD73, CD105 and
CD45 were detected via flow cytometry using the Human MSC Analysis
Kit (cat. no. 562245; BD Biosciences). Data were analyzed using
FlowJo 10.0.7 software (FlowJo LLC).
Adipogenic and chondrogenic
differentiation assays
The hBMSCs were seeded in 6-well plates at a density
of 1×105 cells per well. The hBMSCs were cultured with
adipogenic induction medium (α-MEM containing 10% FBS, 10 mg/ml
insulin, 1 mM dexamethasone, 50 mM 3-isbutyl-1-methylxanthine and
50 µM indomethacin; all from Sigma-Aldrich; Merck KGaA) and
chondrogenic induction medium (α-MEM containing 1% FBS, 1%
insulin-transferrin-sodium selenite, 20 nM dexamethasone, 10 ng/ml
TGF-β and 40 µg/ml proline; all from Sigma-Aldrich; Merck
KGaA) and stained with Oil Red O or Alcian Blue staining solution
(Cyagen Biosciences, Inc.). The images were acquired under a
microscope and stored.
Extraction of TgESPs
The RH strain of T. gondii (kindly provided
by Dr Young-Ha Lee, Department of Infection Biology, Chungnam
National University School of Medicine, Republic of Korea) was
allowed to proliferate in ARPE-19 cells (CRL-2302; American Type
Culture Collection). T. gondii was collected and rinsed in
PBS, centrifuged at 1,000 × g for 5 min at room temperature, and
this was repeated twice. Purified tachyzoites were collected in 1
ml Hank's balanced salt solution (Gibco; Thermo Fisher Scientific,
Inc.) and oscillated at 3.57 × g in a constant temperature shaker
at 37°C for 4 h. After centrifugation at 14,000 × g thrice for 5
min each at 4°C, the supernatant was stored as TgESPs.
Transmission electron microscopy
To visualize vesicle-like structures in TgESPs, the
TgESPs were deposited on a nickel grid and stained with uranyl
acetate and lead citrate, and images of the samples were acquired
using a JEM-1400 transmission electron microscope (JEOL, Ltd.).
Cell viability assay
The effect of TgESPs on the proliferation of hBMSCs
was evaluated using the Cell Counting Kit-8 (CCK-8; Zeta Life). In
brief, hBMSCs were seeded in 96-well plates at a density of 3,000
cells per well and incubated with different concentrations of
TgESPs (0, 1, 10, 20, 30, 40 and 50 µg/ml) for 24, 48 and 72
h. The wells were then filled with 10 µl CCK-8 solution and
incubated in 5% CO2 in a humidified environment for 2 h
at 37°C. The absorbance of the cells at 450 nm was measured using a
microplate reader (BioTek Instruments, Inc.) and a cell
proliferation curve was generated.
Osteogenic differentiation protocol and
alizarin red S staining
The hBMSCs were seeded in 12-well plates at a
density of 1×105 cells per well. Once they reached
>80% confluence, the medium was changed to the osteogenic
induction medium (OIM), i.e. Dulbecco's modified Eagle's medium
(DMEM) with low glucose (LG) content, supplemented with 100 nM
dexamethasone (Sigma-Aldrich; Merck KGaA), 50 µM L-ascorbic
acid-2-phosphate (Sigma-Aldrich; Merck KGaA), 10 mM
β-glycerophosphate (Sigma-Aldrich; Merck KGaA) and 10% FBS (Gibco;
Thermo Fisher Scientific, Inc.). The experimental group was
incubated in OIM supplemented with 1 or 10 µg/ml TgESPs,
whereas the control group was incubated in OIM without TgESPs, and
the inhibitor group was treated with 25 µM Icg-001 (MCE) or
2 mM 2-deoxy-D-glucose (2-DG; Sigma-Aldrich; Merck KGaA) for three
days to induce osteogenic differentiation. The osteogenic induction
medium was changed every three days.
The cellular mineral deposition was evaluated using
alizarin red staining. The cells were fixed with 4%
paraformaldehyde for 15 min at room temperature. Subsequently, they
were washed thrice with PBS, treated with Alizarin Red S staining
solution (Oricell) for 20 min at room temperature and washed thrice
with PBS. The images were acquired and saved using a camera and
microscope.
Total RNA extraction and reverse
transcription-quantitative PCR (RT-qPCR)
Cells were seeded in 6-well plates at a density of
2×105 cells per well. Total RNA was extracted from the
hBMSCs using TRIzol reagent (Invitrogen; Thermo Fisher Scientific,
Inc.) at 7 days after osteogenic induction and reverse-transcribed
into cDNA using HiScript III RT SuperMix (Vazyme), ChamQ Universal
SYBR qPCR Master Mix (Vazyme) was used for qPCR, and both cDNA
synthesis and qPCR were performed according to the manufacturer's
protocol. The qPCR thermocycling conditions were as follows: 95°C
for 10 sec and 60°C for 30 sec, performed for 40 cycles. The mRNA
expression levels of target genes were normalized to those of the
internal control (β-actin) and quantified using the
2−ΔΔCq method (24).
The primers used for PCR are listed in Table SI.
Western blot analysis
Cells were lysed using RIPA buffer (Invitrogen;
Thermo Fisher Scientific, Inc.) 14 days after the induction of
osteogenic differentiation. Protein concentrations were determined
using a BCA protein assay kit (Beyotime Institute of
Biotechnology). For each lane, 20 µg of protein were loaded
on a 10 or 12% SDS-PAGE gel, resolved and electrotransferred to a
polyvinylidene difluoride membrane (Merck Millipore). The membrane
was then placed in 5% skimmed milk (0.5 g skimmed milk powder
dissolved in 10 ml Tris-buffered saline), blocked at room
temperature for 1-2 h, and incubated with a primary antibody at 4°C
overnight. After washing it thrice with Tris-buffered saline
containing Tween 20 (TBS-T), the membrane was incubated with a
horseradish peroxidase-conjugated secondary antibody for 1 h at
room temperature. After washing the membrane thrice with TBST (10
min each), immunoreactive bands were visualized using a
chemiluminescence detection reagent (Merck Millipore). Finally,
protein intensity was quantified using the ImageJ software version
1.8.0 (National Institutes of Health). All the antibodies used for
western blot analysis are listed in Table SII.
Immunofluorescence (IF)
Cells were induced to undergo osteogenic
differentiation in OIM with or without TgESPs for 7 days. In
addition, RUNX family transcription factor 2 (Runx2) expression was
measured using IF. In brief, the cells were fixed in 4%
paraformaldehyde for 20 min, permeabilized in 0.5% Triton X-100
(Sigma-Aldrich; Merck KGaA) for 30 min and blocked in 1% bovine
serum albumin (Beijing Solarbio) for 1 h. The cells were then
washed thrice with PBS (5 min each) and incubated overnight at 4°C
with a specific primary antibody against Runx2. The cells were
subsequently incubated with AlexA-568-conjugated secondary antibody
for 1 h at room temperature. The nuclei were stained with DAPI.
Immunofluorescence was observed using a fluorescence microscope
(Olympus Corporation). Details of all antibodies used for IF are
provided in Table SII.
Seahorse extracellular flux assays
The hBMSCs were cultured in OIM for 7 days. Cells
were harvested following trypsin digestion and re-seeded in
Seahorse XF24 cell culture microplates (Seahorse Bioscience) at a
density of 2×104 cells per well. Cells were cultured
overnight in OIM and the OCR and ECAR were measured using the
Seahorse XF Cell Mito Stress Test kit (Seahorse Bioscience) and
Seahorse XF Glycolysis Rate Assay kit (Seahorse Bioscience),
respectively, according to the manufacturer's instructions. The
Cell Mito Stress Test assesses ATP production and maximal
mitochondrial respiration by successively adding specific
inhibitors, such as oligomycin, carbonyl cyanide
4-(trifluoromethoxy) phenylhydrazone (FCCP, a potent mitochondrial
oxidative phosphorylation uncoupling agent that promotes
mitochondrial membrane depolarization. In this study, we employed
FCCP to investigate the impact of TgESP on cellular energy
metabolism.) and rotenone/antimycin A (Rot/AA). Afterwards, the
glycolysis rate assay was carried out using Rot/AA and 2-DG to
measure ECAR and intracellular glycolysis capacity. The OCR and
ECAR were measured and analyzed using an XF24 Flux Analyser
(Seahorse Bioscience).
Lactic acid measurements
For lactic acid measurement, a lactic acid detection
kit (cat. no. BC2235; Beijing Solarbio) was used according to the
manufacturer's protocol. The culture medium of the cells was
collected, and mixed with working solution of the lactic acid
detection kit. Subsequently, the mixture was incubated with the
color reagent at 37°C in the dark for 20 min. Finally, the
absorbance at 570 nm was measured using a microplate reader (BioTek
Instruments, Inc.) and the lactic acid concentration was calculated
based a standard curve.
Cell wound healing assay
The cell wound healing assay was performed as
previously described (25). In
brief, hBMSCs were seeded at a density of 2×105 cells
per well in 6-well plates and treated with 1 or 10 µg/ml
TgESPs for 24 h. After this treatment, a vertical scratch was made
in the cell monolayer using a 20-µl micropipette tip. The
cells were then washed once with PBS and incubated with serum-free
basal medium. The migration of hBMSCs was observed and recorded
using Leica DMI3000 microscope (Leica Microsystems GmbH) at 0 and
24 h. The cell migration distance was quantified by measuring the
cell distance between the two sides using Photoshop 2021 (Adobe
Inc.).
