Introduction
Cancer is one of the leading causes of death
worldwide, accounting for a considerable number of mortalities
among various human diseases (1). Colorectal cancer (CRC) accounts for
~10% of all cancer cases and ranks as the second leading cause of
cancer-related deaths worldwide (2). While CRC predominantly affects
individuals aged ≥50 years, the incidence and mortality rates among
younger adults <50 years of age have steadily increased over the
past 25 years (3). According to
the World Health Organization (WHO), >1.9 million new cases of
CRC were diagnosed globally in 2020, with >930,000 deaths
attributed to CRC in the same year. The WHO estimates that by 2040,
the incidence of CRC will increase by 63% to 3.2 million cases
annually, with deaths rising by 73% to 1.6 million per year
(2). In the United States,
cancer is the second leading cause of death, with CRC ranking as
the third most prevalent type of cancer (4). Surgical resection remains the
primary treatment option for CRC (5); however, when CRC is not detected at
an early stage or optimal surgical procedures are not feasible,
patient prognosis often worsens. Moreover, resection of primary or
metastatic tumors can sometimes promote tumor recurrence (6). In addition, chemical drugs, such as
5-fluorouracil (5-FU) and capecitabine, which are frequently
employed as anticancer treatments for CRC, often lead to side
effects including diarrhea, nausea and vomiting, and can lead to
cardiac toxicity. Furthermore, patients with genetic disorders,
such as dihydropyrimidine dehydrogenase deficiency, are at an
increased risk of neurotoxicity following the administration of
5-FU and capecitabine (7,8).
Consequently, research into natural compounds derived from plants
or fruits, which exhibit low toxicity in vivo, has gained
momentum as a promising approach for treating chronic diseases such
as cancer (9).
Gossypin (3,3′,4′,5,7,8-hexahydroxyflavone
8-glucoside), a flavone found in Hibiscus vitifolius, is a
secondary metabolite that contributes to plant pigmentation.
Traditionally, gossypin has been recognized for its anxiolytic,
antidiabetic, antioxidant, anti-inflammatory and anticancer
properties (10-15). In addition, recent studies have
also explored its potential cardioprotective effects with regard to
ischemic heart disease (16,17).
Apoptosis is characterized by morphological changes,
such as chromatin condensation, nuclear fragmentation, membrane
blebbing and cell shrinkage. During apoptosis, cells break down
into small, membrane-bound apoptotic bodies, which are removed
through phagocytosis without triggering inflammatory responses,
which is a major advantage of this process (18). Apoptosis is a self-regulatory
mechanism that eliminates abnormal cells, such as those with DNA
damage (19). In cancer,
inducing apoptosis can selectively target and remove cancer cells
without affecting surrounding healthy cells.
Autophagy is a physiological mechanism wherein
cellular organelles are sequestered within autophagosomes, which
then fuse with lysosomes for degradation. This process occurs in
all cells and is regulated in response to stress or nutrient
deprivation (20). Autophagy is
mediated by various autophagy-related (ATG) proteins recruited to
the cell membrane, and LC3 and Beclin 1 serve key roles in
autophagosome formation (21,22). As well as in cancer, autophagy is
an important process in aging, autoimmune diseases, Crohn's disease
and rheumatoid arthritis. While generally considered a survival
mechanism under conditions of cellular stress, previous studies
have suggested that the regulation of autophagy in tumors, combined
with anticancer drugs, can enhance tumor cell death (23,24).
The MAPK pathway is involved in cell survival,
proliferation and metastasis (25). The three main MAPK signaling
pathways include the ERK, JNK and p38 kinase pathways (26). ERK is involved in cell
proliferation, apoptosis and cytoskeletal remodeling, JNK regulates
cell proliferation and death through various targets (25,26), and p38 serves a central role in
cell cycle progression, metastasis and differentiation (27).
The present study used in vitro and in
vivo experiments to investigate whether gossypin induces
apoptosis and autophagy in HT-29 CRC cells, and to determine
whether these processes are mediated via the MAPK/JNK pathway.
Materials and methods
Reagents and antibodies
RPMI-1640 medium used for cell culture was purchased
from Welgene, Inc. Fetal bovine serum (FBS) and penicillin were
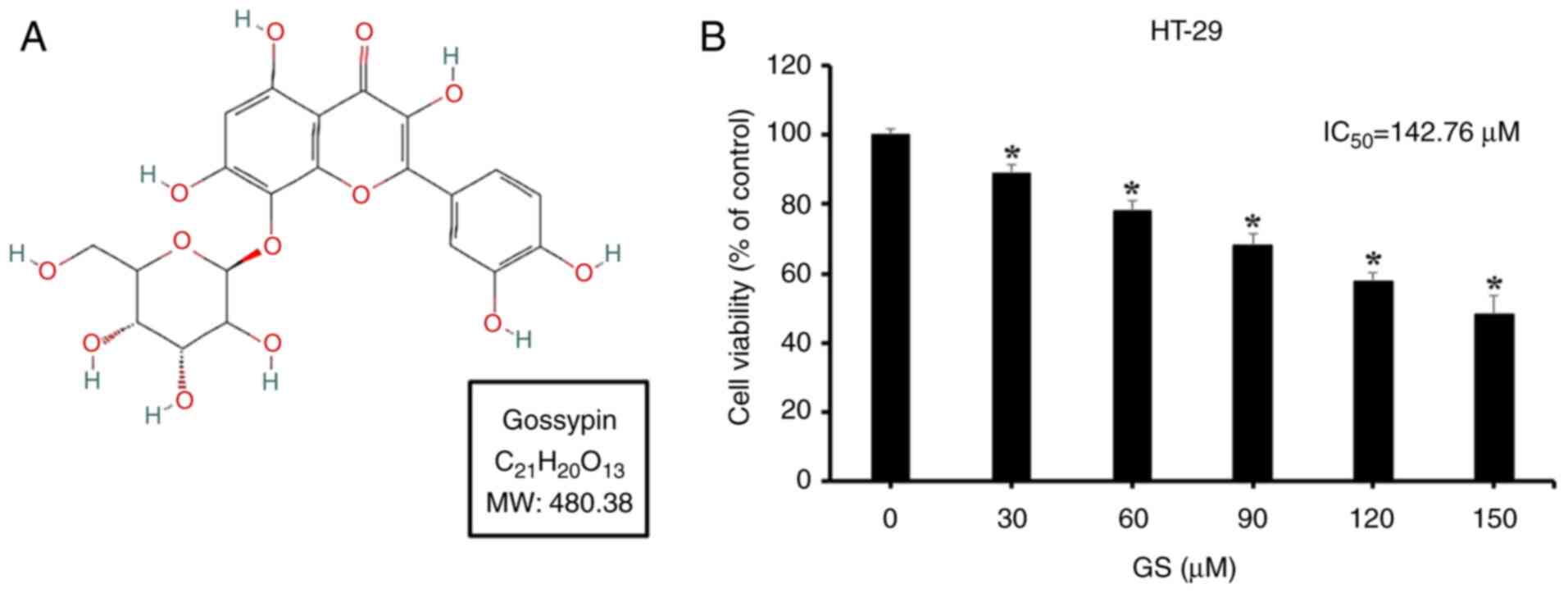
obtained from Gibco; Thermo Fisher Scientific, Inc. Gossypin
(Fig. 1A; purity confirmed by
HPLC: 98.5%) was purchased from Apollo Scientific. General
reagents, dimethyl sulfoxide (DMSO), MTT, DAPI and the JNK
inhibitor SP600125 were procured from MilliporeSigma. The
FITC-Annexin-V detection kit (cat. no. 556547) was procured from BD
Pharmingen; BD Biosciences. Primary antibodies against Bax (rabbit;
1:1,000; cat. no. 2772), Bcl-2 (rabbit; 1:1,000; cat. no. 4223),
PARP (rabbit; 1:1,000; cat. no. 9542), JNK (rabbit; 1:1,000; cat.
no. 9252), phosphorylated (p-)JNK (rabbit, 1:1,000, cat. no. 4668),
ERK (rabbit; 1:1,000; cat. no. 9102), p-ERK (rabbit; 1:1,000; cat.
no. 9101), p38 (rabbit; 1:1,000; cat. no. 9212), p-p38 (rabbit;
1:1,000; cat. no. 9211), mTOR (rabbit; 1:1,1000; cat. no. 2983),
p-mTOR (rabbit; 1:1,000; cat. no. 2971), Beclin 1 (rabbit; 1:1,000;
cat. no. 3738), and LC3 (rabbit; 1:1,000; cat. no. 2775), and the
HRP-conjugated goat anti-rabbit IgG secondary antibody (1:2,000;
cat. no. 7074), were obtained from Cell Signaling Technology, Inc.
Primary anti-β-actin (mouse; 1:1,000; cat. no. sc-47778) and
HRP-conjugated secondary mouse IgG (1:2,000; cat. no. sc-516102)
antibodies were purchased from Santa Cruz Biotechnology, Inc.
Hydroxychloroquine (HCQ) and 3-methyladenine (3-MA) were acquired
from Selleck Chemicals.
Cell culture and treatment
The human CRC cell line HT-29 (cat. no. 30038) was
obtained from the Korean Cell Line Bank; Korean Cell Line Research
Foundation. Cells were cultured in RPMI-1640 medium supplemented
with 5% FBS and 1% penicillin/streptomycin/neomycin at 37°C in an
atmosphere containing 5% CO2. When the bottom surface of
the culture flask reached 80-90% confluence, the cells were washed
with PBS and passaged using a cell scraper. The medium was replaced
every 2-3 days. Gossypin was dissolved in DMSO at concentrations of
30, 60, 90, 120 and 150 µM, and stored at -20°C. HT-29 cells
were treated with gossypin at 37°C in 5% CO2 for 24 h.
