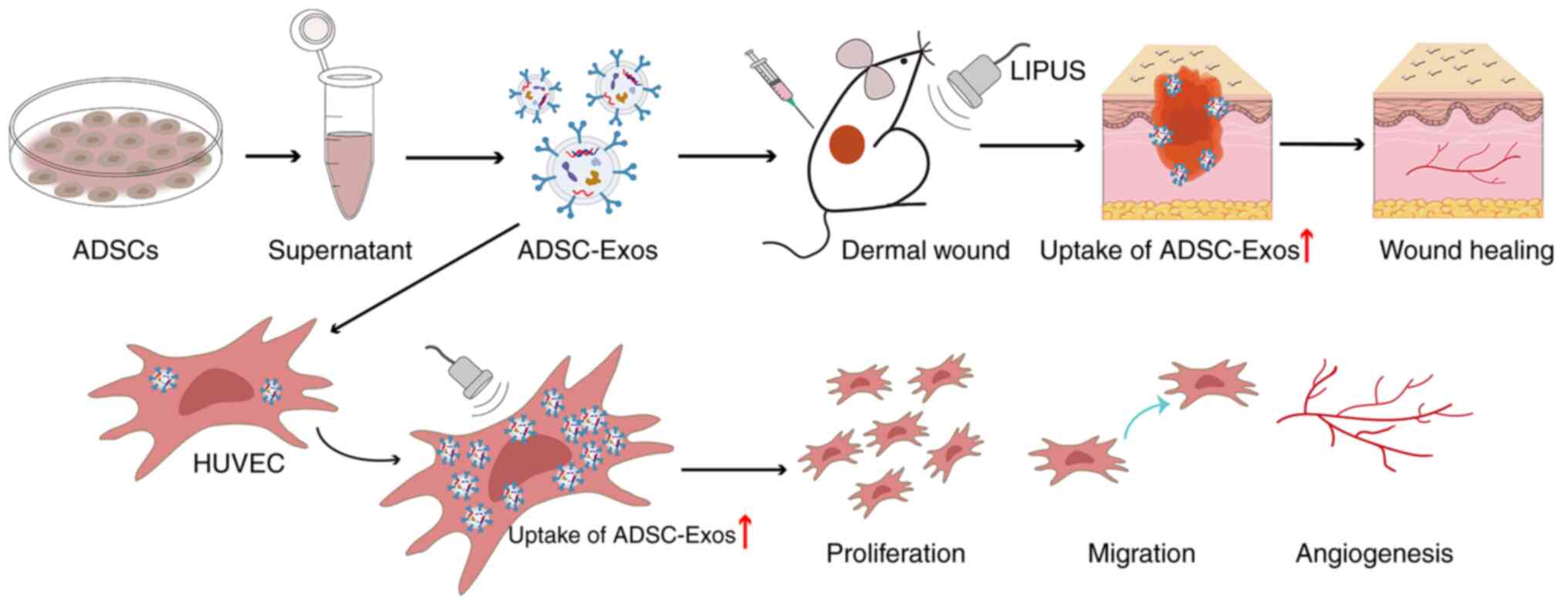
Introduction
Chronic diabetic wounds are a global healthcare
challenge. It is estimated that impaired healing of diabetic wounds
affects ~20% of all patients with diabetes mellitus worldwide
(1). Diabetic foot ulcers (DFUs)
represent the most severe form of diabetic wounds, and have a
number of serious complications, including amputation, poor quality
of life and life-threatening infections (2). Diabetic wounds often take a long
time to heal and may recur after healing. These wounds consume a
significant amount of medical resources (3) and globally, 30% of diabetes-related
treatment costs are spent on DFUs (4). These consequences have serious
public health and clinical implications such as poor prognosis and
dissatisfaction with clinical outcomes (5). It is estimated that 15% of diabetic
patients in the USA will suffer from DFUs in their lifetime
(6). Due to 9.4% of the global
population suffering from diabetes, the number of individuals
receiving DFU treatment may be vast (6). Chronic diabetic wounds cause great
pain for patients and increase the financial burden on the families
of the patient.
Debridement is the main clinical treatment for DFUs
(2). For smaller wounds,
debridement can clear the necrotic tissue, close the wound early
and avoid serious clinical outcomes, such as amputation. However,
due to vascular insufficiency, peripheral neuropathy,
immunosuppression and critical colonization/infection, it is
difficult for debridement to achieve the desired effect (6). Normal wound healing involves the
following four phases: Hemostasis/coagulation, inflammatory,
proliferative and maturation/remodeling phase (7). In diabetes, these physiological
processes are disturbed (8). The
mechanisms that affect diabetic wound healing are complicated as
hyperglycemia seriously hinders the physiological process of normal
wound healing, and impaired angiogenesis is one of the key factors
(9-11). Stem cell-based angiogenesis is an
appealing approach for the treatment of chronic non-healing wounds.
It has been reported that stem cells can enhance wound healing and
tissue regeneration through the regulation of immune responses and
promotion of angiogenesis (12,13). However, due to the low survival
rate of stem cells in diabetic wounds, it is difficult to achieve
satisfactory results.
A growing body of evidence has demonstrated the
therapeutic potential of exosomes derived from adipose-derived stem
cells (ADSC-Exos) for repairing diabetic wounds (14-16). Studies have also shown that
ADSC-Exos have similar therapeutic functions to ADSCs, both of
which can effectively promote the formation of new blood vessels
and restore skin morphology and function (17,18). Compared with ADSC therapy,
ADSC-Exos have certain unique advantages, including higher
stability, better storage and minimal immune rejection (19). Thus, the use of exosomes may be a
promising approach for developing a cell-free alternative to stem
cell therapy. However, due to the low yield of exosomes and their
relatively short half-life in vivo, the clinical
applications of ADSC-Exos still face challenges (20). It is difficult to significantly
increase the yield of ADSC-Exos under the current established
protocols. The number of exosomes produced by exocytosis in the
physiological state of cells is limited, while the contents and
functions of exosomes produced by molecular interference remain to
be determined. However, improving the utilization efficiency of
ADSC-Exos is currently a more realistic approach in improving
efficacy. Increasing exosome uptake by target cells through
external stimulation before metabolic clearance may be an effective
method in improving the utilization efficiency of ADSC-Exos.
Low-intensity pulsed ultrasound (LIPUS) is a type of
specific physical energy that is delivered at a low intensity
(<3 W/cm2) and outputs in the mode of pulsed waves.
LIPUS has minimal thermal effects while maintaining the
transmission of acoustic energy to the target tissue. LIPUS
provides a non-invasive localized mechanical stimulus to cells and
has significant biological effects such as promoting intracellular
signal transduction, enhancing cell proliferation and inhibiting
autophagy (21). In the last 10
years, LIPUS stimulation has emerged as a promising technique in
many therapeutic applications, such as the healing of fresh
fractures, soft tissue regeneration and nerve regulation (22-24). Certain studies have indicated
that LIPUS may participate in cell proliferation, differentiation
and apoptosis by regulating intracellular signals (25-27). However, whether LIPUS plays a
significant role in promoting the uptake of exosomes by target
cells and thus improving the utilization efficiency of exosomes
remains unknown.
In the present study, we hypothesized that LIPUS
could effectively enhance the uptake of ADSC-Exos in vitro
and in vivo, thus markedly improving angiogenesis and
promoting diabetic wound healing (a schematic illustration of this
is shown in Fig. 1). The aim of
the present study was to obtain preliminary evidence of a link
between LIPUS and exosome uptake and to explore how LIPUS enhances
therapeutic angiogenesis of stem cell-derived exosomes to repair
diabetic wounds.
Materials and methods
Isolation and identification of
ADSCs
ADSCs were isolated from the inguinal fat pads of
male SD rats as previously described (28). Briefly, 10 rats were raised in an
SPF environment (temperature, 27°C; humidity, 60-70%; noise, <60
dB) until they were 5 weeks old (110.0±3.0 g). Then, these rats
were euthanized by cervical dislocation, bilateral groin adipose
tissue was obtained under sterile conditions and other tissue such
as blood vessels and blood clots were removed. The adipose tissue
was crushed with scissors and digested at 37°C and 120 rpm/min for
60 min in a constant temperature shaking table. The sample was then
centrifuged at 285 × g at 4°C for 10 min and the supernatant
discarded. The cells were re-suspended and maintained in Dulbecco's
modified Eagle's medium (HyClone; Cytiva) supplemented with 10%
fetal bovine serum (FBS; HyClone; Cytiva) and incubated at 37°C in
a humidified atmosphere with 5% CO2.
ADSCs in the third passage were used for cell
identification. For this, cell morphology was observed with a light
microscope (Olympus Corporation). To identify the multilineage
differentiation potential of ADSCs, osteogenic and adipogenic
differentiation medium kits (Cyagen Biosciences, Inc.) were used
for cell culture and staining, according to the manufacturer's
instructions. Surface antigen expression of ADSCs were also
analyzed by flow cytometry. Briefly, 3×105 cells were
harvested following treatment with 0.25% trypsin-EDTA and washing
twice with PBS. The cells were then incubated for 30 min at 4°C
with a specific monoclonal antibody conjugated to either
fluorescein isothiocyanate or phycoerythrin in 200 μl PBS
for 30 min in the dark at 4°C. Flow cytometry (FACS Calibur; BD
Biosciences) was then performed to determine the expression of
specific cell surface antigens, and FlowJo 10.8.1 (FlowJo LLC)
software was used for analysis. Antibodies specific for CD29 (cat.
no. A14886; eBioscience; Thermo Fisher Scientific, Inc.), CD34 and
CD90 (cat. nos. MA5-17832 and A14726; eBioscience; Thermo Fisher
Scientific, Inc.) were used for the identification of ADSCs.
Extraction and characterization of
ADSC-Exos
ADSCs in the third passage were used for exosome
extraction. Upon reaching 80-90% confluency, the ADSCs were washed
with PBS and cultured in fresh serum-free medium before exosome
extraction. After 48 h of culture, the supernatants were collected.
ADSC-Exos were extracted by differential ultracentrifugation as
described previously, with some modifications (29). Briefly, the supernatant was
centrifuged at 4,000 × g for 15 min to remove the cells and then
centrifuged at 10,000 × g for 30 min to remove the cell debris.
