Introduction
The emergence of systemic chronic inflammation is
significantly influenced by lifestyle, psychosocial elements and
environmental factors. This intricate interplay fosters a spectrum
of non-infectious inflammation-related maladies, including but not
limited to cardiovascular and cerebrovascular diseases, diabetes,
autoimmune disorders, chronic nephritis, chronic liver diseases,
and conditions affecting the osteoarticular and neurodegenerative
systems. These diseases collectively represent substantial
contributors to global morbidity and mortality rates. Addressing
the multifaceted determinants of chronic inflammation is imperative
for developing comprehensive strategies to mitigate the impact of
these prevalent and debilitating health conditions on a global
scale (1).
Studies have shown that pyroptosis is widely
involved in the occurrence and development of non-infectious
inflammation-related diseases and plays an important role (2). The sterile inflammatory response,
devoid of microbial infection, plays a crucial role in organ
development, tissue repair and host defense mechanisms.
Nonetheless, dysregulation of sterile inflammation can precipitate
various inflammatory diseases such as lung inflammation, type 2
diabetes and sterile liver diseases. Pyroptosis is a form of
programmed cell death that is distinct from apoptosis and necrosis.
It is an inflammatory form of cell death triggered by certain
cellular signals, particularly inflammasomes, in response to
infection or cellular stress (3).
During pyroptosis, the cell undergoes a series of morphological
changes, including cell swelling, membrane rupture, and release of
pro-inflammatory cytokines and intracellular contents. This process
is mediated by gasdermin proteins, which forms pores in the cell
membrane, leading to cell lysis and the release of inflammatory
molecules (3,4). Given the pivotal influence of
pyroptosis on orchestrating inflammation, there exists a hypothesis
postulating that pyroptosis may serve as a potential contributor in
several sterile inflammatory diseases.
Stem cell therapy, heralded as a groundbreaking
approach to treat diverse diseases and injuries, holds tremendous
promise for tissue repair and regeneration (5). Their therapeutic prowess lies in
their remarkable adaptability to specific tissue environments,
fostering healing through the generation of functional cells. This
adaptability becomes particularly crucial in scenarios where the
body's natural repair mechanisms prove insufficient, as observed in
degenerative diseases, injuries, or chronic disorders (6–8).
Mesenchymal stem cells (MSCs), for example, exhibit
anti-inflammatory and immunomodulatory properties, contributing to
tissue repair by reducing inflammation and promoting the
regeneration of damaged cells (9).
Previous studies have indicated that the ability of
stem cells to regulate pyroptosis has significant implications for
tissue repair and regenerative medicine (9,10).
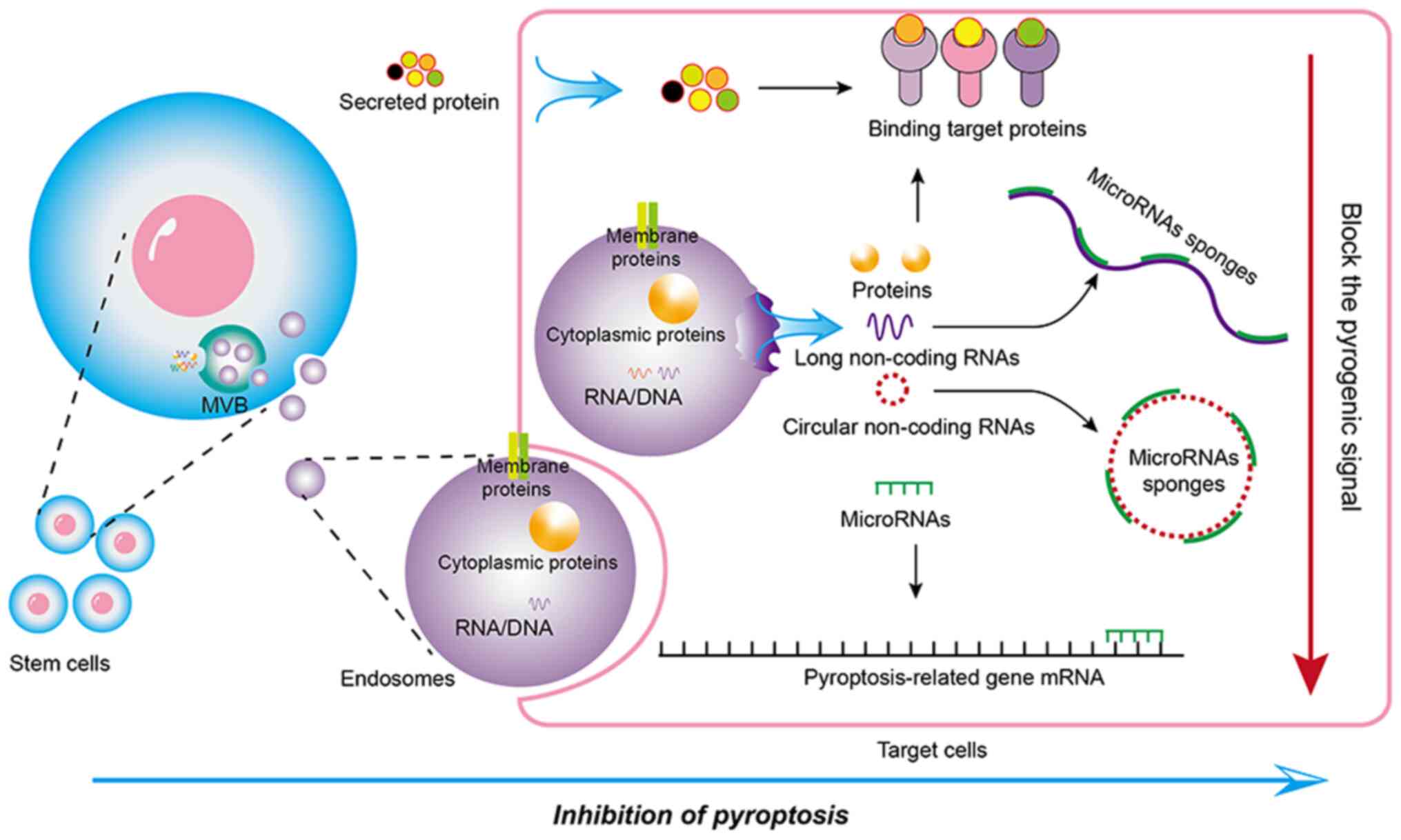
Stem cells can secrete various factors, including anti-inflammatory
cytokines, growth factors and paracrine factors (Fig. 1), which can play a significant
role in reducing inflammation and promoting tissue repair (9,11).
These secreted factors carry a cargo of bioactive molecules, such
as microRNAs, proteins and lipids, which can directly or indirectly
target pyroptosis signaling pathways to influence neighboring cells
and modulate their behavior (12,13). Through intricate signaling
pathways, stem cells modulate pyroptosis to control the
inflammatory response and foster tissue regeneration (14). This regulatory function is crucial
in addressing injuries, degenerative diseases and other conditions
where pyroptosis may contribute to tissue damage (15).
Understanding the interplay between stem cells and
pyroptosis provides insights into innovative therapeutic
strategies, offering the potential for targeted interventions that
harness the regenerative capacities of stem cells to promote tissue
repair while mitigating excessive inflammatory responses. In the
present comprehensive review, it was presented how stem cells
regulate pyroptosis to promote tissue repair in various
diseases.
Canonical and non-canonical pathways of
pyroptosis
Stem cells influence pyroptosis by modulating the
pyroptosis signaling pathway, thereby further promoting tissue
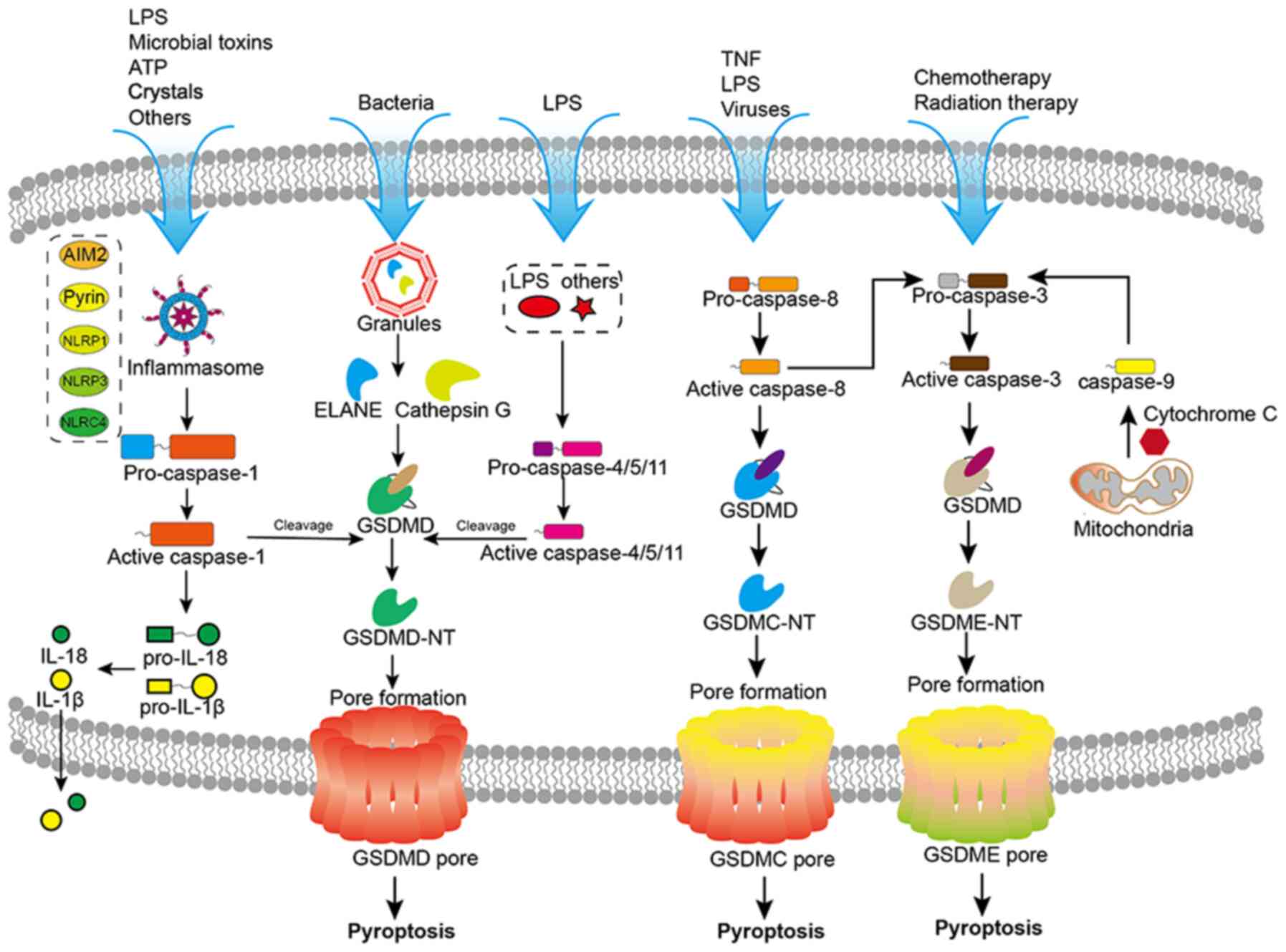
repair or slowing the pathological process (16). Pyroptosis is activated through
distinct pathways, the canonical and non-canonical pathways
(Fig. 2) (17). Damage-associated molecular
patterns (DAMPs), which selectively bind to their complementary
membrane protein receptors, including Toll-like receptors (TLRs)
and nucleotide-binding oligomerization domain-like receptors,
collectively termed pattern recognition receptors (PRRs). Upon
binding to PRRs, DAMPs orchestrate the activation of nuclear factor
kappa B (NF-κB), culminating in the transcriptional upregulation of
numerous pro-inflammatory genes, among which are the essential
components of the inflammasome pathways (18). The assembly of the NOD-like
receptor family, pyrin domain-containing 3 (NLRP3) inflammasome
facilitates the self-cleavage of pro-caspase-1, transforming it
into its enzymatically active form, caspase-1 (19). Subsequently, active caspase-1
cleaves pro-interleukin into its biologically active counterparts,
IL-1β and IL-18 (20).
