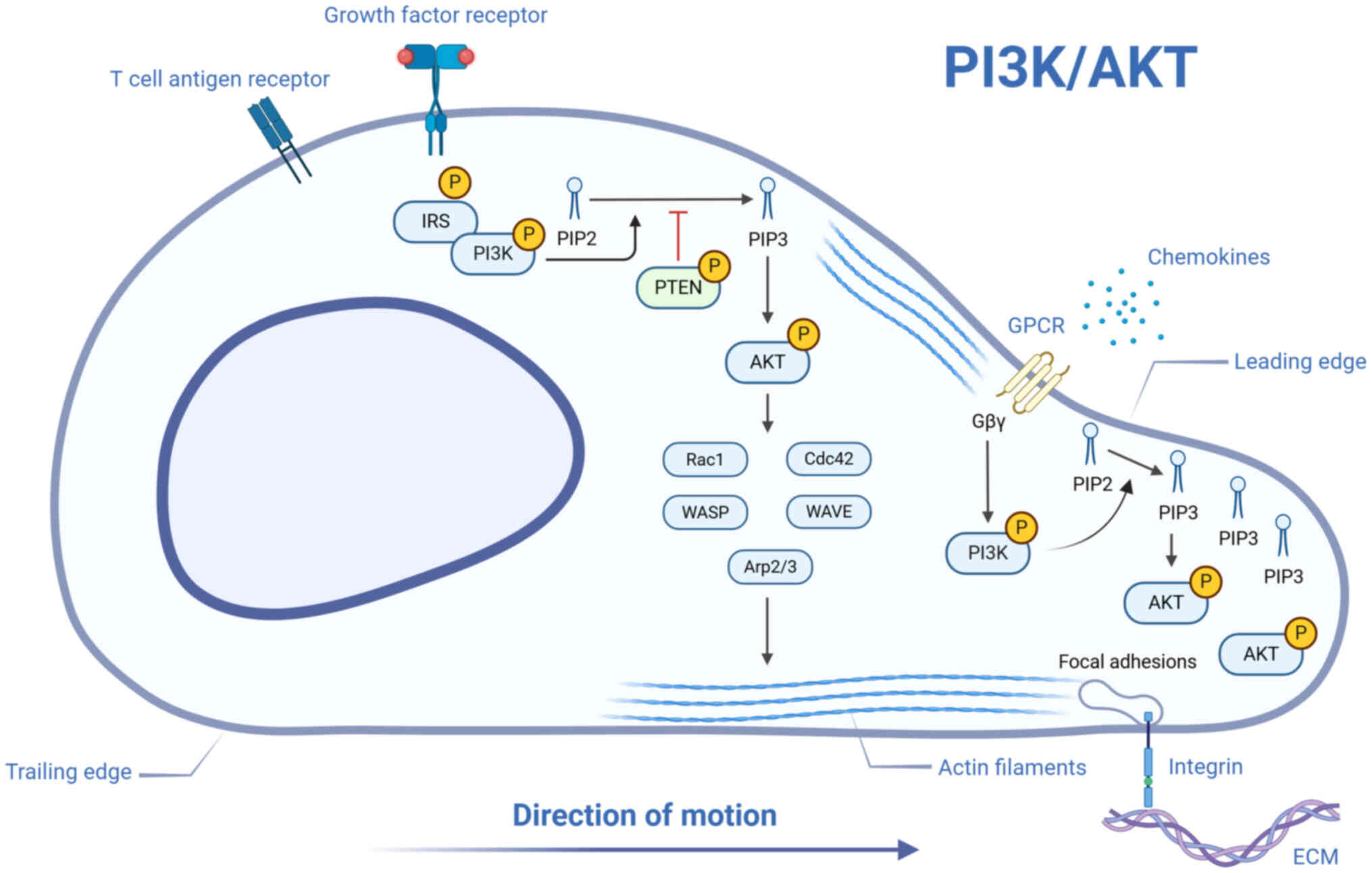
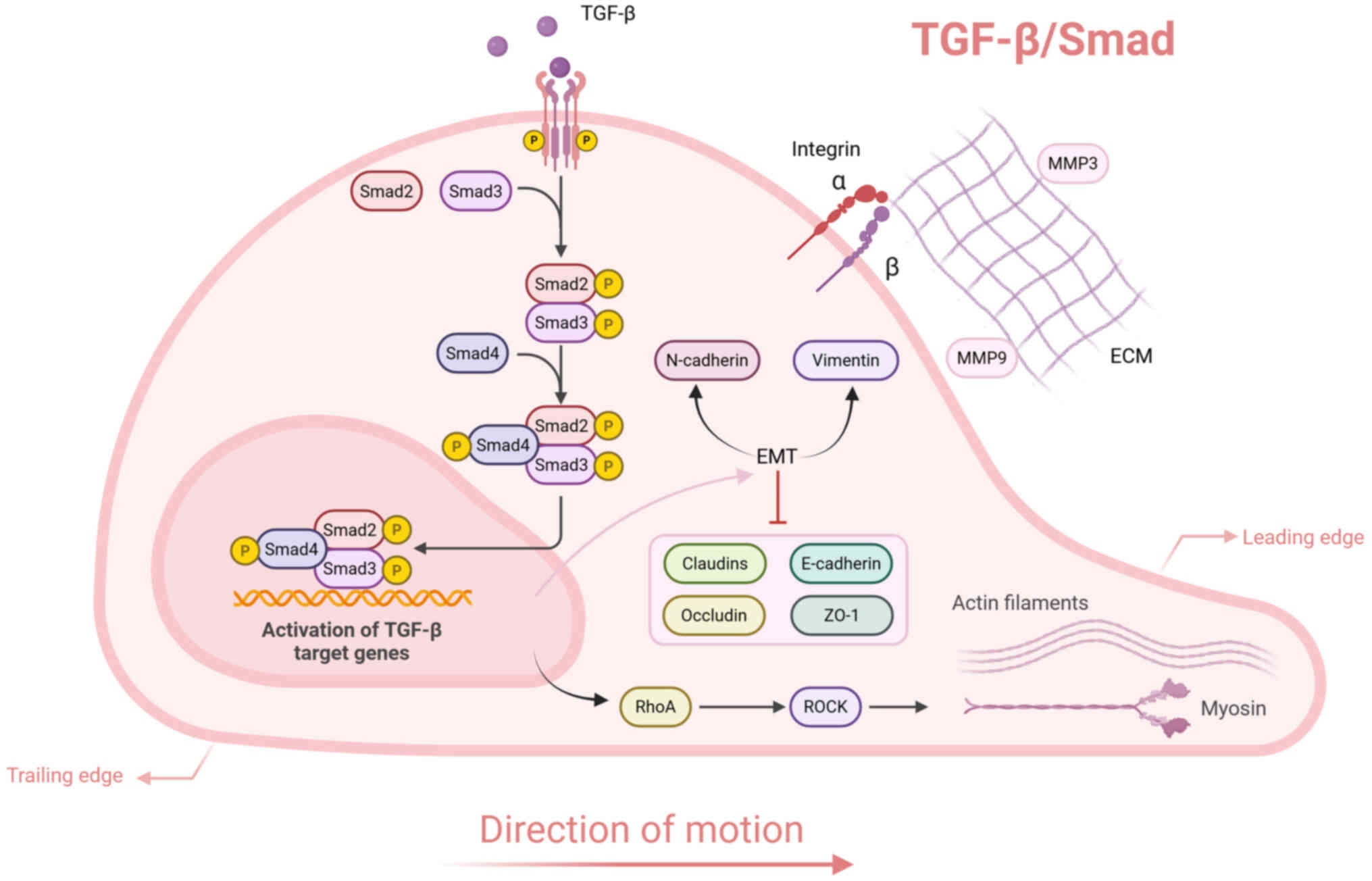
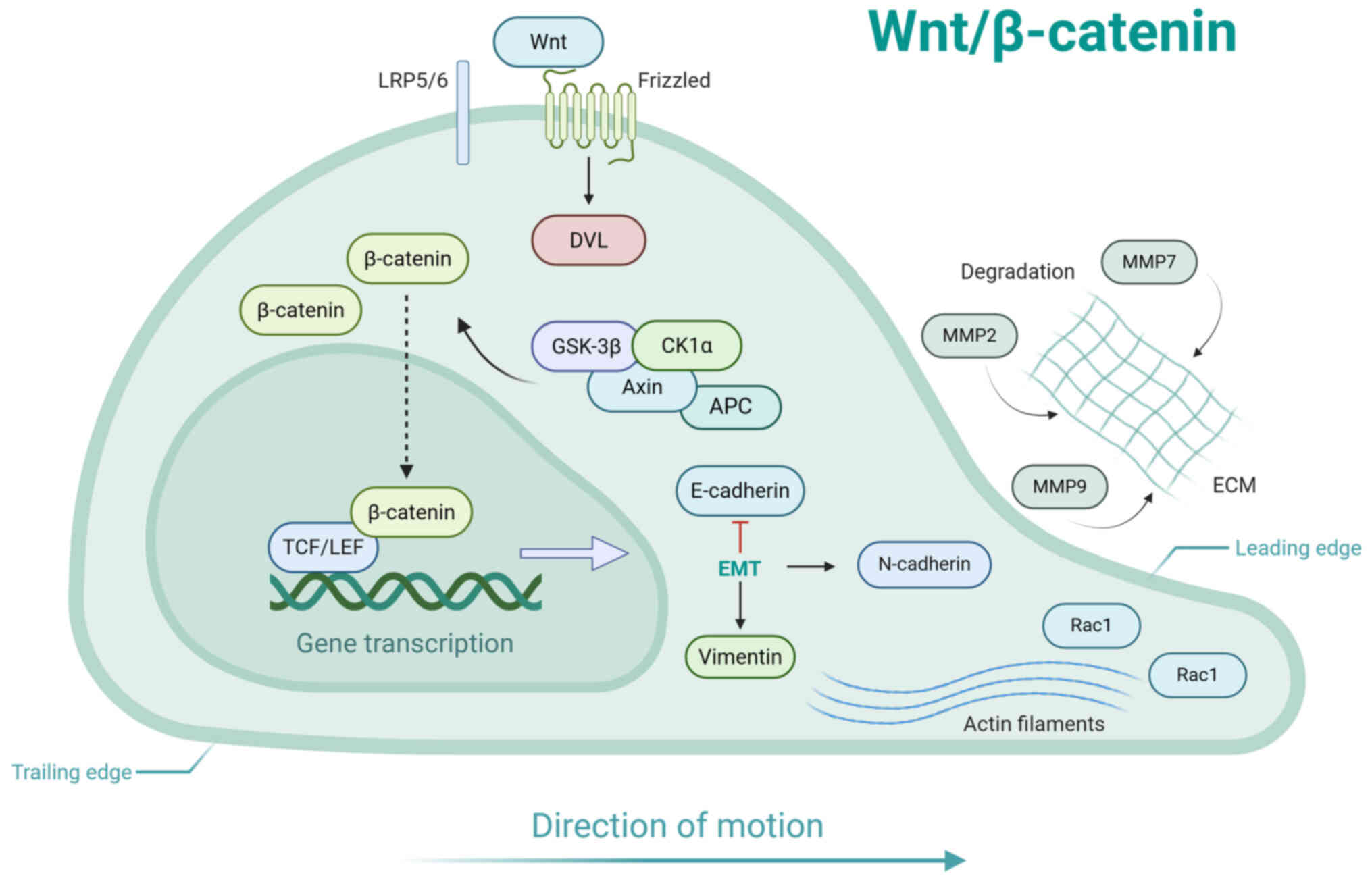
|
1
|
Dwivedi J, Sachan P, Wal P, Wal A and Rai
AK: Current state and future perspective of diabetic wound healing
treatment: Present evidence from clinical trials. Curr Diabetes
Rev. 20:e2808232204052024. View Article : Google Scholar
|
|
2
|
Sun H, Pulakat L and Anderson DW:
Challenges and new therapeutic approaches in the management of
chronic wounds. Curr Drug Targets. 21:1264–1275. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Burgess JL, Wyant WA, Abujamra BA, Kirsner
RS and Jozic I: Diabetic wound-healing science. Medicina (Kaunas).
