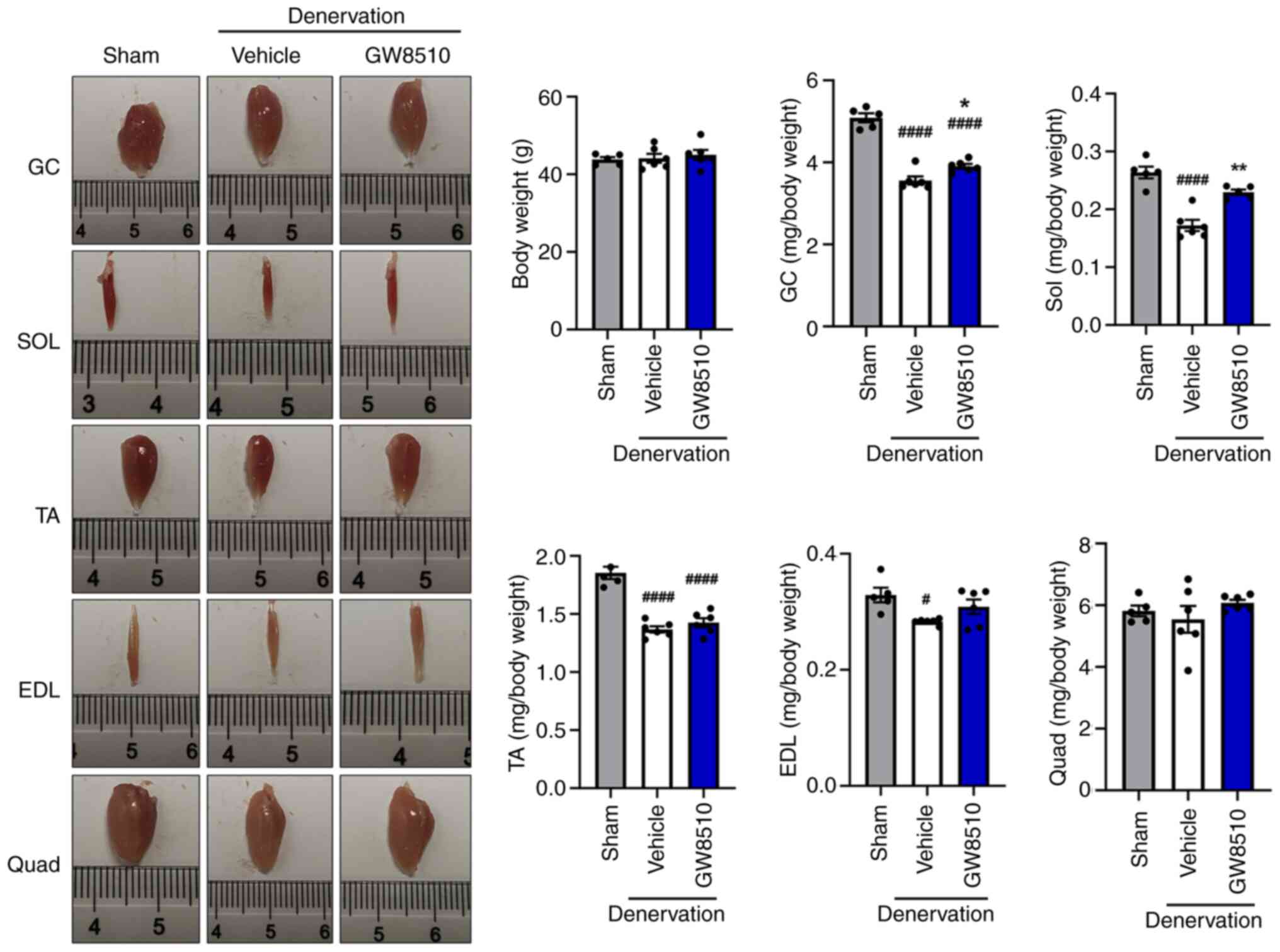
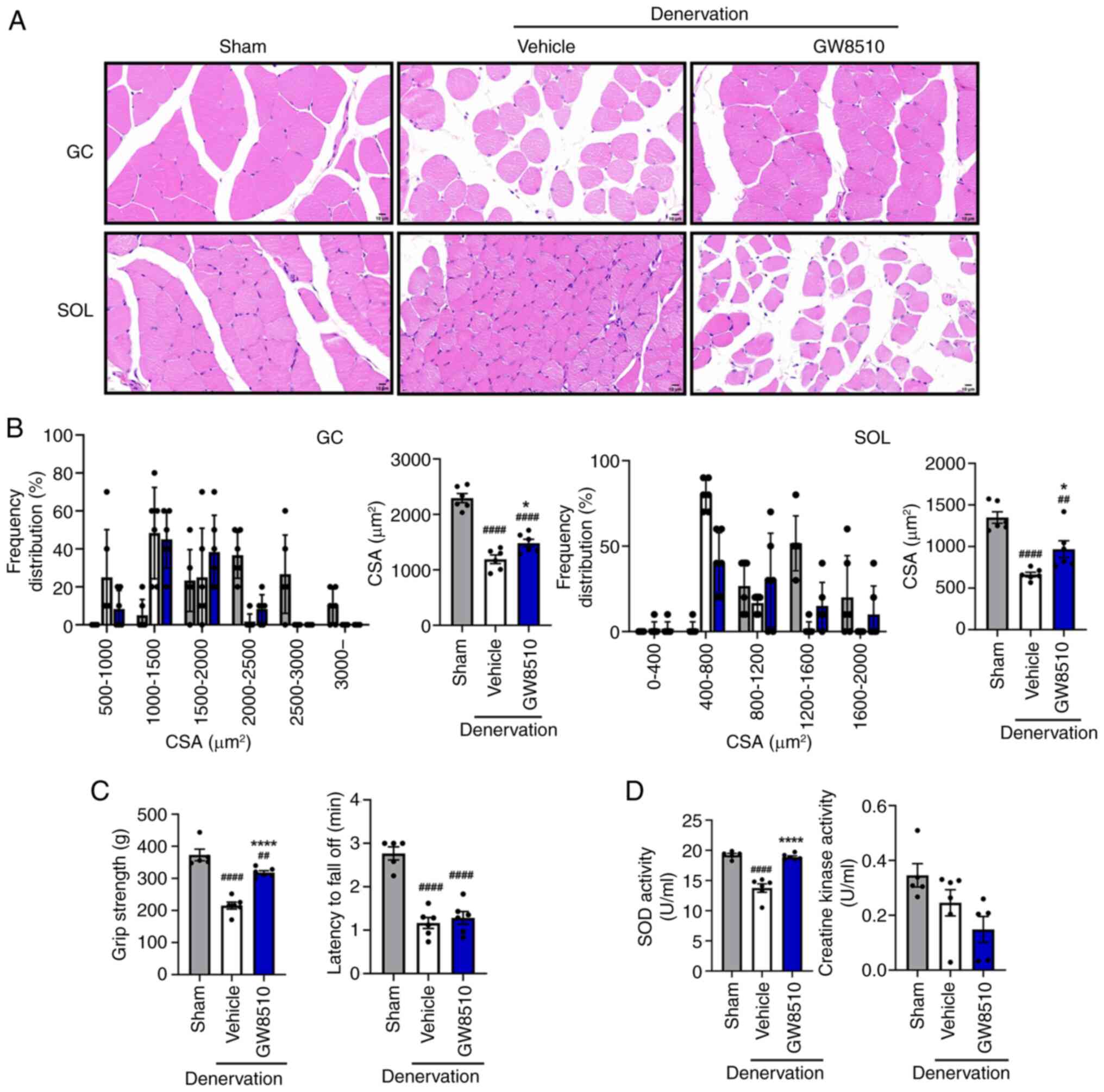
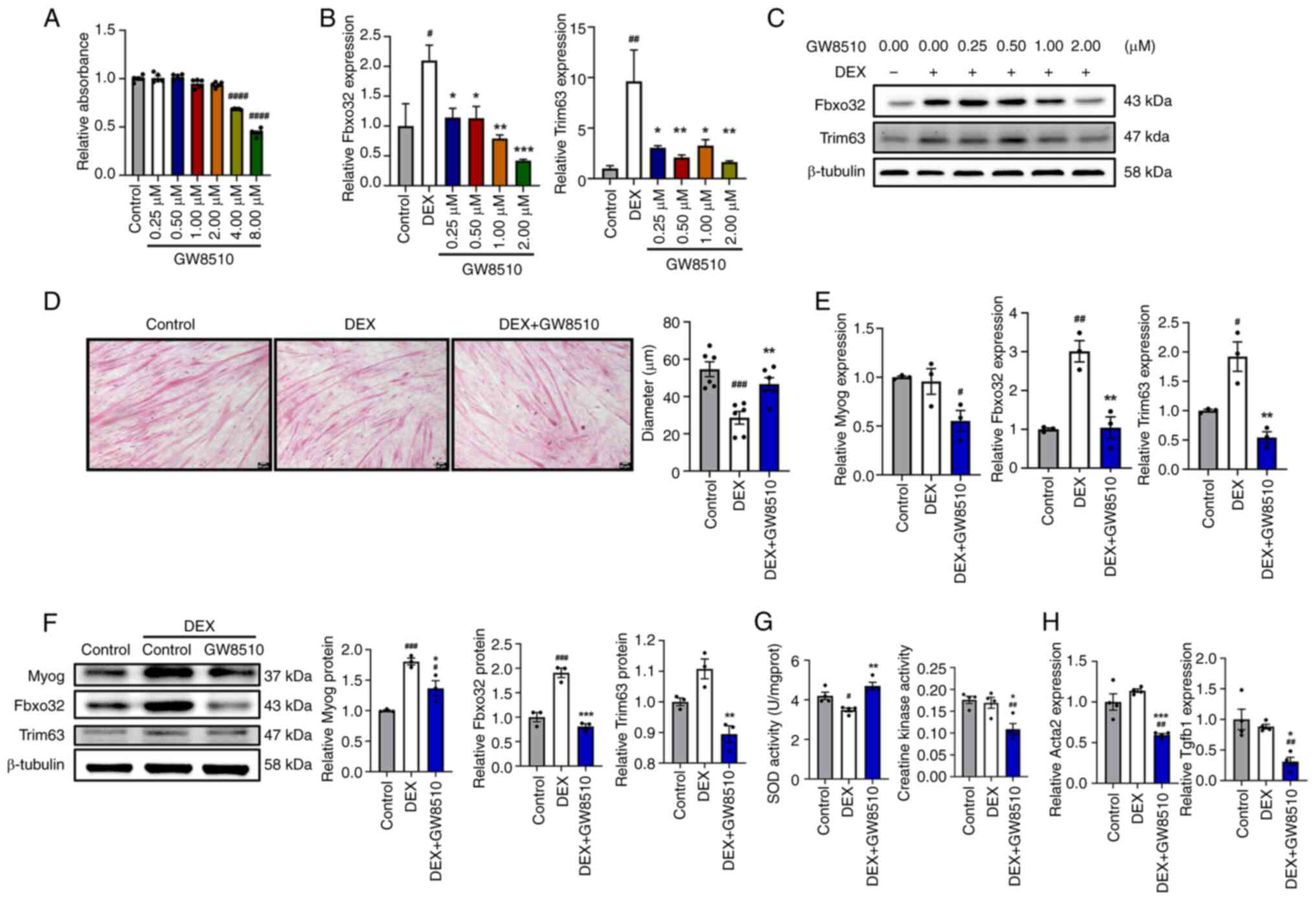
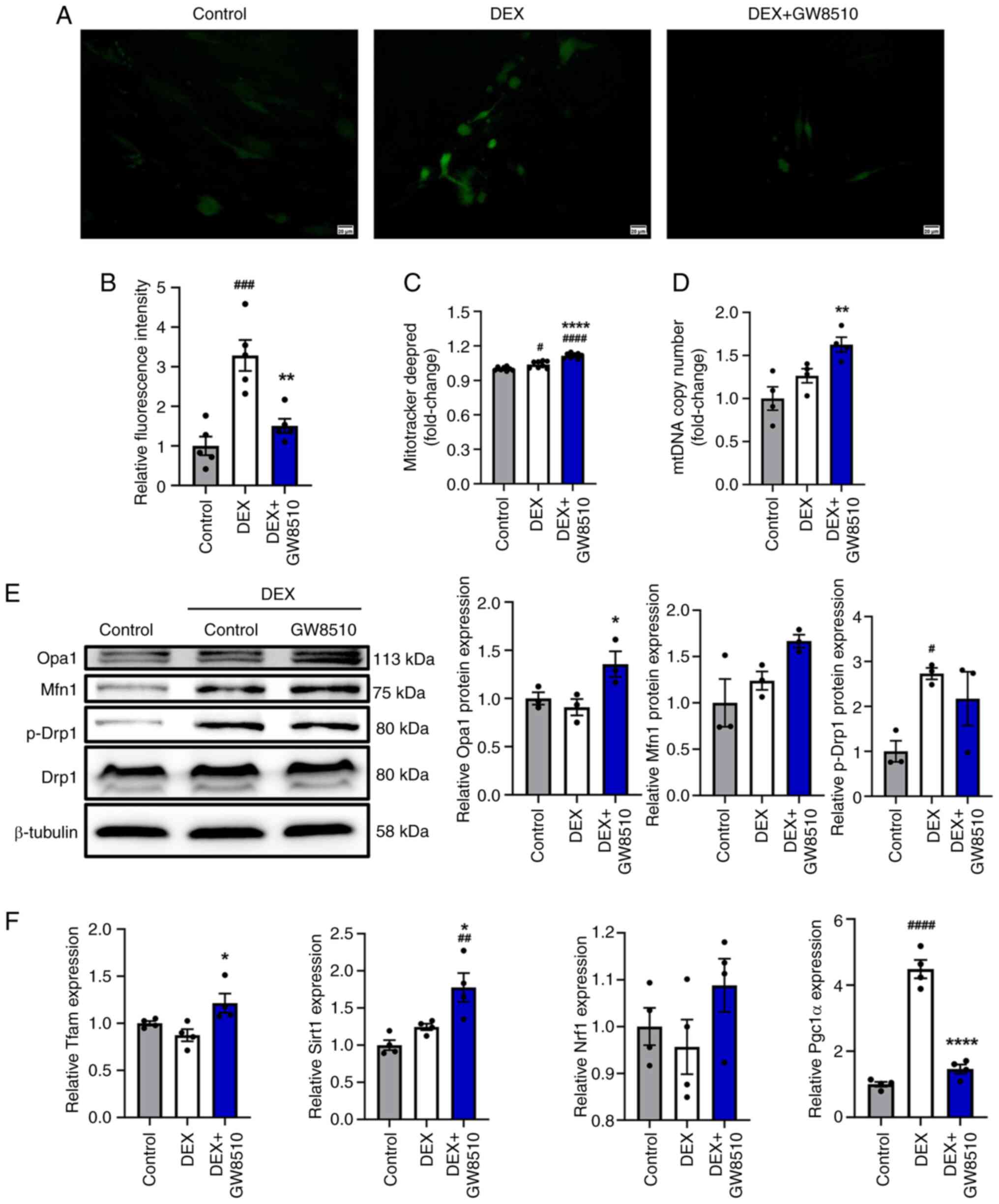
|
1
|
Sayer AA and Cruz-Jentoft A: Sarcopenia
definition, diagnosis and treatment: Consensus is growing. Age
Ageing. 51:afac2202022. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Petrocelli JJ, de Hart N, Lang MJ, Yee EM,
Ferrara PJ, Fix DK, Chaix A, Funai K and Drummond MJ: Cellular
senescence and disrupted proteostasis induced by myotube atrophy
are prevented with low-dose metformin and leucine cocktail. Aging
(Albany NY). 15:1808–1832. 2023. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Paez HG, Pitzer CR and Alway SE:
Age-Related dysfunction in proteostasis and cellular quality
control in the development of sarcopenia. Cells. 12:2492023.
