Introduction
Psoriasis is a chronic autoimmune skin condition
characterized by the aberrant interaction between
hyperproliferative keratinocytes and activated immune cells,
leading to the formation of scales and red patches which
substantial negative effects on patient quality of life (1). However, the underlying mechanisms
of aberrant keratinocytes and activated immune cells in psoriasis
are incompletely understood. Although current treatments include
topical treatments, phototherapy, systemic treatments and
biologics, there is no complete cure for psoriasis (2). Further research is recommended to
investigate novel druggable targets that could enhance treatment
outcomes for psoriasis.
Dendritic cells (DCs) play an important role in the
initiation of psoriasis due to their ability to identify and
present self-nucleotides that emerge from cellular distress,
subsequently leading to T cell activation and proliferation
(3). DCs produce interleukin
(IL)-23, IL-12, tumor necrosis factor-α (TNF-α), and various other
cytokines that significantly enhance the differentiation of naive T
cells into Th (T help)1, Th17, and Th22 subsets. IL-23 plays a
crucial role in sustaining and promoting the proliferation of
pathogenic Th17 cells (4).
Biological therapies targeting the IL-23/IL-17 axis are approved
for clinical use and show excellent efficacy (5,6).
However, the recurrence of psoriasis may still occur following the
administration of IL-23 inhibitors, such as ustekinumab and
guselkumab (7,8). Additionally, side effects
associated with IL-23 inhibitors have been linked to an increased
risk of infections, as evidenced by clinical trials and
post-marketing surveillance (1,9).
Therefore, it is necessary to find safer and more effective drugs
to inhibit the production of inflammatory factors in DCs.
Lobetyolin (LBT; PubChem CID: 14655097) is a
bioactive compound extracted from Codonopsis pilosula (C.
pilosula), commonly known as Dangshen in Chinese (10). There is increasing evidence
suggesting that LBT possesses anti-inflammatory, anti-oxidative,
and xanthine oxidase inhibiting properties (11,12). For example, LBT significantly
reduced serum levels of IL-6, TNF-α and IL-1β while inhibiting
inflammatory cell infiltration in lung and liver tissues during
LPS-induced sepsis (13).
Additionally, LBT protects BV2 microglial cells from oxygen-glucose
deprivation/reperfusion damage by regulating their phenotypic
polarization and reducing inflammatory responses, specifically by
suppressing the production of TNF-α, IL-6, inducible nitric oxide
synthase and CD206 (14).
However, its potential impact on regulating DCs activation and
psoriasis remains unknown.
The present study revealed new insights into LBT's
role in Imiquimod (IMQ)-induced psoriasis-like inflammation. Our
findings showed that LBT notably inhibited psoriasis in mice and
helped maintain skin homeostasis during its progression by
regulating the expression of genes related to keratinocyte
proliferation and differentiation, enhancing the peroxisome
proliferator-activated receptors' (PPAR) signaling pathway, and
upregulating genes and metabolites involved in linoleic acid
metabolism. Moreover, LBT inhibited gene expression linked to
cytokine activity, as well as the IL-17, TNF, and mitogen-activated
protein kinase (MAPK) signaling pathways in IMQ-treated DCs. These
results highlighted LBT's significant ability to reduce IMQ-induced
psoriasis-like skin inflammation by maintaining skin homeostasis
and suppressing inflammatory cytokines in DCs, indicating its
potential as a therapeutic agent for psoriasis.
Materials and methods
Reagents and mice
LBT (HY-N0327) was procured from MedChemExpress. IMQ
(cat. no. tlrl-imq-1) was acquired from InvivoGen. Recombinant
mouse GM-CSF (cat. no. 315-03) was sourced from PeproTech, Inc.
APC-CD45 (cat. no. 109814) antibody was sourced from BioLegend,
Inc. RPMI-1640 medium, along with penicillin, streptomycin, and
fetal bovine serum, were obtained from Gibco-BRL (Thermo Fisher
Scientific, Inc.). TRIzol® reagent was supplied by
Invitrogen; Thermo Fisher Scientific, Inc. The ReverTra Ace qPCR RT
Kit (cat. no. FSQ-201) was purchased from Toyobo Life Science,
while the SYBR Green master Rox (cat. no. 04707516001) was obtained
from Roche. Anti-mouse IL-23 (p19) antibody (cat. no. 513807) was
purchased from BioLegend, Inc. KRT 6A antibody (cat. no.
10590-1-AP) was purchased from Proteintech Group, Inc.
Male C57BL/6 mice (8 weeks-old, average weight, 22
g) were obtained from the Shanghai SLAC Laboratory Animal Center
and housed at the Zhejiang University Laboratory Animal Center with
specific pathogen-free conditions, maintained on a 12/12-h
light-dark cycle with lights activated at 8:00 a.m., in a
controlled environment (23±1°C, 60±5% humidity) with ad
libitum access to standard rodent chow and autoclaved water.
All animal experiments were conducted in accordance with relevant
guidelines and regulations approved by the Institutional Animal
Care and Use Committee at Zhejiang University. The present study
received approval (approval no. 20220283) from the Ethics Committee
of Zhejiang University School of Medicine (Hangzhou, China).
IMQ induced psoriasis-like skin
inflammation in the mice model
A cohort of 196 8-week-old male C57BL/6 mice
(initial n=52, expanded to 196 during revision) were randomized
into eight experimental groups: PBS control, 1 mM LBT, 10 mM LBT,
IMQ alone, IMQ + PBS, IMQ + 1 mM LBT, IMQ + 10 mM LBT, and IMQ +
anti-IL-23p19 antibody (100 µg, i.p.). Psoriasis was induced
by daily topical application of 62.5 mg 5% IMQ cream
(Aldara®) on shaved dorsal/auricular skin for 5
consecutive days, with LBT treatments (1 mM or 10 mM in PBS)
applied topically twice daily. The IMQ challenge elicited
characteristic psoriasiform dermatitis including erythema, edema,
epidermal hyperplasia and scaling. Strict humane endpoints were
enforced, requiring euthanasia by pentobarbital sodium overdose (50
mg/kg, i.p.) followed by cervical dislocation for mice exhibiting
≥20% weight loss, cachexia, prolonged feeding impairment, or
mobility deficits. The cessation of breathing and heartbeat for
more than 2 min post-euthanasia was continuously observed prior to
tissue collection. Throughout the 6-day study period, all animals
underwent at least twice daily (morning and evening) health and
behavioral monitoring to assess disease progression and treatment
efficacy.
Histological analysis
Mouse back skin or ear tissues were fixed in 4%
formaldehyde at 20-25°C (room temperature) for 48 h for proper
tissue fixation. The fixed tissues then underwent dehydration
through a series of alcohol solutions with increasing
concentrations from 75-100%. Next, the tissues were embedded in wax
and sliced into ~5-µm thick sections. After blanching in hot
water, the sections were affixed to glass slides and dried in a
controlled environment at 45°C. Before staining, the wax was
removed using xylene, followed by sequential immersion in
decreasing concentrations of alcohol and rinsing with distilled
water. The slices were dehydrated in 70 and 90% alcohol for 10 min
each, then stained with an alcohol-eosin solution for 2 to 3 min.
Lastly, the stained sections were dehydrated in pure alcohol and
rendered transparent with xylene prior to microscopic examination.
Ki67 staining was performed using anti-Ki67 (Clone SP6; cat. no.
ab16667; Abcam) at 1:200 dilution, incubated overnight at 4°C,
followed by DAB visualization. Stained sections were observed using
a light microscope (IX73; Olympus Corporation). The epidermal
thickness of the skin was calculated as the area of epidermis/the
length of epidermis.
Flow cytometry analysis
Single cells from the ear were isolated following a
previously published protocol (15). The cells were then collected and
processed to obtain a single-cell suspension, with removal of cell
clumps and debris. Subsequently, the suspension was centrifuged and
resuspended in a flow cytometry staining solution for staining with
APC-CD45 antibodies (1:200 dilution), followed by analysis using a
BD Fortessa Cell Analyzer (BD Biosciences). The resulting data were
analyzed using FlowJo v10.6.2 software (FlowJo LLC).
