Introduction
The incidence of cancer is rising globally because
of the aging population; in 2040, new cancer cases are expected to
increase by 47% from 2020 (1). In
particular, the incidence of melanoma, the most fatal type of skin
cancer, has doubled increased over from 1982 to 2011 and in the
absence of new interventions, 112,000 new melanoma cases are
predicted increasing in 2030 (2,3).
Melanoma is a malignant tumor caused by abnormal proliferation of
melanocytes, which produce the skin pigment melanin. Although
melanoma accounts for only 1% of all skin cancers, it accounts for
most skin cancer-related mortality (4,5).
Effective treatment of melanoma involves surgical removal in
combination with radiotherapy and chemotherapy. However, it is
essential to investigate chemotherapeutic methods using natural
substances to minimize cytotoxic effects on healthy cells (6-8).
Alkaloids exhibits various biological activities
such as antiviral, antibacterial, anti-inflammatory anticancer, and
has been developed as an anticancer drug in clinical trials for the
treatment of various malignancies (9,10).
Among these alkaloids, piperlongumine [PL;
5,6-dihydro-1-(2E)-1-oxo-3-(3,4,5-trimethoxyphenyl)-2-propenyl]-2(1H)-pyridinone]
is one of the main compounds found in long pepper (Piper
longum). Long peppers have traditionally been used in the
Ayurvedic Medical System in India and folk therapy in Latin
America. The dry, unripe fruit is used as a tonic and the roots are
used to induce placental expulsion. It has also been used to treat
tumors, malaria, viral infection, bronchitis, cough and asthma
(11). Owing to its various
applications and anticancer properties, studies have been conducted
on the potential of PL as an anticancer agent (12,13). PL is known to have specific
cytotoxicity for tumor cells and little toxicity in normal cells
(14). Although PL is reported to
exert anticancer effects against several types of cancer cell,
including colon (15), lung
(16), stomach (17), prostate (18), and pancreatic cancer (19), research on melanoma is
lacking.
Apoptosis is a mechanism by which injured or
cancerous cells can be removed without affecting nearby healthy
cells (20). Apoptosis leads to
nuclear and cytoplasmic condensation and DNA fragmentation; the
cell forms an apoptotic body, a membrane-bound extracellular
vesicle, and is degraded by neutrophils, macrophages and dendritic
cells (21). At the molecular
level, apoptosis induction to the activation of Fas-associated
death domain, deactivating the anti-apoptotic protein Bcl-2 and
releasing cytochrome c from the mitochondria into the cytoplasm,
where it regulates activity of Bax to cause apoptosis (22,23). In addition, PARP, which restores
damaged DNA in cell stress, is inactivated by caspase-3, resulting
in expression of cleaved-PARP (24).
MAPKs are serine/threonine kinases involved in
apoptosis. The main MAPK pathway includes ERK, JNK and p38. Upon
its activation by growth factors that induce cell differentiation
and proliferation, ERK stimulates expression of anti-apoptotic
proteins and suppresses apoptosis (25). JNK and p38 are activated by stress
and are involved in cell survival and apoptosis. The MAPK pathway
regulates biological activities, including cellular signal
transduction, and serves an important role in cell death and
proliferation (26).
Autophagy occurs before cell death in response to
stress, such as cytotoxicity or chemotherapy. Autophagosomes, which
have double membranes, are formed during autophagy (27). These autophagosomes fuse with
lysosomes to form autolysosomes. Meanwhile, microtubule-associated
protein 1A/1B-light chain 3 (LC3) in the cytoplasm combines with
phosphatidylethanolamine to form an LC3-phosphatidylethanolamine
conjugate (LC3-Ⅱ), which aggregates into autophagosomes (28). mTOR serves a key role in
regulating autophagy; its inactivation triggers autophagy. As
regulating different signaling pathway including Beclin 1, Bcl-2
autophagy induces cell survival or death (29). there is a potential to develop
anticancer agents that use these characteristics (30). Therefore, in the present study,
two melanoma cell lines, A375SM and A375P, were used to assess the
anticancer effects of PL in human melanoma. A375SM has higher
metastaticity (31) and is highly
invasive compared with other melanoma cells (32). The present study aimed to
investigate the effects of PL on the survival of A375SM and A375P
melanoma cells in vitro and whether these effects were
mediated by apoptosis. The present also investigated the induction
of autophagy by PL in melanoma cells and the role of autophagy and
its association with the MAPK pathway.
Materials and methods
Materials and reagents
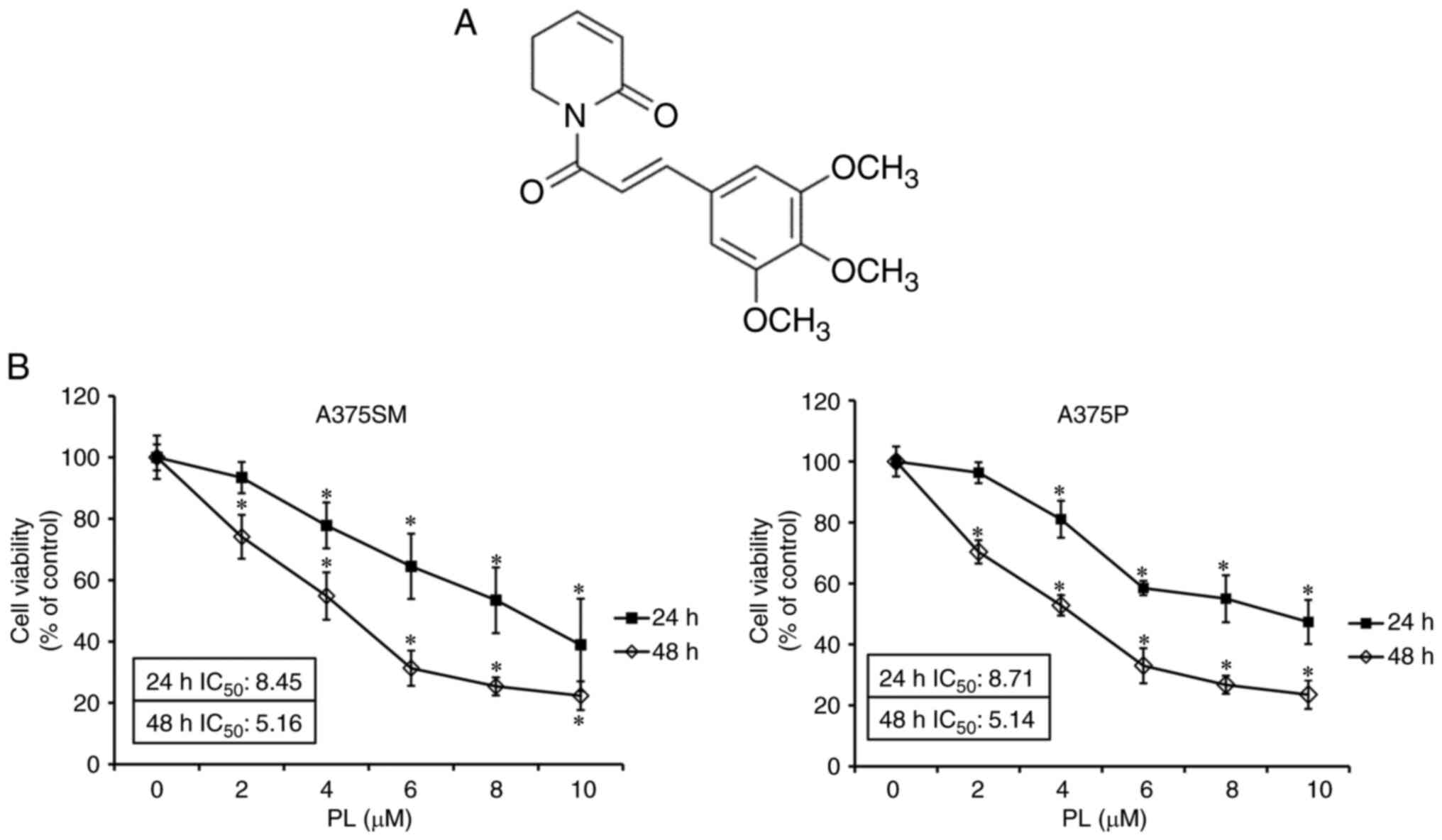
PL (Fig. 1A;
purity, ≥97.0%) was purchased from Shanghai Aladdin Biochemical
Technology Co., Ltd. MTT, DAPI and DMSO were purchased from
Sigma-Aldrich (Merck KGaA). DMSO was used as a solvent for PL and
control group was treated with DMSO alone. FITC Annexin-V detection
kit was purchased from BD Pharmingen™ (BD Biosciences). Antibodies
against PARP (rabbit, 1:1,000, cat. no. #9542), Bax (rabbit, 1:700,
cat. no. #2772), Bcl-2 (rabbit, 1:1,000, cat. no. #4223),
phosphorylated (p-)ERK (rabbit, 1:1,000, cat. no. #9102), ERK
(rabbit, 1:1,000, cat. no. #9101), p-JNK (rabbit, 1:1,000, cat. no.
