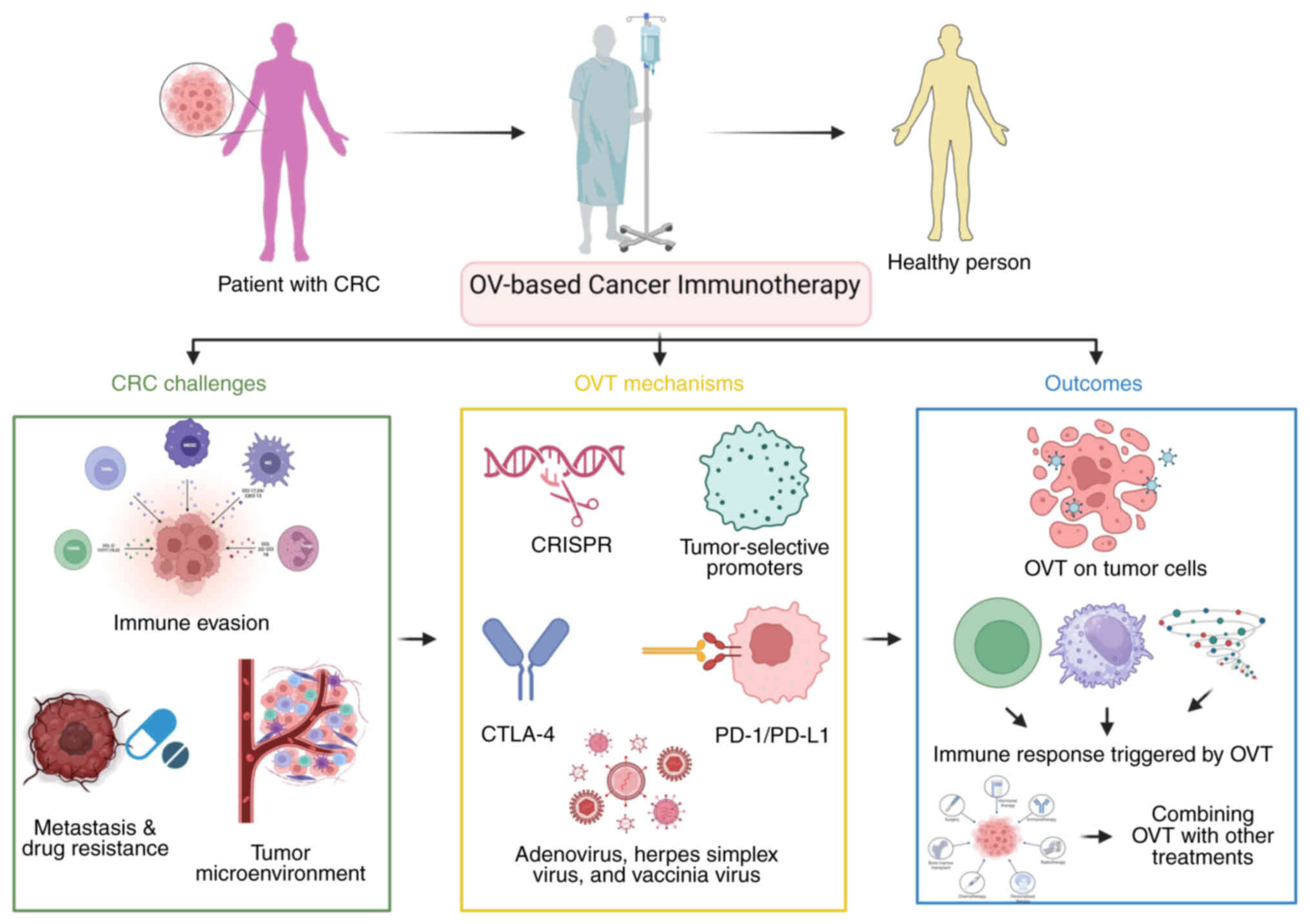
Colorectal cancer (CRC) is one of the most prevalent
cancer types worldwide, and was observed to affect 1.9 million
individuals in 2020 and led to ~930,000 deaths in the same year,
constituting ~10% of cancer-related deaths (1,2).
In particular, CRC incidence seems to be increasing in the younger
population; early-onset CRC cases have been growing and doubling
between 1990 and 2019, which emphasizes the importance of
increasing CRC screening and implementing culturally appropriate
interventions (3,4). CRC incidence is most elevated in
Oceania, including Australia and New Zealand, Europe and in at
least some African areas (2).
At present, CRC treatments are based on the disease
stage and include surgery, systemic chemotherapy, targeted therapy
and radiotherapy. The most distinctive cases of CRC are those that
present with hepatic, pulmonary or peritoneal metastases, for which
radical surgery, hepatic/pulmonary resections and cytoreductive
surgery associated with hyperthermic intraperitoneal chemotherapy
are helpful (5-7). Furthermore, bevacizumab and
cetuximab as systemic therapies add only 3-6 months of survival to
patients with metastatic CRC, and high rates of relapse are still
observed (8,9). Single-agent kinase inhibitors
selective for pathways such as PI3K/Akt/mTOR have proven suboptimal
due to cross-talk between these pathways and poor safety profiles
(10). The specificity of
traditional treatments, such as chemotherapy and radiotherapy, is
very low; they cause significant side effects, and despite
treatment with neoadjuvant therapy, there is a chance of recurrence
in 54% of patients (11-16). Such issues indicate the
importance of a new approach, such as oncolytic viral therapy
(OVT). OVs specifically target and destroy tumor cells and
stimulate strong antitumor immune responses (17-19). One of the most promising
developments is the Food and Drug Administration-approved oncolytic
herpes simplex virus type 1 (HSV-1) for treating melanoma.
Additionally, there is growing interest in the consistent and
clinically significant data reported for the use of vaccinia virus
in treating solid carcinomas (20).
The ability to directly lyse tumors and modulate the
immune response makes OVT worthwhile. For instance, recent
advancements in OVT have shown significant promise in treating CRC.
Notably, the oncolytic vaccinia virus, Pexa-Vec, has been evaluated
in clinical trials specifically targeting patients with refractory
metastatic CRC (21). Early
detection through saliva biomarkers can enhance patient selection
for OVT, allowing timely intervention and improved treatment
success. Understanding oral microbiota may also help optimize OVT
protocols for personalized care (22).
An improved matrix stiffness stress in the TME
restrains immune cell infiltration and immunotherapy
responsiveness; however, it is poorly understood in CRC (23-26). Increasing the understanding of
the mechanisms behind extracellular matrix (ECM) biomechanics could
improve the effectiveness of immune checkpoint blockade (ICB)
therapy and adoptive cell therapy (ACT) (27,28). OVT boosts the upregulation of
tumor-associated antigens (TAAs) and cytokines, thereby enhancing
the effectiveness of ICB. This is especially valuable in tumors
with challenging immune microenvironments, such as
microsatellite-stable (MSS) CRC, which typically shows limited
response to immunotherapy (28-30). Engineered OVs, such as OH2,
demonstrate enhanced specificity and oncolytic potency due to
targeted gene manipulations, particularly when compared with
unmodified or naturally occurring OVs (29). Together with immunotherapy, OVT
has been reported to produce only mild side effects and high rates
of CD8+ T-cell infiltration in other cancer types such
as melanoma and epithelial tumors, including colorectal, bladder
and renal cancer (30,31). Recent research has revealed new
approaches to treating CRC by teasing out the potential
interactions between OVT, immunotherapy and the mechanical
properties of tumors (32). More
specifically, it has shed light on several general strategies to
possibly optimize ICBs, ACTs and tumor cell vaccines for the
treatment of CRC, including the deconstruction of ECM-related
hurdles that prevent OVT strategies from reaching target cancer
cells and improving the existing OVT strategies tailored to each
patient (33).
However, in MSS CRC, the aforementioned improvements
have not yielded long-lasting responses, and the highly
immunosuppressive TME presents unique obstacles that warrant novel
solutions. OVT has been identified as a promising strategy as the
oncolytic capabilities are congruent with the immunomodulatory
properties. However, there are several obstacles to improve virus
delivery, tumor escape mechanisms and the incorporation of OVT into
combined treatment strategies (34). The present review fills the gap
regarding the lack of a comprehensive synthesis of how recent
innovations in genetic engineering, biomarkers and immune
modulation are being translated into clinical applications for OVT
in CRC. This includes addressing barriers such as tumor
heterogeneity, delivery limitations and the integration of OVT with
other therapies, which have hindered broader adoption and
optimization of this treatment modality. A graphical overview of
precision OVT in CRC is provided in Fig. 1. The present review will also
address key questions to develop a roadmap for precision OVT in
CRC, highlighting technological advancements that could transform
the current treatment landscape. The present review discusses not
only the application of engineered OVs in the treatment of immune
suppression and drug resistance but also underscores the potential
of OVT to transform cancer therapy by investigating its value in
becoming a fundamental anticancer weapon.
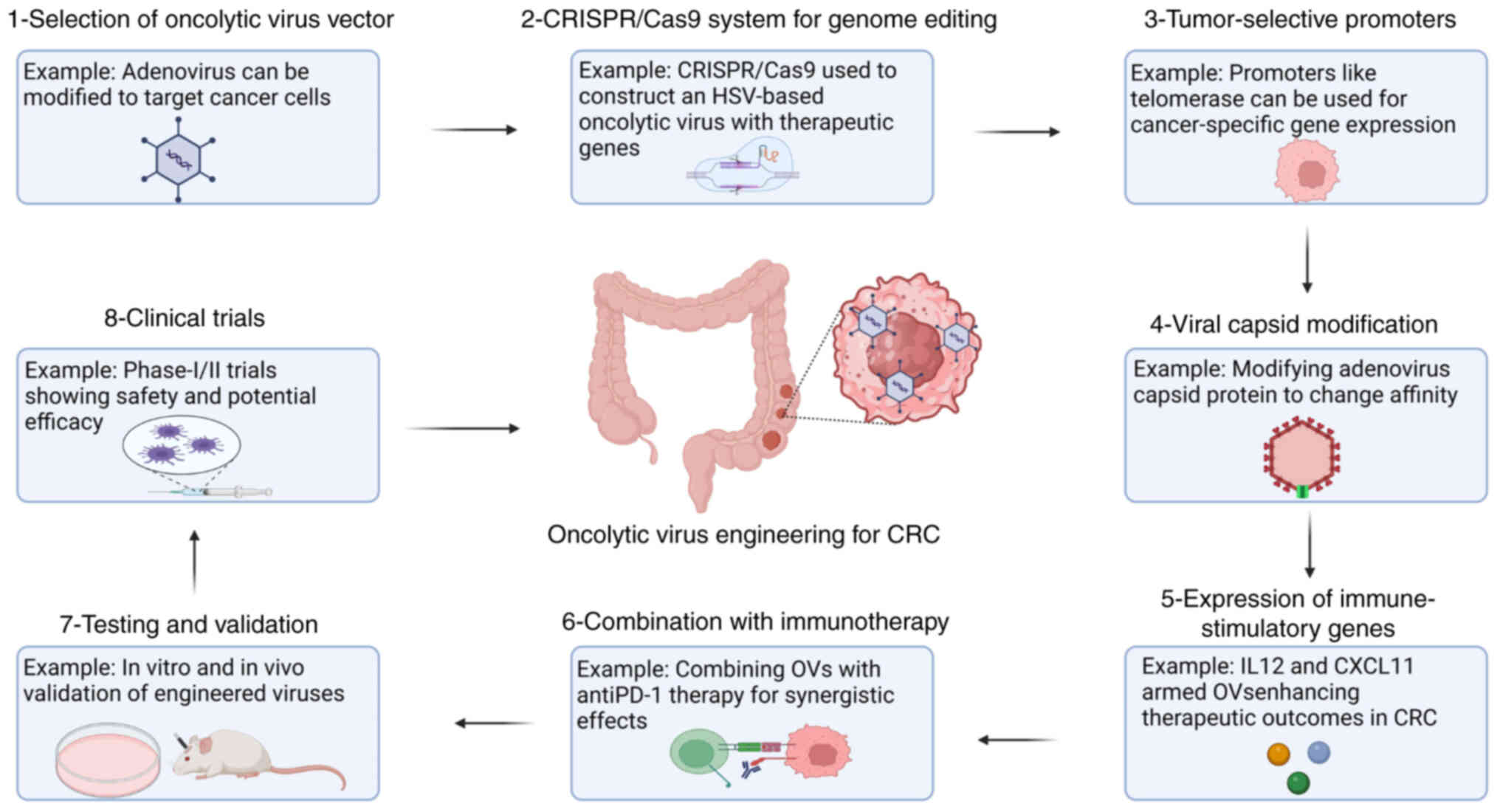
The selection of optimal OVs relies on key criteria
including tumor specificity, replication efficiency and
immune-stimulating capabilities. Genetic engineering approaches,
such as receptor targeting and promoter-driven tropism, enhance
viral selectivity for cancer cells while sparing healthy tissues.
Preclinical screening further refines candidates based on safety
profiles, oncolytic potency and synergy with existing therapies
(35).
For precision OVT in CRC, a virus is designed to
infect and kill cancer cells but not healthy cells. One of the
criteria for selection is replication preference in tumor cells.
For example, TG6002, which was manufactured using the genetically
modified vaccinia virus, has selected replication in cancer cells
due to viral gene deletion. TG6002 converts 5-fluorocytosine to
5-fluorouracil at the tumor site, improving localized therapeutic
effects (36). Similarly,
different types of adenoviruses, such as Ad-PE, int and ins-GCV,
with fabricated integrin-targeting peptides have improved
transduction efficiency and therapeutic activity compared with
controls (37).
In addition to replication preference, other
critical criteria should be considered when selecting OVs for CRC.
First, tumor specificity is paramount; OVs must selectively target
cancer cells while sparing normal tissues to minimize off-target
effects (38). This can be
achieved through genetic modifications that exploit unique TME
features, such as hypoxia or altered receptor expression (39). Second, the ability of OVs to
modulate the immune system is essential. Beyond direct oncolysis,
OVs can stimulate both innate and adaptive immune responses,
generating long-term antitumor immunity (40). Third, safety remains a key
concern, particularly for systemic delivery, where risks of
neutralization by circulating antibodies or off-target infection
must be mitigated (41).
Finally, the potential for combination therapies, such as pairing
OVs with immune checkpoint inhibitors (ICIs) or chemotherapy,
should be evaluated to enhance therapeutic efficacy and overcome
resistance mechanisms (42).
OVs cause tumor cell shedding and immunogenic death,
releasing TAAs into the immune system. This immune activation
combines synergistically with checkpoint inhibitors or T-cell
engagers, as illustrated in preclinical models of CRC (43). The gut microbiome plays a
significant role in CRC progression, and its modulation can
influence the efficacy of immunotherapies, including OVs. Emerging
evidence suggests that specific gut microbiota compositions may
predict treatment response and modulate the anticancer immune
response, although the exact mechanisms remain an active area of
research (44).
OVT has emerged as a promising modality for cancer
treatment, particularly in CRC, where OVs selectively infect and
lyse tumor cells while sparing normal tissues (45). To enhance the efficacy of OVT,
localized delivery systems such as imaging-guided or catheter-based
approaches can be employed to achieve optimal viral density within
the TME while minimizing systemic toxicity (46). Carrier cells, including
mesenchymal stem cells and cytotoxic immune cells such as T cells,
have been explored as 'Trojan horse' delivery vehicles to transport
OVs to tumor sites, leveraging their innate homing capabilities
(47). However, challenges such
as rapid clearance by the reticuloendothelial system and
neutralization by circulating antibodies highlight the need for
innovative strategies, such as engineering OVs to regulate
abnormalities in the TME (such as neovascularization and ECM
stiffness) or combining them with ICIs to amplify antitumor
immunity (21). Recent advances
in genetic editing, viral retargeting and nanotechnology platforms
further underscore the potential of OVs to overcome barriers to
systemic delivery and improve therapeutic outcomes (48). Non-invasive imaging techniques
also play a pivotal role in monitoring viral kinetics and ensuring
safety during treatment (49).
Together, these strategies provide a comprehensive framework for
advancing OVT in CRC.
OVs alter the tumor-associated landscape to enhance
the infiltration of immune cells and reduce the presence of
immunosuppressive cells. This promotes antitumor immune responses
and, more specifically, boosts the effects when used alongside
toxic therapies such as chemotherapy or radiotherapy. For example,
the engineered vaccinia virus, coxsackievirus B3, plus FOLFOXIRI
enhance immunogenicity and CRC survival (50-52).
The selectivity and safety of OVs are critical to
CRC management. Viruses are designed to interact with receptor
molecules upregulated in CRC cells, including CD46 and
intercellular adhesion molecule-1, improving the affinity and viral
replication (53-55). Other sites in CRC tumors,
including specific metabolic pathways and immune checkpoint
suppression, enhance viral survival and replication within an
immunosuppressive environment (56). Genetic modifications make the OVs
more selective, allowing them to target tumor cells instead of
normal cells, thereby enhancing the therapeutic ratio (21).
Genetic engineering approaches have enabled the
fine-tuning of OVs to improve tumor specificity, replication
efficiency and immunogenicity. For example, talimogene
laherparepvec (T-VEC) is an engineered oncolytic HSV-1 designed to
preferentially replicate in tumor cells while inducing antitumor
immune responses (57).
The addition of a heterologous receptor binding
domain) to OVs improves their ability to recognize a greater number
of receptors. This modification significantly enhances the tropism
of these viruses, concentrating on increasing their effectiveness
against tumor cells (58). When
it comes to therapeutic applications, engineered viruses with an
expanded ability to recognize receptors can target and eliminate a
wider range of cancer cells, enhancing their capacity to kill
tumors that might be less susceptible to viral-mediated cell
destruction; it also reduces the dissemination of the OV to the
tumor site and enhances the overall effectiveness of the treatment
process in combating cancer (59). The regulation of transcription
factors has also been studied to selectively enhance viral
replication in cancerous tissues (60).
Anti-angiogenic genes can prevent tumor
angiogenesis, limiting tumor growth and metastasis (61,62). Additionally, immunomodulatory
genes such as interleukin-12 (IL-12) and C-X-C motif chemokine
ligand 11 (CXCL11) notably enhance the effect of the immune system
on the tumor, improving the therapeutic index of OVs. IL-12
promotes T cells producing interferon-γ and improves the
recognition of tumor cells by cytotoxic T lymphocytes (CTLs) and
the intrinsic cytotoxic activity of CTLs. Furthermore, CXCL11 is a
potent chemokine that induces immune cells, including CTLs and T
helper type 1 (Th1), to infiltrate the TME and strengthen the
effective antitumor immune response (63). By combining immunomodulatory
tactics with anti-angiogenic methods that disrupt tumor
angiogenesis and deprive tumors of their blood supply, it is
possible to design a cohesive treatment strategy that addresses
both tumor growth and enhances the immune defense against cancer
(64,65).
