Severe acute respiratory coronavirus 2 (SARS-CoV-2)
is the causative agent of the viral disease known as coronavirus
disease 2019 (COVID-19). This illness was first identified in
December 2019 in Wuhan, China, and has since spread throughout the
globe, culminating in a pandemic (1-3).
COVID-19 is a systemic disease that may present in a broad variety
of clinical manifestations, ranging from patients who are
asymptomatic to those who have significant respiratory symptoms and
even conditions that are life-threatening (3-5).
There are several underlying mechanisms and interactions with
pre-existing conditions, such as obesity among others, that drive
the pathogenesis of the disease, which includes the activation or
dysregulation of localized (for example, vascular) and widespread
inflammation, ultimately resulting in the failure of several organs
and eventually, mortality (2,4,6-16).
With the pandemic now characterized passed the acute
phase, attention is shifting to post-acute sequelae of COVID-19
(PASC), is often referred to as 'long COVID' and possible
preventative and therapeutic approaches are warranted (17,18). PACS comprises from a variety of
symptoms and clinical manifestations, which may include persistent
tiredness, respiratory symptoms (including dyspnea, cough, chest
tightness), joint rigidness, impaired smell and headache, whereas
respiratory, cardiovascular, neurological, cognitive, psychiatric
and gastrointestinal manifestations continue to be the most common
and potentially gravest, presentations of PASC (17,19-21). Recent evidence suggests that a
number of these manifestations may be linked to an unfavorable
impact of the disease on the mitochondrial function of various
tissues and organs (18,22).
Considering the numerous mechanisms and
pathophysiological processes that spread from the deregulation of
the immune system in acute COVID-19 and the potential mitochondrial
basis of long COVID, an ideal and efficient therapeutic option
could be a molecule which functionally behaves as a 'Swiss Army
Knife', such as melatonin (23,24). Indeed, since the SARS-CoV-2 was
classified as a pandemic, numerous studies have proposed that the
use of melatonin should be investigated as a treatment option that
is both safe and likely to be effective with regard to treating the
infection (17,25-27). Its usage is justified not just by
its superior safety profile, but also from its innumerable
beneficial actions as already reviewed extensively elsewhere
(27-30) and it has been demonstrated to
even possess broad-spectrum antiviral drug characteristics
(31,32). Moreover, various potentially
harmful and costly repurposed medicines, such as colchicine,
glucocorticoids, remdesivir and several others, have been advocated
for or utilized as therapeutic options (25,27,33-37). Additionally, despite their
importance, even the presently available vaccinations have major
adverse effects on occasion (38,39). Furthermore, as the virus has
evolved, the efficiency of the immunizations has reduced, several
strains have already been found, and more are expected to emerge,
reducing the efficacy of vaccinations even further (40). All these factors underlie the
need for further therapeutic options despite the various preventive
and already utilized medicinal options.
The present review provides a summary of the
features of melatonin that provide support to its use in the
treatment and/or prevention of SARS-CoV-2 infection and its
complications. The present review initially presents several
actions of melatonin in health and disease, followed by the key
pathophysiological mechanisms of COVID-19 and the potential
mechanisms through which melatonin would interact and mitigate
them, with a focus on long COVID and the mitochondrial functions of
melatonin.
Finally, the results of the available clinical
trials examining the use of melatonin in individuals with COVID-19
are summarized, and future steps on further examining the use of
melatonin are proposed.
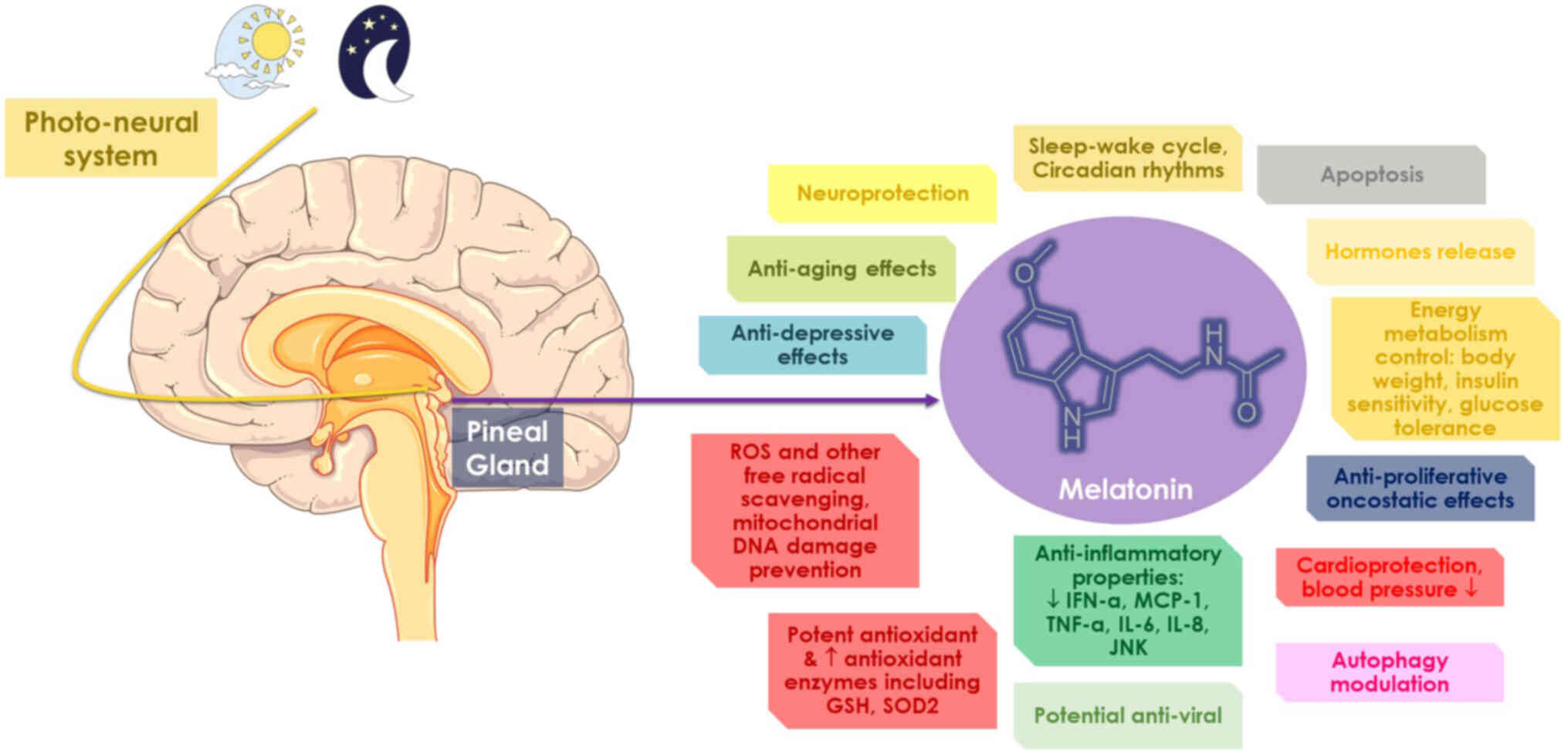
Melatonin is an ubiquitous molecule that can be
found in all living organisms of the animal kingdom, with traces
even found in higher plants, such as fruits, seeds and leaves. The
term 'melatonin' originates from the Greek words 'melas', which
means black or dark, and 'tonos', which means color or tune.
Melatonin is ultimately used to describe the hormone that is
responsible for darkness (41-44). It has been preserved over the
course of evolution, perhaps for these and numerous other
additional features, and it is regarded to be an evolutionarily old
antioxidant, as it has the ability to scavenge free radicals and
stimulate antioxidant enzymes (44-47). Melatonin is primarily synthesized
and secreted (predominantly released at night) by the pineal gland
via the process of hydroxylation of the essential amino acid
tryptophan, whereas tryptophan hydroxylase is responsible for the
formation of 5-hydroxytryptophan (42,43,45,47-49). Serotonin, also known as
5-hydroxytryptamine, is the neurotransmitter that is produced as a
result of this process. Melatonin is the immediate precursor of
serotonin (42,43,45,47,48). Other organs, including the
retina, kidneys, gastrointestinal system, skin and lymphocytes,
produce a modest amount of melatonin (42,43,45,47,48). The role of melatonin in various
biosynthetic metabolic pathways is evident, with different species
having distinct biosynthetic pathways and genes that encode the
enzymes involved in the process of its biosynthesis (42,43,45,47,48). Hydoxyindole-O-methyltransferase,
an enzyme that is indirectly controlled by the photo-neural system,
is responsible for regulating the production of melatonin (42,43,45,47,48). Melatonin is primarily synthesized
at night and is bound to albumin and orosomucoid glycoprotein and
through the process of crossing the blood-brain barrier, it is able
to go to all tissues in the body and regulate brain function
(43,50,51). Melatonin production peaks at 3
months of age and decreases by 80% by the adult stage (43).
Melatonin is primarily considered to govern
physiological processes, such as circadian rhythms in humans, the
sleep-wake cycle, and it may be used as a natural sleep aid
(43,45,52-54). It is a pleiotropic hormone that
regulates several biological processes, including the release of
other hormones, apoptosis and immunological responses (32,49,55,56). The effects of melatonin are
mediated in various cells via either the melatonin receptors type 1
and type 2, G-protein coupled (membrane-independent pathway) or
indirectly (membrane independent) with nuclear orphan receptors
from either the RAR-related orphan receptor α/Z receptor family or
through other pathways, as extensively reviewed elsewhere (57). The oncostatic, anti-inflammatory
and antioxidant characteristics of melatonin indicate that it may
have potential use in the treatment of a variety of disorders
(32,43,58). Both the preventative and
therapeutic benefits of melatonin have been the subject of
substantial research in a variety of neurological conditions,
including Alzheimer's disease, Parkinson's disease, Huntington's
disease, amyotrophic lateral sclerosis, multiple sclerosis and
epilepsy (47,59-62). In lipopolysaccharide-induced
depression, melatonin has been shown to exert antidepressant
effects, which are mediated via the regulation of autophagy
(63). Additionally, it exhibits
anti-aging properties and has the potential for use in the
management and treatment of age-related disorders in human beings
(55,64,65).
Melatonin has been widely investigated for its
anti-proliferative and anti-apoptotic properties on cancer cells,
revealing its oncostatic effects. Melatonin also reduces the loss
of cells, which is a significant benefit (66,67). Melatonin, which has been found in
both in vitro and in vivo studies, has been shown to
inhibit the development of tumors through membrane-independent and
membrane-dependent mechanisms. Melatonin has an effect on cancer
during the initiation phase, such as through DNA repair, and in the
development, progression and metastasis phases, of the
tumorigenesis process (66-68). Melatonin has potent
anti-angiogenic, anti-proliferative and ultimately anti-metastatic
properties that may be used in the treatment of a wide range of
malignancies, particularly those that have a high risk of cancer
spreading to other parts of the body. Additionally, it exerts
synergistic effects with conventional therapy, which increases the
vulnerability of cancer cells to apoptosis (66-68). Melatonin significantly reduces
the adverse effects of cardiotoxic drugs in patients with cancer
and has been shown to have a beneficial effect on coagulopathy
(49). Melatonin has been found
to improve cardiac function and lower blood pressure in patients
who have hypertension, according to clinical data from human
studies and various lines of evidence from animal studies, which
have been reviewed elsewhere (52,60,69-71). Melatonin, a substance that
neutralizes free radicals, has been utilized to mitigate the
harmful effects of certain chemical compounds, such as
methamphetamine (42,50,60,72-74). The use of melatonin as a possible
anti-viral drug for the treatment of viral illnesses, such as Ebola
and COVID-19 has been suggested (27,31,75). As extensively reviewed elsewhere
(31), melatonin exhibits a
plethora of potential antiviral actions in various viral models
(31,75), including the regulation of viral
phase separation and epitranscriptomics in long COVID-19 (17).
Studies have indicated that the anti-inflammatory
properties of melatonin involve suppressing interferon (IFN)-α,
tumor necrosis factor α (TNF-α), interleukin (IL)-6 and IL-8,
inhibiting Janus kinase (JNK) phosphorylation and monocyte
chemoattractant protein-1, and promoting protein degradation for
tight junction integrity, according to numerous studies (56,76-81). During the catastrophic hemorrhage
that occurs during the late phase of Ebola virus infection,
melatonin plays a crucial role in preserving the integrity of the
blood vessels and shielding endothelial cells from damage (31,75,78,82,83). It also exhibits various
biological activities, such as neuroprotective and immunomodulatory
effects, regulatory effects on reproduction, tumor preventive
effects, protective effects on gastrointestinal function and
anti-aging effects (45,84).
