Introduction
The kidney plays a vital role in numerous
physiological processes, including the excretion of metabolic
waste, the regulation of fluid balance and the secretion of
endocrine hormones, which are all fundamental to maintaining
internal homeostasis (1). In
sugar metabolism, the kidneys contribute to acid-base equilibrium
and energy supply by excreting and metabolizing lactate, thereby
supporting glucose and lactate homeostasis (2). The involvement of lactate in kidney
diseases includes intricate metabolic regulation, cellular injury
and clinical monitoring. As a glycolytic product, lactate
accumulates excessively under conditions of impaired clearance in
renal dysfunction, potentially precipitating metabolic acidosis and
directly impacting the function of renal tubular epithelial cells
(3,4). During renal fibrosis, lactate
accelerates fibrotic progression by inducing histone lactylation.
For instance, in advanced glycation end product (AGE)-induced renal
tubular epithelial cells, increased lactate levels enhance histone
H3 lysine 14 lactylation (H3K14la) in diabetic nephropathy (DN),
thereby promoting Krüppel-like factor 5 (KLF5) expression and
driving the epithelial-mesenchymal transition (EMT) in DN (5). In chronic kidney disease (CKD), the
expression of 6-phosphofructo-2-kinase/fructose-2,6-biphosphatase 3
(PFKFB3) is positively correlated with the severity of renal
fibrosis. PFKFB3-mediated glycolytic reprogramming in renal tubules
generates lactate, which enhances H4K12la, facilitating gene
transcription and activation of nuclear factor-κB (NF-κB), thus
subsequently promoting inflammatory responses and exacerbating
renal fibrosis (6). Furthermore,
in acute kidney injury (AKI), DN and CKD, lactate concentrations
and the activity or expression of glycolytic enzymes may exhibit
alterations (4,7). Such metabolic shifts establish
lactate-associated enzymes and lactate kinetics as emerging
biomarkers for disease progression assessment. Consequently,
lactate is not only a significant participant in renal metabolism
but also a potential pathological driver in kidney disease
evolution. Dynamic lactate monitoring offers valuable diagnostic
and therapeutic insights, and targeting lactate metabolism may
represent an innovative approach to renal protection.
Lactate, historically regarded as a negligible
glycolytic byproduct with limited physiological relevance (8), has now emerged as a molecule of
significant biological importance. Beyond its role as a ubiquitous
metabolite, lactate functions as a substrate for epigenetic
modifications, regulating gene transcription and protein function
through lactylation of histone and non-histone proteins (9,10). This discovery illustrates the
capacity of metabolites to regulate gene expression via
post-translational modifications. Lactate, acting as a metabolic
intermediate and signaling molecule, regulates diverse
physiological and pathological processes, including osteoblast
differentiation, inflammatory signal transduction, angiogenesis and
myogenesis, among others (11-14). Notably, targeting the roles of
lactate offers a promising therapeutic strategy for addressing
chronic diseases. An expanding body of research highlights the
relevance of lactate in various cellular functions, with particular
attention on its emerging role in kidney disease.
The present review offers an in-depth evaluation of
lactate and its associated metabolic pathways, providing a
foundation to comprehending its role in renal physiology. Advances
in delineating the involvement of lactate and lactylation in kidney
disorders, specifically AKI, DN and CKD, are thoroughly evaluated.
Moreover, an analysis of therapeutic targets and strategies focused
on lactate metabolism and lactylation is performed, presenting
innovative perspectives for the prevention and treatment of renal
diseases.
Lactate and lactate metabolism
Generation and clearance of lactate
Glycolysis constitutes the primary pathway for
lactate generation in organisms (15) (Fig. 1). Under hypoxic conditions, such
as those experienced during intense physical exertion or within
certain pathological environments, the entry of pyruvate into the
mitochondria for aerobic metabolism is hindered. As a result,
glucose in the cytoplasm undergoes enzymatic reactions leading to
pyruvate formation, which is subsequently reduced to lactate
through lactate dehydrogenase-A (LDHA). Notably, the glycolysis
pathway can generate lactate even under aerobic conditions. This
oxygen concentration-independent process represents a distinct
metabolic mode known as aerobic glycolysis (16). The regulation of glycolysis is
mediated by various enzymes, including LDH, pyruvate kinase M2
(PKM2), glucose transporters (GLUT) and hexokinase 2 (HK2)
(17-20).
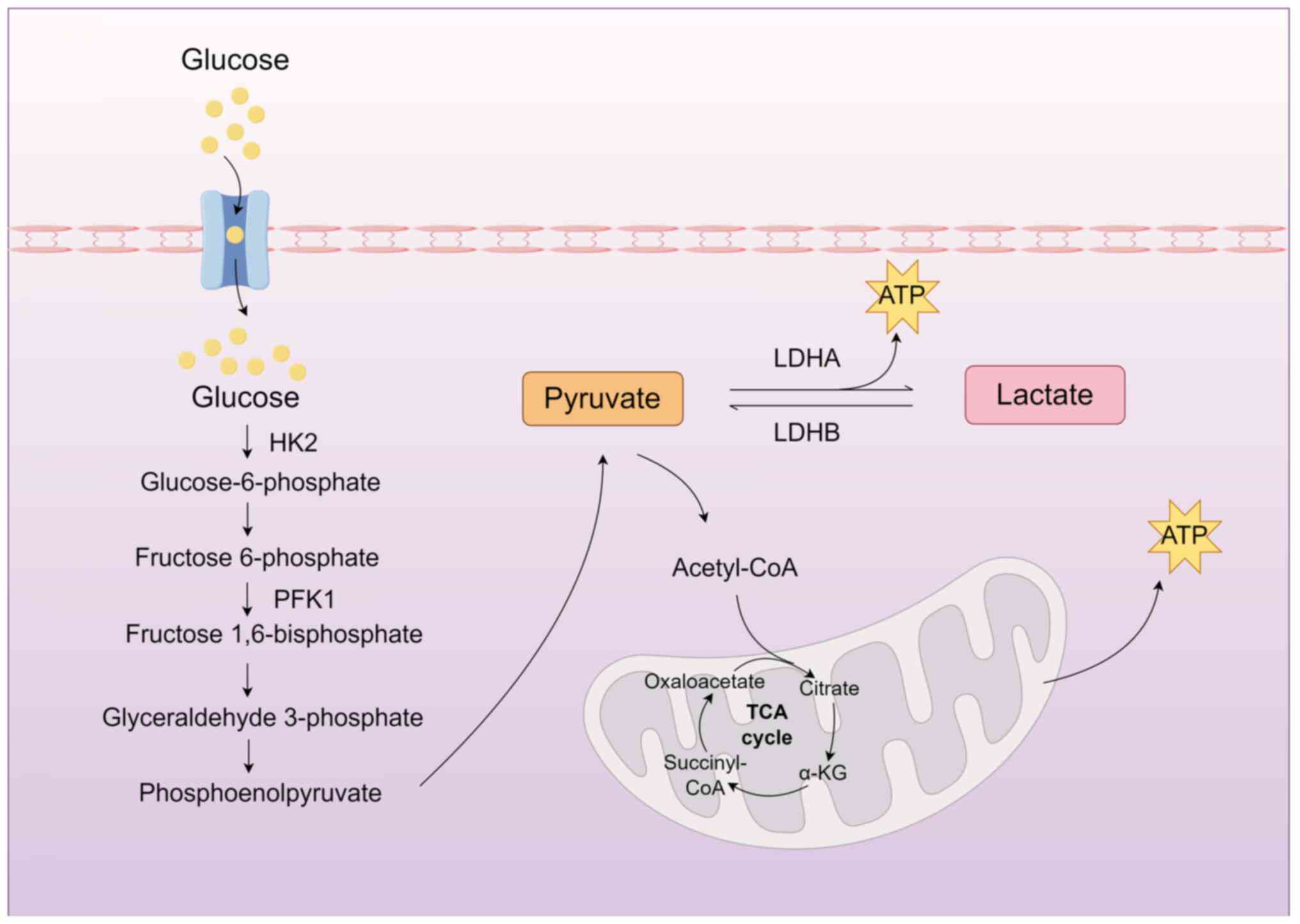 | Figure 1Primary pathways of lactate
production. In the cytoplasm, glucose undergoes sequential
glycolytic enzyme-mediated reactions to generate pyruvate. Under
normoxic conditions, pyruvate translocates into mitochondria to
enter the TCA cycle. By contrast, hypoxic environments promote the
LDHA-catalyzed conversion of pyruvate into lactate. TCA,
tricarboxylic acid; LDHA, lactate dehydrogenase-A; LDHB, lactate
dehydrogenase-B; HK2, hexokinase 2; PFK1, phosphofructokinase-1;
α-KG, α-ketoglutarate; ATP, adenosine triphosphate. The figure was
drawn using Figdraw (figdraw.com). |
Lactate exists in two isomeric forms: L-lactate and
D-lactate. L-lactate, the dominant isomer, is integral to immune
regulation, acid-base balance and signal transduction within
various physiological contexts (21). In eukaryotic cells, L-lactate
represents the chief glycolytic end-product, whereas D-lactate is
present only in trace amounts (22). D-lactate arises predominantly
from intestinal microbiota metabolism, notably by
Lactobacillus and Escherichia coli. In humans,
lactate metabolism primarily depends on L-lactate dehydrogenase,
while D-lactate dehydrogenase activity remains limited, resulting
in slower D-lactate clearance (21,23).
Excessive lactate accumulation correlates with a
heightened risk of lactic acidosis (24). Metabolic homeostasis necessitates
the efficient clearance of lactate from tissues and the bloodstream
(25,26). The primary mechanism for lactate
clearance is its oxidation to pyruvate by LDHB in the presence of
adequate oxygen, followed by mitochondrial entry for participation
in the tricarboxylic acid cycle, yielding CO2, water and
energy (27,28). Elevated lactate concentrations
are also redirected to the liver and kidneys, where lactate
undergoes conversion to glucose via gluconeogenesis. Notably, renal
gluconeogenesis contributes ~40% of total gluconeogenesis during
fasting, a function markedly compromised in CKD, leading to
systemic metabolic imbalances manifesting as hypoglycemia and
hyperlactatemia (7).
Additionally, lactate is enzymatically transformed into fatty
acids, cholesterol, and ketone bodies, or eliminated through the
excretion of urine and sweat (Fig.
2).
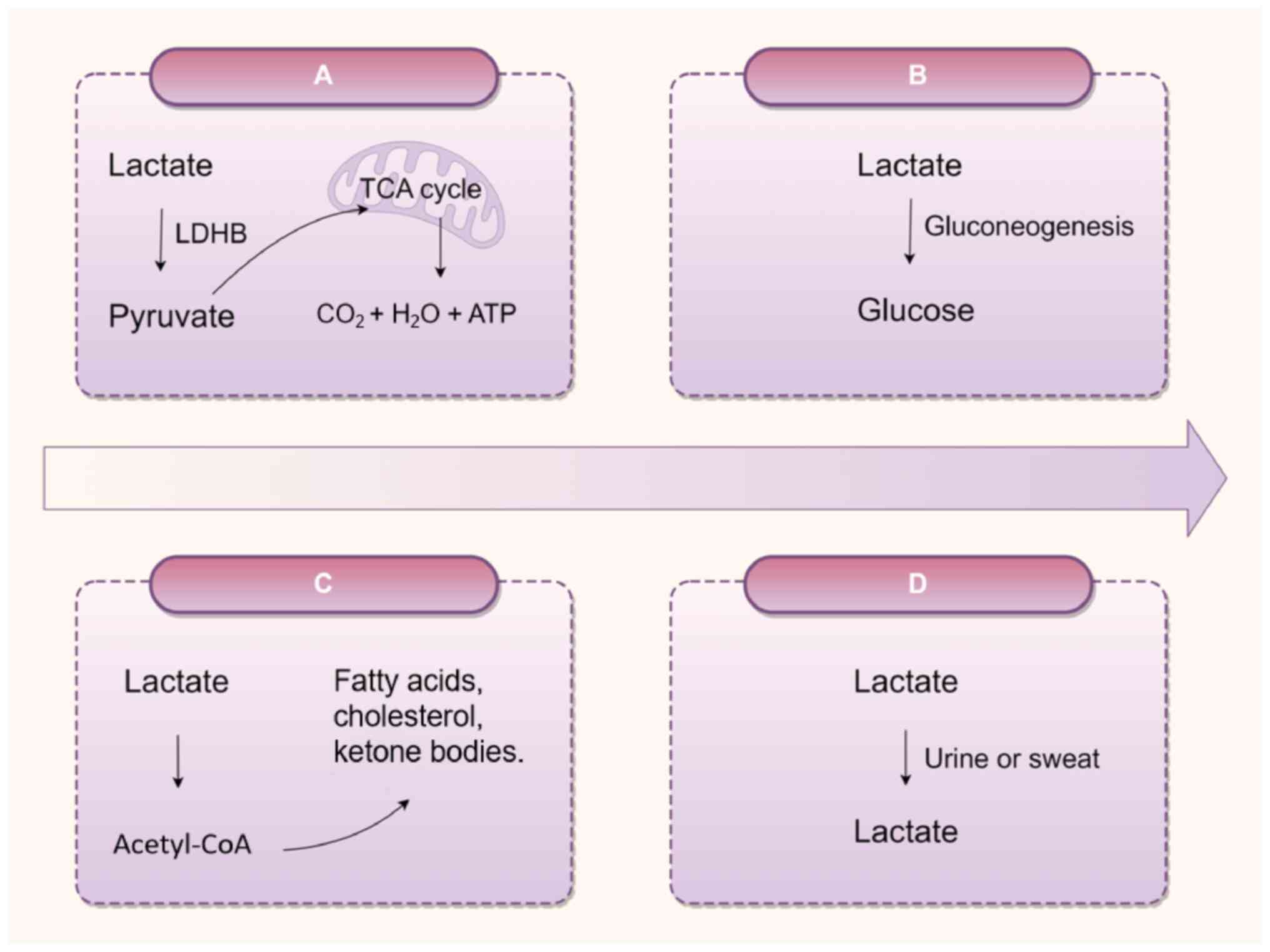 | Figure 2Lactate metabolism. (A) Under
conditions of sufficient oxygen supply, lactate is oxidized to
pyruvate by LDHB, which subsequently enters the mitochondria to
participate in the TCA cycle, generating CO2,
H2O and ATP. (B) Lactate is converted into glucose via
the gluconeogenic pathway. (C) Lactate indirectly participates in
the synthesis of fatty acids, cholesterol or ketone bodies through
the metabolic intermediate acetyl-CoA. (D) When blood lactate
levels are abnormally elevated, lactate may be excreted through
urine; sweat gland cells can secrete trace amounts of lactate,
which is subsequently eliminated via sweat. TCA, tricarboxylic
acid; LDHB, lactate dehydrogenase-B; ATP, adenosine triphosphate.
