Cardiovascular and cerebrovascular diseases (CCVDs),
encompassing ischemic and hemorrhagic conditions affecting the
heart and brain, are primarily caused by adverse lifestyle factors
such as smoking, physical inactivity, poor diet, hypertension,
hyperlipidemia and inadequate glycemic control (1-4).
These diseases have become the leading cause of death worldwide
(5,6). Coronary heart disease is the
predominant type of cardiovascular disease (CVD). Additionally,
conditions such as heart failure (HF), myocardial hypertrophy and
myocarditis also fall under the spectrum of CVDs (7). Cerebrovascular diseases include
ischemic stroke (IS) and hemorrhagic stroke (6). In China, the burden of CCVDs is
particularly severe. The 2022 China Cardiovascular Health and
Disease Report (8) indicated
that there were ~330 million patients with CVD, with CCVDs
accounting for two out of every five deaths. The incidence is
rising, particularly among individuals aged 30-50 years (9,10). CCVDs continue to pose a global
health challenge, contributing to high rates of morbidity and
mortality, while placing substantial medical and financial strain
on healthcare systems (9).
Although pharmacological and surgical interventions can improve
vascular conditions, they fall short in promoting tissue
regeneration and restoring function in areas affected by CCVDs
(11). Thus, identifying novel
therapeutic targets is crucial for the effective management of
these diseases.
MicroRNA-155 (miR-155/miRNA-155) is a highly
conserved non-coding single-stranded RNA encoded by the B-cell
integration cluster (BIC) gene located on chromosome 21 (12). As a key member of the miRNA
family, miR-155 regulates post-transcriptional processes by binding
to the 3'-untranslated region (3'-UTR) of target gene mRNAs through
complementary base pairing (12). As a multifunctional regulatory
molecule, miR-155 is involved in a wide range of biological
processes, including immune responses, inflammation, cell
proliferation and apoptosis, vascular homeostasis, and
tumorigenesis (13-15). Dysregulation of miR-155
expression is closely associated with the onset and progression of
various pathological conditions (such as brain injury, pulmonary
fibrosis and fatty liver disease) (16-18).
miR-155 serves a role in the pathogenesis and
progression of CCVDs, including atherosclerosis (AS), myocardial
hypertrophy, myocardial infarction (MI), ischemia-reperfusion
(I/R), HF, stroke and aneurysm (19-24). The mechanisms of action of
miR-155 include the regulation of inflammation, immunity,
angiogenesis and other key processes (25-28). Currently, miR-155 is considered
to be a promising diagnostic marker and therapeutic target for
CCVDs (29-32). The present review explores the
biogenesis, regulation and function of miR-155, summarizes the
molecular mechanisms by which miR-155 influences the progression of
various CCVDs, and examines therapeutic strategies targeting
miR-155, with the aim of contributing to the understanding of CCVD
pathogenesis and advancing precision treatment approaches. Compared
with previous studies, to the best of our knowledge, the present
review is the first to systematically integrate the dual regulatory
roles of miR-155 in both CVDs and cerebrovascular diseases,
elucidating its contradictory functions in processes such as
inflammation and autophagy through distinct target genes [for
example, endothelial nitric oxide synthase (eNOS) and suppressor of
cytokine signaling 1 (SOCS1)]. The present review also compiles
data on miR-155 inhibitors (such as cobomarsen) from the oncology
field, exploring their cross-disciplinary therapeutic potential,
and proposes an innovative biomimetic nanocarrier strategy [such as
vascular cell adhesion molecule-1 (VCAM-1) antibody-modified
exosomes] to address existing technological gaps in prior
research.
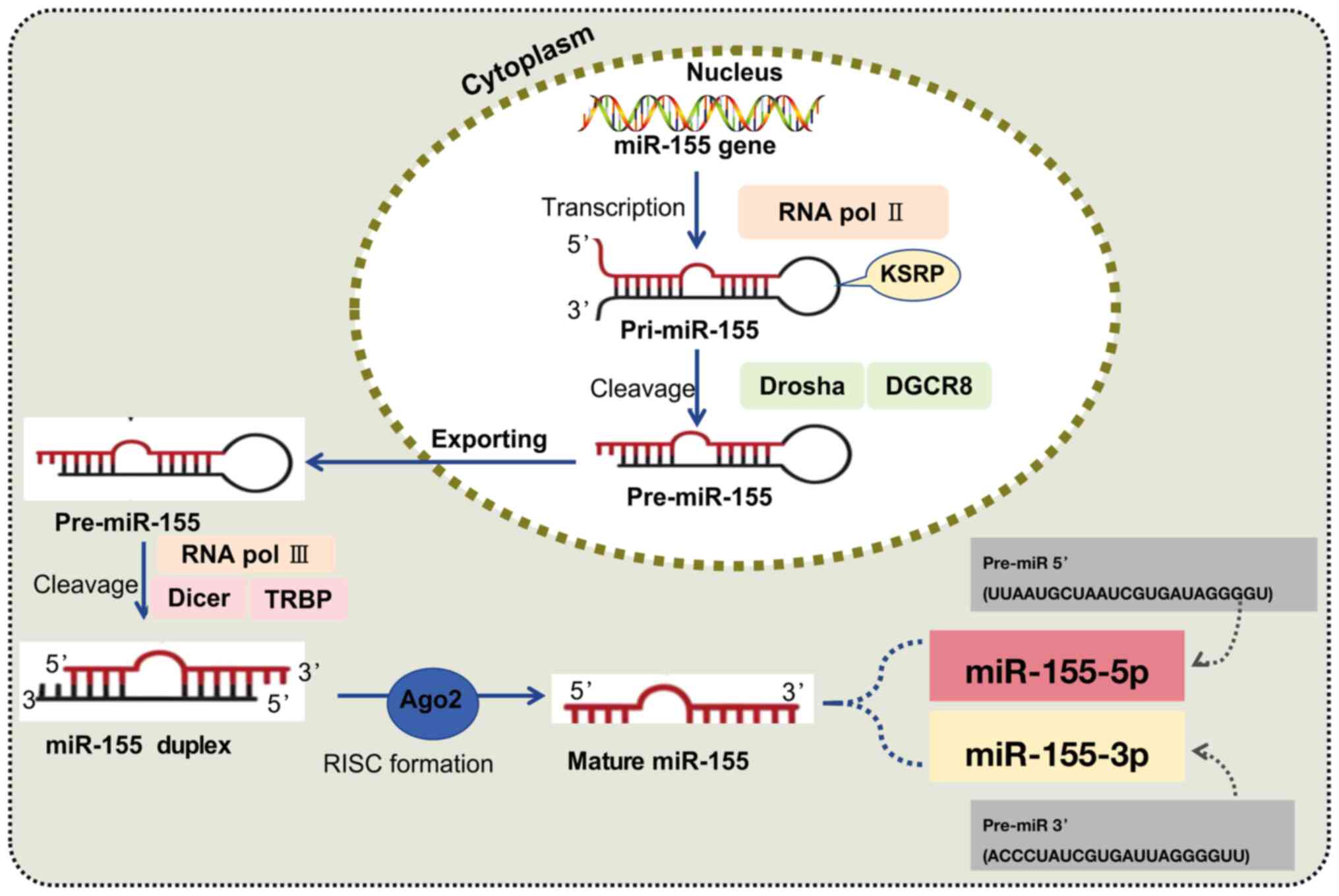
The majority of miRNAs are generated through two
primary steps: First, in the nucleus, the primary transcript
(pri-miRNA) is processed by RNA polymerase II and the
double-stranded RNA-binding protein complex Drosha/DiGeorge
syndrome critical region gene 8 (DGCR8) to produce the miRNA
precursor (pre-miRNA). Second, in the cytoplasm, the pre-miRNA is
further processed by the type III RNA nuclease and the
transactivation-responsive RNA-binding protein complex Dicer/TAR
RNA-binding protein to generate the mature miRNA form (33). The human miR-155 gene is located
within the third exon of the BIC gene on chromosome 21. The mature
sequence of miR-155 is highly conserved across evolution,
specifically targeting the 3'-UTR of target gene mRNAs through its
seed sequence (comprising nucleotides 2-8 at the 5' end), thereby
mediating post-transcriptional gene silencing or mRNA degradation
(34). Argonaute protein 2 binds
to the miR-155 double-stranded complex, forming the core of the
RNA-induced silencing complex and producing single-stranded DNA
molecules (34). The precursor
miRNA hairpin has two arms that can generate biologically active
mature miRNAs. The miRNA derived from the 5' arm is referred to as
miR-155-5p, while that from the 3' arm is referred to as
miR-155-3p. Notably, miR-155-5p exhibits higher biological activity
(34) (Fig. 1). Additionally, miR-155 is
expressed not only in hematopoietic cells but also in a wide range
of tissues, including reproductive tissues, fibroblasts, epithelial
tissues and the central nervous system (35-37).
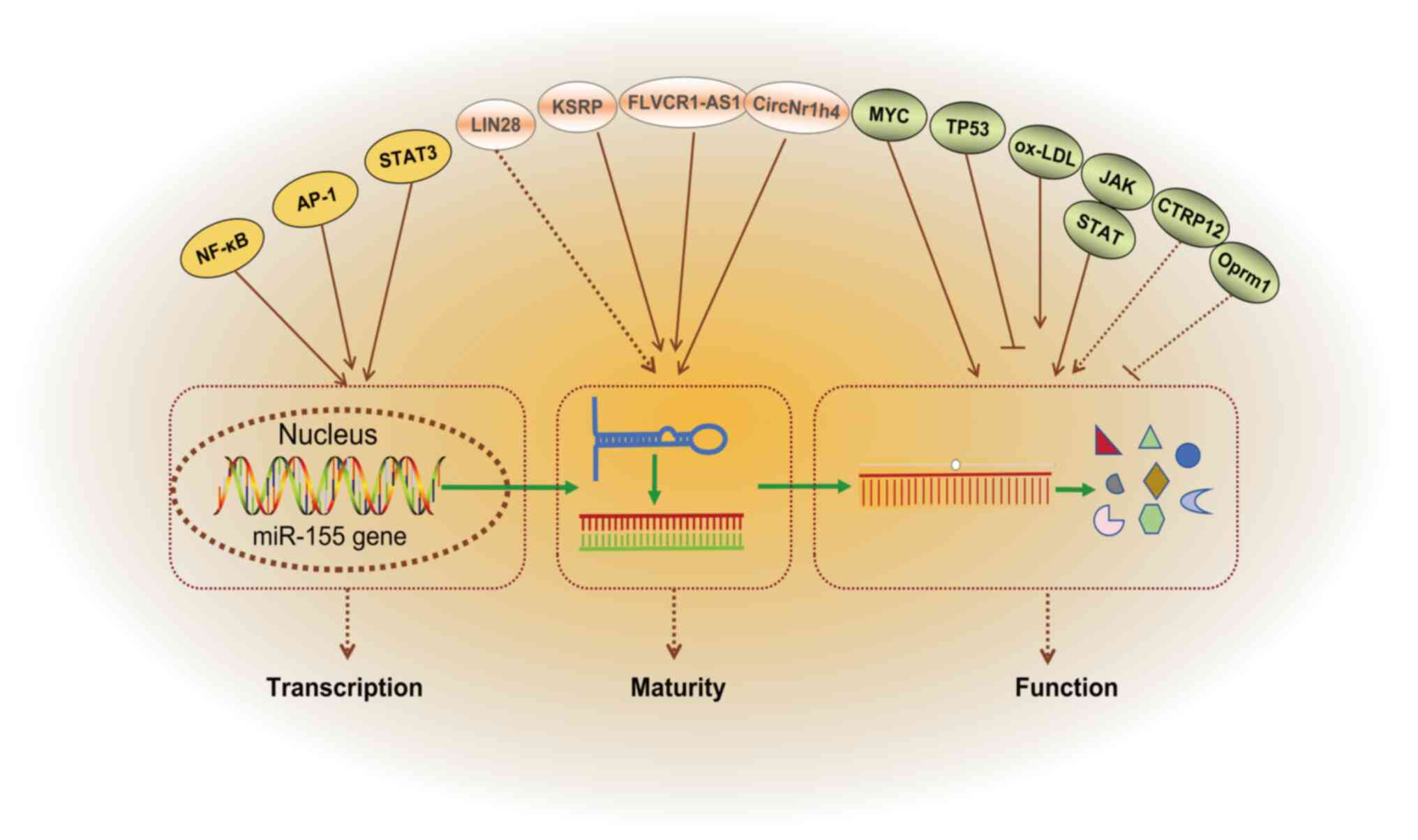
miR-155 is a highly conserved miRNA, and its
expression is tightly regulated at multiple levels, including
transcriptional regulation, post-transcriptional modifications and
epigenetic control (38-40) (Fig. 2). The physiological expression of
miR-155 is essential for immune homeostasis, while pathological
dysregulation contributes to the progression of various diseases,
including AS, stroke and hyperlipidemia (41-43).
The transcription of miR-155 primarily relies on the
synergistic action of cis-regulatory sequences and trans-regulatory
elements in the promoter region (12,44). Activation of toll like receptors
(TLRs) or cytokines (such as TNF-α and IFN-γ) within inflammatory
signaling pathways induces miR-155 expression by activating
transcription factors such as NF-κB and adaptor protein complex-1
(AP-1), which directly bind to the miR-155 promoter region
(38,39). In B and T cells, antigen receptor
(B cell receptor/T cell receptor) signaling activates downstream
transcription factors (such as STAT3) through the PI3K/AKT or MAPK
pathways, promoting miR-155 expression (36,45). Additionally, the transcriptional
activity of the BIC gene can be dynamically regulated by DNA
methylation and histone acetylation. For example, reduced
methylation of the miR-155 promoter region in tumors is closely
associated with its upregulation (46).
The maturation of miR-155 involves the cleavage of
pri-miR-155 to pre-miR-155 and cytoplasmic transport of
pre-miR-155, a process regulated by RNA binding proteins such as
KH-type splicing regulatory protein (KSRP). KSRP binds to the
stem-loop structure of pri-miR-155 and recruits the Drosha-DGCR8
complex to promote the processing of pri-miR-155 into pre-miR-155
(37). Lineage protein 28
(LIN28) family proteins (LIN28A/B), as RNA-binding proteins, can
selectively block the maturation of miRNAs such as let-7 by
inhibiting the processing of pri-miRNAs by Drosha/Dicer complexes
(47). We hypothesized that
LIN28 may regulate miR-155 through a similar mechanism, although
further research is needed to confirm this. Additionally,
competitive endogenous RNAs (ceRNAs) can indirectly modulate the
functional activity of miR-155 by sequestering it via the 'sponge
effect'. For example, long non-coding RNAs (lncRNAs) such as feline
leukemia virus subgroup C receptor 1-antisense RNA 1 can bind to
miR-155, thereby reducing its inhibition of target genes (40). Lu et al (48) identified that circNr1h4 acted as
a ceRNA interacting with miR-155-5p, subsequently influencing the
pathological progression of renal damage in salt-sensitive
hypertensive mouse models.
In disease environments, the expression of miR-155
is often dysregulated due to imbalanced regulatory networks
(49). Specifically, the
activation of cancer-related genes (such as MYC) or suppression of
tumor-suppressor genes (such as TP53) within the tumor
microenvironment can trigger persistently elevated levels of
miR-155, enhancing cellular proliferation and metastasis (50-52). For instance, overexpression of
the BIC gene increases miR-155 levels in diffuse large B-cell
lymphoma (53). During chronic
inflammatory conditions, sustained inflammatory stimuli [such as
high levels of oxidized low-density lipoprotein (ox-LDL) in AS]
maintain miR-155 expression, promoting eNOS production and
exacerbating tissue damage through continuous activation of the
NF-κB signaling pathway (54).
