Introduction
Perimenopause marks a key transitional phase from
reproductive maturity to menopause, characterized by the
progressive decline in ovarian function and reproductive capacity
(1). It typically begins with
noticeable menstrual irregularities and concludes 12 months after
the final menstruation, most commonly occurring between the ages of
40 and 55, although it may start as early as the mid-30s in some
women (2). A hallmark hormonal
change during perimenopause is the gradual decline and fluctuation
of ovarian hormones, particularly estrogen (E2) and progesterone
(3). This hormonal imbalance not
only disrupts the menstrual cycle and ultimately leads to its
cessation but also triggers a range of multisystem symptoms,
including vasomotor disturbances (such as hot flashes and night
sweats), mastalgia, musculoskeletal pain, vaginal dryness, fatigue
and neuropsychiatric symptoms such as anxiety, depression and
cognitive impairment (4-7).
Perimenopausal mood disorders are common yet often
underdiagnosed, exerting notable negative effects on occupational
performance, interpersonal relationships and overall social
functioning (8). Evidence
suggests that these mood disturbances not only reduce quality of
life but also contribute to the development of somatic
comorbidities through dysregulation of the stress axis, endocrine
instability and immune dysfunction (9,10). Current therapeutic options
include psychotherapy (such as cognitive behavioral therapy),
pharmacotherapy (such as antidepressants and anxiolytics) and
hormone replacement therapy (HRT). However, the considerable
variability in individual responses to treatment highlights the
complex and heterogeneous nature of the underlying pathophysiology
(11,12). Understanding the biological
mechanisms of perimenopausal mood disorders is therefore important
to advancing personalized treatment strategies.
Among the emerging mechanisms, mitochondrial
dysfunction has been identified as a central pathological
contributor. Mitochondria are vital organelles responsible for ATP
production and are integral in regulating oxidative stress, calcium
homeostasis, apoptosis and synaptic plasticity (13,14). Increasing evidence has associated
impaired mitochondrial function with various affective disorders,
including major depressive disorder, anxiety and stress-related
conditions (15-17). Disruptions in mitochondrial
homeostasis, characterized by impaired bioenergetics, excessive
reactive oxygen species (ROS) generation and heightened
inflammatory signaling, can damage neuronal structure and function,
particularly in brain regions critical for mood regulation, such as
the hippocampus and prefrontal cortex (18).
During perimenopause, E2 fluctuations exacerbate
these risks. E2 regulates mitochondrial biogenesis, dynamics and
antioxidant defense through both genomic pathways [such as nuclear
receptor-mediated regulation of peroxisome proliferator-activated
receptor γ coactivator 1-α (PGC-1α)] and non-genomic mechanisms
(such as membrane receptor signaling and mitochondrial DNA
protection) (18). As E2levels
decline, mitochondrial instability increases, resulting in greater
neuroenergetic vulnerability. Current interventions targeting these
mechanisms include HRT, mitochondria-targeted pharmacological
agents, antidepressants combined with psychotherapy and
lifestyle-based strategies involving physical activity, dietary
regulation and sleep hygiene (18). These integrated approaches aim to
restore mitochondrial function, reduce oxidative stress and enhance
both emotional and physical well-being in perimenopausal women.
Future research should explore the bidirectional crosstalk between
sex hormones and mitochondrial signaling, paving the way for more
precise and safer therapeutic strategies tailored to individual
pathophysiological profiles.
Perimenopausal mood disorders
Perimenopause denotes the transitional phase from
reproductive to non-reproductive life in women, typically spanning
the 2-8 years preceding menopause and the first year following the
final menstruation (19). This
period is characterized by substantial fluctuations in sex hormone
levels, particularly cyclical declines in E2 and progesterone,
which contribute to both endocrine and clinical heterogeneity
(20-22). These hormonal changes underpin a
range of symptoms, including menstrual irregularities, vasomotor
disturbances and alterations in mood and cognitive function.
Epidemiological characteristics
Extensive evidence highlights a marked increase in
the prevalence of psychiatric symptoms during perimenopause
(23). Epidemiological studies
suggest that ~70% of women experience some form of emotional
disturbance, including irritability, anxiety and depressive
symptoms (24). While some women
report mild mood instability, others may develop moderate-to-severe
depression or anxiety that notably impairs daily functioning and
social adaptation (10). A
meta-analysis of 55 studies revealed a global pooled prevalence of
depressive disorders in perimenopausal women at 33.9% (95% CI:
27.8-40.0%) (25). Another large
population-based study involving 9,141 women revealed that
perimenopausal women are at considerably higher risk of developing
depressive symptoms compared with their premenopausal counterparts
(26).
The onset of perimenopausal mood disorders is
influenced by a complex interaction of biological and psychosocial
risk factors. Low educational attainment, unemployment or part-time
employment, high perceived stress, menstrual irregularity,
constipation poor family relationships (27) and high neuroticism are all
associated with an increased risk of depression (28). By contrast, increased household
income and access to comprehensive healthcare serve as protective
factors (29-32). Notably, a personal history of
affective disorders predicts symptom recurrence or exacerbation
during perimenopause (8,33). In women with bipolar disorder,
perimenopause is often associated with a worsening of mood symptoms
(29-32). One study revealed that 68% of
perimenopausal women diagnosed with bipolar disorder experienced
major depressive episodes, with a markedly higher frequency
compared with their reproductive years (7). Similarly, longitudinal tracking of
13 women with bipolar disorder across premenopausal, perimenopausal
and postmenopausal stages revealed notable mood instability during
the perimenopausal transition (33).
Clinical manifestations
Fluctuating decline in ovarian function during
perimenopause results in considerable hormonal instability,
particularly in E2 and progesterone levels. These endocrine
fluctuations disrupt neurotransmitter systems and neural circuits
involved in emotional regulation, leading to mood symptoms that are
heterogeneous, episodic and often co-occurring with somatic and
cognitive disturbances. Epidemiological studies suggest that
perimenopausal women have a 2- to 4-fold increased risk of
developing major depressive disorder compared with their
premenopausal counterparts (34,35).
Typical depressive symptoms include persistent low
mood, anhedonia, fatigue, impaired concentration, excessive guilt
and pessimism regarding the future. Atypical features, such as
irritability and widespread somatic complaints (such as myalgia),
are also commonly reported (36). Notably, some women show reduced
responsiveness to standard antidepressant therapy. The presence of
severe life stress, a prior history of depression or psychotropic
medication use further elevates the likelihood of progression to
major depressive episodes, which may carry an increased risk of
suicidal ideation and behavior (37,38).
Anxiety is another prevalent symptom cluster, often
characterized by sustained tension, excessive worry, irritability
and distractibility. Autonomic symptoms, such as palpitations,
sweating and tremors, are frequently observed in conjunction with
anxiety (39). In perimenopausal
women, 60-70% report experiencing 'brain fog', a constellation of
mild cognitive deficits, including forgetfulness, reduced attention
and slowed thinking (40,41).
While these symptoms are generally transient, persistent cognitive
decline may heighten the risk for late-life neurodegenerative
disorders, such as Alzheimer's disease and vascular dementia. This
suggests that perimenopause may represent a key window for early
intervention in cognitive aging (42).
Somatic symptoms, including hot flashes, night
sweats, palpitations, headaches and musculoskeletal pain, are also
common and can create a bidirectional feedback loop with mood
disturbances, exacerbating both psychological and physical
symptoms. Sleep disorders are highly prevalent, manifesting as
difficulty falling asleep, frequent nighttime awakenings, early
morning waking and non-restorative sleep (43-45). Poor sleep quality not only
impairs daytime cognitive and physical functioning but is also
associated with anxiety, depression and vasomotor symptoms.
Notably, the interaction between vasomotor symptoms and emotional
states appears to be bidirectional and temporally complex,
collectively contributing to notable declines in quality of life
(46,47).
Pathophysiological mechanisms
Perimenopause represents a phase of profound
endocrine remodeling, during which rapid and irregular fluctuations
in sex hormones, particularly E2 and progesterone, markedly impact
brain regions, such as the hippocampus and prefrontal cortex,
involved in mood regulation (48). Several hormones, including
ovarian steroids, progesterone, testosterone, cortisol and their
neuroactive derivatives, have been implicated in the development
and modulation of perimenopausal mood disorders (22,49).
E2 carries out a key role in this context. Following
menopause, serum E2 levels reduce from premenopausal levels of 5-35
ng/dl to ~1.3 ng/dl (50,51).
This sharp decline is considered a key biological event
contributing to affective instability and cognitive impairment
during the menopausal transition (52,53). E2 exerts neuroprotective and
regulatory effects within the central nervous system (CNS). E2
deficiency induces cerebral hypoperfusion and vasoconstriction,
reducing oxygen supply to brain tissue and exacerbating cognitive
deficits (52). E2 receptors
(ERs) α and β are widely expressed across emotion-related neural
circuits, with particularly high activity in the ventral
corticolimbic-brainstem axis (54,55). ERα primarily regulates nuclear
gene transcription, while ERβ localizes to mitochondrial membranes,
modulating mitochondrial metabolism and cellular stress responses
(56).
E2 deficiency disrupts the synthesis and signaling
of key neurotransmitters, such as acetylcholine, glutamate (Glu)
and neuroprotective peptides, all of which are essential for
cognitive function (57).
Furthermore, E2 modulates dopaminergic and serotonergic systems.
Its withdrawal accelerates dopaminergic neuronal degeneration,
weakening reward circuitry and impairs serotonin (5-HT) synthesis
and reuptake, particularly during the night, contributing to
vasomotor symptoms such as hot flashes and nocturnal sweats
(58,59). Dysfunctional ERβ signaling may
also alter 5-HT transporter (SERT) activity and increase
5-HT1A receptor binding in the amygdala, enhancing the
processing of negative emotions and heightening vulnerability to
depression and anxiety (60,61).
Progesterone levels also fluctuate considerably
during perimenopause due to irregular ovulation and declining
luteal function. One of its key neuroactive metabolites,
allopregnanolone (ALLO), acts as a potent positive allosteric
modulator of the γ-aminobutyric acid (GABA)_A receptor, enhancing
inhibitory neurotransmission and exerting anxiolytic and
antidepressant effects (22,62-64). However, the inconsistent
synthesis of ALLO under conditions of hormonal instability may
destabilize GABAergic signaling, promoting mood lability and
increasing affective vulnerability (65,66). Additionally, concurrent
fluctuations in ovarian hormones and neurosteroids may impair
GABA_A receptor sensitivity, disrupting
hypothalamic-pituitary-adrenal axis homeostasis and heightening
stress responsiveness, thereby elevating susceptibility to anxiety
and depressive symptoms (67,68).
Collectively, these findings emphasize the
multifactorial and hormone-sensitive nature of perimenopausal mood
disorders, shaped by complex interactions among E2, progesterone,
neurosteroids, neurotransmitters and neuroendocrine stress
systems.
Therapeutic strategies
HRT
HRT remains a primary intervention for alleviating
mood disturbances associated with E2 withdrawal in perimenopausal
women. Substantial clinical evidence supports its efficacy in
reducing depressive and anxiety symptoms. In a randomized
controlled trial, 17β-estradiol treatment markedly improved
depressive symptoms compared with placebo (68 vs. 17% remission
rate), highlighting its antidepressant potential (69). HRT has also demonstrated benefits
in improving irritability, sleep disturbances and other affective
symptoms, and, in some cases, serves as an adjunct to psychotropic
medications to enhance treatment response and overall functioning
(54,70).
However, the implementation of HRT requires careful
evaluation of the risk-benefit balance. Although it may reduce the
incidence of hip fractures and endometrial cancer, long-term use is
associated with increased risks of coronary artery disease, stroke,
breast cancer and dementia (71). These risks are especially
relevant for women > 50 years or those using HRT for >5 years
(72). By contrast, initiating
HRT before the age of 50 years may present a more favorable safety
profile. For asymptomatic women, the potential risks may outweigh
the benefits, particularly concerning breast cancer incidence
(71,73).
To overcome the limitations of conventional HRT,
alternative formulations have been developed. Tissue-selective E2
complexes, which combine E2s with selective E2 receptor modulators,
aim to retain therapeutic benefits while minimizing adverse effects
(74). Additionally, plant-based
phytoE2s, such as black cohosh extracts, have been explored for
their potential mood-enhancing effects. While early results are
promising, further research is necessary to confirm their long-term
efficacy and safety in perimenopausal populations (75).
Antidepressant therapy
Selective 5-HT reuptake inhibitors (SSRIs) and
5-HT-norepinephrine (NE) reuptake inhibitors (SNRIs) are considered
first-line pharmacological treatments for perimenopausal depression
and anxiety. These medications function by inhibiting the
presynaptic reuptake of 5-HT and/or NE, thereby increasing synaptic
neurotransmitter availability and enhancing mood regulation
(76). SSRIs specifically target
SERT, while SNRIs inhibit both SERT and the NE transporter, making
them particularly effective for patients with concurrent anxiety
and reduced motivation.
Studies suggest that SSRIs may also have indirect
effects on estradiol levels, potentially enhancing cognitive and
neuroprotective benefits (77).