Exosomes were pelleted by ultracentrifugation at 100,000 × g for 70
min at 4°C to eliminate contaminating proteins. The obtained
ADSC-Exos were resuspended in 1X PBS and stored at -80°C for future
use.
The morphology of the ADSC-Exos was verified by
transmission electron microscopy (TEM; HT7700; Hitachi, Ltd.). For
negative staining, the samples were incubated with 2%
phosphotungstic acid solution for 10 min at room temperature.
Nanoparticle tracking analysis was performed at room temperature
using a ZetaView PMX 110 (Particle Metrix GmbH) to determine the
size distribution and concentration of the exosomes in each sample.
Each sample was diluted to 1×105/ml with PBS, and all
experiments were repeated three times. The expression levels of the
exosomal markers, CD9 and tumor susceptibility gene 101 (Tsg101)
(1:1,000; cat. nos. 20597-1-AP and 28283-1-AP, respectively;
Proteintech, Group, Inc.), were analyzed by western blotting.
Calnexin (1:1,000; 10427-2-AP; Proteintech, Group, Inc.), a
specific protein of the cellar endoplasmic reticulum, was used as a
negative control. After the samples were lysed using RIPA (Biosharp
Life Sciences) buffer (RIPA, PMSF, phosphatase inhibitor, protease
inhibitor and EDTA were formulated at a ratio of 100:1:2:2:2), the
protein concentration of the supernatant was determined by the BCA
method. Protein samples (30 μg per lane) were separated by
SDS-PAGE under reducing conditions on Novex Bis-Tris gels (4-20%
acrylamide) and then transferred to PVDF membranes. The membranes
were then blocked using 5% skimmed milk powder sealing solution
(room temperature for 2 h) and subsequently incubated with the
primary antibody overnight at 4°C. The blots were incubated with
HRP-labeled goat anti-mouse IgG (1:5,000; cat no. SA00001-1;
Proteintech, Group, Inc.) at room temperature for 1 h, then
developed with enhanced chemiluminescence reagents (Pierce; Thermo
Fisher Scientific, Inc.) and exposed using chemiluminescence
apparatus (ChemiDoc Touch; Bio-Rad Laboratories, Inc.).
Construction of a skin wound model in
diabetic mice
The mice were raised in an SPF environment
(temperature, 27°C; humidity, 60-70%; noise, <60 Db; water and
food were provided by the animal testing center in a standard
manner). An animal model of full-thickness skin defects in diabetic
mice was established to investigate the treatment effects of LIPUS
combined with ADSC-Exos. Male C57BL/6 mice (4 weeks old, weighing
12-16 g) were used in this experiment. The mice were fed a high-fat
diet for 2 weeks and then intraperitoneally injected with
streptozotocin (STZ; Sigma-Aldrich; Merck KGaA) at a dose of 50
mg/kg. Blood samples from the caudal vein were collected from the
mice every 2 days. For this, after immobilizing the mouse in a
fixed box, the tail was wiped, and a blood collection needle was
inserted into the tip of the tail. The tail was then pinched to
drip 10 μl blood onto the test paper, and glucose levels
were measured using a glucometer (Roche Diagnostics, Ltd.). Blood
glucose levels >16.7 mM that lasted for >1 week were
considered to indicate the successful establishment of diabetes,
and these animals were eligible for subsequent experiments. A total
of 55 mice were used in this study, 48 of which developed diabetes.
The remaining 7 mice were euthanized by CO2 (30% volume
displacement every min). For wound generation, isoflurane was used
to anesthetize mice at a concentration of 2-3% to induce anesthesia
and 1.5-2% to maintain anesthesia. After shaving the hair and
disinfecting the skin, a 1.0 cm diameter full-thickness wound was
generated on the upper backs of the mice with surgical scissors,
and a sterile alginate gel dressing was used to cover the entire
wound. The humane endpoints followed in the present study included
the development of any serious infections during treatment.
Fortunately, no mice reached this humane endpoint and were
therefore not euthanized before the end of the study.
LIPUS combined with ADSC-Exos
treatment
A total of 48 mice were successfully used to
construct a diabetic wound model and were randomly divided into
four groups (n=12/group). All the mice in the 4 groups received one
round of treatment immediately after wound establishment. Each
group received the following treatments: i) The control group,
subcutaneous injection of 100 μl PBS at four points (25
μl at each point) around the wound on day 0 after wound
establishment; ii) the LIPUS group, treated with PBS as
aforementioned in combination with LIPUS irradiation; iii) the
ADSC-Exos group, treated via subcutaneous injection of 100
μl ADSC-Exo suspension (each 100 μl injection mixture
contained 70 μg ADSC-Exos) at four points (25 μl at
each point) around the wound on day 0 after wound establishment;
and iv) the LIPUS + ADSC-Exos group, treated with ADSC-Exo
suspension as aforementioned in combination with LIPUS
irradiation.
A multichannel ultrasound irradiation machine
(RS232; Ultrasonic Research Institute of Chongqing Medical
University, Chongqing, China) was used for LIPUS irradiation. All
treatments were performed in a sterile environment. A sterile
alginate gel dressing was used to cover the entire wound and the
probe, and a layer of sterile coupling gel was applied between the
transparent dressing on the wound surface and the transducer to
ensure optimal ultrasound exposure. The transducer was fixed, and
the distance between the transducer and the wound surface was
maintained at ~3 mm in all experiments. The central frequency of
the transducer was 1.5 MHz, and the output form was a plane and
pulse wave. The mice were treated with LIPUS at an average
intensity of 300 mW/cm2, a pulse repetition rate of 1
kHz and a duty cycle of 20% for 30 min.
Wound healing evaluation
Images of the wounds were captured using a digital
camera (Canon, Inc.) on days 0, 3, 7, 10 and 14 post-operation.
These time points were chosen based on the procedure of wound
healing, and the extent of wound healing may reflect the duration
of each healing stage (30).
ImageJ software (V1.8.0; National Institutes of Health) was used to
measure the area of the wound. The wound healing rate was
determined as follows: Wound closure rate (%)=(A0-At)/A0 ×100%,
where A0 represents the wound area at day 0, and At represents the
wound area at days 3, 7, 10 or 14.
Histology analysis
All mice of the four groups were anesthetized using
isoflurane (2-3% to induce anesthesia and 1.5-2% to maintain
anesthesia) at day 14 post-operation, and wound tissues were
harvested for histological analysis. The mice were then euthanized
using CO2 (injected into the box at a rate of 30% of the
volume every min; the maximum flow rate did not exceed 0.5 kPa).
The mice were considered dead when they maintained respiratory
arrest, no motion, and pupil dilation for >5 min.
Wound samples were fixed in 4% paraformaldehyde at
room temperature overnight and then dehydrated and embedded in
paraffin. The samples were cut into 5-μm thick sections and
mounted on slides for staining. Hematoxylin and eosin (H&E)
staining (Beyotime Institute of Biotechnology) and Masson trichrome
staining (Beyotime Institute of Biotechnology) were performed
according to the manufacturers' instructions. The length of the
scar and maturity of collagen were observed under a light
microscope (BX21; Olympus Corporation).
Small animal Doppler evaluation of blood
perfusion
A small animal Doppler ultrasound was used in the
present study to evaluate the blood perfusion of diabetic wounds
after treatment. On day 14 post-operation, the mice were
anesthetized using isoflurane (2-3% to induce anesthesia and 1.5-2%
to maintain anesthesia) and shaved. A laser speckle contrast
imaging system (FLPI-2; Moor Instruments, Ltd.) was used to image
the wounds at a constant distance and with the same dimensions to
determine the area. The obtained data were then analyzed using
MoorFLPI-2 Review V5.0 software (Moor Instruments, Ltd.). The mean
perfusion unit (MPU) index was used to evaluate the local blood
perfusion around the wounds in the different groups, and the data
are presented as the ratio of the MPU of the wound area (ROI-1) to
the MPU of the area surrounding the wound (ROI-2).
Immunofluorescence analysis of
angiogenesis
To further evaluate angiogenesis in the wound area,
immunofluorescence staining of the wound sections for CD31 (for
endothelial cells; 1:1,000; cat. no. GB12063-100; Wuhan Servicebio
Technology Co., Ltd.) and α-smooth muscle actin (α-SMA; 1:1,000;
cat. no. GB12063-100; Wuhan Servicebio Technology Co., Ltd.), was
performed on day 14. Briefly, the sections were deparaffinized and
rehydrated, blocked with 5% bovine serum albumin (Beyotime
Institute of Biotechnology) at room temperature for 1 h and
incubated with primary antibodies overnight at 4°C. Then, the
slides were treated with Alexa Fluor 647 or Alexa Fluor 488
secondary antibodies (1:5,000; cat. nos. ab150115 and ab150117;
Abcam) at room temperature for 2 h, and the nuclei were stained
with 4,6-diamidino-2-phenylindole (DAPI; Beyotime Institute of
Biotechnology) at room temperature and observed immediately. All
slides were then observed under a fluorescent microscope (BX21;
Olympus Corporation), and the number of newly formed and mature
vessels was calculated in five random fields per section between
wound edges using ImageJ software.
ADSC-Exos uptake in vivo
To evaluate the effect of LIPUS irradiation on
ADSC-Exos metabolism, full-thickness wounds on 10 diabetic mice (in
addition to the 48 mice described above) were generated as
aforementioned. ADSC-Exos were labeled using DiR (Shanghai Yuanye
Biotechnology Co., Ltd.) and subcutaneously injected at four points
around the wound as aforementioned. In total, 5 mice were
irradiated immediately after injection and the remaining 5 mice
were covered with sterile dressings and returned to the cage. The
distribution of exosome fluorescence in vivo was observed
using a small animal imaging system (PerkinElmer, Inc.) at 1, 2, 6,
24 and 36 h after injection. The fluorescence signal intensity was
analyzed using Living Image software (version 4.4; PerkinElmer,
Inc.).
