Introduction
The ovary plays a crucial role as a manager and
regulator of various female physiological processes associated with
female fertility, including ovulation and sexual hormone secretion
(1). However, the process of
aging indisputably has indisputable detrimental effects on ovarian
function (2). Natural ovarian
aging (NOA) is a degenerative condition that is characterized by
the gradual decline in ovarian function, a process that is
influenced by both genetics and environmental factors (3). The progressive loss of follicles and
oocytes in terms of both quantity and quality as a result of aging
has been reported to be strongly associated with significant
ovarian dysfunction (4). When
this progression is accelerated or advanced, it can lead to
premature insufficiency and insufficient reserve of the ovary,
resulting in pathological damage in females (5). In particular, decreased ovarian
reserve can adversely affect the function of other organs and
tissues, which contribute to reproductive senescence, urogenital
symptoms, decreased bone density and cardiovascular disease
(6). NOA is a physiological
phenomenon which may cause organ dysfunctions and psychological
burdens. Given the marked increase in the global average life
expectancy, there has been a notable rise in the proportion of
postmenopausal women (7). With
improving living standards and increased health awareness, there
are currently aspirations to either attenuate or partially reverse
the process of NOA to enhance the quality of life. In 2015, a study
published in 'Nature' emphasized the importance of addressing aging
seriously, as it serves as a catalyst for a long list of diseases
in the human body (8). Therefore,
the challenge lies in effectively addressing the natural aging
process in the ovary within the field of medicine.
Hormone replacement therapy has traditionally been
applied for addressing menopausal symptoms. However, its
non-fundamental reconstruction of the ovary and associated
side-effects always result in unsatisfactory efficiency (9). Therefore, there is an urgent demand
for a novel therapeutic approach that can fundamentally improve
ovarian function in the management of NOA. With the increasing
utilization of regenerative therapy in medicine, mesenchymal stem
cells (MSCs) and MSC-derived exosomes (MSCs-Exos) have emerged as a
potential means to achieve such a restoration of ovarian function
(10). MSCs possess various
characteristics, including low immunogenicity, and paracrine and
multi-directional differentiation capabilities, enabling them to
reconstruct damaged tissues and organs (11). In particular, human umbilical
cord-derived MSCs (hUCMSCs) can be easily isolated and have high
proliferative capacities (12),
leading to their commercialization by biotechnology companies and
widespread application in basic research, biomedical applications,
clinical trials or even medical therapies (13-15). The paracrine effectors of MSCs,
namely their secretome, have been reported to exert long-term
therapeutic effects by transporting microRNAs (miRNAs/miRs) and
proteins (cytokines or growth factors) to target recipient cells in
injured organs (16,17). Exosomes are major mediators of the
paracrine function of MSCs by facilitating intercellular
communications by delivering a variety of biomolecules (18). With an average diameter of 100 nm,
these exosomes possess the ability to regulate gene expression
through the miRNAs being transported (19).
Follicular atresia and functional failure are
prominent pathological features of NOA (20). Previous research has demonstrated
that the apoptosis of granulosa cells (GCs) within follicles serves
as the main underlying cause of follicle impairment (21). During follicular atresia, there is
an elevation in the expression level of apoptosis-associated genes,
a downregulation in that of anti-apoptotic genes and an increase in
the activity of the caspase family of proteins within GCs (22,23). Therefore, modulating he expression
of apoptotic genes to inhibit GC apoptosis represents a promising
strategy for alleviating the progression of NOA. PTEN has
previously been documented to be an important regulator of various
physiological processes, including proliferation, migration and
apoptosis (24). The involvement
of PTEN in the regulation of apoptosis is particularly
well-documented, since it inhibits AKT activation, a critical
suppressor of apoptosis (25). In
addition, the PTEN/PI3K/AKT signaling pathway plays a crucial role
in governing the recruitment and growth of primordial follicles
(26), where PTEN plays a key
role in the regulation of follicle activation. Previous research
has reported that the inhibition of PTEN expression can promote
follicle activation and restore reproductive function (27). During exosome transplantation,
MSC-Exos can carry miRNAs that have the ability to target and
inhibit PTEN expression, thereby reducing cellular apoptosis and
restoring the functionality of target organs. A previous study
found that miR-144 derived from MSC-Exos blocked cardiomyocyte
apoptosis by targeting PTEN and modulating the AKT pathway
(28). Although previous studies
have demonstrated the potential of MSC-Exos in improving ovarian
function in cases of premature ovarian insufficiency (10,29), limited attention has been paid to
the detection of the effects of MSCs or MSC-Exos on NOA (30). Furthermore, the underlying
mechanisms through which exosomal miRNAs can alleviate ovarian
cells apoptosis in NOA remains poorly understood.
Therefore, the primary aim of the present study was
to evaluate the potential of hUCMSC-derived exosomes (hUCMSC-Exos)
in restoring ovarian function in a mouse model of NOA and to
explore the regulatory effects of exosomes on PTEN expression and
apoptosis both in vivo and in vitro. In addition, the
high-throughput sequencing of exosomes was conducted to identify
highly expressed miRNAs that can target PTEN expression, following
which their effects on GC apoptosis were validated. It is hoped
that the findings of the present study will lay the foundation for
future investigations of hUCMSC-Exos for countering ovarian
aging.
Materials and methods
Identification of hUCMSCs and
exosomes
The hUCMSCs used herein were purchased from Shandong
Qilu Cell Therapy Engineering Technology Co., Ltd. and cultured in
a complete medium at 37°C with 5% CO2. The
identification of hUCMSCs was performed by following a previously
established protocol (31).
Initially, their morphology was confirmed using a light microscope
(Carl Zeiss AG). Subsequently, the osteogenesis differentiation kit
(A1007201, Gibco; Thermo Fisher Scientific, Inc.) and adipogenesis
differentiation kit (A1007001, Gibco; Thermo Fisher Scientific,
Inc.) were utilized for the induction of the differentiation of the
MSCs. Alizarin Red staining and Oil Red O staining were performed
on the cells following the induction of cell differentiation. For
Alizarin Red staining, the cells were fixed using 4%
paraformaldehyde at room temperature for 10-20 min. A staining
solution of 0.1% Alizarin Red in Tris-HCl buffer at pH 4.2
(MilliporeSigma) was applied to the cells for 20-30 min at room
temperature. Following staining, the cells were rinsed with
distilled water until color fading ceased. Similarly, for Oil Red O
staining, the cells were fixed with 4% paraformaldehyde at room
temperature for 10-20 min. A 0.5% Oil Red O staining solution in
isopropanol (MilliporeSigma) was applied to the cells for 15-20 min
at room temperature. Subsequently, the cells were washed with 70%
ethanol until color fading ceased. Staining results were observed
and documented under a microscope (Axio Imager.D2; Carl Zeiss AG).
Finally, flow cytometry (BD FACSCanto II; BD Biosciences) was
utilized to detect the expression of positive surface markers
(CD44, CD73, CD90, and CD105; Biolegend, Inc.) and negative surface
markers (CD34, CD45, and HLA-DR; Biolegend, Inc.) of MSCs.
The extraction of exosomes was successfully
performed following a previously established method (32). Subsequently, the identification of
exosomes was performed in accordance with the standards set by the
International Society for Extracellular Vesicles (33). The size distribution and
concentration of exosomes were determined using the Flow Nano
Analyzer (Particle Metrix GmbH). Additionally, the morphologies of
the exosomes were observed using a transmission electron microscope
(Thermo Fisher Scientific, Inc.). Lastly, the surface markers of
exosomes (CD9, CD63, and CD81; Biolegend, Inc.) were detected using
flow cytometry (BD Biosciences).
Single-cell RNA-seq (scRNA-seq) analysis
of aging primate ovaries
Bioinformatics analysis was performed to investigate
the expression levels of PTEN and apoptosis-related genes in aging
primate ovaries using single-cell data. The scRNA-seq data from the
GSE130664 dataset (34) were
obtained from the Gene Expression Omnibus (GEO) database
(https://www.ncbi.nlm.nih.gov/geo/).
This dataset was derived from 2,601 single cells with high-quality
transcriptomes collected from the ovaries of 4 young and 4 aged
monkeys for scRNA-seq analysis (34). The 'Seurat' R package was utilized
for quality control, normalization, dimensional reduction and
processing of the scRNA-seq expression data. Genes expressed in
fewer than three cells were excluded, whereas cells with <50
genes were removed from the analysis. The expression data were then
normalized, dimensionally reduced and clustered using the
t-distributed stochastic neighbor embedding (t-SNE) method. Cluster
annotation was performed using cell markers from the 'CellMarker'
database (35) and from a
previous study (34). Finally,
the expression of PTEN and apoptosis-related molecules was
presented.
Experimental animals and treatment with
hUCMSC-Exos
A total of 36 female C57BL/6 mice, aged 3 months
(n=12) and 14 months (n=24), were obtained from SPF Biotechnology
(Beijing, China). The principles outlined in the Declaration of
Helsinki and the Guidelines for the Care and Use of Laboratory
Animals of the Chinese Institute of Health were strictly adhered to
in the present study. The authors also complied with the ARRIVE
guidelines. Ethical approval for the animal experiments was
obtained from the Ethical Committee of Second Hospital of Hebei
Medical University (approval no. 2021-AE034). All animal
experiments conducted in the present study followed the guidelines
and regulations specified in this ethical approval. The aging mice
were equally and randomly divided into two groups as follows: the
NOA group and Exos group (n=12 per group). Additionally, a group of
3-month-old mice, referred to as the Young group (n=12), was also
included in the present study. The animals were kept in
individually ventilated cages with autoclaved aspen woodchips and a
mouse house, a maintaining a temperature of 21±2°C, a 12:12-h
light-dark cycle, and a relative humidity of 55±10%. They were
provided with unrestricted access to a commercially prepared
autoclaved dry rodent diet and water. The health of the animals was
monitored on a daily basis through routine visual checks. In the
animal experiments, humane endpoints were established to ensure the
welfare of the animals and to minimize suffering. The humane
endpoints included the following: A 20% decrease in body weight
compared to the pre-study weight; continuous 4-day anorexia with
significant weight loss; persistent diarrhea or vomiting with no
response to treatment; conditions unresponsive to clinical
treatments, such as organ failure, respiratory distress and sepsis;
severe hypothermia or hyperthermia unresponsive to corrective
measures; severe complications resulting from medical/surgical
interventions or other experimental manipulations with no available
corrective measures; severe self-injurious behaviors unmanageable
by behavioral interventions, medical treatments, and/or research
removal. These humane endpoints were implemented in accordance with
the guidelines and regulations of the authors' institution to
ensure the ethical conduct of the research. Following the adaptive
feeding period, the mice in the Exos group received intraperitoneal
injections of 150 µg exosomes twice, with a 7-day interval
between injections, based on observations from a previous study
(32). By contrast, mice in the
NOA group were injected with an equivalent volume of
phosphate-buffered saline (PBS; Wuhan Servicebio Technology Co.,
Ltd.), whilst the mice in the Young group did not receive any
therapeutic interventions. No mice were found dead prior to
euthanasia. At 2 weeks after the injection of the exosomes
(36), the animals were
euthanized by an intraperitoneal injection of an overdose of
pentobarbital (100 mg/kg) in all three groups (n=36). The
confirmation of animal death was determined by examining whether
the heartbeat of the animal had ceased completely and whether the
pupils were dilated. Ovarian tissue and serum samples were then
collected for subsequent experimental analyses. Each data analysis
included at least three replicate molecular experiments, with a
minimum of six animal samples per group in each experiment.
Culture and identification of human
GCs
The human ovarian granulosa cell line, HO-23
(37,38), was obtained from Qingqi (Shanghai)
Biotechnology Development Co., Ltd. (cat. no. BFN60808800) and
cultured in DMEM (Gibco; Thermo Fisher Scientific, Inc.)
supplemented with 10% exosome-depleted FBS (Gibco; Thermo Fisher
Scientific, Inc.) at 37°C and 5% CO2.
The immunofluorescence staining of
follicle-stimulating hormone (FSH) receptor (FSHR) was performed to
identify the human GCs (hGCs), as FSHR is specifically expressed in
the cytoplasm of ovarian GCs. Initially, the complete medium was
removed, and the cells were washed three times with PBS (Wuhan
Servicebio Technology Co., Ltd.). Subsequently, permeabilization
was achieved using 0.3% Triton X-100 (Wuhan Servicebio Technology
Co., Ltd.), followed by blocking with 5% goat serum (Wuhan
Servicebio Technology Co., Ltd.). The cells were then incubated
overnight at 4°C with primary antibody against FSHR (1:800; cat.
no. GB11275-1-100, Wuhan Servicebio Technology Co., Ltd.). On the
following day, the cells were incubated with a FITC-conjugated
secondary antibody (1:400; cat. no. GB22303, Wuhan Servicebio
Technology Co., Ltd.) for 1 h. Finally, the cell nuclei were
stained with DAPI for 10 min at room temperature (cat. No.:
0100-20, SouthernBiotech). Images were captured using a ZEISS
fluorescence microscope (Carl Zeiss AG).
Western blot analysis
Western blot analyses were performed to examine
protein expression in ovarian tissues and GCs. The samples were
first lysed using RIPA lysis buffer (Wuhan Servicebio Technology
Co., Ltd.). The protein concentrations were measured using the BCA
protein assay kit (Beijing Solarbio Science & Technology Co.,
Ltd.), before the protein samples were denatured at 100°C for 10
min. Subsequently, the proteins were separated in SDS-PAGE (10%)
and transferred onto PVDF membranes. In the adjacent lanes of the
target samples, a protein molecular weight marker was utilized. To
conserve the usage of antibodies, we subjected the membrane to
precise trimming based on the protein quantity of the target
molecule, resulting in a final presentation of the membrane in a
strip-like format. These membranes were then blocked with 5%
skimmed milk for 2 h and incubated overnight at 4°C with primary
antibodies against PTEN (1:1,000; cat. no. bs-0748R, BIOSS), Bax
(1:500; cat. no. AF1270, Beyotime Institute of Biotechnology),
Bcl-2 (1:500; cat. no. bs-4563R, BIOSS), caspase-3 (1:500; cat. no.