57:10722021. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Vijayakumar V, Samal SK, Mohanty S and
Nayak SK: Recent advancements in biopolymer and metal
nanoparticle-based materials in diabetic wound healing management.
Int J Biol Macromol. 122:137–148. 2019. View Article : Google Scholar
|
|
5
|
Armstrong DG, Tan TW, Boulton AJM and Bus
SA: Diabetic foot ulcers: A review. JAMA. 330:62–75. 2023.
View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Dasari N, Jiang A, Skochdopole A, Chung J,
Reece EM, Vorstenbosch J and Winocour S: Updates in diabetic wound
healing, inflammation, and scarring. Semin Plast Surg. 35:153–158.
2021. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
GBD 2021 Diabetes Collaborators: Global,
regional, and national burden of diabetes from 1990 to 2021, with
projections of prevalence to 2050: A systematic analysis for the
global burden of disease study 2021. Lancet. 402:203–234. 2023.
View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Feng J, Yao Y, Wang Q, Han X, Deng X, Cao
Y, Chen X, Zhou M and Zhao C: Exosomes: Potential key players
towards novel therapeutic options in diabetic wounds. Biomed
Pharmacother. 166:1152972023. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Peña OA and Martin P: Cellular and
molecular mechanisms of skin wound healing. Nat Rev Mol Cell Biol.
25:599–616. 2024. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Hanson AJ and Quinn MT: Effect of fibrin
sealant composition on human neutrophil chemotaxis. J Biomed Mater
Res. 61:474–481. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Liu C, Lu Y, Du P, Yang F, Guo P, Tang X,
Diao L and Lu G: Mesenchymal stem cells pretreated with
proinflammatory cytokines accelerate skin wound healing by
promoting macrophages migration and M2 polarization. Regen Ther.
21:192–200. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Wang S, Wang K, Xin Y and Lv D: Maggot
excretions/secretions induces human microvascular endothelial cell
migration through AKT1. Mol Biol Rep. 37:2719–2725. 2010.
View Article : Google Scholar
|
|
13
|
Xia W, Li M, Jiang X, Huang X, Gu S, Ye J,
Zhu L, Hou M and Zan T: Young fibroblast-derived exosomal
microRNA-125b transfers beneficial effects on aged cutaneous wound
healing. J Nanobiotechnology. 20:1442022. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Fang PH, Lai YY, Chen CL, Wang HY, Chang
YN, Lin YC, Yan YT, Lai CH and Cheng B: Cobalt protoporphyrin
promotes human keratinocyte migration under hyperglycemic
conditions. Mol Med. 28:712022. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Huang SM, Wu CS, Chiu MH, Yang HJ, Chen GS
and Lan CCE: High-glucose environment induced intracellular
O-GlcNAc glycosylation and reduced galectin-7 expression in
keratinocytes: Implications on impaired diabetic wound healing. J
Dermatol Sci. 87:168–175. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Song Q, An X, Li D, Sodha NR, Boodhwani M,
Tian Y, Sellke FW and Li J: Hyperglycemia attenuates angiogenic
capability of survivin in endothelial cells. Microvasc Res.
78:257–264. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Huttenlocher A: Cell polarization
mechanisms during directed cell migration. Nat Cell Biol.
7:336–337. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Brothers KM, Stella NA, Hunt KM,
Romanowski EG, Liu X, Klarlund JK and Shanks RMQ: Putting on the
brakes: Bacterial impediment of wound healing. Sci Rep.
5:140032015. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Carragher NO, Fincham VJ, Riley D and
Frame MC: Cleavage of focal adhesion kinase by different proteases
during SRC-regulated transformation and apoptosis. Distinct roles
for calpain and caspases. J Biol Chem. 276:4270–4275. 2001.
View Article : Google Scholar
|
|
20
|
Zhao Y, Wang Y, Sarkar A and Wang X:
Keratocytes generate high integrin tension at the trailing edge to
mediate rear de-adhesion during rapid cell migration. iScience.
9:502–512. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Clayton SM, Shafikhani SH and Soulika AM:
Macrophage and neutrophil dysfunction in diabetic wounds. Adv Wound
Care (New Rochelle). 13:463–484. 2024. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Rousselle P, Braye F and Dayan G:
Re-epithelialization of adult skin wounds: Cellular mechanisms and
therapeutic strategies. Adv Drug Deliv Rev. 146:344–365. 2019.
View Article : Google Scholar
|
|
23
|
Rodrigues M, Kosaric N, Bonham CA and
Gurtner GC: Wound healing: A cellular perspective. Physiol Rev.
99:665–706. 2019. View Article : Google Scholar :
|
|
24
|
Su Y and Richmond A: Chemokine regulation
of neutrophil infiltration of skin wounds. Adv Wound Care (New
Rochelle). 4:631–640. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Dahlgren C, Gabl M, Holdfeldt A, Winther M
and Forsman H: Basic characteristics of the neutrophil receptors
that recognize formylated peptides, a danger-associated molecular
pattern generated by bacteria and mitochondria. Biochem Pharmacol.
114:22–39. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Mamun AA, Shao C, Geng P, Wang S and Xiao
J: Recent advances in molecular mechanisms of skin wound healing
and its treatments. Front Immunol. 15:13954792024. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Lämmermann T, Afonso PV, Angermann BR,
Wang JM, Kastenmüller W, Parent CA and Germain RN: Neutrophil
swarms require LTB4 and integrins at sites of cell death in vivo.
Nature. 498:371–375. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Lee WL, Harrison RE and Grinstein S:
Phagocytosis by neutrophils. Microbes Infect. 5:1299–1306. 2003.
View Article : Google Scholar : PubMed/NCBI
|
|
29
|
McLeish KR and Fernandes MJ: Understanding
inhibitory receptor function in neutrophils through the lens of
CLEC12A. Immunol Rev. 314:50–68. 2023. View Article : Google Scholar
|
|
30
|
Fetz AE and Bowlin GL: Neutrophil
extracellular traps: Inflammation and biomaterial preconditioning
for tissue engineering. Tissue Eng Part B Rev. 28:437–450. 2022.
View Article : Google Scholar
|
|
31
|
Bratton DL and Henson PM: Neutrophil
clearance: When the party is over, clean-up begins. Trends Immunol.
32:350–357. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Guo S and DiPietro LA: Factors affecting
wound healing. J Dent Res. 89:219–229. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
de Oliveira S, Rosowski EE and
Huttenlocher A: Neutrophil migration in infection and wound repair:
Going forward in reverse. Nat Rev Immunol. 16:378–391. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Chen WYJ and Rogers AA: Recent insights
into the causes of chronic leg ulceration in venous diseases and
implications on other types of chronic wounds. Wound Repair Regen.
15:434–449. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Isles HM, Herman KD, Robertson AL, Loynes
CA, Prince LR, Elks PM and Renshaw SA: The CXCL12/CXCR4 signaling
axis retains neutrophils at inflammatory sites in zebrafish. Front
Immunol. 10:17842019. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Wood W: Wound healing: Calcium flashes
illuminate early events. Curr Biol. 22:R14–R16. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Niethammer P, Grabher C, Look AT and
Mitchison TJ: A tissue-scale gradient of hydrogen peroxide mediates
rapid wound detection in zebrafish. Nature. 459:996–999. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Razzell W, Evans IR, Martin P and Wood W:
Calcium flashes orchestrate the wound inflammatory response through
DUOX activation and hydrogen peroxide release. Curr Biol.
23:424–429. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Minutti CM, Knipper JA, Allen JE and Zaiss
DM: Tissue-specific contribution of macrophages to wound healing.
Semin Cell Dev Biol. 61:3–11. 2017. View Article : Google Scholar
|
|
40
|
Dutra FF and Bozza MT: Heme on innate
immunity and inflammation. Front Pharmacol. 5:1152014. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Sindrilaru A and Scharffetter-Kochanek K:
Disclosure of the culprits: Macrophages-versatile regulators of
wound healing. Adv Wound Care (New Rochelle). 2:357–368. 2013.
View Article : Google Scholar
|
|
42
|
Gurtner GC, Werner S, Barrandon Y and
Longaker MT: Wound repair and regeneration. Nature. 453:314–321.
2008. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Zhang T, Tai Z, Miao F, Zhao Y, Wang W,
Zhu Q and Chen Z: Bioinspired nanovesicles derived from macrophage
accelerate wound healing by promoting angiogenesis and collagen
deposition. J Mater Chem B. 12:12338–12348. 2024. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Werner S, Krieg T and Smola H:
Keratinocyte-fibroblast interactions in wound healing. J Invest
Dermatol. 127:998–1008. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Rousselle P, Montmasson M and Garnier C:
Extracellular matrix contribution to skin wound
re-epithelialization. Matrix Biol. 75-76:12–26. 2019. View Article : Google Scholar
|
|
46
|
Rousselle P and Beck K: Laminin 332
processing impacts cellular behavior. Cell Adh Migr. 7:122–134.
2013. View Article : Google Scholar :
|
|
47
|
Hamill KJ and McLean WHI: The alpha-3
polypeptide chain of laminin 5: Insight into wound healing
responses from the study of genodermatoses. Clin Exp Dermatol.
30:398–404. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
48
|
Rousselle P, Lunstrum GP, Keene DR and
Burgeson RE: Kalinin: An epithelium-specific basement membrane
adhesion molecule that is a component of anchoring filaments. J
Cell Biol. 114:567–576. 1991. View Article : Google Scholar : PubMed/NCBI
|
|
49
|
Theveneau E and Mayor R: Collective cell
migration of epithelial and mesenchymal cells. Cell Mol Life Sci.
70:3481–3492. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
50
|
Krawczyk WS: A pattern of epidermal cell
migration during wound healing. J Cell Biol. 49:247–263. 1971.
View Article : Google Scholar : PubMed/NCBI
|
|
51
|
Headon D: Reversing stratification during
wound healing. Nat Cell Biol. 19:595–597. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
52
|
Freedberg IM, Tomic-Canic M, Komine M and
Blumenberg M: Keratins and the keratinocyte activation cycle. J
Invest Dermatol. 116:633–640. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
53
|
Veith AP, Henderson K, Spencer A, Sligar
AD and Baker AB: Therapeutic strategies for enhancing angiogenesis
in wound healing. Adv Drug Deliv Rev. 146:97–125. 2019. View Article : Google Scholar :
|
|
54
|
Velnar T and Gradisnik L: Tissue
augmentation in wound healing: The role of endothelial and
epithelial cells. Med Arch. 72:444–448. 2018. View Article : Google Scholar
|
|
55
|
Lamalice L, Le Boeuf F and Huot J:
Endothelial cell migration during angiogenesis. Circ Res.
