Introduction
Bone fractures are common traumatic injuries of the
bone cortex (1). Fracture
healing is a regenerative process that recapitulates a number of
the ontological events of embryonic skeletal development (1,2).
Bone fracture healing is histologically defined in four steps: The
initial inflammation-responsive phase within the first several days
after fracture, the generation of surrounding soft callus and
subsequent hard callus formation, followed by the
osteoblast-induced formation of woven bone on the calcified matrix
and recreation of the appropriate anatomical shape (2). The mechanisms involved in fracture
healing are highly complex and are closely related to the interplay
between cells, the extracellular matrix (ECM) and cytokines
(1). It is well recognised that
multiple cell types, such as chondrocytes, osteoblasts, endothelial
cells, osteocytes and even mesenchymal stem cells, are involved in
bone regeneration via the secretion of growth factors and the
temporal expression of bone healing-related genes, such as bone
sialoprotein and osteocalcin (3).
Osteocytes are the most common functional cell type
involved in fracture healing (4,5).
Osteocytes connect with each other and with other cell types, such
as osteoblasts, bone marrow cells and periosteal cells, through an
abundant dendritic network, which allows migration of signalling
factors among the cells (4).
Therefore, the position and status of osteocytes are critical for
bone metabolism, remodelling and generation (6).
Extracellular signal-regulated kinase (ERK) is a
critical regulator of animal development (7). ERK activation initiates a
phosphorylation cascade within cells, affects gene expression and
causes changes in cell phenotypes, such as proliferation,
differentiation and motility (7). Oxidative stress causes decreased
autophagy of osteocytes, during which inhibition of ERK signalling
impairs autophagosome formation and promotes osteocyte cell death
(8). In addition, activation of
ERK signalling in osteocytes can cause changes in the cytoskeleton,
remodelling of the ECM, and further alteration of tissue structure
and bones (1).
The regulator of G protein signalling (RGS) family
contains key cytosolic proteins that are capable of accelerating
GTPase activity and regulating downstream signalling under various
physiological conditions (9).
For example, RGS18 is associated with platelet production and
reactivity, and participates in the haemostatic response after
injury (10). Plasma levels of
RGS18 are a promising biomarker of gastric cancer (11). Furthermore, RGS18 suppresses
osteoclastogenesis by negatively regulating OGR1/NFAT signalling in
osteoclasts (12). The present
study conducted a bioinformatics analysis of acutely injured
subjects (AIS) and screened for elevated RGS18 expression.
Gene Set Enrichment Analysis (GSEA) and subsequent experimental
verification revealed that ERK signalling is involved in osteocyte
proliferation and apoptosis. The present study provides a novel
target for bone fracture healing.
Materials and methods
Bioinformatics analysis
The GSE93138 and GSE93215 datasets (13) in the Gene Expression Omnibus
(GEO) database (http://www.ncbi.nlm.nih.gov/geo/) were selected for
the present bioinformatics analysis. Differentially expressed genes
were screened using the 'limma' package (14). In the two datasets, peripheral
blood samples were obtained from acutely injured subjects (AIS)
collected over multiple days, and were compared with those obtained
from age- and sex-matched healthy volunteers (HVs). Microarrays
were then performed to compare changes in gene expression between
the AIS and HVs. The AIS were enrolled upon presentation for
fracture care. To comprehensively analyse the basic functions and
participating pathways of the differentially expressed genes, GSEA
was performed using GSEA software (version 4.2.1; https://www.gsea-msigdb.org/gsea/index.jsp) with the
c2.cp.kegg.v7.1.symbols.gmt gene set (https://www.gsea-msigdb.org/gsea/index.jsp) (15,16).
Specimen selection
All experiments were performed with the approval of
the Ethics Committee of The Fifth Affiliated Hospital Jinan
University (approval no. 2022-10.19.1.0; Heyuan, China). Peripheral
blood samples were collected from AIS (<7 days after injury)
(n=10). In total, samples were collected from five male patients
and five female patients, with a median age of 43.4 years (range,
26-54 years). The inclusion criterion was patients aged between 18
and 55 years. The exclusion criteria were: Prior knee injury or
surgery in either knee, posterior cruciate ligament injury,
posterolateral corner injury, lateral collateral ligament injury,
diabetes or other systemic diseases, and a history of inflammatory
arthritis or gout. Peripheral blood samples were also collected
from age- and sex-matched HVs (n=10), and were used as controls.
The peripheral blood samples were collected between November 2022
and March 2023. The expression levels of RGS18 were determined by
reverse transcription-quantitative PCR (RT-qPCR). All donors signed
an informed consent form.
Cell culture
Mouse osteocyte MLO-Y4 cells (Procell Life Science
& Technology Co., Ltd.) were cultured in α-minimal essential
medium (α-MEM; Procell Life Science & Technology Co., Ltd.)
containing 10% foetal bovine serum (FBS; Shanghai Basal Media
Technologies Co., Ltd.) and 1% penicillin-streptomycin. Mouse
osteoblast MC3T3-E1 cells (Nanjing Cobioer Biosciences Co., Ltd.)
were cultured in α-MEM containing 20% FBS, 2 mM L-glutamine, 1 mM
sodium pyruvate, and 1% penicillin-streptomycin. The cells were
maintained at 37°C in an incubator containing 5% CO2 and
95% humidity. The ERK activator
12-O-tetradecanoylphorbol-13-acetate (TPA) and MEK1/2 inhibitor
PD98059 were purchased from MilliporeSigma.
RT-qPCR
Total RNA was extracted from the collected
peripheral blood samples and MLO-Y4 and MC3T3-E1 cells using Trizol
reagent (Beyotime Institute of Biotechnology). RNA then underwent
cDNA synthesis using a Prime Script RT-PCR kit (Takara
Biotechnology Co., Ltd.) according to the manufacturer's protocol.
TB Green Fast qPCR Mix (Takara Biotechnology Co., Ltd.) was used
for qPCR. The following thermocycling conditions were used: 95°C
for 30 sec, followed by 40 cycles at 95°C for 5 sec and 60°C for 10
sec. The expression levels of genes were normalised to GAPDH
using the 2−ΔΔCq method (17). The primer sequences were designed
and synthesised by Shanghai GenePharma Co., Ltd., as follows: Mouse
RGS18, forward 5′-GGC CAA AGA AAC AAG ATG GAG T-3′, reverse
5′-ACA CTC TGC TTT GTG CCG TA-3′; mouse GAPDH, forward
5′-CAG GAG AGT GTT TCC TCG TCC-3′, reverse 5′-GAT GGG CTT CCC GTT
GAT GA-3′; human RGS18, forward 5′-GCA GAG ACA GAA AGA AAC
GCA G-3′, reverse 5′-CTC TTC AGG GGA GAC TCT TGT-3′; and human
GAPDH, forward 5′-CCA TGT TGC AAC CGG GAA G-3′ and reverse
5′-GCC CAA TAC GAC CAA ATC AGA G-3′.