#4668), JNK (rabbit, 1:1,000, cat. no. #9252), p-p38 (rabbit,
1:1,000, cat. no. #9211), p38 (rabbit, 1:1,000, cat. no. #9211),
p-mTOR (rabbit, 1:1,000, cat. no. #2971), Beclin 1 (rabbit,
1:1,000, cat. no. #3738), LC3 (rabbit, 1:1,000, cat. no. #4108),
secondary antibody rabbit IgG (rabbit, 1:1,000, cat. no. #7074),
ERK inhibitor PD98059 and JNK inhibitor SP00615 were purchased from
Cell Signaling Technology, Inc. β-actin (mouse, 1:1,000, cat. no.
sc-47778) and secondary mouse IgG (1:1,000, cat. no. sc-516102)
antibodies were purchased from Santa Cruz Biotechnology, Inc.
Autophagy inhibitor 3-methyladenine (3-MA) and hydroxychloroquine
(HCQ) were purchased from Selleck Chemical.
Cell culture
The melanoma cells A375SM and A375P were purchased
from the Korea Cell Line Bank (Seoul, Korea). DMEM and FBS used for
cell culture were purchased from Welgene, Inc.
Streptomycin/penicillin was purchased from Gibco (Thermo Fisher
Scientific, Inc.). The melanoma cells A375SM and A375P in DMEM
containing 5% FBS and 1% streptomycin/penicillin were cultured in
an incubator at 37°C and 5% CO2. When the cells reached
80-90% density, they were subcultured and the medium was replaced
every 2-3 days.
MTT assay
MTT assay was performed to observe the effect of PL
on A375SM and A375P cell viability. Cells were incubated in a
96-well plate (2×104 cells/ml) for 24 h at 37°C. PL was
added (0, 2, 4, 6, 8 or 10 µm) at 37°C for 24 or 48 h.
Half-maximal inhibitory concentration (IC50) were
calculated from curves constructed by plotting cell viability
versus PL concentration. HCQ (15 or 20 µM), 3-MA (2.5 or 3.5
mM), PD98059 (25 µM) and SP600125 (25 µM) were added
for 3 h pretreatment at 37°C, then PL was added (4 µM) at
37°C for 24 h. The medium was removed and 40 µl MTT solution
was added for 2 h. After removing the MTT solution, 100 µl
DMSO was used to dissolve formazan and the absorbance was measured
at 595 nm with ELISA reader (Bio-Rad Laboratories Inc.).
DAPI staining
DAPI staining was performed to observe morphological
changes in the nucleus due to nuclear condensation of apoptosis.
After incubating 2×105 cells/ml in 60-mm dish for 24 h,
PL (0, 4 or 8 µm) was added at 37°C for 24 h. After that,
the medium containing PL was removed and cells were washed with PBS
and fixed with 4% paraformaldehyde for 5 min at 20°C. Following
rinsing with PBS, DAPI solution was added (2 ml/dish) for 2 min at
20°C and observed at ×200 magnification with a fluorescence
microscope (Zeiss AG).
Annexin V-PI staining
Annexin V-propidium iodide (PI) staining was
performed to analyze the degree of early and late apoptosis induced
by PL in melanoma cells and confirmed using FACS. A375SM and A375P
cells were cultured 70-80% in a 175-cm2 flask at 37°C
for 24 h, then PL was added at concentrations of 0, 4 or 8
µm and cultured at 37°C for 24 h. Cells were removed from
the flask using a cell scraper, centrifuged at 260 × g for 5 min,
4°C to obtain cell pellets, washed with PBS, then centrifuged at
260 × g, 5 min, 4°C. After suspending 2×105 cells/ml in
1X binding buffer, Annexin-V and PI were added to stain for 15 min
at 20°C and measured using FACSCalibur™ flow cytometer (BD
Biosciences) and BD FACSuite™ software version 1.0.6 (BD
Biosciences).
Acridine orange (AO) staining
AO staining was performed to observe acid vesicular
organelles (AVOs), one of the morphological characteristics of
autophagy (33). A375SM and A375P
cells were seeded (2×105 cells/ml) and cultured at 37°C
for 24 h. PL was added at a concentration of 0, 4 or 8 µm in
a CO2 incubator at 37°C for 24 h, the medium containing
PL was removed and cells were washed twice with PBS. After 4%
paraformaldehyde was applied for 15 min for fixation at 20°C, cells
were washed twice with PBS and then 2 ml AO (5 µg/ml) was
added for 10 min at 20°C and cells were viewed with a fluorescence
microscope at ×100 magnification.
Western blot analysis
Western blot was performed to confirm expression of
apoptosis-associated proteins. In a 175-cm2 flask,
A375SM and A375P were incubated 70-80% at 37°C and 5%
CO2 for 24 h. Medium containing 0, 4 or 8 µm PL
was added for 24 h at 37 °C. Cells were suspended using
trypsin-EDTA, then centrifuged at 260 × g, 5 min at 4°C. The cell
pellet was treated with cell lysis buffer (PRO-PREP™ Protein
Extraction Solution; Invitrogen; Thermo Fisher Scientific, Inc.) at
4°C for 20 min and centrifuged at 15,920 × g, 5 min and 4°C. The
extracted protein was quantified with Bradford protein assay
(Bio-Rad Laboratories Inc.). Then, 50-90 µg/lane of protein
sample was loaded per lane was separated according to molecular
weight using 12% SDS-PAGE and transferred to nitrocellulose
membrane. Membrane was blocked with 5% skimmed milk at 20°C for 2
h, then primary antibody was added overnight at 4°C. Horseradish
peroxidase-conjugated anti-rabbit IgG or anti-mouse IgG was added
at 20°C for 2 h. Each protein was identified using Pierce™ enhanced
chemiluminescence (ECL) western blotting substrate (Pierce; Thermo
Fisher Scientific, Inc.) and quantified using Image J Launcher
software version 1.52a (provided by National Center for
Biotechnology Information).
Statistical analysis
All data are presented as the mean ± SD of three
experiments. Comparisons between >2 groups were performed by
one-way ANOVA followed by Dunnett's post hoc test. The difference
between two groups was assessed by unpaired Student's t test. The
data were analyzed with IBM SPSS statistics version27. P<0.05
was considered to indicate a statistically significant
difference.
Results
PL decreases viability of melanoma
cells
A375SM and A375P melanoma cells were incubated PL to
assess survival rates using an MTT assay. Following treatment with
PL, A375SM cells showed survival rates of 77.80 at 4 and 53.46% at
8 µm at 24 h, whereas A375P cells showed survival rates of
80.98 and 55.00, respectively. In addition, the IC50 of
A375SM and A375P was 8.45 and 8.71 µm, respectively, at 24
h. A375SM cells showed survival rates of 54.84 at 4 and 25.39% at 8
µm at 48 h, whereas A375P cells showed survival rates of
52.81 and 26.78, respectively. In addition, IC50 of
A375SM and A375P was 5.16 and 5.14 µm, respectively, at 48 h
(Fig. 1B). These results
demonstrated that PL decreased the survival rates of A375SM and
A735P melanoma cells in a dose- and time- dependent manner. In the
subsequent experiments, PL was used at 4 and 8 µm.