100:782–794. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
56
|
Al Sadoun H: Macrophage phenotypes in
normal and diabetic wound healing and therapeutic interventions.
Cells. 11:24302022. View Article : Google Scholar : PubMed/NCBI
|
|
57
|
Song J, Wu Y, Chen Y, Sun X and Zhang Z:
Epigenetic regulatory mechanism of macrophage polarization in
diabetic wound healing (Review). Mol Med Rep. 31:22025. View Article : Google Scholar
|
|
58
|
Morbidelli L, Genah S and Cialdai F:
Effect of microgravity on endothelial cell function, angiogenesis,
and vessel remodeling during wound healing. Front Bioeng
Biotechnol. 9:7200912021. View Article : Google Scholar : PubMed/NCBI
|
|
59
|
Hsu S, Thakar R, Liepmann D and Li S:
Effects of shear stress on endothelial cell haptotaxis on
micropatterned surfaces. Biochem Biophys Res Commun. 337:401–409.
2005. View Article : Google Scholar : PubMed/NCBI
|
|
60
|
Li S, Huang NF and Hsu S:
Mechanotransduction in endothelial cell migration. J Cell Biochem.
96:1110–1126. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
61
|
Lynch MD and Watt FM: Fibroblast
heterogeneity: Implications for human disease. J Clin Invest.
128:26–35. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
62
|
Talbott HE, Mascharak S, Griffin M, Wan DC
and Longaker MT: Wound healing, fibroblast heterogeneity, and
fibrosis. Cell Stem Cell. 29:1161–1180. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
63
|
Bussone G: Subjectivity in primary
headaches: Insight the causes. Neurol Sci. 38:1–2. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
64
|
Younesi FS, Miller AE, Barker TH, Rossi
FMV and Hinz B: Fibroblast and myofibroblast activation in normal
tissue repair and fibrosis. Nat Rev Mol Cell Biol. 25:617–638.
2024. View Article : Google Scholar : PubMed/NCBI
|
|
65
|
Shao DD, Suresh R, Vakil V, Gomer RH and
Pilling D: Pivotal advance: Th-1 cytokines inhibit, and Th-2
cytokines promote fibrocyte differentiation. J Leukoc Biol.
83:1323–1333. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
66
|
Grieb G, Steffens G, Pallua N, Bernhagen J
and Bucala R: Circulating fibrocytes-biology and mechanisms in
wound healing and scar formation. Int Rev Cell Mol Biol. 291:1–19.
2011. View Article : Google Scholar
|
|
67
|
Park JG, Lim DC, Park JH, Park S, Mok J,
Kang KW and Park J: Benzbromarone induces targeted degradation of
HSP47 protein and improves hypertrophic scar formation. J Invest
Dermatol. 144:633–644. 2024. View Article : Google Scholar
|
|
68
|
Kohlhauser M, Mayrhofer M, Kamolz LP and
Smolle C: An update on molecular mechanisms of scarring-A narrative
review. Int J Mol Sci. 25:115792024. View Article : Google Scholar : PubMed/NCBI
|
|
69
|
Hinz B: Formation and function of the
myofibroblast during tissue repair. J Invest Dermatol. 127:526–537.
2007. View Article : Google Scholar : PubMed/NCBI
|
|
70
|
Luo L, An Y, Geng K, Wan S, Zhang F, Tan
X, Jiang Z and Xu Y: High glucose-induced endothelial STING
activation inhibits diabetic wound healing through impairment of
angiogenesis. Biochem Biophys Res Commun. 668:82–89. 2023.
View Article : Google Scholar : PubMed/NCBI
|
|
71
|
Huang SM, Wu CS, Chiu MH, Wu CH, Chang YT,
Chen GS and Lan CCE: High glucose environment induces M1 macrophage
polarization that impairs keratinocyte migration via TNF-α: An
important mechanism to delay the diabetic wound healing. J Dermatol
Sci. 96:159–167. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
72
|
Lamers ML, Almeida MES, Vicente-Manzanares
M, Horwitz AF and Santos MF: High glucose-mediated oxidative stress
impairs cell migration. PLoS One. 6:e228652011. View Article : Google Scholar : PubMed/NCBI
|
|
73
|
Brubaker AL, Rendon JL, Ramirez L,
Choudhry MA and Kovacs EJ: Reduced neutrophil chemotaxis and
infiltration contributes to delayed resolution of cutaneous wound
infection with advanced age. J Immunol. 190:1746–1757. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
74
|
den Dekker A, Davis FM, Kunkel SL and
Gallagher KA: Targeting epigenetic mechanisms in diabetic wound
healing. Transl Res. 204:39–50. 2019. View Article : Google Scholar :
|
|
75
|
Kasuya A and Tokura Y: Attempts to
accelerate wound healing. J Dermatol Sci. 76:169–172. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
76
|
Lan CCE, Liu IH, Fang AH, Wen CH and Wu
CS: Hyperglycaemic conditions decrease cultured keratinocyte
mobility: Implications for impaired wound healing in patients with
diabetes. Br J Dermatol. 159:1103–1115. 2008.PubMed/NCBI
|
|
77
|
Zhou P, Feng H, Qin W and Li Q: KRT17 from
skin cells with high glucose stimulation promotes keratinocytes
proliferation and migration. Front Endocrinol (Lausanne).
14:12370482023. View Article : Google Scholar : PubMed/NCBI
|
|
78
|
Worsley AL, Lui DH, Ntow-Boahene W, Song
W, Good L and Tsui J: The importance of inflammation control for
the treatment of chronic diabetic wounds. Int Wound J.
20:2346–2359. 2023. View Article : Google Scholar :
|
|
79
|
Li M, Wang T, Tian H, Wei G, Zhao L and
Shi Y: Macrophage-derived exosomes accelerate wound healing through
their anti-inflammation effects in a diabetic rat model. Artif
Cells Nanomed Biotechnol. 47:3793–3803. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
80
|
Nirenjen S, Narayanan J, Tamilanban T,
Subramaniyan V, Chitra V, Fuloria NK, Wong LS, Ramachawolran G,
Sekar M, Gupta G, et al: Exploring the contribution of
pro-inflammatory cytokines to impaired wound healing in diabetes.
Front Immunol. 14:12163212023. View Article : Google Scholar : PubMed/NCBI
|
|
81
|
Goren I, Müller E, Schiefelbein D,
Christen U, Pfeilschifter J, Mühl H and Frank S: Systemic
anti-TNFalpha treatment restores diabetes-impaired skin repair in
ob/ob mice by inactivation of macrophages. J Invest Dermatol.
127:2259–2267. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
82
|
Mirza RE, Fang MM, Ennis WJ and Koh TJ:
Blocking interleukin-1β induces a healing-associated wound
macrophage phenotype and improves healing in type 2 diabetes.
Diabetes. 62:2579–2587. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
83
|
Xu F, Zhang C and Graves DT: Abnormal cell
responses and role of TNF-α in impaired diabetic wound healing.
Biomed Res Int. 2013:7548022013. View Article : Google Scholar
|
|
84
|
Dai J, Shen J, Chai Y and Chen H: IL-1β
impaired diabetic wound healing by regulating MMP-2 and MMP-9
through the p38 pathway. Mediators Inflamm. 2021:66457662021.
View Article : Google Scholar
|
|
85
|
Lan CCE, Wu CS, Huang SM, Wu IH and Chen
GS: High-glucose environment enhanced oxidative stress and
increased interleukin-8 secretion from keratinocytes: New insights
into impaired diabetic wound healing. Diabetes. 62:2530–2538. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
86
|
Wang G, Yang F, Zhou W, Xiao N, Luo M and
Tang Z: The initiation of oxidative stress and therapeutic
strategies in wound healing. Biomed Pharmacother. 157:1140042023.
View Article : Google Scholar
|
|
87
|
Xu Q, Huff LP, Fujii M and Griendling KK:
Redox regulation of the actin cytoskeleton and its role in the
vascular system. Free Radic Biol Med. 109:84–107. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
88
|
Long M, de la Vega MR, Wen Q, Wen Q,
Bharara M, Jiang T, Zhang R, Zhou S, Wong PK, Wondrak GT, et al: An
essential role of NRF2 in diabetic wound healing. Diabetes.
65:780–793. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
89
|
Zhou X, Ruan Q, Ye Z, Chu Z, Xi M, Li M,
Hu W, Guo X, Yao P and Xie W: Resveratrol accelerates wound healing
by attenuating oxidative stress-induced impairment of cell
proliferation and migration. Burns. 47:133–139. 2021. View Article : Google Scholar
|
|
90
|
Gao M, Nguyen TT, Suckow MA, Wolter WR,
Gooyit M, Mobashery S and Chang M: Acceleration of diabetic wound
healing using a novel protease-anti-protease combination therapy.
Proc Natl Acad Sci USA. 112:15226–15231. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
91
|
Jones JI, Nguyen TT, Peng Z and Chang M:
Targeting MMP-9 in diabetic foot ulcers. Pharmaceuticals (Basel).
12:792019. View Article : Google Scholar : PubMed/NCBI
|
|
92
|
Yabluchanskiy A, Ma Y, Iyer RP, Hall ME
and Lindsey ML: Matrix metalloproteinase-9: Many shades of function
in cardiovascular disease. Physiology (Bethesda). 28:391–403.
2013.PubMed/NCBI
|
|
93
|
Ambrozova N, Ulrichova J and Galandakova
A: Models for the study of skin wound healing. The role of Nrf2 and
NF-κB. Biomed Pap Med Fac Univ Palacky Olomouc Czech Repub.
161:1–13. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
94
|
Zubair M and Ahmad J: Role of growth
factors and cytokines in diabetic foot ulcer healing: A detailed
review. Rev Endocr Metab Disord. 20:207–217. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
95
|
Papanas N and Maltezos E: Growth factors
in the treatment of diabetic foot ulcers: New technologies, any
promises? Int J Low Extrem Wounds. 6:37–53. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
96
|
Ong HT and Dilley RJ: Novel non-angiogenic
role for mesenchymal stem cell-derived vascular endothelial growth
factor on keratinocytes during wound healing. Cytokine Growth
Factor Rev. 44:69–79. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
97
|
Ou MY, Tan PC, Xie Y, Liu K, Gao YM, Yang
XS, Zhou SB and Li QF: Dedifferentiated Schwann cell-derived TGF-β3
is essential for the neural system to promote wound healing.
Theranostics. 12:5470–5487. 2022. View Article : Google Scholar :
|
|
98
|
R AS, Nambi N, Radhakrishnan L, Prasad MK
and Ramkumar KM: Neutrophil migration is a crucial factor in wound
healing and the pathogenesis of diabetic foot ulcers: Insights into
pharmacological interventions. Curr Vasc Pharmacol. 30: View Article : Google Scholar : 2024.
|
|
99
|
Michopoulou A, Montmasson M, Garnier C,
Lambert E, Dayan G and Rousselle P: A novel mechanism in wound
healing: Laminin 332 drives MMP9/14 activity by recruiting
syndecan-1 and CD44. Matrix Biol. 94:1–17. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
100
|
Fang WC and Lan CCE: The epidermal
keratinocyte as a therapeutic target for management of diabetic
wounds. Int J Mol Sci. 24:42902023. View Article : Google Scholar : PubMed/NCBI
|
|
101
|
Pilcher BK, Dumin JA, Sudbeck BD, Krane
SM, Welgus HG and Parks WC: The activity of collagenase-1 is
required for keratinocyte migration on a type I collagen matrix. J
Cell Biol. 137:1445–1457. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
102
|
Lan CCE, Wu CS, Kuo HY, Huang SM and Chen
GS: Hyperglycaemic conditions hamper keratinocyte locomotion via
sequential inhibition of distinct pathways: New insights on poor
wound closure in patients with diabetes. Br J Dermatol.