Simultaneously, caspase-1 also cleaves gasdermin D (GSDMD),
resulting in the formation of two fragments: GSDMD-N and GSDMD-C.
GSDMD-N subsequently assembles into pores within the plasma
membrane, initiating pyroptotic cell death and the release of IL-1β
and IL-18 to induce more inflammation (21).
In addition to the canonical pathway, emerging
evidence has demonstrated the involvement of caspase-4, caspase-5
and caspase-11 in pyroptosis, particularly in scenarios involving
conventional or unconventional inflammasome signaling (22–24). These caspases play a dual role in
initiating pyroptotic cell death. Firstly, caspase-4/5/11 can
directly cleave GSDMD (25). This
direct cleavage leads to the formation of GSDMD-N, which assembles
into membrane pores, resulting in pyroptotic cell death (25). Secondly, caspase-4/5/11 can also
stimulate the NLRP3 inflammasome/caspase-1 signaling pathway,
eventually leading to pyroptosis. In this context, caspase-4/5/11
serves as an upstream activator, triggering the assembly of the
NLRP3 inflammasome. This, in turn, leads to the activation of
caspase-1, which subsequently cleaves GSDMD, ultimately culminating
in the execution of pyroptotic cell death (26).
The modulation of pyroptosis by stem cells
for alleviating progression in degenerative diseases
Osteoarthritis (OA)
(OA) is a chronic joint disease marked by the
gradual degradation of articular cartilage (AC), along with
subchondral bone sclerosis, synovial hyperplasia, and the formation
of osteophytes (27). Emerging
evidence suggests that pyroptosis may play a crucial role in the
pathogenesis of OA (6). Recent
research has revealed that inhibiting pyroptosis in chondrocytes
can attenuate the development of OA (28). Excessive inflammation within
chondrocytes is a key factor contributing to chondrocyte survival
and is implicated in the progression of OA. In an adjuvant-induced
arthritis model, acid-sensitive ion channel 1a has been identified
as a mediator of chondrocyte pyroptosis, shedding light on the
underlying mechanisms of OA pathology (29). Furthermore, in knee OA,
fibroblast-like synoviocytes have been recognized as the main
effector cells responsible for synovial fibrosis. Pyroptosis in
these cells has been linked to NOD-like receptor family, pyrin
domain-containing 1 and NLRP3 inflammasomes, underscoring the
significance of pyroptosis in driving fibroblast-like synoviocyte
dysfunction in knee OA (30). An
increase in hypoxia-inducible factor-1α levels has been found to
exacerbate synovial fibrosis by promoting fibroblast-like
synoviocyte pyroptosis (31).
These studies elucidate the critical role of pyroptosis in the
pathophysiological processes of OA.
Recently, adipose-derived MSCs (adMSCs) have been
demonstrated to exert a protective effect by delaying the
development of rat OA. This safeguarding mechanism hinges on the
secretion of sTNFR1 by adMSCs, which possesses highly specific
neutralizing activity against tumor necrosis factor-alpha (TNF-α).
This inhibition of TNF-α serves to curtail chondrocyte pyroptosis,
a pivotal contributor to OA progression (32). In a similar vein, exosomes derived
from bone marrow MSCs (BMSC-Exos) have demonstrated the ability to
mitigate OA by delivering microRNA (miR)-326 to chondrocytes and
cartilage. Through this delivery, BMSC-Exos target HDAC3 and the
STAT1/NF-κB p65 axis, effectively inhibiting chondrocyte and
cartilage pyroptosis (15).
Likewise, extracellular vesicles derived from human umbilical cord
MSCs (hucMSC-EVs) have emerged as a promising avenue for
alleviating OA and maintaining chondrocyte homeostasis. A novel
therapeutic mechanism was revealed, centered around the pivotal
miR-223. This miR directly binds to the 3′-untranslated region of
NLRP3 mRNA, exerting profound anti-inflammatory and
cartilage-protective effects through hucMSC-EVs (33).
Intervertebral disc degeneration
(IVDD)
IVDD represents a chronic and degenerative disease
characterized by dysregulation of the catabolic and anabolic
processes within the extracellular matrix (ECM) and alterations in
the microenvironment of the intervertebral disc (IVD) (34,35). The healthy intervertebral disc is
a complex tissue composed of a soft inner nucleus pulposus (NP)
surrounded by the fibrocartilaginous ring annulus fibrosus and
cartilage endplates (36,37). However, during IVDD, there is an
aberrant expression of matrix metalloproteinases and a reduction in
collagen II secretion from NP cells (NPCs), which disrupts the
delicate balance of the ECM (38,39). Consequently, remodeling of the
IVDD microenvironment occurs, leading to the accumulation of
inflammatory factors and inducing NLRP3-mediated pyroptosis of NPCs
(40,41). This cascade of events initiates a
series of worsening reactions, contributing to the progression and
severity of the disease.
EVs, specifically exosomes, from stem cells play a
critical role in paracrine signaling, effectively protecting NPCs
from apoptosis, promoting ECM synthesis, and mitigating
inflammatory responses in intervertebral discs (42,43). A thermosensitive acellular ECM
hydrogel coupled with adMSCs exosomes has shown promise. This
approach helps maintain early IVDD microenvironment homeostasis and
ameliorates IVDD (44).
In several studies, the mechanism by which EVs from
stem cells inhibit pyroptosis of NPCs has been reported. In IVDD
patients, methyltransferase like 14 (METTL14) expression is
significantly elevated in NPCs (45). METTL14 stabilizes NLRP3 mRNA in a
manner dependent on insulin-like growth factor-binding protein 2
(46,47). Consequently, the increased NLRP3
levels lead to elevated production of IL-1β and IL-18, triggering
pyroptotic cell death in NPCs. However, hope lies in the
therapeutic potential of miRs derived from exosomes of different
stem cell sources. For instance, miR-26a-5p, found in hucMSCs
exosomes, directly degrades METTL14, effectively improving NP cell
viability and protecting them from pyroptosis (47). Similarly, MSCs-derived exosomal
miR-410 plays an essential role in counteracting pyroptosis by
directly binding to NLRP3 mRNA, thereby suppressing the NLRP3
pathway (9). Furthermore,
exosomal miR-302c, which originates from ESCs, exhibits
anti-pyroptotic properties by inhibiting NLRP3 and consequently
alleviating pyroptosis in NPCs (48).
Cartilage regeneration and
reconstruction
The regeneration and reconstruction of AC after a
defect, including OA and IVDD, pose significant challenges, owing
to its inherently restricted intrinsic reparative capabilities
(49,50). When AC sustains injury, the
afflicted tissue releases damage-associated DAMPs (51), subsequently provoking the
neighboring cells and circulating immune cells to secrete
pro-inflammatory chemokines (52). These inflammatory cytokines,
notably IL-1 and TNF-α, exert a profound inhibitory influence on
the proliferation and differentiation of MSCs and chondrocytes.
Notably, studies have documented that IL-1 and TNF-α impede the
differentiation of growth plate chondrocytes and suppress the
expression of chondrogenic-related genes in MSCs (53,54). The crux of addressing AC defects
lies in effectively promoting regeneration at the site and
controlling the inflammatory response (55).
In recent years, there has been a growing interest
in employing an endogenous regenerative approach that mobilizes
resident MSCs to the sites of injury-a promising alternative
strategy. Research has unveiled a bioactive multifunctional
scaffold designed with the aptamer Apt19S as a mediator for the
targeted recruitment of MSCs. This scaffold not only enhances
cellular chondrogenesis but also exerts effective regulation over
the inflammatory response through the incorporation of
Mg2+ (56). Another
noteworthy study has reported that the incorporation of magnesium
hydroxide nanoparticles within a poly (lactic-co-glycolic acid)
scaffold enhances chondrogenesis by inducing the chondrogenic
differentiation of human BMSCs. Furthermore, it diminishes
pyroptosis during the early stages of chondrogenic differentiation
and curtails the release of inflammatory cytokines (57).
Stem cell-based transplantation holds immense
promise as a therapeutic approach for IVDD (58). MSCs transplantation, in
particular, has gained traction as a representative cell therapy
for IVDD (59). However, the
intra-discal injection of ‘naked’ MSCs faces challenges due to the
harsh IVDD microenvironment, resulting in poor survival rates and
altered activity (60,61). To address this, a novel strategy
involving the use of embryo-derived long-term expandable NP
progenitor cells (NPPCs) and esterase-responsive ibuprofen
nano-micelles (PEG-PIB) was devised for synergistic
transplantation. The PEG-PIB micelles were endocytosed to
pre-modify the NPPCs, rendering them adaptable to the harsh IVDD
microenvironment by inhibiting pyroptosis of NPPCs through the
COX2/NF-κB/Caspase-1 signaling pathway. This synergistic
transplantation approach demonstrated effective functional
recovery, histological regeneration, and inhibition of pyroptosis
during the process of IVDD regeneration (62).
Pyroptosis plays a pivotal role in degenerative
diseases, with a specific emphasis on its impact on OA and IVDD
(41,63). Stem cells exhibit dual
functionality in mitigating degenerative diseases. Firstly, they
impede pyroptosis at lesion sites through paracrine pathways,
thereby decelerating the pathological progression. Secondly, the
hostile microenvironment at lesion sites induces pyroptosis in
transplanted stem cells. Several innovative designs are required to
inhibit stem cell pyroptosis, enhancing the efficacy of lesion
repair.
Stem cell-mediated modulation of pyroptosis
for tissue repair in ischemia/reperfusion (I/R) injury
Myocardial I/R injury
Myocardial I/R injury is a complex pathological
process that occurs when blood flow to the heart muscle
(myocardium) is temporarily reduced or interrupted (ischemia),
followed by the restoration of blood flow (reperfusion) (64). This intricate phenomenon is
observed in various cardiac conditions, such as acute myocardial
infarction (heart attack) and coronary artery bypass grafting
(65).
During myocardial I/R, the activation of
inflammasomes and the subsequent release of pro-inflammatory
cytokines can trigger pyroptosis, a highly regulated form of cell
death (66). This cascade of
events contributes to the progression of tissue damage and
inflammation, exacerbating the consequences of myocardial I/R
injury. Understanding the mechanisms underlying pyroptosis in the
context of myocardial I/R injury is of utmost importance for
devising novel therapeutic interventions and mitigating the impact
of cardiovascular diseases (67).