For pretreatment of HT-29 cells, the autophagy inhibitors 3-MA (2
mM) and HCQ (20 µM) were dissolved in the medium and the
cells were pretreated at 37°C and 5% CO2 for 2 h. In
addition, the JNK inhibitor SP600125 (10 µM) was dissolved
in DMSO, added to the medium and used to pretreat HT-29 cells at
37°C and 5% CO2 for 2 h.
MTT assay
The MTT assay was conducted to test the inhibitory
effects of gossypin on HT-29 cell viability. HT-29 cells were
seeded into a 96-well plate at a density of 2×104
cells/ml and incubated for 24 h. Thereafter, the cells were treated
with 0, 30, 60, 90, 120 and 150 µM gossypin and incubated at
37°C in the presence of 5% CO2 for 24 h. After
treatment, 40 µl MTT reagent was added to each well, and the
plate was incubated at 37°C in 5% CO2 for 2 h.
Subsequently, the MTT solution was removed, and 100 µl DMSO
was added to each well to dissolve formazan crystals. Absorbance
was measured at 595 nm using a microplate reader (Bio-Rad
Laboratories, Inc.).
DAPI staining
DAPI staining was performed to observe
apoptosis-associated morphological changes in the nuclei of
gossypin-treated HT-29 cells. HT-29 cells were seeded in a 60-mm
dish at a density of 1×105 cells/ml and incubated at
37°C in 5% CO2 for 24 h. Cells were then treated with 0,
60 and 120 µM gossypin and incubated for another 24 h.
Thereafter, the dishes were washed with PBS, fixed in 4%
formaldehyde at room temperature for 15 min and washed again using
PBS. DAPI solution was then added (2 ml) and the cells were
observed using a fluorescence microscope (Zeiss AG).
Flow cytometry
Annexin V/propidium iodide (PI) staining was
conducted to quantitatively analyze gossypin-induced apoptosis in
HT-29 cells. HT-29 cells were treated with 0, 60 and 120 µM
gossypin at 37°C in 5% CO2 for 24 h. Cells were then
washed with PBS and harvested using a cell scraper, followed by
centrifugation at 260 × g for 5 min at 4°C. The cell pellet was
resuspended in 1X Annexin V binding buffer at a density of
1×106 cells/ml, and the cells were then treated with
FITC-conjugated Annexin V and PI (binding buffer:FITC-conjugated
Annexin V/PI, 20:1, v/v) in the dark at room temperature for 15
min. Subsequently, the samples were analyzed using a FACSCalibur™
flow cytometer (BD Biosciences) with BD FACSuite™ software version
1.0.6 (BD Biosciences).
Western blotting
Western blotting was performed to analyze the
protein expression levels in gossypin-treated HT-29 cells. HT-29
cells were cultured in a 75T flask at 37°C in 5% CO2 for
24 h and then treated with gossypin at concentrations of 0, 60 and
120 µM at 37°C in 5% CO2 for 24 h. Cells were
then harvested using a cell scraper and centrifuged at 260 × g for
5 min at 4°C. The cell pellet was lysed using cell lysis buffer
(PRO-PREP™ Protein Extraction Solution; Invitrogen; Thermo Fisher
Scientific, Inc.) at 4°C for 20 min. The lysate was centrifuged at
15,920 × g for 5 min, and the supernatant was collected. The
protein concentration was measured using a Bradford protein assay.
Protein samples (60 µg/lane) were loaded and separated by
SDS-polyacrylamide gel electrophoresis on 12% gels and were
transferred to nitrocellulose membranes. Membranes were blocked
using 5% skim milk-TBS-Tween 20 (20 mM Tris·HCl, pH 7.5; 150 mM
NaCl; 0.1% Tween 20) at room temperature for 2 h, followed by
overnight incubation with primary antibodies in 5% skim milk-TBST
at 4°C. Membranes were then incubated with secondary antibodies in
5% skim milk-TBST at room temperature for 2 h, the protein bands
were visualized using ECL detection reagents (Pierce; Thermo Fisher
Scientific, Inc.) and band densities were analyzed using ImageJ
Launcher software version 1.52 (National Institutes of Health).
Acridine orange staining
To assess whether gossypin induces autophagy in
HT-29 cells, acridine orange staining was performed. After
culturing HT-29 cells in a 75T flask until they reached 80-90%
confluence, cells were seeded at 1×105 cells/ml in 60-mm
dishes and incubated for 24 h at 37°C in 5% CO2. The
medium was then replaced, and the cells were incubated with
gossypin at 0, 60 and 120 µM for 24 h at 37°C in 5%
CO2. After incubation, the cells were washed with PBS
and fixed in 4% paraformaldehyde solution at room temperature for
15 min. Subsequently, the cells were stained using 2 ml acridine
orange stain solution (Thermo Fisher Scientific, Inc.) at room
temperature for 12 min and were observed using a fluorescence
microscope (Zeiss AG).
Xenograft model
Animal experiments were reviewed and approved by the
Institutional Animal Care and Use Committee (IACUC) of Kongju
National University (approval no. IACUC-KNU_2024-07; Yesan, South
Korea). A total of 10 BALB/c nude female mice (weight, 18-22 g;
age, 4 weeks) were purchased from Nara Biotech Co., Ltd. The mice
were housed under a 12-h light/dark cycle at 23±3°C and 50±10%
humidity. Food and water supplies were restricted for 2 h before
and after administration; however, during the remaining time, food
and water were available ad libitum. The humane endpoints
were as follows: Tumor volume reached 10% of body weight, or if the
mouse exhibited signs of significant pain and distress. No mice met
these humane endpoints in the in vivo experiment and were
humanely euthanized at the conclusion of the planned study. For the
xenograft model, HT-29 cells (1×107 cells/ml) suspended
in RPMI-1641 with 50% FBS were subcutaneously injected into both
shoulders of the mice. After the tumors stabilized, gossypin (100
mg/kg, n=5) or vehicle control (0 mg/kg gossypin, PBS 10 ml/kg,
n=5) was orally administered five times per week for 4 weeks. Tumor
volumes were measured every 3 days using Vernier calipers (Mitutoyo
Corporation) and were calculated using the following formula: Tumor
volume (mm3)=0.5 × [width (mm)]2 × [length
(mm)]. At the end of the experiment, mice were euthanized using
CO2 gas (30% vol/min for 3 min). Mice were exposed to
CO2, and their movement and breathing were observed to
confirm that both had stopped. Afterward, the heartbeat was
confirmed to have stopped, and CO2 exposure was halted.
Euthanasia was verified by confirming that mice did not recover
within 10 min. Euthanized mice were autopsied and the tumors were
weighed.
Hematoxylin and eosin staining
The murine liver and kidneys tissue were fixed in
10% formaldehyde for 24 h at room temperature, embedded in paraffin
and sectioned into 5-µm slices. Sections were deparaffinized
using xylene, hydrated with ethanol, and were then stained with
hematoxylin for 5 min and with eosin for 30 sec at room
temperature. The tissues were observed using an optical microscope
(Olympus Corporation).
Terminal deoxynucleotidyl transferase
dUTP nick end labeling (TUNEL) assay
The TUNEL assay was conducted using a TUNEL
Apoptosis Detection Kit (cat. no. HY-K1091; MedChemExpress). Tumor
tissues were fixed in 10% formaldehyde for 24 h at room
temperature, embedded in paraffin and sectioned into 4-µm
slices. Tumor tissue sections were deparaffinized using xylene and
rehydrated using ethanol. After washing with PBS, the sections were
treated with proteinase K (20 µg/ml, 100 µl per
slide) for 30 min at room temperature. Endogenous peroxidase
activity was inactivated using 0.3% hydrogen peroxide at room
temperature for 30 min, followed by incubation with equilibration
buffer and a mixture of biotinylated Nucl. Mix and rTdT at 37°C for
1 h. Streptavidin HRP was then applied to each slide, followed by
incubation at 37°C for 30 min. After washing with PBS, the slides
were stained with DAB solution at room temperature for 30 min,
counterstained with methyl green at room temperature for 5 min,
mounted and observed using an optical microscope (Olympus
Corporation).
Immunohistochemistry
Immunohistochemistry was performed to analyze the
expression of apoptosis-related proteins in tumor tissues. Tumor
tissues were fixed in 10% formaldehyde for 24 h at room
temperature, embedded in paraffin and sectioned into 4-µm
slices. Tumor tissue sections were deparaffinized using xylene and
rehydrated using ethanol. Antigen retrieval was conducted by
immersing the slides in sodium citrate buffer (pH 6.0) and placing
them in a water bath at 97°C for 20 min, and then the slides were
transferred to distilled water and allowed to cool at room
temperature for 20 min. Endogenous peroxidase was inactivated with
0.3% hydrogen peroxide. After washing with PBS, blocking was
performed using 5% BSA (MP Biomedicals)-TBST at 37°C for 1 h.
Subsequently, the sections were incubated with primary antibodies
against p-JNK (1:100 in 5% skim milk-TBST; cat. no. 4668; Cell
Signaling Technology, Inc.) overnight at 4°C. After washing with
PBS, a HRP-conjugated secondary antibody (cat. no. 8114; Cell
Signaling Technology, Inc.) was applied at room temperature for 2
h, and DAB (cat. no. 8059; Cell Signaling Technology, Inc.)
staining was performed. Slides were counterstained with methyl
green at room temperature for 5 min, mounted and observed using an
optical microscope (Olympus Corporation).
Statistical analysis
All experiments were performed in triplicate and
data are presented as the mean ± standard deviation. Comparisons
among groups were performed using one-way ANOVA followed by
Dunnett's or Tukey's test. Differences between two groups were
analyzed using unpaired Student's t-test. Statistical analysis was
performed using SPSS Statistics Version 27 (IBM Corp.). P<0.05
was considered to indicate a statistically significant
difference.