160:1206–1214. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
103
|
Zhang C, Ponugoti B, Tian C, Xu F,
Tarapore R, Batres A, Alsadun S, Lim J, Dong G and Graves DT: FOXO1
differentially regulates both normal and diabetic wound healing. J
Cell Biol. 209:289–303. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
104
|
Wang Y and Graves DT: Keratinocyte
function in normal and diabetic wounds and modulation by FOXO1. J
Diabetes Res. 2020:37147042020. View Article : Google Scholar : PubMed/NCBI
|
|
105
|
Kaussikaa S, Prasad MK and Ramkumar KM:
Nrf2 activation in keratinocytes: A central role in
diabetes-associated wound healing. Exp Dermatol. 33:e151892024.
View Article : Google Scholar : PubMed/NCBI
|
|
106
|
da Silva L, Carvalho E and Cruz MT: Role
of neuropeptides in skin inflammation and its involvement in
diabetic wound healing. Expert Opin Biol Ther. 10:1427–1439. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
107
|
Pradhan L, Nabzdyk C, Andersen ND, LoGerfo
FW and Veves A: Inflammation and neuropeptides: The connection in
diabetic wound healing. Expert Rev Mol Med. 11:e22009. View Article : Google Scholar : PubMed/NCBI
|
|
108
|
Grande R, Puca V and Muraro R: Antibiotic
resistance and bacterial biofilm. Expert Opin Ther Pat. 30:897–900.
2020. View Article : Google Scholar : PubMed/NCBI
|
|
109
|
Marano RJ, Wallace HJ, Wijeratne D, Fear
MW, Wong HS and O'Handley R: Secreted biofilm factors adversely
affect cellular wound healing responses in vitro. Sci Rep.
5:132962015. View Article : Google Scholar
|
|
110
|
Xing H, Huang Y, Kunkemoeller BH, Dahl PJ,
Muraleetharan O, Malvankar NS, Murrell MP and Kyriakides TR:
Dysregulation of TSP2-Rac1-WAVE2 axis in diabetic cells leads to
cytoskeletal disorganization, increased cell stiffness, and
dysfunction. Sci Rep. 12:224742022. View Article : Google Scholar : PubMed/NCBI
|
|
111
|
Dupont S, Morsut L, Aragona M, Enzo E,
Giulitti S, Cordenonsi M, Zanconato F, Le Digabel J, Forcato M,
Bicciato S, et al: Role of YAP/TAZ in mechanotransduction. Nature.
474:179–183. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
112
|
Brusatin G, Panciera T, Gandin A, Citron A
and Piccolo S: Biomaterials and engineered microenvironments to
control YAP/TAZ-dependent cell behaviour. Nat Mater. 17:1063–1075.
2018. View Article : Google Scholar : PubMed/NCBI
|
|
113
|
Lin MO, Sampath D, Bosykh DA, Wang C, Wang
X, Subramaniam T, Han W, Hong W and Chakraborty S: YAP/TAZ drive
agrin-matrix metalloproteinase 12-mediated diabetic skin wound
healing. J Invest Dermatol. 145:155–170.e2. 2025. View Article : Google Scholar
|
|
114
|
Majumdar R, Steen K, Coulombe PA and
Parent CA: Non-canonical processes that shape the cell migration
landscape. Curr Opin Cell Biol. 57:123–134. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
115
|
Xie L, Feng H, Li S, Meng G, Liu S, Tang
X, Ma Y, Han Y, Xiao Y, Gu Y, et al: SIRT3 mediates the antioxidant
effect of hydrogen sulfide in endothelial cells. Antioxid Redox
Signal. 24:329–343. 2016. View Article : Google Scholar :
|
|
116
|
Meng G, Liu J, Liu S, Song Q, Liu L, Xie
L, Han Y and Ji Y: Hydrogen sulfide pretreatment improves
mitochondrial function in myocardial hypertrophy via a
SIRT3-dependent manner. Br J Pharmacol. 175:1126–1145. 2018.
View Article : Google Scholar
|
|
117
|
Yang S, Xu M, Meng G and Lu Y: SIRT3
deficiency delays diabetic skin wound healing via oxidative stress
and necroptosis enhancement. J Cell Mol Med. 24:4415–4427. 2020.
View Article : Google Scholar : PubMed/NCBI
|
|
118
|
Johan MZ, Pyne NT, Kolesnikoff N,
Poltavets V, Esmaeili Z, Woodcock JM, Lopez AF, Cowin AJ, Pitson SM
and Samuel MS: Accelerated closure of diabetic wounds by efficient
recruitment of fibroblasts upon inhibiting a 14-3-3/ROCK regulatory
axis. J Invest Dermatol. 144:2562–2573.e4. 2024. View Article : Google Scholar : PubMed/NCBI
|
|
119
|
Li Z, Zhang C, Wang L, Zhang Q, Dong Y,
Sha X, Wang B, Zhu Z, Wang W, Wang Y, et al: Chitooligosaccharides
promote diabetic wound healing by mediating fibroblast
proliferation and migration. Sci Rep. 15:5562025. View Article : Google Scholar : PubMed/NCBI
|
|
120
|
Gao Q, Pan L, Li Y and Zhang X:
Astragaloside IV attenuates high glucose-induced human
keratinocytes injury via TGF-β/Smad signaling pathway. J Tissue
Viability. 31:678–686. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
121
|
Dong M, Ma X and Li F: Dedifferentiated
fat cells-derived exosomes (DFATs-Exos) loaded in GelMA accelerated
diabetic wound healing through Wnt/β-catenin pathway. Stem Cell Res
Ther. 16:1032025. View Article : Google Scholar
|
|
122
|
Ridley AJ: Rho GTPase signalling in cell
migration. Curr Opin Cell Biol. 36:103–112. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
123
|
Wu M, Zhang S, Zhang W, Zhou Y, Guo Z,
Fang Y, Yang Y, Shen Z, Lian D, Shen A and Peng J: Qingda granule
ameliorates vascular remodeling and phenotypic transformation of
adventitial fibroblasts via suppressing the TGF-β1/Smad2/3 pathway.
J Ethnopharmacol. 313:1165352023. View Article : Google Scholar
|
|
124
|
Fang H, Yang S, Luo Y, Zhang C, Rao Y, Liu
R, Feng Y and Yu J: Notoginsenoside R1 inhibits vascular smooth
muscle cell proliferation, migration and neointimal hyperplasia
through PI3K/Akt signaling. Sci Rep. 8:75952018. View Article : Google Scholar : PubMed/NCBI
|
|
125
|
Carvalho JR, Fortunato IC, Fonseca CG,
Pezzarossa A, Barbacena P, Dominguez-Cejudo MA, Vasconcelos FF,
Santos NC, Carvalho FA and Franco CA: Non-canonical Wnt signaling
regulates junctional mechanocoupling during angiogenic collective
cell migration. Elife. 8:e458532019. View Article : Google Scholar : PubMed/NCBI
|
|
126
|
Van den Broeke C and Favoreel HW: Actin'
up: Herpesvirus Interactions with Rho GTPase signaling. Viruses.
3:278–292. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
127
|
Qian W, Yamaguchi N, Lis P, Cammer M and
Knaut H: Pulses of RhoA signaling stimulate actin polymerization
and flow in protrusions to drive collective cell migration. Curr
Biol. 34:245–259.e8. 2024. View Article : Google Scholar :
|
|
128
|
Takaya K, Imbe Y, Wang Q, Okabe K, Sakai
S, Aramaki-Hattori N and Kishi K: Rac1 inhibition regenerates
wounds in mouse fetuses via altered actin dynamics. Sci Rep.
14:272132024. View Article : Google Scholar : PubMed/NCBI
|
|
129
|
Shih PC, Tzeng IS, Chen YC and Chen ML:
Gastrodin mitigates ketamine-induced inhibition of F-actin
remodeling and cell migration by regulating the rho signaling
pathway. Biomedicines. 13:6492025. View Article : Google Scholar : PubMed/NCBI
|
|
130
|
Lawson CD and Ridley AJ: Rho GTPase
signaling complexes in cell migration and invasion. J Cell Biol.
217:447–457. 2018. View Article : Google Scholar :
|
|
131
|
Xue C, Li G, Lu J and Li L: Crosstalk
between circRNAs and the PI3K/AKT signaling pathway in cancer
progression. Signal Transduct Target Ther. 6:4002021. View Article : Google Scholar : PubMed/NCBI
|
|
132
|
Teng Y, Fan Y, Ma J, Lu W, Liu N, Chen Y,
Pan W and Tao X: The PI3K/Akt pathway: Emerging roles in skin
homeostasis and a group of non-malignant skin disorders. Cells.
10:12192021. View Article : Google Scholar : PubMed/NCBI
|
|
133
|
Zhang X, Shi L, Xing M, Li C, Ma F and Ma
Y and Ma Y: Interplay between lncRNAs and the PI3K/AKT signaling
pathway in the progression of digestive system neoplasms (Review).
Int J Mol Med. 55:152024. View Article : Google Scholar : PubMed/NCBI
|
|
134
|
Karar J and Maity A: PI3K/AKT/mTOR pathway
in angiogenesis. Front Mol Neurosci. 4:512011. View Article : Google Scholar : PubMed/NCBI
|
|
135
|
Wu X, Sun Q, He S, Wu Y, Du S, Gong L, Yu
J and Guo H: Ropivacaine inhibits wound healing by suppressing the
proliferation and migration of keratinocytes via the PI3K/AKT/mTOR
pathway. BMC Anesthesiol. 22:1062022. View Article : Google Scholar : PubMed/NCBI
|
|
136
|
Deng S, Leong HC, Datta A, Gopal V, Kumar
AP and Yap CT: PI3K/AKT signaling tips the balance of cytoskeletal
forces for cancer progression. Cancers (Basel). 14:16522022.
View Article : Google Scholar : PubMed/NCBI
|
|
137
|
Hsu JW, Bai M, Li K, Yang JS, Chu N, Cole
PA, Eck MJ, Li J and Hsu VW: The protein kinase Akt acts as a coat
adaptor in endocytic recycling. Nat Cell Biol. 22:927–933. 2020.
View Article : Google Scholar : PubMed/NCBI
|
|
138
|
Hannigan M, Zhan L, Li Z, Ai Y, Wu D and
Huang CK: Neutrophils lacking phosphoinositide 3-kinase gamma show
loss of directionality during N-formyl-Met-Leu-Phe-induced
chemotaxis. Proc Natl Acad Sci USA. 99:3603–3608. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
139
|
Najafi M, Farhood B and Mortezaee K:
Extracellular matrix (ECM) stiffness and degradation as cancer
drivers. J Cell Biochem. 120:2782–2790. 2019. View Article : Google Scholar
|
|
140
|
Nakerakanti S and Trojanowska M: The role
of TGF-β receptors in fibrosis. Open Rheumatol J. 6:156–162. 2012.
View Article : Google Scholar :
|
|
141
|
Tu S, Huang W, Huang C, Luo Z and Yan X:
Contextual regulation of TGF-β signaling in liver cancer. Cells.
