Cancer presents a considerable challenge in medical
research due to its diversity and breadth. The proposal of cancer
hallmarks attempts to convert the complexity of cancer into a set
of provisional basic principles, which can help researchers
understand the mechanisms underlying cancer development more
comprehensively and logically. Cancer hallmarks are a set of
capabilities acquired by human cells as they progress from a normal
to a proliferative state, and are key to the malignant development
of tumors (1). The tumor
microenvironment (TME) is considered to carry out an important role
in tumorigenesis and malignant progression. The TME is a dynamic
environment that includes all non-cancerous and non-cellular
components of a tumor, such as fibroblasts, immune cells, the
extracellular matrix (ECM) and various secreted factors such as
IL-6 and IFN-γ (2). Among these
components (POSTN), a matricellular protein initially identified in
bone and periodontal ligaments, has emerged as a multifunctional
regulator in both physiological and pathological contexts (3). POSTN was initially discovered in
1993 (4), and numerous
associated studies have since been conducted (5-11). Initially, POSTN was reported to
be expressed minimally or not at all in the vast majority of mature
tissues but, highly expressed in the context of disease states such
as cancer, inflammation and fibrosis (12,13). Additionally, POSTN regulates the
development and maturation of fibrous-rich embryonic tissues, such
as heart valves and other periodontal ligaments (14,15).
In tumors, POSTN is a key player in tumor hallmarks
such as proliferation, metastasis and immune evasion (12). POSTN achieves this through
interactions with integrins and the activation of underlying
downstream signaling pathways (1). Despite growing evidence associating
POSTN with diverse cancer hallmarks, its isoform-specific
functions, regulatory mechanisms within heterogeneous TME
components and therapeutic potential remain incompletely
characterized (16-22). An increasing number of
preclinical and clinical studies have demonstrated the potential of
POSTN in oncogenic mechanisms and as a biomarker (12,23-28). The present review primarily
focuses on the latest research on the functional diversity of POSTN
and its role in the tumor niche, which provides insights into
therapeutic strategies.
Early studies of POSTN date back to 1993, when a
gene was identified that encodes the 811 amino acid residue
osteoblast-specific factor 2, later renamed as POSTN (4,29-31). The human POSTN gene, located on
chromosome 13, spans 36,262 bp, contains 24 exons and encodes a
protein of 836 amino acids (https://www.ncbi.nlm.nih.gov/gene/10631).
Selective splicing is a tightly regulated process
that contributes to the high complexity of the multicellular
eukaryotic transcriptome at the protein level. Various studies have
revealed that in several species, there is alternative splicing of
the POSTN gene in either insertion or deletion patterns (4,32-34), In humans, exons 17, 18, 19 and 21
of the POSTN gene are selectively spliced, resulting in a variety
of isoforms. Moreover, other isoforms of the POSTN gene, including
10 variants of unknown function, have also been discovered in
various types of cancer such as renal cell carcinoma and non-small
cell lung cancer (32,35). Among the physiologically
expressed POSTN isoforms, PN4 (POSTN lacking exons 17 and 21) is
ubiquitously expressed and predominant (34). Several studies have discovered
new isoforms and explored their possible roles (18,36-41). Additionally, POSTN3 containing
exon 17 and 21 (but not containing exon 12) has been shown to
promote the growth and metastasis of breast cancer tumors through
alternative splicing (34).
Similarly, although POSTN is a suppressor in bladder cancer, POSTN
variant I, which is highly expressed in bladder cancer, does not
exhibit a suppressive effect (42).
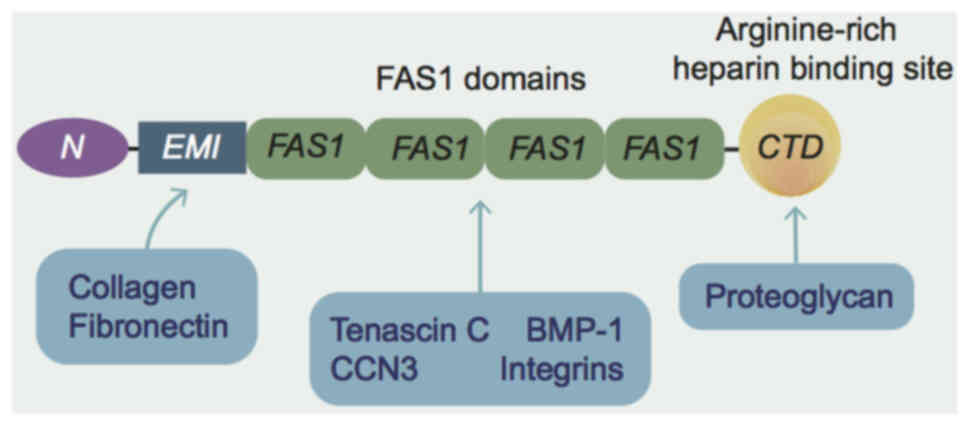
POSTN is a disulfide-linked 90 kDa protein secreted
by osteoblasts and osteoclast-like cell lines (8). The protein consists of six
structural domains, including a cysteine-rich Egf-like module
containing Mucin-like hormone receptor-like structural domain, four
tandemly repeated FAS-1 structural domains and a hydrophilic
C-terminal structural domain (4)
(Fig. 1). The four-repeat
fasciclin structural domain has homology to insect proteins, based
on which the Fas1 structural domain characterizes it as a member of
the fasciclin 1 family. The FAS-1 structural domain has been shown
to interact with fibronectin (43), tenascin-C (44), bone morphogenetic protein-1
(45) and Cellular Communication
Network factor 3 (46). As an
ECM protein, POSTN relies largely on the FAS-1 structural domain to
bind to integrins and thus mediate various signaling pathways
(47). The C-terminal structural
domain, conversely, lacks known functional domains and is
intrinsically disordered (48).
POSTN is a matrix protein that is named after its
preferential expression in mouse periosteum and periodontal
ligaments. The expression of POSTN in these tissues is regulated by
mechanical stresses (49).
Physiologically expressed in collagen-rich connective tissues (such
as, cardiac valves, tendons and lungs), POSTN becomes active during
fetal development but is predominantly absent or minimally
expressed in the majority of adult tissues. It regulates tissue
maturation in embryonic structures such as the periosteum,
periodontal ligaments, liver, kidneys and ureters (14,50). Notably, POSTN drives fetal
cardiomyocyte lineage differentiation and heart valve maturation
(15), positioning it as a
potential biomarker for prenatal non-invasive screening of
congenital heart defects. Additionally, elevated POSTN expression
occurs in stem cell niches of adult organs, including the mammary
gland, intestine, spleen, bone and skin, suggesting
context-dependent roles in tissue homeostasis.
POSTN and its variants are differentially expressed
and are upregulated in inflammation, sites of injury and tumors
(18,51,52). POSTN contributes to pathological
processes such as allergy, inflammation, tissue remodeling,
regeneration, fibrosis and tumor progression. For example, POSTN is
a valuable biomarker of type 2 inflammation (a specific type of
immune response that is primarily mediated by type 2 helper T
cells)as a downstream molecule of IL-4 and IL-13, which has been
closely associated with the pathogenesis of asthma (53), chronic rhinosinusitis (54), allergic dermatitis (55) and pulmonary fibrosis (56). In addition, POSTN is also
involved in the development of myocardial infarction through its
response to TGF-β in inflammation and fibrosis in chronic liver and
kidney disease (57), as well as
through its interaction with other ECM proteins (for example,
fibronectin, heparin and Tenascin-C) (58).
POSTN is expressed in several primary types of
cancer, including breast, bladder, colon, pancreatic and ovarian
cancer, as well as non-small cell lung and oral cancer (13,59) (Table I). For example, POSTN is
expressed at low levels in normal breast tissue, but high
expression of POSTN is often detected in breast cancer, with the
average expression level of the periosteal protein being 20-fold
higher compared with the baseline expression level defined by gene
array data for normal breast tissue (60). POSTN is also abnormally elevated
in metastatic cancer types, most notably liver metastases from
colorectal cancer, which has been associated with a supportive
fibrotic microenvironment promoted by both POSTN and TGF-β1
(61). However, the upregulation
of POSTN expression in tumors is not universal. For example, POSTN
is not expressed in hematologic malignancies such as leukemia and
myeloma (62).
Cancer development is a multistep process initiated
by oncogenic mutations that confer clonal advantage, ultimately
driven by genomic instability (63). This progression is marked by
acquired hallmarks including sustained proliferation, evasion of
growth suppression, resistance to apoptosis, replicative
immortality, angiogenesis, invasion/metastasis, metabolic
reprogramming and immune escape. Emerging features such as
phenotypic plasticity, epigenetic dysregulation, microbiome
interactions and senescence further define tumorigenesis (1). Notably, POSTN overexpression and
its alternatively spliced isoforms (such as, in renal, lung, breast
and gastrointestinal cancer) regulate multiple oncogenic processes
(13,40,64).
Cell proliferation is a process regulated by the
synergistic action of mitogenic growth-promoting and
antiproliferative signals (65).
POSTN has been shown to induce proliferation in a variety of
tumors. For example, POSTN can interact with the classical Wnt
signaling pathway to promote colorectal tumorigenesis (66) and proliferation in neuroblastoma
(67). POSTN from tumor stromal
fibroblasts promotes colorectal carcinogenesis by activating
YAP/TAZ through the Integrin-focal adhesion kinase (FAK)-Src
signaling pathway (19). POSTN
also promotes gastric cancer proliferation studies revealed that
POSTN enhances the proliferation of OCUM-2MLN and OCUM-12 diffuse
gastric cancer cell lines in vitro through activation of
ERK, intuitively, tumor growth in POSTN (−/−) mice is markedly
slower compared with that in wild-type mice (68). POSTN-promoted proliferation of
tumor cells is also exemplified in carcinomas such as the A549 lung
carcinoma cell line (69),
triple-negative breast carcinoma (17) and melanoma (70).
Besides promoting cell proliferation, POSTN also
carries out a key role in activating invasion and metastasis. Tumor
invasion and metastasis are an inefficient process requiring the
overcoming of several rate-limiting steps, the occurrence of which
often portends malignancy and a poor prognosis (71). Due to the heterogeneity of tumor
cells, tumor invasion and metastasis are difficult to treat and
remain one of the major challenges in the field of tumor therapy
(72).
Epithelial-mesenchymal transition (EMT), initially
identified during embryogenesis, is now recognized as a key driver
of developmental processes, disease progression and tumor invasion
(73). POSTN is involved in
tumor invasion and metastasis by regulating EMT, the classical way
of which is that POSTN binds to integrins and activates Akt/PKB and
FAK-mediated signaling pathways (13). Studies demonstrate that stromal
POSTN expression in ovarian cancer promotes tumor invasion and
metastasis via PI3K/Akt pathway activation, mechanistically driving
EMT (27,74,75). POSTN activates STAT3 signaling to
modulate EMT markers such as caveolin-1 and angiogenesis-related
genes such as HIF-1α and vascular endothelial growth factor-A
(VEGF-A), with its knockdown shown to reverse glioblastoma stem
cell (GSC) resistance to anti-VEGF-A therapy (76). POSTN has also been demonstrated
to induce EMT in hepatoblastoma (77) and prostate cancer (78). Moreover, POSTN can inhibit
specific targeted microRNAs (miRNAs) involved in the regulation of
EMT. miRNAs are small endogenous RNAs that post-transcriptionally
regulate gene expression and may target up to one-third of human
mRNAs (79). Song et al
(80) found that the POSTN gene
was markedly upregulated in the gastric cancer cell line SGC-7901
treated with miR-148b mimic, which suggests the possible
involvement of POSTN in the pathways related to the development and
progression of gastric cancer. In lung cancer, POSTN promotes EMT
through the MAPK/miR-381 axis (81).
POSTN promotes cell motility to promote tumor
metastasis. It was demonstrated that secretion of POSTN by von
Hippel-Lindau oncogene non-expressing (VHL-) cells enhanced the
motility of VHL-WT cells and promoted vascular escape in clear cell
renal cell carcinoma tumor cells (82). In a study on colorectal cancer,
POSTN was found to activate FAK and AKT or STAT3, accelerating
metastasis and activating migration of tumor and stromal cells
through stromal remodeling capacity (83). These studies suggest that POSTN
carries out an important role in the development of the TME and
that increased expression of POSTN in the stroma is somewhat
indicative of tumor stage and prognosis (84).
Interestingly, POSTN largely promotes tumor-invasive
metastasis, yet it is regarded as a tumor suppressor in bladder
cancer (85). This may be
associated with the selective sharing of POSTN. Of the two splicing
variations of POSTN (variants I and II) isolated from bladder
cancer, Variant II has key domains that interact with matrix
proteins such as integrins, inhibiting cancer cell invasion and
metastasis. While Variant I, lacking certain exons, has a changed
structure that weakens these interactions, losing its inhibitory
function and potentially promoting cancer progression (42).
