|
1
|
Siegel RL, Giaquinto AN and Jemal A:
Cancer statistics, 2024. CA Cancer J Clin. 74:12–49. 2024.
View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Seckinger A, Delgado JA, Moser S, Moreno
L, Neuber B, Grab A, Lipp S, Merino J, Prosper F, Emde M, et al:
Target expression, generation, preclinical activity, and
pharmacokinetics of the BCMA-T cell bispecific antibody em801 for
multiple myeloma treatment. Cancer Cell. 31:396–410. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Bazarbachi AH, Al Hamed R, Malard F,
Bazarbachi A, Harousseau JL and Mohty M: Induction therapy prior to
autologous stem cell transplantation (ASCT) in newly diagnosed
multiple myeloma: An update. Blood Cancer J. 12:472022. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Wang L, Luo R, Onyshchenko K, Rao X, Wang
M, Menz B, Gaedicke S, Grosu AL, Firat E and Niedermann G: Adding
liposomal doxorubicin enhances the abscopal effect induced by
radiation/αPD1 therapy depending on tumor cell mitochondrial DNA
and cGAS/STING. J Immunother Cancer. 11:e0062352023. View Article : Google Scholar
|
|
5
|
Rahmy S, Cheng X, Wang M, Feng H, Qiu W,
Zhao R and Lu X: Organ-specific regulation of CHD1 by acute PTEN
and p53 loss in mice. Biochem Biophys Res Commun. 525:614–619.
2020. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Wang M, Rao X, Gaedicke S, Wang L, Menz B,
Multhoff G and Niedermann G: Adding a PPARα agonist enhances t
cell-mediated effects of RT in combination with anti-PD-1. Int J
Radiat Oncol Biol Phys. 120(Suppl 1): e409–e410. 2024. View Article : Google Scholar
|
|
7
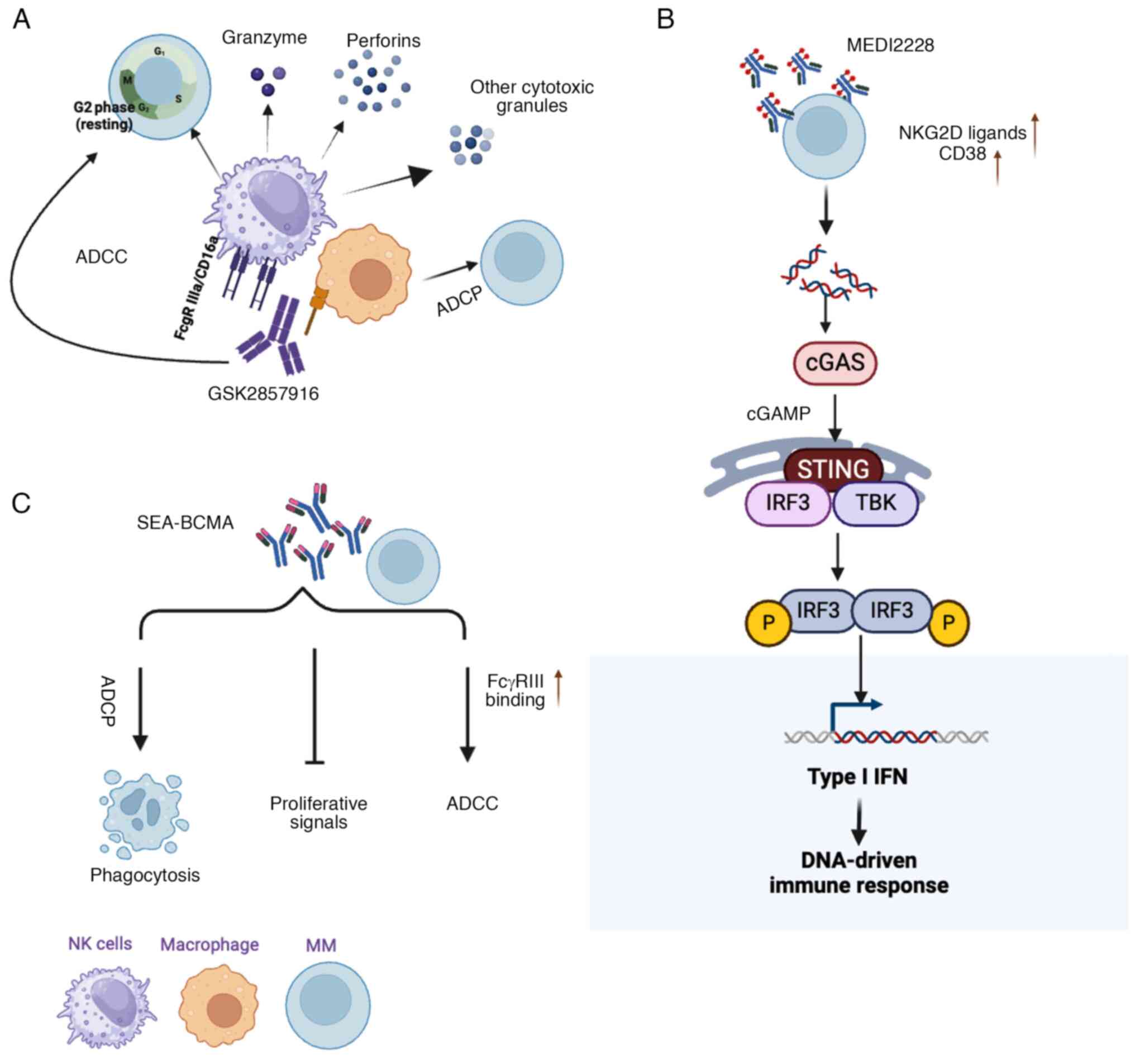
|
Ali SA, Shi V, Maric I, Wang M, Stroncek
DF, Rose JJ, Brudno JN, Stetler-Stevenson M, Feldman SA, Hansen BG,
et al: T cells expressing an anti-B-cell maturation antigen
chimeric antigen receptor cause remissions of multiple myeloma.