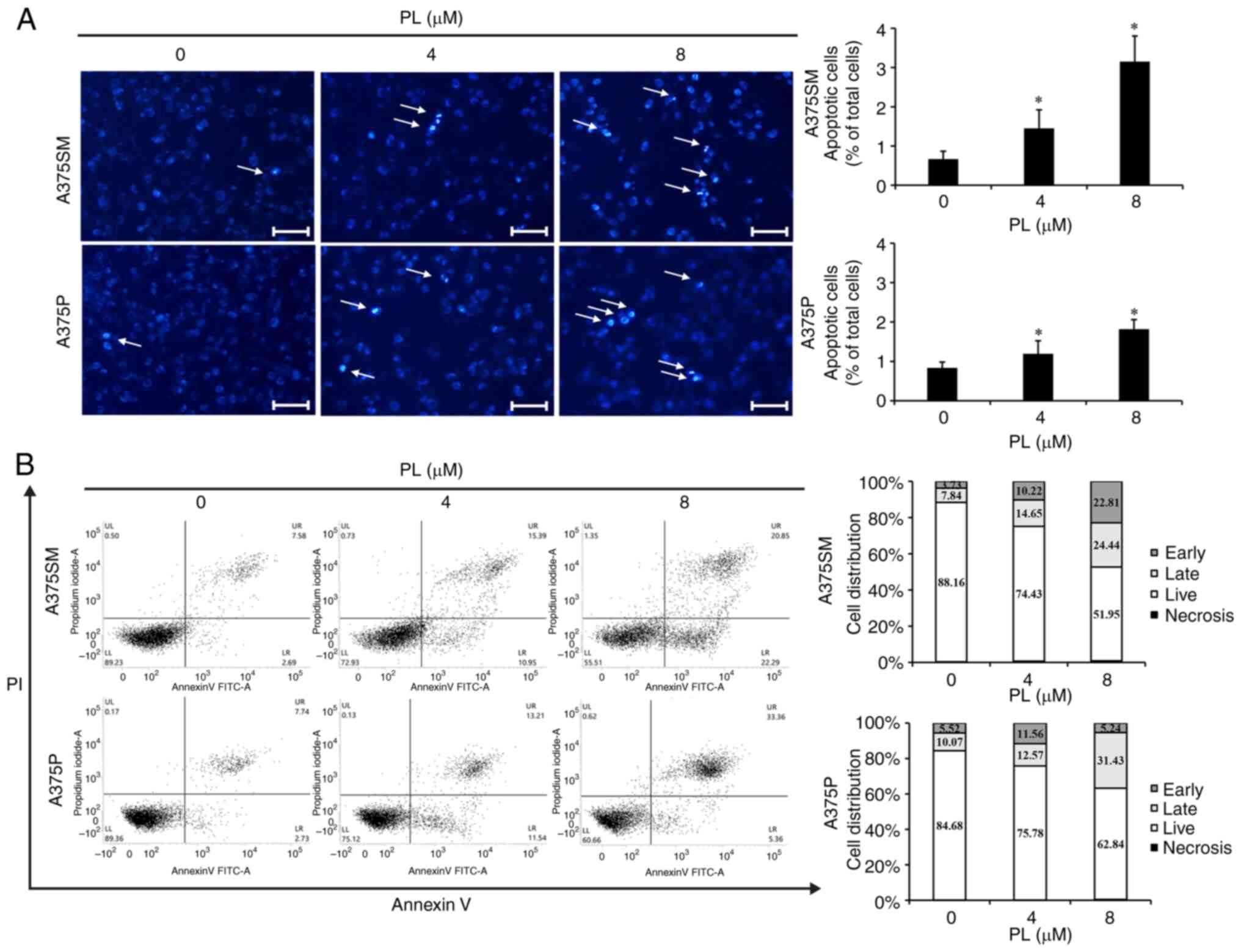
PL induces apoptosis of melanoma
cells
DAPI staining was to confirm whether the reduction
in survival rate was due to apoptosis. Fluorescence microscopy was
used to identify apoptotic bodies, characterized by morphological
changes in nuclear DNA and condensation of the cytoplasm (34). There was a dose-dependent increase
in apoptotic cells for A375SM cells of 0.66% under the control
condition, 1.45% at 4 µm PL, and 3.15% at 8 µm PL;
and for A375P cells, 0.83% under the control condition; 1.19% at 4
µm PL; and 1.81% at 8 µm PL (Fig. 2A).
Apoptosis induced by PL was measured using Annexin
V-PI staining. Both A375SM and A375P cells showed dose-dependent
increases in early/late apoptosis when treated with PL compared
with control (Fig. 2B). These
results suggested that the decrease in A375SM and A375P cell
survival following treatment with PL was due to apoptosis.
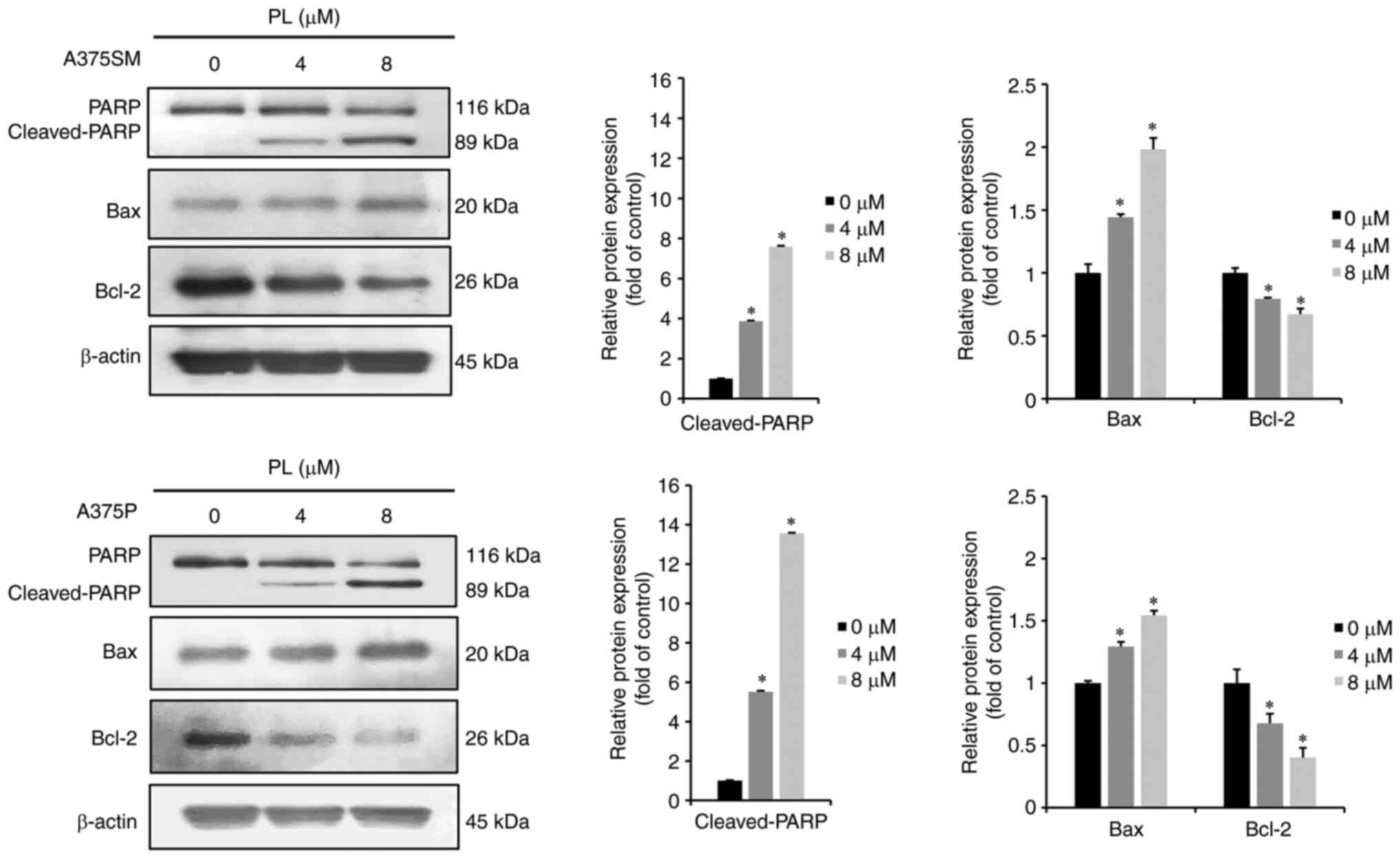
PL expresses apoptosis-associated
proteins in melanoma cells
Western blotting was performed to investigate
expression of apoptosis-related proteins in A375SM and A375P cells
following treatment with PL. When A375SM and A375P melanoma cells
were treated with 0, 4 or 8 µm PL for 24 h, pro-apoptotic
protein cleaved-PARP and Bax increased in a dose-dependent.