Tumor angiogenesis enables nutrient and oxygen
delivery to growing malignancies, with aberrant vascular features,
such as disorganized architecture, sinusoidal vessel formation and
functional heterogeneity, distinguishing tumor vasculature from
normal tissues (86). VEGF is a
central driver of angiogenesis, which binds tyrosine kinase
receptors Flt-1 and Flk-1/KDR to initiate signaling. POSTN has been
identified as a pro-angiogenic factor that facilitates tumor
neovascularization via 'vascular co-option', exploiting
pre-existing vessels in adjacent healthy tissues (87). When the tumor volume is >2
mm3, increased expression of POSTN enhances the
resistance of cancer cells to hypoxic conditions (88). For instance, in colorectal
cancer, POSTN activates the Akt/PKB pathway to increase endothelial
cell survival and vascular proliferation, correlating with
metastasis and serving as a prognostic marker (89). Similarly, ovarian cancer studies
reveal that POSTN overexpression drives angiogenesis and metastatic
spread (11,90-92). Mechanistically, POSTN upregulates
Flk-1/KDR expression in endothelial cells via integrin αvβ3-FAK
signaling, a pathway associated with breast cancer progression
(22,60).
POSTN also promotes lymphangiogenesis, a key step in
metastatic dissemination. Gillot et al (93) demonstrated that POSTN enhances
VEGF-C-mediated lymphangiogenesis and primes lymph nodes for
metastatic colonization. In head and neck squamous cell carcinoma
(HNSCC), POSTN drives lymphatic remodeling through dual mechanisms:
Indirect upregulation of VEGF-C mRNA and direct activation of
Src/Akt pathways. This aligns with observed associations between
POSTN-overexpressing xenografts and lymphatic involvement in HNSCC
(94). Collectively, POSTN
orchestrates tumor vascularization and lymphatic network expansion,
fostering metastatic efficiency.
Apoptosis is a regulated form of programmed cell
death, which carries out dual roles in tumor biology. Its
dysregulation contributes to tumor progression, therapy resistance
and metabolic reprogramming by enabling uncontrolled proliferation
or insufficient apoptosis (95).
Apoptosis also interacts with tumor metabolism to collectively
drive malignancy (96). Notably,
apoptosis can paradoxically support tumor survival through
microenvironmental interactions (97). Emerging concepts such as
pan-apoptosis (combined apoptosis-necroptosis) further highlight
the complexity of apoptosis regulation in cancer immunity (98).
ECM protein POSTN enhances tumor cell survival under
stress conditions (hypoxia, nutrient deprivation). Mechanistically,
POSTN activates β4 integrin/PI3K and Akt/PKB pathways to suppress
apoptosis in pancreatic, colorectal and non-small cell lung cancer
(88,89,99). In hypoxic pancreatic tumors,
POSTN drives a pathological cycle: Hypoxia induces stellate cells
to secrete POSTN, which sustains fibrosis and cellular viability
while exacerbating hypoxia (100). This ECM-mediated adaptation
enables tumor persistence across multiple cancer types by
counteracting environmental stressors and death signals.
Immunomodulation carries out an important role in
tumor development and signaling between POSTN and immune cells can
take place, thus carrying out a role in the immune escape of
tumors. A study noted that GSCs in glioblastoma multiforme (GBM)
secrete POSTN, which can recruit M2-type tumor-associated
macrophages (TAMs) to support GBM growth (101). Similarly, POSTN can promote
ovarian cancer metastasis by enhancing M2 macrophages and
cancer-associated fibroblasts (CAFs) through integrin-mediated
NF-κB and TGF-β2 signaling (74). Wang et al (102) discovered that POSTN+ CAFs can
recruit secreted phosphoprotein 1 (SPP1)+ macrophages and trigger
an increase in SPP1 expression via the IL-6/STAT3 signaling
pathway, thereby enhancing therapeutic resistance in hepatocellular
carcinoma (HCC). In addition, a recent study have shown that
thyroid tumor cells and CAFs can promote tumor development through
crosstalk between IL-4 and POSTN (103). The aforementioned studies may
suggest new targets for cancer inhibition.
Cell senescence is a stress-induced stable growth
arrest that has been regarded as a passive bystander in the
cessation of proliferation. However, research over the past decade
suggests that senescence also serves as the foundation for tissue
development and function (104-107). For tumors, cellular senescence
has two opposing functions. Firstly, senescence halts the cell
cycle, thereby preventing the formation of malignant tumor cells
and promoting tissue regeneration and repair (108). Secondly, senescent cells
present in the TME can induce persistent inflammatory states and
reprogram the TME (109)
through the production of the senescence-associated secretory
phenotype, thereby promoting tumor growth, metastasis and drug
resistance (110). There is
currently evidence suggesting an association between POSTN and
cellular senescence, warranting further investigation. Zhu et
al (111) experiments
indicate that POSTN may promote nucleus pulposus cell senescence
and ECM metabolism by activating the NF-κB and Wnt/β-catenin
signaling pathways, carrying out a notable role in the progression
of disc degeneration. Overexpression of POSTN simultaneously leads
to fibrosis and senescence of cardiomyocytes (112). Similarly, POSTN has been
revealed to promote kidney aging by participating in fibrosis and
lipid metabolism (113). Huang
et al (114) used
RNA-Sequencing technology to examine gene expression changes in
primary skin fibroblasts from healthy controls and patients with
pyrroloquinoline quinone reductase 1 (PYCR1) mutations, and found
that POSTN was one of the most downregulated candidate genes. To
the best of our knowledge, although no published articles have yet
described how POSTN influences cancer development through aging,
the aforementioned discussions provide potential avenues for future
research in this direction.
Epigenetic and metabolic reprogramming are closely
related and mutually regulate each other, driving immune escape or
hindering immune surveillance in certain cases, and carrying out an
important role in tumor progression (115). CAFs are recognized as key
components of the TME, which are stable at the genomic level but
differ considerably from normal stromal precursors (116). The transformation of normal
fibroblasts into CAFs involves extensive methylation changes, which
can be induced by various factors such as TGFβ and pro-inflammatory
cytokines such as leukemia inhibitory factor (117,118). The epigenetic modifications in
CAFs not only activate and maintain their pro-invasive phenotype
(118) but also enhances
glycolysis in tumor metabolism. Becker et al (119) demonstrated that the epigenetic
reprogramming of CAFs disrupts glucose metabolism and promotes
breast cancer progression. Additionally, studies have elucidated
the promotional role of the epigenetics of CAFs in prostate cancer
(120) and bladder urothelial
carcinoma (121). POSTN carries
out an important role as a marker of CAFs in this process. Han
et al (122) reported
that POSTN is one of the DNA methylation biomarkers of
nasopharyngeal carcinoma and patients with high POSTN expression
have shorter overall survival times. The methylation of POSTN is
also associated with the molecular mechanisms of important pathways
associated with glioblastoma formation and patient overall survival
(123).
POSTN is a multifunctional, pleiotropic molecule in
cancer, with its effects exhibiting high context-dependence. It
serves as a potent 'oncogenic factor' while also potentially
assuming the complex role of a 'potential collaborator' under
specific conditions, with the underlying core being the dynamic
changes in TME (124,125). First, cancer of different
tissue origins and molecular subtypes exhibit considerable
differences in their TME. POSTN can be secreted by various cell
types (such as, tumor cells and CAFs) and its effects may depend on
the source cells as well as the specific receptors and downstream
signaling pathways with which it interacts. POSTN typically
promotes immune suppression; however, in certain contexts (such as
specific immunotherapy combinations), the immune cells it recruits
may unexpectedly alter the balance of the TME, potentially
producing effects favorable to therapeutic response. This is not a
direct 'beneficial' effect but an indirect outcome of specific
contextual factors (126).
Additionally, different splice variants of POSTN can have opposed
effects, as demonstrated in bladder cancer (85).
Cancer is driven by the accumulation of mutations in
cancerous cells but is not exclusively genetically determined. The
majority of cancer types (90-95%) occur in close association with
the environmental exposures and lifestyle choices of an individual,
while genetic factors account for only a small proportion (5-10%)
of all cancer cases (127).
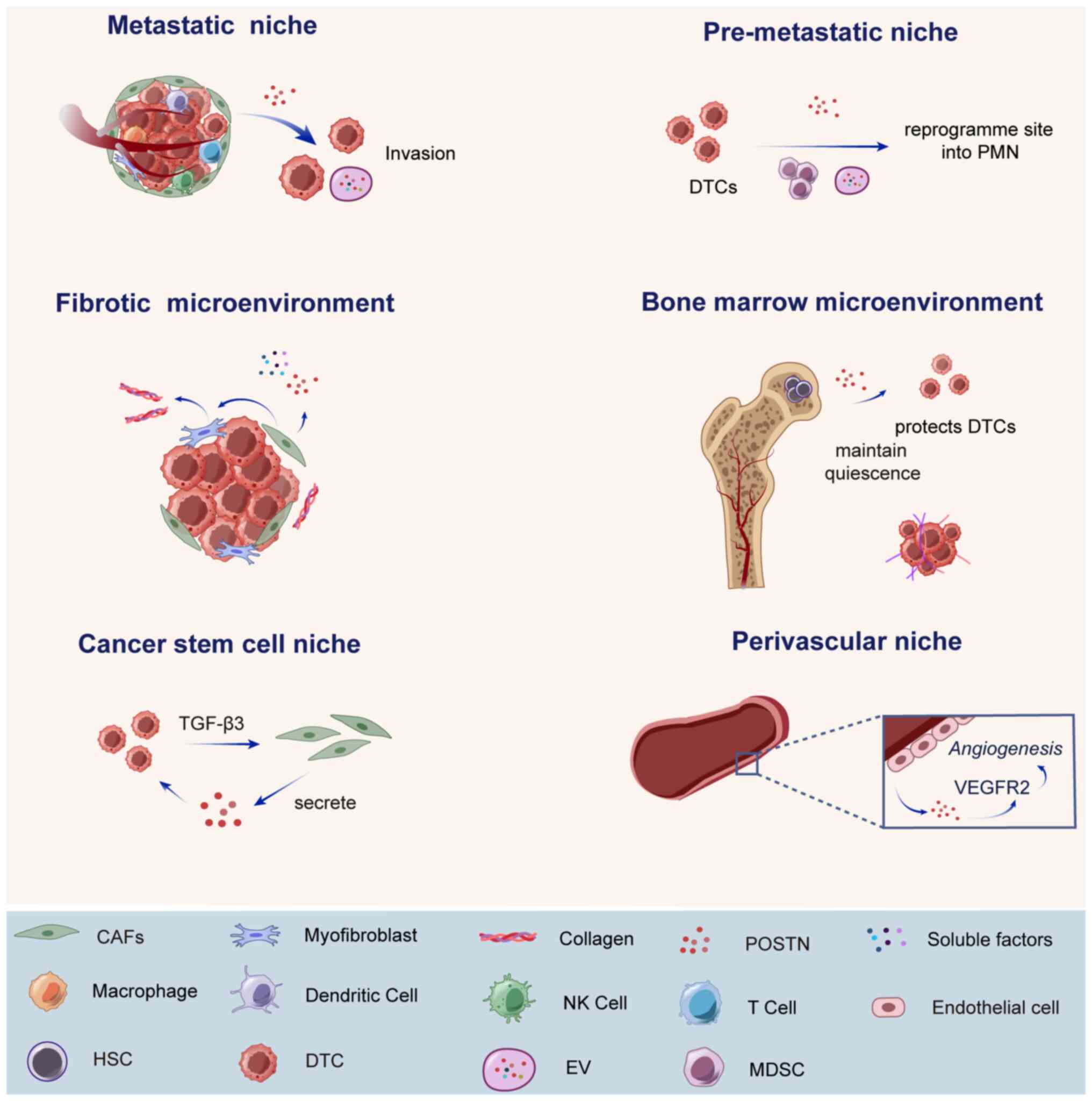
TME refers to all the non-cancerous cells and
non-cellular components in a tumor, which can be regarded as a
special ecological niche where tumor cells are located. The
cellular components include fibroblasts, adipocytes, endothelial
cells, infiltrating immune cells such as lymphocytes and dendritic
cells; while the non-cellular components include ECM and soluble
components such as hormones, growth factors or cytokines (12). These components were previously
considered 'innocent bystanders', but several studies have revealed
that constant, reciprocal and dynamic interactions between tumor
cells and the TME (2,128) are key in the cancer process
(129) (Fig. 2). Current evidence suggests that
POSTN activates key downstream signaling pathways by interacting
with its receptor integrin, remodels the ECM by interacting with
other ECM proteins [such as type I collagen, fibronectin, tenascin
C and thrombospondin 1 (TSP-10)] (44) and remodels TME by cross-talking
with other signaling molecules and recruiting inflammatory and
immune cells (3).
Tumor metastasis is an inefficient process where the
majority of disseminated cells die, with only rare cells
successfully colonizing distant sites. Emerging evidence implicates
cancer stem cells (CSCs) as key drivers of metastasis, facilitated
by interactions with the metastatic niche (130-133). Stromal fibroblast-derived
POSTN, a component of normal stem cell niches, mediates CSC-niche
crosstalk, as demonstrated by Malanchi et al (134). In metastatic tissues, POSTN is
primarily secreted by CAFs, which are activated by paracrine or
autocrine signals to coordinate metastasis through direct
interactions with tumor cells and stromal components (135). For example, in colorectal
cancer, POSTN overexpression in hepatic metastatic niches promotes
tumor cell survival, angiogenesis and metastasis via the Akt/PKB
pathway (89). Similarly,
cervical squamous cell carcinoma studies reveal that POSTN+ CAFs
disrupt lymphatic endothelial barriers by activating
integrin-FAK/Src-VE-calmodulin signaling, facilitating
dissemination (136-138). In ovarian cancer, POSTN drives
metastasis through autocrine NF-κB/TGF-β2 activation in tumor cells
and by recruiting M2 macrophages and CAFs. Stromal POSTN also
enhances metastatic infiltration in skin and head-neck squamous
carcinomas (74). Furthermore,
POSTN can synergize with TNC, for example, POSTN may aggregate Wnt
for stem cells, while TNC may enhance the response of these cells
to Wnt and Notch (139) are
'two sides of the same metastatic ecological niche'.
