Introduction
Colorectal cancer (CRC) is the third most frequent
type of cancer worldwide and one of the most common malignant
tumors of the digestive tract. According to the International
Agency for Research on Cancer (IARC) data, there were ~1.93 million
new cases in 2020, with 935,000 related deaths (1,2).
The onset of CRC is a complex developmental process involving
numerous phases and the involvement of multiple genes, with
atypical early symptoms. Traditional treatment procedures, such as
surgery, chemoradiotherapy and targeted therapy have gained some
success; however, patient survival rates after 5 years are not yet
optimal (3,4). Therefore, it is crucial to explore
the pathogenesis of CRC and to identify novel therapeutic targets
for the diagnosis and treatment of CRC.
The environment in which the tumor cells are
positioned affects the growth of tumors in addition to the genomic
instability and epigenetic alterations of the tumor itself
(5,6). Tumor cells, non-tumor stromal cells
(endothelial cells, tumor-associated fibroblasts and immune cells)
and extracellular components (extracellular matrix, growth factors,
cytokines, etc.) comprise the majority of the tumor
microenvironment (TME), which is the term used to describe the
mesenchymal cells that the tumor locally infiltrates. Chemokines
produced by tumor cells have the capacity to facilitate blood
vessel formation (7-9). Tumor-associated macrophages (TAMs)
are macrophages are considered to be the most numerous and
significant proportion of myeloid cells in the TME. As one of the
key elements of the TME, TAMs are closely linked to the development
of tumor-associated inflammation (10,11). TAMs can affect tumor growth,
invasion and metastasis, as well as vascular formation within the
tumor. Furthermore, TAMs can suppress the development of the
antitumor immune response by secreting a variety of cytokines and
chemokines, thus playing a multifaceted role in shaping the context
of tumor development and progression (12,13).
Within the TME, TAMs are stimulated by various
signals, undergoing polarization into the distinct subtypes, M1 and
M2. The activation of M1-type macrophages, triggered by molecules,
such as lipopolysaccharide (LPS) and interferon-γ (IFN-γ), is
associated with the antitumor response. Conversely, the generation
of M2-type macrophages, mainly induced by interleukin (IL)-4 and
IL-13, promotes tumor immune escape, highlighting a crucial role of
this type of TAMs in cancer progression (14,15). TAMs are generally M2-type
macrophages and are associated with malignant disease progression,
drug resistance, recurrence and metastasis, as well as with a poor
prognosis (16,17). Therefore, the identification and
development of targets capable of modulating the polarization state
of TAMs may prove to be a pivotal strategy with which to limit
tumor growth and proliferation.
Grb2-associated binder 2 (Gab2) is one of the
crucial members of the Gab protein family. This family of proteins
represents a class of substrate molecules that can associate with
tyrosine kinase through the recruitment of signaling molecules rich
in phosphotyrosine domains, participating in the activation and
transduction of numerous signaling pathways, playing a critical
role in cellular physiological processes, such as differentiation,
proliferation, migration and apoptosis (18). Previous studies have discovered a
marked elevated expression of Gab2 in leukemia (19,20) and several human malignancies,
such as breast cancer (BRCA) (21), ovarian cancer (OV) (22,23), hepatocellular carcinoma (HCC)
(24,25), CRC (26) and melanoma (27), indicating its potential
significance in oncologic progression. However, the impact and
mechanisms of action of Gab2 on TAM polarization remain unclear.
Thus, further exploration is required in order to elucidate its
complex role within the TME.
The present study aimed to investigate the
following: i) The expression level of Gab2 within TAMs in tissue
microarrays from patients with CRC, and its association with
patient survival; ii) the effect of Gab2 on TAM polarization in
vitro; and iii) verify the potential role of Gab2 in modulating
TAM polarization and its effects on CRC progression, by utilizing a
mouse subcutaneous transplantation model.
Materials and methods
Tissue microarray and fluorescence
staining
Human CRC tissue microarrays (cat. no. HColA180Su12)
that included 93 paraffin-embedded CRC tissues and 87
para-cancerous tissues were obtained from Shanghai Outdo Biotech
Co., Ltd. The present study was approved by the Ethics Committee of
the Shanghai Outdo Biotech Co., Ltd., and was conducted in
accordance with the ethical standards set out in the Declaration of
Helsinki. All patients or their next of kin provided their informed
consent prior to the study.
The tissue microarrays were dewaxed and hydrated;
antigen retrieval was performed by boiling the tissue sections in
10 mM sodium citrate buffer with pH 6.0 at 100°C for 10 min.
Following this, the tissue sections were blocked and then incubated
overnight at 4°C with the corresponding primary antibodies. The
following antibodies were used in the present study: CD68 (1:100;
cat. no. ab283654; Abcam), Gab2 (1:50; cat. no. 22549-1-AP;
Proteintech Group, Inc.). The tissue sections were then washed
three times with TBST for 5 min each. This was followed by a 2-h
incubation at room temperature in the dark with the following
secondary antibodies: Alexa Fluor 488-conjugated goat anti-rabbit
IgG (H+L) (1:500; cat. no. 4412; Cell Signaling Technology, Inc.)
and Alexa Fluor 555-conjugated goat anti-rabbit IgG (H+L) (1:500;
cat. no. 4413; Cell Signaling Technology, Inc.). Images were
acquired using an Olympus fast, high-resolution, inverted
fluorescence imaging system (Olympus Corporation).
In each segment on the tissue microarray, five
random high-magnification (×400 magnification) fields were analyzed
using a bi-rater semi-quantitative assessment method for scoring.
The expression of Gab2 was evaluated semi-quantitatively as
follows: A score of 0 was given for <5% of cells exhibiting
positive staining, 1 for 6-25%, 2 for 26-50%, and 3 for >50%.
Concurrently, the staining intensity was graded on a scale of 0
(negative), 1 (faint), 2 (moderate), and 3 (strong). Consequently,
the staining index was calculated as the product of the positivity
percentage and intensity score, averaged over five fields of view.
Accordingly, a total score of 0-4 indicated a low Gab2 expression,
and a score of 5-9 indicated a high expression of Gab2.
Mice
BALB/c mice (female, 5-6 weeks old; n=45; weighing
20±1.5 g) were purchased from Chongqing Tengxin Bio-Technology Co.,
Ltd. The animals were housed under specific pathogen-free
conditions in environment with regulated temperature (25±1°C) and
humidity (40-70%) and exposure to a constant 12-h light/dark cycle
in the animal facility at Zunyi Medical University (Zunyi, China).
All animal experiments were performed according to the guidelines
for the Care and Use of Laboratory Animals (Ministry of Health,
China, 1998). The experimental procedures were approved by the
ethical guidelines the Zunyi Medical University Laboratory Animal
Care and Use Committee (permit no. 2018016). All experiments were
repeated three times.
Cells and cell culture
The human monocytic cell line (THP-1) and BALB/c
mouse colon adenocarcinoma cell line (CT26) were obtained from The
Cell Bank of Type Culture Collection of the Chinese Academy of
Sciences. Fetal human colonic mucosa cells (FHC) and human CRC cell
lines (SW620, SW480 and HCT116) were kept in the Immunology
Laboratory of Zunyi Medical University. The THP-1 cells were
cultured in RPMI-1640 medium (cat. no. SH30027.01; HyClone; Cytiva)
supplemented with 10% fetal bovine serum (FBS; Thermo Fisher
Scientific, Inc.), 0.11 g/l sodium pyruvate (cat. no. C0331;
Beyotime Institute of Biotecnology) and 1% penicillin-streptomycin
(cat. no. P1400; Beijing Solarbio Science & Technology Co.,
Ltd.) at 37°C in 5% CO2. The THP-1 monocytes were
differentiated into macrophages with 100 ng/ml phorbol 12-myristate
13-acetate (PMA; cat. no. P1585; MilliporeSigma) for 24 h. The CT26
and HCT116 cells were cultured in high-glucose DMEM (cat. no.
SH30243.01; HyClone; Cytiva) supplemented with 10% FBS and 1%
penicillin-streptomycin. The SW620 and SW480 cells were cultured in
L-15 medium (cat. no. SH30525.01; HyClone; Cytiva) supplemented
with 10% FBS and 1% penicillin-streptomycin. For the primary
culture of BMDMs, bone marrow cells were harvested from 6-to
8-week-old female BALB/c mice (n=3). In brief, bone marrow cells
were isolated from the femur and tibia via fine dissection. Red
blood cells were lysed, and the remaining bone marrow cells were
cultured in high-glucose DMEM containing 10% FBS and 1%
penicillin-streptomycin, supplemented with 20 ng/ml recombinant
murine M-CSF (cat. no. 315-02, PeproTech, Inc.) and maintained at
37°C in 5% CO2. BMDM were harvested after 7 days of
M-CSF-mediated macrophage differentiation. Peritoneal cells were
collected from BALB/c mice, briefly, a total of 3 female BALB/c
mice were sacrificed, and the skin was removed from the abdominal
area. Mice were then injected intraperitoneally with 4-5 ml PBS
using a 4.5 gauge needle. Without extracting the needle, the
abdomen was gently massaged and then as much fluid from the
peritoneum as possible was slowly withdrawn with the syringe.
Following removal, the peritoneal cells were gently washed with PBS
prior to use, and then seeded at a concentration of
2×106/ml in plates containing RPMI-1640 medium
supplemented with 10% FBS and 1% penicillin-streptomycin. The cells
were incubated for 12 h at 37°C in 5% CO2, and
non-adherent cells were then washed out with PBS; the remaining
adherent cells were peritoneal macrophages (PMΦ). Polarization of
PMΦ towards the M1 phenotype was achieved by stimulation with
lipopolysaccharide (LPS; cat. no. L2880-10MG; MilliporeSigma) 100
ng/ml and interferon-γ (IFN-γ; cat. no. 315-05; PeproTech, Inc.) 20
ng/ml for 24 h. On the other hand, polarization towards the M2
phenotype was generated by incubation of macrophages with
interleukin-4 (IL-4; cat. no. 214-4; PeproTech, Inc.) 20 ng/ml for
24 h.
Preparation of tumor-conditioned medium
(TCM)
The CT26 cells were seeded in flasks and cultured in
high-glucose DMEM supplemented with 10% FBS and 1%
penicillin-streptomycin. Upon reaching a confluency >60%, the
medium was replaced with fresh high-glucose DMEM, and incubation
was continued for 48 h at 37°C in 5% CO2. Subsequently,
the supernatant was collected and centrifuged at 12,000 × g for 10
min at 4°C. A sterile 0.22-μm filter was used to filter the
supernatant following centrifugation. The supernatant was then
aliquoted and kept at −80°C for use in further experiments.
