Diabetic wounds are a prevalent and severe
complication of diabetes mellitus, characterized by impaired
healing, increased infection risk and failure to achieve complete
closure (1,2). Among reported cases of diabetes
mellitus, ~25% are at risk of developing diabetic wounds (3). Diabetes disrupts wound healing,
potentially leading to chronic foot ulcers, lower extremity
amputation and increased mortality (4,5).
Moreover, the high treatment costs of diabetic wounds place an
economic burden on healthcare systems (6). The global prevalence of diabetes
was estimated at 529 million cases in 2021, with projections
indicating a potential surge to 1.31 billion by 2050 (7). With the increasing prevalence of
diabetes, the incidence of diabetic wounds is expected to rise,
posing a challenge to public health systems.
The difficulty in diabetic wound healing primarily
arises from a multifactorial combination of neuropathy,
vasculopathy and infection, which collectively exacerbate the
complexity and challenges of wound management (8). At the cellular level, a key factor
influencing wound healing is the efficient and rapid migration of
cells to the wound center (9).
This involves multiple cell types, including neutrophils,
macrophages, keratinocytes, fibroblasts and endothelial cells (ECs)
(10-14). The diabetic microenvironment
impairs the migratory capacity of cells via multiple mechanisms,
thereby contributing to delayed wound healing (15,16). Consequently, understanding of
cell migration regulatory mechanisms is key to formulate novel
therapeutic interventions for diabetic wounds.
The present study aimed to summarize cell migration
dysfunction in diabetic wounds and the mechanisms underlying cell
migration impairment induced by hyperglycemia, chronic
inflammation, oxidative stress and an abnormal microenvironment.
Based on key signaling pathways, non-coding RNAs (ncRNAs) and their
associated molecular networks that regulate cell migration in
diabetic wounds, the present study further explores innovative
therapeutic strategies to enhance cell migration, as well as
therapeutic challenges and future research directions, offering
innovative perspectives to advance diabetic wound clinical
management.
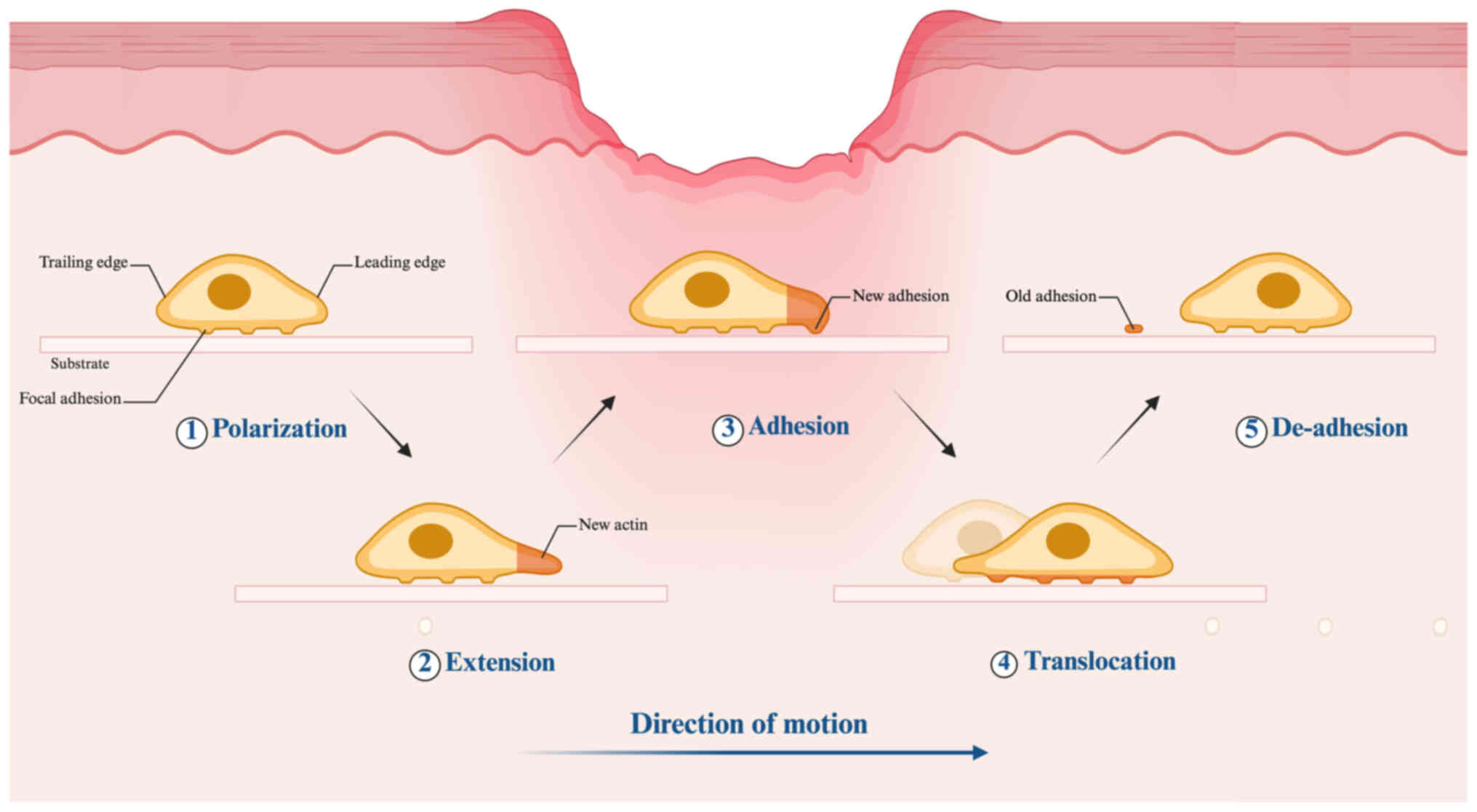
Cell migration is a synchronized and dynamic
phenomenon that generally commences with the polarization of cells
(Fig. 1) (17). Cell polarization occurs when
migrating cells detect chemotactic signals such as chemokines,
growth factors or extracellular matrix (ECM) cues. This process
involves coordinated activity of key molecular players, including
Rho GTPases, integrins, phosphoinositide 3-kinase (PI3K),
microtubules and vesicular transport systems, to establish
front-rear polarity and generate distinct functional patterns
between the leading and trailing edges (18). Actin polymerization at the
leading edge drives membrane protrusion, forming pseudopodial
structures such as lamellipodia and filopodia, facilitating cell
extension. The extended pseudopodia adhere to the ECM through
adhesion molecules such as integrins, forming focal adhesions that
mechanically link the cytoskeleton to the extracellular environment
(19). Through myosin-mediated
contractile forces, the cell body is propelled forward, driving
translocation. Disassembly of adhesion complexes at the trailing
edge permits tail detachment from the substrate, finalizing the
de-adhesion process (20). The
coordinated actions of the anterior and posterior regions complete
one cycle of migration.
Cell migration is a critical and continuous process
in wound healing, integral to every phase of repair. Various cell
types facilitate tissue regeneration, notably the migration of
neutrophils, macrophages, keratinocytes, vascular ECs and
fibroblasts. Through precise migration and functional modulation,
these cells contribute to pathogen clearance, angiogenesis
promotion, ECM secretion and epidermal barrier reconstruction.
Their migration is regulated by signaling molecules and
microenvironmental factors to ensure efficient repair (21,22). Investigating these migration
mechanisms not only enhances understanding of the physiological
principles governing wound healing but also offers a solid
theoretical framework for potential therapeutic strategies for
managing chronic wounds and impaired repair.
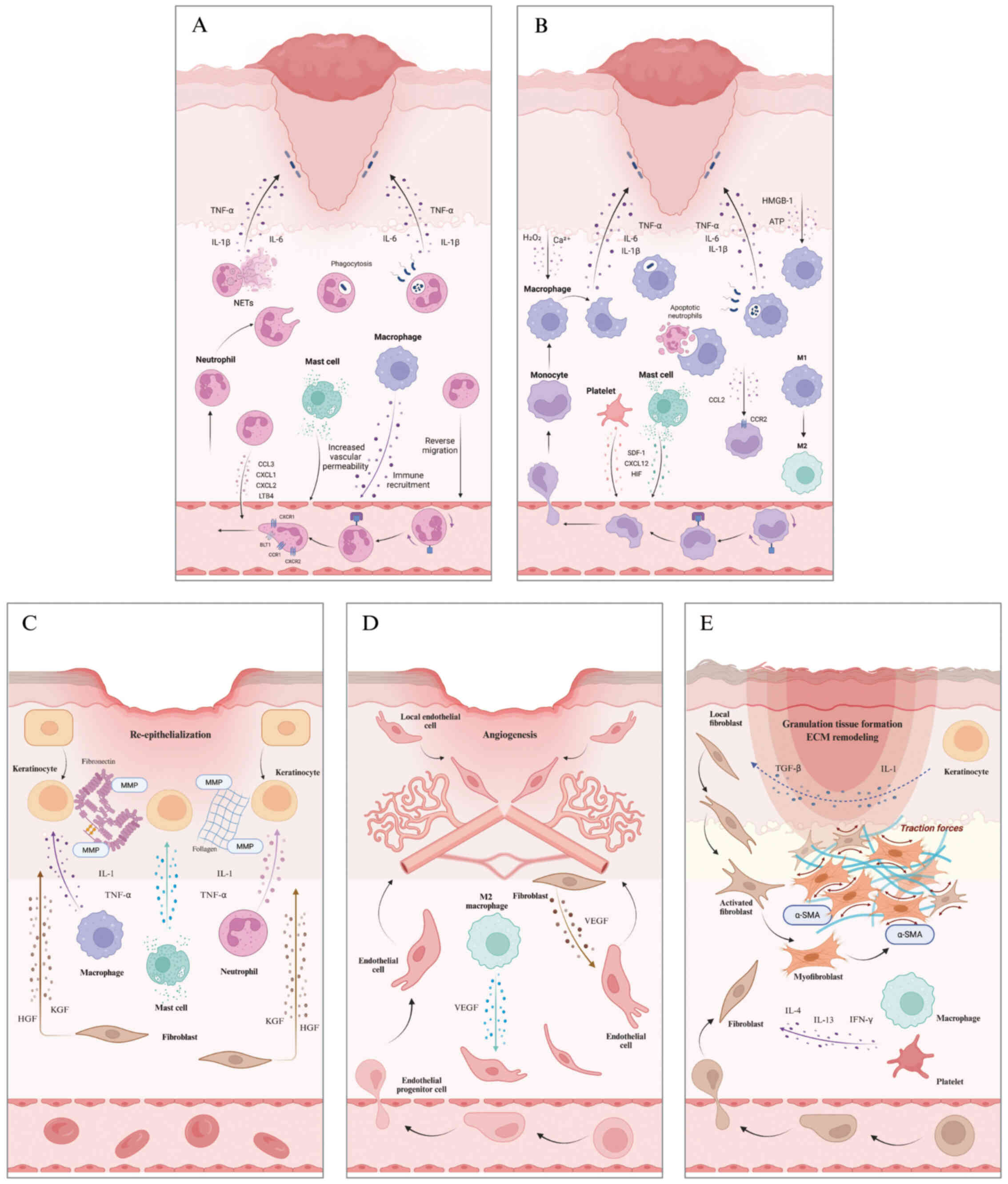
Neutrophils are the initial immune responders during
wound healing, rapidly mobilizing to injury sites via intricate
migratory mechanisms (Fig. 2A).
Neutrophils detect 'find-me' signals, including damage-associated
molecular patterns (DAMPs), hydrogen peroxide, lipid mediators and
chemokines, via surface receptors such as G protein-coupled
receptors (GPCRs), integrins, Fc receptors and pattern recognition
receptors. These signals, released from injured tissues, drive
neutrophil directed migration to the site of damage and trigger
inflammatory responses (23,24). The migration process is
coordinated by ~30 distinct neutrophil receptors and multiple
signaling pathways. Neutrophils recognize fMet-Leu-Phe (fMLP)
released by damaged cells and bacteria through specific formyl
peptide receptors (25). Mast
cells augment vascular permeability and facilitate neutrophil
infiltration via the release of histamine, chemokines and
inflammatory mediators. Macrophages identify DAMPs or
pathogen-associated molecular patterns, leading to their activation
and guidance of neutrophils to the site of injury (26). Local cells and neutrophils
release chemokines, including C-C motif ligand 3 (CCL3) and C-X-C
motif ligands 1/2 (CXCL1/2). These chemokines interact with their
receptors (CCR1, CXCR1, and CXCR2) to guide neutrophil migration
(21). Furthermore, neutrophils
produce leukotriene B4, which binds leukotriene B4 receptor 1 to
drive neutrophil recruitment from distant tissue (27). During migration, neutrophils
utilize adhesion molecules (such as CD11b) and downstream signaling
pathways (such as Src and Rho GTPases) to drive actin remodeling
and membrane extension, enabling transendothelial migration and
deep tissue infiltration through ECM degradation (23,28). At the wound site, neutrophils
perform essential antimicrobial functions through various
mechanisms, including the secretion of toxic granules, production
of reactive oxygen species, phagocytosis of invading pathogens and
the formation of neutrophil extracellular traps (29,30). Additionally, they secrete
proteases to remodel the ECM, recruit immune cells and facilitate
tissue repair. While these functions are essential for combating
infections, they can also lead to bystander effects, causing tissue
damage, particularly in chronic inflammatory conditions (31,32). Upon completion of their task,
neutrophils are cleared by macrophages or re-enter the vasculature
via reverse migration, thereby contributing to the resolution of
inflammation (33). Excessive
neutrophil retention or migration defects may sustain inflammatory
responses and hinder healing processes, contributing to chronic
wound pathogenesis (34). The
CXCL12-CXCR4 axis serves a pivotal role in neutrophil retention at
inflammatory sites. Inhibition of this pathway may enhance
inflammation resolution by inducing neutrophil reverse migration
(35).
Following tissue injury, a coordinated chemokine
cascade drives monocyte and macrophage trafficking to the wound
site (Fig. 2B). Damaged cells
release calcium waves that activate NADPH oxidase, producing
hydrogen peroxide, which, together with calcium, serves as an early
signal to mobilize immune cells to the injury site (36-38). Additionally, DAMPs such as
high-mobility group box 1 and ATP, along with inflammatory
cytokines such as IL-1 and IL-33, activate resident macrophages,
prompting the release of pro-inflammatory factors that establish a
localized inflammatory environment (39). During the initial injury phase,
monocytes expressing CCR2 migrate in response to CCL2 signaling.
These monocytes simultaneously express and respond to CCL7,
promoting the recruitment of myeloid cells (monocytes and
macrophages) to the injury site (21). Furthermore, the degranulation of
platelets and mast cells releases chemokines such as stromal
cell-derived factor 1/CXCL12, while hypoxia-inducible factors
(HIFs) amplify chemotactic signals, promoting the recruitment of
monocytes (23). The clotting
mechanism initiated by blood vessel damage further liberates
compounds such as unbound heme, exacerbating the inflammatory
reaction and drawing monocytes to the site of injury (40). Upon reaching the wound site,
monocytes develop into macrophages and serve essential roles
throughout the healing process. Initially, M1-polarized macrophages
dominate, displaying potent antimicrobial and inflammatory
properties (41). As healing
progresses, macrophages transition to the M2 phenotype,
facilitating inflammation resolution and angiogenesis while
orchestrating collagen deposition and ECM remodeling to promote
tissue regeneration and functional recovery (42,43).
Keratinocyte movement is key for successful wound
closure, facilitating re-epithelialization and barrier function
recovery (Fig. 2C). Following
tissue injury, edge-located keratinocytes respond to inflammatory
mediators (IL-1, TNF-α) by adopting a flattened morphology,
cellular elongation and forming membrane protrusions such as
lamellipodia and filopodia (22). These changes are driven by
cytoskeletal reorganization, which provides the structural support
and mechanical force required for migration. Fibroblasts promote
keratinocyte proliferation, migration and differentiation by
secreting growth factors such as keratinocyte and hepatocyte growth
factors, thereby driving the re-epithelialization of wounds
(44). During motility,
keratinocytes engage with the ECM via integrin receptors such as
αvβ5 and α5β1 while releasing MMPs to remodel temporary matrix
proteins (fibrin, fibronectin), thereby promoting cellular
migration (45-48). Dynamic regulation of cell-cell
and cell-ECM connections, along with increased gap junction
communication, ensures coordinated and efficient migration
(49). Keratinocytes employ
multiple mechanisms for migration, including the 'leapfrog' and
'sliding' models and suprabasal cell dedifferentiation in
collaboration with basal cells (22). The leapfrog mechanism proposes
that suprabasal cells roll over the leading edge basal cells,
undergo dedifferentiation, and subsequently form new migratory
leaders within the cohesive epidermal tongue (50). In the sliding mechanism,
keratinocytes from the basal layer move forward as a cohesive block
at the leading edge, while the overlying cluster of superficial
cells is passively dragged along (51). Suprabasal cell dedifferentiation
refers to the reversal of differentiation in committed suprabasal
keratinocytes, which regain migratory and proliferative capacity to
directly contribute to epidermal regeneration. During migration,
keratinocytes proliferate and differentiate to form new epithelium,
reestablishing the skin barrier. They also regulate local
inflammation, promote ECM remodeling and coordinate the activity of
neighboring cells through autocrine and paracrine signaling
(52). Additionally,
keratinocytes adapt their migratory behavior and functional
characteristics to the dynamic wound microenvironment, providing
key flexibility and support for effective wound repair.
New blood vessel formation (angiogenesis) peaks in
the proliferative stage of repair, delivering oxygen and nutrients
critical for tissue regeneration and functional restoration
(53). EC movement is key for
vascular restructuring and necessary for new blood vessel
formation, primarily mediated by chemotactic, haptotactic and
mechanotactic cues (Fig. 2D)
(54). Chemotaxis in
angiogenesis involves the directional migration of ECs along
chemical gradients of attractants such as VEGF and basic fibroblast
growth factor. This migration is initiated when VEGF binds to VEGF
receptor (R)-2, activating downstream effectors such as PI3K/Akt
and Rho GTPases to remodel the cytoskeleton. VEGFR-2 serves as the
primary regulator of this process (55). Through paracrine signaling, VEGF
is secreted by repair-associated macrophages, keratinocytes and
fibroblasts, driving EC expansion and motility to enhance blood
vessel formation (56,57). Haptotaxis refers to the
directional movement of ECs during angiogenesis, driven by the
interaction of integrins (such as αvβ3 and αvβ5) with ECM
components along a ligand gradient (58). Integrin activation triggers
downstream signaling pathways, including Ras-related C3 botulinum
toxin substrate (Rac) and cell division cycle 42 (Cdc42), which
regulate cytoskeletal remodeling and mechanical force generation to
propel cell movement (59).
Additionally, integrins synergize with growth factor signaling
pathways to enhance migratory efficiency. Mechanotaxis is the
process by which ECs undergo directed migration in response to
mechanical forces, such as shear stress, mediated by integrin
activation and cytoskeletal remodeling (60).
As key mediators of tissue regeneration, fibroblasts
generate and remodel ECM proteins, including collagen, elastin,
fibronectin and laminin, to support structural integrity (61). Chemokines and cytokines secreted
by platelets and inflammatory cells guide fibroblasts to the injury
site during early wound healing (62,63). Keratinocytes support this process
by releasing IL-1 and transforming growth factor (TGF)-β, which
enhance fibroblast migration and activation (Fig. 2E) (44). Fibroblasts recruited to the wound
site synthesize and secrete ECM components, such as collagen,
promoting granulation tissue formation. Under mechanical tension
and cytokine stimulation (TGF-β), these fibroblasts differentiate
into myofibroblasts. This transition is marked by α-smooth muscle
actin expression, which forms stress fibers that generate
contractile forces to draw the wound edges together, aiding in
closure (64). Additionally, a
small population of bone marrow-derived circulating fibroblasts
migrates to the wound bed in response to cytokines such as IL-4,
IL-13 and IFN-γ and differentiates into myofibroblasts (65); however, their contribution to
skin wound healing is relatively minor (66). Persistent fibroblast
overproliferation and hyperactivation drive excessive ECM
deposition, primarily collagen, promoting hypertrophic scar or
keloid formation (67,68). In the final remodeling phase,
fibroblasts deposit type I collagen to replace type III collagen,
reinforcing the ECM and forming a mature, mechanically stable scar.