The figure was drawn using Figdraw (figdraw.com). |
Biological roles of lactate shuttle and
lactate in the kidneys
The lactate shuttle theory systematically
characterizes the intercellular transfer of lactate, highlighting
its function as both an oxidative and gluconeogenic precursor, as
well as a signaling molecule (29,30). This mechanism regulates the
exchange of energy substrates between active cells, such as
skeletal muscle, and recipient cells, including the brain, heart,
liver and kidney, a process governed by the concentration gradients
generated through mitochondrial respiration within recipient cells
(30).
Monocarboxylate transporters (MCTs) are essential to
the lactate shuttle mechanism, mediating lactate transport. The MCT
family comprises 14 distinct members, each characterized by
specific transport properties and tissue-specific expression. Among
them, MCT1 and MCT4 are central to lactate dynamics: MCT1 promotes
lactate flux in accordance with cellular metabolic needs, while
MCT4 predominantly handles lactate efflux (31-33). Acting synergistically, these
transporters maintain lactate homeostasis, pH balance and
intracellular metabolic stability.
In murine kidneys, MCTs mediate the transport of
monocarboxylates between renal tubular cells and the circulatory
system (34). MCT1, MCT2, MCT7
and MCT8 are specifically localized on the basolateral membranes of
the epithelial cells lining the nephron, with MCT1 and MCT8
expressed in proximal tubule cells, and MCT7 and MCT2 in the thick
ascending limb and distal tubule (35). Concurrently, LDHA is
predominantly expressed in the proximal nephron, functioning as a
lactate producer, whereas LDHB is primarily localized to the distal
nephron, serving as a lactate consumer, with both exhibiting
spatial and temporal shifts in response to ischemic injury
(36). Collectively, this
spatial distribution supports the hypothesis that lactate shuttles
from the proximal to the distal nephron, acting either as an energy
substrate or a signaling mediator.
In addition, lactate exerts essential biological
functions within the kidney. Podocytes and their foot processes
constitute critical components of the renal filtration barrier,
governing glomerular permeability. Podocyte injury is widely
recognized as a central pathological mechanism in various kidney
diseases, particularly DN and primary tubular disorders (37,38). Emerging evidence indicates that
podocytes utilize lactate as an energy substrate and possess
intrinsic regulatory systems to maintain lactate homeostasis. Under
glucose-deprived conditions, L-lactate exposure preserves cellular
viability and sustains glycolytic flux, mitigating glycogen
depletion and highlighting the indispensable role of lactate in
supporting podocyte metabolism during metabolic stress (39). Additionally, lactate stimulation
modulates mitochondrial dynamics and respiratory efficiency;
exposure to lactate elevates the mitochondrial DNA-to-nuclear DNA
ratio, thereby enhancing mitochondrial biogenesis and regulating
LDH subtype expression and activity in podocytes (40).
In renal cells, lactate not only undergoes catalysis
to pyruvate, serving as a key intermediary in energy metabolism,
but also functions as a gluconeogenic precursor for endogenous
glucose synthesis. Renal gluconeogenesis predominantly occurs in
the renal cortex, particularly within the proximal tubules, where
the key enzymes required for the gluconeogenic pathway are
expressed (41,42). Renal and hepatic gluconeogenesis
collectively regulate systemic glucose homeostasis, critically
contributing to the regulation of blood glucose levels and
whole-body energy metabolism (43).
In clinical contexts, renal insufficiency impairs
the lactate clearance capacity of the kidney, resulting in lactate
accumulation and subsequent metabolic acidosis (44). This elevation serves as an
indirect marker of renal dysfunction, particularly after excluding
confounding factors such as hypoxia and infection. Therefore,
monitoring lactate levels can indirectly help to assess the
compensatory status of kidney function in patients with kidney
disease. In diabetic populations, urinary lactate levels can
predict clinical outcomes such as doubling of serum creatinine or
the development of kidney failure, and elevated urinary lactate
levels may be a potential biomarker for the risk of kidney disease
progression (4). Thus, the role
of lactate in kidney function not only reflects the metabolic and
excretory capacity of the kidney but is also related to acidosis
and disease prognosis. In clinical practice, a comprehensive
analysis combining lactate levels, kidney function indicators and
etiology should be conducted to optimize intervention strategies
for kidney diseases.
In conclusion, lactate constitutes an essential
metabolic substrate sustaining the energetic demands of renal
cells. Beyond its fundamental bioenergetic contribution, lactate
operates as a central regulator within cellular metabolic and
signaling networks, critically supporting physiological stability
and systemic metabolic balance.
Discovery of lactylation and its
regulation
Lactate metabolism, homeostasis and the lactate
microenvironment significantly regulate cellular and biomolecular
functions. Recent studies have identified lactate as an epigenetic
modulator (11,45,46), capable of inducing
post-translational modifications and influencing gene expression,
thereby causing diverse biological outcomes. Zhang et al
(45) demonstrated that lactate
facilitates transcriptional regulation through lactylation, an
epigenetic mechanism wherein lactate-derived modifications of
histone lysine residues directly promote gene transcription within
chromatin, marking the initial discovery of histone lysine
lactylation. Consequently, lactylation establishes a direct nexus
between cellular metabolic activity and epigenetic governance.
Following the identification of histone lactylation,
the emergence of non-histone lactylation has highlighted its wider
prospects beyond chromatin modification (47). Current evidence implicates
protein lactylation in a range of renal pathologies, including AKI
(48) and DN (49), along with a variety of other
diseases. For instance, H3K18la has been associated with the
progression of arsenite-induced idiopathic pulmonary fibrosis
(50), whereas glutamine
mitigates intervertebral disc degeneration by inhibiting
adenosine-5′-monophosphate-activated protein kinase α (AMPKα)
lactylation through suppression of glycolytic pathways (51). Moreover, lactylation exerts
regulatory effects on critical physiological processes, such as
autophagy, macrophage activation and osteoblast differentiation
(13,52,53). Accordingly, lactylation emerges
as a central mechanism within both renal pathophysiology and
systemic disease contexts.
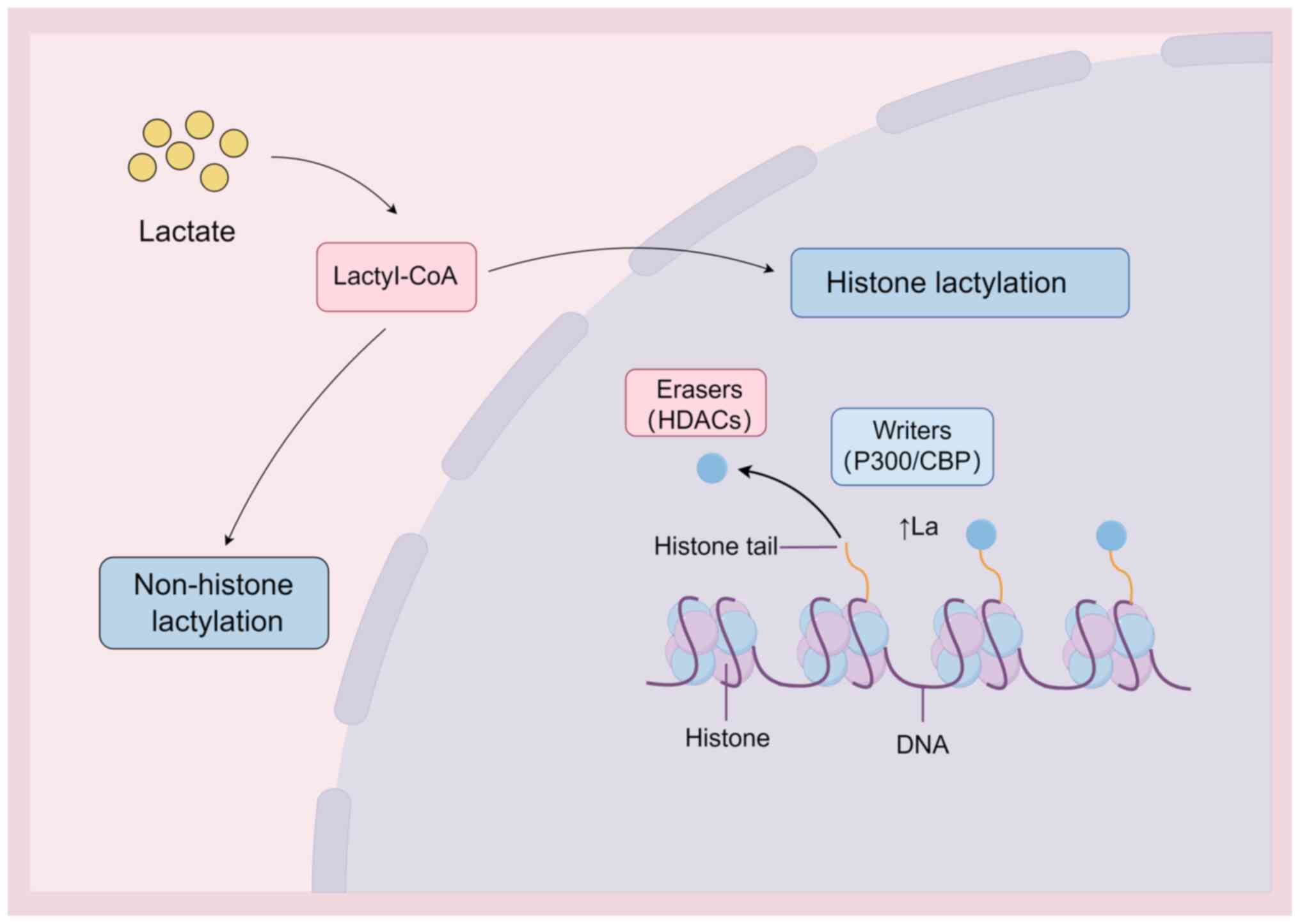
The regulation of lactylation is modulated by a
network of enzymes and regulatory proteins. Emerging evidence
reveals that, analogous to acetylation, lactylation is controlled
by 'writer' proteins such as P300/CREB binding protein and 'eraser'
proteins, including histone deacetylases (HDACs) (13,54,55), and that these proteins are
integral to the dynamic modulation and reversibility of lactylation
(Fig. 3). P300, a major writer
protein, catalyzes lysine lactylation by transferring lactate
groups onto lysine residues. The P300/ASF1A complex integrates
metabolic reprogramming with epigenetic modulation via H3K18la
during atherogenesis driven by endothelial-to-mesenchymal
transition (56). Eraser
proteins, such as HDACs, counteract lactylation by enzymatically
removing lactate groups. Notably, the feedback loop between H3K9la
and HDAC2 in endothelial cells modulates vascular endothelial
growth factor-induced angiogenesis; glycolysis inhibition reduces
H3K9la levels and consequently attenuates neovascularization
(11).
