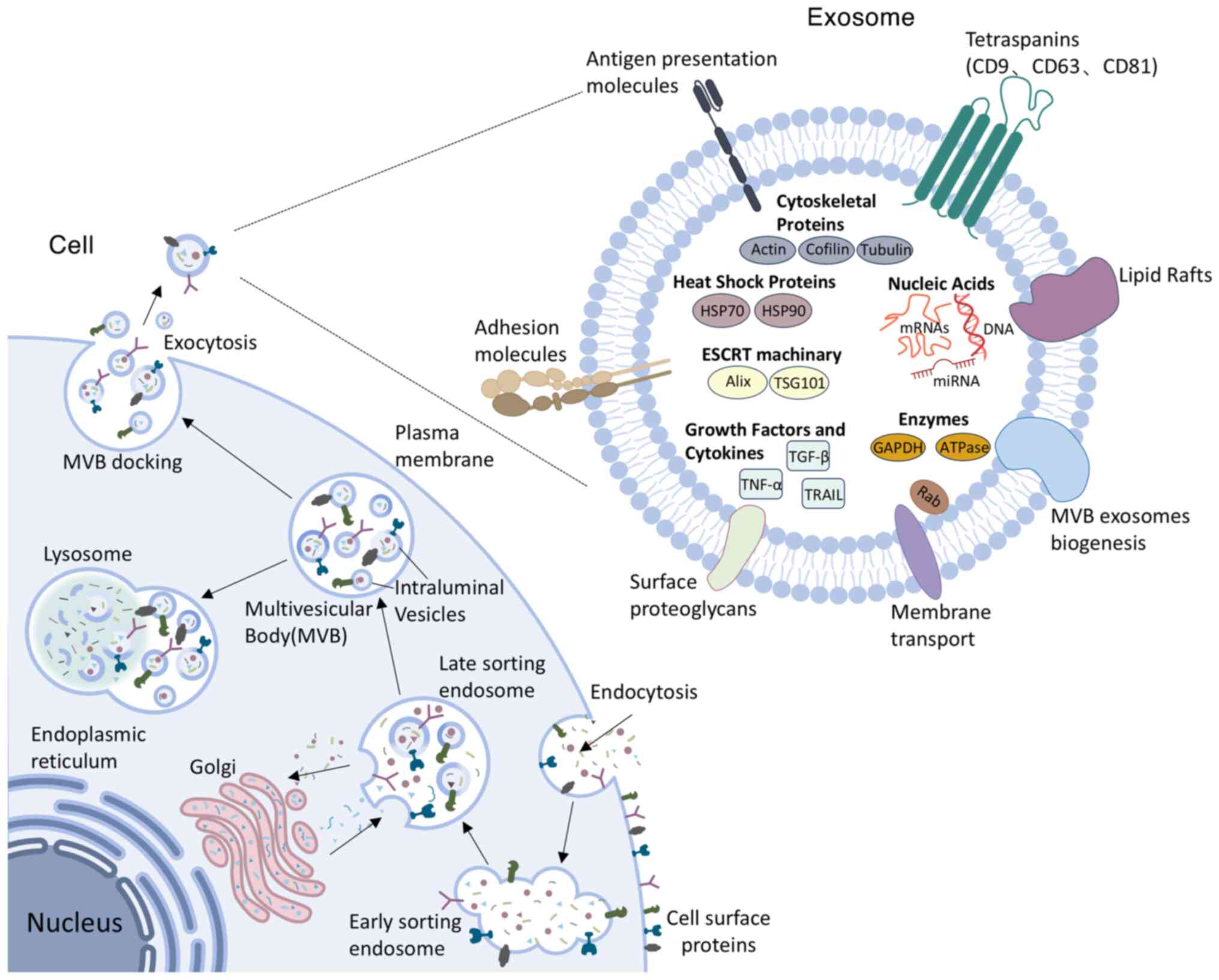
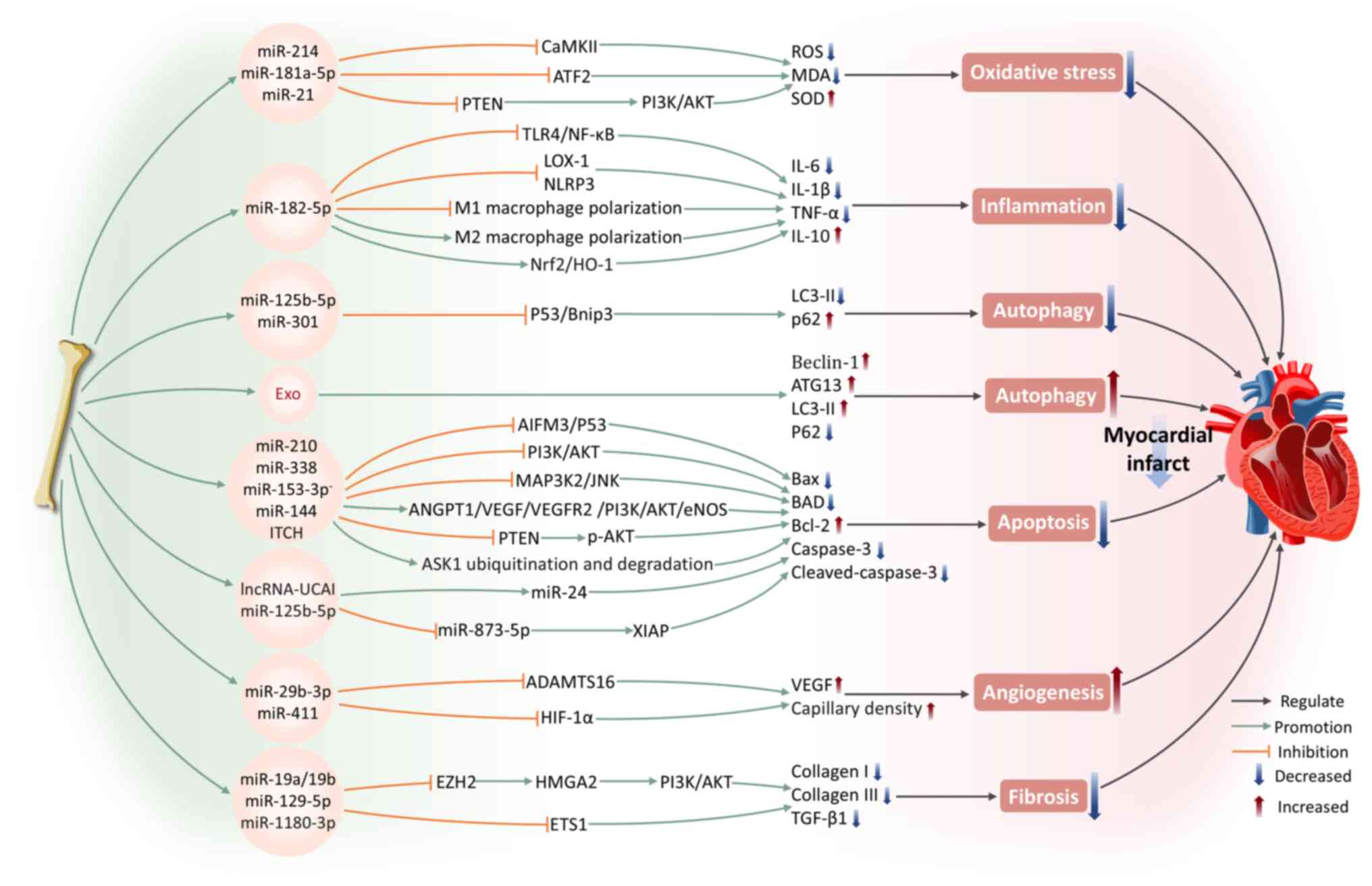
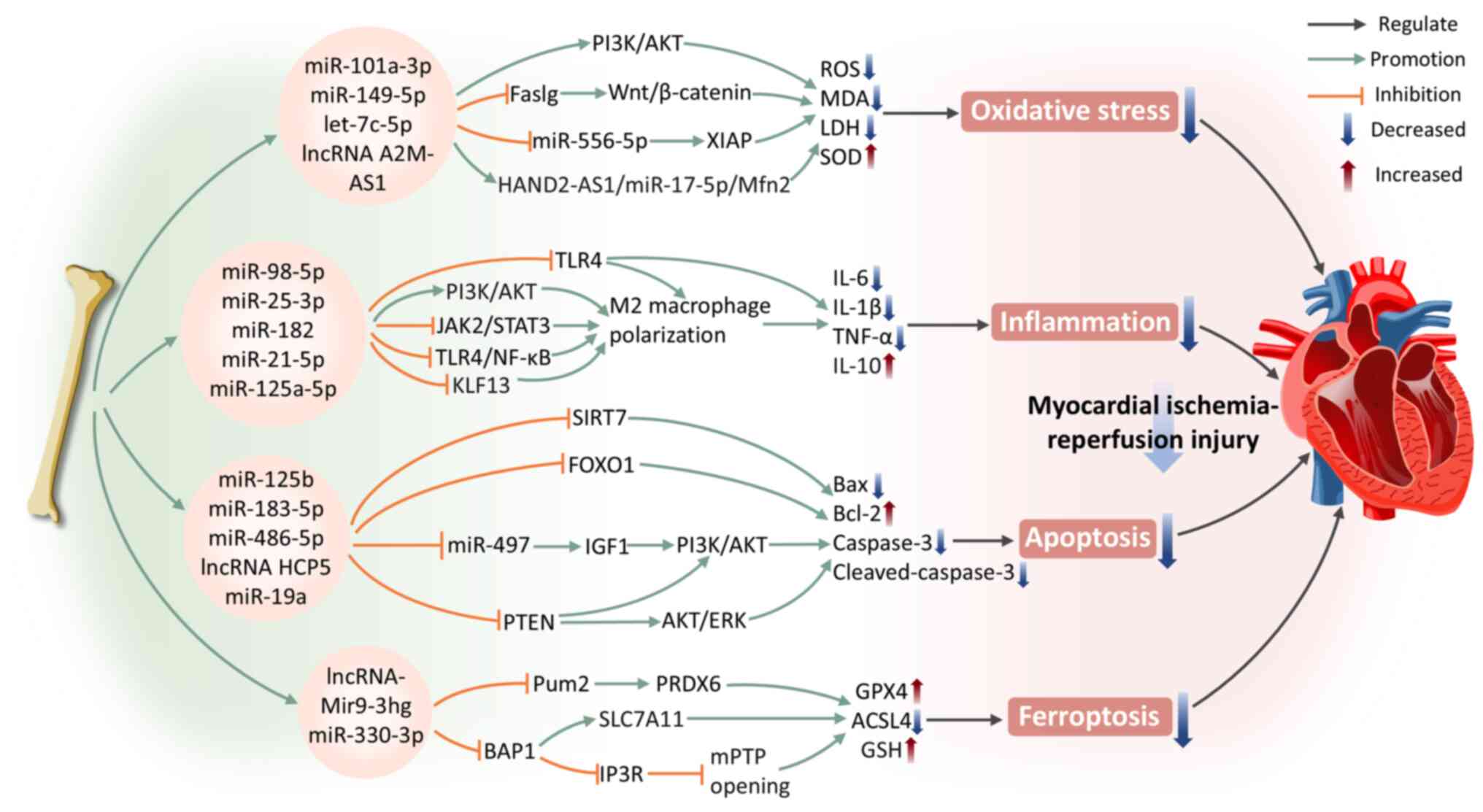
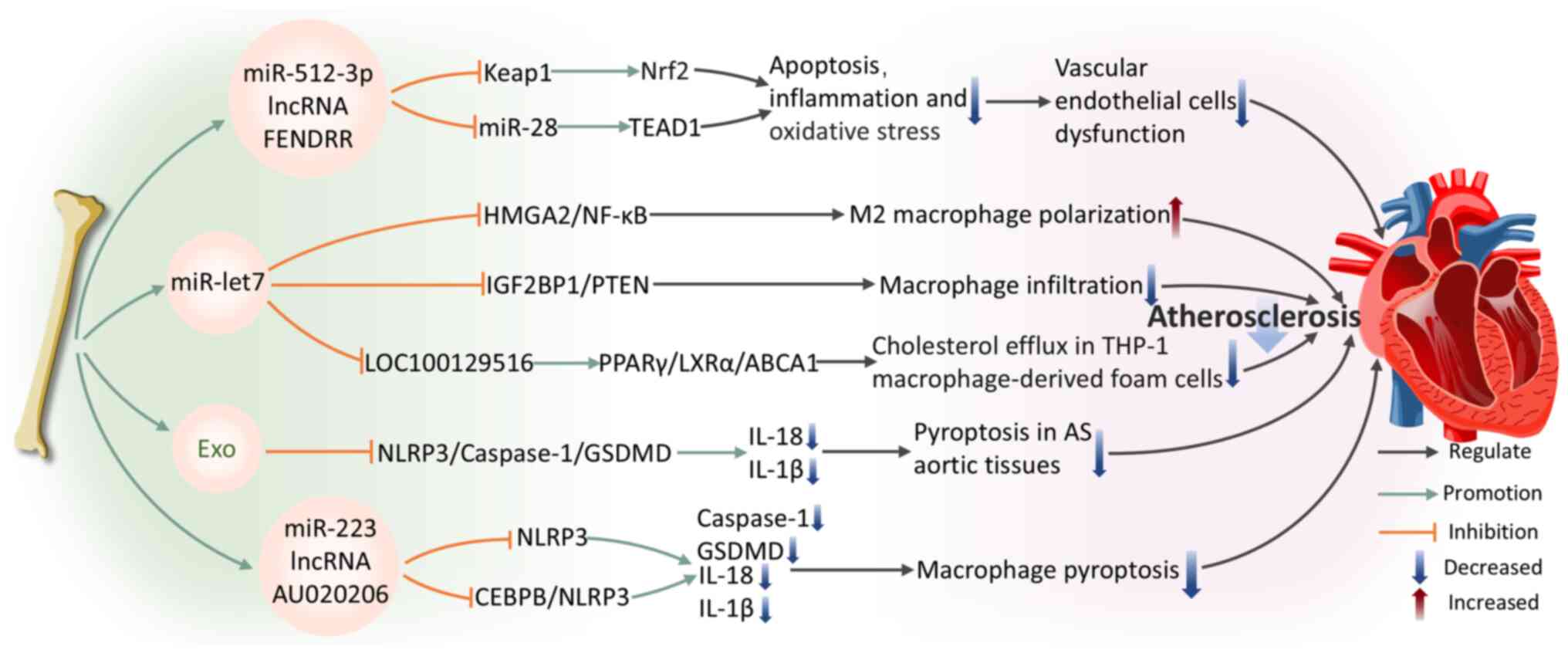
|
1
|
Welsh JA, Goberdhan DCI, O'Driscoll L,
Buzas EI, Blenkiron C, Bussolati B, Cai H, Di Vizio D, Driedonks
TAP, Erdbrügger U, et al: Minimal information for studies of
extracellular vesicles (MISEV2023): From basic to advanced
approaches. J Extracell Vesicles. 13:e124042024. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Patil M, Henderson J, Luong H, Annamalai
D, Sreejit G and Krishnamurthy P: The art of intercellular wireless
communications: Exosomes in heart disease and therapy. Front Cell
Dev Biol. 7:3152019. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Deddens JC, Vrijsen KR, Colijn JM,
Oerlemans MI, Metz CHG, van der Vlist EJ, Nolte-'t Hoen ENM, den
Ouden K, Jansen Of Lorkeers SJ, van der Spoel TI, et al:
Circulating extracellular vesicles contain miRNAs and are released
as early biomarkers for cardiac injury. J Cardiovasc Transl Res.
9:291–301. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Tian Y, Wang TS, Bu H, Shao G, Zhang W and
Zhang L: Role of exosomal miR-223 in chronic skeletal muscle
inflammation. Orthop Surg. 14:644–651. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Homme RP, Zheng Y, Smolenkova I, Singh M
and Tyagi SC: Remote hind-limb ischemia mechanism of preserved
ejection fraction during heart failure. Front Physiol.
12:7453282021. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Zhu LP, Tian T, Wang JY, He JN, Chen T,
Pan M, Xu L, Zhang HX, Qiu XT, Li CC, et al: Hypoxia-elicited
mesenchymal stem cell-derived exosomes facilitates cardiac repair
through miR-125b-mediated prevention of cell death in myocardial
infarction. Theranostics. 8:6163–6177. 2018. View Article : Google Scholar
|
|
7
|
Hayasaka T, Takehara N, Aonuma T, Kano K,
Horiuchi K, Nakagawa N, Tanaka H, Kawabe JI and Hasebe N:
Sarcopenia-derived exosomal micro-RNA 16-5p disturbs cardio-repair
via a pro-apoptotic mechanism in myocardial infarction in mice. Sci
Rep. 11:191632021. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Ranjan P, Dutta RK, Colin K, Li J, Zhang
Q, Lal H, Qin G and Verma SK: Bone marrow-fibroblast progenitor
