Introduction
Intestinal ischemia-reperfusion (IIR) is an acute
condition often caused by cardiopulmonary bypass, small intestinal
transplantation, intestinal obstruction, mesenteric ischemia,
incarcerated hernia, septic shock or trauma with a mortality rate
of >50% and a global incidence rate of >0.001% (1-3).
In America, 30,000 individuals per year experience
ischemia-reperfusion (4).
Furthermore, acute mesenteric ischemia alone accounts for 0.1% of
all hospital admissions globally (5). Despite improvements in treatment
modalities, the mortality rate of acute mesenteric ischemia is
50-80% (5). Therefore,
investigating novel therapeutic strategies to improve the treatment
of IIR is necessary.
During IIR, epithelial cell death and mucosal injury
disrupts the integrity of the mucosal barrier, which increases
intestinal permeability, resulting in systemic inflammatory
response syndrome or shock (6,7).
Previous studies have shown that non-apoptotic cell death
mechanisms, including ferroptosis, pyroptosis and necroptosis,
serve key roles in the pathological process of acute and chronic
gut injury (8,9).
Pyroptosis, a type of proinflammatory programmed
cell death, occurs in multiple types of cells, such as enterocytes,
oligodendrocytes and vascular endothelial cells including those in
the digestive system, under several stress conditions such as
mitochondrial oxidative stress and endoplasmic reticulum stress,
and in response to a large number of metabolites such as
trimethylamine N-oxide and methylglyoxal (10-13). Pyroptotic cells release several
inflammatory cytokines and danger-associated molecular patterns
(DAMPs) through gasdermin D (GSDMD) pores or ruptured membranes,
resulting in the activation of immune cells such as alveolar
macrophages and non-immune cells such as lung endothelial cells
that in turn generate further proinflammatory cytokines (10). This exacerbates inflammation and
induces further pyroptosis, which leads to a positive feedback loop
and host mortality (10,14). For example, interleukin (IL)-1β
and IL-18, released from pyroptotic cells, recruit and activate
immune cells by binding to IL-receptors. This triggers the
production of several proinflammatory cytokines, such as tumor
necrosis factor-α (TNF-α) and interferon-γ (IFN-γ), which amplifies
the proinflammatory response (10). IFN-γ and TNF-α induce pyroptosis
and inflammatory responses through the activation of the janus
kinase/signal transducer and activator of transcription 1/IFN
regulatory factor 1 pathway. However, blocking the activation of
IFN-γ and TNF-α using neutralizing antibodies ameliorates
infectious and autoinflammatory diseases by inhibiting inflammation
and tissue damage (15,16). In addition, as a DAMP,
high-mobility group box 1 not only activates macrophages to
generate inflammatory cytokines, but it also induces pyroptosis in
macrophages, which further exacerbates the inflammatory response
(14,17). Therefore, the initiation of
excessive pyroptosis leads to severe inflammatory consequences,
whereas its inhibition ameliorates the pathogenic effects of
inflammatory diseases. It is generally accepted that the NOD-, LRR-
and pyrin domain-containing protein 3 (NLRP3) inflammasome, which
is an inflammatory regulatory complex, contributes to the
initiation of pyroptosis (18-20). Once the assembly of inflammasomes
is complete, procaspase-1 is recruited and cleaved, and then its
active fragment not only cleaves pro-IL-1β and pro-IL-18 into their
activated forms but it also transforms GSDMD into C- and N-terminal
fragments, which triggers pyroptosis (21,22). In addition, previous studies
demonstrate that NLRP3-GSDMD-dependent pyroptosis contributes to
IIR injury (23-25). Therefore, inhibiting NLRP3
pathway-mediated pyroptosis might be a promising therapeutic option
for IIR injury.
Mitochondrial dysfunction is considered to be one of
the principal contributors to IIR-induced injury. When IIR occurs,
mitochondria inhibit adenosine triphosphate generation, produce
reactive oxygen species (ROS) and increase the amount of
mitochondrial DNA (mtDNA) in the blood circulation, thereby
aggravating cell damage (26,27). Recent studies reveal that
mitochondrial dysfunction contributes to NLRP3 inflammasome
activation-induced pyroptosis during pathological processes, such
as septic shock and hepatic encephalopathy (28,29). Mitochondria-associated membrane
formation during IIR activates the NLRP3 inflammasome and induces
pyroptosis (30). Therefore,
mitochondrial dysfunction may contribute to NLRP3 inflammasome
activation-induced pyroptosis in IIR injury. Sirtuin-3 (SIRT3),
which is mainly expressed in mitochondria, is usually reduced in
models of IR, including IIR (31-33). The activation of SIRT3 protects
against IIR injury by ameliorating mitochondrial damage (34). However, whether SIRT3 can
ameliorate NLRP3 inflammasome activation-triggered pyroptosis by
regulating mitochondrial function during IIR has not been fully
elucidated.
Honokiol (HKL), a natural small-molecule polyphenol
extracted from the herb Magnolia officinalis, has been
identified to possess antitumor, anti-inflammatory and antioxidant
properties (35-37). Furthermore, HKL can directly
target SIRT3 and increase SIRT3 expression levels (38). A previous study demonstrates that
HKL can protect mitochondrial integrity by activating SIRT3 in
cisplatin-induced acute kidney injury (39,40). Therefore, the present study
investigated whether HKL can also ameliorate IIR injury.
Subsequently, the present study investigated the underlying
mechanisms of HKL and its effects on IIR in a rat model of small
IIR and in IEC-6 epithelial cells.
Materials and methods
Rat model of small IIR
In total, 96 8-week-old adult male Sprague-Dawley
rats [Shanghai Jihui Laboratory Animal Breeding Co., Ltd.; license
no. SCXK(Hu)2022-0009] weighing 250-300 g were kept in an
environment with a stable humidity (40-70%) and temperature
(22±2°C), with a time-controlled lighting system and unrestricted
access to water and standard commercial food. The time-controlled
lighting system had a 12 h light/dark cycle. All animal
experimental protocols were approved by the ethics committee of the
Southwest Medical University (Luzhou, China; approval no.
20221128-001). After the rats had adapted to their environment for
5 days, 24 rats were used to investigate different durations of
ischemia to determine the duration that gave the most notable
alteration in intestinal damage after IIR. The rats were randomly
divided into groups (n=6/group), namely: The sham; ischemia for 30
min and reperfusion for 2 h; ischemia for 45 min and reperfusion
for 2 h; or ischemia for 60 min and reperfusion for 2 h groups. The
rat IIR model was established as previously described (41,42). Prior to surgery, a mixture
containing ketamine (55 mg/kg) and xylazine (7.5 mg/kg) was
injected once to anesthetize the rats via intraperitoneal injection
(duration, ~70 min). To ensure that the rats were anesthetized, 1
min after injection the rats were rolled onto their sides once
every min until the righting reflex was lost. Subsequently, 1 min
after the righting reflex was lost, a toe pinch test was applied to
each rat to determine whether the withdrawal reflex was present.
Loss of the righting reflex and toe pinch response was used to
confirm that the rats were anesthetized. A midline laparotomy was
carried out, and then the superior mesenteric artery (SMA) was
exposed and clamped for 30, 45 or 60 min at 22±2°C. During the
surgical period and ischemia, a tail pinch test was carried out
once every 5 min and the palpebral reflex was observed to monitor
the degree of anesthesia. If the rats reacted to the tail pinch
test or the palpebral reflex was observed, anesthesia was
maintained with injections of 20 mg/kg ketamine. After ischemia,
the clamp was removed, and the skin was sutured after the
intestines returned to a pink color. Subsequently, reperfusion was
performed for 2 h, and the rats were allowed to wake up. In the
sham group, the rats underwent the same procedure without SMA
occlusion. In the subsequent experiments, the SMA was clamped for
45 min at 22±2°C, and 42 rats were randomly divided into seven
groups (n=6/group) to determine the appropriate dose of HKL,
namely: The sham; IIR; IIR with 0.5 mg/kg HKL (MedChemExpress) (IIR
+ 0.5 HKL); IIR with 1 mg/kg HKL (IIR + 1 HKL); IIR with 5 mg/kg
HKL (IIR + 5 HKL); IIR with 10 mg/kg HKL (IIR + 10 HKL); or IIR
with 20 mg/kg HKL (IIR + 20 HKL) groups. In addition, 30 rats were
randomly divided into three groups (n=10/group),namely: The sham;
IIR; and IIR + 10 HKL groups, which were used for
immunohistochemical (IHC), intestinal fatty acid-binding protein
(i-FABP), IL-1β and IL-18 tests (n=6/group) or western blotting
tests (n=4/group). For the HKL treatment groups, a solvent mixture
of DMSO and PBS at a volume ratio 1:1 was prepared. HKL was
dissolved in the solvent mixture to prepare a 10 mg/ml stock
solution. The HKL stock solution was added to the solvent mixture
of DMSO and PBS to prepare working solutions according to the
injection dose and total mass of each rat. Each rat was
administered an intraperitoneal injection of 1 ml HKL solution once
daily for 7 days prior to surgery (43-45). All animals from the sham and IIR
groups were given an equal volume of the solvent mixture of DMSO
and PBS via an intraperitoneal injection. Following reperfusion,
rats were anesthetized using a mixture of ketamine (55 mg/kg) and
xylazine (7.5 mg/kg), and peripheral blood was collected from the
abdominal aorta to euthanize all animals. Rats were confirmed dead
when the absence of a heartbeat, pulse, breathing and corneal
reflex was observed for >3 min. The rats were then dissected,
and the small intestine collected.
Hematoxylin and eosin (H&E) staining
and pathological score
For pathological experiments, all of the small
intestinal tissues were fixed in 4% polyformaldehyde for 24 h at
4°C and embedded in paraffin. Following cooling at -20°C, 4
µm-thick sections were prepared using a tissue slicer and
stained with H&E solution (cat. no. G1005-500ML; Wuhan
Servicebio Technology Co., Ltd.). The sections were stained using
hematoxylin for 5 min at room temperature. After washing with
water, the sections were stained using eosin for 15 sec at room
temperature. Following staining, the morphology of the small
intestine was observed using a light microscope (magnification,
×100; Eclipse E100; Nikon Corporation). The destruction of the
small intestinal tissues was evaluated using Chiu's score (46) and ImageJ software (version 1.8.0;
National Institutes of Health). Two independent observers blinded
to the groups determined the Chiu's score.
IHC staining
Small intestinal tissues were fixed in 4%
polyformaldehyde for 24 h at 4°C and embedded in paraffin.
Following cooling at −20°C, sections (4 µm-thick) of
intestinal tissue were prepared and dewaxed using BioDewax and
Clear Solution (cat. no. G1128-500ML; Wuhan Servicebio Technology
Co., Ltd.) and hydrated in ethanol at different concentrations.
Following antigen retrieval for 20 min at 95°C, the tissue sections
were incubated with 3% BSA (cat. no. GC305010-5 g; Wuhan Servicebio
Technology Co., Ltd.) for 30 min at room temperature. Subsequently,
the tissues were incubated with primary antibodies: Anti-tight
junction protein 1 (ZO-1; 1:250; cat. no. 164329; Chengdu
Zen-Bioscience Co., Ltd.); anti-occludin (1:250; cat. no. 502601;
Chengdu Zen-Bioscience Co., Ltd.); anti-NLRP3 (1:300; cat. no.
GB114320-100; Wuhan Servicebio Technology Co., Ltd.); anti-SIRT3
(1:100; cat. no. GB115590-100; Wuhan Servicebio Technology Co.,
Ltd.); anti-cleaved-Caspase-1 (cleavedCleaved-Casp1; 1:200; cat.
no. YC0003; ImmunoWay Biotechnology Company); and anti-GSDMD
N-terminal domain (GSDMD-N; 1:100; cat. no. DF13758; Affinity
Biosciences) at 4°C overnight. The tissue samples were then
incubated with a HRP-conjugated goat anti-rabbit secondary antibody
(1:1,000; cat. no. GB23303; Wuhan Servicebio Technology Co., Ltd.)
at room temperature for 50 min and visualized using a DAB substrate
chromogen kit (cat. no. G1212-200T; Wuhan Servicebio Technology
Co., Ltd.), after the cell nuclei were stained with hematoxylin
solution for 5 min at room temperature. All images were captured
using an inverted light microscope (magnification, ×100 and ×400;
OLYMPUS IX73; Olympus Corporation) and analyzed using ImageJ
software (version 1.8.0; National Institutes of Health).
Hypoxia/reoxygenation (H/R) model and
treatments
A rat intestinal IEC-6 epithelial cell line was
provided by Wuhan Sunncell Biotechnology Co., Ltd. IEC-6 cells were
cultured in DMEM (cat. no. 11965092; Thermo Fisher Scientific,
Inc.) with 10% FBS (cat. no. 13011-8611;Zhejiang Tianhang
Biotechnology Co., Ltd.) and 10 mg/l insulin at 37°C in a 5%
CO2 atmosphere.
To establish the H/R model in vitro, IEC-6
cells were maintained in a Water-Jacketed CO2 incubator
commercial hypoxia system (Thermo Scientific™ 8000; Thermo Fisher
Scientific, Inc.; 94% N2, 5% CO2 and 1%
O2) for 12 h at 37°C. Following hypoxia, IEC-6 cells
were transferred to a normoxic incubator (74% N2, 5%
CO2 and 21% O2) and further cultured for 6 h
at 37°C (47). For the treatment
groups, a solvent mixture of DMSO and PBS at a 1:1 volume ratio was
prepared. HKL was dissolved in the solvent mixture to prepare a 10
mM stock solution. The HKL stock solution was then further diluted
in the solvent mixture of DMSO and PBS to prepare working solutions
according the dose and total volume required. The
mitochondria-targeted antioxidant (Mito-TEMPO; MedChemExpress) was
dissolved in deionized water. HKL (1, 5, 10, 20 or 40 µM) or
Mito-TEMPO (200 µM) solutions were used to treat cells at
37°C for 12 h prior to H/R.
