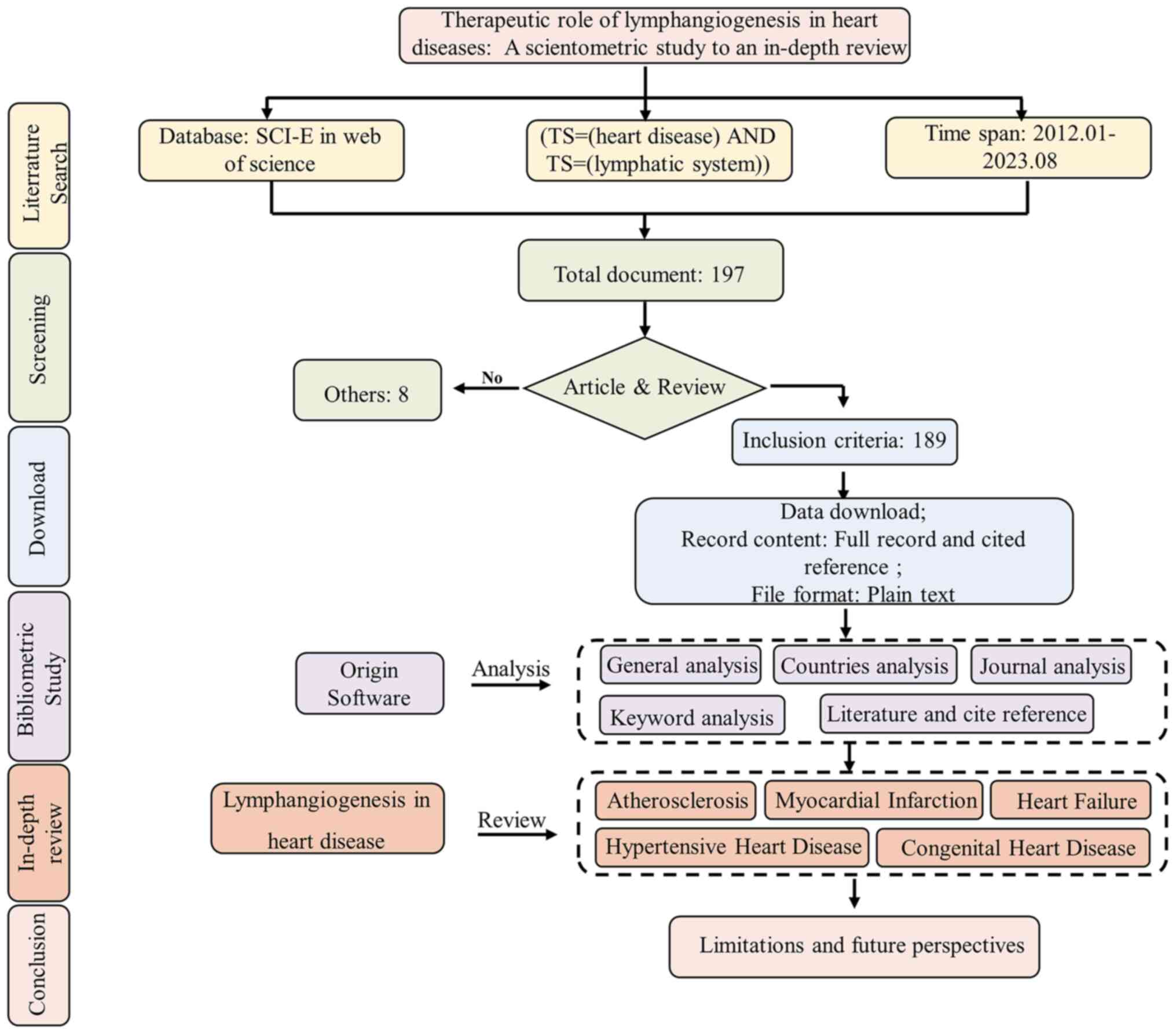
A number of diseases such as atherosclerosis (AS),
myocardial infarction (MI), heart failure (HF), hypertensive heart
disease (HHD) and congenital heart disease (CHD), profoundly impact
cardiac health (1). Despite the
numerous treatment options available, heart disease remains the
leading global cause of mortality, requiring innovative therapeutic
approaches. The data show that there were 4.58 million estimated
heart disease deaths in 2020; the age-standardized mortality rate
(ASMR) was 245.39 per 100,000 in 2020 (2,3).
The discovery of lymphatic marker genes, such as acid receptor 1
(LYVE-1) and vascular endothelial growth factor (VEGF) receptor
(VEGFR)-3, alongside advancements in lymphatic function imaging and
quantification techniques, has brought attention to the role of
lymphatic vessels in the progression of heart disease (4). The lymphatic system is an open,
low-pressure, one-way transmission network between the
extracellular space and the veins that differs from closed,
hypertensive circulatory vascular networks. This extensive
lymphatic network plays a key role in maintaining tissue fluid
homeostasis and monitoring cardiac immunity (5,6).
Obstruction of cardiac lymph flow leads to edema and cardiac
dysfunction (7), underscoring
the clinical significance of elucidating the role of the lymphatic
system in cardiovascular diseases. In the field of information
science, scientometrics, a highly specialized branch of
bibliometrics, offers a quantitative method leveraging mathematical
and statistical approaches to visualize emerging research trends
and hotspots in scientific literature (8). This method aids in identifying
publications, researchers, journals and research institutions that
significantly contribute to a field, and enhances our understanding
of scientific citations (9). In
the present review, scientometrics was used to analyze research
hotspots of lymphatic vessels in heart disease and evaluate the
impact of the relevant literature. Additionally, a detailed review
of molecular mechanisms surrounding these hotspots is presented to
advance the development of drugs targeting lymphatic vessels.
The search in the Web of Science core collection
database revealed 189 publications related to the therapeutic
effects of the lymphatic system on heart disease before August 1,
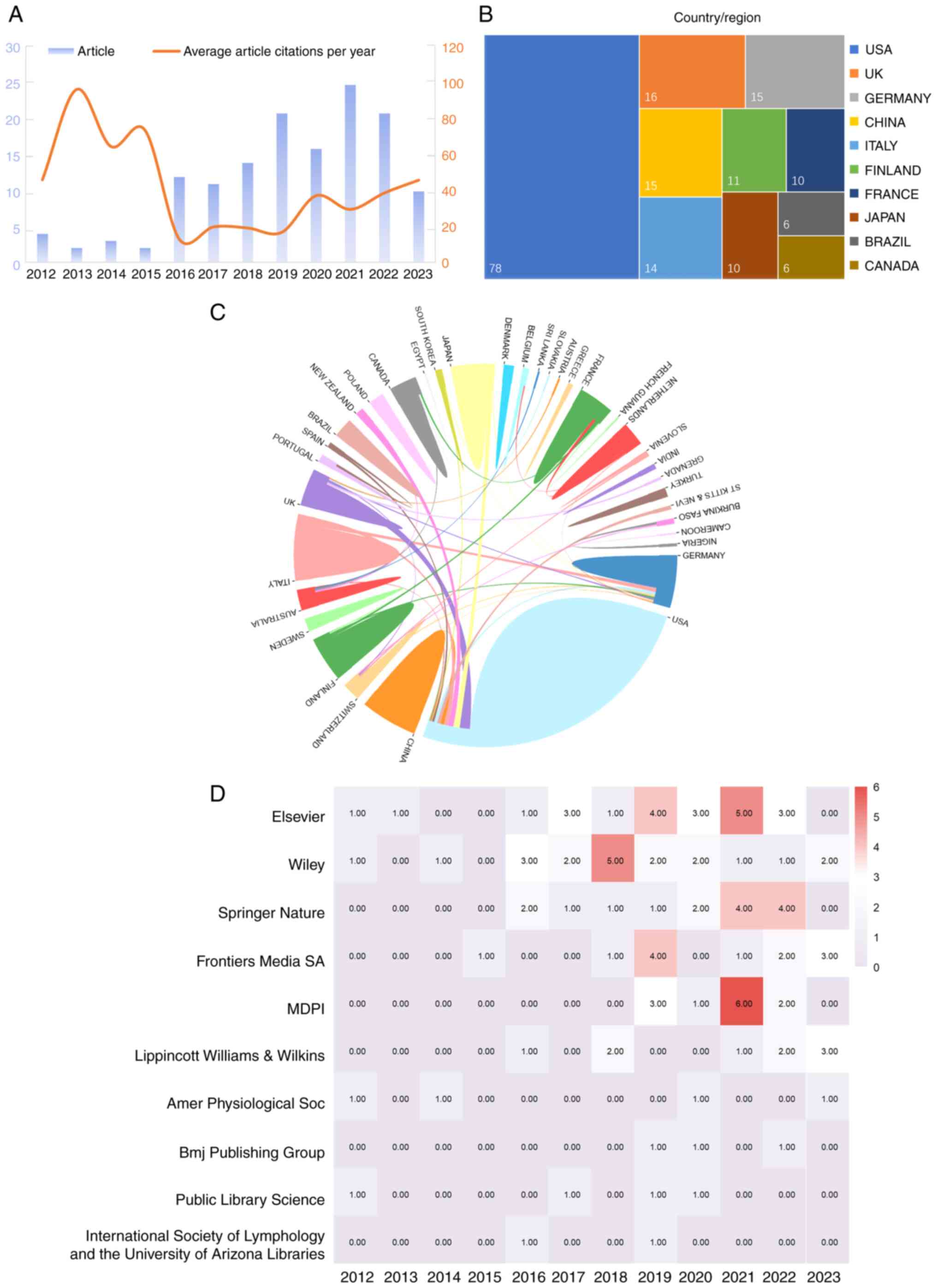
2023. On average, these documents were cited 28.06 times. In 2021,
the highest number of articles was published, while the maximum
number of citations was observed in 2013 (Fig. 2A). The observed trend indicates a
growing body of research on the therapeutic effects of the
lymphatic system on heart disease.
A total of 35 countries were represented in the
publications included in the present review. The USA published the
highest number of published articles, followed by United Kingdom,
Germany, Italy, China, Japan, Finland, France, Brazil and Canada
(Fig. 2B). Collaborative efforts
were evident among several countries (Fig. 2C).