Recent evidence highlights the significant role of
exosomes derived from MSCs in conferring protective effects against
myocardial I/R injury by inhibiting myocardial pyroptosis (7). These exosomes act as carriers of
endogenous molecules, including non-coding RNA, which play a
crucial role in suppressing pyroptosis following I/R (68). Specifically, exosomal miR-320b
targets NLRP3 (69), miR-100-5p
targets FOXO3 (16), and
miR-182-5p targets GSDMD (7),
leading to downregulation of these target proteins and subsequent
inhibition of pyroptosis. Additionally, the lncRNA KLF3-AS1,
competes with miR-138-5p to regulate Sirt1 expression, contributing
to the attenuation of pyroptosis (70). Another lncRNA, XIST, serves as a
competing endogenous RNA of miR-214-3p, leading to upregulation of
its target gene Arl2, which in turn attenuates myocardial
pyroptosis (71). Moreover,
MSC-derived exosomes exhibit their protective role in preventing
ischemic injury through the release of circHIPK3, a circular
non-coding RNA. CircHIPK3 downregulates miR-421, resulting in
increased expression of FOXO3a, which effectively inhibits
pyroptosis and the release of pro-inflammatory cytokines such as
IL-1β and IL-18 (72).
Neuronal I/R injury
Cerebral ischemic stroke stands as the most
prevalent cause of death and disability among central nervous
system (CNS) diseases (73). This
ischemic insult is often accompanied by a robust inflammatory
response. In the face of cerebral I/R injury, various forms of
programmed cell death, including apoptosis and pyroptosis, emerge
as prominent contributors to the ensuing tissue damage (74). Microglia, as the resident immune
cells in the central nervous system, play a pivotal role in the
initiation and progression of I/R injury (75). Notably, prior investigations have
shown that inhibiting microglial pyroptosis in neonatal mice
subjected to cerebral I/R promotes neuronal survival and ultimately
ameliorates brain injury (76).
In the realm of neuroprotection, a distinct class of
MSCs called olfactory mucosa MSCs demonstrate their capacity to
confer neuroprotection by mitigating apoptosis and pyroptosis in
microglial cells, employing an oxygen-glucose
deprivation/reperfusion model that closely mimics I/R conditions
(77). Moreover, emerging
research highlights the significance of MSC-derived exosomes in
inhibiting microglial pyroptosis. These exosomes facilitate
FOXO3a-dependent mitophagy, a cellular process responsible for
clearing damaged mitochondria, thereby mediating neuroprotection
(78). Additionally, MSC-derived
exosomes modulate microglial polarization, shifting them from an M1
phenotype (pro-inflammatory) to an M2 phenotype
(anti-inflammatory), further contributing to the amelioration of
cerebral I/R injury (79).
Another noteworthy finding pertains to BMSCs-derived exosomal
miR193b-5p, which plays a pivotal role in reducing microglia
pyroptosis post-ischemic stroke. The miR targets the absent in
melanoma 2 (AIM2) pathway, effectively suppressing AIM2-mediated
pyroptosis (12).
A recent study suggested that retinal I/R injury may
initiate pyroptosis in retinal neurons (80). Throughout retinal I/R injury,
various cellular processes, including oxidative stress,
mitochondrial dysfunction and activation of inflammasomes,
contribute to the induction of pyroptosis in retinal neurons.
Interventions targeting pyroptosis could prove to be an effective
strategy in preventing I/R-induced retinal damage and subsequent
visual impairment (81). The
neuroprotective effects of intravitreal injection of MSCs and
MSC-conditioned medium in rats during retinal ischemia are
noteworthy (82,83). These effects are primarily
mediated by EVs (84). While both
MSCs and MSC-derived EVs have demonstrated neuroprotective effects
following retinal I/R injury, direct evidence confirming this
protective effect's association with the modulation of pyroptosis
is currently lacking.
Cardiac and cerebral injuries after
cardiopulmonary resuscitation (CPR)
After CPR, cardiac and cerebral injuries often lead
to unfavorable outcomes in cardiac arrest (CA) victims (85). The pathogenesis of these injuries
may involve cell pyroptosis and ferroptosis. However, promisingly,
MSCs have also emerged as a potential therapeutic strategy for
providing cardiac and cerebral protection for I/R injury. MSCs have
been identified to effectively reduce post-resuscitation cardiac
and cerebral pyroptosis and ferroptosis, offering hope for
improving the prognosis and overall outcome of CA patients
(10). Although the mechanism by
which MSCs reduce pyroptosis in post-resuscitation cardiac and
brain cells is unclear, there is evidence that MSCs downregulate
the level of M1 macrophages and upregulate the level of M2
macrophages after CA (86). Given
the link between pyroptosis and inflammation, the modulation of
post-resuscitation cardiac and cerebral pyroptosis by MSCs may
involve the regulation of the inflammatory microenvironment.
Lung I/R injury (LIRI)
LIRI represents a pathological phenomenon triggered
by various clinical conditions, including lung transplantation,
hemorrhagic shock and pulmonary embolism (87). A key contributing factor to LIRI
is the heightened release of IL-1β and IL-6, leading to pyroptosis
in lung epithelial cells (88).
Remarkably, treatment with human BMSCs has shown significant
efficacy in alleviating hypoxia-induced pulmonary epithelial
injury, offering promise for LIRI intervention (89). Recent investigations have
identified a crucial mechanism by which BMSC-derived exosomes exert
their protective effects against LIRI. Specifically, the exosomal
miR-202-5p plays a central role in repressing pulmonary epithelial
pyroptosis, thereby inhibiting the progression of LIRI (90). The targeted molecule of miR-202-5p
is CMPK2, a mitochondrial nucleotide monophosphate kinase known for
its regulatory role in macrophage activation and inflammatory
responses (91).
Hepatic I/R injury (HIRI)
HIRI is a significant contributor to liver injury
and failure following liver surgery. This condition is
characterized by massive cell death, severe inflammation and
oxidative stress (92). adMSCs
and their exosomes have been demonstrated to reduce pyroptosis in
the injured liver and promote the expression of factors associated
with liver regeneration. Additionally, they can inhibit the NF-κB
pathway while activating the Wnt/β-catenin pathway, both of which
play crucial roles in inflammation and tissue repair (93).
These elucidate the crucial role of pyroptosis in
I/R injuries, particularly focusing on cardiac and cerebral
injuries, lung I/R injury, and hepatic I/R injury (85,87,92,94). In summary, stem cell-based
therapies, particularly involving MSCs and their exosomes, showcase
promising outcomes in alleviating I/R injuries.
Stem cell-mediated modulation of pyroptosis
for tissue repair in drugs-induced injury
Doxorubicin (Dox)-induced
cardiotoxicity
Dox is a potent antineoplastic agent widely used for
cancer treatment. However, its use can lead to cardiotoxicity and
muscle toxicity due to the escalation of oxidative stress and
inflammation, ultimately culminating in pyroptosis. Encouragingly,
the protective effects of exosomes derived from ESCs on Dox-induced
myocardial pyroptosis have been previously demonstrated (95). At a mechanistic level, Dox
treatment significantly upregulates the expression of inflammasome
markers, such as TLR4 and NOD-like receptor protein 3 (NLRP3), as
well as pyroptotic markers, including caspase-1, IL-1β and IL-18
(96). Additionally, Dox
treatment leads to an increase in cell signaling proteins, such as
myeloid differentiation primary response 88 (MyD88), phosphorylated
(p-)P38, and p-JUN N-terminal kinase, which play pivotal roles in
inflammation and cell signaling (96,97). Moreover, the treatment with Dox
promotes the pro-inflammatory M1 macrophages and the secretion of
TNF-α cytokine, further contributing to the pro-inflammatory
environment and pyroptotic response (96,97).
However, the adverse effects induced by Dox,
including pyroptosis, inflammation and cell signaling protein
expression, are effectively counteracted by the application of
ESCs-exosomes or ESCs themselves (97). These protective interventions
demonstrate promising potential in mitigating Dox-induced cardiac
and muscle damage by attenuating the pyroptotic response and
dampening the inflammatory signaling (96,98).
Drugs-induced liver injury (DILI)
DILI involves liver damage resulting from the use of
certain medications. Some drugs may trigger the activation of
inflammasomes, leading to the release of pro-inflammatory cytokines
and initiating pyroptotic pathways in liver cells. This process can
exacerbate liver damage and contribute to the progression of
DILI.
Stem cell-based therapy has exhibited promise in
treating liver diseases due to the regenerative and paracrine
secretion properties of stem cells (99). Notably, MSCs have
anti-inflammatory effects and have been investigated for their
therapeutic potential in various liver diseases. For instance, the
injection of interleukin-10 or transplantation of MSCs has been
found to ameliorate liver injury induced by D-galactosamine, as
evidenced by reduced levels of liver injury markers, inflammatory
cytokines and NH3 (100).
Moreover, when adMSCs are preincubated with green tea theanine,
they demonstrate an enhanced therapeutic effect in rats with liver
injury induced by N-nitrosodiethylamine, significantly suppressing
pyroptosis markers, such as caspase-1 and IL-1β (101).
Various drugs can activate inflammasomes, triggering
the release of pro-inflammatory cytokines and initiating pyroptotic
pathways in affected cells. This process exacerbates tissue damage
and contributes to the progression of drug-induced injuries. These
findings highlight the potential of stem cell-based interventions
in mitigating drug-induced injuries by suppressing pyroptosis.
Stem cell-mediated modulation of pyroptosis
for tissue repair in diabetes complications
Diabetes is a chronic metabolic disorder
characterized by elevated blood sugar levels, which can result from
inadequate insulin production or ineffective insulin utilization.
This condition can lead to various complications, including
cardiovascular diseases, kidney damage, nerve damage, and issues
with the skin and eyes (102).
Diabetic skin wounds
In diabetes, skin wounds become a major medical
concern. High blood sugar levels and impaired wound healing
mechanisms can lead to chronic wounds that heal slowly and are
prone to infections (103).
Pyroptosis, a type of cell death triggered by inflammation, can
exacerbate the inflammatory response at the wound site and hinder
tissue repair, further delaying the healing process (104). Hair follicle MSCs (hfMSCs) have
shown promise in promoting skin wound healing in diabetic mice.
Exosomes from hfMSCs containing long non-coding RNA (lncRNA) H19
have been found to enhance the proliferation and migration of HaCaT
cells (a human keratinocyte cell line) and inhibit pyroptosis by
reversing the stimulation of the NLRP3 inflammasome (105). Moreover, the use of
BMSCs-conditioned medium (BMSC-CM) has revealed positive effects in
diabetic foot ulcers. MSC-CM accelerates wound closure, promotes
cell proliferation and angiogenesis (formation of new blood
vessels), enhances cell autophagy (a process that supports cell
survival and renewal), and reduces cell pyroptosis. By modulating
these cellular processes, MSC-CM facilitates a more efficient wound
healing process in diabetic foot ulcers (13).
In regenerative therapy, providing a conducive
microenvironment is crucial to increasing the survival of
transplanted stem cells. However, hyperglycemia can lead to stem
cell death, hindering the effectiveness of stem cell therapy. A
previous study reported that hyperglycemia-induced oxidative stress
contributes to apoptosis and pyroptosis in human cardiac stem cells
(106). Downregulation of NLRP3
in adipose-derived stem cells has been found to improve the effects
of stem cell therapy under hyperglycemic conditions by suppressing
pyroptosis (107).
Diabetic kidney disease (DKD)
DKD is a serious complication of diabetes
characterized by kidney damage and impaired function resulting from
prolonged high blood sugar levels. It stands as a leading cause of
chronic kidney disease and kidney failure worldwide (108). Promisingly, research has
revealed that BMSCs-derived exosomal miR-30e-5p can effectively
inhibit caspase-1-mediated pyroptosis in HK-2 cells induced by high
glucose. The discovery of this regulatory mechanism opens up new
possibilities for treating DKD (109). By attenuating pyroptosis, which
is known to contribute to kidney damage and dysfunction,
BMSCs-derived exosomal miR-30e-5p offers a promising new strategy
for the management and treatment of diabetic kidney disease.