Knockdown of Gab2 in PMΦ cells
The lentiviral interference vector (LV-Gab2-shRNA),
and the negative control viral vector [CON077
(hU6-MCS-Ubiquitin-EGFP-IRES-puromycin)], were designed,
constructed, and packaged by Shanghai Genechem Co., Ltd., with
specifics listed in Table SI.
The recombined GV248 lentiviral vector plasmid or the negative
control lentiviral vector plasmid and pHelper 1.0 plasmid, the
pHelper 2.0 plasmid (Shanghai Genechem Co., Ltd.) were
co-transfected into 293T cells (American Type Culture Collection)
via HitransG enhanced infection solution (Shanghai Genechem Co.,
Ltd.) at 37°C in 5% CO2 for 6 h. The high-glucose DMEM
supplemented with 10% FBS medium was refreshed. The culture
supernatants were collected at 48 h following transfection.
Following centrifugation at 4,000 × g for 10 min at 4°C to remove
cell debris, the supernatant was filtered through 0.45-μm
polyethersulfone low protein-binding filters. The concentrated
viral supernatant was aliquoted and kept at −80°C prior to use. The
lentivirus was diluted with serum-free high-glucose DMEM medium,
followed by the addition of diluted lentiviral particles and
polybrene (final concentration is 8 μg/ml) to PMΦ cells at a
multiplicity of infection (MOI) of 80. Following an 8-12 h at 37°C
in 5% CO2 incubation, the medium was refreshed.
Subsequently, at 72 h post-infection, the PMΦ cells were harvested
for further analysis, and the transfection efficiency was verified
using reverse transcription-quantitative PCR (RT-qPCR) and
immunofluorescence.
Establishment of the mouse subcutaneous
tumor xenograft model
BALB/c wild-type (WT) female mice (5-6 weeks old;
weighing 20±1.5 g) were divided into three groups. In the first
group (CT26 group), a total of 2×105 CT26 cells
suspended in 100 μl PBS were injected subcutaneously into
the left flank of the mice (n=3). In the second group
(Gab2WT-CT26), a 100 μl mixture of PMΦ
(2×104) infected with LV-Con and CT26 (2×105)
cells suspension was injected subcutaneously into the left flank of
the mice (n=3). In the third group (GabDef-CT26), a 100
μl mixture of PMΦ infected with LV-Gab2 (2×104)
and CT26 (2×105) cells suspension was injected
subcutaneously into the left flank of the mice (n=3). Tumor size
was initially evaluated on the 6th day post-injection using
calipers and the length and width of the tumors was then monitored
every 2 days, the tumor volume was calculated according to the
following formula: (length × width2)/2. The health and
behavior of the animals were monitored every 3 days. No mice
succumbed and there were no abnormal signs of humane endpoints over
the course of the experiment. Tumor growth curves were plotted for
each group of mice based on the measurements. On the 21st day
following the implantation of CT26 cells, the mice were sacrificed
via cervical dislocation. Death was confirmed by continuing to
observe the mice for 3 min after the observation of no heartbeat,
respiration and the pupils are dilated, then the tumor tissues were
isolated for subsequent analyses. The humane endpoints for the
experiment were designated as follows: A marked reduction in food
or water intake, labored breathing, an inability to stand and no
response to external stimuli. However, no abnormal signs that were
indicative of humane endpoints of the experiment were observed in
any of the mice during these experiments. All mouse experiments
lasted 1 month and included acclimatization to the feeding
environment, tumor implantation and growth.
Isolation of macrophages from murine
tumor tissue and fluorescence-activated cell sorting
Tumor tissues were extracted from the mice, and the
fascia, fat and necrotic zones were cleared. A single-cell
suspension of tumor tissue was prepared using the Mouse Tumor
Dissociation kit (cat. no. 130-096-730; Miltenyi Biotechnology Co.,
Ltd.) according to the manufacturer's instructions. The resulting
single-cell suspension was resuspended in cold PBS buffer and
centrifuged at 300 × g for 7 min at 4°C. The cell concentration was
adjusted to 6×105 per tube, and the F4/80 antibody (0.5
μg/test; cat. no. 11-4801-85; Invitrogen; Thermo Fisher
Scientific, Inc.) was added and incubated on ice for 30 min,
protected from light. The stained single-cell suspension of tumor
tissue were analyzed, and the macrophages were selectively sorted
utilizing a Beckman Gallios flow cytometer (Beckman Coulter,
Inc.).
Immunofluorescence
The cells on coverslips were fixed in 4%
paraformaldehyde for 15 min at 4°C, blocked with bovine serum
albumin (BSA) for 60 min at room temperature, and then incubated
overnight at 4°C with the appropriate primary antibodies. The
primary antibodies utilized were as follows: F4/80 (1:200; cat. no.
ab6640; Abcam), Gab2 (1:50; cat. no. 22549-1-AP; Proteintech Group,
Inc.), CD206/macrophage mannose receptor (CD206; 1:500; cat. no.
18704-1-AP; Proteintech Group, Inc.) and arginase-1 (Arg-1;
1:10,000; cat. no. 16001-1-AP; Proteintech Group, Inc.).
Subsequently, the coverslips were rinsed three times with cold PBS
for 5 min each time. This was followed by a 2-h incubation at room
temperature in the dark with the following secondary antibodies:
Alexa Fluor 488-conjugated goat anti-rabbit IgG (H+L) (1:500; cat.
no. 4412; Cell Signaling Technology, Inc.) and Alexa Fluor
555-conjugated goat anti-rabbit IgG (H+L) (1:500; cat. no. 4413;
Cell Signaling Technology, Inc.). Images were acquired using an
Olympus fast, high-resolution, inverted fluorescence imaging system
(Olympus Corporation).
RNA extraction and RT-qPCR
Total RNA was isolated from the PMΦ, BMDM,
macrophages sorted from subcutaneously transplantated tumors in
mice (Tu-TAM) and macrophages cultured in TCM (TCM-TAM) using
RNAiso Plus (cat. no. 9108; Takara Biotechnology Co., Ltd.)
according to the manufacturer's instructions. A total of 3
μg RNA was reverse transcribed into cDNA using the RT
reagent kit (cat. no. RR037A; Takara Biotechnology Co., Ltd.). qPCR
was conducted using a Bio-Rad CFX96 detection system (Bio-Rad
Laboratories, Inc.) with 25 μl PCR mix containing 12.5
μl SYBR-Green master mix, 2 μl primer mix, 2
μl cDNA and 8.5 μl deionized water. The RT-qPCR
thermocycling conditions were as follows: Initial denaturation at
95°C for 30 sec, followed by 40 cycles at 95°C for 5 sec and 60°C
for 30 sec. The relative mRNA expression levels of genes were
calculated using the comparative threshold cycle
(2−ΔΔCq) method (28), utilizing GAPDH as the reference
gene. All primers were synthesized by Sangon Biotech (Shanghai)
Co., Ltd., and are listed in Table
SII.
Protein extraction and western blot
analysis
Proteins were extracted from the PMΦ, BMDM, THP-1,
Tu-TAM, TCM-TAM using cell lysis buffer (cat. no. 9803; Cell
Signaling Technology, Inc.), followed by centrifugation at 12,000 ×
g for 10 min at 4°C. The protein concentration was quantified using
the BCA Protein Quantitation kit (cat. no. GK5011; Shanghai Generay
Biological Engineering Co., Ltd.). A total of 30 μg protein
per well was loaded and electrophoresed on a 10% SDS-PAGE gel to
achieve protein separation based on molecular weights. The proteins
were then transferred onto PVDF membranes (cat. no. IPVH00010;
Merck Millipore). The membranes were blocked with 5% skim milk at
room temperature for 2 h and then incubated with specific primary
antibodies overnight at 4°C. The primary antibodies used were as
follows: Gab2 (1:1,000; cat. no. 22549-1-AP; Proteintech Group,
Inc.), CD206 (1:500; cat. no. 18704-1-AP; Proteintech Group, Inc.),
Arg-1 (1:10,000; cat. no. 16001-1-AP; Proteintech Group, Inc.),
ERK1/2 (1:1,000; cat. no. 4695; Proteintech Group, Inc.),
phosphorylated (p-)p44/42 MAPK (ERK1/2) (Thr202/Tyr204; 1:1,000;
cat. no. 9101 Proteintech Group, Inc.), AKT (1:1,000; cat. no.
9272; Proteintech Group, Inc.), p-AKT (Ser473; 1:1,000; cat. no.
4058; Proteintech Group, Inc.), signal transducer and activator of
transcription (STAT)3 (1:1,000; cat. no. 9132; Proteintech Group,
Inc.), p-STAT3 (Tyr705; 1:1,000; cat. no. 9131; Proteintech Group,
Inc.), STAT6 (1:1,000; cat. no. ab32520; Abcam), p-STAT6 (Y641;
1:1,000; cat. no. ab263947; Abcam), GAPDH (1:1,000; cat. no. 2118;
Proteintech Group, Inc.) and then incubated with goat anti-rabbit
IgG (H+L)-HRP [1:5,000; cat. no. abs20147; Aibixin (Shanghai)
Biotechnology Co., Ltd.] for 2 h at room temperature. The proteins
were visualized by enhanced chemiluminescence using the ECL assay
kit (cat. no. WBKLS0100; Merck Millipore). ImageJ software (version
1.8.0; National Institutes of Health) was used to quantify the
protein band intensities.
Hematoxylin and eosin (H&E)
staining
Tumor and lung tissues from the mice in the
xenograft tumor model were subjected to paraffin embedding,
sectioning and H&E staining with the assistance of the
Department of Pathology, Affiliated Hospital of Zunyi Medical
University. Briefly, the tumor and lung tissues were fixed in 10%
neutral formalin for 48 h at room temperature, embedded in
paraffin, and sectioned at a thickness of 3 μm. This was
followed by deparaffinization and rehydration using a series of
laboratory graded alcohol at different percentages (75%; 85%;
95%-I; 95%-II; 95% alcohol-III, dimethyl benzene-I and dimethyl
benzene-II). Alcohol and dimethyl benzene were obtained from
Guizhou Keode Biotechnology Co., Ltd., respectively and the
sections were stained with hematoxylin for 8 min and eosin solution
for 40 sec at room temperature (G1120; Beijing Solarbio Science
& Technology Co., Ltd.), and the tissue sections were rinsed
under running water. Finally, the tumor and lung tissues structures
were observed under a full slide scanning microscope (Olympus
Corporation).