cell-derived small extracellular vesicles promote cardiac fibrosis
via miR-21-5p and integrin subunit αV signalling. J Extracell Biol.
3:e1522024. View Article : Google Scholar
|
|
9
|
Aminzadeh MA, Rogers RG, Fournier M, Tobin
RE, Guan X, Childers MK, Andres AM, Taylor DJ, Ibrahim A, Ding X,
et al: Exosome-mediated benefits of cell therapy in mouse and human
models of duchenne muscular dystrophy. Stem Cell Reports.
10:942–955. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Zhou C, Zhang B, Yang Y, Jiang Q, Li T,
Gong J, Tang H and Zhang Q: Stem cell-derived exosomes: Emerging
therapeutic opportunities for wound healing. Stem Cell Res Ther.
14:1072023. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Caby MP, Lankar D, Vincendeau-Scherrer C,
Raposo G and Bonnerot C: Exosomal-like vesicles are present in
human blood plasma. Int Immunol. 17:879–887. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Milasan A, Tessandier N, Tan S, Brisson A,
Boilard E and Martel C: Extracellular vesicles are present in mouse
lymph and their level differs in atherosclerosis. J Extracell
Vesicles. 5:314272016. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Pisitkun T, Shen RF and Knepper MA:
Identification and proteomic profiling of exosomes in human urine.
Proc Natl Acad Sci USA. 101:13368–13373. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Kagota S, Taniguchi K, Lee SW, Ito Y,
Kuranaga Y, Hashiguchi Y, Inomata Y, Imai Y, Tanaka R, Tashiro K,
et al: Analysis of extracellular vesicles in gastric juice from
gastric cancer patients. Int J Mol Sci. 20:9532019. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Zlotogorski-Hurvitz A, Dayan D, Chaushu G,
Korvala J, Salo T, Sormunen R and Vered M: Human saliva-derived
exosomes: Comparing methods of isolation. J Histochem Cytochem.
63:181–189. 2015. View Article : Google Scholar :
|
|
16
|
McAndrews KM and Kalluri R: Mechanisms
associated with biogenesis of exosomes in cancer. Mol Cancer.
18:522019. View Article : Google Scholar : PubMed/NCBI
|
|
17
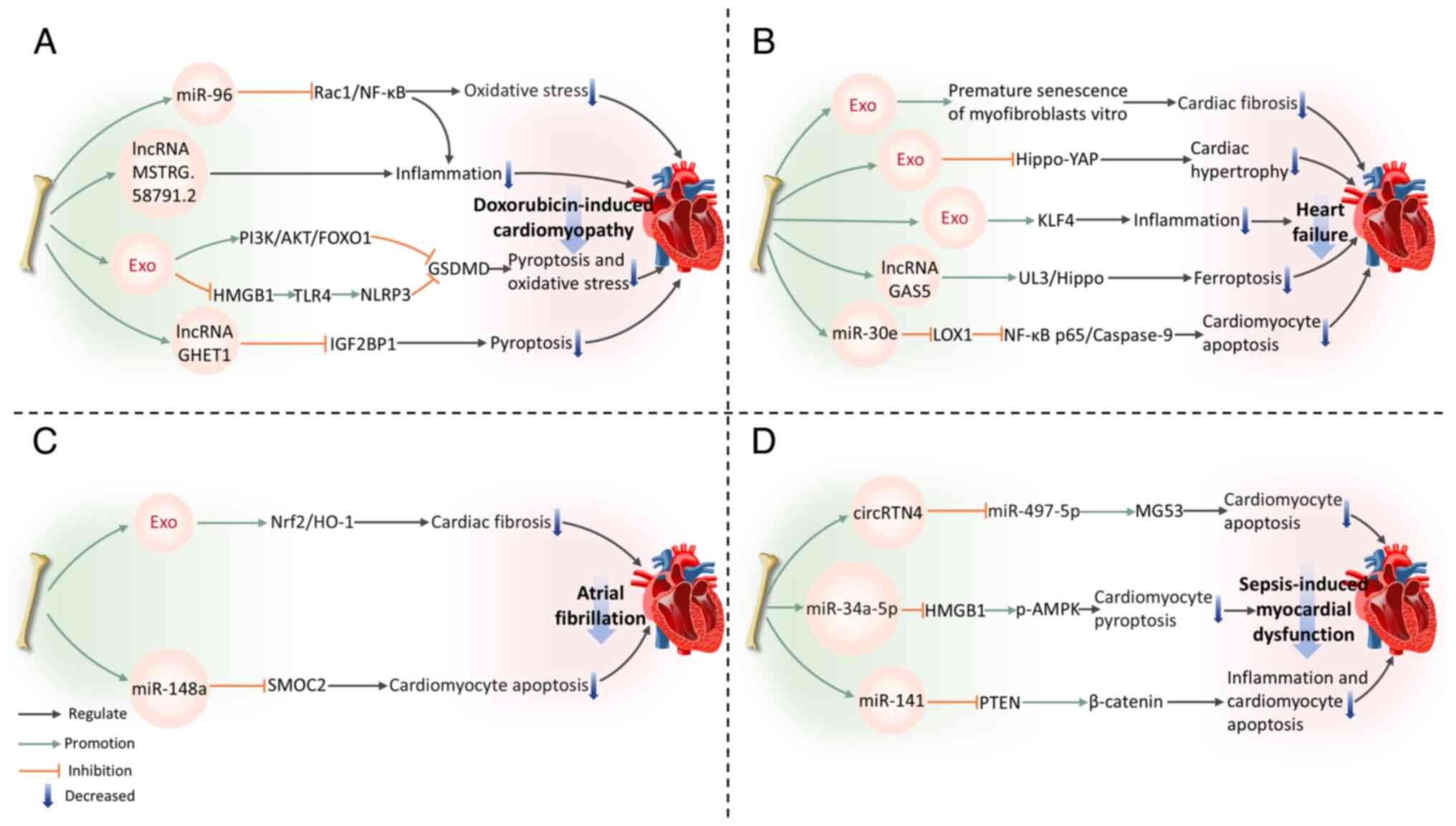
|
Lener T, Gimona M, Aigner L, Börger V,
Buzas E, Camussi G, Chaput N, Chatterjee D, Court FA, Del Portillo
HA, et al: Applying extracellular vesicles based therapeutics in
clinical trials-an ISEV position paper. J Extracell Vesicles.
