Introduction
Cancer pain is the most frequently occurring and
problematic symptom of cancer and is experienced by 66.4% of
patients with advanced cancer (1). Bones are a particularly common site
for cancer metastases. The bone metastasis incidence is ~30-75%
with breast cancer and prostate cancer (2). Bone metastasis leads to
hypercalcemia, pathological skeletal fractures, compression of the
spinal cord and pain (3).
Cancer-induced bone pain (CIBP) is characterized as moderate to
severe pain and is a combination of background, spontaneous and
incidental pain that persists for years (4). According to the World Health
Organization approach to cancer pain treatment, a three-step ladder
provides guidelines for patients with cancer pain, including
nonopioid pain medications and lower or higher potency opioids for
mild to moderate or moderate to severe pain (5). However, ~30% of patients were
dissatisfied with the treatment. (6) Depending on the advanced treatment
and increased diagnoses, almost half of cancer patients live for
longer than 10 years (7). As a
result, this means that an increased number of patients with cancer
are suffering from uncontrolled pain. Elucidating the pathogenesis
of CIBP would be helpful for the development of novel therapies for
pathological pain.
Spinal inflammation plays a major role in chronic
pain. When cancer cells metastasize and grow in bone marrow, bone
structure becomes impaired, inflammatory infiltration in the bone
is increased and the nociceptive signals are stimulated (8). Nociceptive signals enter the spinal
cord serve to activate neuroinflammation, which is characterized by
leukocyte infiltration, glial activation and the release of
pro-inflammatory cytokines and chemokines (9,10). Elevated levels of
neuroinflammation have been observed in patients with chronic pain,
including lumbar radiculopathy and pain-positive human
immunodeficiency virus (11).
Neuroinflammation in the spinal cord is also activated in rodent
models of several types of chronic pain, including inflammation
pain, neuropathic pain, chemotherapy pain and bone cancer pain
(12,13). The NOD-like receptor pyrin
domain-containing 3 (NLRP3) inflammasome mediates caspase-1
activation and proinflammatory cytokine IL-1β secretion and
participates in chronic pain (14). Activated NLRP3 recruits the
apoptosis-associated speck-like protein that contains a CARD (ASC)
and caspase-1 as a means of forming NLRP3 inflammasome (15). The activated caspase-1 cleaves
IL-1β to maturation and secretion (16). Increased NLRP3 and IL-1β
expressions are found in the blood of patients who suffer from
fibromyalgia, which is a type of chronic pain (17). MCC950 is a specific small
molecular inhibitor of NLRP3 inflammasome treatment that reduces
NLRP3, ASC, caspase-1 and IL-1β expression levels to basal levels
in the spinal cord and attenuates mechanical allodynia in the
rodent models of cancer-induced bone pain and oxaliplatin-induced
peripheral neuropathy (18-20). Therefore, inhibiting NLRP
inflammasome-mediated neuroinflammation in the spinal cord is a
potential therapeutic strategy for pathological pain.
Histone deacetylase (HDAC) 6 is a cytoplasmic class
II HDAC that is considered to be a potential therapeutic target for
chronic pain (21). The
dysregulation of HDAC6 has been implicated in peripheral neuropathy
and neuropathic pain (22).
HDAC6 inhibitor SW-100 treatment restores α-tubulin acetylation
loss in sciatic nerve tissue while also alleviating mechanical
allodynia and hyperalgesia in the peripheral neuropathy mouse model
of Charcot-Marie-Tooth disease (23). Cisplatin-induced mechanical
allodynia and spontaneous pain are reversed in chemotherapy-induced
peripheral neuropathy model mice by HDAC6 inhibitor ACY-1083
(24). The genetic deletion of
HDAC6 serves to protect against cisplatin-induced mechanical
allodynia (25). HDAC6 plays a
crucial role at the intersection of pathogenic pathways, which
includes the cellular responses to protein stress, organelle damage
and neuroinflammatory signaling (26). HDAC6 functions as a dynein
adapter and facilitates the retrograde transporting of NLRP3
inflammasome for activation (27). Research on animal models linked
to inflammation has indicated that HDAC6 inhibition serves to
improve neuropathies through the downregulation of NLRP3
inflammasome activation (28).
The application of HDAC6 degrader attenuates the activation of
NLRP3 inflammasome in lipopolysaccharide-induced mice (29). The inhibition of HDAC6 decreases
NLRP3 and IL-1β expressions while also suppressing nicotine-induced
pyroptosis in atherosclerosis model mice (30). The inhibition of HDAC6 by
tubastatin (TSA) attenuates NLRP3 expression and the maturation of
caspase-1 and IL-1β in Parkinson's disease model mice (31). Accordingly, targeting HDAC6 is
promising for the management of chronic pain and
neuroinflammation.
In the present study, HDAC6 inhibitor TSA was
injected into the spinal cords of CIBP rats as a means of
investigating the effect of HDAC6 on cancer pain and changes in
behaviors and spinal inflammation were subsequently detected. The
aim of present study was to clarify the effect of HDAC6 inhibition
has on cancer pain alleviation and providing an analgesic target
for the management of cancer pain.
Materials and methods
Antibodies and reagents
The following antibodies were used in the cell and
tissue analysis: Anti-glial fibrillary acidic protein (GFAP; cat.
no. A19058), anti-complement factor B (CFB) (cat. no. A13243),
anti-NLRP3 (cat. no. A12694), anti-NF-κB (cat. no. A19653),
anti-caspase-1 (cat. no. A16792), anti-TNF-α (cat. no. A11534) and
anti-HDAC6 (cat. no. A1732) were purchased from ABclonal Biotech
Co., Ltd.; anti-IL-1β (cat. no. AF5103), anti-C3 (cat. no.
DF13224), anti-GFAP (cat. no. BF8023), anti-NLRP3 (cat. no. BF8029)
and anti-β-actin (cat. no. AF7018), were purchased from Affinity
Biosciences; and anti-pSer22-HDAC6 (cat. no. YP0922), which was
purchased from ImmunoWay Biotechnology Company. The secondary
antibodies that were used for western blotting were HRP Goat
anti-rabbit IgG (H+L; cat. no. AS014) and HRP Goat Anti-Mouse IgG
(H+L; cat. no. AS003), which were purchased from ABclonal Biotech
Co., Ltd. The secondary antibodies that were used for
immunofluorescence analysis were Alexa Fluor 555-labeled Donkey
Anti-Rabbit IgG (H+L; cat. no. A0453) and Alexa Fluor 488-labeled
Goat Anti-Rabbit IgG (H+L; cat. no. A0423), which were purchased
from Beyotime Institute of Biotechnology. Tubastatin A (cat. no.
S8049) was purchased from Selleck Chemicals. Hematoxylin and eosin
(H&E) staining solution (cat. no. BL735B), phosphate buffer
solution (cat. no. BL550A), 4% paraformaldehyde (PFA; cat. no.
BL539A), 0.3% Triton X-100 (cat. no. BL935A) and ECL detection
reagent (cat. no. BL520A) were all purchased from Biosharp Life
Sciences. RPMI 1640 medium (cat. no. 11875119), fetal bovine serum
(FBS; cat. no. 10091148), 50 U/ml penicillin and 50 μg/ml
streptomycin (cat. no. 15140122), Hank's balance salt solution
(HBSS; cat. no. 14170146) and DMEM medium (cat. no. 11320033) were
all purchased from Gibco (Thermo Fisher Scientific, Inc.). IL-1β
(cat. no. P5106; 10 μg), immunofluorescence blocking
solution (cat. no. P0102), Hoechst 33342 (cat. no. C1027), RIPA
lysis buffer (cat. no. P0013B), Protease inhibitors cocktail (cat.
no. P1005), BCA protein assay kit (cat. no. BL521A), QuickBlock
Blocking Buffer for Western Blot (cat. no. P0252; 500ml) and
protein G agarose (cat. no. P2009) were all purchased from Beyotime
Institute of Biotechnology. The commercial products HDAC6 short
interfering (si) RNA (sc-35544), siRNA Transfection Medium
(sc-36868), siRNA Transfection Reagent (sc-29528) and control siRNA
(sc-37007) were both purchased from Santa Cruz Biotechnology,
Inc.
Animals
A total of 27 male Sprague-Dawley rats that weighed
160-200 g (6-8 weeks) were purchased from Hubei Province
Experimental Animal Center. All the rats were housed with ad
libitum access to water and food in a 12-h light/dark cycle at
a stable environment temperature of 22-24°C and humidity of 55-58%.
All procedures were conducted in accordance with the Chinese
National Guidelines for the ethical review of animal welfare (GB/T
35892-2018, https://std.samr.gov.cn/gb/search/gbDetailed?id=71F772D8283AD3A7E05397BE0A0AB82A)
and they were approved by the Ethics Committee of Hubei University
of Science and Technology (approval number 2020-01-900). The rats
were allowed to acclimatize to their environment for five days
before the experiments started. The 27 rats were divided at random
into the three following groups: Sham-operated (sham) group, CIBP
group and CIBP + TSA group; there were nine rats in each group.
Cell culture and treatment
MRMT-1 rat cells (cat. no. RCB2860; Jennio Biotech
Co, Ltd.) were cultured in RPMI 1640 medium supplemented with 10%
fetal bovine serum, 50 U/ml penicillin and 50 μg/ml
streptomycin in a humidified incubator that contained 5%
CO2 at 37°C. MRMT-1 cells were collected and the pellet
was resuspended in Hank's balance salt solution (HBSS) for
inoculation. Rat glioma cells (C6; cat. no. CBP60888; Jennio
Biotech Co., Ltd.) and U-87 MG cells (cat. no. HTB-14; Jennio
Biotech Co., Ltd.) were cultured in and DMEM medium with 10% fetal
bovine serum, 50 U/ml penicillin and 50 μg/ml streptomycin
at 37°C with 5% CO2. C6 and U-87 MG cells were cultured
in the 24-well plate with sterilized coverslips, stimulated with
IL-1β (5 ng/ml) for 30 min and incubated with 1 μM TSA at
37°C for 24 h for immunofluorescence assay. For western blot
analysis, U-87 cells were treated with MCC950 (1 μM) or TSA
(1 μM) for 24 h. Following 24 h of incubation, the
whole-cell extracts were collected for biochemical analyses.
