Introduction
Osteoporosis is a prevalent age-related chronic
condition characterized by a decrease in bone mass and disrupted
alignment of bone trabeculae, which has been reported to be
influenced by iron levels in the body (1,2).
Consequently, it has been hypothesized that age-associated iron
overload and oxidative stress serve pivotal roles in the
development of osteoporosis.
Iron serves an important role in a wide range of
physiological functions, including oxygen transport, enzyme
reactions, energy production, protein synthesis and DNA repair
(3). Iron metabolism is
rigorously regulated by numerous factors and when this equilibrium
is disrupted, iron overload leads to elevated levels of reactive
oxygen species (ROS) and cytotoxicity, resulting in tissue and
organ dysfunction. Numerous studies have indicated that iron
overload-induced osteoporosis in mice is characterized by a
reduction in volume fraction, thickness and spacing of bone
trabeculae, and these findings have been corroborated in rats
(4,5). Furthermore, excessive iron impedes
bone formation in zebrafish models of iron overload, accompanied by
decreased expression of osteoblast-specific genes, such as alkaline
phosphatase (ALP), collagen type I (Col I), and Runt-related
transcription factor 2 (Runx2) (6). Therefore, mouse, rat or zebrafish
models subjected to iron overload may serve as valuable tools for
investigating the effects of various drugs on osteoporosis.
Iron overload leading to iron toxicity is known to
be closely related to amino acid, lipid and iron metabolism
(7). Divalent iron exhibits
strong reductive properties and can generate excessive ROS through
the Fenton reaction, resulting in oxidative damage to cells
(8). Mitochondria have a pivotal
role in cellular metabolism, with abnormalities in the
tricarboxylic acid cycle and electron transport chain being
responsible for lipid peroxidation (LPO) (9). Mitochondrial ROS (mtROS) serve as
indicators of mitochondrial damage, whereas lipid ROS play a
pivotal role in ferroptosis (10). Oxidative mitochondrial damage
induced by various external stressors, such as iron overload, can
lead to various forms of cell death, including apoptosis (11). The MC3T3-E1 cells are derived from
mouse embryonic skull tissue. It is an osteoblast cell line that
can readily proliferate, maintain normal differentiation and
osteogenic capacity in culture in vitro, making it a common
model used for osteoporosis research (12,13). Therefore, it remains imperative to
investigate whether excessive iron intervention in MC3T3-E1 cells
induces mitochondrial dysfunction and apoptosis, thus impacting
their differentiation into osteoblasts.
The PI3K/Akt axis is involved in the regulation of
various biological processes and cellular mechanisms, such as
angiogenesis (14), epigenetic
regulation of tumors (15), and
regulation of the nervous system and brain injury (16). Activation of the PI3K/Akt
signaling pathway has been shown to inhibit ferroptosis in
osteoblasts as a potential treatment for osteoporosis (17). Additionally, it has been reported
that ROS act as a mediator in the regulation of the PI3K/Akt
signaling pathway to safeguard osteoblasts from apoptosis and
promote their osteogenesis (18,19). Arctiin (ARC), a lignin bioactive
compound initially isolated from Arctium lappa L (20), has been demonstrated to exert
antioxidant and anti-inflammatory effects (21,22). In previous studies, ARC has been
reported to inhibit the migration and invasion of cervical cancer
cells by regulating the PI3K/Akt signaling pathway, and to reduce
inflammation by modulating macrophage polarization (23-25). ARC has also been shown to modulate
osteogenic differentiation and maintain bone homeostasis in the
treatment of osteoporosis (26,27). However, whether ARC can
effectively treat iron overload-induced osteoporosis (IOOP) by
regulating the PI3K/Akt pathway remains to be investigated.
The present study examined the efficacy of ARC in
treating IOOP based on the PI3K/Akt signaling pathway. This study
also aimed to provide new insights into the mechanisms via which
ARC is effective in mitigating IOOP.
Materials and methods
Reagents
Penicillin/streptomycin (P/S), 0.25% Trypsin-EDTA
and MC3T3-E1 mouse embryonic osteoblast precursor cells were
obtained from Thermo Fisher Scientific, Inc. Fetal bovine serum
(FBS) was purchased from Gibco (Thermo Fisher Scientific, Inc.).
α-Minimum Essential Medium (α-MEM) and PBS were obtained from Wuhan
Servicebio Technology Co., Ltd. DAPI, ferric ammonium citrate (FAC;
CAS 1185-57-5), iron-dextran (CAS 9004-66-4), dimethyl sulfoxide
(DMSO; CAS 67-68-5), 0.2% Triton X-100 and Immobilon®
transfer membrane (PVDF membrane) were purchased from
MilliporeSigma. LY294002 (CAS 154447-36-6) was obtained from
MedChemExpress. ARC (HPLC ≥98%, CAS 20362-31-6) was obtained from
Chengdu Desite Biotechnology Co., Ltd., which was dissolved in DMSO
as stock solution and stored at −20°C in a light-proof environment.
The concentration of DMSO in the cell culture reagents was
<0.1%. Alizarin Red S (ARS) Solution was purchased from OriCell
Therapeutics, Co., Ltd. RIPA buffer, MitoSOX Red Mitochondrial
Superoxide Indicator, dichlorodihydrofluorescein diacetate
(DCFH-DA), LPO sensor (BODIPY® C11), the BCIP/NBT ALP
Color Development Kit (cat. no. C3202), BCA Assay Kit, SDS-PAGE Gel
Preparation Kit and the Membrane Potential Assay Kit (JC-1) were
all from Beyotime Institute of Biotechnology. Cell Counting Kit 8
(CCK8) and Calcein-AM were from GlpBio Technology. ECL
Chemiluminescent Substrate Substrate (Extra Ultra Sensitive) Kit
was obtained from Biosharp Life Sciences. The Annexin V-FITC/PI Kit
was purchased from Multi Sciences (Lianke) Biotechnology Co., Ltd.
The hematoxylin and eosin (H&E) staining kit, membrane
regeneration solution reagent and Serum Iron Concentration Assay
Kit (cat. no. BC1735) were from Beijing Solarbio Science &
Technology Co., Ltd. The following western blotting primary
antibodies were obtained from Affinity Biosciences: Col I (cat. no.
AF7001), Runx2 (cat. no. AF5186), Bax (cat. no. AF0120), Bcl-2
(cat. no. AF6139), PI3K (cat. no. AF6241), phosphorylated (p)-PI3K
(cat. no. AF3242), Akt (cat. no. AF6261), p-Akt (cat. no. AF0016),
β-actin (cat. no. AF7018). Goat anti-rabbit IgG H&L secondary
antibody (cat. no. bs-40295G-HRP) was purchased from BIOSS.
Animal experiments
A total of 40 male C57/BL6 mice (age, 49 days;
weight, 20±2 g) were provided by The Animal Experiment Center of
Guangzhou University of Chinese Medicine (Guangzhou, China). The
temperature of the animal laboratory is controlled at 20-26°C, with
a daily temperature difference of 4°C. The relative humidity is
controlled at 40-70%. The mice had constant access to adequate
SPF-grade mouse chow and water and were maintained under a 12-h
light/dark cycle. After 7 days of acclimatization, the mice were
randomly divided into the following four groups: i) Control group,
where mice received a once weekly intraperitoneal injection of
saline equivalent to the volume of iron dextrose applied + a daily
gavage of saline equivalent to the volume to the ARC applied (n=6);
ii) intraperitoneal injection of dextran iron (ID) group, where
mice received a once weekly intraperitoneal injection of dextran
iron + a daily gavage of equivalent volume of saline (n=6); iii) ID
+ ARC-L group, where the mice received a weekly intraperitoneal
injection of dextran iron + a daily gavage of 20 mg/kg ARC (n=6);
and iv) ID + ARC-H group, where the mice received a weekly
intraperitoneal injection of dextran iron + a daily gavage of 40
mg/kg ARC (n=6). In vivo doses of ARC were obtained with
reference to previous literature (28,29). All mice, with the exception of
those in the normal control group, were administered
intraperitoneal doses of 500 mg/kg iron dextran for 3 months. In
particular, dextran iron is administered intraperitoneally 1 month
prior to ARC gavage (30). The
design of the animal experiment protocols in the present study is
shown in Fig. 1A. All mice used
in the present study were raised in the SPF Experimental Animal
Center of the First Affiliated Hospital of Guangzhou University of
Chinese Medicine (Guangzhou, China). All animal experiments were
approved by the Ethics Committee of Guangzhou University of Chinese
Medicine (approval no. TCMF1-2021029; May 20, 2021). Animals were
euthanized at 20 weeks of age through an intraperitoneal injection
of an overdose of pentobarbital (150 mg/kg). Death was confirmed by
continuing to observe the mice for 2 min after the observation of
no heartbeat, respiration and the pupils are dilated.
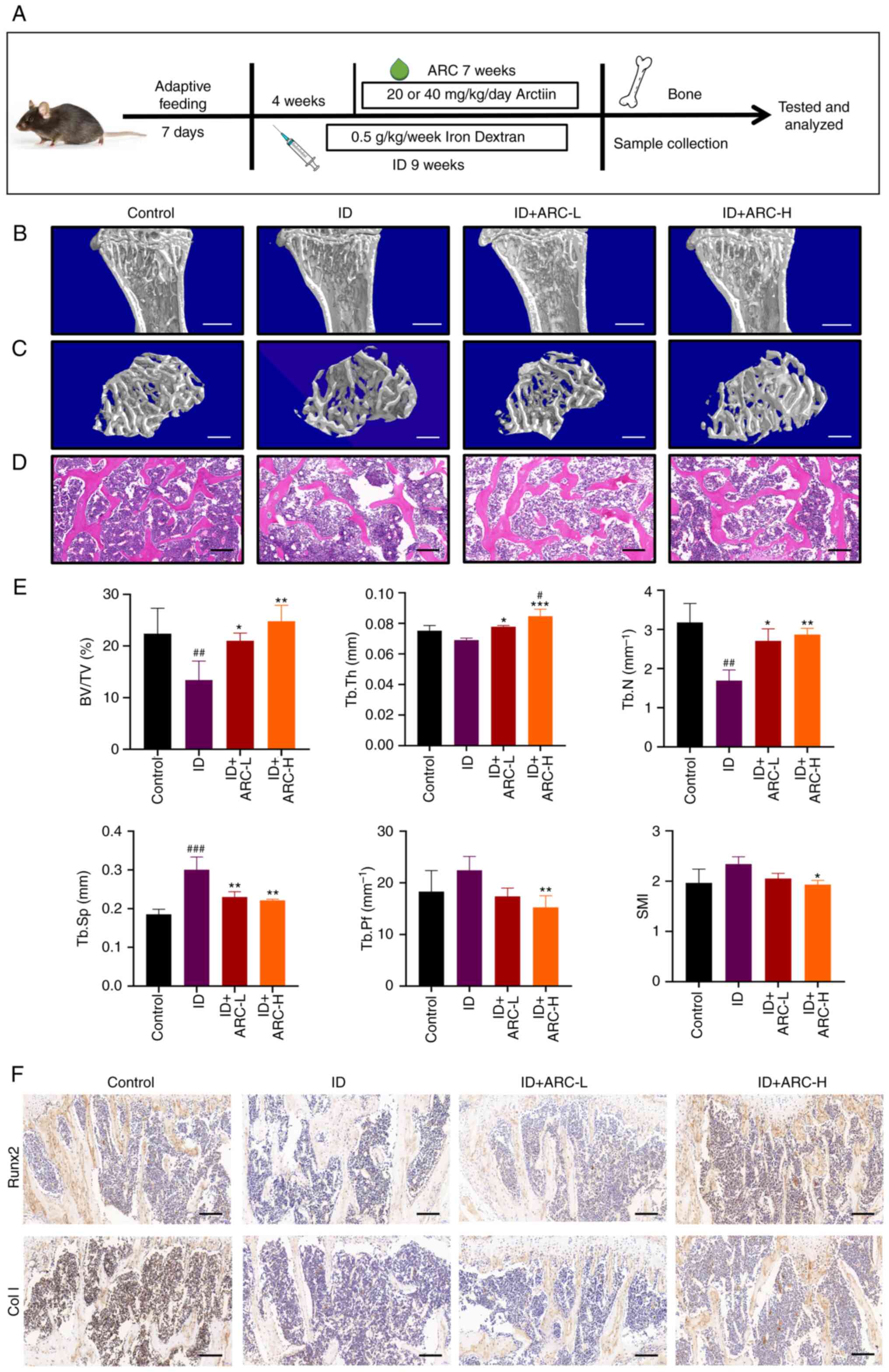 | Figure 1ARC alleviates IOOP in mice. (A)
Modeling method and flow chart of ARC treatment in a mouse model of
IOOP. (B and C) Micro-CT analysis of tibial specimens. Scale bars:
(B) 1 mm and (C) 0.5 mm. (D) Hematoxylin and eosin staining of
excised thin layers of tibial specimens. Scale bar, 0.2 mm. (E)
Bone structural parameters obtained from micro-CT scans, including
SMI, BV/TV, Tb.Th, Tb.Sp, Tb.N and Tb.Pf, were analyzed. (F)
Immunohistochemical analysis of Runx2 and Col I in tibial samples.