Results
Effects of gossypin on the viability of
HT-29 CRC cells
To evaluate the effects of gossypin on cell
viability, an MTT assay was conducted using HT-29 cells. The cells
were divided into six groups and treated with gossypin
concentrations of 0, 30, 60, 90, 120 and 150 µM for 24 h,
Compared with the control group (0 µM), cell viability
decreased in a concentration-dependent manner to 88.8, 78.2, 68.1,
57.5 and 48.0%, respectively, with a statistically significant
difference observed starting at 30 µM (Fig. 1B). Based on the MTT assay
results, concentrations of 60 and 120 µM were selected for
subsequent experiments as moderate and high doses,
respectively.
Induction of apoptosis by gossypin in
HT-29 CRC cells
To determine whether the reduction in cell viability
by gossypin was caused by apoptosis, DAPI staining was performed to
observe morphological changes in cells. Apoptotic cells showed
nuclear condensation and formation of apoptotic bodies, which were
quantified. The proportion of apoptotic cells increased in a
concentration-dependent manner to 0.8, 3.1 and 7.7%, respectively
(Fig. 2A). To determine changes
in the rate of apoptosis, Annexin V and PI were used for double
staining, followed by flow cytometric analysis. The combined ratio
of Annexin V-positive regions (upper-right and lower-right
quadrants) showed a concentration-dependent increase, with values
of 27.0, 36.0 and 51.3% across the respective concentration groups
(Fig. 2B). Additionally, western
blotting was performed to examine the expression levels of
apoptosis-related proteins. The results indicated that, in HT-29
cells, increasing concentrations of gossypin led to enhanced
cleavage of PARP, which contributes to DNA repair, an increase in
the pro-apoptotic protein Bax, and a decrease in the anti-apoptotic
protein Bcl-2 (Fig. 2C).
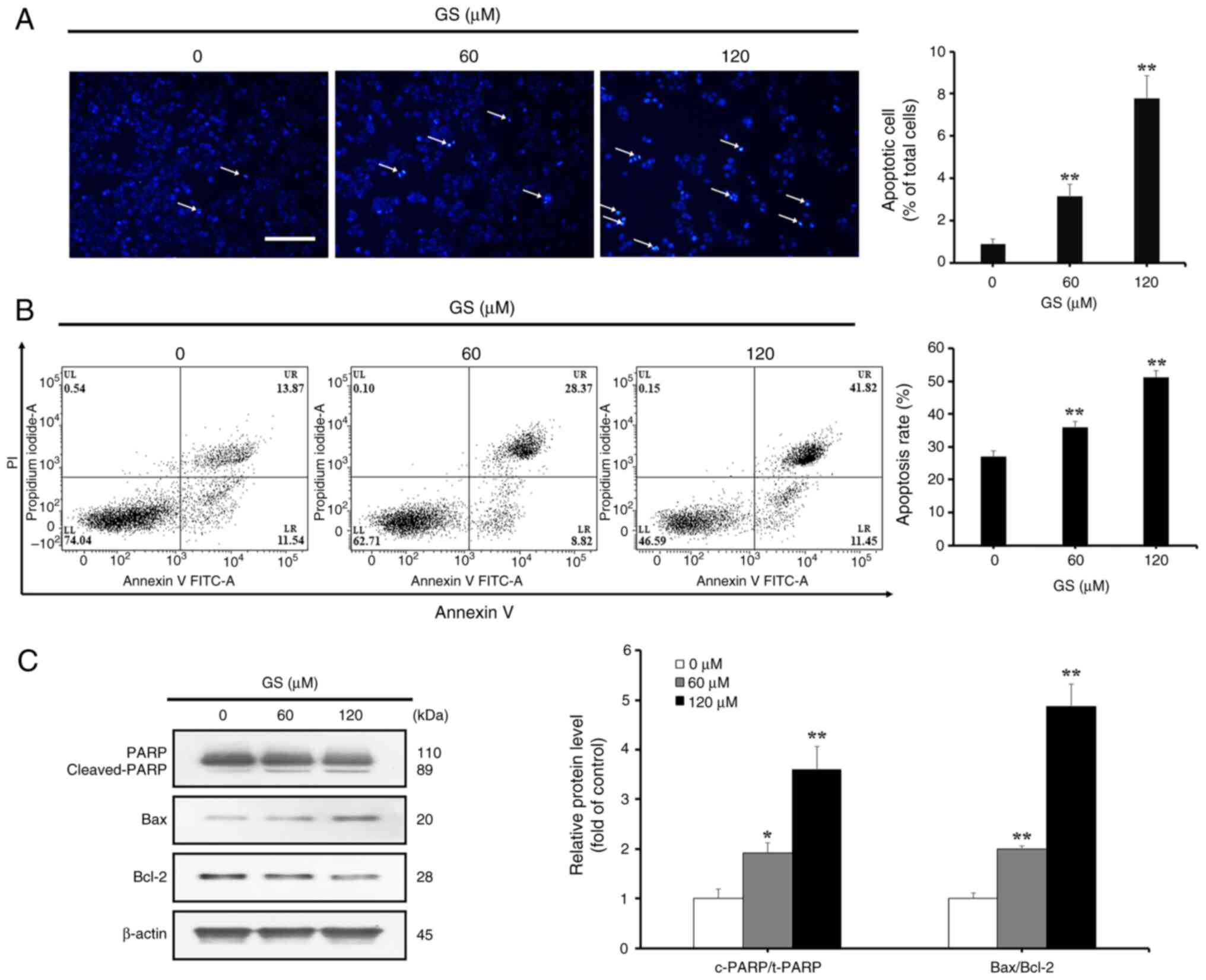 | Figure 2Effects of GS on the apoptosis of
HT-29 colorectal cancer cells. (A) HT-29 cells were treated with GS
(0, 60 and 120 µM) for 24 h, and the cells were then stained
with DAPI. Positive cells were analyzed using a fluorescence
microscope and the arrows indicate chromatin condensation (scale
bar, 100 µm). The average number of DAPI-positive cells is
presented as a percentage of total cells. (B) HT-29 cells were
treated with GS (0, 60 and 120 µM) for 24 h, stained with
Annexin V/PI and analyzed by flow cytometry. The percentage of
apoptotic cells among total cells is shown. (C) HT-29 cells were
treated with GS (0, 60 and 120 µM) for 24 h, and the
expression levels of apoptosis-related proteins, PARP, Bax and
Bcl-2, were measured by western blotting. β-actin was used as a
loading control, and semi-quantification was performed using
ImageJ. Control (0 µM) cells were subjected to treatment
with an equal amount of dimethyl sulfoxide. The results are
representative of three independent experiments and data are
presented as the mean ± standard deviation. *P<0.05,
**P<0.01 vs. control group. GS, gossypin; PI,
propidium iodide. |
Induction of autophagy by gossypin in
HT-29 CRC cells
To investigate whether gossypin induced autophagy,
acridine orange staining was performed to detect acidic vesicular
organelles, a characteristic of autophagy. The results showed an
increase in autophagic vacuole-positive cells with increasing
gossypin concentrations (Fig.
3A). Western blotting was also performed to examine the
expression levels of autophagy-associated proteins. The results
showed decreased expression of p-mTOR, an inhibitor of
autophagosome formation, and increased levels of Beclin 1 and
LC3-II, which are proteins critical for autophagosome formation
(Fig. 3B).
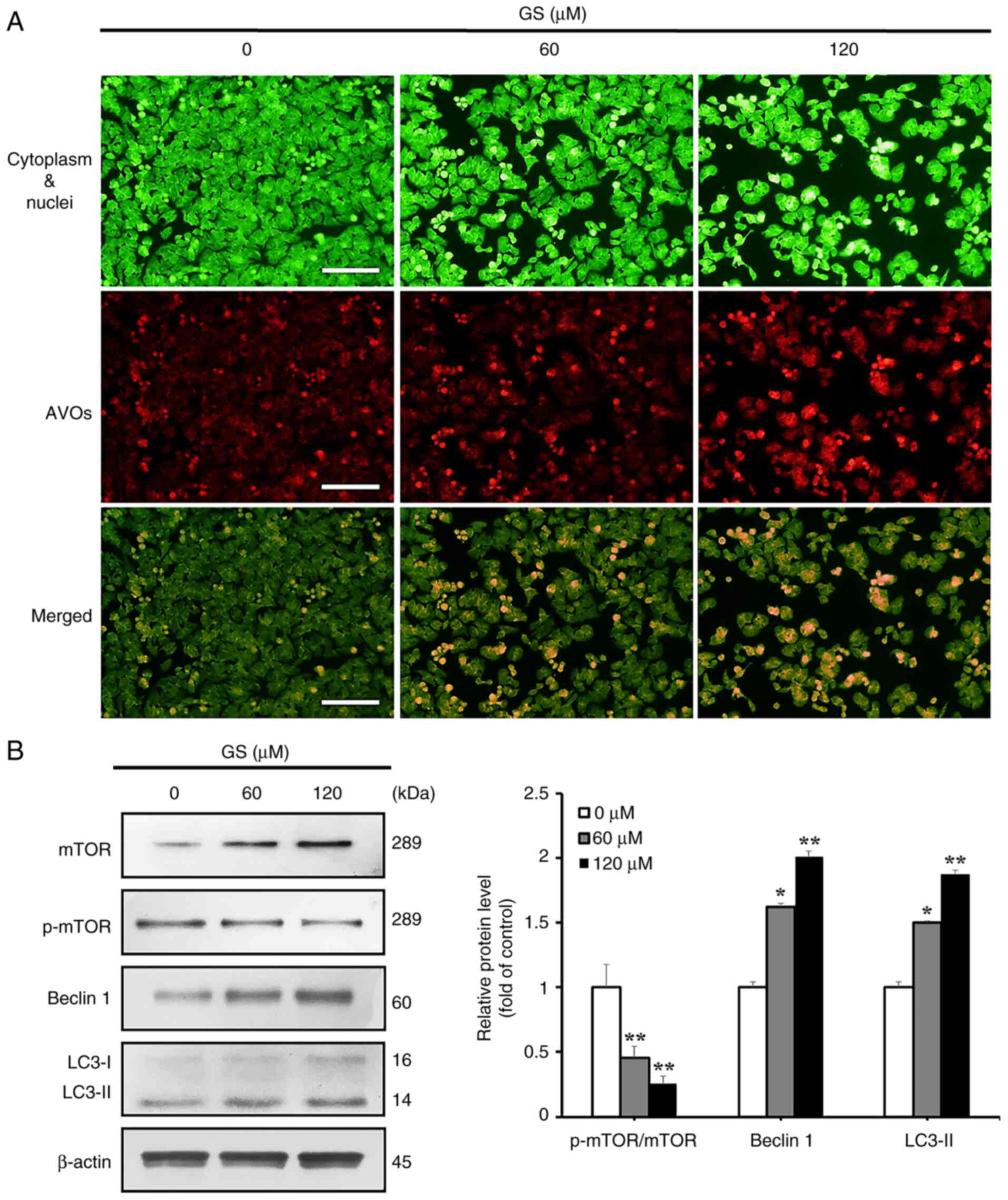 | Figure 3Effect of GS on the induction of
autophagy in HT-29 colorectal cancer cells. HT-29 cells were
treated with GS (0, 60 and 120 µM) for 24 h. (A) AVOs were
visualized via fluorescence microscopy using acridine orange
staining. The cytoplasm and the nuclei-were stained fluorescent
green, and the AVOs were stained fluorescent red (scale bar, 100
µm). (B) Expression levels of autophagy-related proteins,
mTOR, p-mTOR, Beclin 1 and LC3, were measured by western blotting.