Furthermore, in patients with rheumatoid arthritis, synovial
fibroblasts aberrantly activate miR-155 via the Janus kinase
(JAK)-STAT pathway, leading to the release of inflammatory
mediators, including IL-6 and TNF-α (55). Wang et al (56) demonstrated that C1q tumor
necrosis factor related protein 12 (CTRP12) could mitigate AS by
enhancing reverse cholesterol transport and reducing vasculitis
through the miR-155-5p/liver X receptor α (LXRα) pathway. However,
it remains unclear whether CTRP12 directly regulates miR-155-5p
(56). A study (57) utilizing cerebral I/R injury (IRI)
mouse models has demonstrated that upregulated expression levels of
lncRNA opioid receptor Mu 1 (Oprm1) alleviated cellular apoptosis
induced by cerebral IRI via the Oprm1/miR-155/GATA binding protein
3 (GATA3) pathway. However, the direct regulatory relationship
between Oprm1 and miR-155 remains undetermined (57).
miR-155 is extensively involved in physiological and
pathological processes, including immune regulation, inflammatory
responses, tumorigenesis and metabolic homeostasis, by targeting
the expression of downstream genes (36,38,53,54). miR-155 contributes to the
development of CCVDs by regulating the expression of multiple
molecules, some of which are shown in Fig. 3. miR-155 serves a critical role
in both adaptive and innate immunity. One study has shown that in T
cells, miR-155 targets and inhibits the protein expression of
SOCS1, enhances STAT5 signaling, promotes the differentiation of T
helper type 1 (Th1) and T helper type 17 (Th17) cells, and sustains
the anti-infective immune response (36). In the regulation of inflammation,
miR-155 is the only miRNA upregulated by macrophages in response to
various inflammatory stimuli, including virus-related signals [such
as the poly(I) synthetic analog of double-stranded RNA and
interferons IFN-β/γ] and bacteria-related stimuli [for example, TLR
ligands such as lipopolysaccharide, CpG DNA and Pam3Cys-Ser-(Lys)
4]. These stimuli activate miR-155 expression via myeloid
differentiation primary response 88 (MYD88)- or Toll/IL-1 receptor
domain-containing adaptor-inducing interferon-dependent TLR
signaling pathways, while IFN-β/γ indirectly induces miR-155
expression through TNF-α autocrine signaling, involving TNFR1.
miR-155 integrates the TLR, IFN and TNF-α signaling networks,
activating transcription through the JNK/AP-1 pathway (38). miR-155 exhibits notable
carcinogenic properties within the tumor microenvironment. The
overexpression of miR-155 promotes tumor cell proliferation,
invasion and angiogenesis by targeting and inhibiting tumor
suppressor genes, such as tumor protein p53 inducible nuclear
protein 1 (50,51) and SH2-containing inositol
5'-phosphatase 1 (58,59). miR-155 may also exert anticancer
effects in certain solid tumors (for example, breast cancer, liver
cancer and lymphoma) by inhibiting TGF-β signaling (60-63), highlighting its functional
complexity.
As a non-coding RNA molecule, miR-155 serves as a
multidimensional regulator in the pathological processes of CCVDs.
miR-155 participates in key mechanisms such as inflammation,
endothelial dysfunction, oxidative stress and apoptosis by
targeting the expression of downstream genes (64-68). This section will delve into the
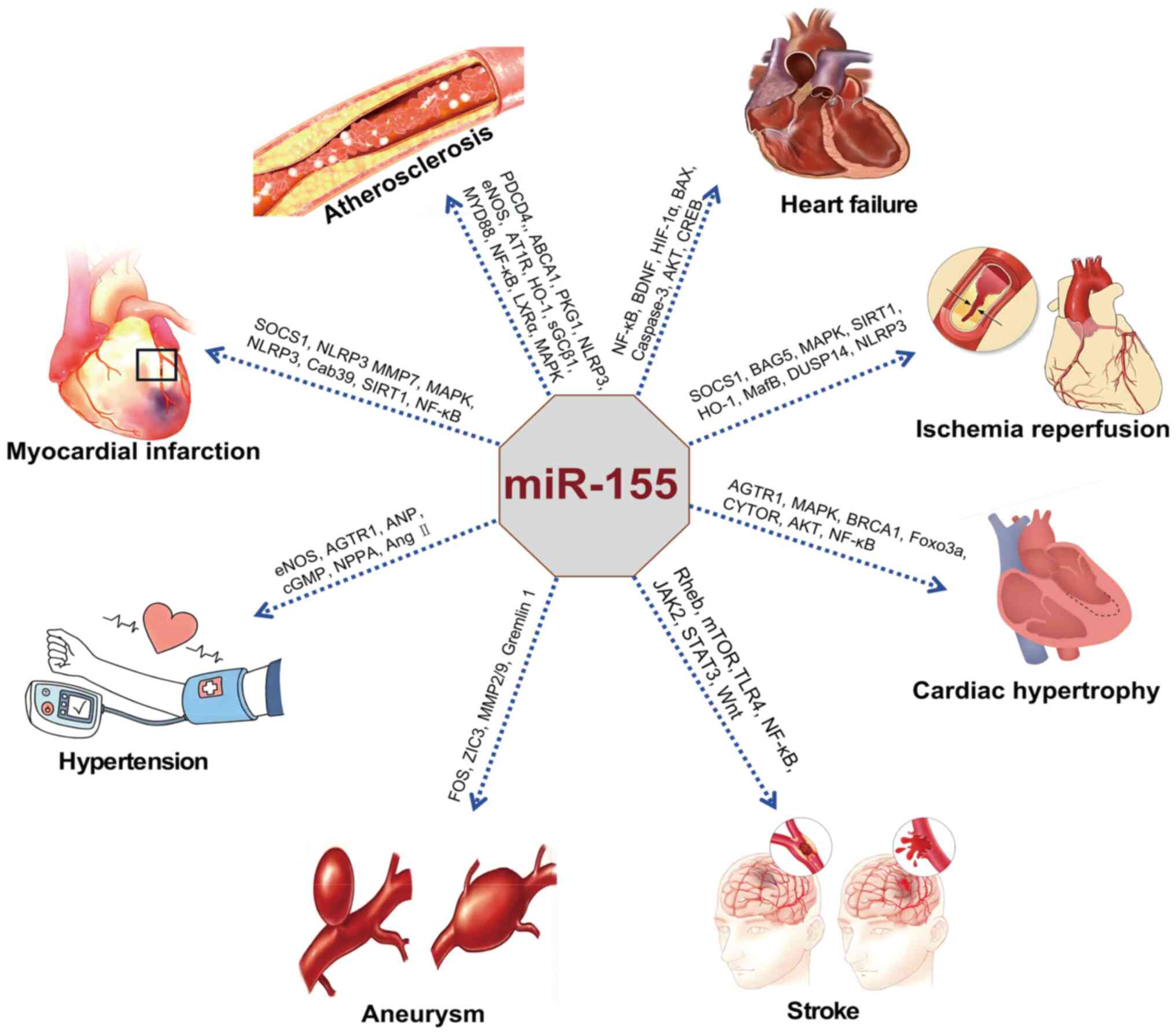
specific molecular mechanisms through which miR-155 influences the
progression of CCVDs, including AS, HF, MI, hypertension, IRI,
stroke and aneurysm (Tables
I-III).
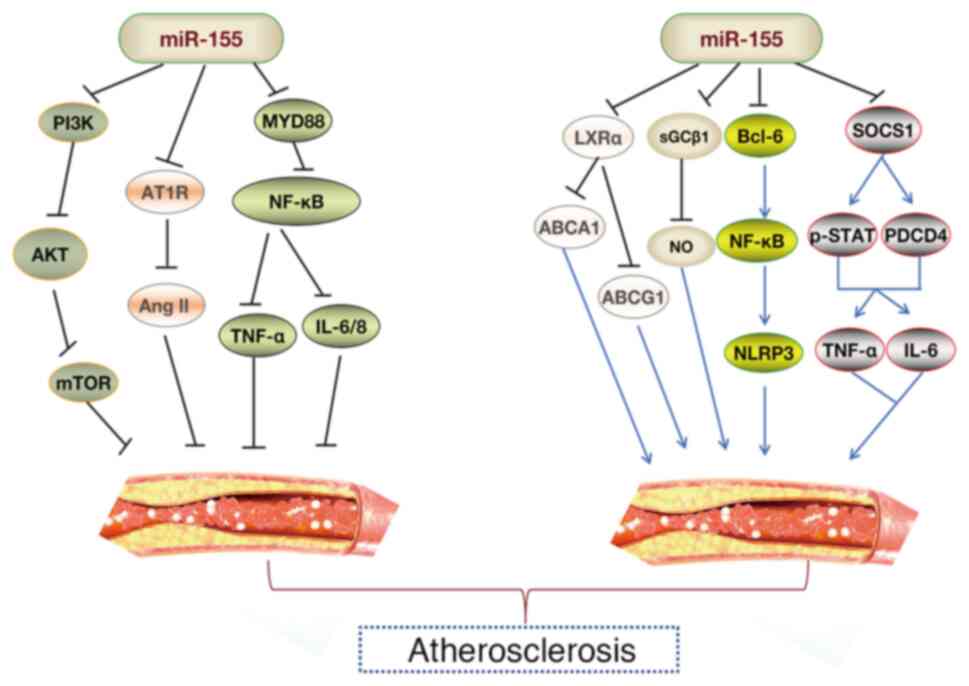
AS represents a pathological condition characterized
by inflammatory responses and lipid accumulation, serving a role in
the progression of CCVDs (69).
The pathogenesis of AS involves a prolonged immunological
inflammatory process, initiated by various pro-inflammatory
mediators interacting with multiple cell types, including
endothelial cells (ECs), vascular smooth muscle cells (VSMCs) and
monocytes/macrophages (25,54,70). miR-155, a versatile miRNA, is
highly expressed in atherosclerotic plaques of both murine and
human subjects (71), emerging
as a critical molecular factor in controlling the initiation and
progression of AS (Fig. 4).
ECs serve as the interface between the blood vessel
wall and the bloodstream, facilitating the exchange of oxygen and
nutrients between the blood and tissues (72). ECs serve a pivotal role in
inflammation responses, thrombosis regulation and vascular tone
modulation, directly influencing CVD progression (72). eNOS generates nitric oxide (NO),
a crucial molecule for maintaining cardiovascular homeostasis.
Aberrant eNOS expression is often linked to endothelial dysfunction
and CVD (73,74). Sun et al (75) demonstrated that miR-155 directly
targeted eNOS, where elevated miR-155 levels reduced eNOS
expression and NO production in HUVECs, impairing
acetylcholine-mediated endothelium-dependent vasodilation in human
mammary arteries. miR-155 is co-expressed with the angiotensin II
type I receptor (AT1R) in ECs and VSMCs. The molecular mechanism
involves miR-155 binding specifically to the 3'-UTR of AT1R mRNA,
inhibiting its translation, and thus, mitigating the pathological
effects of angiotensin II (Ang II) on HUVECs (76). This finding highlights the
pivotal role of the miR-155-AT1R axis in vascular function
regulation. EC apoptosis is a form of endothelial injury and is
closely associated with the development of AS (73,74). Lee et al (77) demonstrated that G protein subunit
α12 protected HUVECs from serum withdrawal-induced apoptosis by
maintaining miR-155 expression. Additionally, another study
demonstrated that overexpression of miR-155 inhibited palmitic
acid-induced apoptosis, reactive oxygen species (ROS) production
and inflammatory cytokine release in HUVECs by suppressing the Wnt
signaling pathway (78).
Endothelial autophagy, as a cytoprotective mechanism, maintains
endothelial homeostasis by clearing damaged organelles and
misfolded proteins, serving a critical role in preventing the onset
and progression of AS (79,80). Under physiological conditions,
ECs utilize autophagy to remove ox-LDL and ROS, thereby preventing
lipid accumulation and inflammatory cytokine release (81). In HUVECs, ox-LDL induces
autophagy and elevates miR-155 expression. Increased miR-155 levels
promote autophagy, while reduced miR-155 expression suppresses
autophagic activity in vascular ECs (82). Further research by Yin et
al (83) indicated that
elevated miR-155 levels enhanced ox-LDL-mediated autophagy in
HUVECs by inhibiting the PI3K/AKT/mTOR signaling pathway. Metabolic
abnormalities and genetic variations in homocysteine (Hcy)
metabolism lead to hyperhomocysteinemia and endothelial
dysfunction, which are both hallmark features of AS and key
contributors to CVD (84).
Research by Witucki and Jakubowski (85) demonstrated that metabolites of
Hcy elevated the expression levels of miR-21, miR-155, miR-216 and
miR-320c, which in turn reduced autophagy in human ECs, a process
critical for maintaining vascular homeostasis. The
stress-responsive enzyme heme oxygenase-1 (HO-1) protects cells
under stress conditions and promotes endothelial anti-inflammatory
and vasodilatory responses (86). Pulkkinen et al (87) showed that miR-155 exerted a
protective effect on endothelial inflammation by reducing the
translation of BTB domain and CNC homolog 1 (BACH1), which induced
HO-1 expression in ECs. Another study demonstrated that both
Abelmoschus esculentus and metformin ameliorated endothelial
inflammation induced by a high-fat diet in rats through enhanced
miR-155 expression, primarily by inhibiting TNF receptor associated
factor 6 and NF-κB p65 activation (88). Yang et al (89) found that miR-155 not only
regulated hypoxia-inducible factor 1α (HIF-1α) expression under
hypoxic conditions but also enhanced the angiogenic potential of
ECs by targeting E2F transcription factor 2. Promoting angiogenesis
is a key feature of healthy ECs.
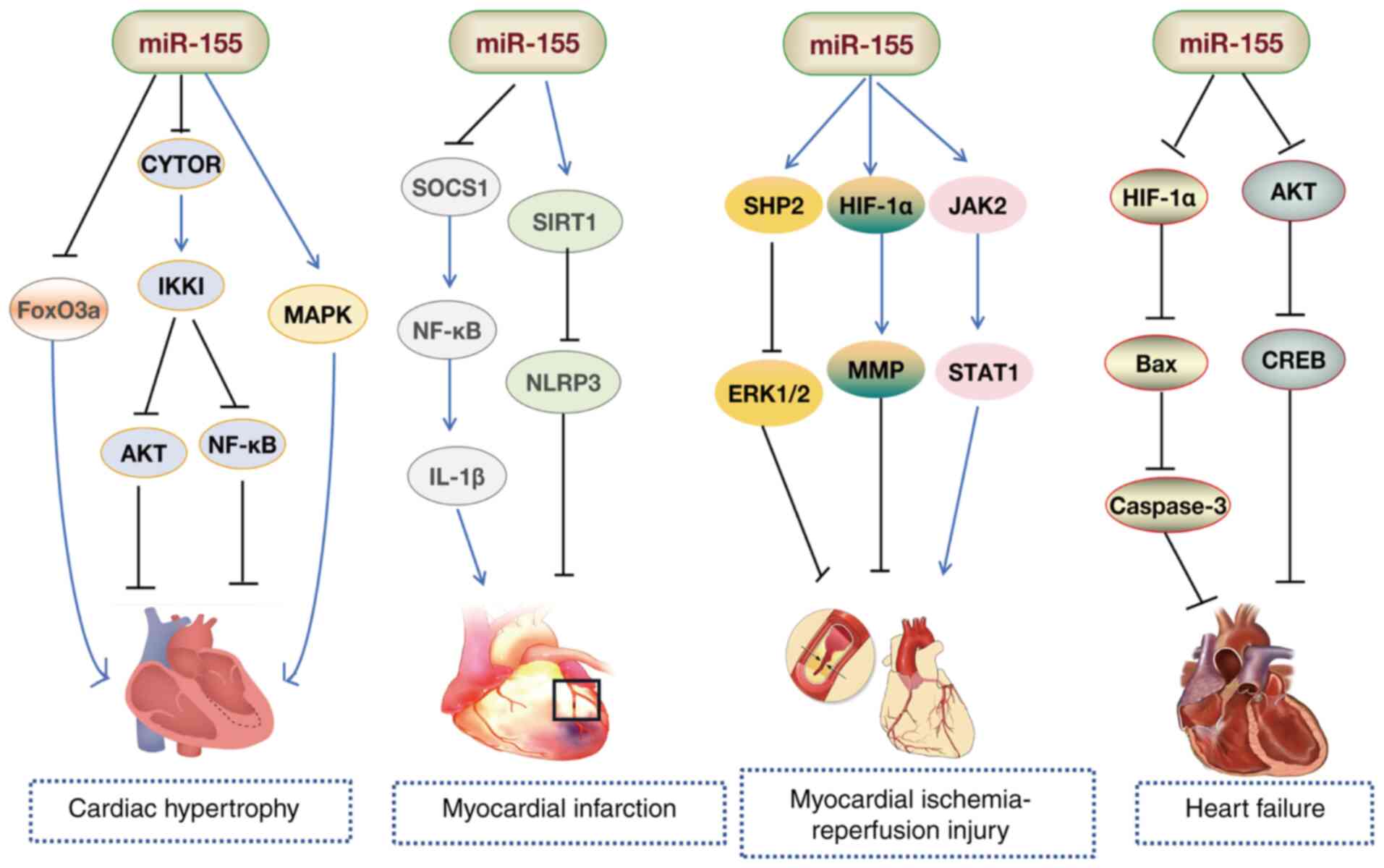
miR-155 is a critical regulator involved in cardiac
inflammation and hypertrophic responses (103-106). Bao and Lin (107) observed a marked increase in
miR-155 levels in the myocardial tissue of mice with Coxsackievirus
B3-induced myocarditis. Functional experiments in vitro
showed that miR-155 mitigated myocardial damage by inhibiting the
NF-κB pathway (107).
