Introduction
Esophageal cancer (EC) is one of the most common and
lethal malignant tumors of the digestive tract (1). It is characterized by aggressive
metastasis, which leads to a poor prognosis and low 5-year survival
rates (2). Esophageal squamous
cell carcinoma (ESCC) is a common histopathological subtype
worldwide and is estimated to account for 90% of EC cases in China
(3-4). According to the specific stage of
the disease, treatment methods primarily include surgery,
chemotherapy and radiotherapy. However, despite thorough
investigations by the research community, the prognosis of the
disease has not improved markedly in the past 20 years and the
disease currently has a 5-year survival rate of <20% (5-6).
Thus far, combined chemotherapy before ESCC surgery
has proven to be beneficial (7-9).
Cisplatin (DDP) and paclitaxel (PTX) are promising
chemotherapeutics that are increasingly being used in ESCC
treatment (10). However, a
large proportion of patients do not respond positively to
chemotherapy and exhibit severe side effects (i.e. bone marrow
suppression, allergic reactions, nausea and vomiting and
cardiotoxicity), which could potentially lead to resistance and
recurrence (11). Therefore, it
is necessary to screen appropriate combination reagents to address
issues of drug resistance and enhance the sensitivity of ESCC to
anticancer therapy.
Multi-drug resistance (MDR) is a key factor
responsible for the failure of tumor chemotherapy, usually because
the increase in drug outflow leads to a decrease in drug
accumulation in tumor cells (12). Luteolin, as one of the most
common flavonoid compounds, is widely present in a number of
plants, such as mint, rosemary, thyme, pine and ferns (13). A previous published article have
shown that luteolin sensitizes DDP-resistant ovarian cell lines and
xenograft models by inducing apoptosis and inhibiting cell
migration and invasion (14).
Luteolin also increases the sensitivity of cells by inhibiting the
Nrf2 pathway in oxaliplatin-resistant colorectal cancer cells
(15). Another study reported
that luteolin enhances the accumulation of p53 and promotes the
therapeutic activity of cisplatin in ovarian cancer cells in a
mouse xenograft model in vivo (16). These results indicate the
potential chemosensitivity of various cancer cells to luteolin and
the applicability of luteolin as an adjuvant in the regulation of
drug sensitivity. However, only a few reports have been published
on whether luteolin can increase chemotherapeutic sensitivity in
PTX-resistant ESCC and the potential mechanism involved. This topic
warrants further research.
The current study investigated the bio-functions of
luteolin in the PTX-resistant esophageal squamous cell line EC1/PTX
and the chemosensitizing effects of luteolin combined with PTX
EC1/PTX in vitro and in vivo. It also explored the
associated molecular mechanisms based on the findings.
Materials and methods
Cell lines and cell culture
ESCC cells (EC1) were obtained from the Cell Bank of
Type Culture Collection of The Chinese Academy of Sciences.
PTX-resistant cell lines EC1/PTX (17) were established by our group.
Cells from the two ESCC cell lines were cultured in RPMI-1640
(Gibco; Thermo Fisher Scientific, Inc.) supplemented with 10% fetal
bovine serum (FBS; Gibco; Thermo Fisher Scientific, Inc.). Cells
were incubated at 37°C in the presence of 5% CO2.
Cell counting kit-8 (CCK-8) assay
A total of 3×103 cells/ml were seeded in
96-well culture plates with five replicate wells in each group. PTX
(6 mg/ml) or luteolin (120 mmol/l) was used to treat the cells for
24 or 48 h. Subsequently, according to the manufacturer's
instructions, 10 μl of CCK-8 reagent (Dojindo Molecular
Technologies, Inc.) was added to each well and the cells were
incubated for another 3 h at 37°C. Cell viability was determined
based on the optical density at 450 nm measured using a microplate
reader (Victor1420; PerkinElmer, Inc.). The experiment was
performed at least three times and in triplicate.
Colony formation assay
A total of 2×103 cells/ml were seeded in
a six-well plate. In each well, 0.1% DMSO and 10, 20, or 40
μM luteolin were added. The cells were incubated at 37°C for
7 days. In accordance with the manufacturer's instructions, the
cells were fixed with 1 ml of 4% paraformaldehyde for 30 min and
stained with 1% crystal violet for 30 min at room temperature. The
number of colonies formed was then determined. Experiments were
repeated at least three times.
Flow cytometry assay
For tests on apoptosis, an Annexin V-FITC apoptosis
detection kit (BD Biosciences) was used according to the
manufacturer's instructions. EC1/PTX cells were treated with
luteolin at different concentrations (10, 20, or 40 μM) for
24 h. Further, cells were stained with 5 μl of Annexin-FITC
and 10 μl of PI and kept for 15 min in the dark at room
temperature. FlowJo 7.6 (FlowJo LLC) software was used to analyze
data. Early apoptotic cells (Annexin V-positive and PI-negative)
and late apoptotic cells (Annexin V-positive and PI-positive) were
both included while calculating the apoptosis rate. Experiments
were performed in triplicate. Iced PBS was used to wash the cells
in the cell cycle distribution assay. Following this, the cells
were fixed in ethanol at −20°C. This was followed by re-suspension
of the cells in PBS and treatment with 40 μg/ml PI, 0.1
mg/ml RNase A and 0.1% Triton X-100 for 30 min in a dark room at
37°C. Then, the cells were analyzed using flow cytometry.
Wound healing
The wound healing assay was conducted in accordance
with a previously published method (18) with minor modifications. Briefly,
ESCC cells adherent in 35 mm dishes were scratched with 10
μl pipette tips and washed twice with PBS. Following this,
0.1% DMSO and 10 μM or 20 μM luteolin were added to
each well. The cells were incubated overnight in serum-free medium
at 37°C in the presence of 5% CO2. ImageJ v1.8.0
software (National Institutes of Health) was used to calculate the
area of scratches and analyze cell mobility in the different
treatment groups.
Transwell assay
Serum-free Opti-Minimum Essential Medium (MEM;
Invitrogen; Thermo Fisher Scientific, Inc.) was used to resuspend
cells at a density of 3×104 cells/ml. Next, 50 μl
of Matrigel (Millipore Sigma) was spread in the chamber and 600
μl of 10% RPMI-1640 medium was added dropwise to the
basolateral chamber under 37°C within 1 h. The chambers were
immersed 4% paraformaldehyde for 30 min and stained with 1% crystal
violet for 1 h at room temperature. The number of stained cells was
counted using an inverted microscope.
Western blotting
RIPA lysis buffer (Cell Signaling Technology, Inc.)
was used to isolate total protein from the cells. A BCA protein
assay kit (Thermo Fisher Scientific, Inc.) was used to measure the
protein concentration according to the manufacturer's instructions.
Then, 50 μg of protein was separated using 10% sodium
dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) and
transferred to polyvinylidene fluoride membranes. The membranes
were subsequently blocked with 5% non-fat milk for 2 h at 37°C.
This was followed by overnight treatment with primary antibodies
against N-cadherin (1:5,000; cat. no. ab76011; Abcam), MMP-2
(1:1,000; cat. no. ab92536; Abcam), Snail (1:1,000; cat. no.
ab216347; Abcam), CD133 (1:2,000; cat. no. Ab222782; Abcam), CD44
(1:1,000; cat. no. ab243894; Abcam), sex determining region Y-box 2
(SOX-2) (1:1,000; cat. no. Ab92494; Abcam), AKT (1:1,000; cat. no.
ab8805; Abcam), phosphorylated (p-)AKT (1:5,000; cat. no. ab81283;
Abcam), Src (1:1,000; cat. no. 2109; Cell Signaling Technology),
p-Src (1:1,000; cat. no. 59548; Cell Signaling Technology),
epidermal growth factor receptor-2 (ErbB2) (1:1,000; cat. no.
ab134182; Abcam), focal adhesion kinase (FAK) (1:2,000; cat. no.
ab40794; Abcam), p-FAK (1:1,000; cat. no. 81298; Abcam), multidrug
resistance protein 1 (MRP1) (1:1,000; cat. no. ab260038; Abcam),
breast cancer resistance protein (BCRP) (1:1,000; cat. no.
ab207732; Abcam), P-gp (1:2,000; cat. no. ab170904; Abcam) and
β-actin (1:5,000; cat. no. ab6276; Abcam) at 4°C. The membranes
were then treated with secondary antibodies Goat Anti-Rabbit IgG
H&L (HRP) (1:10,000; cat. no. ab6721; Abcam) or Goat Anti-Mouse
IgG H&L (HRP) (1:10,000; cat. no. ab6789; Abcam) for 2 h at
room temperature. Protein signals were recorded using a
chemiluminescence (ECL) detection system (Solarbia S&T Co.,
Ltd.). The resulting images were assessed using an imaging system
(Bio-Rad Laboratories, Inc.) and analyzed by a densitometric
analysis software (Image Lab v3.0; Bio-Rad Laboratories, Inc.).
Sphere-formation assay
Single-cell suspensions were plated in ultralow
attachment six-well plates (Corning, Inc.) at 5×103
cells/ml and cultured in modified DMEM without serum
supplementation, as described in the Cell lines and cell
culture subsection. Media were replaced every 3 days. Cell
spheres in each group were observed after 7 days.
ELISA
The levels of enzymes were evaluated using
standardized commercially available kits. The following ELISA assay
kit were purchased from Shanghai Yaji Biological Technology Co.,
Ltd.: VEGFR1 (cat. no. YS01065B), VEGFR2 (cat. no. YS06892B),
VEGFR3 (cat. no. YS06893B), and C-Kit (cat. no. YS02312B). The EGFR
ELISA assay kit (cat. no. H032) was bought from Nanjing Jiancheng
Bioengineering institute. The following kits and antibodies were
purchased from Merck KGaA: RET (cat. no. RAB0987), Flt-3 (cat. no.
RAB1464), ErbB2 (cat. no. RAB0173k), ErbB4 (cat. no. RAB1041), FAK
(cat. no. RAB095), FGFR1 (cat. no. RAB0961), FGFR2 (cat. no.
