Introduction
Adropin, a novel 76-amino acid polypeptide, is
detected in various organs and tissues, including the liver,
endothelial cells, heart, small intestine, pancreas, serum, kidney
and brain (1-3). Initially discovered by Kumar et
al in 2008 (1) during an
investigation into the hypothalamic regulation of liver metabolism,
adropin was identified in the livers of C57BL/6J mice lacking
melanocortin-3 receptors through a microarray analysis of total
gene expression. This encoding gene is associated with energy
homeostasis and lipid metabolism, earning it the designation of the
energy homeostasis-related (ENHO) gene (1).
Adropin functions as a secreted peptide that is
bound to and secreted by cell membranes and as a membrane-bound
protein that facilitates intercellular communication. Extensive
research has demonstrated the significant impact of adropin on
mechanisms related to increased obesity, glucose and lipid
metabolism, as well as insulin resistance (IR) (4-6).
In glucose metabolism, adropin enhances glucose utilization in
mice, regulating the insulin signaling pathway (6,7).
Additionally, adropin treatment mitigates non-alcoholic fatty liver
disease (NAFLD) progression by inhibiting protein phosphatase 2A
(PP2A), activating the AMP-activated protein kinase (AMPK) pathway
and reducing hepatic glucose production in the context of IR
(4). Regarding lipid metabolism,
adropin reduces serum triglycerides (TGs), total cholesterol (TC)
and low-density lipoprotein (LDL) cholesterol levels in
hypertensive rats (6). Adropin
downregulates the expression of lipogenic proteins, sterol
regulatory element-binding protein (SREBP) 1 and adipocyte
differentiation-related protein (ADRP), in diabetic kidney disease
(DKD) mice, improving lipid deposition in renal tissue (8). Additionally, adropin influences
disease development by modulating molecular signaling pathways.
Specifically, it activates the phosphatidylinositol-3 kinase
(PI3K)/protein kinase B (AKT) and extracellular regulated kinase
(ERK)1/2 pathways through vascular endothelial growth factor
receptor 2 (VEGFR2), protecting endothelial cells (9). Moreover, adropin inhibits the
osteogenic differentiation of vascular smooth muscle cells (VSMCs)
and reduces vascular calcification by activating the Janus kinase 2
(JAK2)/signal transducer and activator of transcription 3 (STAT3)
signaling pathway (10).
These findings indicate that adropin participates in
disease development through multiple pathways, including glucose
metabolism, lipid metabolism and molecular signaling pathways.
However, the effects of each pathway are interconnected, making the
impact of adropin on disease mechanisms complex. In previous
reviews, the role of adropin in energy and metabolic disorders has
been described (11-14). Compared with previous reviews,
the present review not only includes a section regarding the
regulation of adropin, but also a summary of some of the associated
molecular signaling pathways. To elucidate the function and
clinical research status of adropin, research on adropin in various
diseases, including acute myocardial infarction (AMI), lung injury,
NAFLD/non-alcoholic steatohepatitis (NASH), kidney disease,
obesity, diabetes, atherosclerosis, polycystic ovary syndrome
(PCOS), multiple sclerosis (MS) and cancer, from the past decade
was systematically reviewed. Compared with previous reviews, the
present review identified several challenges and limitations
associated with adropin research in both animal and clinical
contexts, aiming to offer valuable insights for future
investigations.
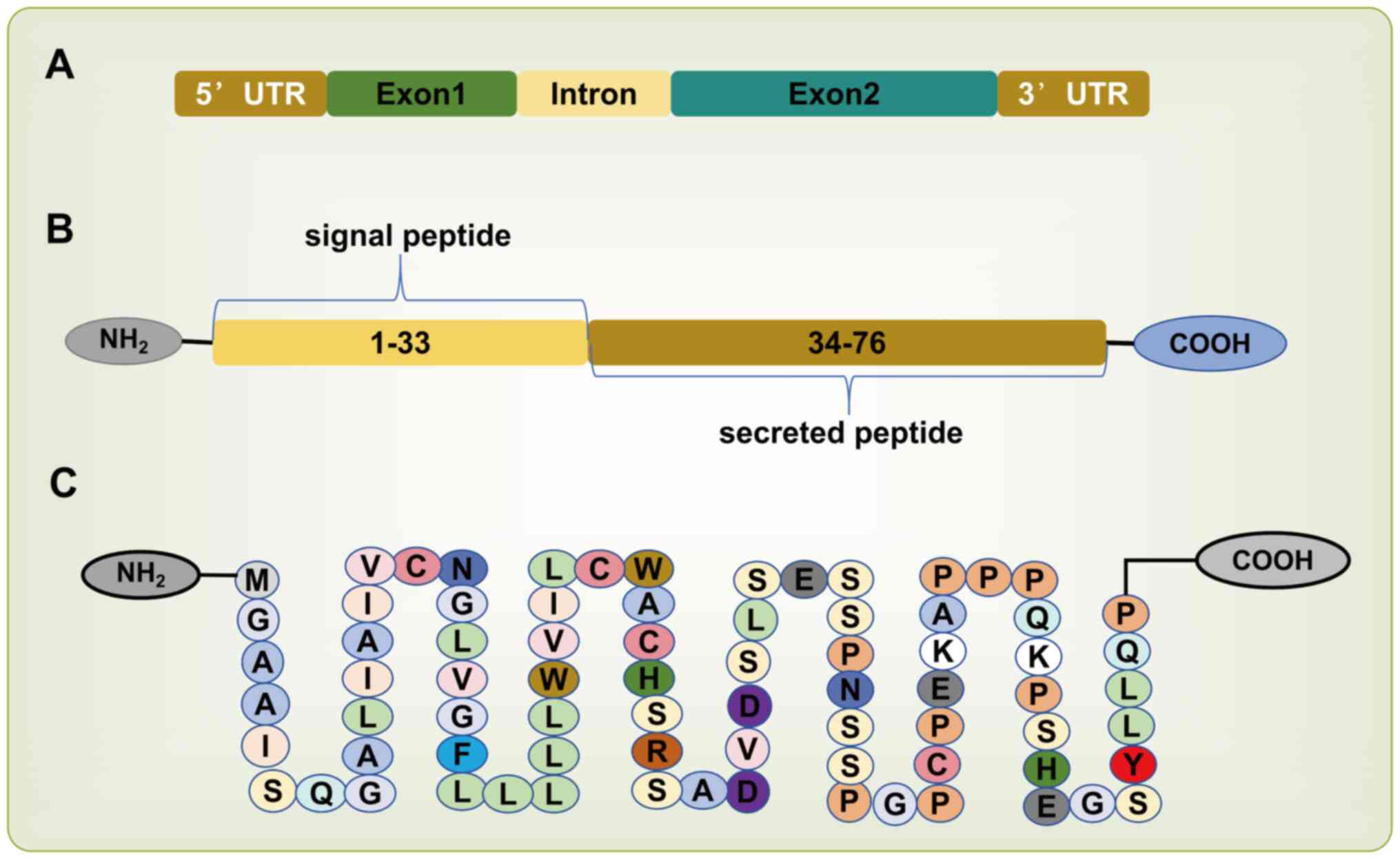
Structure of adropin
The ENHO gene encodes adropin, a polypeptide
consisting of 76 amino acids (MGA AIS QGA LIA IVC NGL VGF LLL LLW
VIL CWA CHS RSA DV DSL SES SPN SSP GPC PEK APP PQK PSH EGS YLL QP).
The ENHO gene, located on chromosome 9p13.3, comprises one intron
and two exons (1). The first 33
amino acids serve as a secretory signal peptide (1), while amino acids 34-76 possess
biological activity (7)
(Fig. 1). Adropin has a
molecular weight of 4.499 Da (1). Structurally, the N-terminal amino
acids 1-9 are cytoplasmic, amino acids 9-30 span the membrane, and
amino acids 30-76 are extracellular (15,16). The amino acid sequence of adropin
is 100% identical across rats, mice and humans (1,17).
Regulation of adropin expression
Kumar et al (1) demonstrated that adropin is
nutritionally regulated in mice. In C57BL/6J mice, adropin
expression increased rapidly with a high-fat diet (HFD) but
decreased in fasted mice, compared with the control group (1). Additionally, mice on a diet high in
fat and low in carbohydrates exhibit higher adropin expression,
whereas those on a diet low in fat and high in carbohydrates show
reduced adropin levels (18).
The rapid upregulation of hepatic ENHO mRNA in response to
macronutrient changes suggests the involvement of intracellular
lipid sensors (18). This
evidence indicates that adropin expression is linked to dietary fat
intake.
Adropin expression is regulated by several factors,
including liver X receptor α (LXRα), retinoic acid receptor-related
orphan receptor (ROR), estrogen receptor α (ERα), STAT3 and
glucagon-like peptide-1 (GLP-1) (16-21) (Fig. 2). LXRα is a nuclear receptor and
a sensor for blood lipids and glucose and plays a notable role in
the regulation of lipid metabolism (19). In HepG2 cells, the LXRα agonist,
GW3965, reduces ENHO expression, an effect that can be blocked by
LXRα-targeting antisense RNA (20). Reduced hepatic ENHO mRNA levels
were observed in diet-induced obese mice treated with the LXRα
agonist, indicating that LXRα regulated liver ENHO expression
(1). Furthermore, another study
found that ENHO expression is rhythmic in the liver of male mice,
peaking during maximum food consumption in the dark phase, which is
related to the transcriptional activation of the circadian clock
genes, RORα/γ (21).
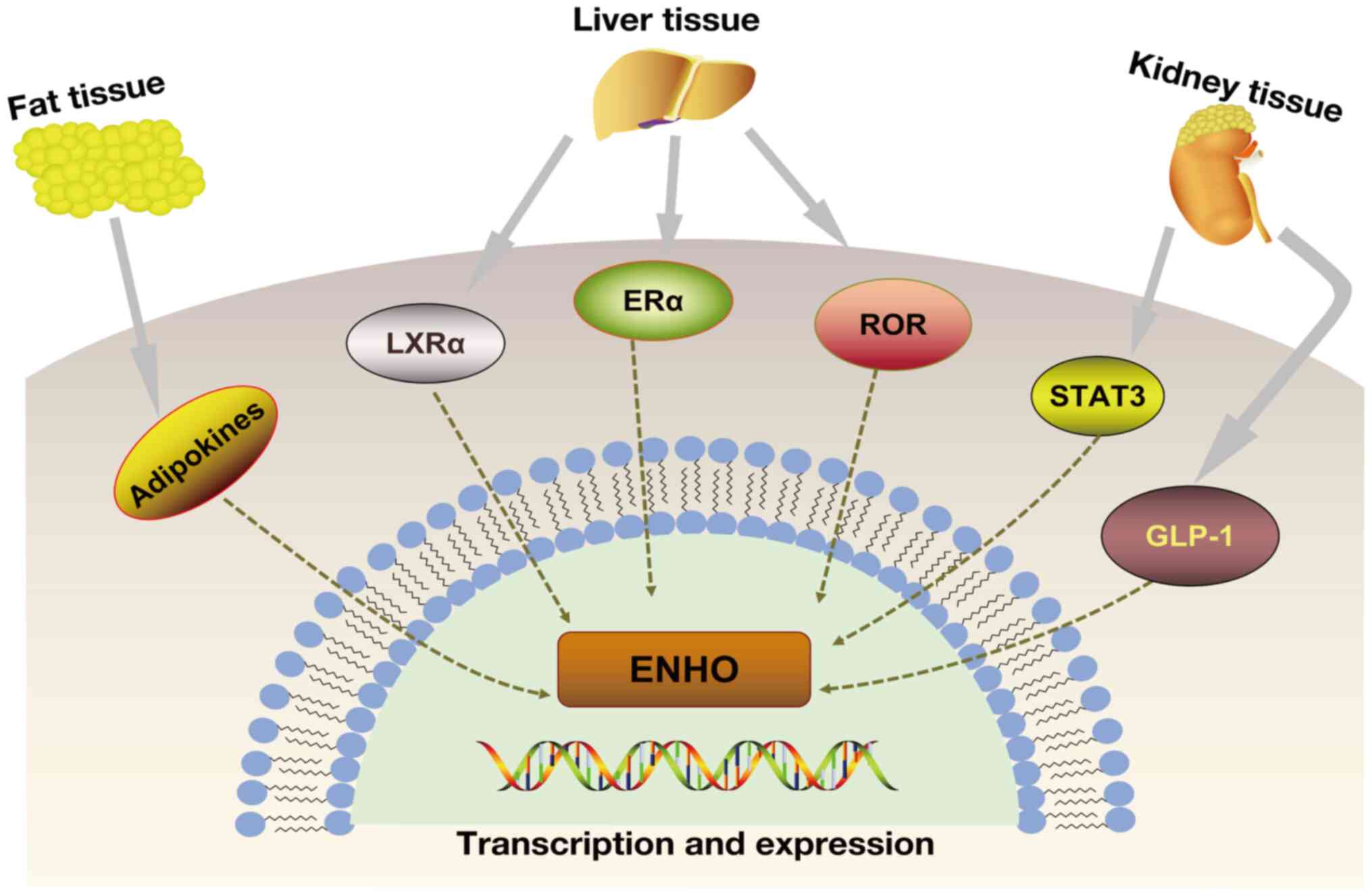 | Figure 2Expression of adropin is affected by
LXRα, ROR, ERα, STAT3 and GLP-1. Dietary fat can affect adropin
levels. In the liver, adropin levels are affected by the LXRα, ROR
and Erα genes. STAT3 and GLP-1 have been found to affect adropin
levels in diabetic nephropathy. The dotted line indicates that it
is uncertain whether it is a direct target modulation. ENHO, energy
homeostasis-associated gene; ERα, estrogen receptor α; LXRα, liver
X receptor α; GLP-1, glucagon-like peptide-1; ROR, retinoic acid
receptor-related orphan receptor; STAT3, signal transducer and
activator of transcription 3. |
Recent research on female mice with NAFLD under HFD
conditions revealed a negative correlation between adropin levels
and both lipogenic gene expression and a fatty liver, which was an
ERα-dependent effect (22).