Due to their favorable efficacy and tolerability profiles, SSRIs
and SNRIs are widely recommended by international guidelines for
managing perimenopausal affective symptoms (71,73). However, considerable
interindividual variability in treatment response persists. Adverse
effects such as nausea, diarrhea, insomnia and sexual dysfunction
may affect some patients, and ~30% may fail to achieve sufficient
symptom relief with a single agent (78). Therefore, personalized medication
selection and continuous monitoring of therapeutic efficacy and
tolerability are key for optimizing patient outcomes.
Mitochondrial homeostasis and its
disruption
Mitochondrial function
Mitochondria are the primary bioenergetic organelles
in eukaryotic cells, responsible for generating ATP through the
tricarboxylic acid (TCA) cycle and oxidative phosphorylation
(OXPHOS) (79,80). This energy production process is
driven by the electron transport chain (ETC) and ATP synthase,
which together convert nutrients into usable cellular energy
(81). Reducing equivalents such
as NADH and flavin adenine dinucleotide (FADH2) donate
electrons to the ETC, ultimately leading to ATP synthesis and
maintaining cellular homeostasis. The TCA cycle metabolizes
pyruvate into carbon dioxide while producing NADH and
FADH2, which fuel the ETC (82).
Mitochondria also exhibit dynamic behavior regulated
by continuous cycles of fission and fusion. These processes are key
for maintaining mitochondrial integrity and function. Fission
allows for the removal of damaged mitochondria and supports
cellular adaptation under stress, while fusion promotes the mixing
of mitochondrial contents and enhances network connectivity to
optimize energy distribution (83).
In the CNS, mitochondria are key not only for
sustaining neuronal energy demands but also for regulating
neurotransmitter synthesis, calcium buffering, oxidative stress
responses and apoptotic signaling (84) (Fig. 1). These functions are
particularly vital in neurons, which have high metabolic demands.
Neurons rely heavily on ATP to maintain resting membrane potential,
recycle synaptic vesicles and support neurotransmitter release.
Mitochondria dynamically redistribute within neurons in response to
local energy needs, often clustering near synaptic terminals during
periods of high activity to meet localized energy demands (85). This spatial and functional
plasticity is essential for maintaining synaptic transmission,
neuronal excitability and overall neurophysiological stability.
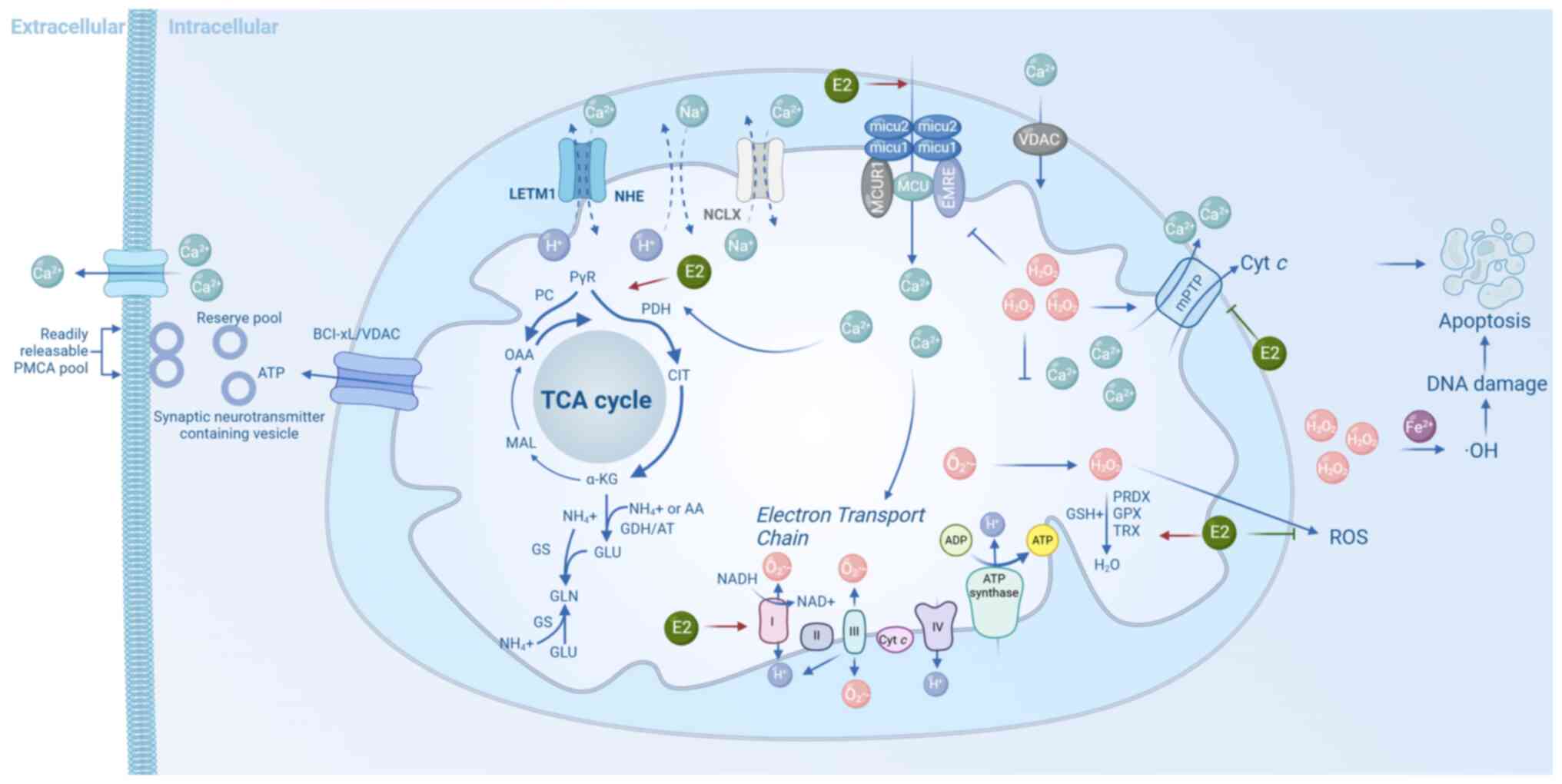 | Figure 1Key mitochondrial processes in
neuronal homeostasis. Mitochondrial Ca2+ uptake via VDAC
and the MCU complex activates TCA cycle dehydrogenases (PDH,
isocitrate dehydrogenase and α-KG dehydrogenase), promoting
NADH/flavin adenine dinucleotide (reduced form) production and ATP
synthesis. Electron leakage from complexes I/III generates
superoxide (O2−•), converted to
H2O2 and detoxified by antioxidant systems
(superoxide dismutase 2, GPX, PRDX, TRX and GSH). Excess ROS
triggers mPTP opening, cyt c release and apoptosis.
Glutamate is synthesized from α-ketoglutarate via GDH or
transaminases, supporting neurotransmission and nitrogen
metabolism. E2 enhances bioenergetics and antioxidant defense (red
arrows), while inhibiting ROS and apoptosis (green arrows). α-KG,
α-ketoglutarate; ATP, adenosine triphosphate; Ca2+,
calcium ion; Cyt c, cytochrome c; E2, 17β-estradiol;
GDH, glutamate dehydrogenase; GPX, glutathione peroxidase; GSH,
reduced glutathione; H2O2, hydrogen peroxide;
MCU, mitochondrial calcium uniporter; mPTP, mitochondrial
permeability transition pore; NADH, nicotinamide adenine
dinucleotide (reduced form); O2−•, superoxide
anion radical; PDH, pyruvate dehydrogenase; PRDX, peroxiredoxin;
ROS, reactive oxygen species; SOD2, superoxide dismutase 2; TCA
cycle, tricarboxylic acid cycle; TRX, thioredoxin; VDAC,
voltage-dependent anion channel. |
Mitochondria and neurotransmitter
regulation
Mitochondria carry out a direct and essential role
in the synthesis, metabolism and release of various
neurotransmitters. Their functional integrity is important for
maintaining synaptic transmission and emotional stability within
the CNS (85).
Glu, the primary excitatory neurotransmitter in the
brain, also serves as a precursor for the inhibitory
neurotransmitter GABA (86). The
synthesis of Glu is highly dependent on mitochondrial metabolism,
particularly through the TCA cycle, which generates α-ketoglutarate
(α-KG), a key substrate for Glu biosynthesis. Glu is then converted
to GABA via Glu decarboxylase, representing a key biochemical shift
from excitation to inhibition (87). In astrocytes, glutamine (Gln),
the major precursor of Glu, is synthesized via Gln synthetase and
transferred to neurons, where it is converted back into Glu by
phosphate-activated glutaminase, further entering mitochondrial
pathways.
Mitochondrial regulation of calcium
homeostasis
Mitochondria function as major intracellular calcium
(Ca2+) buffers, carrying out a key role in maintaining
cytosolic calcium homeostasis (84). Calcium signaling not only
facilitates intracellular signal transduction but also supports
mitochondrial bioenergetics by modulating the mitochondrial
membrane potential (ΔΨm), which is essential for ATP production. In
the mitochondrial matrix, moderate elevations in Ca2+
levels activate key dehydrogenases of the TCA cycle, such as
isocitrate dehydrogenase (IDH) and α-KG dehydrogenase (α-KGDH),
thereby enhancing NADH production, fueling the ETC, and increasing
ATP synthase activity (88).
The precise regulation of mitochondrial calcium flux
is mediated by a network of transmembrane channel complexes. Under
resting conditions, Ca2+ enters the intermembrane space
through voltage-dependent anion channels located on the outer
mitochondrial membrane. It then crosses the inner membrane into the
matrix through the OXPHOS mitochondrial calcium uniporter (MCU)
complex, which includes core subunits such as MCU, MICU1, MCUR1 and
EMRE (89). These components
work together to regulate Ca2+ uptake in a tightly
controlled manner, ensuring efficient and safe calcium accumulation
(90,91). During neuronal excitation,
mitochondrial calcium uptake via the MCU is essential for
sustaining synaptic activity. Calcium buffering within mitochondria
supports synaptic vesicle fusion and neurotransmitter release by
shaping local calcium transients, thereby optimizing the efficiency
and timing of synaptic transmission.
In addition to their intrinsic calcium-handling
systems, mitochondria are functionally and structurally connected
to the ER at specialized membrane contact sites known as
mitochondria-associated membranes. These microdomains facilitate
calcium transfer between organelles. Upon stimulation, inositol
1,4,5-trisphosphate receptors on the ER release stored
Ca2+ near the mitochondrial outer membrane, where it is
taken up via the voltage-dependent anion channel-MCU pathway.
Conversely, sarco/endoplasmic reticulum Ca2+-ATPase
pumps recapture cytosolic Ca2+ back into the ER,
contributing to calcium clearance and recycling (81).
Redox balance and oxidative stress
regulation
Mitochondria carry out a central role in maintaining
cellular redox balance, acting as both the primary source of ROS
and a key platform for their detoxification and stress response.
Under normal physiological conditions, electrons from NADH and
FADH2 are transferred through the mitochondrial ETC to
molecular oxygen, driving ATP synthesis. However, during this
process, especially at Complex I and Complex III, some electrons
may prematurely reduce oxygen, generating superoxide anion
(O2–•), the major intracellular ROS (92,93). Superoxide is rapidly dismutated
by mitochondrial superoxide dismutase (SOD2) into hydrogen peroxide
(H2O2), which can then form highly reactive
hydroxyl radicals (OH•) via Fenton chemistry in the presence of
transition metals such as Fe2+. While ROS at low levels
function as signaling molecules involved in transcriptional
regulation, proliferation, differentiation and immune responses
(94,95), excessive ROS production can
overwhelm the antioxidant defense system, leading to oxidative
stress. This imbalance results in protein oxidation, lipid
peroxidation, DNA damage and mitochondrial dysfunction, ultimately
causing cell death or senescence (92).
The generation of ROS is further exacerbated under
conditions of elevated mitochondrial membrane potential (ΔΨm) or an
imbalanced NADH/NAD+ ratio. To mitigate this,
mitochondria are equipped with robust enzymatic and non-enzymatic
antioxidant systems. In addition to SOD2, enzymes such as
glutathione peroxidase (GPx) and the thioredoxin-2/peroxiredoxin-3
system convert H2O2 into water, preventing
harmful accumulation. Glutathione, a major non-enzymatic
antioxidant, maintains the reduced intracellular environment and
participates in free radical neutralization (96).
ROS also activate nuclear antioxidant signaling
pathways that enhance cellular adaptive capacity. For example, ROS
can promote the dissociation of nuclear factor erythroid 2-related
factor 2 (Nrf2) from its repressor Keap1, enabling its nuclear
translocation and subsequent activation of antioxidant enzyme
genes, including SOD, GPx and heme oxygenase-1 (HO-1) (97). Simultaneously, ROS activate
mitochondrial biogenesis and stress adaptation pathways through
coactivators and transcription factors such as PGC-1α and FOXO3a,
thereby enhancing mitochondrial resilience (98,99).