β-actin was used as a loading control and semi-quantification was
performed using ImageJ. The results are representative of three
independent experiments and data are presented as the mean ±
standard deviation. *P<0.05, **P<0.01
vs. control group. AVOs, acidic vesicular organelles; GS, gossypin;
p-, phosphorylated. |
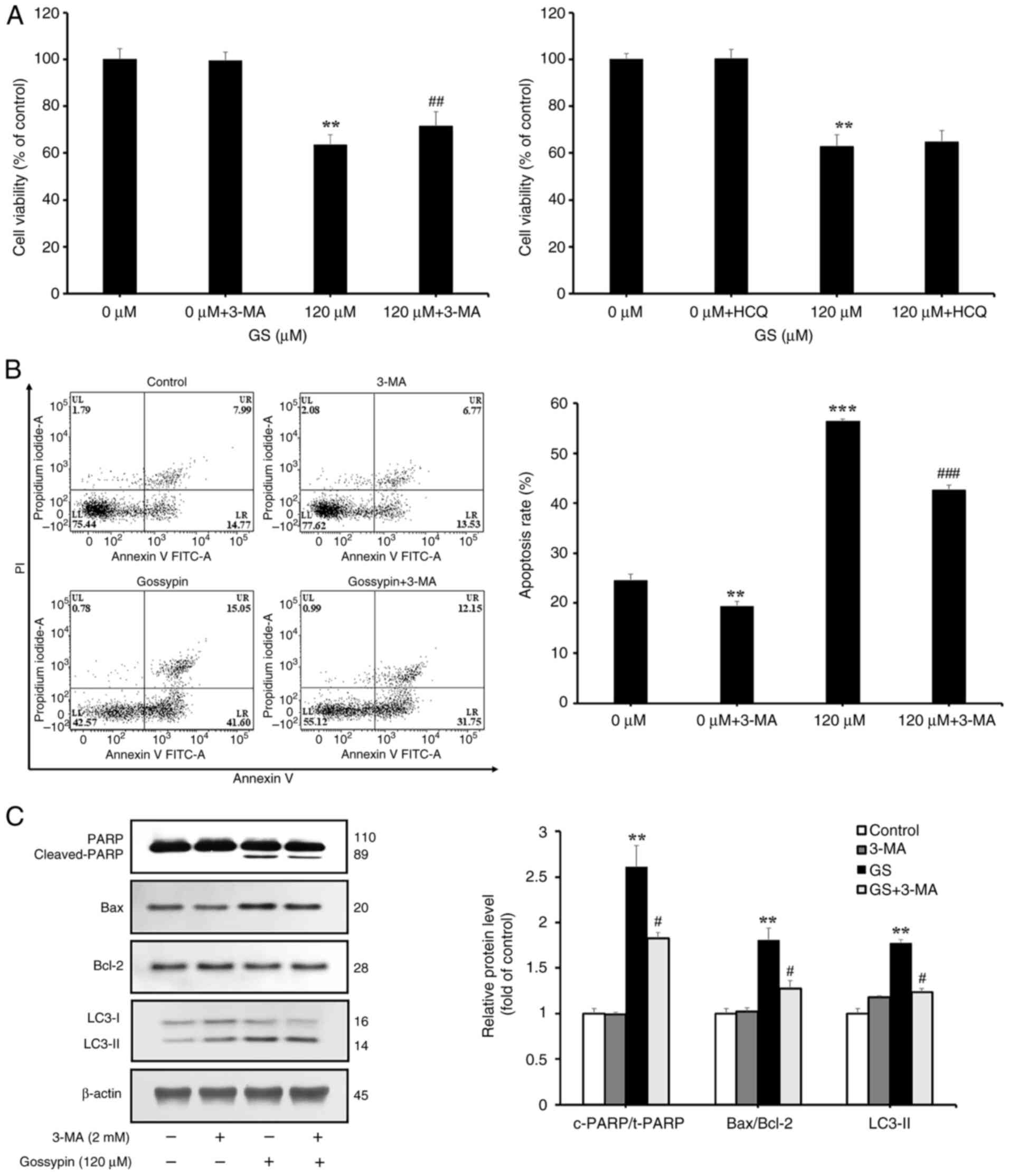
Effects of autophagy inhibition on
gossypin-induced apoptosis
To investigate the induction and inhibition of
apoptosis when gossypin-induced autophagy was suppressed in HT-29
cells, the cells were pretreated with an early-stage autophagy
inhibitor 3-MA and a late-stage autophagy inhibitor HCQ, and cell
viability was assessed using the MTT assay for gossypin.
Subsequently, cell viability, and the expression levels of
autophagy- and apoptosis-related proteins were assessed. Cell
viability was significantly increased in the group treated with
3-MA and gossypin compared with that in cells treated with gossypin
alone, but no significant difference was observed with HCQ
treatment (Fig. 4A). To
determine whether the 3-MA-induced changes in cell viability were
associated with apoptosis, flow cytometric analysis was conducted
following pretreatment with this inhibitor. The results indicated
that Annexin V positivity was significantly lower in the group
pretreated with 3-MA compared with that in cells treated with
gossypin alone (Fig. 4B).
Furthermore, western blotting was performed to confirm that these
changes were associated with apoptosis. Compared with in cells
treated with gossypin alone, the application of 3-MA followed by
gossypin resulted in decreased Bax and cleaved PARP expression, and
increased Bcl-2 expression, indicating a tendency for apoptosis to
be suppressed (Fig. 4C). These
findings confirmed that gossypin-induced autophagy may contribute
to HT-29 cell death.
