Obesity has now become the world's most significant
public health issue, intimately intertwined with an assortment of
metabolic dysregulations, encompassing insulin resistance, type 2
diabetes mellitus and cardiovascular pathologies (1). Over the past few years, there has
been a burgeoning interest in the role of immune cells in obesity
and its associated pathologies, which has risen to prominence as a
pivotal area of investigation (2).
Bariatric surgery, recognized as an effective
intervention for obesity and its associated metabolic disorders,
has become widely utilized in clinical settings (3). Research indicates that the
peripheral immune cells of obese individuals experience substantial
alterations prior to and following bariatric surgery (4). The observed alterations pertain to
the restructuring of immune cell populations, alterations in their
activation levels and changes in the expression patterns of
molecules that regulate immune checkpoints (4). Bariatric surgery is known to elicit
transformations in the circulation of T cells among obese
individuals, notably affecting the regulatory dynamics of cluster
of differentiation (CD)4+ and CD8+T cell
populations. Additionally, alterations in the gut microbiome
following bariatric surgery may influence the progression of
obesity-related diseases by modulating the host's immune system
(5).
Nevertheless, research into the changes in
peripheral immune cells following bariatric surgery remains an area
of ongoing exploration and refinement (6). Variations in sample selection,
detection methods and observation time across different studies
have contributed to some inconsistencies in the findings. Moreover,
the causal link between changes in immune cells and the improvement
of obesity-related diseases, as well as the underlying molecular
mechanisms, remain incompletely understood (7). The risk of complications following
bariatric surgery can now be quantified by four categories of
biomarkers: Pre-operative albumin <3.5 g/dl increases 30-day
mortality and anastomotic leak risk by 1.4-2.3-fold (8); C-reactive protein (CRP) >12
mg/dl plus white blood count >12×109/l on
post-operative day 1 yields an 82% positive predictive value for
major complications (9); rising
oxidative-stress markers [malondialdehyde (MDA),
8-hydroxy-2'-deoxyguanosine (8-OHdG)] and interleukin (IL)-6 peaks
show a dose-response relationship with Clavien-Dindo ≥III
complications (8); and
pre-operative deficiencies in Fe, B12 and 25-hydroxy vitamin D
affect 35-60% of patients, with a 25% anemia rate at one year,
indicating that peri-operative inflammatory and nutritional
biomarkers jointly forewarn of both early and late adverse events
(10).
The present review sought to comprehensively
synthesize the latest research advances regarding the changes in
peripheral immune cells following bariatric surgery. It aims to
investigate the underlying links between these immune cell
alterations and obesity-related diseases and to suggest potential
directions for future research. By doing so, it hopes to offer a
new theoretical foundation and innovative research perspectives for
the management of obesity and its associated conditions.
Following bariatric surgery, there is a noted
augmentation in the population of CD4+T lymphocytes and
the CD4+T cell profile returned to a phenotype
resembling that of lean controls, along with an expansion of T
follicular helper (Tfh) cells. However, no changes were noted in
CD8+T cells (4).
Bariatric surgery altered the subset composition of
CD4+T cells and B cells to more closely resemble that of
lean controls. Furthermore, there was an enhancement in the
capacity of CD4+T cells to synthesize IL-2 and IFN-γ,
observed three months following the surgical procedure.
Nonetheless, the cytokine production capabilities of
CD8+T cells and B cells did not exhibit any significant
alteration three months post-operation (16). However, while reviewing the
literature, it was found that this study indicated a significant
increase in the number of CD8+T cells following
bariatric surgery (4,6). Therefore, the key reason for the
divergent conclusions lies in the different metrics used: One study
focused on the relative proportion of cells within lymphocytes,
whereas others monitored the absolute count in blood (4,6,16). Post-surgical weight loss and
hemoconcentration can raise the total number of cells while keeping
their proportion among lymphocytes unchanged.
Obesity disrupts the subset of regulatory T cells
that provide metabolic protection in visceral adipose tissue (VAT),
leading to heightened VAT inflammation and aggravated insulin
resistance (17). Regulatory T
cells (Tregs) gather in VAT to sustain systemic metabolic balance,
but their numbers decrease during obesity; restoring Treg
cholesterol homeostasis was found to rescue VAT Treg accumulation
in obese mice (15). Patients
with low Treg levels showed more significant metabolic improvement
after surgery, likely due to their more severe preoperative
inflammatory state (18). After
the removal of inflammatory adipose tissue during surgery, the
proportion of Tregs in the remaining fat tissue may increase
relatively, though further research is needed to confirm this
(19).
In B cells, the adipose tissue size in obese
individuals grows with age, causing systemic and intrinsic B cell
inflammation (20). This leads
to a decline in protective B cell responses and an upsurge in
pathogenic B cell responses, which in turn results in increased
secretion of autoantibodies (20). In addition, obese individuals
experience heightened IL-6 production and diminished IL-10
production. Furthermore, immune activation markers like tumor
necrosis factor (TNF)-α and micro-RNAs are found to be elevated in
stimulated B cells and these alterations are inversely associated
with B cell function (21). At 6
months post-bariatric surgery, patients exhibited a unique B cell
profile that is nearly unrecognizable compared with their
preoperative state and is somewhat similar to that of healthy lean
individuals (22). Despite
overall improvements in inflammation and metabolic health,
discrepancies within the B cell compartment in comparison to lean
controls continue to be observed (22).
Following bariatric surgery, the ability of B cells
to produce cytokines in patients with obesity stays at levels
comparable to those before the surgery and does not reach the
levels seen in healthy individuals (16). This alteration in function might
be linked to the persistent inflammation often seen in obesity.
Even though bariatric surgery helps reduce inflammation, it may
take B cells a longer time to regain full function (16). Thus, the juxtaposition of
'structural similarity' with 'functional non-recovery' is not
contradictory; rather, it reflects the staged nature of
post-surgical immune remodeling: First achieving 'form', then
'function', with the latter requiring a more prolonged period of
inflammation resolution.
Among obese individuals, there is a decline in the
expression of activating receptors on NK cells, while inhibitory
receptors on NK cells are more highly expressed. In addition,
studies have shown that in the natural killer cells of overweight
and obese subjects, the expression of the maturation and
differentiation marker CD27 is found to be insufficient (23). Additionally, the functionality of
natural killer cells is markedly diminished in both obese animals
and humans (24). In obesity,
human adipose tissue-resident NK cells exhibit a wide range of
phenotypic variations (25).
NKT cells are capable of detecting lipid antigens
displayed by cells that express CD1d (28). Within adipose tissue, they
interact with a variety of CD1d-expressing cells, such as
adipocytes, macrophages and dendritic cells. Through these
interactions, NKT cells play a pivotal role in orchestrating either
a pro-inflammatory or anti-inflammatory environment. This
environment, in turn, has a significant effect on the progression
of obesity and insulin resistance. The intricate interactions of
NKT cells within adipose tissue help to shape a specialized
microenvironment that exerts influence over both obesity and
insulin resistance. A study revealed that, compared with healthy
individuals, the body weight and waist circumference of patients
with obesity do not affect the proportion of invariant (i)NKT cells
(29). However, excessive intake
of free sugars can diminish the levels of iNKT cells, thereby
leading to immune dysfunction. Particularly, free sugars from solid
foods can reduce the proportion of iNKT cells by 22% (29). The abundance of NKT cells and
iNKT cells markedly escalates subsequent to bariatric surgery,
which implies that these interventions might ameliorate
obesity-associated metabolic derangements through the recalibration
of immune cells residing in adipose tissue (30).
In individuals with obesity, there is a notable rise
in the quantity of monocytes present in the peripheral blood
(31-33). These monocytes are marked by a
high rate of glycolysis and markedly higher mitochondrial oxygen
consumption (32). Additionally,
such changes may result in abnormal cytokine secretion, including
elevated levels of IL-8 (32).
Conversely, the recruited monocytes tend to differentiate into M2
macrophages, which in turn amplifies the inflammatory response
(33). Following bariatric
surgery, the phenotypic alterations of monocytes, including the
proportion of CCR5+ monocytes, showed improvement, yet
they did not completely revert to normal levels (4). Post-bariatric surgery, the
frequency of hCD7+ monocytes in peripheral blood
exhibited a negative correlation with regaining weight (31). Among those who achieved weight
loss and sustained it, a higher proportion of hCD7+
monocytes was observed, whereas individuals who experienced weight
regain had a lower proportion (31). Following bariatric surgery,
monocytes in adipose tissue still retain certain pro-inflammatory
characteristics, which may serve as a potential mechanism
underlying weight regain and metabolic disorders (34).
Macrophages associated with lipids, known as LAMs,
release the cytokine transforming growth factor-β1. This factor,
via interaction with aldehyde dehydrogenase 1 family member A1,
instigates a loss of brown adipocyte characteristics and
facilitates the metamorphosis of brown adipose tissue into white
adipose tissue, a phenomenon often observed in conditions such as
obesity and type 2 diabetes (35). Extracellular vesicles released by
bone marrow macrophages in obese mice can lead to bone loss in lean
mice by enhancing fat formation and suppressing bone formation
through the regulation of skeletal stem/progenitor cells (36). Moreover, a newly identified
subset of adipose tissue macrophages, termed interstitial
adipose-tissue macrophages (iMAMs) has been discovered using
single-nucleus RNA sequencing (37). These iMAMs display traits of
inflammatory and metabolic activation, where protein
disulfide-isomerase A3 plays a key role in sustaining their
migratory and pro-inflammatory functions (37). Following bariatric surgery, there
is a reduction in the number of pro-inflammatory macrophages within
adipose tissue, whereas the count of anti-inflammatory macrophages,
such as M2 macrophages, increases (38). This alteration aids in
diminishing the inflammatory condition of adipose tissue and
enhancing metabolic status (38). Despite the reduction in
pro-inflammatory macrophages following bariatric surgery, the
inflammatory gene expression levels, including those of TNF and
IL-6, persist at higher levels than those observed in individuals
who are healthy (34).
Obesity has the potential to amplify the functional
activity of myeloid-derived suppressor cells (MDSCs) (39-41). Obesity triggers the upregulation
of PD-1 on macrophages, leading to the suppression of anti-tumor
immunity (39). Furthermore,
obesity drives the expansion of MDSCs in ovarian cancer by
elevating IL-6 production, which strengthens the tumor's capacity
to evade immune detection (41).
However, it is regrettable that there is still a lack of research
on myeloid-derived suppressor cells following bariatric
surgery.
