Introduction
Breast cancer is the most common cancer in women
worldwide. The International Agency for Research on Cancer (IARC)
released GLOBOCAN 2018 statistics from 185 countries, which showed
2.3 million new cases (11.7%) and a 6.9% death rate from breast
cancer (1). Despite a 39% drop
in mortality over the past 30 years, it remains the leading cause
of cancer-related death in women (2). A total of 5-11% of patients with
breast cancer have metastases despite surgery, radiation, or
chemotherapy (3). Breast cancer
metastasis is the leading cause of death among women in numerous
countries (4). Compared with
other common metastatic sites, the liver is one of the most common
sites of metastatic recurrence (5). As a common symptom in patients with
liver metastases from breast cancer, ascites is associated with its
progression. Malignant ascites (MA), a common clinical presentation
in patients with advanced tumors, is a dire complication
characterized by ascites and is associated with poor prognosis
(6,7). MA is a very serious and difficult
problem for patients with cancer and is one of the most common
complications of advanced breast cancer. MA is known to create a
pro-inflammatory environment that promotes immunosuppression and
allows tumor cell proliferation and metastasis (8-10). Clearly, there is a need to
identify new and effective treatments for metastatic breast cancer,
and effective reduction of ascites in patients with breast cancer
is critical for this.
Inflammation is one of the typical features of
cancer and is a component of cancer initiation, progression and
metastasis (11). It is now
considered a hallmark of cancer and an attractive target for cancer
therapy. Numerous studies have shown that cancer metastasis is
associated with inflammation (12). For instance, Shen et al
(13) reported that inflammatory
factors such as IL-1 and IL-6 can promote the expression of matrix
metalloproteinases through NF-κB and STAT3, thereby inducing tumor
cell invasion and metastasis, providing an explanation for the role
of inflammation in cancer metastasis. In addition, inflammation is
increasingly recognized as an important component of metastasis in
breast cancer. Studies have identified that controlling
cancer-induced inflammation prevents lung metastases in murine
models of breast cancer (14)
and inflammation triggered by loss of p53 drives breast cancer
metastasis (15). Therefore,
anti-inflammatory is expected to be an effective treatment for
inhibiting breast cancer metastasis.
The nucleotide-binding domain, leucine-rich
containing family, NLR family pyrin domain containing-3 (NLRP3) is
the most studied and best characterized type of inflammasome
(16). It is a sensor protein
that binds to ASC and Pro-caspase-1 to form inflammasomes,
activates caspase-1, and causes the proinflammatory cytokines
interleukin-1β (IL-1β) and interleukin-18 (IL-18) synthesis and
secretion (17). It has
attracted the attention and great interest of numerous research
groups due to its involvement in the development of various types
of tumors (18). Although it was
originally reported that the inflammasome inhibits tumor growth by
activating the immune system (19), increasing evidence has
demonstrated that the activation of the inflammasome leads to
cancer cell growth and promotes the process of cancer metastasis.
For example, Li et al (20) revealed that IL-1β treatment
induced a significant increase in gastric cancer cell
proliferation, while NLRP3 gene silencing significantly inhibited
the growth of gastric cancer cells. Guo et al (21) reported that tumor growth and
metastasis were significantly reduced in NLRP3 knockout mice in a
breast cancer xenograft model. Similarly, Ershaid et al
(22) investigated the
correlation between NLRP3 inflammasome activation in fibroblast and
its effect on breast cancer development and metastasis, and
indicated an upregulation of the NLRP3 pathway for both murine
mammary carcinogenesis and cancer-associated fibroblasts in human
breast cancer conditions. Based on previous studies, the NLRP3
inflammasome plays a key role in cancer metastasis and is
considered a key pathway for cancer therapy.
Euphorbia L. are traditional medicine used in
folk medicine practice (23).
The seeds of Euphorbia lathyris L., a plant of the
Euphorbiaceae family, are traditional Chinese medicinal
materials in China. They have various biological activities,
including anti-inflammatory, antiviral and antitumor activities,
and have been used to treat diseases such as edema, ascites and
cancer (24). The active
ingredients extracted from their seeds can be divided into 21
species, which are Euphorbia factor L1-L21 (EFL1-21). Among them,
Euphorbia factor L2 (EFL2) has attracted increasing attention in
recent years because of its anticancer effect. For instance, Lin
et al (24) confirmed
that EFL2 promoted the apoptosis process of lung cancer cells A549
through mitochondrial channels. Fan et al (25) demonstrated that EFL2 inhibited
tumor growth by inhibiting the proliferation and migration of
SMMC-7721 and Hep G2 cells through STAT3 phosphorylation. In
addition, EFL2 has anti-inflammatory effects; Zhang et al
(26) reported that it can
reduce lipopolysaccharide-induced inflammation in mice by
inhibiting NF-κB activation. However, whether EFL2 exerts an
inhibitory effect on breast cancer metastasis remains unclear.
Based on the anticancer and anti-inflammatory
effects of EFL2, and the critical role of the NLRP3 inflammasome in
cancer metastasis, it was investigated whether EFL2 could exert an
inhibitory effect on breast cancer metastasis through the
inflammasome NLRP3. Therefore, the present study attempted to
explore whether EFL2 could inhibit the generation of breast cancer
ascites by constructing a mouse breast cancer liver metastasis
model, and to further explore whether this inhibition was achieved
by inhibiting the activation of the NLPR3 inflammasome.
Materials and methods
Chemicals and antibodies
Antibodies against NLRP3 (cat. no. 13158), IL-1β
(cat. no. 12703), caspase 1 (cat. no. 24232), cleaved-caspase1
(cat. no. 89332), GAPDH (cat. no. 5174), and Goat Anti-Rabbit IgG
(H+L) HRP (cat. no. 14708) were purchased from Cell Signaling
Technology, Inc. Antibodies specifically against CD4 (cat. no.
Ab183685) and CD8 (cat. no. Ab209775) were purchased from Abcam.
Dulbecco's modified Eagle medium (DMEM) and fetal bovine serum
(FBS) were purchased from Sigma-Aldrich; Merck KGaA. Dimethyl
sulfoxide (DMSO), trypsin, penicillin-streptomycin solution,
multicolor protein marker, Tween 20 and sodium dodecyl
sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) were
purchased from Beijing Solarbio Science & Technology Co., Ltd.
Polyvinylidene fluoride membranes (PVDF) were purchased from
MilliporeSigma and the ECL reagent was purchased from COWIN Biotech
Co., Ltd. BSA was obtained from Shanghai Aladdin Biochemical
Technology Co., Ltd. HiScript II Q RT SuperMix for qPCR, Taq
MasterMix and AceQ qPCR SYBR Green Master Mix were purchased from
Vazyme Biotech, Cho., Ltd.
Animals
A total of 81 female BALB/c mice (27), 4-6 weeks old, weighing 15-18 g
were purchased from the Laboratory Animal Center of Nanjing
University of Chinese Medicine. Mice were kept at 22±2°C with a
12/12-h light/dark cycle and given free access to water and food.
Euthanasia of mice was performed using cervical dislocation and
subsequently tissues were obtained from mice. All animal procedures
were approved by the Institutional Animal Care and Use Committee of
Nanjing University of Chinese Medicine (approval no. 202205A137;
Nanjing, China) and conducted in accordance with the Guidelines of
Accommodation and Care for Animals formulated by the Chinese
Convention for the Protection of Vertebrate Animals Used for
Experimental and Other Scientific Purposes. The minimum number of
animals required to obtain consistent data was used. The humane end
point of animal studies was selected for euthanasia when the animal
has lost >20% of its body weight during the experiment or was
too weak to perform or not tolerating pain, or the mean maximum
diameter of the mouse tumor was ≥2 cm. None of the humane endpoints
considered was encountered in the course of the current study.
Cell culture
4T1 cells were obtained from the Shanghai Institute
of Biochemistry and Cell Biology. Cells were cultured in DMEM
supplemented with 10% FBS, 2 mM L-glutamine and 2%
penicillin/streptomycin. Cells were cultured at 37°C in a
humidified atmosphere with 5% CO2.
Study design
Construction of a breast cancer liver
metastasis model
A total of 200 μl of wild or NLRP3
overexpressed 4T1 cells (resuspended in PBS) with a concentration
of 2.5×107/ml was injected subcutaneously into the back
of the scapula of female BALB/c mice to establish a subcutaneous
tumor model of 4T1 cells in female BALB/c mice. The tumor volume of
the mice inoculated with cancer cells was measured every 3 days
after modeling. When the diameter of the tumor was ≥1 cm, the tumor
was removed, and the tumor was cut into a size of 1 mm3,
and then placed in Matrigel in an ice bath.