Additionally, hepatic ERα induced adropin-inhibited hepatic
lipogenesis and lipid deposition in women with high dietary lipid
intake (22). A concurrent study
indicated that liver adropin regulation involves estrogen.
Ovariectomized mice treated with estrogen exhibited elevated
hepatic ENHO expression, in which estrogen-dependent ERα bound to
the ENHO gene. Adropin treatment also improved metabolic disorders
in the mice (23).
In HepG2 cells treated with a high-glucose medium,
Kuo et al (24) observed
a clear upregulation of the phosphorylated (p-)STAT3/STAT3 ratio,
reactive oxygen species (ROS) expression and ENHO mRNA levels as
the glucose levels increased. This effect was mitigated by
pretreatment with Stattic or STAT3-specific small interfering RNAs.
Similar outcomes were noted in animal models, suggesting that STAT3
is integral to ENHO gene regulation and promotes hepatic adropin
expression in diabetic rats (24).
Li et al (25) found that myricetin
dose-dependently increased plasma β-endorphin and adropin levels,
effects that were inhibited by a GLP-1 receptor antagonist. Further
mechanistic experiments using the HepG2 cell line showed that
myricetin-induced GLP-1 receptor activation modulated adropin
expression. In diabetic rats, the effect of myricetin on plasma
adropin was primarily mediated through endogenous β-endorphin
following GLP-1 receptor activation via acute bolus
injection. Chronic myricetin treatment also enhanced adropin
secretion in diabetic rats, suggesting a link between adropin
expression and GLP-1 (25).
However, as with LXRα, ROR, ERα and STAT3, current studies only
demonstrate that adropin expression is regulated by these upstream
molecules. It remains unclear whether these molecules directly
target and regulate adropin, necessitating further detailed
research.
Association of adropin with the development
of multiple diseases
Adropin significantly influences energy balance
regulation and the metabolism of fatty acids and glucose (26,27). Acting as a membrane-bound
protein, adropin facilitates intercellular communication, and its
involvement spans various diseases, including AMI (28), lung injury (29), NAFLD/NASH (30), kidney disease (31), PCOS (32), obesity (33), diabetes (34), atherosclerosis (35), MS (36) and cancer (37,38) (Fig. 3). The molecular mechanisms
through which adropin contributes to these diseases are detailed in
Table I.
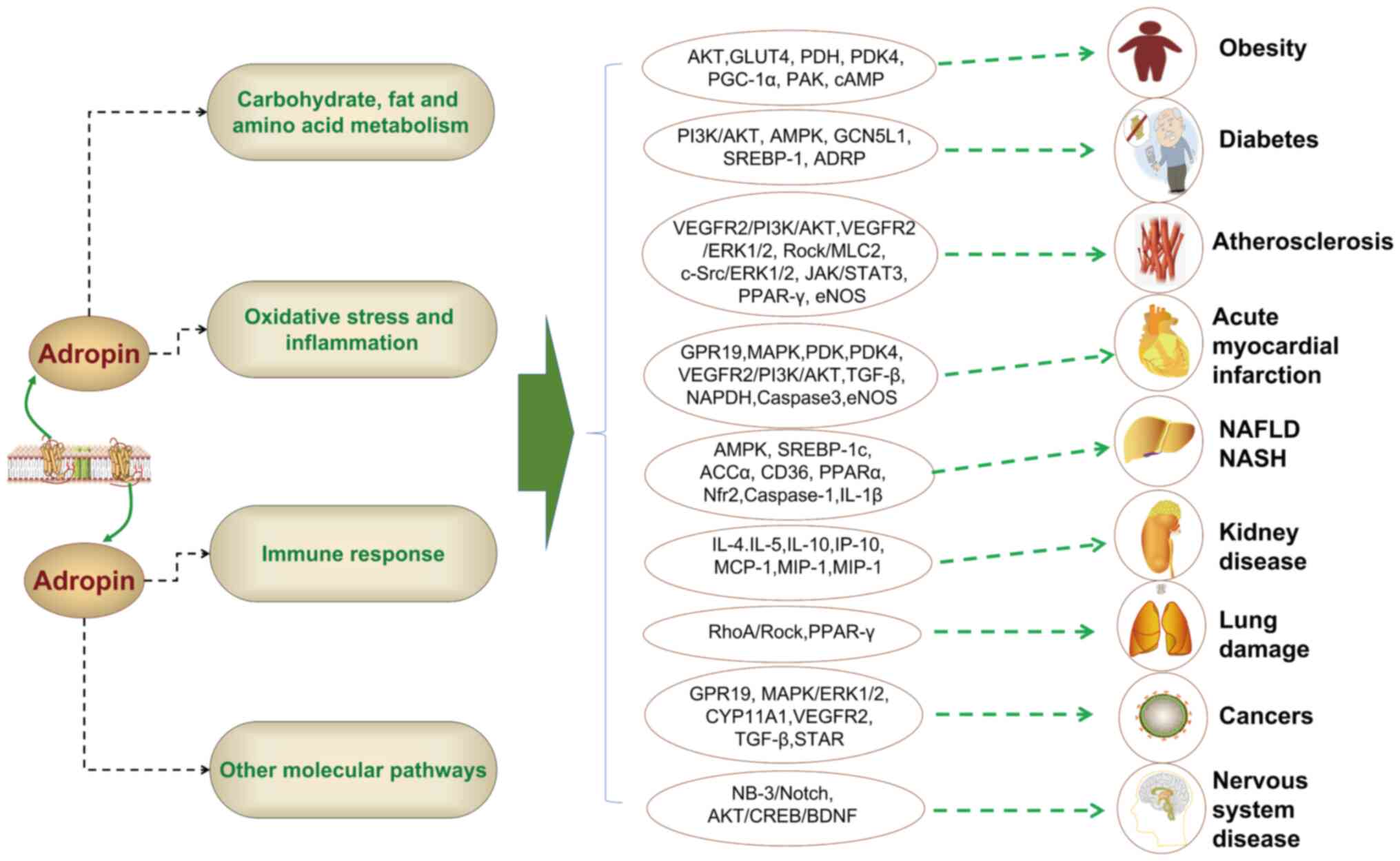 | Figure 3Adropin is involved in the occurrence
of various diseases through multiple mechanisms. Adropin can
participate in the development of metabolic diseases by affecting
carbohydrate, lipid and amino acid metabolism, and can also
participate in the development of non-metabolic diseases by
affecting inflammation, immune response and signaling molecules or
pathways. ADRP, adipocyte differentiation-related protein; ACCα,
acetyl-CoA carboxylase α; AMPK, AMP-activated protein kinase; AKT,
protein kinase B; BDNF, brain-derived neurotrophic factor; CD36,
cluster of differentiation 36; CREB, cyclic AMP response
element-binding protein; cAMP, cyclic adenosine monophosphate;
CYP11A1, cholesterol side-chain cleavage enzyme, cytochrome
P450scc; eNOS, endothelial nitric oxide synthase; ERK1/2,
extracellular regulated kinase 1/2; GLUT4, glucose transporters
type 4; GCN5L1, general control of amino acid synthesis 5 like-1;
GPR19, G-protein coupled receptor 19; MLC2, myosin light chain;
MCP-1, monocyte chemoattractant protein-1; MIP-1, macrophage
inflammatory protein 1; NAFLD, non-alcoholic fatty liver disease;
NASH, non-alcoholic steatohepatitis; NAPDH, nicotinamide adenine
dinucleotide phosphate; Nrf2, nuclear factor erythroid 2-related
factor 2; PI3K, phosphatidylinositol-3 kinase; PDH, pyruvate
dehydrogenase; PDK4, pyruvate dehydrogenase kinase 4; PGC-1,
peroxisome proliferator-activated receptor gamma coactivator-1;
PAK, protein kinase A; PPARγ, peroxisome proliferator-activated
receptor γ; Rock, Rho-associated coiled-coil containing protein
kinase; RhoA, ras homolog gene family member A; STAT3, signal
transducer and activator of transcription 3; STAR, steroidogenesis,
the steroidogenic acute regulatory; SREBP-1, sterol regulatory
element-binding protein 1; TGF-β, transforming growth factor β;
VEGFR2, vascular endothelial growth factor receptor-2. |
 | Table IMechanism of action of adropin in the
development of various diseases. |
Table I
Mechanism of action of adropin in the
development of various diseases.
| Disease | Mechanism | Impact | (Refs.) |
|---|
| AMI |
Adropin→GPR19↑→MAPK↑→PDK-4↓→PDH↑→Glucose
oxidation↑; adropin→VEGFR2↑→PI3K↑→AKT↑→ROS, fibrosis, apoptosis↓;
adropin→eNOS, TGF-β, NADPH, caspase3↓ | Improve | (11,42) |
| Lung damage |
Adropin→RhoA↓→ROCK↓→ROS, inflammation,
fibrosis↓; adropin→PPARγ↓→Macrophage M2
polarization↑→inflammation↓ | Improve | (29,48) |
| NAFLD/NASH |
Adropin→PP2A↓→AMPK↑→Glucose production↓;
adropin→Nrf2↑→ROS↓; adropin→Caspase-1, IL-1β↓→ROS and
inflammation↓; adropin→SREBP-1c, ACCα, CD36↓; PPARα↑ | Improve | (4,38,52,54) |
| Kidney-related
diseases | Adropin→IFN-γ,
IL-4, IL-5, IL-10, IL-12, IL-17A, CXCL2↓; IP-10, MCP-1, MIP-1α,
MIP-2↑ | Improve | (61) |
| PCOS | Still not
clear | Improve | |
| Obesity | Adropin→AKT,
GLUT4↑→insulin sensitivity↑; adropin→PGC-1α↓→ PDK-4 ↓→PDH ↑→Glucose
oxidation↑; adropin→PAK↓→cAMP↓ →blood sugar improvement↑ | Improve | (7,77) |
| Diabetes | Adropin→PI3K↑→AKT↑,
AMPK↑→insulin regulation↑; adropin→TG, TC, ALT, AST, GGT, ALP,
LDL↓ | Improve | (86,92) |
| DKD | Adropin→SREBP-1,
ADRP↓→Mitochondrial damage and lipogenesis↓ | Improve | (8) |
| Diabetic
cardiomyopathy |
Adropin→GCN5L1↑→Glucose oxidation↑;
adropin→Drp1, collagen I, and collagen III↓; Mitofusin-1 and
Mitofusin-2↑ | Improve | (91,103) |
| Diabetic
retinopathy | Still not
clear | Improve | |
|
Atherosclerosis |
Adropin→VEGFR2↑→PI3K↑→AKT↑VEGFR2↑→ERK1/2↑→eNOS↑→NO↑→endothelial
function↑; adropin→Rock↓→MLC2↓→endothelial permeability↓;
adropin→c-Src↓→erk1/2↓→endothelial cell proliferation↓;
adropin→JAK2↑→STAT3↑→Vascular calcification↓;
adropin→TGF-β↓→Smad2/3↓→EMT↓ | Improve | (9,10,107,109,110) |
| MS | TGFβ →
Adropin↓→GPR19↓→fibrosis↑ | | (36) |
| Breast cancer |
Adropin→GPR19↑→MAPK↑→ERK1/2↑→EMT↑→pr
oliferation and metastasis↑ | Promote | (118) |
| Adrenocortical
carcinoma |
Adropin→TGF-β↑→STAR, CYP11A1↓→cortisol and
aldosterone↓ | | (119) |
| PDA | Adropin→VEGFR2,
Ki67, cyclin D1, MMP2↓→cell proliferation and angiogenesis↑ | Promote | (121) |
| Others (nervous
system) |
Adropin→NB-3↑→Notch↑→physical coordination
ability↑; adropin→AKT↑→CREB↑→BDNF↑→Memory capacity↑ | Improve | (16,124) |
AMI
Globally, AMI ranks among the leading causes of both
morbidity and mortality (39). A
report has indicated that adropin enhances cardiac energy
metabolism and improves cardiac efficiency by promoting glucose
oxidation and suppressing fatty acid oxidation in the heart
(40). In cardiomyocytes,
adropin activates G-protein coupled receptor 19 (GPR19), which
leads to MAPK-mediated phosphorylation. This process downregulates
the phosphorylation of pyruvate dehydrogenase kinase 4 (PDK4) and
promotes the phosphorylation of pyruvate dehydrogenase (PDH)
(11). Aydin et al
(41) observed that serum
adropin levels gradually increased as myocardial damage from
infarction worsened within the first 1-24 h in myocardial
infarction rats. Another study demonstrated that adropin treatment
effectively improved cardiac function in mice with
radiation-induced myocardial injury, inhibited oxidative stress and
myocardial fibrosis, reduced cardiomyocyte apoptosis and promoted
microangiogenesis (42). The
primary mechanism involves adropin activating the VEGFR2/PI3K/AKT
pathway. Additionally, adropin treatment decreased the expression
of transforming growth factor β1 (TGF-β1), NOX4 and caspase 3 in
cardiomyocytes while promoting endothelial nitric oxide synthase
(eNOS) expression (42).