Establishment of the CIBP rat model and
TSA administration
In order to establish the rat model of
cancer-induced bone pain, centrifugation at 25°C (845 × g; 2 min)
was used to collect MRMT-1 rat mammary gland carcinoma cells from
the cell suspension. The pellet was then resuspended in HBSS and
kept on ice until use. The rats were anaesthetized with
pentobarbital sodium (50 mg/kg) by intraperitoneal injection for
inoculation. Once the left hind limb had been disinfected with 75%
ethanol and shaved, 5.0×105 MRMT-1 cells in HBSS were
slowly injected into the intramedullary space of the left tibia
bones of the CIBP and CIBP + TSA rats using a 50 μl Hamilton
microsyringe. After the injection, the syringe remained in the
injection site for an additional minute as a means of preventing
tumor cells leakages. The injection site was sealed using bone wax
once the syringe had been removed (32). The sham group rats were injected
with an equivalent volume of the vehicle (HBSS) into the cavity.
Following 14 days of inoculation, an intrathecal injection was
administered with a 25-μl microsyringe, which was inserted
into the intervertebral space of the rats between the L5 and L6
vertebrae. The HDAC6 inhibitor TSA was dissolved before use. The
TSA dosage used in the present study was determined based on
published research (33). The
CIBP + TSA group rats were intrathecally injected with 10 μl
TSA (1 mg/kg). The sham and CIBP group rats received an intrathecal
injection of the same volume of the vehicle (10 μl).
Flinching number analysis
The rats were habituated for a minimum of 30 min
before behavioral experiments were conducted. For the spontaneous
pain test, the number of spontaneous flinches was recorded and
counted for 5 min. This was repeated three times (34).
Paw withdrawal threshold (PWT) test
von Frey filaments, ranging 0.4-26 g (Stoelting Co.)
were used for applying mechanical stimuli to the left hind paw. The
50% PWT was determined using Chaplan's up-down method (35). The filaments were pressed
perpendicularly against the plantar surfaces until the filaments
were bent and brisk withdrawal was considered to be a positive
response. In the case of a positive response occurring, the von
Frey filament with the next lower force was applied and in the case
of a negative response occurring, the filament with the next higher
force was applied. The pattern of positive and negative withdrawal
responses was then converted to 50% PWT.
Rotarod test
An accelerating rotarod (ZS-RDM; Zhongshi Dichuang
Technology Development) was used for assessing motor coordination
and the balance of animals. For three days prior to the
experiments, the rats were trained at a fixed speed of 4
revolutions/min for 10 min, which was repeated three times at
10-min intervals. At the start of the experiment, rotation speed
was set at a fixed value of 10 revolutions/min for 10 sec,
accelerated for 10 sec to a working speed of 20 revolutions/min for
30 sec and then accelerated again for 10 sec. This movement was
continuously carried out for 10 min. The experiments were repeated
three times with intervals of 10 min and the latency to fall of
rats was recorded (36).
Bone X-ray and histological analysis
After the behavioral tests were performed, five rats
from each group were sacrificed using an overdose of pentobarbital
sodium (150 mg/kg) that was administered by intraperitoneal
injection. For bone X-ray assay, legs were placed on X-ray film and
exposed to an X-ray source and the roentgenography of the tibia was
performed. For histological analysis, tibias were harvested, fixed
in 4% PFA and decalcified in 10% decalcification solution. The
decalcified bones were then rinsed, dehydrated, embedded in
paraffin and sectioned into 4 μm sections using a microtome
(cat. no. RM 2165; Leica Microsystems GmbH). The sections were
deparaffinized, rehydrated and H&E staining was conducted in
accordance with standard procedures. The sections were stained with
hematoxylin solution for 3 min and then washed using tap water for
10 sec. The sections were stained with eosin for 3 min and then
washed using tap water for 10 sec. The dehydration and clearing
treatment were conducted by putting the sections into a series of
ethanol (100, 90 and 70%, each for 10 min) and xylene (5 min,
twice). The temperature was 25°C for all procedures. The stained
sections were observed through a fluorescence microscope at ×60
magnification (Olympus IX73; Olympus Corporation) (37). The similar cell density and the
state of three fields in each group were examined.
Spinal cord histology
Following the behavioral tests, five rats from each
group were deeply anesthetized using 60 mg/kg sodium pentobarbital
administered by intraperitoneal injection and perfused with 0.9%
NaCl, followed by 4% PFA. Following the completion of perfusion,
the spinal cords were removed, post-fixed in 4% PFA, embedded in
paraffin as above and cut into 4 μm sections using a
microtome. The sections were stained using the standard H&E
method as aforementioned. H&E images were analyzed using ImageJ
v1.51j8 software (National Institutes of Health). The scoring
criteria of inflammation cell infiltration was as follows: 0
(normal); 1 (lymphocyte infiltration around meninges and blood
vessels); 2 (1-10 lymphocytes in a field); 3 (11-100 lymphocytes in
a field); and 4 (>100 lymphocytes in a field) (38).
Immunofluorescence assay
Following the completion of perfusion, the spinal
cords were removed, post-fixed in 4% PFA, embedded in paraffin as
above and cut into 4 μm sections using a microtome. The
spinal cord sections were deparaffinized, incubated in 3%
H2O2 for 10 min at 25°C to quench endogenous
peroxidase or biotin activity, blocked with immunofluorescence
blocking solution at 25°C for 60 min, incubated with primary
antibodies at 4°C for 24 h and secondary antibodies at 25°C for 60
min, mounted with antifade mounting medium and observed through a
fluorescence microscope (Olympus IX73; Olympus Corporation). The
intensity was analyzed using ImageJ v1.48 software (National
Institutes of Health).
After treatment, the cells were rinsed with
phosphate buffer solution, fixed with 4% PFA, permeabilized with
0.5% Triton X-100, blocked with immunofluorescence blocking
solution for 60 min at 25°C, incubated with primary antibodies at
4°C for 24 h and secondary antibodies at 25°C for 60 min,
counterstained with Hoechst 33342 (1 μg/ml) for 15 min at
25°C, mounted with antifade mounting medium and observed via
fluorescence microscopy (Olympus IX73; Olympus Corporation) for
cell research. The intensity was analyzed using ImageJ v1.48
software (National Institutes of Health).
Western blot analysis
After the behavioral tests were completed, another
five rats from each group were sacrificed with an overdose of
pentobarbital sodium (150 mg/kg), which was administered by
intraperitoneal injection. The spinal cord tissues were collected
and homogenized in ice-cold RIPA lysis buffer that contained a
cocktail of protease inhibitors. After being centrifugated at
13,523 × g at 4°C for 15 min, the supernatant was used for western
blotting. Protein concentration was determined using a BCA protein
assay kit. The supernatant was collected, the protein samples were
loaded at 30 μg per lane and separated by 8-12% SDS-PAGE,
then transferred to PVDF membranes. The membranes were blocked
using QuickBlock blocking buffer for western blot for 15 min at
25°C and incubated with primary antibodies for 24 h at 4°C. The
membranes were rinsed and incubated with secondary antibodies for
60 min at 25°C. The protein bands were visualized using ECL
detection reagent and detected with an iBright 1500 instrument
(Invitrogen). The bands were analyzed using ImageJ v1.48 software
(National Institutes of Health) and β-actin was used as a loading
control.
Following siRNA or drug treatment, the cells were
lysed in ice-cold RIPA lysis buffer that was supplemented with the
protease inhibitors for cell research. The subsequent procedures
were the same as those for tissues.
Immunoprecipitation
The spinal cords were collected and homogenized in
ice-cold RIPA lysis buffer that contained a cocktail of protease
inhibitors. After being centrifugated at 13,523 × g for 20 min at
4°C, the supernatant was collected as a protein sample (50
μl) and the protein G agarose (5 μl) was added and
incubated at 4°C for 60 min with rotation. The samples were then
centrifugated at 13,523 × g for 15 min at 4°C and the supernatants
were transferred to fresh tubes. The primary antibodies (anti-NLRP3
and anti-HDAC6, 1:100) were then added and incubated at 4°C for 24
h. This was followed by the addition of protein G agarose (10
μl) for capturing immune complexes at 4°C with rotation.
After being centrifugated at 13,523 × g for 1 min at 4°C, the
pellets were washed in ice-cold PBS (cat. no. C0221A, Beyotime).
They were then centrifugated at 13,523 × g for 2 min at 4°C and the
supernatant was collected. Protein complexes were analyzed using
immunoblot analysis and the primary antibodies were anti-HDAC6 and
anti-NLRP3.
Transient transfection
Chemically-synthesized HDAC6 siRNA (h) targeting
HDAC6 was purchased from Santa Cruz Biotechnology, Inc. U-87 MG
cells were seeded in 6-well culture dishes with 2 ml
antibiotic-free DMEM/F12 medium that was supplemented with FBS. The
cells were incubated at 37°C in a CO2 incubator until
the cells attained 30-50% confluency. The HDAC6 siRNA and control
siRNA were diluted with siRNA transfection medium. The transfection
reagent was also diluted in siRNA transfection medium. The mixture
of siRNA and transfection reagent was incubated for 15-45 min at
room temperature. For each transfection, 0.2 ml transfection
mixture was added in 0.8 ml transfection medium. After washing the
cells with 2 ml of transfection medium, the cells were transfected
with HDAC6 siRNA (60 pmol) and control siRNA (60 pmol) for 7 h at
37°C. Following incubation, 1 ml DMEM/F12 medium containing 2X
normal serum and antibiotics concentration. was added to each well
without the removal of the transfection mixture. The cells were
then incubated for an additional 24 h. The medium was aspirated and
replaced with fresh 1X normal growth medium. After 24 h, the HDAC6
level in U-87 MG cells were assessed using western blotting.
Negative control siRNA was used for assessing nonspecific
gene-silencing effects.
Statistical analysis
SPSS 21.0 statistics software (IBM Corp.) was used
to perform all statistical analyses. One-way analysis of variance
was used for determining differences in experimental groups
followed by Tukey's test. Results of the behaviors tests are
presented as mean ± SEM. Other experiment data is presented as mean
± SD unless otherwise stated. P<0.05 was considered to indicate
a statistically significant difference.