Statistical analysis
Statistical analyses were conducted using IBM SPSS
21 and GraphPad Prism 7 software. The experimental results are
presented as the mean standard deviation (mean ± SD). A two-tailed
unpaired Student's t-test was used to compare two datasets. For
multiple group comparisons, one-way ANOVA followed by Tukey's post
hoc test was used. The Chi-squared test was used for the evaluation
of categorical data. Survival curves were illustrated using
Kaplan-Meier plots and analyzed using the log-rank test. Univariate
and multivariate Cox regression analysis was used to analyze the
factors affecting the 5-year survival rates of patients with CRC.
P<0.05 was considered to indicate a statistically significant
difference.
Results
Gab2 is upregulated within TAMs in tumor
tissues and is associated with a poor prognosis of patients with
CRC
Firstly, the present study analyzed the mRNA
expression of Gab2 using publicly accessible datasets from The
Cancer Genome Atlas (TCGA). The analysis revealed a significant
elevation in Gab2 mRNA expression in the CRC tumor tissues compared
to the normal tissues (Fig. 1A).
To identify the role of Gab2 in TAMs, the expression levels of Gab2
and CD68 in the CRC tissue array were evaluated using
immunofluorescence staining (Fig.
1B). The results indicated that Gab2 was predominantly
localized in the cytoplasm of TAMs, with a nuclear localization
rarely observed; an elevated expression of Gab2 was found within
TAMs in the tumor tissues compared to TAMs in para-cancerous tissue
(Fig. 1C and Table I). Subsequently, the patients
with CRC were categorized into two groups based on the median
expression level of Gab2 immunofluorescence intensity: A high and
low Gab2 expression within TAMs. Upon further analysis, no
significant differences were found between the two groups as
regards conventional prognostic factors, including sex, age, degree
of histological differentiation, tumor volume size, TNM stage and
clinical stage (Table II).
Notably, the findings indicated that an elevated expression of Gab2
within TAMs was associated with a poor 5-year survival rate of
patients with CRC (Fig. 1D).
Using univariate and multivariate COX regression analyses, it was
revealed that the expression level of Gab2 within TAMs was a
potentially pivotal factor influencing the 5-year survival rate of
patients with CRC (Table
III).
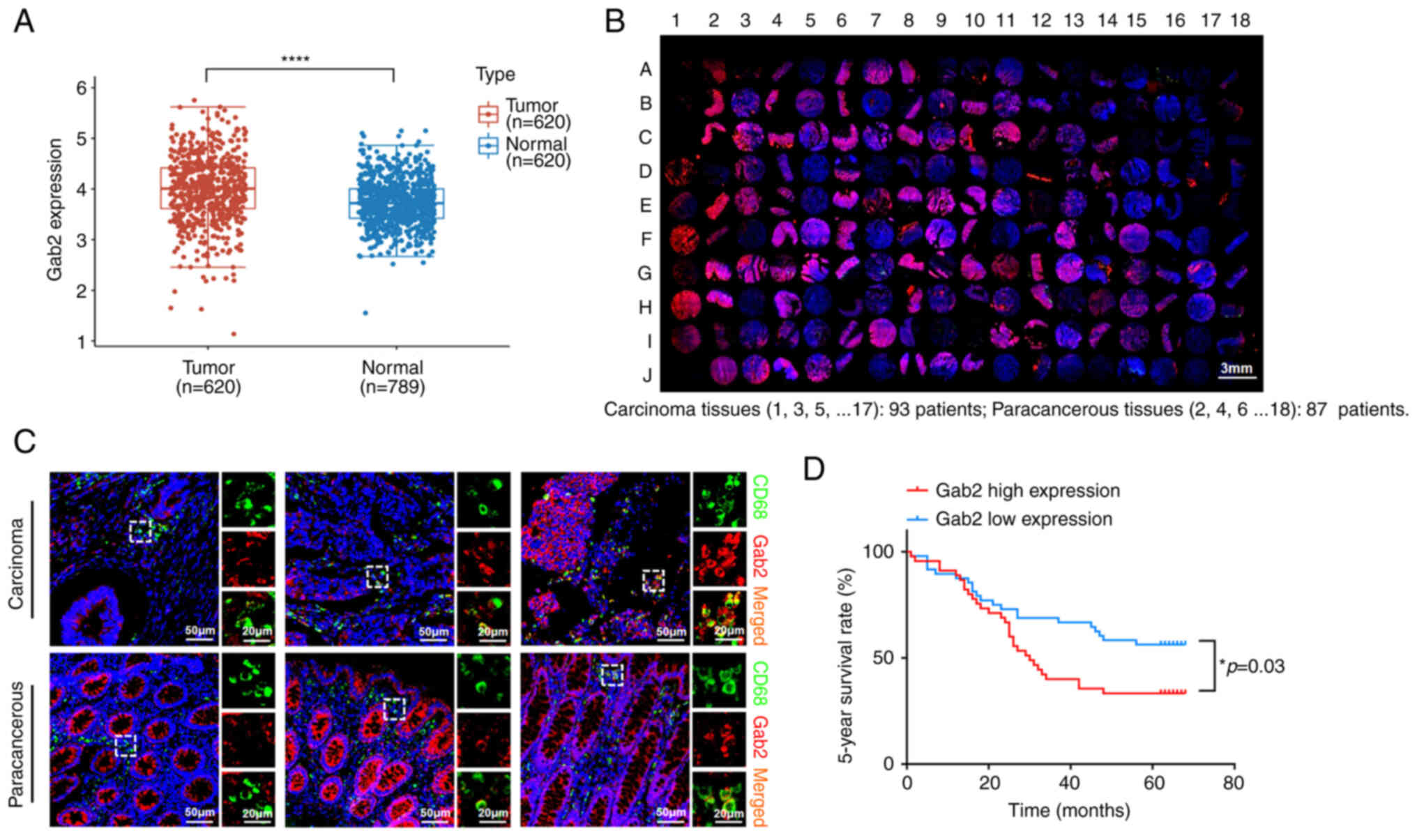 | Figure 1Gab2 is upregulated within TAMs in
tumor tissues and is associated with the poor prognosis of patients
with CRC. (A) Expression level of Gab2 in CRC and adjacent normal
tissues from The Cancer Genome Atlas database.
****P<0.0001, tumor vs. normal. (B) Representative
images of immunohistochemical staining of Gab2 and CD68 in
colorectal carcinoma and para-cancerous tissues. (C) Multiplex
immunofluorescence staining of the macrophage markers, CD68 and
Gab2. CD68 staining is shown in green, Gab2 is shown in red, and
DAPI staining in blue. The panels on the right of each image are
enlarged images of the boxed area in the main images. (D) The
association between Gab2 expression in TAMs and the 5-year survival
rate of patients with CRC. According to the median of the
immunofluorescence intensity score, the patients with CRC were
divided into two groups (Gab2 low expression and Gab2 high
expression). Survival curves were plotted using the Kaplan-Meier
method, and the statistical significance of the difference in
5-year survival rates between the groups was assessed using the
log-rank test. *P<0.05. Gab2, Gab2, Grb2-associated
binder 2; TAMs, tumor-associated macrophages; CRC, colorectal
cancer. |
 | Table IExpression of Gab2 in TAMs from
colorectal carcinoma and para-cancerous tissues. |
Table I
Expression of Gab2 in TAMs from
colorectal carcinoma and para-cancerous tissues.
| Group | No. of
patients | Gab2 expression
| P-value |
|---|
| Positive | Negative | Positive rate |
|---|
| Colorectal
carcinoma | 93 | 70 | 23 | 75.3% | P<0.05 |
| Para-cancerous | 87 | 37 | 50 | 42.5% | |
 | Table IIGab2 expression and
clinicopathological characteristics of patients with CRC. |
Table II
Gab2 expression and
clinicopathological characteristics of patients with CRC.
| Variable | Patient group
(n=93)
| Chi-squared
test | P-value |
|---|
Gab2 low expression
(n=48)
| Gab2 high
expression (n=45)
|
|---|
| No. of
patients | % | No. of
patients | % |
|---|
| Sex | | | | | 0.1229 | 0.7529 |
| Male | 26 | 54.2 | 26 | 57.8 | | |
| Female | 22 | 45.8 | 19 | 42.2 | | |
| Age, years | | | | | 2.3911 | 0.122 |
| <60 | 12 | 25.0 | 18 | 40.0 | | |
| ≥60 | 36 | 75.0 | 27 | 60.0 | | |
| Tumor volume
(cm3) | | | | | 0.2726 | 0.6016 |
| <14 | 25 | 52.1 | 21 | 46.7 | | |
| >14 | 23 | 47.9 | 24 | 53.3 | | |
|
Differentiation | | | | | 0.4593 | 0.489 |
| I-II, II | 40 | 83.3 | 35 | 77.8 | | |
| II-III, III | 8 | 16.7 | 10 | 22.2 | | |
| T stage | | | | | 1.1042 | 0.5757 |
| T2 | 2 | 4.2 | 3 | 6.7 | | |
| T3 | 36 | 75 | 36 | 80 | | |
| T4 | 10 | 20.8 | 6 | 13.3 | | |
| N stage | | | | | 0.8009 | 0.67 |
| N0 | 31 | 64.6 | 25 | 55.6 | | |
| N1 | 13 | 27.1 | 25 | 33.3 | | |
| N2 | 4 | 8.3 | 5 | 11.1 | | |
| M stage | | | | | 0.0044 | 0.9474 |
| M0 | 46 | 95.8 | 43 | 95.6 | | |
| M1 | 2 | 4.2 | 2 | 4.4 | | |
| Clinical stage | | | | | 1.5523 | 0.6703 |
| I | 1 | 2.1 | 3 | 6.7 | | |
| II | 26 | 54.2 | 20 | 44.4 | | |
| III | 19 | 39.6 | 20 | 44.4 | | |
| IV | 2 | 4.2 | 2 | 4.4 | | |
 | Table IIIUnivariate and multivariate analysis
of survival-related factors of patients with CRC. |
Table III
Univariate and multivariate analysis
of survival-related factors of patients with CRC.
| Variable | Univariate analysis
| Multivariate
analysis
|
|---|
| HR | 95% CI | P-value | HR | 95% CI | P-value |
|---|
| Gab2 (high vs.
low) | 1.858 | 1.061-3.255 | 0.03 | 1.936 | 1.077-3.483 | 0.027 |
| Age/years (≥60 vs.