Diabetes poses various complications, including skin
wounds with impaired healing mechanisms. Pyroptosis, an
inflammation-triggered cell death, exacerbates inflammatory
responses in diabetic skin wounds. In conclusion, stem cells,
including hfMSCs and BMSCs, along with their secreted factors and
exosomes, play pivotal roles in mitigating these complications by
inhibiting pyroptosis.
Stem cell-mediated modulation of pyroptosis
for tissue repair in inflammation-related diseases
Inflammatory bowel disease (IBD)
IBD comprises chronic inflammatory disorders
primarily affecting the gastrointestinal tract, including Crohn's
disease and ulcerative colitis (110). Pyroptosis plays a significant
role in contributing to the pathogenesis of IBD (111). In the context of IBD, immune
cells in the gut, such as macrophages and epithelial cells, undergo
pyroptosis, releasing pro-inflammatory molecules that lead to
intestinal inflammation and tissue damage. This process exacerbates
the symptoms of IBD, including abdominal pain, diarrhea and ulcers,
emphasizing the importance of understanding and targeting
pyroptosis as a potential therapeutic approach for managing IBD
(112).
hucMSCs-derived exosomes have emerged as novel
cell-free therapeutic agents for IBD. These exosomes have
demonstrated the ability to inhibit the activation of macrophage
NLRP3 inflammasomes, thereby suppressing the secretion of IL-18,
IL-1β and cleaved caspase-1, resulting in reduced cell pyroptosis.
Furthermore, miR-378a-5p, highly expressed in hucMSCs-derived
exosomes, plays a vital role in promoting colitis repair (113). Additionally, miR-203a-3p.2 in
hucMSC-derived exosomes, acts as an effective mediator in the
interaction with caspase-4 in THP-1 macrophage pyroptosis (114).
Lung injury
Acute lung injury (ALI) and its more severe form,
acute respiratory distress syndrome (ARDS), are characterized by
widespread inflammation and injury to the lungs, often leading to
respiratory failure. Pyroptosis is implicated in various
factors-induced ALI/ARDS, such as lipopolysaccharide and sepsis
(115). Emerging evidence
suggests that pyroptosis is involved in the pathogenesis of
ALI/ARDS, contributing to the amplification of the inflammatory
response in the lungs (116).
Influenza A virus infection triggers an exaggerated immune response
in the host, leading to caspase-3-GSDME-mediated pyroptosis of lung
alveolar epithelial cells. This process contributes to the onset of
a cytokine storm, ultimately resulting in ALI or ARDS. Notably,
BMSCs exhibit a therapeutic effect by mitigating ALI through the
inhibition of caspase-3-GSDME-mediated pyroptosis in lung alveolar
epithelial cells (117).
Furthermore, MSCs-derived exosomes play a crucial role in
inhibiting pyroptosis. This inhibition is achieved through miRNAs
targeting the caspase-1-mediated pathway and proteins with
immunoregulatory functions, thereby suppressing alveolar macrophage
pyroptosis and alleviating ALI (118). Specifically, exosomes derived
from BMSCs act as carriers for delivering miR-125b-5p, which
downregulates STAT3. This, in turn, inhibits macrophage pyroptosis,
demonstrating a potential therapeutic avenue for alleviating
sepsis-associated ALI (119).
Besides, cardiopulmonary bypass (CPB) has been widely used to
support the heart and lung during cardiac surgery. It has been
reported that BMSCs-derived exosomes ameliorate macrophage
infiltration and oxidative stress, and downregulate expression of
pyroptosis-related proteins in CPB-ALI model, as well as promote
YAP interaction with β-catenin and regulate its transcription
activity (120).
Silicosis is induced by prolonged inhalation of
silica particles, resulting in lung cell injury and disruptions to
the intracellular environment. It is characterized by collagen
deposition and the development of pulmonary fibrosis (121). Pyroptosis and apoptosis play
vital roles in the pathogenesis of silicosis (122). Recently, it has been revealed
that BMSCs possess the ability to mitigate silica-induced pulmonary
fibrosis by inhibiting both apoptosis and pyroptosis (123).
Kidney injury
Acute kidney injury (AKI) is characterized by a
sudden and rapid deterioration in kidney function, leading to an
impaired ability to filter waste and fluid from the blood (124). The involvement of pyroptosis has
been recognized in regulating homeostasis in kidney tissues, thus
playing a significant role in the development of AKI (125). It has been reported that EVs
released by BMSCs might carry a specific miR, miR-223-3p, to
mitigate inflammation and pyroptosis induced by AKI through the
modulation of the HDAC2/SNRK axis (126). Furthermore, another study has
demonstrated the beneficial effects of BMSCs in sepsis-induced AKI.
These stem cells promote mitophagy and inhibit apoptosis and
pyroptosis of renal tubular epithelial cells within kidney tissues.
This therapeutic action is attributed to the upregulation of
SIRT1/Parkin, which plays a crucial role in protecting against AKI
in the context of sepsis (127).
Traumatic injury
The CNS is particularly vulnerable to external
mechanical damage. Traumatic brain injury (TBI) can result from
direct impacts or blows to the head, often caused by factors such
as motor vehicle accidents, crush injuries, or assaults (128). The pathophysiology of TBI
involves both primary and secondary injury mechanisms. The primary
injury occurs at the time of impact and involves mechanical damage
to brain tissue. Secondary injury mechanisms follow the primary
injury and involve processes such as inflammation, oxidative stress
and excitotoxicity. In recent developments, exosomes derived from
hucMSCs have shown significant potential in suppressing neuron cell
apoptosis, pyroptosis and ferroptosis in cases of TBI. This
therapeutic effect is mediated through the PINK1/Parkin pathway,
facilitating mitophagy, a process that removes damaged mitochondria
and aids in cellular recovery (129).
Spinal cord injury (SCI) involves damage to the
spinal cord, leading to functional impairment. The trauma induces a
cascade of events, activating pyroptosis-related molecules and
triggering an inflammatory response. Excessive pyroptosis
exacerbates secondary injury processes, contributing to tissue
damage and neurological deficits. Inhibiting the
pyroptosis-regulated cell death and inflammasome components is a
promising therapeutic approach for the treatment of SCI (130). BMSCs have been previously
reported to exhibit a promising therapeutic effect in treating SCI
by mitigating inflammasome-related pyroptosis. The underlying
mechanism involves BMSCs' exosome-derived circ_003564, a circular
RNA, which has been identified to decrease the expression of
inflammasome-related pyroptosis markers, including cleaved
caspase-1, GSDMD, NLRP3, IL-1β and IL-18 in neurons (14). This reduction in pyroptosis
markers indicates a potential protective effect of BMSC-derived
exosomal circ_003564 on neurons in the context of SCI.
Intracerebral hemorrhage (ICH)
Another cerebrovascular disease with high morbidity
and mortality is ICH, often resulting from the rupture of blood
vessels (131). Hemoglobin and
its breakdown products released from erythrocytes during hemorrhage
can activate inflammasomes, initiating the pyroptotic pathway.
Pyroptosis is strongly related to neuroinflammation, which plays a
crucial role in the pathophysiological processes of secondary brain
injury after ICH. Pyroptosis causes the releases of inflammatory
cytokines and DAMPs can exacerbate neuronal injury and affect
surrounding tissue. It has been reported that stem cell-derived
exosomes have demonstrated a protective effect in ICH by inhibiting
pyroptosis. Specifically, exosomal miR-23b from BMSCs exhibits
antioxidant properties through the inhibition of PTEN, thereby
alleviating NLRP3 inflammasome-mediated pyroptosis. This process
promotes neurologic function recovery in rats with ICH (132).
Extensive research demonstrates their effectiveness
in treating diverse inflammation-related diseases by targeting
signals associated with inflammation and pyroptosis (Table I). There is a basis for optimism
that stem cells and the factors they release may exert favorable
effects in various other inflammatory diseases.
 | Table I.Stem cells inhibit pyroptosis in
inflammation-related diseases. |
Table I.
Stem cells inhibit pyroptosis in
inflammation-related diseases.
| Disease types | Classification of
stem cells | Targeted cells | Pyroptotic
signaling molecules | Effectors | (Refs.) |
|---|
| Osteoarthritis | adMSCs | Chondrocyte | TNF-α | sTNFR1 | (32) |
|
| BMSCs | Chondrocyte | HDAC3 | miR-326
(exosomes) | (15) |
|
| hucMSCs | Chondrocyte | NLRP3 | miR-223
(exosomes) | (33) |
| Intervertebral | MSCs | Nucleus
pulposus | METTL14 | miR-26a-5p
(exosomes) | (47) |
| disc
degeneration |
|
|
|
|
|
|
| hucMSCs | Nucleus
pulposus | NLRP3 | miR-410
(exosomes) | (9) |
|
| ESCs | Nucleus
pulposus | NLRP3 | miR-302c
(exosomes) | (48) |
| Myocardial
ischemia/ | MSCs | Myocardial
cell | NLRP3 | miR-320b
(exosomes) | (69) |
| reperfusion
injury |
| Myocardial
cell | GSDMD | miR-182-5p
(exosomes) | (7) |
|
| hucMSCs | Myocardial
cell | FOXO3 | miR-100-5p
(exosomes) | (16) |
|
|
| Myocardial
cell | FOXO3a | Circular non-coding
RNA, | (72) |
|
|
|
|
| circhipk3
(exosomes) |
|
|
| hMSCs | Myocardial
cell | Sirt1 | Long non-coding
RNA, | (70) |
|
|
|
|
| KLF3-AS1
(exosomes) |
|
|
| adMSCs | Myocardial
cell | Arl2 | Long non-coding
RNA, | (71) |
|
|
|
|
| XIST
(exosomes) |
|
| Cerebral
ischemic | omMSCs | Microglial
cells |
| Exosomes | (77,78) |
| stroke | BMSCs | PC12 cells | AIM2 | miR193b-5p
(exosomes) | (12) |
| Lung ischemia- | BMSCs | Pulmonary | CMPK2 | miR-202-5p
(exosomes) | (90) |
| reperfusion
injury |
| epithelial
cells |
|
|
|
| Hepatic
ischemia- | adMSCs | Hepatocyte | NF-κB pathway | Exosomes | (93) |
| reperfusion
injury |
|
| and
Wnt/β-catenin |
|
|
|
|
|
|
pathwaya |
|
|
|
Doxorubicin-induced | ESCs | Myocardial
cell |
| Exosomes | (97) |
| cardiotoxicity |
|
|
|
|
|
| Drugs-induced
liver | ADSCs | Hepatocyte | Caspase-1 and |
| (101) |
| injury |
|
| IL-1βa |
|
|
| Diabetic skin
wounds | hfMSCs | HaCaT cells | NLRP3 | Long non-coding
RNA, | (105) |
|
|
|
|
| H19 (exosomes) |
|
|
| BMSCs |
|
| Bone
marrow-derived | (13) |
|
|
|
|
| mesenchymal
stem |
|
|
|
|
|
| cell-conditioned
medium |
|
| Diabetic
kidney | BMSCs | Renal proximal | ELAVL1 | miR-30e-5p
(exosomes) | (109) |
| disease |
| tubular cells |
|
|
|
| Inflammatory bowel
disease | hucMSCs | THP-1 cells and
mouse peritoneal macrophages | NLRP3 | miR-378a-5p
(exosomes) | (113) |
|
|
| Macrophage | Caspase-4 | miR-203a-3p.2
(exosomes) | (114) |
| Acute lung
injury | BMSCs | Lung cells | YAP/β-catenin
axisa | Exosomes | (120) |
| Silicosis | BMSCs | Lung cells |
|
| (123) |
| Acute kidney
injury | BMSCs | HK-2 cells | HDAC2/SNRK | miR-223-3p
(extracellular | (126) |
|
|
|
|
| vesicles) |
|
|
|
| Renal tubular |
SIRT1/Parkina |
| (127) |
|
|
| epithelial
cells |
|
|
|
| Traumatic brain
injury | hucMSCs | Neuron | PINK1/Parkin | Exosomes | (129) |
| Spinal cord
injury | BMSCs | Neuron |
pathwaya
caspase-1, | Circular non-coding
RNA, |
|
|
|
|
| GSDMD, NLRP3, | circ_003564
(exosomes) | (14) |
|
|
|
| IL-1β and
IL-18a |
|
|
| Intracerebral | BMSCs | Microglial
cells | PTEN | miR-23b
(exosomes) | (132) |
| hemorrhage |
|
|
|
|
|
Prospect
Stem cells are unique cells with the ability to
differentiate into various cell types and self-renew, making them
an attractive option for repairing damaged or diseased tissues
(133). However, its clinical
application also faces numerous challenges and risks (134). On the one hand, the potential
for tumorigenesis, particularly teratoma formation, is a
significant concern associated with the clinical use of ESCs
(135). Teratomas are tumors
that contain a mixture of differentiated tissues from all three
germ layers (ectoderm, mesoderm and endoderm). They can be benign,
but they represent a significant risk associated with the
transplantation of undifferentiated pluripotent cells, including
ESCs (136). While ESC therapy
has immense potential, the risk of teratoma formation underscores
the importance of rigorous safety and quality control measures in
the development and application of these therapies. Researchers and
clinicians are actively working to address these challenges to make
ESC-based treatments safer and more effective (137). On the other hand, autologous
transplantation offers the advantage of using a patient's own
cells, thereby eliminating the risk of immune rejection, but
allogeneic stem cell treatments involve donor cells and carry a
distinct set of potential risks and complications (138). These must be carefully
considered due to the inherent differences between the donor and
recipient, which include factors such as genetic disparities,
tissue compatibility and immunological variations. Overall,
ensuring the safety and efficacy of stem cell treatments, as well
as addressing issues related to immune rejection and ethical
considerations, remain important areas of research (139).