8:12352019. View Article : Google Scholar
|
|
142
|
Zhang K, Zhang H, Xiang H, Liu J, Liu Y,
Zhang X, Wang J and Tang Y: TGF-β1 induces the dissolution of tight
junctions in human renal proximal tubular cells: role of the
RhoA/ROCK signaling pathway. Int J Mol Med. 32:464–468. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
143
|
Gailit J, Welch MP and Clark RA: TGF-beta
1 stimulates expression of keratinocyte integrins during
re-epithelialization of cutaneous wounds. J Invest Dermatol.
103:221–227. 1994. View Article : Google Scholar : PubMed/NCBI
|
|
144
|
Ponugoti B, Xu F, Zhang C, Tian C, Pacios
S and Graves DT: FOXO1 promotes wound healing through the
up-regulation of TGF-β1 and prevention of oxidative stress. J Cell
Biol. 203:327–343. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
145
|
Weber CE, Li NY, Wai PY and Kuo PC:
Epithelial-Mesenchymal transition, TGF-β, and osteopontin in wound
healing and tissue remodeling after injury. J Burn Care Res.
33:311–318. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
146
|
Song P, Gao Z, Bao Y, Chen L, Huang Y, Liu
Y, Dong Q and Wei X: Wnt/β-catenin signaling pathway in
carcinogenesis and cancer therapy. J Hematol Oncol. 17:462024.
View Article : Google Scholar
|
|
147
|
Moon RT, Kohn AD, De Ferrari GV and Kaykas
A: WNT and beta-catenin signalling: Diseases and therapies. Nat Rev
Genet. 5:691–701. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
148
|
Nusse R and Clevers H: Wnt/β-Catenin
signaling, disease, and emerging therapeutic modalities. Cell.
169:985–999. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
149
|
Schaale K, Neumann J, Schneider D, Ehlers
S and Reiling N: Wnt signaling in macrophages: Augmenting and
inhibiting mycobacteria-induced inflammatory responses. Eur J Cell
Biol. 90:553–559. 2011. View Article : Google Scholar
|
|
150
|
Sedgwick AE and D'Souza-Schorey C: Wnt
signaling in cell motility and invasion: Drawing parallels between
development and cancer. Cancers (Basel). 8:802016. View Article : Google Scholar : PubMed/NCBI
|
|
151
|
Wu B, Crampton SP and Hughes CCW: Wnt
signaling induces MMP expression and regulates T cell
transmigration. Immunity. 26:227–239. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
152
|
Xue W, Yang L, Chen C, Ashrafizadeh M,
Tian Y and Sun R: Wnt/β-catenin-driven EMT regulation in human
cancers. Cell Mol Life Sci. 81:792024. View Article : Google Scholar
|
|
153
|
Ono M, Masaki A, Maeda A, Kilts TM, Hara
ES, Komori T, Pham H, Kuboki T and Young MF: CCN4/WISP1 controls
cutaneous wound healing by modulating proliferation, migration and
ECM expression in dermal fibroblasts via α5β1 and TNFα. Matrix
Biol. 68-69:533–546. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
154
|
Repertinger SK, Campagnaro E, Fuhrman J,
El-Abaseri T, Yuspa SH and Hansen LA: EGFR enhances early healing
after cutaneous incisional wounding. J Invest Dermatol.
123:982–989. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
155
|
Wang Y, Wu Z, Tian J, Mi Y, Ren X, Kang J,
Zhang W, Zhou X, Wang G and Li R: Intermedin protects HUVECs from
ischemia reperfusion injury via Wnt/β-catenin signaling pathway.
Ren Fail. 41:159–166. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
156
|
Jun EK, Zhang Q, Yoon BS, Moon JH, Lee G,
Park G, Kang PJ, Lee JH, Kim A and You S: Hypoxic conditioned
medium from human amniotic fluid-derived mesenchymal stem cells
accelerates skin wound healing through TGF-β/SMAD2 and PI3K/Akt
pathways. Int J Mol Sci. 15:605–628. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
157
|
Toraldo G, Bhasin S, Bakhit M, Guo W,
Serra C, Safer JD, Bhawan J and Jasuja R: Topical androgen
antagonism promotes cutaneous wound healing without systemic
androgen deprivation by blocking β-catenin nuclear translocation
and cross-talk with TGF-β signaling in keratinocytes. Wound Repair
Regen. 20:61–73. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
158
|
Wang H, Wang X, Liu X, Zhou J, Yang Q,
Chai B, Chai Y, Ma Z and Lu S: miR-199a-5p plays a pivotal role on
wound healing via suppressing VEGFA and ROCK1 in diabetic ulcer
foot. Oxid Med Cell Longev. 2022:47910592022. View Article : Google Scholar : PubMed/NCBI
|
|
159
|
Yu J, Nam D and Park KS: Substance P
enhances cellular migration and inhibits senescence in human dermal
fibroblasts under hyperglycemic conditions. Biochem Biophys Res
Commun. 522:917–923. 2020. View Article : Google Scholar
|
|
160
|
Huang J, Deng Q, Tsang LL, Chang G, Guo J,
Ruan YC, Wang CC, Li G, Chan HF, Zhang X and Jiang X: Mesenchymal
stem cells from perinatal tissues promote diabetic wound healing
via PI3K/AKT activation. Stem Cell Res Ther. 16:592025. View Article : Google Scholar : PubMed/NCBI
|
|
161
|
Xu S, Jiang C, Yu T and Chen K: A
multi-purpose dressing based on resveratrol-loaded ionic
liquids/gelatin methacryloyl hydrogel for enhancing diabetic wound
healing. Int J Biol Macromol. 283:1367732024. View Article : Google Scholar : PubMed/NCBI
|
|
162
|
Peng Y, Wu S, Tang Q, Li S and Peng C:
KGF-1 accelerates wound contraction through the TGF-β1/Smad
signaling pathway in a double-paracrine manner. J Biol Chem.
294:8361–8370. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
163
|
Oyebode OA and Houreld NN:
Photobiomodulation at 830 nm stimulates migration, survival and
proliferation of fibroblast cells. Diabetes Metab Syndr Obes.
15:2885–2900. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
164
|
Wang L, Chen J, Song J, Xiang Y, Yang M,
Xia L, Yang J, Hou X, Chen L and Wang L: Activation of the
Wnt/β-catenin signalling pathway enhances exosome production by
hucMSCs and improves their capability to promote diabetic wound
healing. J Nanobiotechnology. 22:3732024. View Article : Google Scholar
|
|
165
|
Liu Z, Yang S, Li X, Wang S, Zhang T, Huo
N, Duan R, Shi Q, Zhang J and Xu J: Local transplantation of
GMSC-derived exosomes to promote vascularized diabetic wound
healing by regulating the Wnt/β-catenin pathways. Nanoscale Adv.
5:916–926. 2023. View Article : Google Scholar : PubMed/NCBI
|
|
166
|
Lv Q, Deng J, Chen Y, Wang Y, Liu B and
Liu J: Engineered human adipose stem-cell-derived exosomes loaded
with miR-21-5p to promote diabetic cutaneous wound healing. Mol
Pharm. 17:1723–1733. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
167
|
Yang Y, GuangXuan H, GenMeng W, MengHuan
L, Bo C and XueJie Y: Idiopathic inflammatory myopathy and
non-coding RNA. Front Immunol. 14:12279452023. View Article : Google Scholar : PubMed/NCBI
|
|
168
|
Ballarino M, Morlando M, Fatica A and
Bozzoni I: Non-coding RNAs in muscle differentiation and
musculoskeletal disease. J Clin Invest. 126:2021–2030. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
169
|
Chen L, Shen S and Wang S: LncRNA SNHG16
knockdown promotes diabetic foot ulcer wound healing via sponging
MiR-31-5p. Tohoku J Exp Med. 261:283–289. 2023. View Article : Google Scholar : PubMed/NCBI
|
|
170
|
Peng J, Zhu H, Ruan B, Duan Z and Cao M:
miR-155 promotes m6A modification of SOX2 mRNA through targeted
regulation of HIF-1α and delays wound healing in diabetic foot
ulcer in vitro models. J Diabetes Investig. 16:60–71. 2024.
View Article : Google Scholar
|
|
171
|
Tian M, Tang J, Huang R, Dong J and Jia H:
Circ_072697 knockdown promotes advanced glycation end
products-induced cell proliferation and migration in HaCaT cells
via miR-3150a-3p/KDM2A axis. BMC Endocr Disord. 23:2002023.
View Article : Google Scholar : PubMed/NCBI
|
|
172
|
Wang F, Mei X, Yang Y, Zhang H, Li Z, Zhu
L, Deng S and Wang Y: Non-coding RNA and its network in the
pathogenesis of myasthenia gravis. Front Mol Biosci.
11:13884762024. View Article : Google Scholar : PubMed/NCBI
|
|
173
|
Bhan A and Mandal SS: Long noncoding RNAs:
Emerging stars in gene regulation, epigenetics and human disease.
ChemMedChem. 9:1932–1956. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
174
|
Hong W, Xiong Z, Wang X, Liao X, Liu M,
Jiang Z, Min D, Li J, Guo G and Fu Z: Long noncoding RNA XIST
promotes cell proliferation and migration in diabetic foot ulcers
through the miR-126-3p/EGFR axis. Diabetol Metab Syndr. 16:352024.
View Article : Google Scholar : PubMed/NCBI
|
|
175
|
He M, Tu L, Shu R, Meng Q, Du S, Xu Z and
Wang S: Long noncoding RNA CASC2 facilitated wound healing through
miRNA-155/HIF-1α in diabetic foot ulcers. Contrast Media Mol
Imaging. 2022:62914972022. View Article : Google Scholar
|
|
176
|
Li B, Zhou Y, Chen J, Wang T, Li Z, Fu Y,
Zhai A and Bi C: Long noncoding RNA H19 acts as a miR-29b sponge to
promote wound healing in diabetic foot ulcer. FASEB J.
35:e205262021.
|
|
177
|
Hu M, Wu Y, Yang C, Wang X, Wang W, Zhou
L, Zeng T, Zhou J, Wang C, Lao G, et al: Novel long noncoding RNA
lnc-URIDS delays diabetic wound healing by targeting Plod1.
Diabetes. 69:2144–2156. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
178
|
Gezer U, Özgür E, Cetinkaya M, Isin M and
Dalay N: Long non-coding RNAs with low expression levels in cells
are enriched in secreted exosomes. Cell Biol Int. 38:1076–1079.
2014. View Article : Google Scholar : PubMed/NCBI
|
|
179
|
Li B, Luan S, Chen J, Zhou Y, Wang T, Li
Z, Fu Y, Zhai A and Bi C: The MSC-derived exosomal lncRNA H19
promotes wound healing in diabetic foot ulcers by upregulating PTEN
via MicroRNA-152-3p. Mol Ther Nucleic Acids. 19:814–826. 2020.
View Article : Google Scholar : PubMed/NCBI
|
|
180
|
Han ZF, Cao JH, Liu ZY, Yang Z, Qi RX and
Xu HL: Exosomal lncRNA KLF3-AS1 derived from bone marrow
mesenchymal stem cells stimulates angiogenesis to promote diabetic
cutaneous wound healing. Diabetes Res Clin Pract. 183:1091262022.