Moreover, mitochondrial quality control is
maintained through mitophagy, a process that selectively removes
damaged or ROS-overproducing mitochondria (100). This process is mediated by the
PINK1/Parkin pathway: Upon collapse of the ΔΨm, PINK1 stabilizes on
the outer membrane and recruits the E3 ubiquitin ligase Parkin,
which tags damaged mitochondria for autophagic degradation
(101). This mechanism
effectively prevents the propagation of ROS-induced damage and
preserves cellular homeostasis.
Mitochondria in the balance of cell
survival and apoptosis
Mitochondria are key regulators of the dynamic
balance between neuronal survival and apoptosis, serving as key
checkpoints in determining cell fate. While physiological apoptosis
is essential for eliminating damaged or dysfunctional neurons to
maintain brain homeostasis, aberrant activation of apoptotic
pathways can disrupt neural circuits and contribute to the
pathogenesis of mood disorders and neurodegenerative diseases
(102,103).
Under normal mitochondrial homeostasis,
anti-apoptotic members of the BCL-2 family, such as BCL-2 and
BCL-xL, are anchored in the outer mitochondrial membrane. These
proteins inhibit apoptosis by binding to and neutralizing
pro-apoptotic factors such as Bax and Bak, preventing their
oligomerization and subsequent increase in mitochondrial outer
membrane permeability, thereby promoting neuronal survival
(104). In response to cellular
stressors, including oxidative damage, calcium overload or DNA
damage, pro-apoptotic pathways are activated. For instance,
signaling through the Bad/p53 complex or the JNK-Bim axis promotes
the conformational activation and translocation of Bax to the
mitochondrial outer membrane. This destabilizes the membrane,
leading to a decrease in ΔΨm and the opening of the mitochondrial
permeability transition pore (mPTP) (105). Upon mPTP opening, cytochrome
c (Cyt c) is released from the mitochondrial
intermembrane space into the cytosol, initiating the intrinsic
(mitochondrial) apoptotic pathway. In the cytoplasm, Cyt c
binds to apoptotic protease-activating factor-1 and procaspase-9,
forming the apoptosome. This multiprotein complex activates
executioner caspases, such as caspase-3, initiating a cascade of
proteolytic events that ultimately lead to the programmed death and
clearance of damaged neurons (106).
E2ic regulation of mitochondrial
function
E2 regulates mitochondrial function through both
classical nuclear receptor signaling and rapid non-genomic
pathways, collectively coordinating mitochondrial biogenesis,
morphology, metabolic activity and responses to cellular stress
(Fig. 2) (107).
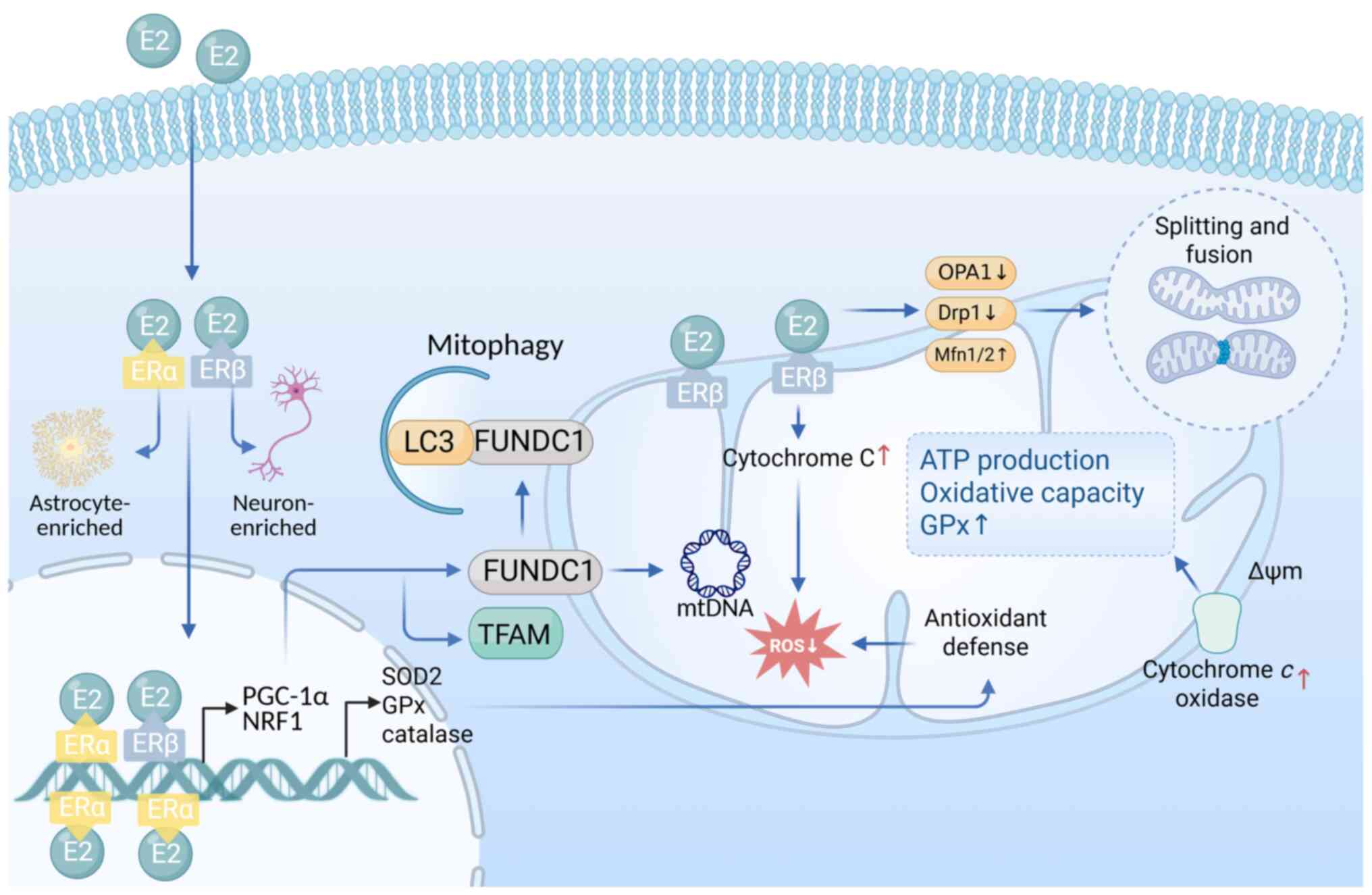 | Figure 2Estrogenic regulation of
mitochondrial function. E2 engages ERα and ERβ to regulate
mitochondrial function via genomic and non-genomic pathways. ERβ is
mainly located in mitochondria in neurons, while ERα shows
predominant nuclear expression in astrocytes. Genomically, it
upregulates TFAM and antioxidant enzymes (SOD2, GPx and catalase),
enhancing oxidative phosphorylation and redox balance.
Non-genomically, E2 stabilizes mitochondrial dynamics (↑Mfn1/2,
OPA1 and ↓Drp1) and promotes mitophagy via ERβ-FUNDC1 signaling.
Drp1, dynamin-related protein 1; E2, 17β-estradiol; ERα, estrogen
receptor α; ERβ, estrogen receptor β; FUNDC1, FUN14 domain
containing 1; GPx, glutathione peroxidase; Mfn1/2, mitofusin 1/2;
OPA1, optic atrophy 1; SOD2, superoxide dismutase 2; TFAM,
transcription factor A, mitochondrial. |
At the transcriptional level, E2 binds to its
receptor isoforms, ERα and ERβ, to activate several nuclear-encoded
mitochondrial regulatory factors, including nuclear respiratory
factor 1 (NRF1) and PGC-1α (107,108). NRF1 and PGC-1α work
synergistically to promote the expression of key genes such as
mitochondrial transcription factor A (TFAM), which facilitates
mtDNA transcription, replication and translation, thus supporting
the structural and functional integrity of OXPHOS complexes
(109-111). Notably, ERβ is also localized
to the mitochondrial membrane, where it exerts direct non-genomic
control over mitochondrial respiration (112,113). By modulating Cyt c
oxidase (complex IV) activity and stabilizing Δψm, ERβ enhances
respiratory efficiency and reduces electron leakage (113).
E2 further upregulates key mitochondrial antioxidant
enzymes, including SOD2, GPx and catalase, thereby enhancing
ROS-scavenging capacity (109).
It may also indirectly reduce ROS production by increasing Cyt
c mRNA and protein expression, thereby strengthening
antioxidant defenses (114).
Beyond metabolic and redox control, E2 influences mitochondrial
morphology by suppressing the recruitment and activation of the
fission-related protein dynamin-related protein 1 (Drp1) and
promoting the expression of fusion proteins such as mitofusin 1/2
(Mfn1/2) and optic atrophy 1 (OPA1) (115). These actions together support
mitochondrial network integrity and ensure efficient energy
distribution (116).
Mitochondrial homeostasis disruption in
perimenopausal mood disorders
During menopause, the abrupt decline in E2 has been
revealed to disrupt mitochondrial homeostasis, leaving measurable
bioenergetic signatures across both central and peripheral tissues
(99,107-112,114-117) (Table I). Clinical studies indicate that
postmenopausal women exhibit a ~30% reduction in ATP production
efficiency, which associates directly with decreased activity of
mitochondrial complex IV, COX (118). Translational neuroimaging
studies, combining 18F-fluorodeoxyglucose positron
emission tomography (18F-FDG PET) and phosphorus-31
magnetic resonance spectroscopy (31P-MRS), reveal a
concurrent decline in cerebral metabolic rate of glucose
consumption (CMRglc) and OXPHOS efficiency in perimenopausal women
(118). Notably, the extent of
metabolic decline is inversely correlated with Beck Depression
Inventory-II (BDI-II) scores, suggesting an association between
impaired brain energetics and depressive symptom severity (119). Furthermore, reduced glucose
utilization in the brain has been associated with decreased Cyt
c oxidase activity in peripheral blood platelets, indicating
a parallel dysfunction of mitochondrial metabolism in both the CNS
and peripheral tissues (120).
This pattern of metabolic disruption appears to result not merely
from chronological aging, but from hormone-driven 'endocrine aging'
that destabilizes mitochondrial function. Collectively, these
findings highlight mitochondrial E2 dependence as a key mechanistic
factor underlying perimenopausal mood disorders.
 | Table IRegulatory effects of estrogen on
mitochondrial function and after its decline. |
Table I
Regulatory effects of estrogen on
mitochondrial function and after its decline.
| Author/s, year | Regulatory
mechanisms | Role of
estrogen | Effects of estrogen
decline | (Refs.) |
|---|
| Gaignard et
al, 2017; Velarde, 2014; Klinge, 2008 | Mitochondrial gene
expression | Activates NRF1 and
PGC-1α via ERα/ERβ, promoting mitochondrial biogenesis and
respiratory complex expression. | Downregulates NRF1
and PGC-1α, impairs mtDNA replication, reduces energy-related gene
expression by ~40%. | (107,110,111) |
| Salnikova et
al, 2021; Kim et al, 2024; Yin et al, 2021 | Fission and fusion
balance | Inhibited Drp1,
promoted the expression of Mfn1/Mfn2 and Opa1, and maintained
mitochondrial homeostasis. | Increases Drp1
activity (~1.5-fold), reduces fusion proteins, leading to
fragmentation and abnormal morphology. | (115-117) |
| Kemper et
al, 2014; Rettberg et al, 2014; Miao et al,
2024 | Respiration and ATP
synthesis | Enhances ETC
activity, supports COX function, stabilizes Δψm, and boosts ATP
production. | Decreases ATP yield
(~30%), OCR and glucose utilization. | (108,112,118) |
| Gujardo-Correa
et al, 2022; Stirone et al, 2005; Miao et al,
2024 | Antioxidant
defense | Induces SOD2, GPx,
and catalase expression, reducing mitochondrial ROS
accumulation. | Decreases SOD2,
increases ROS by 50-80%, causing oxidative damage to membranes,
proteins and DNA. | (109,114,118) |
| Plovanich et
al, 2013; Hunter et al, 2012 | Calcium
homeostasis | Regulates
MCU-mediated Ca2+ influx, supporting ATP synthase
activity and calcium signaling. | MCU dysfunction
reduces Ca2+ uptake (~40%), impairing respiration and
triggering apoptotic pathways. | (126,127) |
| Liu et al,
2021; Li et al, 2023 | Mitophagy | FUNDC1 is
upregulated by PGC-1α to promote mitochondrial clearance of
damage. | Autophagy disorder
leads to damage mitochondrial accumulation and a further increase
in ROS, creating a vicious cycle. | (99,158) |
Mitochondrial bioenergetic
dysfunction
E2 deficiency during perimenopause is a major driver
of mitochondrial bioenergetic impairment, with the ETC being a
primary target. Experimental studies indicate that E2 enhances the
expression and activity of several ETC complexes, including Complex
I (NDUFB8), Complex IV (MTCO1) and Complex V (121). In the absence of E2, the
Erβ-PGC-1α-NRF1 transcriptional axis is downregulated, leading to
reduced expression of OXPHOS subunits, decreased ΔΨm and impaired
coupling between substrate oxidation and ATP synthesis (122). In ovariectomized rats,
mitochondrial oxygen consumption rate in skeletal muscle decreases
by ~25% at rest, with maximal respiratory capacity reduced by up to
≤40% (123). These deficits
associate with diminished expression of Complex I and IV subunits,
highlighting the structural and functional deterioration of the ETC
as a key mechanism underlying energy failure due to E2
deficiency.