Macrophage infiltration is a hallmark of viral myocarditis, and
Zhang et al (108) found
that silencing of miR-155 reduced viral myocarditis-induced cardiac
damage and dysfunction by promoting macrophage M2 phenotype
polarization. Additionally, inhibition of miR-155 improved
experimental autoimmune myocarditis in mice by enhancing the
Th17/regulatory T cell (Treg) immune response (109). These findings suggest that
miR-155 suppression could serve as an effective treatment for
autoimmune myocarditis. Zhang et al (110) demonstrated that Astragalus
mongholicus (Fisch.) Bge alleviated immune imbalances in
peripheral Tregs in children with viral myocarditis by reducing
miR-155 levels. Collectively, these studies (107-109) position miR-155 as a promising
diagnostic biomarker and potential therapeutic target for
cardiomyopathy.
Research has also indicated a decrease in miR-155
levels in animal and patient models of myocardial hypertrophy
(111-113). Overexpression of miR-155
induces hypertrophy in H9C2 cardiomyocytes in vitro
(114). Conversely, inhibition
of miR-155 alleviates myocardial hypertrophy in H9C2 rat
cardiomyocytes by downregulating angiotensin II receptor subtype 1
(AGTR1) and inhibiting the calcium signaling pathway activated by
AGTR1 (115). Seok et al
(116) found that the absence
of miR-155 protected the heart from pathological cardiac
hypertrophy, primarily by reducing jumonji and AT-rich interaction
domain containing 2 expression. Another study revealed that EVs
derived from hypertrophic cardiomyocytes activated the
miR-155-mediated MAPK (ERK, JNK and p38) pathway, inducing
macrophage inflammation and exacerbating myocardial cell damage
(117). Studies have
demonstrated that miR-155-overexpressing macrophages directly
targeted and suppressed FoxO3a expression in cardiomyocytes of
uremic mice via EVs, leading to reduced FoxO3a levels, which
promoted cardiomyocyte pyroptosis, and exacerbated hypertrophy and
fibrosis (118,119). Additionally, Fan et al
(120) demonstrated that
resveratrol, a polyphenol compound, alleviated cardiac hypertrophy
and improved cardiac function by activating BRCA1 in
cardiomyocytes. This mechanism involved BRCA1 activation, which
suppressed miR-155 expression, thereby enhancing FoxO3a expression
and reducing cardiac hypertrophy (120). Furthermore, Yuan et al
(121) demonstrated that
cytoskeleton regulator RNA (CYTOR) deletion decreased IKKI protein
expression, while IKKI deficiency triggered cardiac hypertrophy via
the AKT and NF-κB pathways. miR-155 suppression partially
attenuated the effects of CYTOR. The authors proposed that CYTOR
functions as a ceRNA for miR-155, counteracting miR-155-mediated
inhibitor of nuclear factor κB kinase subunit ε suppression, thus
offering protection against cardiac hypertrophy (121). These studies highlight the
critical role of miR-155 in cardiac hypertrophy (117-121) (Fig. 5).
MI is an acute cardiovascular event resulting from
the rapid reduction or interruption of coronary blood flow, leading
to ischemia, hypoxia and necrosis of myocardial cells. The primary
cause is coronary atherosclerotic plaque rupture or thrombosis
(122). Plaque rupture often
results from an imbalance between macrophage-mediated degradation
and fibroblast repair functions (123). Wang et al (124) found that upregulated miR-155
was predominantly present in macrophages and fibroblasts in the
damaged heart, while pri-miR-155 was exclusively expressed in
macrophages. Mice deficient in miR-155 exhibited increased
proliferation of cardiac fibroblasts and collagen production, along
with reduced inflammation in the damaged heart (124). Notably, suppression of miR-155
reduces nerve growth factor expression by decreasing the phagocytic
activity of M1 macrophages and the inflammation mediated by the
SOCS1/NF-κB pathway, thereby mitigating sympathetic remodeling and
ventricular arrhythmias induced by MI in mice (125,126). Pro-inflammatory
macrophage-mediated degradation of connexin 43 (Cx43) serves a
pivotal role in arrhythmia following MI (127). A study has demonstrated that
miR-155 inhibited the downregulation of macrophage-mediated IL-1β
and MMP7 expression via the SOCS1/NF-κB pathway, reducing Cx43
degradation after MI (128).
Aging impairs the function of human mesenchymal stem
cells (MSCs), reducing their therapeutic potential in MI (129). Hong et al (130) showed that inhibition of
miR-155-5p suppressed MSC aging through the Cab39/AMPK signaling
pathway. This suggests that miR-155-5p could be a novel target for
restoring the vitality of bone marrow MSCs and enhancing their
cardioprotective effects (130). Guo et al (21) also found that miR-155 levels
dynamically increased in the hearts of mice with MI and in neonatal
rat ventricular myocytes injured by hydrogen peroxide
(H2O2). Downregulation of miR-155 promoted
apoptosis induced by MI by targeting RNA binding protein Quaking
(21). Hypoxia/reoxygenation
(H/R)-induced cardiomyocyte apoptosis serves a critical role in MI
pathogenesis. Inhibition of miR-155-5p can prevent NLRP3
inflammasome activation by targeting sirtuin 1 (SIRT1), thus
reducing H/R-induced cardiomyocyte pyroptosis (131). lncRNA XIST promotes cardiac
fibroblast proliferation and extracellular matrix (ECM)
accumulation by acting as a sponge for miR-155-5p, thus
facilitating MI formation (132). Another study demonstrated that
in a mouse model of MI with dyslipidemia, the deletion of the
miR-155 gene did not reduce infarct size or chronic HF but
decreased the density of myofibroblasts in ischemic scars (133). An investigation into
miR-155-based therapeutic strategies has revealed that rosuvastatin
potentially reduces cardiovascular events and inflammatory markers
(INF-γ, TNF-α and IL-6) in patients with acute coronary syndrome by
suppressing the miR-155/Src homology 2-containing inositol
5-phosphatase 1 (SHIP-1) signaling cascade (134). Furthermore, combination of
American ginseng with Danshen increased the serum levels of
hepatocyte growth factor and basic fibroblast growth factor in
acute MI rats, enhanced myocardial microvascular density and CD31
levels, and suppressed the miR-155-5p/HIF-1α/VEGF pathway,
promoting angiogenesis, compared with those in the acute myocardial
infarction model group (135).
These findings highlight the potential of miR-155 as a target for
the treatment of MI (Fig.
5).
IRI refers to the pathological phenomenon where
tissue, such as cardiac or brain tissue, experiences restored blood
flow after a period of ischemia, but this restoration exacerbates
cell damage (136). This
process is commonly observed in vascular recanalization therapies
(such as thrombolysis and percutaneous coronary intervention
surgery), cardiac or cerebrovascular surgeries, and organ
transplantation following acute MI or IS (137,138). IRI poses a challenge in the
field of CCVDs, highlighting the urgent need for the identification
of novel therapeutic targets.
miR-155 expression increases in myocardial tissue
following IRI, which is associated with elevated levels of TNF-α,
IL-1β, CD105 and caspase-3, as well as enhanced leukocyte
infiltration (139). Knockout
of miR-155 reduces inflammatory cell recruitment and decreases ROS
production in white blood cells (139). Notably, miR-155 exacerbates the
inflammatory response, leukocyte infiltration and tissue damage in
IRI by regulating SOCS-1-dependent ROS production (139). Chen et al (20) found that inhibition of miR-155
reduced the MI area by specifically regulating HIF-1α, inhibited
IRI-induced cardiomyocyte apoptosis, maintained MMP levels and
alleviated myocardial injury in rats. Furthermore, inhibition of
miR-155 led to targeted regulation of Bcl-2-associated athanogene 5
and MAPK/JNK signaling, reducing myocardial IRI and the size of MI
(140). Another study found
that, in IRI mice, miR-155 was expressed at high levels, while
SIRT1 expression was low. The expression levels of SIRT1, confirmed
to be a target gene of miR-155, were increased following
sevoflurane treatment, which reduced miR-155 levels, improved
cardiac function, reduced infarct size and inhibited myocardial
cell apoptosis (141). Greco
et al (142) observed
that myocardial IRI-induced EVs exhibited pro-inflammatory
features, exacerbating cardiac injury. The specific molecular
mechanism involved these EVs transferring miR-155-5p to
macrophages, thereby enhancing the inflammatory response via
activation of the JAK2/STAT1 pathway (142). This suggests that targeting EVs
could be a potential therapeutic approach for the management of
IRI. An investigation has demonstrated that miR-155 deletion
improved cardiac ultrasound measurements in IRI mice, with
reductions in MI areas, myocardial fibrosis and cellular apoptosis
(including reducing the expression levels of caspase-3, caspase-4
and caspase-11) (143).
Investigation of the underlying mechanism revealed that decreased
miR-155 expression increased SH2 domain-containing protein tyrosine
phosphatase 2 levels and alleviated IRI-induced necroptosis by
suppressing ERK1/2 pathway activation (143). These findings highlight the
potential of miR-155 as a key therapeutic target in the treatment
of myocardial IRI (Fig. 5).
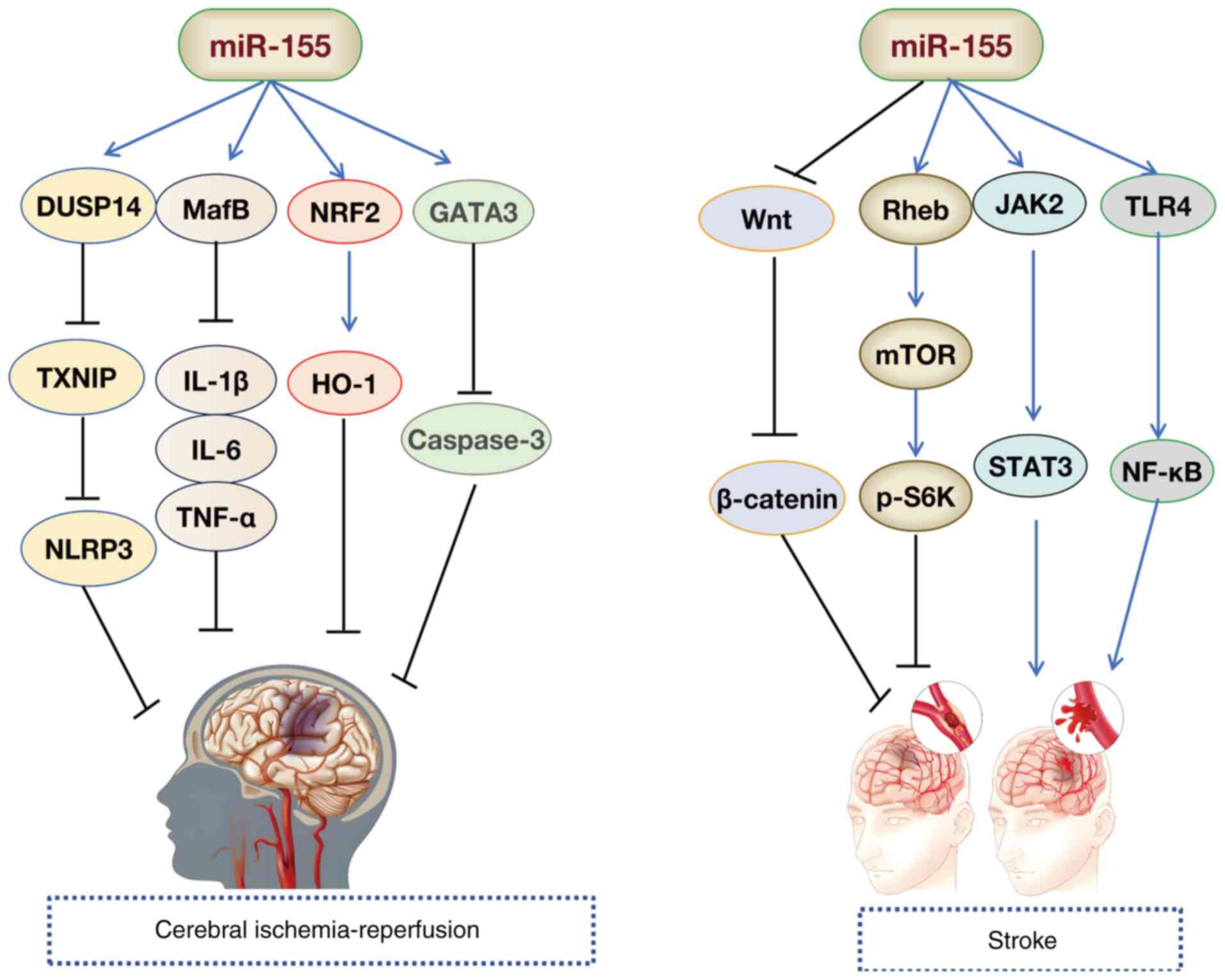
The elimination of the miR-155 gene protects against
brain injury and hemorrhagic transformation induced by I/R
(142). Multiple studies have
corroborated these findings. Jiang et al (144) found that miR-155 deficiency
reduced NO production and eNOS expression by activating the Notch
pathway, thereby alleviating the damage caused by cerebral I/R in
mice with middle cerebral artery occlusion (MCAO). Inhibition of
miR-155 increases cell viability and reduces apoptosis by targeting
the nuclear factor erythroid 2-related factor 2/HO-1 pathway,
preventing neuronal damage induced by cerebral I/R (65). Furthermore, downregulation of
miR-155 mitigates IRI by targeting V-Maf avian musculoaponeurotic
fibrosarcoma oncogene homolog B, improving the neurological
function and inhibiting inflammatory responses [IL-1β, IL-6, TNF-α,
inducible nitric oxide synthase (iNOS) and cyclooxygenase-2]
(145). Another study also
found that in MCAO/reperfusion mouse models and oxygen glucose
deprivation/reoxygenation (OGD/R)-induced SH-SY5Y cells, miR-155-5p
targeted dual specificity phosphatase 14 (DUSP14) by regulating the
NF-κB and MAPK signaling pathways, thereby accelerating cerebral
IRI (146). Suppression of
miR-155-5p reduces cellular apoptosis and cerebral injury (146). Research by Shi et al
(147) revealed that decreased
miR-155-5p expression alleviated cerebral I/R-induced inflammation
and cell death by modulating the DUSP14/thioredoxin interacting
protein/NLRP3 signaling cascade. Notably, increased expression of
lncRNA Oprm1 mitigates apoptosis following cerebral I/R damage via
the Oprm1/miR-155/GATA3 regulatory axis (57). Furthermore, elevated Parkinson's
disease protein 7 expression suppresses miR-155 levels, thereby
enhancing SHP-1 expression and modulating astrocyte activation
during cerebral IRI (148).
These findings suggest potential therapeutic strategies for
managing cerebral IRI (Fig.
6).
HF is a clinical syndrome characterized by a decline
in the pumping function of the heart, leading to an inability to
meet the metabolic needs of the body, with common symptoms
including dyspnea, fatigue and fluid retention (149). The etiology of HF encompasses
conditions such as hypertension, coronary heart disease and
cardiomyopathy (150). The
underlying pathological mechanisms involve myocardial remodeling,
inflammation and fibrosis (151). Li et al (152) utilized bioinformatics and
experimental validation to investigate the potential role of
miR-155 in HF. The authors found that miR-155 may regulate the
viability and apoptosis of H9c2 cardiomyocytes by targeting and
modulating G protein-coupled receptor 18 (152). In the same year, another study
reported that in established rat and cell models of HF,
overexpression of Sirt1 upregulated NF-κB p65 and miR-155,
promoting brain-derived neurotrophic factor (BDNF) expression and
reducing cardiomyocyte apoptosis. The authors proposed that Sirt1
alleviated HF in rats via the NF-κB p65/miR-155/BDNF signaling
cascade (153). Gao et
al (154) found that
schisandrin protected rat cardiomyocytes and prevented congestive
HF by regulating miR-155 expression and mediating the AKT/cAMP
response element-binding protein (CREB) signaling pathway. Another
study revealed that miR-155 expression was downregulated in the
myocardial tissue of mice with HF. Overexpression of miR-155
inhibited myocardial cell apoptosis (via inhibition of Bax and
downstream caspase-3) through HIF-1α, reducing cardiac function
damage in HF mice (155). These
studies (152-154) highlight the critical role of
miR-155 in myocardial protection and suggest its potential for
improving cardiac function through the regulation of specific
molecules (for example, HIF-1α, AKT and CREB) (Fig. 5).