In obese individuals, there is a marked elevation in
the mast cell count within adipose tissue (42,43). Additionally, these mast cells
become activated and show a positive correlation with indicators of
fibrosis, inflammation and diabetes (42). By releasing a cascade of
inflammatory cytokines, chemokines and proteolytic enzymes, these
cells foster both angiogenesis and apoptosis within adipose tissue,
this dual action exacerbates the conditions of obesity and glucose
intolerance (43). Adipocytes
release adipokines such as leptin and adiponectin, which modulate
mast cell activity. Although both substances can induce the
migration of mast cells, leptin amplifies the release of histamine
and cysteinyl leukotrienes, along with the expression of chemokine
cc motif ligand 2, while adiponectin, conversely, encourages the
generation of the anti-inflammatory cytokine IL-10. This
demonstrates how obesity drives chronic inflammation by altering
mast cell behavior (44).
Indeed, the major tissue injury during surgery itself is the heart
and relevant biomarkers reflect this: In heart failure, the heart's
capacity to generate energy via oxidative phosphorylation (using
oxygen) is impaired, forcing it to rely more heavily on glycolysis
or other metabolic pathways. Bariatric surgery may alleviate mast
cell (MC)-driven inflammation through the following pathways:
reducing adipocyte stress→decreasing MC activation; improving the
adipokine profile (such as lowering leptin and increasing
adiponectin)→inhibiting MC-mediated cytokine release; remodeling
the immune microenvironment of adipose tissue→reducing MCs in
visceral fat after surgery (45).
In mice undergoing high-fat diet and fecal
microbiota transplantation linked to obesity, the differentiation
of bone marrow precursor cells into MDSCs is enhanced, whereas
their potential to develop into dendritic cells (DCs) is reduced
(46). This indicates a decline
in dendritic cell populations in individuals with obesity (47). Moreover, in obese mice,
CD103+ DCs in the mesenteric lymph nodes exhibit
elevated expression of dipeptidyl peptidase-4 and TNF-α,
potentially influencing Treg expansion and directly causing Treg
loss via interactions with cyclophilin A (47). Post metabolic surgery, there is
observed a significant elevation in the proportion of myeloid
dendritic cells within the peripheral blood circulation of patients
with obesity, with these levels eventually gravitating towards
normalization after the surgical intervention (48). Following the operation, the total
count of peripheral blood DCs dropped in both patient groups but
later recovered. In the open surgery group, the proportion and
activation level of tolerogenic DCs were relatively lower, whereas
the proportion of immature dendritic cells was comparatively higher
(49).
Diet-induced obese mice show earlier infiltration of
neutrophils and enhanced formation of neutrophil extracellular
traps (NETs) during the process of fracture healing (50). NETs can hinder the process of
bone healing by activating the NOD-like receptor family pyrin
domain containing 3 inflammasome. The activation process exerts a
dual effect on the cellular landscape, simultaneously inhibiting
the osteogenic differentiation of bone marrow mesenchymal stem
cells and driving macrophages toward the M1 polarization phenotype,
a hallmark of pro-inflammatory activity (50). A strong positive correlation
exists between neutrophil count and measures of body mass index,
triglycerides and uric acid levels (51). Transformations in the peripheral
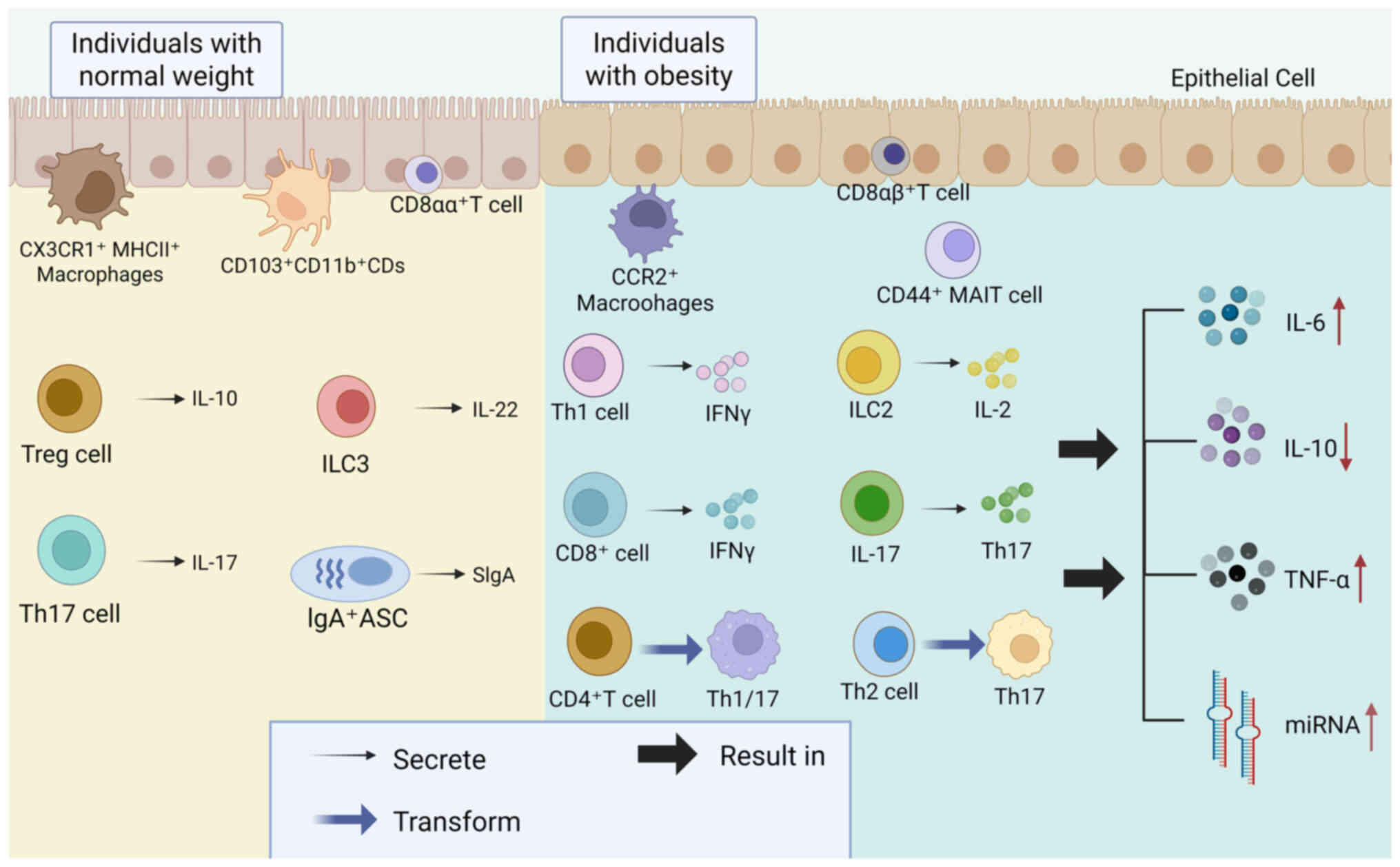
immune cell composition post-bariatric surgery are shown in
Fig. 2. Bariatric surgery
markedly affects neutrophil populations, with low-density
neutrophils showing a substantial reduction within months following
the procedure (52). The dynamic
changes in the neutrophil-to-lymphocyte ratio (NLR) following
bariatric surgery reveal a significant decline in NLR within 3
months post-surgery, driven by a more marked reduction in
neutrophil counts compared with lymphocytes (53).
In patients with obesity, a decrease in eosinophils
within adipose tissue, or their complete absence as seen in
ΔdblGATA knockout mice, results in elevated body weight and
worsened insulin resistance (54,55). Eosinophils enhance the browning
and thermogenic activity of adipose tissue by releasing Th2-type
cytokines, including IL-4 and IL-13 (55). However, excessive activation of
the Th2 response in the liver can contribute to fibrosis and the
progression of liver disease (54). Investigations into the efficacy
of bariatric interventions among subjects afflicted with
eosinophilic esophagitis have demonstrated that procedures such as
the Roux-en-Y gastric bypass and sleeve gastrectomy are notably
successful in facilitating considerable weight reduction. Moreover,
these surgical interventions do not lead to an increase in the
severity of eosinophilic esophagitis symptoms following the
operations (56). However,
direct evidence on eosinophil changes remains scarce, suggesting
that bariatric surgery could potentially benefit eosinophil-related
inflammatory diseases.
Studies have delineated the sophisticated interplay
among bariatric surgery, metabolic well-being and the modulation of
the immune system. Evidence suggests that adiponectin exerts an
anti-inflammatory effect by suppressing the expression of TNF-α, a
mechanism that could be instrumental in the metabolic shifts and
inflammatory responses observed post-bariatric surgery (57). Additionally, it has been
established that obesity may dampen anti-tumor immunity by
elevating PD-1 expression on macrophages, implying that bariatric
surgery could potentially augment the immune system's
tumor-combating prowess through the modification of this pathway
(58).
The prospect of harnessing the immune
microenvironment linked to obesity for the advancement of cancer
therapies has been studied within the scientific community
(59). Discussions have centered
on the potential to target the immune milieu in obese individuals
to refine cancer treatments, a process that may be markedly
influenced by the alterations prompted by bariatric surgery
(59). Moreover, investigations
have probed the impact of obesity and adipose tissue on T-cell
responses and the efficacy of cancer immunotherapy through immune
checkpoint inhibitors, indicating that bariatric surgery could
potentially alter the dynamics between adipose tissue and T-cells,
thereby enhancing the impact of immunotherapeutic interventions
(60). Bariatric surgery
markedly improves immunotherapy response through several converging
immune mechanisms: Post-operative restoration of peripheral Tregs,
expansion of Tfh cells and downregulation of PD-1 relieve T-cell
suppression (4). Concomitant
reduction of PD-1+ macrophages and MDSCs remodels the
tumor microenvironment, converting 'cold' into 'hot' tumors
(59). A systematic review
confirmed polarization from Th1/Th17 toward Th2/Treg profiles with
decreased IL-17 and IFN-γ, thereby enhancing checkpoint-inhibitor
sensitivity and increased Breg cells secreting IL-10 and TGF-β,
further dampening pro-inflammatory responses (61). Additionally, recovery of
MAIT-cell frequencies and diminished IL-17 indicate reprogramming
of the metabolic-immune axis that synergistically strengthens
anti-tumor immunity (62).
Furthermore, the intricate effects of obesity on
T-cell functionality during tumor progression and immune checkpoint
blockade have been subjected to intense examination, underscoring
the necessity for a more nuanced comprehension of how bariatric
surgery could influence these interactions by modulating T-cell
functions to refine immunotherapeutic strategies (63). To a greater extent, the
administration of gut-targeted therapies, such as probiotics and
prebiotics, can markedly optimize the health of the gut microbiota,
thereby augmenting the integrity of the intestinal barrier and
exerting a regulatory effect on immune responses (64).