Cell adhesion assay
The cell adhesion assay was conducted following a
previously reported protocol (26). In brief, hBMSCs were seeded in
6-well plates and treated with 1 and 10 µg/ml TgESPs for 24
h. After this treatment, the cells were digested, resuspended and
seeded at a density of 1×104 cells per well in 96-well
plates coated with polylysine (Beijing Solarbio). After allowing
the cells to attach for 1 h, non-adherent cells were washed off
with PBS. Subsequently, 10 µl MTS assay solution was added
to each well and incubated at 37°C for 2 h. The absorbance was
measured at 490 nm using a microplate reader (BioTek Instruments,
Inc.). The relative cell adhesion rate was thus evaluated.
Statistical analysis
Statistical analyses were performed using GraphPad
Prism 8.0 software (GraphPad Software; Dotmatics). Comparisons of
data between two groups were performed using an unpaired two-tailed
Student's t-test, while data were compared among three or more
groups by one-way ANOVA followed by Tukey's post-hoc test. Values
are expressed as the mean ± standard deviation and were based on
results from at least three independent experiments. P<0.05 was
considered to indicate a statistically significant difference.
Results
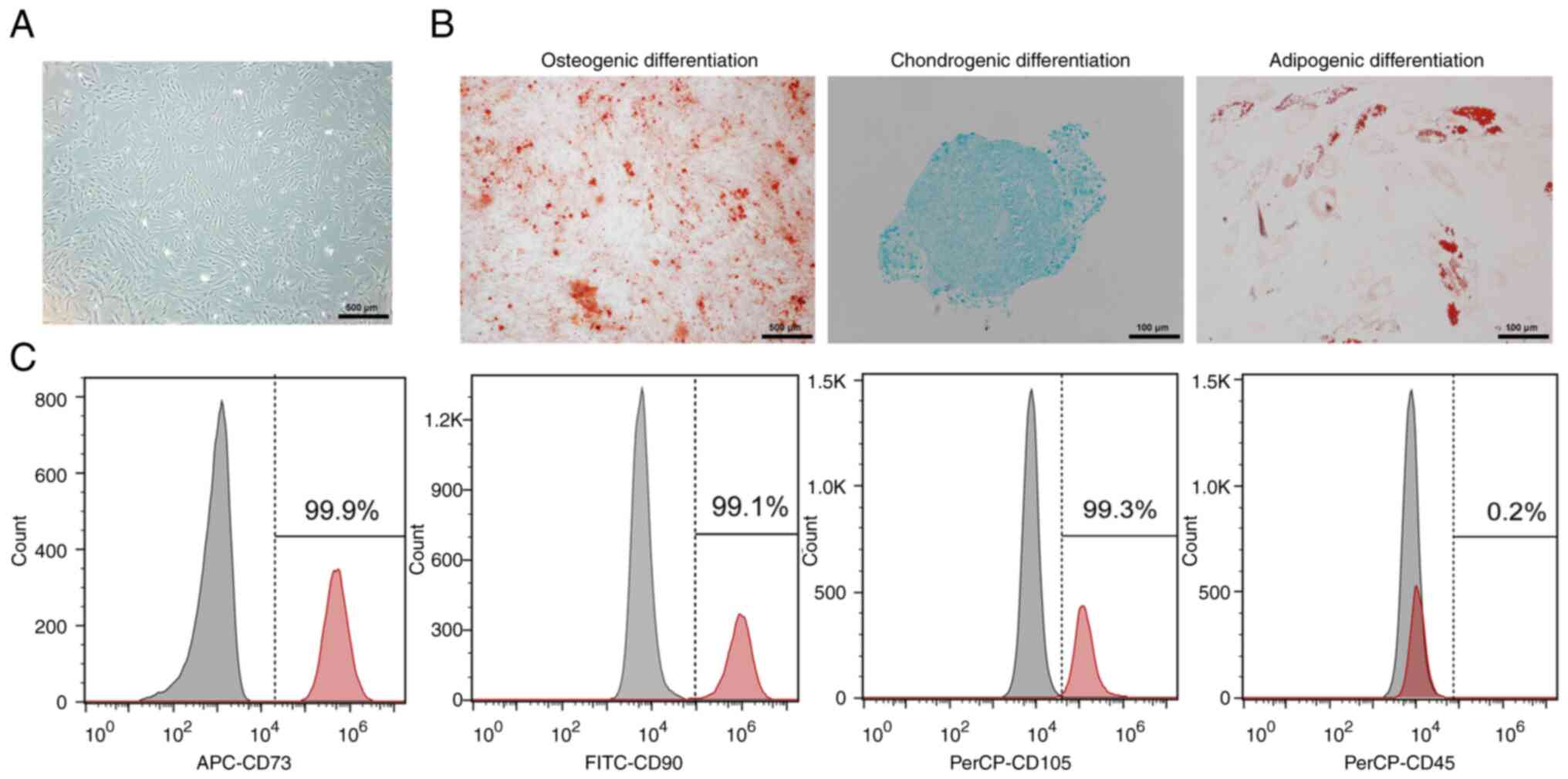
Characterization and phenotypic
identification of hBMSCs
hBMSCs were isolated and cultured from human bone
marrow samples and passaged three to four times. After 10 days of
primary culture, the attached hBMSCs appeared fibroblast-like and
spindle-shaped and grew to 90% confluency (Fig. 1A). Alizarin Red S, Oil Red O and
Alcian blue staining revealed that hBMSCs had the potential for
osteogenic, adipogenic and chondrogenic differentiation,
respectively (Fig. 1B). When the
confluence of hBMSCs in passage three reached 90%, the surface
markers assessed via flow cytometry showed that expression of the
positive markers CD73 (99.9%), CD90 (99.1%) and CD105 (99.3%) was
>95%, while that of the negative marker CD45 (0.2%) was <2%
(Fig. 1C). These features suggest
that the cells isolated from human bone marrow can be considered
hBMSCs.
TgESPs contain secretory vesicles
Ramírez-Flores et al (23) observed that T. gondii can
secrete tubular structures composed of vesicles in vitro,
which disperse into a single exosomes-like vesicle after
centrifugation. In the present study, TgESPs extracted by
centrifugation also contained numerous vesicles (~100-160 nm in
diameter) with the morphological characteristics of exosomes, as
observed under the transmission electron microscope (Fig. 1A). The isolate also contained some
fine particulate matter, which may be other soluble proteins.
TgESPs enhance hBMSC proliferation,
adhesion and migration
To address the effect of TgESPs on hBMSC
proliferation, the CCK-8 assay was performed. The results indicated
that 1, 30, 40 and 50 µg/ml TgESPs had no inhibitory effect
on the proliferation of hBMSCs, while 10 and 20 µg/ml TgESPs
had a positive impact on the growth of hBMSCs after 24 and 48 h of
treatment compared to that in the control group (P<0.05)
(Fig. 2B). Further investigation
of the effect of TgESPs on hBMSC behavior revealed that low
concentrations of TgESPs promoted cell adhesion, and both 1 and 10
µg/ml concentrations enhanced cell migration in hBMSCs
(Fig. S1). These results
indicate that TgESPs not only promote the proliferation of hBMSCs
at low concentrations, but also enhance cell adhesion and
migration.
