Introduction
Peripheral nerve injury (PNI) is a major cause of
long-term disability worldwide, with an annual incidence estimated
between 1.46-2.8% (1), and
accounting for 2.8% of traumatic injuries (2). PNI can impose substantial physical
and socioeconomic burdens in several ways. Firstly, it triggers
rapid skeletal muscle atrophy, with the cross-sectional area (CSA)
of skeletal muscles reduced by up to 75% within months (3). Secondly, prevalent sensory
dysfunction is evident, as 50% of patients experience neuropathic
pain after traumatic injury in the upper extremities. Thirdly, it
incurs a considerable financial burden, as indicated by an average
sick-leave duration of 147 days and socioeconomic costs amounting
to 197€ per day (4).
In the past decades, significant progress has been
made in microsurgical techniques for repairing injured nerves,
which, in some cases, has led to improved outcomes (5). However, functional recovery often
remains suboptimal following PNI. After such nerve injuries, the
injured neurons must regenerate their axons over long distances at
an extremely slow rate of ~1 mm per day (6). At this sluggish pace,
re-establishing functional motor units or reinnervating sensory
organs can take several months or even years, a condition termed
chronic axotomy, which frequently results in poor prognosis
(7). Therefore, identifying
strategies to accelerate axon regeneration and enhance therapeutic
outcomes is of considerable significance.
RhoA, a small GTPase, becomes activated upon binding
to GTP, thereby activating the downstream Rho kinase (also known as
Rho-associated coiled-coil containing protein kinase, ROCK), a
serine/threonine protein kinase existing in two subtypes: ROCK1 and
ROCK2 (8). The RhoA/ROCK pathway
regulates actin and microtubule dynamics by phosphorylating the
myosin light chain (MLC) and LIM kinase (9). During axon growth, inhibition of
the RhoA/ROCK pathway reduces actin contraction, thereby promoting
axon growth and regeneration (10). Additionally, this pathway
influences axonal growth through crosstalk with other signaling
pathways, most notably by inhibiting the PI3K/Akt pathway to impede
axon outgrowth. One investigation demonstrated that ROCK inhibition
could reduce flap necrosis and promote cutaneous nerve regeneration
by activating the PI3K/Akt pathway (11). Another study showed that ROCK
inhibition could significantly enhance axonal regeneration and
remyelination, as well as motor and sensory functional recovery in
a rat sciatic nerve (SN) transection model by activating the
PI3K/Akt pathway (12). However,
the downstream targets of the ROCK/PI3K/Akt pathway involved in
regulating axon regeneration remain undefined.
Numerous studies indicate that well-characterized
downstream molecules of the PI3K/Akt pathway regulating axonal
growth include GSK-3β (13),
mTOR (14) and FoxO3a (15). Among these, GSK-3β directly
participates in the regulation of cytoskeletal microtubule dynamics
(13). Activation of the
PI3K/Akt pathway phosphorylates GSK-3β at serine residues 9
(16), reducing its kinase
activity, resulting in alleviated inhibition on
microtubule-associated proteins, such as CRMP-2, MAP1B and APC
(17-19), thereby stabilizing growth cone
microtubules and promoting axonal extension. In the present study,
it was aimed to investigate whether GSK-3β acts as a downstream
mediator of the PI3K/Akt pathway, contributing to the effects of
ROCK inhibition on axonal regeneration, myelin repair, and
functional recovery following SN injury.
Materials and methods
Animal model and experimental groups
All animal procedures were approved by the
Experimental Animal Ethics Committee of Fujian Medical University
(approval no. 2024-Y-0566; Fuzhou, CHina) and complied with the
National Institutes of Health guidelines and ARRIVE standards. 96
male ICR mice (22±3 g, 6 weeks old) were used and housed under
standard conditions with ad libitum access to food and
water. After anesthesia with sodium pentobarbital [50 mg/kg,
intraperitoneal injection (i.p.)], a SN crush (SNC) injury model
was established by exposing the right SN at the level of the biceps
femoris tendon and applying constant pressure for 1 min using
hemostatic forceps. Mice were randomized into four groups: i)
Experimental group (DMSO, i.p.); ii) Y27632 group (ROCK inhibitor
Y27632); iii) Y + LY group (Y27632 + PI3K inhibitor LY294002); and
iv) Y + LY + SB group (Y27632 + LY294002 + GSK3β inhibitor
SB216763). All compounds were purchased from Shanghai Aladdin
Biochemical Technology Co., Ltd. and administered i.p. at a dose of
10 mg/kg. Additionally, a Sham group in which the SN was exposed
but not crushed was included. Mice were sacrificed with an overdose
of sodium pentobarbital (≥150 mg/kg, i.p.) at specified time points
(days 1, 3, 5, 14, and 30), with six animals per group per time
point for histological or biochemical analyses. Death was confirmed
by the cessation of heartbeat and respiration, as well as absence
of corneal and pedal reflexes.
Dorsal root ganglion (DRG) explant
culture and axotomy
Dorsal root ganglia (DRG) were isolated from E15
Sprague-Dawley rat embryos. For pregnant rats from which embryos
were harvested, the same euthanasia method as aforementioned was
used. Only after confirmation of maternal death were the embryos
collected under sterile conditions. Ganglia were cultured on
six-well plates coated with poly-D-lysine (20 μg/ml) and
laminin (overnight, 0.3 ml/well). The culture medium consisted of
Neurobasal medium supplemented with B-27 (2%), L-glutamine (0.4
mM), glucose (2.5 mg/ml), fetal bovine serum (FBS; 1%, Gibco;
Thermo Fisher Scientific, Inc.), and 2.5S nerve growth factor (10
ng/ml). Cytosine β-D-arabinofuranoside (5 μM),
5-fluoro-2'-deoxyuridine (20 μM), and uridine (20 μM)
were added during the first 2 days to inhibit proliferation of
non-neuronal cells (20).
To facilitate the transection of axons, a customized
polydimethylsiloxane (PDMS) mold was designed. In brief, PDMS mixed
with its curing agent (Sylgard 184; Corning, Inc.) in a ratio of
10:1 was poured into the wells of a six-well plate. After curing, a
thick razor blade was used to carve two grooves intersecting with
each other at the center of each PDMS mold. The long groove, 1.5 mm
in width, was for accommodation of DRG, forcing the axons to grow
bidirectionally, whereas the short groove, 2 mm in width, served as
a slot for placement of a disposable razor blade that could gently
transect the axons when they grew across the intersection. The DRG,
cultured as aforementioned, were placed at ~1.5 mm away from the
intersection, and the transfection was carried out at day 6. After
transection, the DRG were assigned into the same four groups (no
sham group) as in the mice experiment. The concentration was set at
10 μM for each inhibitor. On day 3, using the center of each
DRG as the origin, the lengths of six regenerated axons, spaced at
30° intervals on the injured side, were measured as aforementioned
and averaged to represent the lengths of regenerated axons of each
DRG. Also, proteins were extracted from DRG in each group for
western blotting, and Phalloidin and β-tubulin were used to
respectively label the actin filaments and microtubules in the
growth cones of DRG.
Immunofluorescence staining
SNs, spinal cords, and footpads were harvested at
the designated time points, fixed in 4% paraformaldehyde (PFA) at
room temperature overnight, dehydrated, and embedded in paraffin.
