Ulcerative colitis (UC), a prototypical clinical
subtype of inflammatory bowel disease (IBD), is characterized by
chronic and recurrent non-specific inflammation primarily localized
within the mucosal and submucosal layers of the rectum and colon.
Surveillance data have highlighted a concerning rise in the global
incidence of UC (1,2), a trend that not only severely
compromises the quality of life of patients but also significantly
elevates the risk of colorectal carcinogenesis (3,4).
At present, conventional therapeutic agents such as
5-aminosalicylic acid, glucocorticoids and biologics (such as
anti-TNF and anti-integrin agents) are commonly employed.
Nevertheless, these pharmacotherapeutic interventions are hampered
by notable limitations in terms of efficacy, adverse effects,
individual responsiveness and a high relapse rate (5). Moreover, the economic burden and
psychological impact of long-term treatment further exacerbate
patient stress, underscoring the necessity for more effective and
individualized therapeutic approaches. Despite extensive research
efforts, the precise etiological determinants and comprehensive
pathophysiological mechanisms underlying UC remain incompletely
elucidated. Growing evidence accentuates the pivotal role of
intestinal barrier integrity (6,7),
a sophisticated, multi-layered defense system encompassing the
mucus layer, epithelial cells and intercellular junction complexes
(8), in both the initiation and
progression of UC. Mechanistic investigations have demonstrated
that UC pathogenesis involves intricate interactions among immune
dysregulation, disruption of intestinal epithelial homeostasis,
dysbiosis-driven bacterial colonization and compromised epithelial
barrier function (9).
Pathological alterations in tight junction (TJ)
proteins, such as ZO-1 and occludin, result in abnormal intestinal
permeability, facilitating the translocation of luminal bacteria,
endotoxins and undigested dietary antigens into the lamina propria
(10). These microbial
components and metabolites directly activate lamina propria immune
cells, triggering the robust release of pro-inflammatory cytokines
(11). This chemotactic
signaling recruits additional leukocytes to the inflammatory sites,
initiating a cascade that results in characteristic pathological
manifestations, including tissue erythema, exudation and mucosal
erosion. Notably, this self-perpetuating inflammatory milieu
exacerbates the degradation of TJ proteins (12,13), establishing a detrimental cycle
that perpetuates mucosal injury.
Intestinal epithelial cells (IECs) constitute the
primary defense of the intestinal mucosal barrier, playing a
pivotal role in maintaining mucosal homeostasis through dynamic
cell turnover and selective permeability regulation. Recent
investigations have identified dysregulated IEC death as a key
factor contributing to the perpetuation of colonic inflammation and
the resistance to therapy in UC pathogenesis (14-16). Under physiological conditions,
apoptosis maintains intestinal epithelial homeostasis through
programmed cell turnover. However, an excess of apoptotic activity
exceeding regenerative capacity can lead to extensive depletion of
IECs, causing structural denudation and compromising barrier
integrity (17). In addition to
apoptosis, other cell death mechanisms, including necroptosis,
PANoptosis, pyroptosis, ferroptosis and autophagy, have been
implicated in the pathogenesis of UC (18-22).
Therefore, elucidating the role of IEC death in UC
pathogenesis and systematically consolidating current research
achievements on modulating excessive IEC death through natural
compounds and nanoparticle-mediated drug delivery systems to
alleviate UC progression (particularly by integrating recent
advances in mechanistic pathways, bioactive constituents and
molecular targets) may offer novel perspectives for unraveling UC
etiology. This strategy could facilitate the precise identification
of potential therapeutic targets, thereby paving the way for
innovative methods for clinical UC management. Future research
endeavors should concentrate on developing targeted therapies that
address the multifaceted nature of IEC death and its downstream
inflammatory repercussions, ultimately disrupting the cycle of
mucosal damage and the inflammation characteristic of UC.
IECs are characterized by rapid and continuous
cellular renewal. Apoptosis, a key programmed cell death mechanism,
serves as an essential pathway for the physiological elimination of
aged IECs at the end of their life cycle and plays a critical role
in sustaining intestinal homeostasis (23). However, excessive activation of
apoptosis during UC progression may amplify inflammatory responses
via enhanced cytokine signaling (24). Upon exposure to stress stimuli,
such as the inflammatory microenvironment of UC, pro-apoptotic
proteins Bax and Bak undergo oligomerization and translocate to the
mitochondrial outer membrane, forming transmembrane pores (25). This event triggers mitochondrial
swelling and facilitates the efflux of cytochrome c from the
intermembrane space into the cytosol (26). Released cytochrome c subsequently
binds to apoptotic protease-activating factor-1, assembling the
apoptosome complex that activates caspase-9 through proteolytic
cleavage (27). This initiates a
downstream caspase cascade culminating in the execution phase of
apoptosis via caspase-3 activation and eventual cellular
dismantling (28).
In the dextran sulfate sodium (DSS)-induced mouse
model of UC, intestinal tissues display a pronounced state of
oxidative stress, accompanied by upregulated Bax protein
expression, decreased mitochondrial membrane potential, increased
cytochrome c release, markedly elevated apoptosis of IECs and
impaired intestinal barrier function, which manifests as increased
intestinal permeability and frequent bacterial translocation
(29,30). Clinical investigations have
demonstrated that patients with UC exhibit significantly elevated
expression levels of Fas and Fas ligand (FasL) in intestinal
mucosal tissues compared with healthy controls, with these levels
positively correlating with clinical severity indices (31,32). These findings collectively
suggest that the Fas/FasL-mediated extrinsic apoptosis pathway is
aberrantly activated during UC pathogenesis, driving excessive IEC
apoptosis and thereby compromising barrier integrity. Within the
colonic tissues of patients with UC, the tumor suppressor p53
functions as a pivotal transcriptional regulator orchestrating
multiple apoptosis-related genes (33). Notably, enhanced phosphorylation
of p53 has been shown to be positively correlated with disease
severity (34). Under
inflammatory conditions, interleukin (IL)-1β released from
activated immune cells potentiates p53-mediated apoptotic
signaling, thereby exacerbating epithelial apoptosis and amplifying
mucosal inflammation (35). An
experimental study employing TNF-α-induced murine models revealed
augmented IEC apoptosis in both acute and chronic inflammation
settings (36). This apoptotic
imbalance disrupts epithelial homeostasis, thereby facilitating UC
progression. Moreover, the upregulated expression of METTL3 and
long non-coding RNAs such as MALAT1 under inflammatory conditions
has been mechanistically linked to suppressed cellular viability
and enhanced IEC apoptosis, unveiling novel molecular pathways in
UC pathogenesis (37,38).
Following IEC apoptosis, multiple chemokines are
released to recruit immune cells to intestinal inflammatory sites,
including monocyte chemoattractant protein-1 and IL-8, a process
that plays a central role in the pathogenesis of UC. During disease
progression, persistent inflammatory stimuli and sustained cell
death signaling maintain macrophage activation, thereby
establishing a self-amplifying pathological loop (39,40). In DSS-induced murine UC models, a
rapid elevation of IL-8 levels has been observed in intestinal
tissues post-induction, concomitant with robust neutrophil
infiltration. This pathological progression exhibits temporal
synchronization with IEC apoptosis and necrosis, and these three
pathological events mutually reinforce one another through
feed-forward mechanisms. Collectively, this triad drives the
chronicity and perpetuation of intestinal inflammation (41).
Conventional therapies have been predominantly aimed
at suppressing excessive immune responses but have largely
neglected emerging pathological aspects such as the restoration of
intestinal mucosal barrier integrity and the correction of
dysregulated IEC apoptosis, resulting in suboptimal efficacy and
frequent relapses in certain patient populations (42). These limitations have catalyzed
the exploration of natural compounds and novel targeted
therapeutic. Ginkgetin (GK), a natural compound derived from
Ginkgo biloba with multi-protective properties, has been
shown to ameliorate DSS-induced experimental colitis in murine
models. Mechanistic investigations reveal that GK administration
attenuates IEC apoptosis through inhibition of the EGFR/PI3K/AKT
signaling pathway, accompanied by upregulation of the
anti-apoptotic protein Bcl-2 and downregulation of pro-apoptotic
proteins Bax and caspase-3, thereby restoring intestinal barrier
integrity (43). Similarly, in
acetic acid-induced rat models of UC, Centella asiatica
treatment effectively normalizes dysregulated apoptosis markers,
including elevated Bcl-2 alongside reduced Bax and caspase-3, while
mitigating oxidative stress and attenuating inflammatory responses
(44). Despite consistent
modulation of key biomarkers, studies on Centella asiatica
often lack rigorous dose-response evaluation and thorough long-term
toxicity assessments, which are essential for translational
applicability. Daphnetin exerts protective effects against IEC
apoptosis by inhibiting the regenerating islet-derived protein
3α-dependent Janus kinase 2 (JAK2)/STAT3 signaling pathway,
resulting in reduced expression of pro-apoptotic proteins and
decreased levels of pro-inflammatory cytokines (45). Both lipopolysaccharide
(LPS)-treated human colon adenocarcinoma Caco-2 cell inflammatory
models and DSS-induced UC rat models have demonstrated that the
natural bioactive compound paeoniflorin alleviates UC by modulating
serum metabolites and suppressing the CDC42/JNK signaling pathway,
thereby inhibiting IEC apoptosis (46).
Nonetheless, current investigations primarily
emphasize acute therapeutic efficacy, with limited assessment of
remission durability or relapse prevention in chronic UC contexts.
Emerging as a promising therapeutic approach, exosome-based
interventions have gained attention due to their low immunogenicity
and superior circulatory stability. In murine UC models, caprine
milk-derived exosomes have been shown to attenuate oxidative
stress, suppress apoptosis, restore intestinal barrier integrity
and modulate gut microbiota composition (47). Mechanistically, oxidative stress
acts as a key driver of IEC apoptosis and recent evidence indicates
that mesenchymal stem cell-derived exosomes confer protective
effects by mitigating ROS accumulation in IECs, thereby alleviating
UC-associated tissue damage (48). While exosome therapies offer
multi-modal benefits, current preclinical studies face challenges
including heterogeneity in exosome isolation techniques, lack of
standardized dosing regimens and limited investigation of potential
off-target effects. Furthermore, the precise mechanisms underlying
exosome-mediated immunomodulation, barrier restoration and
concurrent suppression of inflammatory signaling and apoptotic
pathways remain to be fully elucidated.
While active exploration into natural compounds and
biological vectors such as exosomes continues, advances in
pharmaceutical engineering and drug repurposing have concurrently
unveiled innovative avenues for UC therapy. Prior investigations
have substantiated that berberine (BBR), a bioactive constituent of
traditional Chinese medicine, effectively mitigates DSS-induced
colonic inflammation; however, its clinical translation has been
hampered by inherent limitations including poor aqueous solubility
and short half-life (49,50).
In recent years, the advent of nanotechnology-engineered poly
(lactic-co-glycolic acid) nanoparticles encapsulating BBR has
markedly enhanced drug encapsulation efficiency, aqueous solubility
and bioactivity, thereby conferring superior therapeutic outcomes
in UC experimental models (51).
Donepezil, originally developed for Alzheimer's disease management,
is renowned for its neuroprotective, anti-inflammatory and
antioxidant attributes (52,53). Evidence has further elucidated
its capacity to upregulate low-density lipoprotein receptor-related
protein 1 expression, activate AMPK signaling and suppress the
NF-κB inflammatory cascade, thereby attenuating intestinal
inflammation and epithelial apoptosis (54). Analogously, the small-molecule
inhibitor ruxolitinib ameliorates UC pathological progression by
targeting the JAK/STAT3 pathway, effectively suppressing NF-κB
activation, curtailing apoptosis and fostering barrier repair, thus
presenting a promising targeted therapeutic strategy (55). Notwithstanding these
advancements, emerging modalities continue to confront key
challenges, including unresolved optimal dosing regimens,
tissue-specific targeting and sustained long-term efficacy within
the intestinal milieu.
Necroptosis, a distinct form of programmed necrotic
cell death, exerts a pivotal influence on intestinal inflammation.
The core molecular machinery involves sequential activation and
signaling transduction of receptor-interacting protein kinase
(RIPK) 1, RIPK3 and mixed lineage kinase domain-like protein (MLKL)
(56). Under homeostatic
conditions, RIPK1 serves as a central signaling hub that
coordinates cellular stress responses and preserves epithelial
integrity (57). During
intestinal inflammation triggered by pathogenic stimuli or
excessive TNF-α release, TNF-α engagement with tumor necrosis
factor receptor 1 initiates RIPK1 oligomerization via death domain
interactions, culminating in kinase activation (58). RIPK1 is subsequently incorporated
into a complex containing Fas-associated death domain (FADD) and
caspase-8 (complex IIa), in which caspase-8 acts as a critical
molecular switch. Upon activation, caspase-8 cleaves RIPK1 and
RIPK3, thereby promoting apoptotic cell death while concurrently
suppressing necroptosis initiation (59). By contrast, when caspase-8
activity is compromised (for example, by genetic deletion or
pharmacological inhibition) RIPK1 interacts with RIPK3 to
facilitate formation of the RIPK1-RIPK3 complex, known as the
necrosome (60-62). Activated RIPK3 phosphorylates
MLKL, converting it from an inactive monomer to a functional
oligomeric state (63). The
phosphorylated MLKL oligomers acquire membrane-targeting capability
through conformational changes and subsequently translocate to the
plasma membrane, ultimately leading to cellular swelling, membrane
rupture and necroptosis. The resultant released of
damage-associated molecular patterns (DAMPs), including bioactive
molecules such as high mobility group box 1 (HMGB1) and ATP, have
been demonstrated to exert immunomodulatory effects (64). Studies have demonstrated that
selective deficiency or functional impairment of caspase-8 in the
intestinal epithelium markedly increases susceptibility to
necroptosis, exacerbates experimental colitis and further
underscores its protective role in the pathogenesis of UC (65,66).