CRISPR/Cas9 technology offers a precise and
efficient method for editing viral genomes to improve tumor
selectivity (66). For example,
a tissue-specific HSV-1 has been designed by knocking in the murine
IL-12 and CXCL11 cassettes using the ICP34.5 coding region and
knocking out the immunomodulatory gene, ICP47. This double
alteration improves the selectivity of viral replication in tumor
cells and boosts antitumor immunity against CRC. Additionally, the
armed HSV-1 features enhanced tumor selective replication and
initiates an immune response, resulting in potent antitumor
effects. IL-12 favors the Th1 cell development and CTL response,
and CXCL11 has been credited for attracting effector T cells and
natural killer (NK) cells to the tumor site (67). This coaction greatly optimizes
the functional capabilities of immune effector cells and enhances
therapeutic efficacy against tumor cells. This approach may be
considered a promising strategy for improving OVT in the treatment
of CRC (67,68).
The incorporation of telomerase and CEA gene
promoters into OVs ensures targeted viral replication and
therapeutic gene expression exclusively in cancer cells (69). This strategy significantly
reduces the likelihood of side effects by sparing normal tissues,
thereby improving the safety and efficacy of OVT for CRC (54). As a tumor marker with
significantly increased expression in colon cancer, CEA serves as
an ideal target for constructing oncolytic adenoviruses (OAVs)
(70). These viruses are
designed to selectively replicate in and lyse tumor cells while
sparing normal tissues. The CEA promoter has been successfully used
to regulate the expression of therapeutic genes in OAVs, enhancing
their specificity and efficacy against CRC (70,71,72).
OVs represent a novel antitumor strategy that
selectively targets CRC cells while leaving surrounding healthy
tissue intact. However, their clinical application is limited by
challenges related to the safety and efficacy of delivering OVs to
the target tumor tissue. To address these issues, researchers have
developed innovative methods. One approach involves engineering OVs
to carry natural microRNAs (miRNAs), which are potent regulators of
gene expression. By incorporating sequences upregulated in CRC,
miRNAs guide OVs specifically toward cancer cells, minimizing side
effects and enhancing safety (73). Another promising strategy is the
virosomal administration of interferon, where viral particles are
encapsulated in liposomes to protect them from destruction by
antibodies and complement proteins. The lipid membrane of these
liposomes can also be modified with targeting molecules, such as
antibodies or ligands, to direct the OVs to the tumor site. This
improvement significantly enhances the efficiency and effectiveness
of OV delivery (74) Key
strategies for engineering OVs are illustrated in Fig. 2. Although those strategies have
shown promising outcomes in preclinical studies, a number of
improvements and clinical trials are required to confirm their
safety and effectiveness for treating CRC (75,76).
Several types of OVs for CRC treatment are in use
today, which includes the adenoviruses, oHSV2, vaccinia viruses,
reoviruses and the Newcastle disease virus. Adenoviruses have been
highlighted for their biosafety and potential to selectively infect
and lyse cancer cells with therapeutic protein expression,
augmented by immunomodulation when combined with checkpoint
inhibitors (77). Other OVs
include oHSV2, derived from HSV-2, which has exhibited broad
potency in a study inducing CRC cell necrosis and enhancing
adaptive immune responses, improving the survival time of
tumor-bearing mice (78). The
vaccinia virus and reovirus exhibit unique characteristics,
including a heightened ability to infect tumor tissues and
selectively replicate within cancer cells. These properties make
them promising candidates for advanced virotherapy strategies aimed
at improving tumor targeting and therapeutic outcomes (79,80). Newcastle disease virus is
pinpointed for its enhanced effects when applied in conjunction
with other methods, thereby proving the capability of OVs in
improving the treatment of CRC (81,82).
The benefits of adeno-derived oncolytic therapy in
CRC arise from the selective replication of the virus in cancer
cells, sparing normal cells. This selectivity is achieved by
incorporating cancer cell-specific promoters, such as
cyclooxygenase-2, which drive viral replication specifically in
cancerous cells. Consequently, OAVs can selectively infect and kill
cancer cells, with the relative sparing of normal neighboring
tissues (83). Moreover, OAVs
may recode the TME and induce an intensive immune reaction, which
may cooperate with immunotherapies such as anti-programmed cell
death protein 1 (PD-1) (84).
Nevertheless, drawbacks include difficulties in applying high local
concentrations to distant metastatic tumors since conventional
methods may not always be sufficient. In addition, OAVs can prompt
immunogenic cell death (ICD), but the immune responses can
sometimes hinder their effectiveness. Thus, immune clearance
strategies are needed (85).
The safety of HSV, particularly the genetically
modified T-VEC, is favorable. T-VEC has been shown to have low
toxicity in a clinical trial, and side effects, if any, are
controllable. Risks and complications are frequent, but severe
adverse events are rare. The HSV OVT has been shown to explicitly
target tumor cells without affecting normal cells, which has
greatly enhanced the safety of the virus (86). HSV can be administered for
patients with advanced cancer, such as CRC (87). The vaccinia virus, including
modified strains such as JX-594, also demonstrates fairly good
safety indicators. In certain co-housing investigations, it has
been administered with fewer reported serious side effects. JX-594
does not cause severe local reactions even when used with other
chemotherapeutic agents and is compatible with standard clinical
treatment regimens (88,89). Certain research has shown that
HSV can produce systemic tumor regression in CRC-bearing animals
(90). Current clinical trials
are testing its utility when combined with other immunotherapies
and when administered as a monotherapy. Initial findings indicate
that HSV immunomodulatory capabilities can increase antitumor
immunity and have implied the effective treatment of patients with
CRC (86). Since the antitumor
activity is well-known, the vaccinia virus has shown promising
results in phase I clinical trials. For instance, Pexa-Vec has been
demonstrated to be effective in decreasing tumor size and improving
immune effects on CRC cells. Its potential in stimulating the
immune system and directly lysing cancer cells provides enough
merit to warrant more developments regarding CRC therapy (88,89).
Continually propelling the research effort on CRC
vaccines, Ad5GUCY2C-PADRE is a non-replicating plasmid-based
adenoviral vector encoding the GUCY2C antigen fused with the helper
T-cell epitope, PADRE. This adenoviral vector has been shown to
elicit vigorous cytotoxic and humoral immune responses, directly
targeting CRC cells with upregulated GUCY2C expression with little
side effects (91). However, it
was revealed that antibodies targeting the core adenoviral vector
could neutralize adenoviral vectors, which may be an issue in the
immunology of adenoviral vaccines (91). Furthermore, although the
adenoviral vector is known to elicit preexisting immunity in the
patient population, a significant drawback, the oncolytic
adenovirus Ad5 [E1-, E2b]-CEA(6D) holds promise due its ability to
induce immunogenicity against CEA (92). Intratumor influenza vaccines that
increase CD8+ T cells in proficient mismatch repair
(pMMR) CRC are being launched to treat patients (NCT04591379)
(93). Recombinant adenoviruses
can transduce antigens and present oncolytic properties that
activate CTLs (94). There is
also evidence that the Sendai virus and vesicular stomatitis virus
have oncolytic abilities in other preclinical CRC models,
preferring to infect and kill cancer cells (95). Table I summarizes various OVs,
including vaccinia, adenovirus, HSV and Newcastle disease virus,
detailing their engineered constructs, immune responses and
efficacy in preclinical CRC models. These viruses demonstrate
potent anti-tumor effects through mechanisms such as immune
activation, cytokine induction and targeted cytotoxicity.
The advancement of CRC and the continual alteration
of its state depend on mutations disrupting or impairing critical
genes that regulate cell turnover. For instance, KRAS mutations
result in the constitutive activation of RAS proteins, driving
enhanced cell division and survival, thereby promoting
tumorigenesis (96). However,
studies have also shown that APC mutations affect the regulation of
WNT signaling and hence encourage increased division of tumor
cells. Both tropism mutation types are highly associated with CRC
pathogenesis (97) and the
alteration is applicable irrespective of the stage or location of
the tumor. Additionally, loss of p53 function through mutational
inactivation of the tumor-suppressor gene TP53 hampers facets of
the CRC cell's ability to respond to DNA damage, thereby causing
the cells to continue to proliferate (96). SMAD4 loss increases tumor
invasiveness, enhances metastatic properties, induces
chemo-resistance and is associated with a poorer survival,
decreasing the overall survival of patients (98,99).
Due to its high incidence rate in CRC (estimated at
45%), KRAS mutations are considered suitable target candidates for
OVT (100). These mutations are
often associated with resistance to chemotherapy and standard
EGFR-directed therapies, the ability of cancer cells to adapt to
treatment and a poor prognosis (101). KRAS mutations play a critical
role in reprogramming cancer cell metabolism to support rapid
growth and survival. These mutations increase the dependency of
cancer cells on glutamine, an essential nutrient for energy
production and biosynthesis (102). Additionally, KRAS mutations
upregulate nuclear factor erythroid 2-related factor 2 (Nrf2)
signaling, a pathway that regulates antioxidant responses and
metabolic adaptations. By enhancing Nrf2 activity, cancer cells can
manage oxidative stress, maintain redox balance and sustain
metabolic pathways such as glycolysis and glutaminolysis. Targeting
these metabolic vulnerabilities, including glutamine metabolism and
Nrf2 signaling, offers potential therapeutic strategies to combat
KRAS-driven cancer (103,104). For example, Pelareorep (also
known as REOLYSIN), an OVT, can target and kill KRAS-mutated cells,
or any tumor cell. Furthermore, these viruses can induce autophagy
in cancer cells, which may enhance the therapeutic efficacy of this
approach compared with other treatments (105).
Genetic changes in APC and TP53 are significant in
CRC progression. Most of the APC gene abnormalities are primary to
CRC tumorigenesis, whereby the overproduction of β-catenin
stimulates cell proliferation. During the later phases of multistep
carcinogenesis, alterations in TP53 affect critical cellular
functions such as the cell cycle and apoptosis and thus promote
malignancy (106). These
genetic changes elucidate the molecular pathogenesis of CRC and
highlight their potential as effective targets for OVT (107). Adenoviruses with deletions in
the E1B 55kDa gene are OAVs that selectively infect cancer cells
with mutations or deletions in the TP53 gene. The E1B 55kDa
protein, under normal circumstances, binds to and inactivates the
p53 tumor suppressor to enhance replication. The loss of this gene
means OAVs are able to replicate only in cells where p53 function
is compromised, an attribute of numerous types of cancer (71,108,109).
Another critical target is the BRAFV600E mutation,
prevalent in dMMR or MSI-H CRC. While BRAF and MEK inhibitors have
shown success in melanoma (105) and non-small cell lung cancer
(110), CRC requires additional
EGFR blockade due to compensatory EGFR upregulation (111). Integrating targeted therapies
for BRAF mutations with OVT has the potential to improve outcomes
in these subsets of patients with CRC (112).
HER2 (also known as erbB2) amplification is
frequently detected in CRC and can serve as a prognostic and
therapeutic gene; its upregulation is related to tumor progression
and poor prognosis, as well as in breast cancer (113-115). The synergy between
HER2-targeted treatments with OVT has been noted, especially in
HER2-positive CRC subtypes (116).
Since MSI-H, dMMR and TMB are related to sensitivity
and prognosis, these biomarkers are essential for identifying
patients appropriate for immunotherapy. Patients with such features
show improved outcomes when administered ICIs common for CRC
(117). For instance, it has
been identified that CRC tumors with high TMB show increased
responsiveness to immunotherapy, as higher mutational loads
generate more neoantigens, enhancing the immune system's ability to
recognize and attack cancer cells (118). Future research on OVT
biomarkers could contribute to developing individualized
therapeutic strategies, potentially leading to higher effectiveness
in treatment outcomes.
The pro-inflammatory microenvironment of CRC also
yields potential targets for OVT. At present, options for molecular
prognostic markers for CRC, including serum IL-6, IL-8, programmed
cell death ligand 1 (PD-L1), CEA (71,119), CA19-9 and MMP-9, are still
being explored regarding their potential use in immunotherapy
(120). Additionally, PIK3CA
mutations are associated with the growth and survival of CRC,
making them effective targets for OVT. These mutations can improve
the vulnerabilities of the tumor to viral infection, which provides
a new direction for the treatment of CRC (121).
In a number of cases, the CRC TME is marked by the
infiltration of immunosuppressive immune cells, including
regulatory T cells (Tregs), MDSCs and M2 macrophages. These cells
can negatively regulate effector T cells and NK cells, reducing the
antitumor immune response (122). Tumor hypoxia, or low oxygen
tension, is a recurrent phenomenon in the TME of CRC. Hypoxia can
stimulate the release of hypoxia-inducible factor 1-α (HIF-1α),
which is involved in the formation of immunosuppressive factors and
therapy resistance (123-125). ECM components in the TME can be
degraded by enzymes such as MMPs, and this degradation can prevent
immune cells from infiltrating the tumor and promote
immunosuppressive activity (126). CRC cells can also release
PD-L1, which binds to PD-1 on the surface of T cells, thus
suppressing their functioning. The mechanism of this interaction is
one of the primary significant ways the tumor can escape the immune
response in the TME (127,128). The CRC TME can secrete
cytokines such as TGF-β, IL-10 and VEGF, which promote cancer
progression by supporting tumor growth, facilitating the formation
of new blood vessels and enabling immune system evasion.
Furthermore, metabolic changes, including glycolysis and
glutaminolysis, produce immunosuppressive metabolites and consume
nutrients required for immune cells (129).
In CRC, immune cells such as Tregs and MDSCs are
particularly relevant for immune evasion and avoidance (130). Tregs can secrete adenosine and
transfer cAMP to effector T cells, thereby suppressing their
activity. Tregs also outcompete effector T cells for IL-2,
metabolize IL-2 into its biologically inactive form, and inhibit
the production of IL-2 by dendritic cells, which are crucial for
activating effector T cells. Additionally, Tregs can suppress
effector T cells through the induction of apoptosis (131). Both Tregs and MDSCs enhance
each other's proliferation, creating a symbiotic circuit of
immunosuppression within the TME (132,133). Immune cells, such as Tregs and
MDSCs, in the microenvironment of CRC decrease the immune response
needed for the virus to act upon the cancer cells and destroy them
during OVT (134). These cells
can help neutralize the virus and suppress the initiation of
antitumor immunity, lowering the effectiveness of the therapy
(32).
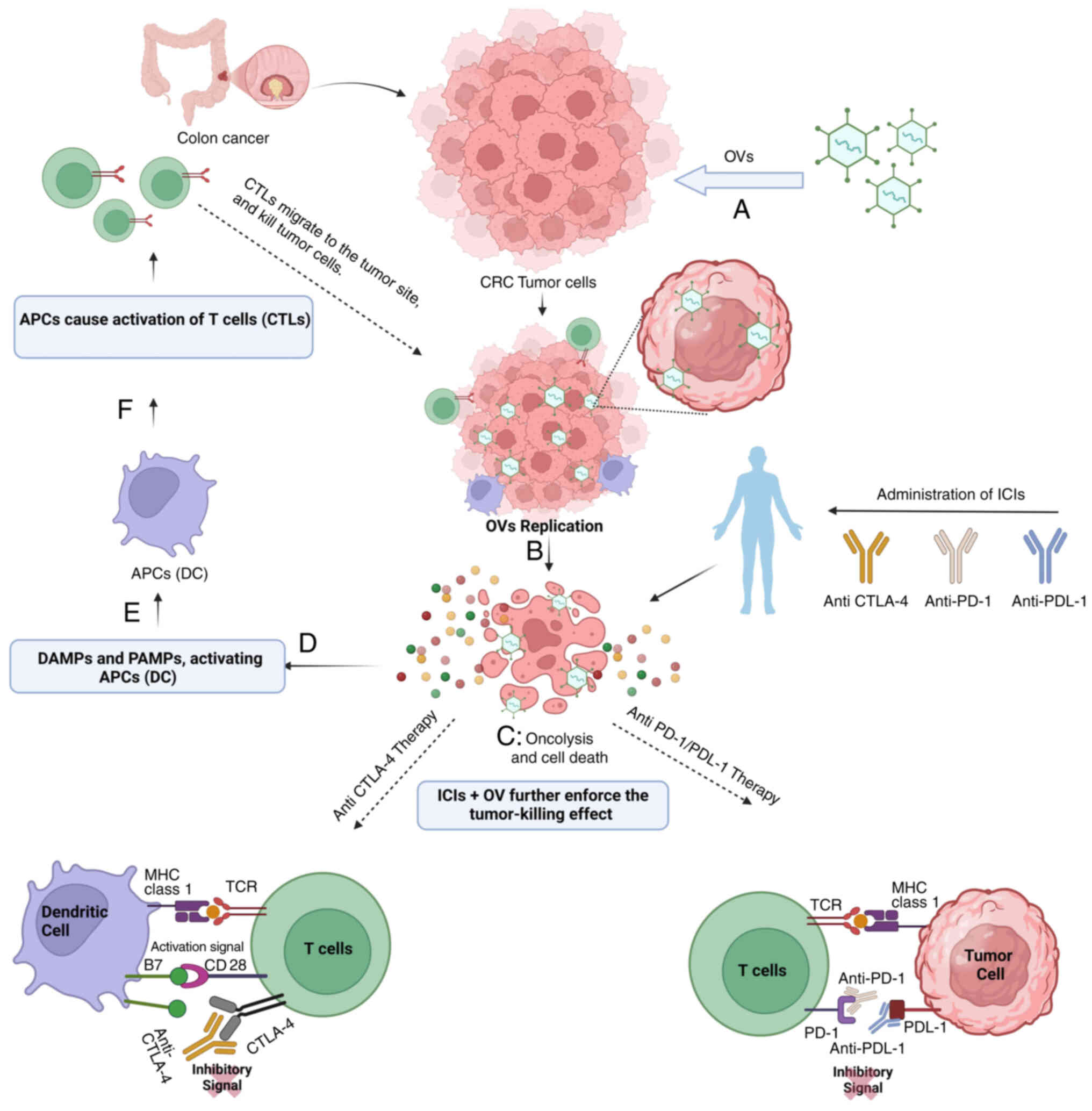
ICIs disrupt the binding between the immune
checkpoint proteins, such as PD-1/PD-L1, CTLA-4 and sTim-3
(135) and their receptors on
the tumor or immune cell surface (134). Cancer cells typically use these
interactions to subvert the immune response system in the body.