CSCs or tumor-initiating cells, drive tumor
heterogeneity through self-renewal, clonal initiation and long-term
repopulation capabilities. The CSC niche preserves these
properties, shields CSCs from immune surveillance and facilitates
metastasis (132). As
highlighted by Malanchi et al (134), CD90+CD24+ breast CSCs from
primary tumors metastasize to the lungs and activate CAFs to
secrete POSTN. This stromal POSTN recruits Wnt ligands into CSCs,
amplifying Wnt signaling to fuel CSC expansion and metastatic
colonization. In basal-like breast carcinoma, POSTN maintains CSC
traits and mesenchymal phenotypes by regulating NF-κB-dependent
cytokines (IL4 and IL8) via ERK signaling, thereby promoting
metastasis (140). Similarly,
ovarian cancer studies show that POSTN enhances CSC stemness,
increasing invasiveness, therapy resistance and recurrence
(20,27,74,90,141). In colon cancer, high Wnt
activity defines CSC stemness (142) and POSTN interacts with Wnt
ligands (Wnt1/Wnt3A) in colon CSCs, suggesting its role in
reinforcing stemness (143).
Further supporting this paradigm, Chen et al (144) identified a POSTN/TGF-β1
positive feedback loop in HCC. Through analysis of 110 HCC patient
tissues and xenograft models, this study demonstrated that
POSTN/TGF-β1 activates AP-2α transcription to sustain HCC CSC
stemness and accelerate malignancy.
Perivascular niche is key for maintaining both
hematopoietic stem cells and CSCs, serving as a dynamic hub in
cancer evolution (83). This
niche context-dependently regulates tumor dormancy, metastatic
spread, stemness and immune evasion, shaping tumor progression
(145). Tumor cells exploit
this niche to invade blood vessels (extravasation), a process
involving endothelial cell contractility (via myosin) and
mechanical interactions with the subendothelial matrix (146). However, disseminated tumor
cells (DTCs) often face suppression in secondary organs, with only
a subset surviving or entering dormancy. DTC 'awakening' represents
a key rate-limiting step for metastasis. Stable vasculature
suppresses proliferation through stromal TSP-1, while sprouting neo
vessels exhibit pro-metastatic properties: Their endothelial cells
upregulate POSTN, tenascin-C, fibronectin and TGF-β1, creating a
microenvironment that drives tumor reactivation (3,147-149).
The perivascular niche also modulates immunity by
facilitating immune cell infiltration. Perivascular TAMs promote
angiogenesis, immunosuppression and tumor survival (150). POSTN recruits TAMs to remodel
this niche, as seen in GBM, where GSCs secrete POSTN to attract and
polarize TAMs toward the pro-tumor M2 subtype. This POSTN-mediated
crosstalk sustains GSC-TAM clustering in perivascular regions,
fostering therapy resistance and recurrence (101,151,152). These dual roles, structural
support for metastasis and immunomodulation, highlight the
perivascular niche as a promising therapeutic target.
PMN, consisting of distinct resident cell types, ECM
components and infiltrating cell populations, is a microenvironment
induced by tumors in distant tissues that can provide a conducive
environment for the proliferation of incoming tumor cells (153). It is currently considered that
distant sites can be reprogrammed into the PMN by extracellular
vesicles (EVs) carrying tumor-secreted factors. Potential
mechanisms include enhanced angiogenesis and vascular remodeling,
stromal remodeling, inhibitory effects of EVs on immune cells and
alteration of the metabolic milieu. Formation of PMN is an
integrative process that extends from the local to the systemic
level (146,154,155).
POSTN can be induced by tumor products that reach
distant tissues first and participate in pre-metastatic ecological
niche formation (134). A study
using lung fibroblasts isolated from the lung tissues of C5BL/6 J
mice revealed that CAF EVs induced pre-metastatic ecotone formation
in the SACC lungs of mice and demonstrated more robust matrix
remodeling than SACC EVs. This may be due to POSTN activation of
the TGF-β signaling pathway in lung fibroblasts (156). Previous studies reveal
multifaceted roles of POSTN in pre-metastatic niche formation:
Tumor-derived EVs deliver lncRNA MALAT1 to mediate M2 macrophage
polarization via the POSTN/Hippo/YAP axis in triple-negative breast
cancer (157), while colorectal
cancer EVs carrying ITGBL1 reprogram fibroblasts to prime
metastatic sites (158). POSTN
facilitates immunosuppression by engaging myeloid-derived
suppressor cells in tumor immune evasion (159) and promotes niche establishment
through trauma-induced secretion in melanoma (160). Exosome-mediated POSTN transfer
modifies distant tissue microenvironments (99), with elevated exosomal POSTN
levels in thyroid cancer patients directly accelerating progression
via niche remodeling (161).
Formation of a fibrotic microenvironment is
associated with a series of events, including tissue injury,
inflammation, myofibroblast differentiation and ultimately the
excessive deposition of ECM components. The ECM comprises fibrous
proteins and glycoproteins, forming a dynamic 3D structure that
regulates cell adhesion, migration, tissue repair and disease
processes through mechanotransduction (162,163). POSTN promotes fibrosis in
pathological and cancer contexts, by interacting with soluble
factors to induce myofibroblast differentiation (61,164). Activated myofibroblasts secrete
a large amount of ECM components, primarily collagen, elastin and
glycosaminoglycans (165).
The formation of fibrotic niches is key for tumor
metastasis and the growth of tumors characterized by
fibroproliferation. POSTN is one of the markers of fibrotic niches
and it primarily functions through interactions with key signaling
pathways such as TGF-β1 (61)
and Wnt/β-catenin (166).
Elevated POSTN levels in melanoma lung metastases associate with
bleomycin-induced pulmonary fibrosis progression (167). In uterine fibroids, POSTN acts
as both a biomarker and a therapeutic target by stimulating
collagen overproduction and myometrial cell transformation
(168,169). Additionally,
metastasis-associated macrophages activate hepatic stellate cells
via granulin secretion, generating POSTN-producing myofibroblasts
that remodel liver stroma for tumor growth (170).
Bone is a frequent metastatic site for solid tumors
(for example, breast, prostate and lung cancer) (171,172). Bone marrow, a semi-solid tissue
within bone cavities, facilitates hematopoiesis via hematopoietic
stem cells and comprises cellular components such as mesenchymal
stromal cells, osteoblasts and immune cells, as well as
non-cellular elements including cytokines and growth factors
(173,174). This microenvironment protects
DTCs during colonization, dormancy and growth, and contributes to
hematologic malignancies such as leukemia and myeloma (175,176).
POSTN is associated with bone metastasis. In breast
cancer, BMSC-derived POSTN promotes bone metastasis, with murine
models showing an 8-fold POSTN increase at metastasis sites
compared with controls (177,178). Contrastingly, C-terminal intact
periosteal protein (iPTN) levels decrease in breast cancer bone
metastases due to histone K cleavage, suggesting iPTN as a
potential biomarker for bone recurrence detection (179). POSTN also drives HNSCC
progression via BMSC-induced PI3K/Akt/mTOR pathway activation
(180). In multiple myeloma,
elevated POSTN levels are associated with advanced disease
severity, such as International Staging System stage 3 (181) and with dysregulation of bone
metabolism, including an inverse relationship with bone formation
markers and a positive correlation with bone resorption markers
(182). Additionally, POSTN
facilitates prostate cancer bone metastasis via integrin-mediated
interactions with osteoblasts and tumor cells (183) and supports B-cell Acute
Lymphoblastic Leukemia cell proliferation in the bone marrow niche
(184).
Although extensive basic research has confirmed
that POSTN carries a central role in various pathological processes
(185-187), its successful translation from
a basic molecular marker to a clinically useful tool remains
challenging. Currently, the practical application value of POSTN as
a clinical biomarker is still under investigation, and there
remains a notable gap between its potential applications and
clinical practice.
Since the pathological expression levels of POSTN
are markedly higher when compared with normal expression levels
(64,188), POSTN has potential as a
diagnostic and prognostic biomarker for diseases. Since the
upregulation of POSTN is not specific to any particular disease,
this may limit its application as a standalone diagnostic
biomarker. Currently, research is focused on combining POSTN with
other biomarkers to develop more precise diagnostic and prognostic
prediction models. A study demonstrated that the combination of
plasma POSTN and CA19.9 considerably improved the sensitivity,
specificity and AUC for diagnosing cholangiocarcinoma compared with
using a single marker, reaching 87 and 91%, and 0.94, respectively
(plasma POSTN: 78 and 85%, and 0.86; CA19.9: 67 and 90%, and 0.86)
(189). Jia et al
(25) demonstrated that, to
distinguish healthy controls from locally advanced BCa, POSTN
exhibited the highest AUC (AUCPOSTN=0.72, AUCCA153=0.57 and
AUCCEA=0.62), and the AUC of CA153 and CEA were markedly improved
when used in combination with POSTN. Furthermore, multiple studies
have demonstrated that elevated serum POSTN levels are associated
with worse overall survival and progression-free survival (7,190-193). In summary, the potential role
of POSTN as a diagnostic and prognostic biomarker holds clinical
value.
With the advent of the era of precision medicine,
accurate identification of subtypes has become increasingly
important. In various types of tumors, the 'POSTN+' subtype is
typically associated with features such as stromal proliferation,
EM and TM characteristics (17,102,126). Patients with this subtype often
exhibit greater invasiveness, increased metastasis risk and worse
response rates to conventional chemotherapy. By contrast, the
'POSTN-' subtype is associated with relatively improved prognosis.
Zhang et al (141)
revealed that the stroma markedly contributes to the molecular
classification of the mesenchymal subtype in high-grade serous
ovarian cancer, with POSTN being one of the genes predominantly
expressed in the stroma. In osteosarcoma, POSTN-positive expression
is notably associated with histological subtype (P=0.000), Enneking
staging (P=0.027) and tumor size (P=0.009) (194).
In invasive basal cell carcinoma (BBC), the two
upregulated genes POSTN and WISP1 were validated at the protein
level as being associated with invasive BCC (195). This subtype classification lays
the foundation for developing precision strategies. A molecular
typing kit based on POSTN expression levels could be developed to
identify high-risk patient groups, guiding more intensive follow-up
and early intervention. In terms of treatment, it provides a basis
for achieving stratified therapy. Patients with high POSTN
expression in this subtype may be the optimal candidate population
for targeted therapies targeting the POSTN pathway itself or its
downstream effector molecules or for immunotherapies targeting the
immunosuppressive microenvironment.
Additionally, new CAF subtypes can be defined based
on POSTN expression levels. Wang et al (102) recently found that high
infiltration of POSTN+ CAFs and SPP1+ macrophages is associated
with resistance to immunotherapy. For example, a study identified
five CAF subtypes in gastric cancer, revealing that the CAF_0
subtype was highly associated with poor prognosis, with POSTN being
the most notable marker gene for this subtype (196). These findings suggest that
targeting specific CAF subpopulations may have potential benefits
for improving clinical responses to immunotherapy.
Targeted therapy for POSTN has progressed from the
proof-of-concept stage to practical application. In preclinical
studies, various targeted strategies have shown great promise. For
example, PNX-001 (a humanized anti-POSTN monoclonal antibody)
effectively blocks the interaction between POSTN and integrin in
preclinical models, inhibits tumor growth and metastasis, and
enhances the delivery of chemotherapy drugs (197). Another neutralizing monoclonal
antibody targeting POSTN (MZ-1) has been shown to have a
considerable growth-inhibitory effect on a subgroup of invasive
ovarian tumors that overexpress POSTN (198). PNDA-3 (human osteopontin-3) is
a DNA aptamer that selectively binds to the FAS-1 domain of POSTN,
disrupting its interaction with its cell surface receptors αvβ3 and
αvβ5 integrins, thereby markedly reducing primary tumor growth and
distant metastasis (199,200).
Due to the increasing number of POSTN-related
diseases, several clinical trials are currently underway. However,
therapies targeting POSTN in the field of oncology remain at a
relatively early stage, with very limited publicly available
clinical data. Key future challenges include: Determining the
optimal treatment window, selecting appropriate biomarkers to
screen for patient populations likely to respond and exploring
combination strategies with other therapies (such as immune
checkpoint inhibitors, anti-angiogenic drugs) to overcome the
complexity of the TME.
Despite considerable progress in POSTN research,
there remain notable knowledge gaps and technical limitations in
this field, which hinder its clinical translation process. First,
there are conflicting data in the existing literature. In certain
types of cancer, high POSTN expression is associated with poor
prognosis [such as, breast cancer (13,128,130,139), glioblastoma (101,123), ovarian cancer (20,27,74,91,141) and liver cancer (13,62,71,89,193), but in other types of cancer
(201,202), such as estrogen
receptor-negative (ER-) or progesterone receptor-negative (PR-)
phenotypes of breast cancer (203), its expression levels show only
a modest association with clinical outcomes (203-205). These inconsistencies may stem
from methodological heterogeneity, differences in patient cohorts
and variations in the cellular origin and tissue localization of
POSTN within the TME. Typically, POSTN is present in the ECM, while
the main presence of POSTN in tumor tissues is in the cancer matrix
(206). Additionally, current
detection methods struggle to distinguish between different POSTN
splice variants, which may possess entirely different or even
opposing biological functions, potentially contributing to the data
inconsistencies (34). Current
research also faces notable technical limitations. The majority of
studies rely on static measurements from tissue biopsies, which
cannot capture the dynamic changes in POSTN expression or its
real-time functional role in treatment response and disease
progression. More importantly, complete inhibition of POSTN may
interfere with important physiological functions, leading to side
effects such as impaired wound healing, abnormal bone fibrosis or
cardiac dysfunction (64).