Blood. 128:1688–1700. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Hu M, Wang L, Su D, Yuan Q, Xiao C, Guo L,
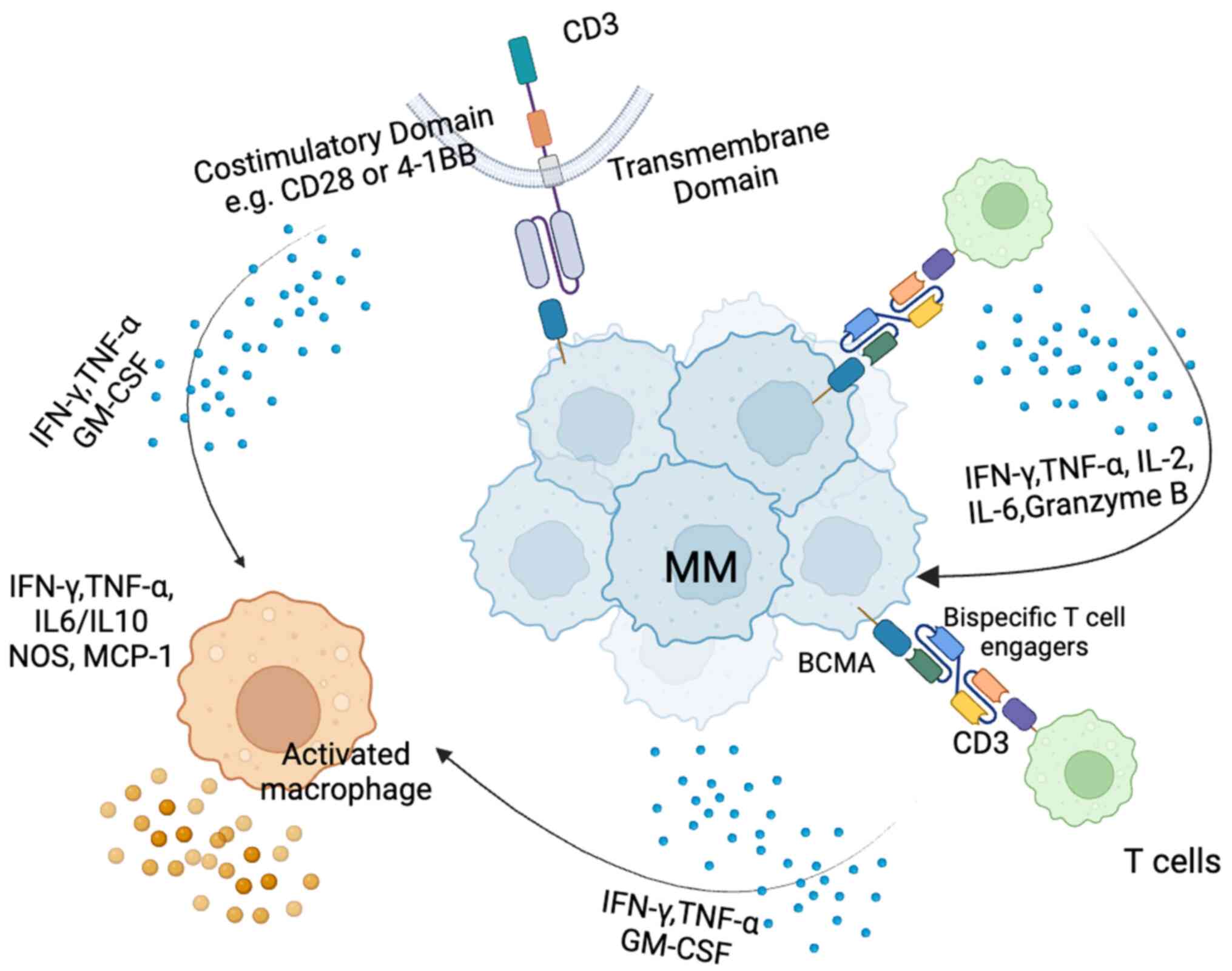
Wang M, Kang C, Zhang J and Zhou T: Evaluation of mycotoxins,
mycobiota and toxigenic fungi in the traditional medicine Radix
Dipsaci. Front Microbiol. 15:14546832024. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Sanchez E, Li M, Kitto A, Li J, Wang CS,
Kirk DT, Yellin O, Nichols CM, Dreyer MP, Ahles CP, et al: Serum
B-cell maturation antigen is elevated in multiple myeloma and
correlates with disease status and survival. Br J Haematol.
158:727–738. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
X R, W M, W G, Z L, W X, C W and W C:
Chemotherapy-induced toxic epidermal necrolysis in a patient with
multiple myeloma, a case report and literature review. Front Oncol.
13:12274482023. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Salem DA, Maric I, Yuan CM, Liewehr DJ,
Venzon DJ, Kochenderfer J and Stetler-Stevenson M: Quantification
of B-cell maturation antigen, a target for novel chimeric antigen
receptor T-cell therapy in Myeloma. Leuk Res. 71:106–111. 2018.
View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Fabi A, Bhargava R, Fatigoni S, Guglielmo
M, Horneber M, Roila F, Weis J, Jordan K and Ripamonti CI; ESMO
Guidelines Committee: Electronic address
clinicalguidelines@esmo.org: Cancer-related fatigue: ESMO clinical
practice guidelines for diagnosis and treatment. Ann Oncol.