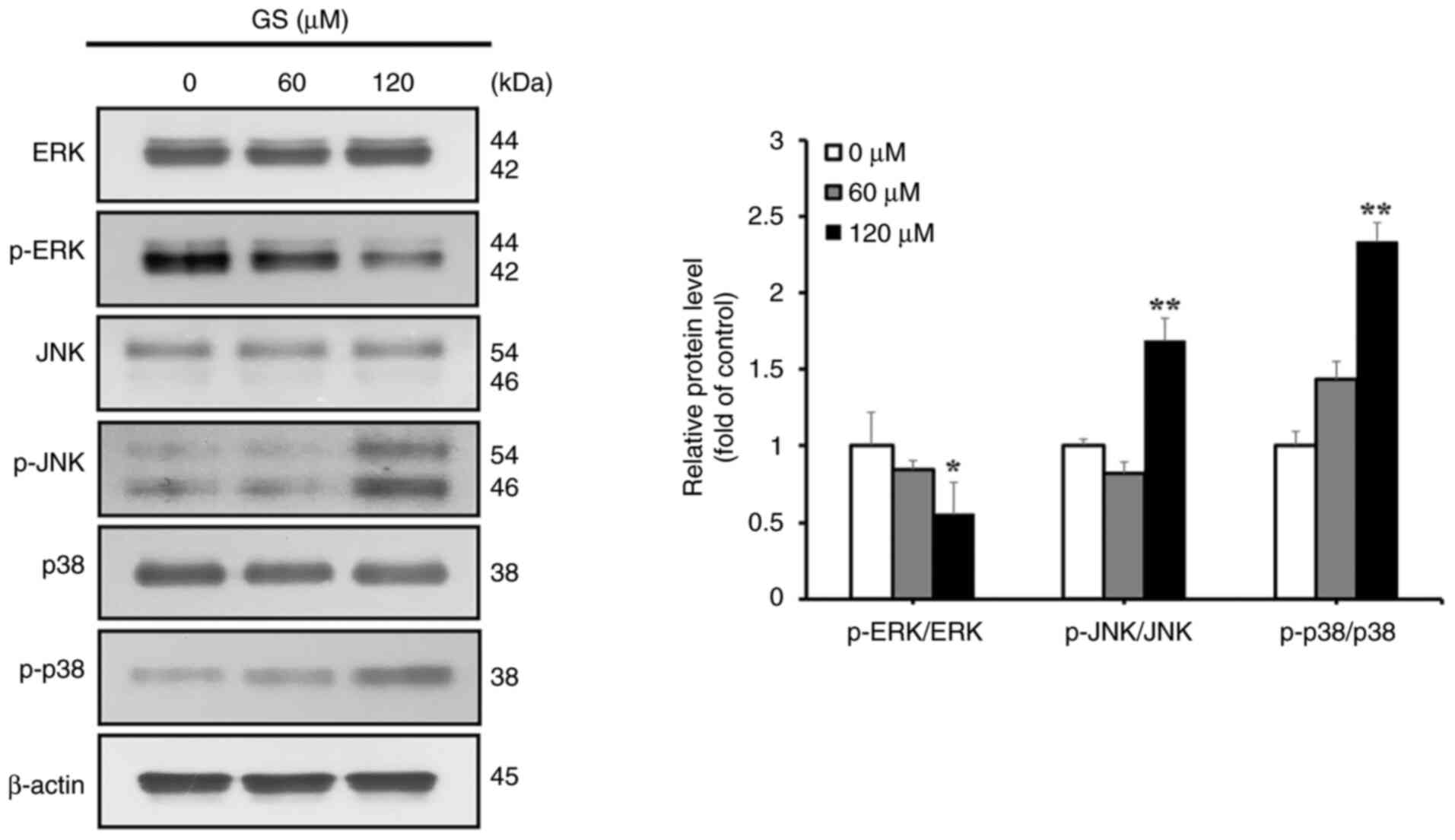
The MAPK/JNK pathway in gossypin-induced
apoptosis and autophagy
To determine whether gossypin-induced apoptosis in
HT-29 cells involved the MAPK pathway, MAPK pathway proteins were
analyzed using western blotting. The results showed decreased p-ERK
expression, and increased p-JNK and p-p38 levels in response to
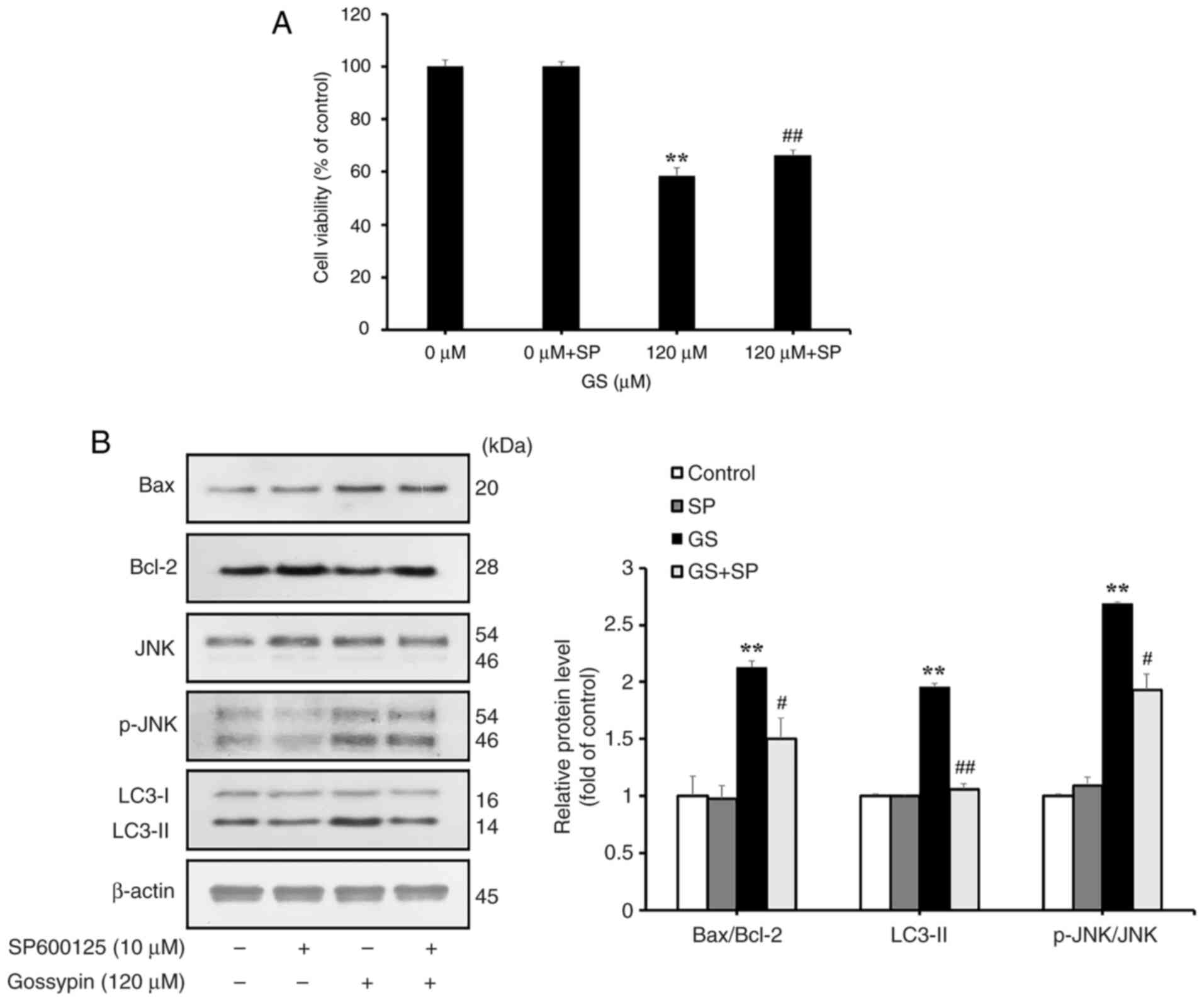
gossypin (Fig. 5). To further
investigate changes in apoptosis and autophagy After JNK inhibition
using SP600125 in HT-29 cells, the resulting changes in cell
viability and the expression levels of apoptosis- and
autophagy-related proteins in response to gossypin were examined
through western blotting. The resulting changes in cell viability,
and the expression levels of apoptosis- and autophagy-related
proteins were examined through western blotting. Cell viability was
significantly increased in the SP600125-treated gossypin group
compared with that in the gossypin-only group (Fig. 6A). The results further showed
that, compared with in the group treated with gossypin alone, the
group treated with SP600125 and gossypin exhibited decreased Bax
expression and increased Bcl-2 expression, indicating suppression
of apoptosis (Fig. 6B).
Additionally, a reduction in LC3-II expression confirmed the
suppression of autophagy in this group. These results suggested
that gossypin-induced apoptosis and autophagy in HT-29 cells may be
mediated through the MAPK/JNK pathway.
Effects of gossypin on HT-29 xenograft
tumors
The anticancer effects of gossypin observed in
vitro were evaluated in vivo using an HT-29 xenograft
model. HT-29 cells were subcutaneously injected into both shoulders
of BALB/c nude mice. Once tumors reached ~90 mm3, the
mice were randomly assigned to two groups, and gossypin (0 and 100
mg/kg) was orally administered for 28 days (five times/week).
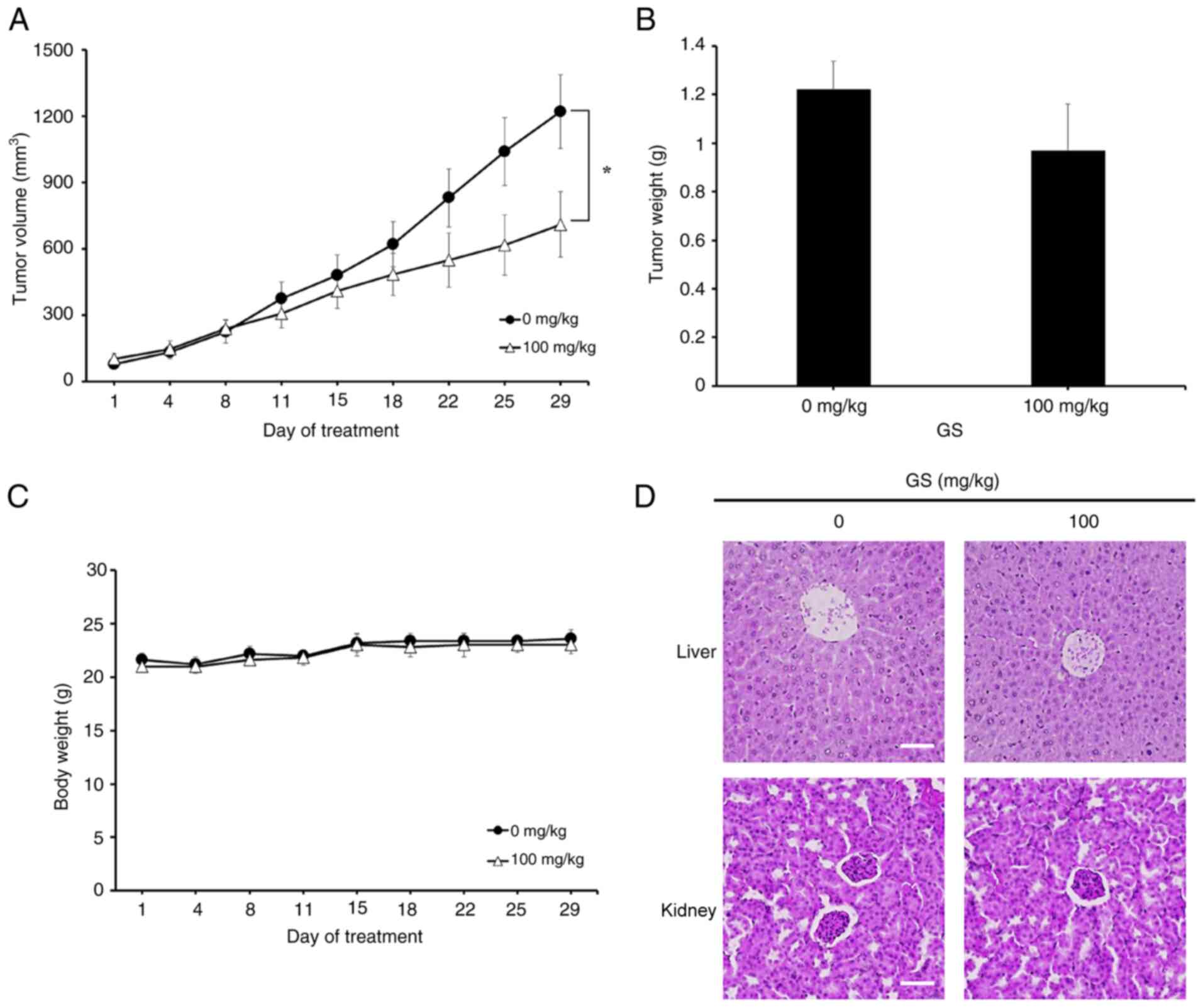
Notably, tumor volume was significantly reduced in the
gossypin-treated group (Figs. 7A
and S1). Tumor weight exhibited
a decreasing trend; however, this change was not statistically
significant. Additionally, there was no notable difference in body
weight between the control group and the gossypin group (Fig. 7B and C). Hematoxylin and eosin
staining was performed to determine whether gossypin administration
in mice induced toxicity in the liver and kidneys, which are
toxicity-sensitive organs. No differences were observed in either
organ between the gossypin-treated and control groups (Fig. 7D).