The identification of protein lactylation introduces
a new framework for linking nutrient metabolism to gene expression.
Continued investigation into lactylation and its regulatory
pathways is anticipated to enhance the understanding of cellular
dynamics and disease mechanisms. Expanding research efforts may
clarify the functional roles of this post-translational
modification and reveal novel therapeutic targets for related
pathologies. Future studies should delineate the interplay between
lactylation, cellular metabolism and other post-translational
modifications, and systematically define the regulatory
architecture of lactylation in kidney disease initiation and
progression. Such advances would substantially enrich the
mechanistic insight into lactate metabolism in kidney disease.
Detection methods of lactylation
Elucidating the biological significance of
lactylation necessitates the development and implementation of
precise detection methodologies.
Mass spectrometry (MS) is currently one of the most
commonly used techniques for detecting lactylation, leveraging
high-resolution instruments to identify mass shifts in proteins or
peptides and thereby accurately pinpointing lactylation sites on
proteins (57). As demonstrated
by Li et al (58) in an
AKI model, MS was successfully employed to identify lactylation
sites on aldehyde dehydrogenase 2 (ALDH2), followed by
immunoprecipitation-MS (IP-MS) analysis to characterize the
interaction between ALDH2 and prohibitin 2 (PHB2). The study
established that ALDH2 lactylation exacerbates mitochondrial
dysfunction in AKI by impairing PHB2-mediated mitophagy (58).
Specific antibodies targeting lactylated lysine
residues constitute indispensable tools for detecting lactylation,
enabling the application of western blotting and IP assays to
assess lactylation expression in cellular and tissue contexts. In a
hypoxia/reoxygenation cell model in the human proximal tubular HK-2
cell line, Zhou et al (59) employed western blotting to detect
histone lactylation and performed chromatin IP (ChIP) to assess its
association with hexokinase 2 (HK2). The results demonstrated
significant enrichment of H3K18la at the HK2 promoter region, which
upregulated HK2 expression and consequently exacerbated renal
ischemia/reperfusion injury. In renal tubular epithelial cells, An
et al (48) demonstrated
through western blot analysis that lactate overproduction mediates
lactylation of mitochondrial fission 1 protein (Fis1) lysine 20
(Fis1 K20la), which subsequently induces mitochondrial dysfunction
and exacerbates sepsis-associated AKI (SA-AKI).
Techniques such as immunofluorescence (IF) and
immunohistochemistry (IHC) further allow for the visualization of
the spatial distribution of lactylation within cells and tissues,
providing an intuitive localization tool for studying lactylation.
Qiao et al (60) employed
western blotting and IF to assess H3K18la expression in the renal
tubular epithelial cells of SA-AKI mice, combined with Cleavage
Under Targets and Tagmentation technology to identify
H3K18la-regulated target genes. The study revealed significant
enrichment of H3K18la at the promoter region of Ras homolog gene
family member A (RhoA), demonstrating that H3K18la upregulation
activates the RhoA/Rho-associated protein kinase/Ezrin signaling
pathway, thereby promoting renal dysfunction (60).
ChIP sequencing (ChIP-seq), a high-throughput method
for analyzing protein-DNA interactions (61), facilitates genome-wide mapping of
lactylation sites. Integration of RNA-seq data provides a
complementary strategy to elucidate transcriptional regulatory
networks influenced by lactylation modifications (62). As demonstrated by Zhang et
al (5) in AGE-induced renal
tubular epithelial cells, IHC and IF analyses of pan-lysine
lactylation (pan-Kla) and H3K14la, combined with integrated
ChIP-seq and RNA-seq profiling, revealed that H3K14la promotes the
EMT process through upregulation of downstream KLF5 expression
(5).
However, research on lactylation modifications
remains in its nascent stages, with methodologies undergoing
continuous refinement. Integrating multiple analytical approaches
can significantly improve the reliability of findings. As
technological advancements progress, future detection methods for
lactylation modifications are expected to achieve greater
sensitivity and efficiency, providing robust support for
elucidating their significant roles in biology.
Function of lactylation in renal cells
The kidney has unique anatomical and physiological
characteristics, with various renal cell types displaying
specialized metabolic adaptations tailored to their specific
functions and microenvironments (63). Consequently, the functional of
lactylation in renal cells is likely determined by cell
type-specific metabolic properties and pathological status,
particularly in injury repair, metabolic regulation and fibrosis
progression.
In AKI, the lactylation of renal tubular epithelial
cells modulates kidney functional by regulating cellular
proliferation and reparative pathways. For instance, heat shock
protein A12A augments the proliferative capacity of renal tubular
epithelial cells by enhancing c-Myc lactylation, thereby promoting
functional restoration following AKI (64). Furthermore, lactylation
influences the progression of SA-AKI, wherein lactate modifies
inflammatory metabolism in renal proximal tubular epithelial cells
via lactylation at H3K18 and Ezrin-K263 residues, consequently
affecting SA-AKI recovery (60).
Excessive lactate accumulation in renal tubular epithelial cells
drives Fis1 K20la. Elevated Fis1 K20la levels promote aberrant
mitochondrial fission, leading to ATP depletion, mitochondrial
reactive oxygen species (ROS) overproduction and mitochondrial
apoptosis, ultimately intensifying SA-AKI pathology (48). Thus, lactylation may regulate
renal tubular epithelial cell survival and functional restoration
in AKI through epigenetic mechanisms.
In DN, lactate derived from renal tubular epithelial
cells promotes KLF5 expression via H3K14la, thereby upregulating
Vimentin and α-smooth muscle actin (α-SMA) expression and
accelerating the EMT process (5). In the CKD model, lactate stimulates
transforming growth factor (TGF)-β1 expression in mouse renal
tubular epithelial cells via H3K18la, which subsequently activates
the Smad3 pathway in macrophages, driving macrophage-myofibroblast
transition and renal fibrosis (65). Furthermore, in DN mice, lactate
accumulation promotes histone pan-lactylation in podocytes, which
downregulates nephrin and zonula occludens-1 levels while
upregulating collagen IV, fibronectin and α-SMA, ultimately
inducing EMT in podocytes (66).
These findings demonstrate that during AKI, renal
tubular epithelial cells are susceptible to lactylation-induced
disturbances in energy metabolism and oxidative stress. By
contrast, under DN or CKD conditions, tubular lactylation appears
to promote renal fibrogenesis. In podocytes, lactylation primarily
drives fibrotic progression. Current evidence reveals the complex
regulatory roles of lactylation in renal cells. However, its
effects in other renal cells, including interstitial fibroblasts,
glomerular mesangial cells, immune cells and pericytes, remain
largely unexplored. The functional heterogeneity of lactylation
among different renal cell populations warrants further
investigation.
Lactate metabolism and lactylation in kidney
diseases
AKI
AKI, a common and severe renal disorder, involves a
sudden decline in renal function, evidenced by decreased glomerular
filtration rate (GFR) and/or diminished urine output, resulting in
metabolic waste accumulation, acid-base disturbances and
electrolyte imbalances. Integrating prognostic biomarkers with
clinical risk factors remains essential for early evaluation and
management of AKI (67).
Clinical evidence has revealed an association
between AKI and impaired gluconeogenesis, along with diminished
lactate clearance (68).
Elevated lactate levels have emerged as reliable predictors for
both the onset and prognosis of AKI (69,70). Gong et al (71) identified increased serum lactate
as an independent risk factor for SA-AKI, where concentrations
≥2.75 mmol/l were linked to a 1.772-fold escalation in AKI risk. In
the postoperative setting, particularly after cardiac surgery,
serum lactate levels independently predict AKI development
(72). Early dynamic monitoring
of lactate trajectories also enables the prediction of continuous
renal replacement therapy requirements following acute type A
aortic dissection surgery (73).
Furthermore, in conditions such as acute decompensated heart
failure (74), ST-segment
elevation myocardial infarction (75) and traumatic brain injury
(76), lactate levels serve as
key indicators for assessing AKI risk, thereby strengthening
opportunities for early diagnosis.
Clinical studies have highlighted the potential of
lactate levels as biomarkers in AKI, prompting further exploration
of the regulatory mechanisms of lactate in this condition. In a
suppurative AKI model induced by cecal ligation and puncture in
C57/B6 mice, lactate-mediated activation of the programmed cell
death protein 1 (PD-1)/programmed death ligand 1 (PD-L1) pathway
triggered lymphocyte apoptosis and subsequent immunosuppression
(Fig. 4), indicating that
blockade of lactate receptors or inhibition of the PD-1/PD-L1 axis
may offer innovative therapeutic strategies for septic AKI
(77). Sepsis-induced aerobic
glycolysis, characterized by increased lactate production and
upregulation of glycolysis-associated genes in renal tissues,
further implicates lactate in AKI pathology. In
lipopolysaccharide-stimulated HK-2 cells, lactate treatment
suppressed sirtuin 3 (SIRT3) and phosphorylated-AMP-activated
protein kinase (p-AMPK) expression, decreased the
microtubule-associated protein 1A/1B-light chain
3-II/microtubule-associated protein 1A/1B-light chain 3-I ratio and
elevated p62 levels, thereby inhibiting autophagy, promoting
apoptosis and intensifying sepsis-induced AKI (SI-AKI) progression
(78) (Fig. 4). Intervention with the aerobic
glycolysis inhibitor 2-deoxy-D-glucose attenuated glycolysis, with
underlying mechanisms likely involving the restoration of autophagy
via modulation of the lactate/SIRT3/AMPK signaling axis, thereby
mitigating SI-AKI (78). Renal
fibroblasts play a crucial role in the regulation, repair and
recovery of renal tubular injury after AKI. The proliferation of
fibroblasts follows the proliferation of renal tubular epithelial
cells. Proliferating renal tubular epithelial cells preferentially
utilize aerobic glycolysis as the primary metabolic pathway during
AKI progression. In the later stages of AKI, lactate generated by
injured tubular epithelial cells is absorbed by interstitial
fibroblasts, promoting fibroblast activation and proliferation
(79). Consequently,
glycolysis-derived lactate from tubular epithelial cells modulates
fibroblast activation and proliferation.