31:713–723. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Friedman KM, Garrett TE, Evans JW, Horton
HM, Latimer HJ, Seidel SL, Horvath CJ and Morgan RA: Effective
targeting of multiple B-cell maturation antigen-expressing
hematological malignances by anti-B-cell maturation antigen
chimeric antigen receptor T cells. Hum Gene Ther. 29:585–601. 2018.
View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Kleber M, Ntanasis-Stathopoulos I and
Terpos E: BCMA in multiple myeloma-A promising key to therapy. J
Clin Med. 10:40882021. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Abramson HN: B-cell maturation antigen
(BCMA) as a target for new drug development in relapsed and/or
refractory multiple myeloma. Int J Mol Sci. 21:51922020. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Yu B, Jiang T and Liu D: BCMA-targeted
immunotherapy for multiple myeloma. J Hematol Oncol. 13:1252020.
View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Tai YT, Acharya C, An G, Moschetta M,
Zhong MY, Feng X, Cea M, Cagnetta A, Wen K, van Eenennaam H, et al:
APRIL and BCMA promote human multiple myeloma growth and
immunosuppression in the bone marrow microenvironment. Blood.
127:3225–3236. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Coquery CM and Erickson LD: Regulatory
roles of the tumor necrosis factor receptor BCMA. Crit Rev Immunol.
32:287–305. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Novak AJ, Darce JR, Arendt BK, Harder B,
Henderson K, Kindsvogel W, Gross JA, Greipp PR and Jelinek DF:
Expression of BCMA, TACI, and BAFF-R in multiple myeloma: A
mechanism for growth and survival. Blood. 103:689–694. 2004.
View Article : Google Scholar
|
|
20
|
Shah N, Chari A, Scott E, Mezzi K and
Usmani SZ: B-cell maturation antigen (BCMA) in multiple myeloma:
Rationale for targeting and current therapeutic approaches.
Leukemia. 34:985–1005. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Oaknin A, Fariñas-Madrid L, García-Duran
C, Martin LP, O'Malley DM, Schilder RJ, Uyar D, Moroney JW, Diaz
JP, Spira AI, et al: Luveltamab tazevibulin (STRO-002), an
anti-folate receptor alpha (FolRα) antibody drug conjugate (ADC),
safety and efficacy in a broad distribution of FolRα expression in
patients with recurrent epithelial ovarian cancer (OC): Update of
STRO-002-GM1 phase 1 dose expansion cohort. J Clin Oncol. 41(Suppl
16): S55082023. View Article : Google Scholar
|
|
22
|
Rao X, Onyshchenko K, Wang L, Wang M and
Niedermann G: Comparison of two triple therapy regimens for
enhancing the abscopal effect in mice. Int J Radiat Oncol Biol
Phys. 117:e2562023. View Article : Google Scholar
|
|
23
|
Dhakal B, Yong K, Harrison SJ, Mateos MV,
Moreau P, van de Donk NWCJ, Sidana S, Popat R, Lendvai N, Lonardi
C, et al: First phase 3 results from CARTITUDE-4: Cilta-cel versus
standard of care (PVd or DPd) in lenalidomide-refractory multiple
myeloma. J Clin Oncol. 41:LBA1062023. View Article : Google Scholar
|
|
24
|
Dhakal B, Szabo A, Chhabra S, Hamadani M,
D'Souza A, Usmani SZ, Sieracki R, Gyawali B, Jackson JL,
Asimakopoulos F and Hari PN: Autologous transplantation for newly
diagnosed multiple myeloma in the era of novel agent induction: A
systematic review and meta-analysis. JAMA Oncol. 4:343–350. 2018.
View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Gopalakrishnan S, D'Souza A, Scott E,
Fraser R, Davila O, Shah N, Gale RP, Kamble R, Diaz MA, Lazarus HM,
et al: Revised international staging system is predictive and
prognostic for early relapse (<24 months) after autologous
transplantation for newly diagnosed multiple myeloma. Biol Blood
Marrow Transplant. 25:683–688. 2019. View Article : Google Scholar
|
|
26
|
Lin J, Zhang G, Lou B, Sun Y, Jia X, Wang
M, Zhou J and Xia Z: Identification of copper metabolism-related
markers in Parkinson's disease. Ann Med. 56:24250642024. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Strimbu K and Tavel JA: What are
biomarkers? Curr Opin HIV AIDS. 5:463–466. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Califf RM: Biomarker definitions and their
applications. Exp Biol Med (Maywood). 243:213–221. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Oliva S, Gambella M, Gilestro M, Muccio
VE, Gay F, Drandi D, Ferrero S, Passera R, Pautasso C, Bernardini
A, et al: Minimal residual disease after transplantation or
lenalidomide-based consolidation in myeloma patients: A prospective
analysis. Oncotarget. 8:5924–5935. 2017. View Article : Google Scholar :
|
|
30
|
Jeong TD, Park CJ, Shim H, Jang S, Chi HS,
Yoon DH, Kim DY, Lee JH, Lee JH, Suh C and Lee KH: Simplified flow
cytometric immunophenotyping panel for multiple myeloma,
CD56/CD19/CD138(CD38)/CD45, to differentiate neoplastic myeloma
cells from reactive plasma cells. Korean J Hematol. 47:260–266.