To further evaluate the uptake of exosomes in the
targeted area, an additional 10 diabetic mice were treated as
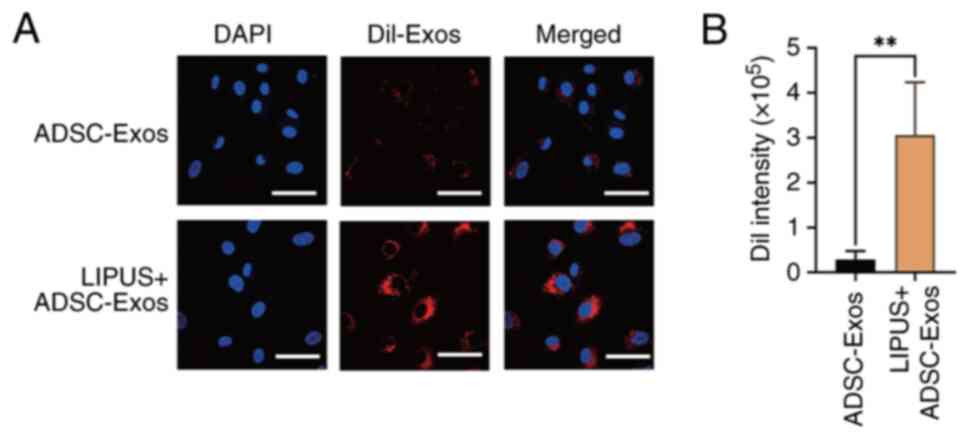
aforementioned, except using DiI-labeled ADSC-Exos. The wound and
surrounding skin tissues were harvested for frozen section
preparation and examination 48 h after DiI-labeled ADSC-Exos
subcutaneous injection and LIPUS treatment, following euthanasia as
aforementioned. After the preparation of frozen sections
(8-μm) in liquid nitrogen, the nuclei were stained with DAPI
at room temperature, and fluorescence was observed immediately by
laser scanning confocal microscopy (FV1200; Olympus Corporation).
ImageJ software was used for analysis.
ADSC-Exos uptake in vitro
To further verify whether LIPUS can promote exosome
uptake in vitro, immortalized human umbilical vein
endothelial cells (HUVECs; cat. no. CL-191 h; Wuhan Saios
Biotechnology Co., Ltd.) were divided into the four following
groups: The control, LIPUS, ADSC-Exos and LIPUS + ADSC-Exos groups.
The LIPUS and ADSC-Exo groups were treated with LIPUS or ADSC-Exos
alone. The LIPUS + ADSC-Exos group was treated with 10 μl
exosomes (100 μg/ml) combined with LIPUS irradiation. The
aforementioned multichannel ultrasound irradiation machine was used
for LIPUS irradiation. The ultrasonic transducer was placed under
the cell culture plate and a coupling agent was used to ensure the
probe fully contacted the plate. The central frequency of the
transducer was 1.5 MHz, and the output form was a plane wave and
pulse wave. HUVECs were treated with LIPUS at an average intensity
of 30 mW/cm2, a pulse repetition rate of 1 kHz and a
duty cycle of 20% for 30 min.
The ADSC-Exos were labeled using a DiI fluorescent
labeling kit (Invitrogen; Thermo Fisher Scientific, Inc.). Briefly,
ADSC-Exos were incubated with 5 μM DiI dye at 37°C for 15
min in the dark and then centrifuged at 12,000 × g for 15 min to
remove free DiI dye. The supernatant was removed, and the labeled
ADSC-Exos were resuspended in PBS before use. Subsequently, the
HUVECs were incubated with DiI-labeled ADSC-Exos at a dose of 5
μg/ml for 6 h. After removing the supernatant and washing
with PBS, the samples were fixed with 4% paraformaldehyde at room
temperature for 15 min and washed with PBS three times. The fixed
samples were stained with 10 μg/ml DAPI (Invitrogen; Thermo
Fisher Scientific, Inc.) at room temperature. After washing with
PBS three times, the uptake of ADSC-Exos by HUVECs was observed
using a fluorescent microscope (Olympus Corporation). The DiI
fluorescence intensity in the HUVECs was quantified using ImageJ
software.
Angiogenesis in vitro
To investigate the effect of LIPUS on angiogenesis
after the cellular uptake of ADSC-Exos, Cell Counting Kit-8
(CCK-8), scratch wound healing and tube formation assays were
conducted.
The CCK-8 (Dojindo Laboratories, Inc.) assay was
conducted to analyze cell viability, following the manufacturer's
protocol. HUVECs were seeded into 96-well plates (5×103
cells/well in 100 μl culture medium) and treated as
aforementioned. After incubation of the HUVECs at 37°C for 24 h, 10
μl of CCK-8 was added to each well. After a further
incubation at 37°C for 4 h, the absorbance was measured at 450 nm
using an automatic microplate reader (PerkinElmer, Inc.). All
experiments were repeated three times.
A scratch wound healing assay was conducted to
assess the migration capability of HUVECs. Briefly,
5×105 HUVECs were seeded into a 6-well cell culture
plate with culture medium supplemented with 10% FBS. When the cell
confluency reached 90%, the cell monolayer was scraped in a
straight line with a 200-μl pipette tip. The cells were
treated as aforementioned and cultured for 24 h in FBS-free medium.
Images of five fields of view were collected at 0 and 24 h using an
inverted microscope (Olympus Corporation), and the decrease in the
wound area was quantified using ImageJ software.
A tube formation assay was conducted to assess the
proangiogenic effects of the different treatments. HUVECs
(5×105 cells) were seeded into Matrigel-coated 6-well
cell culture plates with FBS-free medium and treated as
aforementioned. After incubation for 4 h in 5% CO2 at
37°C, the total number of branching points and the total tube
length of the cells were observed using an inverted microscope
(Olympus Corporation). ImageJ software was used to analyze the
images and count the tubes.
Statistical analysis
All experiments were performed in triplicate. The
data are presented as the mean ± standard deviation. Differences
between two groups were analyzed by unpaired Student's t-tests and
differences between multiple groups were analyzed by one-way ANOVA,
followed by Tukey's post hoc test for pairwise comparisons.
Statistical analysis was conducted using SPSS software (version 26;
IBM Corp.). P<0.05 was considered to indicate a statistically
significant difference.
Results
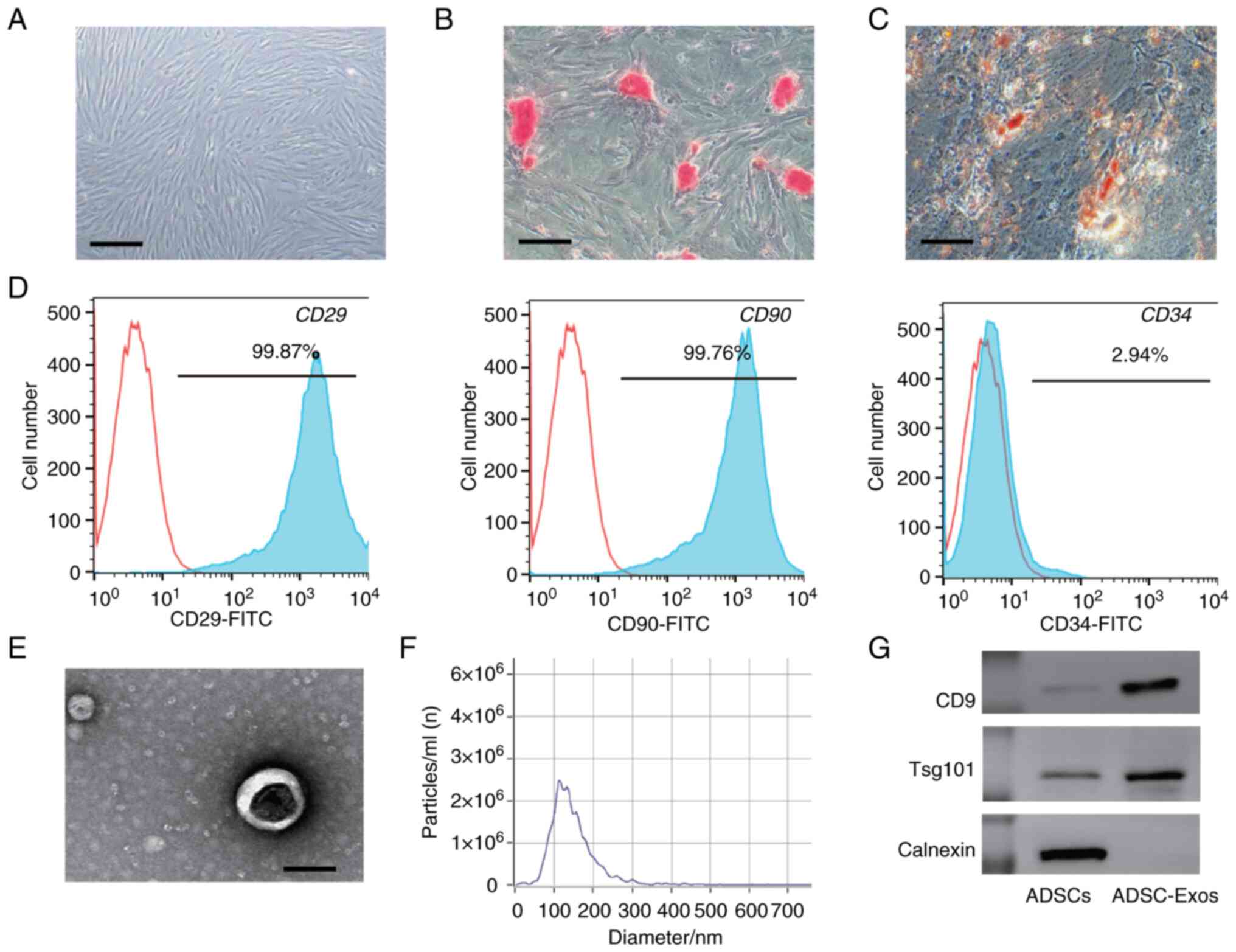
Characterization of ADSC-Exos
The third-passage ADSCs exhibited a spindle-like
morphology, as observed under a light microscope (Fig. 2A). The osteogenic and adipogenic
differentiation ability of the ADSCs (assessed using osteogenic and
adipogenic differentiation medium kits) confirmed the multilineage
differentiation potential of the cell line (Fig. 2B and C). The flow cytometry
analysis of surface marker expression is shown in Fig. 2D. The ADSCs were positive for the
mesenchymal stem cell (MSC) markers, CD29 (99.87%) and CD90
(99.76%), but negative for the hematopoietic stem cell marker, CD34
(2.94%).