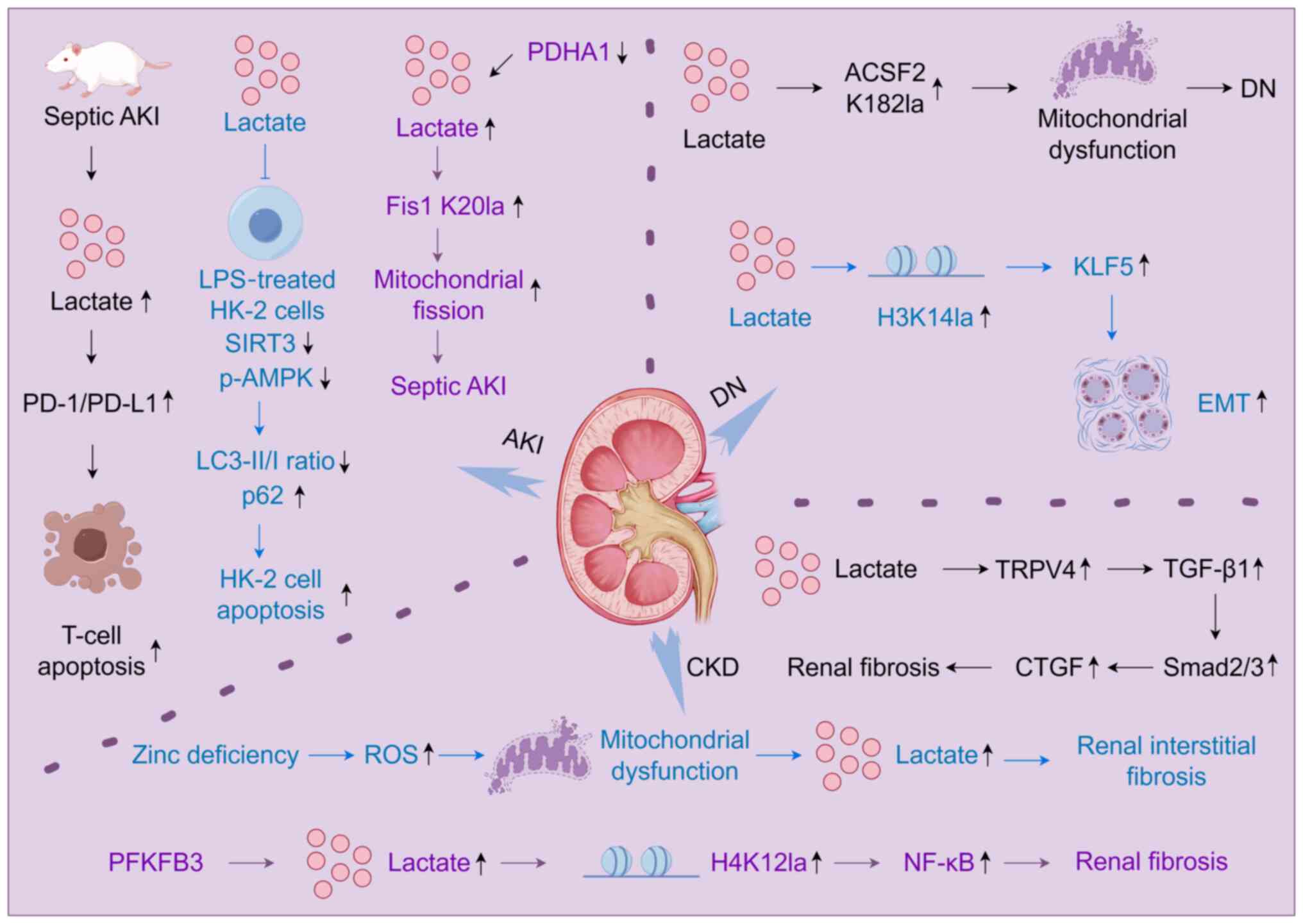 | Figure 4Mechanisms of lactate and lactylation
in renal diseases. In a septic AKI mouse model, lactate activates
the PD-1/PD-L1 pathway, inducing T lymphocyte apoptosis and
contributing to immunosuppression during septic AKI. In
LPS-stimulated HK-2 cells, lactate inhibits autophagy by
downregulating SIRT3 and p-AMPK expression, reducing the LC3-II/I
ratio and elevating p62 levels, thereby promoting HK-2 cell
apoptosis and worsening SI-AKI. High acetylation and inactivation
of PDHA1 in renal tubular epithelial cells drive lactate
accumulation, mediate Fis1 K20la, impair mitochondrial function and
aggravate SI-AKI. In human proximal tubular epithelial cells,
increased lactylation at K182 of ACSF2 induces mitochondrial
dysfunction, accelerates renal tubular injury and advances DN
progression. Elevated lactate concentrations in renal tubular
epithelial cells promote H3K14la, upregulating KLF5 expression and
driving EMT in DN. In spontaneous hypertension, excessive lactate
mediates renal fibrosis through activation of the
TRPV4-TGFβ1-SMAD2/3-CTGF signaling cascade. ROS stress resulting
from zinc deficiency also disrupts mitochondrial function, causing
metabolic stress characterized by abnormal lactate metabolism and
promoting the development of renal interstitial fibrosis. Moreover,
lactate accumulation driven by PFKFB3 activation enhances H4K12la,
augments NF-κB gene transcription and activation, intensifies
inflammatory responses, and accelerates renal fibrosis. LPS,
lipopolysaccharide; SIRT3, sirtuin 3; p-AMPK,
phosphorylated-AMP-activated protein kinase; PDHA1, pyruvate
dehydrogenase E1 component subunit α; Fis1 K20la, mitochondrial
fission 1 protein lysine 20 lactylation; ACSF2, Acyl-CoA synthetase
family member 2; K182la, lysine 182 lactylation; H3K14la, histone
H3 lysine 14 lactylation; EMT, epithelial-mesenchymal transition;
TRPV4, transient receptor potential vanilloid 4; TGF-β1,
transforming growth factor β1; p-Smad2/3, phospho-Smad2/3; TRPV4,
transient receptor potential cation channel subfamily V member 4;
CTGF, connective tissue growth factor; ROS, reactive oxygen
species; PFKFB3,
6-phosphofructo-2-kinase/fructose-2,6-biphosphatase 3; SI-AKI,
sepsis-induced acute kidney injury; PD-1/PD-L1, programmed cell
death protein 1/programmed death ligand 1; HK-2, hexokinase 2;
LC3-II/I, microtubule-associated protein 1A/1B-light chain
3-II/microtubule-associated protein 1A/1B-light chain 3-I ratio;
KLF5, Krüppel-like factor 5; diabetic neuropathy; CKD, chronic
kidney disease. The figure was drawn using Figdraw (figdraw.com). |
The discovery of lactylation has substantially
advanced the understanding of its involvement in AKI. Elevated
lactate levels are established as an independent risk factor for
SA-AKI. Evidence suggests that H3K18la may serve as a biomarker for
diagnosing and gauging the severity of septic shock (80). A recent investigation has reveal
that increased H3K18la expression in SA-AKI contributes to the
progression of renal insufficiency associated with this condition
(60). Moreover,
hyperacetylation and inactivation of pyruvate dehydrogenase E1
component subunit α in renal tubular epithelial cells promote
excessive lactate production, subsequently inducing Fis1
lactylation and accelerating the deterioration of SI-AKI (48) (Fig. 4). These findings elucidate the
epigenetic pathways implicated in AKI pathogenesis.
Although lactate is recognized as a biomarker for
AKI prediction and prognosis assessment, the mechanisms underlying
AKI progression and the specific contributions of lactate require
further elucidation. While H3K18la involvement in SA-AKI has been
characterized, the impact of lactylation in AKI resulting from
other etiologies remains poorly defined. Future research should
focus on delineating the roles and mechanistic pathways of lactate
and lactylation across diverse AKI models.
DN
DN, a severe microvascular complication of diabetes
characterized by proteinuria and progressive GFR decline, is a
leading global contributor to CKD (81). Effective early detection and
intervention depend on the development of robust predictive models
and biomarkers. Accumulating evidence positions lactate as a
promising non-invasive biomarker for early DN diagnosis (82). Jiang et al (83) proposed a DN risk prediction model
incorporating serum lactate levels through a nomogram, offering a
straightforward, economical and highly sensitive method for DN risk
stratification.
Investigations into the role of LDH in DN have
yielded significant insights. Tang et al (84) analyzed data from type 2 diabetes
(T2DM) patients collected between 2009 and 2018 in the National
Nutrition and Health Examination Survey database, utilizing
restricted cubic spline plots to delineate a dose-response
relationship between LDH levels and DN risk, thereby establishing
LDH as a relevant biomarker for DN screening in T2DM populations.
In parallel, Azushima et al (4) applied targeted metabolomics in a DN
mouse model, revealing elevated renal lactate concentrations,
impaired energy metabolism, and increased expression levels of LDHA
and LDHB isoforms. Administration of angiotensin receptor blockers
attenuated albuminuria, suppressed LDH expression, mitigated renal
injury, restored metabolic homeostasis, normalized lactate
concentrations and improved renal ATP content, implicating
metabolic dysregulation and excessive lactate accumulation in the
pathogenesis of DN.
Research into the mechanisms of lactate and
lactylation in DN has demonstrated that enhanced lactylation at
lysine 182 (K182) of ACSF2 in human proximal tubular epithelial
cells induces mitochondrial dysfunction, thereby promoting renal
tubular injury and accelerating DN progression (49) (Fig. 4). Moreover, EMT is widely
recognized as a central contributor to DN development. In renal
tubular epithelial cells exposed to AGEs, a metabolic shift from
oxidative phosphorylation to glycolysis elevates renal lactate
levels. Elevated lactate levels also increase H3K14la, subsequently
upregulating KLF5 expression and further driving EMT in DN
(5) (Fig. 4).
Advances in lactylation research have substantially
expanded the understanding of the epigenetic regulation of DN.
Modulation of the lactate-mediated H3K14la/KLF5 axis or adjustment
of lysine lactylation levels offers a potential pathway for
therapeutic innovation in DN. Although current evidence identifies
lactate and LDH as candidate biomarkers for DN, further
investigations are required to clarify the underlying mechanisms
and enhance their clinical translation.
CKD
CKD constitutes a major global public health
concern, characterized by chronic structural and functional renal
impairments originating from diverse etiologies, including primary
and secondary glomerulonephritis, tubular injury and renovascular
pathologies (85,86).
Lactate metabolism holds clinical relevance in CKD
across several dimensions, including metabolic dysregulation,
prognostic evaluation and complication management. Impaired renal
function, particularly tubular dysfunction, diminishes lactate
clearance in patients with CKD. Evidence indicates that defective
renal gluconeogenesis in CKD alters systemic metabolism, typified
by reduced glucose and elevated lactate levels, increasing
susceptibility to hyperlactatemia. This metabolic disturbance not
only predisposes patients to metabolic acidosis but also correlates
with adverse renal outcomes (7,87). Consequently, abnormal lactate
metabolism is a manifestation of metabolic imbalance in patients
with CKD. Monitoring blood lactate levels may help assess the
status of lactate metabolism in patients with CKD and could
potentially assist in evaluating the prognosis of the disease.
However, its specific clinical application value in monitoring
disease progression, managing complications and assessing prognosis
in CKD still requires further evaluation.
The hallmark pathology of CKD is renal fibrosis,
encompassing glomerulosclerosis, tubular atrophy and interstitial
fibrosis. Dysregulated lactate metabolism has been implicated in
fibrotic progression. In spontaneous hypertension, elevated lactate
concentrations aggravate renal fibrosis via activation of the
transient receptor potential cation channel subfamily V member
4-TGFβ1-SMAD2/3-connective tissue growth factor (CTGF) signaling
cascade (88) (Fig. 4). Zinc, a critical micronutrient
for mitigating oxidative stress and promoting tissue repair, is
essential for maintaining systemic homeostasis. Markedly reduced
plasma zinc concentrations have been reported in patients with CKD
(89), where ROS stress
resulting from zinc deficiency also disrupts mitochondrial
function, causing metabolic stress characterized by abnormal
lactate metabolism and promoting the development of renal
interstitial fibrosis (90)
(Fig. 4).
Enhanced renal glycolysis contributes to CKD
progression (91), with PFKFB3
acting as a critical modulator of this metabolic shift (92). PFKFB3 expression is markedly
upregulated in renal proximal tubular cells following
ischemia-reperfusion injury in mice, displaying a positive
correlation with the extent of renal fibrosis (6). Mechanistically, lactate
accumulation driven by PFKFB3-mediated glycolytic reprogramming
significantly elevates histone lactylation, particularly H4K12la,
which is enriched at the promoters of NF-κB signaling genes,
thereby initiating their transcription and intensifying
inflammatory responses (6)
(Fig. 4). Thus, targeting
PFKFB3-mediated NF-κB signaling in tubular cells represents a
potential therapeutic avenue for CKD. During renal fibrosis,
cellular metabolic reprogramming occurs, with enhanced glycolysis
constituting a major feature of disease progression (93,94). Suppressing glycolytic flux may,
therefore, mitigate fibrosis advancement. However, glycolysis
exerts divergent effects across different renal cells; for
instance, dichloroacetic acid and shikonin inhibit fibroblast
activation while eliciting distinct responses in renal epithelial
cells (95), suggesting that
cell type-specific metabolic modulation could open new therapeutic
possibilities.
Recent investigations further implicate lactate
metabolism in CKD progression, although the underlying mechanisms
and clinical relevance require deeper exploration. Future research
should prioritize identifying specific cell types affected by
lactate dynamics, and delineating the gene networks and regulatory
pathways modulated by lactate. Expanding this area of study could
establish a theoretical framework for innovative therapeutic
strategies and ultimately enhance clinical outcomes.
Prospects and limitations of lactate and its
related biomarkers in nephropathy
Clinical application prospects of
lactate-related biomarkers
The application prospects of lactate and its related
biomarkers in samples of patients with kidney disease include the
potential as diagnostic markers, prognostic assessment tools and
therapeutic targets.
Evidence from a cohort of patients with T2DM
demonstrated that urinary lactate levels maintained a statistically
significant association with end-stage renal disease risk, even
after adjustment for conventional risk factors such as estimated
glomerular filtration rate and urine albumin-to-creatinine ratio,
highlighting their value as predictive biomarkers for disease
advancement (4). Notably,
urinary lactate levels show a significant correlation with
established biomarkers of tubular injury and epithelial stress,
namely kidney injury molecule-1 and Dickkopf-3, suggesting its
potential as a predictive biomarker for disease progression
(4). These findings underscore
the utility of urinary lactate as a potent tool for risk
stratification in DN, where it is particularly adept at identifying
patient populations who, despite presenting with seemingly
acceptable conventional indicators, are actually at a high
risk.
Beyond quantifying lactate concentrations, molecules
involved in lactate metabolism have emerged as promising
biomarkers. H3K14la, a lactate-mediated epigenetic modification,
has been identified as a key participant in EMT progression in DN
through activation of KLF5 expression (5). Theoretically, measuring H3K14la
levels in renal tissues or urinary exosomes could serve as an
indicator of renal fibrosis activity. However, standardized
detection methodologies for this purpose remain unavailable.