AF1213, Beyotime Institute of Biotechnology), caspase-9 (1:1,000;
cat. no. AF1264, Beyotime Institute of Biotechnology) or GAPDH
(1:2,000; cat. no. GB11002-100, Wuhan Servicebio Technology Co.,
Ltd.). The membranes were cut prior to antibody hybridization,
resulting in the absence of the full-length blot. On the following
day, the membranes were incubated with an HRP-conjugated secondary
antibody (1:5,000, cat. no. GB23303, Wuhan Servicebio Technology
Co., Ltd.) for 1 h. After washing the membranes with PBS-Tween-20,
the protein levels were quantified using the ChemiDoc MP Imaging
System (Bio-Rad Laboratories, Inc.). For the statistical analysis
of the western blot data using Image Lab™ software (version 6.0,
Bio-Rad Laboratories, Inc.), at least three replicate experiments
were performed, with each experiment including at least six animal
samples per group.
Reverse transcription-quantitative PCR
(RT-qPCR)
Initially, total RNA was extracted using
TRIzol® reagent (Invitrogen; Thermo Fisher Scientific,
Inc.). The MonScript™ RTIII All-in-One Mix with dsDNase (Monad
Biotech Co., Ltd.) was used for the reverse transcription of total
RNA into cDNA. To perform qPCR, specific primers were obtained from
the primer bank and synthesized by Sangon Biotech Co., Ltd. The
primer sequences are presented in Table I. GoTaq® qPCR Master
Mix (Promega Corporation) was used for qPCR with the Bio-Rad CFX96
detection system, using the thermocycling conditions of 95°C for 10
min, followed by 40 cycles of 95°C for 15 sec and 60°C for 60 sec.
The relative expression of miRNA and mRNA was calculated using the
2−ΔΔCq method (39).
To ensure robust statistical analysis of the RT-qPCR data, a
minimum of three independent replicate experiments were performed.
Each experiment consisted of at least six animal samples per
group.
 | Table IPrimer sequences used for
RT-qPCR. |
Table I
Primer sequences used for
RT-qPCR.
| Gene | Forward primer | Reverse primer |
|---|
| Human PTEN |
5′-TTTGAAGACCATAACCCACCAC-3′ |
5′-ATTACACCAGTTCGTCCCTTTC-3′ |
| Human Bax |
5′-CCCGAGAGGTCTTTTTCCGAG-3′ |
5′-CCAGCCCATGATGGTTCTGAT-3′ |
| Human Bcl-2 |
5′-GGTGGGGTCATGTGTGTGG-3′ |
5′-CGGTTCAGGTACTCAGTCATCC-3′ |
| Human
caspase-3 |
5′-CATGGAAGCGAATCAATGGACT-3′ |
5′-CTGTACCAGACCGAGATGTCA-3′ |
| Human
caspase-9 |
5′-CTCAGACCAGAGATTCGCAAAC-3′ |
5′-GCATTTCCCCTCAAACTCTCAA-3′ |
| Human GAPDH |
5′-ACAACTTTGGTATCGTGGAAGG-3′ |
5′-GCCATCACGCCACAGTTTC-3′ |
| MousePTEN |
5′-CACACTGTTCCTCGTTATGAAGA-3′ |
5′-CTTGAGATCCCGATGGGCAAT-3′ |
| Mouse Bax |
5′-TGAAGACAGGGGCCTTTTTG-3′ |
5′-AATTCGCCGGAGACACTCG-3′ |
| Mouse Bcl-2 |
5′-GCTACCGTCGTGACTTCGC-3′ |
5′-CCCCACCGAACTCAAAGAAGG-3′ |
| Mouse
caspase-3 |
5′-CTGACTGGAAAGCCGAAACTC-3′ |
5′-CGACCCGTCCTTTGAATTTCT-3′ |
| Mouse
caspase-9 |
5′-TCCTGGTACATCGAGACCTTG-3′ |
5′-AAGTCCCTTTCGCAGAAACAG-3′ |
| Mouse GAPDH |
5′-AGGTCGGTGTGAACGGATTTG-3′ |
5′-GGGGTCGTTGATGGCAACA-3′ |
| Human
miR-21-5p |
5′-ACACTCCAGCTGGGTAGCTTATCAGACTGA-3′ |
5′-ATGGTGTCGTGGAGTCG-3′ |
| Human
miR-26a-5p |
5′-ACACTCCAGCTGGGTTCAAGTAATCCAGGA-3′ |
5′-ATGGTGTCGTGGAGTCG-3′ |
| U6 |
5′-CTCGCTTCGGCAGCACA-3 |
5′-AACGCTTCACGAATTTGCGT-3′ |
TUNEL assay
TUNEL assays on the ovarian sections and hGCs were
conducted to assess the level of apoptosis. The procedure involved
permeabilization using 0.3% Triton X-100 (Beijing Solarbio Science
& Technology Co., Ltd.) and incubation with the TUNEL detection
solution (Beyotime Institute of Biotechnology) for both the ovarian
sections and GCs. The nuclei were subsequently labeled with the
DAPI solution (SouthernBiotech) at room temperature. The staining
results were observed under a fluorescence microscope (Carl Zeiss
AG), where the apoptotic cell nuclei appeared green.
5-Ethynyl-2′-deoxyuridine (EdU)
assay
EdU assay was used to estimate the extent of
proliferation in ovarian tissues and hGCs. EdU is a marker of DNA
synthesis that can be administered to animals through injection or
feeding (40). In the present
study, EdU was dissolved in PBS at a concentration of 50 mg/kg and
intraperitoneally injected into the mice. After 4 h, the mice were
rapidly sacrificed (the euthanasia method was the same as the
method described above) and their ovaries were collected.
Paraffin-embedded sections of the ovarian tissues were prepared
using standard procedure (41).
Permeabilization of these slides was achieved using 0.3% Triton
X-100. After being washed with PBS, the slides were stained with
reaction solution (Beyotime Institute of Biotechnology). Cell
nuclei were labeled with Hoechst 33342 at room temperature
(1:1,000; Beyotime Institute of Biotechnology). The slides were
observed using a fluorescence microscope (Carl Zeiss AG), before
the presence of EdU was visualized as a fluorescence signal.
For the EdU assay of the cells, cells in the
logarithmic growth phase were seeded in a six-well plate, treated
with 10 µM EdU (ST067, Beyotime Institute of Biotechnology)
and incubated for 2 h at 37°C. The cells were then fixed with 4%
paraformaldehyde for 30 min at room temperature. Permeabilization
was achieved using 0.3% TritonX-100 and the cells were stained with
EdU reaction solution at room temperature (C0071S, Beyotime
Institute of Biotechnology). Similarly, nuclei were labeled with
Hoechst 33342 (Beyotime Institute of Biotechnology). After
completing these steps, images were captured using a fluorescence
microscope (Carl Zeiss AG).
Tracing experiment of exosomes in
vivo
To investigate the uptake and migration of the
transplanted hUCMSC-Exos in the ovaries of mice, the exosomes were
labeled with 5 µl/Test EvLINK 555 (cat. no. CL012100220;
Tingoscience) and incubated at room temperature for 30 min. The
labeled exosomes were then centrifuged at 10,000 × g for 15 min at
4°C, which was then repeated five times to remove free fluorescent
probes and small molecules. Subsequently, the labeled exosomes were
purified using a CORE400 column (Tianyan Biotech Co., Ltd.)
(http://cesi.sinopae.com/index.html)
in pH 7.4 buffer, before the mice were injected with these purified
labeled exosomes. In total, 5 days later, the ovarian tissues of
mice were obtained for frozen sections. The cell nuclei were
labeled with DAPI at room temperature and images were captured
using a fluorescence microscope (AxioCam HRc; Carl Zeiss AG).
Ovarian morphology and follicle
counts
The ovarian tissues were obtained from the
euthanized mice following the completion of the exosome-mediated
therapy. These tissues were fixed in 4% paraformaldehyde for over
48 h at room temperature. Following the completion of
paraffin-embedding, the ovarian tissues were sliced into
5-µm-thick sections. From each group, 6 mice were randomly
selected and their ovaries were consecutively sectioned. For
hematoxylin and eosin (H&E) (MilliporeSigma) staining, the 10th
slice of all ovaries was selected. During the hematoxylin staining
step, the tissues are exposed to the stain at room temperature for
approximately 5-10 min, allowing for the visualization of cell
nuclei. Conversely, in the subsequent eosin staining step, the
tissues are subjected to the stain at room temperature for 1-3 min,
providing coloration to cytoplasmic components and extracellular
matrix. Additionally, three sections at 200-µm intervals
with the largest cross-sectional area were selected to count the
number of follicles at each stage. Ovarian morphology was observed
using a light microscope (Carl Zeiss AG) and all types of follicles
were counted. The average number of follicles at each stage was
calculated. The stage of individual follicles was determined using
the following criteria: i) The primordial follicle has an oocyte
surrounded by a layer of flat squamous GCs; ii) the primary
follicle has an oocyte surrounded by a single layer of cuboidal
GCs; iii) the secondary follicle has an oocyte surrounded by more
than one layer of cuboidal GCs without a visible antrum; iv) the
mature follicle exhibits a large antral space, prominent cumulus
and a visible oocyte crown; and v) the atretic follicle has a
collapsed follicular wall, a disappearance of oocyte structure and
shrinkage of the zona pellucida.
ELISA
The blood from sacrificed mice was collected and the
serum was obtained by centrifugation of the blood at 5,000 rpm for
10 min at 4°C. The levels of anti-Müllerian hormone (AMH),
estradiol (E2) and FSH in serum were measured using
ELISA Kits (cat. nos. 15775, 1609 and 23789 Meimian Technology Co.,
Ltd.) according to the manufacturer's instructions. Briefly, 50
µl serum from each mouse were added to the coated wells for
incubation at 37°C for 30 min. After washing the wells five times,
HRP-conjugated antibodies were added. Following another round of
washing, color development solution was added and incubated with
the wells at 37°C for 30 min. Finally, the reaction was terminated
using stop buffer and the optical density was measured at a
wavelength of 450 nm using a microplate reader (BioTek Epoch;
BioTek Instruments, Inc.). The concentrations of these hormones
were determined by comparing the readings to the standard curve.
For statistical analysis of the ELISA results, a minimum of three
replicate experiments were conducted, with each experiment
including at least six animal samples per group.
Immunofluorescence staining
Firstly, the paraffin-embedded ovarian sections
(5-µm-thick) underwent antigen retrieval using sodium
citrate buffer (Wuhan Servicebio Technology Co., Ltd.).
Subsequently, permeabilization was performed using 0.3% Triton
X-100 (Beijing Solarbio Science & Technology Co., Ltd.) and
blocking was performed with 5% goat serum (Wuhan Servicebio
Technology Co., Ltd.). The slides were then incubated overnight at
4°C with primary antibodies, including Ki67 (1:600; cat. no.
GB111141-100, Wuhan Servicebio Technology Co., Ltd.) and
proliferating cell nuclear antigen (PCNA; 1:600; cat. no.
GB11010-100, Wuhan Servicebio Technology Co., Ltd.). The following
day, the slides were incubated with the secondary FITC-conjugated
antibody (1:400; cat. no. GB22303, Wuhan Servicebio Technology Co.,
Ltd.) and Cy3-conjugated antibody (1:400; cat. no. GB21303, Wuhan
Servicebio Technology Co., Ltd.) for 1 h. Finally, the cell nuclei
were stained with DAPI for 10 min at room temperature
(SouthernBiotech), and the slides were observed under a
fluorescence microscope (Carl Zeiss AG).
Immunohistochemistry
The ovarian tissue sections underwent the same
procedures in terms of antigen retrieval, permeabilization and
blocking as those for immunofluorescence staining. Subsequently,
primary antibodies (Ki67, 1:800; cat. no. GB111141-100, Wuhan
Servicebio Technology Co., Ltd.) were incubated with the ovarian
tissue sections at 4°C overnight. The following day, the
biotinylated secondary antibodies (1:2,000; cat. no.
GB21303GB23303, Wuhan Servicebio Technology Co., Ltd.) were applied
to the slides and incubated at room temperature for 1 h. Finally,
the slides were stained using a reaction solution containing
diaminobenzidine (ZSGB-BIO). ImageJ software (version 1.53,
National Institutes of Health;) was utilized to analyze the
immunofluorescence staining and the relative density was measured
by normalizing the fluorescence intensity to the Young group.
Transfection with miR-21-5p
inhibitor/mimics
To perform transfection with miR-21-5p
inhibitor/mimics, RNA oligos were obtained from the Genepharma Co.,
Ltd. The sequences are presented in Table II. A total of 100 µl
serum-free Opti-MEM was utilized to dilute 5 µl
Lipofectamine™ 2000® (cat. no. 11668019, Thermo Fisher
Scientific, Inc.), 50 nmol miR-negative control (NC) and 50 nmol
miR-21-5p mimics and inhibitors separately. Following a 5-min
incubation at room temperature, Lipofectamine™ 2000 was mixed with
either miR-NC or miR-21-5p mimics/inhibitor, before the resulting
mixture was then incubated for 20 min at room temperature.
Subsequently, all these reagents were added to the cell medium for
incubation for 6 h at 37°C. The cells were collected at 2 days
following transfection for use in subsequent experiments.
 | Table IISequences for miRNA mimics and
inhibitors. |
Table II
Sequences for miRNA mimics and
inhibitors.
| Gene | Sequences |
|---|
| hsa-miR-21-5p
mimics | Sense:
5′-UAGCUUAUCAGACUGAUGUUGA-3′ |
| Antisense:
5′-AACAUCAGUCUGAUAAGCUAUU-3′ |
| Mimics negative
control | Sense:
5′-UUCUCCGAACGUGUCACGUTT-3′ |
| Antisense:
5′-ACGUGACACGUUCGGAGAATT-3 |
| hsa-miR-21-5p
inhibitor |
5′-UCAACAUCAGUCUGAUAAGCUA-3′ |
| Inhibitor negative
control |
5′-CAGUACUUUUGUGUAGUACAA-3′ |
Establishment of cell apoptosis
model
To investigate the effects of hUCMSC-Exos on GCs
apoptosis, a series of cell-related mechanistic studies were
performed. The cells were divided into three groups for
experimentation. In the cyclophosphamide (CTX) group, hGCs were
co-cultured with 30 µM CTX (Sigma-Aldrich; Merck KGaA) for
24 h. In the Exos group, after the CTX intervention, 30
µg/ml exosomes were added to the medium of hGCs. In the
normal group, which served as the control, the GCs were cultured in
the ordinary cellular medium without any additional
interventions.