Although the role of periosteal proteins in promoting metastasis
and chemotherapy resistance has been well established, it remains
unclear how targeting periosteal proteins interacts with existing
treatment modalities, such as chemotherapy, immunotherapy or
radiotherapy, within the TME.
To overcome these challenges and drive further
development in this field, future research should establish unified
POSTN detection standards and conduct large-scale, multicenter
prospective cohort studies to systematically assess the predictive
and prognostic value of POSTN in different disease stages and
treatment settings. Additionally, novel dynamic imaging
technologies, such as molecular imaging probes capable of
non-invasively and real-time monitoring of POSTN expression and
functional activity within the body, should be actively developed.
In terms of treatment strategies, the focus should be on precision
interventions, including the design of inhibitors that can
specifically block pathological protein interactions (such as
integrin binding) without affecting physiological functions, the
development of drugs targeting disease-specific isoforms and the
exploration of prodrug strategies based on TME activation to
minimize off-target risks to normal tissues. Finally, any clinical
translation research must comprehensively assess the potential
long-term effects of inhibiting POSTN on the regenerative and
reparative functions of key organs such as bones, skin and the
heart during the preclinical stage to ensure treatment safety. By
adopting these recommendations, future research will be able to
more reliably evaluate the clinical efficacy of POSTN and design
safer, more effective targeted treatment strategies, ultimately
bridging the gap between basic research and clinical
application.
As an important matricellular protein, POSTN
carries out multifaceted roles in both physiological and
pathological processes, including embryonic development, allergic
inflammation, fibrotic diseases and tumorigenesis (12,64). Increasing evidence indicates that
POSTN can regulate various cancer hallmarks by interacting with
integrins and modulating key signaling pathways such as
Wnt/β-catenin, PI3K/Akt and TGF-β (27,49,51,61,74,111,166,168,180,188,207). The present review focuses on
the promoting effects of POSTN on tumor growth,
invasion-metastasis, angiogenesis, anti-apoptosis and immune
evasion. Considering the increasingly recognized role of TME, POSTN
also exerts considerable effects on niche regulation, particularly
in the maintenance of CSCs, remodeling of the ECM and
immune-suppressive reprogramming (102,135,140,144,208).
These findings suggest a promising therapeutic and
prognostic potential for targeting POSTN in tumor management. In
terms of cancer treatment, the main approaches include blocking the
POSTN signaling pathway and using POSTN inhibitors. Studies have
shown that Let-7f reduces angiogenesis mimicry in glioma cells by
inhibiting POSTN (209,210). DNA aptamers targeting POSTN
have been revealed to effectively inhibit the growth and metastasis
of breast and gastric cancer in mouse models (209). POSTN can also be combined with
immune checkpoint inhibitors to enhance the therapeutic efficacy of
colorectal tumors (211).
Moreover, selecting appropriate POSTN isoforms is important. For
instance, POSTN-203 may be a more promising target for glioma
because it is associated with low patient survival and contains
multiple predicted human leukocyte antigen-restricted epitopes
(40). POSTN can also serve as a
prognostic biomarker for gastrointestinal malignancies,
hepatobiliary and pancreatic cancer (212), breast cancer, ovarian cancer
(20) and genitourinary cancer
(59).
Key knowledge gaps still exist, including the
functional heterogeneity of POSTN isoforms, the detailed mechanisms
of its molecular interactions and the potential off-target effects
of POSTN-targeted therapies. In summary, future research should
prioritize addressing these challenges to advance POSTN-based
diagnostics and therapeutics and ultimately improve the clinical
outcomes of cancer and associated pathologies.
Not applicable.
YX have made substantial contributions to the
conception of the present review, wrote the main manuscript text,
prepared Figs. 1 and 2, and reviewed the manuscript. LW made
substantial contributions to the conception of the present review
and reviewed the manuscript. Data authentication not applicable.
All authors read and approved the final manuscript.
Not applicable.
Not applicable.
The authors declare that they have no competing
interests.
Not applicable.
The present work was supported by the Open Research Fund of
Hubei Province Key Laboratory of Precision Radiation Oncology
(grant no. 2024ZLJZFL003) and the Key Laboratory of Anesthesiology
and Resuscitation (Huazhong University of Science and Technology),
Ministry of Education (grant no. 2024MZFS010), which are awarded to
Dr Lufang Wang, Department of Obstetrics and Gynecology, Union
Hospital, Tongji Medical College, Huazhong University of Science
and Technology, Wuhan, Hubei 430022, P.R. China.
|
1
|
Hanahan D: Hallmarks of cancer: New
Dimensions. Cancer Discov. 12:31–46. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Xiao Y and Yu D: Tumor microenvironment as
a therapeutic target in cancer. Pharmacol Ther. 221:1077532021.
View Article : Google Scholar :
|
|
3
|
Liu AY, Zheng H and Ouyang G: Periostin, a
multifunctional matricellular protein in inflammatory and tumor
microenvironments. Matrix Biol. 37:150–156. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Takeshita S, Kikuno R, Tezuka K and Amann
E: Osteoblast-specific factor 2: Cloning of a putative bone
adhesion protein with homology with the insect protein fasciclin I.
Biochem J. 294:271–278. 1993. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Horiuchi K, Amizuka N, Takeshita S,
Takamatsu H, Katsuura M, Ozawa H, Toyama Y, Bonewald LF and Kudo A:
Identification and characterization of a novel protein, periostin,
with restricted expression to periosteum and periodontal ligament
and increased expression by transforming growth factor beta. J Bone
Miner Res. 14:1239–1249. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Sasaki H, Dai M, Auclair D, Fukai I,
Kiriyama M, Yamakawa Y, Fujii Y and Chen LB: Serum level of the
periostin, a homologue of an insect cell adhesion molecule, as a
prognostic marker in nonsmall cell lung carcinomas. Cancer.
92:843–848. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Sasaki H, Dai M, Auclair D, Kaji M, Fukai
I, Kiriyama M, Yamakawa Y, Fujii Y and Chen LB: Serum level of the
periostin, a homologue of an insect cell adhesion molecule, in
thymoma patients. Cancer Lett. 172:37–42. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Borg TK and Markwald R: Periostin: More
than just an adhesion molecule. Circ Res. 101:230–231. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Ruan K, Bao S and Ouyang G: The
multifaceted role of periostin in tumorigenesis. Cell Mol Life Sci.
66:2219–2230. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Kudo A: Periostin in fibrillogenesis for
tissue regeneration: Periostin actions inside and outside the cell.
Cell Mol Life Sci. 68:3201–3207. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Zhu M, Fejzo MS, Anderson L, Dering J,
Ginther C, Ramos L, Gasson JC, Karlan BY and Slamon DJ: Periostin
promotes ovarian cancer angiogenesis and metastasis. Gynecol Oncol.
119:337–344. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
González-González L and Alonso J:
Periostin: A matricellular protein with multiple functions in
cancer development and progression. Front Oncol. 8:2252018.
View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Morra L and Moch H: Periostin expression
and Epithelial-mesenchymal transition in cancer: A review and an
update. Virchows Arch. 459:465–475. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Norris RA, Moreno-Rodriguez RA, Sugi Y,
Hoffman S, Amos J, Hart MM, Potts JD, Goodwin RL and Markwald RR:
Periostin regulates atrioventricular valve maturation. Dev Biol.
316:200–213. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Sorocos K, Kostoulias X, Cullen-McEwen L,
Hart AH, Bertram JF and Caruana G: Expression patterns and roles of
periostin during kidney and ureter development. J Urol.
186:1537–1544. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Sun D, Lu J, Tian H, Li H, Chen X, Hua F,
Yang W, Yu J and Chen D: The impact of POSTN on tumor cell behavior
and the tumor microenvironment in lung adenocarcinoma. Int
Immunopharmacol. 145:1137132025. View Article : Google Scholar
|
|
17
|
Lin S, Zhou M, Cheng L, Shuai Z, Zhao M,
Jie R, Wan Q, Peng F and Ding S: Exploring the association of
POSTN+ cancer-associated fibroblasts with
Triple-negative breast cancer. Int J Biol Macromol. 268:1315602024.
View Article : Google Scholar
|
|
18
|
Kanemoto Y, Sanada F, Shibata K,
Tsunetoshi Y, Katsuragi N, Koibuchi N, Yoshinami T, Yamamoto K,
Morishita R, Taniyama Y and Shimazu K: Expression of periostin
alternative splicing variants in normal tissue and breast cancer.
Biomolecules. 14:10932024. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Ma H, Wang J, Zhao X, Wu T, Huang Z, Chen
D, Liu Y and Ouyang G: Periostin promotes colorectal tumorigenesis
through integrin-FAK-Src Pathway-mediated YAP/TAZ activation. Cell
Rep. 30:793–806.e6. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Kujawa KA, Zembala-Nożyńska E, Cortez AJ,
Kujawa T, Kupryjańczyk J and Lisowska KM: Fibronectin and periostin
as prognostic markers in ovarian cancer. Cells. 9:1492020.
View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Pickup MW, Mouw JK and Weaver VM: The
extracellular matrix modulates the hallmarks of cancer. EMBO Rep.
15:1243–1253. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Försti A, Jin Q, Altieri A, Johansson R,
Wagner K, Enquist K, Grzybowska E, Pamula J, Pekala W, Hallmans G,
et al: Polymorphisms in the KDR and POSTN genes: Association with
breast cancer susceptibility and prognosis. Breast Cancer Res
Treat. 101:83–93. 2007. View Article : Google Scholar
|
|
23
|
Szefler S, Corren J, Silverberg JI,
Okragly A, Sun Z, Natalie CR, Zitnik R, Siu K and Blauvelt A:
Lebrikizumab decreases type 2 inflammatory biomarker levels in
patients with asthma: Data from randomized phase 3 trials (LAVOLTA
I and II). Immunotherapy. 16:1211–1216. 2024. View Article : Google Scholar
|
|
24
|
Gan C, Li M, Lu Y, Peng G, Li W, Wang H,
Peng Y, Hu Q, Wei W, Wang F, et al: SPOCK1 and POSTN are valuable
prognostic biomarkers and correlate with tumor immune infiltrates
in colorectal cancer. BMC Gastroenterol. 23:42023. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Jia L, Li G, Ma N, Zhang A, Zhou Y, Ren L
and Dong D: Soluble POSTN is a novel biomarker complementing CA153
and CEA for breast cancer diagnosis and metastasis prediction. BMC
Cancer. 22:7602022. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Alzobaidi N, Rehman S, Naqvi M, Gulati K
and Ray A: Periostin: A potential biomarker and therapeutic target
in pulmonary diseases. J Pharm Pharm Sci. 25:137–148. 2022.
View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Yue H, Li W, Chen R, Wang J, Lu X and Li
J: Stromal POSTN induced by TGF-β1 facilitates the migration and
invasion of ovarian cancer. Gynecol Oncol. 160:530–538. 2021.
View Article : Google Scholar
|
|
28
|
Olsson L, Hammarström ML, Israelsson A,
Lindmark G and Hammarström S: Allocating colorectal cancer patients
to different risk categories by using a Five-biomarker mRNA
combination in lymph node analysis. PLoS One. 15:e02290072020.
View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Sugiura T, Takamatsu H, Kudo A and Amann
E: Expression and characterization of murine osteoblast-specific
factor 2 (OSF-2) in a baculovirus expression system. Protein Expr
Purif. 6:305–311. 1995. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Ducy P, Geoffroy V and Karsenty G: Study
of Osteoblast-specific expression of one mouse osteocalcin gene:
Characterization of the factor binding to OSE2. Connect Tissue Res.
35:7–14. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Ulstrup JC, Jeansson S, Wiker HG and
Harboe M: Relationship of secretion pattern and MPB70 homology with
osteoblast-specific factor 2 to osteitis following Mycobacterium
bovis BCG vaccination. Infect Immun. 63:672–675. 1995. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Hoersch S and Andrade-Navarro MA:
Periostin shows increased evolutionary plasticity in its
alternatively spliced region. BMC Evol Biol. 10:302010. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Nakama T, Yoshida S, Ishikawa K, Kobayashi
Y, Abe T, Kiyonari H, Shioi G, Katsuragi N, Ishibashi T, Morishita
R and Taniyama Y: Different roles played by periostin splice
variants in retinal neovascularization. Exp Eye Res. 153:133–140.
2016. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Ikeda-Iwabu Y, Taniyama Y, Katsuragi N,
Sanada F, Koibuchi N, Shibata K, Shimazu K, Rakugi H and Morishita
R: Periostin short fragment with Exon 17 via aberrant alternative
splicing is required for breast cancer growth and metastasis.