The application of lactate-related biomarkers
extends beyond diagnosis and prognosis, offering potential for
therapeutic target identification. The elucidation of the
H3K14la/KLF5 axis introduces new avenues for anti-fibrotic drug
development, with interventions targeting this pathway representing
a potential strategy to delay DN progression (5). In parallel, therapeutic approaches
aimed at modulating lactate production or clearance, such as
adjusting LDH activity or promoting lactate utilization, may offer
new opportunities for kidney disease management. The refinement and
implementation of these therapeutic strategies will inevitably rely
on the guidance and monitoring of corresponding biomarkers.
Limitations of clinical application
Despite significant progress, current research and
technological applications encounter multiple limitations that
obstruct the clinical translation of biomarkers. Obstacles span the
entire workflow, from sample collection and data analysis to
mechanistic interpretation and clinical validation, demanding
prompt and systematic resolution.
A primary technical barrier lies in the absence of
standardized detection protocols. Studies utilize a wide range of
lactate measurement techniques, including enzymatic electrode
assays, gas chromatography-MS, and high-performance liquid
chromatography, each differing in sensitivity, specificity and
reproducibility, thereby complicating cross-study comparisons
(96,97). Variations in sample types and
analytical strategies further impede the definition of unified
cutoff thresholds.
The invasive nature of sample collection constrains
the clinical applicability of certain biomarkers. Although urinary
lactate measurement offers a relatively non-invasive alternative,
analyses of kidney tissue-specific modifications, such as H3K14la,
continue to rely on renal biopsy specimens, limiting practicality
for routine diagnostics. Even blood-based assays, while less
invasive than tissue sampling, impose additional burdens due to the
need for frequent collection, potentially compromising patient
adherence. While microneedle sensors present a novel approach for
minimally invasive monitoring, their long-term stability and
reusability require further validation (98,99).
Regarding biomarker specificity, elevated lactate
levels commonly arise under diverse pathophysiological conditions,
including tissue hypoxia, hepatic dysfunction, systemic
inflammatory responses and pharmacological influences (78,100). Such conditions may overlap in
patients with renal disease, thereby limiting the utility of
lactate quantification alone to accurately distinguish
renal-specific injuries from systemic disturbances. Moreover,
distinct kidney pathologies, such as DN and ischemic renal injury,
may drive lactate metabolism alterations via different molecular
mechanisms (63). However, the
majority of current studies focus on specific disease types and
lack systematic data for cross-disease comparisons.
Limitations in dynamic monitoring technologies
constrain the clinical implementation of lactate-related
biomarkers. Kidney disease progression often involves dynamic
metabolic fluctuations that single-time-point measurements fail to
capture. Although wearable devices theoretically support continuous
monitoring, practical application remains hindered by challenges
such as signal instability, skin irritation and acquisition
efficiency (99,101).
The translational gap between mechanistic research
and clinical application remains a major obstacle. Findings derived
from basic studies often lack reproducibility in clinical cohorts.
Additionally, current clinical investigations predominantly employ
observational designs. Interventional trials are required to
substantiate the clinical relevance of lactate-related
biomarkers.
Research on lactate is evolving beyond its
characterization as a metabolic byproduct toward its recognition as
a multifunctional signaling molecule. Addressing existing
challenges is essential for refining future research priorities,
advancing detection methodologies and optimizing the clinical
integration of current technologies. These efforts may offer novel
perspectives for the early diagnosis and precision management of
kidney diseases.
Management strategies of kidney diseases
based on lactate metabolism
According to the present review, lactate has
demonstrated predictive utility as a biomarker in kidney disease
and contributes to disease progression; its production is regulated
by critical glycolytic enzymes, including LDH, GLUT, HK2 and PKM2.
Modulation of lactate metabolism through targeting these enzymes,
altering its synthesis, transport and conversion, represents a
potential therapeutic approach for the treatment of kidney
diseases.
PKM2, a key isomer of the pyruvate kinase family,
serves a fundamental role in glycolysis; its expression is markedly
diminished in podocytes from patients with hypertensive nephropathy
and DN (102). Urinary PKM2 has
also emerged as a potential biomarker for predicting SA-AKI
(103), highlighting PKM2 as an
attractive therapeutic target for kidney diseases. TEPP-46, a
small-molecule activator of PKM2, enhances glycolytic flux in
healthy renal tissue (104). In
diabetic mouse models, TEPP-46 restores tubular epithelial
integrity by suppressing EMT and abnormal glycolysis, thereby
attenuating diabetic renal fibrosis (105). Additionally, TEPP-46 restricts
pericyte proliferation, migration and pericyte-myofibroblast
transdifferentiation by limiting nuclear translocation of PKM2,
offering a new approach to preventing the transition from AKI to
CKD (106). Microcystin-RR
(MC-RR), which demonstrates anti-pulmonary fibrosis properties
(107), also exhibits renal
protective effects. Using unilateral ureteral obstruction (UUO)
mouse models and in vitro cell models, Ren et al
(108) demonstrated that MC-RR
directly bound to PKM2, modulated the PKM2-HIF-1α signaling axis,
restored suppressed MMP-7 and MMP-13 expression, and reduced
elevated MMP-9 levels in UUO renal tissue, thereby mitigating renal
fibrosis. Furthermore, several Chinese herbal compounds exert
nephroprotective effects through PKM2 targeting. For instance,
modified Hu-lu-ba-wan ameliorates glomerular injury and podocyte
apoptosis by maintaining PKM2-mediated mitochondrial homeostasis in
DN (109). Similarly, Qian Yang
Yu Yin granules improve hypertensive nephropathy by reprogramming
metabolism through the HIF-1α/PKM2 positive feedback mechanism
(110).
HK2, the principal rate-limiting enzyme of
glycolysis, serves a central function in metabolic regulation.
Evidence indicates that ceria nanoparticles attenuate metabolic
disturbances in renal fibrosis models by diminishing ROS, enhancing
mitochondrial ATP production, and suppressing HK1 and HK2
expression, thereby conferring renal protection (111). LDHA, responsible for catalyzing
the conversion of pyruvate to lactate during glycolysis, is
integral to maintaining cellular energy equilibrium. Curcumin, the
primary curcuminoid in turmeric, mediates renoprotective effects by
inhibiting aerobic glycolysis through the miR-489/LDHA axis,
thereby alleviating glucose fluctuation-induced renal injury in the
293 cell model (112).
Moreover, the glycolysis inhibitor 3-bromopyruvate restrains
TGF-β1-induced fibroblast proliferation in a time- and
dose-dependent manner, significantly downregulating the expression
of aerobic glycolysis-related enzymes, such as HK2, LDHA and PKM2,
while modulating the IL-1 receptor-associated kinase 4/MYC
signaling pathway to mitigate renal fibrosis (113).
Modulating glucose entry into glycolysis represents
a promising therapeutic strategy. The membrane protein GLUT is
integral to the transport of glucose and related substrates across
cell membranes. In HK-2 cells treated with high glucose, the
expression level of GLUT-1 was upregulated (114), and in a streptozotocin-induced
DN rat model, the expression level of GLUT-2 was downregulated
(115). p-Coumaric acid
nanoparticles have been shown to attenuate DN in rats by
controlling hyperglycemia, suppressing inflammation and enhancing
GLUT-2 mRNA expression in nephropathic models (115). Similarly, Huangqi decoction
restores GLUT expression in diabetic kidneys, elevating GLUT4
levels while reducing GLUT1 expression in a dose-dependent manner
(116). A bioflavonoid
combination has further demonstrated renal function improvement in
DN by regulating MMP-9/TIMP-1 expression, upregulating GLUT-4 and
downregulating TGF-β (117).
Regulation of glycolytic enzymes through these interventions may
influence lactate production, transport and metabolism, offering
therapeutic advantages in kidney disease management.
Clinical research into drugs targeting lactate
metabolism or modulating lactylation mechanisms for kidney disease
treatment remains an emerging and promising domain. Pharmacological
or metabolic interventions designed to reduce lactate accumulation
may potentially slow disease progression. Sodium-glucose
cotransporter 2 (SGLT2) inhibitors, such as dapagliflozin,
currently in clinical use, appear to indirectly decrease lactate
levels by enhancing energy metabolism and exert renal protective
effects in patients with DN (118). Levocarnitine mitigates
oxidative stress and attenuates lactate accumulation in patients
with kidney disease, particularly benefiting individuals with
chronic renal failure and L-carnitine deficiency secondary to
hemodialysis (119,120). However, most agents intended to
regulate lactate metabolism or influence lactylation pathways
remain at experimental stages, with clinical validation of their
therapeutic potential still limited. Further research is warranted
to clarify their mechanisms of action, assess safety profiles and
establish therapeutic efficacy to support clinical translation in
kidney disease management.
In clinical practice, dysregulation of lactate
metabolism and lactate-mediated modifications in the kidney
constitute a shared pathophysiological basis for multiple renal
disorders. Effective monitoring requires an integrated evaluation
of blood lactate concentrations, urinary lactate levels and
biomarkers of lactate modifications in renal biopsy specimens.
Management of patients with lactic acidosis emphasizes the
aggressive treatment of underlying conditions, correction of
hypoxic states and amelioration of metabolic perturbations. In the
medication of certain populations, particular caution is warranted
in patients with CKD to avoid nephrotoxins that may enhance lactate
production, such as metformin (121), alongside monitoring of serum
lactate and acid-base status. In the context of SA-AKI, early
goal-directed therapy combined with infection source control can
mitigate lactate accumulation. Pharmacological strategies targeting
lactification currently remain under investigation. Future progress
will depend on interdisciplinary efforts to integrate lactate
metabolic regulation with epigenetic modification mechanisms,
thereby enhancing their translational potential.
Targeting glycolytic enzymes to regulate lactate
metabolism offers a promising therapeutic approach for kidney
diseases but faces multiple substantive challenges. Low target
specificity remains a major concern, as glycolytic enzymes such as
PKM2 and HK2 are extensively involved in systemic metabolism,
raising the risk of unintended metabolic disturbances. The
pronounced heterogeneity among kidney diseases further complicates
therapeutic outcomes, with the same target having variable effects
across different pathological contexts. Insufficient drug delivery
efficiency also limits therapeutic success, particularly for
natural compounds such as curcumin, which exhibit poor renal
targeting due to hepatic first-pass metabolism and systemic
distribution. Additionally, inhibition of a single glycolytic
enzyme may provoke compensatory metabolic pathways, introducing
further complexity into therapeutic modulation. Clinical
translation remains constrained by the reliance on animal and in
vitro models, with minimal validation in human kidney tissues.
Future strategies require the development of highly specific renal
delivery technologies. Integrating multi-omics approaches to
delineate the metabolic-immune-epigenetic networks, alongside the
construction of dynamic biomarker systems through spatial
metabolomics, will be instrumental in mapping the spatiotemporal
heterogeneity of lactate metabolism throughout kidney disease
progression. Furthermore, advancing personalized therapeutic
strategies through clinical trials guided by dynamic biomarkers may
shift the paradigm from merely correcting metabolic imbalances to
reconstructing renal microenvironmental homeostasis.
Conclusion
Lactate and its metabolic pathways exert
multifaceted roles in renal physiology and pathology, functioning
not only as central mediators of energy metabolism but also through
lactylation-driven epigenetic modifications that influence renal
cell inflammation, fibrosis and reparative processes. Current
research has preliminarily established the association between
dysregulated lactate metabolism and kidney diseases, with
interventions targeting lactate pathways exhibiting therapeutic
potential in preclinical models. Nevertheless, prevailing
conclusions largely derive from broad-spectrum metabolic regulation
studies, lacking precise delineation of cell-type specificity,
spatiotemporal dynamics and the molecular network underlying
lactylation-mediated pathological mechanisms, which remain to be
systematically elucidated.
Future research priorities should address the
following areas: i) Development of precision intervention tools,
engineering tissue-specific modulators of lactylation, such as LDHA
inhibitors or lactylation enzyme regulators targeting renal tubular
epithelial cells, to enable selective intervention within renal
parenchymal cells while minimizing systemic metabolic
perturbations. ii) Construction of dynamic models, establishing
animal models capturing the temporal dynamics of lactylation
alterations during kidney disease progression, including
cell-specific lactate sensor transgenic mice to characterize
metabolic heterogeneity across pathological stages. iii)
Integration of interdisciplinary technologies, applying multi-omics
approaches such as metabolomics and spatial transcriptomics to
unravel the interaction networks linking the glycolysis-lactate
axis with immune microenvironmental remodeling and fibrotic
pathways, and identifying key regulatory nodes. Concurrently,
clinical translation challenges must be addressed by developing
non-invasive detection tools for lactate metabolism markers and
validating the safety profiles of therapeutic strategies using
models such as organoids, thereby providing a basis for
personalized treatment. Comprehensive, multidimensional analyses of
cell-specific mechanisms and dynamic lactate metabolic networks are
anticipated to drive the evolution from biomarker discovery to
targeted therapies in kidney disease management.
