Introduction
Achilles tendinitis (AT) commonly arises from
trauma, iatrogenic factors and inflammatory conditions,
particularly spondyloarthritis (1). Tendon ruptures have been reported
in patients with systemic lupus erythematosus, often linked to
corticosteroid therapy (2). In
rheumatoid arthritis, tendinitis may involve the tendon, the
surrounding synovium or both. Tendon involvement occurs in up to
64% of patients with rheumatoid arthritis, although some studies
report lower prevalence rates (3,4).
Typical symptoms of tendinitis include localized pain and
tenderness along the affected tendon, particularly near joints, and
symptoms often worsen with exercise or physical exertion (5). In AT, patients experience
persistent pain and swelling accompanied by a sustained
inflammatory response. Several inflammatory mediators contribute to
the disease pathology, including tumor necrosis factor (TNF)-α,
IL-1β, MMP and metabolic enzymes such as cyclooxygenase-2 (6-10).
Serum TNF-α levels are significantly higher in
patients with rotator cuff tears accompanied by sleep disturbance
compared with those with rotator cuff tears and normal sleep, as
well as patients in a control group with chronic shoulder
instability (11). The
expression level of TNF-α is frequently upregulated in the
subscapular sac tissue and degenerated tendon tissue of patients
with rotator cuff injury (12,13). The overexpression of TNF-α in
tendon sheath injuries can exacerbate the inflammatory response,
thereby leading to more severe tissue damage and increased pain
(12,13). Etanercept is a soluble fusion
protein composed of two human tumor necrosis factor receptor II
(TNFR II, molecular weight 75 kDa) subunits linked to the Fc
portion of human IgG1. It is administered via subcutaneous
injection and displays slow systemic absorption, reaching peak
concentration 2-3 days post-injection (14). Acting as a decoy receptor,
etanercept binds TNF-α and TNF-β with greater affinity than
endogenous soluble TNFRs, thereby blocking their interaction with
native receptors and preventing downstream proinflammatory
signaling (15). By
competitively inhibiting TNF-α binding to its natural receptors,
etanercept disrupts inflammatory, apoptotic and immune-associated
signaling pathways. This blockade effectively reduces inflammation
in a range of pathological conditions (15-17). Experimental results demonstrated
that etanercept treatment alleviated stress-shielding-induced
structural and morphological changes in tendon collagen tissue
(18). Etanercept suppressed the
upregulation of MMP-13 and MMP-3, as well as collagen III
expression levels (18).
Most adult human organs contain regenerative stem
cells (SCs) that reside within specialized microenvironments, or
niches, which are key for maintaining tissue homeostasis and
facilitating repair following injury (19). The function of SCs depends
primarily on their dynamic interaction with the surrounding
microenvironment. Tendon SCs (TSCs) are undifferentiated cells
within tendon tissue, characterized by their ability to self-renew
and differentiate into tenocytes (20). Although studies suggest that TSCs
contribute to tendon repair and regeneration during disease
progression, the underlying molecular mechanisms remain poorly
understood (20-22).
TNF-α is a key cytokine involved in inflammation.
TNF-α not only activates proinflammatory genes but also exacerbates
inflammatory responses by promoting apoptosis, modulating immune
activity and facilitating disease progression (23). Elevated TNF-α levels are commonly
observed in inflammatory diseases, where they influence the
development, senescence and functional properties of mesenchymal
SCs (MSCs) under specific physiological and pathological conditions
(24). However, the role of
TNF-α in the onset and progression of tendon disorders remains
insufficiently defined. While anti-TNF-α biological agents have
proven highly effective in treating chronic inflammation and
autoimmune disease, their effects on TSCs are still unclear
(25). Therefore, investigating
the influence of TNF-α and TNF-α inhibitors on TSCs is key.
The present study aimed to clarify the role of TNF-α
in tendon pathology by examining its effects on TSC senescence and
inflammation at cellular and animal levels, and to assess whether
TNF-α inhibition with Etanercept could mitigate these pathological
processes. This work seeks to provide mechanistic insights into
tendon disorders and validate potential therapeutic strategies.
Materials and methods
Cell isolation and culture
All animal procedures strictly followed
institutional regulations for animal research and were approved by
the Animal Care Committee of Nantong University (Nantong, China;
approval no. S20230522-005). TSCs were isolated from the flexor
digitorum profundus (FDP) tendons of female C57BL/6 mice (n=6,
weight 16-18 g, provided by Nantong University Experimental Animal
Center, Nantong, China; age, 4 weeks). The mice were euthanized
humanely via cervical dislocation. FDP tendons were dissected from
the index, middle and ring digits of the hind limbs, washed in
sterile phosphate-buffered saline (PBS) and finely minced into
small fragments. Tissue was digested using a solution containing 3
mg/ml type I collagenase and 4 mg/ml dispase (Gibco, Thermo Fisher
Scientific, Inc.) for 2 h at 37°C. The cell suspension was filtered
through a 70-µm cell strainer to obtain a single-cell
suspension, which was seeded onto 25-cm2 culture plates.
Cells were cultured in DMEM supplemented with 10% FBS (both Gibco;
Thermo Fisher Scientific, Inc.), 100 U/ml penicillin and 100
µg/ml streptomycin. Cultures were maintained at 37°C in a
humidified atmosphere containing 5% CO2. Medium was
replaced every 3 days to support optimal cell proliferation. Cells
at 85-90% confluence were sub-cultured at a ratio of 1:3 (one part
cells was split into three new culture vessels). Cells were
detached using 0.25% trypsin and transferred to new flasks for
continued expansion and downstream analyses.
Cells were grouped as follows (all treatments were
performed at 37°C): i) TSCs treated with PBS served as the control;
TSCs stimulated with TNF-α at ii) 20 and iii) 40 ng/ml for 6 h,
repeated three times at 48-h intervals and TSCs were stimulated
with TNF-α at iv) 20 and v) 40 ng/ml for 6 h, repeated six times at
24-h intervals. To investigate the potential therapeutic effect of
TNF-α inhibition, TSCs stimulated three times with 20 ng/ml
TNF-α-were co-cultured with a TNF-α antagonist (Etanercept) for 48
h at 37°C. For the therapeutic co-culture assay, TSCs
pre-stimulated three times with 20 ng/ml TNF-α (37°C, 6 h per
stimulation, 48-h intervals) were treated with the TNF-α antagonist
etanercept (recombinant human TNF-α receptor II:IgG Fc fusion
protein, 20 ng/ml, supplied by Sunshine Guojian Pharmaceutical) at
37°C for 48 h. Untreated TSCs served as controls.
Differentiation assay
TSCs were seeded at a density of 1×104
cells/cm2 to initiate differentiation. Mouse TSCs were
induced to undergo osteogenic and adipogenic differentiation using
a commercial differentiation medium kit (cat. no. RASTA-90021; cat.
no. RASTA-90031, Cyagen Biosciences, Inc.). Cells were cultured in
differentiation media at 37°C with 5% CO2 for 28 days.
Following washing with PBS, cells were fixed in 4% PFA) at room
temperature for 15 min. To assess osteogenic differentiation, cells
were stained with Alizarin Red S and underwent an alkaline
phosphatase assay (cat. no. C0148S, cat. no. C3250S, Beyotime
Institute of Biotechnology) at room temperature for 20 min to
evaluate matrix calcification. 0.5% Oil Red O staining (cat. no.
C0157M, Beyotime Institute of Biotechnology) at room temperature
for 20 min was performed to detect intracellular neutral lipid
vacuoles, indicating adipogenic differentiation.
To initiate chondrogenic differentiation, TSCs were
centrifuged in 15 ml conical tubes at 160 × g at room temperature
for 5 min to form cell pellets. Pellets were cultured in
chondrogenic differentiation medium (cat. no. RASTA-90041, Cyagen
Biosciences, Inc.) at 37°C with 5% CO2, replacing the
medium every 3 days for a total of 4 weeks. Pellets were fixed in
4% PFA at room temperature for 24 h, dehydrated through a graded
sucrose series 4°C, 4 h per step, embedded in optimal cutting
temperature compound, and sectioned into 5-µm slices.
Sections were stained with toluidine blue (cat. no. C0637, Beyotime
Institute of Biotechnology) at room temperature for 10 min for
histological evaluation. Images were captured using a light
microscope (Leica).
Senescence associated β-galactosidase
(SA-β-gal) assay
TSC senescence was assessed using the Senescence
β-Galactosidase Staining kit (SA-β-gal assay kit) (cat. no. C0602,
Beyotime Institute of Biotechnology), according to the
manufacturer's instructions. TSCs were cultured at a density of
1×106 cells/well in 6-well plates and fixed with 4% PFA
at room temperature for 10 min following treatment as
aforementioned. Cells were incubated at 37°C overnight with the
SA-β-gal staining solution. Following thorough washing by PBS,
cells were examined under a light microscope (Olympus) at ×10
magnification. Senescent cells exhibited blue staining. To quantify
senescence, 1,000 cells were counted across 20 randomly selected
fields of view/slide and the percentage of SA-β-gal-positive cells
was calculated (26).
Immunofluorescent staining
TSCs were fixed in 4% PFA at 4°C for 1 h, then
washed with 0.1% Triton X-100 in PBS (PBST). Cells were blocked in
PBST containing 10% FBS at room temperature for 30 min and
incubated overnight at 4°C with primary antibodies (1:100)
targeting γ-H2A.X (cat. no. ab81299, anti-rabbit, Abcam), p65 (cat.
no. sc-8008, anti-mouse, Santa Cruz Biotechnology, Inc.), p53 (cat.
no. ab246550, anti-rabbit, Abcam), p21 (cat. no. ab188224,
anti-rabbit, Abcam), Oct4 (cat. no. ab200834, anti-rabbit, Abcam)
and Nanog (cat. no. ab109250, anti-rabbit, Abcam). Following
washing with PBS, secondary antibodies [goat Anti-Mouse IgG H&L
(Alexa Fluor® 488) (ab150113), Goat Anti-Rabbit IgG
H&L (Alexa Fluor® 488) (ab150077), goat Anti-Rabbit
IgG H&L (Alexa Fluor® 568) (ab175471), Abcam] at
1:250 were added for 2 h at room temperature. Nuclei were
counterstained with DAPI (cat. no. ab228549, Abcam) at 1:500 at
room temperature for 30 min and cells were examined using a
fluorescence microscope at ×20 magnification.
Filamentous (F-)actin staining
The Cell Navigator™ F-Actin Labeling kit (cat. no.