Melatonin is also a key factor in the regulation of
energy homeostasis, which includes the regulation of body weight,
insulin sensitivity and glucose tolerance of the body (45,85). It regulates energy metabolism,
affecting intake, flow and expenditure in the energy balance, which
in turn may be critical for preventing a variety of dysmetabolic
conditions, particularly obesity, which in turn can affect the
outcome of patients with COVID-19 (11,86-89). In addition to this, it
synchronizes the needs for energy metabolism with the daily and
yearly cyclical environmental photoperiod by means of its
chronobiotic and seasonal effects (45,85). In experimental
ischemia/reperfusion research, particularly in cases of myocardial
infarction and stroke, melatonin has been shown to successfully
prevent oxidative damage and the pathophysiological repercussions
of such damage are essential (43,82,90,91). Of utmost importance is to further
present the free radical scavenging properties of melatonin, as
these protect against mitochondrial DNA damage induced by reactive
oxygen species (ROS) displaying another of its significant effects
on mitochondrial homeostasis (24,92,93). In preclinical studies, the
administration of melatonin has been shown to increase the activity
of several antioxidant markers/enzymes, including glutathione
peroxidase and superoxide dismutase 2 (SOD2). The latter was
achieved by promoting the function of sirtuin 3, that deacetylates
SOD2, essentially facilitating its activation (24,92,94-97). Whether melatonin is present in
the mitochondria has been debatable (24,92); however, experimental evidence
demonstrates up to 100-fold higher levels of melatonin within the
mitochondria post-administration on mitochondrial membranes
(98). It appears that the
highest concentration of melatonin occurs in the mitochondria,
where the highest amount of ROS and oxidative stress occur
(99). High amounts of melatonin
in the mitochondria may be due to oligopeptide transporters 1/2 or
mitochondria generating their own melatonin, with research
indicating the existence of such enzymes in brain mitochondria
(92,94,100-102). The effects of melatonin on
mitochondria may be mediated via MT1/2 receptors, resulting in
decreased ROS generation, higher antioxidant capabilities, and
therefore, in less neural apoptosis, activating nuclear factor
erythroid 2-related factor 2, as shown in preclinical models
(24,92,103,104). Melatonin additionally prevents
stress-induced cytochrome c release from mitochondrial outer
membranes (100). Finally,
melatonin appears to increase classes of oxidative phosphorylation
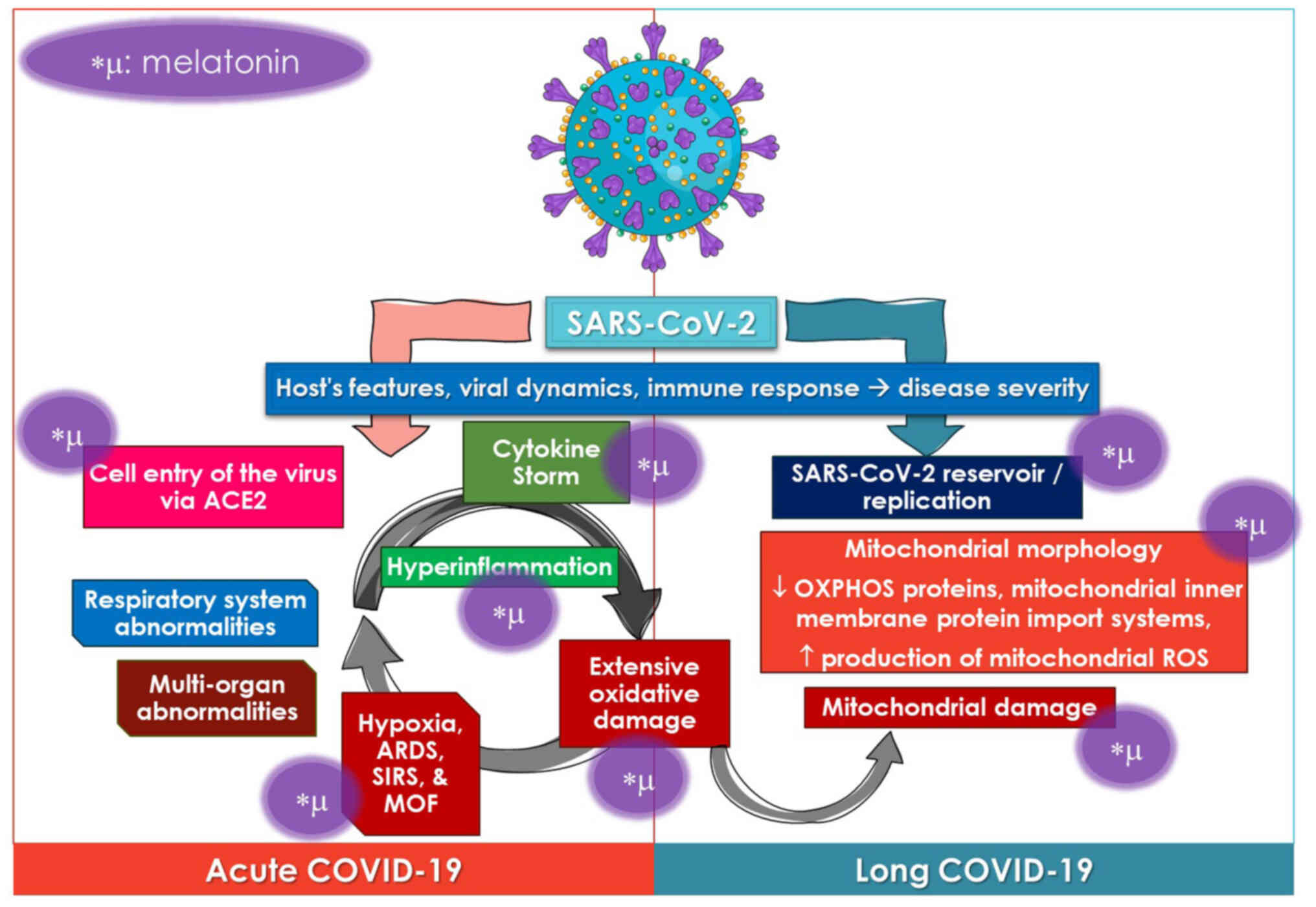
(OXPHOS) proteins, thereby preventing damage (105). All these mitochondria-related
features of melatonin are of key relevance, apart from the acute
phase of COVID-19, which is strongly associated with oxidative
stress, but also long COVID, which will be discussed in the
following section. Based on novel data, melatonin is related to the
mitochondrial dysfunction/downregulation of vital mitochondrial
markers. The physiology of melatonin is summarized in the schematic
diagram in Fig. 1.
Although individuals with COVID-19 often have modest
symptoms, 20% develop substantial to severe illness that requires
hospitalization (106). The
most common include respiratory system abnormalities; however,
several other organs may also be affected (3,7,10-12,33,34). The features of the host, viral
dynamics and immune response are associated with the severity of
the disease and in general, severe COVID-19, as well as a higher
mortality rate are linked to an older age, high body mass index,
and comorbidities such as cardiovascular diseases, diabetes or
cancer (3,8,10,11,87,107,108).
The pathophysiological symptoms of COVID-19 are
partly mediated by the cell entrance of the virus, which is
enhanced by the binding of the viral spike peptides to the
angiotensin converting enzyme 2 (ACE2) receptors in diverse organs
(2,7,8,109). In humans, ACE2 is expressed in
numerous organ systems and tissues, including the lungs (e.g., the
pneumocytes of alveolar sacs), hepatic, cardiac tissue, kidney,
gastrointestinal endothelium, adipose tissue (AT) and vascular
endothelium (3,49,110,111). This wide distribution likely
explains the multisystem involvement of the infection, while also
enhancing the magnitude of the illness in patients afflicted by
SARS-CoV-2 (49). Interstitial
pneumonia, the most prevalent lung involvement in patients with
COVID-19, if left untreated, may lead to a hypoxic status,
resulting in acute respiratory distress syndrome and/or systemic
inflammatory response syndrome and fatal multiorgan failure
(3,6,13,15,37, 108,112,113). These sepsis-related
consequences occur from a pathophysiological perspective, have the
same underlying backgrounds, ignited by the cytokine storm and
hyperinflammatory statuses with significant oxidative damage caused
by the reaction of the host to SARS-CoV-2 (49,114).
It is possible that the widespread extrapulmonary
damage observed in patients with COVID-19 may be attributed to the
presence of ACE2 receptors on cells other than those that lining
the respiratory alveoli (113).
Other organ involvement results in symptoms that are particular to
the organ; for example, gastrointestinal involvement may cause
symptoms such as nausea, vomiting, diarrhea and abdominal pain
(113). Hepatic damage, as
evidenced by increased levels of circulating liver enzymes, is also
prevalent (3). There are several
symptoms that may be associated with peripheral and central nervous
system involvement, and these include headaches and dizziness,
hyposmia or anosmia (indicative of encephalopathy), neuralgia and
Guillain-Barré syndrome (115,116). Hospitalized patients are more
likely to experience thromboembolic events, which have been
established as an independent risk factor for a poor prognosis, and
acute coronary modalities, cardiomyopathies, several types of
arrhythmias, pericarditis and various thromboembolic events
(49,117). Infections caused by SARS-CoV-2
may also result in coagulopathies, thrombocytopenia being the most
prevalent, which play a crucial role in the development of
extrapulmonary complications (8,49). In critically ill patients, deep
venous thrombosis and/or pulmonary embolism are frequent, with
pulmonary embolism being more prevalent in patients in intensive
care units (49). Inflammation,
immunological responses, coagulation cascades and the dysregulation
of the renin-angiotensin system may cause acute kidney damage in
25% of hospitalized patients (8,49,118). Finally, AT from individuals
with obesity is hypothesized to exhibit higher amounts of ACE2,
perhaps serving as a SARS-CoV-2 repository with postponed viral
shedding and may presumably contribute to long COVID (3).
Long COVID refers to patients who have experienced
persistent impairments following infection with COVID-19, including
various organs and tissues (18,119-122). A previous retrospective
analysis of 193,113 participants found an elevated risk for
respiratory impairment and pulmonary function impairment after 6
months in these patients (123). The most prevalent manifestation
is impaired diffusion capacity for carbon monoxide (DLCO) (124). Survivors with a critical
illness had a greater risk of DLCO impairment, lower residual
volume and lower total lung capacity (124,125). Notably, the risk of developing
long COVID appears to differ depending on the various strains.
Studies have found a lower risk of complications, intensive care
unit admission, ventilation requirement and mortality rate in
omicron-infected individuals compared to those infected with other
variants (126). Furthermore,
as compared to the delta variant, the omicron variant has been
shown to be associated with a lower likelihood of developing long
COVID (127).
Mutations in antigenic sites are essential for
antibody and immunological evasion, and chronic symptoms in
patients with long COVID-19 may be partly due to a lessening of the
antibody response to vaccination or to variant resistance (17,122,128,129). Of note, >100 persistent
symptoms were recorded by participants at least 4 weeks after
infection, according to a scoping analysis that included 50 trials
(130). It is possible for the
majority of 'long-haulers' to have a relapse as a result of either
physical or mental stress, and cognitive impairment or memory
issues are common regardless of age (18,131). The establishment of a viral
reservoir in individuals with PASC may potentially be a possible
explanation for the improvement in clinical symptoms that occurred
following the administration of the SARS-CoV-2 immunization
(132). Reservoirs of viruses
are cells or anatomical locations where the virus may persist and
accumulate with better kinetic stability than the primary pool of
viruses that are actively reproducing (17,133,134). There is a increasing evidence
of an association between the presence of viral RNA in probable
SARS-CoV-2 reservoirs in extrapulmonary organs and tissues, and the
continued manifestation of symptoms in PASC (17,18,133,134). Patients who have been diagnosed
with COVID for a long period of time often have reactivated
viruses, which may cause mitochondrial fragmentation and disrupt
energy metabolism (18,135-137). In addition, there is evidence
of oxidative stress, abnormal amounts of mitochondrial proteins and
deficits in tetrahydrobiopterin (138,139).
In addition to the dysregulations of inflammatory
responses, COVID-19 has been connected to mitochondrial function.