Elevated miR-155 levels in the circulation of
hypertensive patients have been found to be positively associated
with inflammatory markers, indicating their involvement in the
pathological process of hypertension through immune regulation
(68,156). Animal models have confirmed
that miR-155 deficiency alleviates perivascular inflammation and
reduces blood pressure (157).
Yang et al (68)
demonstrated that levamlodipine improved vascular inflammation and
endothelial dysfunction by regulating miR-155 in hypertensive rats,
thereby modulating the receptor activator of nuclear factor
κ-B/receptor activator of nuclear factor κ-B ligand/osteoprotegerin
pathway. Additionally, studies have shown that miR-155 regulates
vascular tone and endothelial-dependent vasodilation by targeting
the eNOS and vascular endothelial growth factor signaling pathways
(75,158). Overexpression of miR-155 can
inhibit eNOS activity, reducing NO production, promoting
endothelial dysfunction and contributing to hypertension
development (75). VSMCs are
essential for maintaining vascular function, and
inflammation-induced VSMC dysfunction can also lead to hypertension
(159). Park et al
(26) found that miR-155,
induced by NF-κB, downregulated sGCβ1 expression, impaired the VSMC
contractile phenotype and disrupted NO induced vasodilation,
leading to VSMC functional damage. Another study revealed that in
the tunica media (VSMCs) of hypertensive rats, miR-155 expression
was elevated. Inhibition of miR-155 reduced systolic and diastolic
blood pressure, increased expression of p27 and α-smooth muscle
actin (α-SMA) in the tunica media, and decreased the thickness of
the tunica media. These findings suggest that miR-155 has
therapeutic potential for hypertension, with its expression levels
being positively associated with vascular wall thickness (160).
Ang II is a key peptide hormone in the
renin-angiotensin system, serving a critical role in regulating
vasoconstriction and renal sodium reabsorption (161). The physiological and
pathophysiological effects of Ang II are primarily mediated through
AT1R (162). Zheng et al
(157) found that
overexpression of miR-155 in cells reduced Ang II-induced α-SMA
expression, suggesting that miR-155 may regulate the
differentiation of rat aortic adventitia fibroblasts and inhibit
AT1R expression. Additionally, research has shown that vascular
mineralocorticoid receptors promote vasoconstriction and increase
blood pressure with age by modulating miR-155 (163). Specifically restoring miR-155
in aged mineralocorticoid receptor-intact mice reduces calcium
voltage-gated channel subunit α1 2 and AgtR1 mRNA levels,
alleviating L-type calcium channel-mediated and Ang II-induced
vasoconstriction and oxidative stress (163). Atrial natriuretic peptide
(ANP), secreted by primary atrial myocytes, lowers blood pressure
by increasing cGMP levels, inducing vasodilation, diuresis and
sodium excretion (164).
Vandenwijngaert et al (165) found that compared with
individual use of miR-425 or miR-155, the combination of miR-425
and miR-155 demonstrated greater suppression of natriuretic peptide
A expression and cGMP production in cardiomyocytes. These studies
showed that promoting the expression of miR-155 may represent an
effective strategy for regulating blood pressure in hypertensive
disorders (163-165).
Stroke is the second leading cause of death
worldwide, and IS is a major subtype (166). miR-155 serves a role in the
progression of IS (167,168).
With ongoing research, the molecular mechanisms of miR-155 in
protecting against IS are becoming clearer (Fig. 6). Xing et al (169) used an in vivo rat model
of MCAO and an in vitro oxygen-glucose deprivation cell
model to simulate IS onset. The authors found that inhibition of
miR-155 could protect against IS by promoting the phosphorylation
of S6K through the Ras homolog enriched in brain/mTOR pathway
(169). Ischemia induces
autophagy via miR-155, contributing to nerve damage (170). Yang et al (170) revealed that miR-155-induced
autophagy altered inflammatory responses and exacerbated ischemic
brain injury by regulating the TLR4/NF-κB pathway in ischemic brain
tissue. Another study indicated that miR-155 worsened cellular
damage in IS by activating the TLR4/MYD88 signaling pathway
(22). The study by Adly Sadik
et al (171)
demonstrated that miR-155 may promote inflammatory responses
post-IS by activating the JAK2/STAT3 axis. A recent study found
that miR-155 inhibited the activation of Wnt/β-catenin signaling,
restored the Th17/Treg balance and prevented acute IS in mice
(172). These findings
highlight that miR-155 is involved in multiple signaling pathways
and targets in IS. Knockdown of miR-155 expression and the use of
specific inhibitors to block miR-155 targets may offer a novel
approach for treating IS by interrupting signaling pathway
transmission. Research has shown that geniposide and ginsenoside
Rg1 protect against focal cerebral ischemia in MCAO model rats by
inhibiting miR-155-5p in microglia following ischemic injury
(173). Additionally,
Opa-interacting protein 5-AS1 inhibits oxidative stress and
inflammation by regulating the miR-155-5p/interferon regulatory
factor 2 binding protein 2 axis, alleviating OGD/R-induced damage
in HMC3 and SH-SY5Y cells, offering a novel targeted therapeutic
molecule for IS treatment (174).
Arterial aneurysms are typically asymptomatic in
their early stages, but as the aneurysm expands, the risk of
rupture increases, which can lead to fatal bleeding (175). Based on anatomical location,
aneurysms are classified into abdominal aortic aneurysms (AAAs),
thoracic aortic aneurysms and intracranial aneurysms (IAs)
(176). miR-155 promotes
macrophage infiltration by upregulating pro-inflammatory factors
such as TNF-α and IL-6, suggesting that miR-155 may contribute to
the inflammatory process during aneurysm development (24,177). Zhang et al (24) found that inhibition of miR-155
prevented AAA formation by regulating macrophage inflammation. The
development of AAA was linked to the proliferation and apoptosis of
VSMCs. Their study also found that overexpression of miR-155
increased the levels of MMP-2, MMP-9, iNOS and monocyte
chemoattractant protein-1 in ApoE-/- mouse models,
stimulating VSMC proliferation and migration. Another study found
that miR-155-5p expression was increased in VSMCs damaged by
H2O2 or NaAsO2. Overexpression of
miR-155-5p inhibited VSMC survival and promoted aneurysm formation
by targeting FOS proto-oncogene (FOS) and Zic family member 3
(ZIC3) (178). These findings
suggest that miR-155 could be a therapeutic target for AAA
treatment. The degradation of the ECM in blood vessels is another
key factor in aneurysm formation. miR-155 enhances the activity of
MMP-2/9, accelerating the degradation of collagen and elastin
(24). Inhibition of miR-155 can
alleviate ECM damage and delay AAA progression (24). Yang et al (178) found that reduced miR-155
expression increased the incidence of IA rupture by upregulating
MMP-2 expression, particularly in subjects with the SNP rs767649
genotype. This SNP in the miR-155 promoter reduces its
transcriptional activity, suggesting a genetic predisposition to
increased aneurysm risk (178).
Additionally, another study found that miR-155-5p, derived from
tumor-associated macrophages, can target IA formation and
macrophage infiltration induced by Gremlin1 (179). These findings highlight
miR-155-5p as a potential therapeutic target for IAs.
miR-155, a key regulatory miRNA, serves a pivotal
role in the onset and progression of CCVDs by influencing
pathological and physiological processes such as inflammation,
oxidative stress and apoptosis (180-182). Research has shown that miR-155
is upregulated in the blood and tissues of patients with CCVDs such
as MI (183) and stroke
(171), indicating its
potential as an effective marker for early diagnosis and prognosis
evaluation.
In a clinical study of 89 patients with
inflammatory cardiomyopathy (iCMP), Obradovic et al
(185) observed elevated plasma
miR-155 levels in patients with iCMP compared with those with
dilated cardiomyopathy, suggesting miR-155 as a novel biomarker for
iCMP diagnosis. Previous studies have indicated a marked increase
in miR-155 expression in both myocardial tissue and blood after MI,
which was strongly associated with inflammatory responses (IL-17A,
IL-6 and TNF-α) and myocardial injury (183,186). Notably, Wang et al
(187) found urinary miR-155
levels to be 30-fold higher in the MI group compared with healthy
individuals, suggesting urinary miR-155 as a potential non-invasive
diagnostic biomarker for MI.
miR-155 holds promise as a biomarker for CCVDs,
serving a pivotal role in their pathogenesis and progression.
Alterations in miR-155 expression are strongly associated with
disease severity and clinical prognosis. Quantitative detection of
miR-155 expression can facilitate earlier identification of disease
risk, providing a foundation for timely clinical intervention and
therapeutic management (171,184,185,189). Therefore, further exploration
of the mechanistic pathways of miR-155, along with the development
of miR-155-based diagnostic tools and therapeutic strategies
targeting miR-155, presents substantial potential for breakthroughs
in the prevention and treatment of these diseases.
In the field of precision medicine, innovative
therapies based on miRNAs are gaining increasing attention, with
miR-155 standing out due to its pivotal role in the pathological
and physiological processes of CVDs (19,60,193). As a critical therapeutic
candidate, miR-155 has garnered interest in biomedical research.
While preclinical investigations of miR-155 extend across various
disciplines beyond CCVDs, the mechanistic insights gained have
considerable translational implications for the advancement of
vascular medicine (13,46,58). Notably, cobomarsen, a synthetic
oligonucleotide inhibitor specifically targeting miR-155, has shown
therapeutic promise in oncology. Its action, validated in
hematologic malignancies, demonstrates particular efficacy in
managing non-Hodgkin lymphoma and cutaneous T-cell lymphoma (in a
xenograft NSG mouse model of the activated B-cell subtype of
diffuse large B-cell lymphoma), with ongoing clinical trials
confirming favorable pharmacodynamic profiles (194,195). These studies indicate that
cobomarsen can inhibit tumor growth and regulate critical signaling
pathways, such as the JAK/STAT, MAPK/ERK and PI3K/AKT pathways, to
exert antitumor effects (194,195). In fibrosis research, local
injection of miR-155 antagonists has been shown to inhibit the
Wnt/β-catenin and AKT signaling pathways, thereby reducing skin
collagen deposition and improving fibrosis (196). Experiments have revealed that
this antagomiR-155 targets the regulation of casein kinase 1α and
SHIP-1, blocking key fibrotic pathways (196). Additionally, systemic delivery
of miR-155-5p inhibitors (antigomiR-155) has been shown to reduce
lipid accumulation in macrophages and reduce atherosclerotic plaque
burden in ApoE-/- mice (197). Another study involving
intravenous injection of miR-155 inhibitors starting 48 h
post-distal MCAO in mice demonstrated a reduction in C-C motif
chemokine ligand 3 and C-C motif chemokine ligand 12 cytokine
expression after 7 days, with notable increases in IL-10, IL-4,
IL-6, macrophage inflammatory protein-1α, IL-5 and IL-17 levels
after 14 days (27). These
findings suggest that miR-155 inhibition in stroke models alters
the temporal progression of cytokine expression, potentially
influencing inflammation and tissue repair following cerebral
ischemia (27). Furthermore,
magnetic resonance imaging examination revealed that the miR-155
inhibitor group exhibited a 34% reduction in infarct volume
compared with the control group after 21 days (14.43% in controls
vs. 9.5% in the inhibitor group (42). These findings highlight the
potential of miRNA-based therapies in targeting key pathogenic
pathways in diseases. miR-155-based therapies, in particular, hold
great promise for the treatment of CVDs. With ongoing research and
technological advancements, this novel approach may offer new
options for the treatment of patients with CVD.
miR-155, a highly conserved non-coding RNA,
regulates target gene expression and serves a critical role in the
pathological processes of various CCVDs, including AS, MI, HF,
hypertension and stroke. miR-155 is involved in mechanisms such as
endothelial dysfunction, inflammation, oxidative stress, apoptosis
and fibrosis. Notably, miR-155 exhibits a dual nature, exerting
both pro-inflammatory and anti-inflammatory effects, depending on
the specific disease context and microenvironment. Additionally,
miR-155 holds promise as a diagnostic biomarker for CCVDs and
represents a potential target for gene therapy. For example,
cobomarsen, an anti-miR-155 oligonucleotide, has shown therapeutic
efficacy in a xenograft NSG mouse model of the activated B-cell
subtype of diffuse large B-cell lymphoma (195).
However, current research on miR-155 faces several
challenges. Firstly, the mechanistic complexity of miR-155 remains
incompletely understood. For instance, in AS, overexpression of
miR-155 inhibits palmitic acid-induced apoptosis, ROS production
and pro-inflammatory cytokine release in HUVECs by suppressing the
Wnt signaling pathway (78),
thereby mitigating the progression of AS. However, studies have
also demonstrated that overexpression of miR-155-5p reduces AKT1
levels and its phosphorylation, thereby inhibiting VSMC
proliferation and migration (92) while decreasing VSMC apoptosis
(71), which conversely promotes
AS development. Across different diseases, miR-155 exhibits
functional complexity. In AS, elevated miR-155 levels enhance
ox-LDL-mediated autophagy in HUVECs by inhibiting the PI3K/AKT/mTOR
signaling pathway (83), thereby
alleviating inflammation during AS progression. Conversely,
researchers have found that overexpression of miR-155-5p promotes
aneurysm formation by targeting FOS and ZIC3 to inhibit VSMC
survival (178). These
discrepancies may stem from the multifunctionality of its upstream
and downstream genes. While multiple mRNAs have been identified as
direct targets of miR-155-5p, these targets could also be regulated
by other miRNAs. Furthermore, alterations in miR-155-5p may induce
expression changes in associated upstream genes, such as lncRNAs
and circular RNAs, potentially contributing to its differential
expression patterns in the same disease. This remains a key area
for future research. Secondly, validation of miR-155 target genes
remains insufficient. Numerous studies have demonstrated that
miR-155 can regulate the expression levels of various molecules
(for example, Oprm1 and CTRP12) (23,57), but have not yet confirmed whether
miR-155 directly targets and modulates their expression, with the
intermediate molecules between them remaining unclear. Thirdly,
animal models, predominantly murine models (such as
ApoE- mice), have limitations due to species-specific
differences from human diseases, and clinical data are still
limited. Fourthly, most clinical studies related to miR-155 have
small sample sizes, which compromises the stability and reliability
of the findings and makes it difficult to comprehensively and
accurately reflect the true role of miR-155 in relevant diseases or
physiological processes. Additionally, small-scale studies may fail
to adequately account for inter-individual variations, such as
variations in age, sex, ethnicity, lifestyle and underlying medical
conditions, which influence miR-155 expression and function,
thereby limiting the generalizability and clinical applicability of
the conclusions. Lastly, therapeutic strategies targeting miR-155
are in the early stages. While cobomarsen, a miR-155 inhibitor,
shows efficacy in oncology, its safety, delivery efficiency and
long-term effects in CCVDs require further validation.
The proposed solutions to address these challenges
primarily include: First, utilizing single-cell sequencing and
spatial transcriptomics to elucidate the cell type-specific
functions of miR-155, complemented by the development of humanized
animal models that better replicate human disease
microenvironments. Second, employing multimodal experimental
validation, such as dual-luciferase assays to confirm direct
binding interactions, followed by RT-qPCR and western blot analyses
of target genes (such as Oprm1 and CTRP12) after miR-155
overexpression or inhibition, with subsequent validation in both
cellular and animal models. Third, implementing multicenter
clinical studies incorporating organoid models to assess
therapeutic efficacy and safety. Finally, phase I/II trials should
prioritize high-risk CCVD populations (for example, patients with
familial hypercholesterolemia) (198), integrating dynamic monitoring
technologies such as fluorescent reporter genes for real-time
tracking of miR-155 activity, with dosage adjustments based on
circulating miR-155 levels.
Systemic regulation targeting miR-155 requires
careful consideration of several key issues. First, off-target
effects present significant risks: miR-155 modulates hundreds of
genes (such as eNOS, SOCS1 and Bcl-6), and systemic inhibition or
overexpression may disrupt physiological functions in non-target
tissues, potentially causing immune dysregulation. As a key
regulator of Th1/Th17 cell differentiation, miR-155 suppression
could compromise anti-infective immunity and increase
susceptibility to infections (172,199). Paradoxically, inhibition of
miR-155 could enhance VSMC proliferation, leading to vascular
stenosis or restenosis and disrupting vascular homeostasis
(200). Second, delivery
systems lack specificity: Current technologies, such as lipid
nanoparticles or viral vectors, struggle to precisely target
diseased tissues, risking hepatorenal toxicity (201,202). Third, dose-dependent toxicity
may arise: miR-155 exhibits nonlinear disease associations;
insufficient levels may impair anti-inflammatory effects (41), while excessive expression could
promote fibrosis [for example, cardiac fibrosis (203)]. Future research should
integrate single-cell sequencing and CRISPR screening to clarify
cell-specific miR-155 functions and develop tissue-targeted
delivery systems (such as exosomal vectors) (92,177,180,204,205). Conventional carriers, such as
lipid or viral vectors, face issues such as hepatic sequestration
(causing hepatotoxicity) and poor vascular barrier penetration (for
example, of the blood-brain barrier) (206,207). Innovative solutions include: i)
Bioinspired nanocarriers, including engineering exosomes to
encapsulate miR-155 inhibitors (such as antagomirs) for degradation
protection, with surface-conjugated VCAM-1 antibodies for precise
binding to inflamed endothelium; ii) biomimetic membrane coatings,
such as macrophage membrane-wrapped nanoparticles [for example,
poly(lactide-co-glycolide)-polyethylene glycol cores loaded with
inhibitors] that leverage innate chemotaxis to migrate toward
lesions (such as plaques and infarcted myocardium); and iii)
preclinical validation in large animal models with dynamic
monitoring (such as fluorescent reporters) for real-time dose
adjustments (208-210).