Results
Morphological analysis and behavioral
testing for CIBP rats
The establishment and assessment of CIBP rats
followed the protocol as shown in Fig. 1A. In the present study, a rat
model of CIBP was established by inoculating MRMT-1 cells into the
medullary cavity of the tibia. Following two weeks of surgery,
X-ray, histological analysis and behavioral testing verified the
model. In contrast to the sham group, X-ray radiographs showed
there to be bone damage and destruction in the tibias of CIBP rats
(Fig. 1B). Tibial bone H&E
staining demonstrated that trabecular bone was markedly destroyed
in the CIBP rat compared with the sham group (Fig. 1C). Behavioral tests were
performed on day 4, 7, 11 and 14 after surgery as a means of
confirming whether the inoculation of cancer cells resulted in the
development of pain-related behaviors in CIBP rats. The CIBP rats
exhibited an increase in the number of flinches, proving
spontaneous pain (Fig. 1D).
There was also a decrease in PWT values, indicating increased
mechanical pain sensitivity (Fig.
1E) and a reduced latency to fall, representing impaired motor
coordination (Fig. 1F).
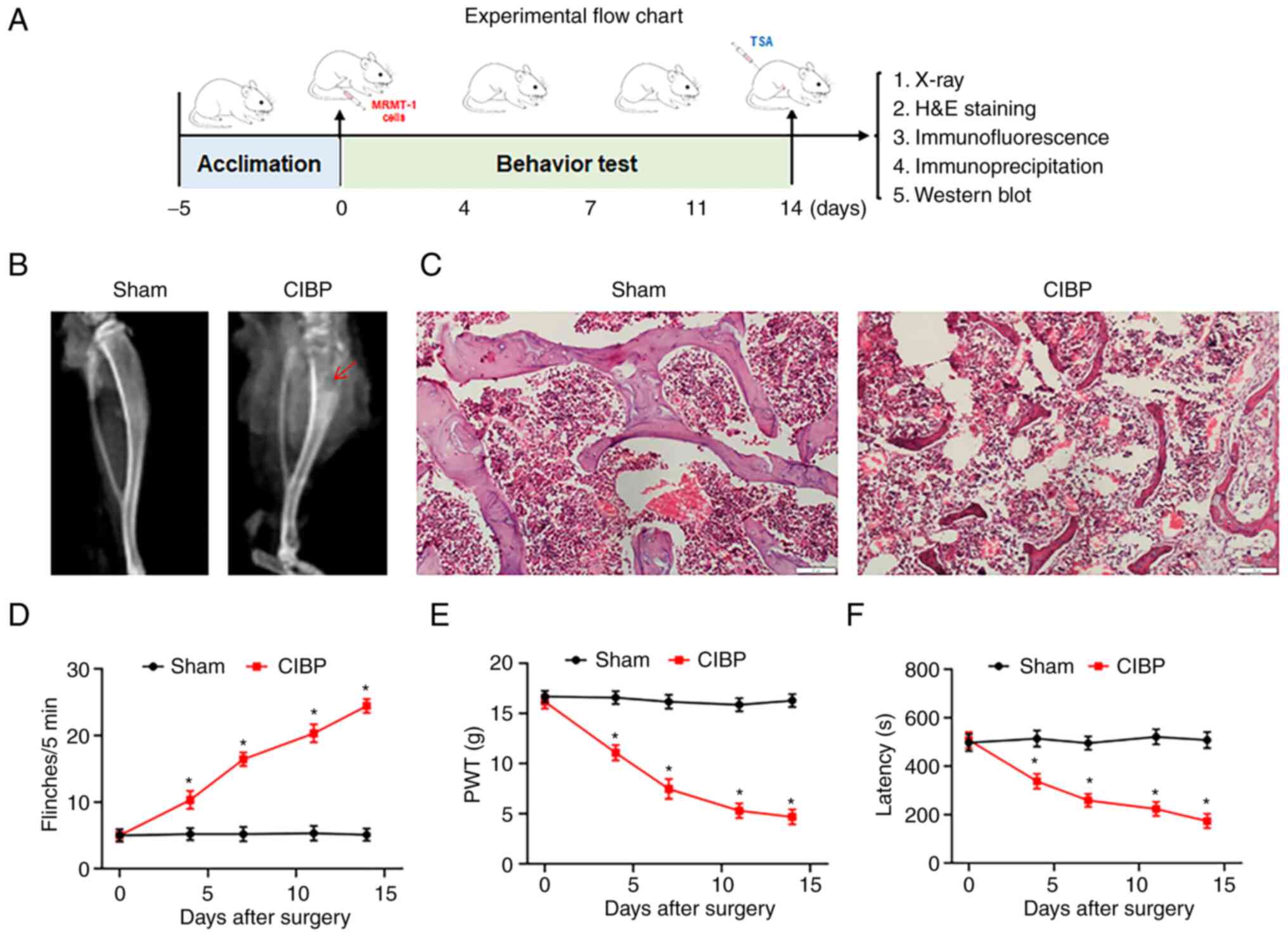 | Figure 1Validation of CIBP rat model. (A)
Schematic diagram of the experimental procedures. On day 0, CIBP
model was established. On day 0, 4, 7, 14 after surgery, the
behavioral tests including spontaneous flinches record, PWT value
test and latency to fall assay were performed. On day 14, X-ray
scan, histology staining, western blotting, immunofluorescence
assays were performed after behavioral tests. (B) Representative
radiographs of the ipsilateral tibia from sham and CIBP rats on day
14. The red arrow indicated areas of bone destruction. n=9. (C)
Representative images of hematoxylin and eosin staining of tibial
sections from sham and CIBP rats. Scale bars, 50 μm; n=3.
(D) The numbers of spontaneous flinches of sham and CIBP rats. (E)
PWT values was assessed by von Frey filaments with the up-and-down
method. (F) The latency to fall of accelerated rotarod motor test
in sham and CIBP rats. Data were expressed as the mean ± SEM (n=9).
*P<0.05 vs. sham group. CIBP, cancer-induced bone
pain; PWT, paw withdrawal threshold. |
TSA treatment inhibits HDAC6 and
alleviates pain behaviors
As the inhibition of HDAC6 has an effect on
neuropathic pain (39),
immunofluorescence and western blot assays were applied in the
present study for analyzing HDAC6 expression and phosphorylation in
the spinal cord tissue. The phosphorylation of HDAC6 at serine 22
(pHDAC6-Ser22) increases HDAC6 deacetylase activity (40). The immunofluorescence images
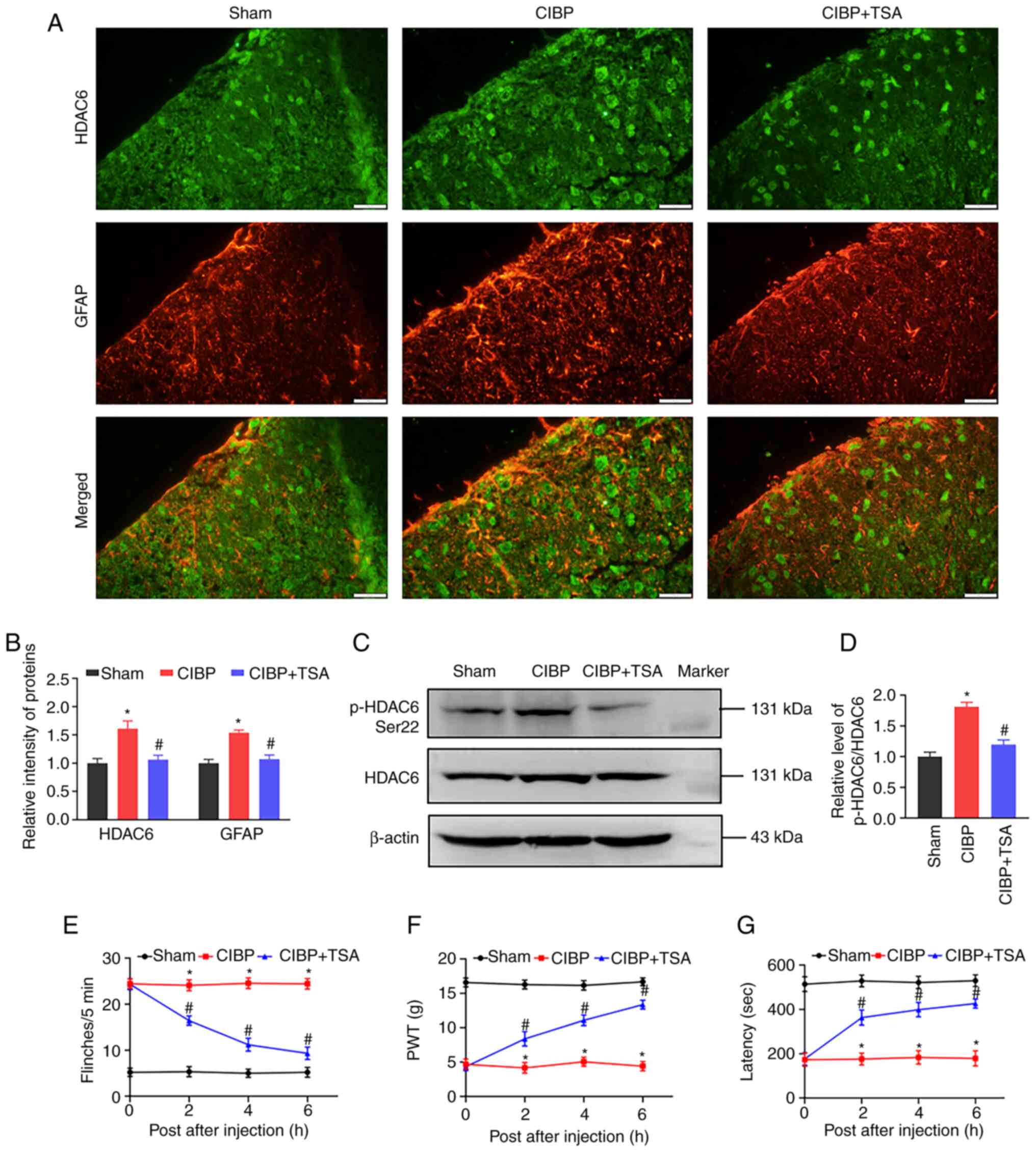
showed co-localization of HDC6 and GFAP in the spinal dorsal horn.
