Introduction
Sepsis is a clinical syndrome of systemic
dysfunctional metabolism triggered by infection (1). There are ~19 million cases of
sepsis worldwide each year, with a mortality rate of 30%. Surveys
have indicated that the incidence of sepsis and its morbidity rate
are increasing annually (2,3).
Sepsis can lead to functional impairment of multiple organs, with
the heart being the most susceptible, accounting for 16-65% of
cases and being the primary cause of death in patients with sepsis
(4-6). The clinical term for sepsis
accompanied by cardiac dysfunction is sepsis-induced cardiomyopathy
(SIC) (7). SIC is a serious
complication characterized by myocardial damage directly caused by
the production of large amounts of inflammatory factors in the body
after infection, by myocardial systolic depression, or by reduced
cardiac output due to impaired myocardial energy metabolism
(8).
Patients with sepsis usually have altered levels or
functions of hormones, including thyroid hormones, insulin and
adrenal glucocorticoids; low levels of triiodothyronine (T3) in
particular are highly associated with mortality in patients with
sepsis (9). Studies have shown
that alterations in thyroid hormone levels or functions are often
associated with liver and cardiovascular diseases (10,11). Thyroid hormones are synthesized
by thyroid follicular epithelial cells and are stored in the
follicular lumen (12); two
biologically active thyroid hormones circulate in the body, namely,
thyroxine and T3, with T3 being more active and having a greater
affinity for receptors in the nuclei of target organs (13). The heart is one of the most
important target organs for the action of thyroid hormones.
Notably, thyroid hormones regulate calcium homeostasis in
cardiomyocytes by modulating calcium channels and pumps,
influencing physiological processes such as cardiomyocyte
electrophysiology, mechanical contraction and energy metabolism
(14,15).
The pathogenesis of SIC is complex and includes
myocardial cell damage, increased release of proinflammatory
factors, mitochondrial dysfunction and an imbalance in calcium
homeostasis (16). Intracellular
calcium cycling serves as a central regulator of myocardial
contraction and diastole, ensuring proper myocardial cell function
(17,18). The sarcoplasmic reticulum (SR)
releases calcium ions into the cell cytoplasm to induce contraction
and then recycles calcium ions back into the SR via the SR calcium
ATPase (SERCA2) to achieve myocyte relaxation (19,20). This complex process is essential
for excitation-contraction coupling to balance Ca2+
homeostasis, and abnormal Ca2+ handling caused by SR
dysfunction in cardiomyocytes results in systolic dysfunction.
Phospholamban (PLN), which is located in the SR, modulates SERCA2
activity and participates in intracellular calcium recycling,
thereby influencing myocardial contractile and diastolic functions
(21,22). When PLN binds to SERCA2, it
decreases the affinity of the enzyme for calcium ions, preventing
calcium re-entry into the SR and causing its accumulation in the
cytoplasm. Phosphorylated-PLN fails to combine with SERCA2,
facilitating calcium recycling from the cytoplasm to the SR. Thus,
PLN maintains intracellular calcium homeostasis and thereby serves
an important regulatory role in cardiac systolic-diastolic function
(23-25). Numerous studies have indicated
that, in addition to causing disturbances in myocardial contraction
and relaxation, intracellular calcium overload can lead to various
adverse effects, such as arrhythmia, cardiomyocyte apoptosis and
mitochondrial dysfunction (26,27).
Current research on the role of PLN in SIC has
focused primarily on its regulation of cardiac contractile
function. Protein kinase A and the state of PLN itself, such as its
protein pentameric form or monomers and the SERCA-PLN complex can
influence the extent of PLN phosphorylation, thereby affecting
cardiac function (28,29). Additionally, it has been reported
that thyroid hormones are important regulatory factors at the
transcriptional level of SERCA2, indirectly affecting the function
of PLN by influencing the activity and expression of SERCA2 in
cardiac muscle (30). However,
in SIC, the specific molecular mechanism underlying the interaction
between thyroid hormones and PLN has been largely overlooked.
Research investigating the relationship between low T3 syndrome
occurring in sepsis and calcium homeostasis is scarce (11).
Given the aforementioned findings, it may be
hypothesized that T3 mitigates SIC by reducing PLN expression and
calcium overload. The present study aimed to explore the
relationship between low levels of T3 and PLN in SIC, and to
further elucidate the specific mechanisms by which T3 regulates the
expression of PLN. Additionally, the study endeavored to assess the
clinical application of PLN in the context of SIC.
Materials and methods
Clinical patients and healthy
controls
Patients who met the clinical diagnostic criteria
for SIC and those who met the diagnostic criteria for sepsis on the
day of hospital admission were included in the disease group and
the disease control group, respectively. Healthy control group
subjects were recruited from the general population upon completion
of patient recruitment from Children's Hospital of Chongqing
Medical University (Chongqing, China). The mean age of the three
groups (healthy, Sepsis and SIC) was as follows: 6.08 (1-15), 2.67 (0-12) and 4.3 (0-16) years,
respectively. The sex proportion (males/females) for each of the
three groups was 60.0/40.0%. Patients and controls with malignant
tumors, organ transplants, chronic viral infections (hepatitis,
HIV), cirrhosis, chronic renal insufficiency or autoimmune
diseases, and those who had used immunosuppressive drugs within the
past 28 weeks were excluded from the study. A total of 33 children
with SIC and 30 children with sepsis were included in the disease
group and the disease control group, respectively, on the day of
admission from Children's Hospital of Chongqing Medical University.
Clinical data including vital signs, routine blood parameters,
cardiac enzyme profiles, and liver and kidney function test data,
were recorded. In addition, 23 healthy children were recruited as
controls at the Physical Examination Center of the Children's
Hospital of Chongqing Medical University. The complete date range
for patient recruitment was from December 2021 to October 2022.
Written informed consent was obtained from the parents of all of
the patients in accordance with the ethical standards of The
Declaration of Helsinki. The present study protocol was approved by
the Clinical Research Ethics Committee of the Children's Hospital
of Chongqing Medical University (approval no. 2021-353).
Establishment of an animal model
The SIC model was established via the
intraperitoneal injection of lipopolysaccharide (LPS; cat. no.
L8880; Beijing Solarbio Science & Technology Co., Ltd.)
dissolved in sterile saline at a dose of 20 mg/kg. A total of 18
male C57BL/6J mice (age, 6-8 weeks; weight, 18-20 g) were purchased
from the Animal Experiment Center of Chongqing Medical University
(SCXK: 2022-0010). All of the mice were maintained under standard
housing conditions: Temperature, 22±1°C; humidity, 50%; 12-h
dark/light cycle; ad libitum access to food and water. After
3 days of acclimation, 18 mice were randomly divided into the
following three groups: The sham group (n=6), the LPS experimental
group (n=6) and the LPS + T3 experimental group (n=6). The sham
group and LPS experimental group were injected intraperitoneally
with saline or LPS solution, respectively, while the LPS + T3 group
was pretreated with 80 mg/kg T3 (cat. no. T162132; Shanghai Aladdin
Biochemical Technology Co., Ltd.) 24 h before injection with LPS.
Since the half-life of T3 has been reported to be 24 h (31), and due to the survival status of
the experimental animals after modeling, the duration of the
experiment was 24 h. Animal healthy behavior and the humane
endpoints, which were defined as a body temperature <33°C,
inactivity of >3 h and/or a drop in weight of >20% of their
original body weight, were monitored every 6 h during the study.
Anesthesia and specific housing conditions were used during
handling and intraperitoneal injection with LPS to ensure minimal
pain, suffering and distress to animals. Each mouse was deeply
anesthetized with an intraperitoneal injection of 10% chloral
hydrate (350 mg/kg) prior to intraperitoneal injection with LPS,
and no mouse exhibited signs of peritonitis following the
administration of 10% chloral hydrate. Following intraperitoneal
injection of LPS, the mice began to exhibit signs of infection,
including ruffled fur, decreased activity, hunched posture and
tachypnea. Subsequently, at 24 h post-injection, the 6 mice from
the LPS group and the 6 mice from the LPS + T3 group were
sacrificed. The mice in the sham group were euthanized 24 h after
intraperitoneal injection of saline. The mice were euthanized by
inhalation of 30% CO2 for 5 min and death was verified
by cervical dislocation. No moving, no breathing and pupil dilation
of mice were assessed to confirm death. At the end of the
experiment and after the mice were sacrificed, blood was collected
from the retro-orbital vein and myocardial tissue samples were
collected. The present study followed the animal experimentation
guidelines (32) and was
approved by the Animal Ethics Committee of the Children's Hospital
of Chongqing Medical University (approval no.
CHCMU-IACUC20210114036).
Establishment of cell models
H9C2 cells, provided by The Cell Bank of Type
Culture Collection of The Chinese Academy of Sciences, were
cultured in DMEM (4.5 g/l glucose; Gibco; Thermo Fisher Scientific,
Inc.) supplemented with 10% fetal bovine serum (Gibco; Thermo
Fisher Scientific, Inc.) in a 5% CO2 humidified
incubator. The cells were divided into four groups: The control
group, the LPS group, the LPS + T3 group and the LPS + SP600125
(SP; MedChemExpress) group. The concentration range was established
according to the literature (33,34), and 80 ng/ml T3 and 10 µM
SP (JNK inhibitor) were selected as the working concentrations The
cells in the control group were cultured in complete medium
containing 10% fetal bovine serum; the cells in the LPS group were
incubated for 24 h after they reached 75% confluence, and the
medium was then replaced with complete DMEM containing 10
µg/ml LPS at 37°C for 24 h to construct an in vitro
model of SIC. In addition, the LPS + T3 and LPS + SP groups were
pretreated with T3 for 24 h before LPS treatment or with SP for 30
min at 37°C, respectively. All of the cell experiments were
independently repeated at least in triplicate.
Detection of serological indicators
Blood samples from patients, as well as mouse blood
obtained from the orbital venous plexus, were centrifuged at 1,000
× g at room temperature for 15 min. According to the manufacturers'
instructions, the levels of PLN, cardiac troponin I (cTnI), and T3
in the serum were determined via human-derived PLN (cat. no.