According to current research, there are significant
differences in immune responses to various weight-loss strategies
(pharmacological, dietary and exercise interventions) and to
biological markers such as age and sex. The differences are showed
in Table I (65-70).
Glycolysis is one of the most studied metabolic
pathways in immune cells, with its high metabolic flux being a
hallmark of activated immune cells, especially in pro-inflammatory
immune cells (71).
Hypoxia-inducible factor-1α (HIF-1α), a key subunit of HIF-1, is
activated in hypoxic environments and can upregulate glycolysis in
immune cells (72). Moreover, it
exerts a substantial influence on oxidative stress, cancer
progression and a myriad of other diseases (72). Research has demonstrated that
inhibiting HIF-1α can effectively diminish glycolysis in immune
cells, including macrophages and T cells (73). This finding suggests a promising
therapeutic strategy for addressing autoimmune diseases, metabolic
disorders and various inflammatory conditions.
Within the intricate landscape of immunometabolic
regulation, the phosphoinositide 3-kinase (PI3K)/Akt signaling
cascade emerges as a pivotal actor orchestrating glycolysis within
immune cells, exerting its influence through downstream effectors
such as 6-phosphofructo-2-kinase/fructose-2,6-biphosphatase 3
(PFKFB3) and glucose transporter type 1 (74-76). PFKFB3, a sentinel enzyme
governing glycolytic flux, is subject to activation by the PI3K/Akt
axis, thereby amplifying the glycolytic program (78). This metabolic amplification can
be attenuated by specific PFKFB3 inhibitors, which have
demonstrated efficacy in diminishing both glycolysis and the
pro-inflammatory vigor of immune cells (74-76).
In the aftermath of bariatric surgery, patients
undergo substantial metabolic transformations that are inextricably
linked to glycolysis. The elevation of bile acid levels
post-surgery precipitates the activation of receptors such as TGR5
and farnesoid X receptor (FXR), thereby exerting a profound
influence on glycolytic metabolism (79). For example, the activation of
TGR5 catalyzes the secretion of glucagon-like peptide-1, which in
turn modulates glucose metabolism (79). Concurrently, an enhancement in
insulin sensitivity is observed, as evidenced by the upregulation
of hepatic lipid oxidation gene expression and the downregulation
of lipogenic gene expression, both of which have a significant
impact on glycolysis (79).
These metabolic modifications present novel avenues for targeted
therapeutic interventions following bariatric surgery.
In the realm of oncology, the metabolic alterations
elicited by bariatric surgery may have a substantial effect on the
glycolytic metabolism of tumor cells. Extant research posits that
tumor cells predominantly engage in aerobic glycolysis as a means
of metabolic reprogramming (80). By targeting glycolysis-related
metabolic enzymes, such as lactate dehydrogenase A (LDH-A) and
hexokinase 2 (HK2), it is possible to curtail tumor progression
(80). For instance, the
inhibition of LDH-A results in a reduction of lactate production,
which subsequently mitigates immune suppression and fortifies
anti-tumor immune responses (80). Moreover, post-surgery
improvements in pancreatic β-cell function lead to more efficient
and coordinated insulin secretion. The regulation of glycolysis can
further optimize insulin secretion and enhance glucose control
(79).
Strategies for glycolysis-targeted treatment
encompass the development of specific metabolic enzyme inhibitors,
such as those targeting LDH-A and HK2, to suppress tumor cell
glycolysis, diminish lactate production and augment anti-tumor
immune responses (81).
Additionally, modulating bile acid levels or activating bile acid
receptors like TGR5 and FXR can indirectly influence glycolytic
metabolism, thereby improving glucose control and insulin
sensitivity. The potential applications of these strategies merit
further exploration (79).
Therapeutic targeting of immunometabolism offers a
novel approach to treat various diseases, including cancer,
autoimmune disorders and inflammatory conditions (82). Several strategies have emerged
from preclinical and clinical studies. Modulation of oxidative
phosphorylation (OXPHOS) is one such strategy. Downregulating
OXPHOS in immune cells, particularly in Tregs has shown potential
in cancer immunotherapy. For instance, targeting FABP5 to reduce
OXPHOS in Tregs can impair their immunosuppressive function,
enhancing anti-tumor immunity (83). Similarly, OXPHOS inhibitors such
as IACS-010759 have demonstrated efficacy in targeting cancer cells
with high Myc activity, although the exact mechanism remains
unclear (84,85). Conversely, upregulating OXPHOS in
immune cells, such as CD8+T cells, can improve their
anti-tumor activity. For example, incorporating the 4-1BB domain
into chimeric antigen receptor T cells upregulates OXPHOS, leading
to enhanced persistence and efficacy in cancer immunotherapy
(86). Additionally, drugs like
NX-13, which upregulate OXPHOS, have shown anti-inflammatory
effects in ulcerative colitis (87,88).
In the realm of cancer therapeutics, the inhibition
of glycolysis in immune cells has garnered significant attention.
However, given the immunosuppressive milieu that characterizes the
cancer microenvironment, the strategy of inhibiting immune cell
glycolysis in cancer, excluding immune cell-derived tumors,
requires nuanced consideration. JHU083, a glutamine antagonist,
exemplifies a dual-action inhibitor capable of suppressing
glycolysis in both tumor cells and effector CD8+T cells
(89). Intriguingly, the
reduction of glycolysis in CD8+T cells precipitates an
upregulation of OXPHOS, thereby activating potent anti-tumor immune
responses (89,90). This phenomenon is inextricably
intertwined with the replenishment of tricarboxylic acid cycle
intermediates, a mechanism that has been thoroughly investigated
and elucidated (90).
In a similar vein, L-arginine imitates the effects
of glutamine antagonists by catalyzing a metabolic transition from
glycolysis to OXPHOS in effector T cells, thereby enhancing their
anti-tumor efficacy (91). HK2,
a crucial rate-limiting enzyme in the glycolytic pathway, has
garnered significant attention as a potential therapeutic target in
cancer treatment (92). The
inhibition of HK2 has been shown to suppress glycolysis, thereby
disrupting the expression of PD-L1 and subsequently reactivating
CD8+T cells (93). In
summary, rather than merely suppressing inflammation, glycolysis
inhibitors have exhibited anti-tumor immune effects by enhancing
OXPHOS. This phenomenon is likely due to the non-specific targeting
of glycometabolism in immune cells by existing inhibitors. In the
aftermath of bariatric surgery, adipose tissue undergoes a marked
enhancement in mitochondrial function, characterized by an
upregulation of gene expression associated with OXPHOS (94). This, in turn, amplifies the
oxidative capacity of adipose tissue, facilitating fatty acid
oxidation and energy expenditure. These metabolic alterations not
only contribute to weight reduction but also exert a salutary
effect on insulin sensitivity and overall metabolic health.
Furthermore, obesity is frequently accompanied by mitochondrial
dysfunction, a condition that bariatric surgery can effectively
ameliorate by diminishing mitochondrial fragmentation and
bolstering mitochondrial OXPHOS function (95). In the context of oncology, OXPHOS
emerges as a pivotal metabolic vulnerability for certain tumor
subtypes, such as lung cancer cells harboring SWI/SNF mutations and
prostate cancer cells with PTEN deficiency, as these cells are
highly reliant on OXPHOS for their proliferation and survival,
OXPHOS inhibitors, including Gboxin and berberine, have been
demonstrated to exert a potent inhibitory effect on tumor growth
(96,97). Additionally, the OXPHOS activity
of tumor cells in obese individuals may be attenuated due to the
improved mitochondrial function, thereby reducing the recurrence
rate of tumors. Consequently, targeting OXPHOS presents a novel and
promising therapeutic strategy for drug-resistant tumors, offering
the potential to enhance anti-tumor efficacy (95). The present review posits that
these findings furnish a theoretical foundation for the development
of therapeutic strategies targeting OXPHOS, which holds promise for
playing a significant role in the treatment of metabolic disorders
and oncology.
Lipid metabolism modulation is another area of
interest. Modulating fatty acid oxidation (FAO) in immune cells has
been explored in common diseases, including cancer, autoimmune
diseases, metabolic disease, atherosclerosis and lung inflammation
(78,86,98-105). Downregulating FAO in M2
macrophages can alleviate immunosuppression in cancer, while
upregulating FAO in macrophages can promote anti-inflammatory
effects in conditions like ulcerative colitis (105). peroxisome
proliferator-activated receptor α activators, such as fibrates,
have shown promise in enhancing FAO and reducing inflammation.
Inhibiting lipid synthesis in immune cells, particularly in Tregs
and CD8+T cells, has been shown to suppress
immunosuppression and pro-inflammatory responses, respectively
(101,106,107). For example, inhibiting SREBP
signaling in Tregs can reduce tumor growth without causing
autoimmune toxicity (108).
Bariatric surgery, by ameliorating lipid metabolism, markedly
reduces serum cholesterol levels, enhances the oxidative capacity
of adipose tissue and promotes fatty acid oxidation and energy
expenditure, thereby alleviating body weight and improving insulin
sensitivity and overall metabolic health (109).
In the context of obesity-associated chronic
low-grade inflammation, both macrophages and T cells are
prominently present within adipose tissue, exerting significant
influence on the inception and propagation of inflammatory
processes (18). Post-bariatric
surgery, a notable regulatory shift occurs in macrophage function,
characterized by a polarization transition from the
pro-inflammatory M1 phenotype to the anti-inflammatory M2
phenotype. This transformation effectively curtails the generation
of inflammatory cytokines (131).
Concurrently, the signaling pathways governing T
cell behavior undergo substantial alterations (132). Specifically, the expression
profiles of several key genes, including CXCR1, CXCR2, CCR7, IL7R
and G-protein coupled receptor 97 (GPR97), are modulated. These
genetic changes subsequently affect the activation and functional
dynamics of T cells, thereby contributing to a mitigated
inflammatory response (133).
Moreover, the cytokine IL-6, which exhibits a
complex dual role in obesity-related inflammation, is subject to
significant changes post-surgery (134). In obese individuals, elevated
IL-6 levels are typically associated with heightened inflammation
and insulin resistance (134).
However, following bariatric intervention, the levels and
functional attributes of IL-6 are reconfigured. The
pro-inflammatory effects of IL-6 are attenuated, while its
beneficial metabolic regulatory functions are preserved. Similarly,
TNF-α, a pivotal pro-inflammatory cytokine intricately linked to
obesity-associated inflammation and insulin resistance, experiences
a marked reduction in concentration following bariatric surgery
(135). This decline in TNF-α
levels plays a crucial role in dampening the overall inflammatory
cascade (135).