ICIs function by blocking inhibitory pathways, such as PD-1/PD-L1
or CTLA-4 interactions, thereby releasing the suppressive signals
on T cells. This enhances T-cell activation and enables them to
more effectively recognize and eliminate cancer cells (136).
Combining OVT and ICIs, the immune response to CRC
is improved via several mechanisms. OVT employs viruses capable of
infecting and killing malignant cells and, at the same time,
disseminating TAAs and stimulating antitumor immunity (55). This can enhance the visibility of
the tumor to the immune system, thus increasing its immunogenicity
(137). Furthermore, OVT can
increase the levels of immune checkpoint proteins on cancer cells,
allowing ICIs to bind and enhance the immune response. In addition,
OVT can alter the tumor-related stroma and make it less
immunosuppressive to cancer cells, thus enabling improved immune
cell infiltration and effector functions (55). This pro-additive synergy
increases the possibility of a more profound and longer lasting
immunological response to CRC (137).
Experimental and clinical data have demonstrated the
synergism between OVT and anti-PD-1/PD-L1 and CTLA-4
immunotherapies in CRC. Previous in vivo investigations have
revealed that OVT may enhance the immunogenicity of tumors, making
tumors more conspicuous to the immune system, and raising the
levels of immune checkpoint proteins that can be modulated by ICIs
(138). According to clinical
trials, these combinations have resulted in objective responses and
disease control with reasonable toxicity (139,140). CTLA-4 and PD-1/PD-L1, together
with the blockade, upregulate T cell activation and treatment
effectiveness, improving the anti-tumor results (141,142).
To maintain the effectiveness of OVT, studies have
highlighted the importance of engineering OVs with
immune-modulatory genes. For instance, incorporating genes for
cytokines such as granulocyte-macrophage colony-stimulating factor
(GM-CSF) or checkpoint inhibitors such as anti-PD-1 can enhance
antitumor immunity and extend the duration of viral activity
(143). Recent findings suggest
that OVs equipped with costimulatory molecules, such as VALO-D102
encoding CD40L and OX40L, can significantly improve tumor growth
control and boost the infiltration of tumor-specific
CD8+ effector T cells, as demonstrated in melanoma
models (54). Enhancing the
efficacy of OVT can also be achieved by targeting the TME through
strategies such as remodeling the ECM or inhibiting
immunosuppressive pathways, particularly those involving PI3Kγ in
macrophages (144).
Additionally, combining OVT with targeted therapies, such as
anti-angiogenic agents (including regorafenib) (145) or EGFR inhibitors (including
cetuximab), enhances viral delivery and counteracts resistance
mechanisms in the TME (146).
The synergistic mechanism of OVT and ICIs is depicted in Fig. 3.
CRC develops resistance to ICIs through mechanisms
such as altered PI3K/AKT/mTOR signaling, often due to PIK3CA
mutations, and interactions within an immunosuppressive TME
(147). To overcome this
resistance, combining OVT with PI3K-γ inhibitors such as copanlisib
or histone deacetylase inhibitors (HDIs) can effectively suppress
oncogenic pathways, enhance viral replication and synergistically
improve T-cell infiltration alongside ICIs (148). Nevertheless, there are problems
with this approach. Despite ICI therapy, 30-50% of patients with
MSI-H CRC develop drug resistance, while single-agent ICIs have
been reported to have little impact in patients with pMMR or MSS
metastatic CRC (149,150). Furthermore, the effectiveness
and safety of PD-1/PD-L1 and CTLA-4 ICIs on advanced CRC are still
inconclusive in clinical studies, and the outcomes are paradoxical
(139,151). By contrast, previous findings
suggest that NK-1R antagonists induce apoptosis in CRC cells
through endoplasmic reticulum stress and calcium release,
activating the PERK/eIF2α/ATF4/CHOP pathway, which enhances
chemotherapy sensitivity and reveals potential biomarkers and
therapeutic targets (152).
Moreover, the heterogeneity of CRC or the TME can modulate the
response to therapeutic intervention, and there may be shortcomings
in identifying patients likely to benefit from this combined
treatment strategy (153).
Viral immunogenicity is essential in the mechanism
of action of OVT for CRC since it establishes the capacity of the
virus to elicit an immune response against cancer cells (154). When OVs infect and lyse cancer
cells, they display antigens that the immune system can see and
thus stimulate T cells and other immune factors (155). This process is termed ICD and
is crucial for priming and reinforcing antitumor responses
(57). OVs can be engineered
genetically to boost this immunogenicity. These changes may concern
the deletion of viral genes that inhibit the host immune response,
the addition of other genes that encode immune-stimulating
products, such as cytokines (IL-12 and IL-15) or antibodies (such
as against PD-1), and the presence of molecules enhancing viral
replication in tumor cells and their spread (156,157). For example, oncolytic HSV-1
containing the humanized anti-PD-1 antibody gene has been reported
to strengthen immune response and suppress tumor progression in
models of CRC (158). Moreover,
cytokine preconditioning parental cells can increase the release or
the antitumor activity of exosomes originating from such parent
cells, thus strengthening the immune response (159). These genetic changes seek to
transform the 'cold', difficult-to-treat tumors that are less
sensitive to ICIs and enhance their ability to stimulate an immune
response (154).
Immune adjuvants play a pivotal role in enhancing
the effectiveness of OVT in CRC by amplifying the immune response.
They stimulate the innate immune system, promoting the recruitment
and maturation of antigen-presenting cells, which is crucial for
initiating adaptive immunity against cancer cells (160). For example, T-VEC produces
GM-CSF, which improves antigen-presenting cell function and elicits
a copious T-cell response at the tumor site (161,162). However, genetic modifications
can be made to increase immunogenicity by endowing OVs with
'micrometals', CD40L and OX40L, which have been found to improve
tumor control and CD8+ effector T cell functions
(163).
New strategies to enhance viral immunogenicity for
OVT include the use of OVs with cytokine-gene or immune-checkpoint
inhibitors (54). For instance,
VALO-D102, an adenovirus that encodes CD40L and OX40L, has a more
robust efficacy on tumor suppression if used with an anti-PD-1
antibody (163). Additionally,
there is evidence of the employment of bispecific T-cell activators
and other antibody formats to increase oncolytic viral productivity
(164,165). These strategies aim to
counteract the suppressive immunological characteristics of tumors
in OVT, thereby enhancing therapeutic effects on CRC.
Furthermore, the emerging therapeutic applications
of exosomes are gaining attention due to their ability to modulate
epithelial-mesenchymal transition (EMT) and other tumor-promoting
processes. Recent advances highlight their potential as effective
delivery platforms for miRNAs or drugs to reverse EMT or enhance
treatment efficacy (166). By
integrating exosome technology with existing OVT strategies there
is potential for improved treatment outcomes in CRC. Additionally,
exosomes play a crucial role as essential mediators of
intercellular communication, regulating EMT. This enables tumor
cells to acquire invasive and metastatic properties, which poses
challenges for effective treatment strategies (167).
The TME in CRC is replete with immunosuppressive
cells such as Tregs and tumor-associated macrophages (TAMs) that
form a massive hurdle to immune effector cells (161). OVT can change this TME by using
ICD, which will release other TAAs and recruit immune cells,
including macrophages and T cells. These immune cells play a
crucial role as they can recognize both the viral infection and
tumor antigens, thereby generating a significantly stronger
antitumor immune response (30).
Macrophages, in particular, when polarized to an M1 phenotype, are
known to support the antitumor immune response (162), whereas T cells, especially CD8
cytotoxic T-lymphocytes, directly kill cancer cells (168). Recent developments in OVT have
focused on the modification of viral specificity and tropism and
the modification of OVs to provide immune-stimulatory molecules to
increase viral immunogenicity (169). For instance, viruses can be
equipped with cytokines such as GM-CSF, which enhance the antitumor
function of lymphocytes, or chemokines, which attract more immune
cells into the TME (170).
Additionally, incorporating ICIs into the virus can counteract the
immunosuppressive state of the TME and enhance the immune targeting
of CRC (32).
Chemotherapy and radiation therapy are known
treatments for the therapy of CRC with the overall goal of
therapeutic mitigation of tumors and the associated symptoms
(21). These drugs act by
causing permanent defects in the DNA of actively dividing cancer
cells, inhibiting growth. Nevertheless, these treatments also
target healthy cells and, therefore, cause side effects and may not
help all patients, particularly those diagnosed with MSS CRC, since
this disease typically does not respond well to immunotherapy
(171-173). In this context, the combination
of OVT with chemotherapy or radiotherapy has emerged as a promising
strategy in CRC treatment. Such combinations aim to enhance
therapeutic efficacy by leveraging complementary mechanisms,
including the induction of ICD and the modulation of the TME
(21). The current research
states that integrating these therapies exploits similar pathways,
including ICD. OVT, chemotherapy and radiotherapy all release
danger associated molecular patterns, which enhance innate and
adaptive immunity (174). This
improves the overall tumouricidal activity and seems beneficial in
permitting lower doses of cytotoxic agents to be deployed in
combination with enzymes, thereby diminishing toxicity. For
instance, genetically modified oncolytic viruses can be programmed
to express enzymes such as cytosine deaminase to transform
prodrugs, such as 5-fluorocytosine, into toxic agents wherever the
virus is replicating but minimize the side effect on other organs
of the body (175).
Targeted therapy for CRC is aimed at proteins or
molecules that directly impact cancer rather than harming all
dividing cells, such as in chemotherapy. These therapies target
critical signaling pathways misregulated in CRC, including SHH/Gli,
Wnt/β-cat, TGF-β/SMA, EGFR and Notch. For example, vismodegib, a
hedgehog (Hh) inhibitor, suppresses the Hh pathway by binding to
the Smoothened receptor and thus induces apoptosis in colon cancer
cells (176). Similarly,
Albring et al (177)
have demonstrated the ability of berberine and its derivatives to
modulate the Wnt/β-catenin signaling cascade.
The combination of targeted agents can improve the
outcomes of OVT in managing CRC. When combined with targeted drugs
that modulate molecular pathways, OVT generates enhanced toxicity
for cancer cells while preserving standard tissue tolerance. In
this regard, the use of monoclonal antibodies to EGFR, such as
cetuximab and panitumumab, improves the response of patients with
CRC to OVT by increasing the sensitivity of cancer cells to the OV
(178). The same drugs can
positively affect tumor vessels; for instance, bevacizumab can
prevent the formation of short and irregular vessels typical for
tumor-related vasculature, increasing the effectiveness of OV
(179). This implies that there
is an opportunity to improve the effectiveness of OVT using
targeted therapies that make cancer cells sensitive to the
treatment while at the same time lowering the general toxicity.
When used together, there is the potential for drug candidates such
as anti-angiogenic agents for CRC and EGFR inhibitors to enhance
the effectiveness of OVT by making cancer cells more sensitive to
treatment while reducing overall toxicity. Certain anti-angiogenic
agents, such as regorafenib (an oral multikinase inhibitor),
combined with immunotherapies such as nivolumab, have been shown to
be beneficial in patients with CRC, especially those with MSS
tumors, which are less sensitive to immunotherapies (180). This combination demonstrated an
overall response rate of ~44% in the REGONIVO study (181).
Bevacizumab is an anti-angiogenic agent recently
licensed in CRC and has demonstrated increased overall survival and
progression-free survival (PFS) in metastatic CRC (182). Furthermore, research has shown
that combining chemotherapy with bevacizumab in patients with
metastatic CRC is influenced by PD-L1 expression, suggesting that
PD-L1 status plays a crucial role in the effectiveness of this
therapeutic approach (183,184). Cetuximab target the EGFR
signaling cascade, which is frequently mutated in CRC, and have
shown efficacy in KRAS wild-type metastatic CRC (185). These potent agents have been
evaluated in several clinical trials for the first-line,
second-line or third-line treatment of metastatic CRCs.
OVT combined with targeted therapies in CRC also
poses certain issues, such as resistance, toxicity and dosage.
Crossover and bypass can result in innate and acquired resistance
due to existing interactions between pathways (186). One major issue with combining
these therapies is that, as the number of treatment sessions
increases, the rate of complications also rises (187). The dosage needs to be carefully
fine-tuned to allow for effective targeted therapy while avoiding
toxicity due to the druggability of targets in the TME, which have
not been entirely elucidated (188). Some factors that should be
weighed include prophylactic considerations, cost/benefit
considerations, patient selection and follow-up, given the
inter-compliant variation results (189,190).
Biomarkers are critical in precision medicine in
CRC due to their guiding role in identifying various aspects of the
tumor molecular profile that define its management. The American
Society of Clinical Oncology, European Society for Medical Oncology
and National Comprehensive Cancer Network recognize mutations
within these genes as essential pharmacogenomic biomarkers
(191-193). Notably, BRAF mutation is
considered to be adverse for survival, highlighting the role of
biomarkers as a guide for the selection of treatments based on
tumor characteristics (194).
In particular, PD-L1, TMB and MSI are established as possible
indicators of ICIs. PD-L1 is currently used to predict response to
anti-PD-1/anti-PD-L1 therapy, while TMB and MSI have some
predictive roles in numerous cancer types, including colon cancer
(195,196). Another molecular biomarker
associated with improved clinical outcomes in ICI therapy for
patients with CRC is MSI status, particularly in tumors that are
MSI-H or dMMR (197). However,
the predictive value of PD-L1 has been criticized since some
patients with low or undetectable PD-L1 expression respond to this
targeted therapy (198). This
indicates that there is a requirement for improved predictive
biomarkers for ICI therapy.
Abnormalities in the RAS, BRAF and EGFR genes are
considered valid prognostic indicators in CRC (199). More specifically, anti-EGFR
receptor therapies fail to show efficiency when KRAS mutations
exist in the malignant cells. Detecting individuals with these
genetic changes is recommended as these treatments can be unhelpful
and expensive (200). In
locally advanced rectal cancer, patients harboring wild-type KRAS
have improved pathological complete response rates with cetuximab,
a targeted agent (201). These
biomarkers may be utilized in individualized treatment planning,
which will benefit patients by reducing toxicities and misguided
treatment costs.
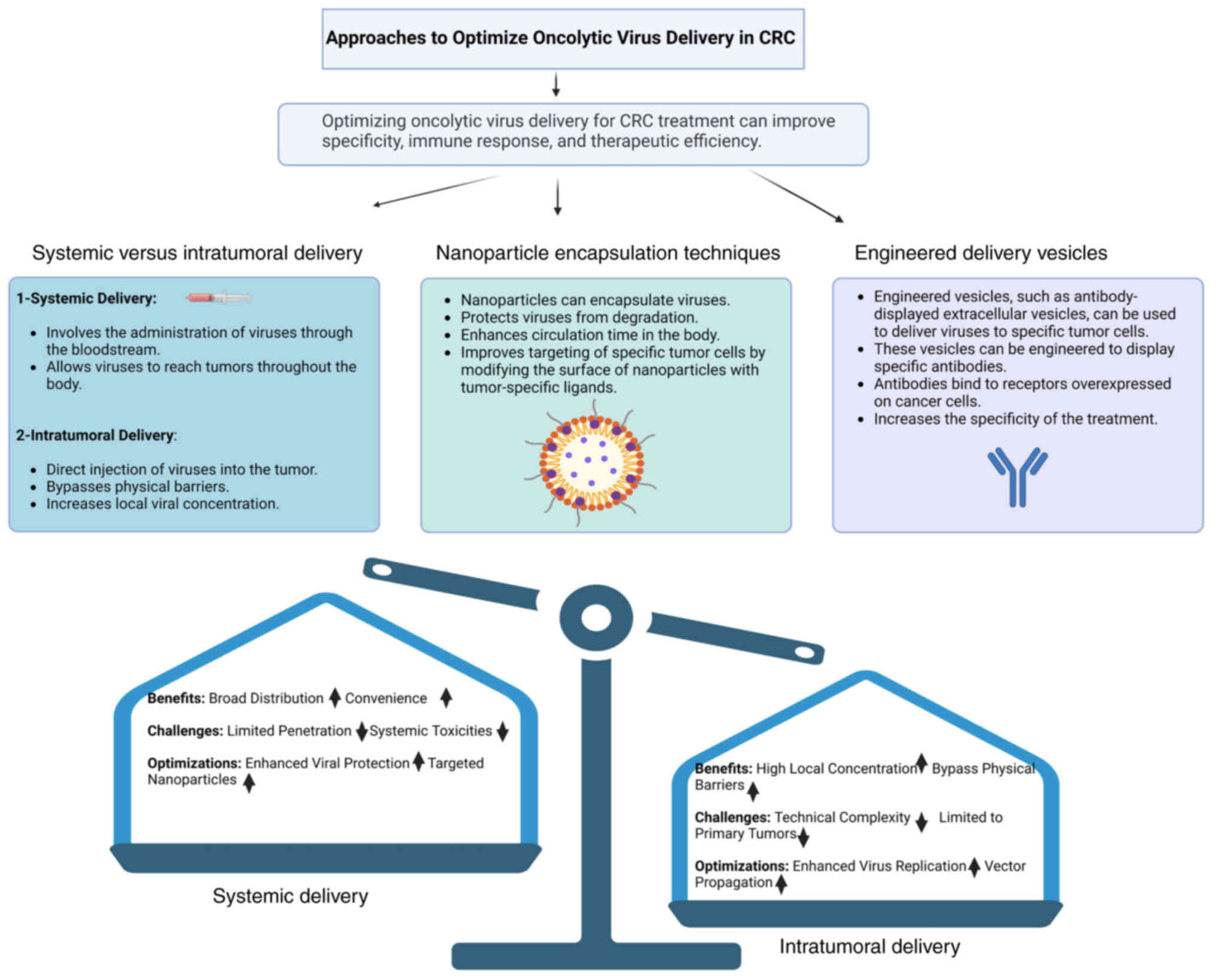
Delivering OVs into CRC tumors is challenging since
CRC tumors are developed and heterogeneous. Although the concept of
systemic delivery of OVs is thought-provoking due to its capability
to target disseminated metastases, the approach is also faced with
challenges such as the short biological half-life OVs in the
circulatory system and the poor ability to target as well as
sediment at the tumor site, thereby reducing the possibility of
delivering OVs to the metastatic deposits. Intratumoral delivery,
while less versatile as it is restricted to accessible tumors, can
garner higher viral shed-load and produce vigorous local antitumor
effects (42). Furthermore,
other factors, such as the secretion of stromal cell-derived
factor-1, can also worsen the mobilization nadir as well as greatly
enhance the number of circulating progenitor cells, but the
responses are typically relatively weak at untreated distal sites
(202).