Yu et al (28) found that patients who had
experienced AMI had significantly lower serum adropin levels
compared with those with stable angina pectoris and normal
controls. In a study from 2022, Adıyaman et al (43) evaluated serum adropin levels and
their link to folic acid and cobalamin in 70 patients with
ST-segment elevation myocardial infarction (STEMI) and 70 controls.
Within the first 24 h, the STEMI group exhibited notably elevated
levels of adropin, troponin and C-reactive protein, while the amine
and folate levels were considerably lower than in the control group
(43). Therefore, patients with
early-stage myocardial infarction may benefit from screening
adropin as a cardiac biomarker. However, the underlying mechanism
behind the negative relationship between adropin, cobalamin and
folic acid levels in patients with myocardial infarction remains
under investigation. Another study by Chang et al (44) divided 163 patients with AMI into
low (n=82) and high (n=80) adropin groups based on serum adropin
levels. The clinical follow-up revealed that the low adropin group
exhibited a higher rate of recurrent myocardial infarction compared
with the high adropin group (44), indicating that adropin levels are
crucial for the long-term prognosis of patients with AMI.
Lung damage
Myeloperoxidase (MPO)-antineutrophil cytoplasmic
autoantibody (ANCA)-associated vasculitis frequently leads to
potentially fatal alveolar hemorrhage or fibrosis (45,46). Gao et al (47) utilized adropin gene knockout
(AdrKO) C57BL/6J mice to explore the pathogenesis of lung injury
linked to MPO-ANCA. The results indicated that AdrKO mice exhibited
reduced phosphorylation of AKT1 (Ser473) and eNOS (Ser1177) and a
loss of regulatory T cells, with the adropin allele mutation
increasing susceptibility to MPO-ANCA-related alveolar hemorrhage.
These results suggest that initial events in MPO-ANCA-associated
lung injury may be mediated at the molecular level by adropin
mutations or deficiency (47).
Additionally, Rizk et al (48) demonstrated that adropin mitigated
diabetic lung injury by inhibiting the ras homolog gene family
member A/Rho-associated coiled-coil containing protein kinase
(Rock) pathway, apoptosis, inflammatory response, oxidative stress
and lung tissue fibrosis. Concurrently, an animal study revealed
that adropin promoted M2 macrophage polarization in the lungs and
reduced the severity of acute pancreatitis-related lung injury by
modulating peroxisome proliferator-activated receptor (PPAR)γ
phosphorylation in lung macrophages (29).
A prospective study involving 57 participants [28
controls and 29 patients with acute pulmonary embolism (PE)]
reported a notably lower level of adropin in the PE group compared
with the control group (49). At
an optimal cut-off value of 213.78 pg/ml, the sensitivity of
adropin was 82% and its specificity was 75%. These results suggest
that adropin could serve as a potential marker to rule out PE,
which warrants further investigation (49).
NAFLD/NASH
NAFLD is a chronic hepatic disorder characterized by
excessive fat accumulation and metabolic disturbances of
carbohydrates and lipids (50,51). Adropin plays a protective role
against disorder in sugar and lipid metabolism (11). A study by Zhang et al
(52) demonstrated that adropin
suppressed TC accumulation in tilapia liver cells and reduced the
expression of SREBP-1c, acetyl-CoA carboxylase α and cluster of
differentiation 36 (CD36), while increasing PPARα expression.
Notably, adropin inhibits liver glucose output and TC accumulation
in tilapia through AMPK activation. Chen et al (4) showed that AdrKO mice exhibited an
unbalanced increase in hepatic glucose production before the onset
of HFD-induced systemic IR. Further research indicated that adropin
treatment might active the AMPK pathway by inhibiting PP2A, thereby
reducing hepatic glucose synthesis under IR conditions (4). Additionally, adropin overexpression
or treatment was found to ameliorate palmitic acid-induced
oxidative stress in hepatocytes (53). In NASH mice, adropin activates
the nuclear factor erythroid 2-related factor 2 signaling pathway
and reduces ROS production by hepatocyte mitochondria, thereby
protecting against liver damage (38). Another notable study revealed
that exercise inhibited HFD-induced or methionine- and
choline-deficient diet-induced NLR family pyrin domain containing 3
(NLRP3) inflammasome (NLRP3, Caspase-1 and IL-1β) in NASH mice,
with serum adropin levels negatively correlated with serum IL-1β
levels (54). Further findings
showed that adropin treatment reduced the expression of Caspase-1
and IL-1β in hepatic cells and palmitic acid-treated Kupffer cells,
as well as decreased ROS levels in PA-stimulated hepatic cells and
Kupffer cells (54). A recent
study confirmed that hepatic adropin levels were inversely related
to lipogenic gene expression and fatty liver in female mice under
HFD conditions, an effect dependent on hepatic ERα (22). Consequently, adropin could be a
therapeutic target for metabolic diseases such as NAFLD/NASH.
Patients with NAFLD/NASH exhibit decreased serum
adropin levels. A study by Kutlu et al (30) including 51 patients with hepatic
steatosis and 30 healthy controls, observed significantly reduced
serum adropin levels in the NAFLD group compared with the healthy
controls and patients with IR. Moreover, adropin levels were
negatively correlated with serum insulin, homeostasis model
assessment of IR (HOMA-IR), urea, γ-glutamyl transferase, TC and TG
levels (30). A subsequent
case-control study (15 normal participants, 26 with NAFLD and 21
with NASH) revealed significantly lower serum adropin levels in
patients with NASH compared with the normal controls and patients
with NAFLD, including an inverse relationship between the
histological severity of NAFLD and serum adropin levels (53). In a study of 62 participants [30
patients with metabolic dysfunction-associated fatty liver disease
(MAFLD) and 32 healthy controls] Li et al (55) showed that patients with MAFLD had
significantly lower circulating adropin levels than healthy
controls, with serum adropin levels that were negatively correlated
with NAFLD activity score, intrahepatic TG and TC (55). Furthermore, liraglutide treatment
for type 2 diabetes mellitus (T2DM) combined with MAFLD resulted in
increased serum adropin levels, which were closely associated with
decreased liver fat content and improved glucose and lipid
metabolism (56). Therefore,
adropin may serve as a potential marker for the positive effects of
liraglutide in treating T2DM and MAFLD.
Kidney-related diseases
Chronic kidney disease (CKD) is a chronic disease
with a high incidence rate that is a general term for most kidney
diseases (57). CKD poses a
serious threat to the health of individuals; therefore, it is of
great significance to study the pathogenesis and treatment targets
of CKD (58-60). Most studies indicate that adropin
is involved in the onset and progression of kidney-related
diseases. In a study on the regulatory effects of adropin and
spexin on systemic inflammation, rats with adenine-induced chronic
kidney failure (CKF) were divided into CKF, CKF + saline, CKF +
adropin, CKF + spexin and CKF + adropin + spexin groups. The
results showed that concurrent treatment with adropin and spexin
reduced urinary protein and 24-h urine output (61). Furthermore, treatment with
adropin alone significantly reduced the levels of granulocyte
colony stimulating factor, IFN-γ, IL-4, IL-5, IL-10, IL-12, IL-17A
and C-X-C motif chemokine ligand 1, while increasing levels of
monocyte chemoattractant protein-1, IFN-γ-induced protein 10,
macrophage inflammatory protein (MIP)-1 and MIP-2 (61). This suggests that adropin exerts
a regulatory impact on both the inflammatory response and renal
function, potentially offering protection against systemic
inflammation and the progression of renal failure.
In clinical studies, Grzegorzewska et al
(62) found that among 50
patients undergoing hemodialysis (HD; 27 males and 23 females) and
26 healthy participants, those undergoing HD exhibited reduced
levels of circulating adropin compared with the controls. A
concurrent study assessing circulating adropin and irisin levels in
48 patients with end-stage renal disease (ESRD) undergoing chronic
hemodialysis (HD) or peritoneal dialysis (PD) and 36 healthy
participants revealed that the adropin levels in patients with ESRD
were negatively related to body weight, extra- and intra-cellular
water and albumin concentration (63). Thus, adropin could serve as a
novel indicator of nutritional status in patients with ESRD
(63), although further research
is needed to elucidate its mechanism of action. Kałużna et
al (64) explored the
relationship between adropin, irisin and cardiac status in 79
patients with ESRD (under HD, PD or kidney transplantation) and 40
healthy controls. The findings indicated a significant positive
correlation between adropin concentration and both cardiac troponin
T and plasma N-terminal probrain natriuretic peptide. Adropin was
also correlated with the left ventricular systolic internal
diameter and relative wall thickness, suggesting that adropin may
become a candidate marker for cardiac dysfunction in patients
undergoing HD (64). Comparative
analysis of adropin concentration changes post-kidney
transplantation revealed that levels were higher in patients before
and after transplantation compared with the controls (65), remaining elevated for 1 year and
significantly dropping 5-7 days post-transplantation (66). The plasma adropin levels peaked
12 months after transplantation (65). Further studies are required to
understand the mechanisms underlying these changes in kidney
transplant recipients. A 2023 cross-sectional study classified
participants into four groups: Control (n=50), CKD stages 1-2
(n=50), CKD stages 3-4 (n=50) and CKD stage 5 (n=50) (67). The results showed that the fourth
group (CKD stage 5) exhibited significantly reduced serum adropin
levels compared with the other three groups (67). Adropin levels are inversely
associated with cardiovascular risk biomarkers, suggesting that low
serum adropin levels may be a predictor of CKD, HD and their
complications (68,69).
PCOS
Multiple clinical studies have demonstrated that
adropin influences the occurrence of PCOS (32,70,71). In a cross-sectional study
involving 152 women with PCOS and 152 non-PCOS controls, serum
adropin levels were significantly reduced in patients with PCOS and
inversely linked to TNF-α levels (70). Another study comparing 60 women
with PCOS to 60 non-PCOS participants found notably lower levels of
adropin in both the serum and follicular fluid (FF) of the PCOS
group. Furthermore, the adropin levels were negatively correlated
with the LDL levels but positively correlated with body mass index
(BMI) and high-density lipoprotein (HDL) levels (71). This suggests that adropin may
impact the pathophysiological processes of PCOS through its role in
human metabolism.