Gossypin-induced apoptosis and autophagy
in tumors
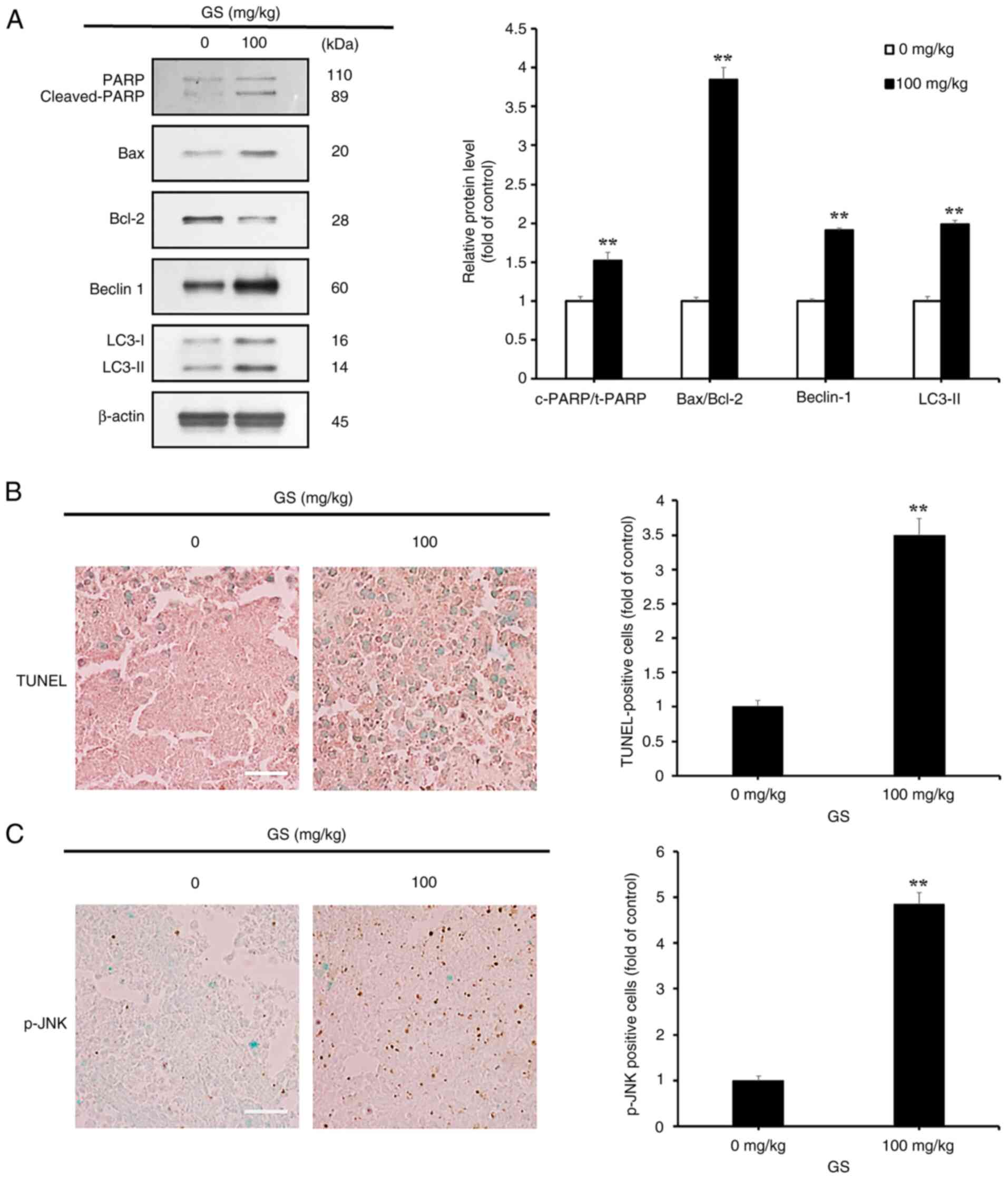
Western blotting was performed to investigate
whether gossypin induced apoptosis and autophagy in CRC tumors
in vivo. The results showed an increased expression of key
apoptosis-related proteins, including cleaved PARP and Bax, and a
decreased expression of Bcl-2 in the gossypin-treated group;
additionally, the levels of key ATG proteins, LC3-II and Beclin 1,
were increased (Fig. 8A). A
TUNEL assay was also conducted on CRC tumors. The gossypin-treated
group exhibited a significantly higher number of TUNEL-positive
cells than the control group; quantification of the images revealed
that the treated group had more than three times the number of
TUNEL-positive cells than the control group (Fig. 8B).
MAPK/JNK pathway activation in
gossypin-treated tumors
Immunohistochemistry was performed to assess p-JNK
expression in tumors. The results showed a clear increase in the
number of p-JNK-positive cells in the gossypin-treated group
compared with that in the control group; quantification of these
p-JNK-positive cells revealed that the treated group had more than
four times the number observed in the control group (Fig. 8C).
Discussion
Gossypin, a flavone found in H. vitifolius,
has previously been studied for its diverse physiological effects
and anticancer properties (10,11,13). However, its role in apoptosis,
autophagy and MAPK pathways in human CRC cell lines remains
underexplored. The present study investigated the anticancer
effects of gossypin on the human CRC cell line HT-29, and assessed
whether these effects were mediated through MAPK/JNK-induced
apoptosis and autophagy.
To determine whether gossypin suppresses the
viability of human CRC cells, HT-29 cells were treated with
gossypin, and cell viability was assessed using an MTT assay. The
results showed a significant concentration-dependent reduction in
cell viability, with a half-maximal inhibitory concentration value
of 142.76 µM. Regarding the treatment duration of gossypin
in human HT-29 CRC cells, a duration of 24 h was selected, based on
prior anticancer studies involving natural products (28-30). Previous studies have demonstrated
that gossypin similarly reduces cell viability in various cancer
cell lines, including lung (A549), breast (MCF-7), gastric (HGC27,
AGS), melanoma (A375, WM1552C, WM793B, SKMEL-31, SKMEL-28),
glioblastoma (U251) and prostate (PC-3) cells, suggesting its
anticancer potential across a range of cell types (13-15,31-33). The toxicity of gossypin in
nontumor cells has also been investigated in a previous study; in
human astrocytes, gossypin showed no toxicity at concentrations
ranging from 0 to 90 µM over 24, 48 and 72 h (32). However, toxicity assessments
involving cell types other than tumors remain inadequate. The
absence of these results presents a major limitation in this
research, and further experiments involving gossypin and nontumor
cells are crucial to evaluate its potential as an anticancer
agent.
To assess whether the reduction in cell viability
was mediated by apoptosis, DAPI and Annexin V/PI staining assays
were performed. Apoptosis leads to chromatin and nuclear
condensation, forming apoptotic bodies, which exhibit blue
fluorescence when stained with DAPI (34). In the current study, HT-29 cells
treated with gossypin exhibited a concentration-dependent increase
in the number of apoptotic bodies. Apoptosis was also investigated
using Annexin V, which binds to phosphatidylserine exposed on the
cell surface during apoptosis, and PI, which stains the nucleus but
cannot penetrate intact cell membranes, marking necrotic or late
apoptotic cells (35,36). After treating HT-29 cells with
gossypin and staining them with Annexin V/PI, flow cytometry
revealed that the total apoptosis rate, combining early and late
apoptosis, was significantly increased in a concentration-dependent
manner. Previous studies using Annexin V/PI staining following
gossypin treatment reported similar trends; apoptosis rates were
shown to be increased with higher gossypin concentrations in human
lung and gastric cancer cell lines (13,15). These findings suggested that
gossypin dose-dependently induces apoptosis in HT-29 cells.
Additionally, apoptosis-related proteins were
examined through western blotting. In mitochondrial
pathway-mediated apoptosis, Bcl-2 family proteins serve a crucial
role; this family includes pro-apoptotic proteins, such as Bax and
Bak, and anti-apoptotic proteins, such as Bcl-2 and Bcl-xL, which
regulate the mitochondrial membrane. During apoptosis, decreases in
Bcl-2 and Bcl-xL expression, and increases in Bax and Bak
expression, disrupt the mitochondrial membrane, enhancing its
permeability. Due to this increased permeability, cytochrome
c is released from the mitochondria, which, in conjunction
with Aparf-1, triggers a caspase cascade that ultimately induces
apoptosis (18). PARP, a protein
essential for DNA repair, is cleaved by activated caspases during
apoptosis (37,38). Western blotting after treating
HT-29 cells with gossypin demonstrated a concentration-dependent
increase in the expression levels of cleaved PARP and the
pro-apoptotic protein Bax, alongside a decrease in the expression
of the anti-apoptotic protein Bcl-2. These findings suggested that
gossypin may induce mitochondrial pathway-mediated apoptosis in
HT-29 cells by promoting PARP cleavage, decreasing Bcl-2 expression
and increasing Bax levels.