To enhance the efficiency of systemic delivery, a
new method involving nanoparticle encapsulation and engineered
vesicles has been proposed. Nanoparticles improve the resistance,
solubility, crossover ability and circulation time of OVs; in other
words, they make viral vectors safer and more effective in clinical
applications (203). A recent
innovation in nanoparticle construction has incorporated more
hierarchical structures, bio-feedback guidance components and
conjugates, which make OVs reach tumor-specific locale more
efficiently (204,205). For example, biomimetic
nanoparticles coated by cell membranes can use immune evasion,
prolonged circulation and disease-specific targeting to enhance the
oncolytic effect of OVs at the tumor site (206). The systemic and intratumoral
delivery methods are compared in Fig. 4.
In summary, different delivery systems of OVs
possess certain limitations, namely, immune clearance and
insufficient tumor accumulation, which can be resolved by
nanoparticle encapsulation and engineered vesicles. Modifications
to nanoparticles, such as surface functionalization with targeting
ligands or the incorporation of stimuli-responsive elements, have
been shown to significantly enhance their ability to deliver OVs
specifically to tumor lesions while minimizing off-target effects
(207). These strategies will
likely enhance the clinical use of OVT in CRC.
Understanding the mechanisms of cancer drug
resistance can guide the optimization of existing targeted
therapies, identify therapeutic targets valuable to discovering new
and improved agents and form the basis of therapeutic advances in
cancer treatment (208). As
with other cancer treatments, CRC cells may interfere with or
become resistant to OVT via several processes. This mechanism
involves the activation of the PI3K-γ/AKT pathway in
tumor-associated myeloid cells (TAMCs), which fosters an
immunosuppressive environment by downregulating cytotoxic
CD8+ T lymphocytes and inhibiting their activity
(209-212). Additionally, CRC cells may have
upregulated expression of immune checkpoint molecules, such as
PD-L1, and thereby inhibit T cell function. The TME is also
involved and the mechanical property of the tumor tissue and matrix
stiffness hampers immune cell infiltration, which is essential for
the effectiveness of OVT (213,214).
Understanding mechanisms of resistance is needed to
outline how to counter this type of adaptive resistance. However,
when the current OVTs are combined with players such as HDIs, the
viral replication, oncolytic activity and recognition of NK cell
activating ligands and TAAs are all boosted (215-217). Inhibition of PI3K-γ can
overcome immuno-suppression in TAMCs and improve the therapeutic
effect of OVT (218). The
aberrant activation of the PI3K/AKT/mTOR signaling pathway in CRC
promotes therapeutic resistance by enhancing cancer cell survival,
metabolic reprogramming and immune evasion through mechanisms such
as PTEN loss or PIK3CA mutations (219). Targeting this pathway with PI3K
inhibitors (such as alpelisib and copanlisib) or mTOR inhibitors
(such as everolimus) may counteract resistance by suppressing
downstream oncogenic signaling and restoring apoptotic sensitivity
(220). Combining these
inhibitors with immune checkpoint blockers (such as pembrolizumab
and nivolumab) could further enhance efficacy by reversing
PI3K/AKT-mediated immunosuppression and reinvigorating antitumor
immunity (221). Additionally,
epigenetic therapies targeting DNA methyltransferases or histone
deacetylases may reactivate tumor suppressor genes silenced by
PI3K/AKT-driven hypermethylation, while miRNA-based strategies
(such as with miR-34a) could downregulate pathway components
(222). Thus, it can be stated
that using selective targeting of specific resistance mechanisms
and combining OVT with other treatment approaches, it is possible
to effectively overcome the adaptive resistance of CRC cells to
OVT.
Potential issues connected with OVT for colon
cancer are related to uncontrolled proliferation of the virus, the
reaction of the immune defense mechanisms, other types of
infections as well as unpredictable side effects. Furthermore,
combined interventions of OVT with other treatments may increase
such possibilities, posing threats to the patient's well-being and
thus mandating close supervision (175). Further studies are being
conducted to determine the correct dosage of OVs. Specific
indicators, such as biomarkers or patient characteristics, are used
to select the most suitable candidates for OVT and to anticipate
possible side effects linked to this innovative treatment protocol
(175).
Measures that help to avoid contact with the OV,
including strict measures for transportation and administration of
the OV, are necessary to minimize the potential exposure. Moreover,
the training of clinicians and information for patients on how to
take care of the area where injections are administered is also
required. Adverse effects surveillance in patients administered OVT
faces challenges such as under-reporting and bias in data
collection, which can obscure the true incidence of adverse events.
Additionally, distinguishing between genuine product-related
effects and coincidental events complicates accurate surveillance
and assessment (57). As
effective as OVT may be for precision therapy in CRC, it must also
be regulated for safety due to possible unintended consequences and
viral transmission. Genetic engineering for specificity, containing
the virus or making the treatment highly supervised, and monitoring
the treatment closely and strictly can guarantee the use of OVs in
cancer therapy.
The gaps in the patient selection criteria for
current OVT clinical trials for CRC are multifaceted. One
significant gap is the historical underrepresentation of specific
populations in clinical trials, limiting the generalizability of
results and affecting the diversity of eligible participants.
Specifically, the strict eligibility criteria often exclude a large
proportion of patients, particularly those who are older, female or
non-Latinx Black, and those with a lower socioeconomic status, thus
limiting the pool of eligible candidates. The lack of broadened
eligibility criteria may potentially exclude patients who could
benefit from the treatment (223). Furthermore, the challenges in
validating combination treatment strategies using patient-derived
organoids highlight the need for more refined and representative
models to guide patient selection in clinical trials (153). Addressing these gaps is crucial
for optimizing patient selection, ensuring more inclusive trials
and improving the clinical applicability of OVT in CRC.
In OVT clinical trials for CRC, selecting
predictive endpoints is crucial for determining success. According
to the latest research, endpoints such as PFS and immune reactivity
are considered highly predictive of success (224). PFS is a critical secondary
endpoint that measures the time from trial randomization to the
occurrence of disease progression or death, and it is a standard
measure of the effectiveness of a treatment in delaying disease
progression. However, immune reactivity is assessed through the
objective response rate, which measures the proportion of patients
achieving a complete or partial response, and the disease control
rate, which includes patients with stable disease for a defined
period (225). These endpoints
are integral to evaluating the immunotherapeutic effects of
OVT.
Recent progress in OVT for CRC has occurred through
efforts to enhance the viral agents' specificity and carcinoid
efficacy by gene engineering and distinct delivery system methods
in the last decade. New strategies include elements of viral
genomes that promote antitumor immune responses and the integration
of OVT with ICIs to overcome TME (226). To illustrate this progress,
Table III summarizes ongoing
and completed clinical trials investigating OVT in CRC,
highlighting the use of engineered viruses, immune-modulating
therapies and innovative delivery systems.
Precision oncology, mainly with the use of virtual
trials and OVT, opens new possibilities in clinical practice.
However, their implementation should also consider compliance with
regulatory requirements concerning the safety and efficacy of
applied approaches, data correctness and ethical standards.
Additionally, patient privacy, data security measures and consent
are essential for successfully approving and developing precision
OVT (227). The application of
precision to OVT for patients has notable challenges, such as
restraints in safety and efficacy, Good Manufacturing Practice
standards and patient consent. Long-term monitoring is necessary
for continuous post-market surveillance (226). The germline genetic profile,
comorbidities and age-specific mutational processes should inform
the creation of these regimens. The modified form of OVT can
increase the efficacy of treatments due to the targeted receptor
contributing to immune evasion, thus leading to improved patient
results (228). Researchers
hope that adjusting the therapy according to the properties of the
tumor will enhance the antitumor immune response and overall
survival. Additionally, the synergistic effects of OVT with other
immunotherapies may be apparent, improving the overall therapeutic
advantage (55).
The use of genetically modified OVs introduces
unique ethical challenges, particularly concerning patient safety
and potential long-term risks. While these viruses are engineered
to selectively target cancer cells and minimize off-target effects,
unintended consequences such as viral shedding, transmission to
non-target tissues or unforeseen immune reactions could pose
significant risks (229).
Ensuring rigorous preclinical testing, transparent informed consent
processes and long-term post-market surveillance is critical to
mitigating these concerns. Additionally, equitable access to
precision OVT must be considered, as high costs and complex
logistical requirements may limit availability in low-resource
settings. Addressing these ethical dimensions will not only
safeguard patients but also foster public trust in this
transformative therapeutic approach.
OVT represents a new paradigm in the field of CRC
treatment, as it has the potential to circumvent the drawbacks
associated with conventional therapies while utilizing targeting
and immune-stimulating properties. During the last decade, genetic
engineering and biomarkers have opened the door for targeted
treatment options, a style that remains novel in CRC due to
intersquamous tumor heterogeneity. Additionally, combining ICIs
with other conventional methods such as chemotherapy and targeted
therapy produces synergistic effects on treatment outcomes.
Nevertheless, the OV technique has certain
limitations, which have posed the following challenges: How best to
deliver viruses, what strategies to counter viral resistance and
the question of safety when the viruses will be used clinically.
These include integrating new, efficient delivery systems
(nanoparticle encapsulation) and creating new biomarkers. Although
OVT holds significant promise, its implementation in low- and
middle-income countries is hindered by notable challenges, such as
inadequate healthcare infrastructure, prohibitive treatment costs
and limited access to advanced therapeutic technologies. To bridge
these gaps, it is crucial to develop innovative strategies,
including cost-effective viral production techniques, simplified
and scalable delivery systems as well as fostering international
collaborations aimed at promoting equitable access to OVT-based
treatments. Such efforts could pave the way for broader global
adoption of this transformative cancer therapy.
Future directions for enhancing translation involve
incorporating artificial intelligence into these findings and
utilizing well-established clinical trials for patient selection.
By addressing these issues through a behavioral/precision OVT
approach, CRC treatment may undergo a radical transformation,
establishing a new standard in the oncology process. With ongoing
research, this innovative treatment modality could eventually
transition from experimental to routine practice, offering millions
of patients a renewed chance at life worldwide.
Not applicable.
Conceptualization was conducted by MHS and YW; MHS,
XJ and YW collected and organized the relevant literature; YX and
QZ conducted a systematic review of the literature to identify key
themes and trends; MHS, QZ, YX, XJ and YW critically evaluated the
sources and synthesized the findings; project administration was
conducted by YW; the original draft was written by MHS; reviewing
and editing the manuscript was performed by MHS, YW and XJ. All
authors read and approved the final version of the manuscript. Data
authentication is not applicable.
Not applicable.
Not applicable.
The authors declare that they have no competing
interests.
Not applicable.
This work was supported by the Natural Science Foundation of
Zhejiang Province (grant no. LGF22C010005) and the Science
Foundation of Zhejiang Sci-Tech University (grant no.
18042291-Y).
|
1
|
Fadlallah H, El Masri J, Fakhereddine H,
Youssef J, Chemaly C, Doughan S and Abou-Kheir W: Colorectal
cancer: Recent advances in management and treatment. World J Clin
Oncol. 15:1136–1156. 2024. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Morgan E, Arnold M, Gini A, Lorenzoni V,
Cabasag CJ, Laversanne M, Vignat J, Ferlay J, Murphy N and Bray F:
Global burden of colorectal cancer in 2020 and 2040: Incidence and
mortality estimates from GLOBOCAN. Gut. 72:338–344. 2023.
View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Pan H, Zhao Z, Deng Y, Zheng Z, Huang Y,
Huang S and Chi P: The global, regional, and national early-onset
colorectal cancer burden and trends from 1990 to 2019: Results from
the global burden of disease study 2019. BMC Public Health.
22:18962022. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Maida M, Dahiya DS, Shah YR, Tiwari A,
Gopakumar H, Vohra I, Khan A, Jaber F, Ramai D and Facciorusso A:
Screening and surveillance of colorectal cancer: A review of the
literature. Cancers (Basel). 16:27462024. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Nordlinger B, Sorbye H, Glimelius B,
Poston GJ, Schlag PM, Rougier P, Bechstein WO, Primrose JN, Walpole
ET, Finch-Jones M, et al: Perioperative FOLFOX4 chemotherapy and
surgery versus surgery alone for resectable liver metastases from
colorectal cancer (EORTC 40983): Long-term results of a randomised,
controlled, phase 3 trial. Lancet Oncol. 14:1208–1215. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Zabaleta J, Iida T, Falcoz PE, Salah S,
Jarabo JR, Correa AM, Zampino MG, Matsui T, Cho S, Ardissone F, et
al: Individual data meta-analysis for the study of survival after
pulmonary metastasectomy in colorectal cancer patients: A history
of resected liver metastases worsens the prognosis. Eur J Surg
Oncol. 44:1006–1012. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Verwaal VJ, van Ruth S, de Bree E, van
Slooten GW, van Tinteren H, Boot H and Zoetmulder FAN: Randomized
trial of cytoreduction and hyperthermic intraperitoneal
chemotherapy versus systemic chemotherapy and palliative surgery in
patients with peritoneal carcinomatosis of colorectal cancer. J
Clin Oncol. 21:3737–3743. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Cercek A, Lumish M, Sinopoli J, Weiss J,
Shia J, Lamendola-Essel M, El Dika IH, Segal N, Shcherba M,
Sugarman R, et al: PD-1 blockade in mismatch repair-deficient,
locally advanced rectal cancer. N Engl J Med. 386:2363–2376. 2022.
View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Chalabi M, Verschoor YL, van den Berg J,
Sikorska K, Beets G, Lent AV, Grootscholten MC, Aalbers A, Buller
N, Marsman H, et al: LBA7 Neoadjuvant immune checkpoint inhibition
in locally advanced MMR-deficient colon cancer: The NICHE-2 study.
Ann Oncol. 33(Suppl 7): S13892022. View Article : Google Scholar
|
|
10
|
Yang J, Nie J, Ma X, Wei Y, Peng Y and Wei
X: Targeting PI3K in cancer: Mechanisms and advances in clinical
trials. Mol Cancer. 18:262019. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Javid H, Hashemian P, Yazdani S, Sharbaf
Mashhad A and Karimi-Shahri M: The role of heat shock proteins in
metastatic colorectal cancer: A review. J Cell Biochem.
123:1704–1735. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Golestaneh M, Firoozrai M, Javid H and
Hashemy SI: The substance P/neurokinin-1 receptor signaling pathway
mediates metastasis in human colorectal SW480 cancer cells. Mol
Biol Rep. 49:4893–4900. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Aapro M, Jelkmann W, Constantinescu SN and
Leyland-Jones B: Effects of erythropoietin receptors and
erythropoiesis-stimulating agents on disease progression in cancer.
Br J Cancer. 106:1249–1258. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
González-Perera I, Gutiérrez-Nicolás F,
Nazco-Casariego GJ, Ramos-Díaz R, Hernández-San Gil R, Pérez-Pérez
JA, González García J and González De La Fuente GA: 5-fluorouracil
toxicity in the treatment of colon cancer associated with the
genetic polymorphism 2846 A>G (rs67376798). J Oncol Pharm Pract.
23:396–398. 2017. View Article : Google Scholar
|
|
15
|
Javid H, Karimi-Shahri M, Khorramdel M,
Mashhad AS, Tabrizi AT, Sathyapalan T, Afshari AR and Sahebkar A:
Probiotics as an adjuvant for management of gastrointestinal
cancers through their anti-inflammatory effects: A mechanistic
review. Curr Med Chem. 30:390–406. 2023. View Article : Google Scholar
|
|
16
|
Ogura A, Konishi T, Cunningham C,
Garcia-Aguilar J, Iversen H, Toda S, Lee IK, Lee HX, Uehara K, Lee
P, et al: Neoadjuvant (Chemo)radiotherapy with total mesorectal
excision only is not sufficient to prevent lateral local recurrence
in enlarged nodes: Results of the multicenter lateral node study of
patients with low cT3/4 rectal cancer. J Clin Oncol. 37:33–43.
2019. View Article : Google Scholar :
|
|
17
|
Ye LY, Li YS, Ge T, Liu LC, Si JX, Yang X,
Fan WJ, Liu XZ, Zhang YN, Wang JW, et al: Engineered luminescent
oncolytic vaccinia virus activation of photodynamic-immune
combination therapy for colorectal cancer. Adv Healthc Mater.
13:e23041362024. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Ruan Z, Chi J, Kong Y, Li C, Ruan X, Zhou
X, Chen Y, Li Y and Luo Z: Natural oncolysis of enterovirus 71 in
antitumor therapy of colorectal cancer. Adv Biol (Weinh).
7:e22003362023. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Komant S, Wang J, Favis N, Alex C, Evans
DH, Noyce RS and Baldwin TA: Oncolytic vaccinia virus as a
precision cancer vaccine platform. bioRxiv. 2024.08.16.608170.
2024.
|
|
20
|
Nasar RT, Uche IK and Kousoulas KG:
Targeting cancers with oHSV-based oncolytic viral immunotherapy.
Curr Issues Mol Biol. 46:5582–5594. 2024. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Ren Y, Miao JM, Wang YY, Fan Z, Kong XB,
Yang L and Cheng G: Oncolytic viruses combined with immune
checkpoint therapy for colorectal cancer is a promising treatment
option. Front Immunol. 13:9617962022. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Wang XL, Xu HW and Liu NN: Oral
microbiota: A new insight into cancer progression, diagnosis and
treatment. Phenomics. 3:535–547. 2023. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Mai Z, Lin Y, Lin P, Zhao X and Cui L:
Modulating extracellular matrix stiffness: A strategic approach to
boost cancer immunotherapy. Cell Death Dis. 15:3072024. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Kalli M, Poskus MD, Stylianopoulos T and
Zervantonakis IK: Beyond matrix stiffness: Targeting force-induced
cancer drug resistance. Trends Cancer. 9:937–954. 2023. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Yuan Z, Li Y, Zhang S, Wang X, Dou H, Yu
X, Zhang Z, Yang S and Xiao M: Extracellular matrix remodeling in
tumor progression and immune escape: From mechanisms to treatments.
Mol Cancer. 22:482023. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Ahmed H, Mahmud AR, Siddiquee MF, Shahriar
A, Biswas P, Shimul MEK, Ahmed SZ, Ema TI, Rahman N, Khan MA, et
al: Role of T cells in cancer immunotherapy: Opportunities and
challenges. Cancer Pathog Ther. 1:116–126. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Noro J, Vilaça-Faria H, Reis RL and
Pirraco RP: Extracellular matrix-derived materials for tissue
engineering and regenerative medicine: A journey from isolation to
characterization and application. Bioact Mater. 34:494–519.