Female BALB/c mice were anesthetized with 50 mg/kg
sodium pentobarbital. The left lobe of the liver was exposed by
laparotomy, and the blind cavity was punctured in the left lobe of
the liver with a 25G syringe needle, then pressed to stop bleeding
and remove blood residue. The tumor mass adhered with Matrigel was
placed in the blind end duct on the liver and sutured, and the
wound was disinfected with active iodine. The mice were placed on a
heating pad and fed a normal diet after waking up. The weight of
the mice was measured every week, and the abdominal circumference
of the mice was recorded. The drug intervention began 10 days after
the operation and was administered for a total of 2 weeks. All mice
were sacrificed on the second day of the last administration. The
volume of ascites, tumor volume and weight of mice were recorded
(28).
Grouping and drug treatments in mice
i) Normal model
The mice were randomly divided into 5 groups, 9 mice
in each group, which were normal mice control group, model group,
EFL2 low-dose group (25 mg/kg/day), EFL2 high-dose group (50
mg/kg/day) and positive Drug doxorubicin group (5 mg/kg/day)
(29). EFL2 was intragastrically
administered once a day, and the positive drug was
intraperitoneally injected once every 3 days. A total of 2 weeks
was the duration of administration, and all mice were sacrificed
the day after the last administration.
ii) NLRP3 overexpression model
Firstly, the 4T1 cell line with NLRP3 overexpression
[puromycin (10 μg/ml) was used for selection] was
constructed; the NLRP3 overexpression lentivirus was provided by
Nanjing Bivoli Medical Research Institute Co., Ltd. The lentivirus
titer was 1.0×108 TU/ml, the transfection MOI value was
20, and the transfection time was 12-24 h. Then the model was
established as aforementioned. The mice were randomly divided into
4 groups with 9 mice in each group, namely model group (WT 4T1
cells), WT 4T1 cells + EFL2 high-dose group (50 mg/kg/day), NLRP3
overexpression 4T1 cells group, and NLRP3 overexpression 4T1 cells
+ EFL2 high dose group (50 mg/kg/day). The mice were
intragastrically administered once a day for a total of 2 weeks,
and all mice were sacrificed on the second day of the last
administration.
Immunohistochemistry
The tumor tissues fixed for 24 h at 4°C in 4%
paraformaldehyde were dehydrated and embedded in paraffin, and the
tissues were cut into 4-μm sections by using a rotary
microtome. Sections were dewaxed and rehydrated with xylene and
graded ethanol in turn. Antigen retrieval was performed using
sodium citrate antigen retrieval solution for 10 min, and then
incubated with 3% hydrogen peroxide at room temperature for 10 min.
After blocking with 5% goat serum (Beyotime Institute of
Biotechnology) blocking solution for 30 min, the primary antibodies
(rabbit anti-CD4:1:1,000; rabbit anti-CD8:1:2,000) were incubated
at 4°C overnight. The primary antibody was discarded the next day,
and the HRP-labeled Goat Anti-Rabbit IgG (H+L) secondary antibody
(1:1,000) was incubated at room temperature for 1 h. Then the
freshly prepared DAB chromogenic solution was used to develop color
for 1-20 min, and the color development was observed under the
light microscope. Sections were immersed in hematoxylin staining
solution for 4 min and washed in tap water for 2 min. Then the acid
differentiation solution was used to differentiate for 15 sec, and
the blue was reversed under running water for 20 min. The sections
were dehydrated with graded ethanol, rendered transparent with
xylene, and mounted with neutral gum. Finally, a light microscope
was used for microscopic examination, and image acquisition and
analysis were performed.
Hematoxylin and eosin staining
The tumor, liver and small intestine tissues fixed
for 24 h at 4°C in 4% paraformaldehyde were dehydrated and embedded
in paraffin, and then the paraffin block was cut into 4-μm
sections using a rotary microtome. Sections were successively
dewaxed with xylene, dehydrated with different concentrations of
ethanol, stained with hematoxylin nucleus, differentiated by
differentiation medium, stained with eosin cytoplasm, dehydrated
with different concentrations of ethanol, cleared with xylene, and
mounted with neutral resin. The tissue sections were observed under
a light microscope.
Western blot analysis
Total protein from tumor tissues in vivo was
extracted using RIPA cell lysis buffer (Beyotime Institute of
Biotechnology) supplemented with PMSF, protease inhibitors and
phosphatase inhibitors. The protein concentration was determined
using the BCA protein quantification kit according to the
manufacturer's instructions. Equal amounts of protein samples (40
μg) were loaded and separated in 10% SDS-PAGE. After
transferring to the PVDF membrane, membranes were blocked with 5%
BSA at room temperature for 1 h and incubated with the following
primary antibodies: NLRP3 (1:1,000), IL-1β (1:1,000), caspase 1
(1:1,000) and cleaved-caspase1 (1:1,000) overnight at 4°C, followed
by incubation with the HRP-labeled Goat Anti-Rabbit IgG (H+L)
secondary antibody (1:3,000) at room temperature for 1 h. Finally,
the visualization of the membranes was performed using enhanced
chemiluminescence (ECL reagent; COWIN Biotech Co., Ltd.). The
membranes were then visualized using an imaging system (Bio-Rad
Laboratories, Inc.) and quantified using the ImageJ 1.8.0 software
(National Institutes of Health).
Immunofluorescence
Tumor tissue (4% paraformaldehyde-fixed) was
dehydrated and embedded in paraffin, followed by sectioning,
deparaffinization, hydration, antigen retrieval and blocking (as
aforementioned in the 'Immunohistochemistry' paragraph), followed
by an overnight primary antibody (NLRP3; 1:500; cat. no. PA5-79740;
Thermo Fisher Scientific, Inc.) incubation at 4°C. After washing 3
times with PBS, sections were incubated with FITC-conjugated
secondary antibody (1:1,000; cat. no. ab150077; Abcam) for 1 h at
37°C. Next, the slices were washed and mounted in a fade-resistant
mounting medium with DAPI (5 μg/ml). Finally, the sections
were evaluated on a fluorescence microscope.
Reverse transcription-quantitative PCR
(RT-qPCR)
Total RNA from mice tumor tissues (10-50 mg) was
prepared by using TRIzol reagent (Vazyme Biotech Co., Ltd). cDNA
was generated by HiScript II Q RT SuperMix for qPCR Kit according
to the manufacturer's instructions. qPCR was performed using AceQ
qPCR SYBR Green Master Mix. The qPCR thermal cycling conditions
were as follows: An initial denaturation step at 95°C for 5 min,
followed by 40 cycles of denaturation at 95°C for 10 sec and
annealing/extension at 60°C for 30 sec. Each sample was subjected
to PCR amplification in triplicate. Data were analyzed with
2−ΔΔCq method (30),
using GAPDH for normalization. The mRNA levels of NLRP3, IL-1β and
GAPDH in tumor tissues were accessed. The sequences of the primers
used are shown in Table I.
 | Table IPrimers for reverse
transcription-quantitative PCR. |
Table I
Primers for reverse
transcription-quantitative PCR.
| Gene name | Primer sequence
(5′→3′) |
|---|
| NLR family pyrin
domain containing-3 | F:
TCCACAATTCTGACCCACAA
R: ACCTCACAGAGGGTCACCAC |
| IL-1β | F:
TTGACGGACCCCAAAAGATG
R: AGGACAGCCCAGGTCAAAG |
| GAPDH | F:
GCCTCCTCCAATTCAACCCT
R: CTCGTGGTTCACACCCATCA |
MTT assay
MTT assay was used to detect the effect of different
treatments on cell viability. Briefly, 4T1 cells were cultured for
24 h in 96-well plates at a density of 8,000 cells/well. The cells
were treated with different concentrations of drug-containing serum
24 h after the cell model was prepared in vitro. After 24 h
of treatment, 20 μl MTT (5 mg/ml) was added to each well,
and the supernatant was discarded for 4 h. After that, 150
μl DMSO was added to each well, and the dirty crystals were
fully dissolved using low-speed oscillations for 10 min. Finally,
OD values were determined by using an enzymic labelled meter at 570
nm wavelength.
EdU cell proliferation assay
4T1 cells and EFL2-treated 4T1 cells were incubated
into 96-well culture plates at a density of 2×103
cells/well and incubated for 24 h. The EdU analysis kit (cat. no.
C6015S; US Everbright Inc.) was used to analyze and evaluate cell
proliferation, and the specific method was carried out following
the instructions provided by the manufacturer. The samples were
analysed with a fluorescence microscope.
Statistical analysis
GraphPad Prism 7.0 software (Dotmatics) was used for
all statistical analyses. The mean ± SD was used to represent the
results. Data were analyzed using one-way analysis of variance
(ANOVA) with Dunnett's multiple comparisons test or Tukey's
multiple comparisons test. P<0.05 and P<0.01 were considered
to indicate a significant and very significant difference,
respectively.
Results
Effects of EFL2 on ascites generation in
breast cancer liver metastasis
To investigate the effects of EFL2 on generation of
ascites in liver metastasis model of breast cancer, the abdominal
circumference, the volume of peritoneal fluid and body weight in
mice, as well as liver tumor volume and weight, were measured.