JL15150; Shanghai Jianglai Biotechnology Co., Ltd.) and T3 (cat.
no. JL13028; Shanghai Jianglai Biotechnology Co., Ltd.) ELISA kits,
and mouse-derived cTnI (cat. no. E-EL-M1203c; Wuhan Elabscience
Biotechnology Co., Ltd.), PLN (cat. no. JL24496; Shanghai Jianglai
Biotechnology Co., Ltd.) and T3 (cat. no. E-EL-0079c;Wuhan
Elabscience Biotechnology Co., Ltd.) ELISA kits. Albumin (ALB) and
creatinine (CREA) were assessed using an automatic biochemical
analyzer (Roche Cobas c701; Roche Diagnostics), procalcitonin (PCT)
was assessed using an automatic chemiluminescence immunoassay
analyzer (Cobas pro e801; Roche Diagnostics), and brain natriuretic
peptide (BNP) was assessed using another automatic
chemiluminescence immunoassay analyzer (Atellica IM 1600;
Siemens).
Histological analysis
After the mice were treated for 24 h to establish an
in vivo model, they were sacrificed with 30% CO2.
The heart tissues were collected, rinsed with isotonic saline, and
fixed in 4% paraformaldehyde for 12 h at room temperature.
Subsequently, the tissues were dehydrated, embedded in paraffin,
and were sectioned (8 µm) and stained with hematoxylin for 6
min and 1% eosin for 1 min, or Masson for 5 min at room
temperature. The sections were then placed under a light microscope
for observation and images were captured.
Cell viability and drug toxicity
assay
Cell viability and drug toxicity were measured using
the Cell Counting Kit-8 (CCK-8) assay. The cells
(4×103/well) were placed in 96-well plates and were
incubated according to the experimental design. Subsequently, 100
µl CCK-8 assay reagent (cat. no. M4839; AbMole BioScience)
was diluted 10 times with serum-free culture medium and was
incubated with the cells for 3 h. The optical density (OD) value
was then detected at 450 nm using a microplate reader (Thermo
Fisher Scientific, Inc.). All OD values were normalized by
converting them to a percentage of the mean control value.
Apoptosis assay
An apoptosis assay kit (cat. no. 556547; BD
Biosciences) was used to detect apoptosis according to the
manufacturer's instructions. All of the cells (including those in
suspension) were collected as a precipitate, and 100 µl 1X
binding buffer diluted in distilled water was added to resuspend
the cell pellet. Subsequently, 5 µl FITC and 5 µl
propidium iodide were added and vortexed sequentially, after which
the samples were incubated at room temperature in the dark for 30
min. Finally, 400 µl 1X binding buffer was added to each
tube, apoptosis was examined by flow cytometry (FACSCanto II;
Becton, Dickinson and Company) and the data were analyzed using
FlowJo (software version 10.8; FlowJo LLC).
Crystal violet staining
The cells were placed in 24-well plates
(5×104/well) and were treated for 24 h. The cells were
then fixed with 1 ml 4% paraformaldehyde for 10 min and were
subjected to staining with crystal violet (cat. no. C0121; Beyotime
Institute of Biotechnology) on a shaker at room temperature for 15
min. The cells were rinsed 3-5 times with PBS, air-dried, were
placed under a light microscope for observation and images were
captured. In addition, the OD value was detected at 570 nm using a
microplate reader. All OD values were normalized by converting them
to a percentage of the mean control value.
Calcium assay
Intracellular calcium levels were detected using the
calcium fluorescent probe Fluo-4AM (cat. no. S1060; Beyotime
Institute of Biotechnology). The Fluo-4AM working solution (5
µM) was prepared according to the manufacturer's
instructions, and the cells were incubated with it for 40 min at
37°C in the dark for fluorescent probe loading. The staining
solution was then discarded, and PBS was added and the cells were
incubated for a further 20 min. The cells were then observed and
images were captured under a confocal microscope, and the mean
fluorescence intensity was detected via flow cytometry (FACSCanto
II) and the data were analyzed using FlowJo (software version
10.8). The values of the experimental groups were normalized to
those of the control group.
Reactive oxygen species (ROS) assay
A ROS assay was performed using DHE (cat. no.
KGAF019; Nanjing KeyGen Biotech Co., Ltd.) and Hoechst 33258 (cat.
no. C1011; Beyotime Institute of Biotechnology) fluorescent probe
kits according to the manufacturers' instructions. Briefly, the
cells were incubated with 5 µM DHE for 30 min at 37°C in the
dark. To measure the levels of ROS, fluorescence images of the
cells were captured under a confocal fluorescence microscope, or
the cell suspension was collected in test tubes, and the mean
fluorescence intensity of DHE was measured by flow cytometry
(FACSCanto II) and the data were analyzed using FlowJo (software
version 10.8). The values of the experimental groups were
normalized to those of the control group.
Plasmid construction and
transfection
The pcDNA3.1(+) plasmid overexpressing c-Jun
contained the coding sequence region of the rat c-Jun gene from
NCBI (https://www.ncbi.nlm.nih.gov/) and
was constructed by Beijing Biomed Gene Technology Co., Ltd. An
empty pcDNA3.1(+) plasmid was used as a negative control. The
overexpression plasmids (900 ng/µl) were transfected into
H9C2 cells (80% confluence) using the PolyJet™ transfection reagent
(cat. no. SL100688; SignaGen Laboratories), according to the
manufacturer's instructions, at 37°C for 6 h. The DNA and PolyJet
were diluted separately at a ratio of 3:1 in serum-free DMEM,
mixed, incubated for 15 min at room temperature and then added to
the cell culture medium. After 12-18 h of transfection, the medium
containing the PolyJet/DNA complex was removed and replaced with
fresh serum-containing complete medium.
Reverse transcription-quantitative PCR
(RT-qPCR)
Total RNA was extracted from cell samples or
myocardial tissue samples using an RNA extraction kit (cat. no.
9109; Takara Bio, Inc.), and single-stranded complementary DNA was
synthesized using an RT kit (cat. no. RK20429; ABclonal Biotech
Co., Ltd.). β-actin was selected as the reference gene according to
previous studies (35,36). qPCR was performed using SYBR qPCR
reagents (cat. no. RK21203; ABclonal Biotech Co., Ltd.), and the
values of the average quantification cycle (Cq) were normalized to
the expression of β-actin; finally, the relative mRNA expression
levels were determined using the 2−ΔΔCq method (37). The values of the experimental
groups were normalized to those of the control group. The following
qPCR cycling conditions were used: 3 min at 95°C, followed by 45
cycles at 95°C for 30 min, 95°C for 5 sec and 60°C for 30 sec. The
primer details are shown in Table
I.
 | Table IQuantitative PCR primer
sequences. |
Table I
Quantitative PCR primer
sequences.
| Name | Primer sequence,
5′-3′ |
|---|
| PLN-Mouse | F:
CCAAGACAGAAGCAGGTGAAGAGAC |
| R:
AAAGTTGACAACAGGCAGCCAAATG |
| PLN-Rat | F:
TTACTCGCTCGGCTATCAGGAGAG |
| R:
ACAATGATGCAGATCAGCAGCAGAC |
| SERCA2-Mouse | F:
GAATTAAGCCCTTCAGCCCAGAGAG |
| R:
AGCATCATTCACACCATCACCAGTC |
| IL-6-Rat | F:
AGTTGCCTTCTTGGGACTGATGTTG |
| R:
GGTATCCTCTGTGAAGTCTCCTCTCC |
| TNF-α-Rat | F:
ATGGGCTCCCTCTCATCAGTTCC |
| R:
CCTCCGCTTGGTGGTTTGCTAC |
| c-Jun-Rat | F:
CTTCTACGACGATGCCCTCAACG |
| R:
AGGTTCAAGGTCATGCTCTGCTTC |
| β-actin-Mouse | F:
CTGAGAGGGAAATCGTGCGTGAC |
| R:
ACCGCTCGTTGCCAATAGTGATG |
| β-actin-Rat | F:
GCTGTGCTATGTTGCCCTAGACTTC |
| R:
GGAACCGCTCATTGCCGATAGTG |
Western blotting (WB)
Proteins were extracted from cells and myocardial
tissues according to the instructions of the protein extraction kit
(cat. no. KGP2100; Nanjing KeyGen Biotech Co., Ltd.), and their
concentrations were determined with a BCA assay kit (cat. no.
KGP902; Nanjing KeyGen Biotech Co., Ltd.). Proteins (60 µg)
were separated by SDS-PAGE on 10 or 15% gels and were transferred
to PVDF membranes, after which the protein samples were blocked
with 5% skim milk powder for 2 h at room temperature. The membranes
were subsequently incubated with the primary antibodies at 4°C
overnight and with an HRP-conjugated secondary antibody for 1 h at
room temperature. Finally, the blots were rinsed three times with
TBS-0.1% Tween (TBST; 5 min each wash) and 200 µl Pico ECL
Ultrasensitive Substrate Chemiluminescent Detection Kit (cat. no.
PA134-01; Beijing Biomed Gene Technology Co;) was added to scan the
blot using the ChemiDoc MP imaging system (Bio-Rad Laboratories,
Inc.), and the band intensities were measured using ImageJ software
(v1.54; National Institutes of Health). The values of the
experimental groups were normalized to those of the control group.
Each experiment was performed in triplicate, and all samples were
normalized to β-actin. The following antibodies diluted with TBST
(1:500) were used: Rabbit anti-PLN (cat. no. ab219626; Abcam),
rabbit anti-phosphorylated (p)-PLN (Ser16) (cat. no. AP0907;
ABclonal Biotech Co., Ltd.), rabbit anti-p-PLN (Thr17) (cat. no.
AF7278; Affinity Biosciences), rabbit anti-SERCA2 (cat. no. 381667;
ZENBIO), rabbit anti-JNK (cat. no. ET1601-28; HUABIO), rabbit
anti-p-JNK (cat. no. ET1609-42; HUABIO), rabbit anti-c-Jun (cat.
no. ET1608-3; HUABIO), rabbit anti-p-c-Jun (cat. no. ET1608-4;
HUABIO), mouse anti-β-actin (cat. no. R23613; ZENBIO), goat
anti-rabbit-HRP (cat. no. SA00001-2 Proteintech Group, Inc.) and
goat anti-mouse-HRP (cat. no. SA00001-1; Proteintech Group,
Inc.).