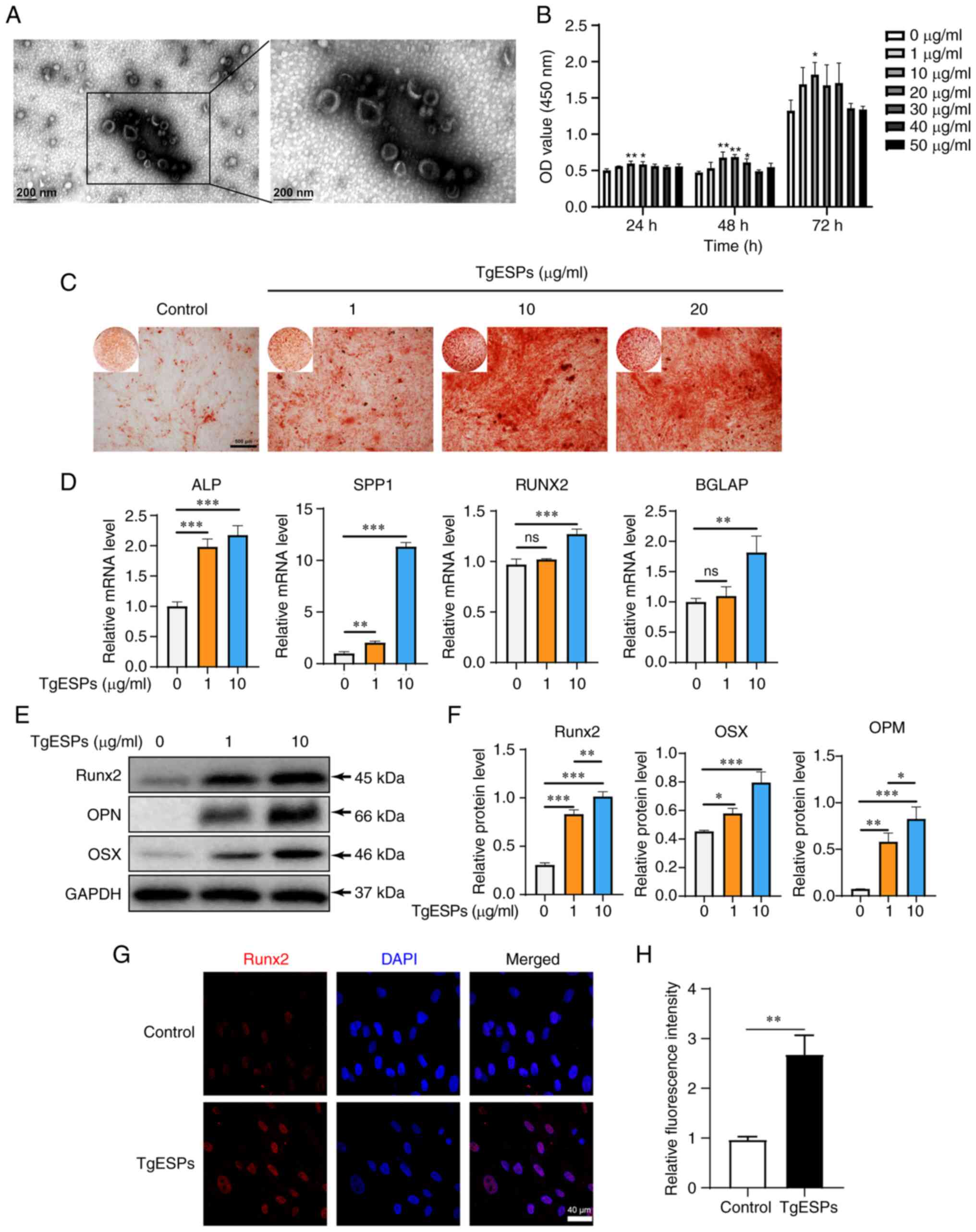 | Figure 2TgESPs promote the proliferation and
osteogenic differentiation of hBMSCs. (A) TgESPs were observed by
transmission electron microscopy (scale bar, 200 nm). (B) A Cell
Counting Kit-8 assay was used to detect the effects of different
concentrations of TgESPs (0, 1, 10, 20, 30, 40 and 50 µg/ml)
on the proliferation of hBMSCs at 24, 48 and 72 h. (C) Alizarin red
S staining was used to assess the TgESP-mediated osteogenic
induction of hBMSCs for 14 days (scale bar, 500 µm). (D)
Reverse transcription-quantitative PCR analysis of the mRNA levels
of osteogenic markers in TgESP-stimulated hBMSCs after 7 days of
osteogenic induction. (E) Western blot analysis of the protein
levels of Runx2, OPN and OSX in TgESP-induced hBMSCs after 14 days
of osteogenic induction. (F) Quantitative analysis of the
expression of each protein using GAPDH as the internal control. (G)
Immunofluorescence analysis of the protein levels of Runx2 after
osteogenic induction for 7 days in hBMSCs treated with 10
µg/ml TgESPs or PBS (scale bar, 40 µm). (H)
Quantification of immunofluorescence in G. *P<0.05;
**P<0.01; ***P<0.001 vs. control group
or as indicated; ns, no significance. TgESPs, Toxoplasma
gondii excretory/secretory proteins; hBMSCs, human bone marrow
mesenchymal stem cells; OD, optical density; Runx2, RUNX family
transcription factor 2; OPN, osteopontin; OSX, osterix; ALP,
alkaline phosphatase; SPP1, secreted phosphoprotein 1; BGLAP, bone
gamma-carboxyglutamate protein. |
TgESPs enhance osteogenic differentiation
of hBMSCs
To understand the effect of TgESPs on the osteogenic
differentiation of hBMSCs, the effects of 0, 1, 10 and 20
µg/ml TgESPs on osteogenic differentiation of hBMSCs were
examined using alizarin red staining. After 14 days of osteogenic
differentiation induction, compared with that in the control group,
TgESP treatment enhanced the osteogenic mineralization ability of
hBMSCs. However, no obvious differences in mineralization were
observed between 10 and 20 µg/ml (Fig. 2C); consequently, two
concentrations, 1 and 10 µg/ml, were used in the subsequent
experiments.
To elucidate the effect of TgESPs on osteogenic
differentiation of hBMSCs in vitro, the expression levels of
the osteogenesis-specific genes alkaline phosphatase (ALP),
secreted phosphoprotein 1 (SPP1), RUNX2 and bone
gamma-carboxyglutamate protein (BGLAP) were determined using
RT-qPCR. The mRNA expression levels of ALP, SPP1, RUNX2 and BGLAP
were significantly increased after seven days of TgESP treatment
(P<0.05) (Fig. 2D). Next, the
effect of TgESPs on the protein levels of Runx2, osteopontin (OPN)
and osterix (OSX) was determined using western blotting and IF. The
results showed that after ESP treatment, the protein expression of
Runx2, OPN and OSX significantly increased in a
concentration-dependent manner (P<0.05) (Fig. 2E-H). These findings indicate that
TgESPs have a positive role in the osteogenic differentiation of
hBMSCs.
TgESPs enhance osteogenic differentiation
of hBMSCs through the β-catenin signaling pathway
The role of the Wnt/β-catenin signaling pathway in
the osteogenic differentiation of BMSCs is crucial. To determine
the mechanism by which TgESPs affect the osteogenic differentiation
of hBMSCs, the expression of the Wnt/β-catenin signaling pathway
was studied after TgESP treatment. First, the protein expression of
LEF1, Dvl segment polarity protein 3 (DVL3), β-catenin and cyclin
D1 was detected using western blotting. The results indicated that
the protein expression of LEF1, DVL3, β-catenin and cyclin D1 was
significantly increased after TgESP treatment (P<0.05) (Fig. 3A and B). This suggests that TgESPs
may affect the Wnt/β-catenin signaling pathway during osteogenic
differentiation of hBMSCs. After Icg-001 treatment, calcium
deposition in hBMSCs was significantly reduced compared to that in
the controls and addition of TgESPs restored the inhibitory effect
of Icg-001 (Fig. 3C). In
addition, the expression levels of osteogenic-specific genes and
proteins, as well as β-catenin, were determined using RT-qPCR,
western blotting and IF. The results suggested that the mRNA and
protein expression of Runx2, OPN, OSX and β-catenin was
significantly inhibited by Icg-001. The inhibition of these genes
and proteins by Icg-001 was reversed due to the repressive effects
of TgESP treatment (Fig.
3D-H).
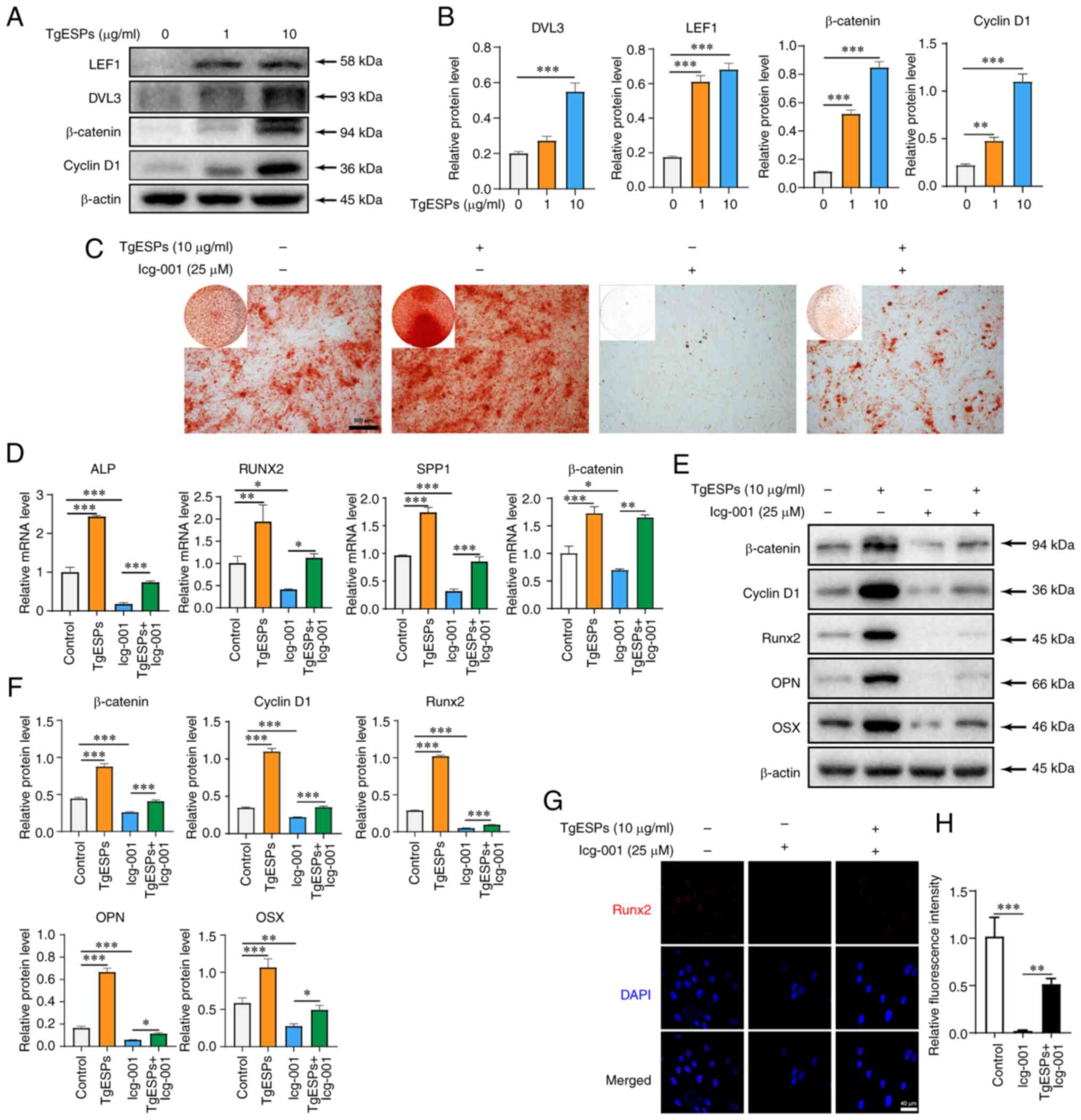 | Figure 3TgESPs positively regulate the
Wnt/β-catenin signaling pathway during osteogenic differentiation
of hBMSCs. (A) Western blot analysis of the levels of LEF1, DVL3,
β-catenin and cyclin D1 proteins in TgESP-induced hBMSCs after 14
days of osteogenic induction. (B) Quantitative analysis of the
expression of each protein. (C) Alizarin red S staining was used to
assess the ability of hBMSCs cells to mineralize with TgESPs (10
µg/ml) in the presence or absence of Icg-001 (25 µM;
scale bar, 500 µm). (D) Reverse transcription-quantitative
PCR analysis of mRNA levels of ALP, RUNX2, SPP1 and β-catenin in
hBMSCs after 7 days of osteogenic induction by TgESPs in the
presence or absence of Icg-001. (E) Western blot analysis of
protein levels of Runx2, OPN, OSX, β-catenin and cyclin D1 in
hBMSCs after 14 days of osteogenic induction by TgESPs in the
presence or absence of Icg-001. (F) Quantitative analysis of the
expression of each protein using β-actin as the internal control.