View Article : Google Scholar
|
|
181
|
Fu W, Liang D, Wu X, Chen H, Hong X, Wang
J, Zhu T, Zeng T, Lin W, Chen S, et al: Long noncoding RNA
LINC01435 impedes diabetic wound healing by facilitating
YY1-mediated HDAC8 expression. iScience. 25:1040062022. View Article : Google Scholar : PubMed/NCBI
|
|
182
|
Roso-Mares A, Andújar I, Corpas TD and Sun
BK: Non-coding RNAs as skin disease biomarkers, molecular
signatures, and therapeutic targets. Hum Genet. 143:801–812. 2024.
View Article : Google Scholar
|
|
183
|
Shirley SN, Watson AE and Yusuf N:
Pathogenesis of inflammation in skin disease: From molecular
mechanisms to pathology. Int J Mol Sci. 25:101522024. View Article : Google Scholar : PubMed/NCBI
|
|
184
|
Tsai HC, Chang GRL, Tung MC, Tu MY, Chen
IC, Liu YH, Cidem A and Chen CM: MicroRNA signature in an in vitro
keratinocyte model of diabetic wound healing. Int J Mol Sci.
25:101252024. View Article : Google Scholar : PubMed/NCBI
|
|
185
|
Zhao X, Xu M, Tang Y, Xie D, Deng L, Chen
M and Wang Y: Decreased expression of miR-204-3p in peripheral
blood and wound margin tissue associated with the onset and poor
wound healing of diabetic foot ulcers. Int Wound J. 20:413–429.
2023. View Article : Google Scholar
|
|
186
|
Zhang HC, Wen T and Cai YZ: Overexpression
of miR-146a promotes cell proliferation and migration in a model of
diabetic foot ulcers by regulating the AKAP12 axis. Endocr J.
69:85–94. 2022. View Article : Google Scholar
|
|
187
|
Wang W, Yang C, Wang XY, Zhou LY, Lao GJ,
Liu D, Wang C, Hu MD, Zeng TT, Yan L and Ren M: MicroRNA-129 and
-335 promote diabetic wound healing by inhibiting Sp1-mediated
MMP-9 expression. Diabetes. 67:1627–1638. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
188
|
Li P, Hong G, Zhan W, Deng M, Tu C, Wei J
and Lin H: Endothelial progenitor cell derived exosomes mediated
miR-182-5p delivery accelerate diabetic wound healing via
down-regulating PPARG. Int J Med Sci. 20:468–481. 2023. View Article : Google Scholar : PubMed/NCBI
|
|
189
|
Wang KX, Zhao LL, Zheng LT, Meng LB, Jin
L, Zhang LJ, Kong FL and Liang F: Accelerated wound healing in
diabetic rat by miRNA-185-5p and its anti-inflammatory activity.
Diabetes Metab Syndr Obes. 16:1657–1667. 2023. View Article : Google Scholar : PubMed/NCBI
|
|
190
|
Wu M, Tu J, Huang J, Wen H, Zeng Y and Lu
Y: Exosomal IRF1-loaded rat adipose-derived stem cell sheet
contributes to wound healing in the diabetic foot ulcers. Mol Med.
29:602023. View Article : Google Scholar : PubMed/NCBI
|
|
191
|
Wu Y, Zhang K, Liu R, Zhang H, Chen D, Yu
S, Chen W, Wan S, Zhang Y, Jia Z, et al: MicroRNA-21-3p accelerates
diabetic wound healing in mice by downregulating SPRY1. Aging
(Albany NY). 12:15436–15445. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
192
|
Wang C, Huang L, Li J, Liu D and Wu B:
MicroRNA miR-145-5p inhibits cutaneous wound healing by targeting
PDGFD in diabetic foot ulcer. Biochem Genet. 62:2437–2454. 2024.
View Article : Google Scholar
|
|
193
|
Zhao X, Xu M, Tang Y, Xie D, Wang Y and
Chen M: Changes in miroRNA-103 expression in wound margin tissue
are related to wound healing of diabetes foot ulcers. Int Wound J.
20:467–483. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
194
|
Zhang P, Song X, Dong Q, Zhou L and Wang
L: miR-27-3p inhibition restore fibroblasts viability in diabetic
wound by targeting NOVA1. Aging (Albany NY). 12:12841–12849. 2020.
View Article : Google Scholar : PubMed/NCBI
|
|
195
|
Zheng L, Song H, Li Y, Li H, Lin G and Cai
Z: Insulin-Induced gene 1-enhance secretion of BMSC exosome
enriched in miR-132-3p promoting wound healing in diabetic mice.
Mol Pharm. 21:4372–4385. 2024. View Article : Google Scholar : PubMed/NCBI
|
|
196
|
Zuo C, Fan P, Yang Y and Hu C: MiR-488-3p
facilitates wound healing through CYP1B1-mediated Wnt/β-catenin
signaling pathway by targeting MeCP2. J Diabetes Investig.
15:145–158. 2024. View Article : Google Scholar
|
|
197
|
Huang H, Zhu W, Huang Z, Zhao D, Cao L and
Gao X: Adipose-derived stem cell exosome NFIC improves diabetic
foot ulcers by regulating miR-204-3p/HIPK2. J Orthop Surg Res.
18:6872023. View Article : Google Scholar : PubMed/NCBI
|
|
198
|
Qiu ZY, Xu WC and Liang ZH: Bone marrow
mesenchymal stem cell-derived exosomal miR-221-3p promotes
angiogenesis and wound healing in diabetes via the downregulation
of forkhead box P1. Diabet Med. 41:e153862024. View Article : Google Scholar : PubMed/NCBI
|
|
199
|
Zhou X, Ye C, Jiang L, Zhu X, Zhou F, Xia
M and Chen Y: The bone mesenchymal stem cell-derived exosomal
miR-146a-5p promotes diabetic wound healing in mice via macrophage
M1/M2 polarization. Mol Cell Endocrinol. 579:1120892024. View Article : Google Scholar
|
|
200
|
Guo E, Wang L, Wu J and Chen Q: Exosomes
from MicroRNA-125b-modified adipose-derived stem cells promote
wound healing of diabetic foot ulcers. Curr Stem Cell Res Ther.
20:409–420. 2024. View Article : Google Scholar : PubMed/NCBI
|
|
201
|
Che D, Xiang X, Xie J, Chen Z, Bao Q and
Cao D: exosomes derived from adipose stem cells enhance
angiogenesis in diabetic wound via miR-146a-5p/JAZF1 axis. Stem
Cell Rev Rep. 20:1026–1039. 2024. View Article : Google Scholar : PubMed/NCBI
|
|
202
|
Ge L, Wang K, Lin H, Tao E, Xia W, Wang F,
Mao C and Feng Y: Engineered exosomes derived from
miR-132-overexpresssing adipose stem cells promoted diabetic wound
healing and skin reconstruction. Front Bioeng Biotechnol.
11:11295382023. View Article : Google Scholar : PubMed/NCBI
|
|
203
|
Sedykh S, Kuleshova A and Nevinsky G: Milk
exosomes: Perspective agents for anticancer drug delivery. Int J
Mol Sci. 21:66462020. View Article : Google Scholar : PubMed/NCBI
|
|
204
|
Munagala R, Aqil F, Jeyabalan J and Gupta
RC: Bovine milk-derived exosomes for drug delivery. Cancer Lett.
371:48–61. 2016. View Article : Google Scholar :
|
|
205
|
Yan C, Chen J, Wang C, Yuan M, Kang Y, Wu
Z, Li W, Zhang G, Machens HG and Rinkevich Y: Milk
exosomes-mediated miR-31-5p delivery accelerates diabetic wound
healing through promoting angiogenesis. Drug Deliv. 29:214–228.
2022. View Article : Google Scholar : PubMed/NCBI
|
|
206
|
Liu R, Zhang L, Zhao X, Liu J, Chang W,
Zhou L and Zhang K: circRNA: Regulatory factors and potential
therapeutic targets in inflammatory dermatoses. J Cell Mol Med.
26:4389–4400. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
207
|
Beňačka R, Szabóová D, Guľašová Z and
Hertelyová Z: Non-Coding RNAs in breast cancer: Diagnostic and
therapeutic implications. Int J Mol Sci. 26:1272024. View Article : Google Scholar
|
|
208
|
Zhang Y, Wu Y, Liu Z, Yang K, Lin H and
Xiong K: Non-coding RNAs as potential targets in metformin therapy
for cancer. Cancer Cell Int. 24:3332024. View Article : Google Scholar : PubMed/NCBI
|
|
209
|
Fu Z, Jiang Z, Liao X, Liu M, Guo G, Wang
X, Yang G, Zhou Z, Hu L and Xiong Z: Upregulation of circ_0080968
in diabetic foot ulcer inhibits wound healing via repressing the
migration and promoting proliferation of keratinocytes. Gene.
883:1476692023. View Article : Google Scholar : PubMed/NCBI
|
|
210
|
Han D, Liu W, Li G and Liu L: Circ_PRKDC
knockdown promotes skin wound healing by enhancing keratinocyte
migration via miR-31/FBN1 axis. J Mol Histol. 52:681–691. 2021.
View Article : Google Scholar : PubMed/NCBI
|
|
211
|
Wang A, Toma MA, Ma J, Li D, Vij M, Chu T,
Wang J, Li X and Landén NX: Circular RNA hsa_circ_0084443 is
upregulated in diabetic foot ulcer and modulates keratinocyte
migration and proliferation. Adv Wound Care (New Rochelle).
9:145–160. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
212
|
Liang ZH, Pan NF, Lin SS, Qiu ZY, Liang P,
Wang J, Zhang Z and Pan YC: Exosomes from mmu_circ_0001052-modified
adipose-derived stem cells promote angiogenesis of DFU via
miR-106a-5p and FGF4/p38MAPK pathway. Stem Cell Res Ther.
13:3362022. View Article : Google Scholar : PubMed/NCBI
|
|
213
|
Huang Q, Chu Z, Wang Z, Li Q, Meng S, Lu
Y, Ma K, Cui S, Hu W, Zhang W, et al: circCDK13-loaded small
extracellular vesicles accelerate healing in preclinical diabetic
wound models. Nat Commun. 15:39042024. View Article : Google Scholar : PubMed/NCBI
|
|
214
|
Chen M, Przyborowski M and Berthiaume F:
Stem cells for skin tissue engineering and wound healing. Crit Rev
Biomed Eng. 37:399–421. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
215
|
Xu ZH, Ma MH, Li YQ, Li LL and Liu GH:
Progress and expectation of stem cell therapy for diabetic wound
healing. World J Clin Cases. 11:506–513. 2023. View Article : Google Scholar : PubMed/NCBI
|
|
216
|
Deng Q, Pan S, Du F, Sang H, Cai Z, Xu X,
Wei Q, Yu S, Zhang J and Li C: The Effect of conditioned medium
from angiopoietin-1 gene-modified mesenchymal stem cells on wound
healing in a diabetic mouse model. Bioengineering (Basel).
