The tendon-bone interface is a transitional region
of soft tissue-to-bone transformation composed of the tendon,
uncalcified fibrocartilage, calcified fibrocartilage and bone. It
is a highly specialized structure (1). The connection between the tendon or
ligament and the bone is established. The tendon-bone interface
transmits the force generated by skeletal muscle contraction to the
bone, providing strong support for joint movement and facilitating
the gradual transfer of intricate mechanical loads in an efficient
manner. In addition, it also plays an essential role in maintaining
homeostasis and intercellular communications. However, due to the
enormous difference in elastic modulus between tendon and bone, the
tendon is a stress-bearing area, which significantly increases the
risk of injury (2). Tendon-bone
insertion (TBI) injuries are a common musculoskeletal disorder,
including the anterior cruciate ligament (ACL) and the rotator cuff
(RC) and Achilles tendon injuries. Injuries of the ACL and RC are
the most common (3). Survey data
indicate that the incidence of RC tears in the general population
is ~20.7%, and increases with age (4). ACL injuries occur in >2 million
patients annually in the United States, accounting for more than
half of all knee injuries (5).
Local tendon swelling, pain and functional impairment typically
accompany TBI injury. This condition severely affects the quality
of life of affected individuals and is a significant cause of pain
and disability (6).
Bone marrow mesenchymal stem cells (BMSCs) are adult
stem cells derived from bone marrow with self-replication and
multiple differentiation potential that can differentiate into
various cell types, including osteoblasts, chondrocytes and
adipocytes, and these entities possess the capacity to efficiently
restore impaired tissues, thus rendering them ideal for
regenerative medicine and tissue engineering repair (7,8).
BMSCs have the advantages of convenient sampling, a potent
proliferative ability, low immunogenicity, easy gene transfection
and strong differentiation potential, and they are the optimal type
of stem cells used in current research and clinical applications.
With further research and the development of related technologies,
BMSCs are widely used in the treatment of orthopedic clinical
diseases. Previous studies have indicated that BMSCs can promote
bone, cartilage, muscle, ligament and tendon healing (9,10). BMSCs possess the ability to
modulate the expression and release of growth factors and cytokines
in the vicinity of the injury site, thereby facilitating the
process of wound healing and regeneration of damaged tissues
(11,12).
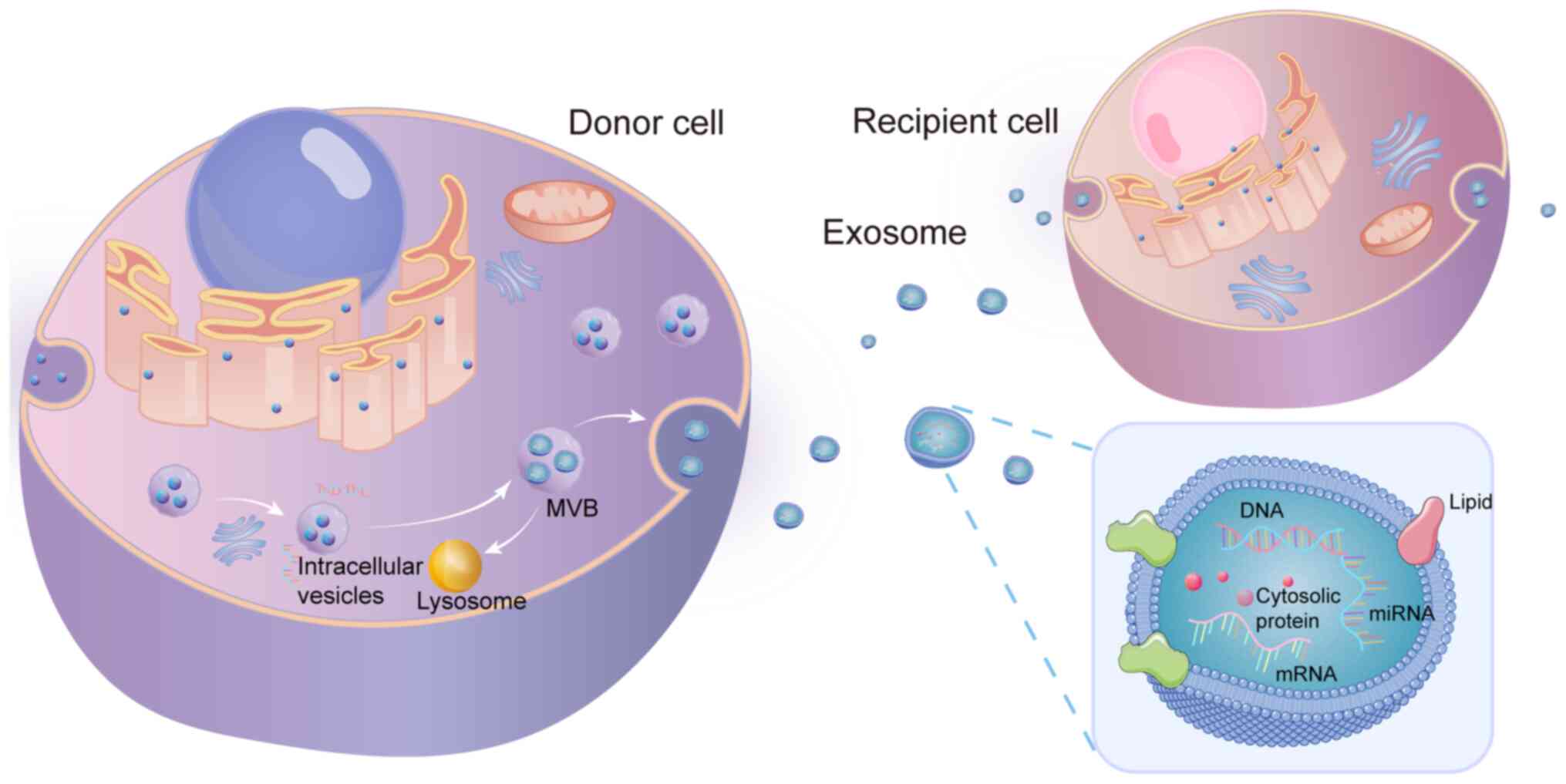
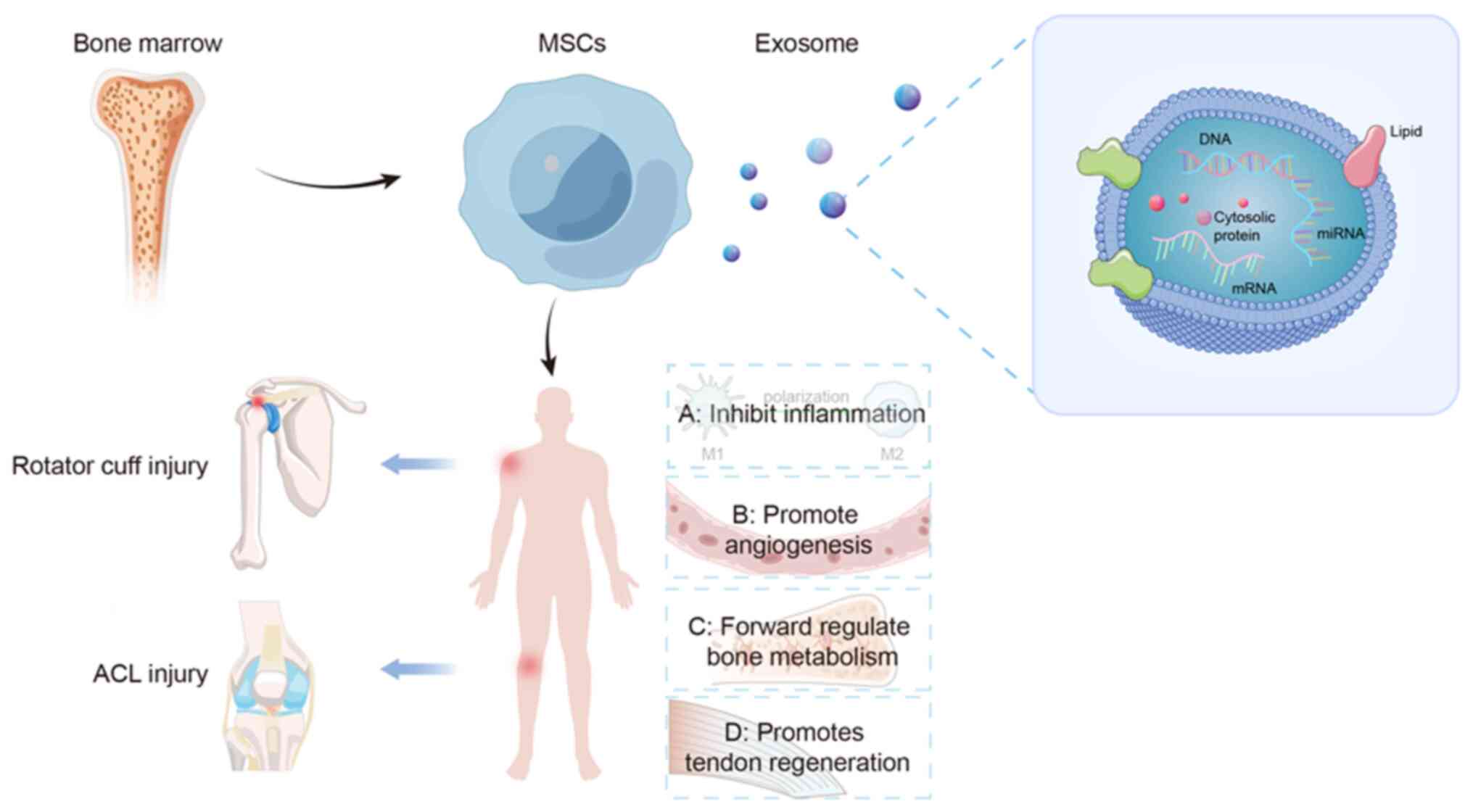
Exosomes (EXOs) are nanoparticles with lipid
bilayers and membrane structures that are naturally released by
cells through cytoplasmic exocytosis (13). Research has indicated that BMSCs
release EXOs containing various bioactive substances in a paracrine
manner to regulate the local microenvironment of tissues and
physiological and pathological activities in cells and may be
critical factors by which BMSCs play a therapeutic role (14).
EXOs are derived from intracellular bodies termed
multivesicular bodies (MVBs) and are nanoscale extracellular
vesicles, with a diameter of ~30 to 150 nm (15,16) and a density between 1.1 and 1.2
g/ml. They are found in various cell types and extracellular
fluids, such as plasma, synovial fluid, urine, amniotic fluid,
saliva, cerebrospinal fluid and breast milk (17-21). The cell membrane invaginates to
form early endosomes, which turn into late endosomes under the
control of various cellular signaling pathways (22-24). Stage 2 refers to the budding of
late endosomes to form MVBs. Stage 3 of the process entails the
amalgamation of MVBs with the plasma membrane, followed by the
selective recruitment of cytoplasmic elements, including proteins,
RNA and lipids, to form intact EXOs. The process of EXO formation
is relatively complex and is regulated by a number of factors, such
as tetraspanins, cholesterol, endosomal sorting complexes
responsible for transport, sphingomyelinases and adhesion
molecules, after which they are released into the microenvironment
(25,26). When EXOs were first discovered,
scholars considered them a way to expel unwanted components from
cells (27). As research
progressed, receptor-ligand interactions with EXOs, and direct
membrane fusions for the delivery of biomolecules to target cells
and mediate intercellular communications have been verified
(28).