Mitochondria play a critical role in the control of immune
responses and cellular metabolism (22,140-143). The shape of the mitochondria is
altered by infection, which results in a reduction in the number of
OXPHOS proteins, a reduction in the number of mitochondrial inner
membrane protein import systems, and an increase in the release of
mitochondrial reactive oxygen species (144-146). The SARS-CoV-2 virus is capable
of binding to a variety of host proteins, with mitochondrial
proteins accounting for up to 16% of the total (22,147-149). Human cells and tissues that
have been infected display a decrease in the amount of proteins and
transcripts of OXPHOS genes, an increase in glycolysis, a
suppression of OXPHOS, an increase in mitochondrial ROS production,
inflammation factors, and an increase in hypoxia inducible
factor-1α (HIF-1α) and its target genes (22,144,150-155). A disruption in the process of
mitochondrial protein synthesis may lead to an imbalance in the
proportion of mitochondrial proteins that are coded by nuclear DNA
and mitochondrial DNA, which has the potential to activate the
integrated stress response and have a number of unfavorable
repercussions (22). Recently,
Guarnieri et al (22)
demonstrated that once the viral titter peaks, this causes a
systemic reaction from the host, which includes the regulation of
mitochondrial gene transcription and glycolysis, ultimately
resulting in an antiviral immune defense mechanism. Nevertheless,
despite the fact that lung clearance and lung mitochondrial
function recovery were documented, mitochondrial function in the
heart, kidney, liver and lymph nodes continues to be damaged, which
may result in severe COVID-19 pathology (22).
Melatonin, which is well-known for its antioxidant
and anti-inflammatory qualities, has the potential to assist in
overcoming the cytokine storm that is associated with virus-related
infections, such as SARS-CoV-2, and may also be able to prevent
mitochondrial-related chronic consequences of the disease. The
anti-inflammatory and antioxidant properties of melatonin may
potentially be beneficial for the treatment of possibly chronic
inflammation in patients with long COVID-19. These views are
discussed in the following section. The effects of melatonin on the
pathophysiological mechanisms of COVID-19 are summarized in the
schematic diagram in Fig. 2.
Melatonin supplementation has the potential to
target and benefit the host by reducing the exaggeration of the
innate immune system, which is essential for improving tolerance
against the invasion of pathogens (156). There is a substantial
association between the immunological response of the host,
particularly the innate immune network, and the symptoms and the
results of viral infections with the host (156,157). The overwhelming inflammatory
response that is triggered by the cytokine storm is responsible for
the majority of the detrimental effects caused by SARS-CoV-2
(36,114,156,157). Consequently, this excessive
production of cytokines is harmful to organs and tissues, which
ultimately results in oxidative damage to several organs (36,114,157,158). A considerable improvement in
the outcomes of patients with SARS-CoV-2 infection may be achieved
by downregulating the innate immune response and reducing the
inflammatory reaction. This provides evidence for the use of this
treatment method in the treatment of patients with severe COVID-19
(77,159).
Melatonin is a potent free radical scavenger and
antioxidant that directly detoxifies a wide range of ROS and
reactive nitrogen species (RNS). These ROS and RNS include hydroxyl
radicals, peroxynitrite anion, hydrogen peroxide, superoxide anion
radicals and hypoochlorous acid (25,27,50,93,160). Its electron-donating
metabolites outperform traditional antioxidants, such as vitamins C
and E, carotenoids, and NADH in reducing other oxidizing compounds
(156,161). Additionally, melatonin has an
advantageous cellular distribution due to its solubility in both
water and lipids, and it may form hydrogen bonds with proteins and
DNA to provide protection (60,161). Additionally, it upregulates the
gene expression levels of several antioxidant enzymes, thus
indirectly enhancing the cellular antioxidant capacity (161,162). By interacting on the
mitochondrial metabolism, melatonin is also able to inhibit the
production of ROS and RNS (60,156).
Melatonin is a potent anti-inflammatory chemical
that functions by rescuing the peroxynitrite anion, which leads to
the inhibition of inflammation that is not specific to any one
substance, such as carrageenan or zymosan (79,163,164). Its anti-inflammatory mechanisms
are diverse, including the suppression of the activity or
downregulation of pro-inflammatory enzymes, such as
cyclooxygenase-2, inducible nitric oxide synthase, eosinophilic
peroxidase and matrix metalloproteinase 2 (MMP)2, which are
responsible for the generation of inflammatory mediators (156,165-167). Furthermore, melatonin has the
ability to inhibit the advancement of the NLR family pyrin domain
containing 3 (NLRP3) inflammasome, which ultimately results in the
activation of caspase-1 and the maturation of IL-1β and IL-18. This
ultimately leads to pyroptosis, a damaging consequence of
inflammation (168-170). Melatonin is able to effectively
prevent the production of NLRP3 inflammasomes and reduce
inflammation, both of which are connected to COVID-19. This affect
is achieved by its interaction with signal transduction pathways
(167-169). Melatonin has the ability to
decrease the phosphorylation of IκBα, therefore reducing the
translocation of NF-κB into the nucleus. This, in turn, helps to
control the cytokine storm that occurs following infection with
COVID-19 and may be associated with damaging inflammation (171-174). The downregulation of melatonin
also stimulates autophagic capacity, which is often accompanied by
a reduction in the creation of inflammasomes. This may speed up the
process of tissue healing from inflammation (174,175).
Melatonin is a hormone that controls the immune
system, reducing the excessive response of both the innate immune
system and fostering the development of adaptive immunity (156,176). Some examples of pathogen
associated molecular pattern receptors are Toll-like receptors
(TLRs), Nod-like receptors (NLRs), AIM2-like receptors, GMP-AMP
synthase (cGAS) and AIM2. These receptors are responsible for
driving the innate immune system, which is the initial line of
defense against the invasion of pathogens (156,177). Innate immune cells are able to
eliminate infections with the assistance of these receptors, which
are able to identify RNA, DNA, proteins and lipids that are
associated with pathogens (156,177). However, their excessive
responses often result in injury to the tissues. Melatonin is able
to suppress the activation of TLR4, TLR9 and cGAS, which results in
a reduction in the innate immune response and a reduction in the
damage to tissue that is caused by infections, ischemia/reperfusion
and other disturbances (156,178-180).
Innate immune cells are directly affected by
melatonin, principally via the negative regulatory functions that
it has (156,181). It does this by preventing ERK
phosphorylation, which in turn prevents neutrophil migration and
the tissue damage that is associated with it (182). The administration of melatonin
lowers mast cell activation, TNF-α and IL-6 production, and
IKK/NF-κB signal transduction in activated mast cells (155,182-185). Treatment with melatonin
reverses the transformation from M2 anti-inflammatory macrophages
to M1 pro-inflammatory subtypes, which assists in the elimination
of SARS-CoV-2 and suppresses the dysfunctional hyper-inflammatory
response that is mediated by M1 macrophages (156,186). Whens physiological
circumstances are met, melatonin has the potential to boost innate
immunity, thus maintaining its protective effects against the
invasion of pathogens (31,187).
Melatonin may also have an effect on COVID-19
infection by preventing the virus from entering cells and
replicating after first entry (17,25,156). There are three enzymes that are
responsible for the entry of SARS-CoV-2 into cells: ACE2,
transmembrane protease serine 2 and A disintegrin and
metalloprotease 17 (188-190). It is possible that melatonin
can target these molecules in order to delay the entry of the
coronavirus into the cells (189). The progression of COVID-19 may
be controlled by the circadian system, while the melatonin
circadian rhythm may also be responsible for this regulation
(155,188-190). It is also possible that
melatonin may influence ACE2 activity in an indirect manner by
binding to calmodulin or MMP9 (191). Recent research has indicated
that melatonin has the potential for use as a therapeutic agent on
ACE2. It has been found that transgenic mice exhibit greater
vulnerability to SARS-CoV-2 infection, as well as delayed clinical
signs and an enhanced survival (192,193). In addition, melatonin has the
potential to decrease the activation of CD147 during a SARS-CoV-2
infection via inhibiting the production of HIF-1A (194). Research has demonstrated that
melatonin may reduce the reproduction of some viruses, such as
swine coronaviruses and Dengue virus, with the effectiveness of
this effect being dose-dependent (195,196). Melatonin may suppress
SARS-CoV-2 replication; however, to date, no animal research has
shown this to be true (197).
It is possible that melatonin inhibits viral replication by
blocking growth factor signaling (27,198,199). Due to its uniqueness and lack
of presence in host cells, the major protease (Mpro) of SARS-CoV-2
has emerged as a possible target for the development of replication
inhibitors (156). According to
the crystal structure of the SARS-CoV-2 Mpro and PF-07321332
complex, melatonin binds to the catalytic amino acid residues of
C145 and H41 via pi-sulfur/conventional hydrogen bonds and
carbon-hydrogen bonds. This suggests that melatonin works as an
effective Mpro inhibitor (156,194,200,201). In the following section, the
limited evidence of the beneficial effects of melatonin on patients
with COVID-19 is discussed, building on these potential advantages
derived from previous clinical or preclinical research.
Previous research on other viral diseases, together
with the possible antiviral properties of melatonin, has led to its
suggestion as a possible therapeutic agent for COVID-19 (17,49,202). Melatonin has been tested in
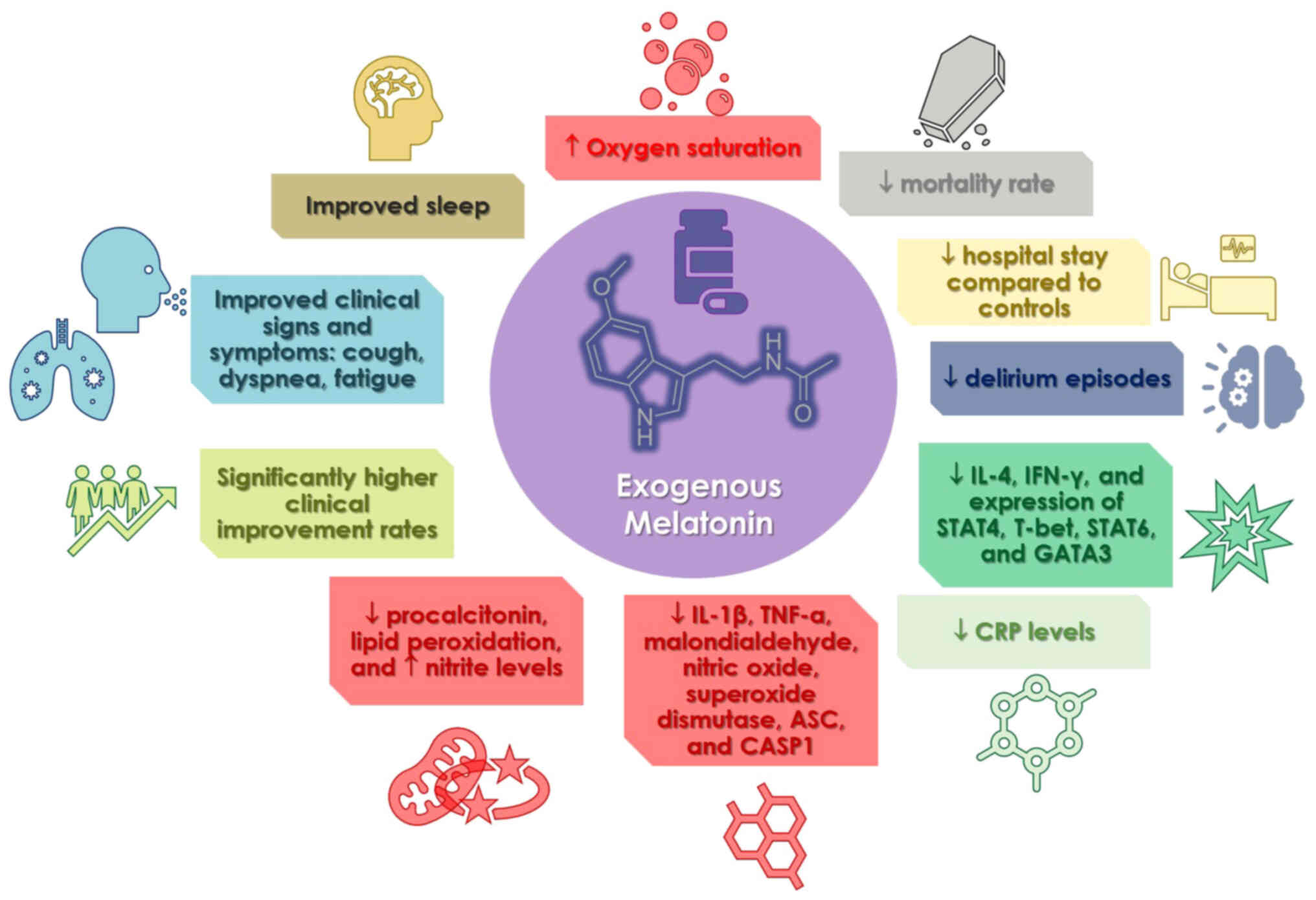
clinical studies for the treatment of COVID-19. The results
revealed that the drug improved sleep quality, reduced the duration
of hospitalization and was useful as a preventative measure
(155,180,202,203). However, the studies are
restricted owing to inadequate financial assistance (melatonin is
affordable and non-patentable) (156).