4:300872015. View Article : Google Scholar
|
|
18
|
Roucourt B, Meeussen S, Bao J, Zimmermann
P and David G: Heparanase activates the syndecan-syntenin-ALIX
exosome pathway. Cell Res. 25:412–428. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Pan BT and Johnstone RM: Fate of the
transferrin receptor during maturation of sheep reticulocytes in
vitro: Selective externalization of the receptor. Cell. 33:967–978.
1983. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Johnstone RM, Adam M, Hammond JR, Orr L
and Turbide C: Vesicle formation during reticulocyte maturation.
Association of plasma membrane activities with released vesicles
(exosomes). J Biol Chem. 262:9412–9420. 1987. View Article : Google Scholar : PubMed/NCBI
|
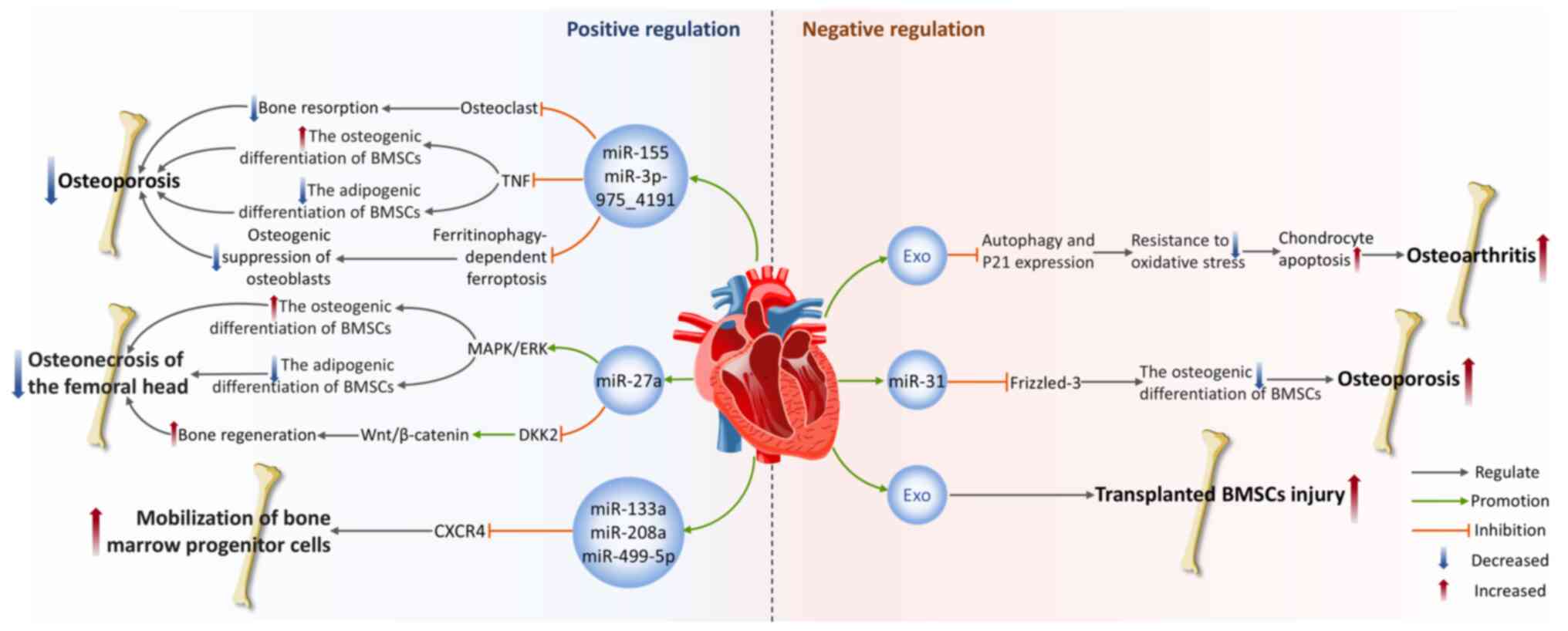
|
21
|
Mulcahy LA, Pink RC and Carter DR: Routes
and mechanisms of extracellular vesicle uptake. J Extracell
Vesicles. 3:2014. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Krylova SV and Feng D: The machinery of
exosomes: Biogenesis, release and uptake. Int J Mol Sci.
24:13372023. View Article : Google Scholar
|
|
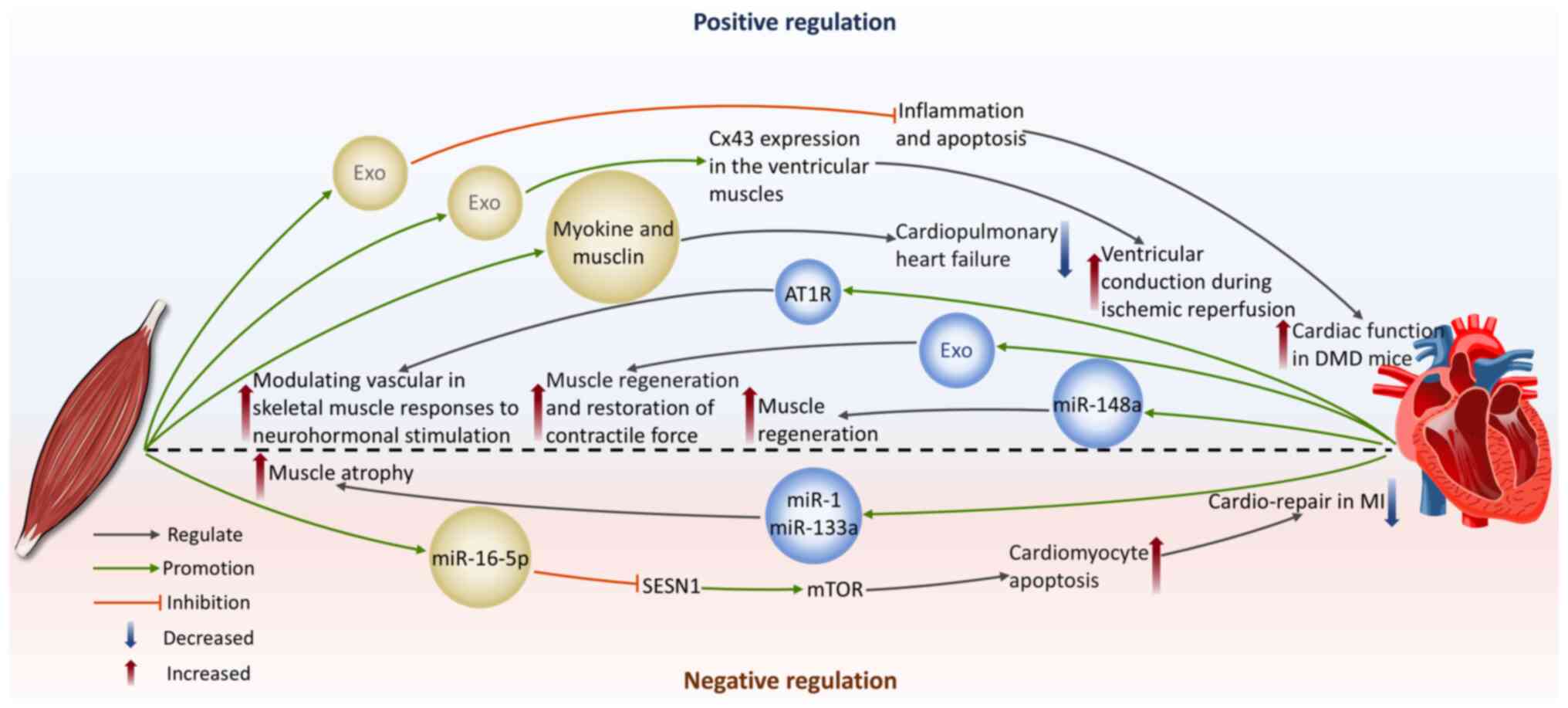
23
|
Li X, Lian Y, Wu Y, Ye Z, Feng J, Zhao Y,
Guo X and Kang J: Neonatal plasma exosomes contribute to
endothelial cell-mediated angiogenesis and cardiac repair after
acute myocardial infarction. Int J Mol Sci. 24:31962023. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Wang Y, Jiang R, Zhao H, Li F, Li Y and
Zhu M: TTN-AS1 delivered by gastric cancer cell-derived exosome
induces gastric cancer progression through in vivo and in vitro
studies. Cell Biol Toxicol. 39:557–571. 2023. View Article : Google Scholar
|
|
25
|
Nambara S, Masuda T, Hirose K, Hu Q, Tobo
T, Ozato Y, Kurashige J, Hiraki Y, Hisamatsu Y, Iguchi T, et al:
Rab27b, a regulator of exosome secretion, is associated with
peritoneal metastases in gastric cancer. Cancer Genomics
Proteomics. 20:30–39. 2023. View Article : Google Scholar :
|
|
26
|
Liu X, Li R, Chen X, Yao J, Wang Q, Zhang
J, Jiang Y and Qu Y: SYT7 is a key player in increasing exosome
secretion and promoting angiogenesis in non-small-cell lung cancer.
Cancer Lett. 577:2164002023. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Boilard E: Extracellular vesicles and
their content in bioactive lipid mediators: More than a sack of
microRNA. J Lipid Res. 59:2037–2046. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Sharma A and Johnson A: Exosome DNA:
Critical regulator of tumor immunity and a diagnostic biomarker. J
Cell Physiol. 235:1921–1932. 2020. View Article : Google Scholar
|
|
29
|
Chennakrishnaiah S, Meehan B, D'Asti E,
Montermini L, Lee TH, Karatzas N, Buchanan M, Tawil N, Choi D,
Divangahi M, et al: Leukocytes as a reservoir of circulating
oncogenic DNA and regulatory targets of tumor-derived extracellular
vesicles. J Thromb Haemost. 16:1800–1813. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Yang Z, Shi J, Xie J, Wang Y, Sun J, Liu
T, Zhao Y, Zhao X, Wang X, Ma Y, et al: Large-scale generation of
functional mRNA-encapsulating exosomes via cellular nanoporation.
Nat Biomed Eng. 4:69–83. 2020. View Article : Google Scholar :
|
|
31
|
Zheng D, Huo M, Li B, Wang W, Piao H, Wang
Y, Zhu Z, Li D, Wang T and Liu K: The role of exosomes and exosomal
MicroRNA in cardiovascular disease. Front Cell Dev Biol.
8:6161612021. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Poulet C, Njock MS, Moermans C, Louis E,
Louis R, Malaise M and Guiot J: Exosomal long non-coding RNAs in
lung diseases. Int J Mol Sci. 21:35802020. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Cao X, Xue LD, Di Y, Li T, Tian YJ and
Song Y: MSC-derived exosomal lncRNA SNHG7 suppresses
endothelial-mesenchymal transition and tube formation in diabetic
retinopathy via miR-34a-5p/XBP1 axis. Life Sci. 272:1192322021.