Exosomal vesicles are rich in lipids, proteins and
various types of nucleic acids, such as DNA, mRNAs, microRNAs
(miRNAs/miRs) and long non-coding RNAs, transcription factors and
cytokine receptors. Organelles, such as ribosomes and mitochondria
are typically absent. EXOs mediate cell-cell and cell-extracellular
matrix (ECM) communications through the biologically active
molecules within them to induce the necessary growth signals and
transcriptional changes to cause phenotypic changes in the local
environment (29,30). Various physiological and
pathological mechanisms have been observed to exert an effect on
recipient cells, including the amelioration of ischemic brain
injury and the stimulation of angiogenesis, which leads to the
acceleration of skin wound healing, thus holding promise as a
potential therapeutic approach for addressing osteoarthritis
(31-33). Depending on the cell type that
produces the EXOs and changes in the cellular microenvironment,
such as physiological and pathological states, the number of EXOs
secreted and the composition of the contents of EXOs varies
(34). For example, BMSC-derived
EXOs (BMSC-EXOs) contain a variety of anti-inflammatory factors and
growth factors, such as transforming growth factor-β (TGF-β),
interleukin (IL)-10 and tumor necrosis factor (TNF)-stimulated gene
((TSG)-6 (35). It has been
demonstrated that BMSC-EXOs contain miR-301a, miR-22 and miR-let-7,
which are involved in immune-related pathways (36). Given their significant osmotic
impact, EXOs possess the ability to traverse the blood-brain and
blood-spinal cord barriers effectively. Moreover, they exhibit a
selective propensity to infiltrate sites of inflammation within
bodily tissues, thereby exerting regulatory control over the
inflammatory response (37,38). In addition, the membrane
transport process is involved in the production of EXOs. Several
proteins that are closely related to this process, such as
tetraspanins (CD9, CD63 and CD81), the membrane proteins, annexin
and flotillin, heat shock proteins (HSP70 and HSP90) and markers of
the endosomal sorting complex required for transport pathway, have
been identified. Several proteins, including lysosomal-associated
membrane protein 1 and TSG101, are highly enriched in various
cell-derived EXOs (39).
Therefore, they are often used as the marker proteins of EXOs and
are widely used to extract and purify EXOs. The morphological and
structural pattern of EXOs is illustrated in Fig. 1.
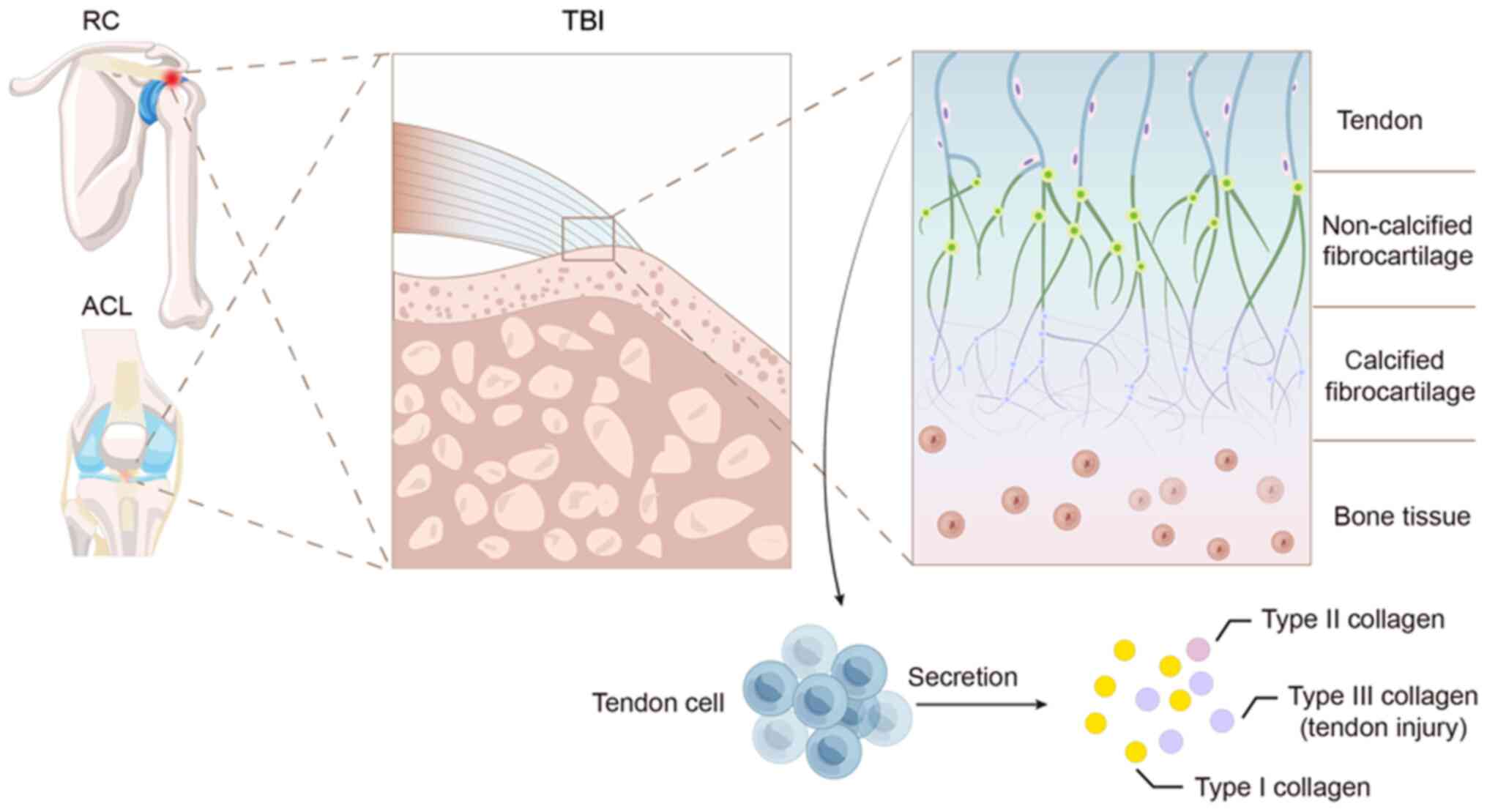
There are two types of TBI: Indirect insertion and
direct insertion. For example, indirect insertion involves the
insertion of the medial collateral ligament into the tibia. The ACL
and RC are anatomical structures that are intricately integrated
into the bone through a gradient structure comprising four
consecutive layers of distinct tissue. These layers can be
categorized from soft to hard, starting with the tendon, followed
by noncalcified fibrocartilage, calcified fibrocartilage and
ultimately bone tissue (40-42). Once the tendon-bone connection is
damaged, restoring the natural anatomical structure is difficult
(Fig. 2).
Tendon cells are responsible for synthesizing and
secreting collagen, and are the basic units of tendon function.
Healthy tendons primarily contain type I collagen, which provides
mechanical strength and durability. Type II collagen is found in
lower amounts and is typically concentrated where tendons connect
to bones. Following a tendon injury, type III collagen is abundant
in scar tissue and is crucial for tendon healing. An imbalance in
type III and type I collagen in the tendons can cause mechanical
damage. Notably, in addition to altering the phenotype and function
of various cells, MSC-EXOs can also increase the content of type
III collagen by increasing the level of TGF-β1, thus promoting
tendon healing (43).
Tendon cells are closely associated with RC disease;
studies have shown that they are involved in tendon repair through
proliferation and migration (44). For example, tendon stem
cell-derived EXOs induce tendon differentiation in mesenchymal stem
cells via TGF-β (45), promote
healing in injured tendons by balancing the synthesis and
degradation of tendon extracellular matrix (6), and can regulate inflammation and
promote high-quality healing of injured tendons (46).
TBI injuries are typically classified as acute and
chronic. If not repaired in time, acute injury can gradually
transform into chronic injury. The overuse or overload of tendons
often leads to injury (42),
while other factors such as age, metabolism and blood pressure can
also exacerbate tendon injury (43). This condition severely affects
the quality of life of affected individuals, and is a major cause
of pain and disability (6). The
distinguishing characteristics of the healing process between
tendons and bones encompass the fusion of bone, heightened
mechanical thresholds and the constricting of the bone tunnel
(47,48). Tendon healing is divided into
four phases: Inflammatory, proliferative, remodeling and maturation
(49). During tendon healing,
the levels of several growth factors, including TGF-β,
platelet-derived growth factor, vascular endothelial growth factor
(VEGF) and insulin-like growth factor-1, are increased and play
critical roles in all stages of the recovery process (50).
TBI healing is very complex for several reasons.
First, the region of fibrocartilage at the site of injury exhibits
a deficiency in cells and blood supply, leading to a delayed or
impaired healing process in TBI (51). Second, the regeneration of new
bone is typically very slow, and strength and stiffness are
correspondingly reduced (2).
Third, during the healing of TBI, an initial inflammatory response
occurs at the junction between the tendon and bone, and a large
number of macrophages infiltrate, fibroblasts release extracellular
matrix to form granulomas, and a large amount of collagen is
deposited; the occurrence of fibrovascular scar tissue formation in
the local area is a consequence that can be observed (52). In contrast to the initial
anatomical arrangement, scar tissue has a significant impact on the
process of new bone generation and the development of the interface
between the tendon and bone. In comparison to the original
physiological system, the presence of scar tissue has notable
implications for the regeneration of new bone. The development of
the tendon-bone interface and its ability to mediate load
transmission and stress dispersion experiences a notable decline,
which leads to decreased biomechanical properties in poorly
regenerated tendons, impaired motor function, a shortened service
life and a significantly reduced quality of healing (8,53).
Non-surgical treatments often need to be revised.
These strategies can only be used to control pain (54). Therefore, in the majority of
cases, TBI injury requires surgical intervention. Thus, for
decades, surgical reconstruction has been considered the standard
treatment for attachment point injuries. The clinical treatment of
ACL and RC injuries often involves tendon/ligament reconstruction
surgery, in which the insertion of the tendon graft into the bone
tunnel is essential for promoting tendon-bone healing. A critical
aspect of this healing process is the regeneration of the
fibrocartilage zone (55,56).
During the surgical procedure of reconstruction, the graft is
carefully positioned and subsequently maneuvered through a bone
tunnel. The indigenous and directly implanted transitional tissue
fails to undergo regeneration, instead giving rise to fibrovascular
scar tissue at the interface of the graft and tunnel (57). Initially, this tissue exhibits a
lack of organization. After 3-4 weeks, vertical fibers that
resemble indirectly inserted threads begin to form. At ~1 year
after surgery, the bone gradually grows into the graft-tunnel
interface, enhancing the strength of graft attachment and
facilitating the integration of the graft with the adjacent bone
(57).
The process of fibrocartilage healing involves the
formation of scar tissue following an injury, which is rich in type
I collagen (40,58,59). The fibrocartilage zone plays a
vital role in the absorption of stress that occurs between the
tendon and bone (2).
