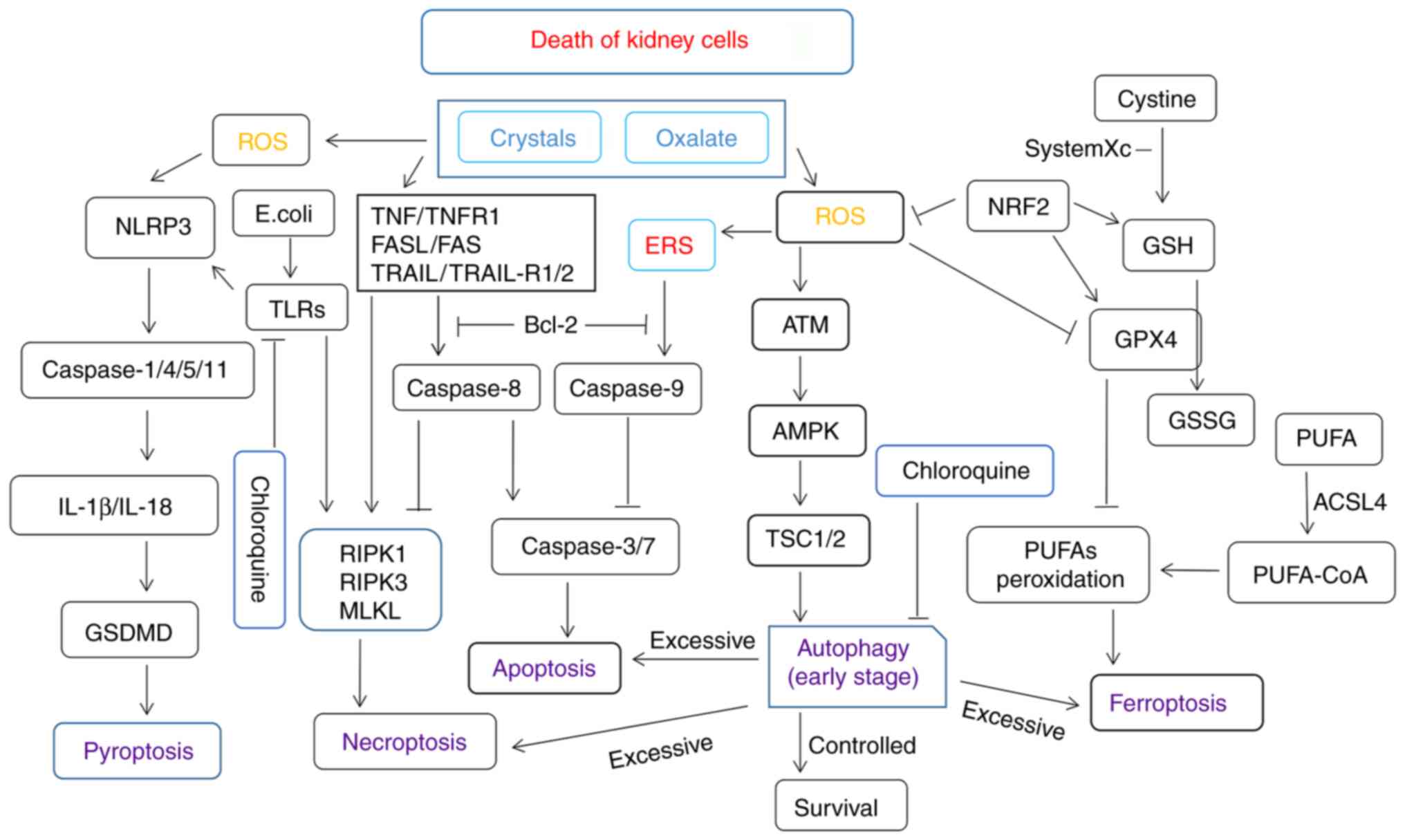
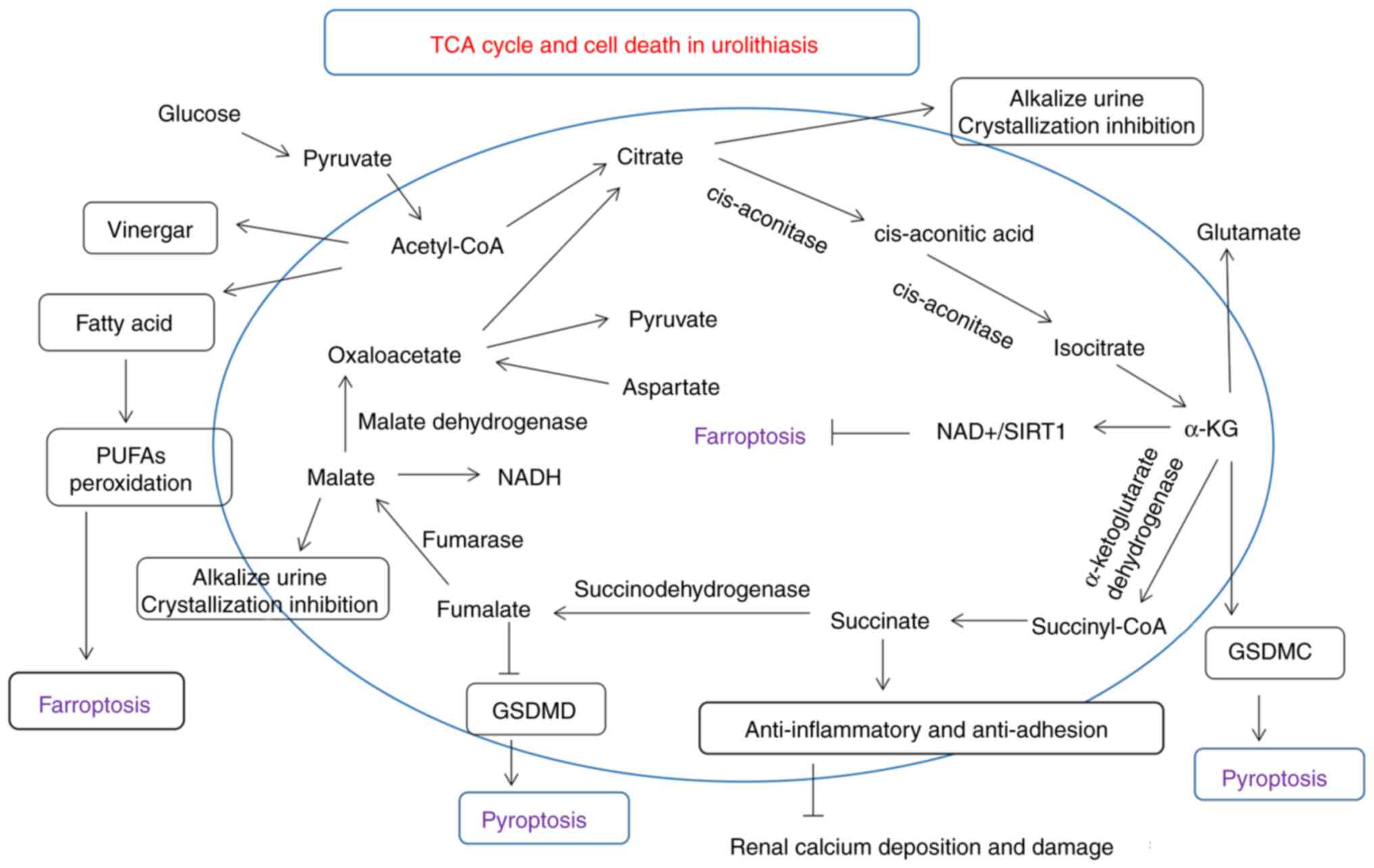
|
1
|
Wigner P, Grębowski R, Bijak M, Szemraj J
and Saluk-Bijak J: The molecular aspect of nephrolithiasis
development. Cells. 10:19262021. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Liu Y, Chen Y, Liao B, Luo D, Wang K, Li H
and Zeng G: Epidemiology of urolithiasis in Asia. Asian J Urol.