Cell transfection
Small interfering RNA (siRNA) targeting RGS18
(si-RGS18) and a plasmid overexpressing RGS18 (pcDNA-RGS18)
were synthesised by Shanghai GenePharma Co., Ltd. Scrambled siRNA
(si-NC) and empty pcDNA3.1 vector were used as negative controls,
respectively. The siRNA sequences were as follows: si-RGS18, sense
5′-GGAGAGACUCAAGCCAGUAGA-3′, antisense 5′-UCUACUGGCUUGAGUCUCUCC-3′;
and si-NC, sense 5′-GAGACAGGGCAGCCAAGUAUA-3′ and antisense
5′-UAUACUUGGCUGCCCUGUCUC-3′. For transfection, MLO-Y4 and
MC3T3-E1cells were seeded in six-well plates at a density of
5×105 cells/well. A total of 5 μl Lipofectamine™
2000 (Invitrogen; Thermo Fisher Scientific, Inc.) and 2 μg
vectors or 100 nM siRNAs was mixed in 100 μl Opti-MEM™
(Gibco; Thermo Fisher Scientific, Inc.) and added to each well.
After 48 h of incubation at 37°C, the medium was replaced with
fresh medium and the cells were cultured for another 24 h.
Cell counting kit 8 (CCK-8) assay
Post-transfection with si-RGS18 or pcDNA-RGS18,
MLO-Y4 and MC3T3-E1 cells were seeded into 96-well plates at a
density of 5,000 cells/well. After incubation for 12, 24, 48 and 72
h at 37°C, CCK-8 solution (Thermo Fisher Scientific, Inc.) was
added and incubated for a further 2 h. Absorbance was measured at
an optical density of 450 nm using a microplate detector (Thermo
Fisher Scientific, Inc.).
5-Ethynyl-2′-deoxyuridine (EdU)
assay
Following transfection, the proliferation of MLO-Y4
and MC3T3-E1 cells was determined using an EdU assay kit (Beyotime
Institute of Biotechnology) according to the manufacturer's
instructions. Briefly, MLO-Y4 and MC3T3-E1 cells (5×103
cells/well in 96-well plates) were stained with EdU (10 μM)
at room temperature for 1 h and then fixed in 4% formaldehyde for
15 min at room temperature (23±2°C), and permeabilised with 0.5%
Triton X-100 for 10 min at room temperature. DAPI (Invitrogen;
Thermo Fisher Scientific, Inc.) was used to label the nuclei for 20
min at room temperature. Images were captured using a fluorescence
microscope (Leica Microsystems, Inc.).
Flow cytometry
Cell cycle arrest and apoptosis were assessed by
flow cytometry. Briefly, MLO-Y4 and MC3T3-E1 cells were transfected
with si-RGS18 or pcDNA-RGS18 as indicated, followed by treatment
with 20 μM PD98059 or 200 nM TPA for 24 h at 37°C. For cell
cycle analysis, 1×106 cells were collected and fixed in
ice-cold 70% ethanol for 12 h at 4°C and then stained with a
mixture of PI (50 μg/ml), 1% Triton-X100 (1%) and DNase-free
RNase (100 μg/ml) at 4°C for 30 min. To assess apoptosis,
1×105 cells were collected and stained using an Annexin
V-FITC/PI double staining kit (Beyotime Institute of
Biotechnology), according to the manufacturer's protocol. The
samples were then assessed by flow cytometry (Accuri-C6™ plus; BD
Biosciences) and the data were analysed using FlowJo™ software
(v7.6.5; FlowJo, LLC).
Western blotting
Total protein lysates were extracted from MLO-Y4 and
MC3T3-E1 cells following transfection using RIPA lysis buffer
(Beijing Solarbio Science & Technology Co., Ltd.) and were
quantified using a BCA kit (Beyotime Institute of Biotechnology).
Equal amounts of protein (30 μg) were separated by SDS-PAGE
on 10-12% gels and transferred onto nitrocellulose membranes. After
blocking with 5% non-fat milk at room temperature for 1 h, the
membranes were probed using primary antibodies against RGS18 (cat.
no. 11866-1-AP; Proteintech Group, Inc.), cyclin D (cat. no.
ab239794; Abcam), cyclin E (cat. no. ab33911; Abcam), cleaved
caspase-3 (cat. no. ab32042; Abcam), cleaved caspase-9 (cat. no.
9509; Cell Signaling Technology, Inc/), ERK1/2 (cat. no. ab17942;
Abcam), phosphorylated (p)-ERK1/2 (cat. no. ab201015; Abcam), p38
(cat. no. ab170099; Abcam), p-p38 (cat. no. ab236527; Abcam),
JNK1/2 (cat. no. ab112501; Abcam), p-JNK1/2 (cat. no. ab4821;
Abcam), ERK5 (cat. no. ab196609; Abcam), p-ERK5 (cat. no. ab5686;
Abcam) (all 1:1,000) and GAPDH (cat. no. 60004-1-Ig; 1:50,000;
Proteintech Group, Inc.) overnight at 4°C. The membranes were then
incubated with the corresponding secondary anti-mouse or
anti-rabbit antibodies conjugated to HRP (cat. nos. ab6789 and
ab205718; both 1:2,000; Abcam) at room temperature for 1 h. Protein
bands of interest were visualised by incubation with an enhanced
chemiluminescence reagent (Pierce; Thermo Fisher Scientific,
Inc.).
Immunostaining
MLO-Y4 and MC3T3-E1 cells were seeded in confocal
dishes at a density of 100,000 cells/well, followed by transfection
with si-RGS18 or pcDNA-RGS18. The cells were then washed with PBS,
fixed in 4% paraformaldehyde at room temperature for 15 min,
permeabilised with 0.5% Triton X-100 at room temperature for 10
min, blocked with 5% BSA at room temperature for 1 h and incubated
with a primary antibody against p-ERK1/2 (cat. no. ab201015; 1:200;
Abcam) at 4°C overnight. Subsequently, the cells were washed with
PBS and incubated with a goat anti-rabbit IgG antibody (Alexa
Fluor® 555) (cat. no. ab150078; 1:200; Abcam) and with
DAPI for 10 min at room temperature. Five random stained areas were
observed under a fluorescence microscope (Leica Microsystems,
Inc.).
Statistical analysis
All experiments were repeated three times and all
data are presented as the mean ± standard deviation and were
analysed using GraphPad Prism 9.5.1 software (Dotmatics).
Statistical differences between two groups were measured using
unpaired Student's t-test, and differences among three or more
groups were analysed using one-way or two-way ANOVA followed by the
Bonferroni post hoc test. P<0.05 was considered to indicate a
statistically significant difference.
