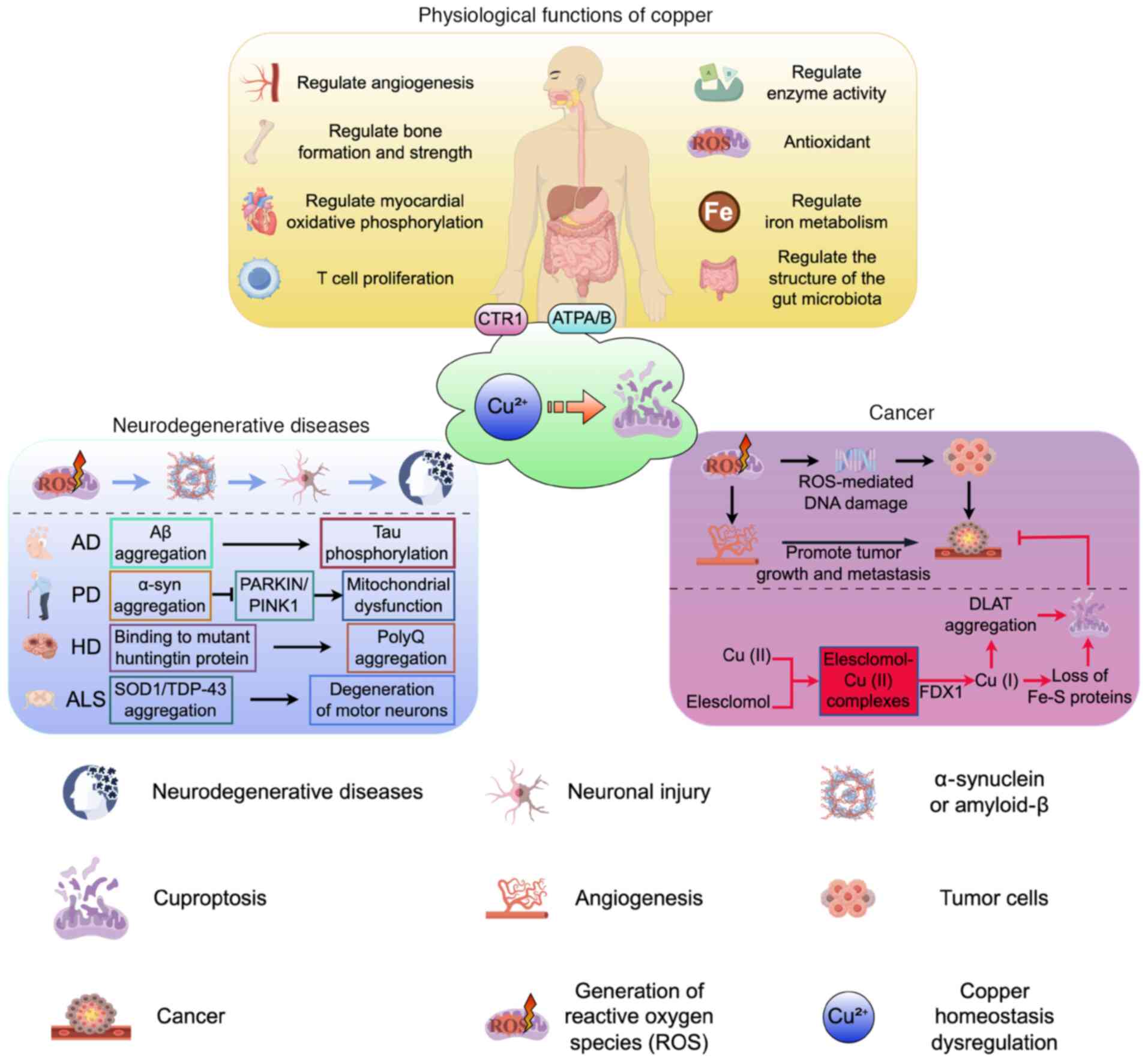
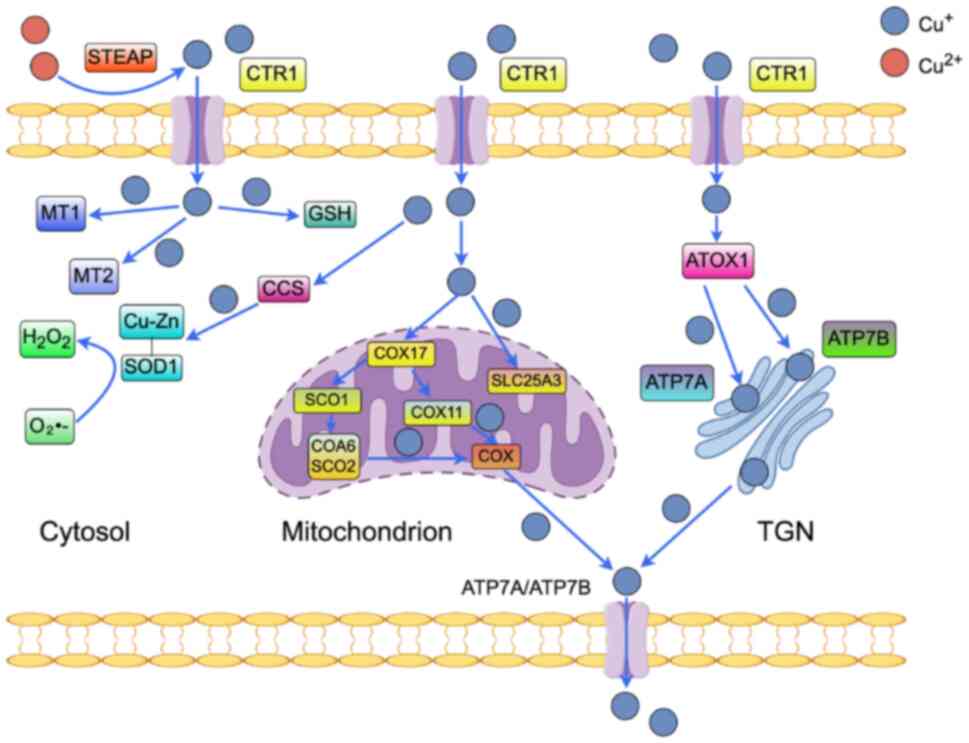
Copper is an essential trace element and the
recommended daily intake for adults ranges from 0.8 to 2.4 mg per
day (1). Copper is required for
almost all the functions of tissues and organs, in particular for
oxidative phosphorylation in the heart and brain, scavenging of
free radicals (2), angiogenesis
(3), bone formation and
regeneration (4,5), modulation of bone strength
(6,7) and the regulation of rest-activity
cycles (8) (Fig. 1). Copper also participates in the
synthesis of copper-dependent enzymes, including superoxide
dismutase (SOD) (9), cytochrome
c oxidase (10), tyrosinase
(11), lysyl oxidase and
dopamine (DA) β-hydroxylase (12,13). Copper enzymes play a crucial role
in key physiological processes such as energy metabolism,
antioxidant defense and neurotransmitter synthesis. Their
dysfunction directly leads to a variety of genetic and acquired
diseases (14,15) (Table I).
Copper homeostasis regulation can be classified into
three processes: Absorption, utilization/storage and excretion.
Disruptions in copper homeostasis can result in impaired
physiological functions and the onset of various related diseases
(Fig. 1). In response to these
challenges, researchers have developed a range of copper-targeted
therapeutic strategies designed to manage such conditions. These
interventions specifically address pathological states caused by
copper deficiency, copper overload or disturbances in copper
metabolism, and they exert their effects by modulating copper
concentration, distribution or biological activity within the body.
These therapeutic approaches show significant potential in the
treatment of hereditary copper metabolism disorders,
neurodegenerative diseases and cancers. This article provides a
comprehensive and timely review of the dual role of copper in human
biology, with a particular emphasis on its implications for health,
neurodegenerative diseases and cancer. This review provides a
theoretical foundation and offers diverse perspectives for the
development of treatments for related diseases.
Copper absorption primarily occurs in the small
intestine, particularly in the duodenum, which serves as the key
site for this process (16). In
dietary sources, copper is predominantly present as Cu(II). The
six-transmembrane epithelial antigen of the prostate proteins
(17), localized on the apical
membrane of small intestinal epithelial cells, possess reductase
activity that facilitates the reduction of Cu(II) to Cu(I).
Subsequently, Cu(I) is taken up by the copper transporter 1 (CTR1),
also known as solute carrier family 31 member 1 (SLC31A1) (18,19), which is expressed on the
intestinal epithelial membrane. Once Cu(I) enters the enterocytes,
it is transported into the bloodstream through vesicular transport
mediated by ATPase copper transporting α (ATP7A) (Fig. 2). In the bloodstream, copper
binds to various proteins, including human serum albumin (20), ceruloplasmin (21), albumin, α-2-macroglobulin
(22), histidine and
transcupreins. These complexes either support copper's
physiological functions through the systemic circulation or enable
its uptake and storage by the liver (23-25).
The high-affinity copper transporter protein CTR1
facilitates the transfer of extracellular Cu(I) into the cell
(26). In mice,
intestinal-specific knockout of CTR1 resulted in severe growth and
developmental defects, leading to embryonic lethality by
mid-gestation. These findings indicate that CTR1-mediated copper
uptake is essential for mammalian copper homeostasis and embryonic
development (27). Additionally,
CTR1 gene expression is regulated by changes in specificity protein
1 (Sp1) activity and extracellular copper concentrations (28,29). Specifically, the human
high-affinity copper transporter hCTR1 is transcriptionally
upregulated during copper deficiency and downregulated under
copper-replete conditions. Elevated hCTR1 levels also suppress its
own expression. Notably, Sp1 modulates hCTR1 expression under
copper stress (30).
Once inside the cell, copper rapidly binds to
co-chaperone proteins such as antioxidant 1 copper chaperone
(Atox1), copper chaperone for SOD (CCS) and cytochrome c oxidase
copper chaperone COX17 (COX17). This interaction is essential for
the safe and targeted delivery of copper to its specific functional
sites, thereby supporting and maintaining critical cellular
processes (29).
Atox1 functions as a key cytoplasmic copper
chaperone, directly binding Cu(I) and mediating its delivery to the
copper-transporting ATPases ATP7A and ATP7B located on the Golgi
membrane (Fig. 2). This process
is essential for the systemic distribution of copper ions and the
maintenance of copper homeostasis (31,32). Importantly, research by Lutsenko
has revealed that Atox1 promotes the synthesis of ceruloplasmin, a
protein critical for iron metabolism, as well as tyrosinases
(33).
The CCS is localized in compartments such as the
cytoplasm and the mitochondrial intermembrane space (34), binds copper and activates SOD1
(Cu/Zn SOD), a copper/zinc-dependent enzyme. In eukaryotes, SOD is
expressed as SOD1 in the cytoplasm and extracellularly, and as
Mn-SOD2 in the mitochondria. The SOD enzymes catalyze the
disproportionation of superoxide (O2•-) to
produce hydrogen peroxide (H2O2), and the
balance between copper and reactive oxygen species (ROS) is
physiologically crucial (35,36). Furthermore, CCS expression is
strongly negatively correlated with copper levels, serving as a
feedback regulatory mechanism to prevent excessive activation of
SOD1 under conditions of copper overload (37).
The COX is associated with oxidative phosphorylation
in mitochondria, and its function is mainly mediated by five
proteins: COX17, synthesis of cytochrome C oxidase 1 (SCO1), SCO2,
cytochrome c oxidase assembly factor 6 (COA6) and COX11, each of
which is essential. COX17 transfers copper ions from the cytoplasm
to the mitochondrial intermembrane space (38), and then, through the formation of
a disulfide bond, transfers them to SCO1 (39). Copper is transported primarily
through the action of COA6 with SCO2 in the maintenance of redox
homeostasis within mitochondria or through COX11 (40).
In addition to these chaperones, copper can be
transported into the mitochondria by the mitochondrial phosphate
transporter SLC25A3 (41).
Another key component is the intracellular copper storage proteins
metallothionein 1 (MT1) and MT2, which have a high affinity for
copper (40), while copper, as a
catalyst for the Fenton reaction, is also capable of binding to
glutathione (GSH) (42). These
proteins are essential for the effective utilization of copper and
the synthesis of key copper enzymes, including SOD, cytochrome c
oxidase, tyrosinase, lysyl oxidase, DA β-hydroxylase, ATPase
copper-transporting α (ATP7A) and ATP7B to maintain normal cellular
functions (40).
The regulation of copper homeostasis is
fundamentally dependent on the copper-transporting ATPases ATP7A
and ATP7B. In response to elevated intracellular copper [Cu(I)]
levels, ATP7A and ATP7B translocate from the trans-Golgi network
(TGN) to vesicles that subsequently fuse with the plasma membrane,
thereby facilitating the efflux of Cu(I) from the cell. Conversely,
when intracellular Cu(I) levels are reduced, these ATPases are
retained within the TGN, where they actively transport Cu(I) into
the TGN lumen to support the maturation of copper-dependent
proteins. Once physiological Cu(I) levels are restored, ATP7A and
ATP7B are recycled back to the TGN, re-establishing their basal
localization and maintaining responsiveness to fluctuations in
cellular copper concentrations (43). ATP7A can mediate copper transport
across polarized cellular barriers, such as the blood-brain barrier
(BBB) and the blood-placenta barrier, thereby enabling copper
export and ensuring that the fetus receives adequate copper during
development. ATP7B, predominantly expressed in the liver, becomes
active under conditions of elevated hepatic copper concentrations
(33). In hepatic cells, ATP7B
plays a crucial role in the regulation of copper homeostasis by
mediating the transport of excess copper into bile, leading to its
excretion via feces, a process essential for maintaining the
systemic copper balance (44).
This protein facilitates copper elimination via bile secretion or
through binding to soluble proteins for delivery to specific
tissues and organs (45).
Copper deficiency is associated with impaired immune
function and reduced T-cell proliferation, which leads to decreased
production of interleukin-2 (IL-2). The capacity of immune cells to
generate superoxide anions and eliminate bacteria is diminished in
mild copper deficiency, and the number of neutrophils in the
peripheral blood is reduced in severe copper deficiency (46,47). Menkes syndrome (MD) is an
X-linked recessive disorder caused by mutations in ATP7A and is
characterized by systemic copper deficiency, primarily due to
impaired intestinal copper uptake, progressive neurodegeneration,
connective tissue disorders, and brittle, kinky hair (48). Infants with severe MD often do
not survive beyond the third year of life, with neurological
deficits, matted hair, developmental delays, genitourinary
abnormalities, skin abnormalities, vascular abnormalities, skeletal
abnormalities and spasticity of the limbs that transform into
muscle weakness of the limbs along with other abnormal signs. Most
patients die within the third year of life from cerebral hemorrhage
due to vascular fragility or infection-related complications
(49). This indicates that
copper plays a crucial role in infant development: Copper imbalance
driven by ATP7A mutations leads to extensive multi-organ
dysfunction in MD, which is manifested as multi-system
abnormalities and fatal outcomes in infants. Another disorder
caused by mutations in ATP7A leading to copper deficiency is
occipital horn syndrome (OHS), which is a phenotype of MD but with
milder symptoms than those of MD (50). Bone exostoses, radial head
dislocations, keloid-like skin lesions and dental abnormalities are
specific to OHS, which can also present with developmental delay
and mild neurological symptoms (14). An early study indicated that the
ATP7A transcript lacking exon 10 encodes a partially functional
protein, and this residual function is associated with a milder
form of occipital horn syndrome (OHS), which is caused by mutations
at the ATP7A splicing site. Unlike the loss-of-function mutations
observed in classical Menkes disease (MD), OHS is characterized by
relatively mild clinical symptoms (50). In 2022, Batzios et al
(51) identified a genetic
disorder of brain copper metabolism caused by CTR1 deficiency,
which presents with hypotonia, global developmental delay, seizures
and rapid cerebral atrophy. Brain CTR1, ATP7A and ATP7B protein
levels may be affected by factors other than gene expression, such
as the rate of copper transport mediated by these proteins. The key
to treating MD is supplementation with additional copper (52-54), which is not replenished orally
because of impaired intestinal absorption caused by ATP7A
deficiency. The most widely used treatment is parenteral or
subcutaneous supplementation with copper histidine (55,56).