Despite being in the early stages of development,
miRNA-based therapies demonstrate substantial potential. As of
August 2025, no miRNA drugs have received global approval, although
~100 candidates are actively being investigated across therapeutic
areas, including oncology, rare diseases, CVDs, metabolic diseases
and inflammatory conditions (211-213). Clinical progress remains
measured, with only a few candidates advancing to clinical trials.
Notably, obefazimod (targeting miR-124) for ulcerative colitis
achieved a 16.4% clinical remission rate in phase III trials
(NCT05507216; July 2025), with the ABTECT-1 subgroup reaching a
clinical remission rate of 19.3% (214). This indicates that it is a
highly promising miRNA drug candidate for the future treatment of
ulcerative colitis. In CVDs, CDR132L (a miR-132 inhibitor)
completed a phase II trial (NCT05350969), demonstrating myocardial
functional recovery in HF (215). Other notable candidates include
miravirsen (miR-122 inhibitor for hepatitis C virus; phase II;
NCT01200420) (216), CWT-001
(miR-29a mimic for tendinopathy; phase II; NCT06192927) and
TTX-MC138 (miR-10b inhibitor for breast/pancreatic cancers; phase
I; NCT06260774). However, safety concerns have stalled some
pipelines. For instance, a trial investigating MRX34 (miR-34a
mimic; NCT01829971) was halted due to severe immune-related adverse
events and is currently under reevaluation (217), highlighting the translational
challenges. No miR-155-targeted therapies have currently entered
clinical trials. However, these clinical studies (214-217) strongly support the general
feasibility of miRNA-based therapeutics, providing robust evidence
for miR-155 as a potential therapeutic target. With deepening
insights into miRNA mechanisms, future development of
miR-155-specific therapies may yield breakthroughs in treating
CCVDs.
In conclusion, the integration of preclinical
research with emerging breakthroughs in miRNA therapeutics across
diverse medical fields positions miR-155 as a promising therapeutic
candidate for CCVDs. This strategic approach may pave the way for
novel therapeutic interventions in cerebrovascular disease. The
expanding understanding of the multifunctional regulation of
miR-155 in CCVD pathophysiology has enhanced the prospects for
precision-targeted therapies, potentially driving paradigm shifts
in vascular medicine. As knowledge of the dual regulatory roles of
this miRNA in both vascular homeostasis and disease progression
deepens, rationally designed miR-155 modulators may ultimately
redefine therapeutic standards for complex cerebrovascular
conditions.
Not applicable.
PW contributed to the conception and design, and
critically revised the manuscript. XZ was responsible for
conceptualization, validation and writing the original draft of the
manuscript.
Not applicable.
Not applicable.
The authors declare that they have no competing
interests.
Not applicable.
The present study was supported by the Liaoning Province Science
and Technology Plan Joint Program (grant no. 2024-MSLH-305), the
Basic Research Projects for Higher Education Institutions of
Liaoning Province in 2022 (grant no. LJKMZ20221337), and the Joint
Project of Shenyang Science and Technology Bureau (grant no.
21-174-9-13).
|
1
|
Magalhães JE and Sampaio Rocha-Filho PA:
Migraine and cerebrovascular diseases: Epidemiology,
pathophysiological, and clinical considerations. Headache.
58:1277–1286. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Zanon Zotin MC, Sveikata L, Viswanathan A
and Yilmaz P: Cerebral small vessel disease and vascular cognitive
impairment: From diagnosis to management. Curr Opin Neurol.
34:246–257. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Gottesman RF and Seshadri S: Risk factors,
lifestyle behaviors, and vascular brain health. Stroke. 53:394–403.
2022. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Nordestgaard LT, Christoffersen M and
Frikke-Schmidt R: Shared risk factors between dementia and
atherosclerotic cardiovascular disease. Int J Mol Sci. 23:97772022.
View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Tsao CW, Aday AW, Almarzooq ZI, Anderson
CAM, Arora P, Avery CL, Baker-Smith CM, Beaton AZ, Boehme AK,
Buxton AE, et al: Heart disease and stroke statistics-2023 update:
A report from the American heart association. Circulation.
147:e93–e621. 2023. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Hilkens NA, Casolla B, Leung TW and de
Leeuw FE: Stroke. Lancet. 403:2820–2836. 2024. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Goldsborough E III, Osuji N and Blaha MJ:
Assessment of cardiovascular disease risk: A 2022 update.
Endocrinol Metab Clin North Am. 51:483–509. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Wang Z, Ma L, Liu M, Fan J and Hu S:
Summary of the 2022 report on cardiovascular health and diseases in
China. Chin Med J (Engl). 136:2899–2908. 2023. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Wang H, Zhang H and Zou Z: Changing
profiles of cardiovascular disease and risk factors in China: A
secondary analysis for the Global Burden of Disease Study 2019.
Chin Med J (Engl). 136:2431–2441. 2023. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Wang W, Liu Y, Liu J, Yin P, Wang L, Qi J,
You J, Lin L, Meng S, Wang F, et al: Mortality and years of life
lost of cardiovascular diseases in China, 2005-2020: Empirical
evidence from national mortality surveillance system. Int J
Cardiol. 340:105–112. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Yun CW and Lee SH: Enhancement of
functionality and therapeutic efficacy of Cell-based therapy using
mesenchymal stem cells for cardiovascular disease. Int J Mol Sci.
20:9822019. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Biasiolo M, Sales G, Lionetti M, Agnelli
L, Todoerti K, Bisognin A, Coppe A, Romualdi C, Neri A and
Bortoluzzi S: Impact of host genes and strand selection on miRNA
and miRNA* expression. PLoS One. 6:e238542011. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Due H, Svendsen P, Bødker JS, Schmitz A,
Bøgsted M, Johnsen HE, El-Galaly TC, Roug AS and Dybkær K: miR-155
as a biomarker in B-cell malignancies. Biomed Res Int.
2016:95130372016. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Chen L, Gao D, Shao Z, Zheng Q and Yu Q:
miR-155 indicates the fate of CD4+ T cells. Immunol Lett.
224:40–49. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Yang X, Zeng X, Shu J, Bao H and Liu X:
MiR-155 enhances phagocytosis of alveolar macrophages through the
mTORC2/RhoA pathway. Medicine (Baltimore). 102:e345922023.
View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Tili E, Croce CM and Michaille JJ:
miR-155: On the crosstalk between inflammation and cancer. Int Rev
Immunol. 28:264–284. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Suofu Y, Wang X, He Y, Li F, Zhang Y,
Carlisle DL and Friedlander RM: Mir-155 knockout protects against
Ischemia/reperfusion-induced brain injury and hemorrhagic
transformation. Neuroreport. 31:235–239. 2020. View Article : Google Scholar
|
|
18
|
Wu L, Pu L and Zhuang Z: miR-155-5p/FOXO3a
promotes pulmonary fibrosis in rats by mediating NLRP3 inflammasome
activation. Immunopharmacol Immunotoxicol. 45:257–267. 2023.
View Article : Google Scholar
|
|
19
|
Cao RY, Li Q, Miao Y, Zhang Y, Yuan W, Fan
L, Liu G, Mi Q and Yang J: The emerging role of MicroRNA-155 in
cardiovascular diseases. Biomed Res Int. 2016:98692082016.
View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Chen JG, Xu XM, Ji H and Sun B: Inhibiting
miR-155 protects against myocardial ischemia/reperfusion injury via
targeted regulation of HIF-1α in rats. Iran J Basic Med Sci.
22:1050–1058. 2019.PubMed/NCBI
|
|
21
|
Guo J, Liu HB, Sun C, Yan XQ, Hu J, Yu J,
Yuan Y and Du ZM: MicroRNA-155 promotes myocardial
infarction-induced apoptosis by targeting RNA-Binding protein QKI.
Oxid Med Cell Longev. 2019:45798062019. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Chen W, Wang L and Liu Z: MicroRNA-155
influences cell damage in ischemic stroke via TLR4/MYD88 signaling
pathway. Bioengineered. 12:2449–2458. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Bai B, Ji Z, Wang F, Qin C, Zhou H, Li D
and Wu Y: CTRP12 ameliorates post-myocardial infarction heart
failure through down-regulation of cardiac apoptosis, oxidative
stress and inflammation by influencing the TAK1-p38 MAPK/JNK
pathway. Inflamm Res. 72:1375–1390. 2023. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Zhang Z, Liang K, Zou G, Chen X, Shi S,
Wang G, Zhang K, Li K and Zhai S: Inhibition of miR-155 attenuates
abdominal aortic aneurysm in mice by regulating macrophage-mediated
inflammation. Biosci Rep. 38:BSR201714322018. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Wu XY, Fan WD, Fang R and Wu GF:
Regulation of microRNA-155 in endothelial inflammation by targeting
nuclear factor (NF)-κB P65. J Cell Biochem. 115:1928–1936.
2014.PubMed/NCBI
|
|
26
|
Park M, Choi S, Kim S, Kim J, Lee DK, Park
W, Kim T, Jung J, Hwang JY, Won MH, et al: NF-κB-responsive miR-155
induces functional impairment of vascular smooth muscle cells by
downregulating soluble guanylyl cyclase. Exp Mol Med. 51:1–12.
2019.
|
|
27
|
Pena-Philippides JC, Caballero-Garrido E,
Lordkipanidze T and Roitbak T: In vivo inhibition of miR-155
significantly alters post-stroke inflammatory response. J
Neuroinflammation. 13:2872016. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Wang X, Han W, Zhang Y, Zong Y, Tan N,
Zhang Y, Li L, Liu C and Liu L: Soluble epoxide hydrolase inhibitor
t-AUCB ameliorates vascular endothelial dysfunction by influencing
the NF-κB/miR-155-5p/eNOS/NO/IκB Cycle in hypertensive rats.
Antioxidants (Basel). 11:13722022. View Article : Google Scholar
|
|
29
|
Faccini J, Ruidavets JB, Cordelier P,
Martins F, Maoret JJ, Bongard V, Ferrières J, Roncalli J, Elbaz M
and Vindis C: Circulating miR-155, miR-145 and let-7c as diagnostic
biomarkers of the coronary artery disease. Sci Rep. 7:429162017.
View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Huang YQ, Huang C, Zhang B and Feng YQ:
Association of circulating miR-155 expression level and
inflammatory markers with white coat hypertension. J Hum Hypertens.
34:397–403. 2020. View Article : Google Scholar
|
|
31
|
Zhang H, Chen G, Qiu W, Pan Q, Chen Y,
Chen Y and Ma X: Plasma endothelial microvesicles and their
carrying miRNA-155 serve as biomarkers for ischemic stroke. J
Neurosci Res. 98:2290–2301. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Jin ZQ: MicroRNA targets and biomarker
validation for diabetes-associated cardiac fibrosis. Pharmacol Res.
174:1059412021. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Sun G, Yan J, Noltner K, Feng J, Li H,
Sarkis DA, Sommer SS and Rossi JJ: SNPs in human miRNA genes affect
biogenesis and function. RNA. 15:1640–1651. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Tam W: Identification and characterization
of human BIC, a gene on chromosome 21 that encodes a noncoding RNA.
Gene. 274:157–167. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Li C, He H, Zhu M, Zhao S and Li X:
Molecular characterisation of porcine miR-155 and its regulatory
roles in the TLR3/TLR4 pathways. Dev Comp Immunol. 39:110–116.
2013. View Article : Google Scholar
|
|
36
|
Rodriguez A, Vigorito E, Clare S, Warren
MV, Couttet P, Soond DR, van Dongen S, Grocock RJ, Das PP, Miska
EA, et al: Requirement of bic/microRNA-155 for normal immune
function. Science. 316:608–611. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Ruggiero T, Trabucchi M, De Santa F, Zupo
S, Harfe BD, McManus MT, Rosenfeld MG, Briata P and Gherzi R: LPS
induces KH-type splicing regulatory protein-dependent processing of
microRNA-155 precursors in macrophages. FASEB J. 23:2898–2908.
2009. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
O'Connell RM, Taganov KD, Boldin MP, Cheng
G and Baltimore D: MicroRNA-155 is induced during the macrophage
inflammatory response. Proc Natl Acad Sci USA. 104:1604–1609. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Guo Q, Zhang H, Zhang B, Zhang E and Wu Y:
Tumor necrosis factor-alpha (TNF-α) enhances miR-155-Mediated
endothelial senescence by targeting sirtuin1 (SIRT1). Med Sci
Monit. 25:8820–8835. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Liu Y, Guo G, Zhong Z, Sun L, Liao L, Wang
X, Cao Q and Chen H: Long non-coding RNA FLVCR1-AS1 sponges miR-155
to promote the tumorigenesis of gastric cancer by targeting c-Myc.
Am J Transl Res. 11:793–805. 2019.PubMed/NCBI
|
|
41
|
Huang RS, Hu GQ, Lin B, Lin ZY and Sun CC:
MicroRNA-155 silencing enhances inflammatory response and lipid
uptake in oxidized Low-density Lipoprotein-stimulated human THP-1
macrophages. J Investig Med. 58:961–967. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Caballero-Garrido E, Pena-Philippides JC,
Lordkipanidze T, Bragin D, Yang Y, Erhardt EB and Roitbak T: In
Vivo inhibition of miR-155 promotes recovery after experimental
mouse stroke. J Neurosci. 35:12446–12464. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Bruen R, Fitzsimons S and Belton O:
miR-155 in the resolution of atherosclerosis. Front Pharmacol.
10:4632019. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Uva P, Da Sacco L, Del Cornò M,
Baldassarre A, Sestili P, Orsini M, Palma A, Gessani S and Masotti
A: Rat mir-155 generated from the lncRNA Bic is 'hidden' in the
alternate genomic assembly and reveals the existence of novel
mammalian miRNAs and clusters. RNA. 19:365–379. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Thai TH, Calado DP, Casola S, Ansel KM,
Xiao C, Xue Y, Murphy A, Frendewey D, Valenzuela D, Kutok JL, et
al: Regulation of the germinal center response by microRNA-155.
Science. 316:604–608. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Schliesser MG, Claus R, Hielscher T, Grimm
C, Weichenhan D, Blaes J, Wiestler B, Hau P, Schramm J, Sahm F, et
al: Prognostic relevance of miRNA-155 methylation in anaplastic
glioma. Oncotarget. 7:82028–82045. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Heo I, Joo C, Cho J, Ha M, Han J and Kim
VN: Lin28 mediates the terminal uridylation of let-7 precursor
MicroRNA. Mol Cell. 32:276–284. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
48
|
Lu C, Chen B, Chen C, Li H, Wang D, Tan Y
and Weng H: CircNr1h4 regulates the pathological process of renal
injury in salt-sensitive hypertensive mice by targeting miR-155-5p.
J Cell Mol Med. 24:1700–1712. 2020. View Article : Google Scholar
|
|
49
|
Pasca S, Jurj A, Petrushev B, Tomuleasa C
and Matei D: MicroRNA-155 implication in M1 polarization and the
impact in inflammatory diseases. Front Immunol. 11:6252020.