View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Bodine SC and Baehr LM: Skeletal muscle
atrophy and the E3 ubiquitin ligases MuRF1 and MAFbx/atrogin-1. Am
J Physiol Endocrinol Metab. 307:E469–E484. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Cong H, Sun L, Liu C and Tien P:
Inhibition of atrogin-1/MAFbx expression by adenovirus-delivered
small hairpin RNAs attenuates muscle atrophy in fasting mice. Hum
Gene Ther. 22:313–324. 2011. View Article : Google Scholar
|
|
6
|
Baehr LM, Furlow JD and Bodine SC: Muscle
sparing in muscle RING finger 1 null mice: Response to synthetic
glucocorticoids. J Physiol. 589:4759–4776. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Stouth DW, vanLieshout TL, Mikhail AI, Ng
SY, Raziee R, Edgett BA, Vasam G, Webb EK, Gilotra KS, Markou M, et
al: CARM1 drives mitophagy and autophagy flux during
fasting-induced skeletal muscle atrophy. Autophagy. 20:1247–1269.
2024. View Article : Google Scholar :
|
|
8
|
Leduc-Gaudet JP, Hussain SNA, Barreiro E
and Gouspillou G: Mitochondrial dynamics and mitophagy in skeletal
muscle health and aging. Int J Mol Sci. 22:81792021. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Abrigo J, Olguín H, Tacchi F,
Orozco-Aguilar J, Valero-Breton M, Soto J, Castro-Sepúlveda M,
Elorza AA, Simon F and Cabello-Verrugio C: Cholic and deoxycholic
acids induce mitochondrial dysfunction, impaired biogenesis and
autophagic flux in skeletal muscle cells. Biol Res. 56:302023.
View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Nascimento CM, Ingles M, Salvador-Pascual
A, Cominetti MR, Gomez-Cabrera MC and Viña J: Sarcopenia, frailty
and their prevention by exercise. Free Radic Biol Med. 132:42–49.
2019. View Article : Google Scholar
|
|
11
|
Fomina-Yadlin D, Kubicek S, Vetere A, He
KH, Schreiber SL and Wagner BK: GW8510 increases insulin expression
in pancreatic alpha cells through activation of p53 transcriptional
activity. PLoS One. 7:e288082012. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Guo K and Walsh K: Inhibition of
myogenesis by multiple cyclin-Cdk complexes. Coordinate regulation
of myogenesis and cell cycle activity at the level of E2F. J Biol
Chem. 272:791–797. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Bhanu NV, Sidoli S, Yuan ZF, Molden RC and
Garcia BA: Regulation of proline-directed kinases and the
trans-histone code H3K9me3/H4K20me3 during human myogenesis. J Biol
Chem. 294:8296–8308. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Rüstem DG, Atay S, Aydin HH and Ak H:
Synergistic interactions between GW8510 and gemcitabine in an in
vitro model of pancreatic cancer. Anticancer Agents Med Chem.