Additionally, E2 regulates the TCA cycle through
epigenetic mechanisms. E2 withdrawal suppresses sirtuin 1-mediated
deacetylation of key metabolic enzymes, resulting in downregulation
of IDH 3α (IDH3α) and α-KGDH, both rate-limiting enzymes in the TCA
cycle (124,125). This suppression limits NADH
production, reducing electron supply to the ETC and slowing TCA
cycle throughput.
E2 also positively regulates the expression and
assembly of the MCU complex. When E2 levels decline <50 pg/ml,
dysfunctional MCU assembly limits mitochondrial Ca2+
uptake, impairing ATP synthase activation and disrupting
calcium-dependent apoptotic signaling (126). Mitochondrial Ca2+ is
essential for activating key dehydrogenases in the TCA cycle, such
as pyruvate dehydrogenase (PDH), IDH and α-KGDH, which facilitate
NADH and FADH2 production. These reducing equivalents
fuel the ETC, enhancing ATP synthesis and supporting mitochondrial
bioenergetics. In perimenopausal women, E2 deficiency reduces
mitochondrial Ca2+ sensitivity, impairing respiratory
efficiency and energy output (127). Moreover, dysregulated
mitochondrial Ca2+ buffering disrupts the spatial and
temporal precision of calcium transients required for synaptic
vesicle release. This leads to inefficient neurotransmission and
contributes to excitatory/inhibitory (E/I) imbalance, often
manifested as elevated extracellular Glu levels and reduced
GABAergic tone. Such imbalances compromise synaptic plasticity,
long-term potentiation and network stability in limbic regions
(128).
Furthermore, E2 deficiency impairs fatty acid
oxidation by downregulating carnitine palmitoyltransferase I, a key
enzyme responsible for mitochondrial fatty acid uptake and
β-oxidation. This defect limits the utilization of alternative
energy substrates and reduces overall metabolic flexibility
(125). E2 also influences
glucose metabolism at multiple regulatory points. Its absence leads
to reduced expression of glucose transporters GLUT1 and GLUT4 in
the brain, and GLUT3 and GLUT4 in peripheral tissues. Additionally,
increased phosphorylation of PDH inhibits its activity, impairing
pyruvate utilization and mitochondrial entry. These combined
effects result in reduced glucose uptake and decreased lactate
production, reflecting a broad suppression of glucose oxidative
capacity (129).
Impaired antioxidant defense
E2 deficiency markedly disrupts mitochondrial
antioxidant defense systems, exacerbating oxidative stress and
contributing to mitochondrial dysfunction. The phenolic hydroxyl
group of E2, particularly on the A-ring, acts as a direct free
radical scavenger by donating hydrogen atoms to neutralize ROS,
thereby exerting non-enzymatic antioxidant effects (118). In perimenopausal women, a
decline in circulating E2 levels is associated with a 50-80%
increase in ROS production, positioning E2 loss as a key driver of
mitochondrial oxidative stress. E2 deficiency downregulates the
Erβ-PGC-1α-NRF1 transcriptional axis, leading to suppressed SOD2
expression and impaired clearance of mitochondrial ROS, initiating
a vicious cycle of oxidative damage and mitochondrial
destabilization (122).
Concurrently, reduced expression of OXPHOS subunits further impairs
mitochondrial electron flow, resulting in decreased ΔΨm and
diminished coupling efficiency. These alterations increase the
NADH/NAD+ ratio, which inhibits Complex I activity and
promotes electron leakage to oxygen, generating additional
superoxide radicals and intensifying oxidative injury (130).
Excessive ROS not only impair ETC function but also
activate pro-inflammatory and pro-apoptotic pathways. For example,
elevated ROS levels can trigger the assembly of NLRP3
inflammasomes, promote mPTP opening and initiate caspase-mediated
apoptosis. These downstream effects contribute to neuroinflammation
and neuronal loss, thereby reinforcing the pathological association
between E2 decline, mitochondrial dysfunction and mood disorders
during perimenopause (131).
Imbalance in mitochondrial dynamics
E2 decline also promotes mitochondrial fission,
resulting in fragmented mitochondria with disorganized cristae and
reduced inter-organelle connectivity. Such fragmentation impairs
substrate and protein exchange across the mitochondrial network,
compromises ΔΨm and reduces coupling efficiency between electron
transport and ATP synthesis.
Mechanistically, E2 deficiency increases the
expression and activation of the fission protein Drp1, particularly
through phosphorylation at Ser616. By contrast, the expression of
key fusion proteins, Mfn1/2 and Opa1, is downregulated (117). This imbalance between enhanced
fission and suppressed fusion leads to structural fragmentation,
metabolic uncoupling and deterioration of respiratory chain
function, all of which contribute to bioenergetic failure.
To illustrate the association between E2 signaling,
mitochondrial integrity and mood regulation, a conceptual framework
outlining the hormone-mitochondria-mood axis is proposed (Fig. 3). As summarized, declining E2
levels during perimenopause disrupt key mitochondrial processes,
such as biogenesis, antioxidant defense, calcium homeostasis,
mitophagy and energy metabolism, through multiple nuclear and
cytoplasmic signaling pathways (132). These impairments collectively
compromise neuronal function and synaptic plasticity in
emotion-related brain regions, such as the hippocampus and
prefrontal cortex, contributing to the onset of affective and
cognitive symptoms (133).
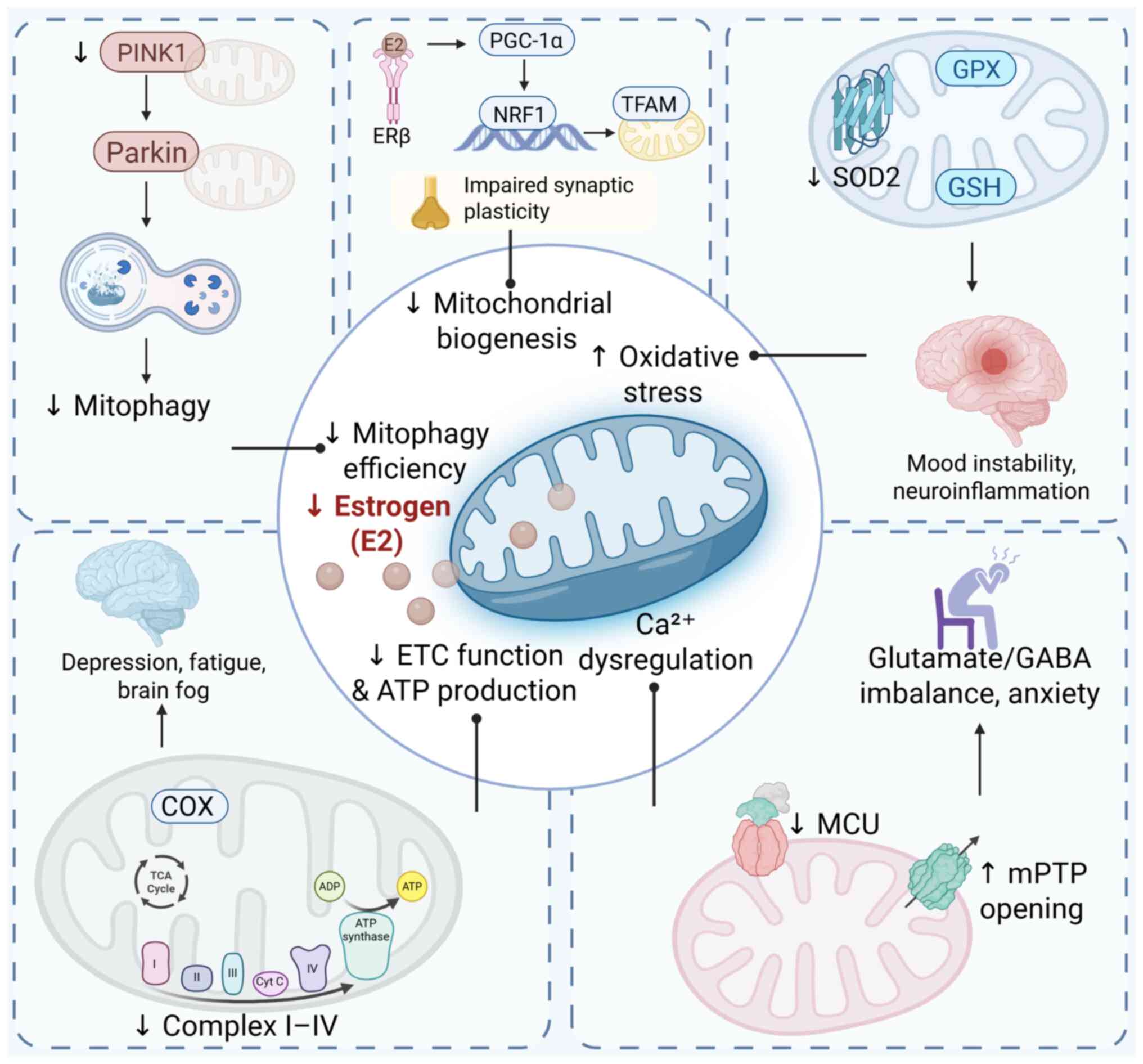 | Figure 3Disruption of the
hormone-mitochondria-mood axis in estrogen deficiency. Estrogen
deficiency induces multifaceted mitochondrial dysfunction in
mood-regulating brain regions, characterized by impaired oxidative
phosphorylation, reduced ATP synthesis, excessive reactive oxygen
species accumulation, dysregulated fission-fusion dynamics and
suppressed mitophagy. These alterations compromise neuronal
bioenergetic capacity and redox homeostasis, contributing to
synaptic dysfunction and neuroinflammatory cascades. This
mechanistic continuum constitutes the hormone-mitochondria-mood
axis, wherein endocrine withdrawal drives mitochondrial
destabilization, ultimately predisposing individuals to affective
and cognitive disturbances during the perimenopausal transition.
ATP, adenosine triphosphate; Ca2+, calcium ion; COX,
cytochrome c oxidase (complex IV); E2, 17β-estradiol; ETC, electron
transport chain; GABA, gamma-aminobutyric acid; GPX, glutathione
peroxidase; GSH, reduced glutathione; MCU, mitochondrial calcium
uniporter; mPTP, mitochondrial permeability transition pore; NRF1,
nuclear respiratory factor 1; Parkin, Parkin RBR E3 ubiquitin
protein ligase; PGC-1α, peroxisome proliferator-activated receptor
gamma coactivator 1-α; PINK1, PTEN-induced putative kinase 1; ROS,
reactive oxygen species; SOD2, superoxide dismutase 2; TCA,
tricarboxylic acid cycle; TFAM, transcription factor A,
mitochondrial. |
Limitations and prospects
Limitations of current research
Clinical confounders and mechanistic
gaps
Perimenopause represents a complex physiological
transition characterized by notable hormonal fluctuations and
multisystem remodeling. During this stage, E2 and progesterone
levels fluctuate along individualized trajectories, profoundly
affecting CNS function and mood regulation pathways (134). While the majority of women
experience varying degrees of mood disturbances, such as
depression, anxiety and cognitive impairment, others may remain
largely asymptomatic. The clinical presentation of perimenopausal
mood disorders reflects the unique interplay between biological
factors and psychosocial influences, encompassing depressive and
anxious symptoms, cognitive decline and somatic complaints. This
variability is likely due to differences in hormone sensitivity,
genetic polymorphisms, psychiatric history and exposure to life
stressors. These confounding factors introduce considerable
variability, complicating the comparability and generalizability of
clinical research findings. Future studies should implement
stratified analyses based on individual stress exposures, assessed
using validated psychological or life event scales, to
differentiate between stress-induced and hormone-driven
mitochondrial alterations.
Although growing evidence implicates mitochondrial
dysfunction, such as impaired ATP production, elevated oxidative
stress, disrupted mitophagy and calcium dysregulation, in the
pathogenesis of perimenopausal mood disorders, the underlying
mechanisms remain poorly understood (135). To date, no fully integrated
pathological cascade has been established associating sex hormone
decline, mitochondrial dysfunction, neurotransmitter imbalance and
affective symptoms. Much of the mechanistic insight stems from
animal models or in vitro systems, which may not accurately
replicate the physiological complexity of human perimenopause.
Additionally, to the best of our knowledge, high-quality,
longitudinal clinical studies specifically targeting perimenopausal
populations are lacking. This gap restricts the development of
robust, evidence-based conclusions and limits the translational
potential of mitochondrial biomarkers and targeted interventions in
this context. Moreover, individual genetic variations in
mitochondrial or E2-responsive genes, such as ESR1, TFAM, PGC-1α
and NRF1, may influence mitochondrial biogenesis, redox regulation
and susceptibility to hormonal decline (136). These polymorphisms may explain
the heterogeneous responses to both E2 loss and therapeutic
interventions across perimenopausal women.