Only a small number of trials have studied the
safety and effectiveness of melatonin and its therapeutic value in
COVID-19, and they were only recently evaluated in a meta-analysis
(202). The most notable
findings were that patients using melatonin had a much higher
clinical improvement rate than the control groups (202). Melatonin administration also
resulted in a reduced death rate, reduced C-reactive protein (CRP)
concentration, and length of hospital stay than the controls
(202). The study concluded
that melatonin had significant benefits on patients with COVID-19
when administered as adjuvant treatment, boosting clinical
improvement and shortening recovery time owing to shorter hospital
stays and mechanical ventilation durations (202). Other research included the
following observations: The case group exhibited lower levels of
IL-4 and IFN-γ in their plasma, as well as lower levels of signal
transducer and activator of transcription (STAT)4, T-bet, STAT6 and
GATA binding protein 3 expression in comparison to the control
group (203). In their study,
Alizadeh et al (204)
discovered that the case group exhibited a reduction in CRP levels
both before and after the ingestion of melatonin. On the other
hand, the control group did not exhibit a significant reduction in
CRP levels. A different case group exhibited an improvement in
clinical signs and symptoms, such as cough, dyspnea and tiredness,
while simultaneously exhibiting a decrease in CRP levels in
comparison to the control group (205). When compared to the control
group, the low dosage of melatonin resulted in a reduction in CRP
levels, lung involvement, a shorter time to discharge from the
hospital, and a shorter period after returning to baseline health
(206). According to the
findings of another study that examined the quality of sleep and
other outcomes of patients with COVID-19, both oxygen saturation
and sleep quality increased (207). Chavarría et al (208) demonstrated that melatonin
supplementation in patients with moderate symptoms resulted in
decreased levels of CRP, IL-6, procalcitonin and lipid
peroxidation, and elevated nitrite levels. In addition, the levels
of numerous pro-inflammatory indicators, such as IL-1β, TNF-α,
malondialdehyde, nitric oxide, superoxide dismutase, ASC and CASP1,
were found to be lower in persons who were administered melatonin
in comparison to the group that served as the control (209). Finally, patients with COVID-19
and insomnia who received prolonged-release melatonin exhibited
improvements in their sleep, a reduction in the number of episodes
of delirium, a shorter length of hospitalization, a shorter stay in
the sub-intensive care unit, and a shorter duration of therapy with
non-invasive ventilation (210). The benefits associated with the
use of melatonin in COVID-19 clinical studies are illustrated in
Fig. 3.
COVID-19 remains a critical global health concern.
Acute COVID pathophysiology linked to the cytokine storm and
oxidative stress, and long COVID research have yielded
mitochondrial dysfunction among other mechanisms, all of which can
be alleviated by providing melatonin (17). The treatment options that have
been proposed include, in addition to enhancing the function of
immune cells, the elimination of autoantibodies, immunosuppressants
and antivirals, as well as agents that possess antioxidant
properties, mitochondrial support and the generation of
mitochondrial energy (18,159). A number of these could be
achieved by including the use of melatonin as an adjuvant
therapeutic option. However, despite promising and with positive
outcomes based on a small number of clinical trials, its actions
need to be investigated further, as an ample amount of the
therapeutic potential of melatonin remains underexplored, also due
to funding limitations (27,202). On the other hand, further
clinical studies that are well-designed are warranted in order to
validate these findings (202).
Of utmost interest would be the design of trials with various time
points primarily examining the acute phase anti-inflammatory
properties and on a longer term, the preventive potential against
mitochondrial damage and long COVID pathology (17). Finally, the factors influencing
the effects of melatonin, including dosage also need to be
thoroughly explored.
Not applicable.
DAS and VEG conceptualized the study. IGL, VEG, RJR
and DAS made a substantial contribution to the interpretation and
analysis of data from the literature to be included in the review,
and wrote and prepared the draft of the manuscript. DAS and RJR
analyzed the data and provided critical revisions. All authors
contributed to manuscript revision, and have read and approved the
final version of the manuscript. Data authentication is not
applicable.
Not applicable.
Not applicable.
DAS is the Editor-in-Chief for the journal, but had
no personal involvement in the reviewing process, or any influence
in terms of adjudicating on the final decision, for this article.
The other authors declare that they have no competing
interests.
The title of the present review was inspired by
William Shakespeare's theatrical masterpiece 'A Midsummer
Night's Dream'.
No funding was received.
|
1
|
Wang D, Hu B, Hu C, Zhu F, Liu X, Zhang J,
Wang B, Xiang H, Cheng Z, Xiong Y, et al: Clinical characteristics
of 138 hospitalized patients with 2019 novel coronavirus-infected
pneumonia in Wuhan, China. JAMA. 323:1061–1069. 2020.
|
|
2
|
Georgakopoulou VE, Makrodimitri S,
Triantafyllou M, Samara S, Voutsinas PM, Anastasopoulou A,
Papageorgiou CV, Spandidos DA, Gkoufa A, Papalexis P, et al:
Immature granulocytes: Innovative biomarker for SARS-CoV-2
infection. Mol Med Rep. 26:2172022.
|
|
3
|
Lempesis IG, Karlafti E, Papalexis P,
Fotakopoulos G, Tarantinos K, Lekakis V, Papadakos SP, Cholongitas
E and Georgakopoulou VE: COVID-19 and liver injury in individuals
with obesity. World J Gastroenterol. 29:908–916. 2023.
|
|
4
|
Guan WJ, Ni ZY, Hu Y, Liang WH, Ou CQ, He
JX, Liu L, Shan H, Lei CL, Hui DSC, et al: Clinical characteristics
of coronavirus disease 2019 in China. N Engl J Med. 382:1708–1720.
2020.
|
|
5
|
Gracia-Ramos AE, Jaquez-Quintana JO,
Contreras-Omana R and Auron M: Liver dysfunction and SARS-CoV-2
infection. World J Gastroenterol. 27:3951–3970. 2021.
|
|
6
|
Gkoufa A, Maneta E, Ntoumas GN,
Georgakopoulou VE, Mantelou A, Kokkoris S and Routsi C: Elderly
adults with COVID-19 admitted to intensive care unit: A narrative
review. World J Crit Care Med. 10:278–289. 2021.
|
|
7
|
Georgakopoulou VE, Gkoufa A, Garmpis N,
Makrodimitri S, Papageorgiou CV, Barlampa D, Garmpi A, Chiapoutakis
S, Sklapani P, Trakas N and Damaskos C: COVID-19 and acute
pancreatitis: A systematic review of case reports and case series.
Ann Saudi Med. 42:276–287. 2022.
|
|
8
|
Georgakopoulou VE, Lembessis P, Skarlis C,
Gkoufa A, Sipsas NV and Mavragani CP: Hematological abnormalities
in COVID-19 disease: Association with type I interferon pathway
activation and disease outcomes. Front Med (Lausanne).
9:8504722022.
|
|
9
|
Madjid M, Safavi-Naeini P, Solomon SD and
Vardeny O: Potential effects of coronaviruses on the cardiovascular
system: A review. JAMA Cardiol. 5:831–840. 2020.
|
|
10
|
Lempesis IG and Georgakopoulou VE:
Implications of obesity and adiposopathy on respiratory infections;
focus on emerging challenges. World J Clin Cases. 11:2925–2933.
2023.
|
|
11
|
Lempesis IG and Georgakopoulou VE:
Physiopathological mechanisms related to inflammation in obesity
and type 2 diabetes mellitus. World J Exp Med. 13:7–16. 2023.
|
|
12
|
Georgakopoulou VE, Bali T, Adamantou M,
Asimakopoulou S, Makrodimitri S, Samara S, Triantafyllou M,
Voutsinas PM, Eliadi I, Karamanakos G, et al: Acute hepatitis and
liver injury in hospitalized patients with COVID-19 infection. Exp
Ther Med. 24:6912022.
|
|
13
|
Mathioudakis N, Zachiotis M, Papadakos S,
Triantafyllou M, Karapanou A, Samara S, Karamanakos G, Spandidos
DA, Papalexis P, Damaskos C, et al: Onodera's prognostic
nutritional index: Comparison of its role in the severity and
outcomes of patients with COVID-19 during the periods of alpha,
delta and omicron variant predominance. Exp Ther Med.
24:6752022.
|
|
14
|
Cholongitas E, Bali T, Georgakopoulou VE,
Kamiliou A, Vergos I, Makrodimitri S, Samara S, Triantafylou M,
Basoulis D, Eliadi I, et al: Comparison of liver function test- and
inflammation-based prognostic scores for coronavirus disease 2019:
A single center study. Eur J Gastroenterol Hepatol. 34:1165–1171.
2022.
|
|
15
|
Georgakopoulou VE, Gkoufa A, Damaskos C,
Papalexis P, Pierrakou A, Makrodimitri S, Sypsa G, Apostolou A,
Asimakopoulou S, Chlapoutakis S, et al: COVID-19-associated acute
appendicitis in adults. A report of five cases and a review of the
literature. Exp Ther Med. 24:4822022.
|
|
16
|
Cholongitas E, Bali T, Georgakopoulou VE,
Giannakodimos A, Gyftopoulos A, Georgilaki V, Gerogiannis D,
Basoulis D, Eliadi I, Karamanakos G, et al: Prevalence of abnormal
liver biochemistry and its impact on COVID-19 patients' outcomes: A
single-center Greek study. Ann Gastroenterol. 35:290–296. 2022.
|
|
17
|
Loh D and Reiter RJ: Melatonin: Regulation
of viral phase separation and epitranscriptomics in post-acute
sequelae of COVID-19. Int J Mol Sci. 23:81222022.
|
|
18
|
Davis HE, McCorkell L, Vogel JM and Topol
EJ: Long COVID: Major findings, mechanisms and recommendations. Nat
Rev Microbiol. 21:133–146. 2023.
|
|
19
|
Mehandru S and Merad M: Pathological
sequelae of long-haul COVID. Nat Immunol. 23:194–202. 2022.
|
|
20
|
Vehar S, Boushra M, Ntiamoah P and Biehl
M: Post-acute sequelae of SARS-CoV-2 infection: Caring for the
'long-haulers'. Cleve Clin J Med. 88:267–272. 2021.
|
|
21
|
Bali T, Georgakopoulou VE, Kamiliou A,
Vergos I, Adamantou M, Vlachos S, Ermidis G, Sipsas NV, Samarkos M
and Cholongitas E: Abnormal liver function tests and coronavirus
disease 2019: A close relationship. J Viral Hepat. 30:79–80.
2023.
|
|
22
|
Guarnieri JW, Dybas JM, Fazelinia H, Kim
MS, Frere J, Zhang Y, Soto Albrecht Y, Murdock DG, Angelin A, Singh
LN, et al: Core mitochondrial genes are down-regulated during
SARS-CoV-2 infection of rodent and human hosts. Sci Transl Med.
15:eabq15332023.
|
|
23
|
Reiter RJ, Tan DX and Galano A: Melatonin:
Exceeding expectations. Physiology (Bethesda). 29:325–333.
2014.
|
|
24
|
Reiter RJ, Sharma R, Rosales-Corral S, de
Campos Zuccari DAP and de Almeida Chuffa LG: Melatonin: A
mitochondrial resident with a diverse skill set. Life Sci.
301:1206122022.
|
|
25
|
Reiter RJ, Sharma R, Tan DX, Neel RL,
Simko F, Manucha W, Rosales-Corral S and Cardinali DP: Melatonin
use for SARS-CoV-2 infection: Time to diversify the treatment
portfolio. J Med Virol. 94:2928–2930. 2022.
|
|
26
|
Romero A, Ramos E, López-Muñoz F,
Gil-Martín E, Escames G and Reiter RJ: Coronavirus disease 2019
(COVID-19) and its neuroinvasive capacity: Is it time for
melatonin? Cell Mol Neurobiol. 42:489–500. 2022.
|
|
27
|
Reiter RJ, Sharma R, Simko F,
Dominguez-Rodriguez A, Tesarik J, Neel RL, Slominski AT,
Kleszczynski K, Martin-Gimenez VM, Manucha W and Cardinali DP:
Melatonin: Highlighting its use as a potential treatment for
SARS-CoV-2 infection. Cell Mol Life Sci. 79:1432022.
|
|
28
|
Mouffak S, Shubbar Q, Saleh E and El-Awady
R: Recent advances in management of COVID-19: A review. Biomed
Pharmacother. 143:1121072021.
|
|
29
|
Wichniak A, Kania A, Siemiński M and
Cubała WJ: Melatonin as a potential adjuvant treatment for COVID-19
beyond sleep disorders. Int J Mol Sci. 22:86232021.
|
|
30
|
Ramos E, López-Muñoz F, Gil-Martín E, Egea
J, Álvarez-Merz I, Painuli S, Semwal P, Martins N, Hernández-Guijo
JM and Romero A: The coronavirus disease 2019 (COVID-19): key
emphasis on melatonin safety and therapeutic efficacy. Antioxidants
(Basel). 10:11522021.
|
|
31
|
Boga JA, Coto-Montes A, Rosales-Corral SA,
Tan DX and Reiter RJ: Beneficial actions of melatonin in the
management of viral infections: A new use for this 'molecular
handyman'? Rev Med Virol. 22:323–338. 2012.
|
|
32
|
Juybari KB, Pourhanifeh MH, Hosseinzadeh
A, Hemati K and Mehrzadi S: Melatonin potentials against viral
infections including COVID-19: Current evidence and new findings.