Scale bar, 0.2 mm. Data are presented as the mean ± SD.
***P<0.001, **P<0.01 and
*P<0.05 vs. ID; ###P<0.001,
##P<0.01 and #P<0.05 vs. Con. ARC,
arctiin; Con, control; ARC-H, ARC-high; ARC-L, ARC-low; BV/TV, bone
volume/tissue volume; Col I, collagen type I; ID, dextran iron;
Runx2, Runt-related transcription factor 2; SMI, structure model
index; Tb.N, trabecular bone number; Tb.Pf, trabecular bone pattern
factor; Tb.Sp, trabecular separation/spacing; Tb.Th, trabecular
thickness; micro-CT, micro-computed tomography. |
Micro-computed tomography (micro-CT)
analysis
The left tibiae of mice after euthanasia from the
aforementioned pentobarbital protocol were collected and fixed in
4% paraformaldehyde for 24 h at room temperature before being
scanning with the Skyscan 1172 micro-CT system (Bruker
Corporation). Subsequently, the data were analyzed using a custom
analysis program (version 1.17.7.2+; CTAn Skyscan; Bruker
Corporation). From the cross marker below the tibial plateau, the
volume of interest consists primarily of a 100-layer transverse
section. Bone microstructural parameters, such as structure model
index (SMI; SMI=Skeletal Muscle Area (SMA)/height2; SMA
was calculated and analyzed from CT images.), ratio of bone
volume/tissue volume (BV/TV), trabecular thickness (Tb.Th),
trabecular separation (Tb.Sp), trabecular number (Tb.N) and
trabecular bone pattern factor (Tb.Pf) were analyzed according to
the manufacturer's protocol.
H&E staining assay
The aforementioned fixed samples were decalcified in
14% EDTA at 37°C for 1 week. After decalcification, the samples
were embedded in paraffin and cut into 5-μm sections. The
sections were stained with hematoxylin for 5 min and eosin stain
for 1 min, both at room temperature, before and a panoramic digital
slide scanner (3DHISTECH Ltd.) was used to assess them.
Immunohistochemistry
The aforementioned paraffin-embedded tissues were
sectioned into 5-μm thin slices, before the slides were
fished out and placed in a 37°C constant-temperature drying oven
for 12 h. The dried slides were sequentially deparaffinized by
immersion in xylene solution followed by a decreasing ethanol
gradient, before being washed with distilled water and PBS. The
sections were then added to a boiling (100°C) citrate antigen
retrieval solution (Beyotime Institute of Biotechnology) for 15
min, prior to cooling and incubation with 3%
H2O2 for 5 min at room temperature to
inactivate endogenous peroxidase. The sections were then blocked
with 10% goat serum (Beyotime Institute of Biotechnology) for 30
min at room temperature. The blocked sections were then incubated
with primary antibodies (all 1:200) against p-PI3K (cat. no.
AF3242; Affinity Biosciences), Col I (cat. no. AF7001; Affinity
Biosciences) or Runx2 (cat. no. AF5186; Affinity Biosciences)
overnight at 4°C, followed by incubation with HRP-labeled Goat
Anti-Rabbit IgG secondary antibodies (1:50; cat. no. A0208;
Beyotime Institute of Biotechnology) for 2 h at room temperature.
The configured DAB coloring solution (20X DAB Substrate Kit; cat.
no. BL732A; Biosharp Life Sciences) was then added dropwise to the
slides according to the manufacturer's protocol and the staining
results were observed under a microscope. The slides were then
rinsed with PBS. Counterstaining was performed with hematoxylin for
1 min at room temperature, the tissue was sealed with neutral gum
and images were captured under a light microscope.
Serum iron concentration assay
A total of 125 μl of each of the Serum Iron
Concentration Assay Kit Reagents 1 and 2 was added to each tube.
Subsequently, 125 μl distilled water was added to a blank
tube, before 125 μl serum [1 week before euthanasia, 0.3 ml
of blood was withdrawn once from the submandibular vein of the mice
(31)], which was let stand for 1
h and then centrifuged at 2,500 × g for 10 min at 4°C to isolate
the supernatant) to be tested was added to the assay tube, and 125
μl standard solution (from the kit) was added to the
standard tube. Subsequently, the tubes were centrifuged at 10,000 ×
g for 10 min at room temperature to obtain the supernatants, the
absorbance of which was measured at 520 nm using a microplate
reader (Thermo Fisher Scientific, Inc.). Finally, serum iron
concentration was calculated using the following formula: Serum
iron content (μg/ml)=125 × (assay tube-distilled water
tube)/(standard tube-distilled water tube).
Cell culture and experimental
protocol
MC3T3-E1 cells were cultured in α-MEM containing 10%
FBS and 1% P/S in a 37°C cell culture incubator with 5%
CO2. Cells were harvested when they reached ~80%
confluence and underwent drug (FAC, ARC and LY294002) intervention
for 48 h at 37°C prior to further experimentation. FAC was prepared
as a 100 mM master mix with PBS, whereas ARC and LY294002 were
prepared as a 10 mM concentration with DMSO. All drugs were stored
at -20°C. All in vitro experiments were repeated three
times.
CCK8 cell viability assay
CCK8 assay was performed to assess the effect of ARC
on cell viability. After treating MC3T3-E1 cells (In 96-well plate
at a density of 5×103 cells per well) with different
concentrations of ARC (0, 2, 4, 8, 12, 16 and 20 μM) for 48
h, 100 μl CCK8 working solution (containing 10 μl
CCK8 reagent and 90 μl serum-free medium) was added to each
well and incubated for 2 h at 37°C in the aforementioned cell
culture environment. The absorbance was then measured at 450 nm
using a microplate reader (Thermo Fisher Scientific, Inc.). To
determine the appropriate dose of FAC, cells in 96-well plates were
treated with different concentrations of FAC (0, 20, 40, 60, 80,
100, 200, 300, 400 and 500 μM) for 48 h at 37°C and the CCK8
assay was performed as aforementioned. The final cellular
intervention dose of FAC was determined to be 500 μM.
Subsequently, to determine the appropriate dosage of ARC, cells in
96-well plates were treated with 500 μM FAC and various
concentrations of ARC (0, 2, 4, 6, 8, 10 and 12 μM) for 48 h
at 37°C and the CCK8 assay was performed as aforementioned.
Grouping of cytology experiments
The following groups were set up for cytology
experiments: i) Control group, where cells were cultured in α-MEM
medium containing 10% FBS; ii) FAC group, where cells were cultured
in α-MEM medium containing 10% FBS and 500 μM FAC; iii) FAC
+ ARC-L group, where cells were cultured in α-MEM medium containing
10% FBS, 500 μM FAC and 4 μM ARC; abd iv) FAC + ARC-H
group, where cells were cultured with α-MEM medium containing 10%
FBS, 500 μM FAC and 8 μM ARC.
Calcein AM assay of intracellular iron
content
The calcein AM staining stock solution was diluted
to a working solution at a concentration of 2 μM in
serum-free medium. MC3T3-E1 cells were inoculated into 24-well
plates at a density of 3×104 overnight at 37°C to
stabilize cell proliferation and were then cultured for 48 h at
37°C in 10% FBS-containing medium containing FAC (500 μM),
with or without ARC (4 and 8 μM). FAC and ARC were added to
α-MEM containing 10% FBS at 37°C. Cells were washed twice with PBS
and were then incubated with calcein AM working solution for 30 min
at 37°C in the dark. The cells were washed twice with PBS, and were
then observed and photographed using a fluorescence microscope
(Leica Microsystems, Inc.) under an emission light wavelength of
494-514 nm.
Analysis of bone mineralization
MC3T3-E1 cells were seeded at a density of
3×104 in 24-well plates at 30% confluence overnight at
37°C in normal α-MEM and were then cultured in osteoblast
differentiation medium containing 1 μM dexamethasone, 100
μg/ml ascorbic acid and 10 mM β-glycerophosphate, with ARC
(4 or 8 μM) and with or without FAC for 7 and 21 days at
37°C (medium changed every 3 days). Subsequently, cells underwent
ALP staining (BCIP/NBT ALP Color Development Kit) and ARS staining.
Briefly, cells were fixed in 4% paraformaldehyde for 20 min at room
temperature before staining, washed twice with double distilled
water and then stained with the BCIP/NBT working solution (500
μl) or ARS solution (500 μl) for 2 h at room
temperature. The staining signals were then recorded using light
microscopy accordingly.
Apoptosis detection assay
Cells were cultured for 48 h at 37°C in 10%
FBS-containing medium containing FAC (500 μM), with or
without ARC (4 and 8 μM). Apoptosis was then analyzed using
the Annexin V-FITC/PI Kit. Cells were washed with PBS after
digestion with 0.25% Trypsin-EDTA for 2 min at 37°C. In the dark,
cells (each aliquot contains ~3×105 cells) were
incubated with Annexin V-FITC and PI for 5 min at room temperature
(Each aliquot of staining contains 5 μl V-FITC and 10
μl PI). A BD FACSCanto II flow cytometer (BD Biosciences)
was then used to detect the cells. The resulting data were
processed by BD FACSDiva™ software (V9.0; BD Biosciences) and the
FlowJo software (V10.6.2; BD Biosciences).
Assessment of ROS levels
After cell treatment [cultured for 48 h at 37°C in
10% FBS-containing medium-containing FAC (500 μM), with or
without ARC (4 and 8 μM)], the cells were incubated for 20
min at 37°C in the dark with serum-free medium containing DCFH-DA,
BODIPY C11 or MitoSOX Red Mitochondrial Superoxide Indicator-probe
(DCFH-DA and BODIPY C11 at a working concentration of 10 μM
and MitoSOX Red Mitochondrial Superoxide Indicator at a working
concentration of 200 nM). The cells were then washed twice with
PBS, before intracellular ROS, lipid ROS or mtROS fluorescence
intensity were detected using a fluorescence microscope.
Alternatively, cells were digested and incubated with the probe for
detection by flow cytometry, using the same flow cytometer and
software as that for Annexin V-FITC/PI assay.
Mitochondrial membrane potential (MMP)
measurements
The harvested cells [The harvested cells were from
6-well plates with 2×105 cells per well. They were first
cultured for 48 h at 37°C in 10% FBS-containing medium containing
FAC (500 μM), with or without ARC (4 and 8 μM)] were
washed twice with PBS, before they were incubated directly with the
JC-1 working solution (the concentration of JC-1 working solution
was 10 μg/ml) for 20 min at 37°C in the dark or were
digested with 0.25% Trypsin-EDTA and then incubated with JC-1 for
20 min. Subsequently, the cells were washed twice and MMP was
examined by fluorescence microscopy or flow cytometry (as
aforementioned), respectively.