Compared with the sham rats, HDAC6 and GFAP intensities increased
in the spinal dorsal horn of CIBP rats. The increased fluorescence
intensities in CIBP rats were reduced by TSA treatment (Fig. 2A and B). Western blot analysis
found the level of pHDAC6-Ser22 in the spinal cord was upregulated
in the CIBP group and decreased following TSA treatment (Fig. 2C and D). The behavioral tests
were then performed. In contrast to the CIBP rats, following the
intrathecal injection of TSA at 2, 4 and 6 h, the TSA-treated CIBP
rats exhibited a decrease in the number of flinches (Fig. 2E), an increase in PWT values
(Fig. 2F) and an elevated
latency to fall (Fig. 2G). These
results demonstrated that spontaneous pain and mechanical
hyperalgesia were alleviated and motor coordination was recovered
in CIBP rats by the administration of TSA.
TSA treatment suppresses spinal
inflammatory response
Neuroinflammation in the spinal cord is associated
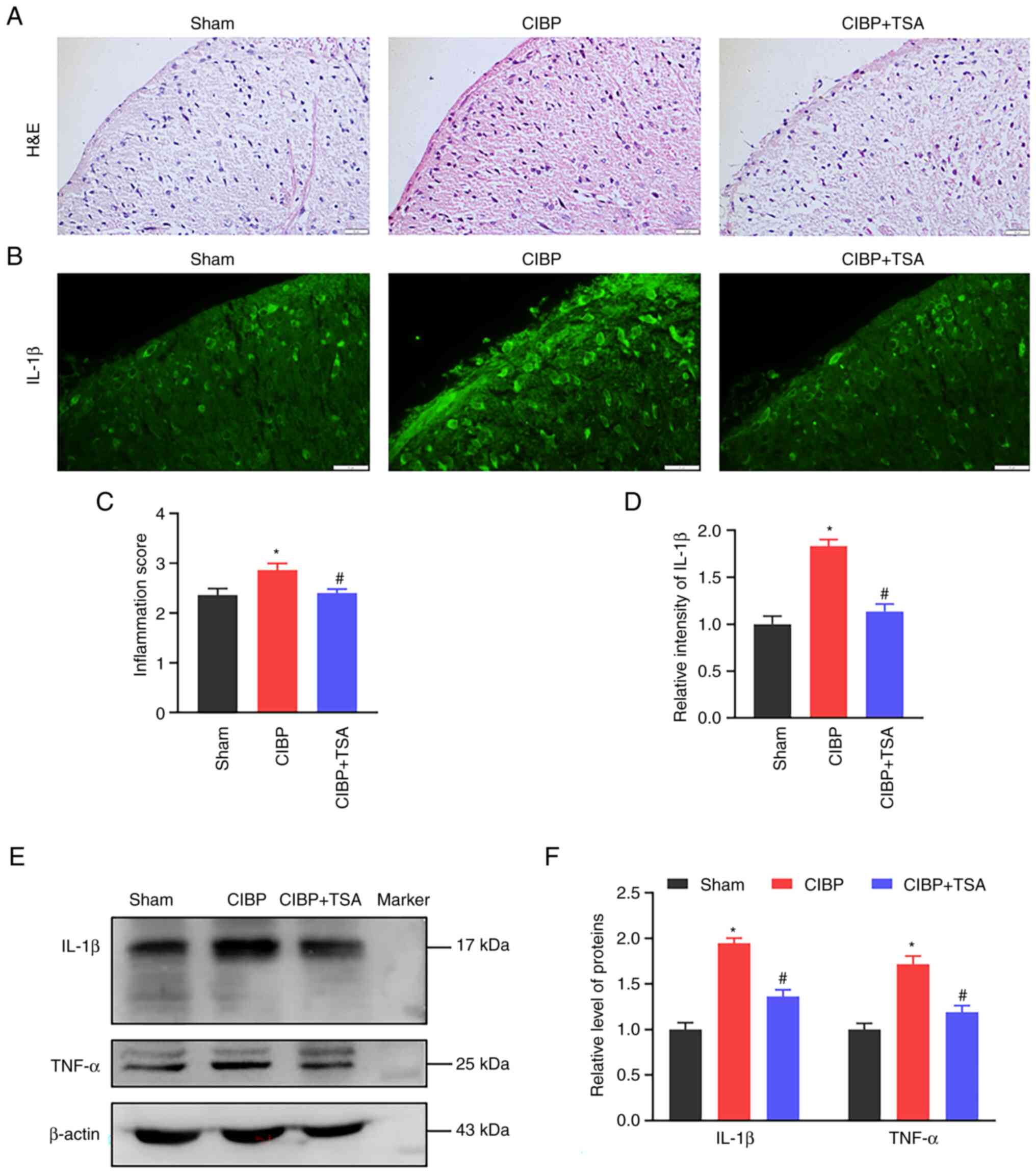
with pain. H&E staining was used in the present study for the
detection of leukocyte infiltration in spinal cord tissue. As shown
in Fig. 3A, an extensive
leukocytes filtration was observed in the spinal dorsal horns of
CIBP rats (P<0.05 vs. sham group, Fig. 3A). The production of cytokines
such as TNF-α and IL-1β is involved in inflammatory response
(41), so the immunofluorescence
analysis of IL-1β intensity in the spinal dorsal horn was
performed, the result showing IL-1β intensity to have robustly
increased in the CIBP rats (P<0.05 vs. sham group; Fig. 3B). Similarly, western blot
analysis showed IL-1β protein levels to be upregulated in the
spinal cords of CIBP rats (P<0.05 vs. sham group; Fig. 3E and F). Following TSA treatment,
the fluorescence intensity and protein levels of IL-1β were both
reduced in the spinal cords (P<0.05 vs. CIBP group, Fig. 3B, D-F). In addition to IL-1β,
another pro-inflammatory cytokine TNF-α was also examined. The
increased expression of TNF-α expression in the CIBP rats was
reduced by TSA treatment (P<0.05 vs. CIBP group, Fig. 3E and F). These results suggest
that TSA treatment decrease the production of inflammatory
cytokines in the spinal cords of the CIBP rats.
TSA treatment reduces spinal astrocytic
activation
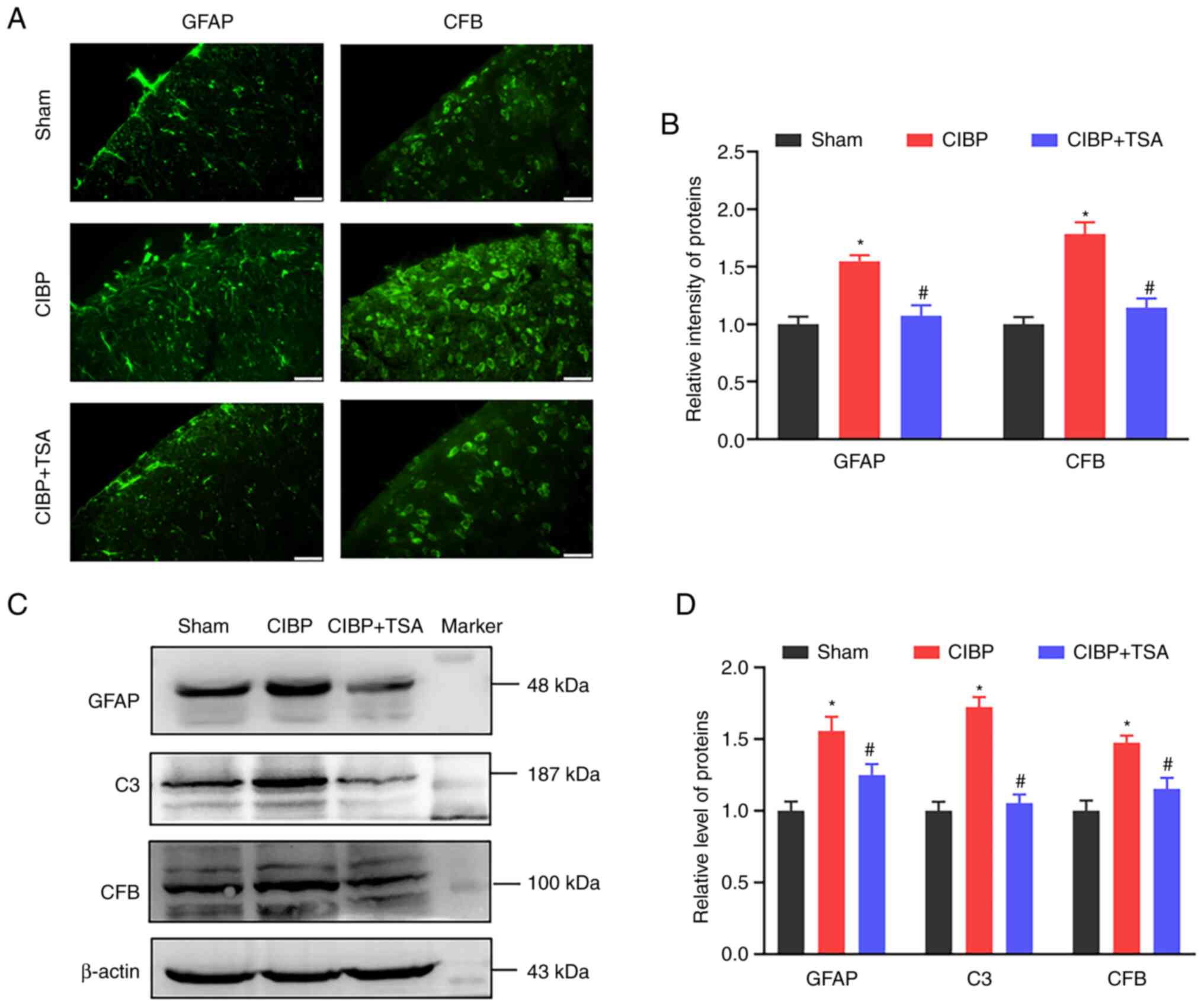
Activated astrocyte promotes inflammatory cytokines
releases, which includes IL-1β and TNF-α. GFAP is the hallmark
intermediate filament protein that is found in astrocytes (42). CFB rapidly amplifies C3
activation (43), which is
linked to astrocyte reactivity and the promotion of the
neuroinflammatory phenotype (44). GFAP, C3 and CFB expression levels
were detected in the present study. Immunofluorescence images
showed that the GFAP and CFB intensities in the spinal dorsal horn
were significantly increased in the CIBP rats (P<0.05 vs. sham
group) and reduced following TSA treatment (P<0.05 vs. CIBP
group; Fig. 4A and B). Western
blot analysis found the GFAP and CFB protein levels to be
upregulated in the spinal cords of the CIBP rats, compared with the
sham group (P<0.05 vs. sham group; Fig. 4C and D). Following TSA treatment,
the upregulated levels of GFAP and CFB were reduced (P<0.05 vs.