In individuals with obesity, visceral adipose tissue
exhibits markedly augmented IL-16 expression, which is intimately
intertwined with a state of smoldering, low-grade inflammation
(136). Following bariatric
surgery, IL-16 undergoes an early, transient surge; yet, as
adiposity and body mass progressively wane, its concentrations
regress to, or even below, pre-operative baselines.
Immunomodulatory interventions that target IL-16 or its processing
proteases could emerge as an adjunct anti-inflammatory strategy
beyond bariatric surgery itself (137). Collectively, these dynamics
intimate that bariatric surgery exerts an indirect but potent
anti-inflammatory effect by alleviating the adipose-tissue burden,
thereby dampening IL-16-driven inflammatory cascades, ameliorating
metabolic homeostasis and mitigating post-surgical inflammatory
sequelae (138).
In addition to these cellular and cytokine changes,
the activity of key signaling pathways is also modulated. The
Toll-like receptor signaling pathway, a primary initiator of
inflammation in adipose tissue, is suppressed post-surgery, leading
to a reduction in inflammatory cytokine production (139). The Janus kinase-signal
transducer and activator of transcription signaling pathway, which
is integral to the regulation of both adipose tissue inflammation
and metabolic homeostasis, also undergoes functional alterations
(140). These changes
collectively contribute to the overall attenuation of the
inflammatory response observed following bariatric surgical
interventions.
Peculiarly, recent studies show that residual
inflammatory 'imprints' can epigenetically reprogram innate and
adaptive immunity, leading to long-term reductions in vaccine
efficacy and immunotherapy success: Prior infection- or
inflammation-induced suppressive programs persist for at least a
month and hinder T-cell expansion and dendritic-cell priming, while
repeated mRNA vaccination in a chronic inflammatory milieu sustains
IFN-α/IL-6 signals that exacerbate inflammation and blunt
subsequent therapeutic responses (141-143).
Obesity is associated with IL-7Rα overexpression,
which disrupts immune cell function (27). Targeting IL-7Rα with specific
antibodies can stabilize T cells, reduce pro-inflammatory cytokines
and control obesity-related inflammation (27). This approach is well-tolerated in
healthy individuals and may be applicable to patients with obesity.
Other immune cell surface molecules, such as CXCR1, CXCR2, CCR7,
IL7R and GPR97, are also being explored as potential targets to
enhance immune cell activation and function, thereby reducing
inflammation (132).
Obesity also alters the gut microbiota and
bariatric surgery can improve its composition (64). Supplementing with specific
probiotics, like those containing Lactobacillus and
Bifidobacterium species, can further lower serum TNF-α
levels and increase postoperative weight loss (64). Probiotics can modulate cytokine
levels by increasing anti-inflammatory cytokines and decreasing
pro-inflammatory cytokines, while simultaneously enhancing
intestinal barrier function, reducing bacterial translocation and
attenuating systemic inflammation (144,145). Additionally, metabolic
reprogramming of immune cells following bariatric surgery,
particularly shifting T cells and B cells from a pro-inflammatory
to an anti-inflammatory state, may help alleviate chronic
inflammation in obesity (118).
Lastly, while inflammation is a necessary part of the body's
defense against injury and infection, surgical procedures and the
anesthetic agents used can either enhance or alter biomarkers
(146,147). Evidence indicates that
anesthesia modality markedly modulates perioperative immunity: A
meta-analysis found propofol TIVA more effectively suppressed
pro-inflammatory cytokines (IL-6, TNF-α) and elevated IL-10 than
sevoflurane, while a hysterectomy trial showed spinal vs. general
anesthesia further reduced postoperative IL-6, CRP and MDA while
preserving antioxidant enzymes. Thus, both propofol over
sevoflurane and spinal over general anesthesia confer
immunoprotection by attenuating systemic inflammation and oxidative
stress (146,147).
New progress has been made in the research of
intracellular target therapy of immune cells following bariatric
surgery. IL-27 enhances the anti-tumor function of CD8+T
cells and inhibits immune suppressor factors by activating the
STAT1/3 signaling pathway (119). It also acts on adipocytes to
promote the expression of uncoupling protein 1 via the
p38MAPK/peroxisome proliferator-activated receptor γ coactivator
1-α pathway, thereby regulating obesity (18). In addition, the pro-inflammatory
characteristics of circulating T cells in patients with obesity are
improved following bariatric surgery, with the recovery of
CD4+ Treg cell levels and increased proliferation and
activation of Tfh and B cells (4). The activation status and expression
changes of immune checkpoint molecules in obesity-related T cell
subsets may serve as potential targets (4). In a study, it was found that
glutamate receptor scaffold proteins (PSD-95 and PICKL) were
involved in the anchoring and transport of glutamate receptors and
their PDZ domains have become therapeutic targets for various
central nervous system diseases (148).
Bariatric surgery is no longer simply a mechanical
restriction or malabsorptive procedure; it functions as a
systems-level 'immuno-metabolic reboot'. By simultaneously
correcting the chronic, low-grade inflammatory tone of obesity and
re-wiring the bioenergetic circuitry of virtually every circulating
immune subset, surgery converts pathologic adipose-immune
cross-talk into a homeostatic dialogue that sustains weight loss
and metabolic remission. Over the next decade, three converging
developments will redefine post-surgical care, besides single-cell
multi-omics and AI-driven trajectory mapping will allow real-time
identification of patients who retain pro-inflammatory immune
'scars', enabling precision rescue therapy before weight regain or
diabetes relapse occurs; pharmacologic fine-tuning of discrete
metabolic checkpoints, glycolytic rate-limiting enzymes, OXPHOS
modulators and glutamine/FAO switches, will be combined with
microbiota-directed interventions to accelerate functional
maturation of Treg, NK and antigen-presenting cell compartments and
these same immuno-metabolic insights will be exported to oncology:
Leveraging the bariatric-induced reduction in PD-1hi macrophages
and MDSCs to convert the obesity-associated 'cold' tumor
microenvironment into one that is highly responsive to
immune-checkpoint blockade. Ultimately, bariatric surgery will
evolve from a last-resort metabolic operation into a programmable
gateway for lifelong immuno-metabolic precision medicine,
delivering not just sustained weight loss, but durable protection
against diabetes, cardiovascular disease and cancer.
Not applicable.
YS and KS were both responsible for the conception
and writing of the present review. RY contributed to the design of
the figures. HX contributed to literature search and collating
references. CL was responsible for language editing and for
critical revision and for making substantial contributions to
conception and design. YD was responsible for preparing analysis
and interpretation of data and stylistic refinement. YR and YZ were
both in charge of the conception and design of the present review.
Data authentication is not applicable. All authors read and
approved the final manuscript.
Not applicable.
Not applicable.
The authors declare that they have no competing
interests.
Not applicable.
The present review was supported by the National Natural Science
Foundation of China (grant no. 82370601) and Natural Science
Foundation of Sichuan Province (grant no. 2025NSFJQ0066).
|
1
|
Schulze MB and Stefan N: Metabolically
healthy obesity: From epidemiology and mechanisms to clinical
implications. Nat Rev Endocrinol. 20:633–646. 2024. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Zhang P, Watari K and Karin M: Innate
immune cells link dietary cues to normal and abnormal metabolic
regulation. Nat Immunol. 26:29–41. 2025. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Moris D, Barfield R, Chan C, Chasse S,
Stempora L, Xie J, Plichta JK, Thacker J, Harpole DH, Purves T, et
al: Immune phenotype and postoperative complications after elective
surgery. Ann Surg. 278:873–882. 2023. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Barbosa P, Pinho A, Lázaro A, Paula D,
Tralhão JG, Paiva A, Pereira MJ, Carvalho E and Laranjeira P:
Bariatric surgery induces alterations in the immune profile of
peripheral blood T cells. Biomolecules. 14:2192024. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Mohammadzadeh N, Razavi S and Ebrahimipour
G: Impact of bariatric surgery on gut microbiota composition in
obese patients compared to healthy controls. AMB Express.
14:1152024. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Rivera-Carranza T, Azaola-Espinosa A,
Bojalil-Parra R, Zúñiga-León E, León-Téllez-Girón A,
Rojano-Rodríguez ME and Nájera-Medina O: Immunometabolic changes
following gastric bypass and sleeve gastrectomy: A comparative
study. Obes Surg. 35:481–495. 2025. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Shaikh SR, Beck MA, Alwarawrah Y and
MacIver NJ: Emerging mechanisms of obesity-associated immune
dysfunction. Nat Rev Endocrinol. 20:136–148. 2024. View Article : Google Scholar
|
|
8
|
Hart A, Sun Y, Titcomb TJ, Liu B, Smith
JK, Correia MLG, Snetselaar LG, Zhu Z and Bao W: Association
between preoperative serum albumin levels with risk of death and
postoperative complications after bariatric surgery: A
retrospective cohort study. Surg Obes Relat Dis. 18:928–934. 2022.
View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Hart JWH, Takken R, Hogewoning CRC, Biter
LU, Apers JA, Zengerink H, Dunkelgrün M and Verhoef C: Markers for
major complications at day-one postoperative in fast-track
metabolic surgery: Updated metabolic checklist. Obes Surg.
33:3008–3016. 2023. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Riva-Moscoso A, Martinez-Rivera RN,
Cotrina-Susanibar G, Príncipe-Meneses FS, Urrunaga-Pastor D,
Salinas-Sedo G and Toro-Huamanchumo CJ: Factors associated with
nutritional deficiency biomarkers in candidates for bariatric
surgery: A cross-sectional study in a peruvian high-resolution
clinic. Nutrients. 14:822021. View Article : Google Scholar
|
|
11
|
Giovenzana A, Bezzecchi E, Bichisecchi A,
Cardellini S, Ragogna F, Pedica F, Invernizzi F, Di Filippo L,
Tomajer V, Aleotti F, et al: Fat-to-blood recirculation of
partially dysfunctional PD-1(+)CD4 Tconv cells is associated with
dysglycemia in human obesity. iScience. 27:1090322024. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Ma Q, Ran H, Li Y, Lu Y, Liu X, Huang H,
Yang W, Yu L, Chen P, Huang X, et al: Circulating Th1/17 cells
serve as a biomarker of disease severity and a target for early
intervention in AChR-MG patients. Clin Immunol. 218:1084922020.