View Article : Google Scholar : PubMed/NCBI
|
|
50
|
Zhang J, Cheng C, Yuan X, He JT, Pan QH
and Sun FY: microRNA-155 acts as an oncogene by targeting the tumor
protein 53-induced nuclear protein 1 in esophageal squamous cell
carcinoma. Int J Clin Exp Pathol. 7:602–610. 2014.PubMed/NCBI
|
|
51
|
Li Y, Zhang L, Dong Z, Xu H, Yan L, Wang
W, Yang Q and Chen C: MicroRNA-155-5p promotes tumor progression
and contributes to paclitaxel resistance via TP53INP1 in human
breast cancer. Pathol Res Pract. 220:1534052021. View Article : Google Scholar : PubMed/NCBI
|
|
52
|
Xiao L, Li X, Mu Z, Zhou J, Zhou P, Xie C
and Jiang S: FTO inhibition enhances the antitumor effect of
temozolomide by targeting MYC-miR-155/23a Cluster-MXI1 feedback
circuit in glioma. Cancer Res. 80:3945–3958. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
53
|
Costinean S, Zanesi N, Pekarsky Y, Tili E,
Volinia S, Heerema N and Croce CM: Pre-B cell proliferation and
lymphoblastic leukemia/high-grade lymphoma in E(mu)-miR155
transgenic mice. Proc Natl Acad Sci USA. 103:7024–7029. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
54
|
Nazari-Jahantigh M, Wei Y, Noels H, Akhtar
S, Zhou Z, Koenen RR, Heyll K, Gremse F, Kiessling F, Grommes J, et
al: MicroRNA-155 promotes atherosclerosis by repressing Bcl6 in
macrophages. J Clin Invest. 122:4190–4202. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
55
|
Kurowska-Stolarska M, Alivernini S,
Ballantine LE, Asquith DL, Millar NL, Gilchrist DS, Reilly J, Ierna
M, Fraser AR, Stolarski B, et al: MicroRNA-155 as a proinflammatory
regulator in clinical and experimental arthritis. Proc Natl Acad
Sci USA. 108:11193–11198. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
56
|
Wang G, Chen JJ, Deng WY, Ren K, Yin SH
and Yu XH: CTRP12 ameliorates atherosclerosis by promoting
cholesterol efflux and inhibiting inflammatory response via the
miR-155-5p/LXRα pathway. Cell Death Dis. 12:2542021. View Article : Google Scholar
|
|
57
|
Jing H, Liu L, Jia Y, Yao H and Ma F:
Overexpression of the long non-coding RNA Oprm1 alleviates
apoptosis from cerebral Ischemia-reperfusion injury through the
Oprm1/miR-155/GATA3 axis. Artif Cells Nanomed Biotechnol.
47:2431–2439. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
58
|
Xue H, Hua LM, Guo M and Luo JM: SHIP1 is
targeted by miR-155 in acute myeloid leukemia. Oncol Rep.
32:2253–2259. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
59
|
Husain K, Villalobos-Ayala K, Laverde V,
Vazquez OA, Miller B, Kazim S, Blanck G, Hibbs ML, Krystal G,
Elhussin I, et al: Apigenin targets MicroRNA-155, enhances SHIP-1
expression, and augments anti-tumor responses in pancreatic cancer.
Cancers (Basel). 14:36132022. View Article : Google Scholar : PubMed/NCBI
|
|
60
|
Faraoni I, Antonetti FR, Cardone J and
Bonmassar E: miR-155 gene: A typical multifunctional microRNA.
Biochim Biophys Acta. 1792:497–505. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
61
|
Johansson J, Berg T, Kurzejamska E, Pang
MF, Tabor V, Jansson M, Roswall P, Pietras K, Sund M, Religa P, et
al: MiR-155-mediated loss of C/EBPβ shifts the TGF-β response from
growth inhibition to epithelial-mesenchymal transition, invasion
and metastasis in breast cancer. Oncogene. 32:5614–5624. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
62
|
Jiang D and Aguiar RC: MicroRNA-155
controls RB phosphorylation in normal and malignant B lymphocytes
via the noncanonical TGF-β1/SMAD5 signaling module. Blood.
123:86–93. 2014. View Article : Google Scholar :
|
|
63
|
Li DP, Fan J, Wu YJ, Xie YF, Zha JM and
Zhou XM: MiR-155 up-regulated by TGF-β promotes
epithelial-mesenchymal transition, invasion and metastasis of human
hepatocellular carcinoma cells in vitro. Am J Transl Res.
9:2956–2965. 2017.
|
|
64
|
Ke F, Wang H, Geng J, Jing X, Fang F, Fang
C and Zhang BH: MiR-155 promotes inflammation and apoptosis via
targeting SIRT1 in hypoxic-ischemic brain damage. Exp Neurol.
362:1143172023. View Article : Google Scholar : PubMed/NCBI
|
|
65
|
Zhai Y, Liu B, Wu L, Zou M, Mei X and Mo
X: Pachymic acid prevents neuronal cell damage induced by
hypoxia/reoxygenation via miR-155/NRF2/HO-1 axis. Acta Neurobiol
Exp (Wars). 82:197–206. 2022.
|
|
66
|
Zhang W, Wang L, Wang R, Duan Z and Wang
H: A blockade of microRNA-155 signal pathway has a beneficial
effect on neural injury after intracerebral haemorrhage via
reduction in neuroinflammation and oxidative stress. Arch Physiol
Biochem. 128:1235–1241. 2022. View Article : Google Scholar
|
|
67
|
Sun L, Ji S and Xing J: Inhibition of
microRNA-155 alleviates neurological dysfunction following
transient global ischemia and contribution of neuroinflammation and
oxidative stress in the hippocampus. Curr Pharm Des. 25:4310–4317.
2019. View Article : Google Scholar : PubMed/NCBI
|
|
68
|
Yang J, Si D, Zhao Y, He C and Yang P:
S-amlodipine improves endothelial dysfunction via the
RANK/RANKL/OPG system by regulating microRNA-155 in hypertension.
Biomed Pharmacother. 114:1087992019. View Article : Google Scholar : PubMed/NCBI
|
|
69
|
Zhu Y, Xian X, Wang Z, Bi Y, Chen Q, Han
X, Tang D and Chen R: Research progress on the relationship between
atherosclerosis and inflammation. Biomolecules. 8:802018.
View Article : Google Scholar : PubMed/NCBI
|
|
70
|
Zheng B, Yin WN, Suzuki T, Zhang XH, Zhang
Y, Song LL, Jin LS, Zhan H, Zhang H, Li JS and Wen JK:
Exosome-Mediated miR-155 transfer from smooth muscle cells to
endothelial cells induces endothelial injury and promotes
atherosclerosis. Mol Ther. 25:1279–1294. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
71
|
González-López P, Ares-Carral C,
López-Pastor AR, Infante-Menéndez J, González Illaness T, Vega de
Ceniga M, Esparza L, Beneit N, Martín-Ventura JL, Escribano Ó and
Gómez-Hernández A: Implication of miR-155-5p and miR-143-3p in the
vascular insulin resistance and instability of human and
experimental atherosclerotic plaque. Int J Mol Sci. 23:102532022.
View Article : Google Scholar : PubMed/NCBI
|
|
72
|
Gimbrone MA Jr and García-Cardeña G:
Endothelial cell dysfunction and the pathobiology of
atherosclerosis. Circ Res. 118:620–636. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
73
|
Ota H, Eto M, Ogawa S, Iijima K, Akishita
M and Ouchi Y: SIRT1/eNOS axis as a potential target against
vascular senescence, dysfunction and atherosclerosis. J Atheroscler
Thromb. 17:431–435. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
74
|
Hong FF, Liang XY, Liu W, Lv S, He SJ,
Kuang HB and Yang SL: Roles of eNOS in atherosclerosis treatment.
Inflamm Res. 68:429–441. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
75
|
Sun HX, Zeng DY, Li RT, Pang RP, Yang H,
Hu YL, Zhang Q, Jiang Y, Huang LY, Tang YB, et al: Essential role
of microRNA-155 in regulating Endothelium-dependent vasorelaxation
by targeting endothelial nitric oxide synthase. Hypertension.
60:1407–1414. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
76
|
Zhu N, Zhang D, Chen S, Liu X, Lin L,
Huang X, Guo Z, Liu J, Wang Y, Yuan W and Qin Y: Endothelial
enriched microRNAs regulate angiotensin II-induced endothelial
inflammation and migration. Atherosclerosis. 215:286–293. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
77
|
Lee HJ, Lee EJ and Seo M: Galpha12
protects vascular endothelial cells from serum Withdrawal-induced
apoptosis through regulation of miR-155. Yonsei Med J. 57:247–253.
2016. View Article : Google Scholar :
|
|
78
|
Zhao Y, Rao W, Wan Y, Yang X, Wang G, Deng
J, Dai M and Liu Q: Overexpression of microRNA-155 alleviates
palmitate-induced vascular endothelial cell injury in human
umbilical vein endothelial cells by negatively regulating the Wnt
signaling pathway. Mol Med Rep. 20:3527–3534. 2019.PubMed/NCBI
|
|
79
|
Wei DH, Jia XY, Liu YH, Guo FX, Tang ZH,
Li XH, Wang Z, Liu LS, Wang GX, Jian ZS and Ruan CG: Cathepsin L
stimulates autophagy and inhibits apoptosis of ox-LDL-induced
endothelial cells: Potential role in atherosclerosis. Int J Mol
Med. 31:400–406. 2013. View Article : Google Scholar
|
|
80
|
Hu M, Ladowski JM and Xu H: The role of
autophagy in vascular endothelial cell health and physiology.
Cells. 13:8252024. View Article : Google Scholar : PubMed/NCBI
|
|
81
|
Jiang X, Ma C, Gao Y, Cui H, Zheng Y, Li
J, Zong W and Zhang Q: Tongxinluo attenuates atherosclerosis by
inhibiting ROS/NLRP3/caspase-1-mediated endothelial cell
pyroptosis. J Ethnopharmacol. 304:1160112023. View Article : Google Scholar
|
|
82
|
Zhang Z, Pan X, Yang S, Ma A, Wang K, Wang
Y, Li T and Liu S: miR-155 Promotes ox-LDL-Induced autophagy in
human umbilical vein endothelial cells. Mediators Inflamm.
2017:91748012017. View Article : Google Scholar : PubMed/NCBI
|
|
83
|
Yin S, Yang S, Pan X, Ma A, Ma J, Pei H,
Dong Y, Li S, Li W and Bi X: MicroRNA-155 promotes ox-LDL-induced
autophagy in human umbilical vein endothelial cells by targeting
the PI3K/Akt/mTOR pathway. Mol Med Rep. 18:2798–2806.
2018.PubMed/NCBI
|
|
84
|
McCully KS: Homocysteine and the
pathogenesis of atherosclerosis. Expert Rev Clin Pharmacol.
8:211–219. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
85
|
Witucki Ł and Jakubowski H: Homocysteine
metabolites inhibit autophagy by upregulating miR-21-5p,
miR-155-5p, miR-216-5p, and miR-320c-3p in human vascular
endothelial cells. Sci Rep. 14:71512024. View Article : Google Scholar : PubMed/NCBI
|
|
86
|
Alonso-Piñeiro JA, Gonzalez-Rovira A,
Sánchez-Gomar I, Moreno JA and Durán-Ruiz MC: Nrf2 and heme
Oxygenase-1 involvement in atherosclerosis related oxidative
stress. Antioxidants (Basel). 10:14632021. View Article : Google Scholar : PubMed/NCBI
|
|
87
|
Pulkkinen KH, Ylä-Herttuala S and Levonen
AL: Heme oxygenase 1 is induced by miR-155 via reduced BACH1
translation in endothelial cells. Free Radic Biol Med.
51:2124–2131. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
88
|
Gou L, Liu G, Ma R, Regmi A, Zeng T, Zheng
J, Zhong X and Chen L: High fat-induced inflammation in vascular
endothelium can be improved by Abelmoschus esculentus and metformin
via increasing the expressions of miR-146a and miR-155. Nutr Metab
(Lond). 17:352020. View Article : Google Scholar : PubMed/NCBI
|
|
89
|
Yang D, Wang J, Xiao M, Zhou T and Shi X:
Role of Mir-155 in controlling HIF-1α level and promoting
endothelial cell maturation. Sci Rep. 6:353162016. View Article : Google Scholar
|
|
90
|
Frismantiene A, Philippova M, Erne P and
Resink TJ: Smooth muscle cell-driven vascular diseases and
molecular mechanisms of VSMC plasticity. Cell Signal. 52:48–64.
2018. View Article : Google Scholar : PubMed/NCBI
|
|
91
|
Choi S, Park M, Kim J, Park W, Kim S, Lee
DK, Hwang JY, Choe J, Won MH, Ryoo S, et al: TNF-α elicits
phenotypic and functional alterations of vascular smooth muscle
cells by miR-155-5p-dependent down-regulation of cGMP-dependent
kinase 1. J Biol Chem. 293:14812–14822. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
92
|
Chen L, Zheng SY, Yang CQ, Ma BM and Jiang
D: MiR-155-5p inhibits the proliferation and migration of VSMCs and
HUVECs in atherosclerosis by targeting AKT1. Eur Rev Med Pharmacol
Sci. 23:2223–2233. 2019.PubMed/NCBI
|
|
93
|
Tong Y, Zhou MH, Li SP, Zhao HM, Zhang YR,
Chen D, Wu YX and Pang QF: MiR-155-5p attenuates vascular smooth
muscle cell oxidative stress and migration via inhibiting BACH1
expression. Biomedicines. 11:16792023. View Article : Google Scholar : PubMed/NCBI
|
|
94
|
Tang Y, Song H, Shen Y, Yao Y, Yu Y, Wei
G, Long B and Yan W: MiR-155 acts as an inhibitory factor in
atherosclerosis-associated arterial pathogenesis by down-regulating
NoxA1 related signaling pathway in ApoE(-/-) mouse. Cardiovasc
Diagn Ther. 11:1–13. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
95
|
He S, Wu C, Xiao J, Li D, Sun Z and Li M:
Endothelial extracellular vesicles modulate the macrophage
phenotype: Potential implications in atherosclerosis. Scand J
Immunol. 87:e126482018. View Article : Google Scholar : PubMed/NCBI
|
|
96
|
Chistiakov DA, Melnichenko AA, Myasoedova
VA, Grechko AV and Orekhov AN: Mechanisms of foam cell formation in
atherosclerosis. J Mol Med (Berl). 95:1153–1165. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
97
|
Zhang F, Zhao J, Sun D and Wei N: MiR-155
inhibits transformation of macrophages into foam cells via
regulating CEH expression. Biomed Pharmacother. 104:645–651. 2018.
View Article : Google Scholar : PubMed/NCBI
|
|
98
|
Li X, Kong D, Chen H, Liu S, Hu H, Wu T,
Wang J, Chen W, Ning Y, Li Y and Lu Z: miR-155 acts as an
anti-inflammatory factor in atherosclerosis-associated foam cell
formation by repressing calcium-regulated heat stable protein 1.
Sci Rep. 6:217892016. View Article : Google Scholar : PubMed/NCBI
|
|
99
|
Zhu J, Chen T, Yang L, Li Z, Wong MM,
Zheng X, Pan X, Zhang L and Yan H: Regulation of microRNA-155 in
atherosclerotic inflammatory responses by targeting MAP3K10. PLoS
One. 7:e465512012. View Article : Google Scholar : PubMed/NCBI
|
|
100
|
Ye J, Guo R, Shi Y, Qi F, Guo C and Yang
L: miR-155 Regulated inflammation response by the SOCS1-STAT3-PDCD4
axis in atherogenesis. Mediators Inflamm. 2016:80601822016.
View Article : Google Scholar : PubMed/NCBI
|
|
101
|
Zheng J, Wang W, Hong T, Yang S, Shen J
and Liu C: Suppression of microRNA-155 exerts an anti-inflammatory
effect on CD4+ T cell-mediated inflammatory response in the
pathogenesis of atherosclerosis. Acta Biochim Biophys Sin
(Shanghai). 52:654–664. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
102
|
Peng Q, Yin R, Zhu X, Jin L, Wang J, Pan X
and Ma A: miR-155 activates the NLRP3 inflammasome by regulating
the MEK/ERK/NF-κB pathway in carotid atherosclerotic plaques in
ApoE(-/-) mice. J Physiol Biochem. 78:365–375. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
103
|
Zhang Y, Li X, Wang D, Jiang X, Zhang M
and Lv K: Serum exosome microRNA panel as a noninvasive biomarker
for molecular diagnosis of fulminant myocarditis. Mol Ther Methods
Clin Dev. 20:142–151. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
104
|
Oh JH, Kim GB and Seok H: Implication of
microRNA as a potential biomarker of myocarditis. Clin Exp Pediatr.
65:230–238. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
105
|
Lewandowski P, Goławski M, Baron M,
Reichman-Warmusz E and Wojnicz R: A systematic review of miRNA and
cfDNA as potential biomarkers for liquid biopsy in myocarditis and
inflammatory dilated cardiomyopathy. Biomolecules. 12L:14762022.
View Article : Google Scholar
|
|
106
|
Aleshcheva G, Baumeier C, Harms D, Bock
CT, Escher F and Schultheiss HP: MicroRNAs as novel biomarkers and
potential therapeutic options for inflammatory cardiomyopathy. ESC
Heart Fail. 10:3410–3418. 2023. View Article : Google Scholar : PubMed/NCBI
|
|
107
|
Bao JL and Lin L: MiR-155 and miR-148a
reduce cardiac injury by inhibiting NF-κB pathway during acute
viral myocarditis. Eur Rev Med Pharmacol Sci. 18:2349–2356.