<60) | 1.105 | 0.597-2.046 | 0.750 | 1.627 | 0.841-3.149 | 0.148 |
| Tumor volume
(cm3) (≥14 vs. <14) | 2.073 | 1.173-3.662 | 0.012 | 1.936 | 1.078-3.474 | 0.027 |
| TNM stage (III-IV
vs. I-II) | 3.263 | 1.845-5.771 | 0.001 | 4.011 | 1.597-10.075 | 0.003 |
| Lymph node
metastasis (yes vs. no) | 2.328 | 1.337-4.063 | 0.003 | 0.741 | 0.307-1.790 | 0.506 |
| Distant metastasis
(yes vs. no) | 2.382 | 0.850-6.672 | 0.099 | 1.288 | 0.435-3.813 | 0.648 |
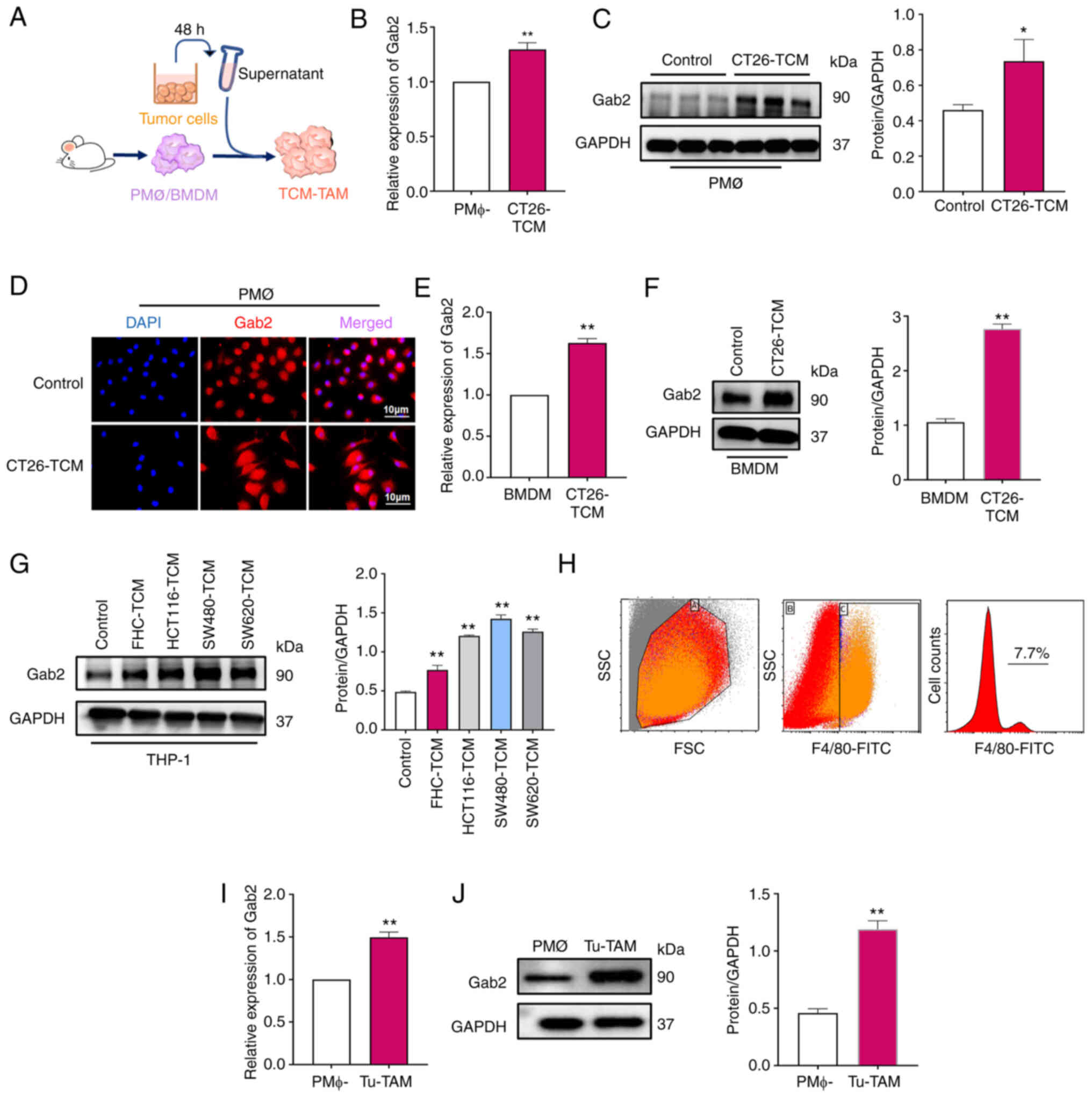
TAMs in tumor tissues exhibit a higher
expression of Gab2 and the M2 phenotype
To elucidate the role of Gab2 within TAMs, its
expression in TAMs was initially investigated using TCM from
various tumor cell lines to culture macrophages to simulate the
TME. Firstly, PMΦ (Fig. 2A-D)
and BMDM (Fig. 2E and F) were
cultured with a TCM from CT26 cells for 24 h to verify Gab2
expression within TAMs. Furthermore, TCM derived from FHC cells and
human CRC cell lines, including HCT116, SW480 and SW620 cells, was
used to culture with PMA-differentiated human THP-1 monocytes to
evaluate Gab2 expression within TAMs (Fig. 2G). Cumulatively, the results
revealed a significantly elevated Gab2 expression within the
TCM-TAMs compared to the normal macrophages or macrophages that
were cultured with TCM from the FHC cell line. To further verify
the expression of Gab2 within TAMs in vivo, a subcutaneously
transplanted tumor model was established using CT26 cells. On the
21st day post-injection, tumor tissues were harvested, and
single-cell suspensions were prepared to analyze and isolate TAMs
for further investigation. It was observed that the proportion of
TAMs was ~7.7% (Fig. 2H).
Notably, a significantly heightened expression of Gab2 was observed
within Tu-TAM compared to PMΦ isolated from tumor-free mice
(Fig. 2I and J). In summary,
these results highlight the elevated expression of Gab2 within TAMs
both in vitro and in vivo, suggesting its potential
role in regulating TAMs.
To determine the polarization phenotype of TAMs, PMΦ
were used as M0 macrophages, which were further polarized into M1
macrophages using LPS and IFN-γ stimulation, or into M2 macrophages
using IL-4 stimulation, thereby representing the two opposite
states of macrophage polarization. Subsequently, the mRNA
expression of M1 markers [inducible nitric oxide synthase
(Inos/Nos2), Il-12 and C-X-C motif chemokine
ligand 9 (Cxcl9)] and M2 markers [Il-10),
Arg-1, chitinase-like protein 3 (Ym-1/Chil3),
resistin-like molecule alpha (Fizz1/Retnla), C-C
motif chemokine ligand 17 (Ccl17) and vascular endothelial
growth factor (Vegf)] in PMΦ, Tu-TAM, TCM-TAM was analyzed
using RT-qPCR. The results revealed the significant suppression of
the M1 markers, Inos and Il-12, in Tu-TAM and TCM-TAM
compared to PMΦ (Fig. 3A). By
contrast, a marked elevation of M2 marker expression was observed
in Tu-TAM, TCM-TAM compared to PMΦ (Fig. 3B). Furthermore, the results of
western blot analysis revealed that the expression of M2 macrophage
markers (CD206 and Arg-1) was significantly upregulated in the
Tu-TAM and TCM-TAM (Fig. 3C).
Taken together, these results indicated that TAMs exhibit an
M2-like phenotype.
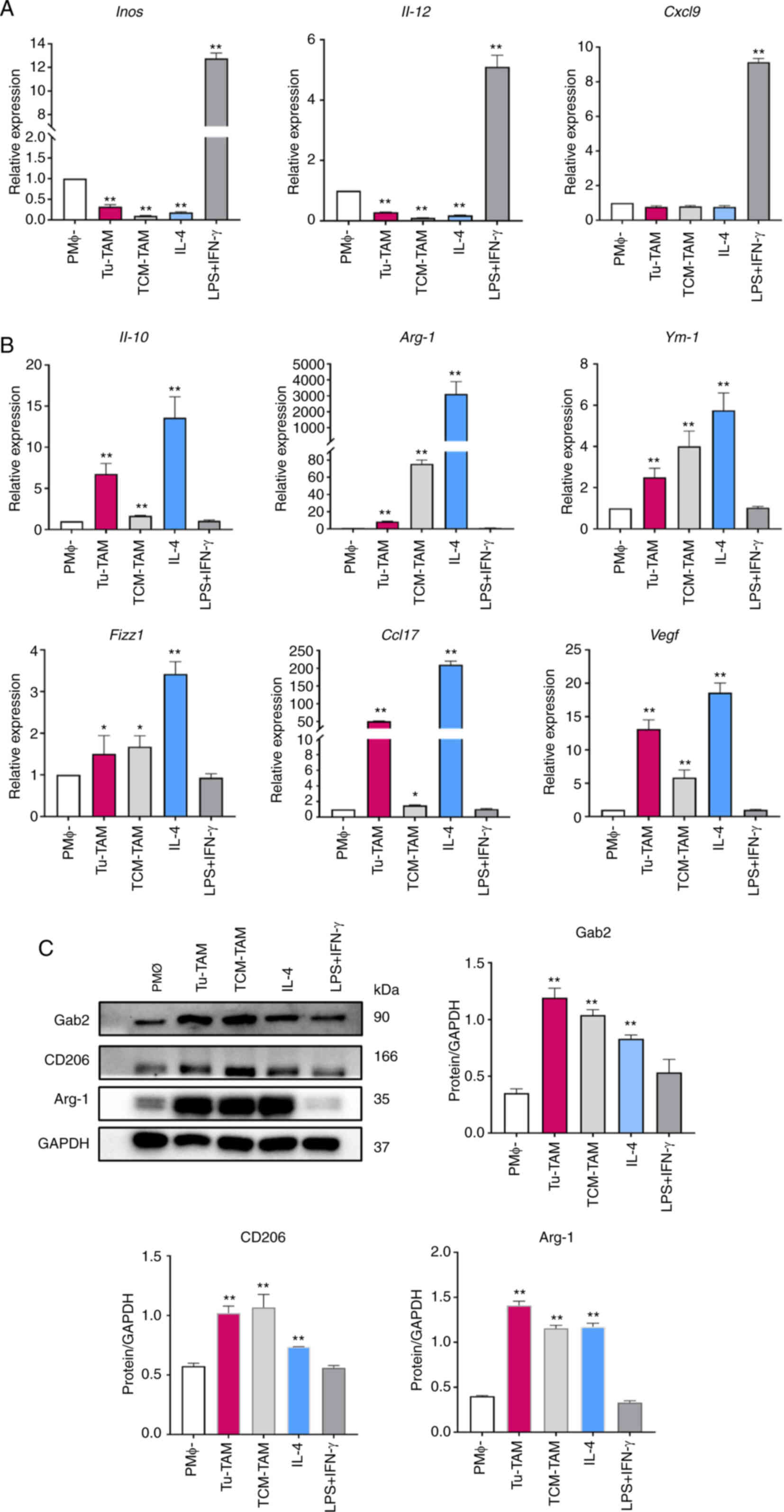 | Figure 3Expression of TAM
polarization-related molecules. PMΦ from BALB/c mice were
stimulated with LPS + IFN-γ and IL-4 for 24 h, serving as an M1/M2
positive control. (A) Evaluation of Inos, Il-12 and
Cxcl9 mRNA expression levels in PMΦ, Tu-TAM, TCM-TAM using
RT-qPCR. **P<0.01, Tu-TAM, TCM-TAM, IL-4, LPS + IFN-γ
vs. PMΦ. (B) Evaluation of Il-10, Arg-1, Ym-1,
Fizz1, Ccl17, Vegf mRNA expression levels in
PMΦ, Tu-TAM, TCM-TAM using RT-qPCR. *P<0.05, Tu-TAM,
TCM-TAM vs. PMΦ. **P<0.01, Tu-TAM, TCM-TAM, IL-4 vs.