Results
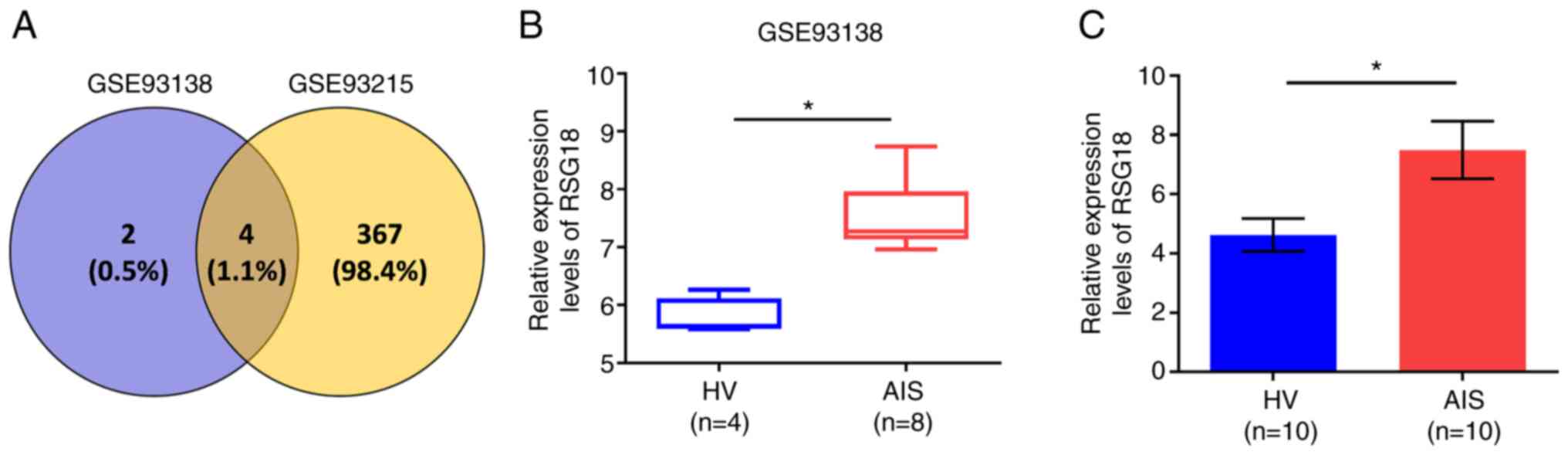
RGS18 expression is increased in AIS
To identify the potential factors associated with
bone fracture, gene expression in AIS and HVs from the GSE93138 and
GSE93215 datasets of the GEO database was analysed. The overlap
analysis identified four genes: KIAA0825, ANXA3,
RGS18 and LIPN that were upregulated in AIS compared
with in HVs (Fig. 1A). Given
that a previous study reported that RGS18 can impair osteoclast
formation (12), RGS18
was selected for further investigation. Further experiments
confirmed that the expression levels of RGS18 were increased
in AIS compared with those in HVs from the GSE93138 dataset
(Fig. 1B) and clinical samples
(Fig. 1C).
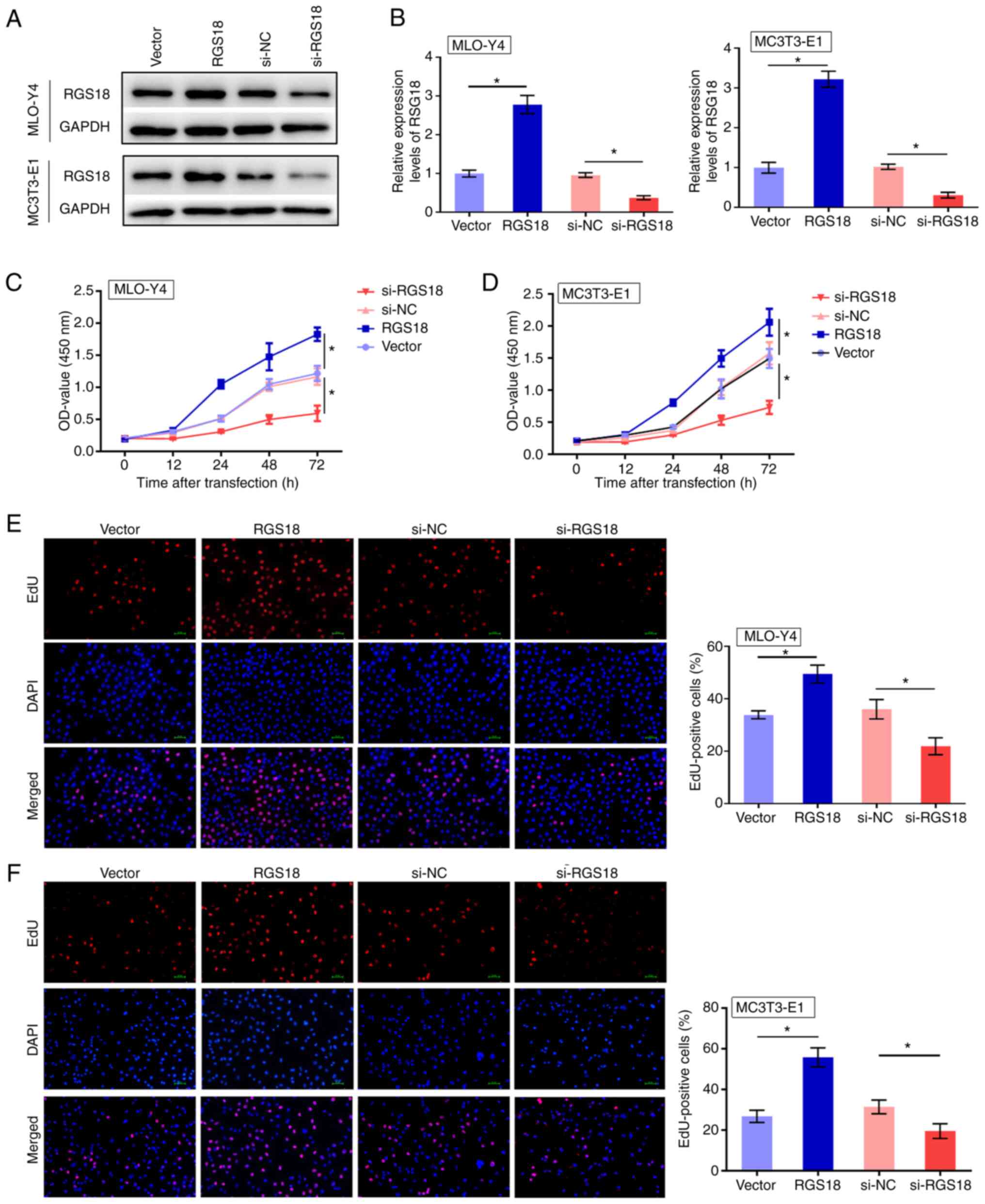
RGS18 promotes osteoclast viability and
proliferation
To assess the function of RGS18 in bone fracture,
MLO-Y4 and MC3T3-E1 osteocytes were transfected with RGS18
overexpression vector or RGS18 siRNA, and the transfection
efficiency was validated by western blot analysis and RT-qPCR
(Fig. 2A and B). The viability
of MLO-Y4 and MC3T3-E1 cells was promoted by RGS18
overexpression, but was suppressed by RGS18 knockdown
(Fig. 2C and D). Furthermore,
overexpression of RGS18 increased and silencing of
RGS18 decreased the number of EdU-positive MLO-Y4 and
MC3T3-E1 cells (Fig. 2E and F),
thus indicating that RGS18 contributes to osteocyte
proliferation.