Immunofluorescence
For permeabilization, the cells [cultured for 48 h
at 37°C in 10% FBS-containing medium containing FAC (500
μM), with or without ARC (4 and 8 μM)] were fixed for
15 min at room temperature with 4% paraformaldehyde, then treated
with 0.2% Triton X-100 for 30 min at room temperature and blocked
with 1% BSA (Biosharp Life Sciences)/10% normal goat serum
(Beyotime Institute of Biotechnology)/0.3 glycine in 0.1% PBS-Tween
for 1 h at room temperature. After incubation with the anti-p-PI3K
primary antibody (1:200; cat. no. AF3242; Affinity Biosciences)
overnight at 4°C, the cells were incubated with a goat anti-rabbit
IgG H&L (Alexa Fluor® 647) secondary antibody (cat.
no. ab150083, abcam) for 1 h at room temperature. The secondary
antibody was prepared in a 1:200 ratio with 4% BSA. The nuclei were
stained with DAPI (the working concentration was 1 μg/ml)
for 3 min at room temperature. Confocal laser scanning microscopy
was used to obtain the immunofluorescence images.
Western blotting
Total protein was extracted from the treated
MC3T3-E1 cells [cultured for 48 h in 10% FBS-containing medium
containing FAC (500 μM), with or without ARC (4 and 8
μM)] using RIPA buffer containing phosphatase inhibitor and
universal protease inhibitor, and the protein concentration was
determined using a BCA assay kit. Subsequently, proteins (20
μg) were separated by SDS-PAGE on 10% gels and were
transferred onto PVDF membranes, which were blocked with Quick
Blocking Solution (Beyotime Institute of Biotechnology) for 15 min
at room temperature according to the manufacturer's protocol. The
membranes were then incubated overnight at 4°C with primary
antibodies (Col I, Runx2, p-PI3K, PI3K, p-Akt, Akt, Bax, Bcl-2,
β-actin; all 1:1,000). The membranes were subsequently washed with
TBS-10% Tween (Beyotime Institute of Biotechnology) and incubated
with the corresponding HRP-conjugated secondary IgG antibodies
(1:5,000) for 1 h at room temperature. Protein strips that had been
coated with secondary antibodies were cut horizontally according to
the protocol of Dual Color Standards (Used to provide standard
protein molecular weights; Bio-Rad Laboratories, Inc.), and were
subsequently visualized with the ECL Chemiluminescent Substrate
Substrate (Extra Ultra Sensitive) Kit and a gel imaging system
(Bio-Rad Laboratories, Inc.). The grayscale values were
semi-quantified using the ImageJ (V.1.8.0-172) software (National
Institutes of Health). The PI3K and Akt blots were washed with
membrane regeneration reagent at room temperature for 10 min to
wash off the primary and secondary antibodies, followed by
re-analysis with the p-PI3K and p-Akt antibodies as
aforementioned.
Statistical analysis
Results are from at least three different
experiments. Mean values are presented as the mean ± SD.
Statistical analysis was performed using SPSS (V25.0) software (IBM
Corp). One-way ANOVA and Tukey's post hoc test (By SPSS) were used
to compare three or more groups. P<0.05 was considered to
indicate a statistically significant difference. All graphs were
generated using GraphPad Prism (version 9.0.2; Dotmatics).
Results
ARC alleviates IOOP in mice
To investigate the effect of ARC on IOOP in
vivo, micro-CT was performed. Notably, micro-CT analysis showed
that mice in the ID group had severe bone loss, thin and
disorganized bone trabeculae and enlarged bone marrow cavities
compared with the control group. By contrast, after gavage of ARC,
the bone mass of mice was restored to some extent and approached
that of the control group (Fig. 1B
and C). The data from the micro-CT scans were then analyzed,
and the two ARC groups exhibited an increase in BV/TV, Tb.Th and
Tb.N, and a decrease in SMI, Tb.Sp and Tb.Pf compared with those in
the ID group (Fig. 1E). These
results were promising and some of the effects were even better in
the ARC-H group than in the control group. Furthermore, after ARC
treatment, H&E staining showed a more pronounced reduction in
adipocytes, recovery of the bone marrow cavity and more orderly
bone trabeculae (Fig. 1D).
Immunohistochemistry can detect the effects of drugs on in
vivo indicators, and the present study examined Runx2 and Col
I, which promote osteogenesis (32,33). The results showed that mice in the
ARC groups exhibited increased Runx2 and Col I expression in the
bone trabeculae and bone marrow compared with that in the ID group
(Fig. 1F). These results
suggested that ARC may have the ability to counteract bone loss
caused by iron overload.
ARC dose-dependently affects the
viability of MC3T3-E1 cells
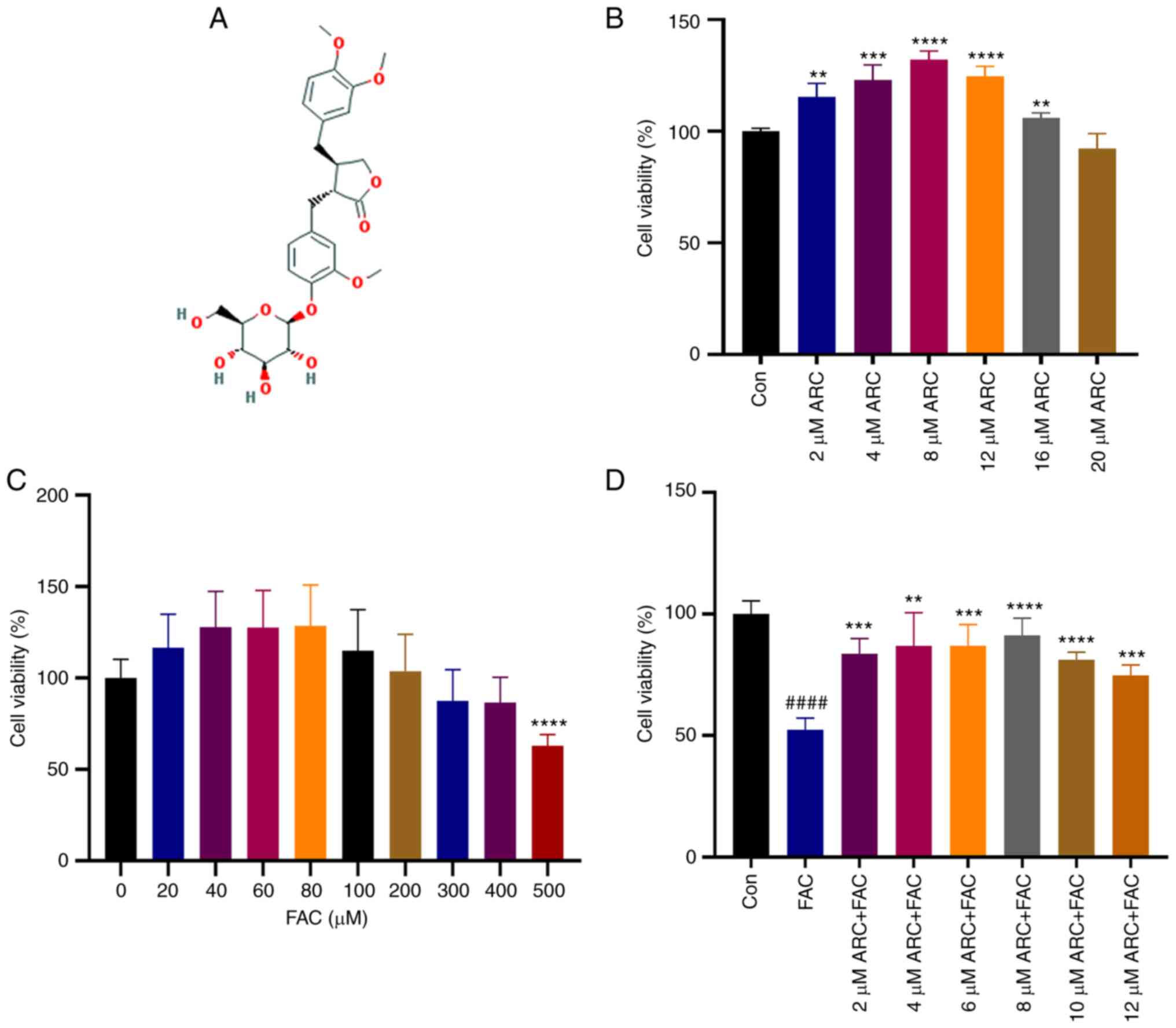
The chemical structural formula of ARC is shown in
Fig. 2A. The effect of ARC on
MC3T3-E1 cell viability was examined using the CCK8 assay, and the
results showed that MC3T3-E1 cells exhibited the best viability
when the concentration of ARC was between 2 and 12 μM
(Fig. 2B); in this concentration
range, ARC had a clear dose-dependent effect on viability. Notably,
slight toxicity to cells was observed when the ARC concentration
exceeded 16 μM. In addition, MC3T3-E1 cells were treated
with different concentrations of FAC to create a model of
intracellular iron overload. The results showed that low doses of
FAC were able to promote cell viability though significance was not
reached, whereas cell viability began to decline when FAC
concentration exceeded 200 μM, and decreased by ~50% when
FAC concentration reached 500 μM. Therefore, 500 μM
FAC was selected as the appropriate concentration for intracellular
iron overload (Fig. 2C). After
the iron overload model was constructed, cell viability was
significantly decreased, and this decrease was reversed by ARC at
concentrations of 2-8 μM (Fig.
2D). Based on these results, it was indicated that ARC may be
able to affect cellular viability in a dose-dependent manner.
ARC reduces intracellular iron
concentration
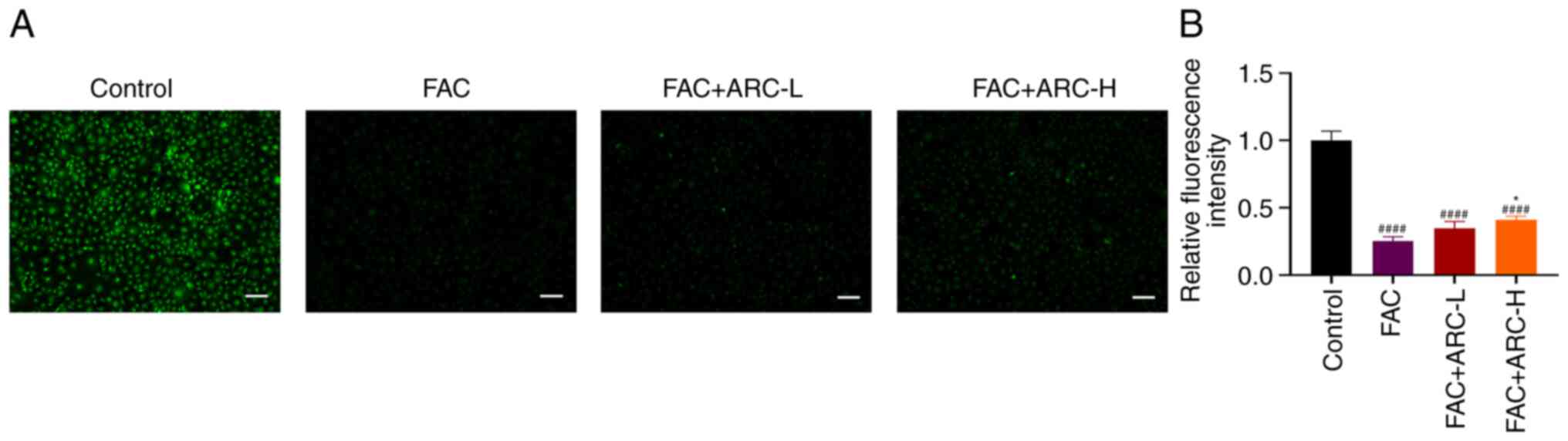
Intracellular iron concentration was examined
according to the method of Ma et al (34). Intracellular Fe3+ is
able to quench the fluorescent system by complexing with calcein
AM. Therefore, there is an inverse relationship between
intracellular iron ion concentration and fluorescence intensity.