Cells. 10:8922021. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
De Oliveira Macena Y, Cezar MEN, Lira CBF,
De Oliveira LBDM, Almeida TN, Costa ADAV, De Araujo BMD, de Almeida
D Junior, Dantas HM, De Mélo EC, et al: The roles of periostin
derived from Cancer-associated fibroblasts in tumor progression and
treatment response. Cancer Metastasis Rev. 44:112024. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Morra L, Rechsteiner M, Casagrande S, Duc
Luu V, Santimaria R, Diener PA, Sulser T, Kristiansen G, Schraml P,
Moch H and Soltermann A: Relevance of periostin splice variants in
renal cell carcinoma. Am J Pathol. 179:1513–1521. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Kuebart T, Oezel L, Gürsoy B, Maus U,
Windolf J, Bittersohl B and Grotheer V: Periostin splice variant
expression in human osteoblasts from osteoporotic patients and its
effects on Interleukin-6 and osteoprotegerin. Int J Mol Sci.
26:9322025. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Litvin J, Selim AH, Montgomery MO, Lehmann
K, Rico MC, Devlin H, Bednarik DP and Safadi FF: Expression and
function of Periostin-isoforms in bone. J Cell Biochem.
92:1044–1061. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Viloria K and Hill NJ: Embracing the
complexity of matricellular proteins: The functional and clinical
significance of splice variation. Biomol Concepts. 7:117–132. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Xiong Z, Sneiderman CT, Kuminkoski CR,
Reinheimer J, Schwegman L, Sever RE, Habib A, Hu B, Agnihotri S,
Rajasundaram D, et al: Transcript-targeted antigen mapping reveals
the potential of POSTN splicing junction epitopes in glioblastoma
immunotherapy. Genes Immun. 26:190–199. 2025. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Wenhua S, Tsunematsu T, Umeda M, Tawara H,
Fujiwara N, Mouri Y, Arakaki R, Ishimaru N and Kudo Y: Cancer
Cell-derived novel periostin isoform promotes invasion in head and
neck squamous cell carcinoma. Cancer Med. 12:8510–8525. 2023.
View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Kim CJ, Isono T, Tambe Y, Chano T, Okabe
H, Okada Y and Inoue H: Role of alternative splicing of periostin
in human bladder carcinogenesis. Int J Oncol. 32:161–169. 2008.
|
|
43
|
Kii I, Nishiyama T and Kudo A: Periostin
promotes secretion of fibronectin from the endoplasmic reticulum.
Biochem Biophys Res Commun. 470:888–893. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Kii I and Ito H: Periostin and its
interacting proteins in the construction of extracellular
architectures. Cell Mol Life Sci. 74:4269–4277. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Maruhashi T, Kii I, Saito M and Kudo A:
Interaction between periostin and BMP-1 promotes proteolytic
activation of lysyl oxidase. J Biol Chem. 285:13294–13303. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Takayama I, Tanabe H, Nishiyama T, Ito H,
Amizuka N, Li M, Katsube KI, Kii I and Kudo A: Periostin is
required for matricellular localization of CCN3 in periodontal
ligament of mice. J Cell Commun Signal. 11:5–13. 2017. View Article : Google Scholar :
|
|
47
|
Orecchia P, Conte R, Balza E, Castellani
P, Borsi L, Zardi L, Mingari MC and Carnemolla B: Identification of
a novel cell binding site of periostin involved in tumour growth.
Eur J Cancer. 47:2221–2229. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
48
|
Rusbjerg-Weberskov CE, Johansen ML, Nowak
JS, Otzen DE, Pedersen JS, Enghild JJ and Nielsen NS: Periostin
C-Terminal is intrinsically disordered and interacts with 143
proteins in an in vitro epidermal model of atopic dermatitis.
Biochemistry. 62:2803–2815. 2023. View Article : Google Scholar : PubMed/NCBI
|
|
49
|
Wang Z, Lin M, Pan Y, Liu Y, Yang C, Wu J,
Wang Y, Yan B, Zhou J, Chen R and Liu C: Periostin+ myeloid cells
improved long bone regeneration in a mechanosensitive manner. Bone
Res. 12:592024. View Article : Google Scholar :
|
|
50
|
Norris RA, Borg TK, Butcher JT, Baudino
TA, Banerjee I and Markwald RR: Neonatal and adult cardiovascular
pathophysiological remodeling and repair: Developmental role of
periostin. Ann N Y Acad Sci. 1123:30–40. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
51
|
Yang L, Guo T, Chen Y and Bian K: The
multiple roles of periostin in Non-neoplastic disease. Cells.
12:502022. View Article : Google Scholar
|
|
52
|
Walker JT, McLeod K, Kim S, Conway SJ and
Hamilton DW: Periostin as a multifunctional modulator of the wound
healing response. Cell Tissue Res. 365:453–465. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
53
|
Matsumoto H: Serum periostin: A novel
biomarker for asthma management. Allergol Int. 63:153–160. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
54
|
Danielides G, Lygeros S, Kanakis M and
Naxakis S: Periostin as a biomarker in chronic rhinosinusitis: A
contemporary systematic review. Int Forum Allergy Rhinol.
12:1535–1550. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
55
|
Maintz L, Welchowski T, Herrmann N, Brauer
J, Traidl-Hoffmann C, Havenith R, Müller S, Rhyner C, Dreher A,
Schmid M, et al: IL-13, periostin and dipeptidyl-peptidase-4 reveal
endotype-phenotype associations in atopic dermatitis. Allergy. Jan
17–2023. View Article : Google Scholar : Epub ahead of
print. View Article : Google Scholar
|
|
56
|
Ono J, Takai M, Kamei A, Azuma Y and
Izuhara K: Pathological roles and clinical usefulness of periostin
in type 2 inflammation and pulmonary fibrosis. Biomolecules.
11:10842021. View Article : Google Scholar : PubMed/NCBI
|
|
57
|
Mael-Ainin M, Abed A, Conway SJ, Dussaule
JC and Chatziantoniou C: Inhibition of periostin expression
protects against the development of renal inflammation and
fibrosis. J Am Soc Nephrol. 25:1724–1736. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
58
|
Qiao B, Liu X, Wang B and Wei S: The role
of periostin in cardiac fibrosis. Heart Fail Rev. 29:191–206. 2024.
View Article : Google Scholar
|
|
59
|
Nuzzo PV, Buzzatti G, Ricci F, Rubagotti
A, Argellati F, Zinoli L and Boccardo F: Periostin: A novel
prognostic and therapeutic target for genitourinary cancer? Clin
Genitourin Cancer. 12:301–311. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
60
|
Shao R, Bao S, Bai X, Blanchette C,
Anderson RM, Dang T, Gishizky ML, Marks JR and Wang XF: Acquired
expression of periostin by human breast cancers promotes tumor
angiogenesis through up-regulation of vascular endothelial growth
factor receptor 2 expression. Mol Cell Biol. 24:3992–4003. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
61
|
Liu B, Wu T, Lin B, Liu X, Liu Y, Song G,
Fan C and Ouyang G: Periostin-TGF-β feedforward loop contributes to
tumour-stroma crosstalk in liver metastatic outgrowth of colorectal
cancer. Br J Cancer. 130:358–368. 2024. View Article : Google Scholar
|
|
62
|
Tilman G, Mattiussi M, Brasseur F, van
Baren N and Decottignies A: Human periostin gene expression in
normal tissues, tumors and melanoma: Evidences for periostin
production by both stromal and melanoma cells. Mol Cancer.
6:802007. View Article : Google Scholar : PubMed/NCBI
|
|
63
|
Zhang S, Xiao X, Yi Y, Wang X, Zhu L, Shen
Y, Lin D and Wu C: Tumor initiation and early tumorigenesis:
Molecular mechanisms and interventional targets. Signal Transduct
Target Ther. 9:1492024. View Article : Google Scholar : PubMed/NCBI
|
|
64
|
Dorafshan S, Razmi M, Safaei S, Gentilin
E, Madjd Z and Ghods R: Periostin: Biology and function in cancer.
Cancer Cell Int. 22:3152022. View Article : Google Scholar : PubMed/NCBI
|
|
65
|
Pavlova NN, Zhu J and Thompson CB: The
hallmarks of cancer metabolism: Still emerging. Cell Metab.
34:355–377. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
66
|
Moniuszko T, Wincewicz A, Koda M,
Domysławska I and Sulkowski S: Role of periostin in esophageal,
gastric and colon cancer. Oncol Lett. 12:783–787. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
67
|
Huang M, Fang W, Farrel A, Li L,
Chronopoulos A, Nasholm N, Cheng B, Zheng T, Yoda H, Barata MJ, et
al: ALK upregulates POSTN and WNT signaling to drive neuroblastoma.
Cell Rep. 43:1139272024. View Article : Google Scholar : PubMed/NCBI
|
|
68
|
Kikuchi Y, Kunita A, Iwata C, Komura D,
Nishiyama T, Shimazu K, Takeshita K, Shibahara J, Kii I, Morishita
Y, et al: The niche component periostin is produced by
cancer-associated fibroblasts, supporting growth of gastric cancer
through ERK activation. Am J Pathol. 184:859–870. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
69
|
Hong L, Sun H, Lv X, Yang D, Zhang J and
Shi Y: Expression of periostin in the serum of NSCLC and its
function on proliferation and migration of human lung
adenocarcinoma cell line (A549) in vitro. Mol Biol Rep.
37:2285–2293. 2010. View Article : Google Scholar
|
|
70
|
Kotobuki Y, Yang L, Serada S, Tanemura A,
Yang F, Nomura S, Kudo A, Izuhara K, Murota H, Fujimoto M, et al:
Periostin accelerates human malignant melanoma progression by
modifying the melanoma microenvironment. Pigment Cell Melanoma Res.
27:630–639. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
71
|
Chambers AF, Groom AC and MacDonald IC:
Dissemination and growth of cancer cells in metastatic sites. Nat
Rev Cancer. 2:563–572. 2002. View
Article : Google Scholar : PubMed/NCBI
|
|
72
|
Parker TM, Henriques V, Beltran A,
Nakshatri H and Gogna R: Cell competition and tumor heterogeneity.
Semin Cancer Biol. 63:1–10. 2020. View Article : Google Scholar
|
|
73
|
Huang Y, Hong W and Wei X: The molecular
mechanisms and therapeutic strategies of EMT in tumor progression
and metastasis. J Hematol Oncol. 15:1292022. View Article : Google Scholar : PubMed/NCBI
|
|
74
|
Lin SC, Liao YC, Chen PM, Yang YY, Wang
YH, Tung SL, Chuang CM, Sung YW, Jang TH, Chuang SE, et al:
Periostin promotes ovarian cancer metastasis by enhancing M2
macrophages and cancer-associated fibroblasts via integrin-mediated
NF-κB and TGF-β2 signaling. J Biomed Sci. 29:1092022. View Article : Google Scholar
|
|
75
|
Cheon DJ, Tong Y, Sim MS, Dering J, Berel
D, Cui X, Lester J, Beach JA, Tighiouart M, Walts AE, et al: A
Collagen-remodeling gene signature regulated by TGF-β signaling is
associated with metastasis and poor survival in serous ovarian
cancer. Clin Cancer Res. 20:711–723. 2014. View Article : Google Scholar
|
|
76
|
Park SY, Piao Y, Jeong KJ, Dong J and de
Groot JF: Periostin (POSTN) Regulates Tumor Resistance to
Antiangiogenic Therapy in Glioma Models. Mol Cancer Ther.
15:2187–2197. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
77
|
Chen L, Tian X, Gong W, Sun B, Li G, Liu
D, Guo P, He Y, Chen Z, Xia Y, et al: Periostin mediates
epithelial-mesenchymal transition through the MAPK/ERK pathway in
hepatoblastoma. Cancer Biol Med. 16:89–100. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
78
|
Tian Y, Choi CH, Li QK, Rahmatpanah FB,
Chen X, Kim SR, Veltri R, Chia D, Zhang Z, Mercola D and Zhang H:
Overexpression of periostin in stroma positively associated with
aggressive prostate cancer. PLoS One. 10:e01215022015. View Article : Google Scholar : PubMed/NCBI
|
|
79
|
Pauley KM and Chan EK: MicroRNAs and their
emerging roles in immunology. Ann N Y Acad Sci. 1143:226–239. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
80
|
Song Y, Sun J, Xu Y, Liu J, Gao P, Chen X,
Zhao J and Wang Z: Microarray analysis of long non-coding RNAs
related to microRNA-148b in gastric cancer. Neoplasma. 64:199–208.
2017. View Article : Google Scholar : PubMed/NCBI
|
|
81
|
Hu WW, Chen PC, Chen JM, Wu YM, Liu PY, Lu
CH, Lin YF, Tang CH and Chao CC: Periostin promotes
epithelial-mesenchymal transition via the MAPK/miR-381 axis in lung
cancer. Oncotarget. 8:62248–62260. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
82
|
Hu J, Tan P, Ishihara M, Bayley NA,
Schokrpur S, Reynoso JG, Zhang Y, Lim RJ, Dumitras C, Yang L, et
al: Tumor heterogeneity in VHL drives metastasis in clear cell
renal cell carcinoma. Signal Transduct Target Ther. 8:1552023.
View Article : Google Scholar : PubMed/NCBI
|
|
83
|
Ueki A, Komura M, Koshino A, Wang C, Nagao
K, Homochi M, Tsukada Y, Ebi M, Ogasawara N, Tsuzuki T, et al:
Stromal POSTN enhances motility of both cancer and stromal cells
and predicts poor survival in colorectal cancer. Cancers (Basel).