Cell transfection
Small interfering (si)-RNA targeting SIRT3 mRNA
(siSIRT3; sense, 5′-GGA CGG AUA AGA CAG ACU AUG DTD T-3′;
antisense, 5′-CAUA GUC UGU CUU AUC CGU CCD TDT-3′; Shanghai
GenePharma Co., Ltd.) and the scrambled negative control (con)
siRNA (siNC; sense, 5′-ACG UGG GAG AAG AUC GUA CAA DTD T-3′;
antisense, 5′-UUG UAC GAU CUU CUC CCA CGU DTD T-3′; Shanghai
GenePharma Co., Ltd.) were transfected into cells using
Lipofectamine® 2000 (cat. no. 11668019; Thermo Fisher
Scientific, Inc.). Briefly, IEC-6 cells (6×105
cells/well) were seeded in a 6-well plate at 37°C overnight for
attachment before being transfected using 12.5 µl of
Lipofectamine® 2000 with 25 nM siSIRT3 or siNC for 48 h
at 37°C. After transfection, cells were immediately used for
subsequent experiments.
Reverse transcription-quantitative
polymerase chain reaction (RT-qPCR)
After exposure to H/R and HKL, IEC-6 cells were
collected and treated with TRIzol® reagent (cat. no.
15596-026; Thermo Fisher Scientific, Inc.). Reverse transcription
was performed using M-MLV Reverse Transcriptase (cat. no. 28025013;
Thermo Fisher Scientific, Inc.) at 42°C for 1 h and 70°C for 10
min. qPCR was performed using TB Green Fast qPCR Mix (cat. no.
RR430A; Takara Biotechnology Co., Ltd.) under the following
conditions: Initial denaturation at 95°C for 15 sec; followed by 40
cycles of 95°C for 5 sec and 60°C for 30 sec. β-actin was used as a
con. The relative expression of SIRT3 mRNA was calculated using the
2−ΔΔcq method (48).
The primer sequences used in the present study were: SIRT3 forward,
5′-GCC TCT ACA GCA ACC TTC-3′ and reverse, 5′-AAG TAG TGA GCG ACA
TTG G-3′; β-actin forward, 5′-CCA TTG AAC ACG GCA TTG-3′ and
reverse, 5′-TAC GAC CAG AGG CAT ACA-3′.
Western blotting
To extract total protein, IEC-6 cells or small
intestinal tissues were treated with RIPA buffer (cat. no. F1053;
Wuhan Frekang Technology Co., Ltd. The protein concentration was
determined using a BCA protein assay kit (cat. no. K001; Wuhan Fine
Biotech Co., Ltd.). Following separation using 10% SDS-PAGE (20
µg of total protein loaded per lane), the proteins were
transferred to a PVDF membrane (cat. no. 36126ES; Shanghai Yeasen
Biotechnology Co., Ltd.) and blocked using 5% non-fat milk for 2 h
at room temperature. The membrane was incubated with primary
antibodies at 4°C overnight. The primary antibodies used were:
Anti-ZO-1 (1:500; cat. no. GB111402-100; Wuhan Servicebio
Technology Co., Ltd.); anti-occludin (1:800; cat. no. GB11149-50;
Wuhan Servicebio Technology Co., Ltd.); anti-SIRT3 (1:300; cat. no.
GB115590-100; Wuhan Servicebio Technology Co., Ltd.); anti-NLRP3
(1:400; cat. no. GB114320-100; Wuhan Servicebio Technology Co.,
Ltd.); anti-cleaved-Casp1 (1:800; cat. no. AF4005; Affinity
Biosciences); anti-Casp1 (1:1,000; cat. no. AF5418; Affinity
Biosciences); anti-GSDMD (1:500; cat. no. GB114198-50; Wuhan
Servicebio Technology Co., Ltd.); anti-GSDMD-N (1:900; cat. no.
DF13758; Affinity Biosciences); and anti-β-actin (1:4,000; cat. no.
30102ES60; Shanghai Yeasen Biotechnology Co., Ltd.). Following
washing with tris buffered saline with tween (0.05%), the membranes
were treated with a HRP-conjugated secondary antibody (1:10,000;
cat. no. GB23303; Wuhan Servicebio Technology Co., Ltd.) at room
temperature for 1 h. The target signals were visualized using a
super ECL detection kit (cat. no. 36208ES60; Shanghai Yeasen
Biotechnology Co., Ltd.) and quantified using ImageJ software
(version 1.8.0; National Institutes of Health).
i-FABP, IL-1β and IL-18 assay
Levels of i-FABP in the serum and culture
supernatant as well as serum IL-1β and IL-18 levels were analyzed
using rat i-FABP ELISA (cat. no. CSB-E08026r; Cusabio Technology,
LLC), rat IL-1β ELISA (cat. no. CSB-E08055r; Cusabio Technology,
LLC) and rat IL-18 ELISA (cat. no. CSB-E04610r; Cusabio Technology,
LLC) kits (49). The standards
and samples were incubated with the biotin-conjugated antibody for
1 h at 37°C, followed by an incubation with HRP-streptavidin for 1
h at 37°C. The tetramethylbenzidine substrate was subsequently
added to all wells. After terminating the reaction, the absorbance
of each well was tested using a microplate reader (Berthold
Tristar2 S LB 942) at 450 nm.
Lactate dehydrogenase (LDH) assay
LDH levels in cell supernatants from each treatment
group were tested using a LDH assay kit (cat. no. C0016; Beyotime
Institute of Biotechnology). Briefly, cell culture plates were
centrifuged at 400 × g for 5 min at room temperature, and the
culture supernatants were obtained. The culture supernatants of
IEC-6 cells were treated with the LDH detection working solution
for 30 min at room temperature in a darkroom. The absorbance was
then determined using a microplate reader (Berthold Tristar2 S LB
942) at 490/630 nm.
Cell viability assay
A Cell Counting Kit-8 (CCK-8; cat. no. C0037;
Beyotime Institute of Biotechnology) was used to determine IEC-6
cell viability. Briefly, IEC-6 cells (4.5×103
cells/well) were seeded in a 96-well plate for 24 h at 37°C.
Following hypoxia for 12 h and reoxygenation for 6 h at 37°C, 10
µl of CCK-8 solution was added for 1 h. Subsequently, the
absorbance of each group was analyzed using a microplate reader
(Berthold Tristar2 S LB 942) at 450 nm.
Immunofluorescent (IF) staining
Following hypoxia for 12 h and reoxygenation for 6 h
at 37°C, IEC-6 cells were fixed with cold 4% paraformaldehyde
solution for 25 min at 4°C, followed by permeabilization with 1%
Triton X-100 working solution. The cells were then treated with 3%
BSA (cat. no. GC305010-5g; Wuhan Servicebio Technology Co., Ltd.)
at room temperature for 45 min. Anti-NLRP3 (1:300; cat. no.
GB114320-100; Wuhan Servicebio Technology Co., Ltd.);
anti-cleaved-Casp1 (1:100; cat. no. YC0003; ImmunoWay); or
anti-GSDMD-N (1:200; cat. no. DF13758; Affinity Biosciences)
antibodies were incubated with cells at 4°C overnight.
Subsequently, an Alexa Fluor® 488-conjugated secondary
antibody (1:400; cat. no. GB25303; Wuhan Servicebio Technology Co.,
Ltd.) was added for 1 h at 37°C. Cell nuclei were stained using a
DAPI solution (cat. no. G1012; Wuhan Servicebio Technology Co.,
Ltd.) for 10 min at room temperature. All images were captured
using an Eclipse C1 Nikon fluorescence microscope (magnification,
×200; Nikon Corporation).
Mitochondrial morphology and
mitochondrial membrane potential (Δψm) analysis
To assess mitochondrial morphology, IEC-6 cells were
washed and stained with Mito-Tracker Green (final concentration,
100 nM; cat. no. C1048; Beyotime Institute of Biotechnology) for 20
min at 37°C. An A1+R Nikon confocal microscope(Nikon
Corporation)was then used to observe mitochondrial morphology
(magnification, ×1,000).
For the Δψm analysis, IEC-6 cells were
labeled with 1 ml of tetramethylrhodamine ethyl ester perchlorate
solution (final concentration, 10 µM; cat. no. C2001S;
Beyotime Institute of Biotechnology) at 37°C for 35 min. Following
washing, the Δψm was determined using an Eclipse C1
Nikon fluorescence microscope (magnification, ×200; Nikon
Corporation).
Mitochondrial ROS analysis
To assess mitochondrial ROS, IEC-6 cells were
co-labeled with MitoSOX Red (final concentration, 5 µM; cat.
no. S0061S; Beyotime Institute of Biotechnology) and Mito-Tracker
Green (final concentration, 100 nM; cat. no. C1048; Beyotime
Institute of Biotechnology) for 20 min at 37°C. Microscopy images
were captured using an Eclipse C1 Nikon fluorescence microscope
(magnification, ×400; Nikon Corporation).
Statistical analysis
Statistical data were analyzed using SPSS software
(version 19.0; IBM Corp.). Data are presented as the mean ±
SD. Statistical graphs were created using GraphPad Prism (version
5; GraphPad; Dotmatics). Difference comparisons between groups were
performed using an unpaired Student's t-test or one-way ANOVA
followed by Tukey's post hoc test. P<0.05 was considered to
indicate a statistically significant difference.
Results
HKL alleviates IIR-induced intestinal
injury in rats
To produce an appropriate rat IIR model, the SMA was
clamped for 30, 45 or 60 min, followed by reperfusion for 2 h
according to previously described methods (41,42). The results revealed that the most
evident alterations in intestinal damage were observed in the rats
after 45 min of ischemia and 2 h of reperfusion, as demonstrated by
an increased Chiu's score (Fig.
S1). Therefore, 45 min of ischemia and 2 h of reperfusion was
used to induce IIR injury in rats for the subsequent in vivo
experiments.
To determine the beneficial effects of HKL (Fig. 1A) treatment on IIR-induced
intestinal injury, five different doses of HKL (0.5, 1, 5, 10 or 20
mg/kg) were used once daily for 7 days prior to surgery (Fig. 1B) based on a previously published
study (50). The results
revealed that the pretreatment of rats with HKL at doses of 0.5, 1,
5, 10 or 20 mg/kg before IIR-induced injury, significantly reduced
the damage to the intestinal mucosa and reduced the Chiu's score
compared with that of the IIR-only group. In addition, the rats
that were pretreated with 10 or 20 mg/kg HKL had reduced damage to
the intestinal mucosa as well as a reduced Chiu's score compared
with those of the 0.5, 1 or 5 mg/kg HKL pretreated rats (Fig. 1C and D). However, 20 mg/kg HKL
did not have a significant effect on improving the damage in the
Chiu score compared with that of HKL at the 10 mg/kg dose (Fig. 1C and D). Therefore, the 10 mg/kg
HKL dose was used in the subsequent experiments. To further
investigate the effect of HKL on IIR-induced intestinal injury, the
concentration of serum i-FABP (a marker of tissue injury) was
determined using ELISA. As shown in Fig. 1E, IIR injury significantly
increased the concentration of serum i-FABP compared with the sham
group. However, in rats with IIR-induced injury, HKL pretreatment
significantly reduced the concentration of serum i-FABP compared
with those without HKL pretreatment. In addition, the effect of HKL
pretreatment on the small intestinal barrier was determined by
analyzing occludin and ZO-1 protein expression levels using IHC
staining and western blotting. The findings revealed that
IIR-induced injury significantly reduced occludin and ZO-1 protein
levels compared with those in the sham group. Furthermore, the
reduced occludin and ZO-1 protein levels that were induced by IIR
injury were significantly reversed with HKL pretreatment (Fig. 1F-H).
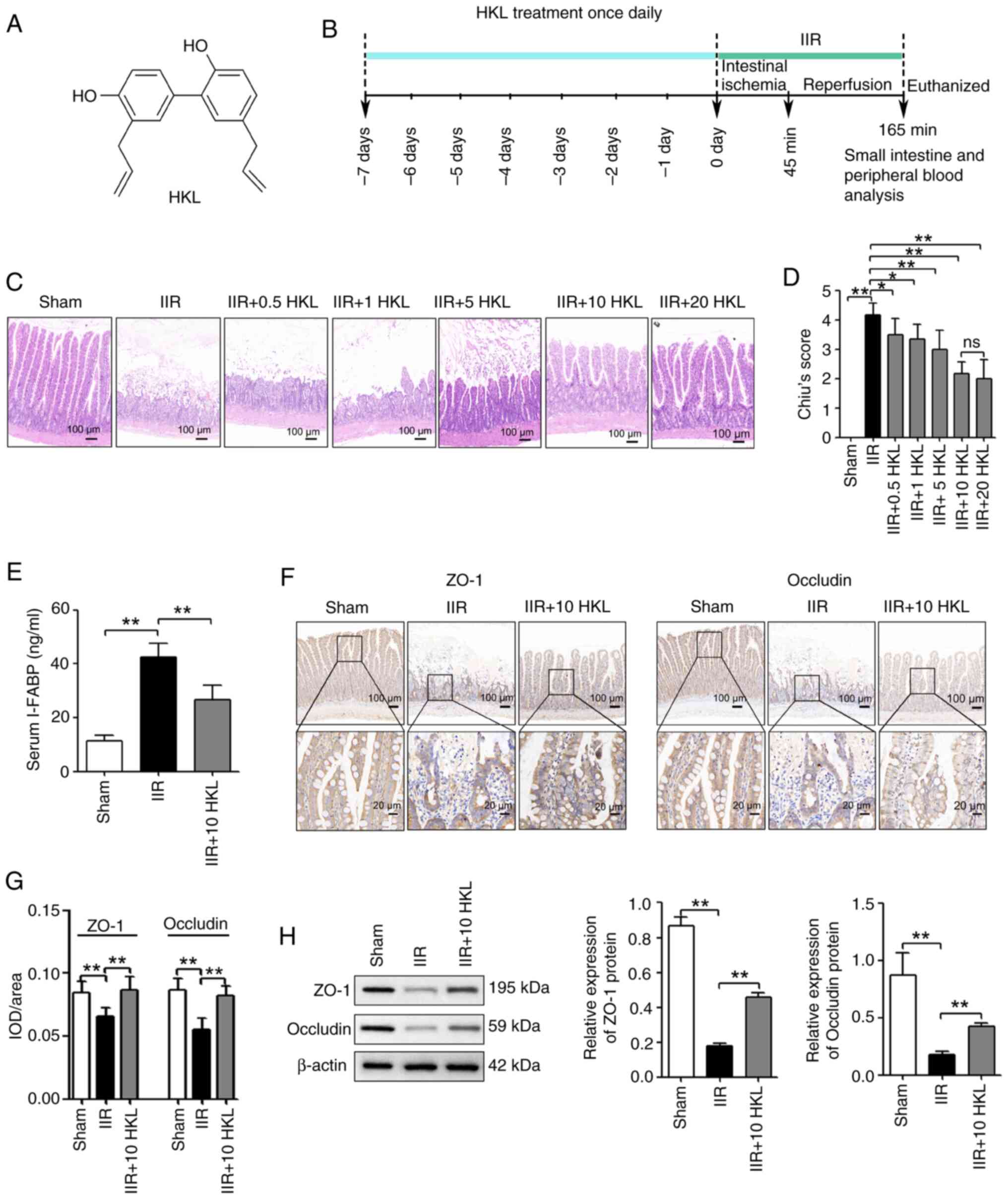 | Figure 1HKL treatment reduces IIR-induced
intestinal injury in rats. (A) Chemical structure of HKL. (B)
Workflow of the in vivo experiments. (C) Hematoxylin and
eosin staining of intestinal tissues from rats after treatment with
0, 0.5, 1, 5, 10 or 20 mg/kg HKL (magnification, ×100; scale bar,
100 µm). (D) Histopathological damage was determined using
Chiu's score (n=6). (E) IIR-induced intestinal injury was measured
using the serum i-FABP levels (n=6). (F) Consecutive sectioning of
the same specimen for IHC staining was used to measure the protein
expression levels of occludin and ZO-1 in the intestinal tissues of
rats in different treatment groups (magnification, ×100 or ×400;
scale bars, 100 or 20 µm; n=6). (G) Quantification of ZO-1
and occludin expression levels after IHC staining. (H) The protein
expression levels of occludin and ZO-1 in the intestinal tissues of
rats in different treatment groups were determined using western
blotting (n=4). *P<0.05 and **P<0.01.