RAB0962). PDGFR-α antibody (cat. no. SAB4502139), PDGFR-β antibody
(cat. no. SAB4502148), SRC antibody (cat. no. SAB4502846), FGFR3
antibody (cat. no. SAB4500888) and FGFR4 antibody (cat. no.
SAB1300137).
Molecular docking
AutoDock Vina v1.1.2 (http://vina.scripps.edu/) was used to determine
ligands and the proteins required for molecular docking. The
crystal structures of target proteins were obtained from the PDB
database (https://www.rcsb.org/). The process
involved a pre-treatment step, including hydrogen removal, amino
acid modification, energy optimization and force field parameter
adjustment. Subsequently, the retrieved ligand structure of
luteolin (https://pubchem.ncbi.nlm.nih.gov/) was docked with the
active sites of target proteins using the vina built-in pyrx
software (https://pyrx.sourceforge.io/), with the affinity
(kcal/mol) representing the combining capacity. Lower the affinity
value, the more stable the ligand binding to the receptor. Visual
analysis was performed using PyMOL (https://pymol.org/2/) and the two-dimensional figures
were visualized using the Discovery Studio 2020 Client (https://discover.3ds.com/discovery-studio-visualizer-download).
Tumor xenografts in nude mice
A total of 25 female 4-6 weeks old athymic BALB/c
nude mice (15-20 g) were obtained from Beijing Vital River
Laboratory Animal Technology Co., Ltd. The animals were previously
administered conventional feed, provided with ad libitum
access to water and housed (temperature, 20-25°C; humidity, 40-60%;
12-h light/dark cycle). In this research, mice was divided into
four groups (NC, LUT, PTX and PTX + LUT) and anesthetized with 1%
pentobarbital sodium. The mice in each group were injected with
2×106 (200 μl) transfected EC1/PTX cells
subcutaneously in the dorsal surface of the right hind leg to
establish xenografts as the NC group. After inoculation, the mice
were injected intraperitoneally with luteolin (20 or 40 mg/kg every
day) in the LUT group, PTX (10 mg/kg every 3 days) in the PTX group
and both drugs in the PTX + LUT group. The tumor size and weight
were recorded. After 22 days, the mice were sacrificed by cervical
dislocation and the tumor tissues were resected, imaged, weighed
and measured.
Hematoxylin and eosin (H&E)
staining
The tumor tissues were excised, immersed immediately
in 10% formaldehyde and maintained at room temperature for 24 h.
The tissues were dehydrated in 70, 80, 90, 95% alcohol for 30 min
respectively, and then in anhydrous alcohol for 60 min for thorough
dehydration. The tissues were embedded in pure paraffin and cut
into 4 μm sections. The dehydrated tissue blocks were placed
in xylene for 60 min for transparent treatment to facilitate
paraffin penetration. The whole morphology was evaluated after
H&E. staining at room temperature for 24 h. The stained
sections were observed under an optical microscope (BX60; Olympus
Corporation). Images were acquired and analysis was performed using
ImageJ v1.8.0 Launcher (National Institutes of Health).
TUNEL staining
TUNEL staining was performed to assess apoptosis in
tumor tissues at 37°C within 30 min. Tissue slices were stained
using an in-situ cell death detection kit (Roche
Diagnostics) according to the manufacturer's instructions. The
percentage of TUNEL-positive nuclei (green nuclei) was
calculated.
Kyoto Encyclopedia of Genes and Genomes
(KEGG) and Gene Ontology (GO) analysis
RStudio v3.6.1 (https://cloud.r-project.org/) was used to visualize GO
terms and KEGG pathways. P<0.05 was set as the cutoff criterion
for significant enrichment.
Statistical analysis
Statistical analysis was performed using the SPSS
21.0 software (IBM Corp.). Data from the experimental results are
expressed as mean ± standard deviation (SD). The data were
statistically analyzed using Independent samples t-test in 2 groups
and one-way analysis of variance (ANOVA) followed by Tukey's post
hoc test was used for ≥3 groups. P<0.05 was considered to
indicate a statistically significant difference.
Results
The inhibitory effects of PTX on the
proliferation of EC1 and EC1/PTX cells
The present study successfully established
PTX-resistant human esophageal squamous cell carcinoma cells
(EC1/PTX). As shown in Fig. 1A,
the IC50 values of PTX in EC1 cells were 0.053±0.001 and
0.030±0.001 μg/ml at 24 and 48 h after PTX treatment,
respectively. Meanwhile, the IC50 values of PTX in
EC1/PTX cells were 0.914±0.044 and 0.540±0.014 μg/ml
(Fig. 1B), respectively. The
aforementioned results indicated that PTX exerted a certain
inhibitory effect on the proliferation of parental and resistant
cells. The resistance indexes (RIs) were 17.387 at 24 h and 17.834
at 48 h, respectively (Table
I).
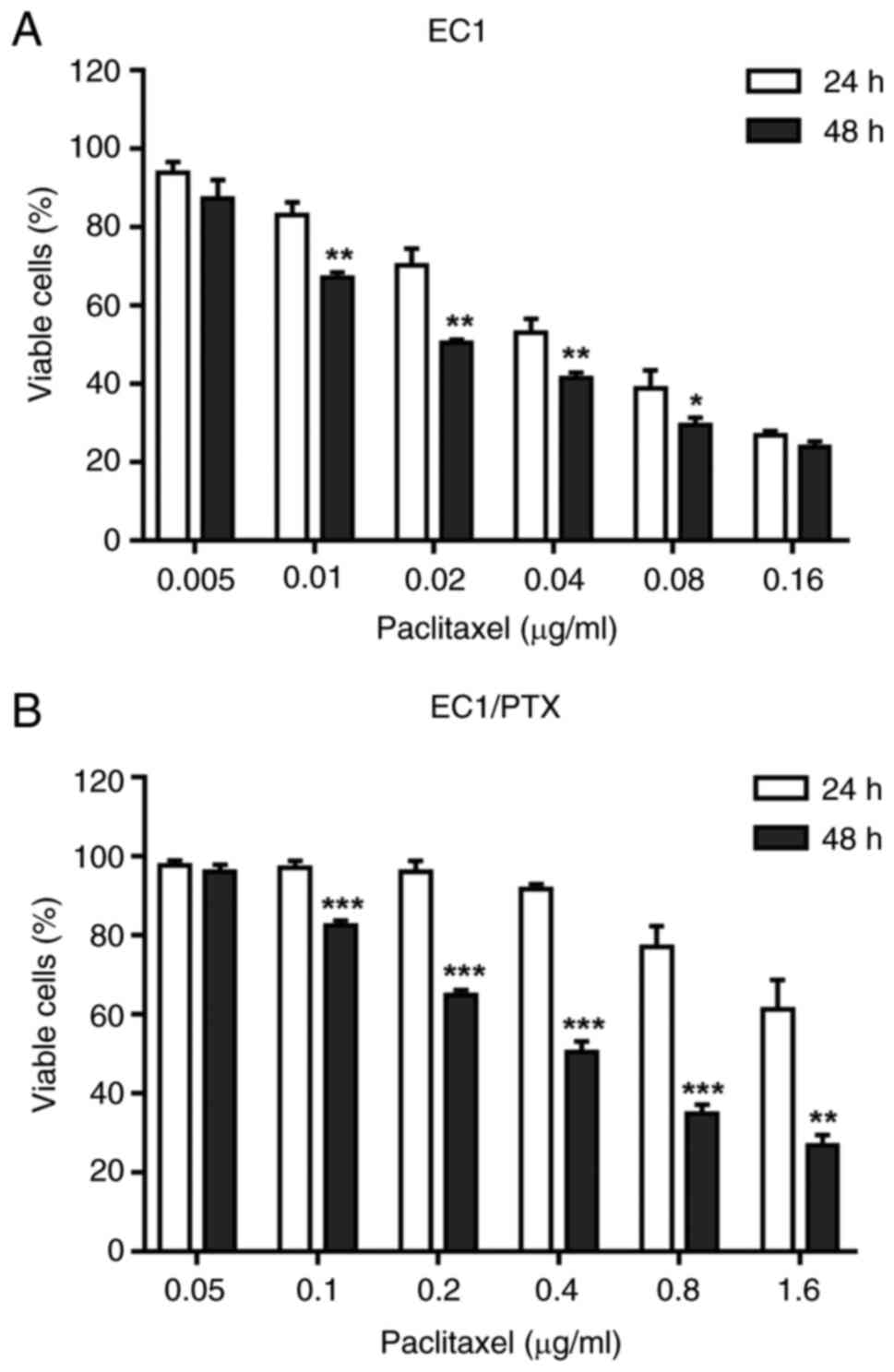 | Figure 1The effects of PTX on the
proliferation of EC1 and EC1/PTX cells. (A) The inhibitory effects
of PTX at different concentrations (0.005, 0.01, 0.02, 0.04, 0.08,
0.16 μg/ml) on the proliferation of EC1 cells. (B) The
inhibitory effects of PTX at different concentrations (0.05, 0.1,
0.2, 0.4, 0.8, 0.16 μg/ml) on the proliferation of EC1/PTX
cells. *P<0.05, **P<0.01, ***P<0.001
vs. DMSO. PTX, paclitaxel. |
 | Table IPTX for IC50 value in
EC1and EC1/PTX cells. |
Table I
PTX for IC50 value in
EC1and EC1/PTX cells.
| Time | PTX/IC50
(mean ± SD, μg/ml)
| RI | P-value |
|---|
| EC1 | EC1/PTX |
|---|
| 24 h | 0.053±0.001 | 0.914±0.044 | 17.387 | <0.01 |
| 48 h | 0.030±0.001 | 0.540±0.014 | 17.834 | <0.001 |
Luteolin inhibits proliferation but
induced cell cycle arrest and apoptosis in PTX-resistant ESCC
cells
The present study used luteolin at different
concentrations (10, 20, 40, 80 and 120 μM) to treat parental
cells (EC1) and PTX-resistant ESCC cells (EC1/PTX) and calculated
the IC50 values of luteolin while determining the cell
survival rate. The IC50 values of luteolin in EC1 cells
were 61.692±1.048 and 29.694±0.997 μM after treatment for 24
and 48 h, respectively. Meanwhile, in the EC1/PTX cells, the
IC50 values were 64.875±1.447 and 32.457±1.104
μM, respectively. Notably, the viability of EC1 and EC1/PTX
cells was inhibited by luteolin in a time- and dose-dependent
manner (Fig. 2A and Table II).