This process may persist for months to years (69).
In wound healing, although different cell types
exhibit distinct migration characteristics, their movement is
regulated by signaling molecules such as chemokines and cytokines,
as well as microenvironmental factors including hypoxia, mechanical
forces and ECM composition (21,23,44,56). These cells sense external cues
via surface receptors, facilitate migration through cytoskeletal
reorganization and dynamic adhesion molecule interactions and
maintain coordinated motility via cell-cell and cell-matrix
communications (23,28). Collectively, these mechanisms
establish an efficient and orderly repair network that drives the
wound healing process.
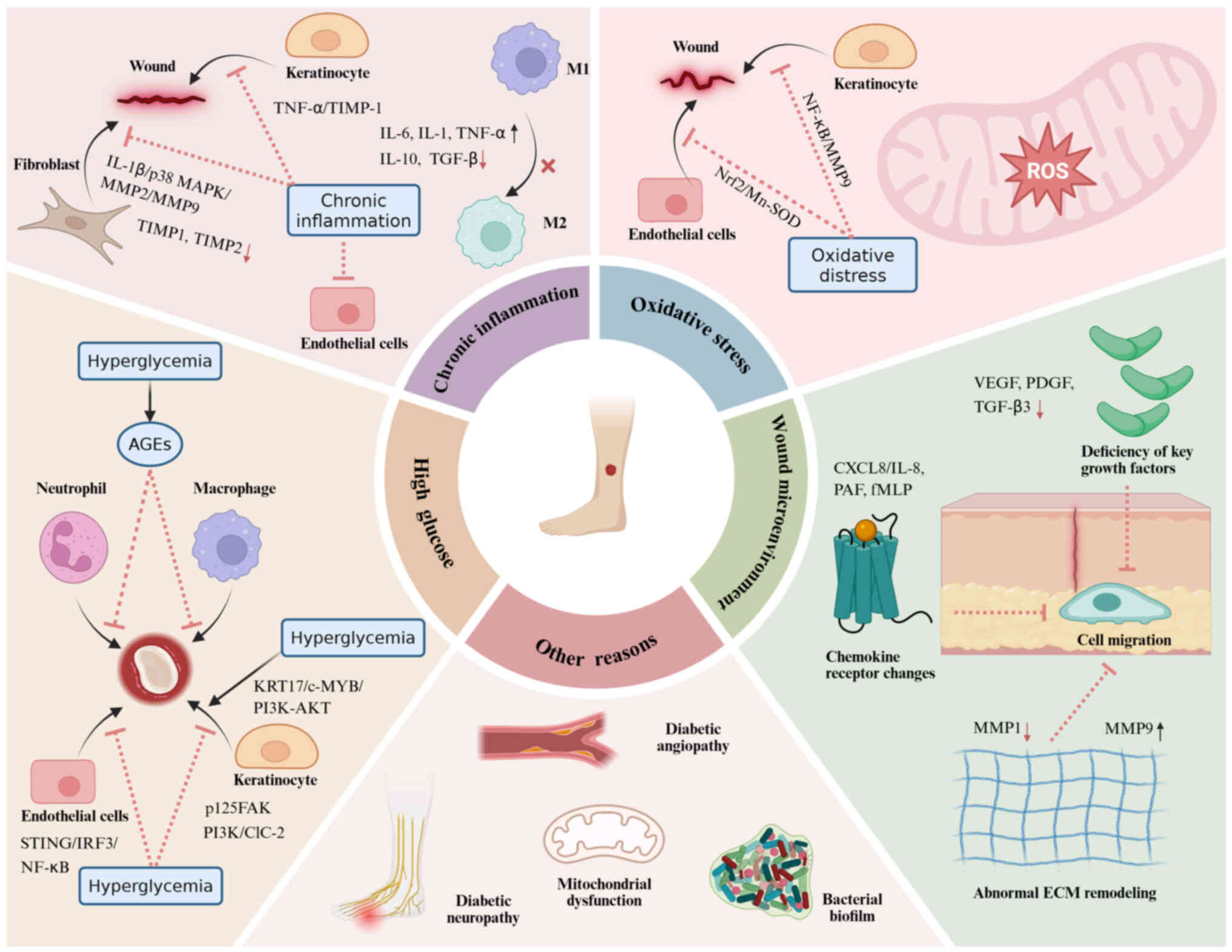
Diabetic patients exhibit impairment in cell
migration function during wound repair (70,71). This impairment may be linked to
the interplay of multiple factors, including hyperglycemia, chronic
inflammatory responses, oxidative stress and an aberrant wound
microenvironment. These factors influence cell migration via
complex biological pathways, ultimately leading to the obstruction
of wound healing and potentially resulting in prolonged refractory
states (Fig. 3).
High glucose may impair cell migration by promoting
the formation of unstable protrusions, decreasing adhesion
maturation, altering RhoA activity to disrupt migration regulation
and enhancing glucose uptake and metabolism to activate the mTOR
pathway (72). These mechanisms
are implicated in various cell types such as neutrophils,
macrophages, keratinocytes and endothelial cells. Chronic elevation
of blood glucose levels results in the buildup of advanced
glycation end products (AGEs) within tissue. AGE-modified proteins
disrupt chemotactic signaling, impairing the migration of
neutrophils to wound sites (73). Under chronic hyperglycemia,
monocyte motility is compromised, resulting in inadequate
macrophage infiltration, decreased phagocytic capacity and
dysregulated polarization from M1 to M2 phenotypes (74). Inadequate migration of
neutrophils and macrophages to the wound site increases the risk of
wound infection (75).
Additionally, high glucose upregulates STING expression and
activates the interferon regulatory factor 3 and NF-κB signaling
pathway, thereby inhibiting EC migration and delaying the healing
of diabetic wounds (70). Under
physiological conditions, keratinocyte proliferation and migration
are key for re-epithelialization during cutaneous wound repair.
Phosphorylated focal adhesion kinase (p125FAK) is a key regulator
of keratinocyte migration. However, hyperglycemia attenuates
p125FAK phosphorylation, compromising keratinocyte migration in
diabetic wound healing. (76).
High glucose also inhibits the PI3K signaling pathway, impairing
the function of ClC-2 chloride channels and suppressing the
migratory capacity of keratinocytes. Conversely,
hyperglycemia-induced upregulation of keratin 17 activates
c-MYB/PI3K/AKT signaling, promoting excessive keratinocyte
proliferation and migration. This dysregulation contributes to
hyperkeratosis and impairs wound healing (77). Thus, high glucose conditions
exert a dual effect, both inhibiting and promoting cell migration
through distinct mechanisms, leading to delayed diabetic wound
healing.
Chronic inflammation is a key pathological mechanism
underlying diabetic wound healing disorder, exerting its effects on
cell migration and delaying the wound repair process through a
variety of mechanisms (78-80). Hyperglycemia impedes macrophage
polarization from the M1 to M2 phenotype, leading to persistent
secretion of pro-inflammatory mediators including IL-6, IL-1 and
TNF-α, alongside decreased production of anti-inflammatory factors
such as IL-10 and TGF-β (57,80). This persistent pro-inflammatory
microenvironment markedly impairs the motility of keratinocytes,
fibroblasts and vascular ECs, extending the inflammatory phase and
impairing wound repair (81-83). Overproduction of TNF-α from
M1-polarized macrophages elevates tissue inhibitor of
metalloproteinases-1 (TIMP-1) expression in keratinocytes,
suppressing motility and ultimately impairing diabetic wound repair
(71). IL-1β is a key
contributor to maintaining a pro-inflammatory state. It activates
the p38 MAPK pathway to upregulate MMP2 and MMP9 expression while
downregulating TIMP1 and TIMP2, thereby altering the levels of ECM
remodeling proteins. This suppresses proliferation and motility of
dermal fibroblasts, thereby delaying wound repair in diabetic
individuals (84).
Oxidative stress reflects a disrupted equilibrium
where oxidation predominates over antioxidant defenses. Tissue in
diabetic hyperglycemic wound microenvironments exhibits heightened
vulnerability to this oxidative imbalance (85). Reactive oxygen species (ROS)
serve a dual role in wound healing: Moderate levels promote tissue
repair, while excess accumulation impairs wound closure and delays
regenerative processes (86).
High concentrations of ROS inhibit cell migration by oxidizing
related proteins such as actin and myosin II, disrupting the
structure and function of the cytoskeleton and impairing cell
contractility (87). High
glucose exacerbates oxidative stress, resulting in excessive Rac1
activation. This overactivation promotes the formation of unstable
protrusions, disrupts cell polarity and impairs adhesion
maturation, collectively decreasing cell migration speed and
directionality, ultimately contributing to defective wound healing
(72). A study has shown that
hyperglycemia exacerbates oxidative stress, impairing the migratory
and proliferative capacity of keratinocytes, thereby impairing
wound repair in diabetic conditions (88). Moreover, oxidative stress
decreases nuclear Nrf2 levels and manganese-superoxide dismutase
(Mn-SOD) expression, thereby weakening the cellular antioxidant
defense system, exacerbating ROS accumulation, impairing EC
proliferation and migration and ultimately delaying tissue
regeneration (89). MMP9 impedes
diabetic wound repair (90,91). In human keratinocytes, ROS
activate NF-κB, upregulating MMP-9 and suppressing keratinocyte
migration, thereby delaying wound closure (92,93).
Alterations in the wound microenvironment, such as
deficiencies in key growth factors, changes in chemokine receptors
and abnormal ECM remodeling, disrupt cell migration and impede
wound healing. Diminished growth factor secretion in diabetic
wounds disrupts cellular migration. During early wound repair,
platelet-derived growth factor (PDGF) recruits fibroblasts,
neutrophils and monocytes to the injury site (94). However, in diabetic wounds, PDGF
and its receptor expression are downregulated, compromising cell
migration and delaying wound closure (95). Similarly, hyperglycemia
suppresses VEGF secretion by macrophages, fibroblasts and
keratinocytes, impairing EC and keratinocyte migration, thereby
hindering vascularization and re-epithelialization and delaying
wound healing (94,96). TGF-β3 has been shown to
facilitate the migration of fibroblasts and keratinocytes;
considering the marked downregulation of TGF-β3 in diabetic wounds,
restoring its activity locally may represent a viable approach to
improving wound regeneration in diabetic patients (97). The alteration of chemokine
receptors is also a key factor in impaired cell migration in
diabetic wounds. Compared with healthy individuals, neutrophils
from diabetic patients exhibit a substantial decrease in chemotaxis
towards the chemokines CXCL8/IL-8, platelet-activating factor and
fMLP (98). This attenuated
chemotactic response may hinder cellular migration to the wound
site, disrupting healing progression. Moreover, in chronic diabetic
wounds, an imbalance in the regulation of MMPs disrupts ECM
remodeling, impairing cell migration and delaying tissue repair
(99,100). Normal function of MMP1 in
keratinocytes is key for their migration on type I collagen
(101). Hyperglycemia may
impair keratinocyte migration via inhibition of the p-Stat-1
pathway and α2β1 integrin-dependent MMP1 activation, contributing
to delayed diabetic wound healing (102). Hyperglycemia upregulates FOXO1,
increasing MMP-9 while decreasing TGF-β1, thereby disrupting ECM
homeostasis, impairing keratinocyte migration and delaying diabetic
wound healing (100,103,104).
Complications of diabetes, including vasculopathy
and neuropathy, are key pathological factors that impair wound
healing by affecting cellular migration. Vascular disease in
diabetic patients contributes to the delayed migration of white
blood cells to injury sites (105). Diabetic neuropathy may disrupt
keratinocyte and immune cell migration by altering neuropeptide
release (substance P and calcitonin gene-related peptide), thereby
impairing wound healing (106,107). Bacterial biofilms are highly
structured, surface-associated microbial aggregates encased in a
self-secreted extracellular polymeric substance, which provides
mechanical stability and protects against environmental stresses
(108). Diabetic wounds exhibit
heightened susceptibility to infection and biofilm formation due to
hyperglycemia-induced immunosuppression. The presence of these
bacteria and their associated biofilms hinders cellular migration
and disrupts the normal wound healing process (18,109). In diabetic wounds, aberrant
mechanical signals may contribute to impaired cell migration
function. Beyond its structural scaffolding role, the ECM also
serves as a platform for initiating and integrating
mechanotransduction signals. Under diabetic conditions, fibroblasts
secrete a thicker and less porous ECM, hindering the migration of
normal fibroblasts. Diabetic fibroblasts exhibit increased cellular
stiffness yet generate markedly reduced traction and contractile
forces within collagen matrices (110). These pathological changes may
impair cell migration, disrupting wound contraction and delaying
healing. Yes-associated protein (YAP) and transcriptional
co-activator with PDZ-binding motif (TAZ) are the central effectors
of the Hippo pathway and serve as the primary nuclear sensors for a
variety of extracellular and intrinsic mechanical signals (111,112). Downregulation of Agrin (a key
component of the ECM) in diabetic wounds may decrease MMP12
expression by inhibiting the nuclear localization of YAP/TAZ and
its positive feedback regulation in keratinocytes (113). This weakens the cell ability to
respond to mechanical stress, impairs their migration efficiency
and ultimately delays wound healing. Mitochondria serve a crucial
role in cell migration by providing ATP and maintaining calcium
homeostasis (114).
Mitochondrial dysfunction in diabetic wounds may be a key factor
impairing cell migration. Sirtuin 3 (SIRT3), a key mitochondrial
deacetylase, regulates energy metabolism and oxidative stress
(115,116). SIRT3 deficiency in diabetic
wounds disrupts mitochondrial structure and function, leading to
oxidative stress, necroptosis, impaired migration of skin
fibroblasts and delayed wound healing (117).
The impaired wound healing in diabetes arises from
multifaceted interactions of various factors that compromise
cellular migratory function. Research has predominantly focused on
conventional mechanisms including metabolic dysregulation,
oxidative stress, inflammatory responses and microenvironmental
alterations (70,79,88,95). However, knowledge remains limited
regarding regulatory pathways such as neural modulation, mechanical
signals, mitochondrial dynamics, epigenetic modification and
microbial community regulation (106,113,117). Future investigations should
broaden the research scope to elucidate the precise roles of these
factors in cellular migration, thereby providing theoretical
foundations for developing targeted therapeutic strategies for
diabetic wounds.
During wound healing, cell migration is controlled
by multiple signaling cascades, including Rho GTPase, PI3K/Akt,
TGF-β/Smad and Wnt/β-catenin pathways (118-121). These signaling axes regulate
key migratory processes such as cytoskeletal dynamics, cell
polarization and force generation through distinct molecular
mechanisms (122-125).
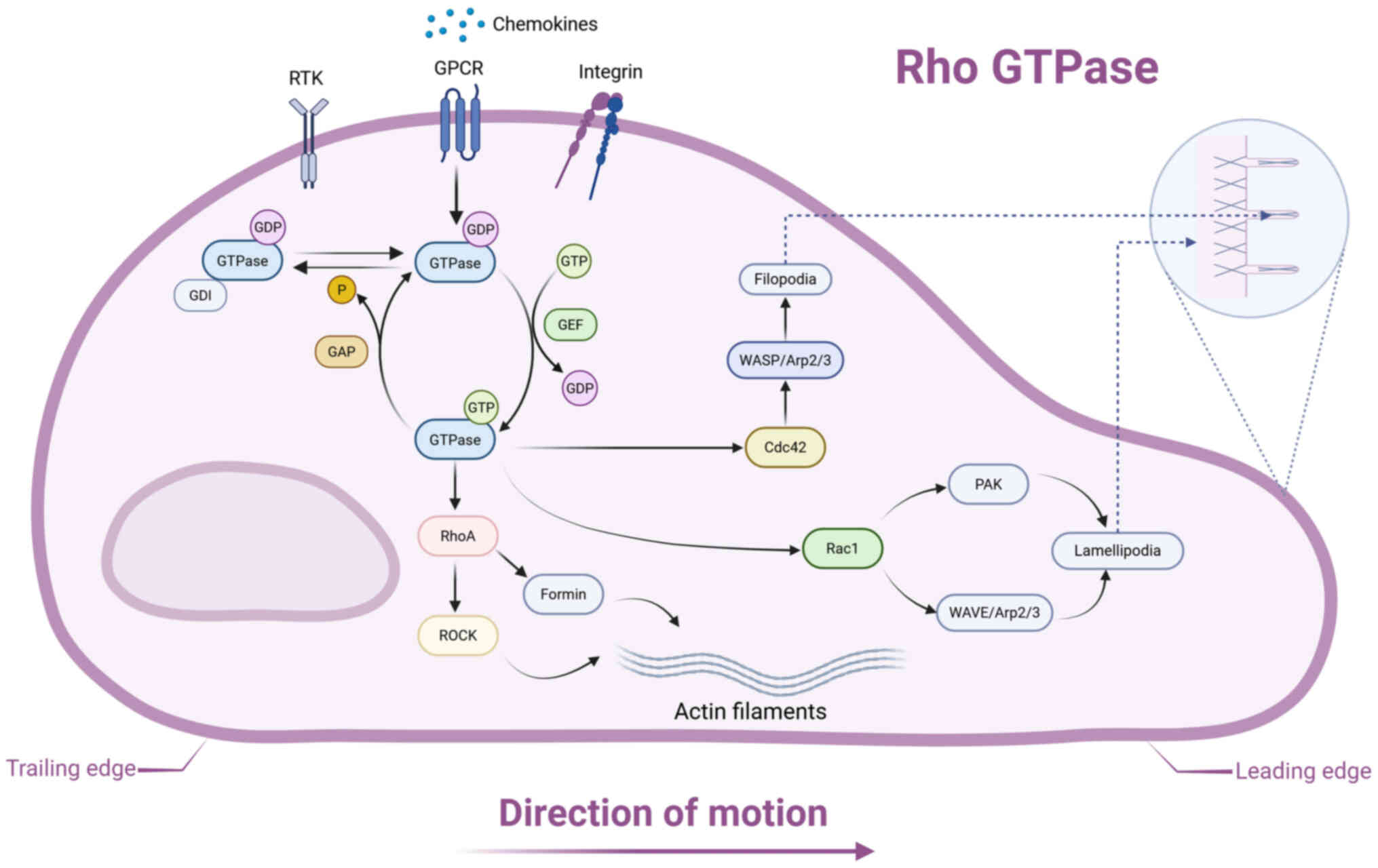
Rho GTPases serve as master regulators of cell
migration, controlling cytoskeletal dynamics to facilitate
directional cell movement (Fig.
4) (122). Rho GTPases are
a distinct subclass within the Ras superfamily, serving as
molecular switches that cycle between their biologically active
GTP-bound conformation and inactive GDP-bound state. The Rho GTPase
regulatory cycle is orchestrated by three protein families: Guanine
nucleotide exchange factors, which promote GDP-to-GTP exchange to
activate Rho GTPases; GTPase-activating proteins, which enhance GTP
hydrolysis, inducing inactivation, and GDP dissociation inhibitors,
which stabilize the inactive GDP-bound form and sequester Rho
GTPases from membranes, thus maintaining quiescence (126).
Recent investigations into cell migration have
largely centered on three key Rho GTPases: RhoA, Rac1 and Cdc42
(127-129). RhoA exhibits activity at both
the leading and trailing edges of migrating cells, where it
orchestrates actomyosin contractility via Rho-associated
coiled-coil forming protein kinase (ROCK) and modulates actin
polymerization via formin family nucleators (130). Rac1 initiates cytoskeletal
remodeling by sequential activation of downstream effectors, first
stimulating PAK kinase and the Wiskott-Aldrich syndrome protein
(WASP) family verprolin-homologous protein (WAVE) regulatory
complex. This signaling cascade induces WAVE-mediated direct
activation of the actin-related protein (Arp) 2/3 complex,
resulting in the nucleation of highly branched actin filament
networks that mechanically drive lamellipodial membrane extension
(122). Cdc42 orchestrates a
distinct morphological response by specifically activating WASP
family proteins, which serve as molecular scaffolds to facilitate
precise Arp2/3 complex assembly and generation of parallel actin
bundles, thereby promoting filopodial protrusion.