Proteomics analyses
The cardiac tissues from three mice in the sham and
LPS groups were collected and used for proteomic analyses. The
essential steps involved in tandem mass tagging (TMT) proteomics
analysis are protein extraction, trypsin digestion, TMT labeling,
HPLC fractionation, LC-MS/MS analysis and data search, which were
performed as previously described (38). The proteomics analysis in the
present study was facilitated by the Jingjie PTM Bioinformatics
Team (Jingjie PTM BioLab Co. Ltd.).
Statistical analysis
Experiments were repeated at least three time,
unless otherwise stated. Data are presented as the mean ± SD and
were statistically analyzed using GraphPad Prism 8.0 (Dotmatics)
and SPSS 27.0 (IBM Corp.) software. Comparisons between two groups
were analyzed using unpaired Students' t-test or Welch's t-test on
the basis of the results of the homogeneity test of variance (F
test). Differences among three or more groups were analyzed using
one-way analysis of variance followed by Tukey's test for pairwise
comparisons or Dunnett's test for comparing each group to a control
group. Receiver operating characteristic (ROC) curve analyses, and
Spearman correlation analyses between PLN, and serum ALB, PCT and
CREA, were performed in SPSS 27.0. P<0.05 was considered to
indicate a statistically significant difference.
Results
Serum T3 levels are significantly
negatively correlated with myocardial PLN levels in sepsis
Hematoxylin and eosin and Masson staining of the
cardiac tissue sections revealed inflammatory cell infiltration and
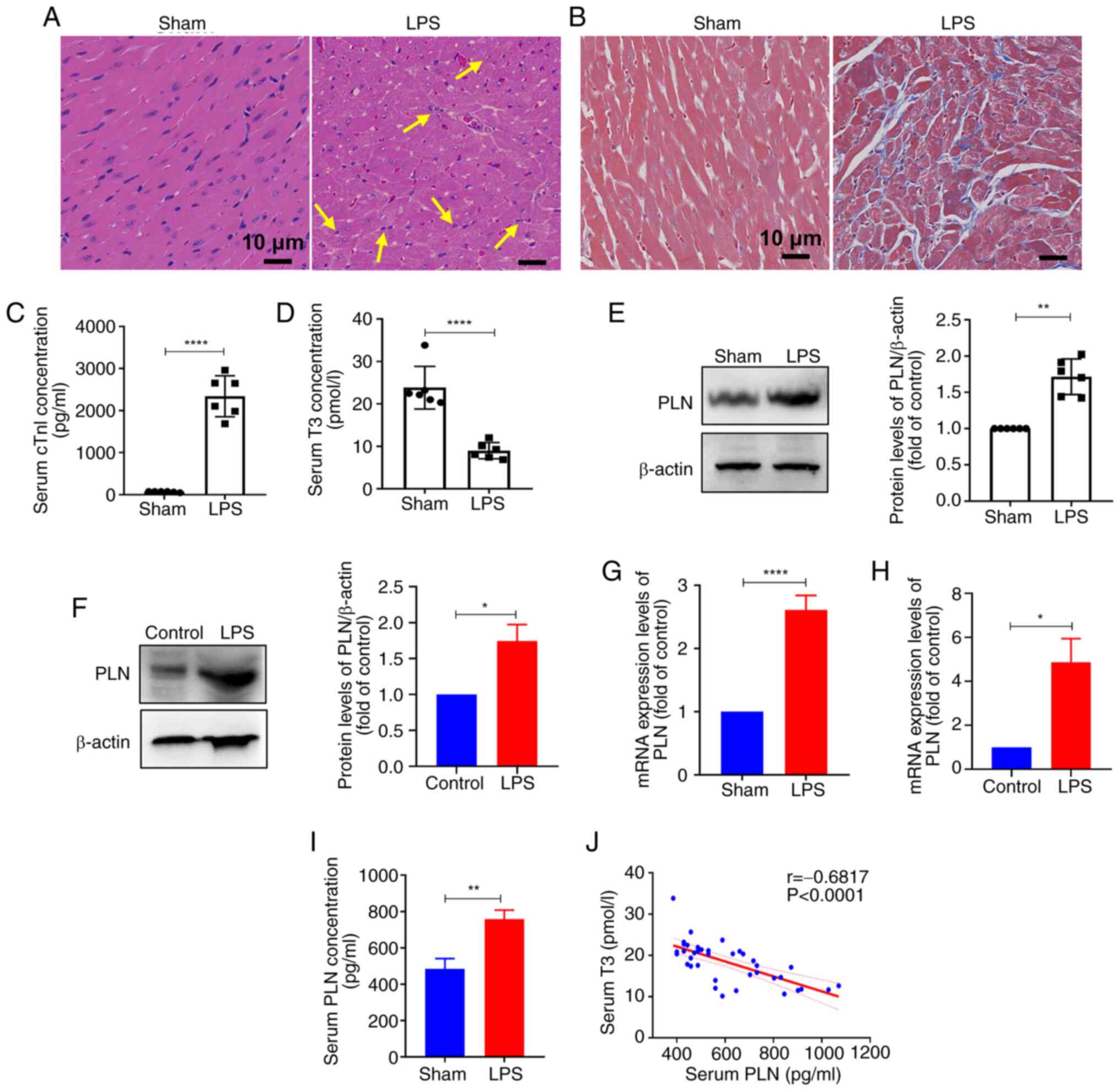
myocardial fibrosis in the LPS group (Fig. 1A and B). The serological levels
of cTnI were significantly greater in the LPS group than those in
the control group (Fig. 1C).
These results indicated that LPS successfully induced SIC in
vivo. Furthermore, serum detection revealed a significant
decrease in the concentration of T3 in response to LPS treatment
(Fig. 1D). Compared with in the
control group cells, the chosen concentration of 80 ng/ml T3 had
the largest effect on cell viability; treatment with this resulted
in a >80% increase in cell viability compared with the control
(Fig. S1). Previous proteomics
analyses of the hearts of normal mice and mice with sepsis (the LPS
group) revealed that the expression of PLN, which is related to T3
regulation of cardiac calcium, was significantly increased in mice
with sepsis (Fig. S2).
Similarly, the results of WB and RT-qPCR further confirmed the
significantly elevated levels of PLN in myocardial tissues and H9C2
cells in the LPS group (Fig.
1E-G). In vitro, the results of proliferation, apoptosis
and inflammation assays revealed that LPS induced inflammatory
damage to cardiomyocytes, as cells treated with LPS exhibited a
decrease in cellular viability, an increased proportion of
apoptotic cells and a reduction in cell growth density compared
with in the control group (Fig.
S3A-D). Moreover, serum PLN concentration was significantly
elevated in the serum of mice with sepsis compared with that in the
control group (Fig. 1I).
Furthermore, analysis of serum T3 and PLN levels revealed a
significant negative correlation between the two parameters in the
mouse model of SIC (Fig. 1J).
PLN is a critical regulator of calcium cycling and contractility in
the heart, and impairs calcium recycling in the endoplasmic
reticulum (30). In the present
study, intracellular calcium overload increased, as indicated by
Fluo-4AM green fluorescence staining, and was accompanied by
abnormally elevated levels of ROS in the LPS group, as indicated by
increased red fluorescence in the nucleus (Fig. S3E-H).
T3 treatment alleviates cardiomyocyte
damage in vitro and in vivo
The results revealed that treatment with T3
significantly increased cell viability, reduced the proportion of
apoptotic cells and promoted cell proliferation under LPS challenge
(Fig. 2A-C). Additionally, T3
treatment significantly reduced the expression levels of the
inflammatory factors interleukin 6 (IL-6) and tumor necrosis factor
α compared with in the LPS group (TNF-α) (Fig. 2D). Furthermore, the results of
the present study revealed that, compared with in the LPS group,
intracellular calcium accumulation was reduced and calcium overload
was effectively alleviated after T3 intervention (Fig. 2E and G). In addition, compared
with in the LPS group, T3 treatment reduced the intracellular ROS
content and alleviated intracellular oxidative stress (Fig. 2F and H).