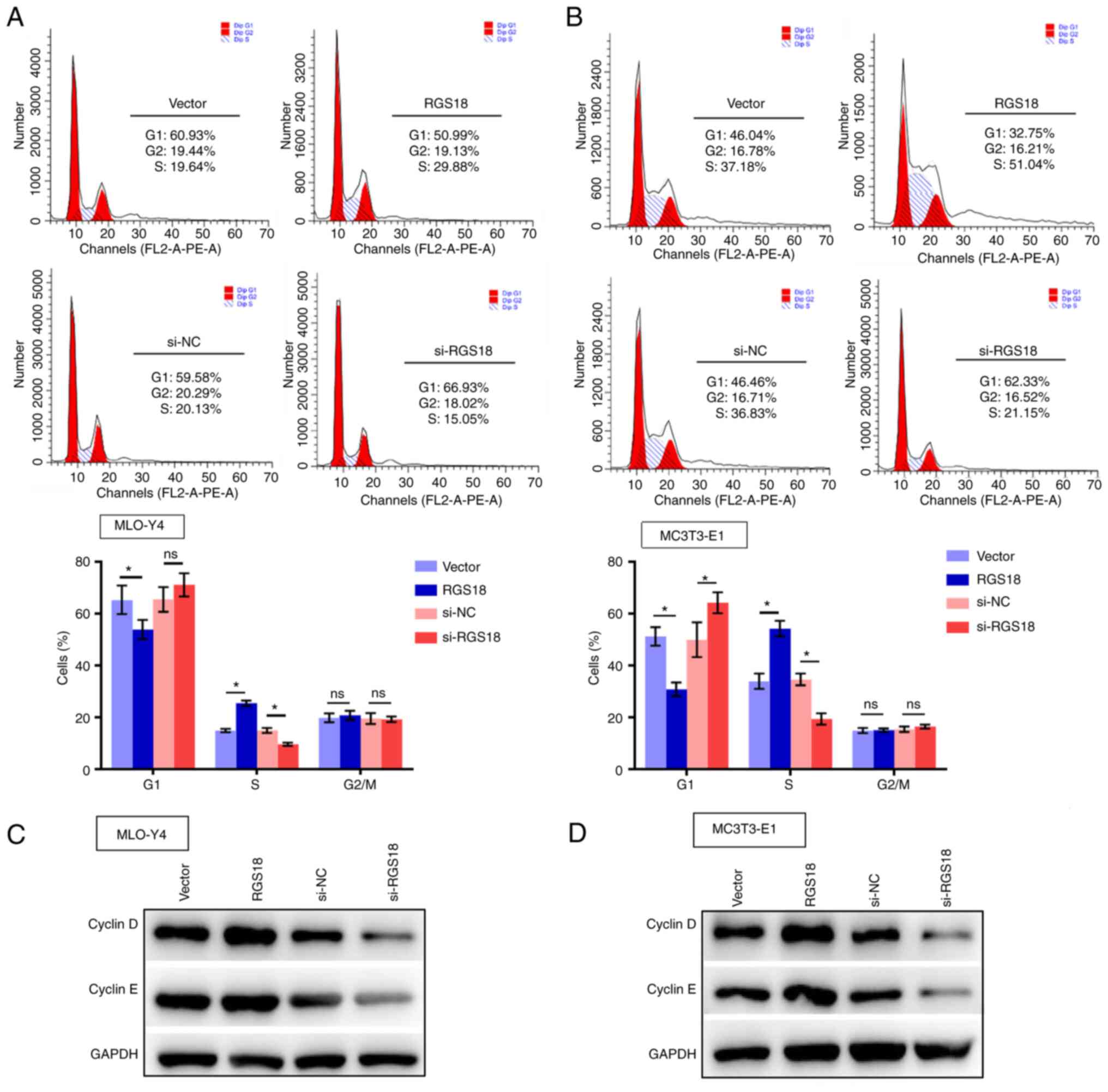
RGS18 promotes osteocyte cell cycle
progression
The present study then examined the effect of RGS18
on osteocyte cell cycle progression. It was revealed that
overexpression of RGS18 reduced the population of MLO-Y4 and
MC3T3-E1 cells in G1 phase, but increased the number of
cells in S phase (Fig. 3A and
B). By contrast, the number of MLO-Y4 and MC3T3-E1 cells in S
phase was decreased by RGS18 knockdown (Fig. 3A and B). Furthermore, the
expression levels of cell cycle-related proteins cyclin D and
cyclin E were increased by RGS18 overexpression but
decreased by RGS18 knockdown in MLO-Y4 and MC3T3-E1 cells
(Fig. 3C and D), implying that
RGS18 contributes to osteocyte cell cycle progression.
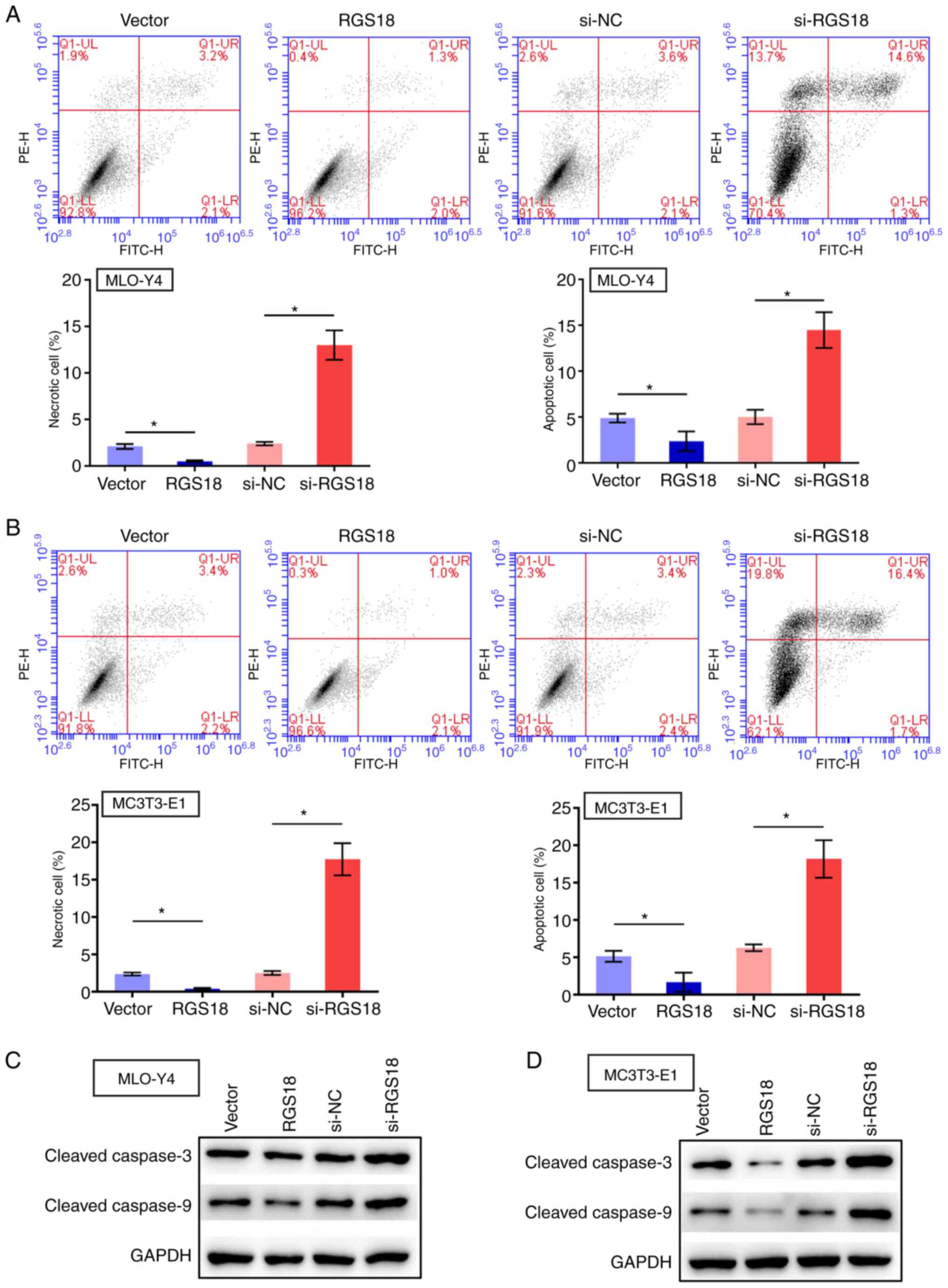
RGS18 suppresses osteocyte apoptosis
The present study also evaluated the function of
RGS18 in modulating osteocyte apoptosis (early + late) and
necrosis. Notably, apoptosis and necrosis of MLO-Y4 and MC3T3-E1
cells was suppressed by RGS18 overexpression, but enhanced
by RGS18 knockdown (Fig. 4A
and B). In addition, the expression levels of apoptosis-related
proteins cleaved caspase-3 and cleaved caspase-9 were inhibited by
RGS18 overexpression and enhanced by RGS18 knockdown
in MLO-Y4 and MC3T3-E1 cells (Fig.