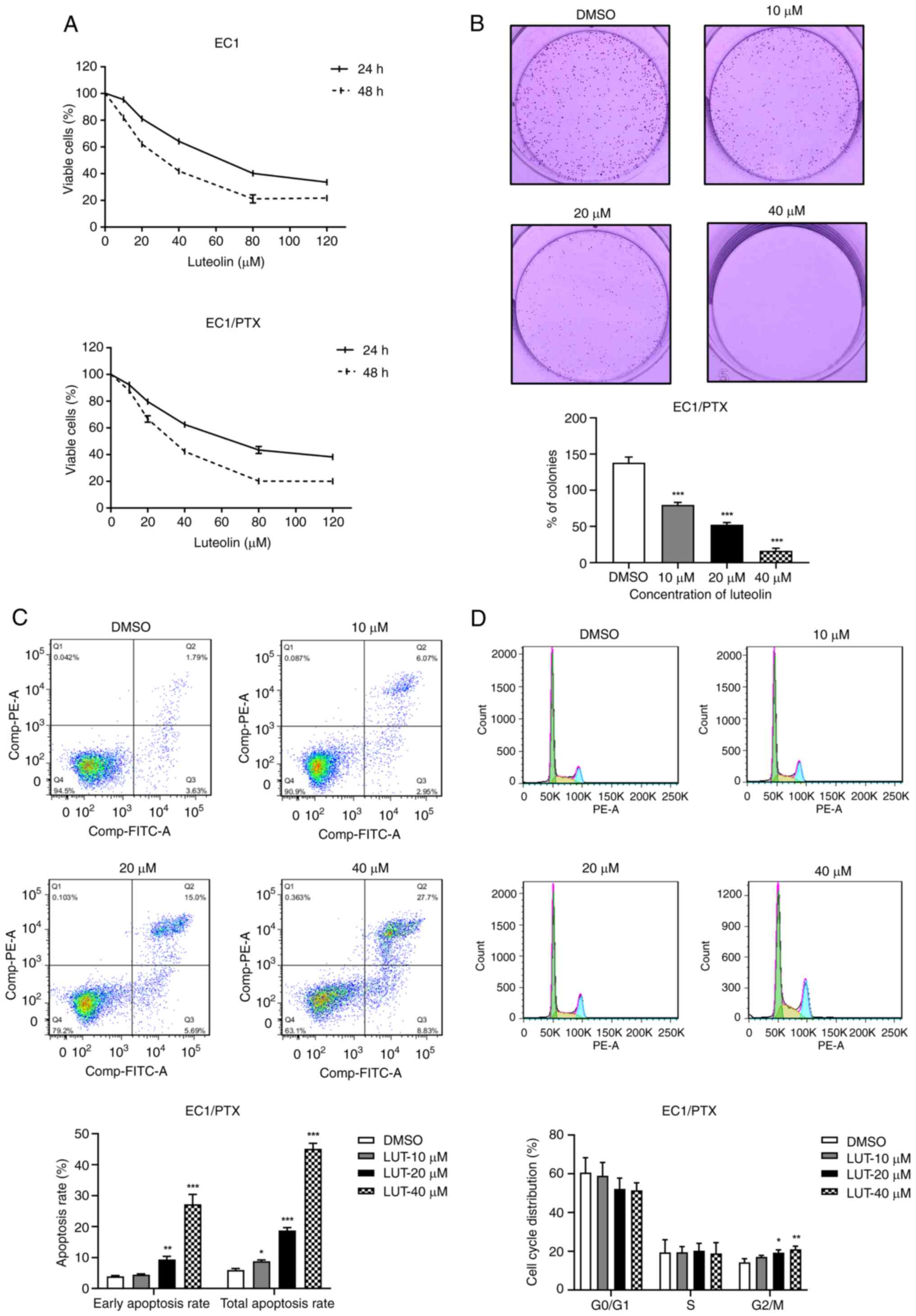 | Figure 2Effects of luteolin on cell
proliferation, cell cycle and apoptosis in PTX-resistant ESCC
cells. (A) The inhibitory effects of luteolin at different
concentrations (10, 20, 40, 80 and 120 μM) on the
proliferation of EC1 cells and EC1/PTX drug-resistant cells. (B)
Luteolin (10, 20 and 40 μM) exerted its effects on EC1/PTX
cells. After 7 days, the cells were stained with 0.1% crystal
violet solution and clone proliferation was analyzed. (C) Luteolin
(10, 20 and 40 μM) acted on EC1/PTX cells for 48 h. The
apoptosis rate of cells in different treatment groups was
determined by flow cytometry after Annexin-FITC/PI staining. (D)
Luteolin (10, 20 and 40 μM) was used to treat EC1/PTX cells
for 24 h and cell cycle distribution was determined by flow
cytometry after PI staining. *P<0.05,
**P<0.01, ***P<0.001 vs. DMSO n=3. PTX,
paclitaxel; ESCC, esophageal squamous cell carcinoma. |
 | Table IILuteolin for IC50 value in
EC1and EC1/PTX cells. |
Table II
Luteolin for IC50 value in
EC1and EC1/PTX cells.
| Cell lines | LUT/IC50
(mean ± SD, mmol/l)
|
|---|
| 24 h | 48 h |
|---|
| EC1 | 61.692±1.048 | 29.694±0.997 |
| EC1/PTX | 64.875±1.447 | 32.457±1.104 |
To detect luteolin-induced changes in the biological
function of PTX-resistant cells, 10, 20 and 40 μM luteolin
was used to treat EC1/PTX cells. Cloning and flow cytometry assays
were used to assess clone formation, cell cycle progression and
apoptosis. As shown in Fig. 2B,
luteolin (10, 20 and 40 μM) could significantly inhibit
colony formation in a dose-dependent manner. The clonogenic
capacities were 138.55±6.32% in the DMSO group, 80.01±2.92% in the
10 μM luteolin group, 52.76±2.54% in the 20 μM
luteolin group and 16.71±3.34% in the 40 μM luteolin group.
In addition, compared to that in the DMSO group, luteolin treatment
significantly increased the total apoptosis rate (including that of
cells in early and late apoptosis) in a dose-dependent manner
(Fig. 2C) and induced cell cycle
distribution in the G2/M phase (Fig. 2D).
Luteolin suppresses the migration,
invasion, epithelialmesenchymal transition (EMT) and dry
spheroidization of PTX-resistant ESCC cells
The present study used 10 and 20 μM luteolin
to treat EC1/PTX cells and examined the migration and invasion
potential of resistant cells in the wound healing and Transwell
assays, individually. The cell migration rate (Fig. 3A) and number of transmembrane
cells (Fig. 3B) in the luteolin
administration group were significantly lesser than those in the
DMSO group. TGF-β1 is widely used to induce EMT in cells. The
present study co-treated the cells with 10 ng/ml TGF-β1 and 10 or
20 μM luteolin and determined the expression level of
EMT-related proteins in the cells by western blotting. TGF-β1
induced the expression of Snail, N-cadherin and MMP-2 proteins,
which was downregulated upon luteolin treatment (Fig. 3C). Subsequently, a cell
microsphere formation experiment was conducted to assess the
spheroidization of cells and expression for stem cell markers.
Compared with luteolin-treated cells, which formed smaller
microspheres and had more debris, DMSO-treated cells formed larger
microspheres and exhibited more rapid proliferation (Fig. 3D). Besides, the expression of
stem cell markers (SOX-2, CD44 and CD133) was significantly
downregulated upon luteolin treatment (Fig. 3E).
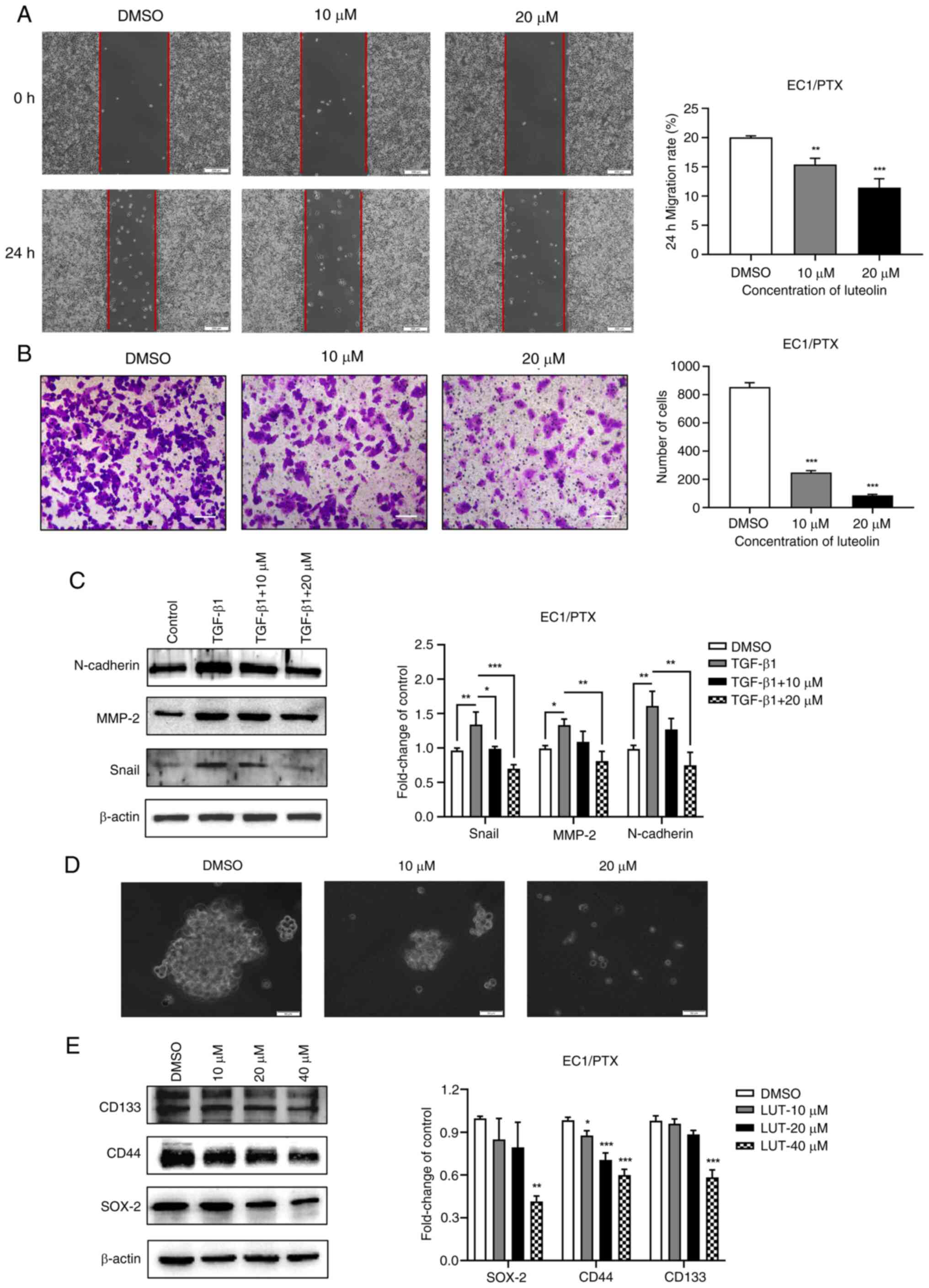 | Figure 3Effects of luteolin on the migration,
invasion, EMT and dry spheroidization of PTX-resistant ESCC cells.