22661, AAT Bioquest) was used to visualize F-actin) in fixed cells,
according to the manufacturer's instructions. TSCs were fixed in 4%
PFA at 4°C for 30 min, followed by washing with PBS at room
temperature. Cells were permeabilized with 0.1% PBST at room
temperature for 5 min and incubated with iFluor™ 488-Phalloidin
working solution at room temperature for 30 min. After washing with
PBS, nuclei were counterstained with DAPI (cat. no. ab228549,
Abcam) at 1:500 at room temperature for 30 min and cells were
examined using a fluorescence microscope at ×20 magnification.
Reactive oxygen species (ROS)
staining
ROS staining kit (cat. no. S0033S, Beyotime
Institute of Biotechnology) was used according to the
manufacturer's instructions. After washing three times with PBS,
TSCs were incubated with ROS-sensitive dye DCFH-DA at room
temperature for 30 min. Following another PBS wash, nuclei were
counterstained with DAPI (cat. no. ab228549, Abcam) at 1:500 at
room temperature for 30 min and cells were examined using a
fluorescence microscope at ×20 magnification.
EdU cell proliferation assay
TSC proliferation was assessed using the Cell-Light™
EdU Apollo In Vitro kit (Guangzhou RiboBio Co., Ltd.), following
the manufacturer's protocol. TSCs in the logarithmic growth phase
were seeded into 24-well plates at a density of 2×104
cells/well and cultured to 70% confluency). Then, 25 µM EdU
medium was added to each well at 37°C for 24 h. Cells were washed
twice with PBS and fixed with 4% PFA at room temperature for 30
min, treated with 2 mg/l glycine at room temperature for 5 min,
washed twice with PBS for 5 min and permeabilized with Triton X-100
(cat. no. P0096, Beyotime Institute of Biotechnology) for 10 min at
room temperature. Apollo® reaction mixture was added at
room temperature for 30 min and cells were washed twice with PBS at
room temperature. Finally, nuclei were stained with DAPI (cat. no.
ab228549, Abcam) at 1:500 at room temperature for 10 min before
washing with PBS and imaging the cells by fluorescence microscopy
at ×10 magnification.
Cell cycle detection
Cell cycle distribution was assessed using the Cell
Cycle Assay kit (Beijing Solarbio Science & Technology Co.,
Ltd.) according to the manufacturer's instructions. Cells were
fixed in pre-chilled 95% ethanol at 4°C for 24 h, washed with PBS
and stained with propidium iodide at 37°C for 30 min. DNA content
was analyzed by flow cytometry using a FACSCalibur system (BD
Biosciences) and cell cycle distribution was quantified using
ModFit LT software (version 5.0, Verity Software House, vsh.com/).
Western blotting
TSCs were lysed for 30 min using RIPA buffer
containing protease inhibitors (cat. no. P0013B, Beyotime Institute
of Biotechnology). Supernatant was collected following
centrifugation at 12,800 × g 4°C for 20 min. Protein concentration
was quantified use BCA assay, samples were mixed with loading
buffer and protein was denatured by heating at 100°C for 10 min.
Protein (20 µg/lane) was separated by 10% or 12% SDS-PAGE
and transferred onto PVDF membranes using a BIO-RAD transfer system
at 300 mA. Membranes were blocked with 5% skimmed milk at room
temperature for 2 h, then incubated overnight at 4°C with primary
antibodies (1:1,000). After washing by Tris-Buffered Saline with
Tween 20 (TBST), membranes were incubated with secondary antibodies
(1:10,000) (cat. no. D110087, D110058, Sangon Biotech) at room
temperature for 2 h. Primary antibodies included GAPDH (cat. no.
10494-1-AP, anti-rabbit, Proteintech Group, Inc.), p65 (cat. no.
sc-8008, anti-mouse, Santa Cruz Biotechnology), p53 (cat. no. ab26,
anti-mouse, Abcam), p21 (cat. no. ab188224, anti-rabbit, Abcam),
phosphorylated p65 (p-p65; cat. no. sc-136548, anti-mouse, Santa
Cruz Biotechnology), γ-H2A.X (cat. no. ab81299, anti-rabbit,
Abcam), CDK2 (cat. no. ab32147, anti-rabbit, Abcam) and cyclin E
(cat. no. 11554-1-AP, anti-rabbit, Proteintech Group, Inc.), H2A.X
(cat. no. 10856-1-AP, anti-rabbit, Proteintech Group, Inc.). The
membranes were incubated with anti-IgG secondary antibodies
conjugated to horseradish peroxidase (HRP). Detection of specific
bands was performed using an ECL Detection kit (Bio-Rad), and
visualization was carried out with the ChemiDoc XRS + System
(Bio-Rad). The densitometry was performed using ImageJ software
(version 1.53t, National Institutes of Health).
Tendinopathy rat model
Rats were housed in a specific pathogen-free (SPF)
facility at temperature 20-26°C, humidity 30-70%, with a 12-h
light/dark cycle and provided ad libitum access to standard rodent
chow and tap water. A total of 30 male Sprague-Dawley rats (age, 8
weeks; weight, 220±9 g, Nantong University Experimental Animal
Center, Nantong, China) were randomly assigned to three treatment
groups (n=10/group), each receiving injections in the Achilles
tendon: i) PBS control; ii) PBS + collagenase type I (5 mg/ml) and
iii) collagenase type I (5 mg/ml)+ etanercept (0.5 mg/kg). At 20
days after collagenase type I (5 mg/ml) treatment twice,
administered 7 days apart, etanercept (0.5 mg/kg) was administered
seven times over 2 weeks. Achilles tendons were harvested for
histopathological analysis to evaluate collagenase-induced
tendinopathy severity and the therapeutic effects of etanercept
(27). The dose of etanercept
(0.5 mg/kg) was selected based on Chen et al (18), who investigated that in a rat
model of stress-shielded tendons, etanercept at 0.5 mg/kg/week for
2 weeks significantly attenuated early tendon degeneration by
reducing collagen fibril disorganization and suppressing matrix
metalloproteinase (MMP)-13, MMP-3, and collagen III expression.
This dosing strategy was adopted to target tumor necrosis factor-α
(TNF-α)-mediated inflammation, the key pathway identified in both
stress-shielding and collagenase-induced tendinopathy models
(18).
Rats were anesthetized with isoflurane (3.0-3.5% in
oxygen, 500-700 ml/min) until completely unresponsive to paw pinch.
Following anesthesia, death was induced by CO2 asphyxia
(40% vol/min for 5 min) followed by cervical dislocation,
consistent with the AVMA Guidelines for the Euthanasia of Animals
(28). Death was verified by the
absence of corneal reflex, breathing and heartbeat.
Histological analyses
Tendon specimens were fixed in 4% PFA at 4°C for 24
h. Samples were dehydrated through graded ethanol, embedded in
paraffin and cut into longitudinal sections of 4.5 µm
thickness. Sections were stained at room temperature with
hematoxylin and eosin (H&E) and Masson's Trichrome to assess
tendon morphology. For H&E staining, tissue sections were
dewaxed and rehydrated. Nuclei were stained with hematoxylin for 5
min. Differentiation was performed in acid alcohol for 30 sec.
Cytoplasmic counterstaining was achieved using eosin for 1 min, and
sections were rapidly dehydrated through ascending ethanol
gradients. Sections were assed using a light microscope ×20 and ×40
magnification. For Masson's Trichrome staining, the sections were
mordanted by immersion in mordant solution at 60°C for 1 h.
Sections were then stained with celestin blue solution for 3 min,
stained with Mayer's hematoxylin for 3 min. Differentiation was
performed in acid solution, followed by a 10-min tap water rinse
for bluing. Sections were stained with Ponceau-fuchsin for 10 min.
Phosphomolybdic acid treatment for 10 min was followed by direct
application of aniline blue solution for 5 min without intermediate
washing. Excess aniline blue was removed with weak acid solution,
and sections were further treated with weak acid for 2 min. Rapid
dehydration was performed in ethanol. Sections were assed using a
light microscope ×10 and ×20 magnification.
For immunohistochemistry, paraffin-embedded sections
were treated with primary antibodies against TNF-α (1:200,
ab183218, Abcam) at 4°C overnight, then incubated with secondary
antibodies enzyme-labeled goat anti-mouse/rabbit IgG polymer
(PV-6000, ZSGB-BIO) for 20 min at room temperature. Tendon
specimens were rapidly frozen in liquid nitrogen and sectioned into
4.5 µm slices. Thawed sections were washed three times with
PBS and fixed in β-galactosidase staining fixing solution (cat. no.
R0757, Shifeng Biological) at room temperature overnight. After
rinsing with PBS, β-Gal Staining A Solution 1 µl, β-Gal
Staining B Solution 1 µl, β-Gal Staining C Solution 93
µl and X-Gal Solution 5 µl) was added for 24 h at
37°C. Sections were assed using a light microscope (29).
For immunofluorescence staining, tendon samples were
incubated with 0.1% PBS-Triton X at room temperature for 15 min to
permeabilize the tissue, followed by blocking (cat. no. P0260,
Beyotime Institute of Biotechnology, Shanghai, China) to prevent
non-specific antibody binding at room temperature for 20 min.
Sections were incubated overnight at 4°C with primary antibodies
against CD44 (cat. no. 15675-1-AP, Proteintech, 1:100) and p53
(cat. no. 60283-2-Ig, Proteintech, 1:100). The samples were
incubated with secondary antibodies [Goat Anti-Mouse IgG H&L
(Alexa Fluor® 568) (ab175473), Goat Anti-Rabbit IgG
H&L (Alexa Fluor® 488) (ab150077), Abcam] at 1:250
for 2 h, followed by nuclear staining with DAPI (cat. no. ab228549,
Abcam) at 1:500 at room temperature. Sections were analyzed using a
fluorescence microscope (BX41, Olympus Corporation).
Statistical analysis
All data are presented as the mean ± SEM from ≥3
independent experiments, each performed in triplicate. Statistical
analyses were conducted using SPSS 16.0 (SPSS, Inc.). For
comparisons involving three or more groups, data were evaluated by
one-way ANOVA followed by Tukey's post hoc test for pairwise
comparisons. For two-group comparisons, Student's unpaired t-test
was used, as all samples were derived from independent experimental
units. P<0.05 was considered to indicate a statistically
significant difference.