Availability of data and materials
Not applicable.
Authors' contributions
XL wrote the manuscript text. HJ conceived and
designed the review. LH and QH edited and revised the manuscript.
All authors read and approved the final version of the manuscript.
Data authentication is not applicable.
Ethics approval and consent to
participate
Not applicable.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
Acknowledgments
Not applicable.
Funding
This study was supported by the National Natural Science
Foundation of China (grant no. 82274307) and the Research Funds of
Center for Xin'an Medicine and Modernization of Traditional Chinese
Medicine of Institute of Health and Medicine (grant no.
2023CXMMTCM018).
References
|
1
|
Silva PHI and Mohebbi N: Kidney metabolism
and acid-base control: Back to the basics. Pflugers Arch.
474:919–934. 2022. View Article : Google Scholar
|
|
2
|
Chen Y, Fry BC and Layton AT: Modeling
glucose metabolism and lactate production in the kidney. Math
Biosci. 289:116–129. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Reddy AJ, Lam SW, Bauer SR and Guzman JA:
Lactic acidosis: Clinical implications and management strategies.
Clevel Clin J Med. 82:615–624. 2015. View Article : Google Scholar
|
|
4
|
Azushima K, Kovalik JP, Yamaji T, Ching J,
Chng TW, Guo J, Liu JJ, Nguyen M, Sakban RB, George SE, et al:
Abnormal lactate metabolism is linked to albuminuria and kidney
injury in diabetic nephropathy. Kidney Int. 104:1135–1149. 2023.
View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Zhang X, Chen J, Lin R, Huang Y, Wang Z,
Xu S, Wang L, Chen F, Zhang J, Pan K and Yin Z: Lactate drives
epithelial-mesenchymal transition in diabetic kidney disease via
the H3K14la/KLF5 pathway. Redox Biol. 75:1032462024. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Wang Y, Li H, Jiang S, Fu D, Lu X, Lu M,
Li Y, Luo D, Wu K, Xu Y, et al: The glycolytic enzyme PFKFB3 drives
kidney fibrosis through promoting histone lactylation-mediated
NF-κB family activation. Kidney Int. 106:226–240. 2024. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Verissimo T, Faivre A, Rinaldi A,
Lindenmeyer M, Delitsikou V, Veyrat-Durebex C, Heckenmeyer C,
Fernandez M, Berchtold L, Dalga D, et al: Decreased renal
gluconeogenesis is a hallmark of chronic kidney disease. J Am Soc
Nephrol. 33:810–827. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Rabinowitz JD and Enerbäck S: Lactate: The
ugly duckling of energy metabolism. Nat Metab. 2:566–571. 2020.
View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Merkuri F, Rothstein M and Simoes-Costa M:
Histone lactylation couples cellular metabolism with developmental
gene regulatory networks. Nat Commun. 15:902024. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Li J, Hou W, Zhao Q, Han W, Cui H, Xiao S,
Zhu L, Qu J, Liu X, Cong W, et al: Lactate regulates major zygotic
genome activation by H3K18 lactylation in mammals. Natl Sci Rev.
11:nwad2952024. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Dai W, Wu G, Liu K, Chen Q, Tao J, Liu H
and Shen M: Lactate promotes myogenesis via activating H3K9
lactylation-dependent up-regulation of Neu2 expression. J Cachexia
Sarcopenia Muscle. 14:2851–2865. 2023. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Fan W, Zeng S, Wang X, Wang G, Liao D, Li
R, He S, Li W, Huang J, Li X, et al: A feedback loop driven by H3K9
lactylation and HDAC2 in endothelial cells regulates VEGF-induced
angiogenesis. Genome Biol. 25:1652024. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Minami E, Sasa K, Yamada A, Kawai R,
Yoshida H, Nakano H, Maki K and Kamijo R: Lactate-induced histone
lactylation by p300 promotes osteoblast differentiation. PLoS One.
18:e02936762023. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Trujillo MN, Jennings EQ, Hoffman EA,
Zhang H, Phoebe AM, Mastin GE, Kitamura N, Reisz JA, Megill E,
Kantner D, et al: Lactoylglutathione promotes inflammatory
signaling in macrophages through histone lactoylation. Mol Metab.
81:1018882024. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Kierans SJ and Taylor CT: Glycolysis: A
multifaceted metabolic pathway and signaling hub. J Biol Chem.
300:1079062024. View Article : Google Scholar
|
|
16
|
Luengo A, Li Z, Gui DY, Sullivan LB,
Zagorulya M, Do BT, Ferreira R, Naamati A, Ali A, Lewis CA, et al:
Increased demand for NAD+ relative to ATP drives aerobic
glycolysis. Mol Cell. 81:691–707.e6. 2021. View Article : Google Scholar :
|
|
17
|
Wang L, Pavlou S, Du X, Bhuckory M, Xu H
and Chen M: Glucose transporter 1 critically controls microglial
activation through facilitating glycolysis. Mol Neurodegener.
14:22019. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Yin X, Choudhury M, Kang JH, Schaefbauer
KJ, Jung MY, Andrianifahanana M, Hernandez DM and Leof EB:
Hexokinase 2 couples glycolysis with the profibrotic actions of
TGF-β. Sci Signal. 12:eaax40672019. View Article : Google Scholar
|
|
19
|
Nishioku T, Anzai R, Hiramatsu S, Terazono
A, Nakao M and Moriyama M: Lactate dehydrogenase A inhibition
prevents RANKL-induced osteoclastogenesis by reducing enhanced
glycolysis. J Pharmacol Sci. 153:197–207. 2023. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Kim E, Hwang Y, Kim H, Kim GU, Ryu YC,
Yoon M and Choi KY: Pyruvate Kinase M2 accelerates cutaneous wound
healing via glycolysis and Wnt/β-catenin signaling. Pharmaceutics.
15:20282023. View Article : Google Scholar
|
|
21
|
Li J, Ma P, Liu Z and Xie J: L- and
D-lactate: Unveiling their hidden functions in disease and health.
Cell Commun Signal. 23:1342025. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Heim CE, Bosch ME, Yamada KJ, Aldrich AL,
Chaudhari SS, Klinkebiel D, Gries CM, Alqarzaee AA, Li Y, Thomas
VC, et al: Lactate production by Staphylococcus aureus biofilm
inhibits HDAC11 to reprogram the host immune response during
persistent infection. Nat Microbiol. 5:1271–1284. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Monroe GR, van Eerde AM, Tessadori F,
Duran KJ, Savelberg SMC, van Alfen JC, Terhal PA, van der Crabben
SN, Lichtenbelt KD, Fuchs SA, et al: Identification of human D
lactate dehydrogenase deficiency. Nat Commun. 10:14772019.
View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Vernon C and LeTourneau JL: Lactic
acidosis: Recognition, kinetics, and associated prognosis. Critical
Care Clinics. 26:255–283. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Emhoff CAW and Messonnier LA: Concepts of
lactate metabolic clearance rate and lactate clamp for metabolic
inquiry: A Mini-review. Nutrients. 15:32132023. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Huang T, Liang Z, Wang K, Miao X and Zheng
L: Novel insights into athlete physical recovery concerning lactate
metabolism, lactate clearance and fatigue monitoring: A
comprehensive review. Front Physiol. 16:14597172025. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Lin Y, Wang Y and Li P: Mutual regulation
of lactate dehydrogenase and redox robustness. Front Physiol.
13:10384212022. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Adeva M, González-Lucán M, Seco M and
Donapetry C: Enzymes involved in l-lactate metabolism in humans.
Mitochondrion. 13:615–629. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Wei T, Guo Y, Huang C, Sun M, Zhou B, Gao
J and Shen W: Fibroblast-to-cardiomyocyte lactate shuttle modulates
hypertensive cardiac remodelling. Cell Biosci. 13:1512023.
View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Brooks GA, Curl CC, Leija RG, Osmond AD,
Duong JJ and Arevalo JA: Tracing the lactate shuttle to the
mitochondrial reticulum. Exp Mol Med. 54:1332–1347. 2022.
View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Zhang L, Xin C, Wang S, Zhuo S, Zhu J, Li
Z, Liu Y, Yang L and Chen Y: Lactate transported by MCT1 plays an
active role in promoting mitochondrial biogenesis and enhancing TCA
flux in skeletal muscle. Sci Adv. 10:eadn45082024. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Contreras-Baeza Y, Sandoval PY, Alarcón R,
Galaz A, Cortés-Molina F, Alegría K, Baeza-Lehnert F, Arce-Molina
R, Guequén A, Flores CA, et al: Monocarboxylate transporter 4
(MCT4) is a high affinity transporter capable of exporting lactate
in high-lactate microenvironments. J Biol Chem. 294:20135–20147.
2019. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Kobayashi M, Narumi K, Furugen A and Iseki
K: Transport function, regulation, and biology of human
monocarboxylate transporter 1 (hMCT1) and 4 (hMCT4). Pharmacol
Ther. 226:1078622021. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Yanase H, Takebe K, Nio-Kobayashi J,
Takahashi-Iwanaga H and Iwanaga T: Cellular expression of a
sodium-dependent monocarboxylate transporter (Slc5a8) and the MCT
family in the mouse kidney. Histochem Cell Biol. 130:957–966. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Becker HM, Mohebbi N, Perna A, Ganapathy
V, Capasso G and Wagner CA: Localization of members of MCT
monocarboxylate transporter family Slc16 in the kidney and
regulation during metabolic acidosis. Am J Physiol Renal Physiol.
299:F141–F154. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Osis G, Traylor AM, Black LM, Spangler D,
George JF, Zarjou A, Verlander JW and Agarwal A: Expression of
lactate dehydrogenase A and B isoforms in the mouse kidney. Am J
Physiol Renal Physiol. 320:F706–F718. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Feng Y, Sun Z, Fu J, Zhong F, Zhang W, Wei
C, Chen A, Liu BC, He JC and Lee K: Podocyte-derived soluble
RARRES1 drives kidney disease progression through direct podocyte
and proximal tubular injury. Kidney Int. 106:50–66. 2024.
View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Zhao Y, Fan S, Zhu H, Zhao Q, Fang Z, Xu
D, Lin W, Lin L, Hu X, Wu G, et al: Podocyte OTUD5 alleviates
diabetic kidney disease through deubiquitinating TAK1 and reducing
podocyte inflammation and injury. Nat Commun. 15:54412024.
View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Szrejder M, Typiak M, Pikul P, Audzeyenka
I, Rachubik P, Rogacka D, Narajczyk M and Piwkowska A: Role of
L-lactate as an energy substrate in primary rat podocytes under
physiological and glucose deprivation conditions. Eur J Cell Biol.
102:1512982023. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Audzeyenka I, Szrejder M, Rachubik P,
Grochowalska K, Kulesza T, Rogacka D, Narajczyk M and Piwkowska A:
Lactate regulates respiratory efficiency and mitochondrial dynamics
in primary rat podocytes. Free Radic Biol Med. 220:312–323. 2024.
View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Dalga D, Verissimo T and de Seigneux S:
Gluconeogenesis in the kidney: In health and in chronic kidney
disease. Clin Kidney J. 16:1249–1257. 2023. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Nakamura M, Satoh N, Horita S and Nangaku
M: Insulin-induced mTOR signaling and gluconeogenesis in renal
proximal tubules: A mini-review of current evidence and therapeutic
potential. Front Pharmacol. 13:10152042022. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Hatano R, Lee E, Sato H, Kiuchi M,
Hirahara K, Nakagawa Y, Shimano H, Nakayama T, Tanaka T and Miki T:
Hepatic ketone body regulation of renal gluconeogenesis. Mol Metab.
84:1019342024. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Zanza C, Facelli V, Romenskaya T,
Bottinelli M, Caputo G, Piccioni A, Franceschi F, Saviano A, Ojetti
V, Savioli G and Longhitano Y: Lactic acidosis related to
pharmacotherapy and human diseases. Pharmaceuticals (Basel).
15:14962022. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Zhang D, Tang Z, Huang H, Zhou G, Cui C,
Weng Y, Liu W, Kim S, Lee S, Perez-Neut M, et al: Metabolic
regulation of gene expression by histone lactylation. Nature.