CIBP group; Fig. 4C and D). In
addition, increased spinal C3 level were also detected in the CIBP
rats (P<0.05 vs. sham group) and TSA treatment reduced C3 levels
in the CIBP rats (P<0.05 vs. CIBP group; Fig. 4C and D). These results suggest
that TSA treatment suppressed astrocytic activation in the spinal
cords of the CIBP rats.
TAS treatment decreases NLRP3
inflammasome activation and NF-κB expression
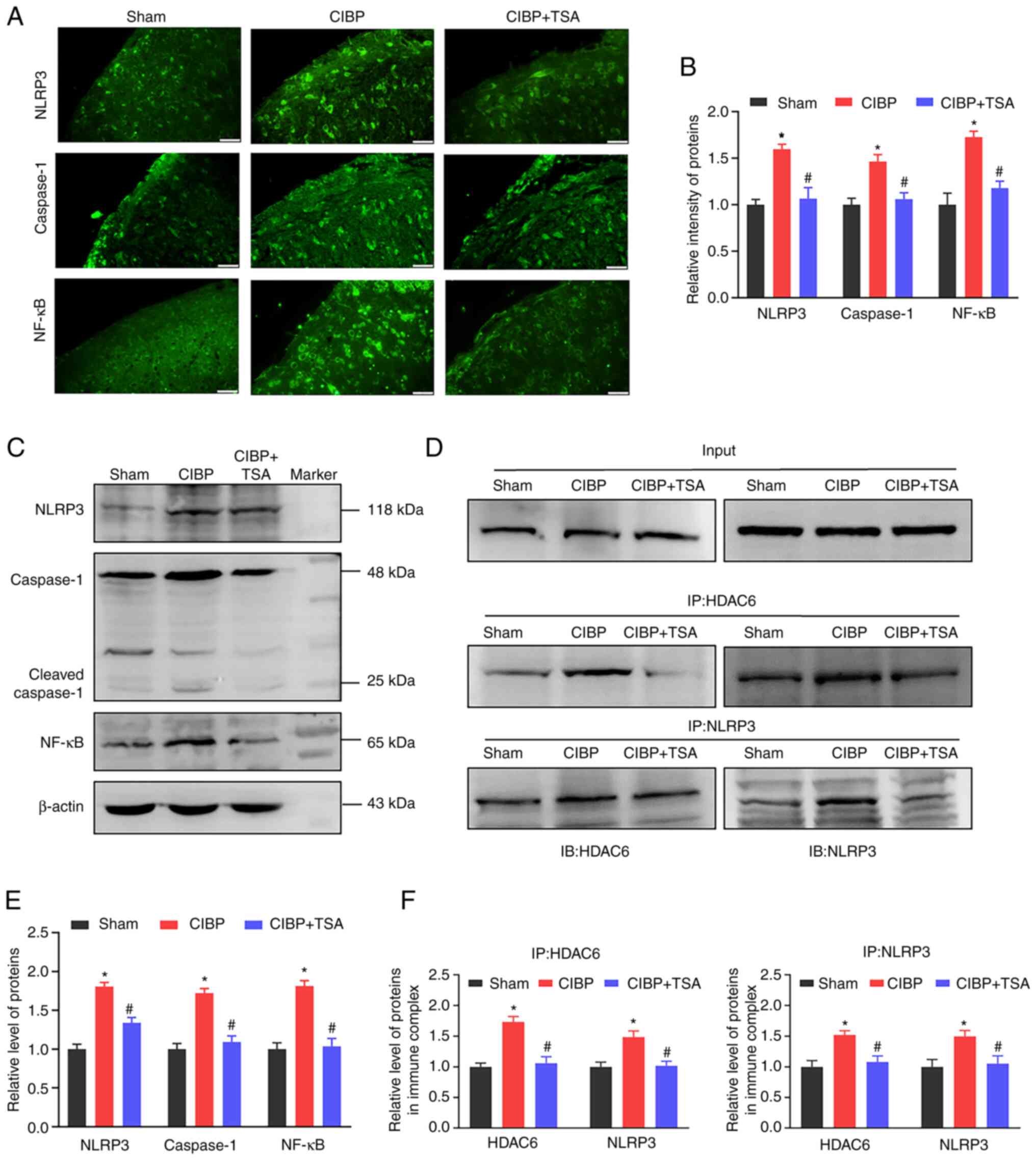
As IL-1β maturation can be catalyzed by NLRP3
inflammasome activation, the effect of TSA on activation of NLRP3
inflammasome was further investigated. Immunofluorescence images
showed the intensities of NLRP3 and caspase-1 to have increased in
the spinal cords of the CIBP rats (P<0.05 vs. sham group) and
TSA treatment reduced these upregulations (P<0.05 vs. CIBP
group; Fig. 5A and B). Western
blot analysis showed the components of NLRP3 inflammasome and
cleaved caspase-1 protein levels to be upregulated in the spinal
cords of the CIBP rats (P<0.05 vs. sham group; Fig. 5C and E). Similarly, TSA treatment
downregulated the NLRP3 and cleaved caspase-1 expression levels in
the CIBP rats (P<0.05 vs. CIBP group; Fig. 5C and E). To provide further
confirmation of the interaction between NLRP3 and HDAC6,
immunoprecipitation was performed. As can be seen in Fig. 5D, input represented a positive
control for demonstrating the existence of HDAC6 and NLRP3 in the
spinal cord tissue extracts. Immune complexes were obtained from
tissue lysates following incubation with anti-HDAC6 or anti-NLRP3
antibodies. The presence of HDAC6 or NLRP3 proteins in the
immunoprecipitants was measured by probing with anti-HDAC6 or
anti-NLRP3 antibodies. The interaction between HDAC6 and NLRP3 was
found to have increased in the spinal cords of the CIBP rats
(P<0.05 vs. sham group) and TSA treatment reduced the
association of HDAC6 to NLRP3 (P<0.05 vs. CIBP group; Fig. 5D and F). In addition to NLRP3
inflammasome, NF-κB also regulates the expression of
pro-inflammatory cytokines. As Fig.
5A shows, NF-κB intensity was increased in the spinal dorsal
horns of the CIBP rats (P<0.05 vs. sham group; Fig. 5A and B). Western blot analysis
demonstrated that the NF-κB protein level was up-regulated in the
spinal cords of the CIBP rats (P<0.05 vs. sham group; Fig. 5C and E). Following TSA treatment,
the upregulated level of NF-κB was found to have reduced in the
spinal cords of the CIBP rats (P<0.05 vs. CIBP group; Fig. 5C and E. These findings
collectively indicated that TSA treatment suppressed the binding
between HDAC6 and NLRP3, decreased NLRP3 inflammasome activation
and reduced the expression of NF-κB.
TSA treatment reduces HDAC6 and NLRP3
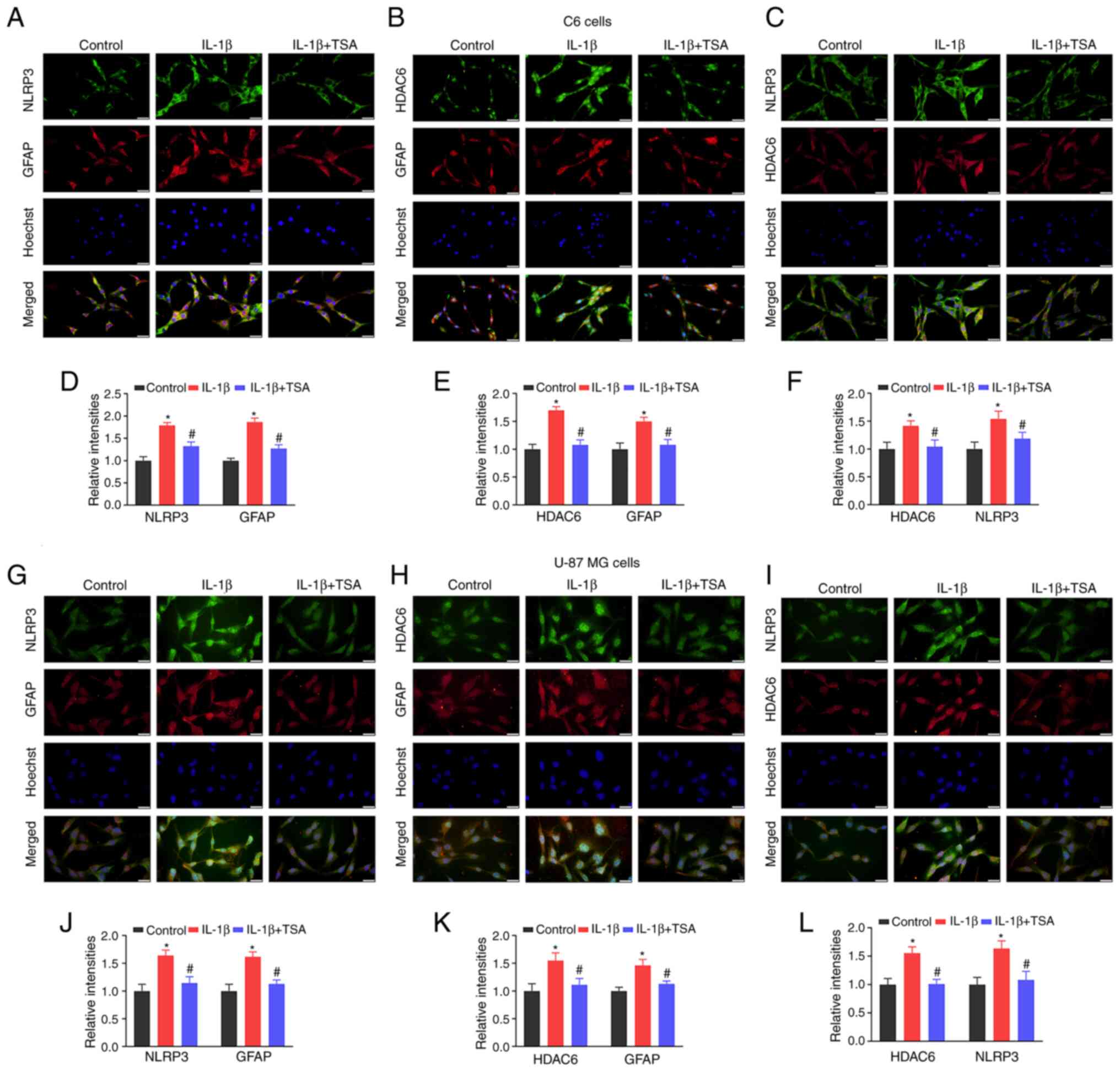
expression in IL-1β-induced C6 and U-87 MG cells
As a means of further confirming that the effects of
HDAC6 on NLRP3 were related to glial cell activation, the
association and expression of HDAC6 and NLRP3 in IL-1β-induced C6
and U-87 MG cells after TSA treatment was investigated.