Conversely, expression of the anti-apoptotic protein Bcl-2
decreased in PL-treated cells (Fig.
3). Thus, PL induced apoptosis in melanoma cells by increasing
cleaved-PARP and Bax expression while decreasing Bcl-2
expression.
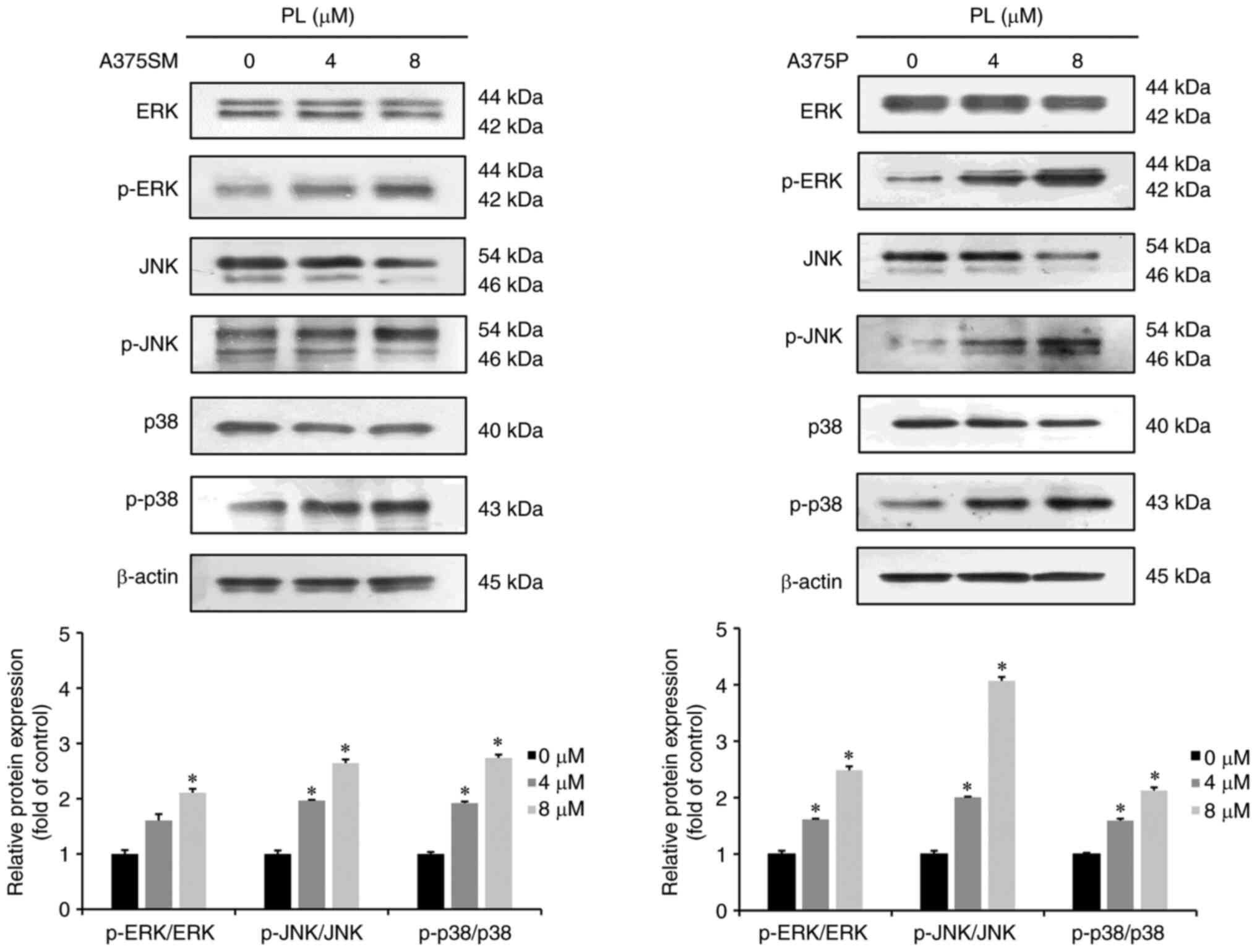
PL expresses MAPK pathway-associated
proteins in melanoma cells
Western blotting was performed to investigate
whether PL-induced apoptosis was mediated by the MAPK pathway.
Compared with the control group, 4 or 8 µM PL-treated A375SM
and A375P cells showed increased expression of p-ERK, p-JNK and
p-p38 in a dose-dependent manner (Fig. 4).
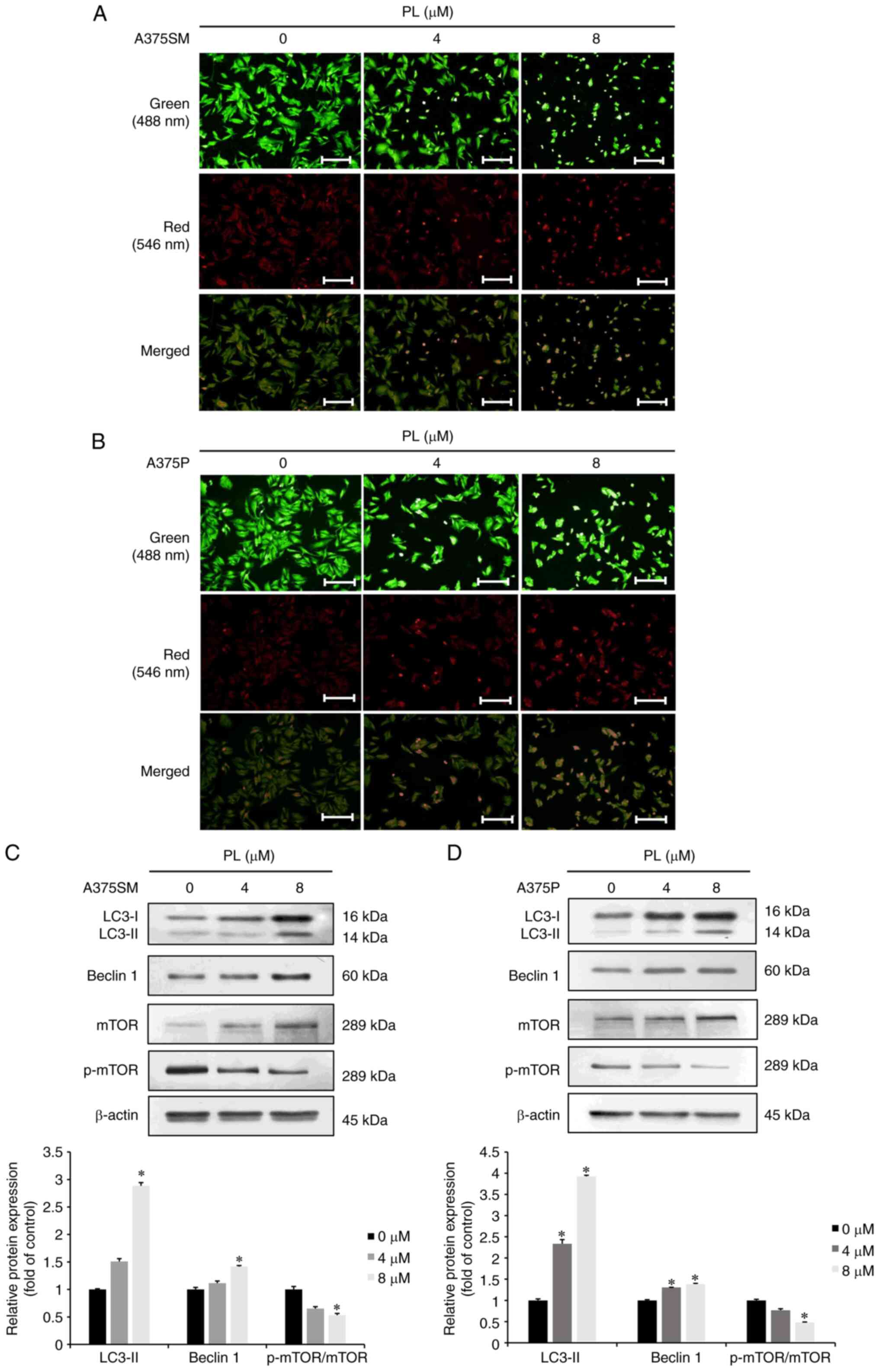
PL induces autophagy in melanoma
cells
AO staining was used to investigate whether PL
induced autophagy in A375SM and A375P cells. When A375SM and A375P
melanoma cells were treated with 4 or 8 µM PL for 24 h, the
expression of AVOs increased compared with that in the control
group (Fig. 5A and B). In
addition, western blotting was performed to investigate whether PL
increased the expression of autophagy-associated proteins in A375SM
and A375P cells. There was an increase in LC3-Ⅱ and Beclin 1
expression and a decrease in p-mTOR expression in PL-treated cells
(Fig. 5C and D). These results
suggested that PL induced autophagy in melanoma cells in a
dose-dependent manner.