4C and D), indicating that RGS18 may suppress osteocyte
apoptosis.
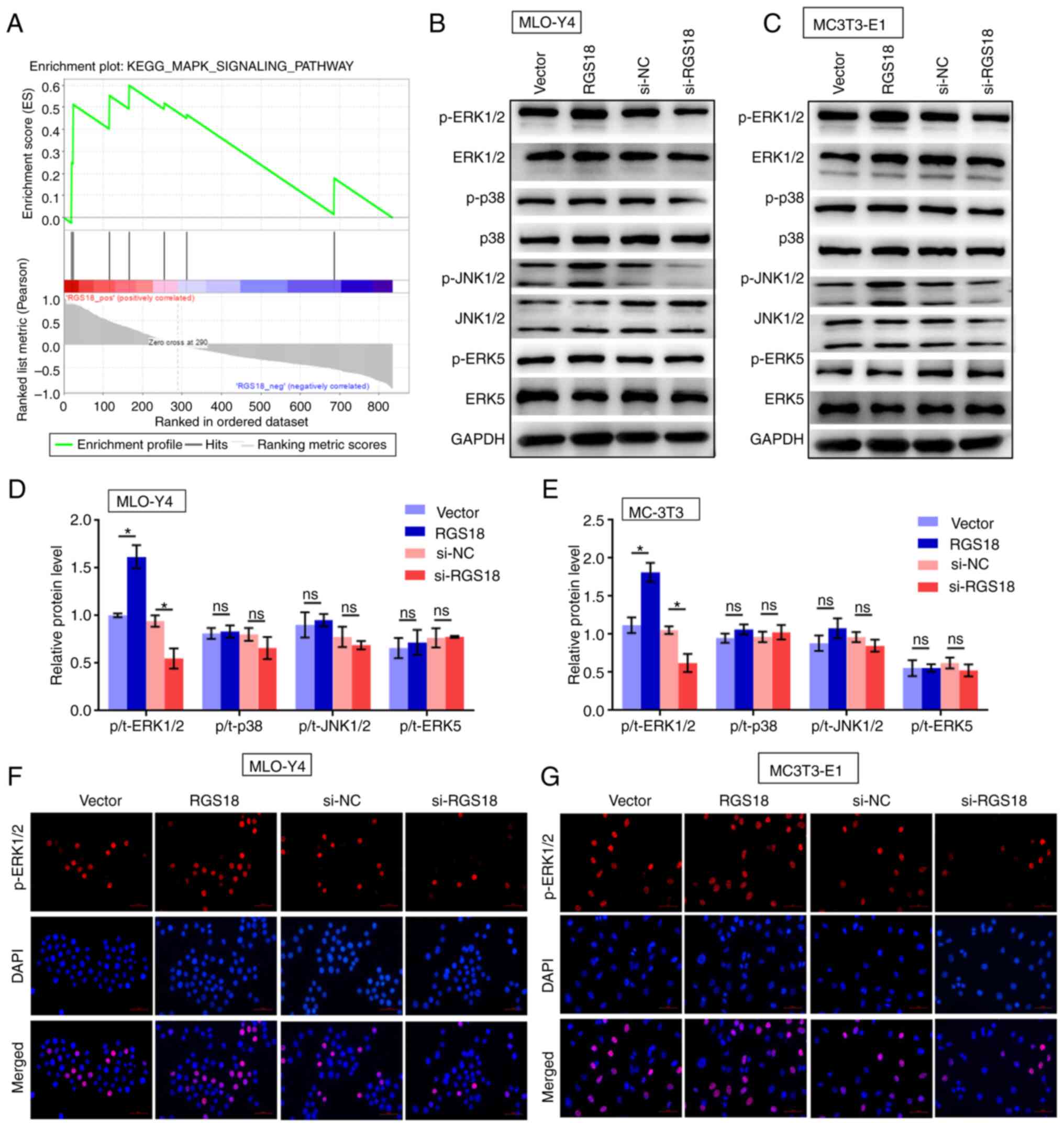
RGS18 stimulates MAPK signalling
The present study explored the potential mechanisms
underlying RGS18-mediated osteocyte function. GSEA of the GSE93138
dataset identified MAPK signalling as one of the RGS18-stimulated
signalling pathways (Fig. 5A).
Considering MAPK signalling is a critical signalling pathway that
is activated due to mechanical stimuli, and results in osteocyte
cytoskeletal changes and ECM remodelling (1), MAPK signalling was selected for
further analysis. Phosphorylation of ERK1/2, but not of p38, JNK1/2
or ERK5, was induced by RGS18 overexpression and was
inhibited by RGS18 knockdown in MLO-Y4 and MC3T3-E1 cells
(Fig. 5B-E). Immunofluorescence
analysis confirmed that the levels of p-ERK1/2 were enhanced by
RGS18 overexpression and reduced by RGS18 knockdown
in MLO-Y4 and MC3T3-E1 cells (Fig.
5F and G).