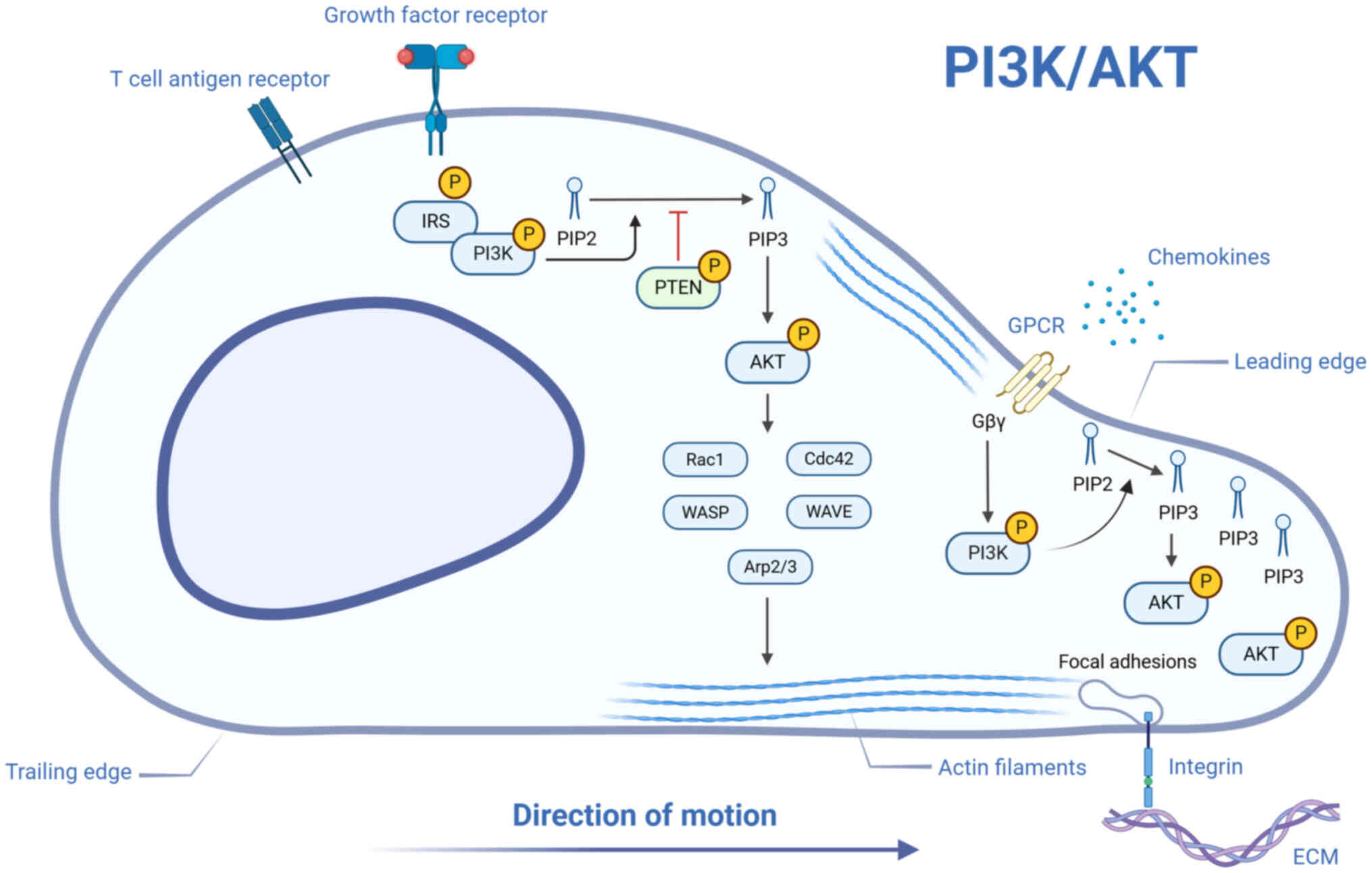
The PI3K/Akt pathway is a central regulator of
fundamental cellular functions, such as proliferative signaling,
migratory behavior and immune modulation (Fig. 5) (131). Initiation of this pathway
occurs through upstream membrane-associated receptors, such as
receptor tyrosine kinases, integrins, antigen/cytokine receptors
and GPCRs, which trigger PI3K activation (132). Following stimulation, PI3K
mediates the phosphorylation of
phosphatidylinositol-4,5-bisphosphate, generating the second
messenger phosphatidylinositol-3,4,5-trisphosphate (PIP3). This
lipid product recruits Akt to the membrane via interaction with its
pleckstrin homology domain, enabling dual phosphorylation
(Thr308/Ser473) and consequent functional activation (133). Once activated, Akt
phosphorylates multiple downstream substrates in both the cytoplasm
and nucleus, thereby modulating cellular motility.
Previous studies have suggested that the PI3K/Akt
axis may contribute to wound repair by modulating the migratory
dynamics of target cells (134,135). Through modulation of
cytoskeletal component equilibrium, this pathway drives cell
migration. Specifically, the PI3K/Akt-mediated signaling cascade
activates Rho family small GTPases, including Rac1 and Cdc42, as
well as actin-polymerization-promoting factors such as
WASP/WAVE-Arp2/3 complexes (136). These components enhance actin
nucleation and branched assembly at the advancing front, fostering
lamellipodia growth and other protrusions that support cell
motility. Secondly, the PI3K/Akt pathway modulates migratory
capacity by regulating the dynamics of cell adhesion. Akt kinase
activity enhances the formation and turnover of integrin-mediated
focal adhesions, thereby allowing migrating cells to maintain
traction at the leading edge while efficiently releasing adhesions
at the rear (137). This
mechanism ensures continuous and coordinated cell movement.
Furthermore, this pathway exerts a key influence on chemotactic
cell migration. When chemokines bind to GPCRs, the Gβγ subunit
rapidly activates PI3K, particularly the PI3Kγ isoform, leading to
increased PIP3 accumulation at the cell membrane leading edge.
Subsequently, PIP3 recruits and activates Akt at the cell front,
locally eliciting downstream pro-migratory effects and guiding the
cell toward areas with higher chemokine concentrations (138).
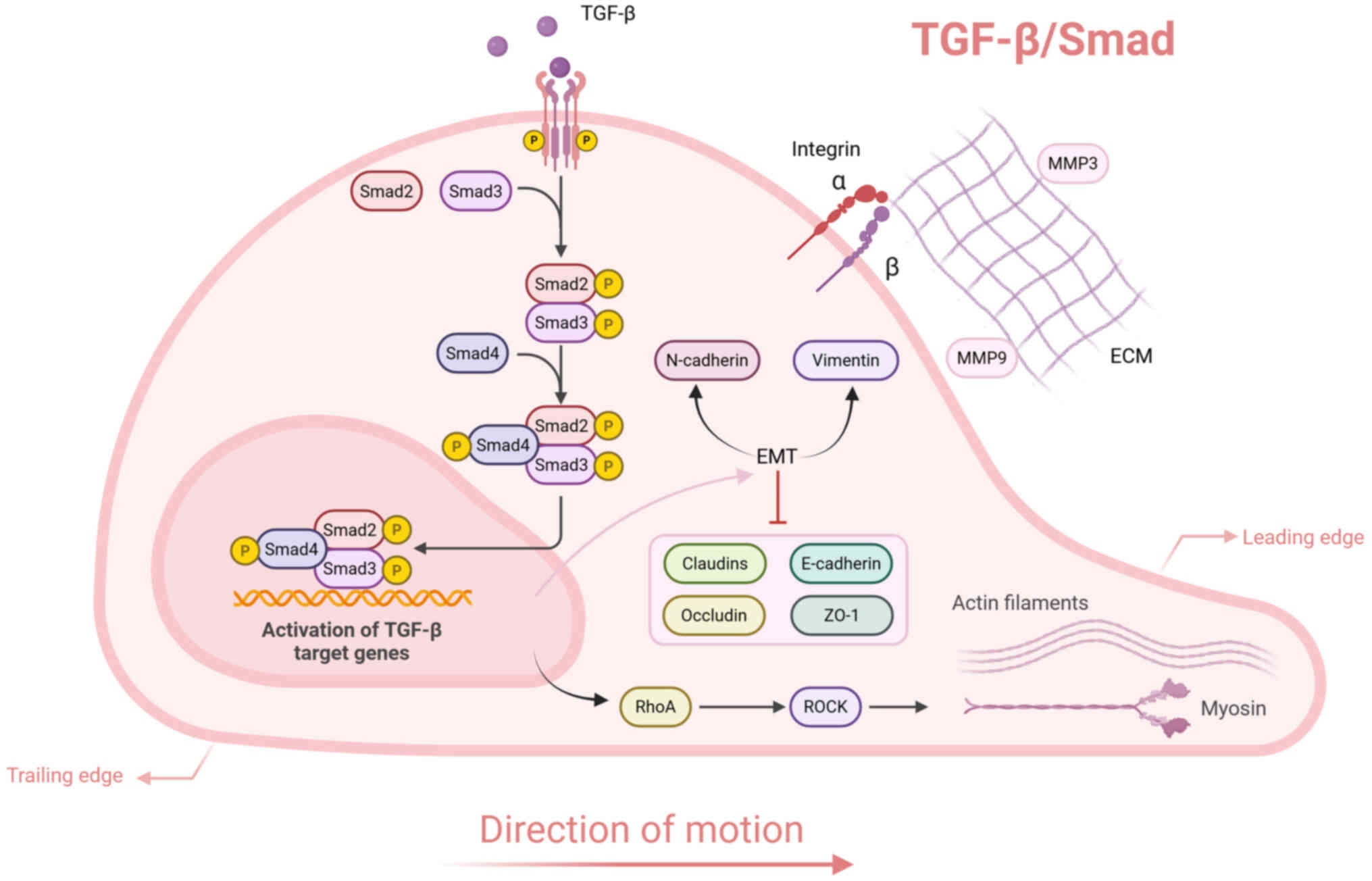
The TGF-β superfamily signaling cascade regulates a
wide range of cell processes, including proliferation, migration
and ECM synthesis and reorganization (139). Central to this pathway is the
TGF-β/Smad axis, which involves TGF-β ligands, cognate receptors
(TGFβRI and TGFβRII) and downstream Smad mediators (Fig. 6). Upon ligand binding, TGFβRII
interacts with TGF-β to assemble a heteromeric receptor complex,
facilitating the recruitment and phosphorylation of TGFβRI
(140). Activated TGFβRI
exhibits kinase function, specifically phosphorylating Smad2 and
Smad3. These phosphorylated Smads dimerize with Smad4, forming a
transcriptionally active oligomeric complex that translocates into
the nucleus to modulate target gene expression (141).
The TGF-β/Smad signaling pathway activates ROCK
through the stimulation of RhoA (142). ROCK facilitates cell migration
by modulating actin cytoskeletal rearrangement and myosin
contractility. TGF-β signaling promotes keratinocyte migration and
facilitates wound healing through the transcriptional regulation of
key ECM-associated molecules. This includes the upregulation of
integrin subunits (α5, αv and β5) as well as specific MMPs,
particularly MMP3 and MMP9 (143,144). The TGF-β/Smad pathway serves as
a key regulator of epithelial-mesenchymal transition (EMT) by
downregulating E-cadherin and tight junction proteins, including
occludin, claudins and zona occludens-1 (145). Simultaneously, elevated
expression of mesenchymal markers, including N-cadherin and
vimentin, reinforces cell motility, promoting the advancement of
EMT.
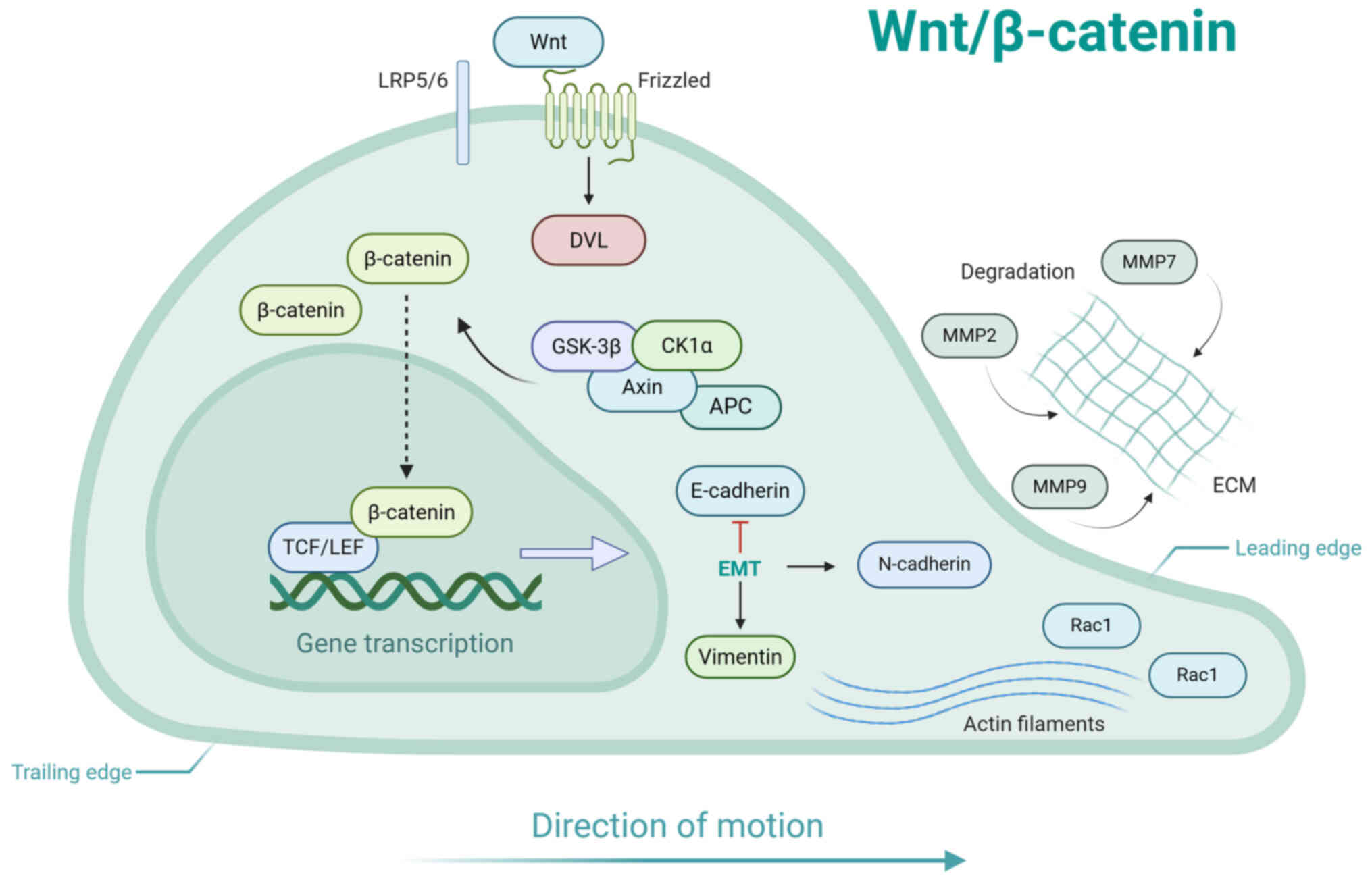
The Wnt/β-catenin signaling pathway, an
evolutionarily conserved regulatory mechanism, governs a spectrum
of biological functions including cellular proliferation,
differentiation, apoptotic regulation and migratory behavior
(Fig. 7) (146). In the absence of Wnt signaling,
cytosolic β-catenin undergoes sequential phosphorylation mediated
by the multiprotein degradation complex, comprising adenomatous
polyposis coli, axin, casein kinase 1 and glycogen synthase
kinase-3β, leading to its proteasomal degradation and thus ensuring
minimal intracellular concentrations (147). Activation occurs upon binding
of Wnt ligands (Wnt3a) to the Frizzled receptor family and its
coreceptor low-density lipoprotein receptor-related protein 5/6,
which disrupts the destabilization complex (148). This stabilization allows
β-catenin to accumulate in the cytoplasm, undergo nuclear
translocation and form complexes with T cell factor/lymphoid
enhancer factor transcription factors, driving transcription of
downstream target genes (149).
Wnt signaling induces localized activation of Rho
family GTPases, including Rac1, at the leading edge of migrating
cells. This stimulates actin polymerization, which is essential for
driving forward cell motility (150). Wnt signaling promotes EMT
through the suppression of E-cadherin and concurrent upregulation
of mesenchymal markers such as vimentin and N-cadherin. This shift
disrupts intercellular adhesion and augments the migratory
potential of cells. In addition, Wnt/β-catenin signaling can
upregulate the expression of MMPs, including MMP2, MMP7 and MMP9,
thereby promoting the degradation of the ECM and facilitating cell
migration (151,152). Activation of Wnt downstream
targets serves a crucial role in cell migration, which
significantly enhances wound healing processes. For example,
Wnt1-inducible signaling pathway protein 1 stimulates proliferation
and directional movement of dermal fibroblasts (153), while epidermal growth factor
receptor (EGFR) activation is essential for keratinocyte
recruitment to wound sites (154). Additionally, VEGF promotes
mitogenic activity and chemotactic migration of ECs during
neovascularization (155).
Crosstalk between signaling pathways may regulate
cell migration during wound healing. Hypoxic conditioned medium
from human amniotic fluid-derived mesenchymal stem cells (MSCs)
promotes fibroblast migration and accelerates wound healing by
modulating the TGF-β/SMAD2 and PI3K/Akt signaling pathways
(156). Toraldo et al
(157) demonstrated that
topical androgen antagonism accelerates keratinocyte migration and
promotes skin wound healing by inhibiting β-catenin nuclear
translocation and its crosstalk with TGF-β signaling in
keratinocytes. Furthermore, the Rho GTPase signaling pathway may
serve as a common downstream node for other pathways to regulate
cell migration (136,142,150). The types of cell migration
regulated by different pathways exhibit distinct characteristics
(Table I) (118-121,158-166).
ncRNAs represent a diverse class of RNA transcripts
that lack protein-coding potential yet serve key roles in
modulating gene expression and orchestrating cell processes
(167). Among these
transcripts, distinct subcategories, including long ncRNAs
(lncRNAs), microRNAs (miRNAs or miRs) and circular RNAs (circRNAs),
have been identified and functionally characterized (168). Emerging evidence underscores
the significance of ncRNAs in controlling migratory behaviors of
cells and their impact on diabetic wound repair (Table II) (169-171). Given their regulatory
versatility, targeting ncRNAs may offer novel therapeutic avenues
for improving wound outcomes in diabetic patients.
miRNAs, a class of evolutionarily conserved small
ncRNAs averaging 21-25 nucleotides in length (182), serve as critical mediators of
post-transcriptional regulation, exerting their effects through
either mRNA destabilization or translational suppression upon
target binding (183). Certain
miRNAs, including miR-3138, miR-204-3p, miR-146a, miR-129, and
miR-335, promote keratinocyte migration through regulation of
downstream targets or key signaling pathways, thereby accelerating
wound healing in diabetic models (184-187). By contrast, elevated expression
of miR-155 and miR-3679-5p suppresses keratinocyte migratory
capacity, contributing to impaired re-epithelialization and
protracted wound closure in diabetic conditions (170,184). Exosomes derived from
endothelial progenitor cells deliver miR-182-5p, which
downregulates PPARG expression, enhances keratinocyte proliferation
and migration under hyperglycemic conditions, decreases apoptotic
activity and ultimately facilitates diabetic wound repair (188). In addition, loading miR-21-5p
into human adipose stem cell-derived exosomes facilitates
keratinocyte proliferation and migration by activating the
Wnt/β-catenin signaling pathway. This improves diabetic wound
repair by concurrently stimulating re-epithelialization, optimizing
collagen deposition and organization, boosting neovascularization
and fostering functional vascular network maturation (166).
In a high-glucose environment, miR-185-5p,
miR-16-5p, and miR-21-3p enhance the migratory capacity of
fibroblasts (189-191). By contrast, miR-145-5p,
miR-103, miR-27-3p, and miR-199a-5p exert a pronounced inhibitory
effect on fibroblast migration in diabetic wounds (192-194,158). Recent research demonstrates
that exosomes derived from insulin-induced gene 1
(Insig1)-overexpressing BMSCs (Insig1-exos), which are highly
enriched with miR-132-3p, markedly enhance dermal fibroblast
migration, proliferation and angiogenic activity under
hyperglycemic conditions (195). Mechanistically, Insig1-exos
modulate key wound repair mediators, including MMP9, PDGF and VEGF,
thereby improving diabetic wound closure in murine models. These
results underscore the key contribution of miR-132-3p in
facilitating cellular motility and tissue regeneration.