2024.PubMed/NCBI
|
|
28
|
Sorokin M, Zolotovskaia M, Nikitin D,
Suntsova M, Poddubskaya E, Glusker A, Garazha A, Moisseev A, Li X,
Sekacheva M, et al: Personalized targeted therapy prescription in
colorectal cancer using algorithmic analysis of RNA sequencing
data. BMC Cancer. 22:11132022. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Hu H, Zhang S, Cai L, Duan H, Li Y, Yang
J, Wang Y and Liu B, Dong S, Fang Z and Liu B: A novel cocktail
therapy based on quintuplet combination of oncolytic herpes simplex
virus-2 vectors armed with interleukin-12, interleukin-15, GM-CSF,
PD1v, and IL-7 x CCL19 results in enhanced antitumor efficacy.
Virol J. 19:742022. View Article : Google Scholar
|
|
30
|
Ribas A, Dummer R, Puzanov I, VanderWalde
A, Andtbacka RHI, Michielin O, Olszanski AJ, Malvehy J, Cebon J,
Fernandez E, et al: Oncolytic virotherapy promotes intratumoral T
cell infiltration and improves Anti-PD-1 immunotherapy. Cell.
170:1109–1119.e10. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Garcia-Carbonero R, Salazar R, Duran I,
Osman-Garcia I, Paz-Ares L, Bozada JM, Boni V, Blanc C, Seymour L,
Beadle J, et al: Phase 1 study of intravenous administration of the
chimeric adenovirus enadenotucirev in patients undergoing primary
tumor resection. J Immunother Cancer. 5:712017. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Zhang H, Ren Y, Wang F, Tu X, Tong Z, Liu
L, Zheng Y, Zhao P, Cheng J, Li J, et al: The long-term
effectiveness and mechanism of oncolytic virotherapy combined with
anti-PD-L1 antibody in colorectal cancer patient. Cancer Gene Ther.
31:1412–1426. 2024. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Berkey SE, Thorne SH and Bartlett DL:
Oncolytic virotherapy and the tumor microenvironment. Tumor Immune
Microenvironment in Cancer Progression and Cancer Therapy. Kalinski
P: Springer International Publishing; Cham: pp. 157–172. 2017
|
|
34
|
Cai L, Chen A and Tang D: A new strategy
for immunotherapy of microsatellite-stable (MSS)-type advanced
colorectal cancer: Multi-pathway combination therapy with
PD-1/PD-L1 inhibitors. Immunology. 173:209–226. 2024. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Hajeri PB, Sharma NS and Yamamoto M:
Oncolytic adenoviruses: Strategies for improved targeting and
specificity. Cancers (Basel). 12:15042020. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Samson A, Smolenschi C, Cassier P, Patel
JV, Hammond C, Kurzawa M, Sainte-Croix S, West E, Sadoun A and
Bendjama K: Abstract CT190: Oncolytic virus TG6002 safety and
activity after intrahepatic artery administration in patients with
liver-dominant metastatic colorectal cancer. Cancer Res. 83(8
Suppl): CT1902023. View Article : Google Scholar
|
|
37
|
Lavilla-Alonso S, Bauerschmitz G,
Abo-Ramadan U, Halavaara J, Escutenaire S, Diaconu I, Tatlisumak T,
Kanerva A, Hemminki A and Pesonen S: Adenoviruses with an αvβ
integrin targeting moiety in the fiber shaft or the HI-loop
increase tumor specificity without compromising antitumor efficacy
in magnetic resonance imaging of colorectal cancer metastases. J
Transl Med. 8:802010. View Article : Google Scholar
|
|
38
|
Naumenko VA, Stepanenko AA, Lipatova AV,
Vishnevskiy DA and Chekhonin VP: Infection of non-cancer cells: A
barrier or support for oncolytic virotherapy? Mol Ther Oncolytics.
24:663–682. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Chen G, Wu K, Li H, Xia D and He T: Role
of hypoxia in the tumor microenvironment and targeted therapy.
Front Oncol. 12:9616372022. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Kingsak M, Meethong T, Jongkhumkrong J,
Cai L and Wang Q: Therapeutic potential of oncolytic viruses in the
era of precision oncology. Biomater Transl. 4:67–84. 2023.
|
|
41
|
Peng Z, Kalim M and Lu Y: Improving
systemic delivery of oncolytic virus by cellular carriers. Cancer
Biol Med. 21:1104–1119. 2025. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Chen L, Zuo M, Zhou Q and Wang Y:
Oncolytic virotherapy in cancer treatment: Challenges and
optimization prospects. Front Immunol. 14:13088902023. View Article : Google Scholar
|
|
43
|
Crupi MJF, Taha Z, Janssen TJA, Petryk J,
Boulton S, Alluqmani N, Jirovec A, Kassas O, Khan ST, Vallati S, et
al: Oncolytic virus driven T-cell-based combination immunotherapy
platform for colorectal cancer. Front Immunol. 13:10292692022.
View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Chen X, Wang G, Qin L, Hu B and Li J:
Intestinal microbiota modulates the antitumor effect of oncolytic
virus vaccines in colorectal cancer. Dig Dis Sci. 69:1228–1241.
2024. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Kontermann RE, Ungerechts G and Nettelbeck
DM: Viro-antibody therapy: Engineering oncolytic viruses for
genetic delivery of diverse antibody-based biotherapeutics. MAbs.
13:19824472021. View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Zheng M, Huang J, Tong A and Yang H:
Oncolytic viruses for cancer therapy: Barriers and recent advances.
Mol Ther Oncolytics. 15:234–247. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Zhu J, Ma J, Huang M, Deng H and Shi G:
Emerging delivery strategy for oncolytic virotherapy. Mol Ther
Oncol. 32:2008092024. View Article : Google Scholar : PubMed/NCBI
|
|
48
|
Song Z, Tao Y, Liu Y and Li J: Advances in
delivery systems for CRISPR/Cas-mediated cancer treatment: A focus
on viral vectors and extracellular vesicles. Front Immunol.
15:14444372024. View Article : Google Scholar : PubMed/NCBI
|
|
49
|
Mainenti PP, Stanzione A, Guarino S, Romeo
V, Ugga L, Romano F, Storto G, Maurea S and Brunetti A: Colorectal
cancer: Parametric evaluation of morphological, functional and
molecular tomographic imaging. World J Gastroenterol. 25:5233–5256.
2019. View Article : Google Scholar : PubMed/NCBI
|
|
50
|
Zhang T, Jiang S, Zhang L, Liu Y, Zheng H,
Zhao H, Du S, Xu Y and Lu X: A bibliometric analysis of oncolytic
virotherapy combined with immunotherapy. Hum Vaccin Immunother.
20:24066212024. View Article : Google Scholar : PubMed/NCBI
|
|
51
|
Chai C, Zhang J, Zhou Y, Yin H, Zhang F,
Diao Y, Zan X, Ma Y, Wang Y, Wu Y and Wang W: The effects of
oncolytic pseudorabies virus vaccine strain inhibited the growth of
colorectal cancer HCT-8 cells in vitro and in vivo. Animals
(Basel). 12:24162022. View Article : Google Scholar : PubMed/NCBI
|
|
52
|
Girod M, Geisler A, Hinze L, Elsner L,
Dieringer B, Beling A, Kurreck J and Fechner H: Combination of
FOLFOXIRI drugs with oncolytic coxsackie B3 virus PD-H
synergistically induces oncolysis in the refractory colorectal
cancer cell line Colo320. Int J Mol Sci. 25:56182024. View Article : Google Scholar : PubMed/NCBI
|
|
53
|
Enow JA, Sheikh HI and Rahman MM: Tumor
tropism of DNA viruses for oncolytic virotherapy. Viruses.
15:22622023. View Article : Google Scholar : PubMed/NCBI
|
|
54
|
Tian Y, Xie D and Yang L: Engineering
strategies to enhance oncolytic viruses in cancer immunotherapy.
Signal Transduct Target Ther. 7:1172022. View Article : Google Scholar : PubMed/NCBI
|
|
55
|
Lin D, Shen Y and Liang T: Oncolytic
virotherapy: Basic principles, recent advances and future
directions. Signal Transduct Target Ther. 8:1562023. View Article : Google Scholar : PubMed/NCBI
|
|
56
|
Robilotti E, Zeitouni NC and Orloff M:
Biosafety and biohazard considerations of HSV-1-based oncolytic
viral immunotherapy. Front Mol Biosci. 10:11783822023. View Article : Google Scholar : PubMed/NCBI
|
|
57
|
Shalhout SZ, Miller DM, Emerick KS and
Kaufman HL: Therapy with oncolytic viruses: progress and
challenges. Nat Rev Clin Oncol. 20:160–177. 2023. View Article : Google Scholar : PubMed/NCBI
|
|
58
|
Alkayyal AA, Darwish M, Ajina R, Alabbas
SY, Alotaibi MA, Alsofyani A, Bokhamseen M, Hakami M, Albaradie OA,
Moglan AM, et al: Repurposing the oncolytic virus VSV∆51M as a
COVID-19 vaccine. Front Bioeng Biotechnol. 11:11508922023.
View Article : Google Scholar
|
|
59
|
Ranki T, Särkioja M, Hakkarainen T, von
Smitten K, Kanerva A and Hemminki A: Systemic efficacy of oncolytic
adenoviruses in imagable orthotopic models of hormone refractory
metastatic breast cancer. Int J Cancer. 121:165–174. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
60
|
Niemann J and Kühnel F: Oncolytic viruses:
Adenoviruses. Virus Genes. 53:700–706. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
61
|
Bolyard C, Yoo JY, Wang PY, Saini U, Rath
KS, Cripe TP, Zhang J, Selvendiran K and Kaur B: Doxorubicin
synergizes with 34.5ENVE to enhance antitumor efficacy against
metastatic ovarian cancer. Clin Cancer Res. 20:6479–6494. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
62
|
Meisen WH, Dubin S, Sizemore ST,
Mathsyaraja H, Thies K, Lehman NL, Boyer P, Jaime-Ramirez AC, Elder
JB, Powell K, et al: Changes in BAI1 and nestin expression are
prognostic indicators for survival and metastases in breast cancer
and provide opportunities for dual targeted therapies. Mol Cancer
Ther. 14:307–314. 2015. View Article : Google Scholar :
|
|
63
|
Medrano RFV, Hunger A, Mendonça SA,
Barbuto JAM and Strauss BE: Immunomodulatory and antitumor effects
of type I interferons and their application in cancer therapy.
Oncotarget. 8:71249–71284. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
64
|
Hirvinen M, Rajecki M, Kapanen M,
Parviainen S, Rouvinen-Lagerström N, Diaconu I, Nokisalmi P,
Tenhunen M, Hemminki A and Cerullo V: Immunological effects of a
tumor necrosis factor alpha-armed oncolytic adenovirus. Hum Gene
Ther. 26:134–144. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
65
|
Zhang B, Wang X and Cheng P: Remodeling of
tumor immune microenvironment by oncolytic viruses. Front Oncol.
10:5613722021. View Article : Google Scholar : PubMed/NCBI
|
|
66
|
Hazafa A, Mumtaz M, Farooq MF, Bilal S,
Chaudhry SN, Firdous M, Naeem H, Ullah MO, Yameen M, Mukhtiar MS
and Zafar F: CRISPR/Cas9: A powerful genome editing technique for
the treatment of cancer cells with present challenges and future
directions. Life Sci. 263:1185252020. View Article : Google Scholar : PubMed/NCBI
|
|
67
|
Zhang N, Li J, Yu J, Wan Y, Zhang C, Zhang
H and Cao Y: Construction of an IL12 and CXCL11 armed oncolytic
herpes simplex virus using the CRISPR/Cas9 system for colon cancer
treatment. Virus Res. 323:1989792023. View Article : Google Scholar :
|
|
68
|
Wang L, Chen Y, Liu X, Li Z and Dai X: The
application of CRISPR/Cas9 technology for cancer immunotherapy:
Current status and problems. Front Oncol. 11:7049992022. View Article : Google Scholar : PubMed/NCBI
|
|
69
|
Ilkow CS and Bell JC: Optimizing oncolytic
virus design: A 'Swiss army knife' approach to create a
systemically delivered therapeutic. Signal Transduct Target Ther.
9:822024. View Article : Google Scholar
|
|
70
|
Xiao B, Ying C, Chen Y, Huang F, Wang B,
Fang H, Guo W, Liu T, Zhou X, Huang B, et al: Doxorubicin
hydrochloride enhanced antitumour effect of CEA-regulated oncolytic
virotherapy in live cancer cells and a mouse model. J Cell Mol Med.
24:13431–13439. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
71
|
Xiao B, Zhang L, Liu H, Fang H, Wang C,
Huang B, Liu X, Zhou X and Wang Y: Oncolytic adenovirus cd55-smad4
suppresses cell proliferation, metastasis, and tumor stemness in
colorectal cancer by regulating wnt/β-catenin signaling pathway.
Biomedicines. 8:5932020. View Article : Google Scholar
|
|
72
|
Zhang Y, Ye M, Huang F, Wang S, Wang H,
Mou X and Wang Y: Oncolytic adenovirus expressing ST13 increases
antitumor effect of tumor necrosis factor-related
apoptosis-inducing ligand against pancreatic ductal adenocarcinoma.
Hum Gene Ther. 31:891–903. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
73
|
Luo Q, Song H, Deng X, Li J, Jian W, Zhao
J, Zheng X, Basnet S, Ge H, Daniel T, et al: A triple-regulated
oncolytic adenovirus carrying MicroRNA-143 exhibits potent
antitumor efficacy in colorectal cancer. Mol Ther Oncolytics.
16:219–229. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
74
|
Toropko M, Chuvpilo S and Karabelsky A:
miRNA-mediated mechanisms in the generation of effective and safe
oncolytic viruses. Pharmaceutics. 16:9862024. View Article : Google Scholar : PubMed/NCBI
|
|
75
|
Yu B, Kang J, Lei H, Li Z, Yang H and
Zhang M: Immunotherapy for colorectal cancer. Front Immunol.
15:14333152024. View Article : Google Scholar : PubMed/NCBI
|
|
76
|
Zhao JL, Lin BL, Luo C, Yi YL, Huang P,
Chen Y, Zhao S, Huang ZJ, Ma XY and Huang L: Challenges and
strategies toward oncolytic virotherapy for leptomeningeal
metastasis. J Transl Med. 22:10002024. View Article : Google Scholar : PubMed/NCBI
|
|
77
|
Oronsky B, Gastman B, Conley AP, Reid C,
Caroen S and Reid T: Oncolytic adenoviruses: The cold war against
cancer finally turns hot. Cancers (Basel). 14:47012022. View Article : Google Scholar : PubMed/NCBI
|
|
78
|
Yin L, Zhao C, Han J, Li Z, Zhen Y, Xiao
R, Xu Z and Sun Y: Antitumor effects of oncolytic herpes simplex
virus type 2 against colorectal cancer in vitro and in vivo. Ther
Clin Risk Manag. 13:117–130. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
79
|
Gong J, Sachdev E, Mita AC and Mita MM:
Clinical development of reovirus for cancer therapy: An oncolytic
virus with immune-mediated antitumor activity. World J Methodol.
6:25–42. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
80
|
Li M, Zhang M, Ye Q, Liu Y and Qian W:
Preclinical and clinical trials of oncolytic vaccinia virus in
cancer immunotherapy: A comprehensive review. Cancer Biol Med.
20:646–661. 2023.PubMed/NCBI
|
|
81
|
Davis D and Lahiri SS: Application of
oncolytic viruses for cure of colorectal cancer. Cancer Res J.
3:76–93. 2015. View Article : Google Scholar
|
|
82
|
Kana SI and Essani K: Immuno-oncolytic
viruses: Emerging options in the treatment of colorectal cancer.
Mol Diagn Ther. 25:301–313. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
83
|
Larocca CJ, Wilber S, Jensen E, Steer C
and Davydova J: 428 Development of an Oncolytic Adenovirus to Treat
Metastatic Colorectal Cancer. J Clin Transl Sci. 7(Suppl 1):
1282023. View Article : Google Scholar :
|
|
84
|
Huang L, Zhao H, Shan M, Chen H, Xu B, He
Y, Zhao Y, Liu Z, Chen J and Xu Q: Oncolytic adenovirus H101
ameliorate the efficacy of anti-PD-1 monotherapy in colorectal
cancer. Cancer Med. 11:4575–4587. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
85
|
Li YS, Ye LY, Luo YX, Zheng WJ, Si JX,
Yang X, Zhang YN, Wang SB, Zou H, Jin KT, et al: Tumor-targeted
delivery of copper-manganese biomineralized oncolytic adenovirus
for colorectal cancer immunotherapy. Acta Biomater. 179:243–255.
2024. View Article : Google Scholar : PubMed/NCBI
|
|
86
|
Scanlan H, Coffman Z, Bettencourt J,
Shipley T and Bramblett DE: Herpes simplex virus 1 as an oncolytic
viral therapy for refractory cancers. Front Oncol. 12:9400192022.
View Article : Google Scholar : PubMed/NCBI
|
|
87
|
Ferrucci PF, Pala L, Conforti F and
Cocorocchio E: Talimogene laherparepvec (T-VEC): An intralesional
cancer immunotherapy for advanced melanoma. Cancers (Basel).
13:13832021. View Article : Google Scholar : PubMed/NCBI
|
|
88
|
Park SH, Breitbach CJ, Lee J, Park JO, Lim
HY, Kang WK, Moon A, Mun JH, Sommermann EM, Maruri Avidal L, et al:
Phase 1b trial of biweekly intravenous pexa-Vec (JX-594), an
oncolytic and immunotherapeutic vaccinia virus in colorectal
cancer. Mol Ther. 23:1532–1540. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
89
|
Harrop R, Drury N, Shingler W, Chikoti P,
Redchenko I, Carroll MW, Kingsman SM, Naylor S, Melcher A, Nicholls
J, et al: Vaccination of colorectal cancer patients with modified
vaccinia ankara encoding the tumor antigen 5T4 (TroVax) given
alongside chemotherapy induces potent immune responses. Clin Cancer
Res. 13:4487–4494. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
90
|
Koch MS, Lawler SE and Chiocca EA: HSV-1
oncolytic viruses from bench to bedside: An overview of current
clinical trials. Cancers (Basel). 12:35142020. View Article : Google Scholar : PubMed/NCBI
|
|
91
|
Snook AE, Baybutt TR, Xiang B, Abraham TS,
Flickinger JC Jr, Hyslop T, Zhan T, Kraft WK, Sato T and Waldman
SA: Split tolerance permits safe Ad5-GUCY2C-PADRE vaccine-induced
T-cell responses in colon cancer patients. J Immunother Cancer.