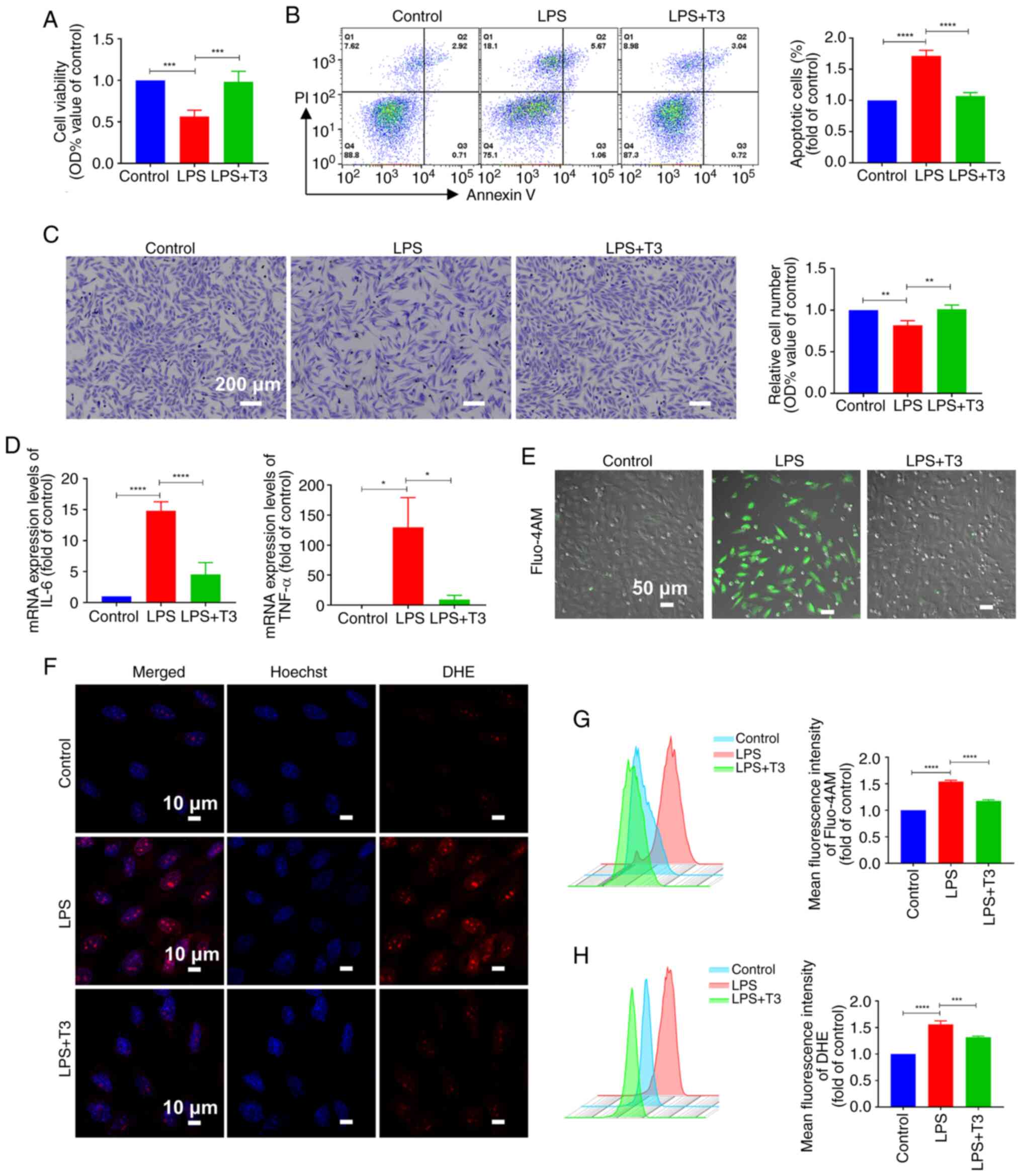 | Figure 2T3 treatment alleviates cardiomyocyte
damage in vitro. (A) Cell Counting Kit-8 assay assessed H9C2
cell viability (n=3). (B) Apoptosis detection and statistical
analysis of H9C2 cells (n=3). (C) Crystal violet staining and
statistical analysis of H9C2 cells (n=3). (D) Quantitative PCR
detected the mRNA expression levels of IL-6 and TNF-α in H9C2 cells
(n=3). (E) Fluo-4AM fluorescence images of H9C2 intracellular
calcium levels. Scale bar, 50 µm; n=3. (F) DHE fluorescence
images of H9C2 intracellular reactive oxygen species levels. Scale
bar, 10 µm; n=3. (G) Flow cytometric detection of (G) Fluo-4
AM and (H) DHE mean fluorescence intensity in H9C2 cells (n=3). All
experiments were repeated three times. Data are presented as the
mean ± standard deviation. *P<0.05,
**P<0.01, ***P<0.001,
****P<0.0001. IL-6, interleukin 6; LPS,
lipopolysaccharide; OD, optical density; PI, propidium iodide; PLN,
phospholamban; T3, triiodothyronine; TNF-α, tumor necrosis factor
α. |
After T3 intervention in the mice, the serum
concentrations of various factors were measured, revealing an
increase in the concentration of T3, and a decrease in cTnI and PLN
concentrations compared with in the LPS group (Fig. 3A). In addition, compared with in
the LPS group, the mRNA expression levels of PLN in myocardial
tissues were reduced, whereas SERCA2 expression was increased after
T3 treatment (Fig. 3B). Tissue
section staining showed that T3 treatment effectively reduced the
infiltration of inflammatory cells in myocardial tissue in the LPS
group (Fig. 3C), decreased the
deposition of collagen fibers and reduced the number of fibroblasts
in myocardial tissue (Fig.
3D).
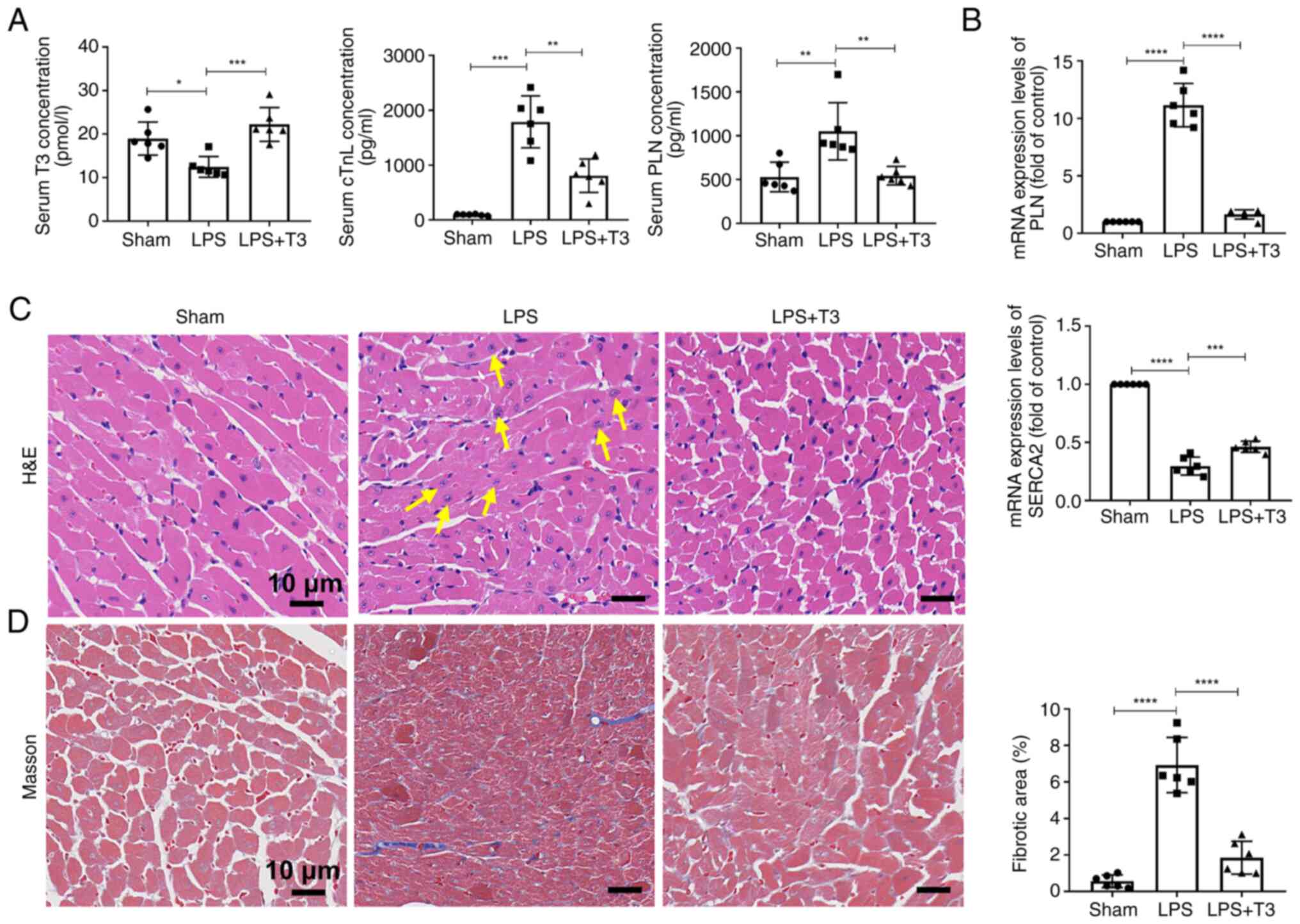 | Figure 3T3 treatment alleviates cardiomyocyte
damage in vivo. (A) Serum ELISA results for mice (n=6). (B)
Quantitative PCR detection of mRNA expression levels in mice (n=6).
(C) H&E staining was applied to mouse myocardial tissue (n=6).
Yellow arrows indicate inflammatory cells. Scale bar, 10 µm.
(D) Masson staining and quantitative analysis were applied to mouse
myocardial tissue (n=6). Scale bar, 10 µm. All experiments
were conducted in triplicate using independent samples. The data
are presented as the mean ± standard deviation.
*P<0.05, **P<0.01,
***P<0.001, ****P<0.0001. cTnI, cardiac
troponin I; H&E, hematoxylin and eosin; LPS,
lipopolysaccharide; PLN, phospholamban; SERCA2, sarcoplasmic
reticulum calcium ATPase; T3, triiodothyronine. |
T3 reduces PLN expression through
JNK/c-Jun signaling pathway inhibition
The specific mechanisms by which T3 regulates PLN
were subsequently investigated. According to the results of WB,
treatment with T3 decreased the protein levels of PLN compared with
in the LPS group in vivo and in vitro (Figs. 4A, B, S4A and B). In addition, it was
revealed that since PLN expression decreased, the p-PLN/PLN ratio
increased after T3 treatment (Fig.
S4C and D). It has previously been reported that T3 can
negatively regulate JNK phosphorylation, thereby inhibiting its
proapoptotic effects (27).
Therefore, the present study examined the levels of p-JNK and
phosphorylation of its downstream target protein, c-Jun, following
T3 intervention, and the results revealed that the phosphorylation
of both JNK and c-Jun was decreased compared with in the LPS group
(Figs. 4A, B, S4A and B).