ADSC-Exos were identified by TEM and western
blotting analysis. According to the TEM images, the ADSC-Exos
exhibited a spherical or cup-shaped structure (Fig. 2E). Particle size measurements
indicated that the median size of the ADSC-Exos was 133 nm
(Fig. 2F). Western blotting
analysis demonstrated that the ADSC-Exos were positive for the
specific exosome surface markers, CD9 and Tsg 101 (Fig. 2G). Collectively, these results
indicated that ADSC-Exos had been successfully harvested.
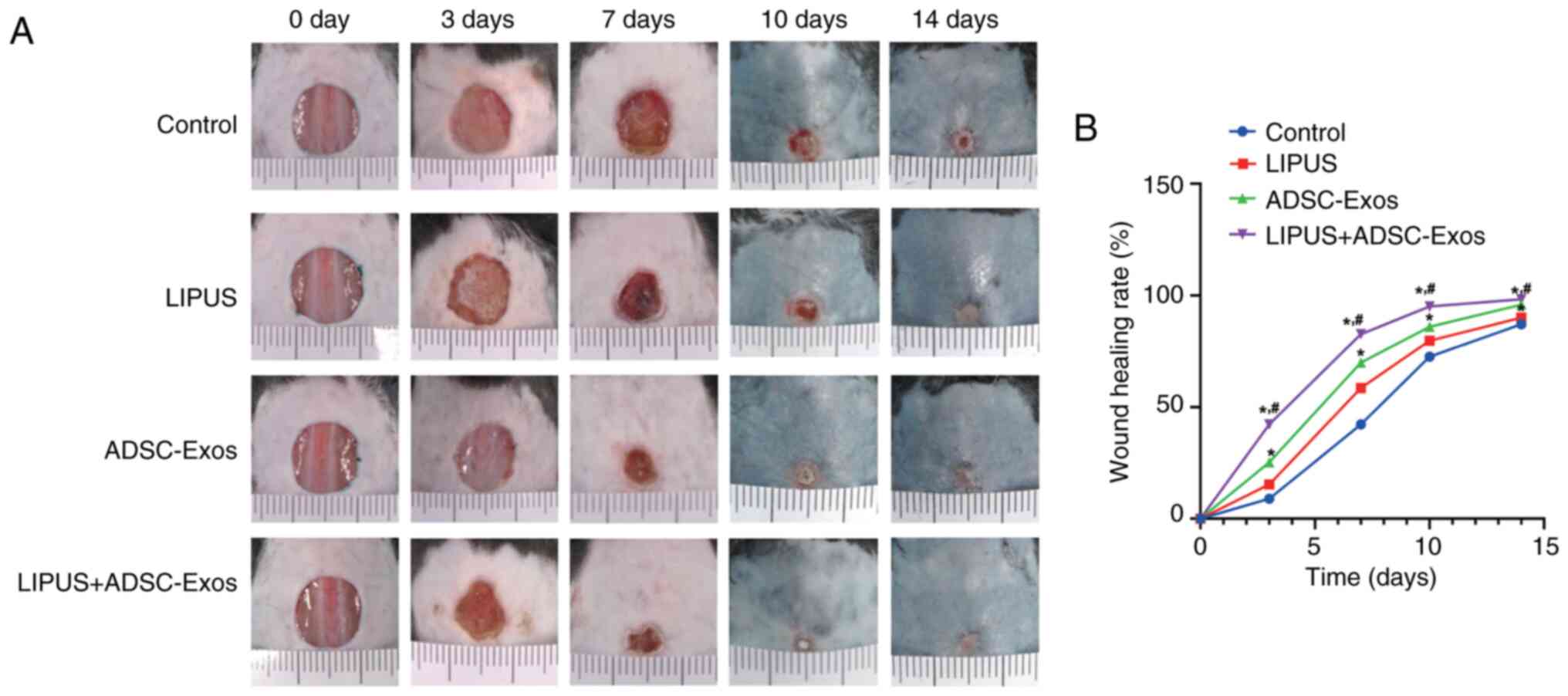
LIPUS combined with ADSC-Exos treatment
accelerates diabetic wound healing
Representative gross observation images of the
diabetic wound healing process at different time points are
displayed in Fig. 3A. From day
10, the mice developed black hairs around the wound area, which
could not be removed, so the skin appeared dark blue. The wounds
treated with LIPUS + ADSC-Exos healed faster than those in the
other groups throughout the entire healing duration. LIPUS, as well
as ADSC-Exo, only treatment also demonstrated faster healing
compared with the diabetic control. On the third day after
treatment, the wound area in the LIPUS + ADSC-Exos group was
significantly reduced, while those in the control and LIPUS groups
were only slightly changed (P<0.05; Fig. 3B). At day 10, only the LIPUS +
ADSC-Exo-treated animals achieved complete wound closure (wound
closure rate >95%). On day 14, the wounds in all groups except
the control group were covered with neo-epidermis. However, the
wound surface of the LIPUS + ADSC-Exo-treated animals was the
flattest. Further quantitative analysis also confirmed that the
wound healing rates at days 3, 7, 10 and 14 were significantly
higher in the LIPUS + ADSC-Exos group compared with the control
group, followed by the rates in the ADSC-Exos group and the LIPUS
group (Fig. 3B).
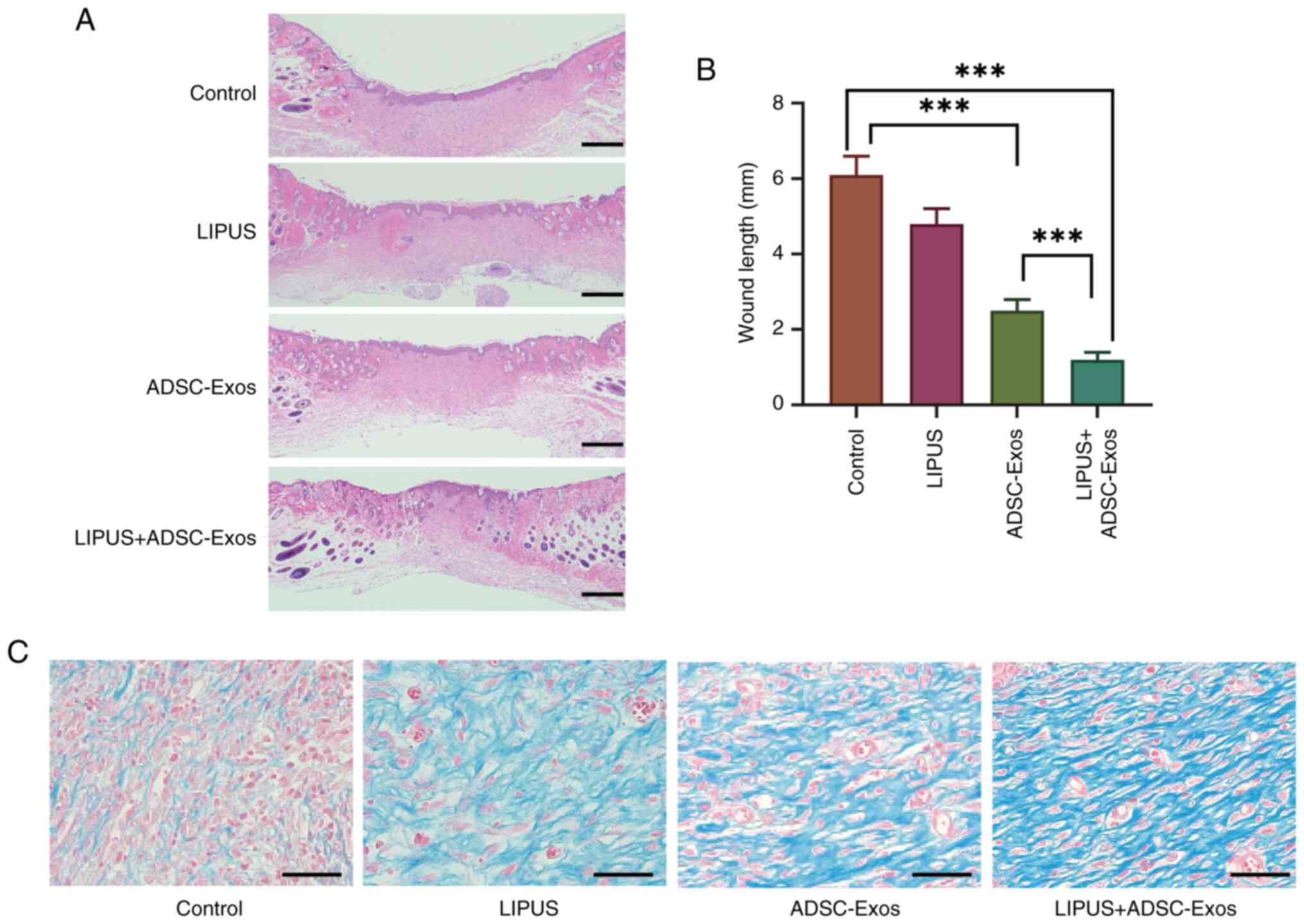
The results of H&E staining of the wound samples
demonstrated that the thickness, differentiation status of the
neo-epidermis and length of the wound area in the LIPUS + ADSC-Exos
group were significantly shorter than those in the control and
ADSC-Exo groups at day 14 (Fig. 4A
and B).
Masson's staining was used to reveal collagen
deposition and remodeling in the diabetic wounds following
different treatments (Fig. 4C).
The amount of collagen in all three treatment groups was notably
greater than in the control group at day 14, and the amount of
collagen fiber was the highest in the LIPUS + ADSC-Exos group.
Moreover, the collagen fibers in the LIPUS + ADSC-Exos group had
more organized structures with higher fiber density and greater
blood vessel formation compared with the LIPUS and ADSC-Exos
groups. Collectively, these results suggested that LIPUS +
ADSC-Exos accelerated collagen deposition and remodeling in the
diabetic wound healing process.