11:12442024. View Article : Google Scholar
|
|
217
|
Liu Z, Wen X, Wang H, Zhou J, Zhao M, Lin
Q, Wang Y, Li J, Li D, Du Z, et al: Molecular imaging of induced
pluripotent stem cell immunogenicity with in vivo development in
ischemic myocardium. PLoS One. 8:e663692013. View Article : Google Scholar : PubMed/NCBI
|
|
218
|
He L, Cai Y, Du H, Shu M and Zhu C:
Adipose stem cell-derived exosomes promote high glucose-induced
wound healing by regulating the TRIM32/STING axis. Arch Dermatol
Res. 316:3232024. View Article : Google Scholar
|
|
219
|
Ding JY, Chen MJ, Wu LF, Shu GF, Fang SJ,
Li ZY, Chu XR, Li XK, Wang ZG and Ji JS: Mesenchymal stem
cell-derived extracellular vesicles in skin wound healing: Roles,
opportunities and challenges. Mil Med Res. 10:362023.PubMed/NCBI
|
|
220
|
Li Y, Zhu Z, Li S, Xie X, Qin L, Zhang Q,
Yang Y, Wang T and Zhang Y: Exosomes: Compositions, biogenesis, and
mechanisms in diabetic wound healing. J Nanobiotechnology.
22:3982024. View Article : Google Scholar : PubMed/NCBI
|
|
221
|
Laiva AL, O'Brien FJ and Keogh MB:
Innovations in gene and growth factor delivery systems for diabetic
wound healing. J Tissue Eng Regen Med. 12:e296–e312. 2018.
View Article : Google Scholar
|
|
222
|
Li Z, Qiu X, Guan G, Shi K, Chen S, Tang
J, Xiao M, Tang S, Yan Y, Zhou J and Xie H: The role of FGF-21 in
promoting diabetic wound healing by modulating high glucose-induced
inflammation. Heliyon. 10:e300222024. View Article : Google Scholar : PubMed/NCBI
|
|
223
|
Tang L, Cai S, Lu X, Wu D, Zhang Y, Li X,
Qin X, Guo J, Zhang X and Liu C: Platelet-Derived growth factor
nanocapsules with tunable controlled release for chronic wound
healing. Small. 20:e23107432024. View Article : Google Scholar : PubMed/NCBI
|
|
224
|
Jeong S, Kim B, Park M, Ban E, Lee SH and
Kim A: Improved diabetic wound healing by EGF encapsulation in
gelatin-alginate coacervates. Pharmaceutics. 12:3342020. View Article : Google Scholar : PubMed/NCBI
|
|
225
|
Wang H, Xu Z, Zhao M, Liu G and Wu J:
Advances of hydrogel dressings in diabetic wounds. Biomater Sci.
9:1530–1546. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
226
|
Serpico L, Iacono SD, Cammarano A and De
Stefano L: Recent advances in stimuli-responsive hydrogel-based
wound dressing. Gels. 9:4512023. View Article : Google Scholar : PubMed/NCBI
|
|
227
|
Liang Y, He J and Guo B: Functional
hydrogels as wound dressing to enhance wound healing. ACS Nano.
15:12687–12722. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
228
|
Miguel SP, Ribeiro MP, Brancal H, Coutinho
P and Correia IJ: Thermoresponsive chitosan-agarose hydrogel for
skin regeneration. Carbohydr Polym. 111:366–373. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
229
|
Bei Z, Zhang L, Li J, Tong Q, Shi K, Chen
W, Yu Y, Sun A, Xu Y, Liu J and Qian Z: A smart
stimulation-deadhesion and antimicrobial hydrogel for repairing
diabetic wounds infected with methicillin-resistant staphylococcus
aureus. Adv Healthc Mater. 13:e23030422024. View Article : Google Scholar
|
|
230
|
Li Y, Fu R, Duan Z, Zhu C and Fan D:
Artificial nonenzymatic antioxidant MXene Nanosheet-anchored
injectable hydrogel as a mild photothermal-controlled oxygen
release platform for diabetic wound healing. ACS Nano.
16:7486–7502. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
231
|
Şeker Ş, Elçin AE and Elçin YM: Current
trends in the design and fabrication of PRP-based scaffolds for
tissue engineering and regenerative medicine. Biomed Mater.
20:202025. View Article : Google Scholar
|
|
232
|
Xu K, Deng S, Zhu Y, Yang W, Chen W, Huang
L, Zhang C, Li M, Ao L, Jiang Y, et al: Platelet rich plasma loaded
multifunctional hydrogel accelerates diabetic wound healing via
regulating the continuously abnormal microenvironments. Adv Healthc
Mater. 12:e23013702023. View Article : Google Scholar : PubMed/NCBI
|
|
233
|
Wei Q, Su J, Meng S, Wang Y, Ma K, Li B,
Chu Z, Huang Q, Hu W, Wang Z, et al: MiR-17-5p-engineered sEVs
encapsulated in GelMA hydrogel facilitated diabetic wound healing
by targeting PTEN and p21. Adv Sci (Weinh). 11:e23077612024.
View Article : Google Scholar : PubMed/NCBI
|
|
234
|
Alberts A, Bratu AG, Niculescu AG and
Grumezescu AM: New perspectives of hydrogels in chronic wound
management. Molecules. 30:6862025. View Article : Google Scholar : PubMed/NCBI
|
|
235
|
Negut I and Bita B: Exploring the
potential of artificial intelligence for hydrogel development-a
short review. Gels. 9:8452023. View Article : Google Scholar : PubMed/NCBI
|
|
236
|
Tan CT, Liang K, Ngo ZH, Dube CT and Lim
CY: Application of 3D bioprinting technologies to the management
and treatment of diabetic foot ulcers. Biomedicines. 8:4412020.
View Article : Google Scholar : PubMed/NCBI
|
|
237
|
Wang M, Song Y, Bisoyi HK, Yang JF, Liu L,
Yang H and Li Q: A liquid crystal elastomer-based unprecedented
two-way shape-memory aerogel. Adv Sci (Weinh). 8:e21026742021.
View Article : Google Scholar : PubMed/NCBI
|
|
238
|
Jeong Y, Patel R and Patel M:
Biopolymer-Based biomimetic aerogel for biomedical applications.
Biomimetics (Basel). 9:3972024. View Article : Google Scholar : PubMed/NCBI
|
|
239
|
Guo N, Xia Y, Zeng W, Chen J, Wu Q, Shi Y,
Li G, Huang Z, Wang G and Liu Y: Alginate-based aerogels as wound
dressings for efficient bacterial capture and enhanced
antibacterial photodynamic therapy. Drug Deliv. 29:1086–1099. 2022.
View Article : Google Scholar : PubMed/NCBI
|
|
240
|
Wang X, Yuan Z, Shafiq M, Cai G, Lei Z, Lu
Y, Guan X, Hashim R, El-Newehy M, Abdulhameed MM, et al: Composite
aerogel scaffolds containing flexible silica nanofiber and
tricalcium phosphate enable skin regeneration. ACS Appl Mater
Interfaces. 16:25843–25855. 2024. View Article : Google Scholar : PubMed/NCBI
|
|
241
|
Vaseghi A, Sadeghizadeh M, Herb M, Grumme
D, Demidov Y, Remmler T and Maleki HH: 3D printing of biocompatible
and antibacterial silica-silk-chitosan-based hybrid aerogel
scaffolds loaded with propolis. ACS Appl Bio Mater. 7:7917–7935.
2024. View Article : Google Scholar : PubMed/NCBI
|
|
242
|
Wu B, Pan W, Luo S, Luo X, Zhao Y, Xiu Q,
Zhong M, Wang Z, Liao T, Li N, et al: Turmeric-Derived
nanoparticles functionalized aerogel regulates multicellular
networks to promote diabetic wound healing. Adv Sci (Weinh).
11:e23076302024. View Article : Google Scholar : PubMed/NCBI
|
|
243
|
Ramos R, Silva JP, Rodrigues AC, Costa R,
Guardão L, Schmitt F, Soares R, Vilanova M, Domingues L and Gama M:
Wound healing activity of the human antimicrobial peptide LL37.
Peptides. 32:1469–1476. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
244
|
Grönberg A, Mahlapuu M, Ståhle M,
Whately-Smith C and Rollman O: Treatment with LL-37 is safe and
effective in enhancing healing of hard-to-heal venous leg ulcers: A
randomized, placebo-controlled clinical trial. Wound Repair Regen.
22:613–621. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
245
|
John JV, Sharma NS, Tang G, Luo Z, Su Y,
Weihs S, Shahriar SMS, Wang G, McCarthy A, Dyke J, et al: Nanofiber
aerogels with precision macrochannels and LL-37-mimic peptides
synergistically promote diabetic wound healing. Adv Funct Mater.
33:22069362023. View Article : Google Scholar : PubMed/NCBI
|
|
246
|
Zhao C, Wu Z, Pan B, Zhang R, Golestani A,
Feng Z, Ge Y and Yang H: Functional biomacromolecules-based
microneedle patch for the treatment of diabetic wound. Int J Biol
Macromol. 267:1316502024. View Article : Google Scholar : PubMed/NCBI
|
|
247
|
Hu F, Gao Q, Liu J, Chen W, Zheng C, Bai
Q, Sun N, Zhang W, Zhang Y and Lu T: Smart microneedle patches for
wound healing and management. J Mater Chem B. 11:2830–2851. 2023.
View Article : Google Scholar : PubMed/NCBI
|
|
248
|
Yin M, Wu J, Deng M, Wang P, Ji G, Wang M,
Zhou C, Blum NT, Zhang W, Shi H, et al: Multifunctional magnesium
organic framework-based microneedle patch for accelerating diabetic
wound healing. ACS Nano. 15:17842–17853. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
249
|
Wang P, Wu J, Yang H, Liu H, Yao T, Liu C,
Gong Y, Wang M, Ji G, Huang P and Wang X: Intelligent microneedle
patch with prolonged local release of hydrogen and magnesium ions
for diabetic wound healing. Bioact Mater. 24:463–476.
2023.PubMed/NCBI
|
|
250
|
Avcil M and Çelik A: Microneedles in drug
delivery: Progress and challenges. Micromachines (Basel).
12:13212021. View Article : Google Scholar : PubMed/NCBI
|
|
251
|
Alsarayreh AZ, Oran SA, Shakhanbeh JM,
Khleifat KM, Al Qaisi YT, Alfarrayeh II and Alkaramseh AM: Efficacy
of methanolic extracts of some medicinal plants on wound healing in
diabetic rats. Heliyon. 8:e100712022. View Article : Google Scholar : PubMed/NCBI
|
|
252
|
Wu JR, Lu YC, Hung SJ, Lin JH, Chang KC,
Chen JK, Tsai WT, Ho TJ and Chen HP: Antimicrobial and
immunomodulatory activity of herb extracts used in burn wound
healing: 'San Huang Powder'. Evid Based Complement Alternat Med.
2021:29000602021. View Article : Google Scholar
|
|
253
|
Soheilifar MH, Dastan D, Masoudi-Khoram N,
Neghab HN, Nobari S, Tabaie SM and Amini R: In vitro and in vivo
evaluation of the diabetic wound healing properties of Saffron
(Crocus Sativus L.) petals. Sci Rep. 14:193732024. View Article : Google Scholar : PubMed/NCBI
|
|
254
|
Huang L, Cai HA, Zhang MS, Liao RY, Huang
X and Hu FD: Ginsenoside Rg1 promoted the wound healing in diabetic
foot ulcers via miR-489-3p/Sirt1 axis. J Pharmacol Sci.
147:271–283. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
255
|
Sun X, Wang X, Zhao Z, Chen J, Li C and
Zhao G: Paeoniflorin accelerates foot wound healing in diabetic
rats though activating the Nrf2 pathway. Acta Histochem.