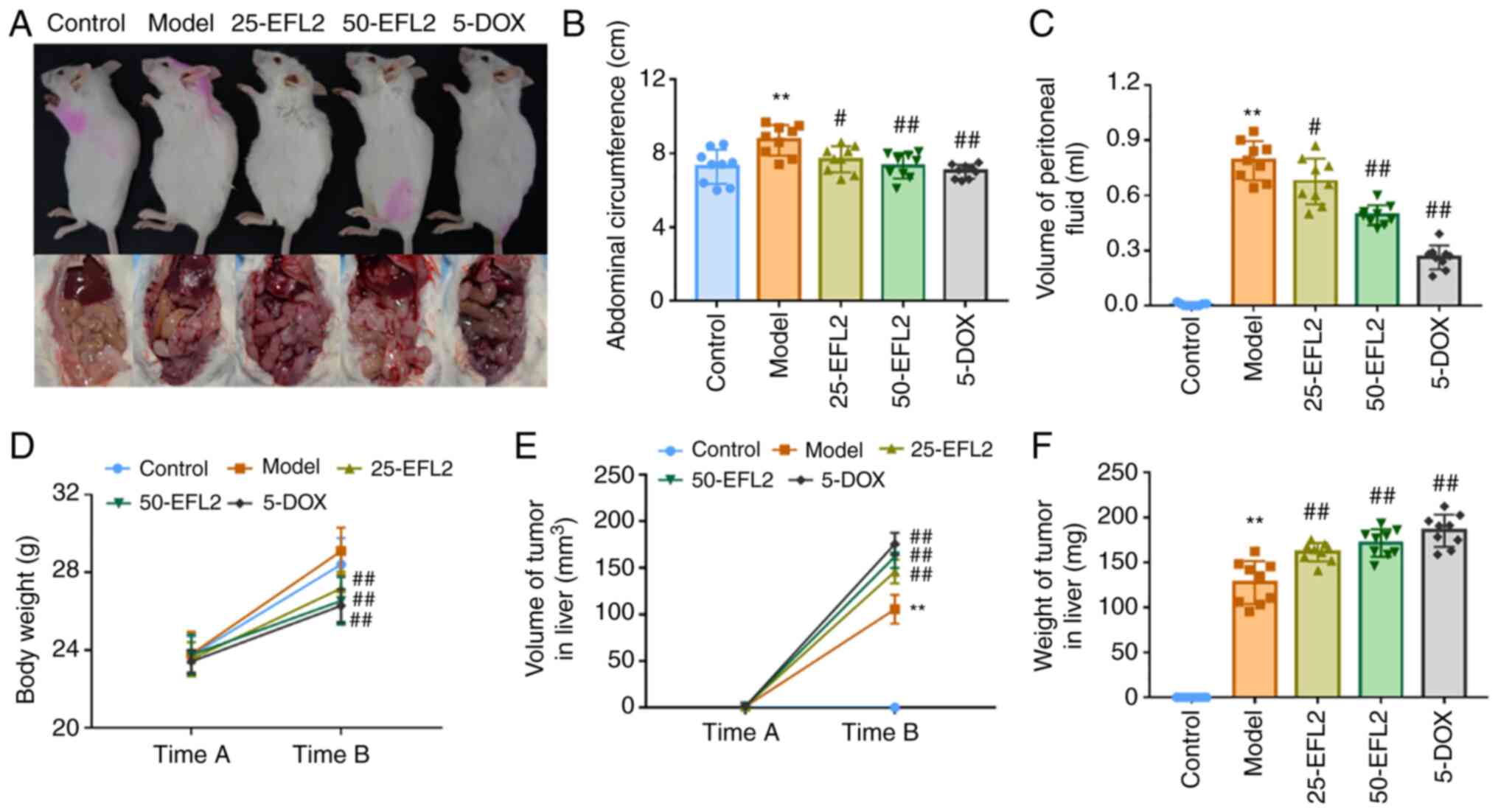
Images of the mice and tumors were captured (Fig. 1A) and compared with the control
group; the abdominal circumference and ascites' volume of the mice
in the model group were significantly increased, but they were
significantly decreased after EFL2 and positive drug treatment
(Fig. 1B and C). The body
weight, liver tumor volume and weight of the mice were then
measured between the two time periods. The weight of mice in the
control group was not significantly different from that in the
model group, but the weight of the mice in the administration group
was significantly lower than that in the model group (Fig. 1D). The tumor volume and weight in
the liver of mice in the model group were considerably smaller than
those in the administration group, and the difference was most
significant with the positive drug group (Fig. 1E and F). These results suggested
that EFL2 could inhibit the generation of ascites in liver
metastasis model of breast cancer. However, because the
tumor-bearing mice tended anorexia, the diet decreased, thus the
weight of the administration group was reduced. The tumor volume
and weight in the liver of the mice in the model group were the
smallest, and the trend was opposite to that of the ascites volume,
which might be due to ascites caused by metastasis of a large
number of solid tumors in the model group, and the migration amount
decreased in each administration group.
EFL2 inhibits liver inflammation and
tumor migration in breast cancer liver metastasis
To investigate the effect of EFL2 on liver tumors,
liver and small intestine tissues as well as liver tumors were
examined using H&E staining. The results of H&E staining of
liver tissue revealed that there were inflammatory cells (the
yellow dotted line circles the symbolic inflammatory cell mass)
infiltration in the liver tissue of tumor-bearing mice, and the
model group had the most, and each administration group attenuated
the infiltration of inflammatory cells to varying degrees (Fig. 2A and D). The H&E staining
results of the small intestine tissue demonstrated that the small
intestine adventitia of the tumor-bearing mice had tumor tissues of
different sizes (black arrows), the model group had the largest
tumor tissue, and each administration group reduced the migration
of tumor cells to the small intestine to varying degrees (Fig. 2B). The results of H&E
staining of tumor tissue showed that the arrangement of tumor cells
in each administration group was more scattered than that in the
model group (Fig. 2C). These
results suggested that inhibition of tumor metastasis by EFL2 may
be associated with reduced tissue inflammation.
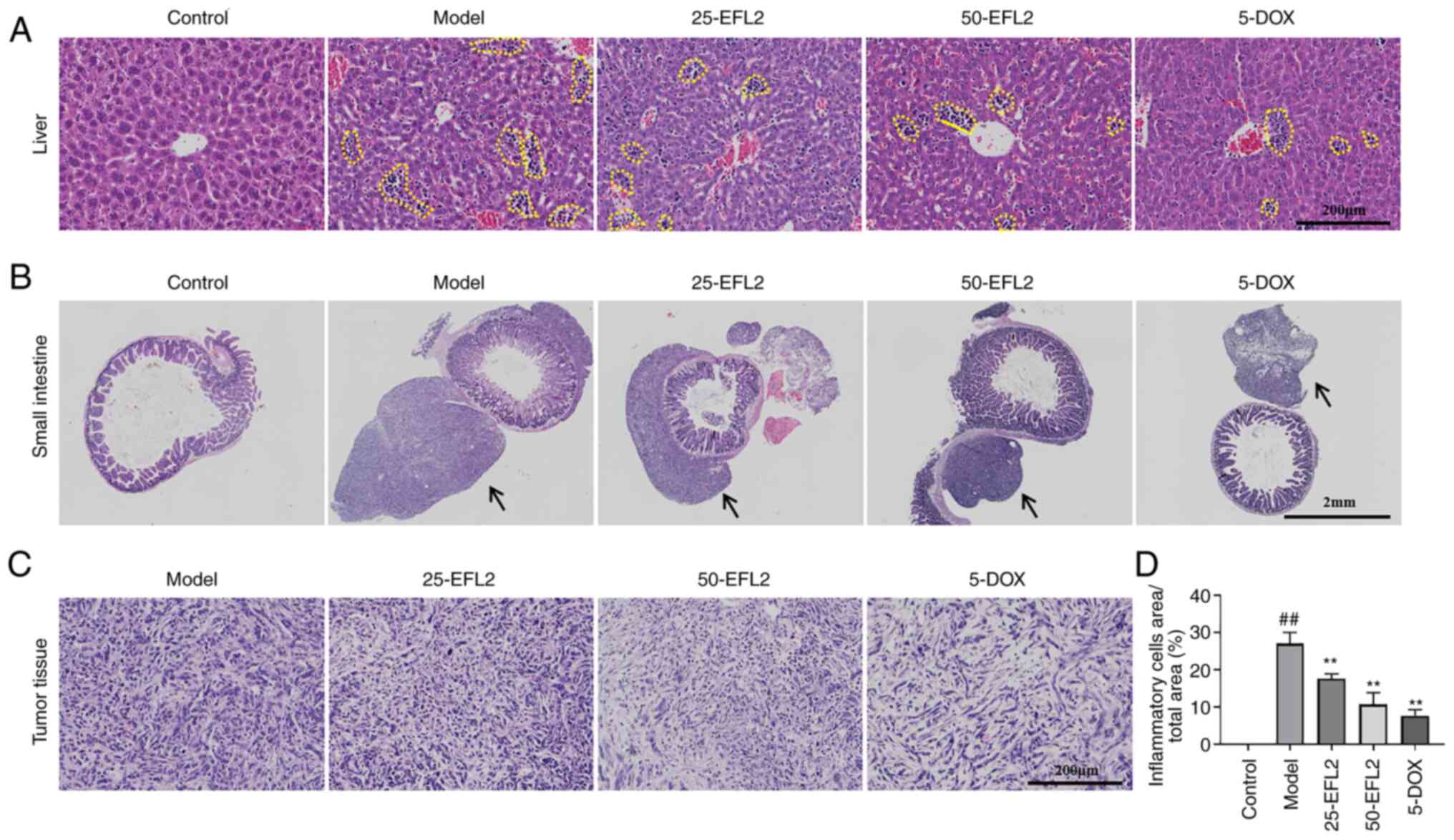 | Figure 2EFL2 inhibits liver inflammation and
tumor migration in breast cancer liver metastasis. H&E staining
of (A) liver tissues (Scale bars, 200 μm), (B) small
intestine tissues (Scale bars, 2 mm), and (C) tumor tissues (Scale
bars, 200 μm) of Control, Model, Low-dose EFL2 (25-EFL2)
treatment, High-dose EFL2 (50-EFL2) treatment, Positive drug
(5-DOX) treatment. (D) Quantitative analysis of the yellow dotted
circle in Fig. 2A (expressed as
a percentage of the total area of the yellow dotted circle) (n=3).