View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Shyu KG, Wang BW, Fang WJ, Pan CM and Lin
CM: Hyperbaric oxygen-induced long non-coding RNA MALAT1 exosomes
suppress MicroRNA-92a expression in a rat model of acute myocardial
infarction. J Cell Mol Med. 24:12945–12954. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Bei Y, Das S, Rodosthenous RS, Holvoet P,
Vanhaverbeke M, Monteiro MC, Monteiro VVS, Radosinska J, Bartekova
M, Jansen F, et al: Extracellular vesicles in cardiovascular
theranostics. Theranostics. 7:4168–4182. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Gupta S and Knowlton AA: HSP60 trafficking
in adult cardiac myocytes: Role of the exosomal pathway. Am J
Physiol Heart Circ Physiol. 292:H3052–H3056. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Lai Z, Liang J, Zhang J, Mao Y, Zheng X,
Shen X, Lin W and Xu G: Exosomes as a delivery tool of
exercise-induced beneficial factors for the prevention and
treatment of cardiovascular disease: A systematic review and
meta-analysis. Front Physiol. 14:11900952023. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Li C, Li X, Shi Z, Wu P, Fu J, Tang J and
Qing L: Exosomes from LPS-preconditioned bone marrow MSCs
accelerated peripheral nerve regeneration via M2 macrophage
polarization: Involvement of TSG-6/NF-κB/NLRP3 signaling pathway.
Exp Neurol. 356:1141392022. View Article : Google Scholar
|
|
39
|
Yu H, Cheng J, Shi W, Ren B, Zhao F, Shi
Y, Yang P, Duan X, Zhang J, Fu X, et al: Bone marrow mesenchymal
stem cell-derived exosomes promote tendon regeneration by
facilitating the proliferation and migration of endogenous tendon
stem/progenitor cells. Acta Biomater. 106:328–341. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Wang Y, Zhao R, Liu D, Deng W, Xu G, Liu
W, Rong J, Long X, Ge J and Shi B: Exosomes derived from
miR-214-enriched bone marrow-derived mesenchymal stem cells
regulate oxidative damage in cardiac stem cells by targeting
CaMKII. Oxid Med Cell Longev. 2018:49712612018. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Wu J, Ji C, Cao F, Lui H, Xia B and Wang
L: Bone marrow mesenchymal stem cells inhibit dendritic cells
differentiation and maturation by microRNA-23b. Biosci Rep.
37:BSR201604362017. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Liu R, Chang W, Wei H and Zhang K:
Comparison of the biological characteristics of mesenchymal stem
cells derived from bone marrow and skin. Stem Cells Int.
2016:36587982016. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Théry C, Zitvogel L and Amigorena S:
Exosomes: Composition, biogenesis and function. Nat Rev Immunol.
2:569–579. 2002. View
Article : Google Scholar : PubMed/NCBI
|
|
44
|
Raposo G and Stoorvogel W: Extracellular
vesicles: Exosomes, microvesicles and friends. J Cell Biol.
200:373–383. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Thygesen K, Alpert JS and White HD; Joint
ESC/ACCF/AHA/WHF Task Force for the Redefinition of Myocardial
Infarction: Universal definition of myocardial infarction. Eur
Heart J. 28:2525–2538. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Frangogiannis NG: Pathophysiology of
myocardial infarction. Compr Physiol. 5:1841–1875. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Shen Y, Liu X, Shi J and Wu X: Involvement
of Nrf2 in myocardial ischemia and reperfusion injury. Int J Biol
Macromol. 125:496–502. 2019. View Article : Google Scholar
|
|
48
|
Hori M and Nishida K: Oxidative stress and
left ventricular remodelling after myocardial infarction.
Cardiovasc Res. 81:457–464. 2009. View Article : Google Scholar
|
|
49
|
Sun Y: Oxidative stress and cardiac
repair/remodeling following infarction. Am J Med Sci. 334:197–205.
2007. View Article : Google Scholar : PubMed/NCBI
|
|
50
|
Liu HY, Yu LF, Zhou TG, Wang YD, Sun DH,
Chen HR and Hou YF: Lipopolysaccharide-stimulated bone marrow
mesenchymal stem cells-derived exosomes inhibit H2O2-induced
cardiomyocyte inflammation and oxidative stress via regulating
miR-181a-5p/ATF2 axis. Eur Rev Med Pharmacol Sci. 24:10069–10077.
2020.PubMed/NCBI
|
|
51
|
Feng J, Yang F, Wu H, Xing C, Xue H, Zhang
L, Zhang C, Hu G and Cao H: Selenium protects against
cadmium-induced cardiac injury by attenuating programmed cell death
via PI3K/AKT/PTEN signaling. Environ Toxicol. 37:1185–1197. 2022.
View Article : Google Scholar : PubMed/NCBI
|
|
52
|
Shi B, Wang Y, Zhao R, Long X, Deng W and
Wang Z: Bone marrow mesenchymal stem cell-derived exosomal miR-21
protects C-kit+ cardiac stem cells from oxidative injury through
the PTEN/PI3K/Akt axis. PLoS One. 13:e01916162018. View Article : Google Scholar : PubMed/NCBI
|
|
53
|
Chen GY and Nuñez G: Sterile inflammation:
Sensing and reacting to damage. Nat Rev Immunol. 10:826–837. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
54
|
Pan W, Zhu Y, Meng X, Zhang C, Yang Y and
Bei Y: Immunomodulation by exosomes in myocardial infarction. J
Cardiovasc Transl Res. 12:28–36. 2019. View Article : Google Scholar
|
|
55
|
Sun C, Li W, Li Y, Chen J, An H, Zeng G,
Wang T, Guo Y and Wang C: MiR-182-5p mediated by exosomes derived
from bone marrow mesenchymal stem cell attenuates inflammatory
responses by targeting TLR4 in a mouse model of myocardial
infraction. Immune Netw. 22:e492022. View Article : Google Scholar
|
|
56
|
Kore RA, Wang X, Ding Z, Griffin RJ,
Tackett AJ and Mehta JL: MSC exosome-mediated cardioprotection in
ischemic mouse heart comparative proteomics of infarct and
peri-infarct areas. Mol Cell Biochem. 476:1691–1704. 2021.
View Article : Google Scholar : PubMed/NCBI
|
|
57
|
de Couto G, Liu W, Tseliou E, Sun B,
Makkar N, Kanazawa H, Arditi M and Marbán E: Macrophages mediate
cardioprotective cellular postconditioning in acute myocardial
infarction. J Clin Invest. 125:3147–3162. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
58
|
Ben-Mordechai T, Palevski D,
Glucksam-Galnoy Y, Elron-Gross I, Margalit R and Leor J: Targeting
macrophage subsets for infarct repair. J Cardiovasc Pharmacol Ther.
20:36–51. 2015. View Article : Google Scholar
|
|
59
|
Xu R, Zhang F, Chai R, Zhou W, Hu M, Liu
B, Chen X, Liu M, Xu Q, Liu N and Liu S: Exosomes derived from
pro-inflammatory bone marrow-derived mesenchymal stem cells reduce
inflammation and myocardial injury via mediating macrophage
polarization. J Cell Mol Med. 23:7617–7631. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
60
|
Ning H, Chen H, Deng J, Xiao C, Xu M, Shan
L, Yang C and Zhang Z: Exosomes secreted by FNDC5-BMMSCs protect
myocardial infarction by anti-inflammation and macrophage
polarization via NF-κB signaling pathway and Nrf2/HO-1 axis. Stem
Cell Res Ther. 12:5192021. View Article : Google Scholar
|
|
61
|
Cătană CS, Atanasov AG and Berindan-Neagoe
I: Natural products with anti-aging potential: Affected targets and
molecular mechanisms. Biotechnol Adv. 36:1649–1656. 2018.
View Article : Google Scholar
|
|
62
|
Xiao C, Wang K, Xu Y, Hu H, Zhang N, Wang
Y, Zhong Z, Zhao J, Li Q, Zhu D, et al: Transplanted mesenchymal
stem cells reduce autophagic flux in infarcted hearts via the
exosomal transfer of miR-125b. Circ Res. 123:564–578. 2018.
View Article : Google Scholar : PubMed/NCBI
|
|
63
|
Kim J, Kundu M, Viollet B and Guan KL:
AMPK and mTOR regulate autophagy through direct phosphorylation of
Ulk1. Nat Cell Biol. 13:132–141. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
64
|
Lavandero S, Troncoso R, Rothermel BA,
Martinet W, Sadoshima J and Hill JA: Cardiovascular autophagy:
Concepts, controversies and perspectives. Autophagy. 9:1455–1466.
2013. View Article : Google Scholar : PubMed/NCBI
|
|
65
|
Li Y, Yang R, Guo B, Zhang H, Zhang H, Liu
S and Li Y: Exosomal miR-301 derived from mesenchymal stem cells
protects myocardial infarction by inhibiting myocardial autophagy.
Biochem Biophys Res Commun. 514:323–328. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
66
|
Zou L, Ma X, Lin S, Wu B, Chen Y and Peng
C: Bone marrow mesenchymal stem cell-derived exosomes protect
against myocardial infarction by promoting autophagy. Exp Ther Med.
18:2574–2582. 2019.PubMed/NCBI
|
|
67
|
Yang Q, Zhong QM, Song MQ, Tong LG and Bai
CZ: Exosomes derived from Danshen decoction-pretreated bone marrow
mesenchymal stem cells alleviate myocardial infarction via
anti-apoptosis and up-regulation of autophagy. Heliyon.
10:e380342024. View Article : Google Scholar : PubMed/NCBI
|
|
68
|
Saraste A, Pulkki K, Kallajoki M,
Henriksen K, Parvinen M and Voipio-Pulkki LM: Apoptosis in human
acute myocardial infarction. Circulation. 95:320–323. 1997.
View Article : Google Scholar : PubMed/NCBI
|
|
69
|
Cheng H, Chang S, Xu R, Chen L, Song X, Wu
J, Qian J, Zou Y and Ma J: Hypoxia-challenged MSC-derived exosomes
deliver miR-210 to attenuate post-infarction cardiac apoptosis.
Stem Cell Res Ther. 11:2242020. View Article : Google Scholar : PubMed/NCBI
|
|
70
|
Fu DL, Jiang H, Li CY, Gao T, Liu MR and
Li HW: MicroRNA-338 in MSCs-derived exosomes inhibits cardiomyocyte
apoptosis in myocardial infarction. Eur Rev Med Pharmacol Sci.
24:10107–10117. 2020.PubMed/NCBI
|
|
71
|
He JG, Li HR, Han JX, Li BB, Yan D, Li HY,
Wang P and Luo Y: GATA-4-expressing mouse bone marrow mesenchymal
stem cells improve cardiac function after myocardial infarction via
secreted exosomes. Sci Rep. 8:90472018. View Article : Google Scholar : PubMed/NCBI
|
|
72
|
Ning W, Li S, Yang W, Yang B, Xin C, Ping
X, Huang C, Gu Y and Guo L: Blocking exosomal miRNA-153-3p derived
from bone marrow mesenchymal stem cells ameliorates hypoxia-induced
myocardial and microvascular damage by targeting the
ANGPT1-mediated VEGF/PI3k/Akt/eNOS pathway. Cell Signal.
77:1098122021. View Article : Google Scholar
|
|
73
|
Wen Z, Mai Z, Zhu X, Wu T, Chen Y, Geng D
and Wang J: Mesenchymal stem cell-derived exosomes ameliorate
cardiomyocyte apoptosis in hypoxic conditions through microRNA144
by targeting the PTEN/AKT pathway. Stem Cell Res Ther. 11:362020.
View Article : Google Scholar : PubMed/NCBI
|
|
74
|
Li X, Hu X, Chen Q and Jiang T: Bone
marrow mesenchymal stem cell-derived exosomes carrying E3 ubiquitin
ligase ITCH attenuated cardiomyocyte apoptosis by mediating
apoptosis signal-regulated kinase-1. Pharmacogenet Genomics.