Studies encompassing patients with UC and animal
models have substantiated that hyperactivation of the necroptosis
pathway represents a pivotal pathogenic determinant, contributing
to both intestinal barrier disruption and aberrant inflammatory
cascades (67,68). Histopathological analyses have
revealed markedly elevated expression levels of RIPK1, RIPK3 and
MLKL in IECs, accompanied by enhanced phosphorylation status,
within the inflammatory lesions of patients with UC and
experimental models (22).
Accumulating evidence indicates that additional regulatory
molecules, including purinergic receptor P2Y14 (P2Y14), retinoic
acid-inducible gene I (RIG-I) and macrophage migration inhibitory
factor (MIF), may participate in the fine-tuned regulation of this
process. For instance, the purinergic receptor P2Y14 is upregulated
in the inflamed colonic mucosa of patients with UC, where it
facilitates RIPK1 transcription via the cAMP/protein kinase A/cAMP
response element-binding protein signaling pathway, thereby
aggravating IEC necroptosis and amplifying intestinal inflammation
(69). Furthermore, upon ligand
engagement, the cytosolic RNA sensor RIG-I upregulates MLKL
expression via interferon signaling, directly facilitating pore
formation (70). Alternatively,
RIG-I may indirectly amplify RIPK3-dependent necroptosis by
eliciting cytokine production, including type I interferons and TNF
(71). Under pathological stress
conditions such as ischemia and inflammation, MIF actively fosters
RIPK1-mediated necroptosis, thereby intensifying tissue injury
(72,73). Collectively, these findings
underscore that necroptosis in UC is governed by a sophisticated,
multifaceted regulatory network. Furthermore, investigations using
the DSS-induced murine colitis model have demonstrated
significantly upregulated expression of A20-binding inhibitor of
NF-κB activation 1, RIPK1, RIPK3 and MLKL in colonic tissues.
Notably, pharmacological blockade with Nec-1s potently inhibits
RIPK1 kinase activity, markedly suppressing IEC necroptosis and
mitigating colonic inflammatory responses (74). Subsequent analyses of clinical
specimens have corroborated a statistically significant positive
correlation between RIPK3 expression in the colonic tissues of
patients with UC and disease severity indices. Concurrently,
genetic ablation of the RIPK3 gene in mice confers notable
protection against IEC apoptosis through blockade of the Toll-like
receptor 4 (TLR4)/myeloid differentiation primary response protein
88 (MyD88)/NF-κB pathway, effectively alleviating experimental
colitis (75).
Contemporary research endeavors are centered on the
development of targeted inhibitors against pivotal necroptosis
regulators, such as RIPK1, RIPK3 and MLKL, with accumulating
evidence demonstrating that pharmacologically modulating these
targets in IEC can effectively ameliorate UC. For instance, the
citrus flavonoid naringenin and curcumin have been shown to
suppress IEC necroptosis by downregulating the mRNA expression of
RIPK3 and MLKL, thereby preserving intestinal barrier integrity and
markedly ameliorating colitis pathology (76,77). A natural chalcone derivative,
ermanin from the genus Leptinella, has exhibited dual
inhibition of RIPK1/3 kinases in the DSS-induced colitis model.
Mechanistic elucidation revealed that it attenuates intestinal
barrier impairment via blockade of MLKL phosphorylation,
demonstrating promising therapeutic potential for UC (78). Further investigations
demonstrated that polysaccharides from pine pollen and their
sulfated derivatives markedly attenuate the inflammatory index in
the colitis model, diminish IEC necroptosis incidence and enhance
mucosal barrier function through augmented mucin 2 secretion,
thereby furnishing robust experimental substantiation for clinical
translation (79). The
traditional Chinese herbal remedy Sargentodoxa cuneata
ameliorates disease manifestations in murine colitis models by
curtailing IEC necroptosis (80).
In addition to the aforementioned natural products,
chemically modified compounds and targeted chemical agents have
likewise unveiled therapeutic utility. For instance, the
myricetin-3-O-β-d-lactose sodium salt derivative
M10, engineered via incorporation of a hydrophilic glycosyl moiety,
displays full aqueous solubility and high stability. A study has
established that oral administration of M10 inhibits necroptosis in
inflamed colonic epithelium by suppressing TNF-α signaling,
exhibiting superior efficacy relative to mesalazine in averting
chronic colitis (81).
Indole-3-carbinol, a naturally occurring dietary agonist of the
aryl hydrocarbon receptor, upon receptor engagement, impedes RIPK1
activation and necrosome assembly in a temporally regulated manner,
thereby reducing IEC apoptosis and ameliorating intestinal
inflammation (82). As a
linchpin modulator of necroptosis and inflammatory cascades, RIPK1
inhibitors potently disrupt inflammatory signaling, attenuate IEC
injury and curtail leukocyte infiltration. Multiple studies have
corroborated that RIPK1 inhibitors such as SZ-15, HtrA2 and
LY3009120 markedly attenuate colonic inflammation, foster tissue
regeneration and ameliorate UC progression, thereby illuminating
the compelling therapeutic prospects of RIPK1-targeted
interventions in UC management (83-85).
Dynamic regulatory nodes orchestrate the delicate
equilibrium between necroptosis and apoptosis, ensuring adaptive
responses to inflammatory stimuli. In the nascent phases of
inflammation, where cellular insult remains modest, the organism
preferentially engages programmed apoptotic pathways. This
non-inflammatory clearance modality safeguards tissue homeostasis
and mitigates excessive immune activation (86). This phase is characterized by
selective engagement of apoptotic cascades, where the extrinsic
apoptotic pathway mediated by the Fas/FasL pathway and the
mitochondrial-dependent intrinsic apoptotic pathway are
preferentially activated to facilitate the orderly clearance of
damaged cells (87). As
inflammatory escalation ensues, perturbations in intracellular
homeostasis, such as caspase inhibition or aberrant upregulation of
anti-apoptotic effectors, precipitate a phenotypic shift in cell
death modality toward necroptosis (88). Notably, DAMPs released during
necroptosis engage pattern recognition receptors on adjacent cells
via paracrine signaling, thereby upregulating death receptor
expression and fostering an apoptosis-prone milieu (89). This ultimately leads to a
positive feedback loop of cell death and inflammation,
significantly exacerbating intestinal epithelial barrier
dysfunction and the spread of inflammation (90). Precise modulation of
apoptosis-associated gene expression is expected to fundamentally
restore the homeostasis of cell death and promote the repair of
intestinal epithelial barrier function. This paradigm unveils a
novel molecular intervention strategy for personalized UC
management, holding notable clinical translational promise.
Nonetheless, effectively implementing these conceptual advances
into practical therapeutics mandates surmounting critical
challenges, including target specificity, efficacious delivery
platforms and inter-individual heterogeneity in gene expression
signatures.
Pyroptosis, a distinct programmed inflammatory cell
death modality, has a molecular foundation based on
inflammasome-dependent activation of select caspase family members,
notably caspase-1 and the caspase-4/5/11 isoforms (91,92). In the pathological evolution of
UC, the NLR family pyrin domain containing 3 (NLRP3) inflammasome
has emerged as a pivotal regulatory hub mediating inflammatory
cascades within the intestinal mucosa. As an intracellular
multiprotein complex, the canonical NLRP3 inflammasome incorporates
three foundational constituents: The pattern recognition receptor
NLRP3, the adaptor protein apoptosis-associated speck-like protein
containing a CARD (ASC) and the procaspase-1 precursor (93). Under homeostatic conditions,
NLRP3 adopts an auto-inhibited conformation with its activation
meticulously governed by an array of modulatory factors, thereby
safeguarding equilibrium in IECs. Upon perturbation of the
intestinal milieu by pathological cues, pathological stimuli
including pathogen-associated molecular patterns (PAMPs), DAMPs or
metabolic stress, the NLRP3 inflammasome initiates its assembly
process (94). In patients with
UC, intestinal dysbiosis results in a marked elevation of LPS
derived from Gram-negative bacteria. Functioning as a canonical
PAMP, LPS initiates nuclear translocation of NF-κB through
engagement with TLR4. Activated NF-κB upregulates NLRP3 gene
expression and fosters synthesis of pro-inflammatory cytokines
(95,96). Consequent to secondary signal
stimulation, intracellular potassium efflux, ROS accumulation or
lysosomal destabilization, the nascent NLRP3 undergoes NACHT
domain-mediated oligomerization, recruiting the adaptor protein ASC
to form multiprotein inflammasome complexes. ASC, through PYD-CARD
domain interplay, facilitates autoproteolytic maturation of
procaspase-1, yielding enzymatically proficient caspase-1
heterotetramers (97). Activated
caspase-1 exerts dichotomous biological effects: i) Orchestrates
extracellular release of mature IL-1β and IL-18 through cleavage of
their pro-forms; and ii) specifically cleaves gasdermin D (GSDMD)
to generate the pore-forming N-terminal domain (GSDMD-N). GSDMD-N
subsequently oligomerizes to form transmembrane pores on the plasma
membrane, triggering cellular osmotic lysis (98). This lytic event facilitates a
large release of DAMPs and HMGB1, thereby engendering a
self-amplifying feedback loop that intensifies intestinal
inflammatory amplification (99).
Pyroptosis in IECs triggers a cascade of immune
responses that disrupt the intestinal immune perturbations that
destabilize the intestinal immune milieu, a phenomenon intimately
linked to UC pathogenesis. In DSS-induced colitis models,
transgenic overexpression of lipocalin-2 in wild-type mice markedly
exacerbates colonic inflammation and epithelial injury, concomitant
with elevated pyroptosis biomarkers specifically localized to the
IECs of colonic mucosa derived from patients with UC (100). Type III interferons (IFN-λ)
have been demonstrated to augment IEC pyroptosis, thereby
undermining mucosal wound repair and curtailing regenerative
potential (101). Danger
signals released during pyroptosis foster dendritic cell maturation
and enhance their antigen-presenting capacity, driving naïve T cell
differentiation toward pro-inflammatory Th1 and Th17 subsets
(102). Concurrently, these
signals attenuate regulatory T cell functionality, engendering an
immunosuppressive imbalance that perpetuates uncontrolled
inflammatory responses (103).
Collectively, these observations delineate IEC pyroptosis as a
perpetuator of intestinal inflammation in UC via a self-sustaining
inflammatory circuit, which fundamentally underpins the therapeutic
refractoriness encountered in clinical practice.
While pyroptosis predominantly exerts
pro-inflammatory and tissue-damaging effects in the pathogenesis of
UC, elucidation of its molecular underpinnings furnishes a
foundational rationale for devising targeted therapeutic
interventions. Phytosterols, a class of plant-derived bioactive
sterols, exhibit therapeutic potential through their prototypical
constituent sitosterol (SIT). Mechanistically, SIT modulates the
NLRP3/caspase-1/GSDMD signaling axis to significantly attenuate IEC
pyroptosis and pro-inflammatory cytokine release, while
concurrently augmenting TJ protein expression to bolster mucosal
barrier integrity (104).
However, most evidence remains preclinical, and the
pharmacokinetics, bioavailability and safety of SIT in chronic UC
contexts remain inadequately characterized. The extract from
Astragalus membranaceus Bunge has been shown to ameliorate
UC by curtailing IEC pyroptosis via upregulation of phospholipase
C-β2 (105). The pathological
characteristics of refractory UC progression primarily encompass a
self-perpetuating cycle linking mucosal barrier disruption and
unrelenting inflammatory amplification. Recent evidence
substantiates that nanocarrier-mediated targeted delivery of
4-octyl itaconate to IECs potently suppresses GSDME-mediated
pyroptosis (14). Further
investigation has revealed that the artemisinin derivative SM934
exhibits dual modulatory effects in experimental colitis models; it
concurrently inhibits programmed cell death modalities and
abrogates caspase-1-dependent pyroptotic cascades, thereby markedly
ameliorating epithelial barrier impairment (106). Nevertheless, the long-term
efficacy, toxicity and dosing regimens for SM934 in chronic models
warrant comprehensive evaluation to surmount translational hurdles.
Schisandrin B attenuates NLRP3 inflammasome activation-mediated
IL-1β secretion and IEC pyroptosis in colitis models by activating
AMPK/Nuclear factor erythroid 2-related factor 2 (Nrf2)-dependent
signaling to mitigate ROS-induced mitochondrial damage, suggesting
its potential as a therapeutic strategy for acute colitis (107). In LPS/ATP-induced in
vitro models of IEC pyroptosis and inflammation utilizing HT29
human colonic carcinoma cells, resveratrol forestalls pyroptosis
onset by impeding NF-κB pathway activation (108). Beyond phytogenic agents,
mesenchymal stem cells (MSCs) and their secreted exosomes have
recently garnered notable attention for therapeutic applications.
Hair follicle-derived MSC exosomes convey differentially expressed
microRNAs (miRNAs) that concurrently suppress both tumor necrosis
factor-related apoptosis-inducing ligand (TRAIL) signaling and
IFN-γ inflammatory pathways, thereby effectively inhibiting IEC
pyroptosis (109). Notably,
bone marrow-derived MSC exosomes selectively suppress
NLRP3/caspase-1 pathway activation via miR-539-5p shuttling,
thereby modulating the pyroptosis-associated molecular network and
ultimately attenuating UC progression (110).
In recent years, contemporary drug discovery
paradigms targeting pyroptosis have diversified, with numerous
chemical and nucleic acid therapeutics evincing robust efficacy.
For instance, miR-141-3p effectively inhibits LPS-induced IEC
pyroptosis by targeting the HSP90 molecular chaperone, while
concurrently alleviating inflammation in DSS-induced murine
colitis, highlighting its promise as a nucleic acid-based
therapeutic approach for UC (111). The broad-spectrum antiviral
nucleotide remdesivir likewise ameliorates IEC pyroptosis and gut
inflammatory cascades by inhibiting the NLRP3 inflammasome and
downstream caspase-1/GSDMD signaling (112). Meanwhile, ruscogenin, a
steroidal sapogenin from Ophiopogon japonicus, has
demonstrated potential in mitigating inflammatory processes by
suppressing NLRP3 inflammasome activation and caspase-1-dependent
canonical pyroptosis (113).