Exosomes derived from stem cells in therapy is their
potential to overcome the limitations associated with stem cell
transplantation, such as potential immune rejection and ethical
concerns. Exosomes can be delivered directly to the target site,
and they have a lower risk of immune response compared with whole
cells. While exosomes hold great promise, further research is
needed to optimize their isolation, characterization and
therapeutic application (8).
Additionally, understanding the precise mechanisms of
exosome-mediated effects will help enhance their efficacy in stem
cell-based therapy and pave the way for more effective regenerative
treatments.
The protective mechanisms employed by stem cells are
remarkably complex, and current understanding only provides a
glimpse of their full potential. In addition to inhibiting
apoptosis, stem cells have been found to suppress pyroptosis and
regulate autophagy (140,141).
While they hold great promise in therapeutic applications, stem
cell therapy is still in its nascent stages and requires extensive
research and clinical practice before it can be fully realized in
clinical settings. Creating an environment that maximizes stem cell
protection and harnessing their potential for therapeutic benefits
necessitate a rigorous and comprehensive approach to furthering
understanding and advancing stem cell-based treatments.
Acknowledgements
Not applicable.
Funding
The present study was supported by the National Natural Science
Fund (grant no. 82002059), the Hunan Provincial Health Commission
Scientific Research Project (grant nos. D202304108322 and
202105011186), the Hunan Innovation Guidance Project for Clinical
Medical Technology (grant no. 2021SK51826), the Hunan Provincial
Natural Science Foundation of China (grant no. 2023JJ30542) and the
Hunan Graduate Student Research and Innovation Project (grant no.
CX20221008).
Availability of data and materials
Not applicable.
Authors' contributions
YiWe and LL conceptualized the study, wrote the
original draft, conducted formal analysis, and wrote, reviewed and
edited the manuscript. YiWa, YC, ZL and CH reviewed and edited the
manuscript. YaW developed methodology. CJ contributed to study
conceptualization, writing the original draft, and writing,
reviewing and editing the manuscript. ZW and JL supervised the
study. All authors have read and approved the final version of the
manuscript. Data authentication is not applicable.
Ethics approval and consent to
participate
Not applicable.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
Glossary
Abbreviations
Abbreviations:
|
AC
|
articular cartilage
|
|
MSCs
|
mesenchymal stem cells
|
|
adMSCs
|
adipose-derived MSCs
|
|
AIM2
|
absent in melanoma 2
|
|
AKI
|
acute kidney injury
|
|
ALI
|
acute lung injury
|
|
BMSCs
|
bone marrow MSCs
|
|
BMSC-CM
|
BMSCs-conditioned medium
|
|
BMSC-Exos
|
exosomes derived from BMSCs
|
|
CA
|
cardiac arrest
|
|
CNS
|
central nervous system
|
|
CPB
|
cardiopulmonary bypass
|
|
CPR
|
cardiopulmonary resuscitation
|
|
DAMPs
|
damage-associated molecular
patterns
|
|
DKD
|
diabetic kidney disease
|
|
DILI
|
drug-induced liver injury
|
|
Dox
|
doxorubicin
|
|
ECM
|
extracellular matrix
|
|
EVs
|
extracellular vesicles
|
|
GSDMD
|
gasdermin D
|
|
hfMSCs
|
hair follicle MSCs
|
|
I/R
|
ischemia/reperfusion;
|
References
|
1
|
Furman D, Campisi J, Verdin E,
Carrera-Bastos P, Targ S, Franceschi C, Ferrucci L, Gilroy DW,
Fasano A, Miller GW, et al: Chronic inflammation in the etiology of
disease across the life span. Nat Med. 25:1822–1832. 2019.
View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Li T, Zheng G, Li B and Tang L:
Pyroptosis: A promising therapeutic target for noninfectious
diseases. Cell Proliferat. 54:e131372021. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Yu P, Zhang X, Liu N, Tang L, Peng C and
Chen X: Pyroptosis: Mechanisms and diseases. Signal Transduct Tar.
6:1282021. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Kovacs SB and Miao EA: Gasdermins:
Effectors of pyroptosis. Trends Cell Biol. 27:673–684. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Dekoninck S and Blanpain C: Stem cell
dynamics, migration and plasticity during wound healing. Nat Cell
Biol. 21:18–24. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Mapp PI and Walsh DA: Mechanisms and
targets of angiogenesis and nerve growth in osteoarthritis. Nat Rev
Rheumatol. 8:390–398. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Yue R, Lu S, Luo Y, Zeng J, Liang H, Qin
D, Wang X, Wang T, Pu J and Hu H: Mesenchymal stem cell-derived
exosomal microRNA-182-5p alleviates myocardial ischemia/reperfusion
injury by targeting GSDMD in mice. Cell Death Discov. 8:2022022.
View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Hade MD, Suire CN and Suo Z: Mesenchymal
stem cell-derived exosomes: Applications in regenerative medicine.
Cells-Basel. 10:19592021. View Article : Google Scholar
|
|
9
|
Zhang J, Zhang J, Zhang Y, Liu W, Ni W,
Huang X, Yuan J, Zhao B, Xiao H and Xue F: Mesenchymal stem
cells-derived exosomes ameliorate intervertebral disc degeneration
through inhibiting pyroptosis. J Cell Mol Med. 24:11742–11754.
2020. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Xu J and Zhang M, Liu F, Shi L, Jiang X,
Chen C, Wang J, Diao M, Khan ZU and Zhang M: Mesenchymal stem cells
alleviate post-resuscitation cardiac and cerebral injuries by
inhibiting cell pyroptosis and ferroptosis in a swine model of
cardiac arrest. Front Pharmacol. 12:7938292021. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Li K, Yan G, Huang H, Zheng M, Ma K, Cui
X, Lu D, Zheng L, Zhu B, Cheng J and Zhao J: Anti-inflammatory and
immunomodulatory effects of the extracellular vesicles derived from
human umbilical cord mesenchymal stem cells on osteoarthritis via
M2 macrophages. J Nanobiotechnol. 20:382022. View Article : Google Scholar
|
|
12
|
Wang Y, Chen H, Fan X, Xu C, Li M, Sun H,
Song J, Jia F, Wei W, Jiang F, et al: Bone marrow mesenchymal stem
cell-derived exosomal miR-193b-5p reduces pyroptosis after ischemic
stroke by targeting AIM2. J Stroke Cerebrovasc. 32:1072352023.
View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Xu YF, Wu YX, Wang HM, Gao CH, Xu YY and
Yan Y: Bone marrow-derived mesenchymal stem cell-conditioned medium
ameliorates diabetic foot ulcers in rats. Clinics (Sao Paulo).
78:1001812023. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Zhao Y, Chen Y, Wang Z, Xu C, Qiao S, Liu
T, Qi K, Tong D and Li C: Bone marrow mesenchymal stem cell exosome
attenuates Inflammasome-Related pyroptosis via delivering
circ_003564 to improve the recovery of spinal cord injury. Mol
Neurobiol. 59:6771–6789. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Xu H and Xu B: BMSC-Derived exosomes
ameliorate osteoarthritis by inhibiting pyroptosis of cartilage via
delivering miR-326 targeting HDAC3 and STAT1//NF-kappaB p65 to
chondrocytes. Mediat Inflamm. 2021:99728052021. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Liang C, Liu Y, Xu H, Huang J, Shen Y,
Chen F and Luo M: Exosomes of human umbilical cord MSCs protect
against Hypoxia/Reoxygenation-Induced pyroptosis of cardiomyocytes
via the miRNA-100-5p/FOXO3/NLRP3 pathway. Front Bioeng Biotech.
8:6158502020. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Broz P, Ruby T, Belhocine K, Bouley DM,
Kayagaki N, Dixit VM and Monack DM: Caspase-11 increases
susceptibility to Salmonella infection in the absence of caspase-1.
Nature. 490:288–291. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Strowig T, Henao-Mejia J, Elinav E and
Flavell R: Inflammasomes in health and disease. Nature.
481:278–286. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
He Y, Hara H and Nunez G: Mechanism and
regulation of NLRP3 inflammasome activation. Trends Biochem Sci.
41:1012–1021. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Cookson BT and Brennan MA:
Pro-inflammatory programmed cell death. Trends Microbiol.
9:113–114. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Chen X, He WT, Hu L, Li J, Fang Y, Wang X,
Xu X, Wang Z, Huang K and Han J: Pyroptosis is driven by
non-selective gasdermin-D pore and its morphology is different from
MLKL channel-mediated necroptosis. Cell Res. 26:1007–1020. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Kayagaki N, Stowe IB, Lee BL, O'Rourke K,
Anderson K, Warming S, Cuellar T, Haley B, Roose-Girma M, Phung QT,
et al: Caspase-11 cleaves gasdermin D for non-canonical
inflammasome signalling. Nature. 526:666–671. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Boise LH and Collins CM:
Salmonella-induced cell death: Apoptosis, necrosis or programmed
cell death? Trends Microbiol. 9:64–67. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Li J and Yuan J: Caspases in apoptosis and
beyond. Oncogene. 27:6194–6206. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Shi J, Zhao Y, Wang K, Shi X, Wang Y,
Huang H, Zhuang Y, Cai T, Wang F and Shao F: Cleavage of GSDMD by
inflammatory caspases determines pyroptotic cell death. Nature.