Publications originated from 53 different types of
publishers, with the top 10 shown in Fig. 2D. Notably, Elsevier emerged as
the most influential publisher, followed by Wiley, Springer Nature,
Frontiers Media SA, MDPI, Lippincott Williams & Wilkins,
American Physiological Society, BMJ Publishing Group, Public
Library Science and International Society of Lymphology and the
University of Arizona Libraries.
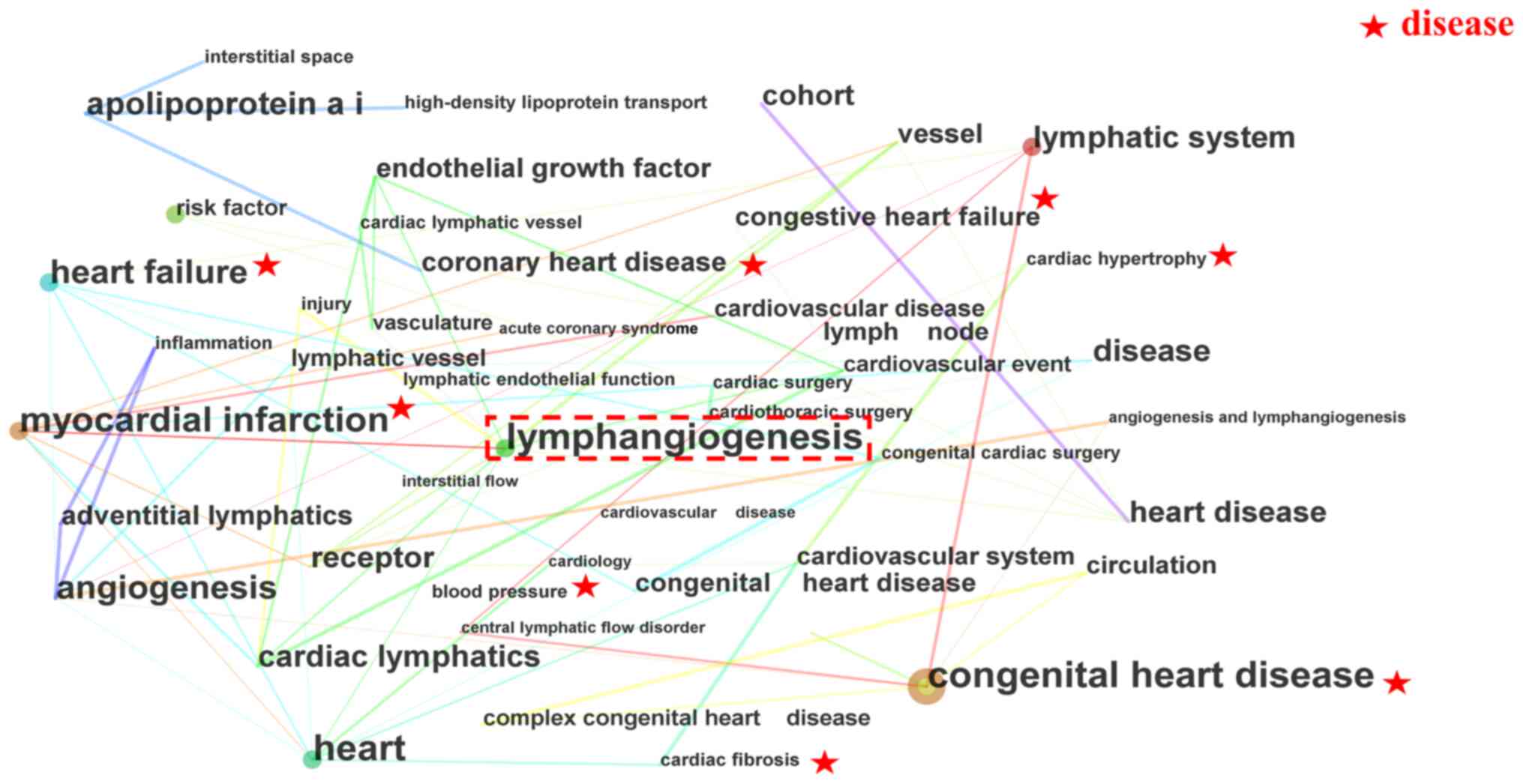
Research key words reflect current trends, hotspots
and the essence of a research document (11). The present review presents a
co-occurrence network of key words (Fig. 3; Table I). In these clusters,
'lymphangiogenesis' emerged as a cross-key word, suggesting its
potential impact on heart disease. The scientometric results
further indicate that the lymphatic vessel is a research hotspot
and potentially a promising treatment avenue for heart diseases,
including MI, HF and CHD, with a focus on the cardiac lymphatic
system.
Citations play a crucial role in acknowledging
sources during the writing or editing of a manuscript, including
both the cited literature and the specific content of the citation
(12). In response to the
analysis of the key words, current research interests and topic
hotspots were explored through literature analysis and references.
Table II presents the top 10
most-cited articles on the therapeutic effect of lymphangiogenesis
on heart diseases. The most influential article highlights the
critical role of the lymphatic vascular system in maintaining
interstitial fluid homeostasis, immune cell transport, nutrient
lipid uptake and transport, and reverse cholesterol transportation
(13). The second most-cited
article underscores that selective stimulation of cardiac
lymphangiogenesis post-MI markedly improves myocardial edema and
restores cardiac function (14).
Additionally, Table III
outlines the top 10 cited references on the therapeutic roles of
lymphangiogenesis in heart diseases. These articles offer a
comprehensive overview of the most influential studies in the field
for further research into the therapeutic effect of
lymphangiogenesis on heart diseases.
The clinical applications of lymphangiogenesis have
been used to improve cardiac function (15). However, The clinical trial have
yet to elucidate the specific signaling pathways regulating
lymphangiogenesis for improved cardiac function. Therefore, animal
experiments would be useful in establishing whether and how
lymphangiogenesis improves cardiac function. The present review
focused extensively on lymphangiogenesis in heart diseases.
Furthermore, despite scientometric analysis indicating that
lymphangiogenesis is a research hotspot and a promising treatment
option for heart disease, a comprehensive and systematic
exploration of relevant mechanisms is lacking. Therefore, in the
current review, existing research at the mechanistic level on
lymphangiogenesis and cardiovascular disease was examined.
AS is a chronic inflammatory disease characterized
by the accumulation of cholesterol, lipoproteins and inflammatory
cells in the arterial vessel wall (16). Lymphatics are present at
atherosclerotic sites in the adventitia of the artery, adjacent to
blood vessels and dilate within atherosclerotic plaques. With a
deeper understanding of lymphatic vessel function, mounting
evidence reveals the critical role of lymphangiogenesis in AS
progression. Particularly, it is increasingly acknowledged that
lymphatics are the main route for transporting high-density
lipoprotein particles in cholesterol to the bloodstream (17). AS promotes lymphangiogenesis
(18), which in turn, alleviates
AS by facilitating reverse cholesterol transport and simultaneously
draining local inflammation (19,20). The number and function of
lymphatic vessels are limited. Consequently, promoting
lymphangiogenesis holds great promise for the treatment of AS.
According to clinical and experimental studies,
lymphangiogenesis in AS is regulated by multiple signaling pathways
and molecules. Clinical evidence indicates that VEGF-C/D levels are
notably and inversely associated with all-cause mortality among
patients with suspected or known AS (21). Early treatment with VEGF-C
inhibited plaque formation, promoting lymphatic molecular transport
and triggering inflammatory cell migration (22). Besides VEGF-C/D, angiopoietin-2
(Angpt2) is associated with angiogenesis and inflammation. Clinical
evidence suggests that the upregulation of Angpt2 may benefit
patients with AS (23). This
effect may be associated with the increase in VEGF-C/D secretion
regulated by Angpt2, promoting lymphangiogenesis around
atherosclerotic coronary arteries (23). R-spondin 2 (RSPO2), an important
factor for regulating cell proliferation and differentiation, has
previously shown potential in promoting lymphangiogenesis (24,25). A study indicated that the
interaction between RSPO2 and G protein-coupled receptor 5 inhibits
Wnt/β-catenin signaling (26),
limiting lymphatic vessel formation, weakening lymphatic drainage
of LDL cholesterol in the arterial wall and promoting AS
development. A further study found that the
phosphatidylinositol-3-hydroxykinase (PI3K)-protein kinase B (AKT)
pathway is a downstream pathway of Wnt/β-catenin performs that
promotes lymphangiogenesis (24). Additionally, the activation of
the C-X-C chemokine receptor type 4 (CXCR4)/chemokine C-X-C motif
ligand 12 (CXCL12) axis exacerbates inflammation by promoting
leukocyte infiltration (27). A
study suggested that the CXCL12/CXCR4 axis regulates cardiac
adventitia lymphangiogenesis, reducing inflammation during AS by
enhancing T-cell drainage (18).