Moreover, in acute severe UC, the methyl-donor betaine effectively
inhibits oxidative stress-induced inflammatory pyroptosis, thereby
expanding the therapeutic repertoire for UC (114). These findings suggest that
precision regulation strategies targeting aberrant pyroptosis in
IECs may disrupt the 'pyroptosis-inflammation-barrier disruption'
positive feedback loop, thereby establishing an innovative
therapeutic paradigm for UC pathological intervention.
Ferroptosis, a unique modality of programmed cell
death, was initially characterized and designated in 2012 by Dixon
et al (115) through
systematic investigation. Distinct from other forms of regulated
cell death, ferroptosis is pathologically defined by the intricate
interplay between iron ion homeostasis dysregulation and lipid
peroxidation accumulation (116). IECs predominantly internalize
iron via transferrin receptor 1-dependent endocytosis (117). Upon endocytosis,
Fe3+ is enzymatically reduced to biologically active
Fe2+ species via metalloreductases such as
Six-transmembrane epithelial antigen of prostate 3 (118,119). During the pathogenesis of UC,
IECs are subjected to a sustained inflammatory milieu, which
elicits dysregulated mobilization of excess Fe2+ from
the labile iron pool and thereby instigates ROS generation through
Fenton reaction cascades (19).
The Fenton reaction employs Fe2+-mediated catalytic
cycles to decompose hydrogen peroxide into highly reactive hydroxyl
radicals. These radicals preferentially attack membrane
phospholipids containing polyunsaturated fatty acids (120), thereby initiating a
self-amplifying chain reaction of lipid peroxidation. Glutathione
peroxidase 4 (GPX4), a pivotal regulator of lipid redox balance,
forms the cornerstone of ferroptosis suppression. Under homeostatic
conditions, GPX4 utilizes reduced glutathione (GSH) to transmute
deleterious lipid hydroperoxides into innocuous lipid alcohols,
effectively suppressing the cascade amplification of lipid
peroxidation (121). Notably,
in UC progression, multiple pathogenic factors synergistically
inhibit GPX4 enzymatic activity, thereby impairing its
detoxification of peroxidation intermediates. Upon decompensation
of the antioxidant defense system, lipid peroxidation products
surpass cellular homeostatic thresholds, ultimately triggering
ferroptosis in IECs through the disruption of membrane structural
integrity (122,123).
As the principal mucosal interface organ, the
intestinal tract exhibits a characteristic oxidative stress
microenvironment due to persistent exposure to exogenous stimuli
including microbiota, metabolites and food-derived antigens
(124). Investigations have
revealed a reciprocal pathophysiological interplay between
ferroptosis in IECs and gut oxidative stress status. In murine
colitis models, inflammation-orchestrated oxidative perturbations
markedly elevate ferroptosis effectors [cyclooxygenase-2 and
acyl-CoA synthetase long-chain family member 4 (ACSL4)] in parallel
with diminished protein abundance of GPX4 and ferritin heavy chain
1. These molecular signatures demonstrate positive correlation with
the extent of IEC injury (125). Pharmacological preconditioning
with ferroptosis inhibitors has been demonstrated to markedly
attenuate above-mentioned histopathological manifestations in
DSS-induced colitis. Integrative multi-omics profiling has unveiled
signature expression patterns of ferroptosis-linked genes in
tissues derived from patients with UC (126,127). Mechanistic investigations have
demonstrated that ACSL4, a master regulator of ferroptosis,
accelerates IEC ferroptosis by activating the NF-κB signaling
pathway, thereby contributing to UC pathogenesis (128).
There have been notable advancements in therapeutic
modalities targeting ferroptosis regulation in IECs, with
innovative interventions conferring antioxidant cytoprotection and
inflammatory attenuation via selective ferroptosis pathway
blockade. Astragalus polysaccharide (APS), the predominant
bioactive polysaccharide from the traditional Chinese herb A.
membranaceus, has demonstrated anti-ferroptotic efficacy across
multiple experimental models. Experimental evidence demonstrated
that APS significantly suppresses ferroptosis progression and
sustains cellular homeostasis in DSS-induced murine colitis models
and as well as RSL3-treated human IECs in vitro (129). Multi-omics analyses has
unveiled that vanillic acid (VA) modulates the ferroptosis axis
through targeted ligation to carbonic anhydrase IX and stromal
interaction molecule 1, effectively restoring intestinal epithelial
barrier integrity and emerging as a novel therapeutic candidate for
UC (130). Nevertheless, the
target fidelity and off-target liabilities of VA within intricate
human milieus necessitate rigorous delineation. Emerging data
implicate that disrupted iron homeostasis exacerbates colitis
progression via dual mechanisms involving ferroptosis activation
and gut microbiota dysbiosis (131,132). Pharmacological investigations
confirm that palmatine, a natural isoquinoline alkaloid, markedly
diminishes colonic iron deposition and ameliorates experimental UC
pathology via multi-target modulation, concurrently suppressing
NF-κB inflammatory signaling, ROS generation and ferroptosis signal
cascades (133). Notably, the
oral iron chelator deferasirox represses IEC ferroptosis,
reprograms gut microbiota architecture and augments short-chain
fatty acid (SCFA) biosynthesis, thereby ameliorating DSS-induced UC
inflammation through multifaceted mechanisms and evincing clinical
promise (134). However, the
use of iron chelators still requires cautious monitoring of dosage
and safety to avoid potential adverse effects (135,136).
Beyond directly targeting the ferroptosis pathway,
certain nutritional factors and metabolites also demonstrate
therapeutic value. Butyrate is a microbiota-derived SCFA depleted
in colitis-afflicted murine cohorts. A study has revealed that
sodium butyrate treatment activates the Nrf2/GPX4 signaling
pathway, thereby inhibiting ferroptosis, alleviating oxidative
stress and inflammatory responses and restoring intestinal barrier
function (137). Furthermore,
vitamin D has been demonstrated to attenuate ferroptosis in
DSS-induced murine models and LPS-stimulated HCT116 cells through
ACSL4 repression, consequently tempering UC severity (138). Additionally, supplementation
with the essential trace element selenium, particularly in the form
of sodium selenite, effectively reduces IEC mortality,
intracellular iron content, lipid ROS and mitochondrial membrane
damage, ultimately attenuating DSS-induced colitis (139). Nevertheless, these benefits,
optimal dosing paradigms, delivery modalities and protracted
efficacy demand validation via robust clinical trials.
While breakthrough advances have been achieved in
natural compound research, exosomes (critical mediators of
intercellular communication) have emerged as possessing unique
biomedical potential in the therapeutics of UC. Human umbilical
cord MSC-derived exosomes can deliver miR-129-5p to specifically
suppress the expression of ACSL4, dually regulating the progression
of lipid peroxidation and the function of the GSH-GPX4 antioxidant
axis (142). A recent study has
demonstrated that endometrial regenerative cell-derived exosomes
enhance GSH biosynthesis and GPX4 enzymatic activity while
coordinately attenuating tissue iron accumulation, malondialdehyde
levels and ACSL4 protein expression, demonstrating marked efficacy
in ameliorating both histopathological damage and clinical
manifestations of colitis (143). An in-depth analysis of the
molecular regulatory network underlying abnormal ferroptosis in
IECs would not only yield precise therapeutic targets for UC but
also promises the advent of innovative pharmacotherapeutics through
targeted modulation of key ferroptosis pathways, thereby improving
intestinal barrier function.
Autophagy, an evolutionarily conserved intracellular
degradation system, orchestrates cellular homeostasis and stress
adaptation via regulated material recycling. Based on substrate
transport mechanisms, autophagy is categorized into three subtypes:
Macroautophagy, microautophagy and chaperone-mediated autophagy
(144). Among these,
macroautophagy predominates as the primary regulatory mechanism,
forming the cornerstone of autophagy research. Upon
microenvironmental stimuli such as nutrient deprivation or
oxidative stress (145,146), endoplasmic reticulum-resident
unfolded protein response sensors synergize with other stress
detectors to trigger a transcriptional cascade of autophagy-related
genes via regulators including transcription factor EB (147). This process initiates with the
formation of a double-membrane structure termed the phagophore in
the cytoplasm (148), which
achieves quality control by selectively enveloping targeted
substrates including damaged organelles and misfolded proteins.
Following subsequent membrane extension and closure, the phagophore
matures into an autophagosome that fuses with lysosomal membranes
to form an autolysosome (149).
Within the autolysosomal lumen, acid hydrolases degrade the
enclosed cargo into recyclable metabolites, such as amino acids and
free fatty acids (150), that
are effluxed into the cytoplasm through lysosomal transporters.
These metabolites are released into the cytoplasm via lysosomal
membrane transporters, where they re-enter biosynthetic pathways or
fuel the tricarboxylic acid cycle, thereby completing the
closed-loop regulation of intracellular material recycling
(151).
In recent years, the regulatory role of autophagy in
UC pathogenesis has garnered substantial attention in
gastroenterology research, with its dual regulatory properties
exhibiting complex biological effects during disease progression.
Pathologically, UC is characterized by dysregulated dynamic
equilibrium of pro-/anti-inflammatory cytokine networks, coupled
with unrelenting inflammatory cascades, aberrant intestinal barrier
permeability and perturbed expression of TJ proteins, collectively
driving disease chronicity (152). Mechanistic investigations have
elucidated that TNF-α-driven inflammatory microenvironments impair
intestinal epithelial barrier integrity via impaired autophagic
flux, manifesting as aberrant claudin-2 expression and TJ
disruption, a process mechanistically linked to autolysosomal
system dysfunction (153).
Analyses of clinical specimens from patients with active UC reveal
notable downregulation of autophagy regulator activating
transcription factor 4 in intestinal mucosa, implicating diminished
autophagic capacity in disease exacerbation (154). Pharmacological autophagy
activation in LPS-stimulated Caco-2 cell models and experimental
colitis animals significantly reduces pro-inflammatory cytokines
and ameliorates oxidative stress indices, underscoring the
therapeutic potential of autophagy modulation in IBD (155,156). In DSS-induced colitis models,
autophagy impairment intensifies intestinal inflammation through
hyperactivation of the NLRP3 inflammasome, thereby promoting
caspase-1 cleavage and maturation of IL-1β/IL-18 (157,158). Crucially, excessive autophagy
activation may induce type II programmed cell death, underscoring
the importance of precise autophagic activity regulation given this
dual-edged effect. In Erbin knockout murine models, autophagy
inhibitor chloroquine mitigates DSS-induced hyperinflammation by
blocking autophagosome-lysosome fusion, with mechanisms involving
downregulation of cell death-associated proteins, thereby
illustrating the context-specific utility of autophagy suppression
(159). Collectively, current
evidence establishes autophagy as a homeostatic modulator in UC,
with its pro-survival and pro-death duality being precisely
controlled by microenvironmental signaling networks.
Pharmacological investigations on the classical
formula Baitouweng decoction have demonstrated that it augments
autophagic flux via AMPKα phosphorylation activation and mTORC1
complex inhibition, thereby restoring intestinal epithelial barrier
integrity and attenuating disease activity index scores in
DSS-induced murine colitis models (160). Mechanistic investigations
reveal that procyanidin A1 augments autophagic activity through the
AMPK/mTOR/p70S6K signaling axis in LPS-stimulated IEC inflammation
models, markedly suppressing pro-inflammatory cytokine secretion
(161). Notably, the probiotic
strain Lactobacillus plantarum OLL2712 activates protective
autophagy in IECs via the MyD88-dependent pathway, thereby
reorganizing TJ proteins to fortify intestinal barrier mechanics
(162). Although
probiotic-mediated autophagy modulation represents a burgeoning
therapeutic avenue, elucidation of strain specificity, dosage
regimens and host microbiome interplay remains imperative. Compound
sophora decoction significantly alleviates DSS-induced intestinal
inflammation by fostering autophagy through suppression of PI3K/AKT
pathway activation (163).
Moreover, the Jianpi Qingchang (JPQC) decoction, composed of nine
traditional Chinese medicinal herbs, alleviates colitis progression
by suppressing endoplasmic reticulum stress-associated excessive
autophagy in IECs within a DSS-induced model (164) Notably, JPQC underscores the
contextual therapeutic merits of both autophagy activation and
inhibition, albeit the dose-dependent dichotomous effects warrant
deeper scrutiny.
Accumulating evidence indicates that disrupting
autophagic homeostasis is a pivotal node in UC pathogenesis, with
pharmacological restoration of autophagic dynamic equilibrium
emerging as a novel therapeutic target. Beyond the aforementioned
Chinese herbal and natural products, diverse active compounds and
biomacromolecules have shown therapeutic promise in UC management
via autophagy modulation. For instance, epimedium polysaccharide
(EPS), a bioactive compound, has been shown to engender autophagy
augmentation via the AMPK/mTOR pathway amid colonic inflammation,
exerting a protective effect in DSS-induced UC models, which
suggests EPS as a potential therapeutic target (165). Upregulation of circular RNA
HECTD1 has been shown to orchestrate HuR-dependent autophagy in
IECs via miR-182-5p sequestration, consequently ameliorating UC
histopathology (166) The Slit
family of glycoproteins (Slit1-3), canonically expressed in neural
and immune compartments, exert regulatory oversight on inflammatory
cascades (167,168). Overexpression of Slit2 has been
shown to sustain intestinal stem cell proliferation and normal
autophagic flux in mice following DSS challenge, thereby mitigating
colonic inflammation and curtailing pro-inflammatory cytokine
secretion (169). These
emerging strategies provide innovative perspectives for UC
intervention through the modulation of autophagy; however, the
majority of current studies remain focused on mechanistic
exploration and are still considerably distant from clinical
application. Future research should focus on enhancing target
specificity, optimizing delivery systems and clarifying the
pharmacodynamic-to-toxicological equilibrium, thereby facilitating
their translation into clinical practice.