LIPUS combined with ADSC-exos treatment
increases angiogenesis in diabetic wounds
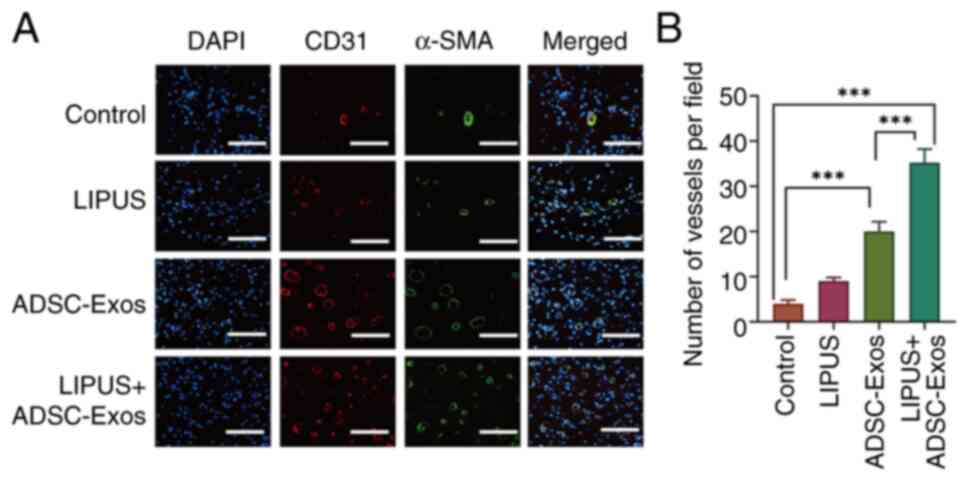
CD31 and α-SMA immunofluorescence staining was
performed to evaluate the newly formed and relatively mature blood
vessels on day 14. Mature blood vessels provide a continuous and
stable supply of blood to tissues and are composed of vascular
endothelial cells and smooth muscle cells. Therefore, the staining
of vascular endothelial cells (against CD31) and smooth muscle
cells (against α-SMA) was used to judge the number of mature
vessels in the present study. As shown in Fig. 5A, little positive staining was
observed in the control group. The LIPUS + ADSC-Exos group
exhibited the highest expression of CD31 and α-SMA, followed by the
ADSC-Exo-treated group, while the LIPUS treatment group showed a
low level of positive staining. The number of vessels connected to
the smooth muscle cells were also counted, according to the α-SMA
staining results (Fig. 5B). The
number of mature vessels in the LIPUS + ADSC-Exos group was
35.12±1.93 per field, which was higher than the numbers observed in
the other three groups. The numbers of mature vessels in the
ADSC-Exos and LIPUS treatment groups were 20.25±1.44 and 9.41±0.98
per field, respectively. These results indicated that LIPUS +
ADSC-Exos treatment efficiently promoted angiogenesis in diabetic
wounds.
LIPUS combined with ADSC-Exos treatment
enhances blood perfusion of diabetic wounds
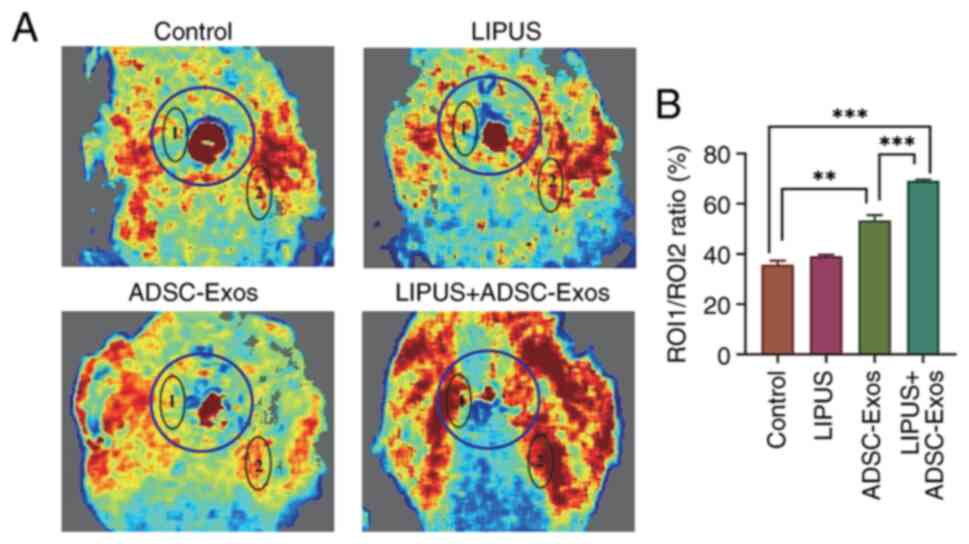
Small animal Doppler ultrasound was used in the
present study to non-invasively monitor blood perfusion of skin
wounds and the surrounding areas before the mice were sacrificed.
At 14 days after treatment, laser specular blood flow imaging
indicated that the ROI-1/ROI-2 ratios of the control, LIPUS,
ADSC-Exos and LIPUS + ADSC-Exos groups were 35.76±1.63, 39.09±0.58,
53.39±2.07 and 69.13±0.53%, respectively (P<0.05; Fig. 6). All three treatments improved
blood perfusion in the wound and surrounding area, but the animals
treated with LIPUS + ADSC-Exos demonstrated the most improvement.
Compared with the control, LIPUS, ADSC-Exos and LIPUS + ADSC-Exos
increased the ROI-1/ROI-2 ratio by 3.33, 17.63 and 33.37%,
respectively. These results suggested that LIPUS + ADSC-Exos
significantly improved blood perfusion around the wound, and the
effect was greater than the sum of the LIPUS and ADSC-Exos
treatments alone.
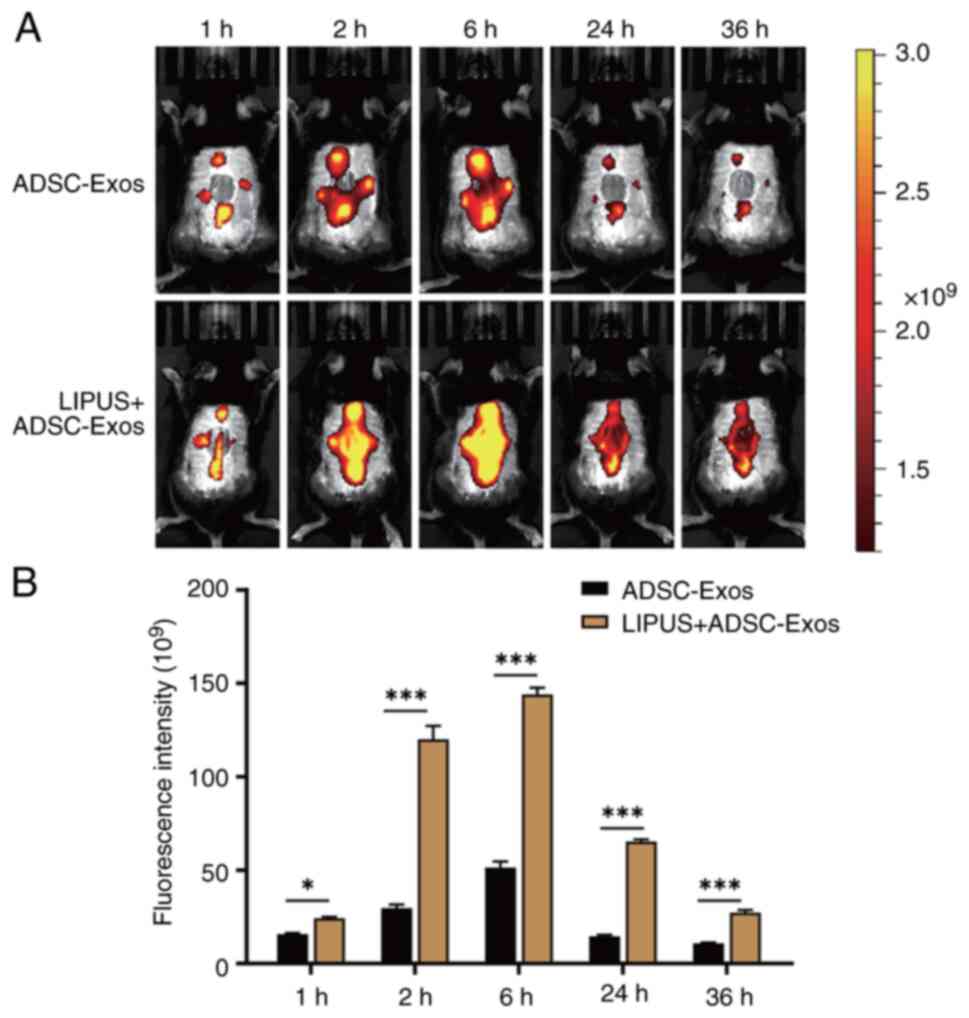
LIPUS promotes uptake of ADSC-Exos in
vivo
To explore whether the effective angiogenesis
therapy of LIPUS + ADSC-Exos was achieved by LIPUS promoting
ADSC-Exo uptake, the distribution and duration of DiR-labeled
ADSC-Exos around the wound were observed via in vivo
imaging. At 2, 6, 24 and 36 h after treatment, the exosome
fluorescence in the LIPUS irradiation group was more widely
distributed, completely covered the wound, was stronger and lasted
longer (Fig. 7). In the group
treated with ADSC-Exos alone, fluorescence was mainly limited to
the four injection sites. At 36 h, strong fluorescence could still
be observed throughout the wound in the mice that received LIPUS
irradiation, while only weak spotted fluorescence was observed in
the group without LIPUS irradiation. Quantitative analysis showed
that the exosome fluorescence intensity in the LIPUS irradiation
group was 1.50, 4.05, 2.81, 4.51 and 5.33-fold higher compared with
the without LIPUS irradiation group at 1, 2, 6, 24 and 36 h,
respectively (Fig. 7B). These
results indicated that LIPUS irradiation improved the distribution
of ADSC-Exos, promoted the uptake of exosomes in the injured area
and prolonged the action time of exosomes.