5:205–214. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Lai Y, Zheng H, Sun X, Lin J, Li Q, Huang
H, Hou Y, Zhong H, Zhang D, Fucai T and He Z: The advances of
calcium oxalate calculi associated drugs and targets. Eur J
Pharmacol. 935:1753242022. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Tang D, Kang R, Berghe TV, Vandenabeele P
and Kroemer G: The molecular machinery of regulated cell death.
Cell Res. 29:347–364. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Vitale I, Pietrocola F, Guilbaud E,
Aaronson SA, Abrams JM, Adam D, Agostini M, Agostinis P, Alnemri
ES, Altucci L, et al: Apoptotic cell death in disease-current
understanding of the NCCD 2023. Cell Death Differ. 30:1097–1154.
2023. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Tsvetkov P, Coy S, Petrova B, Dreishpoon
M, Verma A, Abdusamad M, Rossen J, Joesch-Cohen L, Humeidi R,
Spangler RD, et al: Copper induces cell death by targeting
lipoylated TCA cycle proteins. Science. 375:1254–1261. 2022.
View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Liu X, Nie L, Zhang Y, Yan Y, Wang C,
Colic M, Olszewski K, Horbath A, Chen X, Lei G, et al: Actin
cytoskeleton vulnerability to disulfide stress mediates
disulfidptosis. Nat Cell Biol. 25:404–414. 2023. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Martin-Sanchez D, Fontecha-Barriuso M,
Sanchez-Niño MD, Ramos AM, Cabello R, Gonzalez-Enguita C,
Linkermann A, Sanz AB and Ortiz A: Cell death-based approaches in
treatment of the urinary tract-associated diseases: A fight for
survival in the killing fields. Cell Death Dis. 9:1182018.
View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Yang L, Liu Y, Zhou S, Feng Q, Lu Y, Liu D
and Liu Z: Novel insight into ferroptosis in kidney diseases. Am J
Nephrol. 54:184–199. 2023. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Bayir H, Dixon SJ, Tyurina YY, Kellum JA
and Kagan VE: Ferroptotic mechanisms and therapeutic targeting of
iron metabolism and lipid peroxidation in the kidney. Nat Rev
Nephrol. 19:315–336. 2023. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Sun XY and Ouyang JM: New view in cell
death mode: Effect of crystal size in renal epithelial cells. Cell
Death Dis. 6:e20132015. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Gan QZ, Sun XY, Bhadja P, Yao XQ and
Ouyang JM: Reinjury risk of nano-calcium oxalate monohydrate and
calcium oxalate dihydrate crystals on injured renal epithelial
cells: Aggravation of crystal adhesion and aggregation. Int J
Nanomedicine. 11:2839–2854. 2016.PubMed/NCBI
|
|
13
|
Sanz AB, Sanchez-Niño MD, Ramos AM and
Ortiz A: Regulated cell death pathways in kidney disease. Nat Rev
Nephrol. 19:281–299. 2023. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Abhishek A, Benita S, Kumari M, Ganesan D,
Paul E, Sasikumar P, Mahesh A, Yuvaraj S, Ramprasath T and Selvam
GS: Molecular analysis of oxalate-induced endoplasmic reticulum
stress mediated apoptosis in the pathogenesis of kidney stone
disease. J Physiol Biochem. 73:561–573. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Canela VH, Bowen WS, Ferreira RM, Syed F,
Lingeman JE, Sabo AR, Barwinska D, Winfree S, Lake BB, Cheng YH, et
al: A spatially anchored transcriptomic atlas of the human kidney
papilla identifies significant immune injury in patients with stone
disease. Nat Commun. 14:41402023. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Liu J, Huang J, Gong B, Cheng S, Liu Y,
Chen Y, Feng Q, Li J, Qiu M, Yu G and Liao Y: Polydatin protects
against calcium oxalate crystal-induced renal injury through the
cytoplasmic/mitochondrial reactive oxygen species-NLRP3
inflammasome pathway. Biomed Pharmacother. 167:1156212023.
View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Singh P, Harris PC, Sas DJ and Lieske JC:
The genetics of kidney stone disease and nephrocalcinosis. Nat Rev
Nephrol. 18:224–240. 2022. View Article : Google Scholar
|
|
18
|
Shastri S, Patel J, Sambandam KK and
Lederer ED: Kidney stone pathophysiology, evaluation and
management: Core curriculum 2023. Am J Kidney Dis. 82:617–634.
2023. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Grases F, Rodriguez A and Costa-Bauza A:
Efficacy of mixtures of magnesium, citrate and phytate as calcium
oxalate crystallization inhibitors in urine. J Urol. 194:812–819.