Results
TSCs exhibit SC-like properties and TNF-α
induces senescence in TSCs
TSCs displayed a spindle-shaped, fibroblast-like
morphology and multipotent differentiation capacity toward
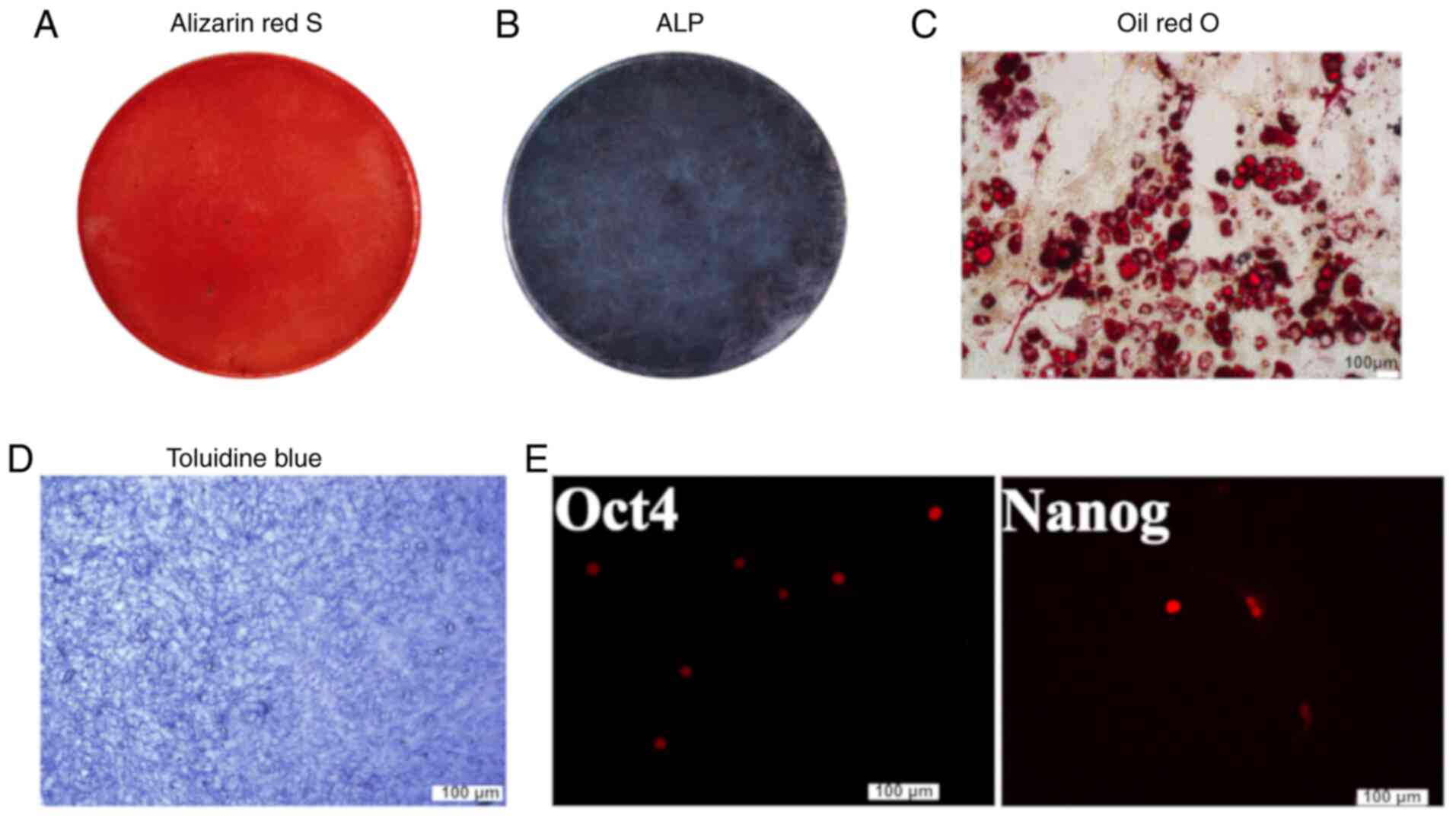
osteogenic, adipogenic and chondrogenic lineages (Fig. 1A-D). The cells also showed
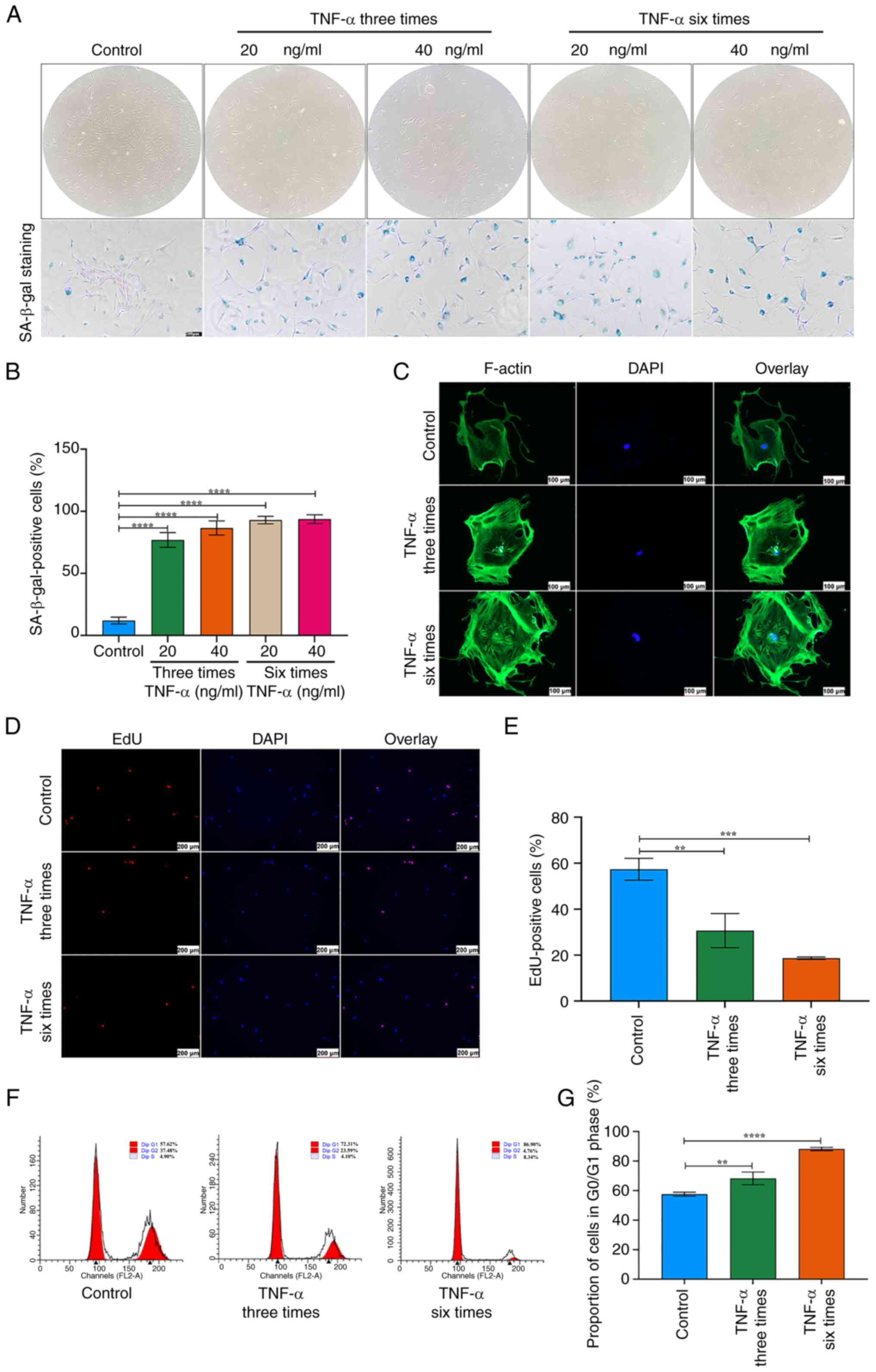
positive expression of stemness markers Oct4 and Nanog (Fig. 1E). TNF-α intensified SA-β-gal
staining, caused progressive cell enlargement and reduced the
typical spindle-shaped morphology. Following treatment with TNF-α,
TSCs exhibited an expanded and flattened phenotype (Fig. 2A). The proportion of
SA-β-gal-positive TSCs increased with higher TNF-α concentrations
and longer stimulation durations (Fig. 2A and B). Immunofluorescence
revealed disrupted F-actin organization and elevated expression
(Fig. 2C). EdU staining showed a
significant decrease in proliferative capacity in TSCs treated with
TNF-α compared with controls (Fig.
2D and E). Since cell cycle arrest is a hallmark of cellular
senescence (30), flow cytometry
was performed on TNF-α-stimulated TSCs, which revealed an increased
proportion of cells in the G0/G1 phase (Fig. 2F and G). Collectively, these
findings indicate that TSC senescence increased with higher TNF-α
concentration and stimulation duration.
TNF-α promotes ROS production and DNA
damage in TSCs
As ROS are inducers of cellular senescence (31), intracellular ROS levels were
measured in TSCs treated with TNF-α. TNF-α significantly elevated
ROS production in TSCs (Fig. 3A and
B). To evaluate the effect of increased ROS on the DNA damage
response, expression of γ-H2A.X, a specific marker of DNA damage
foci, was assessed. Immunofluorescence showed that γ-H2A.X levels
increased following TNF-α stimulation (Fig. 3C and D). The γ-H2A.X expression
increased after TNF-α stimulation, indicating enhanced DNA damage.
H2A.X expression did not differ significantly between the two
groups, as confirmed by western blot analysis (Fig. 3E and F). These results suggested
that TNF-α treatment enhances ROS generation, which is associated
with increased DNA damage in TSCs.