Limitations of current diagnostic and
therapeutic strategies
Although peripheral mitochondrial markers, such as
platelet Cyt c oxidase activity, have been proposed as
minimally invasive proxies for CNS mitochondrial function, their
diagnostic utility remains limited. Differences in metabolic
demand, regulatory environment and cell-type specificity between
platelets and neurons may obscure central pathophysiological
signals (137). Therefore,
future studies should integrate peripheral bioenergetic markers
with neuroimaging and hormonal profiling to establish more
reliable, multidimensional diagnostic tools.
HRT can enhance ΔΨm, increase ATP synthesis
efficiency and upregulate antioxidant enzymes such as SOD2 and GPx,
thereby improving neuronal bioenergetics and redox homeostasis
while attenuating central oxidative stress (138). Notably, the variability in HRT
efficacy may reflect underlying differences in mitochondrial status
among individuals. Women with higher baseline oxidative stress or
impaired mitophagy may exhibit diminished mitochondrial
responsiveness to E2, limiting the therapeutic benefits of HRT
(56). Identifying such
biomarkers could guide personalized interventions.
Mitochondria-targeted therapies, including antioxidants [such as,
coenzyme Q10, N-acetylcysteine (NAC) and α-lipoic acid] and
mitochondrial nutritional supplements, have emerged as promising
alternatives aimed at mitigating oxidative stress and restoring
mitochondrial function.
Additionally, emerging mitochondria-targeted
compounds, such as SS-31 (elamipretide) and MitoQ, have
demonstrated preclinical efficacy in reducing oxidative damage and
restoring mitochondrial function in neuronal models of depression
and E2 deficiency (139).
Although preclinical studies and animal models have revealed
improvements in energy metabolism, reductions in ROS production,
and beneficial behavioral effects in depression and anxiety
paradigms, human studies remain limited. Current clinical trials in
perimenopausal populations face challenges such as small sample
sizes, short intervention durations and a lack of appropriate
control groups, restricting the generalizability and strength of
evidence needed for clinical implementation. Another key limitation
is the prevailing 'one-size-fits-all' approach in current treatment
paradigms, which fails to account for the considerable
interindividual heterogeneity in clinical phenotype, hormonal
responsiveness, mitochondrial status and genetic background
(140).
Future directions
Multi-omics integration and precision
medicine
The advent of large-scale multi-omics approaches,
including metabolomics, epigenomics and neuroimaging, offers
increasing potential to address the heterogeneity of perimenopausal
mood disorders and move toward precision medicine. By integrating
genetic variations, epigenetic modifications, inflammatory profiles
and metabolic signatures with neurofunctional imaging markers,
researchers have begun identifying biologically distinct subtypes
of depression (141). Recent
studies, for instance, have utilized machine learning clustering
based on low-frequency amplitude features from functional magnetic
resonance imaging, combined with multi-omics data, to classify
depression into molecularly distinct subtypes (141,142). These subtypes demonstrate
divergent characteristics in neurodevelopment, synaptic regulation
and immune-inflammatory dysregulation, and associate with
differences in symptom severity.
Extensive evidence has validated the concept of
mechanism-based stratification and highlighted the need for
personalized interventions tailored to individual molecular and
neurobiological profiles (143-145). Moving forward, the integration
of artificial intelligence and machine learning is expected to
enhance data analysis efficiency and support predictive modeling
for disease trajectories and treatment responsiveness in
perimenopausal populations. To further elucidate the
pathophysiological role of mitochondrial dysfunction and inform
treatment development, future studies should integrate omics-based
profiling with dynamic neuroimaging to map the spatiotemporal
relationship between mitochondrial imbalance and affective
symptoms.
Multipathway combination
therapies
Mitochondrial dysfunction in perimenopausal mood
disorders is often accompanied by oxidative stress and neuronal
injury. Antioxidant agents such as coenzyme Q10, vitamin E and NAC
reduce ROS production, enhance mitochondrial ATP levels, and
restore membrane potential (146). These effects contribute to
improved neuroenergetic function and emotional regulation. For
instance, vitamin E has demonstrated efficacy in improving vaginal
health and modulating hormone levels in perimenopausal women, with
a favorable safety profile (147). However, the majority of studies
on coenzyme Q10 and NAC have primarily focused on ovarian function,
and their long-term efficacy and safety in mood-related contexts
remain insufficiently validated (148,149). Natural compounds that activate
the Nrf2 signaling pathway, such as curcumin and resveratrol, have
also demonstrated promise in reducing oxidative stress, mitigating
inflammation and enhancing mitochondrial resilience in preclinical
models (150). ALA upregulates
mitochondrial TFAM, promoting mtDNA replication and mitochondrial
biogenesis, and functions as a cofactor in the ETC, directly
enhancing redox function (151).
Given the multifactorial nature of mitochondrial
imbalance, multipronged therapeutic strategies are likely more
effective than single-target approaches (Table II) (69,71,73,76,78,146,151-153). For example, Nrf2 activators
upregulate endogenous antioxidant defenses and exert
neuroprotective, anti-apoptotic effects, while mitophagy modulators
promote the selective clearance of dysfunctional mitochondria.
Mitophagy deficits are increasingly implicated in depression
pathogenesis; therefore, enhancing mitochondrial quality control
may offer therapeutic benefits. A growing body of preclinical
evidence supports the antidepressant potential of
mitophagy-promoting interventions in animal and cellular models
(154). While multi-pathway
approaches hold promise, priority should be given to interventions
supported by clinical trials, such as phytoE2s (for example
genistein), melatonin or lifestyle strategies such as exercise and
dietary modulation.
 | Table IIComparison of therapeutic strategies
targeting imbalances in mitochondrial homeostasis. |
Table II
Comparison of therapeutic strategies
targeting imbalances in mitochondrial homeostasis.
| Author/s, year | Treatment
strategy | Mechanism of
action | Evidence | Potential
risks/limitations | (Refs.) |
|---|
| Soares et
al, 2001; van Staa et al, 2008; Rossouw et al,
2002 | HRT | Replenishes
estrogen, restores mitochondrial membrane potential, ATP production
and antioxidant defense. | RCTs show notable
symptom reduction in perimenopausal depression (remission, 68 vs.
17%) | Potential long-term
risks: Cardiovascular disease and breast cancer. | (69,71,73) |
| Yan 2014; Wagner
et al, 2012 |
Mitochondria-targeted drugs | Antioxidants
(CoQ10, NAC) reduce ROS; α-lipoic acid enhances energy metabolism
and promotes mitochondrial biogenesis via TFAM. | Animal studies
confirm improved mitochondrial function and gene expression. | Limited clinical
data; long-term safety remains uncertain. | (146,151) |
| Stahl et al,
2005; Parry, 2010 | Antidepressants and
psychological therapy | SSRIs/SNRIs
increase monoamine levels; CBT modifies cognition and alleviates
emotional symptoms. | SSRIs and
phototherapy improve mood; CBT reduces anxiety and sleep
issues. | Side effects (such
as nausea and sexual dysfunction); efficacy varies among
individuals. | (76,78) |
| Shon et al,
2023; Shavaisi et al, 2024 | Lifestyle
interventions | Diet (such as
omega-3 and soy) offers antioxidant/anti-inflammatory effects;
exercise improves metabolism and neuroplasticity. | Improves
progesterone levels, mood, and gut microbiota; reduces
inflammation | Requires long-term
adherence; outcomes vary with individual compliance. | (152,153) |
Nanomedicine and mitochondria-targeted
delivery
To address limitations in drug targeting and
delivery efficiency, the development of mitochondria-targeted
nanomedicine has emerged as a promising strategy. Functionalized
nanoparticles can penetrate both cellular and mitochondrial
membranes, as well as the blood-brain barrier, enabling direct
delivery of therapeutics into neuronal mitochondria. This approach
enhances drug bioavailability, stability and organelle specificity
(155,156).
The structural complexity of mitochondria often
restricts the effective delivery of conventional compounds.
Nanocarriers, such as ligand-functionalized lipid nanoparticles and
MITO-Porter systems, can preferentially accumulate within
mitochondria, facilitating the precise release of antioxidants and
metabolic modulators (157).
However, these experimental strategies are still in the early
stages of development and require further validation before
clinical application.
Conclusion
Perimenopausal mood disorders result from complex
interactions between hormonal decline and mitochondrial
dysfunction. The present review highlights disrupted mitochondrial
homeostasis, including impaired OXPHOS, calcium imbalance, redox
stress and defective mitophagy, as a central mechanism linking E2
loss to neuropsychiatric symptoms. E2 modulates mitochondrial
function through both genomic and non-genomic pathways, positioning
mitochondria as key mediators of hormonal effects on brain health.
While preclinical evidence supports mitochondrial targets, clinical
translation remains hindered by insufficient biomarker-based
stratification and a lack of long-term data. Future research should
integrate multi-omics, neuroimaging and artificial
intelligence-driven subtyping to develop personalized,
mitochondria-targeted interventions. Restoring mitochondrial
integrity presents a novel therapeutic approach for enhancing
emotional and cognitive resilience in perimenopausal women.
Availability of data and materials
Not applicable.
Authors' contributions
YY and HY drafted the initial version of the
manuscript. ZL and LF contributed to the collection, organization,
and critical interpretation of the literature. JL and YL assisted
in preparing figures and tables and participated in manuscript
editing. WL and HC provided intellectual input, supervised the
writing process, and revised the manuscript for important content.
XW conceived the review topic, guided the overall structure, and
finalized the manuscript. All authors read and approved the final
version. Data authentication is not applicable.
Ethics approval and consent to
participate
Not applicable.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
Acknowledgements
Not applicable.
Funding
The present review was funded by Heilongjiang Postdoctoral Fund
(grant no. LBH-Z22283), the Scientific Research Fund of
Heilongjiang University of Chinese Medicine (grant no.
2024XJJ-QNCX020), Heilongjiang Provincial Undergraduate
Universities Basic Scientific Research Fund-Research Project (grant
no. 2024-KYYWF-1389), Heilongjiang Provincial TCM Research Project
(grant no. ZHY2024-233), the Heilongjiang Provincial Natural
Science Foundation (grant no. PL2024H220) and the Heilongjiang
Provincial Chinese Medicine Scientific Research Project (grant no.
HYZ2022-128).
References
|
1
|
Santoro N: Perimenopause: From research to
practice. J Womens Health (Larchmt). 25:332–339. 2016. View Article : Google Scholar
|
|
2
|
Cunningham AC, Pal L, Wickham AP, Prentice
C, Goddard FGB, Klepchukova A and Zhaunova L: Chronicling menstrual
cycle patterns across the reproductive lifespan with real-world
data. Sci Rep. 14:101722024. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Woods NF, Smith-Dijulio K, Percival DB,
Tao EY, Taylor HJ and Mitchell ES: Symptoms during the menopausal
transition and early postmenopause and their relation to endocrine
levels over time: Observations from the seattle midlife women's
health study. J Womens Health (Larchmt). 16:667–677. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Cheng MH, Hsu CY, Wang SJ, Lee SJ, Wang PH
and Fuh JL: The relationship of self-reported sleep disturbance,
mood, and menopause in a community study. Menopause. 15:958–962.
2008. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Wang ST, Gu HY, Huang ZC, Li C, Liu WN and
Li R: Comparative accuracy of osteoporosis risk assessment tools in
postmenopausal women: A systematic review and network
meta-analysis. Int J Nurs Stud. 165:1050292025. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Freitas JPC, Santos JNV, de Moraes DB,
Gonçalves GT, Teixeira LADC, Otoni Figueiró MT, Cunha T, da Silva
Lage VK, Danielewicz AL, Figueiredo PHS, et al: Handgrip strength
and menopause are associated with cardiovascular risk in women with
obesity: A cross-sectional study. BMC Womens Health. 25:1572025.
View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Woods NF and Mitchell ES: Symptoms during
the perimenopause: Prevalence, severity, trajectory, and
significance in women's lives. Am J Med. 118(Suppl 12B): S14–S24.
2005. View Article : Google Scholar
|
|
8
|
Marsh WK, Templeton A, Ketter TA and
Rasgon NL: Increased frequency of depressive episodes during the
menopausal transition in women with bipolar disorder: Preliminary
report. J Psychiatr Res. 42:247–251. 2008. View Article : Google Scholar
|
|
9
|
Marsh WK, Ketter TA and Rasgon NL:
Increased depressive symptoms in menopausal age women with bipolar
disorder: Age and gender comparison. J Psychiatr Res. 43:798–802.
2009. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Truong D and Marsh W: Bipolar disorder in
the menopausal transition. Curr Psychiatry Rep. 21:1302019.
View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Toffol E, Heikinheimo O and Partonen T:
Hormone therapy and mood in perimenopausal and postmenopausal
women: A narrative review. Menopause. 22:564–578. 2015. View Article : Google Scholar
|
|
12
|
Graziottin A and Serafini A: Depression
and the menopause: Why antidepressants are not enough? Menopause
Int. 15:76–81. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Chen H, Lu M, Lyu Q, Shi L, Zhou C, Li M,
Feng S, Liang X, Zhou X and Ren L: Mitochondrial dynamics
dysfunction: Unraveling the hidden link to depression. Biomed
Pharmacother. 175:1166562024. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Khan M, Baussan Y and Hebert-Chatelain E:
Connecting dots between mitochondrial dysfunction and depression.