To assess the function of miR-21-5p in
exosome-mediated therapy, miRNA inhibitor and mimics transfection
experiments were performed. For the miRNA inhibitor test, the
experiments were divided into four groups based on the different
interventions: The CTX group involved hGCs treated with CTX without
exosomes administration; in the Exos group, CTX-treated hGCs were
co-incubated with hUCMSC-Exos; in the Exos + miR-21-inhibitor
group, CTX-treated hGCs were co-cultured with hUCMSC-Exos
transfected with miR-21-inhibitor; in the Exos + miR-21-inhibitor
NC group, which served as a control, hGCs were treated with
hUCMSC-Exos transfected with a NC of the inhibitor. For miRNA
mimics transfection, the cellular experiments were grouped
similarly to those of the miRNA inhibitors, namely the CTX group,
Exos + miR-21-mimics group, Exos + miR-21-mimics NC group and the
Exos group. Exos + miR-21-mimics involved the transfection of
miR-21 mimics and co-incubation with hUCMSC-Exos. In the Exos +
miR-21-mimics NC group, mimics NC was transfected into the cell
models.
Fluorescent imaging of exosome
uptake
To assess the uptake of hUCMSC-Exos by hGCs, a
labeling experiment was performed using Dil dye (Life Technologies;
Thermo Fisher Scientific, Inc.). The hUCMSC-Exos were first labeled
with Dil dye (10 µM) and then incubated with hGCs at 37°C
and 5% CO2 for 24 h. Following incubation, the cells
were washed to remove any non-internalized exosomes. Subsequently,
the hGCs were stained with Calcein-AM (Life Life Technologies;
Thermo Fisher Scientific, Inc.) for 30 min at 37°C. Finally, a
fluorescence microscope (AxioCam HRc; Carl Zeiss AG) was used to
capture images of the labeled exosomes within the hGCs.
Cell Counting Kit-8 (CCK-8) assay
The proliferation of the hGCs was assessed using
CCK-8 assay. After the medium of hGCs was replaced according to the
aforementioned intervention methods, 10 µl CCK-8 solution
(Wuhan Servicebio Technology Co., Ltd.) were added to each well and
incubated with hGCs for 4 h at 37°C. Subsequently, the absorbance
of the medium at 450 nm was measured using a microplate reader
(BioTek Epoch, BioTek Instruments, Inc.). This procedure was
repeated on the following 5 days to evaluate the proliferative
ability of the hGCs.
miRNA sequencing and bioinformatics
analysis
The exosomal miRNAs derived from hUCMSCs were
extracted and the quantity of miRNA was examined. For the
preparation of small RNA sequencing libraries, the TruSeq Small RNA
Sample Prep Kits (Illumina, Inc.) were employed. Once the library
preparation was completed, the constructed library was subjected to
sequencing using the Illumina Hiseq2000/2500 platform with a read
length of 50 pb. Subsequently, the Kyoto Encyclopedia of Genes and
Genomes (KEGG) analysis (42) was
performed to highlight the pathways that were predominantly
targeted by the exosomal-miRNAs.
Statistical analysis
The results of the present study are presented as
the mean ± standard deviation (SD). Statistical analysis was
performed using the SPSS 26.0 software (IBM, Corp.). Specifically,
comparisons between two groups were conducted using a Student's
t-test (unpaired), while one-way ANOVA was used for comparisons
among multiple groups followed by Tukey's post hoc test. A P-value
<0.05 was considered to indicate a statistically significant
difference.
Results
Characterization of hUCMSCs and
exosomes
The features of hUCMSCs and exosomes were identified
in the present study. The morphology of the hUCMSCs was observed
under a microscope' the cells exhibited a fibroblast-like
morphology (Fig. 1A). The hUCMSCs
were then cultured in conditioned medium, prior to induction with
differentiation and were then assessed using Alizarin Red staining
(which revealed calcium deposition) and Oil Red O staining (which
revealed lipid vacuole accumulation) (Fig. 1B). These results indicated that
ex vivo conditional culture systems can effectively induce
hUCMSCs to differentiate into various cell types. The surface
antigen expression profile of the hUCMSCs was then characterized
using flow cytometry. The analysis revealed that the hUCMSCs were
positive for CD73 (99.1%), CD105 (98.4%), CD44 (99.9%) and CD90
(99.8%) expression, while they were negative for CD34 (0.3%), CD45
(0.2%) and HLA-DR (0.2%) expression (Fig. 1C). These surface antigen
characteristics align with those of the established criteria used
for defining hUCMSCs.
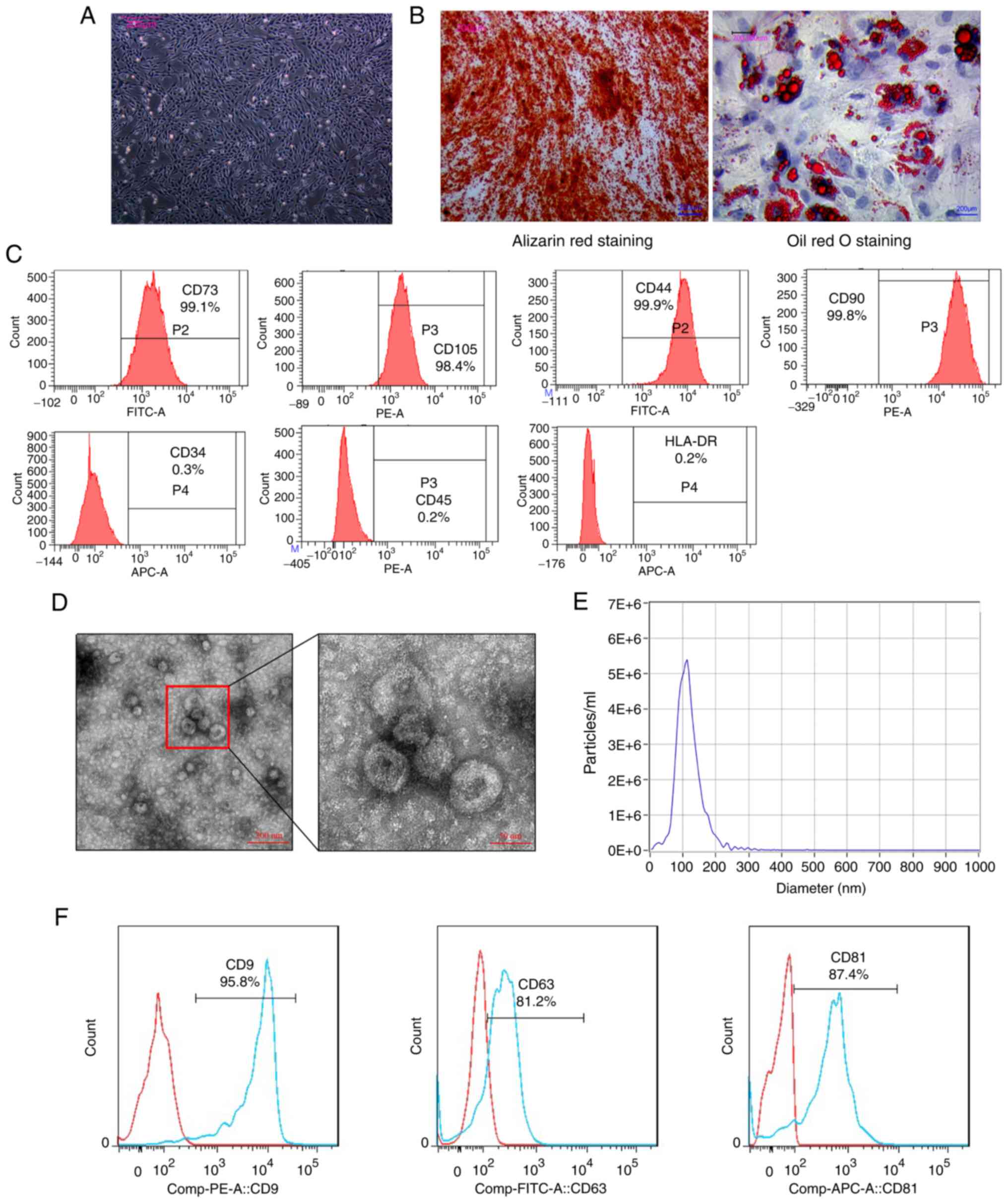 | Figure 1Characterization of hUCMSCs and
hUCMSC-Exos. (A) Fibroblast-like morphology of hUCMSCs. Scale bar,
200 µm. (B) Osteogenic differentiation of hUCMSCs
demonstrated by Alizarin Red staining and adipogenic
differentiation of hUCMSCs demonstrated using Oil Red O staining.
Scale bars, 200 µm. (C) Flow cytometric analysis confirming
positive expression of CD44, CD73, CD90 and CD105 as surface
markers of hUCMSCs, and the negative expression of CD34, CD45 and
HLA-DR. (D) Cup-shaped morphology and distinct membrane structure
of exosomes observed under a transmission electron microscope.
Scale bar, 50 and 200 nm. (E) Size distribution and concentration
analysis of exosomes showing a range of 30 to 150 nm in size and a
high concentration. (F) Flow cytometry demonstrating the positive
expression of CD9, CD63 and CD81 in hUCMSC-Exos. hUCMSCs, human
umbilical cord-derived mesenchymal stem cells; hUCMSC-Exos,
hUCMSC-derived exosomes. |
In accordance with the identification criteria for
extracellular vesicles, exosomes derived from the hUCMSCs were then
characterized (33). Transmission
electron microscopy revealed the cup-like morphology of the
hUCMSC-Exos, displaying a distinctive membrane structure (Fig. 1D). Size distribution and
concentration analysis demonstrated that the concentration of
exosomes was 8.0×1010 particles/ml, where only
extracellular vesicles within the diameter range of 30-150 nm were
classified as exosomes (Fig. 1E).
In addition, the exosomes stained positive for CD9, CD63 and CD81
according to flow cytometry (Fig.
1F). These results suggest that the isolated vesicles derived
from the hUCMSCs met the criteria for exosomes, supporting
subsequent investigations into their therapeutic potential.
scRNA-seq analysis of aging ovaries
To assess the expression profiles of PTEN and
apoptosis-related molecules in aging ovaries, a scRNA-seq analysis
was conducted. The scRNA-seq dataset (GSE130664) consisted of 2,601
single cells obtained from the ovaries of four young and four aged
monkeys (34). The data were
divided into two groups based on age (Old and Young group),
allowing for the analysis of single cells from each group
independently. The t-distributed stochastic neighbor embedding
(t-SNE) method was utilized for dimensional reduction analysis
(43). In the Old group, seven
distinct cell types were identified, whilst the young monkey group
contained eight cell types (Fig.
S1A). Focus was primarily placed on oocytes and GCs for the
present study. The key transcriptional regulators that modulate
cell-type-specific gene regulatory networks, such as FIGLA for
oocytes (44) and NR5A2 for GCs
(45), are illustrated in t-SNE
plots (Fig. S1B). To investigate
the differences in PTEN and apoptotic protein expression between
the two groups, a single-cell atlas displaying PTEN and caspase-3.
The results revealed significantly the elevated expression of PTEN
and caspase-3 in oocytes and GCs from the Old group compared with
that in the Young group (Fig.
S1C).
PTEN expression, apoptosis and
proliferation in animal models of NOA
Animal experiments were subsequently conducted
according to the design summarized in Fig. 2A. Briefly, exosome transplantation
was performed twice in the Exos group, with a 7-day interval
between each transplantation. After completing the treatment
regimen, the mice from all three groups were sacrificed, and their
ovaries and serum were collected for use in subsequent experiments.
Prior to exosome transplantation, a comprehensive comparison of
PTEN expression was conducted in the ovaries of the NOA and the
Young groups, in addition to the extent of apoptosis and
proliferation phenotypes. Western blot analysis and RT-qPCR were
used to determine the levels of PTEN and apoptotic molecules,
including Bax, Bcl-2, caspase-3 and caspase-9. The results of
western blot analysis revealed elevated protein expression levels
of PTEN, Bax, caspase-3 and caspase-9, but a reduced expression of
Bcl-2 in the NOA group compared with the Young group (Fig. 2B). Similarly, the mRNA expression
levels of PTEN, Bax, caspase-3 and caspase-9 were significantly
increased in the NOA group, whilst the expression of Bcl-2 was
notably decreased (Fig. 2C).
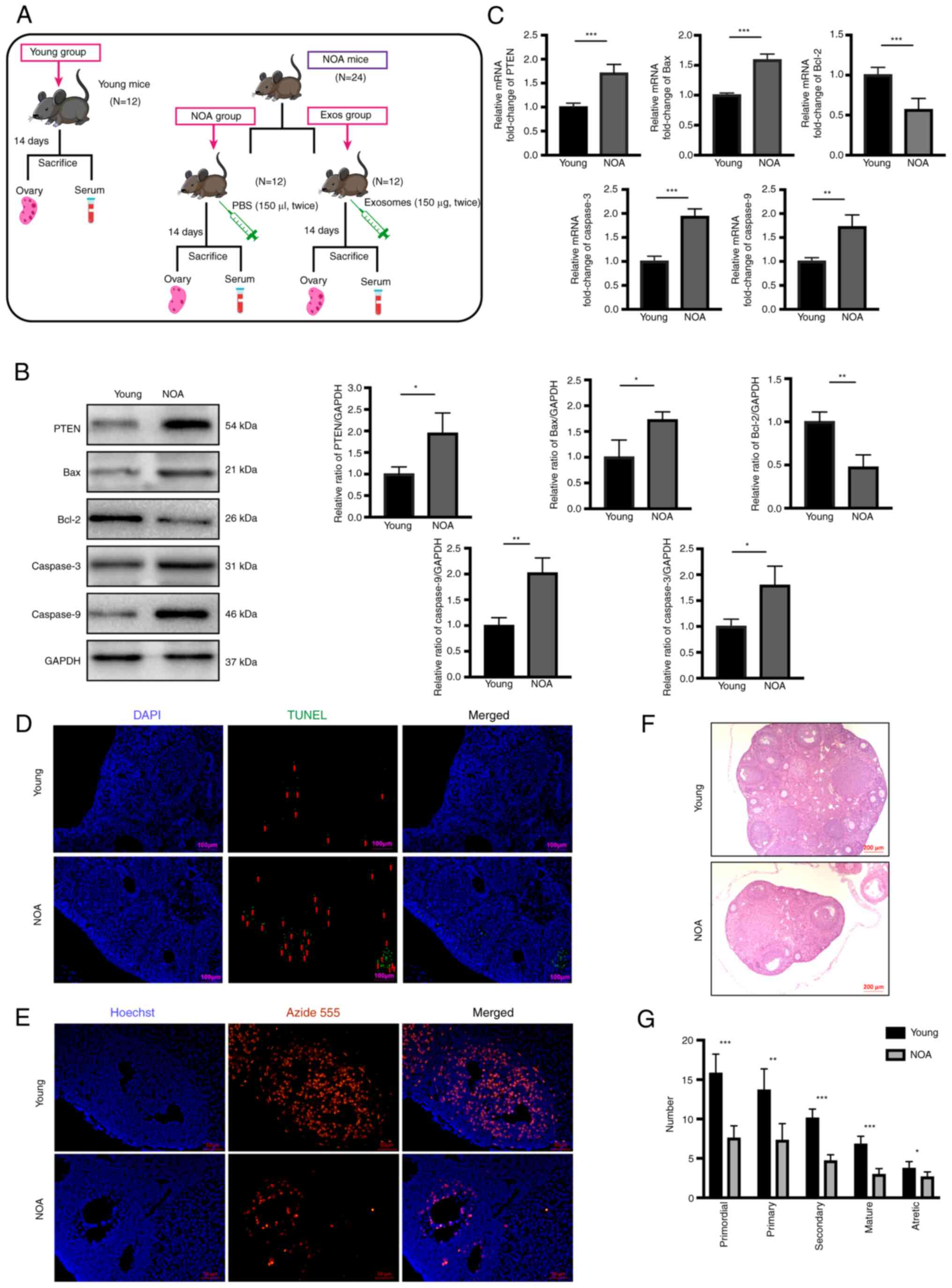 | Figure 2Apoptosis and proliferation levels in
NOA. (A) Experimental timeline. Exosome transplantation was
performed twice in the Exos group at 7-day intervals. At 2 weeks
after treatment, mice from all three groups were sacrificed, and
their ovaries and serum were collected for use in subsequent
experiments. (B) Western blot analysis revealed the increased
protein expression levels of PTEN, Bax, caspase-3 and caspase-9,
and the decreased expression of Bcl-2 in ovarian tissues of the NOA
group compared to the normal group. (C) Reverse
transcription-quantitative PCR revealed significantly elevated mRNA
levels of PTEN, Bax, caspase-3 and caspase-9, and the decreased
expression of Bcl-2 in the ovarian tissues of the NOA group
compared to the normal group. (D) TUNEL assay was performed to
measure apoptosis of ovarian tissue in the NOA and normal groups.