Sections (7 μm) were deparaffinized and rehydrated. Antigen
retrieval was performed in citrate buffer (pH 6.0) using a pressure
cooker. After blocking with 5% normal goat serum for 1 h and
permeabilization (0.3% Triton X-100), sections were incubated with
primary antibodies overnight at 4°C, followed by incubation with
appropriate secondary antibodies for 1 h and DAPI (1 μg/ml)
counterstaining. The same protocol was adapted for DRG samples
after fixation in 4% PFA for 10 min, omitting the deparaffinization
and antigen retrieval steps. The detail of the antibodies used are
listed in Table I.
 | Table IInformation about antibodies used in
the present study. |
Table I
Information about antibodies used in
the present study.
| Antibody name | Manufacturer | Cat. no. | Dilution |
|---|
| Akt1 + Akt 2 +
Akt3 | Abcam | ab179463 | 1:1,000 |
| GAP43 | Proteintech Group,
Inc. | 16971-1-AP | 1:1,000 |
| Goat anti-Mouse IgG
H&L 488 | Beyotime Institute
of Biotechnology | A0428 | 1:500 |
| Goat anti-Rabbit
IgG H&L CY3 | Beyotime Institute
of Biotechnology | A0516 | 1:500 |
| Goat anti-Rabbit
IgG H&L 647 | Beyotime Institute
of Biotechnology | A0468 | 1:500 |
| GSK3β | Beyotime | AF5174 | 1:1,000 |
| HRP-Goat Anti
Mouse | Proteintech Group,
Inc. | SA00001-1 | 1:500 |
| HRP-Goat Anti
Rabbit | Proteintech Group,
Inc. | SA00001-2 | 1:500 |
| Keratin 8 | Wuhan Servicebio
Technology Co., Ltd. | GB15231 | 1:2,000 |
| Ki67 | Wuhan Servicebio
Technology Co., Ltd. | GB111141 | 1:500 |
| MPZ | Beyotime Institute
of Biotechnology | AF7497 | 1:200 |
| NeuN | Proteintech Group,
Inc. | 26975-1-AP | 1:2,000 |
| Neurofilament
200 | MilliporeSigma | N4142 | 1:200 |
| Neurofilament
200 | Wuhan Servicebio
Technology Co., Ltd. | GB12143 | 1:5,000 |
| Phospho-Akt
(Ser473) | Cell Signaling
Technology, Inc. | 9271 | 1:1,000 |
| Phospho-GSK3β
(Ser9) | Cell Signaling
Technology, Inc. | 5558 | 1:1,000 |
| Phospho-PI3K
P85α/β/P55γ | Beyotime Institute
of Biotechnology | AF5905 | 1:1,000 |
| PI3K P85 beta | Beyotime Institute
of Biotechnology | AF1729 | 1:1,000 |
| Rho A | Wuhan Servicebio
Technology Co., Ltd. | GB115177 | 1:1,000 |
| Rhodamine
phalloidin | Invitrogen; Thermo
Fisher Scientific, Inc. | 5210655 | 1:1,000 |
| ROCK1/2 | Abcam | ab45171 | 1:1,000 |
| S100 beta | Wuhan Servicebio
Technology Co., Ltd. | GB15359 | 1:2,000 |
| a-bungarotoxin | Invitrogen; Thermo
Fisher Scientific, Inc. | 2286321 | 1:1,000 |
| β-Tubulin
(Tuj1) | Beyotime Institute
of Biotechnology | AT809 | 1:1,000 |
Whole-mount staining of neuromuscular
junction
On day 30 post-injury, the extensor hallucis longus
muscle was dissected and fixed in 4% PFA for 1 h. Muscle bundles
were teased apart (21) and
permeabilized with 2% Triton X-100 overnight. Axons were stained
with anti-NF-200 antibody, and acetylcholine receptors were labeled
with Alexa 594-conjugated α-bungarotoxin (α-BGT). After imaging,
the reinnervation rate of the endplate was calculated by dividing
the immuno-positive area of NF-200 by the immuno-positive area of
α-BGT in six random myofibers.
Western blotting
Lumbosacral enlargements (LEs; day 3 post-injury in
mice) and pooled DRGs after in vitro axotomy (n=20/group)
were homogenized in NP-40 lysis buffer (cat. no. ST2045; Beyotime
Institute of Biotechnology) with protease and phosphatase
inhibitors. Protein concentrations were determined using the BCA
assay. Equal amounts (20 μg) of protein were separated by
10% SDS-PAGE and transferred to PVDF membranes. After blocking,
membranes were probed with primary and HRP-conjugated secondary
antibodies and visualized using enhanced chemiluminescence
(IGEPAL® CA-630; cat. no. P0018S; Beyotime Institute of
Biotechnology). Band intensity was quantified by ImageJ software
(1.54p; National Institutes of Health) and normalized to loading
controls. The detail of the antibodies used are listed in Table I.
Retrograde tracing with cholera toxin
subunit B (CTB)
A total of 3 weeks after SNC, 1 μl of Alexa
Fluor 594-conjugated CTB (cat. no. C34777; Invitrogen; Thermo
Fisher Scientific, Inc.) was injected 0.5 cm distal to the injury
site in the right SN. A total of 7 days later, lumbosacral segments
were collected, cryo-sectioned (30 μm), and CTB-labeled
neurons were counted across all sections to quantify retrograde
transport.
Functional assessment
Motor function
The Sciatic Function Index (SFI) was assessed at day
30 using footprint analysis. Key measurements included: heel-to-toe
distance (print length, PL); distance between the first and fifth
toes (toe spread, TS); and distance between the second and fourth
toes (intermediary toe spread, IT). Data were collected for both
the normal (N) and experimental (E) hindlimbs. The SFI was
calculated using the formula: SFI= −38.3 × (EPL-NPL)/NPL + 109.5 ×
(ETS-NTS)/NTS + 13.3 × (EIT-NIT)/NIT-8.8.
Sensory function
Mechanical sensitivity was tested with Von Frey
filaments; thermal sensitivity with a hot plate (55±1°C). The paw
withdrawal threshold and latency were recorded as average values
over three trials.
Muscle strength and atrophy
At day 30, after anesthesia with sodium
pentobarbital (50 mg/kg, i.p.), the gastrocnemius muscle was
carefully separated from the surrounding musculature without
damaging the blood supplies and nerves. A suture was tied around
the calcaneal tendon, which was detached from the calcaneus bone.
The other end of the suture was then tied to a tension transducer,
which was connected to a RM6240 Multi-Channel Physiological Signal
Acquisition and Processing System (Chengdu Instrument Factory) for
recording and analysis. The SN under the gluteus maximus was
exposed and a stimulating electrode (Anhui Zhenghua Biological
Instrument and Equipment Co., Ltd.) was placed upon it. The
single-pulse stimulus mode with a pulse width of 0.2 ms was used.
The voltage was started at 0.1 V and gradually increased in 0.05 V
increments until reaching maximum isometric twitch force.
At day 30, the gastrocnemius muscle was dissected
and weighed, and images for macroscopic analysis were captured. The
ratio of the muscle weight on the experimental side to that on the
normal side was calculated for each mouse. Muscles were then
processed for paraffin embedding, and hematoxylin and eosin
(H&E) staining was performed using a commercial kit (Solarbio,
cat. no. G1120). The myofiber CSA was calculated from three
randomly selected mid-belly fields (×20) and averaged using ImageJ
(National Institutes of Health).
RSC96 cell proliferation and migration
assays
For proliferation assay, 3×104 Rat
Schwann Cell Line (RSC96 cells) were cultured in Dulbecco's
Modified Eagle Medium (DMEM; Thermo Fisher Scientific, Inc.)
supplemented with 10% FBS (HyClone; Cytiva), and 1%
penicillin/streptomycin (Beyotime Institute of Biotechnology). The
cells were then divided into the control, Y27632, Y + LY, and Y +
LY + SB groups, with DMSO and the relevant inhibitors added into
the medium as aforementioned, together with 10 μM
5-ethynyl-2'-deoxyuridine (EdU) (Beyotime Institute of
Biotechnology). A total of 24 h later, cells were fixed with 4% PDA
for 15 min at room temperature, permeabilized with PBS containing
0.5% Triton X-100 for 20 min and then incubated with a reaction
solution (cat. no. C0071S; Beyotime Institute of Biotechnology) for
30 min.
For the scratch assay, confluent monolayers were
scraped with a pipette tip. Migration into the scratch area was
monitored at 0 and 24 h using phase-contrast microscopy. Scratch
closure rate was calculated as a percentage of baseline width.
Statistical analysis
Data are presented as the mean ± standard error of
the mean (SEM). One-way ANOVA was used for group comparisons, with
LSD or Dunnett's T3 post-hoc tests depending on variance
homogeneity. Two-way ANOVA was used for DRG axon length analyses.
P<0.05 was considered to indicate a statistically significant
difference. The statistical analysis was performed with GraphPad
Prism 8 (Dotmatics).
Results
Effects of three inhibitors on nerve
regeneration after SNC
Following SNC, NF-200 immunostaining revealed a
complete absence of axons at the clamp site (Fig. 1A and B), confirming successful
model establishment. Compared with the experimental group, the
Y27632 group showed a significantly increased density of
regenerated axons at the clamp site on day 14 post-injury,
calculated by normalizing the NF-200 immuno-positive area to 0.5
mm2 (white box region) (Y27632 vs. experimental,
P=0.0318). Notably, co-treatment with LY294002 significantly
attenuated the pro-regenerative effect of Y27632 (Y + LY vs.
Y27632, P=0.001). Strikingly, supplementation with SB216763
effectively reversed the adverse effects induced by PI3K inhibition
(Y + LY + SB vs. Y + LY, P=0.0316; Fig. 1C and D).