View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Wood S, Branch J, Vasquez P, DeGuzman MM,
Brown A, Sagcal-Gironella AC, Singla S, Ramirez A and Vogel TP:
Th17/1 and ex-Th17 cells are detected in patients with
polyarticular juvenile arthritis and increase following treatment.
Pediatr Rheumatol Online J. 22:322024. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Shirakawa K and Sano M: Drastic
transformation of visceral adipose tissue and peripheral CD4 T
cells in obesity. Front Immunol. 13:10447372023. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Elkins C, Ye C, Sivasami P, Mulpur R,
Diaz-Saldana PP, Peng A, Xu M, Chiang YP, Moll S, Rivera-Rodriguez
DE, et al: Obesity reshapes regulatory T cells in the visceral
adipose tissue by disrupting cellular cholesterol homeostasis. Sci
Immunol. 10:eadl49092025. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Wijngaarden LH, Taselaar AE, Nuijten F,
van der Harst E, Klaassen RA, Kuijper TM, Jongbloed F, Ambagtsheer
G, Klepper M, Ijzermans JNM, et al: T and B cell composition and
cytokine producing capacity before and after bariatric surgery.
Front Immunol. 13:8882782022. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Fernández-Ruiz I: Obesity alters
cholesterol homeostasis in regulatory T cells of visceral adipose
tissue. Nat Rev Cardiol. 22:1462025. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Villarreal-Calderon JR, Cuellar-Tamez R,
Castillo EC, Luna-Ceron E, García-Rivas G and Elizondo-Montemayor
L: Metabolic shift precedes the resolution of inflammation in a
cohort of patients undergoing bariatric and metabolic surgery. Sci
Rep. 11:121272021. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Jalilvand A, Blaszczak A, Bradley D, Liu
J, Wright V, Needleman B, Hsueh W and Noria S: Low visceral adipose
tissue regulatory T cells are associated with higher comorbidity
severity in patients undergoing bariatric surgery. Surg Endosc.
35:3131–3138. 2021. View Article : Google Scholar
|
|
20
|
Frasca D: Obesity accelerates age defects
in human B cells and induces autoimmunity. Immunometabolism.
4:e2200102022. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Artimovič P, Špaková I, Macejková E,
Pribulová T, Rabajdová M, Mareková M and Zavacká M: The ability of
microRNAs to regulate the immune response in ischemia/reperfusion
inflammatory pathways. Genes Immun. 25:277–296. 2024. View Article : Google Scholar
|
|
22
|
Šlisere B, Arisova M, Aizbalte O, Salmiņa
MM, Zolovs M, Levenšteins M, Mukāns M, Troickis I, Meija L,
Lejnieks A, et al: Distinct B cell profiles characterise healthy
weight and obesity pre- and post-bariatric surgery. Int J Obes
(Lond). 47:970–978. 2023. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Naujoks W, Quandt D, Hauffe A, Kielstein
H, Bähr I and Spielmann J: Characterization of surface receptor
expression and cytotoxicity of human NK cells and NK cell subsets
in overweight and obese humans. Front Immunol. 11:5732002020.
View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Bähr I, Spielmann J, Quandt D and
Kielstein H: Obesity-associated alterations of natural killer cells
and immunosurveillance of cancer. Front Immunol. 11:2452020.
View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Haugstøyl ME, Cornillet M, Strand K,
Stiglund N, Sun D, Lawrence-Archer L, Hjellestad ID, Busch C,
Mellgren G, Björkström NK and Fernø J: Phenotypic diversity of
human adipose tissue-resident NK cells in obesity. Front Immunol.
14:11303702023. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Wang YY, Chang EQ, Zhu RL, Liu XZ, Wang
GZ, Li NT, Zhang W, Zhou J, Wang XD, Sun MY and Zhang JQ: An atlas
of dynamic peripheral blood mononuclear cell landscapes in human
perioperative anaesthesia/surgery. Clin Transl Med. 12:e6632022.
View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Gihring A, Gärtner F, Mayer L, Roth A,
Abdelrasoul H, Kornmann M, Elad L and Knippschild U: Influence of
bariatric surgery on the peripheral blood immune system of female
patients with morbid obesity revealed by high-dimensional mass
cytometry. Front Immunol. 14:11318932023. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Satoh M and Iwabuchi K: Contribution of
NKT cells and CD1d-expressing cells in obesity-associated adipose
tissue inflammation. Front Immunol. 15:13658432024. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Alhamawi RM, Almutawif YA, Aloufi BH,
Alotaibi JF, Alharbi MF, Alsrani NM, Alinizy RM, Almutairi WS,
Alaswad WA, Eid HMA and Mumena WA: Free sugar intake is associated
with reduced proportion of circulating invariant natural killer T
cells among women experiencing overweight and obesity. Front
Immunol. 15:13583412024. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Van Kaer L, Parekh VV and Wu L: Invariant
natural killer T cells: Bridging innate and adaptive immunity. Cell
Tissue Res. 343:43–55. 2011. View Article : Google Scholar
|
|
31
|
Zhou HY, Feng X, Wang LW, Zhou R, Sun H,
Chen X, Lu RB, Huang Y, Guo Q and Luo XH: Bone marrow immune cells
respond to fluctuating nutritional stress to constrain weight
regain. Cell Metab. 35:1915–1930.e8. 2023. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Radushev V, Karkossa I, Berg J, von Bergen
M, Engelmann B, Rolle-Kampczyk U, Blüher M, Wagner U, Schubert K
and Rossol M: Dysregulated cytokine and oxidative response in
hyper-glycolytic monocytes in obesity. Front Immunol.
15:14165432024. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Blaszkiewicz M, Gunsch G, Willows JW,
Gardner ML, Sepeda JA, Sas AR and Townsend KL: Adipose tissue
myeloid-lineage neuroimmune cells express genes important for
neural plasticity and regulate adipose innervation. Front
Endocrinol (Lausanne). 13:8649252022. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Hinte LC, Castellano-Castillo D, Ghosh A,
Melrose K, Gasser E, Noé F, Massier L, Dong H, Sun W, Hoffmann A,
et al: Adipose tissue retains an epigenetic memory of obesity after
weight loss. Nature. 636:457–465. 2024. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Sciarretta F, Ninni A, Zaccaria F,
Chiurchiù V, Bertola A, Karlinsey K, Jia W, Ceci V, Di Biagio C, Xu
Z, et al: Lipid-associated macrophages reshape BAT cell identity in
obesity. Cell Rep. 43:1144472024. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Luo JH, Wang FX, Zhao JW, Yang CL, Rong
SJ, Lu WY, Chen QJ, Zhou Q, Xiao J, Wang YN, et al: PDIA3 defines a
novel subset of adipose macrophages to exacerbate the development
of obesity and metabolic disorders. Cell Metab. 36:2262–2280.e5.
2024. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
He C, Hu C, He WZ, Sun YC, Jiang Y, Liu L,
Hou J, Chen KX, Jiao YR, Huang M, et al: Macrophage-derived
extracellular vesicles regulate skeletal stem/progenitor Cell
lineage fate and bone deterioration in obesity. Bioact Mater.
36:508–523. 2024.PubMed/NCBI
|
|
38
|
Yang T, Zhang Y, Duan C, Liu H, Wang D,
Liang Q, Chen X, Ma J, Cheng K, Chen Y, et al: CD300E(+)
macrophages facilitate liver regeneration after splenectomy in
decompensated cirrhotic patients. Exp Mol Med. 57:72–85. 2025.
View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Bader JE, Wolf MM, Lupica-Tondo GL, Madden
MZ, Reinfeld BI, Arner EN, Hathaway ES, Steiner KK, Needle GA,
Hatem Z, et al: Author Correction: Obesity induces PD-1 on
macrophages to suppress anti-tumour immunity. Nature. 631:E162024.
View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Liu W, Li B, Liu D, Zhao B, Sun G and Ding
J: Obesity correlates with the immunosuppressive ILC2s-MDSCs axis
in advanced breast cancer. Immun Inflamm Dis. 12:e11962024.
View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Yang Q, Yu B, Kang J, Li A and Sun J:
Obesity promotes tumor immune evasion in ovarian cancer through
increased production of myeloid-derived suppressor cells via IL-6.
Cancer Manag Res. 13:7355–7363. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Divoux A, Moutel S, Poitou C, Lacasa D,
Veyrie N, Aissat A, Arock M, Guerre-Millo M and Clément K: Mast
cells in human adipose tissue: link with morbid obesity,
inflammatory status, and diabetes. J Clin Endocrinol Metab.
97:E1677–E1685. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Liu J, Divoux A, Sun J, Zhang J, Clément
K, Glickman JN, Sukhova GK, Wolters PJ, Du J, Gorgun CZ, et al:
Genetic deficiency and pharmacological stabilization of mast cells
reduce diet-induced obesity and diabetes in mice. Nat Med.
15:940–945. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Milling S: Adipokines and the control of
mast cell functions: From obesity to inflammation? Immunology.
158:1–2. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Arivazhagan L, Ruiz HH, Wilson RA,
Manigrasso MB, Gugger PF, Fisher EA, Moore KJ, Ramasamy R and
Schmidt AM: An eclectic cast of cellular actors orchestrates innate
immune responses in the mechanisms driving obesity and metabolic
perturbation. Circ Res. 126:1565–1589. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Chen J, Liu X, Zou Y, Gong J, Ge Z, Lin X,
Zhang W, Huang H, Zhao J, Saw PE, et al: A high-fat diet promotes
cancer progression by inducing gut microbiota-mediated leucine
production and PMN-MDSC differentiation. Proc Natl Acad Sci USA.
121:e23067761212024. View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Li C, Wang G, Sivasami P, Ramirez RN,
Zhang Y, Benoist C and Mathis D: Interferon-α-producing
plasmacytoid dendritic cells drive the loss of adipose tissue
regulatory T cells during obesity. Cell Metab. 33:1610–1623.e5.
2021. View Article : Google Scholar
|
|
48
|
Zhang J, Chen X, Liu W, Zhang C, Xiang Y,
Liu S and Zhou Z: Metabolic surgery improves the unbalanced
proportion of peripheral blood myeloid dendritic cells and T
lymphocytes in obese patients. Eur J Endocrinol. 185:819–829. 2021.
View Article : Google Scholar : PubMed/NCBI
|
|
49
|
McAuliffe PF, Efron PA, Scumpia PO, Uchida
T, Mutschlecner SC, Rout WR, Moldawer LL and Cendan JC: Varying
blood monocyte and dendritic cell responses after laparoscopic
versus open gastric bypass surgery. Obes Surg. 15:1424–1431. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
50
|
Zhao X, Wang Q, Wang W and Lu S: Increased
neutrophil extracellular traps caused by diet-induced obesity delay
fracture healing. FASEB J. 38:e701262024. View Article : Google Scholar : PubMed/NCBI
|
|
51
|
Lyu H, Fan N, Wen H, Zhang X, Mao H, Bian
Q and Chen J: Interplay between BMI, neutrophil, triglyceride and
uric acid: A case-control study and bidirectional multivariate
mendelian randomization analysis. Nutr Metab (Lond). 22:72025.