Biomolecules. 13:6952023. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Rezin GT, Amboni G, Zugno AI, Quevedo J
and Streck EL: Mitochondrial dysfunction and psychiatric disorders.
Neurochem Res. 34:1021–1029. 2009. View Article : Google Scholar
|
|
16
|
Iwata K: Mitochondrial involvement in
mental disorders; energy metabolism, genetic, and environmental
factors. Methods Mol Biol. 1916:41–48. 2019. View Article : Google Scholar
|
|
17
|
Jou SH, Chiu NY and Liu CS: Mitochondrial
dysfunction and psychiatric disorders. Chang Gung Med J.
32:370–379. 2009.PubMed/NCBI
|
|
18
|
Beikoghli Kalkhoran S and Kararigas G:
Oestrogenic regulation of mitochondrial dynamics. Int J Mol Sci.
23:11182022. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
No authors listed. Research on the
menopause in the 1990s. Report of a WHO scientific group. World
Health Organ Tech Rep Ser. 866:1–107. 1996.PubMed/NCBI
|
|
20
|
Schmidt PJ, Roca CA, Bloch M and Rubinow
DR: The perimenopause and affective disorders. Semin Reprod
Endocrinol. 15:91–100. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
No authors listed. Clinical challenges of
perimenopause: Consensus opinion of the North American menopause
society. Menopause. 7:5–13. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Gordon JL, Girdler SS, Meltzer-Brody SE,
Stika CS, Thurston RC, Clark CT, Prairie BA, Moses-Kolko E, Joffe H
and Wisner KL: Ovarian hormone fluctuation, neurosteroids, and HPA
axis dysregulation in perimenopausal depression: A novel heuristic
model. Am J Psychiatry. 172:227–236. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Santoro N, Epperson CN and Mathews SB:
Menopausal symptoms and their management. Endocrinol Metab Clin
North Am. 44:497–515. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Cheng MH, Lee SJ, Wang SJ, Wang PH and Fuh
JL: Does menopausal transition affect the quality of life? A
longitudinal study of middle-aged women in Kinmen. Menopause.
14:885–890. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Jia Y, Zhou Z, Xiang F, Hu W and Cao X:
Global prevalence of depression in menopausal women: A systematic
review and meta-analysis. J Affect Disord. 358:474–482. 2024.
View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Badawy Y, Spector A, Li Z and Desai R: The
risk of depression in the menopausal stages: A systematic review
and meta-analysis. J Affect Disord. 357:126–133. 2024. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Oppermann K, Fuchs SC, Donato G, Bastos CA
and Spritzer PM: Physical, psychological, and menopause-related
symptoms and minor psychiatric disorders in a community-based
sample of Brazilian premenopausal, perimenopausal, and
postmenopausal women. Menopause. 19:355–360. 2012. View Article : Google Scholar
|
|
28
|
Li RX, Ma M, Xiao XR, Xu Y, Chen XY and Li
B: Perimenopausal syndrome and mood disorders in perimenopause:
Prevalence, severity, relationships, and risk factors. Medicine
(Baltimore). 95:e44662016. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Soares CN and Taylor V: Effects and
management of the menopausal transition in women with depression
and bipolar disorder. J Clin Psychiatry. 68(Suppl 9): S16–S21.
2007.
|
|
30
|
Timur S and Sahin NH: The prevalence of
depression symptoms and influencing factors among perimenopausal
and postmenopausal women. Menopause. 17:545–551. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Freeman EW: Associations of depression
with the transition to menopause. Menopause. 17:823–827. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Ye B, Zhou Y, Chen M, Chen C, Tan J and Xu
X: The association between depression during perimenopause and
progression of chronic conditions and multimorbidity: Results from
a Chinese prospective cohort. Arch Womens Ment Health. 26:697–705.
2023. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Marsh WK, Ketter TA, Crawford SL, Johnson
JV, Kroll-Desrosiers AR and Rothschild AJ: Progression of female
reproductive stages associated with bipolar illness exacerbation.
Bipolar Disord. 14:515–526. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Bromberger JT, Matthews KA, Schott LL,
Brockwell S, Avis NE, Kravitz HM, Everson-Rose SA, Gold EB, Sowers
M and Randolph JF Jr: Depressive symptoms during the menopausal
transition: The Study of women's health across the nation (SWAN). J
Affect Disord. 103:267–272. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Krajewska-Ferishah K, Kułak-Bejda A,
Szyszko-Perłowska A, Shpakou A, Van Damme-Ostapowicz K and
Chatzopulu A: Risk of depression during menopause in women from
Poland, Belarus, Belgium, and Greece. J Clin Med. 11:33712022.
View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Kulkarni J, Gavrilidis E, Hudaib AR,
Bleeker C, Worsley R and Gurvich C: Development and validation of a
new rating scale for perimenopausal depression-the Meno-D. Transl
Psychiatry. 8:1232018. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Bromberger JT and Kravitz HM: Mood and
menopause: Findings from the study of women's health across the
nation (SWAN) over 10 years. Obstet Gynecol Clin North Am.
38:609–625. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Chen MH, Su TP, Li CT, Chang WH, Chen TJ
and Bai YM: Symptomatic menopausal transition increases the risk of
new-onset depressive disorder in later life: A nationwide
prospective cohort study in Taiwan. PLoS One. 8:e598992013.
View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Bromberger JT, Kravitz HM, Chang Y,
Randolph JF Jr, Avis NE, Gold EB and Matthews KA: Does risk for
anxiety increase during the menopausal transition? Study of women's
health across the nation. Menopause. 20:488–495. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Hogervorst E, Craig J and O'Donnell E:
Cognition and mental health in menopause: A review. Best Pract Res
Clin Obstet Gynaecol. 81:69–84. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Pertesi S, Coughlan G, Puthusseryppady V,
Morris E and Hornberger M: Menopause, cognition and dementia-a
review. Post Reprod Health. 25:200–206. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Sochocka M, Karska J, Pszczołowska M,
Ochnik M, Fułek M, Fułek K, Kurpas D, Chojdak-Łukasiewicz J,
Rosner-Tenerowicz A and Leszek J: Cognitive decline in early and
premature menopause. Int J Mol Sci. 24:65662023. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Coborn J, de Wit A, Crawford S, Nathan M,
Rahman S, Finkelstein L, Wiley A and Joffe H: Disruption of Sleep
continuity during the perimenopause: Associations with female
reproductive hormone profiles. J Clin Endocrinol Metab.
107:e4144–e4153. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Baker FC, de Zambotti M, Colrain IM and
Bei B: Sleep problems during the menopausal transition: Prevalence,
impact, and management challenges. Nat Sci Sleep. 10:73–95. 2018.
View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Zhou Q, Wang B, Hua Q, Jin Q, Xie J, Ma J
and Jin F: Investigation of the relationship between hot flashes,
sweating and sleep quality in perimenopausal and postmenopausal
women: The mediating effect of anxiety and depression. BMC Womens
Health. 21:2932021. View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Aras SG, Grant AD and Konhilas JP:
Clustering of >145,000 symptom logs reveals distinct pre, peri,
and menopausal phenotypes. Sci Rep. 15:6402025. View Article : Google Scholar
|
|
47
|
Cray LA, Woods NF, Herting JR and Mitchell
ES: Symptom clusters during the late reproductive stage through the
early postmenopause: Observations from the seattle midlife women's
health study. Menopause. 19:864–869. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
48
|
Eberling JL, Wu C, Tong-Turnbeaugh R and
Jagust WJ: Estrogen- and tamoxifen-associated effects on brain
structure and function. Neuroimage. 21:364–371. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
49
|
Bromberger JT, Schott LL, Kravitz HM,
Sowers M, Avis NE, Gold EB, Randolph JF Jr and Matthews KA:
Longitudinal change in reproductive hormones and depressive
symptoms across the menopausal transition: Results from the study
of women's health across the nation (SWAN). Arch Gen Psychiatry.
67:598–607. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
50
|
Musial N, Ali Z, Grbevski J, Veerakumar A
and Sharma P: Perimenopause and first-onset mood disorders: A
closer look. Focus (Am Psychiatr Publ). 19:330–337. 2021.PubMed/NCBI
|
|
51
|
Turek J and Gąsior Ł: Estrogen
fluctuations during the menopausal transition are a risk factor for
depressive disorders. Pharmacol Rep. 75:32–43. 2023. View Article : Google Scholar : PubMed/NCBI
|
|
52
|
He L, Guo W, Qiu J, An X and Lu W: Altered
spontaneous brain activity in women during menopause transition and
its association with cognitive function and serum estradiol level.
Front Endocrinol (Lausanne). 12:6525122021. View Article : Google Scholar : PubMed/NCBI
|
|
53
|
Meinhard N, Kessing LV and Vinberg M: The
role of estrogen in bipolar disorder, a review. Nord J Psychiatry.
68:81–87. 2014. View Article : Google Scholar
|
|
54
|
Wharton W, Gleason CE, Olson SRMS,
Carlsson CM and Asthana S: Neurobiological underpinnings of the
estrogen-mood relationship. Curr Psychiatry Rev. 8:247–256. 2012.
View Article : Google Scholar
|
|
55
|
Newhouse P and Albert K: Estrogen, stress,
and depression: A neurocognitive model. JAMA Psychiatry.
72:727–729. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
56
|
Simpkins JW, Yang SH, Sarkar SN and Pearce
V: Estrogen actions on mitochondria-physiological and pathological
implications. Mol Cell Endocrinol. 290:51–59. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
57
|
Metcalf CA, Duffy KA, Page CE and Novick
AM: Cognitive problems in perimenopause: A review of recent
evidence. Curr Psychiatry Rep. 25:501–511. 2023. View Article : Google Scholar : PubMed/NCBI
|
|
58
|
Crandall CJ, Larson JC, Ensrud KE, LaCroix
AZ, Guthrie KA, Reed SD, Bhasin S and Diem S: Are serum estrogen
concentrations associated with menopausal symptom bother among
postmenopausal women? Baseline results from two MsFLASH clinical
trials. Maturitas. 162:23–30. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
59
|
Zhang Z, DiVittorio JR, Joseph AM and
Correa SM: The effects of estrogens on neural circuits that control
temperature. Endocrinology. 162:bqab0872021. View Article : Google Scholar : PubMed/NCBI
|
|
60
|
Amin Z, Canli T and Epperson CN: Effect of
estrogen-serotonin interactions on mood and cognition. Behav Cogn
Neurosci Rev. 4:43–58. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
61
|
Rubinow DR, Schmidt PJ and Roca CA:
Estrogen-serotonin interactions: Implications for affective
regulation. Biol Psychiatry. 44:839–850. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
62
|
Regidor PA: Progesterone in peri-and
postmenopause: A review. Geburtshilfe Frauenheilkd. 74:995–1002.
2014. View Article : Google Scholar : PubMed/NCBI
|
|
63
|
Memi E, Pavli P, Papagianni M, Vrachnis N
and Mastorakos G: Diagnostic and therapeutic use of oral micronized
progesterone in endocrinology. Rev Endocr Metab Disord. 25:751–772.
2024. View Article : Google Scholar : PubMed/NCBI
|
|
64
|
Cao G, Meng G, Zhu L, Zhu J, Dong N, Zhou
X, Zhang S and Zhang Y: Susceptibility to chronic immobilization
stress-induced depressive-like behaviour in middle-aged female mice
and accompanying changes in dopamine D1 and GABAA
receptors in related brain regions. Behav Brain Funct. 17:22021.
View Article : Google Scholar
|
|
65
|
Crowley SK, O'Buckley TK, Schiller CE,
Stuebe A, Morrow AL and Girdler SS: Blunted neuroactive steroid and
HPA axis responses to stress are associated with reduced sleep
quality and negative affect in pregnancy: A pilot study.
Psychopharmacology (Berl). 233:1299–1310. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
66
|
Sanna E, Talani G, Busonero F, Pisu MG,
Purdy RH, Serra M and Biggio G: Brain steroidogenesis mediates
ethanol modulation of GABAA receptor activity in rat hippocampus. J
Neurosci. 24:6521–6530. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
67
|
Hantsoo L, Jagodnik KM, Novick AM, Baweja
R, di Scalea TL, Ozerdem A, McGlade EC, Simeonova DI, Dekel S,
Kornfield SL, et al: The role of the hypothalamic-pituitary-adrenal
axis in depression across the female reproductive lifecycle:
current knowledge and future directions. Front Endocrinol
(Lausanne). 14:12952612023. View Article : Google Scholar : PubMed/NCBI
|
|
68
|
Oyola MG and Handa RJ:
Hypothalamic-pituitary-adrenal and hypothalamic-pituitary-gonadal
axes: Sex differences in regulation of stress responsivity. Stress.