Nevertheless, scar tissue does not possess the inherent gradient
structure and organized alignment of collagen fibers present in the
native tendon-bone interface, resulting in low biomechanical
properties, weak mechanical strength and increased re-tearing rate
(60). There are data indicating
high recurrence rates following TBI; for example, recurrent retear
rates after RC repair are typically 20 to 30%, and can be as high
as 94%, and ACL reconstruction has an average failure rate of 11.7%
(40,61,62). For other commonly injured tendons
and ligaments, such as the distal biceps tendon and lateral ankle
sprain (anterior talofibular ligament, calcaneofibular ligament and
posterior talofibular ligament), the rates of re-rupture were 1.6
and 18.1%, respectively (63).
The most important cause of recurrent retears suboptimal
tendon-bone healing. Promoting the healing of the connection
between tendons and bones is crucial in the treatment of TBI injury
and in preventing the occurrence of another tear after
reconstruction. In contrast to interosseous recovery, the
regeneration of fibrocartilage transitions is restricted, and TBI
regeneration is particularly difficult. Insufficient
osseointegration following surgical reconstruction is one of the
main reasons for unsatisfactory clinical results (64). In addition, joint stiffness and
pain caused by long-term post-operative immobilization can
significantly affect the outcome of surgical treatment.
Over the past few years, there has been a
significant application of growth factors/cytokines, platelet-rich
plasma, physiotherapy, tissue engineering, and different delivery
and induction techniques (65-68). Currently, a diverse range of
growth factors and cytokines, such as TGF-β, bone morphogenetic
protein (BMP) and granulocyte colony-stimulating factor, have been
employed to enhance the healing of tendon grafts within bone
tunnels in different animal models. However, the implementation of
these methods in clinical settings still has certain restrictions.
Of note, retaining these biological factors at the specific
location where the tendon or ligament injury occurred is
challenging, and they are lost or rapidly removed (62).
In recent years, multiple preclinical studies have
demonstrated the role of PRP in promoting TBI interface healing and
improving biomechanical properties (69-71). Several systematic reviews have
reported that PRP can enhance tendon and ligament tissue by
promoting tendon cell proliferation and angiogenesis, thus
significantly promoting healing and controlling pain, and this
treatment has exhibited sound therapeutic effects on RC and lateral
elbow injuries (72-74). However, some studies have
reported the potential limitations of PRP therapy; for example, the
results of a randomized controlled trial suggested that injecting
platelet-rich plasma did not improve tendon function or the quality
of life in patients with severe acute Achilles tendon rupture.
There was no evidence of any benefit from the injection of
platelet-rich plasma (75). In
addition, Bennell et al (76) used PRP therapy to treat patients
with knee osteoarthritis and did not achieve significant efficacy.
In summary, as a new technology, PRP therapy has the advantages of
less trauma and fewer complications. It is a promising treatment
strategy for TBI injury; however, its application scope,
indications and efficacy warrant further investigation.
Stem cell-based cell therapy is anticipated to
emerge as a viable substitute for tissue engineering therapy.
Mesenchymal stem cells can self-renew and differentiate into
various cell types, including adipocytes, osteoblasts and
chondrocytes, and exhibit low immunogenicity when transplanted. In
the context of healing tendons and bones, the stem cells that are
frequently utilized are typically obtained from either the bone
marrow (BMSCs) or adipose tissue (adipose tissue-derived stem
cells). It has been demonstrated that mesenchymal stem cells
derived from the periosteum, synovium and tendon have been
extensively employed to enhance the healing process at locations
where tendons and bones meet (77). Although the results of cell
therapies are promising, they also have some limitations. For
instance, the absence of a standardized protocol for the dosage and
frequency of stem cell therapy hinders the attainment of optimal
outcomes. This poses a significant challenge to the widespread
clinical implementation of stem cells (55,78). The clinical application of stem
cell transplantation therapy still needs to be improved, including
factors such as cell dedifferentiation, low cell survival rate,
immune rejection and ethical issues (79). To address these limitations, it
is necessary to investigate an innovative treatment method that can
effectively meet the clinical requirements and enhance the healing
process of the tendon and bone. The conventional treatment methods
for TBI injuries, as well as their advantages, disadvantages and
clinical applications are presented in Table I.
BMSCs-EXOs have been used to treat diseases of the
respiratory system (such as the treatment of acute lung injury
(ALI), circulatory system (such as the treatment of ischemic
myocardial infarction), digestive system (such as the repair of
liver damage), nervous system diseases (such as the treatment of
brain injury and stroke), urinary system diseases (such as the
treatment of ischemia reperfusion kidney injury), reproductive
system diseases (such as repairing ovarian function) and breast
diseases (such as breast cancer treatment). At present, BMSC-EXOs
have been used in the research of diseases of multiple systems,
providing new insight for the research and application of clinical
drugs in the future. With extensive research on mesenchymal stem
cells in recent years, BMSC-EXOs have also been widely used in the
treatment of motor system diseases, and BMSC-EXOs have been used to
treat TBI injury.
As natural cell products, EXOs have good
biocompatibility, can penetrate biological barriers, exert
therapeutic effects and can be used to treat bone-related diseases.
It has been reported that mesenchymal stem cells from different
sources can be used to promote tendon-bone healing, and in recent
times, a substantial amount of evidence has indicated that the
beneficial impacts of stem cell therapy could potentially occur
through the release of extracellular vesicles termed EXOs from MSCs
via a process known as paracrine signaling (2,58,80-83). This paracrine mechanism, which is
known as stem cell conditioned medium (CM), secretome CM or
secretome, is the medium in which stem cells are cultured and
contains soluble proteins, lipids, nucleic acids and extracellular
vesicles or microvesicles (84,85). These vesicles are further divided
into EXOs and shedding vesicles. A summary of the advances in the
promotion of tendon and bone healing by BMSC-EXOs is presented in
Table II.
EXOs appear to be a more promising treatment option
than stem cell therapy. The benefits of EXOs are mainly
characterized by several aspects: i) EXOs are smaller and have a
simple structure and composition, rendering them easier to separate
and preserve, while exhibiting a lower immunogenicity; ii) EXOs can
block the metastasis of cells that may contain immunogenic
molecules or even mutated or damaged DNA; iii) EXOs can penetrate
any organ and pass through it effortlessly due to their nanoscale
size, unlike large cells that cannot migrate through capillaries to
the injury site; iv) EXOs have the ability to move to different
parts of the body as they have specific molecules on their surfaces
that guide their migration; v) EXOs, being an integral part of the
human body, possess biochemical characteristics that are akin to
their originating cells. As a result, they are capable of evading
phagocytosis, merging with cell membranes and lysosomal fusion
(86,87).
Macrophages are derived from the bone marrow
mononuclear cell line, are essential to the innate immune system
and play an indispensable role in the immune response. Macrophages
are divided into classically activated (M1) macrophages and
alternately activated (M2) macrophages in response to various
environmental stimuli. These two types of macrophages exhibit
significant heterogeneity in phenotype and function. M1 and M2
macrophages are considered to have pro-inflammatory and fibrotic
phenotypes, respectively (88,89). Previous studies have demonstrated
that macrophages play a non-negligible role in the occurrence and
development of TBI injury (90-92). In the early stages following
tissue injury or tendon/ligament reconstruction, macrophages are
recruited in large numbers to the graft-tunnel interface and are
polarized toward the M1 type, inducing inflammatory responses and
engulfing apoptotic cells, and removing cell debris (90). During this period, the expression
of various pro-inflammatory factors, including TNF-α, IL-12, and
inducible nitric oxide synthase, is significantly increased,
thereby amplifying the inflammatory response in the affected area
(90). The presence of
inflammation in the surrounding environment helps attract
additional cells from various locations to travel to the injured
area and assist in the preparation of future tissue healing. In the
advanced phase of the injury, M2 macrophages replace M1 macrophages
in large numbers and secrete anti-inflammatory factors, such as
IL-10 and TGF-β; by doing so, this decreases the localized
inflammation and enhances the local regeneration and repair of
tissues (93). Thus,
accelerating macrophage polarization from M1 to M2 can accelerate
tissue repair (94). If this is
not achieved, it can lead to a prolonged inflammatory phase,
increased apoptosis and decreased cell proliferation, resulting in
slow healing. In addition, this condition can induce the excessive
secretion of ECM by fibroblasts, leading to soft tissue fibrosis
and scar tissue formation at the injury site, which hinders
cartilage regeneration and graft remodeling (95-97). Therefore, regulating the
polarization of macrophages may be key to promoting early
tendon-bone healing.
Previous studies have reported that EXOs regulate
inflammation through macrophage polarization, reduce cell
infiltration and matrix deposition, promote collagen formation, and
improve fiber continuity and alignment during tendon healing
remodeling (46,98,99). In addition, EXOs derived from
mesenchymal stem cells have been shown to modulate macrophage
polarization in several in vitro and in vivo studies.
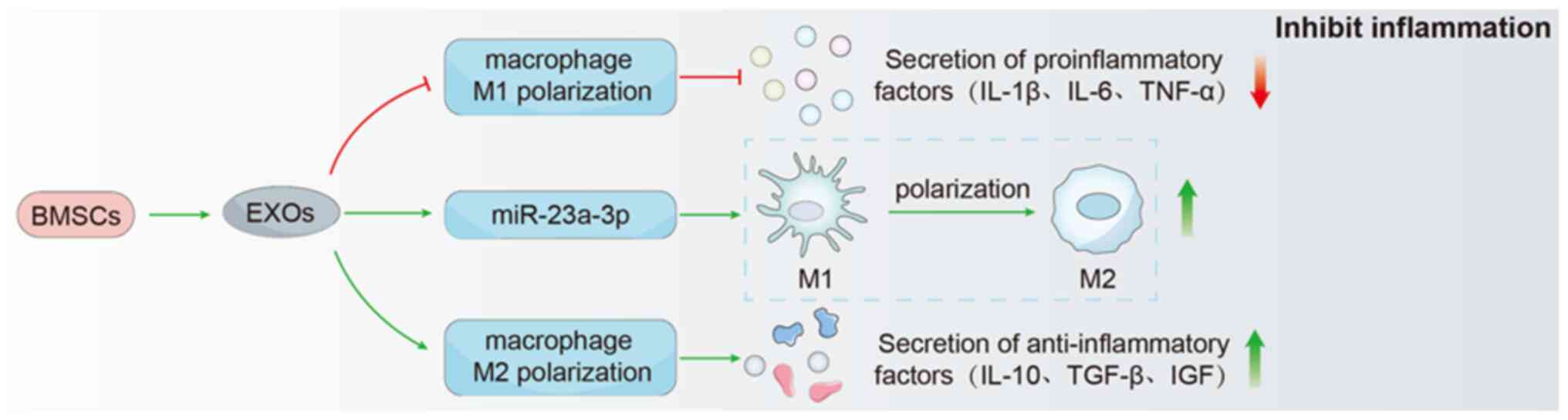
For example, Huang et al (100) discovered that BMSC-EXOs were
able to suppress inflammation by preventing the activation of M1
macrophages and the release of pro-inflammatory substances. Their
study confirmed that BMSC-EXOs have the potential to enhance the
fracture load and stiffness in the reconstructed RC, thereby
inducing angiogenesis and inhibiting inflammation around RC
endpoints, and promoting the healing of tendons and bones following
RC reconstruction in rats. Li et al (8), in their groundbreaking study,
demonstrated that BMSC-EXOs have the ability to induce the
transformation of M1 macrophages into M2 macrophages through the
involvement of miR-23a-3p. This finding suggests that early
treatment with BMSC-EXOs may effectively suppress the inflammatory
response at the interface between the tendon and bone. Furthermore,
it was observed that BMSC-EXOs facilitated the regeneration of
fibrocartilage and expedited the healing process of the tendon-bone
junction following ACL reconstruction. Moreover, miR-23a-3p
overexpression enhanced the therapeutic effect (8). Shi et al (101) demonstrated that BMSC-EXOs
improved the inflammatory microenvironment and promoted
fibrocartilage regeneration at the tendon-bone interface by
increasing M2 macrophage polarization, thereby reducing the
expression of the pro-inflammatory cytokines. IL-1β and IL-6. and
enhancing the expression of the anti-inflammatory cytokines, IL-10,
TGF-β and insulin-like growth factor during tendon healing.