DAPI staining (blue fluorescence) indicates nuclei, while green
fluorescence indicates apoptosis cells. Scale bars, 100 µm.
(E) EdU assay was used to assess proliferation in the ovaries of
the NOA and normal group. DAPI staining indicates nuclei, while red
fluorescence indicates proliferating cells. Scale bars, 50
µm. (F) Hematoxylin and eosin staining of ovaries in the two
different groups. Scale bars, 200 µm. (G) Follicle counts at
various developmental stages between NOA and normal groups. Data
are presented as the mean ± SD. *P<0.05,
**P<0.01 and ***P<0.001, vs. young
group. Data represent three independent experiments in each group.
NOA, natural ovarian aging. |
Additionally, TUNEL and EdU assays were performed on
the ovarian sections to validate the significant differences in
cellular apoptosis and proliferation found between the mice in the
NOA group and the young mice. TUNEL assay revealed a notable
increase in the apoptotic level of the ovaries of mice with NOA
compared with that in the Young group (Fig. 2D). In addition, EdU assay revealed
a significant reduction in ovarian cell proliferation in mice with
NOA compared with those in the Young group (Fig. 2E). H&E staining of the ovarian
tissues also revealed a marked decline in follicle numbers at all
stages in mice with NOA compared with the young mice (Fig. 2F and G). Based on these findings,
it was confirmed that the expression of PTEN and the level of
apoptosis in the ovaries were increased, while the level of
proliferation was decreased, in the aging ovaries of mice with
NOA.
hUCMSC-Exos suppress apoptosis by
negatively regulating PTEN expression in NOA mice
After completing the transplantation of
hUCMSCs-Exos, the expression levels of PTEN and the degree of
apoptosis and proliferation in the mouse ovarian tissue were
explored to determine the therapeutic potential of exosomes.
Specifically, western blot analysis and RT-qPCR were performed to
assess the differences in the expression of PTEN and
apoptosis-related molecules in the ovaries of the three groups. The
results revealed that compared with the NOA group, the protein
expression levels of PTEN and apoptosis-related molecules (Bax,
caspase-3 and caspase-9) were decreased, while those of Bcl-2 were
increased in the Exos group (Fig.
3A). Similarly, the mRNA expression levels of the
aforementioned molecules exhibited a similar trend (Fig. 3B). These exosomal treatment
outcomes suggest that Exos can inhibit PTEN, whilst suppressing
apoptosis in the ovarian tissue in mice with NOA. TUNEL assay was
then performed to further evaluate the extent of apoptosis in the
ovarian tissue in the three groups, revealing a significant
reversal of ovarian apoptosis in mice with NOA following treatment
with hUCMSC-Exos (Fig. 3C).
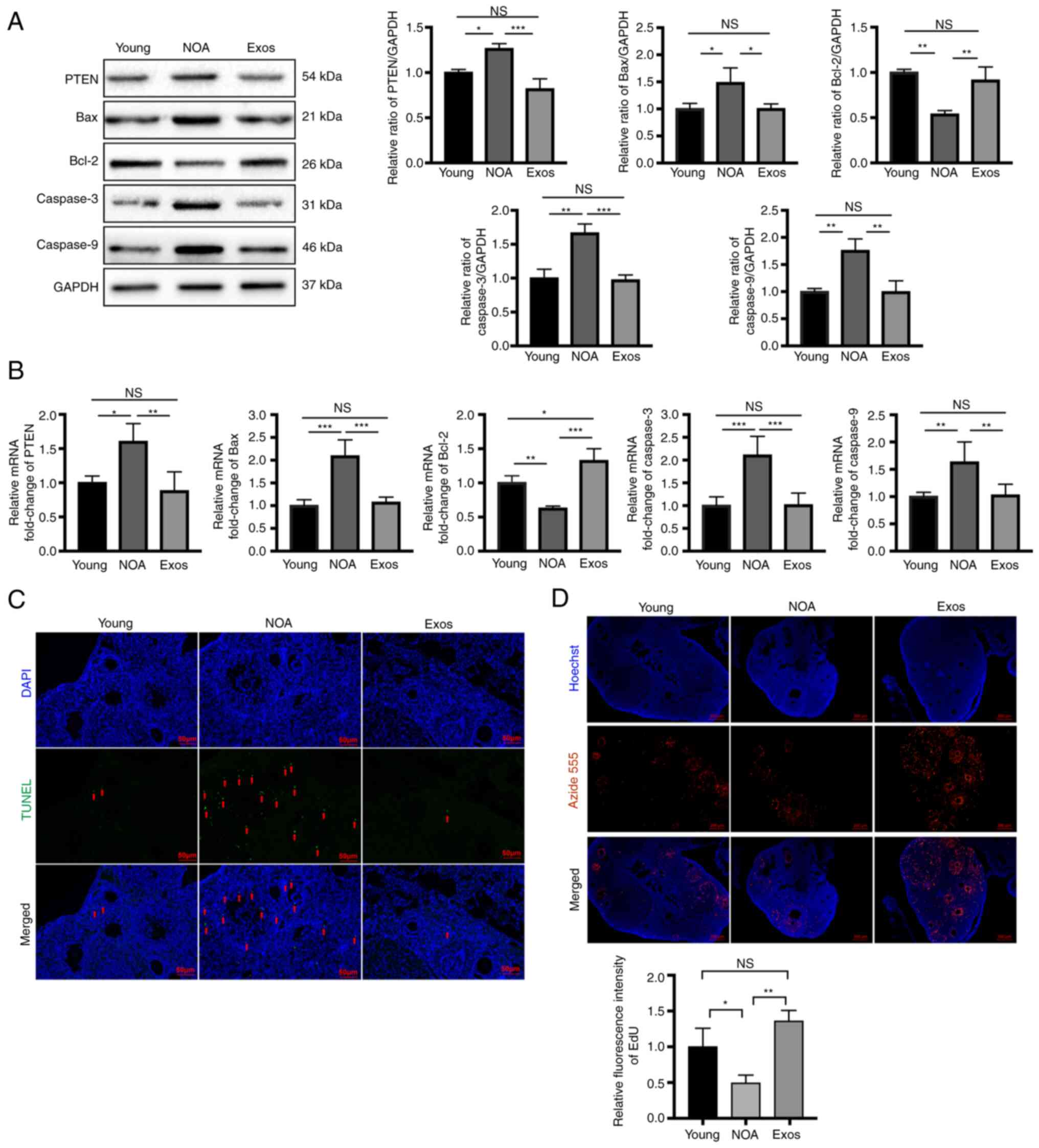 | Figure 3Exosomes suppress apoptosis and
increase proliferation by negatively regulating PTEN expression in
mice with NOA. (A) Following exosomal transplantation, western blot
analysis revealed the decreased protein expression levels of PTEN,
Bax, caspase-3 and caspase-9, and the increased expression of Bcl-2
in the ovarian tissues of the Exos group compared with the NOA
group. (B) Following exosomal transplantation, reverse
transcription-quantitative PCR revealed significantly reduced mRNA
levels of PTEN, Bax, caspase-3 and caspase-9, and the elevated
expression of Bcl-2 in ovarian tissues of the Exos group compared
to the NOA group. (C) TUNEL assay was performed to measure
apoptosis in the ovarian tissue in the three groups. DAPI staining
(blue fluorescence) indicates nuclei, while green fluorescence
indicates cells undergoing apoptosis. Scale bars, 50 µm. (D)
EdU assay was used to assess proliferation in ovaries of the three
different groups. DAPI staining indicates nuclei, while red
fluorescence shows proliferating cells. Scale bars, 200 µm.
Data are presented as the mean ± SD. *P<0.05,
**P<0.01 and ***P<0.001; NS, not
significant. Data represent three independent experiments in each
group. NOA, natural ovarian aging; Exos, exosome-treated group. |
Ovarian cell proliferation was then determined using
EdU assay and immunostaining of proliferative-related molecules.
EdU assay demonstrated a significant increase in proliferation in
the ovarian tissues of mice with NOA following exosomal
transplantation (Fig. 3D).
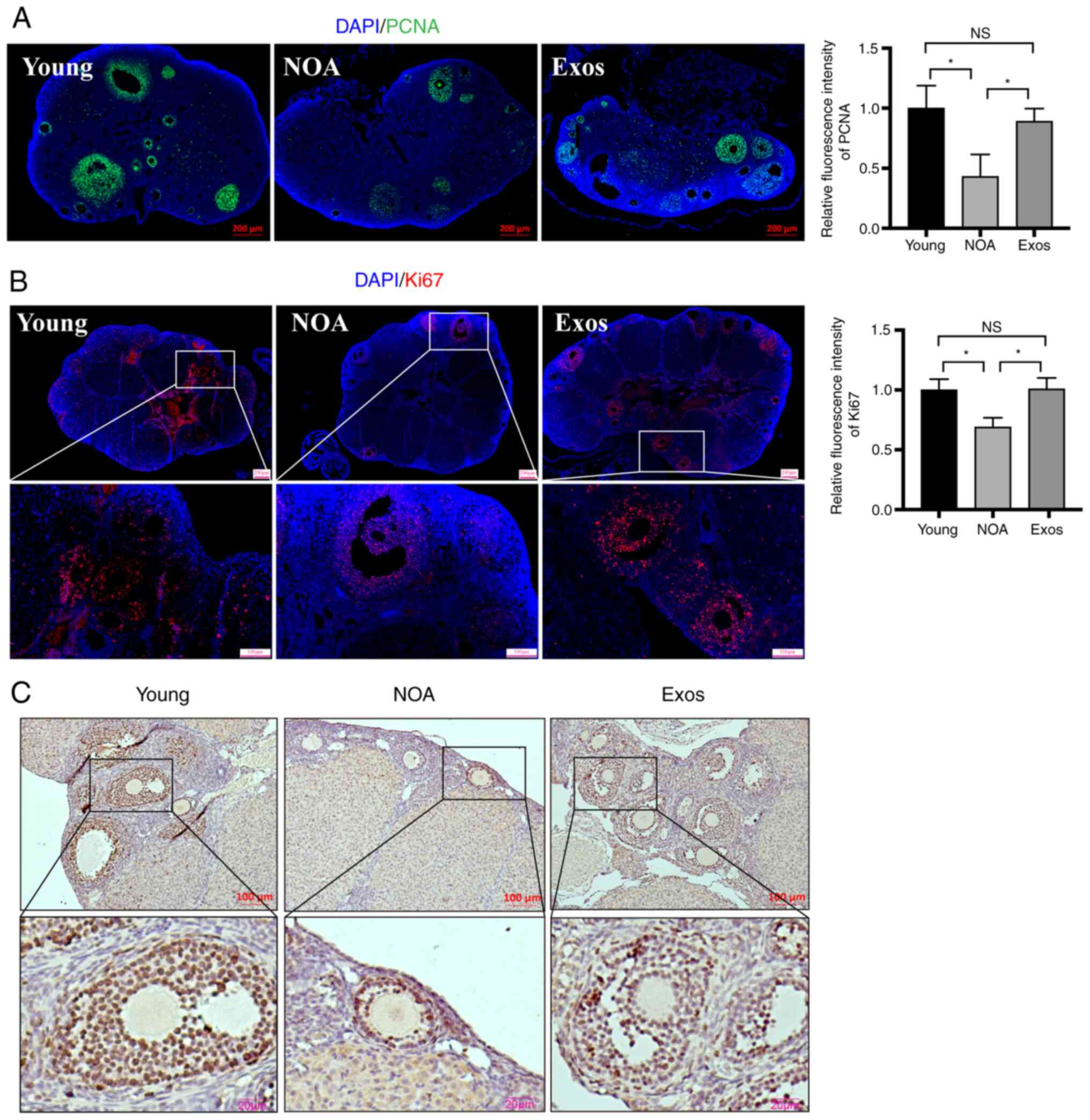
Immunostaining for PCNA and Ki67, markers of cell proliferation,
was performed to further measure the levels of proliferation in the
three groups. Immunofluorescence staining of the ovarian sections
revealed a marked increase in PCNA expression following exosomal
transplantation compared with that in untreated mice with NOA
(Fig. 4A). Similarly, the
immunohistochemical and immunofluorescence staining of Ki67
revealed similar results. The expression of Ki67 in the ovaries of
mice with NOA was found to be significantly increased after the
MSC-derived exosome injection, indicating an improvement in the
proliferation level (Fig. 4B and
C). These experimental findings suggest that the
transplantation of hUMSC-derived exosomes can effectively reduce
the apoptosis of ovarian cells, whilst promoting ovarian cell
proliferation, possibly by targeting PTEN.
hUCMSC-Exos treatment leads to the
recovery of ovarian function in mice with NOA
To confirm the uptake of the injected exosomes by
GCs in vivo, a tracing experiment was conducted. The results
revealed that after 5 days of exosome transplantation, the exosomes
were taken up by the ovaries and internalized by the GCs,
indicating their ability to infiltrate the ovarian cells (Fig. 5A). Subsequently, the status of
ovarian function in the mice with NOA was evaluated.