7:1042019. View Article : Google Scholar : PubMed/NCBI
|
|
92
|
Balint JP, Gabitzsch ES, Rice A, Latchman
Y, Xu Y, Messerschmidt GL, Chaudhry A, Morse MA and Jones FR:
Extended evaluation of a phase 1/2 trial on dosing, safety,
immunogenicity, and overall survival after immunizations with an
advanced-generation Ad5 [E1-, E2b-]-CEA(6D) vaccine in late-stage
colorectal cancer. Cancer Immunol Immunother. 64:977–987. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
93
|
Gögenur M, Balsevicius L, Bulut M, Colak
N, Justesen TF, Fiehn AK, Jensen MB, Høst-Rasmussen K, Cappelen B,
Gaggar S, et al: Neoadjuvant intratumoral influenza vaccine
treatment in patients with proficient mismatch repair colorectal
cancer leads to increased tumor infiltration of CD8+ T cells and
upregulation of PD-L1: A phase 1/2 clinical trial. J Immunother
Cancer. 11:e0067742023. View Article : Google Scholar : PubMed/NCBI
|
|
94
|
Jooss K, Ertl HC and Wilson JM: Cytotoxic
T-lymphocyte target proteins and their major histocompatibility
complex class I restriction in response to adenovirus vectors
delivered to mouse liver. J Virol. 72:2945–2954. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
95
|
Xue Y, Ruan Y, Wang Y, Xiao P and Xu J:
Signaling pathways in liver cancer: Pathogenesis and targeted
therapy. Mol Biomed. 5:202024. View Article : Google Scholar : PubMed/NCBI
|
|
96
|
Calistri D, Rengucci C, Seymour I,
Lattuneddu A, Polifemo AM, Monti F, Saragoni L and Amadori D:
Mutation analysis of p53, K-ras, and BRAF genes in colorectal
cancer progression. J Cell Physiol. 204:484–488. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
97
|
Fodde R: The APC gene in colorectal
cancer. Eur J Cancer. 38:867–871. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
98
|
Mehrvarz Sarshekeh A, Advani S, Overman
MJ, Manyam G, Kee BK, Fogelman DR, Dasari A, Raghav K, Vilar E,
Manuel S, et al: Association of SMAD4 mutation with patient
demographics, tumor characteristics, and clinical outcomes in
colorectal cancer. PLoS One. 12:e01733452017. View Article : Google Scholar : PubMed/NCBI
|
|
99
|
Chung Y, Wi YC, Kim Y, Bang SS, Yang JH,
Jang K, Min KW and Paik SS: The Smad4/PTEN expression pattern
predicts clinical outcomes in colorectal adenocarcinoma. J Pathol
Transl Med. 52:37–44. 2018. View Article : Google Scholar :
|
|
100
|
Vaughn CP, ZoBell SD, Furtado LV, Baker CL
and Samowitz WS: Frequency of KRAS, BRAF, and NRAS mutations in
colorectal cancer. Genes Chromosomes Cancer. 50:307–312. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
101
|
Überall I, Kolář Z, Trojanec R, Berkovcová
J and Hajdúch M: The status and role of ErbB receptors in human
cancer. Exp Mol Pathol. 84:79–89. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
102
|
Kerk SA, Papagiannakopoulos T, Shah YM and
Lyssiotis CA: Metabolic networks in mutant KRAS-driven tumours:
Tissue specificities and the microenvironment. Nat Rev Cancer.
21:510–525. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
103
|
Schmitz KJ, Ademi C, Bertram S, Schmid KW
and Baba HA: Prognostic relevance of autophagy-related markers LC3,
p62/sequestosome 1, Beclin-1 and ULK1 in colorectal cancer patients
with respect to KRAS mutational status. World J Surg Oncol.
14:1892016. View Article : Google Scholar : PubMed/NCBI
|
|
104
|
Jiffry J, Thavornwatanayong T, Rao D,
Fogel EJ, Saytoo D, Nahata R, Guzik H, Chaudhary I, Augustine T,
Goel S and Maitra R: Oncolytic reovirus (pelareorep) induces
autophagy in KRAS-mutated colorectal cancer. Clin Cancer Res.
27:865–876. 2021. View Article : Google Scholar :
|
|
105
|
Goel S, Ocean AJ, Parakrama RY, Ghalib MH,
Chaudhary I, Shah U, Viswanathan S, Kharkwal H, Coffey M and Maitra
R: Elucidation of pelareorep pharmacodynamics in A phase I trial in
patients with KRAS-mutated colorectal cancer. Mol Cancer Ther.
19:1148–1156. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
106
|
Su Z, El Hage M and Linnebacher M:
Mutation patterns in colorectal cancer and their relationship with
prognosis. Heliyon. 10:e365502024. View Article : Google Scholar : PubMed/NCBI
|
|
107
|
Sommariva S, Caviglia G, Ravera S,
Frassoni F, Benvenuto F, Tortolina L, Castagnino N, Parodi S and
Piana M: Computational quantification of global effects induced by
mutations and drugs in signaling networks of colorectal cancer
cells. Sci Rep. 11:196022021. View Article : Google Scholar : PubMed/NCBI
|
|
108
|
Zeng M, Zhang W, Li Y and Yu L: Harnessing
adenovirus in cancer immunotherapy: Evoking cellular immunity and
targeting delivery in cell-specific manner. Biomark Res. 12:362024.
View Article : Google Scholar : PubMed/NCBI
|
|
109
|
Liu CC, Liu JH, Wu SC, Yen CC, Chen WS and
Tsai YC: A novel E1B-55kD-deleted oncolytic adenovirus carrying
mutant KRAS-regulated hdm2 transgene exerts specific antitumor
efficacy on colorectal cancer cells. Mol Cancer Ther. 9:450–460.
2010. View Article : Google Scholar : PubMed/NCBI
|
|
110
|
Planchard D, Besse B, Groen HJM, Souquet
PJ, Quoix E, Baik CS, Barlesi F, Kim TM, Mazieres J, Novello S, et
al: Dabrafenib plus trametinib in patients with previously treated
BRAF(V600E)-mutant metastatic non-small cell lung cancer: An
open-label, multicentre phase 2 trial. Lancet Oncol. 17:984–993.
2016. View Article : Google Scholar : PubMed/NCBI
|
|
111
|
Kopetz S, Grothey A, Yaeger R, Van Cutsem
E, Desai J, Yoshino T, Wasan H, Ciardiello F, Loupakis F, Hong YS,
et al: Encorafenib, binimetinib, and cetuximab in BRAF
V600E-mutated colorectal cancer. N Engl J Med. 381:1632–1643. 2019.
View Article : Google Scholar : PubMed/NCBI
|
|
112
|
Kopetz S, Murphy DA, Pu J, Ciardiello F,
Desai J, Van Cutsem E, Wasan HS, Yoshino T, Saffari H, Zhang X, et
al: Molecular profiling of BRAF-V600E-mutant metastatic colorectal
cancer in the phase 3 BEACON CRC trial. Nat Med. 30:3261–3271.
2024. View Article : Google Scholar : PubMed/NCBI
|
|
113
|
Ivanova M, Venetis K, Guerini-Rocco E,
Bottiglieri L, Mastropasqua MG, Garrone O, Fusco N and Ghidini M:
HER2 in metastatic colorectal cancer: Pathology, somatic
alterations, and perspectives for novel therapeutic schemes. Life
(Basel). 12:14032022.PubMed/NCBI
|
|
114
|
Yonesaka K, Zejnullahu K, Okamoto I, Satoh
T, Cappuzzo F, Souglakos J, Ercan D, Rogers A, Roncalli M, Takeda
M, et al: Activation of ERBB2 signaling causes resistance to the
EGFR-directed therapeutic antibody cetuximab. Sci Transl Med.
3:99ra86. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
115
|
Takegawa N and Yonesaka K: HER2 as an
emerging oncotarget for colorectal cancer treatment after failure
of anti-epidermal growth factor receptor therapy. Clin Colorectal
Cancer. 16:247–251. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
116
|
Liu H, Zhou D, Liu D, Xu X, Zhang K, Hu R,
Xiong P, Wang C, Zeng X, Wang L and Zhang S: Synergistic antitumor
activity between HER2 antibody-drug conjugate and chemotherapy for
treating advanced colorectal cancer. Cell Death Dis. 15:1872024.
View Article : Google Scholar : PubMed/NCBI
|
|
117
|
Li K, Luo H, Huang L, Luo H and Zhu X:
Microsatellite instability: A review of what the oncologist should
know. Cancer Cell Int. 20:162020. View Article : Google Scholar : PubMed/NCBI
|
|
118
|
Schumacher TN and Schreiber RD:
Neoantigens in cancer immunotherapy. Science. 348:69–74. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
119
|
Xiao B, Qin Y, Ying C, Ma B, Wang B, Long
F, Wang R, Fang L and Wang Y: Combination of oncolytic adenovirus
and luteolin exerts synergistic antitumor effects in colorectal
cancer cells and a mouse model. Mol Med Rep. 16:9375–9382. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
120
|
Calu V, Ionescu A, Stanca L, Geicu OI,
Iordache F, Pisoschi AM, Serban AI and Bilteanu L: Key biomarkers
within the colorectal cancer related inflammatory microenvironment.
Sci Rep. 11:79402021. View Article : Google Scholar : PubMed/NCBI
|
|
121
|
Mao C, Yang ZY, Hu XF, Chen Q and Tang JL:
PIK3CA exon 20 mutations as a potential biomarker for resistance to
anti-EGFR monoclonal antibodies in KRAS wild-type metastatic
colorectal cancer: A systematic review and meta-analysis. Ann
Oncol. 23:1518–1525. 2012. View Article : Google Scholar
|
|
122
|
Wu X, Yan H, Qiu M, Qu X, Wang J, Xu S,
Zheng Y, Ge M, Yan L and Liang L: Comprehensive characterization of
tumor microenvironment in colorectal cancer via molecular analysis.
Elife. 12:e860322023. View Article : Google Scholar : PubMed/NCBI
|
|
123
|
Hirata E and Sahai E: Tumor
microenvironment and differential responses to therapy. Cold Spring
Harb Perspect Med. 7:a0267812017. View Article : Google Scholar : PubMed/NCBI
|
|
124
|
Jia Q, Wang A, Yuan Y, Zhu B and Long H:
Heterogeneity of the tumor immune microenvironment and its clinical
relevance. Exp Hematol Oncol. 11:242022. View Article : Google Scholar : PubMed/NCBI
|
|
125
|
Zhang L, Yu X, Zheng L, Zhang Y, Li Y,
Fang Q, Gao R, Kang B, Zhang Q, Huang JY, et al: Lineage tracking
reveals dynamic relationships of T cells in colorectal cancer.
Nature. 564:268–272. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
126
|
Karlsson S and Nyström H: The
extracellular matrix in colorectal cancer and its metastatic
settling-Alterations and biological implications. Crit Rev Oncol
Hematol. 175:1037122022. View Article : Google Scholar
|
|
127
|
Becht E, de Reyniès A, Giraldo NA, Pilati
C, Buttard B, Lacroix L, Selves J, Sautès-Fridman C, Laurent-Puig P
and Fridman WH: Immune and stromal classification of colorectal
cancer is associated with molecular subtypes and relevant for
precision immunotherapy. Clin Cancer Res. 22:4057–4066. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
128
|
Chen DS and Mellman I: Elements of cancer
immunity and the cancer-immune set point. Nature. 541:321–330.
2017. View Article : Google Scholar : PubMed/NCBI
|
|
129
|
Jin MZ and Jin WL: The updated landscape
of tumor microenvironment and drug repurposing. Signal Transduct
Target Ther. 5:1662020. View Article : Google Scholar : PubMed/NCBI
|
|
130
|
Ohta A, Kini R, Ohta A, Subramanian M,
Madasu M and Sitkovsky M: The development and immunosuppressive
functions of CD4(+) CD25(+) FoxP3(+) regulatory T cells are under
influence of the adenosine-A2A adenosine receptor pathway. Front
Immunol. 3:1902012. View Article : Google Scholar : PubMed/NCBI
|
|
131
|
Schmidt A, Oberle N and Krammer PH:
Molecular mechanisms of treg-mediated T cell suppression. Front
Immunol. 3:512012. View Article : Google Scholar : PubMed/NCBI
|
|
132
|
Ghiringhelli F, Puig PE, Roux S, Roux S,
Parcellier A, Schmitt E, Solary E, Kroemer G, Martin F, Chauffert B
and Zitvogel L: Tumor cells convert immature myeloid dendritic
cells into TGF-beta-secreting cells inducing CD4+CD25+ regulatory T
cell proliferation. J Exp Med. 202:919–929. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
133
|
Morello S, Pinto A, Blandizzi C and
Antonioli L: Myeloid cells in the tumor microenvironment: Role of
adenosine. Oncoimmunology. 5:e11085152016. View Article : Google Scholar : PubMed/NCBI
|
|
134
|
Aristin Revilla S, Kranenburg O and Coffer
PJ: Colorectal cancer-infiltrating regulatory T cells: Functional
heterogeneity, metabolic adaptation, and therapeutic targeting.
Front Immunol. 13:9035642022. View Article : Google Scholar : PubMed/NCBI
|
|
135
|
Hong J, Chen X, Chen L, Wang Y, Huang B
and Fang H: Clinical value of combined detection of serum sTim-3
and CEA or CA19-9 for postoperative recurrence of colorectal cancer
diagnosis. Cancer Manag Res. 15:563–572. 2023. View Article : Google Scholar : PubMed/NCBI
|
|
136
|
He X and Xu C: Immune checkpoint signaling
and cancer immunotherapy. Cell Res. 30:660–669. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
137
|
Ganesh K, Stadler ZK, Cercek A, Mendelsohn
RB, Shia J, Segal NH and Diaz LA Jr: Immunotherapy in colorectal
cancer: rationale, challenges and potential. Nat Rev Gastroenterol
Hepatol. 16:361–375. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
138
|
Dong H, Li M, Yang C, Wei W, He X, Cheng G
and Wang S: Combination therapy with oncolytic viruses and immune
checkpoint inhibitors in head and neck squamous cell carcinomas: An
approach of complementary advantages. Cancer Cell Int. 23:12023.
View Article : Google Scholar : PubMed/NCBI
|
|
139
|
Song D, Hou S, Ma N, Yan B and Gao J:
Efficacy and safety of PD-1/PD-L1 and CTLA-4 immune checkpoint
inhibitors in the treatment of advanced colorectal cancer: A
systematic review and meta-analysis. Front Immunol. 15:14853032024.
View Article : Google Scholar : PubMed/NCBI
|
|
140
|
Chen EX, Jonker DJ, Loree JM, Kennecke HF,
Berry SR, Couture F, Ahmad CE, Goffin JR, Kavan P, Harb M, et al:
Effect of combined immune checkpoint inhibition vs best supportive
care alone in patients with advanced colorectal cancer: The
Canadian cancer trials group CO.26 study. JAMA Oncol. 6:831–838.
2020. View Article : Google Scholar : PubMed/NCBI
|
|
141
|
Overman MJ, Lonardi S, Wong KYM, Lenz HJ,
Gelsomino F, Aglietta M, Morse MA, Van Cutsem E, McDermott R, Hill
A, et al: Durable clinical benefit with nivolumab plus ipilimumab
in DNA mismatch repair-deficient/microsatellite instability-high
metastatic colorectal cancer. J Clin Oncol. 36:773–779. 2018.
View Article : Google Scholar : PubMed/NCBI
|
|
142
|
Thibaudin M, Fumet JD, Chibaudel B,
Bennouna J, Borg C, Martin-Babau J, Cohen R, Fonck M, Taieb J,
Limagne E, et al: First-line durvalumab and tremelimumab with
chemotherapy in RAS-mutated metastatic colorectal cancer: a phase
1b/2 trial. Nat Med. 29:2087–2098. 2023. View Article : Google Scholar : PubMed/NCBI
|
|
143
|
Shi T, Song X, Wang Y, Liu F and Wei J:
Combining oncolytic viruses with cancer immunotherapy: Establishing
a new generation of cancer treatment. Front Immunol. 11:6832020.
View Article : Google Scholar : PubMed/NCBI
|
|
144
|
Xu H, Russell SN, Steiner K, O'Neill E and
Jones KI: Targeting PI3K-gamma in myeloid driven tumour immune
suppression: A systematic review and meta-analysis of the
preclinical literature. Cancer Immunol Immunother. 73:2042024.
View Article : Google Scholar : PubMed/NCBI
|
|
145
|
Arai H, Battaglin F, Wang J, Lo JH, Soni
S, Zhang W and Lenz HJ: Molecular insight of regorafenib treatment
for colorectal cancer. Cancer Treat Rev. 81:1019122019. View Article : Google Scholar : PubMed/NCBI
|
|
146
|
Zhou J, Ji Q and Li Q: Resistance to
anti-EGFR therapies in metastatic colorectal cancer: Underlying
mechanisms and reversal strategies. J Exp Clin Cancer Res.
40:3282021. View Article : Google Scholar : PubMed/NCBI
|
|
147
|
Leiphrakpam PD and Are C: PI3K/Akt/mTOR
signaling pathway as a target for colorectal cancer treatment. Int
J Mol Sci. 25:31782024. View Article : Google Scholar : PubMed/NCBI
|
|
148
|
Stefani C, Miricescu D, Stanescu-Spinu II,
Nica RI, Greabu M, Totan AR and Jinga M: Growth factors,
PI3K/AKT/mTOR and MAPK signaling pathways in colorectal cancer
pathogenesis: Where are we now? Int J Mol Sci. 22:102602021.
View Article : Google Scholar : PubMed/NCBI
|
|
149
|
Diaz LA Jr, Shiu KK, Kim TW, Jensen BV,
Jensen LH, Punt C, Smith D, Garcia-Carbonero R, Benavides M, Gibbs
P, et al: Pembrolizumab versus chemotherapy for microsatellite
instability-high or mismatch repair-deficient metastatic colorectal
cancer (KEYNOTE-177): Final analysis of a randomised, open-label,
phase 3 study. Lancet Oncol. 23:659–670. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
150
|
Kalyan A, Kircher S, Shah H, Mulcahy M and
Benson A: Updates on immunotherapy for colorectal cancer. J
Gastrointest Oncol. 9:160–169. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
151
|
Huo G, Liu W, Zhang S and Chen P: Efficacy
of PD-1/PD-L1 plus CTLA-4 inhibitors in solid tumors based on
clinical characteristics: a meta-analysis. Immunotherapy.