Furthermore, it is possible to enhance the biomechanical
characteristics of the healing process between tendons and
bones.
The characteristics of EXOs exhibit potential for
reducing initial inflammatory reactions, which is necessary for
effective tissue healing (102). However, the specific mechanism
through which BMSC-EXOs control the polarization and function of M2
macrophages in TBI injury is not yet completely understood and
warrants further investigation. The mechanisms of BMSC-EXOs in
promoting tendon-bone healing are illustrated in Fig. 3.
After experiencing a traumatic brain injury, there
is a decrease in blood flow to the area where the tendon connects
to the bone. This decrease in blood flow means that important
nutrients that are necessary for the healing of the tendon and bone
are not being delivered properly. As a result, the biomechanical
properties of the tendon and bone are negatively affected, which in
turn affects the overall recovery of the tendon and bone (103). Numerous research studies have
provided evidence that neovascularization plays a crucial role in
facilitating the healing process of tendon-bone. It has been
consistently demonstrated that following RC reconstruction,
tendon-bone healing quality can be effectively improved by
improving the blood supply at the tenodesis point or trough, where
the blood supply is relatively poor (104).
VEGF is a key factor that regulates the natural
formation of new blood vessels in the body. It plays a crucial role
in activating, increasing in number and facilitating the movement
of endothelial cells which line the inside of blood vessels and
improve the circulation of blood to the transplanted tendon, which
in turn facilitates the healing of the tendon-bone connection
(103). Takayama et al
(105) provided evidence that
suppressing the expression of VEGF hindered the process of blood
vessel formation and also hindered the mechanical integrity of
tendon grafts following ACL reconstruction surgery. Furthermore,
the overexpression of VEGF affected the enhancement of the
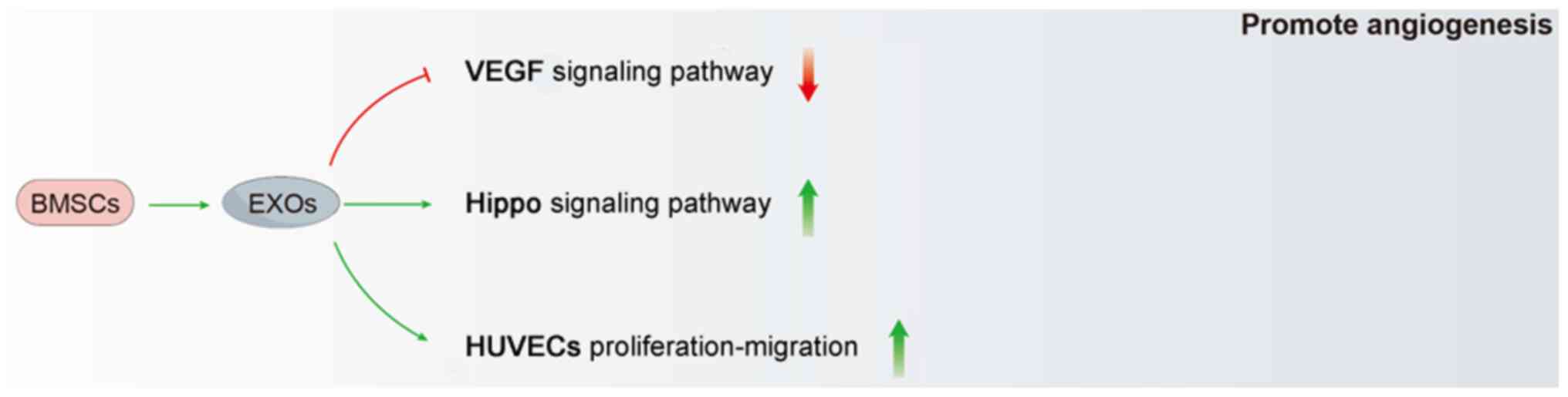
biomechanical strength of tendon grafts (105). Huang et al (100) discovered that BMSC-EXOs have
the ability to enhance the formation of new blood vessels around
the area where the tendon and bone meet. Additionally, they found
that these exosomes can increase the strength and rigidity of the
tendon in rats following reconstruction, as well as stimulate the
growth of the tendon-bone junction (100). During further mechanistic
analyses, the researchers verified that BMSC-EXOs have the ability
to stimulate the VEGF and Hippo signaling pathways. Additionally,
they can enhance the growth, movement and formation of blood
vessels in human umbilical vein endothelial cells in vitro
(100). Notably, the
researchers discovered that the stimulation of the VEGF and Hippo
signaling pathways by BMSC-EXOs could be separate. This indicates
that the activation of the Hippo signaling pathway by BMSC-EXOs
does not solely rely on the VEGF signaling pathway. This suggests
that BMSC-EXOs have extensive and beneficial impacts on enhancing
angiogenesis (Fig. 4).
Bone is a dynamic tissue that undergoes continuous
remodeling by a delicate equilibrium between the creation of new
bone by osteoblasts and the breakdown of old bone by osteoclasts.
The process of bone formation is carefully regulated and involves
the direct transformation of BMSCs into osteoblasts. Following
surgical reconstruction, up to 25% of patients require revision
surgery, partly due to traumatic, technical, bacterial and
biological factors. A significant factor contributing to this issue
is the deterioration of bone surrounding the graft, which directly
affects the secure connection of the transplanted tendon to the
bone tunnel. Therefore, reducing bone loss around the graft may be
a therapeutic strategy with which to promote tendon-bone tunnel
healing and reduce the rate of reconstruction failure.
The healing quality of the grafted tendon-to-bone
tunnel is closely related to the formation of bone around the
graft, which is inseparable from early post-operative angiogenesis.
The blood vessels located at the boundary between the transplanted
tendon and the bone tunnel supply an ample amount of oxygen and
nutrients to the cells. As a result, this has a direct impact on
the formation of new bone around the graft (100,106). Zhang et al (107) examined a rat ACL reconstruction
model and demonstrated that BMSC-EXOs promoted the formation of
blood vessels around the graft and improved bone microstructure,
which accelerated the healing of the transplanted tendon-bone
tunnel following ACL reconstruction.
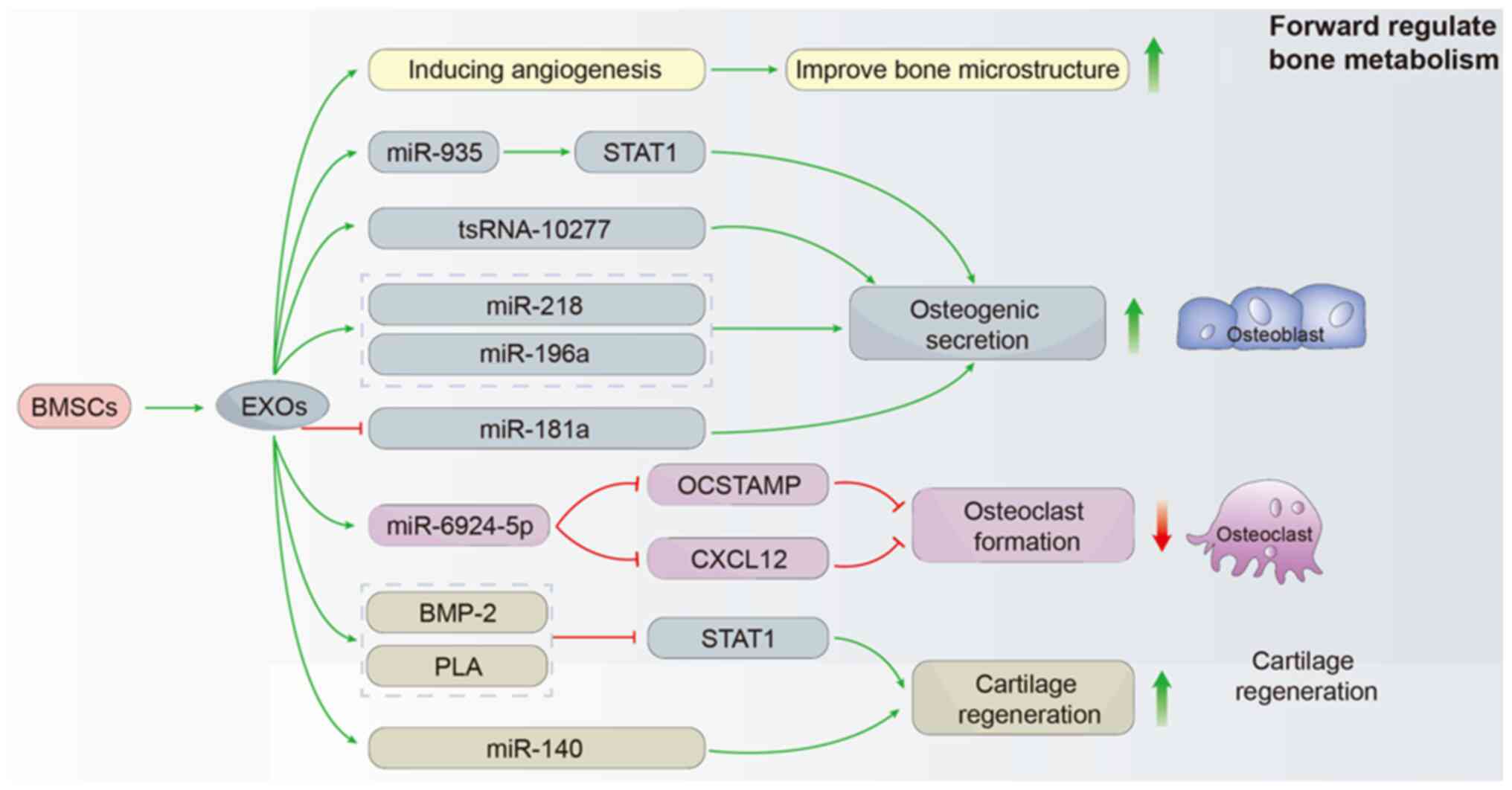
BMSC-EXOs can participate in bone remodeling by
directly regulating the proliferation and activity of osteoblasts.
Fang et al (108) found
that BMSC-EXOs containing tsRNA-10277 altered the adipogenic and
osteogenic potential of BMSCs. Zhang et al (109) found that miR-935-enriched EXOs
produced by BMSCs directly enhanced osteoblast proliferation and
activity by targeting signal transducer and transcription 1
activation to promote osteoblast proliferation and differentiation.