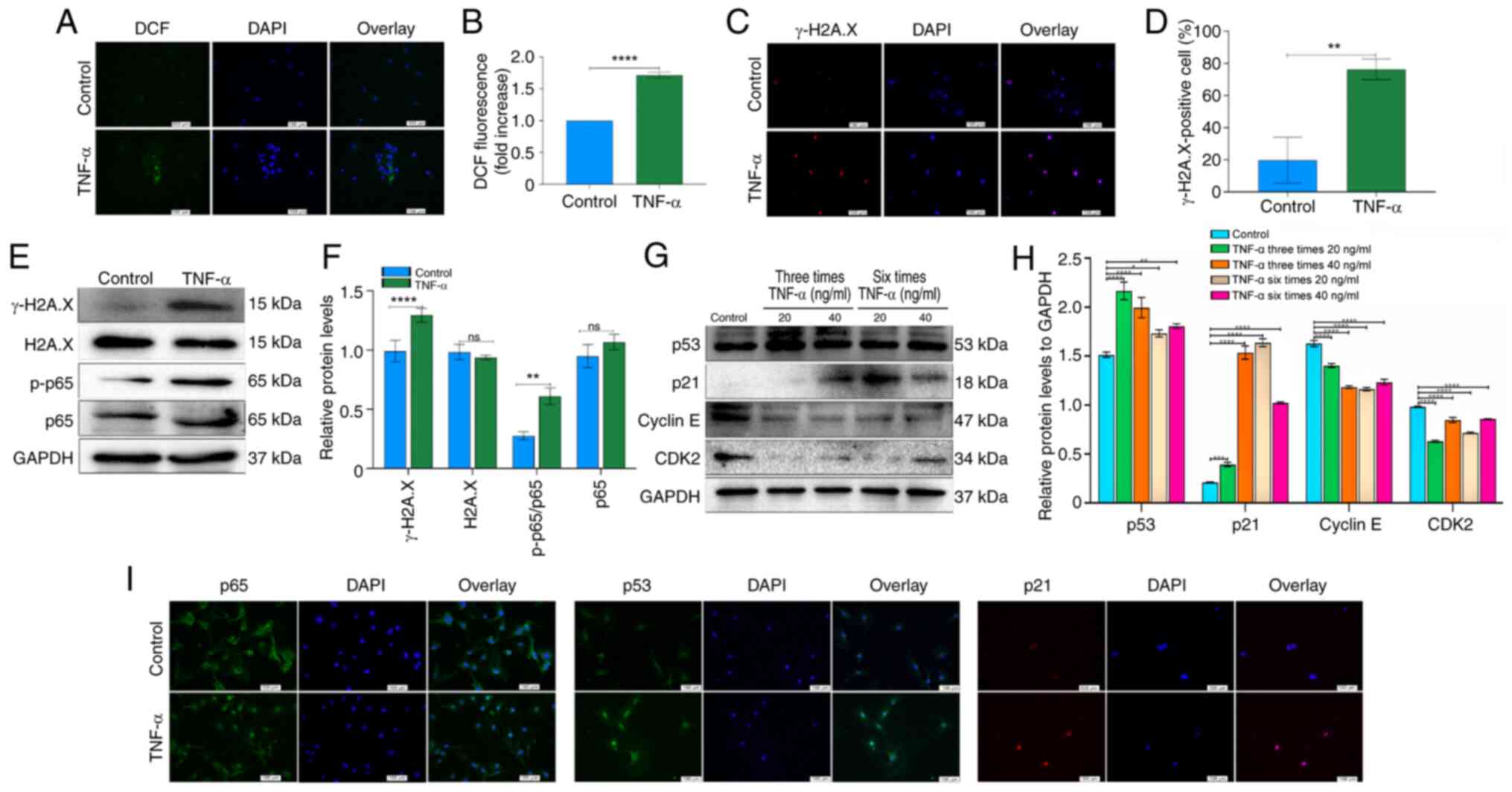 | Figure 3Effects of the NF-κB and
p53/p21/cyclin E/CDK2 signaling pathways on senescence in
TNF-α-treated TSCs. (A) ROS staining of TSCs using DCF fluorescence
probe, showing intracellular ROS distribution. Scale bar=100
µm. (B) Quantitative analysis of DCF fluorescence intensity,
demonstrating TNF-α-induced elevation of ROS levels. (C)
Immunofluorescence staining of γ-H2A.X. Following stimulations with
TNF-α (20 ng/ml, six times), the proportion of γ-H2A.X-positive
TSCs exhibited a considerable increase. Scale bar=100 µm.
(D) Quantitative analysis of γ-H2A.X-positive TSCs following TNF-α
treatment. (E) Expression of γ-H2A.X, H2A.X, p-p65 and p65
following TNF-α stimulation as assessed by western blot. GAPDH was
used as a control. (F) Bar groups showed the relative density of
γ-H2A.X, H2A.X, p-p65 and p65. (G) Expression of p53, p21, cyclin E
and CDK2 following TNF-α stimulation as assessed by western blot.
GAPDH was used as a control. (H) Relative density of p53, p21,
cyclin E and CDK2. (I) Immunofluorescence examination of p65, p53
and p21 expression was consistent with western blotting. Scale
bar=100 µm. *P<0.05, **P<0.01,
***P<0.001, ****P<0.0001. ROS, reactive
oxygen species; TSCs, tendon stem cells; p-, phosphorylation; ns,
not significant. |
TNF-α promotes expression of NF-κB and
p53/p21/cyclin E/CDK2 signaling pathway components during
senescence in TSCs
Following TNF-α stimulation, TSCs exhibited
increased expression of p-p65, while total p65 proteins levels
showed no significant difference (Fig. 3E and F). The ratio of p-p65 to
total p65 demonstrated a significant upregulation (Fig. 3E and F). Compared with the
control group, TNF-α at concentrations of 20 and 40 ng/ml, elevated
the expression of p53 and p21. TNF-α (20 ng/ml, three or six times)
all elevated the expression of p53; however, these changes did not
follow frequency-dependent pattern. TNF-α at 20 ng/ml, administered
either three or six times, significantly elevated p21 expression.
Notably, six administrations induced a higher p21 expression than
three administrations (Fig. 3G and
H). TNF-α suppressed the expression of cyclin E and CDK2 in
TSCs (Fig. 3G and H).
Immunofluorescence staining demonstrated nuclear translocation of
p65 following TNF-α exposure (Fig.
3I). Additionally, immunofluorescence revealed a marked
increase in the number of p53- and p21-positive cells in
TNF-α-treated TSCs (Fig. 3I).
These results indicated that TNF-α promoted TSC senescence by
modulating NF-κB signaling and altering the p53/p21/cyclin E/CDK2
regulatory pathway.
Effects of etanercept on the NF-κB and
p53/p21/cyclin E/CDK2 signaling pathways in TNF-α-Induced TSCs
Senescence
To investigate the role of the NF-κB and
p53/p21/cyclin E/CDK2 signaling in TNF-α–induced senescence, TSCs
were treated with the TNF-α antagonist etanercept. TNF-α increased
ROS fluorescence, while etanercept significantly reduced this
effect (Fig. 4A and B). These
results indicated that etanercept possesses antioxidant activity
capable of attenuating TNF-α-induced oxidative stress. Etanercept
also decreased γ-H2A.X expression and DNA damage in TSCs (Fig. 4C-E). H2A.X expression did not
differ significantly between the two groups, as confirmed by
Western blot analysis (Fig. 4C and
D). Western blot analysis confirmed that etanercept reversed
TNF-α-induced senescence, as evidenced by decreased expression of
p53, p21 and p-p65 (Fig. 4C and
D). By contrast, total p65 levels remained unchanged (Fig. 4C and D). Etanercept inhibited the
nuclear translocation of p65 in response to TNF-α (Fig. 4E). Immunofluorescence staining
also revealed decreased nuclear accumulation of p53 and p21 in the
TNF-α + etanercept group (Fig.
4F). Etanercept, a TNF inhibitor (recombinant Human TNF-α
Receptor II: IgG Fc Fusion Protein) effectively reversed
TNF-α-induced senescence in TSCs. Together, these findings suggest
that etanercept attenuates TNF-α-induced senescence in TSCs by
modulating oxidative stress and inhibiting key components of the
NF-κB and p53/p21 signaling pathways.
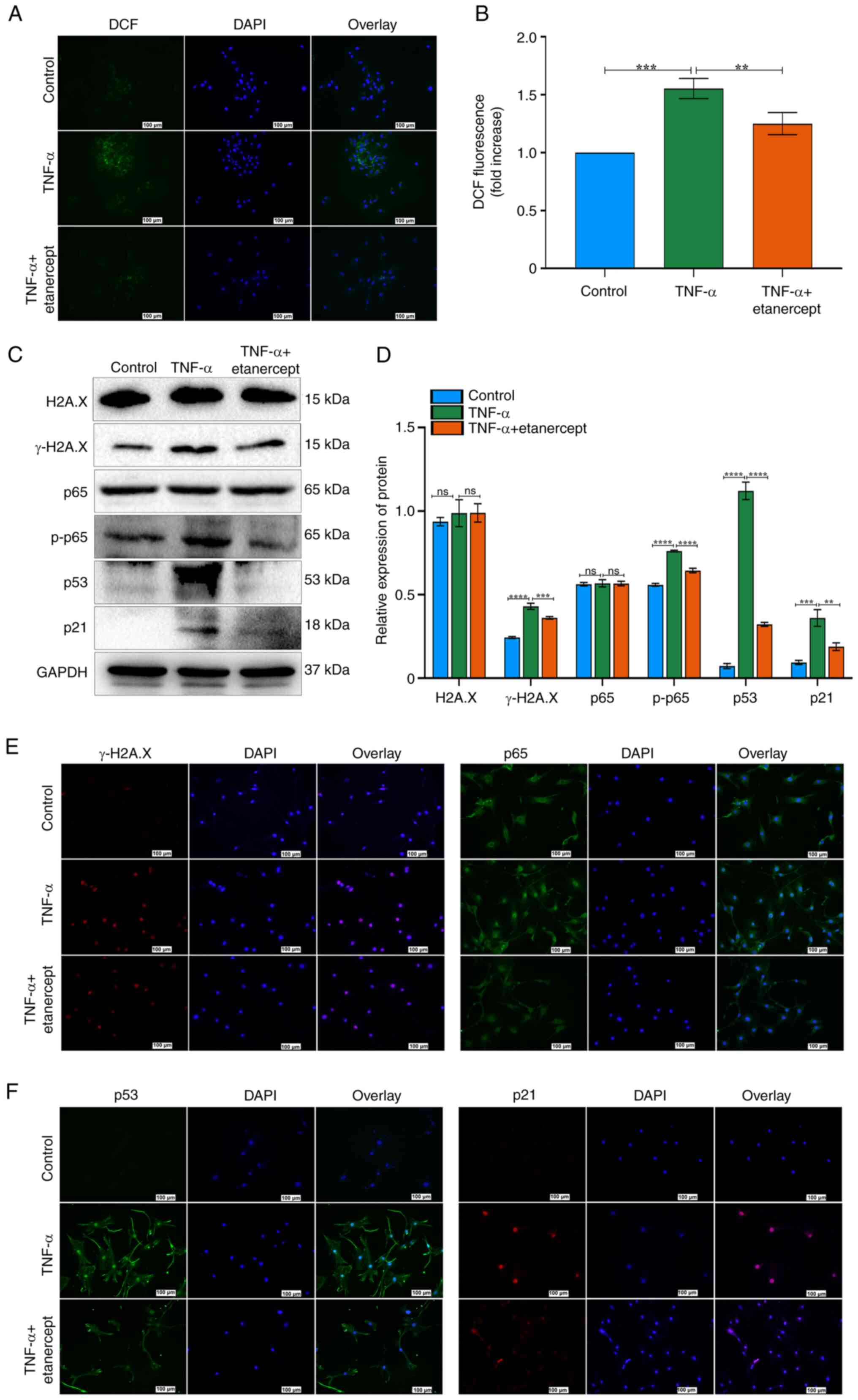 | Figure 4Effects of etanercept on the NF-κB
and p53/p21/cyclin E/CDK2 signaling pathways in tendon stem cell
senescence. (A) ROS staining of TSCs. ROS generation was markedly
elevated following repeated TNF-α stimulation and subsequently
reduced after repeated administration of etanercept. Scale bar=100
µm. (B) Quantitative analysis of DCF fluorescence intensity.