574:575–580. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Zhou Y, Yan J, Huang H, Liu L, Ren L, Hu
J, Jiang X, Zheng Y, Xu L, Zhong F and Li X: The m6A reader IGF2BP2
regulates glycolytic metabolism and mediates histone lactylation to
enhance hepatic stellate cell activation and liver fibrosis. Cell
Death Dis. 15:1892024. View Article : Google Scholar :
|
|
47
|
Wan N, Wang N, Yu S, Zhang H, Tang S, Wang
D, Lu W, Li H, Delafield DG, Kong Y, et al: Cyclic immonium ion of
lactyllysine reveals widespread lactylation in the human proteome.
Nat Methods. 19:854–864. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
48
|
An S, Yao Y, Hu H, Wu J, Li J, Li L, Wu J,
Sun M, Deng Z, Zhang Y, et al: PDHA1 hyperacetylation-mediated
lactate overproduction promotes sepsis-induced acute kidney injury
via Fis1 lactylation. Cell Death Dis. 14:4572023. View Article : Google Scholar : PubMed/NCBI
|
|
49
|
Chen J, Feng Q, Qiao Y, Pan S, Liang L,
Liu Y, Zhang X, Liu D and Liu Z and Liu Z: ACSF2 and lysine
lactylation contribute to renal tubule injury in diabetes.
Diabetologia. 67:1429–1443. 2024. View Article : Google Scholar : PubMed/NCBI
|
|
50
|
Wang P, Xie D, Xiao T, Cheng C, Wang D,
Sun J, Wu M, Yang Y, Zhang A and Liu Q: H3K18 lactylation promotes
the progression of arsenite-related idiopathic pulmonary fibrosis
via YTHDF1/m6A/NREP. J Hazard Mater. 461:1325822024. View Article : Google Scholar
|
|
51
|
Zhang Y, Huang Z, Han W, Wu J, Li S, Qin
T, Zhang C, Shi M, Han S, Gao B, et al: Glutamine suppresses
senescence and promotes autophagy through glycolysis
inhibition-mediated AMPKα lactylation in intervertebral disc
degeneration. Commun Biol. 7:3252024. View Article : Google Scholar
|
|
52
|
Sun W, Jia M, Feng Y and Cheng X: Lactate
is a bridge linking glycolysis and autophagy through lactylation.
Autophagy. 19:3240–3241. 2023. View Article : Google Scholar : PubMed/NCBI
|
|
53
|
Wei Y, Guo H, Chen S and Tang XX:
Regulation of macrophage activation by lactylation in lung disease.
Front Immunol. 15:14277392024. View Article : Google Scholar : PubMed/NCBI
|
|
54
|
Moreno-Yruela C, Zhang D, Wei W, Bæk M,
Liu W, Gao J, Danková D, Nielsen AL, Bolding JE, Yang L, et al:
Class I histone deacetylases (HDAC1-3) are histone lysine
delactylases. Sci Adv. 8:eabi66962022. View Article : Google Scholar : PubMed/NCBI
|
|
55
|
Kikuchi M, Morita S, Wakamori M, Sato S,
Uchikubo-Kamo T, Suzuki T, Dohmae N, Shirouzu M and Umehara T:
Epigenetic mechanisms to propagate histone acetylation by p300/CBP.
Nat Commun. 14:41032023. View Article : Google Scholar : PubMed/NCBI
|
|
56
|
Dong M, Zhang Y, Chen M, Tan Y, Min J, He
X, Liu F, Gu J, Jiang H, Zheng L, et al: ASF1A-dependent
P300-mediated histone H3 lysine 18 lactylation promotes
atherosclerosis by regulating EndMT. Acta Pharm Sin B.
14:3027–3048. 2024. View Article : Google Scholar : PubMed/NCBI
|
|
57
|
Wu X and Tao WA: Uncovering ubiquitous
protein lactylation. Nat Methods. 19:793–794. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
58
|
Li J, Shi X, Xu J, Wang K, Hou F, Luan X
and Chen L: Aldehyde dehydrogenase 2 lactylation aggravates
mitochondrial dysfunction by disrupting PHB2 mediated mitophagy in
acute kidney injury. Adv Sci (Weinh). 12:e24119432024. View Article : Google Scholar : PubMed/NCBI
|
|
59
|
Zhou J, Zhang J, Xu F, Gao H, Wang L, Zhao
Y and Li K: AST-120 alleviates renal ischemia-reperfusion injury by
inhibiting HK2-mediated glycolysis. Mol Med. 30:1332024. View Article : Google Scholar : PubMed/NCBI
|
|
60
|
Qiao J, Tan Y, Liu H, Yang B, Zhang Q, Liu
Q, Sun W, Li Z, Wang Q, Feng W, et al: Histone H3K18 and ezrin
lactylation promote renal dysfunction in Sepsis-associated acute
kidney injury. Adv Sci (Weinh). 11:e23072162024. View Article : Google Scholar : PubMed/NCBI
|
|
61
|
Kumar B, Navarro C, Yung PYK, Lyu J,
Salazar Mantero A, Katsori AM, Schwämmle H, Martin M and Elsässer
SJ: Multiplexed chromatin immunoprecipitation sequencing for
quantitative study of histone modifications and chromatin factors.
Nat Protoc. 20:779–809. 2025. View Article : Google Scholar
|
|
62
|
Zhang L, Xue G, Liu J, Li Q and Wang Y:
Revealing transcription factor and histone modification
co-localization and dynamics across cell lines by integrating
ChIP-seq and RNA-seq data. BMC Genomics. 19:9142018. View Article : Google Scholar
|
|
63
|
Miguel V, Shaw IW and Kramann R:
Metabolism at the crossroads of inflammation and fibrosis in
chronic kidney disease. Nat Rev Nephrol. 21:39–56. 2025. View Article : Google Scholar
|
|
64
|
Li Y, Min X, Zhang X, Cao X, Kong Q, Mao
Q, Cheng H, Gou L, Li Y, Li C, et al: HSPA12A promotes c-Myc
lactylation-mediated proliferation of tubular epithelial cells to
facilitate renal functional recovery from kidney
ischemia/reperfusion injury. Cell Mol Life Sci. 81:4042024.
View Article : Google Scholar : PubMed/NCBI
|
|
65
|
Xiang T, Wang X, Huang S, Zhou K, Fei S,
Zhou B, Yue K, Li Q, Xue S, Dai Y, et al: Inhibition of PKM2 by
shikonin impedes TGF-β1 expression by repressing histone
lactylation to alleviate renal fibrosis. Phytomedicine.
136:1563242025. View Article : Google Scholar
|
|
66
|
Zheng T, Gu YP, Wang JM, Huang TT, Gou LS
and Liu YW: Lactate-triggered histone lactylation contributes to
podocyte epithelial-mesenchymal transition in diabetic nephropathy
in mice. Chem Biol Interact. 408:1114182025. View Article : Google Scholar : PubMed/NCBI
|
|
67
|
Jia L, Sheng X, Zamperetti A, Xie Y,
Corradi V, Chandel S, De Cal M, Montin DP, Caprara C and Ronco C:
Combination of biomarker with clinical risk factors for prediction
of severe acute kidney injury in critically ill patients. BMC
Nephrol. 21:5402020. View Article : Google Scholar : PubMed/NCBI
|
|
68
|
Legouis D, Ricksten S-E, Faivre A,
Verissimo T, Gariani K, Verney C, Galichon P, Berchtold L, Feraille
E, Fernandez M, et al: Altered proximal tubular cell glucose
metabolism during acute kidney injury is associated with mortality.
Nat Metab. 2:732–743. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
69
|
Sklienka P, Maca J, Neiser J, Bursa F,
Sevcik P, Frelich M, Petejova N, Svagera Z, Tomaskova H and Zahorec
R: Physiologic risk factors for early acute kidney injury in
severely injured patients. Bratisl Lek Listy. 121:779–785.
2020.PubMed/NCBI
|
|
70
|
Nasu T, Ueda K, Kawashima S, Okishio Y,
Kunitatsu K, Iwasaki Y and Kato S: Prediction of early acute kidney
injury after trauma using prehospital systolic blood pressure and
lactate levels: A prospective validation study. Injury. 53:81–85.
2022. View Article : Google Scholar
|
|
71
|
Gong C, Jiang Y, Tang Y, Liu F, Shi Y,
Zhou H and Xie K: Elevated serum lactic acid level is an
independent risk factor for the incidence and mortality of
sepsis-associated acute kidney injury. Zhonghua Wei Zhong Bing Ji
Jiu Yi Xue. 34:714–720. 2022.In Chinese. PubMed/NCBI
|
|
72
|
Flores-Salinas HE, Zambada-Gamboa AJ,
Garcia-Garduño TC, Rodríguez-Zavala G, Valle Y, Chávez-Herrera JC,
Martinez-Gutierrez PE, Godinez-Flores A, Jiménez-Limón S and
Padilla-Gutiérrez JR: Association of postoperative serum lactate
levels with acute kidney injury in mexican patients undergoing
cardiac surgery. Clin Pract. 14:1100–1109. 2024. View Article : Google Scholar : PubMed/NCBI
|
|
73
|
Wang Z, Xu J, Kang Y, Liu L, Zhang L and
Wang D: Early dynamic behavior of lactate in predicting continuous
renal replacement therapy after surgery for acute type A aortic
dissection. Front Cardiovasc Med. 9:9486722022. View Article : Google Scholar : PubMed/NCBI
|
|
74
|
Kahyaoglu M, Karaduman A, Geçmen Ç, Candan
Ö, Güner A, Cakmak EO, Bayam E, Yılmaz Y, Çelik M, Izgi IA and
Kirma C: Serum lactate level may predict the development of acute
kidney injury in acute decompensated heart failure. Turk Kardiyol
Dern Ars. 48:683–689. 2020.PubMed/NCBI
|
|
75
|
Zhou X, He Y, Hu L, Zhu Q, Lin Q, Hong X,
Huang W, Shan P and Liang D: Lactate level and lactate clearance
for acute kidney injury prediction among patients admitted with
ST-segment elevation myocardial infarction: A retrospective cohort
study. Front Cardiovasc Med. 9:9302022022. View Article : Google Scholar : PubMed/NCBI
|
|
76
|
Wang R, Wang S, Zhang J, He M and Xu J:
Serum lactate level in early stage is associated with acute kidney
injury in traumatic brain injury patients. Front Surg.
8:7611662021. View Article : Google Scholar
|
|
77
|
Xu J, Ma X, Yu K, Wang R, Wang S, Liu R,
Liu H, Gao H, Yu K and Wang C: Lactate up-regulates the expression
of PD-L1 in kidney and causes immunosuppression in septic Acute
Renal Injury. J Microbiol Immunol Infect. 54:404–410. 2021.
View Article : Google Scholar
|
|
78
|
Tan C, Gu J, Li T, Chen H, Liu K, Liu M,
Zhang H and Xiao X: Inhibition of aerobic glycolysis alleviates
sepsis-induced acute kidney injury by promoting lactate/Sirtuin
3/AMPK-regulated autophagy. Int J Mol Med. 47:192021. View Article : Google Scholar :
|
|
79
|
Shen Y, Jiang L, Wen P, Ye Y, Zhang Y,
Ding H, Luo J, Xu L, Zen K, Zhou Y and Yang J: Tubule-derived
lactate is required for fibroblast activation in acute kidney
injury. Am J Physiol Renal Physiol. 318:F689–F701. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
80
|
Chu X, Di C, Chang P, Li L, Feng Z, Xiao
S, Yan X, Xu X, Li H, Qi R, et al: Lactylated histone H3K18 as a
potential biomarker for the diagnosis and predicting the severity
of septic shock. Front Immunol. 12:7866662021. View Article : Google Scholar
|
|
81
|
Guo W, Song Y, Sun Y, Du H, Cai Y, You Q,
Fu H and Shao L: Systemic immune-inflammation index is associated
with diabetic kidney disease in type 2 diabetes mellitus patients:
Evidence from NHANES 2011-2018. Front Endocrinol (Lausanne).
13:10714652022. View Article : Google Scholar : PubMed/NCBI
|
|
82
|
Roointan A, Gheisari Y, Hudkins KL and
Gholaminejad A: Non-invasive metabolic biomarkers for early
diagnosis of diabetic nephropathy: Meta-analysis of profiling
metabolomics studies. Nutr Metab Cardiovasc Dis. 31:2253–2272.