(G) Immunofluorescence analysis of protein levels of Runx2 in
hBMSCs after 7 days of osteogenic induction by Icg-001 in the
presence or absence of TgESPs (scale bar, 40 µm). (H)
Quantification of immunofluorescence in G. *P<0.05;
**P<0.01; ***P<0.001. TgESPs,
Toxoplasma gondii excretory/secretory proteins; hBMSCs,
human bone marrow mesenchymal stem cells; Runx2, RUNX family
transcription factor 2; OPN, osteopontin; OSX, osterix; ALP,
alkaline phosphatase; SPP1, secreted phosphoprotein 1; BGLAP, bone
gamma-carboxyglutamate protein; LEF1, lymphoid enhancer binding
factor 1; DVL3, dishevelled segment polarity protein 3. |
TgESPs enhance glycolysis during
osteogenic differentiation of hBMSCs
Previous studies suggested that the cellular OCR and
mitochondrial DNA content increase during osteogenic
differentiation of hBMSCs (27,28), and that TgESPs can regulate host
cell metabolism, particularly the TCA cycle (22). Therefore, it was hypothesized that
TgESPs may also regulate the energy metabolism of hBMSCs during
osteogenic differentiation. The results indicated that the OIM
group with induced osteogenic differentiation had significantly
higher OCR in hBMSCs than the LG-DMEM group without induced
osteogenic differentiation. Upon addition of TgESPs, the basal,
maximal, ATP production and respiration rates, as well as the spare
respiratory capacity, were increased. However, no significant
difference in OCR was observed among the induced osteogenic
differentiation groups (Fig. 4A and
B). This observation suggested that TgESPs had no significant
effect on the mitochondrial OXPHOS of hBMSCs. Furthermore, the
glycolysis rate of hBMSCs was measured and it was observed that the
ECAR increased following hBMSC induction and that basal and
compensatory glycolysis were enhanced after TgESP treatment
(Fig. 4C and D). Subsequently,
lactic acid production in cells was detected; the results indicated
that hBMSCs produced significantly more lactic acid after 72 h of
TgESP treatment (Fig. 4E). Next,
to assess the biochemical basis of glycolysis, the gene and protein
expression of several rate-limiting enzymes involved in critical
steps of glycolysis was examined. The mRNA levels of PFKFB3,
PFKFB4, enolase (ENO)3 and LDHA were significantly increased at
three and seven days after osteogenic differentiation induction. In
addition, TgESPs significantly increased the protein expression of
PFKFB3 and LDHA in a concentration-dependent manner (Fig. 4F-H).
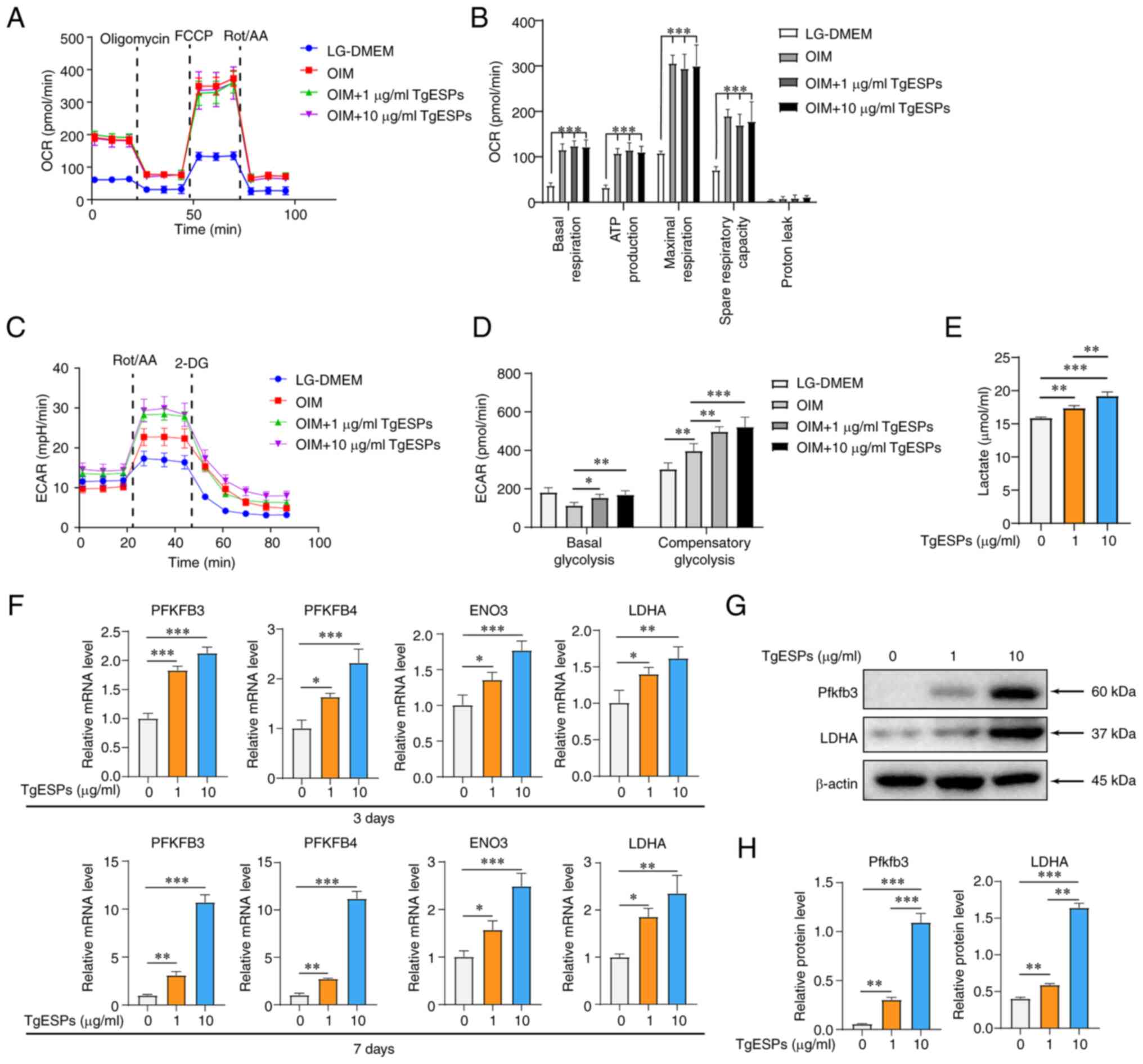 | Figure 4TgESPs enhance aerobic glycolysis
during hBMSC osteogenic differentiation induction. (A) Seahorse
analysis of the OCR of hBMSCs, in which osteogenic differentiation
was induced with TgESPs for 7 days. (B) Basal respiration, ATP
production, maximum respiratory capacity and proton leak in the OCR
assay. (C) Seahorse analysis of the ECAR of hBMSCs, in which
osteogenic differentiation was induced with TgESPs for 7 days. (D)
Basal and compensatory glycolysis in the ECAR assay. (E) Lactate
production by hBMSCs after 3 days of TgESP treatment. (F) Reverse
transcription-quantitative PCR analysis of the mRNA levels of
PFKFB3, PFKFB4, ENO3 and LDHA in TgESP-induced hBMSCs after 3 and 7
days of osteogenic induction. (G) Western blot analysis of the
protein levels of PFKFB3 and LDHA in TgESP-induced hBMSCs after 14
days of osteogenic induction. (H) Quantitative analysis of the
expression of each protein using β-actin as the internal control.
*P<0.05; **P<0.01;
***P<0.001. TgESPs, Toxoplasma gondii
excretory/secretory proteins; hBMSCs, human bone marrow mesenchymal
stem cells; LDHA, lactate dehydrogenase A; PFKFB3,
6-phosphofructo-2-kinase/fructose-2,6-biphosphatase 3; ENO3,
enolase 3; OCR, oxygen consumption rate; ECAR, extracellular
acidification rate; LG, low glucose; Rot/AA, rotenone/antimycin A;
2-DG, 2-deoxy-D-glucose; FCCP, carbonyl cyanide
4-(trifluoromethoxy)phenylhydrazone. |
TgESPs enhance osteogenic differentiation
of hBMSCs through glycolysis
According to the above-mentioned results, TgESPs can
enhance glycolysis during the osteogenic differentiation of hBMSCs.