15:6062023. View Article : Google Scholar : PubMed/NCBI
|
|
84
|
Miyako S, Koma YI, Nakanishi T, Tsukamoto
S, Yamanaka K, Ishihara N, Azumi Y, Urakami S, Shimizu M, Kodama T,
et al: Periostin in Cancer-associated fibroblasts promotes
esophageal squamous cell carcinoma progression by enhancing cancer
and stromal cell migration. Am J Pathol. 194:828–848. 2024.
View Article : Google Scholar : PubMed/NCBI
|
|
85
|
Kim CJ, Sakamoto K, Tambe Y and Inoue H:
Opposite regulation of epithelial-to-mesenchymal transition and
cell invasiveness by periostin between prostate and bladder cancer
cells. Int J Oncol. 38:1759–1766. 2011.PubMed/NCBI
|
|
86
|
Ribatti D, Nico B, Crivellato E and Vacca
A: The structure of the vascular network of tumors. Cancer Lett.
248:18–23. 2007. View Article : Google Scholar
|
|
87
|
Ribatti D, Vacca A and Dammacco F: New
non-angiogenesis dependent pathways for tumour growth. Eur J
Cancer. 39:1835–1841. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
88
|
Baril P, Gangeswaran R, Mahon PC, Caulee
K, Kocher HM, Harada T, Zhu M, Kalthoff H, Crnogorac-Jurcevic T and
Lemoine NR: Periostin promotes invasiveness and resistance of
pancreatic cancer cells to hypoxia-induced cell death: Role of the
beta4 integrin and the PI3k pathway. Oncogene. 26:2082–2094. 2007.
View Article : Google Scholar
|
|
89
|
Bao S, Ouyang G, Bai X, Huang Z, Ma C, Liu
M, Shao R, Anderson RM, Rich JN and Wang XF: Periostin potently
promotes metastatic growth of colon cancer by augmenting cell
survival via the Akt/PKB pathway. Cancer Cell. 5:329–339. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
90
|
Huang Z, Byrd O, Tan S, Hu K, Knight B, Lo
G, Taylor L, Wu Y, Berchuck A and Murphy SK: Periostin facilitates
ovarian cancer recurrence by enhancing cancer stemness. Sci Rep.
13:213822023. View Article : Google Scholar : PubMed/NCBI
|
|
91
|
Mariani A, Wang C, Oberg AL, Riska SM,
Torres M, Kumka J, Multinu F, Sagar G, Roy D and Jung DB: Genes
associated with bowel metastases in ovarian cancer. Gynecol Oncol.
154:495–504. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
92
|
Lozneanu L, Caruntu ID, Amalinei C,
Moscalu M, Gafton B, Marinca MV, Rusu A, Balan R and Giusca SE:
Periostin in ovarian carcinoma: From heterogeneity to prognostic
value. Folia Histochem Cytobiol. 61:1–16. 2023. View Article : Google Scholar : PubMed/NCBI
|
|
93
|
Gillot L, Lebeau A, Baudin L, Pottier C,
Louis T, Durré T, Longuespée R, Mazzucchelli G, Nizet C, Blacher S,
et al: Periostin in lymph node pre-metastatic niches governs
lymphatic endothelial cell functions and metastatic colonization.
Cell Mol Life Sci. 79:2952022. View Article : Google Scholar : PubMed/NCBI
|
|
94
|
Kudo Y, Iizuka S, Yoshida M, Nguyen PT,
Siriwardena SB, Tsunematsu T, Ohbayashi M, Ando T, Hatakeyama D,
Shibata T, et al: Periostin directly and indirectly promotes tumor
lymphangiogenesis of head and neck cancer. PLoS One. 7:e444882012.
View Article : Google Scholar : PubMed/NCBI
|
|
95
|
Pistritto G, Trisciuoglio D, Ceci C,
Garufi A and D'Orazi G: Apoptosis as anticancer mechanism: Function
and dysfunction of its modulators and targeted therapeutic
strategies. Aging (Albany NY). 8:603–619. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
96
|
Vaghari-Tabari M, Ferns GA, Qujeq D,
Andevari AN, Sabahi Z and Moein S: Signaling, metabolism, and
cancer: An important relationship for therapeutic intervention. J
Cell Physiol. 236:5512–5532. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
97
|
Morana O, Wood W and Gregory CD: The
apoptosis paradox in cancer. Int J Mol Sci. 23:13282022. View Article : Google Scholar : PubMed/NCBI
|
|
98
|
Gao L, Shay C and Teng Y: Cell death
shapes cancer immunity: Spotlighting PANoptosis. J Exp Clin Cancer
Res. 43:1682024. View Article : Google Scholar : PubMed/NCBI
|
|
99
|
Ouyang G, Liu M, Ruan K, Song G, Mao Y and
Bao S: Upregulated expression of periostin by hypoxia in
non-small-cell lung cancer cells promotes cell survival via the
Akt/PKB pathway. Cancer Lett. 281:213–219. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
100
|
Erkan M, Reiser-Erkan C, Michalski CW,
Deucker S, Sauliunaite D, Streit S, Esposito I, Friess H and Kleeff
J: Cancer-stellate cell interactions perpetuate the
Hypoxia-fibrosis cycle in pancreatic ductal adenocarcinoma.
Neoplasia. 11:497–508. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
101
|
Zhou W, Ke SQ, Huang Z, Flavahan W, Fang
X, Paul J, Wu L, Sloan AE, McLendon RE, Li X, et al: Periostin
secreted by glioblastoma stem cells recruits M2 Tumour-associated
macrophages and promotes malignant growth. Nat Cell Biol.
17:170–182. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
102
|
Wang H, Liang Y, Liu Z, Zhang R, Chao J,
Wang M, Liu M, Qiao L, Xuan Z, Zhao H and Lu L: POSTN+
cancer-associated fibroblasts determine the efficacy of
immunotherapy in hepatocellular carcinoma. J Immunother Cancer.
12:e0087212024. View Article : Google Scholar :
|
|
103
|
Jin X, Deng Q, Ye S, Liu S, Fu Y, Liu Y,
Wu G, Ouyang G and Wu T: Cancer-associated fibroblast-derived
periostin promotes papillary thyroid tumor growth through
integrin-FAK-STAT3 signaling. Theranostics. 14:3014–3028. 2024.
View Article : Google Scholar : PubMed/NCBI
|
|
104
|
Muñoz-Espín D and Serrano M: Cellular
senescence: From physiology to pathology. Nat Rev Mol Cell Biol.
15:482–496. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
105
|
McHugh D, Durán I and Gil J: Senescence as
a therapeutic target in cancer and age-related diseases. Nat Rev
Drug Discov. 24:57–71. 2025. View Article : Google Scholar
|
|
106
|
Hernandez-Segura A, Nehme J and Demaria M:
Hallmarks of cellular senescence. Trends Cell Biol. 28:436–453.
2018. View Article : Google Scholar : PubMed/NCBI
|
|
107
|
Calcinotto A, Kohli J, Zagato E,
Pellegrini L, Demaria M and Alimonti A: Cellular senescence: Aging,
Cancer, and Injury. Physiol Rev. 99:1047–1078. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
108
|
de Magalhães JP: Cellular senescence in
normal physiology. Science. 384:1300–1301. 2024. View Article : Google Scholar : PubMed/NCBI
|
|
109
|
Fane M and Weeraratna AT: How the ageing
microenvironment influences tumour progression. Nat Rev Cancer.
20:89–106. 2020. View Article : Google Scholar :
|
|
110
|
D'Ambrosio M and Gil J: Reshaping of the
tumor microenvironment by cellular senescence: An opportunity for
senotherapies. Dev Cell. 58:1007–1021. 2023. View Article : Google Scholar : PubMed/NCBI
|
|
111
|
Zhu D, Chen S, Sheng P, Wang Z, Li Y and
Kang X: POSTN promotes nucleus pulposus cell senescence and
extracellular matrix metabolism via activing Wnt/β-catenin and
NF-κB signal pathway in intervertebral disc degeneration. Cell
Signal. 121:1112772024. View Article : Google Scholar
|
|
112
|
Li Q, Liu X and Wei J: Ageing related
periostin expression increase from cardiac fibroblasts promotes
cardiomyocytes senescent. Biochem Biophys Res Commun. 452:497–502.
2014. View Article : Google Scholar : PubMed/NCBI
|
|
113
|
An JN, Kim H, Kim EN, Cho A, Cho Y, Choi
YW, Kim JH, Yang SH, Choi BS, Lim CS, et al: Effects of periostin
deficiency on kidney aging and lipid metabolism. Aging (Albany NY).
13:22649–22665. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
114
|
Huang YW, Chiang MF, Ho CS, Hung PL, Hsu
MH, Lee TH, Chu LJ, Liu H, Tang P and Victor Ng W: A transcriptome
study of progeroid neurocutaneous syndrome reveals POSTN As a new
element in proline metabolic disorder. Aging Dis. 9:1043–1057.
2018. View Article : Google Scholar : PubMed/NCBI
|
|
115
|
Sun L, Zhang H and Gao P: Metabolic
reprogramming and epigenetic modifications on the path to cancer.
Protein Cell. 13:877–919. 2022. View Article : Google Scholar :
|
|
116
|
Halperin C, Hey J, Weichenhan D, Stein Y,
Mayer S, Lutsik P, Plass C and Scherz-Shouval R: Global DNA
methylation analysis of Cancer-associated fibroblasts reveals
extensive epigenetic rewiring linked with RUNX1 upregulation in
breast cancer stroma. Cancer Res. 82:4139–4152. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
117
|
Kehrberg RJ, Bhyravbhatla N, Batra SK and
Kumar S: Epigenetic regulation of cancer-associated fibroblast
heterogeneity. Biochim Biophys Acta Rev Cancer. 1878:1889012023.
View Article : Google Scholar : PubMed/NCBI
|
|
118
|
Albrengues J, Bertero T, Grasset E, Bonan
S, Maiel M, Bourget I, Philippe C, Herraiz Serrano C, Benamar S,
Croce O, et al: Epigenetic switch drives the conversion of
fibroblasts into proinvasive cancer-associated fibroblasts. Nat
Commun. 6:102042015. View Article : Google Scholar : PubMed/NCBI
|
|
119
|
Becker LM, O'Connell JT, Vo AP, Cain MP,
Tampe D, Bizarro L, Sugimoto H, McGow AK, Asara JM, Lovisa S, et
al: Epigenetic reprogramming of Cancer-associated fibroblasts
deregulates glucose metabolism and facilitates progression of
breast cancer. Cell Rep. 31:1077012020. View Article : Google Scholar : PubMed/NCBI
|
|
120
|
Mishra R, Haldar S, Placencio V, Madhav A,
Rohena-Rivera K, Agarwal P, Duong F, Angara B, Tripathi M, Liu Z,
et al: Stromal epigenetic alterations drive metabolic and
neuroendocrine prostate cancer reprogramming. J Clin Invest.
128:4472–4484. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
121
|
Yang M, Wang B, Hou W, Zeng H, He W, Zhang
XK, Yan D, Yu H, Huang L, Pei L, et al: NAD+ metabolism enzyme NNMT
in cancer-associated fibroblasts drives tumor progression and
resistance to immunotherapy by modulating macrophages in urothelial
bladder cancer. J Immunother Cancer. 12:e0092812024. View Article : Google Scholar :
|
|
122
|
Han B, Yang X, Zhang P, Zhang Y, Tu Y, He
Z, Li Y, Yuan J, Dong Y, Hosseini DK, et al: DNA methylation
biomarkers for nasopharyngeal carcinoma. PLoS One. 15:e02305242020.
View Article : Google Scholar : PubMed/NCBI
|
|
123
|
Tang Y, Qing C, Wang J and Zeng Z: DNA
Methylation-based diagnostic and prognostic biomarkers for
glioblastoma. Cell Transplant. 29:9636897209332412020. View Article : Google Scholar : PubMed/NCBI
|
|
124
|
Nabi-Afjadi M, Farzam F, Soltani S,
Golestani N, Asl SS, Aziziyan F, Zalpoor H, Ghasemi F, Dabirmanesh
B and Khajeh K: The association between periostin and tumor
microenvironment: A promising cancer prognostic biomarker and
therapeutic target to combat tumor progression and chemoresistance.
Cancer Cell Int. 25:3202025. View Article : Google Scholar : PubMed/NCBI
|
|
125
|
Gerarduzzi C, Hartmann U, Leask A and
Drobetsky E: The matrix revolution: Matricellular proteins and
restructuring of the cancer microenvironment. Cancer Res.
80:2705–2717. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
126
|
Li Z, Pai R, Gupta S, Currenti J, Guo W,
Di Bartolomeo A, Feng H, Zhang Z, Li Z, Liu L, et al: Presence of
Onco-fetal neighborhoods in hepatocellular carcinoma is associated
with relapse and response to immunotherapy. Nat Cancer. 5:167–186.
2024. View Article : Google Scholar : PubMed/NCBI
|
|
127
|
Anand P, Kunnumakkara AB, Sundaram C,
Harikumar KB, Tharakan ST, Lai OS, Sung B and Aggarwal BB: Cancer
is a preventable disease that requires major lifestyle changes.
Pharm Res. 25:2097–2116. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
128
|
Lu P, Weaver VM and Werb Z: The
extracellular matrix: A dynamic niche in cancer progression. J Cell
Biol. 196:395–406. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
129
|
Kudo A and Kii I: Periostin function in
communication with extracellular matrices. J Cell Commun Signal.