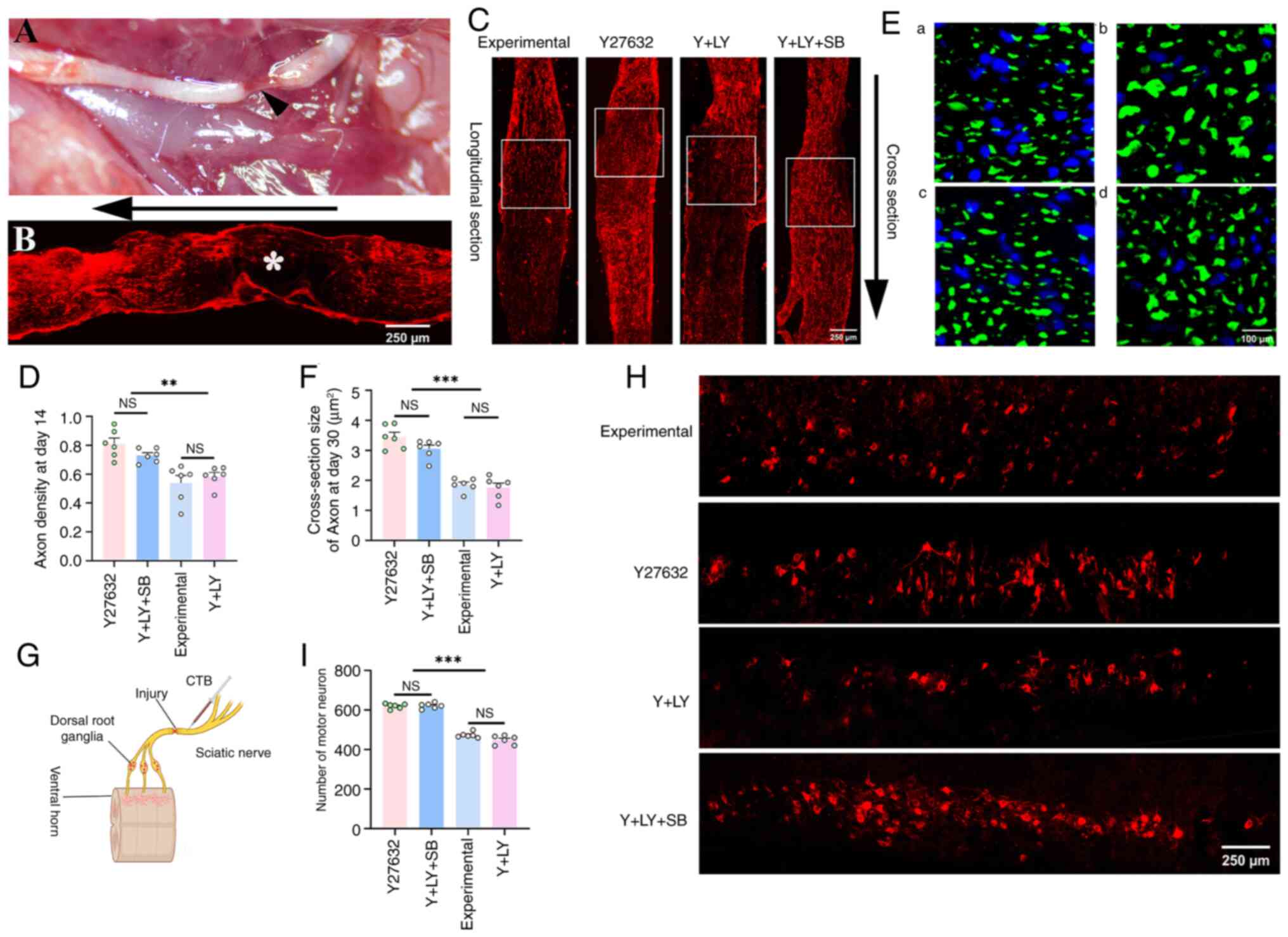 | Figure 1Effects of three inhibitors on nerve
regeneration after SNC. (A) Representative gross image of a SN
after SNC injury. The arrowhead indicates the formation of a nerve
defect, with only the epineurium remaining after hemostat clamping.
(B) The asterisk (*) marks a complete absence of NF-200-positive
staining at the clamped site immediately after injury. The arrow
denotes the proximal-to-distal orientation. (C) Representative
longitudinal sections of injured nerves stained with NF-200 at day
14. (D) Axon density is significantly higher in the Y and Y + LY +
SB groups (n=6). (E) Cross-sections stained with NF-200 on day 30
in all groups. a, b, c and d represent the staining from the
experimental, Y27632, Y + LY, and Y + LY + SB groups, respectively.
(F) The diameter of regenerated axons is significantly larger in
the Y27632 and Y + LY + SB groups (n=6). (G) Schematic diagram of
CTB injection into the SN distal to the clamped site for retrograde
labeling of neurons in the ventral horn. (H and I) CTB-labeled
neurons in the ventral horn are significantly more numerous in the
Y27632 and Y + LY + SB groups (n=6). **P<0.01 and
***P<0.001. SN, sciatic nerve; SNC, SN crush; CTB,
cholera toxin subunit B; NS, not significant (P>0.05). |
Analysis of axonal CSA on day 30 revealed that
Y27632 significantly increased axon diameter compared with the
experimental group (P<0.0001), indicating accelerated
maturation. By contrast, the axons in the Y + LY group reverted to
baseline maturity levels (Y + LY vs. experimental, P=0.9001). This
phenotype was completely reversed upon co-treatment of SB216763 (Y
+ LY + SB vs. Y + LY, P<0.0001; Fig. 1E and F).
Retrograde CTB tracing on day 30 demonstrated that
both the Y27632 group and the Y + LY + SB group exhibited a
remarkably significant increase in CTB-labeled neuron counts
compared with the experimental group and the Y + LY group
(P<0.0001; Fig. 1G-I).
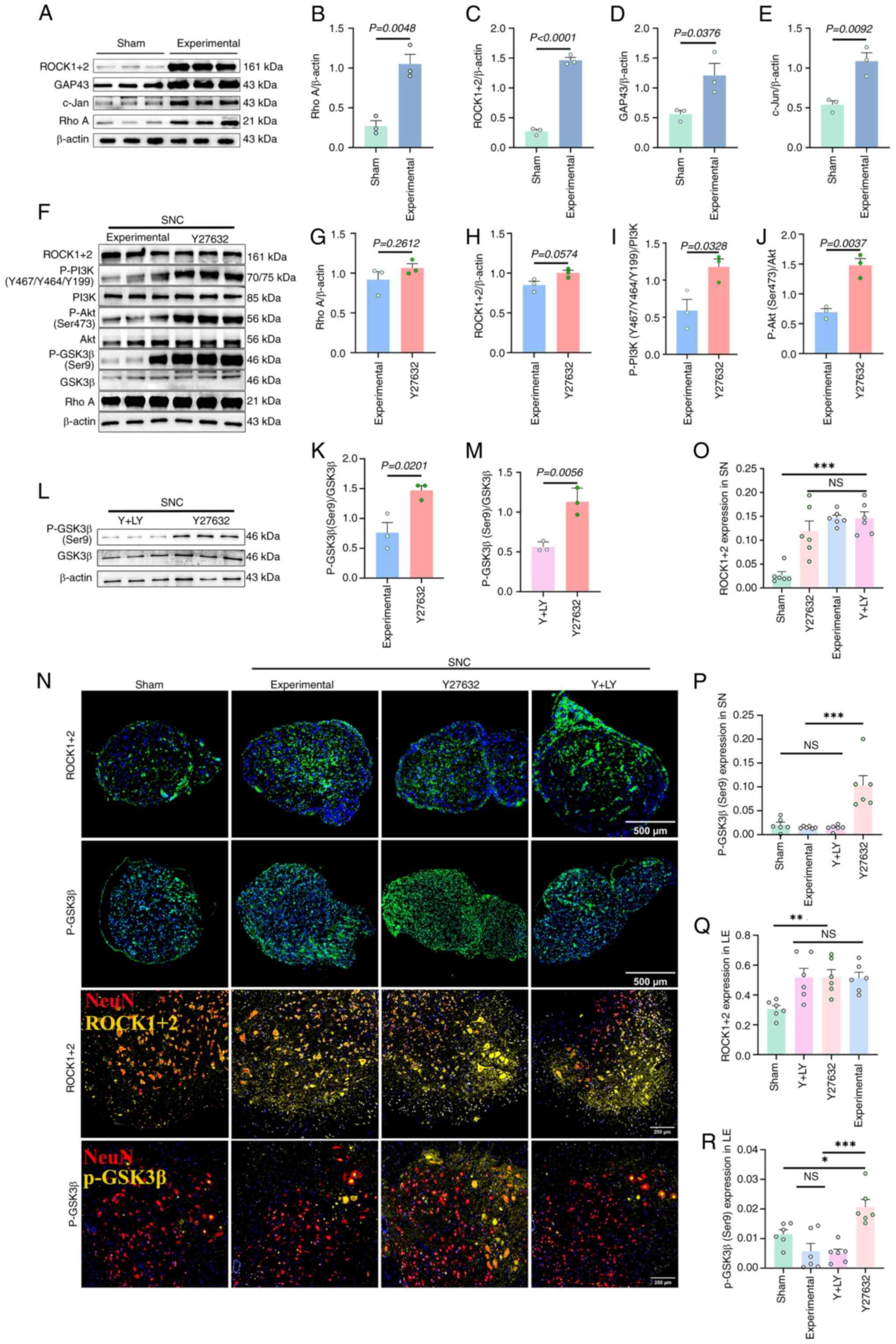
ROCK inhibition promotes phosphorylation
of PI3K/Akt/GSK3β in vivo
Western blot analysis showed that on day 3 after SNC
in mice, the expression of RhoA and ROCK1/2 in the LE were
significantly upregulated (Fig.