Recent studies have demonstrated that miR-488-3p,
miR-199a-5p and miR-204-3p facilitate diabetic wound repair by
enhancing EC migration under hyperglycemia (158,196,197). Moreover, BMSCs release
miR-221-3p and miR-146a-5p, while exosomes from adipose-derived SCs
(ADSCs) deliver miR-125b, miR-146a-5p and miR-132, all of which
collaboratively enhance EC migration, proliferation and angiogenic
functions. These effects contribute to accelerated wound healing in
diabetic models, underscoring their therapeutic promise (198-202). Milk-derived exosomes, obtained
from both bovine colostrum and mature milk, present benefits such
as excellent safety profiles, cost-effectiveness and high
production yields (203). These
natural vesicles exhibit remarkable drug-loading capacity and
robust biological functionality across both in vitro and
in vivo systems, highlighting their potential for
pharmaceutical delivery and immunomodulatory applications (204). Notably, Yan et al
(205) revealed that milk
exosome-encapsulated miR-31-5p exhibits enhanced stability and
cellular internalization (compared with free miR-31-5p mimics),
stimulates EC proliferation, migration and neovascularization
through hypoxia-inducible factor 1 subunit α inhibitor suppression
and ultimately promotes diabetic wound repair.
circRNAs are a unique class of ncRNA molecules
distinguished by their covalently closed-loop conformation, which
confers resistance to exonuclease-mediated degradation (206). circRNAs function as 'sponges'
to inhibit specific miRNAs, preventing their binding to target
mRNAs, thereby serving as endogenous miRNA regulators (inhibitors)
(207). Moreover, circRNAs
modulate post-transcriptional gene expression and transcriptional
processes through interactions with transcription factors and
RNA-binding proteins (208).
circ_072697, circ_0080968, circ_PRKDC and hsa_ circ_0084443 exert
inhibitory effects on keratinocyte migration and impair diabetic
wound healing by suppressing miRNAs or directly modulating target
gene expression (171,209-211). ADSC-derived exosomes carrying
mmu_circ_0001052 downregulate miR-106a-5p, which elevates
fibroblast growth factor 4 (FGF4) levels, triggers the FGF4/p38MAPK
signaling cascade and stimulates HUVEC proliferation, migration and
angiogenic activity in high-glucose environments, collectively
improving diabetic wound healing (212). Huang et al (213) reported that engineered small
extracellular vesicles (sEVs) with circCDK13 overexpression bind
insulin-like growth factor 2 mRNA-binding protein 3, stabilizing
CD44 and c-MYC transcripts to enhance keratinocyte and fibroblast
motility and division, thus accelerating wound closure in diabetic
mouse models.
ncRNAs modulate cell migration via signaling
pathways or downstream targets, presenting a promising intervention
strategy for diabetic wounds. Both lncRNAs and circRNAs serve as
ceRNAs, sequestering specific miRNAs to prevent their suppressive
effects on target mRNAs, thereby indirectly regulating gene
expression (171,174). By contrast, miRNAs directly
bind to mRNAs to induce degradation or translational inhibition,
forming an integrated miRNA-mRNA-functional protein regulatory
network (170,186). Future studies should delineate
the dynamic ncRNA-mediated regulatory networks in diabetic wound
healing to develop precision-based therapeutic approaches for
clinical translation.
SCs are undifferentiated cells characterized by
their capacity for self-renewal and pluripotency, allowing
differentiation into specialized lineages (214). Additionally, they secrete
bioactive factors, modulate inflammatory responses, stimulate
angiogenesis and enhance tissue remodeling, highlighting their
therapeutic potential in regenerative medicine and tissue repair
(Table III) (215). Their low immunogenicity and
diverse sources enhance clinical potential. A recent investigation
revealed that perinatal tissue-derived MSCs potentiate keratinocyte
and EC proliferation and migration via PI3K/Akt pathway activation,
culminating in accelerated healing of diabetic wounds (160). Furthermore, SCs can serve as
carriers for drugs or bioactive molecules, facilitating sustained
release while protecting them from degradation. Administration of
genetically modified umbilical cord MSCs expressing angiopoietin-1
improves wound vascularization in diabetic murine models by
stimulating EC migration and tubulogenesis, leading to faster
healing kinetics (216).
The application of SCs is limited by several
factors, including a low survival rate, difficulty in controlling
differentiation direction and the potential for immune rejection
(217). By contrast, SC-derived
exosomes, characterized by strong targeting ability, well-defined
mechanisms of action and high safety profiles, offer a more precise
and controllable cell-free therapeutic strategy for tissue repair.
Beyond ncRNA-mediated regulation, SC-derived exosomes also promote
cell migration and improve diabetic wound repair by targeting key
signaling pathways. For example, ADSC-exos enhance EC migration and
angiogenesis via the Tripartite motif-containing protein 32
(TRIM32)/STING axis, expediting wound closure in diabetic models
(218). Liu et al
(165) reported that exosomes
isolated from gingival MSCs stimulate EC proliferation, migration
and tube formation by activating the Wnt/β-catenin cascade,
offering a potential therapeutic strategy for diabetic wound
management.
SC therapy has demonstrated substantial therapeutic
potential in diabetic wound repair; however, limitations persist
(215). The precise mechanistic
pathways governing its efficacy remain incompletely characterized,
underscoring the need for further exploration of its molecular and
cellular regulatory networks. Safety concerns need to be addressed
by defining optimal dosages and administration routes to minimize
potential adverse effects. Additionally, the complex processes
involved in SC collection and pretreatment require simplification
to enhance clinical feasibility and efficiency. The preparation and
quality control of exosomes require the establishment of a unified
standard (219). Additionally,
due to the low molecular concentration of natural exosomes and
their limited repair capabilities, exploring pretreatment methods,
genetically engineered exosomes and the integration of exosomes
with biomaterials may represent promising directions for
development (220).
Growth factor therapy for diabetic wound healing is
based on its ability to coordinate key cellular responses and
molecular pathways governing tissue regeneration. Key growth
factors, including PDGF, VEGF, EGF, FGF and TGF, demonstrate potent
pro-healing effects by stimulating cell migration and other
regenerative processes that collectively accelerate wound repair
(221). FGF-21 markedly
improves EC proliferation, migration and angiogenic tube formation
under hyperglycemic conditions, accelerating diabetic wound repair
and underscoring its potential as a therapeutic agent for diabetic
wounds (222). Tang et
al (223) reported that
PDGF-loaded nanocapsules with sustained release properties
efficiently regulate fibroblast migration, proliferation and
neovascularization, contributing to enhanced wound repair in
diabetic models. Jeong et al (224) found that EGF encapsulated
within gelatin-alginate coacervates enhances keratinocyte migration
in vitro and accelerates wound closure in diabetic mice.
Growth factor therapy exhibits potential for
promoting tissue regeneration; however, its clinical translation is
hindered by rapid degradation, poor diffusion efficiency and
insufficient local retention (221). Future studies should prioritize
design of advanced biocompatible biomaterials with tunable
degradation kinetics, alongside optimization of nanoscale delivery
platforms to enable spatiotemporal control of drug release,
targeted accumulation at wound sites and improved pharmacokinetic
profile, which are key for expanding therapeutic utility in
clinical settings.
Hydrogels are three-dimensional polymeric networks
distinguished by high hydration capacity and structural integrity,
formed via cross-linked polymer chains. Owing to their
biocompatibility, low immunogenicity and ability to retain
moisture, hydrogels have gained prominence as an ideal biomaterial
for diabetic wound management (Table IV) (225,226). These materials are capable of
absorbing excess wound exudate, sustaining a moist microenvironment
and preventing anaerobic bacterial proliferation through enhanced
oxygen diffusion, facilitating cellular migration, tissue repair
mechanisms and an accelerated healing trajectory (227,228). Recent studies have demonstrated
that drug-loaded hydrogel dressings accelerate diabetic wound
closure by facilitating cell motility and tissue remodeling
(229,230). Notably, PDGF and cytokines
stimulate ECM production, neovascularization and directed cellular
movement, contributing to enhanced tissue repair (231). Xu et al (232) demonstrated that platelet-rich
plasma-loaded multifunctional hydrogels exhibit dual therapeutic
effects, suppressing excessive inflammation while shifting
macrophage differentiation in favor of the regenerative M2 subset.
This phenotypical modulation enhances migratory activity in both
fibroblasts and vascular ECs, contributing to accelerated wound
repair. This approach presents a clinically viable therapeutic
paradigm for enhancing diabetic wound repair mechanisms. In
addition, the incorporation of engineered sEVs into hydrogels
prolongs their residence within the wound microenvironment,
establishing them as optimal vehicles for bioactive molecule
delivery. Wei et al (233) revealed that miR-17-5p-modified
sEVs encapsulated in gelatin methacryloyl hydrogels enhance ECM
remodeling via PTEN/p21 pathway modulation, thus stimulating both
EC and fibroblast motility. Such a therapeutic strategy markedly
improves the healing kinetics of diabetic wounds.
Hydrogels face limitations in their application.
Due to their time-dependent viscoelastic properties, long-term
structural degradation and stress relaxation under load may occur,
which can impair cell adhesion, migration and proliferation
(234). Additionally,
insufficient mechanical strength, challenges in controlling
degradation rate and narrow functionality further restrict their
practical use. Future integration of artificial intelligence-based
screening with advanced 3D bioprinting platforms may simultaneously
optimize the biomechanical performance, biofunctional
characteristics and therapeutic applicability of next-generation
hydrogels (235,236). Combined with personalized
customization, these advancements may provide more efficient and
precise solutions for wound healing.
Aerogels are ultra-lightweight nanoporous materials
formed by removing the liquid from gel pores to create
interconnected porous structures (237). They exhibit rapid absorption of
exudate while maintaining a moist wound environment and
facilitating efficient gas exchange (238). Compared with conventional
hydrogels and standard wound dressings, aerogels demonstrate
superior structural characteristics, including ultralow density,
minimal thermal conductivity, interconnected macroporosity and an
extensive surface-to-volume ratio, which position them as promising
alternatives for advanced wound management (Table IV) (239). Emerging evidence highlights the
therapeutic potential of aerogel-based drug delivery systems in
accelerating cell migration during wound regeneration (240,241). Wu et al (242) developed a turmeric
nanoparticle-embedded aerogel dressing that demonstrates controlled
drug release kinetics and potent anti-inflammatory and antioxidant
activity, alongside enhanced fibroblast migration and
proliferation. This formulation exhibited remarkable effectiveness
in treating diabetic ulcers. Furthermore, LL-37, a
cathelicidin-derived host defense peptide, serves key biological
functions beyond its antimicrobial effects, including the
modulation of keratinocyte and fibroblast activity to facilitate
cutaneous wound closure (243,244). John et al (245) developed a nanofiber aerogel
scaffold engineered with tailored macrochannels and LL-37
biomimetic peptides for diabetic wound therapy. The results
demonstrated notable stimulation of both keratinocyte and
fibroblast migratory activity and mitotic expansion, alongside
marked enhancement in neovascularization and epidermal
regeneration.
The application of aerogel requires further
enhancement. Its intrinsic porous structure results in inadequate
mechanical properties, low mechanical strength and susceptibility
to fragmentation (238).
Moreover, the intricate preparation process and high production
costs hinder large-scale manufacturing and clinical adoption
(242). In future, it may be
feasible to enhance mechanical strength and flexibility through
composite material design (by integrating with polymers), while
simultaneously optimizing manufacturing processes, reducing
expenses and developing more environmentally friendly and
sustainable preparation methodologies.
Microneedles are miniature, spine-like structures
made from biocompatible materials, typically measuring from tens to
hundreds of microns in size. Microneedle technology facilitates the
penetration of the stratum corneum, enabling drug delivery,
substance extraction or physical therapy targeting deep skin tissue
(Table IV) (246). The microneedle patch integrates
microneedle technology with a patch format, using tiny needle-like
structures distributed on a substrate for transdermal drug
delivery. Given their high exudate absorption capacity, robust
bioadhesive performance and sustained drug release kinetics,
microneedle patches have emerged as a promising therapeutic
modality for chronic wound management (247). Yin et al (248) engineered a microneedle system
incorporating magnesium-based organic frameworks, enabling
efficient transdermal drug transport in diabetic wounds. This
platform significantly augments EC migratory activity, stimulates
neovascularization and accelerates tissue repair processes. Wang
et al (249) developed a
biodegradable poly (lactic-co-glycolic acid) microneedle patch
loaded with magnesium hydride. This platform effectively scavenges
ROS, induces a shift toward pro-regenerative M2 macrophage
phenotypes and stimulates the proliferation and motility of
fibroblasts and ECs, enhancing the healing trajectory of diabetic
wounds.
Microneedle technology encounters several
challenges, including limited drug-loading capacity, high
production cost and insufficient stability in complex wound
environments (246,250). Future advancements are
required, such as optimizing materials and structures for the
design of novel microneedles, assessing drug stability and
enhancing biocompatibility and safety profiles. Additionally, the
combination of micromachining and 3D printing techniques may
streamline the manufacturing process and decrease production
expenses (250).
Phytochemicals derived from medicinal plants
demonstrate multifunctional bioactive properties, including the
stimulation of cellular proliferation and migratory capacity,
potent antimicrobial effects and the induction of
neovascularization, all of which contribute to enhanced tissue
regeneration (Table V) (251,252). Recent research has shown that
topical administration of Crocus sativus L. (saffron) petal
extract markedly accelerates diabetic wound repair by elevating
Collagen type I alpha 1 and VEGF levels, stimulating fibroblast and
EC motility and enhancing overall re-epithelialization in mice
(253). Ginsenoside Rg1 (Rg1),
a principal active component derived from Panax ginseng,
exerts pro-angiogenic effects by stimulating the proliferation and
migration of ECs, thereby facilitating wound repair in diabetic
wounds. Mechanistically, Rg1 downregulates miR-48-3p, elevates
Sirt1 expression and triggers the PI3K/AKT/endothelial nitric oxide
synthase) cascade, collectively enhancing vascular regeneration
(254). Similarly,
paeoniflorin, a key monoterpene glycoside isolated from Paeoniae
alba radix, was demonstrated by Sun et al to attenuate
oxidative damage while promoting keratinocyte proliferation and
motility (255). These
reparative effects are achieved via Nrf2 pathway activation coupled
with increased VEGF and TGF-β1 production, expediting diabetic
wound closure in rats.
Chinese herbal formulas have potential in
facilitating cell migration in diabetic wound healing (256,257). Danggui Sini decoction (DSD), a
TCM formulation, exhibits multi-target pharmacological actions such
as vasodilatory, anti-inflammatory and antioxidant activity
(258). Mechanistic study has
revealed that DSD facilitates diabetic wound repair by augmenting
fibroblast proliferation and migratory capacity, mediated via
regulation of the AGE/RAGE (Receptor for advanced glycation
end-products)/TGF-β/Smad2/3 signaling axis in diabetic foot ulcer
rats (256). Moist exposed burn
ointment, a herbal oil-based preparation, is utilized for burn
management and chronic refractory wound care due to its clinical
effectiveness (259). When
applied to diabetic wounds, as demonstrated by Gong et al
(257), this formulation
accelerates tissue regeneration by stimulating keratinocyte
migration, promoting granulation tissue development and collagen
reorganization and enhancing re-epithelialization.
While TCM demonstrates therapeutic promise in
enhancing diabetic wound repair, several challenges remain to be
resolved, including poorly characterized molecular mechanisms,
intricate multi-component formulations and restricted
administration options. Overcoming these limitations requires
systematic research strategies to elucidate fundamental mechanisms,
optimize bioactive compound extraction protocols, develop novel
delivery systems and validate therapeutic effects through
multicenter clinical studies.
Diabetic foot ulcers are frequently attributed to
inadequate blood supply to the lower limb vessels, leading to
localized hypoxia in the wound and consequently impairing the
healing process. Hyperbaric oxygen therapy (HBOT) serves as an
adjunctive therapy that elevates oxygen concentrations in arterial
blood and tissues (260). This
therapeutic intervention involves the administration of 100% oxygen
in a pressurized chamber, elevating environmental pressure to 2-3
atmospheres absolute (261).
Under hyperbaric conditions, tissue hypoxia is alleviated,
improving oxygenation for key metabolic processes, cellular
proliferation and wound repair. HBOT stimulates fibroblast and EC
activity via HIF-1α pathway activation, which enhances
vascularization and accelerates healing of diabetic wounds
(262).
Negative pressure wound therapy (NPWT) is a
non-surgical therapeutic approach utilizing an airtight dressing
system to achieve localized sub-atmospheric pressure at the wound
bed, facilitating enhanced tissue perfusion and wound closure. This
therapy effectively removes wound exudate and necrotic tissue,
decreases tissue edema, promotes the growth of granulation tissue
and angiogenesis, thereby providing optimal conditions for wound
healing (263,264). Huang et al (265) revealed that NPWT promotes human
dermal fibroblast proliferation and migration via miR-155
downregulation in diabetic wound granulation tissue, concurrently
augmenting FGF7 expression to accelerate wound repair (265). Liu et al (266) demonstrated that NPWT stimulates
keratinocyte proliferation and migration by suppressing
hsa-miR-203, which elevates p63 protein levels in both peripheral
blood and wound edge tissue, contributing to enhanced diabetic
wound healing.
Photobiomodulation (PBM), commonly known as
low-intensity laser therapy, is a non-interventional treatment
approach that employs low-power optical radiation, typically
delivered via lasers or light-emitting diodes (267). PBM enhances wound closure and
tissue regeneration, with optimal therapeutic outcomes depend on
precise selection of wavelength and fluence parameters (268,269). PBM at 830 nm (5
J/cm2 fluence) significantly boosts fibroblast
viability, migration and proliferative capacity via activation of
the TGF-β1/Smad pathway, leading to accelerated healing of diabetic
wounds (163). Cai et al
(270) examined dual-wavelength
(red/blue) phototherapy in diabetic rats, observing substantial
decreases in inflammatory markers and ROS accumulation alongside
enhanced EC activity. This combined approach promotes NO synthesis
and markedly improves wound closure rates.
Filgrastim, a recombinant human granulocyte
colony-stimulating factor analog, promotes both neutrophil
progenitor differentiation and functional maturation (271). Additionally, this cytokine
directs neutrophil trafficking toward inflammatory and infectious
foci, amplifying localized immune defenses via targeted cellular
recruitment. A retrospective analysis of patients with infectious
diabetic wounds demonstrated that those treated with filgrastim
exhibited significantly faster recovery times (272). This indicates the potential
therapeutic value of filgrastim in enhancing infection control and
promoting wound healing via increased neutrophil production and
migration, bolstered immune response and accelerated tissue
repair.
There are limitations in the application of the
aforementioned therapies. The high cost of treatment and reliance
on specialized equipment restrict the widespread adoption of HBOT.
Future research should focus on optimizing treatment parameters,
decreasing cost and investigating combination therapies. NPWT may
induce pain and skin damage, with limited efficacy for infected
wounds. Advances in dressing materials and refined control of
negative pressure are required to minimize adverse reactions
(273). PBM lacks standardized
therapeutic parameters, such as wavelength and fluence, and its
efficacy varies between individuals (268,269). Large-scale clinical trials are
necessary to establish optimal parameters and indications.
Filgrastim may lead to overactivation of neutrophils, potentially
causing increased inflammation and other adverse reactions
(274). Future studies should
aim to optimize dosing regimens and develop novel drugs to enhance
therapeutic outcomes.
The present review summarizes cell migration
dynamics in diabetic wounds, with a focus on cellular mechanisms,
signaling cascades, ncRNA-mediated regulation and their
translational implications for targeted therapies. Emerging
therapies, such as SCs, exosomes, drug-loaded dressings and TCM,
enhance cell migration via ncRNA-mediated signaling (160,275). This establishes regulatory axes
of drug/therapy-ncRNA-signaling pathway/downstream target-cell
migration (166,254). These breakthroughs
substantially enhance understanding of diabetic wound pathological
mechanisms while establishing a framework for targeted therapeutic
development.