H&E-stained images of the ovarian tissue revealed that exosome
delivery led to an increase in the number of follicles in all-stage
and the restoration of follicular morphology within the ovary
(Fig. 5B). To further assess the
efficacy of hUCMSC-Exos in restoring ovarian aging, follicular
numbers at different stages were counted in ovarian tissues from
mice in the three groups. It was observed that exosome
transplantation led to a significant increase in the numbers of
primordial, primary, secondary and mature follicles compared with
the NOA group (Fig. 5C). The
levels of AMH, E2 and FSH in mouse serum were then
measured using ELISA to evaluate the effectiveness of the exosomes
in regulating the levels of reproductive hormones in mice with NOA.
In the mice with NOA, the levels of AMH (Fig. 5D) and E2 (Fig. 5E) were notably decreased, whilst
those of FSH (Fig. 5F) were
elevated, compared with those in the young mice. Following exosomal
treatment, the levels of AMH (Fig.
5D) and E2 (Fig.
5E) in the mice wotj NOA were increased, whereas those of FSH
(Fig. 5F) were decreased.
However, it should be noted that the AMH and FSH levels in the mice
with NOA did not fully recover to the levels observed in the young
mice following the completion of transplantation. These results
suggest that exosomal transplantation can improve ovarian function
in aging mice, although the complete restoration of hormone levels
may not be achieved.
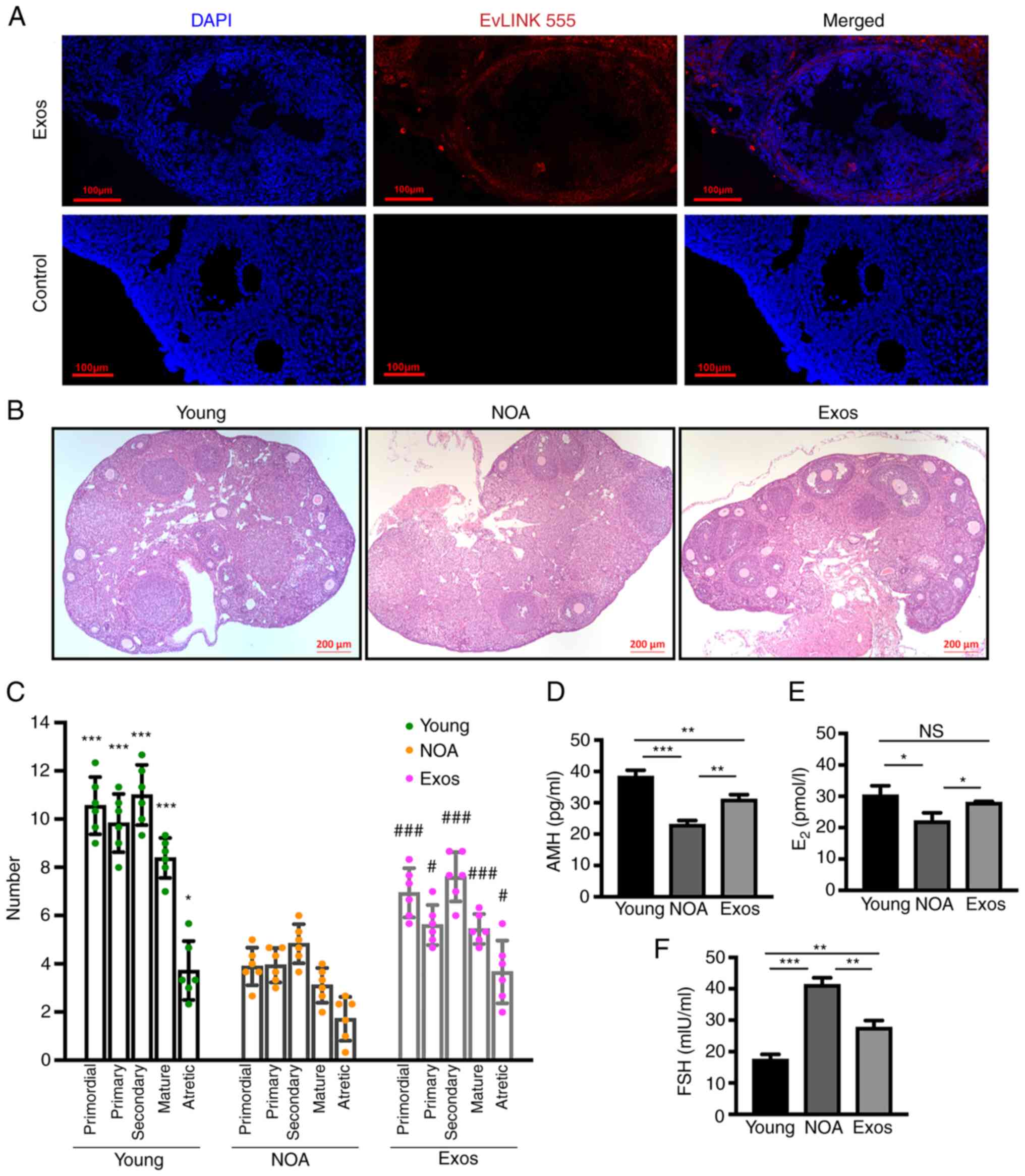 | Figure 5Exosomal treatment recovers ovarian
function in mice with NOA. (A) Tracing experiment demonstrating
uptake of injected exosomes by GCs in vivo. (B) Hematoxylin
and eosin staining of ovaries in the three groups. Scale bars, 200
µm. (C) Quantification of follicles at different stages in
each group. The presented statistical data represent the results of
comparisons between the Young group and the Exo group with the NOA
group. Levels of (D) AMH and (E) E2 were significantly
increased, while (F) the levels of FSH were decreased in the Exos
group compared to the NOA group. Data are presented as the mean ±
SD. *P<0.05, **P<0.01 and
***P<0.001, and #P<0.05 and
###P<0.001 vs. NOA group; NS, not significant. Data
represent three independent experiments in each group. NOA, natural
ovarian aging; Exos, exosome-treated group; GCs, granulosa cells;
AMH, anti-mullerian hormone; E2, estradiol; FSH, follicle
stimulating hormone. |
Identification of hGCs and establishment
of the model of hGC apoptosis
To confirm the specific mechanisms underlying the
effects of hUCMSC-Exos on apoptosis in NOA, the human ovarian
granulosa cell line (HO-23) was used for establishing the cell
model of apoptosis. The HO-23 cell line was established by
transfection with simian virus 40 DNA, Ha-ras oncogen, and a TS
mutant of p53 (p53 Val135) (37,46). Under observation using a light
microscope, the hGCs were observed to be polygonal or elliptical
(Fig. S2A). Immunofluorescence
staining revealed almost all of the cells were positive for FSHR
expression in their cytoplasm, suggesting that this cell line was
indeed ovarian GCs (Fig. S2B).
Subsequently, Dil-labeled hUCMSCs-Exos were co-cultured with hGCs
before Calcein-AM was used to stain the GCs. Through fluorescence
imaging, the uptake of exosomes by hGCs was observed (Fig. S2C).
An apoptosis model of hGCs was then established by
co-culturing the cells with CTX (CTX-hGCs). To verify the
successful establishment of this cell apoptosis model, the
expression of PTEN and apoptosis-related molecules were measured
using western blot analysis and RT-qPCR. Moreover, the apoptotic
and proliferative levels of hGCs were examined using TUNEL, CCK-8
and EdU assays. The protein and mRNA expression levels of PTEN and
Bax were increased, whereas those of Bcl-2 were decreased (Fig. S2D and E). According to the
findings of TUNEL assay, the apoptotic level of the CTX-treated GCs
was notably increased compared with the untreated cells (Fig. S2F and G). Furthermore, from the
results of CCK-8 assay, it was found that the proliferation of the
CTX-GCs was markedly decreased compared with the untreated cells
(Fig. S2H). The results from EdU
assay also revealed that the quantity of proliferative cells
markedly decreased following co-culture with CTX (Fig. S2I and J). These findings proved
that the construction of the model of hGC apoptosis was
successful.
hUCMSC-Exos inhibit the apoptosis of hGCs
by regulating PTEN expression
After completing the establishment of the model of
hGC apoptosis, hUCMSC-Exos were co-cultured with the cell model to
assess the ability and mechanisms of the exosomes in preventing
apoptosis. To investigate the specific role of PTEN-mediated
apoptosis following exosomal treatment, western blot analysis and
RT-qPCR were performed to detect the expression of PTEN and key
molecules of apoptosis. Following treatment with exosomes, the
expression levels of PTEN and pro-apoptotic molecules (Bax,
caspase-3 and caspase-9) were markedly decreased, whereas the
expression level of anti-apoptotic Bcl-2 was notably increased,
according to the detection of protein and mRNA expression (Fig. 6A and B). Additionally, the
apoptotic level of the GCs was assessed using TUNEL assay, whereas
the proliferative level was evaluated using EdU and CCK-8 assays.
Compared with the CTX-treated hGCs, the number of apoptotic hGCs,
which were represented by green fluorescence in TUNEL assay, were
found to be decreased following co-incubation of exosomes (Fig. 6C and D). In addition, the results
of CCK-8 assay revealed that the proliferative level of GCs was
markedly improved following co-culture with hUCMSC-Exos for 5 days
compared with the CTX-treated hGCs (Fig. 6E). EdU assay of the three groups
revealed there were more EdU-positive GCs following co-incubation
with exosomes compared with the CTX group (Fig. 6F and G).
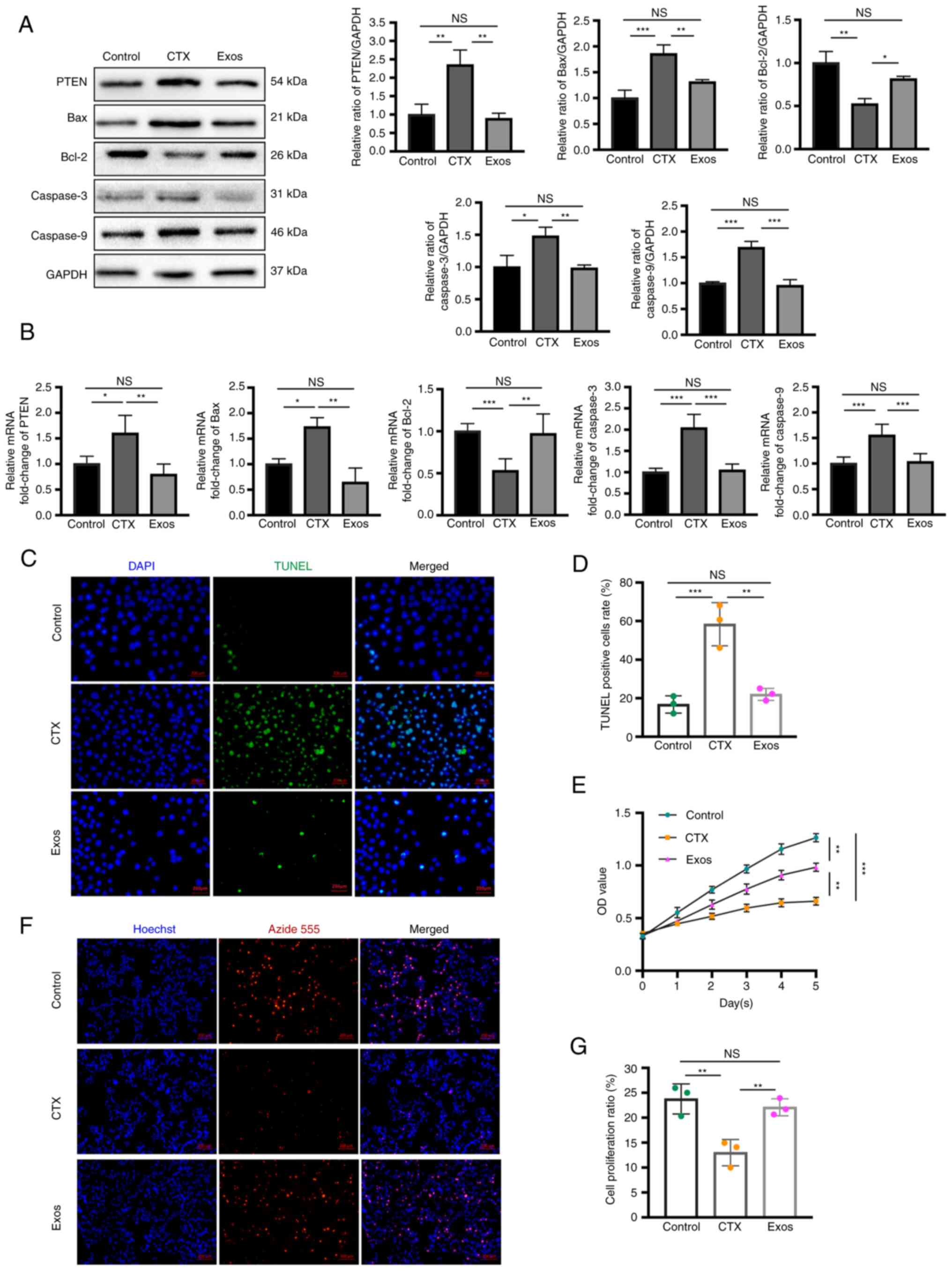 | Figure 6Exosomes suppress the apoptosis of
CTX-treated hGCs by targeting PTEN. (A) Western blot analysis of
hGCs revealed decreased protein expression of PTEN, Bax, caspase-3
and caspase-9, and increased the expression of Bcl-2 following
co-culture with exosomes compared to the CTX group. (B) Reverse
transcription-quantitative PCR demonstrated consistent expression
trends of each molecule in hGCs among the three groups. (C) TUNEL
assay reealed a significant reduction in apoptotic hGCs in the Exos
group compared to CTX-treated hGCs. Scale bars, 200 µm. (D)
The cellular apoptotic rate, calculated from TUNEL staining, was
significantly higher in the Exos group than in the CTX group. (E)
Viability of the hGCs was assessed using CCK-8 assay from days 0 to
5 in the three groups. (F) EdU assay demonstrated a marked increase
in proliferating cells following co-culture of the CTX-treated hGCs
with exosomes. DAPI stained the nucleus with blue fluorescence, and
red fluorescence represented proliferating cells. Scale bars, 200
µm. (G) The cellular proliferation ratio, calculated from
EdU staining, was significantly higher than in the Exos group
compared to the CTX group. Data are presented as the mean ± SD.