Compared with that in the control group, the intensity of cellular
fluorescence was greatly reduced in the FAC group, suggesting a
significant increase in intracellular iron content. ARC was able to
promote intracellular iron ion excretion. However, iron
concentrations in the ARC group were still significantly higher
compared with those in the control group (Fig. 3A and B). The fluorescence
intensity of the FAC group was markedly lower compared with that of
the FAC + ARC-L/H group, suggesting that more Fe3+ was
present in the FAC group. This finding suggested that although ARC
could reduce iron accumulation in iron-overloaded MC3T3-E1 cells,
it is difficult to restore iron concentration to normal levels.
In vivo, serum iron concentrations in mice in the ID group
were similarly significantly higher compared with those in the
control group. Serum iron concentrations in mice in the ID + ARC
group were lower compared with those in the ID group, even though
it was difficult to restore them to normal group levels (Fig. S1).
ARC attenuates the inhibitory effect of
iron overload on the osteogenesis of MC3T3-E1 cells
To investigate the effect of ARC on the osteogenesis
of MC3T3-E1 cells, cells were cultured for 7 days in osteoblast
differentiation medium containing 1 μM dexamethasone, 100
μg/ml ascorbic acid and 10 mM β-glycerophosphate with ARC (4
or 8 μM) with or without FAC at 37°C. Staining with the
BCIP/NBT ALP Staining Kit showed that FAC significantly inhibited
the osteogenic potential of MC3T3-E1 cells; although the difference
in effect between the two concentrations of ARC was not
significant, both were able to significantly reverse the effects of
FAC (Fig. 4A and D).
Subsequently, cells were cultured for 21 days and the formation of
calcium deposits was assessed by ARS staining; the obtained results
were consistent with those of ALP staining (Fig. 4B and E). The expression levels of
osteogenic-related proteins (Runx2 and Col I) were then detected in
cells cultured with normal α-MEM by western blotting. The
expression levels of Col I and Runx2 were significantly reduced in
the model group, a trend that was alleviated after ARC treatment
(Fig. 4C, F and G). Based on
these results, it may be suggested that ARC could reduce the iron
overload-induced inhibition of MC3T3-E1 cell osteogenesis.
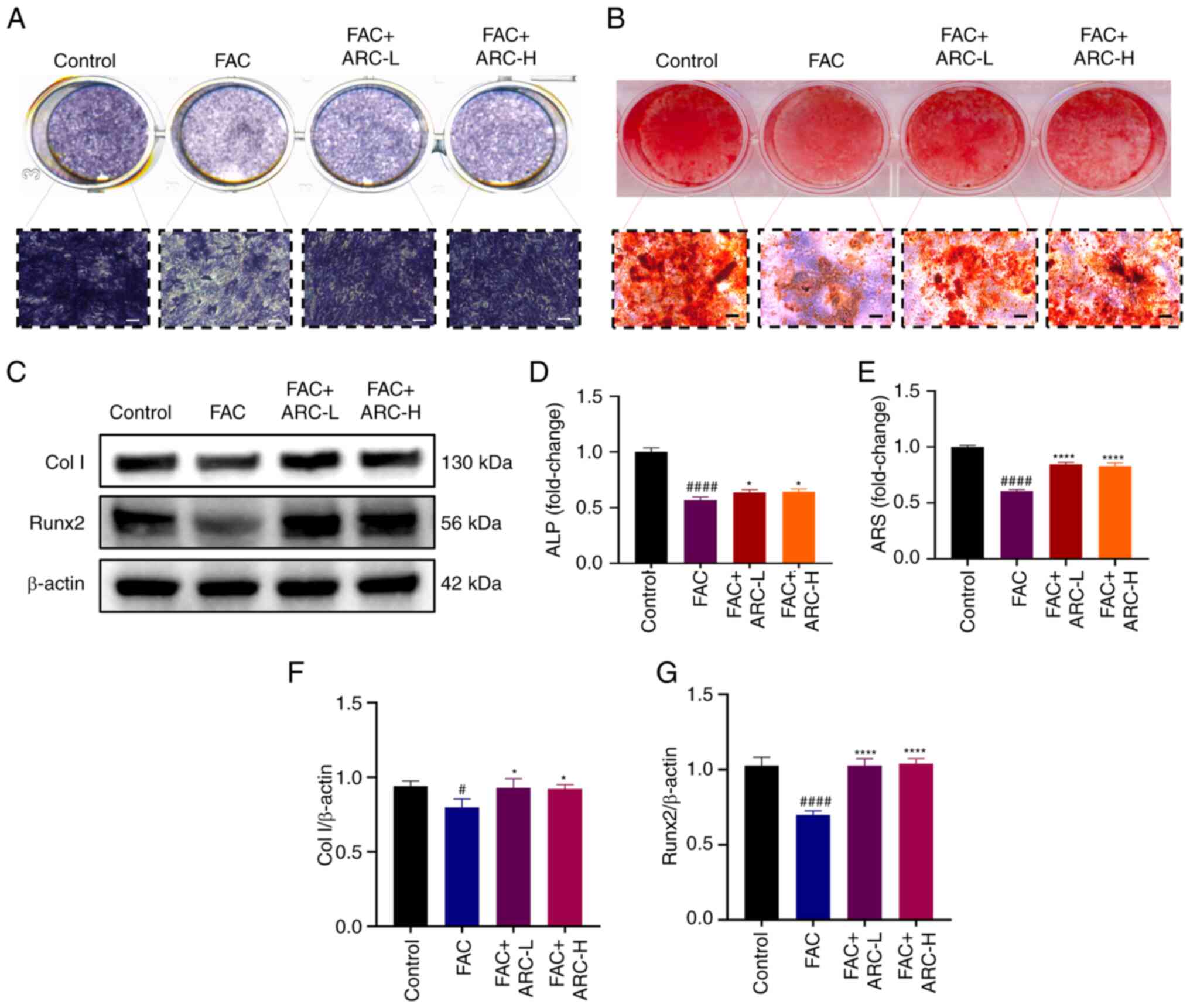 | Figure 4ARC attenuates the inhibitory effect
of iron overload on MC3T3-E1 cell osteogenesis. After FAC
treatment, with or without ARC treatment, MC3T3-E1 cells were
cultured in an osteogenic induction medium containing FAC/ARC
treatment and stained with (A) ALP or (B) ARS on days 7 and 21,
respectively. Scale bar, 100 μm. (C) MC3T3-E1 cells were
harvested after treatment with FAC with or without ARC intervention
for 48 h, before Col I and Runx2 expression levels were measured by
western blotting. (D) Ratio of area of purple nodules of calcium
phosphate to total area after staining. (E) Mineralized nodules
were determined by calculating the ratio of mineralized area to
total area after staining. Expression levels of (F) Col I and (G)
Runx2 were semi-quantified by western blotting. Data are presented
as the mean ± SD. ****P<0.0001 and
*P<0.05 vs. Mod; ####P<0.0001 and
#P<0.05 vs. Con. ALP, alkaline phosphatase; ARC,
arctiin; ARC-H, ARC-high; ARC-L, ARC-low; ARS, Alizarin Red S; Col
I, collagen type I; Con, control; FAC, ferric ammonium citrate;
Mod, model (500 μM FAC); Runx2, Runt-related transcription
factor 2. |
ARC reduces iron overload-induced
apoptosis in MC3T3-E1 cells
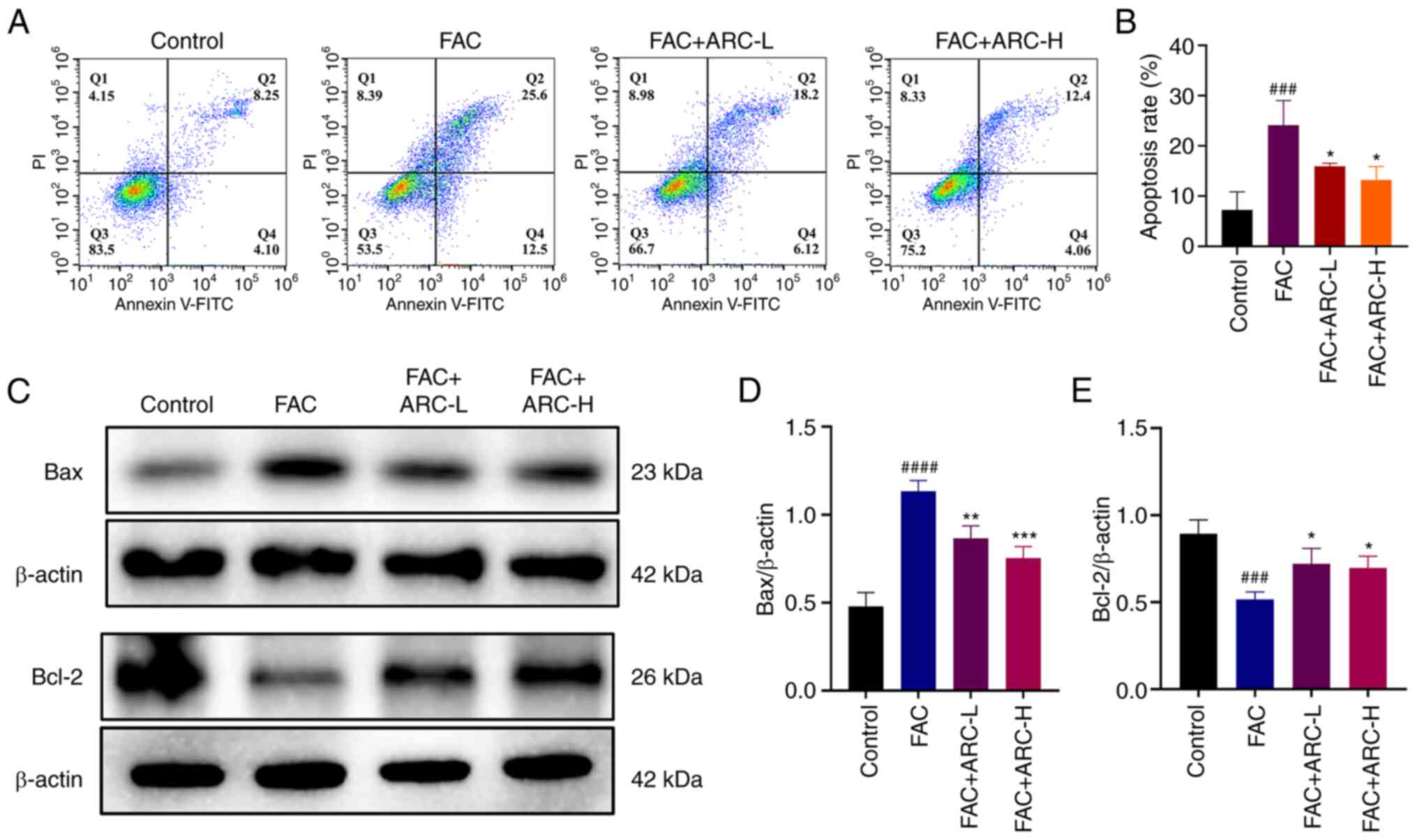
Annexin V-FITC/PI staining was performed to further
verify whether ARC had a protective effect against apoptosis in
MC3T3-E1 cells following high-dose FAC treatment. The results
showed that apoptosis was markedly increased in the iron overload
model compared with that in the control group, with the rate of
late apoptosis increasing from ~8 to ~25% of cells (Fig. 5A and B). By contrast, ARC
treatment, especially at a high concentration, was able to reduce
the apoptotic rate of cells by ~50% compared with that in the model
group, thus significantly attenuating the apoptotic effect of iron
overload on MC3T3-E1 cells. The detection of apoptosis marker
proteins Bcl-2 and Bax by western blotting further verified that
Bcl-2 expression was reduced and Bax expression increased following
intervention with FAC, whereas ARC was able to reverse these
effects (Fig. 5C-E). Based on
these results, it was indicated that ARC was able to attenuate the
apoptosis of MC3T3-E1 cells caused by excess FAC.