2014.PubMed/NCBI
|
|
108
|
Zhang Y, Zhang M, Li X, Tang Z, Wang X,
Zhong M, Suo Q, Zhang Y and Lv K: Silencing MicroRNA-155 attenuates
cardiac injury and dysfunction in viral myocarditis via promotion
of M2 phenotype polarization of macrophages. Sci Rep. 6:226132016.
View Article : Google Scholar : PubMed/NCBI
|
|
109
|
Yan L, Hu F, Yan X, Wei Y, Ma W, Wang Y,
Lu S and Wang Z: Inhibition of microRNA-155 ameliorates
experimental autoimmune myocarditis by modulating Th17/Treg immune
response. J Mol Med (Berl). 94:1063–1079. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
110
|
Zhang Z, Dai X, Qi J, Ao Y, Yang C and Li
Y: Astragalus mongholicus (Fisch.) bge improves peripheral treg
cell immunity imbalance in the children with viral myocarditis by
reducing the levels of miR-146b and miR-155. Front Pediatr.
6:1392018. View Article : Google Scholar : PubMed/NCBI
|
|
111
|
Derda AA, Thum S, Lorenzen JM, Bavendiek
U, Heineke J, Keyser B, Stuhrmann M, Givens RC, Kennel PJ, Schulze
PC, et al: Blood-based microRNA signatures differentiate various
forms of cardiac hypertrophy. Int J Cardiol. 196:115–122. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
112
|
He W, Huang H, Xie Q, Wang Z, Fan Y, Kong
B, Huang D and Xiao Y: MiR-155 knockout in fibroblasts improves
cardiac remodeling by targeting tumor protein p53-Inducible nuclear
protein 1. J Cardiovasc Pharmacol Ther. 21:423–435. 2016.
View Article : Google Scholar
|
|
113
|
Kelly M, Bagnall RD, Peverill RE, Donelan
L, Corben L, Delatycki MB and Semsarian C: A polymorphic miR-155
binding site in AGTR1 is associated with cardiac hypertrophy in
Friedreich ataxia. J Mol Cell Cardiol. 51:848–854. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
114
|
de Oliveira G, Freire PP, Omoto ACM, Cury
SS, Fuziwara CS, Kimura ET, Dal-Pai-Silva M and Carvalho RF:
Osteoglycin post-transcriptional regulation by miR-155 induces
cellular architecture changes in H9c2 cardiomyoblasts. Gene.
676:9–15. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
115
|
Yang Y, Zhou Y, Cao Z, Tong XZ, Xie HQ,
Luo T, Hua XP and Wang HQ: miR-155 functions downstream of
angiotensin II receptor subtype 1 and calcineurin to regulate
cardiac hypertrophy. Exp Ther Med. 12:1556–1562. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
116
|
Seok HY, Chen J, Kataoka M, Huang ZP, Ding
J, Yan J, Hu X and Wang DZ: Loss of MicroRNA-155 protects the heart
from pathological cardiac hypertrophy. Circ Res. 114:1585–1595.
2014. View Article : Google Scholar : PubMed/NCBI
|
|
117
|
Yu H, Qin L, Peng Y, Bai W and Wang Z:
Exosomes derived from hypertrophic cardiomyocytes induce
inflammation in macrophages via miR-155 mediated MAPK Pathway.
Front Immunol. 11:6060452020. View Article : Google Scholar
|
|
118
|
Wang B, Wang ZM, Ji JL, Gan W, Zhang A,
Shi HJ, Wang H, Lv L, Li Z, Tang T, et al: Macrophage-derived
exosomal Mir-155 regulating cardiomyocyte pyroptosis and
hypertrophy in uremic cardiomyopathy. JACC Basic Transl Sci.
5:148–166. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
119
|
Wang Y, Feng T, Duan S, Shi Y, Li S, Zhang
X and Zhang L: miR-155 promotes fibroblast-like synoviocyte
proliferation and inflammatory cytokine secretion in rheumatoid
arthritis by targeting FOXO3a. Exp Ther Med. 19:1288–1296.
2020.PubMed/NCBI
|
|
120
|
Fan Y, Liu L, Fang K, Huang T, Wan L, Liu
Y, Zhang S, Yan D, Li G, Gao Y, et al: Resveratrol Ameliorates
cardiac hypertrophy by Down-regulation of miR-155 through
activation of breast cancer type 1 susceptibility protein. J Am
Heart Assoc. 5:e0026482016. View Article : Google Scholar : PubMed/NCBI
|
|
121
|
Yuan Y, Wang J, Chen Q, Wu Q, Deng W, Zhou
H and Shen D: Long non-coding RNA cytoskeleton regulator RNA
(CYTOR) modulates pathological cardiac hypertrophy through
miR-155-mediated IKKi signaling. Biochim Biophys Acta Mol Basis
Dis. 1865:1421–1427. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
122
|
Murphy A and Goldberg S: Mechanical
complications of myocardial infarction. Am J Med. 135:1401–1409.
2022. View Article : Google Scholar : PubMed/NCBI
|
|
123
|
Bentzon JF, Otsuka F, Virmani R and Falk
E: Mechanisms of plaque formation and rupture. Circ Res.
114:1852–1866. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
124
|
Wang C, Zhang C, Liu L, A X, Chen B, Li Y
and Du J: Macrophage-Derived mir-155-Containing exosomes suppress
fibroblast proliferation and promote fibroblast inflammation during
cardiac injury. Mol Ther. 25:192–204. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
125
|
Hu J, Huang CX, Rao PP, Zhou JP, Wang X,
Tang L, Liu MX and Zhang GG: Inhibition of microRNA-155 attenuates
sympathetic neural remodeling following myocardial infarction via
reducing M1 macrophage polarization and inflammatory responses in
mice. Eur J Pharmacol. 851:122–132. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
126
|
Hu J, Huang CX, Rao PP, Cao GQ, Zhang Y,
Zhou JP, Zhu LY, Liu MX and Zhang GG: MicroRNA-155 inhibition
attenuates endoplasmic reticulum stress-induced cardiomyocyte
apoptosis following myocardial infarction via reducing macrophage
inflammation. Eur J Pharmacol. 857:1724492019. View Article : Google Scholar : PubMed/NCBI
|
|
127
|
Palatinus JA, Valdez S, Taylor L,
Whisenant C, Selzman CH, Drakos SG, Ranjan R, Hong T, Saffitz JE
and Shaw RM: GJA1-20k rescues Cx43 localization and arrhythmias in
arrhythmogenic cardiomyopathy. Circ Res. 132:744–746. 2023.
View Article : Google Scholar : PubMed/NCBI
|
|
128
|
Yang HT, Li LL, Li SN, Wu JT, Chen K, Song
WF, Zhang GB, Ma JF, Fu HX, Cao S, et al: MicroRNA-155 inhibition
attenuates myocardial infarction-induced connexin 43 degradation in
cardiomyocytes by reducing pro-inflammatory macrophage activation.
Cardiovasc Diagn Ther. 12:325–339. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
129
|
Zhu W, Du W, Duan R, Liu Y, Zong B, Jin X,
Dong Z, Wang H, Shahab S, Wang H, et al: miR-873-5p suppression
reinvigorates aging mesenchymal stem cells and improves cardiac
repair after myocardial infarction. ACS Pharmacol Transl Sci.
7:743–756. 2024. View Article : Google Scholar : PubMed/NCBI
|
|
130
|
Hong Y, He H, Jiang G, Zhang H, Tao W,
Ding Y, Yuan D, Liu J, Fan H, Lin F, et al: miR-155-5p inhibition
rejuvenates aged mesenchymal stem cells and enhances
cardioprotection following infarction. Aging Cell. 19:e131282020.
View Article : Google Scholar : PubMed/NCBI
|
|
131
|
Lu Q, Shen Q, Su J, Li X, Xia B and Tang
A: Inhibition of mir-155-5p alleviates cardiomyocyte pyroptosis
induced by hypoxia/reoxygenation via targeting SIRT1-mediated
activation of the NLRP3 inflammasome. J Cardiothorac Surg.
20:1352025. View Article : Google Scholar : PubMed/NCBI
|
|
132
|
Zhang H, Ma J, Liu F and Zhang J: Long
non-coding RNA XIST promotes the proliferation of cardiac
fibroblasts and the accumulation of extracellular matrix by
sponging microRNA-155-5p. Exp Ther Med. 21:4772021. View Article : Google Scholar : PubMed/NCBI
|
|
133
|
Schumacher D, Curaj A, Simsekyilmaz S,
Schober A, Liehn EA and Mause SF: miR155 deficiency reduces
myofibroblast density but fails to improve cardiac function after
myocardial infarction in dyslipidemic mouse model. Int J Mol Sci.
22:54802021. View Article : Google Scholar : PubMed/NCBI
|
|
134
|
Xie W, Li P, Wang Z, Chen J, Lin Z, Liang
X and Mo Y: Rosuvastatin may reduce the incidence of cardiovascular
events in patients with acute coronary syndromes receiving
percutaneous coronary intervention by suppressing miR-155/SHIP-1
signaling pathway. Cardiovasc Ther. 32:276–282. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
135
|
Li X, Liu R, Liu W, Liu X, Fan Z, Cui J,
Wu Y, Yin H and Lin Q: Panax quinquefolium L. and Salvia
miltiorrhiza Bunge. enhances angiogenesis by regulating the
miR-155-5p/HIF-1α/VEGF axis in acute myocardial infarction. Drug
Des Devel Ther. 17:3249–3267. 2023. View Article : Google Scholar :
|
|
136
|
Zhang M, Liu Q, Meng H, Duan H, Liu X, Wu
J, Gao F, Wang S, Tan R and Yuan J: Ischemia-reperfusion injury:
Molecular mechanisms and therapeutic targets. Signal Transduct
Target Ther. 9:122024. View Article : Google Scholar : PubMed/NCBI
|
|
137
|
Zhang Q, Jia M, Wang Y, Wang Q and Wu J:
Cell death mechanisms in cerebral ischemia-reperfusion injury.
Neurochem Res. 47:3525–3542. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
138
|
Liu Y, Li L, Wang Z, Zhang J and Zhou Z:
Myocardial ischemia-reperfusion injury; Molecular mechanisms and
prevention. Microvasc Res. 149:1045652023. View Article : Google Scholar : PubMed/NCBI
|
|
139
|
Eisenhardt SU, Weiss JB, Smolka C,
Maxeiner J, Pankratz F, Bemtgen X, Kustermann M, Thiele JR, Schmidt
Y, Bjoern Stark G, et al: MicroRNA-155 aggravates
ischemia-reperfusion injury by modulation of inflammatory cell
recruitment and the respiratory oxidative burst. Basic Res Cardiol.
110:322015. View Article : Google Scholar : PubMed/NCBI
|
|
140
|
Xi J, Li QQ, Li BQ and Li N: miR-155
inhibition represents a potential valuable regulator in mitigating
myocardial hypoxia/reoxygenation injury through targeting BAG5 and
MAPK/JNK signaling. Mol Med Rep. 21:1011–1020. 2020.PubMed/NCBI
|
|
141
|
Huang G, Hao F and Hu X: Downregulation of
microRNA-155 stimulates sevoflurane-mediated cardioprotection
against myocardial ischemia/reperfusion injury by binding to SIRT1
in mice. J Cell Biochem. 120:15494–15505. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
142
|
Greco R, Demartini C, Zanaboni AM,
Blandini F, Amantea D and Tassorelli C: Endothelial nitric oxide
synthase inhibition triggers inflammatory responses in the brain of
male rats exposed to ischemia-reperfusion injury. J Neurosci Res.
96:151–159. 2018. View Article : Google Scholar
|
|
143
|
Liu M, Fu D, Gao T, Jiang H, Yang P and Li
X: The low expression of miR-155 promotes the expression of SHP2 by
inhibiting the activation of the ERK1/2 pathway and improves cell
pyroptosis induced by I/R in mice. Aging (Albany NY). 16:4778–4788.
2024.PubMed/NCBI
|
|
144
|
Jiang T, Zhou S, Li X, Song J, An T, Huang
X, Ping X and Wang L: MicroRNA-155 induces protection against
cerebral ischemia/reperfusion injury through regulation of the
Notch pathway in vivo. Exp Ther Med. 18:605–613. 2019.PubMed/NCBI
|
|
145
|
Zhang L, Liu C, Huang C, Xu X and Teng J:
miR-155 knockdown protects against cerebral ischemia and
reperfusion injury by targeting MafB. Biomed Res Int.
2020:64582042020. View Article : Google Scholar : PubMed/NCBI
|
|
146
|
Shi Y, Li K, Xu K and Liu QH: MiR-155-5p
accelerates cerebral ischemia-reperfusion injury via targeting
DUSP14 by regulating NF-κB and MAPKs signaling pathways. Eur Rev
Med Pharmacol Sci. 24:1408–1419. 2020.PubMed/NCBI
|
|
147
|
Shi Y, Li Z, Li K and Xu K: miR-155-5p
accelerates cerebral ischemia-reperfusion inflammation injury and
cell pyroptosis via DUSP14/TXNIP/NLRP3 pathway. Acta Biochim Pol.
69:787–793. 2022.PubMed/NCBI
|
|
148
|
Xue Y, Wang Y, Chen T, Peng L, Wang C, Xue
G and Yu S: DJ-1 regulates astrocyte activation through
miR-155/SHP-1 signaling in cerebral ischemia/reperfusion injury. J
Neurochem. 169:e162302025. View Article : Google Scholar
|
|
149
|
Tanai E and Frantz S: Pathophysiology of
heart failure. Compr Physiol. 6:187–214. 2015. View Article : Google Scholar
|
|
150
|
McMurray JJ and Pfeffer MA: Heart failure.
Lancet. 365:1877–1889. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
151
|
Mosterd A and Hoes AW: Clinical
epidemiology of heart failure. Heart. 93:1137–1146. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
152
|
Li J, Su H, Zhu Y, Cao Y and Ma X: ETS2
and microRNA-155 regulate the pathogenesis of heart failure through
targeting and regulating GPR18 expression. Exp Ther Med.
19:3469–3478. 2020.PubMed/NCBI
|
|
153
|
Lin B, Zhao H, Li L, Zhang Z, Jiang N,
Yang X, Zhang T, Lian B, Liu Y, Zhang C, et al: Sirt1 improves
heart failure through modulating the NF-κB p65/microRNA-155/BNDF
signaling cascade. Aging (Albany NY). 13:14482–14498. 2020.
View Article : Google Scholar : PubMed/NCBI
|
|
154
|
Gao L, Li T, Li S, Song Z, Chang Y and
Yuan L: Schisandrin A protects against isoproterenol-induced
chronic heart failure via miR-155. Mol Med Rep. 25:242022.
View Article : Google Scholar
|
|
155
|
Luo Y, Deng X, Chen Q, Cai Y, Bie M, Zhang
Y, Peng L, Yao K, Chen X and Cai H: Up-regulation of miR-155
protects against chronic heart failure by inhibiting HIF-1α. Am J
Transl Res. 15:6425–6436. 2023.
|
|
156
|
Heymans S, Corsten MF, Verhesen W, Carai
P, van Leeuwen RE, Custers K, Peters T, Hazebroek M, Stöger L,
Wijnands E, et al: Macrophage microRNA-155 promotes cardiac
hypertrophy and failure. Circulation. 128:1420–1432. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
157
|
Zheng L, Xu CC, Chen WD, Shen WL, Ruan CC,
Zhu LM, Zhu DL and Gao PJ: MicroRNA-155 regulates angiotensin II
type 1 receptor expression and phenotypic differentiation in
vascular adventitial fibroblasts. Biochem Biophys Res Commun.
400:483–488. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
158
|
Shi L and Fleming I: One miR level of
control: microRNA-155 directly regulates endothelial nitric oxide
synthase mRNA and protein levels. Hypertension. 60:1381–1382. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
159
|
Touyz RM, Alves-Lopes R, Rios FJ, Camargo
LL, Anagnostopoulou A, Arner A and Montezano AC: Vascular smooth
muscle contraction in hypertension. Cardiovasc Res. 114:529–539.
2018. View Article : Google Scholar : PubMed/NCBI
|
|
160
|
Xu D, Liao R, Wang XX and Cheng Z: Effects
of miR-155 on hypertensive rats via regulating vascular mesangial
hyperplasia. Eur Rev Med Pharmacol Sci. 22:7431–7438.