21:2204–2215. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Li ZN, Shu Y, Chen CG, Li XQ, Li MY, Zhao
XH, Wang S and Li J: Acquired tamoxifen resistance is surmounted by
GW8510 through ribonucleotide reductase M2 downregulation-mediated
autophagy induction. Biochem Biophys Res Commun. 528:554–560. 2020.
View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Hsieh YY, Chou CJ, Lo HL and Yang PM:
Repositioning of a cyclin-dependent kinase inhibitor GW8510 as a
ribonucleotide reductase M2 inhibitor to treat human colorectal
cancer. Cell Death Discov. 2:160272016. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Chen P, Wu JN, Shu Y, Jiang HG, Zhao XH,
Qian H, Chen K, Lan T, Chen CG and Li J: Gemcitabine resistance
mediated by ribonucleotide reductase M2 in lung squamous cell
carcinoma is reversed by GW8510 through autophagy induction. Clin
Sci (Lond). 132:1417–1433. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Dong F, Guo W, Zhang L, Wu S, Teraishi F,
Davis JJ and Fang B: Downregulation of XIAP and induction of
apoptosis by the synthetic cyclin-dependent kinase inhibitor GW8510
in non-small cell lung cancer cells. Cancer Biol Ther. 5:165–170.
2006. View Article : Google Scholar
|
|
19
|
Johnson K, Liu L, Majdzadeh N, Chavez C,
Chin PC, Morrison B, Wang L, Park J, Chugh P, Chen HM and D'Mello
SR: Inhibition of neuronal apoptosis by the cyclin-dependent kinase
inhibitor GW8510: Identification of 3′ substituted indolones as a
scaffold for the development of neuroprotective drugs. J Neurochem.
93:538–548. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Wimalasena NK, Le VQ, Wimalasena K,
Schreiber SL and Karmacharya R: Gene expression-based screen for
Parkinson's disease identifies GW8510 as a neuroprotective agent.
ACS Chem Neurosci. 7:857–863. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Asami Y, Aizawa M, Kinoshita M, Ishikawa J
and Sakuma K: Resveratrol attenuates denervation-induced muscle
atrophy due to the blockade of atrogin-1 and p62 accumulation. Int
J Med Sci. 15:628–637. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Kim HJ, Kim SW, Lee SH, Jung DW and
Williams DR: Inhibiting 5-lipoxygenase prevents skeletal muscle
atrophy by targeting organogenesis signalling and insulin-like
growth factor-1. J Cachexia Sarcopenia Muscle. 13:3062–3077. 2022.
View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Chiu HC, Yang RS, Weng TI, Chiu CY, Lan KC
and Liu SH: A ubiquitous endocrine disruptor tributyltin induces
muscle wasting and retards muscle regeneration. J Cachexia
Sarcopenia Muscle. 14:167–181. 2023. View Article : Google Scholar :
|
|
24
|
Chen HJ, Wang CC, Chan DC, Chiu CY, Yang
RS and Liu SH: Adverse effects of acrolein, a ubiquitous
environmental toxicant, on muscle regeneration and mass. J Cachexia
Sarcopenia Muscle. 10:165–176. 2019. View Article : Google Scholar :
|
|
25
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(−Delta Delta C(T)) method. Methods. 25:402–408. 2001.
View Article : Google Scholar
|
|
26
|
Mahdy MAA: Skeletal muscle fibrosis: An
overview. Cell Tissue Res. 375:575–588. 2019. View Article : Google Scholar
|
|
27
|
Kubat GB, Bouhamida E, Ulger O, Turkel I,
Pedriali G, Ramaccini D, Ekinci O, Ozerklig B, Atalay O, Patergnani
S, et al: Mitochondrial dysfunction and skeletal muscle atrophy:
Causes, mechanisms, and treatment strategies. Mitochondrion.
72:33–58. 2023. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Yokokawa T, Mori R, Suga T, Isaka T,
Hayashi T and Fujita S: Muscle denervation reduces mitochondrial
biogenesis and mitochondrial translation factor expression in mice.