Double-label immunofluorescence staining found a colocalization of
HDA6 and NLRP3 in glial cells. As can be seen in Fig. 6, NLRP3 and HDAC6 were colocalized
with astrocyte marker GFAP in both C6 and U-87 MG cells. Compared
with the control group, HDAC6, NLRP3 and GFAP fluorescence
intensities were all increased in IL-1β-induced C6 and U-87 MG
cells (P<0.05; Fig. 6).
Following TSA treatment, the HDAC6, NLRP3 and GFAP intensities were
reduced, compared with the IL-1β group (P<0.05, Fig. 6). These results demonstrated that
TSA treatment decreased NLRP3 inflammasome activation in glial
cells.
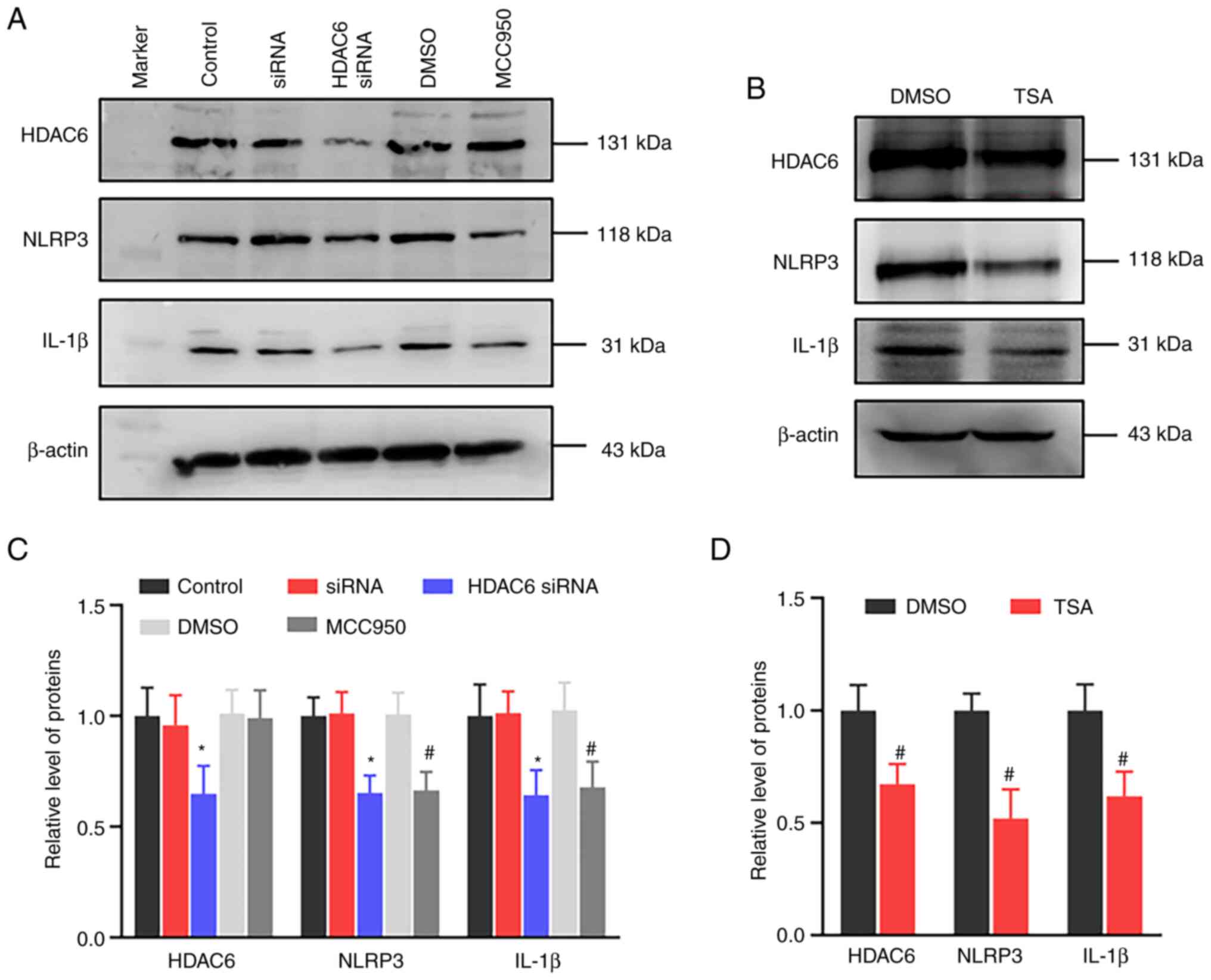
In order to verify the effect of HDAC6 expression
has on the changes in NLRP3 expression and inflammatory factor,
HDAC6 siRNA was used for treating U-87 cells. As Fig. 7 shows, HDAC6 siRNA reduced NLRP3
and downstream inflammatory factor IL-1β expressions. Similarly,
the NLRP3 inhibitor MCC950 and treatment also decreased NLRP3 and
IL-1β expressions. In addition, TSA-treated U-87 MG cells showed
decreased NLRP3 and HDAC6 expressions. The results indicated that
the downregulation of HDAC6 had an inhibitory effect on the
NLRP3-mediated inflammatory factor expression.
Discussion
Neuroinflammation of the spinal cord is an important
mechanism for cancer-induced bone pain generation and maintenance
(7-9). A CIBP rat model was established in
the present study and increased pain sensitivity and impaired motor
ability were observed in the CIBP rats. The present study focused
on the effect HDAC6 has on pain and spinal inflammation. TSA is a
potent and selective HDAC6 inhibitor that has been reported to have
an inhibitory effect on inflammatory response and pain management.
TSA treatment results in reduced IL-6 expression and mediated
inflammatory response while also eliciting anti-inflammatory and
antirheumatic effects in Freund's complete adjuvant-induced
inflammation mouse model and collagen-induced arthritis mouse model
(45). The intravenous
administration of TSA also inhibits HDAC6 activity and limits NLRP3
inflammasome activation and cell pyroptosis in the
ischemia/reperfusion injury model (46). At the same time, TSA treatment
has been found to increase mu-opioid receptor expression,
ameliorate tactile hypersensitivity and enhance the analgesic
effect of morphine in the bone cancer pain rat model (33). TSA treatment decreased IL-1β and
TNF-α expressions and inflammatory infiltration in the spinal cords
of the CIBP rats in the present study. Based on the aforementioned
studies, it can be seen that the analgesic effect of TSA
contributed to the inhibition of spinal inflammation.
The mechanisms of pain that are induced in patients
with bone metastasis include inflammatory and neuropathic
processes. In the present study, increased infiltration of
inflammatory cells and IL-1β expression were observed in the spinal
dorsal horns of the CIBP rats. These changes were involved in the
inflammatory processes. In addition, the relationship between
neuroinflammation and pain behaviors was measured (Fig. 3). The present study and previous
research have shown that the inflammatory signal increases in the
spinal cords of CIBP animals, which is accompanied by hyperalgesia
and pharmacologic inhibition of inflammatory factors expression and
that the inflammatory response could serve to alleviate pain
behaviors (47,48). In the spinal cord, the
inflammation factor is mainly derived from microglia and
astrocytes. The reason that astrocytes were chosen in the present
study is because astrocytic reaction is more persistent, in both
animal models of inflammatory pain and neuropathic pain, compared
with microglia reaction (49).
Cancer-induced bone pain is a type of chronic pain in which both
inflammatory and neuropathic factors are involved. Research on
microglia has further indicated that microgliosis is not always
associated with pain states and neuropathic pain can be reduced
without any microgliosis changes occurring (50). Accordingly, spinal astrocytes
play an important role in chronic pain maintenance. In addition,
the inhibition of GFAP (astrocytic marker) expression and
astrogliosis is associated with a reduction in neuropathic pain
behaviors rather than microglial reaction. The intrathecal
knockdown of GFAP expression in nerve-injured animals also reduces
neuropathic pain behaviors (51).
Studies have indicated that HDAC6 is responsible for
the regulation of NLRP3 inflammasome activation and the expression
of pro-inflammatory cytokines. First, HDAC6 directly binds with
NLRP3 through its ubiquitin-binding domain (52). Second, HDAC6 works as a dynein
adapter and facilitates the assembly of NLRP3 inflammasome. HDAC6
deacetylates α-tubulin through its deacetylase domain and promotes
NLRP3 inflammasome assembly (27). The inhibition of HDAC6
deacetylase activity by tubastatin A, rocilinostat and tubacin
increases α-tubulin acetylation while also blocking the activation
of NLRP3 inflammasome (29).