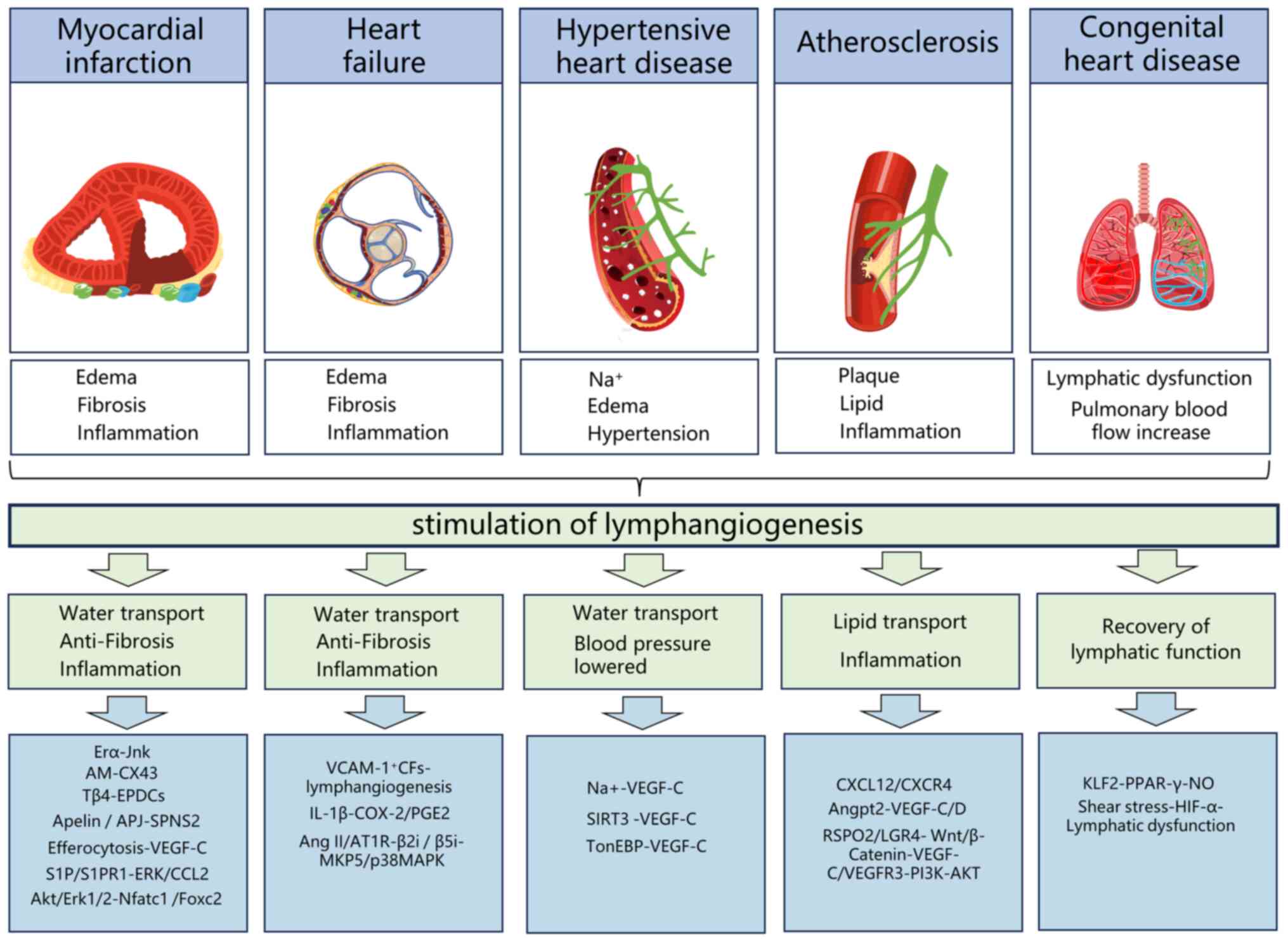
Details are provided in Table
IV and Fig. 4.
The rupture of an unstable atherosclerotic plaque
and subsequent coronary thrombosis often leads to MI (28). MI is often accompanied by
myocardial edema, inflammation and fibrosis (29). Cardiac lymphatic flow begins from
small endocardium lymphatic vessels, traverses through the
myocardium and enters epicardial capillaries. These capillaries
converge into large collecting lymphatic vessels, facilitating the
transportation of excess water and immune cells, and inhibiting
myocardial edema, inflammation and fibrosis (6). Lymphatic vessel formation is
investigated as a potential target for MI in clinical practice
(30). Lymphatic vessels play a
crucial role in heart repair after MI, maintaining tissue fluid
homeostasis and suppressing excessive immune responses (31). The VEGF family and their
endothelial tyrosine kinase receptors induce lymphangiogenesis
(32), but their efficiency is
limited when injected intravenously or intracoronarily after MI due
to short half-lives (33). Thus,
researchers are exploring new pathways new pathways regulating
lymphangiogenesis.
VEGF-C-VEGFR3, sphinogosine-1-phosphate (S1P)/S1P
receptor 1 (S1PR1) is another noteworthy receptor-ligand complex.
S1PR1 is a G-protein-coupled receptor, expressed in lymphatic
endothelial cells, and modulates immune cell transportation
(34). In injured hearts,
S1PR1/S1P activates downstream signals, including extracellular
regulated protein kinases/chemokine ligand 2 (ERK), promoting
lymphangiogenesis in the injured heart, driving macrophage
trafficking, immune modulation, and cardiac repair after MI
(35,36). Estrogen receptor α (ERα)/estrogen
ligand plays a protective role in MI, associated with regulating
lymphangiogenesis (37).
Overexpression of cardiomyocyte-specific ERα promotes
lymphangiogenesis via inducing c-Jun N-terminal kinase
phosphorylation, alleviating ventricular fibrosis (38). Spinster homolog 2 (SPNS2) and
thymosin beta 4 (Tβ4) drive lymphangiogenesis by controlling
lymphatic endothelial cell proliferation and differentiation.
SPNS2, an S1P transporter, contributes to S1P release into
extracellular space and the development of lymphatic vessels
(39,40). Apelin/Apelin receptor signaling
targets SPNS2, promoting lymphatic endothelial cell proliferation
and maintaining lymphatic endothelial cell integrity, limiting
persistent edema and inflammation after MI (41). Tβ4, which is a
43-amino-acid-sequestering peptide in the embryonic heart, is
important for epicardial development and coronary artery formation
(42). Tβ4 promotes the
differentiation of epicardium-derived cells towards lymphatic
endothelial cells, attenuating adverse myocardial remodeling,
enhancing myocardial regeneration, and improving cardiac function
post-MI (43). Connexin43 (Cx43)
may control the function of nascent lymphatic vessels by
determining endothelial cell connections. Cx43 modulates gap
connection coupling between lymphatic endothelial cells and has
been implicated in the pathological process of MI. A previous study
showed that adrenomedullin targeted Cx43 to increase gap
connections between human lymphatic endothelial cells, promoting
the proliferation of the cardiac lymphatic vascular system and
relieving MI-induced edema (44). Additionally, VEGF-C-VEGFR-3
indirectly promotes lymphangiogenesis, polarizing macrophage from
M1 to M2 in the MI area via activation of downstream AKT/ERK1/2 and
calcineurin/nuclear factor of activated T cells 1, forkhead box C2
signaling pathways, accelerating post-MI repair (45). Furthermore, when dead cells are
engulfed by macrophages, more VEGF-C is released, further promoting
myocardial lymphangiogenesis through its receptor (46). Details are provided in Table IV and Fig. 4.
At the end stage of heart disease, the clinical
manifestation is HF, not a single pathological diagnosis but a
clinical syndrome characterized by breathlessness, ankle swelling,
fatigue, pulmonary crackles, peripheral edema and elevated jugular
venous pressure (47,48). Common pathological manifestations
of HF include myocarditis, myocardial edema, and myocardial
fibrosis (49-51). Excessive inflammatory
infiltration forms the biological basis for these manifestations
(52), and edema results as a
major consequence of the inflammatory response in the myocardium
(53). Lymphatics are crucial to
the progression of HF. Cardiac lymphatics play a crucial role in
the progression of HF by adapting their number and function to the
increased demand for inflammatory factors and fluid drainage, thus
alleviating inflammation and edema (54,55).
Most studies suggest that VEGF-C-VEGFR3 signaling,
as the key pathway of lymphangiogenesis, may be a therapeutic
target for HF (45).
Additionally, interleukin-1β (IL-1β), an inflammatory cytokine
associated with MI, has been reported to exert a profound
depressant effect on the contractility of lymphatic muscles
(56). Prostaglandin E2 (PGE2),
the main downstream product of cyclooxygenase-2 activation, induces
relaxation by binding to PGE2 to prostaglandin E receptor 2 or 4,
elevating intracellular cyclic adenosine monophosphate accumulation
(57). A recent study showed
that IL-1β reduced the contractility of cardiac lymphatic muscle
cells through COX-2/PGE2 signal transduction, weakening the
transport of local inflammation in the heart and promoting the
progression of acute myocarditis to HF (58). Furthermore, angiotensin-2 (Ang
II), is an important factor in inducing inflammation in the
vascular system (59).
Angiotensin, serotonin, and endothelin are also known to regulate
lymphatic vessels (60). Ang II
can activate AT1R to promote the expression and activity of
proteasome catalytic subunits β2i and β5i, affecting MKP5 and
VE-cadherin degradation and p38MAPK activation, resulting in
lymphatic endothelial hyperpermeability (61). Moreover, cardiac fibroblasts,
principal contributors to cardiac fibrosis leading to cardiac
remodeling and HF, express vascular cell adhesion molecule-1 and
promote lymphangiogenesis (62).
This mobilizes lymphatic endothelial cells into the infarct zone,
restoring ventricular wall mechanical properties. The mechanism may
involve the formation of lymphatic vessels, inhibition of
interstitial edema, and consequently, reduced inflammation and
myocardial fibrosis (63).
Details are provided in Table
IV and Fig. 4.
HHD is an organic heart disease caused by long-term
poorly controlled hypertension, characterized by cardiac
hypertrophy due to progressively increasing ventricular load
(64). Studies indicated that
high sodium intake contributes to hypertension development
(65). The large accumulation of
sodium leads to electrolyte disorders and promotes edema formation
(66). Lymphatics is a key route
in draining interstitial fluid, and in HHD, lymphangiogenesis is an
effective strategy for reducing cardiac interstitial edema. As a
cytokine regulating lymphangiogenesis, VEGF-C relieves edema caused
by electrolyte imbalances (67).
VEGF-C treatment notably improves cardiac function, preserving
cardiac function, reducing cardiac hypertrophy and fibrosis,
accelerating edema clearance by inducing skin lymphangiogenesis and
enhancing endothelial nitric oxide (NO) synthase expression in
blood vessels to lower blood pressure (68).
Lymphangiogenesis plays an important role not only
in maintaining interstitial fluid balance but also in facilitating
immune cell transport in HHD. The activation of the VEGF-C-VEGFR3
pathway is activated in mononuclear phagocytes, triggered by sodium
salt accumulation, and stimulates skin lymphangiogenesis (69). Systemic overexpression of VEGF-C
enhances lymphangiogenesis within the myocardial interstitium,
attenuating local infiltration of macrophages in the heart
(70). This dual mechanism of
eliminating excess interstitial fluid and transporting immune cells
contributes to the reduction of cardiac decompensation-induced
myocardial edema and inflammation. In HHD, tonicity enhancer
binding protein (TonEBP) and silencing regulatory protein 3 (SIRT3)
play key roles in the upstream regulation of VEGFC-VEGFR3
signaling. TonEBP, a key regulator of cellular responses to
hypertonic stress, binds to the promoter region of VEGF-C,
enhancing its secretion and promoting lymphangiogenesis (71). This, in turn, inhibits left
ventricular remodeling induced by high salt intake (72). SIRT3 is a major mitochondrial
deacetylase closely associated with angiogenesis. A recent study
indicated that SIRT3 activation upregulates the VEGF-C-VEGFR3
signaling axis protein expression, thereby promoting migration and
proliferation of lymphatic endothelial cells (LECs), and
lymphangiogenesis in AngⅡ-induced hypertensive heart models
(73,74). Details are provided in Table IV and Fig. 4.