Mean ± SD as data representation. ##P<0.01 vs. the
control and **P<0.01 vs. the model. The yellow dotted
circles in Fig. 2A represent
symbolic inflammatory cell masses in liver tissue. The black arrows
in Fig. 2B indicate tumor tissue
of different sizes in the outer small intestine of tumor-bearing
mice. EFL2, Euphorbia factor L2; DOX, doxorubicin. |
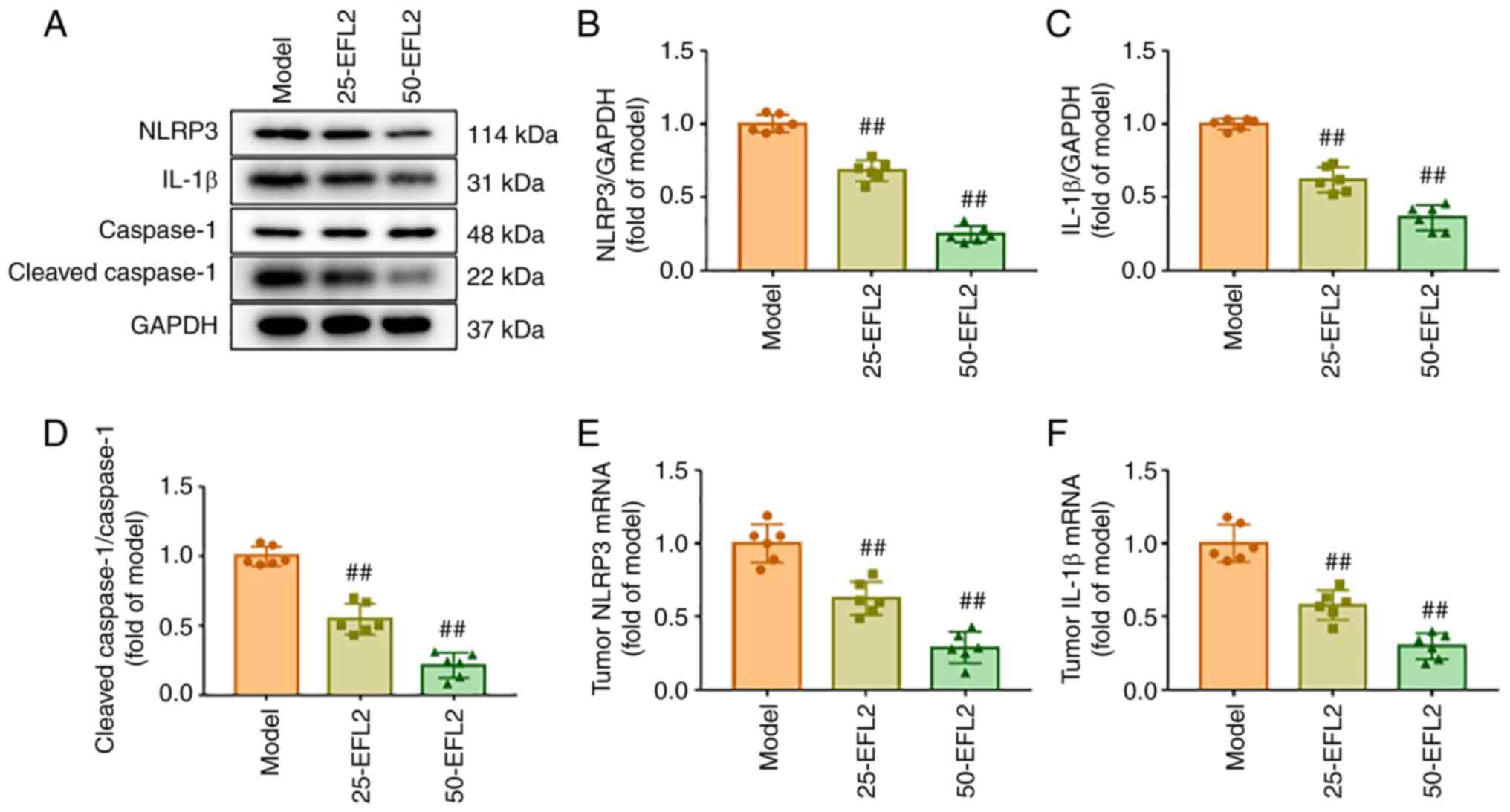
EFL2 inhibits tumor metastasis by
inhibiting NLRP3 in breast cancer liver metastasis
NLRP3 is the most studied and best characterized
type of inflammasome (16). The
aforementioned findings of the present study suggested that the
inhibition of tumor metastasis by EFL2 may be related to the
reduction of tissue inflammation, and that the reduction of
inflammation may be related to NLRP3. To investigate this
hypothesis, the protein expression levels of NLRP3,
cleaved-caspase1, total caspase1 and IL-1β in tumor tissues were
detected using western blotting, and the mRNA transcription of
NLRP3 and IL-1β in tumor tissues were identified using RT-qPCR. The
results of western blotting revealed that compared with the model
group, the expression levels of NLRP3, cleaved-caspase1/caspase1
and IL-1β in tumor tissues were significantly decreased after EFL2
treatment, and the effect of high concentration of EFL2 was more
obvious (Fig. 3A-D). The results
of RT-qPCR were consistent with those of western blotting (Fig. 3E and F). These results suggested
that NLRP3 was involved in the inhibitory effect of EFL2 on tumor
metastasis and tissue inflammation.