526:660–665. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Matikainen S, Nyman TA and Cypryk W:
Function and regulation of noncanonical Caspase-4/5/11
inflammasome. J Immunol. 204:3063–3069. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
O'Neill TW and Felson DT: Mechanisms of
osteoarthritis (OA) pain. Curr Osteoporos Rep. 16:611–616. 2018.
View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Yu H, Yao S, Zhou C, Fu F, Luo H, Du W,
Jin H, Tong P, Chen D, Wu C and Ruan H: Morroniside attenuates
apoptosis and pyroptosis of chondrocytes and ameliorates
osteoarthritic development by inhibiting NF-kappaB signaling. J
Ethnopharmacol. 266:1134472021. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Wu X, Ren G, Zhou R, Ge J and Chen FH: The
role of Ca2+ in acid-sensing ion channel 1a-mediated
chondrocyte pyroptosis in rat adjuvant arthritis. Lab Invest.
99:499–513. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Zhao LR, Xing RL, Wang PM, Zhang NS, Yin
SJ, Li XC and Zhang L: NLRP1 and NLRP3 inflammasomes mediate
LPS/ATP-induced pyroptosis in knee osteoarthritis. Mol Med Rep.
17:5463–5469. 2018.PubMed/NCBI
|
|
31
|
Zhang L, Zhang L, Huang Z, Xing R, Li X,
Yin S, Mao J, Zhang N, Mei W, Ding L and Wang P: Increased HIF-α in
knee osteoarthritis aggravate synovial fibrosis via Fibroblast-Like
synoviocyte pyroptosis. Oxid Med Cell Longev.
2019:63265172019.PubMed/NCBI
|
|
32
|
Xu L, Zhang F, Cheng G, Yuan X, Wu Y, Wu
H, Wang Q, Chen J, Kuai J, Chang Y, et al: Attenuation of
experimental osteoarthritis with human adipose-derived mesenchymal
stem cell therapy: Inhibition of the pyroptosis in chondrocytes.
Inflamm Res. 72:89–105. 2023. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Liu W, Liu A, Li X, Sun Z, Sun Z, Liu Y,
Wang G, Huang D, Xiong H, Yu S, et al: Dual-engineered
cartilage-targeting extracellular vesicles derived from mesenchymal
stem cells enhance osteoarthritis treatment via
miR-223/NLRP3/pyroptosis axis: Toward a precision therapy. Bioact
Mater. 30:169–183. 2023.PubMed/NCBI
|
|
34
|
Sergio MR, Godinho C, Guerra L, Agapito A,
Fonseca F and Costa C: TSH anti-receptor antibodies in Graves'
disease. Acta Medica Port. 9:229–231, (In Portuguese). PubMed/NCBI
|
|
35
|
Buckley CT, Hoyland JA, Fujii K, Pandit A,
Iatridis JC and Grad S: Critical aspects and challenges for
intervertebral disc repair and regeneration-Harnessing advances in
tissue engineering. Jor Spine. 1:e10292018. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Zhao CQ, Wang LM, Jiang LS and Dai LY: The
cell biology of intervertebral disc aging and degeneration. Ageing
Res Rev. 6:247–261. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Basso M, Cavagnaro L, Zanirato A, Divano
S, Formica C, Formica M and Felli L: What is the clinical evidence
on regenerative medicine in intervertebral disc degeneration?
Musculoskelet Surg. 101:93–104. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Binch A, Fitzgerald JC, Growney EA and
Barry F: Cell-based strategies for IVD repair: Clinical progress
and translational obstacles. Nat Rev Rheumatol. 17:158–175. 2021.
View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Francisco V, Pino J, Gonzalez-Gay MA, Lago
F, Karppinen J, Tervonen O, Mobasheri A and Gualillo O: A new
immunometabolic perspective of intervertebral disc degeneration.
Nat Rev Rheumatol. 18:47–60. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Ge Y, Chen Y, Guo C, Luo H, Fu F, Ji W, Wu
C and Ruan H: Pyroptosis and intervertebral disc degeneration:
Mechanistic insights and therapeutic implications. J Inflamm Res.
15:5857–5871. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Luo J, Yang Y, Wang X, Chang X and Fu S:
Role of pyroptosis in intervertebral disc degeneration and its
therapeutic implications. Biomolecules. 12:18042022. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Zhang X, Cai Z, Wu M, Huangfu X, Li J and
Liu X: Adipose stem Cell-Derived exosomes recover impaired matrix
metabolism of torn human rotator cuff tendons by maintaining tissue
homeostasis. Am J Sport Med. 49:899–908. 2021. View Article : Google Scholar
|
|
43
|
Shi Y and Wang Y, Li Q, Liu K, Hou J, Shao
C and Wang Y: Immunoregulatory mechanisms of mesenchymal stem and
stromal cells in inflammatory diseases. Nat Rev Nephrol.
14:493–507. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Xing H, Zhang Z, Mao Q, Wang C, Zhou Y,
Zhou X, Ying L, Xu H, Hu S and Zhang N: Injectable
exosome-functionalized extracellular matrix hydrogel for metabolism
balance and pyroptosis regulation in intervertebral disc
degeneration. J Nanobiotechnol. 19:2642021. View Article : Google Scholar
|
|
45
|
Zhu B, Chen HX, Li S, Tan JH, Xie Y, Zou
MX, Wang C, Xue JB, Li XL, Cao Y and Yan YG: Comprehensive analysis
of N6-methyladenosine (m6A) modification during the degeneration of
lumbar intervertebral disc in mice. J Orthop Transl. 31:126–138.
2021.
|
|
46
|
Shen C, Xuan B, Yan T, Ma Y, Xu P, Tian X,
Zhang X, Cao Y, Ma D, Zhu X, et al: m6A-dependent
glycolysis enhances colorectal cancer progression. Mol Cancer.
19:722020. View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Yuan X, Li T, Shi L, Miao J, Guo Y and
Chen Y: Human umbilical cord mesenchymal stem cells deliver
exogenous miR-26a-5p via exosomes to inhibit nucleus pulposus cell
pyroptosis through METTL14/NLRP3. Mol Med. 27:912021. View Article : Google Scholar : PubMed/NCBI
|
|
48
|
Yu Y, Li W, Xian T, Tu M, Wu H and Zhang
J: Human embryonic stem-cell-derived exosomes repress NLRP3
inflammasome to alleviate pyroptosis in nucleus pulposus cells by
transmitting miR-302c. Int J Mol Sci. 24:76642023. View Article : Google Scholar : PubMed/NCBI
|
|
49
|
Hunziker EB: Articular cartilage repair:
Basic science and clinical progress. A review of the current status
and prospects. Osteoarthr Cartilage. 10:432–463. 2002. View Article : Google Scholar
|
|
50
|
Lin F, Zhang W, Xue D, Zhu T, Li J, Chen
E, Yao X and Pan Z: Signaling pathways involved in the effects of
HMGB1 on mesenchymal stem cell migration and osteoblastic
differentiation. Int J Mol Med. 37:789–797. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
51
|
Bertheloot D and Latz E: HMGB1, IL-1α,
IL-33 and S100 proteins: Dual-function alarmins. Cell Mol Immunol.
14:43–64. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
52
|
Koh TJ and DiPietro LA: Inflammation and
wound healing: The role of the macrophage. Expert Rev Mol Med.
13:e232011. View Article : Google Scholar : PubMed/NCBI
|
|
53
|
Han SA, Lee S, Seong SC and Lee MC:
Effects of CD14 macrophages and proinflammatory cytokines on
chondrogenesis in osteoarthritic synovium-derived stem cells.
Tissue Eng Pt A. 20:2680–2691. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
54
|
Wehling N, Palmer GD, Pilapil C, Liu F,
Wells JW, Muller PE, Evans CH and Porter RM: Interleukin-1beta and
tumor necrosis factor alpha inhibit chondrogenesis by human
mesenchymal stem cells through NF-kappaB-dependent pathways.
Arthritis Rheum. 60:801–812. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
55
|
Yang Z, Li H, Yuan Z, Fu L, Jiang S, Gao
C, Wang F, Zha K, Tian G, Sun Z, et al: Endogenous cell recruitment
strategy for articular cartilage regeneration. Acta Biomater.
114:31–52. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
56
|
Liao Z, Fu L, Li P, Wu J, Yuan X, Ning C,
Ding Z, Sui X, Liu S and Guo Q: Incorporation of magnesium ions
into an Aptamer-Functionalized ECM bioactive scaffold for articular
cartilage regeneration. Acs Appl Mater Inter. 15:22944–22958. 2023.
View Article : Google Scholar : PubMed/NCBI
|
|
57
|
Park KS, Kim BJ, Lih E, Park W, Lee SH,
Joung YK and Han DK: Versatile effects of magnesium hydroxide
nanoparticles in PLGA scaffold-mediated chondrogenesis. Acta
Biomater. 73:204–216. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
58
|
Barakat AH, Elwell VA and Lam KS: Stem
cell therapy in discogenic back pain. J Spine Surg. 5:561–583.
2019. View Article : Google Scholar : PubMed/NCBI
|
|
59
|
Sakai D and Andersson GB: Stem cell
therapy for intervertebral disc regeneration: Obstacles and
solutions. Nat Rev Rheumatol. 11:243–256. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
60
|
Yu C, Li D, Wang C, Xia K, Wang J, Zhou X,
Ying L, Shu J, Huang X, Xu H, et al: Injectable kartogenin and
apocynin loaded micelle enhances the alleviation of intervertebral
disc degeneration by adipose-derived stem cell. Bioact Mater.
6:3568–3579. 2021.PubMed/NCBI
|
|
61
|
Zhou X, Wang J, Fang W, Tao Y, Zhao T, Xia
K, Liang C, Hua J, Li F and Chen Q: Genipin cross-linked type II
collagen/chondroitin sulfate composite hydrogel-like cell delivery
system induces differentiation of adipose-derived stem cells and
regenerates degenerated nucleus pulposus. Acta Biomater.
71:496–509. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
62
|
Xia KS, Li DD, Wang CG, Ying LW, Wang JK,
Yang B, Shu JW, Huang XP, Zhang YA, Yu C, et al: An
esterase-responsive ibuprofen nano-micelle pre-modified embryo
derived nucleus pulposus progenitor cells promote the regeneration
of intervertebral disc degeneration. Bioact Mater. 21:69–85.
2023.PubMed/NCBI
|
|
63
|
Yang J, Hu S, Bian Y, Yao J, Wang D, Liu
X, Guo Z, Zhang S and Peng L: Targeting cell death: Pyroptosis,
ferroptosis, apoptosis and necroptosis in osteoarthritis. Front
Cell Dev Biol. 9:7899482021. View Article : Google Scholar : PubMed/NCBI
|
|
64
|
Tibaut M, Mekis D and Petrovic D:
Pathophysiology of myocardial infarction and acute management
strategies. Cardiovasc Hematol Agents Med Chem. 14:150–159. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
65
|
Zhou M, Yu Y, Luo X, Wang J, Lan X, Liu P,
Feng Y and Jian W: Myocardial Ischemia-reperfusion injury:
Therapeutics from a mitochondria-centric perspective. Cardiology.
146:781–792. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
66
|
Toldo S, Mauro AG, Cutter Z and Abbate A:
Inflammasome, pyroptosis, and cytokines in myocardial
ischemia-reperfusion injury. Am J Physiol-Heart C. 315:H1553–H1568.