*P<0.05, **P<0.01 and
***P<0.001; NS, not significant. Data represent three
independent experiments in each group. CTX, cyclophosphamide; hGCs,
human granulosa cells; Exos, exosome-treated group. |
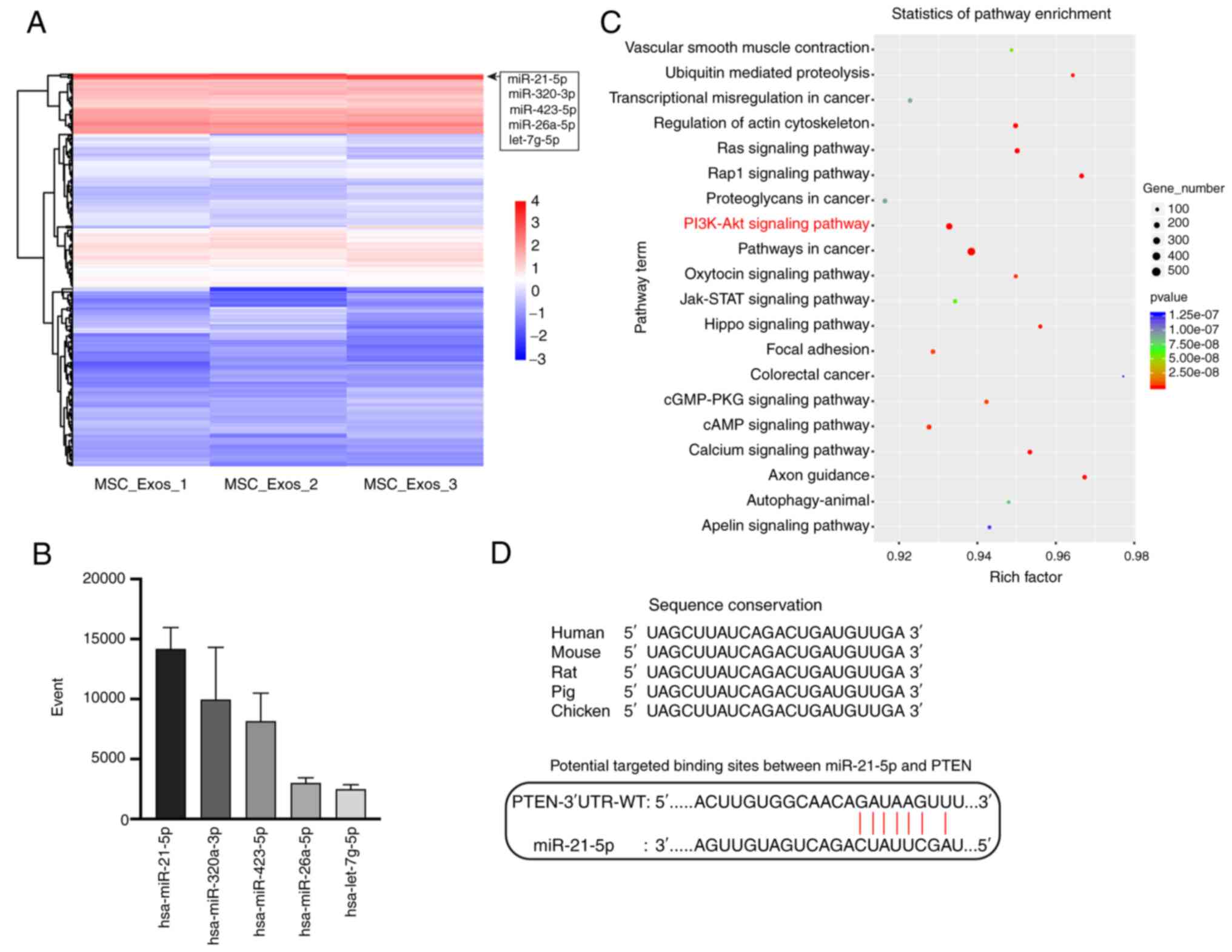
miR-21-5p is highly expressed in
hUCMSC-Exos and is predicted to negatively regulate PTEN
miRNAs are one of the most critical functional
molecules transported by exosomes that can exert regulatory
inter-cellular effects. Herein, to further explore the specific
molecules contained within hUCMSC-Exos that can target PTEN to
inhibit apoptosis, sequencing of the miRNAs contained in the
hUCMSC-Exos was conducted using high-throughput sequencing (Data
S1). Bioinformatics analysis of these sequencing results was then
performed. The heatmap of miRNAs revealed that miR-21-5p, miR-320a,
miR-423-5p, miR-26a-5p and let-7g-5p were the five most highly
expressed miRNAs in the hUCMSC-Exos (Fig. 7A). The individual molecular reads
are presented in Fig. 7B.
Furthermore, enrichment analysis of pathways activated by target
genes of these top expressed miRNAs was then performed, based on
the KEGG database (42). The
PI3K/Akt pathway was found to be one of the significantly regulated
pathways (Fig. 7C), with PTEN
being one of the key regulators of this pathway (47). Through bioinformatics prediction
and a review of the literature (48), miR-21-5p, the most highly
expressed exosomal miRNA, was found to target and inhibit the
expression of PTEN, which is a critical molecule that was verified
by the aforementioned experiments to be significantly overexpressed
in the ovaries in NOA and in the model of cell apoptosis. The
potential seed sequence of miR-21-5p to the mRNA of PTEN is
illustrated in Fig. 7D, where
miR-21-5p was found to be highly conserved among species.
Exosomal miR-21-5p prevents GC apoptosis
by negatively regulating PTEN expression
Following transfection with miR-21-5p mimics and
inhibitor, the expression level of miR-21-5p in MSCs was
significantly altered, with the mimics substantially increasing
miR-21-5p expression (Fig. S3A),
while the inhibitor decreased miR-21-5p expression (Fig. S3B). This confirmed the successful
transfection and functional efficacy of miR-21-5p mimics/inhibitor.
To verify the exosomal miRNA sequencing results, RT-qPCR was
performed to evaluate the expression levels of miR-21-5p in the
hUCMSC-Exos. The results revealed that miR-21-5p expression in the
hUCMSC-Exos was significantly higher compared with that of
miR-26a-5p (Fig. 8A). Following
co-incubation with the hUCMSC-Exos, the hGCs were observed to take
up the exosomes, where intracellular miR-21-5p expression increased
(Fig. 8B). It was previously
reported that miR-21-5p can repress PTEN expression (49). In addition, herein, miR-21-5p was
found to be the most abundant molecule in the hUCMSC-Exos according
to the high-throughput sequencing results. Therefore, it was
hypothesized that exosome-derived miR-21-5p can target PTEN in the
ovarian GCs of mice with NOA, thereby regulating their apoptotic
and proliferative levels. To verify this targeting association,
before the hUCMSC-Exos were used for co-culture with the
CTX-treated hGCs, the inhibitor/mimics or inhibitor/mimics NC of
miR-21-5p were transfected into the cells. Firstly, following
transfection with inhibitor/mimics, miR-21-5p expression in the
different groups was calculated using RT-qPCR after 48 h.
Transfection with the inhibitor markedly inhibited miR-21-5p
expression in the exosomes, whereas the mimics increased its
expression following culture with the hGCs (miR-21-inhibtor/-mimics
group vs. Exos group) (Fig. 8C).
The results from RT-qPCR and western blot analysis revealed that
following co-culture with the exosomes, PTEN expression in the cell
model was notably suppressed compared with that in the untreated
group (Fig. 8D and E). By
contrast, this trend was reversed following transfection with
miR-21-5p inhibitor. The protein expression of apoptosis-related
molecules (Bax and Bcl-2) exhibited a corresponding trend, which
was also reversed following miR-21-5p inhibitor transfection
(Fig. 8E). Finally, miR-21-5p
mimics were used to validate its contributions to the effects of
hUCMSC-Exos treatment. The cells were either transfected with miRNA
mimics or treated with exosomes. The results of western blot
analysis demonstrated that transfection with mimics had the same
effect as exosomal treatment, both of which were able to reduce
PTEN expression and inhibit apoptosis (Fig. 8F).
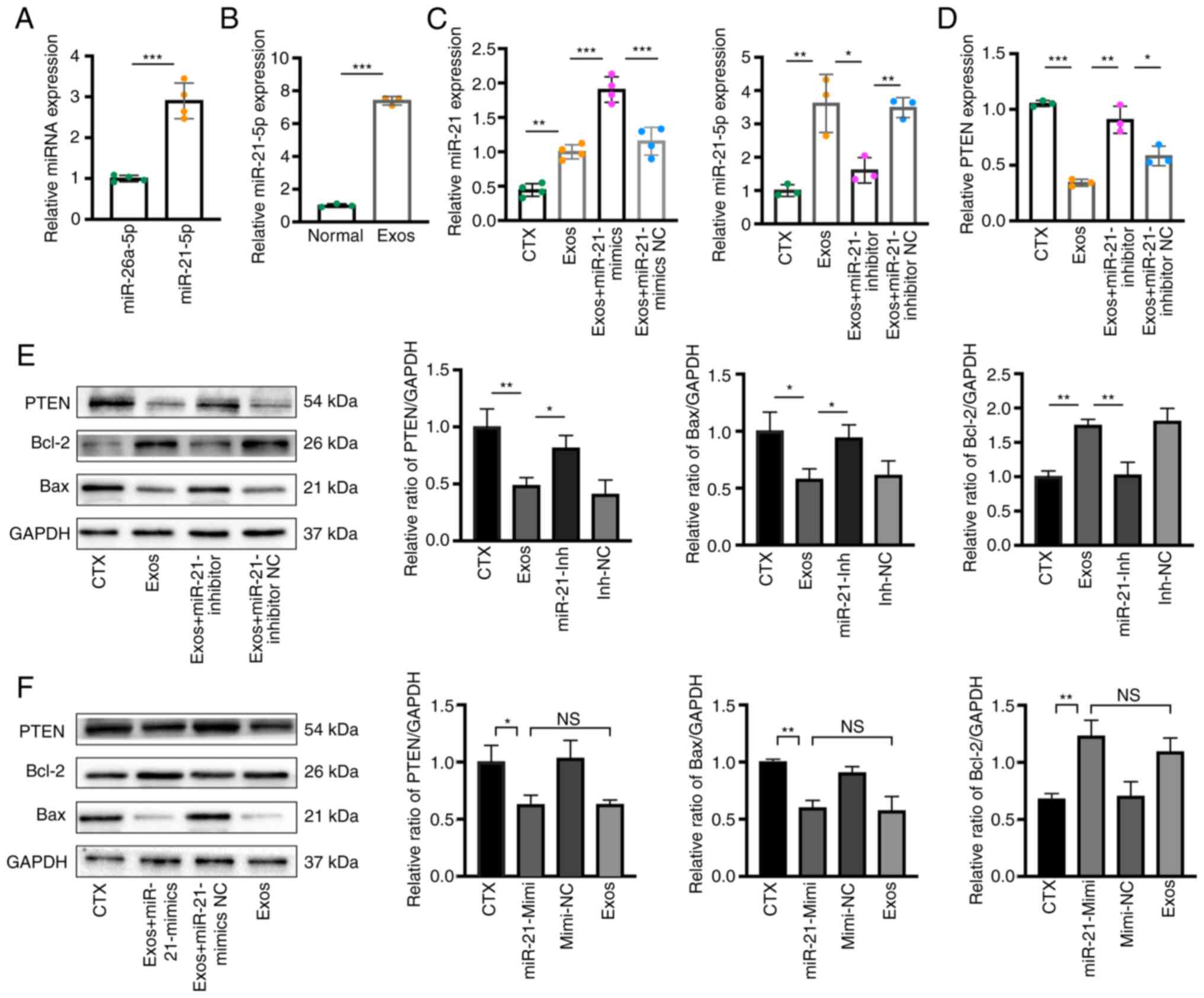 | Figure 8Exosomal miR-21-5p prevents hGC
apoptosis by negatively regulating PTEN. (A) RT-qPCR assays
revealed significantly higher levels of miR-21-5p in exosomes
compared to miR-26a-5p. (B) RT-qPCR assays demonstrated the uptake
of exosomes by hGCs and the increased intracellular levels of
miR-21-5p. (C) RT-qPCR assays revealed that the miR-21-5p inhibitor
significantly inhibited miR-21-5p expression in hGCs, while the
mimics increased its level. (D) RT-qPCR results revealed the mRNA
expression of PTEN in the four groups (the CTX group, Exos group,
Exos + miR-21-inhibitor group, and Exos + miR-21-inhibitor NC). The
inhibitory effect of exosomes on PTEN expression in hGCs was
reversed by the miR-21-5p inhibitor. (E) Western blot analyses
revealed the protein expression of PTEN, Bax, and Bcl-2 in the four
groups. The effect of exosomes in inhibiting the PTEN expression
and apoptotic level in hGCs was reversed by the miR-21-5p
inhibitor. (F) Western blot analyses in the four groups (CTX group,
Exos + miR-21-mimics group, Exos + miR-21-mimics NC group, and Exos
group) demonstrated that the transfection of miR-21-5p mimics
exerted the same inhibitory effect on PTEN and apoptosis levels as
exosomal co-incubation. Data are presented as a percentage or mean
± SD; n=6 per group; *P<0.05, **P<0.01
and ***P<0.001; NS, not significant; hGCs, human
granulosa cells; Exos, exosome-treated group; CTX,
cyclophosphamide; RT-qPCR, reverse transcription-quantitative
PCR. |
Discussion
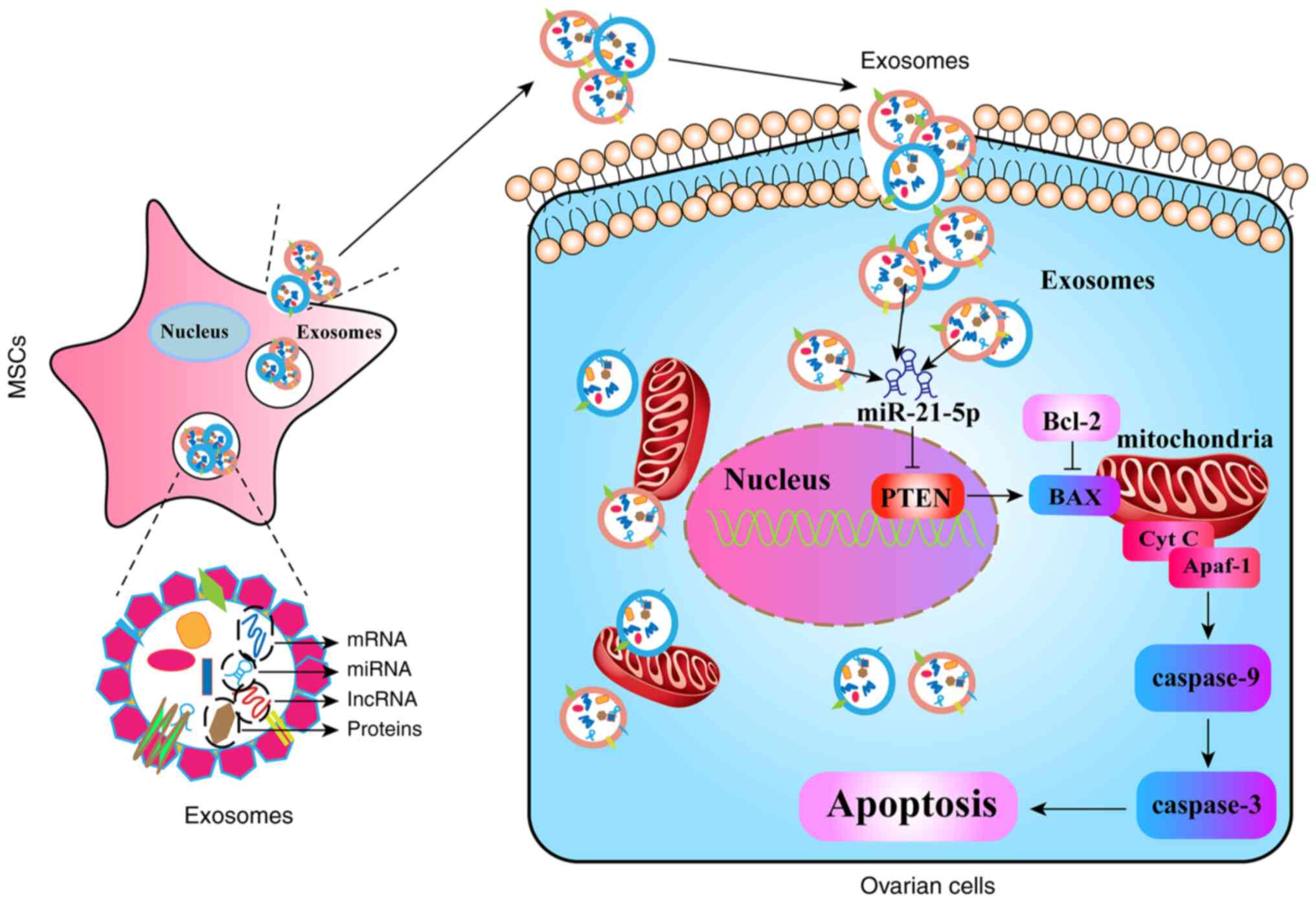
The findings of the present study strongly suggest
the therapeutic potential of hUCMSC-derived exosomes in improving
ovarian function in naturally aging ovaries. In addition,
preliminarily investigations into the therapeutic mechanisms
underlying this novel therapy were performed. Specifically,
exosome-carried miRNAs regulate cell apoptosis by targeting PTEN,
thereby exerting therapeutic functions (Figs. 2-8). Since aging is perceived to be a
physiological process, it has received less attention compared with
common 'diseases'. However, recent advancements in regenerative
medicine have led to the proposal that aging is a treatable
condition, with the slowing of the aging process emerging as a new
frontier in medical development. In females, ovarian aging serves
as the a catalyst and driver of senescence in other vital organs
(50). Consequently, delaying
ovarian aging provides a viable strategy with which to mitigate
dysfunction and even age-related decline in other organs. Various
approaches have been explored to combat ovarian aging, such as the
use of antioxidants and promoting autophagy (51,52). However, the safety and efficacy of
these methods remain controversial, impeding their practical
clinical application. The aim of the present study was to enhance
the current understanding of the underlying mechanisms of ovarian
aging, and to propose and validate an effective method or concept
that can partially or fundamentally restore ovarian function.
Exosomes derived from MSCs have emerged as a promising therapeutic
approach for organ and tissue regeneration in various disorders,
including decreased ovarian function (10,30). Therefore, in the present study,
the effects of exosomes derived from hUCMSCs were examined in a
mouse model of ovarian aging and in cell model (hGCs) of apoptosis.
In addition, the present study explored the underlying mechanisms
of MSC-derived exosomes in alleviating ovarian aging. These
exosomes were found to exhibit the ability to partially restore the
function of aging ovaries. Furthermore, the regulation of apoptosis
by targeting PTEN through exosomal miR-21-5p may be a potential
molecular mechanism of hUCMSC-Exos-mediated ovarian function
recovery in NOA (Fig. 9).
Initially, a comprehensive analysis was conducted,
which revealed a substantial increase in the apoptotic rate within
the ovarian tissue of aged mice compared with that in young mice.
Additionally, a noteworthy elevation in the expression levels of
PTEN was found, a critical regulatory molecule of cellular
apoptosis, in both natural aging mice and cell apoptosis models.
These findings emphasize augmented apoptosis levels and upregulated
PTEN expression as significant factors in the adverse effects of
natural ovarian aging. PTEN has been frequently reported to be an
oncogene that contributes to apoptosis induction in both cancerous
and normal cells (53). Its
essential role in various physiological and pathological processes
is evident through its regulation of apoptotic degrees in different
tissues and organs (54). Through
its modulation of the AKT phosphorylation level, PTEN exerts
control over numerous cellular, including cellular viability,
proliferation and apoptosis (55). Notably, the PTEN-PI3K/AKT pathway
has been reported to play a crucial role in the activation of
primordial follicular and the process of ovarian aging (56). In the present study, following
exosomal treatment, a significant decrease in PTEN expression was