(A) Wound healing assay was used to evaluate the inhibitory effect
of luteolin on the migration potential of EC1/PTX cells
(magnification, ×200). (B) A Transwell chamber was used to
determine the inhibitory effect of luteolin on the invasion
potential of EC1/PTX cells (magnification, ×100). (C) TGF-β1 was
used to induce EMT in EC1/PTX cells and the cells were treated with
10 or 20 μM luteolin. The expression levels of EMT-related
proteins were determined by western blotting. (D) A cell
microsphere formation test was performed to determine the effect of
luteolin on the spheroidizing potential of EC1/PTX cells
(magnification, ×50). (E) After EC1/PTX cells were treated with
luteolin at different concentrations (10, 20 and 40 μM) for
24 h, western blotting was performed to determine the expression
levels of stem cell markers in the cells. *P<0.05,
**P<0.01, ***P<0.001 vs. DMSO n=3. EMT,
epithelial-mesenchymal transition; PTX, paclitaxel; ESCC,
esophageal squamous cell carcinoma. |
Luteolin enhances drug sensitivity and,
combined with PTX, facilitates apoptosis in drug-resistant ESCC
cells
Luteolin (10, 20 and 40 μM) was used to treat
EC1/PTX cells for 24 h. At concentrations of 20 and 40 μM,
luteolin significantly reduced cell growth (Fig. 4A). Based on its low cytotoxicity,
10 μM luteolin was selected for subsequent experiments. The
IC50 value of PTX in EC1/PTX cells decreased from
0.560±0.026 to 0.366±0.007 μM upon luteolin pretreatment, a
reduction by 1.5 folds (Fig.
4B). Next, 10 μM luteolin and 0.2 μg/ml PTX was
used individually and in combination to treat EC1/PTX cells. Cells
were then examined by flow cytometry following Annexin-FITC/PI
staining. The total apoptosis rates were 5.50±0.58, 10.05±1.11,
16.23±0.16 and 24.00±1.04% in the Control, LUT, PTX and PTX + LUT
groups, respectively. Compared to that in the LUT group or PTX
group, the total apoptosis rate in the PTX + LUT group was
significantly higher (Fig.
4C).
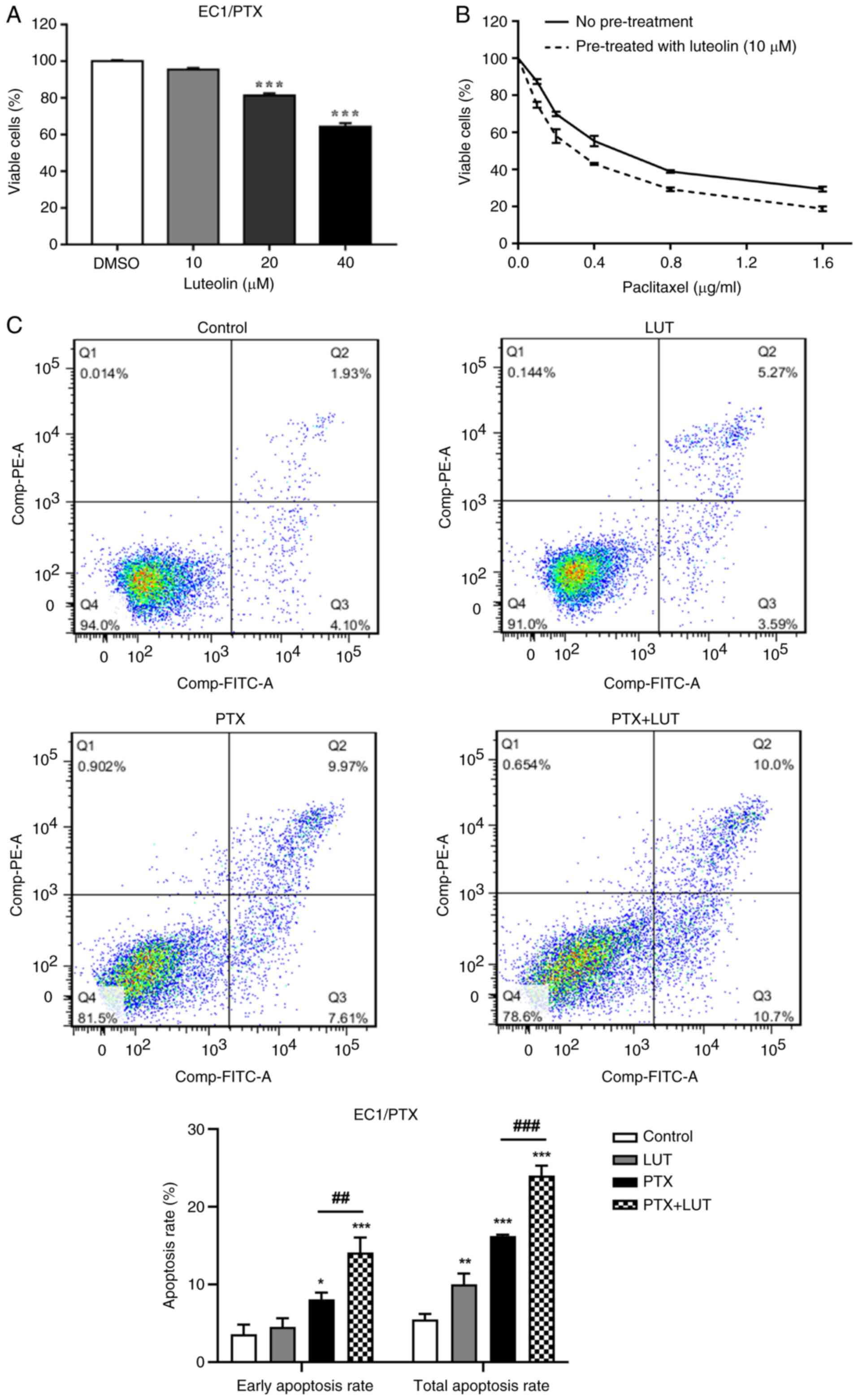 | Figure 4A combination of luteolin and PTX
increased drug sensitivity and apoptosis in PTX-resistant ESCC. (A)
The survival rate of EC1/PTX cells after 24 h of treatment with
luteolin (10, 20 and 40 μM). (B) The effects of luteolin on
the PTX sensitivity of EC1/PTX cells. ***P<0.001 vs.