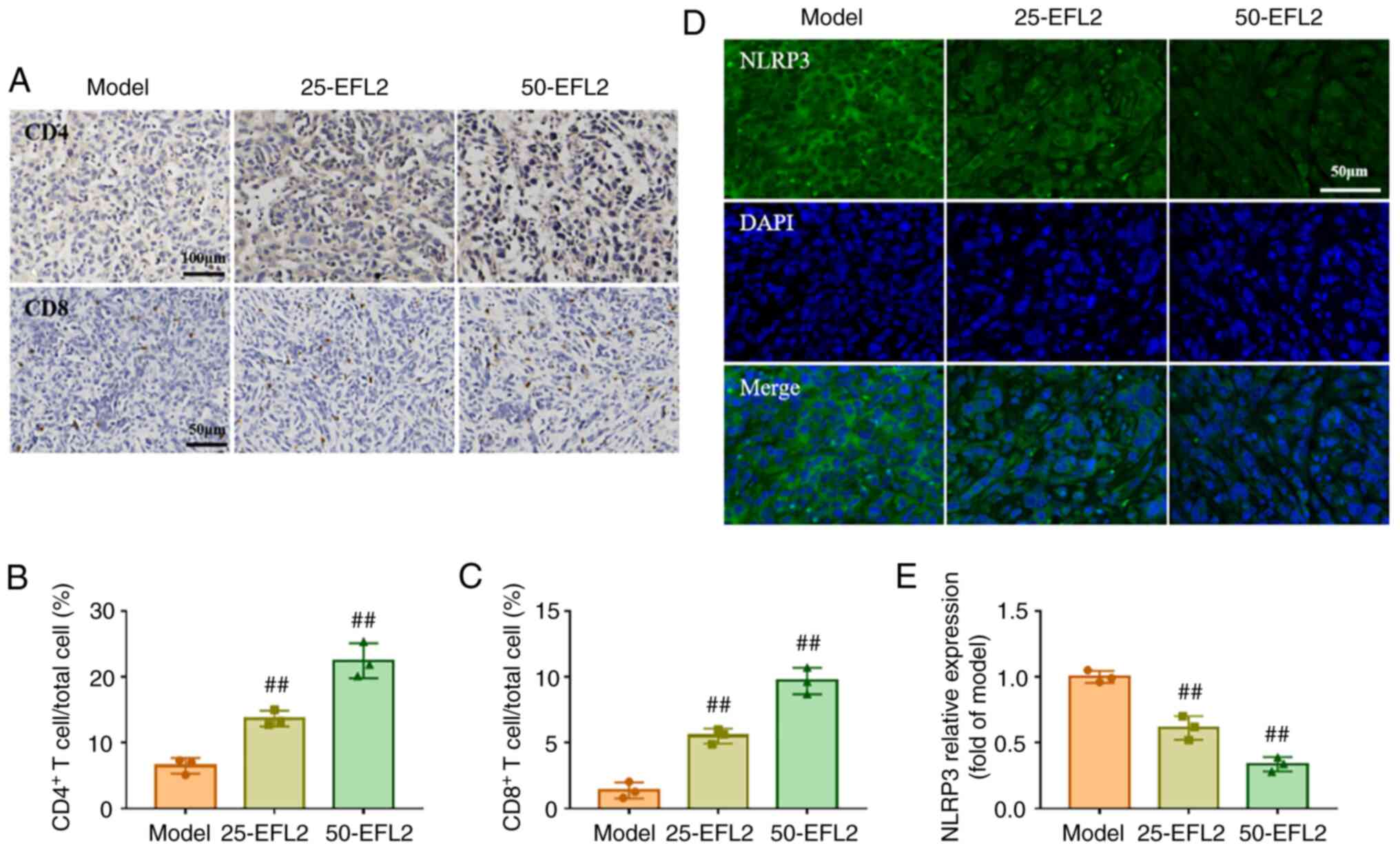
EFL2 enhances immune cell infiltration in
the tumor
The proportion of CD4 and CD8 cells in tumor tissue
was further detected by immunohistochemistry, and the expression of
NLRP3 was detected by immunofluorescence. The immunohistochemical
results demonstrated that compared with the model group, the CD4
and CD8 T cells in the tumor tissue after EFL2 treatment was
greatly increased, and the high concentration of EFL2 was more
significant (Fig. 4A-C). The
immunofluorescence results revealed strong linear staining of NLRP3
and nuclei in the model group. This situation was weak and
discontinuous in EFL2-treated groups. In addition, the expression
level of NLRP3 was significantly decreased in EFL2-treated groups
compared with the model group (Fig.
4D and E). In conclusion, EFL2 inhibited NLRP3 expression and
enhanced immune cell infiltration in tumor tissues, reduced
inflammation, and in turn inhibited tumor cell metastasis.
EFL2 reduces ascites generation in breast
cancer liver metastasis by inhibiting NLRP3 inflammasome
activation
To further verify whether EFL2 inhibits tumor
metastasis by inhibiting the activation of NLRP3, gene
overexpression was used. First, the NLRP3-overexpressing 4T1 cell
line was constructed, followed by tumor seeding. Validation of
overexpression by western blotting, and overexpression of NLRP3 was
effective (Fig. 5A). In
addition, the effect of EFL2 on 4T1 cells was examined in
vitro by MTT assay and Edu experiment. The results of the MTT
assay identified that the IC50 of EFL2 on 4T1 cells was
36.71 μm (Fig. 5B), and
further Edu assay revealed that this concentration of EFL2 could
inhibit the proliferation of 4T1 cells (Fig. 5C). Images of the mice and tumors
were captured (Fig. 5D), and to
investigate whether EFL2 reduced generation of ascites in liver
metastasis model of breast cancer by inhibiting NLRP3 inflammasome
activation, the abdominal circumference, ascites volume, and body
weight of the mice, as well as liver tumor volume and weight, were
measured. Compared with the model group, the abdominal
circumference, volume of peritoneal fluid and body weight of the
EFL2 treatment group were significantly decreased, but there was no
significant difference between the NLRP3 overexpression group and
the NLRP3 overexpression plus EFL2 treatment group (Fig. 5E, F and H). By contrast, compared
with the model group, the liver tumor weight and tumor volume in
the EFL2-treated group were significantly increased, but there was
no significant difference between the NLRP3 overexpression group
and the NLRP3 overexpression plus EFL2 treatment group (Fig. 5G and I). These results further
demonstrated that EFL2 inhibits ascites' generation in liver
metastasis model of breast cancer by inhibiting the activation of
NLRP3.
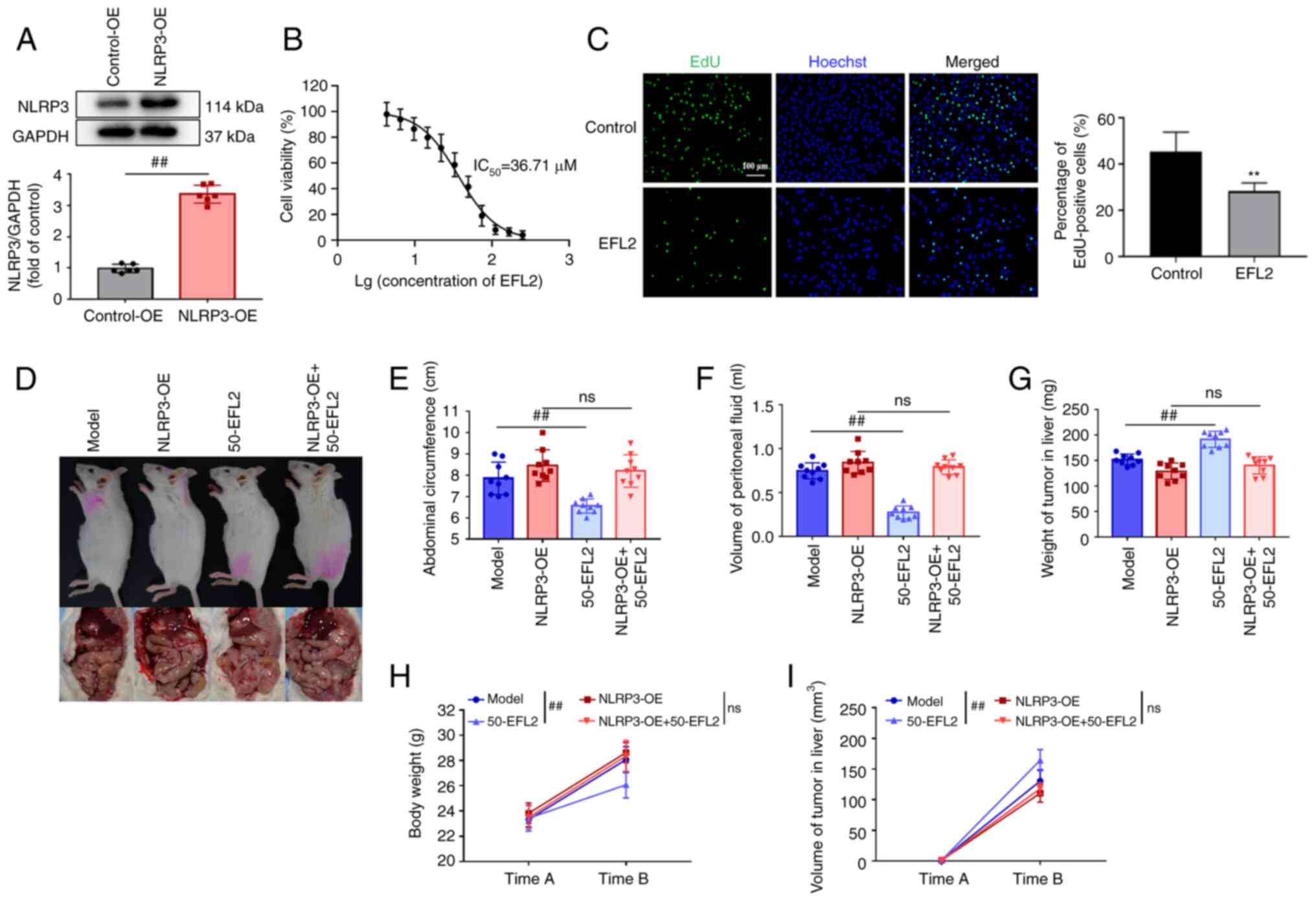 | Figure 5EFL2 reduces generation of ascites in
breast cancer liver metastasis by inhibiting NLRP3 activation. (A)
Analysis of NLRP3 expression in the control group and NLRP3
overexpression group of 4T1 cells using western blotting and
quantitative analysis of the NLRP3 expression level to GAPDH (n=6).