33:117–125. 2023.PubMed/NCBI
|
|
75
|
Zhang CS, Shao K, Liu CW, Li CJ and Yu BT:
Hypoxic preconditioning BMSCs-exosomes inhibit cardiomyocyte
apoptosis after acute myocardial infarction by upregulating
microRNA-24. Eur Rev Med Pharmacol Sci. 23:6691–6699.
2019.PubMed/NCBI
|
|
76
|
Sun L, Zhu W, Zhao P, Wang Q, Fan B, Zhu
Y, Lu Y, Chen Q, Zhang J and Zhang F: Long noncoding RNA UCA1 from
hypoxia-conditioned hMSC-derived exosomes: A novel molecular target
for cardioprotection through miR-873-5p/XIAP axis. Cell Death Dis.
11:6962020. View Article : Google Scholar : PubMed/NCBI
|
|
77
|
Cochain C, Channon KM and Silvestre JS:
Angiogenesis in the infarcted myocardium. Antioxid Redox Signal.
18:1100–1113. 2013. View Article : Google Scholar :
|
|
78
|
Teng X, Chen L, Chen W, Yang J, Yang Z and
Shen Z: Mesenchymal stem cell-derived exosomes improve the
microenvironment of infarcted myocardium contributing to
angiogenesis and anti-inflammation. Cell Physiol Biochem.
37:2415–2424. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
79
|
Zheng J, Zhang X, Cai W, Yang Y, Guo T, Li
J and Dai H: Bone marrow mesenchymal stem cell-derived exosomal
microRNA-29b-3p promotes angiogenesis and ventricular remodeling in
rats with myocardial infarction by targeting ADAMTS16. Cardiovasc
Toxicol. 22:689–700. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
80
|
Yang L, Liu N and Yang Y: Astragaloside
IV-induced BMSC exosomes promote neovascularization and protect
cardiac function in myocardial infarction mice via the
miR-411/HIF-1α axis. J Liposome Res. 34:452–463. 2024. View Article : Google Scholar
|
|
81
|
Talman V and Ruskoaho H: Cardiac fibrosis
in myocardial infarction-from repair and remodeling to
regeneration. Cell Tissue Res. 365:563–581. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
82
|
van den Borne SWM, Diez J, Blankesteijn
WM, Verjans J, Hofstra L and Narula J: Myocardial remodeling after
infarction: The role of myofibroblasts. Nat Rev Cardiol. 7:30–37.
2010. View Article : Google Scholar
|
|
83
|
Jiao W, Hao J, Xie Y, Meng M and Gao W:
EZH2 mitigates the cardioprotective effects of mesenchymal stem
cell-secreted exosomes against infarction via HMGA2-mediated
PI3K/AKT signaling. BMC Cardiovasc Disord. 22:952022. View Article : Google Scholar : PubMed/NCBI
|
|
84
|
Wang S, Li L, Liu T, Jiang W and Hu X:
miR-19a/19b-loaded exosomes in combination with mesenchymal stem
cell transplantation in a preclinical model of myocardial
infarction. Regen Med. 15:1749–1759. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
85
|
Wang S, Dong J, Li L, Wu R, Xu L, Ren Y
and Hu X: Exosomes derived from miR-129-5p modified bone marrow
mesenchymal stem cells represses ventricular remolding of mice with
myocardial infarction. J Tissue Eng Regen Med. 16:177–187. 2022.
View Article : Google Scholar
|
|
86
|
Li C, Zheng C, Pu Y, Zhou H, Li Y, Wang W,
Chen X, Zhang C and Chen Y: Vericiguat enhances the therapeutic
efficacy of mesenchymal stem cells-derived exosomes in acute
myocardial infarction through microRNA-1180-3p/ETS1 pathway. Cell
Signal. 125:1115122025. View Article : Google Scholar
|
|
87
|
Algoet M, Janssens S, Himmelreich U, Gsell
W, Pusovnik M, Van den Eynde J and Oosterlinck W: Myocardial
ischemia-reperfusion injury and the influence of inflammation.
Trends Cardiovasc Med. 33:357–366. 2023. View Article : Google Scholar
|
|
88
|
Hausenloy DJ and Yellon DM: Myocardial
ischemia-reperfusion injury: A neglected therapeutic target. J Clin
Invest. 123:92–100. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
89
|
Xiang M, Lu Y, Xin L, Gao J, Shang C,
Jiang Z, Lin H, Fang X, Qu Y, Wang Y, et al: Role of oxidative
stress in reperfusion following myocardial ischemia and its
treatments. Oxid Med Cell Longev. 2021:66140092021. View Article : Google Scholar : PubMed/NCBI
|
|
90
|
Sousa Fialho MDL, Abd Jamil AH, Stannard
GA and Heather LC: Hypoxia-inducible factor 1 signalling,
metabolism and its therapeutic potential in cardiovascular disease.
Biochim Biophys Acta Mol Basis Dis. 1865:831–843. 2019. View Article : Google Scholar
|
|
91
|
Zhao D, Bu Y, Shao H, Wang J, Li W and Li
Q: Protective effect of exosomes derived from bone marrow
mesenchymal stem cells on hypoxia reperfusion injury of
cardiomyocytes. Cell Mol Biol (Noisy-le-grand). 70:73–80. 2024.
View Article : Google Scholar : PubMed/NCBI
|
|
92
|
Zou L, Ma X, Wu B, Chen Y, Xie D and Peng
C: Protective effect of bone marrow mesenchymal stem cell-derived
exosomes on cardiomyoblast hypoxia-reperfusion injury through the
miR-149/let-7c/Faslg axis. Free Radic Res. 54:722–731. 2020.
View Article : Google Scholar : PubMed/NCBI
|
|
93
|
Yu H, Pan Y, Dai M, Wang X and Chen H:
Mesenchymal stem cell-originated exosomal Lnc A2M-AS1 alleviates
hypoxia/reperfusion-induced apoptosis and oxidative stress in
cardiomyocytes. Cardiovasc Drugs Ther. 37:891–904. 2023. View Article : Google Scholar
|
|
94
|
Li Q, Bu Y, Shao H, Li W, Zhao D and Wang
J: Protective effect of bone marrow mesenchymal stem cell-derived
exosomes on cardiomyoblast hypoxia-reperfusion injury through the
HAND2-AS1/miR-17-5p/Mfn2 axis. BMC Cardiovasc Disord. 23:1142023.
View Article : Google Scholar : PubMed/NCBI
|
|
95
|
Deng J, Zhang T, Li M, Cao G, Wei H, Zhang
Z and Hu T: Irisin-pretreated BMMSCs Secrete exosomes to alleviate
cardiomyocytes pyroptosis and oxidative stress to
hypoxia/reoxygenation injury. Curr Stem Cell Res Ther. 18:843–852.
2023. View Article : Google Scholar
|
|
96
|
Zhang L, Wei Q, Liu X, Zhang T, Wang S,
Zhou L, Zou L, Fan F, Chi H, Sun J and Wang D: Exosomal
microRNA-98-5p from hypoxic bone marrow mesenchymal stem cells
inhibits myocardial ischemia-reperfusion injury by reducing TLR4
and activating the PI3K/Akt signaling pathway. Int Immunopharmacol.
101:1075922021. View Article : Google Scholar : PubMed/NCBI
|
|
97
|
Du J, Dong Y, Song J, Shui H, Xiao C, Hu
Y, Zhou S and Wang S: BMSC-derived exosome-mediated miR-25-3p
delivery protects against myocardial ischemia/reperfusion injury by
constraining M1-like macrophage polarization. Mol Med Rep.
30:1422024. View Article : Google Scholar
|
|
98
|
Zhao J, Li X, Hu J, Chen F, Qiao S, Sun X,
Gao L, Xie J and Xu B: Mesenchymal stromal cell-derived exosomes
attenuate myocardial ischaemia-reperfusion injury through
miR-182-regulated macrophage polarization. Cardiovasc Res.
115:1205–1216. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
99
|
Shen D and He Z: Mesenchymal stem
cell-derived exosomes regulate the polarization and inflammatory
response of macrophages via miR-21-5p to promote repair after
myocardial reperfusion injury. Ann Transl Med. 9:13232021.
View Article : Google Scholar : PubMed/NCBI
|
|
100
|
Gao L, Qiu F, Cao H, Li H, Dai G, Ma T,
Gong Y, Luo W, Zhu D, Qiu Z, et al: Therapeutic delivery of
microRNA-125a-5p oligonucleotides improves recovery from myocardial
ischemia/reperfusion injury in mice and swine. Theranostics.
13:685–703. 2023. View Article : Google Scholar : PubMed/NCBI
|
|
101
|
Chen Q, Liu Y, Ding X, Li Q, Qiu F, Wang
M, Shen Z, Zheng H and Fu G: Bone marrow mesenchymal stem
cell-secreted exosomes carrying microRNA-125b protect against
myocardial ischemia reperfusion injury via targeting SIRT7. Mol
Cell Biochem. 465:103–114. 2020. View Article : Google Scholar :
|
|
102
|
Mao S, Zhao J, Zhang ZJ and Zhao Q:
MiR-183-5p overexpression in bone mesenchymal stem cell-derived
exosomes protects against myocardial ischemia/reperfusion injury by
targeting FOXO1. Immunobiology. 227:1522042022. View Article : Google Scholar : PubMed/NCBI
|
|
103
|
Wang X, Bai L, Liu X, Shen W, Tian H, Liu
W and Yu B: Cardiac microvascular functions improved by MSC-derived
exosomes attenuate cardiac fibrosis after ischemia-reperfusion via
PDGFR-β modulation. Int J Cardiol. 344:13–24. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
104
|
Fresno Vara JA, Casado E, de Castro J,
Cejas P, Belda-Iniesta C and González-Barón M: PI3K/Akt signalling
pathway and cancer. Cancer Treat Rev. 30:193–204. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
105
|
Sun XH, Wang X, Zhang Y and Hui J:
Exosomes of bone-marrow stromal cells inhibit cardiomyocyte
apoptosis under ischemic and hypoxic conditions via miR-486-5p
targeting the PTEN/PI3K/AKT signaling pathway. Thromb Res.
177:23–32. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
106
|
Li KS, Bai Y, Li J, Li SL, Pan J, Cheng
YQ, Li K, Wang ZG, Ji WJ, Zhou Q and Wang DJ: LncRNA HCP5 in
hBMSC-derived exosomes alleviates myocardial ischemia reperfusion
injury by sponging miR-497 to activate IGF1/PI3K/AKT pathway. Int J
Cardiol. 342:72–81. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
107
|
Yu B, Kim HW, Gong M, Wang J, Millard RW,
Wang Y, Ashraf M and Xu M: Exosomes secreted from GATA-4
overexpressing mesenchymal stem cells serve as a reservoir of
anti-apoptotic microRNAs for cardioprotection. Int J Cardiol.
182:349–360. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
108
|
Jiang X, Stockwell BR and Conrad M:
Ferroptosis: Mechanisms, biology and role in disease. Nat Rev Mol
Cell Biol. 22:266–282. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
109
|
Zhang JK, Zhang Z, Guo ZA, Fu Y, Chen XJ,
Chen WJ, Wu HF and Cui XJ: The BMSC-derived exosomal lncRNA
Mir9-3hg suppresses cardiomyocyte ferroptosis in
ischemia-reperfusion mice via the Pum2/PRDX6 axis. Nutr Metab
Cardiovasc Dis. 32:515–527. 2022. View Article : Google Scholar
|
|
110
|
Strzyz P: Iron expulsion by exosomes
drives ferroptosis resistance. Nat Rev Mol Cell Biol. 21:4–5. 2020.