2012. View Article : Google Scholar
|
|
31
|
Bayer-Garner IB, Sanderson RD, Dhodapkar
MV, Owens RB and Wilson CS: Syndecan-1 (CD138) immunoreactivity in
bone marrow biopsies of multiple myeloma: Shed syndecan-1
accumulates in fibrotic regions. Mod Pathol. 14:1052–1058. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Van De Donk NWCJ, Richardson PG and
Malavasi F: CD38 antibodies in multiple myeloma: Back to the
future. Blood. 131:13–29. 2018. View Article : Google Scholar
|
|
33
|
Sanchez E, Gillespie A, Tang G, Ferros M,
Harutyunyan NM, Vardanyan S, Gottlieb J, Li M, Wang CS, Chen H and
Berenson JR: Soluble B-cell maturation antigen mediates
tumor-induced immune deficiency in multiple myeloma. Clin Cancer
Res. 22:3383–3397. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Flores-Montero J, de Tute R, Paiva B,
Perez JJ, Böttcher S, Wind H, Sanoja L, Puig N, Lecrevisse Q,
Vidriales MB, et al: Immunophenotype of normal vs myeloma plasma
cells: Toward antibody panel specifications for MRD detection in
multiple myeloma. Cytometry B Clin Cytom. 90:61–72. 2016.
View Article : Google Scholar
|
|
35
|
Raje N, Berdeja J, Lin Y, Siegel D,
Jagannath S, Madduri D, Liedtke M, Rosenblatt J, Maus MV, Turka A,
et al: Anti-BCMA CAR T-cell therapy bb2121 in relapsed or
refractory multiple myeloma. N Engl J Med. 380:1726–1737. 2019.
View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Chen H, Li M, Xu N, Ng N, Sanchez E, Soof
CM, Patil S, Udd K, Bujarski S, Cao J, et al: Serum B-cell
maturation antigen (BCMA) reduces binding of anti-BCMA antibody to
multiple myeloma cells. Leuk Res. 81:62–66. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Cohen AD, Garfall AL, Stadtmauer EA,
Melenhorst JJ, Lacey SF, Lancaster E, Vogl DT, Weiss BM, Dengel K,
Nelson A, et al: B cell maturation antigen-specific CAR T cells are
clinically active in multiple myeloma. J Clin Invest.
129:2210–2221. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Lee L, Bounds D, Paterson J, Herledan G,
Sully K, Seestaller-Wehr LM, Fieles WE, Tunstead J, McCahon L,
Germaschewski FM, et al: Evaluation of B cell maturation antigen as
a target for antibody drug conjugate mediated cytotoxicity in
multiple myeloma. Br J Haematol. 174:911–922. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Visram A, Soof C, Rajkumar SV, Kumar SK,
Bujarski S, Spektor TM, Kyle RA, Berenson JR and Dispenzieri A:
Serum BCMA levels predict outcomes in MGUS and smoldering myeloma
patients. Blood Cancer J. 11:1202021. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Bujarski S, Soof C, Li M, Wang CS, Sanchez
E, Emamy-Sadr M, Swift R, Rahbari K, Patil S, Spektor TM, et al:
Baseline and early changes in serum B-cell maturation antigen
levels predict progression free survival and response status for
multiple myeloma patients in a phase 1 trial evaluating
ruxolitinib, lenalidomide and methylprednisolone. Blood. 132(Suppl
1): 18942018. View Article : Google Scholar
|
|
41
|
Ghermezi M, Li M, Vardanyan S, Harutyunyan
NM, Gottlieb J, Berenson A, Spektor TM, Andreu-Vieyra C, Petraki S,
Sanchez E, et al: Serum B-cell maturation antigen: A novel
biomarker to predict outcomes for multiple myeloma patients.
Haematologica. 102:785–795. 2017. View Article : Google Scholar :
|
|
42
|
Mailankody S, Htut M, Lee KP, Bensinger W,
Devries T, Piasecki J, Ziyad S, Blake M, Byon J and Jakubowiak A:
JCARH125, anti-BCMA CAR T-cell Therapy for relapsed/refractory
multiple myeloma: Initial proof of concept results from a phase 1/2
multicenter study (EVOLVE). Blood. 132(Suppl 1): 9572018.
View Article : Google Scholar
|
|
43
|
Palumbo A, Avet-Loiseau H, Oliva S,
Lokhorst HM, Goldschmidt H, Rosinol L, Richardson P, Caltagirone S,
Lahuerta JJ, Facon T, et al: Revised international staging system
for multiple myeloma: A report from international myeloma working
group. J Clin Oncol. 33:2863–2869. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Dimopoulos MA, Kastritis E, Michalis E,
Tsatalas C, Michael M, Pouli A, Kartasis Z, Delimpasi S, Gika D,
Zomas A, et al: The international scoring system (ISS) for multiple
myeloma remains a robust prognostic tool independently of patients'
renal function. Ann Oncol. 23:722–729. 2012. View Article : Google Scholar
|
|
45
|
Landgren O and Rajkumar SV: New
developments in diagnosis, prognosis, and assessment of response in
multiple myeloma. Clin Cancer Res. 22:5428–5433. 2016. View Article : Google Scholar
|
|
46
|
Yuregir OO, Sahin FI, Yilmaz Z, Kizilkilic
E, Karakus S and Ozdogu H: Fluorescent in situ hybridization
studies in multiple myeloma. Hematology. 14:90–94. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Huber D, Voith Von Voithenberg L and
Kaigala GV: Fluorescence in situ hybridization (FISH): History,
limitations and what to expect from micro-scale FISH? Micro Nano
Eng. 1:15–24. 2018. View Article : Google Scholar
|
|
48
|
Laurent SA, Hoffmann FS, Kuhn PH, Cheng Q,
Chu Y, Schmidt-Supprian M, Hauck SM, Schuh E, Krumbholz M, Rübsamen
H, et al: γ-Secretase directly sheds the survival receptor BCMA
from plasma cells. Nat Commun. 6:73332015. View Article : Google Scholar
|
|
49
|
Coquery CM, Loo WM, Wade NS, Bederman AG,
Tung KS, Lewis JE, Hess H and Erickson LD: BAFF regulates
follicular helper t cells and affects their accumulation and
interferon-γ production in autoimmunity. Arthritis Rheumatol.