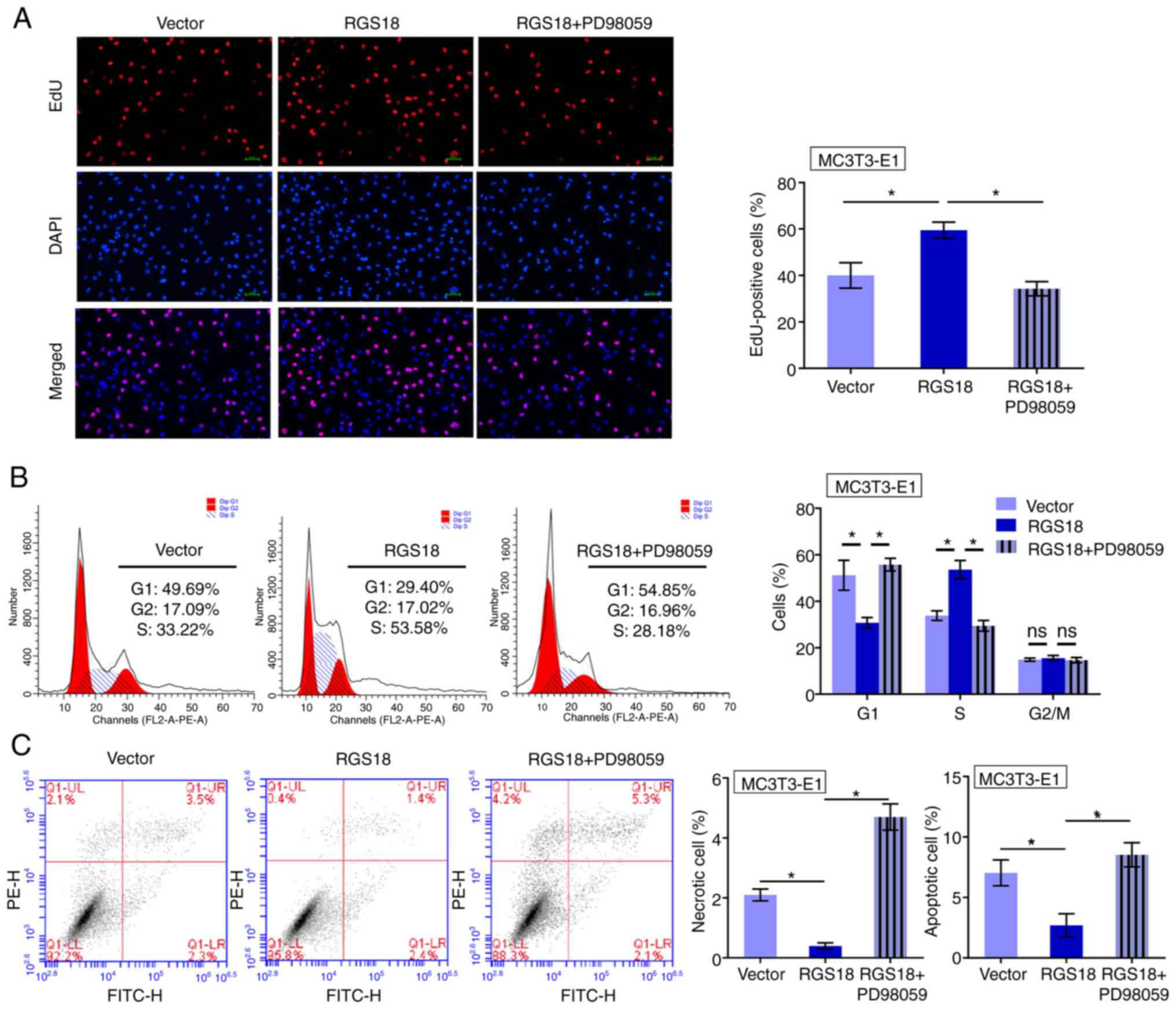
RGS18 promotes osteocyte proliferation
through ERK signalling
The present study evaluated the association between
RGS18 and ERK signalling in the modulation of osteocyte
proliferation. EdU-positive MC3T3-E1 cells were enhanced by
RGS18 overexpression, whereas treatment with the MEK1/2
inhibitor PD98059 blocked this effect (Fig. 6A). Furthermore, overexpression of
RGS18 attenuated the proportion of MC3T3-E1 cells in
G1 phase, but enhanced the number of MC3T3-E1 cells in S
phase, whereas PD98059 reversed this effect (Fig. 6B). In addition, overexpression of
RGS18 suppressed MC3T3-E1 cell apoptosis and necrosis,
whereas treatment with PD98059 reversed this effect (Fig. 6C). Collectively, these data
indicated that RGS18 contributes to osteocyte proliferation by
activating ERK signalling.
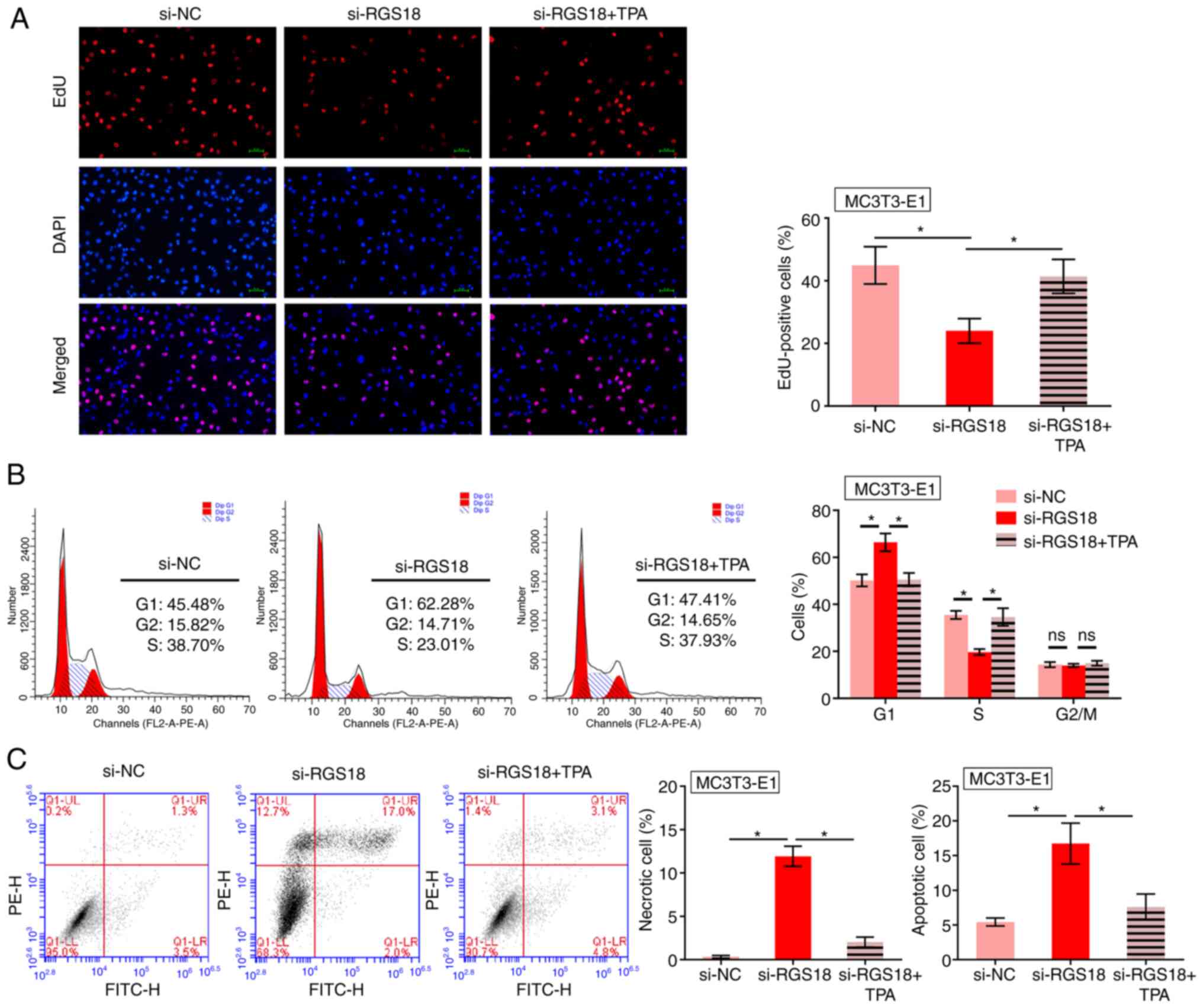
ERK activation reverses RGS18
knockdown-induced osteocyte death
The present study observed that knockdown of
RGS18 decreased the number of EdU-positive MC3T3-E1 cells,
whereas treatment with the ERK1/2 activator TPA reversed this
effect (Fig. 7A). The
distribution of MC3T3-E1 cells in G1 phase was enhanced,
but the number of MC3T3-E1 cells in S phase was reduced by
RGS18 knockdown, whereas TPA treatment reversed these
effects (Fig. 7B). In addition,
the apoptosis and necrosis of MC3T3-E1 cells was promoted by
RGS18 knockdown, whereas treatment with TPA blocked this
effect (Fig. 7C), thus
indicating that ERK activation reversed RGS18
knockdown-induced osteocyte death.