Third, HDAC6 regulates the NF-κB-mediated expression of
pro-inflammatory cytokines. HDAC6 deacetylates the p65 subunit of
NF-κB and decreases its DNA-binding activity (53), which reduces inflammatory gene
expression that includes NLRP3, pro-IL-1β and pro-IL-18 as a
consequence (54). In the
present study, NLRP3 inflammasome activation was observed in the
CIBP rats and was found to be inhibited by TSA treatment. TSA
treatment decreased spinal inflammation via HDAC6/NLRP3 and NF-κB
signaling.
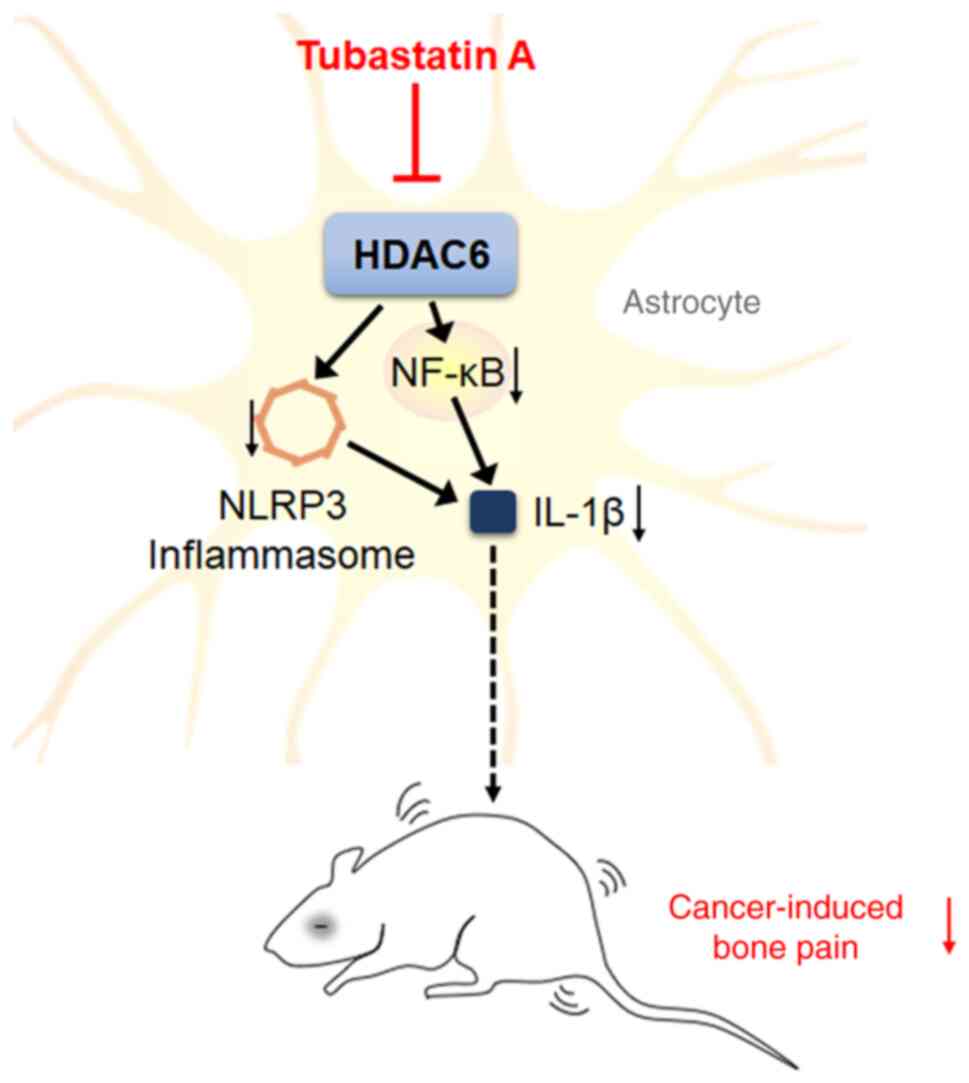
In summary, elevated pain sensitivity, spinal
astrocyte activation and increased neuroinflammatory response were
all found in the CIBP rats. TSA treatment was found to inhibit
HDAC6 activity and reduce NLRP3 and NF-κB mediated inflammatory
response, which consequently causes the alleviation of cancer pain
(Fig. 8). Although the present
study on cancer-induced bone pain rats provided possibilities and
potential target for analgesia, there are still some limitations to
it. First, in the present study, CIBP model was established by
intratibial inoculation of cancer cells. Elsewhere, intracardiac
inoculation is also commonly used. Upon intracardiac inoculation,
most substantial bone destruction tends to occur in the metaphyses
of the distal femur and proximal tibia. Intratibial inoculation is
used when a study focusses on the changes following bone metastasis
(55). Detecting the effect of
TSA on different CIBP models is helpful for the application of TSA
on pain management. Second, in the present study, HDAC6 was
considered as the target protein for CIBP management. However, the
expression of HDAC6 on the genetic level in animals was not
constructed and needed further study.
Availability of data and materials
The datasets generated and analyzed during the
present study are available from the corresponding author on
reasonable request.
Authors' contributions
All authors made a significant contribution to the
present study. MX and LL conceived and designed the study. YH, ZW
and YY performed the experiments, DL, SZ, JD, WM and HZ collected
and analyzed the data. MX and LL drafted and edited the manuscript.
YH, ZW and YY confirm the authenticity of all the raw data. All
authors read and approved the final manuscript.
Ethics approval and consent to
participate
The present study was approved by the Ethics
Committee of Hubei University of Science and Technology (approval
number 2020-01-900). All experimental procedures in the present
study were complied with the local and international guidelines on
ethical use of animals.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
Acknowledgments
Not applicable.
Funding
The present study was supported by the grants from the National
Natural Science Foundation of China (grant nos. 81971066 and
32100823), Research Project of Science and Technology Department of
Hubei Province of China (grant nos. 2022CFB356, 2022CFB791 and
2022SFYF014).
References
|
1
|
Yang J, Wahner-Roedler DL, Zhou X, Johnson
LA, Do A, Pachman DR, Chon TY, Salinas M, Millstine D and Bauer BA:
Acupuncture for palliative cancer pain management: Systematic
review. BMJ Support Palliat Care. 11:264–270. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Jiang W, Rixiati Y, Zhao B, Li Y, Tang C
and Liu J: Incidence, prevalence and outcomes of systemic
malignancy with bone metastases. J Orthop Surg (Hong Kong).
28:23094990209159892020. View Article : Google Scholar
|
|
3
|
Fornetti J, Welm AL and Stewart SA:
Understanding the bone in cancer metastasis. J Bone Miner Res.
33:2099–2113. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Coleman RE, Croucher PI, Padhani AR,
Clézardin P, Chow E, Fallon M, Guise T, Colangeli S, Capanna R and
Costa L: Bone metastases. Nat Rev Dis Primers. 6:832020. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Pfister DG, Spencer S, Adelstein D, Adkins
D, Anzai Y, Brizel DM, Bruce JY, Busse PM, Caudell JJ, Cmelak AJ,
et al: Head and neck cancers, version 2.2020, NCCN clinical
practice guidelines in oncology. J Natl Compr Canc Netw.
18:873–898. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Brozović G, Lesar N, Janev D, Bošnjak T
and Muhaxhiri B: Cancer pain and therapy. Acta Clin Croat. 61(Suppl
2): S103–S108. 2022.
|
|
7
|
Gallaway MS, Townsend JS, Shelby D and
Puckett MC: Pain among cancer survivors. Prev Chronic Dis.
17:E542020. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Falk S and Dickenson AH: Pain and
nociception: Mechanisms of cancer-induced bone pain. J Clin Oncol.
32:1647–1654. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Yam MF, Loh YC, Tan CS, Khadijah Adam S,
Abdul Manan N and Basir R: General pathways of pain sensation and
the major neurotransmitters involved in pain regulation. Int J Mol
Sci. 19:21642018. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Zheng XQ, Wu YH, Huang JF and Wu AM:
Neurophysiological mechanisms of cancer-induced bone pain. J Adv
Res. 35:117–127. 2021. View Article : Google Scholar
|
|
11
|
Shi Y, Gelman BB, Lisinicchia JG and Tang
SJ: Chronic-pain-associated astrocytic reaction in the spinal cord
dorsal horn of human immunodeficiency virus-infected patients. J
Neurosci. 32:10833–10840. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Göbel A, Dell'Endice S, Jaschke N, Pählig
S, Shahid A, Hofbauer LC and Rachner TD: The role of inflammation
in breast and prostate cancer metastasis to bone. Int J Mol Sci.
22:50782021. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Shupp AB, Kolb AD, Mukhopadhyay D and
Bussard KM: Cancer metastases to bone: Concepts, mechanisms and
interactions with bone osteoblasts. Cancers (Basel). 10:1822018.
View Article : Google Scholar
|
|
14
|
Chen R, Yin C, Fang J and Liu B: The NLRP3
inflammasome: An emerging therapeutic target for chronic pain. J
Neuroinflammation. 18:842021. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Moossavi M, Parsamanesh N, Bahrami A,
Atkin SL and Sahebkar A: Role of the NLRP3 inflammasome in cancer.
Mol Cancer. 17:1582018. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Zhang YZ, Zhang YL, Huang Q, Huang C,
Jiang ZL, Cai F and Shen JF: AdipoRon alleviates free fatty
acid-induced myocardial cell injury via suppressing Nlrp3
inflammasome activation. Diabetes Metab Syndr Obes. 12:2165–2179.
2019. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
D'Amico R, Fusco R, Siracusa R,
Impellizzeri D, Peritore AF, Gugliandolo E, Interdonato L, Sforza
AM, Crupi R, Cuzzocrea S, et al: Inhibition of P2X7 purinergic
receptor ameliorates fibromyalgia syndrome by suppressing NLRP3
pathway. Int J Mol Sci. 22:64712021. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Corcoran SE, Halai R and Cooper MA:
Pharmacological inhibition of the Nod-like receptor family pyrin
domain containing 3 inflammasome with MCC950. Pharmacol Rev.