Autophagy is a physiological process that degrades
cellular organelles and molecules via lysosomes to maintain
homeostasis. Autophagy is mediated by ATG proteins, which form
autophagosomes that eventually fuse with lysosomes to create
autolysosomes (39,40). Western blotting was performed in
the present study to investigate whether gossypin induced apoptosis
and autophagy in CRC tumors. Notably, acridine orange staining
showed a dose-dependent increase in acidic vesicular organelles in
HT-29 cells treated with gossypin. Additionally, western blotting
demonstrated a decreased expression of p-mTOR, a factor that
inhibits the initial formation of autophagosomes. Concurrently,
there was an increased expression of Beclin 1, a protein that
promotes autophagy and overall autophagosome formation, as well as
an elevated conversion of LC3-I to LC3-II, which is critical for
the early formation of autophagosomes (22,40). These findings indicated that
gossypin may induce autophagy in the human CRC cell line HT-29.
The dual role of autophagy in cancer includes acting
as both a tumor-suppressing mechanism through organelle removal and
a tumor-promoting mechanism by recycling metabolic by-products to
sustain tumor growth (20). To
understand the mechanism of gossypin-induced autophagy in HT-29
cells, autophagy inhibitors were used to treat cells, followed by a
cell viability assay and western blotting. Previous studies on
HT-29 and HCT-116 CRC cells have demonstrated that inhibiting
autophagy with 3-MA and chloroquine alters cell viability in
response to treatment with Ganoderma lucidum polysaccharide;
3-MA treatment increases cell viability, whereas chloroquine
treatment decreases it. Moreover, apoptosis induction was shown to
be reduced in the 3-MA-pretreated group (41). In the present study, HCQ
treatment did not significantly affect cell viability, whereas
treatment with 3-MA resulted in a marked increase in cell
viability. To explore whether autophagy inhibition affected
apoptosis, flow cytometry and western blotting were performed. The
results indicated a trend toward reduced Annexin V positivity and
decreased expression of apoptosis-related proteins in response to
3-MA. These findings suggested that gossypin-induced autophagy may
serve a role in promoting cell death alongside apoptosis.
Furthermore, the mechanisms of autophagy in cancer cells vary
depending on multiple factors, including the administered drug, the
cell type and the stage of autophagy, highlighting its complex and
context-dependent nature.
The MAPK pathway has a critical role in cell
proliferation, differentiation, apoptosis and angiogenesis
(26). A previous study
demonstrated that treatment of HepG2 human liver cancer cells with
gossypin increases ERK phosphorylation over time, whereas JNK and
p38 phosphorylation levels remain unchanged (42). In the current study, increasing
gossypin concentrations induced phosphorylation of JNK and p38 in
HT-29 cells but decreased ERK phosphorylation. Previous anticancer
studies using natural compounds have demonstrated varying
phosphorylation patterns of MAPK pathway proteins, suggesting that
the phosphorylation of ERK, JNK and p38 may differ depending on
specific conditions in cancer therapy (29,41-43). To investigate the role of
gossypin-induced p-JNK expression in HT-29 cells, the JNK inhibitor
SP600125 was used to treat cells, followed by analyses of cell
viability and the expression of apoptosis- and autophagy-related
proteins using western blotting. Inhibition of the elevated JNK
expression in gossypin-treated HT-29 cells reduced the expression
levels of proteins associated with apoptosis and autophagy.
Previous studies on natural compounds have reported that the
inhibition of elevated p-JNK expression in cancer cells leads to
the suppression of cell death pathways, such as apoptosis,
autophagy and paraptosis (28,29,44), suggesting that p-JNK is involved
in these pathways. Therefore, it was hypothesized that the p-JNK
expression upregulated by gossypin likely acted as a mediator of
apoptosis and autophagy in HT-29 cells. To the best of our
knowledge, the present study is the first to confirm the influence
of the MAPK/JNK pathway in gossypin-treated cancer cells, and the
findings provide foundational evidence that gossypin promotes cell
death in cancer cells via p-JNK. However, it remains challenging to
delineate the relationship between apoptosis and autophagy in
relation to JNK expression induced by treatment with a fixed
concentration of gossypin over only 24 h. Consequently, further
investigations are necessary to explore how variations in JNK
expression, influenced by different concentrations and treatment
durations of gossypin, affect the interplay between apoptosis and
autophagy, and whether these processes occur through distinct
pathways. In addition, the current study exclusively
cross-validated the role of JNK within the MAPK pathway. To
demonstrate that gossypin exerts anticancer effects through the
MAPK pathway in CRC cells, it is essential to investigate the
relationship between decreased p-ERK levels and increased p-p38
levels in relation to apoptosis and autophagy.
To determine whether the anticancer effects of
gossypin observed in vitro also occurred in vivo,
xenograft experiments were conducted. A previous melanoma xenograft
study demonstrated a significant reduction in tumor volume and an
increase in TUNEL-positive cells in gossypin-treated groups
(31). In the present study,
gossypin treatment significantly suppressed tumor volume compared
with that in the control group, although tumor weight only showed a
decreasing trend. No differences were observed in body weight and
the histopathological features of livers and kidneys between the
murine control and treatment groups, suggesting that gossypin
suppressed tumor growth in vivo without toxicity. Western
blotting of tumor samples revealed that, consistent with the in
vitro findings, apoptosis and autophagy were increased in the
gossypin-treated group. Additionally, TUNEL assay results showed
significant increases in the number of TUNEL-positive cells in
tumors of the gossypin-treated group compared with that in the
control group, consistent with previous findings. These results
suggested that the suppression of CRC in the gossypin-treated group
may be mediated by apoptosis and autophagy. Furthermore, the
significant increase in the number of p-JNK-positive cells in the
gossypin-treated group indicated that gossypin-induced apoptosis
and autophagy in CRC tumors were mediated through the JNK pathway.
However, the present in vivo toxicity evaluation of gossypin
was conducted using immune-deficient BALB/c nude mice, rather than
healthy BALB/c wild-type mice, and this evaluation was limited to
the liver and kidneys, which are organs particularly sensitive to
toxicity. Thus, further detailed and systematic experiments are
essential to establish the overall in vivo safety of
gossypin as an anticancer agent. In addition, the dose used in the
present in vivo experiments was based on concentrations
referenced in previous studies (31). Research on the pharmacokinetics
and drug resistance associated with gossypin is lacking; therefore,
comprehensive studies that address clinical limitations are
essential to support the selection of gossypin as a potential
anticancer agent.
In conclusion, the present study investigated
gossypin-induced apoptosis and autophagy in human HT-29 CRC cells.
Gossypin reduced the viability of HT-29 cells through apoptosis,
and induced autophagy by decreasing p-mTOR levels and increasing
Beclin 1 and LC3-II expression. Gossypin-induced autophagy in HT-29
cells activated pro-apoptotic proteins, promoting apoptosis as part
of a cell death mechanism. Furthermore, the present results
confirmed that the induction of apoptosis and autophagy was
regulated via the MAPK/JNK pathway. Additional in vivo
experiments demonstrated that gossypin also induced apoptosis and
autophagy in HT-29 tumors by increasing p-JNK expression. Taken
together, gossypin may induce apoptosis and autophagy in human
HT-29 CRC cells via the MAPK/JNK pathway, suggesting the potential
of gossypin as a natural anticancer agent for CRC therapy.
Supplementary Data
Availability of data and materials
The data generated in the present study may be
requested from the corresponding author.
Authors' contributions
JMM and JYJ designed and conducted experiments, and
collected data. JMM wrote the manuscript. SWL, YSJ, SKK, BKP and
YSP analyzed and interpreted data. SAL, SHJ, MSY, BSK and JYJ
analyzed the results and reviewed the manuscript. JYJ edited the
manuscript and acquired funding. All authors confirm the
authenticity of all the raw data. All authors read and approved the
final version of the manuscript.
Ethics approval and consent to
participate
All animal experiments were conducted in compliance
with the guidelines of the Institutional Animal Care and Use
Committee (IACUC) (approval no. IACUC-KNU_2024-07) of Kongju
National University (Yesan, South Korea).
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
Acknowledgments
Not applicable.
Funding
This work was supported by a research grant of the Kongju
National University in 2024 and the Basic Science Research Program
through the National Research Foundation of Korea funded by the
Ministry of Education, Science and Technology (grant no.
2021R1A2C1010912).
References
|
1
|
Zaorsky NG, Churilla TM, Egleston BL,
Fisher SG, Ridge JA, Horwitz EM and Meyer JE: Causes of death among
cancer patients. Ann Oncol. 28:400–407. 2017. View Article : Google Scholar
|
|
2
|
WHO: Colorectal cancer. 2023, https://www.who.int/newsroom/fact-sheets/detail/colorectal-cancer.
|
|
3
|
Ahmed M: Colon cancer: A clinician's
Perspective in 2019. Gastroenterology Res. 13:1–10. 2020.
View Article : Google Scholar :
|
|
4
|
Siegel RL, Miller KD and Jemal A: Cancer
statistics, 2018. CA Cancer J Clin. 68:7–30. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Vogel JD, Felder SI, Bhama AR, Hawkins AT,
Langenfeld SJ, Shaffer VO, Thorsen AJ, Weiser MR, Chang GJ,
Lightner AL, et al: The American society of colon and rectal
surgeons clinical practice guidelines for the management of colon
cancer. Dis Colon Rectum. 65:148–177. 2022. View Article : Google Scholar
|
|
6
|
Tohme S, Simmons RL and Tsung A: Surgery
for cancer: A trigger for metastases. Cancer Res. 77:1548–1552.