Disruption of intestinal barrier integrity is firmly
established as a central pathological feature of UC, driven by
intricate molecular mechanisms involving a dynamic interplay
between IEC homeostatic imbalance and dysregulation of the immune
microenvironment. The present review summarizes the major
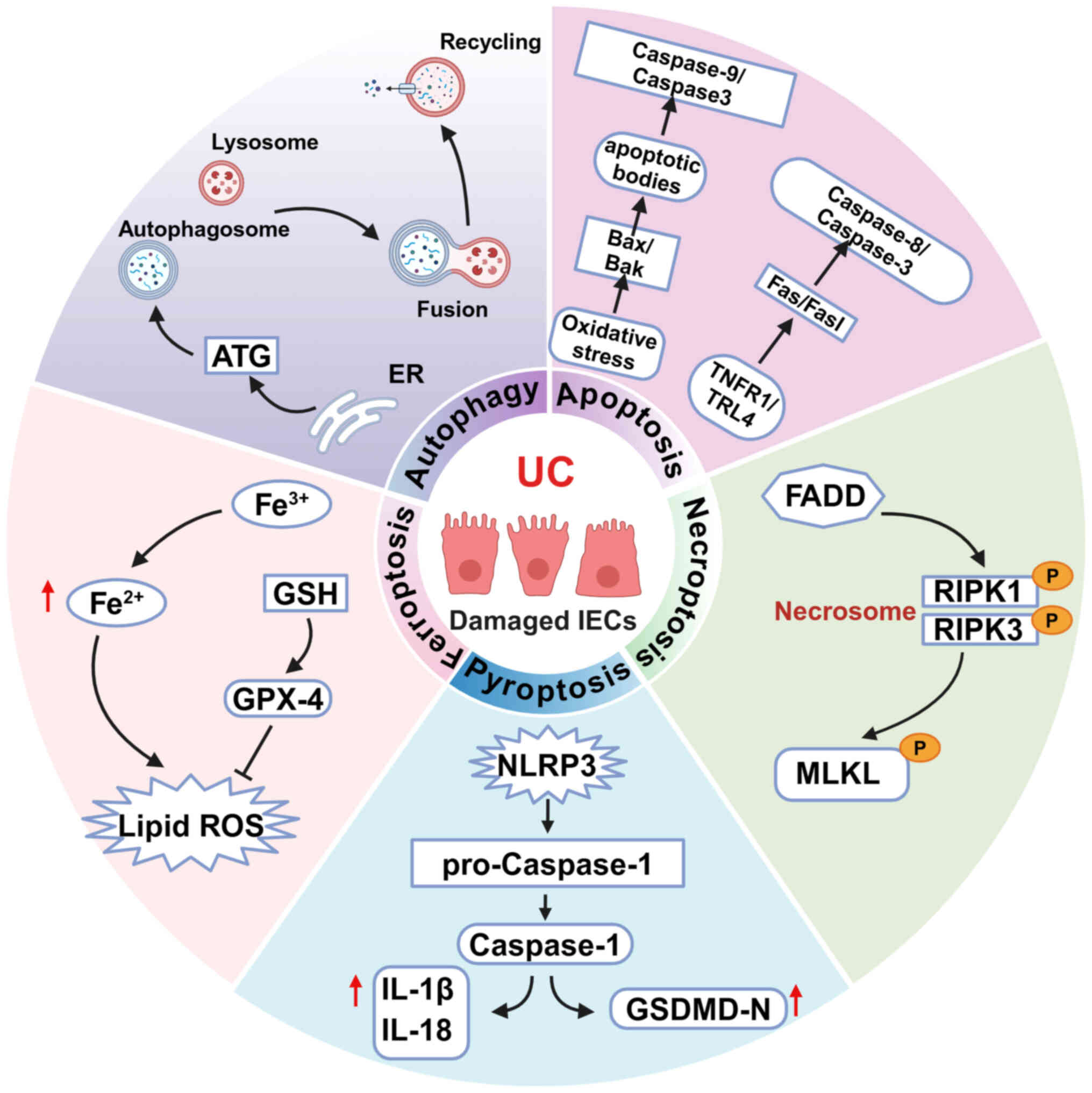
programmed cell death modalities in IECs in UC: Autophagy,
ferroptosis, pyroptosis, apoptosis and necroptosis (Fig. 1). As the principal components of
the intestinal mechanical barrier, dysregulated IEC death directly
impairs TJ complexes, leading to epithelial barrier breakdown and
subsequent hyperactivation of pattern recognition receptor-mediated
innate immune responses. This series of events leads to a
self-sustaining cycle of persistent inflammation and mucosal
injury. Recent progress in single-cell RNA sequencing and
intestinal organoid technologies has yielded unprecedented insights
into the spatiotemporal dynamics of IEC death modalities and their
association with UC pathological phenotypes, providing a molecular
framework for understanding disease heterogeneity and
patient-specific variations (14,17,100,170-172).
Recent studies have unveiled a novel programmed cell
death pathway known as PANoptosis, which merges key molecular
characteristics of pyroptosis, apoptosis and necroptosis to form a
multifaceted death complex (173,174). The regulatory framework of
PANoptosis entails the integration and interplay of multiple
signaling pathways. Research has highlighted the crucial roles of
key regulatory molecules such as Z-DNA binding protein 1 (ZBP1),
RIPK1 and RIPK3 in the PANoptosis pathway (175). The nucleic acid sensor ZBP1,
activated during pathogen infection or cellular stress by
recognizing viral nucleic acids or endogenous DAMPs, undergoes
conformational changes to recruit and phosphorylate RIPK3 (176). Notably, upon activation by
death receptor ligands or PAMPs, RIPK1 initiates PANoptosis
signaling transduction by engaging downstream molecules through its
death domain. Additionally, RIPK1 forms a functional complex with
RIPK3, jointly driving the phosphorylation cascade of MLKL and
ultimately inducing cells to enter the PANoptosis program (177,178). Within IECs, a complex molecular
interplay exists among apoptosis, necroptosis, pyroptosis and the
resulting PANoptosis, all of which play critical roles in the
pathogenesis of IBD. Caspase-8 and its adaptor protein FADD serve
as central molecules interconnecting these three programmed cell
death pathways (59). Upon
excessive stimulation by TNF or TLR signaling, caspase-8 is
activated and regulates cell fate by initiating apoptosis through
the cleavage of downstream caspase-3/7, while also modulating RIPK1
and RIPK3 to inhibit necroptosis (179). Concurrently, caspase-8 can
interact with ASC to activate caspase-1 and cleave GSDMD, thereby
triggering pyroptosis (180).
When caspase-8 function is compromised or inhibited by pathogens,
both apoptotic and pyroptotic pathways are impaired, leading cells
to undergo RIPK3-mediated necroptosis, which exacerbates intestinal
barrier disruption and inflammatory responses. Furthermore, in the
absence of caspase-1 in IECs, inflammasome sensors such as NLRP1b
and NLRC4 can still initiate apoptosis through ASC-dependent
caspase-8 activation (181).
The necroptosis effector MLKL can also promote ASC oligomerization
and caspase-1 activation, thereby linking necroptosis with
pyroptosis (182). These
intricate molecular interactions illustrate that apoptosis,
necroptosis and pyroptosis are interconnected through shared
molecular components, collectively driving epithelial cell death,
barrier dysfunction and exacerbated intestinal inflammation in UC.
Emerging evidence has demonstrated that PANoptosis plays a crucial
regulatory role in the pathogenesis of IBD, particularly UC. In the
DSS-induced murine colitis model, IECs exhibited concurrent
phenotypes of Dynamin related protein 1 mediated mitochondrial
fission and ZBP1-dependent PANoptosis. Notably, analysis of
clinical specimens revealed a significant positive correlation
between the activation levels of PANoptosis in IECs and the
clinical activity index of patients with UC (21,183).
Despite notable advancements in recent years in
elucidating the mechanisms of IEC programmed death in UC, several
crucial issues remain unresolved. For instance, the intricate
interplay between various programmed death pathways, including
apoptosis, necroptosis, pyroptosis and ferroptosis, requires
further elucidation. For instance, the novel multimodal cell death
pathway PANoptosis, which integrates key molecular features of
apoptosis, necroptosis and pyroptosis, necessitates in-depth
exploration of its specific regulatory mechanisms in UC and its
synergistic or antagonistic interactions with other death pathways.
Additionally, the dynamic interactions between programmed death
pathways and the intestinal microenvironment, such as dysbiosis and
metabolic products, demand thorough investigation to unravel the
complex networks in the pathological progression of UC. Given the
intricate interconnections and mutual regulation among these
programmed cell death pathways, combination therapies that target
multiple key nodes of apoptosis, necroptosis and pyroptosis
concurrently may enable precise modulation of the cell death
network, leading to more comprehensive intestinal protection and
inflammation control. Furthermore, emerging strategies such as the
utilization of gene-editing technologies (such as CRISPR/Cas9) to
regulate the expression of critical genes including caspase-8 and
RIPK3, or the application of stem cell and genetically engineered
cell-based therapies to restore compromised epithelial function,
offer promising avenues for future therapeutics. Collectively,
interventions that target programmed cell death in IECs at multiple
levels and from various angles hold significant promise for
mitigating epithelial damage and inflammation in UC, thereby
advancing therapeutic strategies for this disease.
Natural bioactive compounds and
nanoparticle-mediated drug delivery systems have gained significant
attention in biomedical research due to their notable translational
potential, especially in the management of metabolic disorders and
tumor immunomodulation. Their therapeutic efficacy stems from their
unique ability to simultaneously modulate multiple molecular
targets, while maintaining favorable biosafety profiles, offering
innovative and multifaceted strategies for UC treatment. Notably,
an increasing body of evidence highlights the distinct potential of
natural bioactive compounds and nanoparticle-mediated drug delivery
systems in UC therapy, particularly in their capability to preserve
colonic epithelial barrier integrity and mitigate pro-inflammatory
cytokine cascades by precisely regulating programmed cell death
pathways. These compounds and nanoparticles exert their protective
effects by targeting key molecular mechanisms underlying epithelial
dysfunction and immune dysregulation, thereby addressing the dual
pathological axes of UC.
The present review provides a comprehensive analysis
of the central role of IEC death in the pathogenesis of
UC-associated chronic inflammation and mucosal injury. Furthermore,
it consolidates recent progress in the utilization of natural
small-molecule compounds and nanoparticle-mediated drug delivery
systems that selectively target abnormal IEC death to mitigate
barrier dysfunction, as summarized in Table SI. These compounds, sourced from
a variety of natural origins, have exhibited effectiveness in
modulating crucial signaling pathways, presenting promising
therapeutic opportunities for UC management. In terms of clinical
translation, while natural compounds and nanomedicines have
demonstrated significant potential in modulating programmed death
pathways, their targeting and bioavailability encounter challenges.
For instance, the delivery efficiency of nanomedicines is
constrained by the intricate intestinal milieu, and enhancing their
targeting, such as through surface ligand modification or
utilization of intestinal-specific receptors, represents a current
research priority. Additionally, despite the advantages of the
multi-target properties of natural compounds in treating UC, their
potential for unforeseen side effects necessitates further
structural refinement and pharmacological assessment. Future
research should integrate single-cell omics, organoid models and
systems pharmacology techniques to precisely decipher the molecular
mechanisms of programmed death pathways and develop more efficient
and safer targeted therapeutic strategies to surmount existing
treatment bottlenecks and improve the clinical prognosis of
patients with UC.
Looking forward, transformative breakthroughs in UC
therapy will necessitate the development of integrated therapeutic
strategies that concurrently target IEC death pathways and their
downstream hyperinflammatory responses. These strategies aim to
disrupt the self-sustaining cycle of 'barrier damage-inflammation
amplification' that underlies the core of UC pathogenesis. One
approach involves the development of therapeutic agents capable of
synergistically modulating multiple targets within the regulated
cell death network. Given the intricate molecular crosstalk among
different forms of programmed cell death, involving key molecules
such as caspase-8, RIPK3 and GSDMD, inhibitors targeting a single
pathway may be insufficient to fully prevent the dysregulated death
of IECs. The design of small molecules or biologics that
concurrently regulate multiple critical nodes holds potential for
more effectively restoring IEC homeostasis, thereby achieving
comprehensive protection of the intestinal barrier and improved
control of inflammation. Additionally, enhancing targeting
specificity while minimizing systemic side effects is crucial for
successful clinical translation. Employing nanotechnology,
antibody-drug conjugates or oral targeted delivery systems to
selectively accumulate therapeutics within inflamed intestinal
tissues or IECs can enhance local drug concentrations and markedly
reduce off-target effects in other organs. Novel therapeutic
approaches involving gene editing and cell therapy also warrant
exploration. While still in the early stages of investigation,
in vivo or ex vivo editing of dysregulated genes in
IEC (such as caspase-8 or RIPK3) using technologies such as
CRISPR/Cas9, or the administration of genetically engineered stem
cells or organoids to repair damaged epithelium, presents a highly
promising next-generation treatment strategy. These interventions
may provide novel options for patients with refractory UC who are
intolerant to conventional pharmacological or biological therapies.
In summary, future drug development for UC should transcend the
traditional 'single target, single disease' paradigm and adopt
integrated, multi-targeted, multimodal and precision-focused
strategies with an emphasis on local intestinal targeting. By
addressing these unresolved mechanisms and clinical translation
challenges, the present review not only establishes a new
theoretical framework for understanding the pathomechanism of UC
but also establishes a robust basis for the advancement of
innovative therapeutic strategies, offering significant scientific
and clinical implications.
Not applicable.
BW drafted and revised the manuscript. BW and SS
conceptualized the review and contributed to the writing. YL
participated in literature collation and manuscript editing. LG
reviewed and revised the manuscript. All authors contributed to the
manuscript. All authors read and approved the final version of the
manuscript. Data authentication is not applicable.
Not applicable.
Not applicable.