(C) Expression of γ-H2A.X, H2A.X, p53, p21, p-p65 and p65 after
stimulation with TNF-α + etanercept as assessed by western
blotting. GAPDH was used as a control. (D) Bar groups showed the
relative density of γ-H2A.X, H2A.X, p53, p21, p-p65 and p65. (E)
Immunofluorescence of γ-H2A.X and p65 yielded consistent results
with those from western blotting. Scale bar=100 µm. (F)
Immunofluorescence of p53 and p21 yielded consistent results with
those from western blotting. Scale bar=100 µm..
**P<0.01, ***P<0.001,
****P<0.0001. ROS, reactive oxygen species; p-,
phosphorylation; ns, not significant. |
TNF-α antagonists reverse TNF-α-induced
senescence in TSCs
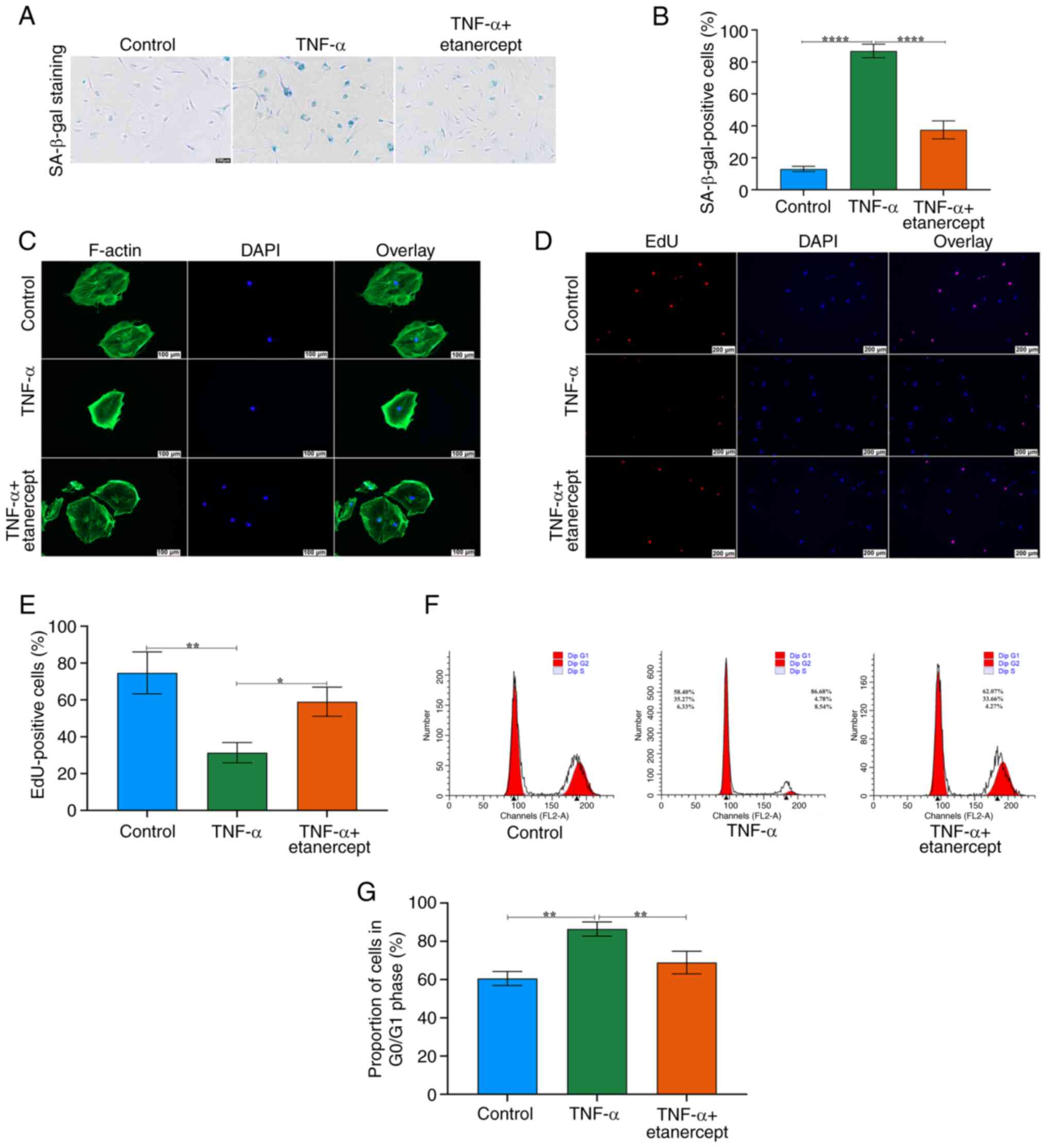
To evaluate the therapeutic potential of TNF-α
inhibition, TSCs pre-treated with TNF-α (20 ng/ml, three cycles)
were co-cultured with Etanercept (20 ng/ml). Etanercept
significantly decreased the number of SA-β-gal-positive cells
(Fig. 5A and B). Etanercept
markedly restored F-actin cytoskeleton integrity disrupted by
TNF-α, normalizing F-actin morphology (Fig. 5C). EdU staining confirmed that
etanercept rescued the proliferative capacity of TSCs (Fig. 5D and E). Etanercept normalized
the ratio of cells in the G1/G0 phase, indicating restoration of
normal cell cycle progression (Fig.
5F and G).
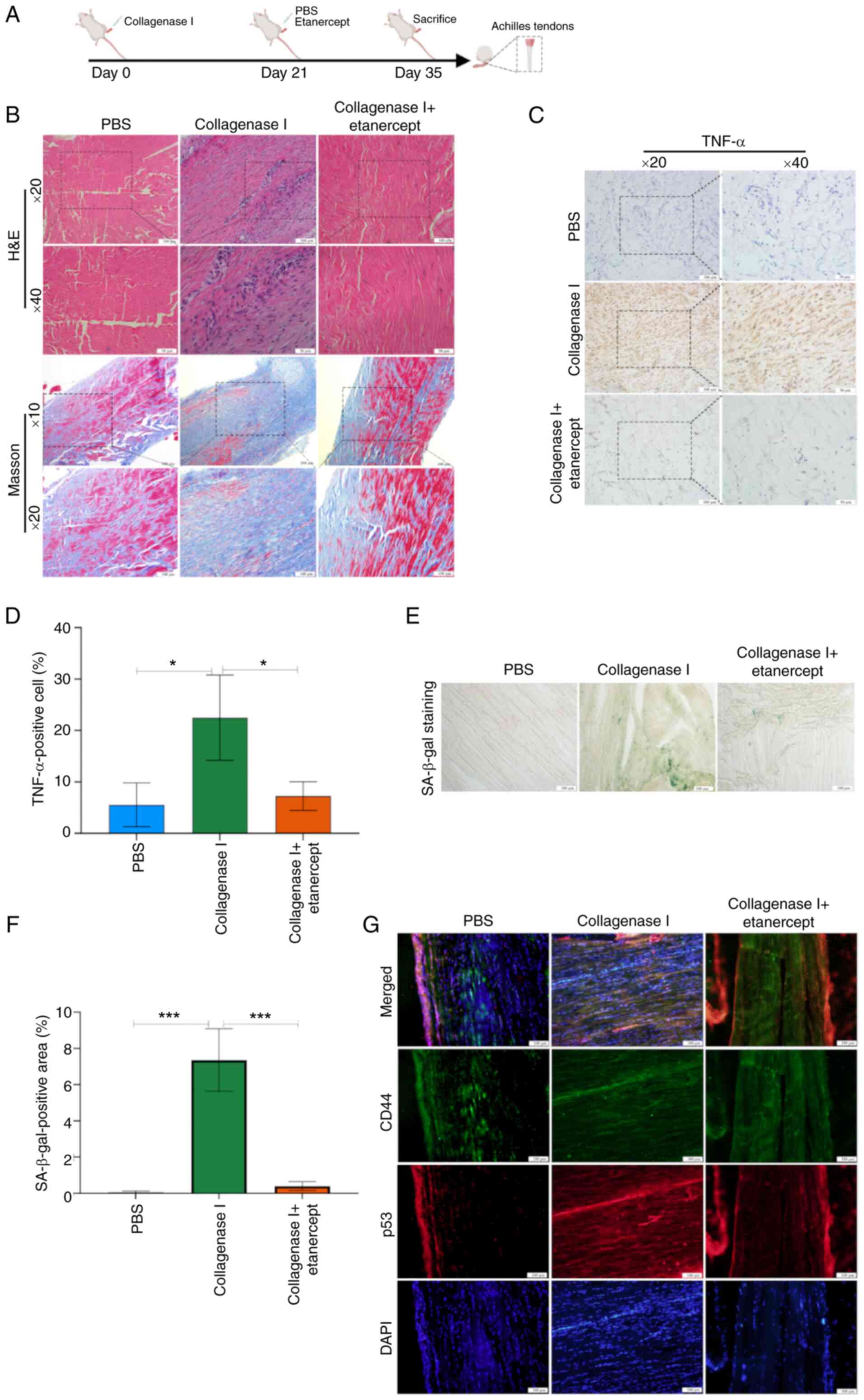
Histological changes in AT rats
To evaluate the therapeutic effects of local
etanercept injection in AT, a rat model was established by
administering collagenase I (Fig.
6A). H&E staining of tendons revealed notable inflammatory
cell infiltration (Fig. 6B). To
assess tendon repair, Masson's trichrome staining was performed 2
weeks after local injection with either PBS or etanercept (Fig. 6B). Tendons in the collagenase I +
PBS group showed disrupted and disorganized collagen fiber
architecture, while etanercept treatment mitigated collagen
degradation and improved fiber alignment (Fig. 6B).
Immunohistochemistry revealed that collagenase I
increased TNF-α protein levels, whereas etanercept significantly
suppressed TNF-α expression (Fig. 6C
and D). Moreover, the proportion of SA-β-gal-positive cells was
significantly elevated in the collagenase I + PBS group compared
with PBS controls, but etanercept treatment decreased levels of
this senescence marker (Fig. 6E and
F). Finally, double immunostaining demonstrated increased p53
expression in CD44-positive TSCs within tendon tissue, confirming
cellular senescence in TSCs (Fig.