Virus Res. 287:1981082020.
|
|
33
|
Georgakopoulou VE, Gkoufa A, Makrodimitri
S, Basoulis D, Tsakanikas A, Karamanakos G, Mastrogianni E,
Voutsinas PM, Spandidos DA, Papageorgiou CV, et al: Early 3-day
course of remdesivir for the prevention of the progression to
severe COVID-19 in the elderly: A single-centre, real-life cohort
study. Exp Ther Med. 26:4622023.
|
|
34
|
Basoulis D, Tsakanikas A, Gkoufa A,
Bitsani A, Karamanakos G, Mastrogianni E, Georgakopoulou VE,
Makrodimitri S, Voutsinas PM, Lamprou P, et al: Effectiveness of
oral nirmatrelvir/ritonavir vs intravenous three-day remdesivir in
preventing progression to severe COVID-19: A single-center,
prospective, comparative, real-life study. Viruses.
15:15152023.
|
|
35
|
Papadopoulou A, Karavalakis G,
Papadopoulou E, Xochelli A, Bousiou Z, Vogiatzoglou A, Papayanni
PG, Georgakopoulou A, Giannaki M, Stavridou F, et al:
SARS-CoV-2-specific T cell therapy for severe COVID-19: a
randomized phase 1/2 trial. Nat Med. 29:2019–2029. 2023.
|
|
36
|
Karlafti E, Paramythiotis D, Pantazi K,
Georgakopoulou VE, Kaiafa G, Papalexis P, Protopapas AA, Ztriva E,
Fyntanidou V and Savopoulos C: Drug-induced liver injury in
hospitalized patients during SARS-CoV-2 infection. Medicina
(Kaunas). 58:18482022.
|
|
37
|
Georgakopoulou VE, Basoulis D, Voutsinas
PM, Makrodimitri S, Samara S, Triantafyllou M, Eliadi I,
Karamanakos G, Papageorgiou CV, Anastasopoulou A, et al: Factors
predicting poor outcomes of patients treated with tocilizumab for
COVID-19-associated pneumonia: A retrospective study. Exp Ther Med.
24:7242022.
|
|
38
|
Gkoufa A, Saridaki M, Georgakopoulou VE,
Spandidos DA and Cholongitas E: COVID-19 vaccination in liver
transplant recipients (Review). Exp Ther Med. 25:2912023.
|
|
39
|
Elrashdy F, Tambuwala MM, Hassan SS, Adadi
P, Seyran M, Abd El-Aziz TM, Rezaei N, Lal A, Aljabali AAA,
Kandimalla R, et al: Autoimmunity roots of the thrombotic events
after COVID-19 vaccination. Autoimmun Rev. 20:1029412021.
|
|
40
|
Taskou C, Sarantaki A, Beloukas A,
Georgakopoulou VE, Daskalakis G, Papalexis P and Lykeridou A:
Knowledge and attitudes of healthcare professionals regarding
perinatal influenza vaccination during the COVID-19 pandemic.
Vaccines (Basel). 11:1682023.
|
|
41
|
Goeser S, Ruble J and Chandler L:
Melatonin: Historical and clinical perspectives. J Pharmaceut Care
Pain Symptom Contr. 5:37–49. 1997.
|
|
42
|
Beyer CE, Steketee JD and Saphier D:
Antioxidant properties of melatonin-an emerging mystery. Biochem
Pharmacol. 56:1265–1272. 1998.
|
|
43
|
Ahmad SB, Ali A, Bilal M, Rashid SM, Wani
AB, Bhat RR and Rehman MU: Melatonin and health: Insights of
melatonin action, biological functions, and associated disorders.
Cell Mol Neurobiol. 43:2437–2458. 2023.
|
|
44
|
Hardeland R, Balzer I, Poeggeler B,
Fuhrberg B, Uría H, Behrmann G, Wolf R, Meyer TJ and Reiter RJ: On
the primary functions of melatonin in evolution: Mediation of
photoperiodic signals in a unicell, photooxidation, and scavenging
of free radicals. J Pineal Res. 18:104–111. 1995.
|
|
45
|
Cipolla-Neto J and Amaral FGD: Melatonin
as a hormone: New physiological and clinical insights. Endocr Rev.
39:990–1028. 2018.
|
|
46
|
Manchester LC, Coto-Montes A, Boga JA,
Andersen LP, Zhou Z, Galano A, Vriend J, Tan DX and Reiter RJ:
Melatonin: An ancient molecule that makes oxygen metabolically
tolerable. J Pineal Res. 59:403–419. 2015.
|
|
47
|
Hardeland R, Reiter R, Poeggeler B and Tan
DX: The significance of the metabolism of the neurohormone
melatonin: Antioxidative protection and formation of bioactive
substances. Neurosci Biobehav Rev. 17:347–357. 1993.
|
|
48
|
Reiter RJ: Pineal melatonin: Cell biology
of its synthesis and of its physiological interactions. Endocr Rev.
12:151–180. 1991.
|
|
49
|
Hosseinzadeh A, Bagherifard A, Koosha F,
Amiri S, Karimi-Behnagh A, Reiter RJ and Mehrzadi S: Melatonin
effect on platelets and coagulation: Implications for a
prophylactic indication in COVID-19. Life Sci. 307:1208662022.
|
|
50
|
Tan DX, Hardeland R, Manchester LC,
Paredes SD, Korkmaz A, Sainz RM, Mayo JC, Fuentes-Broto L and
Reiter RJ: The changing biological roles of melatonin during
evolution: From an antioxidant to signals of darkness, sexual
selection and fitness. Biol Rev Camb Philos Soc. 85:607–623.
2010.
|
|
51
|
Andersen LPH, Gögenur I, Rosenberg J and
Reiter RJ: Pharmacokinetics of melatonin: The missing link in
clinical efficacy? Clin Pharmacokinet. 55:1027–1030. 2016.
|
|
52
|
Reiter RJ, Tan DX and Korkmaz A: The
circadian melatonin rhythm and its modulation: Possible impact on
hypertension. J Hypertens Suppl. 27:S17–S20. 2009.
|
|
53
|
Vriend J and Reiter RJ: Melatonin feedback
on clock genes: A theory involving the proteasome. J Pineal Res.
58:1–11. 2015.
|
|
54
|
Erren TC and Reiter RJ: Melatonin: A
universal time messenger. Neuro Endocrinol Lett. 36:187–192.
2015.
|
|
55
|
Mehrzadi S, Karimi MY, Fatemi A, Reiter RJ
and Hosseinzadeh A: SARS-CoV-2 and other coronaviruses negatively
influence mitochondrial quality control: Beneficial effects of
melatonin. Pharmacol Ther. 224:1078252021.
|
|
56
|
Mauriz JL, Collado PS, Veneroso C, Reiter
RJ and González-Gallego J: A review of the molecular aspects of
melatonin's anti-inflammatory actions: Recent insights and new
perspectives. J Pineal Res. 54:1–14. 2013.
|
|
57
|
Slominski RM, Reiter RJ,
Schlabritz-Loutsevitch N, Ostrom RS and Slominski AT: Melatonin
membrane receptors in peripheral tissues: Distribution and
functions. Mol Cell Endocrinol. 351:152–166. 2012.
|
|
58
|
Gurunathan S, Kang MH, Choi Y, Reiter RJ
and Kim JH: Melatonin: A potential therapeutic agent against
COVID-19. Melatonin Res. 4:30–69. 2021.
|
|
59
|
Naskar A, Prabhakar V, Singh R, Dutta D
and Mohanakumar KP: Melatonin enhances L-DOPA therapeutic effects,
helps to reduce its dose, and protects dopaminergic neurons in
1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine-induced Parkinsonism
in mice. J Pineal Res. 58:262–274. 2015.
|
|
60
|
Reiter RJ, Mayo JC, Tan DX, Sainz RM,
Alatorre-Jimenez M and Qin L: Melatonin as an antioxidant: Under
promises but over delivers. J Pineal Res. 61:253–278. 2016.
|
|
61
|
Ramos E, Patiño P, Reiter RJ, Gil-Martín
E, Marco-Contelles J, Parada E, de Los Rios C, Romero A and Egea J:
Ischemic brain injury: New insights on the protective role of
melatonin. Free Radic Biol Med. 104:32–53. 2017.
|
|
62
|
Sanchez-Barcelo EJ, Rueda N, Mediavilla
MD, Martinez-Cue C and Reiter RJ: Clinical uses of melatonin in
neurological diseases and mental and behavioural disorders. Curr
Med Chem. 24:3851–3878. 2017.
|
|
63
|
Ali T, Rahman SU, Hao Q, Li W, Liu Z, Ali
Shah F, Murtaza I, Zhang Z, Yang X, Liu G and Li S: Melatonin
prevents neuroinflammation and relieves depression by attenuating
autophagy impairment through FOXO3a regulation. J Pineal Res.
69:e126672020.
|
|
64
|
Vriend J and Reiter RJ: Melatonin as a
proteasome inhibitor. Is there any clinical evidence? Life Sci.
115:8–14. 2014.
|
|
65
|
Mayo JC, Sainz RM, González Menéndez P,
Cepas V, Tan DX and Reiter RJ: Melatonin and sirtuins: A 'not-so
unexpected' relationship. J Pineal Res. 62:e123912017.
|
|
66
|
Gurunathan S, Qasim M, Kang MH and Kim JH:
Role and therapeutic potential of melatonin in various type of
cancers. Onco Targets Ther. 14:2019–2052. 2021.
|
|
67
|
Pourhanifeh MH, Mehrzadi S, Kamali M and
Hosseinzadeh A: Melatonin and gastrointestinal cancers: Current
evidence based on underlying signaling pathways. Eur J Pharmacol.
886:1734712020.
|
|
68
|
Reiter RJ, Rosales-Corral SA, Tan DX,
Acuna-Castroviejo D, Qin L, Yang SF and Xu K: Melatonin, a full
service anti-cancer agent: inhibition of initiation, progression
and metastasis. Int J Mol Sci. 18:8432017.
|
|
69
|
Reiter RJ, Tan DX, Paredes SD and
Fuentes-Broto L: Beneficial effects of melatonin in cardiovascular
disease. Ann Med. 42:276–285. 2010.
|
|
70
|
Galano A, Tan DX and Reiter RJ: Melatonin:
A versatile protector against oxidative DNA damage. Molecules.
23:5302018.
|
|
71
|
Simko F, Baka T, Paulis L and Reiter RJ:
Elevated heart rate and nondipping heart rate as potential targets
for melatonin: A review. J Pineal Res. 61:127–137. 2016.
|
|
72
|
Reiter RJ, Tan DX, Kim SJ and Qi W:
Melatonin as a pharmacological agent against oxidative damage to
lipids and DNA. Proc West Pharmacol Soc. 41:229–236. 1998.
|
|
73
|
Reiter RJ, Tan DX, Rosales-Corral S and
Manchester LC: The universal nature, unequal distribution and
antioxidant functions of melatonin and its derivatives. Mini Rev
Med Chem. 13:373–384. 2013.
|
|
74
|
García JJ, López-Pingarrón L,
Almeida-Souza P, Tres A, Escudero P, García-Gil FA, Tan DX, Reiter
RJ, Ramírez JM and Bernal-Pérez M: Protective effects of melatonin
in reducing oxidative stress and in preserving the fluidity of
biological membranes: A review. J Pineal Res. 56:225–237. 2014.
|
|
75
|
Tan DX, Korkmaz A, Reiter RJ and
Manchester LC: Ebola virus disease: Potential use of melatonin as a
treatment. J Pineal Res. 57:381–384. 2014.
|
|
76
|
Reiter RJ, Ma Q and Sharma R: Treatment of
Ebola and other infectious diseases: Melatonin 'goes viral'.