A study investigating the effect of PCOS on adropin
levels observed reduced serum adropin levels in both the lean and
overweight PCOS groups compared with the control group, with no
significant difference between the lean and overweight PCOS groups
(72). Therefore, it is
suggested that BMI is not the cause of the changes in serum adropin
levels. In a larger study involving 67 control participants and 220
patients with PCOS, a significant decrease in adropin levels was
found in both the plasma and FF of patients with PCOS (73). Adropin concentration was
negatively correlated with BMI, free androgen index, HOMA-IR,
androstenedione and TC, and positively correlated with sex hormone
binding globulin and HDL. Additionally, the FF adropin
concentration in the PCOS group was negatively related to HOMA-IR,
isoleucine and valine levels (73). This indicates that reduced
adropin levels in women with PCOS may contribute to IR and abnormal
branched-chain amino acid metabolism. Recent meta-analyses
confirmed that the plasma adropin levels in women with PCOS were
significantly lower than those in non-PCOS individuals, and these
reduced levels were significantly correlated with BMI, dyslipidemia
and IR (74,75). However, further research is
required to elucidate the mechanism by which adropin-related
glucose, lipid and amino acid metabolism affect PCOS.
Obesity
Obesity poses a serious health hazard globally,
leading to metabolic system disorders and worsening health
conditions (76). In HFD-induced
obese mice, adropin has been shown to reduce obesity and improve
blood lipid and sugar homeostasis (6). An early study by Kumar et al
(1) demonstrated that adropin
prevents obesity-related hyperinsulinemia and hepatic steatosis by
regulating glucose and lipids metabolism. In diet-induced obesity
(DIO) mice, Gao et al (7)
found that adropin treatment improved glucose tolerance, insulin
action and metabolic flexibility in utilizing glucose. Adropin
treatment enhanced the phosphorylation of AKT in muscle cells in
response to insulin and increased the expression of glucose
transporter type 4 on cell surfaces, indicating sensitization of
the insulin signaling pathway. Additionally, adropin activated PDH
and downregulated PDK-4, which inhibits PDH. Furthermore, adropin
treatment downregulated peroxisome proliferator-activated receptor
γ coactivator 1-α, which regulates carnitine palmitoyltransferase
1B, CD36 and PDK4 expression (7). Gao et al (77) also investigated the molecular
mechanism underpinning the effect of adropin on liver glucose
metabolism in DIO mice. The results indicated that adropin reduced
the endoplasmic reticulum stress response and c-Jun N-terminal
kinase activity in the liver, thereby enhancing the liver insulin
signaling pathway. Additionally, adropin treatment inhibited
protein kinase A activity, leading to decreased phosphorylation of
the inositol trisphosphate receptor, which mediates calcium efflux
in the endoplasmic reticulum, and cyclic adenosine monophosphate
response element-binding protein, a key transcription factor that
regulates glucose metabolism in the liver. Consequently, blood
glucose regulation in DIO mice improved upon adropin administration
(77). Obesity-related metabolic
dysregulation has been reported to lead to mild cognitive
impairment and increase the risk of dementia (78,79). Ghoshal et al (80) found that overexpression of the
adropin gene improved recognition memory in LDL receptor-deficient
C57BL/6J obese mice. The authors proposed that adropin may enhance
cognitive function in patients with severe metabolic disorders
through pathways related to intercellular communication and
neuronal processes. However, the specific mechanisms remain to be
explored.
Research indicates that serum adropin levels are
significantly decreased in obese children (81-83). Yin et al (84) propose that adropin levels could
serve as a biomarker for predicting metabolic syndrome in obese
children. Another study found that obese prepubertal children had
significantly higher adropin levels compared with their
normal-weight counterparts. Notably, the adropin concentration in
prepubertal girls was higher than in prepubertal boys, whereas
adolescent girls had lower levels than adolescent boys (33). This suggests that adropin levels
are associated with sex hormones that regulate pubertal
development, although further research is needed to confirm
this.
A systematic review and meta-analysis of adults with
obesity revealed that overweight and obese participants had
significantly lower circulating adropin levels than normal-weight
participants, indicating a potential role of adropin in obesity
development (85). A study by
Erman et al (5), which
included 50 obese patients and 22 controls, examined the
relationship between serum adropin and IR. The findings showed that
the serum adropin level was inversely related to BMI, waist
circumference, diastolic blood pressure, fasting blood glucose and
insulin levels. The researchers suggested that serum adropin may
help regulate glucose and lipid metabolism and IR in obese
patients. However, further research is necessary to fully
understand the potential of adropin in regulating metabolic
disorders and its precise mechanisms of action in obese
populations.
Diabetes and its complications
Multiple reports have shown that adropin levels are
associated with the occurrence of diabetes and its complications,
including DKD, diabetic cardiomyopathy and diabetic retinopathy
(DR) (86-88). Adropin levels are closely related
to IR independent of obesity. Moreover, glycemic stability can be
improved through the overexpression or exogenous administration of
adropin (5). Adropin reduces
hepatic glucose synthesis, thereby lowering blood glucose levels in
mice (26).
Diabetes
The metabolic characteristics of patients with
diabetes include typical disorders of glucose and lipid metabolism
(89). Glucose metabolism
disorders are primarily characterized by significantly high blood
sugar, often accompanied by IR and insulin deficiency. In patients
with diabetes, fat synthesis decreases while decomposition
accelerates, leading to lipid metabolism disorders. These disorders
manifest as hypertriglyceridemia, hypercholesterolemia or elevated
LDL levels (89,90). A study found that adropin can
restore glucose oxidation in prediabetic obese mice by regulating
mitochondrial acetyltransferase general control of amino acid
synthesis 5 like-1 (GCN5L1) expression. Adropin regulated
mitochondrial acetyltransferase GCN5L1 expression, altering the
acetylation status and activity of fuel metabolism enzymes to
facilitate glucose utilization (91). He et al (86) discovered that adropin
significantly activated key regulatory molecules of the AMPK,
PI3K/AKT and insulin signaling pathways, including p-AKT, PI3K,
insulin receptor, insulin receptor substrate 1 and AKT. The authors
concluded that adropin exerts anti-diabetic effects in diabetic
rats by modulating the PI3K/AKT and insulin signaling pathways.
Furthermore, adropin was found to regulate lipid metabolism in
diabetic rats, significantly reducing the LDL, very LDL, TG and
cholesterol levels, while increasing HDL in a dose-dependent manner
(86). Similarly, Skrzypski
et al (92) reported that
adropin treatment in T2DM mice reduced liver mass, aspartate
aminotransferase, alanine aminotransferase, serum γ-glutamyl
transferase and alkaline phosphatase, cholesterol and hepatic
triacylglycerol levels.
A study involving 116 patients with T2DM and 60
normal controls in China indicated that serum adropin levels were
downregulated in patients with T2DM, particularly in overweight and
obese individuals (93). Choi
and Yim (94) suggested that
plasma adropin could be a predictive marker for obesity and
obesity-related cancer in Korean patients with T2DM. A 2023
meta-analysis on circulating adropin expression and diabetes also
found reduced adropin levels in patients with diabetes compared
with healthy individuals (95).
These studies suggest that adropin is related to glucose and lipid
homeostasis and insulin sensitivity and serves a role in diabetes
pathogenesis. Additionally, Palizban et al (96) analyzed the rs7903146 allele
frequency in 93 patients with T2DM and 53 healthy individuals,
finding that patients with T2DM carrying rs7903146T/T and
rs7903146C/T had higher adropin levels and had an increased risk of
developing T2DM (96). Another
study found that high-intensity interval training increased plasma
adropin and nitrate/nitrite levels, improving blood pressure and
flow-mediated dilation in patients with T2DM. Increased plasma
adropin levels may lower blood pressure partly by enhancing NO
production (97). Tičinović
Kurir et al (98)
reported that liraglutide treatment in obese male patients with
T2DM significantly increased plasma adropin concentration, reduced
weight and improved IR indicators. Similarly, sitagliptin treatment
significantly enhanced serum adropin expression in patients with
T2DM (99). These reports
suggest that adropin concentration holds considerable promise as a
biomarker for therapeutic improvement in patients with diabetes
following drug treatment.
DKD
DKD is a common clinical complication of diabetes
and the main cause of ESRD (100,101). To explore the correlation
between serum adropin concentration and DKD, Hu and Chen (87) analyzed 245 individuals with T2DM
and 81 healthy controls. The patients with T2DM were divided into
three groups (normoalbuminuria, microalbuminuria and
macroalbuminuria) based on the urinary albumin/creatinine ratio
(ACR). The results demonstrated that the serum adropin
concentration in patients with T2DM was significantly lower than
that in the control group. Furthermore, patients with T2DM
accompanied by microalbuminuria and macroalbuminuria exhibited
lower serum adropin levels than those with normoalbuminuria. Serum
adropin was positively correlated with glomerular filtration rate
and negatively correlated with BMI, blood urea nitrogen, creatinine
and ACR. The study concluded that elevated serum adropin was
associated with improved renal function and serum adropin was
inversely associated with DKD development (87). Another study evaluated serum
adropin levels in patients with T2DM with and without renal
disease. This study included 135 participants (45 patients with
DKD, 45 non-renal diabetic patients and 45 healthy controls), and
adropin levels in fasting venous serum were measured. The results
indicated that patients with DKD exhibited lower serum adropin
levels compared with both the controls and non-renal diabetic
patients (102). Additionally,
serum adropin was negatively correlated with BMI, fasting blood
glucose, glycated hemoglobin, blood urea, creatinine, LDL and ACR,
and positively correlated with HDL and albumin. Therefore, serum
adropin levels could serve as a biomarker for detecting DKD
(102). A recent study showed
that adropin can improve renal lipotoxicity in mice with DKD. The
primary mechanism involves adropin treatment inhibiting ROS
production and protecting mitochondria from damage. Adropin also
reduces ADRP and SREBP-1 lipogenic protein expression in DKD mice,
thereby improving lipid deposition in renal tissue (8). However, further research is needed
to elucidate the precise mechanisms.
Diabetic cardiomyopathy
Adropin has been shown to regulate the expression of
GCN5L1, altering the acetylation activity and status of enzymes
involved in fuel metabolism, thereby facilitating glucose
utilization (91). Under
high-fat conditions, adropin exposure increases myocardial glucose
oxidation (91), potentially
opening novel avenues for treating diabetic cardiomyopathy in the
future. Another study demonstrated that adropin treatment reduced
the perivascular collagen area, collagen volume fraction and the
relative protein expression levels of dynamin-related protein 1,
collagen I and collagen III in diabetic cardiomyopathy rats
(103). Additionally, cardiac
diastolic function and the expression of Mitofusin-1 and
Mitofusin-2 were upregulated in the adropin-treated group (103). Currently, no clinical studies
have reported on the relationship between adropin and diabetic
cardiomyopathy.
DR
To investigate the correlation between serum and
vitreous adropin concentrations and the presence of DR, Li et
al (104) conducted a study
involving 165 patients with T2DM (52 without DR, 69 with
non-proliferative DR and 44 with proliferative DR). The control
group included 68 healthy participants who had undergone vitrectomy
for retinal detachment. The results showed that control
participants had significantly higher serum and vitreous adropin
concentrations compared with patients with diabetes. Patients with
proliferative DR exhibited significantly lower serum and vitreous
adropin concentrations compared with patients with
non-proliferative DR and those with T2DM without DR. Additionally,
patients with non-proliferative DR exhibited lower serum and
vitreous adropin concentrations compared with patients with T2DM
without DR. Logistic regression analysis revealed that serum and
vitreous adropin were associated with a decreased risk of T2DM and
DR, leading to the conclusion that serum and vitreous adropin
concentrations are inversely related to the presence of DR
(104).
In a subsequent study, Li et al (88) measured the concentrations of
plasma cytokines (adropin, copeptin, neprilysin and
chitotriosidase) in 392 patients with TD2M (with or without
retinopathy) and 120 healthy volunteers to predict the risk of DR
in patients with diabetes. It was found that the patients with
concurrent diabetes and retinopathy had reduced levels of adropin
and increased levels of copeptin, neprilysin and chitotriosidase
compared with both the normal controls and patients with diabetes
without retinopathy (88).
However, further in vivo and in vitro studies are
needed to explore the underlying mechanisms.
Atherosclerosis
Low levels of adropin are closely associated with
the occurrence of coronary atherosclerosis (105,106). In the pathophysiology of
atherosclerosis, adropin impacts three critical vascular cells:
VSMCs, macrophages and endothelial cells. Adropin treatment may
reduce atherosclerotic lesion development, irrespective of
metabolic abnormalities or blood pressure (107). Lovren et al (9) demonstrated that adropin protects
endothelial cells by activating the ERK1/2 and PI3K/AKT pathways
via VEGFR2, enhancing the expression of eNOS, increasing NO release
and improving endothelial cell function (9,108). Adropin consistently promotes
capillary formation, proliferation, migration and vascular
permeability and reduces TNF-α-induced apoptosis in endothelial
cells (9). Additionally, adropin
treatment has been shown to reduce endothelial cell permeability by
inhibiting the Rock/myosin light chain 2 pathway (107). In ApoE−/− mice,
adropin treatment reduced the development of atherosclerotic
lesions by regulating plaque stability and vascular elasticity.