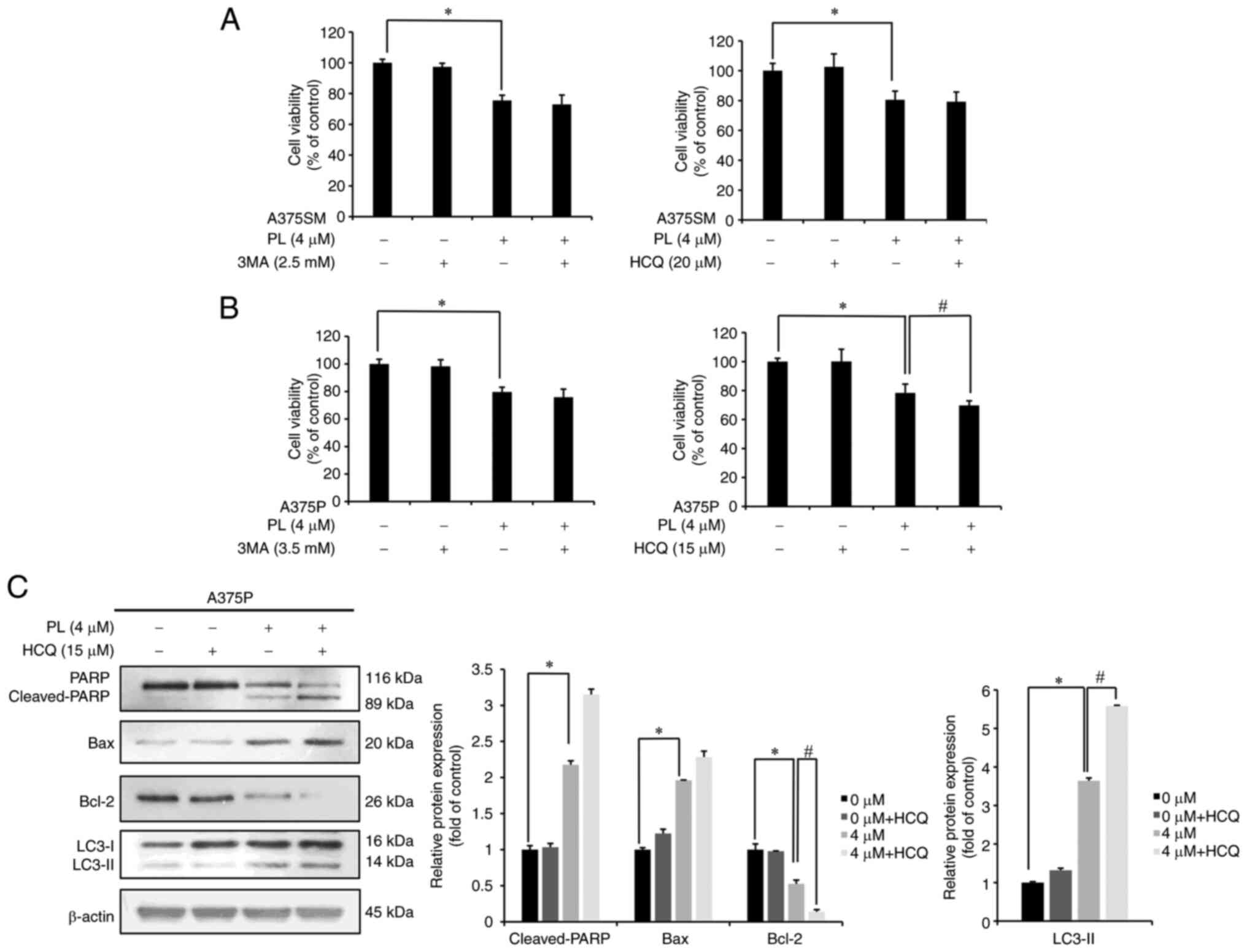
Autophagy induced by PL in melanoma cells
affects apoptosis-associated protein expression
To investigate the role of autophagy, cells were
pretreated with either the autophagy early-stage inhibitor 3-MA or
the autophagy late-stage inhibitor HCQ for 3 h before incubation
with PL for 24 h and MTT assay. The survival rate of A375SM cells
treated with PL was 75.53%, whereas that of PL + 3-MA (2.5
mM)-treated cells was 72.92%. In another set of experiments the
survival rate of PL-treated A375SM cells was 80.54%, whereas that
of PL + HCQ (20 µM)-treated cells was 79.26%, showing an
insignificant decrease (Fig. 6A).
For A375P cells, the survival rate following PL treatment was
78.34%, whereas that after PL + 3-MA (3.5 mM) treatment was 75.80%.
In another experiment set, the survival rate of PL-treated cells
was 79.60%, whereas that of PL + HCQ (15 µM)-treated cells
was 69.66%, demonstrating a significant decrease (Fig. 6B). Based on these results, A375P
cells were selected for further experiments to investigate the
association between apoptosis and autophagy as these cells showed a
significant decrease in survival. Western blotting was performed to
investigate apoptosis-related protein expression in A375P cells
pretreated with HCQ for 3 h followed by PL for 24 h. Compared with
cells treated with PL alone, those treated with PL + HCQ showed
increased Bax expression and significantly decreased Bcl-2
expression. The expression of cleaved-PARP and LC3-Ⅱ was also
significantly increased in PL + HCQ-treated cells compared with
that in PL-treated cells (Fig.
6C). These results suggested that apoptosis increased when
autophagy was suppressed during PL treatment.