(B) The effects of different concentrations of EFL2 on the
viability of 4T1 cells were detected by MTT assay (n=6). (C) Edu
cell proliferation assay was used to detect the effect of 36.71
μm EFL2 on the proliferation of 4T1 cells (Scale bars, 50
μm; n=6), **P<0.01 vs. the control. (D) Gross
appearance and tumor, (E) abdominal circumference, (F) volume of
peritoneal fluid, (G) weight of tumor in the liver, (H) body weight
and (I) volume of tumor in the liver of Model, NLRP3 overexpression
(NLRP3-OE), High-dose EFL2 treatment (50-EFL2) and NLRP3
overexpression plus High-dose EFL2 treatment (NLRP3-OE+50-EFL2)
(n=9). Mean ± SD as data representation. ##P<0.01 vs.
the model. EFL2, Euphorbia factor L2; NLRP3, NLR family pyrin
domain containing-3; OE, overexpression; ns not significant. |
EFL2 inhibits tumor metastasis via
inhibiting NLRP3 activation
The aforementioned experiments proved that EFL2
inhibits the formation of ascites by inhibiting the activation of
NLRP3, but the specific mechanism remains unclear. Therefore, in
order to observe the effect of overexpression of NLRP3 on the
mechanism, liver tumors were detected using immunohistochemistry
and H&E staining, and the expression levels of cleaved-caspase1
and IL-1β in tumor tissues were detected by western blotting.
Immunohistochemical results demonstrated that compared with the
model group, the levels of CD4 and CD8 T cells in the EFL2-treated
group were significantly increased, while there was no significant
difference between the NLRP3 overexpression group and the NLRP3
overexpression plus EFL2 treatment group (Fig. 6A-C). The results of H&E
staining revealed that the tumor cells in the EFL2-treated group
were scattered compared with the model group, and there was no
significant difference between the NLRP3 overexpression group and
the NLRP3 overexpression plus EFL2 treatment group (Fig. 6D). In addition, western blotting
results identified that compared with the model group, the
expression levels of cleaved-caspase1 and IL-1β in the EFL2-treated
group were significantly decreased, while there was no significant
difference between the NLRP3 overexpression group and the NLRP3
overexpression plus EFL2 treatment group (Fig. 6E-G). These results suggested that
EFL2 enhances immune cell infiltration and suppresses tumor cell
metastasis by inhibiting NLRP3 activation, which may be the
mechanism by which EFL2 inhibits generation of ascites in breast
cancer liver metastasis.
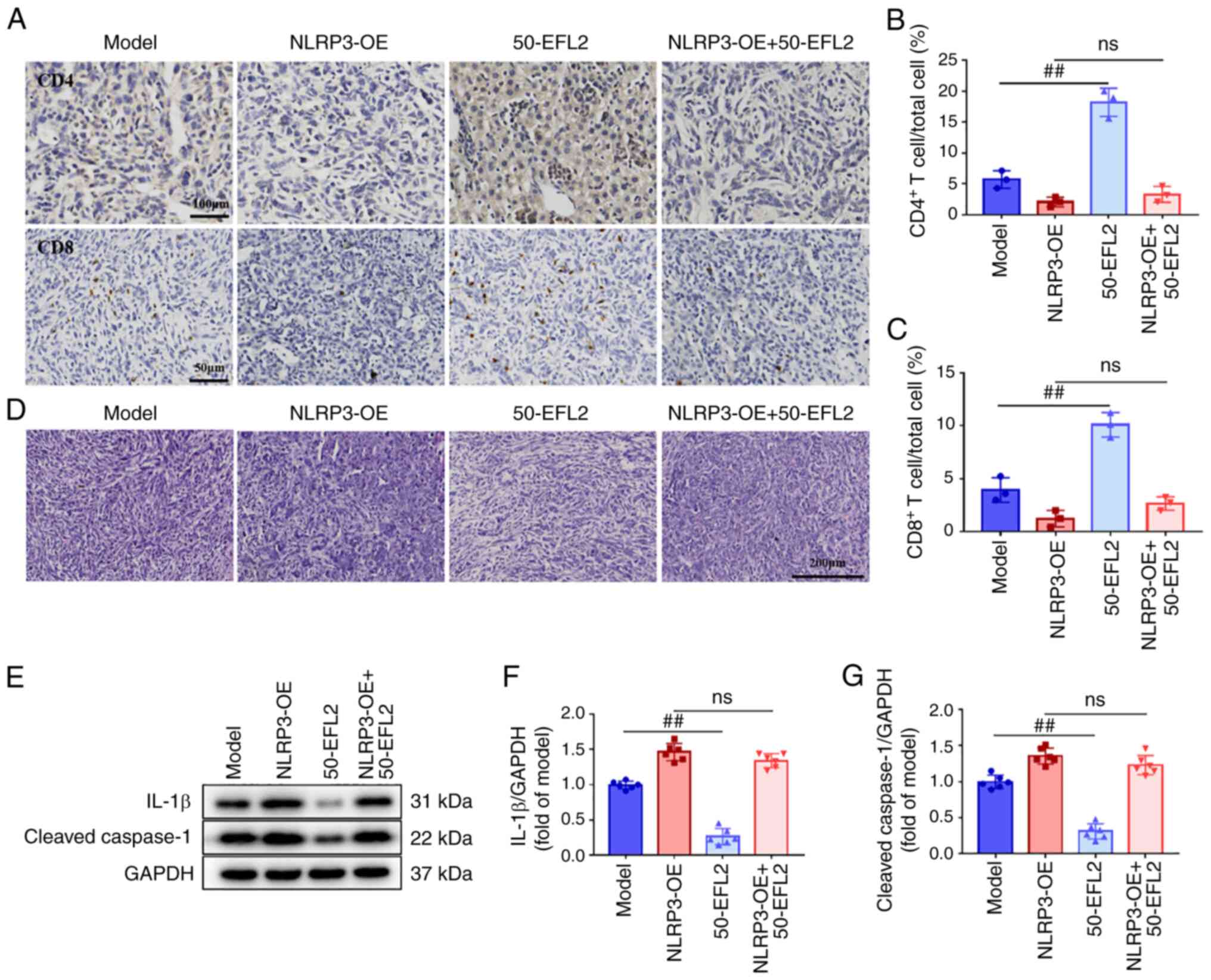 | Figure 6EFL2 inhibits tumor metastasis via
inhibiting NLRP3 activation. (A) The CD4 (Scale bars, 100
μm) and CD8 (Scale bars, 50 μm) T cells in tumor
tissues of Model, NLRP3 overexpression (NLRP3-OE), High-dose EFL2
treatment (50-EFL2) and NLRP3 overexpression plus High-dose EFL2
treatment (NLRP3-OE+50-EFL2) were measured by immunohistochemistry.
(B and C) Quantifications of immunohistochemistry (n=3). (D)
H&E staining of tumor tissues (Scale bars, 200 μm) of
Model, NLRP3 overexpression (NLRP3-OE), High-dose EFL2 treatment
(50-EFL2) and NLRP3 overexpression plus High-dose EFL2 treatment
(NLRP3-OE+50-EFL2). (E) The expression levels of NLRP3 and
cleaved-caspase1 in tumor tissues of Model, NLRP3 overexpression
(NLRP3-OE), High-dose EFL2 treatment (50-EFL2) and NLRP3
overexpression plus High-dose EFL2 treatment (NLRP3-OE+50-EFL2)
were detected by western blotting. (F and G) Normalized and
quantified protein levels to GAPDH (n=6). Mean ± SD as data
representation. ##P<0.01 vs. the model. EFL2,
Euphorbia factor L2; NLRP3, NLR family pyrin domain containing-3;
OE, overexpression; ns not significant. |
Discussion
Breast cancer is a complex disease that has been
found to be the second leading cause of cancer-related death in
women. Although there are numerous effective treatments, the
mortality rate due to breast cancer metastasis is gradually
increasing (31). Nearly all
deaths from breast tumors can be attributed to distant metastasis.
Metastasis is the leading cause of death in the vast majority of
patients with breast cancer. Most drugs treat breast cancer
metastases poorly, highlighting the urgent need to discover new
drug treatments (32).