In addition, miR-218, miR-196a and miR-181a contained in BMSC-EXOs
have been confirmed to exert positive regulatory effects on
osteoblast differentiation (110).
In addition to targeting osteoblasts, BMSC-EXOs can
regulate the activity of osteoclasts, thereby regulating bone
metabolism at the tendon-bone interface. Feng et al
(111) demonstrated that EXOs
containing a high amount of miR-6924-5p, derived from platelet
derived growth factor receptor α (+) BMSCs overexpressing
Scleraxis, could be used as a novel type of nanotherapeutic agent.
These EXOs were able to prevent the formation of osteoclasts by
targeting two specific proteins: Osteoclast stimulatory
transmembrane protein and chemokine (C-X-C motif) ligand 1. In
addition, this treatment could effectively hinder the process of
tunnel osteolysis and enhance the biomechanical stability of
tendon-bone healing (111).
Fibrocartilage is an essential component of the
tendon-bone interface. Recently, Han et al (112) reported that BMP-2 and
polylactic acid delivered by BMSC-EXOs in polyaspartic
acid-polylactic acid-glycolic acid copolymer microcapsules promoted
chondrogenic differentiation through the Smad/RUNX2 pathway,
enhanced tendon interface stiffness and ultimate load strength, and
promoted tendon-bone healing in rabbits with acute RC tears.
BMSC-EXOs that have undergone low-intensity pulsed ultrasound
stimulation (LIPUS) have the potential to enhance the regeneration
of fibrocartilage at the interface between the tendon and bone.
Additionally, they can help reduce the infiltration of fat in the
supraspinatus muscle in a mouse model of RC repair. This is
achieved by delivering miR-140 (113). Cai et al (114) reported that the local injection
of EXOs derived from kartogenin-preconditioned BMSCs had the
potential to efficiently stimulate the development of cartilage,
enhance the maturation of collagen, and facilitate the regeneration
of tissues in the rotator cuffs of rats with chronic RC tears, and
enhance biomechanical properties following RC repair (Fig. 5).
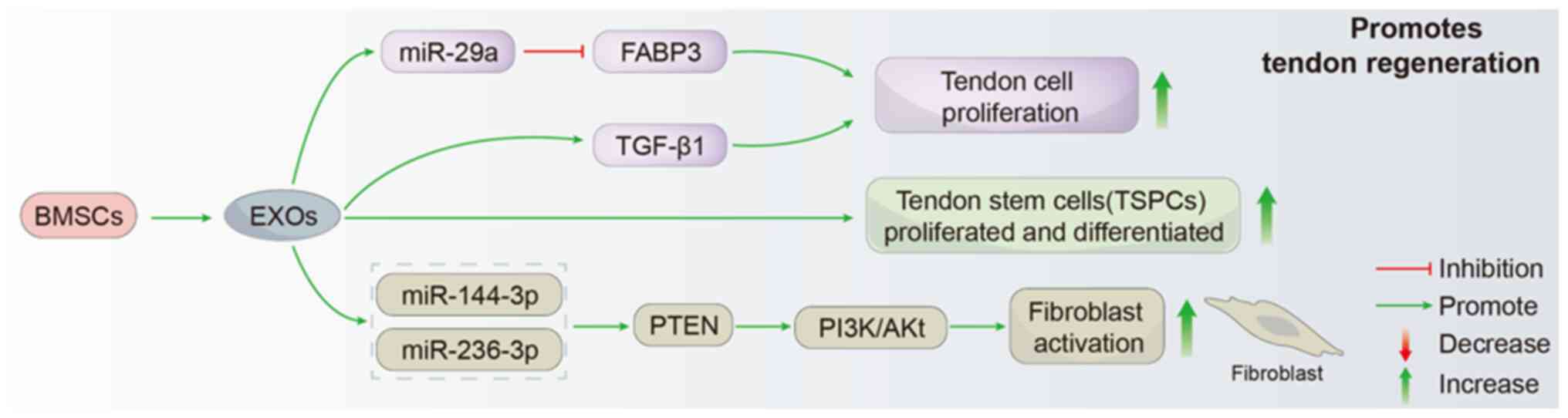
The proliferation and migration of tendon cells are
involved in tendon tissue repair and tendon-bone healing (115). Ample research has validated the
positive impact of BMSC-EXOs in facilitating the restoration of
tendons. For example, Yu et al (116) demonstrated that BMSC-EXOs
promoted the proliferation, migration and tendon differentiation of
tendon stem/progenitor cells (TSPCs) in vitro, and
subsequent in vivo analyses further confirmed that BMSC-EXOs
could be taken up and internalized by rat TSPCs, thereby promoting
the proliferation and migration of TSPCs. This effect was
characterized by improved histological scores of patellar tendons,
the enhanced expression of Mohawk, tending-regulatory protein and
type I collagen, and improved mechanical properties of the new
tendons (116). Subsequent
studies confirmed that exosomes derived from BMSCs promoted the
proliferation, migration and fibrotic activity of rotator cuff
tendon cells, and TGF-β1 was a key molecule that mediated the
effect of exosomes (117).
Pre-treatment of BMSCs with TGF-β1 can also significantly promote
the secretion and release of EXOs from BMSCs. Li et al
(118) first described that
TGF-β1 treatment promoted BMSC-EXO secretion, and miR-29a promoted
tendon cell proliferation, migration and fibrosis by targeting
fatty acid binding protein 3, thereby improving tendon injury and
RC tear. Several previous studies have shown that BMSCs-EXOs can
promote skin wound healing by regulating the activation and
proliferation of fibroblasts (119-121). Recently, Li et al
(122) confirmed that BMSC-EXOs
also play a critical role in promoting tendon-bone healing by
promoting the proliferation and differentiation of fibroblasts. In
addition, the downstream targets of miRNA, which is an active
molecule in BMSCs-EXOs, have also been more fully verified in their
study. According to the study conducted by Li et al
(122), it was confirmed that
miR-144-3p and miR-23b-3p enhanced the proliferation, migration and
collagen synthesis of NIH3T3 fibroblasts through both
bioinformatics analysis and in vitro experiments. Further
analyses demonstrated that miR-144-3p and miR-23b-3p promoted
fibroblast activation through the upregulation of the PTEN and
PI3K/Akt signaling pathways (122). This discovery lays a
theoretical foundation for RC tear therapy and provides a new
avenue for further research (Fig.
6).
EXOs are small soluble vesicles secreted by various
cells that can be found in cell cultures and bodily fluids. Their
lipid bilayers protect proteins and nucleic acids from degradation
in the extracellular environment, and facilitate intercellular
communication by carrying contents across the cell membrane to the
cytoplasm of recipient cells. EXOs play a crucial role in
regulating life activities (123,124). BMSCs have been known to promote
tissue healing and regeneration due to their multipotent stem cell
properties. BMSC-EXOs, which are key products secreted by BMSCs,
have also been shown to possess regenerative qualities similar to
those of parental BMSCs. BMSC-EXOs exhibit great promise in serving
as diagnostic or prognostic biomarkers, drug delivery systems and
carriers for gene therapy in the clinical setting. These findings
provide a new perspective for the study of promoting tendon healing
and bring new opportunities.
In recent years, with increasingly extensive and
in-depth research on the treatment of TBI injury, an increasing
number of new methods have been developed. Numerous studies have
suggested that purified BMSC-EXOs offer several unique advantages
over BMSCs in repairing damaged tissues. They have stable
biological activity, long-term preservation, fast transportation,
strong permeability, good biocompatibility, ease of engineering and
the ability to avoid immune responses and tumorigenesis (8,14,125). Therefore, BMSC-EXO-based
decellularized therapy is a promising therapeutic approach for
maintaining the regenerative properties of BMSCs, while avoiding
the potential downsides associated with cell therapy (126,127).
While the beneficial effects of BMSC-EXOs on
tendon-bone healing have been initially confirmed, further
extensive and in-depth studies are warranted. Clinical trials for
safety and efficacy should be accelerated and performed as soon as
possible. Additionally, the extraction and purification of EXOs
needs to improved, as factors such as high cost, complex
technology, low yield and the ease of destruction limit their broad
clinical applications (128).
The future of EXOs in biomedical engineering is an area that
requires further investigation.
In summary, BMSC-EXOs have a vast potential for use
in tendon-bone healing and repair. This breakthrough presents fresh
possibilities for fundamental scientific investigation, the
diagnosis of medical conditions, and the management of associated
illnesses. However, further research is required in order to fully
comprehend the mechanisms through which BMSC-EXOs promote healing
and improve the clinical application of EXOs (Fig. 7).
Not applicable.
HZ provided a brief introduction to the article. YZ
and GC were responsible for the writing of the manuscript. YZ
prepared the tables. HZ and JY revised the manuscript. All authors
have read and approved the final version of the manuscript. Data
authentication is not applicable.
Not applicable.
Not applicable.
The authors declare that they have no competing
interests.
Not applicable.
The present study was supported by the Shandong Traditional
Chinese Medicine Technology Project (grant no. 2021M156).
|
1
|
Li M, Tang Y, Chen C, Zhou J, Zheng C,
Chen H, Lu H and Qu J: Comparison of bone surface and trough
fixation on bone-tendon healing in a rabbit patella-patellar tendon
injury model. J Orthop Translat. 21:49–56. 2020.
|
|
2
|
Zou J, Yang W, Cui W, Li C, Ma C, Ji X,
Hong J, Qu Z, Chen J, Liu A and Wu H: Therapeutic potential and
mechanisms of mesenchymal stem cell-derived exosomes as bioactive
materials in tendon-bone healing. J Nanobiotechnology.
21:142023.
|
|
3
|
Zou M, Wang J and Shao Z: Therapeutic
potential of exosomes in tendon and tendon-bone healing: A
systematic review of preclinical studies. J Funct Biomater.
14:2992023.
|
|
4
|
Yamamoto A, Takagishi K, Osawa T, Yanagawa
T, Nakajima D, Shitara H and Kobayashi T: Prevalence and risk
factors of a rotator cuff tear in the general population. J
Shoulder Elbow Surg. 19:116–120. 2010.
|
|
5
|
Musahl V and Karlsson J: Anterior cruciate
ligament tear. N Engl J Med. 380:2341–2348. 2019.
|
|
6
|
Wang Y, He G, Guo Y, Tang H, Shi Y, Bian
X, Zhu M, Kang X, Zhou M, Lyu J, et al: Exosomes from tendon stem
cells promote injury tendon healing through balancing synthesis and
degradation of the tendon extracellular matrix. J Cell Mol Med.
23:5475–5485. 2019.
|
|
7
|
Arabpour M, Saghazadeh A and Rezaei N:
Anti-inflammatory and M2 macrophage polarization-promoting effect
of mesenchymal stem cell-derived exosomes. Int Immunopharmacol.
97:1078232021.
|
|
8
|
Li Z, Li Q, Tong K, Zhu J, Wang H, Chen B
and Chen L: BMSC-derived exosomes promote tendon-bone healing after
anterior cruciate ligament reconstruction by regulating M1/M2
macrophage polarization in rats. Stem Cell Res Ther.