The authors declare that they have no competing
interests.
Not applicable.
This work was supported by the Research Foundation of the
Department of Education of Yunnan Province (grant no. 2025Y1195)
and Yunnan Fundamental Research Projects (grant no.
202501AT070405).
|
1
|
Buie MJ, Quan J, Windsor JW, Coward S,
Hansen TM, King JA, Kotze PG, Gearry RB, Ng SC, Mak JWY, et al:
Global hospitalization trends for Crohn's disease and ulcerative
colitis in the 21st century: A systematic review with temporal
analyses. Clin Gastroenterol Hepatol. 21:2211–2221. 2023.
View Article : Google Scholar
|
|
2
|
Ordás I, Eckmann L, Talamini M, Baumgart
DC and Sandborn WJ: Ulcerative colitis. Lancet. 380:1606–1619.
2012. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Leppkes M and Neurath MF: Cytokines in
inflammatory bowel diseases - Update 2020. Pharmacol Res.
158:1048352020. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Le Berre C, Loeuille D and Peyrin-Biroulet
L: Combination therapy with vedolizumab and tofacitinib in a
patient with ulcerative colitis and spondyloarthropathy. Clin
Gastroenterol Hepatol. 17:794–796. 2019. View Article : Google Scholar
|
|
5
|
Abdalla MI and Levesque BG: Progress in
corticosteroid use in the Era of biologics with room for
improvement. Am J Gastroenterol. 116:1187–1188. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Quansah E, Gardey E, Ramoji A,
Meyer-Zedler T, Goehrig B, Heutelbeck A, Hoeppener S, Schmitt M,
Waldner M, Stallmach A and Popp J: Intestinal epithelial barrier
integrity investigated by label-free techniques in ulcerative
colitis patients. Sci Rep. 13:26812023. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Chen Q, Chen T, Xiao H, Wang F, Li C, Hu
N, Bao L, Tong X, Feng Y, Xu Y, et al: APEX1 in intestinal
epithelium triggers neutrophil infiltration and intestinal barrier
damage in ulcerative colitis. Free Radic Biol Med. 225:359–373.
2024. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Peterson LW and Artis D: Intestinal
epithelial cells: Regulators of barrier function and immune
homeostasis. Nat Rev Immunol. 14:141–153. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Jiminez JA, Uwiera TC, Douglas Inglis G
and Uwiera RR: Animal models to study acute and chronic intestinal
inflammation in mammals. Gut Pathog. 7:292015. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Yan H and Ajuwon KM: Butyrate modifies
intestinal barrier function in IPEC-J2 cells through a selective
upregulation of tight junction proteins and activation of the Akt
signaling pathway. PLoS One. 12:e01795862017. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Mehandru S and Colombel JF: The intestinal
barrier, an arbitrator turned provocateur in IBD. Nat Rev
Gastroenterol Hepatol. 18:83–84. 2021. View Article : Google Scholar
|
|
12
|
Su L, Nalle SC, Shen L, Turner ES, Singh
G, Breskin LA, Khramtsova EA, Khramtsova G, Tsai PY, Fu YX, et al:
TNFR2 activates MLCK-dependent tight junction dysregulation to
cause apoptosis-mediated barrier loss and experimental colitis.
Gastroenterology. 145:407–415. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Fukuda T, Majumder K, Zhang H, Turner PV,
Matsui T and Mine Y: Adenine inhibits TNF-α signaling in intestinal
epithelial cells and reduces mucosal inflammation in a dextran
sodium sulfate-induced colitis mouse model. J Agric Food Chem.
64:4227–4234. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Li W, Chen D, Zhu Y, Ye Q, Hua Y, Jiang P,
Xiang Y, Xu Y, Pan Y, Yang H, et al: Alleviating pyroptosis of
intestinal epithelial cells to restore mucosal integrity in
ulcerative colitis by targeting delivery of 4-Octyl-itaconate. ACS
Nano. 18:16658–16673. 2024. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Chi F, Zhang G, Ren N, Zhang J, Du F,
Zheng X, Zhang C, Lin Z, Li R, Shi X and Zhu Y: The anti-alcoholism
drug disulfiram effectively ameliorates ulcerative colitis through
suppressing oxidative stresses-associated pyroptotic cell death and
cellular inflammation in colonic cells. Int Immunopharmacol.
111:1091172022. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Chen Y, Yan W, Chen Y, Zhu J, Wang J, Jin
H, Wu H, Zhang G, Zhan S, Xi Q, et al: SLC6A14 facilitates
epithelial cell ferroptosis via the C/EBPβ-PAK6 axis in ulcerative
colitis. Cell Mol Life Sci. 79:5632022. View Article : Google Scholar
|
|
17
|
Zhang J, Cen L, Zhang X, Tang C, Chen Y,
Zhang Y, Yu M, Lu C, Li M, Li S, et al: MPST deficiency promotes
intestinal epithelial cell apoptosis and aggravates inflammatory
bowel disease via AKT. Redox Biol. 56:1024692022. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Foerster EG, Mukherjee T, Cabral-Fernandes
L, Rocha JDB, Girardin SE and Philpott DJ: How autophagy controls
the intestinal epithelial barrier. Autophagy. 18:86–103. 2022.
View Article : Google Scholar :
|
|
19
|
Xu M, Tao J, Yang Y, Tan S, Liu H, Jiang
J, Zheng F and Wu B: Ferroptosis involves in intestinal epithelial
cell death in ulcerative colitis. Cell Death Dis. 11:862020.
View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Ma ZR, Li ZL, Zhang N, Lu B, Li XW, Huang
YH, Nouhoum D, Liu XS, Xiao KC, Cai LT, et al: Inhibition of
GSDMD-mediated pyroptosis triggered by Trichinella spiralis
intervention contributes to the alleviation of DSS-induced
ulcerative colitis in mice. Parasit Vectors. 16:2802023. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Wang JM, Yang J, Xia WY, Wang YM, Zhu YB,
Huang Q, Feng T, Xie LS, Li SH, Liu SQ, et al: Comprehensive
analysis of PANoptosis-related gene signature of ulcerative
colitis. Int J Mol Sci. 25:3482023. View Article : Google Scholar
|
|
22
|
Akanyibah FA, Zhu Y, Jin T, Ocansey DKW,
Mao F and Qiu W: The function of necroptosis and its treatment
target in IBD. Mediators Inflamm. 2024:72753092024. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Iwanaga T and Takahashi-Iwanaga H:
Disposal of intestinal apoptotic epithelial cells and their fate
via divergent routes. Biomed Res. 43:59–72. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Soroosh A, Fang K, Hoffman JM, Law IKM,
Videlock E, Lokhandwala ZA, Zhao JJ, Hamidi S, Padua DM, Frey MR,
et al: Loss of miR-24-3p promotes epithelial cell apoptosis and
impairs the recovery from intestinal inflammation. Cell Death Dis.
13:82021. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Wolf P, Schoeniger A and Edlich F:
Pro-apoptotic complexes of BAX and BAK on the outer mitochondrial
membrane. Biochim Biophys Acta Mol Cell Res. 1869:1193172022.
View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Sheridan C, Delivani P, Cullen SP and
Martin SJ: Bax- or Bak-induced mitochondrial fission can be
uncoupled from cytochrome C release. Mol Cell. 31:570–585. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Zhou M, Li Y, Hu Q, Bai XC, Huang W, Yan
C, Scheres SH and Shi Y: Atomic structure of the apoptosome:
mechanism of cytochrome c- and dATP-mediated activation of Apaf-1.
Genes Dev. 29:2349–2361. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Albalawi GA, Albalawi MZ, Alsubaie KT,
Albalawi AZ, Elewa MAF, Hashem KS and Al-Gayyar MMH: Curative
effects of crocin in ulcerative colitis via modulating apoptosis
and inflammation. Int Immunopharmacol. 118:1101382023. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Lin S, Zhang X, Zhu X, Jiao J, Wu Y, Li Y
and Zhao L: Fusobacterium nucleatum aggravates ulcerative colitis
through promoting gut microbiota dysbiosis and dysmetabolism. J
Periodontol. 94:405–418. 2023. View Article : Google Scholar
|
|
30
|
Li Y, Ma M, Wang X, Li J, Fang Z, Li J,
Yang B, Lu Y, Xu X and Li Y: Celecoxib alleviates the DSS-induced
ulcerative colitis in mice by enhancing intestinal barrier
function, inhibiting ferroptosis and suppressing apoptosis.
Immunopharmacol Immunotoxicol. 46:240–254. 2024. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Iwamoto M, Makiyama K, Koji T, Kohno S and
Nakane PK: Expression of Fas and Fas-ligand in epithelium of
ulcerative colitis. Nihon Rinsho. 54:1970–1974. 1996.In Japanese.
PubMed/NCBI
|
|
32
|
Souza HS, Tortori CJ, Castelo-Branco MT,
Carvalho AT, Margallo VS, Delgado CF, Dines I and Elia CC:
Apoptosis in the intestinal mucosa of patients with inflammatory
bowel disease: Evidence of altered expression of FasL and perforin
cytotoxic pathways. Int J Colorectal Dis. 20:277–286. 2005.
View Article : Google Scholar
|
|
33
|
Tang R, Jiang L, Ji Q, Kang P, Liu Y, Miao
P, Xu X and Tang M: Resveratrol targeting MDM2/P53/PUMA axis to
inhibit colonocyte apoptosis in DSS-induced ulcerative colitis
mice. Front Pharmacol. 16:15729062025. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Chen J: The Cell-Cycle Arrest and
Apoptotic Functions of p53 in Tumor Initiation and Progression.
Cold Spring Harb Perspect Med. 6:a0261042016. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Eissa N, Hussein H, Diarra A, Elgazzar O,
Gounni AS, Bernstein CN and Ghia JE: Semaphorin 3E regulates
apoptosis in the intestinal epithelium during the development of
colitis. Biochem Pharmacol. 166:264–273. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Parker A, Vaux L, Patterson AM, Modasia A,
Muraro D, Fletcher AG, Byrne HM, Maini PK, Watson AJM and Pin C:
Elevated apoptosis impairs epithelial cell turnover and shortens
villi in TNF-driven intestinal inflammation. Cell Death Dis.
10:1082019. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Yan R, Liang X and Hu J: MALAT1 promotes
colonic epithelial cell apoptosis and pyroptosis by sponging
miR-22-3p to enhance NLRP3 expression. PeerJ. 12:e184492024.
View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Yang L, Wu G, Wu Q, Peng L and Yuan L:
METTL3 overexpression aggravates LPS-induced cellular inflammation
in mouse intestinal epithelial cells and DSS-induced IBD in mice.
Cell Death Discov. 8:622022. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Wu MM, Wang QM, Huang BY, Mai CT, Wang CL,
Wang TT and Zhang XJ: Dioscin ameliorates murine ulcerative colitis
by regulating macrophage polarization. Pharmacol Res.
172:1057962021. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Palmela C, Chevarin C, Xu Z, Torres J,
Sevrin G, Hirten R, Barnich N, Ng SC and Colombel JF:
Adherent-invasive Escherichia coli in inflammatory bowel disease.
Gut. 67:574–587. 2018. View Article : Google Scholar
|
|
41
|
Chen B, Wang Y, Niu Y and Li S: Acalypha
australis L. Extract attenuates DSS-induced ulcerative colitis in
mice by regulating inflammatory factor release and blocking NF-κB
activation. J Med Food. 26:663–671. 2023. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Lasa JS, Olivera PA, Danese S and
Peyrin-Biroulet L: Efficacy and safety of biologics and small
molecule drugs for patients with moderate-to-severe ulcerative
colitis: A systematic review and network meta-analysis. Lancet
Gastroenterol Hepatol. 7:161–170. 2022. View Article : Google Scholar
|
|
43
|
Geng Z, Zuo L, Li J, Yin L, Yang J, Duan
T, Wang L, Zhang X, Song X, Wang Y and Hu J: Ginkgetin improved
experimental colitis by inhibiting intestinal epithelial cell
apoptosis through EGFR/PI3K/AKT signaling. FASEB J. 38:e238172024.
View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Lokman MS, Kassab RB, Salem FAM,
Elshopakey GE, Hussein A, Aldarmahi AA, Theyab A, Alzahrani KJ,
Hassan KE, Alsharif KF, et al: Asiatic acid rescues intestinal
tissue by suppressing molecular, biochemical, and histopathological
changes associated with the development of ulcerative colitis.
Biosci Rep. 44:BSR202320042024. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
He Z, Liu J and Liu Y: Daphnetin
attenuates intestinal inflammation, oxidative stress, and apoptosis
in ulcerative colitis via inhibiting REG3A-dependent JAK2/STAT3
signaling pathway. Environ Toxicol. 38:2132–2142. 2023. View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Hu Q, Xie J, Jiang T, Gao P, Chen Y, Zhang
W, Yan J, Zeng J, Ma X and Zhao Y: Paeoniflorin alleviates
DSS-induced ulcerative colitis by suppressing inflammation,
oxidative stress, and apoptosis via regulating serum metabolites
and inhibiting CDC42/JNK signaling pathway. Int Immunopharmacol.
142(Pt A): 1130392024. View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Gao F, Wu S, Zhang K, Xu Z, Zhang X, Zhu Z
and Quan F: Goat milk exosomes ameliorate ulcerative colitis in
mice through modulation of the intestinal barrier, gut microbiota,
and metabolites. J Agric Food Chem. 72:23196–23210. 2024.
View Article : Google Scholar : PubMed/NCBI
|
|
48
|
Zhu F, Wei C, Wu H, Shuai B, Yu T, Gao F,
Yuan Y, Zuo D, Liu X, Zhang L and Fan H: Hypoxic mesenchymal stem
cell-derived exosomes alleviate ulcerative colitis injury by
limiting intestinal epithelial cells reactive oxygen species
accumulation and DNA damage through HIF-1α. Int Immunopharmacol.