Biochem Biophys Res Commun. 527:146–152. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Romanello V, Guadagnin E, Gomes L, Roder
I, Sandri C, Petersen Y, Milan G, Masiero E, Del Piccolo P, Foretz
M, et al: Mitochondrial fission and remodelling contributes to
muscle atrophy. EMBO J. 29:1774–1785. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Noone J, Rochfort KD, O'Sullivan F and
O'Gorman DJ: SIRT4 is a regulator of human skeletal muscle fatty
acid metabolism influencing inner and outer mitochondrial
membrane-mediated fusion. Cell Signal. 112:1109312023. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Sin J, Andres AM, Taylor DJ, Weston T,
Hiraumi Y, Stotland A, Kim BJ, Huang C, Doran KS and Gottlieb RA:
Mitophagy is required for mitochondrial biogenesis and myogenic
differentiation of C2C12 myoblasts. Autophagy. 12:369–380. 2016.
View Article : Google Scholar :
|
|
32
|
Yeo D, Kang C, Gomez-Cabrera MC, Vina J
and Ji LL: Intensified mitophagy in skeletal muscle with aging is
downregulated by PGC-1alpha overexpression in vivo. Free Radic Biol
Med. 130:361–368. 2019. View Article : Google Scholar
|
|
33
|
Frederick DW, Loro E, Liu L, Davila A Jr,
Chellappa K, Silverman IM, Quinn WJ III, Gosai SJ, Tichy ED, Davis
JG, et al: Loss of NAD homeostasis leads to progressive and
reversible degeneration of skeletal muscle. Cell Metab. 24:269–282.
2016. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Zhang H, Ryu D, Wu Y, Gariani K, Wang X,
Luan P, D'Amico D, Ropelle ER, Lutolf MP, Aebersold R, et al:
NAD+ repletion improves mitochondrial and stem cell
function and enhances life span in mice. Science. 352:1436–1443.
2016. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Carraro V, Combaret L, Coudy-Gandilhon C,
Parry L, Averous J, Maurin AC, Jousse C, Voyard G, Fafournoux P,
Papet I and Bruhat A: Activation of the eIF2α-ATF4 pathway by
chronic paracetamol treatment is prevented by dietary
supplementation with cysteine. Int J Mol Sci. 23:71962022.
View Article : Google Scholar
|
|
36
|
Kjøbsted R, Hingst JR, Fentz J, Foretz M,
Sanz MN, Pehmøller C, Shum M, Marette A, Mounier R, Treebak JT, et
al: AMPK in skeletal muscle function and metabolism. FASEB J.
32:1741–1777. 2018. View Article : Google Scholar :
|
|
37
|
Shan T, Liang X, Bi P and Kuang S:
Myostatin knockout drives browning of white adipose tissue through
activating the AMPK-PGC1α-Fndc5 pathway in muscle. FASEB J.
27:1981–1989. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Rodriguez J, Vernus B, Chelh I,
Cassar-Malek I, Gabillard JC, Sassi AH, Seiliez I, Picard B and
Bonnieu A: Myostatin and the skeletal muscle atrophy and
hypertrophy signaling pathways. Cell Mol Life Sci. 71:4361–4371.
2014. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Fernandez-Marcos PJ and Auwerx J:
Regulation of PGC-1α, a nodal regulator of mitochondrial
biogenesis. Am J Clin Nutr. 93:884s–890s. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Huang Y, Chen K, Ren Q, Yi L, Zhu J, Zhang
Q and Mi M: Dihydromyricetin attenuates dexamethasone-induced
muscle atrophy by improving mitochondrial function via the PGC-1α
pathway. Cell Physiol Biochem. 49:758–779. 2018. View Article : Google Scholar
|
|
41
|
Li Q, Wu J, Huang J, Hu R, You H, Liu L,
Wang D and Wei L: Paeoniflorin ameliorates skeletal muscle atrophy
in chronic kidney disease via AMPK/SIRT1/PGC-1α-mediated oxidative
stress and mitochondrial dysfunction. Front Pharmacol.
13:8597232022. View Article : Google Scholar
|
|
42
|
Cui A, Fan H, Zhang Y, Zhang Y, Niu D, Liu
S, Liu Q, Ma W, Shen Z, Shen L, et al: Dexamethasone-induced
Krüppel-like factor 9 expression promotes hepatic gluconeogenesis
and hyperglycemia. J Clin Invest. 129:2266–2278. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Cheng CF, Ku HC and Lin H: PGC-1α as a
pivotal factor in lipid and metabolic regulation. Int J Mol Sci.