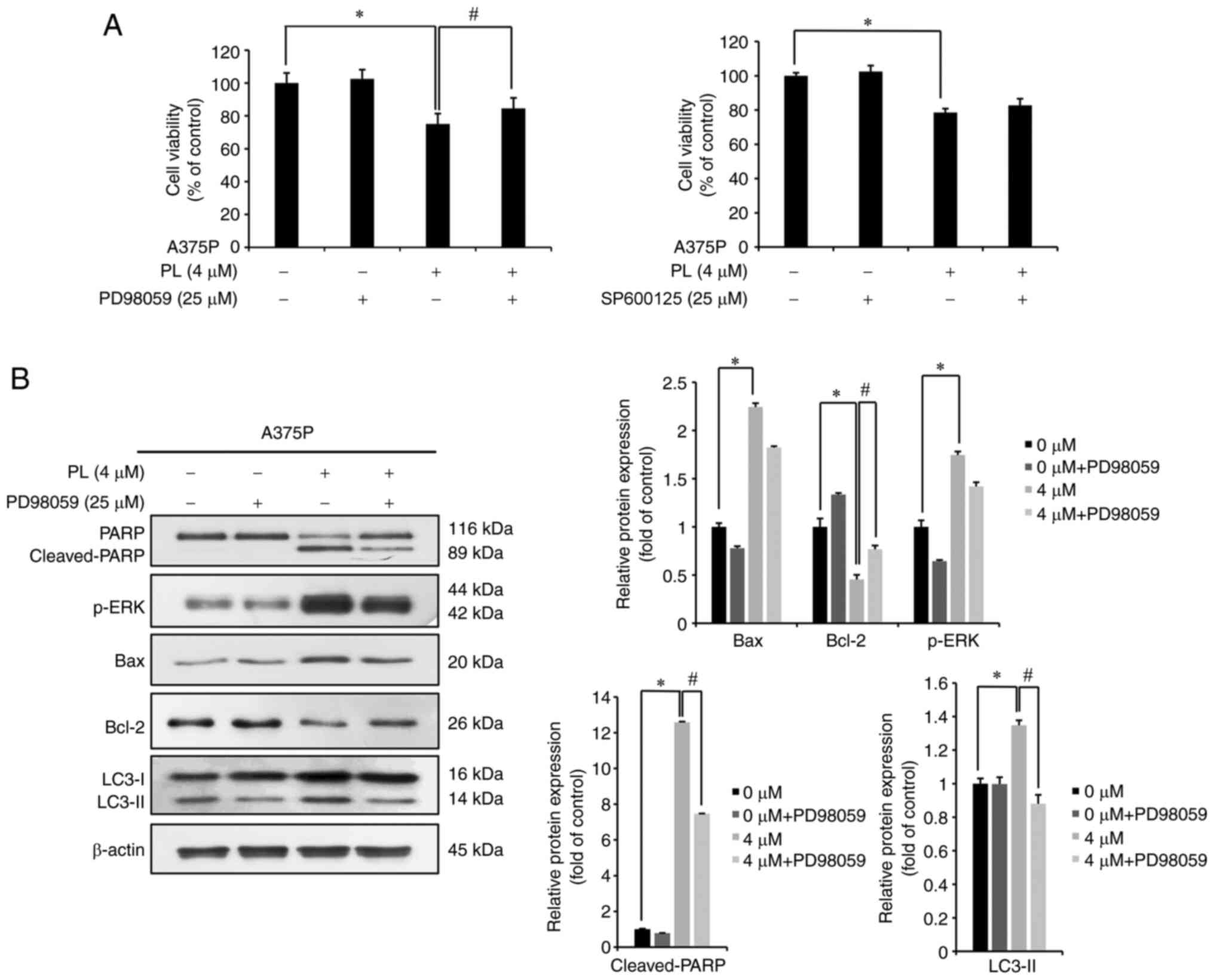
PL induces ERK-mediated apoptosis and
autophagy in melanoma cells
A375P cells were pretreated with 25 µM
PD98059 (ERK inhibitor) or SP600125 (JNK inhibitor) for 3 h,
followed by 0 or 4 µM PL for 24 h and MTT assay. The
survival rate of cells treated only with PL was 75.02% whereas that
of cells treated with PL + PD98059 was 84.53%. The survival rate of
cells treated only with PL (4 µM) was 78.58% whereas that of
cells treated with PL + SP600125 was 82.72% (Fig. 7A). This indicated that, within the
MAPK pathway, ERK was involved in the PL-induced decrease in the
survival of melanoma cells. As ERK had a significant effect on
survival of A375P cells, western blotting was performed after
treatment with PD98059 to investigate the effects of ERK on
apoptosis- and autophagy-related protein expression. The
pro-apoptotic proteins cleaved-PARP and Bax showed significantly
increased expression in PL-treated cells compared with that in the
control cells, whereas both showed insignificantly decreased
expression in PL + PD98059-compared with PL-treated cells. By
contrast, expression of the anti-apoptotic protein Bcl-2 was
significantly decreased in PL-treated cells but increased
significantly in PL + PD98059-treated cells. The representative
autophagy protein LC3-Ⅱ showed significantly decreased expression
in PL + PD98059-treated cells compared with that in PL-treated
cells (Fig. 7B). These findings
suggested that ERK was involved in induction of apoptosis and
autophagy in A375P cells.
Discussion
PL is an amide alkaloid primarily found in Piper
longum and its anticancer effects have been investigated in
several types of cancer (15-19). To the best of our knowledge,
however, studies on the effect of PL on human melanoma cells are
lacking. A375SM has a higher metastatic ability than A375P
(31) and A375SM cells are more
invasive than other melanoma cells (32). Therefore, the anticancer
capability of PL against human melanoma was investigated in A375SM
and A375P. It was hypothesized that A375P cells with lower
metastatic properties would have higher sensitivity to PL than
A375SM cells with higher metastatic properties. The present study
investigated whether the PL-induced reduction in the survival of
A375SM and A375P melanoma cells was due to apoptosis, analyzed the
cytoprotective role of PL-induced autophagy and verified whether
apoptosis and autophagy were affected by the MAPK/ERK pathway.
PL induced a dose- and time-dependent decrease in
the survival of melanoma cells. PL has been shown to exert
anticancer effects against human intestinal cancer cells: In human
intestinal cancer cells INT-407 and HCT-116 treated with PL for 24
and 48 h, the IC50 of INT-407 was 13 at 24 and 9
µm at 48 h and IC50 of HCT-116 was 8 at 24 and 6
µm at 48 h, with the survival rates showing a dose-dependent
decrease (35). Similarly,
PL-treated WRO thyroid cancer cells show a time- and dose-dependent
decrease in cell survival; the IC50 for 24 and 48 h of
treatment with PL is 10.24 and 5.68 µm, respectively
(36). Standard of concentration
was set to medium concentration as 4 µm, when cell survival
rate was 80±5%. High concentration was set at 8 µm when cell
survival rate was 50±5% based on previous studies (35,36). The aforementioned reports are
similar to the present findings and support the conclusion that PL
decreases melanoma cell survival in a dose-dependent manner. PL
does not cause toxicity in normal human cells. There is no
significant change in cell survival following PL treatment of human
kidney cell line HK-2, pancreatic duct epithelial cell line HPDE,
hepatic cell line LO-2 and breast epithelial cell line MCF10A
(37). In addition, a previous
study showed that PL does not decrease in survival rate in normal
cells compared with cancer cells (38) DAPI staining is used to detect
apoptosis as it shows nuclear changes such as condensation by
strongly binding to adenine- and thymine-rich regions. Annexin-V
staining targets areas with loss of membrane phospholipids,
enabling the detection of early apoptosis. PI, which binds to DNA,
cannot penetrate intact cell membranes and indicates cell damage,
representing late apoptosis (39). DAPI and Annexin V-PI staining were
used to verify if the decreased survival rate of melanoma cells
determined by MTT assay was due to apoptosis. In PL-treated human
melanoma cells, DAPI staining revealed a dose-dependent increase in
DNA condensation, which is characteristic of apoptosis. FACS with
Annexin V-PI staining found that early and late apoptosis was
increased in human melanoma cells treated with PL compared with
that in control cells. In a previous study on lung cancer cells
(A549 and NCI-H460) treated with 20 µm PL for 24 h, DAPI
staining revealed an increase in apoptotic bodies, demonstrating
that PL induces apoptosis in lung cancer cells (40). Similarly, in PL (1, 2, and 4
µm)-treated DU145 prostate cancer cells, Annexin V-PI
staining reveals a dose-dependent increase in apoptosis (41). The aforementioned studies support
the present findings that PL induced apoptosis in melanoma cells.
In summary, the decrease in melanoma cell survival induced by PL
was due to apoptosis.
The first stage of apoptosis involves deactivation
of PARP, which in turn prevents DNA repair. PARP cleavage prevents
necrosis and enables caspase-mediated apoptosis (24). Other molecules that regulate
apoptosis include Bcl-2, a protein important for tumor development,
and pro-apoptotic protein Bax (42). The present study observed an
increase in cleaved-PARP and Bax expression and decrease in Bcl-2
expression in PL-treated human melanoma cells. In a previous study,
A546 lung cancer cells treated with PL (6 or 10 µm) for 48 h
showed apoptosis due to increased cleaved-PARP and Bax and
decreased Bcl-2 expression (16).
The present findings showed trends similar to those of previous
studies (42,16), providing evidence that PL
decreases melanoma cell survival by inducing apoptosis.
The MAPK pathway serves an important role in cell
proliferation, differentiation, apoptosis, angiogenesis and tumor
metastasis. JNK and p38 are primarily involved in cell stress and
apoptosis, while ERK is associated with cell proliferation and
differentiation (43). ERK also
serves an important role in halting the cell cycle and inducing
apoptosis after DNA injury (44).
Therefore, the present study investigated the role of the MAPK
pathway in apoptosis induction using western blotting and found
that PL-treated melanoma cells showed dose-dependent increases in
expression of p-ERK, p-JNK, and p-p38. In a previous study, a
natural compound (shikonin) with anticancer properties increased
the expression of p-ERK, p-JNK, and p-p38 in A375SM melanoma cells,
resulting in MAPK pathway-mediated apoptosis (45). Another natural compound
(cudraflavone c) with anticancer properties was found to increase
expression of p-ERK, p-JNK, and p-p38 in A375S2 melanoma cells
(46). Collectively, these
findings indicate that PL induced apoptosis in melanoma cells by
increasing p-ERK, p-JNK and p-p38 expression and activating the
MAPK pathway.