RNA-Seq detects the differentially
expressed genes (DEGs)
Total RNA was isolated using TRIzol™ Reagent (cat.
no. 15596026; Thermo Fisher Scientific, Inc.) according to the
manufacturer's protocol. RNA quantity and integrity were assessed
using the Qubit™ 3.0 Fluorometer (cat. no. Q33216; Thermo Fisher
Scientific, Inc.) and Agilent 5300 Fragment Analyzer System (cat.
no. M5310AA; Agilent Technologies) respectively. RNA samples
demonstrating high purity (A260/A280 ratio 1.8-2.2) and RNA
integrity numbers >7.0 were selected for subsequent library
preparation using Illumina-compatible protocols. mRNA was purified
from 2 µg total RNA using mRNA Capture Beads 2.0 (cat. no.
12629ES; Shanghai Yeasen Biotechnology Co., Ltd.) through two
rounds of poly(A) selection.
Purified mRNA was fragmented in magnesium-containing
fragmentation buffer (cat. no. 12340ES97; Shanghai Yeasen
Biotechnology Co., Ltd.) at 94°C for 5 min. First-strand cDNA was
synthesized using random hexamer-primed reverse transcription
(SuperScript™ IV Reverse Transcriptase; Thermo Fisher Scientific,
Inc.). Second-strand cDNA was generated using a dUTP incorporation
strategy with E. coli DNA Polymerase I, RNase H, and dUTP solution
(cat. no. 12340ES97; Shanghai Yeasen Biotechnology Co., Ltd.).
Blunt-ended cDNA fragments were adenylated at 3′ ends using Klenow
Fragment (3′→5′ exo-) and ligated to Illumina-compatible forked
adapters (IDT) containing T-overhangs. PCR products were
size-selected (400±50 bp inserts) using Hieff NGS DNA Selection
Beads (cat. no. 12601ES75; Shanghai Yeasen Biotechnology Co.,
Ltd.). Strand specificity was maintained through dUTP-based strand
marking and uracil excision. Libraries were sequenced in 2×150 bp
paired-end mode on an Illumina NovaSeq™ X Plus platform (LC-bio
Technologies Hangzhou, Co., Ltd.) following
manufacturer-recommended protocols.
Differential expression analysis of genes was
performed by DESeq2 software (https://bioconductor.org/packages/release/bioc/html/DESeq2.html)
between two different groups (and by edgeR between two samples).
The genes with the parameter of false discovery rate (FDR) below
0.05 and absolute fold change ≥2 were considered DEGs. DEGs were
then subjected to enrichment analysis of Gene Ontology (GO)
functions and Kyoto Encyclopedia of Genes and Genomes (KEGG)
pathways.
RNA isolation and reverse
transcription-quantitative PCR (RT-qPCR)
Total RNA was extracted from skin tissue or DCs
using TRIzol reagent (Takara Biotechnology Co., Ltd.) according to
the manufacturer's instructions. Reverse transcription was
performed with the HiScript III 1st Strand cDNA Synthesis Kit
(+gDNA wiper) (cat. no. R312-01; Vazyme Biotech Co., Ltd.)
according to the manufacturer's instructions. RT-qPCR was conducted
using SYBR Green Master Mix, and the relative difference was
expressed as the fold change compared with control values,
calculated using the comparative cycle method (2−ΔΔCq)
(16). The PCR protocol begins
with an initial denaturation at 95°C for 5 min, followed by 35
cycles of amplification, each consisting of denaturation at 95°C
for 10 sec and a combined annealing/extension step at 60°C for 30
sec. After cycling, a melt curve analysis performed by heating to
95°C for 15 sec, cooling to 60°C for 1 min, and then gradually
ramping to 95°C to assess amplicon specificity. Actb gene
was used as an internal control to normalize the expression of the
target genes across experimental samples. The primer sequences are
listed in Table SI.
Non-targeted metabolomics profiling
analysis
Back-skin tissues from mice treated with IMQ or LBT
were collected and analyzed at Hangzhou Lianchuan Biotechnology Co.
Skin tissue samples (25±1 mg) were collected, mixed with beads and
500 µl of extraction solution (methanol: acetonitrile:
water, 2:2:1 v/v) containing deuterated internal standards. The
mixture was vortexed for 30 sec. The samples were incubated at
-40°C for 1 h to precipitate proteins. Subsequently, the samples
were centrifuged at 12,000 rpm (RCF=13,800 × g, rotor radius=8.6
cm) for 15 min at 4°C. The supernatant was transferred to fresh
glass vials for analysis. Quality control (QC) samples were
prepared by pooling equal volumes of the supernatants from all
biological replicates.
LC-MS/MS analyses were performed using an UHPLC
system (Vanquish; Thermo Fisher Scientific, Inc.) coupled with a
Waters ACQUITY UPLC BEH Amide column (2.1×50 mm, 1.7 µm) and
an Orbitrap Exploris 120 mass spectrometer (Thermo Fisher
Scientific, Inc.). The mobile phase consisted of two solvents: A
(25 mM ammonium acetate and 25 mM ammonia hydroxide in water, pH
9.75) and B (acetonitrile). The auto-sampler temperature was
maintained at 4°C, and the injection volume was 2 µl. The
Orbitrap Exploris 120 mass spectrometer was operated in
information-dependent acquisition (IDA) mode using Xcalibur
software (Thermo Fisher Scientific, Inc.). In this mode, the
acquisition software continuously evaluates the full scan MS
spectrum to select precursor ions for MS/MS fragmentation. The ESI
source conditions were set as follows: sheath gas flow rate at 50
Arb, auxiliary gas flow rate at 15 Arb, capillary temperature at
320°C, full MS resolution at 60,000, MS/MS resolution at 15,000,
collision energy at stepped normalized collision energy (NCE) of
20/30/40, and spray voltage at 3.8 kV (positive mode) or -3.4 kV
(negative mode).
The raw data were converted to the mzXML format
using ProteoWizard and processed with an in-house program developed
in R, utilizing XCMS for peak detection, extraction, alignment and
integration. Subsequently, an in-house MS2 database (https://www.tidymass.org/metid/articles/metabolite_annotation_using_MS2.html)
was employed for metabolite annotation. The annotation cutoff was
set at 0.3. The acquired MS data pretreatments including peak
picking, peak grouping, retention time correction, second peak
grouping, and annotation of isotopes and adducts was performed
using XCMS software. The online KEGG, HMDB database was used to
annotate the metabolites by matching the exact molecular mass data
(m/z) of samples with those from database. Statistical analysis was
performed in R (version 4.0.0) (17). Hypergeometric-based enrichment
analysis with KEGG Pathway was performed to annotate protein
sequences. The software Gene Set Enrichment Analysis (GSEA; v4.1.0)
and MSigDB (https://www.gsea-msigdb.org/gsea/index.jsp) were used
for gene set enrichment analysis to determine whether a set of
genes in a specific KEGG pathway in different situations. Meeting
this condition |NES|>1, NOM p-val<0.05, FDR q-val<0.25
were considered to be significantly different between the two
groups.
BMDCs culture and treatments
Bone marrow cells were isolated from mice and
cultured in RPMI-1640 medium supplemented with 10% FBS and 20 ng/ml
GM-CSF. On day 3, fresh medium with 20 ng/ml GM-CSF was added, and
on day 5, half of the medium was replaced with fresh medium
containing 20 ng/ml GM-CSF. BMDCs were harvested on day 6. All
cells were cultured at 37°C in a 5% CO2 atmosphere.
Before stimulation with IMQ (10 µg/ml) for 3 h, cells were
pre-treated with 10 µM LTB for 24 h, as previously reported
(13).
Enzyme linked immunosorbent assay (ELISA)
assay
The Mouse IL-23 ELISA kit (cat. no. BMS6017; Thermo
Fisher Scientific, Inc.) was used for detecting the level of IL-23
was performed according to the manufacturer's protocols. Briefly,
50 µl of cell supernatant was added to a plate coated with
the capture antibody and incubated at room temperature for 2 h.
Unbound antigens were washed away with TBST (0.05% Tween-20),
followed by the addition of HRP-conjugated detection antibody and a
further 1-h incubation at room temperature. Excess antibodies were
then removed with TBST. Next, 50 µl of TMB chromogenic
substrate was added and incubated for 15 min at room temperature.
The reaction was stopped by adding 25 µl of sulfuric acid,
and absorbance was measured at 450 nm.