This was achieved by suppressing VSMC proliferation by
downregulating the c-Src/ERK1/2 pathway and upregulating the
PI3K/AKT pathway, thereby increasing fibronectin and elastin
expression in VSMCs (109).
Furthermore, adropin enhances the anti-inflammatory activity of
endothelial cells by increasing the expression of PPAR-γ,
decreasing monocyte-endothelial cell adhesion and promoting
monocyte differentiation into anti-inflammatory phenotypes, thus
reducing the inflammatory development of atherosclerosis (109). Li and Xie (110) found that histone deacetylases
11-atherosclerosis (HDAC11-AS1) improved blood lipid levels and
atherosclerosis in ApoE−/− mice on an HFD by negatively
regulating HDAC11. This mechanism involves HDAC11-AS1 enhancing LPL
expression through adropin histone deacetylation. A recent study
has also indicated that adropin restrains the differentiation of
VSMCs into osteogenic cells and reduces vascular calcification via
the JAK2/STAT3 pathway (10).
Moreover, adropin treatment may alleviate atherosclerosis in
ApoE−/−/ENHO−/− mice by inhibiting
endothelial-to-mesenchymal transition through the TGF-β/Smad2/3
signaling pathway (111).
Human studies have also linked decreased serum
adropin levels to coronary atherosclerosis (35). Elevated adropin levels in
overweight participants after high-intensity interval training may
indicate improved endothelial function by enhancing NO-related
signaling pathways (112). Wei
et al (34) examined 503
patients with T2DM and found that increased serum adropin levels
were correlated with a decreased risk of carotid atherosclerosis.
Low circulating adropin levels may thus promote carotid
atherosclerosis (34). These
outcomes suggest that adropin could be a novel target for treating
atherosclerosis.
MS
MS is a chronic autoimmune disease characterized by
progressive neuronal loss, demyelination and central nervous system
inflammation (113,114). Clinical studies have
consistently shown significantly diminished adropin levels in
patients with MS compared with control groups (115,116). However, the molecular
mechanisms linking MS and adropin remain unclear. A study by
Demirdöğen et al (117)
recruited 80 participants (40 patients with MS and 40 healthy
volunteers) to investigate the serum adropin levels in patients
with MS and their correlation with hypothalamic atrophy. The study
found that patients with MS exhibited significantly reduced adropin
levels compared with healthy controls, although no significant
correlation was observed between serum adropin levels and pituitary
diameter or third ventricular diameter. While adropin shows high
potential as a diagnostic marker for MS, comprehensive validation
studies are needed (117). A
2024 report highlighted the critical role of adropin in remodeling
fibrotic tissue in systemic sclerosis. Consistent downregulation of
adropin was observed in the skin of patients with systemic
sclerosis (36). In vivo
and in vitro experiments demonstrated that the profibrotic
cytokine, TGF-β, suppressed adropin expression via a JNK-dependent
pathway. Adropin treatment restored adropin signaling, inhibiting
TGF-β-induced activation of fibroblasts and primary human dermal
fibroblasts, thereby reducing fibrotic tissue remodeling (36). Notably, knocking down the adropin
receptor, GPR19, abolished the anti-fibrotic effects of adropin in
fibroblasts. These insights suggest that TGF-β-induced
downregulation of adropin expression may be a potential
pathological mechanism in systemic sclerosis (36).
Cancer
Research has demonstrated the involvement of
adropin in the biological effects of cancer cells. The pathological
processes involved are not only related to carbohydrate and sterol
metabolism but also the regulation of intercellular pathways. A
previous clinical study including 74 patients (47 patients with
endometrial cancer and 27 healthy participants) demonstrated that
patients with endometrial cancer had significantly reduced serum
adropin levels compared with the control patients (38). However, the specific pathological
mechanism remains unclear.
It is reported that adropin could promote MCF-7
cells to enter the early apoptosis stage and have a protective
effect on breast cancer (37).
Another study showed that adropin-mediated GPR19 activation could
promote mesenchymal-epithelial transition through the MAPK/ERK1/2
pathway, thereby promoting the proliferation and metastasis of
breast tumor cells (118).
However, more research is needed to explore the mechanism of action
in breast cancer.
Stelcer et al (119) found that adropin can activate
TGF-β signaling and reduce the expression levels of steroidogenic
genes (such as steroidogenesis, the steroidogenic acute regulatory
and cholesterol side-chain cleavage enzyme and cytochrome P450scc)
in human adrenal cancer (HAC15) cells, thereby inhibiting cortisol
and aldosterone biosynthesis and secretion. In addition, the
proliferation of adropin-treated HAC15 cells was significantly
enhanced compared with untreated cells. The specific mechanism
involves the regulation of adropin via the ERK1/2 and AKT-dependent
signaling pathways. It was also found that GPR19 expression was
increased in adrenocortical carcinoma compared with normal adrenal
glands (119).
It has also been demonstrated that adropin
expression is likewise diminished in advanced colorectal cancer
(CRC) tumor nest cells (120).
The expression of adropin by cancer cells is negatively related to
the infiltration of macrophages into the CRC tissue matrix.
Nonetheless, promoting adropin expression in tumor macrophages can
enhance tumor invasion and metastasis (120). An investigation into the
effects of adropin treatment on macrophages revealed that low-dose
adropin promoted glucose utilization, while high-dose adropin
increased carnitine palmitoyltransferase 1α expression in
macrophages. As a result, it was hypothesized that fluctuations in
adropin levels within malignant cells or macrophages within tumor
tissues contributed to the progression of CRC to varying degrees.
Therefore, tumor progression in various stages of CRC can be slowed
by elevating or lowering adropin levels (120).
In a recent study in 2023 it was found that adropin
expression was increased in pancreatic ductal adenocarcinoma (PDA)
tissue compared with adjacent tissue (121). The proliferative and migratory
capabilities of PDA cells are significantly enhanced by adropin,
which also increases the expression of cyclin D1, Ki67, p-VEGFR2
and MMP-2. However, these effects were significantly reversed after
knocking down adropin expression or blocking VEGFR2. In addition,
in PDA, adropin upregulation enhances angiogenesis and cancer cell
proliferation in the tumor microenvironment through persistent
activation of the VEGFR2 signaling pathway, thus establishing an
environment conducive to tumor progression (121). These findings suggest that PDA
could potentially be effectively treated by targeting adropin.
It is well known that the functional expression of
genes is affected by the microenvironment. In different cancer
types, adropin is affected by different molecules and pathways or
acts on different downstream molecules to play anticancer or
cancer-promoting effects. Since there are few relevant research
reports, the specific mechanism cannot be elucidated. More research
should be conducted in the future to explore more potential
functions of adropin.
Conclusions and future prospectives
Adropin, a key peptide hormone, is integral to
maintaining energy homeostasis and regulating glucose and fatty
acid metabolism. There is a strong association between low adropin
levels and IR that is independent of obesity (122). Conversely, upregulation or
exogenous administration of adropin can improve glucose homeostasis
(11). In HFD-induced obese
mice, adropin reduces obesity and enhances blood lipid and glucose
homeostasis (6). Additionally,
adropin promotes cardiac glucose oxidation and inhibits cardiac
fatty acid oxidation, thereby improving cardiac energy metabolism
and efficiency (40). Adropin
also influences lipid metabolism by regulating the expression of
genes associated with liver disease and the PPARα receptor, a key
regulator of lipogenesis (123). Moreover, adropin interacts with
the NB-3/Notch signaling pathway to regulate physical activity and
movement coordination (16).
Adropin enhances spatial memory in rats by modulating the
AKT/cyclic AMP response element-binding protein/brain-derived
neurotrophic factor signaling pathway (124). These studies over the past
decade indicate that adropin is involved in both metabolic and
non-metabolic regulation in various diseases. Adropin-based
therapies may emerge as novel treatments for diseases related to
glucose and lipid metabolism.
Adropin, a novel regulatory peptide, presents
numerous challenges for preclinical research. First, its
pharmacokinetics in circulation remain largely unknown, as protein
degradation may limit the efficacy of peptide hormone
administration. Numerous questions about adropin physiology
necessitate further investigation. For instance, the origin of
circulating adropin and whether liver-produced adropin regulates
other tissues require elucidation in both animal models and
patients. Second, adropin expression is influenced by fat and
various molecules, yet the specific regulatory mechanisms remain
unclear. Additionally, lifestyle, diet, weight and body composition
across different races and nationalities may affect adropin levels.
Third, current animal studies suggest the involvement of adropin in
glucose oxidation, lipid metabolism, metabolic diseases,
endothelial function and cardiovascular diseases. However, research
on the function of adropin is still preliminary, with most studies
focusing on mRNA-level gene expression. Notable questions persist,
such as the association between reduced plasma adropin levels and
disease progression, contrasted with high levels observed in kidney
transplant candidates. Changes in adropin levels also appear to
influence CRC progression, though the mechanisms remain undefined.
Given the identical amino acid sequences in humans and mice, animal
study conclusions are likely applicable to humans. A comprehensive
understanding of the function of adropin could lead to its
therapeutic use in various metabolic disorders, warranting
continued exploration. Fourth, mounting evidence links adropin to
inflammatory diseases, where it not only promotes the secretion of
inflammatory cytokines but also indirectly regulates immune cell
phenotypes and behaviors. Additionally, animal studies suggest a
connection between adropin and the circadian clock, although this
area requires further research to clarify its potential
impacts.
Several issues warrant attention in the clinical
research of adropin. First, most published clinical studies on
adropin are limited to observational studies that demonstrate
correlations between plasma adropin levels and diet, various
diseases and metabolic parameters (such as obesity and coronary
heart disease risk). The growing body of evidence from preclinical
studies may inspire future clinical trials to explore the potential
benefits of adropin or its analogs in treating obesity, diabetes,
fatty liver disease and diabetic cardiovascular disease. Second,
reduced plasma levels of adropin appear to be associated with
various pathologies and often correlate with accelerated disease
progression. A number of researchers suggest that adropin could
serve as a serum marker for diseases such as diabetes,
atherosclerosis and AMI. However, each new biomarker must be
thoroughly evaluated for its clinical relevance such as determining
the appropriate patient groups, optimal measurement time points and
whether the biomarker provides additional information beyond the
existing ones. Thus, to establish adropin as a disease biomarker in
various pathologies, prospective large-scale studies in
well-defined populations are necessary. In conclusion, while future
research on adropin faces numerous challenges, exploring its
potential functions and those of its analogs remains highly
valuable for the treatment of obesity, diabetes, cardiovascular
diseases and more.
Availability of data and materials
Not applicable.
Authors' contributions
LC contributed to acquisition, analysis,
interpretation and drafted the manuscript. JL participated in
revising the manuscript. JH and XG contributed to conception and
design and critically revised the manuscript. All authors read and
approve the final version of the manuscript. Data authentication is
not applicable.