Although the mechanisms underlying abnormal cell
migration in diabetic wounds and targeted therapeutic approaches
have seen advancements, notable gaps remain. The majority of
studies emphasize the regulation of individual cell types or
specific signaling pathways, with limited exploration of cellular
interactions and signaling crosstalk (160,165,166,275). Research on the regulation of
cell migration primarily focuses on ncRNAs, whereas other
epigenetic modifications, such as DNA methylation and histone
modification, warrant further investigation (170,177,192,211). Most studies rely on in
vitro experiments or animal models, which differ from the
complex pathological environment of the human body, limiting their
clinical translation and necessitating further validation (170,253). Despite their growing use,
SC/exosome and growth factor therapy, advanced drug-loaded
dressings and TCM intervention lack comprehensive clinical trial
data to confirm their long-term safety and therapeutic efficacy.
Furthermore, given the high heterogeneity of patients, there is a
lack of research on personalized treatment approaches in existing
studies, which restricts broader clinical application (276-278).
Future research should explore crosstalk between
immune cells, fibroblasts, keratinocytes and ECs in diabetic
wounds, focusing on key pathways. The application of organoids or
3D-printed tissue models may facilitate the development of more
accurate models that closely mimic the human pathological
environment (279,280). It is essential to refine the
preparation and delivery technologies for SCs and exosomes, enhance
the manufacturing processes of drug-loaded dressings and design
intelligent dressing delivery systems to improve the precision and
control of therapeutic interventions (281-283). The integration of topical TCM
agents with advanced wound dressings may enhance therapeutic
efficacy, presenting a potential strategy for diabetic wound
management. Large-scale, multi-center clinical trials are required
to validate the efficacy of existing treatments. Integrating
multi-omics techniques with artificial intelligence-based analysis
to explore personalized treatment strategies will aid in achieving
precise intervention tailored to individual patient characteristics
(284). Ultimately, it is
essential to enhance multi-disciplinary collaboration between basic
research and clinical practice, thereby facilitating the
translation of research findings into practical applications and
providing more efficient and safer solutions for the treatment of
diabetic wounds.
Not applicable.
JLS designed the study, wrote the manuscript and
constructed figures. TZ and CW performed the literature review and
created figures. XS, JCS and ZZ revised the manuscript. JCS and ZZ
supervised the study and acquired funding. Data authentication is
not applicable. All authors have read and approved the final
manuscript.
Not applicable.
Not applicable.
The authors declare that they have no competing
interests.
Not applicable.
The present study was supported by the Construction Project of
the TCM Institute of Sore and Ulcer, Tianjin University of
Traditional Chinese Medicine (grant no. 202206), the Construction
Project of the Institute of Traditional Chinese Medicine and
Integrated Chinese and Western Medicine under the Tianjin Municipal
Health Commission (grant no. 202455) and the Research Planning
Projects of the Tianjin Municipal Education Commission (grant no.
2024ZD014).
|
1
|
Dwivedi J, Sachan P, Wal P, Wal A and Rai
AK: Current state and future perspective of diabetic wound healing
treatment: Present evidence from clinical trials. Curr Diabetes
Rev. 20:e2808232204052024. View Article : Google Scholar
|
|
2
|
Sun H, Pulakat L and Anderson DW:
Challenges and new therapeutic approaches in the management of
chronic wounds. Curr Drug Targets. 21:1264–1275. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Burgess JL, Wyant WA, Abujamra BA, Kirsner
RS and Jozic I: Diabetic wound-healing science. Medicina (Kaunas).
57:10722021. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Vijayakumar V, Samal SK, Mohanty S and
Nayak SK: Recent advancements in biopolymer and metal
nanoparticle-based materials in diabetic wound healing management.
Int J Biol Macromol. 122:137–148. 2019. View Article : Google Scholar
|
|
5
|
Armstrong DG, Tan TW, Boulton AJM and Bus
SA: Diabetic foot ulcers: A review. JAMA. 330:62–75. 2023.
View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Dasari N, Jiang A, Skochdopole A, Chung J,
Reece EM, Vorstenbosch J and Winocour S: Updates in diabetic wound
healing, inflammation, and scarring. Semin Plast Surg. 35:153–158.
2021. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
GBD 2021 Diabetes Collaborators: Global,
regional, and national burden of diabetes from 1990 to 2021, with
projections of prevalence to 2050: A systematic analysis for the
global burden of disease study 2021. Lancet. 402:203–234. 2023.
View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Feng J, Yao Y, Wang Q, Han X, Deng X, Cao
Y, Chen X, Zhou M and Zhao C: Exosomes: Potential key players
towards novel therapeutic options in diabetic wounds. Biomed
Pharmacother. 166:1152972023. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Peña OA and Martin P: Cellular and
molecular mechanisms of skin wound healing. Nat Rev Mol Cell Biol.
25:599–616. 2024. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Hanson AJ and Quinn MT: Effect of fibrin
sealant composition on human neutrophil chemotaxis. J Biomed Mater
Res. 61:474–481. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Liu C, Lu Y, Du P, Yang F, Guo P, Tang X,
Diao L and Lu G: Mesenchymal stem cells pretreated with
proinflammatory cytokines accelerate skin wound healing by
promoting macrophages migration and M2 polarization. Regen Ther.
21:192–200. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Wang S, Wang K, Xin Y and Lv D: Maggot
excretions/secretions induces human microvascular endothelial cell
migration through AKT1. Mol Biol Rep. 37:2719–2725. 2010.
View Article : Google Scholar
|
|
13
|
Xia W, Li M, Jiang X, Huang X, Gu S, Ye J,
Zhu L, Hou M and Zan T: Young fibroblast-derived exosomal
microRNA-125b transfers beneficial effects on aged cutaneous wound
healing. J Nanobiotechnology. 20:1442022. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Fang PH, Lai YY, Chen CL, Wang HY, Chang
YN, Lin YC, Yan YT, Lai CH and Cheng B: Cobalt protoporphyrin
promotes human keratinocyte migration under hyperglycemic
conditions. Mol Med. 28:712022. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Huang SM, Wu CS, Chiu MH, Yang HJ, Chen GS
and Lan CCE: High-glucose environment induced intracellular
O-GlcNAc glycosylation and reduced galectin-7 expression in
keratinocytes: Implications on impaired diabetic wound healing. J
Dermatol Sci. 87:168–175. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Song Q, An X, Li D, Sodha NR, Boodhwani M,
Tian Y, Sellke FW and Li J: Hyperglycemia attenuates angiogenic
capability of survivin in endothelial cells. Microvasc Res.
78:257–264. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Huttenlocher A: Cell polarization
mechanisms during directed cell migration. Nat Cell Biol.
7:336–337. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Brothers KM, Stella NA, Hunt KM,
Romanowski EG, Liu X, Klarlund JK and Shanks RMQ: Putting on the
brakes: Bacterial impediment of wound healing. Sci Rep.
5:140032015. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Carragher NO, Fincham VJ, Riley D and
Frame MC: Cleavage of focal adhesion kinase by different proteases
during SRC-regulated transformation and apoptosis. Distinct roles
for calpain and caspases. J Biol Chem. 276:4270–4275. 2001.
View Article : Google Scholar
|
|
20
|
Zhao Y, Wang Y, Sarkar A and Wang X:
Keratocytes generate high integrin tension at the trailing edge to
mediate rear de-adhesion during rapid cell migration. iScience.
9:502–512. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Clayton SM, Shafikhani SH and Soulika AM:
Macrophage and neutrophil dysfunction in diabetic wounds. Adv Wound
Care (New Rochelle). 13:463–484. 2024. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Rousselle P, Braye F and Dayan G:
Re-epithelialization of adult skin wounds: Cellular mechanisms and
therapeutic strategies. Adv Drug Deliv Rev. 146:344–365. 2019.
View Article : Google Scholar
|
|
23
|
Rodrigues M, Kosaric N, Bonham CA and
Gurtner GC: Wound healing: A cellular perspective. Physiol Rev.
99:665–706. 2019. View Article : Google Scholar :
|
|
24
|
Su Y and Richmond A: Chemokine regulation
of neutrophil infiltration of skin wounds. Adv Wound Care (New
Rochelle). 4:631–640. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Dahlgren C, Gabl M, Holdfeldt A, Winther M
and Forsman H: Basic characteristics of the neutrophil receptors
that recognize formylated peptides, a danger-associated molecular
pattern generated by bacteria and mitochondria. Biochem Pharmacol.
114:22–39. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Mamun AA, Shao C, Geng P, Wang S and Xiao
J: Recent advances in molecular mechanisms of skin wound healing
and its treatments. Front Immunol. 15:13954792024. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Lämmermann T, Afonso PV, Angermann BR,
Wang JM, Kastenmüller W, Parent CA and Germain RN: Neutrophil
swarms require LTB4 and integrins at sites of cell death in vivo.
Nature. 498:371–375. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Lee WL, Harrison RE and Grinstein S:
Phagocytosis by neutrophils. Microbes Infect. 5:1299–1306. 2003.
View Article : Google Scholar : PubMed/NCBI
|
|
29
|
McLeish KR and Fernandes MJ: Understanding
inhibitory receptor function in neutrophils through the lens of
CLEC12A. Immunol Rev. 314:50–68. 2023. View Article : Google Scholar
|
|
30
|
Fetz AE and Bowlin GL: Neutrophil
extracellular traps: Inflammation and biomaterial preconditioning
for tissue engineering. Tissue Eng Part B Rev. 28:437–450. 2022.
View Article : Google Scholar
|
|
31
|
Bratton DL and Henson PM: Neutrophil
clearance: When the party is over, clean-up begins. Trends Immunol.
32:350–357. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Guo S and DiPietro LA: Factors affecting
wound healing. J Dent Res. 89:219–229. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
de Oliveira S, Rosowski EE and
Huttenlocher A: Neutrophil migration in infection and wound repair:
Going forward in reverse. Nat Rev Immunol. 16:378–391. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Chen WYJ and Rogers AA: Recent insights
into the causes of chronic leg ulceration in venous diseases and
implications on other types of chronic wounds. Wound Repair Regen.
15:434–449. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Isles HM, Herman KD, Robertson AL, Loynes
CA, Prince LR, Elks PM and Renshaw SA: The CXCL12/CXCR4 signaling
axis retains neutrophils at inflammatory sites in zebrafish. Front
Immunol. 10:17842019. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Wood W: Wound healing: Calcium flashes
illuminate early events. Curr Biol. 22:R14–R16. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Niethammer P, Grabher C, Look AT and
Mitchison TJ: A tissue-scale gradient of hydrogen peroxide mediates
rapid wound detection in zebrafish. Nature. 459:996–999. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Razzell W, Evans IR, Martin P and Wood W:
Calcium flashes orchestrate the wound inflammatory response through
DUOX activation and hydrogen peroxide release. Curr Biol.
23:424–429. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Minutti CM, Knipper JA, Allen JE and Zaiss
DM: Tissue-specific contribution of macrophages to wound healing.
Semin Cell Dev Biol. 61:3–11. 2017. View Article : Google Scholar
|
|
40
|
Dutra FF and Bozza MT: Heme on innate
immunity and inflammation. Front Pharmacol. 5:1152014. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Sindrilaru A and Scharffetter-Kochanek K:
Disclosure of the culprits: Macrophages-versatile regulators of
wound healing. Adv Wound Care (New Rochelle). 2:357–368. 2013.
View Article : Google Scholar
|
|
42
|
Gurtner GC, Werner S, Barrandon Y and
Longaker MT: Wound repair and regeneration. Nature. 453:314–321.
2008. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Zhang T, Tai Z, Miao F, Zhao Y, Wang W,
Zhu Q and Chen Z: Bioinspired nanovesicles derived from macrophage
accelerate wound healing by promoting angiogenesis and collagen
deposition. J Mater Chem B. 12:12338–12348. 2024. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Werner S, Krieg T and Smola H:
Keratinocyte-fibroblast interactions in wound healing. J Invest
Dermatol. 127:998–1008. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Rousselle P, Montmasson M and Garnier C:
Extracellular matrix contribution to skin wound
re-epithelialization. Matrix Biol. 75-76:12–26. 2019. View Article : Google Scholar
|
|
46
|
Rousselle P and Beck K: Laminin 332
processing impacts cellular behavior. Cell Adh Migr. 7:122–134.
2013. View Article : Google Scholar :
|
|
47
|
Hamill KJ and McLean WHI: The alpha-3
polypeptide chain of laminin 5: Insight into wound healing
responses from the study of genodermatoses. Clin Exp Dermatol.
30:398–404. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
48
|
Rousselle P, Lunstrum GP, Keene DR and
Burgeson RE: Kalinin: An epithelium-specific basement membrane
adhesion molecule that is a component of anchoring filaments. J
Cell Biol. 114:567–576. 1991. View Article : Google Scholar : PubMed/NCBI
|
|
49
|
Theveneau E and Mayor R: Collective cell
migration of epithelial and mesenchymal cells. Cell Mol Life Sci.
70:3481–3492. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
50
|
Krawczyk WS: A pattern of epidermal cell
migration during wound healing. J Cell Biol. 49:247–263. 1971.
View Article : Google Scholar : PubMed/NCBI
|
|
51
|
Headon D: Reversing stratification during
wound healing. Nat Cell Biol. 19:595–597. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
52
|
Freedberg IM, Tomic-Canic M, Komine M and
Blumenberg M: Keratins and the keratinocyte activation cycle. J
Invest Dermatol. 116:633–640. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
53
|
Veith AP, Henderson K, Spencer A, Sligar
AD and Baker AB: Therapeutic strategies for enhancing angiogenesis
in wound healing. Adv Drug Deliv Rev. 146:97–125. 2019. View Article : Google Scholar :
|
|
54
|
Velnar T and Gradisnik L: Tissue
augmentation in wound healing: The role of endothelial and
epithelial cells. Med Arch. 72:444–448. 2018. View Article : Google Scholar
|
|
55
|
Lamalice L, Le Boeuf F and Huot J:
Endothelial cell migration during angiogenesis. Circ Res.
100:782–794. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
56
|
Al Sadoun H: Macrophage phenotypes in
normal and diabetic wound healing and therapeutic interventions.
Cells. 11:24302022. View Article : Google Scholar : PubMed/NCBI
|
|
57
|
Song J, Wu Y, Chen Y, Sun X and Zhang Z:
Epigenetic regulatory mechanism of macrophage polarization in
diabetic wound healing (Review). Mol Med Rep. 31:22025. View Article : Google Scholar
|
|
58
|
Morbidelli L, Genah S and Cialdai F:
Effect of microgravity on endothelial cell function, angiogenesis,
and vessel remodeling during wound healing. Front Bioeng
Biotechnol. 9:7200912021. View Article : Google Scholar : PubMed/NCBI
|
|
59
|
Hsu S, Thakar R, Liepmann D and Li S:
Effects of shear stress on endothelial cell haptotaxis on
micropatterned surfaces. Biochem Biophys Res Commun. 337:401–409.
2005. View Article : Google Scholar : PubMed/NCBI
|
|
60
|
Li S, Huang NF and Hsu S:
Mechanotransduction in endothelial cell migration. J Cell Biochem.
96:1110–1126. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
61
|
Lynch MD and Watt FM: Fibroblast
heterogeneity: Implications for human disease. J Clin Invest.
128:26–35. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
62
|
Talbott HE, Mascharak S, Griffin M, Wan DC
and Longaker MT: Wound healing, fibroblast heterogeneity, and
fibrosis. Cell Stem Cell. 29:1161–1180. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
63
|
Bussone G: Subjectivity in primary
headaches: Insight the causes. Neurol Sci. 38:1–2. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
64
|
Younesi FS, Miller AE, Barker TH, Rossi
FMV and Hinz B: Fibroblast and myofibroblast activation in normal
tissue repair and fibrosis. Nat Rev Mol Cell Biol. 25:617–638.
2024. View Article : Google Scholar : PubMed/NCBI
|
|
65
|
Shao DD, Suresh R, Vakil V, Gomer RH and
Pilling D: Pivotal advance: Th-1 cytokines inhibit, and Th-2
cytokines promote fibrocyte differentiation. J Leukoc Biol.
83:1323–1333. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
66
|
Grieb G, Steffens G, Pallua N, Bernhagen J
and Bucala R: Circulating fibrocytes-biology and mechanisms in
wound healing and scar formation. Int Rev Cell Mol Biol. 291:1–19.
2011. View Article : Google Scholar
|
|
67
|
Park JG, Lim DC, Park JH, Park S, Mok J,
Kang KW and Park J: Benzbromarone induces targeted degradation of
HSP47 protein and improves hypertrophic scar formation. J Invest
Dermatol. 144:633–644. 2024. View Article : Google Scholar
|
|
68
|
Kohlhauser M, Mayrhofer M, Kamolz LP and
Smolle C: An update on molecular mechanisms of scarring-A narrative
review. Int J Mol Sci. 25:115792024. View Article : Google Scholar : PubMed/NCBI
|
|
69
|
Hinz B: Formation and function of the
myofibroblast during tissue repair. J Invest Dermatol. 127:526–537.
2007. View Article : Google Scholar : PubMed/NCBI
|
|
70
|
Luo L, An Y, Geng K, Wan S, Zhang F, Tan
X, Jiang Z and Xu Y: High glucose-induced endothelial STING
activation inhibits diabetic wound healing through impairment of
angiogenesis. Biochem Biophys Res Commun. 668:82–89. 2023.
View Article : Google Scholar : PubMed/NCBI
|
|
71
|
Huang SM, Wu CS, Chiu MH, Wu CH, Chang YT,
Chen GS and Lan CCE: High glucose environment induces M1 macrophage
polarization that impairs keratinocyte migration via TNF-α: An
important mechanism to delay the diabetic wound healing. J Dermatol
Sci. 96:159–167. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
72
|
Lamers ML, Almeida MES, Vicente-Manzanares
M, Horwitz AF and Santos MF: High glucose-mediated oxidative stress
impairs cell migration. PLoS One. 6:e228652011. View Article : Google Scholar : PubMed/NCBI
|
|
73
|
Brubaker AL, Rendon JL, Ramirez L,
Choudhry MA and Kovacs EJ: Reduced neutrophil chemotaxis and
infiltration contributes to delayed resolution of cutaneous wound
infection with advanced age. J Immunol. 190:1746–1757. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
74
|
den Dekker A, Davis FM, Kunkel SL and
Gallagher KA: Targeting epigenetic mechanisms in diabetic wound
healing. Transl Res. 204:39–50. 2019. View Article : Google Scholar :
|
|
75
|
Kasuya A and Tokura Y: Attempts to
accelerate wound healing. J Dermatol Sci. 76:169–172. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
76
|
Lan CCE, Liu IH, Fang AH, Wen CH and Wu
CS: Hyperglycaemic conditions decrease cultured keratinocyte
mobility: Implications for impaired wound healing in patients with
diabetes. Br J Dermatol. 159:1103–1115. 2008.PubMed/NCBI
|
|
77
|
Zhou P, Feng H, Qin W and Li Q: KRT17 from
skin cells with high glucose stimulation promotes keratinocytes
proliferation and migration. Front Endocrinol (Lausanne).
14:12370482023. View Article : Google Scholar : PubMed/NCBI
|
|
78
|
Worsley AL, Lui DH, Ntow-Boahene W, Song
W, Good L and Tsui J: The importance of inflammation control for
the treatment of chronic diabetic wounds. Int Wound J.
20:2346–2359. 2023. View Article : Google Scholar :
|
|
79
|
Li M, Wang T, Tian H, Wei G, Zhao L and
Shi Y: Macrophage-derived exosomes accelerate wound healing through
their anti-inflammation effects in a diabetic rat model. Artif
Cells Nanomed Biotechnol. 47:3793–3803. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
80
|
Nirenjen S, Narayanan J, Tamilanban T,
Subramaniyan V, Chitra V, Fuloria NK, Wong LS, Ramachawolran G,
Sekar M, Gupta G, et al: Exploring the contribution of
pro-inflammatory cytokines to impaired wound healing in diabetes.
Front Immunol. 14:12163212023. View Article : Google Scholar : PubMed/NCBI
|
|
81
|
Goren I, Müller E, Schiefelbein D,
Christen U, Pfeilschifter J, Mühl H and Frank S: Systemic
anti-TNFalpha treatment restores diabetes-impaired skin repair in
ob/ob mice by inactivation of macrophages. J Invest Dermatol.
127:2259–2267. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
82
|
Mirza RE, Fang MM, Ennis WJ and Koh TJ:
Blocking interleukin-1β induces a healing-associated wound
macrophage phenotype and improves healing in type 2 diabetes.