Statistical analysis
All statistical analyses were conducted using
GraphPad Prism 8 software (Dotmatics) and are presented as the mean
± SEM. Unpaired Student's t-test was used for comparisons between
two groups, while two-way ANOVA followed by Tukey's honestly
significant difference (HSD) post hoc test was utilized for
comparisons involving more than two groups. P<0.05 was
considered to indicate a statistically significant difference.
Results
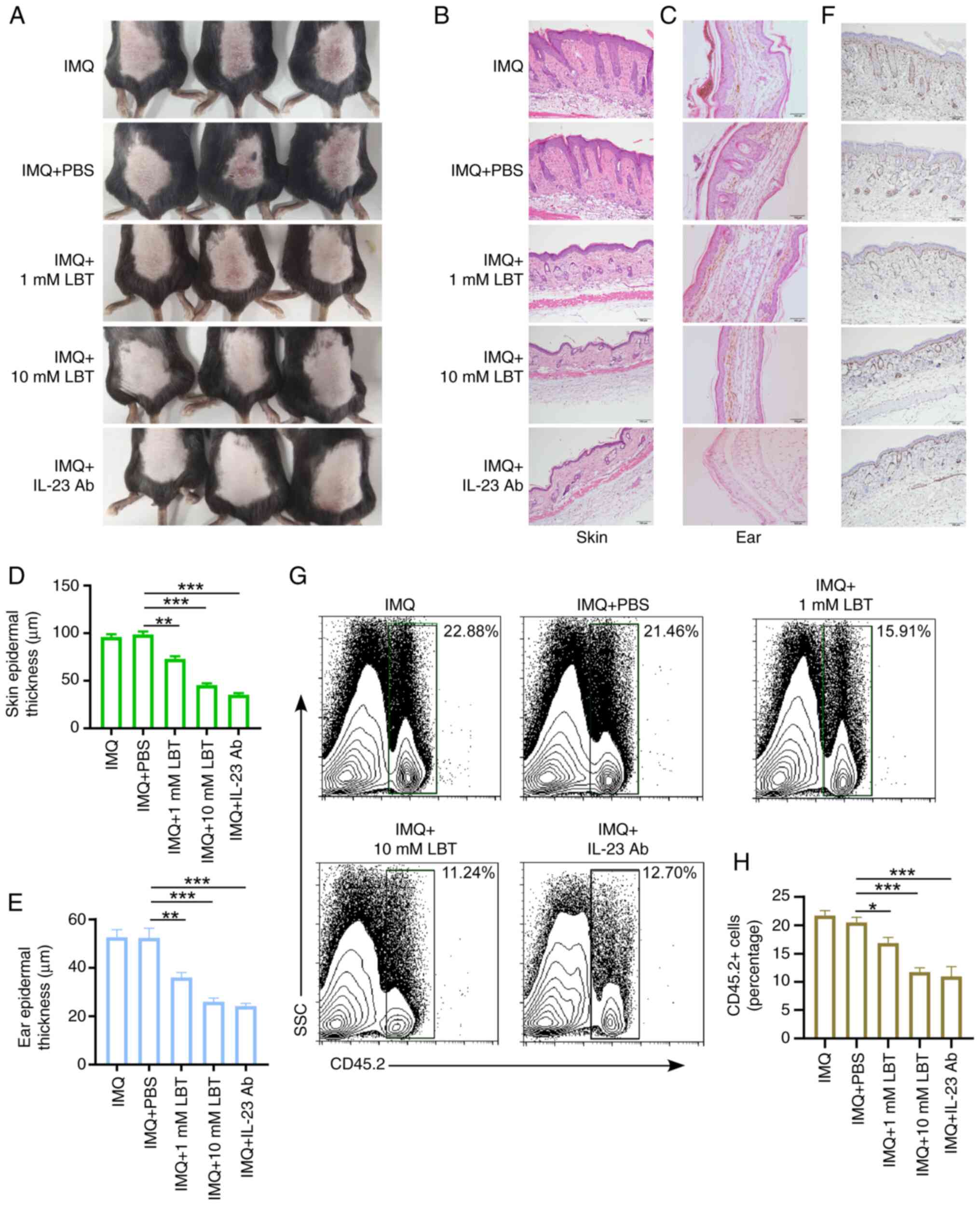
LBT attenuates IMQ-induced psoriasis-like
inflammation in mice
To assess LBT's effect on psoriasis-like skin
inflammation, the IMQ cream was used to induce the psoriasis-like
inflammation in mice. It was observed that IMQ significantly
induced skin inflammation, characterized by pronounced immune cell
infiltration, substantial keratinocyte proliferation, and
thickening of the epithelial layer (Figs. S1A-C and 1A-F). Additionally, 1 or 10 mM LBT was
applied to the dorsal skin and ears of mice treated with 5% IMQ
cream for 5 days, and it was found that LBT significantly
alleviated inflammatory symptoms (Fig. 1A). LBT-treated mice exhibited
less inflammation, epidermal acanthosis, severe swelling, and
keratinocyte proliferation compared with controls in back skin or
ear (Fig. 1B-E). The
proliferation marker Ki67 was also significantly reduced in the
back skin of IMQ-induced psoriasis mice treated with LBT (Fig. 1F). Notably, LBT-treated mice
showed less infiltration of CD45+ immune cells post-IMQ
treatment (Fig. 1G and H).
Anti-mouse IL-23 (p19) antibody was used to treated IMQ-induced
psoriasis-like inflammation. It was also observed LBT and the
anti-IL-23 monoclonal antibody exhibited comparable therapeutic
efficacy in suppressing psoriasis pathogenesis (Fig. 1A-H). These findings demonstrated
that LBT effectively reduces IMQ induced psoriasis-like
inflammation in mice.
LBT regulates the gene expression profile
of extracellular region, keratinization and integral component of
membrane in skin tissue of psoriasis mice
The gene expression profiles modulated by LBT were
investigated in skin tissues from IMQ-induced psoriasis mice using
RNA sequencing. Our analysis revealed that 978 genes were
significantly upregulated, while 678 genes showed notable
downregulation (Fig. 2A). GO
functional enrichment analysis of all DEGs identified the top 20
dysregulated pathways (Fig. 2B).
LBT notably regulated genes related to the extracellular region,
keratinization and integral membrane components (Fig. 3B). Specifically, LBT
significantly suppressed the expression of 113 genes associated
with the extracellular region, including Col12a1,
Krt6a, Lrrc25, Lyn and Ccl2, while it
markedly upregulated 136 genes, such as Card14, Mc5r
and Oxtr (Fig. 2C and D).
LBT supplementation significantly upregulated 30
keratinization-related genes, including Krt79, Lor,
Lce1i, Lce1f and Lce1a2, while downregulating
27 genes, such as Sprr1b, Sprr2g, Krt6a and
Krt16 (Fig. 2E and F).
Additionally, LBT altered the expression of 336 integral membrane
component genes, with 189 showing downregulation (Fig. 2G and H). Notably, the most
significant changes occurred in Slc7a11, Slc7a1,
Ano9 and Oxtr (Fig. 2G
and H). Given that hyperproliferation and abnormal
differentiation of keratinocytes are hallmark features of
psoriasis, it was confirmed that Lce1c and Lor
expression significantly increased, whereas Krt6a and
Krt16 were significantly downregulated following LBT
treatment in IMQ-induced inflammatory skin (Fig. 2I). Immunohistochemical analysis
also revealed decreased KRT6A levels in the ear and back skin of
IMQ-treated mice after LBT treatment (Fig. 2J). Overall, the results
demonstrated that LBT regulates gene expression profiles related to
the extracellular region, keratinization and integral membrane
components.