Copper overload disrupts the expression and function
of antioxidant enzymes and induces oxidative stress, while
oxidative stress and increased ROS levels are thought to be the
main cause of Cu-induced cytotoxicity (57,58). When copper is overloaded,
hydrogen peroxide can be converted into highly reactive hydroxyl
radicals, which can cause DNA and membrane damage (59,60). Excessive copper intake has also
been linked to alterations in the gut microbiota, which in turn can
lead to a range of conditions such as polycystic ovary syndrome
(61). Studies have shown that
increased intracellular Cu activates autophagy through unc-51 like
autophagy activating kinase 1 (ULK1)/ULK2 signaling, thereby
promoting cancer cell growth and survival (62,63). Wilson disease (WD) is an
autosomal recessive disorder caused by mutations in ATP7B that is
primarily due to ATP7B mutations that result in impaired copper
excretion from hepatocytes into bile, a significant increase in
copper levels in hepatocytes and subsequent liver injury; excess
copper is released into the circulation, where it is deposited in
areas such as the brain or the eyeballs, causing damage. WD is
clinically heterogeneous and may present with neurological
dysfunction, acute liver failure and the presence of hemolysis,
rhabdomyolysis, renal tubular injury, leukopenia and
thrombocytopenia (64-66). Long-term management of WD
includes chelating agents such as tetrathiomolybdate (TM) (67), trientine or d-penicillamine
(68-70), as well as zinc salts to reduce
copper absorption (40,71,72).
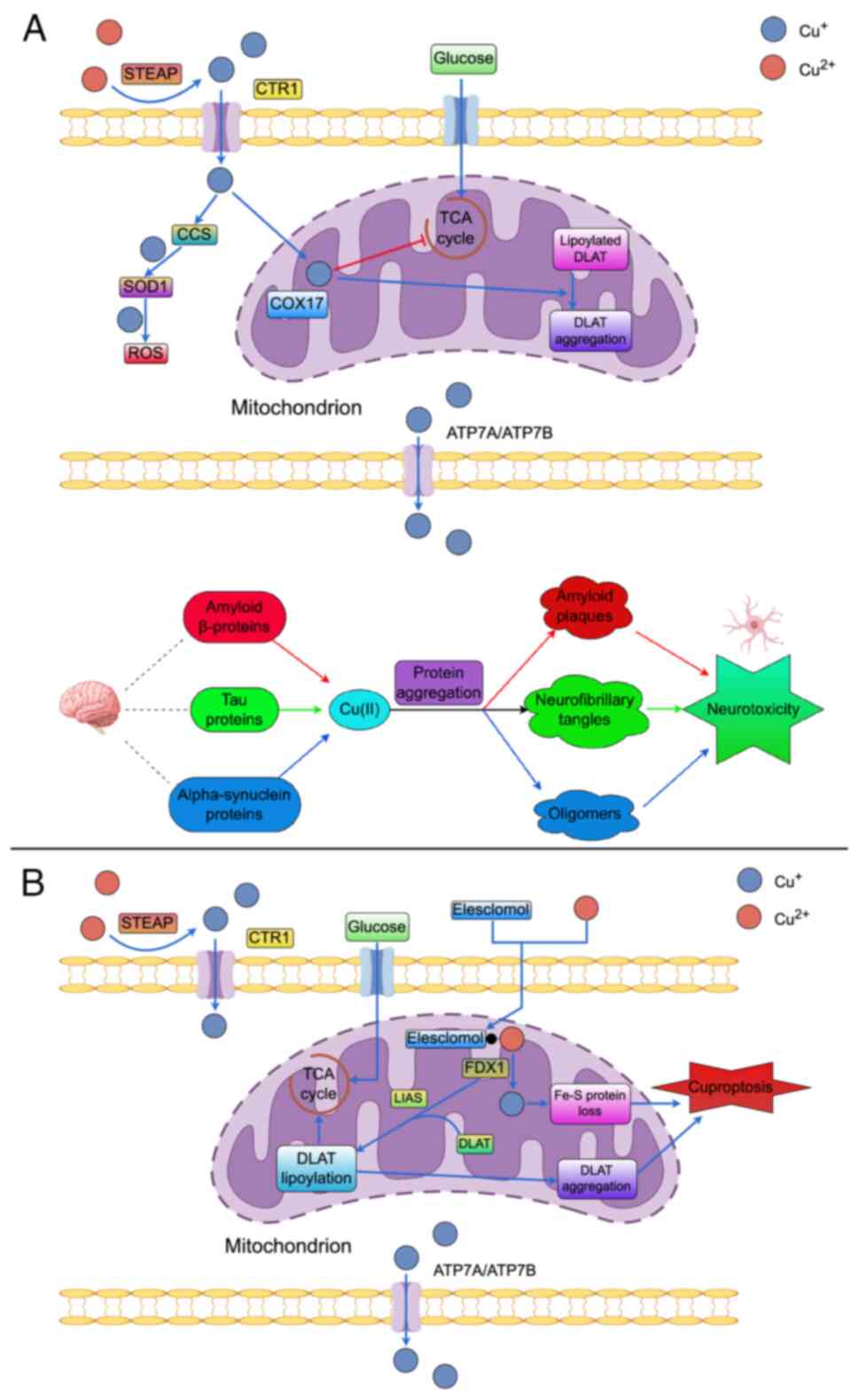
AD is a neurodegenerative disorder characterized by
the presence of β-amyloid (Aβ) plaques and tau protein tangles in
the brain (Fig. 3A). Initially,
these pathological features were regarded as the primary pathogenic
drivers of AD, prompting a focus on therapeutic strategies aimed at
reducing the accumulation of Aβ or tau proteins (73,74). However, drug development
targeting these proteins has faced substantial challenges: >30
Phase III clinical trials have shown that Aβ inhibitors do not
significantly improve cognitive function in patients with AD, and
some interventions have even led to adverse effects following
plaque removal (75-77). This research and development
predicament not only highlights the limitations of traditional
target therapy but also prompts researchers to re-examine and
explore the pathogenesis of AD.
While the precise molecular mechanisms underlying AD
remain elusive, accumulating evidence suggests that dysregulation
of brain metal ions, particularly copper and zinc, may contribute
to the hallmark Aβ neuropathology associated with the disease
(78,79). Transition metals such as copper,
iron and zinc are essential for normal physiological functions;
however, copper has been specifically implicated in promoting Aβ
aggregation and the formation of harmful species. Notably,
individuals with AD exhibit reduced copper levels in brain tissue,
yet elevated concentrations in serum and age-related pigments
(80-82). Copper's role in promoting Aβ
precipitation and generating redox-active species warrants further
investigation. Serum levels of both free and total copper are
significantly elevated in individuals with AD, and increased copper
deposition is observed in the age-related pigments of these
patients (82,83). Following the observation that
copper promotes Aβ precipitation under acidic conditions, the
interactions between copper and Aβ have been increasingly explored
in detail (84-86). Critically, copper can form
complexes with Aβ that drive redox reactions, involving lipids to
induce oxidative stress, ultimately leading to cellular apoptosis
and contributing to cognitive decline (87-89).
HD is an inherited neurodegenerative disorder
characterized by neuropsychiatric symptoms, movement disorders
(most commonly choreoathetosis) and progressive cognitive
impairment. HD is currently treated symptomatically and scientists
have struggled to identify effective disease-modifying therapies.
Metal homeostasis is disrupted during the progression of HD,
although its exact role in the pathogenesis of the disease remains
elusive. Meanwhile, some researchers have observed tissue
abnormalities and deposition of copper (Cu) and zinc (Zn) in
HD-affected brain regions, which may contribute to disease
initiation and progression through mechanisms including
mitochondrial dysfunction, oxidative stress and BBB dysfunction
(110). It has been reported
that copper-regulated genes are upregulated in HD and that copper
binds to the N-terminal fragment of huntingtin, supporting the
involvement of aberrant copper metabolism in HD. Copper has been
shown to accelerate fibril formation of the N-terminal fragment of
huntingtin in vitro via the expanded polyglutamine (polyQ)
stretch (httExon1). Copper has also been found to enhance polyQ
aggregation and toxicity in mammalian cells expressing httExon1.
Studies in a yeast model of HD demonstrated that overexpression of
several genes involved in copper metabolism reduced polyQ-mediated
toxicity. The MT3 gene belongs to the metallothionein family and is
mainly expressed in the central nervous system. It regulates zinc
and copper homeostasis and acts as a neuronal growth inhibitory
factor (111). Overexpression
of MT3 in mammalian cells significantly reduced polyQ aggregation
and toxicity (112). Animal
studies have also shown that copper chelators modulate the early
events of Htt misfolding and reduce neurotoxicity in the
Drosophila HD model (90,113,114). Despite the promising
therapeutic potential of MT3 in HD, its clinical translation
remains limited by technical and mechanistic challenges.
PD is an age-related neurodegenerative disease, and
the role of oxidative stress and mitochondrial dysfunction in the
progression of PD has been widely recognized in relation to the
selective loss of DA neurons in the substantia nigra pars compacta
(SNpc) of the nigrostriatal DA pathway and the reduction of DA
levels in the striatum (115).
The normal substantia nigra contains twice as much copper as other
brain regions, suggesting that copper plays an important role in
this brain area (116). Autopsy
brain samples from patients with PD show increased iron and
decreased copper in the substantia nigra and basal ganglia
(117,118).
At the cellular level, PD is associated with the
overproduction of ROS. While copper may contribute to intracellular
oxidative stress by participating in the Fenton and Haber-Weiss
reactions or by interfering with iron homeostasis, either directly
or indirectly through the formation of hydroxyl radicals,
experimental evidence linking copper deficiency and the formation
of SOD1 aggregates to the progression of PD is also discussed in
terms of its therapeutic implications (119,120). Copper causes a decrease in SOD1
activity by interfering with SOD1 synthesis, leading to a loss of
cellular protection against neuronal oxidative damage (121,122).
It is important to note that mitochondrial
dysfunction represents a central mechanism driving oxidative stress
in PD. Mutations in the phosphatase and tensin homolog-induced
kinase 1 (PINK1) and PARKIN RBR E3 ubiquitin ligase (PARKIN) genes
are associated with familial forms of the disease. Parkin and PINK1
play critical roles in mitochondrial quality control, particularly
in the process of mitophagy, and are essential for the survival of
dopaminergic neurons. Under normal physiological conditions, PINK1
accumulates on damaged mitochondria by sensing changes in the
mitochondrial membrane potential. This accumulation promotes Parkin
recruitment and activates its E3 ubiquitin ligase activity,
resulting in the ubiquitination of damaged mitochondria and the
initiation of autophagic degradation. This pathway limits the
release of ROS and reduces mitochondrial toxicity (123,124).
Dysregulation of copper homeostasis interferes with
the PARKIN/PINK1 pathway through multi-layered mechanisms (125). Excessive copper downregulates
the transcription of PINK1 and PARKIN in cellular models, although
the exact regulatory mechanism (e.g., involvement of NF-κB) remains
to be fully elucidated. Importantly, copper can also impair the
activity of PINK1 and PARKIN proteins by inducing conformational
changes, specifically through binding to the kinase domain of PINK1
and the RING finger domain of PARKIN. Notably, the role of copper
in regulating PINK1/PARKIN is context-dependent: It inhibits
PINK1/PARKIN function under pathological conditions (e.g., copper
excess), while promoting their transcriptional regulation and
autophagic processes under physiological conditions (125,126). It further impairs PINK1 kinase
activity and Parkin E3 ubiquitin ligase activity by coordinating
with cysteine/histidine residues within their respective functional
domains, the kinase domain of PINK1 and the RING finger domain of
Parkin, thereby inducing conformational changes (125). These disruptions prevent Parkin
recruitment to damaged mitochondria, inhibit mitochondrial
substrate ubiquitination and perturb the PARKIN/PINK1-regulated
balance of mitochondrial dynamics, ultimately leading to the
accumulation of dysfunctional mitochondria and exacerbated
oxidative stress. This cascade reflects the pathological shift in
copper's regulatory role in mitophagy under homeostatic imbalance,
which contrasts with its physiological role in promoting
mitophagy-mediated tissue regeneration (126). However, the underlying
mechanisms remain incompletely understood and require further
investigation. In addition, exposure to elevated copper levels has
been shown to accelerate the formation of toxic α-synuclein
aggregates, a key pathological contributor to neuronal loss in PD
(127).
Second, microglia-mediated neuroinflammation is an
important component of the pathogenesis of PD. In animal studies,
researchers have observed inflammatory changes in mouse brain
tissue, including activation of microglia, loss of dopaminergic
neurons and aggregation of α-syn in the substantia nigra. Copper
has been shown to activate BV2 cells via the NF-κB pathway and to
increase ROS levels in BV2 cells (128,129). Sustained copper accumulation in
BV2 cells resulted in a decrease in mitochondrial membrane
potential, a reduction in Parkin and PINK1 expression, an increase
in P62 expression and light chain 3BII/I ratio, and upregulation of
NLR family pyrin domain containing 3/caspase-1/gasdermin D axis
proteins (130,131).
Research on the treatment of PD with copper-based
compounds, such as 8-hydroxyquinoline-2-carboxaldehyde
isonicotinoylhydrazine, a moderate metal-binding compound that
binds to copper ions, has been shown to efficiently compete with
ortho-nucleosides for Cu(I) and Cu(II) binding and to inhibit
protein aggregation in vitro and in cells (132). Copper(II)
diacetylbis(4-methylthiosemicarbazone), also known as CuII(atsm),
is an orally available, biologically active BBB-penetrant compound
that is widely used to selectively label hypoxic tissues in
cellular imaging experiments (133). It has been shown to accumulate
in the striatum of patients with PD at levels that positively
correlate with disease stage (134). Furthermore, CuII(atsm) has
demonstrated therapeutic potential in rescuing dopaminergic neuron
loss and alleviating motor dysfunction in PD models (119,135). In addition, CuII(atsm)
supplementation has been shown to significantly reduce the
misfolding and deposition of wild-type SOD1 protein associated with
PD in a novel mouse model, enhance DA neuron survival and improve
motor function, suggesting its potential as a novel therapeutic
strategy for PD treatment (136). However, further research and
clinical trials are necessary to validate these findings.
ALS is a progressive neurodegenerative disease of
the motor neurons that leads to the worsening of casual muscle
weakness until death from respiratory failure ~3 years after onset
(137). Numerous symptoms of
ALS are associated with signs of copper deficiency, leading to
defects in the vasculature, antioxidant system and mitochondrial
oxidative respiration, but there are also signs of copper toxicity,
such as increased ROS generation and protein aggregation (138).
About 2% of ALS cases have mutations in SOD1, which
neutralizes harmful reactive oxygen superoxide (139). Familial ALS is at times linked
to the gene encoding Cu/Zn-binding SOD. Mutations in ALS are
thought to result in functional enhancement of dismutase activity
(140). Mutations in human SOD1
are found in ~20% of patients with familial ALS. They are
characterized by a conformational dysfunction of the electrostatic
and zinc-binding elements (mutant SOD1-mediated pathogenesis of
ALS). A study using transgenic mice expressing wild-type or mutant
human SOD confirmed that dominant mutations in SOD and the
acquisition of function are important factors in the pathogenesis
of familial ALS (141).
Previous studies have shown that CuII(atsm) in ALS
can restore SOD1 dysfunction caused by copper deficiency (135). Therefore, the use of Cu as a
new target for treating familial progressive ALS becomes possible.
However, further studies are necessary to validate these
findings.
Overall, for copper-related diseases, including both
the copper metabolism disorders discussed earlier and the
neurodegenerative conditions addressed in this chapter, therapeutic
strategies targeting copper homeostasis have been developed, with
drug selection guided by the underlying disease etiology
(deficiency, overload or imbalance). This establishes a clear
pathophysiological and etiological foundation for clinical
intervention (Table II).
According to the most recent estimates provided by
the International Agency for Research on Cancer, ~20 million new
cancer cases, including non-melanoma skin cancers, and 9.7 million
cancer-related deaths were reported globally in 2022. Lung cancer
emerged as the most frequently diagnosed cancer, accounting for
nearly 2.5 million new cases, representing approximately one in
eight cancer diagnoses worldwide (12.4% of all cancers). This was
followed by female breast cancer (11.6%), colorectal cancer (9.6%),
prostate cancer (7.3%) and gastric cancer (4.9%). Furthermore, lung
cancer was identified as the leading cause of cancer-related
mortality, with an estimated 1.8 million deaths (18.7% of total
cancer deaths), followed by colorectal cancer (9.3%), liver cancer
(7.8%), female breast cancer (6.9%) and stomach cancer (6.8%)
(142).
Abnormal accumulation or deficiency of trace
elements plays a significant role in cancer pathogenesis and
progression, with dysregulation of copper metabolism being
particularly notable (143,144). Research suggests that there is
an increased demand for copper during tumor proliferation and
metastasis (40,145). Elevated serum copper levels
have been reported in various cancers, including breast cancer
(146,147), lung cancer (148) and prostate cancer (149). By contrast, reduced serum
copper levels have been observed specifically in hepatocellular
carcinoma and endometrial cancer (150,151), although the underlying
mechanisms remain to be fully elucidated.