ns, not significant; IHC, immunohistochemical; IIR, intestinal
ischemia-reperfusion; HKL, honokiol; i-FABP, intestinal fatty
acid-binding protein; ZO-1, tight junction protein 1; IOD,
integrated optical density. |
HKL alleviates H/R-triggered pyroptosis
in IEC-6 cells
To investigate whether HKL had a beneficial role
in vitro, the cytotoxicity of HKL on IEC-6 cells at five
concentrations (1, 5, 10, 20 or 40 µM) was determined based
on a previously published study (51). As shown in Fig. 2A, 40 µM HKL significantly
reduced the viability of IEC-6 cells following treatment for 12 h.
A H/R model was then established using IEC-6 cells, and a CCK-8
assay was used to investigate the effect of HKL treatment on cell
viability. Compared with the con group, treatment with H/R
significantly reduced the viability of IEC-6 cells. However, the
viability of H/R treated IEC-6 cells was significantly increased
after the cells were pretreated with 5, 10 and 20 µM HKL
compared with those without HKL pretreatment (Fig. 2B). Therefore, 20 µM HKL
was used for the subsequent experiments.
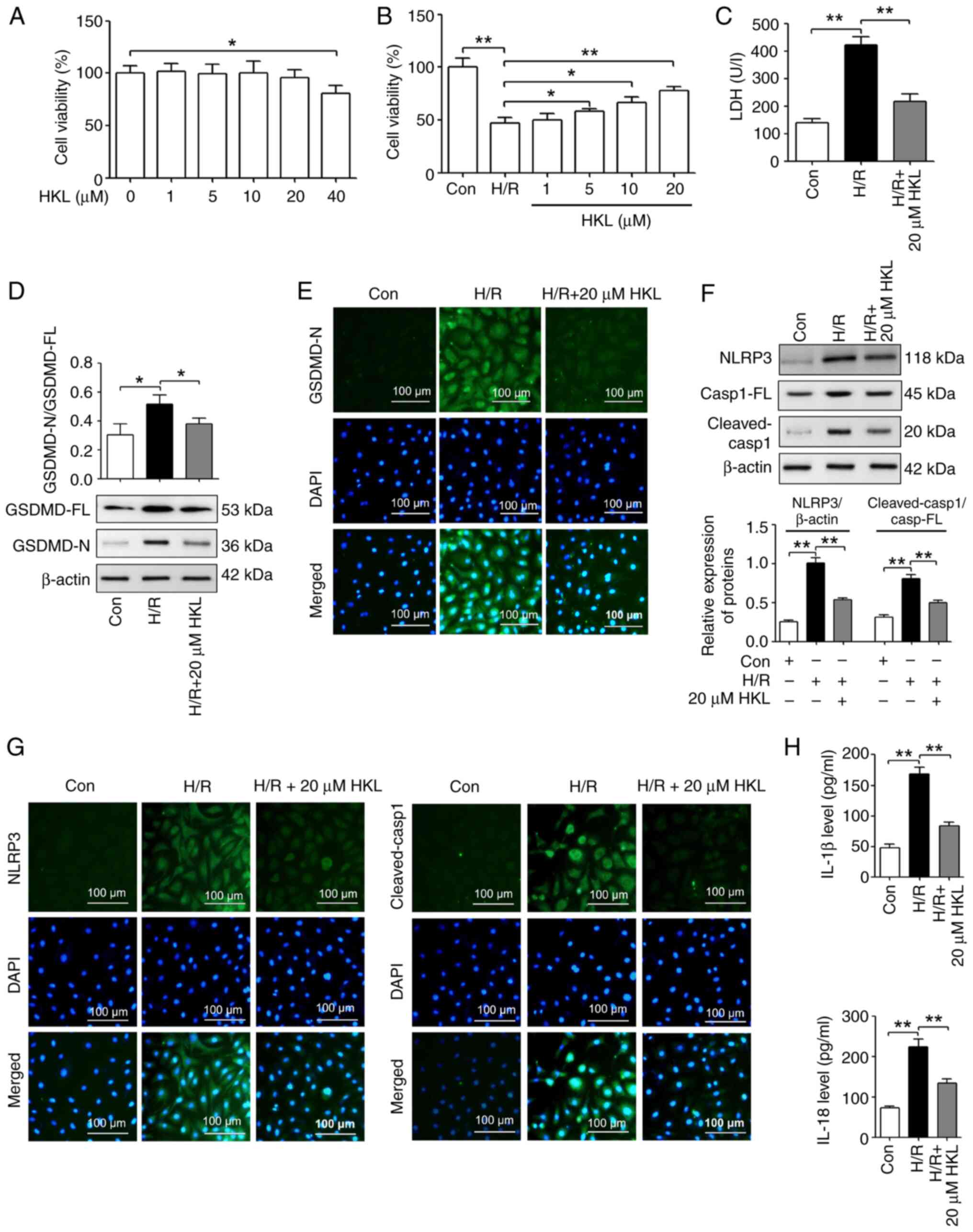 | Figure 2HKL treatment notably inhibits
pyroptosis in IEC-6 cells following H/R. (A) HKL cytotoxicity to
IEC-6 cells was measured using a CCK-8 assay (n=3). (B) The effect
of HKL on the cell viability of H/R-treated IEC-6 cells was
revealed using a CCK-8 assay (n=3). (C) The effect of HKL on cell
injury was investigated by analyzing the LDH levels in cell
supernatants from H/R-treated IEC-6 cells (n=3). (D) Western
blotting assays were used to reveal the effect of HKL on GSDMD-FL
and GSDMD-N expression levels in IEC-6 cells following H/R (n=3).
(E) IF staining assays were used to demonstrate the effect of HKL
on GSDMD-N expression levels in IEC-6 cells following H/R
(magnification, ×200; scale bar, 100 µm; n=3). (F) Western
blotting assays were used to reveal the effects of HKL on the
protein expression levels of NLRP3, Casp1-FL and cleaved-Casp1 in
IEC-6 cells after H/R (n=3). (G) IF staining assays were used to
demonstrate the effects of HKL on the expression levels of NLRP3
and cleaved-Casp1 in IEC-6 cells after H/R (magnification, ×200;
scale bar, 100 µm; n=3). (H) ELISA assays were used to
reveal the effect of HKL on IL-1β and IL-18 protein levels in IEC-6
cells following H/R (n=3). *P<0.05 and
**P<0.01. HKL, honokiol; LDH, lactate dehydrogenase;
GSDMD, gasdermin D; GSDMD-FL, GSDMD-full length; GSDMD-N,
GSDMD-N-terminal domain; H/R, hypoxia/reoxygenation; NLRP3, NOD-,
LRR- and pyrin domain-containing protein 3; IF, immunofluorescent;
IL, interleukin; Con, control; Casp1, caspase-1; Casp1-FL,
Casp1-full length; CCK-8, Cell Counting Kit-8. |
To further investigate whether HKL protected IEC-6
cells following H/R, the LDH (an index of cell injury) levels were
analyzed. Compared with the con group, the LDH levels in the H/R
group were significantly increased. Furthermore, the LDH levels in
the IEC-6 cells treated with H/R and 20 µM HKL were
significantly reduced compared with the cells that were only
treated with H/R (Fig. 2C).
A number of studies show that pyroptosis serves an
important role in IIR-induced injury (23,24). In addition, HKL inhibits NLRP3
inflammasome-mediated pyroptosis, which reduces lipopolysaccharide
(LPS)-induced acute lung injury (50). Therefore, the present H/R model
was used to determine whether HKL affected NLRP3
inflammasome-induced pyroptosis. Western blotting revealed that the
GSDMD-N/GSDMD-full length (GSDMD-FL) ratio was increased in the H/R
group compared with the con group. Furthermore, the
GSDMD-N/GSDMD-FL ratio was significantly reduced in the 20
µM HKL pretreated H/R-induced cells compared with the
H/R-induced cells without HKL pretreatment (Fig. 2D). IF staining experiments
revealed that the protein expression of GSDMD-N was increased in
the H/R group compared with the con group. However, the protein
expression of GSDMD-N was reduced in the 20 µM HKL
pretreated H/R-induced cells compared with the H/R-induced cells
without HKL pretreatment (Fig.
2E). Additionally, the protein levels of NLRP3 and the
cleaved-Casp1/Casp1-full length (Casp1-FL) ratio were significantly
increased in the H/R group compared with those in the con group.
Furthermore, the protein levels of NLRP3 and the
cleaved-Casp1/Casp1-FL ratio were significantly decreased in the 20
µM HKL pretreated H/R-induced cells compared with the
H/R-induced cells without HKL pretreatment (Fig. 2F). IF staining experiments
indicated that the levels of NLRP3 and cleaved-Casp1 were increased
in the H/R group compared with the con group. However, the levels
of NLRP3 and cleaved-Casp1 were reduced in the 20 µM HKL
pretreated H/R-induced cells compared with the H/R-induced cells
without HKL pretreatment (Fig.
2G). Subsequently, the pyroptosis-related inflammatory
products, IL-1β and IL-18, were assessed. The results revealed that
the IL-1β and IL-18 levels in the H/R group were significantly
increased compared with those in the con group. However, the IL-1β
and IL-18 levels were significantly reduced in the 20 µM HKL
pretreated H/R-induced cells compared with the H/R-induced cells
without HKL pretreatment (Fig.
2H).
Inhibition of mitochondrial ROS
alleviates H/R-triggered pyroptosis in IEC-6 cells
Previous studies reveal that mitochondrial
dysfunction activates pyroptosis by regulating the Δψm
and ROS production (52,53). Mitochondria are the predominant
source of ROS production. To investigate the role of mitochondrial
ROS in H/R-induced pyroptosis, mitochondrial ROS production was
inhibited by Mito-TEMPO, which is a mitochondrion-targeted
superoxide dismutase mimic that is usually used to scavenge
superoxide and alkyl radicals (54). As shown in Fig. 3A, 200 µM Mito-TEMPO
reduced the mitochondrial ROS production that was induced by H/R.
Furthermore, mitochondrial fission was significantly increased in
the H/R-induced IEC-6 cells compared with those in the con group.
However, mitochondrial fission was significantly reduced in the
Mito-TEMPO-treated H/R-induced cells compared with those without
Mito-TEMPO treatment (Fig. 3B).
In addition, H/R significantly reduced the Δψm in IEC-6 cells
compared with the con group. This was significantly reversed in
H/R-induced cells with Mito-TEMPO treatment (Fig. 3C).