6G).
Discussion
Previous studies have established TNF-α as a key
mediator in the pathogenesis of AT (18,32-34). Elevated TNF-α levels are
consistently reported in tendon injury models and in the serum and
tissue samples of patients with tendinopathy (18,32-34). Beyond its role in promoting
inflammation, TNF-α is a target for regenerative therapies
(18). Gulotta et al
(35) demonstrated that
polyethylene glycol-conjugated soluble TNF receptor I enhances
collagen deposition and improves biomechanical properties of
healing tendons in a rat rotator cuff repair model, high-lighting
its potential to support tendon regeneration. Similarly, Chen et
al (18) showed that
etanercept decreases inflammation and promotes the proliferation of
tendon-associated cells, contributing to improved tendon healing
outcomes. Despite these findings, the specific effects of TNF-α on
TSCs remain poorly defined. While previous studies have broadly
characterized the role of TNF-α in tendon pathophysiology (36,37), they have not fully explored how
TNF-α influences TSC stemness, proliferation or differentiation at
the molecular level. The present study aimed to systematically
investigate the cellular and molecular responses of TSCs to TNF-α
exposure. The present study assessed TNF-α-induced cellular
senescence, ROS accumulation, DNA damage and the activation of key
signaling cascades, including the NF-κB and p53/p21/cyclin E/CDK2
pathways, and evaluated the therapeutic potential of etanercept in
reversing TNF-α-induced senescence in TSCs. The present findings
provide new mechanistic insight into the role of TNF-α in TSCs
dysfunction and suggest a promising avenue for targeting AT through
TNF-α inhibition.
TSCs exhibit a spindle-shaped, fibroblast-like
morphology and possess the capacity to differentiate into
cartilage, adipose tissue and bone (38). Emerging evidence suggests that
pro-inflammatory cytokines promote cell senescence (39). For example, IL-6 induces
senescence in telomerase-inhibited growth 3 (TIG3) fibroblasts by
activating the STAT3/ IGF binding protein 5 (IGFBP5) signaling
pathway, increasing ROS levels and promoting a senescent phenotype
(40). Similarly, TNF-α drives
senescence in proliferative endothelial progenitor cells via
activation of the p38 MAPK pathway (41). Previous studies have demonstrated
that TNF-α not only amplifies inflammation by upregulating
inflammatory gene expression but also contributes to disease onset
and progression by inducing apoptosis and activating immune
responses in target cells (42,43). The present study used TNF-α
concentrations of 20 and 40 ng/ml based on previous reports and
preliminary experiments (44,45). Research involving MSCs exposed to
inflammatory cytokines has shown that TNF-α concentrations within
this range effectively induce inflammation-associated cell
responses (46). For example,
studies investigating the role of TNF-α in MSCs under inflammatory
conditions used similar concentrations (10-40 ng/ml) to elicit
significant changes in senescence-associated markers, such as p21
and SA-β-galactosidase activity (47,48). Here, increasing the concentration
and frequency (3 vs. 6 times) of TNF-α stimulation led to a
progressive rise in the number of senescent TSCs. This increase was
associated with reduced stemness, enlarged cell morphology,
intensified SA-β-gal staining, impaired proliferative capacity and
an elevated proportion of cells in the G1/G0 phase (49-51). Moreover, TNF-α disrupted cell
cycle progression in TSCs. Notably, treatment with a TNF-α
inhibitor restored both the proliferative ability and SC
characteristics of TSCs, highlighting the potential of TNF-α
inhibition as a therapeutic strategy.
Any signaling pathway that drives inflammation
should also influence inflammation-associated senescence. However,
understanding of the molecular mechanisms underlying inflammatory
senescence remains limited, underscoring the need for further
investigation. NF-κB is a multifaceted nuclear transcription factor
that is broadly expressed in various tissues and cell types
(52). Upon activation, it
regulates the expression of numerous genes involved in immune
responses, inflammation, oxidative stress, cell proliferation and
apoptosis (53). NF-κB also
serves a key role in the intracellular signaling processes
frequently activated in senescent SCs (54). The present in vitro
experiments demonstrated that TNF-α activates NF-κB signaling in
TSCs, as evidenced by increased p-p65 expression. However, the
extent to which NF-κB mediates TSC senescence in vivo
remains unclear. Wang et al (55) reported that inhibiting the IκB
kinase-β) /NF-κB signaling axis reverses inflammaging (chronic
low-grade inflammation associated with aging)-induced TSC
senescence and promotes tendon healing in a rat model. Consistent
with this, the present findings support a link between TNF-α
stimulation and NF-κB activation in TSCs. Whether TNF-α drives TSC
senescence exclusively through NF-κB signaling remains to be
determined. Future studies should use a specific NF-κB inhibitor to
assess the extent of its involvement in TNF-α-induced TSC
senescence. Although prior studies have implicated NF-κB in tendon
degeneration (56,57), the present data suggest that
TNF-α exerts broader effects on the tendon microenvironment,
including promotion of inflammation, disruption of extracellular
matrix organization and impairment of SC function. Future studies
should use genetic or pharmacological strategies to investigate the
contributions of NF-κB and other downstream mediators in
TNF-α-driven tendon pathology.
Comparing etanercept with other TNF-α inhibitors
reveals differences in molecular structure, pharmacokinetics and
side-effect profiles. The distinctive fusion-protein design of
etanercept grants it a longer half-life, allowing for less frequent
dosing than some alternatives, such as methotrexate (58,59). However, choosing a TNF-α
inhibitor depends on factors such as disease type, patient
characteristics and treatment response. Here, etanercept
effectively reversed TNF-α-induced senescence in TSCs and improved
tendon histology in a rat model. Future studies should focus on
optimizing etanercept dosing for tendinitis treatment, assessing
its long-term effects on tendon function and regeneration, and
investigating potential synergistic effects when combined with
growth factors or anti-inflammatory agents to enhance therapeutic
outcomes. Translating these preclinical findings into clinical
trials will be critical to establish the practical efficacy of
etanercept in managing AT.
The present in vivo data offer novel insights
into the pathological role of TNF-α in tendonitis and highlight the
therapeutic potential of TNF-α inhibitors. In the rat AT model,
histological analysis showed that etanercept reduced inflammatory
cell infiltration, collagen fiber disarray and matrix degradation.
These results corroborate previous studies demonstrating that TNF-α
inhibitors effectively decrease tendon inflammation and enhance
tissue repair (18,35). Importantly, the decrease in TNF-α
expression following etanercept treatment confirmed direct
modulation of the TNF-α pathway in tendon tissue. Clinically, TNF-α
inhibitors may offer a promising therapeutic strategy for AT. While
current treatments mainly target symptom relief, inhibiting TNF-α
may address the underlying inflammatory processes that drive tendon
degeneration. Nevertheless, challenges remain before clinical
application. Determining optimal dosing, treatment duration and
long-term safety require further study. Moreover, the diverse
etiologies of tendinitis, including overuse and systemic
inflammation, may demand personalized therapeutic approaches.
Future studies should investigate the long-term
effects of TNF-α and etanercept on TSCs, with a focus on
elucidating the crosstalk between key signaling pathways.
Evaluating the therapeutic potential of etanercept in patients with
AT is required to translate preclinical findings into clinical
application. Additionally, combination therapies involving
etanercept and other pharmacological or biological agents to
enhance therapeutic efficacy should be investigated to identify the
role of TNF-α in tendon pathology and support the development of
more effective, targeted treatment strategies.
In conclusion, the present study demonstrated that
TNF-α induced premature senescence in TSCs, as demonstrated by
increased SA-β-gal activity, disrupted F-actin cytoskeleton and
cell cycle arrest in the G0/G1 phase. Mechanistically, TNF-α
activates the NF-κB signaling pathway, as evidenced by the
significant upregulation of p-p65 and its nuclear translocation in
TSCs. This activation is further linked to the induction of DNA
damage (γ-H2A.X expression) and upregulation of the p53/p21/cyclin
E/CDK2 pathway, which collectively drive cellular senescence. TNF-α
antagonist etanercept reverses the senescent phenotype, as shown by
reduced SA-β-gal-positive cells, restored F-actin cytoskeleton
organization, and normalized cell cycle progression. These effects
are linked to Etanercept's inhibition of ROS production, DNA
damage, and downregulation of NF-κB/p53 signaling. Collectively,
these findings establish a mechanistic link between TNF-α, NF-κB
activation, and TSCs senescence, with therapeutic implications for
Etanercept in tendon degeneration (Fig. 7).