Autophagy serves various physiological roles,
including anti-aging, apoptosis and tumor suppression (39). During autophagy, AVOs are produced
by cells. AVOs protect cells by preventing acidification of the
cytoplasm and providing necessary catabolites for recovery.
However, excess production of AVOs leads to necrosis or apoptosis
(47). The present study used AO
staining to determine whether PL induced autophagy and observed a
dose-dependent increase in AVOs when melanoma cells were treated
with PL. Western blotting to analyze the levels of
autophagy-associated proteins demonstrated PL induced autophagy in
melanoma cells by decreasing p-mTOR and increasing Beclin 1 and
LC3-II expression. Previously, PL was found to induce autophagy by
decreasing p-mTOR and increasing LC3-Ⅱ expression in thyroid cancer
cells and increasing LC3-Ⅱ expression in gallbladder cancer cells
(35,48). These previous results are
consistent with the present study, which strengthens the evidence
that PL induces autophagy in melanoma cells. The present study used
the autophagy inhibitors 3-MA (early-stage inhibitor) and HCQ
(late-stage inhibitor) to investigate the role of PL-induced
autophagy in melanoma cells. Combined treatment with PL + 3-MA
induced no significant change in survival of A375P melanoma cells.
However, PL + HCQ resulted in a significant decrease in cell
survival. Western blot analysis of apoptosis-associated proteins
revealed that PL + HCQ combined treatment increased cleaved-PARP
and Bax expression and decreased Bcl-2 expression. Previously,
endometrioma cells treated with HCQ and a natural anticancer agent
(resveratrol) showed reduced survival and increased cleaved-PARP
expression compared with those treated with only resveratrol, which
indicates that using HCQ with resveratrol) induces apoptosis to a
greater extent than only resveratrol), suggesting that autophagy
has a protective effect against apoptosis (48). These findings are consistent with
those of the present study. The present study showed that
PL-induced autophagy had a cytoprotective effect in A375P cells and
that co-treatment with PL + HCQ enhanced apoptosis to inhibit late
autophagy for a stronger anticancer effect.
The present study investigated the roles of ERK and
JNK by pretreating A375P cells with p-ERK inhibitor PD98059 or
p-JNK inhibitor SP600125, followed by treatment with PL and cell
survival measurement using MTT assay. There was a significant
increase in the survival rate of PD98059-treated cells, suggesting
involvement of the ERK pathway in the reduced survival rate of
PL-treated melanoma cells. In a previous study, 1 h pretreatment
with p-ERK inhibitor UO216 increased the survival rate of
PL-treated colon cancer cells, suggesting that increased p-ERK
levels affect cell survival (49). Here, western blotting revealed
decreased cleaved-PARP and Bax and increased Bcl-2 expression in
melanoma cells treated with PL after 3 h PD98059 pretreatment
compared with that in PL-treated cells. This suggested that when
A375P melanoma cells were treated with PL, inhibition of ERK
activity prevented apoptosis induction. To determine whether
autophagy induction was associated with ERK protein expression,
western blotting was performed on cells pretreated with PD98059 for
3 h and then treated with PL. There was decreased expression of
LC3-Ⅱ, indicating that autophagy occurred downstream of the
MAPK/ERK pathway. However, ERK may not act independently in
PL-induced apoptosis and autophagy in A375P cells. Further studies
are needed to explore more detailed molecular signaling
pathways.
In conclusion, there was no difference between
A375SM and A375P in sensitivity to the concentration of PL. PL
significantly decreased survival of melanoma cells due to apoptosis
resulting from increased Bax and cleaved-PARP and decreased Bcl-2
expression. PL-induced apoptosis was associated with induction of
the MAPK/ERK pathway. Moreover, PL-induced autophagy was identified
based on an increase in AVOs and expression levels of
autophagy-related proteins. Experiments using HCQ revealed the
cytoprotective role of autophagy and the involvement of the
MAPK/ERK pathway. In summary, when PL-induced late autophagy was
inhibited in melanoma cells, the anticancer effects increased and
PL induced apoptosis and autophagy via the MAPK/ERK pathway. The
present study suggested that PL has potential as an anticancer
agent and a greater anticancer effect could be achieved by
regulating ERK expression. However, the present study did not
confirm toxicity in normal cells, assuming that there was no
toxicity in normal cells based on previous studies (37,38). In addition, experiments were only
conducted on melanoma cells in vitro. Therefore, in
vivo research is needed to assess anticancer effects of PL and
confirm its potential clinical application.
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
JSJ, EYC and EJH designed the experiments, curated
data and reviewed manuscript. JSJ wrote the manuscript. MJM and SWL
analyzed and interpreted data. SHJ and JYJ analyzed the results and
reviewed the manuscript. JYJ edited the manuscript and acquired
funding. All the authors have read and approved the final
manuscript. All authors confirmed the authenticity of all the raw
data.
Ethics approval and consent to
participate
Not applicable.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
Acknowledgments
Not applicable.
Funding
The present study was supported by the Basic Science Research
Program through the National Research Foundation of Korea funded by
the Ministry of Education, Science and Technology (grant no.
2021R1A2C1010912).
References
|
1
|
Sung H, Ferlay J, Siegel RL, Laversanne M,
Soerjomataram I, Jemal A and Bray F: Global cancer statistics 2020:
Globocan estimates of incidence and mortality worldwide for 36
cancers in 185 countries. CA Cancer J Clin. 71:209–249. 2021.
View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Erdei E and Torres SM: A new understanding
in the epidemiology of melanoma. Expert Rev Anticancer Ther.
10:1811–1823. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Guy GP Jr, Thomas CC, Thompson T, Watson
M, Massetti GM and Richardson LC; Centers for Disease Control and
Prevention (CDC): Vital signs: Melanoma incidence and mortality
trends and projections-United States, 1982-2030. MMWR Morb Mortal
Wkly Rep. 64:591–596. 2015.PubMed/NCBI
|
|
4
|
Miller AJ and Mihm MC Jr: Melanoma. N Engl
J Med. 355:51–65. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Siegel RL, Miller KD, Fuchs HE and Jemal
A: Cancer statistics, 2022. CA Cancer J Clin. 72:7–33. 2022.
View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Rigel DS and Carucci JA: Malignant
melanoma: Prevention, early detection, and treatment in the 21st
century. CA Cancer J Clin. 50:215–240; quiz 237-240. 2000.
View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Park BR, Park JW, Cho CK, Yoo HS and Lee
YW: A case of breast cancer patient experiencing adriamycin cytoxan
and taxol side effects managed by traditional korean medicine. J
Int Korean Med. 32:451–457. 2011.
|
|
8
|
Zhang QY, Wang FX, Jia KK and Kong LD:
Natural product interventions for chemotherapy and
radiotherapy-induced side effects. Front Pharmacol. 9:12532018.
View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Olofinsan K, Abrahamse H and George BP:
Therapeutic role of alkaloids and alkaloid derivatives in cancer
management. Molecules. 28:55782023. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Ye H, Wang L, Ma L, Ionov M, Qiao G, Huang
J, Cheng L, Zhang Y, Yang X, Cao S and Lin X: Protein kinases as
therapeutic targets to develop anticancer drugs with natural
alkaloids. Front Biosci (Landmark Ed). 26:1349–1361. 2021.
View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Piska K, Gunia-Krzyżak A, Koczurkiewicz P,
Wójcik-Pszczoła K and Pękala E: Piperlongumine (piplartine) as a
lead compound for anticancer agents-synthesis and properties of
analogues: A mini-review. Eur J Med Chem. 156:13–20. 2018.
View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Parama D, Rana V, Girisa S, Verma E,
Daimary UD, Thakur KK, Kumar A and Kunnumakkara AB: The promising
potential of piperlongumine as an emerging therapeutics for cancer.