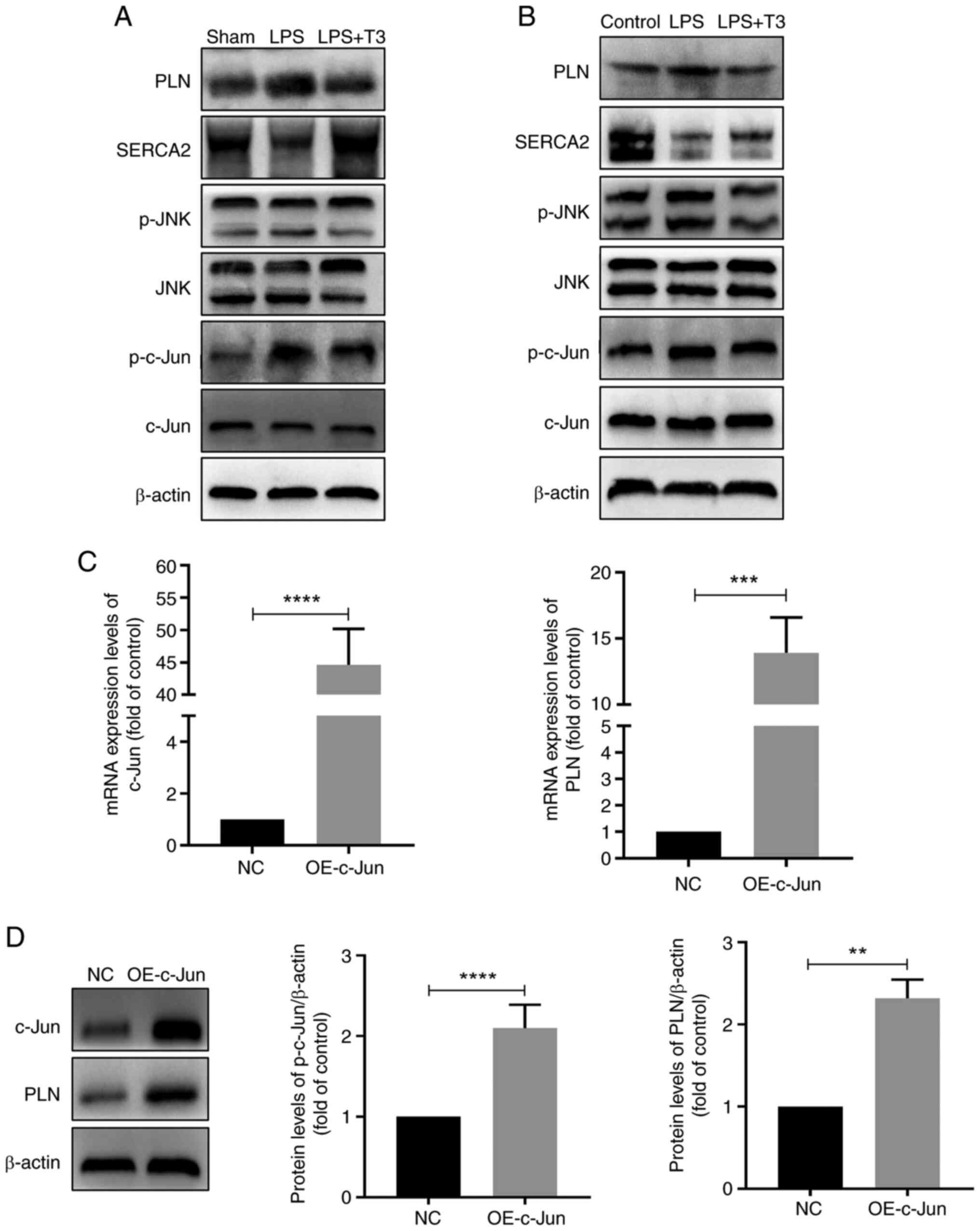 | Figure 4T3 reduces PLN expression through
JNK/c-Jun signaling pathway inhibition. WB detection of protein
levels in the (A) myocardial tissues of mice (n=6) and in (B) H9C2
cells (n=3). (C) Quantitative PCR detection of mRNA expression
levels in H9C2 cells (n=3). (D) WB detection of protein levels in
H9C2 cells (n=3). All in vitro experiments were performed
using triplicate samples and were repeated three times. Data are
presented as the mean ± standard deviation. **P<0.01,
***P<0.001, ****P<0.0001. LPS,
lipopolysaccharide; NC, negative control; OE, overexpression; p-,
phosphorylated; PLN, phospholamban; SERCA2, sarcoplasmic reticulum
calcium ATPase; T3, triiodothyronine; WB, western blotting. |
To further explore the regulatory relationship
between c-Jun and PLN, cells were transfected with a plasmid
overexpressing c-Jun. Overexpression of c-Jun upregulated the mRNA
and protein expression levels of PLN, and decreased the p-PLN/PLN
ratio (Figs. 4C, D, and S4E).
Inhibition of the JNK/c-Jun signaling
pathway reduces cardiomyocyte damage
According to the results of the CCK-8 assay, 10
µM was selected as the working concentration of the JNK
inhibitor SP, as 10 µM SP had the least toxic effect on
cells compared with the control (Fig. S1). Subsequently, it was used to
confirm that T3 alleviates cardiomyocyte damage through the
JNK/c-Jun pathway, as T3 and SP similarly reduced the
phosphorylation levels of JNK and c-Jun (Fig. 5A). Further WB revealed that c-Jun
phosphorylation levels were decreased after SP treatment and that
total PLN protein levels were decreased, whereas the p-PLN/PLN
ratio was increased compared with in the LPS group (Figs. 5A and S5). In comparison with the
LPS group, cell apoptosis was decreased, and the intracellular
synthesis of inflammatory factors was reduced following SP
treatment (Fig. 5B and C).
Furthermore, SP intervention decreased intracellular ROS levels and
reduced intracellular calcium ion accumulation compared with in the
LPS group (Fig. 5D and E).
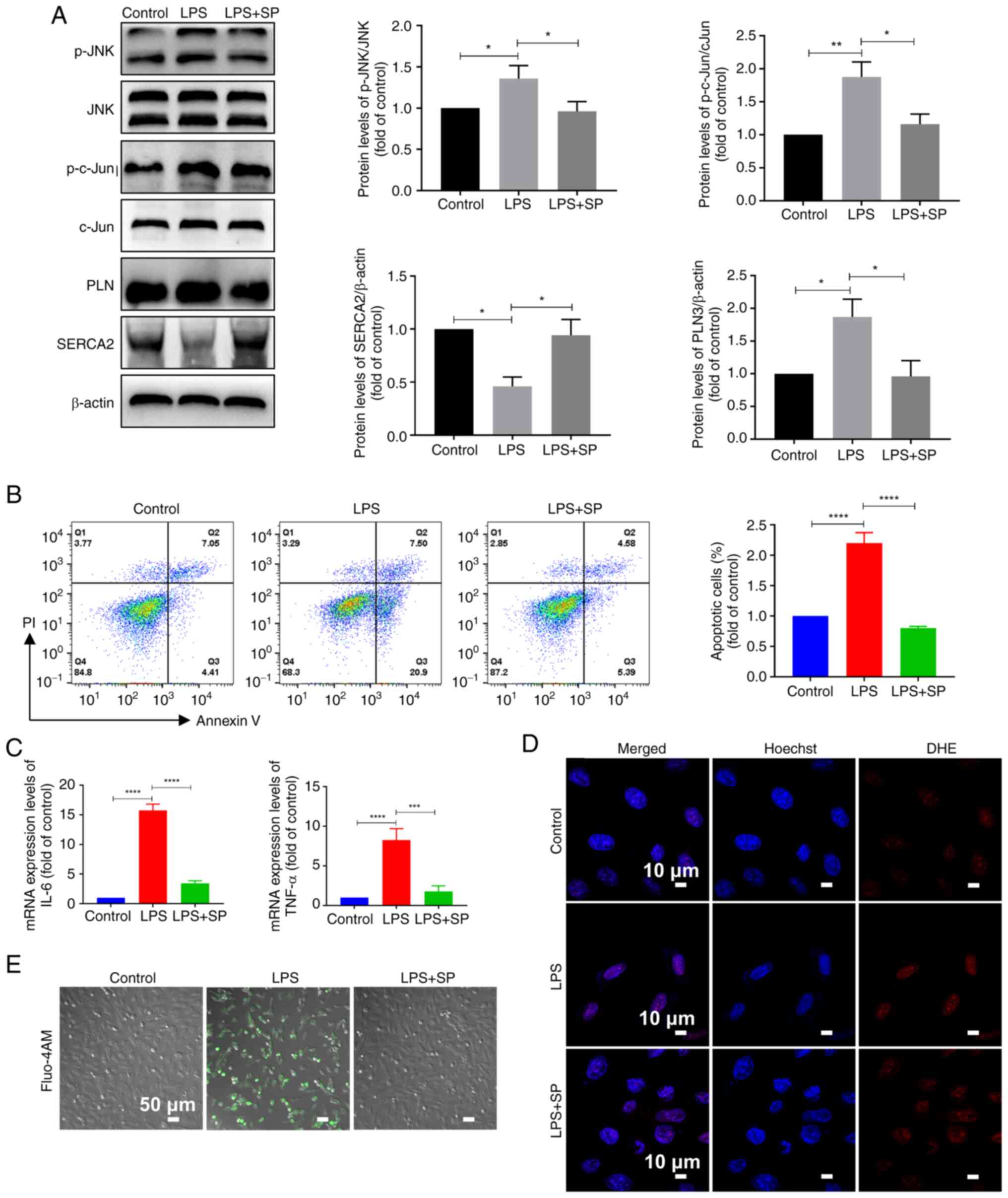 | Figure 5Inhibition of the JNK/c-Jun signaling
pathway improves cardiomyocyte damage. (A) Western blotting
detection of protein levels and semi-quantitative analysis in H9C2
cells (n=3). (B) Apoptosis assay and statistical analysis in H9C2
cells (n=3). (C) Quantitative PCR detection of mRNA expression
levels in H9C2 cells (n=3). (D) Fluorescence images of H9C2
intracellular DHE reactive oxygen species levels. Scale bar, 10
µm; n=3. (E) Fluo-4 AM fluorescence images of H9C2
intracellular calcium levels. Scale bar, 50 µm; n=3. All
experiments were repeated three times. Data are presented as the
mean ± standard deviation. *P<0.05,
**P<0.01, ***P<0.001,
****P<0.0001. IL-6, interleukin 6; LPS,
lipopolysaccharide; p-, phosphorylated; PI, propidium iodide; PLN,
phospholamban; SP, SP600125; TNF-α, tumor necrosis factor α. |
Clinical value of elevated PLN in
patients with SIC
To explore the clinical value of PLN, serum samples
from patients were collected for analysis. The general data of the
patients are listed in Table
SI. The results revealed that in the SIC group, patients had
significantly lower serum T3 levels and significantly higher PLN
levels compared with those in the healthy group (Fig. 6A). ROC curves were constructed on
the basis of the PLN, cTnI and BNP serum contents. PLN had a
greater area under the curve, 0.9503 (95% CI: 0.9014-0.9993),
compared with the other two markers, cTnI [0.9131 (95% CI:
0.8513-0.9748)] and BNP 0.8403 (95% CI: 0.7398-0.9408)], and the
optimal threshold value was determined to be 436.8 pg/ml (Fig. 6B). In addition, PLN was
negatively correlated with serum ALB, whereas it was positively
correlated with serum PCT and serum CREA (Fig. 6C).