15:189–207. 2023. View Article : Google Scholar : PubMed/NCBI
|
|
152
|
Shi Y, Wang X, Meng Y, Ma J, Zhang Q, Shao
G, Wang L, Cheng X, Hong X, Wang Y, et al: A novel mechanism of
endoplasmic reticulum stress- and c-Myc-degradation-mediated
therapeutic benefits of antineurokinin-1 receptor drugs in
colorectal cancer. Adv Sci (Weinh). 8:e21019362021. View Article : Google Scholar : PubMed/NCBI
|
|
153
|
Benboubker V, Ramzy GM, Jacobs S and
Nowak-Sliwinska P: Challenges in validation of combination
treatment strategies for CRC using patient-derived organoids. J Exp
Clin Cancer Res. 43:2592024. View Article : Google Scholar : PubMed/NCBI
|
|
154
|
Sillo TO, Beggs AD, Morton DG and
Middleton G: Mechanisms of immunogenicity in colorectal cancer. Br
J Surg. 106:1283–1297. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
155
|
Hu Z, Li Y, Yang J, Liu J, Zhou H, Sun C,
Tian C, Zhu C, Shao M, Wang S, et al: Improved antitumor
effectiveness of oncolytic HSV-1 viruses engineered with
IL-15/IL-15Rα complex combined with oncolytic HSV-1-aPD1 targets
colon cancer. Sci Rep. 14:236712024. View Article : Google Scholar
|
|
156
|
Bommareddy PK, Shettigar M and Kaufman HL:
Integrating oncolytic viruses in combination cancer immunotherapy.
Nat Rev Immunol. 18:498–513. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
157
|
Liu Z, Ravindranathan R, Kalinski P, Guo
ZS and Bartlett DL: Rational combination of oncolytic vaccinia
virus and PD-L1 blockade works synergistically to enhance
therapeutic efficacy. Nat Commun. 8:147542017. View Article : Google Scholar : PubMed/NCBI
|
|
158
|
Xie X, Lv J, Zhu W, Tian C, Li J, Liu J,
Zhou H, Sun C, Hu Z and Li X: The combination therapy of oncolytic
HSV-1 armed with anti-PD-1 antibody and IL-12 enhances anti-tumor
efficacy. Transl Oncol. 15:1012872022. View Article : Google Scholar
|
|
159
|
Jung I, Shin S, Baek MC and Yea K:
Modification of immune cell-derived exosomes for enhanced cancer
immunotherapy: Current advances and therapeutic applications. Exp
Mol Med. 56:19–31. 2024. View Article : Google Scholar : PubMed/NCBI
|
|
160
|
Fan T, Zhang M, Yang J, Zhu Z, Cao W and
Dong C: Therapeutic cancer vaccines: Advancements, challenges, and
prospects. Signal Transduct Target Ther. 8:4502023. View Article : Google Scholar : PubMed/NCBI
|
|
161
|
Senzer NN, Kaufman H, Amatruda T,
Nemunaitis M, Daniels GA, Glaspy J, Goldsweig H, Coffin RS and
Nemunaitis J: Phase II clinical trial with a second generation,
GM-CSF encoding, oncolytic herpesvirus in unresectable metastatic
melanoma. J Clin Oncol. 27(15 Suppl): S90352009. View Article : Google Scholar
|
|
162
|
Ott PA and Hodi FS: Talimogene
laherparepvec for the treatment of advanced melanoma. Clin Cancer
Res. 22:3127–3131. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
163
|
Ylösmäki E, Ylösmäki L, Fusciello M,
Martins B, Ahokas P, Cojoc H, Uoti A, Feola S, Kreutzman A, Ranki
T, et al: Characterization of a novel OX40 ligand and CD40
ligand-expressing oncolytic adenovirus used in the PeptiCRAd cancer
vaccine platform. Mol Ther Oncolytics. 20:459–469. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
164
|
Huang Q, Cai WQ, Han ZW, Wang MY, Zhou Y,
Cheng JT, Zhang Y, Wang YY, Xin Q, Wang XW, et al: Bispecific T
cell engagers and their synergistic tumor immunotherapy with
oncolytic viruses. Am J Cancer Res. 11:2430–2455. 2021.PubMed/NCBI
|
|
165
|
Guo ZS, Lotze MT, Zhu Z, Storkus WJ and
Song XT: Bi- and tri-specific T cell engager-armed oncolytic
viruses: Next-generation cancer immunotherapy. Biomedicines.
8:2042020. View Article : Google Scholar : PubMed/NCBI
|
|
166
|
Jiang J, Li J, Zhou X, Zhao X, Huang B and
Qin Y: Exosomes regulate the epithelial-mesenchymal transition in
cancer. Front Oncol. 12:8649802022. View Article : Google Scholar : PubMed/NCBI
|
|
167
|
Xiang Z, Hua M, Hao Z, Biao H, Zhu C, Zhai
G and Wu J: The roles of mesenchymal stem cells in gastrointestinal
cancers. Front Immunol. 13:8440012022. View Article : Google Scholar : PubMed/NCBI
|
|
168
|
Rosenberg SA, Yang JC, Sherry RM, Kammula
US, Hughes MS, Phan GQ, Citrin DE, Restifo NP, Robbins PF,
Wunderlich JR, et al: Durable complete responses in heavily
pretreated patients with metastatic melanoma using T-cell transfer
immunotherapy. Clin Cancer Res. 17:4550–4557. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
169
|
Nguyen HM, Bommareddy PK, Silk AW and Saha
D: Optimal timing of PD-1 blockade in combination with oncolytic
virus therapy. Semin Cancer Biol. 86:971–980. 2022. View Article : Google Scholar
|
|
170
|
Pol J, Kroemer G and Galluzzi L: First
oncolytic virus approved for melanoma immunotherapy.
Oncoimmunology. 5:e11156412015. View Article : Google Scholar
|
|
171
|
Overman MJ, Ernstoff MS and Morse MA:
Where we stand with immunotherapy in colorectal cancer: deficient
mismatch repair, proficient mismatch repair, and toxicity
management. Am Soc Clin Oncol Educ Book. 38:239–247. 2018.
View Article : Google Scholar : PubMed/NCBI
|
|
172
|
Creasy JM, Sadot E, Koerkamp BG, Chou JF,
Gonen M, Kemeny NE, Balachandran VP, Kingham TP, DeMatteo RP, Allen
PJ, et al: Actual 10-year survival after hepatic resection of
colorectal liver metastases: What factors preclude cure? Surgery.
163:1238–1244. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
173
|
Yu J, Green MD, Li S, Sun Y, Journey SN,
Choi JE, Rizvi SM, Qin A, Waninger JJ, Lang X, et al: Liver
metastasis restrains immunotherapy efficacy via macrophage-mediated
T cell elimination. Nat Med. 27:152–164. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
174
|
Garg P, Pareek S, Kulkarni P, Horne D,
Salgia R and Singhal SS: Next-generation immunotherapy: Advancing
clinical applications in cancer treatment. J Clin Med. 13:65372024.
View Article : Google Scholar : PubMed/NCBI
|
|
175
|
Jin KT, Du WL, Liu YY, Lan HR, Si JX and
Mou XZ: Oncolytic virotherapy in solid tumors: The challenges and
achievements. Cancers (Basel). 13:5882021. View Article : Google Scholar : PubMed/NCBI
|
|
176
|
Chen Q, Zhang H, Wu M, Wang Q, Luo L, Ma
H, Zhang X and He S: Discovery of a potent hedgehog pathway
inhibitor capable of activating caspase8-dependent apoptosis. J
Pharmacol Sci. 137:256–264. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
177
|
Albring KF, Weidemüller J, Mittag S,
Weiske J, Friedrich K, Geroni MC, Lombardi P and Huber O: Berberine
acts as a natural inhibitor of Wnt/β-catenin
signaling-identification of more active 13-arylalkyl derivatives.
Biofactors. 39:652–662. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
178
|
García-Foncillas J, Sunakawa Y, Aderka D,
Wainberg Z, Ronga P, Witzler P and Stintzing S: Distinguishing
features of cetuximab and panitumumab in colorectal cancer and
other solid tumors. Front Oncol. 9:8492019. View Article : Google Scholar : PubMed/NCBI
|
|
179
|
Xie YH, Chen YX and Fang JY: Comprehensive
review of targeted therapy for colorectal cancer. Signal Transduct
Target Ther. 5:222020. View Article : Google Scholar : PubMed/NCBI
|
|
180
|
Wang D, Zhang H, Xiang T and Wang G:
Clinical application of adaptive immune therapy in MSS colorectal
cancer patients. Front Immunol. 12:7623412021. View Article : Google Scholar : PubMed/NCBI
|
|
181
|
Fukuoka S, Hara H, Takahashi N, Kojima T,
Kawazoe A, Asayama M, Yoshii T, Kotani D, Tamura H, Mikamoto Y, et
al: Regorafenib plus nivolumab in patients with advanced gastric or
colorectal cancer: An open-label, dose-escalation, and
dose-expansion phase Ib trial (REGONIVO, EPOC1603). J Clin Oncol.
38:2053–2061. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
182
|
Hurwitz H, Fehrenbacher L, Novotny W,
Cartwright T, Hainsworth J, Heim W, Berlin J, Baron A, Griffing S,
Holmgren E, et al: Bevacizumab plus irinotecan, fluorouracil, and
leucovorin for metastatic colorectal cancer. N Engl J Med.
350:2335–2342. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
183
|
Qin Y, Ma FY, Zhang Z, Zhao CH and Huang
B: Vascular endothelial growth factor pathway's influence on
bevacizumab efficacy in metastatic colorectal cancer treatment.
World J Gastrointest Oncol. 16:4514–4517. 2024. View Article : Google Scholar : PubMed/NCBI
|
|
184
|
Tilgase A, Olmane E, Nazarovs J, Brokāne
L, Erdmanis R, Rasa A and Alberts P: Multimodality treatment of a
colorectal cancer stage IV patient with FOLFOX-4, bevacizumab,
rigvir oncolytic virus, and surgery. Case Rep Gastroenterol.
12:457–465. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
185
|
Wu Z, Ichinose T, Naoe Y, Matsumura S,
Villalobos IB, Eissa IR, Yamada S, Miyajima N, Morimoto D, Mukoyama
N, et al: Combination of cetuximab and oncolytic virus canerpaturev
synergistically inhibits human colorectal cancer growth. Mol Ther
Oncolytics. 13:107–115. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
186
|
Van Cutsem E, Tabernero J, Lakomy R,
Prenen H, Prausová J, Macarulla T, Ruff P, van Hazel GA, Moiseyenko
V, Ferry D, et al: Addition of aflibercept to fluorouracil,
leucovorin, and irinotecan improves survival in a phase III
randomized trial in patients with metastatic colorectal cancer
previously treated with an oxaliplatin-based regimen. J Clin Oncol.
30:3499–3506. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
187
|
Holch JW, Ricard I, Stintzing S, Modest DP
and Heinemann V: The relevance of primary tumour location in
patients with metastatic colorectal cancer: A meta-analysis of
first-line clinical trials. Eur J Cancer. 70:87–98. 2017.
View Article : Google Scholar
|
|
188
|
Schmoll HJ, Tabernero J, Maroun J, de
Braud F, Price T, Van Cutsem E, Hill M, Hoersch S, Rittweger K and
Haller DG: Capecitabine plus oxaliplatin compared with
fluorouracil/folinic acid as adjuvant therapy for stage iii colon
cancer: Final results of the NO16968 randomized controlled phase
III trial. J Clin Oncol. 33:3733–3740. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
189
|
Lenz HJ, Ou FS, Venook AP, Hochster HS,
Niedzwiecki D, Goldberg RM, Mayer RJ, Bertagnolli MM, Blanke CD,
Zemla T, et al: Impact of consensus molecular subtype on survival
in patients with metastatic colorectal cancer: Results from
CALGB/SWOG 80405 (alliance). J Clin Oncol. 37:1876–1885. 2019.
View Article : Google Scholar : PubMed/NCBI
|
|
190
|
Dienstmann R, Vermeulen L, Guinney J,
Kopetz S, Tejpar S and Tabernero J: Consensus molecular subtypes
and the evolution of precision medicine in colorectal cancer. Nat
Rev Cancer. 17:79–92. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
191
|
Westphalen CB, Martins-Branco D, Beal JR,
Cardone C, Coleman N, Schram AM, Halabi S, Michiels S, Yap C, André
F, et al: The ESMO tumour-agnostic classifier and screener
(ETAC-S): A tool for assessing tumour-agnostic potential of
molecularly guided therapies and for steering drug development. Ann
Oncol. 35:936–953. 2024. View Article : Google Scholar : PubMed/NCBI
|
|
192
|
McKean WB, Moser JC, Rimm D and
Hu-Lieskovan S: Biomarkers in precision cancer immunotherapy:
Promise and challenges. Am Soc Clin Oncol Educ Book. 40:e275–e291.
2020. View Article : Google Scholar : PubMed/NCBI
|
|
193
|
Steuerwald NM, Morris S, Nguyen DG and
Patel JN: Understanding the biology and testing techniques for
pharmacogenomics in oncology: A practical guide for the clinician.
JCO Oncol Pract. 20:1441–1451. 2024. View Article : Google Scholar : PubMed/NCBI
|
|
194
|
Lișcu HD, Verga N, Atasiei DI, Badiu DC,
Dumitru AV, Ultimescu F, Pavel C, Stefan RE, Manole DC and Ionescu
AI: Biomarkers in colorectal cancer: Actual and future
perspectives. Int J Mol Sci. 25:115352024. View Article : Google Scholar :
|
|
195
|
Marcus L, Fashoyin-Aje LA, Donoghue M,
Yuan M, Rodriguez L, Gallagher PS, Philip R, Ghosh S, Theoret MR,
Beaver JA, et al: FDA approval summary: Pembrolizumab for the
treatment of tumor mutational burden-high solid tumors. Clin Cancer
Res. 27:4685–4689. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
196
|
Yarchoan M, Hopkins A and Jaffee EM: Tumor
mutational burden and response rate to PD-1 inhibition. N Engl J
Med. 377:2500–2501. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
197
|
Puccini A, Seeber A and Berger MD:
Biomarkers in metastatic colorectal cancer: Status quo and future
perspective. Cancers (Basel). 14:48282022. View Article : Google Scholar : PubMed/NCBI
|
|
198
|
Sunshine J and Taube JM: PD-1/PD-L1
inhibitors. Curr Opin Pharmacol. 23:32–38. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
199
|
Matsuhashi N, Yamada T, Nagasaka T,
Kataoka K, Sakamoto K, Koda K, Hiramatsu K, Matsuoka H, Kuramochi
H, Ishida H, et al: Impact of RAS and BRAF heterogeneity on the
efficacy of EGFR blockade in patients with metastatic colorectal
cancer. J Clin Oncol. 43(4 Suppl): S2552025. View Article : Google Scholar
|
|
200
|
Ogunwobi OO, Mahmood F and Akingboye A:
Biomarkers in colorectal cancer: Current research and future
prospects. Int J Mol Sci. 21:53112020. View Article : Google Scholar : PubMed/NCBI
|
|
201
|
De Mattia E, Polesel J, Mezzalira S,
Palazzari E, Pollesel S, Toffoli G and Cecchin E: Predictive and
prognostic value of oncogene mutations and microsatellite
instability in locally-advanced rectal cancer treated with
neoadjuvant radiation-based therapy: A systematic review and
meta-analysis. Cancers (Basel). 15:14692023. View Article : Google Scholar : PubMed/NCBI
|
|
202
|
Santollani L, Maiorino L, Zhang YJ,
Palmeri JR, Stinson JA, Duhamel LR, Qureshi K, Suggs JR, Porth OT,
Pinney W III, et al: Local delivery of cell surface-targeted
immunocytokines programs systemic antitumor immunity. Nat Immunol.
25:1820–1829. 2024. View Article : Google Scholar : PubMed/NCBI
|
|
203
|
Zhang Y, Shi X, Shen Y, Dong X, He R, Chen
G, Zhang Y, Tan H and Zhang K: Nanoengineering-armed oncolytic
viruses drive antitumor response: Progress and challenges. MedComm.
5:e7552024. View Article : Google Scholar : PubMed/NCBI
|
|
204
|
Hwang SR, Chakraborty K, An JM, Mondal J,
Yoon HY and Lee Y: Pharmaceutical aspects of nanocarriers for smart
anticancer therapy. Pharmaceutics. 13:18752021. View Article : Google Scholar : PubMed/NCBI
|
|
205
|
Choi JW, Nam JP, Nam K, Lee YS, Yun CO and
Kim SW: Oncolytic adenovirus coated with multidegradable
bioreducible core-cross-linked polyethylenimine for cancer gene
therapy. Biomacromolecules. 16:2132–2143. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
206
|
Yang Y, Liu Q, Wang M, Li L, Yu Y, Pan M,
Hu D, Chu B, Qu Y and Qian Z: Genetically programmable cell
membrane-camouflaged nanoparticles for targeted combination therapy
of colorectal cancer. Signal Transduct Target Ther. 9:1582024.
View Article : Google Scholar : PubMed/NCBI
|
|
207
|
Fang C, Xiao G, Wang T, Song L, Peng B, Xu
B and Zhang K: Emerging nano-/biotechnology drives oncolytic
virus-activated and combined cancer immunotherapy. Research (Wash D
C). 6:01082023.PubMed/NCBI
|
|
208
|
Zhou F, Zhu H and Fu C: Editorial:
Clinical therapeutic development against cancers resistant to
targeted therapies. Front Pharmacol. 12:8168962022. View Article : Google Scholar : PubMed/NCBI
|
|
209
|
Kaufman HL, Kohlhapp FJ and Zloza A:
Erratum: Oncolytic viruses: A new class of immunotherapy drugs. Nat
Rev Drug Discov. 15:6602016. View Article : Google Scholar
|
|
210
|
Lichty BD, Breitbach CJ, Stojdl DF and
Bell JC: Going viral with cancer immunotherapy. Nat Rev Cancer.
14:559–567. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
211
|
Lawler SE, Speranza MC, Cho CF and Chiocca
EA: Oncolytic viruses in cancer treatment: A review. JAMA Oncol.
3:841–849. 2017. View Article : Google Scholar
|
|
212
|
Li X and Cheng Z: Oncolytic viruses in
cancer immunotherapy. Adv Ther. 7:23004452024. View Article : Google Scholar
|
|
213
|
Mariathasan S, Turley SJ, Nickles D,
Castiglioni A, Yuen K, Wang Y, Kadel EE III, Koeppen H, Astarita
JL, Cubas R, et al: TGFβ attenuates tumour response to PD-L1
blockade by contributing to exclusion of T cells. Nature.