Copper can promote tumorigenesis by promoting
oxidative stress that damages DNA and related molecular structures
(152), and contributes to
tumor development by promoting angiogenesis, metastasis and cell
proliferation through multiple mechanisms: Increased intracellular
copper enhances oxidative stress caused by the formation of ROS,
leading to elevated intracellular ROS levels (153), DNA damage and activation of
oncogenes (59), whereas reduced
intracellular copper results in decreased SOD1 activity, nearly
complete loss of resistance to oxidative damage and impaired
maintenance of normal cellular life activities (121).
Copper has also been found to directly bind
ULK1/ULK2 and activate autophagy by Tsang et al (62). Cellular autophagy is inhibited
when CTR1 is deficient, which can suppress oncogene-induced cancer
cell proliferation; autophagosomes are reduced and tumor size is
significantly decreased in mice in which CTR1 is knocked out using
single-guide RNA (62). Copper
promotes cancer by stimulating the MAPK pathway (166). Copper binds to MAPK kinase
(MEK)1/2 and activates downstream ERK1/2 phosphorylation, thereby
promoting tumor proliferation. MEK1/2 activity was inhibited in
BRAFV600E-positive cancer cells, and proliferation was
significantly suppressed after TM treatment, suggesting that MEK1/2
activity is highly correlated with copper levels (167,168). The epidermal growth factor
receptor (EGFR), as a key upstream regulator of the MAPK pathway,
is overexpressed or mutated in various cancers (including ovarian
cancer), driving cell proliferation, survival and metastasis.
Copper binds to and inhibits protein tyrosine phosphatase N2
(PTPN2), leading to the loss of its phosphatase activity. This
inhibition relieves the suppression of EGFR dephosphorylation,
thereby activating EGFR signaling. In turn, activated EGFR
suppresses the transcription of CTR1 via the cAMP responsive
element binding protein (CREB) signaling pathway, forming a
closed-loop regulatory circuit: 'copper-PTPN2-EGFR-CREB-CTR1'.
Fluctuations in intracellular copper levels can thus modulate EGFR
activity through this feedback axis, highlighting a mechanistic
link between copper homeostasis and EGFR signaling (169). Research by Jakhmola et
al (147) revealed the
significant role of EGFR in ovarian cancer through computational
simulation methods and pointed out that the DADETL segment
(including the self-phosphorylated tyrosine 992) is the key region
leading to receptor overexpression. This is similar to the
copper-mediated activation mechanism of the MAPK pathway,
suggesting that copper may indirectly affect tumor development by
regulating EGFR activity or downstream signal transduction. Future
studies are necessary to deeply explore the direct association
between copper and the EGFR pathway, providing new ideas for the
combined application of copper-targeted therapy and EGFR inhibitors
(147).
Copper also mediates the activation of programmed
cell death 1 (PD-1)/programmed cell death ligand 1 (PD-L1) in
cancer cells, enabling immune evasion. When copper chelators are
used, phosphorylation of STAT3 and EGFR is inhibited, promoting
ubiquitination-mediated degradation of PD-L1, and slowing tumor
growth by increasing the number of CD8+ T cells and NK
cells (170,171).
It has been shown that the metabolic profile of
cancer cells differs from that of normal cells in that cancer cells
maintain a higher metabolic rate to support a higher proliferation
rate and to resist cell death signals (172). Cell death involves proteins and
lipids and includes apoptosis (173,174), necroptosis (175), pyroptosis (176) and ferroptosis (177,178). The demand for copper is high
during cell growth and abnormal accumulation of excess copper
within the cell leads to cellular dysfunction and ultimately cell
death (179,180). Mechanisms of copper-induced
toxicity include induction of apoptosis (181-183), caspase-independent cell death
pathways (184-186), induction of ROS or inhibition
of the ubiquitin-proteasome system (129,187-189).
However, the mechanism underlying copper-induced
cell death remained unclear until a study published in Science in
2022 by Tsvetkov et al (190) proposed the concept of
cuproptosis - a novel form of copper-induced cell death that is
distinct from other known forms of cell death (Fig. 3B). This study demonstrated that
copper ionophore-induced cell death is predominantly dependent on
the accumulation of intracellular copper (191,192). Notably, treatment with
inhibitors targeting other established mechanisms of cell death,
including ferroptotic apoptosis (ferrostatin-1), necroptosis
(necrostatin-1) and oxidative stress (N-acetylcysteine), did not
abolish copper ionophore-induced cytotoxicity (190). Genome-wide CRISPR/Cas9
knockdown experiments identified seven specific genes that
significantly mitigated the cytotoxic effects associated with the
copper ionophore elesclomol (193). Among the identified genes was
ferredoxin 1 (FDX1), a reductase known for its role in reducing
Cu(II) to the more toxic Cu(I) and serving as a direct target for
elesclomol (194,195). Additionally, six genes encoding
components of the lipoic acid pathway - specifically
lipoyltransferase 1, lipoic acid synthase (LIAS) and
dihydrolipoamide dehydrogenase - or targets associated with
lipoylated proteins within the pyruvate dehydrogenase complex
[including dihydrolipoyl transacetylase (DLAT), pyruvate
dehydrogenase E1 subunit α1 (PDHA1) and PDHB] were also identified
(196). Importantly, single
gene knockout analyses revealed that FDX1 and LIAS were
particularly effective at diminishing cytotoxicity associated with
copper ion carriers. Furthermore, inhibition of complex I was found
to obstruct copper-induced cell death. This finding suggests that
applying an electron transport chain inhibitor can prevent the
cytotoxic effects induced by copper when administered alongside
elesclomol in both serum-containing and serum-free media. Under
serum-free conditions, cells exhibited significant resistance to
elesclomol-mediated cytotoxicity; substantial toxicity was observed
only when elesclomol-Cu(II) complexes were present. This effect was
inhibited by the addition of TM. Furthermore, buthionine
sulfoximine has been demonstrated to sensitize cells to
copper-induced cell death by inhibiting glutamate cysteine ligase,
which results in a direct depletion of the endogenous copper
chelator GSH. The underlying mechanism involves the role of FDX1 in
promoting the lipoylation of DLAT and dihydrolipoamide
S-succinyltransferase proteins (190,197). Cu(II) binds to lipoylated DLAT,
facilitating the production of insoluble DLAT that promotes cell
death. Conversely, the conversion of Cu(II) to the more toxic Cu(I)
induces cell death and intracellular oxidative stress, leading to
an increase in heat shock protein 70 expression levels (190).
P53 activity maintains the functional integrity of
mitochondrial morphology and structure, and mutations or
deficiencies in p53 lead to significant reductions in cytochrome c
oxidase (COX) activity and respiratory metabolism (198-200). P53 also promotes the conversion
of pyruvate to acetyl coenzyme A by promoting lactate dehydrogenase
A to maintain high pyruvate levels and by promoting
dephosphorylation to activate PDH complexes (201,202). Copper-induced cell death is
also highly dependent on cellular oxidative phosphorylation, the
circulating component that targets tricarboxylic acid, and cells
are more susceptible to copper death when glycolysis is inhibited.
Studies have shown that cells dependent on mitochondrial oxidative
phosphorylation for energy are 1,000-fold more sensitive to copper
ion carriers than glycolysis-dependent cells (190).
New studies have also shown that advanced clear
cell renal cell carcinoma (ccRCC) accumulates Cu and allocates it
to CuCOX. Copper promotes ccRCC growth through a combination of
bioenergetics, biosynthesis and redox oncogenic remodeling of body
homeostasis (203).
Through bioinformatics analysis, more and more
researchers are focusing on the important link between cuproptosis
and the cancer process (128,204). With the discovery of
cuproptosis (205-207), the interactions between copper
ionophores (208,209), copper and mitochondria, and
drugs that had previously been considered as potential anti-tumor
therapies (71), have become
increasingly clear. This has accelerated research into
copper-targeting therapies, with numerous studies on copper
chelators and ionophores demonstrating significant therapeutic
efficacy across various cancer types (Table III).
Copper-targeted therapies exhibit promising
potential in the treatment of cancer and neurodegenerative
diseases, while their immunomodulatory properties merit
comprehensive investigation. Copper ions serve as cofactors for
various enzymes and are involved in the regulation of immune cell
activity and oxidative stress responses, thereby potentially
affecting the functions of critical immune cells, such as dendritic
cells (DCs). A copper-manganese (Cu-Mn) nanocomposite demonstrates
therapeutic potential by selectively releasing Cu2+ in
the acidic tumor microenvironment (TME). The localized accumulation
of Cu2+ induces cuproptosis in cancer cells, promotes
the release of damage-associated molecular patterns, enhances DC
activation - evidenced by a 2.3-fold increase in CD80/CD86
expression - and facilitates increased infiltration of
CD8+ T cells (1.8-fold) in murine melanoma models,
thereby augmenting anti-tumor immune responses. Utilizing the
enhanced permeability and retention effect, the nanocomposite
achieves intratumoral Cu2+ concentrations 5.2-fold
higher than those attained with free chelators, without inducing
off-target toxicity, as indicated by normal liver enzyme levels
(210).
Importantly, DCs also play a critical role in
maintaining immune tolerance and their dysfunction has been
associated with the development of autoimmune disorders. In recent
years, exogenously derived tolerogenic DCs (tolDCs) have emerged as
a novel cell-based immunotherapy approach, designed to restore
immune tolerance in patients with autoimmune disorders. For
instance, Jonny et al (47) conducted a systematic review on
the therapeutic potential of tolDCs in autoimmune diseases,
including systemic lupus erythematosus, demonstrating that the
induction of tolDCs in vitro can effectively modulate T-cell
responses and promote antigen-specific immune tolerance. This
approach aligns with the objectives of copper-regulating therapies
(47).
Recent reviews have highlighted the importance of
metabolic complications in cancer (211-213), such as hypercalcemia, which
affect a significant proportion of patients with advanced
malignancies and contribute to morbidity and treatment challenges.
While hypercalcemia involves dysregulation of calcium homeostasis
often mediated by parathyroid hormone-related protein and
osteoclast activation, it shares with copper dysregulation a common
theme of metal ion imbalance in cancer pathophysiology.
Understanding these parallel mechanisms may provide synergistic
insights into targeting ion homeostasis as a therapeutic strategy.
For instance, just as bisphosphonates and denosumab are used to
manage cancer-related hypercalcemia by inhibiting bone resorption
(143), copper-chelation and
ionophore-based therapies are being explored to modulate copper
levels and induce selective cancer cell death (214,215). This complementary approach
underscores the broader potential of metal-targeting therapies in
oncology.
Among metal elements that perform essential
biological functions, such as iron, zinc, and copper, copper
demonstrates distinct advantages due to its unique chemical
properties. Copper, iron and zinc fulfil distinct roles in cellular
redox processes. The Cu+/Cu2+ redox couple
functions at a high reduction potential, facilitating efficient
catalytic activity in essential enzymatic reactions such as
cytochrome c oxidase-mediated mitochondrial respiration and
SOD-dependent antioxidant defence. Unlike iron - which can induce
non-specific oxidative damage through Fenton reactions - or zinc,
which contributes to structural and catalytic stability without
participating in redox cycling, copper supports tightly regulated
electron transfer and dynamic, chaperone-mediated signaling
processes via proteins such as Atox1. These unique properties
position copper as a specialized redox messenger capable of
mediating compartmentalized signaling pathways that are
inaccessible to iron or zinc (216,217).
Copper-based targeted therapeutic strategies,
including chelators, ionophores, and copper nanoparticles (CuNPs)
(218,219), have made notable progress in
clinical translational research (Table IV). Chelators primarily correct
copper metabolic imbalances by sequestering excess copper ions
(189), ionophores exploit
copper-dependent pathological processes by facilitating the
transport of copper ions across biological membranes (220) and CuNPs integrate the
biological activity of controlled copper ion release with the
targeted delivery capabilities of nanocarriers, thereby expanding
their application scenarios in precision therapy (221).
TM is a primary copper chelator under investigation
for its role in copper-induced cell death. Preclinical studies
indicate that TM exerts antitumor effects by inhibiting
angiogenesis and directly suppressing tumor cell proliferation
(222-224). Mechanistically, copper
depletion - achieved either through TM treatment or knockdown of
the copper transporter SLC31A1 - inhibits the progression of oral
squamous cell carcinoma (OSCC) by selectively targeting cancer
stem-like cells and reducing the stability of the histone
methyltransferase EZH2. This identifies a novel 'copper-dependent
proliferation' pathway that is essential for tumor growth. Notably,
a recent clinical study evaluating TM combined with anti-PD-1
therapy in patients with locally advanced OSCC reported an
objective response rate (ORR) of 57.8% and a 12-month overall
survival (OS) rate of 80.0%, highlighting its potential to overcome
immunotherapy resistance (225).
Beyond cuproptosis, copper also modulates
ferroptosis. In pancreatic cancer models, copper promotes
ferroptosis by inducing Tax1 binding protein 1-mediated autophagic
degradation of GSH peroxidase 4 (GPX4). By contrast, copper
chelators such as TM protect against ferroptosis-related tissue
damage (e.g., in acute pancreatitis) by promoting GPX4
ubiquitination and aggregation through direct binding to cysteine
residues (C107/C148) (226).
Consistent with these findings, the chelator bathocuproine sulfonic
acid enhances ferroptosis via copper depletion-mediated
mitochondrial dysfunction and impairment of antioxidant defense
(227).
Given copper's involvement in fibrosis and
angiogenesis, TM has also been evaluated in related pathological
conditions. In a bleomycin-induced pulmonary fibrosis model, TM
downregulated VEGF expression and improved lung function (228). Its anti-inflammatory effects,
mediated by the inhibition of copper-dependent cytokines, may
further contribute to its antitumor efficacy by reducing the
infiltration of pro-angiogenic immune cells (229). Additionally, NRF2 activation
suppresses angiogenesis by inducing autophagy in vascular
endothelial cells, thereby disrupting the energy supply to cancer
cells (230).
The therapeutic potential of copper chelation
extends beyond oncology. In patients with WD with reproductive
dysfunction, engineered extracellular vesicles delivering a
copper-chelating ligand significantly reduced
non-ceruloplasmin-bound copper (42.7%) and urinary copper (47.7%),
normalized gonadotropin levels in 87.5% of patients and exhibited a
favorable safety profile (6.25% mild gastrointestinal events)
(231). In oncology clinical
settings, trientine combined with radiotherapy improved the ORR in
hepatocellular carcinoma (65.5 vs. 37.9% with radiotherapy alone)
and achieved a 12-month OS rate of 75.9% (232-234). For HER2-negative metastatic
breast cancer (235-237), a polymer-based nano-copper
chelator delivering paclitaxel achieved an ORR of 44.4% and a
6.8-fold increase in intratumoral drug accumulation (238). In case of drug-resistant
Pseudomonas aeruginosa pneumonia, nebulized copper-chelator
nanoparticles achieved an 82.1% sputum bacterial clearance rate and
significantly shortened antipyretic time, while maintaining a
favorable safety profile (239).
Elesclomol, a highly selective copper ionophore,
binds Cu(II) to form a complex that is rapidly transported into
mitochondria (184). Within the
mitochondria, FDX1 facilitates the reduction and dissociation of
the elesclomol-Cu(II) complex, leading to the generation of ROS
(240). Notably, elesclomol
also operates through an FDX1-independent pathway that enables an
extracellular Cu(II) release pathway, distinguishing it from other
ionophores (241). This
sustained copper delivery mechanism results in a chronic elevation
of intracellular copper and ROS levels, ultimately inducing cell
death (242).
The anticancer effects of elesclomol have been
demonstrated across various malignancies. In colorectal cancer
(CRC) models, elesclomol promotes ATP7A degradation (243), leading to mitochondrial copper
accumulation, oxidative stress and the subsequent degradation of
SLC7A11 - a key component of the cystine/glutamate transporter -
thereby inducing ferroptosis (178,184). Beyond CRC, a novel natural
copper ionophore, Pochonin D (PoD), was identified in
triple-negative breast cancer (TNBC). PoD covalently binds to
Cys173 on peroxiredoxin 1 (PRDX1), inhibiting its peroxidase
activity, promoting copper accumulation and ROS generation, and
ultimately triggering copper-dependent cell death. This study
unveils PRDX1 as a critical mediator in copper-induced cell death
and highlights a new regulatory axis between copper metabolism and
oxidative stress (244).