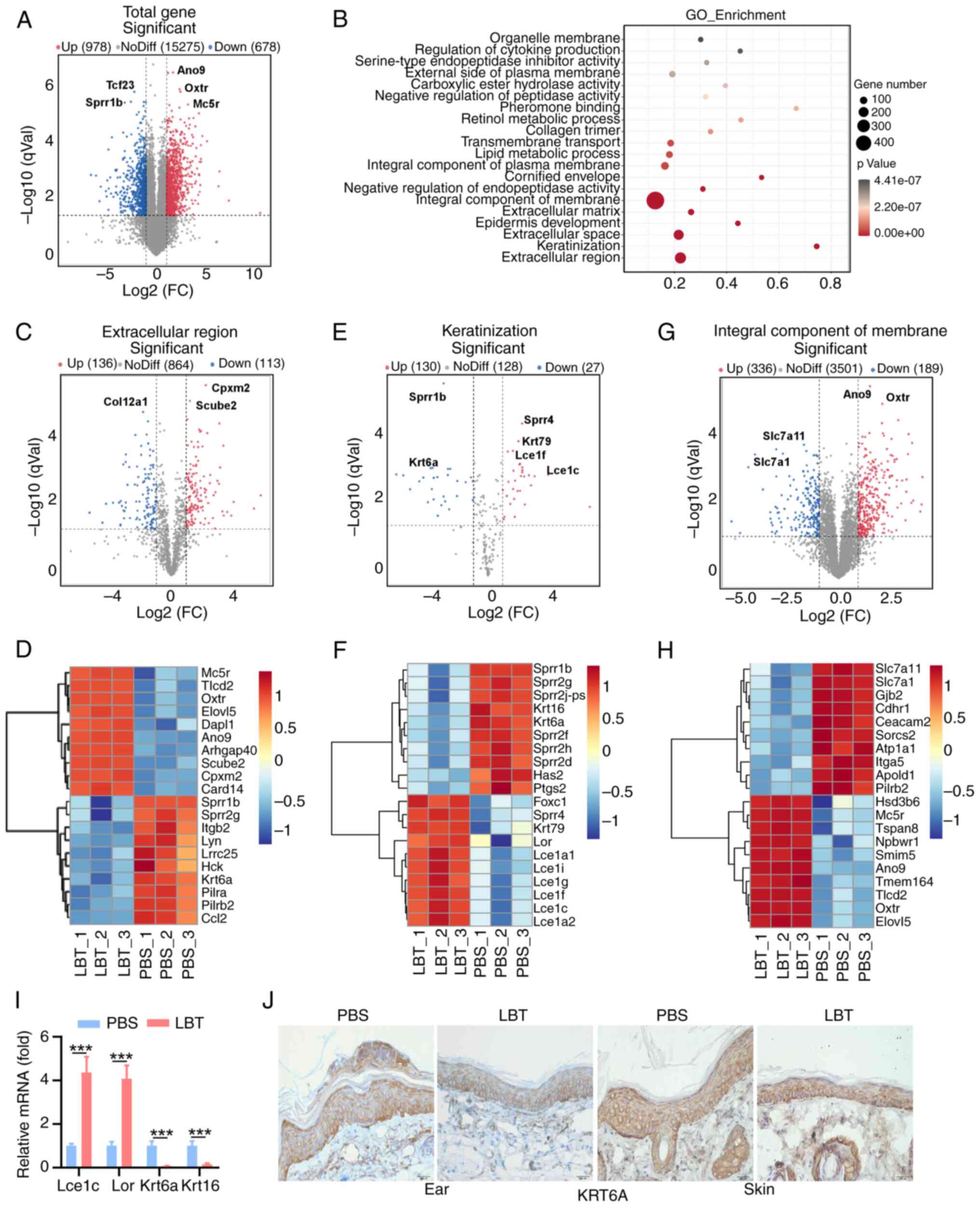 | Figure 2LBT regulates the gene expression
profile of extracellular region, keratinization and integral
component of membrane in skin tissue of psoriasis mice. The mRNAs
were extracted from the back skin tissue of IMQ-induced psoriatic
mice that had been treated with either LBT or PBS for a duration of
five days. Subsequently, these samples were subjected to RNA
sequencing. (A) Volcano plots showed all genes from the data
obtained by RNA sequencing. (B) Scatter plot showed the top 20 most
significantly changed GO terms. (C and D) Volcano plots identified
the extracellular region (B), and the heat map highlighted the top
20 significantly altered DEGs with FPKM values >0 (C). (E and F)
Volcano plots revealed (E) keratinization, and (F) the heat map
showcased the top 20 significantly altered DEGs with FPKM values
>0. (G and H) Volcano plots indicated the (G) integral component
of the membrane, and (H) the heat map displayed the top 20
significantly altered DEGs with FPKM values >0. (I) Reverse
transcription-quantitative PCR analysis detected Lce1c,
Lor, Krt6a, Krt16 mRNA level in the above back
skin tissue. (J) Immunohistochemical staining of KRT6a in ear or
back skin sections obtained from IMQ-induced psoriasis mice treated
with 10 mM LBT or PBS for 5 days. Scale bar, 200 µm. Data
are representative of three independent experiments. P-values were
determined by two-way ANOVA. ***P<0.001. LBT,
lobetyolin; IMQ, Imiquimod; DEGs, differentially expressed
genes. |
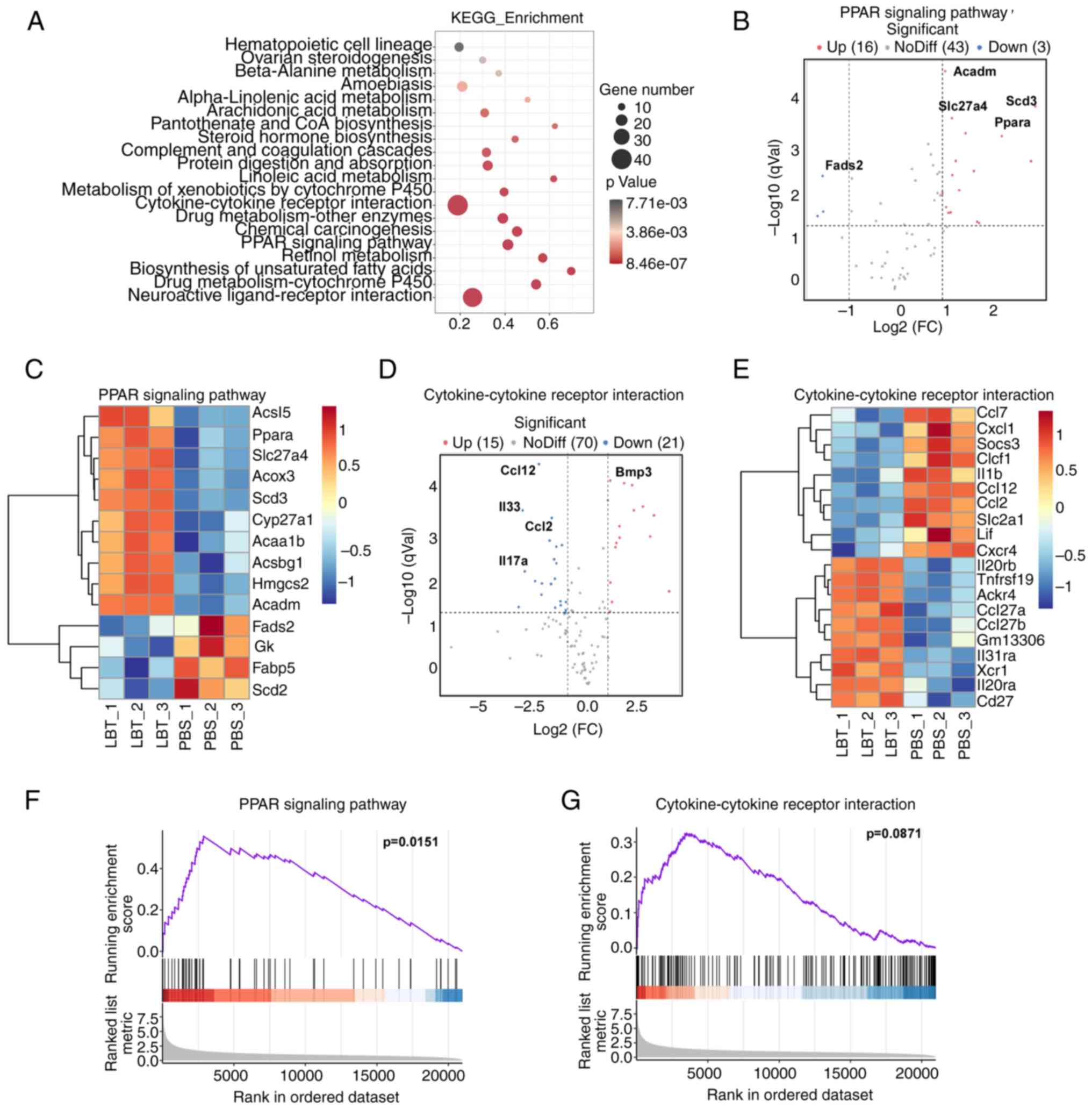
KEGG analysis indicates that LBT
regulates gene expression related to cytokine-cytokine receptor
interactions and the PPAR signaling pathway in skin tissue of
psoriasis mice
To investigate the effects of LBT on IMQ-induced
psoriasis in mice, KEGG pathway enrichment analysis was conducted,
revealing that LBT primarily influenced gene expression in
neuroactive ligand-receptor interactions, cytokine-cytokine
receptor interactions, and the PPAR signaling pathway, among others
(Fig. 3A). Specifically, LBT
upregulated 16 genes related to the PPAR signaling pathway,
including Acadm, Scd3, Slc27a4 and
Ppara, while downregulating 3 genes, notably Fads2
(Fig. 3B and C). LBT treatment
resulted in the upregulation of 15 genes and a significant
downregulation of 21 genes associated with cytokine-cytokine
receptor interactions, with Ccl12, Il33, Ccl2
and Ccl4 being the most notably reduced (Fig. 3D and E). To further explore the
mechanisms by which LBT influences psoriasis progression, GSEA
analysis was employed to predict KEGG downstream pathways. The
current findings indicated that LBT significantly activated the
PPAR signaling pathway, while no significant effects were observed
in the cytokine-cytokine receptor interaction pathway (Fig. 3F and G).