The rapid advancements in modernization research
within Traditional Chinese Medicine (TCM) have garnered
international recognition for its efficacy in disease treatment.
TCM is characterized by personalized treatment, fewer side effects
and a focus on treating both symptoms and root causes and promoting
self-healing ability of the body. A study reported the explicitly
regulatory effects of TCM on lymphatic vessels, particularly in
promoting lymphangiogenesis (83).
Several studies highlight the efficacy of Chinese
herbal extracts and monomers in alleviating lymphedema by promoting
lymphangiogenesis. Ishii et al (88) reported that piper retrofractum
extract and piperine, isolated from piper retrofractum, notably
enhanced the proliferation, migration and tube formation in human
dermal lymphatic microvascular endothelial cells, thereby promoting
lymphangiogenesis through the AKT and ERK pathways and effectively
alleviating lymphedema. T total saponin of Panax notoginseng (PNS)
can markedly promote lymphangiogenesis, and its mechanism may be
related to the activation of the ERK1/2, PI3K and P38-MAPK
signaling pathways, which stimulate the release of VEGF-C. PNS is a
promising therapeutic option for managing secondary lymphedema or
other lymphatic system disorder (89).
Notably, there is a limited number of TCM studies
addressing the regulation of lymphangiogenesis in the treatment of
cardiovascular diseases (90).
Studies have indicated that salvianolic acid B (SAB), a
water-soluble phenolic compound isolated from Danshen, exhibits
therapeutic effects on dilated cardiomyopathy, which is associated
with lymphangiogenesis deficiency (91). Mechanistically, SAB promotes the
proliferation and migration of LECs by upregulating the
TNF-α/NF-κB/VEGF-C signaling pathway, thus regulating the formation
of lymphatic vessels and inhibiting the development of the
pathological process of dilated heart disease (92).
The lymphatic vessel emerges as a key conduit for
local inflammation, playing an important role in various diseases,
including osteoarthritis, lymphedema and cardiovascular disease
(31,93). Notably, a study also highlighted
the rich distribution of lymphatic vessels in the cornea (94). Furthermore, the discovery of
meningeal lymphatic vessels challenges the prevailing notion of the
absence of lymphatic systems in the brain. This discovery opens up
new avenues for efficiently eliminating metabolic products produced
by brain parenchyma (95),
offering potential treatment ideas for Alzheimer's disease,
insomnia and meningitis (96-98).
A study indicated a growing interest in the
regulation of lymphatic vessels by TCM (99). Although current research
primarily focuses on arthritis and lymphedema, there are numerous
promising signs that TCM can improve microcirculation in the eyes
and brain, while also regulating local oxidative stress and immune
responses (100,101). These findings prompt us to
consider the potential role of TCM in regulating lymphatic vessels,
providing a promising perspective for further exploration.
According to TCM theory, all normal bodily fluids,
including inter-tissue fluids and fluids within internal organs
fluids such as gastric and intestinal fluids, are categorized as
body fluids (102). The
generation, distribution and excretion of body fluids are
intricately balanced in a healthy body. An imbalance between body
fluid generation and excretion can lead to insufficient body fluid
and symptoms such as thirst and dry skin (103). TCM syndromes, including phlegm
dampness or phlegm retention, stem from abnormal fluid distribution
and slow flow within the body (104).
Understanding the biological basis of TCM syndrome
has long presented a scientific challenge. Some authors propose
that the biological basis of phlegm retention or phlegm dampness
lies in the accumulation of tissue fluid (105), hyperlipidemia (106), aggregation of adhesion factors
(107) and localized immune
inflammation (108). Modern
medicine recognizes lymphatic functions that involve the clearance
of low-density lipoprotein in macrophages, transportation of immune
cells and drainage of tissue fluid (109). Phlegm and dampness in TCM can
also be understood as local inflammation, lipid accumulation and
fluid retention resulting from the obstruction of lymphatic reflux
(110). Through extensive study
of phlegm and dampness, it is hypothesized that obstruction of
lymphatic reflux might offer a more appropriate explanation.
Scientometrics is a branch of information science
that uses quantitative analysis and statistics to study scholarly
literature, including publications, citations and author
collaboration. Researchers can use it to identify research trends
in a specific area, assess the impact of related research,
understand research progress and identify partners. In the present
review, scientometric analysis was applied to identify new research
hotspots in cardiovascular-related diseases, specifically analyzing
the therapeutic role of lymphangiogenesis. The lymphatic system, as
another important circulatory pathway alongside blood circulation,
facilitates the circulation of body fluids together with the blood
circulatory system. It achieves this by transporting lymphatic
fluid containing water and macromolecules, contributing to overall
body metabolism, while maintaining the stability of internal
lymphangiogenesis (111).
Currently, it is well established that the VEGF family is one of
the potent lymphangiogenic factors (112). Additional factors, including
IL-6 (113), platelet-derived
growth factor (114),
neuropilin-2 (115) and
calcium-binding EGF domain-containing protein 1 (116) have also been identified for
their role in stimulating lymphangiogenesis. Various indirect
factors, such as activated macrophages secreting lymphatic
vessel-promoting factors, also influence lymphangiogenesis
(117). Considerable attention
is currently directed towards the VEGF-C/D-VEGFR3 pathway as a
potential therapeutic target. In relevant experiments, recombinant
VEGF-C-C156S has demonstrated the ability to promote
lymphangiogenesis and improve prognosis after MI. Furthermore,
certain proteins, such as Polydom (118) and Notch (119) play an important role in
lymphangiogenesis. Therefore, gaining a better understanding of the
cellular and molecular mechanisms underlying specific lymphatic
vessel diseases, coupled with the development of new drugs, holds
the potential to limit the development and progression of heart
diseases.
The therapeutic strategy aimed at specifically
targeting lymphangiogenesis in treating heart disease has been
thoroughly validated in animal models. This includes significant
improvements in lipid transport, cardiac function, and immune
response observed after heart transplantation through the
administration of VEGF-C (120-122). However, clinical studies on the
treatment of heart disease with lymphatic neovascularization,
particularly involving VEGF, remain limited. An injection of VEGF-2
into the endocardium has shown notable improvement in angina
pectoris symptoms and was proven to be relatively safe. Conducting
a larger phase III trial is deemed necessary for further validation
(123). AdVEGF-DΔNΔC
gene therapy is safe, well tolerated, and notably improves
myocardial perfusion reserve, enhancing the quality of life for
patients with MI during year 1 of follow-up (124). Moreover, researchers are
actively exploring changes in drug dosage forms to target lesion
sites and enhance drug efficacy. This includes innovative
approaches such as using nano and hyaluronic acid hydrogel-wrapped
VEGF to achieve site-specific delivery. This method of drug
delivery not only avoids the short half-life associated with
intravenous VEGF injection, but also alleviates the disadvantages
of easy degradation in vivo, making it suitable for clinical
lymphangiogenesis treatment (125,126). In conclusion, the exploration
of lymphatic neovascularization factors and related pathways, along
with the development of corresponding therapeutic drugs and dosage
forms, represents a promising therapeutic strategy for heart
disease treatment.
TCM, as a natural medicine, holds a marked advantage
in regulating lymphangiogenesis. Current research indicates that
TCM exerts remarkable anti-inflammatory effects by regulating
lymphatic neovascularization (99). A major effect of TCM lies in its
ability to regulate lymphatic vessel function, including lipid
transport, tissue fluid homeostasis and immunity (127). The structure of lymphatic
vessels plays a crucial role in their function. The mature
lymphatic network comprises initial lymphatics, precollectors and
collecting ducts (128). Due to
structural differences, these vessels serve distinct functions.
Blind-ended initial lymphatics take up lymphatic fluid containing
fluid, macromolecules and immune cells (129). Precollectors and collecting
ducts possess valvular structures that effectively prevent lymph
fluid reflux (130). At
present, TCM research on lymphatic vessels mainly focuses on their
quantity and function, with limited attention to their structures
and mechanisms (99).
Consequently, future research should focus on further exploring the
structure of lymphatic vessels to enhance our understanding of the
way TCM operates. Although lymphangiogenesis is gradually
recognized as a novel target for intervention in cardiovascular
diseases, relatively few studies have been reported on the TCM for
the treatment of cardiovascular diseases focusing on
lymphangiogenesis (90) By
contrast, TCM has been reported to treat arthritis and lymphedema
by promoting lymphangiogenesis (131,132). Since lymphangiogenesis helps to
impede the pathological processes of cardiovascular diseases,
including fibrosis, edema and plaque formation by reversing
cholesterol transport, draining local inflammation and eliminating
excessive interstitial fluid, the role of TCM in the regulation of
lymphangiogenesis will be a promising research direction. While the
majority of drugs are absorbed into the bloodstream after further
breakdown in the intestines, lymphatic vessels are widely
distributed throughout the intestinal tract, ultimately directing
lymph into the veins. It would be of interest to investigate the
presence of small molecules of TCM in lymphatic fluid, enabling
direct administration of drugs through lymphatic vessels. This
approach could notably reduce the first-pass effect of drugs,
thereby enhancing their efficacy.
Not applicable.
BL carried out investigation and visualization, and
wrote the original draft of the manuscript. WY conceptualized the
study, acquired funding, developed methodology and wrote the
original draft of the manuscript. DS carried out investigation and
data curation. SY carried out investigation and used software. CL
acquired funding, reviewed the manuscript and completed language
editing. LL developed methodology, supervised the project, and
reviewed and edited the manuscript. LY conceptualized the study,
supervised the project, and reviewed and edited the manuscript. All
authors have read and approved the final version of the manuscript.
Data authentication not applicable.
Not applicable.
Not applicable.
The authors declare that they have no known
competing financial interests or personal relationships that could
have appeared to influence the work reported in this paper.
The authors would like to thank Shanghai Tengyun
Biotechnology Co., Ltd. for providing technical assistance and
valuable tools for data analysis and visualization using the Hiplot
Pro platform (https://hiplot.com.cn/).
The present study was supported by the National Natural Science
Foundation of China (grant nos. 82204867 and 82174205) and Tianjin
Municipal Health Commission (grant no. 2023145).
|
1
|
Wang J, Duan Y, Sluijter JP and Xiao J:
Lymphocytic subsets play distinct roles in heart diseases.