13:2952022.
|
|
9
|
Yokoya S, Mochizuki Y, Natsu K, Omae H,
Nagata Y and Ochi M: Rotator cuff regeneration using a
bioabsorbable material with bone marrow-derived mesenchymal stem
cells in a rabbit model. Am J Sports Med. 40:1259–1268. 2012.
|
|
10
|
Rodeo SA, Potter HG, Kawamura S, Turner
AS, Kim HJ and Atkinson BL: Biologic augmentation of rotator cuff
tendon-healing with use of a mixture of osteoinductive growth
factors. J Bone Joint Surg Am. 89:2485–2497. 2007.
|
|
11
|
Anz AW, Hackel JG, Nilssen EC and Andrews
JR: Application of biologics in the treatment of the rotator cuff,
meniscus, cartilage, and osteoarthritis. J Am Acad Orthop Surg.
22:68–79. 2014.
|
|
12
|
Jungebluth P, Alici E, Baiguera S,
Blomberg P, Bozóky B, Crowley C, Einarsson O, Gudbjartsson T, Le
Guyader S, Henriksson G, et al: Tracheobronchial transplantation
with a stem-cell-seeded bioartificial nanocomposite: A
proof-of-concept study. Lancet. 378:1997–2004. 2011.
|
|
13
|
Nooshabadi VT, Mardpour S,
Yousefi-Ahmadipour A, Allahverdi A, Izadpanah M, Daneshimehr F, Ai
J, Banafshe HR and Ebrahimi-Barough S: The extracellular
vesicles-derived from mesenchymal stromal cells: A new therapeutic
option in regenerative medicine. J Cell Biochem. 119:8048–8073.
2018.
|
|
14
|
Kourembanas S: Exosomes: Vehicles of
intercellular signaling, biomarkers, and vectors of cell therapy.
Annu Rev Physiol. 77:13–27. 2015.
|
|
15
|
Abels ER and Breakefield XO: Introduction
to extracellular vesicles: Biogenesis, RNA cargo selection,
content, release, and uptake. Cell Mol Neurobiol. 36:301–312.
2016.
|
|
16
|
Xiang XN, Zhu SY, He HC, Yu X, Xu Y and He
CQ: Mesenchymal stromal cell-based therapy for cartilage
regeneration in knee osteoarthritis. Stem Cell Res Ther.
13:142022.
|
|
17
|
Lobb RJ, Becker M, Wen SW, Wong CS,
Wiegmans AP, Leimgruber A and Möller A: Optimized exosome isolation
protocol for cell culture supernatant and human plasma. J Extracell
Vesicles. 4:270312015.
|
|
18
|
Xu X, Liang Y, Li X, Ouyang K, Wang M, Cao
T, Li W, Liu J, Xiong J, Li B, et al: Exosome-mediated delivery of
kartogenin for chondrogenesis of synovial fluid-derived mesenchymal
stem cells and cartilage regeneration. Biomaterials.
269:1205392021.
|
|
19
|
McKiernan J, Donovan MJ, O'Neill V,
Bentink S, Noerholm M, Belzer S, Skog J, Kattan MW, Partin A,
Andriole G, et al: A novel urine exosome gene expression assay to
predict high-grade prostate cancer at initial biopsy. JAMA Oncol.
2:882–889. 2016.
|
|
20
|
Lässer C, Alikhani VS, Ekström K, Eldh M,
Paredes PT, Bossios A, Sjöstrand M, Gabrielsson S, Lötvall J and
Valadi H: Human saliva, plasma and breast milk exosomes contain
RNA: Uptake by macrophages. J Transl Med. 9:92011.
|
|
21
|
Jia L, Qiu Q, Zhang H, Chu L, Du Y, Zhang
J, Zhou C, Liang F, Shi S, Wang S, et al: Concordance between the
assessment of Aβ42, T-tau, and P-T181-tau in peripheral blood
neuronal-derived exosomes and cerebrospinal fluid. Alzheimers
Dement. 15:1071–1080. 2019.
|
|
22
|
Latifkar A, Hur YH, Sanchez JC, Cerione RA
and Antonyak MA: New insights into extracellular vesicle biogenesis
and function. J Cell Sci. 132:jcs2224062019.
|
|
23
|
Zakeri Z, Salmaninejad A, Hosseini N,
Shahbakhsh Y, Fadaee E, Shahrzad MK and Fadaei S: MicroRNA and
exosome: Key players in rheumatoid arthritis. J Cell Biochem.
120:10930–10944. 2019.
|
|
24
|
van Niel G, D'Angelo G and Raposo G:
Shedding light on the cell biology of extracellular vesicles. Nat
Rev Mol Cell Biol. 19:213–228. 2018.
|
|
25
|
Raposo G and Stoorvogel W: Extracellular
vesicles: Exosomes, microvesicles, and friends. J Cell Biol.
200:373–383. 2013.
|
|
26
|
Baglio SR, Rooijers K, Koppers-Lalic D,
Verweij FJ, Pérez Lanzón M, Zini N, Naaijkens B, Perut F, Niessen
HW, Baldini N and Pegtel DM: Human bone marrow- and
adipose-mesenchymal stem cells secrete exosomes enriched in
distinctive miRNA and tRNA species. Stem Cell Res Ther.
6:1272015.
|
|
27
|
Johnstone RM, Adam M, Hammond JR, Orr L
and Turbide C: Vesicle formation during reticulocyte maturation.
Association of plasma membrane activities with released vesicles
(exosomes). J Biol Chem. 262:9412–9420. 1987.
|
|
28
|
Mosquera-Heredia MI, Morales LC, Vidal OM,
Barceló E, Silvera-Redondo C, Vélez JI and Garavito-Galofre P:
Exosomes: Potential disease biomarkers and new therapeutic targets.
Biomedicines. 9:10612021.
|
|
29
|
Kalluri R and LeBleu VS: The biology,
function, and biomedical applications of exosomes. Science.
367:eaau69772020.
|
|
30
|
Théry C, Zitvogel L and Amigorena S:
Exosomes: Composition, biogenesis and function. Nat Rev Immunol.
2:569–579. 2002.
|
|
31
|
Qiu X, Liu J, Zheng C, Su Y, Bao L, Zhu B,
Liu S, Wang L, Wang X, Wang Y, et al: Exosomes released from
educated mesenchymal stem cells accelerate cutaneous wound healing
via promoting angiogenesis. Cell Prolif. 53:e128302020.
|
|
32
|
Keshtkar S, Azarpira N and Ghahremani MH:
Mesenchymal stem cell-derived extracellular vesicles: Novel
frontiers in regenerative medicine. Stem Cell Res Ther.
9:632018.
|
|
33
|
Ren Y, Zhang S, Wang Y, Jacobson DS,
Reisdorf RL, Kuroiwa T, Behfar A, Moran SL, Steinmann SP and Zhao
C: Effects of purified exosome product on rotator cuff tendon-bone
healing in vitro and in vivo. Biomaterials. 276:1210192021.
|
|
34
|
Fang WH, Agrawal DK and Thankam FG: 'Smart
exosomes': A smart approach for tendon regeneration. Tissue Eng
Part B Rev. 28:613–625. 2022.
|
|
35
|
Wang Z, Wu Y, Zhao Z, Liu C and Zhang L:
Study on transorgan regulation of intervertebral disc and
extra-skeletal organs through exosomes derived from bone marrow
mesenchymal stem cells. Front Cell Dev Biol. 9:7411832021.
|
|
36
|
Ma X, Becker Buscaglia LE, Barker JR and
Li Y: MicroRNAs in NF-kappaB signaling. J Mol Cell Biol. 3:159–166.
2011.
|
|
37
|
van den Boorn JG, Schlee M, Coch C and
Hartmann G: SiRNA delivery with exosome nanoparticles. Nat
Biotechnol. 29:325–326. 2011.
|
|
38
|
EL Andaloussi S, Mäger I, Breakefield XO
and Wood MJ: Extracellular vesicles: Biology and emerging
therapeutic opportunities. Nat Rev Drug Discov. 12:347–357.
2013.
|
|
39
|
Vlassov AV, Magdaleno S, Setterquist R and
Conrad R: Exosomes: Current knowledge of their composition,
biological functions, and diagnostic and therapeutic potentials.
Biochim Biophys Acta. 1820:940–948. 2012.
|
|
40
|
Chen H, Li S, Xiao H, Wu B, Zhou L, Hu J
and Li H: Effect of exercise intensity on the healing of the
bone-tendon interface: A mouse rotator cuff injury model study. Am
J Sports Med. 49:2064–2073. 2021.
|
|
41
|
Chen W, Sun Y, Gu X, Cai J, Liu X, Zhang
X, Chen J, Hao Y and Chen S: Conditioned medium of human bone
marrow-derived stem cells promotes tendon-bone healing of the
rotator cuff in a rat model. Biomaterials. 271:1207142021.
|
|
42
|
Cheng P, Han P, Zhao C, Zhang S, Wu H, Ni
J, Hou P, Zhang Y, Liu J, Xu H, et al: High-purity magnesium
interference screws promote fibrocartilaginous entheses
regeneration in the anterior cruciate ligament reconstruction
rabbit model via accumulation of BMP-2 and VEGF. Biomaterials.
81:14–26. 2016.
|
|
43
|
Hu J, Chen Y, Huang Y and Su Y: Human
umbilical cord mesenchymal stem cell-derived exosomes suppress
dermal fibroblasts-myofibroblats transition via inhibiting the
TGF-β1/Smad 2/3 signaling pathway. Exp Mol Pathol.
115:1044682020.
|
|
44
|
Lundgreen K, Lian OB, Engebretsen L and
Scott A: Tenocyte apoptosis in the torn rotator cuff: A primary or
secondary pathological event? Br J Sports Med. 45:1035–1039.
2011.
|
|
45
|
Xu T, Xu M, Bai J, Lin J, Yu B, Liu Y, Guo
X, Shen J, Sun H, Hao Y and Geng D: Tenocyte-derived exosomes
induce the tenogenic differentiation of mesenchymal stem cells
through TGF-β. Cytotechnology. 71:57–65. 2019.
|
|
46
|
Zhang M, Liu H, Cui Q, Han P, Yang S, Shi
M, Zhang T, Zhang Z and Li Z: Tendon stem cell-derived exosomes
regulate inflammation and promote the high-quality healing of
injured tendon. Stem Cell Res Ther. 11:4022020.
|
|
47
|
Sun Y, Chen W, Hao Y, Gu X, Liu X, Cai J,
Liu S, Chen J and Chen S: Stem cell-conditioned medium promotes
graft remodeling of midsubstance and intratunnel incorporation
after anterior cruciate ligament reconstruction in a rat model. Am
J Sports Med. 47:2327–2337. 2019.
|
|
48
|
Kuang GM, Yau WP, Lu WW and Chiu KY:
Osteointegration of soft tissue grafts within the bone tunnels in
anterior cruciate ligament reconstruction can be enhanced. Knee
Surg Sports Traumatol Arthrosc. 18:1038–1051. 2010.
|
|
49
|
Kovacevic D and Rodeo SA: Biological
augmentation of rotator cuff tendon repair. Clin Orthop Relat Res.
466:622–633. 2008.
|
|
50
|
Molloy T, Wang Y and Murrell G: The roles
of growth factors in tendon and ligament healing. Sports Med.