2018. View Article : Google Scholar : PubMed/NCBI
|
|
67
|
Qiu Z, Lei S, Zhao B, Wu Y, Su W, Liu M,
Meng Q, Zhou B, Leng Y and Xia ZY: NLRP3 inflammasome
activation-mediated pyroptosis aggravates myocardial
ischemia/reperfusion injury in diabetic rats. Oxid Med Cell Longev.
2017:97432802017. View Article : Google Scholar : PubMed/NCBI
|
|
68
|
Kalluri R and LeBleu VS: The biology,
function, and biomedical applications of exosomes. Science.
367:eaau69772020. View Article : Google Scholar : PubMed/NCBI
|
|
69
|
Tang J, Jin L, Liu Y, Li L, Ma Y, Lu L, Ma
J, Ding P, Yang X, Liu J and Yang J: Exosomes derived from
mesenchymal stem cells protect the myocardium against
ischemia/reperfusion injury through inhibiting pyroptosis. Drug Des
Devel Ther. 14:3765–7375. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
70
|
Mao Q, Liang XL, Zhang CL, Pang YH and Lu
YX: LncRNA KLF3-AS1 in human mesenchymal stem cell-derived exosomes
ameliorates pyroptosis of cardiomyocytes and myocardial infarction
through miR-138-5p/Sirt1 axis. Stem Cell Res Ther. 10:3932019.
View Article : Google Scholar : PubMed/NCBI
|
|
71
|
Yan B, Liu T, Yao C, Liu X, Du Q and Pan
L: LncRNA XIST shuttled by adipose tissue-derived mesenchymal stem
cell-derived extracellular vesicles suppresses myocardial
pyroptosis in atrial fibrillation by disrupting miR-214-3p-mediated
Arl2 inhibition. Lab Invest. 101:1427–1438. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
72
|
Yan B, Zhang Y, Liang C, Liu B, Ding F,
Wang Y, Zhu B, Zhao R, Yu XY and Li Y: Stem cell-derived exosomes
prevent pyroptosis and repair ischemic muscle injury through a
novel exosome/circHIPK3/ FOXO3a pathway. Theranostics.
10:6728–6742. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
73
|
Feigin VL, Norrving B, George MG, Foltz
JL, Roth GA and Mensah GA: Prevention of stroke: A strategic global
imperative. Nat Rev Neurol. 12:501–512. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
74
|
Jayaraj RL, Azimullah S, Beiram R, Jalal
FY and Rosenberg GA: Neuroinflammation: Friend and foe for ischemic
stroke. J Neuroinflamm. 16:1422019. View Article : Google Scholar
|
|
75
|
Madore C, Yin Z, Leibowitz J and Butovsky
O: Microglia, lifestyle stress, and neurodegeneration. Immunity.
52:222–240. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
76
|
Li W, Shen N, Kong L, Huang H, Wang X,
Zhang Y, Wang G, Xu P and Hu W: STING mediates microglial
pyroptosis via interaction with NLRP3 in cerebral ischaemic stroke.
Stroke Vasc Neurol. svn-2023-002320. 2023.doi:
10.1136/svn-2023-002320. View Article : Google Scholar
|
|
77
|
Huang Y, Tan F, Zhuo Y, Liu J, He J, Duan
D, Lu M and Hu Z: Hypoxia-preconditioned olfactory mucosa
mesenchymal stem cells abolish cerebral
ischemia/reperfusion-induced pyroptosis and apoptotic death of
microglial cells by activating HIF-1alpha. Aging (Albany Ny).
12:10931–10950. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
78
|
Hu Z, Yuan Y, Zhang X, Lu Y, Dong N, Jiang
X, Xu J and Zheng D: Human umbilical cord mesenchymal stem
Cell-Derived exosomes attenuate Oxygen-Glucose
Deprivation/Reperfusion-Induced microglial pyroptosis by promoting
FOXO3a-dependent mitophagy. Oxid Med Cell Longev. 2021:62197152021.
View Article : Google Scholar : PubMed/NCBI
|
|
79
|
Liu X, Zhang M, Liu H, Zhu R, He H, Zhou
Y, Zhang Y, Li C, Liang D, Zeng Q and Huang G: Bone marrow
mesenchymal stem cell-derived exosomes attenuate cerebral
ischemia-reperfusion injury-induced neuroinflammation and
pyroptosis by modulating microglia M1/M2 phenotypes. Exp Neurol.
341:1137002021. View Article : Google Scholar : PubMed/NCBI
|
|
80
|
Huang Y, Wang S, Huang F, Zhang Q, Qin B,
Liao L, Wang M, Wan H, Yan W, Chen D, et al: c-FLIP regulates
pyroptosis in retinal neurons following oxygen-glucose
deprivation/recovery via a GSDMD-mediated pathway. Ann Anat.
235:1516722021. View Article : Google Scholar : PubMed/NCBI
|
|
81
|
Zhou Z, Shang L, Zhang Q, Hu X, Huang JF
and Xiong K: DTX3L induced NLRP3 ubiquitination inhibit R28 cell
pyroptosis in OGD/R injury. Bba-Mol Cell Res.
1870:1194332023.PubMed/NCBI
|
|
82
|
Dreixler JC, Poston JN, Balyasnikova I,
Shaikh AR, Tupper KY, Conway S, Boddapati V, Marcet MM, Lesniak MS
and Roth S: Delayed administration of bone marrow mesenchymal stem
cell conditioned medium significantly improves outcome after
retinal ischemia in rats. Invest Ophth Vis Sci. 55:3785–3796. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
83
|
Mathew B, Poston JN, Dreixler JC, Torres
L, Lopez J, Zelkha R, Balyasnikova I, Lesniak MS and Roth S:
Bone-marrow mesenchymal stem-cell administration significantly
improves outcome after retinal ischemia in rats. Graef Arch Clin
Exp. 255:1581–1592. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
84
|
Mathew B, Ravindran S, Liu X, Torres L,
Chennakesavalu M, Huang CC, Feng L, Zelka R, Lopez J, Sharma M and
Roth S: Mesenchymal stem cell-derived extracellular vesicles and
retinal ischemia-reperfusion. Biomaterials. 197:146–1460. 2019.
View Article : Google Scholar : PubMed/NCBI
|
|
85
|
Neumar RW, Nolan JP, Adrie C, Aibiki M,
Berg RA, Bottiger BW, Callaway C, Clark RS, Geocadin RG, Jauch EC,
et al: Post-cardiac arrest syndrome: Epidemiology, pathophysiology,
treatment, and prognostication. A consensus statement from the
International Liaison Committee on Resuscitation (American Heart
Association, Australian and New Zealand Council on Resuscitation,
European Resuscitation Council, Heart and Stroke Foundation of
Canada, InterAmerican Heart Foundation, Resuscitation Council of
Asia, and the Resuscitation Council of Southern Africa); the
American Heart Association Emergency Cardiovascular Care Committee;
the Council on Cardiovascular Surgery and Anesthesia; the Council
on Cardiopulmonary, Perioperative, and Critical Care; the Council
on Clinical Cardiology; and the Stroke Council. Circulation.
118:2452–2483. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
86
|
Yu Y, Wang D, Li H, Fan J, Liu Y, Zhao X,
Wu J and Jing X: Mesenchymal stem cells derived from induced
pluripotent stem cells play a key role in immunomodulation during
cardiopulmonary resuscitation. Brain Res. 1720:1462932019.
View Article : Google Scholar : PubMed/NCBI
|
|
87
|
Weyker PD, Webb CA, Kiamanesh D and Flynn
BC: Lung ischemia reperfusion injury: A bench-to-bedside review.
Semin Cardiothorac Vasc Anesth. 17:28–43. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
88
|
Fei L, Jingyuan X, Fangte L, Huijun D, Liu
Y, Ren J, Jinyuan L and Linghui P: Preconditioning with rHMGB1
ameliorates lung ischemia-reperfusion injury by inhibiting alveolar
macrophage pyroptosis via the Keap1/Nrf2/HO-1 signaling pathway. J
Transl Med. 18:3012020. View Article : Google Scholar : PubMed/NCBI
|
|
89
|
Shologu N, Scully M, Laffey JG and O'Toole
D: Human mesenchymal stem cell secretome from bone marrow or
Adipose-Derived tissue sources for treatment of hypoxia-induced
pulmonary epithelial injury. Int J Mol Sci. 19:29962018. View Article : Google Scholar : PubMed/NCBI
|
|
90
|
Sun ZL, You T, Zhang BH, Liu Y and Liu J:
Bone marrow mesenchymal stem cell-derived exosomes miR-202-5p
inhibited pyroptosis to alleviate lung ischemic-reperfusion injury
by targeting CMPK2. Kaohsiung J Med Sci. 39:688–698. 2023.
View Article : Google Scholar : PubMed/NCBI
|
|
91
|
Luo Y, Zheng D, Mou T, Pu J, Huang Z, Chen
W, Zhang Y and Wu Z: CMPK2 accelerates liver ischemia/reperfusion
injury via the NLRP3 signaling pathway. Exp Ther Med. 22:13582021.
View Article : Google Scholar : PubMed/NCBI
|
|
92
|
Hirao H, Nakamura K and Kupiec-Weglinski
JW: Liver ischaemia-reperfusion injury: A new understanding of the
role of innate immunity. Nat Rev Gastro Hepat. 19:239–256. 2022.
View Article : Google Scholar
|
|
93
|
Piao C, Sang J, Kou Z, Wang Y, Liu T, Lu
X, Jiao Z and Wang H: Effects of exosomes derived from
adipose-derived mesenchymal stem cells on pyroptosis and
regeneration of injured liver. Int J Mol Sci. 23:120652022.
View Article : Google Scholar : PubMed/NCBI
|
|
94
|
Shi H, Gao Y, Dong Z, Yang J, Gao R, Li X,
Zhang S, Ma L, Sun X, Wang Z, et al: GSDMD-Mediated cardiomyocyte
pyroptosis promotes Myocardial I/R injury. Circ Res. 129:383–396.
2021. View Article : Google Scholar : PubMed/NCBI
|
|
95
|
Tavakoli DZ, Singla R, Johnson T, Kukreja
R and Singla DK: Exosomes derived from embryonic stem cells inhibit
doxorubicin and inflammation-induced pyroptosis in muscle cells.
Can J Physiol Pharm. 96:304–307. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
96
|
Tavakoli DZ and Singla DK: Embryonic stem
cell-derived exosomes inhibit doxorubicin-induced
TLR4-NLRP3-mediated cell death-pyroptosis. Am J Physiol Heart Circ
Physiol. 317:H460–H471. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
97
|
Singla DK, Johnson TA and Tavakoli DZ:
Exosome treatment enhances anti-inflammatory M2 macrophages and
reduces inflammation-induced pyroptosis in doxorubicin-induced
cardiomyopathy. Cells-Basel. 8:12242019. View Article : Google Scholar
|
|
98
|
Dessouki F, Kukreja RC and Singla DK: Stem
Cell-Derived Exosomes ameliorate Doxorubicin-Induced muscle
toxicity through counteracting pyroptosis. Pharmaceuticals (Basel).
13:4502020. View Article : Google Scholar : PubMed/NCBI
|
|
99
|
Wang J, Sun M, Liu W, Li Y and Li M: Stem
cell-based therapies for liver diseases: An overview and update.
Tissue Eng Regen Med. 16:107–118. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
100
|
Wang J, Ren H, Yuan X, Ma H, Shi X and
Ding Y: Interleukin-10 secreted by mesenchymal stem cells
attenuates acute liver failure through inhibiting pyroptosis.