20:476–494. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
69
|
Soares CN, Almeida OP, Joffe H and Cohen
LS: Efficacy of estradiol for the treatment of depressive disorders
in perimenopausal women: A double-blind, randomized,
placebo-controlled trial. Arch Gen Psychiatry. 58:529–534. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
70
|
Behrman S and Crockett C: Severe mental
illness and the perimenopause. BJPsych Bull. 48:1–7.
2023.PubMed/NCBI
|
|
71
|
van Staa TP, Cooper C, Barlow D and
Leufkens HGM: Individualizing the risks and benefits of
postmenopausal hormone therapy. Menopause. 15:374–381. 2008.
View Article : Google Scholar
|
|
72
|
Minelli C, Abrams KR, Sutton AJ and Cooper
NJ: Benefits and harms associated with hormone replacement therapy:
Clinical decision analysis. BMJ. 328:3712004. View Article : Google Scholar : PubMed/NCBI
|
|
73
|
Rossouw JE, Anderson GL, Prentice RL,
LaCroix AZ, Kooperberg C, Stefanick ML, Jackson RD, Beresford SA,
Howard BV, Johnson KC, et al: Risks and benefits of estrogen plus
progestin in healthy postmenopausal women: Principal results from
the women's health initiative randomized controlled trial. JAMA.
288:321–333. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
74
|
Fait T and Vrablik M: Hormone replacement
therapy (HRT) shortages for treating menopause: What can clinicians
do to relieve symptoms and concerns? Sci. 6:462024. View Article : Google Scholar
|
|
75
|
Poluzzi E, Piccinni C, Raschi E, Rampa A,
Recanatini M and De Ponti F: Phytoestrogens in postmenopause: The
state of the art from a chemical, pharmacological and regulatory
perspective. Curr Med Chem. 21:417–436. 2014. View Article : Google Scholar :
|
|
76
|
Stahl SM, Grady MM, Moret C and Briley M:
SNRIs: Their pharmacology, clinical efficacy, and tolerability in
comparison with other classes of antidepressants. CNS Spectr.
10:732–747. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
77
|
Farach FJ, Pruitt LD, Jun JJ, Jerud AB,
Zoellner LA and Roy-Byrne PP: Pharmacological treatment of anxiety
disorders: Current treatments and future directions. J Anxiety
Disord. 26:833–843. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
78
|
Parry BL: Optimal management of
perimenopausal depression. Int J Womens Health. 2:143–151. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
79
|
Wang Y, Wang Y, Yue G and Zhao Y: Energy
metabolism disturbance in migraine: From a mitochondrial point of
view. Front Physiol. 14:11335282023. View Article : Google Scholar : PubMed/NCBI
|
|
80
|
Li W, Chen M, Gong Y, Lin F and Sun C:
Effects of dexmedetomidine on oxidative stress, programmed cell
death, liver function, and expression of peripheral immune cells in
patients with primary liver cancer undergoing hepatectomy. Front
Physiol. 14:11597462023. View Article : Google Scholar : PubMed/NCBI
|
|
81
|
Chandel NS: Mitochondria. Cold Spring Harb
Perspect Biol. 13:a0405432021. View Article : Google Scholar : PubMed/NCBI
|
|
82
|
Martínez-Reyes I and Chandel NS:
Mitochondrial TCA cycle metabolites control physiology and disease.
Nat Commun. 11:1022020. View Article : Google Scholar : PubMed/NCBI
|
|
83
|
Fields M, Marcuzzi A, Gonelli A, Celeghini
C, Maximova N and Rimondi E: Mitochondria-targeted antioxidants, an
innovative class of antioxidant compounds for neurodegenerative
diseases: Perspectives and limitations. Int J Mol Sci. 24:37392023.
View Article : Google Scholar : PubMed/NCBI
|
|
84
|
Su B, Wang X, Zheng L, Perry G, Smith MA
and Zhu X: Abnormal mitochondrial dynamics and neurodegenerative
diseases. Biochim Biophys Acta. 1802:135–142. 2010. View Article : Google Scholar
|
|
85
|
Faria-Pereira A and Morais VA: Synapses:
The brain's energy-demanding sites. Int J Mol Sci. 23:36272022.
View Article : Google Scholar : PubMed/NCBI
|
|
86
|
Dienel GA, Schousboe A, McKenna MC and
Rothman DL: A tribute to Leif Hertz: The historical context of his
pioneering studies of the roles of astrocytes in brain energy
metabolism, neurotransmission, cognitive functions, and
pharmacology identifies important, unresolved topics for future
studies. J Neurochem. 168:461–495. 2024. View Article : Google Scholar
|
|
87
|
Vos M, Lauwers E and Verstreken P:
Synaptic mitochondria in synaptic transmission and organization of
vesicle pools in health and disease. Front Synaptic Neurosci.
2:1392010. View Article : Google Scholar : PubMed/NCBI
|
|
88
|
Glancy B and Balaban RS: Role of
mitochondrial Ca2+ in the regulation of cellular energetics.
Biochemistry. 51:2959–2973. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
89
|
Krols M, van Isterdael G, Asselbergh B,
Kremer A, Lippens S, Timmerman V and Janssens S:
Mitochondria-associated membranes as hubs for neurodegeneration.
Acta Neuropathol. 131:505–523. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
90
|
Tassone A, Meringolo M, Ponterio G, Bonsi
P, Schirinzi T and Martella G: Mitochondrial bioenergy in
neurodegenerative disease: Huntington and Parkinson. Int J Mol Sci.
24:72212023. View Article : Google Scholar : PubMed/NCBI
|
|
91
|
Giorgi C, Missiroli S, Patergnani S,
Duszynski J, Wieckowski MR and Pinton P: Mitochondria-associated
membranes: Composition, molecular mechanisms, and
physiopathological implications. Antioxid Redox Signal.
22:995–1019. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
92
|
Murphy MP: How mitochondria produce
reactive oxygen species. Biochem J. 417:1–13. 2009. View Article : Google Scholar
|
|
93
|
Tönnies E and Trushina E: Oxidative
stress, synaptic dysfunction, and Alzheimer's disease. J Alzheimers
Dis. 57:1105–1121. 2017. View Article : Google Scholar
|
|
94
|
Kim KH and Lee CB: Socialized
mitochondria: Mitonuclear crosstalk in stress. Exp Mol Med.
56:1033–1042. 2024. View Article : Google Scholar : PubMed/NCBI
|
|
95
|
Kowalczyk P, Sulejczak D, Kleczkowska P,
Bukowska-Ośko I, Kucia M, Popiel M, Wietrak E, Kramkowski K,
Wrzosek K and Kaczyńska K: Mitochondrial oxidative stress-a
causative factor and therapeutic target in many diseases. Int J Mol
Sci. 22:133842021. View Article : Google Scholar : PubMed/NCBI
|
|
96
|
Forceville X, Van Antwerpen P and Preiser
JC: Selenocompounds and sepsis: Redox bypass hypothesis for early
diagnosis and treatment: Part A-early acute phase of sepsis: An
extraordinary redox situation (leukocyte/endothelium interaction
leading to endothelial damage). Antioxid Redox Signal. 35:113–138.
2021. View Article : Google Scholar : PubMed/NCBI
|
|
97
|
Tu W, Wang H, Li S, Liu Q and Sha H: The
anti-inflammatory and anti-oxidant mechanisms of the keap1/Nrf2/ARE
signaling pathway in chronic diseases. Aging Dis. 10:637–651. 2019.
View Article : Google Scholar : PubMed/NCBI
|
|
98
|
Ferber EC, Peck B, Delpuech O, Bell GP,
East P and Schulze A: FOXO3a regulates reactive oxygen metabolism
by inhibiting mitochondrial gene expression. Cell Death Differ.
19:968–979. 2012. View Article : Google Scholar :
|
|
99
|
Liu L, Li Y, Wang J, Zhang D, Wu H, Li W,
Wei H, Ta N, Fan Y, Liu Y, et al: Mitophagy receptor FUNDC1 is
regulated by PGC-1α/NRF1 to fine tune mitochondrial homeostasis.
EMBO Rep. 22:e506292021. View Article : Google Scholar
|
|
100
|
Tirichen H, Yaigoub H, Xu W, Wu C, Li R
and Li Y: Mitochondrial reactive oxygen species and their
contribution in chronic kidney disease progression through
oxidative stress. Front Physiol. 12:6278372021. View Article : Google Scholar : PubMed/NCBI
|
|
101
|
Huang E, Qu D, Huang T, Rizzi N, Boonying
W, Krolak D, Ciana P, Woulfe J, Klein C, Slack RS, et al:
PINK1-mediated phosphorylation of LETM1 regulates mitochondrial
calcium transport and protects neurons against mitochondrial
stress. Nat Commun. 8:13992017. View Article : Google Scholar : PubMed/NCBI
|
|
102
|
Giménez-Palomo A, Dodd S, Anmella G,
Carvalho AF, Scaini G, Quevedo J, Pacchiarotti I, Vieta E and Berk
M: The role of mitochondria in mood disorders: From physiology to
pathophysiology and to treatment. Front Psychiatry. 12:5468012021.
View Article : Google Scholar : PubMed/NCBI
|
|
103
|
Fakhri S, Abdian S, Zarneshan SN, Akkol
EK, Farzaei MH and Sobarzo-Sánchez E: Targeting mitochondria by
plant secondary metabolites: A promising strategy in combating
Parkinson's disease. Int J Mol Sci. 22:125702021. View Article : Google Scholar : PubMed/NCBI
|
|
104
|
Vogler M, Braun Y, Smith VM, Westhoff MA,
Pereira RS, Pieper NM, Anders M, Callens M, Vervliet T, Abbas M, et
al: The BCL2 family: From apoptosis mechanisms to new advances in
targeted therapy. Signal Transduct Target Ther. 10:912025.
View Article : Google Scholar : PubMed/NCBI
|
|
105
|
de Oliveira MR, Nabavi SF, Habtemariam S,
Erdogan Orhan I, Daglia M and Nabavi SM: The effects of baicalein
and baicalin on mitochondrial function and dynamics: A review.
Pharmacol Res. 100:296–308. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
106
|
Shen X, Sun P, Zhang H and Yang H:
Mitochondrial quality control in the brain: The physiological and
pathological roles. Front Neurosci. 16:10751412022. View Article : Google Scholar : PubMed/NCBI
|
|
107
|
Gaignard P, Liere P, Thérond P, Schumacher
M, Slama A and Guennoun R: Role of sex hormones on brain
mitochondrial function, with special reference to aging and
neurodegenerative diseases. Front Aging Neurosci. 9:4062017.
View Article : Google Scholar : PubMed/NCBI
|
|
108
|
Kemper MF, Stirone C, Krause DN, Duckles
SP and Procaccio V: Genomic and non-genomic regulation of PGC1
isoforms by estrogen to increase cerebral vascular mitochondrial
biogenesis and reactive oxygen species protection. Eur J Pharmacol.
723:322–329. 2014. View Article : Google Scholar :
|
|
109
|
Guajardo-Correa E, Silva-Agüero JF, Calle
X, Chiong M, Henríquez M, García-Rivas G, Latorre M and Parra V:
Estrogen signaling as a bridge between the nucleus and mitochondria
in cardiovascular diseases. Front Cell Dev Biol. 10:9683732022.
View Article : Google Scholar : PubMed/NCBI
|
|
110
|
Velarde MC: Mitochondrial and sex steroid
hormone crosstalk during aging. Longev Healthspan. 3:22014.
View Article : Google Scholar : PubMed/NCBI
|
|
111
|
Klinge CM: Estrogenic control of
mitochondrial function and biogenesis. J Cell Biochem.
105:1342–1351. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
112
|
Rettberg JR, Yao J and Brinton RD:
Estrogen: A master regulator of bioenergetic systems in the brain
and body. Front Neuroendocrinol. 35:8–30. 2014. View Article : Google Scholar :
|
|
113
|
Kobayashi A, Azuma K, Ikeda K and Inoue S:
Mechanisms underlying the regulation of mitochondrial respiratory
chain complexes by nuclear steroid receptors. Int J Mol Sci.
21:66832020. View Article : Google Scholar : PubMed/NCBI
|
|
114
|
Stirone C, Duckles SP, Krause DN and
Procaccio V: Estrogen increases mitochondrial efficiency and
reduces oxidative stress in cerebral blood vessels. Mol Pharmacol.
68:959–965. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
115
|
Salnikova D, Orekhova V, Grechko A,
Starodubova A, Bezsonov E, Popkova T and Orekhov A: Mitochondrial
dysfunction in vascular wall cells and its role in atherosclerosis.
Int J Mol Sci. 22:89902021. View Article : Google Scholar : PubMed/NCBI
|
|
116
|
Kim SO, Albrecht ED and Pepe GJ: Estrogen
promotes fetal skeletal muscle mitochondrial distribution and ATP
synthase activity important for insulin sensitivity in offspring.
Endocrine. 85:417–427. 2024. View Article : Google Scholar : PubMed/NCBI
|
|
117
|
Yin L, Luo M, Wang R, Ye J and Wang X:
Mitochondria in sex hormone-induced disorder of energy metabolism
in males and females. Front Endocrinol (Lausanne). 12:7494512021.