Ethics approval and consent to
participate
Not applicable.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
Abbreviations:
|
ACR
|
albumin/urine creatinine ratio
|
|
ADRP
|
adipocyte differentiation-related
protein
|
|
AMPK
|
AMP-activated protein kinase
|
|
AKT
|
protein kinase B
|
|
BMI
|
body mass index
|
|
CD36
|
cluster of differentiation 36
|
|
CKD
|
chronic kidney disease
|
|
CRC
|
colorectal cancer
|
|
DIO
|
diet-induced obesity
|
|
eNOS
|
endothelial nitric oxide synthase
|
|
ENHO
|
energy homeostasis-associated
gene
|
|
ERα
|
estrogen receptor α
|
|
ERK1/2
|
extracellular regulated kinase
1/2
|
|
ESRD
|
end-stage renal disease
|
|
FF
|
follicular fluid
|
|
GCN5L1
|
general control of amino acid
synthesis 5 like-1
|
|
GPR19
|
G-protein coupled receptor 19
|
|
GLP-1
|
glucagon-like peptide-1
|
|
HDL
|
high-density lipoprotein
|
|
HFD
|
high-fat diet
|
|
HD
|
hemodialysis
|
|
IR
|
insulin resistance
|
|
LDL
|
low-density lipoprotein
|
|
LXRα
|
liver X receptor α
|
|
MIP-1/2
|
macrophage inflammatory protein
1/2
|
|
MAFLD
|
dysfunction-associated fatty liver
disease
|
|
NAFLD
|
non-alcoholic fatty liver disease
|
|
NASH
|
non-alcoholic steatohepatitis
|
|
NLRP3
|
NLR family pyrin domain containing
3
|
|
PI3K
|
phosphatidylinositol-3 kinase
|
|
PDH
|
pyruvate dehydrogenase
|
|
PD
|
peritoneal dialysis
|
|
PDK4
|
pyruvate dehydrogenase kinase 4
|
|
PPARγ
|
peroxisome proliferator-activated
receptor γ
|
|
PDA
|
pancreatic ductal adenocarcinoma
|
|
PP2A
|
protein phosphatase 2A
|
|
Rock
|
Rho-associated coiled-coil containing
protein kinase
|
|
ROR
|
retinoic acid receptor-related orphan
receptor
|
|
ROS
|
reactive oxygen species
|
|
STAT3
|
signal transducer and activator of
transcription 3
|
|
SREBP-1
|
sterol regulatory element-binding
protein 1
|
|
TGF-β
|
transforming growth factor β
|
|
TC
|
total cholesterol
|
|
TG
|
triglycerides
|
|
T2DM
|
type 2 diabetes mellitus
|
|
VEGFR2
|
vascular endothelial growth factor
receptor-2
|
|
VSMC
|
vascular smooth muscle cell
|
Acknowledgements
Not applicable.
Funding
This work was supported by grants from Pudong New Area
Traditional Chinese Medicine Brand Multiplication Plan-Chronic
Nephropathy (grant no. PDZY-2021-0302), Construction of He Liqun's
famous TCM studio (grant no. PDZY-2022-0703), Clinical Observation
on the Efficacy of Guben Tongluo Formula in Treating Chronic Kidney
Disease Phase 1-3 (grant no. PW2022D-12) and Pilot Project of
Inheritance, Innovation and Development of Traditional Chinese
Medicine in Pudong New Area (grant no. YC-2023-0602).
References
|
1
|
Kumar KG, Trevaskis JL, Lam DD, Sutton GM,
Koza RA, Chouljenko VN, Kousoulas KG, Rogers PM, Kesterson RA,
Thearle M, et al: Identification of adropin as a secreted factor
linking dietary macronutrient intake with energy homeostasis and
lipid metabolism. Cell Metab. 8:468–481. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Marczuk N, Cecerska-Heryć E, Jesionowska A
and Dołęgowska B: Adropin-physiological and pathophysiological
role. Postepy Hig Med Dosw (Online). 70:981–988. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Aydin S, Kuloglu T, Aydin S, Eren MN,
Yilmaz M, Kalayci M, Sahin I, Kocaman N, Citil C and Kendir Y:
Expression of adropin in rat brain, cerebellum, kidneys, heart,
liver, and pancreas in streptozotocin-induced diabetes. Mol Cell
Biochem. 380:73–81. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Chen X, Chen S, Shen T, Yang W, Chen Q,
Zhang P, You Y, Sun X, Xu H, Tang Y, et al: Adropin regulates
hepatic glucose production via PP2A/AMPK pathway in
insulin-resistant hepatocytes. FASEB J. 34:10056–10072. 2020.
View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Erman H, Ozdemir A, Sitar ME, Cetin SI and
Boyuk B: Role of serum adropin measurement in the assessment of
insulin resistance in obesity. J Investig Med. 69:1318–1323. 2021.
View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Akcılar R, Emel Koçak F, Şimşek H, Akcılar
A, Bayat Z, Ece E and Kökdaşgil H: The effect of adropin on lipid
and glucose metabolism in rats with hyperlipidemia. Iran J Basic
Med Sci. 19:245–251. 2016.
|
|
7
|
Gao S, McMillan RP, Zhu Q, Lopaschuk GD,
Hulver MW and Butler AA: Therapeutic effects of adropin on glucose
tolerance and substrate utilization in diet-induced obese mice with
insulin resistance. Mol Metab. 4:310–324. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Yu M, Wang D, Zhong D, Xie W and Luo J:
adropin carried by reactive oxygen species-responsive nanocapsules
ameliorates renal lipid toxicity in diabetic mice. ACS Appl Mater
Interfaces. 14:37330–37344. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Lovren F, Pan Y, Quan A, Singh KK, Shukla
PC, Gupta M, Al-Omran M, Teoh H and Verma S: Adropin is a novel
regulator of endothelial function. Circulation. 122(11 Suppl):
S185–S192. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Wang L, Jin F, Wang P, Hou S, Jin T, Chang
X and Zhao L: Adropin inhibits vascular smooth muscle cell
osteogenic differentiation to alleviate vascular calcification via
the JAK2/STAT3 signaling pathway. Biomed Res Int.
2022:91222642022.PubMed/NCBI
|
|
11
|
Ali II, D'Souza C, Singh J and Adeghate E:
Adropin's role in energy homeostasis and metabolic disorders. Int J
Mol Sci. 23:83182022. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Jasaszwili M, Billert M, Strowski MZ,
Nowak KW and Skrzypski M: Adropin as A Fat-Burning hormone with
multiple functions-review of a decade of research. Molecules.
25:5492020. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Li L, Xie W, Zheng XL, Yin WD and Tang CK:
A novel peptide adropin in cardiovascular diseases. Clin Chim Acta.
453:107–113. 2016. View Article : Google Scholar
|
|
14
|
Niepolski L and Grzegorzewska AE: Salusins
and adropin: New peptides potentially involved in lipid metabolism
and atherosclerosis. Adv Med Sci. 61:282–287. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Petersen TN, Brunak S, von Heijne G and
Nielsen H: SignalP 4.0: discriminating signal peptides from
transmembrane regions. Nat Methods. 8:785–786. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Wong CM, Wang Y, Lee JT, Huang Z, Wu D, Xu
A and Lam KS: Adropin is a brain membrane-bound protein regulating
physical activity via the NB-3/Notch signaling pathway in mice. J
Biol Chem. 289:25976–25986. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Zhang C, Yan Y, Zhang Q and Jiang Q:
Molecular cloning and characterization of the novel adropin from
tilapia (Oreochromis niloticus): Involvement in the control of food
intake. Neuropeptides. 88:1021652021. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Ganesh Kumar K, Zhang J, Gao S, Rossi J,
McGuinness OP, Halem HH, Culler MD, Mynatt RL and Butler AA:
Adropin deficiency is associated with increased adiposity and
insulin resistance. Obesity (Silver Spring). 20:1394–1402. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Bindesbøll C, Fan Q, Nørgaard RC,
MacPherson L, Ruan HB, Wu J, Pedersen TA, Steffensen KR, Yang X,
Matthews J, et al: Liver X receptor regulates hepatic nuclear
O-GlcNAc signaling and carbohydrate responsive element-binding
protein activity. J Lipid Res. 56:771–785. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Stayrook KR, Rogers PM, Savkur RS, Wang Y,
Su C, Varga G, Bu X, Wei T, Nagpal S, Liu XS and Burris TP:
Regulation of human 3 alpha-hydroxysteroid dehydrogenase (AKR1C4)
expression by the liver X receptor alpha. Mol Pharmacol.
73:607–612. 2008. View Article : Google Scholar
|
|
21
|
Ghoshal S, Stevens JR, Billon C, Girardet
C, Sitaula S, Leon AS, Rao DC, Skinner JS, Rankinen T, Bouchard C,
et al: Adropin: An endocrine link between the biological clock and
cholesterol homeostasis. Mol Metab. 8:51–64. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Meda C, Dolce A, Vegeto E, Maggi A and
Della Torre S: ERα-Dependent regulation of adropin predicts sex
differences in liver homeostasis during high-fat diet. Nutrients.
14:32622022. View Article : Google Scholar
|
|
23
|
Stokar J, Gurt I, Cohen-Kfir E, Yakubovsky
O, Hallak N, Benyamini H, Lishinsky N, Offir N, Tam J and
Dresner-Pollak R: Hepatic adropin is regulated by estrogen and
contributes to adverse metabolic phenotypes in ovariectomized mice.
Mol Metab. 60:1014822022. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Kuo FY, Cheng KC, Li Y, Cheng JT and Tsai
CC: Promotion of adropin expression by hyperglycemia is associated
with STAT3 activation in diabetic rats. Diabetes Metab Syndr Obes.
13:2269–2277. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Li YX, Cheng KC, Liu IM and Niu HS:
Myricetin Increases circulating adropin level after activation of
glucagon-like peptide 1 (GLP-1) receptor in type-1 diabetic rats.
Pharmaceuticals (Basel). 15:1732022. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Thapa D, Xie B, Manning JR, Zhang M,
Stoner MW, Huckestein BR, Edmunds LR, Zhang X, Dedousis NL,
O'Doherty RM, et al: Adropin reduces blood glucose levels in mice
by limiting hepatic glucose production. Physiol Rep. 7:e140432019.
View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Jasaszwili M, Pruszyńska-Oszmałek E,
Wojciechowicz T, Strowski MZ, Nowak KW and Skrzypski M: Adropin
slightly modulates lipolysis, lipogenesis and expression of
adipokines but not glucose uptake in rodent adipocytes. Genes
(Basel). 12:9142021. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Yu HY, Zhao P, Wu MC, Liu J and Yin W:
Serum adropin levels are decreased in patients with acute
myocardial infarction. Regul Pept. 190-191:46–49. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Ding F, Liu G, Gao F, Zheng Z, Hong Y,
Chen Y and Weng S: Adropin attenuates pancreatitis-associated lung
injury through PPARγ phosphorylation-related macrophage
polarization. Int J Mol Med. 52:952023. View Article : Google Scholar
|
|
30
|
Kutlu O, Altun Ö, Dikker O, Aktaş Ş, Özsoy
N, Arman Y, Özgün Çil E, Özcan M, Aydın Yoldemir Ş, Akarsu M, et
al: Serum adropin levels are reduced in adult patients with
nonalcoholic fatty liver disease. Med Princ Pract. 28:463–469.
2019. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Berezina TA, Obradovic Z, Boxhammer E,
Berezin AA, Lichtenauer M and Berezin AE: Adropin predicts chronic
kidney disease in type 2 diabetes mellitus patients with chronic
heart failure. J Clin Med. 12:22312023. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Kuliczkowska-Płaksej J, Mierzwicka A,
Jończyk M, Stachowska B, Urbanovych A and Bolanowski M: Adropin in
women with polycystic ovary syndrome. Endokrynol Pol. 70:151–156.
2019. View Article : Google Scholar
|
|
33
|
Herrero L, de Dios O, Gavela-Pérez T,
Riestra P, Jois A, Soriano-Guillén L and Garcés C: Opposite
association of adropin concentrations with obesity in prepubertal
children compared with adolescents. Obesity (Silver Spring).
28:1736–1741. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Wei W, Liu H, Qiu X, Zhang J, Huang J,
Chen H, Qiu S, Lin R, Li S and Tu M: The association between serum
adropin and carotid atherosclerosis in patients with type 2
diabetes mellitus: A cross-sectional study. Diabetol Metab Syndr.
14:272022. View Article : Google Scholar
|
|
35
|
Zhao LP, You T, Chan SP, Chen JC and Xu
WT: Adropin is associated with hyperhomocysteine and coronary
atherosclerosis. Exp Ther Med. 11:1065–1070. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Liang M, Dickel N, Györfi AH,
SafakTümerdem B, Li YN, Rigau AR, Liang C, Hong X, Shen L, Matei
AE, et al: Attenuation of fibroblast activation and fibrosis by
adropin in systemic sclerosis. Sci Transl Med. 16:eadd65702024.
View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Tuna BG, Atalay PB, Altunbek M, Kalkan BM
and Dogan S: Effects of chronic and intermittent calorie
restriction on adropin levels in breast cancer. Nutr Cancer.
69:1003–1010. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Nergiz S, Altinkaya SO, Kurt Ömürlü İ,
Yuksel H, Küçük M and Demircan Sezer S: Circulating adropin levels
in patients with endometrium cancer. Gynecol Endocrinol.