113(Pt A): 1094262022. View Article : Google Scholar : PubMed/NCBI
|
|
49
|
Li H, Fan C, Lu H, Feng C, He P, Yang X,
Xiang C, Zuo J and Tang W: Protective role of berberine on
ulcerative colitis through modulating enteric glial
cells-intestinal epithelial cells-immune cells interactions. Acta
Pharm Sin B. 10:447–461. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
50
|
Gao C, Liu L, Zhou Y, Bian Z, Wang S and
Wang Y: Novel drug delivery systems of Chinese medicine for the
treatment of inflammatory bowel disease. Chin Med. 14:232019.
View Article : Google Scholar : PubMed/NCBI
|
|
51
|
Liu C, Gong Q, Liu W, Zhao Y, Yan X and
Yang T: Berberine-loaded PLGA nanoparticles alleviate ulcerative
colitis by targeting IL-6/IL-6R axis. J Transl Med. 22:9632024.
View Article : Google Scholar : PubMed/NCBI
|
|
52
|
Buck A, Rezaei K, Quazi A, Goldmeier G,
Silverglate B and Grossberg GT: The donepezil transdermal system
for the treatment of patients with mild, moderate, or severe
Alzheimer's disease: A critical review. Expert Rev Neurother.
24:607–614. 2024. View Article : Google Scholar : PubMed/NCBI
|
|
53
|
Gwon HJ, Cho W, Choi SW, Lim DS,
Tanriverdi EÇ, Abd El-Aty AM, Jeong JH and Jung TW: Donepezil
improves skeletal muscle insulin resistance in obese mice via the
AMPK/FGF21-mediated suppression of inflammation and ferroptosis.
Arch Pharm Res. 47:940–953. 2024. View Article : Google Scholar : PubMed/NCBI
|
|
54
|
Li A, Zhang J, Chen K, Wang J, Xu A and
Wang Z: Donepezil attenuates inflammation and apoptosis in
ulcerative colitis via regulating LRP1/AMPK/NF-κB signaling. Pathol
Int. 73:549–559. 2023. View Article : Google Scholar : PubMed/NCBI
|
|
55
|
Li C, Xu Y, Gao T, Zhang S, Lin Z, Gu S,
Fang Y, Yuan X, Yu S, Jiang Q, et al: Ruxolitinib alleviates
inflammation, apoptosis, and intestinal barrier leakage in
ulcerative colitis via STAT3. Inflamm Bowel Dis. 29:1191–1201.
2023. View Article : Google Scholar : PubMed/NCBI
|
|
56
|
Tang R, Xu J, Zhang B, Liu J, Liang C, Hua
J, Meng Q, Yu X and Shi S: Ferroptosis, necroptosis, and pyroptosis
in anticancer immunity. J Hematol Oncol. 13:1102020. View Article : Google Scholar : PubMed/NCBI
|
|
57
|
Newton K: RIPK1 and RIPK3: Critical
regulators of inflammation and cell death. Trends Cell Biol.
25:347–353. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
58
|
Degterev A, Ofengeim D and Yuan J:
Targeting RIPK1 for the treatment of human diseases. Proc Natl Acad
Sci USA. 116:9714–9722. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
59
|
Zhang W, Zhu C, Liao Y, Zhou M, Xu W and
Zou Z: Caspase-8 in inflammatory diseases: A potential therapeutic
target. Cell Mol Biol Lett. 29:1302024. View Article : Google Scholar : PubMed/NCBI
|
|
60
|
Mifflin L, Ofengeim D and Yuan J:
Receptor-interacting protein kinase 1 (RIPK1) as a therapeutic
target. Nat Rev Drug Discov. 19:553–571. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
61
|
Zhou Y, Xiang Y, Liu S, Li C, Dong J, Kong
X, Ji X, Cheng X and Zhang L: RIPK3 signaling and its role in
regulated cell death and diseases. Cell Death Discov. 10:2002024.
View Article : Google Scholar : PubMed/NCBI
|
|
62
|
Yuan J, Amin P and Ofengeim D: Necroptosis
and RIPK1-mediated neuroinflammation in CNS diseases. Nat Rev
Neurosci. 20:19–33. 2019. View Article : Google Scholar :
|
|
63
|
Karlowitz R and van Wijk SJL: Surviving
death: emerging concepts of RIPK3 and MLKL ubiquitination in the
regulation of necroptosis. FEBS J. 290:37–54. 2023. View Article : Google Scholar
|
|
64
|
Mohammed S, Thadathil N, Selvarani R,
Nicklas EH, Wang D, Miller BF, Richardson A and Deepa SS:
Necroptosis contributes to chronic inflammation and fibrosis in
aging liver. Aging Cell. 20:e135122021. View Article : Google Scholar : PubMed/NCBI
|
|
65
|
Jia Z, Xu C, Shen J, Xia T, Yang J and He
Y: The natural compound celastrol inhibits necroptosis and
alleviates ulcerative colitis in mice. Int Immunopharmacol.
29:552–559. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
66
|
Lehle AS, Farin HF, Marquardt B, Michels
BE, Magg T, Li Y, Liu Y, Ghalandary M, Lammens K, Hollizeck S, et
al: Intestinal inflammation and dysregulated immunity in patients
with inherited caspase-8 deficiency. Gastroenterology. 156:275–278.
2019. View Article : Google Scholar
|
|
67
|
Pierdomenico M, Negroni A, Stronati L,
Vitali R, Prete E, Bertin J, Gough PJ, Aloi M and Cucchiara S:
Necroptosis is active in children with inflammatory bowel disease
and contributes to heighten intestinal inflammation. Am J
Gastroenterol. 109:279–287. 2014. View Article : Google Scholar
|
|
68
|
Liu L, Liang L, Yang C, Zhou Y and Chen Y:
Extracellular vesicles of Fusobacterium nucleatum compromise
intestinal barrier through targeting RIPK1-mediated cell death
pathway. Gut Microbes. 13:1–20. 2021. View Article : Google Scholar
|
|
69
|
Liu C, Wang H, Han L, Zhu Y, Ni S, Zhi J,
Yang X, Zhi J, Sheng T, Li H and Hu Q: Targeting P2Y(14)R protects
against necroptosis of intestinal epithelial cells through
PKA/CREB/RIPK1 axis in ulcerative colitis. Nat Commun. 15:20832024.
View Article : Google Scholar : PubMed/NCBI
|
|
70
|
Dunker W, Ye X, Zhao Y, Liu L, Richardson
A and Karijolich J: TDP-43 prevents endogenous RNAs from triggering
a lethal RIG-I-dependent interferon response. Cell Rep.
35:1089762021. View Article : Google Scholar : PubMed/NCBI
|
|
71
|
Maelfait J, Liverpool L and Rehwinkel J:
Nucleic acid sensors and programmed cell death. J Mol Biol.
432:552–568. 2020. View Article : Google Scholar :
|
|
72
|
Li Y, Zou C, Chen C, Li S, Zhu Z, Fan Q,
Pang R, Li F, Chen Z, Wang Z, et al: Myeloid-derived MIF drives
RIPK1-mediated cerebromicrovascular endothelial cell death to
exacerbate ischemic brain injury. Proc Natl Acad Sci USA.
120:e22190911202023. View Article : Google Scholar : PubMed/NCBI
|
|
73
|
Soppert J, Kraemer S, Beckers C, Averdunk
L, Möllmann J, Denecke B, Goetzenich A, Marx G, Bernhagen J and
Stoppe C: Soluble CD74 reroutes MIF/CXCR4/AKT-mediated survival of
cardiac myofibroblasts to necroptosis. J Am Heart Assoc.
7:e0093842018. View Article : Google Scholar : PubMed/NCBI
|
|
74
|
Bao J, Ye B and Ren Y: ABIN1 inhibits
inflammation through necroptosis-dependent pathway in ulcerative
colitis. Genet Res (Camb). 2022:93135592022. View Article : Google Scholar : PubMed/NCBI
|
|
75
|
Duan C, Xu X, Lu X, Wang L and Lu Z: RIP3
knockdown inhibits necroptosis of human intestinal epithelial cells
via TLR4/MyD88/NF-κB signaling and ameliorates murine colitis. BMC
Gastroenterol. 22:1372022. View Article : Google Scholar
|
|
76
|
Zhong Y, Tu Y, Ma Q, Chen L, Zhang W, Lu
X, Yang S, Wang Z and Zhang L: Curcumin alleviates experimental
colitis in mice by suppressing necroptosis of intestinal epithelial
cells. Front Pharmacol. 14:11706372023. View Article : Google Scholar : PubMed/NCBI
|
|
77
|
Zhang J, Lei H, Hu X and Dong W:
Hesperetin ameliorates DSS-induced colitis by maintaining the
epithelial barrier via blocking RIPK3/MLKL necroptosis signaling.
Eur J Pharmacol. 873:1729922020. View Article : Google Scholar : PubMed/NCBI
|
|
78
|
Shen X, Chen H, Wen T, Liu L, Yang Y, Xie
F and Wang L: A natural chalcone cardamonin inhibits necroptosis
and ameliorates dextran sulfate sodium (DSS)-induced colitis by
targeting RIPK1/3 kinases. Eur J Pharmacol. 954:1758402023.
View Article : Google Scholar : PubMed/NCBI
|
|
79
|
Li Z, Wang H, Wang Z and Geng Y: Pine
pollen polysaccharides' and sulfated polysaccharides' effects on UC
mice through modulation of cell tight junctions and RIPK3-dependent
necroptosis pathways. Molecules. 27:76822022. View Article : Google Scholar : PubMed/NCBI
|
|
80
|
Wang Y, Zhang B, Liu S, Xu E and Wang Z:
The traditional herb Sargentodoxa cuneata alleviates DSS-induced
colitis by attenuating epithelial barrier damage via blocking
necroptotic signaling. J Ethnopharmacol. 319(Pt 3): 1173732024.
View Article : Google Scholar
|
|
81
|
Zhou XL, Yang J, Qu XJ, Meng J, Miao RR
and Cui SX: M10, a Myricetin-3-O-b-D-lactose sodium salt, prevents
ulcerative colitis through inhibiting necroptosis in mice. Front
Pharmacol. 11:5573122020. View Article : Google Scholar : PubMed/NCBI
|
|
82
|
Peng C, Wu C, Xu X, Pan L, Lou Z, Zhao Y,
Jiang H, He Z and Ruan B: Indole-3-carbinol ameliorates necroptosis
and inflammation of intestinal epithelial cells in mice with
ulcerative colitis by activating aryl hydrocarbon receptor. Exp
Cell Res. 404:1126382021. View Article : Google Scholar : PubMed/NCBI
|
|
83
|
Zeng YS, Peng J, Gao XF, Tian D, Zhan W,
Liu J, Hu XJ, Huang S, Tian ST, Qiu L, et al: A novel
gut-restricted RIPK1 inhibitor, SZ-15, ameliorates DSS-induced
ulcerative colitis. Eur J Pharmacol. 937:1753812022. View Article : Google Scholar : PubMed/NCBI
|
|
84
|
Zhang C, He A, Liu S, He Q, Luo Y, He Z,
Chen Y, Tao A and Yan J: Inhibition of HtrA2 alleviated dextran
sulfate sodium (DSS)-induced colitis by preventing necroptosis of
intestinal epithelial cells. Cell Death Dis. 10:3442019. View Article : Google Scholar : PubMed/NCBI
|
|
85
|
Zhang C, Luo Y, He Q, Liu S, He A and Yan
J: A pan-RAF inhibitor LY3009120 inhibits necroptosis by preventing
phosphorylation of RIPK1 and alleviates dextran sulfate
sodium-induced colitis. Clin Sci (Lond). 133:919–932. 2019.
View Article : Google Scholar : PubMed/NCBI
|
|
86
|
Kayagaki N, Webster JD and Newton K:
Control of cell death in health and disease. Annu Rev Pathol.
19:157–180. 2024. View Article : Google Scholar
|
|
87
|
Krzyzowska M, Shestakov A, Eriksson K and
Chiodi F: Role of Fas/FasL in regulation of inflammation in vaginal
tissue during HSV-2 infection. Cell Death Dis. 2:e1322011.
View Article : Google Scholar : PubMed/NCBI
|
|
88
|
Ma D, Wang X, Liu J, Cui Y, Luo S and Wang
F: The development of necroptosis: What we can learn. Cell Stress
Chaperones. 28:969–987. 2023. View Article : Google Scholar : PubMed/NCBI
|
|
89
|
Nakano H, Murai S and Moriwaki K:
Regulation of the release of damage-associated molecular patterns
from necroptotic cells. Biochem J. 479:677–685. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
90
|
Ma M, Jiang W and Zhou R: DAMPs and
DAMP-sensing receptors in inflammation and diseases. Immunity.
57:752–771. 2024. View Article : Google Scholar : PubMed/NCBI
|
|
91
|
Chen M, Rong R and Xia X: Spotlight on
pyroptosis: role in pathogenesis and therapeutic potential of
ocular diseases. J Neuroinflammation. 19:1832022. View Article : Google Scholar : PubMed/NCBI
|
|
92
|
Wei X, Xie F, Zhou X, Wu Y, Yan H, Liu T,
Huang J, Wang F, Zhou F and Zhang L: Role of pyroptosis in
inflammation and cancer. Cell Mol Immunol. 19:971–992. 2022.
View Article : Google Scholar : PubMed/NCBI
|
|
93
|
Fu J and Wu H: Structural mechanisms of
NLRP3 inflammasome assembly and activation. Annu Rev Immunol.
41:301–316. 2023. View Article : Google Scholar : PubMed/NCBI
|
|
94
|
Biasizzo M and Kopitar-Jerala N: Interplay
Between NLRP3 Inflammasome and Autophagy. Front Immunol.
11:5918032020. View Article : Google Scholar : PubMed/NCBI
|
|
95
|
Que X, Zheng S, Song Q, Pei H and Zhang P:
Fantastic voyage: The journey of NLRP3 inflammasome activation.