ARC reduces iron overload-induced
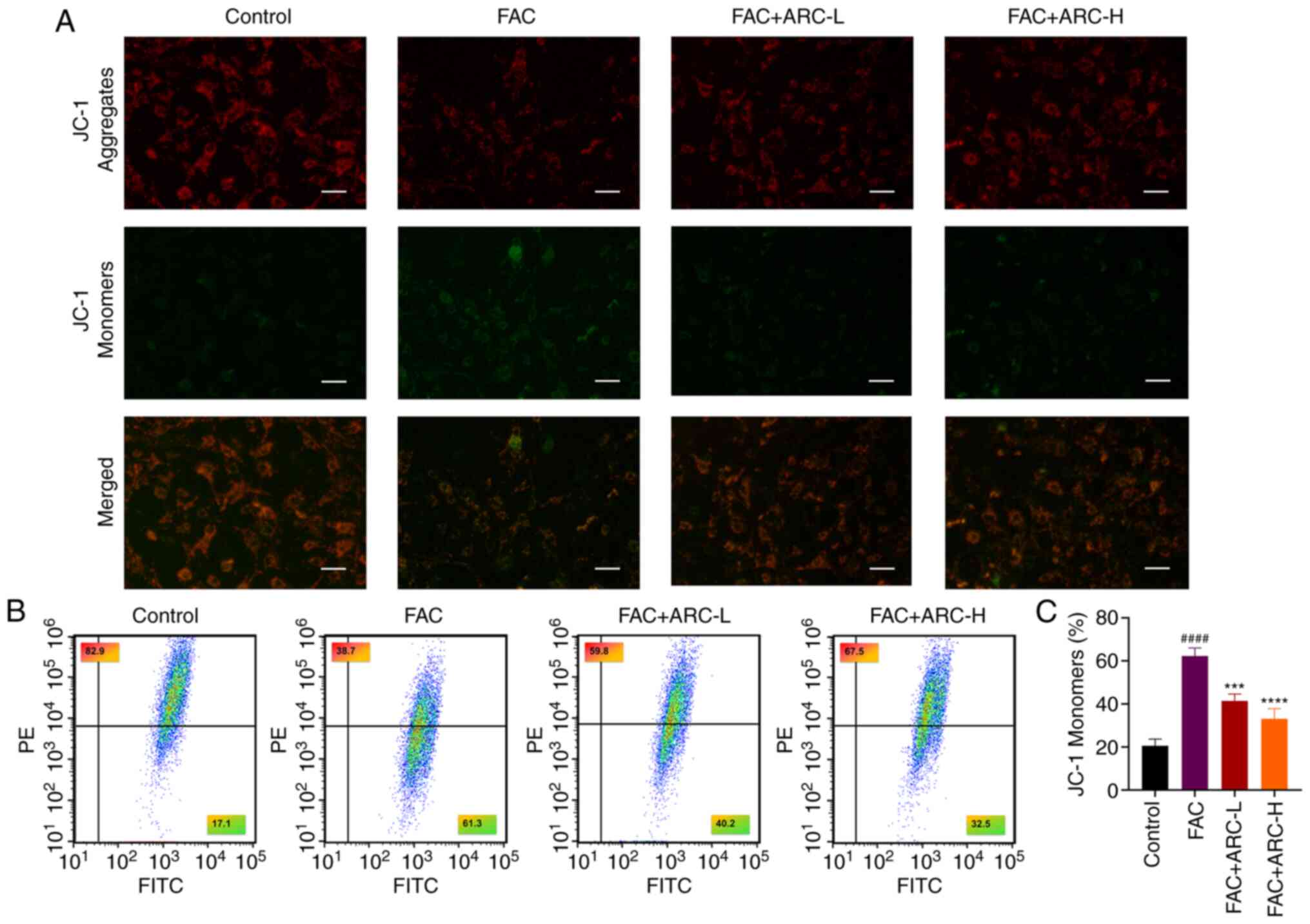
mitochondrial damage
When cells are exposed to stimuli (including the
accumulation of oxidants), mitochondria inevitably initiate a
stress response. The ability to respond appropriately to this
stimulus determines two different consequences for mitochondria.
When mitochondrial function is dysfunctional, it affects the MMP
and accelerates apoptosis (11).
Therefore, the present study next examined MMP using the JC-1 assay
to assess mitochondrial function and to determine whether ARC
inhibits MMP collapse. The results showed that after induction of
iron overload, the green fluorescence intensity of JC-1 monomers
increased and the red fluorescence intensity of JC-1 aggregates
decreased, indicating that MMP was decreased and mitochondrial
function was impaired at this time (Fig. 6A). Notably, when mitochondrial
function is in a dysregulated state, this indicates that the cell
is in a dysregulated state of oxidant accumulation. Notably, the
results of the ARC-L group and ARC-H group were similar to those of
the control group, suggesting that a high concentration of ARC was
able to improve these conditions. The present study verified the
changes in MMP by flow cytometry; the obtained results were
consistent with those observed under microscopy (Fig. 6B and C). Taken together, ARC
improved the mitochondrial function affected by excess FAC.
ARC activates the PI3K/Akt pathway in
iron overload-induced MC3T3-E1 cells
The effect of ARC on the PI3K/Akt pathway in
MC3T3-E1 cells after FAC treatment was detected by western blotting
and immunofluorescence. Notably, PI3K/Akt pathway phosphorylation
was significantly inhibited after FAC treatment; however, ARC was
able to significantly activate their phosphorylation (Fig. 7A and B). Subsequent in
vitro immunofluorescence and in vivo
immunohistochemistry analyses of p-PI3K verified these results
(Fig. 7C and D).
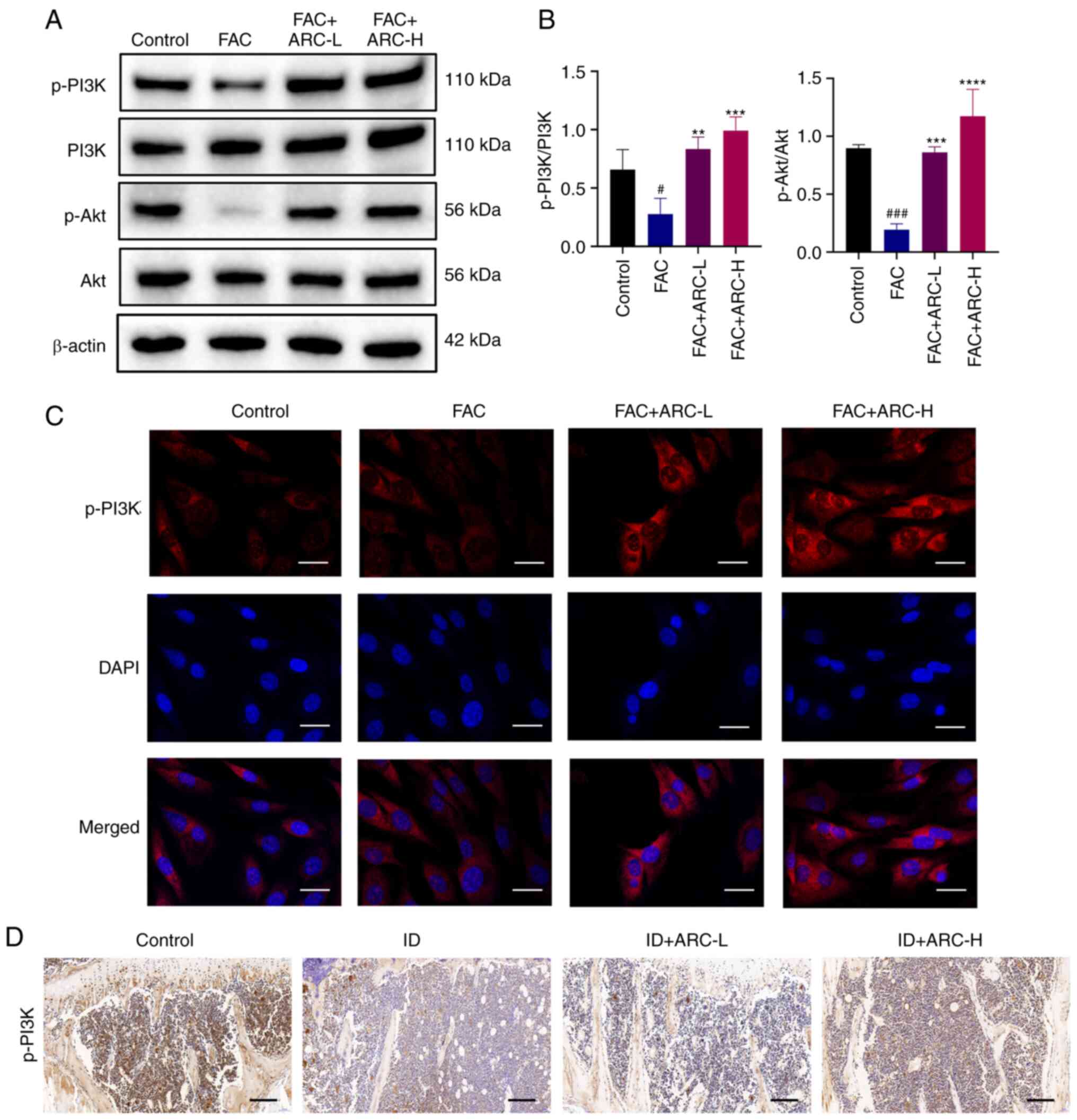 | Figure 7ARC induces activation of the
PI3K/Akt pathway in iron overload-induced MC3T3-E1 cells. (A)
Expression levels of p-PI3K/PI3K and Akt/p-Akt were analyzed by
western blotting. (B) Statistical analysis of blots. (C)
Immunofluorescence analysis of p-PI3K (red) and DAPI-stained
nuclear fluorescence (blue) were visualized by confocal microscopy.
Scale bar, 5 μm. (D) Thin layers of tibial specimens were
immunohistochemically stained for p-PI3K. Scale bar, 0.2 mm. Data
are presented as the mean ± SD. ****P<0.0001,
***P<0.001 and **P<0.01 vs. Mod;
###P<0.001 and #P<0.05 vs. Con. Con.
ARC, arctiin; ARC-H, ARC-high; ARC-L, ARC-low; Con, control; FAC,
ferric ammonium citrate; ID, dextran iron; Mod, model (500
μM FAC); p-, phosphorylated. |
To further validate the effect of ARC on the
PI3K/Akt pathway, the PI3K/Akt pathway inhibitor LY294002 was used
for reverse validation. Following treatment with LY294002, the
ARC-induced phosphorylation of the PI3K/Akt pathway, and expression
of the osteogenic marker proteins Col I and Runx2 and
anti-apoptotic protein Bcl-2 were again inhibited (Fig. 8A-D). By contrast, the apoptosis
marker protein Bax, which was reduced by ARC, was increased by
LY294002 (Fig. 8C and D).
Subsequently, the osteogenic capacity of cells was assessed, and
ARS staining showed that treatment with LY294002 further reduced
the ability of MC3T3-E1 cells to generate calcified nodules
(Fig. 8E and G). Apoptosis was
also detected. Notably, the apoptotic rate of cells was again
elevated in the presence of LY294002 (Fig. 8F and H). These findings indicated
that the anti-apoptotic and bone-producing ability of ARC was
clearly diminished following treatment with a PI3K inhibitor. Based
on this, ARC may be able to treat apoptosis and osteogenic
differentiation of cells under iron overload by regulating the
PI3K/Akt pathway.