View Article : Google Scholar : PubMed/NCBI
|
|
52
|
Roberts CF and Sheu EG: Low density, high
impact? Neutrophil changes in obesity and bariatric surgery.
EBioMedicine. 79:1039882022. View Article : Google Scholar : PubMed/NCBI
|
|
53
|
Chi PJ, Wu KT, Chen PJ, Chen CY, Su YC,
Yang CY and Chen JH: The serial changes of Neutrophile-Lymphocyte
Ratio and correlation to weight loss after Laparoscopic Sleeve
Gastrectomy. Front Surg. 9:9398572022. View Article : Google Scholar : PubMed/NCBI
|
|
54
|
Hu Y and Chakarov S: Eosinophils in
obesity and obesity-associated disorders. Discov Immunol.
2:kyad0222023. View Article : Google Scholar
|
|
55
|
Oliveira MC, Silveira ALM, de Oliveira
ACC, Lana JP, Costa KA, Vieira É LM, Pinho V, Teixeira MM,
Merabtene F, Marcelin G, et al: Eosinophils protect from metabolic
alterations triggered by obesity. Metabolism. 146:1556132023.
View Article : Google Scholar : PubMed/NCBI
|
|
56
|
Deiss-Yehiely N, Lidor A and Hillman L:
Outcomes of patients with eosinophilic esophagitis undergoing
bariatric surgery. J Gastrointest Surg. 28:1706–1708. 2024.
View Article : Google Scholar : PubMed/NCBI
|
|
57
|
Yuan B, Huang L, Yan M, Zhang S, Zhang Y,
Jin B, Ma Y and Luo Z: Adiponectin downregulates TNF-α expression
in degenerated intervertebral discs. Spine (Phila Pa 1976).
43:E381–E389. 2018. View Article : Google Scholar
|
|
58
|
Bader JE, Wolf MM, Lupica-Tondo GL, Madden
MZ, Reinfeld BI, Arner EN, Hathaway ES, Steiner KK, Needle GA,
Hatem Z, et al: Obesity induces PD-1 on macrophages to suppress
anti-tumour immunity. Nature. 630:968–975. 2024. View Article : Google Scholar : PubMed/NCBI
|
|
59
|
Desharnais L, Walsh LA and Quail DF:
Exploiting the obesity-associated immune microenvironment for
cancer therapeutics. Pharmacol Ther. 229:1079232022. View Article : Google Scholar
|
|
60
|
Pasquarelli-do-Nascimento G, Machado SA,
de Carvalho JMA and Magalhães KG: Obesity and adipose tissue impact
on T-cell response and cancer immune checkpoint blockade therapy.
Immunother Adv. 2:ltac0152022. View Article : Google Scholar : PubMed/NCBI
|
|
61
|
Villarreal-Calderón JR, Cuéllar RX,
Ramos-González MR, Rubio-Infante N, Castillo EC,
Elizondo-Montemayor L and García-Rivas G: Interplay between the
adaptive immune system and insulin resistance in weight loss
induced by bariatric surgery. Oxid Med Cell Longev.
2019:39407392019. View Article : Google Scholar : PubMed/NCBI
|
|
62
|
Conroy MJ, Dunne MR, Donohoe CL and
Reynolds JV: Obesity-associated cancer: An immunological
perspective. Proc Nutr Soc. 75:125–138. 2016. View Article : Google Scholar
|
|
63
|
Wang Z, Aguilar EG, Luna JI, Dunai C,
Khuat LT, Le CT, Mirsoian A, Minnar CM, Stoffel KM, Sturgill IR, et
al: Paradoxical effects of obesity on T cell function during tumor
progression and PD-1 checkpoint blockade. Nat Med. 25:141–151.
2019. View Article : Google Scholar :
|
|
64
|
Galyean S, Sawant D and Shin AC:
Immunometabolism, micronutrients, and bariatric surgery: The use of
transcriptomics and microbiota-targeted therapies. Mediators
Inflamm. 2020:88620342020. View Article : Google Scholar : PubMed/NCBI
|
|
65
|
Chen DB and Wang W: Human placental
microRNAs and preeclampsia. Biol Reprod. 88:1302013. View Article : Google Scholar : PubMed/NCBI
|
|
66
|
Mehrdad M, Norouzy A, Safarian M, Nikbakht
HA, Gholamalizadeh M and Mahmoudi M: The antiviral immune defense
may be adversely influenced by weight loss through a calorie
restriction program in obese women. Am J Transl Res.
13:10404–10412. 2021.PubMed/NCBI
|
|
67
|
Ji J, Fotros D, Sohouli MH, Velu P, Fatahi
S and Liu Y: The effect of a ketogenic diet on inflammation-related
markers: a systematic review and meta-analysis of randomized
controlled trials. Nutr Rev. 83:40–58. 2025. View Article : Google Scholar
|
|
68
|
Nemet I and Monnier VM: Vitamin C
degradation products and pathways in the human lens. J Biol Chem.
286:37128–37136. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
69
|
Zhu R, Craciun I, Bernhards-Werge J, Jalo
E, Poppitt SD, Silvestre MP, Huttunen-Lenz M, McNarry MA, Stratton
G, Handjiev S, et al: Age- and sex-specific effects of a long-term
lifestyle intervention on body weight and cardiometabolic health
markers in adults with prediabetes: results from the diabetes
prevention study PREVIEW. Diabetologia. 65:1262–1277. 2022.
View Article : Google Scholar : PubMed/NCBI
|
|
70
|
Potenza L, Vallerini D, Barozzi P, Riva G,
Gilioli A, Forghieri F, Candoni A, Cesaro S, Quadrelli C, Maertens
J, et al: Mucorales-Specific T cells in patients with hematologic
malignancies. PLoS One. 11:e01491082016. View Article : Google Scholar : PubMed/NCBI
|
|
71
|
Cheng SC, Quintin J, Cramer RA, Shepardson
KM, Saeed S, Kumar V, Giamarellos-Bourboulis EJ, Martens JH, Rao
NA, Aghajanirefah A, et al: mTOR- and HIF-1α-mediated aerobic
glycolysis as metabolic basis for trained immunity. Science.
345:12506842014. View Article : Google Scholar
|
|
72
|
Ke X, Fei F, Chen Y, Xu L, Zhang Z, Huang
Q, Zhang H, Yang H, Chen Z and Xing J: Hypoxia upregulates CD147
through a combined effect of HIF-1α and Sp1 to promote glycolysis
and tumor progression in epithelial solid tumors. Carcinogenesis.
33:1598–1607. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
73
|
Liu L, Wang Y, Bai R, Yang K and Tian Z:
MiR-186 inhibited aerobic glycolysis in gastric cancer via HIF-1α
regulation. Oncogenesis. 6:e3182017. View Article : Google Scholar
|
|
74
|
Zhou Z, Plug LG, Patente TA, de
Jonge-Muller ESM, Elmagd AA, van der Meulen-de Jong AE, Everts B,
Barnhoorn MC and Hawinkels LJAC: Increased stromal PFKFB3-mediated
glycolysis in inflammatory bowel disease contributes to intestinal
inflammation. Front Immunol. 13:9660672022. View Article : Google Scholar : PubMed/NCBI
|
|
75
|
Hu X, Xu Q, Wan H, Hu Y, Xing S, Yang H,
Gao Y and He Z: PI3K-Akt-mTOR/PFKFB3 pathway mediated lung
fibroblast aerobic glycolysis and collagen synthesis in
lipopolysaccharide-induced pulmonary fibrosis. Lab Invest.
100:801–811. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
76
|
He Q, Yin J, Zou B and Guo H: WIN55212-2
alleviates acute lung injury by inhibiting macrophage glycolysis
through the miR-29b-3p/FOXO3/PFKFB3 axis. Mol Immunol. 149:119–128.
2022. View Article : Google Scholar : PubMed/NCBI
|
|
77
|
Zhai GY, Qie SY, Guo QY, Qi Y and Zhou YJ:
sDR5-Fc inhibits macrophage M1 polarization by blocking the
glycolysis. J Geriatr Cardiol. 18:271–280. 2021.PubMed/NCBI
|
|
78
|
Hao S, Zhang S, Ye J, Chen L, Wang Y, Pei
S, Zhu Q, Xu J, Tao Y, Zhou N, et al: Goliath induces inflammation
in obese mice by linking fatty acid β-oxidation to glycolysis. EMBO
Rep. 24:e569322023. View Article : Google Scholar
|
|
79
|
Sandoval DA and Patti ME: Glucose
metabolism after bariatric surgery: Implications for T2DM remission
and hypoglycaemia. Nat Rev Endocrinol. 19:164–176. 2023. View Article : Google Scholar
|
|
80
|
Zhou D, Duan Z, Li Z, Ge F, Wei R and Kong
L: The significance of glycolysis in tumor progression and its
relationship with the tumor microenvironment. Front Pharmacol.
13:10917792022. View Article : Google Scholar :
|
|
81
|
DeBerardinis RJ and Chandel NS:
Fundamentals of cancer metabolism. Sci Adv. 2:e16002002016.
View Article : Google Scholar : PubMed/NCBI
|
|
82
|
Cadassou O and Jordheim LP: OXPHOS
inhibitors, metabolism and targeted therapies in cancer. Biochem
Pharmacol. 211:1155312023. View Article : Google Scholar : PubMed/NCBI
|
|
83
|
Pan Y, Tian T, Park CO, Lofftus SY, Mei S,
Liu X, Luo C, O'Malley JT, Gehad A, Teague JE, et al: Survival of
tissue-resident memory T cells requires exogenous lipid uptake and
metabolism. Nature. 543:252–256. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
84
|
Donati G, Nicoli P, Verrecchia A,
Vallelonga V, Croci O, Rodighiero S, Audano M, Cassina L, Ghsein A,
Binelli G, et al: Oxidative stress enhances the therapeutic action
of a respiratory inhibitor in MYC-driven lymphoma. EMBO Mol Med.