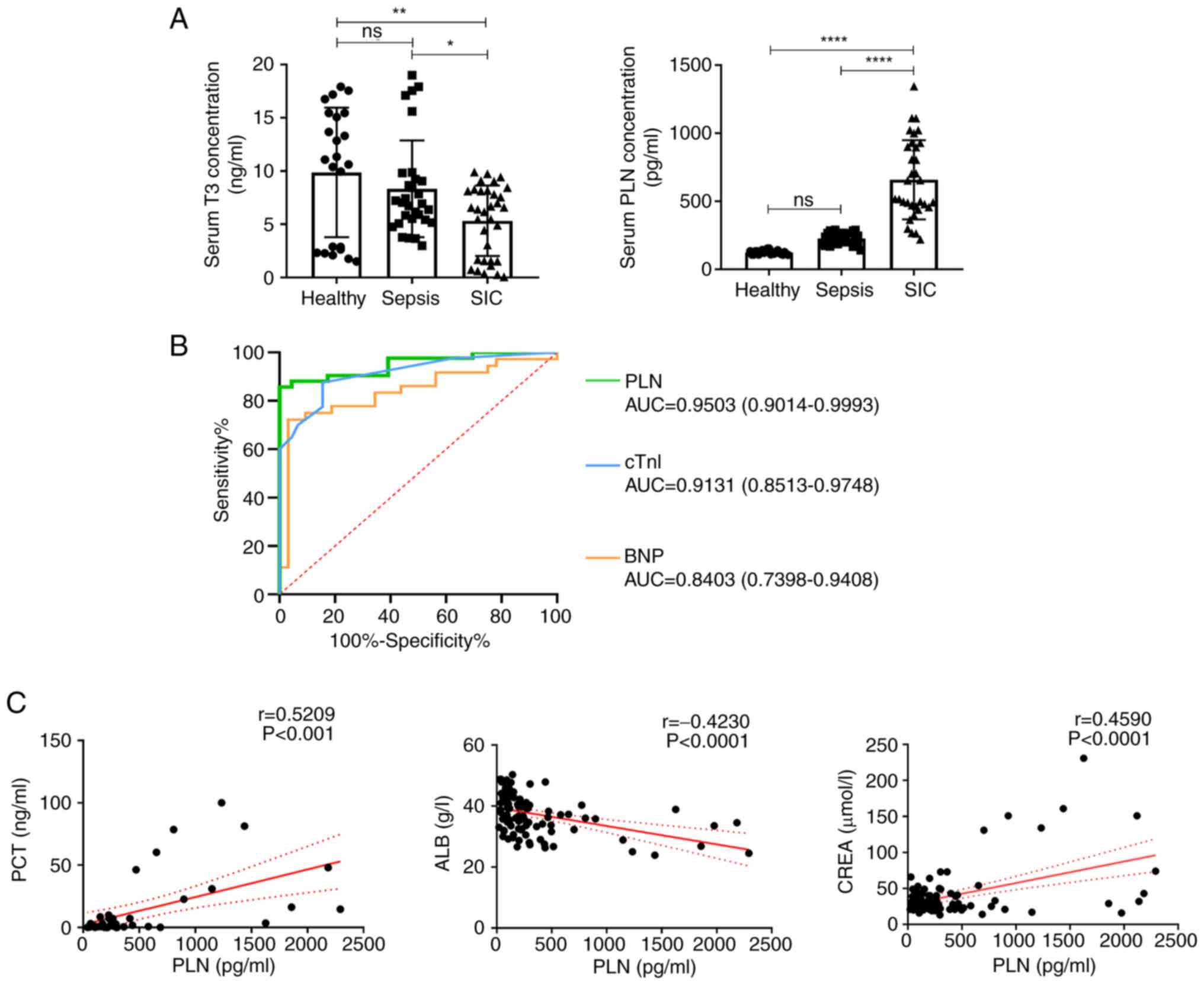 | Figure 6Clinical value of elevated PLN in
patients with SIC. (A) Levels of T3 and PLN in each group of
patients were measured by ELISA. (B) Receiver operating
characteristic curves were constructed according to the serum
levels of PLN, cTnI and BNP. (C) Correlation curves of serum PLN
with PCT, ALB and CREA. Data are presented as the mean ± standard
deviation. ns, no significance; *P<0.05,
**P<0.01, ****P<0.0001. ALB, albumin;
AUC, area under the curve; BNP, brain natriuretic peptide; CREA,
creatinine; cTnI, cardiac troponin I; PCT, procalcitonin; PLN,
phospholamban; SIC, sepsis-induced cardiomyopathy; T3,
triiodothyronine |
Discussion
SIC is a critical condition involving the heart, and
is associated with varying degrees of myocardial damage during the
progression of sepsis, often leading to a poor prognosis and high
mortality rate (7). The
pathological mechanisms of SIC are complex and include inflammatory
damage to cardiomyocytes, the release of nitric oxide and ROS,
mitochondrial dysfunction and abnormal calcium regulation (16). Thyroid hormones are important
indicators of disease severity and mortality, and patients with
sepsis often experience thyroid dysfunction (39). In addition to their classical
metabolic regulatory roles, thyroid hormones exert cardioprotective
and reparative effects (40).
Consistent with these findings, significantly low levels of T3 were
observed in patients with SIC, as well as in in vivo and
in vitro models of SIC, in the present study, and
supplementation with T3 mitigated myocardial tissue lesions while
reducing cardiomyocyte calcium overload and ROS levels.
PLN is a small transmembrane protein localized on
the SR of cardiomyocytes that serves a key regulatory role in
inhibiting Ca2+ transport, and thereby crucially
influences cardiac contractile and diastolic functions (41). In cardiomyocytes, PLN reduces the
affinity of SERCA2 for Ca2+, whereas the phosphorylation
of PLN relieves this effect and promotes calcium recycling back
into the SR (36). This process
is vital for regulating myocardial contraction and diastole.
Recently, Stege et al (42) revealed a prominent role for
abnormal PLN protein distribution and SR/endoplasmic reticulum
disorganization in the underlying mechanism of cardiomyopathy.
In the present study, abnormally elevated levels of
PLN were observed in SIC. This elevation may have hindered the
effective recycling of extracellular calcium into the SR, leading
to calcium overload in cardiomyocytes and impairing cardiomyocyte
diastolic function. This effect may be attributed to alterations in
the phosphorylation levels of the Ser16 and Thr17 sites of PLN.
Furthermore, the experimental results indicated that T3 decreased
PLN expression and altered the phosphorylation ratio of PLN in
cardiomyocytes in vivo and in vitro, which is
consistent with the findings of previous studies (43,44); however, the specific mechanism is
unknown.
It has previously been reported that T3 can protect
against heart ischemia/reperfusion injury by modulating various
intracellular kinase signaling pathways (45). The balance between the
proapoptotic and prosurvival kinase signaling pathways is critical
in myocardial injury and remodeling. It has previously been shown
that T3 can significantly reduce the phosphorylation of the
proapoptotic protein kinase JNK (46). Consistent with these findings,
the current study revealed that activated JNK phosphorylation in
SIC was significantly reduced by T3 treatment, indicating that T3
may inhibit the JNK pathway. JNK is a branch of the MAPK pathway
that serves crucial roles in cell proliferation, migration,
survival, senescence and stress responses. Despite its dual roles
in apoptosis and survival regulation (47), the findings of the present study
suggested that JNK inhibition may effectively prevent apoptosis and
promote cell survival. c-Jun is the primary downstream target
molecule of JNK and is a component of the activator protein-1
transcriptional complex, which is modulated by various protein
kinases and has a regulatory role in apoptosis and stress responses
(48). The JNK/c-Jun signaling
pathway is directly associated with the development of various
diseases, and its activation has been shown to have protective
effects on ischemia/reperfusion injury in the liver, lung, brain
and heart (49-51). The phosphorylation of c-Jun is a
critical indicator of its activation (52). Only a few studies, such as Chin
et al (53) and Song
et al (54), have
suggested a relationship between JNK/c-Jun and PLN in the context
of myocardial ischemia/reperfusion or myocardial hypertrophy. In
the present study, the results revealed increased c-Jun
phosphorylation following JNK activation, indicating the activation
of the JNK/c-Jun signaling pathway in SIC. Notably, concurrent
changes in PLN activity were also observed upon inhibition of the
JNK/c-Jun signaling pathway. Specifically, inhibition of this
pathway led to decreased PLN expression and altered phosphorylation
at its regulatory sites. To further investigate the relationship
between the JNK/c-Jun pathway and PLN, plasmid transfection
experiments were conducted to clarify whether c-Jun may act as an
upstream transcription factor regulating PLN synthesis. The results
showed that the mRNA and protein levels of PLN were found to be
consistent with the expression changes of c-Jun.
The unknown cardiac dysfunction underlying SIC,
combined with the complexities of the cardiovascular system, has
resulted in the absence of standardized diagnostic criteria in
clinical practice (55,56). PLN is specifically expressed in
myocardial tissues and has a small molecular weight of 6 kDa in its
monomeric form and 25 kDa in its pentameric form (57). This molecular weight is much
lower than that of CK at 86 kDa or cTnI at 37 kDa, making PLN more
readily released into the bloodstream during myocardial injury and
demonstrating superior sensitivity as a biomarker in serum. The
area under the ROC curve was 0.9503 for PLN, with a 95% confidence
interval of 0.9014-0.9993, indicating that PLN has excellent
diagnostic performance for SIC. Furthermore, serum PLN levels were
revealed to be correlated with markers of liver and kidney injury,
as well as inflammatory indicators. Therefore, it may be concluded
that PLN holds clinical value in pediatric patients to a certain
extent.
In summary, the present study identified abnormally
decreased T3 and elevated PLN concentrations, with these
concentrations showing a negative correlation in a mouse disease
model. Moreover, elevated PLN levels were associated with impaired
intracellular calcium recycling, leading to increased intracellular
oxidative stress levels and subsequent cardiomyocyte injury.
Exogenous supplementation with T3 reversed calcium overload and
oxidative stress by inhibiting PLN and its downstream calcium
regulatory factors, thereby reducing myocardial injury. The present
study also revealed that T3 treatment suppressed the expression and
activity of JNK and c-Jun, indicating that T3 may regulate PLN to
mitigate myocardial injury through the JNK/c-Jun signaling
pathway.
Notably, the current study has several limitations.