2021. View Article : Google Scholar : PubMed/NCBI
|
|
83
|
Jiang C, Ma X, Chen J, Zeng Y, Guo M, Tan
X, Wang Y, Wang P, Yan P, Lei Y, et al: Development of serum
lactate Level-based nomograms for predicting diabetic kidney
disease in type 2 diabetes mellitus patients. Diabetes Metab Syndr
Obes. 17:1051–1068. 2024. View Article : Google Scholar : PubMed/NCBI
|
|
84
|
Tang L, Yang Q, Ma R, Zhou P, Peng C, Xie
C, Liang Q, Wu T, Gao W, Yu H, et al: Association between lactate
dehydrogenase and the risk of diabetic kidney disease in patients
with type 2 diabetes. Front Endocrinol (Lausanne). 15:13699682024.
View Article : Google Scholar : PubMed/NCBI
|
|
85
|
Muiru AN, Hsu JY, Zhang X, Appel LJ, Chen
J, Cohen DL, Drawz PE, Freedman BI, Go AS, He J, et al: Risk for
chronic kidney disease progression after acute kidney injury:
Findings from the chronic renal insufficiency cohort study. Ann
Intern Med. 176:961–968. 2023. View Article : Google Scholar : PubMed/NCBI
|
|
86
|
Chesnaye NC, Ortiz A, Zoccali C, Stel VS
and Jager KJ: The impact of population ageing on the burden of
chronic kidney disease. Nat Rev Nephrol. 20:569–585. 2024.
View Article : Google Scholar : PubMed/NCBI
|
|
87
|
Tanemoto M: Gap acidosis except lactic
acidosis develops and progresses during chronic kidney disease
stage G5. Clin Exp Nephrol. 23:1045–1049. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
88
|
Zhao B, Xu Y, Chen Y, Cai Y, Gong Z, Li D,
Kuang H, Liu X, Zhou H, Liu G and Yin Y: Activation of TRPV4 by
lactate as a critical mediator of renal fibrosis in spontaneously
hypertensive rats after moderate- and high-intensity exercise.
Front Physiol. 13:9270782022. View Article : Google Scholar : PubMed/NCBI
|
|
89
|
Nagy A, Pethő D, Gáll T, Zavaczki E,
Nyitrai M, Posta J, Zarjou A, Agarwal A, Balla G and Balla J: Zinc
Inhibits HIF-Prolyl Hydroxylase Inhibitor-Aggravated VSMC
calcification induced by high phosphate. Front Physiol.
10:15842020. View Article : Google Scholar : PubMed/NCBI
|
|
90
|
Huang Z, Liao Y, Zheng Y, Ye S, Zhang Q,
Yu X, Liu X and Li N: Zinc deficiency causes glomerulosclerosis and
renal interstitial fibrosis through oxidative stress and increased
lactate metabolism in rats. Biol Trace Elem Res. 203:2084–2098.
2025. View Article : Google Scholar :
|
|
91
|
Li M, Jia F, Zhou H, Di J and Yang M:
Elevated aerobic glycolysis in renal tubular epithelial cells
influences the proliferation and differentiation of podocytes and
promotes renal interstitial fibrosis. Eur Rev Med Pharmacol Sci.
22:5082–5090. 2018.PubMed/NCBI
|
|
92
|
Jiang A, Liu J, Wang Y and Zhang C:
cGAS-STING signaling pathway promotes hypoxia-induced renal
fibrosis by regulating PFKFB3-mediated glycolysis. Free Radic Biol
Mede. 208:516–529. 2023. View Article : Google Scholar
|
|
93
|
Ding H, Jiang L, Xu J, Bai F, Zhou Y, Yuan
Q, Luo J, Zen K and Yang J: Inhibiting aerobic glycolysis
suppresses renal interstitial fibroblast activation and renal
fibrosis. Am J Physiol Renal Physiol. 313:F561–F575. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
94
|
Li X, Ma TK, Wang M, Zhang XD, Liu TY, Liu
Y, Huang ZH, Zhu YH, Zhang S, Yin L, et al: YY1-induced
upregulation of LncRNA-ARAP1-AS2 and ARAP1 promotes diabetic kidney
fibrosis via aberrant glycolysis associated with EGFR/PKM2/HIF-1α
pathway. Front Pharmacol. 14:10693482023. View Article : Google Scholar
|
|
95
|
Wei Q, Su J, Dong G, Zhang M, Huo Y and
Dong Z: Glycolysis inhibitors suppress renal interstitial fibrosis
via divergent effects on fibroblasts and tubular cells. Am J
Physiol Renal Physiol. 316:F1162–F1172. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
96
|
Md Shakhih MF, Rosslan AS, Noor AM,
Ramanathan S, Lazim AM and Wahab AA: Review-enzymatic and
Non-enzymatic electrochemical sensor for lactate detection in human
biofluids. J Electrochem Soc. 168:0675022021. View Article : Google Scholar
|
|
97
|
Henry H, Marmy Conus N, Steenhout P,
Béguin A and Boulat O: Sensitive determination of D-lactic acid and
L-lactic acid in urine by high-performance liquid
chromatography-tandem mass spectrometry. Biomed Chromatogr.
26:425–428. 2012. View Article : Google Scholar
|
|
98
|
Bollella P, Sharma S, Cass AEG and
Antiochia R: Microneedle-based biosensor for minimally-invasive
lactate detection. Biosens Bioelectron. 123:152–159. 2019.
View Article : Google Scholar
|
|
99
|
Xie Y, Li K, Liu J, Zhou Y, Zhang C, Yu Y,
Wang J, Su L and Zhang X: A smart lab on a wearable microneedle
patch with convolutional neural network-enhanced colorimetry for
early warning of syndrome of inappropriate antidiuretic hormone
secretion. Aggregate. 6:e6712025. View Article : Google Scholar
|
|
100
|
Yao S, Chai H, Tao T, Zhang L, Yang X, Li
X, Yi Z, Wang Y, An J, Wen G, et al: Role of lactate and lactate
metabolism in liver diseases (Review). Int J Mol Med. 54:592024.
View Article : Google Scholar : PubMed/NCBI
|
|
101
|
Sun J, Dai W, Guo Q, Gao Y, Chen J, Chen
JL, Mao G, Sun H and Peng YK: Self-powered wearable electrochemical
sensor based on composite conductive hydrogel medium for detection
of lactate in human sweat. Biosens Bioelectron. 277:1173032025.
View Article : Google Scholar : PubMed/NCBI
|
|
102
|
Chen Z, Zhu Z, Liang W, Luo Z, Hu J, Feng
J, Zhang Z, Luo Q, Yang H and Ding G: Reduction of anaerobic
glycolysis contributes to angiotensin II-induced podocyte injury
with foot process effacement. Kidney Int. 103:735–748. 2023.
View Article : Google Scholar : PubMed/NCBI
|
|
103
|
Jiajun W, Kaifeng G and Jing Z: Urinary
PKM2, a marker predicating acute kidney injury in patients with
sepsis. Int Urol Nephrol. 56:3039–3045. 2024. View Article : Google Scholar : PubMed/NCBI
|
|
104
|
Bertelsen LB, Hansen ESS, Sadowski T, Ruf
S and Laustsen C: Hyperpolarized pyruvate to measure the influence
of PKM2 activation on glucose metabolism in the healthy kidney. NMR
Biomed. 34:e45832021. View Article : Google Scholar : PubMed/NCBI
|
|
105
|
Liu H, Takagaki Y, Kumagai A, Kanasaki K
and Koya D: The PKM2 activator TEPP-46 suppresses kidney fibrosis
via inhibition of the EMT program and aberrant glycolysis
associated with suppression of HIF-1α accumulation. J Diabetes
Investig. 12:697–709. 2021. View Article : Google Scholar
|
|
106
|
Chen Y, Bai X, Chen J, Huang M, Hong Q,
Ouyang Q, Sun X, Zhang Y, Liu J, Wang X, et al: Pyruvate kinase M2
regulates kidney fibrosis through pericyte glycolysis during the
progression from acute kidney injury to chronic kidney disease.
Cell Prolif. 57:e135482024. View Article : Google Scholar :
|
|
107
|
Wang J, Ren Y, Zheng X, Kang J, Huang Z,
Xu L and Wang Y: Anti-fibrotic effects of low toxic Microcystin-RR
on Bleomycin-induced pulmonary fibrosis: A comparison with
Microcystin-LR. Front Pharmacol. 12:6759072021. View Article : Google Scholar : PubMed/NCBI
|
|
108
|
Ren Y, Wang J, Guo W, Chen J, Wu X, Gu S,
Xu L, Wu Z and Wang Y: Renoprotection of Microcystin-RR in
unilateral ureteral Obstruction-induced renal fibrosis: Targeting
the PKM2-HIF-1α pathway. Front Pharmacol. 13:8303122022. View Article : Google Scholar
|
|
109
|
Gong M, Guo Y, Dong H, Wu F, He Q, Gong J
and Lu F: Modified Hu-lu-ba-wan protects diabetic glomerular
podocytes via promoting PKM2-mediated mitochondrial dynamic
homeostasis. Phytomedicine. 123:1552472024. View Article : Google Scholar
|
|
110
|
Qian L, Ren S, Xu Z, Zheng Y, Wu L, Yang
Y, Wang Y, Li J, Yan S and Fang Z: Qian yang yu yin granule
improves renal injury of hypertension by regulating metabolic
reprogramming mediated by HIF-1α/PKM2 positive feedback loop. Front
Pharmacol. 12:6674332021. View Article : Google Scholar
|
|
111
|
Wang M, Zeng F, Ning F, Wang Y, Zhou S, He
J, Li C, Wang C, Sun X, Zhang D, et al: Ceria nanoparticles
ameliorate renal fibrosis by modulating the balance between
oxidative phosphorylation and aerobic glycolysis. J
Nanobiotechnology. 20:32022. View Article : Google Scholar : PubMed/NCBI
|
|
112
|
Fu X, Zhang J, Huang X, Mo Z, Sang Z, Duan
W and Huang W: Curcumin antagonizes glucose fluctuation-induced
renal injury by inhibiting aerobic glycolysis via the miR-489/LDHA
pathway. Mediators Inflamm. 2021:61045292021. View Article : Google Scholar : PubMed/NCBI
|
|
113
|
Yu H, Zhu J, Chang L, Liang C, Li X and
Wang W: 3-Bromopyruvate decreased kidney fibrosis and fibroblast
activation by suppressing aerobic glycolysis in unilateral ureteral
obstruction mice model. Life Sci. 272:1192062021. View Article : Google Scholar : PubMed/NCBI
|
|
114
|
Han W, Wang C, Yang Z, Mu L, Wu M, Chen N,
Du C, Duan H and Shi Y: SRT1720 retards renal fibrosis via
inhibition of HIF1A/GLUT1 in diabetic nephropathy. J Endocrinol.
241:85–98. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
115
|
Venkatesan A, Roy A, Kulandaivel S,
Natesan V and Kim SJ: p-Coumaric acid nanoparticles ameliorate
diabetic nephropathy via regulating mRNA expression of KIM-1 and
GLUT-2 in Streptozotocin-induced diabetic rats. Metabolites.
12:11662022. View Article : Google Scholar : PubMed/NCBI
|
|
116
|
Chen X, Wang H, Jiang M, Zhao J, Fan C,
Wang Y and Peng W: Huangqi (astragalus) decoction ameliorates
diabetic nephropathy via IRS1-PI3K-GLUT signaling pathway. Am J
Transl Res. 10:2491–2501. 2018.PubMed/NCBI
|
|
117
|
Ritu, Xiong Y, Sharma HP, Goyal RK, Narwal
S, Berwal A, Jain S, Priya M, Singh M, Agarwal G, et al:
Bioflavonoid combination attenuates diabetes-induced nephropathy in
rats via modulation of MMP-9/TIMP-1, TGF-β, and GLUT-4-associated
pathways. Heliyon. 10:e332172024. View Article : Google Scholar
|
|
118
|
Zhang J, Ding T, Zhang X, Tang D and Wang
J: Dapagliflozin relieves renal injury in a diabetic nephropathy
model by inducing autophagy through regulation of
miR-30e-5p/AKT/mTOR pathway. Trop J Pharm Res. 21:2115–2123. 2022.
View Article : Google Scholar
|
|
119
|
Fatouros IG, Douroudos I, Panagoutsos S,
Pasadakis P, Nikolaidis MG, Chatzinikolaou A, Sovatzidis A,
Michailidis Y, Jamurtas AZ, Mandalidis D, et al: Effects of
L-carnitine on oxidative stress responses in patients with renal
disease. Med Sci Sports Exerc. 42:1809–1818. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
120
|
Sharma B and Yadav DK: L-Carnitine and
chronic kidney disease: A comprehensive review on nutrition and
health perspectives. J Pers Med. 13:2982023. View Article : Google Scholar : PubMed/NCBI
|
|
121
|
Wen YK: Impact of acute kidney injury on
metformin-associated lactic acidosis. Int Urol Nephrol. 41:967–972.
2009. View Article : Google Scholar : PubMed/NCBI
|