PMΦ. (C) Evaluation of Arg-1, CD206 protein levels in PMΦ, Tu-TAM,
TCM-TAM using western blot analysis. **P<0.01,
Tu-TAM, TCM-TAM, IL-4 vs. PMΦ. TAMs, tumor-associated macrophages;
PMΦ, peritoneal macrophages; LPS, LPS, lipopolysaccharide; IFN-γ,
interferon-γ; IL, interleukin; Inos, inducible nitric oxide
synthase; Cxcl9, C-X-C motif chemokine ligand 9; Tu-TAM,
macrophages sorted from subcutaneously transplanted tumors in mice;
TCM, tumor-conditioned medium; RT-qPCR, reverse
transcription-quantitative PCR; Arg-1, arginase-1;
Ym-1, Chil3/chitinase-like protein 3; Fizz1,
Retnla/resistin-like molecule alpha; Vegf, vascular
endothelial growth factor; Ccl17, C-C motif chemokine ligand
17; Gab2, Gab2, Grb2-associated binder 2. |
Silencing of Gab2 expression impedes the
M2 polarization of TAMs
To further investigate the effects of Gab2 on TAM
polarization, PMΦ were infected with LV-CON and three Gab2
lentiviral interference vectors (LV-Gab2-sh1, LV-Gab2-sh2 and
LV-Gab2-sh3). The transfection efficiency was examined using
RT-qPCR and immunofluorescence staining. It was observed that
transfection at an MOI of 80 achieved >80% infection efficiency
while simultaneously maintaining the normal cell confluence and
showing no signs of unusual morphological changes (Fig. 4A). The analyses revealed that
among the three shRNAs, LV-Gab2-sh3 effectively suppressed Gab2
expression in PMΦ (Fig. 4B and
C). Consequently, LV-Gab2-sh3 was selected for use in
subsequent experiments. LV-Gab2-sh3 was used to suppress Gab2
expression in PMΦ; these cells were then incubated with TCM for 24
h to investigate the expression of TAM polarization-related
molecules. The results demonstrated that the suppression of Gab2
expression in PMΦ significantly decreased the mRNA levels of M2
macrophage markers compared to the LV-CON control group.
Furthermore, it was evident that the mRNA levels of TCM-induced
M2-associated molecules, including Il-10, Arg-1,
Ym-1, Fizz1, Ccl17 and Vegf were
significantly reduced in the cells in which Gab2 expression was
suppressed (Fig. 4E). However,
the expression levels of M1 markers, such as Inos, were
increased, whereas Il-12 and Cxcl9 remained
relatively unaltered (Fig. 4D).
As was expected, the results of western blot analysis confirmed
that the suppression of Gab2 expression significantly led to a
notable reduction in the expression of M2-associated molecules,
such as CD206 and Arg-1 (Fig.
4F). Taken together, these data indicate that the
downregulation of Gab2 expression serves as a significant barrier
to the M2 polarization of TAMs.
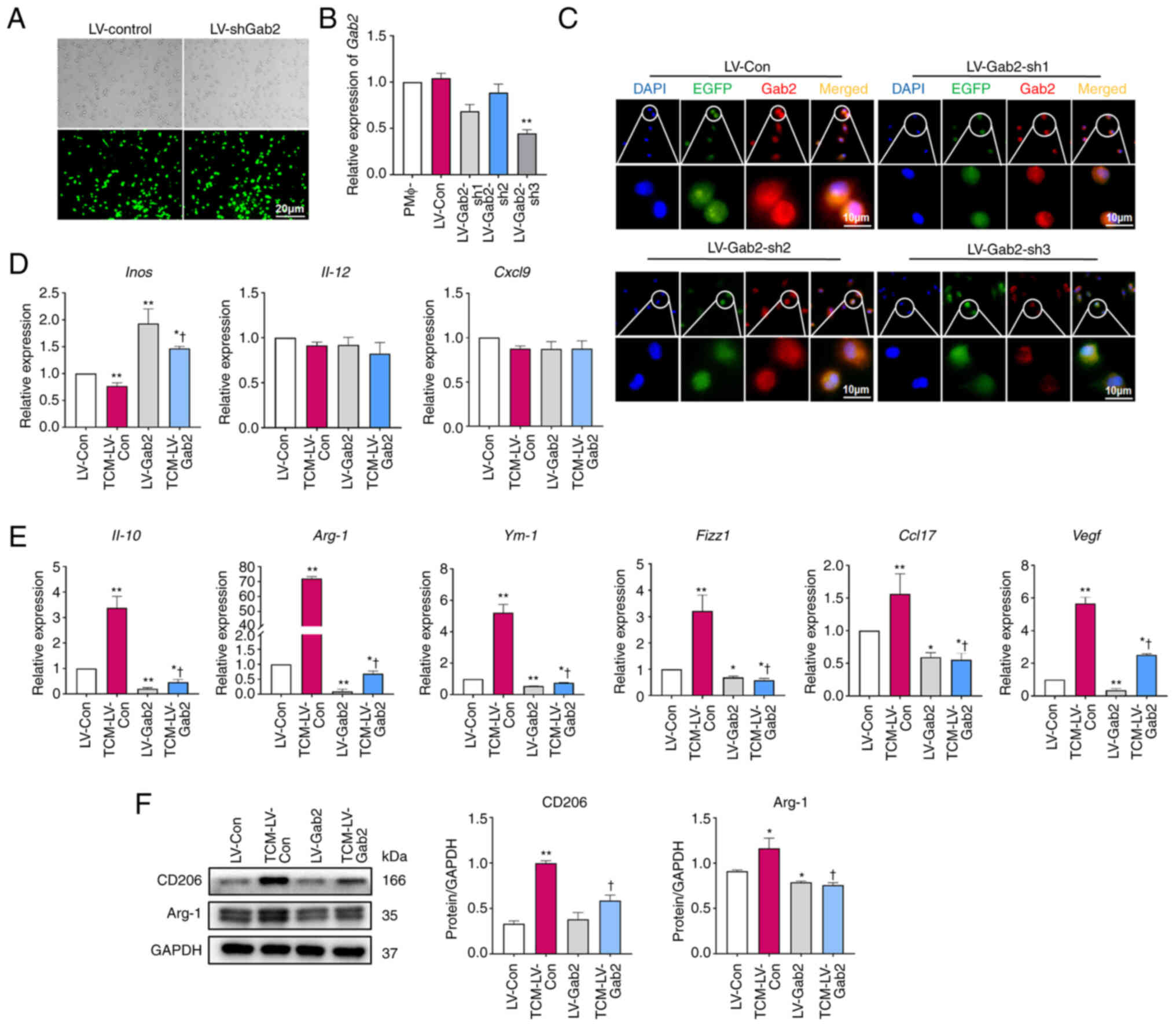 | Figure 4Suppression of Gab2 expression
impedes the M2 polarization of TAMs. (A) Visualization of EGFP
expression in PMΦ following lentivirus infection. Scale bar, 50
μm. (B and C) The expression of Gab2 in PMΦ post-lentivirus
infection. Scar bar, 10 μm. **P<0.01, LV-Gab2
vs. respective control (D) Effect of the suppression of Gab2
expression on the molecules related to TAM M1 polarization.
*P<0.05, TCM-LV-Con vs. LV-Con.
**P<0.01, LV-Gab2 vs. LV-Con. †P<0.05,
TCM-LV-Gab2 vs. TCM-LV-Con. (E) Effect of the suppression of Gab2
expression on TAM M2 polarization markers. *P<0.05,
LV-Gab2 vs. LV-Con. **P<0.01, TCM-LV-Con, LV-Gab2 vs.
PMΦ. †P<0.05, TCM-LV-Gab2 vs. TCM-LV-Con. (F) Effect
of the suppression of Gab2 expression on TAM M2 polarization
markers. *P<0.05, TCM-LV-Con, LV-Gab2 vs. LV-Con.