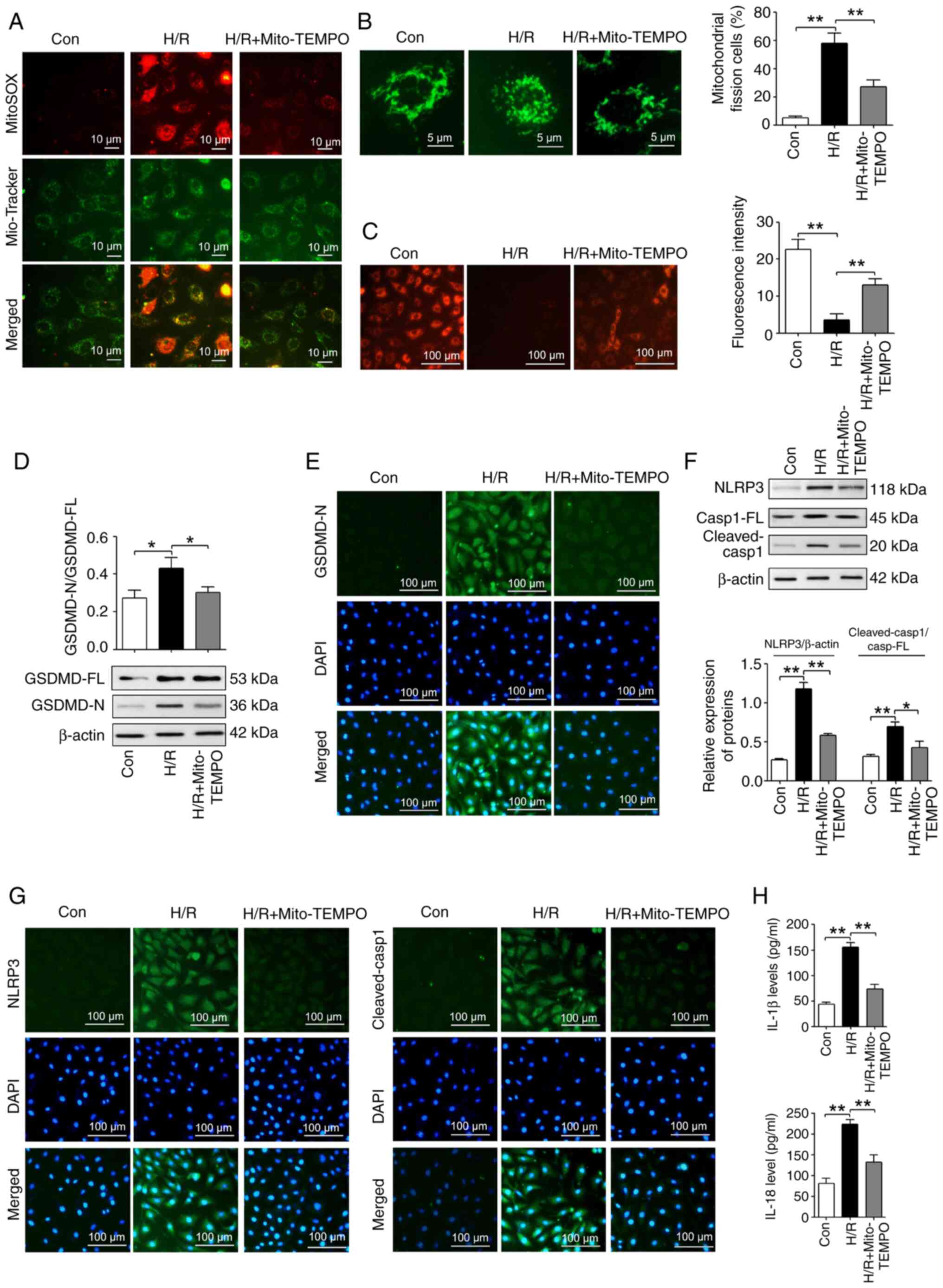 | Figure 3Inhibiting mitochondrial ROS with
Mito-TEMPO reduces pyroptosis in IEC-6 cells following H/R. (A)
MitoSOX Red and Mito-Tracker Green co-staining was used to analyze
the production of mitochondrial ROS in different treatment groups
(Mito-TEMPO, 200 µM; magnification, ×400; scale bar, 10
µm). (B) Mitochondrial fission and integrity in different
treatment groups were measured using Mito-Tracker Green staining
(Mito-TEMPO, 200 µM; magnification, ×1,000; scale bar, 5
µm; n=3). (C) The mitochondrial membrane potential was
analyzed in different treatment groups using tetramethylrhodamine
ethyl ester perchlorate staining (Mito-TEMPO, 200 µM;
magnification, ×200; scale bar, 100 µm; n=3). (D) The
GSDMD-FL and GSDMD-N expression levels in different treatment
groups were measured using western blotting assays (Mito-TEMPO, 200
µM; n=3). (E) The GSDMD-N expression levels were measured in
different treatment groups using IF staining assays (Mito-TEMPO,
200 µM; magnification, ×200; scale bar, 100 µm; n=3).
(F) Western blotting assays were used to reveal the expression
levels of NLRP3, Casp1-FL and cleaved-Casp1 in different treatment
groups (Mito-TEMPO, 200 µM; n=3). (G) IF staining assays
were used to measure the expression levels of NLRP3 and
cleaved-Casp1 in different treatment groups (Mito-TEMPO, 200
µM; magnification, ×200; scale bar, 100 µm; n=3). (H)
ELISA assays were used to reveal the IL-1β and IL-18 protein levels
in different treatment groups (Mito-TEMPO, 200 µM; n=3).
*P<0.05 and**P<0.01. Con, control; H/R,
hypoxia/reoxygenation; GSDMD, gasdermin D; GSDMD-FL, GSDMD-full
length; GSDMD-N, GSDMD-N-terminal domain; NLRP3, NOD-, LRR- and
pyrin domain-containing protein 3; IF, immunofluorescent; IL,
interleukin; Casp1, caspase-1; Casp1-FL, Casp1-full length; ROS,
reactive oxygen species. |
Western blotting indicated that the GSDMD-N/GSDMD-FL
ratio was significantly increased in the H/R group compared with
the con group. However, Mito-TEMPO treatment significantly reversed
this H/R-induced change (Fig.
3D). IF staining experiments demonstrated that the protein
expression of GSDMD-N was increased in the H/R group compared with
the con group, while Mito-TEMPO treatment reversed this H/R-induced
change in the GSDMD-N levels (Fig.
3E). Additionally, western blotting assays revealed that
Mito-TEMPO treatment significantly reversed the H/R-induced
increases in the NLRP3 protein levels and cleaved-Casp-1/Casp-1-FL
ratio (Fig. 3F). Furthermore, IF
staining indicated that Mito-TEMPO treatment reversed the
H/R-induced increases in NLRP3 and cleaved-Casp-1 protein levels
(Fig. 3G). In addition,
Mito-TEMPO treatment reversed the H/R-induced increases in the
IL-1β and IL-18 protein levels (Fig.
3H).
HKL alleviates the H/R-induced
mitochondrial dysfunction in IEC-6 cells
A previous study indicates that HKL alleviates
amyotrophic lateral sclerosis by improving mitochondrial function
and morphology (37). Therefore,
the effect of HKL on mitochondrial dysfunction in the H/R model was
investigated. MitoSOX Red and Mito-Tracker Green co-staining was
carried out to evaluate the production of mitochondrial ROS. As
shown in Fig. 4A, mitochondrial
ROS generation was increased in H/R-induced IEC-6 cells compared
with those in the con group. However, 20 µM HKL treatment
reversed this H/R-induced increase in the ROS accumulation in the
mitochondria. Mito-Tracker Green staining revealed that the
mitochondrial fission was significantly increased in the
H/R-treated IEC-6 cells compared with those in the con group, and
20 µM HKL treatment significantly reversed the H/R-induced
increases in the mitochondrial fission and integrity (Fig. 4B). In addition, the H/R-induced
reduction in the Δψm was significantly reversed with 20
µM HKL treatment (Fig.
4C).
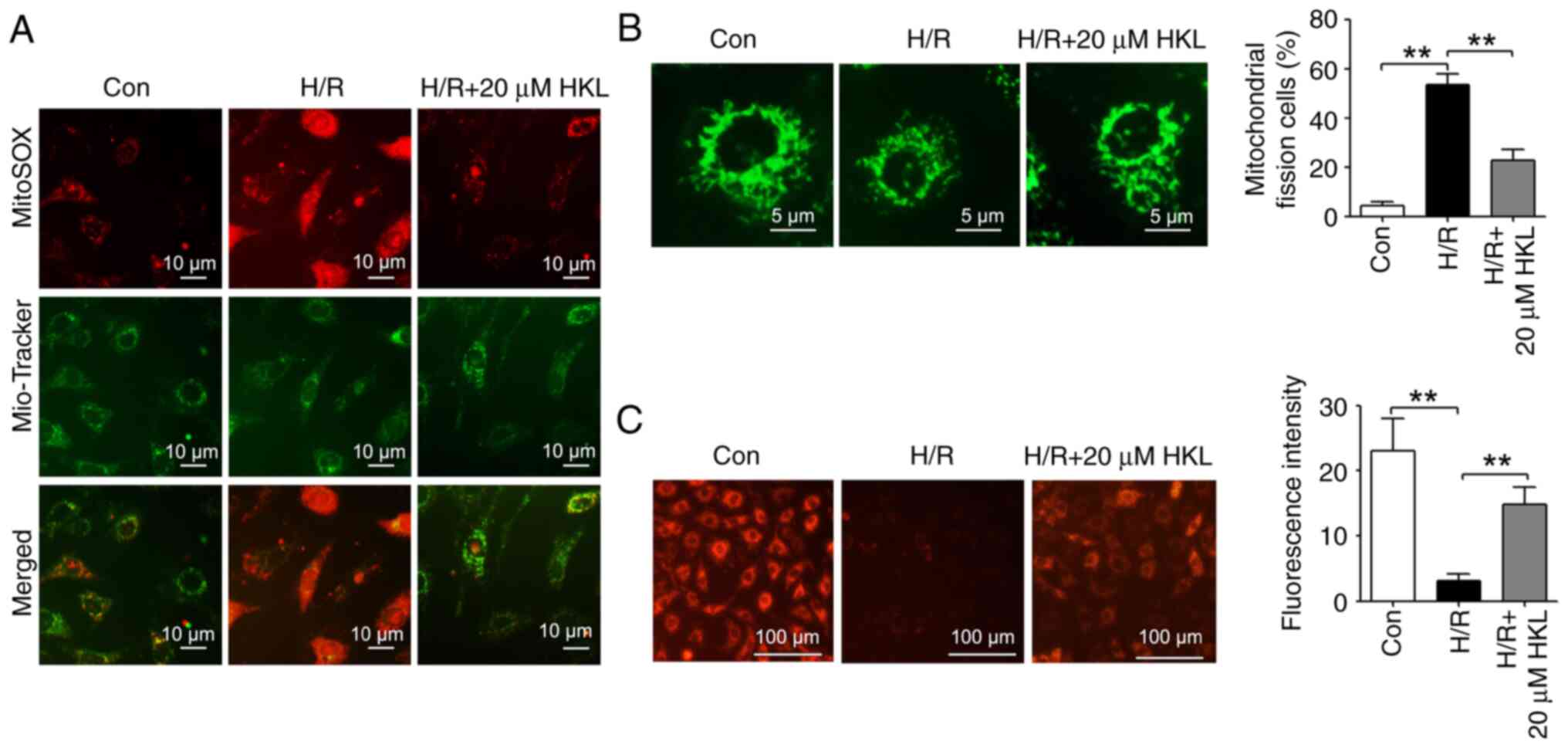 | Figure 4HKL reduces mitochondrial dysfunction
in IEC-6 cells following H/R. (A) MitoSOX Red and Mito-Tracker
Green co-staining was used to investigate the effect of HKL on the
generation of mitochondrial reactive oxygen species in IEC-6 cells
following H/R (magnification, ×400; scale bar, 10 µm). (B)
The effects of HKL on mitochondrial fission and integrity were
demonstrated using Mito-Tracker Green staining in IEC-6 cells after
H/R (magnification, ×1,000; scale bar, 5 µm; n=3). (C) The
mitochondrial membrane potential was analyzed in different
treatment groups using tetramethylrhodamine ethyl ester perchlorate
staining (magnification, ×200; scale bar, 100 µm; n=3).
**P<0.01. Con, control; H/R, hypoxia/reoxygenation;
HKL, honokiol. |
HKL alleviates H/R-induced mitochondrial
dysfunction by inducing the expression of SIRT3 protein in IEC-6
cells
SIRT3 is a mitochondrial protein that is involved in
regulating mitochondrial function (55). In intracerebral hemorrhage, HKL
protects mitochondrial function by increasing the expression of
SIRT3 protein (40). Therefore,
the effects of HKL treatment on SIRT3 in the present H/R cell model
was investigated. SIRT3 levels were revealed to be significantly
reduced in the H/R-induced cells compared with the con group.
However, 20 µM HKL treatment significantly reversed the
H/R-induced reduction in the SIRT3 levels (Fig. 5A). A previous study demonstrates
that HKL also increases the mRNA expression of SIRT3 during cardiac
hypertrophy (38). In the
present study, 20 µM HKL treatment also increased the SIRT3
mRNA levels in IEC-6 cells after H/R inhibition (Fig. S2).
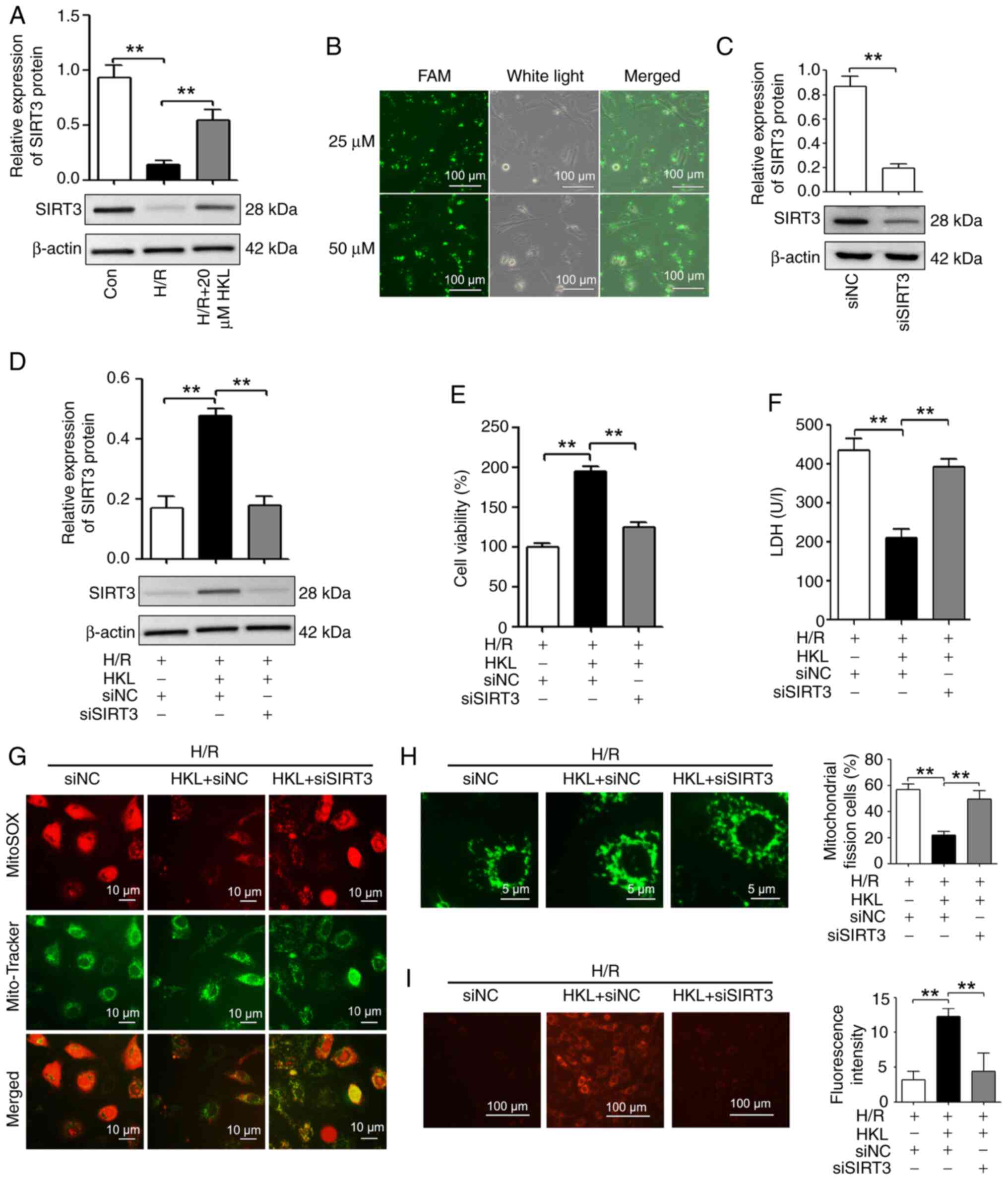 | Figure 5HKL (20 µM) reduces
mitochondrial dysfunction by inducing SIRT3 in IEC-6 cells
following H/R. (A) Western blotting was used to measure the SIRT3
expression levels in different treatment groups (n=3). (B) The
transfection efficiency of siRNAs was demonstrated using
FAM-labeled siNC (magnification, ×200; scale bar, 100 µm).