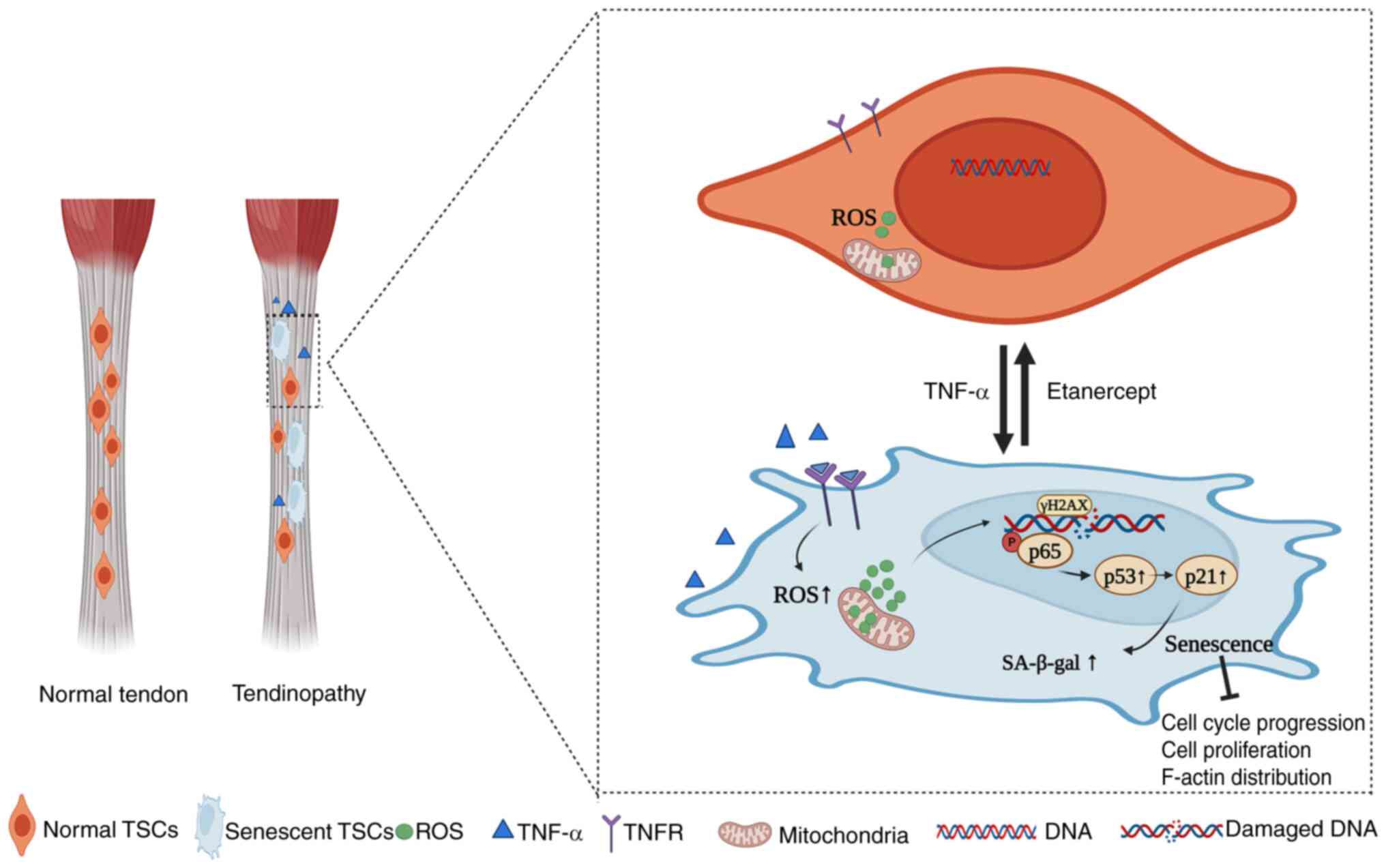 | Figure 7Impact of TNF-α on TSCs in normal
tendon tissues. Under physiological conditions, TSCs exhibit
typical functionality, characterized by regular cell cycles, intact
F-actin structures, low levels of ROS and normal expressions of
transcription factors. Following stimulation by TNF-α, TSCs
experience increased ROS production and DNA damage, activation of
the NF-κB signaling pathway (resulting in elevated levels of p-p65
and p65, leading to p65 translocation to the nucleus) and
modulation of the p53/p21/cyclin E/CDK2 signaling pathways
(resulting in upregulation of p53 and p21 and downregulation of
cyclin E and CDK2). These changes induce senescence in TSCs,
characterized by alterations such as enlarged cell volume and
disrupted F-actin structures. Etanercept, a TNF-α inhibitor,
mitigates these effects by binding to TNF-α, thereby inhibiting the
activation of signaling pathways. This inhibition leads to reduced
ROS levels, mitigates DNA damage, decreases expression of p53, p21
and p-p65, normalizes cyclin E and CDK2 expression and ultimately
reverses senescence in TSCs, thereby restoring normal cellular
functions. Figure created using BioRender (app.biorender.com/illustrations). TSC, tendon stem
cells; ROS, reactive oxygen species; F-actin, filamentous-actin;
p-, phosphorylation; TNFR, tumor necrosis factor receptor;
SA-β-gal, senescence-associated β-galactosidase. |
Availability of data and materials
The data generated in the present study may be
requested from the corresponding author.
Authors' contributions
HG and HC conceived the study, designed and
performed experiments and wrote and edited the manuscript. QL
designed and performed experiments. ZG and GF conceived the study,
performed experiments and edited the manuscript. All authors have
read and approved the final manuscript. ZG and GF confirm the
authenticity of all the raw data.
Ethics approval and consent to
participate
The present study was approved by Animal Experiment
Center of Nantong University (approval no. S20230522-005).
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
Acknowledgments
Not applicable.
Funding
The present study was supported by grants from the Chinese
National Natural Science Foundation (grant nos. 82071838 and
82201983), the Science and Technology Project of Nantong City
(grant nos. JC2021187 and MS22022104), Jiangsu Provincial Medical
Key Discipline Cultivation Unit (grant no. JSDW202205), Jiangsu
Provincial Research Hospital (grant no. YJXYY202204), The Second
Level of the Sixth Jianghai Elite Training Project in Nantong City
(grant no. 2022, III-602) and the 14th Five-Year Science and
Education and Strong Health Engineering Medical Talents Program of
Nantong City (grant no. 2021-2025).
References
|
1
|
Iagnocco A, Riente L, Delle Sedie A,
Filippucci E, Salaffi F, Meenagh G, Scirè CA, Grassi W, Montecucco
C, Bombardieri S and Valesini G: Ultrasound imaging for the
rheumatologist. XXII. Achilles tendon involvement in
spondyloarthritis. A multi-centre study using high frequency
volumetric probe. Clin Exp Rheumatol. 27:547–551. 2009.
|
|
2
|
Alves EM, Macieira JC, Borba E, Chiuchetta
FA and Santiago MB: Spontaneous tendon rupture in systemic lupus
erythematosus: Association with Jaccoud's arthropathy. Lupus.
19:247–254. 2010. View Article : Google Scholar
|
|
3
|
Järvinen TA, Kannus P, Paavola M, Järvinen
TL, Józsa L and Järvinen M: Achilles tendon injuries. Curr Opin
Rheumatol. 13:150–155. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Lin YJ, Anzaghe M and Schülke S: Update on
the pathomechanism, diagnosis, and treatment options for rheumatoid
arthritis. Cells. 9:8802020. View Article : Google Scholar
|
|
5
|
Mobasheri A and Shakibaei M: Is tendinitis
an inflammatory disease initiated and driven by pro-inflammatory
cytokines such as interleukin 1β? Histol Histopathol. 28:955–964.
2013.
|
|
6
|
Sharma P and Maffulli N: Tendon injury and
tendinopathy: Healing and repair. J Bone Joint Surg Am. 87:187–202.
2005.
|
|
7
|
Schulze-Tanzil G, Al-Sadi O, Wiegand E,
Ertel W, Busch C, Kohl B and Pufe T: The role of pro-inflammatory
and immunoregulatory cytokines in tendon healing and rupture: New
insights. Scand J Med Sci Sports. 21:337–351. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Jeong C, Kim SE, Shim KS, Kim HJ, Song MH,
Park K and Song HR: Exploring the in vivo anti-inflammatory actions
of simvastatin-loaded porous microspheres on inflamed tenocytes in
a collagenase-induced animal model of achilles tendinitis. Int J
Mol Sci. 19:8202018. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Alfredson H, Lorentzon M, Bäckman S,
Bäckman A and Lerner UH: cDNA-arrays and real-time quantitative PCR
techniques in the investigation of chronic Achilles tendinosis. J
Orthop Res. 21:970–975. 2003. View Article : Google Scholar
|
|
10
|
Choi S, Song MH, Shim KS, Kim HJ, Lim YM,
Song HR, Park K and Kim SE: Therapeutic efficacy of intratendinous
delivery of dexamethasone using porous microspheres for
amelioration of inflammation and tendon degeneration on achilles
tendinitis in rats. Biomed Res Int. 2020:50520282020. View Article : Google Scholar
|
|
11
|
Cho CH and Kim DH, Baek EH and Kim DH:
Serum levels of TNF-α are increased in patients with rotator cuff
tear and sleep disturbance. Diagnostics (Basel). 11:22152021.
View Article : Google Scholar
|
|
12
|
Stengaard K, Hejbøl EK, Jensen PT, Degn M,
Ta TML, Stensballe A, Andersen DC, Schroder HD, Lambertsen KL and
Frich LH: Early-stage inflammation changes in supraspinatus muscle
after rotator cuff tear. J Shoulder Elbow Surg. 31:1344–1356. 2022.
View Article : Google Scholar
|
|
13
|
Frich LH, Fernandes LR, Schrøder HD,
Hejbøl EK, Nielsen PV, Jørgensen PH, Stensballe A and Lambertsen
KL: The inflammatory response of the supraspinatus muscle in
rotator cuff tear conditions. J Shoulder Elbow Surg. 30:e261–e275.
2021. View Article : Google Scholar
|
|
14
|
Weinblatt ME, Kremer JM, Bankhurst AD,
Bulpitt KJ, Fleischmann RM, Fox RI, Jackson CG, Lange M and Burge
DJ: A trial of etanercept, a recombinant tumor necrosis factor
receptor:Fc fusion protein, in patients with rheumatoid arthritis
receiving methotrexate. N Engl J Med. 340:253–259. 1999. View Article : Google Scholar
|
|
15
|
Moreland LW, Baumgartner SW, Schiff MH,
Tindall EA, Fleischmann RM, Weaver AL, Ettlinger RE, Cohen S,
Koopman WJ, Mohler K, et al: Treatment of rheumatoid arthritis with
a recombinant human tumor necrosis factor receptor (p75)-Fc fusion
protein. N Engl J Med. 337:141–147. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Pennica D, Kohr WJ, Fendly BM, Shire SJ,
Raab HE, Borchardt PE, Lewis M and Goeddel DV: Characterization of
a recombinant extracellular domain of the type 1 tumor necrosis
factor receptor: Evidence for tumor necrosis factor-alpha induced
receptor aggregation. Biochemistry. 31:1134–1141. 1992. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Mease PJ, Goffe BS, Metz J, VanderStoep A,
Finck B and Burge DJ: Etanercept in the treatment of psoriatic
arthritis and psoriasis: A randomised trial. Lancet. 356:385–390.
2000. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Chen K, Li P, Zhao H, Yan X and Ma Y:
Effects of tumor necrosis factor inhibitor on stress-shielded
tendons. Orthopedics. 40:49–55. 2017. View Article : Google Scholar
|
|
19
|
Marhaba R, Klingbeil P, Nuebel T,
Nazarenko I, Buechler MW and Zoeller M: CD44 and EpCAM:
Cancer-initiating cell markers. Curr Mol Med. 8:784–804. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Wang JHC and Komatsu I: Tendon stem cells:
Mechanobiology and development of tendinopathy. Adv Exp Med Biol.