View Article : Google Scholar
|
|
111
|
Shen K, Wang X, Wang Y, Jia Y, Zhang Y,
Wang K, Luo L, Cai W, Li J, Li S, et al: miR-125b-5p in adipose
derived stem cells exosome alleviates pulmonary microvascular
endothelial cells ferroptosis via Keap1/Nrf2/GPX4 in sepsis lung
injury. Redox Biol. 62:1026552023. View Article : Google Scholar : PubMed/NCBI
|
|
112
|
Xiao Z, Li S, Wu X, Chen X, Yan D and He
J: GATA-4 overexpressing BMSC-derived exosomes suppress H/R-induced
cardiomyocyte ferroptosis. iScience. 27:1107842024. View Article : Google Scholar : PubMed/NCBI
|
|
113
|
Lusis AJ: Atherosclerosis. Nature.
407:233–241. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
114
|
Falk E: Pathogenesis of atherosclerosis. J
Am Coll Cardiol. 47(Suppl 8): C7–C12. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
115
|
Hang L, Peng Y, Xiang R, Li X and Li Z:
Ox-LDL causes endothelial cell injury through ASK1/NLRP3-mediated
inflammasome activation via endoplasmic reticulum stress. Drug Des
Devel Ther. 14:731–744. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
116
|
Pirillo A, Norata GD and Catapano AL:
LOX-1, OxLDL and atherosclerosis. Mediators Inflamm.
2013:1527862013. View Article : Google Scholar
|
|
117
|
Chen S, Zhou H, Zhang B and Hu Q: Exosomal
miR-512-3p derived from mesenchymal stem cells inhibits oxidized
low-density lipoprotein-induced vascular endothelial cells
dysfunction via regulating Keap1. J Biochem Mol Toxicol. 35:1–11.
2021. View Article : Google Scholar
|
|
118
|
Zhang N, Luo Y, Zhang H, Zhang F, Gao X
and Shao J: Exosomes derived from mesenchymal stem cells ameliorate
the progression of atherosclerosis in ApoE−/− mice via
FENDRR. Cardiovasc Toxicol. 22:528–544. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
119
|
Wei Y, Lan B, Zheng T, Yang L, Zhang X,
Cheng L, Tuerhongjiang G, Yuan Z and Wu Y: GSDME-mediated
pyroptosis promotes the progression and associated inflammation of
atherosclerosis. Nat Commun. 14:9292023. View Article : Google Scholar : PubMed/NCBI
|
|
120
|
Li J, Xue H, Li T, Chu X, Xin D, Xiong Y,
Qiu W, Gao X, Qian M, Xu J, et al: Exosomes derived from
mesenchymal stem cells attenuate the progression of atherosclerosis
in ApoE−/− mice via miR-let7 mediated infiltration and
polarization of M2 macrophage. Biochem Biophys Res Commun.
510:565–572. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
121
|
Sun L, He X, Zhang T, Han Y and Tao G:
Knockdown of mesenchymal stem cell-derived exosomal LOC100129516
suppresses the symptoms of atherosclerosis via upregulation of the
PPARγ/LXRα/ABCA1 signaling pathway. Int J Mol Med. 48:2082021.
View Article : Google Scholar
|
|
122
|
Zhao R, Feng J and He G: miR-613 regulates
cholesterol efflux by targeting LXRα and ABCA1 in PPARγ activated
THP-1 macrophages. Biochem Biophys Res Commun. 448:329–334. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
123
|
Xu Y, Lai F, Xu Y, Wu Y, Liu Q, Li N, Wei
Y, Feng T, Zheng Z, Jiang W, et al: Mycophenolic acid induces
ATP-binding cassette transporter A1 (ABCA1) expression through the
PPARγ-LXRα-ABCA1 pathway. Biochem Biophys Res Commun. 414:779–782.
2011. View Article : Google Scholar : PubMed/NCBI
|
|
124
|
Bergsbaken T, Fink SL and Cookson BT:
Pyroptosis: Host cell death and inflammation. Nat Rev Microbiol.
7:99–109. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
125
|
Shi J, Gao W and Shao F: Pyroptosis:
Gasdermin-mediated programmed necrotic cell death. Trends Biochem
Sci. 42:245–254. 2017. View Article : Google Scholar
|
|
126
|
Galluzzi L, Vitale I, Aaronson SA, Abrams
JM, Adam D, Agostinis P, Alnemri ES, Altucci L, Amelio I, Andrews
DW, et al: Molecular mechanisms of cell death: Recommendations of
the nomenclature committee on cell death 2018. Cell Death Differ.
25:486–541. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
127
|
Bai Z, Hu H, Hu F, Ji J and Ji Z: Bone
marrow mesenchymal stem cellsderived exosomes stabilize
atherosclerosis through inhibiting pyroptosis. BMC Cardiovasc
Disord. 23:4412023. View Article : Google Scholar : PubMed/NCBI
|
|
128
|
Lin Y, Liu M, Chen E, Jiang W, Shi W and
Wang Z: Bone marrow-derived mesenchymal stem cells microvesicles
stabilize atherosclerotic plaques by inhibiting NLRP3-mediated
macrophage pyroptosis. Cell Biol Int. 45:820–830. 2021. View Article : Google Scholar
|
|
129
|
Zhang N, Luo Y, Shao J, Sun H, Ma K and
Gao X: Exosomal long non-coding RNA AU020206 alleviates macrophage
pyroptosis in atherosclerosis by suppressing CEBPB-mediated NLRP3
transcription. Exp Cell Res. 438:1140542024. View Article : Google Scholar : PubMed/NCBI
|
|
130
|
Damiani RM, Moura DJ, Viau CM, Caceres RA,
Henriques JAP and Saffi J: Pathways of cardiac toxicity: Comparison
between chemotherapeutic drugs doxorubicin and mitoxantrone. Arch
Toxicol. 90:2063–2076. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
131
|
Matusik K, Kamińska K,
Sobiborowicz-Sadowska A, Borzuta H, Buczma K and
Cudnoch-Jędrzejewska A: The significance of the apelinergic system
in doxorubicin-induced cardiotoxicity. Heart Fail Rev. 29:969–988.
2024. View Article : Google Scholar : PubMed/NCBI
|
|
132
|
Renu K, V G A, P B TP and Arunachalam S:
Molecular mechanism of doxorubicin-induced cardiomyopathy-an
update. Eur J Pharmacol. 818:241–253. 2018. View Article : Google Scholar
|
|
133
|
Lei B, Wu X, Xia K, Sun H and Wang J:
Exosomal Micro-RNA-96 derived from bone marrow mesenchymal stem
cells inhibits doxorubicin-induced myocardial toxicity by
inhibiting the Rac1/nuclear factor-κB signaling pathway. J Am Heart
Assoc. 10:e0205892021. View Article : Google Scholar
|
|
134
|
Tian C, Yang Y, Li B, Liu M, He X, Zhao L,
Song X, Yu T and Chu XM: Doxorubicin-induced cardiotoxicity may be
alleviated by bone marrow mesenchymal stem cell-derived exosomal
lncRNA via inhibiting inflammation. J Inflamm Res. 15:4467–4486.
2022. View Article : Google Scholar : PubMed/NCBI
|
|
135
|
Shi J, Zhao Y, Wang K, Shi X, Wang Y,
Huang H, Zhuang Y, Cai T, Wang F and Shao F: Cleavage of GSDMD by
inflammatory caspases determines pyroptotic cell death. Nature.
526:660–665. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
136
|
Li Y, Li YJ and Zhu ZQ: To re-examine the
intersection of microglial activation and neuroinflammation in
neurodegenerative diseases from the perspective of pyroptosis.
Front Aging Neurosci. 15:12842142023. View Article : Google Scholar : PubMed/NCBI
|
|
137
|
Zeng H, Yang Y, Tou F, Zhan Y, Liu S, Zou
P, Chen Y and Shao L: Bone marrow stromal cell-derived exosomes
improve oxidative stress and pyroptosis in doxorubicin-induced
myocardial injury in vitro by regulating the transcription of GSDMD
through the PI3K-AKT-Foxo1 pathway. Immun Inflamm Dis. 11:e8102023.
View Article : Google Scholar : PubMed/NCBI
|
|
138
|
Ali SA and Singla DK: Mesenchymal stem
cell-derived exosomes ameliorate doxorubicin-induced
cardiotoxicity. Pharmaceuticals (Basel). 17:932024. View Article : Google Scholar : PubMed/NCBI
|
|
139
|
Zhai X, Zhou J, Huang X, Weng J, Lin H,
Sun S, Chi J and Meng L: LncRNA GHET1 from bone mesenchymal stem
cell-derived exosomes improves doxorubicin-induced pyroptosis of
cardiomyocytes by mediating NLRP3. Sci Rep. 14:190782024.
View Article : Google Scholar
|
|
140
|
Tanai E and Frantz S: Pathophysiology of
heart failure. Compr Physiol. 6:187–214. 2015. View Article : Google Scholar
|
|
141
|
Chen F, Li X, Zhao J, Geng J, Xie J and Xu
B: Bone marrow mesenchymal stem cell-derived exosomes attenuate
cardiac hypertrophy and fibrosis in pressure overload induced
remodeling. In Vitro Cell Dev Biol Anim. 56:567–576. 2020.
View Article : Google Scholar : PubMed/NCBI
|
|
142
|
Ren Y, Wu Y, He W, Tian Y and Zhao X:
Exosomes secreted from bone marrow mesenchymal stem cells suppress
cardiomyocyte hypertrophy through Hippo-YAP pathway in heart
failure. Genet Mol Biol. 46:e202202212023. View Article : Google Scholar : PubMed/NCBI
|
|
143
|
Ren Y and Zhao X: Bone marrow mesenchymal
stem cells-derived exosomal lncRNA GAS5 mitigates heart failure by
inhibiting UL3/Hippo pathway-mediated ferroptosis. Eur J Med Res.
29:3032024. View Article : Google Scholar : PubMed/NCBI
|
|
144
|
Pu L, Kong X, Li H and He X: Exosomes
released from mesenchymal stem cells overexpressing microRNA-30e
ameliorate heart failure in rats with myocardial infarction. Am J
Transl Res. 13:4007–4025. 2021.PubMed/NCBI
|
|
145
|
Han Y, Bi Y, Zhang D and Liu Y: The
exosomes derived from bone marrow mesenchymal stem cells alleviate
inflammatory injury in heart failure disease by enhancing the
expression of KLF4. Immun Inflamm Dis. 13:e701612025. View Article : Google Scholar : PubMed/NCBI
|
|
146
|
Beyer C, Tokarska L, Stühlinger M,
Feuchtner G, Hintringer F, Honold S, Fiedler L, Schönbauer MS,
Schönbauer R and Plank F: Structural cardiac remodeling in atrial
fibrillation. JACC Cardiovasc Imaging. 14:2199–2208. 2021.