15:e169102023. View Article : Google Scholar : PubMed/NCBI
|
|
85
|
Purhonen J, Klefström J and Kallijärvi J:
MYC-an emerging player in mitochondrial diseases. Front Cell Dev
Biol. 11:12576512023. View Article : Google Scholar : PubMed/NCBI
|
|
86
|
Kawalekar OU, O'Connor RS, Fraietta JA,
Guo L, McGettigan SE, Posey AD Jr, Patel PR, Guedan S, Scholler J,
Keith B, et al: Distinct signaling of coreceptors regulates
specific metabolism pathways and impacts memory development in CAR
T cells. Immunity. 44:7122016. View Article : Google Scholar : PubMed/NCBI
|
|
87
|
Leber A, Hontecillas R, Zoccoli-Rodriguez
V, Bienert C, Chauhan J and Bassaganya-Riera J: Activation of NLRX1
by NX-13 alleviates inflammatory bowel disease through
immunometabolic mechanisms in CD4(+) T cells. J Immunol.
203:3407–3415. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
88
|
Verstockt B, Vermeire S, Peyrin-Biroulet
L, Mosig R, Feagan BG, Colombel JF, Siegmund B, Rieder F, Schreiber
S, Yarur A, et al: The safety, tolerability, pharmacokinetics, and
clinical efficacy of the NLRX1 agonist NX-13 in active ulcerative
colitis: Results of a phase 1b study. J Crohns Colitis. 18:762–772.
2024. View Article : Google Scholar :
|
|
89
|
Leone RD, Zhao L, Englert JM, Sun IM, Oh
MH, Sun IH, Arwood ML, Bettencourt IA, Patel CH, Wen J, et al:
Glutamine blockade induces divergent metabolic programs to overcome
tumor immune evasion. Science. 366:1013–1021. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
90
|
Praharaj M, Shen F, Lee AJ, Zhao L,
Nirschl TR, Theodros D, Singh AK, Wang X, Adusei KM, Lombardo KA,
et al: Metabolic reprogramming of tumor-associated macrophages
using glutamine antagonist JHU083 drives tumor immunity in
myeloid-rich prostate and bladder cancers. Cancer Immunol Res.
12:854–875. 2024. View Article : Google Scholar : PubMed/NCBI
|
|
91
|
Geiger R, Rieckmann JC, Wolf T, Basso C,
Feng Y, Fuhrer T, Kogadeeva M, Picotti P, Meissner F, Mann M, et
al: L-arginine modulates T cell metabolism and enhances survival
and anti-tumor activity. Cell. 167:829–842.e13. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
92
|
Wiel C, Le Gal K, Ibrahim MX, Jahangir CA,
Kashif M, Yao H, Ziegler DV, Xu X, Ghosh T, Mondal T, et al: BACH1
stabilization by antioxidants stimulates lung cancer metastasis.
Cell. 178:330–345.e22. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
93
|
Guo D, Tong Y, Jiang X, Meng Y, Jiang H,
Du L, Wu Q, Li S, Luo S, Li M, et al: Aerobic glycolysis promotes
tumor immune evasion by hexokinase2-mediated phosphorylation of
IκBα. Cell Metab. 34:1312–1324.e6. 2022. View Article : Google Scholar
|
|
94
|
van der Kolk BW, Muniandy M, Kaminska D,
Alvarez M, Ko A, Miao Z, Valsesia A, Langin D, Vaittinen M,
Pääkkönen M, et al: Differential mitochondrial gene expression in
adipose tissue following weight loss induced by diet or bariatric
surgery. J Clin Endocrinol Metab. 106:1312–1324. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
95
|
Xia W, Veeragandham P, Cao Y, Xu Y, Rhyne
TE, Qian J, Hung CW, Zhao P, Jones Y, Gao H, et al: Obesity causes
mitochondrial fragmentation and dysfunction in white adipocytes due
to RalA activation. Nat Metab. 6:273–289. 2024. View Article : Google Scholar : PubMed/NCBI
|
|
96
|
Wu C, Liu Y, Liu W, Zou T, Lu S, Zhu C, He
L, Chen J, Fang L, Zou L, et al: NNMT-DNMT1 axis is essential for
maintaining cancer cell sensitivity to oxidative phosphorylation
inhibition. Adv Sci (Weinh). 10:e22026422022. View Article : Google Scholar : PubMed/NCBI
|
|
97
|
He P, Feng J, Xia X, Sun Y, He J, Guan T,
Peng Y, Zhang X, Liu M, Pang X and Chen Y: Discovery of a potent
and oral available complex I OXPHOS inhibitor that abrogates tumor
growth and circumvents MEKi resistance. J Med Chem. 66:6047–6069.
2023. View Article : Google Scholar : PubMed/NCBI
|
|
98
|
Wu MM, Wang QM, Huang BY, Mai CT, Wang CL,
Wang TT and Zhang XJ: Dioscin ameliorates murine ulcerative colitis
by regulating macrophage polarization. Pharmacol Res.
172:1057962021. View Article : Google Scholar : PubMed/NCBI
|
|
99
|
Wu L, Zhang X, Zheng L, Zhao H, Yan G,
Zhang Q, Zhou Y, Lei J, Zhang J, Wang J, et al: RIPK3 orchestrates
fatty acid metabolism in tumor-associated macrophages and
hepatocarcinogenesis. Cancer Immunol Res. 8:710–721. 2020.
View Article : Google Scholar : PubMed/NCBI
|
|
100
|
Shriver LP and Manchester M: Inhibition of
fatty acid metabolism ameliorates disease activity in an animal
model of multiple sclerosis. Sci Rep. 1:792011. View Article : Google Scholar :
|
|
101
|
Cao D, Khan Z, Li X, Saito S, Bernstein
EA, Victor AR, Ahmed F, Hoshi AO, Veiras LC, Shibata T, et al:
Macrophage angiotensin-converting enzyme reduces atherosclerosis by
increasing peroxisome proliferator-activated receptor α and
fundamentally changing lipid metabolism. Cardiovasc Res.
119:1825–1841. 2023. View Article : Google Scholar : PubMed/NCBI
|
|
102
|
Nomura M, Liu J, Yu ZX, Yamazaki T, Yan Y,
Kawagishi H, Rovira II, Liu C, Wolfgang MJ, Mukouyama YS and Finkel
T: Macrophage fatty acid oxidation inhibits atherosclerosis
progression. J Mol Cell Cardiol. 127:270–276. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
103
|
Hinshaw DC, Hanna A, Lama-Sherpa T, Metge
B, Kammerud SC, Benavides GA, Kumar A, Alsheikh HA, Mota M, Chen D,
et al: Hedgehog signaling regulates metabolism and polarization of
mammary tumor-associated macrophages. Cancer Res. 81:5425–5437.
2021. View Article : Google Scholar : PubMed/NCBI
|
|
104
|
Liu PS, Chen YT, Li X, Hsueh PC, Tzeng SF,
Chen H, Shi PZ, Xie X, Parik S, Planque M, et al: CD40 signal
rewires fatty acid and glutamine metabolism for stimulating
macrophage anti-tumorigenic functions. Nat Immunol. 24:452–462.
2023. View Article : Google Scholar : PubMed/NCBI
|
|
105
|
An L, Lu M, Xu W, Chen H, Feng L, Xie T,
Shan J, Wang S and Lin L: Qingfei oral liquid alleviates
RSV-induced lung inflammation by promoting fatty-acid-dependent
M1/M2 macrophage polarization via the Akt signaling pathway. J
Ethnopharmacol. 298:1156372022. View Article : Google Scholar : PubMed/NCBI
|
|
106
|
Bougarne N, Weyers B, Desmet SJ, Deckers
J, Ray DW, Staels B and De Bosscher K: Molecular actions of PPARα
in lipid metabolism and inflammation. Endocr Rev. 39:760–802. 2018.
View Article : Google Scholar : PubMed/NCBI
|
|
107
|
Wang D, Liu B, Tao W, Hao Z and Liu M:
Fibrates for secondary prevention of cardiovascular disease and
stroke. Cochrane Database Syst Rev. 2015:Cd0095802015.PubMed/NCBI
|
|
108
|
Lim SA, Wei J, Nguyen TM, Shi H, Su W,
Palacios G, Dhungana Y, Chapman NM, Long L, Saravia J, et al: Lipid
signalling enforces functional specialization of T(reg) cells in
tumours. Nature. 591:306–311. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
109
|
Fu Y, Zou T, Shen X, Nelson PJ, Li J, Wu
C, Yang J, Zheng Y, Bruns C, Zhao Y, et al: Lipid metabolism in
cancer progression and therapeutic strategies. MedComm. 2:27–59.
2020. View Article : Google Scholar
|
|
110
|
Altman BJ, Stine ZE and Dang CV: From
Krebs to clinic: Glutamine metabolism to cancer therapy. Nat Rev
Cancer. 16:619–34. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
111
|
Cluntun AA, Lukey MJ, Cerione RA and
Locasale JW: Glutamine metabolism in cancer: Understanding the
heterogeneity. Trends Cancer. 3:169–180. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
112
|
Hensley CT, Wasti AT and DeBerardinis RJ:
Glutamine and cancer: Cell biology, physiology, and clinical
opportunities. J Clin Invest. 123:3678–3684. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
113
|
Martinez-Outschoorn UE, Peiris-Pagés M,
Pestell RG, Sotgia F and Lisanti MP: Cancer metabolism: A
therapeutic perspective. Nat Rev Clin Oncol. 14:1132017. View Article : Google Scholar : PubMed/NCBI
|
|
114
|
Oh MH, Sun IH, Zhao L, Leone RD, Sun IM,
Xu W, Collins SL, Tam AJ, Blosser RL, Patel CH, et al: Targeting
glutamine metabolism enhances tumor-specific immunity by modulating
suppressive myeloid cells. J Clin Invest. 130:3865–3884. 2020.
View Article : Google Scholar : PubMed/NCBI
|
|
115
|
Pillai R, LeBoeuf SE, Hao Y, New C, Blum
JLE, Rashidfarrokhi A, Huang SM, Bahamon C, Wu WL, Karadal-Ferrena
B, et al: Glutamine antagonist DRP-104 suppresses tumor growth and
enhances response to checkpoint blockade in KEAP1 mutant lung
cancer. Sci Adv. 10:eadm98592024. View Article : Google Scholar : PubMed/NCBI
|
|
116
|
Liu N, Chen L, Yan M, Tao Q, Wu J, Chen J,
Chen X, Zhang W and Peng C: Eubacterium rectale improves the
efficacy of anti-PD1 immunotherapy in melanoma via
l-serine-mediated NK cell activation. Research (Wash D C).