**P<0.01, TCM-LV-Con vs. PMΦ. †P<0.05,
TCM-LV-Gab2 vs. LV-Con. Gab2, Gab2, Grb2-associated binder 2; TAMs,
tumor-associated macrophages; PMΦ, peritoneal macrophages; IL,
interleukin; Arg-1, arginase-1; Ym-1,
Chil3/chitinase-like protein 3; Fizz1, Retnla/resistin-like
molecule alpha; Vegf, vascular endothelial growth factor;
Ccl17, C-C motif chemokine ligand 17. |
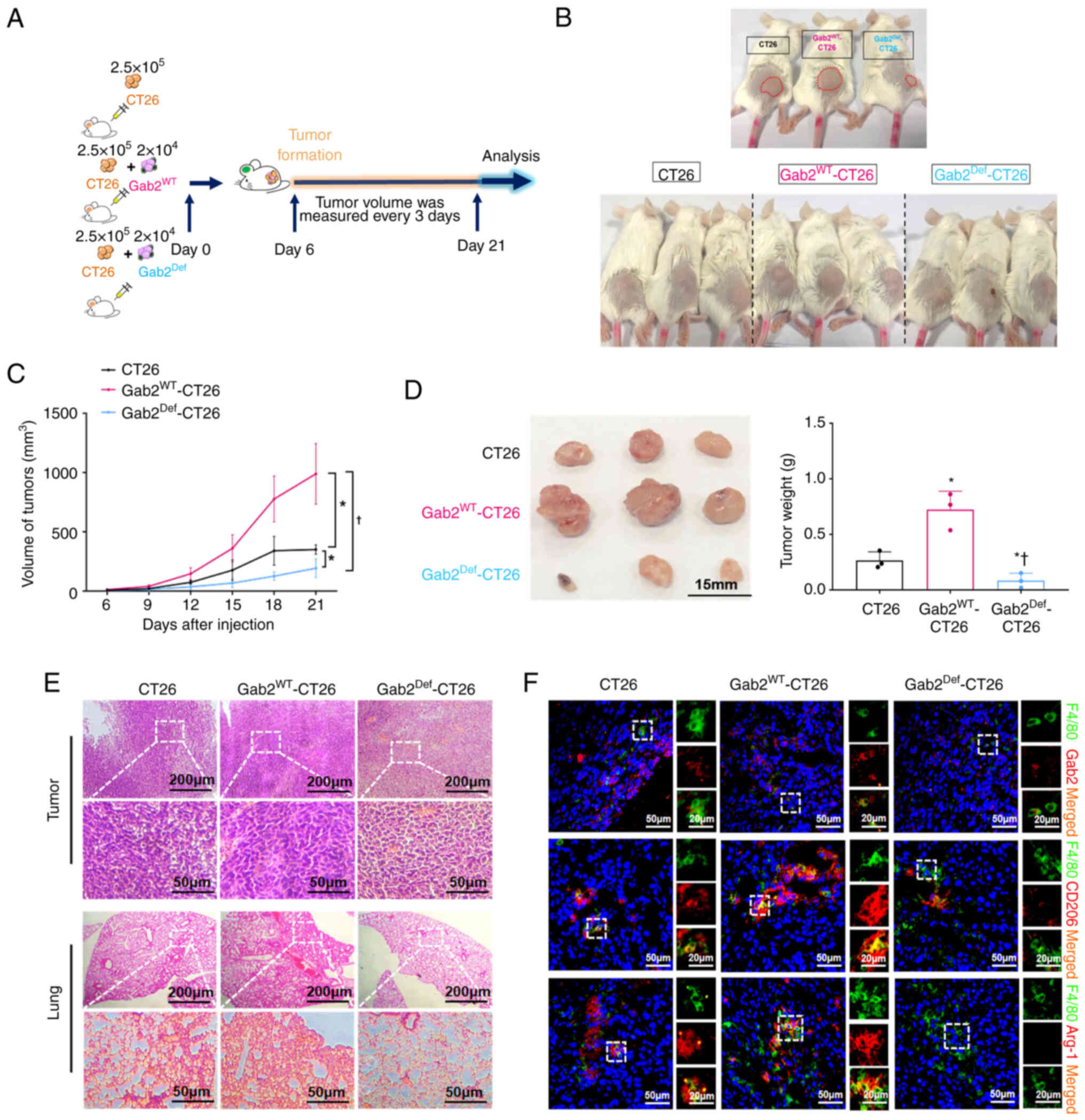
Suppression of Gab2 expression reduces
TAM-mediated CRC tumorigenesis
To explore the role of Gab2 in modulating TAM
polarization and its effects on CRC progression, a CRC xenograft
mouse model was established using CT26 cells, GabWT-CT26
cells and Gab2Def-CT26 cells subcutaneously injected
into the left flanks of mice. On the 21st day post-injection, the
mice were euthanized, and tumor tissues were dissected and weighed
(Fig. 5A and B). Notably, the
Gab2Def-CT26 group demonstrated a significant inhibition
in subcutaneous tumor progression, displaying a marked reduction in
tumor volume compared to the CT26 and Gab2WT-CT26
groups. Specifically, the Gab2WT-CT26 group exhibited
significant increases in both tumor volume and weight (Fig. 5C and D). Histological analyses
revealed that the Gab2Def-CT26 group exhibited a
reduction in abnormal enlargement and hyperchromatism in the tumor
nuclei. Additionally, there was a significant decrease in the
population of heteromorphic cells in the tumor tissue, along with
the most reduced infiltration of metastatic tumor cells within the
lung tissue compared to CT26 and Gab2WT-CT26 groups
(Fig. 5E). Furthermore,
immunofluorescence analysis demonstrated that the expression of
Gab2, CD206 and Arg-1 within TAMs in the tumor tissues was markedly
reduced in the Gab2Def-CT26 group, compared to the CT26
and GabWT-CT26 groups (Fig. 5F). Collectively, these
observations underscore that Gab2 plays a pro-tumorigenic role in
CRC, establishing that its suppression can effectively reduce the
TAM-mediated promotion of CRC tumorigenesis.
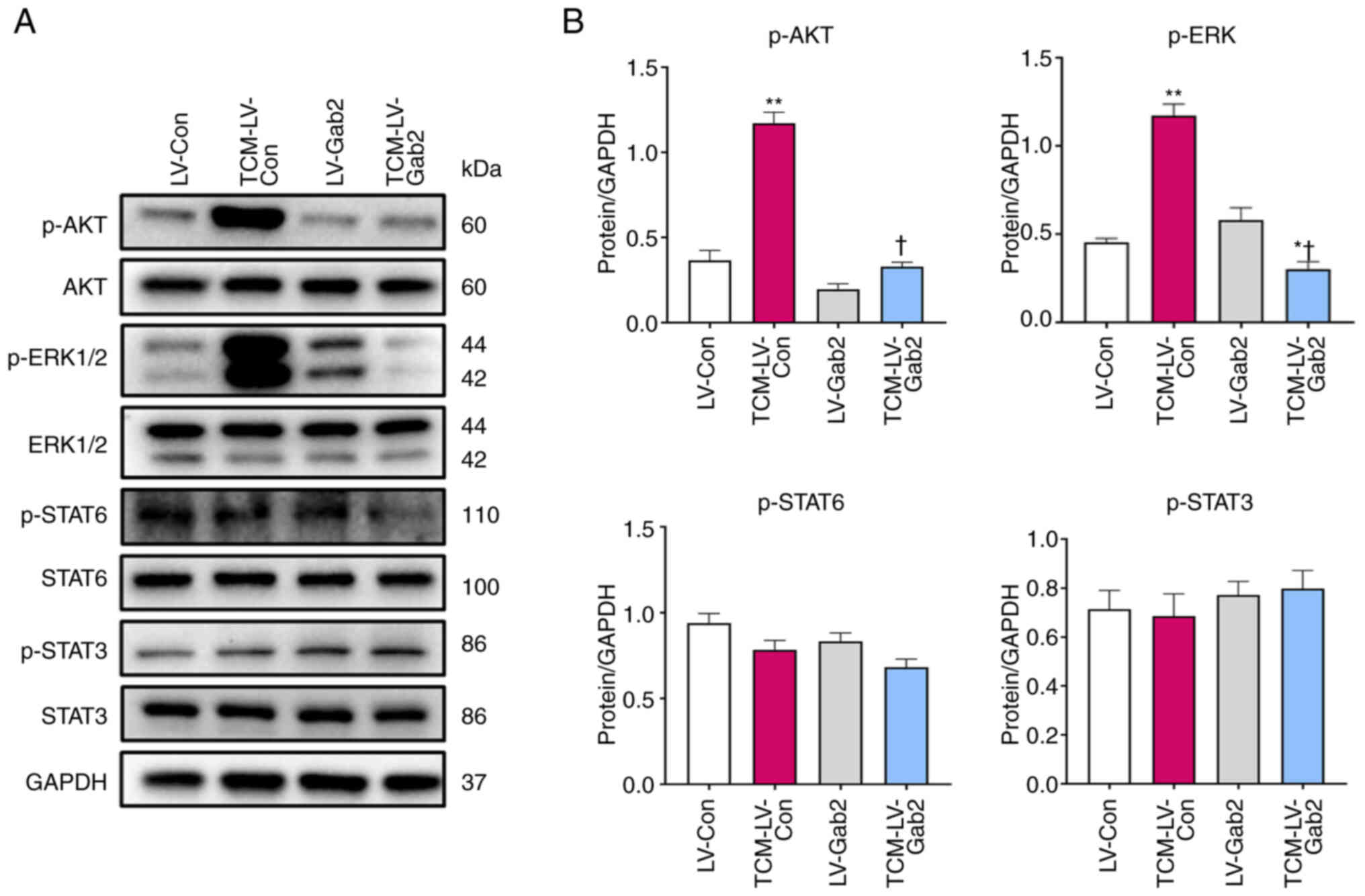
Gab2 induces M2-like macrophage
polarization through the AKT/ERK signaling pathway
The results indicated that the suppression of Gab2
expression impedes TAM polarization into M2-like macrophages,
consequently inhibiting CRC growth in mice. Adams et al
(29) demonstrated that Gab2 was
essential for two major signal transduction pathways in cancer,
namely the PI3K-AKT and ERK signaling pathways, orchestrating
numerous key cellular processes. Therefore, the present study
evaluated the protein expression and phosphorylation levels of AKT,
ERK1/2, STAT3 and STAT6 in signaling pathways associated with
macrophage polarization using western blot analysis. The results
revealed significantly increased levels of p-AKT and p-ERK1/2 in
the TCM-LV-Con compared with the LV-Con group. Conversely, the
TCM-LV-Gab2 group exhibited notably decreased levels of p-AKT and
p-ERK1/2 compared to the TCM-LV-Con group, while no significant
variations were observed in the levels of p-STAT6 and p-STAT3
(Fig. 6B). On the whole, these
findings indicate that the suppression of Gab2 expression
significantly hinders the transition of TAMs into an M2-like
macrophage state, and culminates in altered phosphorylation levels
of key signaling molecules AKT and ERK1/2, serving as a promising
target for the treatment of CRC.
Discussion
The pivotal role of Gab2, a molecule associated with
tumor growth, progression and metastasis (29,18), has been highlighted in recent
studies examining its dysregulated expression across several human
cancers, including BRCA (21),
OV (22,23), HCC (24,25), CRC (26) and melanoma (27), as well as its potential as a
novel oncogene. A previous study by the authors demonstrated a high
expression of Gab2 in CRC tissues and cell lines, particularly in
specimens from patients with CRC with metastases (26). It has also been found that the
upregulated expression of Gab2 promotes the proliferation, invasion
and metastasis of CRC, indicating its crucial role in the
occurrence and development of CRC; this underscores the prospective
value of Gab2 as a prognostic predictor for patients with CRC
(30,31). TAMs are closely related to the
occurrence and development of CRC, notably through polarization
transitions that are critical for maintaining the homeostasis of
TME (32). Guo et al
(33) revealed that Gab2
participated in the IL-4-induced M2-like macrophage polarization in
bleomycin-induced fibrotic lungs. However, the role of Gab2 in
regulating TAM polarization remains largely unexplored. Therefore,
an in-depth study of the Gab2 regulation of TAM polarization could
uncover Gab2 as a promising predictive biomarker and a feasible
therapeutic target for CRC.