Genes Dis. 11:819–829. 2024. View Article : Google Scholar
|
|
96
|
Nabavi-Rad A, Sadeghi A, Asadzadeh Aghdaei
H, Yadegar A, Smith SM and Zali MR: The double-edged sword of
probiotic supplementation on gut microbiota structure in
Helicobacter pylori management. Gut Microbes. 14:21086552022.
View Article : Google Scholar : PubMed/NCBI
|
|
97
|
Wang Y, Yuan H, Shen D, Liu S, Kong W,
Zheng K, Yang J and Ge L: Artemisinin attenuated ischemic stroke
induced pyroptosis by inhibiting ROS/TXNIP/NLRP3/Caspase-1
signaling pathway. Biomed Pharmacother. 177:1168942024. View Article : Google Scholar : PubMed/NCBI
|
|
98
|
Zhang MY, Jiang YX, Yang YC, Liu JY, Huo
C, Ji XL and Qu YQ: Cigarette smoke extract induces pyroptosis in
human bronchial epithelial cells through the ROS/NLRP3/caspase-1
pathway. Life Sci. 269:1190902021. View Article : Google Scholar : PubMed/NCBI
|
|
99
|
Burdette BE, Esparza AN, Zhu H and Wang S:
Gasdermin D in pyroptosis. Acta Pharm Sin B. 11:2768–2782. 2021.
View Article : Google Scholar : PubMed/NCBI
|
|
100
|
Yang Y, Li S, Liu K, Zhang Y, Zhu F, Ben
T, Chen Z and Zhi F: Lipocalin-2-mediated intestinal epithelial
cells pyroptosis via NF-κB/NLRP3/GSDMD signaling axis adversely
affects inflammation in colitis. Biochim Biophys Acta Mol Basis
Dis. 1870:1672792024. View Article : Google Scholar
|
|
101
|
Jena KK, Mambu J, Boehmer D, Sposito B,
Millet V, de Sousa Casal J, Muendlein HI, Spreafico R, Fenouil R,
Spinelli L, et al: Type III interferons induce pyroptosis in gut
epithelial cells and impair mucosal repair. Cell.
187:7533–7550.e23. 2024. View Article : Google Scholar : PubMed/NCBI
|
|
102
|
Liu X, Zhou M, Dai Z, Luo S, Shi Y, He Z
and Chen Y: Salidroside alleviates ulcerative colitis via
inhibiting macrophage pyroptosis and repairing the
dysbacteriosis-associated Th17/Treg imbalance. Phytother Res.
37:367–382. 2023. View Article : Google Scholar
|
|
103
|
Zhang S, Zhong R, Tang S, Chen L and Zhang
H: Metabolic regulation of the Th17/Treg balance in inflammatory
bowel disease. Pharmacol Res. 203:1071842024. View Article : Google Scholar : PubMed/NCBI
|
|
104
|
Zhang D, Ge F, Ji J, Li YJ, Zhang FR, Wang
SY, Zhang SJ, Zhang DM and Chen M: β-sitosterol alleviates dextran
sulfate sodium-induced experimental colitis via inhibition of
NLRP3/Caspase-1/GSDMD-mediated pyroptosis. Front Pharmacol.
14:12184772023. View Article : Google Scholar
|
|
105
|
Shen J, Zhao Y and Cui W: Astragalus
mongholicus Bunge extract improves ulcerative colitis by promoting
PLCB2 to inhibit colonic epithelial cell pyroptosis. J
Ethnopharmacol. 334:1185542024. View Article : Google Scholar : PubMed/NCBI
|
|
106
|
Shao M, Yan Y, Zhu F, Yang X, Qi Q, Yang
F, Hao T, Lin Z, He P, Zhou Y, et al: Artemisinin analog SM934
alleviates epithelial barrier dysfunction via inhibiting apoptosis
and caspase-1-mediated pyroptosis in experimental colitis. Front
Pharmacol. 13:8490142022. View Article : Google Scholar : PubMed/NCBI
|
|
107
|
Zhang W, Wang W, Shen C, Wang X, Pu Z and
Yin Q: Network pharmacology for systematic understanding of
Schisandrin B reduces the epithelial cells injury of colitis
through regulating pyroptosis by AMPK/Nrf2/NLRP3 inflammasome.
Aging (Albany NY). 13:23193–23209. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
108
|
Zhao P, Ning J, Huang J and Huang X:
Mechanism of resveratrol on LPS/ATP-induced pyroptosis and
inflammatory response in HT29 cells. Autoimmunity. 57:24270942024.
View Article : Google Scholar : PubMed/NCBI
|
|
109
|
Chang Y, Zhang Y, Jiang Y, Zhao L, Lv C,
Huang Q, Guan J and Jin S: From hair to colon: Hair
follicle-derived MSCs alleviate pyroptosis in DSS-Induced
ulcerative colitis by releasing exosomes in a paracrine manner.
Oxid Med Cell Longev. 2022:90975302022. View Article : Google Scholar : PubMed/NCBI
|
|
110
|
Wang D, Xue H, Tan J, Liu P, Qiao C, Pang
C and Zhang L: Bone marrow mesenchymal stem cells-derived exosomes
containing miR-539-5p inhibit pyroptosis through NLRP3/caspase-1
signalling to alleviate inflammatory bowel disease. Inflamm Res.
71:833–846. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
111
|
Yan R, Liang X and Hu J: miR-141-3p
alleviates ulcerative colitis by targeting SUGT1 to inhibit colonic
epithelial cell pyroptosis. Autoimmunity. 56:22209882023.
View Article : Google Scholar : PubMed/NCBI
|
|
112
|
Oraby MA, Abdel Mageed SS, Amr Raouf A,
Abdelshafy DA, Ahmed EF, Khalil RT, Mangoura SA and Fadaly DS:
Remdesivir ameliorates ulcerative colitis-propelled cell
inflammation and pyroptosis in acetic acid rats by restoring
SIRT6/FoxC1 pathway. Int Immunopharmacol. 137:1124652024.
View Article : Google Scholar : PubMed/NCBI
|
|
113
|
Li J, Wu H, Zhou J, Jiang R, Zhuo Z, Yang
Q, Chen H and Sha W: Ruscogenin attenuates ulcerative colitis in
mice by inhibiting caspase-1-dependent pyroptosis via the
TLR4/NF-κB signaling pathway. Biomedicines. 12:9892024. View Article : Google Scholar
|
|
114
|
Chen L, Liu D, Mao M, Liu W, Wang Y, Liang
Y, Cao W and Zhong X: Betaine ameliorates acute sever ulcerative
colitis by inhibiting oxidative stress induced inflammatory
pyroptosis. Mol Nutr Food Res. 66:e22003412022. View Article : Google Scholar : PubMed/NCBI
|
|
115
|
Dixon SJ, Lemberg KM, Lamprecht MR, Skouta
R, Zaitsev EM, Gleason CE, Patel DN, Bauer AJ, Cantley AM, Yang WS,
et al: Ferroptosis: An iron-dependent form of nonapoptotic cell
death. Cell. 149:1060–1072. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
116
|
Mou Y, Wang J, Wu J, He D, Zhang C, Duan C
and Li B: Ferroptosis, a new form of cell death: Opportunities and
challenges in cancer. J Hematol Oncol. 12:342019. View Article : Google Scholar : PubMed/NCBI
|
|
117
|
Chen AC, Donovan A, Ned-Sykes R and
Andrews NC: Noncanonical role of transferrin receptor 1 is
essential for intestinal homeostasis. Proc Natl Acad Sci USA.
112:11714–11719. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
118
|
Lomphithak T, Sae-Fung A, Sprio S,
Tampieri A, Jitkaew S and Fadeel B: Exploiting the ferroaddiction
of pancreatic cancer cells using Fe-doped nanoparticles.
Nanomedicine. 55:1027142024. View Article : Google Scholar : PubMed/NCBI
|
|
119
|
Cai H, Chen S, Zhu Y, Zhuang S, Wang J,
Niu X, Cui T, Huang H, Ao R, Yu M, et al: A pH/STEAP
cascade-responsive nanomedicine with self-supplied peroxide for
precise chemodynamic therapy. Adv Healthc Mater. 14:e25007522025.
View Article : Google Scholar : PubMed/NCBI
|
|
120
|
Yang WS, Kim KJ, Gaschler MM, Patel M,
Shchepinov MS and Stockwell BR: Peroxidation of polyunsaturated
fatty acids by lipoxygenases drives ferroptosis. Proc Natl Acad Sci
USA. 113:E4966–E4975. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
121
|
Yang WS, SriRamaratnam R, Welsch ME,
Shimada K, Skouta R, Viswanathan VS, Cheah JH, Clemons PA, Shamji
AF, Clish CB, et al: Regulation of ferroptotic cancer cell death by
GPX4. Cell. 156:317–331. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
122
|
Sun SP, Lu YF, Li H, Weng CY, Chen JJ, Lou
YJ, Lyu D and Lyu B: AMPK activation alleviated dextran sulfate
sodium-induced colitis by inhibiting ferroptosis. J Dig Dis.
24:213–223. 2023. View Article : Google Scholar : PubMed/NCBI
|
|
123
|
Guo M, Du X and Wang X: Inhibition of
ferroptosis: A new direction in the treatment of ulcerative colitis
by traditional Chinese medicine. J Ethnopharmacol. 324:1177872024.
View Article : Google Scholar : PubMed/NCBI
|
|
124
|
Huang F, Zhang S, Li X, Huang Y, He S and
Luo L: STAT3-mediated ferroptosis is involved in ulcerative
colitis. Free Radic Biol Med. 188:375–385. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
125
|
Chen Y, Zhang P, Chen W and Chen G:
Ferroptosis mediated DSS-induced ulcerative colitis associated with
Nrf2/HO-1 signaling pathway. Immunol Lett. 225:9–15. 2020.
View Article : Google Scholar : PubMed/NCBI
|
|
126
|
Zhu J, Wu Y, Ge X, Chen X and Mei Q:
Discovery and validation of ferroptosis-associated genes of
ulcerative colitis. J Inflamm Res. 17:4467–4482. 2024. View Article : Google Scholar : PubMed/NCBI
|
|
127
|
Yokote A, Imazu N, Umeno J, Kawasaki K,
Fujioka S, Fuyuno Y, Matsuno Y, Moriyama T, Miyawaki K, Akashi K,
et al: Ferroptosis in the colon epithelial cells as a therapeutic
target for ulcerative colitis. J Gastroenterol. 58:868–882. 2023.
View Article : Google Scholar : PubMed/NCBI
|
|
128
|
Lam IH, Chan CI, Han M, Li L and Yu HH:
ACSL4 mediates inflammatory bowel disease and contributes to
LPS-induced intestinal epithelial cell dysfunction by activating
ferroptosis and inflammation. Open Med (Wars). 19:202409932024.
View Article : Google Scholar : PubMed/NCBI
|
|
129
|
Chen Y, Wang J, Li J, Zhu J, Wang R, Xi Q,
Wu H, Shi T and Chen W: Astragalus polysaccharide prevents
ferroptosis in a murine model of experimental colitis and human
Caco-2 cells via inhibiting NRF2/HO-1 pathway. Eur J Pharmacol.
911:1745182021. View Article : Google Scholar
|
|
130
|
Ni J, Zhang L, Feng G, Bao W, Wang Y,
Huang Y, Chen T, Chen J, Cao X, You K, et al: Vanillic acid
restores homeostasis of intestinal epithelium in colitis through
inhibiting CA9/STIM1-mediated ferroptosis. Pharmacol Res.
202:1071282024. View Article : Google Scholar : PubMed/NCBI
|
|
131
|
Long D, Mao C, Huang Y, Xu Y and Zhu Y:
Ferroptosis in ulcerative colitis: Potential mechanisms and
promising therapeutic targets. Biomed Pharmacother. 175:1167222024.
View Article : Google Scholar : PubMed/NCBI
|
|
132
|
Wu X, Zhao L, Yu Z and Zhang K:
Buddlejasaponin IVb Alleviates DSS-Induced ulcerative colitis
through the Nrf2/GPX4 pathway and gut microbiota modulation. J
Agric Food Chem. 72:23183–23195. 2024. View Article : Google Scholar : PubMed/NCBI
|
|
133
|
Ji W, Zhang Y, Qian X, Hu C and Huo Y:
Palmatine alleviates inflammation and modulates ferroptosis against
dextran sulfate sodium (DSS)-induced ulcerative colitis. Int
Immunopharmacol. 143(Pt 2): 1133962024. View Article : Google Scholar : PubMed/NCBI
|
|
134
|
Wu Y, Ran L, Yang Y, Gao X, Peng M, Liu S,
Sun L, Wan J, Wang Y, Yang K, et al: Deferasirox alleviates
DSS-induced ulcerative colitis in mice by inhibiting ferroptosis
and improving intestinal microbiota. Life Sci. 314:1213122023.
View Article : Google Scholar
|
|
135
|
Porter JB: A risk-benefit assessment of
iron-chelation therapy. Drug Saf. 17:407–421. 1997. View Article : Google Scholar
|
|
136
|
Di Paola A, Tortora C, Argenziano M,
Marrapodi MM and Rossi F: Emerging roles of the iron chelators in
inflammation. Int J Mol Sci. 23:79772022. View Article : Google Scholar : PubMed/NCBI
|
|
137
|
Chen H, Qian Y, Jiang C, Tang L, Yu J,
Zhang L, Dai Y and Jiang G: Butyrate ameliorated ferroptosis in
ulcerative colitis through modulating Nrf2/GPX4 signal pathway and
improving intestinal barrier. Biochim Biophys Acta Mol Basis Dis.
1870:1669842024. View Article : Google Scholar
|
|
138
|
Gao S, Sun C and Kong J: Vitamin D
attenuates ulcerative colitis by inhibiting ACSL4-mediated
ferroptosis. Nutrients. 15:48452023. View Article : Google Scholar : PubMed/NCBI
|
|
139
|
Shi J, Ji S, Xu M, Wang Y and Shi H:
Selenium inhibits ferroptosis in ulcerative colitis through the
induction of Nrf2/Gpx4. Clin Res Hepatol Gastroenterol.