2017. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Sara JD, Kaur J, Khodadadi R, Rehman M,
Lobo R, Chakrabarti S, Herrmann J, Lerman A and Grothey A:
5-fluorouracil and cardiotoxicity: A review. Ther Adv Med Oncol.
10:17588359187801402018. View Article : Google Scholar
|
|
8
|
Maillard M, Eche-Gass A, Ung M, Brice A,
Marsili S, Montastruc M, Puisset F and Thomas F: Severe toxicity of
capecitabine in a patient with DPD deficiency after a safe FEC-100
experience: Why we should test DPD deficiency in all patients
before high-dose fluoropyrimidines. Cancer Chemother Pharmacol.
87:579–583. 2021. View Article : Google Scholar
|
|
9
|
Huang M, Lu JJ and Ding J: Natural
products in cancer therapy: Past, present and future. Nat Prod
Bioprospect. 11:5–13. 2021. View Article : Google Scholar
|
|
10
|
Patel K, Kumar V, Verma A and Patel DK:
Gossypin: A phytochemical of multispectrum potential. J Coast Life
Med. 5:365–370. 2017. View Article : Google Scholar
|
|
11
|
Song B, Shen X, Tong C, Zhang S, Chen Q,
Li Y and Li S: Gossypin: A flavonoid with diverse pharmacological
effects. Chem Biol Drug Des. 101:131–137. 2023. View Article : Google Scholar
|
|
12
|
Huang H, Wang J, Hussain SA,
Gangireddygari VSR and Fan Y: Gossypin exert lipopolysaccharide
induced lung inflammation via alteration of Nrf2/HO-1 and NF-κB
signaling pathway. Environ Toxicol. 38:1786–1799. 2023. View Article : Google Scholar
|
|
13
|
Lee SU and Kim YH: Anticancer effects and
molecular mechanisms of gossypin on phosphatidylinositol
3-kinase/protein kinase B inhibition in human nonsmall cell lung
cancer cell line, A549. Nat Prod Commun.
18:1934578X2311944202023.
|
|
14
|
Çiçek M, Çınar İ and Aksak S: Gossypin
suppresses cell growth by cytotoxic effect and induces apoptosis in
MCF-7 cells. Med Rec. 4:21–26. 2022.
|
|
15
|
Wang L, Wang X, Chen H, Zu X, Ma F, Liu K,
Bode AM, Dong Z and Kim DJ: Gossypin inhibits gastric cancer growth
by direct targeting of AURKA and RSK2. Phytother Res. 33:640–650.
2019. View
Article : Google Scholar
|
|
16
|
Cinar I, Yayla M, Tavaci T, Toktay E, Ugan
RA, Bayram P and Halici H: In vivo and in vitro cardioprotective
effect of gossypin against isoproterenol-induced myocardial
infarction injury. Cardiovasc Toxicol. 22:52–62. 2022. View Article : Google Scholar
|
|
17
|
Cheng G, Zhang J, Jia S, Feng P, Chang F,
Yan L, Gupta P and Wu H: Cardioprotective effect of gossypin
against myocardial ischemic/reperfusion in rats via alteration of
oxidative stress, inflammation and gut microbiot. J Inflamm Res.
15:1637–1651. 2022. View Article : Google Scholar
|
|
18
|
Nagata S: Apoptosis and clearance of
apoptotic cells. Annu Rev Immunol. 36:489–517. 2018. View Article : Google Scholar
|
|
19
|
Li LY, Guan YD, Chen XS, Yang JM and Cheng
Y: DNA repair pathways in cancer therapy and resistance. Front
Pharmacol. 11:6292662021. View Article : Google Scholar
|
|
20
|
Yang Y and Klionsky DJ: Autophagy and
disease: Unanswered questions. Cell Death Differ. 27:858–871. 2020.
View Article : Google Scholar
|
|
21
|
Yu L, Chen Y and Tooze SA: Autophagy
pathway: Cellular and molecular mechanisms. Autophagy. 14:207–215.
2018. View Article : Google Scholar :
|
|
22
|
Yamamoto H, Zhang S and Mizushima N:
Autophagy genes in biology and disease. Nat Rev Genet. 24:382–400.
2023. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Marinković M, Šprung M, Buljubašić M and
Novak I: Autophagy modulation in cancer: Current knowledge on
action and therapy. Oxid Med Cell Longev. 2018:80238212018.
View Article : Google Scholar
|
|
24
|
Kriel J and Loos B: The good, the bad and
the autophagosome: Exploring unanswered questions of
autophagy-dependent cell death. Cell Death Differ. 26:640–652.
2019. View Article : Google Scholar :
|
|
25
|
Shi A, Liu L, Li S and Qi B: Natural
products targeting the MAPK-signaling pathway in cancer: Overview.
J Cancer Res Clin Oncol. 150:62024. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Guo YJ, Pan WW, Liu SB, Shen ZF, Xu Y and
Hu LL: ERK/MAPK signalling pathway and tumorigenesis. Exp Ther Med.
19:1997–2007. 2020.
|
|
27
|
Kudaravalli S, den Hollander P and Mani
SA: Role of p38 MAP kinase in cancer stem cells and metastasis.
Oncogene. 41:3177–3185. 2022. View Article : Google Scholar
|
|
28
|
Huang CF, Liu SH, Ho TJ, Lee KI, Fang KM,
Lo WC, Liu JM, Wu CC and Su CC: Quercetin induces tongue squamous
cell carcinoma cell apoptosis via the JNK activation-regulated
ERK/GSK-3α/β-mediated mitochondria-dependent apoptotic signaling
pathway. Oncol Lett. 23:782022. View Article : Google Scholar
|
|
29
|
Kim NY, Mohan CD, Sethi G and Ahn KS:
Cannabidiol activates MAPK pathway to induce apoptosis, paraptosis,
and autophagy in colorectal cancer cells. J Cell Biochem.
125:e305372024. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Zhai F, Wang Y and Yan M: Polyphenols from
morchella sextelata induce apoptosis of colorectal cancer cells
through ROS-mediated endogenous mitochondrial apoptosis pathway in
vitro. J Food Biochem. 2025:77777902025. View Article : Google Scholar
|
|
31
|
Bhaskaran S, Dileep KV, Deepa SS,
Sadasivan C, Klausner M, Krishnegowda NK, Tekmal RR, VandeBerg JL
and Nair HB: Gossypin as a novel selective dual inhibitor of V-RAF
murine sarcoma viral oncogene homolog B1 and cyclin-dependent
kinase 4 for melanoma. Mol Cancer Ther. 12:361–372. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Shi L, Chen J, Wang YY, Sun G, Liu JN,
Zhang JX, Yan W, Qian CF, Liu N, Fu Z, et al: Gossypin induces G2/M
arrest in human malignant glioma U251 cells by the activation of
Chk1/Cdc25C pathway. Cell Mol Neurobiol. 32:289–296. 2012.
View Article : Google Scholar
|
|
33
|
Cinar I: Apoptosis-inducing activity and
antiproliferative effect of gossypin on PC-3 prostate cancer cells.
Anticancer Agents Med Chem. 21:445–450. 2021. View Article : Google Scholar
|
|
34
|
Hauser P, Wang S and Didenko VV: Apoptotic
bodies: Selective detection in extracellular vesicles. Methods Mol
Biol. 1554:193–200. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Miller E: Apoptosis measurement by annexin
v staining. Methods Mol Med. 88:191–202. 2004.
|
|
36
|
Riccardi C and Nicoletti I: Analysis of
apoptosis by propidium iodide staining and flow cytometry. Nat
Protoc. 1:1458–1461. 2006. View Article : Google Scholar
|
|
37
|
Boulares AH, Yakovlev AG, Ivanova V,
Stoica BA, Wang G, Iyer S and Smulson M: Role of poly(ADP-ribose)
polymerase (PARP) cleavage in apoptosis. Caspase 3-resistant PARP
mutant increases rates of apoptosis in transfected cells. J Biol
Chem. 274:22932–22940. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Chen A: PARP inhibitors: Its role in
treatment of cancer. Chin J Cancer. 30:463–471. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Santana-Codina N, Mancias JD and Kimmelman
AC: The role of autophagy in cancer. Annu Rev Cancer Biol. 1:19–39.
2017. View Article : Google Scholar
|
|
40
|
Badadani M: Autophagy mechanism,
regulation, functions, and disorders. Int Sch Res Notices.
2012:9270642012.
|
|
41
|
Pan H, Wang Y, Na K, Wang Y, Wang L, Li Z,
Guo C, Guo D and Wang X: Autophagic flux disruption contributes to
Ganoderma lucidum polysaccharide-induced apoptosis in human
colorectal cancer cells via MAPK/ERK activation. Cell Death Dis.
10:4562019. View Article : Google Scholar
|
|
42
|
Lu N, Li Y, Qin H, Zhang YL and Sun CH:
Gossypin up-regulates LDL receptor through activation of ERK
pathway: A signaling mechanism for the hypocholesterolemic effect.
J Agric Food Chem. 56:11526–11532. 2008. View Article : Google Scholar
|
|
43
|
Kwak AW, Kim WK, Lee SO, Yoon G, Cho SS,
Kim KT, Lee MH, Choi YH, Lee JY, Park JW and Shim JH: Licochalcone
B induces ROS-dependent apoptosis in oxaliplatin-resistant
colorectal cancer cells via p38/JNK MAPK signaling. Antioxidants
(Basel). 12:6562023. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Zhang X, Jiang J, Chen Z and Cao M:
Silibinin inhibited autophagy and mitochondrial apoptosis in
pancreatic carcinoma by activating JNK/SAPK signaling. Pathol Res
Pract. 215:1525302019. View Article : Google Scholar
|