Melatonin Res. 3:43–57. 2020.
|
|
77
|
Reiter RJ, Sharma R, Ma Q, Liu C, Manucha
W, González P and Dominguez-Rodriguez A: Metabolic plasticity of
activated immune cells: Advantages for suppression of COVID-19
disease by melatonin. Melatonin Res. 3:362–379. 2020.
|
|
78
|
Reiter RJ, Sharma R, Ma Q,
Dominquez-Rodriguez A, Marik PE and Abreu-Gonzalez P: Melatonin
inhibits COVID-19-induced cytokine storm by reversing aerobic
glycolysis in immune cells: A mechanistic analysis. Med Drug
Discov. 6:1000442020.
|
|
79
|
Sarkar S, Chattopadhyay A and
Bandyopadhyay D: Multiple strategies of melatonin protecting
against cardiovascular injury related to inflammation: A
comprehensive overview. Melatonin Res. 4:1–29. 2021.
|
|
80
|
Esposito E and Cuzzocrea S:
Antiinflammatory activity of melatonin in central nervous system.
Curr Neuropharmacol. 8:228–242. 2010.
|
|
81
|
Hardeland R, Cardinali DP, Brown GM and
Pandi-Perumal SR: Melatonin and brain inflammaging. Prog Neurobiol.
127-128:46–63. 2015.
|
|
82
|
Farez MF, Mascanfroni ID, Méndez-Huergo
SP, Yeste A, Murugaiyan G, Garo LP, Balbuena Aguirre ME, Patel B,
Ysrraelit MC, Zhu C, et al: Melatonin contributes to the
seasonality of multiple sclerosis relapses. Cell. 162:1338–1352.
2015.
|
|
83
|
Dominguez-Rodriguez A, Abreu-Gonzalez P,
Marik PE and Reiter RJ: Melatonin, cardiovascular disease and
COVID-19: A potential therapeutic strategy? Melatonin Res.
3:318–321. 2020.
|
|
84
|
Ozdemir G, Ergün Y, Bakariş S, Kılınç M,
Durdu H and Ganiyusufoğlu E: Melatonin prevents retinal oxidative
stress and vascular changes in diabetic rats. Eye (Lond).
28:1020–1027. 2014.
|
|
85
|
Gheban BA, Rosca IA and Crisan M: The
morphological and functional characteristics of the pineal gland.
Med Pharm Rep. 92:226–234. 2019.
|
|
86
|
Cipolla-Neto J, Amaral F, Afeche SC, Tan
DX and Reiter RJ: Melatonin, energy metabolism, and obesity: A
review. J Pineal Res. 56:371–381. 2014.
|
|
87
|
Lempesis IG, Hoebers N, Essers Y, Jocken
JWE, Dineen R, Blaak EE, Manolopoulos KN and Goossens GH: Distinct
inflammatory signatures of upper and lower body adipose tissue and
adipocytes in women with normal weight or obesity. Front Endocrinol
(Lausanne). 14:12057992023.
|
|
88
|
Lempesis IG, Varrias D, Sagris M, Attaran
RR, Altin ES, Bakoyiannis C, Palaiodimos L, Dalamaga M and
Kokkinidis DG: Obesity and peripheral artery disease: Current
evidence and controversies. Curr Obes Rep. 12:264–279. 2023.
|
|
89
|
Lempesis IG, Apple SJ, Duarte G,
Palaiodimos L, Kalaitzopoulos DR, Dalamaga M and Kokkinidis DG:
Cardiometabolic effects of SGLT2 inhibitors on polycystic ovary
syndrome. Diabetes Metab Res Rev. 39:e36822023.
|
|
90
|
Lempesis IG, Tsilingiris D, Liu J and
Dalamaga M: Of mice and men: Considerations on adipose tissue
physiology in animal models of obesity and human studies. Metabol
Open. 15:1002082022.
|
|
91
|
Simko F, Hrenak J, Dominguez-Rodriguez A
and Reiter RJ: Melatonin as a putative protection against
myocardial injury in COVID-19 infection. Expert Rev Clin Pharmacol.
13:921–924. 2020.
|
|
92
|
Mirza-Aghazadeh-Attari M, Reiter RJ,
Rikhtegar R, Jalili J, Hajalioghli P, Mihanfar A, Majidinia M and
Yousefi B: Melatonin: An atypical hormone with major functions in
the regulation of angiogenesis. IUBMB Life. 72:1560–1584. 2020.
|
|
93
|
Melhuish Beaupre LM, Brown GM, Gonçalves
VF and Kennedy JL: Melatonin's neuroprotective role in mitochondria
and its potential as a biomarker in aging, cognition and
psychiatric disorders. Transl Psychiatry. 11:3392021.
|
|
94
|
Reiter RJ, Rosales-Corral S, Tan DX, Jou
MJ, Galano A and Xu B: Melatonin as a mitochondria-targeted
antioxidant: One of evolution's best ideas. Cell Mol Life Sci.
74:3863–3881. 2017.
|
|
95
|
Reiter RJ, Ma Q and Sharma R: Melatonin in
mitochondria: Mitigating clear and present dangers. Physiology
(Bethesda). 35:86–95. 2020.
|
|
96
|
Wakatsuki A, Okatani Y, Shinohara K,
Ikenoue N, Kaneda C and Fukaya T: Melatonin protects fetal rat
brain against oxidative mitochondrial damage. J Pineal Res.
30:22–28. 2001.
|
|
97
|
Reiter RJ, Tan DX, Rosales-Corral S,
Galano A, Jou MJ and Acuna-Castroviejo D: Melatonin mitigates
mitochondrial meltdown: Interactions with SIRT3. Int J Mol Sci.
19:24392018.
|
|
98
|
Han L, Wang H, Li L, Li X, Ge J, Reiter RJ
and Wang Q: Melatonin protects against maternal obesity-associated
oxidative stress and meiotic defects in oocytes via the
SIRT3-SOD2-dependent pathway. J Pineal Res. 63:e124312017.
|
|
99
|
Mar tín M, Macías M, Escames G, León J and
Acuña-Castroviejo D: Melatonin but not vitamins C and E maintains
glutathione homeostasis in t-butyl hydroperoxide-induced
mitochondrial oxidative stress. FASEB J. 14:1677–1679. 2000.
|
|
100
|
Venegas C, García JA, Escames G, Ortiz F,
López A, Doerrier C, García-Corzo L, López LC, Reiter RJ and
Acuña-Castroviejo D: Extrapineal melatonin: Analysis of its
subcellular distribution and daily fluctuations. J Pineal Res.
52:217–227. 2012.
|
|
101
|
Suofu Y, Li W, Jean-Alphonse FG, Jia J,
Khattar NK, Li J, Baranov SV, Leronni D, Mihalik AC, He Y, et al:
Dual role of mitochondria in producing melatonin and driving GPCR
signaling to block cytochrome c release. Proc Natl Acad Sci USA.
114:E7997–E8006. 2017.
|
|
102
|
He C, Wang J, Zhang Z, Yang M, Li Y, Tian
X, Ma T, Tao J, Zhu K, Song Y, et al: Mitochondria synthesize
melatonin to ameliorate its function and improve mice oocyte's
quality under in vitro conditions. Int J Mol Sci. 17:9392016.
|
|
103
|
Tan DX and Reiter RJ: Mitochondria: The
birth place, battle ground and the site of melatonin metabolism in
cells. Melatonin Res. 2:44–66. 2019.
|
|
104
|
Chumboatong W, Thummayot S, Govitrapong P,
Tocharus C, Jittiwat J and Tocharus J: Neuroprotection of
agomelatine against cerebral ischemia/reperfusion injury through an
antiapoptotic pathway in rat. Neurochem Int. 102:114–122. 2017.
|
|
105
|
de Vries HE, Witte M, Hondius D,
Rozemuller AJ, Drukarch B, Hoozemans J and van Horssen J:
Nrf2-induced antioxidant protection: A promising target to
counteract ROS-mediated damage in neurodegenerative disease? Free
Radic Biol Med. 45:1375–1383. 2008.
|
|
106
|
Martin M, Macias M, Escames G, Reiter RJ,
Agapito MT, Ortiz GG and Acuña-Castroviejo D: Melatonin-induced
increased activity of the respiratory chain complexes I and IV can
prevent mitochondrial damage induced by ruthenium red in vivo. J
Pineal Res. 28:242–248. 2000.
|
|
107
|
Sheleme T, Bekele F and Ayela T: Clinical
presentation of patients infected with coronavirus disease 19: A
systematic review. Infect Dis (Auckl). 13:11786337209520762020.
|
|
108
|
Lempesis IG, Georgakopoulou VE, Papalexis
P, Chrousos GP and Spandidos DA: Role of stress in the pathogenesis
of cancer (Review). Int J Oncol. 63:1242023.
|
|
109
|
Georgakopoulou VE, Gkoufa A, Bougea A,
Basoulis D, Tsakanikas A, Makrodimitri S, Karamanakos G, Spandidos
DA, Angelopoulou E and Sipsas NV: Characteristics and outcomes of
elderly patients with Parkinson's disease hospitalized due to
COVID-19-associated pneumonia. Med Int (Lond). 3:342023.
|
|
110
|
Bourgonje AR, Abdulle AE, Timens W,
Hillebrands JL, Navis GJ, Gordijn SJ, Bolling MC, Dijkstra G, Voors
AA, Osterhaus AD, et al: Angiotensin-converting enzyme 2 (ACE2),
SARS-CoV-2 and the pathophysiology of coronavirus disease 2019
(COVID-19). J Pathol. 251:228–248. 2020.
|
|
111
|
Ozkurt Z and Çınar Tanrıverdi E: COVID-19:
Gastrointestinal manifestations, liver injury and recommendations.
World J Clin Cases. 10:1140–1163. 2022.
|
|
112
|
Zhang Q, Xiang R, Huo S, Zhou Y, Jiang S,
Wang Q and Yu F: Molecular mechanism of interaction between
SARS-CoV-2 and host cells and interventional therapy. Signal
Transduct Target Ther. 6:2332021.
|
|
113
|
Georgakopoulou VE, Gkoufa A, Tsakanikas A,
Makrodimitri S, Karamanakos G, Basoulis D, Voutsinas PM, Eliadi I,
Bougea A, Spandidos DA, et al: Predictors of COVID-19-associated
mortality among hospitalized elderly patients with dementia. Exp
Ther Med. 26:3952023.
|
|
114
|
Thakur V, Ratho RK, Kumar P, Bhatia SK,
Bora I, Mohi GK, Saxena SK, Devi M, Yadav D and Mehariya S:
Multi-organ involvement in COVID-19: Beyond pulmonary
manifestations. J Clin Med. 10:4462021.
|
|
115
|
Martín Giménez VM, de las Heras N, Ferder
L, Lahera V, Reiter RJ and Manucha W: Potential Effects of
melatonin and micronutrients on mitochondrial dysfunction during a
cytokine storm typical of oxidative/inflammatory diseases.
Diseases. 9:302021.
|
|
116
|
Bougea A, Georgakopoulou VE, Palkopoulou
M, Efthymiopoulou E, Angelopoulou E, Spandidos DA and Zikos P:
New-onset non-motor symptoms in patients with Parkinson's disease
and post-COVID-19 syndrome: A prospective cross-sectional study.
Med Int (Lond). 3:232023.
|
|
117
|
Ahmad I and Rathore FA: Neurological
manifestations and complications of COVID-19: A literature review.
J Clin Neurosci. 77:8–12. 2020.
|
|
118
|
Long B, Brady WJ, Koyfman A and Gottlieb
M: Cardiovascular complications in COVID-19. Am J Emerg Med.
38:1504–1507. 2020.
|
|
119
|
Legrand M, Bell S, Forni L, Joannidis M,
Koyner JL, Liu K and Cantaluppi V: Pathophysiology of
COVID-19-associated acute kidney injury. Nat Rev Nephrol.
17:751–764. 2021.
|
|
120
|
Thaweethai T, Jolley SE, Karlson EW,
Levitan EB, Levy B, McComsey GA, McCorkell L, Nadkarni GN,
Parthasarathy S, Singh U, et al: Development of a definition of
postacute sequelae of SARS-CoV-2 infection. JAMA. 329:1934–1946.
2023.
|
|
121
|
Efstathiou V, Stefanou MI, Demetriou M,
Siafakas N, Makris M, Tsivgoulis G, Zoumpourlis V, Kympouropoulos
SP, Tsoporis JN, Spandidos DA, et al: Long COVID and
neuropsychiatric manifestations (Review). Exp Ther Med.
23:3632022.
|
|
122
|
Efstathiou V, Stefanou MI, Demetriou M,
Siafakas N, Katsantoni E, Makris M, Tsivgoulis G, Zoumpourlis V,
Kympouropoulos SP, Tsoporis JN, et al: New-onset neuropsychiatric
sequelae and 'long-COVID'syndrome (Review). Exp Ther Med.