CuNPs have emerged as promising agents for
therapeutic applications due to their tunable physicochemical
properties and multifunctional capabilities. For instance, a CuO
nanoplatform co-loaded with elesclomol potently induces
copper-dependent cell death and enhances antitumor immunity. When
combined with PD-1 inhibitors, this strategy achieves an 85% tumor
suppression rate in melanoma models (219). In prostate cancer, the
combination of elesclomol and CuCl2 induces DLAT
aggregation and inhibits protective autophagy through mTOR
activation, thereby synergizing with docetaxel chemotherapy
(245). The application of
CuNPs extends beyond oncology. Electrospun nanofiber membranes
embedded with CuNPs exhibit broad-spectrum antibacterial activity
against pathogenic Escherichia coli (246). Furthermore, CuNPs have
demonstrated neuroprotective effects in models of AD by improving
cognitive function (247), and
ultra-small CuNPs embedded in hydrogels have been shown to
significantly accelerate diabetic wound healing (248). Despite these promising
findings, the clinical translation of CuNPs requires further
optimization to address challenges related to long-term stability
(249), targeting specificity
and scalable manufacturing (250,251).
In summary, copper-targeting approaches introduce
novel concepts and hold substantial promise in disease diagnosis
and treatment, representing a potential therapeutic strategy.
However, further comprehensive research and validation are required
for its clinical application to refine therapeutic protocols,
enhance treatment efficacy and minimize adverse effects.
The cellular uptake of copper-related therapeutics
is tightly regulated by key transporters: CTR1 and the P-type
ATPases ATP7A and ATP7B. As the primary mediator of copper influx,
CTR1 also facilitates the entry of platinum-based drugs into tumor
cells, a process that is critical for achieving therapeutic
concentrations. By contrast, ATP7A and ATP7B act as copper efflux
transporters, regulating the extrusion and subcellular
sequestration of excess copper and platinum drugs (250). Dysregulation of CTR1 and
ATP7A/B, whether through altered expression (e.g., CTR1
downregulation in drug-resistant tumors) or genetic mutations,
disrupts copper homeostasis and impairs drug accumulation. For
instance, ATP7A loss-of-function mutations cause MD (48), whereas ATP7B mutations underlie
WD (251). In neurodegenerative
diseases (such as AD), abnormal activity of copper-related
transporters can disrupt brain copper homeostasis. Excess copper
promotes Aβ protein aggregation and ROS overproduction, thereby
exacerbating neuronal damage (79,252).
Copper-targeted therapy is significantly limited by
the TME. In the hypoxic microenvironment of solid tumors (253), HIF-1α biaxially induces
resistance to copper-dependent cell death by upregulating pyruvate
dehydrogenase kinase 1/3-mediated degradation of DLAT and promoting
MT2A-mediated chelation of free copper. This results in resistance
to copper ionophores in nearly 60% of clinical samples, with the
IC50 of elesclomol increasing by 7.2-fold under hypoxic
conditions (254). Meanwhile,
vascular abnormalities, elevated interstitial pressure and
infiltration of immunosuppressive cells within the TME
significantly impair drug delivery. For instance, in hepatocellular
carcinoma, multidrug resistance mechanisms, such as the
overexpression of ATP-binding cassette transporters, act
synergistically with these TME-associated barriers to reduce
intratumoral drug accumulation (255).
Copper, an essential micronutrient, maintains
precise systemic homeostasis through coordinated regulatory
mechanisms. However, long-term administration of copper-related
therapeutics (e.g., copper ionophores and chelators), disrupts this
balance. Excess copper catalyzes the generation of ROS via the
Fenton reaction, causing oxidative damage to lipids, proteins and
DNA, and ultimately leads to cytotoxicity (249).
Although cuproptosis induced by copper ionophores
exhibits anti-tumor potential, preclinical studies have
demonstrated that prolonged high-dose treatment elicits severe
toxicity in normal tissues. For instance, mitochondrial-targeted
copper-depleting nanoparticles effectively inhibit the growth of
TNBC but still pose toxicity risks to healthy mice, thereby
narrowing the therapeutic window (256). Additionally, the hypoxic TME
promotes resistance to cuproptosis through HIF-1α activation, which
further compromises therapeutic efficacy (254).
Copper is the third most abundant trace element in
the body after iron and zinc. It is essential for processes such as
oxidative phosphorylation, free radical scavenging, angiogenesis,
bone metabolism, neurotransmission and the synthesis of
copper-dependent enzymes. On the other hand, both copper overload
and deficiency can lead to impaired cellular function or even cell
death, and their prolonged presence can trigger associated
dysfunctions or neurodegenerative disorders such as hepatomegaly,
MD, AD and PD.
It has been found that there are interactions among
copper, iron and zinc, and the use of combination drug formulations
for the treatment of related diseases may represent a new
therapeutic strategy. In the treatment of diseases associated with
copper overload, strategies such as reducing dietary copper intake,
administering oral zinc to inhibit copper absorption or employing
copper chelators to decrease intracellular copper levels may
represent promising therapeutic approaches.
In the context of cancer, copper levels in cancer
cells can exceed the tolerance threshold of normal cells, leading
to significant adverse effects. Conversely, reducing intracellular
copper levels can diminish the synthesis of the copper-dependent
enzyme SOD1, thereby substantially impairing the organism's
antioxidant capacity. Copper-induced cell death exhibits greater
sensitivity in cells reliant on oxidative phosphorylation than in
those dependent on glycolysis for energy production. Thus,
converting the metabolic pathway of cancer cells from glycolysis to
oxidative phosphorylation, coupled with exploiting the
characteristic of elevated intracellular copper, may offer a
promising new approach to cancer therapy. Comprehensive studies of
the mechanisms of copper-induced cell death may help to identify
more effective therapeutic agents, thus providing new insights and
approaches for the treatment of related diseases and improving the
prognosis of affected patients.
Copper regulation therapy has demonstrated
significant potential in the diagnosis and treatment of diseases.
Copper chelating agents, copper ion carriers and copper-based
nanoparticles have shown therapeutic effects across various disease
models. For instance, clinical studies have shown that the
combination of the copper chelating agent TM with anti-PD-1 therapy
achieves promising outcomes in the treatment of locally advanced
OSCC. The copper ion carrier elesclomol exhibits anticancer effects
in multiple malignant tumor models. CuNPs have yielded remarkable
results in cancer therapy, antibacterial applications,
neuroprotection and wound healing. However, the clinical
application of this therapeutic approach faces several challenges.
These include drug uptake dependence on key transporters such as
CTR1, ATP7A and ATP7B, whose dysregulation can compromise
therapeutic efficacy; the influence of the TME, including hypoxia
and vascular abnormalities, which may limit treatment outcomes; and
the potential toxicity associated with long-term copper exposure,
which also narrows the therapeutic window.
Not applicable.
All authors contributed to the study conception and
design. TD and HM were responsible for article design and
manuscript writing. TD and XJ were involved in the design of the
images. JT, JZ and YT were involved in the design of the study and
funding application. All authors read and approved the final
manuscript. Data authentication is not applicable.
Not applicable.
Not applicable.
The authors declare that they have no competing
interests.
Not applicable.
This work was supported by the Science and Technology Support
Program of Guizhou Province [grant nos. QKH-ZK (2023)506 and
QKH-MS(2025)373], the Education Department of Guizhou Province
[grant no. QJJ(2022)242] and Zunyi City Science and Technology and
Big Data Bureau & Zunyi Medical University joint project [grant
nos. HZ(2023)175, HZ(2023)189, (2021)1350-011 and
(2021)1350-025].
|
1
|
Bost M, Houdart S, Oberli M, Kalonji E,
Huneau JF and Margaritis I: Dietary copper and human health:
Current evidence and unresolved issues. J Trace Elem Med Biol.
35:107–115. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Huang F, Lu X, Kuai L, Ru Y, Jiang J, Song
J, Chen S, Mao L, Li Y, Li B, et al: Dual-Site Biomimetic Cu/Zn-MOF
for atopic dermatitis catalytic therapy via suppressing
FcүR-mediated phagocytosis. J Am Chem Soc. 146:3186–3199. 2024.
View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Ash D, Sudhahar V, Youn SW, Okur MN, Das
A, O'Bryan JP, McMenamin M, Hou Y, Kaplan JH, Fukai T and
Ushio-Fukai M: The P-type ATPase transporter ATP7A promotes
angiogenesis by limiting autophagic degradation of VEGFR2. Nat
Commun. 12:30912021. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Ding H, Gao YS, Wang Y, Hu C, Sun Y and
Zhang C: Dimethyloxaloylglycine increases the bone healing capacity
of adipose-derived stem cells by promoting osteogenic
differentiation and angiogenic potential. Stem Cells Dev.
23:990–1000. 2014. View Article : Google Scholar :
|
|
5
|
Akhtar H, Alhamoudi FH, Marshall J, Ashton
T, Darr JA, Rehman IU, Chaudhry AA and Reilly G: Synthesis of
cerium, zirconium, and copper doped zinc oxide nanoparticles as
potential biomaterials for tissue engineering applications.
Heliyon. 10:e291502024. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Han J, Luo J, Wang C, Kapilevich L and
Zhang XA: Roles and mechanisms of copper homeostasis and
cuproptosis in osteoarticular diseases. Biomed Pharmacother.
174:1165702024. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Wei M, Huang Q, Dai Y, Zhou H, Cui Y, Song
W, Di D, Zhang R, Li C, Wang Q and Jing T: Manganese, iron, copper,
and selenium co-exposure and osteoporosis risk in Chinese adults. J
Trace Elem Med Biol. 72:1269892022. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Xiao T, Ackerman CM, Carroll EC, Jia S,
Hoagland A, Chan J, Thai B, Liu CS, Isacoff EY and Chang CJ: Copper
regulates rest-activity cycles through the locus
coeruleus-norepinephrine system. Nat Chem Biol. 14:655–663. 2018.
View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Benatar M, Robertson J and Andersen PM:
Amyotrophic lateral sclerosis caused by SOD1 variants: From genetic
discovery to disease prevention. Lancet Neurol. 24:77–86. 2025.
View Article : Google Scholar
|
|
10
|
Horvath R, Kemp JP, Tuppen HA, Hudson G,
Oldfors A, Marie SK, Moslemi AR, Servidei S, Holme E, Shanske S, et
al: Molecular basis of infantile reversible cytochrome c oxidase
deficiency myopathy. Brain. 132(Pt 11): 3165–3174. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Qu Y, Zhan Q, Du S, Ding Y, Fang B, Du W,
Wu Q, Yu H, Li L and Huang W: Catalysis-based specific detection
and inhibition of tyrosinase and their application. J Pharm Anal.
10:414–425. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Foehr R, Anderson K, Dombrowski O, Foehr A
and Foehr ED: Dysregulation of extracellular matrix and lysyl
oxidase in Ehlers-Danlos syndrome type IV skin fibroblasts.
Orphanet J Rare Dis. 19:92024. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Wassenberg T, Deinum J, van Ittersum FJ,
Kamsteeg EJ, Pennings M, Verbeek MM, Wevers RA, van Albada ME, Kema
IP, Versmissen J, et al: Clinical presentation and long-term
follow-up of dopamine beta hydroxylase deficiency. J Inherit Metab
Dis. 44:554–565. 2021. View Article : Google Scholar
|
|
14
|
De Feyter S, Beyens A and Callewaert B:
ATP7A-related copper transport disorders: A systematic review and
definition of the clinical subtypes. J Inherit Metab Dis.
46:163–173. 2023. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Stalke A, Behrendt A, Hennig F, Gohlke H,
Buhl N, Reinkens T, Baumann U, Schlegelberger B, Illig T, Pfister
ED and Skawran B: Functional characterization of novel or yet
uncharacterized ATP7B missense variants detected in patients with
clinical Wilson's disease. Clin Genet. 104:174–185. 2023.
View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Li Y, Ma J, Wang R, Luo Y, Zheng S and
Wang X: Zinc transporter 1 functions in copper uptake and
cuproptosis. Cell Metab. 36:2118–2129 e6. 2024. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Ohgami RS, Campagna DR, McDonald A and
Fleming MD: The Steap proteins are metalloreductases. Blood.
108:1388–1394. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Wyman S, Simpson RJ, McKie AT and Sharp
PA: Dcytb (Cybrd1) functions as both a ferric and a cupric
reductase in vitro. FEBS Lett. 582:1901–1906. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Kleven MD, Dlakic M and Lawrence CM:
Characterization of a Single b-type Heme, FAD, and metal binding
sites in the transmembrane domain of six-transmembrane epithelial
antigen of the prostate (STEAP) family proteins. J Biol Chem.
290:22558–22569. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Kirsipuu T, Zadorožnaja A, Smirnova J,
Friedemann M, Plitz T, Tõugu V and Palumaa P: Copper(II)-binding
equilibria in human blood. Sci Rep. 10:56862020. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Linder MC: Ceruloplasmin and other copper
binding components of blood plasma and their functions: An update.
Metallomics. 8:887–905. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Moriya M, Ho YH, Grana A, Nguyen L,
Alvarez A, Jamil R, Ackland ML, Michalczyk A, Hamer P, Ramos D, et
al: Copper is taken up efficiently from albumin and
alpha2-macroglobulin by cultured human cells by more than one
mechanism. Am J Physiol Cell Physiol. 295:C708–C721. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Liu N, Lo LS, Askary SH, Jones L, Kidane
TZ, Trang T, Nguyen M, Goforth J, Chu YH, Vivas E, et al:
Transcuprein is a macroglobulin regulated by copper and iron
availability. J Nutr Biochem. 18:597–608. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Wang Y, Hodgkinson V, Zhu S, Weisman GA
and Petris MJ: Advances in the understanding of mammalian copper
transporters. Adv Nutr. 2:129–137. 2011. View Article : Google Scholar :
|
|
25
|
Chen L, Li N, Zhang M, Sun M, Bian J, Yang
B, Li Z, Wang J, Li F, Shi X, et al: APEX2-based proximity labeling
of Atox1 Identifies CRIP2 as a nuclear copper-binding protein that
regulates autophagy activation. Angew Chem Int Ed Engl.
60:25346–25355. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
González-Ballesteros MM, Sánchez-Sánchez
L, Espinoza-Guillén A, Espinal-Enríquez J, Mejía C, Hernández-Lemus
E and Ruiz-Azuara L: Antitumoral and antimetastatic activity by
mixed chelate copper(II) Compounds (Casiopeínas®) on
triple-negative breast cancer, in vitro and in vivo models. Int J
Mol Sci. 25:88032024. View Article : Google Scholar
|
|
27
|
Kuo YM, Gybina AA, Pyatskowit JW,
Gitschier J and Prohaska JR: Copper transport protein (Ctr1) levels
in mice are tissue specific and dependent on copper status. J Nutr.
136:21–26. 2006. View Article : Google Scholar
|
|
28
|
Vizcaíno C, Mansilla S and Portugal J: Sp1
transcription factor: A long-standing target in cancer
chemotherapy. Pharmacol Ther. 152:111–124. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Xue Q, Kang R, Klionsky DJ, Tang D, Liu J
and Chen X: Copper metabolism in cell death and autophagy.
Autophag. 19:2175–2195. 2023. View Article : Google Scholar
|
|
30
|
Liang ZD, Tsai WB, Lee MY, Savaraj N and
Kuo MT: Specificity protein 1 (sp1) oscillation is involved in
copper homeostasis maintenance by regulating human high-affinity
copper transporter 1 expression. Mol Pharmacol. 81:455–464. 2012.
View Article : Google Scholar :
|
|
31
|
Ohse VA, Klotz LO and Priebs J: Copper
homeostasis in the model organism C. elegans. Cells. 13:7272024.
View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Hatori Y and Lutsenko S: The role of
copper chaperone Atox1 in coupling redox homeostasis to
intracellular copper distribution. Antioxidants (Basel). 5:252016.
View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Lutsenko S, Barnes NL, Bartee MY and
Dmitriev OY: Function and regulation of human copper-transporting
ATPases. Physiol Rev. 87:1011–1046. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Sturtz LA, Diekert K, Jensen LT, Lill R
and Culotta VC: A fraction of yeast Cu,Zn-superoxide dismutase and
its metallochaperone, CCS, localize to the intermembrane space of
mitochondria. A physiological role for SOD1 in guarding against
mitochondrial oxidative damage. J Biol Chem. 276:38084–38089. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Wang Y, Branicky R, Noë A and Hekimi S:
Superoxide dismutases: Dual roles in controlling ROS damage and
regulating ROS signaling. J Cell Biol. 217:1915–1928. 2018.