2015. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Wang Z, Zhang Y, Zhang J, Deng Q and Liang
H: Recent advances on the mechanisms of kidney stone formation
(review). Int J Mol Med. 48:1492021. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Letavernier E, Bouderlique E, Zaworski J,
Martin L and Daudon M: Pseudoxanthoma elasticum, kidney stones and
pyrophosphate: From a rare disease to urolithiasis and vascular
calcifications. Int J Mol Sci. 20:63532019. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Dedinszki D, Szeri F, Kozák E, Pomozi V,
Tőkési N, Mezei TR, Merczel K, Letavernier E, Tang E, Le Saux O, et
al: Oral administration of pyrophosphate inhibits connective tissue
calcification. EMBO Mol Med. 9:1463–1470. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Robinson TE, Hughes EAB, Wiseman OJ,
Stapley SA, Cox SC and Grover LM: Hexametaphosphate as a potential
therapy for the dissolution and prevention of kidney stones. J
Mater Chem B. 8:5215–5224. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Zeng G, Zhu W, Robertson WG, Penniston KL,
Smith D, Pozdzik A, Tefik T, Prezioso D, Pearle MS, Chew BH, et al:
International alliance of urolithiasis (IAU) guidelines on the
metabolic evaluation and medical management of urolithiasis.
Urolithiasis. 51:42022. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Liu BC, Tang TT, Lv LL and Lan HY: Renal
tubule injury: A driving force toward chronic kidney disease.
Kidney Int. 93:568–579. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Honarpisheh M, Foresto-Neto O, Desai J,
Steiger S, Gómez LA, Popper B, Boor P, Anders HJ and Mulay SR:
Phagocytosis of environmental or metabolic crystalline particles
induces cytotoxicity by triggering necroptosis across a broad range
of particle size and shape. Sci Rep. 7:155232017. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Guo H, Wang M, Shang Y, Zhang B, Zhang S,
Liu X, Cao P, Fan Y and Tan K: Apoptosis-related prognostic
biomarkers and potential targets for acute kidney injury based on
machine learning algorithm and in vivo experiments. Apoptosis.
29:303–320. 2024. View Article : Google Scholar
|
|
28
|
Klinkhammer BM, Buchtler S, Djudjaj S,
Bouteldja N, Palsson R, Edvardsson VO, Thorsteinsdottir M, Floege
J, Mack M and Boor P: Current kidney function parameters
overestimate kidney tissue repair in reversible experimental kidney
disease. Kidney Int. 102:307–320. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Kumar R, Soni H, Afolabi JM, Kanthakumar
P, Mankuzhy PD, Iwhiwhu SA and Adebiyi A: Induction of reactive
oxygen species by mechanical stretch drives endothelin production
in neonatal pig renal epithelial cells. Redox Biol. 55:1023942022.
View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Li J, Lin Q, Shao X, Li S, Zhu X, Wu J,
Mou S, Gu L, Wang Q, Zhang M, et al: HIF1α-BNIP3-mediated mitophagy
protects against renal fibrosis by decreasing ROS and inhibiting
activation of the NLRP3 inflammasome. Cell Death Dis. 14:2002023.
View Article : Google Scholar
|
|
31
|
Li Y, Yuan Y, Huang ZX, Chen H, Lan R,
Wang Z, Lai K, Chen H, Chen Z, Zou Z, et al: GSDME-mediated
pyroptosis promotes inflammation and fibrosis in obstructive
nephropathy. Cell Death Differ. 28:2333–2350. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Zhang B, Chen X, Ru F, Gan Y, Li B, Xia W,
Dai G, He Y and Chen Z: Liproxstatin-1 attenuates unilateral
ureteral obstruction-induced renal fibrosis by inhibiting renal
tubular epithelial cells ferroptosis. Cell Death Dis. 12:8432021.
View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Jung HD, Cho S and Lee JY: Update on the
effect of the urinary microbiome on urolithiasis. Diagnostics
(Basel). 13:9512023. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
An L, Wu W, Li S, Lai Y, Chen D, He Z,
Chang Z, Xu P, Huang Y, Lei M, et al: Escherichia coli aggravates
calcium oxalate stone formation via PPK1/flagellin-mediated renal
oxidative injury and inflammation. Oxid Med Cell Longev.
2021:99496972021. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Wang M, Lin X, Yang X and Yang Y: Research
progress on related mechanisms of uric acid activating NLRP3
inflammasome in chronic kidney disease. Ren Fail. 44:615–624. 2022.
View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Yifan Z, Luming S, Wei C, Luwei X, Zheng X
and Ruipeng J: Cystine crystal-induced reactive oxygen species
associated with NLRP3 inflammasome activation: Implications for the
pathogenesis of cystine calculi. Int Urol Nephrol. 54:3097–3106.
2022. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Mayayo-Vallverdú C, López de Heredia M,
Prat E, González L, Espino Guarch M, Vilches C, Muñoz L, Asensi MA,
Serra C, Llebaria A, et al: The antioxidant l-Ergothioneine
prevents cystine lithiasis in the Slc7a9-/- mouse model
of cystinuria. Redox Biol. 64:1028012023. View Article : Google Scholar
|
|
38
|
Rao CY, Sun XY and Ouyang JM: Effects of
physical properties of nano-sized hydroxyapatite crystals on
cellular toxicity in renal epithelial cells. Mater Sci Eng C Mater
Biol Appl. 103:1098072019. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Yuan J and Ofengeim D: A guide to cell
death pathways. Nat Rev Mol Cell Bio. Dec;–18. 2023.Epub ahead of
print.
|
|
40
|
Ai Y, Meng Y, Yan B, Zhou Q and Wang X:
The biochemical pathways of apoptotic, necroptotic, pyroptotic, and
ferroptotic cell death. Mol Cell. 84:170–179. 2024. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Sun Y, Kang J, Guan X, Xu H, Wang X and
Deng Y: Regulation of endoplasmic reticulum stress on the damage
and apoptosis of renal tubular epithelial cells induced by calcium
oxalate crystals. Urolithiasis. 49:291–299. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Howles SA and Thakker RV: Genetics of
kidney stone disease. Nat Rev Urol. 17:407–421. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Cil O, Chu T, Lee S, Haggie PM and Verkman
AS: Small-molecule inhibitor of intestinal anion exchanger SLC26A3
for treatment of hyperoxaluria and nephrolithiasis. JCI Insight.
7:e1533592022. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Ming S, Tian J, Ma K, Pei C, Li L, Wang Z,
Fang Z, Liu M, Dong H, Li W, et al: Oxalate-induced apoptosis
through ERS-ROS-NF-κB signalling pathway in renal tubular
epithelial cell. Mol Med. 28:882022. View Article : Google Scholar
|
|
45
|
Wu D, Huang LF, Chen XC, Huang XR, Li HY,
An N, Tang JX, Liu HF and Yang C: Research progress on endoplasmic
reticulum homeostasis in kidney diseases. Cell Death Dis.