Therefore, in the present study, the effect of glycolysis on the
osteogenic differentiation of hBMSCs was investigated in further
detail. After treatment with 2-DG (an inhibitor of glycolysis), the
ECAR and lactate production in hBMSCs were significantly inhibited;
however, the ECAR and lactate secretion in hBMSCs were restored
after simultaneous treatment with TgESPs and 2-DG (Fig. 5A and C). Quantification of the
ECAR data showed that basic and compensatory glycolysis was able to
reverse the inhibition effect of 2-DG after TgESP treatment
(Fig. 5B). Alizarin red staining
indicated that calcium deposition in the 2-DG group was much lower
than that in the control group and TgESP group, but it increased
again in the TgESPs+2-DG group as compared with that in the 2-DG
group (Fig. 5D). Furthermore,
RT-qPCR and western blotting results indicated that 2-DG inhibited
the rate-limiting enzymes of glycolysis, while TgESPs reversed this
inhibition (Fig. 5E, F and H). In
addition, it was found that 2-DG inhibited the mRNA and protein
expression of Runx2, OPN and OSX, while TgESPs reversed the
inhibitory effect of 2-DG on osteogenic differentiation (Fig. 5E-I). These results suggest that
TgESPs can affect glycolysis in hBMSCs and enhance their osteogenic
differentiation ability.
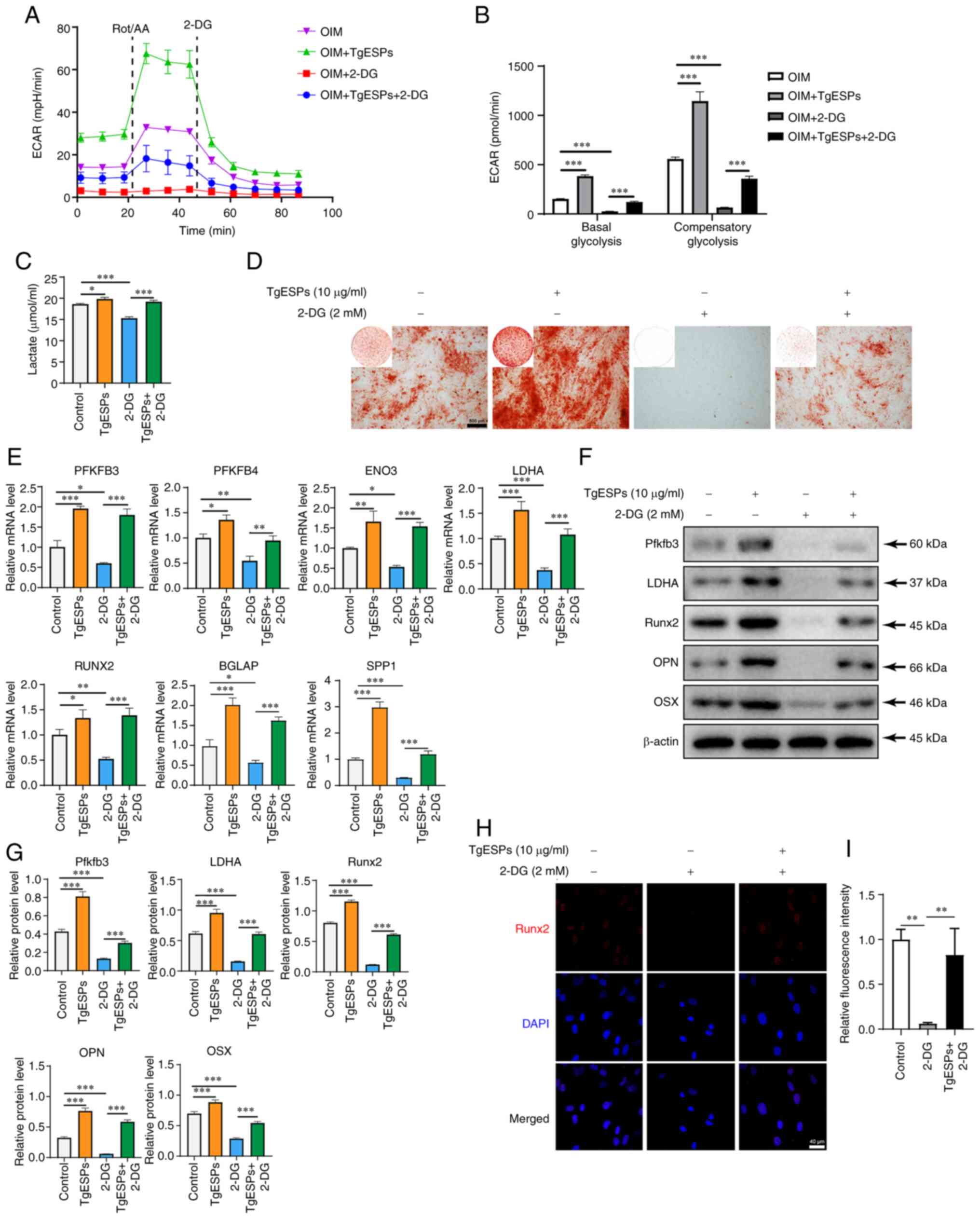 | Figure 5TgESPs promote the osteogenic
differentiation of hBMSCs via aerobic glycolysis. (A) Seahorse
analysis of ECAR showed that in the presence or absence of 2-DG (2
mM), the addition of TgESPs (10 µg/ml) hBMSCs induced
osteogenic differentiation over 7 days. (B) Basal and compensatory
glycolysis in the ECAR assay. (C) Lactate production in hBMSCs
after three days of TgESP treatment in the presence or absence of
2-DG. (D) Alizarin red S staining was used to assess the ability of
hBMSCs to mineralize with TgESPs in the presence or absence of 2-DG
(scale bar, 500 µm). (E) Reverse transcription-quantitative
PCR analysis of mRNA levels of PFKFB3, PFKFB4, ENO3, LDHA, RUNX2,
BGLAP and SPP1 in hBMSCs after 7 days of osteogenic induction by
TgESPs in the presence or absence of 2-DG. (F) Western blot
analysis of protein levels of PFKFB3, LDHA, RUNX2, OPN and OSX in
hBMSCs after 14 days of osteogenic induction by TgESPs in the
presence or absence of 2-DG. (G) Quantitative analysis of the
expression of each protein using β-actin as the internal control.
(H) Immunofluorescence analysis of protein levels of Runx2 in
hBMSCs after 7 days of osteogenic induction by 2-DG in the presence
or absence of TgESPs (scale bar, 40 µm). (I) Quantitative
analysis of the protein levels in H. *P<0.05;
**P<0.01; ***P<0.001. TgESPs,
Toxoplasma gondii excretory/secretory proteins; hBMSCs,
human bone marrow mesenchymal stem cells; LDHA, lactate
dehydrogenase A; PFKFB3,
6-phosphofructo-2-kinase/fructose-2,6-biphosphatase 3; Runx2, RUNX
family transcription factor 2; OPN, osteopontin; OSX, osterix; ALP,
alkaline phosphatase; ENO3, enolase 3; ECAR, extracellular
acidification rate; Rot/AA, rotenone/antimycin A; 2-DG,
2-deoxy-D-glucose. |
TgESPs regulate glycolysis of hBMSCs
through β-catenin
The regulation of glycolysis in MSCs has been
reported to be regulated by the Wnt/β-catenin signaling pathway
(29). Therefore, it was
hypothesized in the present study that TgESPs can affect glycolysis
through the Wnt/β-catenin signaling pathway during the osteogenic
differentiation of hBMSCs. Upon assessing the glycolytic rate, both
basal and compensatory glycolysis were found to be significantly
lower in the OIM+Icg-001 group than those in the OIM group
(P<0.05). In addition, TgESP treatment was observed to reverse
the inhibitory effect of Icg-001 on glycolysis (Fig. 6A and B). Furthermore, cellular
lactate production showed the same trend as that described above
(Fig. 6C). Next, the gene and
protein expression of rate-limiting enzymes in the critical step of
glycolysis were examined. The mRNA expression of PFKFB3, PFKFB4,
ENO3 and LDHA was significantly reduced in the Icg-001 group
(P<0.05), but addition of TgESPs increased the expression of
these genes again (Fig. 6D).
Furthermore, the inhibition of the PFKFB3 and LDHA proteins caused
by Icg-001 was partially reversed by TgESPs (Fig. 6E and F). These results suggest
that β-catenin is crucial in regulating downstream glycolysis and
promoting the osteogenic differentiation of hBMSCs.