(C) The knockdown efficiency of siSIRT3 was revealed using western
blotting (n=3). (D) Western blotting was used to demonstrate the
effect of siSIRT3 on the SIRT3 levels in IEC-6 cells after exposure
to H/R and HKL (n=3). (E) The cell viability of IEC-6 cells after
different treatments was measured using a Cell Counting Kit-8 assay
(n=3). (F) The cell injury of IEC-6 cells after different
treatments was investigated by analyzing the LDH levels (n=3). (G)
The generation of mitochondrial reactive oxygen species was
revealed using MitoSOX Red and Mito-Tracker Green co-staining in
IEC-6 cells after different treatments (magnification, ×400; scale
bar, 10 µm). (H) Mitochondrial fission and integrity were
revealed using Mito-Tracker Green staining in H/R-induced IEC-6
cells following HKL and siSIRT3 treatment (magnification, ×1,000;
scale bar, 5 µm; n=3). (I) The mitochondrial membrane
potential was analyzed in different treatment groups using
tetramethylrhodamine ethyl ester perchlorate staining
(magnification, ×200; scale bar, 100 µm; n=3).
**P<0.01. Con, control; H/R, hypoxia/reoxygenation;
SIRT3, sirtuin 3; FAM, fluorescein amidite; LDH, lactate
dehydrogenase; si, small interfering RNA; NC, negative control;
HKL, honokiol. |
To determine whether siRNAs were transfected into
IEC-6 cells, fluorescein amidite (FAM)-labeled siNC at doses of 25
and 50 µM was examined. As shown in Fig. 5B, cells were fluorescent after
transfection with 25 and 50 µM FAM-labeled siNC, which
indicated that transfection efficiency was >90%. As the
transfection efficiency was comparable using 25 or 50 µM
FAM-labeled siNC, siRNAs at doses of 25 µM were used for
subsequent transfections. The knockdown efficiency of the SIRT3
siRNA was then examined. The results revealed that the expression
of SIRT3 was significantly reduced in the siSIRT3 group compared
with the siNC group (Fig.
5C).
To determine whether HKL affected mitochondrial
function after H/R via SIRT3 in IEC-6 cells, SIRT3 levels were
reduced using a SIRT3 specific siRNA (Fig. 5D). SIRT3 inhibition significantly
reversed the HKL-induced differences in cell viability and LDH
levels in the H/R model (Fig. 5E and
F). Furthermore, the results demonstrated that HKL was unable
to suppress the H/R-induced overproduction of mitochondrial ROS
when SIRT3 was silenced in IEC-6 cells (Fig. 5G). Additionally, HKL treatment
did not alleviate the H/R-induced mitochondrial fission in IEC-6
cells following SIRT3 silencing (Fig. 5H). In addition, HKL treatment
reversed the H/R-induced reduction of the Δψm in IEC-6
cells; however, this HKL-induced increase in the Δψm was
reduced when SIRT3 was silenced (Fig. 5I). Therefore, these results
indicated that SIRT3 may be involved in the HKL-induced protection
against mitochondrial dysfunction in the present H/R model.
HKL alleviates pyroptosis by increasing
the expression of SIRT3 protein in H/R-induced cells
Due to the association between mitochondrial
function and pyroptosis, the potential role of SIRT3 in the
HKL-induced pyroptosis inhibition in IEC-6 cells was investigated.
Fig. 6A shows that the
HKL-induced reduction of the GSDMD-N/GSDMD-FL ratio was
significantly reversed in the present H/R model following SIRT3
silencing. IF staining experiments also demonstrated that the
HKL-induced reduction of GSDMD-N was reversed in the present H/R
model following SIRT3 silencing (Fig. 6B). Additionally, the results
revealed that the HKL-induced decrease in the NLRP3 protein levels
and the cleaved-Casp1/Casp1-FL ratio in the present H/R model was
significantly reversed following SIRT3 inhibition (Fig. 6C). IF staining experiments also
demonstrated that the HKL-induced downregulation of NLRP3 and
cleaved-Casp1 protein levels in the present H/R model were reversed
following SIRT3 inhibition (Fig.
6D). In addition, HKL treatment significantly decreased the
H/R-induced increases in the IL-1β and IL-18 levels in IEC-6 cells;
however, SIRT3 knockdown significantly reversed the effect of HKL
(Fig. 6E). Therefore, these
results suggested that SIRT3 may be involved in the HKL-mediated
pyroptosis inhibition in IEC-6 cells.
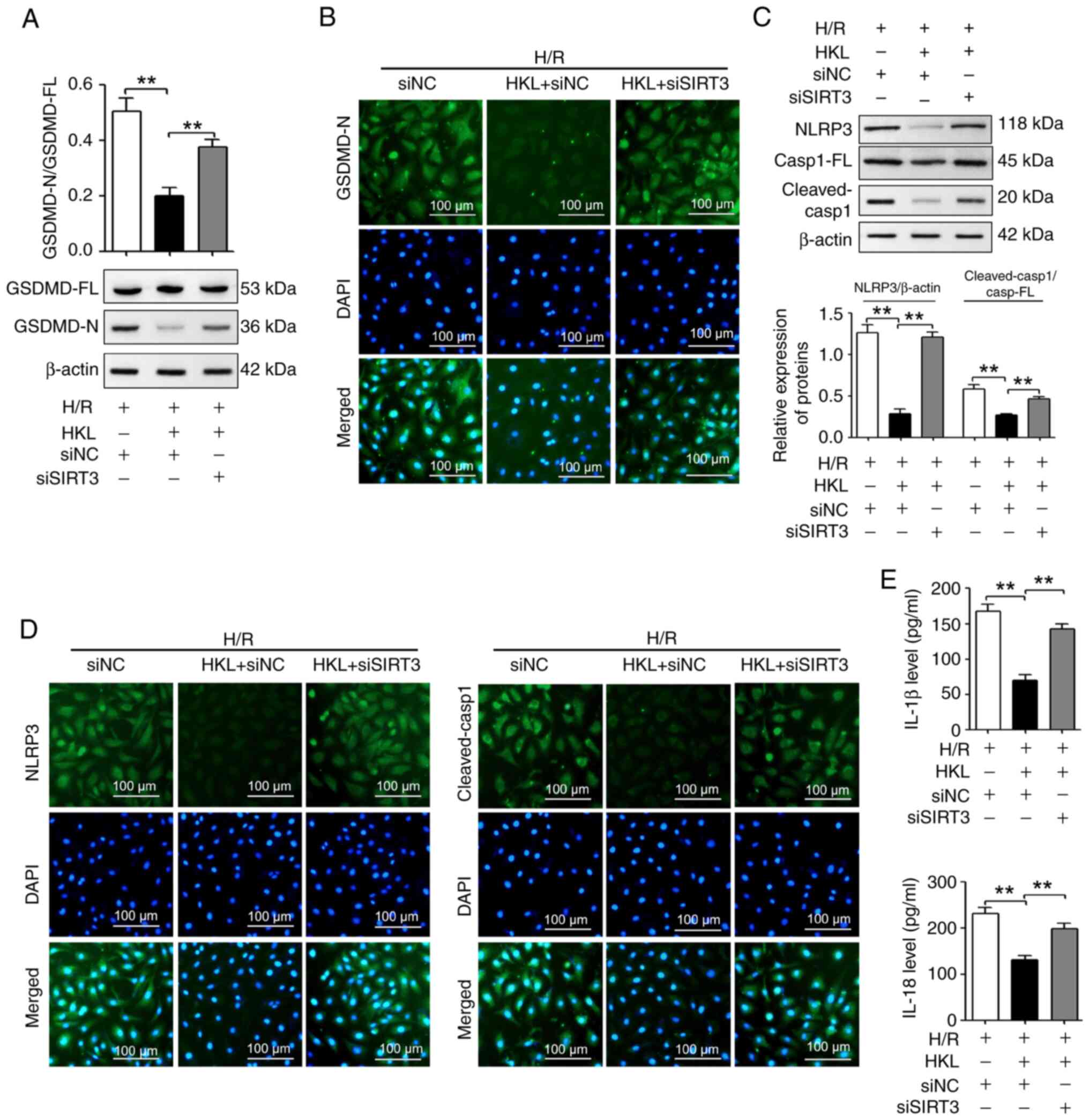 | Figure 6HKL (20 µM)reduces pyroptosis
by increasing the SIRT3 protein levels in IEC-6 cells following
H/R. (A) Western blotting revealed the GSDMD-FL and GSDMD-N protein
levels (n=3). (B) The GSDMD-N protein levels were measured using
immunofluorescent staining assays (magnification, ×200; scale bar,
100 µm; n=3). (C) NLRP3, Casp1-FL and cleaved-Casp1 protein
levels were revealed using western blotting assays (n=3). (D) NLRP3
and cleaved-Casp1 protein levels were measured using
immunofluorescent staining assays in H/R-induced IEC-6 cells with
different treatments (magnification, ×200; scale bar, 100
µm; n=3). (E) ELISA assays were used to analyze the IL-1β
and IL-18 protein levels in different treatment groups (n=3).
**P<0.01. H/R, hypoxia/reoxygenation; SIRT3, sirtuin
3; si, small interfering RNA; NC, negative control; GSDMD,
gasdermin D; GSDMD-FL, GSDMD-full length; GSDMD-N, GSDMD-N-terminal
domain; NLRP3, NOD-, LRR- and pyrin domain-containing protein 3;
IL, interleukin; Casp1, caspase-1; Casp1-FL, Casp1-full length;
HKL, honokiol. |
HKL alleviates IIR-induced pyroptosis by
increasing the expression of SIRT3 protein in vivo
IHC staining experiments revealed that SIRT3 was
significantly decreased in the intestinal tissues of IIR-induced
rats compared with the sham group. However, 10 mg/kg HKL treatment
significantly reversed this IIR-induced change (Fig. 7A and B). In addition, 10 mg/kg
HKL treatment significantly reversed the IIR-induced changes in
NLRP3, cleaved-Casp1 and GSDMD-N protein levels in the intestinal
tissues of rats (Fig. 7A and B).
Furthermore, western blotting assays indicated that, compared with
the sham group, IIR significantly reduced the SIRT3 protein levels
and significantly increased the NLRP3 protein levels as well as the
cleaved-Casp1/Casp1-FL and GSDMD-N/GSDMD-FL ratios in the
intestinal tissues of rats (Fig.
7C). However, 10 mg/kg HKL treatment significantly reversed
these IIR-induced changes (Fig.
7C). Compared with the sham group, IIR significantly increased
the serum IL-1β and IL-18 levels in rats; however, 10 mg/kg HKL
treatment significantly reversed these IIR-induced effects
(Fig. 7D). Taken together, these
findings suggested that HKL may inhibit pyroptosis by regulating
the expression of SIRT3 protein in IIR-induced injury.