View Article : Google Scholar
|
|
118
|
Miao C, Zhao Y, Chen Y, Wang R, Ren N, Liu
Q, Dou X and Zhang Q: He's Yangchao recipe improves premature
ovarian insufficiency by regulating mitochondrial biogenesis of
granulose cells via ERβ/PGC1α/TFAM pathway. Zhejiang Da Xue Xue Bao
Yi Xue Ban. 53:358–367. 2024.In English, Chinese. PubMed/NCBI
|
|
119
|
Mosconi L, Berti V, Dyke J, Schelbaum E,
Jett S, Loughlin L, Jang G, Rahman A, Hristov H, Pahlajani S, et
al: Menopause impacts human brain structure, connectivity, energy
metabolism, and amyloid-beta deposition. Sci Rep. 11:108672021.
View Article : Google Scholar : PubMed/NCBI
|
|
120
|
Mosconi L, Berti V, Quinn C, McHugh P,
Petrongolo G, Osorio RS, Connaughty C, Pupi A, Vallabhajosula S,
Isaacson RS, et al: Perimenopause and emergence of an Alzheimer's
bioenergetic phenotype in brain and periphery. PLoS One.
12:e01859262017. View Article : Google Scholar : PubMed/NCBI
|
|
121
|
Lejri I, Grimm A and Eckert A:
Mitochondria, estrogen and female brain aging. Front Aging
Neurosci. 10:1242018. View Article : Google Scholar : PubMed/NCBI
|
|
122
|
Zhao W, Hou Y, Song X, Wang L, Zhang F,
Zhang H, Yu H and Zhou Y: Estrogen deficiency induces mitochondrial
damage prior to emergence of cognitive deficits in a postmenopausal
mouse model. Front Aging Neurosci. 13:7138192021. View Article : Google Scholar : PubMed/NCBI
|
|
123
|
Duan P, Liu Y, Lin X, Ren J, He J, Liu X
and Xie J: Extracellular matrix stiffness induces mitochondrial
morphological heterogeneity via AMPK activation. Sichuan Da Xue Xue
Bao Yi Xue Ban. 55:47–52. 2024.In Chinese. PubMed/NCBI
|
|
124
|
Tsuchiya T, Takei A, Tsujikado K and
Inukai T: Effects of androgens and estrogens on sirtuin 1 gene
expression in human aortic endothelial cells. Saudi Med J.
41:361–368. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
125
|
Chen JQ, Brown TR and Russo J: Regulation
of energy metabolism pathways by estrogens and estrogenic chemicals
and potential implications in obesity associated with increased
exposure to endocrine disruptors. Biochim Biophys Acta.
1793:1128–1143. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
126
|
Plovanich M, Bogorad RL, Sancak Y, Kamer
KJ, Strittmatter L, Li AA, Girgis HS, Kuchimanchi S, De Groot J,
Speciner L, et al: MICU2, a paralog of MICU1, resides within the
mitochondrial uniporter complex to regulate calcium handling. PLoS
One. 8:e557852013. View Article : Google Scholar : PubMed/NCBI
|
|
127
|
Hunter JC, Machikas AM and Korzick DH:
Age-dependent reductions in mitochondrial respiration are
exacerbated by calcium in the female rat heart. Gend Med.
9:197–206. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
128
|
Sayehmiri F, Motamedi F, Batool Z, Naderi
N, Shaerzadeh F, Zoghi A, Rezaei O, Khodagholi F and Pourbadie HG:
Mitochondrial plasticity and synaptic plasticity crosstalk; in
health and Alzheimer's disease. CNS Neurosci Ther. 30:e148972024.
View Article : Google Scholar : PubMed/NCBI
|
|
129
|
Alemany M: Estrogens and the regulation of
glucose metabolism. World J Diabetes. 12:1622–1654. 2021.
View Article : Google Scholar : PubMed/NCBI
|
|
130
|
Arjmand S, Ilaghi M, Sisakht AK, Guldager
MB, Wegener G, Landau AM and Gjedde A: Regulation of mitochondrial
dysfunction by estrogens and estrogen receptors in Alzheimer's
disease: A focused review. Basic Clin Pharmacol Toxicol.
135:115–132. 2024. View Article : Google Scholar : PubMed/NCBI
|
|
131
|
Zong Y, Li H, Liao P, Chen L, Pan Y, Zheng
Y, Zhang C, Liu D, Zheng M and Gao J: Mitochondrial dysfunction:
Mechanisms and advances in therapy. Signal Transduct Target Ther.
9:1242024. View Article : Google Scholar : PubMed/NCBI
|
|
132
|
Bencker C, Gschwandtner L, Nayman S,
Grikšienė R, Nguyen B, Nater UM, Guennoun R, Sundström-Poromaa I,
Pletzer B, Bixo M and Comasco E: Progestagens and progesterone
receptor modulation: Effects on the brain, mood, stress, and
cognition in females. Front Neuroendocrinol. 76:1011602025.
View Article : Google Scholar
|
|
133
|
Lee JHA, Chen Q and Zhuo M: Synaptic
plasticity in the pain-related cingulate and insular cortex.
Biomedicines. 10:27452022. View Article : Google Scholar : PubMed/NCBI
|
|
134
|
Genazzani AR, Bernardi F, Pluchino N,
Begliuomini S, Lenzi E, Casarosa E and Luisi M: Endocrinology of
menopausal transition and its brain implications. CNS Spectr.
10:449–457. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
135
|
Jiang M, Wang L and Sheng H: Mitochondria
in depression: The dysfunction of mitochondrial energy metabolism
and quality control systems. CNS Neurosci Ther. 30:e145762024.
View Article : Google Scholar : PubMed/NCBI
|
|
136
|
Chen JQ, Cammarata PR, Baines CP and Yager
JD: Regulation of mitochondrial respiratory chain biogenesis by
estrogens/estrogen receptors and physiological, pathological and
pharmacological implications. Biochim Biophys Acta. 1793:1540–1570.
2009. View Article : Google Scholar : PubMed/NCBI
|
|
137
|
Wong-Riley MTT: Bigenomic regulation of
cytochrome c oxidase in neurons and the tight coupling between
neuronal activity and energy metabolism. Adv Exp Med Biol.
748:283–304. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
138
|
Mosconi L, Jett S, Nerattini M, Andy C,
Yepez CB, Zarate C, Carlton C, Kodancha V, Schelbaum E, Williams S,
et al: In vivo brain estrogen receptor expression by neuroendocrine
aging and relationships with gray matter volume, bio-energetics,
and clinical symptomatology. Res Sq. [Preprint] rs.3.rs-2573335.
2023.
|
|
139
|
Tung C, Varzideh F, Farroni E, Mone P,
Kansakar U, Jankauskas SS and Santulli G: Elamipretide: A review of
its structure, mechanism of action, and therapeutic potential. Int
J Mol Sci. 26:9442025. View Article : Google Scholar : PubMed/NCBI
|
|
140
|
Liang L, Chen J, Xiao L, Wang Q and Wang
G: Mitochondrial modulators in the treatment of bipolar depression:
A systematic review and meta-analysis. Transl Psychiatry. 12:42022.
View Article : Google Scholar : PubMed/NCBI
|
|
141
|
Tang L, Tang R, Zheng J, Zhao P, Zhu R,
Tang Y, Zhang X, Gong X and Wang F: Dissecting biological
heterogeneity in major depressive disorder based on neuroimaging
subtypes with multi-omics data. Transl Psychiatry. 15:722025.
View Article : Google Scholar : PubMed/NCBI
|
|
142
|
Sun X, Sun J, Lu X, Dong Q, Zhang L, Wang
W, Liu J, Ma Q, Wang X, Wei D, et al: Mapping neurophysiological
subtypes of major depressive disorder using normative models of the
functional connectome. Biol Psychiatry. 94:936–947. 2023.
View Article : Google Scholar : PubMed/NCBI
|
|
143
|
Bousman CA, Stevenson JM, Ramsey LB,
Sangkuhl K, Hicks JK, Strawn JR, Singh AB, Ruaño G, Mueller DJ,
Tsermpini EE, et al: Clinical pharmacogenetics implementation
consortium (CPIC) guideline for CYP2D6, CYP2C19, CYP2B6, SLC6A4,
and HTR2A genotypes and serotonin reuptake inhibitor
antidepressants. Clin Pharmacol Ther. 114:51–68. 2023. View Article : Google Scholar : PubMed/NCBI
|
|
144
|
Cooper CM, Chin Fatt CR, Jha M, Fonzo GA,
Grannemann BD, Carmody T, Ali A, Aslan S, Almeida JRC, Deckersbach
T, et al: Cerebral blood perfusion predicts response to sertraline
versus placebo for major depressive disorder in the EMBARC trial.
EClinicalMedicine. 10:32–41. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
145
|
Uher R, Tansey KE, Dew T, Maier W, Mors O,
Hauser J, Dernovsek MZ, Henigsberg N, Souery D, Farmer A and
McGuffin P: An inflammatory biomarker as a differential predictor
of outcome of depression treatment with escitalopram and
nortriptyline. Am J Psychiatry. 171:1278–1286. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
146
|
Yan LJ: Positive oxidative stress in aging
and aging-related disease tolerance. Redox Biol. 2:165–169. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
147
|
Parnan Emamverdikhan A, Golmakani N,
Tabassi SA, Hassanzadeh M, Sharifi N and Shakeri MT: A survey of
the therapeutic effects of vitamin E suppositories on vaginal
atrophy in postmenopausal women. Iran J Nurs Midwifery Res.
21:475–481. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
148
|
Lin G, Li X, Jin Yie SL and Xu L: Clinical
evidence of coenzyme Q10 pretreatment for women with diminished
ovarian reserve undergoing IVF/ICSI: A systematic review and
meta-analysis. Ann Med. 56:23894692024. View Article : Google Scholar : PubMed/NCBI
|
|
149
|
Fang YQ, Ding H, Li T, Zhao XJ, Luo D, Liu
Y and Li Y: N-acetylcysteine supplementation improves
endocrine-metabolism profiles and ovulation induction efficacy in
polycystic ovary syndrome. J Ovarian Res. 17:2052024. View Article : Google Scholar : PubMed/NCBI
|
|
150
|
Liang G, Kow ASF, Yusof R, Tham CL, Ho YC
and Lee MT: Menopause-associated depression: Impact of oxidative
stress and neuroinflammation on the central nervous system-a
review. Biomedicines. 12:1842024. View Article : Google Scholar : PubMed/NCBI
|
|
151
|
Wagner AE, Ernst IMA, Birringer M, Sancak
O, Barella L and Rimbach G: A combination of lipoic acid plus
coenzyme Q10 induces PGC1α, a master switch of energy metabolism,
improves stress response, and increases cellular glutathione levels
in cultured C2C12 skeletal muscle cells. Oxid Med Cell Longev.
2012:8359702012. View Article : Google Scholar
|
|
152
|
Shon J, Seong Y, Choi Y and Kim Y, Cho MS,
Ha E, Kwon O and Kim Y, Park YJ and Kim Y: Meal-based intervention
on health promotion in middle-aged women: A pilot study. Nutrients.
15:21082023. View Article : Google Scholar : PubMed/NCBI
|
|
153
|
Shavaisi F, Heydarpour S, Jalilian N,
Jalali A and Rezaei M: The effects of positive psychology and
physical activity on depression, anxiety, and stress among students
with premenstrual syndrome: A single-blind, randomized controlled
trial. BMC Womens Health. 24:4992024. View Article : Google Scholar : PubMed/NCBI
|
|
154
|
Xu W, Gao W, Guo Y, Xue F, Di L, Fang S,
Fan L, He Y, Zhou Y, Xie X and Pang X: Targeting mitophagy for
depression amelioration: A novel therapeutic strategy. Front
Neurosci. 17:12352412023. View Article : Google Scholar : PubMed/NCBI
|
|
155
|
Buck AC, Maarman GJ, Dube A and Bardien S:
Mitochondria targeted nanoparticles for the treatment of
mitochondrial dysfunction-associated brain disorders. Front Bioeng
Biotechnol. 13:15637012025. View Article : Google Scholar : PubMed/NCBI
|
|
156
|
Ge M, Ding Y, Hu T, Chen Y, Shahin V, Li
B, Huang T, Qian Y, Zhou Z, Tao Y, et al: Nanomedicine-enabled
next-generation therapeutics for spinal cord injury. Mater Today.
86:522–547. 2025. View Article : Google Scholar
|
|
157
|
Buchke S, Sharma M, Bora A, Relekar M,
Bhanu P and Kumar J: Mitochondria-targeted, nanoparticle-based
drug-delivery systems: Therapeutics for mitochondrial disorders.
Life (Basel). 12:6572022.PubMed/NCBI
|
|
158
|
Li M, Yu Y, Xue K, Li J, Son G, Wang J,
Qian W, Wang S, Zheng J, Yang C and Ge J: Genistein mitigates
senescence of bone marrow mesenchymal stem cells via ERRα-mediated
mitochondrial biogenesis and mitophagy in ovariectomized rats.
Redox Biol. 61:1026492023. View Article : Google Scholar
|

