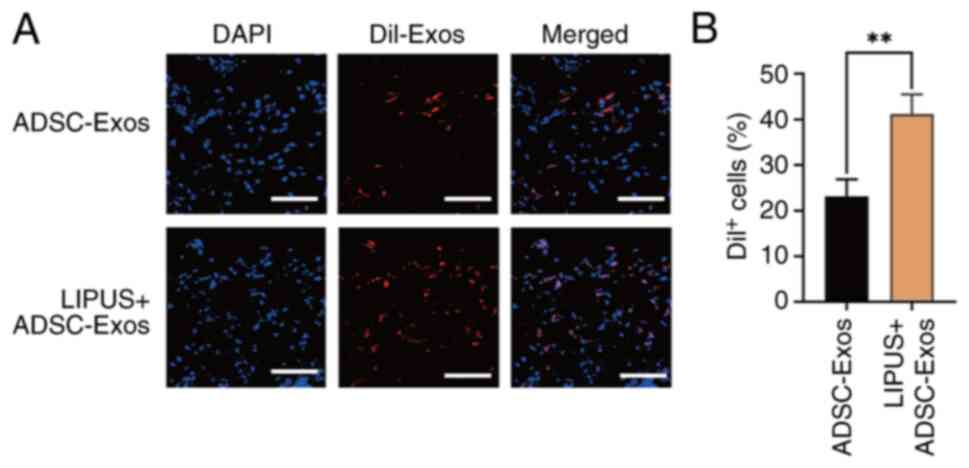
Confocal microscopy demonstrated that the percentage
of cells with visible DiI fluorescence around the wound was
41.20±1.11% after LIPUS irradiation, which was higher than the
non-irradiated group (23.16±1.16%) (Fig. 8). These results confirmed that
LIPUS promoted the uptake of exosomes in the wound area, which may
be why LIPUS + ADSC-Exos treatment promoted angiogenesis and
accelerated diabetic wound repair.
LIPUS promotes cellular uptake of
ADSC-Exos in vitro
To verify that LIPUS + ADSC-Exo therapy promoted
angiogenesis by promoting exosome uptake in vitro, ADSC-Exos
were labelled with DiI. The efficiency of exosome uptake in the
different treatment groups was then compared by measuring the
fluorescence intensity in the HUVECs. Representative confocal
microscopy images shown in Fig.
9A demonstrated that the nuclei of HUVECs were labeled in blue
(DAPI) and that the red fluorescence of DiI-labeled ADSC-Exos was
clearly observed in the cytoplasm. Quantitative analysis shown in
Fig. 9B demonstrated that the
DiI fluorescence intensity of HUVECs in the LIPUS + ADSC-Exos group
was 10.93-fold higher than the ADSC-Exos group, suggesting that
LIPUS significantly increased the uptake of exosomes by HUVECs.
LIPUS enhances angiogenesis by promoting
the cellular uptake of ADSC-Exos in vitro
The angiogenic effects of HUVECs were evaluated
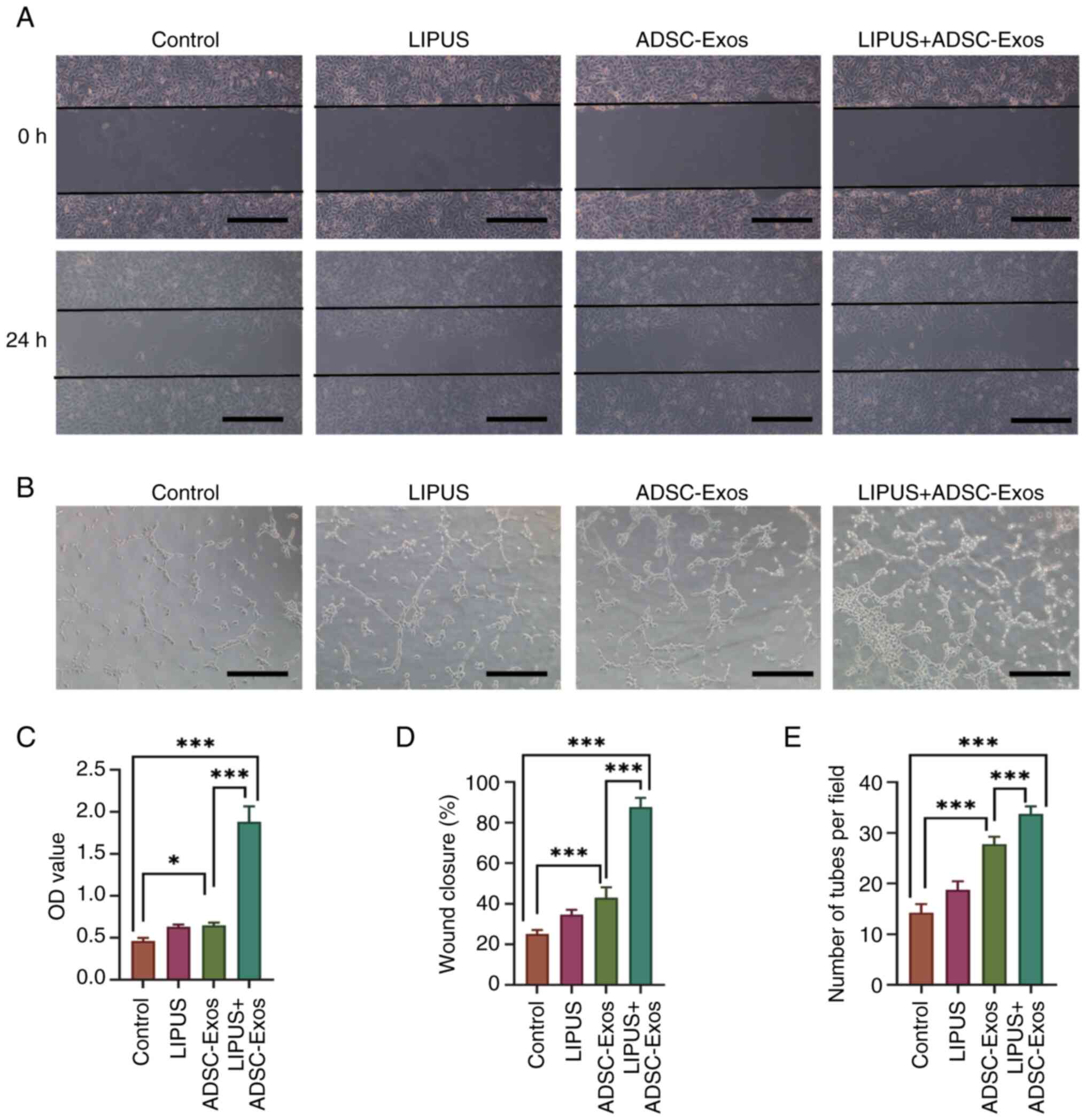
through CCK-8, scratch wound healing and tube formation assays. The
CCK-8 assay demonstrated that the OD value in the LIPUS + ADSC-Exos
group was 1.88±0.14, which was higher than that of the ADSC-Exos
(0.65±0.03), LIPUS (0.63±0.03) and control (0.46±0.04) groups
(Fig. 10C). The CCK-8 results
therefore indicated that HUVECs treated with LIPUS + ADSC-Exos
exhibited a much higher viability than HUVECs in the other
treatment groups. The scratch wound healing assay demonstrated that
the LIPUS + ADSC-Exos group had the smallest gap following wound
closure and therefore the greatest cell migration ability (Fig. 10A and D). The results of the
tube formation assay indicated that the LIPUS + ADSC-Exos group
exhibited better tube formation (characterized by a greater total
tube length and complete tubular structure) than the other three
groups (Fig. 10B and E).
Compared with the LIPUS and ADSC-Exos groups, the LIPUS + ADSC-Exos
treatment group formed more lumens, had larger lumen circumferences
and more mature structures. Collectively, these results confirmed
that LIPUS + ADSC-Exos treatment effectively promoted angiogenesis
in vitro.
Discussion
In the present study, the therapeutic effects of
LIPUS on ADSC-Exo-mediated diabetic wound healing were
investigated. The results demonstrated that treatment with
ADSC-Exos alone moderately contributed to diabetic wound healing,
but the combination of ADSC-Exos and LIPUS significantly promoted
angiogenesis and accelerated cutaneous wound healing. In
vivo imaging demonstrated that LIPUS promoted the uptake of
ADSC-Exos in the wound area and improved the utilization efficiency
of exosomes. The in vitro results further confirmed that
LIPUS enhanced the uptake efficiency of ADSC-Exos by 10.93-fold and
significantly increased the proliferation, migration and tubular
formation abilities of HUVECs. Therefore, the present study, to the
best of our knowledge, illustrated for the first time that LIPUS
can improve ADSC-Exo-mediated diabetic wound healing by promoting
cellular uptake of exosomes and enhancing angiogenesis.
Diabetic wounds are one of the most common and
serious complications of diabetes mellitus. Impaired angiogenesis
is an important reason for the prolonged wound healing time in
diabetic patients (31). A
number of methods, including different types of dressings,
tissue-engineered skin substitutes, growth factors and hyperbaric
oxygen, have been used in the treatment of non-healing diabetic
wounds. However, the results have not been completely satisfactory.
Accumulating evidence has suggested that stem cell-derived exosomes
have great potential in promoting diabetic wound healing through
the induction of therapeutic angiogenesis. Hu et al
(32) demonstrated that exosomes
derived from bone MSCs (BMSCs) that were pretreated with
pioglitazone accelerated diabetic wound healing by promoting
angiogenesis through PI3K/AKT/endothelial nitric oxide synthase
pathway activation. In addition, Yan et al (33) found that human umbilical cord
MSC-derived exosomes accelerated diabetic wound healing by
ameliorating oxidative stress and promoting angiogenesis. Although
various stem cell-derived exosomes potentially exert therapeutic
effects on diabetic wound healing, their biological properties are
different. A comparative study demonstrated that BMSC-derived
exosomes mainly promoted proliferation, whereas ADSC-derived
exosomes had a major effect on angiogenesis (34). Therefore, ADSC-Exos were chosen
as the therapeutic agent for angiogenesis in the present study.
Although ADSC-Exos are safe, effective and
convenient to use, their low yield and short half-life make it
difficult to meet the requirements of clinical treatment. As the
exosome yield cannot be significantly increased, improving its
utilization efficiency is the only feasible approach. Previous
studies have shown that LIPUS, which acts as a low-intensity
mechanical stimulus, can increase cell deformation and membrane
permeability, promote metabolite exchange, regulate cell function
and exert a variety of biological effects (35-37). However, whether LIPUS can enhance
the utilization efficiency of exosomes remains unknown.
The results of the present study demonstrated that
LIPUS combined with ADSC-Exos treatment significantly promoted the
repair of diabetic wounds. Compared with ADSC-Exos alone, the
average wound healing time in the combined treatment group was 4
days shorter, and the new skin was more mature and complete.
Immunofluorescence and small animal Doppler ultrasound indicated
that the combined therapy significantly increased angiogenesis and
blood perfusion in the wound area. These results suggested that the
combined therapy accelerated the healing of diabetic wounds mainly
by promoting angiogenesis. However, whether the effective
angiogenesis of LIPUS + ADSC-Exos therapy is achieved by LIPUS
promoting ADSC-Exos uptake still needs to be explored further.