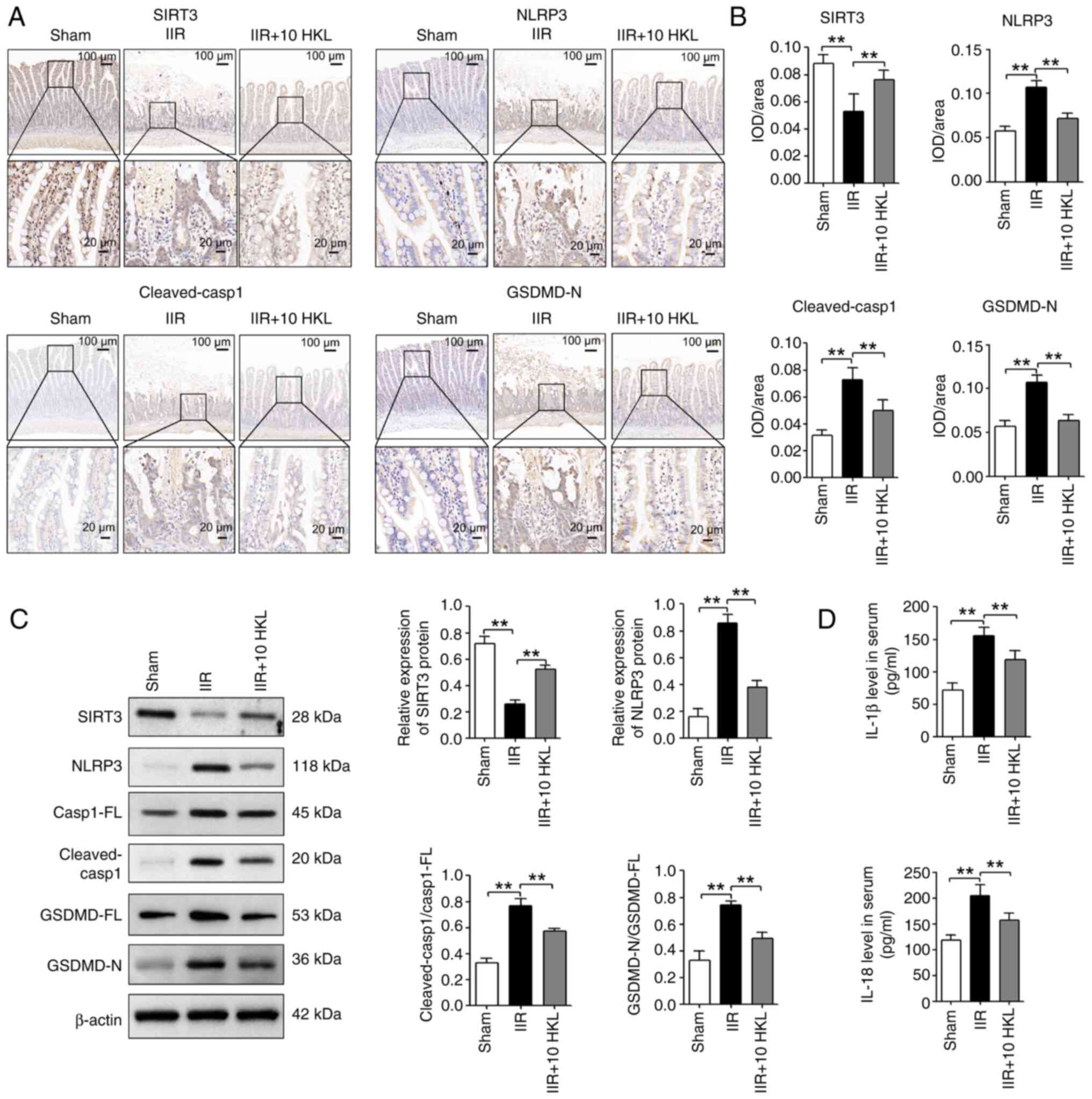 | Figure 7HKL (10 mg/kg) reduces IIR-induced
pyroptosis by increasing the SIRT3 protein levels in rats. (A)
SIRT3, NLRP3, cleaved-Casp1 and GSDMD-N protein levels were
measured using immunohistochemical staining assays and consecutive
sectioning of the same specimen in the same area of the intestinal
tissues from different treatment groups (magnification, ×100 or
×400; scale bars, 100 or 20 µm; n=6). (B) Quantification of
SIRT3, NLRP3, cleaved-Casp1 and GSDMD-N expression levels after
immunohistochemical staining. (C) SIRT3, NLRP3, Casp1-FL,
cleaved-Casp1, GSDMD-FL and GSDMD-N protein levels were revealed
using western blotting in rats following IIR and 10 mg/kg HKL
treatment (n=4). (D) Serum IL-1β and IL-18 levels were analyzed
using ELISA assays in rats after IIR and 10 mg/kg HKL treatment
(n=6). **P<0.01. HKL, honokiol; IIR, intestinal
ischemia-reperfusion; SIRT3, sirtuin 3; GSDMD, gasdermin D;
GSDMD-FL, GSDMD-full length; GSDMD-N, GSDMD-N-terminal domain;
NLRP3, NOD-, LRR- and pyrin domain-containing protein 3; IL,
interleukin; Casp1, caspase-1; Casp1-FL, Casp1-full length; IOD,
integrated optical density. |
Discussion
Previous studies indicate that HKL possesses
protective functions against a number of ischemia-reperfusion
injuries, such as myocardial ischemia-reperfusion injury, renal
ischemia-reperfusion injury and brain ischemia-reperfusion injury
(45,56,57). However, to the best of our
knowledge, the present study was the first to investigate the role
of HKL in IIR injury. In the present study, a rat IIR model was
established, and the effect of HKL on IIR-induced injury was
examined. It was revealed that, compared with IIR-induced injury
alone, HKL pretreatment notably reduced the damage to the
intestinal mucosa, the Chiu's score and the serum i-FABP
concentration, and increased the levels of intestinal barrier
proteins. To further investigate the effect of HKL on IIR-induced
injury in vitro, a H/R cell model was created to mimic
IIR-induced injury. HKL treatment ameliorated the H/R-induced
decrease in the viability of IEC-6 cells and reversed the
H/R-induced levels of LDH. Taken together, the present study
indicated the beneficial role of HKL in IIR-induced injury;
however, the detailed mechanisms need to be further
investigated.
Under normal physiological conditions, pyroptosis
serves a role in the resistance of pathogen infection (11). However, excessive pyroptosis
occurs in a number of pathological processes, such as myocardial
infarction and septicemia, and induces cell death and inflammatory
responses (10). Accumulating
evidence indicates that pyroptosis is associated with
ischemia-reperfusion injury in different organs (58). For example, during cerebral
ischemia-reperfusion injury, pyroptosis is induced in astrocytes
and promotes inflammation, and suppressing pyroptosis improves
sensorimotor function, spatial learning and memory function, and
reduces infarct volume and brain edema (59). When IIR occurs, pyroptosis is
also notably increased, whereas intestinal barrier disruption and
cell death are reduced by inhibiting pyroptosis (23). In addition, HKL improves the
aberrant interactions between tubular epithelial cells and renal
resident macrophages by inhibiting pyroptosis in lupus nephritis
(60). The results of the
present study indicated that IIR induced pyroptosis, as evidenced
by increased levels of GSDMD-N (a marker of pyroptosis). Therefore,
it was hypothesized that HKL may ameliorate IIR-induced injury by
inhibiting pyroptosis. The results of the present study revealed
that HKL treatment inhibited the H/R-induced change in the GSDMD-N
protein level. Under pathological conditions, including
ischemia-reperfusion injury, the canonical pathway of pyroptosis
involves the activation of the NLRP3 inflammasome, which increases
cleaved-Casp1 production, resulting in the cleaving of pro-IL-18
and pro-IL-1β into their mature forms and the induction of
pyroptosis (58,61). The present study revealed that
H/R treatment increased the levels of NLRP3, cleaved-Casp1, IL-1β
and IL-18 in IEC-6 cells, and that this change was reversed by HKL.
Additionally, a study by Liu et al (50) reveals that HKL treatment improves
acute lung injury by suppressing NLRP3 inflammasome-induced
pyroptosis. These results indicated that HKL treatment may
ameliorate IIR injury by reversing NLRP3 inflammasome-induced
pyroptosis.
Mitochondrial damage and dysfunction are involved in
IIR-induced injury (26,27,42). During IIR, mitochondrial
dysfunction generates excessive ROS, and the increase in ROS
induces mtDNA damage, which subsequently leads to increased ROS
production and mitochondrial dysfunction, thereby inducing cell
death (26). Evidence suggests
that the overproduction of ROS by damaged mitochondria can induce
pyroptosis (62). The results of
the present study indicated that inhibiting mitochondrial ROS
reduced the H/R-induced mitochondrial fission and reduction of the
Δψm. Therefore, this indicated that mitochondrial ROS
inhibition may improve IIR-induced pyroptosis. The results of the
present study revealed that reducing mitochondrial ROS production
with Mito-TEMPO reduced pyroptosis, which was evidenced by
decreased NLRP3, cleaved-Casp1 and GSDMD-N protein levels, as well
as reduced IL-1β and IL-18 levels. Consistent with these results,
phospholipase C ε1 promotes cardiomyocyte pyroptosis by inducing
defective mitochondrial function in doxorubicin-induced
cardiotoxicity (63). To the
best of our knowledge, the present study was the first to provide
evidence that mitochondrial dysfunction may be involved in
pyroptosis during IIR-induced injury.
Since ROS production induced by mitochondrial
dysfunction can induce pyroptosis, drugs that protect mitochondrial
function may inhibit ROS-induced pyroptosis. HKL has a protective
role in a number of pathological processes by mitigating
mitochondrial dysfunction (45,64). For example, HKL exerts
cardioprotection by inhibiting mitochondrial dysfunction-induced
apoptosis in myocardial ischemia-reperfusion injury (45). Therefore, it was hypothesized
that HKL may reduce ROS-induced pyroptosis by improving
mitochondrial dysfunction in IIR-induced injury. In the present
study, the results of the in vitro experiments provided
evidence to support this hypothesis. HKL treatment notably
alleviated H/R-induced mitochondrial fission, reduced mitochondrial
ROS production and reversed the reduction of the
Δψm.
Finally, the results of the present study suggested
that the SIRT3/NLRP3 pathway may be important for HKL
mediated-pyroptosis in IIR injury. SIRT3, a mitochondrial
deacetylase, is involved in regulating mitochondrial function,
cellular energy homeostasis and mitochondrial ROS production
(56,65-67). In a number of
ischemia-reperfusion injuries, including IIR injury, SIRT3
expression levels are reduced; however, increasing the SIRT3
expression levels ameliorates ischemia-reperfusion injury by
preserving mitochondrial function and reducing mitochondrial ROS
production (68-70). A recent study reveals that SIRT3
activation by Liguzinediol alleviates gasdermin E-mediated
pyroptosis by suppressing mitochondrial ROS production, which
inhibits doxorubicin-associated cardiotoxicity (71). In the progression of
atherosclerosis, melatonin inhibits NLRP3 inflammasome-mediated
macrophage pyroptosis by decreasing ROS production by activating
the SIRT3/Forkhead box O3 pathway (72). In trimethylamine-N-oxide-induced
vascular inflammation, the downregulation of SIRT3 leads to
mitochondrial ROS production by suppressing the activation of
superoxide dismutase 2, which results in NLRP3
inflammasome-mediated pyroptosis (73). Additionally, results from
molecular docking reveal that HKL can directly bind to SIRT3
protein with a low binding energy (-4.45 kcal mol−1) at
GLU371 and THR380 (74). HKL can
bind to SIRT3 and promote SIRT3 deacetylase activity by increasing
its affinity for NAD, which reduces the acetylation of
manganese-containing superoxide dismutase and reverses ROS
synthesis and cardiac hypertrophy in mice (38).
As HKL can inhibit NLRP3 inflammasome-mediated
pyroptosis by ameliorating mitochondrial dysfunction-induced
mitochondrial ROS production during IIR-induced injury, it was
hypothesized that HKL would inhibit IIR-induced pyroptosis by
regulating the SIRT3/ROS axis. In the present study, HKL treatment
notably increased the levels of SIRT3 after the H/R and IIR-induced
SIRT3 downregulation in vitro and in vivo. In
addition, knocking down SIRT3 using siRNA reduced the protective
effects of HKL on the viability and mitochondrial dysfunction of
IEC-6 cells. Knocking down SIRT3 also reversed the HKL-induced
reductions in the LDH levels and mitochondrial ROS production.
Furthermore, silencing SIRT3 in the present H/R model reversed the
HKL-induced reductions of GSDMD-N, NLRP3 and cleaved-Casp1 as well
as the reduced IL-1β and IL-18 levels. Therefore, these data
indicated that the SIRT3/NLRP3 pathway may be important for the HKL
mediated pyroptosis inhibition in IIR injury.
The findings of the present study, as well as those
of previous studies, indicated that the SIRT3 protein levels were
increased following HKL treatment (40,75,76). In addition, a previous study
revealed that treatment with HKL also increased the mRNA expression
of SIRT3 during cardiac hypertrophy (38). The results of the present study
also revealed that HKL treatment increased the SIRT3 mRNA levels in
IEC-6 cells after H/R inhibition. The mechanism underlying the
HKL-induced increase to the SIRT3 mRNA levels remains unclear,
which was a limitation of the present study. Previous studies
indicate that nuclear factor erythroid 2-related factor 2
(Nrf2)-induced SIRT3 mRNA transcription is involved in alleviating
mitochondrial dysfunction in stress conditions (77,78). In acute lung injury, HKL reverses
the LPS-induced inhibition of Nrf2, which reduces oxidative stress
and pyroptosis (47). Therefore,
these findings indicated that HKL may promote SIRT3 mRNA
transcription by regulating Nrf2. In addition, HKL maintains the
post-translational activation of SIRT3 and upregulates the SIRT3
mRNA level by activating peroxisome proliferator-activated
receptor-γ coactivator-1α in cardiac hypertrophy (38). Therefore, to further investigate
the positive feedback mechanism regulating SIRT3 mRNA
transcription, an in-depth study is required. Another limitation of
the present study is that the mechanism of the SIRT3-regulated
mitochondrial dysfunction and mitochondrial ROS production in IIR
injury also remains unclear. The underlying regulatory mechanism
should be investigated in future studies.
In conclusion, the present study was, to the best
of our knowledge, the first to indicate that IIR-induced
mitochondrial dysfunction triggered pyroptosis via the NLRP3 axis,
and that HKL treatment may have protective effects by upregulating
SIRT3 and alleviating mitochondrial dysfunction (Fig. 8).