67:773–784. 2015. View Article : Google Scholar :
|
|
50
|
Oliva S, Bruinink DHO, Rihova L,
D'Agostino M, Pantani L, Capra A, van der Holt B, Troia R, Petrucci
MT, Villanova T, et al: Minimal residual disease assessment by
multiparameter flow cytometry in transplant-eligible myeloma in the
EMN02/HOVON 95 MM trial. Blood Cancer J. 11:1062021. View Article : Google Scholar : PubMed/NCBI
|
|
51
|
Sanchez E, Tanenbaum EJ, Patil S, Li M,
Soof CM, Vidisheva A, Waterman GN, Hekmati T, Tang G, Wang CS, et
al: The clinical significance of B-cell maturation antigen as a
therapeutic target and biomarker. Expert Rev Mol Diagn. 18:319–329.
2018. View Article : Google Scholar : PubMed/NCBI
|
|
52
|
Montes De Oca R, Alavi AS, Vitali N,
Bhattacharya S, Blackwell C, Patel K, Seestaller-Wehr L, Kaczynski
H, Shi H, Dobrzynski E, et al: Belantamab mafodotin (GSK2857916)
drives immunogenic cell death and immune-mediated antitumor
responses in vivo. Mol Cancer Ther. 20:1941–1955. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
53
|
Trudel S, Lendvai N, Popat R, Voorhees PM,
Reeves B, Libby EN, Richardson PG, Hoos A, Gupta I, Bragulat V, et
al: Antibody-drug conjugate, GSK2857916, in relapsed/refractory
multiple myeloma: an update on safety and efficacy from dose
expansion phase I study. Blood Cancer J. 9:372019. View Article : Google Scholar : PubMed/NCBI
|
|
54
|
Touzeau C, Moreau P and Dumontet C:
Monoclonal antibody therapy in multiple myeloma. Leukemia.
31:1039–1047. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
55
|
Lassiter G, Bergeron C, Guedry R, Cucarola
J, Kaye AM, Cornett EM, Kaye AD, Varrassi G, Viswanath O and Urits
I: Belantamab mafodotin to treat multiple myeloma: A comprehensive
review of disease, drug efficacy and side effects. Curr Oncol.
28:640–660. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
56
|
Trudel S, Lendvai N, Popat R, Voorhees PM,
Reeves B, Libby EN, Richardson PG, Anderson LD Jr, Sutherland HJ,
Yong K, et al: Targeting B-cell maturation antigen with GSK2857916
antibody-drug conjugate in relapsed or refractory multiple myeloma
(BMA117159): A dose escalation and expansion phase 1 trial. Lancet
Oncol. 19:1641–1653. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
57
|
Tai YT, Mayes PA, Acharya C, Zhong MY, Cea
M, Cagnetta A, Craigen J, Yates J, Gliddon L, Fieles W, et al:
Novel anti-B-cell maturation antigen antibody-drug conjugate
(GSK2857916) selectively induces killing of multiple myeloma.
Blood. 123:3128–3138. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
58
|
Lee HC, Raje NS, Landgren O, Upreti VV,
Wang J, Avilion AA, Hu X, Rasmussen E, Ngarmchamnanrith G, Fujii H
and Spencer A: Phase 1 study of the anti-BCMA antibody-drug
conjugate AMG 224 in patients with relapsed/refractory multiple
myeloma. Leukemia. 35:255–258. 2021. View Article : Google Scholar
|
|
59
|
Taft D, Henderson C, O'Day C, Yu C, Li H,
Ho P and Van Epps H: Pharmacodynamics of SEA-BCMA, a nonfucosylated
antibody targeting BCMA, in patients with relapsed/refractory
multiple myeloma. Blood. 138(Suppl 1): 11972021. View Article : Google Scholar
|
|
60
|
Kumar SK, Migkou M, Bhutani M, Spencer A,
Ailawadhi S, Kalff A, Walcott F, Pore N, Gibson D, Wang F, et al:
Phase 1, first-in-human study of MEDI2228, a BCMA-targeted ADC in
patients with relapsed/refractory multiple myeloma. Blood.
136(Suppl 1): S26–S27. 2020. View Article : Google Scholar
|
|
61
|
Xing L, Wang S, Liu J, Yu T, Chen H, Wen
K, Li Y, Lin L, Hsieh PA, Cho SF, et al: BCMA-specific ADC MEDI2228
and daratumumab induce synergistic myeloma cytotoxicity via
IFN-driven immune responses and enhanced CD38 expression. Clin
Cancer Res. 27:5376–5388. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
62
|
Hoffman JE, Lipe B, Melear J, Liedtke M,
Schroeder MA, Niesvizky R, Tomasson MH, Yasenchak CA, Green DJ, Li
H, et al: SEA-BCMA, an investigational nonfucosylated monoclonal
antibody: Ongoing results of a phase 1 study in patients with
relapsed/refractory multiple myeloma (SGNBCMA-001). Blood.