Compelling evidence confirms that inflammation plays
an important role in tumor metastasis (33-35). Lunasin attenuates
obesity-associated 4T1 breast cancer cell metastasis through
anti-inflammatory properties, as previously demonstrated (36). In addition, studies have shown
that inflammation is associated with immune response evasion and
poor prognosis in cancer (37,38). Based on the close link between
inflammation and cancer metastasis and the effect of inflammation
on poor cancer prognosis, it was aimed to determine whether EFL2
has an effect on ascites in liver metastases from breast cancer. By
constructing a breast cancer liver metastasis model, it was found
that compared with the model group, the production of ascites after
EFL2 treatment was significantly reduced, indicating that EFL2 can
effectively inhibit the production of ascites in the breast cancer
liver metastasis model. However, the weight of the mice in the
control group was larger than that of the administration groups and
there was no significant difference with the model group, which may
be due to the tendency of anorexia in the tumor-bearing mice to
reduce their diet and body weight. In addition, the tumor volume
and weight in the liver of the mice in the model group were smaller
than those in the administration group, which was the opposite of
the ascites' volume. This may be due to a large number of solid
tumor metastases in the liver of the model group and the decrease
of metastases in each administration group. Detection of the liver,
small intestine and tumor tissue by H&E staining demonstrated
that EFL2 attenuated inflammatory cell infiltration and tumor cell
migration.
To date, numerous studies have shown that activation
of inflammatory signaling pathways is critical for cancer
development and metastasis (39). For example, the IL-6/JAK/STAT3
pathway is abnormally overactivated in numerous types of cancer,
and this overactivation is often associated with poor clinical
prognosis (40). Liang et
al (41) confirmed that
cancer-derived exosome TRIM59 promotes lung cancer progression by
regulating macrophage NLRP3 inflammasome activation. It has been
reported that inflammasomes are aberrantly expressed and activated
in a variety of malignancies and play an important role in tumor
development (42). Among them,
NLRP3, as the most studied and best characterized inflammasome
type, plays an important role in cancer metastasis. Shao et
al (43) reported that NLRP3
can promote the metastasis of colorectal cancer cells by regulating
epithelial-mesenchymal transition. Moreover, it has been previously
revealed that the NLRP3 inflammasome in macrophages drives
colorectal cancer metastasis to the liver (44). In addition, it has been
identified that inhibition of the NLRP3 inflammasome in the tumor
microenvironment inhibits the metastatic potential of cancer cells
(45). Therefore, in the present
study, the mRNA transcription and protein expression levels of
NLRP3 and related molecules were investigated in tumor tissues, and
the proportions of CD4 and CD8 T cells were examined. It was found
that the mRNA transcription and protein expression levels of NLRP3
and its related molecules were significantly decreased in a
dose-dependent manner by EFL2 treatment. Furthermore, it was
demonstrated that EFL2 led to an increase in CD4 and CD8 T cells
and enhanced immune cell infiltration. Next, in vivo
overexpression experiments of NLRP3 showed that overexpression of
NLRP3 significantly attenuated the inhibitory effect of EFL2,
suggesting that these beneficial effects of EFL2 may be related to
the downregulation of NLRP3 signaling.
In conclusion, the current experimental data
suggested that EFL2 has a significant inhibitory effect on ascites
of breast cancer liver metastasis in vivo, possibly by
inhibiting tumor cell metastasis by downregulating the expression
of NLRP3. Therefore, the application of EFL2 in combination with
current conventional adjuvant therapy may provide a new therapeutic
strategy for breast cancer patients with liver metastases. However,
further studies are needed to confirm the present results and
evaluate the role of EFL2 in breast cancer liver metastasis.
In summary, it was demonstrated in the present study
that EFL2 had a significant inhibitory effect on ascites of breast
cancer liver metastasis in vivo, possibly inhibiting tumor
cell metastasis by downregulating NLRP3 expression. These findings
suggested that the natural drug EFL2 may be an effective drug
treatment option for breast cancer liver metastasis. However, the
mechanism of action of EFL2 on tumor cells in vitro was not
explored; and the in vivo study was not particularly
in-depth. In future studies, this question shall be further
investigated by the authors.
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
DJ performed investigation and data curation. XL
developed methodology. RT wrote the original draft of the
manuscript and contributed to the acquisition of data. YZ curated
data. LZ acquired funding and contributed to the conception or
design of the study. All authors read and approved the final
version of the manuscript. DJ and LZ confirm the authenticity of
all the raw data.
Ethics approval and consent to
participate
The animal study protocol was approved (approval no.
202205A037) by Nanjing University of Chinese Medicine Laboratory
Animal Center (Nanjing, China).
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
Acknowledgments
Not applicable.
Funding
The present study was supported by the Natural Science
Foundation of the Higher Education Institutions of Jiangsu (grant
no. 20KJB360013), the Science and Technology Support Project of
Suzhou (grant no. SYS2020080) and Suzhou Basic Research Program
(grant no. SKY2023042).
References
|
1
|
Bray F, Ferlay J, Soerjomataram I, Siegel
RL, Torre LA and Jemal A: Global cancer statistics 2018: GLOBOCAN
estimates of incidence and mortality worldwide for 36 cancers in
185 countries. CA Cancer J Clin. 68:394–424. 2018.
|
|
2
|
DeSantis CE, Ma J, Goding Sauer A, Newman
LA and Jemal A: Breast cancer statistics, 2017, racial disparity in
mortality by state. CA Cancer J Clin. 67:439–448. 2017.
|
|
3
|
Tao L, Chu L, Wang LI, Moy L, Brammer M,
Song C, Green M, Kurian AW, Gomez SL and Clarke CA: Occurrence and
outcome of de novo metastatic breast cancer by subtype in a large,
diverse population. Cancer Causes Control. 27:1127–1138. 2016.
|
|
4
|
Hazem RM, Mohamed AA, Ghareb N, Mehanna
ET, Mesbah NM, Abo-Elmatty DM and Elgawish MS: Anti-cancer activity
of two novel heterocyclic compounds through modulation of VEGFR and
miR-122 in mice bearing Ehrlich ascites carcinoma. Eur J Pharmacol.
892:1737472021.
|
|
5
|
Guo W, Zhang S and Liu S: Establishment of
a novel orthotopic model of breast cancer metastasis to the lung.
Oncol Rep. 33:2992–2998. 2015.
|
|
6
|
Kipps E, Tan DS and Kaye SB: Meeting the
challenge of ascites in ovarian cancer: New avenues for therapy and
research. Nat Rev Cancer. 13:273–282. 2013.
|
|
7
|
Sangisetty SL and Miner TJ: Malignant
ascites: A review of prognostic factors, pathophysiology and
therapeutic measures. World J Gastrointest Surg. 4:87–95. 2012.
|
|
8
|
Mikuła-Pietrasik J, Uruski P, Szubert S,
Moszyński R, Szpurek D, Sajdak S, Tykarski A and Książek K:
Biochemical composition of malignant ascites determines high
aggressiveness of undifferentiated ovarian tumors. Med Oncol.
33:942016.
|
|
9
|
Yin T, Wang G, He S, Shen G, Su C, Zhang
Y, Wei X, Ye T, Li L, Yang S, et al: Malignant pleural effusion and
ascites induce epithelial-mesenchymal transition and cancer
stem-like cell properties via the vascular endothelial growth
factor (VEGF)/Phosphatidylinositol 3-Kinase (PI3K)/Akt/Mechanistic
target of rapamycin (mTOR) pathway. J Biol Chem. 291:26750–26761.
2016.
|
|
10
|
Vargas-Villarreal J, Cruz-Ramos M,
Espino-Ojeda A, Gutierrez-Hermosillo H, Díaz De Leon-Gonzalez E,
Monsivais-Diaz O, Palacios-Corona R, Martinez-Armenta CA,
González-Salazar F, Moreno-Treviño MG and Guzman-De La Garza FJ:
Acellular fraction from malignant effusions has cytotoxicity in
breast cancer cells. Mol Clin Oncol. 14:1062021.
|
|
11
|
Gupta V, Yull F and Khabele D: Bipolar
tumor-associated macrophages in ovarian cancer as targets for
therapy. Cancers (Basel). 10:3662018.
|
|
12
|
Song XD, Wang YN, Zhang AL and Liu B:
Advances in research on the interaction between inflammation and
cancer. J Int Med Res. 48:3000605198953472020.
|
|
13
|
Shen Y, Guo D, Weng L, Wang S, Ma Z, Yang
Y, Wang P, Wang J and Cai Z: Tumor-derived exosomes educate
dendritic cells to promote tumor metastasis via
HSP72/HSP105-TLR2/TLR4 pathway. Oncoimmunology. 6:e13625272017.
|
|
14
|
Holl EK, Frazier V, Landa K, Boczkowski D,
Sullenger B and Nair SK: Controlling cancer-induced inflammation
with a nucleic acid scavenger prevents lung metastasis in murine
models of breast cancer. Mol Ther. 29:1772–1781. 2021.
|
|
15
|
Wellenstein MD, Coffelt SB, Duits DEM, van
Miltenburg MH, Slagter M, de Rink I, Henneman L, Kas SM, Prekovic
S, Hau CS, et al: Loss of p53 triggers WNT-dependent systemic
inflammation to drive breast cancer metastasis. Nature.