The present study reports a novel biological role of
Gab2, emphasizing its critical involvement in promoting the
alternative activation of TAMs, and thereby promoting CRC growth.
The findings presented herein revealed that a high Gab2 expression
within TAMs was associated with diminished 5-year survival rates of
patients with CRC, indicating the potential effects of Gab2 on the
long-term prognosis of patients with CRC. Notably, Cox analysis
suggested the Gab2 expression levels in TAMs as a potential pivotal
factor in affecting the 5-year survival rate of patients with CRC.
However, it did not reveal any significant association between an
elevated expression of Gab2 in TAMs and conventional prognostic
factors, such as sex, age, the degree of histological
differentiation, tumor volume size, TNM stage and clinical stage.
This lack of an association calls for more in-depth investigations,
urging for a comprehensive exploration of the complex network of
prognostic factors in CRC. The inconsistencies observed in the
present study suggest at the existence of more detailed
interactions that govern the outcomes of patients with CRC, which
may include factors beyond the traditional prognostic indicators.
This presents an opportunity to further examine the intricate
association between Gab2 expression and other unknown variables
that may significantly influence the survival outcomes of patients
with CRC.
The use of a TCM is a pivotal aspect of the
experimental approach used herein, as it serves to replicate the
intricate TME in the in vitro experiments. TCM, enriched
with various cytokines, growth factors and other signaling
molecules secreted by tumor cells, facilitates the simulation of
the complex interactions occurring in the TME. This simulated
environment enabled the study of the crucial role of Gab2 in TAMs,
providing a more realistic representation of the in vivo
conditions. To further investigate the role of Gab2 within TAMs,
the present study examined its expression in TAMs using TCM from
various tumor cell lines to culture macrophages to mimic the TME.
The results revealed an elevated expression of Gab2 in TCM-TAMs
compared to normal macrophages. It was noted that the majority of
TAMs exhibit an M2-like macrophage phenotype, a feature associated
with poor outcomes of patients with CRC (34). In the TME, macrophage
polarization within tumor tissues is regulated by various signals
derived from tumor cells. Influenced by these cytokine signals,
TAMs undergo a transition into the M1 and M2 phenotypes (9,35). M1-like macrophages are
characterized by the secretion of pro-inflammatory cytokines and
have a potent tumor-killing capacity (36). Conversely, M2-like macrophages
express immune-related factors, including CD206, Arg-1, Ym-1,
Fizz1, IL-10, IL-13, TGF-β, VEGF, matrix metalloproteinases (MMPs),
promoting tumor progression and immunosuppression (37-39). The findings of the present study
established that TAMs in CRC display characteristics similar to
M2-type macrophages and that the suppression of Gab2 expression
resulted in decreased M2-associated molecules, consequently
inhibiting the M2 polarization of TAMs.
The main causes of the mortality of patients with
CRC are post-operative recurrence and distant organ metastasis,
with the liver and lungs being the principal metastatic sites
(40). Compared with liver
metastasis, patients with lung metastasis exhibit a less favorable
treatment response, resulting in poorer prognosis and shortened
survival periods (41).
Consequently, tumor invasion and metastasis significantly diminish
the survival duration and impede the quality of life of patients
with CRC (42). In the present
study, using a murine model of CRC, it was confirmed that the
suppression of Gab2 inhibited TAM-mediated CRC tumorigenesis.
Specifically, the Gab2Def-CT26 group exhibited
significant tumor growth inhibition, evidenced by a substantial
decrease in tumor size, alongside a noticeable reduction in
abnormal enlargement and hyperchromatism in the tumor nuclei.
Additionally, there was a significant decrease in the population of
heteromorphic cells in the tumor tissue, along with the most
reduced infiltration of metastatic tumor cells within the lung
tissue compared to the CT26 and Gab2WT-CT26 groups.
Hence, these results verified the pro-tumorigenic role of Gab2,
demonstrating that the suppression of its expression within TAMs
inhibits TAM-mediated CRC tumorigenesis.
In eukaryotic cells, multiple signaling pathways,
such as the AKT and ERK pathways, are interconnected through
complex networks to regulate various cellular processes, including
gene expression, cell survival, apoptosis and cell differentiation
(43,44). Gab2 functions as an adaptor
protein orchestrating several intracellular signaling pathways,
acting as a key facilitator of the PI3K/AKT and SHP2/ERK pathways,
which regulate tumor cell growth, differentiation, migration and
apoptosis (29). Specifically,
the authors previously demonstrated that Gab2 facilitated
epithelial-to-mesenchymal-transition and CRC metastasis through the
activation of the mitogen-activated protein kinase (MEK)/ERK/MMP
signaling pathway and promoted CRC growth and vascularization
through the upregulation of VEGF expression mediated by the
ERK/c-Myc signaling pathway (30,31). Furthermore, Cheng et al
(45) found that the inhibition
of PI3K, MEK or Jak2 significantly inhibited the Gab2-mediated
proliferation and migration of HepG2 cells. Horst et al
(27) found that Gab2 promoted
melanoma cell migration and invasion by activating AKT signaling
and enhancing melanoma growth and metastasis in vivo. Wang
et al (46) confirmed
that Gab2 overexpression promoted migration and invasion through
activation of the PI3K pathway, and inhibited E-calmodulin
expression in OV cells. Gong et al (19) demonstrated that Gab2 promoted
acute myeloid leukemia growth and migration through the
SHP2/ERK/CREB signaling pathway. There is evidence to suggest that
Gab2 is a central player in engaging various signaling pathways
across different types of cancers (30). The present study demonstrated
that Gab2 regulates TAM polarization by upregulating the expression
of p-AKT and p-ERK.
In conclusion, the present study demonstrated the
pivotal role of Gab2 in regulating TAM polarization, providing
further insight into the development of immunotherapeutic
strategies targeting TAMs. Despite the promising findings, the
present study is not without limitations. The emergence of various
drugs and inhibitors to modulate TAM polarization is notable. Here
are a certain strategies that the authors are considering for
future studies, such as: i) Utilizing a Cre-loxP system to
conditionally downregulate Gab2 expression specifically in
macrophages, allowing for the precise evaluation of the functional
consequences of the suppression of Gab2 expression on TAM
polarization and CRC progression; ii) exploring the possibility of
developing Gab2-specific small molecule antagonists, which can be
directed to tumors to bi-directionally regulate CRC cells and
macrophages, thereby affecting tumor growth and metastasis; iii)
using antisense oligonucleotides designed to specifically bind to
Gab2 mRNA, preventing its translation into the protein. These
strategies, alone or in combination with other therapeutic
treatments, could improve the development of novel clinical
therapies targeting Gab2 in CRC, aiming to lay the groundwork for
novel antitumor therapeutics.
Supplementary Data
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author upon reasonable
request.
Authors' contributions
XG and RL performed the experiments, analyzed the
data and wrote the manuscript. MQ performed the experiments and
analyzed the data. WZ participated in writing and reviewing the
original manuscript, and made critical revisions to the manuscript,
as well as directing the animal and cell experiments. LW, PD and JC
conceptualized the study, contributed to the formal analysis, the
visualization of the study, and made critical revisions to the
manuscript. JL and JF conceived and designed the experiments,
analyzed the data and wrote the manuscript. All authors reviewed
the manuscript. All the authors confirm the authenticity of all the
raw data. All the authors have carefully reviewed the manuscript,
and have read and approved the final manuscript.
Ethics approval and consent to
participate
The present study was approved by the Ethics
Committee of the Shanghai Outdo Biotech Co., Ltd, and was conducted
in accordance with the ethical standards set out in the Declaration
of Helsinki. All patients or their next of kin provided their
informed consent prior to the study. The animals were housed under
specific pathogen-free conditions at Zunyi Medical University. All
animal experiments were performed according to the Guidelines for
the Care and Use of Laboratory Animals (Ministry of Health, China,
1998). The experimental procedures were approved in accordance with
the ethical guidelines of the Zunyi Medical University Laboratory
Animal Care and Use Committee (permit no. 2018016).
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
Abbreviations:
|
IARC
|
International Agency for Research on
Cancer
|
|
CRC
|
colorectal cancer
|
|
Gab2
|
Grb2-associated binder 2
|
|
TME
|
tumor microenvironment
|
|
TAMs
|
tumor-associated macrophages
|
|
LPS
|
lipopolysaccharide
|
|
IFN-γ
|
interferon-γ
|
|
IL
|
interleukin
|
|
BRCA
|
breast cancer
|
|
OV
|
ovarian cancer
|
|
HCC
|
hepatocellular carcinoma
|
|
PMΦ
|
peritoneal macrophages
|
|
MOI
|
multiplicity of infection
|
|
Tu-TAM
|
macrophages sorted from subcutaneously
transplanted tumors in mice
|
|
TCM
|
tumor-conditioned medium
|
|
TCM-TAM
|
macrophages cultured in
tumor-conditioned medium
|
|
CD206
|
CD206/macrophage mannose receptor
|
|
Arg-1
|
arginase-1
|
|
Ym-1
|
Chil3/chitinase-like protein 3
|
|
Fizz1
|
Retnla/resistin-like molecule
alpha
|
|
VEGF
|
vascular endothelial growth
factor
|
|
MMP
|
matrix metalloproteinase
|
Acknowledgments
The authors would like to sincerely thank Dr Ding
Chenbo for his valuable suggestions (Shanghai Jiao Tong University,
Shanghai, China).
Funding
The present study was supported by the Program of the Natural
Science Foundation of Zhejiang Province (grant no. LY20H160017),
the Chinese Medicine Study Foundation of Zhejiang Province (grant
no. 2020ZB292) and the PhD research startup foundation of Lishui
People's Hospital (grant no. 2020bs01).
References
|
1
|
Morgan E, Arnold M, Gini A, Lorenzoni V,
Cabasag CJ, Laversanne M, Vignat J, Ferlay J, Murphy N and Bray F:
Global burden of colorectal cancer in 2020 and 2040: Incidence and
mortality estimates from GLOBOCAN. Gut. 72:338–344. 2023.
View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Sung H, Ferlay J, Siegel RL, Laversanne M,
Soerjomataram I, Jemal A and Bray F: Global Cancer Statistics 2020:
GLOBOCAN Estimates of incidence and mortality worldwide for 36
cancers in 185 countries. CA Cancer J Clin. 71:209–249. 2021.