2A-C). Moreover, upregulation of regeneration-associated
proteins GAP-43 and c-Jun confirmed ongoing repair processes
(Fig. 2D and E). Treatment with
Y27632 did not produce significant changes in RhoA or ROCK1/2
expression, but significantly enhanced the phosphorylation levels
of PI3K, Akt and GSK3β compared with the experimental group
(Fig. 2F-K). By contrast,
co-treatment with LY294002 led to an ~50% reduction in GSK3β
phosphorylation (Fig. 2L and
M).
Immunofluorescence confirmed these findings: On day
3 after SNC, ROCK1/2 expression in the SN (calculated as the ratio
of the ROCK1/2 immuno-positive area over the cross-section area of
each nerve) and the LE (calculated from dividing the
ROCK1/2-positive area by the NeuN-positive area) increased by
4.4-fold and 0.5-fold, respectively (SN: P=0.0002; LE: P=0.0333).
Furthermore, Y27632 treatment could significantly elevate the
expression of P-GSK3β in the SN and LE (P<0.01) (Fig. 2N-R).
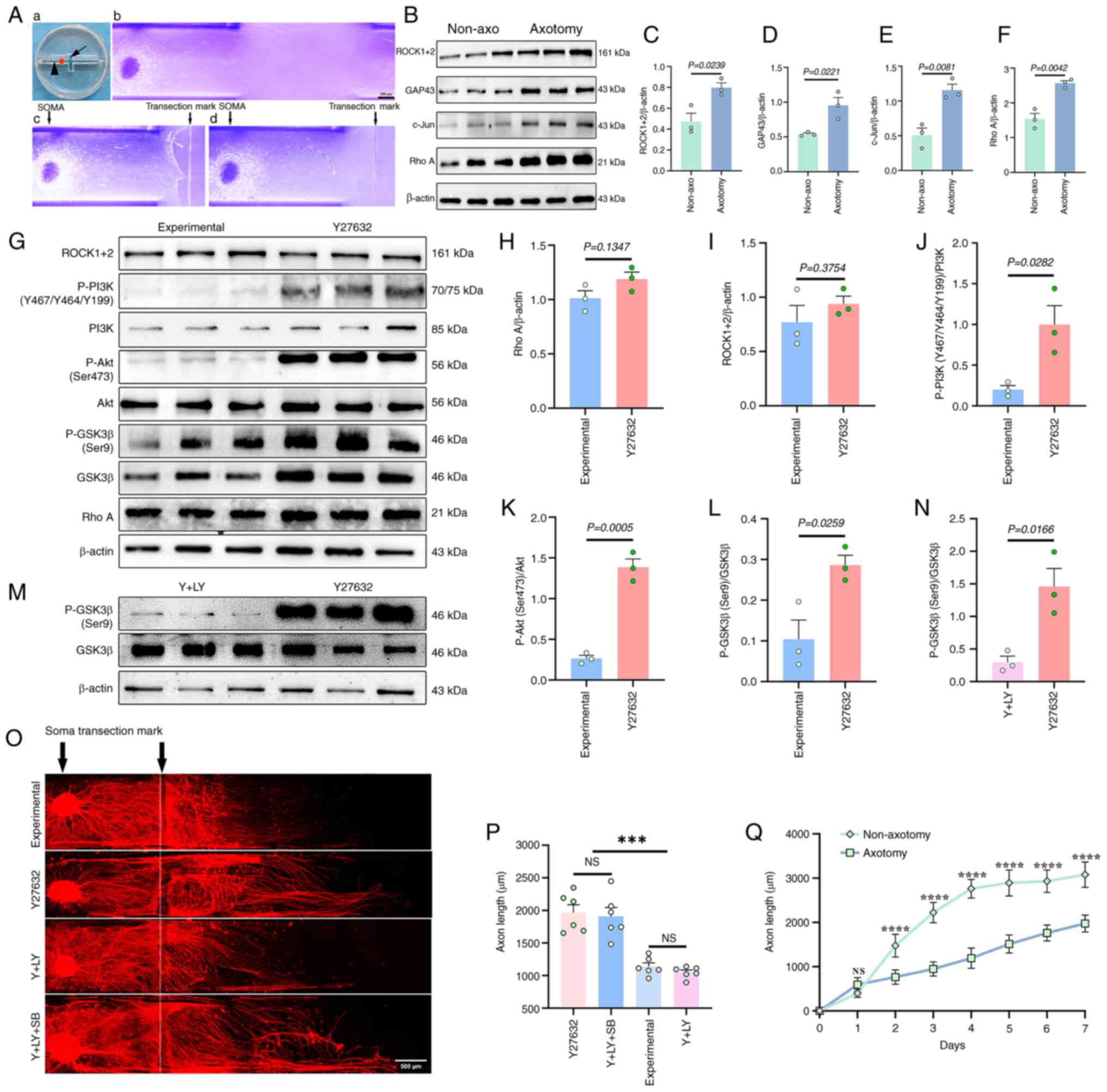
ROCK inhibition promotes PI3K/Akt/GSK3β
phosphorylation and axon regeneration in vitro
In the DRG axotomy model, robust regenerated axons
could be observed extending across the transection mark by day 1
(Fig. 3A). Western blotting on
day 3 after axotomy confirmed significant upregulation of RhoA,
ROCK1/2, GAP43 and c-Jun (Fig.
3B-F), consistent with in vivo axotomy responses.
Compared with non-axotomized controls, axon growth was
significantly reduced after axotomy (Fig. 3Q).
Further analysis demonstrated that Y27632 treatment
significantly elevated phosphorylation levels of PI3K, Akt and
GSK3β (Fig. 3G-L), while
LY294002 treatment reduced P-GSK3β levels by 3.9-fold (Fig. 3M and N). Morphometric analysis
showed that Y27632 increased the length of regenerated axons from
1,139±33 to 1,966±56 μm, which was suppressed by LY294002
co-treatment (1,050±34 μm), but restored by further
co-treatment with SB216763 (1,909±35 μm; P<0.0001;
Fig. 3O and P).