DMSO. (C) Luteolin, PTX and luteolin plus PTX were used to treat
EC1/PTX cells for 48 h. After Annexin-FITC/PI staining, the
apoptosis rate of cells in the different treatment groups was
determined by flow cytometry. *P<0.05,
**P<0.01, ***P<0.001 vs. Control,
##P<0.01, ###P<0.001 vs. PTX, n=3. PTX,
paclitaxel; ESCC, esophageal squamous cell carcinoma. |
The PI3K/Akt signaling pathway may be
involved in PTX-resistant ESCC
To explore the mechanism underlying the reversal of
drug resistance in EC1/PTX cells by luteolin, whole-transcriptome
next-generation sequencing was performed on parental and
drug-resistant cells to screen RNA exhibiting differentiated
expression. High-throughput sequencing analysis was performed to
obtain the Volcano plot and heatmap. These plots showed the
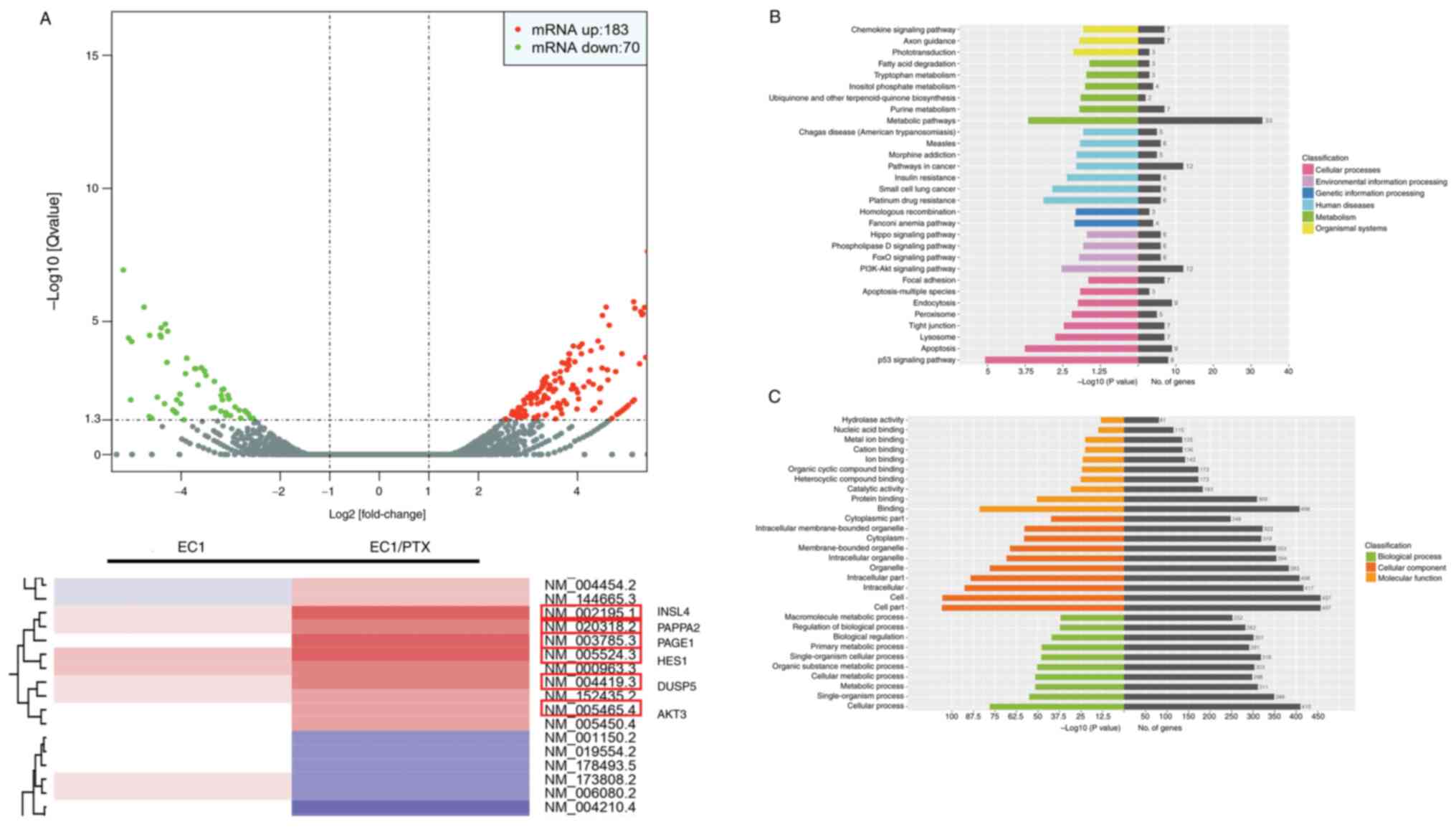
differential expression of mRNAs. Compared to the parent cells,
PTX-resistant ESCC cells showed 253 abnormally expressed mRNA
molecules, of which 183 were upregulated, whereas 70 were
downregulated. Among these, AKT3, INSL4, PAPPA2, PAGE1, HES1 and
DUSP5 mRNAs showed significant upregulation, which was accompanied
by a multiple of difference log2 (fold change) >3.00
(Fig. 5A). This suggested that
AKT3, as a key molecule in the PI3K/Akt signaling pathway, may be
significantly upregulated in PTX-resistant ESCC cells. Following
this, Kyoto Encyclopedia of Genes and Genomes biological pathway
enrichment analysis was performed using the differentially
expressed genes. The results showed that certain signaling
pathways, including the PI3K/Akt, p53, apoptosis and
metabolism-related pathways, among others, were closely associated
with drug resistance in ESCC cells (Fig. 5B). Furthermore, the Gene Ontology
enrichment analysis results showed that the changes (signal
transduction, apoptosis, cell metabolism and other functions) were
significantly associated with PTX resistance (Fig. 5C). This finding suggested that
the PI3K/Akt signaling pathway may be involved in PTX resistance in
ESCC cells. Subsequently, the inhibitory activity of luteolin on
tyrosine kinases was screened using ELISA. Each tyrosine
kinase-specific inhibitor was used as a control. Luteolin inhibited
the activities of various tyrosine kinases, which included FAK,
ErbB2 and Src kinase. The rates of inhibition of FAK, Src and ErbB2
activities upon treatment with 1,000 nM luteolin were 76.1±9.7,
57.4±18.3 and 100.0±0.0%, respectively (Table III). Thus, luteolin can inhibit
key kinases in the FAK/Src/PI3K/Akt signaling pathway.
 | Table IIIInhibition rate of tyrosine kinase
activity (%). |
Table III
Inhibition rate of tyrosine kinase
activity (%).
| Kinases |
Inhibition
rate of different concentration (nM) compounds on tyrosine kinase
activity (%)
|
|---|
Luteolin
| SU11248
| BIBW2992
| Dasatinib
| PF562271
| PF562271
|
|---|
| 1,000 | SD | 100 | SD | 1,000 | SD | 1,000 | SD | 1,000 | SD | 1,000 | SD | 1,000 | SD |
|---|
| VEGFR-1 | 81.4 | 5.1 | 18.8 | 13.2 | 90.8 | 3.8 | | | | | | | | |
| VEGFR-2 | 70.9 | 6.8 | 31.1 | 4.1 | 95.1 | 0.9 | | | | | | | | |
| VEGFR-3 | 99.9 | 0.2 | 37.8 | 8.0 | 99.9 | 0.1 | | | | | | | | |
| PDGFR-α | 57.1 | 15.3 | 65.5 | 11.7 | 92.7 | 4.1 | | | | | | | | |
| PDGFR-β | 73.4 | 4.2 | 43.5 | 6.2 | 92.2 | 1.9 | | | | | | | | |
| RET | 99.9 | 0.2 | 45.7 | 7.4 | 99.8 | 0.3 | | | | | | | | |
| C-Kit | 99.7 | 0.5 | 63.1 | 2.6 | 99.7 | 0.7 | | | | | | | | |
| Flt-3 | 91.2 | 6.0 | 56.9 | 15.3 | 99.9 | 0.2 | | | | | | | | |
| EGFR | 84.9 | 3.6 | 25.6 | 17.2 | | | 100.0 | 0.1 | | | | | | |
| ErbB2 | 100.0 | 0.0 | 62.0 | 21.0 | | | 97.1 | 5.9 | | | | | | |
| ErbB4 | 91.2 | 2.1 | 33.0 | 7.1 | | | 100.0 | 0.0 | | | | | | |
| Src | 57.4 | 18.3 | 2.1 | 2.5 | | | | | 99.8 | 0.3 | | | | |
| FAK | 76.1 | 9.7 | 42.4 | 20.4 | | | | | | | 99.2 | 0.8 | | |
| FGFR1 | 94.6 | 6.3 | 35.0 | 11.8 | | | | | | | | | 100.0 | 0.0 |
| FGFR2 | 86.5 | 4.6 | 72.1 | 6.5 | | | | | | | | | 100.0 | 0.0 |
| FGFR3 | 82.0 | 8.7 | 8.6 | 14.6 | | | | | | | | | 100.0 | 0.0 |
| FGFR4 | 77.5 | 1.3 | 46.3 | 8.5 | | | | | | | | | 98.4 | 0.5 |
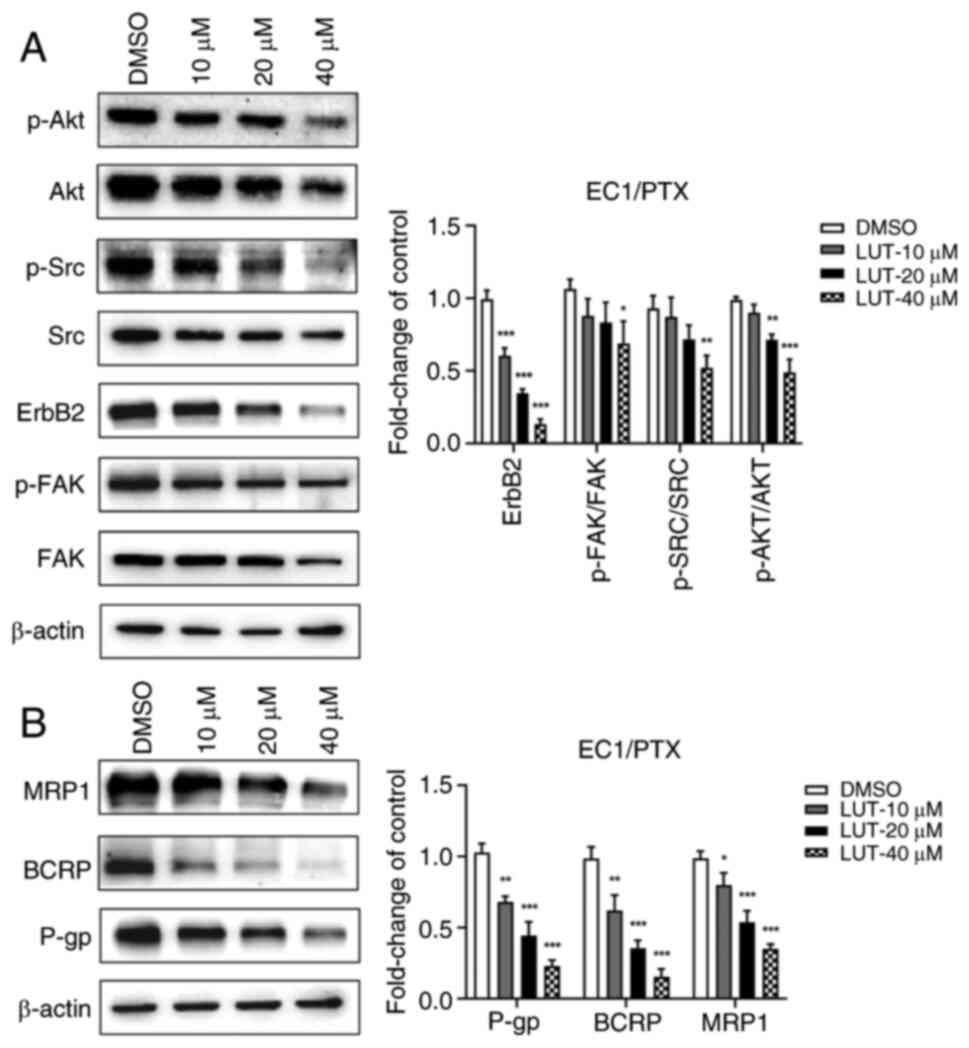
Luteolin reduces the expression of
associated proteins of the FAK/Src/PI3K/Akt pathway and
resistance-related proteins
To evaluate whether luteolin could reverse
PTX-induced resistance by regulating the PI3K/Akt signaling
pathway, EC1/PTX cells were treated with 10, 20 and 40 μM
luteolin and the expression of associated proteins in the
FAK/Src/PI3K/Akt pathway and resistance-related proteins was
measured. Compared to that in the DMSO group, the expression of
p-FAK (Tyr397)/FAK, ErbB2, p-Src (Tyr416)/Src and p-Akt
(Ser473)/Akt was significantly downregulated in the
luteolin-treated group (Fig.