19:34472018. View Article : Google Scholar
|
|
44
|
Zhang HJ, Wang BH, Wang X, Huang CP, Xu
SM, Wang JL, Huang TE, Xiao WL, Tian XL, Lan XQ, et al: Handelin
alleviates cachexia- and aging-induced skeletal muscle atrophy by
improving protein homeostasis and inhibiting inflammation. J
Cachexia Sarcopenia Muscle. 15:173–188. 2024. View Article : Google Scholar
|
|
45
|
Ozaki Y, Ohashi K, Otaka N, Kawanishi H,
Takikawa T, Fang L, Takahara K, Tatsumi M, Ishihama S, Takefuji M,
et al: Myonectin protects against skeletal muscle dysfunction in
male mice through activation of AMPK/PGC1α pathway. Nat Commun.
14:46752023. View Article : Google Scholar
|
|
46
|
Parker SB, Eichele G, Zhang P, Rawls A,
Sands AT, Bradley A, Olson EN, Harper JW and Elledge SJ:
p53-independent expression of p21Cip1 in muscle and other
terminally differentiating cells. Science. 267:1024–1027. 1995.
View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Wang J and Walsh K: Resistance to
apoptosis conferred by Cdk inhibitors during myocyte
differentiation. Science. 273:359–361. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
48
|
Tang H, Macpherson P, Marvin M, Meadows E,
Klein WH, Yang XJ and Goldman D: A histone deacetylase 4/myogenin
positive feedback loop coordinates denervation-dependent gene
induction and suppression. Mol Biol Cell. 20:1120–1131. 2009.
View Article : Google Scholar :
|
|
49
|
Moresi V, Williams AH, Meadows E, Flynn
JM, Potthoff MJ, McAnally J, Shelton JM, Backs J, Klein WH,
Richardson JA, et al: Myogenin and class II HDACs control
neurogenic muscle atrophy by inducing E3 ubiquitin ligases. Cell.
143:35–45. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
50
|
Sakellariou GK, Pearson T, Lightfoot AP,
Nye GA, Wells N, Giakoumaki II, Vasilaki A, Griffiths RD, Jackson
MJ and McArdle A: Mitochondrial ROS regulate oxidative damage and
mitophagy but not age-related muscle fiber atrophy. Sci Rep.
6:339442016. View Article : Google Scholar : PubMed/NCBI
|
|
51
|
Chen X, Ji Y, Liu R, Zhu X, Wang K, Yang
X, Liu B, Gao Z, Huang Y, Shen Y, et al: Mitochondrial dysfunction:
Roles in skeletal muscle atrophy. J Transl Med. 21:5032023.
View Article : Google Scholar : PubMed/NCBI
|
|
52
|
Bellanti F, Romano AD, Lo Buglio A,
Castriotta V, Guglielmi G, Greco A, Serviddio G and Vendemiale G:
Oxidative stress is increased in sarcopenia and associated with
cardiovascular disease risk in sarcopenic obesity. Maturitas.
109:6–12. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
53
|
Whitley BN, Engelhart EA and Hoppins S:
Mitochondrial dynamics and their potential as a therapeutic target.
Mitochondrion. 49:269–283. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
54
|
Zeviani M and Carelli V: Mitochondrial
retinopathies. Int J Mol Sci. 23:2102021. View Article : Google Scholar
|
|
55
|
Wang T, Sun F, Li C, Nan P, Song Y, Wan X,
Mo H, Wang J, Zhou Y, Guo Y, et al: MTA1, a novel ATP synthase
complex modulator, enhances colon cancer liver metastasis by
driving mitochondrial metabolism reprogramming. Adv Sci (Weinh).
10:e23007562023. View Article : Google Scholar : PubMed/NCBI
|
|
56
|
Xu Y and Xiao W: NAD+: An old but
promising therapeutic agent for skeletal muscle ageing. Ageing Res
Rev. 28:1021062023. View Article : Google Scholar
|
|
57
|
Sonntag T, Ancel S, Karaz S, Cichosz P,
Jacot G, Giner MP, Sanchez-Garcia JL, Pannérec A, Moco S,
Sorrentino V, et al: Nicotinamide riboside kinases regulate
skeletal muscle fiber-type specification and are rate-limiting for
metabolic adaptations during regeneration. Front Cell Dev Biol.
10:10496532022. View Article : Google Scholar : PubMed/NCBI
|
|
58
|
Li Y, Ma X, Li J, Yang L, Zhao X, Qi X,
Zhang X, Zhou Q and Shi W: Corneal denervation causes epithelial
apoptosis through inhibiting NAD+ biosynthesis. Invest Ophthalmol
Vis Sci. 60:3538–3546. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
59
|
Hsu CC, Peng D, Cai Z and Lin HK: AMPK
signaling and its targeting in cancer progression and treatment.