found in both aging ovaries and cellular models. Therefore, it was
hypothesized that the exosomes contain specific miRNA molecules
capable of targeting and regulating PTEN, thereby exerting
downstream functions. Therefore, the modulation of PTEN expression
and function was likely to be a vital mechanism through which
hUCMSC-derived exosomes can regulate apoptosis in aged ovaries.
Even though a number of studies focusing on the
therapeutic effects of MSC-Exos have been performed, the inherent
therapeutic mechanisms and the molecular components of MSC-derived
exosomes have been poorly elucidated. Exosomes are generally 30-150
nm in size and are lipidic micro-vesicles. They are typically
secreted by cells into the extracellular environment (57). These vesicles selectively
incorporate particular non-coding RNAs, allowing for intercellular
communication by through exosomal transport between neighboring
cells. Through this mechanism, exosomes can potentially play
crucial roles in regulating physiological processes and disease
progression. Among the various components of exosomes, miRNAs are
particularly important for their biological functions, as they can
modulate ~50% of the gene expression and function in the human body
(58). miRNAs are highly abundant
in MSCs-Exos and possess regulatory properties in target-damaged
organs, by suppressing the translation and activation of target
genes by binding to the 3′-UTR of their corresponding mRNAs
(59-61). Against ovarian dysfunction, miRNAs
derived from MSC-Exos have been documented to play a vital role in
restoring ovarian function through multiple mechanisms, including
promoting proliferation and inhibiting apoptosis (62). While some studies have utilized
MSC-Exos to address premature ovarian insufficiency (10,29), only a limited number of studies
have explored the potential of these for restoring ovarian function
in naturally aging ovaries (30).
A previous study achieved satisfactory results in managing NOA
using human amniotic MSCs (30).
However, that study only provided a basic exploration of the
therapeutic mechanism and did not utilize MSC-derived exosomes
(30). Therefore, the present
study was performed to assess the effects of MSC-derived exosomes
by administering them to naturally aged ovaries and investigated
the underlying mechanisms at the molecular regulatory level.
To elucidate the molecular mechanisms underlying
exosome-mediated treatment and examine the effects of hUCMSC-Exos
on PTEN expression, miRNA sequencing of hUCMSCs-Exos was performed
before the results were screened for the most abundant miRNA
molecules that can target PTEN. This precise sequencing method of
exosomal miRNAs was used to avoid ambiguity. High-throughput
sequencing and laboratory experiments revealed that miR-21-5p was
abundantly expressed in the hUCMSC-Exos. Previous studies have
reported the ability of miR-21 to modulate the expression and
function of intracellular PTEN, establishing PTEN as a downstream
target of miR-21 (48,49,63). Consequently, the present study
focused on investigating the linear regulatory association between
exosomal miR-21, PTEN and apoptosis. Bioinformatics analysis
further demonstrated that miR-21-5p effectively regulated and
inhibit the function of PTEN molecules. Subsequent experiments
confirmed that the increased expression of miR-21-5p and the
decreased expression of PTEN in GCs were attributed to the cellular
uptake of exosomes. Notably, the positive results of exosomes in
targeting PTEN and inhibiting apoptosis were reversed by
transfection with miR-21-5p inhibitor, while transfection with
miR-21-5p mimics alone also effectively suppressed the onset of
apoptosis. These findings suggest that miR-21-5p contained within
hUCMSC-derived exosomes targets PTEN to prevent apoptosis,
highlighting its role in the molecular mechanisms underlying the
effects mediated by exosomal treatment for NOA.
The ability of MSC-derived exosomes to regulate the
expression of genes associated with ovarian aging renders them a
highly promising therapeutic strategy for alleviating natural
ovarian dysfunction. In comparison with previous research (30), the present study utilized
MSC-derived exosomes and employed high-throughput miRNA sequencing,
which enabled investigations into their molecular mechanisms with
precision. Consequently, the present study showcases notable
innovations in the field of MSC-derived exosomal therapy for
ovarian aging. The positive outcomes hold significant value in
establishing the theoretical framework for MSC- and
MSC-Exos-mediated therapy, laying a solid foundation for future
clinical applications. In the future, targeted therapy based on
specialized miRNA may emerge as a promising avenue to combat and
slow down ovarian aging.
Despite the encouraging results obtained in this
study, there are several limitations that should be acknowledged,
and further work is necessary. The route, dose, and frequency of
exosomal transplantation were tentatively determined based on
preliminary experiments and previous studies. Standardization of
these parameters is crucial before clinical application to ensure
its effectiveness and consistency. In addition, since fertility
restoration is not the primary focus in naturally aging
individuals, the fertility status of the NOA model was not
evaluated. Due to concerns regarding the potential adverse effects
of anesthesia and the technical challenges associated with
collecting blood samples from live mice, serum was not collected
from the same mice before and 14 days following exosome
transplantation in vivo in the present study. Future studies
are required to systematically establish the criteria and protocols
for exosome administration, utilizing in vivo and in
vitro experiments to thoroughly evaluate their therapeutic
efficacy. Additionally, inflammatory factors were not specifically
investigated to assess the contributions of immune reactions in
this cross-species transplantation study. Subsequent research is
required to include the detection of immune indicators to confirm
the safety of cross-species transplantation of MSC-exos. The
present study also did not investigate the effects of various
concentration gradients of exosomal transplantation. Thus, further
studies are warranted to determine the dose gradient to be used as
a core parameter to further strengthen the validity and reliability
of the present findings.
In conclusion, the present study provides evidence
that exosomes derived from hUCMSCs have the ability to enhance the
function of naturally aging ovaries. The underlying molecular
mechanisms involve the regulation of cellular apoptosis through the
inhibition of PTEN expression by exosomal miR-21-5p in ovarian
cells. The positive experimental results strongly support the
potential of hUCMSC-Exos transplantation as a promising therapy for
improving ovarian function in the context of natural aging.
Supplementary Data
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
ZL, YL, YD, JZ and XH conceived and designed the
study. ZL, YL, YaT and QL conducted the experiments and wrote the
manuscript. HZ, SY and YiT were responsible for the identification
of the mesenchymal stem cells and exosomes. YD, WS and TY were
responsible for data analysis and for figure preparation/creation.
WS, YaT and XH discussed and revised the manuscript. XH and ZL
reviewed the manuscript. All authors have read and approved the
final manuscript. ZL and YD confirm the authenticity of all the raw
data.
Ethics approval and consent to
participate
The principles outlined in the Declaration of
Helsinki and the Guidelines for the Care and Use of Laboratory
Animals of the Chinese Institute of Health were strictly adhered to
in the present study. The authors also complied with the ARRIVE
guidelines. Ethical approval for the animal experiments was
obtained from the Ethical Committee of Second Hospital of Hebei
Medical University (approval no. 2021-AE034). All animal
experiments conducted in the present study followed the guidelines
and regulations specified in this ethical approval.
Patient consent for publication
Not applicable.
Competing interests
The authors HZ, SY and YiT were affiliated with Qilu
Cell Therapy Technology Co., Ltd. The remaining authors affirm that
the research was conducted without any commercial or financial
associations that may be perceived as a potential conflict of
interest.
Acknowledgments
Not applicable.
Funding
The present study was financially supported by the Excellent
Clinical Medicine Talent Training Project funded by the Hebei
Provincial Government in 2022 (funding project title: 'Umbilical
cord mesenchymal stem cell-derived exosomes regulate the level of
epigenetic modification to restore ovarian function'.
References
|
1
|
Edson MA, Nagaraja AK and Matzuk MM: The
mammalian ovary from genesis to revelation. Endocr Rev. 30:624–712.
2009. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Nelson SM, Telfer EE and Anderson RA: The
ageing ovary and uterus: New biological insights. Hum Reprod
Update. 19:67–83. 2013. View Article : Google Scholar
|
|
3
|
Broekmans FJ, Soules MR and Fauser BC:
Ovarian aging: Mechanisms and clinical consequences. Endocr Rev.
30:465–493. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Park SU, Walsh L and Berkowitz KM:
Mechanisms of ovarian aging. Reproduction. 162:R19–R33. 2021.
View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Lew R: Natural history of ovarian function
including assessment of ovarian reserve and premature ovarian
failure. Best Pract Res Clin Obstet Gynaecol. 55:2–13. 2019.