6A). A similar tendency was observed in the expression of the
drug-resistance-related proteins P-gp, BCRP and MRP1 (Fig. 6B).
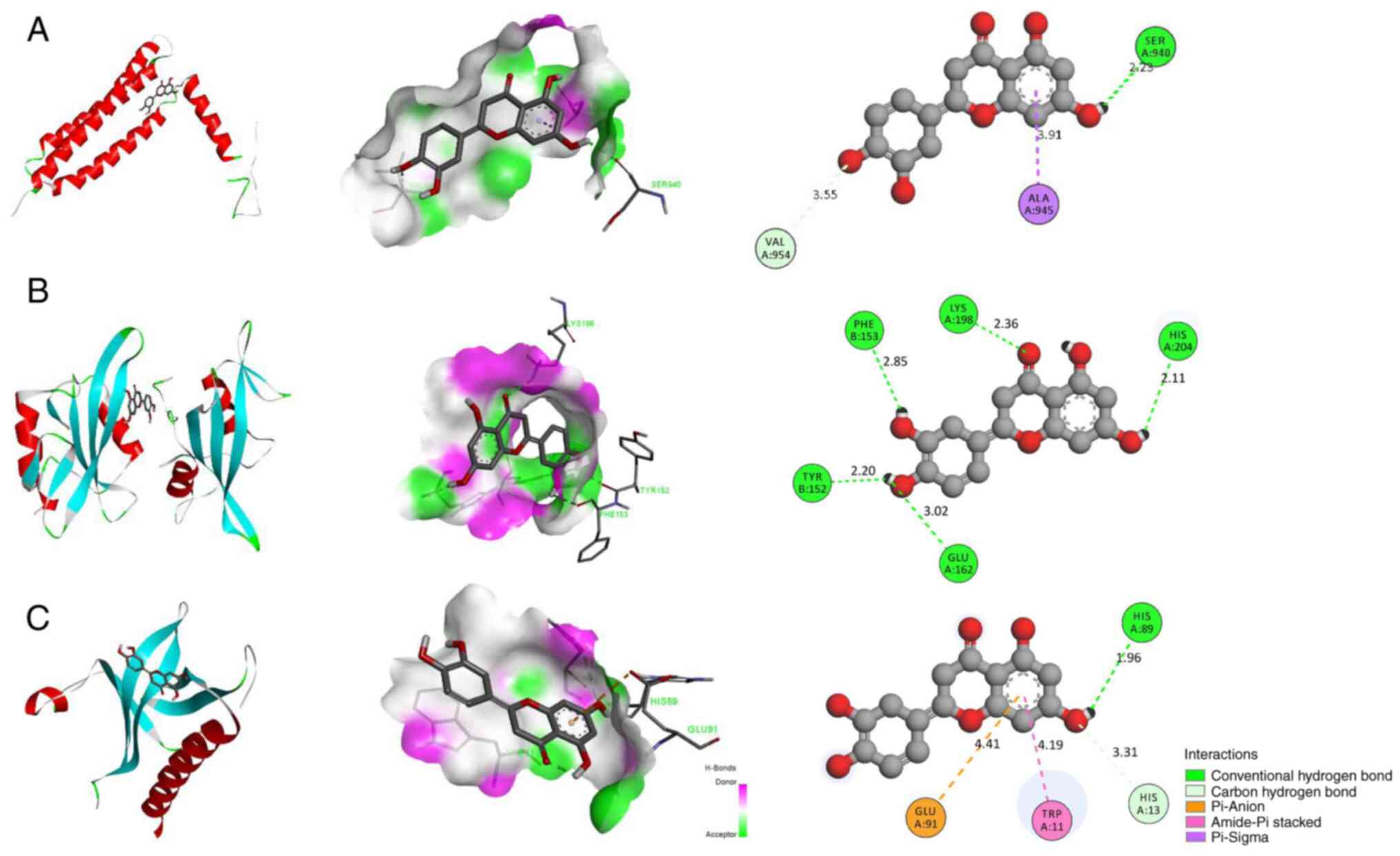
Luteolin can bind to the active sites of
FAK, SRC and AKT
Luteolin was linked to the active sites of FAK, SRC
and AKT. Luteolin formed hydrogen bonds with THR152, GLU162,
HIS211, HISS204 and LYS198 in SRC (1A07), with a binding energy of
−7.7 kcal/mol (Fig. 7A).
Luteolin formed hydrogen bonds with HIS89 in AKT (1H10). Meanwhile,
it showed hydrophobic interactions with the residues HIS13, TRP11
and GLU91, with a binding energy of −6.2 kcal/mol (Fig. 7B). Luteolin formed hydrogen bonds
with SER940 in FAK (1K04) and formed hydrophobic bonds with ALA945
and VAL954, with a binding energy of −6.6 kcal/mol (Fig. 7C). The lower the binding energy,
the higher the docking effect. Collectively, the results indicated
that luteolin had a strong binding ability to the active sites of
FAK, SRC and AKT.
Luteolin combined with PTX inhibits
tumorigenesis in xenografts in nude mice in vivo
To explore the sensitization effects of luteolin
in vivo, a nude mouse xenograft model was developed using
EC1/PTX cells. Each group of cells was treated with 0.9% v/v NS, 20
mg/kg luteolin, 40 mg/kg luteolin, 10 mg/kg PTX and 10 mg/kg PTX +
20 mg/kg luteolin. As shown in Fig.
8A, the body weight of nude mice in each group increased
marginally during the treatment period, but there were no
significant differences in the body weights of mice between the
treatment and NC groups. The tumor volume in the NC group increased
rapidly, whereas the tumor growth rate in the luteolin group or PTX
group was relatively slow and the tumor growth rate in the PTX +
LUT-20 mg/kg group was the slowest (Fig. 8B). From the 16th to the 22nd day
of treatment, the tumor volume in the luteolin or PTX group was
significantly reduced compared with that in the NC group and the
tumor volume in the combination group was the lowest. After 22 days
of treatment, the average tumor mass in the PTX group and LUT-40
mg/kg group was significantly smaller than that in the NC group.
The value showed no significant difference from that in the LUT-20
mg/kg group and showed no obvious toxicity in vivo. Compared
with the two single-treatment groups, the combined treatment group
had a significantly lower average tumor mass (Fig. 8C-D). H&E staining showed that
tumor cells in the NC and LUT groups were uniformly stained and
arranged regularly and densely, exhibiting a clear clumping growth
trend. By contrast, tumor cells in the PTX and PTX + LUT groups had
an irregular morphology, with the nuclear chromatin being partially
dense and concentrated with nuclear pyknosis. The degree of
apoptosis in the PTX + LUT group was the highest among the four
groups (Fig. 8E). In addition,
the TUNEL-positive area with green fluorescence in the PTX + LUT
group was considerably larger than that in the PTX group (Fig. 8F). Thus, combined treatment with
luteolin and PTX led to a synergistic effect on the inhibition of
drug resistance in EC1 cells in vivo.