Theranostics. 9:4030–4046. 2019.
|
|
2
|
Brownrigg JR, Leo V, Rose J, Low E,
Richards S, Carr-White G and Elliott PM: Epidemiology of
cardiomyopathies and incident heart failure in a population-based
cohort study. Heart. 108:1383–1391. 2022.
|
|
3
|
Wang W, Liu Y, Liu J, Yin P, Wang L, Qi J,
You J, Lin L, Meng S, Wang F and Zhou M: Mortality and years of
life lost of cardiovascular diseases in China, 2005-2020: Empirical
evidence from national mortality surveillance system. Int J
Cardiol. 340:105–112. 2021.
|
|
4
|
Harris NR, Bálint L, Dy DM, Nielsen NR,
Méndez HG, Aghajanian A and Caron KM: The ebb and flow of cardiac
lymphatics: A tidal wave of new discoveries. Physiol Rev.
103:391–432. 2023.
|
|
5
|
Monaghan RM, Page DJ, Ostergaard P and
Keavney BD: The physiological and pathological functions of VEGFR3
in cardiac and lymphatic development and related diseases.
Cardiovasc Res. 117:1877–1890. 2021.
|
|
6
|
Vuorio T, Ylä-Herttuala E, Laakkonen JP,
Laidinen S, Liimatainen T and Ylä-Herttuala S: Downregulation of
VEGFR3 signaling alters cardiac lymphatic vessel organization and
leads to a higher mortality after acute myocardial infarction. Sci
Rep. 8:167092018.
|
|
7
|
Aspelund A, Robciuc MR, Karaman S, Makinen
T and Alitalo K: Lymphatic system in cardiovascular medicine. Circ
Res. 118:515–530. 2016.
|
|
8
|
Nair AS: Scientometrics in medical
journals: Indices, their pros and cons. Indian J Anaesth.
63:955–957. 2019.
|
|
9
|
Martinez P, Al-Hussein M and Ahmad R: A
scientometric analysis and critical review of computer vision
applications for construction. Autom Constr. 107:1029472019.
|
|
10
|
Chen C: CiteSpace: A practical guide for
mapping scientific literature. New York: Nova Science Publishers;
2016
|
|
11
|
Bilal, Hysa E, Akbar A, Yasmin F, Rahman
AU and Li S: Virtual learning during the covid-19 pandemic: A
bibliometric review and future research agenda. Risk Manag Healthc
Policy. 15:1353–1368. 2022.
|
|
12
|
Force MM and Robinson NJ: Encouraging data
citation and discovery with the data citation index. J Comput Aided
Mol Des. 28:1043–1048. 2014.
|
|
13
|
Dori Y, Keller MS, Rome JJ, Gillespie MJ,
Glatz AC, Dodds K, Goldberg DJ, Goldfarb S, Rychik J and Itkin M:
Percutaneous lymphatic embolization of abnormal pulmonary lymphatic
flow as treatment of plastic bronchitis in patients with congenital
heart disease. Circulation. 133:1160–1670. 2016.
|
|
14
|
Henri O, Pouehe C, Houssari M, Galas L,
Nicol L, Edwards-Lévy F, Henry JP, Dumesnil A, Boukhalfa I, Banquet
S, et al: Selective stimulation of cardiac lymphangiogenesis
reduces myocardial edema and fibrosis leading to improved cardiac
function following myocardial infarction. Circulation.
133:1484–1497. 2016.
|
|
15
|
Fudim M, Salah HM, Sathananthan J, Bernier
M, Pabon-Ramos W, Schwartz RS, Rodés-Cabau J, Côté F, Khalifa A,
Virani SA and Patel MR: Lymphatic dysregulation in patients with
heart failure: JACC review topic of the week. J Am Coll Cardiol.
78:66–76. 2021.
|
|
16
|
Liu MM, Peng J, Guo YL, Zhu CG, Wu NQ, Xu
RX, Dong Q, Cui CJ and Li JJ: SORBS2 as a molecular target for
atherosclerosis in patients with familial hypercholesterolemia. J
Transl Med. 20:2332022.
|
|
17
|
Oliver G, Kipnis J, Randolph GJ and Harvey
NL: The lymphatic vasculature in the 21st century: Novel functional
roles in homeostasis and disease. Cell. 182:270–296. 2020.
|
|
18
|
Rademakers T, van der Vorst EP,
Daissormont IT, Otten JJ, Theodorou K, Theelen TL, Gijbels M,
Anisimov A, Nurmi H, Lindeman JH, et al: Adventitial lymphatic
capillary expansion impacts on plaque T cell accumulation in
atherosclerosis. Sci Rep. 7:452632017.
|
|
19
|
Nielsen NR, Rangarajan KV, Mao L, Rockman
HA and Caron KM: A murine model of increased coronary sinus
pressure induces myocardial edema with cardiac lymphatic dilation
and fibrosis. Am J Physiol Heart Circ Physiol. 318:H895–H907.
2020.
|
|
20
|
Chakraborty A, Scogin CK, Rizwan K, Morley
TS and Rutkowski JM: Characterizing lymphangiogenesis and
concurrent inflammation in adipose tissue in response to VEGF-D.
Front Physiol. 11:3632020.
|
|
21
|
Wada H, Suzuki M, Matsuda M, Ajiro Y,
Shinozaki T, Sakagami S, Yonezawa K, Shimizu M, Funada J, Takenaka
T, et al: VEGF-C and mortality in patients with suspected or known
coronary artery disease. J Am Heart Assoc. 7:e103552018.
|
|
22
|
Milasan A, Smaani A and Martel C: Early
rescue of lymphatic function limits atherosclerosis progression in
Ldlr−/− mice. Atherosclerosis. 283:106–119. 2019.
|
|
23
|
Drosos I, Pavlaki M, Ortega Carrillo MDP,
Kourkouli A, Buschmann K, Konstantinou F, Gogiraju R, Bochenek ML,
Chalikias G, Tortopidis C, et al: Increased lymphangiogenesis and
lymphangiogenic growth factor expression in perivascular adipose
tissue of patients with coronary artery disease. J Clin Med.
8:10002019.
|
|
24
|
Singla B, Lin HP, Chen A, Ahn W, Ghoshal
P, Cherian-Shaw M, White J, Stansfield BK and Csányi G: Role of
R-spondin 2 in arterial lymphangiogenesis and atherosclerosis.
Cardiovasc Res. 117:1489–1509. 2021.
|
|
25
|
Okura T, Ohkawara B, Takegami Y, Ito M,
Masuda A, Seki T, Ishiguro N and Ohno K: Mianserin suppresses
R-spondin 2-induced activation of Wnt/β-catenin signaling in
chondrocytes and prevents cartilage degradation in a rat model of
osteoarthritis. Sci Rep. 9:28082019.
|
|
26
|
Wu C, Qiu S, Lu L, Zou J, Li WF, Wang O,
Zhao H, Wang H, Tang J, Chen L, et al: RSPO2-LGR5 signaling has
tumour-suppressive activity in colorectal cancer. Nat Commun.
5:31492014.
|
|
27
|
Yu SJ, Wu KJ, Wang YS, Song JS, Wu CH, Jan
JJ, Bae E, Chen H, Shia KS and Wang Y: Protective effect of CXCR4
antagonist CX807 in a rat model of hemorrhagic stroke. Int J Mol
Sci. 21:70852020.
|
|
28
|
Reinhardt SW, Lin CJ, Novak E and Brown
DL: Noninvasive cardiac testing vs clinical evaluation alone in
acute chest pain: A secondary analysis of the ROMICAT-II randomized
clinical trial. JAMA Intern Med. 178:212–219. 2018.
|
|
29
|
Ibanez B, Aletras AH, Arai AE, Arheden H,
Bax J, Berry C, Bucciarelli-Ducci C, Croisille P, Dall'Armellina E,
Dharmakumar R, et al: Cardiac MRI endpoints in myocardial
infarction experimental and clinical trials: JACC scientific expert
panel. J Am Coll Cardiol. 74:238–256. 2019.
|
|
30
|
Klaourakis K, Vieira JM and Riley PR: The
evolving cardiac lymphatic vasculature in development, repair and
regeneration. Nat Rev Cardiol. 18:368–379. 2021.
|
|
31
|
Liu X, De la Cruz E, Gu X, Balint L,
Oxendine-Burns M, Terrones T, Ma W, Kuo HH, Lantz C, Bansal T, et
al: Lymphoangiocrine signals promote cardiac growth and repair.
Nature. 588:705–711. 2020.
|
|
32
|
Lohela M, Bry M, Tammela T and Alitalo K:
VEGFs and receptors involved in angiogenesis versus
lymphangiogenesis. Curr Opin Cell Biol. 21:154–165. 2009.
|
|
33
|
Hendel RC, Henry TD, Rocha-Singh K, Isner
JM, Kereiakes DJ, Giordano FJ, Simons M and Bonow RO: Effect of
intracoronary recombinant human vascular endothelial growth factor
on myocardial perfusion: Evidence for a dose-dependent effect.
Circulation. 101:118–121. 2000.
|
|
34
|
Eken A, Yetkin MF, Vural A, Okus FZ, Erdem
S, Azizoglu ZB, Haliloglu Y, Cakir M, Turkoglu EM, Kilic O, et al:
Fingolimod alters tissue distribution and cytokine production of
human and murine innate lymphoid cells. Front Immunol.
10:2172019.
|
|
35
|
Li Q, Zhou C, Zhao K, Duan Y, Yue J, Liu
X, Wu J and Deng S: Lymphatic endothelial sphingosine 1-phosphate
receptor 1 enhances macrophage clearance via lymphatic system
following myocardial infarction. Front Cardiovasc Med.
9:8721022022.
|
|
36
|
B Gowda S, Gowda D, Kain V, Chiba H, Hui
SP, Chalfant CE, Parcha V, Arora P and Halade GV:
Sphingosine-1-phosphate interactions in the spleen and heart
reflect extent of cardiac repair in mice and failing human hearts.
Am J Physiol Heart Circ Physiol. 321:H599–H611. 2021.
|
|
37
|
Du M, Shan J, Feng A, Schmull S, Gu J and
Xue S: Oestrogen receptor β activation protects against myocardial
infarction via Notch1 signalling. Cardiovasc Drugs Ther.