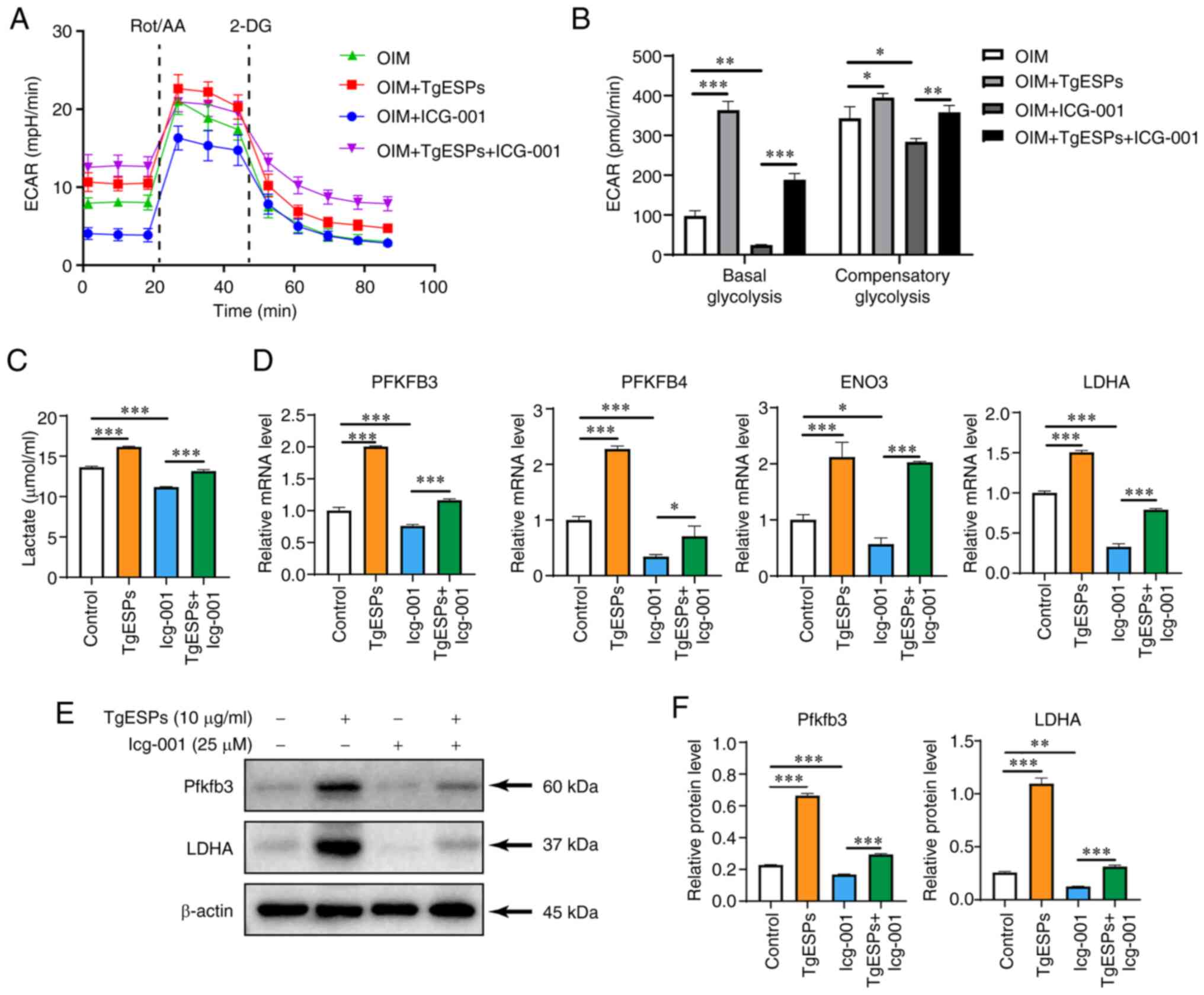 | Figure 6TgESPs regulate aerobic glycolysis
through the Wnt/β-catenin signaling pathway. (A) Seahorse analysis
of ECAR in the presence or absence of Icg-001 with the addition of
TgESPs to hBMSCs for inducing osteogenic differentiation for 7
days. (B) Basal and compensatory glycolysis in the ECAR assay. (C)
Lactate production in hBMSCs after three days of TgESP treatment in
the presence or absence of Icg-001. (D) Reverse
transcription-quantitative PCR analysis of mRNA levels of PFKFB3,
PFKFB4, ENO3 and LDHA in hBMSCs after 7 days of osteogenic
induction by TgESPs in the presence or absence of Icg-001. (E)
Western blot analysis of protein levels of PFKFB3 and LDHA in
hBMSCs after 14 days of osteogenic induction by TgESPs in the
presence or absence of Icg-001. (F) Quantitative analysis of the
expression of each protein using β-actin as the internal control.
*P<0.05; **P<0.01;
***P<0.001. TgESPs, Toxoplasma gondii
excretory/secretory proteins; hBMSCs, human bone marrow mesenchymal
stem cells; PFKFB3,
6-phosphofructo-2-kinase/fructose-2,6-biphosphatase 3; ENO3,
enolase 3; LDHA, lactate dehydrogenase A; ECAR, extracellular
acidification rate; Rot/AA, rotenone/antimycin A; 2-DG,
2-deoxy-D-glucose. |
Discussion
In the present study, it was observed that TgESPs
enhanced the expression of osteogenesis-specific marker genes and
proteins, thereby promoting the osteogenic differentiation of
hBMSCs. In addition, activation of the Wnt/β-catenin signaling
pathway during osteogenic differentiation and enhanced cellular
glycolysis were observed. To further validate the relationship
between Wnt/β-catenin signaling and glycolysis, the inhibitory
effects of β-catenin were evaluated. It was observed that Icg-001,
an inhibitor of β-catenin, and 2-DG, an inhibitor of glycolysis,
partially inhibited the increase in osteogenic differentiation of
hBMSCs caused by TgESPs. These results suggest that TgESPs can
promote osteogenic differentiation of hBMSCs through activation of
the Wnt/β-catenin signaling pathway and glycolysis.
As an abundant tissue in the body, the bone has
numerous different functions and has an instrumental role in human
movement, hematopoiesis and the provision of calcium and phosphorus
(30). However, bone regeneration
is a complex and carefully orchestrated process involving various
cells, including vascular endothelial cells, MSCs, inflammatory
cells and fibroblasts (31).
Accordingly, stem cells have a role in the healing of fractures and
bone defects have a crucial role in fracture and bone defect
healing. Osteoblasts are directly differentiated from BMSCs and are
particularly critical during early bone formation and fracture
repair. They synthesize proteoglycans and type I collagen to form
unmineralized osteoid tissues (32). Therefore, an increasing number of
researchers are working on methods to differentiate BMSCs into
osteoblasts to promote bone regeneration (33).
In translational medicine, BMSCs are widely used as
promising seed cells for accelerating bone healing. According to a
report, exosome transplantation from BMSCs promotes angiogenesis
and bone healing in rats with femoral nonunion (34). Therefore, biologically active
molecules that can provide an optimal osteogenic microenvironment
for BMSCs are expected to accelerate bone formation in future
clinical applications (35). In
the present study, it was observed that TgESPs enhanced cell
differentiation toward osteoblasts while promoting the
proliferation of BMSCs. This observation indicated their potential
use in bone tissue engineering for the treatment of bone
defects.
Previous studies have identified TgESPs as a complex
group of proteins secreted by T. gondii and antibodies
against these secreted antigens have been used as diagnostic
markers for toxoplasmosis infection (36). However, recent research has
expanded the focus to investigate the components of TgESPs and
their effects on cellular functions (23). Studies have revealed that TgESPs
can influence the dissemination of T. gondii within tissues
(37,38). The present study also investigated
the effects of TgESPs on cellular behavior of hBMSCs, including
cell adhesion and migration. Our results revealed that low
concentrations of TgESPs promoted cell adhesion, and both 1 and 10
µg/ml concentrations enhanced cell migration in hBMSCs.
These findings suggest that TgESPs may regulate cell-to-cell
interactions and promote cell migration in BMSCs.
MSCs require a balance between bone formation and
resorption to maintain bone homeostasis, and the integrity of
energy metabolism is crucial in this process. According to most
studies, stem cells rely mainly on glycolysis for metabolism
(39). This may be because
glycolysis provides MSCs with the major cofactors and substrates
required for their biosynthesis and proliferation (40). Furthermore, MSCs are thought to be
dependent on anaerobic energy metabolism, which may be explained by
the long-term evolutionary adaptation of stem cells to their anoxic
niche (41). However, during BMSC
differentiation, there is a high demand for energy sources.
Therefore, the energy acquisition pathway of BMSCs shifts from
glycolysis to oxidative mitochondrial metabolism, generating large
amounts of energy through OXPHOS in mitochondria to meet
differentiation requirements (27). By contrast, the mode of energy
metabolism in osteoblasts is dominated by aerobic glycolysis,
wherein the overexpression of hypoxia-inducible factor-1α activates
glycolysis, leading to increased osteoblast and bone formation in
mice (42). Early osteogenic
differentiation requires the synthesis of collagen to upregulate
OXPHOS. In addition, with the increase in OXPHOS, reactive oxygen
species levels tend to increase, which may shift cellular
metabolism to glycolysis (13,43). Thus, both mitochondrial oxidative
metabolism and glycolysis, which are both modes of energy
metabolism, are essential for osteogenic differentiation. In the
present study, it was found that mitochondrial oxidative metabolism
was upregulated and basal glycolysis was downregulated in BMSCs
during early osteogenic differentiation. This result is consistent
with previous findings (44).
Furthermore, both basal and compensatory glycolysis were
upregulated in BMSCs after TgESP treatment, whereas the
mitochondrial oxidative metabolism remained unchanged.
Subsequently, the glycolysis of BMSCs was inhibited, which resulted
in a decrease in glycolysis in conjunction with a decrease in
osteogenic differentiation. However, addition of TgESPs restored
the glycolytic and osteogenic capacities of BMSCs. This observation
indicates that TgESPs can regulate glycolysis in BMSCs and promote
their osteogenic differentiation through glycolysis.
In addition, the signaling pathways that mediate the
regulation of glycolysis by TgESPs were investigated. Previously,
most developmental signaling pathways were shown to regulate the
osteogenic differentiation of MSCs, but their effects on cellular
energy metabolism need to be further investigated. The
Wnt/β-catenin signaling pathway is critical for differentiation and
development and has an instrumental role in bone homeostasis
(45). A recent study indicated
that activation of the Wnt/β-catenin signaling pathway can restore
osteoblasts from a state of impaired glycolysis and osteogenic
differentiation (46).
Furthermore, the Wnt/β-catenin signaling pathway can regulate
cellular glycolytic metabolism during osteogenic differentiation
and elevate the levels of essential glycolytic enzymes to induce
aerobic glycolysis, further enhancing bone formation in vivo
(29). It was also noted that the
levels of β-catenin expression were markedly increased during
osteogenic differentiation in the TgESP group compared to the
control group. In addition, TgESPs were able to partially reverse
the inhibition of β-catenin after blocking β-catenin expression
with Icg-001 and a similar phenomenon was observed for glycolysis
in BMSCs. The present findings indicate that TgESPs are capable of
elevating glycolysis via the Wnt/β-catenin signaling pathway, which
subsequently stimulates the osteogenic differentiation of BMSCs. In
the present investigation, it was noted that the increase in
osteogenic differentiation in BMSCs was only partially suppressed
by β-catenin inhibition, implying that TgESPs may also exert their
effects through other mechanisms. To date, the role of TgESPs in
the osteogenic differentiation of MSCs has remained elusive. As
such, future research should aim to uncover the ways in which
TgESPs influence the osteogenic differentiation process through
additional signaling pathways.