920:53–62. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Zhang M, Liu H, Cui Q, Han P, Yang S, Shi
M, Zhang T, Zhang Z and Li Z: Tendon stem cell-derived exosomes
regulate inflammation and promote the high-quality healing of
injured tendon. Stem Cell Res Ther. 11:4022020. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Yang Z, Cao H, Gao S, Yang M, Lyu J and
Tang K: Effect of tendon stem cells in
chitosan/β-glycerophosphate/collagen hydrogel on achilles tendon
healing in a rat model. Med Sci Monit. 23:4633–4643. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Jang DI, Lee AH, Shin HY, Song HR, Park
JH, Kang TB, Lee SR and Yang SH: The role of tumor necrosis factor
alpha (TNF-α) in autoimmune disease and current TNF-α inhibitors in
therapeutics. Int J Mol Sci. 22:27192021. View Article : Google Scholar
|
|
24
|
Zhao Y, Zhu XY, Song T, Zhang L, Eirin A,
Conley S, Tang H, Saadiq I, Jordan K, Lerman A and Lerman LO:
Mesenchymal stem cells protect renal tubular cells via TSG-6
regulating macrophage function and phenotype switching. Am J
Physiol Renal Physiol. 320:F454–F463. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Leone GM, Mangano K, Petralia MC,
Nicoletti F and Fagone P: Past, present and (foreseeable) future of
biological anti-TNF alpha therapy. J Clin Med. 12:16302023.
View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Mohamad Kamal NS, Safuan S, Shamsuddin S
and Foroozandeh P: Aging of the cells: Insight into cellular
senescence and detection methods. Eur J Cell Biol. 99:1511082020.
View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Perucca Orfei C, Lovati AB, Viganò M,
Stanco D, Bottagisio M, Di Giancamillo A, Setti S and de Girolamo
L: Dose-related and time-dependent development of
collagenase-induced tendinopathy in rats. PLoS One.
11:e01615902016. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
AVMA Guidelines for the Euthanasia of
Animals: 2020 edition. Available from: https://www.avma.org/resources-tools/avmapolicies/avma-guidelines-euthanasia-animals.pdf.
|
|
29
|
Debacq-Chainiaux F, Erusalimsky JD,
Campisi J and Toussaint O: Protocols to detect
senescence-associated beta-galactosidase (SA-betagal) activity, a
biomarker of senescent cells in culture and in vivo. Nat Protoc.
4:1798–1806. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Hernandez-Segura A, Nehme J and Demaria M:
Hallmarks of cellular senescence. Trends Cell Biol. 28:436–453.
2018. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Qi Z, Yang W, Xue B, Chen T, Lu X, Zhang
R, Li Z, Zhao X, Zhang Y, Han F, et al: ROS-mediated lysosomal
membrane permeabilization and autophagy inhibition regulate
bleomycin-induced cellular senescence. Autophagy. 20:2000–2016.
2024. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Gaida JE, Alfredson H, Forsgren S and Cook
JL: A pilot study on biomarkers for tendinopathy: Lower levels of
serum TNF-α and other cytokines in females but not males with
achilles tendinopathy. BMC Sports Sci Med Rehabil. 8:52016.
View Article : Google Scholar
|
|
33
|
Fredberg U and Ostgaard R: Effect of
ultrasound-guided, peritendinous injections of adalimumab and
anakinra in chronic Achilles tendinopathy: A pilot study. Scand J
Med Sci Sports. 19:338–344. 2009. View Article : Google Scholar
|
|
34
|
Kemoun G and Defebvre L: Gait disorders in
Parkinson disease. Clinical description, analysis of posture,
initiation of stabilized gait. Presse Med. 30:452–459. 2001.In
French. PubMed/NCBI
|
|
35
|
Gulotta LV, Kovacevic D, Cordasco F and
Rodeo SA: Evaluation of tumor necrosis factor α blockade on early
tendon-to-bone healing in a rat rotator cuff repair model.
Arthroscopy. 27:1351–1357. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Kwan KHL, Yeung KWK, Liu X, Wong KKY, Shum
HC, Lam YW, Cheng SH, Cheung KMC and To MKT: Silver nanoparticles
alter proteoglycan expression in the promotion of tendon repair.
Nanomedicine. 10:1375–1383. 2014. View Article : Google Scholar
|
|
37
|
Yang J, He J and Yang L: Advanced
glycation end products impair the repair of injured tendon: A study
in rats. BMC Musculoskelet Disord. 25:7002024. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Bi Y, Ehirchiou D, Kilts TM, Inkson CA,
Embree MC, Sonoyama W, Li L, Leet AI, Seo BM, Zhang L, et al:
Identification of tendon stem/progenitor cells and the role of the
extracellular matrix in their niche. Nat Med. 13:1219–1227. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Shang D, Liu H and Tu Z: Pro-inflammatory
cytokines mediating senescence of vascular endothelial cells in
atherosclerosis. Fundam Clin Pharmacol. 37:928–936. 2023.
View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Kojima H, Kunimoto H, Inoue T and Nakajima
K: The STAT3-IGFBP5 axis is critical for IL-6/gp130-induced
premature senescence in human fibroblasts. Cell Cycle. 11:730–739.
2012. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Zhang Y, Herbert BS, Rajashekhar G, Ingram
DA, Yoder MC, Clauss M and Rehman J: Premature senescence of highly
proliferative endothelial progenitor cells is induced by tumor
necrosis factor-alpha via the p38 mitogen-activated protein kinase
pathway. FASEB J. 23:1358–1365. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Leppkes M, Roulis M, Neurath MF, Kollias G
and Becker C: Pleiotropic functions of TNF-α in the regulation of
the intestinal epithelial response to inflammation. Int Immunol.
26:509–515. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Jacobson EC, Jain L, Vickers MH, Olins AL,
Olins DE, Perry JK and O'Sullivan JM: TNF-α differentially
regulates cell cycle genes in promyelocytic and granulocytic
HL-60/S4 cells. G3 (Bethesda). 9:2775–2786. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Huang Y, Zuo F, Wu J and Wu S: TNF-α
regulated bidirectional interaction between bone marrow mesenchymal
stem cells and articular chondrocytes. Cartilage. Nov 20–2024.Epub
ahead of print. View Article : Google Scholar
|
|
45
|
Zhu L, Dissanayaka WL, Green DW and Zhang
C: Stimulation of EphB2/ephrin-B1 signalling by tumour necrosis
factor alpha in human dental pulp stem cells. Cell Prolif.
48:231–238. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Li W, Liu Q, Shi J, Xu X and Xu J: The
role of TNF-α in the fate regulation and functional reprogramming
of mesenchymal stem cells in an inflammatory microenvironment.
Front Immunol. 14:10748632023. View Article : Google Scholar
|
|
47
|
Fan P, Yu XY, Chen CH, Gao JW, Xu YZ, Xie
XH and Wang YT: Parkin-mediated mitophagy protects against
TNF-α-induced stress in bone marrow mesenchymal stem cells. Exp
Gerontol. 164:1118292022. View Article : Google Scholar
|
|
48
|
Jung YH, Chae CW, Chang HS, Choi GE, Lee
HJ and Han HJ: Silencing SIRT5 induces the senescence of UCB-MSCs
exposed to TNF-α by reduction of fatty acid β-oxidation and
anti-oxidation. Free Radic Biol Med. 192:1–12. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
49
|
Zhou Z, Akinbiyi T, Xu L, Ramcharan M,
Leong DJ, Ros SJ, Colvin AC, Schaffler MB, Majeska RJ, Flatow EL
and Sun HB: Tendon-derived stem/progenitor cell aging: Defective
self-renewal and altered fate. Aging Cell. 9:911–915. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
50
|
Tan Q, Lui PPY and Rui YF: Effect of in
vitro passaging on the stem cell-related properties of
tendon-derived stem cells-implications in tissue engineering. Stem
Cells Dev. 21:790–800. 2012. View Article : Google Scholar
|
|
51
|
Nie D, Zhang J, Zhou Y, Sun J, Wang W and
Wang JHC: Rapamycin treatment of tendon stem/progenitor cells
reduces cellular senescence by upregulating autophagy. Stem Cells
Int. 2021:66382492021. View Article : Google Scholar : PubMed/NCBI
|
|
52
|
Boyce BF, Yao Z and Xing L: Functions of
nuclear factor kappaB in bone. Ann N Y Acad Sci. 1192:367–375.
2010. View Article : Google Scholar : PubMed/NCBI
|
|
53
|
Alharbi KS, Fuloria NK, Fuloria S, Rahman
SB, Al-Malki WH, Javed Shaikh MA, Thangavelu L, Singh SK, Rama Raju
Allam VS, Jha NK, et al: Nuclear factor-kappa B and its role in
inflammatory lung disease. Chem Biol Interact. 345:1095682021.
View Article : Google Scholar : PubMed/NCBI
|
|
54
|
Bashiri Dezfouli A, Salar-Amoli J,
Pourfathollah AA, Yazdi M, Nikougoftar-Zarif M, Khosravi M and
Hassan J: Doxorubicin-induced senescence through NF-κB affected by
the age of mouse mesenchymal stem cells. J Cell Physiol.
235:2336–2349. 2020. View Article : Google Scholar
|
|
55
|
Wang C, Zhou Z, Song W, Cai Z, Ding Z,
Chen D, Xia F and He Y: Inhibition of IKKβ/NF-κB signaling
facilitates tendinopathy healing by rejuvenating inflamm-aging
induced tendon-derived stem/progenitor cell senescence. Mol Ther
Nucleic Acids. 27:562–576. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
56
|
Abraham AC, Shah SA, Golman M, Song L, Li
X, Kurtaliaj I, Akbar M, Millar NL, Abu-Amer Y, Galatz LM and
Thomopoulos S: Targeting the NF-kappaB signaling pathway in chronic
tendon disease. Sci Transl Med. 11:eaav43192019. View Article : Google Scholar
|
|
57
|
Best KT, Nichols AEC, Knapp E, Hammert WC,
Ketonis C, Jonason JH, Awad HA and Loiselle AE: NF-κB activation
persists into the remodeling phase of tendon healing and promotes
myofibroblast survival. Sci Signal. 13:eabb72092020. View Article : Google Scholar
|
|
58
|
Keystone EC, Kavanaugh AF, Sharp JT,
Tannenbaum H, Hua Y, Teoh LS, Fischkoff SA and Chartash EK:
Radiographic, clinical, and functional outcomes of treatment with
adalimumab (a human anti-tumor necrosis factor monoclonal antibody)
in patients with active rheumatoid arthritis receiving concomitant
methotrexate therapy: A randomized, placebo-controlled, 52-week
trial. Arthritis Rheum. 50:1400–1411. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
59
|
Genovese MC, Bathon JM, Martin RW,
Fleischmann RM, Tesser JR, Schiff MH, Keystone EC, Wasko MC,
Moreland LW, Weaver AL, et al: Etanercept versus methotrexate in
patients with early rheumatoid arthritis: Two-year radiographic and
clinical outcomes. Arthritis Rheum. 46:1443–1450. 2002. View Article : Google Scholar : PubMed/NCBI
|