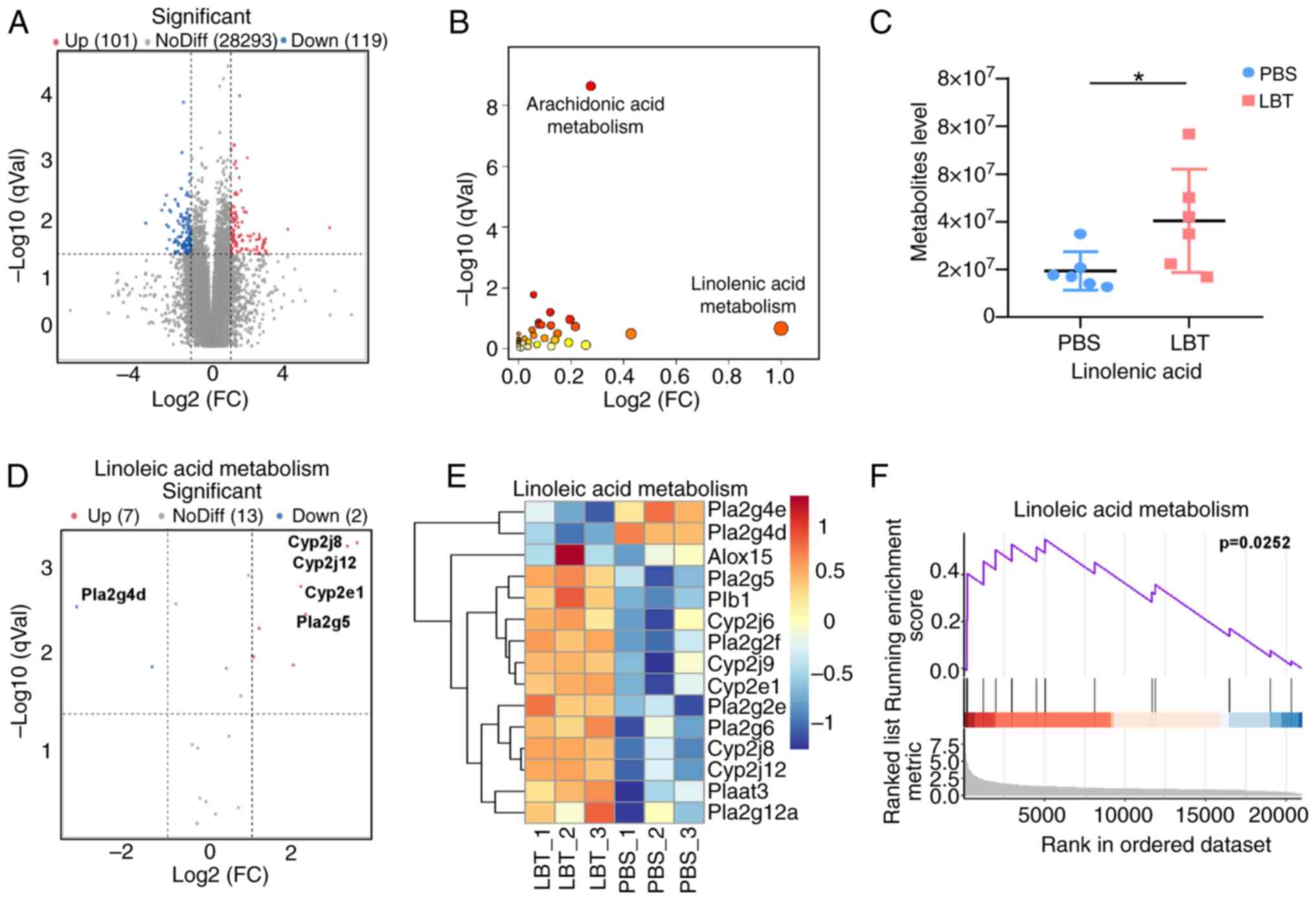
LBT could promote expression of the genes
and metabolites related to linoleic metabolism in skin tissue of
psoriasis mice
After using RNA-Seq to determine that LBT
significantly regulates metabolic gene expression, non-targeted
metabolomics were conducted to assess metabolite changes. A total
of 119 downregulated and 101 upregulated metabolites were
identified (Fig. 4A). KEGG
enrichment analysis indicated that LBT primarily affects
arachidonic acid and linoleic acid metabolism (Fig. 4B). Additionally, linoleic acid
levels were significantly increased in IMQ-induced psoriasis mice
following LBT treatment (Fig.
4C). RNA-Seq revealed that LBT upregulated seven genes,
including Cyp2j8, Cyp2j12 and Cyp2e1, while
downregulating two genes (Fig. 4D
and E). GSEA analysis showed that LBT significantly activated
linoleic acid metabolism (Fig.
4F). Thus, the present findings demonstrated that LBT enhances
linoleic acid metabolism by increasing the expression of associated
genes.
LBT could inhibit expression of the genes
related to cytokine activity, the IL-17, TNF and MAPK signaling
pathways in IMQ-treated DCs
DCs play a vital role in the initiation and
maintenance of psoriasis. The effects of LBT on peritoneal
macrophages were analyzed after LPS stimulation using RNA-seq. Our
results identified 201 DEGs in DCs treated with IMQ. Among these,
48 genes were markedly upregulated, while 153 were downregulated
(Fig. 5A). KEGG pathway analysis
indicated major alterations in the TNF signaling pathway,
cytokine-cytokine interaction and IL-17 signaling pathway (Fig. 5B). GO analysis revealed
considerable changes in cytokine-related genes in LBT-treated DCs
under IMQ stimulation (Fig. 5C).
Downregulation of14 genes linked to cytokine activity was observed,
including Il23a, Cxcl1, and Tnf (Fig. 5D). Key genes in the TNF signaling
pathway, such as Jun, Tnf and Cxcl1, were
significantly downregulated in LBT-treated DCs after IMQ
stimulation (Fig. 5E), alongside
a reduction in MAPK signaling pathway genes including Jun,
Tnf and Dusp8 (Fig.
5F). To confirm the downregulation of genes in the IL-17, TNF
and MAPK signaling pathways, RT-qPCR was performed, and it was
found that the mRNA levels of Il23, Tnf,
Cxcl2, Il12b, Dusp1, Gadd45a,
Nr4a1 and Jun were significantly reduced.
Additionally, an ELISA assay indicated decreased IL-23A production
in LBT-pre-treated DCs. These findings suggested that LBT
effectively modulates gene expression related to cytokine activity,
the IL-17, TNF and MAPK signaling pathways.