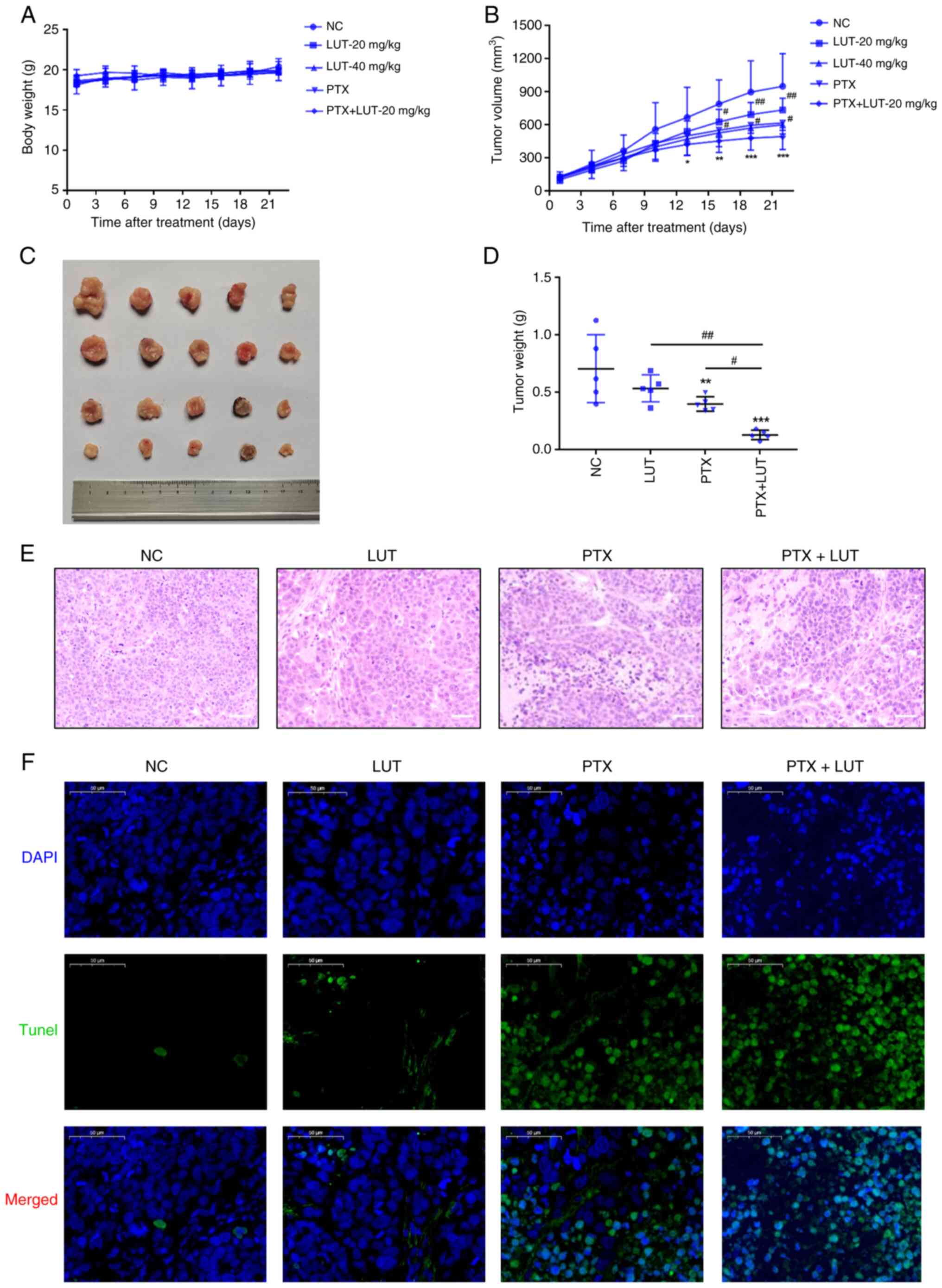 | Figure 8Effect of treatment with luteolin
combined with PTX on the xenografts in nude mice and apoptosis
in vivo. Luteolin combined with PTX was used to treat
xenografts in nude mice. (A) The body weights of nude mice were
measured. (B) The tumor volumes were measured. (C) The images of
tumors were acquired. (D) The average tumor mass was measured.
**P<0.01 vs. NC, #P<0.05 vs. 20 mg/kg,
n=5. (E) Hematoxylin and eosin staining assay for cell morphology
in different groups (magnification, ×100). (F) TUNEL assay for
apoptosis in different groups (magnification, ×50).
*P<0.05, **P<0.01,
***P<0.001 vs. NC, #P<0.05,
##P<0.01 vs. PTX + LUT, n=5. PTX, paclitaxel; LUT,
luteolin; NC, normal control. |
Luteolin suppresses the expression of
FAK/Src/PI3K/Akt pathway and resistance-related proteins in
vivo
The expression of p-FAK (Tyr397)/FAK, ErbB2, p-Src
(Tyr416)/Src and p-Akt (Ser473)/Akt (Fig. 9A) and resistance proteins (P-gp,
BCRP and MRP1) (Fig. 9B) was
significantly downregulated in a dose-dependent manner in response
to luteolin treatment. These results suggested that luteolin may
reverse PTX resistance in cells by inhibiting the expression of
drug-resistant proteins mediated by the FAK/Src/PI3K/Akt signaling
pathway.
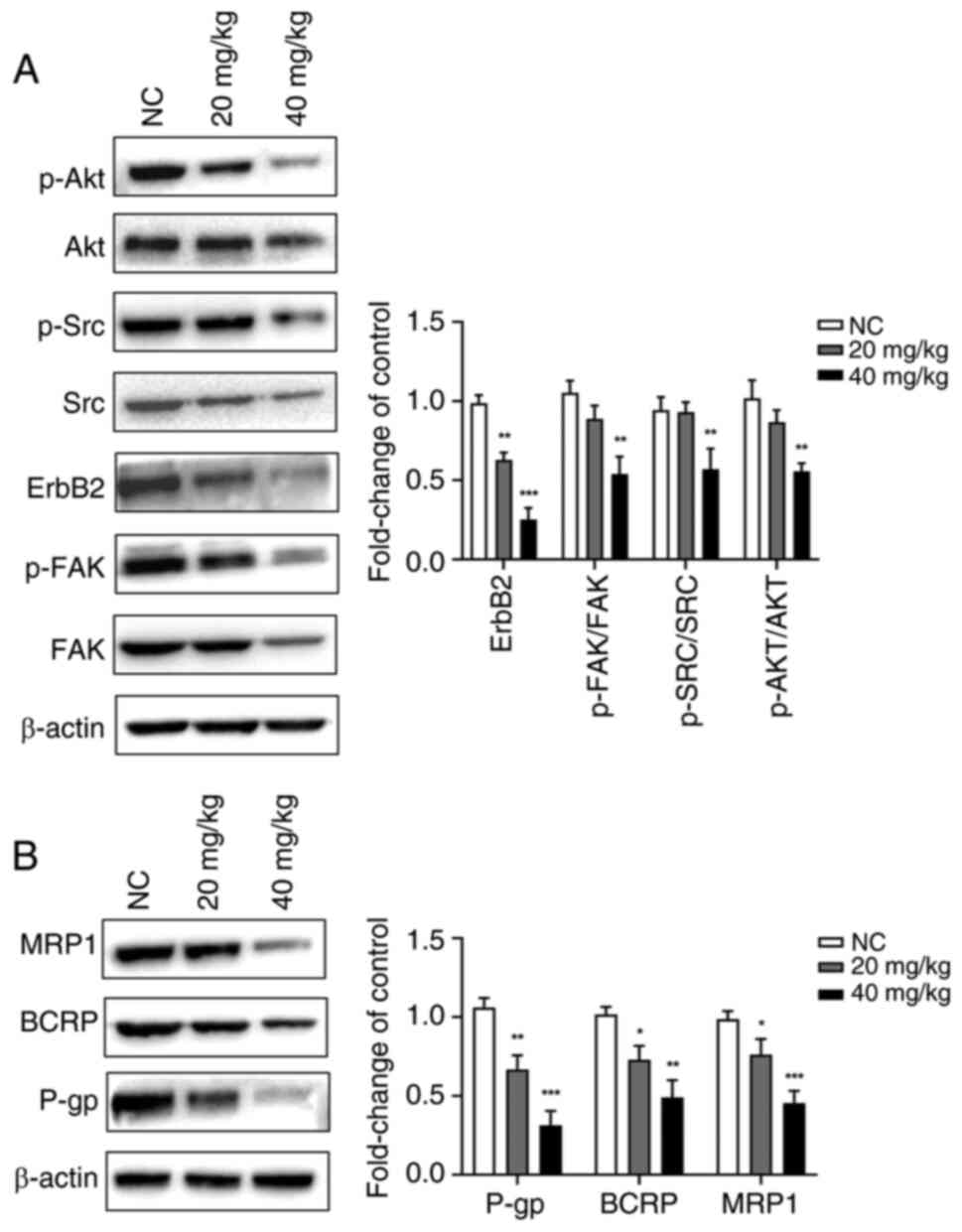 | Figure 9Effect of luteolin on the expression
patterns of proteins associated with the FAK/Src/PI3K/Akt pathway
and drug resistance in vivo. Luteolin (20 or 40 mg/kg) was
used to treat xenografts in nude mice. (A) Proteins associated with
the FAK/Src/PI3K/Akt pathway were detected by western blotting. (B)
The resistance-associated proteins were detected by western
blotting. *P<0.05, **P<0.01,
***P<0.001 vs. NC, n=3. FAK, focal adhesion kinase;
PTX, paclitaxel; ESCC, esophageal squamous cell carcinoma; p-,
phosphorylated; MRP1, multidrug resistance protein 1; BCRP, breast
cancer resistance protein; NC, normal control. |
Discussion
Luteolin can inhibit the proliferation of various
tumor cells in vitro and hinder tumor progression by
inducing cell cycle arrest, facilitating apoptosis and inhibiting
invasion, metastasis and angiogenesis. The present study examined
the biological effects of luteolin on PTX resistance in ESCC cells.
Luteolin inhibited proliferation, clone formation, migration,
invasion and EMT in the cells but induced cell cycle arrest and
apoptosis. It also reduced sphere formation and stemness in EC1/PTX
cells.
In recent years, luteolin has been shown to play a
vital role in chemotherapeutic sensitization by regulating the
biological characteristics of drug-resistant tumor cells, thereby
increasing the drug sensitivity in these cells and improving the
therapeutic effect of cellular chemotherapeutics. For example, Tsai
et al (19) showed that
luteolin suppressed breast cancer stemness and enhanced
chemosensitivity via an Nrf2-mediated pathway. In another study,
luteolin combined with low-dose PTX synergistically restricted EMT
and induced apoptosis in esophageal carcinoma in vitro and
in vivo. Ma et al (20) showed that luteolin potentiated
the oxaliplatin-induced inhibitory effects on cell proliferation in
gastric cancer by inducing G2/M cell cycle arrest and
apoptosis. In the current study, luteolin treatment combined with
PTX treatment significantly increased the total apoptosis rate
compared to that achieved with PTX or luteolin administration. This
suggested that luteolin, as an adjuvant, increased the
chemosensitivity of PTX-resistant ESCC cells.