View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Miriyala S, Spasojevic I, Tovmasyan A,
Salvemini D, Vujaskovic Z, St Clair D and Batinic-Haberle I:
Manganese superoxide dismutase, MnSOD and its mimics. Biochim
Biophys Acta. 1822:794–814. 2012. View Article : Google Scholar :
|
|
37
|
Boyd SD, Calvo JS, Liu L, Ullrich MS,
Skopp A, Meloni G and Winkler DD: The yeast copper chaperone for
copper-zinc superoxide dismutase (CCS1) is a multifunctional
chaperone promoting all levels of SOD1 maturation. J Biol Chem.
294:1956–1966. 2019. View Article : Google Scholar :
|
|
38
|
Ding Y, Chen Y, Wu Z, Yang N, Rana K, Meng
X, Liu B, Wan H and Qian W: SsCox17, a copper chaperone, is
required for pathogenic process and oxidative stress tolerance of
Sclerotinia sclerotiorum. Plant Sci. 322:1113452022. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Banci L, Bertini I, Ciofi-Baffoni S,
Hadjiloi T, Martinelli M and Palumaa P: Mitochondrial copper(I)
transfer from Cox17 to Sco1 is coupled to electron transfer. Proc
Natl Acad Sci USA. 105:6803–6808. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Ge EJ, Bush AI, Casini A, Cobine PA, Cross
JR, DeNicola GM, Dou QP, Franz KJ, Gohil VM, Gupta S, et al:
Connecting copper and cancer: From transition metal signalling to
metalloplasia. Nat Rev Cancer. 22:102–113. 2022. View Article : Google Scholar :
|
|
41
|
McCann C, Quinteros M, Adelugba I, Morgada
MN, Castelblanco AR, Davis EJ, Lanzirotti A, Hainer SJ, Vila AJ,
Navea JG and Padilla-Benavides T: The mitochondrial Cu(+)
transporter PiC2 (SLC25A3) is a target of MTF1 and contributes to
the development of skeletal muscle in vitro. Front Mol Biosci.
9:10379412022. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
van Rensburg MJ, van Rooy M, Bester MJ,
Serem JC, Venter C and Oberholzer HM: Oxidative and haemostatic
effects of copper, manganese and mercury, alone and in combination
at physiologically relevant levels: An ex vivo study. Hum Exp
Toxicol. 38:419–433. 2019. View Article : Google Scholar
|
|
43
|
Ruturaj, Mishra M, Saha S, Maji S,
Rodriguez-Boulan E, Schreiner R and Gupta A: Regulation of the
apico-basolateral trafficking polarity of the homologous
copper-ATPases ATP7A and ATP7B. J Cell Sci. 137:jcs2612582024.
View Article : Google Scholar
|
|
44
|
Guttmann S, Nadzemova O, Grünewald I,
Lenders M, Brand E, Zibert A and Schmidt HH: ATP7B knockout
disturbs copper and lipid metabolism in Caco-2 cells. PLoS One.
15:e02300252020. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Dmitriev OY and Patry J: Structure and
mechanism of the human copper transporting ATPases: Fitting the
pieces into a moving puzzle. Biochim Biophys Acta Biomembr.
1866:1843062024. View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Percival SS: Copper and immunity. Am J
Clin Nutr. 67(5 Suppl): 1064S–1068S. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Jonny, Sitepu EC, Nidom CA, Wirjopranoto
S, Sudiana IK, Ansori ANM and Putranto TA: Ex vivo-generated
tolerogenic dendritic cells: Hope for a definitive therapy of
autoimmune diseases. Curr Issues Mol Biol. 46:4035–4048. 2024.
View Article : Google Scholar : PubMed/NCBI
|
|
48
|
Backal A, Velinov M, Garcia J and Louis
CL: Novel, likely pathogenic variant in ATP7A associated with
Menkes disease diagnosed with ultrarapid genome sequencing. BMJ
Case Rep. 17:e2597922024. View Article : Google Scholar : PubMed/NCBI
|
|
49
|
Tümer Z and Møller LB: Menkes disease. Eur
J Hum Genet. 18:511–518. 2010. View Article : Google Scholar :
|
|
50
|
Moller LB, Mogensen M, Weaver DD and
Pedersen PA: Occipital horn syndrome as a result of splice site
mutations in ATP7A. no activity of ATP7A splice variants missing
exon 10 or exon 15. Front Mol Neurosci. 14:5322912021. View Article : Google Scholar : PubMed/NCBI
|
|
51
|
Batzios S, Tal G, DiStasio AT, Peng Y,
Charalambous C, Nicolaides P, Kamsteeg EJ, Korman SH, Mandel H,
Steinbach PJ, et al: Newly identified disorder of copper metabolism
caused by variants in CTR1, a high-affinity copper transporter. Hum
Mol Genet. 31:4121–4130. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
52
|
Garnica A, Chan WY and Rennert O:
Copper-histidine treatment of Menkes disease. J Pediatr.
125:336–338. 1994. View Article : Google Scholar : PubMed/NCBI
|
|
53
|
Moini M, To U and Schilsky ML: Recent
advances in Wilson disease. Transl Gastroenterol Hepatol. 6:212021.
View Article : Google Scholar : PubMed/NCBI
|
|
54
|
Weiss KH, Członkowska A, Hedera P and
Ferenci P: WTX101 - an investigational drug for the treatment of
Wilson disease. Expert Opin Investig Drugs. 27:561–567. 2018.
View Article : Google Scholar : PubMed/NCBI
|
|
55
|
Kreuder J, Otten A, Fuder H, Tümer Z,
Tønnesen T, Horn N and Dralle D: Clinical and biochemical
consequences of copper-histidine therapy in Menkes disease. Eur J
Pediatr. 152:828–832. 1993. View Article : Google Scholar : PubMed/NCBI
|
|
56
|
Christodoulou J, Danks DM, Sarkar B,
Baerlocher KE, Casey R, Horn N, Tümer Z and Clarke JT: Early
treatment of Menkes disease with parenteral copper-histidine:
Long-term follow-up of four treated patients. Am J Med Genet.
76:154–164. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
57
|
Ramchandani D, Berisa M, Tavarez DA, Li Z,
Miele M, Bai Y, Lee SB, Ban Y, Dephoure N, Hendrickson RC, et al:
Copper depletion modulates mitochondrial oxidative phosphorylation
to impair triple negative breast cancer metastasis. Nat Commun.
12:73112021. View Article : Google Scholar : PubMed/NCBI
|
|
58
|
Xu B, Wang S, Li R, Chen K, He L, Deng M,
Kannappan V, Zha J, Dong H and Wang W: Disulfiram/copper
selectively eradicates AML leukemia stem cells in vitro and in vivo
by simultaneous induction of ROS-JNK and inhibition of NF-κB and
Nrf2. Cell Death Dis. 8:e27972017. View Article : Google Scholar
|
|
59
|
Theophanides T and Anastassopoulou J:
Copper and carcinogenesis. Crit Rev Oncol Hematol. 42:57–64. 2002.
View Article : Google Scholar : PubMed/NCBI
|
|
60
|
Zhong CC, Zhao T, Hogstrand C, Chen F,
Song CC and Luo Z: Copper (Cu) induced changes of lipid metabolism
through oxidative stress-mediated autophagy and Nrf2/PPARγ
pathways. J Nutr Biochem. 100:1088832022. View Article : Google Scholar
|
|
61
|
Wang Q, Sun Y, Zhao A, Cai X, Yu A, Xu Q,
Liu W, Zhang N, Wu S, Chen Y and Wang W: High dietary copper intake
induces perturbations in the gut microbiota and affects host
ovarian follicle development. Ecotoxicol Environ Saf.
255:1148102023. View Article : Google Scholar : PubMed/NCBI
|
|
62
|
Tsang T, Posimo JM, Gudiel AA, Cicchini M,
Feldser DM and Brady DC: Copper is an essential regulator of the
autophagic kinases ULK1/2 to drive lung adenocarcinoma. Nat Cell
Biol. 22:412–424. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
63
|
Conforti RA, Delsouc MB, Zorychta E,
Telleria CM and Casais M: Copper in gynecological diseases. Int J
Mol Sci. 24:175782023. View Article : Google Scholar : PubMed/NCBI
|
|
64
|
Compston A: Progressive lenticular
degeneration: a familial nervous disease associated with cirrhosis
of the liver, by S. A. Kinnier Wilson, (From the National Hospital,
and the Laboratory of the National Hospital, Queen Square, London)
Brain 1912: 34; 295-509. Brain. 132:1997–2001. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
65
|
Kipker N, Alessi K, Bojkovic M, Padda I
and Parmar MS: Neurological-type wilson disease: Epidemiology,
clinical manifestations, diagnosis, and management. Cureus.
15:e381702023.PubMed/NCBI
|
|
66
|
Członkowska A, Litwin T, Dusek P, Ferenci
P, Lutsenko S, Medici V, Rybakowski JK, Weiss KH and Schilsky ML:
Wilson disease. Nat Rev Dis Primers. 4:212018. View Article : Google Scholar
|
|
67
|
Kirk FT, Munk DE, Swenson ES, Quicquaro
AM, Vendelbo MH, Larsen A, Schilsky ML, Ott P and Sandahl TD:
Effects of tetrathiomolybdate on copper metabolism in healthy
volunteers and in patients with Wilson disease. J Hepatol.
80:586–595. 2024. View Article : Google Scholar
|
|
68
|
Bruha R, Marecek Z, Pospisilova L,
Nevsimalova S, Vitek L, Martasek P, Nevoral J, Petrtyl J, Urbanek
P, Jiraskova A and Ferenci P: Long-term follow-up of Wilson
disease: natural history, treatment, mutations analysis and
phenotypic correlation. Liver Int. 31:83–91. 2011. View Article : Google Scholar
|
|
69
|
Członkowska A, Litwin T, Karliński M,
Dziezyc K, Chabik G and Czerska M: D-penicillamine versus zinc
sulfate as first-line therapy for Wilson's disease. Eur J Neurol.
21:599–606. 2014. View Article : Google Scholar
|
|
70
|
Brewer GJ: Practical recommendations and
new therapies for Wilson's disease. Drugs. 50:240–249. 1995.
View Article : Google Scholar : PubMed/NCBI
|
|
71
|
Xie J, Yang Y, Gao Y and He J:
Cuproptosis: Mechanisms and links with cancers. Mol Cancer.
22:462023. View Article : Google Scholar : PubMed/NCBI
|
|
72
|
Członkowska A and Litwin T: Wilson disease
- currently used anticopper therapy. Handb Clin Neurol.
142:181–191. 2017. View Article : Google Scholar
|
|
73
|
Lambert C, Beraldo H, Lievre N,
Garnier-Suillerot A, Dorlet P and Salerno M: Bis(thiosemicarbazone)
copper complexes: Mechanism of intracellular accumulation. J Biol
Inorg Chem. 18:59–69. 2013. View Article : Google Scholar
|
|
74
|
Ayton S and Bush AI: β-amyloid: The known
unknowns. Ageing Res Rev. 65:1012122021. View Article : Google Scholar
|
|
75
|
Egan MF, Kost J, Voss T, Mukai Y, Aisen
PS, Cummings JL, Tariot PN, Vellas B, van Dyck CH, Boada M, et al:
Randomized Trial of Verubecestat for Prodromal Alzheimer's Disease.
N Engl J Med. 380:1408–1420. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
76
|
Doody RS, Thomas RG, Farlow M, Iwatsubo T,
Vellas B, Joffe S, Kieburtz K, Raman R, Sun X, Aisen PS, et al:
Phase 3 trials of solanezumab for mild-to-moderate Alzheimer's
disease. N Engl J Med. 370:311–321. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
77
|
Salloway S, Sperling R, Fox NC, Blennow K,
Klunk W, Raskind M, Sabbagh M, Honig LS, Porsteinsson AP, Ferris S,
et al: Two phase 3 trials of bapineuzumab in mild-to-moderate
Alzheimer's disease. N Engl J Med. 370:322–333. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
78
|
Scheltens P, De Strooper B, Kivipelto M,
Holstege H, Chételat G, Teunissen CE, Cummings J and van der Flier
WM: Alzheimer's disease. Lancet. 397:1577–1590. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
79
|
Mazur T, Malik M and Bienko DC: The impact
of chelating compounds on Cu(2+), Fe(2+)/(3+), and Zn(2+) ions in
Alzheimer's disease treatment. J Inorg Biochem. 257:1126012024.
View Article : Google Scholar : PubMed/NCBI
|
|
80
|
Xu J, Church SJ, Patassini S, Begley P,
Waldvogel HJ, Curtis MA, Faull RLM, Unwin RD and Cooper GJS:
Evidence for widespread, severe brain copper deficiency in
Alzheimer's dementia. Metallomics. 9:1106–1119. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
81
|
Rembach A, Hare DJ, Lind M, Fowler CJ,
Cherny RA, McLean C, Bush AI, Masters CL and Roberts BR: Decreased
copper in Alzheimer's disease brain is predominantly in the soluble
extractable fraction. Int J Alzheimers Dis.
2013:6232412013.PubMed/NCBI
|
|
82
|
Scholefield M, Church SJ, Xu J and Cooper
GJS: Metallomic analysis of brain tissues distinguishes between
cases of dementia with Lewy bodies, Alzheimer's disease, and
Parkinson's disease dementia. Front Neurosci. 18:14123562024.
View Article : Google Scholar : PubMed/NCBI
|
|
83
|
Squitti R, Simonelli I, Ventriglia M,
Siotto M, Pasqualetti P, Rembach A, Doecke J and Bush AI:
Meta-analysis of serum non-ceruloplasmin copper in Alzheimer's
disease. J Alzheimers Dis. 38:809–822. 2014. View Article : Google Scholar
|
|
84
|
Jiao Y and Yang P: Mechanism of copper(II)
inhibiting Alzheimer's amyloid beta-peptide from aggregation: A
molecular dynamics investigation. J Phys Chem B. 111:7646–7655.
2007. View Article : Google Scholar : PubMed/NCBI
|
|
85
|
Gomes LM, Vieira RP, Jones MR, Wang MC,
Dyrager C, Souza-Fagundes EM, Da Silva JG, Storr T and Beraldo H:
8-Hydroxyquinoline Schiff-base compounds as antioxidants and
modulators of copper-mediated Aβ peptide aggregation. J Inorg
Biochem. 139:106–116. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
86
|
Pedersen JT, Østergaard J, Rozlosnik N,
Gammelgaard B and Heegaard NH: Cu(II) mediates kinetically
distinct, non-amyloidogenic aggregation of amyloid-beta peptides. J
Biol Chem. 286:26952–26963. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
87
|
Reybier K, Ayala S, Alies B, Rodrigues JV,
Bustos Rodriguez S, La Penna G, Collin F, Gomes CM, Hureau C and
Faller P: Free Superoxide is an intermediate in the production of
H2O2 by Copper(I)-Aβ Peptide and O2. Angew Chem Int Ed Engl.
55:1085–1089. 2016. View Article : Google Scholar
|
|
88
|
Fanlo-Ucar H, Picón-Pagès P,
Herrera-Fernández V, Ill-Raga G and Muñoz FJ: The dual role of
amyloid beta-peptide in oxidative stress and inflammation:
Unveiling Their connections in Alzheimer's disease etiopathology.
Antioxidants (Basel). 13:12082024. View Article : Google Scholar : PubMed/NCBI
|
|
89
|
Xia Y, Dore V, Fripp J, Bourgeat P, Laws
SM, Fowler CJ, Rainey-Smith SR, Martins RN, Rowe C, Masters CL, et
al: Association of basal forebrain atrophy with cognitive decline
in early Alzheimer disease. Neurology. 103:e2096262024. View Article : Google Scholar : PubMed/NCBI
|
|
90
|
Lang M, Fan Q, Wang L, Zheng Y, Xiao G,
Wang X, Wang W, Zhong Y and Zhou B: Inhibition of human
high-affinity copper importer Ctr1 orthologous in the nervous
system of Drosophila ameliorates Aβ42-induced Alzheimer's
disease-like symptoms. Neurobiol Aging. 34:2604–2612. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
91
|
Ceccom J, Coslédan F, Halley H, Francès B,
Lassalle JM and Meunier B: Copper chelator induced efficient
episodic memory recovery in a non-transgenic Alzheimer's mouse
model. PLoS One. 2012(7): e431052012. View Article : Google Scholar
|
|
92
|
Quinn JF, Harris CJ, Cobb KE, Domes C,
Ralle M, Brewer G and Wadsworth TL: A copper-lowering strategy
attenuates amyloid pathology in a transgenic mouse model of
Alzheimer's disease. J Alzheimers Dis. 21:903–914. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
93
|
Chen C, Jiang X, Li Y, Yu H, Li S, Zhang
Z, Xu H, Yang Y, Liu G, Zhu F, et al: Low-dose oral copper
treatment changes the hippocampal phosphoproteomic profile and
perturbs mitochondrial function in a mouse model of Alzheimer's
disease. Free Radic Biol Med. 135:144–156. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
94
|
Yao D, Jing T, Niu L, Huang X, Wang Y,
Deng X and Wang M: Amyloidogenesis induced by diet cholesterol and
copper in a model mouse for Alzheimer's disease and protection
effects of zinc and fluvastatin. Brain Res Bull. 143:1–8. 2018.