34:165–178. 2020.
|
|
38
|
Mahmoodzadeh S, Leber J, Zhang X, Jaisser
F, Messaoudi S, Morano I, Furth PA, Dworatzek E and Regitz-Zagrosek
V: Cardiomyocyte-specific estrogen receptor alpha increases
angiogenesis, lymphangiogenesis and reduces fibrosis in the female
mouse heart post-myocardial infarction. J Cell Sci Ther.
5:1532014.
|
|
39
|
Jeya PJ, Weigel C, Müller T, Heller R,
Spiegel S and Gräler MH: Inflammatory conditions disrupt
constitutive endothelial cell barrier stabilization by alleviating
autonomous secretion of sphingosine 1-phosphate. Cells.
9:9282020.
|
|
40
|
Nagahashi M, Abe M, Sakimura K, Takabe K
and Wakai T: The role of sphingosine-1-phosphate in inflammation
and cancer progression. Cancer Sci. 109:3671–3678. 2018.
|
|
41
|
Tatin F, Renaud-Gabardos E, Godet AC,
Hantelys F, Pujol F, Morfoisse F, Calise D, Viars F, Valet P, Masri
B, et al: Apelin modulates pathological remodeling of lymphatic
endothelium after myocardial infarction. Jci Insight.
2:e938872017.
|
|
42
|
Poh KK, Lee PSS, Djohan AH, Galupo MJ,
Songco GG, Yeo TC, Tan HC, Richards AM and Ye L: Transplantation of
endothelial progenitor cells in obese diabetic rats following
myocardial infarction: Role of thymosin beta-4. Cells.
9:9492020.
|
|
43
|
Wang YL, Yu SN, Shen HR, Wang HJ, Wu XP,
Wang QL, Zhou B and Tan YZ: Thymosin β4 released from
functionalized self-assembling peptide activates epicardium and
enhances repair of infarcted myocardium. Theranostics.
11:4262–4280. 2021.
|
|
44
|
Trincot CE, Xu W, Zhang H, Kulikauskas MR,
Caranasos TG, Jensen BC, Sabine A, Petrova TV and Caron KM:
Adrenomedullin induces cardiac lymphangiogenesis after myocardial
infarction and regulates cardiac edema via connexin 43. Circ Res.
124:101–113. 2019.
|
|
45
|
Lin QY, Zhang YL, Bai J, Liu JQ and Li HH:
VEGF-C/VEGFR-3 axis protects against pressure-overload induced
cardiac dysfunction through regulation of lymphangiogenesis. Clin
Transl Med. 11:e3742021.
|
|
46
|
Glinton KE, Ma W, Lantz C, Grigoryeva LS,
DeBerge M, Liu X, Febbraio M, Kahn M, Oliver G and Thorp EB:
Macrophage-produced VEGFC is induced by efferocytosis to ameliorate
cardiac injury and inflammation. J Clin Invest.
132:e1406852022.
|
|
47
|
McDonagh TA, Metra M, Adamo M, Gardner RS,
Baumbach A, Bohm M, Burri H, Butler J, Čelutkienė J, Chioncel O, et
al: 2021 ESC guidelines for the diagnosis and treatment of acute
and chronic heart failure. Eur Heart J. 42:3599–3726. 2021.
|
|
48
|
Schwinger RHG: Pathophysiology of heart
failure. Cardiovasc Diagn Ther. 11:263–276. 2021.
|
|
49
|
Weckbach LT, Uhl A, Boehm F, Seitelberger
V, Huber BC, Kania G, Brunner S and Grabmaier U: Blocking LFA-1
aggravates cardiac inflammation in experimental autoimmune
myocarditis. Cells. 8:12672019.
|
|
50
|
Tkacz K, Rolski F, Czepiel M, Działo E,
Siedlar M, Eriksson U, Kania G and Błyszczuk P: Haploinsufficient
Rock1+/− and Rock2+/− mice are not protected
from cardiac inflammation and postinflammatory fibrosis in
experimental autoimmune myocarditis. Cells. 9:7002020.
|
|
51
|
Zheng H, Liu X, Katsurada K and Patel KP:
Renal denervation improves sodium excretion in rats with chronic
heart failure: Effects on expression of renal ENaC and AQP2. Am J
Physiol Heart Circ Physiol. 317:H958–H968. 2019.
|
|
52
|
Maier A, Braig M, Jakob K, Bienert T,
Schäper M, Merkle A, Wadle C, Menza M, Neudorfer I, Bojti I, et al:
Molecular magnetic resonance imaging of activated platelets allows
noninvasive detection of early myocarditis in mice. Sci Rep.
10:132112020.
|
|
53
|
Huang B, Miao H, Yuan Y, Qiu F, Liu X, Liu
Z, Zhang H, Zhao Q, Wang M, Dong H and Zhang Z: PEDF decreases
cardiomyocyte edema during oxygen-glucose deprivation and recovery
via inhibiting lactate accumulation and expression of AQP1. Int J
Mol Med. 43:1979–1990. 2019.
|
|
54
|
Card CM, Yu SS and Swartz MA: Emerging
roles of lymphatic endothelium in regulating adaptive immunity. J
Clin Invest. 124:943–952. 2014.
|
|
55
|
Kajiya K and Detmar M: An important role
of lymphatic vessels in the control of uvb-induced edema formation
and inflammation. J Invest Dermatol. 126:919–921. 2006.
|
|
56
|
Li N, Zhu L, Sun L and Shao G: The effects
of novel coronavirus (SARS-CoV-2) infection on cardiovascular
diseases and cardiopulmonary injuries. Stem Cell Res.
51:1021682021.
|
|
57
|
Hata AN and Breyer RM: Pharmacology and
signaling of prostaglandin receptors: Multiple roles in
inflammation and immune modulation. Pharmacol Ther. 103:147–166.
2004.
|
|
58
|
Al-Kofahi M, Omura S, Tsunoda I, Sato F,
Becker F, Gavins FNE, Woolard MD, Pattillo C, Zawieja D, Muthuchamy
M, et al: IL-1β reduces cardiac lymphatic muscle contraction via
COX-2 and PGE2 induction: Potential role in myocarditis. Biomed
Pharmacother. 107:1591–1600. 2018.
|
|
59
|
Forrester SJ, Booz GW, Sigmund CD, Coffman
TM, Kawai T, Rizzo V, Scalia R and Eguchi S: Angiotensin II signal
transduction: An update on mechanisms of physiology and
pathophysiology. Physiol Rev. 98:1627–1738. 2018.
|
|
60
|
Doan TN, Bernard FC, McKinney JM, Dixon JB
and Willett NJ: Endothelin-1 inhibits size dependent lymphatic
clearance of PEG-based conjugates after intra-articular injection
into the rat knee. Acta Biomater. 93:270–281. 2019.
|
|
61
|
Bai J, Yin L, Yu WJ, Zhang YL, Lin QY and
Li HH: Angiotensin II induces cardiac edema and hypertrophic
remodeling through lymphatic-dependent mechanisms. Oxid Med Cell
Longev. 2022:50440462022.
|
|
62
|
Qu X, Song X, Yuan W, Shu Y, Wang Y, Zhao
X, Gao M, Lu R, Luo S, Zhao W, et al: Expression signature of
lncRNAs and their potential roles in cardiac fibrosis of
post-infarct mice. Biosci Rep. 36:e003372016.
|
|
63
|
Iwamiya T, Segard BD, Matsuoka Y and
Imamura T: Human cardiac fibroblasts expressing VCAM1 improve heart
function in postinfarct heart failure rat models by stimulating
lymphangiogenesis. PLoS One. 15:e02378102020.
|
|
64
|
Simko F, Pechanova O, Repova K, Aziriova
S, Krajcirovicova K, Celec P, Tothova L, Vrankova S, Balazova L,
Zorad S and Adamcova M: Lactacystin-induced model of hypertension
in rats: Effects of melatonin and captopril. Int J Mol Sci.
18:16122017.
|
|
65
|
Pan L, Sun X, Che H and Li M: CTRP9
mitigates vascular endothelial cell injury in patients with
hypertensive heart disease by inhibiting PI3K/Akt/mTOR axis. Am J
Transl Res. 14:6596–6603. 2022.
|
|
66
|
Sterns RH: Disorders of plasma
sodium-causes, consequences, and correction. N Engl J Med.
372:55–65. 2015.
|
|
67
|
Balasubbramanian D, Baranwal G, Clark MCC,
Goodlett BL, Mitchell BM and Rutkowski JM: Kidney-specific
lymphangiogenesis increases sodium excretion and lowers blood
pressure in mice. J Hypertens. 38:874–885. 2020.
|
|
68
|
Wiig H, Schröder A, Neuhofer W, Jantsch J,
Kopp C, Karlsen TV, Boschmann M, Goss J, Bry M, Rakova N, et al:
Immune cells control skin lymphatic electrolyte homeostasis and
blood pressure. J Clin Invest. 123:2803–2815. 2013.
|
|
69
|
Machnik A, Dahlmann A, Kopp C, Goss J,
Wagner H, van Rooijen N, Eckardt KU, Müller DN, Park JK, Luft FC,
et al: Mononuclear phagocyte system depletion blocks interstitial
tonicity-responsive enhancer binding protein/vascular endothelial
growth factor C expression and induces salt-sensitive hypertension
in rats. Hypertension. 55:755–761. 2010.
|
|
70
|
Yang GH, Zhou X, Ji WJ, Zeng S, Dong Y,
Tian L, Bi Y, Guo ZZ, Gao F, Chen H, et al: Overexpression of
VEGF-C attenuates chronic high salt intake-induced left ventricular
maladaptive remodeling in spontaneously hypertensive rats. Am J
Physiol Heart Circ Physiol. 306:H598–H609. 2014.
|
|
71
|
Ye BJ, Lee HH, Yoo EJ, Lee CY, Lee JH,
Kang HJ, Jeong GW, Park H, Lee-Kwon W, Choi SY and Kwon HM: TonEBP
in dendritic cells mediates pro-inflammatory maturation and
Th1/Th17 responses. Cell Death Dis. 11:4212020.
|
|
72
|
Yang GH, Zhou X, Ji WJ, Liu JX, Sun J,
Dong Y, Jiang TM and Li YM: VEGF-C-mediated cardiac
lymphangiogenesis in high salt intake accelerated progression of
left ventricular remodeling in spontaneously hypertensive rats.