Discussion
Bone fracture healing is a complex regenerative
process involving various cells, such as chondrocytes, osteoblasts,
endothelial cells and mesenchymal stem cells, and released factors,
such as bone sialoprotein and osteocalcin (3). Osteocytes serve a crucial role in
the modulation of metabolism, remodelling and bone generation
during fracture healing. In the present study, the role of RGS18 in
the regulation of bone fractures and osteocytes was uncovered.
RGS18 is a member of the RGS family, and
participates in multiple physiological and pathological processes.
It has been reported that RGS18 inhibits platelet activation, and
induces platelet production and survival (10). RGS18 also functions as a
myeloerythroid lineage-related modulator of G protein signalling in
megakaryocytes (18). In
addition, RGS18 regulates cilia-related mechanosensory processes
(19) and serves as a negative
modulator of osteoclastogenesis by regulating NFAT signalling
(12). In the present study, it
was revealed that RGS18 was more highly expressed in samples
from AIS compared with those from HVs. Cell viability and
proliferation of osteocytes were promoted by RGS18
overexpression, but were suppressed by RGS18 knockdown.
Furthermore, overexpression of RGS18 increased the number of
S-phase osteocytes, and RGS18 knockdown resulted in the
opposite effect. Osteocyte apoptosis was suppressed by RGS18
overexpression but induced by RGS18 knockdown. These data
suggested that RGS18 contributes to osteocyte proliferation,
indicating a potential function of RGS18 in the regulation of bone
fracture healing. The present findings elucidate a novel function
of RGS18 in bone fracture healing and osteocytes. The effects of
RGS18 on bone-fracture healing should be confirmed in future in
vitro and in vivo studies.
Regarding the underlying mechanism, GSEA of the
GSE93138 dataset revealed that RGS18 could stimulate MAPK
signalling. Phosphorylation of ERK1/2 was induced by RGS18
overexpression in osteocytes. Furthermore, the present study
confirmed that treatment with the MEK1/2 inhibitor PD98059 reversed
the RGS18 overexpression-induced osteocyte proliferation and
PD98059 reversed the RGS18 overexpression-inhibited
osteocyte apoptosis. Moreover, the ERK1/2 activator TPA reversed
the RGS18 knockdown-induced suppression of osteocyte
proliferation and the RGS18 knockdown-induced osteocyte
apoptosis. These data suggested that RGS18 promotes osteocyte
proliferation by activating ERK signalling. The present findings
provide novel insights into the mechanism by which RGS18
contributes to bone fracture healing via stimulating ERK
signalling. ERK signalling participates in the regulation of
osteocyte function during bone fracture healing. It has been
reported that HMGB1 contributes to bone fracture healing in a rat
tibial fracture model by activating ERK signalling (20). High glucose levels inhibit the
expression of connexin 43, and suppress hemichannel function and
gap junctions in osteocytes by stimulating ERK signalling (21). Overexpression of Lgr5 in
mesenchymal stem cells promotes fracture healing by modulating
mitochondrial dynamics and ERK signalling (22). Furthermore, inhibition of
microRNA-21 can enhance bone fracture healing by activating
the ERK pathway (23). These
findings suggested that ERK signalling may be one of the mechanisms
by which RGS18 mediates bone fracture healing, and other potential
mechanisms should be explored to increase the understanding of
RGS18-regulated bone fracture healing.
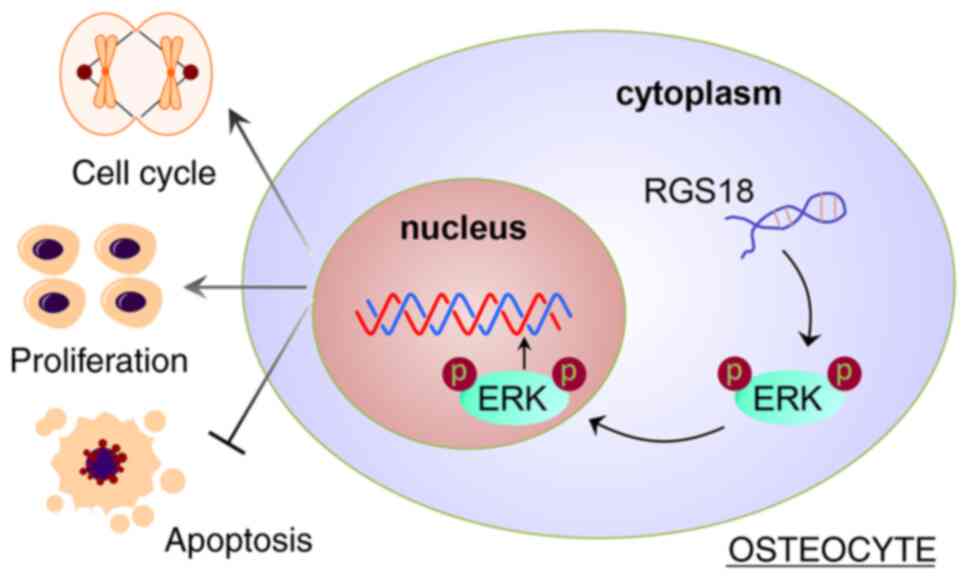
In conclusion, RGS18 may contribute to osteocyte
proliferation and bone fracture healing by activating ERK
signalling (Fig. 8). RGS18 may
be a key factor in promoting osteoblast proliferation, and this
study provides a theoretical basis for further development of
fracture healing treatments.