6:01272023.PubMed/NCBI
|
|
117
|
Liu Y, Du Z, Li T, Zhang J, Cheng Y, Huang
J, Yang J, Wen L, Tian M, Yang M and Chen C: Lycorine eliminates
B-cell acute lymphoblastic leukemia cells by targeting PSAT1
through the serine/glycine metabolic pathway. Eur J Pharmacol.
961:1761622023. View Article : Google Scholar : PubMed/NCBI
|
|
118
|
Apostolova P and Pearce EL: Lactic acid
and lactate: Revisiting the physiological roles in the tumor
microenvironment. Trends Immunol. 43:969–977. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
119
|
Ma J, Tang L, Tan Y, Xiao J, Wei K, Zhang
X, Ma Y, Tong S, Chen J, Zhou N, et al: Lithium carbonate
revitalizes tumor-reactive CD8(+) T cells by shunting lactic acid
into mitochondria. Nat Immunol. 25:552–561. 2024. View Article : Google Scholar : PubMed/NCBI
|
|
120
|
Raud B, McGuire PJ, Jones RG, Sparwasser T
and Berod L: Fatty acid metabolism in CD8(+) T cell memory:
Challenging current concepts. Immunol Rev. 283:213–231. 2018.
View Article : Google Scholar : PubMed/NCBI
|
|
121
|
Ferraz-Bannitz R, Welendorf CR, Coelho PO,
Salgado W Jr, Nonino CB, Beraldo RA and Foss-Freitas MC: Bariatric
surgery can acutely modulate ER-stress and inflammation on
subcutaneous adipose tissue in non-diabetic patients with obesity.
Diabetol Metab Syndr. 13:192021. View Article : Google Scholar : PubMed/NCBI
|
|
122
|
Hatami M, Javanbakht MH, Haghighat N,
Sohrabi Z, Yavar R, Pazouki A and Farsani GM: Energy expenditure
related biomarkers following bariatric surgery: A prospective
six-month cohort study. BMC Surg. 24:1292024. View Article : Google Scholar : PubMed/NCBI
|
|
123
|
Vargas-Mendoza N, Morales-González A,
Madrigal-Santillán EO, Madrigal-Bujaidar E, Álvarez-González I,
García-Melo LF, Anguiano-Robledo L, Fregoso-Aguilar T and
Morales-Gonzalez JA: Antioxidant and adaptative response mediated
by Nrf2 during physical exercise. Antioxidants (Basel). 8:1962019.
View Article : Google Scholar : PubMed/NCBI
|
|
124
|
Lo YC, Lee CF and Powell JD: Insight into
the role of mTOR and metabolism in T cells reveals new potential
approaches to preventing graft rejection. Curr Opin Organ
Transplant. 19:363–371. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
125
|
de Lange P, Lombardi A, Silvestri E,
Cioffi F, Giacco A, Iervolino S, Petito G, Senese R, Lanni A and
Moreno M: Physiological approaches targeting cellular and
mitochondrial pathways underlying adipose organ senescence. Int J
Mol Sci. 24:116762023. View Article : Google Scholar : PubMed/NCBI
|
|
126
|
Biobaku F, Ghanim H, Monte SV, Caruana JA
and Dandona P: Bariatric surgery: Remission of inflammation,
cardiometabolic benefits, and common adverse effects. J Endocr Soc.
4:bvaa0492020. View Article : Google Scholar : PubMed/NCBI
|
|
127
|
Greto VL, Cvetko A, Štambuk T, Dempster
NJ, Kifer D, Deriš H, Cindrić A, Vučković F, Falchi M, Gillies RS,
et al: Extensive weight loss reduces glycan age by altering IgG
N-glycosylation. Int J Obes (Lond). 45:1521–1531. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
128
|
Pribić T, Das JK, Đerek L, Belsky DW,
Orenduff M, Huffman KM, Kraus WE, Deriš H, Šimunović J, Štambuk T,
et al: A 2-year calorie restriction intervention may reduce
glycomic biological age biomarkers-a pilot study. NPJ Aging.
11:712025. View Article : Google Scholar
|
|
129
|
Haran A, Bergel M, Kleiman D, Hefetz L,
Israeli H, Weksler-Zangen S, Agranovich B, Abramovich I,
Ben-Haroush Schyr R, Gottlieb E and Ben-Zvi D: Differential effects
of bariatric surgery and caloric restriction on hepatic one-carbon
and fatty acid metabolism. iScience. 26:1070462023. View Article : Google Scholar : PubMed/NCBI
|
|
130
|
Kim ER, Yun JH, Kim HJ, Park HY, Heo Y,
Park YS, Park DJ and Koo SK: Evaluation of hormonal and circulating
inflammatory biomarker profiles in the year following bariatric
surgery. Front Endocrinol (Lausanne). 14:11716752023. View Article : Google Scholar : PubMed/NCBI
|
|
131
|
Savulescu-Fiedler I, Mihalcea R,
Dragosloveanu S, Scheau C, Baz RO, Caruntu A, Scheau AE, Caruntu C
and Benea SN: The interplay between obesity and inflammation. Life
(Basel). 14:8562024.PubMed/NCBI
|
|
132
|
Poitou C, Perret C, Mathieu F, Truong V,
Blum Y, Durand H, Alili R, Chelghoum N, Pelloux V, Aron-Wisnewsky
J, et al: Bariatric surgery induces disruption in inflammatory
signaling pathways mediated by immune cells in adipose tissue: A
RNA-Seq study. PLoS One. 10:e01257182015. View Article : Google Scholar : PubMed/NCBI
|
|
133
|
Smith-Garvin JE, Koretzky GA and Jordan
MS: T cell activation. Annu Rev Immunol. 27:591–619. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
134
|
Hafida S, Mirshahi T and Nikolajczyk BS:
The impact of bariatric surgery on inflammation: Quenching the fire
of obesity? Curr Opin Endocrinol Diabetes Obes. 23:373–378. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
135
|
Popko K, Gorska E, Stelmaszczyk-Emmel A,
Plywaczewski R, Stoklosa A, Gorecka D, Pyrzak B and Demkow U:
Proinflammatory cytokines Il-6 and TNF-α and the development of
inflammation in obese subjects. Eur J Med Res. 15(Suppl 2):
S120–S122. 2010. View Article : Google Scholar
|
|
136
|
Reyes-Farias M, Fernández-García P,
Corrales P, González L, Soria-Gondek A, Martínez E, Pellitero S,
Tarascó J, Moreno P, Sumoy L, et al: Interleukin-16 is increased in
obesity and alters adipogenesis and inflammation in vitro. Front
Endocrinol (Lausanne). 15:13463172024. View Article : Google Scholar : PubMed/NCBI
|
|
137
|
Niewold TB, Lehman JS, Gunnarsson I, Meves
A and Oke V: Role of interleukin-16 in human diseases: a novel
potential therapeutic target. Front Immunol. 16:15240262025.
View Article : Google Scholar : PubMed/NCBI
|
|
138
|
Jensen RT, Thuesen ACB, Huang Y, Stinson
SE, Juel HB, Madsbad S, Bendtsen F, Hansen T and Pedersen JS:
Changes in inflammatory markers following bariatric surgery and the
impact of the surgical procedure: A 12-month longitudinal study.
Obes Surg. 35:2626–2637. 2025. View Article : Google Scholar : PubMed/NCBI
|
|
139
|
Liu Y, Jin J, Chen Y, Chen C, Chen Z and
Xu L: Integrative analyses of biomarkers and pathways for adipose
tissue after bariatric surgery. Adipocyte. 9:384–400. 2020.
View Article : Google Scholar : PubMed/NCBI
|
|
140
|
McKernan K, Varghese M, Patel R and Singer
K: Role of TLR4 in the induction of inflammatory changes in
adipocytes and macrophages. Adipocyte. 9:212–222. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
141
|
Teymournejad O, Li Z, Beesetty P, Yang C
and Montgomery CP: Toxin expression during Staphylococcus aureus
infection imprints host immunity to inhibit vaccine efficacy. NPJ
Vaccines. 8:32023. View Article : Google Scholar : PubMed/NCBI
|
|
142
|
Hajam IA, Tsai CM, Gonzalez C, Caldera JR,
Lázaro Díez M, Du X, Aralar A, Lin B, Duong W and Liu GY:
Pathobiont-induced suppressive immune imprints thwart T cell
vaccine responses. Nat Commun. 15:103352024. View Article : Google Scholar : PubMed/NCBI
|
|
143
|
Trougakos IP, Terpos E, Alexopoulos H,
Politou M, Paraskevis D, Scorilas A, Kastritis E, Andreakos E and
Dimopoulos MA: Adverse effects of COVID-19 mRNA vaccines: The spike
hypothesis. Trends Mol Med. 28:542–554. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
144
|
Wang Y, Zheng Y, Kuang L, Yang K, Xie J,
Liu X, Shen S, Li X, Wu S, Yang Y, et al: Effects of probiotics in
patients with morbid obesity undergoing bariatric surgery: A
systematic review and meta-analysis. Int J Obes (Lond).
47:1029–1042. 2023. View Article : Google Scholar : PubMed/NCBI
|
|
145
|
Komorniak N, Kaczmarczyk M, Łoniewski I,
Martynova-Van Kley A, Nalian A, Wroński M, Kaseja K, Kowalewski B,
Folwarski M and Stachowska E: Analysis of the efficacy of diet and
short-term probiotic intervention on depressive symptoms in
patients after bariatric surgery: A randomized double-blind placebo
controlled pilot study. Nutrients. 15:49052023. View Article : Google Scholar : PubMed/NCBI
|
|
146
|
O'Bryan LJ, Atkins KJ, Lipszyc A, Scott
DA, Silbert BS and Evered LA: Inflammatory biomarker levels after
propofol or sevoflurane anesthesia: A meta-analysis. Anesth Analg.
134:69–81. 2022. View Article : Google Scholar
|
|
147
|
Hashemian M, Sahebdad-Khabisi S, Honarvar
Z, Torabinejad Z, Taravati H, Mohammadi FD and Amirkhosravi L:
Effects of spinal versus general anesthesia on serum oxidative
stress markers and cytokine release after abdominal hysterectomy: A
non-randomized trial. Sci Rep. 15:302472025. View Article : Google Scholar : PubMed/NCBI
|
|
148
|
Fadahunsi N, Petersen J, Metz S, Jakobsen
A, Vad Mathiesen C, Silke Buch-Rasmussen A, Kurgan N, Kjærgaard
Larsen J, Andersen RC, Topilko T, et al: Targeting postsynaptic
glutamate receptor scaffolding proteins PSD-95 and PICK1 for
obesity treatment. Sci Adv. 10:eadg26362024. View Article : Google Scholar : PubMed/NCBI
|