View Article : Google Scholar : PubMed/NCBI
|
|
147
|
Paliwal N, Ali RL, Salvador M, O'Hara R,
Yu R, Daimee UA, Akhtar T, Pandey P, Spragg DD, Calkins H and
Trayanova NA: Presence of left atrial fibrosis may contribute to
aberrant hemodynamics and increased risk of stroke in atrial
fibrillation patients. Front Physiol. 12:6574522021. View Article : Google Scholar : PubMed/NCBI
|
|
148
|
Xu L, Fan Y, Wu L, Zhang C, Chu M, Wang Y
and Zhuang W: Exosomes from bone marrow mesenchymal stem cells with
overexpressed Nrf2 inhibit cardiac fibrosis in rats with atrial
fibrillation. Cardiovasc Ther. 2022:26878072022. View Article : Google Scholar : PubMed/NCBI
|
|
149
|
Zhang W, Man Y and Chen Z: microRNA-148a
in exosomes derived from bone marrow mesenchymal stem cells
alleviates cardiomyocyte apoptosis in atrial fibrillation by
inhibiting SMOC2. Mol Biotechnol. 64:1076–1087. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
150
|
Martensson J and Bellomo R: Sepsis-induced
acute kidney injury. Crit Care Clin. 31:649–660. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
151
|
Hoste EAJ, Kellum JA, Selby NM, Zarbock A,
Palevsky PM, Bagshaw SM, Goldstein SL, Cerda J and Chawla LS:
Global epidemiology and outcomes of acute kidney injury. Nat Rev
Nephrol. 14:607–625. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
152
|
Huang M, Cai S and Su J: The pathogenesis
of sepsis and potential therapeutic targets. Int J Mol Sci.
20:53762019. View Article : Google Scholar : PubMed/NCBI
|
|
153
|
van der Poll T, van de Veerdonk FL,
Scicluna BP and Netea MG: The immunopathology of sepsis and
potential therapeutic targets. Nat Rev Immunol. 17:407–420. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
154
|
Li J, Jiang R, Hou Y and Lin A:
Mesenchymal stem cells-derived exosomes prevent sepsis-induced
myocardial injury by a CircRTN4/miR-497-5p/MG53 pathway. Biochem
Biophys Res Commun. 618:133–140. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
155
|
Pei Y, Xie S, Li J and Jia B: Bone
marrow-mesenchymal stem cell-derived exosomal microRNA-141 targets
PTEN and activates β-catenin to alleviate myocardial injury in
septic mice. Immunopharmacol Immunotoxicol. 43:584–593. 2021.
View Article : Google Scholar : PubMed/NCBI
|
|
156
|
Li T, Zhao Y, Cao Z, Shen Y, Chen J, Huang
X, Shao Z, Zeng Y, Chen Q, Yan X, et al: Exosomes derived from
apelin-pretreated mesenchymal stem cells ameliorate sepsis-induced
myocardial dysfunction by alleviating cardiomyocyte pyroptosis via
delivery of miR-34a-5p. Int J Nanomedicine. 20:687–703. 2025.
View Article : Google Scholar : PubMed/NCBI
|
|
157
|
Pan L, Huang C, Jin X, Wu J, Jin K, Lin J,
Wang Y, Li J, Yin C, Wang X, et al: Cardiac secreted HSP90α
exacerbates pressure overload myocardial hypertrophy and heart
failure. Redox Biol. 79:1034662025. View Article : Google Scholar
|
|
158
|
Schirone L, Forte M, Palmerio S, Yee D,
Nocella C, Angelini F, Pagano F, Schiavon S, Bordin A, Carrizzo A,
et al: A review of the molecular mechanisms underlying the
development and progression of cardiac remodeling. Oxid Med Cell
Longev. 2017:39201952017. View Article : Google Scholar : PubMed/NCBI
|
|
159
|
Schena GJ, Murray EK, Hildebrand AN,
Headrick AL, Yang Y, Koch KA, Kubo H, Eaton D, Johnson J, Berretta
R, et al: Cortical bone stem cell-derived exosomes' therapeutic
effect on myocardial ischemia-reperfusion and cardiac remodeling.
Am J Physiol Heart Circ Physiol. 321:H1014–H1029. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
160
|
Gao H, Zhang L, Wang Z, Yan K, Zhao L and
Xiao W: Research progress on transorgan regulation of the
cardiovascular and motor system through cardiogenic exosomes. Int J
Mol Sci. 23:57652022. View Article : Google Scholar : PubMed/NCBI
|
|
161
|
Deanfield JE, Halcox JP and Rabelink TJ:
Endothelial function and dysfunction: testing and clinical
relevance. Circulation. 115:1285–1295. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
162
|
Subarajan P, Arceo-Mendoza RM and Camacho
PM: Postmenopausal osteoporosis: A review of latest guidelines.
Endocrinol Metab Clin North Am. 53:497–512. 2024. View Article : Google Scholar : PubMed/NCBI
|
|
163
|
Song H, Li X, Zhao Z, Qian J, Wang Y, Cui
J, Weng W, Cao L, Chen X, Hu Y and Su J: Reversal of osteoporotic
activity by endothelial cell-secreted bone targeting and
biocompatible exosomes. Nano Lett. 19:3040–3048. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
164
|
Yang RZ, Xu WN, Zheng HL, Zheng XF, Li B,
Jiang LS and Jiang SD: Exosomes derived from vascular endothelial
cells antagonize glucocorticoid-induced osteoporosis by inhibiting
ferritinophagy with resultant limited ferroptosis of osteoblasts. J
Cell Physiol. 236:6691–6705. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
165
|
Wang J, Xie X, Li H, Zheng Q, Chen Y, Chen
W, Chen Y, He J and Lu Q: Vascular endothelial cells-derived
exosomes synergize with curcumin to prevent osteoporosis
development. iScience. 27:1096082024. View Article : Google Scholar : PubMed/NCBI
|
|
166
|
Zhao D, Zhang F, Wang B, Liu B, Li L, Kim
SY, Goodman SB, Hernigou P, Cui Q, Lineaweaver WC, et al:
Guidelines for clinical diagnosis and treatment of osteonecrosis of
the femoral head in adults (2019 version). J Orthop Translat.
21:100–110. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
167
|
Wu H, Chen G, Zhang G, Lv Q, Gu D and Dai
M: Mechanism of vascular endothelial cell-derived exosomes modified
with vascular endothelial growth factor in steroid-induced femoral
head necrosis. Biomed Mater. 18:0250172023. View Article : Google Scholar
|
|
168
|
Maeda K, Kobayashi Y, Koide M, Uehara S,
Okamoto M, Ishihara A, Kayama T, Saito M and Marumo K: The
regulation of bone metabolism and disorders by Wnt signaling. Int J
Mol Sci. 20:55252019. View Article : Google Scholar : PubMed/NCBI
|
|
169
|
Zhou B, Peng K, Wang G, Chen W, Liu P,
Chen F and Kang Y: miR-483-3p promotes the osteogenesis of human
osteoblasts by targeting Dikkopf 2 (DKK2) and the Wnt signaling
pathway. Int J Mol Med. 46:1571–1581. 2020.PubMed/NCBI
|
|
170
|
Zhang G, Liu R, Dang X, Liu J and Jiao H:
Experimental study on improvement of osteonecrosis of femoral head
with exosomes derived from miR-27a-overexpressing vascular
endothelial cells. Zhongguo Xiu Fu Chong Jian Wai Ke Za Zhi.
35:356–365. 2021.In Chinese. PubMed/NCBI
|
|
171
|
Cheng M, Yang J, Zhao X, Zhang E, Zeng Q,
Yu Y, Yang L, Wu B, Yi G, Mao X, et al: Circulating myocardial
microRNAs from infarcted hearts are carried in exosomes and
mobilise bone marrow progenitor cells. Nat Commun. 10:9592019.
View Article : Google Scholar : PubMed/NCBI
|
|
172
|
Yang RZ, Zheng HL, Xu WN, Zheng XF, Li B,
Jiang LS and Jiang SD: Vascular endothelial cell-secreted exosomes
facilitate osteoarthritis pathogenesis by promoting chondrocyte
apoptosis. Aging (Albany NY). 13:4647–4662. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
173
|
Weilner S, Schraml E, Wieser M, Messner P,
Schneider K, Wassermann K, Micutkova L, Fortschegger K, Maier AB,
Westendorp R, et al: Secreted microvesicular miR-31 inhibits
osteogenic differentiation of mesenchymal stem cells. Aging Cell.
15:744–754. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
174
|
Hu M, Guo G, Huang Q, Cheng C, Xu R, Li A,
Liu N and Liu S: The harsh microenvironment in infarcted heart
accelerates transplanted bone marrow mesenchymal stem cells injury:
The role of injured cardiomyocytes-derived exosomes. Cell Death
Dis. 9:3572018. View Article : Google Scholar : PubMed/NCBI
|
|
175
|
Duan D, Goemans N, Takeda S, Mercuri E and
Aartsma-Rus A: Duchenne muscular dystrophy. Nat Rev Dis Primers.
7:132021. View Article : Google Scholar : PubMed/NCBI
|
|
176
|
Kamdar F and Garry DJ:
Dystrophin-deficient cardiomyopathy. J Am Coll Cardiol.
67:2533–2546. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
177
|
Wang Z, Su X, Ashraf M, Kim IM, Weintraub
NL, Jiang M and Tang Y: Regenerative Therapy for cardiomyopathies.
J Cardiovasc Transl Res. 11:357–365. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
178
|
Su X, Shen Y, Jin Y, Jiang M, Weintraub N
and Tang Y: Purification and transplantation of myogenic progenitor
cell derived exosomes to improve cardiac function in duchenne
muscular dystrophic mice. J Vis Exp. View
Article : Google Scholar : 2019.
|
|
179
|
Su X, Jin Y, Shen Y, Ju C, Cai J, Liu Y,
Kim IM, Wang Y, Yu H, Weintraub NL, et al: Exosome-derived
dystrophin from allograft myogenic progenitors improves cardiac
function in duchenne muscular dystrophic mice. J Cardiovasc Transl
Res. 11:412–419. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
180
|
Su X, Shen Y, Jin Y, Weintraub NL and Tang
YL: Identification of critical molecular pathways involved in
exosome-mediated improvement of cardiac function in a mouse model
of muscular dystrophy. Acta Pharmacol Sin. 42:529–535. 2021.
View Article : Google Scholar :
|
|
181
|
Heusch G, Bøtker HE, Przyklenk K,
Redington A and Yellon D: Remote ischemic conditioning. J Am Coll
Cardiol. 65:177–195. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
182
|
Hu T, Duan R, Gao H, Bai X, Huang X, Yan
X, An L, Ma Y, Chen R, Hong S and Gan M: Exosomes from myoblasts
induced by hypoxic preconditioning improved ventricular conduction
by increasing Cx43 expression in hypothermia ischemia reperfusion
hearts. Cytotechnology. 76:533–546. 2024. View Article : Google Scholar : PubMed/NCBI
|
|
183
|
Cruz-Jentoft AJ and Sayer AA: Sarcopenia.
Lancet. 393:2636–2646. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
184
|
Cruz-Jentoft AJ, Bahat G, Bauer J, Boirie
Y, Bruyère O, Cederholm T, Cooper C, Landi F, Rolland Y, Sayer AA,
et al: Sarcopenia: Revised European consensus on definition and
diagnosis. Age Ageing. 48:16–31. 2019. View Article : Google Scholar :
|
|
185
|
Bekfani T, Pellicori P, Morris DA, Ebner
N, Valentova M, Steinbeck L, Wachter R, Elsner S, Sliziuk V,
Schefold JC, et al: Sarcopenia in patients with heart failure with
preserved ejection fraction: Impact on muscle strength, exercise
capacity and quality of life. Int J Cardiol. 222:41–46. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
186
|
Anker SD and Sharma R: The syndrome of
cardiac cachexia. Int J Cardiol. 85:51–66. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
187
|
Coats AJ: Research on cachexia, sarcopenia
and skeletal muscle in cardiology. J Cachexia Sarcopenia Muscle.