24:7052022.
|
|
123
|
Daugherty SE, Guo Y, Heath K, Dasmariñas
MC, Jubilo KG, Samranvedhya J, Lipsitch M and Cohen K: Risk of
clinical sequelae after the acute phase of SARS-CoV-2 infection:
Retrospective cohort study. BMJ. 373:n10982021.
|
|
124
|
Huang L, Li X, Gu X, Zhang H, Ren L, Guo
L, Liu M, Wang Y, Cui D, Wang Y, et al: Health outcomes in people 2
years after surviving hospitalisation with COVID-19: A longitudinal
cohort study. Lancet Respir Med. 10:863–876. 2022.
|
|
125
|
Huang C, Huang L, Wang Y, Li X, Ren L, Gu
X, Kang L, Guo L, Liu M, Zhou X, et al: 6-Month consequences of
COVID-19 in patients discharged from hospital: A cohort study.
Lancet. 397:220–232. 2021.
|
|
126
|
E E, R F, Öi E, Im L, M L, S R, E W, C J,
M H and A M: Impaired diffusing capacity for carbon monoxide is
common in critically ill Covid-19 patients at four months
post-discharge. Respir Med. 182:1063942021.
|
|
127
|
Antonelli M, Pujol JC, Spector TD,
Ourselin S and Steves CJ: Risk of long COVID associated with delta
versus omicron variants of SARS-CoV-2. Lancet. 399:2263–2264.
2022.
|
|
128
|
Quaglia F, Salladini E, Carraro M,
Minervini G, Tosatto SCE and Le Mercier P: SARS-CoV-2 variants
preferentially emerge at intrinsically disordered protein sites
helping immune evasion. FEBS J. 289:4240–4250. 2022.
|
|
129
|
Lipsitch M, Krammer F, Regev-Yochay G,
Lustig Y and Balicer RD: SARS-CoV-2 breakthrough infections in
vaccinated individuals: Measurement, causes and impact. Nat Rev
Immunol. 22:57–65. 2022.
|
|
130
|
Hayes LD, Ingram J and Sculthorpe NF: More
than 100 persistent symptoms of SARS-CoV-2 (long COVID): A scoping
review. Front Med (Lausanne). 8:7503782021.
|
|
131
|
Davis HE, Assaf GS, McCorkell L, Wei H,
Low RJ, Re'em Y, Redfield S, Austin JP and Akrami A: Characterizing
long COVID in an international cohort: 7 Months of symptoms and
their impact. EClinicalMedicine. 38:1010192021.
|
|
132
|
Gaebler C, Wang Z, Lorenzi JCC, Muecksch
F, Finkin S, Tokuyama M, Cho A, Jankovic M, Schaefer-Babajew D,
Oliveira TY, et al: Evolution of antibody immunity to SARS-CoV-2.
Nature. 591:639–644. 2021.
|
|
133
|
Proal AD and VanElzakker MB: Long COVID or
post-acute sequelae of COVID-19 (PASC): An overview of biological
factors that may contribute to persistent symptoms. Front
Microbiol. 12:6981692021.
|
|
134
|
Kalkeri R, Goebel S and Sharma GD:
SARS-CoV-2 shedding from asymptomatic patients: Contribution of
potential extrapulmonary tissue reservoirs. Am J Trop Med Hyg.
103:18–21. 2020.
|
|
135
|
Zubchenko S, Kril I, Nadizhko O, Matsyura
O and Chopyak V: Herpesvirus infections and post-COVID-19
manifestations: A pilot observational study. Rheumatol Int.
42:1523–1530. 2022.
|
|
136
|
Su Y, Yuan D, Chen DG, Ng RH, Wang K, Choi
J, Li S, Hong S, Zhang R, Xie J, et al: Multiple early factors
anticipate post-acute COVID-19 sequelae. Cell. 185:881–895.e20.
2022.
|
|
137
|
Schreiner P, Harrer T, Scheibenbogen C,
Lamer S, Schlosser A, Naviaux RK and Prusty BK: Human herpesvirus-6
reactivation, mitochondrial fragmentation, and the coordination of
antiviral and metabolic phenotypes in myalgic
encephalomyelitis/chronic fatigue syndrome. Immunohorizons.
4:201–215. 2020.
|
|
138
|
Peluso MJ, Deeks SG, Mustapic M,
Kapogiannis D, Henrich TJ, Lu S, Goldberg SA, Hoh R, Chen JY,
Martinez EO, et al: SARS-CoV-2 and mitochondrial proteins in
neural-derived exosomes of COVID-19. Ann Neurol. 91:772–781.
2022.
|
|
139
|
Villaume WA: Marginal BH4 deficiencies,
iNOS, and self-perpetuating oxidative stress in post-acute sequelae
of Covid-19. Med Hypotheses. 163:1108422022.
|
|
140
|
Saleh J, Peyssonnaux C, Singh KK and Edeas
M: Mitochondria and microbiota dysfunction in COVID-19
pathogenesis. Mitochondrion. 54:1–7. 2020.
|
|
141
|
Singh KK, Chaubey G, Chen JY and
Suravajhala P: Decoding SARS-CoV-2 hijacking of host mitochondria
in COVID-19 pathogenesis. Am J Physiol Cell Physiol. 319:C258–C267.
2020.
|
|
142
|
Marchi S, Guilbaud E, Tait SWG, Yamazaki T
and Galluzzi L: Mitochondrial control of inflammation. Nat Rev
Immunol. 23:159–173. 2023.
|
|
143
|
West AP and Shadel GS: Mitochondrial DNA
in innate immune responses and inflammatory pathology. Nat Rev
Immunol. 17:363–375. 2017.
|
|
144
|
Wang P, Luo R, Zhang M, Wang Y, Song T,
Tao T, Li Z, Jin L, Zheng H, Chen W, et al: A cross-talk between
epithelium and endothelium mediates human alveolar-capillary injury
during SARS-CoV-2 infection. Cell Death Dis. 11:10422020.
|
|
145
|
Cortese M, Lee JY, Cerikan B, Neufeldt CJ,
Oorschot VMJ, Köhrer S, Hennies J, Schieber NL, Ronchi P, Mizzon G,
et al: Integrative imaging reveals SARS-CoV-2-induced reshaping of
subcellular morphologies. Cell Host Microbe. 28:853–866.e5.
2020.
|
|
146
|
Hui DS, I Azhar E, Madani TA, Ntoumi F,
Kock R, Dar O, Ippolito G, Mchugh TD, Memish ZA, Drosten C, et al:
The continuing 2019-nCoV epidemic threat of novel coronaviruses to
global health-the latest 2019 novel coronavirus outbreak in Wuhan,
China. Int J Infect Dis. 91:264–266. 2020.
|
|
147
|
Gordon DE, Hiatt J, Bouhaddou M, Rezelj
VV, Ulferts S, Braberg H, Jureka AS, Obernier K, Guo JZ, Batra J,
et al: Comparative host-coronavirus protein interaction networks
reveal pan-viral disease mechanisms. Science. 370:eabe94032020.
|
|
148
|
Gordon DE, Jang GM, Bouhaddou M, Xu J,
Obernier K, White KM, O'Meara MJ, Rezelj VV, Guo JZ, Swaney DL, et
al: A SARS-CoV-2 protein interaction map reveals targets for drug
repurposing. Nature. 583:459–468. 2020.
|
|
149
|
Stukalov A, Girault V, Grass V, Karayel O,
Bergant V, Urban C, Haas DA, Huang Y, Oubraham L, Wang A, et al:
Multilevel proteomics reveals host perturbations by SARS-CoV-2 and
SARS-CoV. Nature. 594:246–252. 2021.
|
|
150
|
Li S, Ma F, Yokota T, Garcia G Jr, Palermo
A, Wang Y, Farrell C, Wang YC, Wu R, Zhou Z, et al: Metabolic
reprogramming and epigenetic changes of vital organs in
SARS-CoV-2-induced systemic toxicity. JCI insight.
6:e1450272021.
|
|
151
|
Bojkova D, Klann K, Koch B, Widera M,
Krause D, Ciesek S, Cinatl J and Münch C: Proteomics of
SARS-CoV-2-infected host cells reveals therapy targets. Nature.
583:469–472. 2020.
|
|
152
|
Miller B, Silverstein A, Flores M, Cao K,
Kumagai H, Mehta HH, Yen K, Kim SJ and Cohen P: Host mitochondrial
transcriptome response to SARS-CoV-2 in multiple cell models and
clinical samples. Sci Rep. 11:32021.
|
|
153
|
Medini H, Zirman A and Mishmar D: Immune
system cells from COVID-19 patients display compromised
mitochondrial-nuclear expression co-regulation and rewiring toward
glycolysis. iScience. 24:1034712021.
|
|
154
|
Codo AC, Davanzo GG, Monteiro LB, de Souza
GF, Muraro SP, Virgilio-da-Silva JV, Prodonoff JS, Carregari VC, de
Biagi Junior CAO, Crunfli F, et al: Elevated glucose levels favor
SARS-CoV-2 infection and monocyte response through a
HIF-1α/glycolysis-dependent axis. Cell Metab. 32:437–446.e5.
2020.
|
|
155
|
Tian M, Liu W, Li X, Zhao P, Shereen MA,
Zhu C, Huang S, Liu S, Yu X, Yue M, et al: HIF-1α promotes
SARS-CoV-2 infection and aggravates inflammatory responses to
COVID-19. Signal Transduct Target Ther. 6:3082021.
|
|
156
|
Tan DX and Reiter RJ: Mechanisms and
clinical evidence to support melatonin's use in severe COVID-19
patients to lower mortality. Life Sci. 294:1203682022.
|
|
157
|
Huang PY, Wu JY, Liu TH, Tsai YW, Chen PT,
Liao CT and Toh HS: The clinical efficacy of melatonin in the
treatment of patients with COVID-19: A systematic review and
meta-analysis of randomized controlled trials. Front Med
(Lausanne). 10:11712942023.
|
|
158
|
Fara A, Mitrev Z, Rosalia RA and Assas BM:
Cytokine storm and COVID-19: A chronicle of pro-inflammatory
cytokines. Open Biol. 10:2001602020.
|
|
159
|
Reiter RJ, Abreu-Gonzalez P, Marik PE and
Dominguez-Rodriguez A: Therapeutic algorithm for use of melatonin
in patients with COVID-19. Front Med (Lausanne). 7:2262020.
|
|
160
|
Tan DX, Manchester LC, Terron MP, Flores
LJ and Reiter RJ: One molecule, many derivatives: A never-ending
interaction of melatonin with reactive oxygen and nitrogen species?
J Pineal Res. 42:28–42. 2007.
|
|
161
|
Tan DX, Hardeland R, Manchester LC,
Poeggeler B, Lopez-Burillo S, Mayo JC, Sainz RM and Reiter RJ:
Mechanistic and comparative studies of melatonin and classic
antioxidants in terms of their interactions with the ABTS cation
radical. J Pineal Res. 34:249–259. 2003.
|
|
162
|
Rodriguez C, Mayo JC, Sainz RM, Antolín I,
Herrera F, Martín V and Reiter RJ: Regulation of antioxidant
enzymes: A significant role for melatonin. J Pineal Res. 36:1–9.
2004.
|
|
163
|
Cuzzocrea S, Zingarelli B, Costantino G
and Caputi AR: Protective effect of melatonin in a non-septic shock
model induced by zymosan in the rat. J Pineal Res. 25:24–33.
1998.
|
|
164
|
Cuzzocrea S, Zingarelli B, Gilad E, Hake
P, Salzman AL and Szabó C: Protective effect of melatonin in
carrageenan-induced models of local inflammation: Relationship to
its inhibitory effect on nitric oxide production and its
peroxynitrite scavenging activity. J Pineal Res. 23:106–116.
1997.
|
|
165
|
El-Sokkary GH, Omar HM, Hassanein AFMM,
Cuzzocrea S and Reiter RJ: Melatonin reduces oxidative damage and
increases survival of mice infected with Schistosoma mansoni. Free
Radic Biol Med. 32:319–332. 2002.
|
|
166
|
Costantino G, Cuzzocrea S, Mazzon E and
Caputi AP: Protective effects of melatonin in zymosan-activated
plasma-induced paw inflammation. Eur J Pharmacol. 363:57–63.
1998.
|
|
167
|
Kong J, Zhang Y, Liu S, Li H, Liu S, Wang
J, Qin X, Jiang X, Yang J, Zhang C and Zhang W: Melatonin
attenuates angiotensin II-induced abdominal aortic aneurysm through
the down-regulation of matrix metalloproteinases. Oncotarget.