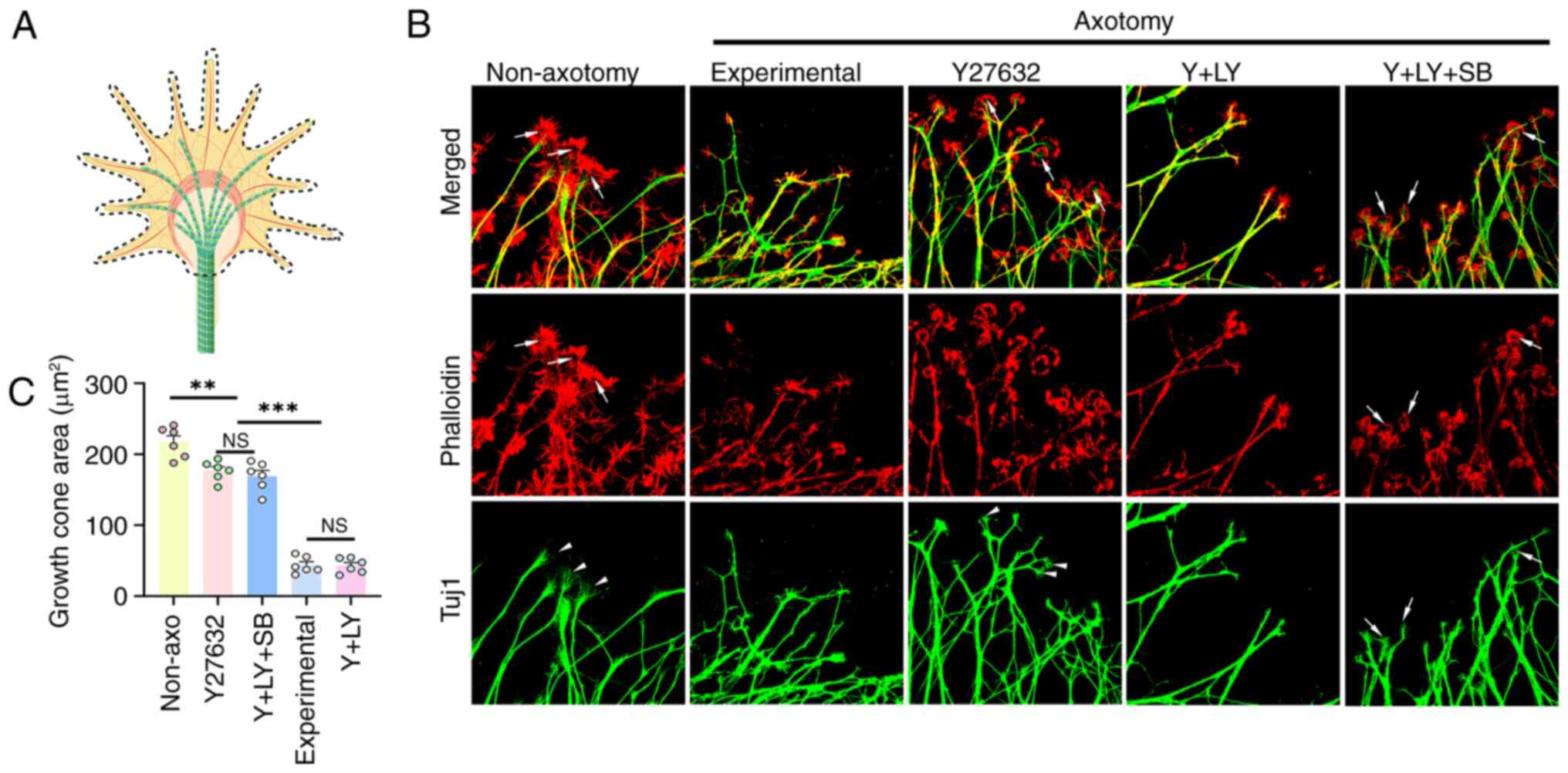
ROCK/PI3K/Akt/GSK3β pathway regulates
growth cone morphology
The morphology of the growth cones in the
experimental group was dramatically altered after axotomy. For the
growth cones in the non-axotomy group, the phalloidin-positive
actin in the periphery was broad, and the Tuj1-positive microtubule
often splayed out at the end like a broom. Also, there was often a
transition zone negative both in phalloidin and Tuj1. In
comparison, after axotomy, the actin-rich area in the periphery was
dramatically reduced, collapsing towards the microtubules,
eliminating the transition zone, and the microtubules did not splay
out at the end, resulting in a fivefold decrease in growth cone
area (P<0.001). Y27632 treatment partially restored the growth
cones morphology, including re-emergence of the transition zone and
microtubule splaying. Statistically, Y27632 treatment enabled a
3-fold increase in the size of the growth cones, though still
smaller than that in the non-axotomy group (Y27632 vs. Non-axotomy,
P=0.002). This recovery was reversed by LY294002 and rescued by
SB216763 (Fig. 4A-C).
ROCK/PI3K/Akt/GSK3β pathway regulates the
recovery of motor and sensory functions
In terms of motor function, Y27632 increased
gastrocnemius muscle recovery compared with the experimental and Y
+ LY groups (both P<0.001). SB216763 reversed the effect of
LY294002 (Y + LY + SB vs. Y27632; P=0.2737) (Fig. 5A and B). Analysis of wet weight
ratio showed that the Y27632 group had a 34.3% increase in
gastrocnemius muscle weight compared with the experimental group
(P=0.0045), while co-administration with LY294002 caused the weight
to decrease by 28.9% (P=0.0015). Further co-administration with
SB216763 reversed the inhibitory effect of LY294002, resulting in a
44.2% weight increase (P=0.0007). Cross-sectional H&E staining
further confirmed this pattern. Further morphological investigation
revealed that the reinnervation rate of the acetylcholine receptors
in the Y27632 group was increased by 0.5-fold compared with the
experimental group (P<0.0001), and there was no statistically
significant difference between the Y27632 group and the Y + LY + SB
group (P=0.3684) (Fig.
5C-F).
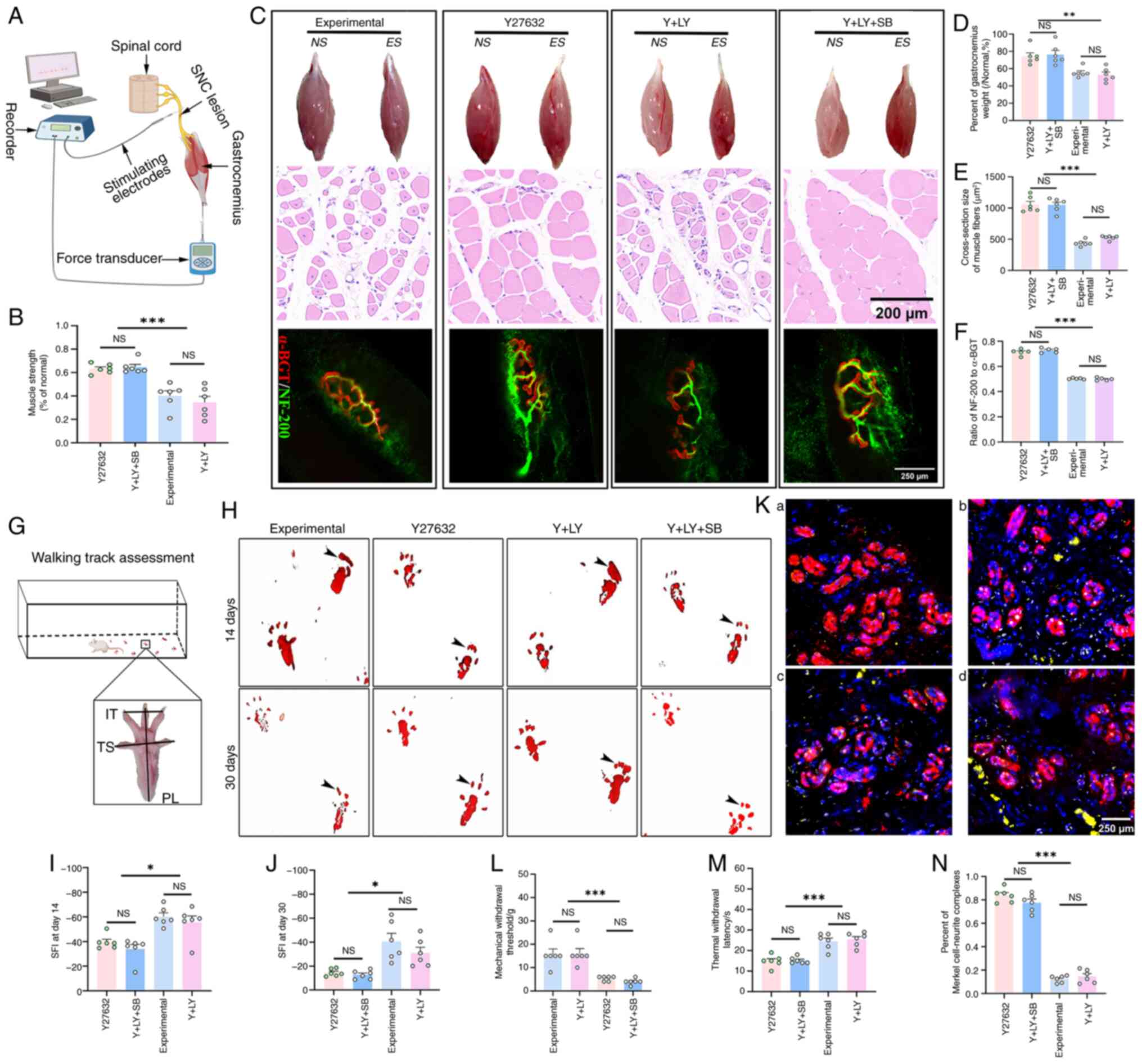 | Figure 5ROCK/PI3K/Akt/GSK3β pathway regulates
locomotor and sensory recovery after sciatic nerve crush. (A)
Experimental setup for muscle strength testing. (B) Gastrocnemius
muscle strength ratio ES/NS was significantly greater in the Y27632
and Y + LY + SB groups (n=6). (C) Top row: Representative
appearance of the gastrocnemius muscles on the NS and ES. Middle
row: Representative cross-sections of the gastrocnemius muscles in
ES. Bottom row: Representative neuromuscular junctions of the
extensor hallucis longus on the ES. (D and E) The wet weight ratio
and myofiber size were significantly larger in the Y27632 and Y +
LY + SB groups (n=6). (F) The reinnervation rate of the
acetylcholine receptor was significantly higher in the Y27632 and Y
+ LY + SB groups (n=6). (G) Schematic illustration evaluating the
locomotor function using the sciatic function index. (H-J)
Representative footprints demonstrated significantly improved
recovery of locomotor function in the Y27632 and Y + LY + SB
groups, as indicated by improved toe-spreading ability. (K)
Representative images of reconnection between NF-200-labeled axons
(Yellow) and Keratin 8-labeled Merkel cells (Red). Panels a, b, c
and d represent typical images from the control, Y27632, Y + LY,
and Y + LY + SB groups, respectively. (L) The reinnervation rate of
the Merkel cells in the footpads was significantly higher in the
Y27632 and Y + LY + SB groups (n=6). (M and N) Paw-licking latency
and mechanical pain thresholds significantly improved in the Y27632
and Y + LY + SB groups (n=6). *P<0.05,
**P<0.01 and ***P<0.001. ES,
experimental side; NS, normal side; NS, not significant
(P>0.05). |
Gait analysis found that the SFI of the Y27632 group
was significantly improved compared with that of the experimental
group (P=0.0027) and the Y + LY group (P=0.0195) on day 14, and
there was no significant difference compared with the Y + LY + SB
group (P=0.6012), and this pattern persisted until day 30 after
injury (Fig. 5G-J).