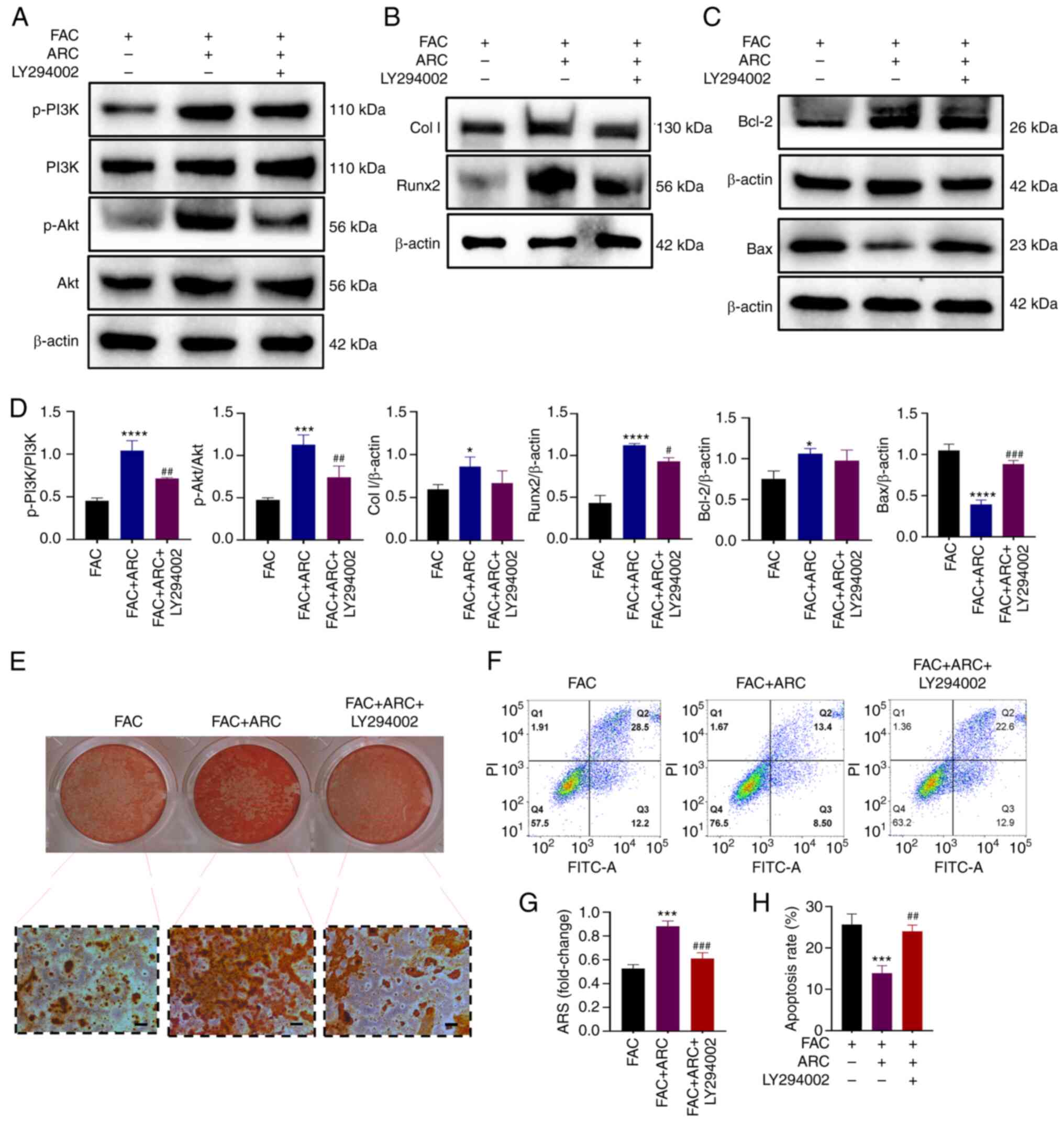 | Figure 8PI3K pathway inhibitors can impair
the anti-apoptotic and bone-producing abilities of ARC. (A)
Expression levels of p-PI3K/PI3K, Akt/p-Akt were analyzed by
western blotting. (B) Expression levels of Col I and Runx2 were
analyzed by western blotting. (C) Expression levels of Bcl-2 and
Bax were analyzed by western blotting. (D) Statistical analysis of
blots. (E) After FAC treatment, with or without ARC and LY294002,
MC3T3-E1 cells were cultured in an osteogenic induction medium
containing FAC/ARC and stained with ARS on day 21. Scale bar, 100
μm. (F) Cells were treated with FAC for 48 h with or without
ARC and LY294002 intervention, after which Annexin V-FITC/PI
staining was performed to detect apoptosis. (G) Mineralized nodules
were quantified by calculating the ratio of mineralized area to
total area after staining. (H) Statistical analysis of percentage
of late apoptosis (Q2) in (F) Data are presented as the mean ± SD.
****P<0.0001, ***P<0.001 and
*P<0.05 vs. FAC; ###P<0.001,
##P<0.01 and #P<0.05 vs. FAC + ARC.
ARC, arctiin; ARS, Alizarin Red S; Col I, collagen type I; FAC,
ferric ammonium citrate; p-, phosphorylated; Runx2, Runt-related
transcription factor 2. |
ARC reduces the accumulation of
intracellular ROS and LPO caused by iron overload
The aforementioned findings confirm the ability of
ARC to alleviate iron overload-induced apoptosis through the
PI3K/Akt pathway; however, iron overload is also known to increase
intracellular ROS and LPO through the Fenton response, causing
cytotoxic effects and further inducing apoptosis. The present study
subsequently investigated the effect of ARC on oxidation in cells
with iron overload. A DCFH-DA probe was used to detect the
accumulation of ROS. Based on microscopic observations and flow
cytometry measurements, the fluorescence intensity was
significantly higher following high-dose FAC treatment, thus
demonstrating the accumulation of ROS under iron overload, whereas
ARC was able to reduce this in a dose-dependent manner (Fig. 9A, D and E).
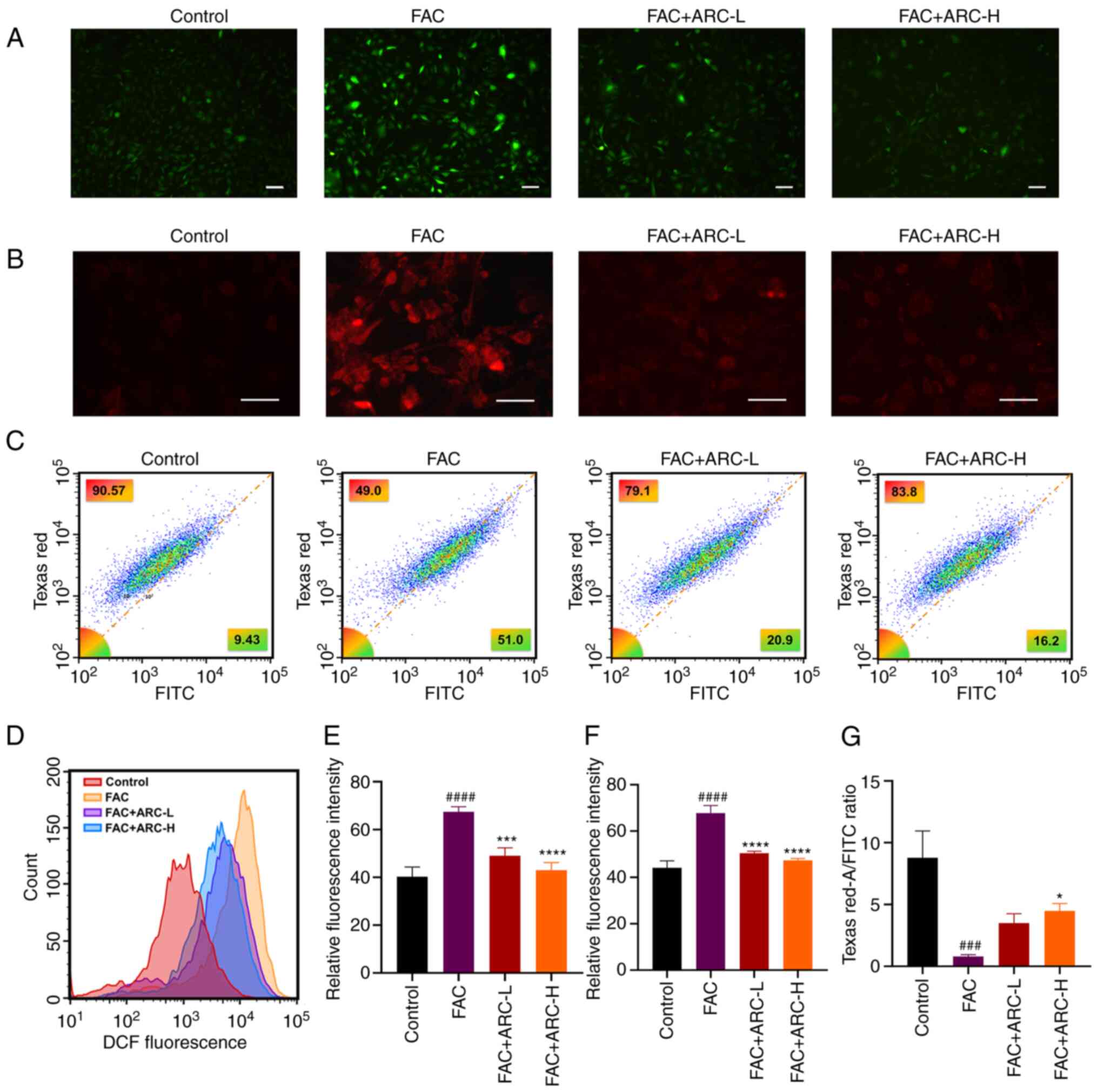 | Figure 9ARC reduces the accumulation of
intracellular reactive oxygen species and LPO due to iron overload.
(A) Intracellular ROS levels were detected by microscopy. Scale
bar, 100 μm. (B) MC3T3-E1 cells were incubated with the
MitoSOX Red Mitochondrial Superoxide Indicator and visualized by
confocal microscopy. Scale bar, 50 μm. (C) Intracellular LPO
levels were detected by flow cytometry after staining with the C11
BODIPY fluorescent probe. (D) Intracellular ROS levels were
detected by flow cytomrtry. (E) Statistical analysis of (A). (F)
Statistical analysis of staining. (G) Semi-quantification of the
ratio of LPO content Texas Red-A/FITC by flow cytometry. Data are
presented as the mean ± SD. ****P<0.0001,
***P<0.001 and *P<0.05 vs. Mod;
####P<0.0001 and ###P<0.001 vs. Con.
ARC, arctiin; ARC-H, ARC-high; ARC-L, ARC-low; Con, control; FAC,
ferric ammonium citrate; LPO, lipid peroxidation; Mod, model (500
μM FAC). |
Cells were also detected with the MitoSOX Red
Mitochondrial Superoxide Indicator Probe in the same manner, to
detect the accumulation of mtROS, and similar results to those
obtained with the ROS assay were determined (Fig. 9B and F).
Intracellular LPO content under iron overload was
detected by flow cytometry, and was revealed to be significantly
increased by FAC compared with that in the control and ARC groups
(Fig. 9C and G). These
experimental results suggested that ARC may directly reduce the
intracellular levels of ROS, mtROS and LPO, and improve cell
survival.