8:14283–14293. 2017.
|
|
168
|
Zhang Y and Li X, Grailer JJ, Wang N, Wang
M, Yao J, Zhong R, Gao GF, Ward PA, Tan DX and Li X: Melatonin
alleviates acute lung injury through inhibiting the NLRP3
inflammasome. J Pineal Res. 60:405–414. 2016.
|
|
169
|
Kaivola J, Nyman TA and Matikainen S:
Inflammasomes and SARS-CoV-2 infection. Viruses. 13:25132021.
|
|
170
|
Kucia M, Ratajczak J, Bujko K, Adamiak M,
Ciechanowicz A, Chumak V, Brzezniakiewicz-Janus K and Ratajczak MZ:
An evidence that SARS-Cov-2/COVID-19 spike protein (SP) damages
hematopoietic stem/progenitor cells in the mechanism of pyroptosis
in Nlrp3 inflammasome-dependent manner. Leukemia. 35:3026–3029.
2021.
|
|
171
|
Davies DA, Adlimoghaddam A and Albensi BC:
The effect of COVID-19 on NF-κB and neurological manifestations of
disease. Mol Neurobiol. 58:4178–4187. 2021.
|
|
172
|
Manik M and Singh RK: Role of toll-like
receptors in modulation of cytokine storm signaling in
SARS-CoV-2-induced COVID-19. J Med Virol. 94:869–877. 2022.
|
|
173
|
Li HW, Ying P, Cai QQ, Yang ZH and Wu XL:
Exogenous melatonin alleviates hemorrhagic shock-induced hepatic
ischemic injury in rats by inhibiting the NF-κB/IκBα signaling
pathway. Mol Med Rep. 23:3412021.
|
|
174
|
Qin M, Liu Y, Sun M, Li X, Xu J, Zhang L
and Jiang H: Protective effects of melatonin on the white matter
damage of neonatal rats by regulating NLRP3 inflammasome activity.
Neuroreport. 32:739–747. 2021.
|
|
175
|
Cao S, Shrestha S, Li J, Yu X, Chen J, Yan
F, Ying G, Gu C, Wang L and Chen G: Melatonin-mediated mitophagy
protects against early brain injury after subarachnoid hemorrhage
through inhibition of NLRP3 inflammasome activation. Sci Rep.
7:24172017.
|
|
176
|
Tan DX and Hardeland R: Targeting host
defense system and rescuing compromised mitochondria to increase
tolerance against pathogens by melatonin may impact outcome of
deadly virus infection pertinent to COVID-19. Molecules.
25:44102020.
|
|
177
|
Sygitowicz G and Sitkiewicz D: Molecular
mechanisms of organ damage in sepsis: An overview. Braz J Infect
Dis. 24:552–560. 2020.
|
|
178
|
Luo J, Song J, Zhang H, Zhang F, Liu H, Li
L, Zhang Z, Chen L, Zhang M, Lin D, et al: Melatonin mediated
Foxp3-downregulation decreases cytokines production via the TLR2
and TLR4 pathways in H. pylori infected mice. Int Immunopharmacol.
64:116–122. 2018.
|
|
179
|
Xu X, Wang G, Ai L, Shi J, Zhang J and
Chen YX: Melatonin suppresses TLR9-triggered proinflammatory
cytokine production in macrophages by inhibiting ERK1/2 and AKT
activation. Sci Rep. 8:155792018.
|
|
180
|
Feng R, Adeniran SO, Huang F, Li Y, Ma M,
Zheng P and Zhang G: The ameliorative effect of melatonin on
LPS-induced Sertoli cells inflammatory and tight junctions damage
via suppression of the TLR4/MyD88/NF-κB signaling pathway in
newborn calf. Theriogenology. 179:103–116. 2022.
|
|
181
|
Tan DX and Reiter RJ: Melatonin reduces
the mortality of severely-infected COVID-19 patients. Melatonin
Res. 4:613–616. 2021.
|
|
182
|
Ren DL, Sun AA, Li YJ, Chen M, Ge SC and
Hu B: Exogenous melatonin inhibits neutrophil migration through
suppression of ERK activation. J Endocrinol. 227:49–60. 2015.
|
|
183
|
Maldonado MD, Mora-Santos M, Naji L,
Carrascosa-Salmoral MP, Naranjo MC and Calvo JR: Evidence of
melatonin synthesis and release by mast cells. Possible modulatory
role on inflammation. Pharmacol Res. 62:282–287. 2010.
|
|
184
|
Muxel SM, Pires-Lapa MA, Monteiro AWA,
Cecon E, Tamura EK, Floeter-Winter LM and Markus RP: NF-κB drives
the synthesis of melatonin in RAW 264.7 macrophages by inducing the
transcription of the arylalkylamine-N-acetyltransferase (AA-NAT)
gene. PLoS One. 7:e520102012.
|
|
185
|
Maldonado MD, García-Moreno H,
González-Yanes C and Calvo JR: Possible involvement of the
inhibition of NF-κB factor in anti-inflammatory actions that
melatonin exerts on mast cells. J Cell Biochem. 117:1926–1933.
2016.
|
|
186
|
Hasan MZ, Islam S, Matsumoto K and Kawai
T: Meta-analysis of single-cell RNA-seq data reveals phenotypic
switching of immune cells in severe COVID-19 patients. Comput Biol
Med. 137:1047922021.
|
|
187
|
Cardinali DP, Brown GM and Pandi-Perumal
SR: An urgent proposal for the immediate use of melatonin as an
adjuvant to anti-SARS-CoV-2 vaccination. Melatonin Res. 4:206–212.
2021.
|
|
188
|
Zipeto D, Palmeira JDF, Argañaraz GA and
Argañaraz ER: ACE2/ADAM17/TMPRSS2 interplay may be the main risk
factor for COVID-19. Front Immunol. 11:5767452020.
|
|
189
|
Shukla M, Htoo HH, Wintachai P, Hernandez
JF, Dubois C, Postina R, Xu H, Checler F, Smith DR, Govitrapong P
and Vincent B: Melatonin stimulates the nonamyloidogenic processing
of βAPP through the positive transcriptional regulation of ADAM10
and ADAM17. J Pineal Res. 58:151–165. 2015.
|
|
190
|
Zlacká J, Stebelová K, Zeman M and
Herichová I: Interactions of renin-angiotensin system and COVID-19:
The importance of daily rhythms in ACE2, ADAM17 and TMPRSS2
expression. Physiol Res. 70(S2): S177–S194. 2021.
|
|
191
|
Hazra S, Chaudhuri AG, Tiwary BK and
Chakrabarti N: Matrix metallopeptidase 9 as a host protein target
of chloroquine and melatonin for immunoregulation in COVID-19: A
network-based meta-analysis. Life Sci. 257:1180962020.
|
|
192
|
Cecon E, Izabelle C, Poder SL, Real F, Zhu
A, Tu L, Ghigna MR, Klonjkowski B, Bomsel M, Jockers R and Dam J:
Therapeutic potential of melatonin and melatonergic drugs on
K18-hACE2 mice infected with SARS-CoV-2. J Pineal Res.
72:e127722022.
|
|
193
|
Cecon E, Fernandois D, Renault N, Coelho
CFF, Wenzel J, Bedart C, Izabelle C, Gallet S, Le Poder S,
Klonjkowski B, et al: Melatonin drugs inhibit SARS-CoV-2 entry into
the brain and virus-induced damage of cerebral small vessels. Cell
Mol Life Sci. 79:3612022.
|
|
194
|
Loh D: The potential of melatonin in the
prevention and attenuation of oxidative hemolysis and myocardial
injury from cd147 SARS-CoV-2 spike protein receptor binding.
Melatonin Res. 3:380–416. 2020.
|
|
195
|
Morchang A, Malakar S, Poonudom K,
Noisakran S, Yenchitsomanus PT and Limjindaporn T: Melatonin
inhibits dengue virus infection via the sirtuin 1-mediated
interferon pathway. Viruses. 13:6592021.
|
|
196
|
Zhai X, Wang N, Jiao H, Zhang J, Li C, Ren
W, Reiter RJ and Su S: Melatonin and other indoles show antiviral
activities against swine coronaviruses in vitro at pharmacological
concentrations. J Pineal Res. 71:e127542021.
|
|
197
|
Tesarik J: Melatonin attenuates growth
factor receptor signaling required for SARS-CoV-2 replication.
Melatonin Res. 3:534–537. 2020.
|
|
198
|
Reiter RJ, Cardinali DP, Neel RL,
Rodriguez AD, Brown GM and Tesarik J: Rationale for the continued
use of melatonin to combat the delta variant of SARS-CoV-2.
Melatonin Res. 4:495–500. 2021.
|
|
199
|
Klann K, Bojkova D, Tascher G, Ciesek S,
Münch C and Cinatl J: Growth factor receptor signaling inhibition
prevents SARS-CoV-2 replication. Mol Cell. 80:164–174 e4. 2020.
|
|
200
|
Behl T, Kaur I, Aleya L, Sehgal A, Singh
S, Sharma N, Bhatia S, Al-Harrasi A and Bungau S: CD147-spike
protein interaction in COVID-19: Get the ball rolling with a novel
receptor and therapeutic target. Sci Total Environ.
808:1520722022.
|
|
201
|
Wang K, Chen W, Zhang Z, Deng Y, Lian JQ,
Du P, Wei D, Zhang Y, Sun XX, Gong L, et al: CD147-spike protein is
a novel route for SARS-CoV-2 infection to host cells. Signal
Transduct Target Ther. 5:2832020.
|
|
202
|
Mohamed Taha A, Adel Abdelkader Saed S,
Hossam-Eldin Moawad M, Abd El-Tawab Moawad W, Al-Hejazi T, Mousa Y,
Sharma R and Reiter RJ: Safety and efficacy of melatonin as an
adjuvant therapy in COVID-19 patients: Systematic review and
meta-analysis. Adv Med Sci. 68:341–352. 2023.
|
|
203
|
Hosseini A, Esmaeili Gouvarchin Ghaleh H,
Aghamollaei H, Fasihi Ramandi M, Alishiri G, Shahriary A,
Hassanpour K, Tat M and Farnoosh G: Evaluation of Th1 and Th2
mediated cellular and humoral immunity in patients with COVID-19
following the use of melatonin as an adjunctive treatment. Eur J
Pharmacol. 904:1741932021.
|
|
204
|
Alizadeh Z, Keyhanian N, Ghaderkhani S,
Dashti-Khavidaki S, Shokouhi Shoormasti R and Pourpak Z: A Pilot
study on controlling coronavirus disease 2019 (COVID-19)
inflammation using melatonin supplement. Iran J Allergy Asthma
Immunol. 20:494–499. 2021.
|
|
205
|
Darban M, Malek F, Memarian M, Gohari A,
Kiani A, Emadi A, Lavvaf S and Bagheri B: Efficacy of high dose
vitamin C, melatonin and zinc in iranian patients with acute
respiratory syndrome due to coronavirus infection: A pilot
randomized trial. J Cell Mol Anesth. 6:164–167. 2021.
|
|
206
|
Farnoosh G, Akbariqomi M, Badri T, Bagheri
M, Izadi M, Saeedi-Boroujeni A, Rezaie E, Ghaleh HEG, Aghamollaei
H, Fasihi-Ramandi M, et al: Efficacy of a low dose of melatonin as
an adjunctive therapy in hospitalized patients with COVID-19: A
randomized, double-blind clinical trial. Arch Med Res. 53:79–85.
2022.
|
|
207
|
Mousavi SA, Heydari K, Mehravaran H,
Saeedi M, Alizadeh-Navaei R, Hedayatizadeh-Omran A and Shamshirian
A: Melatonin effects on sleep quality and outcomes of COVID-19
patients: An open-label, randomized, controlled trial. J Med Virol.
94:263–271. 2022.
|
|
208
|
Chavarría AP, Vázquez RRV, Cherit JGD,
Bello HH, Suastegui HC, Moreno-Castañeda L, Alanís Estrada G,
Hernández F, González-Marcos O, Saucedo-Orozco H, et al:
Antioxidants and pentoxifylline as coadjuvant measures to standard
therapy to improve prognosis of patients with pneumonia by
COVID-19. Comput Struct Biotechnol J. 19:1379–1390. 2021.
|
|
209
|
Esmaeili Gouvarchin Ghaleh H, Hosseini A,
Aghamollaei H, Fasihi-Ramandi M, Alishiri G, Saeedi-Boroujeni A,
Hassanpour K, Mahmoudian-Sani MR and Farnoosh G: NLRP3 inflammasome
activation and oxidative stress status in the mild and moderate
SARS-CoV-2 infected patients: Impact of melatonin as a medicinal
supplement. Z Naturforsch C J Biosci. 77:37–42. 2021.
|
|
210
|
Bologna C, Madonna P and Pone E: Efficacy
of prolonged-release melatonin 2 mg (PRM 2 mg) prescribed for
insomnia in hospitalized patients for COVID-19: A retrospective
observational study. J Clin Med. 10:58572021.
|