First, the clinical sample size was small. Although the findings
revealed increased PLN levels and decreased T3 levels across the
three experimental groups, there was no negative correlation
between PLN and T3 levels in clinical pediatric patients (data not
shown). This discrepancy may be attributed to the incomplete
development of the thyroid gland in children. There are significant
differences in thyroid hormone levels between children and adults,
while these variations tend to approach the adult reference ranges
as age increases (58). Children
have a significantly higher demand for thyroid hormones due to
their growth requirements, which far exceeds that of adults.
Additionally, because their thyroid glands are not yet fully
mature, their hormonal regulatory capabilities are weaker.
Consequently, the fluctuations in hormone levels under stress
conditions are more pronounced in children compared with in adults.
Furthermore, several factors such as obesity, smoking and lifestyle
can influence thyroid hormone levels as age increases (59). To address this limitation, a
larger sample size and a multicenter clinical trial are required to
further validate the scientific hypothesis. Second, additional
robust evidence, such as c-Jun knockdown, is needed to strengthen
the credibility of this mechanism which involves the JNK/c-Jun
signaling pathway in the regulatory mechanisms of T3. Thus, more
direct experimental results including dual-luciferase reporter
assays and other methodologies are needed to substantiate the
interaction between c-Jun and PLN. Addressing these limitations
will contribute to a more comprehensive understanding of the
mechanisms underlying the observed effects of T3 and PLN on SIC.
Finally, Figs. 2, 5 and S3 were derived from several
distinct experiments, where experimental conditions and procedures
may introduce a certain degree of error, hence the rate of
apoptosis between H9C2 cells treated with LPS were not completely
the same among these figures. Thus, in the analysis of each
dataset, the data were normalized to the control group to minimize
the impact of the difference to the greatest extent possible.
In conclusion, the present study demonstrated a
negative correlation between low T3 levels and elevated PLN levels
in a mouse model of SIC. The correlation may stem from the
influence of PLN on intracellular calcium cycling and oxidative
stress levels in cardiomyocytes, which are countered by T3 to
improve cardiomyocyte function through the inhibition of PLN
phosphorylation. Moreover, the findings of the current study
suggested that the JNK/c-Jun signaling pathway may serve a crucial
role in mediating the negative regulatory effects of T3 on PLN.
Additionally, PLN was identified as a novel biomarker for SIC,
which has significant potential for early diagnosis of SIC in
pediatric patients.
Supplementary Data
Availability of data and materials
The data generated in the present study may be
requested from the corresponding author. The data generated in the
present study may be found in the iProX database under accession
number PXD059227 or at the following URL: https://www.iprox.cn/page/project.html?id=IPX0010782000.
Authors' contributions
QX and QY analyzed the patient data regarding SIC
and performed experiments on cell models. JZ contributed to study
conception and design, BT analyzed and interpretated the data, and
both were involved in drafting the manuscript or revising it. HXi,
RW and HL performed the histological examination of the heart, and
were major contributors in writing the manuscript. TC and HXu made
substantial contributions to the conception and design of the
research, analysis and interpretation of data, and the acquisition
of funding. TC and HXu confirm the authenticity of all the raw
data. All authors read and approved the final version of the
manuscript.
Ethics approval and consent to
participate
The clinical study protocol was approved by the
Clinical Research Ethics Committee of the Children's Hospital of
Chongqing Medical University (approval no. 2021-353). Written
informed consent was obtained from the parents of all patients. The
animal experiments were approved by the Animal Ethics Committee of
Children's Hospital of Chongqing Medical University (approval no.
CHCMU-IACUC20210114036).
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
Acknowledgments
The authors would like to thank Dr Qin Zhou and Mrs.
Li Zhao (Department of Pediatric Research Institute, Children's
Hospital of Chongqing Medical University) for their pathology help
and assistance.
Funding
This work was supported by the China Postdoctoral Science
Foundation (grant no. 2023M730447), the Chongqing Science &
Technology Commission (grant no. CSTB2023NSCQ-MSX0603), the
Chongqing Postdoctoral Research Program Special Grant (grant no.
2023CQBSHTB3048), the Chongqing Municipal Talent Program (grant no.
cstc2024ycjh-bgzxm0024) and the Chongqing Medical Scientific
Research Project (Joint project of Chongqing Health Commission and
Science and Technology Bureau; grant no. 2025GDRC008).
References
|
1
|
Singh S, Mohan S and Singhal R:
Definitions for sepsis and septic shock. JAMA. 316:4582016.
View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Gohil SK, Cao C, Phelan M, Tjoa T, Rhee C,
Platt R and Huang SS: Impact of policies on the rise in sepsis
incidence, 2000-2010. Clin Infect Dis. 62:695–703. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Mahla RS, Vincent JL and Sakr Y; ICON and
SOAP Investigators: Sepsis is a global burden to human health:
Incidences are underrepresented: Discussion on 'comparison of
European ICU patients in 2012 (ICON) versus 2002 (SOAP)'. Intensive
Care Med. 44:1197–1198. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Suzuki T, Suzuki Y, Okuda J, Kurazumi T,
Suhara T, Ueda T, Nagata H and Morisaki H: Sepsis-induced cardiac
dysfunction and β-adrenergic blockade therapy for sepsis. J
Intensive Care. 5:222017. View Article : Google Scholar
|
|
5
|
Allison SJ: Sepsis: NET-induced
coagulation induces organ damage in sepsis. Nat Rev Nephrol.
13:1332017. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Weiss SL, Peters MJ, Alhazzani W, Agus
MSD, Flori HR, Inwald DP, Nadel S, Schlapbach LJ, Tasker RC, Argent
AC, et al: Surviving sepsis campaign international guidelines for
the management of septic shock and sepsis-associated organ
dysfunction in children. Intensive Care Med. 46(Suppl 1): S10–S67.
2020. View Article : Google Scholar
|
|
7
|
Hollenberg SM and Singer M:
Pathophysiology of sepsis-induced cardiomyopathy. Nat Rev Cardiol.
18:424–434. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Ehrman RR, Sullivan AN, Favot MJ, Sherwin
RL, Reynolds CA, Abidov A and Levy PD: Pathophysiology,
echocardiographic evaluation, biomarker findings, and prognostic
implications of septic cardiomyopathy: A review of the literature.
Crit Care. 22:1122018. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Melis MJ, Miller M, Peters VBM and Singer
M: The role of hormones in sepsis: An integrated overview with a
focus on mitochondrial and immune cell dysfunction. Clin Sci
(Lond). 137:707–725. 2023. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Das BK, Agarwal P, Agarwal JK and Mishra
OP: Serum cortisol and thyroid hormone levels in neonates with
sepsis. Indian J Pediatr. 69:663–665. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Vidart J, Axelrud L, Braun AC, Marschner
RA and Wajner SM: Relationship among low T3 levels, type 3
deiodinase, oxidative stress, and mortality in sepsis and septic
shock: Defining patient outcomes. Int J Mol Sci. 24:39352023.
View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Jongejan RMS, Meima ME, Visser WE,
Korevaar TIM, Van Den Berg SAA, Peeters RP and de Rijke YB: Binding
characteristics of thyroid hormone distributor proteins to thyroid
hormone metabolites. Thyroid. 32:990–999. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Salas-Lucia F and Bianco AC: T3 levels and
thyroid hormone signaling. Front Endocrinol (Lausanne).
13:10446912022. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Cokkinos DV and Chryssanthopoulos S:
Thyroid hormones and cardiac remodeling. Heart Fail Rev.
21:365–372. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Mastorci F, Sabatino L, Vassalle C and
Pingitore A: Cardioprotection and thyroid hormones in the clinical
setting of heart failure. Front Endocrinol (Lausanne). 10:9272020.
View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Wang R, Xu Y, Fang Y, Wang C, Xue Y, Wang
F, Cheng J, Ren H, Wang J, Guo W, et al: Pathogenetic mechanisms of
septic cardiomyopathy. J Cell Physiol. 237:49–58. 2022. View Article : Google Scholar
|
|
17
|
Shankar TS, Ramadurai DKA, Steinhorst K,
Sommakia S, Badolia R, Thodou Krokidi A, Calder D, Navankasattusas
S, Sander P, Kwon OS, et al: Cardiac-specific deletion of voltage
dependent anion channel 2 leads to dilated cardiomyopathy by
altering calcium homeostasis. Nat Commun. 12:45832021. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Capasso JM, Sonnenblick EH and Anversa P:
Chronic calcium channel blockade prevents the progression of
myocardial contractile and electrical dysfunction in the
cardiomyopathic Syrian hamster. Circ Res. 67:1381–1393. 1990.
View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Selnø ATH, Sumbayev VV and Gibbs BF:
IgE-dependent human basophil responses are inversely associated
with the sarcoplasmic reticulum Ca2+-ATPase (SERCA).
Front Immunol. 13:10522902023. View Article : Google Scholar
|
|
20
|
Wang L, Myles RC, Lee IJ, Bers DM and
Ripplinger CM: Role of reduced sarco-endoplasmic reticulum
Ca2+-ATPase function on sarcoplasmic reticulum
Ca2+ alternans in the intact rabbit heart. Front
Physiol. 12:6565162021. View Article : Google Scholar
|
|
21
|
Carlson CR, Aronsen JM, Bergan-Dahl A,
Moutty MC, Lunde M, Lunde PK, Jarstadmarken H, Wanichawan P,
Pereira L, Kolstad TRS, et al: AKAP18δ anchors and regulates CaMKII
activity at phospholamban-SERCA2 and RYR. Circ Res. 130:27–44.
2022. View Article : Google Scholar
|
|
22
|
Hamstra SI, Whitley KC, Baranowski RW,
Kurgan N, Braun JL, Messner HN and Fajardo VA: The role of
phospholamban and GSK3 in regulating rodent cardiac SERCA function.
Am J Physiol Cell Physiol. 319:C694–C699. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Kranias EG and Hajjar RJ: Modulation of
cardiac contractility by the phospholamban/SERCA2a regulatome. Circ
Res. 110:1646–1660. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Sivakumaran V, Stanley BA, Tocchetti CG,
Ballin JD, Caceres V, Zhou L, Keceli G, Rainer PP, Lee DI, Huke S,
et al: HNO enhances SERCA2a activity and cardiomyocyte function by
promoting redox-dependent phospholamban oligomerization. Antioxid
Redox Signal. 19:1185–1197. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Lygren B, Carlson CR, Santamaria K,
Lissandron V, McSorley T, Litzenberg J, Lorenz D, Wiesner B,
Rosenthal W, Zaccolo M, et al: AKAP complex regulates Ca2+
re-uptake into heart sarcoplasmic reticulum. EMBO Rep. 8:1061–1067.