Clin Exp Hypertens. 39:740–747. 2017.
|
|
73
|
Zhang C, Li N, Suo M, Zhang C, Liu J, Liu
L, Qi Y, Zheng X, Xie L, Hu Y and Bu P: Sirtuin 3 deficiency
aggravates angiotensin II-induced hypertensive cardiac injury by
the impairment of lymphangiogenesis. J Cell Mol Med. 25:7760–7771.
2021.
|
|
74
|
Hosios AM and Vander Heiden MG:
Endothelial cells Get β-ox-ed in to support lymphangiogenesis. Dev
Cell. 40:118–119. 2017.
|
|
75
|
Li X, Qiu J, Pan M, Zheng D, Su Y, Wei M,
Kong X, Sun W and Zhu J: Correlation between congenital heart
disease complicated with pulmonary artery hypertension and
circulating endothelial cells as well as endothelin-1. Int J Clin
Exp Pathol. 8:10743–10751. 2015.
|
|
76
|
Kelly B, Mohanakumar S and Hjortdal VE:
Diagnosis and management of lymphatic disorders in congenital heart
disease. Curr Cardiol Rep. 22:1642020.
|
|
77
|
Li D, Sheppard SE, March ME, Battig MR,
Surrey LF, Srinivasan AS, Matsuoka LS, Tian L, Wang F, Seiler C, et
al: Genomic profiling informs diagnoses and treatment in vascular
anomalies. Nat Med. 29:1530–1539. 2023.
|
|
78
|
Boehme JT, Morris CJ, Chiacchia SR, Gong
W, Wu KY, Kameny RJ, Raff GW, Fineman JR, Maltepe E and Datar SA:
HIF-1α promotes cellular growth in lymphatic endothelial cells
exposed to chronically elevated pulmonary lymph flow. Sci Rep.
11:14682021.
|
|
79
|
Scallan JP, Hill MA and Davis MJ:
Lymphatic vascular integrity is disrupted in type 2 diabetes due to
impaired nitric oxide signalling. Cardiovasc Res. 107:89–97.
2015.
|
|
80
|
Hwang J, Kleinhenz DJ, Lassegue B,
Griendling KK, Dikalov S and Hart CM: Peroxisome
proliferator-activated receptor-gamma ligands regulate endothelial
membrane superoxide production. Am J Physiol Cell Physiol.
288:C899–C905. 2005.
|
|
81
|
Choi D, Park E, Jung E, Seong YJ, Hong M,
Lee S, Burford J, Gyarmati G, Peti-Peterdi J, Srikanth S, et al:
ORAI1 activates proliferation of lymphatic endothelial cells in
response to laminar flow through Krüppel-like factors 2 and 4. Circ
Res. 120:1426–1439. 2017.
|
|
82
|
Morris CJ, Kameny RJ, Boehme J, Gong W, He
Y, Zhu T, Maltepe E, Raff GW, Fineman JR and Datar SA:
KLF2-mediated disruption of PPAR-γ signaling in lymphatic
endothelial cells exposed to chronically increased pulmonary lymph
flow. Am J Physiol Heart Circ Physiol. 315:H173–H181. 2018.
|
|
83
|
Yu J, Mao L, Guan L, Zhang Y and Zhao J:
Ginsenoside Rg1 enhances lymphatic transport of intrapulmonary
silica via VEGF-C/VEGFR-3 signaling in silicotic rats. Biochem
Biophys Res Commun. 472:182–188. 2016.
|
|
84
|
Allard B, Cousineau I, Allard D, Buisseret
L, Pommey S, Chrobak P and Stagg J: Adenosine A2a receptor promotes
lymphangiogenesis and lymph node metastasis. Oncoimmunology.
8:16014812019.
|
|
85
|
Han H, Ma Y, Wang X, Yun R, Lu S, Xia M,
Wang Y, Shi Q, Zhai W, Liang Q and Xu H: Fang-Ji-Huang-Qi-Tang
attenuates degeneration of early-stage KOA mice related to
promoting joint lymphatic drainage function. Evid Based Complement
Alternat Med. 2020:34716812020.
|
|
86
|
Chen Y, Li J, Li Q, Wang T, Xing L, Xu H,
Wang Y, Shi Q, Zhou Q and Liang Q: Du-Huo-Ji-Sheng-Tang attenuates
inflammation of TNF-Tg mice related to promoting lymphatic drainage
function. Evid Based Complement Alternat Med. 2016:70676912016.
|
|
87
|
Hou T, Liu Y, Wang X, Jiao D, Xu H, Shi Q,
Wang Y, Li W, Wu T and Liang Q: Ginsenoside Rg1 promotes lymphatic
drainage and improves chronic inflammatory arthritis. J
Musculoskelet Neuronal Interact. 20:526–534. 2020.
|
|
88
|
Ishii M, Miyata H, Ikeda N, Tagawa T and
Nishimura M: Piper retrofractum extract and its component piperine
promote lymphangiogenesis via an AKT- and ERK-dependent mechanism.
J Food Biochem. 46:e142332022.
|
|
89
|
Li J, Chen Y, Zhang L, Xing L, Xu H, Wang
Y, Shi Q and Liang Q: Total saponins of panaxnotoginseng promotes
lymphangiogenesis by activation vegf-c expression of lymphatic
endothelial cells. J Ethnopharmacol. 193:293–302. 2016.
|
|
90
|
Peng L, Dong Y, Fan H, Cao M, Wu Q and
Wang Y, Zhou C, Li S, Zhao C and Wang Y: Traditional Chinese
medicine regulating lymphangiogenesis: A literature review. Front
Pharmacol. 11:12592020.
|
|
91
|
Benvenuti LA, da Silva AMG and Aiello VD:
Quantification of lymphatic vessels in dilated and chronic chagasic
cardiomyopathy. Arq Bras Cardiol. 94:564–567. 2010.In
Portuguese.
|
|
92
|
Peng L, Ma M, Dong Y, Wu Q, An S, Cao M,
Wang Y, Zhou C, Zhou M, Wang X, et al: Kuoxin Decoction promotes
lymphangiogenesis in zebrafish and in vitro based on network
analysis. Front Pharmacol. 13:9151612022.
|
|
93
|
Shi J, Liang Q, Zuscik M, Shen J, Chen D,
Xu H, Wang YJ, Chen Y, Wood RW, Li J, et al: Distribution and
alteration of lymphatic vessels in knee joints of normal and
osteoarthritic mice. Arthritis Rheumatol. 66:657–666. 2014.
|
|
94
|
Lou B, Wu W, Zeng L, Zhou W, Zhang X, Zhou
X, Liu Z, Liu K, Gu X, Chen X, et al: Alleviating experimental
allergic eye disease by inhibiting pro-lymphangiogenic VEGFR3
signal. Ocul Surf. 26:1–12. 2022.
|
|
95
|
Ahn JH, Cho H, Kim JH, Kim SH, Ham JS,
Park I, Suh SH, Hong SP, Song JH, Hong YK, et al: Meningeal
lymphatic vessels at the skull base drain cerebrospinal fluid.
Nature. 572:62–66. 2019.
|
|
96
|
Da Mesquita S, Papadopoulos Z, Dykstra T,
Brase L, Farias FG, Wall M, Jiang H, Kodira CD, de Lima KA, Herz J,
et al: Meningeal lymphatics affect microglia responses and anti-Aβ
immunotherapy. Nature. 593:255–260. 2021.
|
|
97
|
Nedergaard M and Goldman SA: Glymphatic
failure as a final common pathway to dementia. Science. 370:50–56.
2020.
|
|
98
|
Li X, Qi L, Yang D, Hao S, Zhang F, Zhu X,
Sun Y, Chen C, Ye J, Yang J, et al: Meningeal lymphatic vessels
mediate neurotropic viral drainage from the central nervous system.
Nat Neurosci. 25:577–587. 2022.
|
|
99
|
Zhou M and Wang Y, Zhou C, Peng L, Liang Q
and Wang Y: A research progress of Traditional Chinese medicine
acting on the lymphangiogenesis and lymphatic drainage function.
Mod Tradit Chin Med Materia Medica-World Sci Tech. 24:802–809.
2022.In Chinese.
|
|
100
|
Qi SM, Zhang JT, Zhu HY, Wang Z and Li W:
Review on potential effects of traditional Chinese medicine on
glaucoma. J Ethnopharmacol. 304:1160632023.
|
|
101
|
Zhang J, Li Q and Zhou YY: Research
progress on mechanism of traditional Chinese medicine
polysaccharides in preventing and treating Alzheimer's disease.
Chin Tradit Herb Drugs. 53:7553–7565. 2022.In Chinese.
|
|
102
|
Zhang QM, Wang YG, Zhang JX, Han XY and
Tian X: Functional properties and biological basis of essence, qi,
blood and body fluid. Glob Tradit Chin Med. 14:841–847. 2021.
|
|
103
|
Cui JK, Chen X and Jiang Q: Discussion on
the treatment of Sjogren's syndrome from the metabolism of body
fluid. J Basic Chin Med. 25:1662–1663. 2019.In Chinese.
|
|
104
|
Jiang L, Zhao JX, Zhang YF, Meng FZ, Jian
J and Liu JT: Discussion on the treatment of diabetic kidney
disease edema in traditional Chinese medicine based on the theory
of qi, blood and water. China J Tradit Chin Med Pharm. 36:887–890.
2021.In Chinese.
|
|
105
|
Fang Y, Huang K and Li X: Preliminary
study on the blood circulation characteristics of phlegm syndrome.
Hubei J Tradit Chin Med. 1:33–34. 1992.In Chinese.
|
|
106
|
Wei D, Liu M and Shi X: A study on the
distribution of traditional Chinese medicine syndromes of elevated
blood glucose in the acute stage of stroke. J Emerg Tradit Chin
Med. 10:91–92. 2001.In Chinese.
|
|
107
|
Wang J, Yan C, Deng Z and Pan Y: Exploring
the mechanism of phlegm syndrome from the abnormal metabolism of
adhesion molecules. Chin J Integr Tradit West Med. 20:296–297.