View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Krul MF, Elferink MAG, Kok NFM, Dekker E,
Lansdorp-Vogelaar I, Meijer GA, Nagtegaal ID, Breekveldt ECH, Ruers
TJM, van Leerdam ME and Kuhlmann KFD: Initial impact of national
CRC screening on incidence and advanced colorectal cancer. Clin
Gastroenterol Hepatol. 21:797–807. 2023. View Article : Google Scholar
|
|
4
|
Wele P, Wu X and Shi H: Sex-dependent
differences in colorectal cancer: With a focus on obesity. Cells.
11:36882022. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Barkley D, Moncada R, Pour M, Liberman DA,
Dryg I, Werba G, Wang W, Baron M, Rao A, Xia B, et al: Cancer cell
states recur across tumor types and form specific interactions with
the tumor microenvironment. Nat Genet. 54:1192–1201. 2022.
View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Elhanani O, Ben-Uri R and Keren L: Spatial
profiling technologies illuminate the tumor microenvironment.
Cancer Cell. 41:404–420. 2023. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Luo H, Xia X, Huang LB, An H, Cao M, Kim
GD, Chen HN, Zhang WH, Shu Y, Kong X, et al: Pan-cancer single-cell
analysis reveals the heterogeneity and plasticity of
cancer-associated fibroblasts in the tumor microenvironment. Nat
Commun. 13:66192022. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Xu W, Wu Y, Liu W, Anwaier A, Tian X, Su
J, Huang H, Wei G, Qu Y, Zhang H and Ye D: Tumor-associated
macrophage-derived chemokine CCL5 facilitates the progression and
immunosuppressive tumor microenvironment of clear cell renal cell
carcinoma. Int J Biol Sci. 18:4884–4900. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Chen D, Zhang X, Li Z and Zhu B: Metabolic
regulatory crosstalk between tumor microenvironment and
tumor-associated macrophages. Theranostics. 11:1016–1030. 2021.
View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Christofides A, Strauss L, Yeo A, Cao C,
Charest A and Boussiotis VA: The complex role of tumor-infiltrating
macrophages. Nat Immunol. 23:1148–1156. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Mantovani A, Allavena P, Marchesi F and
Garlanda C: Macrophages as tools and targets in cancer therapy. Nat
Rev Drug Discov. 21:799–820. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Pan Y, Yu Y, Wang X and Zhang T:
Tumor-associated macrophages in tumor immunity. Front Immunol.
11:5830842020. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Cassetta L and Pollard JW: A timeline of
tumour-associated macrophage biology. Nat Rev Cancer. 23:238–257.
2023. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Boutilier AJ and Elsawa SF: Macrophage
polarization states in the tumor microenvironment. Int J Mol Sci.
22:69952021. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Wang H, Yung MMH, Ngan HYS, Chan KKL and
Chan DW: The impact of the tumor microenvironment on macrophage
polarization in cancer metastatic progression. Int J Mol Sci.
22:65602021. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Hwang I, Kim JW, Ylaya K, Chung EJ, Kitano
H, Perry C, Hanaoka J, Fukuoka J, Chung JY and Hewitt SM:
Tumor-associated macrophage, angiogenesis and lymphangiogenesis
markers predict prognosis of non-small cell lung cancer patients. J
Transl Med. 18:4432020. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Li H, Luo F, Jiang X, Zhang W, Xiang T,
Pan Q, Cai L, Zhao J, Weng D, Li Y, et al: CircITGB6 promotes
ovarian cancer cisplatin resistance by resetting tumor-associated
macrophage polarization toward the M2 phenotype. J Immunother
Cancer. 10:e0040292022. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Ding CB, Yu WN, Feng JH and Luo JM:
Structure and function of Gab2 and its role in cancer (Review). Mol
Med Rep. 12:4007–4014. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Gong R, Li H, Liu Y, Wang Y, Ge L, Shi L,
Wu G, Lyu J, Gu H and He L: Gab2 promotes acute myeloid leukemia
growth and migration through the SHP2-Erk-CREB signaling pathway. J
Leukoc Biol. 112:669–677. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Spohr C, Poggio T, Andrieux G, Schönberger
K, Cabezas-Wallscheid N, Boerries M, Halbach S, Illert AL and
Brummer T: Gab2 deficiency prevents Flt3-ITD driven acute myeloid
leukemia in vivo. Leukemia. 36:970–982. 2022. View Article : Google Scholar :
|
|
21
|
Zhang P, Chen Y, Gong M, Zhuang Z, Wang Y,
Mu L, Wang T, Pan J, Liu Y, Xu J, et al: Gab2 ablation reverses the
stemness of HER2-overexpressing breast cancer cells. Cell Physiol
Biochem. 50:52–65. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Davis SJ, Sheppard KE, Anglesio MS, George
J, Traficante N, Fereday S, Intermaggio MP, Menon U, Gentry-Maharaj
A, Lubinski J, et al: Enhanced GAB2 expression is associated with
improved survival in high-grade serous ovarian cancer and
sensitivity to PI3K inhibition. Mol Cancer Ther. 14:1495–1503.
2015. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Duckworth C, Zhang L, Carroll SL, Ethier
SP and Cheung HW: Overexpression of GAB2 in ovarian cancer cells
promotes tumor growth and angiogenesis by upregulating chemokine
expression. Oncogene. 35:4036–4047. 2016. View Article : Google Scholar :
|
|
24
|
Hu X, He B, Zhou L, Xie H and Zheng S:
Expression pattern and clinical significance of Gab2 protein in
hepatocellular carcinoma. Clin Lab. 62:1087–1092. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Liu R, Sun Y, Chen S, Hong Y and Lu Z:
FOXD3 and GAB2 as a pair of rivals antagonistically control
hepatocellular carcinogenesis. FEBS J. 289:4536–4548. 2022.
View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Ding C, Luo J, Yu W, Gao S, Yang L, Chen C
and Feng J: Gab2 is a novel prognostic factor for colorectal cancer
patients. Int J Clin Exp Pathol. 8:2779–2786. 2015.PubMed/NCBI
|
|
27
|
Horst B, Gruvberger-Saal SK, Hopkins BD,
Bordone L, Yang Y, Chernoff KA, Uzoma I, Schwipper V, Liebau J,
Nowak NJ, et al: Gab2-mediated signaling promotes melanoma
metastasis. Am J Pathol. 174:1524–1533. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(-Delta Delta C(T)) method. Methods. 25:402–408. 2001.
View Article : Google Scholar
|
|
29
|
Adams SJ, Aydin IT and Celebi JT: GAB2-a
scaffolding protein in cancer. Mol Cancer Res. 10:1265–1270. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Ding C, Luo J, Li L, Li S, Yang L, Pan H,
Liu Q, Qin H, Chen C and Feng J: Gab2 facilitates
epithelial-to-mesenchymal transition via the MEK/ERK/MMP signaling
in colorectal cancer. J Exp Clin Cancer Res. 35:52016. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Ding C, Luo J, Fan X, Li L, Li S, Wen K,
Feng J and Wu G: Elevated Gab2 induces tumor growth and
angiogenesis in colorectal cancer through upregulating VEGF levels.
J Exp Clin Cancer Res. 36:562017. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Wu K, Lin K, Li X, Yuan X, Xu P, Ni P and
Xu D: Redefining tumor-associated macrophage subpopulations and
functions in the tumor microenvironment. Front Immunol.
11:17312020. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Guo X, Li T, Xu Y, Xu X, Zhu Z, Zhang Y,
Xu J, Xu K, Cheng H, Zhang X and Ke Y: Increased levels of Gab1 and
Gab2 adaptor proteins skew interleukin-4 (IL-4) signaling toward M2
macrophage-driven pulmonary fibrosis in mice. J Biol Chem.
292:14003–14015. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Cheng Y, Zhu Y, Xu J, Yang M, Chen P, Xu
W, Zhao J, Geng L and Gong S: PKN2 in colon cancer cells inhibits
M2 phenotype polarization of tumor-associated macrophages via
regulating DUSP6-Erk1/2 pathway. Mol Cancer. 17:132018. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Gao J, Liang Y and Wang L: Shaping
polarization of tumor-associated macrophages in cancer
immunotherapy. Front Immunol. 13:8887132022. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Kashfi K, Kannikal J and Nath N:
Macrophage reprogramming and cancer therapeutics: Role of
iNOS-derived NO. Cells. 10:31942021. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Zhang Q and Sioud M: Tumor-associated
macrophage subsets: Shaping polarization and targeting. Int J Mol
Sci. 24:74932023. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Bingle L, Brown NJ and Lewis CE: The role
of tumour-associated macrophages in tumour progression:
Implications for new anticancer therapies. J Pathol. 196:254–265.
2002. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Wang X, Yuwen TJ, Zhong Y, Li ZG and Wang
XY: A new method for predicting the prognosis of colorectal cancer
patients through a combination of multiple tumor-associated
macrophage markers at the invasive front. Heliyon. 9:e132112023.
View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Shen T, Liu JL, Wang CY, Rixiati Y, Li S,
Cai LD, Zhao YY and Li JM: Targeting erbin in B cells for therapy
of lung metastasis of colorectal cancer. Signal Transduct Target
Ther. 6:1152021. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Hou J, Zhang Y and Zhu Z: Gene
heterogeneity in metastasis of colorectal cancer to the lung. Semin
Cell Dev Biol. 64:58–64. 2017. View Article : Google Scholar
|
|
42
|
Malki A, ElRuz RA, Gupta I, Allouch A,
Vranic S and Al Moustafa AE: Molecular mechanisms of colon cancer
progression and metastasis: Recent insights and advancements. Int J
Mol Sci. 22:1302020. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Ye Q, Cai W, Zheng Y, Evers BM and She QB:
ERK and AKT signaling cooperate to translationally regulate
survivin expression for metastatic progression of colorectal
cancer. Oncogene. 33:1828–1839. 2014. View Article : Google Scholar :
|
|
44
|
Hijazi M, Casado P, Akhtar N,
Alvarez-Teijeiro S, Rajeeve V and Cutillas PR: eEF2K activity
determines synergy to cotreatment of cancer cells with PI3K and MEK
inhibitors. Mol Cell Proteomics. 21:1002402022. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Cheng J, Zhong Y, Chen S, Sun Y, Huang L,
Kang Y, Chen B, Chen G, Wang F, Tian Y, et al: Gab2 mediates
hepatocellular carcinogenesis by integrating multiple signaling
pathways. FASEB J. 31:5530–5542. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Wang Y, Sheng Q, Spillman MA, Behbakht K
and Gu H: Gab2 regulates the migratory behaviors and E-cadherin
expression via activation of the PI3K pathway in ovarian cancer
cells. Oncogene. 31:2512–2520. 2012. View Article : Google Scholar :
|