View Article : Google Scholar : PubMed/NCBI
|
|
95
|
Maynard CJ, Cappai R, Volitakis I,
Laughton KM, Masters CL, Bush AI and Li QX: Chronic exposure to
high levels of zinc or copper has little effect on brain metal
homeostasis or Abeta accumulation in transgenic APP-C100 mice. Cell
Mol Neurobiol. 29:757–767. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
96
|
Acevedo KM, Hung YH, Dalziel AH, Li QX,
Laughton K, Wikhe K, Rembach A, Roberts B, Masters CL, Bush AI and
Camakaris J: Copper promotes the trafficking of the amyloid
precursor protein. J Biol Chem. 286:8252–8262. 2011. View Article : Google Scholar :
|
|
97
|
Borchardt T, Camakaris J, Cappai R,
Masters CL, Beyreuther K and Multhaup G: Copper inhibits
beta-amyloid production and stimulates the non-amyloidogenic
pathway of amyloid-precursor-protein secretion. Biochem J.
344:461–467. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
98
|
Afsar A, Chacon Castro MDC, Soladogun AS
and Zhang L: Recent development in the understanding of molecular
and cellular mechanisms underlying the etiopathogenesis of
Alzheimer's disease. Int J Mol Sci. 24:72582023. View Article : Google Scholar : PubMed/NCBI
|
|
99
|
Wang Y, Chen Y, Zhang J, Yang Y, Fleishman
JS, Wang Y, Wang J, Chen J, Li Y and Wang H: Cuproptosis: A novel
therapeutic target for overcoming cancer drug resistance. Drug
Resist Updat. 72:1010182024. View Article : Google Scholar
|
|
100
|
Kumar V, Singh AP, Wheeler N, Galindo CL
and Kim JJ: Safety profile of D-penicillamine: A comprehensive
pharmacovigilance analysis by FDA adverse event reporting system.
Expert Opin Drug Saf. 20:1443–1450. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
101
|
Lee EJ, Woo MH, Moon JS and Ko JS:
Efficacy and safety of D-penicillamine, trientine, and zinc in
pediatric Wilson disease patients. Orphanet J Rare Dis. 19:2612024.
View Article : Google Scholar : PubMed/NCBI
|
|
102
|
Perez DR, Sklar LA and Chigaev A:
Clioquinol: To harm or heal. Pharmacol Ther. 199:155–163. 2019.
View Article : Google Scholar : PubMed/NCBI
|
|
103
|
Relkin N: Testing the mettle of PBT2 for
Alzheimer's disease. Lancet Neurol. 7:762–763. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
104
|
Daniel KG, Chen D, Orlu S, Cui QC, Miller
FR and Dou QP: Clioquinol and pyrrolidine dithiocarbamate complex
with copper to form proteasome inhibitors and apoptosis inducers in
human breast cancer cells. Breast Cancer Res. 7:R897–R908. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
105
|
Crouch PJ, Hung LW, Adlard PA, Cortes M,
Lal V, Filiz G, Perez KA, Nurjono M, Caragounis A, Du T, et al:
Increasing Cu bioavailability inhibits Abeta oligomers and tau
phosphorylation. Proc Natl Acad Sci USA. 106:381–386. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
106
|
Schimmer AD: Clioquinol - a novel
copper-dependent and independent proteasome inhibitor. Curr Cancer
Drug Targets. 11:325–331. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
107
|
Costello LC and Franklin RB: A proposed
efficacious treatment with clioquinol (Zinc Ionophore) and
Cabergoline (Prolactin Dopamine Agonist) for the treatment of
terminal androgen-independent prostate cancer. Why and How? J Clin
Res Oncol. Feb 27–2019.Epub ahead of print. PubMed/NCBI
|
|
108
|
Donnelly PS, Caragounis A, Du T, Laughton
KM, Volitakis I, Cherny RA, Sharples RA, Hill AF, Li QX, Masters
CL, et al: Selective intracellular release of copper and zinc ions
from bis(thiosemicarbazonato) complexes reduces levels of Alzheimer
disease amyloid-beta peptide. J Biol Chem. 283:4568–4577. 2008.
View Article : Google Scholar
|
|
109
|
Drew SC: Chelator PBT2 forms a ternary
Cu(2+) Complex with β-Amyloid That has high stability but low
specificity. Int J Mol Sci. 24:92672023. View Article : Google Scholar
|
|
110
|
Scholefield M, Patassini S, Xu J and
Cooper GJS: Widespread selenium deficiency in the brain of cases
with Huntington's disease presents a new potential therapeutic
target. Ebiomedicine. 97:1048242023. View Article : Google Scholar : PubMed/NCBI
|
|
111
|
Upadhyay A, Chhangani D, Rao NR, Kofler J,
Vassar R, Rincon-Limas DE and Savas JN: Amyloid fibril proteomics
of AD brains reveals modifiers of aggregation and toxicity. Mol
Neurodegener. 18:612023. View Article : Google Scholar : PubMed/NCBI
|
|
112
|
Hands SL, Mason R, Sajjad MU, Giorgini F
and Wyttenbach A: Metallothioneins and copper metabolism are
candidate therapeutic targets in Huntington's disease. Biochem Soc
Trans. 38:552–558. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
113
|
Zhu Z, Song M, Ren J, Liang L, Mao G and
Chen M: Copper homeostasis and cuproptosis in central nervous
system diseases. Cell Death Dis. 15:8502024. View Article : Google Scholar : PubMed/NCBI
|
|
114
|
Cherny RA, Ayton S, Finkelstein DI, Bush
AI, McColl G and Massa SM: PBT2 Reduces Toxicity in a C. elegans
Model of polyQ aggregation and extends lifespan, reduces striatal
atrophy and improves motor performance in the R6/2 mouse model of
Huntington's disease. J Huntingtons Dis. 1:211–219. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
115
|
Rodriguez-Oroz MC, Jahanshahi M, Krack P,
Litvan I, Macias R, Bezard E and Obeso JA: Initial clinical
manifestations of Parkinson's disease: Features and
pathophysiological mechanisms. Lancet Neurol. 8:1128–1139. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
116
|
Davies KM, Hare DJ, Cottam V, Chen N,
Hilgers L, Halliday G, Mercer JF and Double KL: Localization of
copper and copper transporters in the human brain. Metallomics.
5:43–51. 2013. View Article : Google Scholar
|
|
117
|
Dexter DT, Wells FR, Lees AJ, Agid F, Agid
Y, Jenner P and Marsden CD: Increased nigral iron content and
alterations in other metal ions occurring in brain in Parkinson's
disease. J Neurochem. 52:1830–1836. 1989. View Article : Google Scholar : PubMed/NCBI
|
|
118
|
Ayton S, Lei P, Duce JA, Wong BX,
Sedjahtera A, Adlard PA, Bush AI and Finkelstein DI: Ceruloplasmin
dysfunction and therapeutic potential for Parkinson disease. Ann
Neurol. 73:554–559. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
119
|
Bisaglia M and Bubacco L: Copper ions and
Parkinson's disease: Why Is homeostasis so relevant? Biomolecules.
10:1952020. View Article : Google Scholar : PubMed/NCBI
|
|
120
|
Hemmati-Dinarvand M, Saedi S, Valilo M,
Kalantary-Charvadeh A, Alizadeh Sani M, Kargar R, Safari H and
Samadi N: Oxidative stress and Parkinson's disease: Conflict of
oxidant-antioxidant systems. Neurosci Lett. 709:1342962019.
View Article : Google Scholar : PubMed/NCBI
|
|
121
|
Trist BG, Davies KM, Cottam V, Genoud S,
Ortega R, Roudeau S, Carmona A, De Silva K, Wasinger V, Lewis SJG,
et al: Amyotrophic lateral sclerosis-like superoxide dismutase 1
proteinopathy is associated with neuronal loss in Parkinson's
disease brain. Acta Neuropathol. 134:113–127. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
122
|
Zhang MS, Liang JH, Yang MJ, Ren YR, Cheng
DH, Wu QH, He Y and Yin J: Low serum superoxide dismutase is
associated with a high risk of cognitive impairment after mild
acute ischemic stroke. Front Aging Neurosci. 14:8341142022.
View Article : Google Scholar : PubMed/NCBI
|
|
123
|
Quinn PMJ, Moreira PI, Ambrósio AF and
Alves CH: PINK1/PARKIN signalling in neurodegeneration and
neuroinflammation. Acta Neuropathol Commun. 8:1892020. View Article : Google Scholar : PubMed/NCBI
|
|
124
|
Cummins N and Götz J: Shedding light on
mitophagy in neurons: What is the evidence for PINK1/Parkin
mitophagy in vivo? Cell Mol Life Sci. 75:1151–1162. 2018.
View Article : Google Scholar
|
|
125
|
Aschner M, Skalny AV, Lu R, Martins AC,
Tizabi Y, Nekhoroshev SV, Santamaria A, Sinitskiy AI and Tinkov AA:
Mitochondrial pathways of copper neurotoxicity: Focus on
mitochondrial dynamics and mitophagy. Front Mol Neurosci.
17:15048022024. View Article : Google Scholar : PubMed/NCBI
|
|
126
|
Ling Z, Ge X, Jin C, Song Z, Zhang H, Fu
Y, Zheng K, Xu R and Jiang H: Copper doped bioactive glass promotes
matrix vesicles-mediated biomineralization via osteoblast mitophagy
and mitochondrial dynamics during bone regeneration. Bioact Mater.
46:195–212. 2024.
|
|
127
|
Synhaivska O, Bhattacharya S, Campioni S,
Thompson D and Nirmalraj PN: Single-particle resolution of
copper-associated annular α-synuclein oligomers reveals potential
therapeutic targets of neurodegeneration. ACS Chem Neurosci.
13:1410–1421. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
128
|
Triscott J, Rose Pambid M and Dunn SE:
Concise review: Bullseye: Targeting cancer stem cells to improve
the treatment of gliomas by repurposing disulfiram. Stem Cells.
33:1042–1046. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
129
|
Kannappan V, Ali M, Small B, Rajendran G,
Elzhenni S, Taj H, Wang W and Dou QP: Recent advances in
repurposing disulfiram and disulfiram derivatives as
copper-dependent anticancer agents. Front Mol Biosci. 8:7413162021.
View Article : Google Scholar : PubMed/NCBI
|
|
130
|
Zhou Q, Zhang Y, Lu L, Zhang H, Zhao C, Pu
Y and Yin L: Copper induces microglia-mediated neuroinflammation
through ROS/NF-κB pathway and mitophagy disorder. Food Chem
Toxicol. 168:1133692022. View Article : Google Scholar
|
|
131
|
Chidambaram SB, Anand N, Varma SR,
Ramamurthy S, Vichitra C, Sharma A, Mahalakshmi AM and Essa MM:
Superoxide dismutase and neurological disorders. IBRO Neurosci Rep.
16:373–394. 2024. View Article : Google Scholar : PubMed/NCBI
|
|
132
|
Cukierman DS, Pinheiro AB,
Castiñeiras-Filho SL, da Silva AS, Miotto MC, De Falco A, de P
Ribeiro T, Maisonette S, da Cunha AL, Hauser-Davis RA, et al: A
moderate metal-binding hydrazone meets the criteria for a
bioinorganic approach towards Parkinson's disease: Therapeutic
potential, blood-brain barrier crossing evaluation and preliminary
toxicological studies. J Inorg Biochem. 170:160–168. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
133
|
Vavere AL and Lewis JS: Cu-ATSM: A
radiopharmaceutical for the PET imaging of hypoxia. Dalton Trans.
4893–4902. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
134
|
Ikawa M, Okazawa H, Kudo T, Kuriyama M,
Fujibayashi Y and Yoneda M: Evaluation of striatal oxidative stress
in patients with Parkinson's disease using [62Cu]ATSM PET. Nucl Med
Biol. 38:945–951. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
135
|
Hung LW, Villemagne VL, Cheng L, Sherratt
NA, Ayton S, White AR, Crouch PJ, Lim S, Leong SL, Wilkins S, et
al: The hypoxia imaging agent CuII(atsm) is neuroprotective and
improves motor and cognitive functions in multiple animal models of
Parkinson's disease. J Exp Med. 209:837–854. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
136
|
Rowlands BD, Trist BG, Karozis C, Schaffer
G, Mor D, Harwood R, Rosolen SA, Cottam V, Persson-Carboni F,
Richardson M, et al: Copper supplementation mitigates
Parkinson-like wild-type SOD1 pathology and nigrostriatal
degeneration in a novel mouse model. Acta Neuropathol Commun.
13:1332025. View Article : Google Scholar : PubMed/NCBI
|
|
137
|
Al-Chalabi A and Hardiman O: The
epidemiology of ALS: A conspiracy of genes, environment and time.
Nat Rev Neurol. 9:617–628. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
138
|
Min JH, Sarlus H and Harris RA: Copper
toxicity and deficiency: The vicious cycle at the core of protein
aggregation in ALS. Front Mol Neurosci. 17:14081592024. View Article : Google Scholar : PubMed/NCBI
|
|
139
|
Moriyama H and Yokota T: Recent progress
of antisense oligonucleotide therapy for
superoxide-dismutase-1-mutated amyotrophic lateral sclerosis: Focus
on tofersen. Genes (Basel). 15:13422024. View Article : Google Scholar : PubMed/NCBI
|
|
140
|
Jerusalem F, Pohl C, Karitzky J and Ries
F: ALS. Neurology. 47(Suppl 4): S218–S220. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
141
|
Gurney ME, Pu H, Chiu AY, Dal Canto MC,
Polchow CY, Alexander DD, Caliendo J, Hentati A, Kwon YW, Deng HX,
et al: Motor neuron degeneration in mice that express a human Cu,
Zn superoxide dismutase mutation. Science. 264:1772–1775. 1994.
View Article : Google Scholar : PubMed/NCBI
|
|
142
|
Bray F, Laversanne M, Sung H, Ferlay J,
Siegel RL, Soerjomataram I and Jemal A: Global cancer statistics
2022: GLOBOCAN estimates of incidence and mortality worldwide for
36 cancers in 185 countries. CA Cancer J Clin. 74:229–263.
2024.PubMed/NCBI
|
|
143
|
Ansori ANM, Widyananda MH, Antonius Y,
Murtadlo AAA, Kharisma VD, Wiradana PA, Sahadewa S, Durry FD,
Maksimiuk N, Rebezov M and Zainul R: A review of cancer-related
hypercalcemia: Pathophysiology, current treatments, and future
directions. J Med Pharm Chem Res. 6:944–952. 2024.
|
|
144
|
Wu T, Sempos CT, Freudenheim JL, Muti P
and Smit E: Serum iron, copper and zinc concentrations and risk of
cancer mortality in US adults. Ann Epidemiol. 14:195–201. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
145
|
Hyvönen MT, Ucal S, Pasanen M, Peräniemi
S, Weisell J, Khomutov M, Khomutov AR, Vepsäläinen J, Alhonen L and
Keinänen TA: Triethylenetetramine modulates polyamine and energy
metabolism and inhibits cancer cell proliferation. Biochem J.
473:1433–1441. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
146
|
Pavithra V, Sathisha TG, Kasturi K,
Mallika DS, Amos SJ and Ragunatha S: Serum levels of metal ions in
female patients with breast cancer. J Clin Diagn Res. 9:BC25–c27.
2015.PubMed/NCBI
|
|
147
|
Jakhmola V, Parashar T, Ghildiyal P,
Ansori ANM, Sharma RK, Rao NGR, Kalra K, Singh N, Nainwal N, Singh
RK, et al: An in silico study to explore the role of EGFR in
ovarian cancer. Pharmacog J. 14:817–821. 2022. View Article : Google Scholar
|
|
148
|
Zhang X and Yang Q: Association between
serum copper levels and lung cancer risk: A meta-analysis. J Int
Med Res. 46:4863–4873. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
149
|
Saleh SAK, Adly HM, Abdelkhaliq AA and
Nassir AM: Serum levels of selenium, zinc, copper, manganese, and
iron in prostate cancer patients. Curr Urol. 14:44–49. 2020.
View Article : Google Scholar : PubMed/NCBI
|
|
150
|
Atakul T, Altinkaya SO, Abas BI and
Yenisey C: Serum copper and zinc levels in patients with
endometrial cancer. Biol Trace Elem Res. 195:46–54. 2020.