14:4732023. View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Sharma M, Naura AS and Singla SK: A
deleterious interplay between endoplasmic reticulum stress and its
functional linkage to mitochondria in nephrolithiasis. Free Radical
Bio Med. 168:70–80. 2021. View Article : Google Scholar
|
|
47
|
Wu Y, Zhang J, Li C, Hu H, Qin B, Wang T,
Lu Y and Wang S: The activation of ROS/NF-κB/MMP-9 pathway promotes
calcium-induced kidney crystal deposition. Oxid Med Cell Longev.
2021:88363552021. View Article : Google Scholar
|
|
48
|
Yiu AJ, Ibeh CL, Roy SK and Bandyopadhyay
BC: Melamine induces Ca2+-sensing receptor activation
and elicits apoptosis in proximal tubular cells. Am J Physiol Cell
Physiol. 313:C27–C41. 2017. View Article : Google Scholar
|
|
49
|
Wu CF, Liu CC, Tsai YC, Chen CC, Wu MT and
Hsieh TJ: Diminishment of Nrf2 antioxidative defense aggravates
nephrotoxicity of melamine and oxalate coexposure. Antioxidants
(Basel). 10:14642021. View Article : Google Scholar : PubMed/NCBI
|
|
50
|
Peng Y, Fang Z, Liu M, Wang Z, Li L, Ming
S, Lu C, Dong H, Zhang W, Wang Q, et al: Testosterone induces renal
tubular epithelial cell death through the HIF-1alpha/BNIP3 pathway.
J Transl Med. 17:622019. View Article : Google Scholar
|
|
51
|
Gombedza FC, Shin S, Kanaras YL and
Bandyopadhyay BC: Abrogation of store-operated Ca2+
entry protects against crystal-induced ER stress in human proximal
tubular cells. Cell Death Discov. 5:1242019. View Article : Google Scholar
|
|
52
|
Yan L, Chen J and Fang W: Exosomes derived
from calcium oxalate-treated macrophages promote apoptosis of HK-2
cells by promoting autophagy. Bioengineered. 13:2442–2450. 2022.
View Article : Google Scholar : PubMed/NCBI
|
|
53
|
Khan SR, Canales BK and
Dominguez-Gutierrez PR: Randall's plaque and calcium oxalate stone
formation: Role for immunity and inflammation. Nat Rev Nephrol.
17:417–433. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
54
|
He J, Cao Y, Zhu Q, Wang X, Cheng G, Wang
Q, He R, Lu H, Weng Y, Mao G, et al: Renal macrophages monitor and
remove particles from urine to prevent tubule obstruction.
Immunity. 57:106–123.e7. 2024. View Article : Google Scholar : PubMed/NCBI
|
|
55
|
Liu Q, Liu Y, Guan X, Wu J, He Z, Kang J,
Tao Z and Deng Y: Effect of M2 macrophages on injury and apoptosis
of renal tubular epithelial cells induced by calcium oxalate
crystals. Kidney Blood Press Res. 44:777–791. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
56
|
Lu H, Sun X, Jia M, Sun F, Zhu J, Chen X,
Chen K and Jiang K: Rosiglitazone suppresses renal crystal
deposition by ameliorating tubular injury resulted from oxidative
stress and inflammatory response via promoting the Nrf2/HO-1
pathway and shifting macrophage polarization. Oxid Med Cell Longev.
2021:55271372021. View Article : Google Scholar : PubMed/NCBI
|
|
57
|
Xi J, Chen Y, Jing J, Qi W and Zhang Y:
LncRNA LINC01197 inhibited the formation of calcium oxalate-induced
kidney stones by regulating miR-516b-5p/SIRT3/FOXO1 signaling
pathway. Cell Tissue Res. 392:553–563. 2023. View Article : Google Scholar : PubMed/NCBI
|
|
58
|
Xi J, Jing J, Zhang Y, Liang C, Hao Z,
Zhang L and Chen Y: SIRT3 inhibited the formation of calcium
oxalate-induced kidney stones through regulating NRF2/HO-1
signaling pathway. J Cell Biochem. 120:8259–8271. 2019. View Article : Google Scholar
|
|
59
|
Li Y, Ding T, Hu H, Zhao T, Zhu C, Ding J,
Yuan J and Guo Z: LncRNA-ATB participates in the regulation of
calcium oxalate crystal-induced renal injury by sponging the
miR-200 family. Mol Med. 27:1432021. View Article : Google Scholar : PubMed/NCBI
|
|
60
|
Su B, Han H, Ji C, Hu W, Yao J, Yang J,
Fan Y and Li J: MiR-21 promotes calcium oxalate-induced renal
tubular cell injury by targeting PPARA. Am J Physiol Renal Physiol.
319:F202–F214. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
61
|
Cabuzu D, Ramakrishnan SK, Moor MB,
Harmacek D, Auberson M, Durussel F and Bonny O: Loss of Ecrg4
improves calcium oxalate nephropathy. PLoS One. 17:e2759722022.
View Article : Google Scholar
|
|
62
|
Gao X, Peng Y, Fang Z, Li L, Ming S, Dong
H, Li R, Zhu Y, Zhang W, Zhu B, et al: Inhibition of EZH2
ameliorates hyperoxaluria-induced kidney injury through the
JNK/FoxO3a pathway. Life Sci. 291:1202582022. View Article : Google Scholar
|
|
63
|
Zhou Z, Zhou X, Zhang Y, Yang Y, Wang L
and Wu Z: Butyric acid inhibits oxidative stress and inflammation
injury in calcium oxalate nephrolithiasis by targeting CYP2C9. Food
Chem Toxicol. 178:1139252023. View Article : Google Scholar : PubMed/NCBI
|
|
64
|
Song Q, Song C, Chen X, Xiong Y, Li L,
Liao W, Xue L and Yang S: FKBP5 deficiency attenuates calcium
oxalate kidney stone formation by suppressing cell-crystal
adhesion, apoptosis and macrophage M1 polarization via inhibition
of NF-κB signaling. Cell Mol Life Sci. 80:3012023. View Article : Google Scholar
|
|
65
|
Xun Y, Zhou P, Yang Y, Li C, Zhang J, Hu
H, Qin B, Zhang Z, Wang Q, Lu Y and Wang S: Role of Nox4 in high
calcium-induced renal oxidative stress damage and crystal
deposition. Antioxid Redox Sign. 36:15–38. 2022. View Article : Google Scholar
|
|
66
|
Thomas K, Zondler L, Ludwig N, Kardell M,
Lüneburg C, Henke K, Mersmann S, Margraf A, Spieker T, Tekath T, et
al: Glutamine prevents acute kidney injury by modulating oxidative
stress and apoptosis in tubular epithelial cells. JCI Insight.