Semin Cancer Biol. 85:52–68. 2022. View Article : Google Scholar :
|
|
60
|
Guo Y, Meng J, Tang Y, Wang T, Wei B, Feng
R, Gong B, Wang H, Ji G and Lu Z: AMP-activated kinase α2
deficiency protects mice from denervation-induced skeletal muscle
atrophy. Arch Biochem Biophys. 600:56–60. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
61
|
Paul PK, Gupta SK, Bhatnagar S, Panguluri
SK, Darnay BG, Choi Y and Kumar A: Targeted ablation of TRAF6
inhibits skeletal muscle wasting in mice. J Cell Biol.
191:1395–1411. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
62
|
Kang MJ, Moon JW, Lee JO, Kim JH, Jung EJ,
Kim SJ, Oh JY, Wu SW, Lee PR, Park SH and Kim HS: Metformin induces
muscle atrophy by transcriptional regulation of myostatin via HDAC6
and FoxO3a. J Cachexia Sarcopenia Muscle. 13:605–620. 2022.
View Article : Google Scholar :
|
|
63
|
Xu M, Chen X, Huang Z, Chen D, Chen H, Luo
Y, Zheng P, He J, Yu J and Yu B: Procyanidin B2 promotes skeletal
slow-twitch myofiber gene expression through the AMPK signaling
pathway in C2C12 myotubes. J Agric Food Chem. 68:1306–1314. 2020.
View Article : Google Scholar : PubMed/NCBI
|
|
64
|
Mounier R, Lantier L, Leclerc J,
Sotiropoulos A, Pende M, Daegelen D, Sakamoto K, Foretz M and
Viollet B: Important role for AMPKalpha1 in limiting skeletal
muscle cell hypertrophy. FASEB J. 23:2264–2273. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
65
|
Nielsen JN, Mustard KJ, Graham DA, Yu H,
MacDonald CS, Pilegaard H, Goodyear LJ, Hardie DG, Richter EA and
Wojtaszewski JF: 5′-AMP-activated protein kinase activity and
subunit expression in exercise-trained human skeletal muscle. J
Appl Physiol (1985). 94:631–641. 2003. View Article : Google Scholar
|
|
66
|
Campos JC, Bozi LH, Krum B, Bechara LR,
Ferreira ND, Arini GS, Albuquerque RP, Traa A, Ogawa T, van der
Bliek AM, et al: Exercise preserves physical fitness during aging
through AMPK and mitochondrial dynamics. Proce Natl Acad Sci USA.
120:e22047501202023. View Article : Google Scholar
|
|
67
|
Wu L, Zhou M, Li T, Dong N, Yi L, Zhang Q
and Mi M: GLP-1 regulates exercise endurance and skeletal muscle
remodeling via GLP-1R/AMPK pathway. Biochimica et biophysica acta
Mol Cell Res. 1869:1193002022. View Article : Google Scholar
|
|
68
|
Gibala MJ, McGee SL, Garnham AP, Howlett
KF, Snow RJ and Hargreaves M: Brief intense interval exercise
activates AMPK and p38 MAPK signaling and increases the expression
of PGC-1alpha in human skeletal muscle. J Appl Physiol (1985).
106:929–934. 2009. View Article : Google Scholar
|
|
69
|
Wenz T: Mitochondria and PGC-1α in aging
and age-associated diseases. J Aging Res. 2011:8106192011.
View Article : Google Scholar
|
|
70
|
Jäger S, Handschin C, St-Pierre J and
Spiegelman BM: AMP-activated protein kinase (AMPK) action in
skeletal muscle via direct phosphorylation of PGC-1alpha. Proc Natl
Acad Sci USA. 104:12017–12022. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
71
|
Trevino MB, Zhang X, Standley RA, Wang M,
Han X, Reis FCG, Periasamy M, Yu G, Kelly DP, Goodpaster BH, et al:
Loss of mitochondrial energetics is associated with poor recovery
of muscle function but not mass following disuse atrophy. Am J
Physiol Endocrinol Metab. 317:E899–E910. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
72
|
Sandri M, Lin J, Handschin C, Yang W,
Arany ZP, Lecker SH, Goldberg AL and Spiegelman BM: PGC-1alpha
protects skeletal muscle from atrophy by suppressing FoxO3 action
and atrophy-specific gene transcription. Proc Natl Acad Sci USA.
103:16260–16265. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
73
|
Kang D and Hamasaki N: Mitochondrial
transcription factor A in the maintenance of mitochondrial DNA:
Overview of its multiple roles. Ann N Y Acad Sci. 1042:101–108.
2005. View Article : Google Scholar : PubMed/NCBI
|
|
74
|
Penna F, Costamagna D, Fanzani A, Bonelli
G, Baccino FM and Costelli P: Muscle wasting and impaired
myogenesis in tumor bearing mice are prevented by ERK inhibition.
PLoS One. 5:e136042010. View Article : Google Scholar : PubMed/NCBI
|