33:381–394. 2003.
|
|
51
|
Sharma P and Maffulli N: Tendon injury and
tendinopathy: Healing and repair. J Bone Joint Surg Am. 87:187–202.
2005.
|
|
52
|
Chamberlain CS, Leiferman EM, Frisch KE,
Duenwald-Kuehl SE, Brickson SL, Murphy WL, Baer GS and Vanderby R:
Interleukin-1 receptor antagonist modulates inflammation and
scarring after ligament injury. Connect Tissue Res. 55:177–186.
2014.
|
|
53
|
Xu Y and Murrell GAC: The basic science of
tendinopathy. Clin Orthop Relat Res. 466:1528–1538. 2008.
|
|
54
|
Sevivas N, Teixeira FG, Portugal R, Araújo
L, Carriço LF, Ferreira N, Vieira da Silva M, Espregueira-Mendes J,
Anjo S, Manadas B, et al: Mesenchymal stem cell secretome: A
potential tool for the prevention of muscle degenerative changes
associated with chronic rotator cuff tears. Am J Sports Med.
45:179–188. 2017.
|
|
55
|
Xu Y, Zhang WX, Wang LN, Ming YQ, Li YL
and Ni GX: Stem cell therapies in tendon-bone healing. World J Stem
Cells. 13:753–775. 2021.
|
|
56
|
Shengnan Q, Bennett S, Wen W, Aiguo L and
Jiake X: The role of tendon derived stem/progenitor cells and
extracellular matrix components in the bone tendon junction repair.
Bone. 153:1161722021.
|
|
57
|
Hao ZC, Wang SZ, Zhang XJ and Lu J: Stem
cell therapy: A promising biological strategy for tendon-bone
healing after anterior cruciate ligament reconstruction. Cell
Prolif. 49:154–162. 2016.
|
|
58
|
Lui PPY: Mesenchymal stem cell-derived
extracellular vesicles for the promotion of tendon repair-an update
of literature. Stem Cell Rev Rep. 17:379–389. 2021.
|
|
59
|
Patel S, Caldwell JM, Doty SB, Levine WN,
Rodeo S, Soslowsky LJ, Thomopoulos S and Lu HH: Integrating soft
and hard tissues via interface tissue engineering. J Orthop Res.
36:1069–1077. 2018.
|
|
60
|
Connor DE, Paulus JA, Dabestani PJ,
Thankam FK, Dilisio MF, Gross RM and Agrawal DK: Therapeutic
potential of exosomes in rotator cuff tendon healing. J Bone Miner
Metab. 37:759–767. 2019.
|
|
61
|
Diebold G, Lam P, Walton J and Murrell
GAC: Relationship between age and rotator cuff retear: A study of
1,600 consecutive rotator cuff repairs. J Bone Joint Surg Am.
99:1198–1205. 2017.
|
|
62
|
Wang J, Xu J, Wang X, Sheng L, Zheng L,
Song B, Wu G, Zhang R, Yao H, Zheng N, et al: Magnesium-pretreated
periosteum for promoting bone-tendon healing after anterior
cruciate ligament reconstruction. Biomaterials. 268:1205762021.
|
|
63
|
Lim WL, Liau LL, Ng MH, Chowdhury SR and
Law JX: Current progress in tendon and ligament tissue engineering.
Tissue Eng Regen Med. 16:549–571. 2019.
|
|
64
|
Ménétrey J, Duthon VB, Laumonier T and
Fritschy D: 'Biological failure' of the anterior cruciate ligament
graft. Knee Surg Sports Traumatol Arthrosc. 16:224–231. 2008.
|
|
65
|
Mihelic R, Pecina M, Jelic M, Zoricic S,
Kusec V, Simic P, Bobinac D, Lah B, Legovic D and Vukicevic S: Bone
morphogenetic protein-7 (osteogenic protein-1) promotes tendon
graft integration in anterior cruciate ligament reconstruction in
sheep. Am J Sports Med. 32:1619–1625. 2004.
|
|
66
|
Murray MM, Spindler KP, Ballard P, Welch
TP, Zurakowski D and Nanney LB: Enhanced histologic repair in a
central wound in the anterior cruciate ligament with a
collagen-platelet-rich plasma scaffold. J Orthop Res. 25:1007–1017.
2007.
|
|
67
|
Cervellin M, de Girolamo L, Bait C, Denti
M and Volpi P: Autologous platelet-rich plasma gel to reduce
donor-site morbidity after patellar tendon graft harvesting for
anterior cruciate ligament reconstruction: A randomized, controlled
clinical study. Knee Surg Sports Traumatol Arthrosc. 20:114–120.
2012.
|
|
68
|
Lu H, Liu F, Chen C, Wang Z, Chen H, Qu J,
Zhang T, Xu D and Hu J: Low-intensity pulsed ultrasound stimulation
for tendon-bone healing: A dose-dependent study. Am J Phys Med
Rehabil. 97:270–277. 2018.
|
|
69
|
Ersen A, Demirhan M, Atalar AC, Kapicioğlu
M and Baysal G: Platelet-rich plasma for enhancing surgical rotator
cuff repair: Evaluation and comparison of two application methods
in a rat model. Arch Orthop Trauma Surg. 134:405–411. 2014.
|
|
70
|
Zhang M, Zhen J, Zhang X, Yang Z, Zhang L,
Hao D and Ren B: Effect of autologous platelet-rich plasma and
gelatin sponge for tendon-to-bone healing after rabbit anterior
cruciate ligament reconstruction. Arthroscopy. 35:1486–1497.
2019.
|
|
71
|
Zhang J, Li F, Augi T, Williamson KM,
Onishi K, Hogan MV, Neal MD and Wang JH: Platelet HMGB1 in
platelet-rich plasma (PRP) promotes tendon wound healing. PLoS One.
16:e02511662021.
|
|
72
|
Chen X, Jones IA, Park C and Vangsness CT
Jr: The efficacy of platelet-rich plasma on tendon and ligament
healing: A systematic review and meta-analysis with bias
assessment. Am J Sports Med. 46:2020–2032. 2018.
|
|
73
|
Kim CH, Park YB, Lee JS and Jung HS:
Platelet-rich plasma injection vs operative treatment for lateral
elbow tendinosis: A systematic review and meta-analysis. J Shoulder
Elbow Surg. 31:428–436. 2022.
|
|
74
|
Chen X, Jones IA, Togashi R, Park C and
Vangsness CT Jr: Use of platelet-rich plasma for the improvement of
pain and function in rotator cuff tears: A systematic review and
meta-analysis with bias assessment. Am J Sports Med. 48:2028–2041.
2020.
|
|
75
|
Keene DJ, Alsousou J, Harrison P, Hulley
P, Wagland S, Parsons SR, Thompson JY, O'Connor HM, Schlüssel MM,
Dutton SJ, et al: Platelet rich plasma injection for acute Achilles
tendon rupture: PATH-2 randomised, placebo controlled, superiority
trial. BMJ. 367:l61322019.
|
|
76
|
Bennell KL, Paterson KL, Metcalf BR, Duong
V, Eyles J, Kasza J, Wang Y, Cicuttini F, Buchbinder R, Forbes A,
et al: Effect of intra-articular platelet-rich plasma vs placebo
injection on pain and medial tibial cartilage volume in patients
with knee osteoarthritis: The RESTORE randomized clinical trial.
JAMA. 326:2021–2030. 2021.
|
|
77
|
Lui PPY, Wong OT and Lee YW: Application
of tendon-derived stem cell sheet for the promotion of graft
healing in anterior cruciate ligament reconstruction. Am J Sports
Med. 42:681–689. 2014.
|
|
78
|
Akbari A, Jabbari N, Sharifi R, Ahmadi M,
Vahhabi A, Seyedzadeh SJ, Nawaz M, Szafert S, Mahmoodi M, Jabbari
E, et al: Free and hydrogel encapsulated exosome-based therapies in
regenerative medicine. Life Sci. 249:1174472020.
|
|
79
|
Lu V, Tennyson M, Zhang J and Khan W:
Mesenchymal stem cell-derived extracellular vesicles in tendon and
ligament repair-A systematic review of in vivo studies. Cells.
10:25532021.
|
|
80
|
Liu Q, Yu Y, Reisdorf RL, Qi J, Lu CK,
Berglund LJ, Amadio PC, Moran SL, Steinmann SP, An KN, et al:
Engineered tendon-fibrocartilage-bone composite and bone
marrow-derived mesenchymal stem cell sheet augmentation promotes
rotator cuff healing in a non-weight-bearing canine model.
Biomaterials. 192:189–198. 2019.
|
|
81
|
Rothrauff BB, Smith CA, Ferrer GA,
Novaretti JV, Pauyo T, Chao T, Hirsch D, Beaudry MF, Herbst E, Tuan
RS, et al: The effect of adipose-derived stem cells on enthesis
healing after repair of acute and chronic massive rotator cuff
tears in rats. J Shoulder Elbow Surg. 28:654–664. 2019.
|
|
82
|
Utsunomiya H, Sekiya I and Uchida S:
Editorial commentary: Are we ready to apply stem cell therapy in
rotator cuff tear surgery? Arthroscopy. 36:86–87. 2020.
|
|
83
|
Sun H, Pratt RE, Hodgkinson CP and Dzau
VJ: Sequential paracrine mechanisms are necessary for the
therapeutic benefits of stem cell therapy. Am J Physiol Cell
Physiol. 319:C1141–C1150. 2020.
|
|
84
|
Pawitan JA: Prospect of stem cell
conditioned medium in regenerative medicine. Biomed Res Int.
2014:9658492014.
|
|
85
|
Driscoll J and Patel T: The mesenchymal
stem cell secretome as an acellular regenerative therapy for liver
disease. J Gastroenterol. 54:763–773. 2019.
|
|
86
|
Riau AK, Ong HS, Yam GHF and Mehta JS:
Sustained delivery system for stem cell-derived exosomes. Front
Pharmacol. 10:13682019.
|
|
87
|
Malekpour K, Hazrati A, Zahar M, Markov A,
Zekiy AO, Navashenaq JG, Roshangar L and Ahmadi M: The potential
use of mesenchymal stem cells and their derived exosomes for
orthopedic diseases treatment. Stem Cell Rev Rep. 18:933–951.
2022.
|
|
88
|
Gordon S, Plüddemann A and Martinez
Estrada F: Macrophage heterogeneity in tissues: Phenotypic
diversity and functions. Immunol Rev. 262:36–55. 2014.
|
|
89
|
McWhorter FY, Wang T, Nguyen P, Chung T
and Liu WF: Modulation of macrophage phenotype by cell shape. Proc
Natl Acad Sci USA. 110:17253–17258. 2013.
|
|
90
|
Kawamura S, Ying L, Kim HJ, Dynybil C and
Rodeo SA: Macrophages accumulate in the early phase of tendon-bone
healing. J Orthop Res. 23:1425–1432. 2005.
|
|
91
|
Geng R, Lin Y, Ji M, Chang Q, Li Z, Xu L,
Zhang W and Lu J: MFG-E8 promotes tendon-bone healing by regualting
macrophage efferocytosis and M2 polarization after anterior
cruciate ligament reconstruction. J Orthop Translat. 34:11–21.
2022.
|
|
92
|
Chen Z, Jin M, He H, Dong J, Li J, Nie J,
Wang Z, Xu J and Wu F: Mesenchymal stem cells and macrophages and
their interactions in tendon-bone healing. J Orthop Translat.