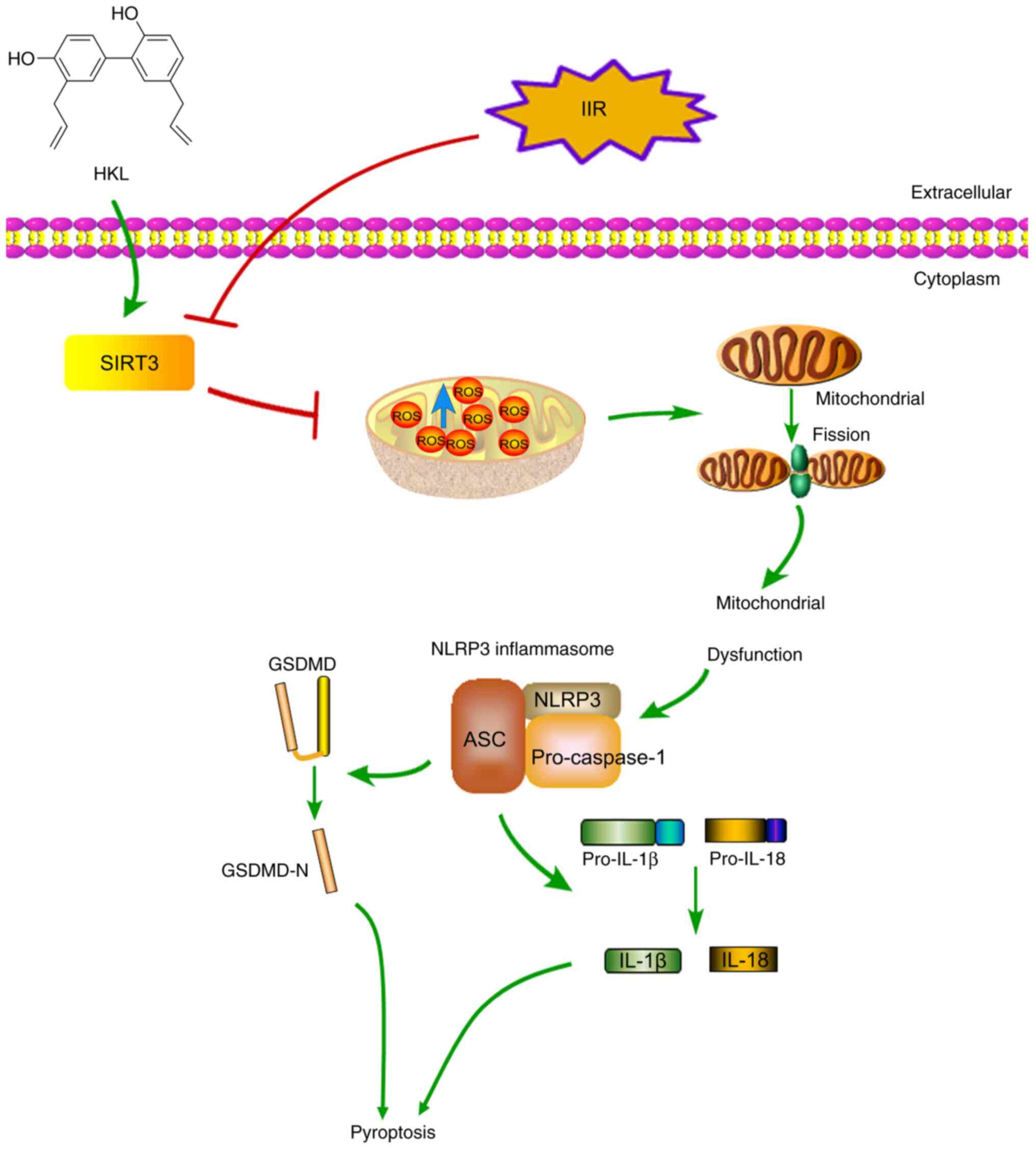 | Figure 8Schematic diagram of the suggested
protective mechanism of HKL on IIR-induced injury in rats. HKL
inhibited IIR-induced pyroptosis by alleviating mitochondrial
dysfunction through the upregulation of SIRT3 protein expression
levels. HKL, honokiol; SIRT3, sirtuin 3; GSDMD, gasdermin D;
GSDMD-N, GSDMD-N-terminal domain; NLRP3, NOD-, LRR- and pyrin
domain-containing protein 3; IL, interleukin; ROS, reactive oxygen
species; ASC, apoptosis-associated speck-like protein; IIR,
intestinal ischemia-reperfusion. |
Supplementary Data
Availability of data and materials
The data generated in the present study may be
requested from the corresponding author.
Authors' contributions
KW, CX and WG designed the study. KW, ZZ, QW and WG
performed the animal experiments. KW, WL, JP, LW and WG performed
the cell experiments. KW, CX, WL, JP and XM analyzed data. KW, CX
and WG wrote the first draft of the manuscript. WL, JP, ZZ, QW, CX,
LW, XM and WG revised the manuscript. KW, CX and WG confirm the
authenticity of all the raw data. All authors read and approved the
final version of the manuscript.
Ethics approval and consent to
participate
The animal experimental protocols were approved by
the Ethics Committee of Southwest Medical University (Luzhou,
China; approval no. 20221128-001).
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
Acknowledgments
Not applicable.
Funding
This study was supported by the Sichuan Provincial
Administration of Traditional Chinese Medicine 2023 Traditional
Chinese Medicine Research Special Project (grant no. 2023MS038),
the Sichuan Provincial Administration of Traditional Chinese
Medicine 2024 Traditional Chinese Medicine Research Special Project
(grant no. 2024MS151) and the Southwest Medical University
Integrated Traditional Chinese and Western Medicine Special Project
(grant no. 2023ZYYJ07).
References
|
1
|
Jin B, Li G, Zhou L and Fan Z: Mechanism
involved in acute liver injury induced by intestinal
Ischemia-reperfusion. Front Pharmacol. 13:9246952022. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Tassopoulos A, Chalkias A, Papalois A,
Iacovidou N and Xanthos T: The effect of antioxidant
supplementation on bacterial translocation after intestinal
ischemia and reperfusion. Redox Rep. 22:1–9. 2017. View Article : Google Scholar
|
|
3
|
Taylor LM Jr and Moneta GL: Intestinal
ischemia. Ann Vasc Surg. 5:403–406. 1991. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Slone EA and Fleming SD: Membrane lipid
interactions in intestinal ischemia/reperfusion-induced Injury.
Clin Immunol. 153:228–240. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Stamatakos M, Stefanaki C, Mastrokalos D,
Arampatzi H, Safioleas P, Chatziconstantinou C, Xiromeritis C and
Safioleas M: Mesenteric ischemia: Still a deadly puzzle for the
medical community. Tohoku J Exp Med. 216:197–204. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Li G, Wang S and Fan Z: Oxidative stress
in intestinal ischemia-reperfusion. Front Med (Lausanne).
8:7507312022. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Zhang X, Wu J, Liu Q, Li X, Li S, Chen J,
Hong Z, Wu X, Zhao Y and Ren J: mtDNA-STING pathway promotes
necroptosis-dependent enterocyte injury in intestinal ischemia
reperfusion. Cell Death Dis. 11:10502020. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Subramanian S, Geng H and Tan XD: Cell
death of intestinal epithelial cells in intestinal diseases. Sheng
Li Xue Bao. 72:308–324. 2020.PubMed/NCBI
|
|
9
|
Li Y, Feng D, Wang Z, Zhao Y, Sun R, Tian
D, Liu D, Zhang F, Ning S, Yao J and Tian X: Ischemia-induced ACSL4
activation contributes to ferroptosis-mediated tissue injury in
intestinal ischemia/reperfusion. Cell Death Differ. 26:2284–2299.
2019. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Wei Y, Yang L, Pandeya A, Cui J, Zhang Y
and Li Z: Pyroptosis-induced inflammation and tissue damage. J Mol
Biol. 434:1673012022. View Article : Google Scholar :
|
|
11
|
Rao Z, Zhu Y, Yang P, Chen Z, Xia Y, Qiao
C, Liu W, Deng H, Li J, Ning P, et al: Pyroptosis in inflammatory
diseases and cancer. Theranostics. 12:4310–4329. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Ji X, Tian L, Niu S, Yao S and Qu C:
Trimethylamine N-oxide promotes demyelination in spontaneous
hypertension rats through enhancing pyroptosis of oligodendrocytes.
Front Aging Neurosci. 14:9638762022. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Wang Y, Chen J, Zheng Y, Jiang J, Wang L,
Wu J, Zhang C and Luo M: Glucose metabolite methylglyoxal induces
vascular endothelial cell pyroptosis via NLRP3 inflammasome
activation and oxidative stress in vitro and in vivo. Cell Mol Life
Sci. 81:4012024. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Vasudevan SO, Behl B and Rathinam VA:
Pyroptosis-induced inflammation and tissue damage. Semin Immunol.
69:1017812023. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Guo Q, Wu Y, Hou Y, Liu Y, Liu T, Zhang H,
Fan C, Guan H, Li Y, Shan Z and Teng W: Cytokine secretion and
pyroptosis of thyroid follicular cells mediated by enhanced NLRP3,
NLRP1, NLRC4, and AIM2 inflammasomes are associated with autoimmune
thyroiditis. Front Immunol. 9:11972018. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Karki R, Sharma BR, Tuladhar S, Williams
EP, Zalduondo L, Samir P, Zheng M, Sundaram B, Banoth B, Malireddi
RKS, et al: Synergism of TNF-α and IFN-γ triggers inflammatory cell
death, tissue damage, and mortality in SARS-CoV-2 infection and
cytokine shock syndromes. Cell. 184:149–168.e17. 2021. View Article : Google Scholar
|
|
17
|
Li Y, Yuan Y, Huang ZX, Chen H, Lan R,
Wang Z, Lai K, Chen H, Chen Z, Zou Z, et al: GSDME-mediated
pyroptosis promotes inflammation and fibrosis in obstructive
nephropathy. Cell Death Differ. 28:2333–2350. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Coll RC, Schroder K and Pelegrín P: NLRP3
and pyroptosis blockers for treating inflammatory diseases. Trends
Pharmacol Sci. 43:653–668. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Bai B, Yang Y, Wang Q, Li M, Tian C, Liu
Y, Aung LHH, Li PF, Yu T and Chu XM: NLRP3 inflammasome in
endothelial dysfunction. Cell Death Dis. 11:7762020. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Huang Y, Xu W and Zhou R: NLRP3
inflammasome activation and cell death. Cell Mol Immunol.
18:2114–2127. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Zhang L, Ai C, Bai M, Niu J and Zhang Z:
NLRP3 Inflammasome/Pyroptosis: A key driving force in diabetic
cardiomyopathy. Int J Mol Sci. 23:106322022. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Kovacs SB and Miao EA: Gasdermins:
Effectors of pyroptosis. Trends Cell Biol. 27:673–684. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Jia Y, Cui R, Wang C, Feng Y, Li Z, Tong
Y, Qu K, Liu C and Zhang J: Metformin protects against intestinal
ischemia-reperfusion injury and cell pyroptosis via
TXNIP-NLRP3-GSDMD pathway. Redox Biol. 32:1015342020. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Li W, Yang K, Li B, Wang Y, Liu J, Chen D
and Diao Y: Corilagin alleviates intestinal
ischemia/reperfusion-induced intestinal and lung injury in mice via
inhibiting NLRP3 inflammasome activation and pyroptosis. Front
Pharmacol. 13:10601042022. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Chen Y, Han B, Guan X, Du G, Sheng B, Tang
X, Zhang Q, Xie H, Jiang X, Tan Q, et al: Enteric fungi protect
against intestinal ischemia-reperfusion injury via inhibiting the
SAA1-GSDMD pathway. J Adv Res. 61:223–237. 2024. View Article : Google Scholar
|
|
26
|
Hu Q, Ren J, Li G, Wu J, Wu X, Wang G, Gu
G, Ren H, Hong Z and Li J: The mitochondrially targeted antioxidant
MitoQ protects the intestinal barrier by ameliorating mitochondrial
DNA damage via the Nrf2/ARE signaling pathway. Cell Death Dis.
9:4032018. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Liao S, Luo J, Kadier T, Ding K, Chen R
and Meng Q: Mitochondrial DNA release contributes to intestinal
ischemia/reperfusion injury. Front Pharmacol. 13:8549942022.
View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Zhang Y, Lv Y, Zhang Q, Wang X, Han Q,
Liang Y, He S, Yuan Q, Zheng J, Xu C, et al: ALDH2 attenuates
myocardial pyroptosis through breaking down Mitochondrion-NLRP3
inflammasome pathway in septic shock. Front Pharmacol.
14:11258662023. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Cheon SY, Kim MY, Kim J, Kim EJ, Kam EH,
Cho I, Koo BN and Kim SY: Hyperammonemia induces microglial NLRP3
inflammasome activation via mitochondrial oxidative stress in
hepatic encephalopathy. Biomed J. 46:1005932023. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Li Z, Wang B, Tian L, Zheng B, Zhao X and
Liu R: Methane-rich saline suppresses ER-mitochondria contact and
activation of the NLRP3 inflammasome by regulating the PERK
signaling pathway to ameliorate intestinal Ischemia-reperfusion
injury. Inflammation. 47:376–389. 2024. View Article : Google Scholar
|
|
31
|
Mao H, Zhang Y, Xiong Y, Zhu Z, Wang L and
Liu X: Mitochondria-targeted antioxidant mitoquinone maintains
mitochondrial homeostasis through the Sirt3-dependent pathway to
mitigate oxidative damage caused by renal ischemia/reperfusion.
Oxid Med Cell Longev. 2022:22135032022. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Zhang Q, Liu XM, Hu Q, Liu ZR, Liu ZY,
Zhang HG, Huang YL, Chen QH, Wang WX and Zhang XK: Dexmedetomidine
inhibits mitochondria damage and apoptosis of enteric glial cells
in experimental intestinal ischemia/reperfusion injury via
SIRT3-dependent PINK1/HDAC3/p53 pathway. J Transl Med. 19:4632021.
View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Shen X, Shi H, Chen X, Han J, Liu H, Yang
J, Shi Y and Ma J: Esculetin alleviates inflammation, oxidative
stress and apoptosis in intestinal ischemia/reperfusion injury via
targeting SIRT3/AMPK/mTOR signaling and regulating autophagy. J
Inflamm Res. 16:3655–3667. 2023. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Wang Z, Sun R, Wang G, Chen Z, Li Y, Zhao
Y, Liu D, Zhao H, Zhang F, Yao J, et al: SIRT3-mediated
deacetylation of PRDX3 alleviates mitochondrial oxidative damage
and apoptosis induced by intestinal ischemia/reperfusion injury.