In the present study, to observe the metabolism of
exosomes in vivo, ADSC-Exos were labeled with DiR. In
vivo imaging demonstrated that LIPUS promoted the distribution
of ADSC-Exos in the wound area, increased the fluorescence
intensity of target tissue and prolonged the duration of the
exosomes. Quantitative analysis showed that the fluorescence
intensity of DiR-labeled ADSC-Exos in the LIPUS irradiation group
was 5.33-fold higher than in the non-irradiation group 36 h after
treatment. This result indicated that LIPUS effectively increased
the uptake of exosomes and reduced metabolic clearance, thus
improving the utilization efficiency of exosomes in vivo. In
summary, these results suggested that LIPUS irradiation may improve
the uptake of ADSC-Exos, thus enhancing therapeutic angiogenesis
and accelerating the repair of diabetic wounds.
Immortalized HUVECs cell line is widely used as the
cell model in vascular endothelial cell experiments. Immortalized
HUVECs are the closest to original human endothelial cells and have
the ability to form tubes, which is the basic characteristic of
endothelial vascularization. In addition, HUVECs have stem cell
potential and remain stable for 50 generations (38). While aortic and coronary
endothelial cell lines are more consistent with the characteristics
of cardiovascular endothelial cells, they are mainly used in the
study of atherosclerosis and are not suitable for simulating the
peripheral microvascular conditions caused by diabetes. In the
present study, to further verify the underlying mechanism by which
LIPUS promotes the cellular uptake of exosomes and enhances
angiogenesis in vitro, ADSC-Exos were labeled with the
fluorescent dye, DiI, and the uptake of exosomes by immortalized
HUVECs was observed. The results demonstrated that LIPUS
significantly increased the cellular uptake of exosomes by
10.8-fold in vitro and that DiI-labeled ADSC-Exos were
mainly localized in the cytoplasm. Furthermore, compared with
ADSC-Exo treatment alone, the combination of ADSC-Exos with LIPUS
irradiation significantly increased the proliferation, migration
and tube formation abilities of HUVECs in vitro. These
results confirmed that LIPUS promoted the uptake of ADSC-Exos and
improved the utilization efficiency of exosomes, thus enhancing
therapeutic angiogenesis.
Certain mechanisms have been proposed that may
explain why LIPUS increased the cellular uptake of ADSC-Exos in
vivo and in vitro. For example, LIPUS irradiation
produces a stable cavitation effect in the target area, which
causes microbubbles (as the gas nucleus in the liquid environment)
to continuously expand and shrink, thus forming a microstream
(39). The microstream can cause
exosomes to spread in different directions and therefore expand the
distribution of exosomes in the wound area. In the present study,
fluorescently labeled ADSC-Exos were subcutaneously injected at
four points around the wound area and, 2 h after LIPUS irradiation,
ADSC-Exos diffused in different directions and covered the entire
wound area. In the group treated with ADSC-Exos alone, fluorescence
was mainly limited to the four injection sites. In addition, a
stable cavitation effect can promote contact between exosomes and
target cells and increase the permeability of cell membranes, thus
significantly improving the endocytosis of target cells (40). Rapid diffusion and endocytosis
significantly increase exosome uptake and reduce immune clearance,
thus improving exosome utilization efficiency. In the present
study, in vivo imaging demonstrated that 36 h after LIPUS
irradiation, a large amount of fluorescence covering the wound was
still observed in the combined treatment group, while only weak
dot-like fluorescence was observed in the group treated with
exosomes alone. This result confirmed that LIPUS reduced the immune
clearance of exosomes and prolonged the duration of exosome
treatment.
LIPUS, a non-invasive localized mechanical stimulus,
exerts a variety of therapeutic biophysical effects and has been
used to treat a number of diseases such as ligament trauma,
fracture, arthromeningitis and neurodegenerative disorders
(41). The use of LIPUS for
improving bone regeneration has been approved by the US Food and
Drug Administration for human application. However, the molecular
mechanisms of the therapeutic effects of LIPUS remain unclear. A
study has suggested that LIPUS 'massages' cells and can activate
multiple signaling pathways through energy transmission (42). Razavi et al (43) found that LIPUS downregulated
pro-inflammatory cytokines (IL-1β, IL-2, IL-6, IFN-γ, IFN-γ-induced
protein 10 and TNF-α), and Burks et al (44) suggested that LIPUS exposure
induced the upregulation of some signaling [such as VEGF,
fibroblast growth factor (GF), placental GF, hepatocyte GF and
stromal derived factor-1α] and cell adhesion (such as intercellular
adhesion molecule 1 and vascular cell adhesion molecule 1)
molecules on muscle vasculature. Yoon et al (45) have suggested that pulsed focused
ultrasound can mediate MSC homing by mechanically opening transient
receptor potential cation channel subfamily C member 1 on the
plasma membrane, causing an influx of sodium and calcium that
depolarizes the membrane and activates the voltage-gated calcium
channel, causing further calcium influx and activating NF-κB and
cyclooxygenase-2. These studies suggest that LIPUS may promote
wound healing by inhibiting the inflammatory pathway and enhancing
the regeneration of tissue. Since LIPUS can activate cellular
signaling pathways and since exosomes are important molecules in
intercellular communication, we hypothesize that the combination of
LIPUS and exosomes might exert synergistic effects that enhance
intercellular communication. However, this hypothesis requires
further exploration.
Although the results of the present study revealed
that LIPUS significantly improved the therapeutic effect of
ADSC-Exos in diabetic wound repair by enhancing the cellular uptake
of exosomes, there were also some limitations to the study. First,
the etiology and pathophysiological processes of diabetes are
complex, and it is difficult to construct an animal model that
conforms to the pathophysiology of human diabetes. Spontaneous
models of diabetes such as NOD mice, and single-gene obesity models
such as Lepob/ob mice, are commonly used in research,
but these particular strains may not develop the corresponding
complications and are very expensive (46). In the present study,
intraperitoneal injection of STZ after a period of high-fat feeding
had a high success rate of diabetes induction in mice and could be
used to evaluate drug efficacy. However, this model still may not
represent the true condition of patients with diabetes. Second, in
addition to improving the efficiency of ADSC-Exos uptake, LIPUS is
likely to exert synergistic effects with ADSC-Exos. The mechanisms
underlying these synergistic effects may be complicated and further
studies are needed to elucidate them.
In conclusion, the present study explored a novel
method to enhance the therapeutic effects of ADSC-Exos in diabetic
wound healing by combining administration with LIPUS. The results
demonstrated that LIPUS significantly improved the uptake of
exosomes in vivo and in vitro, thus improving the
utilization efficiency of ADSC-Exos and enhancing angiogenesis. As
a non-invasive and convenient technology, LIPUS may provide a
promising strategy for enhancing exosome-mediated diabetic wound
repair.
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
QZ and SC made contributions to the conception of
the study. QD initiated and designed the research. FZ and JL
performed the experiments and acquired the raw data. LY, BG, HW and
NJ contributed to data analysis and interpretation. QD and FZ wrote
the first draft of the manuscript, and SC and QZ critiqued and
modified the manuscript. QD and FZ confirm the authenticity of all
the raw data. All authors have read and approved the final version
of the manuscript.
Ethics approval and consent to
participate
All animal experiments were conducted in accordance
with the National Institutes of Health Guide for the Care and Use
of Laboratory Animals (NIH Publications No. 8023, revised 1978).
The animal protocols were approved by The Laboratory Animal Care
and Use Committee of Renmin Hospital, Wuhan University (Wuhan,
China; approval nos. 20210210 and 20210506).
Patient consent for publication
Not applicable.
Competing interests
All authors declare that they have no competing
interests.
Abbreviations:
|
DFUs
|
diabetic foot ulcerations
|
|
ADSCs
|
adipose-derived stem cells
|
|
ADSC-Exos
|
exosomes derived from ADSCs
|
|
LIPUS
|
low-intensity pulsed ultrasound
|
|
HUVECs
|
human umbilical vein endothelial
cells
|
|
TEM
|
transmission electron microscopy
|
|
DAPI
|
4,6-diamidino-2-phenylindole
|
|
H&E
|
hematoxylin and eosin
|
|
α-SMA
|
α-smooth muscle actin
|
|
CCK-8
|
Cell Counting Kit-8
|
|
MPU
|
mean perfusion unit
|
Acknowledgments
Not applicable.
Funding
This study was supported by The National Natural Science
Foundation of China (grant nos. 81901759, 81971624, 81901757 and
82102045).
References
|
1
|
Saeedi P, Petersohn I, Salpea P, Malanda
B, Karuranga S, Unwin N, Colagiuri S, Guariguata L, Motala AA,
Ogurtsova K, et al: Global and regional diabetes prevalence
estimates for 2019 and projections for 2030 and 2045: Results from
the International Diabetes Federation Diabetes Atlas, 9th edition.
Diabetes Res Clin Pract. 157:1078432019. View Article : Google Scholar
|
|
2
|
International Working Group on the
Diabetic Foot: IWGDF Guidelines on the prevention and management of
diabetic foot disease. International Working Group on the Diabetic
Foot; Maastricht: 2019
|
|
3
|
National Institute of Health: Diabetic
Foot Consortium. Diabetic Foot Consortium; 2020
|
|
4
|
International Working Group of the
Diabetic Foot: Diabetic Foot-Epidemiology, Psychosocial, and
Economic Factors. IWGoD; Maastricht: 2012
|
|
5
|
CDC Diabetes Report Card: Services UDoHaH.
Centers for Disease Control Prevention; Arlington, VA: 2017
|
|
6
|
Dayya D, O'Neill OJ, Huedo-Medina TB,
Habib N, Moore J and Iyer K: Debridement of Diabetic Foot Ulcers.
Adv Wound Care (New Rochelle). 11:666–686. 2022. View Article : Google Scholar
|
|
7
|
Baronski S and Ayello EA: Wound Care
Essentials Principles and Practice. Wilkins LW: Philadelphia:
Wolters Kluwer; 2008
|
|
8
|
den Dekker A, Davis FM, Kunkel SL and
Gallagher KA: Targeting epigenetic mechanisms in diabetic wound
healing. Transl Res. 204:39–50. 2019. View Article : Google Scholar :
|
|
9
|
Wilkinson HN and Hardman MJ: Wound
healing: Cellular mechanisms and pathological outcomes. Open Biol.