There are certain limitations to the present study.
First, the results were not validated in vivo due to the
fact that cell proliferation and differentiation may vary under
different cultural conditions, leading to potentially different
results. Furthermore, there are three main ways of cellular energy
metabolism, namely glucose, fatty acid and amino acid metabolism,
although in the present study, only changes in glucose metabolism
were detected, while the potential mechanisms by which TgESPs
regulate fatty acid and amino acid metabolism were not explored
further. Finally, although the composition of TgESPs has been
determined (23), the specific
proteins responsible for the effect were not clearly identified in
the present study. Therefore, further studies need to clarify which
specific components of TgESPs regulate BMSCs.
In conclusion, the present study indicated that
TgESPs enhance the osteogenic differentiation of hBMSCs. They
increase the expression of glycolytic enzymes and lactate
production under adequate oxygen conditions, leading to an increase
in aerobic glycolysis (Fig. 7).
Furthermore, TgESPs regulate this process by modulating the
Wnt/β-catenin signaling pathway, thus stimulating osteogenic
differentiation.
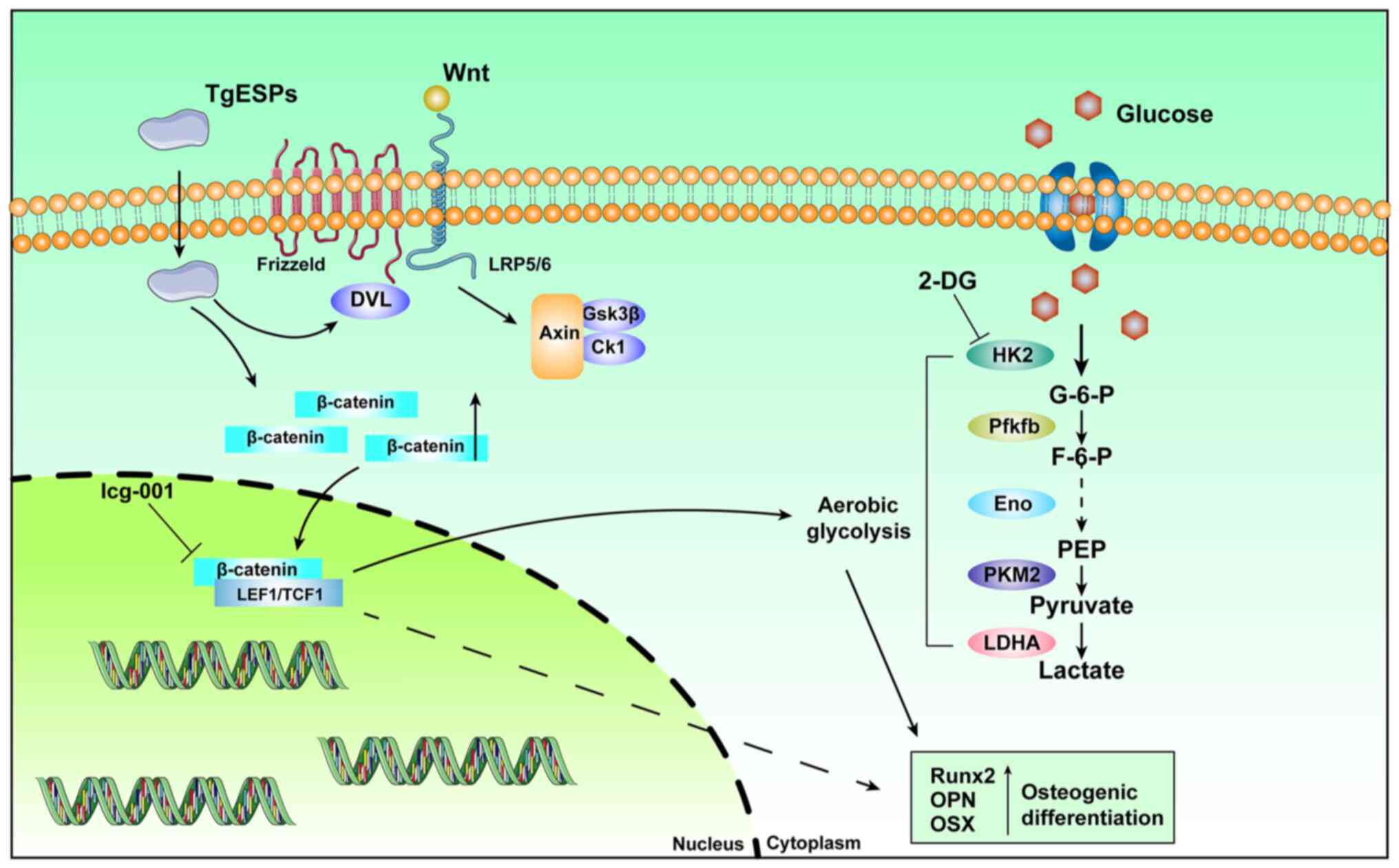 | Figure 7Illustration of a hypothetical model
in which TgESPs regulate aerobic glycolysis to promote osteogenic
differentiation through the Wnt/β-catenin signaling pathway.
TgESPs, Toxoplasma gondii excretory/secretory proteins;
2-DG, 2-deoxy-D-glucose; HK2, hexokinase-2; LDHA, lactate
dehydrogenase A; PFKFB3,
6-phosphofructo-2-kinase/fructose-2,6-biphosphatase 3; PKM2,
pyruvate kinase M2; G-6-P, glucose-6-phosphate; PEP,
phospho-enol-pyruvate; Runx2, RUNX family transcription factor 2;
OPN, osteopontin; OSX, osterix; ENO, enolase; LEF1, lymphoid
enhancer binding factor 1; DVL3, dishevelled segment polarity
protein 3; GSK3β, glycogen synthase kinase 3β; Ck1, casein kinase
1. |
Supplementary Data
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
JC and JQ designed the research. ZC and TL collected
the bone marrow samples. WZ, MD, ZZ, XW, ZZ and FG performed the
experiments and analyzed the data. JC, JQ and WZ wrote and revised
the manuscript. WZ and JC checked and confirmed the authenticity of
all the raw data. All authors have read and approved the final
manuscript.
Ethics approval and consent to
participate
The hBMSCs were all derived from the bone marrow of
patients with traumatic femur fractures who had provided written
informed consent. The protocol was approved by the Ethics Committee
of the Affiliated Hospital of Guangdong Medical University
(Zhanjiang, China).
Patient consent for publication
Not applicable.
Competing interests
All authors declare that they have no competing
interests.
Acknowledgments
The authors thank Dr Young-Ha Lee (Department of
Infection Biology, Chungnam National University School of Medicine,
Republic of Korea) for providing the RH strain of T.
gondii.
Funding
This work was supported by the Special fund for Affiliated
Hospital of Guangdong Medical University 'Clinical Medicine +'
CnTech Co-construction Platform (grant no. CLP2021A001) and the
Competitive Allocation Project of Special Funds for Science and
Technology Development in Zhanjiang City (grant nos. 2019A01032 and
2022A01198).
References
|
1
|
Thormann U, Ray S, Sommer U, Elkhassawna
T, Rehling T, Hundgeburth M, Henß A, Rohnke M, Janek J, Lips KS, et
al: Bone formation induced by strontium modified calcium phosphate
cement in critical-size metaphyseal fracture defects in
ovariectomized rats. Biomaterials. 34:8589–8598. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Giannoudis PV, Dinopoulos H and Tsiridis
E: Bone substitutes: An update. Injury. 36(Suppl 3): S20–S27. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Sheng G: The developmental basis of
mesenchymal stem/stromal cells (MSCs). BMC Dev Biol. 15:442015.
View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Saeed H, Ahsan M, Saleem Z, Iqtedar M,
Islam M, Danish Z and Khan AM: Mesenchymal stem cells (MSCs) as
skeletal therapeutics-an update. J Biomed Sci. 23:412016.
View Article : Google Scholar
|
|
5
|
Ullah I, Subbarao RB and Rho GJ: Human
mesenchymal stem cells-current trends and future prospective.
Biosci Rep. 35:e001912015. View Article : Google Scholar
|
|
6
|
Pasin L, Boraso S and Tiberio I:
Initiation of renal-replacement therapy in the intensive care unit.
N Engl J Med. 375:1899–1902. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Xu Z, He J, Zhou X, Zhang Y, Huang Y, Xu N
and Yang H: Down-regulation of LECT2 promotes osteogenic
differentiation of MSCs via activating Wnt/beta-catenin pathway.
Biomed Pharmacother. 130:1105932020. View Article : Google Scholar
|
|
8
|
Ahmadzadeh A, Norozi F, Shahrabi S,
Shahjahani M and Saki N: Wnt/β-catenin signaling in bone marrow
niche. Cell Tissue Res. 363:321–335. 2016. View Article : Google Scholar
|
|
9
|
Canalis E: MANAGEMENT OF ENDOCRINE
DISEASE: Novel anabolic treatments for osteoporosis. Eur J
Endocrinol. 178:R33–R44. 2018. View Article : Google Scholar
|
|
10
|
Liu D, Chen L, Zhao H, Vaziri ND, Ma SC
and Zhao YY: Small molecules from natural products targeting the
Wnt/β-catenin pathway as a therapeutic strategy. Biomed
Pharmacother. 117:1089902019. View Article : Google Scholar
|
|
11
|
Nusse R and Clevers H: Wnt/β-catenin
signaling, disease, and emerging therapeutic modalities. Cell.