7:e1631612022. View Article : Google Scholar : PubMed/NCBI
|
|
67
|
Li Y, Lu X, Yu Z, Wang H and Gao B:
Meta-data analysis of kidney stone disease highlights ATP1A1
involvement in renal crystal formation. Redox Biol. 61:1026482023.
View Article : Google Scholar : PubMed/NCBI
|
|
68
|
Ye QL, Wang DM, Wang X, Zhang ZQ, Tian QX,
Feng SY, Zhang ZH, Yu DX, Ding DM and Xie DD: Sirt1 inhibits kidney
stones formation by attenuating calcium oxalate-induced cell
injury. Chem Biol Interact. 347:1096052021. View Article : Google Scholar : PubMed/NCBI
|
|
69
|
Ji N, Qi Z, Wang Y, Yang X, Yan Z, Li M,
Ge Q and Zhang J: Pyroptosis: A new regulating mechanism in
cardiovascular disease. J Inflamm Res. 14:2647–2666. 2021.
View Article : Google Scholar : PubMed/NCBI
|
|
70
|
Vande WL and Lamkanfi M: Drugging the
NLRP3 inflammasome: From signalling mechanisms to therapeutic
targets. Nat Rev Drug Discov. 23:43–66. 2024. View Article : Google Scholar
|
|
71
|
Que X, Zheng S, Song Q, Pei H and Zhang P:
Fantastic voyage: The journey of NLRP3 inflammasome activation.
Genes Dis. 11:819–829. 2023. View Article : Google Scholar : PubMed/NCBI
|
|
72
|
Darisipudi MN and Knauf F: An update on
the role of the inflammasomes in the pathogenesis of kidney
diseases. Pediatr Nephrol. 31:535–544. 2016. View Article : Google Scholar
|
|
73
|
Chen Y, Yang S, Kong H, Wang Q, Chen S,
Wang X, Chen L and Qi S: Oxalate-induced renal pyroptotic injury
and crystal formation mediated by NLRP3-GSDMD signaling in vitro
and in vivo. Mol Med Rep. 28:2092023. View Article : Google Scholar
|
|
74
|
Gu Y, Shen Y, Chen W, He H, Ma Y, Mei X,
Ju D and Liu H: Protective effects of interleukin-22 on
oxalate-induced crystalline renal injury via alleviating
mitochondrial damage and inflammatory response. Appl Microbiol
Biot. 106:2637–2649. 2022. View Article : Google Scholar
|
|
75
|
Zhang Y, Wang S, Dai X, Liu T, Liu Y, Shi
H, Yin J, Xu T, Zhang Y, Zhao D, et al: Simiao San alleviates
hyperuricemia and kidney inflammation by inhibiting NLRP3
inflammasome and JAK2/STAT3 signaling in hyperuricemia mice. J
Ethnopharmacol. 312:1165302023. View Article : Google Scholar : PubMed/NCBI
|
|
76
|
Gan XG, Wang ZH and Xu HT: Mechanism of
miRNA-141-3p in calcium oxalate-induced renal tubular epithelial
cell injury via NLRP3-mediated pyroptosis. Kidney Blood Press Res.
47:300–308. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
77
|
Ding T, Zhao T, Li Y, Liu Z, Ding J, Ji B,
Wang Y and Guo Z: Vitexin exerts protective effects against calcium
oxalate crystal-induced kidney pyroptosis in vivo and in vitro.
Phytomedicine. 86:1535622021. View Article : Google Scholar : PubMed/NCBI
|
|
78
|
Song Z, Zhang Y, Gong B, Xu H, Hao Z and
Liang C: Long noncoding RNA LINC00339 promotes renal tubular
epithelial pyroptosis by regulating the miR-22-3p/NLRP3 axis in
calcium oxalate-induced kidney stone. J Cell Biochem.
120:10452–10462. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
79
|
Liu J, Yang K, Jin Y, Liu Y, Chen Y, Zhang
X, Yu S, Song E, Chen S, Zhang J, et al: H3 relaxin protects
against calcium oxalate crystal-induced renal inflammatory
pyroptosis. Cell Prolif. 53:e129022020. View Article : Google Scholar : PubMed/NCBI
|
|
80
|
Yifan Z, Benxiang N, Zheng X, Luwei X,
Liuhua Z, Yuzheng G and Ruipeng J: Ceftriaxone Calcium crystals
induce acute kidney injury by NLRP3-mediated inflammation and
oxidative stress injury. Oxid Med Cell Longev. 2020:64284982020.
View Article : Google Scholar : PubMed/NCBI
|
|
81
|
Sundaram B, Pandian N, Mall R, Wang Y,
Sarkar R, Kim HJ, Malireddi RKS, Karki R, Janke LJ, Vogel P and
Kanneganti TD: NLRP12-PANoptosome activates PANoptosis and
pathology in response to heme and PAMPs. Cell. 186:2783–2801. 2023.
View Article : Google Scholar : PubMed/NCBI
|
|
82
|
Mulay SR, Shi C, Ma X and Anders HJ: Novel
insights into crystal-induced kidney injury. Kidney Dis (Basel).
4:49–57. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
83
|
Hou B, Liu M, Chen Y, Ni W, Suo X, Xu Y,
He Q, Meng X and Hao Z: Cpd-42 protects against calcium oxalate
nephrocalcinosis-induced renal injury and inflammation by targeting
RIPK3-mediated necroptosis. Front Pharmacol. 13:10411172022.
View Article : Google Scholar : PubMed/NCBI
|
|
84
|
Sedmaki K, Karnam K, Sharma P, Mahale A,
Routholla G, Ghosh B and Prakash Kulkarni O: HDAC6 inhibition
attenuates renal injury by reducing IL-1β secretion and RIP kinase
mediated necroptosis in acute oxalate nephropathy. Int
Immunopharmacol. 110:1089192022. View Article : Google Scholar
|
|
85
|
Prajapati S, Tomar B, Srivastava A,
Narkhede YB, Gaikwad AN, Lahiri A and Mulay SR:
6,7-Dihydroxycoumarin ameliorates crystal-induced necroptosis
during crystal nephropathies by inhibiting MLKL phosphorylation.
Life Sci. 271:1191932021. View Article : Google Scholar : PubMed/NCBI
|
|
86
|
Mulay SR, Eberhard JN, Desai J, Marschner
JA, Kumar SV, Weidenbusch M, Grigorescu M, Lech M, Eltrich N,
Müller L, et al: Hyperoxaluria requires TNF receptors to initiate
crystal adhesion and kidney stone disease. J Am Soc Nephrol.