In terms of sensory function, the morphological
study demonstrated that Y27632 treatment increased the
co-localization rate of Merkel cells and axons (calculated by the
dividing the area of the axons labeled by NF-200 staining by that
of the Merkel cells labeled by Keratin-8 staining) in the footpads
by 5.8-fold (P<0.0001), and the treatment of SB216763 could
reverse the inhibitory effect of LY294002 (P<0.0001), restoring
the co-localization rate to 92.7% of the level of Y27632 treatment
alone (P=0.1843, Fig. 5K and L).
The morphological improvement in innervation in footpads translated
to similar patterns of mechanical tactile and thermal pain
sensation in the four groups (Fig.
5M and N).
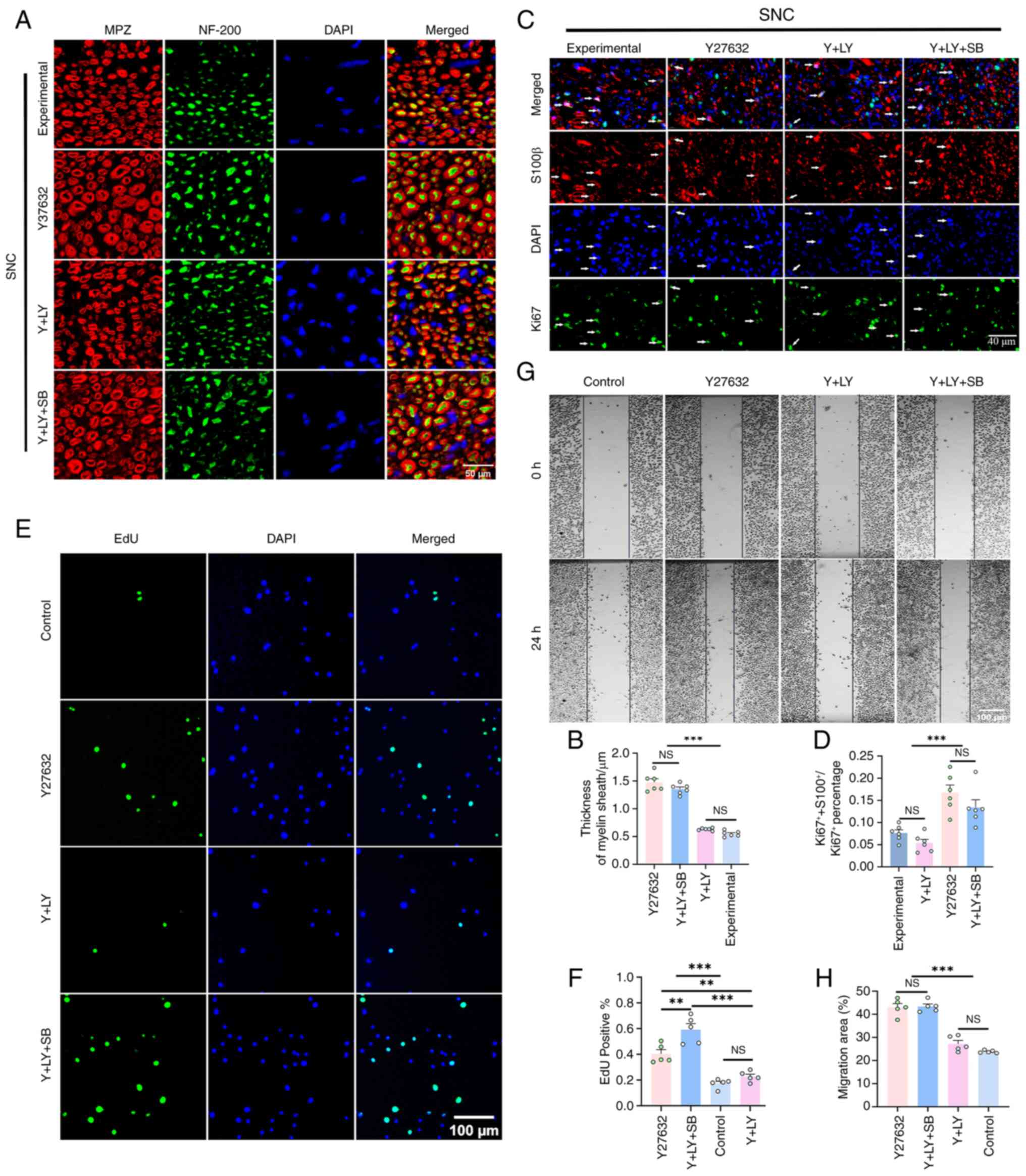
ROCK/PI3K/Akt/GSK3β axis promotes
remyelination and Schwann cell activity
Myelin sheath staining revealed that following
Y27632 treatment, myelin sheath thickness in the injured area was
1.7- and 1.3-fold greater than that of the experimental and Y + LY
groups, respectively (both P<0.001). The co-treatment with
SB216763 also demonstrated a comparable remyelination-promoting
effect, with the thickness increasing by 1.1-fold relative to the Y
+ LY group (P<0.001; Fig. 6A and
B). Furthermore, the proliferation rate of Schwann cells
(calculated by dividing the number of S100β/Ki67 positive cells by
the number of Ki67 positive cells in three random fields taken
under ×20 objective magnification) was significantly elevated by
1.1-fold on day 5 after SNC in the Y27632 group when compared with
the experimental group (P<0.001). Similarly, this effect was
abolished by LY294002 co-treatment and rescued by further SB216763
co-treatment (Fig. 6C and
D).
In vitro, Y27632 enhanced EdU+
RSC96 cell proliferation by 1.3-fold (P=0.0003) and accelerated
scratch closure rate by 0.8-fold (P<0.001). These effects were
completely reversed by LY294002 and restored by SB216763, which
even increased proliferation beyond Y27632 alone (P<0.005;
Fig. 6E-H).
Discussion
The present study demonstrated that inhibition of
ROCK via Y27632 significantly enhances axonal regeneration,
remyelination, and functional recovery after axotomy.