169:985–999. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
van Gastel N and Carmeliet G: Metabolic
regulation of skeletal cell fate and function in physiology and
disease. Nat Metab. 3:11–20. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Guntur AR, Le PT, Farber CR and Rosen CJ:
Bioenergetics during calvarial osteoblast differentiation reflect
strain differences in bone mass. Endocrinology. 155:1589–1595.
2014. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Lee SY and Long F: Notch signaling
suppresses glucose metabolism in mesenchymal progenitors to
restrict osteoblast differentiation. J Clin Invest. 128:5573–5586.
2018. View
Article : Google Scholar : PubMed/NCBI
|
|
15
|
Liu H and Rosen CJ: Nitric oxide and bone:
The phoenix rises again. J Clin Invest. 131:e1470722021. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Vallee A, Lecarpentier Y and Vallee JN:
The key role of the WNT/β-catenin pathway in metabolic
reprogramming in cancers under normoxic conditions. Cancers
(Basel). 13:55572021. View Article : Google Scholar
|
|
17
|
Pate KT, Stringari C, Sprowl-Tanio S, Wang
K, TeSlaa T, Hoverter NP, McQuade MM, Garner C, Digman MA, Teitell
MA, et al: Wnt signaling directs a metabolic program of glycolysis
and angiogenesis in colon cancer. EMBO J. 33:1454–1473. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Deng L, Yi S, Yin X, Li Y and Luan Q: MFN2
knockdown promotes osteogenic differentiation of iPSC-MSCs through
aerobic glycolysis mediated by the Wnt/β-catenin signaling pathway.
Stem Cell Res Ther. 13:1622022. View Article : Google Scholar
|
|
19
|
Gomez S, Adalid-Peralta L, Palafox-Fonseca
H, Cantu-Robles VA, Soberon X, Sciutto E, Fragoso G, Bobes RJ,
Laclette JP, Yauner L and Ochoa-Leyva A: Genome analysis of
Excretory/Secretory proteins in Taenia solium reveals their
abundance of antigenic regions (AAR). Sci Rep. 5:96832015.
View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Garg G and Ranganathan S: Helminth
secretome database (HSD): A collection of helminth
excretory/secretory proteins predicted from expressed sequence tags
(ESTs). BMC Genomics. 13(Suppl 7): S82012. View Article : Google Scholar
|
|
21
|
Liu W and Chen YH: High epitope density in
a single protein molecule significantly enhances antigenicity as
well as immunogenicity: A novel strategy for modern vaccine
development and a preliminary investigation about B cell
discrimination of monomeric proteins. Eur J Immunol. 35:505–514.
2005. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Ma Z, Alhameed AM, Kaminga AC, Lu B, Li X,
Zhang J and Wu X: Bioinformatics of excretory/secretory proteins of
Toxoplasma gondii strain ME49. Microb Pathog. 140:1039512020.
View Article : Google Scholar
|
|
23
|
Ramirez-Flores CJ, Cruz-Miron R,
Mondragon-Castelan ME, Gonzalez-Pozos S, Rios-Castro E and
Mondragon-Flores R: Proteomic and structural characterization of
self-assembled vesicles from excretion/secretion products of
Toxoplasma gondii. J Proteomics. 208:1034902019. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(-Delta Delta C(T)) method. Methods. 25:402–408. 2001.
View Article : Google Scholar
|
|
25
|
Muscella A, Vetrugno C, Cossa LG and
Marsigliante S: TGF-β1 activates RSC96 Schwann cells migration and
invasion through MMP-2 and MMP-9 activities. J Neurochem.
153:525–538. 2020. View Article : Google Scholar
|
|
26
|
Shibuya T, Honma M, Fujii M, Iinuma S and
Ishida-Yamamoto A: Podoplanin suppresses the cell adhesion of
epidermal keratinocytes via functional regulation of
beta1-integrin. Arch Dermatol Res. 311:45–53. 2019. View Article : Google Scholar
|
|
27
|
Forni MF, Peloggia J, Trudeau K, Shirihai
O and Kowaltowski AJ: Murine mesenchymal stem cell commitment to
differentiation is regulated by mitochondrial dynamics. Stem Cells.
34:743–755. 2016. View Article : Google Scholar
|
|
28
|
Wanet A, Remacle N, Najar M, Sokal E,
Arnould T, Najimi M and Renard P: Mitochondrial remodeling in
hepatic differentiation and dedifferentiation. Int J Biochem Cell
Biol. 54:174–185. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Esen E, Chen J, Karner CM, Okunade AL,
Patterson BW and Long F: WNT-LRP5 signaling induces Warburg effect
through mTORC2 activation during osteoblast differentiation. Cell
Metab. 17:745–755. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Florencio-Silva R, Sasso GR, Sasso-Cerri
E, Simoes MJ and Cerri PS: Biology of bone tissue: Structure,
function, and factors that influence bone cells. Biomed Res Int.
2015:4217462015. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Bahney CS, Zondervan RL, Allison P,
Theologis A, Ashley JW, Ahn J, Miclau T, Marcucio RS and Hankenson
KD: Cellular biology of fracture healing. J Orthop Res. 37:35–50.
2019. View Article : Google Scholar :
|
|
32
|
Hankenson KD, Gagne K and Shaughnessy M:
Extracellular signaling molecules to promote fracture healing and
bone regeneration. Adv Drug Deliv Rev. 94:3–12. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Garg P, Mazur MM, Buck AC, Wandtke ME, Liu
J and Ebraheim NA: Prospective review of mesenchymal stem cells
differentiation into osteoblasts. Orthop Surg. 9:13–19. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Zhang L, Jiao G, Ren S, Zhang X, Li C, Wu
W, Wang H, Liu H, Zhou H and Chen Y: Exosomes from bone marrow
mesenchymal stem cells enhance fracture healing through the
promotion of osteogenesis and angiogenesis in a rat model of
nonunion. Stem Cell Res Ther. 11:382020. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Gao C, Seuntjens J, Kaufman GN, Tran-Khanh
N, Butler A, Li A, Wang H, Buschmann MD, Harvey EJ and Henderson
JE: Mesenchymal stem cell transplantation to promote bone healing.
J Orthop Res. 30:1183–1189. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Saadatnia G, Mohamed Z, Ghaffarifar F,
Osman E, Moghadam ZK and Noordin R: Toxoplasma gondii excretory
secretory antigenic proteins of diagnostic potential. APMIS.
120:47–55. 2012. View Article : Google Scholar
|
|
37
|
Ramirez-Flores CJ, Cruz-Miron R,
Lagunas-Cortes N, Mondragon-Castelan M, Mondragon-Gonzalez R,
Gonzalez-Pozos S and Mondragon-Flores R: Toxoplasma gondii
excreted/secreted proteases disrupt intercellular junction proteins
in epithelial cell monolayers to facilitate tachyzoites
paracellular migration. Cell Microbiol. 23:e132832021. View Article : Google Scholar
|
|
38
|
Ramirez-Flores CJ, Cruz-Miron R, Arroyo R,
Mondragon-Castelan ME, Nopal-Guerrero T, Gonzalez-Pozos S,
Rios-Castro E and Mondragon-Flores R: Characterization of
metalloproteases and serine proteases of Toxoplasma gondii
tachyzoites and their effect on epithelial cells. Parasitol Res.
118:289–306. 2019. View Article : Google Scholar
|
|
39
|
Rafalski VA, Mancini E and Brunet A:
Energy metabolism and energy-sensing pathways in mammalian
embryonic and adult stem cell fate. J Cell Sci. 125:5597–5608.
2012. View Article : Google Scholar
|
|
40
|
Wanet A, Arnould T, Najimi M and Renard P:
Connecting mitochondria, metabolism, and stem cell fate. Stem Cells
Dev. 24:1957–1971. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Hsu YC, Wu YT, Yu TH and Wei YH:
Mitochondria in mesenchymal stem cell biology and cell therapy:
From cellular differentiation to mitochondrial transfer. Semin Cell
Dev Biol. 52:119–131. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Regan JN, Lim J, Shi Y, Joeng KS, Arbeit
JM, Shohet RV and Long F: Up-regulation of glycolytic metabolism is
required for HIF1alpha-driven bone formation. Proc Natl Acad Sci
USA. 111:8673–8678. 2014. View Article : Google Scholar
|
|
43
|
Tormos KV, Anso E, Hamanaka RB, Eisenbart
J, Joseph J, Kalyanaraman B and Chandel NS: Mitochondrial complex
III ROS regulate adipocyte differentiation. Cell Metab. 14:537–544.
2011. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Li B, Shi Y, Liu M, Wu F, Hu X, Yu F, Wang
C and Ye L: Attenuates of NAD(+) impair BMSC osteogenesis and
fracture repair through OXPHOS. Stem Cell Res Ther. 13:772022.
View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Maupin KA, Droscha CJ and Williams BO: A
comprehensive overview of skeletal phenotypes associated with
alterations in wnt/β-catenin signaling in humans and mice. Bone
Res. 1:27–71. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Yang YY, Zhou YM, Xu JZ, Sun LH, Tao B,
Wang WQ, Wang JQ, Zhao HY and Liu JM: Lgr4 promotes aerobic
glycolysis and differentiation in osteoblasts via the canonical
Wnt/beta-catenin pathway. J Bone Miner Res. 36:1605–1620. 2021.
View Article : Google Scholar : PubMed/NCBI
|