12:301–308. 2018. View Article : Google Scholar :
|
|
130
|
Wang Z and Ouyang G: Periostin: A bridge
between cancer stem cells and their metastatic niche. Cell Stem
Cell. 10:111–112. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
131
|
Celià-Terrassa T and Jolly MK: Cancer stem
cells and Epithelial-to-mesenchymal transition in cancer
metastasis. Cold Spring Harb Perspect Med. 10:a0369052020.
View Article : Google Scholar
|
|
132
|
Plaks V, Kong N and Werb Z: The cancer
stem cell niche: How essential is the niche in regulating stemness
of tumor cells? Cell Stem Cell. 16:225–238. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
133
|
Babaei G, Aziz SG and Jaghi NZZ: EMT
cancer stem cells and autophagy; The three main axes of metastasis.
Biomed Pharmacother. 133:1109092021. View Article : Google Scholar
|
|
134
|
Malanchi I, Santamaria-Martínez A, Susanto
E, Peng H, Lehr HA, Delaloye JF and Huelsken J: Interactions
between cancer stem cells and their niche govern metastatic
colonization. Nature. 481:85–89. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
135
|
Costa A, Kieffer Y, Scholer-Dahirel A,
Pelon F, Bourachot B, Cardon M, Sirven P, Magagna I, Fuhrmann L,
Bernard C, et al: Fibroblast heterogeneity and immunosuppressive
environment in human breast cancer. Cancer Cell. 33:463–479.e10.
2018. View Article : Google Scholar : PubMed/NCBI
|
|
136
|
Wei WF, Chen XJ, Liang LJ, Yu L, Wu XG,
Zhou CF, Wang ZC, Fan LS, Hu Z, Liang L and Wang W: Periostin+
cancer-associated fibroblasts promote lymph node metastasis by
impairing the lymphatic endothelial barriers in cervical squamous
cell carcinoma. Mol Oncol. 15:210–227. 2021. View Article : Google Scholar
|
|
137
|
Bhattacharjee S, Hamberger F, Ravichandra
A, Miller M, Nair A, Affo S, Filliol A, Chin L, Savage TM, Yin D,
et al: Tumor restriction by type I collagen opposes Tumor-promoting
effects of Cancer-associated fibroblasts. J Clin Invest.
131:e1469872021. View Article : Google Scholar : PubMed/NCBI
|
|
138
|
Han CC, Liu TY and Yin R: Biomarkers for
Cancer-associated fibroblasts. Biomark Res. 8:642020. View Article : Google Scholar : PubMed/NCBI
|
|
139
|
Oskarsson T and Massagué J: Extracellular
matrix players in metastatic niches. EMBO J. 31:254–256. 2012.
View Article : Google Scholar :
|
|
140
|
Lambert AW, Wong CK, Ozturk S, Papageorgis
P, Raghunathan R, Alekseyev Y, Gower AC, Reinhard BM, Abdolmaleky
HM and Thiagalingam S: Tumor Cell-derived periostin regulates
cytokines that maintain breast cancer stem cells. Mol Cancer Res.
14:103–113. 2016. View Article : Google Scholar :
|
|
141
|
Zhang Q, Wang C and Cliby WA:
Cancer-associated stroma significantly contributes to the
mesenchymal subtype signature of serous ovarian cancer. Gynecol
Oncol. 152:368–374. 2019. View Article : Google Scholar :
|
|
142
|
Vermeulen L, De Sousa EMF, van der Heijden
M, Cameron K, de Jong JH, Borovski T, Tuynman JB, Todaro M, Merz C,
Rodermond H, et al: Wnt activity defines colon cancer stem cells
and is regulated by the microenvironment. Nat Cell Biol.
12:468–476. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
143
|
Li P, Yang R and Gao WQ: Contributions of
Epithelial-mesenchymal transition and cancer stem cells to the
development of castration resistance of prostate cancer. Mol
Cancer. 13:552014. View Article : Google Scholar : PubMed/NCBI
|
|
144
|
Chen G, Wang Y, Zhao X, Xie XZ, Zhao JG,
Deng T, Chen ZY, Chen HB, Tong YF, Yang Z, et al: A positive
feedback loop between Periostin and TGFβ1 induces and maintains the
stemness of hepatocellular carcinoma cells via AP-2α activation. J
Exp Clin Cancer Res. 40:2182021. View Article : Google Scholar
|
|
145
|
Nowosad A, Marine JC and Karras P:
Perivascular niches: Critical hubs in cancer evolution. Trends
Cancer. 9:897–910. 2023. View Article : Google Scholar : PubMed/NCBI
|
|
146
|
Liu Z, Chen J, Ren Y, Liu S, Ba Y, Zuo A,
Luo P, Cheng Q, Xu H and Han X: Multi-stage mechanisms of tumor
metastasis and therapeutic strategies. Signal Transduct Target
Ther. 9:2702024. View Article : Google Scholar : PubMed/NCBI
|
|
147
|
Erez N: Cancer: Angiogenic awakening.
Nature. 500:37–38. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
148
|
Weis SM and Cheresh DA: A wake-up call for
hibernating tumour cells. Nat Cell Biol. 15:721–723. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
149
|
Ghajar CM, Peinado H, Mori H, Matei IR,
Evason KJ, Brazier H, Almeida D, Koller A, Hajjar KA, Stainier DY,
et al: The perivascular niche regulates breast tumour dormancy. Nat
Cell Biol. 15:807–817. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
150
|
Lewis CE, Harney AS and Pollard JW: The
multifaceted role of perivascular macrophages in tumors. Cancer
Cell. 30:3652016. View Article : Google Scholar : PubMed/NCBI
|
|
151
|
Pietras A, Katz AM, Ekström EJ, Wee B,
Halliday JJ, Pitter KL, Werbeck JL, Amankulor NM, Huse JT and
Holland EC: Osteopontin-CD44 signaling in the glioma perivascular
niche enhances cancer stem cell phenotypes and promotes aggressive
tumor growth. Cell Stem Cell. 14:357–369. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
152
|
Shi Y, Ping YF, Zhang X and Bian XW:
Hostile takeover: Glioma stem cells recruit TAMs to support tumor
progression. Cell Stem Cell. 16:219–220. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
153
|
Liu Y and Cao X: Characteristics and
Significance of the Pre-metastatic Niche. Cancer Cell. 30:668–681.
2016. View Article : Google Scholar : PubMed/NCBI
|
|
154
|
Li Y, Zheng Y, Tan X, Du Y, Wei Y and Liu
S: Extracellular vesicle-mediated pre-metastatic niche formation
via altering host microenvironments. Front Immunol. 15:13673732024.
View Article : Google Scholar : PubMed/NCBI
|
|
155
|
Peinado H, Zhang H, Matei IR, Costa-Silva
B, Hoshino A, Rodrigues G, Psaila B, Kaplan RN, Bromberg JF, Kang
Y, et al: Pre-metastatic niches: Organ-specific homes for
metastases. Nat Rev Cancer. 17:302–317. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
156
|
Kong J, Tian H, Zhang F, Zhang Z, Li J,
Liu X, Li X, Liu J, Li X, Jin D, et al: Extracellular vesicles of
carcinoma-associated fibroblasts creates a pre-metastatic niche in
the lung through activating fibroblasts. Mol Cancer. 18:1752019.
View Article : Google Scholar : PubMed/NCBI
|
|
157
|
Wang X, Jian Q, Zhang Z, Gu J, Wang X and
Wang Y: Effect of tumor-derived extracellular vesicle-shuttled
lncRNA MALAT1 on proliferation, invasion and metastasis of
triple-negative breast cancer by regulating macrophage M2
polarization via the POSTN/Hippo/YAP axis. Transl Oncol.
49:1020762024. View Article : Google Scholar : PubMed/NCBI
|
|
158
|
Ji Q, Zhou L, Sui H, Yang L, Wu X, Song Q,
Jia R, Li R, Sun J, Wang Z, et al: Primary tumors release
ITGBL1-rich extracellular vesicles to promote distal metastatic
tumor growth through fibroblast-niche formation. Nat Commun.
11:12112020. View Article : Google Scholar : PubMed/NCBI
|
|
159
|
Pitt JM, Marabelle A, Eggermont A, Soria
JC, Kroemer G and Zitvogel L: Targeting the tumor microenvironment:
Removing obstruction to anticancer immune responses and
immunotherapy. Ann Oncol. 27:1482–1492. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
160
|
Fukuda K, Sugihara E, Ohta S, Izuhara K,
Funakoshi T, Amagai M and Saya H: Periostin is a key niche
component for wound metastasis of melanoma. PLoS One.
10:e01297042015. View Article : Google Scholar : PubMed/NCBI
|
|
161
|
Wang Y, Li Q, Yang X, Guo H, Ren T, Zhang
T, Ghadakpour P and Ren F: Exosome-mediated communication in
thyroid cancer: Implications for prognosis and therapeutic targets.
Biochem Genet. 63:2673–2697. 2025. View Article : Google Scholar
|
|
162
|
Mouw JK, Ou G and Weaver VM: Extracellular
matrix assembly: A multiscale deconstruction. Nat Rev Mol Cell
Biol. 15:771–785. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
163
|
Boulter L, Bullock E, Mabruk Z and Brunton
VG: The fibrotic and immune microenvironments as targetable drivers
of metastasis. Br J Cancer. 124:27–36. 2021. View Article : Google Scholar :
|
|
164
|
Sonnenberg-Riethmacher E, Miehe M and
Riethmacher D: Periostin in allergy and inflammation. Front
Immunol. 12:7221702021. View Article : Google Scholar : PubMed/NCBI
|
|
165
|
Tomasek JJ, Gabbiani G, Hinz B, Chaponnier
C and Brown RA: Myofibroblasts and mechano-regulation of connective
tissue remodelling. Nat Rev Mol Cell Biol. 3:349–363. 2002.
View Article : Google Scholar : PubMed/NCBI
|
|
166
|
Xu X, Chang W, Yuan J, Han X, Tan X, Ding
Y, Luo Y, Cai H, Liu Y, Gao X, et al: Periostin expression in
intra-tumoral stromal cells is prognostic and predictive for
colorectal carcinoma via creating a cancer-supportive niche.
Oncotarget. 7:798–813. 2016. View Article : Google Scholar :
|
|
167
|
Ashley SL, Wilke CA, Kim KK and Moore BB:
Periostin regulates fibrocyte function to promote myofibroblast
differentiation and lung fibrosis. Mucosal Immunol. 10:341–351.
2017. View Article : Google Scholar :
|
|
168
|
Kiesler ZG, Hunter MI, Balboula AZ and
Patterson AL: Periostin's role in uterine leiomyoma development: A
Mini-review on the potential periostin poses as a pharmacological
intervention for uterine leiomyoma. Arch Gynecol Obstet.
309:1825–1831. 2024. View Article : Google Scholar : PubMed/NCBI
|
|
169
|
Jamaluddin MFB, Ko YA, Kumar M, Brown Y,
Bajwa P, Nagendra PB, Skerrett-Byrne DA, Hondermarck H, Baker MA,
Dun MD, et al: Proteomic profiling of human uterine fibroids
reveals upregulation of the extracellular matrix protein periostin.
Endocrinology. 159:1106–1118. 2018. View Article : Google Scholar
|
|
170
|
Nielsen SR, Quaranta V, Linford A, Emeagi
P, Rainer C, Santos A, Ireland L, Sakai T, Sakai K, Kim YS, et al:
Macrophage-secreted granulin supports pancreatic cancer metastasis
by inducing liver fibrosis. Nat Cell Biol. 18:549–560. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
171
|
Clézardin P, Coleman R, Puppo M, Ottewell
P, Bonnelye E, Paycha F, Confavreux CB and Holen I: Bone
metastasis: Mechanisms, therapies, and biomarkers. Physiol Rev.
101:797–855. 2021. View Article : Google Scholar
|
|
172
|
Jackett KN, Browne AT, Aber ER, Clements M
and Kaplan RN: How the bone microenvironment shapes the
pre-metastatic niche and metastasis. Nat Cancer. 5:1800–1814. 2024.
View Article : Google Scholar : PubMed/NCBI
|
|
173
|
Muscarella AM, Aguirre S, Hao X, Waldvogel
SM and Zhang XH: Exploiting bone niches: Progression of
disseminated tumor cells to metastasis. J Clin Invest.
131:e1437642021. View Article : Google Scholar : PubMed/NCBI
|
|
174
|
Baccin C, Al-Sabah J, Velten L, Helbling
PM, Grünschläger F, Hernández-Malmierca P, Nombela-Arrieta C,
Steinmetz LM, Trumpp A and Haas S: Combined single-cell and spatial
transcriptomics reveal the molecular, cellular and spatial bone
marrow niche organization. Nat Cell Biol. 22:38–48. 2020.
View Article : Google Scholar
|
|
175
|
Chen F, Han Y and Kang Y: Bone marrow
niches in the regulation of bone metastasis. Br J Cancer.
124:1912–1920. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
176
|
Shafat MS, Gnaneswaran B, Bowles KM and
Rushworth SA: The bone marrow microenvironment-Home of the leukemic
blasts. Blood Rev. 31:277–286. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
177
|
Weilbaecher KN, Guise TA and McCauley LK:
Cancer to bone: A fatal attraction. Nat Rev Cancer. 11:411–425.