122:1516492020. View Article : Google Scholar : PubMed/NCBI
|
|
256
|
Zhang S, Xu Y, Junior CZ, Chen X and Zhu
J: Dang-Gui-Si-Ni decoction facilitates wound healing in diabetic
foot ulcers by regulating expression of AGEs/RAGE/TGF-β/Smad2/3.
Arch Dermatol Res. 316:3382024. View Article : Google Scholar
|
|
257
|
Gong Y, Jiang Y, Huang J, He Z and Tang Q:
Moist exposed burn ointment accelerates diabetes-related wound
healing by promoting re-epithelialization. Front Med (Lausanne).
9:10420152022. View Article : Google Scholar
|
|
258
|
Ding R, Wang Y, Zhu JP, Lu WG, Wei GL, Gu
ZC, An ZT and Huo JG: Danggui Sini decoction protects against
oxaliplatin-induced peripheral neuropathy in rats. J Integr
Neurosci. 19:663–671. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
259
|
Mabvuure NT, Brewer CF, Gervin K and Duffy
S: The use of moist exposed burn ointment (MEBO) for the treatment
of burn wounds: A systematic review. J Plast Surg Hand Surg.
54:337–343. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
260
|
Zhao D, Luo S, Xu W, Hu J, Lin S and Wang
N: Efficacy and safety of hyperbaric oxygen therapy used in
patients with diabetic foot: A meta-analysis of randomized clinical
trials. Clin Ther. 39:2088–2094.e2. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
261
|
Damineni U, Divity S, Gundapaneni SRC,
Burri RG and Vadde T: Clinical outcomes of hyperbaric oxygen
therapy for diabetic foot ulcers: A systematic review. Cureus.
17:e786552025.PubMed/NCBI
|
|
262
|
Huang X, Liang P, Jiang B, Zhang P, Yu W,
Duan M, Guo L, Cui X, Huang M and Huang X: Hyperbaric oxygen
potentiates diabetic wound healing by promoting fibroblast cell
proliferation and endothelial cell angiogenesis. Life Sci.
259:1182462020. View Article : Google Scholar : PubMed/NCBI
|
|
263
|
Borys S, Hohendorff J, Koblik T, Witek P,
Ludwig-Slomczynska AH, Frankfurter C, Kiec-Wilk B and Malecki MT:
Negative-pressure wound therapy for management of chronic
neuropathic noninfected diabetic foot ulcerations-short-term
efficacy and long-term outcomes. Endocrine. 62:611–616. 2018.
View Article : Google Scholar : PubMed/NCBI
|
|
264
|
Chen L, Zhang S, Da J, Wu W, Ma F, Tang C,
Li G, Zhong D and Liao B: A systematic review and meta-analysis of
efficacy and safety of negative pressure wound therapy in the
treatment of diabetic foot ulcer. Ann Palliat Med. 10:10830–10839.
2021. View Article : Google Scholar : PubMed/NCBI
|
|
265
|
Huang Y, Yu Z, Xu M, Zhao X, Tang Y, Luo
L, Deng D and Chen M: Negative pressure wound therapy promotes
wound healing by down-regulating miR-155 expression in granulation
tissue of diabetic foot ulcers. Sci Rep. 15:67332025. View Article : Google Scholar : PubMed/NCBI
|
|
266
|
Liu L, Chen R, Jia Z, Li X, Tang Y, Zhao
X, Zhang S, Luo L, Fang Z, Zhang Y and Chen M: Downregulation of
hsa-miR-203 in peripheral blood and wound margin tissue by negative
pressure wound therapy contributes to wound healing of diabetic
foot ulcers. Microvasc Res. 139:1042752022. View Article : Google Scholar
|
|
267
|
Houreld NN: Shedding light on a new
treatment for diabetic wound healing: A review on phototherapy.
ScientificWorldJournal. 2014:3984122014. View Article : Google Scholar : PubMed/NCBI
|
|
268
|
Houreld N and Abrahamse H: Low-intensity
laser irradiation stimulates wound healing in diabetic wounded
fibroblast cells (WS1). Diabetes Technol Ther. 12:971–978. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
269
|
Gupta A, Dai T and Hamblin MR: Effect of
red and near-infrared wavelengths on low-level laser (light)
therapy-induced healing of partial-thickness dermal abrasion in
mice. Lasers Med Sci. 29:257–265. 2014. View Article : Google Scholar
|
|
270
|
Cai W, Hamushan M, Zhang Y, Xu Z, Ren Z,
Du J, Ju J, Cheng P, Tan M and Han P: Synergistic effects of
photobiomodulation therapy with combined wavelength on diabetic
wound healing in vitro and in vivo. Photobiomodul Photomed Laser
Surg. 40:13–24. 2022.
|
|
271
|
Frampton JE, Lee CR and Faulds D:
Filgrastim. A review of its pharmacological properties and
therapeutic efficacy in neutropenia. Drugs. 48:731–760. 1994.
View Article : Google Scholar : PubMed/NCBI
|
|
272
|
Edmonds M, Gough A, Solovera J and
Standaert B: Filgrastim in the treatment of infected diabetic foot
ulcers. Clin Drug Investig. 17:275–286. 1999. View Article : Google Scholar
|
|
273
|
Orgill DP and Bayer LR: Negative pressure
wound therapy: Past, present and future. Int Wound J. 10:15–19.
2013. View Article : Google Scholar : PubMed/NCBI
|
|
274
|
Yoon D, Byun HJ, Oh SJ, Park JH and Lee
DY: A case of cutaneous leukocytoclastic vasculitis associated with
granulocyte colony-stimulating factor: An unusual presentation. Ann
Dermatol. 32:164–167. 2020. View Article : Google Scholar
|
|
275
|
Song P, Liang Q, Ge X, Zhou D, Yuan M, Chu
W and Xu J: Adipose-derived stem cell exosomes promote scar-free
healing of diabetic wounds via miR-204-5p/TGF-β1/Smad pathway. Stem
Cells Int. 2025:63448442025. View Article : Google Scholar
|
|
276
|
Van Netten JJ, Woodburn J and Bus SA: The
future for diabetic foot ulcer prevention: A paradigm shift from
stratified healthcare towards personalized medicine. Diabetes Metab
Res Rev. 36(Suppl 1): e32342020. View Article : Google Scholar : PubMed/NCBI
|
|
277
|
Ng GW, Gan KF, Liew H, Ge L, Ang G, Molina
J, Sun Y, Prakash PS, Harish KB and Lo ZJ: A Systematic review and
classification of factors influencing diabetic foot ulcer treatment
adherence, in accordance with the WHO dimensions of adherence to
long-term therapies. Int J Low Extrem Wounds.
20:153473462412339622024. View Article : Google Scholar
|
|
278
|
Zhang YW, Sun L, Wang YN and Zhan SY: Role
of macrophage polarization in diabetic foot ulcer healing: A
bibliometric study. World J Diabetes. 16:997552025. View Article : Google Scholar : PubMed/NCBI
|
|
279
|
Choudhury S, Dhoke NR, Chawla S and Das A:
Bioengineered MSCCxcr2 transdifferentiated keratinocyte-like
cell-derived organoid potentiates skin regeneration through ERK1/2
and STAT3 signaling in diabetic wound. Cell Mol Life Sci.
81:1722024. View Article : Google Scholar :
|
|
280
|
de Souza A, Martignago CCS, Santo GDE,
Sousa KDSJ, Cruz MA, Amaral GO, Parisi JR, Estadella D, Ribeiro DA,
Granito RN, et al: 3D printed wound constructs for skin tissue
engineering: A systematic review in experimental animal models. J
Biomed Mater Res B Appl Biomater. 111:1419–1433. 2023. View Article : Google Scholar : PubMed/NCBI
|
|
281
|
Yuan T, Tan M, Xu Y, Xiao Q, Wang H, Wu C,
Li F and Peng L: All-in-one smart dressing for simultaneous
angiogenesis and neural regeneration. J Nanobiotechnology.
21:382023. View Article : Google Scholar : PubMed/NCBI
|
|
282
|
Mirani B, Hadisi Z, Pagan E, Dabiri SMH,
van Rijt A, Almutairi L, Noshadi I, Armstrong DG and Akbari M:
Smart dual-sensor wound dressing for monitoring cutaneous wounds.
Adv Healthc Mater. 12:e22032332023. View Article : Google Scholar : PubMed/NCBI
|
|
283
|
Su R, Wang L, Han F, Bian S, Meng F, Qi W,
Zhai X, Li H, Wu J, Pan X, et al: A highly stretchable smart
dressing for wound infection monitoring and treatment. Mater Today
Bio. 26:1011072024. View Article : Google Scholar : PubMed/NCBI
|
|
284
|
Lin B, Ma Y and Wu S: Multi-Omics and
artificial intelligence-guided data integration in chronic liver
disease: Prospects and challenges for precision medicine. OMICS.
26:415–421. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
285
|
Wang Z, Xu H, Xue B, Liu L, Tang Y, Wang Z
and Yao K: MSC-derived exosomal circMYO9B accelerates diabetic
wound healing by promoting angiogenesis through the
hnRNPU/CBL/KDM1A/VEGFA axis. Commun Biol. 7:17002024. View Article : Google Scholar : PubMed/NCBI
|
|
286
|
Liu J, Qu M, Wang C, Xue Y, Huang H, Chen
Q, Sun W, Zhou X, Xu G and Jiang X: A dual-cross-linked hydrogel
patch for promoting diabetic wound healing. Small. 18:e21061722022.
View Article : Google Scholar : PubMed/NCBI
|
|
287
|
Wu C, Long L, Zhang Y, Xu Y, Lu Y, Yang Z,
Guo Y, Zhang J, Hu X and Wang Y: Injectable conductive and
angiogenic hydrogels for chronic diabetic wound treatment. J
Control Release. 344:249–260. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
288
|
Liu W, Zhai X, Zhao X, Cai Y, Zhang X, Xu
K, Weng J, Li J and Chen X: Multifunctional double-layer and dual
drug-loaded microneedle patch promotes diabetic wound healing. Adv
Healthc Mater. 12:e23002972023. View Article : Google Scholar : PubMed/NCBI
|
|
289
|
Xiong W, Zhang X, Zhou J, Chen J, Liu Y,
Yan Y, Tan M, Huang H, Si Y and Wei Y: Astragaloside IV promotes
exosome secretion of endothelial progenitor cells to regulate
PI3KR2/SPRED1 signaling and inhibit pyroptosis of diabetic
endothelial cells. Cytotherapy. 26:36–50. 2024. View Article : Google Scholar
|
|
290
|
Lei T, Gao Y, Duan Y, Cui C, Zhang L and
Si M: Panax notoginseng saponins improves healing of high
glucose-induced wound through the GSK-3β/β-catenin pathway. Environ
Toxicol. 37:1867–1877. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
291
|
Lu Y, Ding X, Qi F, Ru Y, Kuai L, Chen S,
Yang Y, Li X, Li F, Li B, et al: Quyu Shengji formula facilitates
diabetic wound healing via inhibiting the expression of
prostaglandin transporter. Evid Based Complement Alternat Med.
2021:88499352021. View Article : Google Scholar : PubMed/NCBI
|
|
292
|
Liu Y, Mo J, Liang F, Jiang S, Xiong J,
Meng X and Mo Z: Pien-tze-huang promotes wound healing in
streptozotocin-induced diabetes models associated with improving
oxidative stress via the Nrf2/ARE pathway. Front Pharmacol.
14:10626642023. View Article : Google Scholar : PubMed/NCBI
|