31:730–735. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Reed GW, Rossi JE and Cannon CP: Acute
myocardial infarction. Lancet. 389:197–210. 2017. View Article : Google Scholar
|
|
40
|
Altamimi TR, Gao S, Karwi QG, Fukushima A,
Rawat S, Wagg CS, Zhang L and Lopaschuk GD: Adropin regulates
cardiac energy metabolism and improves cardiac function and
efficiency. Metabolism. 98:37–48. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Aydin S, Kuloglu T, Aydin S, Kalayci M,
Yilmaz M, Çakmak T and Eren MN: Elevated adropin: A candidate
diagnostic marker for myocardial infarction in conjunction with
troponin-I. Peptides. 58:91–97. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Li B, Wang Z, He Y, Chen T, Zhang Y, Yuan
X and Li P: Adropin improves radiation-induced myocardial injury
via VEGFR2/PI3K/Akt pathway. Oxid Med Cell Longev.
2022:82302142022.PubMed/NCBI
|
|
43
|
Adıyaman M, Canpolat Erkan RE, Kaya İ and
Aba Adıyaman Ö: Serum adropin level in the early period of
ST-Elevation myocardial infarction and its relationship with
cobalamin and folic acid. Cureus. 14:e327482022.
|
|
44
|
Chang X, Jin F, Wang L, Jiang Y, Wang P,
Liu J and Zhao L: Adropin-A new player in energy regulation
predicts long-term prognosis of patients with acute myocardial
infarction. Heliyon. 9:e178032023. View Article : Google Scholar
|
|
45
|
Foucher P, Heeringa P, Petersen AH,
Huitema MG, Brouwer E, Tervaert JW, Prop J, Camus P, Weening JJ and
Kallenberg CG: Antimyeloperoxidase-associated lung disease. An
experimental model. Am J Respir Crit Care Med. 160:987–994. 1999.
View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Saeki T, Fujita N, Kourakata H, Yamazaki H
and Miyamura S: Two cases of hypertrophic pachymeningitis
associated with myeloperoxidase antineutrophil cytoplasmic
autoantibody (MPO-ANCA)-positive pulmonary silicosis in tunnel
workers. Clin Rheumatol. 23:76–80. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Gao F, Fang J, Chen F, Wang C, Chen S,
Zhang S, Lv X, Zhang J, He Q, Weng S, et al: Enho mutations causing
low adropin: A possible pathomechanism of MPO-ANCA Associated lung
injury. EBioMedicine. 9:324–335. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
48
|
Rizk FH, El-Saka MH, Ibrahim RR, El-Deeb
OS, Ibrahim HA, El Saadany AA, Mashal SS, Ammar L, Abdelsattar AM
and Barhoma RA: Possible mitigating effect of adropin on lung
injury in diabetic rats: Targeting the role of Rho A/Rho-associated
kinase pathway. Biofactors. 49:928–939. 2023. View Article : Google Scholar : PubMed/NCBI
|
|
49
|
Orun S, Celikkol A, Basol BI and Yeniay E:
Diagnostic accuracy of adropin as a preliminary test to exclude
acute pulmonary embolism: a prospective study. BMC Pulm Med.
22:3512022. View Article : Google Scholar : PubMed/NCBI
|
|
50
|
Byrne CD and Targher G: NAFLD: A
multisystem disease. J Hepatol. 62(1 Suppl): S47–S64. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
51
|
Younossi Z, Tacke F, Arrese M, Chander
Sharma B, Mostafa I, Bugianesi E, Wai-Sun Wong V, Yilmaz Y, George
J, Fan J and Vos MB: Global perspectives on nonalcoholic fatty
liver disease and nonalcoholic steatohepatitis. Hepatology.
69:2672–2682. 2019. View Article : Google Scholar
|
|
52
|
Zhang C, Zhang Q, Huang Z and Jiang Q:
Adropin inhibited tilapia hepatic glucose output and triglyceride
accumulation via AMPK activation. J Endocrinol. 246:109–122. 2020.
View Article : Google Scholar : PubMed/NCBI
|
|
53
|
Chen X, Sun X, Shen T, Chen Q, Chen S,
Pang J, Mi J, Tang Y, You Y, Xu H and Ling W: Lower adropin
expression is associated with oxidative stress and severity of
nonalcoholic fatty liver disease. Free Radic Biol Med. 160:191–198.
2020. View Article : Google Scholar : PubMed/NCBI
|
|
54
|
Yang W, Liu L, Wei Y, Fang C, Liu S, Zhou
F, Li Y, Zhao G, Guo Z, Luo Y and Li L: Exercise suppresses NLRP3
inflammasome activation in mice with diet-induced NASH: A plausible
role of adropin. Lab Invest. 101:369–380. 2021. View Article : Google Scholar
|
|
55
|
Li N, Xie G, Zhou B, Qu A, Meng H, Liu J
and Wang G: Serum adropin as a potential biomarker for predicting
the development of type 2 diabetes mellitus in individuals with
metabolic dysfunction-associated fatty liver disease. Front
Physiol. 12:6961632021. View Article : Google Scholar : PubMed/NCBI
|
|
56
|
Zhang L, Wu X, Li X, Chang X, Ding X, Wang
Q, Jiang T, Wang G and Liu J: Longitudinal changes in serum adropin
levels and liver fat content during liraglutide treatment in newly
diagnosed patients with type 2 diabetes mellitus and metabolic
dysfunction-associated fatty liver disease. Acta Diabetol.
60:971–979. 2023. View Article : Google Scholar : PubMed/NCBI
|
|
57
|
Zhu T, Du Y, Xuan M, Guo C and Rao X:
Clinical characteristics and Chinese Medicine therapy of chronic
kidney disease combined with cardiovascular disease. Integr Med
Nephrol Androl. 10:e000232023. View Article : Google Scholar
|
|
58
|
Song Z and Gong X: research progress on
the potential mechanisms of acute kidney injury and chronic kidney
disease induced by proton pump inhibitors. Integr Med Nephrol
Androl. 10:e000272023. View Article : Google Scholar
|
|
59
|
Tao P, Huo J and Chen L: Bibliometric
analysis of the relationship between gut microbiota and chronic
kidney disease from 2001-2022. Integr Med Nephrol Androl.
11:e000172024. View Article : Google Scholar
|
|
60
|
Zhang HQ, Wu S, Chen X, Fang YX, Lan QM,
Zhou ZJ, Qiao YH, Li J, Zhao YR, Pei M and Yang B: Potential
efficacy and mechanism of medicinal plants on chronic kidney
disease-associated vascular calcification: A review. Tradit Med
Res. 9:512024. View Article : Google Scholar
|
|
61
|
Memi G and Yazgan B: Adropin and spexin
hormones regulate the systemic inflammation in adenine-induced
chronic kidney failure in rat. Chin J Physiol. 64:194–201. 2021.
View Article : Google Scholar : PubMed/NCBI
|
|
62
|
Grzegorzewska AE, Niepolski L, Mostowska
A, Warchoł W and Jagodziński PP: Involvement of adropin and
adropin-associated genes in metabolic abnormalities of hemodialysis
patients. Life Sci. 160:41–46. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
63
|
Kałużna M, Hoppe K, Schwermer K, Ibrahim
AY, Pawlaczyk K and Ziemnicka K: Adropin and irisin levels in
relation to nutrition, body composition, and insulin resistance in
patients with end-stage renal disease on chronic hemodialysis and
peritoneal dialysis. Pol Arch Med Wewn. 126:474–482. 2016.
|
|
64
|
Kałużna M, Pawlaczyk K, Schwermer K, Hoppe
K, Człapka-Matyasik M, Ibrahim AY, Sawicka-Gutaj N, Minczykowski A,
Ziemnicka K, Oko A and Ruchała M: Adropin and irisin: New
biomarkers of cardiac status in patients with end-stage renal
disease? A preliminary study. Adv Clin Exp Med. 28:347–353. 2019.
View Article : Google Scholar
|
|
65
|
Cecerska-Heryć E, Adamiak D, Serwin N,
Grygorcewicz B and Dołęgowska B: Comparative analysis of adropin
concentration changes in response to kidney transplantation. Eur J
Intern Med. 84:112–114. 2021. View Article : Google Scholar
|
|
66
|
Maciorkowska M, Musiałowska D and Małyszko
J: Adropin and irisin in arterial hypertension, diabetes mellitus
and chronic kidney disease. Adv Clin Exp Med. 28:1571–1575. 2019.
View Article : Google Scholar : PubMed/NCBI
|
|
67
|
Kaur R, Krishan P, Kumari P, Singh T,
Singh V, Singh R and Ahmad SF: Clinical significance of adropin and
afamin in evaluating renal function and cardiovascular health in
the presence of CKD-MBD biomarkers in chronic kidney disease.
Diagnostics (Basel). 13:31582023. View Article : Google Scholar : PubMed/NCBI
|
|
68
|
Liu F, Cui B, Zhao X, Wu Y, Qin H, Guo Y,
Wang H, Lu M, Zhang S, Shen J, et al: Correlation of serum adropin
levels with risk factors of cardiovascular disease in hemodialysis
patients. Metab Syndr Relat Disord. 19:401–408. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
69
|
Boric-Skaro D, Mizdrak M, Luketin M,
Martinovic D, Tokic D, Vilovic M, Supe-Domic D, Kurir TT and Bozic
J: Serum adropin levels in patients on hemodialysis. Life (Basel).
11:3372021.PubMed/NCBI
|
|
70
|
Kume T, Calan M, Yilmaz O, Kocabas GU,
Yesil P, Temur M, Bicer M and Calan OG: A possible connection
between tumor necrosis factor alpha and adropin levels in
polycystic ovary syndrome. J Endocrinol Invest. 39:747–754. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
71
|
Bousmpoula A, Kouskouni E, Benidis E,
Demeridou S, Kapeta-Kourkouli R, Chasiakou A and Baka S: Adropin
levels in women with polycystic ovaries undergoing ovarian
stimulation: Correlation with lipoprotein lipid profiles. Gynecol
Endocrinol. 34:153–156. 2018. View Article : Google Scholar
|
|
72
|
Inal ZO, Erdem S, Gederet Y, Duran C,
Kucukaydin Z, Kurku H and Sakarya DK: The impact of serum adropin
and ischemia modified albumin levels based on BMI in PCOS.
Endokrynol Pol. 69:135–141. 2018.PubMed/NCBI
|
|
73
|
Ye Z, Zhang C and Zhao Y: Potential
effects of adropin on systemic metabolic and hormonal abnormalities
in polycystic ovary syndrome. Reprod Biomed Online. 42:1007–1014.
2021. View Article : Google Scholar : PubMed/NCBI
|
|
74
|
Varikasuvu SR, Reddy EP, Thangappazham B,
Varshney S, Das VL and Munikumar M: Adropin levels and its
associations as a fat-burning hormone in patients with polycystic
ovary syndrome: A correlational meta-analysis. Gynecol Endocrinol.
37:879–884. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
75
|
Ke Y, Hu J, Zhu Y, Wang Y, Chen S and Liu
S: Correlation between circulating adropin levels and patients with
PCOS: An updated systematic review and meta-analysis. Reprod Sci.
29:3295–3310. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
76
|
Engin A: The definition and prevalence of
obesity and metabolic syndrome. Adv Exp Med Biol. 960:1–17. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
77
|
Gao S, Ghoshal S, Zhang L, Stevens JR,
McCommis KS, Finck BN, Lopaschuk GD and Butler AA: The peptide
hormone adropin regulates signal transduction pathways controlling
hepatic glucose metabolism in a mouse model of diet-induced
obesity. J Biol Chem. 294:13366–13377. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
78
|
Dye L, Boyle NB, Champ C and Lawton C: The
relationship between obesity and cognitive health and decline. Proc
Nutr Soc. 76:443–454. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
79
|
Miller AA and Spencer SJ: Obesity and
neuroinflammation: A pathway to cognitive impairment. Brain Behav
Immun. 42:10–21. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
80
|
Ghoshal S, Banerjee S, Zhang J, Niehoff
ML, Farr SA and Butler AA: Adropin transgenesis improves
recognition memory in diet-induced obese LDLR-deficient C57BL/6J
mice. Peptides. 146:1706782021. View Article : Google Scholar : PubMed/NCBI
|
|
81
|
Altincik A and Sayin O: Evaluation of the
relationship between serum adropin levels and blood pressure in
obese children. J Pediatr Endocrinol Metab. 28:1095–1100. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
82
|
Chen RM, Yuan X, Ouyang Q, Lin XQ, Ai ZZ,
Zhang Y and Yang XH: Adropin and glucagon-like peptide-2 are
associated with glucose metabolism in obese children. World J
Pediatr. 15:565–571. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
83
|
Yuan X, Chen R, Ouyang Q, Lin X, Ai Z,
Zhang Y and Yang X: Novel associations of serum adropin and
lipopolysaccharide-binding protein versus lipid profiles in
childhood obesity. J Pediatr Endocrinol Metab. 33:265–270. 2020.