10:2002232020. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Geng K, Ma X, Jiang Z, Huang W, Gao C, Pu
Y, Luo L and Xu Y and Xu Y: Innate immunity in diabetic wound
healing: Focus on the mastermind hidden in chronic inflammatory.
Front Pharmacol. 12:6539402021. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Wang Y, Cao Z, Wei Q, Ma K, Hu W, Huang Q,
Su J, Li H, Zhang C and Fu X: VH298-loaded extracellular vesicles
released from gelatin methacryloyl hydrogel facilitate diabetic
wound healing by HIF-1α-mediated enhancement of angiogenesis. Acta
Biomater. 147:342–355. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Deptuła M, Brzezicka A, Skoniecka A,
Zieliński J and Pikuła M: Adipose-derived stromal cells for
nonhealing wounds: Emerging opportunities and challenges. Med Res
Rev. 41:2130–2171. 2021. View Article : Google Scholar
|
|
13
|
Nalisa DL, Moneruzzaman M, Changwe GJ,
Mobet Y, Li LP, Ma YJ and Jiang HW: Stem cell therapy for diabetic
foot ulcers: Theory and practice. J Diabetes Res. 2022:60287432022.
View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Li X, Xie X, Lian W, Shi R, Han S, Zhang
H, Lu L and Li M: Exosomes from adipose-derived stem cells
overexpressing Nrf2 accelerate cutaneous wound healing by promoting
vascularization in a diabetic foot ulcer rat model. Exp Mol Med.
50:1–14. 2018.
|
|
15
|
Lv Q, Deng J, Chen Y, Wang Y, Liu B and
Liu J: Engineered human adipose stem-cell-derived exosomes loaded
with miR-21-5p to promote diabetic cutaneous wound healing. Mol
Pharm. 17:1723–1733. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
An Y, Lin S, Tan X, Zhu S, Nie F, Zhen Y,
Gu L, Zhang C, Wang B, Wei W, et al: Exosomes from adipose-derived
stem cells and application to skin wound healing. Cell Prolif.
54:e129932021. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Wang J, Wu H, Peng Y, Zhao Y, Qin Y, Zhang
Y and Xiao Z: Hypoxia adipose stem cell-derived exosomes promote
high-quality healing of diabetic wound involves activation of
PI3K/Akt pathways. J Nanobiotechnology. 19:2022021. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Luo H, Wang Y, Su Y, Liu D, Xiao H, Wu M,
Zhao Y and Xue F: Paracrine effects of adipose-derived stem cells
in cutaneous wound healing in streptozotocin-induced diabetic rats.
J Wound Care. 31(Suppl 3): S29–S38. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Zhou C, Zhang B, Yang Y, Jiang Q, Li T,
Gong J, Tang H and Zhang Q: Stem cell-derived exosomes: Emerging
therapeutic opportunities for wound healing. Stem Cell Res Ther.
14:1072023. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Rezaie J, Feghhi M and Etemadi T: A review
on exosomes application in clinical trials: Perspective, questions,
and challenges. Cell Commun Signal. 20:1452022. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Poolman RW, Agoritsas T, Siemieniuk RA,
Harris IA, Schipper IB, Mollon B, Smith M, Albin A, Nador S, Sasges
W, et al: Low intensity pulsed ultrasound (LIPUS) for bone healing:
A clinical practice guideline. BMJ. 356:j5762017. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Elmajee M, Munasinghe C, Nasser AAH,
Nagappa S and Mahmood A: The perceptions of clinicians using
low-intensity pulsed ultrasound (LIPUS) for orthopaedic pathology:
A national qualitative study. Injury. 53:3214–3219. 2022.
View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Lai WC, Iglesias BC, Mark BJ and Wang D:
Low-intensity pulsed ultrasound augments tendon, Ligament, and
bone-soft tissue healing in preclinical animal models: A systematic
review. Arthroscopy. 37:2318–2333. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Sabbagh A, Beccaria K, Ling X, Marisetty
A, Ott M, Caruso H, Barton E, Kong LY, Fang D, Latha K, et al:
Opening of the blood-brain barrier using low-intensity pulsed
utrasound enhances responses to immunotherapy in preclinical glioma
models. Clin Cancer Res. 27:4325–4337. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Truong TT, Chiu WT, Lai YS, Huang H, Jiang
X and Huang CC: Ca2+ signaling-mediated low-intensity pulsed
ultrasound-induced proliferation and activation of motor neuron
cells. Ultrasonics. 124:1067392022. View Article : Google Scholar
|
|
26
|
Wu Y, Gao Q, Zhu S, Wu Q, Zhu R, Zhong H,
Xing C, Qu H, Wang D, Li B, et al: Low-intensity pulsed ultrasound
regulates proliferation and differentiation of neural stem cells
through notch signaling pathway. Biochem Biophys Res Commun.
526:793–798. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Wang X, Lin Q, Zhang T, Wang X, Cheng K,
Gao M, Xia P and Li X: Low-intensity pulsed ultrasound promotes
chondrogenesis of mesenchymal stem cells via regulation of
autophagy. Stem Cell Res Ther. 10:412019. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Bunnell BA, Flaat M, Gagliardi C, Patel B
and Ripoll C: Adipose-derived stem cells: Isolation, expansion and
differentiation. Methods. 45:115–120. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Hsu HH, Wang AYL, Loh CYY, Pai AA and Kao
HK: Therapeutic potential of exosomes derived from diabetic adipose
stem cells in cutaneous wound healing of db/db mice. Pharmaceutics.
14:12062022. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Foster DS, Januszyk M, Yost KE, Chinta MS,
Gulati GS, Nguyen AT, Burcham AR, Salhotra A, Ransom RC, Henn D, et
al: Integrated spatial multiomics reveals fibroblast fate during
tissue repair. Proc Natl Acad Sci USA. 118:e21100251182021.
View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Geng X, Qi Y, Liu X, Shi Y, Li H and Zhao
L: A multifunctional antibacterial and self-healing hydrogel laden
with bone marrow mesenchymal stem cell-derived exosomes for
accelerating diabetic wound healing. Biomater Adv. 133:1126132022.
View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Hu Y, Tao R, Chen L, Xiong Y, Xue H, Hu L,
Yan C, Xie X, Lin Z, Panayi AC, et al: Exosomes derived from
pioglitazone-pretreated MSCs accelerate diabetic wound healing
through enhancing angiogenesis. J Nanobiotechnology. 19:1502021.
View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Yan C, Xv Y, Lin Z, Endo Y, Xue H, Hu Y,
Hu L, Chen L, Cao F, Zhou W, et al: Human umbilical cord
mesenchymal stem cell-derived exosomes accelerate diabetic wound
healing via ameliorating oxidative stress and promoting
angiogenesis. Front Bioeng Biotechnol. 10:8298682022. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Pomatto M, Gai C, Negro F, Cedrino M,
Grange C, Ceccotti E, Togliatto G, Collino F, Tapparo M, Figliolini
F, et al: Differential therapeutic effect of extracellular vesicles
derived by bone marrow and adipose mesenchymal stem cells on wound
healing of diabetic ulcers and correlation to their cargoes. Int J
Mol Sci. 22:38512021. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Xia P, Shi Y, Wang X and Li X: Advances in
the application of low-intensity pulsed ultrasound to mesenchymal
stem cells. Stem Cell Res Ther. 13:2142022. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Lucchetti D, Perelli L, Colella F,
Ricciardi-Tenore C, Scoarughi GL, Barbato G, Boninsegna A, De Maria
R and Sgambato A: Low-intensity pulsed ultrasound affects growth,
differentiation, migration, and epithelial-to-mesenchymal
transition of colorectal cancer cells. J Cell Physiol.
235:5363–5377. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Huang D, Gao Y, Wang S, Zhang W, Cao H,
Zheng L, Chen Y, Zhang S and Chen J: Impact of low-intensity pulsed
ultrasound on transcription and metabolite compositions in
proliferation and functionalization of human adipose-derived
mesenchymal stromal cells. Sci Rep. 10:136902020. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Cao Y, Gong Y, Liu L, Zhou Y, Fang X,
Zhang C, Li Y and Li J: The use of human umbilical vein endothelial
cells (HUVECs) as an in vitro model to assess the toxicity of
nanoparticles to endothelium: A review. J Appl Toxicol.
37:1359–1369. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Cheng M, Li F, Han T, Yu ACH and Qin P:
Effects of ultrasound pulse parameters on cavitation properties of
flowing microbubbles under physiologically relevant conditions.
Ultrason Sonochem. 52:512–521. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Atherton P, Lausecker F, Harrison A and
Ballestrem C: Low-intensity pulsed ultrasound promotes cell
motility through vinculin-controlled Rac1 GTPase activity. J Cell
Sci. 130:2277–2291. 2017.PubMed/NCBI
|
|
41
|
Jiang X, Savchenko O, Li Y, Qi S, Yang T,
Zhang W and Chen J: A Review of Low-Intensity Pulsed Ultrasound for
Therapeutic Applications. IEEE Trans Biomed Eng. 66:2704–2718.
2019. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Harrison A and Alt V: Low-intensity pulsed
ultrasound (LIPUS) for stimulation of bone healing-A narrative
review. Injury. 52(Suppl): S91–S96. 2021. View Article : Google Scholar
|
|
43
|
Razavi M, Zheng F, Telichko A, Ullah M,
Dahl J and Thakor AS: Effect of pulsed focused ultrasound on the
native pancreas. Ultrasound Med Biol. 46:630–638. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Burks SR, Ziadloo A, Hancock HA, Chaudhry
A, Dean DD, Lewis BK, Frenkel V and Frank JA: Investigation of
cellular and molecular responses to pulsed focused ultrasound in a
mouse model. PLoS One. 6:e247302011. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Yoon CW, Jung H, Goo K, Moon S, Koo KM,
Lee NS, Weitz AC and Shung KK: Low-intensity ultrasound modulates
Ca2+ dynamics in human mesenchymal stem cells via connexin 43
hemichannel. Ann Biomed Eng. 46:48–59. 2018. View Article : Google Scholar
|
|
46
|
King AJ: The use of animal models in
diabetes research. Br J Pharmacol. 166:877–894. 2012. View Article : Google Scholar : PubMed/NCBI
|