572:538–542. 2019.
|
|
16
|
Tengesdal IW, Menon DR, Osborne DG, Neff
CP, Powers NE, Gamboni F, Mauro AG, D'Alessandro A, Stefanoni D,
Henen MA, et al: Targeting tumor-derived NLRP3 reduces melanoma
progression by limiting MDSCs expansion. Proc Natl Acad Sci USA.
118:e20009151182021.
|
|
17
|
Sun R, Gu J, Chang X, Liu F, Liang Y, Yang
X, Liang L and Tang D: Metabonomics study on orthotopic
transplantion mice model of colon cancer treated with astragalus
membranaceus-curcuma wenyujin in different proportions via
UPLC-Q-TOF/MS. J Pharm Biomed Anal. 193:1137082021.
|
|
18
|
Ratajczak MZ, Bujko K, Cymer M, Thapa A,
Adamiak M, Ratajczak J, Abdel-Latif AK and Kucia M: The Nlrp3
inflammasome as a 'rising star' in studies of normal and malignant
hematopoiesis. Leukemia. 34:1512–1523. 2020.
|
|
19
|
Zaki MH, Vogel P, Body-Malapel M, Lamkanfi
M and Kanneganti TD: IL-18 production downstream of the Nlrp3
inflammasome confers protection against colorectal tumor formation.
J Immunology. 185:4912–4920. 2010.
|
|
20
|
Li S, Liang X, Ma L, Shen L, Li T, Zheng
L, Sun A, Shang W, Chen C, Zhao W and Jia J: MiR-22 sustains NLRP3
expression and attenuates H. pylori-induced gastric carcinogenesis.
Oncogene. 37:884–896. 2018.
|
|
21
|
Guo B, Fu S, Zhang J, Liu B and Li Z:
Targeting inflammasome/IL-1 pathways for cancer immunotherapy. Sci
Rep. 6:361072016.
|
|
22
|
Ershaid N, Sharon Y, Doron H, Raz Y, Shani
O, Cohen N, Monteran L, Leider-Trejo L, Ben-Shmuel A, Yassin M, et
al: NLRP3 inflammasome in fibroblasts links tissue damage with
inflammation in breast cancer progression and metastasis. Nat
Commun. 10:43752019.
|
|
23
|
Tang J, Cheng X, Yi S, Zhang Y, Tang Z,
Zhong Y, Zhang Q, Pan B and Luo Y: Euphorbia factor L2
ameliorates the progression of K/BxN serum-induced arthritis by
blocking TLR7 mediated IRAK4/IKKβ/IRF5 and NF-kB signaling
pathways. Front Pharmacol. 12:7735922021.
|
|
24
|
Lin M, Tang S, Zhang C, Chen H, Huang W,
Liu Y and Zhang J: Euphorbia factor L2 induces apoptosis in A549
cells through the mitochondrial pathway. Acta Pharm Sin B. 7:59–64.
2017.
|
|
25
|
Fan L, Zhu H, Tao W, Liu L, Shan X, Zhao M
and Sun D: Euphorbia factor L2 inhibits TGF-β-induced cell growth
and migration of hepatocellular carcinoma through AKT/STAT3.
Phytomedicine. 62:1529312019.
|
|
26
|
Zhang Q, Zhu S, Cheng X, Lu C, Tao W,
Zhang Y, William BC, Cao X, Yi S, Liu Y, et al: Euphorbia factor L2
alleviates lipopolysaccharide-induced acute lung injury and
inflammation in mice through the suppression of NF-κB activation.
Biochem Pharmacol. 155:444–454. 2018.
|
|
27
|
Tallón de Lara P, Castañón H, Vermeer M,
Núñez N, Silina K, Sobottka B, Urdinez J, Cecconi V, Yagita H,
Movahedian Attar F, et al: CD39+PD-1+CD8+ T cells mediate
metastatic dormancy in breast cancer. Nat Commun. 12:7692021.
|
|
28
|
Lim HI, Yamamoto J, Han Q, Sun YU, Nishino
H, Tashiro Y, Sugisawa N, Tan Y, Choi HJ, Nam SJ, et al: Response
of triple-negative breast cancer liver metastasis to oral
recombinant methioninase in a patient-derived orthotopic xenograft
(PDOX) model. In Vivo. 34:3163–3169. 2020.
|
|
29
|
Zhang S, Liu X, Abdulmomen Ali Mohammed S,
Li H, Cai W, Guan W, Liu D, Wei Y, Rong D, Fang Y, et al: Adaptor
SH3BGRL drives autophagy-mediated chemoresistance through promoting
PIK3C3 translation and ATG12 stability in breast cancers.
Autophagy. 18:1822–1840. 2022.
|
|
30
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(-Delta Delta C(T)) method. Methods. 25:402–408. 2001.
|
|
31
|
Nirgude S, Mahadeva R, Koroth J, Kumar S,
Kumar KSS, Gopalakrishnan V, S Karki SS and Choudhary B: ST09, a
novel curcumin derivative, blocks cell migration by inhibiting
matrix metalloproteases in breast cancer cells and inhibits tumor
progression in EAC mouse tumor models. Molecules. 25:44992020.
|
|
32
|
Papageorgis P, Ozturk S, Lambert AW,
Neophytou CM, Tzatsos A, Wong CK, Thiagalingam S and Constantinou
AI: Targeting IL13Ralpha2 activates STAT6-TP63 pathway to suppress
breast cancer lung metastasis. Breast Cancer Res. 17:982015.
|
|
33
|
De Simone V, Franzè E, Ronchetti G,
Colantoni A, Fantini MC, Di Fusco D, Sica GS, Sileri P, MacDonald
TT, Pallone F, et al: Th17-type cytokines, IL-6 and TNF-α
synergistically activate STAT3 and NF-kB to promote colorectal
cancer cell growth. Oncogene. 34:3493–3503. 2015.
|
|
34
|
Mishra DK, Rocha HJ, Miller R and Kim MP:
Immune cells inhibit the tumor metastasis in the 4D cellular lung
model by reducing the number of live circulating tumor cells. Sci
Rep. 8:165692018.
|
|
35
|
Yang C, Wang Z, Li L, Zhang Z, Jin X, Wu
P, Sun S, Pan J, Su K, Jia F, et al: Aged neutrophils form
mitochondria-dependent vital NETs to promote breast cancer lung
metastasis. J Immunother Cancer. 9:e0028752021.
|
|
36
|
Hsieh CC, Wang CH and Huang YS: Lunasin
attenuates obesity-associated metastasis of 4T1 breast cancer cell
through anti-inflammatory property. Int J Mol Sci. 17:21092016.
|
|
37
|
Monkkonen T and Debnath J: Inflammatory
signaling cascades and autophagy in cancer. Autophagy. 14:190–198.
2018.
|
|
38
|
Zhao S, Shen W, Du R, Luo X, Yu J, Zhou W,
Dong X, Gao R, Wang C, Yang H and Wang S: Three
inflammation-related genes could predict risk in prognosis and
metastasis of patients with breast cancer. Cancer Med. 8:593–605.
2019.
|
|
39
|
Lu Z, Long Y, Li J, Li J, Ren K, Zhao W,
Wang X, Xia C, Wang Y, Li M, et al: Simultaneous inhibition of
breast cancer and its liver and lung metastasis by blocking
inflammatory feed-forward loops. J Control Release. 338:662–679.
2021.
|
|
40
|
Johnson DE, O'Keefe RA and Grandis JR:
Targeting the IL-6/JAK/STAT3 signalling axis in cancer. Nat Rev
Clin Oncol. 15:234–248. 2018.
|
|
41
|
Liang M, Chen X, Wang L, Qin L, Wang H,
Sun Z, Zhao W and Geng B: Cancer-derived exosomal TRIM59 regulates
macrophage NLRP3 inflammasome activation to promote lung cancer
progression. J Exp Clin Cancer Res. 39:1762020.
|
|
42
|
Wang H, Luo Q, Feng X, Zhang R, Li J and
Chen F: NLRP3 promotes tumor growth and metastasis in human oral
squamous cell carcinoma. BMC Cancer. 18:5002018.
|
|
43
|
Shao X, Lei Z and Zhou C: NLRP3 promotes
colorectal cancer cell proliferation and metastasis via regulating
epithelial mesenchymal transformation. Anticancer Agents Med Chem.
20:820–827. 2020.
|
|
44
|
Deng Q, Geng Y, Zhao L, Li R, Zhang Z, Li
K, Liang R, Shao X, Huang M, Zuo D, et al: NLRP3 inflammasomes in
macrophages drive colorectal cancer metastasis to the liver. Cancer
Lett. 442:21–30. 2019.
|
|
45
|
Lee HE and Lee JY, Yang G, Kang HC, Cho
YY, Lee HS and Lee JY: Inhibition of NLRP3 inflammasome in tumor
microenvironment leads to suppression of metastatic potential of
cancer cells. Sci Rep. 9:122772019.
|