Availability of data and materials
The datasets used and/or analysed during the current
study are available from the corresponding author on reasonable
request. For bioinformatics analysis, the datasets generated and/or
analysed during the current study are available in the GEO database
(GSE93138: https://www.ncbi.nlm.nih.gov/geo/query/acc.cgi?acc=GSE93138
and GSE93215: https://www.ncbi.nlm.nih.gov/geo/query/acc.cgi?acc=GSE93215).
Authors' contributions
YM conceived and designed the study. YM, SQQ and QW
performed experiments. YM and JLZ performed data analysis and
interpretation. All authors confirm the authenticity of all the raw
data. All authors read and approved the final manuscript.
Ethics approval and consent to
participate
The present study follows The Declaration of
Helsinki. The protocol of this research was approved by the Ethics
Committee of The Fifth Affiliated Hospital Jinan University (ethics
approval no. 2022-10.19.1.0). All donors signed informed consent
forms.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
Acknowledgments
Not applicable.
Funding
No funding was received.
References
|
1
|
Camal Ruggieri IN, Cícero AM, Issa JPM and
Feldman S: Bone fracture healing: Perspectives according to
molecular basis. J Bone Miner Metab. 39:311–331. 2021. View Article : Google Scholar
|
|
2
|
McBrien CS Jr: Meta-bone fracture repair
via minimally invasive plate osteosynthesis. Vet Clin North Am
Small Anim Pract. 50:207–212. 2020. View Article : Google Scholar
|
|
3
|
Lin X, Patil S, Gao YG and Qian A: The
bone extracellular matrix in bone formation and regeneration. Front
Pharmacol. 11:7572020. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Andreev D, Liu M, Weidner D, Kachler K,
Faas M, Grüneboom A, Schlötzer-Schrehardt U, Muñoz LE, Steffen U,
Grötsch B, et al: Osteocyte necrosis triggers osteoclast-mediated
bone loss through macrophage-inducible C-type lectin. J Clin
Invest. 130:4811–4830. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Choy MHV, Wong RMY, Chow SKH, Li MC, Chim
YN, Li TK, Ho WT, Cheng JCY and Cheung WH: How much do we know
about the role of osteocytes in different phases of fracture
healing? A systematic review. J Orthop Translat. 21:111–121. 2020.
View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Ru JY and Wang YF: Osteocyte apoptosis:
The roles and key molecular mechanisms in resorption-related bone
diseases. Cell Death Dis. 11:8462020. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Patel AL and Shvartsman SY: Outstanding
questions in developmental ERK signaling. Development.
145:dev1438182018. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Kar R, Riquelme MA, Hua R and Jiang JX:
Glucocorticoid-Induced autophagy protects osteocytes against
oxidative stress through activation of MAPK/ERK signaling. JBMR
Plus. 3:e100772019. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Masuho I, Balaji S, Muntean BS, Skamangas
NK, Chavali S, Tesmer JJG, Babu MM and Martemyanov KA: A global map
of G protein signaling regulation by RGS proteins. Cell.
183:503–521 e519. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
DeHelian D, Gupta S, Wu J, Thorsheim C,
Estevez B, Cooper M, Litts K, Lee-Sundlov MM, Hoffmeister KM, Poncz
M, et al: RGS10 and RGS18 differentially limit platelet activation,
promote platelet production, and prolong platelet survival. Blood.
136:1773–1782. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Su C, Li H, Peng Z, Ke D, Fu H and Zheng
X: Identification of plasma RGS18 and PPBP mRNAs as potential
biomarkers for gastric cancer using transcriptome arrays. Oncol
Lett. 17:247–255. 2019.PubMed/NCBI
|
|
12
|
Iwai K, Koike M, Ohshima S, Miyatake K,
Uchiyama Y, Saeki Y and Ishii M: RGS18 acts as a negative regulator
of osteoclastogenesis by modulating the acid-sensing OGR1/NFAT
signaling pathway. J Bone Miner Res. 22:1612–1620. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
McKinley TO, Lisboa FA, Horan AD, Gaski GE
and Mehta S: Precision medicine applications to manage multiply
injured patients with orthopaedic trauma. J Orthop Trauma. 33(Suppl
6): S25–S29. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Ritchie ME, Phipson B, Wu D, Hu Y, Law CW,
Shi W and Smyth GK: limma powers differential expression analyses
for RNA-sequencing and microarray studies. Nucleic Acids Res.
43:e472015. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Subramanian A, Tamayo P, Mootha VK,
Mukherjee S, Ebert BL, Gillette MA, Paulovich A, Pomeroy SL, Golub
TR, Lander ES and Mesirov JP: Gene set enrichment analysis: A
knowledge-based approach for interpreting genome-wide expression
profiles. Proc Natl Acad Sci USA. 102:15545–15550. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Mootha VK, Lindgren CM, Eriksson KF,
Subramanian A, Sihag S, Lehar J, Puigserver P, Carlsson E,
Ridderstråle M, Laurila E, et al: PGC-1alpha-responsive genes
involved in oxidative phosphorylation are coordinately
downregulated in human diabetes. Nat Genet. 34:267–273. 2003.
View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(-Delta Delta C(T)) method. Methods. 25:402–408. 2001.
View Article : Google Scholar
|
|
18
|
Yowe D, Weich N, Prabhudas M, Poisson L,
Errada P, Kapeller R, Yu K, Faron L, Shen M, Cleary J, et al: RGS18
is a myeloerythroid lineage-specific regulator of
G-protein-signalling molecule highly expressed in megakaryocytes.
Biochem J. 359:109–118. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Louwette S, Labarque V, Wittevrongel C,
Thys C, Metz J, Gijsbers R, Debyser Z, Arnout J, Van Geet C and
Freson K: Regulator of G-protein signaling 18 controls
megakaryopoiesis and the cilia-mediated vertebrate mechanosensory
system. FASEB J. 26:2125–2136. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Chen MQ and Luan JJ: HMGB1 promotes bone
fracture healing through activation of ERK signaling pathway in a
rat tibial fracture model. Kaohsiung J Med Sci. 35:550–558. 2019.
View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Yang L, Zhou G, Li M, Li Y, Yang L, Fu Q
and Tian Y: High glucose downregulates connexin 43 Expression and
its gap junction and hemichannel function in osteocyte-like MLO-Y4
cells through activation of the p38MAPK/ERK signal pathway.
Diabetes Metab Syndr Obes. 13:545–557. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Lin W, Xu L, Pan Q, Lin S, Feng L, Wang B,
Chen S, Li Y, Wang H, Li Y, et al: Lgr5-overexpressing mesenchymal
stem cells augment fracture healing through regulation of Wnt/ERK
signaling pathways and mitochondrial dynamics. FASEB J.
33:8565–8577. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Sheng J, Liang WD, Xun CH, Xu T, Zhang J
and Sheng WB: Downregulation of miR-21 promotes tibial fracture
healing in rabbits through activating ERK pathway. Eur Rev Med
Pharmacol Sci. 23:10204–10210. 2019.PubMed/NCBI
|