2011. View Article : Google Scholar : PubMed/NCBI
|
|
178
|
Contié S, Voorzanger-Rousselot N, Litvin
J, Clézardin P and Garnero P: Increased expression and serum levels
of the stromal cell-secreted protein periostin in breast cancer
bone metastases. Int J Cancer. 128:352–360. 2011. View Article : Google Scholar
|
|
179
|
Gineyts E, Bonnet N, Bertholon C, Millet
M, Pagnon-Minot A, Borel O, Geraci S, Bonnelye E, Croset M, Suhail
A, et al: The C-terminal intact forms of periostin (iPTN) are
surrogate markers for osteolytic lesions in experimental breast
cancer bone metastasis. Calcif Tissue Int. 103:567–580. 2018.
View Article : Google Scholar : PubMed/NCBI
|
|
180
|
Liu C, Feng X, Wang B, Wang X, Wang C, Yu
M, Cao G and Wang H: Bone marrow mesenchymal stem cells promote
head and neck cancer progression through Periostin-mediated
phosphoinositide 3-kinase/Akt/mammalian target of rapamycin. Cancer
Sci. 109:688–698. 2018. View Article : Google Scholar :
|
|
181
|
Ling W, Johnson SK, Mehdi SJ, Alapat DV,
Bauer M, Zangari M, Schinke C, Thanendrarajan S, van Rhee F and
Yaccoby S: EDNRA-expressing mesenchymal cells are expanded in
myeloma interstitial bone marrow and associated with disease
progression. Cancers (Basel). 15:45192023. View Article : Google Scholar : PubMed/NCBI
|
|
182
|
Terpos E, Christoulas D, Kastritis E,
Bagratuni T, Gavriatopoulou M, Roussou M, Papatheodorou A,
Eleutherakis-Papaiakovou E, Kanellias N, Liakou C, et al: High
levels of periostin correlate with increased fracture rate, diffuse
MRI pattern, abnormal bone remodeling and advanced disease stage in
patients with newly diagnosed symptomatic multiple myeloma. Blood
Cancer J. 6:e4822016. View Article : Google Scholar : PubMed/NCBI
|
|
183
|
Sun CY, Mi YY, Ge SY, Hu QF, Xu K, Guo YJ,
Tan YF, Zhang Y, Zhong F and Xia GW: Tumor- and Osteoblast-derived
periostin in prostate cancer bone metastases. Front Oncol.
11:7957122021. View Article : Google Scholar
|
|
184
|
Ma Z, Zhao X, Deng M, Huang Z, Wang J, Wu
Y, Cui D, Liu Y, Liu R and Ouyang G: Bone marrow mesenchymal
stromal cell-derived periostin promotes B-ALL progression by
modulating CCL2 in leukemia cells. Cell Rep. 26:1533–1543.e4. 2019.
View Article : Google Scholar : PubMed/NCBI
|
|
185
|
Izuhara K, Nunomura S, Nanri Y, Ogawa M,
Ono J, Mitamura Y and Yoshihara T: Periostin in inflammation and
allergy. Cell Mol Life Sci. 74:4293–4303. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
186
|
Kii I: Practical application of periostin
as a biomarker for pathological conditions. Adv Exp Med Biol.
1132:195–204. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
187
|
Conway SJ, Izuhara K, Kudo Y, Litvin J,
Markwald R, Ouyang G, Arron JR, Holweg CT and Kudo A: The role of
periostin in tissue remodeling across health and disease. Cell Mol
Life Sci. 71:1279–1288. 2014. View Article : Google Scholar :
|
|
188
|
Lu S, Peng L, Ma F, Chai J, Hua Y, Yang W
and Zhang Z: Increased expression of POSTN predicts poor prognosis:
A potential therapeutic target for gastric cancer. J Gastrointest
Surg. 27:233–249. 2023. View Article : Google Scholar
|
|
189
|
Chu LJ, Tsai CJ, Chien CC, Hsiao YC, Yu
JS, Tsai JY and Yeh TS: Plasma POSTN derived from bile proteome is
a promising biomarker for cholangiocarcinoma with efficacy
comparable and complementary to CA19.9. United European
Gastroenterol J. 13:1155–1170. 2025. View Article : Google Scholar : PubMed/NCBI
|
|
190
|
Rachner TD, Göbel A, Hoffmann O, Erdmann
K, Kasimir-Bauer S, Breining D, Kimmig R, Hofbauer LC and Bittner
AK: High serum levels of periostin are associated with a poor
survival in breast cancer. Breast Cancer Res Treat. 180:515–524.
2020. View Article : Google Scholar : PubMed/NCBI
|
|
191
|
Dong D, Zhang L, Jia L, Ji W, Wang Z, Ren
L, Niu R and Zhou Y: Identification of serum periostin as a
potential diagnostic and prognostic marker for colorectal cancer.
Clin Lab. 64:973–981. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
192
|
Lv Y, Wang W, Jia WD, Sun QK, Huang M,
Zhou HC, Xia HH, Liu WB, Chen H, Sun SN and Xu GL: High
preoparative levels of serum periostin are associated with poor
prognosis in patients with hepatocellular carcinoma after
hepatectomy. Eur J Surg Oncol. 39:1129–1135. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
193
|
Fujimoto K, Kawaguchi T, Nakashima O, Ono
J, Ohta S, Kawaguchi A, Tonan T, Ohshima K, Yano H, Hayabuchi N, et
al: Periostin, a matrix protein, has potential as a novel
serodiagnostic marker for cholangiocarcinoma. Oncol Rep.
25:1211–1216. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
194
|
Hu F, Shang XF, Wang W, Jiang W, Fang C,
Tan D and Zhou HC: High-level expression of periostin is
significantly correlated with tumour angiogenesis and poor
prognosis in osteosarcoma. Int J Exp Pathol. 97:86–92. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
195
|
Villani R, Murigneux V, Alexis J, Sim SL,
Wagels M, Saunders N, Soyer HP, Parmentier L, Nikolaev S, Fink JL,
et al: Subtype-specific analyses reveal infiltrative basal cell
carcinomas are highly interactive with their environment. J Invest
Dermatol. 141:2380–2390. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
196
|
Zhao Z, Mak TK, Shi Y, Li K, Huo M and
Zhang C: Integrative analysis of Cancer-associated fibroblast
signature in gastric cancer. Heliyon. 9:e192172023. View Article : Google Scholar : PubMed/NCBI
|
|
197
|
Kyutoku M, Taniyama Y, Katsuragi N,
Shimizu H, Kunugiza Y, Iekushi K, Koibuchi N, Sanada F, Oshita Y
and Morishita R: Role of periostin in cancer progression and
metastasis: Inhibition of breast cancer progression and metastasis
by anti-periostin antibody in a murine model. Int J Mol Med.
28:181–186. 2011.PubMed/NCBI
|
|
198
|
Zhu M, Saxton RE, Ramos L, Chang DD,
Karlan BY, Gasson JC and Slamon DJ: Neutralizing monoclonal
antibody to periostin inhibits ovarian tumor growth and metastasis.
Mol Cancer Ther. 10:1500–1508. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
199
|
Lee YJ, Kim IS, Park SA, Kim Y, Lee JE,
Noh DY, Kim KT, Ryu SH and Suh PG: Periostin-binding DNA aptamer
inhibits breast cancer growth and metastasis. Mol Ther.
21:1004–1013. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
200
|
Liu GX, Xi HQ, Sun XY and Wei B: Role of
periostin and its antagonist PNDA-3 in gastric cancer metastasis.
World J Gastroenterol. 21:2605–2613. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
201
|
Halin Bergström S, Semenas J, Nordstrand
A, Thysell E, Wänman J, Crnalic S, Widmark A, Thellenberg-Karlsson
C, Andersson P, Gidlund S, et al: Morphological heterogeneities in
prostate cancer bone metastases are related to molecular subtypes
and prognosis. Clin Exp Metastasis. 49:422025.
|
|
202
|
Zeng L, Li L, Liao X, Zhang L, Yin C, Chen
X and Sun J: Population-based high-dimensional analyses identify
multiple intrinsic characters for cancer vaccines against lung
squamous cell carcinoma. Med Oncol. 41:422024. View Article : Google Scholar : PubMed/NCBI
|
|
203
|
Ratajczak-Wielgomas K, Grzegrzolka J,
Piotrowska A, Matkowski R, Wojnar A, Rys J, Ugorski M and Dziegiel
P: Expression of periostin in breast cancer cells. Int J Oncol.
51:1300–1310. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
204
|
Yang T, Deng Z, Pan Z, Qian Y, Yao W and
Wang J: Prognostic value of periostin in multiple solid cancers: A
systematic review with Meta-analysis. J Cell Physiol.
235:2800–2808. 2020. View Article : Google Scholar
|
|
205
|
Li M, Ma G, Li X and Zhou Q: The
prognostic value of periostin expression in Non-small cell lung
cancer: A Meta-analysis. Med Sci Monit. 28:e9368982022. View Article : Google Scholar : PubMed/NCBI
|
|
206
|
Morra L, Rechsteiner M, Casagrande S, von
Teichman A, Schraml P, Moch H and Soltermann A: Characterization of
periostin isoform pattern in non-small cell lung cancer. Lung
Cancer. 76:183–190. 2012. View Article : Google Scholar
|
|
207
|
Utispan K, Sonongbua J, Thuwajit P,
Chau-In S, Pairojkul C, Wongkham S and Thuwajit C: Periostin
activates integrin α5β1 through a PI3K/AKT-dependent pathway in
invasion of cholangiocarcinoma. Int J Oncol. 41:1110–1118. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
208
|
Wu Y, Li S, Yu H, Zhang S, Yan L, Guan X,
Xu W, Wang Z, Lv A, Tian X, et al: Integrative Single-cell and
spatial transcriptomics analysis reveals ECM-remodeling
Cancer-associated Fibroblast-derived POSTN as a key mediator in
pancreatic ductal adenocarcinoma progression. Int J Biol Sci.
21:3573–3596. 2025. View Article : Google Scholar : PubMed/NCBI
|
|
209
|
Xue H, Gao X, Xu S, Zhang J, Guo X, Yan S,
Li T, Guo X, Liu Q and Li G: MicroRNA-Let-7f reduces the
vasculogenic mimicry of human glioma cells by regulating
periostin-dependent migration. Oncol Rep. 35:1771–1777. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
210
|
Yan S, Han X, Xue H, Zhang P, Guo X, Li T,
Guo X, Yuan G, Deng L and Li G: Let-7f inhibits glioma cell
proliferation, migration, and invasion by targeting periostin. J
Cell Biochem. 116:1680–1692. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
211
|
Wei T, Wang K, Liu S, Fang Y, Hong Z, Liu
Y, Zhang H, Yang C, Ouyang G and Wu T: Periostin deficiency reduces
PD-1+ tumor-associated macrophage infiltration and enhances
anti-PD-1 efficacy in colorectal cancer. Cell Rep. 42:1120902023.
View Article : Google Scholar
|
|
212
|
Zhang W, Wu FH, Wang J, Wei HZ, Wang TW
and Zhang YC: Periostin: A predictable molecule to prognosis and
chemotherapy responses of gastrointestinal and
hepato-biliary-pancreatic malignant tumors? Neoplasma. 69:491–503.
2022. View Article : Google Scholar : PubMed/NCBI
|
|
213
|
Ishibashi Y, Mochizuki S, Horiuchi K,
Tsujimoto H, Kouzu K, Kishi Y, Okada Y and Ueno H: Periostin
derived from cancer-associated fibroblasts promotes esophageal
squamous cell carcinoma progression via ADAM17 activation. Biochim
Biophys Acta Mol Basis Dis. 1869:1666692023. View Article : Google Scholar : PubMed/NCBI
|
|
214
|
Mikheev AM, Mikheeva SA, Trister AD,
Tokita MJ, Emerson SN, Parada CA, Born DE, Carnemolla B, Frankel S,
Kim DH, et al: Periostin is a novel therapeutic target that
predicts and regulates glioma malignancy. Neuro Oncol. 17:372–382.
2015. View Article : Google Scholar :
|
|
215
|
Chen G, Nakamura I, Dhanasekaran R, Iguchi
E, Tolosa EJ, Romecin PA, Vera RE, Almada LL, Miamen AG,
Chaiteerakij R, et al: Transcriptional induction of periostin by a
sulfatase 2-TGFβ1-SMAD signaling axis mediates tumor angiogenesis
in hepatocellular carcinoma. Cancer Res. 77:632–645. 2017.
View Article : Google Scholar
|
|
216
|
Shang Y, Liang Y, Zhang B, Wu W, Peng Y,
Wang J, Zhang M and Niu C: Periostin-mediated activation of NF-κB
signaling promotes tumor progression and chemoresistance in
glioblastoma. Sci Rep. 15:139552025. View Article : Google Scholar
|
|
217
|
Wu J, Li J, Xu H, Qiu N, Huang X and Li H:
Periostin drives extracellular matrix degradation, stemness, and
chemoresistance by activating the MAPK/ERK signaling pathway in
triple-negative breast cancer cells. Lipids Health Dis. 22:1532023.
View Article : Google Scholar : PubMed/NCBI
|
|
218
|
Wang Y, Wang B, Cao W and Xu X: PTX3
activates POSTN and promotes the progression of glioblastoma via
the MAPK/ERK signalling axis. Biochem Biophys Res Commun.
703:1496652024. View Article : Google Scholar : PubMed/NCBI
|