View Article : Google Scholar
|
|
6
|
Chon SJ, Umair Z and Yoon MS: Premature
ovarian insufficiency: Past, present, and future. Front Cell Dev
Biol. 9:6728902021. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Seli E: Ovarian aging. Semin Reprod Med.
33:375–376. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Check Hayden E: Anti-ageing pill pushed as
bona fide drug. Nature. 522:265–266. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Sullivan SD, Sarrel PM and Nelson LM:
Hormone replacement therapy in young women with primary ovarian
insufficiency and early menopause. Fertility Sterility.
106:1588–1599. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Ding C, Zhu L, Shen H, Lu J, Zou Q, Huang
C, Li H and Huang B: Exosomal miRNA-17-5p derived from human
umbilical cord mesenchymal stem cells improves ovarian function in
premature ovarian insufficiency by regulating SIRT7. Stem Cells.
38:1137–1148. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Pittenger MF, Mackay AM, Beck SC, Jaiswal
RK, Douglas R, Mosca JD, Moorman MA, Simonetti DW, Craig S and
Marshak DR: Multilineage potential of adult human mesenchymal stem
cells. Science. 284:143–147. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Yin N, Wu C, Qiu J, Zhang Y, Bo L, Xu Y,
Shi M, Zhu S, Yang G and Mao C: Protective properties of heme
oxygenase-1 expressed in umbilical cord mesenchymal stem cells help
restore the ovarian function of premature ovarian failure mice
through activating the JNK/Bcl-2 signal pathway-regulated autophagy
and upregulating the circulating of CD8+CD28−
T cells. Stem Cell Res Ther. 11:492020. View Article : Google Scholar
|
|
13
|
Martinez-Carrasco R, Sanchez-Abarca LI,
Nieto-Gomez C, Martin Garcia E, Sanchez-Guijo F, Argueso P, Aijón
J, Hernández-Galilea E and Velasco A: Subconjunctival injection of
mesenchymal stromal cells protects the cornea in an experimental
model of GVHD. Ocul Surf. 17:285–294. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Oliva J: Therapeutic properties of
mesenchymal stem cell on organ ischemia-reperfusion injury. Int J
Mol Sci. 20:55112019. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Salado-Manzano C, Perpina U, Straccia M,
Molina-Ruiz FJ, Cozzi E, Rosser AE and Canals JM: Is the
immunological response a bottleneck for cell therapy in
neurodegenerative diseases? Front Cell Neurosci. 14:2502020.
View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Sajeesh S, Broekelman T, Mecham RP and
Ramamurthi A: Stem cell derived extracellular vesicles for vascular
elastic matrix regenerative repair. Acta Biomater. 113:267–278.
2020. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Ranganath SH, Levy O, Inamdar MS and Karp
JM: Harnessing the mesenchymal stem cell secretome for the
treatment of cardiovascular disease. Cell Stem Cell. 10:244–258.
2012. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Valadi H, Ekstrom K, Bossios A, Sjostrand
M, Lee JJ and Lotvall JO: Exosome-mediated transfer of mRNAs and
microRNAs is a novel mechanism of genetic exchange between cells.
Nat Cell Biol. 9:654–659. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Kalluri R and LeBleu VS: The biology,
function, and biomedical applications of exosomes. Science.
367L:eaau69772020. View Article : Google Scholar
|
|
20
|
Yao J, Ma Y, Zhou S, Bao T, Mi Y, Zeng W,
Li J and Zhang C: Metformin prevents follicular atresia in aging
laying chickens through activation of PI3K/AKT and calcium
signaling pathways. Oxid Med Cell Longev. 2020:36480402020.
View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Tilly JL, Kowalski KI, Johnson AL and
Hsueh AJ: Involvement of apoptosis in ovarian follicular atresia
and postovulatory regression. Endocrinology. 129:2799–2801. 1991.
View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Shen M, Lin F, Zhang J, Tang Y, Chen WK
and Liu H: Involvement of the up-regulated FoxO1 expression in
follicular granulosa cell apoptosis induced by oxidative stress. J
Biol Chem. 287:25727–25740. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Matsuda F, Inoue N, Manabe N and Ohkura S:
Follicular growth and atresia in mammalian ovaries: Regulation by
survival and death of granulosa cells. J Reprod Dev. 58:44–50.
2012. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Yamada KM and Araki M: Tumor suppressor
PTEN: Modulator of cell signaling, growth, migration and apoptosis.
J Cell Sci. 114:2375–2382. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Stambolic V, Suzuki A, de la Pompa JL,
Brothers GM, Mirtsos C, Sasaki T, Ruland J, Penninger JM,
Siderovski DP and Mak TW: Negative regulation of PKB/Akt-dependent
cell survival by the tumor suppressor PTEN. Cell. 95:29–39. 1998.
View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Maidarti M, Anderson RA and Telfer EE:
Crosstalk between PTEN/PI3K/Akt signalling and DNA damage in the
oocyte: Implications for primordial follicle activation, oocyte
quality and ageing. Cells. 9:2002020. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Hsueh AJ, Kawamura K, Cheng Y and Fauser
BC: Intraovarian control of early folliculogenesis. Endocr Rev.
36:1–24. 2015. View Article : Google Scholar :
|
|
28
|
Wen Z, Mai Z, Zhu X, Wu T, Chen Y, Geng D
and Wang J: Mesenchymal stem cell-derived exosomes ameliorate
cardiomyocyte apoptosis in hypoxic conditions through microRNA144
by targeting the PTEN/AKT pathway. Stem Cell Res Ther. 11:362020.
View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Ding C, Qian C, Hou S, Lu J, Zou Q, Li H
and Huang B: Exosomal miRNA-320a is released from hAMSCs and
regulates SIRT4 to prevent reactive oxygen species generation in
POI. Mol Ther Nucleic Acids. 21:37–50. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Ding C, Zou Q, Wang F, Wu H, Chen R, Lv J,
Ling M, Sun J, Wang W, Li H and Huang B: Human amniotic mesenchymal
stem cells improve ovarian function in natural aging through
secreting hepatocyte growth factor and epidermal growth factor.
Stem Cell Res Ther. 9:552018. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Dominici M, Le Blanc K, Mueller I,
Slaper-Cortenbach I, Marini F, Krause D, Deans R, Keating A,
Prockop DJ and Horwitz E: Minimal criteria for defining multipotent
mesenchymal stromal cells. The International Society for Cellular
Therapy position statement. Cytotherapy. 8:315–357. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Li Z, Zhang M, Zheng J, Tian Y, Zhang H,
Tan Y, Li Q, Zhang J and Huang X: Human umbilical cord mesenchymal
stem Cell-derived exosomes improve ovarian function and
proliferation of premature ovarian insufficiency by regulating the
hippo signaling pathway. Front Endocrinol (Lausanne).
12:7119022021. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Lotvall J, Hill AF, Hochberg F, Buzas EI,
Di Vizio D, Gardiner C, Gho YS, Kurochkin IV, Mathivanan S,
Quesenberry P, et al: Minimal experimental requirements for
definition of extracellular vesicles and their functions: A
position statement from the international society for extracellular
vesicles. J Extracell Vesicles. 3:269132014. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Wang S, Zheng Y, Li J, Yu Y, Zhang W, Song
M, Liu Z, Min Z, Hu H, Jing Y, et al: Single-cell transcriptomic
atlas of primate ovarian aging. Cell. 180:585–600.e19. 2020.
View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Zhang X, Lan Y, Xu J, Quan F, Zhao E, Deng
C, Luo T, Xu L, Liao G, Yan M, et al: CellMarker: A manually
curated resource of cell markers in human and mouse. Nucleic Acids
Res. 47:D721–D728. 2019. View Article : Google Scholar :
|
|
36
|
Liu M, Qiu Y, Xue Z, Wu R, Li J, Niu X,
Yuan J, Wang Y and Wu Q: Small extracellular vesicles derived from
embryonic stem cells restore ovarian function of premature ovarian
failure through PI3K/AKT signaling pathway. Stem Cell Res Ther.
11:32020. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Hosokawa K, Dantes A, Schere-Levy C,
Barash A, Yoshida Y, Kotsuji F, Vlodavsky I and Amsterdam A:
Induction of Ad4BP/SF-1, steroidogenic acute regulatory protein,
and cytochrome P450scc enzyme system expression in newly
established human granulosa cell lines. Endocrinology.
139:4679–4687. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Havelock JC, Rainey WE and Carr BR:
Ovarian granulosa cell lines. Mol Cell Endocrinol. 228:67–78. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(-Delta Delta C(T)) method. Methods. 25:402–408. 2001.
View Article : Google Scholar
|
|
40
|
Salic A and Mitchison TJ: A chemical
method for fast and sensitive detection of DNA synthesis in vivo.
Proc Natl Acad Sci USA. 105:2415–2420. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Park CM, Reid PE, Walker DC and MacPherson
BR: A simple, practical 'swiss roll' method of preparing tissues
for paraffin or methacrylate embedding. J Microsc. 145:115–120.
1987. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Kanehisa M and Goto S: KEGG: Kyoto
encyclopedia of genes and genomes. Nucleic Acids Res. 28:27–30.
2000. View Article : Google Scholar
|
|
43
|
Lake BB, Ai R, Kaeser GE, Salathia NS,
Yung YC, Liu R, Wildberg A, Gao D, Fung HL, Chen S, et al: Neuronal
subtypes and diversity revealed by single-nucleus RNA sequencing of
the human brain. Science. 352:1586–1590. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Soyal SM, Amleh A and Dean J: FIGalpha, a
germ cell-specific transcription factor required for ovarian
follicle formation. Development. 127:4645–4654. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Meinsohn MC, Morin F, Bertolin K,
Duggavathi R, Schoonjans K and Murphy BD: The orphan nuclear
receptor liver homolog Receptor-1 (Nr5a2) regulates ovarian
granulosa cell proliferation. J Endocr Soc. 2:24–41. 2018.
View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Sasson R, Tajima K and Amsterdam A:
Glucocorticoids protect against apoptosis induced by serum
deprivation, cyclic adenosine 3′,5′-monophosphate and p53
activation in immortalized human granulosa cells: Involvement of
Bcl-2. Endocrinology. 142:802–811. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Carracedo A and Pandolfi PP: The PTEN-PI3K
pathway: Of feedbacks and cross-talks. Oncogene. 27:5527–5541.
2008. View Article : Google Scholar : PubMed/NCBI
|
|
48
|
Meng F, Henson R, Wehbe-Janek H, Ghoshal
K, Jacob ST and Patel T: MicroRNA-21 regulates expression of the
PTEN tumor suppressor gene in human hepatocellular cancer.
Gastroenterology. 133:647–658. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
49
|
Zhang JG, Wang JJ, Zhao F, Liu Q, Jiang K
and Yang GH: MicroRNA-21 (miR-21) represses tumor suppressor PTEN
and promotes growth and invasion in non-small cell lung cancer
(NSCLC). Clin Chim Acta. 411:846–852. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
50
|
Zhang J, Chen Q, Du D, Wu T, Wen J, Wu M,
Zhang Y, Yan W, Zhou S, Li Y, et al: Can ovarian aging be delayed
by pharmacological strategies? Aging (Albany NY). 11:817–832. 2019.
View Article : Google Scholar : PubMed/NCBI
|
|
51
|
Sugiyama M, Kawahara-Miki R, Kawana H,
Shirasuna K, Kuwayama T and Iwata H: Resveratrol-induced
mitochondrial synthesis and autophagy in oocytes derived from early
antral follicles of aged cows. J Reprod Dev. 61:251–259. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
52
|
Yan F, Zhao Q, Li Y, Zheng Z, Kong X, Shu
C, Liu Y and Shi Y: The role of oxidative stress in ovarian aging:
A review. J Ovarian Res. 15:1002022. View Article : Google Scholar : PubMed/NCBI
|
|
53
|
Weng LP, Smith WM, Dahia PL, Ziebold U,
Gil E, Lees JA and Eng C: PTEN suppresses breast cancer cell growth
by phosphatase activity-dependent G1 arrest followed by cell death.
Cancer Res. 59:5808–5814. 1999.PubMed/NCBI
|
|
54
|
Huang H, Potter CJ, Tao W, Li DM, Brogiolo
W, Hafen E, Sun H and Xu T: PTEN affects cell size, cell
proliferation and apoptosis during Drosophila eye development.
Development. 126:5365–5372. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
55
|
Song MS, Salmena L and Pandolfi PP: The
functions and regulation of the PTEN tumour suppressor. Nat Rev Mol
Cell Biol. 13:283–296. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
56
|
Llarena N and Hine C: Reproductive
longevity and aging: Geroscience approaches to maintain long-term
ovarian fitness. J Gerontol A Biol Sci Med Sci. 76:1551–1560. 2021.
View Article : Google Scholar :
|
|
57
|
Ferguson SW and Nguyen J: Exosomes as
therapeutics: The implications of molecular composition and
exosomal heterogeneity. J Control Release. 228:179–190. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
58
|
Lu J and Clark AG: Impact of microRNA
regulation on variation in human gene expression. Genome Res.
22:1243–1254. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
59
|
Bushati N and Cohen SM: microRNA
functions. Annu Rev Cell Dev Biol. 23:175–205. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
60
|
Ferguson SW, Wang J, Lee CJ, Liu M,
Neelamegham S, Canty JM and Nguyen J: The microRNA regulatory
landscape of MSC-derived exosomes: A systems view. Sci Rep.
8:14192018. View Article : Google Scholar : PubMed/NCBI
|
|
61
|
Cai Y, Yu X, Hu S and Yu J: A brief review
on the mechanisms of miRNA regulation. Genomics Proteomics
Bioinformatics. 7:147–154. 2009. View Article : Google Scholar
|
|
62
|
Yang M, Lin L, Sha C, Li T, Zhao D, Wei H,
Chen Q, Liu Y, Chen X, Xu W, et al: Bone marrow mesenchymal stem
cell-derived exosomal miR-144-5p improves rat ovarian function
after chemotherapy-induced ovarian failure by targeting PTEN. Lab
Invest. 100:342–352. 2020. View Article : Google Scholar
|
|
63
|
Fu X, He Y, Wang X, Peng D, Chen X, Li X
and Wang Q: Overexpression of miR-21 in stem cells improves ovarian
structure and function in rats with chemotherapy-induced ovarian
damage by targeting PDCD4 and PTEN to inhibit granulosa cell
apoptosis. Stem Cell Res Ther. 8:1872017. View Article : Google Scholar : PubMed/NCBI
|