Discussion
Trace amounts of iron are essential for cell
survival; however, an excess of iron promotes the generation of
ROS, leading to cellular apoptosis (35). Iron overload is a pivotal factor
contributing to the development of osteoporosis. It disrupts
cellular redox systems, hampers bone remodeling, and impacts bone
quality, microstructure and biomechanical properties, ultimately
resulting in bone loss and fractures (36-38). It has previously been reported
that ARC can stimulate the differentiation of MC3T3-E1 cells by
modulating cell cycle proteins (27); however, to the best of our
knowledge, its effects in the context of IOOP have not been
reported. The present study provided the first in vivo
evidence of the significant role of ARC in IOOP therapy using an
iron overload animal model created through intraperitoneal
injection of dextran iron. ARC effectively enhanced bone trabeculae
and histological parameters in mice subjected to excess dextran
iron. Additionally, by treating MC3T3-E1 cells with FAC an in
vitro iron overload cell model was also established, and it was
observed that ARC not only promoted ALP production and the
formation of calcified nodules, but also increased the expression
levels of proteins associated with osteogenic differentiation.
According to these in vivo and in vitro
osteogenesis-promoting phenotypic findings, the present study next
proceeded to the next step of mechanistic validation. Endogenous
apoptosis predominantly occurs through the mitochondrial and
endoplasmic reticulum pathways (39,40). When a cell encounters internal
apoptosis-inducing factors, such as DNA damage, it can activate the
mitochondrial apoptosis pathway, ultimately leading to apoptosis
(40). In this pathway, Bcl-2
family proteins regulate mitochondrial outer membrane permeability
by modulating membrane potential (41). Upon receiving apoptotic signals,
Bax relocalizes to the mitochondrial surface, reducing membrane
potential and releasing apoptotic factors. The PI3K/Akt signaling
pathway regulates Bax and other Bcl-2 family proteins (42,43), signifying a close interplay
between mitochondrial dysfunction and the PI3K/Akt pathway. Several
reports have shown that activation of the PI3K/Akt pathway promotes
osteoblast proliferation and differentiation, and mitigates
apoptosis and ferroptosis in MC3T3-E1 cells (17,44,45). Abdurahman et al (46) demonstrated in a mouse model of
osteoporosis that activating the PI3K/Akt pathway enhances
osteogenesis by increasing angiogenesis. In the present study, ARC
successfully restored the diminished MMP caused by excess iron and
subsequently mitigated the apoptosis induced by damaged
mitochondria. ARC also elevated the phosphorylation levels of PI3K
and AKT. To validate these findings, LY294002, a PI3K inhibitor,
was used. Western blotting showed that LY294002 again inhibited the
ARC-activated PI3K/Akt pathway, reversed the increased expression
levels of Col I, Runx2 and Bcl-2 proteins induced by ARC, in
addition to reversing the decreased Bax expression induced by ARC.
Although ARC was effective in promoting osteogenesis and inhibiting
apoptosis, its efficacy was significantly diminished or disappeared
following treatment with LY294002. Similarly, as determined by
immunohistochemistry, p-PI3K and Runx2 were more broadly
distributed in the bone trabeculae of ARC-treated mice. Therefore,
these findings indicated that ARC exerts its anti-apoptotic and
osteogenic effects by activating the PI3K/Akt pathway.
Oxidative stress involves multiple factors, with
excess iron being a significant contributor. Iron-sulfur clusters,
a primary source of intracellular ROS, primarily interact with
ROS-producing enzymes, such as NADPH oxidase and xanthine oxidase,
to regulate ROS levels (47).
When the body experiences iron overload, these enzymes are
substantially upregulated, leading to elevated levels of ROS and,
ultimately, impaired cellular function and death. Furthermore, ROS
generation is facilitated by iron through Fenton reactions
(48). Deng et al
(19) demonstrated that increased
ROS content inhibits the activation of the PI3K/Akt signaling
pathway in osteoblasts. Notably, the ROS-PI3K/AKT cascade response
can lead to apoptosis (49). The
present study established that ARC can regulate apoptosis and
promote osteogenesis via the PI3K/Akt pathway. However, oxidative
accumulation is equally capable of inducing cell death,
particularly in the context of iron overload characterized by
intracellular oxidative accumulation. Therefore, the present study
further investigated the oxidative stress phenotype. It was
observed that ARC could reduce intracellular oxidative stress
induced by iron overload, thereby decreasing the production of ROS,
mtROS and LPO; this may directly slow the rate of apoptosis through
its antioxidant capacity.
Although the present findings suggested that ARC
could promote iron excretion in vivo and in vitro,
this effect was not significant. These findings indicated that ARC
counteracts the adverse effects of iron overload primarily through
other mechanisms, as explored in the present study. Additionally,
although ARC can activate the PI3K/Akt signaling pathway and
exhibit antioxidant effects, the present study did not investigate
the relationship between them in-depth, thus further exploration is
required. Finally, the present study was not able to conduct
further evaluation of the effects of oral ARC, as well as
injectable iron dextran, on other organs in the body, such as the
liver and kidney. This is a limitation of the present study, as it
could not be determined whether ARC has a therapeutic effect or
exhibits dose toxicity on these organs.
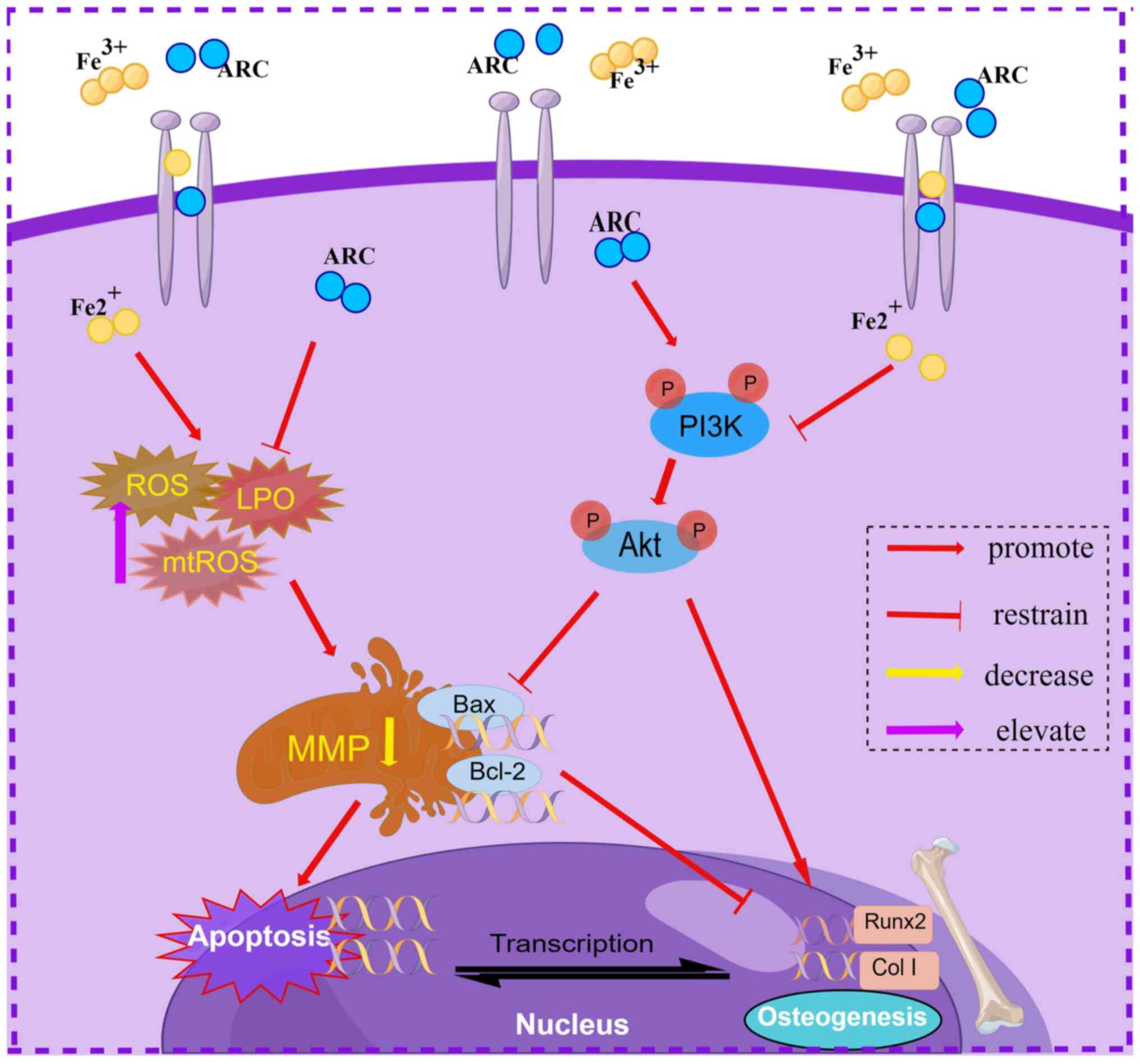
In conclusion, the present study demonstrated that
ARC has potential in mitigating IOOP in vitro and in
vivo. ARC was shown to interact with the PI3K/Akt pathway,
enabling anti-apoptotic properties and facilitating bone
differentiation. In addition, it could directly mitigate iron
overload-induced oxidative stress, shielding cells from damage
(Fig. 10). These findings
indicated that ARC may present a novel therapeutic approach for
addressing IOOP.
Supplementary Data
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
ML conceptualized the study, participated in data
analysis and wrote the original manuscript. ZP participated in data
analysis and organization, wrote and reviewed the original
manuscript and conceptualized the study. QH conceived the study,
participated in writing and reviewing the original manuscript. ML,
ZP and QH co-conceived the study, adapted the experimental ideas
based on the results, wrote the manuscript, participated in data
analysis and organization, and made critical revisions to the
manuscript, as well as directing the animal and cell experiments.
JX was responsible for animal experiments, data analysis and
organization. BC was responsible for flow cytometry experiments and
fluorescence experiments, data analysis and organization. FW was
responsible for cell culture and fluorescence experiments, data
analysis and organization. PK was responsible for cell induction
experiments, in data analysis and organization. HL was responsible
for cell protein imprinting experiments, data analysis and
organization. JL was responsible for cell protein imprinting
experiments, participating in data analysis and organization. JZ
was responsible for western blotting experiments and participated
in data analysis and organization. SL was responsible for animal
experiments and participated in data analysis and organization. JY
was responsible for animal experiments and participated in data
analysis and organization. HW and CZ conceived the study, wrote,
reviewed the original manuscript and was responsible for obtaining
funding. All authors read and approved the final manuscript. ML and
ZP confirm the authenticity of all the raw data.
Ethics approval and consent to
participate
The present study was approved by the Ethics
Committee of Guangzhou University of Chinese Medicine (approval no.
TCMF1-2021029; May 20, 2021).
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
Abbreviations:
|
ALP
|
alkaline phosphatase
|
|
ARC
|
arctiin
|
|
ARS
|
Alizarin Red S
|
|
BV/TV
|
bone volume/tissue volume
|
|
Col I
|
collagen type I
|
|
FAC
|
ferric ammonium citrate
|
|
IOOP
|
iron overload-induced
osteoporosis
|
|
LPO
|
lipid peroxidation
|
|
MMP
|
mitochondrial membrane potential
|
|
mtROS
|
mitochondrial reactive oxygen
species
|
|
Runx2
|
Runt-related transcription factor
2
|
|
SMI
|
structure model index
|
|
Tb.N
|
trabecular bone number
|
|
Tb.Pf
|
trabecular bone pattern factor
|
|
Tb.Sp
|
trabecular separation/spacing
|
|
Tb.Th
|
trabecular thickness
|
|
micro-CT
|
micro-computed tomography
|
Acknowledgments
Not applicable.
Funding
The present study was supported by the National Natural Science
Foundation of China (grant no. 82074462), the Major Projects of
Guangdong Education Department for Foundation Research and Applied
Research (grant no. 2021KTSCX021), the Guangzhou Municipal Science
and Technology Project (grant no. 202201020314) and the
Collaborative Innovation Team Project of Guangzhou University of
Chinese Medicine (grant no. 2021xk53).