3:219–223. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
188
|
Loncar G, Springer J, Anker M, Doehner W
and Lainscak M: Cardiac cachexia: hic et nunc. J Cachexia
Sarcopenia Muscle. 7:246–260. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
189
|
Marbán E: A mechanistic roadmap for the
clinical application of cardiac cell therapies. Nat Biomed Eng.
2:353–361. 2018. View Article : Google Scholar
|
|
190
|
Rogers RG, Fournier M, Sanchez L, Ibrahim
AG, Aminzadeh MA, Lewis MI and Marbán E: Disease-modifying
bioactivity of intravenous cardiosphere-derived cells and exosomes
in mdx mice. JCI Insight. 4:e1257542019. View Article : Google Scholar : PubMed/NCBI
|
|
191
|
Pironti G, Strachan RT, Abraham D, Mon-Wei
Yu S, Chen M, Chen W, Hanada K, Mao L, Watson LJ and Rockman HA:
Circulating exosomes induced by cardiac pressure overload contain
functional angiotensin II type 1 receptors. Circulation.
131:2120–2130. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
192
|
Wang XH: MicroRNA in myogenesis and muscle
atrophy. Curr Opin Clin Nutr Metab Care. 16:258–266. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
193
|
Murach KA and McCarthy JJ: MicroRNAs,
heart failure and aging: Potential interactions with skeletal
muscle. Heart Fail Rev. 22:209–218. 2017. View Article : Google Scholar :
|
|
194
|
Melman YF, Shah R and Das S: MicroRNAs in
heart failure: Is the picture becoming less miRky? Circ Heart Fail.
7:203–214. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
195
|
McCarthy JJ and Esser KA: MicroRNA-1 and
microRNA-133a expression are decreased during skeletal muscle
hypertrophy. J Appl Physiol (1985). 102:306–313. 2007. View Article : Google Scholar
|
|
196
|
Kuwabara Y, Ono K, Horie T, Nishi H, Nagao
K, Kinoshita M, Watanabe S, Baba O, Kojima Y, Shizuta S, et al:
Increased microRNA-1 and microRNA-133a levels in serum of patients
with cardiovascular disease indicate myocardial damage. Circ
Cardiovasc Genet. 4:446–454. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
197
|
Kolhe R, Hunter M, Liu S, Jadeja RN,
Pundkar C, Mondal AK, Mendhe B, Drewry M, Rojiani MV, Liu Y, et al:
Gender-specific differential expression of exosomal miRNA in
synovial fluid of patients with osteoarthritis. Sci Rep.
7:20292017. View Article : Google Scholar : PubMed/NCBI
|
|
198
|
Zhao Y and Xu J: Synovial fluid-derived
exosomal lncRNA PCGEM1 as biomarker for the different stages of
osteoarthritis. Int Orthop. 42:2865–2872. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
199
|
Rezaie J, Feghhi M and Etemadi T: A review
on exosomes application in clinical trials: Perspective, questions
and challenges. Cell Commun Signal. 20:1452022. View Article : Google Scholar
|
|
200
|
Tai YL, Chen KC, Hsieh JT and Shen TL:
Exosomes in cancer development and clinical applications. Cancer
Sci. 109:2364–2374. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
201
|
Gong L, Zhou H, Zhang S, Wang C, Fu K, Ma
C, Zhang Y, Peng C and Li Y: CD44-targeting drug delivery system of
exosomes loading forsythiaside A combats liver fibrosis via
regulating NLRP3-mediated pyroptosis. Adv Healthc Mater.
12:e22022282023. View Article : Google Scholar : PubMed/NCBI
|
|
202
|
Kim MS, Haney MJ, Zhao Y, Mahajan V,
Deygen I, Klyachko NL, Inskoe E, Piroyan A, Sokolsky M, Okolie O,
et al: Development of exosome-encapsulated paclitaxel to overcome
MDR in cancer cells. Nanomedicine. 12:655–664. 2016. View Article : Google Scholar
|
|
203
|
Dumontel B, Susa F, Limongi T, Vighetto V,
Debellis D, Canta M and Cauda V: Nanotechnological engineering of
extracellular vesicles for the development of actively targeted
hybrid nanodevices. Cell Biosci. 12:612022. View Article : Google Scholar : PubMed/NCBI
|
|
204
|
Tang A, Shu Q, Jia S, Lai Z and Tian J:
Adipose mesenchymal stem cell-derived exosomes as nanocarriers for
treating musculoskeletal disorders. Int J Nanomedicine.
19:13547–13562. 2024. View Article : Google Scholar : PubMed/NCBI
|
|
205
|
Liu L, Chen S, Song Y, Cui L, Chen Y, Xia
J, Fan Y and Yang L and Yang L: Hydrogels empowered mesenchymal
stem cells and the derived exosomes for regenerative medicine in
age-related musculoskeletal diseases. Pharmacol Res.
213:1076182025. View Article : Google Scholar : PubMed/NCBI
|
|
206
|
Yao J, Huang K, Zhu D, Chen T, Jiang Y,
Zhang J, Mi L, Xuan H, Hu S, Li J, et al: A minimally invasive
exosome spray repairs heart after myocardial infarction. ACS Nano.
15:11099–11111. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
207
|
Chen P, Zheng L, Wang Y, Tao M, Xie Z, Xia
C, Gu C, Chen J, Qiu P, Mei S, et al: Desktop-stereolithography 3D
printing of a radially oriented extracellular matrix/mesenchymal
stem cell exosome bioink for osteochondral defect regeneration.
Theranostics. 9:2439–2459. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
208
|
Li Q, Xu Y, Lv K, Wang Y, Zhong Z, Xiao C,
Zhu K, Ni C, Wang K, Kong M, et al: Small extracellular vesicles
containing miR-486-5p promote angiogenesis after myocardial
infarction in mice and nonhuman primates. Sci Transl Med.
13:eabb02022021. View Article : Google Scholar : PubMed/NCBI
|
|
209
|
Spiller KL and Koh TJ: Macrophage-based
therapeutic strategies in regenerative medicine. Adv Drug Deliv
Rev. 122:74–83. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
210
|
de Couto G, Gallet R, Cambier L,
Jaghatspanyan E, Makkar N, Dawkins JF, Berman BP and Marbán E:
Exosomal MicroRNA transfer into macrophages mediates cellular
postconditioning. Circulation. 136:200–214. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
211
|
Zavatti M, Beretti F, Casciaro F, Bertucci
E and Maraldi T: Comparison of the therapeutic effect of amniotic
fluid stem cells and their exosomes on monoiodoacetate-induced
animal model of osteoarthritis. Biofactors. 46:106–117. 2020.
View Article : Google Scholar
|
|
212
|
Zheng Y, Fu L, Zhang Z, Wu J, Yuan X, Ding
Z, Ning C, Sui X, Liu S and Guo Q: Three-dimensional bioprinting of
growth differentiation factor 5-preconditioned mesenchymal stem
cell-derived exosomes facilitates articular cartilage endogenous
regeneration. ACS Nano. 19:15281–15301. 2025. View Article : Google Scholar : PubMed/NCBI
|
|
213
|
Maffulli N, Wong J and Almekinders LC:
Types and epidemiology of tendinopathy. Clin Sports Med.
22:675–692. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
214
|
Song K, Jiang T, Pan P, Yao Y and Jiang Q:
Exosomes from tendon derived stem cells promote tendon repair
through miR-144-3p-regulated tenocyte proliferation and migration.
Stem Cell Res Ther. 13:802022. View Article : Google Scholar : PubMed/NCBI
|
|
215
|
Guo H, Huang B, Wang Y, Zhang Y, Ma Q and
Ren Y: Bone marrow mesenchymal stem cells-derived exosomes improve
injury of hippocampal neurons in rats with depression by
upregulating microRNA-26a expression. Int Immunopharmacol.
82:1062852020.Epub ahead of print. View Article : Google Scholar : PubMed/NCBI
|
|
216
|
Pomatto M, Gai C, Negro F, Cedrino M,
Grange C, Ceccotti E, Togliatto G, Collino F, Tapparo M, Figliolini
F, et al: Differential therapeutic effect of extracellular vesicles
derived by bone marrow and adipose mesenchymal stem cells on wound
healing of diabetic ulcers and correlation to their cargoes. Int J
Mol Sci. 22:38512021. View Article : Google Scholar : PubMed/NCBI
|
|
217
|
Liu X, Zhang M, Liu H, Zhu R, He H, Zhou
Y, Zhang Y, Li C, Liang D, Zeng Q and Huang G: Bone marrow
mesenchymal stem cell-derived exosomes attenuate cerebral
ischemia-reperfusion injury-induced neuroinflammation and
pyroptosis by modulating microglia M1/M2 phenotypes. Exp Neurol.
341:1137002021. View Article : Google Scholar : PubMed/NCBI
|
|
218
|
Zhou Y, Zhao B, Zhang XL, Lu YJ, Lu ST,
Cheng J, Fu Y, Lin L, Zhang NY, Li PX, et al: Combined topical and
systemic administration with human adipose-derived mesenchymal stem
cells (hADSC) and hADSC-derived exosomes markedly promoted
cutaneous wound healing and regeneration. Stem Cell Res Ther.
12:2572021. View Article : Google Scholar : PubMed/NCBI
|
|
219
|
Dave KM, Venna VR, Rao KS, Stolz DB, Brady
B, Quaicoe VA, Maniskas ME, Hildebrand EE, Green D, Chen M, et al:
Mitochondria-containing extracellular vesicles from mouse vs human
brain endothelial cells for ischemic stroke therapy. J Control
Release. 373:803–822. 2024. View Article : Google Scholar : PubMed/NCBI
|
|
220
|
Lotfy A, AboQuella NM and Wang H:
Mesenchymal stromal/stem cell (MSC)-derived exosomes in clinical
trials. Stem Cell Res Ther. 14:662023. View Article : Google Scholar : PubMed/NCBI
|
|
221
|
Shin DI, Jin YJ, Noh S, Yun HW, Park DY
and Min BH: Exosomes secreted during myogenic differentiation of
human fetal cartilage-derived progenitor cells promote skeletal
muscle regeneration through miR-145-5p. Tissue Eng Regen Med.
21:487–497. 2024. View Article : Google Scholar : PubMed/NCBI
|
|
222
|
Guo R, Wu Z, Liu A, Li Q, Han T and Shen
C: Hypoxic preconditioning-engineered bone marrow mesenchymal stem
cell-derived exosomes promote muscle satellite cell activation and
skeletal muscle regeneration via the miR-210-3p/KLF7 mechanism. Int
Immunopharmacol. 142:1131432024. View Article : Google Scholar : PubMed/NCBI
|
|
223
|
Xu N, Cui G, Zhao S, Li Y, Liu Q, Liu X,
Zhao C, Feng R, Kuang M and Han S: Therapeutic effects of
mechanical stress-induced C2C12-derived exosomes on
glucocorticoid-induced osteoporosis through miR-92a-3p/PTEN/AKT
signaling pathway. Int J Nanomedicine. 18:7583–7603. 2023.
View Article : Google Scholar : PubMed/NCBI
|
|
224
|
Chen W, Zheng H, Liao Q, Zeng S, Bai R,
Shi J, Jiang Y, Wang T, Jia H, Liang W, et al: Zhuang-Gu-Fang
promotes osteoblast differentiation via myoblasts and
myoblast-derived exosomal miRNAs:miR-5100, miR-126a-3p,
miR-450b-5p, and miR-669a-5p. Phytomedicine. 130:1557182024.
View Article : Google Scholar : PubMed/NCBI
|