138(Suppl 1): S27402021. View Article : Google Scholar
|
|
63
|
Nooka AK, Moreau P, Usmani SZ, Garfall A,
van de Donk NWCJ, San-Miguel JF, Rocafiguera AO, Chari A, Karlin L,
Mateos MV, et al: Teclistamab, a B-cell maturation antigen (BCMA) x
CD3 bispecific antibody, in patients with relapsed/refractory
multiple myeloma (RRMM): Updated efficacy and safety results from
MajesTEC-1. J ClinOncol. 40(16Suppl): S80072022. View Article : Google Scholar
|
|
64
|
Huehls AM, Coupet TA and Sentman CL:
Bispecific T-cell engagers for cancer immunotherapy. Immunol Cell
Biol. 93:290–296. 2015. View Article : Google Scholar
|
|
65
|
Friedrich MJ, Neri P, Kehl N, Michel J,
Steiger S, Kilian M, Leblay N, Maity R, Sankowski R, Lee H, et al:
The pre-existing T cell landscape determines the response to
bispecific T cell engagers in multiple myeloma patients. Cancer
Cell. 41:711–725.e6. 2023. View Article : Google Scholar
|
|
66
|
Ellerman D: Bispecific T-cell engagers:
Towards understanding variables influencing the in vitro potency
and tumor selectivity and their modulation to enhance their
efficacy and safety. Methods. 154:102–117. 2019. View Article : Google Scholar
|
|
67
|
Verkleij CPM, Broekmans MEC, Van Duin M,
Frerichs KA, Kuiper R, de Jonge AV, Kaiser M, Morgan G, Axel A,
Boominathan R, et al: Preclinical activity and determinants of
response of the GPRC5DxCD3 bispecific antibody talquetamab in
multiple myeloma. Blood Adv. 5:2196–2215. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
68
|
Atamaniuk J, Gleiss A, Porpaczy E, Kainz
B, Grunt TW, Raderer M, Hilgarth B, Drach J, Ludwig H, Gisslinger
H, et al: Overexpression of G protein-coupled receptor 5D in the
bone marrow is associated with poor prognosis in patients with
multiple myeloma. Eur J Clin Invest. 42:953–960. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
69
|
Shah Z, Malik MN, Batool SS, Kotapati S,
Akhtar A, Rehman OU, Ghani M, Sadiq M, Akbar A, Ashraf A, et al:
Bispecific T-cell engager (BiTE) antibody based immunotherapy for
treatment of relapsed refractory multiple myeloma (RRMM): A
systematic review of preclinical and clinical trials. Blood.
134(Suppl 1): S55672019. View Article : Google Scholar
|
|
70
|
Usmani SZ, Garfall AL, Van De Donk NWCJ,
Nahi H, San-Miguel JF, Oriol A, Rosinol L, Chari A, Bhutani M,
Karlin L, et al: Teclistamab, a B-cell maturation antigen x CD3
bispecific antibody, in patients with relapsed or refractory
multiple myeloma (MajesTEC-1): A multicentre, open-label,
single-arm, phase 1 study. Lancet. 398:665–674. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
71
|
Mateos MV, Bahlis NJ, Costa LJ, Perrot A,
Pei L, Rubin ML, Lantz K, Sun W, Jaffe M, Kobos R and Nooka AK:
MajesTEC-3: Randomized, phase 3 study of teclistamab plus
daratumumab versus investigator's choice of daratumumab,
pomalidomide, and dexamethasone or daratumumab, bortezomib, and
dexamethasone in patients with relapsed/refractory multiple
myeloma. J Clin Oncol. 40(16 Suppl): TPS80722022. View Article : Google Scholar
|
|
72
|
Madduri D, Rosko A, Brayer J, Zonder J,
Bensinger WI, Li J, Xu L, Adriaens L, Chokshi D, Zhang W, et al:
REGN5458, a BCMA x CD3 bispecific monoclonal antibody, induces deep
and durable responses in patients with relapsed/refractory multiple
myeloma (RRMM). Blood. 136(Suppl 1): S41–S42. 2020. View Article : Google Scholar
|
|
73
|
Lee HC, Bumma N, Richter JR, Dhodapkar MV,
Hoffman JE, Suvannasankha A, Zonder JA, Shah MR, Lentzsch S, Maly
JJ, et al: LINKER-MM1 study: Linvoseltamab (REGN5458) in patients
with relapsed/refractory multiple myeloma. J Clin Oncol. 41(Suppl
16): S80062023. View Article : Google Scholar
|
|
74
|
Rodríguez Otero P, Joseph NS, Kumar SK,
Lee HC, Leleu X, Manier S, Dimopoulos MA, Mateos MV, Oriol A, Bumma
N, et al: Trial in progress: REGN5458, a BCMAxCD3 bispecific
antibody, in a phase Ib multi-cohort study of combination regimens
for patients with relapsed/refractory multiple myeloma. Blood.
140(Suppl 1): S4444–S4446. 2022. View Article : Google Scholar
|
|
75
|
Solh M, Bahlis N, Raje N, Costello C,
Dholaria B, Levy M, Tomasson M, Dube H, Damore M, Lon H, et al:
Efficacy and safety of elranatamab (PF-06863135), A B-cell
maturation antigen (BCMA)-CD3 bispecific antibody, in patients with
relapsed or refractory multiple myeloma. Hematol Transfus Cell
Ther. 43(Suppl 1): S195–S196. 2021. View Article : Google Scholar
|
|
76
|
Raje NS, Jakubowiak A, Gasparetto C,
Cornell RF, Krupka HI, Navarro D, Forgie AJ, Navarro D, Forgie AJ,
Udata C, et al: Safety, clinical activity, pharmacokinetics, and
pharmacodynamics from a phase I study of PF-06863135, a B-cell
maturation antigen (BCMA)-CD3 bispecific antibody, in patients with
relapsed/refractory multiple myeloma (RRMM). Blood. 134(Suppl 1):
S18692019. View Article : Google Scholar
|
|
77
|
Bahlis NJ, Costello CL, Raje NS, Levy MY,
Dholaria B, Solh M, Tomasson MH, Damore MA, Jiang S, Basu C, et al:
Elranatamab in relapsed or refractory multiple myeloma: The
MagnetisMM-1 phase 1 trial. Nat Med. 29:2570–2576. 2023. View Article : Google Scholar : PubMed/NCBI
|
|
78
|
Lesokhin AM, Tomasson MH, Arnulf B, Bahlis
NJ, Miles Prince H, Niesvizky R, Rodrίguez-Otero P, Martinez-Lopez
J, Koehne G, Touzeau C, et al: Elranatamab in relapsed or
refractory multiple myeloma: Phase 2 MagnetisMM-3 trial results.