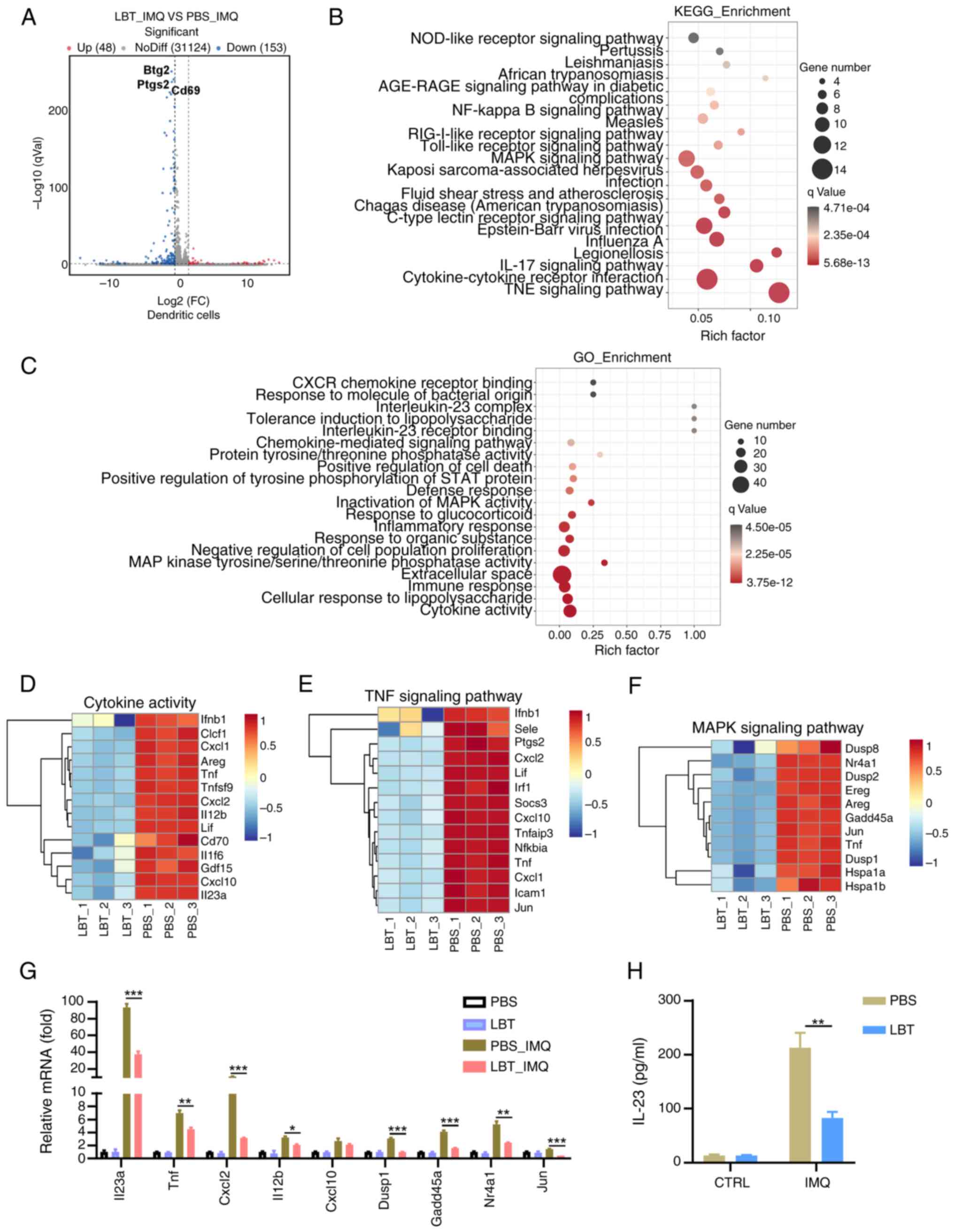 | Figure 5LBT could inhibit expression of the
genes related to cytokine activity, IL-17 signaling pathway, TNF
signaling pathway and MAPK signaling pathway in IMQ-treated DCs.
The mRNAs were extracted from DCs treated with either LBT or PBS
for 24 h, followed by stimulation with 10 µg/ml IMQ for 1 h,
after which the samples underwent RNA sequencing. (A) Volcano plots
showed all genes from the data obtained by RNA sequencing. (B and
C) Scatter plot revealed the top 20 most significantly changed (B)
KEGG and (C) GO terms. (D-F) Heat map displayed significantly
changed differentially expressed genes related to (D) cytokine
activity, (E) TNF signaling pathway and (F) MAPK signaling pathway.
(G) Reverse transcription-quantitative PCR analysis detected the
Il23, Tnf, Cxcl2, Il-12b, Cxcl10, Dusp1,
Gadd45a, Nr4a1 and Jun mRNA level in DCs treated with
either LBT or PBS for 24 h, then stimulated with 10 mg/ml IMQ for 1
h. (H) ELISA analysis measured IL-23 levels in DCs treated with
either LBT or PBS for 24 h, followed by 24-h stimulation with 10
µg/ml IMQ. Data are representative of three independent
experiments. P-values were determined by two-way ANOVA.
*P<0.05 **P<0.01 and
***P<0.001. LBT, lobetyolin; IMQ, Imiquimod; DCs,
dendritic cells; KEGG, Kyoto Encyclopedia of Genes and Genomes; GO,
Gene Ontology. |
Discussion
Psoriasis is a common chronic inflammatory skin
condition that significantly impacts individuals, healthcare
systems and society (18). As
the immunological mechanisms and pathogenesis of psoriasis are
better understood, treatment options have greatly improved.
Systemic agents targeting TNF-α, IL-17 and IL-23-such as
infliximab, secukinumab and Ustekinumab-have shown effective and
safe results (19,20). However, these treatments can
cause severe side effects and often lead to disease recurrence
after therapy ends. Therefore, it is crucial to develop strategies
to prevent psoriasis recurrence post-treatment. LBT, derived from
Codonopsis pilosula, has demonstrated cardioprotective,
anti-inflammatory, antioxidant and antitumor effects (21). It protects mice from LPS-induced
sepsis by downregulating pro-inflammatory cytokines such as TNF-α,
IL-6 and IL-1β in macrophages (13). Additionally, LBT exhibits
significant anticancer properties in gastric and breast cancer
cells by inhibiting cell proliferation and inducing apoptosis
through ASCT2 downregulation (22). It also shows notable
cardioprotective and anti-arrhythmic activities (23). In the present study, it was found
that LBT alleviates symptoms and regulates gene expression
associated with keratinization, cytokine-receptor interactions,
PPAR signaling and linoleic metabolism in mice with IMQ-induced
psoriasis-like inflammation.
Psoriatic lesions feature epidermal acanthosis,
hyperkeratosis and parakeratosis, resulting from increased
keratinocyte proliferation and abnormal terminal differentiation
(24). Keratins (KRTs), which
form the intermediate filament network in epithelial cells, are
essential for maintaining keratinocyte structural stability
(25). Research indicates three
KRTs associated with hyperproliferation in psoriatic epidermis: K6,
K16 and K17 (26). Key epidermal
barrier proteins, filaggrin and loricrin (LOR), show decreased
expression in both lesional and non-lesional skin of psoriasis
patients (27,28). The late cornified envelope (LCE)
genes, located in the epidermal differentiation complex on
chromosome1, encode18 largely function-unknown proteins, with the
deletion of LCE3B and LCE3C being a well-established psoriasis risk
factor linked to the major risk gene HLA-C*06 (29,30). All LCE groups (1,2,5, and 6) were
significantly downregulated in psoriatic skin or IMQ-induced
psoriasiform dermatitis mice (31). The current findings reveal
increased expression of Lce1c and Lor, while
Krt6a and Krt16 were notably downregulated after LBT
treatment. This suggests that LBT may regulate keratinocyte
proliferation and differentiation. Further investigation is needed
to explore LBT's role in keratinocytes.
Advancements in metabolomic and bioinformatic
analyses have highlighted metabolism as a key factor in psoriasis
pathogenesis. Various forms of cellular metabolism-such as
glycolysis, the tricarboxylic acid cycle, lipid metabolism and
amino acid metabolism-regulate keratinocytes and related immune
cells (32). Lipid metabolism,
particularly involving polyunsaturated fatty acids (PUFAs) like
α-linolenic acid (ALA) and linoleic acid (LA), is crucial for both
structural integrity and homeostasis in the stratum corneum, the
outermost epidermal layer composed of corneocytes within a lipid
matrix (33). LA is vital for
skin barrier function as it is incorporated into ω-hydroxylated
ceramides, which bind covalently to corneocytes and support lipid
matrix organization (34).