Diabetes. 62:2579–2587. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
83
|
Xu F, Zhang C and Graves DT: Abnormal cell
responses and role of TNF-α in impaired diabetic wound healing.
Biomed Res Int. 2013:7548022013. View Article : Google Scholar
|
|
84
|
Dai J, Shen J, Chai Y and Chen H: IL-1β
impaired diabetic wound healing by regulating MMP-2 and MMP-9
through the p38 pathway. Mediators Inflamm. 2021:66457662021.
View Article : Google Scholar
|
|
85
|
Lan CCE, Wu CS, Huang SM, Wu IH and Chen
GS: High-glucose environment enhanced oxidative stress and
increased interleukin-8 secretion from keratinocytes: New insights
into impaired diabetic wound healing. Diabetes. 62:2530–2538. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
86
|
Wang G, Yang F, Zhou W, Xiao N, Luo M and
Tang Z: The initiation of oxidative stress and therapeutic
strategies in wound healing. Biomed Pharmacother. 157:1140042023.
View Article : Google Scholar
|
|
87
|
Xu Q, Huff LP, Fujii M and Griendling KK:
Redox regulation of the actin cytoskeleton and its role in the
vascular system. Free Radic Biol Med. 109:84–107. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
88
|
Long M, de la Vega MR, Wen Q, Wen Q,
Bharara M, Jiang T, Zhang R, Zhou S, Wong PK, Wondrak GT, et al: An
essential role of NRF2 in diabetic wound healing. Diabetes.
65:780–793. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
89
|
Zhou X, Ruan Q, Ye Z, Chu Z, Xi M, Li M,
Hu W, Guo X, Yao P and Xie W: Resveratrol accelerates wound healing
by attenuating oxidative stress-induced impairment of cell
proliferation and migration. Burns. 47:133–139. 2021. View Article : Google Scholar
|
|
90
|
Gao M, Nguyen TT, Suckow MA, Wolter WR,
Gooyit M, Mobashery S and Chang M: Acceleration of diabetic wound
healing using a novel protease-anti-protease combination therapy.
Proc Natl Acad Sci USA. 112:15226–15231. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
91
|
Jones JI, Nguyen TT, Peng Z and Chang M:
Targeting MMP-9 in diabetic foot ulcers. Pharmaceuticals (Basel).
12:792019. View Article : Google Scholar : PubMed/NCBI
|
|
92
|
Yabluchanskiy A, Ma Y, Iyer RP, Hall ME
and Lindsey ML: Matrix metalloproteinase-9: Many shades of function
in cardiovascular disease. Physiology (Bethesda). 28:391–403.
2013.PubMed/NCBI
|
|
93
|
Ambrozova N, Ulrichova J and Galandakova
A: Models for the study of skin wound healing. The role of Nrf2 and
NF-κB. Biomed Pap Med Fac Univ Palacky Olomouc Czech Repub.
161:1–13. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
94
|
Zubair M and Ahmad J: Role of growth
factors and cytokines in diabetic foot ulcer healing: A detailed
review. Rev Endocr Metab Disord. 20:207–217. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
95
|
Papanas N and Maltezos E: Growth factors
in the treatment of diabetic foot ulcers: New technologies, any
promises? Int J Low Extrem Wounds. 6:37–53. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
96
|
Ong HT and Dilley RJ: Novel non-angiogenic
role for mesenchymal stem cell-derived vascular endothelial growth
factor on keratinocytes during wound healing. Cytokine Growth
Factor Rev. 44:69–79. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
97
|
Ou MY, Tan PC, Xie Y, Liu K, Gao YM, Yang
XS, Zhou SB and Li QF: Dedifferentiated Schwann cell-derived TGF-β3
is essential for the neural system to promote wound healing.
Theranostics. 12:5470–5487. 2022. View Article : Google Scholar :
|
|
98
|
R AS, Nambi N, Radhakrishnan L, Prasad MK
and Ramkumar KM: Neutrophil migration is a crucial factor in wound
healing and the pathogenesis of diabetic foot ulcers: Insights into
pharmacological interventions. Curr Vasc Pharmacol. 30: View Article : Google Scholar : 2024.
|
|
99
|
Michopoulou A, Montmasson M, Garnier C,
Lambert E, Dayan G and Rousselle P: A novel mechanism in wound
healing: Laminin 332 drives MMP9/14 activity by recruiting
syndecan-1 and CD44. Matrix Biol. 94:1–17. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
100
|
Fang WC and Lan CCE: The epidermal
keratinocyte as a therapeutic target for management of diabetic
wounds. Int J Mol Sci. 24:42902023. View Article : Google Scholar : PubMed/NCBI
|
|
101
|
Pilcher BK, Dumin JA, Sudbeck BD, Krane
SM, Welgus HG and Parks WC: The activity of collagenase-1 is
required for keratinocyte migration on a type I collagen matrix. J
Cell Biol. 137:1445–1457. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
102
|
Lan CCE, Wu CS, Kuo HY, Huang SM and Chen
GS: Hyperglycaemic conditions hamper keratinocyte locomotion via
sequential inhibition of distinct pathways: New insights on poor
wound closure in patients with diabetes. Br J Dermatol.
160:1206–1214. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
103
|
Zhang C, Ponugoti B, Tian C, Xu F,
Tarapore R, Batres A, Alsadun S, Lim J, Dong G and Graves DT: FOXO1
differentially regulates both normal and diabetic wound healing. J
Cell Biol. 209:289–303. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
104
|
Wang Y and Graves DT: Keratinocyte
function in normal and diabetic wounds and modulation by FOXO1. J
Diabetes Res. 2020:37147042020. View Article : Google Scholar : PubMed/NCBI
|
|
105
|
Kaussikaa S, Prasad MK and Ramkumar KM:
Nrf2 activation in keratinocytes: A central role in
diabetes-associated wound healing. Exp Dermatol. 33:e151892024.
View Article : Google Scholar : PubMed/NCBI
|
|
106
|
da Silva L, Carvalho E and Cruz MT: Role
of neuropeptides in skin inflammation and its involvement in
diabetic wound healing. Expert Opin Biol Ther. 10:1427–1439. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
107
|
Pradhan L, Nabzdyk C, Andersen ND, LoGerfo
FW and Veves A: Inflammation and neuropeptides: The connection in
diabetic wound healing. Expert Rev Mol Med. 11:e22009. View Article : Google Scholar : PubMed/NCBI
|
|
108
|
Grande R, Puca V and Muraro R: Antibiotic
resistance and bacterial biofilm. Expert Opin Ther Pat. 30:897–900.
2020. View Article : Google Scholar : PubMed/NCBI
|
|
109
|
Marano RJ, Wallace HJ, Wijeratne D, Fear
MW, Wong HS and O'Handley R: Secreted biofilm factors adversely
affect cellular wound healing responses in vitro. Sci Rep.
5:132962015. View Article : Google Scholar
|
|
110
|
Xing H, Huang Y, Kunkemoeller BH, Dahl PJ,
Muraleetharan O, Malvankar NS, Murrell MP and Kyriakides TR:
Dysregulation of TSP2-Rac1-WAVE2 axis in diabetic cells leads to
cytoskeletal disorganization, increased cell stiffness, and
dysfunction. Sci Rep. 12:224742022. View Article : Google Scholar : PubMed/NCBI
|
|
111
|
Dupont S, Morsut L, Aragona M, Enzo E,
Giulitti S, Cordenonsi M, Zanconato F, Le Digabel J, Forcato M,
Bicciato S, et al: Role of YAP/TAZ in mechanotransduction. Nature.
474:179–183. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
112
|
Brusatin G, Panciera T, Gandin A, Citron A
and Piccolo S: Biomaterials and engineered microenvironments to
control YAP/TAZ-dependent cell behaviour. Nat Mater. 17:1063–1075.
2018. View Article : Google Scholar : PubMed/NCBI
|
|
113
|
Lin MO, Sampath D, Bosykh DA, Wang C, Wang
X, Subramaniam T, Han W, Hong W and Chakraborty S: YAP/TAZ drive
agrin-matrix metalloproteinase 12-mediated diabetic skin wound
healing. J Invest Dermatol. 145:155–170.e2. 2025. View Article : Google Scholar
|
|
114
|
Majumdar R, Steen K, Coulombe PA and
Parent CA: Non-canonical processes that shape the cell migration
landscape. Curr Opin Cell Biol. 57:123–134. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
115
|
Xie L, Feng H, Li S, Meng G, Liu S, Tang
X, Ma Y, Han Y, Xiao Y, Gu Y, et al: SIRT3 mediates the antioxidant
effect of hydrogen sulfide in endothelial cells. Antioxid Redox
Signal. 24:329–343. 2016. View Article : Google Scholar :
|
|
116
|
Meng G, Liu J, Liu S, Song Q, Liu L, Xie
L, Han Y and Ji Y: Hydrogen sulfide pretreatment improves
mitochondrial function in myocardial hypertrophy via a
SIRT3-dependent manner. Br J Pharmacol. 175:1126–1145. 2018.
View Article : Google Scholar
|
|
117
|
Yang S, Xu M, Meng G and Lu Y: SIRT3
deficiency delays diabetic skin wound healing via oxidative stress
and necroptosis enhancement. J Cell Mol Med. 24:4415–4427. 2020.
View Article : Google Scholar : PubMed/NCBI
|
|
118
|
Johan MZ, Pyne NT, Kolesnikoff N,
Poltavets V, Esmaeili Z, Woodcock JM, Lopez AF, Cowin AJ, Pitson SM
and Samuel MS: Accelerated closure of diabetic wounds by efficient
recruitment of fibroblasts upon inhibiting a 14-3-3/ROCK regulatory
axis. J Invest Dermatol. 144:2562–2573.e4. 2024. View Article : Google Scholar : PubMed/NCBI
|
|
119
|
Li Z, Zhang C, Wang L, Zhang Q, Dong Y,
Sha X, Wang B, Zhu Z, Wang W, Wang Y, et al: Chitooligosaccharides
promote diabetic wound healing by mediating fibroblast
proliferation and migration. Sci Rep. 15:5562025. View Article : Google Scholar : PubMed/NCBI
|
|
120
|
Gao Q, Pan L, Li Y and Zhang X:
Astragaloside IV attenuates high glucose-induced human
keratinocytes injury via TGF-β/Smad signaling pathway. J Tissue
Viability. 31:678–686. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
121
|
Dong M, Ma X and Li F: Dedifferentiated
fat cells-derived exosomes (DFATs-Exos) loaded in GelMA accelerated
diabetic wound healing through Wnt/β-catenin pathway. Stem Cell Res
Ther. 16:1032025. View Article : Google Scholar
|
|
122
|
Ridley AJ: Rho GTPase signalling in cell
migration. Curr Opin Cell Biol. 36:103–112. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
123
|
Wu M, Zhang S, Zhang W, Zhou Y, Guo Z,
Fang Y, Yang Y, Shen Z, Lian D, Shen A and Peng J: Qingda granule
ameliorates vascular remodeling and phenotypic transformation of
adventitial fibroblasts via suppressing the TGF-β1/Smad2/3 pathway.
J Ethnopharmacol. 313:1165352023. View Article : Google Scholar
|
|
124
|
Fang H, Yang S, Luo Y, Zhang C, Rao Y, Liu
R, Feng Y and Yu J: Notoginsenoside R1 inhibits vascular smooth
muscle cell proliferation, migration and neointimal hyperplasia
through PI3K/Akt signaling. Sci Rep. 8:75952018. View Article : Google Scholar : PubMed/NCBI
|
|
125
|
Carvalho JR, Fortunato IC, Fonseca CG,
Pezzarossa A, Barbacena P, Dominguez-Cejudo MA, Vasconcelos FF,
Santos NC, Carvalho FA and Franco CA: Non-canonical Wnt signaling
regulates junctional mechanocoupling during angiogenic collective
cell migration. Elife. 8:e458532019. View Article : Google Scholar : PubMed/NCBI
|
|
126
|
Van den Broeke C and Favoreel HW: Actin'
up: Herpesvirus Interactions with Rho GTPase signaling. Viruses.
3:278–292. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
127
|
Qian W, Yamaguchi N, Lis P, Cammer M and
Knaut H: Pulses of RhoA signaling stimulate actin polymerization
and flow in protrusions to drive collective cell migration. Curr
Biol. 34:245–259.e8. 2024. View Article : Google Scholar :
|
|
128
|
Takaya K, Imbe Y, Wang Q, Okabe K, Sakai
S, Aramaki-Hattori N and Kishi K: Rac1 inhibition regenerates
wounds in mouse fetuses via altered actin dynamics. Sci Rep.
14:272132024. View Article : Google Scholar : PubMed/NCBI
|
|
129
|
Shih PC, Tzeng IS, Chen YC and Chen ML:
Gastrodin mitigates ketamine-induced inhibition of F-actin
remodeling and cell migration by regulating the rho signaling
pathway. Biomedicines. 13:6492025. View Article : Google Scholar : PubMed/NCBI
|
|
130
|
Lawson CD and Ridley AJ: Rho GTPase
signaling complexes in cell migration and invasion. J Cell Biol.
217:447–457. 2018. View Article : Google Scholar :
|
|
131
|
Xue C, Li G, Lu J and Li L: Crosstalk
between circRNAs and the PI3K/AKT signaling pathway in cancer
progression. Signal Transduct Target Ther. 6:4002021. View Article : Google Scholar : PubMed/NCBI
|
|
132
|
Teng Y, Fan Y, Ma J, Lu W, Liu N, Chen Y,
Pan W and Tao X: The PI3K/Akt pathway: Emerging roles in skin
homeostasis and a group of non-malignant skin disorders. Cells.
10:12192021. View Article : Google Scholar : PubMed/NCBI
|
|
133
|
Zhang X, Shi L, Xing M, Li C, Ma F and Ma
Y and Ma Y: Interplay between lncRNAs and the PI3K/AKT signaling
pathway in the progression of digestive system neoplasms (Review).
Int J Mol Med. 55:152024. View Article : Google Scholar : PubMed/NCBI
|
|
134
|
Karar J and Maity A: PI3K/AKT/mTOR pathway
in angiogenesis. Front Mol Neurosci. 4:512011. View Article : Google Scholar : PubMed/NCBI
|
|
135
|
Wu X, Sun Q, He S, Wu Y, Du S, Gong L, Yu
J and Guo H: Ropivacaine inhibits wound healing by suppressing the
proliferation and migration of keratinocytes via the PI3K/AKT/mTOR
pathway. BMC Anesthesiol. 22:1062022. View Article : Google Scholar : PubMed/NCBI
|
|
136
|
Deng S, Leong HC, Datta A, Gopal V, Kumar
AP and Yap CT: PI3K/AKT signaling tips the balance of cytoskeletal
forces for cancer progression. Cancers (Basel). 14:16522022.
View Article : Google Scholar : PubMed/NCBI
|
|
137
|
Hsu JW, Bai M, Li K, Yang JS, Chu N, Cole
PA, Eck MJ, Li J and Hsu VW: The protein kinase Akt acts as a coat
adaptor in endocytic recycling. Nat Cell Biol. 22:927–933. 2020.
View Article : Google Scholar : PubMed/NCBI
|
|
138
|
Hannigan M, Zhan L, Li Z, Ai Y, Wu D and
Huang CK: Neutrophils lacking phosphoinositide 3-kinase gamma show
loss of directionality during N-formyl-Met-Leu-Phe-induced
chemotaxis. Proc Natl Acad Sci USA. 99:3603–3608. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
139
|
Najafi M, Farhood B and Mortezaee K:
Extracellular matrix (ECM) stiffness and degradation as cancer
drivers. J Cell Biochem. 120:2782–2790. 2019. View Article : Google Scholar
|
|
140
|
Nakerakanti S and Trojanowska M: The role
of TGF-β receptors in fibrosis. Open Rheumatol J. 6:156–162. 2012.
View Article : Google Scholar :
|
|
141
|
Tu S, Huang W, Huang C, Luo Z and Yan X:
Contextual regulation of TGF-β signaling in liver cancer. Cells.
8:12352019. View Article : Google Scholar
|
|
142
|
Zhang K, Zhang H, Xiang H, Liu J, Liu Y,
Zhang X, Wang J and Tang Y: TGF-β1 induces the dissolution of tight
junctions in human renal proximal tubular cells: role of the
RhoA/ROCK signaling pathway. Int J Mol Med. 32:464–468. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
143
|
Gailit J, Welch MP and Clark RA: TGF-beta
1 stimulates expression of keratinocyte integrins during
re-epithelialization of cutaneous wounds. J Invest Dermatol.
103:221–227. 1994. View Article : Google Scholar : PubMed/NCBI
|
|
144
|
Ponugoti B, Xu F, Zhang C, Tian C, Pacios
S and Graves DT: FOXO1 promotes wound healing through the
up-regulation of TGF-β1 and prevention of oxidative stress. J Cell
Biol. 203:327–343. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
145
|
Weber CE, Li NY, Wai PY and Kuo PC:
Epithelial-Mesenchymal transition, TGF-β, and osteopontin in wound
healing and tissue remodeling after injury. J Burn Care Res.
33:311–318. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
146
|
Song P, Gao Z, Bao Y, Chen L, Huang Y, Liu
Y, Dong Q and Wei X: Wnt/β-catenin signaling pathway in
carcinogenesis and cancer therapy. J Hematol Oncol. 17:462024.
View Article : Google Scholar
|
|
147
|
Moon RT, Kohn AD, De Ferrari GV and Kaykas
A: WNT and beta-catenin signalling: Diseases and therapies. Nat Rev
Genet. 5:691–701. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
148
|
Nusse R and Clevers H: Wnt/β-Catenin
signaling, disease, and emerging therapeutic modalities. Cell.
169:985–999. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
149
|
Schaale K, Neumann J, Schneider D, Ehlers
S and Reiling N: Wnt signaling in macrophages: Augmenting and
inhibiting mycobacteria-induced inflammatory responses. Eur J Cell
Biol. 90:553–559. 2011. View Article : Google Scholar
|
|
150
|
Sedgwick AE and D'Souza-Schorey C: Wnt
signaling in cell motility and invasion: Drawing parallels between
development and cancer. Cancers (Basel). 8:802016. View Article : Google Scholar : PubMed/NCBI
|
|
151
|
Wu B, Crampton SP and Hughes CCW: Wnt
signaling induces MMP expression and regulates T cell
transmigration. Immunity. 26:227–239. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
152
|
Xue W, Yang L, Chen C, Ashrafizadeh M,
Tian Y and Sun R: Wnt/β-catenin-driven EMT regulation in human
cancers. Cell Mol Life Sci. 81:792024. View Article : Google Scholar
|
|
153
|
Ono M, Masaki A, Maeda A, Kilts TM, Hara
ES, Komori T, Pham H, Kuboki T and Young MF: CCN4/WISP1 controls
cutaneous wound healing by modulating proliferation, migration and
ECM expression in dermal fibroblasts via α5β1 and TNFα. Matrix
Biol. 68-69:533–546. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
154
|
Repertinger SK, Campagnaro E, Fuhrman J,
El-Abaseri T, Yuspa SH and Hansen LA: EGFR enhances early healing
after cutaneous incisional wounding. J Invest Dermatol.
123:982–989. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
155
|
Wang Y, Wu Z, Tian J, Mi Y, Ren X, Kang J,
Zhang W, Zhou X, Wang G and Li R: Intermedin protects HUVECs from
ischemia reperfusion injury via Wnt/β-catenin signaling pathway.