FAK is a type of non-receptor tyrosine kinase that
can promote focal adhesion and EMT when phosphorylated, thereby
regulating cell invasion and metastasis (21-22). ErbB2, also known as HER2, is a
member of the epidermal growth factor receptor family of
transmembrane tyrosine kinase receptors. The aberrant expression of
ErbB2 usually contributes to malignant transformation and has been
linked to cell invasion, lymph node metastasis, poor prognosis and
chemotherapy tolerance (23,24). The activation of both ErbB2
(recruited by FAK) and FAK triggers Src (a non-receptor tyrosine
kinase, encoded by the viral oncogene SRC) and the subsequent
activation of Src downstream signaling pathways (e.g. PI3K/AKT and
MAPK) induces tumor apoptosis, promoting tumor invasion, metastasis
and chemoresistance. The next-generation sequencing results of ESCC
drug-resistant cells (EC1/PTX) and its parental cells (EC1) were
analyzed and it was found that a series of signaling pathways
(e.g., PI3K/Akt, p53, apoptosis and metabolic pathways) and
functional changes (signal transduction, apoptosis and cell
metabolism) were significantly related to drug resistance.
Meanwhile, among mRNAs exhibiting differential expression, AKT3, as
the key molecule in the PI3K/Akt pathway, was significantly
upregulated in drug-resistant ESCC cells.
Subsequently, based on its inhibitory effects on
in vitro tyrosine kinase activity, luteolin can function as
a multi-target kinase inhibitor owing to its ability to suppress
the activity of multiple tyrosine kinases. Interestingly, the
kinase activities of FAK, ErbB2 and Src could be inhibited to
different degrees and luteolin could inhibit key kinases in the
FAK/Src/PI3K/Akt pathway. This may be a crucial method for
reversing drug resistance in ESCC.
The present study also assessed whether luteolin
increased the chemotherapeutic drug sensitivity of drug-resistant
ESCC cells by blocking the FAK/Src/PI3K/Akt signaling pathway. The
results of the in vivo and in vitro experiments
showed that luteolin restricted the expression levels of p-FAK
(Tyr397)/FAK, ErbB2, p-Src (Tyr416)/Src, p-Akt (Ser473)/Akt, P-gp,
BCRP and MRP1. This indicated that the FAK/Src/PI3K/Akt signaling
pathway is involved in drug resistance in ESCC cells. Meanwhile,
molecular docking and visualization experiments were performed
using luteolin with FAK, SRC and AKT proteins from the PI3K-Akt
signaling pathway. Luteolin exhibited good binding ability to the
active sites of FAK, SRC and AKT. Therefore, luteolin may increase
drug sensitivity in ESCC-resistant cells by inhibiting the
FAK/Src/PI3K/Akt signaling pathway.
It was concluded that luteolin inhibits
tumorigenesis in drug-resistant tumor cells potentially through
modulation of the FAK/Src/PI3K/Akt signaling pathway. However, the
present study had certain limitations. In future research, it is
planned to validate its findings using different subtypes of
drug-resistant ESCC cell lines and further investigate specific
binding sites between luteolin and key kinases through molecular
docking analysis. These results could serve as a foundation for
considering luteolin as a clinical adjuvant and a promising agent
for reversing drug resistance in ESCC cells.
Availability of data and materials
The data generated in the present study may be
requested from the corresponding author.
Authors' contributions
ZY and TF designed the study and wrote the first
draft of the manuscript. HL revised the manuscript. TF, YS and NG
conducted the bioinformatics analysis. ZY, PG and YH developed the
methods and performed the validation. YL, YS and PG participated in
data analysis and tabulation. NG and HL performed the statistical
analysis. ZY and HL confirmed the authenticity of all the raw data.
All authors read and approved the final manuscript.
Ethics approval and consent to
participate
Not applicable.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
Acknowledgements
Not applicable.
Funding
The present study was supported by the General program of Henan
Natural Science Foundation (grant no. 212300410393), Henan Science
and Technology Research Project (grant no. 232102310298) and Henan
Province Medical Science and Technology Research Program Joint
Construction Project (grant no. LHGJ20210696).
References
|
1
|
Bray F, Ferlay J, Soerjomataram I, Siegel
RL, Torre LA and Jemal A: Global cancer statistics 2018: GLOBOCAN
estimates of incidence and mortality worldwide for 36 cancers in
185 countries. CA Cancer J Clin. 68:394–424. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Zhang Y: Epidemiology of esophageal
cancer. World J Gastroenterol. 19:5598–5606. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Abnet CC, Arnold M and Wei WQ:
Epidemiology of esophageal squamous cell carcinoma.
Gastroenterology. 154:360–373. 2018. View Article : Google Scholar
|
|
4
|
Siegel R, Naishadham D and Jemal A: Cancer
statistics, 2013. CA Cancer J Clin. 63:11–30. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Lin Y, Totsuka Y, He Y, Kikuchi S, Qiao Y,
Ueda J, Wei W, Inoue M and Tanaka H: Epidemiology of esophageal
cancer in Japan and China. J Epidemiol. 23:233–242. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Hiripi E, Jansen L, Gondos A, Emrich K,
Holleczek B, Katalinic A, Luttmann S, Nennecke A and Brenner H;
Gekid Cancer Survival Working Group: Survival of stomach and
esophagus cancer patients in Germany in the early 21st century.
Acta Oncol. 51:906–914. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
van Hagen P, Hulshof MC, van Lanschot JJ,
Steyerberg EW, van Berge Henegouwen MI, Wijnhoven BP, Richel DJ,
Nieuwenhuijzen GA, Hospers GA, Bonenkamp JJ, et al: Preoperative
chemoradiotherapy for esophageal or junctional cancer. N Engl J
Med. 366:2074–2084. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Gebski V, Burmeister B, Smithers BM, Foo
K, Zalcberg J and Simes J; Australasian Gastro-Intestinal Trials
Group: Survival benefits from neoadjuvant chemoradiotherapy or
chemotherapy in oesophageal carcinoma: A meta-analysis. Lancet
Oncol. 8:226–234. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Ando N, Kato H, Igaki H, Shinoda M, Ozawa
S, Shimizu H, Nakamura T, Yabusaki H, Aoyama N, Kurita A, et al: A
randomized trial comparing postoperative adjuvant chemotherapy with
cisplatin and 5-fluorouracil versus preoperative chemotherapy for
localized advanced squamous cell carcinoma of the thoracic
esophagus (JCOG9907). Ann Surg Oncol. 19:68–74. 2012. View Article : Google Scholar
|
|
10
|
Liu Y, Ren Z, Yuan L, Xu S, Yao Z, Qiao L
and Li K: Paclitaxel plus cisplatin vs. 5-fluorouracil plus
cisplatin as first-line treatment for patients with advanced
squamous cell esophageal cancer. Am J Cancer Res. 6:2345–2350.
2016.PubMed/NCBI
|
|
11
|
Hummel R, Sie C, Watson DI, Wang T, Ansar
A, Michael MZ, Van der Hoek M, Haier J and Hussey DJ: MicroRNA
signatures in chemotherapy resistant esophageal cancer cell lines.
World J Gastroenterol. 20:14904–1412. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Limtrakul P, Anuchapreeda S and Buddhasukh
D: Modulation of human multidrug-resistance MDR-1 gene by natural
curcuminoids. BMC Cancer. 4:132004. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Lopez-Lazaro M: Distribution and
biological activities of the flavonoid luteolin. Mini Rev Med Chem.
9:31–59. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Shi R, Huang Q, Zhu X, Ong YB, Zhao B, Lu
J, Ong CN and Shen HM: Luteolin sensitizes the anticancer effect of
cisplatin via c-Jun NH2-terminal kinase-mediated p53
phosphorylation and stabilization. Mol Cancer Ther. 6:1338–1347.
2007. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Chian S, Li YY, Wang XJ and Tang XW:
Luteolin sensitizes two oxaliplatin-resistant colorectal cancer
cell lines to chemotherapeutic drugs via inhibition of the Nrf2
pathway. Asian Pac J Cancer Prev. 15:2911–2916. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Pandurangan AK, Dharmalingam P, Sadagopan
SK and Ganapasam S: Luteolin inhibits matrix metalloproteinase 9
and 2 in azoxymethane-induced colon carcinogenesis. Hum Exp
Toxicol. 33:1176–1185. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Carsten VF and Cardinale PJ: The
overdenture-a review. N Y State Dent J. 44:331–334. 1978.PubMed/NCBI
|
|
18
|
Wu YT, Chen L, Tan ZB, Fan HJ, Xie LP,
Zhang WT, Chen HM, Li J, Liu B and Zhou YC: Luteolin inhibits
vascular smooth muscle cell proliferation and migration by
inhibiting TGFBR1 signaling. Front Pharmacol. 9:10592018.
View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Tsai KJ, Tsai HY, Tsai CC, Chen TY, Hsieh
TH, Chen CL, Mbuyisa L, Huang YB and Lin MW: Luteolin inhibits
breast cancer stemness and enhances chemosensitivity through the
Nrf2-mediated pathway. Molecules. 26:64522021. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Ma J, Chen X, Zhu X, Pan Z, Hao W, Li D,
Zheng Q and Tang X: Luteolin potentiates low-dose
oxaliplatin-induced inhibitory effects on cell proliferation in
gastric cancer by inducing G2/M cell cycle arrest and apoptosis.
Oncol Lett. 23:162022. View Article : Google Scholar
|
|
21
|
Lee BY, Timpson P, Horvath LG and Daly RJ:
FAK signaling in human cancer as a target for therapeutics.
Pharmacol Ther. 146:132–149. 2015. View Article : Google Scholar
|
|
22
|
Seguin L, Desgrosellier JS, Weis SM and
Cheresh DA: Integrins and cancer: Regulators of cancer stemness,
metastasis and drug resistance. Trends Cell Biol. 25:234–240. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Vadlamudi RK, Sahin AA, Adam L, Wang RA
and Kumar R: Heregulin and HER2 signaling selectively activates
c-Src phosphorylation at tyrosine 215. FEBS Lett. 543:76–80. 2003.
View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Yu D and Hung MC: Overexpression of ErbB2
in cancer and ErbB2-targeting strategies. Oncogene. 19:6115–6121.
2000. View Article : Google Scholar
|