28:761–768. 2017. View Article : Google Scholar :
|
|
87
|
Sun S, Shen J, Jiang J, Wang F and Min J:
Targeting ferroptosis opens new avenues for the development of
novel therapeutics. Signal Transduct Target Ther. 8:3722023.
View Article : Google Scholar : PubMed/NCBI
|
|
88
|
Chen X, Kang R, Kroemer G and Tang D:
Broadening horizons: The role of ferroptosis in cancer. Nat Rev
Clin Oncol. 18:280–296. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
89
|
He Z, Liao W, Song Q, Li B, Liu J, Xiong
Y, Song C and Yang S: Role of ferroptosis induced by a high
concentration of calcium oxalate in the formation and development
of urolithiasis. Int J Mol Med. 47:289–301. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
90
|
Ye Z, Xia Y, Li L, Li B, Chen L, Yu W,
Ruan Y, Rao T, Zhou X and Cheng F: p53 deacetylation alleviates
calcium oxalate deposition-induced renal fibrosis by inhibiting
ferroptosis. Biomed Pharmacother. 164:1149252023. View Article : Google Scholar : PubMed/NCBI
|
|
91
|
Song Q, Liao W, Chen X, He Z, Li D, Li B,
Liu J, Liu L, Xiong Y, Song C and Yang S: Oxalate activates
autophagy to induce ferroptosis of renal tubular epithelial cells
and participates in the formation of kidney stones. Oxid Med Cell
Longev. 2021:66303432021. View Article : Google Scholar : PubMed/NCBI
|
|
92
|
Xie J, Ye Z, Li L, Xia Y, Yuan R, Ruan Y
and Zhou X: Ferrostatin-1 alleviates oxalate-induced renal tubular
epithelial cell injury, fibrosis and calcium oxalate stone
formation by inhibiting ferroptosis. Mol Med Rep. 26:2562022.
View Article : Google Scholar :
|
|
93
|
Martin-Saiz L, Guerrero-Mauvecin J,
Martin-Sanchez D, Fresnedo O, Gómez MJ, Carrasco S, Cannata-Ortiz
P, Ortiz A, Fernandez JA and Sanz AB: Ferrostatin-1 modulates
dysregulated kidney lipids in acute kidney injury. J Pathol.
257:285–299. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
94
|
Xia C, Xing X, Zhang W, Wang Y, Jin X,
Wang Y, Tian M, Ba X and Hao F: Cysteine and homocysteine can be
exploited by GPX4 in ferroptosis inhibition independent of GSH
synthesis. Redox Biol. 69:1029992024. View Article : Google Scholar :
|
|
95
|
Ide S, Ide K, Abe K, Kobayashi Y, Kitai H,
McKey J, Strausser SA, O'Brien LL, Tata A, Tata PR and Souma T: Sex
differences in resilience to ferroptosis underlie sexual dimorphism
in kidney injury and repair. Cell Rep. 41:1116102022. View Article : Google Scholar : PubMed/NCBI
|
|
96
|
Chu LK, Cao X, Wan L, Diao Q, Zhu Y, Kan
Y, Ye LL, Mao YM, Dong XQ, Xiong QW, et al: Autophagy of OTUD5
destabilizes GPX4 to confer ferroptosis-dependent kidney injury.
Nat Commun. 14:83932023. View Article : Google Scholar : PubMed/NCBI
|
|
97
|
Song J, Wang H, Sheng J, Zhang W, Lei J,
Gan W, Cai F and Yang Y: Vitexin attenuates chronic kidney disease
by inhibiting renal tubular epithelial cell ferroptosis via NRF2
activation. Mol Med. 29:1472023. View Article : Google Scholar : PubMed/NCBI
|
|
98
|
Lee J and Roh JL: SLC7A11 as a gateway of
metabolic perturbation and ferroptosis vulnerability in cancer.
Antioxidants (Basel). 11:24442022. View Article : Google Scholar : PubMed/NCBI
|
|
99
|
Zhao J, Wu Y, Zhou K, Huang M, Sun Y, Kang
J, Su Q, Zhao Y, Liu Q and Li C: Ferroptosis in calcium oxalate
kidney stone formation and the possible regulatory mechanism of
ANKRD1. Biochim Biophys Acta Mol Cell Res. 1870:1194522023.
View Article : Google Scholar : PubMed/NCBI
|
|
100
|
Hao W, Zhang H, Hong P, Zhang X, Zhao X,
Ma L, Qiu X, Ping H, Lu D and Yin Y: Critical role of
VHL/BICD2/STAT1 axis in crystal-associated kidney disease. Cell
Death Dis. 14:6802023. View Article : Google Scholar : PubMed/NCBI
|
|
101
|
Chen X, Li J, Kang R, Klionsky DJ and Tang
D: Ferroptosis: Machinery and regulation. Autophagy. 17:2054–2081.
2021. View Article : Google Scholar :
|
|
102
|
Liu Q, Tang J, Chen Z, Wei L, Chen J and
Xie Z: Polyunsaturated fatty acids ameliorate renal stone-induced
renal tubular damage via miR-93-5p/Pknox1 axis. Nutrition.
105:1118632023. View Article : Google Scholar
|
|
103
|
Li L, Ye Z, Xia Y, Li B, Chen L, Yan X,
Yuan T, Song B, Yu W, Rao T, et al: YAP/ACSL4 pathway-mediated
ferroptosis promotes renal fibrosis in the presence of kidney
stones. Biomedicines. 11:26922023. View Article : Google Scholar : PubMed/NCBI
|
|
104
|
Mishima E, Ito J, Wu Z, Nakamura T, Wahida
A, Doll S, Tonnus W, Nepachalovich P, Eggenhofer E, Aldrovandi M,
et al: A non-canonical vitamin K cycle is a potent ferroptosis
suppressor. Nature. 608:778–783. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
105
|
Nishizawa H, Yamanaka M and Igarashi K:
Ferroptosis: Regulation by competition between NRF2 and BACH1 and
propagation of the death signal. FEBS J. 290:1688–1704. 2023.
View Article : Google Scholar
|
|
106
|
Dong C, Song C, He Z, Song Q, Song T, Liu
J, Xiong Y, Su X, Zhou J, Yang S and Liao W: Protective efficacy of
Schizandrin B on ameliorating nephrolithiasis via regulating
GSK3β/Nrf2 signaling-mediated ferroptosis in vivo and in vitro. Int
Immunopharmacol. 117:1100422023. View Article : Google Scholar
|
|
107
|
Zhou D, Wu Y, Yan H, Shen T, Li S, Gong J,
Li G, Mai H, Wang D and Tan X: Gallic acid ameliorates calcium
oxalate crystal-induced renal injury via upregulation of Nrf2/HO-1
in the mouse model of stone formation. Phytomedicine.