View Article : Google Scholar
|
|
151
|
Fang AP, Chen PY, Wang XY, Liu ZY, Zhang
DM, Luo Y, Liao GC, Long JA, Zhong RH, Zhou ZG, et al: Serum copper
and zinc levels at diagnosis and hepatocellular carcinoma survival
in the Guangdong liver cancer cohort. Int J Cancer. 144:2823–2832.
2019. View Article : Google Scholar
|
|
152
|
Lun X, Wells JC, Grinshtein N, King JC,
Hao X, Dang NH, Wang X, Aman A, Uehling D, Datti A, et al:
Disulfiram when combined with copper enhances the therapeutic
effects of temozolomide for the treatment of glioblastoma. Clin
Cancer Res. 22:3860–3875. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
153
|
Li Y, Chen F, Chen J, Chan S, He Y, Liu W
and Zhang G: Disulfiram/copper induces antitumor activity against
both nasopharyngeal cancer cells and cancer-associated fibroblasts
through ROS/MAPK and ferroptosis pathways. Cancers (Basel).
12:1382020. View Article : Google Scholar : PubMed/NCBI
|
|
154
|
Mcauslan BR and Reilly W: Endothelial cell
phagokinesis in response to specific metal ions. Exp Cell Res.
130:147–157. 1980. View Article : Google Scholar : PubMed/NCBI
|
|
155
|
Narayanan G, R BS, Vuyyuru H, Muthuvel B
and Konerirajapuram Natrajan S: CTR1 Silencing inhibits
angiogenesis by limiting copper entry into endothelial cells. PLoS
One. 8:e719822013. View Article : Google Scholar : PubMed/NCBI
|
|
156
|
Mandinov L, Mandinova A, Kyurkchiev S,
Kyurkchiev D, Kehayov I, Kolev V, Soldi R, Bagala C, de Muinck ED,
Lindner V, et al: Copper chelation represses the vascular response
to injury. Proc Natl Acad Sci USA. 100:6700–6705. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
157
|
Nurzynska A, Klimek K, Swierzycka I, Palka
K and Ginalska G: Porous curdlan-based hydrogels modified with
copper ions as potential dressings for prevention and management of
bacterial wound infection-an in vitro assessment. Polymers (Basel).
12:18932020. View Article : Google Scholar : PubMed/NCBI
|
|
158
|
Badet J, Soncin F, Guitton JD, Lamare O,
Cartwright T and Barritault D: Specific binding of angiogenin to
calf pulmonary artery endothelial cells. Proc Natl Acad Sci USA.
86:8427–8431. 1989. View Article : Google Scholar : PubMed/NCBI
|
|
159
|
Zhao YN, Chen LH, Yang XL, Dong JY, Wu WB,
Chen D, Geng RM, Ke NW and Liu J: Inhibition of copper
transporter-1 by ammonium tetrathiocarbolybdate in the treatment of
pancreatic cancer. Sichuan Da Xue Xue Bao Yi Xue Ban. 51:643–649.
2020.In Chinese. PubMed/NCBI
|
|
160
|
Pan Q, Bao LW and Merajver SD:
Tetrathiomolybdate inhibits angiogenesis and metastasis through
suppression of the NFkappaB signaling cascade. Mol Cancer Res.
1:701–706. 2003.PubMed/NCBI
|
|
161
|
Pan Q, Kleer CG, van Golen KL, Irani J,
Bottema KM, Bias C, De Carvalho M, Mesri EA, Robins DM, Dick RD, et
al: Copper deficiency induced by tetrathiomolybdate suppresses
tumor growth and angiogenesis. Cancer Res. 62:4854–4859.
2002.PubMed/NCBI
|
|
162
|
Li Y, Fang M, Xu Z and Li X:
Tetrathiomolybdate as an old drug in a new use: As a
chemotherapeutic sensitizer for non-small cell lung cancer. J Inorg
Biochem. 233:1118652022. View Article : Google Scholar : PubMed/NCBI
|
|
163
|
Crowe A, Jackaman C, Beddoes KM, Ricciardo
B and Nelson DJ: Rapid copper acquisition by developing murine
mesothelioma: Decreasing bioavailable copper slows tumor growth,
normalizes vessels and promotes T cell infiltration. PLoS One.
8:e736842013. View Article : Google Scholar : PubMed/NCBI
|
|
164
|
Cosimo RD, Scarpelli A, Lappano R, Pisano
A, Santolla MF, De Marco P, Cirillo F, Cappello AR, Dolce V,
Belfiore A, et al: Copper activates HIF-1α/GPER/VEGF signalling in
cancer cells. Oncotarget. 6:34158–34177. 2015. View Article : Google Scholar
|
|
165
|
Mammoto T, Jiang A, Jiang E, Panigrahy D,
Kieran MW and Mammoto A: Role of collagen matrix in tumor
angiogenesis and glioblastoma multiforme progression. Am J Pathol.
183:1293–1305. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
166
|
Baldari S, Di Rocco G, Heffern MC, Su TA,
Chang CJ and Toietta G: Effects of copper chelation on BRAF(V600E)
positive colon carcinoma cells. Cancers (Basel). 11:6592019.
View Article : Google Scholar : PubMed/NCBI
|
|
167
|
Brady DC, Crowe MS, Turski ML, Hobbs GA,
Yao X, Chaikuad A, Knapp S, Xiao K, Campbell SL, Thiele DJ and
Counter CM: Copper is required for oncogenic BRAF signalling and
tumorigenesis. Nature. 509:492–496. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
168
|
Brady DC, Crowe MS, Greenberg DN and
Counter CM: Copper chelation inhibits BRAFV600E-driven
melanomagenesis and counters resistance to BRAFV600E and MEK1/2
inhibitors. Cancer Res. 77:6240–6252. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
169
|
Ross MO, Xie Y, Owyang RC, Ye C, Zbihley
ONP, Lyu R, Wu T, Wang P, Karginova O, Olopade OI, et al: PTPN2
copper-sensing relays copper level fluctuations into EGFR/CREB
activation and associated CTR1 transcriptional repression. Nat
Commun. 15:69472024. View Article : Google Scholar : PubMed/NCBI
|
|
170
|
Voli F, Valli E, Lerra L, Kimpton K,
Saletta F, Giorgi FM, Mercatelli D, Rouaen JRC, Shen S, Murray JE,
et al: Intratumoral copper modulates PD-L1 expression and
influences tumor immune evasion. Cancer Res. 80:4129–4144. 2020.
View Article : Google Scholar : PubMed/NCBI
|
|
171
|
Xu M, Casio M, Range DE, Sosa JA and
Counter CM: Copper chelation as targeted therapy in a mouse model
of oncogenic BRAF-Driven papillary thyroid cancer. Clin Cancer Res.
24:4271–4281. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
172
|
Akins NS, Nielson TC and Le HV: Inhibition
of glycolysis and glutaminolysis: An emerging drug discovery
approach to combat cancer. Curr Top Med Chem. 18:494–504. 2018.
View Article : Google Scholar : PubMed/NCBI
|
|
173
|
Carneiro BA and El-Deiry WS: Targeting
apoptosis in cancer therapy. Nat Rev Clin Oncol. 17:395–417. 2020.
View Article : Google Scholar : PubMed/NCBI
|
|
174
|
Achmad AB, Proboningrat A, Ansori ANM,
Fadholly A, Rochmi SE, Samsudin RR, Hidayatik N, Hendarti GA and
Jayanti S: Stem bark ethanolic extract of Pinus merkusii induces
caspase 9-mediated apoptosis in HeLa cells. Open Vet J.
14:2628–2633. 2024. View Article : Google Scholar : PubMed/NCBI
|
|
175
|
Weinlich R, Oberst A, Beere HM and Green
DR: Necroptosis in development, inflammation and disease. Nat Rev
Mol Cell Biol. 18:127–136. 2017. View Article : Google Scholar
|
|
176
|
Bergsbaken T, Fink SL and Cookson BT:
Pyroptosis: Host cell death and inflammation. Nat Rev Microbiol.
7:99–109. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
177
|
Dixon SJ, Lemberg KM, Lamprecht MR, Skouta
R, Zaitsev EM, Gleason CE, Patel DN, Bauer AJ, Cantley AM, Yang WS,
et al: Ferroptosis: An iron-dependent form of nonapoptotic cell
death. Cell. 149:1060–1072. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
178
|
Gao W, Huang Z, Duan J, Nice EC, Lin J and
Huang C: Elesclomol induces copper-dependent ferroptosis in
colorectal cancer cells via degradation of ATP7A. Mol Oncol.
15:3527–3544. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
179
|
Pan C, Ji Z, Wang Q, Zhang Z, Wang Z, Li
C, Lu S and Ge P: Cuproptosis: Mechanisms, biological significance,
and advances in disease treatment-A systematic review. CNS Neurosci
Ther. 30:e700392024. View Article : Google Scholar : PubMed/NCBI
|
|
180
|
Huang Z, Wang L, Chen L, Zhang Y and Shi
P: Induction of cell cycle arrest via the p21, p27-cyclin E,A/Cdk2
pathway in SMMC-7721 hepatoma cells by clioquinol. Acta Pharm.
65:463–471. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
181
|
Tsvetkov P, Detappe A, Cai K, Keys HR,
Brune Z, Ying W, Thiru P, Reidy M, Kugener G, Rossen J, et al:
Mitochondrial metabolism promotes adaptation to proteotoxic stress.
Nat Chem Biol. 15:681–689. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
182
|
Tardito S, Bassanetti I, Bignardi C,
Elviri L, Tegoni M, Mucchino C, Bussolati O, Franchi-Gazzola R and
Marchiò L: Copper binding agents acting as copper ionophores lead
to caspase inhibition and paraptotic cell death in human cancer
cells. J Am Chem Soc. 133:6235–6242. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
183
|
Tardito S, Barilli A, Bassanetti I, Tegoni
M, Bussolati O, Franchi-Gazzola R, Mucchino C and Marchiò L:
Copper-dependent cytotoxicity of 8-hydroxyquinoline derivatives
correlates with their hydrophobicity and does not require caspase
activation. J Med Chem. 55:10448–10459. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
184
|
Nagai M, Vo NH, Shin Ogawa L, Chimmanamada
D, Inoue T, Chu J, Beaudette-Zlatanova BC, Lu R, Blackman RK,
Barsoum J, et al: The oncology drug elesclomol selectively
transports copper to the mitochondria to induce oxidative stress in
cancer cells. Free Radic Biol Med. 52:2142–2150. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
185
|
Shimada K, Reznik E, Stokes ME,
Krishnamoorthy L, Bos PH, Song Y, Quartararo CE, Pagano NC, Carpizo
DR, deCarvalho AC, et al: Copper-binding small molecule induces
oxidative stress and cell-cycle arrest in
glioblastoma-patient-derived cells. Cell Chem Biol. 25:585–594 e7.
2018. View Article : Google Scholar : PubMed/NCBI
|
|
186
|
Yip NC, Fombon IS, Liu P, Brown S,
Kannappan V, Armesilla AL, Xu B, Cassidy J, Darling JL and Wang W:
Disulfiram modulated ROS-MAPK and NFĸB pathways and targeted breast
cancer cells with cancer stem cell-like properties. Br J Cancer.
104:1564–1574. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
187
|
Chen D, Cui QC, Yang H and Dou QP:
Disulfiram, a clinically used anti-alcoholism drug and
copper-binding agent, induces apoptotic cell death in breast cancer
cultures and xenografts via inhibition of the proteasome activity.
Cancer Res. 66:10425–10433. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
188
|
Liu N, Huang H, Dou QP and Liu J:
Inhibition of 19S proteasome-associated deubiquitinases by
metal-containing compounds. Oncoscience. 2:457–466. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
189
|
Baldari S, Di Rocco G and Toietta G:
Current biomedical use of copper chelation therapy. Int J Mol Sci.
21:10692020. View Article : Google Scholar : PubMed/NCBI
|
|
190
|
Tsvetkov P, Coy S, Petrova B, Dreishpoon
M, Verma A, Abdusamad M, Rossen J, Joesch-Cohen L, Humeidi R,
Spangler RD, et al: Copper induces cell death by targeting
lipoylated TCA cycle proteins. Science. 375:1254–1261. 2022.
View Article : Google Scholar : PubMed/NCBI
|
|
191
|
Li P, Sun Q, Bai S, Wang H and Zhao L:
Combination of the cuproptosis inducer disulfiram and anti-PD-L1
abolishes NSCLC resistance by ATP7B to regulate the HIF-1 signaling
pathway. Int J Mol Med. 53:192024. View Article : Google Scholar :
|
|
192
|
Huang N, Feng Y, Liu Y, Zhang Y, Liu L,
Zhang B, Zhang T, Su Z, Xue L and Wu ZB: Disulfiram mediated
anti-tumour effect in pituitary neuroendocrine tumours by inducing
cuproptosis. Int Immunopharmacol. 134:1121592024. View Article : Google Scholar : PubMed/NCBI
|
|
193
|
Guo B, Yang F, Zhang L, Zhao Q, Wang W,
Yin L, Chen D, Wang M, Han S, Xiao H and Xing N: Cuproptosis
induced by ROS responsive nanoparticles with elesclomol and copper
combined with αPD-L1 for enhanced cancer immunotherapy. Adv Mater.
35:e22122672023. View Article : Google Scholar
|
|
194
|
Sun L, Zhang Y, Yang B, Sun S, Zhang P,
Luo Z, Feng T, Cui Z, Zhu T, Li Y, et al: Lactylation of METTL16
promotes cuproptosis via m(6)A-modification on FDX1 mRNA in gastric
cancer. Nat Commun. 14:65232023. View Article : Google Scholar : PubMed/NCBI
|
|
195
|
Buccarelli M, D'Alessandris QG, Matarrese
P, Mollinari C, Signore M, Cappannini A, Martini M, D'Aliberti P,
De Luca G, Pedini F, et al: Elesclomol-induced increase of
mitochondrial reactive oxygen species impairs glioblastoma
stem-like cell survival and tumor growth. J Exp Clin Cancer Res.
40:2282021. View Article : Google Scholar : PubMed/NCBI
|
|
196
|
Solmonson A and DeBerardinis RJ: Lipoic
acid metabolism and mitochondrial redox regulation. J Biol Chem.
293:7522–7530. 2018. View Article : Google Scholar :
|
|
197
|
Harris IS, Endress JE, Coloff JL, Selfors
LM, McBrayer SK, Rosenbluth JM, Takahashi N, Dhakal S, Koduri V,
Oser MG, et al: Deubiquitinases maintain protein homeostasis and
survival of cancer cells upon glutathione depletion. Cell Metab.
29:1166–1181.e6. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
198
|
Zhou S, Kachhap S and Singh KK:
Mitochondrial impairment in p53-deficient human cancer cells.
Mutagenesis. 18:287–292. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
199
|
Matoba S, Kang JG, Patino WD, Wragg A,
Boehm M, Gavrilova O, Hurley PJ, Bunz F and Hwang PM: p53 regulates
mitochondrial respiration. Science. 312:1650–1653. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
200
|
Saleem A, Adhihetty PJ and Hood DA: Role
of p53 in mitochondrial biogenesis and apoptosis in skeletal
muscle. Physiol Genomics. 37:58–66. 2009. View Article : Google Scholar
|
|
201
|
Zhang C, Lin M, Wu R, Wang X, Yang B,
Levine AJ, Hu W and Feng Z: Parkin, a p53 target gene, mediates the
role of p53 in glucose metabolism and the Warburg effect. Proc Natl
Acad Sci USA. 108:16259–16264. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
202
|
Contractor T and Harris CR: p53 negatively
regulates transcription of the pyruvate dehydrogenase kinase Pdk2.
Cancer Res. 72:560–567. 2012. View Article : Google Scholar
|
|
203
|
Bischoff ME, Shamsaei B, Yang J, Secic D,
Vemuri B, Reisz JA, D'Alessandro A, Bartolacci C, Adamczak R,
Schmidt L, et al: Copper drives remodeling of metabolic state and
progression of clear cell renal cell carcinoma. Cancer Discov.
15:401–426. 2025. View Article : Google Scholar :
|
|
204
|
Gan Y, Liu T, Feng W, Wang L, Li LI and
Ning Y: Drug repositioning of disulfiram induces endometrioid
epithelial ovarian cancer cell death via the both apoptosis and
cuproptosis pathways. Oncol Res. 31:333–343. 2023. View Article : Google Scholar : PubMed/NCBI
|
|
205
|
Liu X, Luo B, Wu X and Tang Z: Cuproptosis
and cuproptosis-related genes: Emerging potential therapeutic
targets in breast cancer. Biochim Biophys Acta Rev Cancer.