48:1024672024. View Article : Google Scholar : PubMed/NCBI
|
|
140
|
Gao BB, Wang L, Li LZ, Fei ZQ, Wang YY,
Zou XM, Huang MC, Lei SS and Li B: Beneficial effects of oxymatrine
from Sophora flavescens on alleviating Ulcerative colitis by
improving inflammation and ferroptosis. J Ethnopharmacol.
332:1183852024. View Article : Google Scholar : PubMed/NCBI
|
|
141
|
Li W, Wang Y, Zhang Y, Fan Y, Liu J, Zhu
K, Jiang S and Duan J: Lizhong decoction ameliorates ulcerative
colitis by inhibiting ferroptosis of enterocytes via the
Nrf2/SLC7A11/GPX4 pathway. J Ethnopharmacol. 326:1179662024.
View Article : Google Scholar : PubMed/NCBI
|
|
142
|
Wei Z, Hang S, Wiredu Ocansey DK, Zhang Z,
Wang B, Zhang X and Mao F: Human umbilical cord mesenchymal stem
cells derived exosome shuttling mir-129-5p attenuates inflammatory
bowel disease by inhibiting ferroptosis. J Nanobiotechnology.
21:1882023. View Article : Google Scholar : PubMed/NCBI
|
|
143
|
Zhu Y, Qin H, Sun C, Shao B, Li G, Qin Y,
Kong D, Ren S, Wang H, Wang Z, et al: Endometrial regenerative
cell-derived exosomes attenuate experimental colitis through
downregulation of intestine ferroptosis. Stem Cells Int.
2022:30141232022. View Article : Google Scholar : PubMed/NCBI
|
|
144
|
Takabatake Y, Kimura T, Takahashi A and
Isaka Y: Autophagy and the kidney: health and disease. Nephrol Dial
Transplant. 29:1639–1647. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
145
|
Anding AL and Baehrecke EH: Autophagy in
cell life and cell death. Curr Top Dev Biol. 114:67–91. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
146
|
Mizushima N and Komatsu M: Autophagy:
renovation of cells and tissues. Cell. 147:728–741. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
147
|
Klionsky DJ, Cregg JM, Dunn WA Jr, Emr SD,
Sakai Y, Sandoval IV, Sibirny A, Subramani S, Thumm M, Veenhuis M
and Ohsumi Y: A unified nomenclature for yeast autophagy-related
genes. Dev Cell. 5:539–545. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
148
|
Mizushima N: A brief history of autophagy
from cell biology to physiology and disease. Nat Cell Biol.
20:521–527. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
149
|
Yu L, Chen Y and Tooze SA: Autophagy
pathway: Cellular and molecular mechanisms. Autophagy. 14:207–215.
2018. View Article : Google Scholar :
|
|
150
|
Shen HM and Mizushima N: At the end of the
autophagic road: An emerging understanding of lysosomal functions
in autophagy. Trends Biochem Sci. 39:61–71. 2014. View Article : Google Scholar
|
|
151
|
Takeshige K, Baba M, Tsuboi S, Noda T and
Ohsumi Y: Autophagy in yeast demonstrated with proteinase-deficient
mutants and conditions for its induction. J Cell Biol. 119:301–311.
1992. View Article : Google Scholar : PubMed/NCBI
|
|
152
|
Liu Z and Wang H: Probiotics alleviate
inflammatory bowel disease in mice by regulating intestinal
microorganisms-bile acid-NLRP3 inflammasome pathway. Acta Biochim
Pol. 68:687–693. 2021.PubMed/NCBI
|
|
153
|
Zhang C, Yan J, Xiao Y, Shen Y, Wang J, Ge
W and Chen Y: Inhibition of autophagic degradation process
contributes to claudin-2 expression increase and epithelial tight
junction dysfunction in TNF-α treated cell monolayers. Int J Mol
Sci. 18:572017.
|
|
154
|
Hu X, Deng J, Yu T, Chen S, Ge Y, Zhou Z,
Guo Y, Ying H, Zhai Q, Chen Y, et al: ATF4 deficiency promotes
intestinal inflammation in mice by reducing uptake of glutamine and
expression of antimicrobial peptides. Gastroenterology.
156:1098–1111. 2019. View Article : Google Scholar
|
|
155
|
Chen Z, Gu Q and Chen R: miR-146a-5p
regulates autophagy and NLRP3 inflammasome activation in epithelial
barrier damage in the in vitro cell model of ulcerative colitis
through the RNF8/Notch1/mTORC1 pathway. Immunobiology.
228:1523862023. View Article : Google Scholar : PubMed/NCBI
|
|
156
|
Zhou M, Xu W, Wang J, Yan J, Shi Y, Zhang
C, Ge W, Wu J, Du P and Chen Y: Boosting mTOR-dependent autophagy
via upstream TLR4-MyD88-MAPK signalling and downstream NF-κB
pathway quenches intestinal inflammation and oxidative stress
injury. EBioMedicine. 35:345–360. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
157
|
Martinon F, Burns K and Tschopp J: The
inflammasome: a molecular platform triggering activation of
inflammatory caspases and processing of proIL-beta. Mol Cell.
10:417–426. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
158
|
Takahama M, Akira S and Saitoh T:
Autophagy limits activation of the inflammasomes. Immunol Rev.
281:62–73. 2018. View Article : Google Scholar
|
|
159
|
Shen T, Li S, Cai LD, Liu JL, Wang CY, Gan
WJ, Li XM, Wang JR, Sun LN, Deng M, et al: Erbin exerts a
protective effect against inflammatory bowel disease by suppressing
autophagic cell death. Oncotarget. 9:12035–12049. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
160
|
Pan SM, Wang CL, Hu ZF, Zhang ML, Pan ZF,
Zhou RY, Wang XJ, Huang SW, Li YY, Wang Q, et al: Baitouweng
decoction repairs the intestinal barrier in DSS-induced colitis
mice via regulation of AMPK/mTOR-mediated autophagy. J
Ethnopharmacol. 318(Pt A): 1168882024. View Article : Google Scholar
|
|
161
|
Zhang H, Lang W, Liu X, Bai J, Jia Q and
Shi Q: Procyanidin A1 alleviates DSS-induced ulcerative colitis via
regulating AMPK/mTOR/p70S6K-mediated autophagy. J Physiol Biochem.
78:213–227. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
162
|
Watanabe-Yasuoka Y, Gotou A, Shimizu S and
Sashihara T: Lactiplantibacillus plantarum OLL2712 Induces
Autophagy via MYD88 and strengthens tight junction integrity to
promote the barrier function in intestinal epithelial cells.
Nutrients. 15:26552023. View Article : Google Scholar : PubMed/NCBI
|
|
163
|
Liu Y, Deng S, Sun L, He H, Zhou Q, Fan H,
Yang C and Yang J: Compound sophorae decoction mitigates
DSS-induced ulcerative colitis by activating autophagy through
PI3K-AKT pathway: A integrative research combining network
pharmacology and in vivo animal model validation. J Ethnopharmacol.
337:1188852025. View Article : Google Scholar
|
|
164
|
Qiao D, Liu X, Zhang Y, Zhang Z, Tang Y,
Chen Q, Shi Y, Chen Y, Tang Z and Dai Y: Jianpi-Qingchang decoction
alleviates ulcerative colitis by modulating endoplasmic reticulum
stress-related autophagy in intestinal epithelial cells. Biomed
Pharmacother. 158:1141332023. View Article : Google Scholar
|
|
165
|
Zhao L, Jiang T, Zhang Y and Shen Z:
Epimedium polysaccharides ameliorate ulcerative colitis by
inhibiting oxidative stress and regulating autophagy. J Sci Food
Agric. 105:2655–2670. 2025. View Article : Google Scholar
|
|
166
|
Xu Y, Tian Y, Li F, Wang Y, Yang J, Gong
H, Wan X and Ouyang M: Circular RNA HECTD1 mitigates ulcerative
colitis by promoting enterocyte autophagy Via miR-182-5p/HuR axis.
Inflamm Bowel Dis. 28:273–288. 2022. View Article : Google Scholar
|
|
167
|
Chen B, Carr L and Dun XP: Dynamic
expression of Slit1-3 and Robo1-2 in the mouse peripheral nervous
system after injury. Neural Regen Res. 15:948–958. 2020. View Article : Google Scholar
|
|
168
|
Wang L, Zheng J, Pathak JL, Chen Y, Liang
D, Yang L, Sun H, Zhong M, Wu L, Li L, et al: SLIT2 overexpression
in periodontitis intensifies inflammation and alveolar bone loss,
possibly via the activation of MAPK pathway. Front Cell Dev Biol.
8:5932020. View Article : Google Scholar : PubMed/NCBI
|
|
169
|
Xie J, Li L, Deng S, Chen J, Gu Q, Su H,
Wen L, Wang S, Lin C, Qi C, et al: Slit2/Robo1 mitigates
dss-induced ulcerative colitis by activating autophagy in
intestinal stem cell. Int J Biol Sci. 16:1876–1887. 2020.
View Article : Google Scholar : PubMed/NCBI
|
|
170
|
Wei S, Zhang J, Wu X, Chen M, Huang H,
Zeng S, Xiang Z, Li X and Dong W: Fusobacterium nucleatum
Extracellular Vesicles Promote Experimental Colitis by Modulating
Autophagy via the miR-574-5p/CARD3 Axis. Inflamm Bowel Dis.
29:9–26. 2023. View Article : Google Scholar
|
|
171
|
Wang XJ, Zhang D, Yang YT, Li XY, Li HN,
Zhang XP, Long JY, Lu YQ, Liu L, Yang G, et al: Suppression of
microRNA-222-3p ameliorates ulcerative colitis and
colitis-associated colorectal cancer to protect against oxidative
stress via targeting BRG1 to activate Nrf2/HO-1 signaling pathway.
Front Immunol. 14:10898092023. View Article : Google Scholar : PubMed/NCBI
|
|
172
|
Liang H, Zhang F, Wang W, Zhao W, Zhou J,
Feng Y, Wu J, Li M, Bai X, Zeng Z, et al: Heat shock transcription
factor 2 promotes mitophagy of intestinal epithelial cells through
PARL/PINK1/Parkin pathway in ulcerative colitis. Front Pharmacol.
13:8934262022. View Article : Google Scholar : PubMed/NCBI
|
|
173
|
Zhu P, Ke ZR, Chen JX, Li SJ, Ma TL and
Fan XL: Advances in mechanism and regulation of PANoptosis:
Prospects in disease treatment. Front Immunol. 14:11200342023.
View Article : Google Scholar : PubMed/NCBI
|
|
174
|
Qi Z, Zhu L, Wang K and Wang N:
PANoptosis: Emerging mechanisms and disease implications. Life Sci.
333:1221582023. View Article : Google Scholar : PubMed/NCBI
|
|
175
|
Sun X, Yang Y, Meng X, Li J, Liu X and Liu
H: PANoptosis: Mechanisms, biology, and role in disease. Immunol
Rev. 321:246–262. 2024. View Article : Google Scholar
|
|
176
|
You YP, Yan L, Ke HY, Li YP, Shi ZJ, Zhou
ZY, Yang HY, Yuan T, Gan YQ, Lu N, et al: Baicalin inhibits
PANoptosis by blocking mitochondrial Z-DNA formation and
ZBP1-PANoptosome assembly in macrophages. Acta Pharmacol Sin.
46:430–447. 2025. View Article : Google Scholar
|
|
177
|
Shi C, Cao P, Wang Y, Zhang Q, Zhang D,
Wang Y, Wang L and Gong Z: PANoptosis: A cell death characterized
by pyroptosis, apoptosis, and necroptosis. J Inflamm Res.
16:1523–1532. 2023. View Article : Google Scholar : PubMed/NCBI
|
|
178
|
Malireddi RKS, Kesavardhana S, Karki R,
Kancharana B, Burton AR and Kanneganti TD: RIPK1 distinctly
regulates yersinia-induced inflammatory cell death, PANoptosis.
Immunohorizons. 4:789–796. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
179
|
Newton K, Wickliffe KE, Dugger DL,
Maltzman A, Roose-Girma M, Dohse M, Kőműves L, Webster JD and Dixit
VM: Cleavage of RIPK1 by caspase-8 is crucial for limiting
apoptosis and necroptosis. Nature. 574:428–431. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
180
|
Zheng Z, Deng W, Bai Y, Miao R, Mei S,
Zhang Z, Pan Y, Wang Y, Min R, Deng F, et al: The lysosomal
rag-ragulator complex licenses RIPK1 and caspase-8-mediated
pyroptosis by yersinia. Science. 372:eabg02692021. View Article : Google Scholar
|
|
181
|
Van Opdenbosch N, Van Gorp H, Verdonckt M,
Saavedra PHV, de Vasconcelos NM, Gonçalves A, Vande Walle L, Demon
D, Matusiak M, Van Hauwermeiren F, et al: Caspase-1 engagement and
TLR-Induced c-FLIP expression suppress ASC/Caspase-8-Dependent
apoptosis by inflammasome sensors NLRP1b and NLRC4. Cell Rep.
21:3427–3444. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
182
|
Gutierrez KD, Davis MA, Daniels BP, Olsen
TM, Ralli-Jain P, Tait SW, Gale M Jr and Oberst A: MLKL activation
triggers NLRP3-mediated processing and release of IL-1β
independently of gasdermin-D. J Immunol. 198:2156–2164. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
183
|
Ye Z, Deng M, Yang Y, Song Y, Weng L, Qi
W, Ding P, Huang Y, Yu C, Wang Y, et al: Epithelial mitochondrial
fission-mediated PANoptosis is crucial for ulcerative colitis and
its inhibition by saquinavir through Drp1. Pharmacol Res.
210:1075382024. View Article : Google Scholar : PubMed/NCBI
|