106:1544292022. View Article : Google Scholar : PubMed/NCBI
|
|
108
|
Li Y, Zhang J, Liu H, Yuan J, Yin Y, Wang
T, Cheng B, Sun S and Guo Z: Curcumin ameliorates
glyoxylate-induced calcium oxalate deposition and renal injuries in
mice. Phytomedicine. 61:1528612019. View Article : Google Scholar : PubMed/NCBI
|
|
109
|
Zhu J, Wang Q, Li C, Lu Y, Hu H, Qin B,
Xun Y, Zhu Y, Wu Y, Zhang J and Wang S: Inhibiting inflammation and
modulating oxidative stress in oxalate-induced nephrolithiasis with
the Nrf2 activator dimethyl fumarate. Free Radical Bio Med.
134:9–22. 2019. View Article : Google Scholar
|
|
110
|
Ushimoto C, Sugiki S, Kunii K, Inoue S,
Kuroda E, Akai R, Iwawaki T and Miyazawa K: Dynamic change and
preventive role of stress response via Keap1-Nrf2 during renal
crystal formation. Free Radic Bio Med. 207:120–132. 2023.
View Article : Google Scholar
|
|
111
|
Song Q, He Z, Li B, Liu J, Liu L, Liao W,
Xiong Y, Song C, Yang S and Liu Y: Melatonin inhibits
oxalate-induced endoplasmic reticulum stress and apoptosis in HK-2
cells by activating the AMPK pathway. Cell Cycle. 19:2600–2610.
2020. View Article : Google Scholar : PubMed/NCBI
|
|
112
|
Zhou J, Meng L, He Z, Song Q, Liu J, Su X,
Wang C, Ke H, Dong C, Liao W and Yang S: Melatonin exerts a
protective effect in ameliorating nephrolithiasis via targeting
AMPK/PINK1-Parkin mediated mitophagy and inhibiting ferroptosis in
vivo and in vitro. Int Immunopharmacol. 124:1108012023. View Article : Google Scholar : PubMed/NCBI
|
|
113
|
Su X, Song C, He Z, Song Q, Meng L, Dong
C, Zhou J, Ke H, Xiong Y, Liu J, et al: Ambra1 in exosomes secreted
by HK-2 cells damaged by supersaturated oxalate induce mitophagy
and autophagy-ferroptosis in normal HK-2 cells to participate in
the occurrence of kidney stones. Biochim Biophys Acta Mol Cell Res.
1871:1196042024. View Article : Google Scholar
|
|
114
|
Khan MA, Nag P, Grivei A, Giuliani KTK,
Wang X, Diwan V, Hoy W, Healy H, Gobe G and Kassianos AJ: Adenine
overload induces ferroptosis in human primary proximal tubular
epithelial cells. Cell Death Dis. 13:1042022. View Article : Google Scholar : PubMed/NCBI
|
|
115
|
Liu S, Yao S, Yang H, Liu S and Wang Y:
Autophagy: Regulator of cell death. Cell Death Dis. 14:6482023.
View Article : Google Scholar : PubMed/NCBI
|
|
116
|
Lee S, Hwang N, Seok BG, Lee S, Lee SJ and
Chung SW: Autophagy mediates an amplification loop during
ferroptosis. Cell Death Dis. 14:4642023. View Article : Google Scholar : PubMed/NCBI
|
|
117
|
Bhatia D and Choi ME: Autophagy and
mitophagy: Physiological implications in kidney inflammation and
diseases. Am J Physiol Renal Physiol. 325:F1–F21. 2023. View Article : Google Scholar : PubMed/NCBI
|
|
118
|
Duan X, Kong Z, Mai X, Lan Y, Liu Y, Yang
Z, Zhao Z, Deng T, Zeng T, Cai C, et al: Autophagy inhibition
attenuates hyperoxaluria-induced renal tubular oxidative injury and
calcium oxalate crystal depositions in the rat kidney. Redox Biol.
16:414–425. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
119
|
Sun Y, Kang J, Tao Z, Wang X, Liu Q, Li D,
Guan X, Xu H, Liu Y and Deng Y: Effect of endoplasmic reticulum
stress-mediated excessive autophagy on apoptosis and formation of
kidney stones. Life Sci. 244:1172322020. View Article : Google Scholar
|
|
120
|
Kang J, Sun Y, Deng Y, Liu Q, Li D, Liu Y,
Guan X, Tao Z and Wang X: Autophagy-endoplasmic reticulum stress
inhibition mechanism of superoxide dismutase in the formation of
calcium oxalate kidney stones. Biomed Pharmacother. 121:1096492020.
View Article : Google Scholar
|
|
121
|
Kumar P, Laurence E, Crossman DK, Assimos
DG, Murphy MP and Mitchell T: Oxalate disrupts monocyte and
macrophage cellular function via Interleukin-10 and mitochondrial
reactive oxygen species (ROS) signaling. Redox Biol. 67:1029192023.
View Article : Google Scholar : PubMed/NCBI
|
|
122
|
Nakamura S, Shigeyama S, Minami S, Shima
T, Akayama S, Matsuda T, Esposito A, Napolitano G, Kuma A,
Namba-Hamano T, et al: LC3 lipidation is essential for TFEB
activation during the lysosomal damage response to kidney injury.
Nat Cell Biol. 22:1252–1263. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
123
|
Wu Y, Xun Y, Zhang J, Hu H, Qin B, Wang T,
Wang S, Li C and Lu Y: Resveratrol attenuates oxalate-induced renal
oxidative injury and calcium oxalate crystal deposition by
regulating TFEB-induced autophagy pathway. Front Cell Dev Biol.
9:6387592021. View Article : Google Scholar : PubMed/NCBI
|
|
124
|
Dong F, Jiang S, Tang C, Wang X, Ren X,
Wei Q, Tian J, Hu W, Guo J, Fu X, et al: Trimethylamine N-oxide
promotes hyperoxaluria-induced calcium oxalate deposition and
kidney injury by activating autophagy. Free Radic Bio Med.
179:288–300. 2022. View Article : Google Scholar
|
|
125
|
Alaygut D, Ozturk I, Ulu S and Gungor O:
NETosis and kidney disease: What do we know? Int Urol Nephrol.