Redox Biol. 28:1013432020. View Article : Google Scholar
|
|
35
|
Chen HH, Chang PC, Chen C and Chan MH:
Protective and therapeutic activity of honokiol in reversing motor
deficits and neuronal degeneration in the mouse model of
Parkinson's disease. Pharmacol Rep. 70:668–676. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Huang K, Chen Y, Zhang R, Wu Y, Ma Y, Fang
X and Shen S: Honokiol induces apoptosis and autophagy via the
ROS/ERK1/2 signaling pathway in human osteosarcoma cells in vitro
and in vivo. Cell Death Dis. 9:1572018. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Zhou Y, Tang J, Lan J, Zhang Y, Wang H,
Chen Q, Kang Y, Sun Y, Feng X, Wu L, et al: Honokiol alleviated
neurodegeneration by reducing oxidative stress and improving
mitochondrial function in mutant SOD1 cellular and mouse models of
amyotrophic lateral sclerosis. Acta Pharm Sin B. 13:577–597. 2023.
View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Pillai VB, Samant S, Sundaresan NR,
Raghuraman H, Kim G, Bonner MY, Arbiser JL, Walker DI, Jones DP,
Gius D and Gupta MP: Honokiol blocks and reverses cardiac
hypertrophy in mice by activating mitochondrial Sirt3. Nat Commun.
6:66562015. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Zhang Y, Wen P, Luo J, Ding H, Cao H, He
W, Zen K, Zhou Y, Yang J and Jiang L: Sirtuin 3 regulates
mitochondrial protein acetylation and metabolism in tubular
epithelial cells during renal fibrosis. Cell Death Dis. 12:8472021.
View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Mao RW, He SP, Lan JG and Zhu WZ: Honokiol
ameliorates cisplatin-induced acute kidney injury via inhibition of
mitochondrial fission. Br J Pharmacol. 179:3886–3904. 2022.
View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Gao Y, Chen H, Cang X, Chen H, Di Y, Qi J,
Cai H, Luo K and Jin S: Transplanted hair follicle mesenchymal stem
cells alleviated small intestinal ischemia-reperfusion injury via
intrinsic and paracrine mechanisms in a rat model. Front Cell Dev
Biol. 10:10165972022. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Almoiliqy M, Wen J, Xu B, Sun YC, Lian MQ,
Li YL, Qaed E, Al-Azab M, Chen DP, Shopit A, et al: Cinnamaldehyde
protects against rat intestinal ischemia/reperfusion injuries by
synergistic inhibition of NF-κB and p53. Acta Pharmacol Sin.
41:1208–1222. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Zhang B, Zhai M, Li B, Liu Z, Li K, Jiang
L, Zhang M, Yi W, Yang J, Yi D, et al: Honokiol ameliorates
myocardial ischemia/reperfusion injury in type 1 diabetic rats by
reducing oxidative stress and apoptosis through activating the
SIRT1-Nrf2 signaling pathway. Oxid Med Cell Longev.
2018:31598012018. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Zheng J, Shi L, Liang F, Xu W, Li T, Gao
L, Sun Z, Yu J and Zhang J: Sirt3 ameliorates oxidative stress and
mitochondrial dysfunction after intracerebral hemorrhage in
diabetic rats. Front Neurosci. 12:4142018. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Lv L, Kong Q, Li Z and Zhang Y, Chen B, Lv
L and Zhang Y: Honokiol provides cardioprotection from myocardial
ischemia/reperfusion injury (MI/RI) by inhibiting mitochondrial
apoptosis via the PI3K/AKT signaling pathway. Cardiovasc Ther.
2022:10016922022. View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Cong R, Sun L, Yang J, Cui H, Ji X, Zhu J,
Gu JH and He B: Protein O-GlcNAcylation alleviates small intestinal
injury induced by ischemia-reperfusion and oxygen-glucose
deprivation. Biomed Pharmacother. 138:1114772021. View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Han X, Yao W, Liu Z, Li H, Zhang ZJ, Hei Z
and Xia Z: Lipoxin A4 preconditioning attenuates intestinal
ischemia reperfusion injury through keap1/Nrf2 pathway in a lipoxin
A4 receptor independent manner. Oxid Med Cell Longev.
2016:93036062016. View Article : Google Scholar : PubMed/NCBI
|
|
48
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(-Delta Delta C(T)) method. Methods. 25:402–408. 2001.
View Article : Google Scholar
|
|
49
|
Li Y, Xu B, Xu M, Chen D, Xiong Y, Lian M,
Sun Y, Tang Z, Wang L, Jiang C and Lin Y: 6-Gingerol protects
intestinal barrier from ischemia/reperfusion-induced damage via
inhibition of p38 MAPK to NF-κB signalling. Pharmacol Res.
119:137–148. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
50
|
Liu Y, Zhou J, Luo Y, Li J, Shang L, Zhou
F and Yang S: Honokiol alleviates LPS-induced acute lung injury by
inhibiting NLRP3 inflammasome-mediated pyroptosis via Nrf2
activation in vitro and in vivo. Chin Med. 16:1272021. View Article : Google Scholar : PubMed/NCBI
|
|
51
|
Qiu L, Xu R, Wang S, Li S, Sheng H, Wu J
and Qu Y: Honokiol ameliorates endothelial dysfunction through
suppression of PTX3 expression, a key mediator of IKK/IκB/NF-κB, in
atherosclerotic cell model. Exp Mol Med. 47:e1712015. View Article : Google Scholar
|
|
52
|
Wang Y, Shi P, Chen Q, Huang Z, Zou D,
Zhang J, Gao X and Lin Z: Mitochondrial ROS promote macrophage
pyroptosis by inducing GSDMD oxidation. J Mol Cell Biol.
11:1069–1082. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
53
|
Zheng Z, Bian Y, Zhang Y, Ren G and Li G:
Metformin activates AMPK/SIRT1/NF-κB pathway and induces
mitochondrial dysfunction to drive caspase3/GSDME-mediated cancer
cell pyroptosis. Cell Cycle. 19:1089–1104. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
54
|
Feng WQ, Zhang YC, Xu ZQ, Yu SY, Huo JT,
Tuersun A, Zheng MH, Zhao JK, Zong YP and Lu AG: IL-17A-mediated
mitochondrial dysfunction induces pyroptosis in colorectal cancer
cells and promotes CD8 + T-cell tumour infiltration. J Transl Med.
21:3352023. View Article : Google Scholar
|
|
55
|
Zhang J, Xiang H, Liu J, Chen Y, He RR and
Liu B: Mitochondrial Sirtuin 3: New emerging biological function
and therapeutic target. Theranostics. 10:8315–8342. 2020.
View Article : Google Scholar : PubMed/NCBI
|
|
56
|
Park EJ, Dusabimana T, Je J, Jeong K, Yun
SP, Kim HJ, Kim H and Park SW: Honokiol protects the kidney from
renal ischemia and reperfusion injury by upregulating the
glutathione biosynthetic enzymes. Biomedicines. 8:3522020.
View Article : Google Scholar : PubMed/NCBI
|
|
57
|
Xu G, Dong R, Liu J, Zhao L, Zeng Y, Xiao
X, An J, Huang S, Zhong Y, Guang B and Yang T: Synthesis,
characterization and in vivo evaluation of honokiol bisphosphate
prodrugs protects against rats' brain ischemia-reperfusion injury.
Asian J Pharm Sci. 14:640–648. 2019.
|
|
58
|
Zheng Y, Xu X, Chi F and Cong N:
Pyroptosis: A newly discovered therapeutic target for
ischemia-reperfusion injury. Biomolecules. 12:16252022. View Article : Google Scholar : PubMed/NCBI
|
|
59
|
Li J, Xu P, Hong Y, Xie Y, Peng M, Sun R,
Guo H, Zhang X, Zhu W, Wang J and Liu X: Lipocalin-2-mediated
astrocyte pyroptosis promotes neuroinflammatory injury via NLRP3
inflammasome activation in cerebral ischemia/reperfusion injury. J
Neuroinflammation. 20:1482023. View Article : Google Scholar : PubMed/NCBI
|
|
60
|
Ma Q, Xu M, Jing X, Qiu J, Huang S, Yan H,
Yin L, Lou J, Zhao L, Fan Y and Qiu P: Honokiol suppresses the
aberrant interactions between renal resident macrophages and
tubular epithelial cells in lupus nephritis through the
NLRP3/IL-33/ST2 axis. Cell Death Dis. 14:1742023. View Article : Google Scholar : PubMed/NCBI
|
|
61
|
Feng Y, Li M, Yangzhong X, Zhang X, Zu A,
Hou Y, Li L and Sun S: Pyroptosis in inflammation-related
respiratory disease. J Physiol Biochem. 78:721–737. 2022.
View Article : Google Scholar : PubMed/NCBI
|
|
62
|
Zheng D, Liu J, Piao H, Zhu Z, Wei R and
Liu K: ROS-triggered endothelial cell death mechanisms: Focus on
pyroptosis, parthanatos, and ferroptosis. Front Immunol.
13:10392412022. View Article : Google Scholar : PubMed/NCBI
|
|
63
|
Tuersuntuoheti M, Peng F, Li J, Zhou L,
Gao H and Gong H: PLCE1 enhances mitochondrial dysfunction to
promote GSDME-mediated pyroptosis in doxorubicin-induced
cardiotoxicity. Biochem Pharmacol. 223:1161422024. View Article : Google Scholar : PubMed/NCBI
|
|
64
|
Kerr M, Miller JJ, Thapa D, Stiewe S, Timm
KN, Aparicio CNM, Scott I, Tyler DJ and Heather LC: Rescue of
myocardial energetic dysfunction in diabetes through the correction
of mitochondrial hyperacetylation by honokiol. JCI Insight.
5:e1403262020. View Article : Google Scholar : PubMed/NCBI
|
|
65
|
Ji Z, Liu GH and Qu J: Mitochondrial
sirtuins, metabolism, and aging. J Genet Genomics. 49:287–298.
2022. View Article : Google Scholar
|
|
66
|
Dikalov S and Dikalova A: Mitochondrial
deacetylase Sirt3 in vascular dysfunction and hypertension. Curr
Opin Nephrol Hypertens. 31:151–156. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
67
|
Shen Y, Wu Q, Shi J and Zhou S: Regulation
of SIRT3 on mitochondrial functions and oxidative stress in
Parkinson's disease. Biomed Pharmacother. 132:1109282020.
View Article : Google Scholar : PubMed/NCBI
|
|
68
|
Wang X, Shen T, Lian J, Deng K, Qu C, Li
E, Li G, Ren Y, Wang Z, Jiang Z, et al: Resveratrol reduces
ROS-induced ferroptosis by activating SIRT3 and compensating the
GSH/GPX4 pathway. Mol Med. 29:1372023. View Article : Google Scholar : PubMed/NCBI
|
|
69
|
Xie X, Xu Q and Zhou D: Sirtuin-3
activates the mitochondrial unfolded protein response and reduces
cerebral ischemia/reperfusion injury. Int J Biol Sci. 19:4327–4339.
2023. View Article : Google Scholar
|
|
70
|
Zhao H, Luo Y, Chen L, Zhang Z, Shen C, Li
Y and Xu R: Sirt3 inhibits cerebral ischemia-reperfusion injury
through normalizing Wnt/β-catenin pathway and blocking
mitochondrial fission. Cell Stress Chaperones. 23:1079–1092. 2018.
View Article : Google Scholar : PubMed/NCBI
|
|
71
|
Zhu W, Lian N, Wang J, Zhao F, Liu B,
Sheng J, Zhang C, Zhou X, Gao W, Xie C, et al: Liguzinediol
potentiates the metabolic remodeling by activating the AMPK/SIRT3
pathway and represses Caspase-3/GSDME-mediated pyroptosis to
ameliorate cardiotoxicity. Chin Med. 19:852024. View Article : Google Scholar : PubMed/NCBI
|
|
72
|
Cong L, Liu X, Bai Y, Qin Q, Zhao L, Shi
Y, Bai Y and Guo Z: Melatonin alleviates pyroptosis by regulating
the SIRT3/FOXO3α/ROS axis and interacting with apoptosis in
Atherosclerosis progression. Biol Res. 56:622023. View Article : Google Scholar
|
|
73
|
Chen ML, Zhu XH, Ran L, Lang HD, Yi L and
Mi MT: Trimethylamine-N-Oxide induces vascular inflammation by
activating the NLRP3 inflammasome through the SIRT3-SOD2-mtROS
signaling pathway. J Am Heart Assoc. 6:e0063472017. View Article : Google Scholar : PubMed/NCBI
|
|
74
|
Zhang Y, Liu Y, Hou M, Xia X, Liu J, Xu Y,
Shi Q, Zhang Z, Wang L, Shen Y, et al: Reprogramming of
Mitochondrial Respiratory Chain Complex by Targeting SIRT3-COX4I2
Axis Attenuates Osteoarthritis Progression. Adv Sci (Weinh).
10:e22061442023. View Article : Google Scholar : PubMed/NCBI
|
|
75
|
Chang Y, Wang C, Zhu J, Zheng S, Sun S, Wu
Y, Jiang X, Li L, Ma R and Li G: SIRT3 ameliorates
diabetes-associated cognitive dysfunction via regulating
mitochondria-associated ER membranes. J Transl Med. 21:4942023.
View Article : Google Scholar : PubMed/NCBI
|
|
76
|
Zheng X, Gao J, Zhao M, Han L, Zhang D,
Wang K and Cui J: Honokiol attenuates mitochondrial fission and
cell apoptosis by activating Sirt3 in intracerebral hemorrhage.
Chin Med J (Engl). 136:719–731. 2023. View Article : Google Scholar : PubMed/NCBI
|
|
77
|
Yao Y, Ren Z, Yang R, Mei Y, Dai Y, Cheng
Q, Xu C, Xu X, Wang S, Kim KM, et al: Salidroside reduces
neuropathology in Alzheimer's disease models by targeting
NRF2/SIRT3 pathway. Cell Biosci. 12:1802022. View Article : Google Scholar : PubMed/NCBI
|
|
78
|
Li Y, Feng L, Xie D, Luo Y, Lin M, Gao J,
Zhang Y, He Z, Zhu YZ and Gong Q: Icariside II mitigates myocardial
infarction by balancing mitochondrial dynamics and reducing
oxidative stress through the activation of Nrf2/SIRT3 signaling
pathway. Eur J Pharmacol. 956:1759872023. View Article : Google Scholar : PubMed/NCBI
|






