Dietary supplementation with ALA has been shown to reduce T cell
signaling activation, thereby decreasing inflammatory cytokine
secretion in psoriatic patients (35). Clinically, n-3 PUFAs are commonly
used to alleviate psoriasis symptoms (36). Plasma samples from 56 psoriatic
patients revealed significantly reduced total PUFAs, along with
lower levels of α-linolenic acid (C18:3n3) and linoleic acid
(C18:2n6) (37). This indicates
that dysregulation of LA metabolism is closely linked to psoriasis
onset. The present study demonstrated that LA levels and gene
expression related to LA metabolism significantly increased after
LBT treatment in the skin of IMQ-induced psoriasis mice, suggesting
that LBT may modulate LA metabolism in the skin. Further research
is necessary to explore LBT's effects on LA metabolism in
keratinocytes.
Communication between immune cells and keratinocytes
via cytokines and their receptors is crucial in psoriasis
pathogenesis. Immune cells primarily produce cytokines including
TNF-α, IFN-γ, IL-23/IL-17A and IL-22, which activate keratinocytes
and initiate various signaling pathways, leading to excessive
keratinocyte proliferation and the production of antimicrobial
proteins, cytokines, chemokines and growth factors (38). Notably, TNF-α, IL-17A and IL-23
are central to psoriasis, as therapies targeting these cytokines
are the most effective (39). In
the present study, LBT treatment significantly downregulated the
expression of Il17a, Il1b, Ccl12, Il33,
Ccl2 and Ccl4 in the skin of IMQ-induced psoriasis
mice. Additionally, a significant inhibition of Il23a,
Tnf and Cxcl2 gene expression was observed in DCs
following IMQ treatment. Thus, the current findings indicated that
LBT can modulate inflammatory cytokine production in DCs and
mitigate psoriasis progression.
In the present study, it was also identified that
LBT promotes the expression of genes associated with the PPAR
signaling pathway. PPARs, comprising three subtypes (PPARα, PPARβ/δ
and PPARγ), regulate genes involved in glucose and lipid
metabolism, inflammation and differentiation (40). They are crucial for maintaining
skin barrier permeability, inhibiting keratinocyte growth,
promoting terminal differentiation, and regulating skin
inflammation (41). For
instance, activating PPAR-γ enhances cell differentiation, reduces
proliferation, and modulates immune responses, alleviating
inflammation in TNF-α-induced fibroblast-like synoviocytes in
rheumatoid arthritis (42).
PPAR-γ may regulate abnormal lipid metabolism, inflammatory
cytokines and keratinocytes in psoriasis' pathogenesis (43), leading us to hypothesize that LBT
may suppress psoriasis development by upregulating PPAR.
Additionally, it was found that LBT significantly downregulated
genes related to the MAPK signaling pathway, which plays a key role
in psoriasis pathogenesis and regulating keratinocyte proliferation
and immune responses (44). P38
and ERK1/2 are activated in psoriatic epidermis, and p38 activation
leads to psoriasis-like dermatitis in mice, whereas ERK inhibition
reduces IMQ-induced psoriasiform lesions (44,45). JNK/c-Jun signaling is necessary
for the transcription of CCL2 and IL-23 in dendritic cells
(46). In the present study, it
was found that LBT could notably downregulate c-Jun expression,
inhibiting IL-23 and TNF-α production.
Supplementary Data
Availability of data and materials
The data generated in the present study may be found
in the Sequence Read Archive under accession number PRJNA1188620 or
at the following URL: https://www.ncbi.nlm.nih.gov/sra/?term=PRJNA1188620
and in the Open Archive for Miscellaneous Data under accession
number OMIX009890 or at the following URL: https://ngdc.cncb.ac.cn/omix/release/OMIX009890.
Authors' contributions
JP and JieZ conceived and designed the study. JH,
CF, YX and SC performed the experiments. JiaZ, JC, HC, JP and YS
analyzed the data. JH was a major contributor in writing the
manuscript. JiaZ and JieZ assume overall responsibility for the
manuscript. JieZ and JH confirm the authenticity of all the raw
data. JZ was responsible for supervision and funding acquisition.
All authors read and approved the final version of the
manuscript.
Ethics approval and consent to
participate
The present study was approved (approval no.
20220283) by the Ethics Committee of Zhejiang University School of
Medicine (Hangzhou, China).
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
Acknowledgments
The authors acknowledge the support from the
Immunology Institute of Zhejiang University and the Public
Technology Platform of Zhejiang University School of Medicine.
Funding
The present study was supported by the Natural Science
Foundation of Zhejiang (grant no. LY22H110003).
References
|
1
|
Sieminska I, Pieniawska M and Grzywa TM:
The immunology of psoriasis-current concepts in pathogenesis. Clin
Rev Allergy Immunol. 66:164–191. 2024. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Ahmad A, Akhtar J, Ahmad M, Islam A,
Badruddeen, Khan MI, Siddiqui S and Srivastava A: Curcumin nanogel
preparations: A promising alternative for psoriasis treatment. Curr
Drug Metab. 25:179–187. 2024. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Brunner SM, Ramspacher A, Rieser C,
Leitner J, Heil H, Ablinger M, Tevini J, Wimmer M, Koller A, Piñón
Hofbauer J, et al: Topical diacerein decreases skin and splenic
CD11c+ dendritic cells in psoriasis. Int J Mol Sci.
24:43242023. View Article : Google Scholar
|
|
4
|
Song Y, Zhao X, Qu H, Su Y, He R, Chen L,
Fang L, Li J, Zou Z, He J, et al: Epigenetic regulation of IL-23 by
E3 ligase FBXW7 in dendritic cells is critical for psoriasis-like
inflammation. J Immunol. 211:1701–1713. 2023. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Mohanakrishnan R, Beier S and Deodhar A:
IL-23 inhibition for the treatment of psoriatic arthritis. Expert
Opin Biol Ther. 22:59–65. 2022. View Article : Google Scholar
|
|
6
|
Fiechter RH, de Jong HM, van Mens LJJ,
Fluri IA, Tas SW, Baeten DLP, Yeremenko NG and van de Sande MGH:
IL-12p40/IL-23p40 blockade with ustekinumab decreases the synovial
inflammatory infiltrate through modulation of multiple signaling
pathways including MAPK-ERK and Wnt. Front Immunol. 12:6116562021.
View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Carmona-Rocha E and Puig L: The biological
basis of disease recurrence in psoriasis. Ital J Dermatol Venerol.
158:279–291. 2023.PubMed/NCBI
|
|
8
|
Bubna AK and Viplav V: Revisiting
risankizumab: A newer biologic drug in dermatology. Ital J Dermatol
Venerol. 159:543–554. 2024.PubMed/NCBI
|
|
9
|
Megna M, Lauletta G, Tommasino N, Salsano
A, Battista T, Ruggiero A, Martora F and Potestio L: Management of
psoriasis patients with serious infectious diseases. Adv Ther.
41:2099–2111. 2024. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Feng YJ, Wang XX, Zhuang PY, Zhang DY, Gao
L, Chen JM and Han G: Study on chemical constituents of Codonopsis
pilosula. Zhongguo Zhong Yao Za Zhi. 42:135–139. 2017.In Chinese.
PubMed/NCBI
|
|
11
|
Yue J, Xiao Y and Chen W: Insights into
genus codonopsis: From past achievements to future perspectives.
Crit Rev Anal Chem. 54:3345–3376. 2024. View Article : Google Scholar
|
|
12
|
Hu J, Wang D, Wang F and Lin P: Lobetyolin
suppresses the proliferation of hepatocellular carcinoma through
activating DUSP1-ERK1/2 signaling pathway. Biol Pharm Bull.
47:1751–1758. 2024. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Chen Z, Su Y, Ding J, He J, Lai L and Song
Y: Lobetyolin protects mice against LPS-induced sepsis by
downregulating the production of inflammatory cytokines in
macrophage. Front Pharmacol. 15:14051632024. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Wang J, Liu X, Wei W, Yang J, Li Q, Chu S,
Liu P, Zhang J and He W: Regulation of oxygen-glucose
deprivation/reperfusion-induced inflammatory responses and M1-M2
phenotype switch of BV2 microglia by lobetyolin. Metab Brain Dis.