55:1985–1994. 2023. View Article : Google Scholar : PubMed/NCBI
|
|
126
|
Makki MS, Winfree S, Lingeman JE, Witzmann
FA, Worcester EM, Krambeck AE, Coe FL, Evan AP, Bledsoe S,
Bergsland KJ, et al: A precision medicine approach uncovers a
unique signature of neutrophils in patients with brushite kidney
stones. Kidney Int Rep. 5:663–677. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
127
|
Cai Z, Wu X, Song Z, Sun S, Su Y, Wang T,
Cheng X, Yu Y, Yu C, Chen E, et al: Metformin potentiates
nephrotoxicity by promoting NETosis in response to renal
ferroptosis. Cell Discov. 9:1042023. View Article : Google Scholar : PubMed/NCBI
|
|
128
|
Malireddi RKS, Kesavardhana S and
Kanneganti TD: ZBP1 and TAK1: Master regulators of NLRP3
inflammasome/pyroptosis, apoptosis, and necroptosis (PAN-optosis).
Front Cell Infect Microbiol. 9:4062019. View Article : Google Scholar : PubMed/NCBI
|
|
129
|
Hadian K and Stockwell BR: The therapeutic
potential of targeting regulated non-apoptotic cell death. Nat Rev
Drug Discov. 22:723–742. 2023. View Article : Google Scholar : PubMed/NCBI
|
|
130
|
Peerapen P and Thongboonkerd V: Kidney
stone prevention. Adv Nutr. 14:555–569. 2023. View Article : Google Scholar : PubMed/NCBI
|
|
131
|
Baltazar P, de Melo Junior AF, Fonseca NM,
Lança MB, Faria A, Sequeira CO, Teixeira-Santos L, Monteiro EC,
Campos Pinheiro L, Calado J, et al: Oxalate (dys)metabolism:
Person-to-person variability, kidney and cardiometabolic toxicity.
Genes (Basel). 14:17192023. View Article : Google Scholar : PubMed/NCBI
|
|
132
|
Marengo SR and Romani AMP: Oxalate in
renal stone disease: The terminal metabolite that just won't go
away. Nat Clin Pract Nephrol. 4:368–377. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
133
|
Grocholski C, Derain Dubourg L,
Guebre-Egziabher F, Acquaviva-Bourdain C, Abid N, Bacchetta J,
Chambrier C and Lemoine S: Oxalate: From physiology to pathology.
Nephrol Ther. 19:201–214. 2023.In French. View Article : Google Scholar : PubMed/NCBI
|
|
134
|
Kim D, Rimer JD and Asplin JR:
Hydroxycitrate: A potential new therapy for calcium urolithiasis.
Urolithiasis. 47:311–320. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
135
|
Lunyera J, Diamantidis CJ, Bosworth HB,
Patel UD, Bain J, Muehlbauer MJ, Ilkayeva O, Nguyen M, Sharma B, Ma
JZ, et al: Urine tricarboxylic acid cycle signatures of early-stage
diabetic kidney disease. Metabolomics. 18:52021. View Article : Google Scholar : PubMed/NCBI
|
|
136
|
Chen L, Min J and Wang F: Copper
homeostasis and cuproptosis in health and disease. Signal Transduct
Target Ther. 7:3782022. View Article : Google Scholar : PubMed/NCBI
|
|
137
|
Humphries F, Shmuel-Galia L,
Ketelut-Carneiro N, Li S, Wang B, Nemmara VV, Wilson R, Jiang Z,
Khalighinejad F, Muneeruddin K, et al: Succination inactivates
gasdermin D and blocks pyroptosis. Science. 369:1633–1637. 2020.
View Article : Google Scholar : PubMed/NCBI
|
|
138
|
Zhang XZ, Lei XX, Jiang YL, Zhao LM, Zou
CY, Bai YJ, Li YX, Wang R, Li QJ, Chen QZ, et al: Application of
metabolomics in urolithiasis: The discovery and usage of succinate.
Signal Transduct Target Ther. 8:412023. View Article : Google Scholar : PubMed/NCBI
|
|
139
|
Sun K, Zhi Y, Ren W, Li S, Zhou X, Gao L
and Zhi K: The mitochondrial regulation in ferroptosis signaling
pathway and its potential strategies for cancer. Biomed
Pharmacother. 169:1158922023. View Article : Google Scholar : PubMed/NCBI
|
|
140
|
Duan X, Zhang T, Ou L, Kong Z, Wu W and
Zeng G: 1H NMR-based metabolomic study of metabolic
profiling for the urine of kidney stone patients. Urolithiasis.
48:27–35. 2020. View Article : Google Scholar
|
|
141
|
Hernandez Y, Costa-Bauza A, Calvó P,
Benejam J, Sanchis P and Grases F: Comparison of two dietary
supplements for treatment of uric acid renal lithiasis: Citrate vs
Citrate + theobromine. Nutrients. 12:20122020. View Article : Google Scholar
|
|
142
|
Eisner BH, Asplin JR, Goldfarb DS, Ahmad A
and Stoller ML: Citrate, malate and alkali content in commonly
consumed diet sodas: Implications for nephrolithiasis treatment. J
Urol. 183:2419–2423. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
143
|
Zhu W, Liu Y, Lan Y, Li X, Luo L, Duan X,
Lei M, Liu G, Yang Z, Mai X, et al: Dietary vinegar prevents kidney
stone recurrence via epigenetic regulations. EBioMedicine.
45:231–250. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
144
|
Zhang JY, Zhou B, Sun RY, Ai YL, Cheng K,
Li FN, Wang BR, Liu FJ, Jiang ZH, Wang WJ, et al: The metabolite
α-KG induces GSDMC-dependent pyroptosis through death receptor
6-activated caspase-8. Cell Res. 31:980–997. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
145
|
Kumar P, Saini K, Saini V and Mitchell T:
Oxalate alters cellular bioenergetics, redox homeostasis,
antibacterial response, and immune response in macrophages. Front
Immunol. 12:6948652021. View Article : Google Scholar : PubMed/NCBI
|
|
146
|
Cai W, Wannemuehler Y, Dell'anna G,
Nicholson B, Barbieri NL, Kariyawasam S, Feng Y, Logue CM, Nolan LK
and Li G: A novel two-component signaling system facilitates
uropathogenic Escherichia coli's ability to exploit abundant host
metabolites. PLoS Pathog. 9:e10034282013. View Article : Google Scholar : PubMed/NCBI
|
|
147
|
Yu H, Gan D, Luo Z, Yang Q, An D, Zhang H,
Hu Y, Ma Z, Zeng Q, Xu D and Ren H: α-Ketoglutarate improves
cardiac insufficiency through NAD+-SIRT1
signaling-mediated mitophagy and ferroptosis in pressure
overload-induced mice. Mol Med. 30:152024. View Article : Google Scholar
|