Explor Target Antitumor Ther. 2:323–354. 2021.PubMed/NCBI
|
|
13
|
Tripathi SK and Biswal BK: Piperlongumine,
a potent anticancer phytotherapeutic: Perspectives on contemporary
status and future possibilities as an anticancer agent. Pharmacol
Res. 156:1047722020. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Zhu P, Qian J, Xu Z, Meng C, Zhu W, Ran F,
Zhang W, Zhang Y and Ling Y: Overview of piperlongumine analogues
and their therapeutic potential. Eur J Med Chem. 220:1134712021.
View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Kumar S and Agnihotri N: Piperlongumine
targets NF-κB and its downstream signaling pathways to suppress
tumor growth and metastatic potential in experimental colon cancer.
Mol Cell Biochem. 476:1765–1781. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Wang F, Mao Y, You Q, Hua D and Cai D:
Piperlongumine induces apoptosis and autophagy in human lung cancer
cells through inhibition of PI3K/AKT/mTOR pathway. Int J
Immunopathol Pharmacol. 28:362–373. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Song B, Zhan H, Bian Q and Gu J:
Piperlongumine inhibits gastric cancer cells via suppression of the
JAK1,2/STAT3 signaling pathway. Mol Med Rep. 13:4475–4480. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Golovine KV, Makhov PB, Teper E, Kutikov
A, Canter D, Uzzo RG and Kolenko VM: Piperlongumine induces rapid
depletion of the androgen receptor in human prostate cancer cells.
Prostate. 73:23–30. 2013. View Article : Google Scholar
|
|
19
|
Dhillon H, Chikara S and Reindl KM:
Piperlongumine induces pancreatic cancer cell death by enhancing
reactive oxygen species and DNA damage. Toxicol Rep. 1:309–318.
2014. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Kerr JF: History of the events leading to
the formulation of the apoptosis concept. Toxicology.
181-182:471–474. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Xu X, Lai Y and Hua ZC: Apoptosis and
apoptotic body: Disease message and therapeutic target potentials.
Biosci Rep. 39:BSR201809922019. View Article : Google Scholar :
|
|
22
|
Fulda S and Debatin KM: Extrinsic versus
intrinsic apoptosis pathways in anticancer chemotherapy. Oncogene.
25:4798–4811. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Hongmei Z: Extrinsic and intrinsic
apoptosis signal pathway review. Apoptosis and Medicine. InTech.
2012. View Article : Google Scholar
|
|
24
|
Mullen P: PARP cleavage as a means of
assessing apoptosis. Methods Mol Med. 88:171–181. 2004.
|
|
25
|
Sun Y, Liu WZ, Liu T, Feng X, Yang N and
Zhou HF: Signaling pathway of MAPK/ERK in cell proliferation,
differentiation, migration, senescence and apoptosis. J Recept
Signal Transduct Res. 35:600–604. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Yue J and López JM: Understanding MAPK
signaling pathways in apoptosis. Int J Mol Sci. 21:23462020.
View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Mizushima N: Autophagy: Process and
function. Genes Dev. 21:2861–2873. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Tanida I, Ueno T and Kominami E: LC3 and
autophagy. Methods Mol Biol. 445:77–88. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Paquette M, El-Houjeiri L and Pause A:
mTOR pathways in cancer and autophagy. Cancers (Basel). 10:182018.
View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Janku F, McConkey DJ, Hong DS and Kurzrock
R: Autophagy as a target for anticancer therapy. Nat Rev Clin
Oncol. 8:528–539. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Sriramarao P and Bourdon MA: Melanoma cell
invasive and metastatic potential correlates with endothelial cell
reorganization and tenascin expression. Endothelium. 4:85–97. 1996.
View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Hazarika P, McCarty MF, Prieto VG, George
S, Babu D, Koul D, Bar-Eli M and Duvic M: Up-regulation of
Flotillin-2 is associated with melanoma progression and modulates
expression of the thrombin receptor protease activated receptor 1.
Cancer Res. 64:7361–7369. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Xi G, Hu X, Wu B, Jiang H, Young CY, Pang
Y and Yuan H: Autophagy inhibition promotes paclitaxel-induced
apoptosis in cancer cells. Cancer Letters. 307:141–148. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Taatjes DJ, Sobel BE and Budd RC:
Morphological and cytochemical determination of cell death by
apoptosis. Histochem Cell Biol. 129:33–43. 2008. View Article : Google Scholar
|
|
35
|
Rawat L, Hegde H, Hoti SL and Nayak V:
Piperlongumine induces ROS mediated cell death and synergizes
paclitaxel in human intestinal cancer cells. Biomed Pharmacother.
128:1102432020. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Lin TH, Kuo CH, Zhang YS, Chen PT, Chen
SH, Li YZ and Lee YR: Piperlongumine induces cellular apoptosis and
autophagy via the ROS/AKT signaling pathway in human follicular
thyroid cancer cells. Int J Mol Sci. 24:80482023. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Afolabi LO, Bi J, Chen L and Wan X: A
natural product, Piperlongumine (PL), increases tumor cells
sensitivity to NK cell killing. Int Immunopharmacol. 96:1076582021.
View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Choi DG, Venkatesan J and Shim MS:
Selective anticancer therapy using pro-oxidant drug-loaded
chitosan-fucoidan nanoparticles. Int J Mol Sci. 20:32202019.
View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Atale N, Gupta S, Yadav UC and Rani V:
Cell-death assessment by fluorescent and nonfluorescent cytosolic
and nuclear staining techniques. J Micros. 255:7–19. 2014.
View Article : Google Scholar
|
|
40
|
Zheng J, Son DJ, Gu SM, Woo JR, Ham YW,
Lee HP, Kim WJ, Jung JK and Hong JT: Piperlongumine inhibits lung
tumor growth via inhibition of nuclear factor kappa B signaling
pathway. Sci Rep. 6:263572016. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Zhang DF, Yang ZC, Chen JQ, Jin XX, Qiu
YD, Chen XJ, Shi HY, Liu ZG, Wang MS, Liang G and Zheng XH:
Piperlongumine inhibits migration and proliferation of
castration-resistant prostate cancer cells via triggering
persistent DNA damage. BMC Complementary Med Ther. 21:1–15.
2021.
|
|
42
|
Scott WL and Athena WL: Apoptosis in
cancer. Carcinogenesis. 21:485–495. 2000. View Article : Google Scholar
|
|
43
|
Guo YJ, Pan WW, Liu SB, Shen ZF, Xu Y and
Hu LL: ERK/MAPK signalling pathway and tumorigenesis. Exp Ther Med.
19:1997–2007. 2023.
|
|
44
|
Chen SY, Huang HY, Lin HP and Fang CY:
Piperlongumine induces autophagy in biliary cancer cells via
reactive oxygen species-activated ERK signaling pathway. Int J Mol
Med. 44:1687–1696. 2019.PubMed/NCBI
|
|
45
|
Lee JH, Han SH, Kim YM, Kim SH, Yoo ES,
Woo JS, Jung GH, Jung SH, Kim BS and Jung JY: Shikonin inhibits
proliferation of melanoma cells by MAPK pathway-mediated induction
of apoptosis. Biosci Rep. 41:BSR202038342023. View Article : Google Scholar
|
|
46
|
Lee CW, Yen FL, Ko HH, Li SY, Chiang YC,
Lee MH, Tsai MH and Hsu LF: Cudraflavone C induces apoptosis of
A375.S2 melanoma cells through mitochondrial ROS production and
MAPK activation. Int J Mol Sci. 18:15082017. View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Paglin S, Hollister T, Delohery T, Hackett
N, McMahill M, Sphicas E, Domingo D and Yahalom J: A novel response
of cancer cells to radiation involves autophagy and formation of
acidic vesicles. Cancer Res. 61:439–444. 2023.
|
|
48
|
Fukuda T, Oda K, Wada-Hiraike O, Sone K,
Inaba K, Ikeda Y, Makii C, Miyasaka A, Kashiyama T, Tanikawa M, et
al: Autophagy inhibition augments resveratrol-induced apoptosis in
ishikawa endometrial cancer cells. Oncol Lett. 12:2560–2566. 2023.
View Article : Google Scholar
|
|
49
|
Randhawa H, Kibble K, Zeng H, Moyer MP and
Reindl KM: Activation of ERK signaling and induction of colon
cancer cell death by piperlongumine. Toxicol In Vitro.
27:1626–1633. 2013. View Article : Google Scholar : PubMed/NCBI
|