References
|
1
|
Li Y, Bai B and Zhang Y: Bone
abnormalities in young male rats with iron intervention and
possible mechanisms. Chem Biol Interact. 279:21–26. 2018.
View Article : Google Scholar
|
|
2
|
Manolagas SC: From estrogen-centric to
aging and oxidative stress: A revised perspective of the
pathogenesis of osteoporosis. Endocr Rev. 31:266–300. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Harrison PM, Fischbach FA, Hoy TG and
Haggis GH: Ferric oxyhydroxide core of ferritin. Nature.
216:1188–1190. 1967. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Li GF, Pan YZ, Sirois P, Li K and Xu YJ:
Iron homeostasis in osteoporosis and its clinical implications.
Osteoporos Int. 23:2403–2408. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Tsay J, Yang Z, Ross FP,
Cunningham-Rundles S, Lin H, Coleman R, Mayer-Kuckuk P, Doty SB,
Grady RW, Giardina PJ, et al: Bone loss caused by iron overload in
a murine model: Importance of oxidative stress. Blood.
116:2582–2589. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Chen B, Yan YL, Liu C, Bo L, Li GF, Wang H
and Xu YJ: Therapeutic effect of deferoxamine on iron
overload-induced inhibition of osteogenesis in a zebrafish model.
Calcif Tissue Int. 94:353–360. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Stockwell BR, Friedmann Angeli JP, Bayir
H, Bush AI, Conrad M, Dixon SJ, Fulda S, Gascón S, Hatzios SK,
Kagan VE, et al: Ferroptosis: A regulated cell death nexus linking
metabolism, redox biology, and disease. Cell. 171:273–285. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Nishizaki D and Iwahashi H: Baicalin
inhibits the fenton reaction by enhancing electron transfer from Fe
(2+) to dissolved oxygen. Am J Chin Med. 43:87–101. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Gao M, Yi J, Zhu J, Minikes AM, Monian P,
Thompson CB and Jiang X: Role of mitochondria in ferroptosis. Mol
Cell. 73:354–363.e3. 2019. View Article : Google Scholar :
|
|
10
|
Feng H and Stockwell BR: Unsolved
mysteries: How does lipid peroxidation cause ferroptosis? PLoS
Biol. 16:e20062032018. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Hamacher-Brady A: CMLS forum reviews:
Mitochondrial damage control. Cell Mol Life Sci. 78:3763–3765.
2021. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Yin N, Zhu L, Ding L, Yuan J, Du L, Pan M,
Xue F and Xiao H: MiR-135-5p promotes osteoblast differentiation by
targeting HIF1AN in MC3T3-E1 cells. Cell Mol Biol Lett. 24:512019.
View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Izumiya M, Haniu M, Ueda K, Ishida H, Ma
C, Ideta H, Sobajima A, Ueshiba K, Uemura T, Saito N and Haniu H:
Evaluation of MC3T3-E1 cell osteogenesis in different cell culture
Media. Int J Mol Sci. 22:77522021. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Karar J and Maity A: PI3K/AKT/mTOR Pathway
in Angiogenesis. Front Mol Neurosci. 4:512011. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Yang Q, Jiang W and Hou P: Emerging role
of PI3K/AKT in tumor-related epigenetic regulation. Semin Cancer
Biol. 59:112–124. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Xu F, Na L, Li Y and Chen L: Roles of the
PI3K/AKT/mTOR signalling pathways in neurodegenerative diseases and
tumours. Cell Biosci. 10:542020. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Hao J, Bei J, Li Z, Han M, Ma B, Ma P and
Zhou X: Qing'e Pill inhibits osteoblast ferroptosis via ATM
serine/threonine kinase (ATM) and the PI3K/AKT pathway in primary
osteoporosis. Front Pharmacol. 13:9021022022. View Article : Google Scholar
|
|
18
|
Chen L, Liu P, Feng X and Ma C:
Salidroside suppressing LPS-induced myocardial injury by inhibiting
ROS-mediated PI3K/Akt/mTOR pathway in vitro and in vivo. J Cell Mol
Med. 21:3178–3189. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Deng S, Dai G, Chen S, Nie Z, Zhou J, Fang
H and Peng H: Dexamethasone induces osteoblast apoptosis through
ROS-PI3K/AKT/GSK3β signaling pathway. Biomed Pharmacother.
110:602–608. 2019. View Article : Google Scholar
|
|
20
|
Cai E, Han J, Yang L, Zhang W, Zhao Y,
Chen Q, Guo M and He X: Novel method of preparation and activity
research on arctigenin from fructus arctii. Pharmacogn Mag.
14:87–94. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Gao Q, Yang M and Zuo Z: Overview of the
anti-inflammatory effects, pharmacokinetic properties and clinical
efficacies of arctigenin and arctiin from Arctium lappa L. Acta
Pharmacol Sin. 39:787–801. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Liu X, Wang J, Dou P, Zhang X, Ran X, Liu
L and Dou D: The ameliorative effects of arctiin and arctigenin on
the oxidative injury of lung induced by Silica via
TLR-4/NLRP3/TGF-β signaling pathway. Oxid Med Cell Longev.
2021:55989802021.
|
|
23
|
Hyam SR, Lee IA, Gu W, Kim KA, Jeong JJ,
Jang SE, Han MJ and Kim DH: Arctigenin ameliorates inflammation in
vitro and in vivo by inhibiting the PI3K/AKT pathway and polarizing
M1 macrophages to M2-like macrophages. Eur J Pharmacol. 708:21–29.
2013. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Lee CY, Hsin MC, Chen PN, Lin CW, Wang PH,
Yang SF and Hsiao YH: Arctiin Inhibits cervical cancer cell
migration and invasion through suppression of S100A4 expression via
PI3K/Akt pathway. Pharmaceutics. 14:3652022. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Zhou M, Li G, Zhu L, Zhou H and Lu L:
Arctiin attenuates high glucose-induced human retinal capillary
endothelial cell proliferation by regulating
ROCK1/PTEN/PI3K/Akt/VEGF pathway in vitro. J Cell Mol Med.
24:5695–5706. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Chen D, Ye Z, Wang C, Wang Q, Wang H, Kuek
V, Wang Z, Qiu H, Yuan J, Kenny J, et al: Arctiin abrogates
osteoclastogenesis and bone resorption via suppressing
RANKL-induced ROS and NFATc1 activation. Pharmacol Res.
159:1049442020. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Liu Z and Wu Y: Arctiin elevates
osteogenic differentiation of MC3T3-E1 cells by modulating cyclin
D1. Bioengineered. 13:10866–10874. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Zhou B, Weng G, Huang Z, Liu T and Dai F:
Arctiin prevents LPS-Induced acute lung injury via inhibition of
PI3K/AKT signaling pathway in mice. Inflammation. 41:2129–2135.
2018. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Li G, Park JN, Park HJ, Suh JH and Choi
HS: High cholesterol-induced bone loss is attenuated by arctiin via
an action in osteoclasts. Nutrients. 14:44832022. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
He Q, Yang J, Pan Z, Zhang G, Chen B, Li
S, Xiao J, Tan F, Wang Z, Chen P and Wang H: Biochanin A protects
against iron overload associated knee osteoarthritis via regulating
iron levels and NRF2/System xc-/GPX4 axis. Biomed Pharmacother.
157:1139152023. View Article : Google Scholar
|
|
31
|
Fernández I, Peña A, Del Teso N, Pérez V
and Rodríguez-Cuesta J: Clinical biochemistry parameters in
C57BL/6J mice after blood collection from the submandibular vein
and retroorbital plexus. J Am Assoc Lab Anim Sci. 49:202–206.
2010.PubMed/NCBI
|
|
32
|
Long JR, Liu PY, Lu Y, Xiong DH, Zhao LJ,
Zhang YY, Elze L, Recker RR and Deng HW: Association between COL1A1
gene polymorphisms and bone size in Caucasians. Eur J Hum Genet.
12:383–388. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Kim WJ, Shin HL, Kim BS, Kim HJ and Ryoo
HM: RUNX2-modifying enzymes: Therapeutic targets for bone diseases.
Exp Mol Med. 52:1178–1184. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Ma Y, Abbate V and Hider RC:
Iron-sensitive fluorescent probes: Monitoring intracellular iron
pools. Metallomics. 7:212–222. 2015. View Article : Google Scholar
|
|
35
|
Andrews NC: Disorders of iron metabolism.
N Engl J Med. 341:1986–1995. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Fung EB, Harmatz PR, Milet M, Coates TD,
Thompson AA, Ranalli M, Mignaca R, Scher C, Giardina P, Robertson
S, et al: Fracture prevalence and relationship to endocrinopathy in
iron overloaded patients with sickle cell disease and thalassemia.
Bone. 43:162–168. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Jomova K and Valko M: Advances in
metal-induced oxidative stress and human disease. Toxicology.
283:65–87. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Jeney V: Clinical impact and cellular
mechanisms of iron overload-associated bone loss. Front Pharmacol.
8:772017. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Hu H, Tian M, Ding C and Yu S: The C/EBP
homologous protein (CHOP) transcription factor functions in
endoplasmic reticulum stress-induced apoptosis and microbial
infection. Front Immunol. 9:30832019. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Bock FJ and Tait SWG: Mitochondria as
multifaceted regulators of cell death. Nat Rev Mol Cell Biol.
21:85–100. 2020. View Article : Google Scholar
|
|
41
|
Green DR: The mitochondrial pathway of
apoptosis part II: The BCL-2 protein family. Cold Spring Harb
Perspect Biol. 14:a0410462022. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Simonyan L, Renault TT, Novais MJ, Sousa
MJ, Côrte-Real M, Camougrand N, Gonzalez C and Manon S: Regulation
of Bax/mitochondria interaction by AKT. FEBS Lett. 590:13–21. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Rahmani M, Nkwocha J, Hawkins E, Pei X,
Parker RE, Kmieciak M, Leverson JD, Sampath D, Ferreira-Gonzalez A
and Grant S: Cotargeting BCL-2 and PI3K Induces BAX-Dependent
mitochondrial apoptosis in AML cells. Cancer Res. 78:3075–3086.
2018. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Gu YX, Du J, Si MS, Mo JJ, Qiao SC and Lai
HC: The roles of PI3K/Akt signaling pathway in regulating MC3T3-E1
preosteoblast proliferation and differentiation on SLA and SLActive
titanium surfaces. J Biomed Mater Res A. 101:748–754. 2013.
View Article : Google Scholar
|
|
45
|
Ma P, Gu B, Ma J, E L, Wu X, Cao J and Liu
H: Glimepiride induces proliferation and differentiation of rat
osteoblasts via the PI3-kinase/Akt pathway. Metabolism. 59:359–366.
2010. View Article : Google Scholar
|
|
46
|
Abdurahman A, Li X, Li J, Liu D, Zhai L,
Wang X, Zhang Y, Meng Y, Yokota H and Zhang P: Loading-driven
PI3K/Akt signaling and erythropoiesis enhanced angiogenesis and
osteogenesis in a postmenopausal osteoporosis mouse model. Bone.
157:1163462022. View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Dixon SJ and Stockwell BR: The role of
iron and reactive oxygen species in cell death. Nat Chem Biol.
10:9–17. 2014. View Article : Google Scholar
|
|
48
|
Gammella E, Recalcati S and Cairo G: Dual
Role of ROS as signal and stress agents: Iron tips the balance in
favor of toxic effects. Oxid Med Cell Longev. 2016:86290242016.
View Article : Google Scholar : PubMed/NCBI
|
|
49
|
Wan J, Cheng W, Xing X, He Y, Tang P, Feng
Y, Liu S, Lu X and Zhong L: A SERS-Based dual-parameter monitoring
nanoprobe of ROS and PI3K/Akt during Ginsenoside Rg3-induced cell
apoptosis. Biosensors (Basel). 13:2122023. View Article : Google Scholar : PubMed/NCBI
|