Ren Fail. 41:159–166. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
156
|
Jun EK, Zhang Q, Yoon BS, Moon JH, Lee G,
Park G, Kang PJ, Lee JH, Kim A and You S: Hypoxic conditioned
medium from human amniotic fluid-derived mesenchymal stem cells
accelerates skin wound healing through TGF-β/SMAD2 and PI3K/Akt
pathways. Int J Mol Sci. 15:605–628. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
157
|
Toraldo G, Bhasin S, Bakhit M, Guo W,
Serra C, Safer JD, Bhawan J and Jasuja R: Topical androgen
antagonism promotes cutaneous wound healing without systemic
androgen deprivation by blocking β-catenin nuclear translocation
and cross-talk with TGF-β signaling in keratinocytes. Wound Repair
Regen. 20:61–73. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
158
|
Wang H, Wang X, Liu X, Zhou J, Yang Q,
Chai B, Chai Y, Ma Z and Lu S: miR-199a-5p plays a pivotal role on
wound healing via suppressing VEGFA and ROCK1 in diabetic ulcer
foot. Oxid Med Cell Longev. 2022:47910592022. View Article : Google Scholar : PubMed/NCBI
|
|
159
|
Yu J, Nam D and Park KS: Substance P
enhances cellular migration and inhibits senescence in human dermal
fibroblasts under hyperglycemic conditions. Biochem Biophys Res
Commun. 522:917–923. 2020. View Article : Google Scholar
|
|
160
|
Huang J, Deng Q, Tsang LL, Chang G, Guo J,
Ruan YC, Wang CC, Li G, Chan HF, Zhang X and Jiang X: Mesenchymal
stem cells from perinatal tissues promote diabetic wound healing
via PI3K/AKT activation. Stem Cell Res Ther. 16:592025. View Article : Google Scholar : PubMed/NCBI
|
|
161
|
Xu S, Jiang C, Yu T and Chen K: A
multi-purpose dressing based on resveratrol-loaded ionic
liquids/gelatin methacryloyl hydrogel for enhancing diabetic wound
healing. Int J Biol Macromol. 283:1367732024. View Article : Google Scholar : PubMed/NCBI
|
|
162
|
Peng Y, Wu S, Tang Q, Li S and Peng C:
KGF-1 accelerates wound contraction through the TGF-β1/Smad
signaling pathway in a double-paracrine manner. J Biol Chem.
294:8361–8370. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
163
|
Oyebode OA and Houreld NN:
Photobiomodulation at 830 nm stimulates migration, survival and
proliferation of fibroblast cells. Diabetes Metab Syndr Obes.
15:2885–2900. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
164
|
Wang L, Chen J, Song J, Xiang Y, Yang M,
Xia L, Yang J, Hou X, Chen L and Wang L: Activation of the
Wnt/β-catenin signalling pathway enhances exosome production by
hucMSCs and improves their capability to promote diabetic wound
healing. J Nanobiotechnology. 22:3732024. View Article : Google Scholar
|
|
165
|
Liu Z, Yang S, Li X, Wang S, Zhang T, Huo
N, Duan R, Shi Q, Zhang J and Xu J: Local transplantation of
GMSC-derived exosomes to promote vascularized diabetic wound
healing by regulating the Wnt/β-catenin pathways. Nanoscale Adv.
5:916–926. 2023. View Article : Google Scholar : PubMed/NCBI
|
|
166
|
Lv Q, Deng J, Chen Y, Wang Y, Liu B and
Liu J: Engineered human adipose stem-cell-derived exosomes loaded
with miR-21-5p to promote diabetic cutaneous wound healing. Mol
Pharm. 17:1723–1733. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
167
|
Yang Y, GuangXuan H, GenMeng W, MengHuan
L, Bo C and XueJie Y: Idiopathic inflammatory myopathy and
non-coding RNA. Front Immunol. 14:12279452023. View Article : Google Scholar : PubMed/NCBI
|
|
168
|
Ballarino M, Morlando M, Fatica A and
Bozzoni I: Non-coding RNAs in muscle differentiation and
musculoskeletal disease. J Clin Invest. 126:2021–2030. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
169
|
Chen L, Shen S and Wang S: LncRNA SNHG16
knockdown promotes diabetic foot ulcer wound healing via sponging
MiR-31-5p. Tohoku J Exp Med. 261:283–289. 2023. View Article : Google Scholar : PubMed/NCBI
|
|
170
|
Peng J, Zhu H, Ruan B, Duan Z and Cao M:
miR-155 promotes m6A modification of SOX2 mRNA through targeted
regulation of HIF-1α and delays wound healing in diabetic foot
ulcer in vitro models. J Diabetes Investig. 16:60–71. 2024.
View Article : Google Scholar
|
|
171
|
Tian M, Tang J, Huang R, Dong J and Jia H:
Circ_072697 knockdown promotes advanced glycation end
products-induced cell proliferation and migration in HaCaT cells
via miR-3150a-3p/KDM2A axis. BMC Endocr Disord. 23:2002023.
View Article : Google Scholar : PubMed/NCBI
|
|
172
|
Wang F, Mei X, Yang Y, Zhang H, Li Z, Zhu
L, Deng S and Wang Y: Non-coding RNA and its network in the
pathogenesis of myasthenia gravis. Front Mol Biosci.
11:13884762024. View Article : Google Scholar : PubMed/NCBI
|
|
173
|
Bhan A and Mandal SS: Long noncoding RNAs:
Emerging stars in gene regulation, epigenetics and human disease.
ChemMedChem. 9:1932–1956. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
174
|
Hong W, Xiong Z, Wang X, Liao X, Liu M,
Jiang Z, Min D, Li J, Guo G and Fu Z: Long noncoding RNA XIST
promotes cell proliferation and migration in diabetic foot ulcers
through the miR-126-3p/EGFR axis. Diabetol Metab Syndr. 16:352024.
View Article : Google Scholar : PubMed/NCBI
|
|
175
|
He M, Tu L, Shu R, Meng Q, Du S, Xu Z and
Wang S: Long noncoding RNA CASC2 facilitated wound healing through
miRNA-155/HIF-1α in diabetic foot ulcers. Contrast Media Mol
Imaging. 2022:62914972022. View Article : Google Scholar
|
|
176
|
Li B, Zhou Y, Chen J, Wang T, Li Z, Fu Y,
Zhai A and Bi C: Long noncoding RNA H19 acts as a miR-29b sponge to
promote wound healing in diabetic foot ulcer. FASEB J.
35:e205262021.
|
|
177
|
Hu M, Wu Y, Yang C, Wang X, Wang W, Zhou
L, Zeng T, Zhou J, Wang C, Lao G, et al: Novel long noncoding RNA
lnc-URIDS delays diabetic wound healing by targeting Plod1.
Diabetes. 69:2144–2156. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
178
|
Gezer U, Özgür E, Cetinkaya M, Isin M and
Dalay N: Long non-coding RNAs with low expression levels in cells
are enriched in secreted exosomes. Cell Biol Int. 38:1076–1079.
2014. View Article : Google Scholar : PubMed/NCBI
|
|
179
|
Li B, Luan S, Chen J, Zhou Y, Wang T, Li
Z, Fu Y, Zhai A and Bi C: The MSC-derived exosomal lncRNA H19
promotes wound healing in diabetic foot ulcers by upregulating PTEN
via MicroRNA-152-3p. Mol Ther Nucleic Acids. 19:814–826. 2020.
View Article : Google Scholar : PubMed/NCBI
|
|
180
|
Han ZF, Cao JH, Liu ZY, Yang Z, Qi RX and
Xu HL: Exosomal lncRNA KLF3-AS1 derived from bone marrow
mesenchymal stem cells stimulates angiogenesis to promote diabetic
cutaneous wound healing. Diabetes Res Clin Pract. 183:1091262022.
View Article : Google Scholar
|
|
181
|
Fu W, Liang D, Wu X, Chen H, Hong X, Wang
J, Zhu T, Zeng T, Lin W, Chen S, et al: Long noncoding RNA
LINC01435 impedes diabetic wound healing by facilitating
YY1-mediated HDAC8 expression. iScience. 25:1040062022. View Article : Google Scholar : PubMed/NCBI
|
|
182
|
Roso-Mares A, Andújar I, Corpas TD and Sun
BK: Non-coding RNAs as skin disease biomarkers, molecular
signatures, and therapeutic targets. Hum Genet. 143:801–812. 2024.
View Article : Google Scholar
|
|
183
|
Shirley SN, Watson AE and Yusuf N:
Pathogenesis of inflammation in skin disease: From molecular
mechanisms to pathology. Int J Mol Sci. 25:101522024. View Article : Google Scholar : PubMed/NCBI
|
|
184
|
Tsai HC, Chang GRL, Tung MC, Tu MY, Chen
IC, Liu YH, Cidem A and Chen CM: MicroRNA signature in an in vitro
keratinocyte model of diabetic wound healing. Int J Mol Sci.
25:101252024. View Article : Google Scholar : PubMed/NCBI
|
|
185
|
Zhao X, Xu M, Tang Y, Xie D, Deng L, Chen
M and Wang Y: Decreased expression of miR-204-3p in peripheral
blood and wound margin tissue associated with the onset and poor
wound healing of diabetic foot ulcers. Int Wound J. 20:413–429.
2023. View Article : Google Scholar
|
|
186
|
Zhang HC, Wen T and Cai YZ: Overexpression
of miR-146a promotes cell proliferation and migration in a model of
diabetic foot ulcers by regulating the AKAP12 axis. Endocr J.
69:85–94. 2022. View Article : Google Scholar
|
|
187
|
Wang W, Yang C, Wang XY, Zhou LY, Lao GJ,
Liu D, Wang C, Hu MD, Zeng TT, Yan L and Ren M: MicroRNA-129 and
-335 promote diabetic wound healing by inhibiting Sp1-mediated
MMP-9 expression. Diabetes. 67:1627–1638. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
188
|
Li P, Hong G, Zhan W, Deng M, Tu C, Wei J
and Lin H: Endothelial progenitor cell derived exosomes mediated
miR-182-5p delivery accelerate diabetic wound healing via
down-regulating PPARG. Int J Med Sci. 20:468–481. 2023. View Article : Google Scholar : PubMed/NCBI
|
|
189
|
Wang KX, Zhao LL, Zheng LT, Meng LB, Jin
L, Zhang LJ, Kong FL and Liang F: Accelerated wound healing in
diabetic rat by miRNA-185-5p and its anti-inflammatory activity.
Diabetes Metab Syndr Obes. 16:1657–1667. 2023. View Article : Google Scholar : PubMed/NCBI
|
|
190
|
Wu M, Tu J, Huang J, Wen H, Zeng Y and Lu
Y: Exosomal IRF1-loaded rat adipose-derived stem cell sheet
contributes to wound healing in the diabetic foot ulcers. Mol Med.
29:602023. View Article : Google Scholar : PubMed/NCBI
|
|
191
|
Wu Y, Zhang K, Liu R, Zhang H, Chen D, Yu
S, Chen W, Wan S, Zhang Y, Jia Z, et al: MicroRNA-21-3p accelerates
diabetic wound healing in mice by downregulating SPRY1. Aging
(Albany NY). 12:15436–15445. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
192
|
Wang C, Huang L, Li J, Liu D and Wu B:
MicroRNA miR-145-5p inhibits cutaneous wound healing by targeting
PDGFD in diabetic foot ulcer. Biochem Genet. 62:2437–2454. 2024.
View Article : Google Scholar
|
|
193
|
Zhao X, Xu M, Tang Y, Xie D, Wang Y and
Chen M: Changes in miroRNA-103 expression in wound margin tissue
are related to wound healing of diabetes foot ulcers. Int Wound J.
20:467–483. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
194
|
Zhang P, Song X, Dong Q, Zhou L and Wang
L: miR-27-3p inhibition restore fibroblasts viability in diabetic
wound by targeting NOVA1. Aging (Albany NY). 12:12841–12849. 2020.
View Article : Google Scholar : PubMed/NCBI
|
|
195
|
Zheng L, Song H, Li Y, Li H, Lin G and Cai
Z: Insulin-Induced gene 1-enhance secretion of BMSC exosome
enriched in miR-132-3p promoting wound healing in diabetic mice.
Mol Pharm. 21:4372–4385. 2024. View Article : Google Scholar : PubMed/NCBI
|
|
196
|
Zuo C, Fan P, Yang Y and Hu C: MiR-488-3p
facilitates wound healing through CYP1B1-mediated Wnt/β-catenin
signaling pathway by targeting MeCP2. J Diabetes Investig.
15:145–158. 2024. View Article : Google Scholar
|
|
197
|
Huang H, Zhu W, Huang Z, Zhao D, Cao L and
Gao X: Adipose-derived stem cell exosome NFIC improves diabetic
foot ulcers by regulating miR-204-3p/HIPK2. J Orthop Surg Res.
18:6872023. View Article : Google Scholar : PubMed/NCBI
|
|
198
|
Qiu ZY, Xu WC and Liang ZH: Bone marrow
mesenchymal stem cell-derived exosomal miR-221-3p promotes
angiogenesis and wound healing in diabetes via the downregulation
of forkhead box P1. Diabet Med. 41:e153862024. View Article : Google Scholar : PubMed/NCBI
|
|
199
|
Zhou X, Ye C, Jiang L, Zhu X, Zhou F, Xia
M and Chen Y: The bone mesenchymal stem cell-derived exosomal
miR-146a-5p promotes diabetic wound healing in mice via macrophage
M1/M2 polarization. Mol Cell Endocrinol. 579:1120892024. View Article : Google Scholar
|
|
200
|
Guo E, Wang L, Wu J and Chen Q: Exosomes
from MicroRNA-125b-modified adipose-derived stem cells promote
wound healing of diabetic foot ulcers. Curr Stem Cell Res Ther.
20:409–420. 2024. View Article : Google Scholar : PubMed/NCBI
|
|
201
|
Che D, Xiang X, Xie J, Chen Z, Bao Q and
Cao D: exosomes derived from adipose stem cells enhance
angiogenesis in diabetic wound via miR-146a-5p/JAZF1 axis. Stem
Cell Rev Rep. 20:1026–1039. 2024. View Article : Google Scholar : PubMed/NCBI
|
|
202
|
Ge L, Wang K, Lin H, Tao E, Xia W, Wang F,
Mao C and Feng Y: Engineered exosomes derived from
miR-132-overexpresssing adipose stem cells promoted diabetic wound
healing and skin reconstruction. Front Bioeng Biotechnol.
11:11295382023. View Article : Google Scholar : PubMed/NCBI
|
|
203
|
Sedykh S, Kuleshova A and Nevinsky G: Milk
exosomes: Perspective agents for anticancer drug delivery. Int J
Mol Sci. 21:66462020. View Article : Google Scholar : PubMed/NCBI
|
|
204
|
Munagala R, Aqil F, Jeyabalan J and Gupta
RC: Bovine milk-derived exosomes for drug delivery. Cancer Lett.
371:48–61. 2016. View Article : Google Scholar :
|
|
205
|
Yan C, Chen J, Wang C, Yuan M, Kang Y, Wu
Z, Li W, Zhang G, Machens HG and Rinkevich Y: Milk
exosomes-mediated miR-31-5p delivery accelerates diabetic wound
healing through promoting angiogenesis. Drug Deliv. 29:214–228.
2022. View Article : Google Scholar : PubMed/NCBI
|
|
206
|
Liu R, Zhang L, Zhao X, Liu J, Chang W,
Zhou L and Zhang K: circRNA: Regulatory factors and potential
therapeutic targets in inflammatory dermatoses. J Cell Mol Med.
26:4389–4400. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
207
|
Beňačka R, Szabóová D, Guľašová Z and
Hertelyová Z: Non-Coding RNAs in breast cancer: Diagnostic and
therapeutic implications. Int J Mol Sci. 26:1272024. View Article : Google Scholar
|
|
208
|
Zhang Y, Wu Y, Liu Z, Yang K, Lin H and
Xiong K: Non-coding RNAs as potential targets in metformin therapy
for cancer. Cancer Cell Int. 24:3332024. View Article : Google Scholar : PubMed/NCBI
|
|
209
|
Fu Z, Jiang Z, Liao X, Liu M, Guo G, Wang
X, Yang G, Zhou Z, Hu L and Xiong Z: Upregulation of circ_0080968
in diabetic foot ulcer inhibits wound healing via repressing the
migration and promoting proliferation of keratinocytes. Gene.
883:1476692023. View Article : Google Scholar : PubMed/NCBI
|
|
210
|
Han D, Liu W, Li G and Liu L: Circ_PRKDC
knockdown promotes skin wound healing by enhancing keratinocyte
migration via miR-31/FBN1 axis. J Mol Histol. 52:681–691. 2021.
View Article : Google Scholar : PubMed/NCBI
|
|
211
|
Wang A, Toma MA, Ma J, Li D, Vij M, Chu T,
Wang J, Li X and Landén NX: Circular RNA hsa_circ_0084443 is
upregulated in diabetic foot ulcer and modulates keratinocyte
migration and proliferation. Adv Wound Care (New Rochelle).
9:145–160. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
212
|
Liang ZH, Pan NF, Lin SS, Qiu ZY, Liang P,
Wang J, Zhang Z and Pan YC: Exosomes from mmu_circ_0001052-modified
adipose-derived stem cells promote angiogenesis of DFU via
miR-106a-5p and FGF4/p38MAPK pathway. Stem Cell Res Ther.
13:3362022. View Article : Google Scholar : PubMed/NCBI
|
|
213
|
Huang Q, Chu Z, Wang Z, Li Q, Meng S, Lu
Y, Ma K, Cui S, Hu W, Zhang W, et al: circCDK13-loaded small
extracellular vesicles accelerate healing in preclinical diabetic
wound models. Nat Commun. 15:39042024. View Article : Google Scholar : PubMed/NCBI
|
|
214
|
Chen M, Przyborowski M and Berthiaume F:
Stem cells for skin tissue engineering and wound healing. Crit Rev
Biomed Eng. 37:399–421. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
215
|
Xu ZH, Ma MH, Li YQ, Li LL and Liu GH:
Progress and expectation of stem cell therapy for diabetic wound
healing. World J Clin Cases. 11:506–513. 2023. View Article : Google Scholar : PubMed/NCBI
|
|
216
|
Deng Q, Pan S, Du F, Sang H, Cai Z, Xu X,
Wei Q, Yu S, Zhang J and Li C: The Effect of conditioned medium
from angiopoietin-1 gene-modified mesenchymal stem cells on wound
healing in a diabetic mouse model. Bioengineering (Basel).
11:12442024. View Article : Google Scholar
|
|
217
|
Liu Z, Wen X, Wang H, Zhou J, Zhao M, Lin
Q, Wang Y, Li J, Li D, Du Z, et al: Molecular imaging of induced
pluripotent stem cell immunogenicity with in vivo development in
ischemic myocardium. PLoS One. 8:e663692013. View Article : Google Scholar : PubMed/NCBI
|
|
218
|
He L, Cai Y, Du H, Shu M and Zhu C:
Adipose stem cell-derived exosomes promote high glucose-induced
wound healing by regulating the TRIM32/STING axis. Arch Dermatol
Res. 316:3232024. View Article : Google Scholar
|
|
219
|
Ding JY, Chen MJ, Wu LF, Shu GF, Fang SJ,
Li ZY, Chu XR, Li XK, Wang ZG and Ji JS: Mesenchymal stem
cell-derived extracellular vesicles in skin wound healing: Roles,
opportunities and challenges. Mil Med Res. 10:362023.PubMed/NCBI
|
|
220
|
Li Y, Zhu Z, Li S, Xie X, Qin L, Zhang Q,
Yang Y, Wang T and Zhang Y: Exosomes: Compositions, biogenesis, and
mechanisms in diabetic wound healing. J Nanobiotechnology.
22:3982024. View Article : Google Scholar : PubMed/NCBI
|
|
221
|
Laiva AL, O'Brien FJ and Keogh MB:
Innovations in gene and growth factor delivery systems for diabetic
wound healing. J Tissue Eng Regen Med. 12:e296–e312. 2018.
View Article : Google Scholar
|
|
222
|
Li Z, Qiu X, Guan G, Shi K, Chen S, Tang
J, Xiao M, Tang S, Yan Y, Zhou J and Xie H: The role of FGF-21 in
promoting diabetic wound healing by modulating high glucose-induced
inflammation. Heliyon. 10:e300222024. View Article : Google Scholar : PubMed/NCBI
|
|
223
|
Tang L, Cai S, Lu X, Wu D, Zhang Y, Li X,
Qin X, Guo J, Zhang X and Liu C: Platelet-Derived growth factor
nanocapsules with tunable controlled release for chronic wound
healing. Small. 20:e23107432024. View Article : Google Scholar : PubMed/NCBI
|
|
224
|
Jeong S, Kim B, Park M, Ban E, Lee SH and
Kim A: Improved diabetic wound healing by EGF encapsulation in
gelatin-alginate coacervates. Pharmaceutics. 12:3342020. View Article : Google Scholar : PubMed/NCBI
|
|
225
|
Wang H, Xu Z, Zhao M, Liu G and Wu J:
Advances of hydrogel dressings in diabetic wounds. Biomater Sci.