Mechanistically, these effects are mediated by activation of the
PI3K/Akt signaling pathway and subsequent phosphorylation-mediated
inhibition of GSK3β. These findings not only deepen our
understanding of the molecular basis of peripheral nerve
regeneration but also offer compelling evidence for the therapeutic
potential of pharmacological modulation of the ROCK/PI3K/Akt/GSK3β
signaling axis.
Western blot and immunofluorescence analyses in SNC
model in mice demonstrated that Y27632 increased the
phosphorylation levels of PI3K, Akt and GSK3β. Co-administration of
the PI3K inhibitor LY294002 significantly suppressed GSK3β
phosphorylation, validating the pathway's linearity and dependency.
These molecular changes were associated with enhanced axonal
regrowth and myelin sheath thickness, indicating that activation of
this signaling axis not only initiates but sustains regenerative
processes. However, this is one limitation that needs to be
acknowledged here: the expression of proteins analyzed in mice was
extracted from the LE, which sends motor axons into the SN. To
strengthen the study, the expression of proteins in the
L4-6 DRG, which contribute sensory axons to the SN,
should also be examined. However, challenges were faced in
precisely identifying the L4-6 DRG in mice.
Additionally, the small size of murine DRGs presented significant
obstacles for cryo-sectioning. This limitation needs to be
addressed in future studies.
Histological and behavioral assessments further
confirmed that Y27632 treatment translated to significant
improvements in sensorimotor function. In terms of motor recovery,
Y27632 enhanced neuromuscular junction reinnervation, reduced
muscle atrophy, and increased gastrocnemius contractile strength.
These results suggest that axons regenerated under ROCK inhibition
are capable of functionally reconnecting with their target muscles.
In terms of sensory function, Y27632 restores the integrity of
Merkel complexes, which are formed by Merkel cells connecting with
axons from slowly adapting type I Aβ low-threshold mechanoreceptor
neurons, primarily responsible for tactile discrimination of object
shape, curvature, and texture (22,23). The enhanced re-connection between
Merkel cells and axons exemplifies improved growth of axons into
the plantar skin, probably attributable to shortened thermal pain
latency and reduced mechanical tactile threshold.
Importantly, the beneficial effects of Y27632 were
largely abolished by PI3K inhibition and subsequently rescued by
co-treatment with the GSK3β inhibitor SB216763. These findings
identify GSK3β as a critical downstream effector mediating the
pro-regenerative effects of ROCK inhibition. To the best of the
authors' knowledge, this is the first study to delineate a
functional ROCK → PI3K/Akt → GSK3β signaling cascade that governs
axon regeneration and remyelination following PNI.
The regulation of the ROCK/PI3K/Akt/GSK3β pathway on
axon regeneration can also be replicated in the in vitro DRG
axotomy model, which is often adopted for the study of axon
degeneration and regeneration (24,25). In this model, the upregulation of
c-Jun and GAP-43, two common RAGs, along with upregulation of RhoA
and ROCK mirrors the transcriptional programs that occur after
nerve crush injury in mice (26-29), making it an ideal in vitro
model for the investigation of molecular pathways relating to axon
regeneration. In this context, Y27632 significantly promoted axonal
elongation and growth cone expansion in a PI3K/GSK3β-dependent
manner. One noteworthy point is that in this in vitro
axotomy model, the original anatomical location of the DRG, whether
from cervical, thoracic, or lumbar, is irrelevant, as each
extracted DRG underwent axotomy, and treated with various agents as
described. Another noteworthy point is that the in vivo
experiments were performed in adult mice while the in vitro
DRG axotomy model used embryonic rat ganglia. Although these differ
in species and developmental stage, the core signaling modules
investigated, RhoA/ROCK, PI3K/Akt and GSK3β, are evolutionarily
conserved across vertebrates and across developmental contexts
(10,30,31).
The growth cone, a specialized structure at the tip
of extending axons, plays a pivotal role in sensing guidance cues
and mediating cytoskeletal rearrangements (32,33). It is composed of actin at the
periphery and microtubules at the core. As demonstrated in the
present study, the size of growth cones after axotomy is
drastically reduced after injury, shrinking the thickness of the
actin-rich periphery, eliminating the transition zone and
broom-like end of the microtubule, probably due to the upregulation
of the RhoA/ROCK pathway. The transition zone is abundant in
myosin, which interacts with the actin to generate the force
required to advance the growth cone along the substrate (34). Additionally, it has been reported
that splaying out of microtubule into the periphery allows for
interplay between the actin and microtubule and is conducive to
axon growth. Therefore, the disappearance of the transition zone
and microtubule splaying out is likely responsible for the
significant slowing down of axon outgrowth after axotomy.
As expected, Y27632 can significantly expand the
size of the growth cones, restoring the transition zone, and
partially restores the broom-like appearance of the microtubule
ends. The size of the growth cones then shrinks after co-treatment
with LY294002 and expands again after further co-treatment with
SB216763, suggesting the involvement of the ROCK/PI3K/Akt/GSK3β
pathway in regulating the morphogenesis of the growth cones. Since
it has been shown that Y27632 treatment can significantly increase
the phosphorylation of GSK3β in DRG, and it is well established
that increased phosphorylation of GSK3β can dephosphorylate MAPs,
such as CRMP-2, MAP1B and APC, increasing their microtubule-binding
affinity and thereby stabilizing microtubules to promote axon
growth, it can be hypothesized that Y27632 may promote axon
regeneration through stabilizing microtubules mediated by
PI3K/Akt/GSK3β. While ROCK inhibition is well known to decrease MLC
phosphorylation and reduce actomyosin contractility, how the
PI3K/Akt/GSK3β axis intersects with actomyosin dynamics remains
unclear and warrants future investigation.
The current data also suggest that ROCK inhibition
promotes Schwann cell proliferation, migration, and myelin sheath
formation, all of which are essential for peripheral nerve
regeneration. These effects were also dependent on the
PI3K/Akt/GSK3β pathway. Notably, previous in vitro studies
have reported conflicting results regarding the role of ROCK
inhibition in Schwann cell-mediated myelination, with some
describing aberrant or discontinuous myelin formation (35). By contrast, the current in
vivo data consistently show enhanced remyelination and
functional recovery. These discrepancies may reflect
context-dependent differences between in vitro co-culture
systems and the more complex in vivo microenvironment.
A key limitation of the present study is the
reliance on systemic administration of pharmacological inhibitors,
which may have off-target effects. Although dosages were carefully
matched across groups, these compounds are not neuron-specific and
may influence other signaling cascades in peripheral or central
tissues. Future studies should employ cell type-specific
conditional knockout models to further dissect the roles of ROCK,
PI3K and GSK3β in distinct neural populations, such as neurons
versus Schwann cells.
In conclusion, the novelty of the present study lies
in identification of GSK3β as a critical downstream effector of
ROCK inhibition in the context of peripheral nerve regeneration.
Although previous studies have shown that ROCK inhibition can
activate PI3K/Akt signaling (11,12), the present study is, to the best
of the authors' knowledge, the first to demonstrate that
PI3K/Akt-mediated phosphorylation and inactivation of GSK3β are
required for the regenerative effects on both axon and myelin
sheath of ROCK inhibition. This elucidation of the downstream
pathway lays an important foundation for exploring pharmaceutical
agents targeting different nodes in this pathway individually or
combinatorially for improving therapeutic effects of PNI.
Availability of data and materials
The data generated in the present study may be
requested from the corresponding author.
Authors' contributions
SD and ZL were involved in the conceptualization of
the study, performed the experiments, analyzed the data, and
drafted the initial manuscript. FF contributed to the experimental
design and retrograde tracing experiments. BZ performed in
vitro experiments. ZR and HW provided expertise in clinical
relevance and functional recovery assessments. QZ contributed to
the histological and immunofluorescence analyses. YZ conceptualized
the study, supervised the study, acquired funding, provided
critical resources, interpretated the data, reviewed and edited the
manuscript. All authors read and approved the final version of the
manuscript. SD and YZ confirm the authenticity of all the raw
data.
Ethics approval and consent to
participate
All animal procedures were approved (approval no.
2024-Y-0566) by the Institutional Animal Care and Use Committee of
Fujian Medical University (Fuzhou, China) and complied with the
National Institutes of Health guidelines and ARRIVE standards.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
Use of artificial intelligence tools
During the preparation of this work, artificial
intelligence tools were used to improve the readability and
language of the manuscript or to generate images, and subsequently,
the authors revised and edited the content produced by the
artificial intelligence tools as necessary, taking full
responsibility for the ultimate content of the present
manuscript.