2007. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Steinberg C, Roston TM, Van der Werf C,
Sanatani S, Chen SRW, Wilde AAM and Krahn AD:
RYR2-ryanodinopathies: From calcium overload to calcium deficiency.
Europace. 25:euad1562023. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Strubbe-Rivera JO, Schrad JR, Pavlov EV,
Conway JF, Parent KN and Bazil JN: The mitochondrial permeability
transition phenomenon elucidated by cryo-EM reveals the genuine
impact of calcium overload on mitochondrial structure and function.
Sci Rep. 11:10372021. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Reddy LG, Autry JM, Jones LR and Thomas
DD: Co-reconstitution of phospholamban mutants with the Ca-ATPase
reveals dependence of inhibitory function on phospholamban
structure. J Biol Chem. 274:7649–7655. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Qin J, Zhang J, Lin L, Haji-Ghassemi O,
Lin Z, Woycechowsky KJ, Van Petegem F, Zhang Y and Yuchi Z:
Structures of PKA-phospholamban complexes reveal a mechanism of
familial dilated cardiomyopathy. Elife. 11:e753462022. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Feyen DAM, Perea-Gil I, Maas RGC,
Harakalova M, Gavidia AA, Arthur Ataam J, Wu TH, Vink A, Pei J,
Vadgama N, et al: Unfolded protein response as a compensatory
mechanism and potential therapeutic target in PLN R14del
cardiomyopathy. Circulation. 144:382–392. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Sinha RA and Yen PM: Metabolic messengers:
Thyroid hormones. Nat Metab. 6:639–650. 2024. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Percie du Sert N, Hurst V, Ahluwalia A,
Alam S, Avey MT, Baker M, Browne WJ, Clark A, Cuthill IC, Dirnagl
U, et al: The ARRIVE guidelines 2.0: Updated guidelines for
reporting animal research. PLoS Boil. 18:e30004102020. View Article : Google Scholar
|
|
33
|
Feng X, Wang L, Zhou R, Zhou R, Chen L,
Peng H, Huang Y, Guo Q, Luo X and Zhou H: Senescent immune cells
accumulation promotes brown adipose tissue dysfunction during
aging. Nat Commun. 14:32082023. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Jiang J, Zhou D, Zhang A, Yu W, Du L, Yuan
H, Zhang C, Wang Z, Jia X, Zhang ZN and Luan B: Thermogenic
adipocyte-derived zinc promotes sympathetic innervation in male
mice. Nat Metab. 5:481–494. 2023. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Mahmood SR, Xie X, Hosny El Said N, Venit
T, Gunsalus KC and Percipalle P: β-actin dependent chromatin
remodeling mediates compartment level changes in 3D genome
architecture. Nat Commun. 12:52402021. View Article : Google Scholar
|
|
36
|
Hunter T and Garrels JI: Characterization
of the mRNAs for alpha-, beta- and gamma-actin. Cell. 12:767–781.
1977. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(-Delta Delta C(T)) method. Methods. 25:402–408. 2001.
View Article : Google Scholar
|
|
38
|
Zhang L and Elias JE: Relative protein
quantification using tandem mass tag mass spectrometry. Methods Mol
Biol. 1550:185–198. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Pantos C, Malliopoulou V, Paizis I,
Moraitis P, Mourouzis I, Tzeis S, Karamanoli E, Cokkinos DD,
Carageorgiou H, Varonos D and Cokkinos DV: Thyroid hormone and
cardioprotection: study of p38 MAPK and JNKs during ischaemia and
at reperfusion in isolated rat heart. Mol Cell Biochem.
242:173–180. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Lieder HR, Braczko F, Gedik N, Stroetges
M, Heusch G and Kleinbongard P: Cardioprotection by
post-conditioning with exogenous triiodothyronine in isolated
perfused rat hearts and isolated adult rat cardiomyocytes. Basic
Res Cardiol. 116:272021. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Vafiadaki E, Haghighi K, Arvanitis DA,
Kranias EG and Sanoudou D: Aberrant PLN-R14del protein interactions
intensify SERCA2a inhibition, driving impaired Ca2+
handling and arrhythmogenesis. Int J Mol Sci. 23:69472022.
View Article : Google Scholar
|
|
42
|
Stege NM, de Boer RA, Makarewich CA, van
der Meer P and Silljé HHW: Reassessing the mechanisms of PLN-R14del
cardiomyopathy: From calcium dysregulation to S/ER malformation.
JACC Basic Transl Sci. 9:1041–1052. 2024. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Dong J, Gao C, Liu J, Cao Y and Tian L:
TSH inhibits SERCA2a and the PKA/PLN pathway in rat cardiomyocytes.
Oncotarget. 7:39207–39215. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Gaique TG, Lopes BP, Souza LL, Paula GSM,
Pazos-Moura CC and Oliveira KJ: Cinnamon intake reduces serum T3
level and modulates tissue-specific expression of thyroid hormone
receptor and target genes in rats. J Sci Food Agric. 96:2889–2895.
2016. View Article : Google Scholar
|
|
45
|
Mattiazzi A, Tardiff JC and Kranias EG:
Stress seats a new guest at the table of PLN/SERCA and their
partners. Circ Res. 128:471–473. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Zeng B, Liao X, Liu L, Zhang C, Ruan H and
Yang B: Thyroid hormone mediates cardioprotection against
postinfarction remodeling and dysfunction through the
IGF-1/PI3K/AKT signaling pathway. Life Sci. 267:1189772021.
View Article : Google Scholar : PubMed/NCBI
|
|
47
|
de Castro AL, Fernandes RO, Ortiz VD,
Campos C, Bonetto JH, Fernandes TR, Conzatti A, Siqueira R, Tavares
AV, Schenkel PC, et al: Thyroid hormones improve cardiac function
and decrease expression of pro-apoptotic proteins in the heart of
rats 14 days after infarction. Apoptosis. 21:184–194. 2016.
View Article : Google Scholar
|
|
48
|
Brennan A, Leech JT, Kad NM and Mason JM:
Selective antagonism of cJun for cancer therapy. J Exp Clin Cancer
Res. 39:1842020. View Article : Google Scholar : PubMed/NCBI
|
|
49
|
Vernia S, Morel C, Madara JC,
Cavanagh-Kyros J, Barrett T, Chase K, Kennedy NJ, Jung DY, Kim JK,
Aronin N, et al: Excitatory transmission onto AgRP neurons is
regulated by cJun NH2-terminal kinase 3 in response to metabolic
stress. Elife. 5:e100312016. View Article : Google Scholar : PubMed/NCBI
|
|
50
|
Manieri E, Folgueira C, Rodríguez ME,
Leiva-Vega L, Esteban-Lafuente L, Chen C, Cubero FJ, Barrett T,
Cavanagh-Kyros J, Seruggia D, et al: JNK-mediated disruption of
bile acid homeostasis promotes intrahepatic cholangiocarcinoma.
Proc Natl Acad Sci USA. 117:16492–16499. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
51
|
Yang J, Do-Umehara HC, Zhang Q, Wang H,
Hou C, Dong H, Perez EA, Sala MA, Anekalla KR, Walter JM, et al:
miR-221-5p-mediated downregulation of JNK2 aggravates acute lung
injury. Front Immunol. 12:7009332021. View Article : Google Scholar : PubMed/NCBI
|
|
52
|
Jaeschke A, Karasarides M, Ventura JJ,
Ehrhardt A, Zhang C, Flavell RA, Shokat KM and Davis RJ: JNK2 is a
positive regulator of the cJun transcription factor. Mol Cell.
23:899–911. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
53
|
Chin KY, Silva LS, Darby IA, Ng DCH and
Woodman OL: Protection against reperfusion injury by
3′,4′-dihydroxyflavonol in rat isolated hearts involves inhibition
of phospholamban and JNK2. Int J Cardiol. 254:265–271. 2018.
View Article : Google Scholar : PubMed/NCBI
|
|
54
|
Song Q, Schmidt AG, Hahn HS, Carr AN,
Frank B, Pater L, Gerst M, Young K, Hoit BD, McConnell BK, et al:
Rescue of cardiomyocyte dysfunction by phospholamban ablation does
not prevent ventricular failure in genetic hypertrophy. J Clin
Invest. 111:859–867. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
55
|
Sundqvist A, Voytyuk O, Hamdi M, Popeijus
HE, van der Burgt CB, Janssen J, Martens JWM, Moustakas A, Heldin
CH, Ten Dijke P and van Dam H: JNK-dependent cJun phosphorylation
mitigates TGFβ- and EGF-induced pre-malignant breast cancer cell
invasion by suppressing AP-1-mediated transcriptional responses.
Cells. 8:14812019. View Article : Google Scholar
|
|
56
|
Martin L, Derwall M, Al Zoubi S,
Zechendorf E, Reuter DA, Thiemermann C and Schuerholz T: The septic
heart: Current understanding of molecular mechanisms and clinical
implications. Chest. 155:427–437. 2019. View Article : Google Scholar
|
|
57
|
Funk F, Kronenbitter A, Hackert K, Oebbeke
M, Klebe G, Barth M, Koch D and Schmitt JP: Phospholamban
pentamerization increases sensitivity and dynamic range of cardiac
relaxation. Cardiovasc Res. 119:1568–1582. 2023. View Article : Google Scholar : PubMed/NCBI
|
|
58
|
Babić Leko M, Gunjača I, Pleić N and
Zemunik T: Environmental factors affecting thyroid-stimulating
hormone and thyroid hormone levels. Int J Mol Sci. 22:65212021.
View Article : Google Scholar
|
|
59
|
Silvestri E, Lombardi A, de Lange P,
Schiavo L, Lanni A, Goglia F, Visser TJ and Moreno M: Age-related
changes in renal and hepatic cellular mechanisms associated with
variations in rat serum thyroid hormone levels. Am J Physiol
Endocrinol Metab. 294:E1160–E1168. 2008. View Article : Google Scholar : PubMed/NCBI
|