9:1530–1546. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
226
|
Serpico L, Iacono SD, Cammarano A and De
Stefano L: Recent advances in stimuli-responsive hydrogel-based
wound dressing. Gels. 9:4512023. View Article : Google Scholar : PubMed/NCBI
|
|
227
|
Liang Y, He J and Guo B: Functional
hydrogels as wound dressing to enhance wound healing. ACS Nano.
15:12687–12722. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
228
|
Miguel SP, Ribeiro MP, Brancal H, Coutinho
P and Correia IJ: Thermoresponsive chitosan-agarose hydrogel for
skin regeneration. Carbohydr Polym. 111:366–373. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
229
|
Bei Z, Zhang L, Li J, Tong Q, Shi K, Chen
W, Yu Y, Sun A, Xu Y, Liu J and Qian Z: A smart
stimulation-deadhesion and antimicrobial hydrogel for repairing
diabetic wounds infected with methicillin-resistant staphylococcus
aureus. Adv Healthc Mater. 13:e23030422024. View Article : Google Scholar
|
|
230
|
Li Y, Fu R, Duan Z, Zhu C and Fan D:
Artificial nonenzymatic antioxidant MXene Nanosheet-anchored
injectable hydrogel as a mild photothermal-controlled oxygen
release platform for diabetic wound healing. ACS Nano.
16:7486–7502. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
231
|
Şeker Ş, Elçin AE and Elçin YM: Current
trends in the design and fabrication of PRP-based scaffolds for
tissue engineering and regenerative medicine. Biomed Mater.
20:202025. View Article : Google Scholar
|
|
232
|
Xu K, Deng S, Zhu Y, Yang W, Chen W, Huang
L, Zhang C, Li M, Ao L, Jiang Y, et al: Platelet rich plasma loaded
multifunctional hydrogel accelerates diabetic wound healing via
regulating the continuously abnormal microenvironments. Adv Healthc
Mater. 12:e23013702023. View Article : Google Scholar : PubMed/NCBI
|
|
233
|
Wei Q, Su J, Meng S, Wang Y, Ma K, Li B,
Chu Z, Huang Q, Hu W, Wang Z, et al: MiR-17-5p-engineered sEVs
encapsulated in GelMA hydrogel facilitated diabetic wound healing
by targeting PTEN and p21. Adv Sci (Weinh). 11:e23077612024.
View Article : Google Scholar : PubMed/NCBI
|
|
234
|
Alberts A, Bratu AG, Niculescu AG and
Grumezescu AM: New perspectives of hydrogels in chronic wound
management. Molecules. 30:6862025. View Article : Google Scholar : PubMed/NCBI
|
|
235
|
Negut I and Bita B: Exploring the
potential of artificial intelligence for hydrogel development-a
short review. Gels. 9:8452023. View Article : Google Scholar : PubMed/NCBI
|
|
236
|
Tan CT, Liang K, Ngo ZH, Dube CT and Lim
CY: Application of 3D bioprinting technologies to the management
and treatment of diabetic foot ulcers. Biomedicines. 8:4412020.
View Article : Google Scholar : PubMed/NCBI
|
|
237
|
Wang M, Song Y, Bisoyi HK, Yang JF, Liu L,
Yang H and Li Q: A liquid crystal elastomer-based unprecedented
two-way shape-memory aerogel. Adv Sci (Weinh). 8:e21026742021.
View Article : Google Scholar : PubMed/NCBI
|
|
238
|
Jeong Y, Patel R and Patel M:
Biopolymer-Based biomimetic aerogel for biomedical applications.
Biomimetics (Basel). 9:3972024. View Article : Google Scholar : PubMed/NCBI
|
|
239
|
Guo N, Xia Y, Zeng W, Chen J, Wu Q, Shi Y,
Li G, Huang Z, Wang G and Liu Y: Alginate-based aerogels as wound
dressings for efficient bacterial capture and enhanced
antibacterial photodynamic therapy. Drug Deliv. 29:1086–1099. 2022.
View Article : Google Scholar : PubMed/NCBI
|
|
240
|
Wang X, Yuan Z, Shafiq M, Cai G, Lei Z, Lu
Y, Guan X, Hashim R, El-Newehy M, Abdulhameed MM, et al: Composite
aerogel scaffolds containing flexible silica nanofiber and
tricalcium phosphate enable skin regeneration. ACS Appl Mater
Interfaces. 16:25843–25855. 2024. View Article : Google Scholar : PubMed/NCBI
|
|
241
|
Vaseghi A, Sadeghizadeh M, Herb M, Grumme
D, Demidov Y, Remmler T and Maleki HH: 3D printing of biocompatible
and antibacterial silica-silk-chitosan-based hybrid aerogel
scaffolds loaded with propolis. ACS Appl Bio Mater. 7:7917–7935.
2024. View Article : Google Scholar : PubMed/NCBI
|
|
242
|
Wu B, Pan W, Luo S, Luo X, Zhao Y, Xiu Q,
Zhong M, Wang Z, Liao T, Li N, et al: Turmeric-Derived
nanoparticles functionalized aerogel regulates multicellular
networks to promote diabetic wound healing. Adv Sci (Weinh).
11:e23076302024. View Article : Google Scholar : PubMed/NCBI
|
|
243
|
Ramos R, Silva JP, Rodrigues AC, Costa R,
Guardão L, Schmitt F, Soares R, Vilanova M, Domingues L and Gama M:
Wound healing activity of the human antimicrobial peptide LL37.
Peptides. 32:1469–1476. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
244
|
Grönberg A, Mahlapuu M, Ståhle M,
Whately-Smith C and Rollman O: Treatment with LL-37 is safe and
effective in enhancing healing of hard-to-heal venous leg ulcers: A
randomized, placebo-controlled clinical trial. Wound Repair Regen.
22:613–621. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
245
|
John JV, Sharma NS, Tang G, Luo Z, Su Y,
Weihs S, Shahriar SMS, Wang G, McCarthy A, Dyke J, et al: Nanofiber
aerogels with precision macrochannels and LL-37-mimic peptides
synergistically promote diabetic wound healing. Adv Funct Mater.
33:22069362023. View Article : Google Scholar : PubMed/NCBI
|
|
246
|
Zhao C, Wu Z, Pan B, Zhang R, Golestani A,
Feng Z, Ge Y and Yang H: Functional biomacromolecules-based
microneedle patch for the treatment of diabetic wound. Int J Biol
Macromol. 267:1316502024. View Article : Google Scholar : PubMed/NCBI
|
|
247
|
Hu F, Gao Q, Liu J, Chen W, Zheng C, Bai
Q, Sun N, Zhang W, Zhang Y and Lu T: Smart microneedle patches for
wound healing and management. J Mater Chem B. 11:2830–2851. 2023.
View Article : Google Scholar : PubMed/NCBI
|
|
248
|
Yin M, Wu J, Deng M, Wang P, Ji G, Wang M,
Zhou C, Blum NT, Zhang W, Shi H, et al: Multifunctional magnesium
organic framework-based microneedle patch for accelerating diabetic
wound healing. ACS Nano. 15:17842–17853. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
249
|
Wang P, Wu J, Yang H, Liu H, Yao T, Liu C,
Gong Y, Wang M, Ji G, Huang P and Wang X: Intelligent microneedle
patch with prolonged local release of hydrogen and magnesium ions
for diabetic wound healing. Bioact Mater. 24:463–476.
2023.PubMed/NCBI
|
|
250
|
Avcil M and Çelik A: Microneedles in drug
delivery: Progress and challenges. Micromachines (Basel).
12:13212021. View Article : Google Scholar : PubMed/NCBI
|
|
251
|
Alsarayreh AZ, Oran SA, Shakhanbeh JM,
Khleifat KM, Al Qaisi YT, Alfarrayeh II and Alkaramseh AM: Efficacy
of methanolic extracts of some medicinal plants on wound healing in
diabetic rats. Heliyon. 8:e100712022. View Article : Google Scholar : PubMed/NCBI
|
|
252
|
Wu JR, Lu YC, Hung SJ, Lin JH, Chang KC,
Chen JK, Tsai WT, Ho TJ and Chen HP: Antimicrobial and
immunomodulatory activity of herb extracts used in burn wound
healing: 'San Huang Powder'. Evid Based Complement Alternat Med.
2021:29000602021. View Article : Google Scholar
|
|
253
|
Soheilifar MH, Dastan D, Masoudi-Khoram N,
Neghab HN, Nobari S, Tabaie SM and Amini R: In vitro and in vivo
evaluation of the diabetic wound healing properties of Saffron
(Crocus Sativus L.) petals. Sci Rep. 14:193732024. View Article : Google Scholar : PubMed/NCBI
|
|
254
|
Huang L, Cai HA, Zhang MS, Liao RY, Huang
X and Hu FD: Ginsenoside Rg1 promoted the wound healing in diabetic
foot ulcers via miR-489-3p/Sirt1 axis. J Pharmacol Sci.
147:271–283. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
255
|
Sun X, Wang X, Zhao Z, Chen J, Li C and
Zhao G: Paeoniflorin accelerates foot wound healing in diabetic
rats though activating the Nrf2 pathway. Acta Histochem.
122:1516492020. View Article : Google Scholar : PubMed/NCBI
|
|
256
|
Zhang S, Xu Y, Junior CZ, Chen X and Zhu
J: Dang-Gui-Si-Ni decoction facilitates wound healing in diabetic
foot ulcers by regulating expression of AGEs/RAGE/TGF-β/Smad2/3.
Arch Dermatol Res. 316:3382024. View Article : Google Scholar
|
|
257
|
Gong Y, Jiang Y, Huang J, He Z and Tang Q:
Moist exposed burn ointment accelerates diabetes-related wound
healing by promoting re-epithelialization. Front Med (Lausanne).
9:10420152022. View Article : Google Scholar
|
|
258
|
Ding R, Wang Y, Zhu JP, Lu WG, Wei GL, Gu
ZC, An ZT and Huo JG: Danggui Sini decoction protects against
oxaliplatin-induced peripheral neuropathy in rats. J Integr
Neurosci. 19:663–671. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
259
|
Mabvuure NT, Brewer CF, Gervin K and Duffy
S: The use of moist exposed burn ointment (MEBO) for the treatment
of burn wounds: A systematic review. J Plast Surg Hand Surg.
54:337–343. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
260
|
Zhao D, Luo S, Xu W, Hu J, Lin S and Wang
N: Efficacy and safety of hyperbaric oxygen therapy used in
patients with diabetic foot: A meta-analysis of randomized clinical
trials. Clin Ther. 39:2088–2094.e2. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
261
|
Damineni U, Divity S, Gundapaneni SRC,
Burri RG and Vadde T: Clinical outcomes of hyperbaric oxygen
therapy for diabetic foot ulcers: A systematic review. Cureus.
17:e786552025.PubMed/NCBI
|
|
262
|
Huang X, Liang P, Jiang B, Zhang P, Yu W,
Duan M, Guo L, Cui X, Huang M and Huang X: Hyperbaric oxygen
potentiates diabetic wound healing by promoting fibroblast cell
proliferation and endothelial cell angiogenesis. Life Sci.
259:1182462020. View Article : Google Scholar : PubMed/NCBI
|
|
263
|
Borys S, Hohendorff J, Koblik T, Witek P,
Ludwig-Slomczynska AH, Frankfurter C, Kiec-Wilk B and Malecki MT:
Negative-pressure wound therapy for management of chronic
neuropathic noninfected diabetic foot ulcerations-short-term
efficacy and long-term outcomes. Endocrine. 62:611–616. 2018.
View Article : Google Scholar : PubMed/NCBI
|
|
264
|
Chen L, Zhang S, Da J, Wu W, Ma F, Tang C,
Li G, Zhong D and Liao B: A systematic review and meta-analysis of
efficacy and safety of negative pressure wound therapy in the
treatment of diabetic foot ulcer. Ann Palliat Med. 10:10830–10839.
2021. View Article : Google Scholar : PubMed/NCBI
|
|
265
|
Huang Y, Yu Z, Xu M, Zhao X, Tang Y, Luo
L, Deng D and Chen M: Negative pressure wound therapy promotes
wound healing by down-regulating miR-155 expression in granulation
tissue of diabetic foot ulcers. Sci Rep. 15:67332025. View Article : Google Scholar : PubMed/NCBI
|
|
266
|
Liu L, Chen R, Jia Z, Li X, Tang Y, Zhao
X, Zhang S, Luo L, Fang Z, Zhang Y and Chen M: Downregulation of
hsa-miR-203 in peripheral blood and wound margin tissue by negative
pressure wound therapy contributes to wound healing of diabetic
foot ulcers. Microvasc Res. 139:1042752022. View Article : Google Scholar
|
|
267
|
Houreld NN: Shedding light on a new
treatment for diabetic wound healing: A review on phototherapy.
ScientificWorldJournal. 2014:3984122014. View Article : Google Scholar : PubMed/NCBI
|
|
268
|
Houreld N and Abrahamse H: Low-intensity
laser irradiation stimulates wound healing in diabetic wounded
fibroblast cells (WS1). Diabetes Technol Ther. 12:971–978. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
269
|
Gupta A, Dai T and Hamblin MR: Effect of
red and near-infrared wavelengths on low-level laser (light)
therapy-induced healing of partial-thickness dermal abrasion in
mice. Lasers Med Sci. 29:257–265. 2014. View Article : Google Scholar
|
|
270
|
Cai W, Hamushan M, Zhang Y, Xu Z, Ren Z,
Du J, Ju J, Cheng P, Tan M and Han P: Synergistic effects of
photobiomodulation therapy with combined wavelength on diabetic
wound healing in vitro and in vivo. Photobiomodul Photomed Laser
Surg. 40:13–24. 2022.
|
|
271
|
Frampton JE, Lee CR and Faulds D:
Filgrastim. A review of its pharmacological properties and
therapeutic efficacy in neutropenia. Drugs. 48:731–760. 1994.
View Article : Google Scholar : PubMed/NCBI
|
|
272
|
Edmonds M, Gough A, Solovera J and
Standaert B: Filgrastim in the treatment of infected diabetic foot
ulcers. Clin Drug Investig. 17:275–286. 1999. View Article : Google Scholar
|
|
273
|
Orgill DP and Bayer LR: Negative pressure
wound therapy: Past, present and future. Int Wound J. 10:15–19.
2013. View Article : Google Scholar : PubMed/NCBI
|
|
274
|
Yoon D, Byun HJ, Oh SJ, Park JH and Lee
DY: A case of cutaneous leukocytoclastic vasculitis associated with
granulocyte colony-stimulating factor: An unusual presentation. Ann
Dermatol. 32:164–167. 2020. View Article : Google Scholar
|
|
275
|
Song P, Liang Q, Ge X, Zhou D, Yuan M, Chu
W and Xu J: Adipose-derived stem cell exosomes promote scar-free
healing of diabetic wounds via miR-204-5p/TGF-β1/Smad pathway. Stem
Cells Int. 2025:63448442025. View Article : Google Scholar
|
|
276
|
Van Netten JJ, Woodburn J and Bus SA: The
future for diabetic foot ulcer prevention: A paradigm shift from
stratified healthcare towards personalized medicine. Diabetes Metab
Res Rev. 36(Suppl 1): e32342020. View Article : Google Scholar : PubMed/NCBI
|
|
277
|
Ng GW, Gan KF, Liew H, Ge L, Ang G, Molina
J, Sun Y, Prakash PS, Harish KB and Lo ZJ: A Systematic review and
classification of factors influencing diabetic foot ulcer treatment
adherence, in accordance with the WHO dimensions of adherence to
long-term therapies. Int J Low Extrem Wounds.
20:153473462412339622024. View Article : Google Scholar
|
|
278
|
Zhang YW, Sun L, Wang YN and Zhan SY: Role
of macrophage polarization in diabetic foot ulcer healing: A
bibliometric study. World J Diabetes. 16:997552025. View Article : Google Scholar : PubMed/NCBI
|
|
279
|
Choudhury S, Dhoke NR, Chawla S and Das A:
Bioengineered MSCCxcr2 transdifferentiated keratinocyte-like
cell-derived organoid potentiates skin regeneration through ERK1/2
and STAT3 signaling in diabetic wound. Cell Mol Life Sci.
81:1722024. View Article : Google Scholar :
|
|
280
|
de Souza A, Martignago CCS, Santo GDE,
Sousa KDSJ, Cruz MA, Amaral GO, Parisi JR, Estadella D, Ribeiro DA,
Granito RN, et al: 3D printed wound constructs for skin tissue
engineering: A systematic review in experimental animal models. J
Biomed Mater Res B Appl Biomater. 111:1419–1433. 2023. View Article : Google Scholar : PubMed/NCBI
|
|
281
|
Yuan T, Tan M, Xu Y, Xiao Q, Wang H, Wu C,
Li F and Peng L: All-in-one smart dressing for simultaneous
angiogenesis and neural regeneration. J Nanobiotechnology.
21:382023. View Article : Google Scholar : PubMed/NCBI
|
|
282
|
Mirani B, Hadisi Z, Pagan E, Dabiri SMH,
van Rijt A, Almutairi L, Noshadi I, Armstrong DG and Akbari M:
Smart dual-sensor wound dressing for monitoring cutaneous wounds.
Adv Healthc Mater. 12:e22032332023. View Article : Google Scholar : PubMed/NCBI
|
|
283
|
Su R, Wang L, Han F, Bian S, Meng F, Qi W,
Zhai X, Li H, Wu J, Pan X, et al: A highly stretchable smart
dressing for wound infection monitoring and treatment. Mater Today
Bio. 26:1011072024. View Article : Google Scholar : PubMed/NCBI
|
|
284
|
Lin B, Ma Y and Wu S: Multi-Omics and
artificial intelligence-guided data integration in chronic liver
disease: Prospects and challenges for precision medicine. OMICS.
26:415–421. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
285
|
Wang Z, Xu H, Xue B, Liu L, Tang Y, Wang Z
and Yao K: MSC-derived exosomal circMYO9B accelerates diabetic
wound healing by promoting angiogenesis through the
hnRNPU/CBL/KDM1A/VEGFA axis. Commun Biol. 7:17002024. View Article : Google Scholar : PubMed/NCBI
|
|
286
|
Liu J, Qu M, Wang C, Xue Y, Huang H, Chen
Q, Sun W, Zhou X, Xu G and Jiang X: A dual-cross-linked hydrogel
patch for promoting diabetic wound healing. Small. 18:e21061722022.
View Article : Google Scholar : PubMed/NCBI
|
|
287
|
Wu C, Long L, Zhang Y, Xu Y, Lu Y, Yang Z,
Guo Y, Zhang J, Hu X and Wang Y: Injectable conductive and
angiogenic hydrogels for chronic diabetic wound treatment. J
Control Release. 344:249–260. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
288
|
Liu W, Zhai X, Zhao X, Cai Y, Zhang X, Xu
K, Weng J, Li J and Chen X: Multifunctional double-layer and dual
drug-loaded microneedle patch promotes diabetic wound healing. Adv
Healthc Mater. 12:e23002972023. View Article : Google Scholar : PubMed/NCBI
|
|
289
|
Xiong W, Zhang X, Zhou J, Chen J, Liu Y,
Yan Y, Tan M, Huang H, Si Y and Wei Y: Astragaloside IV promotes
exosome secretion of endothelial progenitor cells to regulate
PI3KR2/SPRED1 signaling and inhibit pyroptosis of diabetic
endothelial cells. Cytotherapy. 26:36–50. 2024. View Article : Google Scholar
|
|
290
|
Lei T, Gao Y, Duan Y, Cui C, Zhang L and
Si M: Panax notoginseng saponins improves healing of high
glucose-induced wound through the GSK-3β/β-catenin pathway. Environ
Toxicol. 37:1867–1877. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
291
|
Lu Y, Ding X, Qi F, Ru Y, Kuai L, Chen S,
Yang Y, Li X, Li F, Li B, et al: Quyu Shengji formula facilitates
diabetic wound healing via inhibiting the expression of
prostaglandin transporter. Evid Based Complement Alternat Med.
2021:88499352021. View Article : Google Scholar : PubMed/NCBI
|
|
292
|
Liu Y, Mo J, Liang F, Jiang S, Xiong J,
Meng X and Mo Z: Pien-tze-huang promotes wound healing in
streptozotocin-induced diabetes models associated with improving
oxidative stress via the Nrf2/ARE pathway. Front Pharmacol.
14:10626642023. View Article : Google Scholar : PubMed/NCBI
|