38:2627–2644. 2023. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Yang W, He R, Qu H, Lian W, Xue Y, Wang T,
Lin W, Zhu P, Xia M, Lai L and Wang Q: FXYD3 enhances IL-17A
signaling to promote psoriasis by competitively binding TRAF3 in
keratinocytes. Cell Mol Immunol. 20:292–304. 2023. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(-Delta Delta C(T)) method. Methods. 25:402–408. 2001.
View Article : Google Scholar
|
|
17
|
Domingo-Almenara X and Siuzdak G:
Metabolomics data processing using XCMS. Methods Mol Biol.
2104:11–24. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Francis L, Capon F, Smith CH, Haniffa M
and Mahil SK: Inflammatory memory in psoriasis: From remission to
recurrence. J Allergy Clin Immunol. 154:42–50. 2024. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Katsiaunis A and Lipner SR: Devices for
treatment of nail psoriasis. Ital J Dermatol Venerol. 159:561–565.
2024.PubMed/NCBI
|
|
20
|
Mrowietz U, Lauffer F, Sondermann W,
Gerdes S and Sewerin P: Psoriasis as a systemic disease. Dtsch
Arztebl Int. 121:467–472. 2024.PubMed/NCBI
|
|
21
|
Hou YY, Qi SM, Leng J, Shen Q, Tang S,
Zhang JT, Hu JN, Jiang S and Li W: Lobetyolin, a Q-marker isolated
from radix platycodi, exerts protective effects on
cisplatin-induced cytotoxicity in HEK293 cells. J Nat Med.
77:721–734. 2023. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Bailly C: Anticancer properties of
lobetyolin, an essential component of radix codonopsis (Dangshen).
Nat Prod Bioprospect. 11:143–153. 2021. View Article : Google Scholar :
|
|
23
|
Ni SH, OuYang XL, Liu X, Lin JH, Li Y, Sun
SN, Deng JP, Han XW, Zhang XJ, Li H, et al: A molecular phenotypic
screen reveals that lobetyolin alleviates cardiac dysfunction in
5/6 nephrectomized mice by inhibiting osteopontin. Phytomedicine.
107:1544122022. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Kouris A, Platsidaki E, Kouskoukis C and
Christodoulou C: Psychological parameters of psoriasis.
Psychiatriki. 28:54–59. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Cohen E, Johnson CN, Wasikowski R, Billi
AC, Tsoi LC, Kahlenberg JM, Gudjonsson JE and Coulombe PA:
Significance of stress keratin expression in normal and diseased
epithelia. iScience. 27:1088052024. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Romashin DD, Tolstova TV, Varshaver AM,
Kozhin PM, Rusanov AL and Luzgina NG: Keratins 6, 16, and 17 in
health and disease: A summary of recent findings. Curr Issues Mol
Biol. 46:8627–8641. 2024. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Kircik L, Alexis AF, Andriessen A,
Blattner C, Glick BP, Lynde CW and Gold LS: Psoriasis and skin
barrier dysfunction: The role of gentle cleansers and moisturizers
in treating psoriasis. J Drugs Dermatol. 22:773–778. 2023.
View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Graubard R, Perez-Sanchez A and Katta R:
Stress and skin: An overview of mind body therapies as a treatment
strategy in dermatology. Dermatol Pract Concept. 11:e20210912021.
View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Niehues H, Tsoi LC, van der Krieken DA,
Jansen PAM, Oortveld MAW, Rodijk-Olthuis D, van Vlijmen IMJJ,
Hendriks WJAJ, Helder RW, Bouwstra JA, et al: Psoriasis-associated
late cornified envelope (LCE) proteins have antibacterial activity.
J Invest Dermatol. 137:2380–2388. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Coto E, Santos-Juanes J, Coto-Segura P,
Díaz M, Soto J, Queiro R and Alvarez V: Mutation analysis of the
LCE3B/LCE3C genes in psoriasis. BMC Med Genet. 11:452010.
View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Utsunomiya A, Chino T, Utsunomiya N, Luong
VH, Tokuriki A, Naganuma T, Arita M, Higashi K, Saito K, Suzuki N,
et al: Homeostatic function of dermokine in the skin barrier and
inflammation. J Invest Dermatol. 140:838–849.e9. 2020. View Article : Google Scholar
|
|
32
|
Solis ER and Jameson JM: Skin deep:
Epithelial cell metabolism and chronic skin inflammation. Immunity.
57:1451–1453. 2024. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Simard M, Morin S, Ridha Z and Pouliot R:
Current knowledge of the implication of lipid mediators in
psoriasis. Front Immunol. 13:9611072022. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Simard M, Tremblay A, Morin S, Martin C,
Julien P, Fradette J, Flamand N and Pouliot R: α-Linolenic acid and
linoleic acid modulate the lipidome and the skin barrier of a
tissue-engineered skin model. Acta Biomater. 140:261–274. 2022.
View Article : Google Scholar
|
|
35
|
Hu X, Que W, Hirano H, Wang Z, Nozawa N,
Ishii T, Ishizuka M, Ito H, Takahashi K, Nakajima M, et al:
5-Aminolevulinic acid/sodium ferrous citrate enhanced the antitumor
effects of programmed cell death-ligand 1 blockade by regulation of
exhausted T cell metabolism in a melanoma model. Cancer Sci.
112:2652–2663. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Kristensen S, Schmidt EB, Schlemmer A,
Rasmussen C, Johansen MB and Christensen JH: Beneficial effect of
n-3 polyunsaturated fatty acids on inflammation and analgesic use
in psoriatic arthritis: A randomized, double blind,
placebo-controlled trial. Scand J Rheumatol. 47:27–36. 2018.
View Article : Google Scholar
|
|
37
|
Łuczaj W, Gęgotek A and Skrzydlewska E:
Analytical approaches to assess metabolic changes in psoriasis. J
Pharm Biomed Anal. 205:1143592021. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Kamata M and Tada Y: Crosstalk:
Keratinocytes and immune cells in psoriasis. Front Immunol.
14:12863442023. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Kim H, Choi MR, Jeon SH, Jang Y and Yang
YD: Pathophysiological roles of ion channels in epidermal cells,
immune cells, and sensory neurons in psoriasis. Int J Mol Sci.
25:27562024. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Wagner N and Wagner KD: The role of PPARs
in disease. Cells. 9:23672020. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Wagner N and Wagner KD: Recent insights
into the role of PPARs in disease. Cells. 12:15722023. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Li XF, Yin SQ, Li H, Yang YL, Chen X, Song
B, Wu S, Wu YY, Wang H and Li J: PPAR-γ alleviates the inflammatory
response in TNF-α-induced fibroblast-like synoviocytes by binding
to p53 in rheumatoid arthritis. Acta Pharmacol Sin. 44:454–464.
2023. View Article : Google Scholar
|
|
43
|
Lin X, Meng X, Song Z and Lin J:
Peroxisome proliferator-activator receptor γ and psoriasis,
molecular and cellular biochemistry. Mol Cell Biochem.
477:1905–1920. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
An Y and Zhang Q, Ren Y, Yang S and Zhang
Q: BML-111 modulates and alleviates p38/MAPK signaling pathway and
Th1/Th2/Th17 cytokine response in murine psoriasis-like dermatitis.
Discov Med. 36:2026–2036. 2024. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Long Q, Ma T, Wang Y, Chen S, Tang S, Wang
T, Zhou Y, Xu K, Wan P and Cao Y: Orientin alleviates the
inflammatory response in psoriasis like dermatitis in BALB/c mice
by inhibiting the MAPK signaling pathway. Int Immunopharmacol.
134:1122612024. View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Novoszel P, Holcmann M, Stulnig G, De Sa
Fernandes C, Zyulina V, Borek I, Linder M, Bogusch A, Drobits B,
Bauer T, et al: Psoriatic skin inflammation is promoted by
c-Jun/AP-1-dependent CCL2 and IL-23 expression in dendritic cells.
EMBO Mol Med. 13:e124092021. View Article : Google Scholar : PubMed/NCBI
|