Nat Med. 29:2259–2267. 2023. View Article : Google Scholar : PubMed/NCBI
|
|
79
|
Weisel K, D'souza A, Hurd D, Voorhees P,
Teipel R, Chung A, Rodriguez C, Tuchman S, Korde N, Safah H, et al:
P862: A phase 1 first-in-human monotherapy study of ABBV-383, A
BCMA x CD3 bispecific T-CELL–redirecting antibody, in
relapsed/refractory multiple myeloma. Hemasphere. 7(Suppl):
e00363072023. View Article : Google Scholar :
|
|
80
|
D'Souza A, Shah N, Rodriguez C, Voorhees
PM, Weisel K, Bueno OF, Pothacamury RK, Freise KJ, Yue S, Ross JA,
et al: A phase I first-in-human study of ABBV-383, a B-cell
maturation antigen x CD3 bispecific T-cell redirecting antibody, in
patients with relapsed/refractory multiple myeloma. J Clin Oncol.
40:3576–3586. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
81
|
Vij R, Kumar SK, D'Souza A, Mckay JT,
Voorhees PM, Chung A, Tuchman SA, Korde N, Weisel K, Teipel R, et
al: Updated safety and efficacy results of Abbv-383, a BCMA x CD3
bispecific T-cell redirecting antibody, in a first-in-human phase 1
study in patients with relapsed/refractory multiple myeloma. Blood.
142(Suppl 1): S33782023. View Article : Google Scholar
|
|
82
|
Kumar S, D'Souza A, Shah N, Rodriguez C,
Voorhees PM, Bueno OF, Buelow B, Freise KJ, Yue S, Pothacamury RK,
et al: A phase 1 first-in-human study of Tnb-383B, a BCMA x CD3
bispecific T-cell redirecting antibody, in patients with
relapsed/refractory multiple myeloma. Blood. 138(Suppl 1):
S9002021. View Article : Google Scholar
|
|
83
|
Harrison SJ, Minnema MC, Lee HC, Spencer
A, Kapoor P, Madduri D, Larsen J, Ailawadhi S, Kaufman JL, Raab MS,
et al: A phase 1 first in human (FIH) study of AMG 701, an
anti-B-cell maturation antigen (BCMA) half-life extended (HLE)
BiTE® (bispecific T-cell engager) molecule, in
relapsed/refractory (RR) multiple myeloma (MM). Blood. 136(Suppl
1): S28–S29. 2020. View Article : Google Scholar
|
|
84
|
Goldstein RL, Goyos A, Li CM, Deegen P,
Bogner P, Sternjak A, Thomas O, Klinger M, Wahl J, Friedrich M, et
al: AMG 701 induces cytotoxicity of multiple myeloma cells and
depletes plasma cells in cynomolgus monkeys. Blood Adv.
4:4180–4194. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
85
|
Costa LJ, Wong SW, Bermúdez A, de la Rubia
J, Mateos MV, Ocio EM, Rodríguez-Otero P, San-Miguel L, Li S,
Sarmiento R, et al: First clinical study of the B-cell maturation
antigen (BCMA) 2+1 T cell engager (TCE) CC-93269 in patients (Pts)
with relapsed/refractory multiple myeloma (RRMM): Interim results
of a phase 1 multicenter trial. Blood. 134(Suppl 1): S1432019.
View Article : Google Scholar
|
|
86
|
Wong SW, Bar N, Victoria Mateos M, Ribas
P, Hansson M, Paris L, Hofmeister C, Rodriguez-Otero P, Bermúdez
MA, Santoro A, et al: P883: Alnuctamab (ALNUC; BMS-986349;
CC-93269), A BCMA x CD3 T-cell engager, in patients (PTS) with
relapsed/refractory multiple myeloma (RRMM): Latest results from a
phase 1 first-in-human clinical study. Hemasphere. 7(Suppl 1):
e12207452023. View Article : Google Scholar
|
|
87
|
Wong SW, Bar N, Paris L, Hofmeister CC,
Hansson M, Santoro A, Mateos MV, Rodríguez-Otero P, Lund J, Encinas
C, et al: Alnuctamab (ALNUC; BMS-986349; CC-93269), a B-cell
maturation antigen (BCMA) x CD3 T-cell engager (TCE), in patients
(pts) with relapsed/refractory multiple myeloma (RRMM): Results
from a phase 1 first-in-human clinical study. Blood. 140(Suppl 1):
S400–S402. 2022. View Article : Google Scholar
|
|
88
|
Roex G, Timmers M, Wouters K,
Campillo-Davo D, Flumens D, Schroyens W, Chu Y, Berneman ZN, Lion
E, Luo F and Anguille S: Safety and clinical efficacy of BCMA
CAR-T-cell therapy in multiple myeloma. J Hematol Oncol.
13:1642020. View Article : Google Scholar : PubMed/NCBI
|
|
89
|
Xu Y, Zhang C, Cai D, Zhu R and Cao Y:
Exosomal miR-155-5p drives widespread macrophage M1 polarization in
hypervirulent Klebsiella pneumoniae-induced acute lung injury via
the MSK1/p38-MAPK axis. Cell Mol Biol Lett. 28:922023. View Article : Google Scholar : PubMed/NCBI
|
|
90
|
Sperling AS, Derman BA, Nikiforow S, Im
SY, Ikegawa S, Prabhala RH, Rodriguez DH, Li Y, Quinn DS, Pearson
D, et al: Updated phase I study results of PHE885, a T-charge
manufactured BCMA-directed CAR-T cell therapy, for patients (pts)
with r/r multiple myeloma (RRMM). J Clin Oncol. 41(Suppl 16):
S80042023. View Article : Google Scholar
|
|
91
|
Teoh PJ and Chng WJ: CAR T-cell therapy in
multiple myeloma: More room for improvement. Blood Cancer J.