2018.PubMed/NCBI
|
|
161
|
Lai KN, Leung JCK and Tang SCW: The
renin-angiotensin system. Contrib Nephrol. 170:135–144. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
162
|
Forrester SJ, Booz GW, Sigmund CD, Coffman
TM, Kawai T, Rizzo V, Scalia R and Eguchi S: Angiotensin II signal
transduction: An update on mechanisms of physiology and
pathophysiology. Physiol Rev. 98:1627–1738. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
163
|
DuPont JJ, McCurley A, Davel AP, McCarthy
J, Bender SB, Hong K, Yang Y, Yoo JK, Aronovitz M, Baur WE, et al:
Vascular mineralocorticoid receptor regulates microRNA-155 to
promote vasoconstriction and rising blood pressure with aging. JCI
Insight. 1:e889422016. View Article : Google Scholar : PubMed/NCBI
|
|
164
|
Theilig F and Wu Q: ANP-induced signaling
cascade and its implications in renal pathophysiology. Am J Physiol
Renal Physiol. 308:F1047–1055. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
165
|
Vandenwijngaert S, Ledsky CD, Agha O, Wu
C, Hu D, Bagchi A, Domian IJ, Buys ES, Newton-Cheh C and Bloch DB:
MicroRNA-425 and microRNA-155 cooperatively regulate atrial
natriuretic peptide expression and cGMP production. PLoS One.
13:e01966972018. View Article : Google Scholar : PubMed/NCBI
|
|
166
|
Stonesifer C, Corey S, Ghanekar S,
Diamandis Z, Acosta SA and Borlongan CV: Stem cell therapy for
abrogating stroke-induced neuroinflammation and relevant secondary
cell death mechanisms. Prog Neurobiol. 158:94–131. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
167
|
Putaala J: Ischemic stroke in young
adults. Continuum (Minneap Minn). 26:386–414. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
168
|
Choi GH, Ko KH, Kim JO, Kim J, Oh SH, Han
IB, Cho KG, Kim OJ, Bae J and Kim NK: Association of miR-34a,
miR-130a, miR-150 and miR-155 polymorphisms with the risk of
ischemic stroke. Int J Mol Med. 38:345–356. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
169
|
Xing G, Luo Z, Zhong C, Pan X and Xu X:
Influence of miR-155 on cell apoptosis in rats with ischemic
stroke: Role of the ras homolog enriched in brain (Rheb)/mTOR
pathway. Med Sci Monit. 22:5141–5153. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
170
|
Yang Y, Zhang N, Wang S and Wen Y:
MicroRNA-155 regulates inflammatory response in ischemic cerebral
tissues through autophagy. Curr Neurovasc Res. 15:103–110. 2018.
View Article : Google Scholar : PubMed/NCBI
|
|
171
|
Adly Sadik N, Ahmed Rashed L and Ahmed
Abd-El Mawla M: Circulating miR-155 and JAK2/STAT3 axis in acute
ischemic stroke patients and its relation to Post-ischemic
inflammation and associated ischemic stroke risk factors. Int J Gen
Med. 14:1469–1484. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
172
|
Huang W, Hong Q, Wang H, Zhu Z and Gong S:
MicroRNA-155 inhibition activates Wnt/β-Catenin signaling to
restore Th17/Treg cell balance and protect against acute ischemic
stroke. eNeuro. 12:ENEURO.0347-24.2024. 2025. View Article : Google Scholar
|
|
173
|
Wang J, Li D, Hou J and Lei H: Protective
effects of geniposide and ginsenoside Rg1 combination treatment on
rats following cerebral ischemia are mediated via microglial
microRNA-155-5p inhibition. Mol Med Rep. 17:3186–3193. 2018.
|
|
174
|
Zhang JK, Li Y, Yu ZT, Jiang JW, Tang H,
Tu GL and Xia Y: OIP5-AS1 inhibits oxidative stress and
inflammation in ischemic stroke through miR-155-5p/IRF2BP2 axis.
Neurochem Res. 48:1382–1394. 2023.
|
|
175
|
Bossone E and Eagle KA: Epidemiology and
management of aortic disease: Aortic aneurysms and acute aortic
syndromes. Nat Rev Cardiol. 18:331–348. 2021. View Article : Google Scholar
|
|
176
|
Spin JM, Li DY, Maegdefessel L and Tsao
PS: Non-coding RNAs in aneurysmal aortopathy. Vascul Pharmacol.
114:110–121. 2019. View Article : Google Scholar
|
|
177
|
Hu J, Huang S, Liu X, Zhang Y, Wei S and
Hu X: miR-155: An important role in inflammation response. J
Immunol Res. 2022:74372812022. View Article : Google Scholar : PubMed/NCBI
|
|
178
|
Yang X, Peng J, Pang J, Wan W and Chen L:
A functional polymorphism in the promoter region of miR-155
predicts the risk of intracranial hemorrhage caused by rupture
intracranial aneurysm. J Cell Biochem. 120:18618–18628. 2019.
View Article : Google Scholar : PubMed/NCBI
|
|
179
|
Feng Z, Zhang X, Li L, Wang C, Feng M,
Zhao K, Zhao R, Liu J and Fang Y: Tumor-associated
macrophage-derived exosomal microRNA-155-5p stimulates intracranial
aneurysm formation and macrophage infiltration. Clin Sci (Lond).
133:2265–2282. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
180
|
Zhang RL, Wang WM, Li JQ, Li RW, Zhang J,
Wu Y and Liu Y: The role of miR-155 in cardiovascular diseases:
Potential diagnostic and therapeutic targets. Int J Cardiol
Cardiovasc Risk Prev. 24:2003552025.PubMed/NCBI
|
|
181
|
Yang WW, Li QX, Wang F, Zhang XR, Zhang
XL, Wang M, Xue D, Zhao Y and Tang L: Exosomal miR-155-5p promote
the occurrence of carotid atherosclerosis. J Cell Mol Med.
28:e701872024. View Article : Google Scholar : PubMed/NCBI
|
|
182
|
Eshraghi R, Rafiei M, Hadian Jazi Z,
Shafie D, Raisi A and Mirzaei H: MicroRNA-155 and exosomal
microRNA-155: Small pieces in the cardiovascular diseases puzzle.
Pathol Res Pract. 257:1552742024. View Article : Google Scholar
|
|
183
|
Kazimierczyk E, Eljaszewicz A, Zembko P,
Tarasiuk E, Rusak M, Kulczynska-Przybik A, Lukaszewicz-Zajac M,
Kaminski K, Mroczko B, Szmitkowski M, et al: The relationships
among monocyte subsets, miRNAs and inflammatory cytokines in
patients with acute myocardial infarction. Pharmacol Rep. 71:73–81.
2019. View Article : Google Scholar
|
|
184
|
Fitzsimons S, Oggero S, Bruen R, McCarthy
C, Strowitzki MJ, Mahon NG, Ryan N, Brennan EP, Barry M, Perretti
M, et al: microRNA-155 is decreased during atherosclerosis
regression and is increased in urinary extracellular vesicles
during atherosclerosis progression. Front Immunol. 11:5765162020.
View Article : Google Scholar
|
|
185
|
Obradovic D, Rommel KP, Blazek S, Klingel
K, Gutberlet M, Lücke C, Büttner P, Thiele H, Adams V, Lurz P, et
al: The potential role of plasma miR-155 and miR-206 as circulatory
biomarkers in inflammatory cardiomyopathy. ESC Heart Fail.
8:1850–1860. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
186
|
Yao R, Ma Y, Du Y, Liao M, Li H, Liang W,
Yuan J, Ma Z, Yu X, Xiao H and Liao Y: The altered expression of
inflammation-related microRNAs with microRNA-155 expression
correlates with Th17 differentiation in patients with acute
coronary syndrome. Cell Mol Immunol. 8:486–495. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
187
|
Wang Q, Liu L, Chen X, Wang T, Zhou H,
Huang H, Qing L and Luo P: Noninvasive prognosis of postmyocardial
infarction using urinary miRNA ultratrace detection based on
Single-Target DNA-functionalized AuNPs. ACS Appl Mater Interfaces.
14:3633–3642. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
188
|
Tijsen AJ, Pinto YM and Creemers EE:
Non-cardiomyocyte microRNAs in heart failure. Cardiovasc Res.
93:573–582. 2012. View Article : Google Scholar
|
|
189
|
Ikitimur B, Cakmak HA, Coskunpinar E,
Barman HA and Vural VA: The relationship between circulating
microRNAs and left ventricular mass in symptomatic heart failure
patients with systolic dysfunction. Kardiol Pol. 73:740–746. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
190
|
Ding H, Wang Y, Hu L, Xue S, Wang Y, Zhang
L, Zhang Y, Qi H, Yu H, Aung LHH, et al: Combined detection of
miR-21-5p, miR-30a-3p, miR-30a-5p, miR-155-5p, miR-216a and miR-217
for screening of early heart failure diseases. Biosci Rep.
40:BSR201916532020. View Article : Google Scholar : PubMed/NCBI
|
|
191
|
Kin K, Miyagawa S, Fukushima S, Shirakawa
Y, Torikai K, Shimamura K, Daimon T, Kawahara Y, Kuratani T and
Sawa Y: Tissue- and plasma-specific MicroRNA signatures for
atherosclerotic abdominal aortic aneurysm. J Am Heart Assoc.
1:e0007452012. View Article : Google Scholar
|
|
192
|
Biros E, Moran CS, Wang Y, Walker PJ,
Cardinal J and Golledge J: microRNA profiling in patients with
abdominal aortic aneurysms: The significance of miR-155. Clin Sci
(Lond). 126:795–803. 2014. View Article : Google Scholar
|
|
193
|
Mashima R: Physiological roles of miR-155.
Immunology. 145:323–333. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
194
|
Seto AG, Beatty X, Lynch JM, Hermreck M,
Tetzlaff M, Duvic M and Jackson AL: Cobomarsen, an oligonucleotide
inhibitor of miR-155, co-ordinately regulates multiple survival
pathways to reduce cellular proliferation and survival in cutaneous
T-cell lymphoma. Br J Haematol. 183:428–444. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
195
|
Anastasiadou E, Seto AG, Beatty X,
Hermreck M, Gilles ME, Stroopinsky D, Pinter-Brown LC, Pestano L,
Marchese C, Avigan D, et al: Cobomarsen, an oligonucleotide
inhibitor of miR-155, slows DLBCL tumor cell growth in vitro and in
vivo. Clin Cancer Res. 27:1139–1149. 2021. View Article : Google Scholar :
|
|
196
|
Yan Q, Chen J, Li W, Bao C and Fu Q:
Targeting miR-155 to treat experimental scleroderma. Sci Rep.
6:203142016. View Article : Google Scholar : PubMed/NCBI
|
|
197
|
Tian FJ, An LN, Wang GK, Zhu JQ, Li Q,
Zhang YY, Zeng A, Zou J, Zhu RF, Han XS, et al: Elevated
microRNA-155 promotes foam cell formation by targeting HBP1 in
atherogenesis. Cardiovasc Res. 103:100–110. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
198
|
Tokgozoglu L and Kayikcioglu M: Familial
hypercholesterolemia: Global burden and approaches. Curr Cardiol
Rep. 23:1512021. View Article : Google Scholar : PubMed/NCBI
|
|
199
|
Yao R, Ma YL, Liang W, Li HH, Ma ZJ, Yu X
and Liao YH: MicroRNA-155 modulates Treg and Th17 cells
differentiation and Th17 cell function by targeting SOCS1. PLoS
One. 7:e460822012. View Article : Google Scholar : PubMed/NCBI
|
|
200
|
Zhao L, Ouyang Y, Bai Y, Gong J and Liao
H: miR-155-5p inhibits the viability of vascular smooth muscle cell
via targeting FOS and ZIC3 to promote aneurysm formation. Eur J
Pharmacol. 853:145–152. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
201
|
Ertl HCJ: Immunogenicity and toxicity of
AAV gene therapy. Front Immunol. 13:9758032022. View Article : Google Scholar : PubMed/NCBI
|
|
202
|
Kahil N, Abouzeinab NS, Hussein MAA and
Khalil MI: Intraperitoneal hepatorenal toxicity of zinc oxide and
nickel oxide nanoparticles in rats: A systematic review.
Nanotoxicology. 18:583–598. 2024. View Article : Google Scholar : PubMed/NCBI
|
|
203
|
Wei Y, Yan X, Yan L, Hu F, Ma W, Wang Y,
Lu S, Zeng Q and Wang Z: Inhibition of microRNA-155 ameliorates
cardiac fibrosis in the process of angiotensin II-induced cardiac
remodeling. Mol Med Rep. 16:7287–7296. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
204
|
Tang H, Mao J, Ye X, Zhang F, Kerr WG,
Zheng T and Zhu Z: SHIP-1, a target of miR-155, regulates
endothelial cell responses in lung fibrosis. FASEB J. 34:2011–2023.
2020. View Article : Google Scholar : PubMed/NCBI
|
|
205
|
Kalkusova K, Taborska P, Stakheev D and
Smrz D: The role of miR-155 in antitumor immunity. Cancers (Basel).
14:54142022. View Article : Google Scholar : PubMed/NCBI
|
|
206
|
Ghoumari AM, Rixe O, Yarovoi SV, Zerrouqi
A, Mouawad R, Poynard T, Opolon P, Khayat D and Soubrane C: Gene
transfer in hepatocarcinoma cell lines: In vitro optimization of a
virus-free system. Gene Ther. 3:483–490. 1996.PubMed/NCBI
|
|
207
|
Wu J, Wu GY and Zern MA: The prospects of
hepatic drug delivery and gene therapy. Expert Opin Investig Drugs.
7:1795–1817. 1998. View Article : Google Scholar
|
|
208
|
Golubovic A, Tsai S and Li B: Bioinspired
lipid nanocarriers for RNA delivery. ACS Bio Med Chem Au.
3:114–136. 2023. View Article : Google Scholar : PubMed/NCBI
|
|
209
|
Xu F, Reiser M, Yu X, Gummuluru S, Wetzler
L and Reinhard BM: Lipid-mediated targeting with membrane-wrapped
nanoparticles in the presence of corona formation. ACS Nano.
10:1189–1200. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
210
|
Streeter SS, Hebert KA, Bateman LM, Ray
GS, Dean RE, Geffken KT, Resnick CT, Austin DC, Bell JE, Sparks MB,
et al: Current and future applications of fluorescence guidance in
orthopaedic surgery. Mol Imaging Biol. 25:46–57. 2023. View Article : Google Scholar :
|
|
211
|
Brillante S, Volpe M and Indrieri A:
Advances in MicroRNA therapeutics: From preclinical to clinical
studies. Hum Gene Ther. 35:628–648. 2024. View Article : Google Scholar : PubMed/NCBI
|
|
212
|
Miao Y, Fu C, Yu Z, Yu L, Tang Y and Wei
M: Current status and trends in small nucleic acid drug
development: Leading the future. Acta Pharm Sin B. 14:3802–3817.
2024. View Article : Google Scholar : PubMed/NCBI
|
|
213
|
Nappi F: Non-coding RNA-targeted therapy:
A state-of-the-Art review. Int J Mol Sci. 25:36302024. View Article : Google Scholar : PubMed/NCBI
|
|
214
|
Vermeire S, Nitcheu J, Gineste P, Flatres
A, Santo J, Scherrer D, Peyrin-Biroulet L, Dulai PS, Danese S,
Dubinsky M, et al: Obefazimod in patients with moderate-to-severely
active ulcerative colitis: Efficacy and safety analysis from the
96-week open-label maintenance phase 2b study. J Crohns Colitis.
19:jjaf0742025. View Article : Google Scholar : PubMed/NCBI
|
|
215
|
Bauersachs J, Solomon SD, Anker SD,
Antorrena-Miranda I, Batkai S, Viereck J, Rump S, Filippatos G,
Granzer U, Ponikowski P, et al: Efficacy and safety of CDR132L in
patients with reduced left ventricular ejection fraction after
myocardial infarction: Rationale and design of the HF-REVERT trial.
Eur J Heart Fail. 26:674–682. 2024. View Article : Google Scholar : PubMed/NCBI
|
|
216
|
Janssen HL, Reesink HW, Lawitz EJ, Zeuzem
S, Rodriguez-Torres M, Patel K, van der Meer AJ, Patick AK, Chen A,
Zhou Y, et al: Treatment of HCV infection by targeting microRNA. N
Engl J Med. 368:1685–1694. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
217
|
Hong DS, Kang YK, Borad M, Sachdev J,
Ejadi S, Lim HY, Brenner AJ, Park K, Lee JL, Kim TY, et al: Phase 1
study of MRX34, a liposomal miR-34a mimic, in patients with
advanced solid tumours. Br J Cancer. 122:1630–1637. 2020.
View Article : Google Scholar : PubMed/NCBI
|