554:544–548. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
214
|
Lv D, Fei Y, Chen H, Wang J, Han W, Cui B,
Feng Y, Zhang P and Chen J: Crosstalk between T lymphocyte and
extracellular matrix in tumor microenvironment. Front Immunol.
15:13407022024. View Article : Google Scholar : PubMed/NCBI
|
|
215
|
Fox CR and Parks GD: Histone deacetylase
inhibitors enhance cell killing and block interferon-beta synthesis
elicited by infection with an oncolytic parainfluenza virus.
Viruses. 11:4312019. View Article : Google Scholar : PubMed/NCBI
|
|
216
|
Jennings VA, Scott GB, Rose AMS, Scott KJ,
Migneco G, Keller B, Reilly K, Donnelly O, Peach H, Dewar D, et al:
Potentiating oncolytic virus-induced immune-mediated tumor cell
killing using histone deacetylase inhibition. Mol Ther.
27:1139–1152. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
217
|
Roulstone V, Pedersen M, Kyula J,
Mansfield D, Khan AA, McEntee G, Wilkinson M, Karapanagiotou E,
Coffey M, Marais R, et al: BRAF- and MEK-targeted small molecule
inhibitors exert enhanced antimelanoma effects in combination with
oncolytic reovirus through ER stress. Mol Ther. 23:931–942. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
218
|
De Henau O, Rausch M, Winkler D, Campesato
LF, Liu C, Cymerman DH, Budhu S, Ghosh A, Pink M, Tchaicha J, et
al: Overcoming resistance to checkpoint blockade therapy by
targeting PI3Kγ in myeloid cells. Nature. 539:443–447. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
219
|
Choi HJ, Chung TW, Kang SK, Lee YC, Ko JH,
Kim JG and Kim CH: Ganglioside GM3 modulates tumor suppressor
PTEN-mediated cell cycle progression-transcriptional induction of
p21(WAF1) and p27(kip1) by inhibition of PI-3K/AKT pathway.
Glycobiology. 16:573–583. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
220
|
Kazanets A, Shorstova T, Hilmi K, Marques
M and Witcher M: Epigenetic silencing of tumor suppressor genes:
Paradigms, puzzles, and potential. Biochim Biophys Acta.
1865:275–288. 2016.PubMed/NCBI
|
|
221
|
Wallace BD, Wang H, Lane KT, Scott JE,
Orans J, Koo JS, Venkatesh M, Jobin C, Yeh LA, Mani S and Redinbo
MR: Alleviating cancer drug toxicity by inhibiting a bacterial
enzyme. Science. 330:831–835. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
222
|
Jung G, Hernández-Illán E, Moreira L,
Balaguer F and Goel A: Epigenetics of colorectal cancer: Biomarker
and therapeutic potential. Nat Rev Gastroenterol Hepatol.
17:111–130. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
223
|
Kaur M, Frahm F, Lu Y, Ascha MS, Guadamuz
JS, Dotan E, Gottesman AS, Leybovich BC, Sondhi A, Zhao Y, et al:
Broadening eligibility criteria and diversity among patients for
cancer clinical trials. NEJM Evid. 3:EVIDoa23002362024. View Article : Google Scholar : PubMed/NCBI
|
|
224
|
Veen T, Kanani A, Lea D and Søreide K:
Clinical trials of neoadjuvant immune checkpoint inhibitors for
early-stage operable colon and rectal cancer. Cancer Immunol
Immunother. 72:3135–3147. 2023. View Article : Google Scholar : PubMed/NCBI
|
|
225
|
Li C, Ferro A, Mhatre SK, Lu D, Lawrance
M, Li X, Li S, Allen S, Desai J, Fakih M, et al: Hybrid-control arm
construction using historical trial data for an early-phase,
randomized controlled trial in metastatic colorectal cancer. Commun
Med (Lond). 2:902022. View Article : Google Scholar : PubMed/NCBI
|
|
226
|
Qi Z, Gu J, Qu L, Shi X, He Z, Sun J, Tan
L and Sun M: Advancements of engineered live oncolytic
biotherapeutics (microbe/virus/cells): Preclinical research and
clinical progress. J Control Release. 375:209–235. 2024. View Article : Google Scholar : PubMed/NCBI
|
|
227
|
Xu Z, Li W, Dong X, Chen Y, Zhang D, Wang
J, Zhou L and He G: Precision medicine in colorectal cancer:
Leveraging multi-omics, spatial omics, and artificial intelligence.
Clin Chim Acta. 559:1196862024. View Article : Google Scholar : PubMed/NCBI
|
|
228
|
Cornish AJ, Gruber AJ, Kinnersley B, Chubb
D, Frangou A, Caravagna G, Noyvert B, Lakatos E, Wood HM, Thorn S,
et al: The genomic landscape of 2,023 colorectal cancers. Nature.
633:127–136. 2024. View Article : Google Scholar : PubMed/NCBI
|
|
229
|
Gujar S, Pol JG, Kim Y, Lee PW and Kroemer
G: Antitumor benefits of antiviral immunity: An underappreciated
aspect of oncolytic virotherapies. Trends Immunol. 39:209–221.
2018. View Article : Google Scholar
|
|
230
|
Su Y, Su C and Qin L: Current landscape
and perspective of oncolytic viruses and their combination
therapies. Transl Oncol. 25:1015302022. View Article : Google Scholar : PubMed/NCBI
|
|
231
|
Bi Y, Sun L, Gao D, Ding C, Li Z, Li Y,
Cun W and Li Q: High-efficiency targeted editing of large viral
genomes by RNA-guided nucleases. PLoS Pathog. 10:e10040902014.
View Article : Google Scholar : PubMed/NCBI
|
|
232
|
Hsu PD, Lander ES and Zhang F: Development
and applications of CRISPR-Cas9 for genome engineering. Cell.
157:1262–1278. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
233
|
Cornall AM, Phillips S, Cummins E, Garland
SM and Tabrizi SN: In vitro assessment of the effect of
vaccine-targeted human papillomavirus (HPV) depletion on detection
of non-vaccine HPV types: Implications for post-vaccine
surveillance studies. J Virol Methods. 214:10–14. 2015. View Article : Google Scholar
|
|
234
|
Monge C, Xie C, Myojin Y, Coffman K,
Hrones DM, Wang S, Hernandez JM, Wood BJ, Levy EB, Juburi I, et al:
Phase I/II study of PexaVec in combination with immune checkpoint
inhibition in refractory metastatic colorectal cancer. J Immunother
Cancer. 11:e0056402023. View Article : Google Scholar : PubMed/NCBI
|
|
235
|
Zhang B, Huang J, Tang J, Hu S, Luo S, Luo
Z, Zhou F, Tan S, Ying J, Chang Q, et al: Intratumoral OH2, an
oncolytic herpes simplex virus 2, in patients with advanced solid
tumors: A multicenter, phase I/II clinical trial. J Immunother
Cancer. 9:e0022242021. View Article : Google Scholar : PubMed/NCBI
|
|
236
|
Zhang L, Pakmehr SA, Shahhosseini R,
Hariri M, Fakhrioliaei A, Karkon Shayan F, Xiang W and Karkon
Shayan S: Oncolytic viruses improve cancer immunotherapy by
reprogramming solid tumor microenvironment. Med Oncol. 41:82023.
View Article : Google Scholar : PubMed/NCBI
|
|
237
|
Fakih M, Raghav KPS, Chang DZ, Larson T,
Cohn AL, Huyck TK, Cosgrove D, Fiorillo JA, Tam R, D'Adamo D, et
al: Regorafenib plus nivolumab in patients with mismatch
repair-proficient/microsatellite stable metastatic colorectal
cancer: A single-arm, open-label, multicentre phase 2 study.
EClinicalMedicine. 58:1019172023. View Article : Google Scholar : PubMed/NCBI
|
|
238
|
Wang N, Wang J, Zhang Z, Cao H, Yan W, Chu
Y, Chard Dunmall LS and Wang Y: A novel vaccinia virus enhances
anti-tumor efficacy and promotes a long-term anti-tumor response in
a murine model of colorectal cancer. Mol Ther Oncolytics. 20:71–81.
2020. View Article : Google Scholar
|
|
239
|
Chen L, Chen H, Ye J, Ge Y, Wang H, Dai E,
Ren J, Liu W, Ma C, Ju S, et al: Intratumoral expression of
interleukin 23 variants using oncolytic vaccinia virus elicit
potent antitumor effects on multiple tumor models via tumor
microenvironment modulation. Theranostics. 11:6668–6681. 2021.
View Article : Google Scholar : PubMed/NCBI
|
|
240
|
Deng L, Yang X, Fan J, Ding Y, Peng Y, Xu
D, Huang B and Hu Z: IL-24-Armed oncolytic vaccinia virus exerts
potent antitumor effects via multiple pathways in colorectal
cancer. Oncol Res. 28:579–590. 2021. View Article : Google Scholar :
|
|
241
|
Li J, O'Malley M, Sampath P, Kalinski P,
Bartlett DL and Thorne SH: Expression of CCL19 from oncolytic
vaccinia enhances immunotherapeutic potential while maintaining
oncolytic activity. Neoplasia. 14:1115–1121. 2012. View Article : Google Scholar
|
|
242
|
Flanagan K, Glover RT, Hörig H, Yang W and
Kaufman HL: Local delivery of recombinant vaccinia virus expressing
secondary lymphoid chemokine (SLC) results in a CD4 T-cell
dependent antitumor response. Vaccine. 22:2894–2903. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
243
|
Bereta M, Bereta J, Park J, Medina F, Kwak
H and Kaufman HL: Immune properties of recombinant vaccinia virus
encoding CD154 (CD40L) are determined by expression of virally
encoded CD40L and the presence of CD40L protein in viral particles.
Cancer Gene Ther. 11:808–818. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
244
|
Warner SG, Kim SI, Chaurasiya S, O'Leary
MP, Lu J, Sivanandam V, Woo Y, Chen NG and Fong Y: A novel chimeric
poxvirus encoding hNIS Is tumor-tropic, imageable, and synergistic
with radioiodine to sustain colon cancer regression. Mol Ther
Oncolytics. 13:82–92. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
245
|
Xing M, Wang X, Chi Y and Zhou D: Gene
therapy for colorectal cancer using adenovirus-mediated full-length
antibody, cetuximab. Oncotarget. 7:28262–28272. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
246
|
Rong Y, Ning Y, Zhu J, Feng P, Zhu W, Zhao
X, Xiong Z, Ruan C, Jin J, Wang H, et al: Oncolytic adenovirus
encoding decorin and CD40 ligand inhibits tumor growth and liver
metastasis via immune activation in murine colorectal tumor model.
Mol Biomed. 5:392024. View Article : Google Scholar : PubMed/NCBI
|
|
247
|
Nie ZL, Pan YQ, He BS, Gu L, Chen LP, Li
R, Xu YQ, Gao TY, Song GQ, Hoffman AR, et al: Gene therapy for
colorectal cancer by an oncolytic adenovirus that targets loss of
the insulin-like growth factor 2 imprinting system. Mol Cancer.
11:862012. View Article : Google Scholar : PubMed/NCBI
|
|
248
|
Hecht JR, Raman SS, Chan A, Kalinsky K,
Baurain JF, Jimenez MM, Garcia MM, Berger MD, Lauer UM, Khattak A,
et al: Phase Ib study of talimogene laherparepvec in combination
with atezolizumab in patients with triple negative breast cancer
and colorectal cancer with liver metastases. ESMO Open.
8:1008842023. View Article : Google Scholar : PubMed/NCBI
|
|
249
|
Tian L, Liu T, Jiang S, Cao Y, Kang K, Su
H, Ren G, Wang Z, Xiao W and Li D: Oncolytic Newcastle disease
virus expressing the co-stimulator OX40L as immunopotentiator for
colorectal cancer therapy. Gene Ther. 30:64–74. 2023. View Article : Google Scholar
|
|
250
|
Vigil A, Park MS, Martinez O, Chua MA,
Xiao S, Cros JF, Martínez-Sobrido L, Woo SL and García-Sastre A:
Use of reverse genetics to enhance the oncolytic properties of
Newcastle disease virus. Cancer Res. 67:8285–8292. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
251
|
Li X, Wang Z, Zhang S, Yao Q, Chen W and
Liu F: Ruxolitinib induces apoptosis of human colorectal cancer
cells by downregulating the JAK1/2-STAT1-Mcl-1 axis. Oncol Lett.
21:3522021. View Article : Google Scholar : PubMed/NCBI
|
|
252
|
Ghonime MG and Cassady KA: Combination
therapy using ruxolitinib and oncolytic HSV renders resistant
MPNSTs susceptible to virotherapy. Cancer Immunol Res. 6:1499–1510.
2018. View Article : Google Scholar : PubMed/NCBI
|
|
253
|
Hong YS, Hong SW, Kim SM, Jin DH, Shin JS,
Yoon DH, Kim KP, Lee JL, Heo DS, Lee JS and Kim TW: Bortezomib
induces G2-M arrest in human colon cancer cells through
ROS-inducible phosphorylation of ATM-CHK1. Int J Oncol. 41:76–82.
2012.PubMed/NCBI
|
|
254
|
Kim Y, Lee J, Lee D and Othmer HG:
Synergistic effects of bortezomib-OV therapy and anti-invasive
strategies in glioblastoma: A mathematical model. Cancers (Basel).
11:2152019. View Article : Google Scholar : PubMed/NCBI
|
|
255
|
Li X, Hu W, Shen J, Li M and Gong W:
Targeting proteasome enhances anticancer activity of oncolytic
HSV-1 in colorectal cancer. Virology. 578:13–21. 2023. View Article : Google Scholar
|
|
256
|
Dinu IM, Mihăilă M, Diculescu MM, Croitoru
VM, Turcu-Stiolica A, Bogdan D, Miron MI, Lungulescu CV,
Alexandrescu ST, Dumitrașcu T, et al: Bevacizumab treatment for
metastatic colorectal cancer in real-world clinical practice.
Medicina (Kaunas). 59:3502023. View Article : Google Scholar : PubMed/NCBI
|
|
257
|
Saoudi González N, Ros J, Baraibar I,
Salvà F, Rodríguez-Castells M, Alcaraz A, García A, Tabernero J and
Élez E: Cetuximab as a key partner in personalized targeted therapy
for metastatic colorectal cancer. Cancers (Basel). 16:4122024.
View Article : Google Scholar : PubMed/NCBI
|
|
258
|
Yang S, Li X, Guan W, Qian M, Yao Z, Yin X
and Zhao H: NVP-BKM120 inhibits colon cancer growth via
FoxO3a-dependent PUMA induction. Oncotarget. 8:83052–83062. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
259
|
Wang L, Ning J, Wakimoto H, Wu S, Wu CL,
Humphrey MR, Rabkin SD and Martuza RL: Oncolytic herpes simplex
virus and PI3K inhibitor BKM120 synergize to promote killing of
prostate cancer stem-like cells. Mol Ther Oncolytics. 13:58–66.
2019. View Article : Google Scholar : PubMed/NCBI
|
|
260
|
Soltantoyeh T, Akbari B, Shahosseini Z,
Mirzaei HR and Hadjati J: Simultaneous targeting of Tim3 and A2a
receptors modulates MSLN-CAR T cell antitumor function in a human
cervical tumor xenograft model. Front Immunol. 15:13629042024.
View Article : Google Scholar : PubMed/NCBI
|
|
261
|
Chin K, Chand VK and Nuyten DSA: Avelumab:
Clinical trial innovation and collaboration to advance anti-PD-L1
immunotherapy. Ann Oncol. 28:1658–1666. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
262
|
Redman JM, O'Sullivan Coyne G, Reed CT,
Madan RA, Strauss J, Steinberg SJ, Marté J, Cordes L, Heery C and
Gulley JL: Avelumab in patients with metastatic colorectal cancer.
Oncologist. 28:823–e804. 2023. View Article : Google Scholar : PubMed/NCBI
|
|
263
|
Español-Rego M, Fernández-Martos C, Elez
E, Foguet C, Pedrosa L, Rodríguez N, Ruiz-Casado A, Pineda E, Cid
J, Cabezón R, et al: A phase I-II multicenter trial with Avelumab
plus autologous dendritic cell vaccine in pre-treated mismatch
repair-proficient (MSS) metastatic colorectal cancer patients;
GEMCAD 1602 study. Cancer Immunol Immunother. 72:827–840. 2023.
View Article : Google Scholar :
|
|
264
|
Zhu WM and Middleton MR: Combination
therapies for the optimisation of bispecific T-cell engagers in
cancer treatment. Immunother Adv. 3:ltad0132023. View Article : Google Scholar : PubMed/NCBI
|
|
265
|
Heidbuechel JPW and Engeland CE: Oncolytic
viruses encoding bispecific T cell engagers: A blueprint for
emerging immunovirotherapies. J Hematol Oncol. 14:632021.
View Article : Google Scholar : PubMed/NCBI
|
|
266
|
Puca E, Schmitt-Koopmann C, Furter M,
Murer P, Probst P, Dihr M, Bajic D and Neri D: The targeted
delivery of interleukin-12 to the carcinoembryonic antigen
increases the intratumoral density of NK and CD8+ T cell
in an immunocompetent mouse model of colorectal cancer. J
Gastrointest Oncol. 11:803–811. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
267
|
Li Q, Oduro PK, Guo R, Li R, Leng L, Kong
X, Wang Q and Yang L: Oncolytic viruses: Immunotherapy drugs for
gastrointestinal malignant tumors. Front Cell Infect Microbiol.
12:9215342022. View Article : Google Scholar : PubMed/NCBI
|
|
268
|
Sumransub N, Vantanasiri K, Prakash A and
Lou E: Advances and new frontiers for immunotherapy in colorectal
cancer: Setting the stage for neoadjuvant success? Mol Ther
Oncolytics. 22:1–12. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
269
|
Yoo SY, Bang SY, Jeong SN, Kang DH and Heo
J: A cancer-favoring oncolytic vaccinia virus shows enhanced
suppression of stem-cell like colon cancer. Oncotarget.
7:16479–16489. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
270
|
Shebbo S, Binothman N, Darwaish M, Niaz
HA, Abdulal RH, Borjac J, Hashem AM and Mahmoud AB: Redefining the
battle against colorectal cancer: A comprehensive review of
emerging immunotherapies and their clinical efficacy. Front
Immunol. 15:13502082024. View Article : Google Scholar : PubMed/NCBI
|