11:842021. View Article : Google Scholar : PubMed/NCBI
|
|
92
|
Wehrli M, Gallagher K, Chen YB, Leick MB,
McAfee SL, El-Jawahri AR, DeFilipp Z, Horick N, O'Donnell P,
Spitzer T, et al: Single-center experience using anakinra for
steroid-refractory immune effector cell-associated neurotoxicity
syndrome (ICANS). J Immunother Cancer. 10:e0038472022. View Article : Google Scholar : PubMed/NCBI
|
|
93
|
Lam N, Trinklein ND, Buelow B, Patterson
GH, Ojha N and Kochenderfer JN: Anti-BCMA chimeric antigen
receptors with fully human heavy-chain-only antigen recognition
domains. Nat Commun. 11:2832020. View Article : Google Scholar : PubMed/NCBI
|
|
94
|
Mehta P, Cron RQ, Hartwell J, Manson JJ
and Tattersall RS: Silencing the cytokine storm: The use of
intravenous anakinra in haemophagocytic lymphohistiocytosis or
macrophage activation syndrome. Lancet Rheumatol. 2:e358–e367.
2020. View Article : Google Scholar : PubMed/NCBI
|
|
95
|
Minakata D, Ishida T, Ando K, Suzuki R,
Tanaka J, Hagiwara S, Ananthakrishnan R, Kuwayama S, Nishio M,
Kanda Y and Suzuki K: Phase 2 results of idecabtagene vicleucel
(ide-cel, bb2121) in Japanese patients with relapsed and refractory
multiple myeloma. Int J Hematol. 117:729–737. 2023. View Article : Google Scholar : PubMed/NCBI
|
|
96
|
Wang D, Wang J, Hu G, Wang W, Xiao Y, Cai
H, Jiang L, Meng L, Yang Y, Zhou X, et al: A phase 1 study of a
novel fully human BCMA-targeting CAR (CT103A) in patients with
relapsed/refractory multiple myeloma. Blood. 137:2890–2901. 2021.
View Article : Google Scholar : PubMed/NCBI
|
|
97
|
Li C, Wang D, Yu Q, Li Z, Wang W, Hu G, Mu
W, Li C, An N, Long X, et al: Long-term follow-up of fully human
BCMA-targeting CAR (CT103A) in patients with relapsed/refractory
multiple myeloma. Blood. 142(Suppl 1): S48542023. View Article : Google Scholar
|
|
98
|
Du J, Fu W, Jiang H, Dong B, Gao L, Liu L,
Ge J, He A, Li L, Lu J, et al: P869: Updated results of a phase I,
open-label study of bcma/cd19 dual-targeting fastcar-T GC012F for
patients with relapsed/refractory multiple myeloma (RRMM).
Hemasphere. 7(Suppl 1): e84060bf2023. View Article : Google Scholar :
|
|
99
|
Doucet L, Cailleteau A, Vaugier L,
Gourmelon C, Bureau M, Salaud C, Roualdes V, Samarut E, Aumont M,
Zenatri M, et al: Prognostic value of hPG80 (circulating
progastrin) in IDH-wild type glioblastoma treated with
radio-chemotherapy. J Clin Oncol. 40(Suppl 16): S20492022.
View Article : Google Scholar
|
|
100
|
Mailankody S, Ghosh A, Staehr M, Purdon
TJ, Roshal M, Halton E, Diamonte C, Pineda J, Anant P, Bernal Y, et
al: Clinical responses and pharmacokinetics of MCARH171, a
human-derived bcma targeted CAR T cell therapy in
relapsed/refractory multiple myeloma: Final results of a phase I
clinical trial. Blood. 132(Suppl 1): S9592018. View Article : Google Scholar
|
|
101
|
Yamaguchi Y, Gibson J, Ou K, Lopez LS, Ng
RH, Leggett N, Jonsson VD, Zarif JC, Lee PP, Wang X, et al: PD-L1
blockade restores CAR T cell activity through IFN-γ-regulation of
CD163+ M2 macrophages. J Immunother Cancer. 10:e0044002022.
View Article : Google Scholar
|
|
102
|
Ledergor G, Fan Z, Wu K, McCarthy E,
Hyrenius-Wittsten A, Starzinski A, Chang H, Bridge M, Kwek S,
Cheung A, et al: CD4+ CAR T-cell exhaustion associated with early
relapse of multiple myeloma after BCMA CAR T-cell therapy. Blood
Adv. 8:3562–3575. 2024. View Article : Google Scholar : PubMed/NCBI
|
|
103
|
Zwirner NW, Domaica CI and Fuertes MB:
Regulatory functions of NK cells during infections and cancer. J
Leukoc Biol. 109:185–194. 2021. View Article : Google Scholar
|
|
104
|
Padovan E, Terracciano L, Certa U, Jacobs
B, Reschner A, Bolli M, Spagnoli GC, Borden EC and Heberer M:
Interferon stimulated gene 15 constitutively produced by melanoma
cells induces e-cadherin expression on human dendritic cells.
Cancer Res. 62:3453–3458. 2002.PubMed/NCBI
|
|
105
|
Li W, Zhang B, Cao W, Zhang W, Li T, Liu
L, Xu L, Gao F, Wang Y, Wang F, et al: Identification of potential
resistance mechanisms and therapeutic targets for the relapse of
BCMA CAR-T therapy in relapsed/refractory multiple myeloma through
single-cell sequencing. Exp Hematol Oncol. 12:442023. View Article : Google Scholar : PubMed/NCBI
|