Acknowledgements
The authors gratefully thank Ms Ling Lin and Mr Xi
Lin from the public technology service center (Fujian medical
university, Fuzhou, China) for technical assistance.
Funding
The present study was supported by Fujian provincial fund for
joint scientific innovation (grant nos. 2024Y9096 and 2024Y9644)
and Fujian Natural Science Fund (grant nos. 2025J01689 and
2025J01091).
References
|
1
|
Lavorato A, Aruta G, De Marco R, Zeppa P,
Titolo P, Colonna MR, Galeano M, Costa AL, Vincitorio F, Garbossa D
and Battiston B: Traumatic peripheral nerve injuries: A
classification proposal. J Orthop Traumatol. 24:202023. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Chiono V and Tonda-Turo C: Trends in the
design of nerve guidance channels in peripheral nerve tissue
engineering. Prog Neurobiol. 131:87–104. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Yadav A and Dabur R: Skeletal muscle
atrophy after sciatic nerve damage: Mechanistic insights. Eur J
Pharmacol. 970:1765062024. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Miclescu A, Straatmann A, Gkatziani P,
Butler S, Karlsten R and Gordh T: Chronic neuropathic pain after
traumatic peripheral nerve injuries in the upper extremity:
Prevalence, demographic and surgical determinants, impact on health
and on pain medication. Scand J Pain. 20:95–108. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Sulaiman W and Gordon T: Neurobiology of
peripheral nerve injury, regeneration, and functional recovery:
from bench top research to bedside application. Ochsner J.
13:100–108. 2013.PubMed/NCBI
|
|
6
|
Wariyar SS and Ward PJ: Application of
electrical stimulation to enhance axon regeneration following
peripheral nerve injury. Bio Protoc. 13:e48332023. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Guo S, Moore RM, Charlesworth MC, Johnson
KL, Spinner RJ, Windebank AJ and Wang H: The proteome of distal
nerves: Implication in delayed repair and poor functional recovery.
Neural Regen Res. 17:1998–2006. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Liu J, Wada Y, Katsura M, Tozawa H, Erwin
N, Kapron CM, Bao G and Liu J: Rho-associated coiled-coil kinase
(ROCK) in molecular regulation of angiogenesis. Theranostics.
8:6053–6069. 2018. View Article : Google Scholar
|
|
9
|
Guan G, Cannon RD, Coates DE and Mei L:
Effect of the Rho-Kinase/ROCK signaling pathway on cytoskeleton
components. Genes (Basel). 14:2722023. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Fujita Y and Yamashita T: Axon growth
inhibition by RhoA/ROCK in the central nervous system. Front
Neurosci. 8:3382014. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Wang H, Fang F, Chen S, Jing X, Zhuang Y
and Xie Y: Dual efficacy of Fasudil at improvement of survival and
reinnervation of flap through RhoA / ROCK / PI3K / Akt pathway. Int
Wound J. 19:2000–2011. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Wang H, Fang F, Jing X, Xu D, Ren Z, Dou
S, Xie Y and Zhuang Y: Augmentation of functional recovery via
ROCK/PI3K/AKT pathway by Fasudil Hydrochloride in a rat sciatic
nerve transection model. J Orthop Transl. 47:74–86. 2024.
|
|
13
|
Zhang J, Yang S-G and Zhou F-Q: Glycogen
synthase kinase 3 signaling in neural regeneration in vivo. J Mol
Cell Biol. 15:mjad0752024. View Article : Google Scholar :
|
|
14
|
Ma Q, Chen G, Li Y, Guo Z and Zhang X: The
molecular genetics of PI3K/PTEN/AKT/mTOR pathway in the
malformations of cortical development. Genes Dis. 11:1010212024.
View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Li D, Qu Y, Mao M, Zhang X, Li J, Ferriero
D and Mu D: Involvement of the PTEN-AKT-FOXO3a pathway in neuronal
apoptosis in developing rat brain after hypoxia-ischemia. J Cereb
Blood Flow Metab. 29:1903–1913. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Kitagishi Y, Nakanishi A, Ogura Y and
Matsuda S: Dietary regulation of PI3K/AKT/GSK-3β pathway in
Alzheimer's disease. Alzheimers Res Ther. 6:352014. View Article : Google Scholar
|
|
17
|
Barnat M, Benassy M-N, Vincensini L,
Soares S, Fassier C, Propst F, Andrieux A, von Boxberg Y and
Nothias F: The GSK3-MAP1B pathway controls neurite branching and
microtubule dynamics. Mol Cell Neurosci. 72:9–21. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Leibinger M, Hilla AM, Andreadaki A and
Fischer D: GSK3-CRMP2 signaling mediates axonal regeneration
induced by Pten knockout. Commun Biol. 2:3182019. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Juanes MA, Isnardon D, Badache A,
Brasselet S, Mavrakis M and Goode BL: The role of APC-mediated
actin assembly in microtubule capture and focal adhesion turnover.
J Cell Biol. 218:3415–3435. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Shekarabi M, Robinson JA and Burdo TH:
Isolation and culture of dorsal root ganglia (DRG) from rodents.
Methods Mol Biol. 2311:177–184. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Schüler SC, Dumontier S, Rigaux J and
Bentzinger CF: Visualization of the skeletal muscle stem cell niche
in fiber bundles. Curr Protoc. 1:e2632021. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Bataille A, Le Gall C, Misery L and
Talagas M: Merkel cells are multimodal sensory cells: A review of
study methods. Cells. 11:38272022. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Fleming MS and Luo W: The anatomy,
function, and development of mammalian Aβ low-threshold
mechanoreceptors. Front Biol (Beijing). 8:408–420. 2013. View Article : Google Scholar
|
|
24
|
Assessing axonal degeneration in embryonic
dorsal root ganglion neurons in vitro. Methods in Molecular
Biology. Springer US; New York, NY: pp. 41–54. 2020
|
|
25
|
George E, Glass J and Griffin J:
Axotomy-induced axonal degeneration is mediated by calcium influx
through ion-specific channels. J Neurosci. 15:6445–6452. 1995.
View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Joshi AR, Bobylev I, Zhang G, Sheikh KA
and Lehmann HC: Inhibition of Rho-kinase differentially affects
axon regeneration of peripheral motor and sensory nerves. Exp
Neurol. 263:28–38. 2015. View Article : Google Scholar
|
|
27
|
Liu K, Tedeschi A, Park KK and He Z:
Neuronal intrinsic mechanisms of axon regeneration. Annu Rev
Neurosci. 34:131–152. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Hiraga A, Kuwabara S, Doya H, Kanai K,
Fujitani M, Taniguchi J, Arai K, Mori M, Hattori T and Yamashita T:
Rho-kinase inhibition enhances axonal regeneration after peripheral
nerve injury. J Peripher Nerv Syst. 11:217–224. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Saijilafu, Hur E-M, Liu C-M, Jiao Z, Xu
W-L and Zhou F-Q: PI3K-GSK3 signalling regulates mammalian axon
regeneration by inducing the expression of Smad1. Nat Commun.
4:26902013. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Hemmings BA and Restuccia DF: PI3K-PKB/Akt
Pathway. Cold Spring Harb Perspect Biol. 4:a0111892012. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Zhang X, Zhao G, Yang F, Li C, Lin W, Dai
H, Zhai L, Xi X, Yuan Q and Huo J: Transcriptional regulation
analysis provides insight into the function of GSK3β gene in
Diannan small-ear pig spermatogenesis. Genes (Basel). 15:6552024.
View Article : Google Scholar
|
|
32
|
Alfadil E, Bradke F and Dupraz S: In situ
visualization of axon growth and growth cone dynamics in acute ex
vivo embryonic brain slice cultures. J Vis Exp. 176:1–24. 2021.
|
|
33
|
Nakajima C, Sawada M, Umeda E, Takagi Y,
Nakashima N, Kuboyama K, Kaneko N, Yamamoto S, Nakamura H, Shimada
N, et al: Identification of the growth cone as a probe and driver
of neuronal migration in the injured brain. Nat Commun.
15:18772024. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Santos TE, Schaffran B, Broguière N, Meyn
L, Zenobi-Wong M and Bradke F: Axon growth of CNS neurons in three
dimensions is amoeboid and independent of adhesions. Cell Rep.
32:1079072020. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Melendez-Vasquez CV, Einheber S and Salzer
JL: Rho kinase regulates schwann cell myelination and formation of
associated axonal domains. J Neurosci. 24:3953–3963. 2004.
View Article : Google Scholar : PubMed/NCBI
|