39:63–73. 2023.
|
|
93
|
Klinkert K, Whelan D, Clover AJP, Leblond
AL, Kumar AHS and Caplice NM: Selective M2 macrophage depletion
leads to prolonged inflammation in surgical wounds. Eur Surg Res.
58:109–120. 2017.
|
|
94
|
Sindrilaru A, Peters T, Wieschalka S,
Baican C, Baican A, Peter H, Hainzl A, Schatz S, Qi Y, Schlecht A,
et al: An unrestrained proinflammatory M1 macrophage population
induced by iron impairs wound healing in humans and mice. J Clin
Invest. 121:985–997. 2011.
|
|
95
|
Mirza R, DiPietro LA and Koh TJ: Selective
and specific macrophage ablation is detrimental to wound healing in
mice. Am J Pathol. 175:2454–2462. 2009.
|
|
96
|
Janssen RP and Scheffler SU:
Intra-articular remodelling of hamstring tendon grafts after
anterior cruciate ligament reconstruction. Knee Surg Sports
Traumatol Arthrosc. 22:2102–2108. 2014.
|
|
97
|
Li S, Xu Z, Wang Z, Xiang J, Zhang T and
Lu H: Acceleration of bone-tendon interface healing by
low-intensity pulsed ultrasound is mediated by macrophages. Phys
Ther. 101:pzab0552021.
|
|
98
|
Chamberlain CS, Kink JA, Wildenauer LA,
McCaughey M, Henry K, Spiker AM, Halanski MA, Hematti P and
Vanderby R: Exosome-educated macrophages and exosomes
differentially improve ligament healing. Stem Cells. 39:55–61.
2021.
|
|
99
|
Wang C, Zhang Y, Zhang G, Yu W and He Y:
Adipose stem cell-derived exosomes ameliorate chronic rotator cuff
tendinopathy by regulating macrophage polarization: From a mouse
model to a study in human tissue. Am J Sports Med. 49:2321–2331.
2021.
|
|
100
|
Huang Y, He B, Wang L, Yuan B, Shu H,
Zhang F and Sun L: Bone marrow mesenchymal stem cell-derived
exosomes promote rotator cuff tendon-bone healing by promoting
angiogenesis and regulating M1 macrophages in rats. Stem Cell Res
Ther. 11:4962020.
|
|
101
|
Shi Y, Kang X, Wang Y, Bian X, He G, Zhou
M and Tang K: Exosomes derived from bone marrow stromal cells
(BMSCs) Enhance tendon-bone healing by regulating macrophage
polarization. Med Sci Monit. 26:e9233282020.
|
|
102
|
Fatima F, Ekstrom K, Nazarenko I, Maugeri
M, Valadi H, Hill AF, Camussi G and Nawaz M: Non-coding RNAs in
mesenchymal stem cell-derived extracellular vesicles: Deciphering
regulatory roles in stem cell potency, inflammatory resolve, and
tissue regeneration. Front Genet. 8:1612017.
|
|
103
|
Yoshikawa T, Tohyama H, Katsura T, Kondo
E, Kotani Y, Matsumoto H, Toyama Y and Yasuda K: Effects of local
administration of vascular endothelial growth factor on mechanical
characteristics of the semitendinosus tendon graft after anterior
cruciate ligament reconstruction in sheep. Am J Sports Med.
34:1918–1925. 2006.
|
|
104
|
Fealy S, Adler RS, Drakos MC, Kelly AM,
Allen AA, Cordasco FA, Warren RF and O'Brien SJ: Patterns of
vascular and anatomical response after rotator cuff repair. Am J
Sports Med. 34:120–127. 2006.
|
|
105
|
Takayama K, Kawakami Y, Mifune Y,
Matsumoto T, Tang Y, Cummins JH, Greco N, Kuroda R, Kurosaka M,
Wang B, et al: The effect of blocking angiogenesis on anterior
cruciate ligament healing following stem cell transplantation.
Biomaterials. 60:9–19. 2015.
|
|
106
|
Sivaraj KK and Adams RH: Blood vessel
formation and function in bone. Development. 143:2706–2715.
2016.
|
|
107
|
Zhang T, Yan S, Song Y, Chen C, Xu D, Lu B
and Xu Y: Exosomes secreted by hypoxia-stimulated bone-marrow
mesenchymal stem cells promote grafted tendon-bone tunnel healing
in rat anterior cruciate ligament reconstruction model. J Orthop
Translat. 36:152–163. 2022.
|
|
108
|
Fang S, He T, Jiang J, Li Y and Chen P:
Osteogenic effect of tsRNA-10277-loaded exosome derived from bone
mesenchymal stem cells on steroid-induced osteonecrosis of the
femoral head. Drug Des Devel Ther. 14:4579–4591. 2020.
|
|
109
|
Zhang Y, Cao X, Li P, Fan Y, Zhang L, Ma
X, Sun R, Liu Y and Li W: microRNA-935-modified bone marrow
mesenchymal stem cells-derived exosomes enhance osteoblast
proliferation and differentiation in osteoporotic rats. Life Sci.
272:1192042021.
|
|
110
|
Xie Y, Chen Y, Zhang L, Ge W and Tang P:
The roles of bone-derived exosomes and exosomal microRNAs in
regulating bone remodelling. J Cell Mol Med. 21:1033–1041.
2017.
|
|
111
|
Feng W, Jin Q, Ming-Yu Y, Yang H, Xu T,
You-Xing S, Xu-Ting B, Wan C, Yun-Jiao W, Huan W, et al:
MiR-6924-5p-rich exosomes derived from genetically modified
Scleraxis-overexpressing PDGFRα(+) BMMSCs as novel nanotherapeutics
for treating osteolysis during tendon-bone healing and improving
healing strength. Biomaterials. 279:1212422021.
|
|
112
|
Han L, Liu H, Fu H, Hu Y, Fang W and Liu
J: Exosome-delivered BMP-2 and polyaspartic acid promotes tendon
bone healing in rotator cuff tear via Smad/RUNX2 signaling pathway.
Bioengineered. 13:1459–1475. 2022.
|
|
113
|
Wu B, Chen H, Shi X, Wang L, Zhang T, Guan
C, Huang T, Yang Y, Hu J and Lu H: Exosomes derived from bone
marrow mesenchymal stem cell preconditioned by low-intensity pulsed
ultrasound stimulation promote bone-tendon interface fibrocartilage
regeneration and ameliorate rotator cuff fatty infiltration. Res
Sq. 2021.
|
|
114
|
Cai J, Xu J, Ye Z, Wang L, Zheng T, Zhang
T, Li Y, Jiang J and Zhao J: Exosomes derived from
kartogenin-preconditioned mesenchymal stem cells promote cartilage
formation and collagen maturation for enthesis regeneration in a
rat model of chronic rotator cuff tear. Am J Sports Med.
51:1267–1276. 2023.
|
|
115
|
Berger DR, Centeno CJ and Steinmetz NJ:
Platelet lysates from aged donors promote human tenocyte
proliferation and migration in a concentration-dependent manner.
Bone Joint Res. 8:32–40. 2019.
|
|
116
|
Yu H, Cheng J, Shi W, Ren B, Zhao F, Shi
Y, Yang P, Duan X, Zhang J, Fu X, et al: Bone marrow mesenchymal
stem cell-derived exosomes promote tendon regeneration by
facilitating the proliferation and migration of endogenous tendon
stem/progenitor cells. Acta Biomater. 106:328–341. 2020.
|
|
117
|
Li J, Liu ZP, Xu C and Guo A:
TGF-β1-containing exosomes derived from bone marrow mesenchymal
stem cells promote proliferation, migration and fibrotic activity
in rotator cuff tenocytes. Regen Ther. 15:70–76. 2020.
|
|
118
|
Li J, Wang ZH and Sun YH: TGF-β1
stimulated mesenchymal stem cells-generated exosomal miR-29a
promotes the proliferation, migration and fibrogenesis of tenocytes
by targeting FABP3. Cytokine. 162:1560902023.
|
|
119
|
Xiong QH, Zhao L, Wan GQ, Hu YG and Li XL:
Engineered BMSCs-derived exosomal miR-542-3p promotes cutaneous
wound healing. Endocr Metab Immune Disord Drug Targets. 23:336–346.
2023.
|
|
120
|
Wu D, Kang L, Tian J, Wu Y, Liu J, Li Z,
Wu X, Huang Y, Gao B, Wang H, et al: Exosomes derived from bone
mesenchymal stem cells with the stimulation of
Fe3O4 nanoparticles and static magnetic field
enhance wound healing through upregulated miR-21-5p. Int J
Nanomedicine. 15:7979–7993. 2020.
|
|
121
|
Pomatto M, Gai C, Negro F, Cedrino M,
Grange C, Ceccotti E, Togliatto G, Collino F, Tapparo M, Figliolini
F, et al: Differential therapeutic effect of extracellular vesicles
derived by bone marrow and adipose mesenchymal stem cells on wound
healing of diabetic ulcers and correlation to their cargoes. Int J
Mol Sci. 22:38512021.
|
|
122
|
Li FQ, Chen WB, Luo ZW, Chen YS, Sun YY,
Su XP, Sun JM and Chen SY: Bone marrow mesenchymal stem
cell-derived exosomal microRNAs target PI3K/Akt signaling pathway
to promote the activation of fibroblasts. World J Stem Cells.
15:248–267. 2023.
|
|
123
|
Gatti S, Bruno S, Deregibus MC, Sordi A,
Cantaluppi V, Tetta C and Camussi G: Microvesicles derived from
human adult mesenchymal stem cells protect against
ischaemia-reperfusion-induced acute and chronic kidney injury.
Nephrol Dial Transplant. 26:1474–1483. 2011.
|
|
124
|
Kordelas L, Rebmann V, Ludwig AK, Radtke
S, Ruesing J, Doeppner TR, Epple M, Horn PA, Beelen DW and Giebel
B: MSC-derived exosomes: A novel tool to treat therapy-refractory
graft-versus-host disease. Leukemia. 28:970–973. 2014.
|
|
125
|
Di Rocco G, Baldari S and Toietta G:
Towards therapeutic delivery of extracellular vesicles: Strategies
for in vivo tracking and biodistribution analysis. Stem Cells Int.
2016:50296192016.
|
|
126
|
Sevivas N, Teixeira FG, Portugal R,
Direito-Santos B, Espregueira-Mendes J, Oliveira FJ, Silva RF,
Sousa N, Sow WT, Nguyen LTH, et al: Mesenchymal stem cell secretome
improves tendon cell viability in vitro and tendon-bone healing in
vivo when a tissue engineering strategy is used in a rat model of
chronic massive rotator cuff tear. Am J Sports Med. 46:449–459.
2018.
|
|
127
|
Gaspar D, Spanoudes K, Holladay C, Pandit
A and Zeugolis D: Progress in cell-based therapies for tendon
repair. Adv Drug Deliv Rev. 84:240–256. 2015.
|
|
128
|
Gao H, Zhang L, Wang Z, Yan K, Zhao L and
Xiao W: Research progress on transorgan regulation of the
cardiovascular and motor system through cardiogenic exosomes. Int J
Mol Sci. 23:57652022.
|