73:968–1000. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Chen SP, Zhou YQ, Wang XM, Sun J, Cao F,
HaiSam S, Ye DW and Tian YK: Pharmacological inhibition of the
NLRP3 inflammasome as a potential target for cancer-induced bone
pain. Pharmacol Res. 147:1043392019. View Article : Google Scholar
|
|
20
|
Starobova H, Monteleone M, Adolphe C,
Batoon L, Sandrock CJ, Tay B, Deuis JR, Smith AV, Mueller A, Nadar
EI, et al: Vincristine-induced peripheral neuropathy is driven by
canonical NLRP3 activation and IL-1β release. J Exp Med.
218:e202014522021. View Article : Google Scholar
|
|
21
|
Seidel C, Schnekenburger M, Dicato M and
Diederich M: Histone deacetylase 6 in health and disease.
Epigenomics. 7:103–118. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
English K and Barton MC: HDAC6: A key link
between mitochondria and development of peripheral neuropathy.
Front Mol Neurosci. 14:6847142021. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Shen S, Picci C, Ustinova K, Benoy V,
Kutil Z, Zhang G, Tavares MT, Pavlíček J, Zimprich CA, et al:
Tetrahydroquinoline-capped histone deacetylase 6 inhibitor SW-101
ameliorates pathological phenotypes in a charcot-marie-tooth type
2A mouse model. J Med Chem. 64:4810–4840. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Van Helleputte L, Kater M, Cook DP, Eykens
C, Rossaert E, Haeck W, Jaspers T, Geens N, Vanden Berghe P,
Gysemans C, et al: Inhibition of histone deacetylase 6 (HDAC6)
protects against vincristine-induced peripheral neuropathies and
inhibits tumor growth. Neurobiol Dis. 111:59–69. 2018. View Article : Google Scholar
|
|
25
|
Krukowski K, Ma J, Golonzhka O, Laumet GO,
Gutti T, van Duzer JH, Mazitschek R, Jarpe MB, Heijnen CJ and
Kavelaars A: HDAC6 inhibition effectively reverses
chemotherapy-induced peripheral neuropathy. Pain. 158:1126–1137.
2017. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Prior R, Van Helleputte L, Klingl YE and
Van Den Bosch L: HDAC6 as a potential therapeutic target for
peripheral nerve disorders. Expert Opin Ther Targets. 22:993–1007.
2018. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Chang P, Li H, Hu H, Li Y and Wang T: The
role of HDAC6 in autophagy and NLRP3 inflammasome. Front Immunol.
12:7638312021. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Yan S, Wei X, Jian W, Qin Y, Liu J, Zhu S,
Jiang F, Lou H and Zhang B: Pharmacological inhibition of HDAC6
attenuates NLRP3 inflammatory response and protects dopaminergic
neurons in experimental models of parkinson's disease. Front Aging
Neurosci. 12:782020. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Magupalli VG, Negro R, Tian Y, Hauenstein
AV, Di Caprio G, Skillern W, Deng Q, Orning P, Alam HB, Maliga Z,
et al: HDAC6 mediates an aggresome-like mechanism for NLRP3 and
pyrin inflammasome activation. Science. 369:eaas89952020.
View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Wu X, Zhang H, Qi W, Zhang Y, Li J, Li Z,
Lin Y, Bai X, Liu X, Chen X, et al: Nicotine promotes
atherosclerosis via ROS-NLRP3-mediated endothelial cell pyroptosis.
Cell Death Dis. 9:1712018. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Godena VK, Brookes-Hocking N, Moller A,
Shaw G, Oswald M, Sancho RM, Miller CC, Whitworth AJ and De Vos KJ:
Increasing microtubule acetylation rescues axonal transport and
locomotor deficits caused by LRRK2 Roc-COR domain mutations. Nat
Commun. 5:52452014. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Yao FD, Yang JQ, Huang YC, Luo MP, Yang
WJ, Zhang B and Liu XJ: Antinociceptive effects of ginsenoside Rb1
in a rat model of cancer-induced bone pain. Exp Ther Med.
17:3859–3866. 2019.PubMed/NCBI
|
|
33
|
Hou X, Weng Y, Ouyang B, Ding Z, Song Z,
Zou W, Huang C and Guo Q: HDAC inhibitor TSA ameliorates mechanical
hypersensitivity and potentiates analgesic effect of morphine in a
rat model of bone cancer pain by restoring μ-opioid receptor in
spinal cord. Brain Res. 1669:97–105. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Liu M, Cheng X, Yan H, Chen J, Liu C and
Chen Z: MiR-135-5p alleviates bone cancer pain by regulating
astrocyte-mediated neuroinflammation in spinal cord through
JAK2/STAT3 signaling pathway. Mol Neurobiol. 58:4802–4815. 2021.
View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Dixon WJ: Efficient analysis of
experimental observations. Annu Rev Pharmacol Toxicol. 20:441–62.
1980. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Shi X, Bai H, Wang J, Wang J, Huang L, He
M, Zheng X, Duan Z, Chen D, Zhang J, et al: Behavioral assessment
of sensory, motor, emotion and cognition in rodent models of
intracerebral hemorrhage. Front Neurol. 12:6675112021. View Article : Google Scholar
|
|
37
|
Song ZP, Xiong BR, Guan XH, Cao F,
Manyande A, Zhou YQ, Zheng H and Tian YK: Minocycline attenuates
bone cancer pain in rats by inhibiting NF-κB in spinal astrocytes.
Acta Pharmacol Sin. 37:753–762. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Song S, Guo R, Mehmood A, Zhang L, Yin B,
Yuan C, Zhang H, Guo L and Li B: Liraglutide attenuate central
nervous inflammation and demyelination through AMPK and
pyroptosis-related NLRP3 pathway. CNS Neurosci Ther. 28:422–434.
2022. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Chen C, Liu A, Lu Q, Luo L, Li J, Ke J,
Liu Y and Feng X: HDAC6 inhibitor ACY-1215 improves neuropathic
pain and its comorbidities in rats of peripheral nerve injury by
regulating neuroinflammation. Chem Biol Interact. 353:1098032022.
View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Mazzetti S, De Leonardis M, Gagliardi G,
Calogero AM, Basellini MJ, Madaschi L, Costa I, Cacciatore F,
Spinello S, Bramerio M, et al: Phospho-HDAC6 gathers into protein
aggregates in parkinson's disease and atypical parkinsonisms. Front
Neurosci. 14:6242020. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Dong ZB, Wang YJ, Wan WJ, Wu J, Wang BJ,
Zhu HL, Xie M and Liu L: Resveratrol ameliorates
oxaliplatin-induced neuropathic pain via anti-inflammatory effects
in rats. Exp Ther Med. 24:5862022. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Hol EM and Pekny M: Glial fibrillary
acidic protein (GFAP) and the astrocyte intermediate filament
system in diseases of the central nervous system. Curr Opin Cell
Biol. 32:121–130. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Vallee KJ and Fields JA: Caloric
restriction mimetic 2-deoxyglucose reduces inflammatory signaling
in human astrocytes: Implications for therapeutic strategies
targeting neurodegenerative diseases. Brain Sci. 12:3082022.
View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Gharagozloo M, Smith MD, Jin J, Garton T,
Taylor M, Chao A, Meyers K, Kornberg MD, Zack DJ, Ohayon J, et al:
Complement component 3 from astrocytes mediates retinal ganglion
cell loss during neuroinflammation. Acta Neuropathol. 142:899–915.
2021. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Mao Y, Zhou J, Liu X, Gu E, Zhang Z and
Tao W: Comparison of different histone deacetylase inhibitors in
attenuating inflammatory pain in rats. Pain Res Manag.
2019:16489192019. View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Xu J, Zhao X, Jiang X, He L, Wu X, Wang J,
Chen Q, Li Y and Zhang M: Tubastatin A Improves post-resuscitation
myocardial dysfunction by inhibiting NLRP3-mediated pyroptosis
through enhancing transcription factor EB signaling. J Am Heart
Assoc. 11:e0242052022. View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Zhao J, Yan Y, Zhen S, Yu L, Ding J, Tang
Q, Liu L, Zhu H and Xie M: LY294002 alleviates bone cancer pain by
reducing mitochondrial dysfunction and the inflammatory response.
Int J Mol Med. 51:422023. View Article : Google Scholar : PubMed/NCBI
|
|
48
|
Chen J, Cong X, Zhan X, Zhou Z and Zheng
W: Effects of parecoxib on pain threshold and inflammatory factors
IL-1β, IL-6 and TNF-α in spinal cord of rats with bone cancer pain.
J Coll Physicians Surg Pak. 29:528–531. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
49
|
Ji RR, Berta T and Nedergaard M: Glia and
pain: Is chronic pain a gliopathy? Pain. 154(Suppl 1): S10–S28.
2013. View Article : Google Scholar : PubMed/NCBI
|
|
50
|
Chen G, Zhang YQ, Qadri YJ, Serhan CN and
Ji RR: Microglia in pain: Detrimental and Protective roles in
pathogenesis and resolution of pain. Neuron. 100:1292–1311. 2018.
View Article : Google Scholar : PubMed/NCBI
|
|
51
|
Ji RR, Donnelly CR and Nedergaard M:
Astrocytes in chronic pain and itch. Nat Rev Neurosci. 20:667–685.
2019. View Article : Google Scholar : PubMed/NCBI
|
|
52
|
Hwang I, Lee E, Jeon SA and Yu JW: Histone
deacetylase 6 negatively regulates NLRP3 inflammasome activation.
Biochem Biophys Res Commun. 467:973–8. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
53
|
Yang CJ, Liu YP, Dai HY, Shiue YL, Tsai
CJ, Huang MS and Yeh YT: Nuclear HDAC6 inhibits invasion by
suppressing NF-κB/MMP2 and is inversely associated with metastasis
of non-small cell lung cancer. Oncotarget. 6:30263–30276. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
54
|
Barter MJ, Butcher A, Wang H, Tsompani D,
Galler M, Rumsby EL, Culley KL, Clark IM and Young DA: HDAC6
regulates NF-κB signalling to control chondrocyte IL-1-induced MMP
and inflammatory gene expression. Sci Rep. 12:66402022. View Article : Google Scholar
|
|
55
|
Wright LE, Ottewell PD, Rucci N,
Peyruchaud O, Pagnotti GM, Chiechi A, Buijs JT and Sterling JA:
Murine models of breast cancer bone metastasis. Bonekey Rep.
5:8042016. View Article : Google Scholar : PubMed/NCBI
|