View Article : Google Scholar : PubMed/NCBI
|
|
84
|
Yin C, Zhang H, Zhang M and Xiao Y:
Adropin and apelin-12 efficiently predict metabolic syndrome in
obese children. Pediatr Diabetes. 21:1132–1139. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
85
|
Soltani S, Kolahdouz-Mohammadi R, Aydin S,
Yosaee S, Clark CCT and Abdollahi S: Circulating levels of adropin
and overweight/obesity: A systematic review and meta-analysis of
observational studies. Hormones (Athens). 21:15–22. 2022.
View Article : Google Scholar
|
|
86
|
He L, Zhang FJ, Li HY, Li L, Song LG, Mao
Y, Li J, Liu HM, Li FL, Xu LY, et al: Anti-diabetic role of adropin
in streptozotocin induced diabetic rats via alteration of PI3K/Akt
and insulin signaling pathway. J Oleo Sci. 70:657–664. 2021.
View Article : Google Scholar : PubMed/NCBI
|
|
87
|
Hu W and Chen L: Association of serum
adropin concentrations with diabetic nephropathy. Mediators
Inflamm. 2016:60382612016. View Article : Google Scholar : PubMed/NCBI
|
|
88
|
Li B, Li N, Guo S, Zhang M, Li J, Zhai N,
Wang H and Zhang Y: The changing features of serum adropin,
copeptin, neprilysin and chitotriosidase which are associated with
vascular endothelial function in type 2 diabetic retinopathy
patients. J Diabetes Complications. 34:1076862020. View Article : Google Scholar : PubMed/NCBI
|
|
89
|
Jiang S, Young JL, Wang K, Qian Y and Cai
L: Diabetic-induced alterations in hepatic glucose and lipid
metabolism: The role of type 1 and type 2 diabetes mellitus
(Review). Mol Med Rep. 22:603–611. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
90
|
Jones JG: Hepatic glucose and lipid
metabolism. Diabetologia. 59:1098–1103. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
91
|
Thapa D, Xie B, Zhang M, Stoner MW,
Manning JR, Huckestein BR, Edmunds LR, Mullett SJ, McTiernan CF,
Wendell SG, et al: Adropin treatment restores cardiac glucose
oxidation in pre-diabetic obese mice. J Mol Cell Cardiol.
129:174–178. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
92
|
Skrzypski M, Kołodziejski PA,
Pruszyńska-Oszmałek E, Wojciechowicz T, Janicka P, Krążek M, Małek
E, Strowski MZ and Nowak KW: Daily treatment of mice with type 2
diabetes with adropin for four weeks improves glucolipid profile,
reduces hepatic lipid content and restores elevated hepatic enzymes
in serum. Int J Mol Sci. 23:98072022. View Article : Google Scholar : PubMed/NCBI
|
|
93
|
Zang H, Jiang F, Cheng X, Xu H and Hu X:
Serum adropin levels are decreased in Chinese type 2 diabetic
patients and negatively correlated with body mass index. Endocr J.
65:685–691. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
94
|
Choi HN and Yim JE: Plasma adropin as a
potential marker predicting obesity and obesity-associated cancer
in korean patients with type 2 diabetes mellitus. J Cancer Prev.
23:191–196. 2018. View Article : Google Scholar
|
|
95
|
Soltani S, Beigrezaei S, Malekahmadi M,
Clark CCT and Abdollahi S: Circulating levels of adropin and
diabetes: A systematic review and meta-analysis of observational
studies. BMC Endocr Disord. 23:732023. View Article : Google Scholar : PubMed/NCBI
|
|
96
|
Palizban AA, Yazdani AH and
Jahanbani-Ardakani H: Role of rs7903146 polymorphism and adropin
serum level in patients with diabetes mellitus; a case-control
study from Isfahan, Iran. Arch Physiol Biochem. 128:378–381. 2022.
View Article : Google Scholar
|
|
97
|
Davoodi M, Hesamabadi BK, Ariabood E,
Izadi MR, Ghardashi-Afousi A, Bigi MAB, Asvadi-Fard M and Gaeini
AA: Improved blood pressure and flow-mediated dilatation via
increased plasma adropin and nitrate/nitrite induced by
high-intensity interval training in patients with type 2 diabetes.
Exp Physiol. 107:813–824. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
98
|
Tičinović Kurir T, Miličević T, Novak A,
Vilović M and Božić J: Adropin-potential link in cardiovascular
protection for obese male type 2 diabetes mellitus patients treated
with liraglutide. Acta Clin Croat. 59:344–350. 2020.
|
|
99
|
Wang Q, An Y, Zhang L, Zhang Y, Wang G and
Liu J: Regulation of Adropin by Sitagliptin monotherapy in
participants with newly diagnosed type 2 diabetes. BMC Endocr
Disord. 22:3062022. View Article : Google Scholar : PubMed/NCBI
|
|
100
|
Wang X, Liu C, Jiang H, Chen BC, Yang X,
Xiao M, Xie YQ and Li K: Network pharmacology and verification
experiment-based prediction of active components and potential
targets of Alpiniae Oxyphyllae Fructus-Saposhnikoviae Radix
(Yizhiren-Fangfeng) for treatment of diabetic kidney disease.
Tradit Med Res. 8:262023. View Article : Google Scholar
|
|
101
|
Liu SM, Yan ZJ, Xiao M and Xie YQ:
Mechanistic study of lipid metabolism disorders in diabetic kidney
disease treated with GLQMP based on network pharmacology, molecular
docking and in vitro experiments. Tradit Med Res. 9:112024.
View Article : Google Scholar
|
|
102
|
Es-Haghi A, Al-Abyadh T and Mehrad-Majd H:
The clinical value of serum adropin level in early detection of
diabetic nephropathy. kidney Blood Press Res. 46:734–740. 2021.
View Article : Google Scholar : PubMed/NCBI
|
|
103
|
Liu M, Ai J, Shuai Z, Tang K, Li Z and
Huang Y: Adropin alleviates myocardial fibrosis in diabetic
cardiomyopathy rats: A preliminary study. Front Cardiovasc Med.
8:6885862021. View Article : Google Scholar : PubMed/NCBI
|
|
104
|
Li S, Sun J, Hu W, Liu Y, Lin D, Duan H
and Liu F: The association of serum and vitreous adropin
concentrations with diabetic retinopathy. Ann Clin Biochem.
56:253–258. 2019. View Article : Google Scholar
|
|
105
|
Yang C, DeMars KM and Candelario-Jalil E:
Age-Dependent decrease in adropin is associated with reduced levels
of endothelial nitric oxide synthase and increased oxidative stress
in the rat brain. Aging Dis. 9:322–330. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
106
|
Shahdadian F, Saneei P, Lotfi K, Feizi A,
Askari G and Safavi SM: Association of plant-based diets with
adropin, atherogenic index of plasma, and metabolic syndrome and
its components: A cross-sectional study on adults. Front Nutr.
10:10777092023. View Article : Google Scholar : PubMed/NCBI
|
|
107
|
Yang C, DeMars KM, Hawkins KE and
Candelario-Jalil E: Adropin reduces paracellular permeability of
rat brain endothelial cells exposed to ischemia-like conditions.
Peptides. 81:29–37. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
108
|
Jurrissen TJ, Ramirez-Perez FI,
Cabral-Amador FJ, Soares RN, Pettit-Mee RJ, Betancourt-Cortes EE,
McMillan NJ, Sharma N, Rocha HNM, Fujie S, et al: Role of adropin
in arterial stiffening associated with obesity and type 2 diabetes.
Am J Physiol Heart Circ Physiol. 323:H879–H891. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
109
|
Sato K, Yamashita T, Shirai R, Shibata K,
Okano T, Yamaguchi M, Mori Y, Hirano T and Watanabe T: Adropin
contributes to anti-atherosclerosis by suppressing
monocyte-endothelial cell adhesion and smooth muscle cell
proliferation. Int J Mol Sci. 19:12932018. View Article : Google Scholar : PubMed/NCBI
|
|
110
|
Li L and Xie W: LncRNA HDAC11-AS1
suppresses atherosclerosis by inhibiting HDAC11-Mediated adropin
histone deacetylation. J Cardiovasc Transl Res. 15:1256–1269. 2022.
View Article : Google Scholar : PubMed/NCBI
|
|
111
|
Ying T, Wu L, Lan T, Wei Z, Hu D, Ke Y,
Jiang Q and Fang J: Adropin inhibits the progression of
atherosclerosis in ApoE(−/−)/Enho(−/−) mice by regulating
endothelial-to-mesenchymal transition. Cell Death Discov.
9:4022023. View Article : Google Scholar
|
|
112
|
Abbasian S, Ravasi AA, Soori R and Aydin
S, Choobineh S and Aydin S: High-intensity interval training
ameliorates endothelial dysfunction through adropin, nitric oxide,
MR-proADM, and copeptin changes in overweight subjects. Hormones
(Athens). 21:707–717. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
113
|
Kamma E, Lasisi W, Libner C, Ng HS and
Plemel JR: Central nervous system macrophages in progressive
multiple sclerosis: Relationship to neurodegeneration and
therapeutics. J Neuroinflammation. 19:452022. View Article : Google Scholar : PubMed/NCBI
|
|
114
|
McGinley MP, Goldschmidt CH and Rae-Grant
AD: Diagnosis and treatment of multiple sclerosis: A review. JAMA.
325:765–779. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
115
|
Cinkir U, Bir LS, Topsakal S, Avci Cicek E
and Tekin S: Investigation of blood leptin and adropin levels in
patients with multiple sclerosis: A CONSORT-clinical study.
Medicine (Baltimore). 100:e272472021. View Article : Google Scholar : PubMed/NCBI
|
|
116
|
Algul S and Ozcelik O: Evaluating the
energy regulatory hormones of nesfatin-1, irisin, adropin and
preptin in multiple sclerosis. Mult Scler Relat Disord.
68:1042212022. View Article : Google Scholar : PubMed/NCBI
|
|
117
|
Demirdöğen F, Akdağ T, Gündüz ZB and
Odabaş FO: Investigation of serum adropin levels and its
relationship with hypothalamic atrophy in patients with multiple
sclerosis. Mult Scler Relat Disord. 67:1039992022. View Article : Google Scholar
|
|
118
|
Rao A and Herr DR: G protein-coupled
receptor GPR19 regulates E-cadherin expression and invasion of
breast cancer cells. Biochim Biophys Acta Mol Cell Res.
1864:1318–1327. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
119
|
Stelcer E, Milecka P, Komarowska H, Jopek
K, Tyczewska M, Szyszka M, Lesniczak M, Suchorska W, Bekova K,
Szczepaniak B, et al: Adropin stimulates proliferation and inhibits
adrenocortical steroidogenesis in the human adrenal carcinoma
(HAC15) cell line. Front Endocrinol (Lausanne). 11:5613702020.
View Article : Google Scholar : PubMed/NCBI
|
|
120
|
Jia L, Liao L, Jiang Y, Hu X, Lu G, Xiao
W, Gong W and Jia X: Low-dose adropin stimulates inflammasome
activation of macrophage via mitochondrial ROS involved in
colorectal cancer progression. BMC Cancer. 23:10422023. View Article : Google Scholar : PubMed/NCBI
|
|
121
|
Hu J, Wu Q, Ding Q, Wu W, Li Q and Zheng
Z: High level of adropin promotes the progression of pancreatic
ductal adenocarcinoma. Curr Cancer Drug Targets. 24:629–641. 2024.
View Article : Google Scholar
|
|
122
|
Butler AA and Havel PJ: Adropin and
insulin resistance: Integration of endocrine, circadian, and stress
signals regulating glucose metabolism. Obesity (Silver Spring).
29:1799–1801. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
123
|
Smati S, Régnier M, Fougeray T, Polizzi A,
Fougerat A, Lasserre F, Lukowicz C, Tramunt B, Guillaume M, Burnol
AF, et al: Regulation of hepatokine gene expression in response to
fasting and feeding: Influence of PPAR-α and insulin-dependent
signalling in hepatocytes. Diabetes Metab. 46:129–136. 2020.
View Article : Google Scholar
|
|
124
|
Ozkan A, Aslan MA, Sinen O, Munzuroglu M,
Derin N, Parlak H, Bulbul M and Agar A: Effects of adropin on
learning and memory in rats tested in the Morris water maze.
Hippocampus. 32:253–263. 2022. View Article : Google Scholar : PubMed/NCBI
|