1878:1890132023. View Article : Google Scholar : PubMed/NCBI
|
|
206
|
Liu T, Zhou Z, Zhang M, Lang P, Li J, Liu
Z, Zhang Z, Li L and Zhang L: Cuproptosis-immunotherapy using PD-1
overexpressing T cell membrane-coated nanosheets efficiently treats
tumor. J Control Release. 362:502–512. 2023. View Article : Google Scholar : PubMed/NCBI
|
|
207
|
Lu X, Deng W, Wang S, Zhao S, Zhu B, Bai
B, Mao Y, Lin J, Yi Y, Xie Z, et al: PEGylated
Elesclomol@Cu(II)-based Metal-organic framework with effective
nanozyme performance and cuproptosis induction efficacy for
enhanced PD-L1-based immunotherapy. Mater Today Bio. 29:1013172024.
View Article : Google Scholar
|
|
208
|
Hasinoff BB, Wu X, Yadav AA, Patel D,
Zhang H, Wang DS, Chen ZS and Yalowich JC: Cellular mechanisms of
the cytotoxicity of the anticancer drug elesclomol and its complex
with Cu(II). Biochem Pharmacol. 93:266–276. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
209
|
Jiao Y, Hannafon BN and Ding WQ:
Disulfiram's anticancer activity: Evidence and mechanisms.
Anticancer Agents Med Chem. 16:1378–1384. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
210
|
Xie L, Gong J, He Z, Zhang W and Wang H,
Wu S, Wang X, Sun P, Cai L, Wu Z and Wang H: A copper-manganese
based nanocomposite induces cuproptosis and potentiates anti-tumor
immune responses. Small. 21:e24121742025. View Article : Google Scholar : PubMed/NCBI
|
|
211
|
Deng L, Liu T, Liu CA, Zhang Q, Song MM,
Lin SQ, Wang YM, Zhang QS and Shi HP: The association of metabolic
syndrome score trajectory patterns with risk of all cancer types.
Cancer. 130:2150–2159. 2024. View Article : Google Scholar : PubMed/NCBI
|
|
212
|
Song M, Liu T, Liu H, Zhang Q, Zhang Q,
Wang Y, Ma X, Cao L and Shi H: Association between metabolic
syndrome, C-reactive protein, and the risk of primary liver cancer:
A large prospective study. BMC Cancer. 22:8532022. View Article : Google Scholar : PubMed/NCBI
|
|
213
|
Gathirua-Mwangi WG, Song Y, Monahan PO,
Champion VL and Zollinger TW: Associations of metabolic syndrome
and C-reactive protein with mortality from total cancer,
obesity-linked cancers and breast cancer among women in NHANES III.
Int J Cancer. 143:535–542. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
214
|
Springer C, Humayun D and Skouta R:
Cuproptosis: Unraveling the mechanisms of copper-induced cell death
and its implication in cancer therapy. Cancers (Basel). 16:6472024.
View Article : Google Scholar : PubMed/NCBI
|
|
215
|
Chen Z, Li Y, Yin Y, Song M, Wang F and
Jiang G: Cu Nanowires trigger efficient cuproptosis via special
intracellular distribution and excessive Cu Ion release. Nano Lett.
24:11446–11453. 2024. View Article : Google Scholar : PubMed/NCBI
|
|
216
|
Shao R, Visser I, Fros JJ and Yin X:
Versatility of the zinc-finger antiviral protein (ZAP) As a
modulator of viral infections. Int J Biol Sci. 20:4585–4600. 2024.
View Article : Google Scholar : PubMed/NCBI
|
|
217
|
Zhang N, Yu X, Xie J and Xu H: New
insights into the role of ferritin in iron homeostasis and
neurodegenerative diseases. Mol Neurobiol. 58:2812–2823. 2021.
View Article : Google Scholar : PubMed/NCBI
|
|
218
|
Ermini ML and Voliani V: Antimicrobial
nano-agents: The copper age. ACS Nano. 15:6008–6029. 2021.
View Article : Google Scholar : PubMed/NCBI
|
|
219
|
Lu X, Chen X, Lin C, Yi Y, Zhao S, Zhu B,
Deng W, Wang X, Xie Z, Rao S, et al: Elesclomol loaded copper oxide
nanoplatform triggers cuproptosis to enhance antitumor
immunotherapy. Adv Sci (Weinh). 11:e23099842024. View Article : Google Scholar : PubMed/NCBI
|
|
220
|
Zheng P, Zhou C, Lu L, Liu B and Ding Y:
Elesclomol: A copper ionophore targeting mitochondrial metabolism
for cancer therapy. J Exp Clin Cancer Res. 41:2712022. View Article : Google Scholar : PubMed/NCBI
|
|
221
|
Woźniak-Budych MJ, Staszak K and Staszak
M: Copper and copper-based nanoparticles in medicine-perspectives
and challenges. Molecules. 28:66872023. View Article : Google Scholar
|
|
222
|
Brewer GJ: Tetrathiomolybdate anticopper
therapy for Wilson's disease inhibits angiogenesis, fibrosis and
inflammation. J Cell Mol Med. 7:11–20. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
223
|
Kim YJ, Tsang T, Anderson GR, Posimo JM
and Brady DC: Inhibition of BCL2 family members increases the
efficacy of copper chelation in BRAFV600E-Driven melanoma. Cancer
Res. 80:1387–1400. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
224
|
Ishida S, McCormick F, Smith-McCune K and
Hanahan D: Enhancing tumor-specific uptake of the anticancer drug
cisplatin with a copper chelator. Cancer Cell. 17:574–583. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
225
|
Lin X, Chen W, Li B, Zhao Z, Yu Z, Zhao
XY, Zhou X, Feng Z, Lin C and Cao W: Targeting intratumoral copper
inhibits tumor progression via p62-Mediated EZH2 degradation and
potentiates Anti-PD-1 immunotherapy in oral squamous cell
carcinoma. Adv Sci (Weinh). Jul 28–2025.Epub ahead of print.
View Article : Google Scholar
|
|
226
|
Xue Q, Yan D, Chen X, Li X, Kang R,
Klionsky DJ, Kroemer G, Chen X, Tang D and Liu J: Copper-dependent
autophagic degradation of GPX4 drives ferroptosis. Autophagy.
19:1982–1996. 2023. View Article : Google Scholar : PubMed/NCBI
|
|
227
|
Li F, Wu X, Liu H, Liu M, Yue Z, Wu Z, Liu
L and Li F: Copper depletion strongly enhances ferroptosis via
mitochondrial perturbation and reduction in antioxidative
mechanisms. Antioxidants (Basel). 11:20842022. View Article : Google Scholar : PubMed/NCBI
|
|
228
|
Derseh HB, Perera KUE, Dewage SNV, Stent
A, Koumoundouros E, Organ L, Pagel CN and Snibson KJ:
Tetrathiomolybdate treatment attenuates bleomycin-induced
angiogenesis and lung pathology in a sheep model of pulmonary
fibrosis. Front Pharmacol. 12:7009022021. View Article : Google Scholar : PubMed/NCBI
|
|
229
|
Brewer GJ: The promise of copper lowering
therapy with tetrathiomolybdate in the cure of cancer and in the
treatment of inflammatory disease. J Trace Elem Med Biol.
28:372–378. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
230
|
Zhang M, Qiu H, Mao L, Wang B, Li N, Fan
Y, Weng P, Hu S, Dong X, Qin X, et al: Ammonium tetrathiomolybdate
triggers autophagy-dependent NRF2 activation in vascular
endothelial cells. Cell Death Dis. 13:7332022. View Article : Google Scholar : PubMed/NCBI
|
|
231
|
Wang T, Lu W, Cheng Z, Wang L, Jiang Z,
Yue Y, Jiang P, Xia Z, He L, Wang F, et al: Oral engineered
extracellular vesicles based on ion exchange strategy for
multipronged management of Wilson's disease complicated with
reproductive dysfunction therapy. Adv Sci (Weinh). 12:e016892025.
View Article : Google Scholar : PubMed/NCBI
|
|
232
|
Ke Y, Wu C, Zeng Y, Chen M, Li Y, Xie C,
Zhou Y, Zhong Y and Yu H: Radiosensitization of clioquinol combined
with zinc in the nasopharyngeal cancer stem-like cells by
inhibiting autophagy in vitro and in vivo. Int J Biol Sci.
16:777–789. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
233
|
Yoshiji H, Kuriyama S, Yoshii J, Ikenaka
Y, Noguchi R, Yanase K, Namisaki T, Yamazaki M, Tsujinoue H, Imazu
H and Fukui H: The copper-chelating agent, trientine, attenuates
liver enzyme-altered preneoplastic lesions in rats by angiogenesis
suppression. Oncol Rep. 10:1369–1373. 2003.PubMed/NCBI
|
|
234
|
Yang M, Wu X, Hu J, Wang Y, Wang Y, Zhang
L, Huang W, Wang X, Li N, Liao L, et al: COMMD10 inhibits HIF1α/CP
loop to enhance ferroptosis and radiosensitivity by disrupting
Cu-Fe balance in hepatocellular carcinoma. J Hepatol. 76:1138–1150.
2022. View Article : Google Scholar : PubMed/NCBI
|
|
235
|
Wang Y, Li W, Patel SS, Cong J, Zhang N,
Sabbatino F, Liu X, Qi Y, Huang P, Lee H, et al: Blocking the
formation of radiation-induced breast cancer stem cells.
Oncotarget. 5:3743–3755. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
236
|
Wu L, Meng F, Dong L, Block CJ, Mitchell
AV, Wu J, Jang H, Chen W, Polin L, Yang Q, et al: Disulfiram and
BKM120 in combination with chemotherapy impede tumor progression
and delay tumor recurrence in tumor initiating cell-rich TNBC. Sci
Rep. 9:2362019. View Article : Google Scholar : PubMed/NCBI
|
|
237
|
Sun T, Yang W, Toprani SM, Guo W, He L,
DeLeo AB, Ferrone S, Zhang G, Wang E, Lin Z, et al: Induction of
immunogenic cell death in radiation-resistant breast cancer stem
cells by repurposing anti-alcoholism drug disulfiram. Cell Commun
Signal. 18:362020. View Article : Google Scholar : PubMed/NCBI
|
|
238
|
Zhou P, Qin J, Zhou C, Wan G, Liu Y, Zhang
M, Yang X, Zhang N and Wang Y: Multifunctional nanoparticles based
on a polymeric copper chelator for combination treatment of
metastatic breast cancer. Biomaterials. 195:86–99. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
239
|
Hu H, Hua S, Lu F, Zhang W, Zhang Z, Cui
J, Lei X, Xia J, Xu F and Zhou M: Mucous permeable nanoparticle for
inducing cuproptosis-like death in broad-spectrum bacteria for
nebulized treatment of acute pneumonia. Adv Sci (Weinh).
12:e24085802025. View Article : Google Scholar : PubMed/NCBI
|
|
240
|
Wu A, Yin N, Li Z, Zhang X, Zhang Z, Zhong
T, Xia F, Pan J, Wang D, Liu L and Dong J: FDX1 facilitates
elesclomol-induced cuproptosis and promotes glioblastoma
development via transcription factor NFKB1. Biochem Pharmacol.
241:1171862025. View Article : Google Scholar : PubMed/NCBI
|
|
241
|
Wang W, Lu K, Jiang X, Wei Q, Zhu L, Wang
X, Jin H and Feng L: Ferroptosis inducers enhanced cuproptosis
induced by copper ionophores in primary liver cancer. J Exp Clin
Cancer Res. 42:1422023. View Article : Google Scholar : PubMed/NCBI
|
|
242
|
Zulkifli M, Spelbring AN, Zhang Y, Soma S,
Chen S, Li L, Le T, Shanbhag V, Petris MJ, Chen TY, et al:
FDX1-dependent and independent mechanisms of elesclomol-mediated
intracellular copper delivery. Proc Natl Acad Sci USA.
120:e22167221202023. View Article : Google Scholar : PubMed/NCBI
|
|
243
|
Chisholm CL, Wang H, Wong AH,
Vazquez-Ortiz G, Chen W, Xu X and Deng CX: Ammonium
tetrathiomolybdate treatment targets the copper transporter ATP7A
and enhances sensitivity of breast cancer to cisplatin. Oncotarget.
7:84439–84452. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
244
|
Feng L, Wu TZ, Guo XR, Wang YJ, Wang XJ,
Liu SX, Zhang R, Ma Y, Tan NH, Bian JL and Wang Z: Discovery of
natural resorcylic acid lactones as novel potent copper ionophores
covalently targeting PRDX1 to induce cuproptosis for
triple-negative breast cancer therapy. ACS Cent Sci. 11:357–370.
2025. View Article : Google Scholar : PubMed/NCBI
|
|
245
|
Wen H, Qu C, Wang Z, Gao H, Liu W, Wang H,
Sun H, Gu J, Yang Z and Wang X: Cuproptosis enhances docetaxel
chemosensitivity by inhibiting autophagy via the DLAT/mTOR pathway
in prostate cancer. FASEB J. 37:e231452023. View Article : Google Scholar : PubMed/NCBI
|
|
246
|
Ahire JJ, Neveling DP and Dicks LMT:
Polyacrylonitrile (PAN) nanofibres spun with copper nanoparticles:
An anti-Escherichia coli membrane for water treatment. Appl
Microbiol Biotechnol. 102:7171–7181. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
247
|
Cendrowska-Pinkosz M, Krauze M, Juśkiewicz
J, Fotschki B and Ognik K: The influence of copper nanoparticles on
neurometabolism marker levels in the brain and intestine in a rat
model. Int J Mol Sci. 24:113212023. View Article : Google Scholar : PubMed/NCBI
|
|
248
|
Geng X, Liu K, Wang J, Su X, Shi Y and
Zhao L: Preparation of ultra-small copper nanoparticles-loaded
self-healing hydrogels with antibacterial, inflammation-suppressing
and angiogenesis-enhancing properties for promoting diabetic wound
healing. Int J Nanomedicine. 18:3339–3358. 2023. View Article : Google Scholar : PubMed/NCBI
|
|
249
|
Li L, Tai Z, Liu W, Luo Y, Wu Y, Lin S,
Liu M, Gao B and Liu JX: Copper overload impairs hematopoietic stem
and progenitor cell proliferation via prompting HSF1/SP1
aggregation and the subsequently downregulating FOXM1-Cytoskeleton
axis. iScience. 26:1064062023. View Article : Google Scholar : PubMed/NCBI
|
|
250
|
Petruzzelli R and Polishchuk RS: Activity
and trafficking of copper-transporting ATPases in tumor development
and defense against platinum-based drugs. Cells. 8:10802019.
View Article : Google Scholar : PubMed/NCBI
|
|
251
|
Nayagam JS, Jeyaraj R, Foskett P, Dhawan
A, Ala A, Joshi D, Bomford A and Thompson RJ: ATP7B genotype and
chronic liver disease treatment outcomes in wilson disease: Worse
survival with loss-of-function variants. Clin Gastroenterol
Hepatol. 21:1323–1329.e4. 2023. View Article : Google Scholar
|
|
252
|
Wang Y, Li D, Xu K, Wang G and Zhang F:
Copper homeostasis and neurodegenerative diseases. Neural Regen
Res. 20:3124–3143. 2025. View Article : Google Scholar :
|
|
253
|
Chan N, Willis A, Kornhauser N, Ward MM,
Lee SB, Nackos E, Seo BR, Chuang E, Cigler T, Moore A, et al:
Influencing the tumor microenvironment: A phase II study of copper
depletion using tetrathiomolybdate in patients with breast cancer
at high risk for recurrence and in preclinical models of lung
metastases. Clin Cancer Res. 23:666–676. 2017. View Article : Google Scholar
|
|
254
|
Yang Z, Su W, Wei X, Pan Y, Xing M, Niu L,
Feng B, Kong W, Ren X, Huang F, et al: Hypoxia inducible factor-1α
drives cancer resistance to cuproptosis. Cancer Cell.
43:937–954.e9. 2025. View Article : Google Scholar
|
|
255
|
Zhang J, Han H, Wang L, Wang W, Yang M and
Qin Y: Overcoming the therapeutic resistance of hepatomas by
targeting the tumor microenvironment. Front Oncol. 12:9889562022.
View Article : Google Scholar : PubMed/NCBI
|
|
256
|
Chen L, Ma S, Wu H, Zheng L, Yi Y, Liu G,
Li B, Sun J, Du Y, Wang B, et al: Zonated copper-driven breast
cancer progression countered by a copper-depleting nanoagent for
immune and metabolic reprogramming. Adv Sci (Weinh).
12:e24124342025. View Article : Google Scholar : PubMed/NCBI
|