2000.In Chinese.
|
|
108
|
Yu S, Liu C, Zhou L, Zhong Y, Xiong N, Xu
J, Liu C, Cheng S and Wang P: Wendantang treats inflammation in
obesity (Syndrome of Phlegm-dampness) by regulatingPI3K/Akt/mTOR
pathway-mediated adipocyte autophagy. Chin J Exp Tradit Med
Formulae. 29:1–10. 2023.In Chinese.
|
|
109
|
Yi GH: Lymphatic vessels: A potential path
to intervene the development of atherosclerosis. Chin J
Arterioscler. 26:973–979. 2018.In Chinese.
|
|
110
|
Mikami T, Koyama A, Hashimoto K, Maegawa
J, Yabuki Y, Kagimoto S, Kitayama S, Kaneta T, Yasumura K,
Matsubara S and Iwai T: Pathological changes in the lymphatic
system of patients with secondary upper limb lymphoedema. Sci Rep.
9:84992019.
|
|
111
|
Mukherjee A, Hooks J, Nepiyushchikh Z and
Dixon JB: Entrainment of lymphatic contraction to oscillatory flow.
Sci Rep. 9:58402019.
|
|
112
|
Huang SW, Yang HY, Huang WJ, Chen WC, Yu
MC, Wang SW, Hsu YF and Hsu MJ: WMJ-S-001, a novel aliphatic
hydroxamate-based compound, suppresses lymphangiogenesis through
p38mapk-p53-survivin signaling cascade. Front Oncol.
9:11882019.
|
|
113
|
Shinriki S, Jono H, Ueda M, Ota K, Ota T,
Sueyoshi T, Oike Y, Ibusuki M, Hiraki A, Nakayama H, et al:
Interleukin-6 signalling regulates vascular endothelial growth
factor-C synthesis and lymphangiogenesis in human oral squamous
cell carcinoma. J Pathol. 225:142–150. 2011.
|
|
114
|
Zhang N, Hu H, Fu Y, He F, Wang L, Zhuang
S and Ding M: The overexpression of PDGF-BB and its receptor is
correlated with lymphatic metastasis in patients with non-small
cell lung cancer. Int J Clin Exp Pathol. 11:6010–6017. 2018.
|
|
115
|
Caunt M, Mak J, Liang WC, Stawicki S, Pan
Q, Tong RK, Kowalski J, Ho C, Reslan HB, Ross J, et al: Blocking
neuropilin-2 function inhibits tumor cell metastasis. Cancer Cell.
13:331–342. 2008.
|
|
116
|
Song J, Chen W, Cui X, Huang Z, Wen D,
Yang Y, Yu W, Cui L and Liu CY: CCBE1 promotes tumor
lymphangiogenesis and is negatively regulated by TGFβ signaling in
colorectal cancer. Theranostics. 10:2327–2341. 2020.
|
|
117
|
Haemmerle M, Keller T, Egger G, Schachner
H, Steiner CW, Stokic D, Neumayer C, Brown MK, Kerjaschki D and
Hantusch B: Enhanced lymph vessel density, remodeling, and
inflammation are reflected by gene expression signatures in dermal
lymphatic endothelial cells in type 2 diabetes. Diabetes.
62:2509–2529. 2013.
|
|
118
|
Morooka N, Futaki S, Sato-Nishiuchi R,
Nishino M, Totani Y, Shimono C, Nakano I, Nakajima H, Mochizuki N
and Sekiguchi K: Polydom is an extracellular matrix protein
involved in lymphatic vessel remodeling. Circ Res. 120:1276–1288.
2017.
|
|
119
|
Choi D, Park E, Yu RP, Cooper MN, Cho IT,
Choi J, Yu J, Zhao L, Yum JI, Yu JS, et al: Piezo1-regulated
mechanotransduction controls flow-activated lymphatic expansion.
Circ Res. 131:e2–e21. 2022.
|
|
120
|
Klotz L, Norman S, Vieira JM, Masters M,
Rohling M, Dubé KN, Bollini S, Matsuzaki F, Carr CA and Riley PR:
Cardiac lymphatics are heterogeneous in origin and respond to
injury. Nature. 522:62–67. 2015.
|
|
121
|
Lim HY, Thiam CH, Yeo KP, Bisoendial R,
Hii CS, McGrath KC, Tan KW, Heather A, Alexander JS and Angeli V:
Lymphatic vessels are essential for the removal of cholesterol from
peripheral tissues by SR-BI-mediated transport of HDL. Cell Metab.
17:671–684. 2013.
|
|
122
|
Dashkevich A, Raissadati A, Syrjälä SO,
Zarkada G, Keränen MA, Tuuminen R, Krebs R, Anisimov A, Jeltsch M,
Leppanen VM, et al: Ischemia-reperfusion injury enhances lymphatic
endothelial VEGFR3 and rejection in cardiac allografts. Am J
Transplant. 16:1160–1172. 2016.
|
|
123
|
Losordo DW, Vale PR, Hendel RC, Milliken
CE, Fortuin FD, Cummings N, Schatz RA, Asahara T, Isner JM and
Kuntz RE: Phase 1/2 placebo-controlled, double-blind,
dose-escalating trial of myocardial vascular endothelial growth
factor 2 gene transfer by catheter delivery in patients with
chronic myocardial ischemia. Circulation. 105:2012–2018. 2002.
|
|
124
|
Hartikainen J, Hassinen I, Hedman A,
Kivelä A, Saraste A, Knuuti J, Husso M, Mussalo H, Hedman M,
Rissanen TT, et al: Adenoviral intramyocardial VEGF-DΔNΔC gene
transfer increases myocardial perfusion reserve in refractory
angina patients: A phase I/IIa study with 1-year follow-up. Eur
Heart J. 38:2547–2555. 2017.
|
|
125
|
O'Dwyer J, Murphy R, Dolan EB, Kovarova L,
Pravda M, Velebny V, Heise A, Duffy GP and Cryan SA: Development of
a nanomedicine-loaded hydrogel for sustained delivery of an
angiogenic growth factor to the ischaemic myocardium. Drug Deliv
Transl Res. 10:440–454. 2020.
|
|
126
|
O'Dwyer J, Murphy R, González-Vázquez A,
Kovarova L, Pravda M, Velebny V, Heise A, Duffy GP and Cryan SA:
Translational studies on the potential of a VEGF
nanoparticle-loaded hyaluronic acid hydrogel. Pharmaceutics.
13:7792021.
|
|
127
|
Zheng W, Aspelund A and Alitalo K:
Lymphangiogenic factors, mechanisms, and applications. J Clin
Invest. 124:878–887. 2014.
|
|
128
|
Hu D, Li L, Li S, Wu M, Ge N, Cui Y, Lian
Z, Song J and Chen H: Lymphatic system identification,
pathophysiology and therapy in the cardiovascular diseases. J Mol
Cell Cardiol. 133:99–111. 2019.
|
|
129
|
Hwang SD, Song JH, Kim Y, Lim JH, Kim MY,
Kim EN, Hong YA, Chung S, Choi BS, Kim YS and Park CW: Inhibition
of lymphatic proliferation by the selective VEGFR-3 inhibitor
SAR131675 ameliorates diabetic nephropathy in db/db mice. Cell
Death Dis. 10:2192019.
|
|
130
|
Rahbar E, Weimer J, Gibbs H, Yeh AT,
Bertram CD, Davis MJ, Hill MA, Zawieja DC and Moore JE Jr: Passive
pressure-diameter relationship and structural composition of rat
mesenteric lymphangions. Lymphat Res Biol. 10:152–163. 2012.
|
|
131
|
Fang Y, Wang XY, You JF, Liang QQ and
Zheng WC: Jia-Wei-Niu-Bang-Zi-Tang promotes lymphangiogenesis in
TNF-α transgenic mice and alleviates rheumatoid arthritis. Chin
Pharmacol Bulletin. 37:1469–1474. 2021.In Chinese.
|
|
132
|
Feng X, Sun H, Gao F and Ma W: Observation
of curative effect of the treatment of lymphedema of upper limb
after breast cancer operation with fumigation and washing of
traditional Chinese medicine combined with acupoint application of
Jiawei Jinhuang ointment. J Mod Oncol. 31:1252–1256. 2023.In
Chinese.
|
|
133
|
Lohrke J, Frenzel T, Endrikat J, Alves FC,
Grist TM, Law M, Lee JM, Leiner T, Li KC, Nikolaou K, et al: 25
years of contrast-enhanced MRI: Developments, current challenges
and future perspectives. Adv Ther. 33:1–28. 2016.
|
|
134
|
Quintanilla M, Montero-Montero L, Renart J
and Martin-Villar E: Podoplanin in inflammation and cancer. Int J
Mol Sci. 20:7072019.
|
|
135
|
Aldea R, Weller RO, Wilcock DM, Carare RO
and Richardson G: Cerebrovascular smooth muscle cells as the
drivers of intramural periarterial drainage of the brain. Front
Aging Neurosci. 11:12019.
|
|
136
|
Brakenhielm E and Alitalo K: Cardiac
lymphatics in health and disease. Nat Rev Cardiol. 16:56–68.
2019.
|
|
137
|
Gordon-Walker TT, Bove K and Veldtman G:
Fontan-associated liver disease: A review. J Cardiol. 74:223–232.
2019.
|
|
138
|
Savla JJ, Itkin M, Rossano JW and Dori Y:
Post-operative chylothorax in patients with congenital heart
disease. J Am Coll Cardiol. 69:2410–2422. 2017.
|
|
139
|
Goumans MJ, Zwijsen A, Ten Dijke P and
Bailly S: Bone morphogenetic proteins in vascular homeostasis and
disease. Cold Spring Harb Perspect Biol. 10:a0319892018.
|
|
140
|
Nash LA, Burns JK, Chardon JW, Kothary R
and Parks RJ: Spinal muscular atrophy: More than a disease of motor
neurons? Curr Mol Med. 16:779–792. 2016.
|
|
141
|
Melenotte C, Protopopescu C, Million M,
Edouard S, Carrieri MP, Eldin C, Angelakis E, Djossou F, Bardin N,
Fournier PE, et al: Clinical features and complications of coxiella
burnetii infections from the french national reference center for Q
fever. JAMA Netw Open. 1:e1815802018.
|
|
142
|
Vieira JM, Norman S, Villa Del Campo C,
Cahill TJ, Barnette DN, Gunadasa-Rohling M, Johnson LA, Greaves DR,
Carr CA, Jackson DG and Riley PR: The cardiac lymphatic system
stimulates resolution of inflammation following myocardial
infarction. J Clin Invest. 128:3402–3412. 2018.
|
|
143
|
Chavhan GB, Amaral JG, Temple M and Itkin
M: MR lymphangiography in children: Technique and potential
applications. Radiographics. 37:1775–1790. 2017.
|