Hepatol Res. 48:E194–E202. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
101
|
Chen TS, Lai YA, Lai YJ and Chien CT:
Adipose stem cells preincubated with theanine exert liver
regeneration through increase of stem cell paracrine VEGF and
suppression of ROS, pyroptosis as well as autophagy markers in
liver damage induced by N-nitrosodiethylamine. Life Sci.
308:1209692022. View Article : Google Scholar : PubMed/NCBI
|
|
102
|
Forbes JM and Cooper ME: Mechanisms of
diabetic complications. Physiol Rev. 93:137–188. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
103
|
Kunkemoeller B, Bancroft T, Xing H, Morris
AH, Luciano AK, Wu J, Fernandez-Hernando C and Kyriakides TR:
Elevated thrombospondin 2 contributes to delayed wound healing in
diabetes. Diabetes. 68:2016–223. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
104
|
Mu X, Wu X, He W, Liu Y, Wu F and Nie X:
Pyroptosis and inflammasomes in diabetic wound healing. Front
Endocrinol. 13:9507982022. View Article : Google Scholar : PubMed/NCBI
|
|
105
|
Yang H, Zhang Y, Du Z, Wu T and Yang C:
Hair follicle mesenchymal stem cell exosomal lncRNA H19 inhibited
NLRP3 pyroptosis to promote diabetic mouse skin wound healing.
Aging (Albany Ny). 15:791–809. 2023. View Article : Google Scholar : PubMed/NCBI
|
|
106
|
Yadav SK, Kambis TN, Kar S, Park SY and
Mishra PK: MMP9 mediates acute hyperglycemia-induced human cardiac
stem cell death by upregulating apoptosis and pyroptosis in vitro.
Cell Death Dis. 11:1862020. View Article : Google Scholar : PubMed/NCBI
|
|
107
|
Luo C, Peng Y, Zhou X, Fan J, Chen W,
Zhang H and Wei A: NLRP3 downregulation enhances engraftment and
functionality of adipose-derived stem cells to alleviate erectile
dysfunction in diabetic rats. Front Endocrinol. 13:9132962022.
View Article : Google Scholar : PubMed/NCBI
|
|
108
|
Alicic RZ, Rooney MT and Tuttle KR:
Diabetic kidney disease: Challenges, progress, and possibilities.
Clin J Am Soc Nephro. 12:2032–2045. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
109
|
Lv J, Hao YN, Wang XP, Lu WH, Xie LY and
Niu D: Bone marrow mesenchymal stem cell-derived exosomal
miR-30e-5p ameliorates high-glucose induced renal proximal tubular
cell pyroptosis by inhibiting ELAVL1. Renal Failure.
45:21770822023. View Article : Google Scholar : PubMed/NCBI
|
|
110
|
Zhang YZ and Li YY: Inflammatory bowel
disease: Pathogenesis. World J Gastroentero. 20:91–99. 2014.
View Article : Google Scholar
|
|
111
|
Zhen Y and Zhang H: NLRP3 Inflammasome and
inflammatory bowel disease. Front Immunol. 10:2762019. View Article : Google Scholar : PubMed/NCBI
|
|
112
|
Zhang S, Liang Y, Yao J, Li DF and Wang
LS: Role of pyroptosis in inflammatory bowel disease (IBD): From
gasdermins to DAMPs. Front Pharmacol. 13:8335882022. View Article : Google Scholar : PubMed/NCBI
|
|
113
|
Cai X, Zhang ZY, Yuan JT, Ocansey D, Tu Q,
Zhang X, Qian H, Xu WR, Qiu W and Mao F: hucMSC-derived exosomes
attenuate colitis by regulating macrophage pyroptosis via the
miR-378a-5p/NLRP3 axis. Stem Cell Res Ther. 12:4162021. View Article : Google Scholar : PubMed/NCBI
|
|
114
|
Xu Y, Tang X, Fang A, Yan J, Kofi WOD,
Zhang X and Mao F: HucMSC-Ex carrying miR-203a-3p.2 ameliorates
colitis through the suppression of caspase11/4-induced macrophage
pyroptosis. Int Immunopharmacol. 110:1089252022. View Article : Google Scholar : PubMed/NCBI
|
|
115
|
Jiao Y, Zhang T, Zhang C, Ji H, Tong X,
Xia R, Wang W, Ma Z and Shi X: Exosomal miR-30d-5p of neutrophils
induces M1 macrophage polarization and primes macrophage pyroptosis
in sepsis-related acute lung injury. Crit Care. 25:3562021.
View Article : Google Scholar : PubMed/NCBI
|
|
116
|
Feng Y, Li M, Yangzhong X, Zhang X, Zu A,
Hou Y, Li L and Sun S: Pyroptosis in inflammation-related
respiratory disease. J Physiol Biochem. 78:721–737. 2022.
View Article : Google Scholar : PubMed/NCBI
|
|
117
|
Zhang M, Xu G, Zhou X, Luo M, Ma N, Wang
X, Wang Z, Tang H, Wang X, Li Y, et al: Mesenchymal stem cells
ameliorate H9N2-induced acute lung injury by inhibiting
caspase-3-GSDME-mediated pyroptosis of lung alveolar epithelial
cells. Eur J Pharmacol. 960:1761482023. View Article : Google Scholar : PubMed/NCBI
|
|
118
|
Liu P, Yang S, Shao X, Li C, Wang Z, Dai H
and Wang C: Mesenchymal stem cells-derived exosomes alleviate acute
lung injury by inhibiting alveolar macrophage pyroptosis. Stem Cell
Transl Med. szad0942024.doi: 10.1093/stcltm/szad094 (Epub ahead of
print). View Article : Google Scholar
|
|
119
|
Tao Y, Xu X, Yang B, Zhao H and Li Y:
Mitigation of sepsis-induced acute lung injury by BMSC-Derived
exosomal miR-125b-5p through STAT3-Mediated suppression of
macrophage pyroptosis. Int J Nanomed. 18:7095–7113. 2023.
View Article : Google Scholar : PubMed/NCBI
|
|
120
|
Zhang T, Lu L, Li M, Zhang D, Yu P, Zhang
X, Zhang Z and Lei C: Exosome from BMMSC attenuates cardiopulmonary
bypass-induced acute lung injury via YAP/beta-catenin pathway:
Downregulation of pyroptosis. Stem Cells. 40:1122–1133. 2022.
View Article : Google Scholar : PubMed/NCBI
|
|
121
|
Li C, Lu Y, Du S, Li S, Zhang Y, Liu F,
Chen Y, Weng D and Chen J: Dioscin exerts protective effects
against crystalline silica-induced pulmonary fibrosis in mice.
Theranostics. 7:4255–4275. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
122
|
Song M, Wang J, Sun Y, Pang J, Li X, Liu
Y, Zhou Y, Yang P, Fan T, Liu Y, et al: Inhibition of gasdermin
D-dependent pyroptosis attenuates the progression of silica-induced
pulmonary inflammation and fibrosis. Acta Pharm Sin B.
12:1213–1224. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
123
|
Zhao Q, Hao C, Wei J, Huang R, Li C and
Yao W: Bone marrow-derived mesenchymal stem cells attenuate
silica-induced pulmonary fibrosis by inhibiting apoptosis and
pyroptosis but not autophagy in rats. Ecotox Environ Safe.
216:1121812021. View Article : Google Scholar : PubMed/NCBI
|
|
124
|
Levey AS and James MT: Acute Kidney
Injury. Ann Intern Med. 167:ITC66–ITC80. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
125
|
Li N, Wang Y, Wang X, Sun N and Gong YH:
Pathway network of pyroptosis and its potential inhibitors in acute
kidney injury. Pharmacol Res. 175:1060332022. View Article : Google Scholar : PubMed/NCBI
|
|
126
|
Xie Z, Tang J, Chen Z, Wei L, Chen J and
Liu Q: Human bone marrow mesenchymal stem cell-derived
extracellular vesicles reduce inflammation and pyroptosis in acute
kidney injury via miR-223-3p/HDAC2/SNRK. Inflamm Res. 72:553–576.
2023. View Article : Google Scholar : PubMed/NCBI
|
|
127
|
Guo J, Wang R and Liu D: Bone
marrow-derived mesenchymal stem cells ameliorate sepsis-induced
acute kidney injury by promoting mitophagy of renal tubular
epithelial cells via the SIRT1/Parkin axis. Front Endocrinol.
12:6391652021. View Article : Google Scholar : PubMed/NCBI
|
|
128
|
Reddi S, Thakker-Varia S, Alder J and
Giarratana AO: Status of precision medicine approaches to traumatic
brain injury. Neural Regen Res. 17:2166–2171. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
129
|
Zhang L, Lin Y, Bai W, Sun L and Tian M:
Human umbilical cord mesenchymal stem cell-derived exosome
suppresses programmed cell death in traumatic brain injury via
PINK1/Parkin-mediated mitophagy. Cns Neurosci Ther. 29:2236–2258.
2023. View Article : Google Scholar : PubMed/NCBI
|
|
130
|
Al MA, Wu Y, Monalisa I, Jia C, Zhou K,
Munir F and Xiao J: Role of pyroptosis in spinal cord injury and
its therapeutic implications. J Adv Res. 28:97–109. 2021.
View Article : Google Scholar
|
|
131
|
Sheth KN: Spontaneous intracerebral
hemorrhage. New Engl J Med. 387:1589–1596. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
132
|
Hu LT, Wang BY, Fan YH, He ZY and Zheng
WX: Exosomal miR-23b from bone marrow mesenchymal stem cells
alleviates oxidative stress and pyroptosis after intracerebral
hemorrhage. Neural Regen Res. 18:560–567. 2023. View Article : Google Scholar : PubMed/NCBI
|
|
133
|
Giri TK, Alexander A, Agrawal M and Saraf
S and Saraf S: Ajazuddin: Current status of stem cell therapies in
tissue repair and regeneration. Curr Stem Cell Res Ther.
14:117–126. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
134
|
Yamanaka S: Pluripotent stem cell-based
cell therapy-promise and challenges. Cell Stem Cell. 27:523–531.
2020. View Article : Google Scholar : PubMed/NCBI
|
|
135
|
Masuda S: Risk of teratoma formation after
transplantation of induced pluripotent stem cells. Chest.
141:1120–1121. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
136
|
Chour T, Tian L, Lau E, Thomas D, Itzhaki
I, Malak O, Zhang JZ, Qin X, Wardak M, Liu Y, et al: Method for
selective ablation of undifferentiated human pluripotent stem cell
populations for cell-based therapies. JCI Insight. 6:e1420002021.
View Article : Google Scholar : PubMed/NCBI
|
|
137
|
Alessandrini M, Preynat-Seauve O, De Bruin
K and Pepper MS: Stem cell therapy for neurological disorders. S
Afr Med J. 109:70–77. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
138
|
Trounson A and McDonald C: Stem cell
therapies in clinical trials: Progress and challenges. Cell Stem
Cell. 17:11–22. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
139
|
Liu XY, Yang LP and Zhao L: Stem cell
therapy for Alzheimer's disease. World J Stem Cells. 12:787–802.
2020. View Article : Google Scholar : PubMed/NCBI
|
|
140
|
Al-Ghadban S and Bunnell BA: Adipose
Tissue-Derived stem cells: Immunomodulatory effects and therapeutic
potential. Physiology. 35:125–133. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
141
|
Li Q, Wang Z, Xing H, Wang Y and Guo Y:
Exosomes derived from miR-188-3p-modified adipose-derived
mesenchymal stem cells protect Parkinson's disease. Mol Ther
Nucleic Acids. 23:1334–1344. 2021. View Article : Google Scholar : PubMed/NCBI
|